Abstract
While chemotherapy remains a common cancer treatment, it is associated with debilitating side effects (e.g., anorexia, weight loss, and fatigue) that adversely affect patient quality of life and increase mortality. However, the mechanisms underlying taxane chemotherapy-induced side effects, and effective treatments to ameliorate them, are not well-established. Here, we tested the longitudinal relationship between a clinically-relevant paclitaxel regimen, inflammation, and sickness behaviors (loss of body mass, anorexia, fever, and fatigue) in adult, female mice. Furthermore, we sought to identify the extent to which voluntary exercise (wheel running) attenuates paclitaxel-induced sickness behaviors and underlying central pathways. Body mass and food intake decreased following six doses of chemotherapy treatment relative to vehicle controls, lasting less than 5 days after the last dose. Paclitaxel treatment also transiently decreased locomotion (open field test), voluntary wheel running, home-cage locomotion, and core body temperature without affecting motor coordination (rotarod task). Circulating interleukin (IL)-6 and hypothalamic Il1b gene expression remained elevated in chemotherapy-treated mice at least 3 days after the last dose. Exercise intervention did not ameliorate fatigue or inflammation, but hastened recovery from paclitaxel-induced weight loss. Body mass recovery was associated with the wheel running-induced recovery of body composition, paclitaxel-induced alterations to hypothalamic melanocortin signaling and associated peripheral circulating hormones (ghrelin and leptin). The present findings demonstrate the benefits of exercise on faster recovery from paclitaxel-induced body mass loss and deficits in melanocortin signaling and suggests the development of therapies targeting the melanocortin pathway to reduce paclitaxel-induced weight loss.
Keywords: neuroinflammation, cytokines, antineoplastic, ghrelin, leptin, exercise
1. INTRODUCTION
Chemotherapy drugs are instrumental components of cancer treatment that kill neoplastic tumor cells. Nevertheless, these drugs also kill dividing healthy cells and thereby contribute to a wide range of well-documented chemotherapy-related toxicities, including severe weight loss [1, 2], anorexia [1, 2], and fatigue [3, 4]. Cancer-related fatigue is different from other forms of fatigue as it is neither caused by increased activity levels nor restored by rest, and is comprised of central (motivational) and peripheral (metabolic and muscle-driven) components [3, 5, 6]. These “sickness behavior” side effects of chemotherapy persist during and following treatment [3, 4] resulting in frequent patient reports of severely decreased quality of life [3]. One common chemotherapeutic drug, paclitaxel, is a taxane agent that binds to microtubules to prevent proper mitotic spindle formation and is a “mainstay” treatment for breast, ovarian, and other cancers (reviewed in [7]). Compared to other chemotherapeutic drugs (e.g., cyclophosphamide [8, 9], doxorubicin [8, 9], 5-fluorouracil [9, 10], methotrexate [11]), the causal role and underlying mechanisms of taxane drugs in physiological and behavioral toxicities is poorly understood.
Systemic inflammation resulting from immune cell signaling triggered by chemotherapy-induced cell death, is posited as a primary contributor to weight loss, anorexia, and fatigue [8]. Taxane drugs, including paclitaxel, are associated with increased circulating concentrations of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α in cancer patients and preclinical models [12–14]. Indeed, these paclitaxel-induced increases in peripheral cytokines are significantly correlated with increased fatigue in cancer patients [13, 15, 16]. Peripheral inflammation can be translated into central inflammation and sickness behaviors through multiple pathways: circulating cytokines can activate endothelial cells and immune cells near more permeable portions (e.g., near circumventricular organs) of the blood-brain barrier, resulting in cytokine release in the brain; peripheral cytokines can activate vagal afferents, leading to neural activation of brain nuclei controlling sickness behavior; and peripheral cytokines leading to prostaglandin E2 production in brain endothelial cells, resulting in prostaglandin-induced fever (reviewed in [17]). However, the temporal dynamics of the onset and duration of paclitaxel-induced sickness behaviors, and the understanding of differences between a single dose versus more clinically-relevant multiple doses is neither well-understood nor well-modeled in the current literature. Centrally-mediated fatigue is difficult to quantify due to the need to distinguish lethargy versus a lack of motivation for locomotion, and methods of assessment have varied in clinical and preclinical studies [13, 18]. Thus, a comprehensive analysis of fatigue (passive versus active motivation, central versus peripheral contributions to fatigue) throughout paclitaxel treatment is warranted.
One potential mechanism by which inflammation leads to anorexia and fatigue is through the melanocortin pathway (reviewed in [19] and [20]), however this pathway has not yet been investigated in the context of chemotherapy. In one potential pathway, inflammation arising from paclitaxel treatment increases circulating leptin [21], which activates pro-opiomelanocortin (POMC)-expressing neurons in the arcuate nucleus of the hypothalamus [22] to release α-melanocyte-stimulating hormone (α-MSH). Neurons in the paraventricular nucleus of the hypothalamus are then activated by α-MSH, ultimately resulting in reduced food intake behavior [19, 20]. The counterbalance to this pathway occurs by elevated circulating ghrelin, which promotes orexigenic signaling that results in increased food intake (reviewed in [23]). Ghrelin is synthesized in the stomach and is secreted into circulation, where it binds to the growth hormone secretagogue receptor (GHSR) in target tissues. When ghrelin binds to the GHSR on neuropeptide Y (NPY) neurons in the arcuate nucleus, POMC neurons are silenced and food intake is stimulated [23]. Orexigenic neurons have shown to be inhibited by other classes of chemotherapeutic drugs, and in addition to affecting food intake, orexin-A/hypocretin-1 administration reverses fatigue induced by a cocktail of doxorubicin, cyclophosphamide, and 5-fluorouracil chemotherapies [8].
Physical exercise has been investigated as a potential treatment to improve symptoms associated with chemotherapy-induced toxicities in clinical studies [24, 25]. Doxorubicin and 5-fluorouracil/methotrexate-induced cognitive deficits [26, 27] and decreases in muscle mass in rodent models [2, 10, 28] have been shown to be ameliorated by exercise. Moreover, physical exercise reduces both central and peripheral inflammation in clinical and preclinical models [29, 30], and activates orexin neurons to stimulate food intake [31]. However, the extent to which exercise reverses paclitaxel-induced fatigue, anorexia, and inflammation has not been determined.
In the present study, we examined the effects of paclitaxel chemotherapy in mice over single or multiple doses in order to understand the time course of chemotherapy-induced sickness behaviors and inflammation, including multiple facets of fatigue behavior. We then tested the capacity of exercise, in the form of voluntary wheel running, to ameliorate longitudinal paclitaxel-induced fatigue, anorexia, body mass loss, and their underlying mechanisms. Our aim of the present study was to determine the extent to which paclitaxel increases peripheral inflammation and alters melanocortin signaling, and the extent to which these changes induce murine sickness behaviors.
2. MATERIALS AND METHODS
2.1. Mice.
Female, 7-8-week-old, nulliparous C57BL/6 mice (Charles River, Wilmington, MA, USA) were acclimated to a 14 h light-10 h dark light cycle (lights on: 0000 EST, lights off: 1400 EST) in a temperature-controlled vivarium (22 ± 1 °C) for one week prior to experiments. Female mice were exclusively used to control for sex differences and to create a more clinically-relevant model for studying the effects of paclitaxel in breast cancer patients. Mice were handled at least 3 times prior to any procedures to reduce handling-induced stress during subsequent behavioral testing. Standard rodent chow (Harlan 7912) and water was provided ad libitum throughout all studies, with the exception that mice were fasted 3 h prior to euthanasia in Experiment 3. Experiments were conducted with approval from the Ohio State Institutional Animal Use and Care Committee and in accordance with guidelines established in the Guide for the Care and Use of Laboratory Animals [32]. All efforts were made to reduce individual rodent suffering and the total number of mice used in all experiments.
2.2. Chemotherapy and sickness behaviors.
Paclitaxel (Sigma-Aldrich, St. Louis, MO, USA) was dissolved and injected as described previously [33]. Briefly, paclitaxel was dissolved in 1:1 100% EtOH:Cremophor EL (Millipore, Burlington, MA, USA) and then diluted 1:1 with sterile PBS. Unless otherwise stated, mice were given a series of six intraperitoneal, 100 μL injections of chemotherapy (30 mg/kg body mass) or vehicle every other day for 11 days as a mouse equivalent of a clinically-relevant treatment regimen used to treat breast cancer in women (4-8 cycles of chemotherapy). Body mass and food intake were recorded every 48 h, at least two days prior to injections (baseline measurements), and on the days of drug injections to assess the time course of chemotherapy-induced body mass loss and anorexia during and following treatment. Average daily food intake was calculated and body mass was measured and presented as a percent change from baseline.
2.3. Experimental design overview.
2.3.1: Chemotherapy and sickness behavior.
Mice were psuedorandomized into three paradigms of chemotherapy/vehicle treatment with balanced initial body masses: one dose of chemotherapy (n = 8) or vehicle (n = 7), six injections with short-term observation (n = 10/group), or six injections with long-term observation (n = 10/group, timeline in Fig. 1A). In the single-dose group, fatigue in an open field was assessed 6 h after the injection and 3 days later mice were euthanized and tissues were harvested. In the six-dose short-term group, mice were injected with vehicle or chemotherapy every other day over an 11-day period. Six hours after the final treatment, all mice were observed for fatigue by the open field test, and all mice were euthanized three days later. In the six-dose long-term group, vehicle- and chemotherapy-treated mice were injected every other day for 11 days. Six days after the final injection, mice were tested for motor coordination using the rotarod test [34] followed by fatigue in the open field test (5 d later), and tissue collection (3 d later). Weight loss and anorexia (i.e., food intake) measures of sickness behavior were collected in all mice throughout the experiments. Central inflammation relevant to sickness behavior was assessed via hypothalamic gene expression and peripheral inflammation was assessed via circulating cytokines/chemokine concentrations after euthanasia via trunk blood for all mice.
Figure 1. Paclitaxel-induced sickness behaviors.
