Abstract
Haemodynamic instability predisposes patients to cardiac complications in non-cardiac surgery. Esmolol, a short-acting cardioselective beta-adrenergic blocker might be efficient in perioperative cardiac protection, but could affect other vital organs, such as the kidneys, and post-discharge survival. We performed a systematic review on the use of esmolol for perioperative cardiac protection. We searched PubMed, Ovid Medline and Cochrane Central Register for Controlled trials. Eligible randomized controlled studies (RCTs) reported a perioperative esmolol intervention with at least one of the primary (major cardiac or renal complications during the first 30 postoperative days) or secondary (postoperative adverse effects and all-cause mortality) outcomes. We included 196 adult patients from three RCTs. Esmolol significantly reduced postoperative myocardial ischaemia, RR =0.43 [95% confidence interval, CI: 0.21–0.88], p = .02. No association with clinically significant bradycardia and hypotension compared to patients receiving control treatment could be confirmed (RR =7.4 [95% CI: 0.29–139.81], p = .18 and RR =2.21 [95% CI: 0.34–14.36], p = .41, respectively). No differences regarding other outcomes were observed. No study reported postoperative renal outcomes. Esmolol seems promising for the prevention of perioperative myocardial ischaemia. However, the association with bradycardia and hypotension remains unclear. Randomized trials investigating the effect of β1-selective blockade on clinically relevant outcomes and non-cardiac vital organs are warranted.
Key messages
Short-acting cardioselective esmolol seems efficient in the prevention of perioperative myocardial ischaemia.
The possibly increased risk of bradycardia and hypotension with short-acting intravenous beta blockade could not be confirmed or refuted by available data. Future adequately powered trials investigating the effect of β1-selective blockade on clinically relevant outcomes and non-cardiac vital organs are warranted.
Keywords: Esmolol, beta blockers, beta-1 selective blockers, myocardial ischaemia, acute kidney injury, prevention, perioperative care, non-cardiac surgery
Introduction
Perioperative myocardial infarction (PMI) and acute kidney injury (AKI) are serious complications causing morbidity, mortality and substantial costs of medical care. Large studies in non-cardiac surgery patients with prospective ischaemia screening have shown a 5–7% incidence for PMI [1–6] and associated mortality of 12–40% [1,7,8]. Postoperative AKI accounts for 18–47% of all hospital-acquired AKI and carries a poor prognosis with a mortality rate of 25–90% [9].
The perioperative and patient-dependent risk factors of PMI and AKI are overlapping in many respects. One of the main risk factors is haemodynamic instability associated with surgery. This commonly manifests as tachycardia and hypotension leading to hypoperfusion and subsequent injury to the heart and kidneys. These complications may be avoided by using a management regime targeting optimal individualized blood pressure and heart rate levels [10].
The safety and efficacy of perioperative beta-blockade are controversial. Several studies suggest that beta-blockers reduce perioperative myocardial ischaemia and may decrease the risk of PMI and cardiovascular death in high-risk patients [11–14]. On the other hand, in the POISE study, perioperative beta-blocker prophylaxis was associated with serious adverse outcomes [3]. Dose titration, as recommended in current ACC/AHA guidelines, may optimize the beta-blockade and consequently decrease the undesired effects of the treatment [15,16]. The choice of beta-blocking agent may also have affected the results of the previous studies. The metabolism of metoprolol succinate [3] may lead to significant differences in the circulating metoprolol concentrations between individual patients, rendering it unsuitable for prophylactic use [17].
In a recent systematic review and meta-analysis on the effects of beta-blockers in vascular and endovascular surgery [18] the intervention did not improve the postoperative outcome in high-risk patients generally expected to benefit from reduced perioperative myocardial stress and oxygen demand. Contrary to an oral fixed-dose regimen, intravenous beta-blockade targeting prespecified heart rate and blood pressure levels might be efficient in reducing myocardial ischaemia and serious arrhythmias without predisposing end organs to hypoperfusion. In a previous systematic review and meta-analysis on esmolol by Landoni and colleagues, published almost 10 years ago, esmolol seemed to reduce myocardial ischaemia in non-cardiac surgery without increasing episodes of bradycardia and hypotension [19]. However, data regarding patient-centred outcomes and extra-cardiac effects of esmolol are limited. We, therefore, performed a systematic review of the published randomized controlled trials (RCTs) and conducted a meta-analysis on the effects of perioperative esmolol on cardiac and renal outcomes.
Methods
Search strategy
We searched three major databases, PubMed, Ovid Medline and the Cochrane Central Register for Controlled trials for original articles presenting randomized controlled trials (RCTs). Ovid Medline is an extensive medical library containing more than 100 databases such as Embase. We did not apply restrictions regarding the publication date or language. Non-English articles were translated before further analysis. We designed the search strategy to be highly comprehensive and sensitive by including keywords “Intravenous OR infusion OR bolus OR boluses AND esmolol OR adrenergic beta-1 receptor antagonists AND surgery”. The expanding feature of PubMed was exploited; thus searching for “surgery” implies searching for “surgery [text word]” and “surgery [medical subject heading]”. Furthermore, we hand-searched the reference lists of relevant articles for further potentially relevant studies.
