Abstract
Cardiovascular disease is a leading cause of death. Proneurotensin is a biomarker associated with the development of cardiovascular disease, cardiovascular mortality, and all-cause mortality. We assessed the association of fasting proneurotensin with mortal events by gender and race (black–white) in a US population. Using a case-cohort subpopulation of the Reasons for Geographic and Racial Differences in Stroke study, fasting proneurotensin was measured on a 1,046-person subcohort and in 651 participants with incident coronary heart disease. Higher proneurotensin was associated with all-cause mortality (hazard ratio [HR] 1.6 per interquartile range, 95% confidence interval [CI] 1.3 to 1.9) and cardiovascular mortality (HR 1.8, 95% CI 1.2 to 2.6). For all-cause and cardiovascular mortality, association was stronger in women (HR 1.9, 95% CI 1.4 to 2.6 and HR 2.5, 95% CI 1.4 to 4.7, respectively) than men (HR 1.4, 95% CI 1.0 to 1.8 and HR 1.4, 95% CI 0.9 to 2.3, respectively), although this difference was not significant. Proneurotensin predicted all-cause mortality in both races and was not predictive of cardiovascular mortality in whites but was in blacks. Proneurotensin was not associated with incident coronary heart disease events. Elevated proneurotensin levels predicted all-cause and cardiovascular mortality in both genders, with a trend toward stronger association in women. Associations were similar in blacks and whites. In conclusion, proneurotensin may be a useful biomarker for all-cause and cardiovascular mortality regardless of race, and it is potentially specific in women.
Cardiovascular disease (CVD) is the leading cause of death in the United States (US) in men and women.1,2 Women are underrepresented in CVD trials, and outcomes may not apply to them.3–5 Risk factors for CVD are similar between genders, but their prevalence differs between genders and current risk prediction models may not adequately reflect risk in women.2,6,7 Neurotensin is a 13-amino-acid regulatory peptide found in the central nervous, gastrointestinal, and cardiovascular systems.8–12 Proneurotensin is a stable precursor fragment released in equimolar amounts as neurotensin.13 Elevated fasting proneurotensin levels are associated with the development of CVD, coronary heart disease (CHD), cardiovascular mortality, and all-cause mortality and may be more predictive in women.14,15 Previous studies were predominantly white participants, so further study is needed in other groups.16 We aimed to confirm the association between proneurotensin concentration with mortality and CVD in a US population of blacks and whites, and if proneurotensin specifically predicts CVD and mortality in women and in blacks.
Methods
Data for the analysis was from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study, a large national longitudinal cohort study. The details and design of the REGARDS study have been previously described.17,18 Briefly, REGARDS is a large prospective cohort study evaluating risk factors for racial and geographic disparities in stroke mortality in the United States. From 2003 to 2007, 30,239 black and white community-dwelling adults ≥45 years of age were enrolled by mail and telephone from all 48 contiguous US states with oversampling of blacks and the Southeastern United States because of higher stroke mortality. Written consent was obtained from all participants during in-person evaluation.
The prognostic ability of proneurotensin for all-cause mortality and cardiovascular morality was tested in a subcohort. The prognostic ability for incident CHD was examined with a case-cohort sample. The case-cohort sample included all cases of CHD and a stratified cohort random sample, as previously described.19 If a suspected event was identified during follow-up, details of the event were collected including medical records and death certificates, and next of kin were interviewed to ascertain circumstances around the time of death. All events were centrally adjudicated as previously described.18,20,21 Cases of CHD included 651 individuals with incident events, and the stratified cohort random sample included 1,046 participants. Stratification of the cohort random sample was performed to assure balance for age, gender, and race.
The outcomes of all-cause mortality and cardiovascular mortality were assessed in the cohort random sample using a nested case-control design. Cardiovascular mortality was defined as death due to myocardial infarction (MI), stroke, heart failure, sudden cardiac death, and other cardiovascular causes. Adjudication of cardiovascular mortality was completed through December 31, 2012. All-cause mortality was completed through December 31, 2015, and follow-up was truncated at 10 years from enrollment. Incident CHD in the case-cohort sample included fatal and nonfatal MI. Adjudication of CHD outcomes was complete through December 31, 2012.
