Abstract
Background:
The emergence of reactive stroma is a hallmark of PCa progression and a potential source for prognostic and diagnostic markers of PCa. Collagen is a main component of reactive stroma and changes systematically and quantitatively to reflect the course of PCa, yet has remained undefined due to a lack of tools that can define collagen protein structure. Here we use a novel collagen-targeting proteomics approach to investigate zonal regulation of collagen type proteins in PCa prostatectomies.
Methods:
Prostatectomies from 9 patients were divided into zones containing 0, 5, 20, 70–80% glandular tissue and 0, 5, 25, 70% by mass of prostate cancer tumor following the McNeal model. Tissue sections from zones were graded by a pathologist for Gleason score, percent tumor present, percent prostatic intraepithelial neoplasia and/or inflammation. High resolution accurate mass (HRAM) collagen targeting proteomics was done on a select subset of tissue sections from patient-matched tumor or nontumor zones. Imaging mass spectrometry was used to investigate collagen type regulation corresponding to pathologist defined regions.
Results:
Complex collagen proteomes were detected from all zones. COL17A and COL27A increased in zones of inflammation compared to zones with tumor present. COL3A1, COL4A5, and COL8A2 consistently increased in zones with tumor content, independent of tumor size. Collagen hydroxylation of proline (HYP) was altered in tumor zones compared to zones with inflammation and no tumor. COL3A1 and COL5A1 showed significant changes in HYP peptide ratios within tumor compared to zones of inflammation (2.59 ± 0.29 p-value 0.015; 3.75 ± 0.96 p-value 0.036, respectively). By imaging mass spectrometry COL3A1 showed defined localization and regulation to tumor pathology. COL1A1 and COL1A2 showed gradient regulation corresponding to PCa pathology across zones. Pathologist defined tumor regions showed significant increases in COL1A1 HYP modifications compared to COL1A2 HYP modifications. Certain COL1A1 and COL1A2 peptides could discriminate between pathologist defined tumor and inflammatory regions.
Conclusions:
Site-specific post-translational regulation of collagen structure by proline hydroxylation may be involved in reactive stroma associated with PCa progression. Translational and post-translational regulation of collagen protein structure has potential for new markers to understand PCa progression and outcomes.
Keywords: prostate cancer, prostate adenocarcinoma, collagen, proline hydroxylation, imaging mass spectrometry, proteomics, peptide imaging, formalin-fixed, paraffin-embedded tissue imaging, tissue imaging, MALDI imaging mass spectrometry
Introduction
For prostate cancer (PCa), the regions defined by tumor stroma and more normal regions adjacent to tumor stroma have emerged as novel sources for prognostic and diagnostic tissue biomarkers 1–9. In PCa tissues, stroma morphologically and functionally changes to become reactive stroma with a significantly altered gene regulatory network compared to normal prostate stroma 8,10,11. Reactive stroma is a maladaptation of normal tissue repair with altered extracellular matrix remodeling, growth factors, cytokines, and immune recruitment 8,10–12. Reactive stroma emerges concomitant with pre-malignant prostate intraepithelial neoplasia, co-evolves with PCa throughout the processes of hyperplasia, inflammation, and tumor expansion, and contributes to therapeutic resistance 10,11,13–15. Collagen regulation is a key histopathological feature that distinguishes reactive stroma from normal stroma and correlates with PCa progression 7,16–19. PCa progression is accompanied by quantitative and systematic pathological changes in collagen content during PCa progression with increasing alignment of collagen fibers and increased transcription of specific collagens, particularly fibrillar collagens type 1 and type 3 8,10,16,20–22. However, it is unknown how the translational and post-translational regulation of the molecular changes in collagen structure contribute to PCa pathogenesis in the tumor microenvironment.
In many cancer types, translational regulation of very specific collagen types contributes to expansion of the tumor microenvironment, promotes tumor cell migration into the surrounding tissues, alters cell adhesion, and induces endothelial mesenchymal transition 23–26. Multiple dynamically regulated collagen post-translational modifications (PTMs) that alter cell signaling, including non-enzymatic glycation, lysine crosslinking, N- and O-linked glycosylation, and tyrosine sulfation 27–29. A main collagen PTM is 4-hydroxylation of proline (HYP), regulated by co-factors of iron, oxygen, and ascorbic acid, and dependency on nutritional status and oxidative stress 28,30–33. The enzymes prolyl hydroxylases P4HA1, P4HA2, P4HA3 drive HYP modification on collagen and expression patterns of the enzyme vary by cancer cell type33,34. Regulation of the prolyl hydroxylases are linked to metastasis, may alter cancer stemness properties and can increase sensitivity of cancer cells to drug treatment 35–39. Variability in HYP site localization and regulation within the collagen structure alters protein folding, changes collagen triple helical configuration and affects protease access to the collagen structure 28,29,31,40. Mammalian cells recognize very specific sites of collagen hydroxylated prolines through cell-fibril interactions with integrins and tyrosine kinase discoidin domain receptors, and this alters cell processes of adhesion, proliferation and migration41–44.
Analysis of collagen sub-types and structural features of collagen in tissue sections can be challenging in that the stroma and extracellular matrix components are largely insensitive to standard trypsin-based proteomic analysis. There is a lack of antibody reagents specific to collagen sub-type or collagen-post-translational modifications. We have recently developed a collagenase-based imaging mass spectrometry analysis workflow that specifically targets analysis of stroma and extracellular matrix composition in formalin-fixed paraffin-embedded tissues from tissue microarray cores, biopsies and full tissue sections45–47. In the current study, we hypothesized that clinical and pathological features of PCa prostatectomy tissues may be characterized by translational and post-translational regulation of collagen types. To test this, prostatectomies were divided by zone of tumor grade and inflammation. Thin tissue sections from the selected prostatectomies were annotated by a pathologist for tumor content, pre-malignant prostate intraepithelial neoplasia (PIN) and inflammation. Selected tissue sections were analyzed by high mass accuracy liquid chromatographic proteomics to measure and sequence collagen type proteomic signature. Regulation of collagen structure by pathology was tested using collagen targeting imaging mass spectrometry on the same tissue sections 45–48. Major findings are that additional collagen type proteins beyond the primary fibrillar collagens type I and III are regulated between high grade PCa and inflammatory zones. We further identify that distinct sites of the collagen post-translational modification hydroxylation of proline uniquely regulated within the prostate tumor and not in adjacent tissue. Defining pathological regulation of collagen type expression provides new information towards understanding PCa.
Methods
Materials and Reagents
Xylenes, 200 proof ethanol, methanol, HPLC grade water were purchased from Fisher Scientific (Pittsburgh, PA, USA). Hematoxylin (Gill’s Hematoxylin Type 2 and Eosin Y, intensified, Cancer Diagnostics) purchased through Thermo Fisher Scientific (Waltham, MA, USA). Collagenase type III (C. histolyticum) was purchased from Worthington (Lakewood, NJ, USA). Acetonitrile, α-Cyano-4-hydroxycinnamic acid (CHCA), trifluoroacetic acid (TFA), ammonium bicarbonate, ammonium phosphate monobasic, calcium chloride, and Trizma® base were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Tissue Procurement
Tissue sections were procured through the Biorepository & Tissue Analysis Shared Resource (BTASR) at the Hollings Cancer Center at the Medical University of South Carolina (MUSC) with Institutional Review Board approval. Tissues came from prostatectomies divided into zones containing 0, 5, 20, 70–80% glandular tissue and 0, 5, 25, 70% by mass of prostate cancer tumor following the McNeal model 49. Data for each sample included Gleason grade, percent tumor present, percent PIN and/or inflammation, and serum PSA levels at time of diagnosis (Figure 1).
Figure 1.
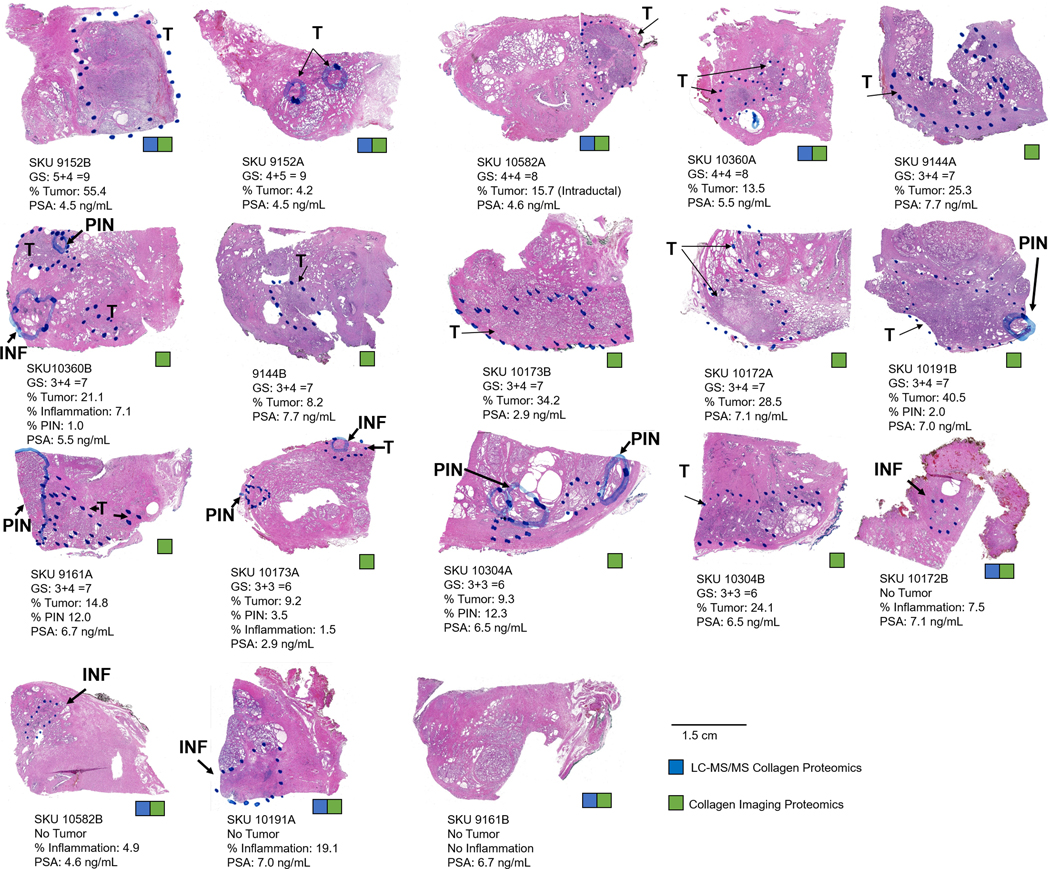
Pathological features of the prostatectomy zones used in the study. Each tissue is annotated by a KKU and same SKUs indicate that the tissue is from the same patient. For this study, collagen targeting sequencing proteomics was done on a select group of tumor or nontumor tissue (blue square). Collagen targeting imaging mass spectrometry was done on all tissue sections. INF- inflammation, PIN-prostate intraepithelial neoplasia.
