Abstract
Congenital aphantasia is a recently characterized variation of experience defined by the inability to form voluntary visual imagery, in individuals who are otherwise high performing. Because of this specific deficit to visual imagery, individuals with aphantasia serve as an ideal group for probing the nature of representations in visual memory, particularly the interplay of object, spatial, and symbolic information. Here, we conducted a large-scale online study of aphantasia and revealed a dissociation in object and spatial content in their memory representations. Sixty-one individuals with aphantasia and matched controls with typical imagery studied real-world scene images, and were asked to draw them from memory, and then later copy them during a matched perceptual condition. Drawings were objectively quantified by 2,795 online scorers for object and spatial details. Aphantasic participants recalled significantly fewer objects than controls, with less color in their drawings, and an increased reliance on verbal scaffolding. However, aphantasic participants showed high spatial accuracy equivalent to controls, and made significantly fewer memory errors. These differences between groups only manifested during recall, with no differences between groups during the matched perceptual condition. This object-specific memory impairment in individuals with aphantasia provides evidence for separate systems in memory that support object versus spatial information. The study also provides an important experimental validation for the existence of aphantasia as a variation in human imagery experience.
Keywords: Mental imagery, Object Information, Spatial Information, False Memory, Memory Recall
1. Introduction
Visual imagery, the ability to form visual mental representations of objects or scenes that are not physically in front of us, is a common human cognitive experience, which has been difficult to characterize and quantify. What is the nature of the images that come to mind when forming mental representations of absent items, and are these even visual in nature? What might these representations look like if one lacks visual imagery? Aphantasia is a recently characterized variation in experience, defined by an inability to create voluntary visual mental images, although semantic memory and vision is reported to remain intact (Zeman, Dewar, & Della Sala, 2015; Keogh & Pearson, 2018). Aphantasia is still largely uncharacterized, with many of its studies based on case studies or employing small samples of individuals with congenital aphantasia (Zeman et al., 2015; Keogh & Pearson, 2018; Jacobs, Schwarzkopf, & Silvanto, 2018; Brons, 2019; Dawes, Keogh, Andrillon & Pearson, 2020), with few case studies of acquired aphantasia (e.g. Zeman et al., 2010; see also, Botez, Olivier, Vezina, Botez & Kaufman, 1985). Here, using an online crowd-sourced drawing task designed to quantify the content of visual memories (Bainbridge, Hall, & Baker, 2019), we examine the nature of aphantasics’ mental representations of visual stimuli within a large sample, and reveal differences in behavior for object and spatial imagery.
Although a first study describes individuals with an absence of mental imagery in the 19th century (Galton, 1880), the variation in experience has only recently been defined and named as aphantasia, and there has been very little formal investigation, with only six published studies (Zeman et al., 2015; Keogh & Pearson, 2018; Jacobs et al., 2018; Brons, 2019; Dawes et al., 2020; Zeman et al., 2020). This is arguably because most individuals with aphantasia can lead functional, ordinary lives, with many individuals realizing their imagery experience differed from the majority only in adulthood. The current method for identifying if an individual has aphantasia is through subjective self-report, using the Vividness of Visual Imagery Questionnaire (Marks, 1973). However, recent research has begun quantifying the experience using objective measures such as priming during binocular rivalry (Keogh & Pearson, 2018) and skin conductance during reading (Wicken et al., Unpublished results). Since its identification, several prominent figures have come forth describing their experience with aphantasia, including physicist Nicholas Watkins (Watkins, 2018), Firefox co-creator Blake Ross (Ross, 2016), and Ed Catmull, co-founder of Pixar and recently retired president of Walt Disney Animation Studios (Gallagher, 2019), leading to broader recognition of the experience.
Like prosopagnosia (Behrmann & Avidan, 2005), aphantasia is considered to be congenital in the majority of cases, because participants report that they have always experienced a lack of imagery (although it can also be acquired through trauma; Zeman et al., 2010; Thorudottir et al., 2020). A single-participant aphantasia case study found no significant difference from controls in a visual imagery task (judging the location of a target in relation to an imagined shape) nor its matched version of a working memory task, except at the hardest level of difficulty (Jacobs et al., 2018). However, individuals with aphantasia show significantly less imagery-based priming in a binocular rivalry task (Keogh & Pearson, 2018; Pearson, 2019), and show diminished physiological responses to fearful text as compared with controls (Wicken et al., Unpublished results). A recent self-report study has shown that individuals with aphantasia experience less rich autobiographical memories, with some but not all reporting decreased imagery in other sensory domains (Dawes et al., 2020; Zeman et al., 2020). While these studies have observed differences between individuals with aphantasia and controls, the nature of aphantasics’ mental representations during visual recall is still unknown. Understanding these differences in representation between individuals with aphantasia and controls could shed light on broader questions of what information (visual, spatial, symbolic) makes up a memory, and how this information compares to the initial perceptual trace. As individuals with aphantasia are selectively impaired only with imagery but not perception, this suggests perception and imagery do not reply upon identical neural substrates and representations (Dijkstra, Bosch, & van Gerven, 2019). Although this does not exclude the possibility of some overlap in the two processes, this acts as further evidence towards a growing body of work demonstrating key differences between imagery and perception (Lee, Kravitz, & Baker, 2012; Favila, Lee, & Kuhl, 2020; Bainbridge, Hall, & Baker, 2020). Examination into aphantasia thus has wide-reaching potential implications for the understanding of the way we form mental representations of our world.
The nature and content of our visual imagery has proven very difficult to quantify. Several studies in psychology have developed tasks to objectively study the cognitive process of mental imagery through visual working memory or priming (e.g., Marmor & Zaback, 1976; Keogh & Pearson 2011). The difficulty in objectively quantifying the imagery experience led to a long-standing debate within the imagery literature over the nature of images, and specifically whether visual imagery representations are depictive and picture-like in nature (Kosslyn, 1980; Kosslyn 2005) or symbolic, “propositional” representations (Pylyshyn, 1981; Pylyshyn, 2003). Neuropsychological research, especially in neuroimaging, has led to large leaps in our understanding of visual imagery. Studies examining the role and activation of the primary visual cortex during imagery tasks have been interpreted as supporting the depictive nature of imagery (Ishai, Ungerleider, & Haxby, 2000; Kosslyn, Ganis, & Thompson, 2001; Schacter et al., 2012; Pearson & Kosslyn, 2015). However, neuropsychological studies have identified patients with dissociable impairments in perception versus imagery (Behrmann, 2000; Bartolomeo, 2008), and recent neuroimaging work has suggested there may be systematically related yet separate cortical areas for perception and imagery, and that the neural representation during imagery may lack much of the richer, elaborative processing of the initial perceptual trace (Lee et al., 2012; Xiao et al., 2017; Silson et al., 2019; Favila, et al., 2020; Bainbridge, Hall, & Baker, 2020). Combined with research identifying situations where propositional encoding dominates spatial imagery (e.g., Stevens & Coupe, 1978), researchers have concluded that there is a role for both propositional and depictive elements in the imagery process (e.g., Denis & Cocude, 1989). In their case study, Jacobs and colleagues (2018) argue that differences in performance between aphantasic participant AI and neurotypical controls may result from different strategies, including a heavier reliance on propositional encoding, relying on a spatial or verbal code. Thus, ideally a task that measures both depictive (visual) and propositional (symbolic) elements of a mental representation could directly compare the strategies used by aphantasic and control participants. In a recent study, impressive levels of both object and spatial detail could be quantified by drawings made by neurotypical adults in a drawing-based visual memory experiment (Bainbridge et al., 2019). The amount of detail included in these memory drawings far surpassed the amount of detail recalled in a matched verbal memory task, suggesting that this drawing task specifically taps into visual mental representations of an item. Such drawings allow a more direct look at the information within one’s mental representation of a visual image, in contrast to verbal descriptions or recognition-based tasks. Thus, a drawing task may allow us to identify what fundamental differences exist between individuals with aphantasia and typical imagery, and in turn inform us of what information exists within imagery.
In the current study, we examine the visual memory representations of individuals with congenital aphantasia and typical imagery (controls) for real-world scene images. Through online crowd-sourcing, we leverage the power of the internet to identify and recruit large numbers of both aphantasic (VVIQ ≤ 25) and controls (≥ 40) for a memory drawing task. We also recruit over 2,700 online scorers to objectively quantify these drawings for object details, spatial details, and errors in the drawings. We discover a selective impairment in aphantasic participants for object memory, with significantly fewer visual details and evidence for increased verbal scaffolding. In contrast, for the items that they remember, aphantasic participants show spatial accuracy at the same high level of precision as controls. Aphantasic participants also show fewer memory errors and memory correction as compared to controls. These results add to a growing body evidence for two separate systems that support object information versus spatial information in memory.
2. Materials and Methods
2.1. Participants
N=123 adults participated in the main online drawing recall experiment, while 2,795 adults participated in online scoring experiments on Amazon Mechanical Turk (AMT) of the drawings from the main experiment. Aphantasic participants for the main experiment were recruited from aphantasia-specific online forums, including “Aphantasia (Non-Imager/Mental Blindness) Awareness Group”, “Aphantasia!” and Aphantasia discussion pages on Reddit. Control participants for the main experiment were recruited from the population at the University of Westminster, online social media sites such as Facebook and Twitter pages for the University of Westminster Psychology, and “Participate in research” pages on Reddit. Scoring participants were recruited from the general population of AMT.
