Abstract
Cellular senescence is a primary aging process and tumor suppressive mechanism characterized by irreversible growth arrest, apoptosis resistance, production of a senescence-associated secretory phenotype (SASP), mitochondrial dysfunction, and alterations in DNA and chromatin. In pre-clinical aging models, accumulation of senescent cells is associated with multiple chronic diseases and disorders, geriatric syndromes, multi-morbidity, and accelerated aging phenotypes. In animals, genetic and pharmacologic reduction of senescent cell burden results in the prevention, delay, and/or alleviation of a variety of aging-related diseases and sequelae. Early clinical trials have thus far focused on safety and target engagement of senolytic agents that clear senescent cells. We hypothesize that these pharmacologic interventions may have transformative effects on geriatric medicine.
Targeting cellular senescence as an intervention in primary aging
Cellular and molecular processes that account for primary aging include chronic, low-grade, non-microbial inflammation, cellular senescence (see Glossary), accumulation of damaged macromolecules (DNA, proteins, carbohydrates, lipids), as well as stem and progenitor cell dysfunction [1]. These changes lead to pathophysiological manifestations of tissue atrophy and loss, denervation, hypertrophy, decreased perfusion, decreased responsiveness to external signals (i.e. stimuli and stressors), decreased regenerative responses to injury, fatty transformation, fibrosis, and changes in homeostatic set points. At the level of the organism, clinical findings present as functional decline, decreased resilience, increased vulnerability, reduced survival, and multi-morbidity.
Substantial progress has been made towards targeting cellular senescence as a fundamental aging process with encouraging results that support the geroscience hypothesis [2]. In pre-clinical models, clearance of senescent cells results in improvement in age-related phenotypes, including geriatric syndromes, chronic diseases, and poor resilience [3–15]. Interventions that genetically or pharmacologically remove senescent cells in animal models of aging offer proof-of-concept that they can be translated for human conditions associated with aging, especially those with heavy senescent cell burden (see Clinician’s Corner). There are many potential interventions proposed to intervene in primary aging processes that may be distinct from cellular senescence; however, these processes are not necessarily mutually exclusive and, in fact, may be interdependent (Figure 1). This suggests that any intervention that targets a single fundamental aging process could affect multiple processes that impact aging.
Clinician’s Corner.
Aging is the most important risk factor for chronic diseases as well as physical and cognitive declines.
Senescent cell burden contributes to age-onset chronic diseases, geriatric syndromes, conditions of accelerated aging (e.g., premature ovarian failure), and pathophysiological processes where there are focal accumulations of senescent cells (e.g., osteoarthritis).
Senescent cells accumulate over time and tissue-specific burden depends upon the net consequences of (1) the type and amount of exposure to inducers of senescence, (2) immune system clearance, and (3) bystander effects of existing senescent cells on non-senescent cells through paracrine and systemic actions.
Targeting senescent cell burden, or any primary aging process, should prevent, delay, or otherwise alleviate multiple age-related conditions. Since senescent cells take weeks to re-accumulate, it may be possible to deliver senolytic drugs intermittently (and infrequently) with the potential to reduce unwanted side effects.
First generation senolytic agents are currently available repurposed drugs and nutraceuticals, but clinical use of these compounds awaits safety, target engagement, and efficacy studies to determine the minimal/optimal amounts and dosage frequency needed to clear senescent cells.
Unlike other clinical trials that focus on recruitment of relatively healthy older adults with a single or few co-morbidities, clinical studies using senotherapeutic drugs hope to recruit older adults with multi-morbidity, geriatric syndromes, or relatively poor physical functioning.
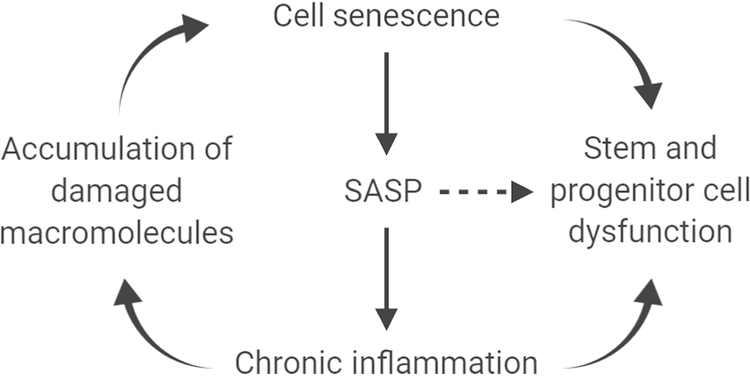
Figure 1. Interdependency of fundamental aging processes.
Cellular senescence can occur in many cell types, including stem and progenitor cells, causing impairments in differentiated function and tissue regeneration. Through production of the SASP, chronic inflammation can mediate deteriorative changes and result in the accumulation of damaged macromolecules. The SASP can also promote paracrine and systemic bystander effects (⇢) that cause stem and progenitor cell dysfunction. This schema is a framework that can be tested in a hypothesis-driven fashion.