Study selection
Two authors (A.O. and L.V.) independently assessed the titles and abstracts of the identified articles. All studies considered as potentially pertinent by at least one of the assessors were retrieved as complete articles. The following inclusion criteria were used for the final selection of the reports: Randomized, parallel-group clinical trials investigating perioperative esmolol intervention compared to placebo, standard treatment, or any other medication, including adult patients (aged over 18 years) undergoing non-cardiac surgery. We did not apply any restrictions regarding the type of anaesthesia. Figure 1 describes the study selection process. A.O. and L.V. independently assessed all the retrieved complete articles for compliance to selection criteria and selected relevant reports for final analysis. Discrepancies were resolved by discussion and in the case of disagreement the last author (E.W.) was consulted. We used the PICO approach for study selection, data extraction and data analysis and synthesis: patients (P), intervention (I), control (C) and outcome (O).
Figure 1.
Flowchart of study selection. RCT: randomized controlled trial; ESM: electronic supplementary material.
Patients (P)
We extracted the number of patients randomized to each treatment arm and their baseline demographic and clinical characteristics (age, gender, comorbidities, information about pre-existing beta-blocker medication, type of surgery and American Society of Anesthesiologists (ASA) classification. To assess the representativeness of the cohort, we recorded the exclusion criteria of each individual study and information about dropouts and withdrawals. In case of missing data, at least two separate attempts in order to contact the study authors were made. We used an individual patient as the unit of analysis and the final analysis was based on intention-to-treat data from the individual clinical studies. As the primary and secondary outcome measures in this study are dichotomous variables, we calculated a risk ratio (RR) as the summary measures. The RR is the ratio of the risk of an event in the esmolol group compared to the no esmolol group. We assessed heterogeneity among studies using the Cochrane Q test (Chi-squared). Inconsistency across studies was quantified by calculating I-squared, which was interpreted the following guide: 0%–40% may not be important, 30%–60% may represent moderate heterogeneity, 50%–90% may represent substantial heterogeneity, and 75%–100% represent considerable heterogeneity. We used fixed effect or random effects modelling for analysis, as appropriate. The random effects model was applied if considerably heterogeneity among the studies is identified. The results were reported in a forest plot with 95% confidence intervals (CIs). We initially planned to conduct subgroup analyses including patients with high perioperative cardiac and/or renal risk and patients preoperatively on beta-blocker medication. However, as the number of included RCTs was small and the reporting of outcomes in specific subgroups heterogeneous, the analyses were regarded as methodologically inappropriate and were left out of the final meta-analysis.
Intervention (I)
We extracted the following data about esmolol intervention: timing and duration of the treatment, bolus or infusion type of administration and the treatment group’s esmolol dosage. Furthermore, we recorded the data about potential unplanned co-interventions.
Control (C) group
We extracted the number of patients randomized into the no-esmolol group and their baseline demographic and clinical characteristics, similarly to the patients randomized into esmolol group. We did not apply restrictions as to the potential treatment of the control group, instead, placebo, standard treatment, or treatment with oral beta-blockers or any other medication started for perioperative cardiac/renal prophylaxis was considered as comparator. Subgroup analyses were conducted including the different treatment protocols of the control group.
Outcomes (O)
The primary outcomes were major cardiac or renal complications during the first 30 postoperative days. The outcomes are specified as follows: myocardial infarction (MI), myocardial ischaemia, cardiac arrest, cardiac death, heart failure, unstable angina pectoris (UAP), new-onset arrhythmias, acute kidney injury (AKI), composite of renal events (AKI, need for renal replacement therapy (RRT), or worsening/development of chronic kidney failure) and composite of cardiac events (MI, UAP, heart failure, new-onset arrhythmias or cardiac death). The secondary outcomes were clinically significant bradycardia and/or hypotension, bronchospasm, stroke, neurologic sequelae, serious infection/sepsis and all-cause mortality. Although our primary focus was the effect of esmolol on postoperative outcomes, based on preliminary searches, we expected a small number of eligible studies, and thus, decided to include additional studies with only intraoperative follow-up reporting the predefined outcome measures [20–30]. These studies were excluded from the meta-analysis and their characteristics are presented in the electronic supplementary material (ESM).
Data completeness, the risk of bias and quality of evidence assessment
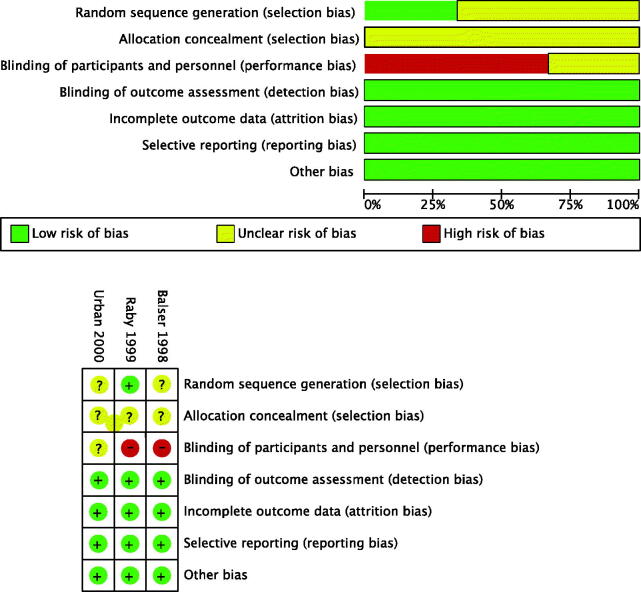
We assessed the completeness of data by calculating a data completeness score based on 36 data items (Supplemental Table 1) with a maximum of 38 points: 12 for patient population (P1–P11; two points for a multi-center study, one point for the rest), four for intervention (I1–I4; one point for each), five for control group (C1–C5), and 17 for outcomes (O1–O16; two points for a reported 30-day outcome, one point for the rest). This type of methodological scoring has been used in other reviews as well, for instance by Efendijev and colleagues [31]. The selection of data items and the scoring vary between reviews depending on respective designs and aims. In addition, we assessed the risk of bias of the selected studies using the Cochrane tool [32] for assessing the risk of bias of randomized trials. The Cochrane tool includes the following domains: adequate random sequence generation, adequate allocation concealment, blinding of participants and personnel, blinding of outcome assessment, reporting of attritions or exclusions and re-inclusion, and selective outcome reporting. Figure 3 illustrates the estimated risk of bias of the selected studies. A.O. and L.V. individually assessed the completeness of data and risk of bias of each selected study. Divergences were resolved by discussion and in the case of disagreement, E.W. acted as an adjudicator.