Tissue Preparation for Imaging Mass Spectrometry
Each selected formalin-fixed, paraffin-embedded (FFPE) tissue block was sectioned into 5-μm thick sections by MUSC BTASR and two serial sections stained with hematoxylin and eosin. Stained tissue sections were digitally scanned (Nanozoomer, Hamamatsu). Serial sections were annotated for pathology and one section with pathological annotation was retained for reference. For the remaining serial section, coverslips were removed by overnight incubation in xylenes. The same FFPE sections of prostate tissue were prepared for imaging mass spectrometry as previously described 45–48. Briefly, tissues were heated, dewaxed, antigen retrieved at pH 3 and sprayed with a recombinant glycosidase (PNGase F Prime™, N-zyme Scientifics) to deglycosylate proteins and improve enzymatic access to collagens 48. Released N-glycans were removed from the tissues using an aqueous high pH, low pH strategy 50. Tissue was then antigen retrieved using 10 mM Tris buffer pH 9, and a thin layer of collagenase type III (collagenase) was applied by automated sprayer (M3 TM-Sprayer, HTXImaging, Chapel Hill, NC, USA). Collagenase spraying used parameters of 45°C, 10 psi, 25 μL/min, 1200 velocity, and 15 passes with a 3.0 mm offset. Samples were collagenase digested in ≥80% relative humidity at 37.5°C for five hours followed by application of CHCA matrix prepared as 7 mg/mL in 50% acetonitrile, 1% TFA with a spiked standard of 200 femtomole/microliter [Glu1]-Fibrinopeptide B human (Glufib) (Sigma-Aldrich, St. Louis, MO, USA). CHCA was applied by automated sprayer with parameters of 79°C, 10 psi, 70 μL/min, 1300 velocity, and 14 passes with a 2.5 mm offset. Slides were rapidly dipped (<1 s) in cold 5 mM ammonium phosphate, monobasic to limit matrix cluster formation 51 and dried in a desiccator prior to imaging mass spectrometry data acquisition.
Imaging Mass Spectrometry Acquisition and Image Analyses
PCa tissue sections were analyzed using a MALDI-FT-ICR (solariX™ Legacy 7.0 Tesla, Bruker Scientific, LLC, Bremen, Germany) in positive ion mode, collecting 200 laser shots per pixel. Transients of 512 kilowords were acquired in broadband mode over mass to charge (m/z) 600–3,000 with a calculated on-tissue mass resolution at full width half maximum of 81,000 at m/z 1400. Lockmass (GluFib peptide m/z 1570.6768) was maintained at 10 ppm during tissue imaging. All data were analyzed using linear interpolation without denoising normalized to total ion current using SCiLS Lab software 2019 Pro Build 7.02.10901 (Bruker Scientific, LLC, Bremen, Germany). Digital images of H&E stained tissue sections marked by the pathologist were uploaded and co-registered to the imaging mass spectrometry data. Comparisons were made based on overall tissue characterization or pathological regions of tumor, prostatic intraepithelial neoplasia or inflammation. Statistical significance of extracted peak intensities compared between regions was determined using exact p-values calculated by the Mann-Whitney test (GraphPad, Prism; version 8.3.0). Percent composition tumor, prostatic intraepithelial neoplasia, or inflammation (INF) was calculated based on pathologist annotated area compared to total area using ImageJ52. Peptide identifications were made by high mass accuracy (≤ 3 ppm) matches from LC-proteomic data done on the same tissue sections.
Chromatographic Proteomics
After imaging, matrix was removed from the tissue sections using ethanol washes 50. Tissue sections were scraped into centrifuge tubes and digested with collagenase overnight in solution as previously described 45. Collagenase peptides were dried down and desalted by solid phase extraction using C18 StageTip (Thermo Scientific). Peptides were separated and analyzed on an EASY nLC 1200 System (ThermoScientific) in-line with the Orbitrap Fusion Lumos Tribrid mass spectrometer (ThermoScientific) with instrument control software v. 4.2.28.14. Peptides were pressure loaded at 1,180 bar, and separated on a C18 reversed phase column (Acclaim PepMap RSLC, 75 μm x 50 cm (C18, 2 μm, 100 Å), ThermoFisher) using a gradient of 2% to 35% B in 180 min (Solvent A: 0.1% FA; Solvent B: 80% ACN/ 0.1% FA) at a flow rate of 300 nL/min. The column was thermostated at 45 °C. Mass spectra were acquired in data-dependent mode with a high resolution (60,000) FTMS survey scan, mass range of m/z 375–1620, followed by tandem mass spectra (MS/MS) of the most intense precursors with a cycle time of 3 s. The automatic gain control target value was 4.0e5 for the survey MS scan. Fragmentation was performed with a precursor isolation window of 1.6 m/z, a maximum injection time of 50 ms, and HCD collision energy of 35%; the fragments were detected in the Orbitrap at a 15,000 resolution. Monoisotopic-precursor selection was set to “peptide”. Apex detection was not enabled. Precursors were dynamically excluded from resequencing for 20 sec and a mass tolerance of 10 ppm. Advanced peak determination was not enabled. Precursor ions with charge states that were undetermined or > 7 were excluded. For protein analysis, tandem mass spectra were searched using both MASCOT (Version 2.4.01) and SEQUEST HT via Proteome Discoverer 1.4 (Thermo Scientific, Waltham, MA, USA) against a subset of human protein sequences downloaded on May 7, 2017 from UniProtKB (SwissProt) containing 1,783 entries (keywords used: collagen, elastin, aggrecan, gelatin, osteonectin, perlecan, plasminogen, and fibronectin). Search parameters were unspecified enzyme, precursor mass tolerances of ± 20 ppm, and fragment mass tolerances ± 0.8 Da. Methionine oxidation, asparagine and glutamine deamidation were used as variable modifications. Data were uploaded into Scaffold v4.8.1 (Proteomesoftware, Portland OR, USA) with protein probabilities ≥99% including two or more peptides. Hydroxyproline site modification probabilities were reported using MaxQuant version 1.6.3.3 53 searched against the same human database. Protein localization and function relative to collagen regulation was determined using Uniprot 54 via incorporated databases (Reactome) and primary publications. Accurate mass comparisons between image data and proteomic data (≤ 3 ppm) were done by calculating accurate mass of a peptide using Protein Prospector version 5.22.0 (Baker, P.R. and Clauser, K.R. http://prospector.ucsf.edu, University of California at San Francisco, San Francisco CA, USA). Gene Ontology55 or manual literature searches were used to annotate non-collagen extracellular matrix proteins for potential role in collagen regulation.
Results
Overview.
Pathology tissue blocks of prostatectomies from nine PCa patients were evaluated for localization and regulation of collagen types. Tissues were selected to represent pathological variation by PCa progression. Data from each tissue section included an H&E stain with pathological annotation of tumor, prostatic intraepithelial neoplasia (PIN), and inflammation (Supplemental Figure 1). Prostate specific antigen (PSA) levels were recorded. Chromatographic proteomics was used to quantify collagen by type on a select subset (the top highest-grade tumors and 4 glandular zones with or without inflammation). Collagen imaging mass spectrometry data was used to measure signals from collagen peptides that localized with specific pathologies.
Pathological characterization of prostatectomies.
