Highlights
-
•
The electron-stream effect is responsible of an out-of-field dose when performing partial breast irradiation at the MR-linac.
-
•
The electron-stream effect is located to the patient́s chin and, for lateral targets, to the arm.
-
•
The electron-stream effect is effectively minimized by a bolus.
-
•
Novel concepts and clinical trials for breast cancer at the 1.5 T MR-linac can be tested.
Keywords: Partial breast irradiation, MR linear accelerator, MRL, Electron stream effect, Toxicity, Cosmesis, PROMs
Abstract
Introduction
The hybrid magnetic resonance linear accelerator (MRL) has the potential to test novel concepts in breast cancer patients such as daily MR-guided real-time plan adaptation. Before starting clinical trials, preparatory studies for example of the MR-dependent electron stream effect (ESE) are necessary.
Material and Methods
To prospectively investigate the ESE, data from 11 patients treated with partial breast irradiation (PBI) at the 1.5 T MRL were evaluated. A bolus was placed on the chin and in vivo dosimetry results were compared with the dose simulated by the treatment planning system (TPS). The same measurements were carried out for three patients treated at a conventional linac. Toxicity and cosmesis were evaluated.
Results
Median doses measured and simulated on top/ underneath the bolus were 1.91 / 0.62 Gy and 2.82 / 0.63 Gy, respectively. Median differences between calculations and measurements were 0.8 Gy and 0.1 Gy. At the conventional linac, median measured doses on top/ underneath the bolus were 0.98 and 1.37 Gy. No acute toxicity exceeding grade 2 was recorded. Cosmesis was good or excellent and patient reported outcome measures were mostly scored as none or mild.
Conclusion
The dose due to the ESE is low, correctly predicted by the TPS and effectively minimized by a bolus.
1. Introduction
The majority of patients with breast cancer presents with localized disease and have overall survival rates at five years of more than 99% [1]. Clinical research focusses on reducing acute and late toxicities while maintaining the excellent tumour control and survival rates.
Based on the observation that most relapses occur in the tumour bed [2], [3], partial breast irradiation (PBI) was implemented initially as brachytherapy [4], [5], [6], intraoperative radiotherapy [7], [8] and more recently as external beam radiotherapy [9]. PBI represents today a treatment option for low-risk breast cancer patients recommended by international guidelines [10], [11], [12].
Recently, linear accelerators with an integrated magnetic resonance image-guidance device (MRL) have become clinically available. The potential of this new technology has been demonstrated for tumours of different anatomical regions [13], [14], [15], [16], [17], [18], [19]. In early breast cancer, this hybrid system may allow a precise target visualization and daily MR-guided real-time plan adaptation which might facilitate concepts such as neoadjuvant radiotherapy [20]. MRL technology comes with many new challenges as, for example, the MR-specific electron stream effect (ESE). Due to the specific location of the target, the interaction of secondary electrons with the magnetic field is responsible for the ESE [21], [22], [23], [24], [25], [26], which might increase the out-of-field dose, especially in the chin region, as already reported for low-field (0.35 T) magnetic resonance image guidance systems. Before starting clinical trials to test novel treatment concepts in high field MRL, preparatory studies for example to characterize the ESE are necessary. We have reported the first-in-human PBI using the 1.5 T MRL including the description of the ESE in this patient [27]. Based on this observation in a single patient, we extended the prospective evaluation of the ESE to a group of patients treated with PBI at the 1.5 T MRL. To put the data in context, we have performed parallel measurements in patients treated at a conventional linac. Treatment data, toxicity, patient reported outcomes (PROMs) and cosmesis are reported. Furthermore, we examine on phantoms which factors might affect the ESE.
2. Material and methods
2.1. Patient inclusion criteria, treatment planning workflow and follow up
Eleven patients with low risk breast cancer were consecutively treated with PBI at the 1.5 T MRL Unity (Elekta AB; Stockholm, Sweden). Data were collected prospectively as part of a phase 2 feasibility study (NCT04172753, 659/2017BO1). All patients signed informed consent. Definition of low risk (“suitable group”) was based on the GEC-ESTRO [28] and the updated ASTRO criteria [29]: patients older than 50 years with unicentric, unifocal invasive not lobular breast cancer without extensive intraductal component, without lymphovascular invasion and resected with a minimum of 2 mm. Patients with tumours larger than 2 cm and hormone negative (according to ASTRO guidelines), DCIS (according to GEC-ESTRO and, partially, ASTRO guidelines), Her2 positive, with proliferation index greater than 25% and Grade 3 were excluded. Only patients with clips in the tumour bed were enrolled. PBI at the conventional linac was performed with volumetric modulated arc radiotherapy (VMAT) using cone beam computed tomography (CBCT) for daily position verification according to our institutional standard.