Figure 3.
Summary of the risk of bias of the selected studies.
We assessed quality of data by using the GRADE approach [33]. GRADE evaluates the body of evidence of an outcome according to the following parameters: Risk of bias, presence of reporting bias, inconsistency of data (heterogeneity), indirectness of data (was the outcome of interest tested in a population of interest for the intervention of interest), and imprecision of data (is the sample size smaller than optimal information size and/or is the CI wide, covering zones of no effect (RR 1.0), potential harm (RR 1.25) and potential benefit (RR 0.75)). Based on these parameters, GRADE classifies the level of confidence regarding an outcome as very low, low, moderate or high.
To further investigate the relevance of the results and the strength of evidence of the meta-analysis, we performed trial sequential analysis (TSA) that helps to evaluate if the detected effect holds based on the cumulative evidence and how many subjects would be needed to define that information size is optimal [34,35].
Results
The last search of the databases was conducted on 2 May 2017. The selection of pertinent reports is presented in Figure 1. Database searches yielded a total of 249 reports, published between 1973 and 2017, of which 56 full-texts were reviewed. Ten reports were added to the review based on searches of the reference lists. After reviewing the remaining 66 full-texts, 52 reports were further excluded, 37 (56%) of which not reporting the desired outcome data. Of the remaining 14 reports, 11 presented only intraoperative outcome data and are summarized in the ESM. Finally, we included three reports presenting original data from three RCTs into the final analysis. The studies were published in 1998–2000 and enrolled patients between 1997 and 2000.
Study characteristics
The characteristics of the selected studies are presented in Table 1. The selected three studies included 196 patients of whom 101 received esmolol and 96 received control drug or placebo. Placebo was given to 66 of 96 (68.8%) of the patients in the control group; the remaining 30 patients received diltiazem. In one of the studies [36], one patient was included at two separate times and both times were randomized to receive the control drug, hence from this study 64 patients were taken into analysis. However, outcomes were analyzed and reported using only 63 patients. Two of the studies were open and one double-blinded. The types of surgeries included peripheral vascular, arthroplasty, abdominal, thoracic, neuro-, and general surgery. All the procedures were major surgeries carrying a potential perioperative cardiac risk. Furthermore, all the patients included in the meta-analysis presented with an increased perioperative cardiac risk, having either preoperative myocardial ischaemia, documented coronary artery disease (CAD) or risk factors for CAD, or major surgery requiring postoperative intensive care unit (ICU) admission [11,36,37]. In two studies, esmolol was infused without a loading bolus, and in one study patients received a loading bolus of 12.5–250 mg followed by an infusion. The duration of the intervention varied from 12 to 48 hours between the studies. Esmolol was titrated according to the patients’ postoperative heart rate; the target heart rate was set differently in each study (Table 1). All studies had a single-centre design and an inadequate sample size to reach any statistically significant results in clinical outcome variables.
Table 1.
Characteristics of the studies included in the Meta-analysis.
| Raby | Urban | Balser | ||||
|---|---|---|---|---|---|---|
| Journal | Anesth Analg | Anesth Analg | Anesthesiology | |||
| Year of publication | 1999 | 2000 | 1998 | |||
| Study design | Double-blind | Open | Open | |||
| No. of patients (esmolol) | 15 | 52 | 34 | |||
| No. of patients (control) | 11 | 55 | 30b | |||
| No. of groups | 2 | 2 | 2 | |||
| Preoperative beta-blocker administration,% of randomised patients (esmolol/control) | 33/36 | 27/29 | 39/30 | |||
| Intervention | Esmolol 100–300 µg/kg/min | Esmolol 250 mg/h | Esmolol with a bolus of 12.5–250 mg followed by an infusion of 50–150 µg/kg/min | |||
| Length of administration | 48 hours | 18–24 hoursa | 12 hours | |||
| Starting time of intervention | After surgery | After surgery | After surgery | |||
| Control | Placebo | Placebo | Diltiazem with a bolus of 20–45 mg followed by an infusion of 10–15 mg/h | |||
| Haemodynamic target | Postoperative HR 20% below the ischaemic threshold (minimum of 60 bpm) | Postoperative HR below 80 bpm | Postoperative HR between 80–100 bpm | |||
| No. of patients treated with any beta-blockers in the control group | 9 (81.8) | 18 (32.7) | 0 | |||
| Type of surgery | Peripheral vascular | Orthopaedic | Non-cardiacc | |||
| Urgency |
NR |
|
Elective |
NR |
||
| Outcomes n (%) |
Esmolol |
Control |
Esmolol |
Control |
Esmolol |
Control |
| MI | 0 | 1 (9.1) | 1 (1.9) | 3 (5.5) | NR | NR |
| Myocardial ischaemia | 5 (33.3) | 8 (72.7) | 3 (5.8) | 8 (14.5) | NR | NR |
| Cardiac arrest | NR | NR | NR | NR | NR | NR |
| Cardiac death | 0 | 0 | NR | NR | NR | NR |
| Heart failure | 0 | 0 | NR | NR | NR | NR |
| UAP | 1 (6.6) | 0 | NR | NR | NR | NR |
| New-onset arrhythmias | NR | NR | NR | NR | NR | NR |
| Composite of cardiac events (1) | 1 (6.7) | 1 | 7 (13.5) | 12 (21.8) | NR | NR |
| AKI | NR | NR | NR | NR | NR | NR |
| Composite of renal events (2) | NR | NR | NR | NR | NR | NR |
| Bradycardia | NR | NR | 3 (5.8) | 0 | 0 | 0 |
| Hypotension | NR | NR | 1 (1.9) | 0 | 2 (5.9) | 1 (3.3) |
| Stroke | NR | NR | NR | NR | NR | NR |
| Bronchospasm | NR | NR | NR | NR | NR | NR |
| Comatose symptoms | NR | NR | NR | NR | NR | NR |
| Serious infection/sepsis | NR | NR | NR | NR | NR | NR |
| All-cause mortality | NR | NR | NR | NR | 10.5 (30.8) | 11 (37.9) |
(1) MI, unstable angina, heart failure, new-onset arrhythmias, cardiac death; (2) AKI, need for RRT, worsening development of chronic kidney failure.