A cohort of prostatectomies from 9 consented patients was examined for localization and regulation of collagen types. Median age was 65.9, 95% CI [62.2, 69.3]. Race was primarily Caucasian (8 Caucasian, 1 African-American). Prostatectomies were divided by % tumor composition and glandular zone following the McNeal model 49. One zone representing high grade tumor and one zone representing lower grade tumor or no tumor were selected for analysis. PCa is a heterogenous cancer type and PCa tumors carry multiple grades of cancer cells accompanied by changes in glandular morphology. Pathological grading of PCa was done using the 2014 World Health Organization grading system with five prognostic grade groups 56. This system reports Gleason scoring by primary (x) and secondary (y) cancer cell grading with five grades possible dependent on the sum of x+y. In the selected cohort, pathologies from all 2014 WHO grade groups were represented (Figure 1) with the majority having a grade of 2 (3+4 =7). Median percent tumor size was 18.4% of total area, 95% CI [10.8, 26.0]. Prostatic intraepithelial neoplasia, a precursor of malignant cells 57, was present in five tissue sections (10360B, 10191B, 9161A, 10173A, and 10304A). Three tissues had no tumor and regions of inflammation (10304B, 10172B, and 10582B, percent of inflammation 7.5, 4.9, and 19.1, respectively). One tissue showed no tumor and no inflammation with compacted glandular stroma (9161B). Serum levels of prostate specific antigen (PSA) are reported as an additional pathological readout. PSA levels > 4.0 ng/mL have been used to recommend prostate biopsies58, although research has demonstrated that PCa may be present with low levels of serum PSA or may not be present in men with higher levels of serum PSA 59. In the cohort, serum PSA varied from 2.0 to 7.7 ng/mL (median 5.5, 95% CI [3.6–7.4]. Notably, a PSA reading of 2.0 ng/mL was reported for a tissue with a >3 cm tumor present (10191B). In summary, the cohort represented suitable pathological variation towards probing collagen type and distribution across the disease severity spectrum of PCa.
Complexity of collagen type protein varies with PCa pathology.
Collagen targeting proteomics was used to determine regulation of collagen types in PCa. The top four high grade tissues were compared with three tissue sections containing inflammation and the single tissue section that represented compacted glandular stroma and no tumor or inflammation. A total of 45 proteins were identified with 100% probability and ≥2 unique peptides. Within the identified protein population, a total of 19 collagen type proteins were represented (Figure 2A, Supplemental Table 1). Additional extracellular matrix proteins included vimentin (VIM), lumican (LUM), decorin (DC), biglycan (BGN), transforming growth factor beta induced (TGFBI), and ADAM Metallopeptidase Domain 15 (ADAM15). Bioinformatics from gene ontology biological processes and primary literature searches reported that the detected non-collagen proteins were involved in collagen regulation with primary processes of collagen fiber regulation and collagen binding (Figure 2 B, Supplemental Table 2). Collagen composition was clustered by percent composition and varied for each tissue sections (Figure 2C). Primary collagens were the fibrillar collagens collagen alpha-1(I) chain (COL1A1), collagen alpha-2(I) chain (COL1A2) and collagen alpha-1(III) chain (COL3A1). Collagen type IV (α1, α2, α3), collagen type V (α1, α2, α3), and collagen type VI (α1, α2, α3) were the next abundant collagen types and changed in abundancy by patient tissue and potentially within a single prostate. For instance, SKU 9152 (A&B) and 10582 (A&B) represent two patient pairs. The high-grade tumor 9152A (55.4% tumor) showed increased composition of collagen type VI compared to pair 9152B that had very low tumor content (4.2% tumor). Similarly, SKU pair 10582 A (15.7% tumor) and 10582 B (no tumor and 4.9% inflammation by area) showed variation in percent composition of collagen type 1(alpha XI) α1, α2 (COL11A1 and COL11A2) with unique peptides from COL11A2 detected in non-tumor 10582B. Collagen alpha-1 (II) (COL2A1), a cartilaginous collagen associated with mineralization, was represented by an average of 6.3 exclusive unique peptides per tissue (tumor or inflammation) and average total spectral counts over 264. Trends for regulation of collagen type proteins were compared between high grade tumor (n=4) and tissue sections with inflammation (n=3) (Figure 2 D–F). Collagen types XVIIα1 (COL17A1) and XXVII α1 (COL27A1) trended to decreasing in PCa compared to tissue with inflammation (p-values 0.06, 0.004 and 0.07). Collagen types COL3A1, COL4A5, and collagen α2(VIII) COL8A2 trended to increasing in PCa (p-values 0.07, 0.08, and 0.01 respectively). Overall, the data depicts that collagen type composition changes by pathological grading while data from SKU-pairs suggests that collagen composition is dynamically regulated across the prostate during PCa.
Figure 2.
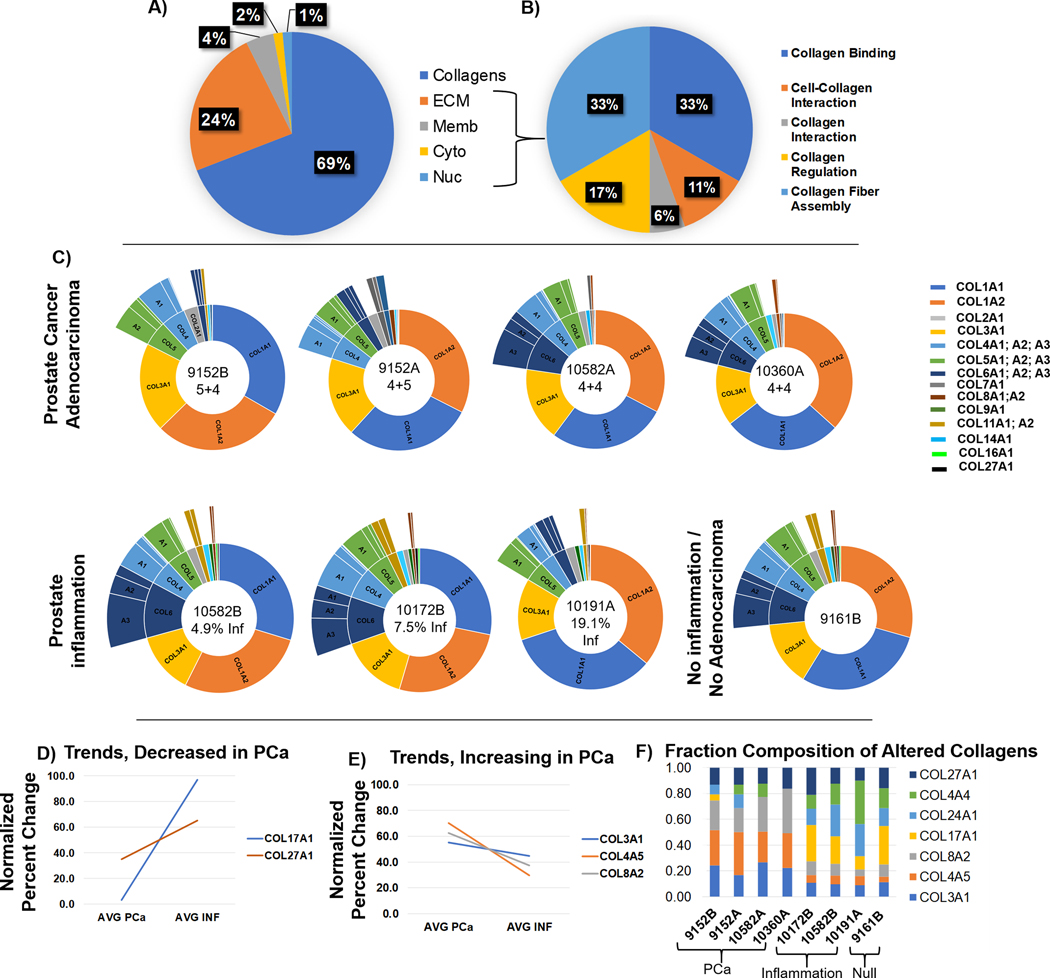
Collagen composition from high mass accuracy collagen sequencing proteomics. The normalized total spectra from unique peptides is used to illustrate composition. A) Total cellular localization of collagen and other proteins identified by the study. B) Non-collagen proteins participate in binding and regulation of collagen, contributing to tissue homeostasis. C) Collagen type distribution by hierarchical sunburst analysis which arranges collagen types by abundance. Center of each sunburst reports SKU, grade of tumor, or percent inflammation. Zones with inflammation shows more abundance of different collagen types compared to tumor zones. D) Collagen types with trends that decrease in PCa as evaluated by t-test <0.01 when comparing tumor to inflammatory zones. E) Collagen types with trends that increase in PCa as evaluated by t-test <0.01 when comparing tumor to inflammatory zones. F) Evaluation of fractional composition by zone type.
Collagen hydroxylation of proline (HYP) is regulated in PCa.
HYP is an important post-translational regulation of collagen that stabilizes collagen structure, protects against degradation, and facilitates cell-fiber recognition through integrins and discoidin domain receptors (DDRs)28,33,42,43. Changes in HYP site regulation may alter collagen organization to facilitate cell processes, e.g., invasion, migration, remodeling44. While previous studies have shown PCa upregulation of prolyl hydroxylases, the specific site regulation of HYP within the collagen structure has not been reported in PCa. Secondary database searches for HYP demonstrated that the number of HYP peptides varied by patient and pathological status of the tissue (Figure 3, Supplemental Table 3). Comparison of number of peptides with HYP modifications to unmodified peptides showed that per patient, primary collagens COL1A2 and COL3A1 had ratios of HYP/unmodified peptides ≥1.5 (Figure 3A). COL3A1 had significant increases in ratios of HYP/unmodified peptides in tumor versus nontumor tissue (2.59 ± 0.29 tumor versus 1.92 ± 0.10 no tumor, p-value 0.015). COL5A1 also showed an increase in ratios of HYP peptides to unmodified peptides (3.75 ± 0.96 tumor versus 1.94 ± 0.58 no tumor, p-value 0.036). Alignment of full-length collagen sequence with collagen helical domain (pfam PF01391) and fibrillar collagen C-terminal domain (pfam PF01410) showed that the triple helical domain had significant sequence coverage (Figures 4B). Many identified peptides showed more than one HYP site per peptide and HYP varied by patient and tumor status. Previous reports suggest that collagen types transcriptional regulation may be associated with PSA levels60. Analyses between collagen type peptides and PSA levels reported there were no identified peptides with a positive correlation to PSA levels, while three peptides showed a significant negative correlation to PSA levels (Table 1). Interestingly, all three of the peptides were modified by hydroxylation of proline and all three were within the triple helical regions. Together, this data suggests that HYP regulation of the collagen structure is a dynamic and likely patient specific response in PCa with a potential influence on collagen structure by PSA status.
