Abstract
Many types of mycotoxins are found in food sources contaminated with fungi, and if these are ingested in large quantities or over a long period, they can affect the health of humans and domestic animals. Berberine (BBR) is a plant alkaloid with multiple pharmacological functions. This study aimed to investigate the effect of different levels of the plant alkaloid BBR on reducing toxic effects of aflatoxin B1 (AFB) and ochratoxin A (OTA) in broilers by examining performance characteristics, blood biochemistry, antioxidant systems, ileum morphology, and histopathology of the liver. The experiment was performed with 288 Ross 308 broilers reared in floor pens for 42 d in a randomized design with 9 treatments. Each treatment was replicated 4 times, and each replicate contained 8 chicks. Experimental treatments included (1) negative control diet with no additives (NC); (2) NC + 2 ppm AFB (positive control AFB; PCAFB); (3) NC + 2 ppm OTA (positive control OTA; PCOTA); (4) PCAFB + 200 mg/kg BBR; (5) PCAFB + 400 mg/kg BBR; (6) PCAFB + 600 mg/kg BBR; (7) PCOTA + 200 mg/kg BBR; (8) PCOTA + 400 mg/kg BBR; and (9) PCOTA + 600 mg/kg BBR. Compared with NC, feeding PCAFB and PCOTA diets reduced average daily feed intake, weight gain, serum concentrations of superoxide dismutase, glutathione peroxidase, and the length and width of ileum villi (P < 0.05). At the same time, these parameters increased in birds fed PCAFB or PCOTA diets supplemented with 600 mg/kg of BBR (P < 0.05). Feeding PCAFB and PCOTA diets increased feed conversion ratio (FCR), serum aspartate aminotransferase (AST), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), and gamma-glutamyl transferase (GGT) activities, serum urea, and liver lesions compared with NC. By contrast, compared with PCAFB and PCOTA, adding 600 mg/kg BBR decreased FCR, AST, LDH, ALT, and GGT activities, urea, and liver lesions (P < 0.05). Overall, supplementation with 600 mg/kg BBR may improve growth performance, liver function, and antioxidant status of broilers fed diets contaminated with AFB and OTA.
Key words: berberine, aflatoxicosis, ochratoxicosis, liver lesion, broiler
Introduction
In many parts of the world, poultry can be exposed to mycotoxin contaminants, which are fungal metabolites or molds grown on stored farm products or cereals. Even at low concentrations, mycotoxins are harmful to poultry and can alter normal metabolic functions in various organs (Wild and Gong, 2010). Aflatoxins are a group of mycotoxins produced by Aspergillus flavus and Aspergillus parasiticus (Devegowda and Murthy, 2005). Aflatoxins have many adverse effects on poultry, including weight loss and increased feed conversion ratio (FCR), liver, spleen, and pancreas enlargement, liver cell necrosis, anemia, and increased susceptibility to invasive infectious agents (Devegowda and Murthy, 2005; Denli and Perez, 2010).
Ochratoxins are the second major group of mycotoxins that were discovered after aflatoxins (Bennett and Klich, 2003), and they are classified as the most toxic mycotoxin for domestic poultry (Denli and Perez, 2010). Nowadays, ochratoxins are of interest among mycotoxins because they not only affect the health and economic performance of domestic animals but are also capable of threatening the health of the human community (Iqbal et al., 2014).
Food crops can become contaminated both before and after harvesting. Preharvest contamination with mycotoxins is mainly limited to maize, cottonseed, peanuts, and tree nuts. Postharvest contamination can be found in a variety of other crops such as coffee, rice, and spices. Improper storage under conditions that favor mold growth, such as warm and humid storage environments, can typically lead to levels of contamination much higher than those found in the field. In developed countries, mean aflatoxin dietary exposures are generally less than 1 ng/kg body weight (BW) per day. By contrast, estimates for some sub-Saharan African countries exceed 100 ng/kg BW per day, although these latter estimates are often based on very few data. Estimates of dietary exposure to aflatoxin M1 have rarely exceeded 1 ng/kg BW per day in any country, although up to 6.5 and 8.8 ng/kg BW per day for young children and breastfed infants have been reported (WHO, 2018). To date, in the European Union, the amount of ochratoxin A (OTA) is limited to a small number of products, such as sunflower seeds, pumpkin seeds, peanuts, and processed products thereof and was estimated to be 10 μg/kg (EU, 2002). Removal of mycotoxins from contaminated feeds is a critical aspect of nutritional research. A variety of physical, chemical, and biological methods to eliminate aflatoxins have been somewhat successful. A standard method is to use non–nutritive-absorbing materials in the diet that bind toxins such as aflatoxin or ochratoxin and reduce their uptake from the gastrointestinal tract (Jindal et al., 1994). Several compounds have been used to reduce aflatoxin or ochratoxin toxicity in poultry feed. Zeolites (Zavala-Franco et al., 2018), sodium bentonite (Gallo and Francesco, 2010), and mannose oligosaccharides (Sun et al., 2019) have been used to neutralize aflatoxin B1 (AFB), and yeast cell wall has been used to decontaminate OTA (Piotrowska and Masek, 2015).
In some studies, use of medicinal plants to reduce the toxic effects of aflatoxin in feed has been investigated (Loi et al., 2020). Berberine (BBR) is a plant alkaloid with a long history of use in traditional Chinese and Indian medicine (Tang et al., 2009). This alkaloid is found in the roots, rhizomes, and shoots of many plants, including Coptis chinesis and Berberis vulgaris (Vuddanda et al., 2010). Berberine exerts side effects on carbohydrate and fat metabolism. It increased mRNA expression of the insulin receptor in human liver and skeletal muscle cell cultures and reduced insulin resistance in a rat model of diabetes (Kong et al., 2009), prevented the destruction of pancreatic cells, especially β-cells, against oxidative stress in diabetic rats (Zhou et al., 2009), and interfered with potassium and calcium absorption in isolated rat hepatocytes (Wang et al., 2004). Free radicals are induced by peroxidation of unsaturated fatty acids in cell membranes and by cell membrane defects. As a result, compounds that have antioxidant properties and remove free radicals can have hepatoprotective properties (Farghali et al., 2015). Berberine inhibited oxidative stress and inflammation in a variety of tissues, including liver, adipose, kidney, and pancreas. Mechanisms of the antioxidant and anti-inflammatory activities of BBR interacted. These involved multiple signaling pathways and cellular kinases, such as the nuclear factor-kB (NF-kB), AMP-activated protein kinase, mitogen-activated protein kinase, and nuclear factor erythroid-2-related factor-2 (Nrf2) pathways (Li et al., 2014). Thus, we sought to induce experimental aflatoxicosis and ochratoxicosis by contaminating feed with high levels of AFB and OTA in broilers so that the efficacy of BBR in reducing effects of acute aflatoxicosis and ochratoxicosis on performance-related indices, blood biochemistry, antioxidant system, ileum morphometry, and histopathology of broiler chickens could be determined.
Materials and methods
Toxin Production
Standard vials of A. flavus and A. parasiticus were used to produce AFB. Yeast extract medium, prepared as described by Shotwell et al. (1966), was used to propagate the fungus and after fermentation on rice, AFB was produced. Standard Aspergillus extracellular vials cultured on wheat were used to produce OTA (Trenk et al., 1971). Toxin concentrations of AFB and OTA were measured by high-performance liquid chromatography at Mabna Veterinary Laboratory (Karaj, Iran).