Plasma collected at baseline was retrieved from storage for proneurotensin assessment in the case-cohort sample. Assays were performed blinded to clinical data at an independent laboratory (ICI Immunochemical Intelligence GmbH, Berlin, Germany) using a 1-step sandwich immunoassay based on a chemiluminescence-label and coated-tube technique (SphingoTec, Hennigsdorf, Germany). The assay has a functional sensitivity of 10 pmol/L determined as the lowest concentration measurable with an interassay precision of maximally 20% coefficient of variation. The limit of detection was 1.9 pmol/L. The interassay coefficient of variation in the proneurotensin concentration range observed in the present study was 5% to 9%.
To replicate the analysis in the Malmö Diet and Cancer (MDC) study of proneurotensin, a multivariable model was tested using the same covariates of age, gender, systolic blood pressure (SBP), body mass index (BMI), antihypertensive therapy, diabetes mellitus (DM), current cigarette smoking, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol.14 To take advantage of the unique features of REGARDS, the variables race, region, annual household income, and education were added. Assessment of variables is as previously described, with cigarette smoking defined as current use regardless of frequency.18
Group comparisons of continuous variables were performed using the Kruskal–Wallis test, and categorical data were compared using the parametric chi-squared test. Proneurotensin was natural log-transformed. For all-cause and cardiovascular mortality, Cox proportional-hazards regression was used to analyze the effect of risk factors on survival in univariable and multivariable analyses. The proportional hazards assumptions were tested for all variables. To test for added predictive value of proneurotensin, we used the likelihood ratio chi-squared test for nested models. For continuous variables, hazard ratios (HRs) were standardized to describe the HR for a biomarker difference of 1 interquartile range. Ninety-five percent confidence intervals (CIs) for risk factors and significance levels based on the Wald chi-squared test. The predictive value of each model was assessed by the model likelihood ratio chi-squared statistic. The concordance index was calculated. Survival curves plotted by the Kaplan–Meier method were used for illustrative purposes. Time-dependent AUC values were determined from censored survival data using the Kaplan–Meier method.22 Time-dependent AUCs were adjusted for age, but further analysis with other variables was limited by the small number of events. Analyses were repeated within each gender and race. Interaction testing was performed for proneurotensin with gender and race. For the outcome of CHD, participants with prevalent CHD were excluded. Statistical methods were the same as those described earlier. All statistical tests were assessed with a 2-sided p value < 0.05 for significance. The statistical analyses were performed using R version 2.5.1 (http://www.r-project.org, library Design, Hmisc) and Statistical Package for the Social Sciences version 22.0 (SPSS Inc., Chicago, Illinois).
Results
A total of 651 CHD events occurred during follow-up. Of these, 93 were excluded for not fasting and 1 was missing a blood sample, leaving 557 cases. Excluded cases were similar to those included except for a higher prevalence of DM (32.1% for included cases vs 44.1% in excluded cases, p = 0.03). The cohort random sample consisted of 1,046 participants, of which 164 lacked fasting samples and 1 was missing a blood sample, leaving 881 participants. Excluded participants had a higher prevalence of DM (20.5% for included participants vs 27.9% in excluded participants, p = 0.04). Of the 881 included participants, 17 (1.9%) had a CHD event.
The average age of the cohort random sample was 67.4 years (SD 12.2 years) with 49% men and 50% black (Table 1A). Men had higher SBP and tobacco use, whereas women had higher BMI, HDL cholesterol, and antihypertensive therapy use. The average age of the CHD cases was 68.2 years (SD 9.2 years) with 64% men and 41% black.
Table 1.