HR: heart rate; bpm: beats per minute; NR: not reported; MI: myocardial infarction; UAP: unstable angina pectoris; AKI: acute kidney injury.
On the first postoperative morning esmolol was switched to oral metoprolol (starting dose 25 mg twice a day) that was titrated to keep HR below 80 bpm and continued for the next 48 hours.
bOne patient included at two separate times.
cSurgeries including major abdominal, urologic, thoracic, vascular, neurosurgery, and general surgery operations.
Data synthesis
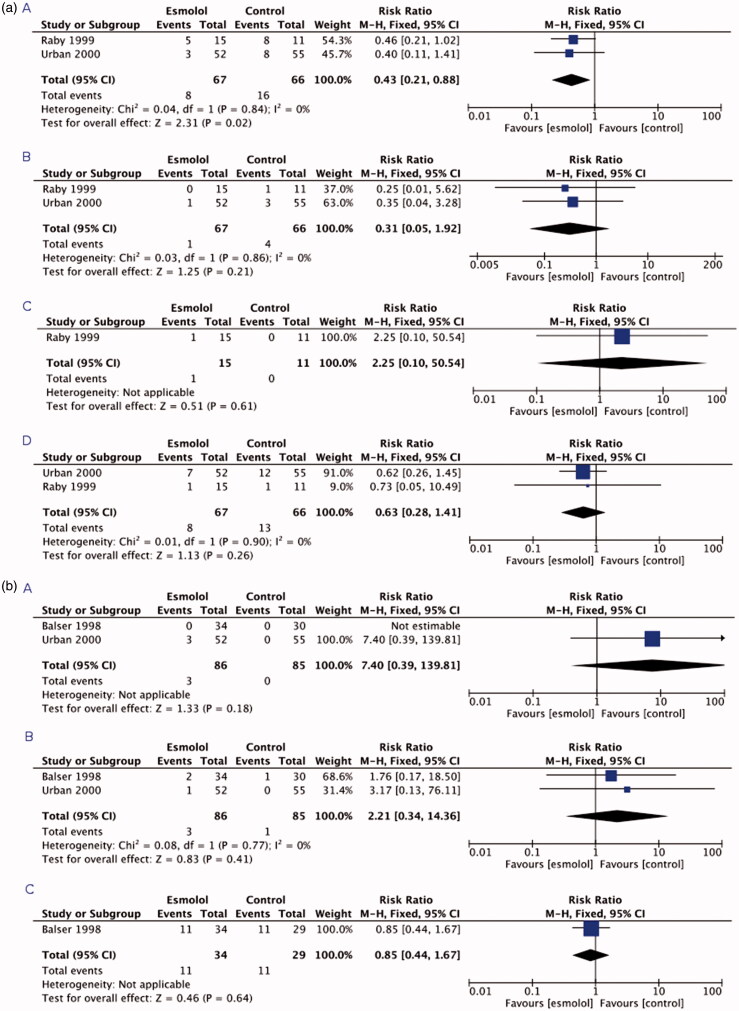
Figure 2 (a,b) graphically demonstrate the results of the meta-analysis. The main findings are summarized in Table 2. Two [11,37] of the three selected studies reported the incidence of myocardial ischaemia, PMI and a composite of cardiac events, and in both studies, esmolol was compared to placebo. The incidence of UAP was reported only in one [11] of the three studies in which esmolol was compared to placebo. None of the studies reported the incidence of AKI or composite of renal events.
Figure 2.
(A) Forest plots of the comparisons (panel A) myocardial ischaemia, (panel B) myocardial infarction, (panel C) unstable angina pectoris, and (panel D) a composite of cardiac events. (B) Forest plots of the comparisons (panel A) bradycardia, (panel B) hypotension, and (panel C) all-cause mortality.
Table 2.
