Abstract
Bone tissue engineering (BTE) encompasses the field of biomaterials, cells, and bioactive molecules to successfully guide the growth and repair of bone tissue. Current BTE strategies rely on delivering osteogenic molecules or cells via scaffolding materials. However, growth factor- and stem cell-based treatments have several limitations, such as source restriction, low stability, difficulties in predicting long-term efficacy, and high costs, among others. These issues have promoted the development of material-based therapy with properties of accessibility, high stability, tunable efficacy, and low-cost production. Hydrogels are widely used in BTE applications because of their unique hydrophilic nature and tunable physicochemical properties to mimic the native bone environment. However, current hydrogel materials are not ideal candidates due to minimal osteogenic capability on their own. Therefore, recent studies of BTE hydrogels attempt to counterbalance these issues by modifying their biophysical properties. In this article, we review recent progress in the design of hydrogels to instruct osteogenic potential, and present strategies developed to precisely control its bone healing properties.
Graphical Abstract
Introduction
Bone is a highly mineralized tissue with a solid intercellular matrix. It has a biphasic extracellular matrix (ECM) that is composed of both an inorganic mineral and an organic phase, consisting of 90% collagen and 10% non-collagenous proteins, including growth factors1. Bone also preserves bone marrow producing mesenchymal stem cells and possesses a set of bone cells that include osteoblasts, osteocytes, and osteoclasts2.
Bone remodeling is a dynamic process that involves recruitment of cells, cytokines, and growth factors. However, a self-remodeling is not achievable with the severe defects or in the presence of complications, such as osteoporosis, osteoarthritis, or osteogenesis imperfecta3. Common treatments such as autografts or allografts are difficult to utilize extensively because of their source limitations, potential for infection, and adverse immune responses. Therefore, the demand for effective bone repair strategy is growing and has led to increased research in bone tissue engineering (BTE). The success of BTE is governed by several key factors, such as the osteogenic scaffold, osteogenic cells, and osteogenic stimulating factors. A treatment based on each component or a combined strategy is appealing, but the field of BTE is currently dominated by growth factor-based treatment. A treatment using bone morphogenetic proteins (BMPs) is well-established because of its high efficacy; however it is associated with severe side effects when supraphysiological doses are administered and poor delivery efficiency using currently available delivery system4.
A hydrogel is a viscoelastic polymeric scaffold with significant water uptake ability mimicking bone ECM5. An aqueous three-dimensional environment provides the hydrogel with unique properties, such as injectability, swelling, and surface absorption. A hydrogel has potential to entrap and attract native cells by providing anchoring sites on its mesh network. It is able to deliver proteins or small molecule drugs locally, and its ion adsorption ability initiates mineralization on the hydrogel surface to provide a bone-like environment. Therefore, hydrogel is a promising candidate for use as an osteogenic scaffold, and as an osteogenic carrier for delivery of cells or stimulating factors.
The ideal attributes of a hydrogel for BTE are 1) biocompatibility, 2) biodegradability, 3) in-situ gel formation ability, 4) integration with surrounding tissues, 5) delivery of cells or drugs, and 6) osteogenic properties. These properties can be diversified by a choice of hydrogel polymer backbone, crosslinking, and various functionalizations. Advances in engineering hydrogels have provided many candidate hydrogels with the above-mentioned properties; however, osteogenic capacity is still very limited. Improvement of osteogenic performance of a hydrogel enables the simple biomaterials based (cell- and growth factor-free) strategy of BTE. Osteogenic performance of a hydrogel requires a delicate balance of osteoinduction, osteoconduction, and osseointegration over a long period of bone healing6. However, many hydrogels used in bone repair have insufficient intrinsic osteogenic function, leading to limited healing in clinical settings7. In this article, we will focus on reviewing the fundamentals and various state-of-the-art approaches used to design an osteogenic hydrogel system, and the ongoing challenges and subsequent opportunities in the field.
1. Hydrogel fundamentals
The use of hydrogels is attractive in BTE due to its ability to mimic the bone ECM with high water contents that could provide suitable microenvironments to encapsulate cells or bioactive molecules. Additionally, hydrogels with injectable capability enable filling irregular bone defects with a good integration into the surrounding tissues, while avoiding complex surgery. However, there are challenges concerning osteoconductivity, mechanical stability, and the loss of encapsulated bioactive components that need to be further investigated for successful BTE. The characteristics of a hydrogel can be modulated by its source and crosslinking methods. Polymeric hydrogels are widely used in tissue engineering because of their tunable physicochemical properties. Crosslinking is usually required to protect a hydrogel from rapid degradation and improve its stability. In this section, we will introduce polymer sources and crosslinking technologies being used to construct BTE hydrogels and discuss their advantages and limitations for use in bone repair (Figure 1).
Figure 1.
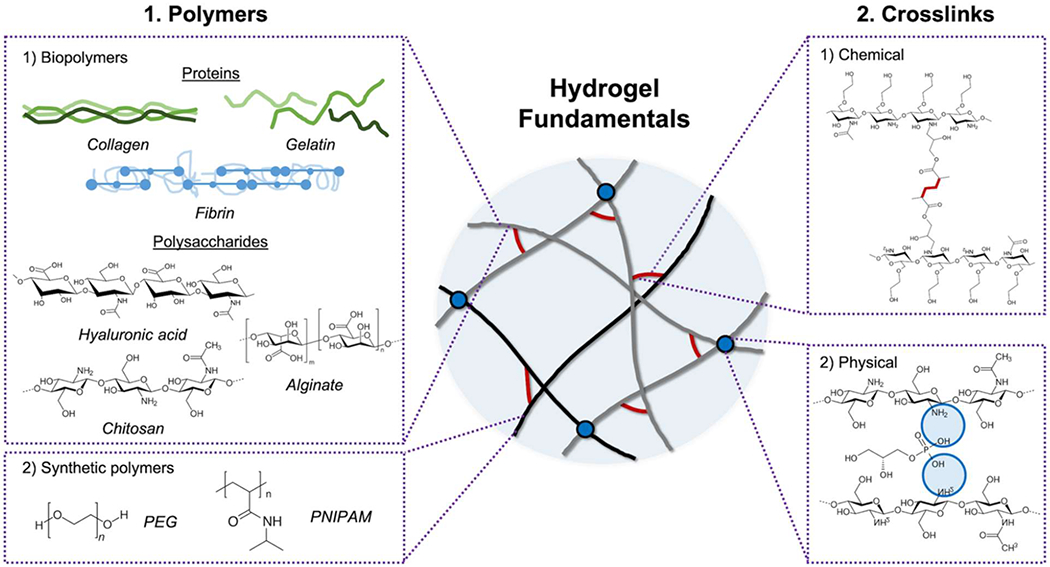
Scheme of hydrogel fundamentals. Hydrogel is formed by crosslinking of polymer network, and its physicochemical properties can be modulated by the choices of polymers and crosslinking methods. Various types of biopolymers, including proteins (collagen, gelatin, and fibrin) and polysaccharides (hyaluronic acid, chitosan, and alginate), and synthetic polymers are widely used in BTE hydrogels. Diverse crosslinking technologies (chemical and physical) support the mechanical stability of hydrogels. The representative scheme of the chemical and physical crosslink is a radical crosslinking of methacrylated glycol chitosan and an ionic crosslinking of chitosan with glycerol phosphate, respectively.
1.1. Polymers used as backbones of hydrogels for BTE
Polymers are promising materials to prepare BTE hydrogels and can be classified into natural and synthetic polymers by their sources. The main advantages and drawbacks are listed in Table 1. No single material meets all requirements for successful bone regeneration, requiring further modifications to obtain desired biophysical properties.
Table 1.
Comparative table of polymers to fabricate hydrogels for bone regeneration
| Polymer | Advantages | Drawbacks |
|---|---|---|
| Collagen | - Most abundant ECM in bone - Providing cell attachment sites - Natural growth factor reservoir |
- Potentially provoking immune response - Hard to control the quality due to heterogeneity of sources |
| Gelatin | - Mimicking bone ECM - Lower immunogenicity than collagen - Easy to functionalize |
- Fast degradation - Low mechanical strength |
| Fibrin | - Providing cell attachment sites - Easy to tune mechanical strength - Fast gelation |
- Significant shrinking during gelation - Fast degradation - Low bone specific bioactivity |
| Hyaluronic acid | - Interacting with growth factors - Low immunogenicity - Easy to functionalize |
- Low mechanical stability without crosslinking - Fast degradation |
| Chitosan | - Inherent antibacterial properties - Easy to functionalize - Low immunogenicity |
- Requiring additional modification to improve solubility |
| Alginate | - Fast gelation - Easy to functionalize |
- Possibility to lose structure by cation leaching |
| Nucleic acid | - Easy to control structure due to its specific basepairing properties | - Difficult to make a bulk hydrogel |
| PEG | - Easy to functionalize - Stable in physiological condition |
- Nondegradable - Low cell adhesion - Some immunogenicity |
| PNIPAM | - Temperature sensitive - Low immunogenicity |
- Nondegradable - Weak mechanical strength |
1.1.1. Biopolymers
Biopolymers derived from natural macromolecules are an advantageous hydrogel source in BTE resulting from their biocompatibility, biodegradability, and similarity to natural bone ECM. Natural proteins, such as collagen, gelatin, or fibrin, and polysaccharides, such as hyaluronic acid, chitosan, or alginate, are extensively used in the field. These biopolymers can be used alone, or blended with other polymers to form a composite depending on the application.
1) Collagen is the main constructional protein in bone ECM and type I collagen is the most abundant among the five common types of collagen, I, II, III, IV, and V. The molecular weight of collagen is approximately 300 kDa with a long triple helix fibril structure. This elongated structure provides mechanical strength by hierarchical assembly. The integration of hydroxyapatite in fibril networks can enhance the mechanical properties of the materials, as well as provide biochemical cues. Collagen is known for successful cell attachment and differentiation of osteoprogenitor cells, which makes it widely used in BTE8. In the context of bone regeneration, type I collagen has been found to be a regulator of osteogenic differentiation of mesenchymal stem cells (MSCs) derived from rat bone marrow or dental pulp in vitro and in a rat calvarial defect9, 10. Collagen matrices are already being used in clinical practice to deliver BMP-2 for bone regeneration but premature leakage of BMP-2 could induce ectopic bone formation11. There are also continued concerns about the widespread use of collagen due to its challenging purification process, a risk of disease transmission and immune response12–14.
2) Gelatin originates from collagen and is usually referred to an irreversibly hydrolyzed collagen. Because of hydrolysis, the molecular weight of gelatin varies from 20 to 220 kDa, which is smaller than collagen. It undergoes undemanding degradation, which leads to lower immunogenicity in comparison to collagen. Gelatin possesses similar amino acid profiles to collagen and thus exhibits the ability to support cellular attachment and growth15. Different gelatin processing from collagen allows various physicochemical properties, providing versatile platforms for tissue engineering scaffolds or delivery vehicles16. On the other hand, the functional and bioactive properties of gelatin may differ from native collagen. Moreover, lower mechanical strength is a recurring issue in gelatin applications. A recent gelatin hydrogel was specifically designed to possess macroporosity and was shown to support robust endochondral ossification of human MSCs in a mouse subcutaneous model17. The compressive modulus of the engineered bone constructs was 10-fold higher compared to conventional gelatin hydrogels17.
3) Fibrin, a main part of the hemostatic clot, is formed by rapid reaction of thrombin protease on fibrinogen. Its mechanical properties and stability are easily tunable by adjusting fibrinogen and thrombin concentration. Fibrin is already being used widely as tissue adhesives and its intrinsic angiogenic properties make fibrin matrix attractive biomaterials for BTE18, 19. Fibrin prepared from platelet-rich plasma was successfully used in combination with dental implants to enhance osseointegration in elderly patients20. However, the specific effects of the growth factors present in platelets on bone repair is poorly understood.
4) Hyaluronic acid (HA) is a linear glycosaminoglycan with alternating β1→4 and β1→3 glycosidic bonds of D-glucuronic acid and N-acetyl-D-glucosamine. Because of the presence of repeating carboxylic acids in the HA backbone, it is negatively charged at physiological pH. It can bind to proteins, such as CD44, noncovalently and affects the diffusion of cells and proteins21. It is also known to have low immunogenicity, anti-inflammatory, antioxidant properties, and can induce the migration of MSCs22, which make it as an interesting candidate for BTE applications. A recent study demonstrated enhanced bone formation capability of bone graft materials when mixed with HA in a rat mandibular defect while minimal bone healing was observed in a defect treated with HA alone23. In addition, HA is used as a carrier for commercially available demineralized bone allografts to improve its handling properties24.
5) Chitosan is a linear cationic polysaccharide with β1→4 linkages of D-glucosamine and N-acetyl-D-glucosamine. It has an intrinsic ability to interact with cells and can be degraded by chitosanases25 or lysozymes26. The degree of deacetylation of chitosan alters the free amine ratio in the chitosan backbone, which influences the charge and provides further modification potential. The cationic nature of chitosan makes it an attractive candidate in BTE applications because chitosan can bind oppositely charged red blood cells or bacterial cell walls to exert hemostasis and antimicrobial properties in the infected wound site27. Chitosan provided an osteocompatible environment to promote the proliferation of osteoblasts and calcium deposition28. Chitosan also form a polyelectrolyte complex with many anionic glycosaminoglycans such as heparin that is known to modulate the bioactivity of growth factors such as BMP-2 important to bone regeneration29. Various bioconjugation techniques can modify the amine groups to other functional moieties such as methacrylate30 or bone-specific phosphate groups31.
6) Alginate is a linear anionic polysaccharide composed of both a β1→4 linkage for D-mannuronate and an α1→4 linkage for L-guluronate. Alginate can rapidly form a gel, with the addition of divalent cationic ions, such as Ca2+ or Zn2+, which create ionic bridges between chains via the egg-box model32. The fast gelation property of alginate makes it widely used in calcium containing solutions mimicking the bone environment33. Despite its low cost and convenient gelation, alginate presents no cell-binding motifs, limiting cell attachment, and undergoes slow degradation due to the lack of specific enzymes to degrade it in vivo34. These properties limit the use of alginate on its own for BTE. A study demonstrated that cell-laden alginate hydrogels induced inferior bone formation compared to type I collagen hydrogels in a porcine calvarial defect35.
1.1.2. Synthetic polymers
Synthetic polymers have multiple benefits, such as low batch-to-batch variability, convenience for functionalization with specific bioactive moieties, and relatively stable structural properties. The widely used synthetic polymers for BTE hydrogels are polyethylene glycol (PEG) and poly(N-isopropylacrylamide) (PNIPAM).
1). Polyethylene glycol (PEG)
PEG is a water-soluble polymer with a chemical formula of H-[OCH2CH2]n–OH, also known as polyethylene oxide (PEO). The molecular weight of PEG is directly linked to its physical properties, such as viscosity and it is available in different forms, such as branched or star-shaped PEG. It is biologically inert and safe, as a result, it is widely used in biomedical applications such as an excipient of pharmaceutical drugs36. PEG hydrogels were evaluated as barrier membranes for guided bone regeneration for the treatment of periodontal bone defects37. PEG hydrogel membranes were found to be as effective as conventional collagen membranes at enhancing bone growth at implant sites37. Furthermore, the solubility of PEG is dependent on its molecular weight, which makes PEG gels more attractive for use as controlled delivery vehicles to mediate sustained release of osteoinductive factors36, 38.
2). Poly(N-isopropylacrylamide) (PNIPAM)
PNIPAM is a temperature-responsive polymer with a chemical formula of [C6H11NO]n. It has a lower critical solution temperature (LCST) phase transition at 32 °C, such that it is highly solvated below LCST and can reversibly interchange to shrink above its LCST, because of the hydrophobic aggregation of isopropyl groups39. The LCST of PNIPAM is close to the human body temperature, as a result, it is widely used as a thermo-gel in biomedical applications40. In spite of its distinct thermo-responsive properties, the use of PNIPAM hydrogels is limited for BTE applications due to its intrinsic nature of poor mechanical strength and biodegradability39. For this reason, PNIPAM is often used in copolymers with other polymer blocks to produce hydrogels with improved physicochemical properties. Gelatin was grafted with PNIPAM to create biodegradable in situ gelling systems and injection of the resultant hydrogels encapsulating BMSCs successfully induced new bone formation in a rat cranial model41.
1.2. Crosslinks to form hydrogel networks for BTE
Crosslinking is an essential process to fabricate a stable hydrogel structure, which cannot be dissolved in aqueous conditions and maintains good mechanical strength until the desired time point. A variety of techniques including both chemical and physical approaches are used to crosslink hydrogels (Table 2). Besides the efficiency of crosslinking, it is also very important to consider a mild reaction environment, which will not damage the encapsulated cells or drugs.
Table 2.
Comparative table of crosslinking methods to fabricate hydrogels for bone regeneration
| Crosslinking | Advantages | Drawbacks |
|---|---|---|
| Photo-radical |
- Fast and mild reaction by not altering pH or temperature enormously - Forming a stable crosslinked network |
- May damage cells or bioactive molecules depending on the light sources - Irreversible reaction |
| Schiff base |
- Reversible reaction with self-healing properties - Mild reaction condition |
- Relatively low stability of pseudocovalent bond - Need to prepare two separate reactive polymers |
| Michael addition |
- Forming a stable crosslinked network - Self-healing properties under certain conditions such as excess presence of thiols |
- Potential side reaction to form unexpected disulfide bonds - Need to prepare two separate reactive polymers |
| Diels-Alder |
- Specific bio-orthogonal reaction - No side reactions and byproducts |
- Need to prepare two separate reactive polymers - Considered as an irreversible reaction under physiological condition (reversible at extremely high temperature above 800K) |
Enzyme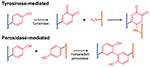
|
- Fast and mild reaction with controlled gelation kinetics - Forming a stable crosslinked network |
- Short half-life of enzymes - Enzyme potentially involving in inflammatory reaction by activating cytokines - Irreversible reaction |
| Ionic |
- Fast and mild reaction - Reversible reaction with self-healing properties |
- Relatively low stability - Possibly disrupt the crosslinking by ion bleaching |
| Hydrogen bond |
- Fast and mild reaction - Reversible reaction with self-healing properties |
- Relatively low stability - Require multiple multivalent hydrogen bond to form hydrogel |
| Hydrophobic |
- Reversible reaction with self-healing properties - Can form a fixed geometry using a host-guest interaction |
- Relatively low stability - Potentially form a brittle network by pushing water from hydrogel network |
1.2.1. Chemical crosslinks
Chemical crosslinking creates covalent bonds at the inter- and intra-molecular level. It can be initiated by a radical reaction, a linkage between specific functional groups, or enzymatic activity. Covalent bonds are more stable and stronger than non-covalent bonds, leading to the excellent stability and mechanical properties under physiological conditions.