Participant group membership was confirmed by their score on the Vividness of Visual Imagery Questionnaire (VVIQ), a self-report measure of the vividness of one’s visual mental images (Marks, 1973). Scores on the VVIQ range from 16 to 80. Although aphantasia is currently determined by scores on the VVIQ (e.g., Zeman et al., 2015; Jacobs et al., 2017; Dawes et al., 2020; Zeman et al., 2020), there is currently no agreed cut-off to classify an experience as aphantasic or not. Some studies have used a cut off of 32 (e.g. Dawes et al., 2020; Wicken et al., Unpublished Results). Recently others have begun to take a more conservative approach in an attempt to distinguish between the extreme of aphantasia (no imagery experience) and self-reports of limited imagery experience (e.g. Zeman et al., 2020). Where it is addressed at all, classification of “typical” imagery experience also varies within aphantasic research (Keogh & Pearson 2017; Zeman et al., 2020). The VVIQ was not developed as a clinical tool, and as such there is limited normative date on “normal” imagery experience in the general population. In a meta-analysis, McKelvie (1995) suggested that the population mean VVIQ was 59.2 (SD = 11.07). He also identified a low-imagery group, for whom the mean score was 49.6 (SD = 9.04). In this study, aphantasia was defined by VVIQ scores ≤ 25 (M = 16.87, SD = 2.16), a particularly conservative cut-off to ensure we were specifically studying those with incredibly low imagery. Control participants had VVIQ scores ≥ 40 (M = 60.10, SD = 8.62), which are in line with the mean VVIQ scores found within the meta-anlysis of ‘normal’ imagery experience (McKelvie, 1995). Eight participants were removed from the analyses for having scores between 26 and 39. Some participants skipped questions in the VVIQ, likely due to mis-clicks on the online interface or fatigue at the end of the experiment. Two participants skipped over 25% of the questions on the VVIQ, and were removed from the analyses. Of the remaining aphantasic participants, four skipped one question, one skipped two questions, and one skipped three questions. Of the remaining control participants, five skipped one question, and one skipped three questions. None of these small errors were enough to change the group membership of these participants (regardless of how they might have answered these questions), and their data were retained for the analyses. There were 61 aphantasic and 52 control participants in total for the final analyses.
No personally identifiable information was collected from any participants, and participants had to acknowledge participation in order to continue, following the guidelines approved by the University of Westminster Psychology Ethics Committee (ETH1718-2345) and the National Institutes of Health Office of Human Subjects Research Protections (18-NIMH-00696).
2.2. Main Experiment: Drawing Recall Experiment
The Drawing Recall Experiment was a fully online memory experiment that consisted of five sections ordered: 1) study phase, 2) recall drawing phase, 3) recognition phase, 4) copied drawing (perception) phase, and 5) questionnaires and demographics. The methods of the experiment are summarized in Fig. 1. The experiment was programmed in a standard text editor, using HTML, Javascript, and CSS, and participant submissions were saved to a web server using PHP and a MySQL server-side database. Participants saw the experiment as a standard web page. The drawing tool was adapted from open source Javascript plugin wPaint (http://wpaint.websanova.com/). All code and drawing data, as well as a tutorial on how to code similar online experiments from the ground up, can be downloaded from the Open Science Framework (https://osf.io/cahyd/).
Fig. 1.
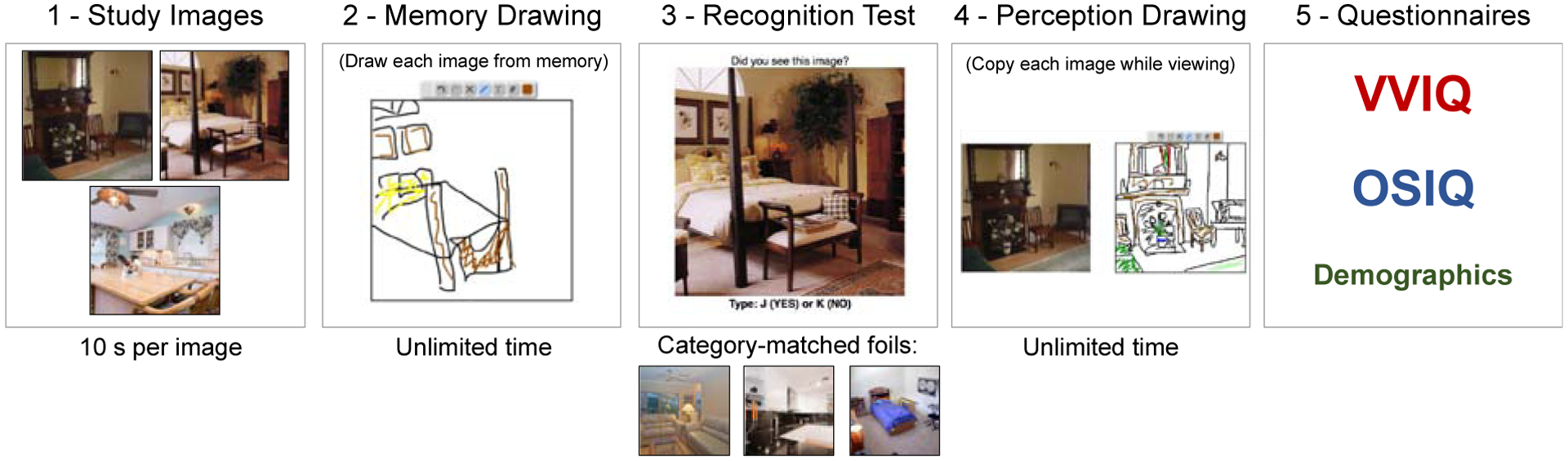
The experimental design of the online experiment. Participants 1) studied three separate scene photographs presented sequentially, 2) drew them from memory, 3) completed a recognition task, 4) copied the images while viewing them, and then 5) filled out the VVIQ and OSIQ questionnaires in addition to demographics questions. The whole experiment took approximately 30 minutes.
First, for the study phase, participants were told to study three images in as much detail as possible. The images were presented at 500 × 500 pixels. They were shown each image for 10 s, presented in a randomized order with a 1 s interstimulus interval (ISI). These three images (see Fig 1a) were selected from a previously validated memory drawing study (Bainbridge et al., 2019), as the images with the highest recall success, highest number of objects, and several unique elements compared to a canonical representation of its category. For example, the kitchen scene does not include several typical kitchen components such as a refrigerator, microwave, or stove, and does include more idiosyncratic objects such as a ceramic chef, zebra-printed chairs, and a ceiling fan. This is important as we want to assess the ability to recall unique visual information beyond just a coding of the category name (e.g., just drawing a typical kitchen). Participants were not informed what they would do after studying the images, to prevent targeted memory strategies.
Second, the recall drawing phase tested what visual memory representations participants had for these images through drawing. Participants were presented with a blank square with the same dimensions as the original images and told to draw an image from memory in as much detail as possible using their mouse. Participants drew using an interface like a simple paint program. They could draw with a pen in multiple colors, erase lines, and undo or redo actions. They were given unlimited time and could draw the images in any order. They were also instructed that they could write labels for any unclear items (e.g., indicate that a specific scribble is a chair). Once a participant finished a drawing, they then moved onto another blank square to start a new drawing. They were asked to create three drawings from memory, and could not go back to edit previous drawings. As they were drawing, their mouse movements were recorded to track timing and erasing behavior. These drawings were later quantified by online scorers in a series of separate experiments (see Section 2.3 below).
Third, the recognition phase tested whether there was visual recognition memory for these specific images. Participants viewed images and were told to indicate whether they had seen each image before or not. The images consisted of the three images presented in the study phase as well as three new foil images of the same scene categories (kitchen, bedroom, living room). Matched foils were used so that recognition performance could not rely on recognizing the category type alone. All images were presented at 500 × 500 pixels. Participants were given unlimited time to view the image and respond, and a fixation cross appeared between each image for 200 ms.
Fourth, the copied drawing phase had participants copy the drawings while viewing them, in order to see how participants perceive each image in the absence of a memory task. This phase provides an estimate of the participant’s drawing ability and ability to use this drawing interface with a computer mouse to create drawings. This phase also measures the maximum information one might draw for a given image (e.g., you won’t draw every plate stacked in a cupboard). Participants saw each image from the study phase presented next to a blank square. They were instructed to copy the image in as much detail as possible, resulting in a “perception drawing”. The blank square used the same interface as the recall drawing phase. When they were done, they could continue onto the next image, until they copied all three images from the study phase. The images were tested in a random order, and participants had as much time as they wanted to draw each image, but could not go back to any completed drawings.
Finally, participants filled out three questionnaires at the end. They completed the previously mentioned VVIQ (Marks, 1973), which was mainly used to determine participant group membership. Participants also completed the more recent Object and Spatial Imagery Questionnaire (OSIQ) (Blajenkova, Kozhevnikov, & Motes, 2006), which measures visual imagery preference for object information and spatial information, providing a score between 15–75 for each subscore (object, spatial). Finally, participants provided basic demographics, basic information about their computer interface, and their experience with art. In these final questions, they indicated which component of the experiment was most difficult, and were able to write comments on why they found it difficult.
2.3. Online Scoring Experiments
In order to objectively and rapidly score the 655 drawings produced in the Drawing Recall Experiment, we conducted online crowd-sourced scoring experiments with a set of 2,795 participants on AMT, an online platform used for crowd-sourcing of tasks. None of these participants took part in the Drawing Recall Experiment. For all online scoring experiments, scorers could participate in as many trials as they wanted, and were compensated for their time. Scorers did not know the nature or origin of the drawings; they did not know these drawings related to a study of aphantasia and that the drawings came from different groups of people.
