Abstract
Background
Despite significant advances in haemodialysis (HD) in recent decades, current dialysis techniques are limited by inadequate removal of uraemic solutes such as middle molecules and protein-bound uraemic toxins. Novel medium cut-off (MCO) membrane or ‘expanded haemodialysis’ (HDx) provides diffusive removal of conventional and large middle molecular weight uraemic toxins, with marginal albumin leak.
Methods
This prospective, open-label, controlled, cross-over pilot study compared HDx (novel MCO membrane Theranova® 400) and conventional HD in 20 prevalent HD patients. Biochemical, dialysis adequacy and safety measures (adverse events, infections and hospitalization frequency) were recorded. Ten patients underwent conventional HD high-flux dialyser and 10 patients underwent HDx for 3 months, and the patients then switched and received the other treatment for a further 3 months.
Results
Treatment with HDx was associated with a significant reduction in serum albumin concentration [median (interquartile range) reduction −0.45 g/dL (−0.575 to −0.05); P = 0.025]. However, median albumin levels were ≥3.5 g/dL and no patients had clinical symptoms of hypoalbuminaemia or needed intravenous albumin administration. The number of infections was lower in patients treated with HDx (n = 7/19) compared with patients treated with HD (n = 14/20; P = 0.03). Patients treated with HDx had reduced levels of interleukin (IL)-1β (from 0.06 ± 0.02 pg/mL versus 0.28 ± 0.18 pg/mL with HD) and IL-6 (6.45 ± 1.57 pg/mL versus 9.48 ± 2.15 pg/mL), while tumour necrosis factor-α levels remain unchanged.
Conclusions
This study demonstrates that the chronic use of the novel MCO dialyser Theranova® appears to be safe and well-tolerated, without serious side effects or hypoalbuminaemia, as well as fewer infections. These results need to be confirmed in larger randomized clinical trials.
Keywords: haemodialysis, infection, uraemic toxins
INTRODUCTION
Haemodialysis (HD) has made important advances in recent decades, leading to longer survival and better quality of life in patients with end-stage renal disease (ESRD). However, rates of hospitalization and mortality in dialysis patients are still higher than in the general population [1]. Patients treated with dialysis are hospitalized on average twice a year [2], mainly due to infectious diseases or vascular access issues [3]. Annual mortality in dialysis patients is ∼15–20% [4], and the main causes of death are cardiovascular diseases (50%), septicaemia and infections (11%), and malignancies (4%). These data confirm that HD is still a suboptimal treatment. Indeed, current dialysis techniques still have important limitations in adequately removing some of the uraemic solutes such as middle molecules and protein-bound uraemic toxins [1].
Middle molecules are organic compounds characterized by a molecular weight >500 kDa, which can accumulate in ESRD and exert many toxic effects. The retention of middle molecules is associated with the development of cardiovascular disease, chronic inflammatory disease, chronic kidney disease–mineral and bone disorder (CKD-MBD), secondary immunodeficiency, amyloidosis and protein-energy wasting [5–9]. Therefore, a better clearance of these toxins could lead to improved long-term outcomes in patients with ESRD.
Several attempts have been made to enhance the removal of conventional and large middle molecular weight uraemic toxins through dialysis. Online haemodiafiltration provides a good clearance of middle molecules, but the use of this technique is limited by the need for high blood flows and accurate monitoring of devices [10]. Also, high cut-off membranes can be used to remove efficiently middle molecules from the bloodstream, but this treatment is also associated with protein loss, and its chronic use leads to hypoalbuminaemia [2]. Instead, the new medium cut-off (MCO) membranes provide diffusive and to some extent convective removal of solutes of molecular weight up to 45 kDa, with only marginal albumin leak.
The MCO membranes have a tight pore size distribution resulting in a steep sieving curve, with the values of molecular weight retention onset and molecular weight cut-off very close to each other, and with a cut-off value close to but lower than that of albumin [1]. Due to these novel membrane characteristics, the treatment with MCO membranes ‘expands’ the spectrum of uraemic toxins that can be removed by HD, and therefore this novel treatment modality is called ‘expanded HD’ (HDx).
The aim of this pilot study is to compare HDx (using the new MCO membrane Theranova® 400) and bicarbonate dialysis in prevalent HD patients based on haematochemical values, inflammatory markers, parameters of dialysis adequacy, incidence of adverse events, incidence of infections, number and causes of hospitalization.
MATERIALS AND METHODS
Patients and study design
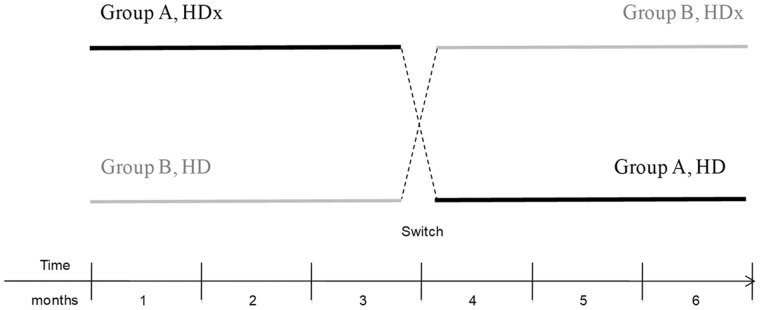
Twenty prevalent HD patients participated in this prospective, open-label, controlled, cross-over pilot study. The study was undertaken in our Dialysis Unit, University of Milan, Italy from 1 October 2017 to 31 December 2018. Consecutively, unselected male and female adult patients with ESRD on HD were eligible for participation in the study. Participation in the study was voluntary. Patients presenting with cachexia or cancer were excluded. Patients were discretionarily divided into two groups (A and B), with similar mean age, male–female ratio and dialytic vintage. This was a cross-over design whereby patients in Group A were treated with Theranova dialyser (HDx) for the first 3 months of the study, and then switched to conventional bicarbonate dialysis for the remaining 3 months. Patients in Group B were treated with bicarbonate dialysis for the first 3 months of the study, and then switched to HDx for the next 3 months (Figure 1). Sera samples from both groups were collected at 1, 2 and 3 months. This study complies with the ethical standards of the 1975 Declaration of Helsinki and the study was approved by the local Ethical Committee. This study is registered at ClinicalTrials.gov. The registration identification number is NCT03169400. All patients provided written informed consent before inclusion in the study, in compliance with Italian Legislative Decree (L. 675/1996).