1). Radical chemistry
Radical crosslinking is initiated by radical initiators, which can generate free radicals in the presence of various stimuli, such as photo-, thermal-, or redox reactions. A photoreaction, such as UV- or visible-light is widely applied in tissue engineering hydrogel formation, because of its mild reaction conditions in comparison to the extreme changes in temperature or pH. Photoinitiators can be chosen differently depending on light sources with differences in excitation wavelength. Common photoinitiators are Irgacure 295930, 42 or Irgacure 65143 used for UV crosslinking (250-370 nm), and camphorquinone42, 44 or riboflavin45 used for visible light crosslinking (400-700 nm). Radical reactive groups in the polymer backbone can be activated by free radicals to form a network structure in hydrogels. An unsaturated vinyl group is usually introduced into a polymer as a radical reactive group. Amine, carboxyl, or hydroxyl groups in the polymer backbone can be converted to acrylate or methacrylate groups by simple bioconjugation.
Gelatin and HA modified with methacrylate functional groups have been extensively explored as modified natural ECM components for BTE applications. For example, photo-crosslinked gelatin methacryloyl (GelMA) hydrogels encapsulating osteogenic and angiogenic cells were effective for the formation of vascularized bone tissue constructs46. Moreover, bioactivity of osteogenic growth factors such as BMP-2 was significantly enhanced when delivered from GelMA hydrogels compared to exogenous BMP-247. There has also been an increased use of photo-crosslinkable hydrogels in combination with 3D bioprinting techniques for complex bone regeneration. Researchers took advantages of the fast gelation ability of photopolymerization and produced cell-laden complex 3D hydrogel constructs via 3D printing systems based on stereolithography. Photosensitive methacrylate HA was successfully printed into porous and anatomically shaped bone constructs under UV light radiation with the high level of cell survival incorporated48. However, photo-crosslinkable hydrogels have not been translated into clinical practice due to concerns associated with oxygen inhibition in polymerization reactions and the nature of a random chain growth mode, which may decrease curing efficiency and polymer network homogeneity with the production of unreacted toxic residues49, 50. Moreover, UV irradiation is known to have harmful effects on host tissues including oxidative damage to DNA and premature aging. Alternative visible light or near-infrared has been demonstrated to be safe with deeper tissue penetration.
2). Click chemistry
Click chemistry that allows for very selective and specific reactions with a high yield of desired covalent bonds51, 52 has been widely utilized in hydrogel network formation. Numerous functional moieties can be involved in this reaction; the established reactions are, (a) Schiff base, (b) Michael addition, and (c) Diels-Alder reaction.
(a). Schiff base reaction
The Schiff reaction is a condensation reaction used to form a covalent imine type bond via nucleophilic addition of an amine to an electrophilic aldehyde or ketone under physiological conditions53. Additionally, aldehyde groups can bind to tissues firmly because of the abundant amine groups on the tissue surface54. Among imine type bonds, hydrazone and oxime are more stable than imine because of mesomeric effects55. The resulting bond is biocompatible, pH-responsive, and reversible, which is advantageous for application in dynamic hydrogels with self-healing properties. Compared to other chemical crosslinking, the Schiff reaction enables rapid gelation under very mild conditions without initiators or light irradiation. A recent study demonstrated that injectable hydrogels based on glycol chitosan and oxidized HA successfully induced osteogenesis and bone formation in vitro and in a rat calvarial defect56. In addition, dynamic covalent bonds in Schiff base crosslinking hydrogels add advantage of using this system for 3D printing bioinks due to its shear thinning and cytoprotective ability during extrusion and subsequent self-healing to form stabilized printed constructs57.
(b). Michael addition
A Michael type reaction is a nucleophilic addition to unsaturated carbonyl groups. Typically, amines, thiols, or phosphines act as nucleophiles, and alkynes or olefins act as acceptors in the reaction. It is often seen in the coupling of a thiol and a vinyl containing polymers, or crosslinker in a basic environment. However, it can also form undesired disulfide bonds as a side reaction. A pair of acrylated HA and tetrathiolated PEG was used to form a hydrogel to deliver BMP-2 and cells58. The thiol Michael reaction is much slower compared to Schiff base crosslinking, requiring excessive modification with thiol groups to form stable hydrogels. A drawback with the thiol-Michael click chemistry is unwanted thiol oxidation that could affect protein stability by breaking disulfide bridges in the protein. A recent study found that crosslinking chemistry could influence the bioactivity of osteogenic growth factors loaded in a hydrogel by evaluating two HA hydrogels loaded with BMP-2, HA-thiol-michael and HA-hydrazone gels59. In this study, BMP-2 delivered from HA-hydrazone gels promoted a better ectopic bone formation in a rat subcutaneous model, indicating a detrimental effect of thiol-based crosslinking on the bioactivity of growth factors.
(c). Diels-Alder reaction
The Diels-Alder reaction is a cycloaddition of an electron donating diene and an electron withdrawing alkene. It is a one-step, catalyst-free, bio-orthogonal reaction without byproduct formation. It is usually applied in hydrogel formation with polymers modified with furan and maleimide derivatives. A previous study demonstrated the potential of Diels-Alder crosslinking hydrogels for sustained drug release and osteogenic differentiation of human MSCs based on maleimide PEG and furan dexamethasone60. However, Diels-Alder click chemistry is less effective for use as BTE hydrogels due to its relatively longer gelation time and substantial degradation under physiological conditions. A recent study reported an improved Diels-Alder reaction with significantly increased gelation rates and hydrolytic stability by replacing the furans with more electron-rich fulvene dienes61.
3). Enzyme-catalyzed chemistry
Enzymatic crosslinking has proven to be specific, efficient, cell-friendly, with mild reaction conditions, which are beneficial for in situ gelation62. Transglutaminase, binding with calcium cofactor, catalyzes post-translational modifications and promotes amide formation between carboxamide and amine. A fibrin gel formed by factor XIII, a transglutaminase isoenzyme, could deliver BMP as a bone substitute63. There has been a more extensive use of the transglutaminase crosslinking strategy since more cost-effective microbial transglutaminase (mTG) was discovered. mTG-crosslinked gelatin has been shown to support proliferation and osteogenic differentiation of MSCs as well as the release of osteogenic growth factors for potential BTE64, 65. Another promising enzymatic crosslinking approach is through a peroxidase-catalyzed reaction due to its ability to fine-tune the various physico-mechanical properties of formed hydrogels66. Peroxidase, in the presence of hydrogen peroxide, catalyzes the oxidation of various organic substrates, such as tyramine, phenol or aniline, and creates polymer-phenol conjugates. A recent study illustrated versatile manipulation for the gelation rate, stiffness and degradation behavior of tyramine-modified HA and chondroitin sulfate by varying the concentration of horseradish peroxidase and hydrogen peroxide67. Such an enzyme catalyzed hydrogel promoted osteogenic differentiation of BMSCs in vitro and bone regeneration in a rat femoral defect67.
1.2.2. Physical crosslinks
Physical crosslinks are driven by non-covalent reversible intermolecular interactions. It does not require any chemical agents, which may be potentially toxic to the encapsulated cells or biologies. Additionally, it exhibits stimulus-responsive properties by responding to changes in the surrounding environment, such as a change in ionic concentration, temperature, or pH.
1). Ionic interactions
Electrostatic interactions, driven by opposite electric charges attracting each other, can crosslink a network. A polyanionic polymer can be crosslinked with a cationic ion, and a polycationic polymer can be crosslinked with an anionic ion. Alginate crosslinks with divalent cations including calcium or magnesium to form an egg-box structure at room temperature in physiological pH68. Chitosan also rapidly crosslinks with anions such as glycerol phosphate69. Moreover, a pair of polyanionic and polycationic polymers can easily form a polyelectrolyte complex, such as alginate-chitosan70, collagen-chitosan71, and chondroitin sulfate-chitosan72 for BTE applications. Although ionically crosslinked gels can be prepared under very mild process conditions without covalent modification, the fast gelation rate may lead to inhomogeneous precipitation, making it difficult to achieve injectable or moldable preparations. Several studies have been conducted on control over the gelation kinetics of hydrogels based on retarded gelation-inhibiting buffers73, 74. Another challenge to ionic gelling is its unpredictable long-term stability in vivo due to rapid ion exchange and gel dissolution in a physiological medium. Moreover, crosslinked ions released from the gel may cause unwanted biological reactions.
2). Hydrogen bond
A hydrogen bond is a specific dipole-dipole interaction between hydrogens and electronegative atoms, such as oxygen, nitrogen, or sulfur. It is an essential interaction for stabilization of the three-dimensional structure of DNA, proteins, and other synthetic polymers. A DNA-based hydrogel with tunable properties is available by controlling DNA sequences and its nanostructures75, 76. A supramolecular polymer, P(NAGA-VPA), was based on copolymerization of N-acryloyl glycinamide and vinylphosphonic acid directly crosslinked by hydrogen bonding between glycinamide groups in an aqueous solution77. This hydrogel mineralized in situ to improve mechanical strength and deliver BMP-2 to support bone regeneration in rat forelimb defects77. Hydrogen bond-based hydrogels are weak and fragile compared to covalently crosslinked or other physical hydrogels. Recent developments seek multiple hydrogen bonding motifs in preparing hydrogels with improved structural integrity. In particular, ureido-pyrimidinone (UPy) is a well-studied quadruple hydrogel bonding unit that can form strong intermolecular bonds compared to a single hydrogel bond78. A previous study developed injectable hydrogels with rapid self-integrating properties by grafting UPy to a dextran backbone and the resultant hydrogels successfully regenerated both bone and cartilage tissues in a mouse subcutaneous model79.
3). Hydrophobic interactions
Non-polar groups in a hydrophilic polymer tend to aggregate and fold the network to form a hydrophobically associated hydrogel. A hydrophobic interaction is also triggered by an external stimulus, such as temperature. A gelation of PNIPAM can be initiated by the aggregation of isopropyl moieties at a temperature above its LCST (32 °C), and reversibly interchange its gelation state at this point39, 40. Poly(N-acryloyl glycinamide) is an example of an upper critical solution temperature (UCST) induced hydrogel, and its composite hydrogel with transforming growth factor β-1 (TGFβ-1) and β-tricalcium phosphate (β-TCP) was effective in rat osteochondral regeneration80. A hydrogel can be designed to possess hydrophobic guest and host molecules, adamantane and cyclodextrin groups, to direct crosslinking in a fixed geometry81. A pair of cyclodextrin modified gelatin and aromatic residue possessing gelatin could form a stable hydrogel by guest-host interaction, and supported bone regeneration in a rat calvarial defect82. Although gelation caused by hydrophobic interactions occurs under mild conditions with a low risk of damaging encapsulated cells and bioactive agents, there are some disadvantages to this approach for applications as BTE scaffolds. Thermosensitive hydrogels exhibit a slow response rate to temperature changes, high shrinkage after dehydration, and poor mechanical properties. Many efforts have been made to modify the hydrogel features, including interpenetrating polymer networks, nanocomposites, etc41, 83. Hydrophobic domains are often introduced to polymer chains to create high-toughness hydrogels84, 85. However, incorporation of hydrophobic units could lead to nonuniform arrangement or phase separation of hydrophobic domains.
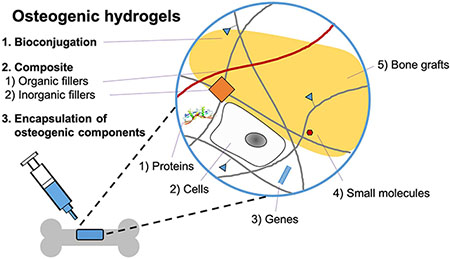
2. Biofunctionalization of hydrogels
Matrix remodeling is mediated by encapsulated cells, followed by the process of cell attachment, spreading, migration, differentiation, and ECM deposition86. Although a hydrogel already has advantages for providing a suitable microenvironment for cells because of its hydrophilic and three-dimensional organized nature, it is still necessary to present osteogenic microenvironments for applications in BTE (Figure 2). In the following sections, we will discuss the several strategies used to customize hydrogel microenvironments to specifically guide bone regeneration (Table 3 and Figure 3).
Figure 2.
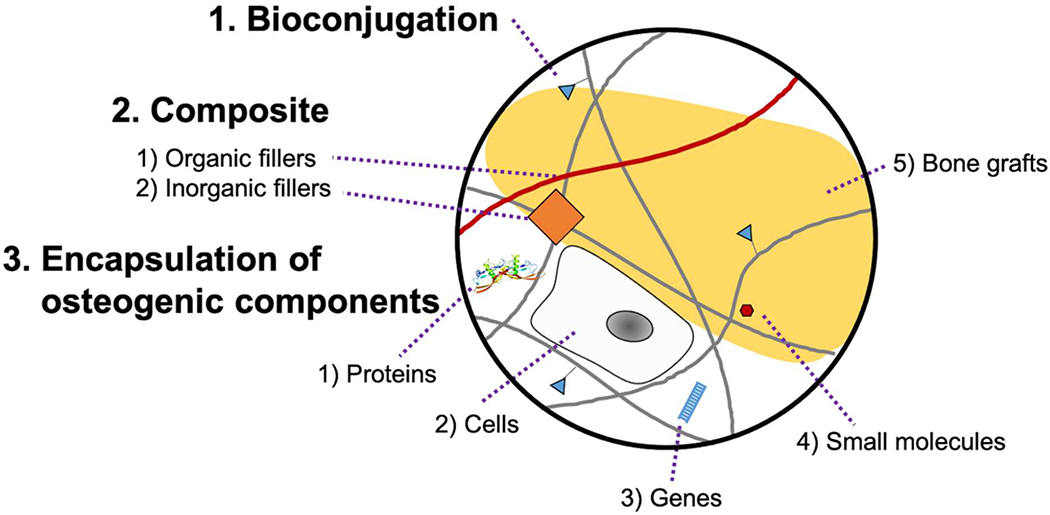
Scheme of hydrogel biofunctionalization. The osteogenic properties of hydrogels are enhanced by various modifications including bioconjugation, composite formation, and encapsulation of osteogenic components. Bioconjugation enables covalent tethering of numerous functional groups. Composite hydrogel allows non-covalent incorporation of functional organic or inorganic fillers. Moreover, diverse osteogenic components (proteins, cells, genes, small molecules, or bone grafts) can be encapsulated into hydrogel network.
Table 3.
Biofunctionalization of hydrogel
| Biofunctionaliz ation | Function | Application |
|---|---|---|
| RGD peptide | - Binding ligand of integrin - Regulate cell attachment and spreading - Mediate osteoblast differentiation |
- Alginate91 - Chitosan31 - PEG134 |
| Catechol group | - Amino acid in mussel adhesive - Enhance cell adhesion - Adsorb hydroxyapatite and induce mineralization |
-HA95 - Alginate90 |
| Calcium-binding group | - Negatively charted PO43− or COOH - Nucleate minerals by capturing calcium ions |
- Chitosan31 - OPF98 - Poly acrylamide99 |
| Heparin | - High binding affinity on growth factors - Enhance BMP function by stabilization and protection from antagonists |
- Fibrin103, 104 - Chitosan105 |
| BMP/BMP-derived peptide | - Key regulating factor in osteogenesis - Various application in bone repair application |
- PEG-PCL107 - Fibrin-HA108 - Alginate109 |
| Nucleic acids | - Modulate cellular function in gene level - siRNA, miRNA, DNA etc. - Carrier free delivery of genes by direct hydrogel conjugation |
|
| Calcium phosphate | - Precursor of hydroxyapatite crystal | - Chitosan-gelatin114 - PEG116 - Gellan gum118 |
| Nanoclay | - 2D silicate sheets adsorbing biomolecules - MMT, LAPONITE® etc - Induce osteogenesis by enabling osteogenic signaling |
- MMT-chitosan121 - Laponite-HA122 - Laponite-chitosan123 |
| Bioactive glass | - Osteoinductive glass-ceramic surfaces - Possess numerous silicon-OH groups |
- Gelatin124 - Chitosan-silk fibrin125 |
| Bone cement | Contain tricalcium silicate and calcium sulfate hemihydrate - Enable apatite mineralization |
- Alginate127 |
| Polymeric fillers | - Incorporate biomolecules mimicking polymers - Can form a stable IPN structure for better mechanical support |
- Fibrin-HA129 - Chitosan-polysulfonate29 - PEG-PLGA132 |
Figure 3.
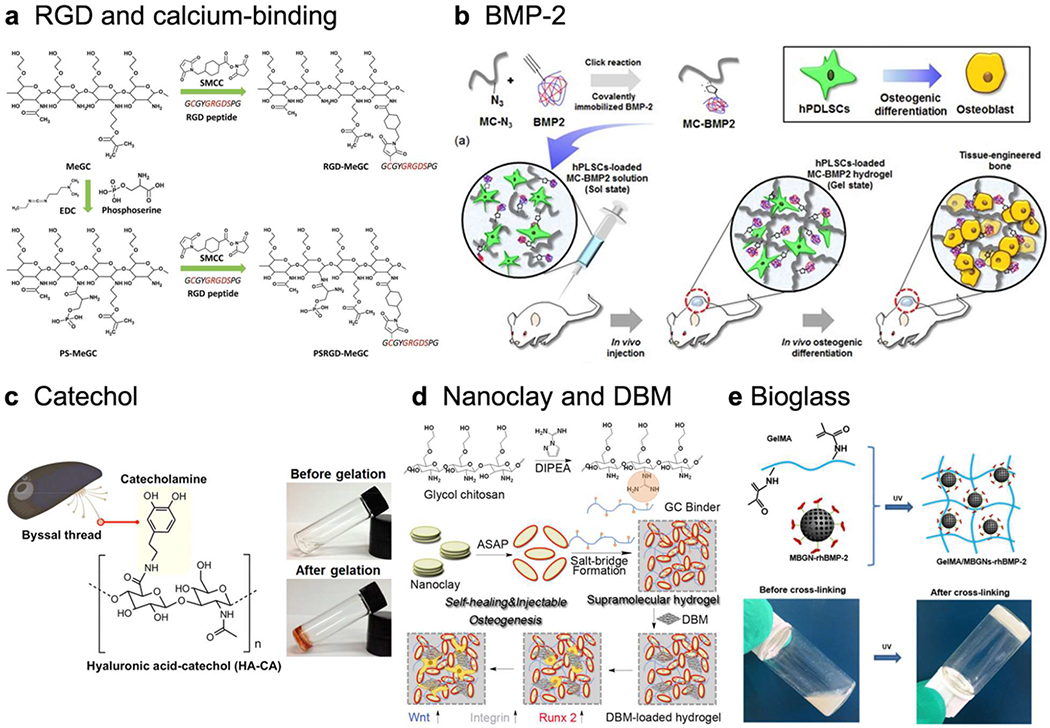
Biofunctionalization of hydrogel, a) Chitosan hydrogel modified with both cell RGD peptide, a cell binding site, and phosphoserine, a calcium binding group. Adapted with permission from 31. Copyright 2016 The Royal Society of Chemistry, b) BMP-2 immobilized hydrogel inducing osteogenesis of human periodontal ligament stem cells. Reprinted with permission from 107 without changes under the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/). Copyright 2017 Springer Nature, c) Mussel-inspired catechol functionalized HA hydrogel and its gelation. Adapted with permission from 95. Copyright 2016 American Chemical Society, d) Supramolecular chitosan hydrogel functionalized with guanidine group, nanoclay, and DBM activating Wnt/β-catenin signaling. Adapted with permission from 123. Copyright 2020 American Chemical Society, e) Gelatin hydrogel incorporated with rhBMP-2 grafted mesoporous bioglass nanoparticles and its crosslinking. Adapted with permission from 124. Copyright 2020 American Chemical Society.