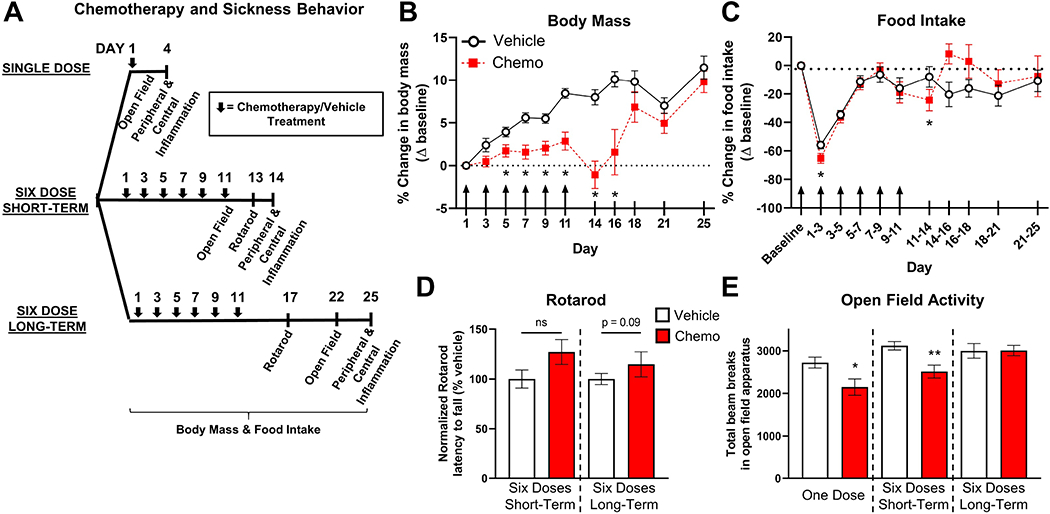
A. Timeline for the assessment of chemotherapy-induced sickness behaviors. B. Chemotherapy (30 mg/kg i.p.) significantly reduced body mass by the third treatment dose, which returned to the mass of vehicle control mice by one week post-treatment. C. Chemotherapy slightly reduced the amount of food consumed during treatment. Mice that received chemotherapy consumed slightly more food 7 d post-treatment. D. Chemotherapy-treated mice exhibited similar motor coordination to mice injected with vehicle. E. Paclitaxel caused sickness behavior 3 d after a single injection and 3 d after six injections as measured by the open field test. Sickness behavior ceased by 11 d following chemotherapy. Arrows indicate days of chemotherapy/vehicle injections. *p < 0.05; **p < 0.01.
2.3.2: Chemotherapy and fatigue.
Sixteen mice (n = 8/group) were implanted with electronic telemetry devices in their peritoneal cavity (see “Voluntary wheel running and telemetry recording”, timeline in Fig. 3A). Following surgery, all mice were single-housed in cages containing running wheels to simultaneously record voluntary wheel running (motivated behavior fatigue), body temperature, and total locomotion (home cage fatigue). Following two weeks of recovery from surgery, core body temperature, total locomotion, and voluntary wheel running were continuously analyzed for a 3-day baseline period. Next, mice were pseudorandomized into two groups based on equivalent body masses and injected with either vehicle or chemotherapy over an 11-day drug treatment period, and voluntary wheel running, body temperature, and total locomotion were continuously analyzed during the 11-day treatment and 20-day recovery periods.
Figure 3. Paclitaxel transiently induces fatigue in a 14:10 h light-dark cycle.
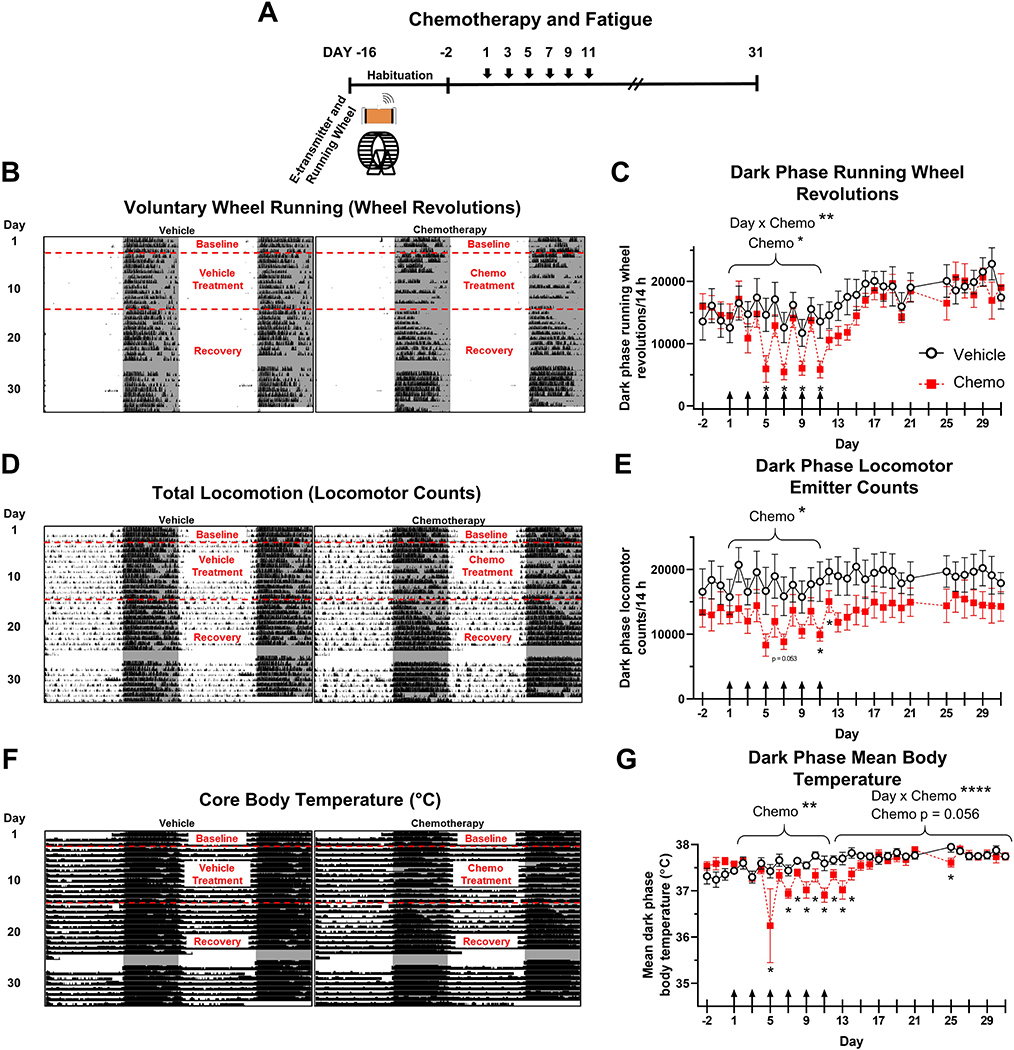
A. Timeline of chemotherapy and fatigue experiment. B. Representative double-plotted running wheel actograms from a vehicle and a chemotherapy-treated mouse. Shading indicates dark phase. C. Quantification of running wheel revolutions during the dark phase. D. Representative total locomotion actograms from vehicle and chemotherapy-treated mice. E. Quantification of total locomotion during the dark phase. F. Representative core body temperature actograms from vehicle and chemotherapy-treated mice. G. Quantification of mean body temperature during dark phase. Arrows in C, E, and G indicate days of chemotherapy/vehicle injections. *p < 0.05; **p < 0.01; ****p < 0.0001.
2.3.3: Exercise effects on chemotherapy-induced behavioral deficits.
Seventy-two mice were singly-housed and pseudorandomly assigned into two groups with balanced initial body masses (timeline in Fig. 4A). The first group (n = 36) had access to a functional running wheel as a form of voluntary exercise (“Running”), whereas wheels of the second group (n = 36) were secured with a metal bolt that prevented wheel rotation, and therefore prevented exercise (“Locked”). All mice were acclimated to the wheels for 10-11 days and then pseudorandomized into chemotherapy or vehicle subgroups based on body mass: Vehicle Locked, Vehicle Running, Chemotherapy Locked, and Chemotherapy Running (n = 18/group).
Figure 4. Exercise intervention effects on body mass, food intake, and open field test.
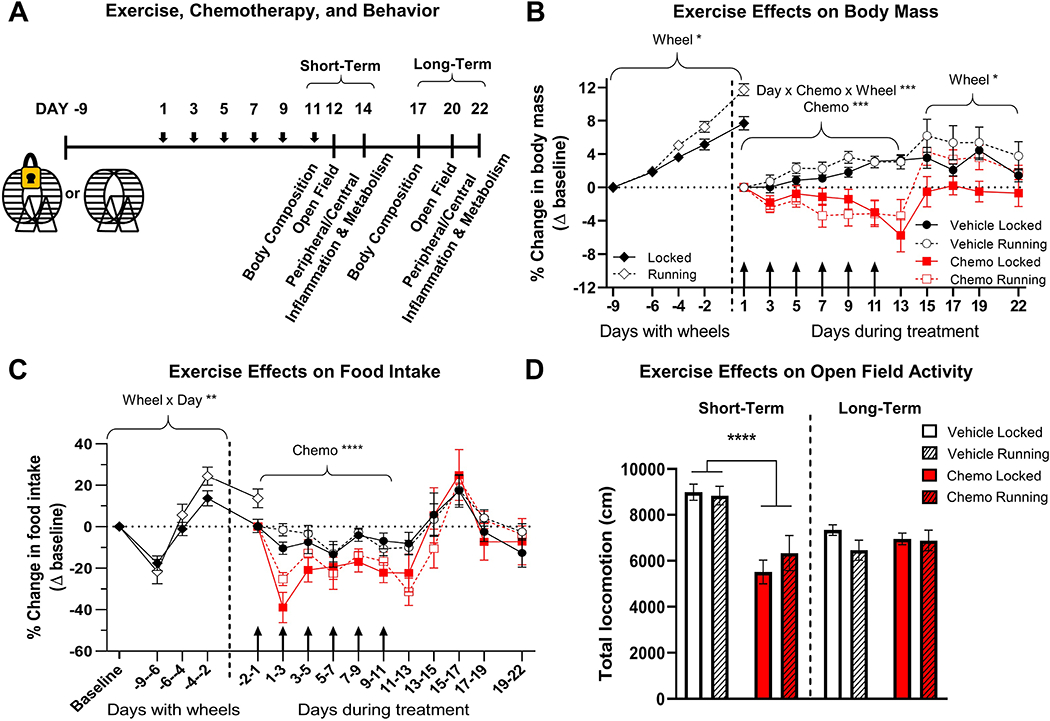
A. Timeline of exercise intervention for paclitaxel-induced behavioral and physiological changes. B. Percent change in body mass compared to baseline. Baseline for locked and running groups was the body mass before wheels were added to cages, and baseline for chemotherapy and vehicle treatment was the body mass immediately before the first dose (10-11 days after wheels were added). C. The percent change in food intake as compared to baseline. Food intake measured on day indicated on x-axis as average per 24 h over the previous 2-4 d and calculated as a percent of baseline. D. Fatigue as measured by total locomotion over 15 min in an open-field arena. Total wheel revolutions were used as a covariate in all analyses to control for unequal exercise performance between vehicle and chemotherapy groups. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Following the 6-dose chemotherapy or vehicle injections, mice were analyzed for short-term (n = 12/group) and long-term (n = 6/group) effects of exercise on behavior. A subset of short-term mice (n = 6/group) were analyzed for body fat and muscle composition (see “Body composition analysis” below) on the day of the sixth dose of chemotherapy. Short-term mice were then observed for sickness behavior using the open field test the next day and were sacrificed two days later to analyze peripheral and central inflammation. Long-term mice were scanned for body composition 6 days following the final dose of chemotherapy, sickness behavior was assessed 9 days following chemotherapy using the open field test, and mice were sacrificed 11 days following chemotherapy to assess levels of central and peripheral inflammation.