Figure 3.
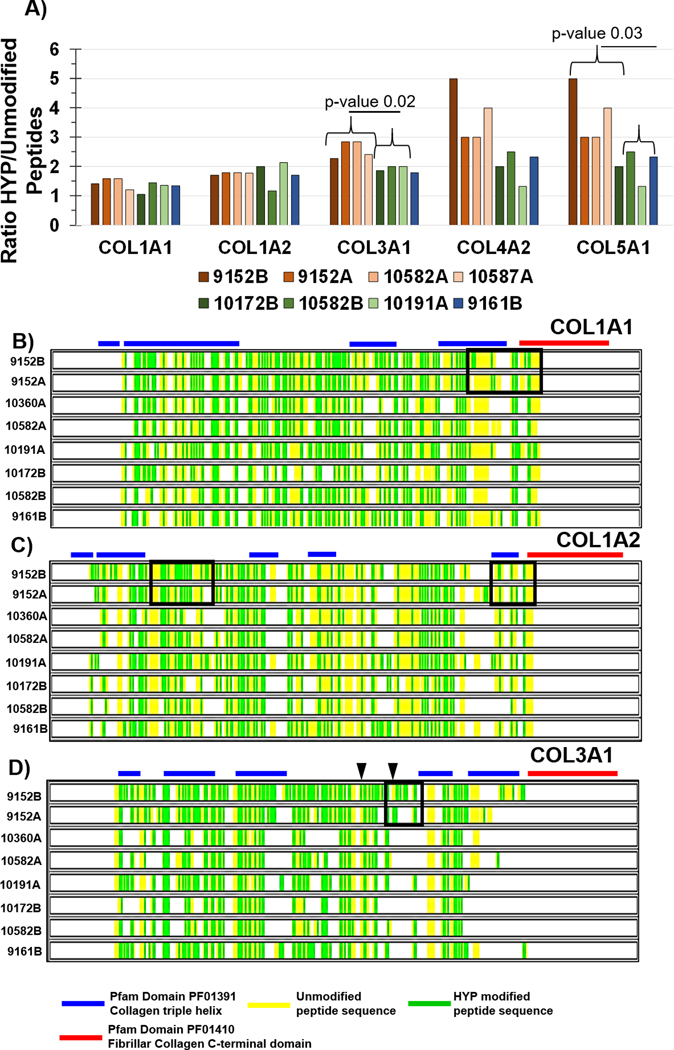
Analyses of collagen hydroxylation of proline by proteomic sequencing. Peptides with negative log probability scores ≥ 100 were used in comparison. A) Comparison of ratio of peptides having one or more hydroxylated proline to unmodified peptides per collagen type. COL3A1 and COL5A1 show higher amounts of HYP in tumor zones than in inflammatory zones, p-value is Mann-Whitney test. B) Sequence coverage of COL1A1, where green highlights hydroxylated prolines. Boxed in region highlights differential hydroxylated proline sites spanning triple helical domains and fibrillar collagen c-terminal domains from same-patient zones with high amount of tumor (9152B, 55.4% tumor) and low amount of tumor 9152A (4.2% tumor). C) COL1A2 sequence coverage where green highlights hydroxylated proline sites. Boxed in region highlights potential changes between zones with high amount of tumor versus low tumor in the same patient. D) COL3A1 sequence coverage where green indicates hydroxylated proline site. COL3A1 has 145 potential sites of 4-hydroxyproline (source, uniprot). Black arrows highlight regions differentially modified with high tumor content (9152B) compared to low tumor content (9152A). Zones were grouped by SKUs as follows: High grade tumor = 9152B (55.4% tumor), 9152A (4.2% tumor), 10360A (13.5% tumor) and 10582A (15.7% tumor with intraductal features). Inflammation = 10191A (19.1% inflammation), 10172B (7.5% inflammation), 10582B (4.9% inflammation). No tumor and no inflammation = SKU 9161B.
Figure 4.
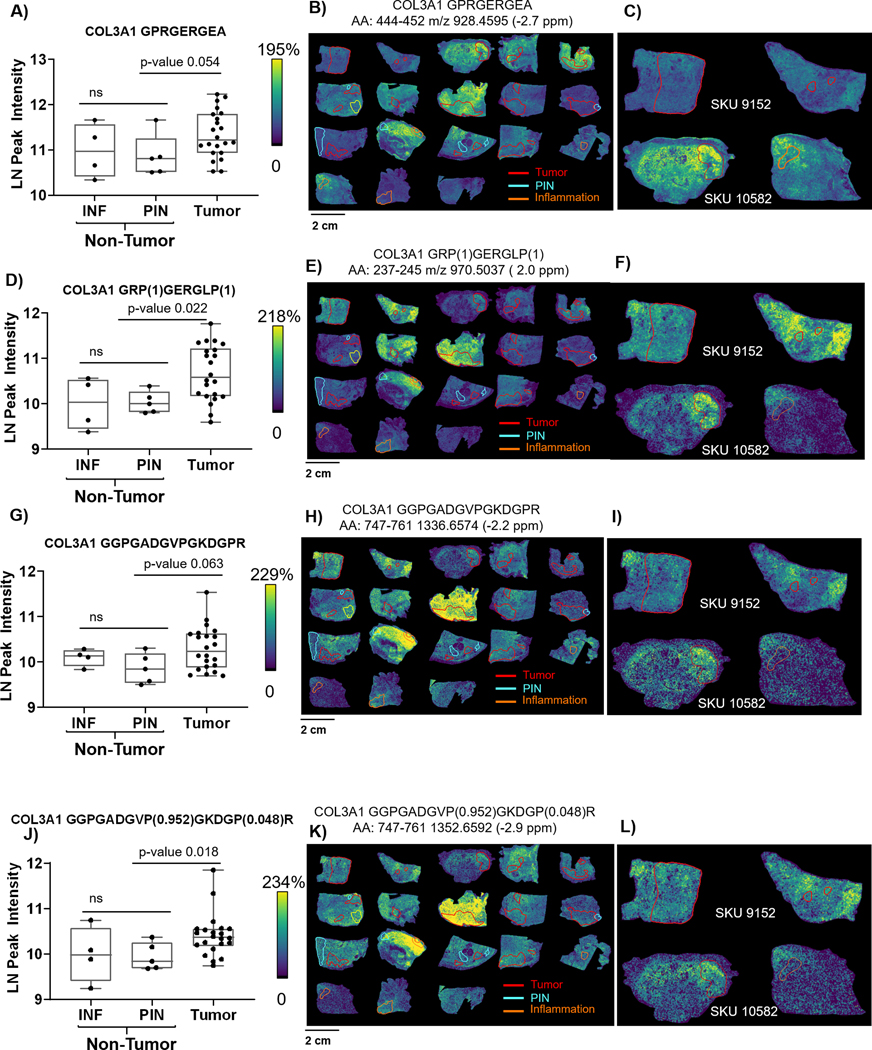
Translational and post-translational regulation of COL3A1 structure across PCa pathology by imaging mass spectrometry. Hydroxylation of proline is annotated by site probability in parenthesis from the highest scoring peptide. Abbreviation AA indicates amino acid residues that are sampled from the collagen structure. INF- inflammation, PIN-prostate intraepithelial neoplasia. A, D, G, J) COL3A1 per pathological region show significant changes in expression between tumor and PIN. Inflammation expression is more diffuse and may vary dependent on distance to the tumor and grade of tumor within the prostatectomy. B, E, H, K) Overall patterns of each peptide with tumor, PIN or inflammation annotated. C,F,I L) Comparison of high grade tumor (9152) to high grade tumor with intraductal features (SKU 10582). SKU 10582 shows increased expression levels of each peptide within the tumor site and with gradients extending from the tumor dependent on what portion of the COL3A1 structure is sampled. J-L) highlight that it is specific prolines that are differentially modified by pathology. Proline 749 is not modified, while proline 755 has the highest observed site probability at 95%; proline 760 presents with a low site probability at 4.8%.
Table 1.
Peptides showing negative correlation with PSA values. Precursor mass under 3 parts per million (PPM) was used to match between high mass accuracy chromatography sequences and the high mass accuracy image data.
| Collagen Type | Sequencea | Modifiedb | AA position | Peptide Scorec | Theoretical M+H | Observed m/z | Mass Error (PPM) | Correlation Coefficient | p-valued |
|---|---|---|---|---|---|---|---|---|---|
| COL3A1 | GQRGEP(1)GP | 371 | 365–373 | 102.0 | 941.4435 | 941.4428 | 2.1 | −0.77 | 0.01 |
| COL3A1 | GEP(1)GGP(1)G ADGVPGKDGPR |
746;749 | 744–761 | 94.4 | 1651.7671 | 1651.7650 | 1.3 | −0.71 | 0.03 |
| COL1A1 | GESGREGAP(1) GAEGSP(1)GRD GSP(1) |
1018; 1024; 1030 |
1010–1030 | 176.5 | 1974.8384 | 1974.8370 | 0.7 | −0.72 | 0.03 |
Abbreviations: AA- amino acid.
hydroxyproline site probabilities listed in parenthesis;
Amino acid site modification;
peptide score is −log probability
Spearman’s Correlation; p-value of the correlation test; Mass error in parts per million (PPM).