FIGURE 1.
Study design.
Dialysis materials
All treatments were provided by Fresenius® 5008 (Fresenius 5008, Fresenius Medical Care, Bad Homburg, Germany). A novel membrane, Theranova® 400 (Baxter, USA), was used for patients undergoing HDx, while various other membranes (FX8, FX10, FX80, FX100, BK1.6, BG2.1), based on clinical needs, were used in patients undergoing bicarbonate dialysis.
Clinical outcome measures
Hypotension was defined as an episode of at least one symptomatic blood pressure reduction [decrease in systolic blood pressure (SBP) by ≥20 mmHg or a decrease in mean arterial pressure by 10 mmHg] at any time during the dialysis session, recorded by nurse staff into clinical records [11]. An infective event was defined as an episode characterized by clinical signs and symptoms necessitating antibiotic therapy. Hospitalization was defined as any hospital admission resulting in inpatient status for 24 h or longer.
Determination of cytokine levels
Levels of serum inflammatory cytokines were quantified using Quantikine HS ELISA kits for human interleukin (IL)-6 (HS600C) and for IL-1β (HSLB00D) (R&D Systems, Minneapolis, MN, USA). Briefly, frozen human sera were thawed at 4°C and centrifuged at 16 000g for 2 min and 100 µL were used for the assay. Following the addition of substrate, colorimetric change was determined by the absorbance value measured at 450 nm.
Statistical analysis
Data are presented as mean ± SD for continuous variables, and median and interquartile range (IQR) for not normally distributed variables. Comparisons between Groups A and B were performed by the Fisher’s exact test for categorical variables or Mann–Whitney U test for continuous variables. Comparisons of continuous variables between treatments within the same groups were performed by the Wilcoxon signed-rank test. The association between changes in serum albumin levels, as dependent variable, and treatment, time and time × treatment interaction (predictors) was performed using a regression model. The frequency of clinical outcome measures (hypotension, infection and hospitalization) was arbitrarily categorized into subclasses. The association between the frequency of events (dependent variable) and treatment, time and their interaction (predictors) was subsequently analysed by generalized linear models (for dichotomous dependent variables) or ordinal logistic regression models (for dependent variable with more than two levels). A P < 0.05 was considered statistically significant. All analysis was performed using R 3.2.1 GUI 1.6 and STATA 11.2 software (Statacorp LP Inc., College Station, TX, USA).
RESULTS
Patients
Baseline characteristics of patients are shown in Table 1. The majority of patients were male (n = 16, 76%) and mean age was 71 ± 13 years with median dialysis vintage of 27 months. Co-morbid diseases such as hypertension (86%), dyslipidaemia (71%) and diabetes (48%) were highly prevalent in these patients. Both groups of patients were well-matched for age, dialysis vintage and comorbid diseases.
Table 1.
Baseline clinical characteristics of patients
| Characteristic | All (n = 21) | Group A (n = 10) | Group B (n = 11) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||
| Male gender, n (%) | 16 (76) | 7 (70) | 9 (82) | 0.635 | ||||||
| Age, years | 71 ± 13 | 71 ± 15 | 71 ± 13 | 0.916 | ||||||
| Current smoker, n (%) | 3 (14) | 2 (20) | 1 (9) | 0.587 | ||||||
| Ex-smoker, n (%) | 4 (19) | 1 (10) | 3 (27) | 0.597 | ||||||
| Dialysis vintage, months | 27 (15–37) | 27 (18–33) | 18(14–39) | 0.805 | ||||||
| Access, n (%) | ||||||||||
| Fistula | 8 (38) | 3 (30) | 5 (45) | 0.659 | ||||||
| Central venous catheter | 13 (62) | 7 (70) | 6 (55) | 0.659 | ||||||
| Comorbid diseases | ||||||||||
| Hypertension, n (%) | 18 (86) | 9 (90) | 9 (82) | 1.000 | ||||||
| Diabetes, n (%) | 10 (48) | 5 (50) | 5 (45) | 1.000 | ||||||
| Dyslipidaemia, n (%) | 15 (71) | 8 (80) | 7 (64) | 0.635 | ||||||
| BMI (kg/m²) | 23.9 ± 2.9 | 23.5 ± 3.3 | 24.4 ± 2.5 | 0.870 | ||||||
Data are presented as mean ± SD unless and otherwise specified. Dialysis vintage is presented as median (IQR).
BMI, body mass index.
Drop-outs
During the third month of the study, one patient in Group B was relocated to another dialysis centre. This patient was still included in the final analysis. At the time of switching treatment, one patient could not change to HDx because of previous allergic reactions to polysulphone and therefore, another prevalent HD patient was enrolled for the second part of the study. One patient died during the fourth month of the study. During the fifth month of the study, one patient needed a lower flux and discontinued treatment with HDx. Taking into consideration the aforementioned drop-out rate, biochemical parameters were available at the beginning and at the end of each study period in 10 patients in Group A. For Group B, biochemical parameters were available for 10 patients for the entire first period and at the beginning of the second study period, while they were available for 8 patients during the second study period.