(a) Characteristics for cohort random sample and CHD cases in whole and separated by sex. (b) Study population characteristics for cohort random sample by proneurotensin quartiles
| a | ||||||
|---|---|---|---|---|---|---|
| Random Cohort |
CHD Cases |
|||||
| All | Men | Women | All | Men | Women | |
| Variable | (n = 881) | (n = 431) | (n = 450) | (n = 557) | (n = 355) | (n = 202) |
| Age (years), mean (SD) | 67.4 ± 12.2 | 67.4 ± 12.3 | 67.3 ± 12.1 | 68.2 ± 9.2 | 68.3 ± 9.1 | 68 ± 9.4 |
| Men | 49% | - | - | 64% | - | - |
| Systolic blood pressure, mean (SD) | 128 ± 17.3 | 130 ± 17.2 | 127 ± 17.2 | 135 ± 19.4 | 133 ± 18.3 | 137 ± 21.1 |
| Body mass index (kg/m2), mean (SD) | 28.7 ± 5.8 | 28.2 ± 5.0 | 29.1 ± 6.5 | 29.2 ± 6.3 | 28.2 ± 4.7 | 31.0 ± 8.0 |
| Antihypertensive therapy | 52% | 49% | 54% | 59% | 53% | 70% |
| Diabetes mellitus | 19% | 19% | 19% | 30% | 28% | 34% |
| LDL cholesterol (mg/dL), mean (SD) | 113 ± 33.9 | 113 ± 35.0 | 113 ± 32.7 | 119 ± 36.5 | 116 ± 35.3 | 124 ± 37.9 |
| HDL cholesterol (mg/dL), mean (SD) | 52 ± 16.3 | 46 ± 13.6 | 58 ± 16.2 | 47 ± 14.3 | 44 ± 13.4 | 53 ± 14.0 |
| Current smoker | 14% | 17% | 12% | 20% | 21% | 19% |
| Black | 50% | 50% | 50% | 41% | 34% | 54% |
| Stroke belt | 35% | 35% | 34% | 39% | 36% | 44% |
| Stroke buckle | 18% | 17% | 20% | 17% | 19% | 15% |
| Other | 47% | 48% | 46% | 44% | 46% | 42% |
| Annual income <$20,000 | 18% | 14% | 22% | 24% | 16% | 37% |
| Education < high school | 15% | 16% | 14% | 17% | 14% | 22% |
| Proneurotensin (pmol/L), median (IQR) | 168 [105–272] | 157 [99–262] | 177 [113–279] | 175 [102–292] | 151 [95–261] | 214 [135–316] |
| All-cause mortality | 23% | 27% | 19% | 54% | 55% | 53% |
| Cardiovascular mortality | 6.2% | 8.1% | 4.4% | 38% | 37% | 40% |
| Coronary Heart Disease Cases | 1.9% | 3% | 0.9% | 100% | 100% | 100% |
| b | ||||||
| Quartiles | ||||||
| Quartile range (pmol/L) | All | 0–105] | 105–168] | 168–272] | 272–1650] | p-value |
| Age, median (IQR) | 68 [57–77] | 67 [56–78] | 70 [60–78] | 66 [55–75] | 68 [57–76] | 0.087 |
| Men | 49% | 56% | 48% | 46% | 46% | 0.091 |
| Systolic blood pressure, median (IQR) | 126 [118–139] | 124 [117–134] | 127 [119–139] | 125 [118–137] | 129 [118–142] | 0.078 |
| Body mass index (kg/m2), median (IQR) | 28.1 [24.4–31.6] | 27.1 [23.8–30.5] | 27.7 [24.3–31.4] | 28.3 [24.7–31.6] | 29.4 [25.6–33.9] | <0.001 |
| Antihypertensive therapy | 54% | 45% | 54% | 50% | 65% | 0.001 |
| Diabetes mellitus | 19% | 12% | 13.4% | 20% | 31% | <0.001 |
| LDL cholesterol (mg/dL), median (IQR) | 110 [90–134.8] | 113.5 [91–135.5] | 114 [96–139] | 109 [88–132] | 106 [83.5–127] | 0.020 |
| HDL cholesterol (mg/dL), median (IQR) | 49 [40–61] | 47 [39–58.8] | 50 [41–61] | 51 [43–62] | 49 [41–63] | 0.114 |
| Current smoker | 14% | 10% | 13% | 16% | 18% | 0.071 |
| Black | 50% | 39% | 43% | 56% | 64% | <0.001 |
| Region: | 0.569 | |||||
| Stroke belt | 35% | 34% | 33% | 32% | 39% | |
| Stroke buckle | 18% | 19% | 21% | 21% | 14% | |
| Other | 47% | 48% | 46% | 47% | 47% | |
| Annual income <$20,000 | 15% | 12% | 18% | 11% | 20% | 0.021 |
| Education < high school | 18% | 13% | 21% | 16% | 21% | 0.062 |
| Cardiovascular mortality | 6.2% | 3.6% | 5.9% | 5.9% | 9.5% | 0.079 |
| All-cause mortality | 23% | 18% | 24% | 21% | 29% | 0.034 |
In the cohort random sample, multiple characteristics were associated with increasing proneurotensin quartile (Table 1B). BMI, antihypertensive therapy use, prevalence of DM, percentage of participants with education less than a high school diploma, and percentage of black participants all increased with increasing proneurotensin quartile, whereas low-density lipoprotein cholesterol decreased across quartiles. All-cause mortality significantly increased as proneurotensin quartile increased, whereas cardiovascular death did not.