Cellular senescence
Cellular senescence has been described as a major mechanism for aging at the cellular level, although likely not the only mechanism. There are multiple inducers of cellular senescence, including DNA damage, reactive metabolites, inflammation, oncogenes, mitogens, proteotoxic stress, and damage-associated molecular patterns [16–18]. A DNA damage response (DDR) is central for the induction of cellular senescence [19]. The DDR can be caused by telomere shortening or dysfunction, mutations, radiation and alkylating agents. Reactive metabolites include reactive oxygen species, fatty acids, elevated glucose, and ceramides. Protein aggregation, unfolded protein responses, and mTOR activation are associated with proteotoxic stress and loss of proteostasis. These inducers ultimately mediate the cellular senescence phenotype through mechanisms that converge on pathways that activate p16INK4a/Rb and/or p53/p21, depending on the inducer and cell type [20]. Hallmarks of the cellular senescence phenotype include irreversible growth arrest, a senescence-associated secretory phenotype (SASP) (Box 1), apoptosis resistance, mitochondrial dysfunction [21], persistent DNA damage foci [22] and epigenetic remodeling, which includes formation of senescence-associated heterochromatin foci [23]. Transposons (“jumping genes”) can be produced within senescent cells that re-insert into senescent cell DNA, contributing to the senescent cell phenotype and exacerbating their SASP [24, 25].
Box 1. The senescence-associated secretory phenotype (SASP).
The SASP consists of pro-inflammatory cytokines, chemokines, and extracellular matrix-degrading proteins that have deleterious paracrine and systemic effects, including inducing senescence in otherwise healthy cells as a bystander effect [11, 95–97]. Factors that are comprised in the SASP vary depending on the cell type from which senescent cells were derived, the inducer(s) of senescence, microenvironmental signals, and the approach to their suppression. The SASP can be modified or normalized using glucocorticoids, rapamycin, metformin, reverse transcriptase inhibitors (e.g., lamivudine) or JAK1/2 inhibitors [13, 27, 55, 98–100]. Depending on the kind of inhibition, not all components of the SASP are down-regulated. Identification of cell-specific SASP factors may identify unique signatures of senescent cells that could be used as surrogate biomarkers for target engagement and clinical intervention. Unlike senolytic agents which can be given intermittently to reduce senescent cell burden, SASP inhibitors, senomodulators, or senostatic agents that modify or suppress the SASP may need to be given on a more regular schedule, since senescent cells are not being cleared.
Although senescent cells may have beneficial effects in a transient fashion (Box 2), they accumulate chronically in virtually all tissues with aging, promoting detrimental consequences. The smallest amount of senescent cell burden required to promote local tissue dysfunction and/or have deleterious effects at distant locations is unknown. However, a relatively low abundance of senescent cells (e.g., ~10–15%) are present in the skin of old primates [26], and transplanting even a small number of senescent cells into younger mice is sufficient to cause tissue dysfunction [12]. Strategies for decreasing senescent cell burden include targeting networks of anti-apoptotic regulators that confer senescent cell survival, reducing the inflammatory SASP, and genetically clearing senescent cells by activating apoptotic signals driven by p16Ink4a or p21Cip1 promoter elements in transgenic animals [3–5, 8, 11–14, 27–34].
Box 2. Potential beneficial effects of cellular senescence.
Cellular senescence is a tumor suppressive mechanism [101], but it may have other beneficial physiological roles in wound healing and in development (i.e., embryogenesis). In contrast to senescent cells that accumulate slowly with aging, other senescent-like cells may function transiently in a remodeling role, with their removal facilitated by the immune system. Senescence may also be beneficial in limiting the fibrotic response produced secondary to liver damage [102], and in promoting stem cell function by inducing cell plasticity and facilitating tissue regeneration [103]. Activation of p16Ink4a in pancreatic beta cells promotes glucose-stimulated insulin release and in diabetic mice results in better glucose control [104]. The SASP may reinforce senescent cell growth arrest of precancerous cells through an autocrine feedback loop [105–107] and also induce senescence of neighboring noncancerous (but dividing) cells through a paracrine mechanism that protects against the same stressors that increase the risk of malignant transformation [95, 108, 109]. The effects of senescence are likely to be context-specific and pleiotropic and so intermittent clearance of senescent cells may help to avoid targeting of those that are beneficial in the short term.
Genetic models of senescent cell clearance
Elimination of even a relatively small proportion of existing senescent cells (~30% reduction) using a “suicide” transgene, INK-ATTAC, that permits inducible elimination of p16Ink4a-expressing senescent cells upon administration of a drug (AP20187; Figure 2), extends healthspan and prevents the development of multiple age-related morbidities in both progeroid and normal chronologically-aged mice [3, 8, 11, 35]. Using mice harboring the p16Ink4a-promoter driven ATTAC suicide transgene (Figure 2) it is possible to test the contributions of p16Ink4a- driven cellular senescence in mediating age-related phenotypes.