HD treatment
HD treatment parameters during follow-up are summarized in Table 2. In Group B, dialytic parameters at the end of the sixth month were limited to seven patients due to two drop-outs and the lack of dialytic parameters at the end of the study in one patient.
Table 2.
Dialytic parameters of Groups A and B during treatment with HDx (black) and HD (grey)
|
Kt/V |
QB (mL/min) |
ΔBW (kg) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Month | Group A | Group B | P-value | Group A | Group B | P-value | Group A | Group B | P-value |
| 1 | 1.18 (1.14–1.20) | 1.03 (0.95–1.15) | 0.121 | 297 (286–301) | 283 (248–309) | 0.796 | 2.39 (2.09–2.61) | 2.01 (1.35–2.91) | 0.739 |
| 2 | 1.16 (1.12–1.23) | 1.02 (0.99–1.13) | 0.089 | 295 (283–301) | 292 (264–307) | 0.971 | 2.23 (1.81–2.58) | 2.51 (1.39–3.13) | 0.739 |
| 3 | 1.17 (1.12–1.18) | 1.02 (0.93–1.09) | 0.031 | 295 (289–307) | 293 (246–311) | 0.762 | 2.03 (1.67–2.37) | 2.61 (1.90–3.22) | 0.257 |
| 4 | 1.11 (1.09–1.15) | 1.07 (0.90–1.21) | 0.657 | 301 (291–310) | 289 (263–304) | 0.515 | 2.06 (1.78–2.58) | 2.98 (2.38–3.29) | 0.286 |
| 5 | 1.12 (1.10–1.18) | 1.10 (1.01–1.11) | 0.435 | 296 (288–310) | 284 (265–298) | 0.407 | 2.00 (1.58–2.41) | 3.23 (3.08–3.44) | 0.089 |
| 6 | 1.12 (1.03–1.15) | 1.12 (1.01–1.22) | 0.769 | 296 (275–304) | 280 (256–302) | 0.475 | 2.06 (1.36–2.65) | 3.02 (2.09–3.45) | 0.222 |
Values are presented as median (IQR). P-values refer to difference between Groups A and B at each month (Mann–Whitney U test).
ΔBW, body weight gain during the long interval; QB, blood flow.
No significant differences between the two groups were observed throughout the study, except for higher Kt/V values in Group A, which attained statistical significance only within the third month of the first study period. A non-significant decrease in inter-dialysis weight gain during HDx treatment in Group A and increase under HD treatment in Group B was also observed. A non-significant trend towards higher SBP was detected in Group B prior to treatment (Table 3), without further variations throughout the study by both HD and HDx treatments (Table 3).
Table 3.
Blood pressure parameters in Groups A and B during treatment with HDx (black) and HD (grey)
| Month | Group A | Group B | P-value | Group A | Group B | P-value |
|---|---|---|---|---|---|---|
| SBP start (mmHg) | DBP start (mmHg) | |||||
| 1 | 136 (126–140) | 145 (140–152) | 0.063 | 63 (57–70) | 61 (59–68) | 0.796 |
| 2 | 138 (121–147) | 151 (140–168) | 0.052 | 66 (61–70) | 64 (58–68) | 0.739 |
| 3 | 136 (124–149) | 148 (142–156) | 0.063 | 63 (55–69) | 64 (62–68) | 0.796 |
| 4 | 133 (122–146) | 142 (134–159) | 0.145 | 61 (57–66) | 64 (62–72) | 0.451 |
| 5 | 133 (114–145) | 146 (138–150) | 0.133 | 64 (57–69) | 63 (60–73) | 1.000 |
| 6 | 131 (115–141) | 143 (139–148) | 0.161 | 62 (58–68) | 64 (62–69) | 0.601 |
| SBP end (mmHg) | DBP end (mmHg) | |||||
| 1 | 139 (120–150) | 154 (148–161) | 0.105 | 67 (56–74) | 71 (64–74) | 0.491 |
| 2 | 131 (125–146) | 157 (137–169) | 0.123 | 65 (61–73) | 72 (66–79) | 0.345 |
| 3 | 133 (116–159) | 155 (136–168) | 0.143 | 67 (60–78) | 72 (64–79) | 0.529 |
| 4 | 146 (120–159) | 151 (141–166) | 0.515 | 70 (60–73) | 74 (72–78) | 0.155 |
| 5 | 140 (117–159) | 150 (138–155) | 0.669 | 69 (63–75) | 74 (69–77) | 0.601 |
| 6 | 138 (117–162) | 148 (135–153) | 0.962 | 68 (59–74) | 70 (65–73) | 0.845 |
Values are presented as median (IQR). P-values refer to difference between Groups A and B at each month (Mann–Whitney U test).
DBP, diastolic blood pressure.