2.3.1. Object Selection Study
AMT scorers were asked to indicate which objects from the original images were in each drawing. This allows us to systematically measure how many and what types of objects exist in the drawings. They were presented with one drawing and five photographs of the original image with a different object highlighted in red. They had to click on all object images that were contained in the original drawing. Five scorers were recruited per object, with 909 unique scorers in total. An object was determined to exist in the drawing if at least 3 out of 5 scorers selected it.
2.3.2. Object Location Study
For each object, AMT scorers were asked to place and resize an oval around that object in the drawing, in order to get information on the location and size accuracy of the objects in the drawings. AMT scorers were instructed on which object to circle in the drawing by the original image with the object highlighted in red, and only objects selected in the Object Selection Study were used. Five scorers were recruited per object, with 1,310 unique scorers in total. Object location and size (in both the x and y directions) were taken as the median pixel values across the five scorers.
2.3.3. Object Details Study
AMT scorers indicated what details existed in the specific drawings. In a first AMT experiment, five scorers per object (N=304 total) saw each object from the original images and were asked to list 5 unique traits about the object (e.g., shape, material, pattern, style). A list of unique traits was then created for each object in the images. In a second AMT experiment, scorers were then shown each object in the drawings (highlighted by the ellipse drawn in the Object Location Study), and had to indicate whether that trait described the drawn object or not. Five scorers were recruited per trait per drawn object, with 777 unique scorers in total.
2.3.4. False Objects Study
AMT scorers were asked to indicate “false objects” in the drawings—what objects were drawn in the drawing that didn’t exist in the original image? Scorers were shown a drawing and its corresponding image and were asked to write down a list of all false objects. Nine scorers were recruited per drawing, with 337 unique scorers in total. An object was counted as a false object if at least three scorers listed it.
2.4. Additional Drawing Scoring Metrics and Analyses
In addition to the Online Scoring Experiments (Section 2.3), other attributes were collected for the drawings. A blind scorer (the corresponding author) viewed each drawing presented in a random order (without participant or condition information visible) and coded yes or no for if the drawing 1) contained any color, 2) contained any text, and 3) contained any erasures. Erasures were quantified by viewing the mouse movements used for drawing the image, to see if lines were drawn and then erased, and did not make it into the final image.
Throughout this manuscript, whenever parametric statistical tests were used to compare groups, we first confirmed the measures were not significantly different from a normal distribution, using the Kolmogorov-Smirnov test of goodness-of-fit.
3. Results
With these memory and perceptual drawings, we can then make direct comparisons in the types of detail, amounts of detail, and types of errors that may differ between aphantasic and control participants . First, we examine the demographic measures between the two groups, such as age, gender, art ability, and ratings on the OSIQ. Second, we turn to objective quantification of the drawings, and explore differences in the objects drawn by aphantasic and control participants and text-based strategies. Third, we compare spatial accuracy in the drawings between these two groups. Finally, we compare the presence of memory errors, quantifying the number of falsely inserted additional objects.
3.1. No demographic differences between groups, but reported differences in object and spatial imagery
First, we analyzed whether there were demographic differences between the groups. There was a significant difference in age between groups with aphantasic participants generally older than controls (aphantasic: M=41.88 years, SD=13.88, Range=18 to 74 years; control: M=32.12 years, SD=15.26, Range=18 to 75 years; t(107)=3.49, p=6.95 × 10−4). To ensure the effects we report are not simply due to age differences, we also ran all of the following analyses using a sub-sampled set of aphantasic and control participants with matched age distributions (Supplementary Material 1). All main results replicated even when controlling for age, indicating that the results reported in this manuscript are due to imagery differences, and not age differences between groups. There was no significant difference in gender proportion between the two groups (aphantasic: 62.3% female; control: 59.6% female; Pearson’s chi-square test for proportions: χ2=0.08, p=0.771), even though a previous study reported a sample comprising of predominantly males (Zeman et al., 2015).
Second, we investigated the relationship of the VVIQ score and OSIQ (Fig. 2), a questionnaire developed to separate abilities to perform imagery with individual objects versus spatial relations amongst objects (Blajenkova et al., 2006). Controls scored significantly higher on the OSIQ than aphantasic participants (t(103) = 12.70, p=8.55 × 10−23, effect size Cohen’s d=2.48). There was a significant correlation between VVIQ score and OSIQ score for control participants (M=89.73, SD=10.97; Spearman rank-correlation test: ρ=0.54, p=7.70 × 10−5), but only marginally for aphantasic participants (OSIQ M score=62.88, SD=10.65; ρ=0.26, p=0.052). When broken down by OSIQ subscale, there was a significant difference between groups in questions relating to object imagery (t(103)=20.00, p=3.01 × 10−37, d=3.80), but not spatial imagery (t(103)=−0.33, p=0.742). Indeed, a 2-way ANOVA (participant group × subscale) reveals a main effect of participant group (F(1,206)=154.97, p~0, effect size ηp2=0.43), subscale (F(1,206)=40.11, p=1.48 × 10−9, ηp2=0.16), and a significant interaction (F(1,206)=167.94, p~0, ηp2=0.45), confirming a difference in self-reported ratings for object imagery and spatial imagery respectively. This difference in self-reported object imagery and spatial imagery has been reported a previous study (Keogh & Pearson, 2018), and suggests a potential difference between the two imagery subsystems.
Figure 2. Experimental paradigm and basic demographics.
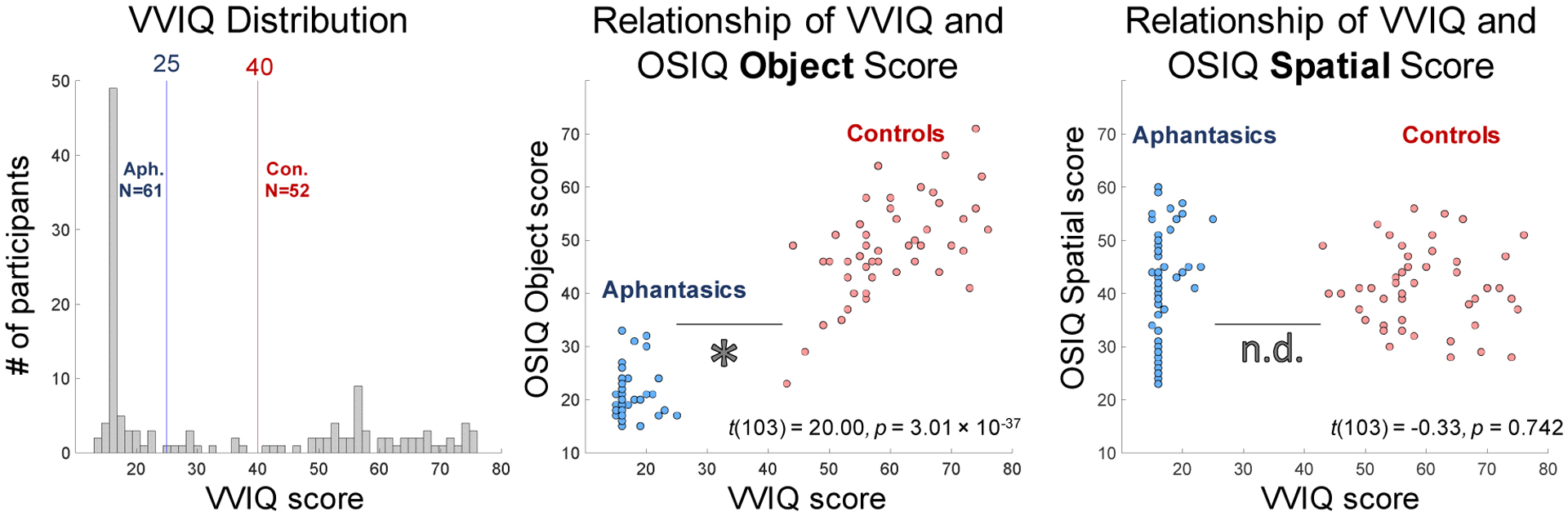
a) b) (Left) A histogram of the distribution of participants across the VVIQ. Aphantasic participants were selected as those scoring 25 and below (N=61) and controls were selected as those scoring 40 and above (N=52), while those in between were removed from the analyses (N=8). While the range of the VVIQ is from 16 to 80, some participants (N=10 out of 121 total) skipped 1–3 questions, leading to some participants scoring below 16. These skipped questions did not affect group membership. (Middle) A scatterplot of total VVIQ score plotted against total OSIQ Object component score for participants meeting criterion. Each point represents a participant, with aphantasic participants in blue and controls in red. There was a significant difference in OSIQ Object score between the two groups. (Right) A scatterplot of total VVIQ score plotted against OSIQ Spatial component score. There was no difference in OSIQ Spatial score between the two groups. Both the OSIQ Object component and Spatial components have a range of 15 to 75 points.