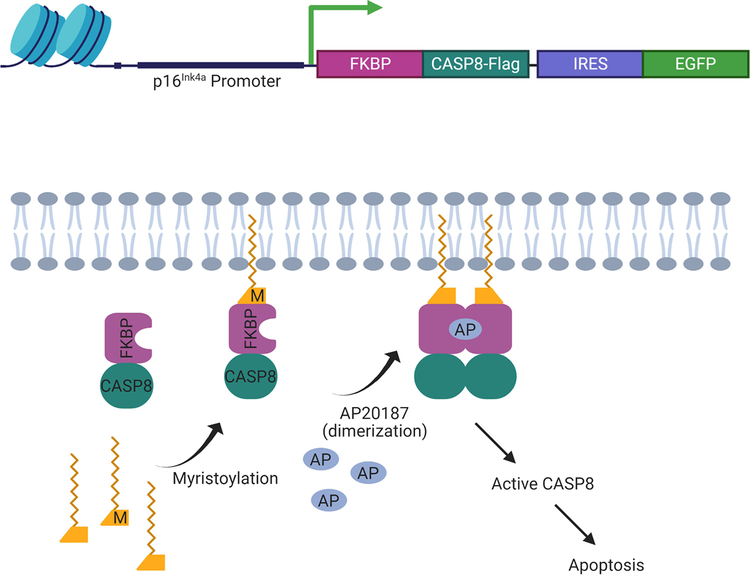
Figure 2. A genetic model for senescent cell clearance.
Upper panel: schematic diagram of the INK-ATTAC construct. Lower panel: mechanism of apoptosis activation in p16Ink4a-positive senescent cells upon administration of AP20187 to INK-ATTAC mice. A fusion protein consisting of FK506-binding protein (FKBP) and caspase 8 (CASP8) is driven by a transcriptionally active element of the p16Ink4a promoter in senescent cells. A small-molecule compound (AP20187) without known off-target effects causes dimerization of FKBP-CASP8 and results in caspase-dependent apoptosis of senescent cells that are positive for p16Ink4a. Monitoring of p16Ink4a-positive senescent cells is accomplished by markers such as enhanced green fluorescent protein (EGFP). Use of internal ribosome entry sites (IRES) improves detection of auto-fluorescent reporter gene products such EGFP. Figure based on information first described by Baker et al. [3].
Current models of senescent cell clearance have been limited to the mouse. However, the rat has tremendous potential for use in geroscience research, owning to its aging physiology being closer to humans compared to mice, especially with respect to onset of certain age-related conditions, as well as its larger size, which permits serial sampling and imaging studies not feasible in the mouse [36–41]. The rat is particularly amendable to measurements of cognitive-behavioral, endocrine (e.g., glucose tolerance), muscle, and cardiovascular endpoints, and there is extensive historical data on physiological changes with aging [42–46]. With advancements in genetic modification technologies in the rat [47–51], it is now possible to create rat models of senescent cell clearance. For example, it would be very useful to produce a rat model where p16Ink4a-positive cells can selectively be eliminated. Results using this rat model would more closely model the effects of cell senescence (and its abrogation) toward human physiology.
Senotherapeutic agents to reduce senescent cell burden
While genetic models of senescent cell clearance provide proof-of-principle for the health and life span benefits of reducing the burden of old cells and the SASP, translation into humans has relied on the identification and development of senotherapeutic drugs, although efforts to develop senolytics actually began before and independently from developing the transgenic mice. Depending on whether these agents mostly promote senescent cell death or normalization of the SASP (i.e. without killing senescent cells), they have been described as senolytics or SASP inhibitors/senomorphics/senostatics, respectively (Figure 3, Key Figure) [12–14, 27–30, 32, 34, 52–56].
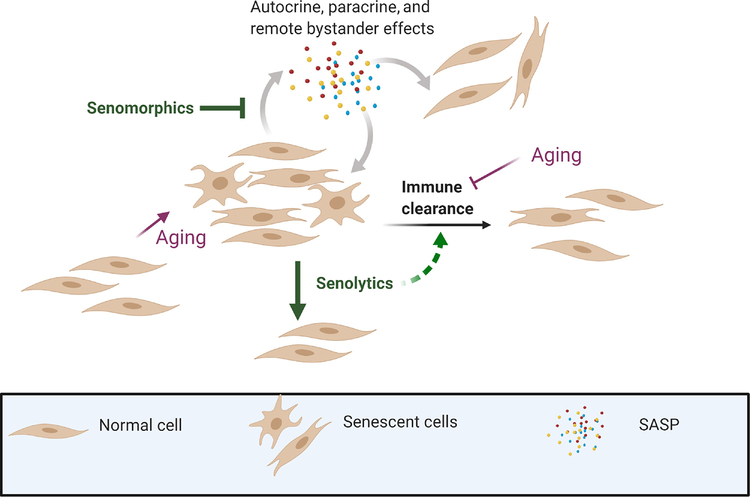
Figure 3. Pharmacologic reduction in senescent cell burden.
SASP inhibitors (senomorphic/ senostatic agents) do not kill senescent cells but suppress or normalize the SASP. Immune clearance mitigates accumulation of senescent cells but diminishes with aging. Senolytic agents kill senescent cells by targeting apoptosis resistance pathways and may also improve immune clearance by removing senescent immune cells.