COL3A1 post-translational modification of hydroxylated proline increases in tumor zones.
To understand where collagen structure regulation corresponding with PCa pathological localization, imaging mass spectrometry was used to map primary collagen peptides across the tissue, comparing by pathological region. Annotated pathological regions included 5 regions of inflammation (INF) and six regions of prostatic intraepithelial neoplasia (PIN). Inflammation region on SKU 10173 was surrounded by tumor and intensity expression levels correlated with those of the tumor (Spearman’s correlation 0.979 p-value <0.001) suggesting that the region of inflammation was under the influence of the tumor field (Supplemental Figure 2). COL3A1 peptide intensities matched by high mass accuracy appeared with a diffuse gradient that increased in tumor regions compared to nontumor regions (Figure 4). This correlated with LC-proteomics data where tissues with tumor had elevated expression levels of COL3A1. Certain COL3A1 peptides showed differential patterns between zones, suggesting differential regulation of COL3A1 structure throughout prostatic tissue. For instance, COL3A1 amino acid positions 444–452 (peptide 444–452) appeared lower in regions surrounding high grade tumor (Figure 4B, C). However, in SKU 10582 which had tumor with intraductal features, COL3A1 peptide 444–452 appeared elevated in the primary tumor region with increased expression in regions of inflammation. Other COL3A1 peptides also increased in intensity localized to tumor (Figure 4C, F, I, J). The intensity distribution of these peptides varied in tissue adjacent to the tumor (Figure 4C compared to 4F). For tissue with inflammation, COL3A1 increases appeared to be adjacent to regions of inflammation. Generally, both inflammation and PIN regions had significantly lower COL3A1 intensity than in tumor (Table 2). However, the tumor with intraductal features showed overall increased intensity compared to other high grade tumors. This suggests that in PCa tissue, COL3A1 expression may vary dependent on distance from the tumor and, potentially, the type of tumor. HYP peptides matched by mass accuracy in this study had similar pathological distributions to unmodified COL3A1 peptides. As an example, the unmodified peptide at amino acid sequence 747–761 with triple prolines (Figures 4G–I) had a positive correlation with expression levels of the double HYP modified peptide (Spearman’s correlation 0.706, p-value <0.001; Figure 5J–L). The combined LC and imaging proteomics data suggested that HYP site localization on the collagen structure is very specific. The sequencing data from LC-proteomics showed that HYP at amino acid position 755 had an overall higher probability of site modification (0.95) compared to prolines at position 749 (null probability of HYP modification) and proline at position 761 (0.048 probability of modification) (Figure 4K–L). This appeared with intensity in the tumor with intraductal features and adjacent to high grade tumor regions (SKU 9152, Figure 4L). Overall, COL3A1 is regulated in defined pathological regions at the translational level and at the post-translational level through hydroxylation of proline.
Table 2.
COL3A1 peptides mapping to image data used for comparison between tumor and non-tumor (where non-tumor is prostatic intraepithelial neoplasia, PIN). Peptide peak intensities were extracted from area of pathology annotation (tumor n=11; inflammation n= 4; PIN =5). Precursor mass error under 3 parts per million (PPM) was used to match between high mass accuracy chromatography sequences and the high mass accuracy image data.
| Collagen α−1(III) chain (COL3A1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sequencea | Modifiedb | AA position | Peptide Scorec | Theoretical M+H | Observed m/z | Mass Error (PPM) | PIN/Tumor Ratio | p-valued |
| GPRGERGEA | None | 444–452 | 121.6 | 928.4595 | 928.4620 | −2.7 | 2.98 ± 0.13 | 0.054 |
| GKSGDRGESGPA | None | 1059–1070 | 168.6 | 1117.5232 | 1117.5246 | −1.3 | 1.96 ± 0.09 | 0.280 |
| GGPGADGVPGKDGPR | None | 747–761 | 237.5 | 1336.6604 | 1336.6574 | 2.2 | 3.25 ± 0.10 | 0.002 |
| GRP(1)GERGLP(1) | 239;245 | 237–245 | 170.1 | 970.5056 | 970.5037 | 2.0 | 4.38 ± 0.18 | 0.022 |
| GRDGNPGSDGLP(1) | 1022 | 1011–1022 | 130.1 | 1157.5182 | 1157.5145 | 2.8 | 2.85 ± 0.14 | 0.145 |
| GSP(1)GERGETGP(1)P(1) | 797;805;806 | 795–806 | 120.9 | 1188.5127 | 1188.5133 | −0.5 | 2.49 ± 0.10 | 0.072 |
| GGPGADGVP(0.952)GKDGP(0.048)R | 755;760 | 747–761 | 116.2 | 1352.6553 | 1352.6592 | −2.9 | 2.87 ± 0.11 | 0.018 |
| GARGNDGARGSDGQP(1) | 332 | 318–332 | 101.9 | 1430.6367 | 1430.6389 | −1.5 | 2.73 ± 0.11 | 0.063 |
Abbreviations: AA- amino acid.
hydroxyproline site probabilities listed in parenthesis;
Amino acid site modification;
peptide score is −log probability
Mann-Whitney U
Figure 5.
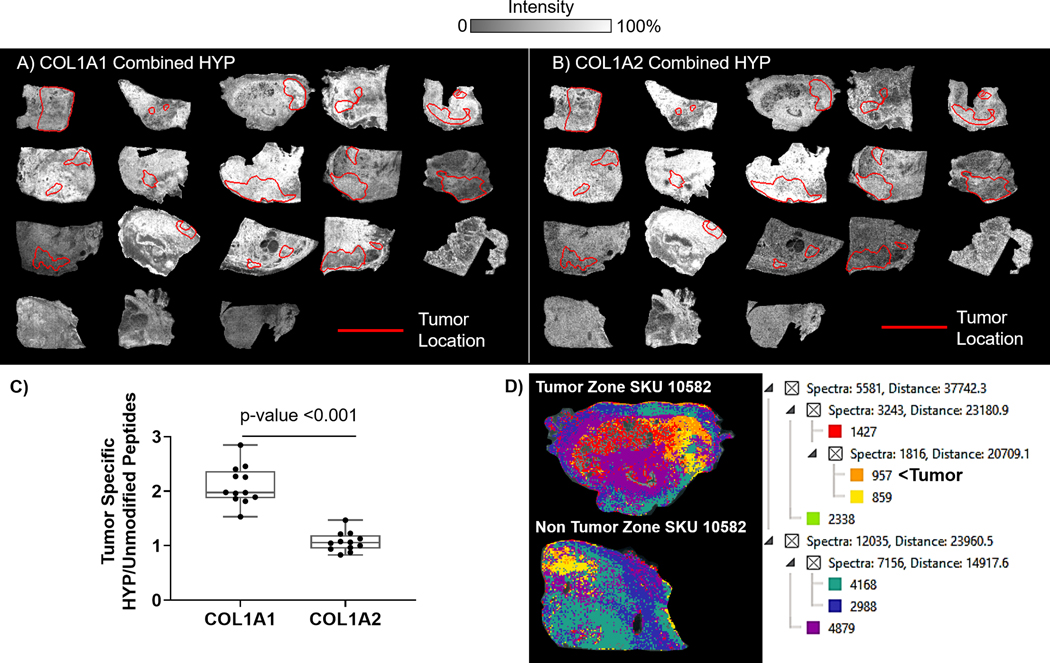
Regulation of COL1A1 and COL1A2 hydroxylated prolines (HYP) localized to pathology. Heatmaps highlight changes in expression levels across tissue. Amino acid positions are indicated in parenthesis. A) Combined ion map for COL1A1 top ten most abundant HYP modified peptides. B) Combined ion map for COL1A2 top ten most abundant HYP modified peptides. C) Tumor localized comparison of HYP/ Unmodified peptide ratios. COL1A1 shows higher ratios of hydroxylated proline when compared to COL1A2. D) Analysis of COL1A1 and COL1A2 composition within same patient tumor or nontumor zones. Analysis uses image segmentation which COL1A1 and COL1A2 clusters confidently identified peptides based on intensity and localization. Clusters are shown by color, tumor is highlighted by spectral cluster with orange color sampled by 957 spectra.
Collagen α−1(I) and collagen α−1(II) structures are regulated with PCa pathology.