Third, we investigated whether aphantasic and control participants reported different levels of comfort or familiarity with art, which may influence their drawing performance. When asked to rate their artistic abilities on a scale from 1 (very poor) to 5 (very good), aphantasic and control participants showed no significant difference in their ratings (aphantasic: M=2.30, SD=1.34; control: M=2.52, SD=0.99; non-parametric Wilcoxon rank sum test: Z=1.23, p=0.219). Both aphantasic and control participants also reported taking art classes in the past (39.34% of aphantasic participants, 37.74% of controls). When asked to list occupation, many aphantasic participants (13.11%) reported being employed within industries involving artistic abilities, such as sculpting, visual arts, makeup art, and interior decoration. In contrast, surprisingly none of the control participants reported being employed in artistic fields (instead with occupations such as software developer, patent attorney, librarian, sales associate). That being said, these occupational differences should not be over-interpreted as we did not explicitly aim to sample a broad set of occupations. However, overall, aphantasic and control participants in the current sample did not show strong differences in their propensity for, or interest in, art.
Finally, given the focus of the current experiment on visual recall, we also compared measures of visual recognition performance. Both groups performed near ceiling at visual recognition of the images they studied, with no significant difference between groups in recognition hit rate (control: M=0.96, SD=0.12; aphantasic: M=0.97, SD=0.12; Wilcoxon rank-sum test: Z=1.09, p=0.274), or false alarm rate (control: M=0.02, SD=0.12; aphantasic: M=0, SD=0; Wilcoxon rank-sum test: Z=1.10, p=0.273). These results indicate that there is no evidence for a deficit in aphantasic participants for recognizing images within this element of the task, even with lures from the same semantic scene category. That being said, this recognition task may not have been challenging enough to highlight potential underlying differences between groups.
3.2. Diminished object information for aphantasics
Next, we turned to analyzing the drawings made by the participants to reveal objective measures of the mental representations of these two groups. Looking at overall number of drawings made, while a small number of participants could not recall all three images, there was no significant difference between groups in number of images drawn from memory (control: M=2.92, SD=0.27; aphantasic: M=2.89, SD=0.37; Wilcoxon rank-sum test: Z=0.42, p=0.678). Example drawings can be seen in Fig. 3.
Figure 3. Example drawings.

Example drawings made by aphantasic and control participants from memory and perception (i.e., copying the image) showing the range of performance. The memory and perception drawings connected by arrows are from the same participant, and every row is from a different participant. Low memory examples show participants who drew the fewest from memory but the most from perception. High memory examples show participants who drew the highest amounts of detail from both memory and perception. These examples are all circled in the scatterplot of Fig. 4. The key question is whether there are meaningful differences between these two sets of participants’ drawings.
To score level of object information, AMT workers (N=5 per object) identified whether each of the objects in an image was present in each drawing of that image (Fig. 4). A 2-way ANOVA of participant group (aphantasic / control) × drawing type (memory / perception drawing, repeated measure) looking at number of objects drawn per image showed no significant overall effect of participant group (F(1,223)=0.26, p=0.613), but a significant effect of drawing type (F(1,223)=507.03, p~0, ηp2=0.82), and more importantly, a significant statistical interaction (F(1,223)=9.25, p=0.0029, ηp2=0.08). Targeted post-hoc independent t-tests revealed that when drawing from memory, controls drew significantly more objects (M=6.32 objects per image, SD=3.07) than aphantasic participants (M=4.98, SD=2.54; t(111)=2.53, p=0.013, d=0.47) across the experiment. In contrast, when copying a drawing (perception drawing), aphantasic participants on average drew more objects from the images than controls, but with no significant difference (control: M=18.00 objects per image, SD=5.81; aphantasic: M=20.07, SD=7.26; t(111)=1.74, p=0.085). These results suggest that aphantasic participants are showing a specific deficit in recalling object information during memory.
Figure 4. Comparison of object information in drawings between aphantasic and control participants.
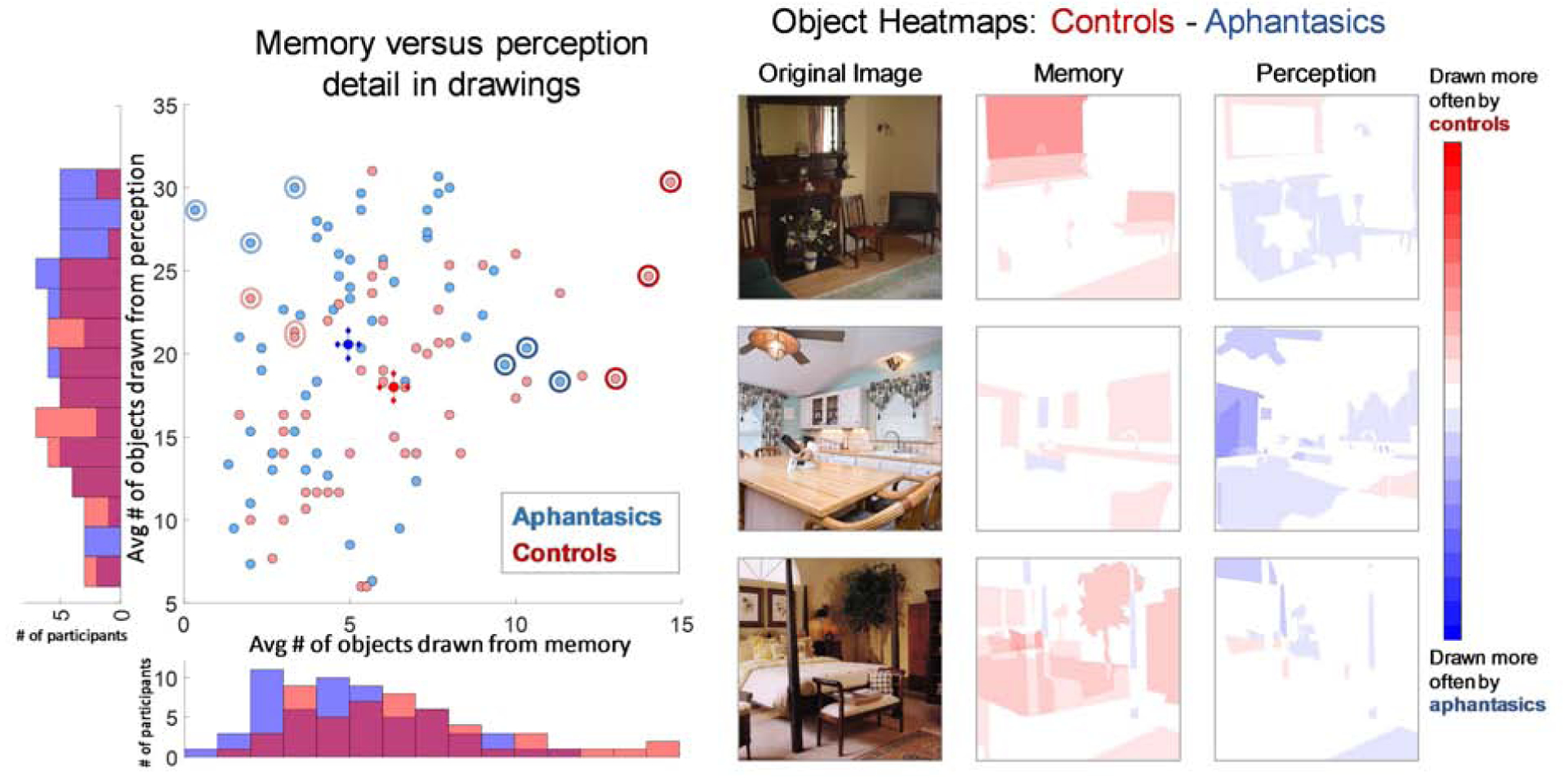
(Left) A scatterplot of each participant as a point, showing average number of objects drawn from memory across the three images (x-axis), versus average number of objects drawn from perception across the three images (y-axis). Aphantasic participants are in blue, while control participants are in red. The bright blue circle indicates average aphantasic performance, while the bright red circle indicates average control performance, with crosshairs for both indicating standard error of the mean for memory and perception respectively. Histograms on the axes show the number of participants who drew each number of objects. Controls drew significantly more objects from memory, although with a tendency towards fewer from perception. The circled light blue and red points are the participants with the lowest memory performance shown in Fig. 3, while the circled dark blue and red points are the participants with the highest memory performance shown in Fig. 3. (Right) Heatmaps of which objects for each image tended to be drawn more by controls (red) or aphantasic participants (blue). Pixel value represents the proportion of control participants who drew that object in the image subtracted by the proportion of aphantasic participants who drew that object (with a range of −1 to 1). Controls remembered more objects (i.e., there is more red in the memory heatmaps), even though aphantasic participants tended to copy more objects (i.e., there is more blue in the perception heatmaps).
Given that some participants tended to draw few objects even when copying from an image, we also investigated a corrected measure, taken as the number of objects drawn from memory divided by the number of objects drawn from perception, for each image for each participant. Drawings from perception with fewer than 5 objects were not included in the analysis, to remove any low-effort trials. Aphantasic participants drew a significantly smaller proportion of objects from memory than control participants (aphantasic: M=0.261, SD=0.165; control: M=0.369, SD=0.162; Wilcoxon rank-sum test: Z=4.09, p=4.24 × 10−5, effect size r=0.39). We also investigated the correlation within groups between the number of objects drawn from memory and the number drawn from perception. There was a significant correlation for both groups, where the more one draws from perception, the more one also tends to draw from memory (Pearson correlation; aphantasic: r=0.34, p=0.0075; control: r=0.40, p=0.0035). We also assessed the relationship between performance in the task and self-reported object imagery in the OSIQ. Across groups, there was a significant correlation between proportion of objects drawn from memory and OSIQ object score (Spearman’s rank correlation: ρ=0.33, p=7.18 × 10−4), although these correlations were not significant when separated by participant group (p>0.10).