Senescent cells can resist apoptotic stimuli, thus increasing the likelihood of their survival through anti-apoptotic defenses (Box 3). In some respects, senescent cells are like non-dividing cancer cells, including apoptosis resistance and metabolic shifts [7, 11, 28, 57–59]. Many first-generation senolytics are in fact repurposed cancer therapeutics. Others are bioflavonoids. Senolytics have been used to prevent, delay, or alleviate a plethora of age-onset diseases and syndromes in pre-clinical models. Combinations of senolytics that target more than one pathway conferring apoptosis resistance will conceptually clear larger numbers and types of senescent cell subpopulations, and appears to be the case for the combination of the first generation senolytics dasatinib and quercetin [59]. Dasatinib is an orally bioavailable synthetic small molecule-inhibitor of SRC-family protein-tyrosine kinases used in the therapy of chronic myelogenous leukemia positive for the Philadelphia chromosome. Quercetin is a bioflavonoid and anti-oxidant found in many food sources.
Box 3. Apoptosis resistance networks.
The networks that senescent cells employ to become apoptosis resistant include those involving ephrins/dependence receptors, PI3K/AKT, Bcl-2 (Bcl-xl, Bcl-2, Bcl-w), p53/ FOXO4/ p21/ serpine (PAI-1&2), HIF-1α, and HSP90 [28, 32]. Senolytics target different senescent cell populations, depending on the pathways used to confer apoptosis resistance [59]. For example, dasatinib interferes with apoptosis resistance networks containing dependence receptors, Src and tyrosine kinases, and targets senescent primary preadipocytes (i.e. adipose-derived stem cells). Quercetin interferes with networks containing the Bcl-2 family, p53/p21/Serpine, and PI3K/AKT, and targets senescent endothelial cells and mesenchymal stem cells. In combination, dasatinib and quercetin target those senescent cell types that they individually are effective against, but also target senescent lung and embryonic fibroblasts.
In terms of alleviating age-related phenotypes, minimizing the SASP using Janus kinase/signal transducer and activator of transcription (JAK/STAT) inhibition is very similar to eliminating or reducing the burden of senescent cells genetically (e.g., using INK-ATTAC mice) or pharmacologically with senolytic agents [4, 11, 13, 60]. Single or intermittent doses of senolytics appear to attenuate at least some age- or senescence-related conditions, which may not be the case for SASP-inhibitors, since they do not clear senescent cells. This is related to the time it takes for senescent cells to re-accumulate after they are removed. Based on studies in salamander limb regeneration [61] and in bleomycin-induced senescence in young mice [62], turnover of senescent cells is estimated to be about four weeks. Thus, intermittent treatment with senolytics may be a reasonable approach in humans, potentially reducing side effects. Because senescent cells do not replicate, concerns over possible drug resistance seem unlikely. At younger ages, senescent cells are cleared by the host immune system [63–66], and thus to the extent that senotherapeutic or other agents may also delay or mitigate immune senescence or adverse effects of non-immune senescent cells on immune cell function, clearance of senescent cells may be further optimized (Figure 3).
The use of senotherapeutic drugs could potentially be effective in treating a variety of conditions primarily or secondarily mediated by accumulation of senescent cells [6, 33, 67–87]. These include multi-morbidity, conditions of premature or accelerated aging (e.g., childhood cancer survivors, bone marrow transplant survivors, progeroid syndromes, diabetes due to obesity, human immunodeficiency virus-related dementia and frailty, premature ovarian failure), and conditions with localized cellular senescence (e.g., osteoarthritis, glaucoma, macular degeneration, fracture non-union, radiotherapy-induced bystander effects, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease and tobacco-related lung injury, atherosclerosis, preeclampsia, hyperoxia-induced neonatal lung injury and airway hyper-reactivity) and the geriatric syndromes. Otherwise fatal conditions for which pathogenesis is related to high senescent cell burden are also candidates for treatment with senotherapeutics (e.g., idiopathic pulmonary fibrosis, primary sclerosing cholangitis). Senolytics and/or senomorphics could also be used prophylactically in the setting of clinical stressors that are known to generate senescent cells, such as chemotherapy, radiotherapy, elective surgery, bone marrow transplantation, rehabilitation after myocardial infarction, and hypoxia-reoxygenation as well as other stresses associated with arteriovenous fistulae for hemodialysis.
Early results from small pilot clinical studies suggest minimal adverse effects of senotherapeutic agents and clearance of senescent cells in humans [88–91]. However, with the first generation senolytic dasatinib contraindications exist, including QTc interval > 450 msec, hepatic insufficiency, drug interactions with CYP3A4 inhibitors, CYP3A4 inducers, antacids, H2 antagonists/proton pump inhibitors, and other cautions as indicated by the full prescribing information. At doses and frequency used for anti-cancer effects, dasatinib can cause myelosuppression, bleeding-related events, fluid retention, cardiovascular events, pulmonary arterial hypertension, QT prolongation, severe dermatologic reactions, cell lysis syndrome, and embryo-fetal toxicity, but it is uncertain how these potential complications will relate to the intermittent and infrequent administration of dasatinib used for senescent cell clearance. Similarly, the first generation senolytic quercetin has contraindications with respect to potential drug-drug interactions (e.g., strong inhibitors of CYP3A4) and common side effects (e.g., headache), but again it is not clear how these possible adverse events will be manifested with intermittent dosing regimens.