Animal Husbandry and Growth Performance
The research was carried out at the Research Hall of Bidmeshk, the poultry research farm that has been licensed by the Veterinary Organization of South Khorasan Province, Birjand, Iran, under the supervision of the Department of Animal Sciences, University of Birjand, Birjand, Iran. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Birjand. One-day-old vaccinated Ross broilers were obtained from a local commercial hatchery (South Poultry Production Complex, South Khorasan Province, Birjand, Iran) and randomly divided between 36 pens (1.2 m × 1 m; 8 birds per pen) equipped with a nipple drinker and manual feeder. The study was conducted in a completely randomized design with 9 dietary groups, each replicated 4 times (n = 4). To ensure severe effects of toxins were observed so that efficacy of BBR in mitigating destructive effects of acute aflatoxicosis and ochratoxicosis could be evaluated, a suprapharmacological dose of 2 ppm was utilized for this study. This is also a dose that has been used by other groups investigating acute toxicosis (Martinez-de-Anda et al., 2010; Chen et al., 2016; Hameed et al., 2017; Ruan et al., 2019). Experimental treatments included (1) negative control diet with no additives (NC); (2) NC + 2 ppm AFB (positive control AFB; PCAFB); (3) NC + 2 ppm OTA (positive control OTA; PCOTA); (4) PCAFB + 200 mg/kg BBR; (5) PCAFB + 400 mg/kg BBR; (6) PCAFB + 600 mg/kg BBR; (7) PCOTA + 200 mg/kg BBR; (8) PCOTA + 400 mg/kg BBR; and (9) PCOTA + 600 mg/kg BBR. Berberine was purchased from Bulk Supplement Factory (USA). The temperature was maintained at 32°C during the first week and then reduced by 3°C per week thereafter. Continuous lighting was used through 42 d. The corn-soybean meal diet (Table 1) met or exceeded the nutritional requirements recommended by the NRC (1994) and were based on recommendations of the primary breeder (Aviagen, 2014) for starter (1–10 d), grower (11–24 d), and finisher (25–42 d) phases. Water and feed were available ad libitum. The analyzed mycotoxin levels (AFB and OTA) in the diets measured by high-performance liquid chromatography are given in Table 2. Mortality was recorded, and at the end of each diet phase (days 10, 24, and 42), pen weight and feed consumption were determined and average daily gain (ADG), average daily feed intake (ADFI), and mortality-corrected FCR were calculated.
Table 1.
Composition of the experimental basal diets (%, as fed basis unless otherwise stated).
| Item (g/kg as fed) | Starter (1–10 d) | Grower (11–24 d) | Finisher (25–42 d) |
|---|---|---|---|
| Corn | 49.77 | 49.40 | 54.31 |
| Soybean | 37.94 | 41.32 | 35.98 |
| Fishmeal | 5.00 | 0.00 | 0.00 |
| Fat | 3.66 | 5.45 | 6.30 |
| Dicalcium phosphate | 1.23 | 1.38 | 1.21 |
| Oyster shell | 1.18 | 1.23 | 1.12 |
| Multivitamin1 | 0.25 | 0.25 | 0.25 |
| Multimineral2 | 0.25 | 0.25 | 0.25 |
| Salt | 0.40 | 0.40 | 0.40 |
| DL-methionine | 0.27 | 0.27 | 0.15 |
| L-lysine | 0.05 | 0.05 | 0.03 |
| Total | 100 | 100 | 100 |
| Calculated analysis | |||
| Energy (kcal/kgr) | 3,010.00 | 3,100.00 | 3,200 |
| Protein | 23.00 | 21.50 | 19.50 |
| Met + Cys | 0.95 | 0.91 | 0.74 |
| Lysine | 1.35 | 1.19 | 1.00 |
| Tryptophan | 0.30 | 0.29 | 0.26 |
| Calcium | 0.96 | 0.87 | 0.78 |
| Phosphorus | 0.48 | 0.43 | 0.39 |
Provided per kilogram of diet: vitamin A, 44,000 IU; vitamin D3, 7,200 IU; vitamin E (all-rac-α-tocopherol), 1.4 mg; riboflavin, 6 mg; nicotinamide, 80 mg; choline chloride, 1,200 mg; calcium pantothenate, 20 mg; pyridoxine·HCl, 8 mg; biotin, 0.08 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.032 mg.
Provided per kilogram of diet: Cu (from copper sulfate), 8.0 mg; Mn (from manganese sulfate), 64.5 mg; Zn (from zinc oxide), 33.8 mg; I (from calcium iodate), 3.5 mg; Se (from sodium selenite), 0.8 mg.
Table 2.
Analyzed mycotoxins levels in contaminated diets (μg/kg diet).
| Item | Starter (1–10 d) | Grower (11–24 d) | Finisher (25–42 d) |
|---|---|---|---|
| NC1 | 25 | 19 | 35 |
| AFB contaminated diets | 1,908 | 1,930 | 1,990 |
| OTA contaminated diets | 2,010 | 1,960 | 1,935 |
Abbreviations: AFB, aflatoxin B1; OTA, ochratoxin A.
NC, negative control diet with no additives.
Serum Biochemical Parameters
At 42 d of age, 2 chicks weighing close to the pen average BW (mean ± 1 SD) were selected from each of the 4 replicate pens for each dietary treatment (8 birds/treatment), and blood samples were taken from the brachial vein to determine circulating levels of several biochemical parameters. Serum creatinine (CR), urea (UR), malondialdehyde (MDA), and aspartate aminotransferase (AST), lactate dehydrogenase, alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), superoxide dismutase (SOD), and glutathione peroxidase (GPX) activities were measured using commercial ELISA kits following the manufacturer's protocol (Pars Azmoon, Iran) in an autoanalyzer (Chem 200, Gesan, Italy).
Histological Analyses
After blood collection, birds were humanely euthanized for tissue collection. The liver was removed and weighed, and a fragment (0.5 cm2) of the right lobe was collected for histology. A fragment (0.5 cm2) was collected from the center of the ileum for histology. Liver and ileum fragments were placed in 10% formalin and stored at ambient temperature for 72 h, after which samples were rinsed with distilled water transferred to a tissue processing cassette (approximately 2 × 3 cm), and placed into a tissue automatic processor (MK1110, Pooyan Teb Khadem, Iran) for dehydration (50, 70, 80, 90, and 100% ethanol), clearing (xylene for 300 min), and paraffin embedding (56–58% paraffin for 150 min in first step and 200 min in second step). At the end of tissue processing, a paraffin block was prepared for each fixed specimen. From each block, a 6-sided transverse section was prepared with a semiautomatic microtome (MK1120, Pooyan Teb Khadem, Iran) and stained with hematoxylin and eosin to determine tissue properties using light microscopy (Olympus BX51) and Image Pro Plus v 4.5 software (Sakamoto et al., 2000).
Previously described methods (Ishak et al., 1995) were used to evaluate liver lesions. Samples were examined for fat degeneration, necrosis of liver cells, bile duct proliferation, fibrosis, cellular infiltration, nucleus size, and hyperplasia. Each of the lesions or cases was given a score of 0 to 3 in terms of lesion severity (0 = no lesion, 1 = mild, 2 = moderate, 3 = severe). Scores of each of the above categorized lesions were summed for each sample to obtain the final score.
Ileum tissue traits evaluated included villi height, villi width, and crypt depth. The distance between the tip of each villi and the junction with the crypt was considered as the height of the villi, and the width was measured at the widest point. The depth of the crypt was measured from the villi-crypt intersection to the basement membrane (Sakamoto et al., 2000; Laudadio et al., 2012).
Statistical Analysis
As 2 birds were sampled from each pen, the average values of all parameters were used for statistical analysis so that the experimental unit was pen (n = 4). All data were analyzed using SAS software and the general linear model. The mean of experimental groups was compared using the Tukey method at 5% probability level (P < 0.05).
Results
Growth Performance
The effect of different experimental treatments on ADG (g/bird/d) of chickens during different diet phases and throughout the whole experimental period is presented in Table 3. In the starter phase (1–10 d), birds fed PCAFB had lower ADG than those in the NC treatment (P < 0.05) but did not differ from birds in the remaining treatments. In the grower phase (11–24 d), both PCAFB- and PCOTA-fed birds had reduced ADG compared with NC-fed birds (P < 0.05). During this period, supplementing PCAFB and PCOTA diets with 400 or 600 mg/kg BBR partially or completely reversed this effect so that ADG was greater than that in PCAFB and PCOTA diets alone and not different from the NC diet in the case of PCAFB at both levels and PCOTA at the highest level (P < 0.05). In the finisher phase (25–42 d), PCAFB and PCOTA treatments had reduced ADG compared with NC (P < 0.05) and increasing the level of BBR in contaminated diets partially reversed this effect. Supplementation at all levels increased ADG compared with birds fed PCAFB and PCOTA diets, but they still had a smaller ADG than NC birds (P < 0.05). The results of the whole period (1–42 d) showed that PCAFB and PCOTA diets reduced ADG of birds compared to the NC diet (P < 0.05), and although supplementation with all levels of BBR partially reversed this, adding 600 mg/kg BBR was most effective (P < 0.05).