2.1. Bioconjugation
Bioconjugation covalently couples functional groups onto the polymer backbone to fabricate novel cell-responsive hydrogels. Because of strong covalent bonds, it can stably deliver bioactive molecules to the localized area. These functional groups can be a variety of peptides, proteins, polysaccharides, specifically charged moieties, and genes.
2.1.1. RGD peptide
The arginine-glycine-aspartic acid (RGD) peptide is an integrin binding ligand, which regulates cell attachment and spreading87. While some biopolymers like collagen or fibrin already contain abundant RGD groups in their structure, many other polymeric materials such as hyaluronic acid, alginate, chitosan, and PEG lack this ligand. RGD peptide also triggers intracellular signaling, which mediates osteoblast differentiation88, 89. Therefore, RGD conjugation significantly improved cell attachment, spreading, and osteogenesis in these hydrogels31, 90–92.
2.1.2. Catechol group
Catecholic amino acid present in mussel adhesive is an attractive functional moiety for enhancing cell adhesion. The role of catechol in wet-adhesion is well known through the mechanism of π-π stacking, coacervation, or oxidation93. The catechol group can be adsorbed onto hydroxyapatite, which is a key component of matrix mineralization94. Therefore, catechol-functionalized polymeric hydrogels such as hyaluronic acid95 or alginate96 have high potential in BTE.
2.1.3. Calcium-binding groups
Matrix mineralization is a unique process in bone tissue that fills the organic matrix with calcium-based nanocrystals. Negatively charged PO43− or COOH groups can attract calcium ions, which are appealing candidates for bioconjugation97. Phosphate groups containing phosphoserine conjugated chitosan hydrogel could nucleate minerals on the hydrogel surface and support bone repair in a mouse calvarial defect31. Oligo[poly(ethylene glycol) fumarate] hydrogel functionalized with phosphate groups also allowed calcium uptake and exhibited osteoconductivity98. Carboxyl group functionalized polyacrylamide hydrogel could capture calcium ions by decreasing its diffusion rate and initiating chelate formation99.
2.1.4. Heparin
Heparin is a highly sulfated ECM molecule with great binding affinity for growth factors, such as BMPs. It forms a stable complex with growth factors to increase their bioactivities, and also regulates BMP function by interacting with its antagonists, such as noggin100–102. By conjugating heparin onto a hydrogel backbone, it is possible to achieve a specific interaction with BMP to demonstrate the overall osteogenic ability of the hydrogel. Heparin conjugated fibrin hydrogel was studied for the sustained delivery of BMP-2 and improved bone regeneration in a mouse calvarial defect103, 104. Heparin conjugated chitosan hydrogel enhanced BMP bioactivity by protecting BMP from physiological stressors and its antagonist, noggin105.
2.1.5. BMP or BMP-derived peptides
BMP is a key regulating factor in osteogenesis, which is commonly used with various delivery strategies for bone repair106. However, a high dose application of BMP-2 because of its short half-life and poor vehicle ability produces many side effects4. Therefore, direct immobilization of BMP-2 in a hydrogel scaffold is an appealing strategy to prolong BMP-2 stability and release. BMP immobilized methoxy polyethylene glycol-polycaprolactone block copolymer hydrogel could mineralize calcium and induce osteogenic differentiation in a mouse subcutaneous implant107. BMP conjugated fibrin-hyaluronic acid hydrogel showed no side effects, such as heterotopic bone formation in a goat mild intervertebral disc degeneration model108. However, direct conjugation of BMP is costly. Therefore, a peptide that mimics BMP is an alternative strategy to keep the osteogenic potency of BMP protein with a simpler conjugation. BMP-2 mimicking peptide conjugated alginate hydrogel could initiate BMP-2 signaling and increase mineral deposition of murine MSCs109.
2.1.6. Nucleic acids
Nucleic acids can modulate cellular function at the gene level by enabling specific protein expression with the addition of DNA and mRNA, or knocking down target genes with the incorporation of siRNA and miRNA. Delivery of nucleic acids using nanoparticles is an extensively studied field. In comparison to nanoparticle-based delivery, carrier-free delivery of genes is not a typical strategy because of its low cellular uptake. However, lipophilic modification of genes could effectively improve siRNA uptake110, such that it enables direct delivery of naked genes by conjugation in a hydrogel network. Recent studies showed that covalently tethered siRNA in a dextran hydrogel could control and prolong its localized release and improve RNA bioactivity111.
2.2. Composite-hydrogel
Beside chemical conjugation of hydrogels, a composite strategy driven by non-chemical interactions is also beneficial for hydrogel functionalization. Composite-hydrogels can exhibit synergistic effects by counterbalancing the drawbacks of each of the combined materials112. A hydrogel network provides a continuous phase in composite-hydrogels, and a dispersed phase created by other incorporated molecules can add bioactivities as well as reinforcement113.
2.2.1. Inorganic fillers
Inorganic substance in bone tissue plays a role in supporting and protecting the structure of the organic portion. A composite hydrogel constructed with inorganic fillers and an organic hydrogel mimics native bone structure.
1). Calcium phosphate
The main organic and inorganic composition of bone is collagen and hydroxyapatite, a crystal form of calcium phosphate. Therefore, direct incorporation of calcium phosphate in various hydrogels is a common fabrication method for BTE applications. This approach is seen in diverse hydrogels, such as chitosan-gelatin114, whey protein isolate-gelatin115, PEG116, polyacrylic acid-polyaspartic acid117, and methacrylated gellan gum118. Calcium phosphate can be self-assembled in a hydrogel network by incorporating calcium intriguing molecules, such as poly-L-glutamic acid119 or 2D black phosphorus nanosheets120. These hydrogels have increased mechanical strength with better mineralization compared with the unmodified hydrogels.
2). Nanoclay
Clays and clay minerals, such as montmorillonite and laponite, are 2D silicate sheets with large surface area, which can adsorb biomolecules such as proteins, peptides, or protons. A montmorillonite-chitosan nanocomposite hydrogel could induce osteogenic differentiation of the encapsulated cells and enhance bone healing in a mouse calvarial defect model without additional therapeutic agents or stem cells121. Laponite-bisphosphonate functionalized HA hydrogel could facilitate self-assembly of hydrogel and BMP-2 localization in vivo122. Laponite-guanidylated chitosan hydrogel induced osteoinductivity by enabling osteogenic differentiation via activation of the Wnt/β-catenin signaling pathway123. Addition of nanoclay molecules in hydrogels enhanced stiffness and released biochemical cues for osteogenesis over time during degradation.
3). Bioactive glass
Bioactive glass is an osteoinductive glass-ceramic surface decorated with silicon-OH groups. The surface of bioactive glass can deposit calcium phosphate and lead hydroxyapatite formation. The functional groups on the surface of bioactive glass can be used in grafting BMP-2 and form a composite hydrogel with methacrylate gelatin to promote bone regeneration in a rat calvarial defect124. It can form a composite hydrogel with chitosan-silk fibrin-glycerophosphate and support bone repair in a rat calvarial defect125. Bioactive glass can be mixed with other ceramic powders to produce bonelike granules and combined with a dextrin hydrogel for application in goat tibial fractures126.
4). Others
Bone cement consisting of tricalcium silicate and calcium sulfate hemihydrate in an alginate hydrogel enabled apatite mineralization with excellent cytocompatibility of rat bone marrow stem cells127. Another inorganic filler, such as eggshell, was also able to form a composite with a gelatin hydrogel and successfully regulate osteogenic differentiation of human dental pulp stem cells128.
2.2.2. Organic fillers
Composite-hydrogels can be fabricated using not only inorganic but also organic fillers, such as various polymers. A polymer-based composite-hydrogel has an entangled network because of the elongated structure of the polymer, which can support physical stiffness, and influence the swelling and deswelling response.
A polymer-based composite-hydrogel can be fabricated with a simple mixture of different polymers. Usually polymers with different surface charges can be stably incorporated because of electrostatic binding leading to stable network formation. A composite-hydrogel of fibrin and HA minimized cell-contractile forces, which accelerated fibrin degradation and maintained a three-dimensional structure that supported cell proliferation129. Another composite-hydrogel of chitosan and heparin-mimicking sulfonated polymers, such as poly-vinylsulfonic acid or poly-4-styrenesulfonic acid, were stably incorporated in a chitosan network with homogenous distribution29. These polysulfonate fillers were also able to stabilize and enhance BMP activity similar to heparin. There are also other examples of polymer-polymer composite hydrogels in BTE, such as chitosan-collagen71 or chitosan-alginate70 composites.
Polymer-based composite-hydrogels can be diversified using various fabrication techniques, such as electrospinning or self-assembly. Electrospinning is a typical method for generating nanofibers, with a high aspect ratio, greater than 50130, in comparison to bulk native polymer chains, with an aspect ratio of 2-3131. Therefore, nanofiber incorporation in hydrogels can be a reinforcing material, which enhances the mechanical properties of the composite-hydrogel. Poly (lactic-co-glycolic acid) (PLGA) stiffened PEG hydrogel could improve the mechanical strength of the hydrogel compared with unmodified hydrogel and support cell viability and proliferation132. A nanofiber is also able to form a porous structure, which is favorable for cell adhesion and migration. Chitosan nanofiber incorporated PEG hydrogel could provide mechanical strength and support osteoblast attachment and growth133.
2.2.3. Interphase in composite-hydrogels
A composite-hydrogel has two or more heterogenous phases, and the interaction of hydrogel and fillers at the interphase determines the long-term stability and mechanical support of the composite-hydrogel. The nature of the interphase depends on the two inorganic and organic filler types. There are three types of interphase between hydrogel and inorganic filler, 1) filler dispersed in hydrogel network, 2) filler acting as a crosslinker, and 3) filler adsorbed on the hydrogel surface. There are two types of interphase between hydrogel and organic filler, 4) interpenetrating network (IPN) and 5) semi-IPN.
Inorganic fillers in a composite-hydrogel can support mechanical stiffness by providing additional crosslinking points in the polymer network. It is possible to have no interactions with the polymer network in 1) or hinder crosslinking between polymer chains by changing polymer dispersion by adsorbing onto the surface corresponding to 3). Nanoclay121 or hydroxyapatite135 are good examples that can provide additional anchoring points in a hydrogel network corresponding to 2) by intercalation chemistry. However, when the addition of hydroxyapatite increased, it decreased the elasticity of the hydrogel network and showed heterogenous mechanical properties at mesolength and macroscopic range scale136.
Organic fillers in a composite-hydrogel can also stiffen mechanical strength by presenting additional conflicting points in the hydrogel network. IPN structure is generated by two independently crosslinked networks corresponding to 4), or entrapping a linear polymer in a crosslinking network corresponding to 5). Two independent networks can be formed by different crosslinking technologies, such as a combination of chemical and physical crosslinks. A mixture of gelatin methacrylamide and alignate successfully formed a double-crosslinked IPN structure using step by step UV irradiation and calcium ion absorption137.
2.3. Hydrogels as delivery vehicles for bioactive molecules
Multiple bioactive molecules including growth factors (large proteins, cytokines, hormones), genes, and small molecule drugs play an important role in cellular signaling and tissue development. Each category of molecules has its own physicochemical characteristic and requires an accurate delivery system. A hydrogel could be a candidate for a delivery carrier, but many molecules still need additional vehicles for maintaining a proper release profile138.
An osteoinductive growth factor, BMP-2, was tactically integrated in a hydrogel via various vehicles including liposomes139, microfibers140, nanofibers141, poly(phosphazene) nanoparticles142, coacervates, and gelatin microparticles143. These BMP-2 incorporated hydrogels showed great osteoinductive potentials in vitro and in vivo. Parathyroid hormone (PTH) is an FDA approved anabolic treatment of osteoporosis, which can improve bone mineral density in female patients144. A PTH-PEG hydrogel coated onto a poly(propylene fumarate) scaffold supported bone healing in rat femoral defects by bridging full bone or a combination of bone and cartilage145.
Gene transfection is an attractive strategy for inducing osteogenesis. Both viral and non-viral vectors, which help effective transfection, incorporate homogenously in a hydrogel network for osteoinductive functionalization. RNAi strategy using siRNA or miRNA to downregulate a BMP antagonist, such as noggin or chordin, could enhance osteogenic differentiation in vitro and in vivo146, 147. There are several studies that integrate these siRNAs in a bulk hydrogel to promote osteogenesis including siNoggin-chitosan-calcium phosphate in a chitosan hydrogel148, siNoggin-sterosome in a chitosan hydrogel149, siNoggin-PEI in a PEG hydrogel150, and miRNA-20a-PEI in a PEG hydrogel151. Additionally, siRNA152 or miRNA153 sequestration could be carried out by hydrophobic moieties in RNA and HA hydrogel, which successfully control and prolong its release.
Multiple small molecule drugs have been studied for applications in bone repair, such as dexamethasone, phenamil, simvastatin, purmorphamine, and hydroxycholesterol. Many small molecule drugs are hydrophobic, which makes it difficult to directly integrate them in a hydrophilic hydrogel network, therefore, appropriate carriers are required to disperse these molecules homogenously in a hydrogel. These drugs could be applied in various forms with great osteogenic properties like nanodiamond-dexamethasone in a gelatin hydrogel154, liposome-phenamil in a chitosan hydrogel155, maltodextrin based micelle-simvastatin in a maltodextrin hydrogel156, mesophorous silicate nanoparticle-purmorphamine in a HA hydrogel157, and sterosome formed with hydroxycholesterol and sterylamine in a chitosan hydrogel158.
Demineralized bone matrix (DBM) is an allograft processed to remove the mineral of native bone to better expose osteoinductive factors such as BMP-2. However, the use of DBM is limited because of its handling issues and batch-to-batch variability in osteoinductivity159, 160. Although commercially available DBM products commonly use HA, sodium alginate, glycerol, and calcium sulfate as delivery carriers, these are still easily dispersed in the body and can potentially induce inflammation and lower the osteogenic effects161, 162. Several studies have investigated a new carrier hydrogel to deliver DBM and improve its osteogenic efficacy such as pentenoate-modified hyaluronic acid163, heparinized chitosan105, and laponite-chitosan hydrogel123.
3. Strategic approaches to improve osteogenic capacity
A conventional hydrogel provides a homogenous and steady microenvironment, which enables the stable incorporation of cells and bioactive molecules initially. However, actual tissue growth is not a static but dynamic process involving surrounding tissue architecture, cells, and bioactive molecules. Therefore, hydrogels reflecting these physicochemical dynamics are required for advanced BTE. Various strategies to improve osteogenic capacity will be discussed in the following section (Figure 4).
Figure 4.
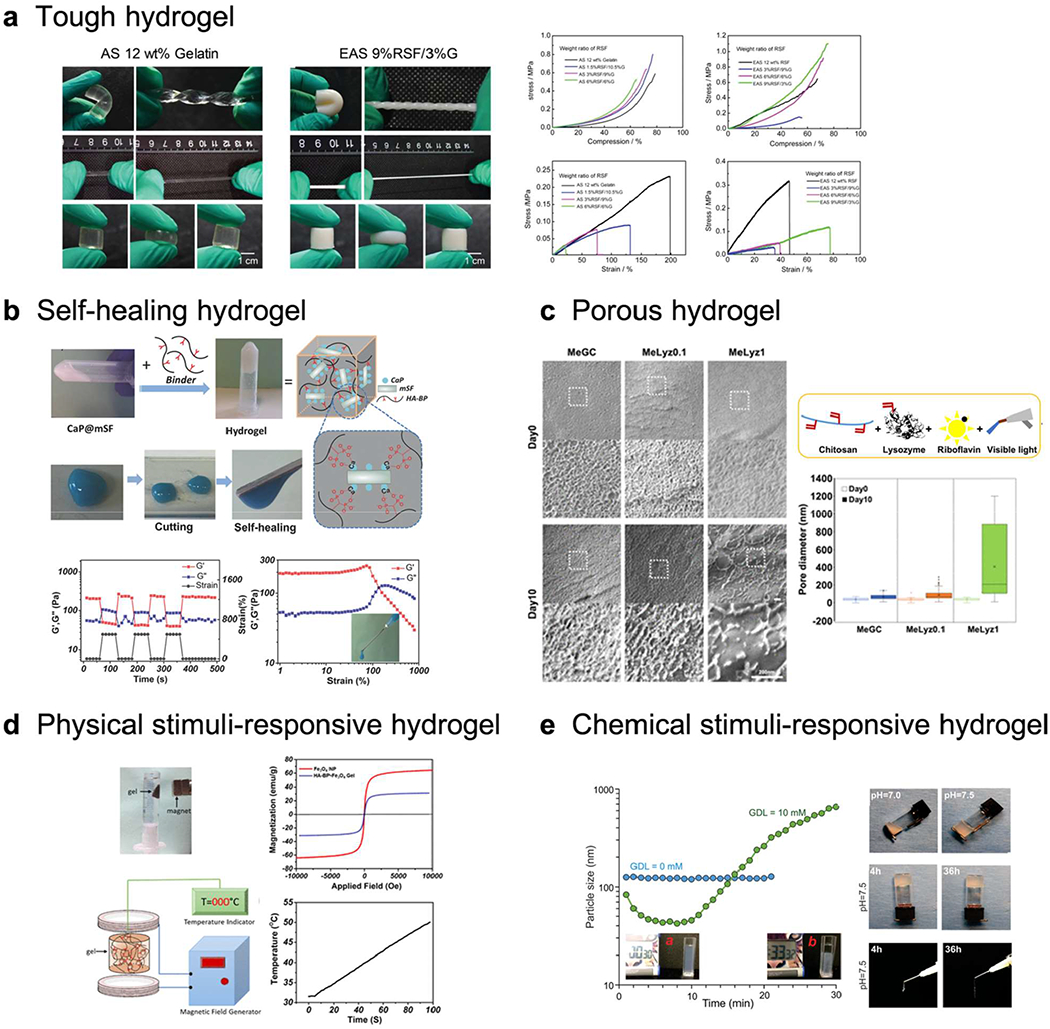
Various strategies to improve the osteogenic potentials of hydrogels, a) Tough hydrogel. Double-crosslinked (physical and chemical) regenerated silk fibroin/gelatin (RSF/G) hydrogel with enhanced mechanical properties (AS: ammonium sulfate, EAS: enzyme-treated AS). Adapted with permission from 164. Copyright 2019 Wiley-VCH. b) Self-healing hydrogel. Silk fibroin-calcium phosphate-HA hydrogel and its self-healing property. Adapted with permission from 165. Copyright 2017 Wiley-VCH. c) Porous hydrogel. Lysozyme conjugated hydrogel with tunable degradation to generate porous hydrogel. Adapted with permission from 166. Copyright 2018 American Chemical Society, d) Magnetic-responsive hydrogel. HA hydrogel incorporating FesCE nanoparticles and its heat generation under magnetic stimulus. Adapted with permission from 167. Copyright 2019 American Chemical Society, e) pH-responsive hydrogel. Carboxymethyl chitosan (CMCh)-amorphous calcium phosphate (ACP) hybrid hydrogel demonstrating pH triggered self-assembly. Adapted with permission from 168. Copyright 2019 American Chemical Society.