Patients received on the same day the planning CT (Brilliance Big Bore, Philips, Eindhoven, The Netherlands) and the planning MR (1.5 T Unity MRL, Elekta, AB, Stockholm, Sweden) in supine position with the wing board device and free breathing. A rigid automatic registration with, if needed, manual modification was used. Target definition and fractionation schedule were based on the IMPORT LOW protocol [9], as previously described [27]. Briefly, the clinical target volume (CTV) was defined including the surgical clips, the seroma, the visible tumour bed as well as the postoperative architectural changes of the surrounding breast gland, without additional margins. The planning target volume (PTV) was created adding 10 mm radial margin, limited to 5 mm from the skin surface and 7 mm posteriorly, according to the standard of our institute for PBI at the conventional linac. Ipsilateral and contralateral breast, heart, ventricles, left coronary artery, right coronary artery (according to [30]) and the lungs were considered organs at risk. The prescribed dose was 40.05 Gy (ICRU) in 15 fractions. Step and Shoot IMRT plans for the MRL were created using Monaco 5.4 (Elekta AB, Stockholm, Sweden). For all patients, a back-up VMAT plan for the conventional linac (Beam Modulator, Elekta AB, Stockholm, Sweden) was generated using our in-house treatment planning system Hyperion (Hyperion Version 4.13). For all fractions, treatment plans were adapted based on the MR of the day. Before and during treatment delivery, the organ motion was monitored with real-time MR-imaging (motion monitoring during “beam-on”), using the PTV as reference volume. Workflow of PBI planning and treatment at the 1.5 T MRL is shown in supplementary Fig. 1. The first clinical follow up visit took place three months after treatment, then one year after treatment. Acute toxicity was scored by the treating physician according to CTCAE V4 and cosmetic results were scored on a three point scale as excellent, good or poor. In addition, PRO-CTCAE™-questionnaires [31] were collected weekly during treatment and one year after, each item was scored on a five point scale, namely none (0), mild (1), moderate (2), severe (3) and very severe (4). One year after treatment, patients were asked to complete additional PROM questionnaires, namely Body Image Scale (BIS), EORTC QLQ-C30, EORTC QLQ-B23, FACIT-F, and FACT-B. Items of the FACIT-F and FACT-B were scored in accordance to the PRO-CTCAE on a five point scale. Items of the BIS, EORTC QLQ-C30 and QLQ-B23 were scored on a four point scale, namely not at all (1), a little (2), quite a bit (3) and very much (4). In the analysis of the PROMs, we considered for all the questionnaires a score of 3 or 4 relevant.
2.2. Electron stream effect evaluation
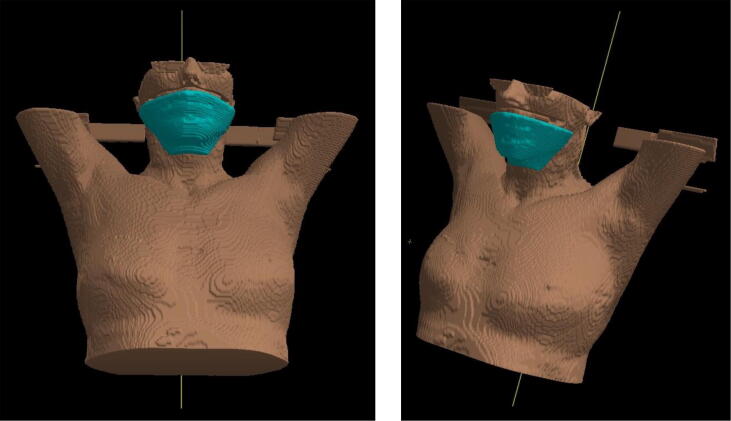
Based on previous publications [21], [22], [23] demonstrating in low-field and phantom measurements that the MR-specific ESE results in an out-of-field dose of the chin, we placed a 1 cm bolus (Unger Medizintechnik GmbH Co. KG, Mülheim-Körlich, Germany) on the chin area. Since the ESE was shown to be a phenomenon that deflects electrons scattered from the high dose region into air in the direction of the magnetic field, the bolus has been positioned horizontally in cranio-caudal direction with respect to the high dose region (Fig. 1). In vivo dosimetry was performed during one treatment fraction using Grafchromic® EBT3 (Grafchromic, Ashland Specialty Ingredients, NJ, USA) films which were positioned on top and underneath of the bolus. Films were 2x5cm2 large and placed in correspondence of the areas of expected higher ESE dose according to Monacós calculations. As previously described [27], the measured dose was averaged in an area of 1.5 × 4 cm2 of the radiochromic films. The simulated dose was calculated in the TPS on top and underneath the bolus using a margin of 3 mm thickness (corresponding to the dose grid resolution). The simulated dose was averaged in an equivalent area of the highest scoring dose voxels. Results of the in vivo dosimetry and the simulated dose were compared using the Wilcoxon signed rank test. For comparison with CB-CT position verification, in vivo film dosimetry was performed in three patients treated with PBI at the conventional linac (Synergy, Elekta AB, Stockholm, Sweden), the same way we performed dosimetry at the MRL, namely during one fraction with a bolus placed on patient́s chin and films placed on top and underneath the bolus (here the measured dose represents a sum of dose scattered by the linac and dose from the CB-CT). PBI treatments at the conventional linac were performed with 6 MV VMAT with two half arcs (e.g. from 0 to 180° and back, depending on patients anatomy and tumour location). Regarding the CBCT protocols, for right breast: start 20°, stop 180°, 120 KV, 366 frames, 585.6 mAs, nominal dose 12 mGy. For left breast: start 340°, stop 180°, 120 KV, 366 frames, 585.6 mAs, nominal dose 12 mGy
Fig. 1.