Table 3.
Effect of dietary aflatoxin B1 (AFB) or ochratoxin A (OTA) contamination with or without berberine (BBR) supplementation on average daily gain (ADG; g/bird/d).
| Treatment | Starter (1–10 d) | Grower (11–24 d) | Finisher (25–42 d) | Total (1–42 d) |
|---|---|---|---|---|
| NC1 | 20.16a | 59.53a | 66.68a | 53.22a |
| PCAFB1 | 17.14b | 32.14d | 30.55f | 27.89f |
| PCOTA1 | 17.31a,b | 27.23d | 28.22f | 25.29f |
| PCAFB+200 BBR2 | 18.35a,b | 36.67c,d | 47.56c,d | 36.97d,e |
| PCAFB+400 BBR2 | 18.00a,b | 50.11a,b | 47.72c,d | 41.44c,d |
| PCAFB+600 BBR2 | 19.69a,b | 56.19a | 57.36b | 48.00b |
| PCOTA+200 BBR2 | 17.37a,b | 37.87c,d | 39.42e | 33.65e |
| PCOTA+400 BBR2 | 19.20a,b | 44.01b,c | 40.79d,e | 36.72d,e |
| PCOTA+600 BBR2 | 19.03a,b | 51.16a,b | 59.14b,c | 43.93b,c |
| P-Value | 0.0001 | 0.0001 | 0.0001 | 0.0050 |
| SEM | 0.59 | 2.33 | 1.51 | 1.09 |
a–fSuperscripts within columns indicate significant differences between treatments (P < 0.05).
NC, negative control diet with no additives; PCAFB, NC + 2 ppm AFB (positive control AFB); PCOTA, NC + 2 ppm OTA (positive control OTA).
Berberine supplementation (mg/kg diet).
Table 4 presents the results of the effects of experimental treatments on ADFI (g/bird/d) of chickens during different diet phases and throughout the experiment. In all experimental periods, PCAFB and PCOTA treatments had a lower ADFI than NC (P < 0.05). In the starter phase, BBR supplementation of contaminated diets did not increase ADFI. During the grower period, supplementation of 400 or 600 mg/kg BBR to PCAFB diets completely reversed the reduction so that ADFI was higher than in the PCAFB diet (P < 0.05) but not different from NC (P > 0.05). Similarly, supplementation of BBR at all levels completely reversed the negative effect of OTA contamination on ADFI, and this was increased relative to PCOTA treatment (P < 0.05) and not different from the NC treatment (P > 0.05). During the finisher phase, the addition of different levels of BBR to feeds contaminated with toxins increased feed intake in these treatments compared with PCAFB and PCOTA treatments (P < 0.05), and the at the 600 mg/kg level this did not differ from NC (P > 0.05). During the whole experiment (1–42 d), ADFI was reduced in PCAFB and PCOTA treatments compared with the NC treatment (P < 0.05). Addition of BBR improved ADFI compared with PCAFB and PCOTA diets (P < 0.05), and at the highest level, they also did not differ from NC treatment (P > 0.05).
Table 4.
Effect of dietary aflatoxin B1 (AFB) or ochratoxin A (OTA) contamination with or without berberine (BBR) supplementation on average daily feed intake (ADFI; g/bird/d).
| Treatment | Starter (1–10 d) | Grower (11–24 d) | Finisher (25–42 d) | Total (1–42 d) |
|---|---|---|---|---|
| NC1 | 32.25a | 107.14a | 135.41a | 101.42a |
| PCAFB1 | 24.37b | 63.48c | 69.44d | 56.72c |
| PCOTA1 | 24.00b | 55.80c | 65.83d | 52.53e |
| PCAFB+200 BBR2 | 25.75b | 72.94b,c | 108.33b,c | 76.87d |
| PCAFB+400 BBR2 | 25.12b | 97.41a | 107.77b,c | 84.64b,c,d |
| PCAFB+600 BBR2 | 26.72b | 106.33a | 120.20a,b | 93.32a,b |
| PCOTA+200 BBR2 | 25.25b | 87.76a,b | 91.73c | 74.58d |
| PCOTA+400 BBR2 | 28.25a,b | 98.89a | 97.08c | 81.29c,d |
| PCOTA+600 BBR2 | 26.52b | 104.10a | 115.62b | 90.57a,b,c |
| P-Value | 0.0007 | 0.0001 | 0.0011 | 0.0001 |
| SEM | 1.12 | 4.36 | 3.55 | 2.32 |
a–dSuperscripts within columns indicate significant differences between treatments (P < 0.05).
NC, negative control diet with no additives; PCAFB, NC + 2 ppm AFB (positive control AFB); PCOTA, NC + 2 ppm OTA (positive control OTA).
Berberine supplementation (mg/kg diet).
The effects of experimental treatments on mortality at different ages and the whole experimental period are presented in Table 5. In the starter phase, PCAFB-fed birds had higher mortality than NC-fed birds (P < 0.05), and addition of BBR to the contaminated diets completely or partially reversed this. In the grower period, PCAFB- and PCOTA-fed birds had higher mortality than NC-fed birds (P < 0.05), and supplementation with BBR decreased mortality in birds fed the PCAFB and PCOTA diets (P < 0.05). During the finisher phase, the NC treatment again had lower mortality relative to PCAFB and PCOTA treatments (P < 0.05), and addition of BBR to both contaminated diets reversed the negative effects, with higher levels being more effective (P < 0.05). Cumulatively, the NC treatment had the lowest mortality compared with other treatments (P < 0.05), and in chicks fed PCAFB and PCOTA diets, addition of 600 mg/kg of BBR decreased mortality, so that it was either not different from NC (PCAFB + 600 mg/kg BBR; P > 0.05) or still higher than NC (PCOTA + 600 mg/kg; P < 0.05) but less than PCOTA alone (P < 0.05).
Table 5.
Effect of dietary aflatoxin B1 (AFB) or ochratoxin A (OTA) contamination with or without berberine (BBR) supplementation on mortality (%).
| Treatment | Starter (1–10 days) | Grower (11–24 d) | Finisher (25–42 d) | Total (1–42 d) |
|---|---|---|---|---|
| NC1 | 3.15b | 3.11d | 3.18f | 3.15f |
| PCAFB1 | 5.59a | 12.31a | 15.55a | 11.15a |
| PCOTA1 | 3.92a,b | 9.39b | 15.60a | 9.64b |
| PCAFB+200 BBR2 | 0.00c | 6.25c | 12.37b | 6.20c |
| PCAFB+400 BBR2 | 0.00c | 6.25c | 9.34c | 5.19d |
| PCAFB+600 BBR2 | 0.00c | 3.13d | 6.26e | 3.15f |
| PCOTA+200 BBR2 | 0.00c | 6.27c | 9.28c | 5.18d |
| PCOTA+400 BBR2 | 0.00c | 6.26c | 7.30d | 4.52e |
| PCOTA+600 BBR2 | 3.09b | 3.13d | 6.26e | 4.16e |
| P-Value | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| SEM | 0.35 | 0.06 | 0.08 | 0.12 |
a–cSuperscripts within columns indicate significant differences between treatments (P < 0.05).
NC, negative control diet with no additives; PCAFB, NC + 2 ppm AFB (positive control AFB); PCOTA, NC + 2 ppm OTA (positive control OTA).
Berberine supplementation (mg/kg diet).