Haematological and biochemical parameters
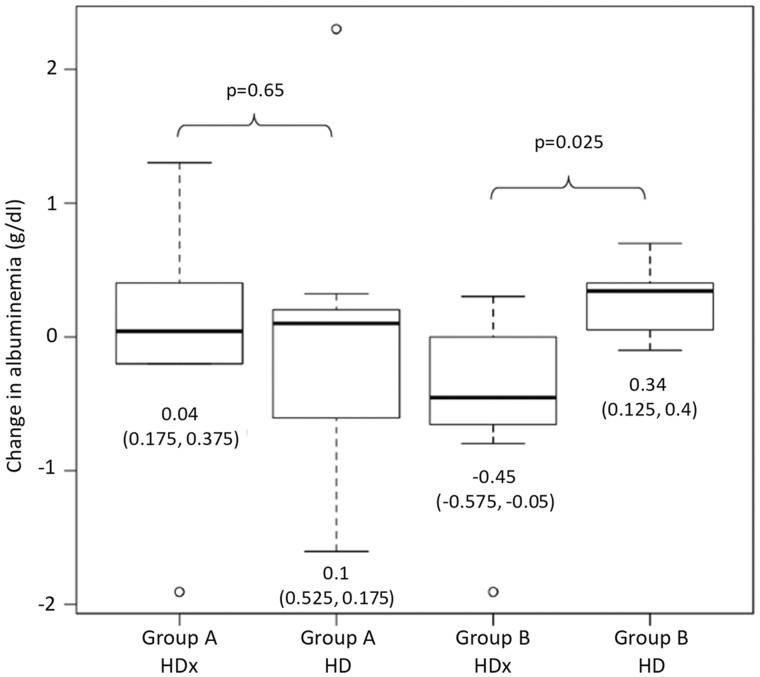
Haematological parameters did not significantly change during the study and did not differ between the treatment groups, with the exception of a greater iron saturation detected in the Group B at the end of the study (Table 4). Serum albumin levels were maintained in patients in Group A during both treatments, while patients in Group B showed a decrease in albumin concentration following treatment with HDx compared with HD. A median (IQR) reduction in circulating albumin of −0.45 g/dL (−0.575 to −0.05) was observed within Group B during the HDx period compared with an increase of 0.34 g/dL (0.125–0.40) under HD (P = 0.025; Figure 2), after exclusion of two patients without available albumin levels at the end of the study. In a multivariate regression model, the change in albumin levels was not predicted by treatment (P = 0.50), study period (P = 0.38) and their interaction (P = 0.67).
Table 4.
Haematological and biochemical parameters at the beginning of the study, at cross-over and at the end of the study
| Parameter | All (n = 20) | Group A (n = 10) | Group B (n = 10) | P-value |
|---|---|---|---|---|
| Baseline | ||||
| Haemoglobin, g/dL | 11.0 (10.4–11.7) | 11.5 (10.6–12.2) | 10.7 (9.9–11.1) | 0.19 |
| Haematocrit, vol% | 35.1 (34.0–37.7) | 37.6 (34.4–38.4) | 34.7 (31.8–35.5) | 0.16 |
| MCV, fL | 98 (93–100) | 98 (91–101) | 97 (94–99) | 1.00 |
| WBC, ×10³/µL | 6.76 (5.90–8.18) | 6.93 (5.88–8.05) | 6.76 (6.26–8.25) | 0.85 |
| Platelets, ×10³/µL | 217 (184.5–255.3) | 199 (155.3–290.5) | 225.5 (206.5–243.5) | 0.39 |
| Ferritin, ng/mL | 233 (149–405) | 313 (201–416) | 210 (139–237) | 0.39 |
| Iron, µg/dL | 52 (44–65) | 63 (52–68) | 45 (35–53) | 0.10 |
| Iron saturation, % | 24 (19–30) | 26 (21–29) | 22 (18–30) | 0.68 |
| Albumin, g/dL | 3.65 (3.00–3.80) | 3.75 (3.10–3.80) | 3.47 (3.05–3.80) | 0.65 |
| Calcium, mg/dL | 8.9 (8.7–9.2) | 8.9 (8.7–9.3) | 8.9 (8.4–9.1) | 0.76 |
| Phosphate, mg/dL | 5.1 (4.2–5.8) | 5.1 (3.7–6.2) | 5.1 (4.8–5.4) | 0.79 |
| PTH, pg/mL | 398 (104–658) | 408 (116–618) | 398 (162–675) | 0.58 |
| Arterial pH | 7.32 (7.28–7.35) | 7.33 (7.30–7.37) | 7.31 (7.27–7.35) | 0.45 |
| Sodium, mEq/L | 136 (135–139) | 137 (135–139) | 136 (135–137) | 0.57 |
| Potassium, mEq/L | 5.0 (4.7–5.3) | 4.9 (4.4–5.2) | 5.0 (4.8–5.4) | 0.60 |
| Bicarbonate, mEq/L | 20.2 (18.4–23.3) | 22.0 (18.5–23.6) | 19.0 (18.5–22.9) | 0.73 |
| ALT, UI/L | 18 (17–24) | 17 (16–22) | 18 (17–30) | 0.54 |
| AST, UI/L | 13 (11–21) | 13 (11–20) | 12 (12–19) | 0.97 |
| Switch over (3 months) | ||||
| Haemoglobin, g/dL | 11.8 (11.0–12.3) | 12.1 (10.9–12.4) | 11.7 (11.5–12.3) | 0.71 |
| Haematocrit, vol% | 37.8 (35.4–40.0) | 38.5 (35.4–39.9) | 37.2 (36.1–40.0) | 1.00 |
| MCV, fL | 100 (93–103) | 98 (93–103) | 100 (94–102) | 0.87 |
| WBC, ×10³/µL | 7.54 (6.25–9.33) | 6.87 (5.81–8.16) | 8.38 (6.58–9.65) | 0.22 |
| Platelets, ×10³/µL | 207 (188–278) | 192 (150.5–283) | 217 (202–254) | 0.31 |
| Ferritin, ng/mL | 225 (56–431) | 191 (65–364) | 290 (55–443) | 0.72 |
| Iron, µg/dL | 50 (41–60) | 51 (44–57) | 48 (37–60) | 0.74 |
| Iron saturation, % | 21 (14–25) | 20 (15–24) | 21 (14–25) | 0.97 |
| Albumin, g/dL | 3.70 (3.30–3.95) | 3.60 (3.35–3.80) | 3.80 (3.30–4.20) | 0.37 |