On-going clinical trials will continue to primarily address safety and target engagement of senescent cells, with secondary endpoints that may give insight into efficacy of senolytics and senomorphics. See Resources for representative clinical trials that are assessing senotherapeutic drugs (I-V). Larger clinical trials will be needed to better understand the potential for senolytics and senomorphics as therapeutic interventions and to more fully evaluate off-target effects. Limitations of current clinical trials include small recruitment sizes, the paucity of measurable, clinically-relevant outcomes appropriate for older adults, as well as the need for biomarkers that follow pharmacokinetic, pharmacodynamic, and mechanistic properties of study drugs and could serve as surrogate endpoints [92–94].
Concluding Remarks
Multiple interventions that target primary aging processes are currently being explored. Senescent cell burden represents one fundamental aging process that has been carefully studied and whose targeting at the pre-clinical level by genetic and pharmacologic reduction has yielded compelling findings that support the geroscience hypothesis (see Outstanding Questions). Translation of promising pharmacologic interventions in the form of senotherapeutic agents has begun to assess safety and target engagement. Future challenges to the clinical use of these agents include optimizing clinical trial design by consideration of appropriate primary endpoints, recruitment of at-risk or symptomatic older adults, biomarker discovery, and demonstrating effectiveness in alleviating cellular senescence-associated conditions.
Outstanding Questions.
Do senescent cells serve any beneficial functions, such as providing an inflammatory milieu during tissue healing?
What are the tissue-specific thresholds for senescent cell burden that once exceeded, promote tissue dysfunction and systemic deleterious bystander effects?
Does the clearance of senescent cells reduce the influence of other primary aging processes?
How infrequently can senolytic agents be given to minimize senescent cell burden?
How can combinations of senotherapeutic agents be devised to optimize senescent cell clearance?
Can immune system modulation synergize with senotherapeutic agents promote senescent cell clearance?
Will senolytics synergize with disease-specific treatments to yield a more than additive beneficial effect, for example in cardiac disease?
Are paracrine and other bystander effects the primary mechanism for the pathophysiological consequences of senescent cell accumulation?
Do immune clearance mechanisms have the same accessibility to different senescent cell niches (e.g., fat versus bone versus nevi)?
Reduction in senescent cell burden could be transformative to clinical practice, especially geriatric medicine. Clinically relevant primary endpoints for older adults will likely include aspects of both objective and subjective physical functioning, since these are predictive of morbidity and mortality, contribute to risks of cognitive decline and injury, are prominent components of geriatric syndromes, and are consistent with measureable improvements being made in the short-term. Biomarker discovery will be facilitated by larger clinical trials, measurement of changes in multiple analytes in multiple target specimens, and replication of biomarker feasibility and utility across multiple sites within a single study and among different studies. In the longer term, it should be possible to assess the delays in onset of chronic diseases and geriatric syndromes with compression of morbidity, using interventions based on reduction of senescent cell burden and other interventions in the aging process.
Highlights.
Accumulation of senescent cells is a fundamental aging process that contributes to age-onset disease, compromises health span, and shortens life span.
Senescent cells have been cleared genetically in animal models and pharmacologically in animals and humans.
Reduction in senescent cell burden can be accomplished pharmacologically by targeting anti-apoptotic networks or the senescence-associated secretory phenotype.
Early, proof-of-principle pilot clinical studies on senolytic agents are addressing safety and target engagement.
Acknowledgements
This work was supported by the Robert and Arlene Kogod Professorship (RJP); National Institutes of Health P01 AG062413 (SK, JLK, TT, RJP), P01 AG004875 (SK), R01 AG048792 (SK), R37 AG 013925 (JLK), and R33 061456 (JLK), as well as the Connor Group (JLK), Robert and Theresa Ryan Foundation (JLK), the Noaber Foundation (JLK), and the Ted Nash Foundation (JFP).
Glossary
- Apoptosis
programed cell death during normal growth, development, and removal of potentially harmful cells, such as those that are pre-cancerous or virus-infected
- Cellular senescence
process by which cells undergo irreversible growth arrest, usually secondary to toxic insults or unchecked mitogenic signals. Senescent cells are characterized by several dramatic changes including epigenetic remodeling of chromatin, changes in the abundance and functionality of organelles and the enhanced secretion of pro-inflammatory molecules; however, many of these alterations are not universal and will depend on the cell-type and type of inducing stimuli
- Compression of morbidity
reduction in the lifetime burden of illness when onset of chronic disease is postponed to a greater extent than the increase in life expectancy
- Damage-associated molecular patterns (DAMPS)
repertoire of molecules released from damaged or necrotic cells that activate the innate immune system by binding to pattern recognition receptors. They promote pathological inflammatory responses and induce cellular senescence
- Geroscience hypothesis
proposition that since aging underlies most chronic disease and debilitating states, interventions that retard primary aging processes would also concomitantly prevent, delay, or alleviate multiple age-related conditions
- JAK/STAT
in mammals, the principal regulatory mechanism for a wide array of cytokines and growth factors. The JAK pathway is more highly active in senescent cells and its inhibition can suppress the SASP
- Progeroid
resembling the aging phenotype in older individuals, usually in reference to pre-mature or accelerated aging
- p16INK4a/Rb pathway