The effect of experimental treatments on mortality-corrected FCR is presented in Table 6. In the starter phase, NC-fed birds unexpectedly had the highest FCR (P < 0.05), whereas FCR did not differ among birds fed the other diets. In the grower period, PCAFB- and PCOTA-fed birds control treatments had higher FCR than NC-fed birds (P < 0.05). During this period, supplementation with BBR did not improve FCR in birds fed the PCAFB diet (P > 0.05) and actually worsened FCR in birds fed the PCOTA diet at 200 and 400 mg/kg (P < 0.05). Supplementation of PCOTA diets with 600 mg/kg BBR to PCOTA diets improved FCR relative to the lower levels (P < 0.05), but it was not different from the PCOTA diet alone (P > 0.05). During the finisher phase, the NC treatment had improved FCR relative to PCAFB and PCOTA treatments (P < 0.05). Addition of 600 mg/kg BBR to the PCAFB diet completely reversed the negative effect of feeding the PCAFB diet, so that FCR in this group was improved relative to PCAFB alone (P < 0.05) and not different from the NC diet (P > 0.05). Lower BBR supplementation to this diet did not improve FCR relative to PCAFB only (P > 0.05). Birds fed the PCOTA diet supplemented with 600 mg/kg of BBR had a better FCR than the lower levels (P < 0.05), but this still did not differ from PCOTA alone (P > 0.05). The NC treatment had the lowest FCR compared with other treatments throughout the 1–42 d period. In chicks fed PCAFB diets, addition of 600 mg/kg of BBR improved FCR so that it was not different from NC (P > 0.05) and lower than other PCAFB diets (P < 0.05). Similar to the grower phase, PCOTA diets supplemented with 200 or 400 mg/kg BBR had a worse FCR than PCOTA alone and 600 mg/kg lowered this to levels not different from PCOTA alone (P > 0.05).
Table 6.
Effect of dietary aflatoxin B1 (AFB) or ochratoxin A (OTA) contamination with or without berberine (BBR) supplementation on FCR (g feed intake/g body weight gain).
| Treatment | Starter (1–10 d) | Grower (11–24 d) | Finisher (25–42 d) | Total (1–42 d) |
|---|---|---|---|---|
| NC1 | 1.60a | 1.80c | 2.03c | 1.90c |
| PCAFB1 | 1.42b | 1.97b | 2.27a,b | 2.03b |
| PCOTA1 | 1.38b | 2.05b | 2.33a,b | 2.07b |
| PCAFB+200 BBR2 | 1.40b | 1.99b | 2.27a,b | 2.07b |
| PCAFB+400 BBR2 | 1.39b | 1.94b,c | 2.25b | 2.04b |
| PCAFB+600 BBR2 | 1.35b | 1.89b,c | 2.09c | 1.94c |
| PCOTA+200 BBR2 | 1.45a,b | 2.31a | 2.32a,b | 2.21a |
| PCOTA+400 BBR2 | 1.47a,b | 2.24a | 2.37a | 2.21a |
| PCOTA+600 BBR2 | 1.39b | 2.03b | 2.21b | 2.06b |
| P-Value | 0.0079 | 0.0001 | 0.0001 | 0.0001 |
| SEM | 0.03 | 0.03 | 0.02 | 0.01 |
a–cSuperscripts within columns indicate significant differences between treatments (P < 0.05).
NC, negative control diet with no additives; PCAFB, NC + 2 ppm AFB (positive control AFB); PCOTA, NC + 2 ppm OTA (positive control OTA).
Berberine supplementation (mg/kg diet).
Serum Biochemistry
The effect of different treatments on the biochemistry of broiler serum at 42 d of age is presented in Table 7. Both PCAFB and PCOTA diets increased serum CR values, whereas only the PCAFB diet increased serum UR, as compared with the NC diet (P < 0.05). Supplementation of 600 mg/kg BBR and 400 or 600 mg/kg BBR to PCAFB diets completely reversed effects on CR and UR, respectively, so that levels in these treatments were lower than in the positive control diets (P < 0.05) and not different from the NC diet (P > 0.05). Adding 400 or 600 mg/kg BBR to PCOTA diets normalized serum CR also, and levels in birds fed these diets were reduced from PCOTA alone (P < 0.05) to levels not different from the NC treatment (P > 0.05). There was no influence of BBR supplementation at any level on serum UR in birds fed PCOTA diets (P > 0.05).
Table 7.
Effect of dietary aflatoxin B1 (AFB) or ochratoxin A (OTA) contamination with or without berberine (BBR) supplementation on serum blood biochemistry at 42 d of age.
| Treatment | CR (mg/dL) | UR (mg/dL) | AST (mg/dL) | ALT (mg/dL) | GGT (mg/dL) | MDA (U/mg protein) | SOD (U/mg protein) | GPX (U/mg protein) |
|---|---|---|---|---|---|---|---|---|
| NC1 | 0.270e | 4.00b | 183.25c | 7.25c | 17.15c | 2.08c | 248.75a | 48.00a |
| PCAFB1 | 0.347a,b | 6.00a | 280.30a | 11.50a | 30.30a | 2.35a | 217.50c | 28.75d |
| PCOTA1 | 0.380a | 5.50a,b | 264.17a | 12.25a | 27.30a,b | 2.33a | 220.00b,c | 27.25d |
| PCAFB+200 BBR2 | 0.340a,b,c | 4.50a,b | 230.70b | 9.75a,b,c | 24.60b | 2.27a,b | 228.25b,c | 30.00c,d |
| PCAFB+400 BBR2 | 0.345b,c,d,e | 4.25b | 196.07c | 8.08b,c | 20.17c | 2.23a,b | 227.00b,c | 31.50c,d |
| PCAFB+600 BBR2 | 0.287c,d,e | 4.26b | 183.90c | 7.50b,c | 17.47c | 2.17b,c | 227.75b,c | 38.00b |
| PCOTA+200 BBR2 | 0.330a,b,c,d | 5.25a,b | 196.87c | 10.25a,b | 24.17b | 2.28a,b | 221.75b,c | 30.50c,d |
| PCOTA+400 BBR2 | 0.305b,c,d,e | 4.25b | 189.72c | 8.05b,c | 19.72c | 2.24a,b | 226.75b,c | 31.75c,d |
| PCOTA+600 BBR2 | 0.282d,e | 4.09b | 186.97c | 8.00b,c | 19.47c | 2.23a,b | 230.00b | 35.00b,c |
| P-Value | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0009 | 0.0001 | 0.0005 | 0.0001 |
| SEM | 0.011 | 0.0346 | 4.714 | 0.585 | 0.79 | 0.02 | 2.52 | 1.21 |
a–eSuperscripts within columns indicate significant differences between treatments (P < 0.05).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, creatinine; GGT, gamma-glutamyl transferase; GPX, glutathione peroxidase; MDA, malondialdehyde; SOD, superoxide dismutase; UR, urea.
NC, negative control diet with no additives; PCAFB, NC + 2 ppm AFB (positive control AFB); PCOTA, NC + 2 ppm OTA (positive control OTA).
Berberine supplementation (mg/kg diet).
Serum levels of AST, ALT, and GGT enzyme activities in PCAFB and PCOTA treatments were higher than in the NC treatment (P < 0.05), and supplementation with BBR at multiple levels partially or fully reversed these effects. Addition of 200 mg/kg BBR to PCAFB diet reduced serum AST activity as compared with PCAFB alone, but this activity was still elevated compared with the NC diet (P < 0.05). Addition of higher levels of BBR to PCAFB diets and all 3 levels of BBR to PCOTA reduced AST activity to levels not different from that in the NC group (P > 0.05). Supplementation of 400 or 600 mg/kg BBR to both PCAFB and PCOTA diets reversed the increase in ALT activity, so levels were not different from birds fed the NC diet. Activity of GGT was reduced to intermediate levels in both PCAFB and PCOTA groups when these diets were supplemented with 200 mg/kg BBR, as compared with the positive control or NC diets (P < 0.05). Higher amounts of BBR supplementation to both PCAFB and PCOTA groups reduced GGT activity so that it was not different from the NC group (P > 0.05).
Levels of serum MDA were elevated, whereas SOD and GPX activities were reduced, in PCAFB- and PCOTA-fed birds as compared with NC-fed birds (P < 0.05). Only 600 mg/kg BBR addition to PCAFB diets partially restored levels of MDA and GPX activity so they were intermediate between PCAFB and NC diets (P < 0.05). Similarly, only PCOTA diets supplemented with 600 mg/kg BBR partially normalized GPX activity to levels between PCOTA and NC diets (P < 0.05). Birds fed PCOTA diets with all levels of BBR still had higher MDA in serum than NC birds (P < 0.05), and these were not different than birds fed PCOTA alone (P > 0.05). Likewise, no level of BBR supplementation influenced effects of PCAFB and PCOTA diets on SOD activity (P > 0.05), and levels remained lower than those in birds fed the NC diet (P < 0.05).