In the cohort random sample, there were 201 deaths. The unadjusted standardized HR for proneurotensin was 1.5 per interquartile range higher concentration (Table 2). Proneurotensin remained significant in both genders with a numerically higher HR in women than men, whereas the HR for mortality was similar by race (Table 2). In the multivariable analysis, proneurotensin remained a significant predictor of all-cause mortality with an HR of 1.6 (Table 2). Similar to the unadjusted models, women had a higher HR than men and similar HRs by race. Interaction testing showed that the differences by gender (p = 0.51) and race (p = 0.60) were not statistically significant. Findings were similar when analyzed with the covariates from the MDC study alone (data not shown).
Table 2.
Baseline fasting proneurotensin and risk of all-cause mortality and cardiovascular mortality in the cohort random sample
| Univariate |
Multivariable* |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | Gender | HR | 95% CI | p-value | Events | HR | 95% CI | p-value | |
| All-cause mortality | 201 | All | 1.5 | 1.2–1.8 | <0.01 | 187 | 1.6 | 1.3–1.9 | <0.01 |
| 114 | Male | 1.4 | 1.0–1.8 | 0.02 | 105 | 1.4 | 1.0–1.8 | 0.04 | |
| 87 | Female | 1.7 | 1.3–2.2 | <0.01 | 82 | 1.9 | 1.4–2.6 | <0.01 | |
| Interaction | 0.51 | ||||||||
| 108 | Black | 1.5 | 1.1–1.9 | <0.01 | 100 | 1.6 | 1.2–2.1 | <0.01 | |
| 93 | White | 1.4 | 1.1–1.9 | 0.01 | 87 | 1.7 | 1.2–2.3 | <0.01 | |
| Interaction | 0.60 | ||||||||
| Cardiovascular Mortality | 55 | All | 1.7 | 1.2–2.4 | <0.01 | 52 | 1.8 | 1.2–2.6 | <0.01 |
| 35 | Male | 1.4 | 0.9–2.3 | 0.12 | 33 | 1.4 | 0.8–2.4 | 0.23 | |
| 20 | Female | 2.5 | 1.4–4.4 | <0.01 | 19 | 2.5 | 1.3–4.7 | <0.01 | |
| Interaction | 0.86 | ||||||||
| 37 | Black | 1.6 | 1.1–2.5 | 0.03 | 35 | 1.9 | 1.2–3.1 | 0.01 | |
| 18 | White | 1.4 | 0.8–2.7 | 0.27 | 17 | 1.7 | 0.8–3.5 | 0.11 | |
| Interaction | 0.20 | ||||||||
| Incident Coronary Heart Disease | 512 | All | 1.1 | 1.0–1.2 | 0.15 | 512 | 1.1 | 1.0–1.2 | 0.24 |
| 326 | Male | 1.1 | 0.9–1.2 | 0.42 | 313 | 1.1 | 0.9–1.3 | 0.27 | |
| 186 | Female | 1.3 | 1.0–1.6 | 0.02 | 177 | 1.1 | 0.9–1.4 | 0.35 | |
| 222 | Black | 1.2 | 1.0–1.4 | 0.07 | 210 | 1.1 | 0.9–1.3 | 0.43 | |
| 290 | White | 1.1 | 0.9–1.3 | 0.31 | 280 | 1.1 | 0.9–1.3 | 0.42 | |
Covariates for multivariate analysis include age, sex, systolic blood pressure, body mass index, antihypertensive therapy, diabetes mell use, LDL cholesterol, HDL cholesterol, race, region, education, and annual income mellitus current tobacco
HR = standardized hazard ratio; CI = confidence interval.
In a multivariable model, significant variables included age, proneurotensin, and smoking. BMI was significant in women, whereas income was significant in men. Adding proneurotensin modestly improved prognostic power in the whole cohort (Table 3). When analyzed by gender, although proneurotensin significantly improved the model in men, the change in the concordance index was marginal, whereas the change for women was significant and sizeable (Table 3).
Table 3.