3.1. To enhance mechanical performance
3.1.1. Tough hydrogels
A hydrogel typically has low mechanical strength, which makes it difficult to apply in load-bearing tissues like bone. Mechanical strength of tissues vary from 0.01-1.0 kPa for fat, brain, or natural ECM, 1-10 kPa for skin, lung, or kidney, 10-100 kPa for muscle, 100-1,000 kPa for cartilage, and over 1,000 kPa for bone169. Many hydrogel matrices can diversify their mechanical properties to 0.1-100 kPa by simple modification169. Although it is impractical to achieve over 1,000 kPa of initial mechanical strength in a hydrogel environment, the fate of stem cells could be modulated by relatively soft tuning of mechanical properties of the hydrogel.
Multiple studies have already demonstrated that neurogenesis, myogenesis, and osteogenesis of stem cells varied by mechanical strength of the 2D hydrogel surface, 0.1–1 kPa, 8–17 kPa, and 25–40 kPa170–173. In a 3D environment, matrix stiffness also influenced the fate of stem cells such that hMSCs are likely differentiated to neuronal cells at 1 kPa, while they are transformed to a glial lineage at 10 kPa174. Murine MSCs also differentiated to an adipogenic lineage at 2.5-5 kPa and an osteogenic lineage at 11-30 kPa, respectively175. In general, softer hydrogels are favorable for the differentiation of soft tissues, such as brain, fat, and muscle, while stiffer hydrogels are favorable for the differentiation of hard tissues, such as cartilage and bone175.
Many studies have investigated various strategies to overcome the mechanical weakness176 of hydrogels by enhancing homogeneity of the hydrogel network, dissipating energy, or a combined approach. Homogeneity of a hydrogel network can evenly distribute the stress and is achieved by various functionalization techniques. Energy dissipation can prevent macrocrack propagation and is acquired by network entanglement, such as with an IPN structure. A double-network hydrogel composed of both rigid-brittle and soft-stretchable materials is tough-rigid177. A brittle network dissipates energy during deformation, and the elastic network allows it to return to its original form. A combined approach can be carried out by various composite-hydrogel forms.
An alginate hydrogel functionalized with RGD, methacrylate, and dopamine has shown enhanced tensile strength and great bone repair capacity in a rat peri-implantitis model178. A silk fibroin and gelatin hydrogel with dual crosslinking network also showed great improvement in compressive and tensile strength with promising bone healing ability in a rat calvarial defect. A composite-hydrogel of acrylamide and urethacrylate dextran with hydroxyapatite mineralization exhibited strengthened mechanical properties with osteoconductivity179. A ternary composite-hydrogel with chitosan, graphene oxide, and hydroxyapatite provided an oriented microstructure with high mechanical strength and good biocompatibility180.
3.1.2. Self-healing hydrogels
Self-healing hydrogel recovers its mechanical properties upon destruction, which is beneficial for preserving the structure in a dynamic tissue healing environment. Briefly, the self-healing mechanism depends on crack closure ability mediated by mass transfer and reconnection of broken networks in a hydrogel matrix. Various mechanisms that mediate self-healing properties are studied including noncovalent and dynamic covalent bonds181.
A silk fibroin-calcium phosphate-HA composite-hydrogel exhibited self-healing properties by metal-ligand coordination and supported bone healing in a rat calvarial defect165. Laponite nanosilicate is a well-known thixotropic modulator and showed self-healing properties by forming composite-hydrogels with gelatin182, chitosan123, and HA122. A DNA-alginate composite-hydrogel with laponite provided two self-healable links, a dynamic imine bond and electrostatic interactions with nanosilicate183. Both Diels-Alder and cyclodextrin-adamantane interactions provide dynamic covalent bonds that support self-recovery of hydrogels. A chondroitin sulfate-PEG hydrogel with both Diels-Alder and guest-host interactable moieties could form a dual crosslinked network and support bone repair in a mouse limb defect61 and a rat cranial defect184.
3.2. To mimic the tissue architecture
Native bone tissue is a highly complex structure composed of nanofibrous entanglement and microporous channel arranged in a macroscopic network. The defined structure of hydrogels can affect cell-matrix interactions in the presence of soluble cues at nano-, micro-, and macro-scales for bone repair and regeneration.
3.2.1. To mimic nanoscale tissue structure
Polymeric hydrogels often incorporate linear nanofibers but the simple linear structure is not sufficient to provide a stable hydrogel network for cell encapsulation or growth factor delivery. Therefore, self-assembling peptides were widely used to create stable 3D hydrogels driven by physicochemical crosslinking of amphiphilic peptides185. In addition, their 3D structure could be easily modulated by altering the sequence of peptides. A recent study has shown that the nanofiber hydrogel made by synthetic D-form and L-form self-assembling peptides formed stable hydrogels and facilitated the release of growth factors to promote the rat femoral bone repair186. Additionally, various electrospinning techniques have been applied to improve the alignments, orders, and structure of nanofibers in the hydrogels187. Another study reported an electrospun organic-inorganic hybrid hydrogel that could constantly mineralize on its surface and promote osteogenic differentiation188.
3.2.2. To mimic microscale tissue structure
Tissue reformation requires mass transport to support cell migration, infiltration, and proliferation which lead to differentiation and vascularization over time. However, a traditional nano-porous hydrogel network interferes with these activities because of restricted room for cell movement. Modulation of a conventional nanoporous hydrogel with various functionalizations or recent progress to fabricate hydrogel microparticles are widely applied to produce microporous structures.
The conventional method to generate a porous hydrogel is the use of freeze-drying technology or the incorporation of a porogen. A hybrid hydrogel of silk fibroin and organosilane fabricated by unidirectional freeze-drying showed high porosity and bone-type anisotropic structure. It could trigger osteoblast proliferation and exhibited successful osseointegration in rat femur189. An alginate hydrogel incorporating hydrolytically degradable porogens could deliver MSCs and recruit endogenous cells to regenerate a critical-sized rat femoral defect34.
A nanoporous hydrogel network was able to enlarge its pore size by degradation of the hydrogel network. A lysozyme conjugated chitosan hydrogel could increase its pore size by degradation and induce cell migration throughout the bulk hydrogel166. It was also able to increase osteogenic differentiation and bone healing in a mouse calvarial defect. A nanocomposite-hydrogel also enables the generation of a microporous structure by changing network orientation. A chitosan-MMT hydrogel-composite exhibited an interconnected microporous network by intercalation chemistry and the presence of nanosilicates also successfully promoted bone healing in a mouse calvarial defect121. A composite-hydrogel including chitosan, HA, and hydroxyapatite also exhibited porous structure associated with hydroxyapatite aggregation in a hydrogel network with good cytocompatibility190.
3.2.3. To mimic macroscale tissue structure
3D printing is an emerging technique to construct BTE hydrogels. It allows fabricating a precise, complex, and highly customizable architecture mimicking native bone microstructure, and provides great cell support and infiltration191. The physicochemical properties of hydrogels can be readily controlled by different 3D printing processes and the selection of bioinks. The current printing techniques are based on laser, nozzle, or inkjet. Hydrogel inks can be prepared by polymer composite or reinforced filler192, which are beneficial to improve mechanical properties of the final products. The hydrogel consisting of chitosan and hydroxyapatite could be successfully 3D printed and supported the osteogenic differentiation of preosteoblasts193.
Moreover, 3D printing enables the construction of more complicated structures such as layered, graded, multicellular, or even customized shape of hydrogels. For example, bone defects in the subchondral region requires a sophisticated approach to treat the complex osteochondral tissue with appropriate mechanical loading. A 3D printed tri-layered hydrogel with diverse gelatin and hydroxyapatite ratio was successfully integrated with the surrounding tissue and enhanced the osteochondral regeneration in a rabbit osteochondral defect model194. A 3D printing of thermosensitive copolymerized N-acryloyl glycinamide, and N-[tris(hydroxymethyl)methyl] acrylamide could prepare a gradient hydrogel due to rapid sol-gel transition of the materials195. The 3D printed gradient hydrogels promoted cartilage and subchondral bone regeneration in a rat model195. Additionally, the hierarchical haversian bone structure could be built up by 3D printing196. A recent study exhibited great potential of 3D printing to arrange the spatial distribution of different types of cells and create multicellular tissue, thereby increasing bone repair with blood vessel formation196. Lastly, cell-laden-hydrogels can be an ideal candidate for bioinks to be used in 3D bioprinting due to its highly cell-friendly nature. Bioinks with different properties can readily be obtained by blending different biopolymers. Although 3D bioprinting enables fabrication of customized bone grafts, many challenging points still remain to obstruct the clinical translation; the restricted size of printed constructs, a lack of long-term stability, and slow host tissue integration, among others197.
Recent advances in 3D printing technology also open the field of 4D printing. 4D printing incorporates the fourth dimension, time, by facilitating stimuli-responsive smart hydrogels. The 4D printed constructs transform their shapes and orientations by responding to the external stimuli, which is also beneficial to on-demand biomedical applications such as drug delivery198.
3.3. To enhance the release of bioactive molecules
A hydrogel implanted in bone tissue encounters diverse stimuli derived from physical, chemical, and biological cues. Without proper response to these stimuli, the hydrogel might undergo unexpected consequences, such as fast degradation or burst release of encapsulated cells and bioactive molecules. Additionally, because of the dynamic remodeling environment, a hydrogel sustains defects, which may disrupt the structure and lead to failure of the implant. Therefore, an optimal stimuli-responsive hydrogel must be engineered and advances made in engineering hydrogels have provided a number of potential candidates. Lastly, these stimuli-responsive hydrogels also enable 4D cell culture mimicking the dynamic heterogeneity of an in vivo environment199.
3.3.1. Physical stimuli-responsive hydrogels
Physical stimuli include various signals including light, temperature, and magnetic field. Photoresponsive hydrogels usually incorporate photoreactive molecules or functional groups in the hydrogel backbone. A variety of photo-crosslinkable hydrogels modified with vinyl groups are good examples200. Photodegradation is also a common mechanism used to modulate hydrogel structure. For instance, black phosphorous nanosheet incorporated gelatin hydrogel could enhance the phosphate release profile with light activation, including natural and near infrared light, and improved bone healing in a rabbit calvarial defect201. An azobenzene derived crosslinker reversibly changed its chemical isomerization with UV and visible light activation to act as a photo-switchable crosslinker and modulated hydrogel stiffness by light activation202.
A thermo-responsive hydrogel is also very commonly used in bone tissue engineering. PNIPAM based thermal gel is a well-known thermo-crosslinkable hydrogel40. PNIPAM conjugated HA hydrogel exhibited reversible gelation203, and effectively delivered bioactive microvascular fragments with enhanced vascularization in a mouse femur defect model204, 205. Alginate conjugated with temperature-responsive poly(ε-caprolactone-co-lactide)-b-poly(ethylene glycol)-b-poly(ε-caprolactone-co-lactide) and O-phosphorylethanolamine transformed to a stable hydrogel at 37 °C and showed in situ mineralization in a rat subcutaneous injection with BMP-2 loading206.
A magnetic-responsive hydrogel incorporates paramagnetic or ferromagnetic molecules in a hydrogel network. A super paramagnetic Fe3O4 nanoparticle incorporated hydrogel of methoxy(polyethylene glycol)-polyalanine and hydroxyapatite could enhance osteogenic gene expression under both static and moving magnetic fields207. Iron oxide, Fe3O4, incorporated bisphosphonate modified HA hydrogel exhibited heat-generation controlled by a magnetic field and showed good biocompatibility167. In addition, a recent study has shown that a ferrogel loaded with BMP-2 could successfully control the timing of the protein release using remote magnetic stimulation in an immediate or delayer manner208.
3.3.2. Chemical and biological stimuli-responsive hydrogels
Chemical and biological stimuli involve alterations in pH or enzymes. A pH-responsive hydrogel contains functional moieties, which can accept or donate protons with pH alteration. A composite hydrogel of chitosan and PEG reinforced with bone ash could modulate incorporated amoxicillin release at different pH 1.2 and 7.4, which are close to the gastric and intestinal environment pHs, respectively because of the polyelectrolyte properties of chitosan209. A chitosan-calcium phosphate-glucono δ-lactone composite-hydrogel demonstrated pH-triggered self-assembly and induced ectopic bone formation in mouse with BMP incorporation168.
Enzyme-responsive hydrogels containing substrates are triggered by endogenous enzyme activity in the human body. Chondroitin sulfate-PEG hydrogel modified with transglutaminase factor XIII specific substrate sequences could tune hydrogel network properties by altering transglutaminase concentration210. It also exhibited controlled release of BMP-2 and promoted the osteogenic differentiation of BMSCs. Matrix metalloproteinase (MMP) cleavable crosslinker is also a well-known enzyme-sensitive group widely used in BTE. MMP-sensitive PEG hydrogels could support cell maturation from osteoblast to osteocyte and bone ECM deposition211.
4. Evaluation of osteogenic hydrogels
Osteogenic potential of the newly developed hydrogel can be evaluated under diverse conditions including in vitro, ex vivo, in vivo, and clinical settings. However, direct translation to the clinical assessment costs hugely, and it is sometimes difficult to understand the results because of the complexity of the system. Therefore, researchers have investigated various preclinical models to assist the clinical studies. In this section, we will discuss the characteristics of each evaluation model.
4.1. In vitro evaluation
The intimate interaction of hydrogels with bone tissue is critical to the success of implantation. Therefore, it is necessary to evaluate molecular and cellular level phenomena in the immediate environment of bone forming cells212. Bone tissue possesses a variety of cells including osteoblasts, osteoclasts, mesenchymal stem cells, and different types of immune cells. Osteoblastic cell lines are well-established and available for in vitro investigation. It usually begins with a typical characterization of cell adhesion and growth, followed by the analysis of osteogenic differentiation phenotype markers. However, true bone regeneration is not a one-pot reaction, but followed by a dynamic sequential order or crosstalk between different cell types.
Bone response to implanted biomaterials is a highly concerted process involving blood clotting, secretion of inflammatory cytokines, initiation of vascularization, and woven bone formation. To simulate these complex dynamic environments in vitro, recent studies have tested biomaterials with co-cultures of immune cells, osteoclasts, and endothelial cells, or step-by-step inclusion of stimulating factors213. The effect of a periosteal ECM derived hydrogel on the different stages of bone healing was evaluated in vitro by demonstrating its ability to promote the M1-to-M2 transition of macrophages and induce angiogenesis and osteogenesis214. The bioactivity of a silica-collagen hydrogel was characterized in a human co-culture model by evaluating its ability to control the ratio of osteoblasts and osteoclasts215. A collagen-fibrin gel was tested in the co-culture system of endothelial cells and MSCs to recapitulate vascular formation in vitro216.
Additionally, advances in nano- and pico-technology provide new methods to characterize the surface and internal morphology of hydrogels in vitro. Conventional methods to characterize materials are occasionally not appropriate for hydrogels due to their high-water content or soft nature. Cryo-imaging has been adopted to observe the hydrated hydrogel network and its mesh size, which is difficult to detect after dehydration166, 217. Additionally, the development of spectroscopy such as Brillouin or Raman enabled nondestructive observation of nanostructured hydrogel networks in a noninvasive manner136. Diverse nanoindentation methods including atomic force microscopy could measure poroelastic properties of hydrogels218, 219.
4.2. Ex vivo evaluation
Even though both in vitro and ex vivo models represent the tests conducting outside of the living body, in vitro is usually related to the test tube experiments and ex vivo covers more broad range. Traditionally, ex vivo bone organ culture was accompanied by the retrieval of bone tissue and treatment with biomaterials, cells, or stimulating factors220. The cytoarchitecture and intercellular connections are well retained in ex vivo systems, which can mimic and replace ethically challenging in vivo models221. For instance, an organotypic chick femur defect culture system was adopted as a critical size defect model to evaluate bone augmenting abilities of hydrogels releasing osteogenic growth factors222, 223. Human ex vivo bone defect model prepared by cylinder-cut of femoral heads also remained viable for 28 days and exhibited bone repair processes with the incorporation of collagen hydrogels224. Moreover, recent development of microfluidics systems create the organs-on-a-chip models which bridge the gap between traditional ex vivo and in vivo studies225. Bone-on-a-chip enables the spontaneous growth of miniaturized bone and periodic sampling in a physiologically relevant model226. The ex vivo bone models allow rapid screening of therapies, high reproducibility, and low cost, which are beneficial in comparison to in vivo studies. However, ex vivo models are not appropriate for long-term culture and do not completely mimic the in vivo state in the perspective of nutrients supply or missing mechanical stimulation.
4.3. In vivo evaluation
In vivo models have been the long-established sources to elucidate safety and activity of biomaterials in a living organism prior to human clinical trials. The in vivo bone repair ability of osteogenic hydrogels can be studied using diverse animal models. Animal studies are more suitable for estimating results before translating them to clinical settings in comparison to in vitro experiments. Animal models are selected using multiple criteria including size, cost, handling, surgery resistance, infection, biological similarity to humans, and bone structure and composition etc. Diverse animal models to assess bone healing capacity will be discussed in the following section (Table 4).
Table 4.
In vivo evaluation of various hydrogels
| Animal | Defect | Hydrogels | Size | Time | Outcome |
|---|---|---|---|---|---|
| Mouse | Calvarial | ciiitosan-MMT121 | 3 mm | 6 weeks | - Recruited native cells - Bone volume increased to 46% |
| Calvarial | PEG-RGD-Lys-glutamine receptor237 | 4 mm | 12 weeks | - Recruit mesenchymal progenitor cells - Bone volume increased to 1 mm3 |
|
| Femur | Decellularized adipose tissue-hydroxyapatite238 | 3 mm | 12 weeks | - Increased collagen I and osteopontin expression - Bone volume increased to 5 mm3 |
|
| Ectopic | Gelatin239 | N/A | 4 weeks | - Coated with cells in osteogenic media - Increased collagen I and osteopontin expression |
|
| Ectopic | FIA-Laponite122 | N/A | 6 weeks | - Sustained retention of BMP-2 in vivo | |
| Rat | Calvarial | Pentonate-HA-devitalized tendon240 | 7.5 mm | 8 weeks | - Enhanced compressive modulus and calcium - Bone volume increased to 42% |
| Calvarial | Gelatin-PEO-PPO-PEO241 | 8 mm | 12 weeks | - Comparable with autologous bone graft - Bone surface area increased to 80% |
|
| Femur | Gelatin-Alginate242 | 3 mm | 3 weeks | - Mg particle as a foaming agent induced vascularization - Bone volume increased to 60% |
|
| Mandible | PNFPAM-PLGA-PEG-mesoporous silica nanoparticles243 | 5 mm | 10 weeks | - Incorporated with miRNA222 and aspirin - Bone volume increased to 22% |
|
| Ectopic | HA244 | N/A | 8 weeks | - Acidic F1A enhance BMP-2 sequestering and improve bone formation in vivo | |
| Rabbit | Mandible | Chitosan-Collagen245 | 8 mm | 12 weeks | - Incorporated with BMP-2 in gelatin microsphere - Bone volume increased to 55% |
| Femur | Polyacrylamide-Dextran-Flydroxyapatite179 | 5 mm | 12 weeks | - Inorganic-organic interaction in hydrogel enhanced mechanical properties - Bone volume increased to 8 mm3 |
|
| Pig | Mandible | Alginate-HA240 | 10 mm | 9 weeks | - Delivered BMP-2 and hBMSCs, and enhanced bone healing |
| Femur | Polyhydroxyethyl methacrylate-Collagen-DBM247 | 10 mm | 8 weeks | - Collagen stimulated bone healing by changing biological properties of synthetic polymer and enhanced mineralization | |
| Sheep | Iliac crest | ROD-Alginate-HA248 | 15 mm | 12 weeks | - Incorporated with ovine MSCs - Bone volume increased to 40% |
| Femur | Keratin-Hydroxyapatite249 | 10 mm | 4 weeks | - Dental implant coated with hydrogel was implanted - Bone-implant contact increased to 58.1% |
4.3.1. Choice of animals
Various animal models ranging from small animals, such as rodents and rabbits, to large animals, such as dog, goat, sheep, and pig, are employed to evaluate the osteogenic properties of hydrogels. Rodent models are preferred because of their numerous advantages in mouse and rat species, respectively. A mouse model is commonly used in biological research because of the diversity in the available strains and ease of use with relatively high cost efficiency227. However, it has a size limitation, which presents challenges for surgery and makes it difficult to perform experiments and create proper load-bearing defects. A rat model may overcome the size issue of mice with similar organ proportions to humans. However, it has limited strain diversity compared with mice, and it is less cost efficient to maintain because of its larger size. A rabbit model has a larger bone structure but is still limited in size compared with a dog, sheep, or pig. Large mammals have a much larger skeleton which resembles the high load-bearing human bone environment, but each model also has limitations in the existence of gaps between human bone composition and healing rate.