p16 is an inhibitor of cyclin-dependent kinases (CDKs) that prevents CDK4/6 binding of cyclin D and formation of an active protein complex that phosphorylates retinoblastoma protein (pRB). If Rb is phosphorylated by CDKs it dissociates from E2F family transcription factors (E2F 1–3), enters the nucleus, and promotes transcription of target genes necessary for G1 to S phase cell cycle transition. For clarity, we have simplified this pathway, however, it should be noted that other players may be involved, including several non-E2F target genes of Rb. p16 hypermethylation, mutation, or deletion leads to dysregulation of cell cycle progression. Increased expression of p16 is associated with cell senescence
- p53/p21 pathway
p53-dependent cell cycle arrest is the primary response to DNA damage. p21, an inhibitor of cyclin-dependent kinases, is the primary mediator of downstream cell cycle arrest due to p53 activation. The p21 protein functions as a regulator of cell cycle progression at both G1 and S phases. Activation of p21 is associated with cell senescence
- Telomere
region of repetitive nucleotide sequences at chromosome ends, and associated with a protein complex to form a protective loop structure that prevents against deterioration or fusion with adjacent chromosomes. With replication cycles telomeres grow shorter or dysfunctional and when critically short or dysfunctional are associated with a DNA damage response that triggers cellular senescence
- Transposons
elements within DNA that can be translated into RNA, be reverse-transcribed into DNA, and re-inserted at new sites in chromosomes (“jumping genes”). Transposons can disrupt chromosomal structure and elicit responses within cells that contribute to inflammation and the SASP
Footnotes
Disclaimer Statement
JLK and TT have a financial interest related to this opinion piece. Patents on senolytic drugs are held by Mayo Clinic. Research findings cited in this opinion piece was previously reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. No conflicts of interest, financial or otherwise, are declared by the other authors.
Resources
- Alleviation by Fisetin of Frailty, Inflammation, and Related Measures in Older Adults (AFFIRM-LITE) https://clinicaltrials.gov/ct2/show/NCT03675724
- Alleviation by Fisetin of Frailty, Inflammation, and Related Measures in Older Women (AFFIRM) https://clinicaltrials.gov/ct2/show/NCT03430037
- Inflammation and Stem Cells in Diabetic and Chronic Kidney Disease https://clinicaltrials.gov/ct2/show/NCT03325322
- Vaccination Efficacy With Metformin in Older Adults (VEME) https://clinicaltrials.gov/ct2/show/NCT03996538?
- Targeting Pro-Inflammatory Cells in Idiopathic Pulmonary Fibrosis: a Human Trial (IPF) https://clinicaltrials.gov/ct2/show/NCT02874989
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirkland JL (2016) Translating the Science of Aging into Therapeutic Interventions. Cold Spring Harb Perspect Med 6 (3), a025908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kennedy BK et al. (2014) Geroscience: linking aging to chronic disease. Cell 159 (4), 709–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker DJ et al. (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479 (7372), 232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farr JN et al. (2017) Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23 (9), 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moncsek A et al. (2018) Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2(−/−) ) mice. Hepatology 67 (1), 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nath KA et al. (2018) The murine dialysis fistula model exhibits a senescence phenotype: pathobiological mechanisms and therapeutic potential. Am J Physiol Renal Physiol 315 (5), F1493–F1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmer AK et al. (2019) Targeting senescent cells alleviates obesity-induced metabolic dysfunction. Aging Cell 18 (3), e12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roos CM et al. (2016) Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell 15 (5), 973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suvakov S et al. (2019) Targeting senescence improves angiogenic potential of adipose-derived mesenchymal stem cells in patients with preeclampsia. Biol Sex Differ 10 (1), 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M et al. (2017) Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J Gerontol A Biol Sci Med Sci 72 (6), 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M et al. (2015) Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4, e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M et al. (2018) Senolytics improve physical function and increase lifespan in old age. Nat Med 24 (8), 1246–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M et al. (2015) JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 112 (46), E6301–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yousefzadeh MJ et al. (2018) Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y et al. (2014) Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 17 (4), 324–8. [DOI] [PubMed] [Google Scholar]
- 16.Liu J et al. (2019) Roles of Telomere Biology in Cell Senescence, Replicative and Chronological Ageing. Cells 8 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapman J et al. (2019) Mitochondrial dysfunction and cell senescence: deciphering a complex relationship. FEBS Lett 593 (13), 1566–1579. [DOI] [PubMed] [Google Scholar]
- 18.Childs BG et al. (2014) Senescence and apoptosis: dueling or complementary cell fates? EMBO Rep 15 (11), 1139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.d’Adda di Fagagna F et al. (2003) A DNA damage checkpoint response in telomere-initiated senescence. Nature 426 (6963), 194–8. [DOI] [PubMed] [Google Scholar]
- 20.Rayess H et al. (2012) Cellular senescence and tumor suppressor gene p16. Int J Cancer 130 (8), 1715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passos JF et al. (2007) Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol 5 (5), e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodier F et al. (2009) Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol 11 (8), 973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narita M et al. (2003) Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113 (6), 703–16. [DOI] [PubMed] [Google Scholar]
- 24.De Cecco M et al. (2013) Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell 12 (2), 247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Cecco M et al. (2019) L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature 566 (7742), 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herbig U et al. (2006) Cellular senescence in aging primates. Science 311 (5765), 1257. [DOI] [PubMed] [Google Scholar]