C statistic of proneurotensin, multivariate model with proneurotensin, and with the addition of proneurotensin to the model
| Outcome | Gender | proNT | Basic Model* | Model + proNT* | p-value (added Chi2) |
|---|---|---|---|---|---|
| All-cause Mortality | All | 0.581 | 0.752 | 0.761 | <0.0001 (19.6) |
| Male | 0.552 | 0.720 | 0.722 | 0.038 (4.3) | |
| Female | 0.632 | 0.774 | 0.793 | <0.0001 (18.4) | |
| Cardiovascular Mortality | All | 0.622 | 0.770 | 0.784 | 0.004 (8.6) |
| Male | 0.581 | 0.749 | 0.746 | 0.216 (1.5) | |
| Female | 0.718 | 0.710 | 0.764 | 0.003 (8.8) |
The multivariate models were made with age, sex, systolic blood pressure, body mass index, antihypertensive therapy, diabetes mellitus, current tobacco use, LDL cholesterol, HDL cholesterol, race, region, education level and income. Significant variables in all-cause mortality model included age, proneurotensin, and smoking while body mass index was only significant in women and income was only significant in men. For cardiovascular mortality, age and proneurotensin where significant in the entire cohort as well as each sex, while systolic blood pressure, education, and race were only significant when all participants were analyzed but not when analyzed by sex. The p-value refers to the added chi square of proneurotensin on top of the basic model.
proNT = proneurotensin
bootstrap corrected c index for multivariable models.
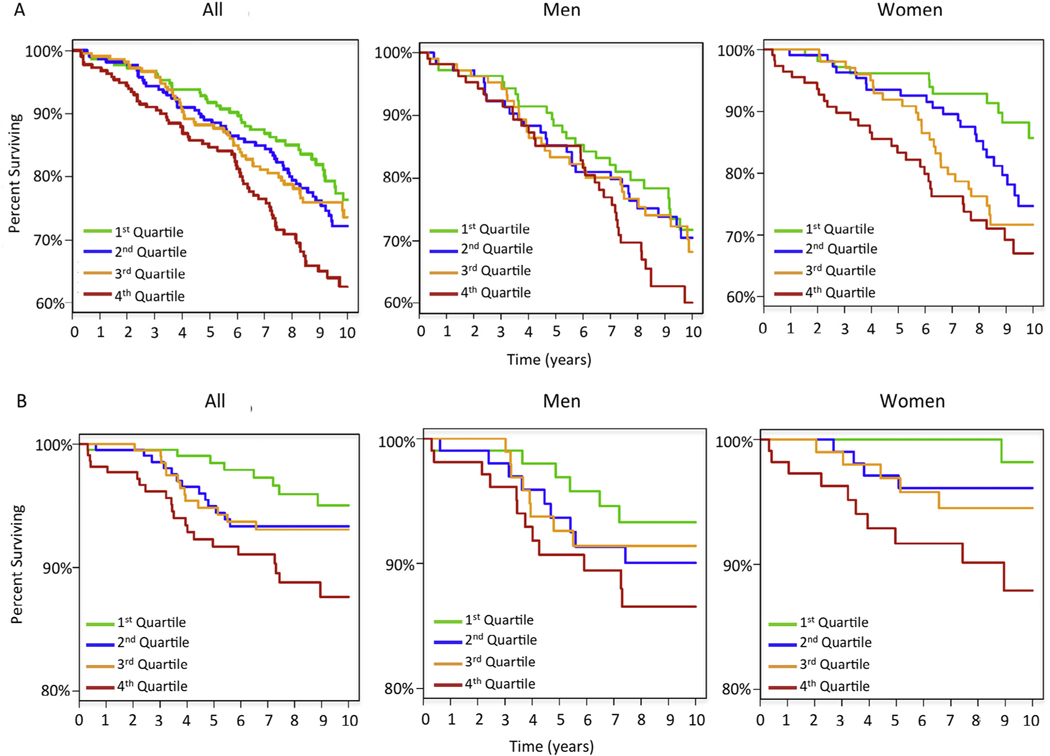
Figure 1 illustrates the predictive performance of proneurotensin quartiles by the Kaplan–Meier plot. There was an increased mortality in women in the highest quartile with early separation from other quartiles. When proneurotensin was analyzed by time to all-cause mortality in women, there was a time-dependent change in the HR with proneurotensin having better prognostic ability for short-term than long-term all-cause mortality (Table 4A). Within the first 2 years, the HR for proneurotensin was 4.0 (p <0.01, concordance index 0.78). This time-dependent effect was not seen in men (Table 4). Findings were similar when adjusted for age.