The primary fibrillar type collagen type 1 forms the structure of all human tissue and organs 61; tissue homeostasis is controlled by localized translational and post-translational regulation of the collagen structure that becomes imbalanced in disease. Localization of Collagen α−1(I) chain (COL1A1) and collagen α−1(II) chain (COL1A2) peptides were investigated as indicators of structural variation due to PCa pathology. By imaging mass spectrometry, distinctive gradient changes were observed for peptides from COL1A1 and COL1A2 structures (Supplemental Figure 3). Notably, these gradients were not all overlapping, suggesting very specific structural components of collagen 1 type proteins are modulated in PCa pathology. Confidentially identified HYP peptides from COL1A1 and COL1A2 further demonstrated differential patterning in the combined ion images (Fig. 5A&B). HYP modified peptides generally had gradients with more intensity localized around or in tumor sites rather than in nontumor zones. Quantification of identified HYP peptide/Unmodified peptides within tumor regions established that there were significant differences in ratios of tumor localized HYP peptides for COL1A and COL1A2 (COL1A1 1.77 ± 0.04; COL1A2 1.44 ± 0.18, p-value 0.029) (Figure 5C). By in silico analysis, putative hydroxyproline sites on each chain reported 129 sites for COL1A1 and 16 sites for COL1A2. The variation in potential sites compared to actual findings in PCa tissue suggests a potentially higher complexity in hydroxyproline site regulation within collagen 1 type structures that occurs within tumor localized regions. Segmentation mapping of all identified COL1A1 and COL1A2 peptides was used to cluster COL1A1 and COL1A2 peptides to pathology based on localization and intensity (Figure 5D). Here, clustering reported distinct patterns in specific tumor regions, particularly SKU 10582 (intraductal features) and 9161 (high cell density), which were not observed in the nontumor zones. Additionally, area under the receiver operating curve reported that certain COL1A1 and COL1A2 peptides could distinguish between pathologist define regions of inflammation and tumor (Table 3). The combined data implies that collagen type 1 structures are differentially regulated by PCA tumor and, with larger studies, could become useful in the development of markers reporting emergence of tumor.
Table 3.
Example of COL1A1 and COL1A2 collagen peptides mapping to image data. Area under the receiver operating curve was used to determine if the peptides could discriminate between pathologist defined tumor and inflammation. AUC ≥0.700 was used as a test result indicating high sensitivity and specificity in distinguishing between same patient inflammation and tumor regions, p-value was <0.001.
| Collagen α−1(I) chain (COL1A1) | |||||||
|---|---|---|---|---|---|---|---|
| Sequencea | Modifiedb | AA position | Peptide Scorec | Theoretical M+H | Observed m/z | Mass Error (PPM) | AUC INF vs Tumor |
| GPVGPVGAR | No | 1076–1084 | 167.3 | 809.4628 | 809.4617 | 1.4 | 0.743 |
| GPRGETGPA | No | 908–916 | 154.7 | 841.4163 | 841.4165 | −0.29 | 0.751 |
| GPAGERGEQ | No | 626–634 | 168.3 | 900.4170 | 900.4181 | −1.2 | |
| GPAGARGNDGATGAA | No | 317–331 | 163.5 | 1242.5822 | 1242.5799 | 1.8 | 0.740 |
| GPAGERGSP(1) | 514 | 505–514 | 143.4 | 843.3955 | 843.3953 | 0.26 | 0.786 |
| GPAGQDGRP(1) | 565 | 556–565 | 116.4 | 870.4064 | 870.4074 | −1.1 | 0.738 |
| GKDGLNGLP(1) | 1159 | 1150–1159 | 115.7 | 886.4629 | 886.4606 | 2.6 | 0.873 |
| GPAGRP(1)GEVGP (0.003)P(0.997) |
919;925 | 913–925 | 209.7 | 1122.5538 | 1122.5520 | 1.6 | 0.887 |
| GPRGLP(1)GER | 307 | 301–310 | 242.1 | 954.5116 | 954.5089 | 2.8 | 0.896 |
| Collagen α−2(I) chain (COL1A2) | |||||||
| GPVGRTGEVGAV | No | 826–837 | 152.3 | 1098.5902 | 1098.5925 | −2.1 | 0.755 |
| GERGLHGEF | No | 571–579 | 121.6 | 1001.4799 | 1001.4829 | −3.0 | 0.765 |
| GPAGATGDRGEAGAAGPA | No | 685–702 | 188.1 | 1482.6932 | 1482.6972 | −2.7 | |
| GPAGARGSDGSVGPV | No | 229–243 | 182.4 | 1283.6339 | 1283.6338 | 0.04 | 0.798 |
| GLVGEP(1)GPA | 348 | 343–351 | 139.3 | 1082.6317 | 1082.6347 | −2.8 | 0.884 |
| GSP(1)GERGEVGPA | 711 | 709–720 | 112.9 | 1343.6186 | 1343.6217 | −2.3 | 0.798 |
Abbreviations: AA- amino acid; I, m/z – mass to charge; Mass error in PPM- parts per million; INF- Inflammation.
Sequences with hydroxyproline site probabilities listed in parenthesis if present;
Proline site modification;
peptide score is (-log probability).
Discussion
Prostate cancer has been linked to collagen regulation at the genetic and transcription level 8,10,11 with few studies on translational and post-translational regulation of collagen. Stroma morphologically and functionally changes to become reactive stroma, a maladaptation of normal tissue processes that promotes the emergence and progression of prostatic cancer 8,10. Reactive stroma is characterized by upregulation of the primary fibrillar collagens type 1 and 3 accompanied by increasing alignment of collagen fiber structures 8,10,16,20–22. In the current study, we used our novel collagen targeting imaging method45 that works on all types of formalin-fixed, paraffin-embedded tissue samples to investigate translational and post-translational modification of collagens across pathologically defined zones of prostatectomies. A major finding within this study is that the aggregate collagen type protein profiles of prostate tissue quantitatively vary by zone within same patient prostatectomies. We also report that post-translational regulation of the primary fibrillar collagens type I and III varies based on pathological localization within the prostatic gland. Additionally, the method reported extracellular matrix proteins interacting with the collagenome. These findings provide important information on heterogeneity within the prostatic gland and links to disease sensitive changes in collagen structure as potential biomarkers of PCa.
Changes in collagen type regulation observed between tumor zones and zones with inflammation involve a continuum of collagen signaling across initiation and progression of PCa. In PCa, initial prostatic stroma changes are accompanied by collagen deposition and closing of glandular regions by transformation of prostate stromal cells into myofibroblasts. A desmoplastic response, called reactive stroma, contributes to transformation of prostate stromal fibroblasts into myofibroblasts, in part through TGFβ1 driven pathways10,20,62. Myofibroblasts in turn quantitatively increase collagen deposition and remodeling results in re-alignment of collagen fibers with increasing tumor grade 7,63. Remodeling also results in the release and production of repair response, notifying the immune system to recruit inflammatory cells to the site of collagen deposition10,62. Adaptive immune responses may in turn, result in specific remodeling facilitated by immune cells64,65. Our proteomic analysis showed that collagen types of the prostatic gland are regulated across inflammation and tumor tissue. Zones of inflammation showed an abundant and complex mix of collagen types with trends of increasing type XVII (COL17A1) and XXVII (COL27A1). COL17A1 is a favorable prognostic marker in breast cancer 66 with expression occurring in basal cell carcinoma of the skin 67. The role of COL27A1 in cancer is poorly characterized, yet COL27A1 increases during processes of embryogenesis and in adult cartilage 68,69. From these data, we suggest that COL17A1 and COL27A1 may be part of the reactive stroma response when tumor is present in the prostate tissue. The decreased collagen complexity in tumor zones was accompanied by increased collagens types III (COL3A1), IV (COL4A5), and VIII (COL8A2). Increased COL3A1 corresponds to previous reports of increased COL3A1 by prostate myofibroblasts through TGFβ1 pathways20. Decreases in basement membrane expression of COL4A5 were previously observed in invasive prostate carcinoma70. This contrasts with our finding that COL4A5 increases in tumor zones compared to zones of inflammation. It is possible that elevation of COL4A5 represents a later “stage” of reactive stroma and may have some dependence on the percent, type and grade of tumor found per zone. Additionally, remodeling could involve other types of post-translational modifications or truncation products not found in our study 71. We detected increases in collagen type VIII (COL8A2) in tumor zones compared to inflammation. Interestingly, collagen type VIII has been shown to be induced by physical cell to cell contact in PCa bone metastasis72. Recent work has shown that collagen type VIII is a potential biomarker of prostate cancer, and is elevated in the serum of prostate cancer patients compared to normal controls73. We propose that reactive stroma may be staged by collagen type composition and regulation; identifying exact contributors of early stages compared to tumor adjacent stroma may lead to the most effective therapeutic targets. These specific proline sites are currently being assessed in larger prostate cancer tissue cohorts to evaluate their biomarker potential.
Collagen structure modulation includes diverse translational and post-translational regulation of various portions of the structure to form networks and fibrils that are essential for tissue homeostasis and are deregulated in disease 28,61,74. In this study, we focused on PCa post-translational regulation of collagen by hydroxylation of proline (HYP). Significant increases in HYP site regulation were found on the fibrillar COL3A1 when measured in tumor zones compared to nontumor zones. This specific observation of overall HYP increased in tumor matches evidence that proly-4-hydroxylases are upregulated by decreases in oxygen tension, a potential tipping point in the cancer-regulated re-alignment of collagen fibers that promotes cancer fibroblast migration from the tumor site30,35,75. HYP sites on fibrillar collagens COL1A1, COL1A2 and COL3A1 were localized to tumor regions with gradients extending outside of the tumor margin that may possibly follow oxygen tension gradients due to dense glandular stroma. An important finding in the current work is that certain collagen prolines but not all prolines were modified by hydroxylation of proline within the PCa tumor microenvironment. Furthermore, not all HYP sites were significantly regulated between tumor and nontumor tissue. This suggests that it is very specific prolines within the collagen structure that may be susceptible to disease modification, supporting that post-translational changes to collagen structure may be biomarkers useful for PCa.