Liver Histopathology
Changes in the liver of chickens fed PCAFB and PCOTA diets indicated degeneration, which was seen as cellular swelling in hepatocytes, fatty degeneration, and vascular changes (Figure 1). The central veins were dilated and damaged in some areas. Around the portal vein, some areas of tissue exhibited mild vacuolar degeneration. Fat accumulation in the liver was seen as specific vacuoles in the cytoplasm of birds fed the PCAFB diet, which can lead to yellow pigmentation and liver enlargement. The influence of dietary treatment on relative liver weight (%BW) and severity of liver tissue lesions are presented in Table 8. The highest relative weight of the liver was in positive control treatments, which had a significant difference with negative control treatments (P < 0.05). Adding 600 mg/kg of BBR to the AFB-contaminated diet reduced relative liver weight compared to PCAFB (P < 0.05). The use of BBR in AFB-contaminated diets significantly reduced the rate of pathological liver lesions compared with a positive control (P < 0.05), but there were no significant differences between different levels of BBR (P > 0.05). In the treatment with OTA plus 600 mg/kg of BBR, decreased pathological liver lesions were observed compared with the positive controls (P < 0.05).
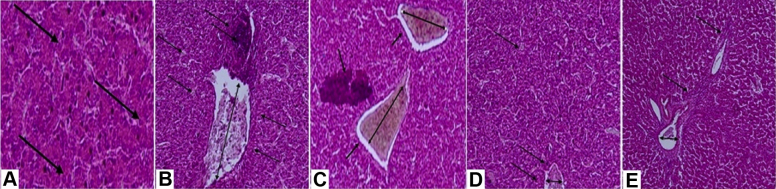
Figure 1.
Liver histopathology in chickens fed diets contaminated with aflatoxin B1 (AFB) or ochratoxin A (OTA) in the absence and presence of (BBR) supplementation. Tissues were hematoxylin and eosin stained and imaged at 200X magnification. (A) Negative control (NC) diet containing no additives with normal hepatocytes (arrows). (B) Positive control diet contaminated with AFB (PCAFB). Evidence of necrosis (single arrows in dark area, top center), inflammation and infiltration of heterophils and lymphocytes around portal vein (single arrows around portal vein pointing to white spots, left and right sides), and enlargement and congestion of the portal vein (double-headed arrow, center) is apparent. (C) Positive control diet contaminated with OTA (PCOTA). Evidence of necrosis (single arrow in dark area, left center), inflammation and infiltration of heterophils and lymphocytes around portal vein (single arrows around portal vein pointing to white spots, top right and center), and enlargement and congestion of the portal vein (double-headed arrow, top right and center) is apparent. (D) PCAFB +600 mg/kg BBR. Inflammation around the portal vein (single arrows, bottom center), diameter of the portal vein (double-headed arrow, bottom center), and fatty deposits (single arrow, top left) are reduced. (E) PCOTA +600 mg/kg BBR. Inflammation around the portal vein (single arrows, center and top right) and diameter of the portal vein (double-headed arrow, bottom left) are reduced.
Table 8.
Effect of dietary aflatoxin B1 (AFB) or ochratoxin A (OTA) contamination with or without berberine (BBR) supplementation on liver weight and lesion score at 42 d of age.
| Treatment | Weight (% body weight) | Lesion score |
|---|---|---|
| NC1 | 1.93c | 1.25e |
| PCAFB1 | 2.83a,b | 6.50a |
| PCOTA1 | 2.90a | 6.00a,b |
| PCAFB+200 BBR2 | 2.34a,b,c | 4.75b,c,d |
| PCAFB+400 BBR2 | 2.26a,b,c | 4.50c,d |
| PCAFB+600 BBR2 | 2.14b,c | 3.75d |
| PCOTA+200 BBR2 | 2.64a,b,c | 5.50a,b,c |
| PCOTA+400 BBR2 | 2.54a,b,c | 5.00b,c,d |
| PCOTA+600 BBR2 | 2.49a,b,c | 4.25c,d |
| P-Value | 0.0003 | 0.0001 |
| SEM | 0.155 | 0.304 |
a–dSuperscripts within columns indicate significant differences between treatments (P < 0.05).
NC, negative control diet with no additives; PCAFB, NC + 2 ppm AFB (positive control AFB); PCOTA, NC + 2 ppm OTA (positive control OTA).
Berberine supplementation (mg/kg diet).
Ileal Micromorphometry
The effect of experimental treatments on microarchitecture of the ileum is presented in Table 9 and Figure 2. Villus height, villus width, crypt depth, and villus height:crypt depth ratio in PCAFB and PCOTA treatments were all lower than in the NC diet (P < 0.05), and addition of BBR to the positive control diets ameliorated some of these effects. For villus height, 400 and 600 mg/kg BBR added to PCAFB diets increased villus height relative to PCAFB alone (P < 0.05) and 400 mg/kg BBR was not different from NC diet (P > 0.05). All levels of BBR supplementation to PCOTA diets increased villus height relative to PCOTA alone (P < 0.05), and at the highest level, this was not different from NC (P > 0.05). The highest level of BBR (600 mg/kg) increased villus width and crypt depth when added to PCAFB diets (P < 0.05); otherwise, supplementation did not have an effect relative to positive control diets alone (P > 0.05). Addition of 400 mg/kg BBR to the PCAFB diet or 200 and 400 mg/kg BBR to the PCOTA diet increased villus height:crypt depth ratio above contaminated diets alone (P < 0.05). These changes resulted in villus height:crypt depth ratios that were numerically higher but not different than the NC diet (P > 0.05). The epithelial cells in the apical region of ileum villus appeared to be shedding in the PCAFB and PCOTA groups (Figures 2B and 2C). No pathological changes were observed in the tissues examined in NC, PCAFB +600 mg/kg BBR, and PCOCT +600 mg/kg BBR group during the experiment (Figures 2A, 2D, and 2E), demonstrating further the protective effect of this level of BBR supplementation.
Table 9.
Effect of aflatoxin B1 (AFB) or ochratoxin A (OTA) contamination with or without berberine (BBR) supplementation on ileum micromorphometry.
| Treatment | Villus height (μm) | Villus width (μm) | Crypt Depth (μm) |
Ratio (villus height: Crypt Depth) |
|---|---|---|---|---|
| NC1 | 122.25a | 45.00a | 38.25a | 3.20a,b |
| PCAFB1 | 83.50c | 31.75d | 28.25c | 2.24c |
| PCOTA1 | 64.75c | 33.75c,d | 27.00c | 2.39c |
| PCAFB+200 BBR2 | 76.00c | 34.00c,d | 28.75c | 2.64b,c |
| PCAFB+400 BBR2 | 110.50a,b | 35.00b,c,d | 29.50b,c | 3.74a |
| PCAFB+600 BBR2 | 103.75b | 39.00b | 33.75b | 3.07c,d |
| PCOTA+200 BBR2 | 103.00b | 35.00b,c,d | 28.50c | 3.61a |
| PCOTA+400 BBR2 | 114.50b | 37.25b,c | 29.50b,c | 3.88a |
| PCOTA+600 BBR2 | 113.25a,b | 38.25b,c | 31.25b,c | 3.63a,b,c |
| P-Value | 0.0001 | 0.0008 | 0.0100 | 0.0001 |
| SEM | 3.79 | 0.99 | 0.79 | 0.13 |
a–cSuperscripts within columns indicate significant differences between treatments (P < 0.05).
NC, negative control diet with no additives; PCAFB, NC + 2 ppm AFB (positive control AFB); PCOTA, NC + 2 ppm OTA (positive control OTA).
Berberine supplementation (mg/kg diet).
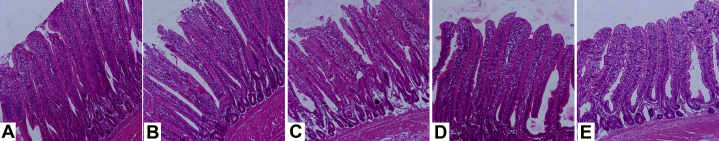
Figure 2.