Next, we examined whether there was a difference in visual detail within objects, by quantifying differences between groups in color and amount of time spent on the drawings. Significantly more memory drawings by controls contained color than those by aphantasic participants (control: 38.2%, aphantasic: 21.6%; Pearson’s chi-square test for proportions: χ2=10.09, p=0.0015, effect size φ=0.18), while there was no significant difference for perception drawings (control: 46.2%, aphantasic: 39.4%, χ2=1.46, p=0.227). Control participants also spent significantly longer time on their memory drawings than aphantasic participants (control: M= 119.41 s per image, SD=68.88 s; aphantasic: M=71.22 s, SD=49.17 s; t(110) = 4.31, p=3.56 × 10−5, d=0.81). For the perception drawings, there was no significant difference between groups in the amount of time they spent on their drawings (control: M=272.33 s, SD=214.17 s; aphantasic: M=295.18 s, SD=304.54 s; p=0.654). These differences in time spent on memory drawing could reflect controls spending more time because they drew more objects from memory. However, even if we normalize total drawing time by number of objects drawn to get an estimate of average time spent per object, controls spent significantly more time per object than aphantasic participants when drawing from memory (Wilcoxon rank sum test: Z=2.09, p=0.037, r=0.20), but not when drawing during perception (Z=0.75, p=0.454). This implies that aphantasic participants not only spent less time per drawing, but also less time on the details for each object. Finally, we investigated other forms of object detail, by having AMT workers (N=777) judge whether different object descriptors (e.g., material, texture, shape, aesthetics; generated by 304 separate AMT workers) applied to each drawn object. This task did not identify differences between groups for the memory drawings (t(110)=0.21, p=0.833), although objects were significantly more detailed when copied than when drawn from memory for both aphantasic (memory: M=42.4% descriptors per object applied, SD=5.1%; copied: M=45.9%, SD=4.1%; t(119)=4.12, p=6.92 × 10−5, d=0.76) and control participants (memory: M=42.2%, SD=5.6%; copied: M=47.0%, SD=3.9%; t(100)=5.06, p=1.92 × 10−6, d=0.99). However, it is possible this task may have required information that was too fine-grained than could be measured from these drawings (e.g., judging the material and texture of a drawn chair).
In sum, these results present concrete evidence that aphantasic recall fewer objects than control participants, and these objects contain less visual detail (i.e., color, less time spent for drawing) within their memory representations.
3.3. Aphantasics show greater dependence on symbolic representations
While aphantasic participants show decreased object information in their memory drawings, they are still able to successfully draw some objects from memory (4.98 objects per image on average). Do these drawings reveal evidence for alternative, non-visual strategies that may have supported this level of performance? To test this question, we quantified the amount of text used to label objects included in the participants’ drawings. Note that while labeling was allowed (the instructions stated: “Please draw or label anything you are able to remember”), it was effortful as it required drawing the letters with the mouse. We found that significantly more memory drawings by aphantasic participants contained text than those by control participants (aphantasic: 29.6%, control: 16.0%; χ2=7.57, p=0.0059, φ=0.16). Further, there was no significant difference between groups for perception drawings (aphantasic: 2.9%, control: 0.8%; χ2=1.77, p=0.184). These results imply that aphantasic participants may have relied upon symbolic representations (Pylyshyn, 1981), rather than pictoral, to support their memory.
One question is whether aphantasic participants just prefer writing over drawing, and so prioritized time or effort on writing text over drawing objects. To elaborate, it is possible that aphantasic participants expend their effort on writing text, and then do not want to spend further time on drawing objects even if they might have object information in memory. If this were the case, then drawings that contain text should contain fewer objects. However, we found there was no significant difference in number of objects between aphantasic memory drawings with text and without (independent samples t-test by drawing: t(174)=0.07, p=0.947). There was also no significant difference for their drawings made during perception (t(171)=0.35, p=0.726), nor were there differences for controls (memory drawings: t(150)=0.004, p=0.997; perception drawings: t(152)=1.50, p=0.135). These results indicate that the usage of text was not a trade-off with object memory; aphantasic participants preferred to include text in their memory drawings regardless of how many objects they recalled.
Comments by aphantasic participants at the end of the experiment supported their use of symbolic strategies. When asked what they thought was difficult about the task, one participant noted, “Because I don’t have any images in my head, when I was trying to remember the photos, I have to store the pieces as words. I always have to draw from reference photos.” Another aphantasic stated, “I had to remember a list of objects rather than the picture,” and another said, “When I saw the images, I described them to myself and drew from that description, so I… could only hold 7–9 details in memory.” In contrast, control participants largely commented on their lack of confidence in their drawing abilities: e.g., “I am very uncoordinated so making things look right was frustrating”; “I can see the picture in my mind, but I am terrible at drawing.”
3.4. Aphantasics and controls show equally high spatial accuracy in memory
While aphantasic participants show an impairment in memory for object information, do they also show an impairment in spatial placement of the objects? To test this question, AMT workers (N=5 per object) drew an ellipse around the drawn version of each object, allowing us to quantify the size and location accuracy of each drawn object (Fig. 5). When drawing from memory, there was no significant difference between groups in object location error in the x-direction (aphantasic: M pixel error=63.86, SD=31.59; control: M=60.63, SD=28.45; t(111)=0.57, p=0.572) nor the y-direction (aphantasic: M=65.43, SD=29.89; control: M=69.10, SD=29.72; t(111)=0.65, p=0.515). However, this lack of difference was not due to difficulty in spatial accuracy; both groups’ drawings were highly spatially accurate, with all average errors in location less than 10% of the size of the images themselves. Similarly, there was also no significant difference in drawn object size error in terms of width (aphantasic: M pixel error=23.00, SD=10.95; control: M=24.89, SD=13.58; t(111)=0.82, p=0.413) and height (aphantasic: M=26.75; SD=14.15; control: M=22.82; SD=11.05; t(111)=1.62, p=0.107), and these sizes were highly accurate in both groups (average errors less than 4% of the image size). There was no correlation between a participant’s level of object location or size error and ratings on the OSIQ spatial questions (all p>0.30). In all, these results show that both aphantasic and control participants have highly accurate memories for spatial location, with no observable differences between groups.
Figure 5. Average object locations and sizes recalled by aphantasics and controls.
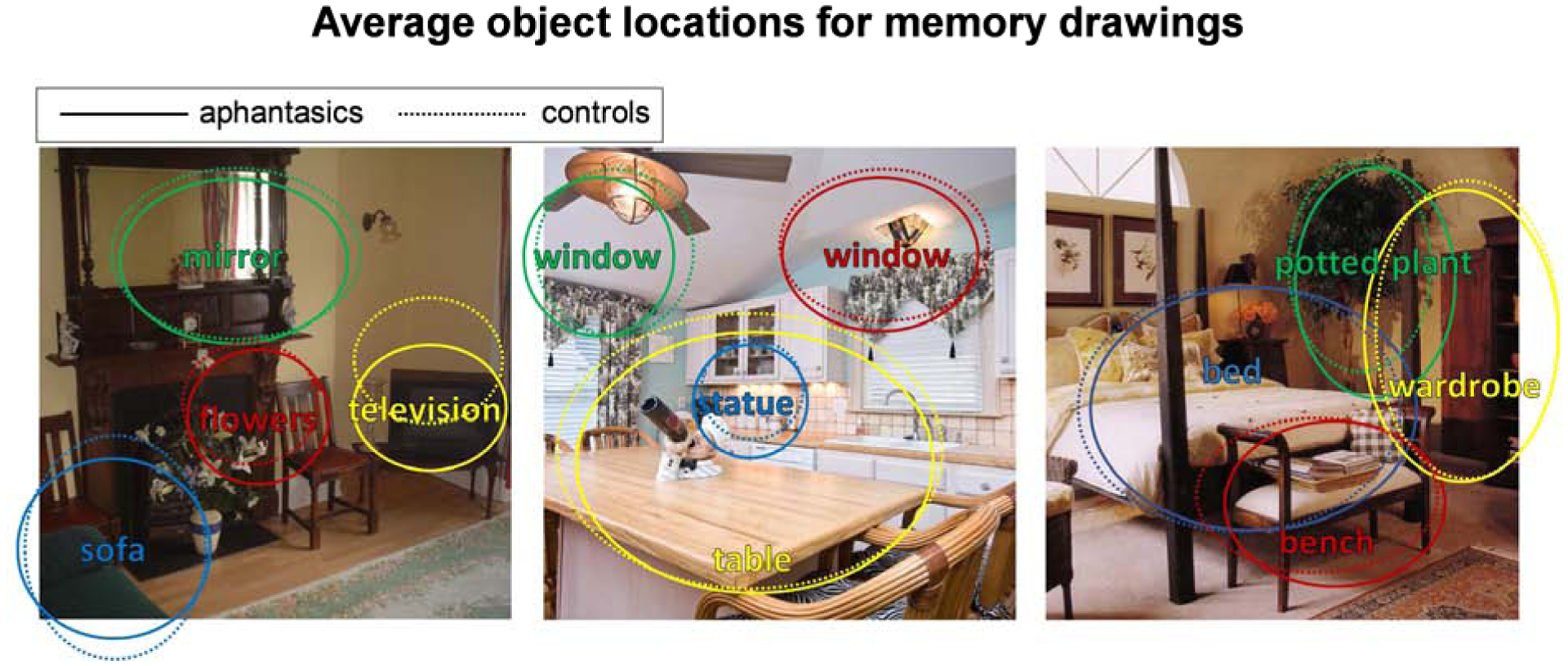
Average object locations and sizes for memory drawings of four of the main objects from each image, made by aphantasic participants (solid lines) and control participants (dashed lines). Even though these objects were drawn from memory, their location and size accuracy was still very high. Importantly, aphantasic and control participants showed no significant differences in object location or size accuracy.