- 27.Laberge RM et al. (2012) Glucocorticoids suppress selected components of the senescence-associated secretory phenotype. Aging Cell 11 (4), 569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y et al. (2015) The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14 (4), 644–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang J et al. (2016) Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med 22 (1), 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Y et al. (2016) Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell 15 (3), 428–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baar MP et al. (2017) Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell 169 (1), 132–147 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuhrmann-Stroissnigg H et al. (2017) Identification of HSP90 inhibitors as a novel class of senolytics. Nat Commun 8 (1), 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon OH et al. (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23 (6), 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrott KM et al. (2017) Apigenin suppresses the senescence-associated secretory phenotype and paracrine effects on breast cancer cells. Geroscience 39 (2), 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogrodnik M et al. (2017) Cellular senescence drives age-dependent hepatic steatosis. Nat Commun 8, 15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Febo M (2011) Technical and conceptual considerations for performing and interpreting functional MRI studies in awake rats. Front Psychiatry 2, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallagher M et al. (2011) Mindspan: lessons from rat models of neurocognitive aging. ILAR J 52 (1), 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iannaccone PM and Jacob HJ (2009) Rats! Dis Model Mech 2 (5–6), 206–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacob HJ (1999) Functional genomics and rat models. Genome Res 9 (11), 1013–6. [DOI] [PubMed] [Google Scholar]
- 40.Mashimo T et al. (2005) Rat Phenome Project: the untapped potential of existing rat strains. J Appl Physiol (1985) 98 (1), 371–9. [DOI] [PubMed] [Google Scholar]
- 41.Shimoyama M et al. (2016) Exploring human disease using the Rat Genome Database. Dis Model Mech 9 (10), 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bader M (2010) Rat models of cardiovascular diseases. Methods Mol Biol 597, 403–14. [DOI] [PubMed] [Google Scholar]
- 43.Carter CS et al. (2002) Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci 57 (5), B193–7. [DOI] [PubMed] [Google Scholar]
- 44.Ellenbroek B and Youn J (2016) Rodent models in neuroscience research: is it a rat race? Dis Model Mech 9 (10), 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.King AJ (2012) The use of animal models in diabetes research. Br J Pharmacol 166 (3), 877–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Like AA et al. (1982) Spontaneous autoimmune diabetes mellitus in the BB rat. Diabetes 31 (Suppl 1 Pt 2), 7–13. [DOI] [PubMed] [Google Scholar]
- 47.Aitman TJ et al. (2008) Progress and prospects in rat genetics: a community view. Nat Genet 40 (5), 516–22. [DOI] [PubMed] [Google Scholar]
- 48.Brown AJ et al. (2013) Whole-rat conditional gene knockout via genome editing. Nat Methods 10 (7), 638–40. [DOI] [PubMed] [Google Scholar]
- 49.Flister MJ et al. (2015) 2015 Guidelines for Establishing Genetically Modified Rat Models for Cardiovascular Research. J Cardiovasc Transl Res 8 (4), 269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hermsen R et al. (2015) Genomic landscape of rat strain and substrain variation. BMC Genomics 16, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meek S et al. (2017) From engineering to editing the rat genome. Mamm Genome 28 (7–8), 302–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SR et al. (2019) Increased renal cellular senescence in murine high-fat diet: effect of the senolytic drug quercetin. Transl Res 213, 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nogueira-Recalde U et al. (2019) Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine 45, 588–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tchkonia T et al. (2013) Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123 (3), 966–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiley CD et al. (2018) Small-molecule MDM2 antagonists attenuate the senescence-associated secretory phenotype. Sci Rep 8 (1), 2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Y et al. (2017) New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY) 9 (3), 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwon SM et al. (2019) Metabolic features and regulation in cell senescence. BMB Rep 52 (1), 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wiley CD and Campisi J (2016) From Ancient Pathways to Aging Cells-Connecting Metabolism and Cellular Senescence. Cell Metab 23 (6), 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirkland JL and Tchkonia T (2017) Cellular Senescence: A Translational Perspective. EBioMedicine 21, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu M et al. (2016) Perspective: Targeting the JAK/STAT pathway to fight age-related dysfunction. Pharmacol Res 111, 152–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yun MH et al. (2015) Recurrent turnover of senescent cells during regeneration of a complex structure. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karin O et al. (2019) Senescent cell turnover slows with age providing an explanation for the Gompertz law. Nat Commun 10 (1), 5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Munoz DP et al. (2019) Targetable mechanisms driving immunoevasion of persistent senescent cells link chemotherapy-resistant cancer to aging. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pereira BI et al. (2019) Senescent cells evade immune clearance via HLA-E-mediated NK and CD8(+) T cell inhibition. Nat Commun 10 (1), 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prata L et al. (2019) Senescent cell clearance by the immune system: Emerging therapeutic opportunities. Semin Immunol, 101275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vicente R et al. (2016) Cellular senescence impact on immune cell fate and function. Aging Cell 15 (3), 400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cupit-Link MC et al. (2017) Biology of premature ageing in survivors of cancer. ESMO Open 2 (5), e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de Magalhaes JP and Passos JF (2018) Stress, cell senescence and organismal ageing. Mech Ageing Dev 170, 2–9. [DOI] [PubMed] [Google Scholar]