| Calcium, mg/dL | 9.0 (8.6–9.5) | 9.3 (8.6–9.5) | 8.7 (8.7–9.0) | 0.57 |
| Phosphate, mg/dL | 5.0 (3.8–6.6) | 5.0 (3.4–6.3) | 5.4 (4.0–6.4) | 0.65 |
| PTH, pg/mL | 541 (166–774) | 543 (203–660) | 541 (144–804) | 0.45 |
| Arterial pH | 7.31 (7.30–7.37) | 7.31 (7.30–7.38) | 7.32 (7.31–7.36) | 0.84 |
| Sodium, mEq/L | 137 (136–139) | 137 (136–138) | 136 (136–140) | 0.68 |
| Potassium, mEq/L | 4.8 (4.6–5.2) | 4.7 (4.6–5.0) | 5.2 (4.8–5.5) | 0.10 |
| Bicarbonate, mEq/L | 20.1 (19.1–21.1) | 20.1 (19.0–20.9) | 20.1 (19.3–21.0) | 0.97 |
| ALT, UI/L | 21 (16–25) | 19 (14–22) | 21 (17–26) | 0.37 |
| AST, UI/L | 15 (12–20) | 17 (11–20) | 14 (13–19) | 0.90 |
| End of study (6 months) | ||||
| All (n = 20) | Group A (n = 10) | Group B (n = 8) | ||
| Haemoglobin, g/dL | 10.4 (9.9–11.3) | 10.8 (9.6–11.3) | 10.3 (10.1–11.2) | 0.93 |
| Haematocrit, vol% | 32.9 (31.7–36.2) | 34.1 (31.6–36.0) | 32.6 (32–35) | 0.57 |
| MCV, fL | 100 (95–103) | 100 (96–103) | 99 (94–102) | 0.79 |
| WBC, ×10³/µL | 5.99 (5.45–8.00) | 5.71 (4.81–8.44) | 6.88 (5.89–7.88) | 0.37 |
| Platelets, ×10³/µL | 182 (143.5–244.2) | 161.5 (117–297.8) | 199.5 (148–242.8) | 0.63 |
| Ferritin, ng/mL | 339 (142–534) | 190 (138–459) | 432 (273–561) | 0.25 |
| Iron, µg/dL | 59 (49–79) | 54 (43–60) | 72 (63–82) | 0.11 |
| Iron saturation, % | 26 (22–37) | 22 (19–25) | 36 (27–41) | 0.01 |
| Albumin, g/dL | 3.50 (3.40–3.85) | 3.50 (3.43–3.70) | 3.6 (2.98–3.90) | 1.00 |
| Calcium, mg/dL | 9.2 (8.8–9.4) | 9.3 (8.9–9.4) | 9.1 (7.7–9.4) | 0.47 |
| Phosphate, mg/dL | 4.6 (3.7–6.2) | 4.6 (3.9–5.5) | 4.8 (3.5–6.5) | 0.89 |
| PTH, pg/mL | 248 (135–474) | 167 (128–363) | 287 (210–704) | 0.32 |
| Arterial pH | 7.34 (7.32–7.37) | 7.35 (7.33–7.40) | 7.32 (7.29–7.37) | 0.17 |
| Sodium, mEq/L | 137 (135–139) | 138 (136–139) | 136 (133–139) | 0.82 |
| Potassium, mEq/L | 5.1 (4.5–5.4) | 5.1 (4.7–5.3) | 4.9 (4.4–5.5) | 0.97 |
| Bicarbonate, mEq/L | 22.0 (21.0–23.4) | 22.1 (21.5–23.5) | 21.7 (18.6–22.6) | 0.26 |
| ALT, UI/L | 20 (16–22) | 19 (16–22) | 20 (18–28) | 0.50 |
| AST, UI/L | 14 (12–16) | 14 (11–16) | 15 (12–17) | 0.82 |
Data are presented as median (IQR). P-values refer to difference between Groups A and B at each month (Mann–Whitney U test).
ALT, alanine transaminase; AST, aspartate transaminase; MCV, mean corpuscular volume; PTH, parathyroid hormone; WBC, white blood cells.
FIGURE 2.
Change in albuminaemia in Groups A and B during treatment with HDx and HD. Values are presented as median (IQR).
Inflammatory cytokines
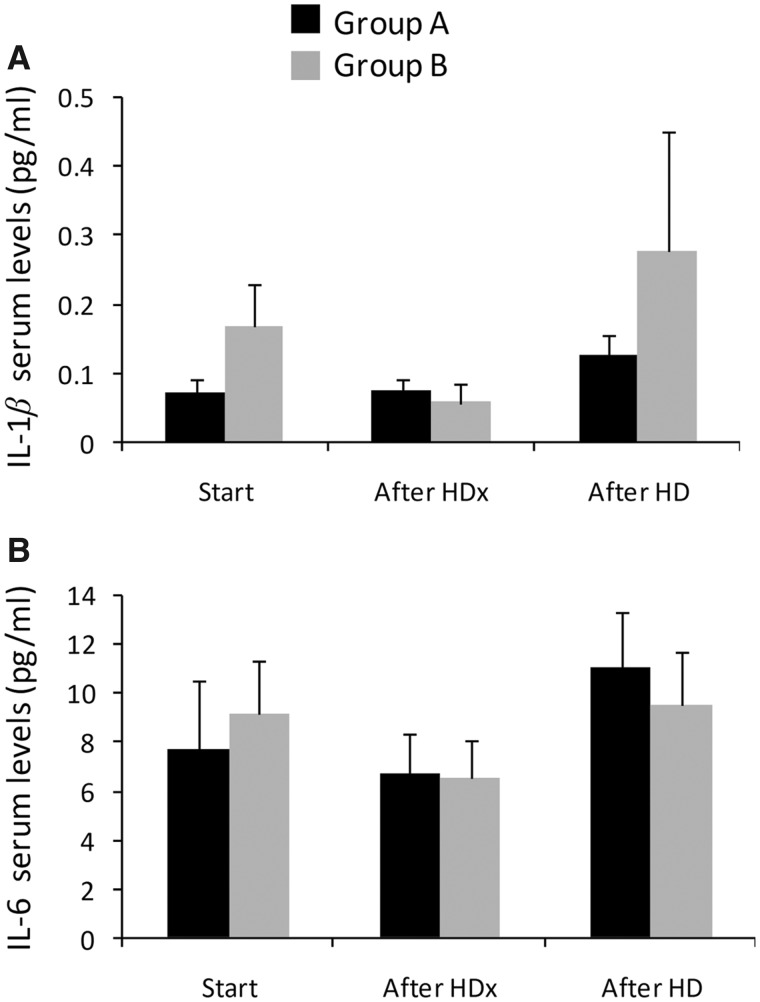
Levels of sera IL-1β were higher in Group A under HD compared with HDx, but this difference was not statistically significant (0.12 pg/mL versus 0.07 ± 0.016 pg/mL; P = 0.16). Similarly, IL-1β levels were reduced under HDx versus HD in Group B, but this difference was not statistically significant (0.057 ± 0.027 pg/mL versus 0.28 ± 0.17 pg/mL; P = 0.16) (Figure 3A). IL-6 levels slightly increased following HD compared with patients under HDx in Group A (10.9 ± 2.3 pg/mL versus 6.7 ± 1.7 pg/mL; P = 0.15) and reduced in HDx patients versus those under HD in Group B (6.5 ± 1.6 pg/mL versus 9.5 ± 2.2 pg/mL; P = 0.28), but this difference did not attain statistical significance (Figure 3B).