Figure 1.
Kaplan–Meier plots for all-cause mortality (A) and cardiovascular mortality (B) separated by proneurotensin quartiles in the entire cohort random sample as well as separated by genders.
Table 4.
Time dependent hazard ratios for proneurotensin for all-cause and cardiovascular mortality for men and women
| Women |
Men |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | FU | Events | HR | 95% CI | C-Index | p-value | Events | HR | 95% CI | C-Index | p-value |
| All-cause mortality | 10 years | 87 | 1.7 | 1.3–2.2 | 0.632 | <0.001 | 114 | 1.4 | 1.0–1.8 | 0.552 | 0.022 |
| 5 years | 36 | 2.2 | 1.5–3.3 | 0.669 | <0.001 | 59 | 1.2 | 0.8–1.7 | 0.540 | 0.336 | |
| 2 years | 9 | 4.0 | 1.8–8.9 | 0.783 | 0.0006 | 16 | 1.1 | 0.5–2.1 | 0.535 | 0.837 | |
| Cardiovascular Mortality | 10 years | 20 | 2.4 | 1.4–4.0 | 0.718 | 0.0016 | 35 | 1.4 | 0.9–2.3 | 0.581 | 0.121 |
| 5 years | 14 | 3.4 | 1.8–6.4 | 0.764 | 0.0002 | 25 | 1.5 | 0.9–2.7 | 0.603 | 0.122 | |
| 2 years | 3 | 9.5 | 1.9–47.6 | 0.959 | 0.0021 | 4 | 0.6 | 0.2–2.5 | 0.500 | 0.504 | |
FU = follow up; HR = standardized hazard ratio; CI = confidence interval.
In the cohort random sample, there were 55 (6.2%) adjudicated cardiovascular deaths. Variables significant for cardiovascular mortality in univariate analysis included male gender, age, SBP, antihypertensive therapy, race, and proneurotensin. The unadjusted standardized HR for proneurotensin was 1.7 per interquartile range (Table 2). When analyzed by gender, the HR was smaller in men than in women, whereas associations were similar by race. In multivariable analysis, proneurotensin remained a significant predictor of cardiovascular mortality, with an HR of 1.8 (Table 2). The HR was higher in women than in men. The association was similar in blacks and whites. Interaction testing for gender was not statistically significant (p = 0.86). Findings were similar when analyzed with the covariates from the MDC study.
In the multivariable model, variables predictive of cardiovascular mortality included age and proneurotensin. The addition of proneurotensin to age modestly improved the prognostic ability in the overall cohort (Table 3). Proneurotensin did not improve the model in men but largely increased the prognostic ability of the model for women (Table 3).
Figure 1 illustrates the Kaplan–Meier plot for cardiovascular mortality by proneurotensin quartiles. The HR for proneurotensin was stronger for short-term cardiovascular mortality than for long-term cardiovascular mortality in women (Table 4). This was not seen for men (Table 4). Results were similar when adjusted for age.
In the case-cohort sample, 223 participants with prevalent CHD were excluded, leaving 512 incident CHD events and 698 without an event during follow-up. The HR for proneurotensin was not increased in unadjusted or multivariable analysis (Table 2). An exploratory analysis was done by gender and race. Proneurotensin predicted CHD in univariate analysis for women but not for men, but this significance was lost in multivariable analysis (Table 2). Proneurotensin did not predict CHD for either race in univariate or multivariate analysis (Table 2).
Discussion
In this national study of US blacks and whites, fasting proneurotensin was prognostic for all-cause mortality and cardiovascular mortality. Proneurotensin remained a significant predictor of all-cause mortality in both genders with a numerically stronger association in women. Race did not affect prognostic ability. Proneurotensin was a significant predictor of cardiovascular mortality in women only. It was a significant predictor in blacks but not in whites, although a low event rate in white subjects may have contributed to this finding. Although there was a sizable numerical difference in the HR between genders for both mortality outcomes, they were not statistically significant. Proneurotensin was not associated with the development of CHD.
These findings add to previous studies of proneurotensin.14,15 In the MDC study, elevated proneurotensin was associated with an increased risk of all-cause mortality and cardiovascular death, mainly in women, from a cohort of over 4,600 individuals.14 Our study showed that proneurotensin had a larger HR for all-cause mortality and cardiovascular death in women than in men. Our findings enhance previous studies by using a diverse population of blacks and whites from the 48 contiguous states of the United States.