2.4. Behavioral testing.
Behavioral tests were performed to comprehensively assess chemotherapy-induced changes in fatigue, as well as locomotor coordination. All tests occurred during the dark, active phase (1400-2000 EST) under dim red light unless otherwise specified.
2.4.1: Rotarod.
Locomotor coordination capacity was tested using the well-validated rotarod test [34]. Mice (n = 11-12/group) were tested using a rotarod apparatus with the following dimensions: 388.5 mm height from spindle to bottom of apparatus, spindle diameter 398.8 mm (Columbus Instruments, Columbus, OH, USA, model #: 0207-003M). All mice were first acclimated to the stationary spindle for 30 s followed by slow rotation (4 rpm) of the spindle for 90 s. A single trial was then performed with a base speed of 5 rpm with 0.3 rpm/s acceleration until the mouse fell from the spindle; if a mouse fell within the first 3 s of the trial the trial was immediately repeated. Two trials were conducted 20 min apart with mice returning to home cages between trials, and the average latency to fall was recorded. All trials were completed under white light between 0730-1330 h.
2.4.2. Open Field.
Mice were placed into one corner of a 40.6 x 40.6 cm photobeam arena (San Diego Instruments, San Diego, CA, USA) that was lightly covered with corncob bedding, and allowed to explore for 15 min to measure general locomotion. The apparatus was cleaned with 70% ethanol between mice. Measures of locomotion were analyzed using PAS Data Reporter (San Diego Instruments) and reported as ambulatory beam breaks or XY Distance traveled.
2.5. Tissue collections.
All mice were euthanized during the dark phase (1400-2000 h) under dim red light to analyze central and peripheral inflammation. Mice were rapidly decapitated and trunk blood was collected in heparin-lined Natleson capillary tubes (Kimble Chase, Vineland, NJ, USA). Whole blood was kept on ice and then centrifuged at 4 °C at 2500 rpm for 20 min, and plasma was collected and stored at −80 °C until analyzed for cytokine, chemokine, and metabolic hormone concentrations. Brains were rapidly dissected and a roughly 0.5 cm3 cube of hypothalamic tissue was snap frozen on dry ice. Hypothalamic samples were then stored at −80 °C until RNA was extracted to analyze central inflammation using quantitative RT-PCR.
2.6. Voluntary wheel running and telemetry recording.
Electronic telemetry devices (G2 E-mitters, Starr Life Sciences, Oakmont, PA) were surgically implanted into the peritoneal cavity using an aseptic technique as previously described [35]. Mice recovered from surgery with in-cage running wheels (Starr-Life Sciences) with a 120.7 mm diameter and 50.8 mm width for 1 week prior to data collection. Following this recovery period, cages were placed on receiving platforms (ER4000, Starr Life Sciences) to continuously record core body temperature and total locomotion. Glass probes recorded voluntary wheel running at 1-min sampling intervals using VitalView 5.1 software (Starr Life Sciences). ClockLab Analysis 6 (Actimetrics, Wilmette, IL, USA) generated 24-h graphs of wheel running, locomotion, and body temperature. All wheel running, locomotion, and body temperature graphs were double-plotted to better visualize trends in chemotherapy-induced fatigue (average dark phase running wheel revolutions and locomotor emitter counts) and fever (°C). In Experiment 2, a recording error prevented data acquisition on days 25-27 (11-13 d following chemotherapy) but did not affect statistical differences in recovery from fatigue measures. VitalView software was used to ensure that voluntary wheel running occurred when analyzing effects of exercise on body composition and central and peripheral inflammation.
2.7. Body composition analysis.
Body composition measurements of fat and lean muscle were taken 1 – 2 h after the final injections of vehicle or chemotherapy using an EchoMRI analyzer (EchoMRI LLC., Houston, TX, USA) per manufacture protocol. Briefly, mice were placed in a cylinder with a loose-fitting plunger to keep the mouse from jumping and were then inserted into the instrument while measurements were taken for 1 – 3 min. Each animal was measured twice and the average of those results were reported.
2.8. Plasma cytokine, chemokine, and metabolic hormone concentrations.
Circulating cytokine and chemokine (IL-1β, IL-6, IL-10, TNF-α, and CXCL1 (KC/GRO)) and metabolic hormone (ghrelin, insulin, and leptin) concentrations were measured in plasma using a custom U-PLEX immunoassay (Meso Scale Discovery, Rockville, MD, USA) according to the manufacturer’s instructions. Plasma samples were thawed on ice and ran in duplicate on a QuickPlex SQ 120 instrument (Meso Scale Discovery) and samples that were determined to be undetectable for a particular cytokine/chemokine were quantified as 0 pg/ml (IL-1β: n = 40/49 vehicle, n = 38/51 chemotherapy; IL-6: n = 25/48 vehicle, n = 7/50 chemotherapy). Intraassay variation for all analytes was calculated as IL-1β < 20%, IL-6 < 15%, IL-10 < 15%, CXCL1: < 5%, TNF-α < 10%; ghrelin: < 5%; insulin: < 5%; leptin: < 10%.
2.9. Quantitative RT-PCR.
Total RNA was extracted from the hypothalamus using Qiagen RNeasy Mini kits (Qiagen, Germantown, MD, USA) and reverse transcribed using qScript cDNA Supermix (Quantabio, Beverly, MA, USA). Expression of pro-inflammatory cytokines (Il1b, Il6, Tnfa), an endothelial and leukocyte cellular adhesion molecule (Icam1), and metabolic gene expression (Ghsr, Hcrtr1, Npy, Pome) in the brain were assessed using TaqMan Fast Advanced Master Mix (Thermo Fisher Scientific) and TaqMan probes (Gapdh: Mm99999915_g1. Ghsr: Mm00616415_m1, Hcrtr1: Mm01185776_m1, Icam1: Mm00516023_m1, Il1b: Mm00434228_m1, Il6: Mm00446190_m1, Npy: Mm01410146_m1, Pomc: Mm00435874_m1, Tnfa: Mm00443258_m1,). Gene expression was normalized to Gapdh and represented as 2−ΔT; values did not differ between mice among all conditions examined. Outliers were removed from 2−ΔT values.
2.10. Statistical analyses.
Analyses of open field locomotion, open field central tendency, hypothalamic gene expression, and protein concentration were calculated using Student’s t-test when distributions were normal; otherwise, nonparametric Mann-Whitney tests were used. Repeated measures ANCOVAs were used to calculate differences in voluntary wheel running, total locomotion, and core body temperature with the averages of three baseline days used as covariates, and Student’s t-tests were computed on to detect differences between groups on specific days (IBM SPSS Statistics, version 26, IBM Corporation, Armonk, NY, USA). Repeated measures ANOVAs with post-hoc Bonferroni’s multiple comparisons tests or paired t-tests were used to analyze changes in body mass and food intake between groups by calculating daily food intake (amount of food consumed / days between measurements) and percent body mass change from baseline (difference in body mass measurements / baseline body mass x 100%) (GraphPad Prism 8.2.1, GraphPad Software, Inc., San Diego, CA, USA). Multivariate (two-way) ANOVAs were used to calculate effects of running wheels and drug treatment, and Bonferroni’s multiple comparisons test was used to determine statistically significant differences between groups (GraphPad Prism). The exercise intervention study utilized ANCOVAs using total wheel revolutions throughout the experiment as the covariate to control for the variation in the amount of exercise performed between chemotherapy and vehicle groups. Grubb’s Test (α = 0.05) was used to determine outliers (GraphPad Prism). Values are presented as mean ± standard error of the mean (SEM) and statistical significance was reported at the level of p < 0.05.
3. RESULTS
3.1. Paclitaxel induces sickness behaviors but does not affect locomotor coordination.
Mice treated with paclitaxel failed to increase body mass over time from the third dose of chemotherapy (Day 5, Fig. 1B) through five days following the final dose of chemotherapy (Day 16, F1, 38 = 16.69, p < 0.05, post-hoc comparisons: p < 0.05 for Days 7, 9, 11, and 14, Fig. 1B). However, food intake remained relatively consistent between groups over time, with an interaction between treatment and time (based on a slight decrease after the first and final paclitaxel doses) and a compensatory increase in chemotherapy-treated mice five days after the final paclitaxel dose (time x treatment: F10, 292 = 2.70, p < 0.005, Fig. 1C). A six-dose regimen of paclitaxel failed to inhibit locomotor coordination compared to control mice as measured by latency to fall during the rotarod test (t21 = 1.79, p > 0.05, Fig. 1D). In the open field test, fatigue was evident three days following a single dose of chemotherapy compared to vehicle-treated controls as measured by reduced activity (t13 = 2.41, p < 0.05, Fig. 1E). Chemotherapy continued to reduce open field activity three days after the six-dose treatment regimen (t17 = 3.25, p < 0.005), which returned to control baseline activity levels by 11 days post-treatment (t18 = 0.03, p > 0.05, Fig. 1E).