4.3.2. Bone regeneration models
In vivo bone healing studies can be divided into two models, orthotopic and ectopic bone formation. An orthotopic bone formation model usually derives from bone fracture healing. It allows for an actual bone remodeling environment with endogenous MSCs, osteogenic growth factors, and mechano-transduction. It is composed of multiple models such as the calvarial, long bone, partial cortical, or cancellous bone defect228. In contrast, ectopic bone formation models including subcutaneous, intramuscular, or kidney capsule implantation have relatively low load-bearing environments with low bone-related cytokines229. Taking into account the differences in each model, an appropriate animal model can be chosen to evaluate a new osteogenic hydrogel.
A calvarial defect model is predominantly applied in rodent species by creating a critical-sized hole, with a diameter of 5 mm for rat and 3 mm for mouse, and implantation of a tissue-engineered hydrogel230. The surgical procedure is relatively simple and it does not require any additional support to hold the implanted hydrogel because of the stable position of calvaria. However, it is unable to provide a load-bearing environment and is difficult to use for long-term studies because of the relatively short life-period of the rodent models, the most widespread animal species used for calvarial surgery. It also has a large difference in size and skeletal loading system in comparison to humans. Additionally, the biological consequences of bone growth are different in the calvarial models compared with other bone areas. Although common bone growth derives from embryonic mesoderm via a cartilage intermediate (endochondral ossification), calvarial bone growth is influenced by the neural crest and mesoderm (intramembranous ossification)231.
The maxillofacial/Mandible defect model is also used in rodents and other small animal models232–234 Maxillofacial defects are usually found in dental clinics and occur by tooth loss, apical cysts, or periodontis etc. The environment of the maxillofacial bone is different from the calvarial area in blood supply, mucosal covering, microorgamisms, and mechanical loading etc235. Therefore, the mandible defect model establishes its unique scope of bone regeneration in comparison to calvarial or long bone defect models.
Long bone segmental defects in large animals are more similar to the clinical setting than calvarial defects. It is usually executed by removing a segment of the long bone by using a drill or a saw. Fixation devices are often used externally or internally because of the large defect size and mobility. The physiological bone condition of humans is close to dogs, sheep, goats, and pigs; the most widely used model is sheep because of its similar weight to adult humans and relatively low social concern and handling issues236. Although the long bone defect model is closer to the physiological condition of humans, the standardized critical defect conditions are still disputable because of the complicated aspects of a model depending on animal species, age, defect location, presence of periosteum, or metabolic conditions.
The ectopic bone formation model provides a relatively controlled in vivo conditions compared with the fracture healing models229. Therefore, it is beneficial for assessing a new osteoinductive hydrogel, growth factors, and bone-forming stem cells by ruling out the impact of surrounding osseous environments. However, it has poor robust bone growth potentially affected by the lack of blood flux and orthotopic environment. It is essential to understand the benefits of each model and choose an appropriate system to evaluate a newly designed hydrogel.
4.3.3. Mouse models for bone disease research
In addition to simple bone regeneration from the fractures, it is also necessary to evaluate bone healing under more severe complications such as disease models. Multiple bone disorders have seriously impacted the general population, specifically for aging communities. There are many age-related bone diseases including osteoporosis, osteodystrophy, osteopetrosis, and bone cancer230. We will not discuss the details of each bone disorder, but we will explore how to develop a specific animal model to understand the disease mechanisms. Bone disorders can be generated naturally or can be induced by genetic modification. However, a naturally-occurring model is very limited in number as well as conditions, so a genetically modified animal model is preferable. A genetically-modified mouse model has numerous advantages for use in bone disease studies such as availability, well-understood genome, which is similar to the human genome, low cost, high reproductive potential, and ease of handling. There are other animals available for bone disorder research; however, the mouse model is preferred in many applications because of its unique advantages.
4.4. Clinical translation
After pre-clinical studies using in vitro, ex vivo, and in vivo models, a new osteogenic hydrogel can be referred for clinical translation. Bone graft substitutes, including osteogenic hydrogels, categorized in implants and prosthetics by FDA guidelines, which require them to proceed as class II-510(k), or III-Premarket Approvals (PMA) medical devices approvals and clearances depending on the specific indications for use. There are 184 bone graft substitutes with FDA 510(k) clearance and 96 products with PMA clearance from 2015 to 2019, which were identified by searching the FDA database. These data suggest that numerous new bone substitute products are made available reflecting market demand. Many biomaterial-based products incorporate both inorganic and organic substances, such as the mixture of hydroxyapatite or silicate granules in various hydrogel matrices. Osteogenic properties of these products are mainly governed by inorganic components and the hydrogel matrix plays a role in the improvement of handling properties. Therefore, many of these products have localized surface functionalization, which potentially limits bone healing7, 250. The enhancement of overall osteogenic properties in a hydrogel matrix can broaden opportunities for the production of new bone substitute products.
5. Challenges and opportunities
In this review, we have discussed the latest approaches for designing new osteogenic hydrogels. Numerous new materials have been studied, but only a limited number of products are moved into clinical applications because of several critical challenges.
First and foremost, the mechanical strength of a hydrogel is insufficient for application in large bone defect areas. The soft, elastic, water-enriched environment of a hydrogel is beneficial for attracting native cells or growth factors, but the instability from weak mechanical strength is a key issue for applications in clinical settings. However, many researchers have investigated methods to improve the mechanical properties of hydrogels, which makes it possible to broaden the potential applications.
Additionally, many of the commercially available bone graft products are growth factor-based treatments delivering osteogenic proteins via carrier materials, such as collagen sponges, calcium phosphate, and hydrogels. A growth factor-based treatment is an attractive strategy, but it also carries well-known clinical side effects mainly because of the supraphysiological doses administered. Hydrogels currently used as carriers have limited function for physically enclosing the osteogenic molecules to the defect sites, but do not interactively communicate with the molecules. A simple modification of a hydrogel carrier can enhance the stability and bioactivity of proteins, which can potentially improve the efficacy of growth factor-based treatment.
Lastly, many commercially available biomaterial-based treatments rely on inorganic substances in the composite-hydrogel. The limited number of accessible inorganic molecules such as calcium phosphate or silicate molecules hinder extensive use. These inorganic substances are usually more cost-effective than biologies; however, their osteogenic efficacy is less favorable. The localized distribution of inorganic substances in the composite-hydrogel also limits their osteogenic features. Therefore, the improvement of osteogenic properties of a hydrogel can potentially enhance the overall bone healing capacity of new composite-hydrogel materials.
Although many ongoing challenges remain, multiple interdisciplinary studies are needed to engineer new osteogenic hydrogels that can overcome the current challenges and expand future opportunities.
Acknowledgements
This work was supported by grants from the National Institutes of Health (R01 DE027332), the Department of Defense (W81XWH-18-1-0337), and the MTF Biologics.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Berendsen AD; Olsen BR, Bone development. Bone 2015, 80, 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner UH, Osteoblasts, Osteoclasts, and Osteocytes: Unveiling Their Intimate-Associated Responses to Applied Orthodontic Forces. Seminars in Orthodontics 2012, 18, (4), 237–248. [Google Scholar]
- 3.Grant SF; Reid DM; Blake G; Herd R; Fogelman I; Ralston SH, Reduced bone density and osteoporosis associated with a polymorphic Sp1 binding site in the collagen type I alpha 1 gene. Nat Genet 1996, 14, (2), 203–5. [DOI] [PubMed] [Google Scholar]
- 4.James AW; LaChaud G; Shen J; Asatrian G; Nguyen V; Zhang XL; Ting K; Soo C, A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Engineering Part B-Reviews 2016, 22, (4), 284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elisseeff J; Puleo C; Yang F; Sharma B, Advances in Skeletal Tissue Engineering with Hydrogels. Orthod Craniofac Res 2005, 8, (3), 150–61. [DOI] [PubMed] [Google Scholar]
- 6.Albrektsson T; Johansson C, Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001, 10 Suppl 2, S96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra R; Bishop T; Valerio IL; Fisher JP; Dean D, The potential impact of bone tissue engineering in the clinic. Regen Med 2016, 11, (6), 571–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen BB; Moriarty RA; Kamalitdinov T; Etheridge JM; Fisher JP, Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J Biomed Mater Res A 2017, 105, (4), 1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hesse E; Hefferan TE; Tarara JE; Haasper C; Meller R; Krettek C; Lu L; Yaszemski MJ, Collagen type I hydrogel allows migration, proliferation, and osteogenic differentiation of rat bone marrow stromal cells. J Biomed Mater Res A 2010, 94, (2), 442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamieh F; Collignon A-M; Coyac BR; Lesieur J; Ribes S; Sadoine J; Llorens A; Nicoletti A; Letourneur D; Colombier M-L; Nazhat SN; Bouchard P; Chaussain C; Rochefort GY, Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Scientific Reports 2016, 6, (1), 38814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiger M; Li RH; Friess W, Collagen sponges for bone regeneration with rhBMP-2. Advanced Drug Delivery Reviews 2003, 55, (12), 1613–1629. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L; Niu X; Sun L; She Z; Tan R; Wang W, Immune response of bovine sourced cross-linked collagen sponge for hemostasis. Journal of Biomaterials Applications 2017, 32, (7), 920–931. [DOI] [PubMed] [Google Scholar]
- 13.Widdowson JP; Picton AJ; Vince V; Wright CJ; Mearns-Spragg A, In vivo comparison of jellyfish and bovine collagen sponges as prototype medical devices. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2018, 106, (4), 1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy JP Jr; Schade W; Siegle RJ; Vanderveen EE; Zachary CB; Waldinger TP; Swanson NA, Immune responses to bovine collagen implants: Significance of pretreatment serology. Journal of the American Academy of Dermatology 1987, 16, (5), 955–960. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Guillén MC; Giménez B; López-Caballero ME; Montero MP, Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocolloids 2011, 25, (8), 1813–1827. [Google Scholar]
- 16.Zhang Z; Li G; Shi B, Physicochemical properties of collagen, gelatin and collagen hydrolysate derived from bovine limed split wastes. Journal of the Society of Leather Technologists and Chemists 2006, 90, (1), 23–28. [Google Scholar]
- 17.Conrad B; Hayashi C; Yang F, Gelatin-Based Microribbon Hydrogels Guided Mesenchymal Stem Cells to Undergo Endochondral Ossification In Vivo with Bone-Mimicking Mechanical Strength. Regenerative Engineering and Translational Medicine 2019. [Google Scholar]
- 18.Spotnitz WD; Prabhu R, Fibrin Sealant Tissue Adhesive-Review and Update. 2005, 15, (3), 245–270. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed TAE; Dare EV; Hincke M, Fibrin: A Versatile Scaffold for Tissue Engineering Applications. Tissue Engineering Part B: Reviews 2008, 14, (2), 199–215. [DOI] [PubMed] [Google Scholar]
- 20.Cortese A; Pantaleo G; Borri A; Caggiano M; Amato M, Platelet-rich fibrin (PRF) in implant dentistry in combination with new bone regenerative technique in elderly patients. International Journal of Surgery Case Reports 2016, 28, 52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aruffo A; Stamenkovic I; Melnick M; Underhill CB; Seed B, CD44 is the principal cell surface receptor for hyaluronate. Cell 1990,61,(7), 1303–1313. [DOI] [PubMed] [Google Scholar]
- 22.Zhu H; Mitsuhashi N; Klein A; Barsky LW; Weinberg K; Barr ML; Demetriou A; Wu GD, The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells 2006, 24, (4), 928–35. [DOI] [PubMed] [Google Scholar]
- 23.Koca C; Komerik N; Ozmen O, Comparison of efficiency of hyaluronic acid and/or bone grafts in healing of bone defects. Nigerian Journal of Clinical Practice 2019, 22, (6), 754–762. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz Z; Goldstein M; Raviv E; Hirsch A; Ranly DM; Boyan BD, Clinical evaluation of demineralized bone allograft in a hyaluronic acid carrier for sinus lift augmentation in humans: a computed tomography and histomorphometric study. Clinical Oral Implants Research 2007, 18, (2), 204–211. [DOI] [PubMed] [Google Scholar]
- 25.Vårum KM; Holme HK; Izume M; Stokke BT; Smidsrød O, Determination of Enzymatic Hydrolysis Specificity of Partially N-Acetylated Chitosans. Biochim Biophys Acta 1996, 1291, (1), 5–15. [DOI] [PubMed] [Google Scholar]
- 26.Vårum KM; Myhr MM; Hjerde RJ; Smidsrød O, In Vitro Degradation Rates of Partially N-Acetylated Chitosans in Human Serum. CarbohydrRes 1997, 299, (1–2), 99–101. [DOI] [PubMed] [Google Scholar]
- 27.LogithKumar R; KeshavNarayan A; Dhivya S; Chawla A; Saravanan S; Selvamurugan N, A review of chitosan and its derivatives in bone tissue engineering. Carbohydr Polym 2016, 151, 172–188. [DOI] [PubMed] [Google Scholar]
- 28.Seol Y-J; Lee J-Y; Park Y-J; Lee Y-M; Ku Y; Rhyu I-C; Lee S-J; Han S-B; Chung C-P, Chitosan sponges as tissue engineering scaffolds for bone formation. Biotechnology Letters 2004, 26, (13), 1037–1041. [DOI] [PubMed] [Google Scholar]
- 29.Kim S; Cui ZK; Kim PJ; Jung LY; Lee M, Design of Hydrogels to Stabilize and Enhance Bone Morphogenetic Protein Activity by Heparin Mimetics. Acta Biomater 2018, 72, 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amsden BG; Sukarto A; Knight DK; Shapka SN, Methacrylated Glycol Chitosan as a Photopolymerizable Biomaterial. Biomacromolecules 2007, 8, (12), 3758–3766. [DOI] [PubMed] [Google Scholar]
- 31.Kim S; Cui ZK; Fan JB; Fartash A; Aghaloo TL; Lee M, Photocrosslinkable Chitosan Hydrogels Functionalized with the RGD Peptide and Phosphoserine to Enhance Osteogenesis. Journal of Materials Chemistry B 2016, 4, (31), 5289–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bidarra SJ; Barrias CC; Granja PL, Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomaterialia 2014, 10, (4), 1646–1662. [DOI] [PubMed] [Google Scholar]
- 33.Kolambkar YM; Dupont KM; Boerckel JD; Huebsch N; Mooney DJ; Hutmacher DW; Guldberg RE, An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 2011, 32, (1), 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garske DS; Schmidt-Bleek K; Ellinghaus A; Dienelt A; Gu L; Mooney DJ; Duda GN; Cipitria A, Alginate Hydrogels for In Vivo Bone Regeneration: The Immune Competence of the Animal Model Matters. Tissue Engineering Part A 2020, 26, (15–16), 852–862. [DOI] [PubMed] [Google Scholar]
- 35.Chang SCN; Chung H-Y; Tai C-L; Chen PKT; Lin T-M; Jeng L-B, Repair of large cranial defects by hBMP-2 expressing bone marrow stromal cells: Comparison between alginate and collagen type I systems. Journal of Biomedical Materials Research Part A 2010, 94A, (2), 433–441. [DOI] [PubMed] [Google Scholar]
- 36.D’souza AA; Shegokar R, Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv 2016, 13, (9), 1257–75. [DOI] [PubMed] [Google Scholar]
- 37.Jung RE; Häig GA; Thoma DS; Hämmerle CHF, A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants. Clinical Oral Implants Research 2009, 20, (2), 162–168. [DOI] [PubMed] [Google Scholar]
- 38.Burdick JA; Mason MN; Hinman AD; Thorne K; Anseth KS, Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. Journal of Controlled Release 2002, 83, (1), 53–63. [DOI] [PubMed] [Google Scholar]