Gender differences in the diagnostic and prognostic ability of biomarkers are known.16,23 The findings of our study and the MDC study highlight this for proneurotensin. Less studied is the influence of race on a biomarker.24,25 Race did not influence proneurotensin’s prognostic ability for all-cause mortality. Proneurotensin was prognostic for cardiovascular mortality in blacks but not in whites, which may have been due to a low event rate in whites. Further study is needed to determine if race influences proneurotensin prognostic ability.
The pathophysiologic link between proneurotensin and CVD is unknown. Potentially an elevated proneurotensin reflects metabolic derangements that increase the risk of CVD.14 As neurotensin is involved in fat metabolism, elevated fasting levels of proneurotensin may be analogous to insulin resistance for fat metabolism.11 This may explain the association between proneurotensin and the development of DM.14 As DM is one of the strongest risk factors for CVD in women, proneurotensin could be a unique biomarker specific to women with the ability to predict the development of diabetes, CVD, and death.26,27
A novel finding was that proneurotensin strongly predicted earlier events of mortality and cardiovascular death compared with later events. This suggests serial measurements of proneurotensin could be helpful in risk stratification. The small number of events during the shorter follow-up times and only a single assessment of proneurotensin limit the confidence of this finding. A large cohort study with serial assessment of proneurotensin is needed.
Proneurotensin was not associated with the development of CHD in this study. Previous studies found proneurotensin to predict the development of CVD defined as MI or stroke.14 Women have a higher lifetime risk of stroke, and this combined outcome may have had more events in women yielding enough power to reveal proneurotensin’s prognostic ability.28 Our outcome consisted of fatal CHD and nonfatal MI and may have missed CVD events in women. Further study is needed to determine which specific cardiovascular outcomes proneurotensin may predict.
A possible limitation to using proneurotensin is its association with other risk factors of CVD and mortality. Increasing quartiles of proneurotensin were associated with increasing SBP, BMI, DM, smoking, and lower income. Proneurotensin may be redundant for these risk factors; however, the ability of a single biomarker to integrate the biological influences of multiple risk factors could also be seen as a strength. Other limitations include the exclusion of almost 15% of participants because of nonfasting samples.As mentioned previously, the time-dependent AUC was based on a small number of events during the shorter follow-up times, making the analysis exploratory. Lastly, we had a relatively small number of cardiovascular deaths, so confirmation of our findings is needed.
In conclusion, fasting proneurotensin was predictive of all-cause mortality and cardiovascular mortality in both black and white women. We found a time-dependent change in the prognostic ability of proneurotensin that suggests a potential role for serial assessment of proneurotensin. Proneurotensin did not predict CHD, differing from previous studies of proneurotensin. Proneurotensin may be a useful biomarker for all-cause and cardiovascular mortality specifically in women regardless of race.
Acknowledgment:
The authors thank the investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of investigators and institutions can be found at http://www.regardsstudy.org.