3.2. Paclitaxel increases peripheral and central inflammation.
Three days after a single dose of paclitaxel, circulating IL-6 concentrations trended towards increased levels compared to control mice (U = 13, p = 0.08, Fig. 2A), but circulating concentrations of IL-1β, IL-10, TNF-α, and CXCL1 were unchanged (p > 0.05). Six doses of paclitaxel increased circulating IL-6 and TNF-α 3d after chemotherapy relative to controls (IL-6: U = 6, p < 0.005; TNF-α: t16 = 2.77, p < 0.05, Fig. 2A), which resolved by 15 days postchemotherapy (p > 0.05 for all markers, Fig. 2A). Although circulating concentrations of IL-1β tended to increase 3 days after six doses of chemotherapy, this increase did not reach statistical significance (p = 0.08). IL-10 and CXCL1 were not altered by chemotherapy treatment at any time point tested (p > 0.05 in all cases).
Figure 2. Paclitaxel-induced inflammatory responses in circulation and hypothalamus.
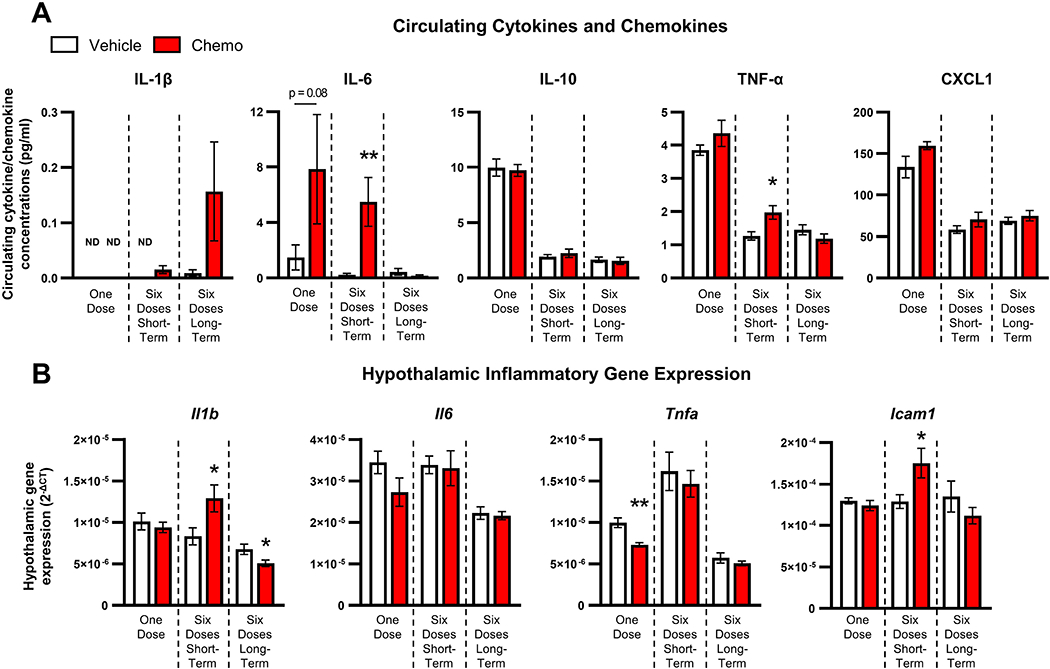
A. Circulating IL-6 tended to increase 3 days following one dose of chemotherapy treatment, but IL-1β, IL-10, TNF-α, and CXCL1 levels remained unchanged. Circulating IL-6 and TNF-α concentrations increased 3 days after 6 doses of paclitaxel and were restored by 15 days following treatment. IL-1β, IL-10, and CXCL1 levels were unchanged by paclitaxel. B. Hypothalamic Tnfa gene expression decreased following one chemotherapy dose, while hypothalamic Il1b and Icam1 gene expression increased 3 days after six paclitaxel injections. Eleven days following six chemotherapy doses, hypothalamic Il1b gene expression decreased. ND: not detected; *p < 0.05; **p < 0.01.
Paclitaxel treatment altered the hypothalamic mRNA expression of multiple markers of neuroinflammation. Three days after a single injection of paclitaxel, Tnfa expression was significantly reduced (t12 = 4.18, p < 0.005, Fig. 2B), whereas Il1b, Il6, and Icam1 were unchanged (p > 0.05). Six paclitaxel injections increased hypothalamic Il1b and Icam1 gene expression (Il1b: t17 = 2.29, p < 0.05; Icam1: t16 = 2.19, p < 0.05, Fig. 2B) 3 days post-treatment, whereas Tnfa and Il6 remained unchanged relative to controls (p > 0.05). However, 15 days after 6 injections of paclitaxel, there were no increases in hypothalamic inflammatory gene expression, and Il1b was significantly decreased (t17 = 2.24, p < 0.05, Fig. 2B).
3.3. Paclitaxel induces fatigue and fever.
Mice treated with chemotherapy demonstrated fatigue as measured by multiple behavioral assessments. Paclitaxel-treated mice exhibited less nocturnal voluntary wheel running acutely during the period of chemotherapy treatment (effect of drug: F1,11 = 6.38, p < 0.05, interaction of day x drug: F1,10 = 3.046, p < 0.005, Fig. 3B–C), with a significant reduction of wheel revolutions specifically on Days 5, 7, 9, and 11 (i.e., days of administration of doses 3-6, p < 0.05 in each case). Wheel running returned to vehicle-like levels on the days in between injections. During the recovery period (Days 12-31), total wheel revolutions increased over time but did not differ between groups (effect of day: F1,16 = 2.55, p < 0.005, Fig 3B–C).
In-cage, total locomotion as measured by implanted e-transmitter was modestly reduced by chemotherapy during the treatment period relative to controls (paclitaxel effect: F1,9 = 6.64, p < 0.05, Fig. 3D–E) with significantly reduced activity on days 7, 11, and 12 (Day 7: p = 0.053; Days 11 and 12: p < 0.05). Paclitaxel did not reduce total locomotor activity from Days 13-31 relative to vehicle-treated controls (p > 0.05, Fig. 3D–E). Chemotherapy dramatically reduced core body temperature during the treatment period (F1,10 = 12.68, p < 0.01, Fig. 3F–G) and throughout recovery (effect of drug: p = 0.056, day x drug interaction: F1,16 = 3.99, p < 0.0001) compared with controls, with particularly low temperatures on Days 5, 7-14, and 25 following the initial injection (p < 0.05 in all cases).
3.4. Exercise ameliorates paclitaxel-induced weight loss and affects select aspects of inflammatory and melanocortin signaling.
Mice given access to unlocked running wheels prior to treatment gained more weight over this 10-day period than mice given access to locked running wheels (effect of wheel F1,68 = 6.46, p < 0.05, effect of time F2.581,175.5 = 186.7, p < 0.0001, Fig. 4B). Paclitaxel-treated micelost weight relative to vehicle-treated controls over the course of drug treatment (Days 1-11), and access to wheels did not affect this loss of body mass (Days 1-11, effect of drug: F1,17 = 21.21, p < 0.0005, day x drug x wheel interaction F6,88 = 4.61, p < 0.0005, Fig. 4B). After chemotherapy treatment ceased, mice with locked running wheels gained weight to return to baseline levels; however, chemotherapy-treated mice with unlocked running wheels gained more weight, gaining the same proportion of body mass as vehicle-treated mice (Days 13 – 22, effect of wheel: F1,5 = 6.690, p < 0.05, effect of day: F3,15 = 4.304, p < 0.05, Fig. 4B).
Prior to treatment, mice with unlocked wheels ate more food than mice with locked running wheels (day x wheel interaction: F4,272 = 3.98, p < 0.005, Fig. 4C). Mice treated with chemotherapy exhibited a reduction in food intake over the course of treatment (Days 1 – 13) that was not significantly affected by access to running wheels (effect of drug F1,17 = 30.43, p < 0.0001, Fig. 4C). Mice with access to functional wheels reduced voluntary wheel running over the course of chemotherapy treatment compared to controls, consistent with the previous experiment (Supplementary Fig. 1). Similarly to previous experiments, a six-dose paclitaxel regimen reduced locomotion in the open field relative to vehicle-treated controls in mice with locked wheels; however, access to a functional running wheel did not ameliorate this measure of fatigue (effect of drug: F1,20 = 31.42, p < 0.0001, Fig. 4D). No differences in open field locomotion were observed 9 days following chemotherapy regardless of either treatment or exercise status (effects of drug or wheel: p > 0.05, Fig. 4D).
Three days following the final injection, voluntary running increased circulating IL-1β concentrations in both vehicle- and chemotherapy-treated mice (effect of wheels: F1, 39 = 4.67, p < 0.05, interaction: p = 0.09, Fig. 5A); by 11 d following injections, IL-1β did not differ among groups. Circulating IL-6 trended towards increased levels three days following paclitaxel treatment (p = 0.09) and were significantly increased by 11 days (F1, 17 = 7.80, p < 0.05, Fig. 5A). Furthermore, a trend towards an interaction between chemotherapy and running wheels was observed with circulating IL-10 three days post-treatment (interaction: p = 0.06, Fig. 5A) with decreased circulating IL-10 in mice with running wheels administered chemotherapy compared to vehicle treatment (p < 0.01). Running wheels tended to increase IL-10 11 days following injections regardless of treatment, but did not reach statistical significance (p = 0.07). CXCL1 concentrations were decreased by chemotherapy 3 days following injections regardless of access to a functioning running wheel (F1, 41 = 43.38, p < 0.0001, Fig. 5A), which was restored by 11 days following treatment (p > 0.05). However, in this experiment circulating TNF-α levels did not change regardless of drug treatment, (p > 0.05, Fig. 5A) but between groups with functional running wheels TNF-α was decreased by paclitaxel compared to controls (p < 0.05).