Summary of the main results.
| Number of trials | Esmolol n/N (%) | Control n/N (%) | NNT/NNH [95% CI] | RR [95% CI] | p – effect | p – heterogeneity | I2 (%) | Quality of the evidence (GRADE) | |
|---|---|---|---|---|---|---|---|---|---|
| Myocardial ischaemia | 2 | 8/67 (11.9) | 16/66 (24.2) | 9 [4–159]b | 0.43 [0.21–0.88] | .02 | .84 | 0 | Moderate +++- |
| PMI | 2 | 1/67 (1.5) | 4/66 (6.1) | 22 [9–53]b | 0.31 [0.05–1.92] | .21 | .86 | 0 | Low ++-- |
| UAP | 1 | 1/15 (6.7) | 0/11 | 15 [5–17]a,b | 2.25 [0.10–50.5] | .61 | NA | NA | Low ++-- |
| Composite of cardiac events | 2 | 8/67 (11.9) | 13/66 (19.7) | 13 [5–22]b | 0.63 [0.28–1.41] | .26 | .90 | 0 | Low ++-- |
| Bradycardia | 2 | 3/86 (3.5) | 0/85 | 29 [14–257]a,b | 7.4 [0.29–139.8] | .18 | NA | NA | Low ++-- |
| Hypotension | 2 | 3/86 (3.5) | 1/85 (1.2) | 44 [15–46]a,b | 2.21 [0.34–14.4] | .41 | .77 | NA | Low ++-- |
| All-cause mortality | 1 | 11/34 (32.4) | 11/29 (37.9) | 18 [3–6]b | 0.85 [0.44–1.67] | .64 | NA | NA | Low ++-- |
n/N: events/ randomized patients; NNT: number needed to treat; RR: risk ratio; CI: confidence interval; PMI: perioperative myocardial infarction; UAP: unstable angina pectoris; NA: not applicable; GRADE: GRADE Working Group grades of evidence [33].
NNH: number needed to harm.
bCI for absolute risk reduction extends from a negative to a positive number.
Patients receiving esmolol did not present with more bradycardia and hypotension compared to patients receiving control treatment (Table 2). These outcomes were reported in two [36,37] of the three studies and esmolol was compared to placebo (64.7% of the controls) or diltiazem (35.3% of the controls). Only one study [36], in which esmolol was compared to diltiazem, reported all-cause mortality. None of the studies reported the incidence of bronchospastic symptoms, neurologic sequelae, stroke, or serious infections/sepsis. E-mail enquiries to the authors of the three RCTs confirmed that information (e.g. unpublished observations) regarding the missing outcome measures was not available.
The completeness of reported data and risk of bias
Supplemental Table 2 presents the calculated data completeness scores of each study. The two investigators (A.O. and L.V.) initially disagreed on 19 out of 108 (17.6%) of the registered data items, mostly regarding the reported outcome measures. All these discrepancies were reconciled in the final evaluation. The average total data completeness score was 19.7 (range 18–22) of 38. The studies reported 55.3–57.9% of the required data, of which data regarding the predefined outcomes (O1–O16) were most frequently missing. None of the studies reported the effect of esmolol on postoperative renal function or 30-day survival. Furthermore, none of the studies systematically recorded the adverse effects potentially associated with beta-blocker treatment (O12–O16).
The summary and results of the risk of bias assessment are graphically presented in Figure 3. After the first evaluation, there was a disagreement on 8 of 21 (38.1%) items, mainly regarding the blinding of participants and personnel. A consensus was received in the final evaluation.
The trial sequential analysis confirmed that the detected effect on myocardial ischemia is significant, but only about 10% of optimal information size of 1000 participant required to detect 20% relative change in case that median control group event rate is assumed to be as observed. For other outcomes, there were no significant findings and required sizes much larger and should be evaluated after more studies become available.
Discussion
In this systematic review comprising three RCTs enrolling 196 patients and investigating the effect of target controlled esmolol on postoperative cardiac outcome, we found that perioperative esmolol infusion, titrated according to individual haemodynamic target, significantly decreased postoperative myocardial ischaemia. No clear evidence was found regarding the increase in clinically significant bradycardia and hypotension in patients receiving esmolol compared to patients receiving control treatment. All studies included in the meta-analysis defined bradycardia as a heart rate below 60 bpm [11,36,37]. Furthermore, in one study [36] even higher heart rates were considered as bradycardia if clinical features were suggestive to hypoperfusion (low blood pressure, changes in mental status) were present. Only two studies defined hypotension [36,37] and in those hypotension was defined as a blood pressure level leading the treating physician to discontinue the study drug and administer fluid boluses and/or vasoactive medications. There was no significant heterogeneity among the study populations or interventional treatments. Of note, 27 (28.1%) of the 96 patients randomized to the control group received alternative beta-blocker treatment during the time of intervention. We found no reports investigating the effect of esmolol on postoperative renal function in adult non-cardiac surgery patients.
In all studies included in the meta-analysis, patients were perioperatively continuously monitored with either Holter or telemetry monitoring devices. Two studies [11,37] systematically screened for myocardial ischaemia and both defined the ischemic episode as horizontal or down-sloping ST-segment depression over 1 mm from the baseline lasting over 1 min. The ischaemic episodes were detected by Holter or telemetry devices. The definition of myocardial ischaemia is in accordance with other studies having used continuous electrocardiographic monitoring to detect perioperative ischaemia [38]. Further, this type of silent, transient myocardial ischaemia has been shown to associate with cardiac complications and worse clinical outcome [38]. Today, however, perioperative myocardial ischaemia is most commonly screened by repeated cardiac biomarker, primarily troponin, measurements. Two studies [11,37] reported the incidence of myocardial infarction. In both studies, serum creatine phosphokinase-MB isoenzyme (CK MB) concentration was used for diagnostics. In addition, one study required electrocardiographic changes suggestive to myocardial ischaemia (new ST-segment depression/elevation, T-inversions, Q-waves, and/or bundle branch block) [37] to establish the diagnosis whereas the other did not [11]. It is important to note, that the definition of myocardial infarction used in the studies differs from that of the current clinical practice, both in terms of the used biomarker and assessment of other ischemic features [39].