3D reconstruction of the bolus position on patient́s chin.
2.3. Phantom plans comparison
The aim of this investigation was to study the ESE in different geometrical scenarios: position and volume of the breast as well as target position within the breast. For this, we used the Rando Alderson Phantom (Radiology Support Device, Long beach, CA, USA) and artificial breast prosthesis with different size (130 and 275 ml; Mesmo Sensitive/ POLYtxt, Opticon, Sublime line, Polythec Health & Aesthetics GmbH, Dieburg, Germany). To test if the breast position, and not only the breast volume, has an impact on the dose to the chin, three different planning CTs and planning MRs were performed: 1. 130 ml- prosthesis in a caudal position, 2. 275 ml- prosthesis in a caudal position and 3. 275 ml- prosthesis in a cranial position. To test if the tumour location has an impact on the dose to the chin, we contoured for each of the three scenarios a central and a lateral located target. CTV was defined as a circle of 23 mm diameter and PTV obtained as for patients treatment (10 mm in all direction, but 7 mm posteriorly and 5 mm distant from the skin). Plans for each breast volume and position and tumour location were created with Monaco 5.4 and the dose to the chin in the presence of the magnetic field was calculated.
3. Results
Patient, tumour and treatment characteristics are summarized in Table 1. Step and Shoot IMRT plans with five to seven segments per beam were used. Treatment plans calculated with the TPS Monaco showed in all patients out-of-field dose spots in the chin area or the upper arm. Mean doses, for the sum of all 15 fractions and 40.05 Gy PTV dose, measured on top and underneath the bolus were 2.69 ± 1.95 SD and 0.56 ± 0.39 Gy, respectively (Table 2). Mean values calculated with the TPS on top and underneath the bolus were 3.11 ± 1.29 and 0.63 ± 0.25 Gy, respectively (Table 2). There was a good concordance between calculated and measured values, namely a mean difference on top of the bolus of 0.42 ± 1.08 Gy and underneath the bolus of 0.07 ± 0.45 Gy (Table 2, supplementary Fig. 2).
Table 1.
tumour characteristics, treatment time, acute toxicity and early cosmetic results for all the patients treated at the 1.5 MRL.