FIGURE 3.
Levels of (A) IL-1β and (B) IL-6 prior to and after HD and HDx treatments. Data are presented as mean and standard error of the mean.
Hypotension
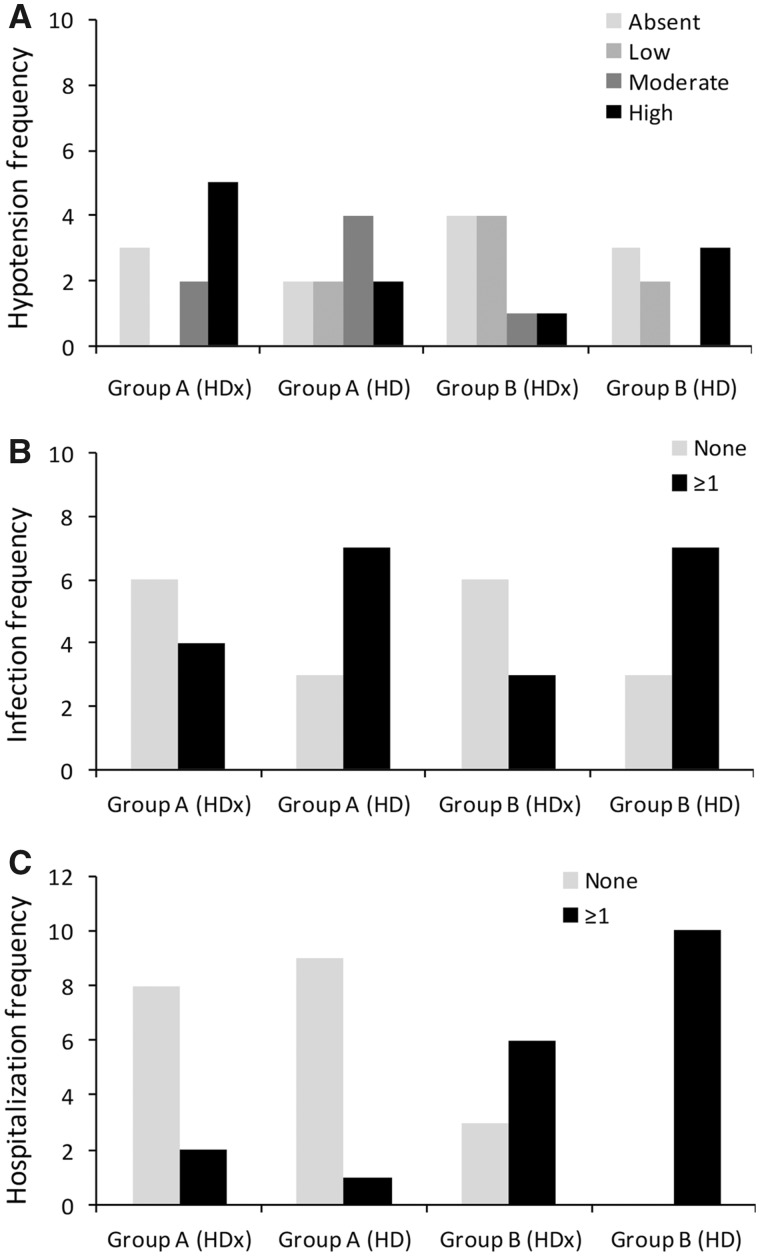
Data concerning intra-dialytic hypotension were available in 10 patients for Group A for both study periods, 10 patients of Group B during the first study period and 8 patients of Group B during the second study period (due to the two aforementioned drop-outs). A total of 110 hypotensive episodes were recorded in a total of 16 individual patients. The frequency of hypotension per patient was categorized into four classes: absent, low (one episode/patient), middle (two to three episodes/patient) and high (more than four episodes/patient) (Figure 4A). In eight patients, there was a higher incidence of intra-dialytic symptomatic hypotensive events during HDx compared with five events recorded for HD (Figure 4A). However, the total number of absent/low hypotensive events was 11 during HD versus 9 in HDx, and patients in both treatment groups had the same number of moderate or high hypotensive events (n = 9). Multivariate analysis revealed that HD was associated with a non-significant trend towards a lower risk of hypotension, independently from time and time × treatment interaction [odds ratio (OR) 0.22, 95% confidence interval (CI) 0.04–1.20; P = 0.074]. The effect of treatment was lost in a model including group effect (data not shown).