Figure 5. Circulating and hypothalamic inflammation following exercise intervention.
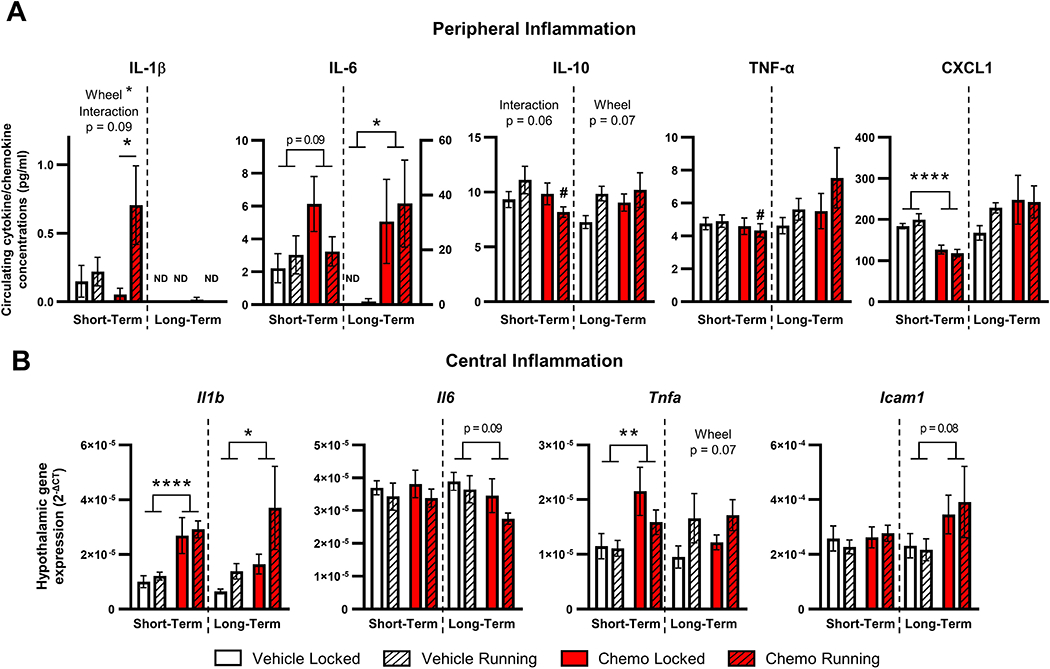
A. Circulating cytokines and chemokine concentrations from 3 days (short-term) and 11 days (long-term) following chemotherapy. B. Hypothalamic inflammatory gene expression at short- and long-term time points. Total wheel revolutions were used as a covariate in all analyses to control for unequal exercise performance between vehicle and chemotherapy groups. ND: not detected; *p < 0.05; **p < 0.01; ****p < 0.0001; #p < 0.05 between vehicle and chemotherapy running groups.
Based on our earlier study in which paclitaxel-induced decreased body mass was associated with elevated hypothalamic neuroinflammatory gene expression, the capacity for voluntary wheel running to ameliorate this neuroinflammation was assessed. Three days following the final dose of chemotherapy, Il1b and Tnfa was elevated in the hypothalamus of chemotherapy-treated mice regardless of running wheel access (Il1b: F1,26 = 22.07, p < 0.0001, Tnfa: F1,31 = 7.94, p < 0.01, Fig. 5B). In the mice evaluated after a longer-term recovery from chemotherapy treatment, Il1b and Icam1 gene expression in the hypothalamus was higher in chemotherapy-treated mice than vehicle-treated mice, with a trend of potentiated Il1b expression in mice with access to running wheels (Il1b drug effect: F1,17 = 4.74, p < 0.05, wheel effect: p = 0.08; Icam1 drug effect: F1,17 = 3.48, p = 0.08, Fig. 5B). Also observed in long-term recovery mice was a moderate increase in hypothalamic Tnfa gene expression in mice with access to running wheels, which was not significantly affected by chemotherapy treatment (wheel effect: F1,18 = 3.838, p = 0.07, Fig. 5B).
EchoMRI analysis was used to provide more detail into the weight difference between chemotherapy mice with access to locked versus freely-moving running wheels. Running wheels decreased the relative percentage of body fat of vehicle-treated, but not chemotherapy-treated mice measured immediately (short-term) after the 6th dose (wheel effect: F1,18 = 12.14, p < 0.005, wheel x drug interaction: F1,18 = 9.240, p < 0.01, post-hoc comparison, p < 0.005, Fig. 6A). In contrast, by 11 days after the last injection (long-term), running wheel access was sufficient to significantly decrease the percentage of body fat in both chemotherapy- and vehicle-treated mice (wheel effect: F1,19 = 49.19, p < 0.0001, Fig. 6A). Statistically significant differences in lean muscle mass based on chemotherapy or running wheels were not observed in either short- or long-term groups (Supplemental Fig. 2).
Figure 6. Peripheral and central mediators of appetite control following exercise intervention.
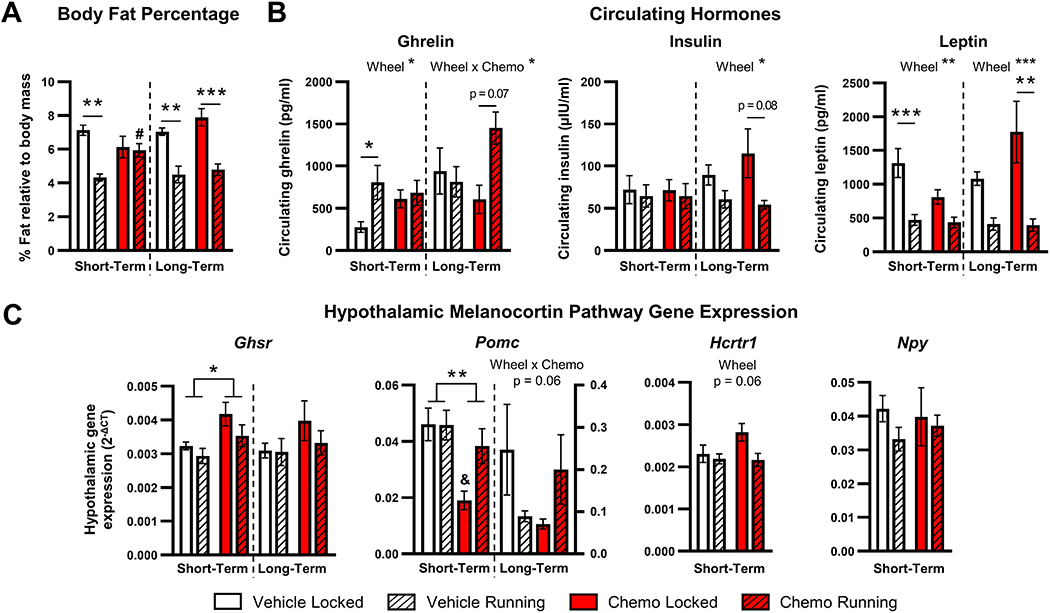
A. Body fat percentage as measured by Echo-MRI in mice 3 days (short-term) and 11 days (long-term) following the last dose of chemotherapy. B. Circulating hormones from short- and long-term groups of mice. C. Hypothalamic ghrelin receptor (Ghsr) and POMC (Pomc) gene expression from short- and long-term mice and orexin receptor (Hcrtr1) and NPY (Npy) in short-term mice. Total wheel revolutions were used as a covariate in all analyses to control for unequal exercise performance between vehicle and chemotherapy groups. *p < 0.05; **p < 0.01; ****p < 0.0001; #p < 0.05 between vehicle and chemotherapy running groups; &p < 0.05 between vehicle and chemotherapy locked groups.
As running wheels did not attenuate inflammation while body mass decreased, changes in metabolic hormones and associated melanocortin pathway hypothalamic gene expression were measured. Three days following injections, voluntary wheel running increased circulating ghrelin which was driven by the vehicle-treated mice (effect of wheels: F1,43 = 4.87, p < 0.05, post-hoc comparison: p < 0.05, Fig. 6B). Conversely, leptin was decreased by wheel access, which was also driven by the vehicle-treated groups (effect of wheels: F1,43 = 20.52, p < 0.0001, post-hoc comparison: p < 0.0005, Fig. 6B). Indeed, the effects of exercise on ghrelin and leptin were absent within chemotherapy-treated mice (p > 0.05, Fig. 6B). Circulating insulin was comparable among all 4 groups (p > 0.05, Fig. 6B). Eleven days following treatment, ghrelin rebounded in chemotherapy-treated mice with functional running wheels (interaction: F1,14 = 5.65, p < 0.05, post-hoc comparison: p = 0.07, Fig. 6B). At this long-term recovery time point, exercise sufficiently decreased both circulating insulin (effect of wheels: F1,14 = 8.77, p < 0.05) and leptin (effect of wheels: F1,44 = 22.09, p < 0.0005), which was again driven primarily by the chemotherapy-treated groups (insulin post-hoc comparison: p = 0.056, leptin post-hoc comparison: p < 0.005, Fig. 6B).
Next, central responses to these peripheral hormones were assessed. Chemotherapy increased hypothalamic ghrelin receptor (Ghsr) gene expression in mice 3 days after the final treatment with chemotherapy, regardless of access to running wheels (F1,23 = 7.58, p < 0.05, Fig. 6C). Chemotherapy also decreased hypothalamic Pomc gene expression (F1,19 = 8.67, p < 0.01, Fig. 6C), driven largely by the sedentary mice (post-hoc comparison, p < 0.05, Fig. 6C), whereas exercise in chemotherapy-treated mice restored Pomc to vehicle-treated levels. In contrast, hypothalamic orexin receptor (Hcrtr1) and neuropeptide Y (Npy) was not altered by chemotherapy or exercise (p > 0.05, Fig. 6C). When hypothalamic expression of food intake genes were assessed 11 days after the last dose of chemotherapy (long-term), there were no observed statistically-significant differences among treatment groups (Fig. 6C).