| Patient | Laterality | Tumour location | T stage | Grade | Ki67 (%) | Mean in-room time (range, minutes) | Acute toxicity | Toxicity 3 months | Toxicity 1 year | Cosmesis 1 year |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | right | upper lateral | pT1c | 1 | 5 | 33.5 (25–44) | G1 breast edema, erythema, warm feeling, pain and fatigue | G1 breast edema | G1 breast oedema | good |
| #2 | left | upper central | pT1c | 2 | 5–10 | 26.2 (20–38) | G1 erythema and fatigue | none | none | excellent |
| #3 | left | upper lateral | pT1c | 1 | 10 | 26.9 (20–41) | G1 erythema, warm feeling and pain | G1 breast edema | G1 hyperpigmentation | good |
| #4 | right | lower medial | pT1c | 2 | 5 | 26.3 (20–32) | G2 breast edema, G1 erythema, warm feeling, pain and fatigue | G1 erythema, warm feeling and fatigue | none | good |
| #5 | right | upper medial | pT1c | 2 | 20 | 22.8 (19–29) | G1 erythema, warm feeling and pain | G1 hyperpigmentation | G1 hyperpigmentation | good |
| #6 | right | upper central | pT1a | 1 | 10 | 23.9 (21–33) | G2 breast edema, G1 erythema, pain and fatigue | G1 breast edema, erythema and warm feeling | none | excellent |
| #7 | right | upper lateral | pT1c | 2 | 5–10 | 22.9 (19–29) | G1 erythema | G1 erythema | G1 fibrosis | excellent |
| #8 | left | lower medial | pT1b | 2 | 15–20 | 25.1 (19–31) | G1 erythema, warm feeling, pain and fatigue | G1 hyperpigmentation, fibrosis and fatigue | none | excellent |
| #9 | right | upper lateral | pT1c | 1 | 5 | 23.5 (19–26) | G1 erythema and warm feeling | none | none | good |
| #10 | left | upper central | pT1c | 2 | 15–20 | 25.1 (20–33) | G1 erythema, warm feeling and pain | G1 edema; exanthema, in few days resolved | none | excellent |
| #11 | left | upper medial | pT1c | 2 | 10 | 27.3 (20–52) | G1 erythema, warm feeling and fatigue | none | none | excellent |
Table 2.
Doses measured with the in vivo dosimetry and calculated by the TPS Monaco on the chin on top and underneath the bolus for the 11 patients treated at the MRL. *Data of patient #9 were excluded because the measured value was more than 1.5 fold lower from the quartile Q(25)–Q(75) and median of the entire cohort (probably because film was not correctly placed). IQA = interquartile range.
| Patient | Dose measured on top of the bolus on chin (Gy) | Dose measured underneath the bolus on chin (Gy) | Dose calculated on top of the bolus on chin (Gy) | Dose calculated underneath the bolus on chin (Gy) | Absolute difference outside the bolus on chin (Gy) | Absolute difference underneath the bolus on chin (Gy) |
|---|---|---|---|---|---|---|
| #1MRL | 1.76 | 0.51 | 2.70 | 0.68 | 0.95 | 0.17 |
| #2MRL | 1.31 | 1.01 | 0.53 | 0.29 | −0.78 | −0.72 |
| #3MRL | 1.25 | 1.40 | 1.03 | 0.51 | −0.22 | −0.89 |
| #4MRL | 6.60 | 0.30 | 5.20 | 0.30 | −1.40 | 0.00 |
| #5MRL | 1.20 | 0.86 | 2.31 | 1.25 | 1.11 | 0.40 |
| #6MRL | 2.43 | 0.00 | 4.46 | 0.54 | 2.03 | 0.54 |
| #7MRL | 3.15 | 0.11 | 4.73 | 0.52 | 1.58 | 0.42 |
| #8MRL | 1.38 | 0.81 | 2.94 | 0.83 | 1.56 | 0.02 |
| #9MRL* | 0.41 | 0.00 | 5.53 | 0.28 | 5.13 | 0.28 |
| #10MRL | 5.81 | 0.09 | 4.48 | 1.02 | −1.33 | 0.93 |
| #11MRL | 2.06 | 0.72 | 2.70 | 0.36 | 0.65 | −0.36 |
| mean | 2.69 | 0.56 | 3.11 | 0.63 | 0.42 | 0.07 |
| Stand. Dev.* | 1.95 | 0.39 | 1.29 | 0.25 | 1.08 | 0.45 |
| Median* | 1.91 | 0.62 | 2.82 | 0.53 | 0.80 | 0.10 |
| IQA* | 1.6 | 0.69 | 2.07 | 0.4 | 2.09 | 0.69 |
At the conventional linac median doses for the three patients measured on top and underneath the bolus were 0.98 Gy (range, 0.75–1.17 Gy) and 1.37 Gy (range, 0.9–1.62 Gy), respectively (Table 3).
Table 3.
Doses measured with the in vivo dosimetry for the 3 patients treated at the conventional linac and doses calculated by Monaco for the 3 patients with out-of-field dose on the arm treated at the MRL.