FIGURE 4.
Frequency of (A) hypotensive events, (B) infections and (C) hospitalizations in Groups A and B following HD and HDx treatments.
Infections
Data related to infective episodes were available for 10 patients in Group A for both study periods, 9 patients in Group B during the first study period and 10 patients in Group B during the second study. A total of 27 infective episodes were reported among 10 individual patients. Detailed frequency of infection is summarized in Supplementary data, Table S1. The frequency of infection per patient was categorized into two classes: none and one or more infection/patient (Figure 4B). The total number of infections was lower during treatment with HDx than with HD (n = 7/19 versus n = 14/20; P = 0.03). As shown in Figure 4B, a higher risk of developing one or more infection was observed during HD (n = 6/19 versus n = 14/20; P = 0.026) treatment than during HDx (OR 5.04, 95% CI 1.35–21.16; P = 0.020), independently of the effect of time and groups.
Hospitalization
Patients hospitalized included 10 patients in Group A during both study periods, 9 patients in Group B during the first study period and 10 patients in Group B during the second study period. A total of 19 hospitalizations were recorded. Causes of hospitalization are summarized in Supplementary data, Table S2. The frequency of hospitalizations was categorized into the following two classes: none or one or more episode/patient. The total number of hospitalizations was higher during treatment with HD than with HDx (n = 11/19 versus n = 8/19; P = 0.53). Hospitalization rate was also higher in Group B than in Group A for both arms of the study (Figure 4C). In a logistic regression model, HD was associated with a non-significant increase in the risk of hospitalization (OR 5.99, 95% CI 0.811–44.221; P = 0.079).
DISCUSSION
The main finding that emerged from this study confirmed that chronic HD with MCO membranes is not associated with hypoalbuminaemia. Indeed, the reduction in median level of albuminaemia in patients of Group B during the second part of the study [from 3.8 (3.3–4.2) g/dL to 3.6 (2.98–3.9) g/dL] was not associated with treatment after multivariate analysis. Moreover, this reduction did not appear to have any clinical relevance. This result is consistent with other studies. Zickler et al. [12] described a reduction in albumin levels during the first 4 weeks of treatment with HDx, followed by an increase during the subsequent 8 weeks of the same treatment. Instead, no differences in mean albumin levels were found in other studies undertaken by Ronco et al. [13], Teatini and Longhena [14], Belmouaz et al. [15] and Arrascue et al. [16].
Therefore, we can hypothesize that the quantity of albumin lost during HDx (estimated by Baxter as 1.2–3.9 g/treatment) can easily be replaced by hepatic synthesis and could also be beneficial for CKD patients in whom high levels of urea can promote carbamylation of serum albumin. Carbamylated albumin may be considered as a uraemic toxin since it seems to be associated with higher mortality, accelerated renal fibrosis and higher erythropoietin resistance in patients with ESRD [9, 17, 18].
The other important result that emerged from our study was the difference in the incidence of infections during the two different treatments. Although difficult to interpret, because of the small sample size and potential bias, this result is very encouraging since infectious diseases are the most common cause of hospitalization and the second most common cause of death in HD patients [3, 19]. These patients are more prone to infections than the general population, mainly because of the high prevalence of diabetes mellitus, the presence of an indwelling catheter and a condition of acquired immune dysfunction due to the retention of uraemic toxins and chronic inflammation.
We can propose different pathways through which HDx could play a role in reducing the rate of infections in HD patients. One of these is that treatment with MCO membranes may reduce inflammation, as supported by the lowering of the concentration of inflammatory cytokines in our patients undergoing HDx. These findings are consistent with results of previous studies in which HDx appeared to be associated with a lowering of the concentration of C-reactive protein [20] and the reduction of transcription of pro-inflammatory cytokines in peripheral leucocytes [12]. Another possible explanation for the decrease of infectious events could be that HDx reduces serum concentration of free light chains, since their retention in CKD patients is associated with in vitro inhibition of leucocytes chemotaxis [21].
In addition to an improvement in the immunological response, lower concentrations of inflammatory cytokines induced by treatment with MCO membranes may also lead to other beneficial effects in patients with ESRD. In these patients, systemic inflammation is associated with increased mortality due to cardiovascular disease, and with osteoporosis, depression, metabolic and nutritional derangements, and poor quality of life [22, 23]. In particular, there is strong evidence linking chronic inflammation to the development of cardiovascular diseases. Indeed, IL-1β seems to be associated with left ventricular hypertrophy in dialysis patients [24] and with the progression of atherosclerosis in patients with ischaemic heart disease, in whom serum concentrations of IL-1β correlate with plaque severity [25]. Elevated levels of IL-6 are also associated with cardiovascular mortality and left ventricular hypertrophy in HD patients [26].
The present data revealed a non-significant trend towards higher risk of hospitalization with HD. However, the incidence of hospitalizations remains difficult to interpret due to the global higher hospitalization rate observed within Group B and to the weak clinical relationship linking the causes of hospitalization to dialysis modality. Although there was slightly higher incidence of symptomatic hypotensive events in the HDx group (8 events versus 5 events), the total number of absent/low hypotensive events was 11 in the HD group versus 9 in the HDx group, and both groups had the same number of moderate or high hypotensive events (n = 9). The risk of hypotension in patients, particularly with cardiovascular disease, should be monitored, independent of dialysis type. A larger sample size will be needed to verify whether hypotension rate could be different among different dialysis methods.