3.5. Aphantasics draw fewer false objects than controls
Finally, we quantified the amount of error in participants’ drawings from memory by group. AMT workers (N=5 per drawing) viewed a drawing and its corresponding image and wrote down all objects in the drawings that were not present in the original image (essentially quantifying false object memories). Significantly more memory drawings by controls contained false objects than drawings by aphantasic participants (control: 14 drawings, aphantasic: 3 drawings; Pearson chi-square test: χ2=9.35, p=0.002, φ=0.18); examples can be seen in Fig. 6. This is not just because controls drew more objects overall and were thus more likely to draw false objects. If we also look at proportion of total objects drawn by each group that were false objects, significantly more objects drawn by controls were false objects than those drawn by aphantasic participants (χ2=6.37, p=0.012, φ=0.06). This indicates that control participants were making more memory errors, even after controlling for the fewer number of objects drawn overall by aphantasic participants. Interestingly, all aphantasic errors (see Fig. 6) were transpositions from another image and drawn in the correct location as the original object (a tree from the bedroom to the living room, a window from the kitchen to the living room, and a ceiling fan from the kitchen to the bedroom). In contrast, several false memories from controls were objects that did not exist across any image but instead appeared to be filled in based on the scene category (e.g., a piano in the living room, a dresser in the bedroom, logs in the living room). No perception drawings by participants from either group contained false objects.
Figure 6. False object memories in the drawings.
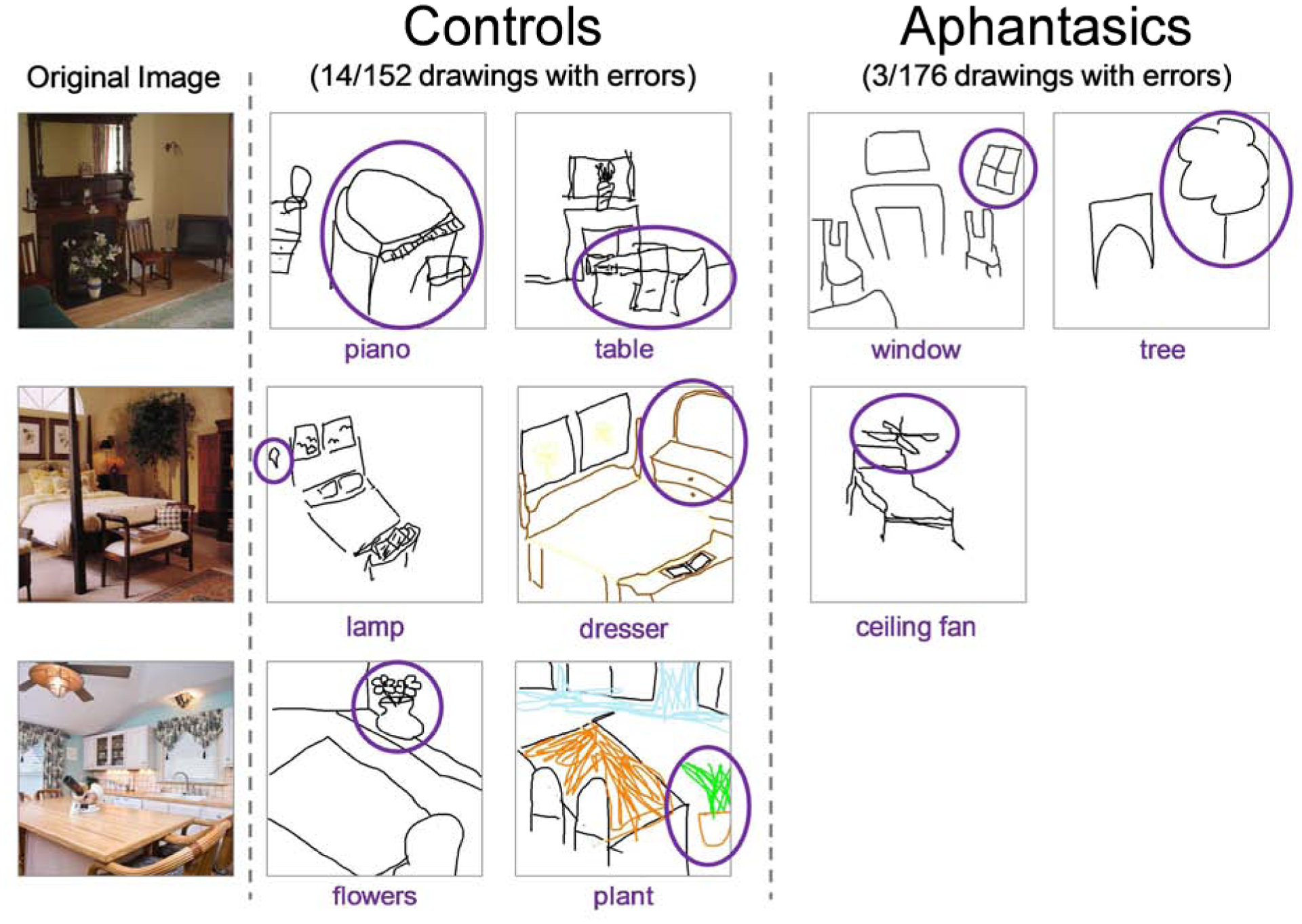
Examples of the false object memories made by participants in their memory drawings, with the inaccurate objects circled. Control participants made significantly more errors, with only 3 out of 176 total aphantasic drawings containing a falsely remembered object. Note that all aphantasic errors were also transpositions from other images.
As another metric of memory error, we also coded whether a drawing was edited or not, based on tracked mouse movements. A drawing was scored as edited if at least one line was drawn and then erased during the drawing. Significantly more memory drawings by control participants had editing than those by aphantasic participants (aphantasic: 28.4%, control: 46.6%; χ2=10.72, p=0.0011, φ=0.19). There was no significant difference in editing between groups for the perception drawings (aphantasic: 37.6%, control: 47.7%; χ2=3.17, p=0.075), indicating these differences are likely not due to differences in effort.
4. Discussion
Through a drawing task with a large online sample, we conducted an in-depth characterization of memory and perceptual drawings of real-world scenes made by individuals with aphantasia, who self-report the inability to form voluntary visual imagery. We discover that aphantasic participants show impairments in object memory, drawing fewer objects, containing less color, and spending less time drawing each object. Further, we find evidence for greater dependence on symbolic information in the task, with more text in their drawings and common self-reporting of verbal strategies. However, aphantasic participants show no impairments in spatial memory, positioning objects at accurate locations with the correct sizes. Further, aphantasic participants show significantly fewer errors in memory, with fewer falsely recalled objects, and less correction of their drawings. Importantly, we observe no significant differences between control and aphantasic participants when drawing directly from an image, indicating these differences are specific to memory and not driven by differences in effort, drawing ability, or perceptual processing. Indeed, aphantasic participants reported an equal confidence in their art abilities compared to controls, and many had experience with art classes and art-based careers.
Collectively, these results point to a dissociation in imagery between object-based information and spatial information. In addition to selective deficits in object memory over spatial memory, aphantasic participants subjectively report weaker object imagery compared to spatial imagery in the OSIQ. This supports subjective self-report of intact spatial imagery in the smaller dataset (N=15) of Keogh & Pearson (2017), which first reported differences in OSIQ measures and have since been replicated (Dawes et al., 2020). Further, in the current study, participants’ self-reported object imagery abilities correlated with the number of objects they drew from memory. These consistent results both confirm the OSIQ as a meaningful measure, while also demonstrating how such deficits can be captured by a behavioral measure such as drawing. While a similar dissociation between object and spatial memory has been observed in other paradigms and populations (Farah & Hammond, 1988), the current study provides further evidence for this dissociation. Cognitive decline from aging and dementia have shown selective deficits in object identification versus object localization (Reagh et al., 2016), owing to changes in the medial temporal lobe, where the perirhinal cortex is thought to contribute to object detail recollection, while the parahippocampal cortex contributes to scene detail recollection (Staresina, Duncan, & Davachi, 2011). The neocortex is also considered to be organized along separate visual processing pathways, with ventral regions primarily coding information about visual features, and parietal regions coding spatial information (Farah, Hammond, Levine, & Calvanio, 1988; Ungerleider & Haxby, 1994; Corballis, 1997; Carlesimo, Perri, Turriziani, Tomaiuolo, & Caltagirone, 2001; Kravitz, Saleem, Baker, & Mishkin, 2011). These findings also suggest interesting parallels between the imagery experience of individuals with aphantasia and individuals who are congenitally blind, who perform similarly to typically sighted individuals on a variety of spatial imagery tasks (Kerr, 1983; Zimler & Keenan, 1983; Eardley & Pring, 2007; Cattaneo et al., 2008), suggesting that they utilize spatial representations in the absence of visual representations of the stimuli. This may be the same for individuals with aphantasia who use spatial representations (i.e., spatial imagery), despite the absence of visual memory representations of these scenes. Neuroimaging of individuals with aphantasia will be an important next step, to see whether these impairments manifest in decreased volume or connectivity of regions specific to the imagery of visual details, such as anterior regions within inferotemporal cortex (Ishai et al., 2000; O’Craven & Kanwisher, 2000; Lee et al., 2012; Bainbridge et al., 2020) or medial parietal regions implicated in memory recall (Buckner, Andrews-Hanna, & Schacter, 2008; Vilberg & Rugg, 2008; Ranganath & Ritchey, 2012; Silson et al., 2019).