| Patient | Dose measured on top of the bolus on chin (Gy) | Dose measured underneath the bolus on chin (Gy) |
|---|---|---|
| #1conv.lin. | 0,98 | 0,9 |
| #2conv.lin. | 1,17 | 1,62 |
| #3conv.lin. | 0,75 | 1,37 |
| median | 0,98 | 1,37 |
| Patient | Dose calculated on top of the bolus on arm (Gy) | Dose calculated underneath the bolus on arm (Gy) |
| #3MRL | 0,76 | 0,48 |
| #7MRL | 3,3 | 0,45 |
| #8MRL | 2,2 | 0,45 |
| median | 2,2 | 0,45 |
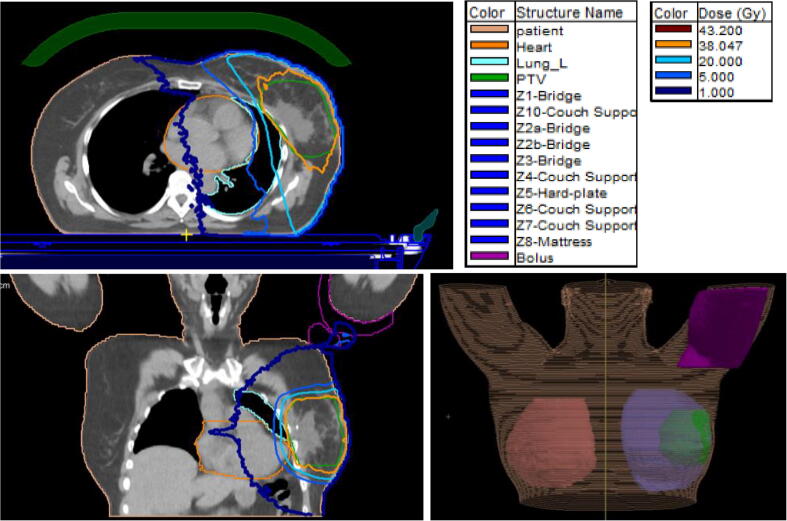
The TPS showed in three patients with laterally located tumours an out-of-field dose as well at the arm. The median calculated doses were 2.2 Gy (range: 0.76–3.3 Gy) and 0.45 Gy (0.45–0.48 Gy) on top and underneath the bolus placed at the arm (Table 3 and Fig. 2).
Fig. 2.
Example of out-of-field dose on the arm for a patient with lateral located tumour. Left the PTV in green and the isodoses are shown, in orange the 95% isodose (region of interest and isodose legend displayed in the figure). Upper left: sagittal, lower left: coronal image. Lower right a three dimensional reconstruction of the patient, including ipsilateral, controlateral breast, PTV and bolus on the right arm is shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The phantom simulation showed a higher chin dose when the target was located medially. A median dose on top of the bolus of 1.35 Gy (range, 0.49–1.6 Gy) for lateral targets and 3.66 Gy (range, 1.71–4 Gy) for medial targets was calculated. Doses were minimized by the bolus to a median value of 0.14 Gy both for lateral (range, 0.1–0.2) and medial (range 0.13–0.32 Gy) targets (Table 4).
Table 4.
Doses calculated by Monaco in the phantom according to breast size, breast position and target position.
| Phantom | Dose calculated on top of the bolus on chin (Gy) | Dose calculated underneath the bolus on chin (Gy) |
|---|---|---|
| Small Breast lateral | 0,49 | 0,1 |
| Small Breast medial | 1,71 | 0,13 |
| Large Breast lateral | 1,6 | 0,14 |
| Large Breast medial | 3,66 | 0,14 |
| Large High Breast lateral | 1,35 | 0,2 |
| Large High Breast medial | 4 | 0,32 |
| Median lateral | 1,35 | 0,14 |
| Median medial | 3,66 | 0,14 |
Regarding feasibility, a total of 162 fractions were delivered at the 1.5 T MRL. In one patient, two fractions were performed at the conventional linac with the back-up plan because of technical problems. In two other patients the delivery of one fraction had to be interrupted due to technical reasons and the fraction had to be completed the next day at the 1.5 T MRL. For all patients, daily plan adaption with virtual couch shift (adapt-to-position, ATP) [32] was used. The mean in-room treatment time was 25.5 min (range: 19 – 52 min, details in supplementary Fig. 3).
Treatment was well tolerated by all patients: nine patients experienced grade 1 and two patients grade 2 toxicity at the end of the treatment. There was no > grade 1 erythema at all. Importantly, the skin reactions were located only in the high-dose irradiated breast region whereas no erythema was observed in the chin or arm areas. Three months after the end of treatment grade 1 was scored in 8 patients and no ≥ grade 2 toxicity was observed (Table 1). Cosmetic outcomes one year after radiotherapy were scored as good in five patients and excellent in six patients. With 7% of missing data, items of the PRO-CTCAE questionnaire were scored as none or mild by 88.1%, 90.8%, 91.5% and 93% at week one, week two, week three and one year after radiotherapy. Insomnia and fatigue were leading symptoms (supplementary Fig. 4). The other PROM questionnaires (1% of missing data) revealed that 5% of the items were scored as 3 or 4, namely severe or very severe for FACIT-F and FACT-B and “quite a bit or very much” for BIS, EORTC QLQ-C30 and QLQ-B23. Items more often scored as 3 or 4 were hot flashes (4/11 patients), no interest in sex (5/9 patients, 2 patients did not answer), sexual activity not enjoyable (5/9 patients, 2 patients did not answer) and arm / shoulder pain (5/11 patients). Specifically regarding the BIS, one patient reported 4 items with a score or 3 or 4, while all the other patients reported one or none 3 or 4 scores. Scar dissatisfaction was the item within the BIS with most of 3 or 4 scores (in 3/11 patients).