- 39.Haq MA; Su Y; Wang D, Mechanical properties of PNIPAM based hydrogels: A review. Mater Sci Eng C Mater Biol Appl 2017, 70, (Pt1), 842–855. [DOI] [PubMed] [Google Scholar]
- 40.Xu X; Liu Y; Fu W; Yao M; Ding Z; Xuan J; Li D; Wang S; Xia Y; Cao M, Poly(N-isopropylacrylamide)-Based Thermoresponsive Composite Hydrogels for Biomedical Applications. Polymers (Basel) 2020, 12, (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren Z; Wang Y; Ma S; Duan S; Yang X; Gao P; Zhang X; Cai Q, Effective Bone Regeneration Using Thermosensitive Poly(N-Isopropylacrylamide) Grafted Gelatin as Injectable Carrier for Bone Mesenchymal Stem Cells. ACS Applied Materials & Interfaces 2015, 7, (34), 19006–19015. [DOI] [PubMed] [Google Scholar]
- 42.Bryant SJ; Nuttelman CR; Anseth KS, Cytocompatibility of UV and visible light photoinitiating systems on cultured NIH/3T3 fibroblasts in vitro. J Biomater Sci Polym Ed 2000, 11, (5), 439–57. [DOI] [PubMed] [Google Scholar]
- 43.Fairbanks BD; Singh SP; Bowman CN; Anseth KS, Photodegradable, Photoadaptable Hydrogels via Radical-Mediated Disulfide Fragmentation Reaction. Macromolecules 2011, 44, (8), 2444–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsuda T; Magoshi T, Preparation of vinylated polysaccharides and photofabrication of tubular scaffolds as potential use in tissue engineering. Biomacromolecules 2002, 3, (5), 942–50. [DOI] [PubMed] [Google Scholar]
- 45.Hu J; Hou Y; Park H; Choi B; Hou S; Chung A; Lee M, Visible Light Crosslinkable Chitosan Hydrogels for Tissue Engineering. Acta Biomater 2012, 8, (5), 1730–1738. [DOI] [PubMed] [Google Scholar]
- 46.Kazemzadeh-Narbat M; Rouwkema J; Annabi N; Cheng H; Ghaderi M; Cha B-H; Apamathi M; Khalilpour A; Byambaa B; Jabbari E; Tamayol A; Khademhosseini A, Engineering Photocrosslinkable Bicomponent Hydrogel Constmcts for Creating 3D Vascularized Bonq. Advanced Healthcare Materials 2017, 6, (10), 1601122. [DOI] [PubMed] [Google Scholar]
- 47.Samorezov JE; Headley EB; Everett CR; Alsberg E, Sustained presentation of BMP-2 enhances osteogenic differentiation of human adipose-derived stem cells in gelatin hydrogels. Journal of Biomedical Materials Research Part A 2016, 104, (6), 1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poldervaart MT; Goversen B; de Ruijter M; Abbadessa A; Melchels FPW; Oner FC; Dhert WJA; Vermonden T; Alblas J, 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS One 2017, 12, (6), e0177628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taki K; Watanabe Y; Ito H; Ohshima M, Effect of Oxygen Inhibition on the Kinetic Constants of the UV-Radical Photopolymerization of Diurethane Dimethacrylate/Photoinitiator Systems. Macromolecules 2014, 47, (6), 1906–1913. [Google Scholar]
- 50.Tibbitt MW; Kloxin AM; Sawicki LA; Anseth KS, Mechanical Properties and Degradation of Chain and Step-Polymerized Photodegradable Hydrogels. Macromolecules 2013, 46, (7), 2785–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolb HC; Finn MG; Sharpless KB, Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angewandte Chemie International Edition 2001, 40, (11), 2004–2021. [DOI] [PubMed] [Google Scholar]
- 52.Lummerstorfer T; Hoffmann H, Click Chemistry on Surfaces: 1,3-Dipolar Cycloaddition Reactions of Azide-Terminated Monolayers on Silica. The Journal of Physical Chemistry B 2004, 108, (13), 3963–3966. [Google Scholar]
- 53.Xu J; Liu Y; Hsu S. h., Hydrogels Based on Schiff Base Linkages for Biomedical Applications. Molecules 2019, 24, (16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murakami Y; Yokoyama M; Okano T; Nishida H; Tomizawa Y; Endo M; Kurosawa H, A novel synthetic tissue-adhesive hydrogel using a crosslinkable polymeric micelle. J BiomedMater Res A 2007, 80, (2), 421–7. [DOI] [PubMed] [Google Scholar]
- 55.Belowich ME; Stoddart JF, Dynamic inline chemistry. Chemical Society Reviews 2012, 41, (6), 2003–2024. [DOI] [PubMed] [Google Scholar]
- 56.Lee SJ; Nah H; Heo DN; Kim K-H; Seok JM; Heo M; Moon H-J; Lee D; Lee JS; An SY; Hwang Y-S; Ko WK; Kim SJ; Sohn S; Park SA; Park S-Y; Kwon IK, Induction of osteogenic differentiation in a rat calvarial bone defect model using an In situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogel. Carbon 2020, 168, 264–277. [Google Scholar]
- 57.Wang LL; Highley CB; Yeh Y-C; Galarraga JH; Uman S; Burdick JA, Three-dimensional extrusion bioprinting of single- and double-network hydrogels containing dynamic covalent crosslinks. Journal of Biomedical Materials Research Part A 2018, 106, (4), 865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim J; Kim IS; Cho TH; Lee KB; Hwang SJ; Tae G; Noh I; Lee SH; Park Y; Sun K, Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials 2007, 28, (10), 1830–1837. [DOI] [PubMed] [Google Scholar]
- 59.Paidikondala M; Wang S; Hilbom J; Larsson S; Varghese OP, Impact of Hydrogel Cross-Linking Chemistry on the in Vitro and in Vivo Bioactivity of Recombinant Human Bone Morphogenetic Protein-2. ACS Applied Bio Materials 2019, 2, (5), 2006–2012. [DOI] [PubMed] [Google Scholar]
- 60.Koehler KC; Alge DL; Anseth KS; Bowman CN, A Diels–Alder modulated approach to control and sustain the release of dexamethasone and induce osteogenic differentiation of human mesenchymal stem cells. Biomaterials 2013, 34, (16), 4150–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madl CM; Heilshorn SC, Rapid Diels-Alder Cross-linking of Cell Encapsulating Hydrogels. Chemistry of Materials 2019, 31, (19), 8035–8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moreira Teixeira LS; Feijen J; van Blitterswijk CA; Dijkstra PJ; Karperien M, Enzyme-catalyzed crosslinkable hydrogels: Emerging strategies for tissue engineering. Biomaterials 2012, 33, (5), 1281–1290. [DOI] [PubMed] [Google Scholar]
- 63.Karfeld-Sulzer LS; Siegenthaler B; Ghayor C; Weber FE, Fibrin Hydrogel Based Bone Substitute Tethered with BMP-2 and BMP-2/7 Heterodimers. Materials (Basel) 2015, 8, (3), 977–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang G; Xiao Z; Ren X; Long H; Qian H; Ma K; Guo Y, Enzymatically crosslinked gelatin hydrogel promotes the proliferation of adipose tissue-derived stromal cells. PeerJ 2016, 4, e2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Echave MC; Pimenta-Lopes C; Pedraz JL; Mehrali M; Dolatshahi-Pirouz A; Ventura F; Orive G, Enzymatic crosslinked gelatin 3D scaffolds for bone tissue engineering. International Journal of Pharmaceutics 2019, 562, 151–161. [DOI] [PubMed] [Google Scholar]
- 66.Bae JW; Choi JH; Lee Y; Park KD, Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications. Journal of Tissue Engineering and Regenerative Medicine 2015, 9, (11), 1225–1232. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y; Chen H; Zhang T; Zan Y; Ni T; Cao Y; Wang J; Liu M; Pei R, Injectable hydrogels from enzyme-catalyzed crosslinking as BMSCs-laden scaffold for bone repair and regeneration. Materials Science and Engineering: C 2019, 96, 841–849. [DOI] [PubMed] [Google Scholar]
- 68.Tonnesen HH; Karlsen J, Alginate in drug delivery systems. Drug Development and Industrial Pharmacy 2002, 28, (6), 621–630. [DOI] [PubMed] [Google Scholar]
- 69.Ahmadi R; de Bruijn JD, Biocompatibility and gelation of chitosan-glycerol phosphate hydrogels. J Biomed Mater Res A 2008, 86, (3), 824–32. [DOI] [PubMed] [Google Scholar]
- 70.Park D-J; Choi B-H; Zhu S-J; Huh J-Y; Kim B-Y; Lee S-H, Injectable bone using chitosan-alginate gel/mesenchymal stem cells/BMP-2 composites. Journal of Cranio-Maxillofacial Surgery 2005, 33, (1), 50–54. [DOI] [PubMed] [Google Scholar]
- 71.Arakawa C; Ng R; Tan S; Kim S; Wu B; Lee M, Photopolymerizable Chitosan-Collagen Hydrogels for Bone Tissue Engineering. J Tissue Eng Regen Med 2017, 11, (1), 164–174. [DOI] [PubMed] [Google Scholar]
- 72.Fan M; Ma Y; Tan H; Jia Y; Zou S; Guo S; Zhao M; Huang H; Ling Z; Chen Y; Hu X, Covalent mid injectable chitosan-chondroitin sulfate hydrogels embedded with chitosan microspheres for drug delivery and tissue engineering. Materials Science and Engineering: C 2017, 71,67–74. [DOI] [PubMed] [Google Scholar]
- 73.Mayr J; Saldías C; Díaz Díaz D, Release of small bioactive molecules from physical gels. Chemical Society Reviews 2018, 47, (4), 1484–1515. [DOI] [PubMed] [Google Scholar]
- 74.Bassett DC; Håti AG; Melø TB; Stokke BT; Sikorski P, Competitive ligand exchange of crosslinking ions for ionotropic hydrogel formation. Journal of Materials Chemistry B 2016, 4, (37), 6175–6182. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez-Castanon J; Bianchi S; Saglimbeni F; Di Leonardo R; Sciortino F, Microrheology of DNA hydrogel gelling and melting on cooling. Soft Matter 2018, 14, (31), 6431–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bomboi F; Romano F; Leo M; Fernandez-Castanon J; Cerbino R; Bellini T; Bordi F; Filetici P; Sciortino F, Re-entrant DNA gels. Nat Commun 2016, 7, 13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X; Xu B; Gao F; Zheng P; Liu W, Repair of volumetric bone defects with a high strength BMP-loaded-mineralized hydrogel tubular scaffold. Journal of Materials Chemistry B 2017, 5, (28), 5588–5596. [Google Scholar]
- 78.Sijbesma RP; Beijer FH; Brunsveld L; Folmer BJB; Hirschberg JHKK; Lange RFM; Lowe JKL; Meijer EW, Reversible Polymers Formed from Self-Complementary Monomers Using Quadmple Hydrogen Bonding. Science 1997, 278, (5343), 1601. [DOI] [PubMed] [Google Scholar]
- 79.Hou S; Wang X; Park S; Jin X; Ma PX, Rapid Self-Integrating, Injectable Hydrogel for Tissue Complex Regeneration. Advanced Healthcare Materials 2015, 4, (10), 1491–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Z; Liu W, Poly(N-acryloyl glycinamide): a fascinating polymer that exhibits a range of properties from UCST to high-strength hydrogels. Chem Commun (Camb) 2018, 54, (75), 10540–10553. [DOI] [PubMed] [Google Scholar]
- 81.Rodell CB; Kaminski AL; Burdick JA, Rational Design of Network Properties in Guest-Host Assembled and Shear-Thinning Hyaluronic Acid Hydrogels. Biomacromolecules 2013, 14, (11), 4125–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng Q; Wei K; Lin S; Xu Z; Sun Y; Shi P; Li G; Bian L, Mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host-guest interactions assist cell infiltration and in situ tissue regeneration. Biomaterials 2016, 101, 217–228. [DOI] [PubMed] [Google Scholar]
- 83.Amiryaghoubi N; Noroozi Pesyan N; Fathi M; Omidi Y, Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. International Journal of Biological Macromolecules 2020, 162, 1338–1357. [DOI] [PubMed] [Google Scholar]
- 84.Jiang H; Duan L; Ren X; Gao G, Hydrophobic association hydrogels with excellent mechanical and self-healing properties. European Polymer Journal 2019, 112, 660–669. [Google Scholar]
- 85.Zhao D; Huang J; Zhong Y; Li K; Zhang L; Cai J, High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Advanced Functional Materials 2016, 26, (34), 6279–6287. [Google Scholar]
- 86.Aheame M, Introduction to cell-hydrogel mechanosensing. Interface Focus 2014, 4, (2), 20130038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruoslahti E; Pierschbacher MD, New Perspectives in Cell Adhesion: RGD and Integrins. Science 1987, 238, (4826), 491–7. [DOI] [PubMed] [Google Scholar]
- 88.García AJ; Reyes CD, Bio-adhesive Surfaces to Promote Osteoblast Differentiation and Bone Formation. Journal of Dental Research 2005, 84, (5), 407–413. [DOI] [PubMed] [Google Scholar]
- 89.Visser R; Arrabal PM; Santos-Ruiz L; Fernandez-Barranco R; Becerra J; Cifuentes M, A Collagen-Targeted Biomimetic RGD Peptide to Promote Osteogenesis. Tissue Engineering Part A 2014, 20, (1-2), 34–44. [DOI] [PubMed] [Google Scholar]
- 90.Yang F; Williams CG; Wang D.-a.; Lee H; Manson PN; Elisseeff J, The effect of incorporating RGD adhesive peptide in polyethylene glycol diacrylate hydrogel on osteogenesis of bone marrow stromal cells. Biomaterials 2005, 26, (30), 5991–5998. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y; Peng W; Liu X; Zhu M; Sun T; Peng Q; Zeng Y; Feng B; Zhi W; Weng J; Wang J, Study of bilineage differentiation of human-bone-marrow-derived mesenchymal stem cells in oxidized sodium alginate/N-succinyl chitosan hydrogels and synergistic effects of RGD modification and low-intensity pulsed ultrasound. Acta Biomaterialia 2014, 10, (6), 2518–2528. [DOI] [PubMed] [Google Scholar]
- 92.Lam J; Truong NF; Segura T, Design of cell–matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomaterialia 2014, 10, (4), 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang W; Wang R; Sun Z; Zhu X; Zhao Q; Zhang T; Cholewinski A; Yang F; Zhao B; Pinnaratip R; Forooshani PK; Lee BP, Catechol-functionalized hydrogels: biomimetic design, adhesion mechanism, and biomedical applications. Chemical Society Reviews 2020, 49, (2), 433–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chirdon WM; O’Brien WJ; Robertson RE, Adsorption of catechol and comparative solutes on hydroxyapatite. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2003, 66B, (2), 532–538. [DOI] [PubMed] [Google Scholar]
- 95.Park H-J; Jin Y; Shin J; Yang K; Lee C; Yang HS; Cho S-W, Catechol-Functionalized Hyaluronic Acid Hydrogels Enhance Angiogenesis and Osteogenesis of Human Adipose-Derived Stem Cells in Critical Tissue Defects. Biomacromolecules 2016, 17, (6), 1939–1948. [DOI] [PubMed] [Google Scholar]
- 96.Yan S; Wang W; Li X; Ren J; Yun W; Zhang K; Li G; Yin J, Preparation of mussel-inspired injectable hydrogels based on dual-functionalized alginate with improved adhesive, self-healing, and mechanical properties. Journal of Materials Chemistry B 2018, 6, (40), 6377–6390. [DOI] [PubMed] [Google Scholar]
- 97.Gkioni K; Leeuwenburgh SC; Douglas TE; Mikos AG; Jansen JA, Mineralization of hydrogels for bone regeneration. Tissue Eng Part B Rev 2010, 16, (6), 577–85. [DOI] [PubMed] [Google Scholar]
- 98.George MN; Liu X; Miller Ii AL; Xu H; Lu L, Phosphate functionalization and enzymatic calcium mineralization synergistically enhance oligo[poly(ethylene glycol) fumarate] hydrogel osteoconductivity for bone tissue engineering. Journal of Biomedical Materials Research Part A 2020, 108, (3), 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yokoi T; Kawashita M; Ohtsuki C, Biomimetic mineralization of calcium phosphates in polymeric hydrogels containing carboxyl groups. Journal of Asian Ceramic Societies 2013, 1, (2), 155–162. [Google Scholar]
- 100.Zhao BH; Katagiri T; Toyoda H; Takada T; Yanai T; Fukuda T; Chung UI; Koike T; Takaoka K; Kamijo R, Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. Journal of Biological Chemistry 2006, 281, (32), 23246–23253. [DOI] [PubMed] [Google Scholar]
- 101.Viviano BL; Paine-Saunders S; Gasiunas N; Gallagher J; Saunders S, Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J Biol Chem 2004, 279, (7), 5604–11. [DOI] [PubMed] [Google Scholar]
- 102.Paine-Saunders S; Viviano BL; Economides AN; Saunders S, Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem 2002, 277, (3), 2089–96. [DOI] [PubMed] [Google Scholar]
- 103.Yang HS; La WG; Cho YM; Shin W; Yeo GD; Kim BS, Comparison between heparin-conjugated fibrin and collagen sponge as bone morphogenetic protein-2 carriers for bone regeneration. Exp Mol Med 2012, 44, (5), 350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang HS; La W-G; Bhang SH; Jeon J-Y; Lee JH; Kim B-S, Heparin-Conjugated Fibrin as an Injectable System for Sustained Delivery of Bone Morphogenetic Protein-2. Tissue Engineering P art A 2009, 16, (4), 1225–1233. [DOI] [PubMed] [Google Scholar]
- 105.Kim S; Fan J; Lee C-S; Chen C; Bubukina K; Lee M, Heparinized chitosan stabilizes the bioactivity of BMP-2 and potentiates the osteogenic efficacy of demineralized bone matrix. Journal of Biological Engineering 2020, 14, (1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seeherman H; Wozney JM, Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine & Growth Factor Reviews 2005, 16, (3), 329–345. [DOI] [PubMed] [Google Scholar]
- 107.Park SH; Kwon JS; Lee BS; Park JH; Lee BK; Yun J-H; Lee BY; Kim JH; Min BH; Yoo TH; Kim MS, BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Scientific Reports 2017, 7, (1), 6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peeters M; Detiger SEL; Karfeld-Sulzer LS; Smit TH; Yayon A; Weber LE; Helder MN, BMP-2 and BMP-2/7 Heterodimers Conjugated to a Fibrin/Hyaluronic Acid Hydrogel in a Large Animal Model of Mild Intervertebral Disc Degeneration. BioResearch Open Access 2015, 4, (1), 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Madl CM; Mehta M; Duda GN; Heilshorn SC; Mooney DJ, Presentation of BMP-2 Mimicking Peptides in 3D Hydrogels Directs Cell Fate Commitment in Osteoblasts and Mesenchymal Stem Cells. Biomacromolecules 2014, 15, (2), 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wolfrum C; Shi S; Jayaprakash KN; Jayaraman M; Wang G; Pandey RK; Rajeev KG; Nakayama T; Charrise K; Ndungo EM; Zimmermann T; Koteliansky V; Manoharan M; Stoffel M, Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nature Biotechnology 2007, 25, (10), 1149–1157. [DOI] [PubMed] [Google Scholar]
- 111.Nguyen MK; Huynh CT; Gilewski A; Wilner SE; Maier KE; Kwon N; Levy M; Alsberg E, Covalently tethering siRNA to hydrogels for localized, controlled release and gene silencing. Sci Adv 2019, 5, (8), eaax0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Utech S; Boccaccini AR, A review of hydrogel-based composites for biomedical applications: enhancement of hydrogel properties by addition of rigid inorganic fillers. Journal of Materials Science 2016, 51, (1), 271–310. [Google Scholar]