Histological structures of ileum at 42 d of age. Tissues were hematoxylin and eosin stained and imaged at 200X magnification. (A) Negative control (NC) diet containing no additives with normal epithelial cells in the apical region of villi. (B) Positive control diet contaminated with AFB (PCAFB). The apical region of epithelial cells was shedding and the villus height was decreased. (C) Positive control diet contaminated with OTA (PCOTA). Similar to image B, the villus height was decreased and apical region of epithelial cells were shedding. (D) PCAFB +600 mg/kg BBR. Cell destruction around apical region was less and the height of villus was longer than the PCAFB group. (E) PCOTA +600 mg/kg BBR. Destruction around the apical region of ileum villi was less than PCOTA and the height of villi was more than the PCOTA diet.
Discussion
Growth Performance
There is a consensus among researchers that weight loss, reduced feed intake, and increased FCR occur in broilers fed fungal toxin-contaminated diets. Reports indicate that marked changes in internal organs appear at levels of contamination above 0.5 mg/kg AFB (Yunus et al., 2011; Chen et al., 2014; Naseem et al., 2018). Weight loss due to AFB intake is attributed to reduced protein production, impaired nutrient absorption, and impaired production and secretion of digestive enzymes (Yunus et al., 2011). In addition, AFB decreases activity of some pancreatic enzymes, including amylase and trypsin, which are essential for the digestive process (Richard et al., 1983). Therefore, reduced dry matter and protein digestibility and reduced nutrient availability can lead to weight loss caused by aflatoxin-contaminated diets. Because FCR is influenced by 2 factors, feed intake and BW gain, the results of FCR were consistent with the results of feed intake and BW gain, respectively. There was a significant difference in FCR between chicks fed the NC diet and chicks fed positive control diets contaminated with AFB and OTA. The poor FCR in chickens fed diets contaminated with toxins was mainly due to the low BW gain and to a lesser extent the reduced feed intake in this group. Aflatoxin-contaminated foods reduce activity of important enzymes in the digestion of carbohydrates, proteins, lipids, and nucleic acids in broilers (Vieira, 2003). In particular, aflatoxin inhibits digestibility of fat by reducing both enzyme activity and bile acid production necessary for their digestion and absorption. Although it was not measured in the current experiment, poor nutrient digestibility may have contributed to the reduction in BW gain in birds fed diets contaminated with AFB or OTA. Reduced feed intake observed here due to diet contamination with AFB is in line with reports by most researchers. Tedesco et al. (2004) reported that diets infused with 0.8 mg/kg aflatoxin reduced feed intake during the experiment. Chand et al. (2011) stated that feeding broilers infected with 80 μg/kg aflatoxin reduced feed intake, BW, and FCR.
Addition of different levels of BBR to chickens challenged with AFB and OTA reduced the negative effect of these toxins on broiler feed intake. It has been shown that BBR minimizes the impact of aflatoxin by various mechanisms. In chicks receiving BBR, B-cell lymphoma 2 (BCL2) family of conserved proteins can inhibit mitochondrial permeability and release of apoptosis proteins from the mitochondria, ultimately inhibiting apoptosis or necrosis (Cory and Adams, 2005). By contrast, proapoptotic proteins such as BCL-2-associated X (BAX) can induce apoptosis or necrosis by mitochondrial degradation (Cory and Adams, 2005). Aflatoxin B1 inhibits expression of BCL2 and stimulates expression of BAX proteins (Yang et al., 2013), and BBR has been shown to reduce deleterious effects of AFB by inhibiting BAX expression and increasing expression of BCL2-protecting proteins (Zhou et al., 2009). Another explanation for the improvement in production and reduction in mortality rate could be the presence of useful substances in BBR and its antioxidant and antimicrobial effects that inhibit pathogenic and nonbeneficial gastrointestinal microbes, allowing it to act as a protector in challenging conditions (Cernakova et al., 2002). In general, BBR may have pharmacological activities, including inhibition of microorganism metabolic processes, inhibition of intestinal gram-negative bacteria (Cernakova et al., 2002), inhibition of intestinal ion secretion (Feng et al., 2019), inhibition of intestinal smooth muscle contraction (Feng et al., 2010; Gu et al., 2011), reduction of inflammation (Chen et al., 2016), and inhibition of cytokine storms (Zhou et al., 2009) that decrease severity of aflatoxicosis and ochratoxicosis in chicks fed diets contaminated with toxins.
Serum Biochemistry
Feeding broilers AFB-contaminated diets significantly increased detection of liver enzymes in the serum. Increased liver enzyme activity was reported as an indicator of serological susceptibility in poisoning and kidney disease (Shi et al., 2006). Serum ALT, AST, and alkaline phosphatase (ALP) levels are specific for liver damage and indicative of degenerative changes in liver tissue. They are used as a marker for changes in cell viability and cell membrane permeability resulting from liver damage (Ortatatli et al., 2005). These enzymes are normally found within hepatocytes, but with damage to the hepatocytes and their apoptosis due to toxins, these enzymes are released into the bloodstream and their levels in circulation increase (Fan et al., 2015). When the liver becomes damaged, hepatocytes, liver stellate cells, sinusoidal endothelial cells, and Kupffer cells also produce excessive amounts of inflammatory factors. In this study, evaluation of serum ALT, AST, and GGT indicated that their levels were elevated in serum of birds fed diets contaminated with AFB and OTA and that BBR supplementation partially or completely reversed these effects. This suggests that BBR can ameliorate effects of liver damage due to the toxins. Consistent with these results, a carbon tetrachloride-induced liver failure model has also been shown to inhibit substantially the increase in activity of ALT, AST, and ALP enzymes (Domitrovic and Jakovac, 2010). Studies have shown that BBR inhibits Th17 differentiation by activating extracellular signal-regulated kinase 1/2 activity. In addition, BBR decreased interleukin (IL)-17A production by regulating mitogen-activated protein kinase activity (Cui et al., 2009). Furthermore, a study of palmitate-stimulated HepG2 cells, a human liver cell line, showed that BBR reduced release of inflammatory factors IL-6 and tumor necrosis factor alpha (TNF-α) from these cells, which significantly increased under palmitate stimulation (Lou et al., 2011). In addition, it has been reported that exposure of the liver to toxins causes oxidative stress that can lead to release of TNF-α from Kupffer cells and damaged hepatocytes, thereby enhancing inflammation and liver damage. Berberine suppressed the proinflammatory cascade initiated by TNF-α and liver injury (Domitrovic et al., 2011). In accordance with the evidence provided by various researchers about the inflammatory factors released by the liver due to the toxins and the anti-inflammatory activity of BBR, it can be said that the anti-inflammatory effect of BBR could reduce the release of inflammatory factors such as IL-1, IL-6, and TNF-α. It blocks hepatocyte damage by the toxins, thereby preventing the inflammatory responses initiated by these factors that cause further tissue damage to the liver.
Creatinine is a byproduct of normal muscle metabolism that is freely filtered in kidney glomeruli and normally has no tubular resorption. Therefore, the amount of CR in the blood depends on the glomerular filtration rate. Normal levels of CR and UR in plasma indicate normal kidney function and it has been shown that AFB and OTA increased serum CR and blood urea nitrogen concentrations by impairing renal function and thereby decreasing glomerular filtration rate (Martinez-de-Anda et al., 2010). Evaluation of plasma CR and UR in diets containing BBR and AFB or OTA showed that the use of BBR reduced CR and UR levels compared with the PCAFB and PCOTA diets without it. Consistently, results of the beneficial effects of BBR on renal function in rats with diabetic nephropathy have shown that eating BBR reduces blood urea nitrogen and serum CR levels, suggesting improved kidney function (Fan et al., 2019).