4. Discussion
In this study, ESE was investigated in eleven patients treated with PBI at the 1.5 T MRL. ESE with out-of-field-dose to the chin potentially represents a particular challenge for PBI at the 1.5 T MRL because of the target location, i.e. a convex superficial region. Malkov et al. [23] studied factors influencing ESE using a phantom and a water panel with different surface inclinations (10, 30 and 45°), magnetic field conditions (0, 0.35 and 1.5 T) as well as distance from the isocenter. Higher doses up to 39% were measured when surfaces were more inclined and, surprisingly, with lower magnetic field strengths. The lower doses with 1.5 T were explained by the larger radius of curvature of the electron trajectories in the 0.35 T. Moreover, even though a higher out-of-field dose was measured when the panel was closer to the isocenter, the difference was very small, without revealing a large variation of the ESE dose. Interestingly, even at 0 T an up to 9% out-of-field dose was detected. Furthermore, a good agreement between the Monte Carlo simulated out-of-field ESE dose and the EBT-3 film measurements on phantoms were observed [24]. Similarly, good accordance of simulated and measured dose was reported for the electron return effect [33].
MR guided PBI has been clinically performed in low-field MRL [34], [35] and the ESE has been documented in this setting [21]. We have performed the first-in-human PBI treatment at the 1.5 T MRL [27] and documented an ESE in this first patient. As the ESE has not been systematically evaluated for 1.5 T, the present study aims to prospectively characterize the ESE in a group of consecutive patients resembling interpatient heterogeneity in anatomy and target position. This study was designed as a preparatory trial for future clinical trials testing novel concepts at the 1.5 MRL. Overall, our data suggest the ESE to the chin is low (and comparable to CB-CT based PBI), correctly predicted by the TPS and efficiently prevented by a 1 cm bolus. Minimizing radiation dose to normal tissues is of particular importance for breast cancer patients because these patients have an excellent oncological outcome and may experience very late side effects. Although with a very limited follow-up and patient number, physician and patient reported outcome measurements indicated that the PBI at the 1.5 T was well tolerated.
The phantom measurements indicated that geometric factors may influence the magnitude of the ESE to the chin: higher doses were documented when the target was located medially, while breast size had no impact on ESE dose to the chin. In line with Malkov et al. [23], cranio-caudal position of the target did not affect ESE. In addition, TPS simulations in our study revealed that lateral targets might result in a ESE dose to the arms which again can be minimized by a 1 cm bolus.
Our study has a number of limitations. First, it suffers from inherent inaccuracies of the methods including in vivo dosimetry and low dose simulation in TPS. Geographical mismatch of the area used for in vivo dosimetry and dose simulation might further limit the comparison of the read-out parameters. The impact of this uncertainty on the results of our study is aggravated by the fact that only one film measurement was performed per patient and therefore no information on intra-patient reproducibility could be obtained. Instead, the study was designed to evaluate interpatient variability of the ESE. Second, the number of patients is small and follow-up is very limited. Given the fact that only a small number of patients can be treated at the MRL at a given time, an external validation of our early and limited observations would be of great value. Nevertheless, we feel that our data might give important information for the safety of patients and the design of future interventional trials in breast cancer using the 1.5 MRL. Of note, the phantom analysis was performed to study geometric effects and only calculated doses were used in this part of the study.
Our initial experience with regard to the MR daily adaption procedure, in-room-time and feasibility suggest that the 1.5 MRL appears to be suitable to test novel treatment concepts in breast cancer patients, i.e. neoadjuvant MR-guided radiotherapy. The dosimetric results may lead to protocol recommendations to manage ESE in such trials.