Patient specific variability was observed both in stromal pathology and with the study on translation and post-translational modification of collagen. A main variability appeared to be with localization of the HYP site within the collagen structure. While this is likely dependent in some ways upon the amount and grade of tumor within the zone, it is possible that secondary factors play a role in which prolines are hydroxylated. Proline metabolism alone has a critical role in tissue homeostasis through wound healing, oxidative stress regulation and immune response 76–79. By serum analysis, aggressive and non-aggressive PCa have been significantly linked to negative regulation metabolites within the proline metabolic pathway80. Inclusion of proline in a metabolite panel with PSA has been suggested to improve PCa diagnosis81 and the current study found negative correlation of proline site regulation correlating with PSA measurements. HYP modification requires cofactors of iron, oxygen, and nutritional intake of ascorbic acid. Losses of collagen HYP lead to defects in normal tissue development & integrity, wound healing, and result in diseases such as scurvy 37,82,83. The socioeconomic status of the patient thus plays a large role in tissue homeostasis through multiple points that regulate collagen proline, particularly through nutritional access, immune response, and life stress. Additionally, racial status is a significant factor in PCa progression. African American men show a high risk for PCa, clinically present with higher grade PCa at a younger age, and are twice as likely to die from PCa when compared to other races 84–86. Although not specifically addressed in this first report, initial data suggests racial differences between collagen organization and proline hydroxylation in the prostate tumor microenvironment. A specific prostate cancer racial tissue cohort87 is currently under evaluation for collagen and proline hydroxylation characterization aligned with clinical, socioeconomic and behavioral data.
Conclusions
A main issue in the treatment of prostate cancer is whether PCa will remain indolent for many years or become malignant. New biomarkers are continually being sought towards prognosis and diagnosis of PCa. Translational and post-translational collagen regulation and re-alignment is a key pathological feature that systematically changes during emerging reactive stroma to tumor growth yet remains mostly unexplored. We show here that very specific collagen types are deposited in inflammatory zones compared to tumor zones. We additionally demonstrate that certain portions of the primary fibrillar collagen structure are post-translationally altered by hydroxylation of proline in tumor zones and are quantitatively decreased in nontumor regions. This highlights that subtle changes in collagen structure contribute to disease. Hydroxylation of proline is a nexus of collagen organization that is responsive to social and economic factors through nutritional, oxidative stress and metabolic factors. It is quite possible that very specific sites of proline modification within the collagen structure could be markers of risk for aggressive PCa and contribute to progression. Multiple studies are ongoing to extend this pilot study and determine how collagen type proteins contribute to risk and influence PCa aggressiveness. Translational and post-translational collagen regulation is a rich source for a new understanding of PCa.
Supplementary Material
Acknowledgements
PMA is supported by P20GM103542 (NIH/NIGMS), HL007260 (NHLBI), R21 CA240148 and in part by pilot research funding from the Hollings Cancer Center Support Grant P30 CA138313 at the Medical University of South Carolina. CHH, RRD, and PMA are additionally supported by U54MD010706. Additional support to RRD and CHH provided by the South Carolina Centers of Economic Excellence SmartState program. The Mass Spectrometry Facility and Redox Proteomics Core is supported by the University and P20GM103542 (NIH/NIGMS) with shared instrumentation S10 OD010731 and S10 OD025126 (NIH/OD) to LEB.
Abbreviations:
- PCa -ECM
extracellular matrix
- ECM IMS
extracellular matrix imaging mass spectrometry
- LUAD
lung adenocarcinoma
- MALDI IMS
matrix-assisted laser desorption / ionization imaging mass spectrometry
- TMA
tissue microarray
Footnotes
COI: The authors declare no potential conflicts of interest.
Disclosure Statement
There are no conflicts of interest involving the authors and this work.
References
- 1.Shen H. et al. Epidermal growth factor-containing fibulin-like extracellular matrix protein 1 (EFEMP1) acts as a potential diagnostic biomarker for prostate cancer. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 23, 216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucarelli G. et al. Spondin-2, a secreted extracellular matrix protein, is a novel diagnostic biomarker for prostate cancer. The Journal of Urology 190, 2271–2277 (2013). [DOI] [PubMed] [Google Scholar]
- 3.D’Antonio KB et al. Extracellular matrix associated protein CYR61 is linked to prostate cancer development. The Journal of Urology 183, 1604–1610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochette A. et al. Asporin is a stromally expressed marker associated with prostate cancer progression. British Journal of Cancer 116, 775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ni W-D et al. Tenascin-C is a potential cancer-associated fibroblasts marker and predicts poor prognosis in prostate cancer. Biochemical and Biophysical Research Communications 486, 607–612 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen F. et al. Up-regulation of biglycan is associated with poor prognosis and PTEN deletion in patients with prostate cancer. Neoplasia 19, 707–715 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling Y. et al. Second harmonic generation (SHG) imaging of cancer heterogeneity in ultrasound guided biopsies of prostate in men suspected with prostate cancer. Journal of Biophotonics 10, 911–918 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Tuxhorn JA et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clinical Cancer Research 8, 2912–2923 (2002). [PubMed] [Google Scholar]
- 9.Randall EC et al. Molecular characterization of prostate cancer with associated Gleason score using mass spectrometry imaging. Molecular Cancer Research 17, 1155–1165 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barron DA & Rowley DR The reactive stroma microenvironment and prostate cancer progression. Endocrine-related Cancer 19, R187-R204 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planche A. et al. Identification of prognostic molecular features in the reactive stroma of human breast and prostate cancer. PloS one 6, e18640 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nissen NI, Karsdal M & Willumsen N Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. Journal of Experimental & Clinical Cancer Research 38, 115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josson S, Matsuoka Y, Chung LWK, Zhau HE & Wang R Tumor–stroma co-evolution in prostate cancer progression and metastasis. Semin Cell Dev Biol 21, 26–32 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levesque C & Nelson PS Cellular constituents of the prostate stroma: Key contributors to prostate cancer progression and therapy resistance. Cold Spring Harbor Perspectives in Medicine, a030510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuxhorn JA, Ayala GE & Rowley DR Reactive stroma in prostate cancer progression. The Journal of Urology 166, 2472–2483 (2001). [PubMed] [Google Scholar]
- 16.Tyekucheva S. et al. Stromal and epithelial transcriptional map of initiation progression and metastatic potential of human prostate cancer. Nature Communications 8, 420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen MK et al. Integrative metabolic and transcriptomic profiling of prostate cancer tissue containing reactive stroma. Scientific Reports 8, 14269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonçalves BF et al. Key participants of the tumor microenvironment of the prostate: An approach of the structural dynamic of cellular elements and extracellular matrix components during epithelial–stromal transition. Acta Histochemica 117, 4–13 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Penet M-F et al. Structure and Function of a Prostate Cancer Dissemination–Permissive Extracellular Matrix. Clinical Cancer Research 23, 2245–2254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R. et al. Long noncoding RNA DNM3OS promotes prostate stromal cells transformation via the miR-29a/29b/COL3A1 and miR-361/TGFβ1 axes. Aging (Albany NY) 11, 9442 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long Q. et al. Global transcriptome analysis of formalin-fixed prostate cancer specimens identifies biomarkers of disease recurrence. Cancer Research 74, 3228–3237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao H. et al. Genome‐wide characterization of gene expression variations and DNA copy number changes in prostate cancer cell lines. The Prostate 63, 187–197 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz M & Christofori G EMT, the cytoskeleton, and cancer cell invasion. Cancer and Metastasis Reviews 28, 15–33.PMID: 19169796. (2009). [DOI] [PubMed] [Google Scholar]
- 24.Fang M, Yuan J, Peng C & Li Y Collagen as a double-edged sword in tumor progression. Tumor Biology 35, 2871–2882.PMID: 24338768 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vellinga TT et al. Collagen-rich stroma in aggressive colon tumors induces mesenchymal gene expression and tumor cell invasion. Oncogene 35, 5263–5271. PMID: 26996663 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Kalluri R The biology and function of fibroblasts in cancer. Nature Reviews Cancer 16, 582–598. PMID: 27550820 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Myllyharju J & Kivirikko KI Collagens, modifying enzymes and their mutations in humans, flies and worms. TRENDS in Genetics 20, 33–43.PMID: 14698617 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Shoulders MD & Raines RT Collagen structure and stability. Annual Review of Biochemistry 78, 929–958. PMID: 19344236. (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bella J Collagen structure: new tricks from a very old dog. Biochemical Journal 473, 1001–1025.PMID: 27060106. (2016). [DOI] [PubMed] [Google Scholar]
- 30.Tang L. et al. Global Metabolic Profiling Identifies a Pivotal Role of Proline and Hydroxyproline Metabolism in Supporting Hypoxic Response in Hepatocellular Carcinoma. Clinical Cancer Research 24, 474–485.PMID: 29084919. (2018). [DOI] [PubMed] [Google Scholar]
- 31.Wedemeyer WJ, Welker E & Scheraga HA Proline cis− trans isomerization and protein folding. Biochemistry 41, 14637–14644. PMID: 12475212. (2002). [DOI] [PubMed] [Google Scholar]
- 32.Heart Disease and Stroke Statistic, 2005. Update.