Aflatoxins, during metabolic processing in the liver, produce intracellular reactive oxygen species (ROS) such as superoxide anion, hydrogen peroxide, and hydroxyl radicals that cause oxidative damage. Important antioxidants such as SOD and GPX remove ROS from cells (Wills, 1966). Oxidative stress occurs when ROS levels exceed the capacity of the cell's antioxidant defense system. Aflatoxin B1 can produce hepatic lipid peroxidation and damage the antioxidant system by producing free radicals and altering the concentration of MDA (Yang et al., 2012), an end-product of lipid peroxidation that increases during oxidative stress and an essential indicator of antioxidant ability. Various studies have reported an increase in MDA concentration and a decrease in SOD and GPX concentrations in chickens fed feed contaminated with aflatoxin and ochratoxin (Fouad et al., 2019). Aflatoxin B1 disrupts the digestion and absorption of nutrients and metabolism of fats by damaging the digestive tract, liver, and pancreas. As a result, the absorption of vitamins that have antioxidant properties, such as vitamins C, E, and A, could be reduced so that oxidation within tissues abnormally increases (Decoudu et al., 1992). Improvement of MDA concentration in birds receiving BBR demonstrates that it could be a useful protective tool against oxidative damage due to aflatoxicosis and ochratoxicosis.
Liver Histopathology
In the histopathological examination of liver from birds fed PCAFB and PCOTA diets, apoptosis of hepatocytes with vascular congestion in the central vein and infiltration of inflammatory cells, including lymphocytes into the portal space, were observed. Several different death factors, such as transforming growth factor beta 1, apoptosis antigen 1/95 ligand, Fas receptor, and TNF-α, have been known to induce active cell death in the liver (Schulte-Hermann et al., 1997). Liver sinusoidal cells have been shown to produce apoptotic factors such as IL-1, TNF-α, transforming growth factor beta 1, and IL-6 in pathological conditions such as inflammation and cell metastasis (Dini et al., 1995). Oxidative stress also activates the mitochondrial pathway of apoptosis in the liver by increasing proapoptotic signal molecules such as BAX and decreasing antiapoptotic proteins such as BCL2. Lowering BCL2 and simultaneously stimulating an increase in BAX reduces the ratio of BCL2 to BAX. The decrease in the ratio of BCL2 to BAX results in the activation and translocation of proapoptotic signal molecules to the mitochondria. Transfer of these molecules to mitochondria by opening the mitochondrial permeable transfer pore releases apoptotic inducer proteins such as cytochrome C into the cytosol. Cytochrome C activates caspase peptidases, which eventually cause apoptosis. Inhibition of oxidative stress by hydroxyl radical sweepers reduces the mitochondrial pathway of apoptosis (Guha et al., 2006). In addition, previous studies have shown that toxins reduce antioxidant enzymatic activity, increase neutrophil and lymphocyte accumulation, and increase oxidative stress and lipid peroxidation in liver tissue (Hameed et al., 2017; Fouad et al., 2019). Thus, toxins can induce apoptosis through inflammatory mechanisms, including neutrophil migration, increased expression of IL-6 and TNF-α, as well as oxidative stress. Histopathological examination of the liver tissue showed that application of 600 mg/kg BBR to PCACT-fed birds reduced apoptosis in hepatocytes. Research on hepatocytes has shown that BBR decreases release of IL-6 and TNF-α from palmitate-stimulated HepG2 cells (Lou et al., 2011). Therefore, it can be speculated that the anti-inflammatory activity of BBR was able to reduce lesions caused by aflatoxin by reducing inflammatory cytokine production.
Ileum Micromorphometry
The villus height decreased in the ileum area of broilers fed diets infused with AFB or OTA compared with the controls. In another study, villus height was reduced with aflatoxin contamination (Aboutalebi, 2013). These observations may be influenced by the gut microbial population, which could have adverse effects on the intestinal surface area for absorption. Shorter and thinner villi in chickens fed aflatoxin are due to impaired protein synthesis and reduced epithelial cell proliferation (Yang et al., 2012; Chen et al., 2016). The results of the present study are consistent with the reviews of Awad et al. (2006) and Wu et al. (2015) that showed the harmful effects of mycotoxins on intestinal morphology. However, a recent study by Chen et al. (2016) reported that villus height and crypt depth in chickens fed 1.5 mg/kg aflatoxin-contaminated feed did not differ significantly from the control treatment.
Berberine prevents oxidative damage by reducing lipid peroxidation and increasing cellular antioxidants (Domitrovic et al., 2011). As a result, it protects intestinal epithelial cells against oxidative factors caused by toxins and increases the growth of epithelial cells. In addition, BBR in rats prevented intestinal mucosal injury caused by LPS-mediated endotoxemia and improved intestinal mucosa, likely through intestinal glutamine transport and increased glutaminase activity (Niu et al., 2011). Therefore, the positive effect of BBR on ileum micromorphometry in chickens fed contaminated diets could be attributed to the protective role of BBR. In this study, AFB and OTA appeared to destroy cells in the upper villi area. This might be due to disrupted protein synthesis and cell proliferation, as has been shown previously (Yang et al., 2013). Morphological changes observed here were similar to several previous studies. For example, Zhang et al. (2016) found that 0.3 mg/kg AFB could induce shedding of epithelial cells on the tip of the jejunal villus; Wang et al. (2019) reported that 0.6 mg/kg AFB treatment–induced histopathological injuries in intestinal villi; Solcan et al. (2015) indicated that high amounts of OTA cause destruction and injury of the intestinal mucosa of chickens, and crypt hyperplasia related to villous atrophy; and Ruan et al. (2019) reported ducks fed diets contaminated with OTA (2 mg/kg feed) showed villous blunting and epithelial denudation along with a related decrease in villous length. Evidence has shown that BBR can improve intestinal physiological function by reducing inflammatory factors (Chen et al., 2020), reducing oxidative stress (Cui et al., 2018), and regulating gastrointestinal microbiota (Li et al., 2020). Matsumoto et al. (2019) found that BBR increased the number of goblet cells, one of the critical secretory cells in the intestine. Together, these findings indicate the role of BBR in maintaining the replacement of intestinal epithelial cells and balance of the intestinal environment (Zhang et al., 2021), all which might contribute to the reduced destructive effects of toxins on villi in the ileum.
Supplementation of AFB- and OTA-contaminated diets with BBR improved growth performance and reduced vascular congestion, inflammatory cell infiltration into the liver portal space, and hepatocyte apoptosis. Furthermore, it protected against toxin-induced damage to the ileal epithelium. These findings suggest that BBR supplementation could be a useful dietary strategy to prevent effects of aflatoxicosis and ochratoxicosis in broiler chickens.
Acknowledgments
The authors are grateful for the financial support of the University of Birjand.
Disclosures
The authors declare no conflicts of interest.