- 113.De Santis R; Guarino V; Ambrosio L, 10 - Composite biomaterials for bone repair In Bone Repair Biomaterials (Second Edition), Pawelec KM; Planell JA, Eds. Woodhead Publishing: 2019; pp 273–299. [Google Scholar]
- 114.Nie L; Wu Q; Long H; Hu K; Li P; Wang C; Sun M; Dong J; Wei X; Suo J; Hua D; Liu S; Yuan H; Yang S, Development of chitosan/gelatin hydrogels incorporation of biphasic calcium phosphate nanoparticles for bone tissue engineering. Journal of Biomaterials Science, Polymer Edition 2019, 30, (17), 1636–1657. [DOI] [PubMed] [Google Scholar]
- 115.Dziadek M; Kudlackova R; Zima A; Slosarczyk A; Ziabka M; Jelen P; Shkarina S; Cecilia A; Zuber M; Baumbach T; Surmeneva MA; Surmenev RA; Bacakova L; Cholewa-Kowalska K; Douglas TEL, Novel multicomponent organic-inorganic WPI/gelatin/CaP hydrogel composites for bone tissue engineering. Journal of Biomedical Materials Research Part A 2019, 107, (11), 2479–2491. [DOI] [PubMed] [Google Scholar]
- 116.Schweikle M; Bjørnøy SH; van Helvoort ATJ; Haugen HJ; Sikorski P; Tiainen H, Stabilisation of amorphous calcium phosphate in polyethylene glycol hydrogels. Acta Biomaterialia 2019, 90, 132–145. [DOI] [PubMed] [Google Scholar]
- 117.Yao S; Xu Y; Zhou Y; Shao C; Liu Z; Jin B; Zhao R; Cao H; Pan H; Tang R, Calcium Phosphate Nanocluster-Loaded Injectable Hydrogel for Bone Regeneration. ACS Applied Bio Materials 2019, 2, (10), 4408–4417. [DOI] [PubMed] [Google Scholar]
- 118.Vieira S; da Silva Morais A; Garet E; Silva-Correia J; Reis RL; González-Fernández Á; Miguel Oliveira J, Self-mineralizing Ca-enriched methacrylated gellan gum beads for bone tissue engineering. Acta Biomaterialia 2019, 93, 74–85. [DOI] [PubMed] [Google Scholar]
- 119.Kuang L; Ma X; Ma Y; Yao Y; Tariq M; Yuan Y; Liu C, Self-Assembled Injectable Nanocomposite Hydrogels Coordinated by in Situ Generated CaP Nanoparticles for Bone Regeneration. ACS Applied Materials & Interfaces 2019, 11, (19), 17234–17246. [DOI] [PubMed] [Google Scholar]
- 120.Wang Z; Zhao J; Tang W; Hu L; Chen X; Su Y; Zou C; Wang J; Lu WW; Zhen W; Zhang R; Yang D; Peng S, Multifunctional Nanoengineered Hydrogels Consisting of Black Phosphorus Nanosheets Upregulate Bone Formation. Small 2019, 15, (41), 1901560. [DOI] [PubMed] [Google Scholar]
- 121.Cui Z-K; Kim S; Baljon JJ; Wu BM; Aghaloo T; Lee M, Microporous Methacrylated Glycol Chitosan-Montmorillonite Nanocomposite Hydrogel for Bone Tissue Engineering. Nature Communications 2019, 10, (1), 3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim Y-H; Yang X; Shi L; Lanham SA; Hilborn J; Oreffo ROC; Ossipov D; Dawson JI, Bisphosphonate nanoclay edge-site interactions facilitate hydrogel self-assembly and sustained growth factor localization. Nature Communications 2020, 11, (1), 1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X; Fan J; Lee C-S; Kim S; Chen C; Lee M, Supramolecular Hydrogels Based on Nanoclay and Guanidine-Rich Chitosan: Injectable and Moldable Osteoinductive Carriers. ACS Applied Materials & Interfaces 2020, 12, (14), 16088–16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xin T; Mao J; Liu L; Tang J; Wu L; Yu X; Gu Y; Cui W; Chen L, Programmed Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Inorganic Ion Composite Hydrogel as Artificial Periosteum. ACS Applied Materials & Interfaces 2020, 12, (6), 6840–6851. [DOI] [PubMed] [Google Scholar]
- 125.Wu J; Zheng K; Huang X; Liu J; Liu H; Boccaccini AR; Wan Y; Guo X; Shao Z, Thermally triggered injectable chitosan/silk fibroin/bioactive glass nanoparticle hydrogels for in-situ bone formation in rat calvarial bone defects. Acta Biomaterialia 2019, 91, 60–71. [DOI] [PubMed] [Google Scholar]
- 126.Pereira I; Fraga S; Maltez L; Requicha J; Guardão L; Oliveira J; Prada J; Alves H; Santos JD; Teixeira JP; Pereira JE; Soares R; Gama FM, In vivo systemic toxicity assessment of an oxidized dextrin-based hydrogel and its effectiveness as a carrier and stabilizer of granular synthetic bone substitutes. Journal of Biomedical Materials Research Part A 2019, 107, (8), 1678–1689. [DOI] [PubMed] [Google Scholar]
- 127.Ji M; Ding Z; Chen H; Peng H; Yan Y, Design of novel organic-inorganic composite bone cements with high compressive strength, in vitro bioactivity and cytocompatibility. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2019, 107, (7), 2365–2377. [DOI] [PubMed] [Google Scholar]
- 128.Huang K; Hou J; Gu Z; Wu J, Egg-White-/Eggshell-Based Biomimetic Hybrid Hydrogels for Bone Regeneration. ACS Biomaterials Science & Engineering 2019, 5, (10), 5384–5391. [DOI] [PubMed] [Google Scholar]
- 129.Lee F; Kurisawa M, Formation and stability of interpenetrating polymer network hydrogels consisting of fibrin and hyaluronic acid for tissue engineering. Acta Biomaterialia 2013, 9, (2), 5143–5152. [DOI] [PubMed] [Google Scholar]
- 130.Iwamoto S; Lee S-H; Endo T, Relationship between aspect ratio and suspension viscosity of wood cellulose nanofibers. Polymer Journal 2014, 46, (1), 73–76. [Google Scholar]
- 131.Latinwo F; Schroeder CM, Model systems for single molecule polymer dynamics. Soft Matter 2011, 7, (18), 7907–7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bilgili HK; Onak G; Karaman O In Development and Characterization of Nanofiber-Reinforced Hydrogel for Bone Regeneration, 2019 Medical Technologies Congress (TIPTEKNO), 3-5 Oct. 2019, 2019; 2019; pp 1–4. [Google Scholar]
- 133.Nitta S; Komatsu A; Ishii T; Ohnishi M; Inoue A; Iwamoto H, Fabrication and characterization of water-dispersed chitosan nanofiber/poly(ethylene glycol) diacrylate/calcium phosphate-based porous composites. Carbohydrate Polymers 2017, 174, 1034–1040. [DOI] [PubMed] [Google Scholar]
- 134.Burdick JA; Anseth KS, Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 2002, 23, (22), 4315–4323. [DOI] [PubMed] [Google Scholar]
- 135.Nazeer MA; Yilgör E; Yilgör I, Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydrate Polymers 2017, 175, 38–46. [DOI] [PubMed] [Google Scholar]
- 136.Meng Z; Thakur T; Chitrakar C; Jaiswal MK; Gaharwar AK; Yakovlev VV, Assessment of Local Heterogeneity in Mechanical Properties of Nanostructured Hydrogel Networks. ACS Nano 2017, 11, (8), 7690–7696. [DOI] [PubMed] [Google Scholar]
- 137.Pacelli S; Rampetsreiter K; Modaresi S; Subham S; Chakravarti AR; Lohfeld S; Detamore MS; Paul A, Fabrication of a Double-Cross-Linked Interpenetrating Polymeric Network (IPN) Hydrogel Surface Modified with Polydopamine to Modulate the Osteogenic Differentiation of Adipose-Derived Stem Cells. ACS Applied Materials & Interfaces 2018, 10, (30), 24955–24962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dang M; Saunders L; Niu X; Fan Y; Ma PX, Biomimetic delivery of signals for bone tissue engineering. Bone Research 2018, 6, (1), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu L; Xiang Y; Wang Z; Yang X; Yu X; Lu Y; Deng L; Cui W, Adhesive liposomes loaded onto an injectable, self-healing and antibacterial hydrogel for promoting bone reconstruction. NPG Asia Materials 2019, 11, (1), 81. [Google Scholar]
- 140.Ning H; Wu X; Wu Q; Yu W; Wang H; Zheng S; Chen Y; Li Y; Su J, Microfiber-Reinforced Composite Hydrogels Loaded with Rat Adipose-Derived Stem Cells and BMP-2 for the Treatment of Medication-Related Osteonecrosis of the Jaw in a Rat Model. ACS Biomaterials Science & Engineering 2019, 5, (5), 2430–2443. [DOI] [PubMed] [Google Scholar]
- 141.Tan J; Zhang M; Hai Z; Wu C; Lin J; Kuang W; Tang H; Huang Y; Chen X; Liang G, Sustained Release of Two Bioactive Factors from Supramolecular Hydrogel Promotes Periodontal Bone Regeneration. ACS Nano 2019, 13, (5), 5616–5622. [DOI] [PubMed] [Google Scholar]
- 142.Seo B-B; Koh J-T; Song S-C, Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials 2017, 122, 91–104. [DOI] [PubMed] [Google Scholar]
- 143.Kim S; Kim J; Gajendiran M; Yoon M; Hwang MP; Wang Y; Kang B-J; Kim K, Enhanced Skull Bone Regeneration by Sustained Release of BMP-2 in Interpenetrating Composite Hydrogels. Biomacromolecules 2018, 19, (11), 4239–4249. [DOI] [PubMed] [Google Scholar]
- 144.Wojda SJ; Donahue SW, Parathyroid hormone for bone regeneration. Journal of Orthopaedic Research 2018, 36, (10), 2586–2594. [DOI] [PubMed] [Google Scholar]
- 145.Wojda SJ; Marozas IA; Anseth KS; Yaszemski MJ; Donahue SW, Thiol-ene Hydrogels for Local Delivery of PTH for Bone Regeneration in Critical Size defects. Journal of Orthopaedic Research 2020, 38, (3), 536–544. [DOI] [PubMed] [Google Scholar]
- 146.Wan DC; Pomerantz JH; Brunet LJ; Kim JB; Chou YF; Wu BM; Harland R; Blau HM; Longaker MT, Noggin suppression enhances in vitro osteogenesis and accelerates in vivo bone formation. J Biol Chem 2007, 282, (36), 26450–9. [DOI] [PubMed] [Google Scholar]
- 147.Heliotis M; Tsiridis E, Suppression of bone morphogenetic protein inhibitors promotes osteogenic differentiation: therapeutic implications. Arthritis Res Ther 2008, 10, (4), 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Choi B; Cui ZK; Kim S; Fan J; Wu BM; Lee M, Glutamine-chitosan modified calcium phosphate nanoparticles for efficient siRNA delivery and osteogenic differentiation. J Mater Chem B Mater Biol Med 2015, 3, (31), 6448–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cui Z-K; Fan J; Kim S; Bezouglaia O; Fartash A; Wu BM; Aghaloo T; Lee M, Delivery of siRNA via cationic Sterosomes to enhance osteogenic differentiation of mesenchymal stem cells. Journal of Controlled Release 2015, 217, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huynh CT; Liu F; Cheng Y; Coughlin KA; Alsberg E, Thiol-Epoxy “Click” Chemistry to Engineer Cytocompatible PEG-Based Hydrogel for siRNA-Mediated Osteogenesis of hMSCs. ACS Applied Materials & Interfaces 2018, 10, (31), 25936–25942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nguyen MK; Jeon O; Dang PN; Huynh CT; Varghai D; Riazi H; McMillan A; Herberg S; Alsberg E, RNA interfering molecule delivery from in situ forming biodegradable hydrogels for enhancement of bone formation in rat calvarial bone defects. Acta Biomaterialia 2018, 75, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang LL; Chung JJ; Li EC; Uman S; Atluri P; Burdick JA, Injectable and protease-degradable hydrogel for siRNA sequestration and triggered delivery to the heart. Journal of Controlled Release 2018, 285, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang LL; Liu Y; Chung JJ; Wang T; Gaffey AC; Lu M; Cavanaugh CA; Zhou S; Kanade R; Atluri P; Morrisey EE; Burdick JA, Sustained miRNA delivery from an injectable hydrogel promotes cardiomyocyte proliferation and functional regeneration after ischaemic injury. Nature Biomedical Engineering 2017, 1, (12), 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pacelli S; Maloney R; Chakravarti AR; Whitlow J; Basu S; Modaresi S; Gehrke S; Paul A, Controlling Adult Stem Cell Behavior Using Nanodiamond-Reinforced Hydrogel: Implication in Bone Regeneration Therapy. Scientific Reports 2017, 7, (1), 6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cui ZK; Sun JA; Baljon JJ; Fan J; Kim S; Wu BM; Aghaloo T; Lee M, Simultaneous delivery of hydrophobic small molecules and siRNA using Sterosomes to direct mesenchymal stem cell differentiation for bone repair. Acta Biomater 2017, 58, 214–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yan S; Ren J; Jian Y; Wang W; Yun W; Yin J, Injectable Maltodextrin-Based Micelle/Hydrogel Composites for Simvastatin-Controlled Release. Biomacromolecules 2018, 19, (12), 4554–4564. [DOI] [PubMed] [Google Scholar]
- 157.Li R; Li J; Xu J; Hong Wong DS; Chen X; Yuan W; Bian L, Multiscale reconstruction of a synthetic biomimetic micro-niche for enhancing and monitoring the differentiation of stem cells. Biomaterials 2018, 173, 87–99. [DOI] [PubMed] [Google Scholar]
- 158.Cui ZK; Kim S; Baljon JJ; Doroudgar M; Lafleur M; Wu BM; Aghaloo T; Lee M, Design and Characterization of a Therapeutic Non-phospholipid Liposomal Nanocarrier with Osteoinductive Characteristics To Promote Bone Formation. ACS Nano 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ladd AL; Pliam NB, Use of bone-graft substitutes in distal radius fractures. J Am Acad Orthop Surg 1999, 7, (5), 279–90. [DOI] [PubMed] [Google Scholar]
- 160.Kuhls R; Werner-Rustner M; Küchler I; Soost F, Human demineralised bone matrix as a bone substitute for reconstruction of cystic defects of the lower jaw. Cell Tissue Bank 2001, 2, (3), 143–53. [DOI] [PubMed] [Google Scholar]
- 161.Shehadi JA; Elzein SM, Review of commercially available demineralized bone matrix products for spinal fusions: A selection paradigm. Surg Neurolint 2017, 8, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang H; Yang L; Yang XG; Wang F; Feng JT; Hua KC; Li Q; Hu YC, Demineralized Bone Matrix Carriers and their Clinical Applications: An Overview. Orthop Surg 2019, 11, (5), 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Townsend JM; Sali G; Homburg HB; Cassidy NT; Sanders ME; Fung K-M; Andrews BT; Nudo RJ; Bohnstedt BN; Detamore MS, Thiolated bone and tendon tissue particles covalently bound in hydrogels for in vivo calvarial bone regeneration. Acta Biomaterialia 2020, 104, 66–75. [DOI] [PubMed] [Google Scholar]
- 164.Jiang L-B; Su D-H; Ding S-L; Zhang Q-C; Li Z-F; Chen F-C; Ding W; Zhang S-T; Dong J, Salt-Assisted Toughening of Protein Hydrogel with Controlled Degradation for Bone Regeneration. Advanced Functional Materials 2019, 29, (26), 1901314. [Google Scholar]
- 165.Shi L; Wang F; Zhu W; Xu Z; Fuchs S; Hilborn J; Zhu L; Ma Q; Wang Y; Weng X; Ossipov DA, Self-Healing Silk Fibroin-Based Hydrogel for Bone Regeneration: Dynamic Metal-Ligand Self-Assembly Approach. Advanced Functional Materials 2017, 27, (37), 1700591. [Google Scholar]
- 166.Kim S; Cui ZK; Koo B; Zheng J; Aghaloo T; Lee M, Chitosan-Lysozyme Conjugates for Enzyme-Triggered Hydrogel Degradation in Tissue Engineering Applications. ACS Appl Mater Interfaces 2018, 10, (48), 41138–41145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shi L; Zeng Y; Zhao Y; Yang B; Ossipov D; Tai C-W; Dai J; Xu C, Biocompatible Injectable Magnetic Hydrogel Formed by Dynamic Coordination Network. ACS Applied Materials & Interfaces 2019, 11, (49), 46233–46240. [DOI] [PubMed] [Google Scholar]
- 168.Zhao C; Qazvini NT; Sadati M; Zeng Z; Huang S; De La Lastra AL; Zhang L; Feng Y; Liu W; Huang B; Zhang B; Dai Z; Shen Y; Wang X; Luo W; Liu B; Lei Y; Ye Z; Zhao L; Cao D; Yang L; Chen X; Athiviraham A; Lee MJ; Wolf JM; Reid RR; Tirrell M; Huang W; de Pablo JJ; He T-C, A pH-Triggered, Self-Assembled, and Bioprintable Hybrid Hydrogel Scaffold for Mesenchymal Stem Cell Based Bone Tissue Engineering. ACS Applied Materials & Interfaces 2019, 11, (9), 8749–8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vining KH; Mooney DJ, Mechanical forces direct stem cell behaviour in development and regeneration. Nature Reviews Molecular Cell Biology 2017, 18, (12), 728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.González-Díaz EC; Varghese S, Hydrogels as Extracellular Matrix Analogs. Gels 2016, 2, (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sun M; Chi G; Li P; Lv S; Xu J; Xu Z; Xia Y; Tan Y; Xu J; Li L; Li Y, Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. International Journal of Medical Sciences 2018, 15, (3), 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Huebsch N; Arany PR; Mao AS; Shvartsman D; Ali OA; Bencherif SA; Rivera-Feliciano J; Mooney DJ, Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 2010, 9, (6), 518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Madl CM; Heilshom SC, Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche. Annual Review of Biomedical Engineering 2018, 20, (1), 21–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Her GJ; Wu H-C; Chen M-H; Chen M-Y; Chang S-C; Wang T-W, Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages. Acta Biomaterialia 2013, 9, (2), 5170–5180. [DOI] [PubMed] [Google Scholar]
- 175.Ma Y; Lin M; Huang G; Li Y; Wang S; Bai G; Lu TJ; Xu F, 3D Spatiotemporal Mechanical Microenvironment: A Hydrogel-Based Platform for Guiding Stem Cell Fate. Advanced Materials 2018, 30, (49), 1705911. [DOI] [PubMed] [Google Scholar]
- 176.Fuchs S; Shariati K; Ma M, Specialty Tough Hydrogels and Their Biomedical Applications. Advanced Healthcare Materials 2020, 9, (2), 1901396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gong JP, Materials both Tough and Soft. Science 2014, 344, (6180), 161. [DOI] [PubMed] [Google Scholar]
- 178.Hasani-Sadrabadi MM; Sarrion P; Pouraghaei S; Chau Y; Ansari S; Li S; Aghaloo T; Moshaverinia A, An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med 2020, 12, (534). [DOI] [PubMed] [Google Scholar]
- 179.Fang J; Li P; Lu X; Fang L; Lu X; Ren F, A strong, tough, and osteoconductive hydroxyapatite mineralized polyacrylamide/dextran hydrogel for bone tissue regeneration. Acta Biomaterialia 2019, 88, 503–513. [DOI] [PubMed] [Google Scholar]
- 180.Yu P; Bao R-Y; Shi X-J; Yang W; Yang M-B, Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydrate Polymers 2017, 155, 507–515. [DOI] [PubMed] [Google Scholar]