- 69.Demaria M et al. (2017) Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov 7 (2), 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farr JN et al. (2016) Identification of Senescent Cells in the Bone Microenvironment. J Bone Miner Res 31 (11), 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guida JL et al. (2019) Measuring Aging and Identifying Aging Phenotypes in Cancer Survivors. J Natl Cancer Inst. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.LeBrasseur NK et al. (2015) Cellular Senescence and the Biology of Aging, Disease, and Frailty. Nestle Nutr Inst Workshop Ser 83, 11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lecot P et al. (2016) Context-dependent effects of cellular senescence in cancer development. Br J Cancer 114 (11), 1180–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lewis-McDougall FC et al. (2019) Aged-senescent cells contribute to impaired heart regeneration. Aging Cell 18 (3), e12931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Menon R et al. (2016) Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY) 8 (2), 216–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morty RE and Prakash YS (2019) Senescence in the lung: is this getting old? Am J Physiol Lung Cell Mol Physiol 316 (5), L822–L825. [DOI] [PubMed] [Google Scholar]
- 77.Ness KK et al. (2015) Frailty in childhood cancer survivors. Cancer 121 (10), 1540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ness KK et al. (2018) Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J Clin Oncol 36 (21), 2206–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ogrodnik M et al. (2019) Obesity-Induced Cellular Senescence Drives Anxiety and Impairs Neurogenesis. Cell Metab 29 (5), 1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Palmer AK et al. (2019) Cellular senescence: at the nexus between ageing and diabetes. Diabetologia 62 (10), 1835–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parikh P et al. (2019) Hyperoxia-induced Cellular Senescence in Fetal Airway Smooth Muscle Cells. Am J Respir Cell Mol Biol 61 (1), 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parikh P et al. (2019) Cellular senescence in the lung across the age spectrum. Am J Physiol Lung Cell Mol Physiol 316 (5), L826–L842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patil P et al. (2019) Systemic clearance of p16(INK4a) -positive senescent cells mitigates age-associated intervertebral disc degeneration. Aging Cell 18 (3), e12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rocca WA et al. (2018) Loss of Ovarian Hormones and Accelerated Somatic and Mental Aging. Physiology (Bethesda) 33 (6), 374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rocca WA et al. (2017) Bilateral Oophorectomy and Accelerated Aging: Cause or Effect? J Gerontol A Biol Sci Med Sci 72 (9), 1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rocca WA et al. (2016) Accelerated Accumulation of Multimorbidity After Bilateral Oophorectomy: A Population-Based Cohort Study. Mayo Clin Proc 91 (11), 1577–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schafer MJ et al. (2017) Cellular senescence mediates fibrotic pulmonary disease. Nat Commun 8, 14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hickson LJ et al. (2019) Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 47, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hickson LJ et al. (2020) Corrigendum to ‘Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease’ EBioMedicine 47 (2019) 446–456. EBioMedicine 52, 102595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Justice JN et al. (2019) Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 40, 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh M et al. (2016) Effect of Low-Dose Rapamycin on Senescence Markers and Physical Functioning in Older Adults with Coronary Artery Disease: Results of a Pilot Study. J Frailty Aging 5 (4), 204–207. [DOI] [PubMed] [Google Scholar]
- 92.Burd CE et al. (2016) Barriers to the Preclinical Development of Therapeutics that Target Aging Mechanisms. J Gerontol A Biol Sci Med Sci 71 (11), 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Justice J et al. (2016) Frameworks for Proof-of-Concept Clinical Trials of Interventions That Target Fundamental Aging Processes. J Gerontol A Biol Sci Med Sci 71 (11), 1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Niedernhofer LJ et al. (2017) Molecular pathology endpoints useful for aging studies. Ageing Res Rev 35, 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Acosta JC et al. (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15 (8), 978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coppe JP et al. (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coppe JP et al. (2008) Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6 (12), 2853–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laberge RM et al. (2015) MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat Cell Biol 17 (8), 1049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moiseeva O et al. (2013) Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation. Aging Cell 12 (3), 489–98. [DOI] [PubMed] [Google Scholar]
- 100.Wiley CD et al. (2016) Mitochondrial Dysfunction Induces Senescence with a Distinct Secretory Phenotype. Cell Metab 23 (2), 303–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krtolica A et al. (2001) Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A 98 (21), 12072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krizhanovsky V et al. (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134 (4), 657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ritschka B et al. (2017) The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev 31 (2), 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Helman A et al. (2016) p16(Ink4a)-induced senescence of pancreatic beta cells enhances insulin secretion. Nat Med 22 (4), 412–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Acosta JC et al. (2008) Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133 (6), 1006–18. [DOI] [PubMed] [Google Scholar]
- 106.Kuilman T et al. (2008) Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133 (6), 1019–31. [DOI] [PubMed] [Google Scholar]
- 107.Wajapeyee N et al. (2008) Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132 (3), 363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hubackova S et al. (2012) IL1- and TGFbeta-Nox4 signaling, oxidative stress and DNA damage response are shared features of replicative, oncogene-induced, and drug-induced paracrine ‘bystander senescence’. Aging (Albany NY) 4 (12), 932–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nelson G et al. (2012) A senescent cell bystander effect: senescence-induced senescence. Aging Cell 11 (2), 345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]