Declaration of Competing Interest
The MR-Linac research program is funded by the German Research Council (to D.T. and D.Z., ZI 736/2-1). Radiation Oncology Tübingen receives financial and technical support from Elekta AB (Stockholm, Sweden) under a research agreement. C.D.C. is supported by the Medical Faculty Tübingen (TüFF).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2020.12.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Mannino M., Yarnold J. Accelerated partial breast irradiation trials: diversity in rationale and design. Radiother Oncol. 2009;91(1):16–22. doi: 10.1016/j.radonc.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Salvadori B., Marubini E., Miceli R., Conti A.R., Cusumano F., Andreola S. Reoperation for locally recurrent breast cancer in patients previously treated with conservative surgery. Br J Surg. 1999;86(1):84–87. doi: 10.1046/j.1365-2168.1999.00961.x. [DOI] [PubMed] [Google Scholar]
- 4.Hattangadi J.A., Powell S.N., MacDonald S.M., Mauceri T., Ancukiewicz M., Freer P. Accelerated partial breast irradiation with low-dose-rate interstitial implant brachytherapy after wide local excision: 12-year outcomes from a prospective trial. Int J Radiat Oncol Biol Phys. 2012;83(3):791–800. doi: 10.1016/j.ijrobp.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polgár C., Ott O.J., Hildebrandt G., Kauer-Dorner D., Knauerhase H., Major T. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(2):259–268. doi: 10.1016/S1470-2045(17)30011-6. [DOI] [PubMed] [Google Scholar]
- 6.Wilkinson J.B., Beitsch P.D., Shah C., Arthur D., Haffty B.G., Wazer D.E. Evaluation of current consensus statement recommendations for accelerated partial breast irradiation: a pooled analysis of William Beaumont Hospital and American Society of Breast Surgeon MammoSite Registry Trial Data. Int J Radiat Oncol Biol Phys. 2013;85(5):1179–1185. doi: 10.1016/j.ijrobp.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Veronesi U., Orecchia R., Maisonneuve P., Viale G., Rotmensz N., Sangalli C. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14(13):1269–1277. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 8.Vaidya J.S., Wenz F., Bulsara M., Tobias J.S., Joseph D.J., Keshtgar M. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014;383(9917):603–613. doi: 10.1016/S0140-6736(13)61950-9. [DOI] [PubMed] [Google Scholar]
- 9.Coles C.E., Griffin C.L., Kirby A.M., Titley J., Agrawal R.K., Alhasso A. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390(10099):1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Interdisziplinaere S3-Leitlinie fuer die Frueherkennung, Diagnostik, Therapie und Nachsorge des Mammakarzinoms. 2017:144.
- 11.Cardoso F.K.S., Ohno S., Penault-Liorca F., Poortmans P. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019 doi: 10.1093/annonc/mdz189. [DOI] [PubMed] [Google Scholar]
- 12.TRCoR FoCO. Postoperative radiotherapy for breast cancer: UK consensus statements. 2016:22–3.
- 13.Werensteijn-Honingh A.M., Kroon P.S., Winkel D., Aalbers E.M., van Asselen B., Bol G.H. Feasibility of stereotactic radiotherapy using a 1.5T MR-linac: multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol. 2019;134:50–54. doi: 10.1016/j.radonc.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Murray J., Tree A.C. Prostate cancer - advantages and disadvantages of MR-guided RT. Clin Transl Radiat Oncol. 2019;18:68–73. doi: 10.1016/j.ctro.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gani C., Boldrini L., Valentini V. Online MR guided radiotherapy for rectal cancer. New opportunities. Clin Transl Radiat Oncol. 2019;18:66–67. doi: 10.1016/j.ctro.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiloiro G., Boldrini L., Meldolesi E., Re A., Cellini F., Cusumano D. MR-guided radiotherapy in rectal cancer: first clinical experience of an innovative technology. Clin Transl Radiat Oncol. 2019;18:80–86. doi: 10.1016/j.ctro.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finazzi T., Palacios M.A., Haasbeek C.J.A., Admiraal M.A., Spoelstra F.O.B., Bruynzeel A.M.E. Stereotactic MR-guided adaptive radiation therapy for peripheral lung tumors. Radiother Oncol. 2020;144:46–52. doi: 10.1016/j.radonc.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Paulson E.S., Ahunbay E., Chen X., Mickevicius N.J., Chen G.-P., Schultz C. 4D-MRI driven MR-guided online adaptive radiotherapy for abdominal stereotactic body radiation therapy on a high field MR-Linac: implementation and initial clinical experience. Clin Transl Radiat Oncol. 2020;23:72–79. doi: 10.1016/j.ctro.