- 33.Gorres KL & Raines RT Prolyl 4-hydroxylase. Critical Reviews in Biochemistry and Molecular Biology 45, 106–124. PMCID: PMC2841224. (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annunen P, Autio-Harmainen H & Kivirikko KI The novel type II prolyl 4-hydroxylase is the main enzyme form in chondrocytes and capillary endothelial cells, whereas the type I enzyme predominates in most cells. J Biol Chem 273, 5989–5992. PMID: 9497309. (1998). [DOI] [PubMed] [Google Scholar]
- 35.Xiong G, Deng L, Zhu J, Rychahou PG & Xu R Prolyl-4-hydroxylase α subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer 14, 1PMCID: PMC3880410. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilkes DM et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Research 73, 3285–3296. PMCID: PMC3674184. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rappu P, Salo AM, Myllyharju J & Heino J Role of prolyl hydroxylation in the molecular interactions of collagens. Essays in biochemistry 63, 325–335. PMCID: PMC6744578. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winslow S, Lindquist KE, Edsjö A & Larsson C The expression pattern of matrix-producing tumor stroma is of prognostic importance in breast cancer. BMC Cancer 16, 841 PMCID: PMC5095990. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizwan A. et al. Metastatic breast cancer cells in lymph nodes increase nodal collagen density. Scientific Reports 5, 10002. PMCID: PMC4423440. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krane SM The importance of proline residues in the structure, stability and susceptibility to proteolytic degradation of collagens. Amino Acids 35, 703–710. PMID: 18431533. (2008). [DOI] [PubMed] [Google Scholar]
- 41.Sipilä KH et al. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J Biol Chem 293, 7645–7658. PMCID: PMC5961056. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilamaran M. et al. A self-assembly and higher order structure forming triple helical protein as a novel biomaterial for cell proliferation. Biomaterials Science 7, 2191–2199. PMID: 30900708. (2019). [DOI] [PubMed] [Google Scholar]
- 43.Hamaia S & Farndale RW in I Domain Integrins 127–142. PMID: 25023172. (Springer, 2014). [Google Scholar]
- 44.Davidenko N. et al. Selecting the correct cellular model for assessing of the biological response of collagen-based biomaterials. Acta Biomaterialia 65, 88–101. PMID: 25023172. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angel PM et al. Mapping Extracellular Matrix Proteins in Formalin-Fixed, Paraffin-embedded Tissues by MALDI Imaging Mass Spectrometry. Journal of Proteome Research 17, 635–646. PMID: 29161047 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angel PM et al. Extracellular Matrix Imaging of Breast Tissue Pathologies by MALDI Imaging Mass Spectrometry. Proteomics Clinical Applications 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Angel PM et al. Extracellular Matrix Alterations in Low Grade Lung Adenocarcinoma Compared to Normal Lung Tissue by Imaging Mass Spectrometry. Journal of Mass Spectrometry In press (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clift CL, Mehta AS, Drake RR & Angel PM Multiplexed Imaging Mass Spectrometry of Histological Staining, N-glycan and Extracellular Matrix from One Tissue Section: A Tool for Fibrosis Research. Methods in Molecular Biology In press (2019). [DOI] [PubMed] [Google Scholar]
- 49.McNeal JE, Redwine EA, Freiha FS & Stamey TA Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. The American Journal of Surgical Pathology 12, 897–906 (1988). [DOI] [PubMed] [Google Scholar]
- 50.Angel PM, Mehta A, Norris-Caneda K & Drake RR in Methods in Molecualr Biology: Tissue Proteomics Vol. doi: 10.1007/7651_2017_81. [Epub ahead of print]. PMID: 29058228 (Humana Press, 2017). [DOI] [Google Scholar]
- 51.Smirnov IP et al. Suppression of α-cyano-4-hydroxycinnamic acid matrix clusters and reduction of chemical noise in MALDI-TOF mass spectrometry. Analytical Chemistry 76, 2958–2965. PMID: 15144210. (2004). [DOI] [PubMed] [Google Scholar]
- 52.Collins TJ ImageJ for microscopy. Biotechniques 43, 25–30. PMID: 17936939. (2007). [DOI] [PubMed] [Google Scholar]
- 53.Tyanova S, Temu T & Cox J The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nature Protocols 11, 2301–2319 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Apweiler R. et al. UniProt: the universal protein knowledgebase. Nucleic Acids Research 47, D506–515 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ashburner M. et al. Gene ontology: tool for the unification of biology. Nature Genetics 25, 25–29. PMCID: PMC3037419. (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epstein JI et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. The American Journal of Surgical Pathology 40, 244–252 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Brawer MK Prostatic intraepithelial neoplasia: an overview. Reviews in Urology 7, S11 (2005). [PMC free article] [PubMed] [Google Scholar]
- 58.Cooner WH et al. Prostate cancer detection in a clinical urological practice by ultrasonography, digital rectal examination and prostate specific antigen. The Journal of Urology 143, 1146–1152 (1990). [DOI] [PubMed] [Google Scholar]
- 59.Thompson IM et al. Prevalence of prostate cancer among men with a prostate-specific antigen level≤ 4.0 ng per milliliter. New England Journal of Medicine 350, 2239–2246 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Banyard J. et al. Collagen XXIII expression is associated with prostate cancer recurrence and distant metastases. Clinical Cancer Research 13, 2634–2642 (2007). [DOI] [PubMed] [Google Scholar]
- 61.Ricard-Blum S The collagen family. Cold Spring Harbor Perspectives in Biology 3, a004978.PMID: 21421911 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiao SL, Chu GC-Y & Chung LWK Regulation of prostate cancer progression by the tumor microenvironment. Cancer Letters 380, 340–348 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Osorio CFEM, Costa WS, Gallo CBM & Sampaio FJB Expression of stromal elements of prostatic adenocarcinoma in different gleason scores. Acta Cirurgica Brasileira 34 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kitamura T, Qian B-Z & Pollard JW Immune cell promotion of metastasis. Nature Reviews Immunology 15, 73PMCID: PMC4470277. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jansen CS, Prokhnevska N & Kissick HT The requirement for immune infiltration and organization in the tumor microenvironment for successful immunotherapy in prostate cancer. Urologic Oncology 37, 543–555 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yodsurang V. et al. Identification of a novel p53 target, COL17A1, that inhibits breast cancer cell migration and invasion. Oncotarget 8, 55790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parikka M, Kainulainen T, Tasanen K, Bruckner‐Tuderman L & Salo T Altered expression of collagen XVII in ameloblastomas and basal cell carcinomas. Journal of Oral Pathology & Medicine 30, 589–595 (2001). [DOI] [PubMed] [Google Scholar]
- 68.Plumb DA et al. Collagen XXVII is developmentally regulated and forms thin fibrillar structures distinct from those of classical vertebrate fibrillar collagens. J Biol Chem 282, 12791–12795 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hjorten R. et al. Type XXVII collagen at the transition of cartilage to bone during skeletogenesis. Bone 41, 535–542 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dehan P. et al. Loss of type IV collagen alpha 5 and alpha 6 chains in human invasive prostate carcinomas. The American Journal of Pathology 151, 1097 (1997). [PMC free article] [PubMed] [Google Scholar]
- 71.Tanjore H & Kalluri R The role of type IV collagen and basement membranes in cancer progression and metastasis. The American Journal of Pathology 168, 715 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang J, Levenson AS & Satcher RL Identification of a unique set of genes altered during cell-cell contact in an in vitro model of prostate cancer bone metastasis. International Journal of Molecular Medicine 17, 849–856 (2006). [PubMed] [Google Scholar]
- 73.Hansen NUB et al. Type VIII collagen is elevated in diseases associated with angiogenesis and vascular remodeling. Clinical Biochemistry 49, 903–908 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Fidler AL, Boudko SP, Rokas A & Hudson BG The triple helix of collagens–an ancient protein structure that enabled animal multicellularity and tissue evolution. J Cell Sci 131, jcs203950. PMCID: PMC5963836. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zurlo G, Guo J, Takada M, Wei W & Zhang Q New insights into protein hydroxylation and its important role in human diseases. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1866, 208–220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu G. et al. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino acids 40, 1053–1063. PMCID: PMC3773366. (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li P & Wu G Roles of dietary glycine, proline, and hydroxyproline in collagen synthesis and animal growth. Amino Acids 50, 29–38 (2018). [DOI] [PubMed] [Google Scholar]
- 78.Liang X, Zhang L, Natarajan SK & Becker DF Proline mechanisms of stress survival. Antioxidants & Redox Signaling 19, 998–1011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phang JM, Donald SP, Pandhare J & Liu Y The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids 35, 681–690 (2008). [DOI] [PubMed] [Google Scholar]
- 80.Huang J. et al. Serum metabolomic profiling of prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. British Journal of Cancer 115, 1087–1095 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Heger Z. et al. Determination of common urine substances as an assay for improving prostate carcinoma diagnostics. Oncology Reports 31, 1846–1854 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Mussini E, Hutton JJ & Udenfriend S Collagen proline hydroxylase in wound healing, granuloma formation, scurvy, and growth. Science 157, 927–929 (1967). [DOI] [PubMed] [Google Scholar]
- 83.Albaugh VL, Mukherjee K & Barbul A Proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. The Journal of Nutrition 147, 2011–2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grizzle WE et al. Self‐Identified African Americans and prostate cancer risk: West African genetic ancestry is associated with prostate cancer diagnosis and with higher Gleason sum on biopsy. Cancer Medicine 8, 6915–6922 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mahal BA, Berman RA, Taplin M-E & Huang FW Prostate cancer–specific mortality across Gleason scores in black vs nonblack men. JAMA 320, 2479–2481 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Byun JS, Park S, Caban A, Jones A & Gardner K Linking Race, Cancer Outcomes, and Tissue Repair. The American Journal of Pathology 188, 317–328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bedolla RG et al. Receptor tyrosine kinase recepteur d’origine nantais as predictive marker for aggressive prostate cancer in African Americans. Molecular Carcinogenesis 58, 854–861 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