Limitations
Limitations of our study include the small sample size, high number of drop-outs, absence of a wash-out period prior to switching treatment with no possibility to correct for any potential carry-over effect. In addition, parameters of dialysis adequacy suggested under-treatment and uncontrolled medical therapies for anaemia, CKD-MBD, hypertension and water balance.
Conclusions and future perspectives
Despite these limitations, this study allowed us to confirm the safety and tolerability of HDx chronic treatment over a period of 3 months. Furthermore, it validated the ability of these new membranes to reduce the serum concentration of soluble inflammatory mediators. Modulating inflammation, chronic HDx treatment may play a key role in improving immunological function and reducing cardiovascular burden in HD patients. These results encourage further trials with longer treatment periods and larger sample size.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank all patients who participated in this study.
FUNDING
This study was supported by an unrestricted grant from Baxter (Deerfield, IL, USA).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Ronco C. The rise of expanded hemodialysis. Blood Purif 2017; 44: I–VIII [DOI] [PubMed] [Google Scholar]
- 2. Yu X. The evolving patterns of uremia: unmet clinical needs in dialysis. Contrib Nephrol 2017; 191: 1–7 [DOI] [PubMed] [Google Scholar]
- 3. Maduell F, Moreso F, Pons M. et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013; 24: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. 2017 USRDS Annual Data Report: Executive Summary. Am J Kidney Dis 2018; 71: S1–S8 [Google Scholar]
- 5. Cozzolino M, Galassi A, Pivari F. et al. The cardiovascular burden in end-stage renal disease. Contrib Nephrol 2017; 191: 44–57 [DOI] [PubMed] [Google Scholar]
- 6. Massy ZA, Liabeuf S.. Middle-molecule uremic toxins and outcomes in chronic kidney disease. Contrib Nephrol 2017; 191: 8–17 [DOI] [PubMed] [Google Scholar]
- 7. Barreto FC, Barreto DV, Canziani M.. Uremia retention molecules and clinical outcomes. Contrib Nephrol 2017; 191: 18–31 [DOI] [PubMed] [Google Scholar]
- 8. Wolley MJ, Hutchison CA.. Large uremic toxins: an unsolved problem in end-stage kidney disease. Nephrol Dial Transplant 2018; 33: iii6–iii11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cozzolino M, Mangano M, Stucchi A. et al. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant 2018; 33: iii28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ronco C. Hemodiafiltration: technical and clinical issues. Blood Purif 2015; 40: 2–11 [DOI] [PubMed] [Google Scholar]
- 11. Assimon MM, Flythe JE.. Definitions of intradialytic hypotension. Semin Dial 2017; 30: 464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zickler D, Schindler R, Willy K. et al. Medium cut-off (MCO) membranes reduce inflammation in chronic dialysis patients—a randomized controlled clinical trial. PLoS One 2017; 12: e0169024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ronco C, Marchionna N, Brendolan A. et al. Expanded haemodialysis: from operational mechanism to clinical results. Nephrol Dial Transplant 2018; 33 (Suppl 3): iii41–iii47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Teatini U, Longhena GR.. Removal evaluation of a new dialyzers with medium cut-off membrane in HD treatments. Nephrol Dial Transplant 2017; 32 (Suppl 3): iii622–iii623 [Google Scholar]
- 15. Belmouaz M, Diolez J, Bauwens M. et al. Comparison of hemodialysis with medium cut-off dialyzer and on-line hemodiafiltration on the removal of small and middle size molecules: a pilot study. Nephrol Dial Transplant 2017; 32 (Suppl 3): iii623–iii624 [PubMed] [Google Scholar]
- 16. Arrascue FH, Guzman GP, Lopez et al. Effects of clinical and dialytic parameters with a new medium cut off membrane dialyzer in conventional hemodialysis compared to a high flux dialyzer in online hemodifiltration. Nephrol Dial Transplant 2018; (Suppl 1): i505 [Google Scholar]
- 17. Berg AH, Drechsler C, Wenger J. et al. Carbamylation of serum albumin as a risk factor for mortality in patients with kidney failure. Sci Transl Med 2013; 5: 175ra29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalim S, Tamez H, Wenger J. et al. Carbamylation of serum albumin and erythropoietin resistance in end stage kidney disease. Clin J Am Soc Nephrol 2013; 8: 1927–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Jager DJ, Grootendorst DC, Jager KJ. et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 2009; 302: 1782–1789 [DOI] [PubMed] [Google Scholar]
- 20. Gernone G, Montemurro M, Capurso D. et al. Mid-term evaluation of the new medium cut-off filter (Theranova) on removal efficiency and quality of life. Nephrol Dial Transplant 2018; 33 (Suppl 1): i513–i514 [Google Scholar]
- 21. Cohen G, Haag-Weber M, Mai B. et al. Effect of immunoglobulin light chains from hemodialysis and continuous ambulatory peritoneal dialysis patients on polymorphonuclear leukocyte functions. J Am Soc Nephrol 1995; 6: 1592–1599 [DOI] [PubMed] [Google Scholar]
- 22. Jankowska M, Cobo G, Lindholm B. et al. Inflammation and protein-energy wasting in the uremic milieu. Contrib Nephrol 2017; 191: 58–71 [DOI] [PubMed] [Google Scholar]
- 23. Cobo G, Lindholm B, Stenvinkel P.. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant 2018; 33 (Suppl 3): iii35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta J, Dominic EA, Fink JC. et al. Association between inflammation and cardiac geometry in chronic kidney disease: findings from the CRIC study. PLoS One 2015; 10: e0124772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galea J, Armstrong J, Gadsdon P. et al. Interleukin-1β in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol 1996; 16: 1000. [DOI] [PubMed] [Google Scholar]
- 26. Rao M, Guo D, Perianayagam MC. et al. Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 2005; 45: 324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.