Beta-blockers in non-cardiac surgery have been extensively studied during the past decades. Earlier studies have shown that beta-blockers may reduce major cardiac events in non-cardiac surgery [40,41]. This conclusion, however, has later been questioned in randomized controlled trials [3,42] and systematic reviews [18,43,44]. Le Manach and colleagues conducted an observational study on the impact of perioperative bleeding on the protective effect of beta-blockers in 1801 patients undergoing infrarenal aortic reconstruction. Although beta-blocker use reduced the risk for postoperative myocardial infarction, it was also associated with an increased frequency of multiple organ dysfunction syndromes and in-hospital deaths. This association was seen especially in patients with severe perioperative bleeding [42]. These observations could likely be explained by decreased cardiac output associating with chronic beta-blocker use, leading into a fail-to-compensate situation, hypoperfusion and multiple organ dysfunction in presence of severe bleeding. This highlights the importance of individualized perioperative treatment and continuous monitoring of haemodynamic parameters in high-risk patients undergoing major surgery. The oral fixed-dose beta-blockade used e.g. in the study by Le Manach and colleagues does not allow rapid titration or discontinuation of the treatment. In the current meta-analysis, we found no significant evidence of the increase of bradycardia or hypotension in esmolol-treated patients. This emphasizes the need for future clinical trials to determine possible positive and negative effects of perioperative intravenous beta-blockade on non-cardiac vital organs. Thus far, only a few studies, all focusing on oral beta-blockers, have been published on the association of perioperative beta-blockers with renal failure in high-risk patients [42,45,46].
No recommendations regarding treatment of perioperative myocardial infarction exist. Moreover, none of the investigated prevention methods has reached a high-class of recommendation according to the European and North American guidelines [47–49]. Perioperative cardiac complications carry a poor prognosis [50] and are costly [51]. Furthermore, recent evidence suggests that myocardial infarction with cardiac biomarker release and other ischemic features are just a tip of an iceberg in a spectrum of perioperative ischemic complications. A mere postoperative cardiac troponin release associates with significantly increased short- and long-term mortality [52]. Trials aiming to reduce these complications are needed, as only few studies on short-acting intravenous beta-blockade and its effects on clinically significant outcomes in non-cardiac surgery exist. This may partly be explained by challenges in conducting the perioperative monitoring that is essential for such intervention. Recently, several manufacturers have launched light, wireless haemodynamic monitoring devices. If these prove feasible at surgical wards, intensified routine monitoring would possibly enable clinical studies about the effect of intravenous beta-blockade on clinically relevant patient-related outcomes.
Contrary to data from non-cardiac surgery, more data on the use of esmolol in cardiac surgery are available. Zangrillo and colleagues showed that esmolol significantly reduced the incidence of perioperative myocardial ischaemia and arrhythmias in patients undergoing cardiac surgery. However, all the included studies were small and no significant differences in the incidence of myocardial infarction or mortality were discovered [53].
The quality of evidence regarding perioperative beta blockade in non-cardiac surgery remains low to moderate. A recent large meta-analysis by Blessberger and colleagues showed that although beta-blockers reduce the incidence of myocardial infarction in non-cardiac surgery patients, risk for all-cause mortality increases [44]. However, the authors combined oral and intravenous beta-blockade in the primary analyses and further, in non-cardiac surgery group 73% of the patients came from the POISE study [3]. In the light of accumulating evidence, the serious adverse effects (stroke, increased all-cause mortality) offset the potential cardioprotective effects of preoperatively started oral fixed-dose beta blockers. However, much less is known about the effects of short-acting intravenous beta-blockade, adjusted according to individual haemodynamic targets. This systematic review and meta-analysis attempted to elucidate this topic. According to the data available, esmolol seems to reduce postoperative myocardial ischemia but its effects on major postoperative cardiac and renal events and mortality remain unknown. Adequately powered clinical trials are needed to answer this question.
The current review has some important limitations to consider when interpreting the results. Firstly, because of the low number of eligible studies and a relatively small number of patients included in these studies, the statistical power of the review was inadequate. TSA analysis confirmed this for all outcomes. Secondly, we did not apply any restrictions regarding the dose or timing of esmolol intervention. However, in all included studies, esmolol was administered postoperatively with comparable infusion rates, thus, diverse administration protocols do not confound the results. Finally, we combined studies that compared esmolol to placebo to those comparing esmolol to another drug. This approach potentially affected the results of bradycardia and hypotension analyses.
In conclusion, esmolol seems promising in the prevention of perioperative myocardial ischaemia and the serious complications associated with prolonged ischaemia. Whether patients receiving esmolol perioperatively are at a higher risk for bradycardia and hypotension remains unclear. Thus, future adequately powered trials investigating the effect of esmolol on clinically relevant patient-related outcomes, such as PMI, AKI and long-term mortality, are warranted.