4. DISCUSSION
The present study thoroughly examined the effects of paclitaxel on sickness behaviors and peripheral and central inflammation in a mouse model and the capacity for voluntary exercise to ameliorate these deleterious effects. While behavioral side effects and toxicities associated with other chemotherapeutics have been extensively studied [2, 36], a paucity of information on the commonly-prescribed class of taxanes (e.g., paclitaxel) has limited our understanding of how these drugs specifically contribute to toxicities in cancer patients. Multiple measures of paclitaxel-induced sickness behaviors (fatigue, fever, anorexia, body mass loss) were examined for the first time in a dose-response manner. Both longitudinal and cross-sectional changes were assessed, with a particular emphasis on comparing various facets of fatigue behaviors in mice (open field locomotion, in-cage locomotion, and voluntary wheel running).
We first observed paclitaxel-induced loss of body mass starting at the third injection. Notably, paclitaxel-induced anorexia was observed at the time of the second dose but did not persist for the same duration as decreased body mass, which persisted for up to a week post-treatment. Weight loss has been reported in tumor-bearing mice receiving paclitaxel treatment [37, 38], and the paclitaxel-induced decreased body mass observed in the present study was consistent with another study in tumor-naïve mice [18], although these studies required more doses to observe decreased body mass compared to the work described here. Our initial assessments of fatigue also included a transient reduction of locomotion in an open field arena after paclitaxel. This is likely indicative of centrally-mediated fatigue rather than changes to locomotor coordination, as paclitaxel-treated mice did not exhibit impaired rotarod performance which was consistent with other rodent studies reporting intact motor coordination at similar paclitaxel doses [39, 40]. Lean muscle mass as assessed via EchoMRI was not decreased by paclitaxel treatment, furthering supporting the notion that the observed fatigue was centrally mediated and not due to muscle wasting.
The effects of paclitaxel on inflammatory markers in circulation and the hypothalamus were then investigated, as increased peripheral inflammation is correlated with increased fatigue in cancer patients receiving paclitaxel [13, 15]. Increased peripheral inflammation is also associated with anorexia and loss of body mass in tumor models [38], and increased fatigue occurs following an immune challenge caused by lipopolysaccharide treatment [35], Circulating IL-6 and TNF-α were elevated 3 days following a six-dose paclitaxel regimen which correlated with elevated hypothalamic transcripts for Il1b (coding for the pro-inflammatory cytokine IL-1β) and Icam1 (coding for intercellular adhesion molecule 1, a molecule present on endothelial cells necessary for monocyte recruitment and induced by IL-1β; [41]). Conversely, a single paclitaxel dose was insufficient to induce markers of inflammation three days after treatment. One probable explanation for this dose response is a cumulative inflammatory response after repeated paclitaxel immune “challenges” caused by inflammatory signals released in response to chemotherapy-induced cell death. For example, microglia (resident macrophages in the brain) can be “primed” by earlier immune challenges (e.g., aging or neurodegeneration) and results in an exaggerated immune response upon a second immune challenge [42]. As we have previously demonstrated brain region-specific alterations in microglial activation following paclitaxel treatment [33], it is possible that microglia activity could underlie these changes in central inflammation following repeated chemotherapy doses. Furthermore, reduced hypothalamic Il1b 11 d after six doses and Tnfa 3 d after one dose may indicate compensatory inactivation or cell death of microglia, as previously suggested [33]. Future studies are needed to assess the potentially dynamic response of peripheral and central immune cells over time and doses, as paclitaxel is rapidly pumped from the brain into circulation by P-glycoprotein [7]. Furthermore, neither one or six doses of paclitaxel induced a strong inflammatory response in the blood of all mice as indicated by larger error bars for IL-1β and IL-6. The reasons for this heterogeneous response among chemotherapy-treated mice merit further investigation and may represent individual differences in responses to chemotherapy observed in cancer patients [13].
In subsequent experiments, multiple continuous behavioral and physiological measurements were assessed in combination with cross-sectional assessments to examine paclitaxel effects on fatigue and sickness behaviors longitudinally. This multi-faceted approach of measuring central fatigue was designed to differentiate among a potential lack of motivation to exercise (voluntary running wheels; [43]), lethargy in performing basic functions in the home cage (eating, drinking; e-mitters), and/or a lack of exploration of a novel environment (open field). The use of comprehensive measures of murine fatigue behavior was intended to model methods used to assess fatigue in cancer patients through motivated behavior assessment (i.e., self-reported questionnaires; [44, 45]) and objective activity assessment (i.e., actigraphy; [44]). Paclitaxel has previously been shown to increase nociception [46], increase anxiety-like behavior [46], and decrease average body temperature and total locomotion in the weeks following treatment in mice [18]. However, the present data generate a much higher resolution of the daily dynamics of paclitaxel-induced sickness behaviors and recovery, compared to previous reports that collapsed full weeks of data [18, 46]. Indeed, here paclitaxel dramatically decreased voluntary wheel running during the active phase on days of injection beginning on the day of the third dose, consistent with previous reports of decreased rodent running wheel activity following paclitaxel treatment [18, 39]. However, the present experimental approach was uniquely sensitive enough to detect that wheel running, home cage locomotion, and body temperature all quickly rebounded on the initial “rest” days in between paclitaxel doses, with a dampening of this rebound effect over time. Notably, despite some rebounding, mean core body temperature remained significantly decreased on days between chemotherapy doses and remained low for several days after the final paclitaxel dose. This hypothermic response is consistent with previous work demonstrating that lipopolysaccharide-induced hypothermia in mice as part of the febrile-like response is based on the temperature of the room [35, 47]. Thermoregulatory issues (e.g., hot flashes) are also reported in breast cancer survivors [48]. These longitudinal analyses suggest cumulative effects of paclitaxel treatment on various sickness behaviors that can linger beyond active treatment. By comparison, the reduction in home cage locomotion observed following paclitaxel was more modest than that of wheel running. This difference likely indicates that paclitaxel decreases the motivation to engage in non-essential movement (e.g., exercising) while prioritizing movement needed to achieve essential functions, like eating and drinking. In addition to paclitaxel, longitudinal effects of 5-fluorouracil [49] and a cocktail of cyclophosphamide/doxorubicin/5-fluorouracil [8, 9] on fatigue have been described in mice, but the underlying mechanisms and duration of this fatigue is poorly understood. Additional work to understand why paclitaxel-induced decreases in core body temperature persisted longer than changes to locomotion and voluntary wheel running is merited.
In humans, the benefits of exercise during chemotherapy treatment extend into multiple aspects of patient quality of life. Physical training can improve chemotherapy-associated fatigue, prevent nausea, vomiting, and pain associated with chemotherapy, and improve mental health measures after chemotherapy [24, 25, 50]. Notably, a recent study failed to report fatigue amelioration by exercise in paclitaxel-treated breast cancer patients, though this study was underpowered [51]. Furthermore, patients who exercise during chemotherapy may also return to work earlier and work for longer hours than those patients who did not exercise [25]. Thus, the potential for voluntary, moderate exercise (wheel running) was investigated as a possible therapy for paclitaxel-induced weight loss, fatigue, and inflammation. While weight loss persisted during paclitaxel administration in these experiments, exercise accelerated robust gains in body mass during the recovery period. In contrast, exercise did not ameliorate deficits in food intake or locomotion in the open field (i.e., fatigue). Wheel running during cisplatin chemotherapy, however, ameliorates cisplatin-induced anorexia but not decreased body mass in mice [2]. Additional studies assessing the capacity for exercise to ameliorate other measures of paclitaxel-induced fatigue or sickness behavior (e.g., home cage locomotion, core body temperature) are warranted. Studies assessing the ability of exercise to improve or prevent the behavioral side effects of other chemotherapy drugs have focused on cognitive function or neuropathic pain. For example, intensive treadmill exercise attenuates the loss of muscle mass and strength due to non-taxane chemotherapies in rodents, and restores deficiencies in cognitive function in doxorubicin-treated rats [26, 28]. Voluntary wheel running also improves measures of spatial and working memory in methotrexate and 5-fluoruracil treated rats [27]. In the present study, paclitaxel prevented wheel running-induced body fat loss in the short term but resolved this body composition benefit after cessation of paclitaxel treatment. Lean muscle mass was not significantly altered by paclitaxel treatment in the present study, in contrast to clinical studies describing skeletal mass following a combination of paclitaxel and cisplatin treatment [5]. The consistent muscle mass among treatment groups regardless of exercise intervention indicated that the fatigue observed was unlikely to be derived from peripheral muscle fatigue as seen in cancer patients [5, 6]. Finally, when assessing exercise as an intervention for chemotherapy side effects, it is important to account for potential discrepancies in exercise exposure between groups due to chemotherapy-induced fatigue. Indeed, the present analyses for the exercise intervention experiments used wheel running revolutions as a covariate for all dependent variables assessed.
The precise mechanisms underlying the favorable effects of exercise on chemotherapy are understudied [52]. While exercise has been shown to be an anti-inflammatory intervention in other contexts (e.g. trauma, reviewed in [53]), only a modest exercise-induced decrease in IL-6 and increase in IL-1β were observed in circulation, while no reductions of paclitaxel-induced hypothalamic inflammation were observed due to wheel running. Although the hypothalamus was investigated in this study, further work analyzing the capacity for exercise to ameliorate paclitaxel-induced hippocampal inflammation is warranted, as voluntary wheel running has been previously shown to reduce hippocampal Il1b expression [54]. Notably, the modest exercise-induced reduction of circulating IL-6 in this murine study is similar to that observed in a breast cancer patient study following physical exercise intervention, indicating that improved behavioral effects of exercise following chemotherapy treatment are likely independent of IL-6 signaling [55]. In addition, a possible variable confounding the potential for exercise to reduce inflammation is the fact that paclitaxel-treated mice did not run as much as vehicle-treated control mice during the treatment period. More rigorous exercise, such as forced treadmill running, may exhibit more pronounced anti-inflammatory effects compared to the reduced voluntary wheel running observed in this study [56]. The discrepancy between the benefits of exercise on different chemotherapy-induced sickness behaviors (e.g., decreased body mass, but not fatigue) suggests different underlying mechanisms for each of these ailments.