- 181.Talebian S; Mehrali M; Taebnia N; Pennisi CP; Kadumudi FB; Foroughi J; Hasany M; Nikkhah M; Akbari M; Orive G; Dolatshahi-Pirouz A, Self-Healing Hydrogels: The Next Paradigm Shift in Tissue Engineering? Adv Sci (Weinh) 2019, 6, (16), 1801664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xavier JR; Thakur T; Desai P; Jaiswal MK; Sears N; Cosgriff-Hemandez E; Kaunas R; Gaharwar AK, Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano 2015, 9, (3), 3109–18. [DOI] [PubMed] [Google Scholar]
- 183.Basu S; Pacelli S; Paul A, Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomaterialia 2020, 105, 159–169. [DOI] [PubMed] [Google Scholar]
- 184.Bai X; Lü S; Cao Z; Ni B; Wang X; Ning P; Ma D; Wei H; Liu M, Dual crosslinked chondroitin sulfate injectable hydrogel formed via continuous Diels-Alder (DA) click chemistry for bone repair. Carbohydrate Polymers 2017, 166, 123–130. [DOI] [PubMed] [Google Scholar]
- 185.Rivas M; Del Valle LJ; Alemán C; Puiggalí J, Peptide Self-Assembly into Hydrogels for Biomedical Applications Related to Hydroxyapatite. Gels (Basel, Switzerland) 2019, 5, (1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.He B; Ou Y; Chen S; Zhao W; Zhou A; Zhao J; Li H; Jiang D; Zhu Y, Designer bFGF-incorporated d-form self-assembly peptide nanofiber scaffolds to promote bone repair. Materials Science and Engineering: C 2017, 74, 451–458. [DOI] [PubMed] [Google Scholar]
- 187.Ma B; Xie J; Jiang J; Shuler FD; Bartlett DE, Rational design of nanofiber scaffolds for orthopedic tissue repair and regeneration. Nanomedicine (London, England) 2013, 8, (9), 1459–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu W; Bi W; Sun Y; Wang L; Yu X; Cheng R; Yu Y; Cui W, Biomimetic organic-inorganic hybrid hydrogel electrospinning periosteum for accelerating bone regeneration. Materials Science and Engineering: C 2020, 110, 110670. [DOI] [PubMed] [Google Scholar]
- 189.Maleki H; Shahbazi M-A; Montes S; Hosseini SH; Eskandari MR; Zaunschirm S; Verwanger T; Mathur S; Milow B; Krammer B; Hüsing N, Mechanically Strong Silica-Silk Fibroin Bioaerogel: A Hybrid Scaffold with Ordered Honeycomb Micromorphology and Multiscale Porosity for Bone Regeneration. ACS Applied Materials & Interfaces 2019, 11, (19), 17256–17269. [DOI] [PubMed] [Google Scholar]
- 190.Huang Y; Zhang X; Wu A; Xu H, An injectable nano-hydroxyapatite (n-HA)/glycol chitosan (G-CS)/hyaluronic acid (HyA) composite hydrogel for bone tissue engineering. RSC Advances 2016, 6, (40), 33529–33536. [Google Scholar]
- 191.Seyednejad H; Gawlitta D; Kuiper RV; de Bruin A; van Nostrum CF; Vermonden T; Dhert WJ; Hennink WE, In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials 2012, 33, (17), 4309–18. [DOI] [PubMed] [Google Scholar]
- 192.Wang X; Jiang M; Zhou Z; Gou J; Hui D, 3D printing of polymer matrix composites: A review and prospective. Composites Part B: Engineering 2017, 110, 442–458. [Google Scholar]
- 193.Demirtaş TT; Irmak G; Gümüşderelioğlu M, A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, (3), 035003. [DOI] [PubMed] [Google Scholar]
- 194.Liu J; Li L; Suo H; Yan M; Yin J; Fu J, 3D printing of biomimetic multi-layered GelMA/nHA scaffold for osteochondral defect repair. Materials & Design 2019, 171, 107708. [Google Scholar]
- 195.Gao F; Xu Z; Liang Q; Liu B; Li H; Wu Y; Zhang Y; Lin Z; Wu M; Ruan C; Liu W, Direct 3D Printing of High Strength Biohybrid Gradient Hydrogel Scaffolds for Efficient Repair of Osteochondral Defect. Advanced Functional Materials 2018, 28, (13), 1706644. [Google Scholar]
- 196.Zhang M; Lin R; Wang X; Xue J; Deng C; Feng C; Zhuang H; Ma J; Qin C; Wan L; Chang J; Wu C, 3D printing of Haversian bone-mimicking scaffolds for multicellular delivery in bone regeneration. Science Advances 2020, 6, (12), eaaz6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ashammakhi N; Hasan A; Kaarela O; Byambaa B; Sheikhi A; Gaharwar AK; Khademhosseini A, Advancing Frontiers in Bone Bioprinting. Advanced Healthcare Materials 2019, 8, (7), 1801048. [DOI] [PubMed] [Google Scholar]
- 198.Wan Z; Zhang P; Liu Y; Lv L; Zhou Y, Four-dimensional bioprinting: Current developments and applications in bone tissue engineering. Acta Biomaterialia 2020, 101, 26–42. [DOI] [PubMed] [Google Scholar]
- 199.Ruskowitz ER; DeForest CA, Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nature Reviews Materials 2018, 3, (2), 17087. [Google Scholar]
- 200.Choi JR; Yong KW; Choi JY; Cowie AC, Recent advances in photo-crosslinkable hydrogels for biomedical applications. BioTechniques 2019, 66, (1), 40–53. [DOI] [PubMed] [Google Scholar]
- 201.Huang K; Wu J; Gu Z, Black Phosphorus Hydrogel Scaffolds Enhance Bone Regeneration via a Sustained Supply of Calcium-Free Phosphorus. ACS Applied Materials & Interfaces 2019, 11, (3), 2908–2916. [DOI] [PubMed] [Google Scholar]
- 202.Lee IN; Dobre O; Richards D; Ballestrem C; Curran JM; Hunt JA; Richardson SM; Swift J; Wong LS, Photoresponsive Hydrogels with Photoswitchable Mechanical Properties Allow Time-Resolved Analysis of Cellular Responses to Matrix Stiffening. ACS Applied Materials & Interfaces 2018, 10, (9), 7765–7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.D’Este M; Alini M; Eglin D, Single step synthesis and characterization of thermoresponsive hyaluronan hydrogels. Carbohydrate Polymers 2012, 90, (3), 1378–1385. [DOI] [PubMed] [Google Scholar]
- 204.D’Este M; Sprecher CM; Milz S; Nehrbass D; Dresing I; Zeiter S; Alini M; Eglin D, Evaluation of an injectable thermoresponsive hyaluronan hydrogel in a rabbit osteochondral defect model. Journal of Biomedical Materials Research Part A 2016, 104, (6), 1469–1478. [DOI] [PubMed] [Google Scholar]
- 205.Orth M; Altmeyer MAB; Scheuer C; Braun BJ; Holstein JH; Eglin D; D’Este M; Histing T; Laschke MW; Pohlemann T; Menger MD, Effects of locally applied adipose tissue-derived microvascular fragments by thermoresponsive hydrogel on bone healing. Acta Biomaterialia 2018, 77, 201–211. [DOI] [PubMed] [Google Scholar]
- 206.Kim SH; Thambi T; Giang Phan VH; Lee DS, Modularly engineered alginate bioconjugate hydrogel as biocompatible injectable scaffold for in situ biomineralization. Carbohydrate Polymers 2020, 233, 115832. [DOI] [PubMed] [Google Scholar]
- 207.Huang W-S; Chu IM, Injectable polypeptide hydrogel/inorganic nanoparticle composites for bone tissue engineering. PLOS ONE 2019, 14, (1), e0210285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Madani SZM; Reisch A; Roxbury D; Kennedy SM, A Magnetically Responsive Hydrogel System for Controlling the Timing of Bone Progenitor Recruitment and Differentiation Factor Deliveries. ACS Biomaterials Science & Engineering 2020, 6, (3), 1522–1534. [DOI] [PubMed] [Google Scholar]
- 209.Aycan D; Alemdar N, Development of pH-responsive chitosan-based hydrogel modified with bone ash for controlled release of amoxicillin. Carbohydrate Polymers 2018, 184, 401–407. [DOI] [PubMed] [Google Scholar]
- 210.Anjum F; Lienemann PS; Metzger S; Biernaskie J; Kallos MS; Ehrbar M, Enzyme responsive GAG-based natural-synthetic hybrid hydrogel for tunable growth factor delivery and stem cell differentiation. Biomaterials 2016, 87, 104–117. [DOI] [PubMed] [Google Scholar]
- 211.Aziz AH; Wilmoth RL; Ferguson VL; Bryant SJ, IDG-SW3 Osteocyte Differentiation and Bone Extracellular Matrix Deposition Are Enhanced in a 3D Matrix Metalloproteinase-Sensitive Hydrogel. ACS Applied Bio Materials 2020, 3, (3), 1666–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bizios R, Mini-review: Osteoblasts: An in vitro model of bone-implant interactions. Biotechnol Bioeng 1994, 43, (7), 582–5. [DOI] [PubMed] [Google Scholar]
- 213.Kohli N; Ho S; Brown SJ; Sawadkar P; Sharma V; Snow M; García-Gareta E, Bone remodelling in vitro: Where are we headed?: -A review on the current understanding of physiological bone remodelling and inflammation and the strategies for testing biomaterials in vitro. Bone 2018, 110, 38–46. [DOI] [PubMed] [Google Scholar]
- 214.Qiu P; Li M; Chen K; Fang B; Chen P; Tang Z; Lin X; Fan S, Periosteal matrix-derived hydrogel promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis. Biomaterials 2020, 227, 119552. [DOI] [PubMed] [Google Scholar]
- 215.Heinemann S; Heinemann C; Wenisch S; Alt V; Worch H; Hanke T, Calcium phosphate phases integrated in silica/collagen nanocomposite xerogels enhance the bioactivity and ultimately manipulate the osteoblast/osteoclast ratio in a human co-culture model. Acta Biomaterialia 2013, 9, (1), 4878–4888. [DOI] [PubMed] [Google Scholar]
- 216.Rao RR; Peterson AW; Ceccarelli J; Putnam AJ; Stegemann JP, Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 2012, 15, (2), 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Marmorat C; Arinstein A; Koifman N; Talmon Y; Zussman E; Rafailovich M, Cryo-Imaging of Hydrogels Supermolecular Structure. Scientific Reports 2016, 6, (1), 25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lai Y; Hu Y, Probing the swelling-dependent mechanical and transport properties of polyacrylamide hydrogels through AFM-based dynamic nanoindentation. Soft Matter 2018, 14, (14), 2619–2627. [DOI] [PubMed] [Google Scholar]
- 219.Oyen ML, Mechanical characterisation of hydrogel materials. International Materials Reviews 2014, 59, (1), 44–59. [Google Scholar]
- 220.Bellido T; Delgado-Calle J, Ex Vivo Organ Cultures as Models to Study Bone Biology. JBMR Plus 2020, 4, (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Abubakar AA; Noordin MM; Azmi TI; Kaka U; Loqman MY, The use of rats and mice as animal models in ex vivo bone growth and development studies. Bone Joint Res 2016, 5, (12), 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Smith EL; Kanczler JM; Gothard D; Roberts CA; Wells JA; White LJ; Qutachi O; Sawkins MJ; Peto H; Rashidi H; Rojo L; Stevens MM; El Haj AJ; Rose FRAJ; Shakesheff KM; Oreffo ROC, Evaluation of skeletal tissue repair, Part 1: Assessment of novel growth-factor-releasing hydrogels in an ex vivo chick femur defect model. Acta Biomaterialia 2014, 10, (10), 4186–4196. [DOI] [PubMed] [Google Scholar]
- 223.Smith EL; Kanczler JM; Gothard D; Roberts CA; Wells JA; White LJ; Qutachi O; Sawkins MJ; Peto H; Rashidi H; Rojo L; Stevens MM; El Haj AJ; Rose FRAJ; Shakesheff KM; Oreffo ROC, Evaluation of skeletal tissue repair, Part 2: Enhancement of skeletal tissue repair through dual-growth-factor-releasing hydrogels within an ex vivo chick femur defect model. Acta Biomaterialia 2014, 10, (10), 4197–4205. [DOI] [PubMed] [Google Scholar]
- 224.Klüter T; Hassan R; Rasch A; Naujokat H; Wang F; Behrendt P; Lippross S; Gerdesmeyer L; Eglin D; Seekamp A; Fuchs S, An Ex Vivo Bone Defect Model to Evaluate Bone Substitutes and Associated Bone Regeneration Processes. Tissue Engineering Part C: Methods 2019, 26, (1), 56–65. [DOI] [PubMed] [Google Scholar]
- 225.McLean IC; Schwerdtfeger LA; Tobet SA; Henry CS, Powering ex vivo tissue models in microfluidic systems. Lab Chip 2018, 18, (10), 1399–1410. [DOI] [PubMed] [Google Scholar]
- 226.Hao S; Ha L; Cheng G; Wan Y; Xia Y; Sosnoski DM; Mastro AM; Zheng S-Y, A Spontaneous 3D Bone-On-a-Chip for Bone Metastasis Study of Breast Cancer Cells. Small 2018, 14, (12), 1702787. [DOI] [PubMed] [Google Scholar]
- 227.Vandamme TF, Use of rodents as models of human diseases. Journal of pharmacy & bioallied sciences 2014, 6, (1), 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.McGovern JA; Griffin M; Hutmacher DW, Animal models for bone tissue engineering and modelling disease. Disease Models & Mechanisms 2018, 11, (4), dmm033084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Scott MA; Levi B; Askarinam A; Nguyen A; Rackohn T; Ting K; Soo C; James AW, Brief Review of Models of Ectopic Bone Formation. Stem Cells and Development 2011, 21, (5), 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bigham-Sadegh A; Oryan A, Selection of animal models for pre-clinical strategies in evaluating the fracture healing, bone graft substitutes and bone tissue regeneration and engineering. Connective Tissue Research 2015, 56, (3), 175–194. [DOI] [PubMed] [Google Scholar]
- 231.Nakamura T; Shirakata Y; Shinohara Y; Miron RJ; Hasegawa-Nakamura K; Fujioka-Kobayashi M; Noguchi K, Comparison of the effects of recombinant human bone morphogenetic protein-2 and -9 on bone formation in rat calvarial critical-size defects. Clinical Oral Investigations 2017, 21, (9), 2671–2679. [DOI] [PubMed] [Google Scholar]
- 232.Fan J; Pi-Anfruns J; Guo M; Im DCS; Cui ZK; Kim S; Wu BM; Aghaloo TL; Lee M, Small molecule-mediatedtribbles homolog 3 promotes bone formation induced by bone morphogenetic protein-2. Sci Rep 2017, 7, (1), 7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Fan J; Guo M; Im CS; Pi-Anfruns J; Cui ZK; Kim S; Wu BM; Aghaloo TL; Lee M, Enhanced Mandibular Bone Repair by Combined Treatment of Bone Morphogenetic Protein 2 and Small-Molecule Phenamil. Tissue Eng Part A 2017, 23, (5–6), 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fan J; Park H; Lee MK; Bezouglaia O; Fartash A; Kim J; Aghaloo T; Lee M, Adipose-derived stem cells and BMP-2 delivery in chitosan-based 3D constructs to enhance bone regeneration in a rat mandibular defect model. Tissue Eng Part A 2014, 20, (15–16), 2169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Liu G; Guo Y; Zhang L; Wang X; Liu R; Huang P; Xiao Y; Chen Z; Chen Z, A standardized rat burr hole defect model to study maxillofacial bone regeneration. Acta Biomaterialia 2019, 86, 450–464. [DOI] [PubMed] [Google Scholar]
- 236.Newman E; Turner AS; Wark JD, The potential of sheep for the study of osteopenia: Current status and comparison with other animal models. Bone 1995, 16, (4, Supplement), S277–S284. [DOI] [PubMed] [Google Scholar]
- 237.Lienemann PS; Vallmajo-Martin Q; Papageorgiou P; Blache U; Metzger S; Kivelio A-S; Milleret V; Sala A; Hoehnel S; Roch A; Reuten R; Koch M; Naveiras O; Weber FE; Weber W; Lutolf MP; Ehrbar M, Smart Hydrogels forthe Augmentation of Bone Regeneration by Endogenous Mesenchymal Progenitor Cell Recruitment. Advanced Science 2020, 7, (7), 1903395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mohiuddin OA; Campbell B; Poche JN; Ma M; Rogers E; Gaupp D; Harrison MAA; Bunnell BA; Hayes DJ; Gimble JM, Decellularized Adipose Tissue Hydrogel Promotes Bone Regeneration in Critical-Sized Mouse Femoral Defect Model. Frontiers in Bioengineering and Biotechnology 2019, 7, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Fang X; Xie J; Zhong L; Li J; Rong D; Li X; Ouyang J, Biomimetic gelatin methacrylamide hydrogel scaffolds for bone tissue engineering. J Mater Chem B 2016, 4, (6), 1070–1080. [DOI] [PubMed] [Google Scholar]
- 240.Townsend JM; Andrews BT; Feng Y; Wang J; Nudo RJ; Van Kampen E; Gehrke SH; Berkland CJ; Detamore MS, Superior calvarial bone regeneration using pentenoate-functionalized hyaluronic acid hydrogels with devitalized tendon particles. Acta Biomaterialia 2018, 71, 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lohmann P; Willuweit A; Neffe AT; Geisler S; Gebauer TP; Beer S; Coenen HH; Fischer H; Hermanns-Sachweh B; Lendlein A; Shah NJ; Kiessling F; Langen KJ, Bone regeneration induced by a 3D architectured hydrogel in a rat critical-size calvarial defect. Biomaterials 2017, 113, 158–169. [DOI] [PubMed] [Google Scholar]
- 242.Tang Y; Lin S; Yin S; Jiang F; Zhou M; Yang G; Sun N; Zhang W; Jiang X, In situ gas foaming based on magnesium particle degradation: A novel approach to fabricate injectable macroporous hydrogels. Biomaterials 2020, 232, 119727. [DOI] [PubMed] [Google Scholar]
- 243.Lei L; Liu Z; Yuan P; Jin R; Wang X; Jiang T; Chen X, Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. Journal of Materials Chemistry B 2019, 7, (16), 2722–2735. [DOI] [PubMed] [Google Scholar]
- 244.Yan HJ; Casalini T; Hulsart-Billstrom G; Wang S; Oommen OP; Salvalaglio M; Larsson S; Hilborn J; Varghese OP, Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials 2018, 161, 190–202. [DOI] [PubMed] [Google Scholar]
- 245.Song W-Y; Liu G-M; Li J; Luo Y-G, Bone morphogenetic protein-2 sustained delivery by hydrogels with microspheres repairs rabbit mandibular defects. Tissue Engineering and Regenerative Medicine 2016, 13, (6), 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Jung SW; Byun J-H; Oh SH; Kim TH; Park J-S; Rho G-J; Lee JH, Multivalent ion-based in situ gelling polysaccharide hydrogel as an injectable bone graft. Carbohydrate Polymers 2018, 180, 216–225. [DOI] [PubMed] [Google Scholar]
- 247.Smetana K; Stol M; Korbelar P; Novak M; Adam M, Implantation of p(HEMA)-collagen composite into bone. Biomaterials 1992, 13, (9), 639–642. [DOI] [PubMed] [Google Scholar]
- 248.Ingavle GC; Gionet-Gonzales M; Vorwald CE; Bohannon LK; Clark K; Galuppo LD; Leach JK, Injectable mineralized microsphere-loaded composite hydrogels for bone repair in a sheep bone defect model. Biomaterials 2019, 197, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Duncan WJ; Greer PFC; Lee M-H; Loch C; Gay JHA, Wool-derived keratin hydrogel enhances implant osseointegration in cancellous bone. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2018, 106, (6), 2447–2454. [DOI] [PubMed] [Google Scholar]
- 250.Shah NV; Meislin R, Current state and use of biological adhesives in orthopedic surgery. Orthopedics 2013, 36, (12), 945–56. [DOI] [PubMed] [Google Scholar]