Supplementary Material
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- 1.Devereaux PJ, Xavier D, Pogue J, et al. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154:523–528. [DOI] [PubMed] [Google Scholar]
- 2.Botto F, Alonso-Coello P, Chan MT, et al. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564–578. [DOI] [PubMed] [Google Scholar]
- 3.POISE Study Group, Devereaux PJ, Yang H, Yusuf S, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371:1839–1847. [DOI] [PubMed] [Google Scholar]
- 4.Nagele P, Brown F, Gage BF, et al. High-sensitivity cardiac troponin T in prediction and diagnosis of myocardial infarction and long-term mortality after noncardiac surgery. Am Heart J. 2013;166:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Waes JA, Nathoe HM, de Graaff JC, et al. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127:2264–2271. [DOI] [PubMed] [Google Scholar]
- 6.Badner NH, Knill RL, Brown JE, et al. Myocardial infarction after noncardiac surgery. Anesthesiology. 1998;88:572–578. [DOI] [PubMed] [Google Scholar]
- 7.Devereaux PJ, Goldman L, Yusuf S, et al. Surveillance and prevention of major perioperative ischemic cardiac events in patients undergoing noncardiac surgery: a review. CMAJ. 2005;173:779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davenport DL, Ferraris VA, Hosokawa P, et al. Multivariable predictors of postoperative cardiac adverse events after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg. 2007;204:1199–1210. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael P, Carmichael AR. Acute renal failure in the surgical setting. ANZ J Surg. 2003;73:144–153. [DOI] [PubMed] [Google Scholar]
- 10.Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318:1346–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raby KE, Brull SJ, Timimi F, et al. The effect of heart rate control on myocardial ischemia among high-risk patients after vascular surgery. Anesth Analg. 1999;88:477–482. [DOI] [PubMed] [Google Scholar]
- 12.London MJ, Hur K, Schwartz GG, et al. Association of perioperative beta-blockade with mortality and cardiovascular morbidity following major noncardiac surgery. JAMA. 2013;309:1704–1713. [DOI] [PubMed] [Google Scholar]
- 13.Welten GM, Chonchol M, Hoeks SE, et al. Beta-blockers improve outcomes in kidney disease patients having noncardiac vascular surgery. Kidney Int. 2007;72:1527–1534. [DOI] [PubMed] [Google Scholar]
- 14.Feringa HH, Bax JJ, Schouten O, et al. Beta-blockers improve in-hospital and long-term survival in patients with severe left ventricular dysfunction undergoing major vascular surgery. Eur J Vasc Endovasc Surg. 2006;31:351–358. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the 2002 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–e157. [DOI] [PubMed] [Google Scholar]
- 16.Antman EM, Hand M, Armstrong PW, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 writing group to review new evidence and update the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction, writing on behalf of the 2004 writing committee. Circulation. 2008;117:296–329. [DOI] [PubMed] [Google Scholar]
- 17.Ismail R, Teh LK. The relevance of CYP2D6 genetic polymorphism on chronic metoprolol therapy in cardiovascular patients. J Clin Pharm Ther. 2006;31:99–109. [DOI] [PubMed] [Google Scholar]
- 18.Hajibandeh S, Hajibandeh S, Antoniou SA, et al. Effect of beta-blockers on perioperative outcomes in vascular and endovascular surgery: a systematic review and meta-analysis. Br J Anaesth. 2017;118:11–21. [DOI] [PubMed] [Google Scholar]
- 19.Landoni G, Turi S, Biondi-Zoccai G, et al. Esmolol reduces perioperative ischemia in noncardiac surgery: a meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth. 2010;24:219–229. [DOI] [PubMed] [Google Scholar]
- 20.Atlee JL, Dhamee MS, Olund TL, et al. The use of esmolol, nicardipine, or their combination to blunt hemodynamic changes after laryngoscopy and tracheal intubation. Anesth Analg. 2000;90:280–285. [DOI] [PubMed] [Google Scholar]
- 21.Gold MI, Sacks DJ, Grosnoff DB, et al. Use of esmolol during anesthesia to treat tachycardia and hypertension. Anesth Analg. 1989;68:101–104. [DOI] [PubMed] [Google Scholar]
- 22.Jangra K, Malhotra SK, Gupta A, et al. Comparison of quality of the surgical field after controlled hypotension using esmolol and magnesium sulfate during endoscopic sinus surgery. J Anaesthesiol Clin Pharmacol. 2016;32:325–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanitz DD, Ebert TJ, Kampine JP. Intraoperative use of bolus doses of esmolol to treat tachycardia. J Clin Anesth. 1990;2:238–242. [DOI] [PubMed] [Google Scholar]
- 24.Kol IO, Kaygusuz K, Yildirim A, et al. Controlled hypotension with desflurane combined with esmolol or dexmedetomidine during tympanoplasty in adults: a double-blind, randomized, controlled trial. Curr Ther Res Clin Exp. 2009;70:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korpinen R, Simola M, Saarnivaara L. Effect of esmolol on the heart rate, arterial pressure and electrocardiographic changes during laryngomicroscopy. Acta Anaesthesiol Scand. 1997;41:371–375. [DOI] [PubMed] [Google Scholar]
- 26.Louizos AA, Hadzilia SJ, Davilis DI, et al. Administration of esmolol in microlaryngeal surgery for blunting the hemodynamic response during laryngoscopy and tracheal intubation in cigarette smokers. Ann Otol Rhinol Laryngol. 2007;116:107–111. [DOI] [PubMed] [Google Scholar]
- 27.Miller DR, Martineau RJ, Wynands JE, et al. Bolus administration of esmolol for controlling the haemodynamic response to tracheal intubation: the Canadian multicentre trial. Can J Anaesth. 1991;38:849–858. [DOI] [PubMed] [Google Scholar]
- 28.Oxorn D, Knox JW, Hill J. Bolus doses of esmolol for the prevention of perioperative hypertension and tachycardia. Can J Anaesth. 1990;37:206–209. [DOI] [PubMed] [Google Scholar]
- 29.Parnass SM, Rothenberg DM, Kerchberger JP, et al. A single bolus dose of esmolol in the prevention of intubation-induced tachycardia and hypertension in an ambulatory surgery unit. J Clin Anesth. 1990;2:232–237. [DOI] [PubMed] [Google Scholar]
- 30.Singh PP, Dimich I, Sampson I, et al. A comparison of esmolol and labetalol for the treatment of perioperative hypertension in geriatric ambulatory surgical patients. Can J Anaesth. 1992;39:559–562. [DOI] [PubMed] [Google Scholar]
- 31.Efendijev I, Nurmi J, Castren M, et al. Incidence and outcome from adult cardiac arrest occurring in the intensive care unit: a systematic review of the literature. Resuscitation. 2014;85:472–479. [DOI] [PubMed] [Google Scholar]
- 32.Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration , 2011. Available from http://Handbook.cochrane.org. In: Higgins JPT, Altman DG, Sterne JAC (editors), ed. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, green S (editors). Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane collaboration, 2011. Available from www.handbook.cochrane.org.; 2011.