Given the contribution of the melanocortin system to inflammation-induced anorexia and weight loss ([57], reviewed in [58]), and since peripheral and central inflammation was not reduced with exercise, we investigated paclitaxel-induced metabolic changes in the periphery and hypothalamus as a possible pathway by which exercise induced body mass recovery. In the periphery, exercise in vehicle-treated mice increased circulating orexigenic ghrelin and reduced body fat and associated leptin compared to sedentary controls, which is consistent with previous reports [54]. Notably, 3 days of recovery after paclitaxel treatment was not sufficient to increase plasma ghrelin levels (to stimulate food intake) but did increase the gene expression of the hypothalamic receptor for ghrelin (Ghsr). In contrast, previous reports indicate that cisplatin and methotrexate chemotherapies increase circulating ghrelin [1, 59] and that exercise increases hypothalamic Ghsr expression [60]. Furthermore, these effects of paclitaxel on ghrelin signaling in the brain appeared to successfully inhibit POMC signaling, which was reversed by exercise and associated with recovery from body mass deficits three days after the final dose of chemotherapy. However, at least 11 d of recovery from paclitaxel in combination with exercise resulted in significant increases in orexigenic ghrelin and was associated with the observed improvements in weight gain. Indeed, by 11 d after chemotherapy the expected exercise-induced effects (increased circulating ghrelin, decreased circulating leptin/insulin) were restored in paclitaxel-treated mice, suggesting that the benefits of exercise were masked during active chemotherapy treatment, but apparent during recovery. Taken together, this temporal pattern suggests an initial benefit of exercise on central orexigenic signals (Pomc) in chemotherapy-treated mice, followed by a subsequent, compensatory recovery in peripheral hormones regulating food intake. An earlier assessment would be necessary to understand how these metabolic pathways differ during active treatment compared to during recovery. Previous work characterized the role of another metabolic hormone, orexin, in chemotherapy-related fatigue using a cocktail of cyclophosphamide, doxorubicin, and 5-fluorouracil as orexin also regulates arousal and appetite [8]. As the present study established paclitaxel-induced effects on fatigue and orexigenic signaling, further work characterizing the potential role of melanocortin signaling in paclitaxel-induced fatigue is merited.
While the scope of this study is limited to analyzing the effects of central and peripheral inflammation and melanocortin signaling on paclitaxel-induced sickness behavior, this work could be expanded upon in multiple ways. First, it would be useful to assess the extent to which other motivational behaviors (e.g., sucrose preference test, social preference tests) are affected by paclitaxel. By doing so, the paclitaxel-induced deficits in voluntary wheel running may be definitively characterized as evidence of multiple forms of motivational alterations and/or sickness behaviors caused by chemotherapy. Future studies should also investigate the mechanistic changes of paclitaxel-induced decreases in body mass. As changes to total lean muscle mass were not observed in the present study, understanding the etiology of paclitaxel-induced body mass deficits may highlight the differences between the relative contributions of paclitaxel chemotherapy and tumor biology to cancer-induced cachexia observed in human patients [5, 61].
5. CONCLUSIONS
Together, the present study determined a time course of paclitaxel-induced inflammation and sickness behaviors, and identified exercise as a potential measure to protect against paclitaxel-related weight loss via metabolic pathways. These findings provide insight into various causes of paclitaxel-induced sickness behaviors independently of tumors and identifies a possible mechanism of how exercise intervention may improve cancer patient quality of life.
Supplementary Material
HIGHLIGHTS.
Repeated paclitaxel injections induce febrile and various fatigue responses
Repeated paclitaxel injections induce transient peripheral and central inflammation
Voluntary exercise accelerates recovery from paclitaxel-induced cachexia
Exercise improves recovery from paclitaxel-dysregulated melanocortin signaling
ACKNOWLEDGEMENTS
The authors thank Dr. Michelle Basso, Lesley Fisher, Browning Haynes, Ashley Lahoud, Jasskiran Kaur, Wesley Wang, and Jaimie Gray for their technical assistance. We also thank Dr. Stacey Meeker, Megan Fleming, and Cindy Fairbanks for animal husbandry. We would like to acknowledge the Small Animal Imaging Core at The Ohio State University who provided access and use of the EchoMRI analyzer used in this study. This work was supported by The Ohio State University Medical Center (L.P.), a Pelotonia Graduate Fellowship (K.S.), a Pelotonia Undergraduate Fellowship (S.V.), and National Institutes of Health grant CA216290 (L.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors do not declare any conflicts of interest.
REFERENCES
- [1].Francois M, Takagi K, Legrand R, Lucas N, Beutheu S, Bole-Feysot C, Cravezic A, Tennoune N, do Rego JC, Coeffier M, Inui A, Dechelotte P, Fetissov SO, Increased Ghrelin but Low Ghrelin-Reactive Immunoglobulins in a Rat Model of Methotrexate Chemotherapy-Induced Anorexia, Front Nutr 3 (2016) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hojman P, Fjelbye J, Zerahn B, Christensen JF, Dethlefsen C, Lonkvist CK, Brandt C, Gissel H, Pedersen BK, Gehl J, Voluntary exercise prevents cisplatin-induced muscle wasting during chemotherapy in mice, PloS one 9(9) (2014) e109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Berger AM, Gerber LH, Mayer DK, Cancer-related fatigue: implications for breast cancer survivors, Cancer 118(8 Suppl) (2012) 2261–9. [DOI] [PubMed] [Google Scholar]
- [4].Tabrizi FM, Alizadeh S, Cancer Related Fatigue in Breast Cancer Survivors: in Correlation to Demographic Factors, Maedica (Buchar) 12(2) (2017) 106–111. [PMC free article] [PubMed] [Google Scholar]
- [5].Bezjak A, Tu D, Bacon M, Osoba D, Zee B, Stuart G, Roy JA, Piccart M, Eisenhauer E, Quality of life in ovarian cancer patients: comparison of paclitaxel plus cisplatin, with cyclophosphamide plus cisplatin in a randomized study, J Clin Oncol 22(22) (2004) 4595–603. [DOI] [PubMed] [Google Scholar]
- [6].Bohlen J, McLaughlin SL, Hazard-Jenkins H, Infante AM, Montgomery C, Davis M, Pistilli EE, Dysregulation of metabolic-associated pathways in muscle of breast cancer patients: preclinical evaluation of interleukin-15 targeting fatigue, J Cachexia Sarcopenia Muscle 9(4) (2018) 701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rowinsky EK, The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents, Annu Rev Med 48 (1997) 353–74. [DOI] [PubMed] [Google Scholar]
- [8].Weymann KB, Wood LJ, Zhu X, Marks DL, A role for orexin in cytotoxic chemotherapy-induced fatigue, Brain Behav Immun 37 (2014) 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Elsea CR, Kneiss JA, Wood LJ, Induction of IL-6 by Cytotoxic Chemotherapy Is Associated With Loss of Lean Body and Fat Mass in Tumor-free Female Mice, Biol Res Nurs 17(5) (2015) 549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ballaro R, Beltra M, De Lucia S, Pin F, Ranjbar K, Hulmi JJ, Costelli P, Penna F, Moderate exercise in mice improves cancer plus chemotherapy-induced muscle wasting and mitochondrial alterations, FASEB J 33(4) (2019) 5482–5494. [DOI] [PubMed] [Google Scholar]
- [11].Gibson EM, Nagaraja S, Ocampo A, Tam LT, Wood LS, Pallegar PN, Greene JJ, Geraghty AC, Goldstein AK, Ni L, Woo PJ, Barres BA, Liddelow S, Vogel H, Monje M, Methotrexate Chemotherapy Induces Persistent Tri-glial Dysregulation that Underlies Chemotherapy-Related Cognitive Impairment, Cell 176(1-2) (2019) 43–55 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE, Duska LR, Fuller AF, Goodman AK, Nikrui N, MacNeill KM, Matulonis UA, Preffer FI, Seiden MV, Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel, Int J Gynecol Cancer 10(1) (2000) 33–41. [DOI] [PubMed] [Google Scholar]
- [13].Pusztai L, Mendoza TR, Reuben JM, Martinez MM, Willey JS, Lara J, Syed A, Fritsche HA, Bruera E, Booser D, Valero V, Arun B, Ibrahim N, Rivera E, Royce M, Cleeland CS, Hortobagyi GN, Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy, Cytokine 25(3) (2004) 94–102. [DOI] [PubMed] [Google Scholar]
- [14].Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D, Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes, Br J Cancer 87(1) (2002) 21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR, Inflammatory biomarkers for persistent fatigue in breast cancer survivors, Clin Cancer Res 12(9) (2006) 2759–66. [DOI] [PubMed] [Google Scholar]
- [16].Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, Jasmin C, Levi F, Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer, Clin Cancer Res 11(5)(2005) 1757–64. [DOI] [PubMed] [Google Scholar]
- [17].Dantzer R, Cytokine, sickness behavior, and depression, Immunol Allergy Clin North Am 29(2) (2009) 247–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ray MA, Trammell RA, Verhulst S, Ran S, Toth LA, Development of a mouse model for assessing fatigue during chemotherapy, Comp Med 61(2) (2011) 119–30. [PMC free article] [PubMed] [Google Scholar]
- [19].Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW, Central nervous system control of food intake and body weight, Nature 443(7109) (2006) 289–95. [DOI] [PubMed] [Google Scholar]
- [20].Schwartz MW, Woods SC, Porte D Jr., Seeley RJ, Baskin DG, Central nervous system control of food intake, Nature 404(6778) (2000) 661–71. [DOI] [PubMed] [Google Scholar]