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunlop A., Mitchell A., Tree A., Barnes H., Bower L., Chick J. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Transl Radiat Oncol. 2020;23:35–42. doi: 10.1016/j.ctro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groot Koerkamp M.L., Vasmel J.E., Russell N.S., Shaitelman S.F., Anandadas C.N., Currey A. On behalf of the breast tumor site group of the international MR-linac atlantic consortium. Optimizing MR-guided radiotherapy for breast cancer patients. Front Oncol. 2020;10(10):1107. doi: 10.3389/fonc.2020.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J.M., Shin K.H., Kim J.-i., Park S.-Y., Jeon S.H., Choi N. Air-electron stream interactions during magnetic resonance IGRT: skin irradiation outside the treatment field during accelerated partial breast irradiationLuft-Elektronenstrahl-Interaktionen in der Magnetresonanztomographie-geführten IGRT: Hautbestrahlung außerhalb des Zielgebiets während einer akzelerierten Teilbrustbestrahlung. Strahlenther Onkol. 2018;194(1):50–59. doi: 10.1007/s00066-017-1212-z. [DOI] [PubMed] [Google Scholar]
- 22.Hackett S.L., van Asselen B., Wolthaus J.W.H., Bluemink J.J., Ishakoglu K., Kok J. Spiraling contaminant electrons increase doses to surfaces outside the photon beam of an MRI-linac with a perpendicular magnetic field. Phys Med Biol. 2018;63(9):095001. doi: 10.1088/1361-6560/aaba8f. [DOI] [PubMed] [Google Scholar]
- 23.Malkov V.N., Hackett S.L., Wolthaus J.W.H., Raaymakers B.W., van Asselen B. Monte Carlo simulations of out-of-field surface doses due to the electron streaming effect in orthogonal magnetic fields. Phys Med Biol. 2019;64(11):115029. doi: 10.1088/1361-6560/ab0aa0. [DOI] [PubMed] [Google Scholar]
- 24.Malkov V.N., Hackett S.L., van Asselen B., Raaymakers B.W., Wolthaus J.W.H. Monte Carlo simulations of out-of-field skin dose due to spiralling contaminant electrons in a perpendicular magnetic field. Med Phys. 2019;46(3):1467–1477. doi: 10.1002/mp.13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim A., Lim-Reinders S., McCann C., Ahmad S.B., Sahgal A., Lee J. Magnetic field dose effects on different radiation beam geometries for hypofractionated partial breast irradiation. J Appl Clin Med Phys. 2017;18(6):62–70. doi: 10.1002/acm2.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richter SPS, Mönnich D, Dohm OS, Thorwarth D. Influence of a transverse magnetic field on the dose deposited by a 6 MV linear accelerator. Curr Direct Biomed Eng 2017;3:281–5.
- 27.Nachbar M., Monnich D., Boeke S., Gani C., Weidner N., Heinrich V. Partial breast irradiation with the 1.5 T MR-Linac: first patient treatment and analysis of electron return and stream effects. Radiother Oncol. 2019;145:30–35. doi: 10.1016/j.radonc.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Polgar C., Van Limbergen E., Potter R., Kovacs G., Polo A., Lyczek J. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Correa C., Harris E.E., Leonardi M.C., Smith B.D., Taghian A.G., Thompson A.M. Accelerated partial breast irradiation: executive summary for the update of an ASTRO Evidence-Based Consensus Statement. Pract Radiat Oncol. 2017;7(2):73–79. doi: 10.1016/j.prro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Feng M., Moran J.M., Koelling T., Chughtai A., Chan J.L., Freedman L. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79(1):10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.https://healthcaredelivery.cancer.gov/pro-ctcae.
- 32.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M. Adaptive radiotherapy: the Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han EYWZ, Lee HJ, dela Cruz Paulino A, Lee C. Measurement of Electron Return Effect and Skin Dose Reduction by a Bolus in an Anthropomorphic Physical Phantom under a Magnetic Resonance Guided Linear Accelerator (MR-LINAC) System. Int J Med Phys Clin Eng Radiat Oncol 2018.
- 34.Henke L.E., Contreras J.A., Green O.L., Cai B., Kim H., Roach M.C. Magnetic resonance image-guided radiotherapy (MRIgRT): a 4.5-year clinical experience. Clin Oncol (R Coll Radiol) 2018;30:720–727. doi: 10.1016/j.clon.2018.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Acharya S., Fischer-Valuck B.W., Mazur T.R., Curcuru A., Sona K., Kashani R. Magnetic resonance image guided radiation therapy for external beam accelerated partial-breast irradiation: evaluation of delivered dose and intrafractional cavity motion. Int J Radiat Oncol Biol Phys. 2016;96:785–792. doi: 10.1016/j.ijrobp.2016.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.