Disclosures
Funding was from U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS) National Institutes of Health (NIH), Department of Health and Human Service with additional funding from NHLBI R01 HL080477 and SphingoTec, Hennigsdorf, Germany. The content is solely the responsibility of the authors and does not represent the official views of the NINDS or the NIH. The NINDS did not have any role in the design and conduct of the study, collection, management, analysis, and interpretation of the data, or preparation or approval of the manuscript.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK, American Heart Association. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 2011;57:1404–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim ES, Carrigan TP, Menon V. Enrollment of women in National Heart, Lung, and Blood Institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J Am Coll Cardiol 2008;52:672–673. [DOI] [PubMed] [Google Scholar]
- 4.Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, Dolor RJ, Douglas PS, Mark DB, Newby LK. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010;3:135–142. [DOI] [PubMed] [Google Scholar]
- 5.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA 2006;295:306–313. [DOI] [PubMed] [Google Scholar]
- 6.Mosca L, Barrett-Connor E, Wenger NK. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 2011;124:2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallam T, Watson KE. Predictors of cardiovascular risk in women. Womens Health (Lond) 2013;9:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem 1973;248:6854–6861. [PubMed] [Google Scholar]
- 9.Kitabgi P, Carraway R, Leeman SE. Isolation of a tridecapeptide from bovine intestinal tissue and its partial characterization as neurotensin. J Biol Chem 1976;251:7053–7058. [PubMed] [Google Scholar]
- 10.Caceda R, Kinkead B, Nemeroff CB. Neurotensin: role in psychiatric and neurological diseases. Peptides 2006;27:2385–2404. [DOI] [PubMed] [Google Scholar]
- 11.Kitabgi P. Prohormone convertases differentially process pro-neurotensin/neuromedin N in tissues and cell lines. J Mol Med (Berl) 2006;84:628–634. [DOI] [PubMed] [Google Scholar]
- 12.Osadchii OE. Emerging role of neurotensin in regulation of the cardiovascular system. Eur J Pharmacol 2015;762:184–192. [DOI] [PubMed] [Google Scholar]
- 13.Ernst A, Hellmich S, Bergmann A. Proneurotensin 1–117, a stable neurotensin precursor fragment identified in human circulation. Peptides 2006;27:1787–1793. [DOI] [PubMed] [Google Scholar]
- 14.Melander O, Maisel AS, Almgren P, Manjer J, Belting M, Hedblad B, Engstrom G, Kilger U, Nilsson P, Bergmann A, Orho-Melander M. Plasma proneurotensin and incidence of diabetes, cardiovascular disease, breast cancer, and mortality. JAMA 2012;308:1469–1475. [DOI] [PubMed] [Google Scholar]
- 15.Januzzi JL Jr, Lyass A, Liu Y, Gaggin H, Trebnick A, Maisel AS, D’Agostino RB Sr, Wang TJ, Massaro J, Vasan RS. Circulating proneurotensin concentrations and cardiovascular disease events in the community: the Framingham Heart Study. Arterioscler Thromb Vasc Biol 2016;36:1692–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniels LB, Maisel AS. Cardiovascular biomarkers and sex: the case for women. Nat Rev Cardiol 2015;12:588–596. [DOI] [PubMed] [Google Scholar]
- 17.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 18.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G, REGARDS Investigators. Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012;308:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson NC, Cushman M, Judd SE, Kissela BM, Safford MM, Howard G, Zakai NA. Associations of coagulation factors IX and XI levels with incident coronary heart disease and ischemic stroke: the REGARDS study. J Thromb Haemost 2017;15:1086–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, Howard G. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olubowale OT, Safford MM, Brown TM, Durant RW, Howard VJ, Gamboa C, Glasser SP, Rhodes JD, Levitan EB. Comparison of expert adjudicated coronary heart disease and cardiovascular disease mortality with the national death index: results from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–344. [DOI] [PubMed] [Google Scholar]
- 23.Lew J, Sanghavi M, Ayers CR, McGuire DK, Omland T, Atzler D, Gore MO, Neeland I, Berry JD, Khera A, Rohatgi A, de Lemos JA. Sex-based differences in cardiometabolic biomarkers. Circulation 2017;135:544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W, Cao J, Steffen BT, Post WS, Stein JH, Tattersall MC, Kaufman JD, McConnell JP, Hoefner DM, Warnick R, Tsai MY. Race is a key variable in assigning lipoprotein(a) cutoff values for coronary heart disease risk assessment: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gijsberts CM, den Ruijter HM, Asselbergs FW, Chan MY, de Kleijn DP, Hoefer IE. Biomarkers of coronary artery disease differ between Asians and Caucasians in the general population. Glob Heart 2015;10:301–311, e311. [DOI] [PubMed] [Google Scholar]
- 26.Leifheit-Limson EC, D’Onofrio G, Daneshvar M, Geda M, Bueno H, Spertus JA, Krumholz HM, Lichtman JH. Sex differences in cardiac risk factors, perceived risk, and health care provider discussion of risk and risk modification among young patients with acute myocardial infarction: the VIRGO study. J Am Coll Cardiol 2015;66:1949–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regensteiner JG, Golden S, Huebschmann AG, Barrett-Connor E, Chang AY, Chyun D, Fox CS, Kim C, Mehta N, Reckelhoff JF, Reusch JE, Rexrode KM, Sumner AE, Welty FK, Wenger NK, Anton B, American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Functional Genomics and Translational Biology, Council on Hypertension. Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation 2015;132:2424–2447. [DOI] [PubMed] [Google Scholar]
- 28.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke 2006;37:345–350. [DOI] [PubMed] [Google Scholar]