- [21].Mastronardi CA, Walczewska A, Yu WH, Karanth S, Parlow AF, McCann SM, The possible role of prolactin in the circadian rhythm of leptin secretion in male rats, Proc Soc Exp Biol Med 224(3) (2000) 152–8. [DOI] [PubMed] [Google Scholar]
- [22].Sergeyev V, Broberger C, Hokfelt T, Effect of LPS administration on the expression of POMC, NPY, galanin, CART and MCH mRNAs in the rat hypothalamus, Brain Res Mol Brain Res 90(2) (2001) 93–100. [DOI] [PubMed] [Google Scholar]
- [23].Mason BL, Wang Q, Zigman JM, The central nervous system sites mediating the orexigenic actions of ghrelin, Annu Rev Physiol 76 (2014) 519–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Adamsen L, Quist M, Andersen C, Moller T, Herrstedt J, Kronborg D, Baadsgaard MT, Vistisen K, Midtgaard J, Christiansen B, Stage M, Kronborg MT, Rorth M, Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial, BMJ 339 (2009) b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, Meerum Terwogt JM, van Bochove A, Lustig V, van den Heiligenberg SM, Smorenburg CH, Hellendoorn-van Vreeswijk JA, Sonke GS, Aaronson NK, Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial, J Clin Oncol 33(17) (2015) 1918–27. [DOI] [PubMed] [Google Scholar]
- [26].Park HS, Kim CJ, Kwak HB, No MH, Heo JW, Kim TW, Physical exercise prevents cognitive impairment by enhancing hippocampal neuroplasticity and mitochondrial function in doxorubicin-induced chemobrain, Neuropharmacology 133 (2018) 451–461. [DOI] [PubMed] [Google Scholar]
- [27].Winocur G, Wojtowicz JM, Huang J, Tannock IF, Physical exercise prevents suppression of hippocampal neurogenesis and reduces cognitive impairment in chemotherapy-treated rats, Psychopharmacology (Berl) 231(11) (2014) 2311–20. [DOI] [PubMed] [Google Scholar]
- [28].Park SS, Park HS, Jeong H, Kwak HB, No MH, Heo JW, Yoo SZ, Kim TW, Treadmill Exercise Ameliorates Chemotherapy-Induced Muscle Weakness and Central Fatigue by Enhancing Mitochondrial Function and Inhibiting Apoptosis, Int Neurourol J 23(Suppl 1) (2019) S32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Paolucci EM, Loukov D, Bowdish DME, Heisz JJ, Exercise reduces depression and inflammation but intensity matters, Biol Psychol 133 (2018) 79–84. [DOI] [PubMed] [Google Scholar]
- [30].Spielman LJ, Little JP, Klegeris A, Physical activity and exercise attenuate neuroinflammation in neurological diseases, Brain research bulletin 125 (2016) 19–29. [DOI] [PubMed] [Google Scholar]
- [31].Shiuchi T, Miyatake Y, Otsuka A, Chikahisa S, Sakaue H, Sei H, Role of orexin in exercise-induced leptin sensitivity in the mediobasal hypothalamus of mice, Biochem Biophys Res Commun 514(1) (2019) 166–172. [DOI] [PubMed] [Google Scholar]
- [32].NRC, R. Institute for Laboratory Animal, P. National Academies, Guide for the care and use of laboratory animals, National Academies Press, Washington, D.C., 2011. [Google Scholar]
- [33].Loman BR, Jordan KR, Haynes B, Bailey MT, Pyter LM, Chemotherapy-induced neuroinflammation is associated with disrupted colonic and bacterial homeostasis in female mice, Sci Rep 9(1) (2019) 16490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jones BJ, Roberts DJ, The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod, J Pharm Pharmacol 20(4) (1968) 302–4. [DOI] [PubMed] [Google Scholar]
- [35].Santos JC, Bever SR, Pereira-da-Silva G, Pyter LM, Tumor resection ameliorates tumor-induced suppression of neuroinflammatory and behavioral responses to an immune challenge in a cancer survivor model, Sci Rep 9(1) (2019) 752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zombeck JA, Fey EG, Lyng GD, Sonis ST, A clinically translatable mouse model for chemotherapy-related fatigue, Comp Med 63(6) (2013) 491–7. [PMC free article] [PubMed] [Google Scholar]
- [37].Faiao-Flores F, Suarez JA, Pardi PC, Maria DA, DM-1, sodium 4-[5-(4-hydroxy-3-methoxyphenyl)-3-oxo-penta-1,4-dienyl]-2-methoxy-phenolate: a curcumin analog with a synergic effect in combination with paclitaxel in breast cancer treatment, Tumour Biol 33(3) (2012) 775–85. [DOI] [PubMed] [Google Scholar]
- [38].Tohgo A, Kumazawa E, Akahane K, Asakawa A, Inui A, Anticancer drugs that induce cancer-associated cachectic syndromes, Expert Rev Anticancer Ther 2(1) (2002) 121–9. [DOI] [PubMed] [Google Scholar]
- [39].Griffiths LA, Duggett NA, Pitcher AL, Flatters SJL, Evoked and Ongoing Pain-Like Behaviours in a Rat Model of Paclitaxel-Induced Peripheral Neuropathy, Pain Res Manag 2018 (2018)8217613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Salat K, Filipek B, Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice, J Zhejiang Univ Sci B 16(3) (2015) 167–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Niraula A, Wang Y, Godbout JP, Sheridan JF, Corticosterone Production during Repeated Social Defeat Causes Monocyte Mobilization from the Bone Marrow, Glucocorticoid Resistance, and Neurovascular Adhesion Molecule Expression, The Journal of neuroscience : the official journal of the Society for Neuroscience 38(9) (2018) 2328–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH, Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration, The Journal of neuroscience : the official journal of the Society for Neuroscience 25(40) (2005) 9275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rhodes JS, Garland T Jr., Gammie SC, Patterns of brain activity associated with variation in voluntary wheel-running behavior, Behav Neurosci 117(6) (2003) 1243–56. [DOI] [PubMed] [Google Scholar]
- [44].Ancoli-Israel S, Liu L, Rissling M, Natarajan L, Neikrug AB, Palmer BW, Mills PJ, Parker BA, Sadler GR, Maglione J, Sleep, fatigue, depression, and circadian activity rhythms in women with breast cancer before and after treatment: a 1-year longitudinal study, Support Care Cancer 22(9) (2014) 2535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schmidt ME, Semik J, Habermann N, Wiskemann J, Ulrich CM, Steindorf K, Cancer-related fatigue shows a stable association with diurnal cortisol dysregulation in breast cancer patients, Brain Behav Immun 52 (2016) 98–105. [DOI] [PubMed] [Google Scholar]
- [46].Toma W, Kyte SL, Bagdas D, Alkhlaif Y, Alsharari SD, Lichtman AH, Chen ZJ, Del Fabbro E, Bigbee JW, Gewirtz DA, Damaj MI, Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse, Neuropharmacology 117 (2017) 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oka T, Oka K, Kobayashi T, Sugimoto Y, Ichikawa A, Ushikubi F, Narumiya S, Saper CB, Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors, J Physiol 551(Pt 3) (2003) 945–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Carpenter JS, Gilchrist JM, Chen K, Gautam S, Freedman RR, Hot flashes, core body temperature, and metabolic parameters in breast cancer survivors, Menopause 11(4) (2004) 375–81. [DOI] [PubMed] [Google Scholar]
- [49].Dougherty JP, Wolff BS, Cullen MJ, Saligan LN, Gershengorn MC, Taltirelin alleviates fatigue-like behavior in mouse models of cancer-related fatigue, Pharmacol Res 124 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K, Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial, Int J Cancer 137(2) (2015) 471–80. [DOI] [PubMed] [Google Scholar]
- [51].Vollmers PL, Mundhenke C, Maass N, Bauerschlag D, Kratzenstein S, Rocken C, Schmidt T, Evaluation of the effects of sensorimotor exercise on physical and psychological parameters in breast cancer patients undergoing neurotoxic chemotherapy, J Cancer Res Clin Oncol 144(9) (2018) 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Repka CP, Hayward R, Effects of an Exercise Intervention on Cancer-Related Fatigue and Its Relationship to Markers of Oxidative Stress, Integr Cancer Ther 17(2) (2018) 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA, The anti inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease, Nat Rev Immunol 11(9) (2011) 607–15. [DOI] [PubMed] [Google Scholar]
- [54].Soch A, Bradburn S, Sominsky L, De Luca SN, Murgatroyd C, Spencer SJ, Effects of exercise on adolescent and adult hypothalamic and hippocampal neuroinflammation, Hippocampus 26(11) (2016) 1435–1446. [DOI] [PubMed] [Google Scholar]
- [55].van Vulpen JK, Schmidt ME, Velthuis MJ, Wiskemann J, Schneeweiss A, Vermeulen RCH, Habermann N, Ulrich CM, Peeters PHM, van der Wall E, May AM, Steindorf K, Effects of physical exercise on markers of inflammation in breast cancer patients during adjuvant chemotherapy, Breast Cancer Res Treat 168(2) (2018) 421–431. [DOI] [PubMed] [Google Scholar]
- [56].Smith TTG, Barr-Gillespie AE, Klyne DM, Harris MY, Amin M, Paul RW, Cruz GE, Zhao H, Gallagher S, Barbe MF, Forced treadmill running reduces systemic inflammation yet worsens upper limb discomfort in a rat model of work-related musculoskeletal disorders, BMC Musculoskelet Disord 21(1) (2020) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Marks DL, Ling N, Cone RD, Role of the central melanocortin system in cachexia, Cancer Res 61(4) (2001) 1432–8. [PubMed] [Google Scholar]
- [58].Gautron L, Laye S, Neurobiology of inflammation-associated anorexia, Front Neurosci 3 (2009) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Malik NM, Moore GB, Kaur R, Liu YL, Wood SL, Morrow RW, Sanger GJ, Andrews PL, Adaptive upregulation of gastric and hypothalamic ghrelin receptors and increased plasma ghrelin in a model of cancer chemotherapy-induced dyspepsia, Regul Pept 148(1-3) (2008) 33–8. [DOI] [PubMed] [Google Scholar]
- [60].Gomez-Pinilla F, Ying Z, Differential effects of exercise and dietary docosahexaenoic acid on molecular systems associated with control of allostasis in the hypothalamus and hippocampus, Neuroscience 168(1) (2010) 130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Gilliam LA, St Clair DK, Chemotherapy-induced weakness and fatigue in skeletal muscle: the role of oxidative stress, Antioxid Redox Signal 15(9) (2011) 2543–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