- 33.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61:64–75. [DOI] [PubMed] [Google Scholar]
- 35.Thorlund K, Engstrrom J, Wetterslev J, et al. User manual for Trial Sequential Analysis (TSA). Copenhagen (Denmark): Copenhagen Trial Unit; 2018. [cited Sep 18]. Available from: http://www.ctu.dk/tsa/ [Google Scholar]
- 36.Balser JR, Martinez EA, Winters BD, et al. Beta-adrenergic blockade accelerates conversion of postoperative supraventricular tachyarrhythmias. Anesthesiology. 1998;89:1052–1059. [DOI] [PubMed] [Google Scholar]
- 37.Urban MK, Markowitz SM, Gordon MA, et al. Postoperative prophylactic administration of beta-adrenergic blockers in patients at risk for myocardial ischemia. Anesth Analg. 2000;90:1257–1261. [DOI] [PubMed] [Google Scholar]
- 38.Landesber G, Mosseri M, Wolf Y, et al. Perioperative myocardial ischemia and infarction: identification by continuous 12-lead electrocardiogram with online ST-segment monitoring. Anesthesiology. 2002;96:264–270. [DOI] [PubMed] [Google Scholar]
- 39.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Glob Heart. 2012;7:275–295. [DOI] [PubMed] [Google Scholar]
- 40.Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter study of perioperative ischemia research group. N Engl J Med. 1996;335:1713–1720. [DOI] [PubMed] [Google Scholar]
- 41.Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology. 1998;88:7–17. [DOI] [PubMed] [Google Scholar]
- 42.Le Manach Y, Collins GS, Ibanez C, et al. Impact of perioperative bleeding on the protective effect of beta-blockers during infrarenal aortic reconstruction. Anesthesiology. 2012;117:1203–1211. [DOI] [PubMed] [Google Scholar]
- 43.Mostafaie K, Bedenis R, Harrington D. Beta-adrenergic blockers for perioperative cardiac risk reduction in people undergoing vascular surgery. Cochrane Database Syst Rev. 2015;1:CD006342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blessberger H, Kammler J, Domanovits H, et al. Perioperative beta-blockers for preventing surgery-related mortality and morbidity. Cochrane Database Syst Rev. 2018;3:CD004476. PMID 29533470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matyal R, Mahmood F, Panzica P, et al. Sex-related differences in outcome after high-risk vascular surgery after the administration of beta-adrenergic-blocking drugs. J Cardiothorac Vasc Anesth. 2008;22:354–360. [DOI] [PubMed] [Google Scholar]
- 46.Yang H, Raymer K, Butler R, et al. The effects of perioperative beta-blockade: results of the metoprolol after vascular surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152:983–990. [DOI] [PubMed] [Google Scholar]
- 47.Kristensen SD, Knuuti J, Saraste A, et al. ESC/ESA 2014 Guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35:2383–2431. [DOI] [PubMed] [Google Scholar]
- 48.Fleisher LA, Fleishmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64:e77–e137. [DOI] [PubMed] [Google Scholar]
- 49.American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Society of Echocardiography, American Society of Nuclear Cardiology, et al. 2009 ACCF/AHA focused update on perioperative beta blockade. J Am Coll Cardiol. 2009;54:2102–2128. [DOI] [PubMed] [Google Scholar]
- 50.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. [DOI] [PubMed] [Google Scholar]
- 51.Udeh BL, Dalton JE, Hata JS, et al. Economic trends from 2003 to 2010 for perioperative myocardial infarction: a retrospective, cohort study. Anesthesiology. 2014;121:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mauermann E, Puelacher C, Lurati Buse G. Myocardial injury after noncardiac surgery: an underappreciated problem and current challenges. Curr Opin Anaesthesiol. 2016;29:403–412. [DOI] [PubMed] [Google Scholar]
- 53.Zangrillo A, Turi S, Crescenzi G, et al. Esmolol reduces perioperative ischemia in cardiac surgery: a meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth. 2009;23:625–632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.