Further investigations into aphantasia will also provide critical insight to the nature of imagery, and how it compares to different forms of memory. While aphantasic participants show an impairment during recall performance, no evidence has shown impairments in visual recognition, supporting converging evidence towards a neural dissociation in the processes of quick, automatic visual recognition and slower, elaborative visual recall (Jacoby, 1991; Holdstock et al., 2002; Staresina & Davachi, 2006; Barbeau, Pariente, Felician, & Puel, 2011; Bainbridge et al., 2019). That being said, the recognition task in the current experiment had low difficulty, testing foil images of the same semantic category, but without other matched detail (e.g., identities of objects). Future work could study whether individuals with aphantasia are impaired at more fine-grained recognition tasks, where object and spatial detail within an image are selectively manipulated. Aphantasic participants also report fully intact verbal recall abilities, and our results suggest that they may be using symbolic strategies (i.e., representing information through a symbolic or verbal code), in combination with accurate spatial representations, to compensate for their lack of visual imagery. In fact, in the current study, aphantasic participants’ drawings from memory contained more text than those of control participants, potentially indicating a verbal coding of their memories to perform the task. Imagery of a visual stimulus thus may not necessarily be visual in nature; while forming a visual representation of the scene or object may be one way to undertake the task, there may be other, non-visual strategies to complete the task. Even in neurotypical adults, imagery-based representations in the brain may differ from perceptual representations of the same items (Winlove et al., 2018; Bainbridge et al., 2020). This contrasts with sensory reinstatement accounts proposing that the same neurons code both perception and imagery stimulus representations (e.g., Johnson & Johnson, 2014; Schultz et al., 2019). Further neuroimaging investigations will lead to an understanding of the neural mechanisms underlying these different strategies. The current study also grouped non-aphantasics into a single group, although the opposite experience of hyperphantasia (highly detailed photographic visual imagery) may be an equally important variation of experience to test. In a recent study, individuals with hyperphantasia performed significantly more accurately than aphantasic participants within a behavioural task suggested to involve object imagery, with no differences in performance evident between aphantasic and neurotypical control participants who had mid-range VVIQ scores (Milton et al; Unpublished Results). In the current study, one participant scored 76 on the VVIQ (which falls within the proposed cut-off for hyperphantasia, Zeman et al., 2020), but a larger sample will be needed for a more in-depth investigation to examine between these imagery extremes. Further, drawing may be a potentially sensitive behavioral tool for examining visual memory representations within individuals across the visual imagery vividness spectrum. It is also possible that the current study contained both participants with congenital aphantasia and participants with acquired aphantasia. However, given that acquired aphantasia is rare (see Zeman et al., 2010), and that congenital aphantasia is thought to be experienced by approximately 2% of the population (Zeman et al., 2015), we would expect the majority of participants in this study experienced aphantasia that was congenital in nature.
Further, aphantasic participants exhibited lower errors in memory (e.g., fewer falsely recalled objects compared to controls), which could possibly reflect higher accuracy in symbolic memory versus controls, to compensate for visual memory difficulties. Individuals with aphantasia may serve as an ideal group to probe the difference between visual and verbal memory and their interaction in both behavior and the brain. Additionally, while aphantasia has thus far only been quantified in the visual domain, preliminary work suggests that the experience may extend to other modalities (Zeman et al., 2015; Dawes et al., 2020; Zeman et al., 2020;). Using a multimodal approach, researchers may be able to pinpoint neural differences in aphantasia across other sensory modalities, for instance, the auditory domain which has been shown to have several characteristics similar to the visual domain (Halpern, 1988; Clarke, Bellmann, Meuli, Assal, & Steck, 2000; Bunzeck, Wuestenberg, Lutz, Heinze, & Jancke, 2005).
Finally, these results serve as essential evidence to suggest that aphantasia is a valid experience, at least in part, defined by the inability to form voluntary visual images with a selective impairment in object imagery. It was proposed by some researchers that aphantasia may be more psychogenic and metacognitive, rather than neurogenic and perceptual (de Vito & Bortolomeo, 2016) However, differences in self-report on imagery measures (e.g. Dawes et al., 2020; Zeman et al., 2020) and objective measures (e.g. Keogh & Pearson 2017; Wicken et al., Unpublished Results) between individuals with aphantasia and typical imagery are well established within a number of studies. In the current study, we observe evidence for a selective impairment in object imagery for aphantasic participants compared to controls. Importantly, if the source of such an impairment was metacognitive, we would expect decreased performance in spatial accuracy, decreased performance in the perceptual drawing task, or low ratings in all questions of the OSIQ rather than solely the object imagery component. However, in all of these cases, aphantasic participants performed identically with controls. In fact, aphantasic participants even showed higher memory precision than control participants on some measures, including significantly fewer memory errors and fewer editing in their drawings. Further, the correlations between the VVIQ, OSIQ, and drawn object information lend validity to the self-reported questionnaires in capturing true behavioral deficits. This being said, while we observed a deficit in object memory for aphantasic participants, it was not a complete elimination of object memory abilities. Aphantasic participants were still able to draw five objects per image from memory. While this moderate performance could be due to some preserved ability at object memory, this performance could also reflect the use of verbal lists of objects combined with intact, accurate spatial memory to reconstruct a scene (see Dawes et al., 2020). Future work will need to directly compare visual and verbal strategies, and push the limits to see what occurs when there is more visual detail than can be supported by verbal strategies.
In conclusion, leveraging the wide reach of the internet, we have conducted an in-depth and large scale study of the nature of aphantasics’ mental representations for visual images. In so doing, we have provided an important experimental validation for the differing imagery experiences reported by individuals with aphantasia. These individuals have a unique mental experience that can provide essential insights into the nature of imagery, memory, and perception. The drawings provided by aphantasic participants reveal a complex, nuanced story that show impaired object memory, but intact verbal and spatial memory during recall of real-world scene images. Collectively, these results suggest a dissocation in object and spatial information in visual memory.
Supplementary Material
Highlights.
Aphantasia (impairment in visual imagery) is recently characterized & understudied
We tested visual recall of 61 aphantasics with an online memory drawing task
Aphantasics drew fewer objects (& less detail) from memory than controls
However, aphantasic drawings showed high spatial precision and few false insertions
These results suggest an object-specific memory impairment in aphantasia
5. Acknowledgements
This research was supported (in part) by the Intramural Research Program of the National Institute of Mental Health (ZIA-MH-002909). All study data, drawings, and code will be publicly available on the Open Science Framework at https://osf.io/cahyd/. No part of the study procedures or analyses were pre-registered prior to the research being conducted. We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the current study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: None
6. References
- Bainbridge WA, Hall EH, & Baker CI (2019). Highly diagnostic and detailed content of visual memory revealed during free recall of real-world scenes. Nature Communications 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge WA, Hall EH, & Baker CI (2020). Distinct representational structure and localization for visual encoding and recall during visual imagery. Cerebral Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau EJ, Pariente J, Felician O, & Puel M (2011). Visual recognition memory: A double anato-functional dissociation. Hippocampus 21, 929–934. [DOI] [PubMed] [Google Scholar]
- Bartolomeo P (2008). The neural correlates of visual mental imagery: An ongoing debate. Cortex 44, 107–108. [DOI] [PubMed] [Google Scholar]
- Behrmann M (2000) The mind’s eye mapped onto the brain’s matter. Current Directions in the Psychological Science 9, 50–54. [Google Scholar]
- Behrmann M & Avidan G (2005) Congenital prosopagnosia: face-blind from birth. Trends in Cognitive Sciences 9, 180–187. [DOI] [PubMed] [Google Scholar]
- Blajenkova O, Kozhevnikov M, & Motes MA (2006). Object-spatial imagery: A new self-report imagery questionnaire. Applied Cognitive Psychology 20, 239–263. [Google Scholar]
- Botez MI, Olivier M, Vezina JL, Botez T, & Kaufman B (1985). Defective revisualization: dissociation between cognitive and imagistic thought case report and short review of the literature. Cortex, 21, 375–389. [DOI] [PubMed] [Google Scholar]
- Brain R (1954). Loss of Visualization. Proceedings of the Royal Society of Medicine, 47, 288–290. [PMC free article] [PubMed] [Google Scholar]
- Brons L (2019). Aphantasia, SDAM, and Episodic Memory. Annals of the Japan Association for Philosophy of Science 28, 9–32. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, & Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Wuestenberg T, Lutz K, Heinze H-J, & Jancke L (2005). Scanning silence: mental imagery of complex sounds. Neuroimage 26, 1119–1127. [DOI] [PubMed] [Google Scholar]
- Carlesimo G, Perri R, Turriziani P, Tomaiuolo F, & Caltagirone C (2001). Remembering what but not where: independence of spatial and visual working memory in the human brain. Cortex 37, 519–534. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Vecchi T, Cornoldi C, Mammarella I, Bonino D, Ricciardi E, & Pietrini P (2008). Imagery and spatial processes in blindness and visual impairment. Neuroscience & Biobehavioral Reviews 32, 1346–1360. [DOI] [PubMed] [Google Scholar]
- Clarke S, Bellmann A, Meuli RA, Assal G, & Steck AJ (2000). Auditory agnosia and auditory spatial deficits following left hemispheric lesions: evidence for distinct processing pathways. Neuropsychologia 38, 797–807. [DOI] [PubMed] [Google Scholar]
- Corballis M (1997). Mental rotation and the right hemisphere. Brain and Language 57, 100–121. [DOI] [PubMed] [Google Scholar]
- Dawes AJ, Keogh R, Andrillion T, & Pearson J (2020). A cognitive profile of multi-sensory imagery, memory and dreaming in aphantasia. Scientific Reports, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M, & Cocude M (1989). Scanning visual images generated from verbal descriptions. European Journal of Cognitive Psychology 1, 293–307. [Google Scholar]
- Dijkstra N, Bosch SE, & van Gerven MAJ (2019). Shared neural mechanisms of visual perception and imagery. Trends in Cognitive Sciences 23, 423–434. [DOI] [PubMed] [Google Scholar]
- Eardley AF, & Pring L (2007). Spatial processing, mental imagery, and creativity in individuals with and without sight. European Journal of Cognitive Psychology 19(1), 37–58. [Google Scholar]
- Farah MJ, & Hammond KM (1988). Visual and spatial mental imagery: Dissociable systems of representations. Cognitive Psychology 20, 439–462. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Hammond KM, Levine DN, & Calvanio R (1988). Visual and spatial mental imagery: dissociable systems of representation. Cognitive Psychology 20, 439–462. [DOI] [PubMed] [Google Scholar]
- Favila SE, Lee H, & Kuhl BA (2020). Transforming the concept of memory reactivation. Trends in Neurosciences, 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher J (2019). Aphantasia: Ex-Pixar chief Ed Catmull says “my mind’s eye is blind”. BBC News, at https://www.bbc.com/news/health-47830256. [Google Scholar]
- Galton F (1880). Statistics of Mental Imagery. Mind 5, 301–318. [Google Scholar]
- Halpern A (1988). Mental scanning in auditory imagery for songs. Journal of Experimental Psychology: Learning, Memory, and Cognition 14, 434–443. [DOI] [PubMed] [Google Scholar]
- Holdstock JS, Mayes AR, Roberts N, Cezayirli E, Isaac CL, O’Reilly RC, & Norman KA (2002). Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus 12, 341–351. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider L, & Haxby J (2000). Distributed neural systems for the generation of visual images. Neuron 28, 979–990. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Schwarzkopf DS, & Silvanto J (2018). Visual working memory performance in aphantasia. Cortex 105, 61–73. [DOI] [PubMed] [Google Scholar]
- Jacoby LL (1991). A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language 30, 513–541. [Google Scholar]
- Keogh R, & Pearson J (2011). Mental Imagery and Visual Working Memory. PLoS One, 6(12), e29221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh R, & Pearson J (2018). The blind mind: No sensory visual imagery in aphantasia. Cortex 105, 53–60. [DOI] [PubMed] [Google Scholar]
- Kerr NH (1983). The role of vision in “visual imagery” experiments: Evidence from the congenitally blind. Journal of Experimental Psychology: General 112, 265. [DOI] [PubMed] [Google Scholar]
- Kosslyn S (1980). Image and Mind. Harvard University Press. [Google Scholar]
- Kosslyn SM (2005). Mental images and the brain. Cognitive Neuropsychology 22, 333–347. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, & Thompson WL (2001). Neural foundations of imagery. Nature Reviews Neuroscience 2, 635–642. [DOI] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, & Mishkin M (2011). A new neural framework for visuospatial processing. Nature Reviews Neuroscience 12, 217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kravitz DJ, & Baker CI (2012). Disentangling visual imagery and perception of real-world objects. Neuroimage 59, 4064–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DF (1973). Visual imagery differences in the recall of pictures. British Journal of Psychology. 64, 17–24. [DOI] [PubMed] [Google Scholar]
- Marmor GS, & Zaback LA (1976). Mental rotation by the blind: Does mental rotation depend on visual imagery? Journal of Experimental Psychology: Human Perception and Performance 2, 515–521. [DOI] [PubMed] [Google Scholar]
- Milton F, Fulford J, Dance C, Gaddum J, Heuerman-Williamson B, Jones K, Knight K, MacKisack M, Winlove C, Zeman A (Unpublished Results). Behavioral and neural signatures of visual imagery vividness extremes: Aphantasia vs. Hyperphantasia. 10.31234/OSF.IO/J2ZPN [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Craven KM, & Kanwisher N (2000). Mental imagery of faces and places activates corresponding stimulus-specific brain regions. Journal of Cognitive Neuroscience 12, 1013–1023. [DOI] [PubMed] [Google Scholar]
- Pearson J (2019). The human imagination: the cognitive neuroscience of visual mental imagery. Nature Reviews Neuroscience 20, 624–634. [DOI] [PubMed] [Google Scholar]
- Pearson J, & Kosslyn SM (2015). The heterogeneity of mental representation: Ending the imagery debate. Proceedings of the National Academy of Sciences of the United States of America. 112, 10089–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylyshyn Z (1981). The imagery debate: Analogue media versus tacit knowledge. American Psychological Association 88, 16–45. [Google Scholar]
- Pylyshyn Z (2003). Return of the mental image: are there really pictures in the brain? Trends in Cognitive Sciences 7, 113–118. [DOI] [PubMed] [Google Scholar]
- Ranganath C, & Ritchey M (2012). Two cortical systems for memory-guided behaviour. Nature Reviews Neuroscience 13, 713–726. [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Ho HD, Leal SL, Noche JA, Chun A, Murray EA, & Yassa MA (2016). Greater loss of object than spatial mnemonic discrimination in aged adults. Hippocampus 26, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B (2016). Aphantasia: How it feels to be blind in your mind. Facebook, at https://www.facebook.com/notes/blake-ross/aphantasia-how-it-feels-to-be-blind-in-your-mind/10156834777480504/. [Google Scholar]
- Schacter DL, Addis DR, Hassabis D, Martin VC, Spreng RN, & Szpunar KK (2012) The future of memory: Remembering, imagining, and the brain. Neuron 76, 677–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz H, Tibon R, LaRocque KF, Gagnon SA, Wagner AD, & Staresina BP (2019). Content tuning in the Medial Temporal Lobe Cortex: Voxels that perceive, retrieve. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silson EH, Gilmore AW, Kalinowski SE, Steel A, Kidder A, Martin A, & Baker CI (2019). A posterior-anterior distinction between scene perception and scene construction in human medial parietal cortex. Journal of Neuroscience 39, 705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2006). Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience 26, 9162–9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, & Davachi L (2011). Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. Journal of Neuroscience 31, 8739–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A, & Coupe P (1978). Distortions in judged spatial relations. Cognitive Psychology 10, 422–437. [DOI] [PubMed] [Google Scholar]
- Thorudottir S, Sigurdardottir HM, Rice GE, Kerry SJ, Robotham RJ, Leff AP, & Starrfelt R (2020). The Architect Who Lost the Ability to Imagine: The Cerebral Basis of Visual Imagery. Brain Sciences 10, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, & Haxby JV (1994). ‘What’ and ‘where’ in the human brain. Current Opinion in Neurobiology 4, 157–165. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, & Rugg MD (2008). Memory retrieval and the parietal cortex: A review of evidence from a dual-process perspective. Neuropsychologia 46, 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vito S, & Bortolomeo P (2016). Refusing to imagine? On the possibility of psychogenic aphantasia. A commentary on Zeman et al. (2015). Cortex 74, 334–335. [DOI] [PubMed] [Google Scholar]
- Watkins NW (2018). (A)phantasia and severly deficient autobiographical memory: Scientific and personal perspectives. Cortex 105, 41–52. [DOI] [PubMed] [Google Scholar]
- Wicken M, Keogh R, & Pearson J (Unpublished results). The critical role of mental imagery in human emotion: insights from Aphantasia. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winlove CIP, Milton F, Ranson J, Fulford J, MacKisack M, Macpherson F, & Zeman A (2018). The neural correlates of visual imagery: A coordinate-based meta-analysis. Cortex 105, 4–25. [DOI] [PubMed] [Google Scholar]
- Xiao X, Dong Q, Gao J, Men W, Poldrack RA, & Xue G (2017). Transformed neural pattern reinstatement during episodic memory retrieval. Journal of Neuroscience 37, 2986–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman A, Della Sala S, Torrens LA, Gountouna V-E, McGonigle DJ, & Logie RH (2010). Loss of imagery phenomenology with intact visuo-spatial task performance: a case of ‘blind imagination’. Neuropsychologia 48, 145–155. [DOI] [PubMed] [Google Scholar]
- Zeman A, Milton F, Della Sala S, Dewar M, Frayling T, Gaddum J, … Winlove C (2020). Phantasia–The psychological significance of lifelong visual imagery vividness extremes. Cortex. 10.1016/j.cortex.2020.04.003 [DOI] [PubMed] [Google Scholar]
- Zeman AZJ, Dewar MT, & Della Sala S (2015). Lives without imagery - congenital aphantasia. Cortex 73, 378–380. [DOI] [PubMed] [Google Scholar]
- Zimler J, & Keenan JM (1983). Imagery in the congenitally blind: How visual are visual images?. Journal of Experimental Psychology: Learning, Memory, and Cognition 9, 269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