References
- Aboutalebi N. Toxic effects of aflatoxin B1 on duodenum tissue. J. Am. Sci. 2013;9:115–117. [Google Scholar]
- Aviagen, W . Aviagen; Huntsville, AL: 2014. Ross 308 Broiler Nutrition Specifications. [Google Scholar]
- Awad W.A., Böhm J., Razzazi-Fazeli E., Ghareeb K., Zentek J. Effect of addition of a Probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological Alterations of intestinal villi of broiler chickens. Poult. Sci. 2006;85:974–979. doi: 10.1093/ps/85.6.974. [DOI] [PubMed] [Google Scholar]
- Bennett J.W., Klich M. Mycotoxins. Clin. Microbiol. Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernakova M., Kostalova D., Kettmann V., Plodova M., Tóth J., Drimal J. Potential antimutagenic activity of berberine, a constituent of Mahonia aquifolium. BMC Complement. Altern. Med. 2002;2:2. doi: 10.1186/1472-6882-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chand N., Muhammad D., Durrani F., Qureshi M., Sahibzada S. Protective effects of Milk Thistle (Silybum marianum) against aflatoxin B1 in broiler chicks. Asian-Australas. J. Anim. Sci. 2011;24:1011–1018. [Google Scholar]
- Chen X., Horn N., Applegate T.J. Efficiency of hydrated sodium calcium aluminosilicate to ameliorate the adverse effects of graded levels of aflatoxin B1 in broiler chicks. Poult. Sci. 2014;93:2037–2047. doi: 10.3382/ps.2014-03984. [DOI] [PubMed] [Google Scholar]
- Chen W., Wei S., Yu Y., Xue H., Yao F., Zhang M., Xiao J., Hatch G.M., Chen L. Pretreatment of rats with increased bioavailable berberine attenuates cerebral ischemia-reperfusion injury via down regulation of adenosine-5'monophosphate kinase activity. Eur. J. Pharmacol. 2016;779:80–90. doi: 10.1016/j.ejphar.2016.03.015. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhang F., Li R., Liu Y., Wang X., Zhang X., Xu C., Li Y., Guo Y., Yao Q. Berberine regulates fecal metabolites to ameliorate 5-fluorouracil induced intestinal mucositis through modulating gut microbiota. Biomed. Pharmacother. 2020;124:1–11. doi: 10.1016/j.biopha.2020.109829. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J.M. Killing cancer cells by flipping the Bcl-2/Bax switch. Cancer Cell. 2005;8:5–6. doi: 10.1016/j.ccr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Cui G., Qin X., Zhang Y., Gong Z., Ge B., Zang Y.Q. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J. Biol. Chem. 2009;284:28420–28429. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H.-X., Hu Y.-N., Li J.-W., Yuan K. ypoglycemic echanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxid. Med. Cell. Longev. 2018:1–3. doi: 10.1155/2018/8930374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoudu S., Cassand P., Daubèze M., Frayssinet C., Narbonne J.F. Effect of vitamin A dietary intake on in vitro and in vivo activation of aflatoxin B1. Mutat. Res. 1992;269:269–278. doi: 10.1016/0027-5107(92)90209-k. [DOI] [PubMed] [Google Scholar]
- Denli M., Perez J.F. Ochratoxins in feed, a risk for animal and human health: control strategies. Toxins (Basel) 2010;2:1065–1077. doi: 10.3390/toxins2051065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devegowda G., Murthy T.N.K. Mycotoxins: their effects in poultry and some practical solutions. In: Diaz D., editor. Mycotoxin Blue Book. Nottingham Univ. Press; Nottingham, UK: 2005. pp. 25–56. [Google Scholar]
- Dini L., Lentini A., Diez G.D., Rocha M., Falasca L., Serafino L., Vidal-Vanaclocha F. Phagocytosis of apoptotic bodies by liver endothelial cells. J. Cell Sci. 1995;108:967–973. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- Domitrovic R., Jakovac H. Antifibrotic activity of anthocyanidin delphinidin in carbon tetrachloride-induced hepatotoxicity in mice. Toxicology. 2010;272:1–10. doi: 10.1016/j.tox.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Domitrovic R., Jakovac H., Blagojevic G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl4-intoxicated mice. Toxicology. 2011;280:33–43. doi: 10.1016/j.tox.2010.11.005. [DOI] [PubMed] [Google Scholar]
- European Union Assessment of dietary intake of ochratoxin A by the population of EU member States.Report of Experts Participating in Task 3.2.7 reports on Tasks for Scientific Cooperation. 2002. http://ec.europa.eu/food/fs/scoop/index_en.html
- Fan J., Zhang K., Jin Y., Li B., Gao S., Zhu J., Cui R. Pharmacological effects of berberine on mood disorders. J. Cell. Mol. Med. 2019;23:21–28. doi: 10.1111/jcmm.13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Zhao L., Ji C., Li X., Jia R., Xi L., Zhang J., Ma Q. Protective effects of Bacillus subtilis ANSB060 on serum biochemistry, histopathological changes and antioxidant enzyme activities of broilers fed Moldy peanut meal naturally contaminated with aflatoxins. Toxins (Basel) 2015;7:3330–3343. doi: 10.3390/toxins7083330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farghali H., Canova N.K., Zakhari S. Hepatoprotective properties of extensively studied medicinal plant active constituents: possible common mechanisms. Pharm. Biol. 2015;53:781–791. doi: 10.3109/13880209.2014.950387. [DOI] [PubMed] [Google Scholar]
- Feng Y., Siu K.Y., Ye X., Wang N., Yuen M.F., Leung C.H., Tong Y., Kobayashi S. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin. Med. 2010;5:33. doi: 10.1186/1749-8546-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Sureda A., Jafari S., Memariani Z., Tewari D., Annunziata G., Barrea L., Hassan S.T.S., smejkal K., Malanik M., Sychrova A., Barreca D., Ziberna L., Mahomoodally M.F., Zengin G., Xu S., Nabavi S.M., Shen A.-Z. Berberine in Cardiovascular and metabolic diseases: from mechanisms to Therapeutics. Theranostics. 2019;9:1923–1951. doi: 10.7150/thno.30787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouad A.M., Ruan D., El-Senousey H.K., Chen W., Jiang S., Zheng C. Harmful effects and control strategies of aflatoxin b1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry. Toxins (Basel) 2019;11:176. doi: 10.3390/toxins11030176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo A., Francesco M. In vitro models to evaluate the capacity of different sequestering agents to adsorb aflatoxins. Ital. J. Anim. Sci. 2010;9:109–116. [Google Scholar]
- Gu L., Li N., Gong J., Li Q., Zhu W., Li J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J. Infect. Dis. 2011;203:1602–1612. doi: 10.1093/infdis/jir147. [DOI] [PubMed] [Google Scholar]
- Guha M., Kumar S., Choubey V., Maity P., Bandyopadhyay U. Apoptosis in liver during malaria: role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006;20:1224–1226. doi: 10.1096/fj.05-5338fje. [DOI] [PubMed] [Google Scholar]
- Hameed M.R., Khan M.Z., Saleemi M.K., Khan A., Akhtar M., Hassan Z.-u., Hussain Z. Study of ochratoxin A (OTA)-induced oxidative stress markers in broiler chicks. Toxin. 2017;36:270–274. [Google Scholar]
- Iqbal S.Z., Nisar S., Asi M.R., Jinap S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control. 2014;43:98–103. [Google Scholar]
- Ishak K., Baptista A., Bianchi L., Callea F., De Groote J., Gudat F., Denk H., Desmet V., Korb G., MacSween R.N., Philips M.J., Portmann B.G., Paulsen H., Scheuer P.J., Schmid M., Thaler H. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Jindal N., Mahipal S.K., Mahajan N.K. Toxicity of aflatoxin B1 in broiler chicks and its reduction by activated charcoal. Res. Vet. Sci. 1994;56:37–40. doi: 10.1016/0034-5288(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Kong W.J., Zhang H., Song D.Q., Xue R., Zhao W., Wei J., Wang Y.M., Shan N., Zhou Z.X., Yang P., You X.F., Li Z.R., Si S.Y., Zhao L.X., Pan H.N., Jiang J.D. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism. 2009;58:109–119. doi: 10.1016/j.metabol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Laudadio V., Passantino L., Perillo A., Lopresti G., Passantino A., Khan R.U., Tufarelli V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012;91:265–270. doi: 10.3382/ps.2011-01675. [DOI] [PubMed] [Google Scholar]
- Li C., Ai G., Wang Y., Lu Q., Luo C., Tan L., Lin G., Liu Y., Li Y., Zeng H., Chen J., Lin Z., Xian Y., Huang X., Xie J., Su Z. Oxyberberine, a novel gut microbiota-mediated metabolite of berberine, possesses superior anti-colitis effect: impact on intestinal epithelial barrier, gut microbiota profile and TLR4-MyD88-NF-κB pathway. Pharmacol. Res. 2020;152:1–16. doi: 10.1016/j.phrs.2019.104603. [DOI] [PubMed] [Google Scholar]
- Li Z., Geng Y.N., Jiang J.D., Kong W.J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evid. Based Complement. Alternat. Med. 2014;2014:1–12. doi: 10.1155/2014/289264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi M., Paciolla C., Logrieco A.F., Mule G. Plant Bioactive compounds in Pre- and Postharvest Management for aflatoxins reduction. Front. Microbiol. 2020;11:243. doi: 10.3389/fmicb.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou T., Zhang Z., Xi Z., Liu K., Li L., Liu B., Huang F. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation. 2011;34:659–667. doi: 10.1007/s10753-010-9276-2. [DOI] [PubMed] [Google Scholar]
- Martinez-de-Anda A., Valdivia A., Jaramillo-Juarez F., Reyes J., Ortiz R., Quezada T., de Luna M., Rodríguez M. Effects of aflatoxin chronic intoxication in renal function of laying hens. Poult. Sci. 2010;89:1622–1628. doi: 10.3382/ps.2010-00763. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Nakanishi Y., Yoshioka T., Yamaga Y., Masuda T., Fukunaga Y., Sono M., Yoshikawa T., Nagao M., Araki O., Ogawa S., Goto N., Hiramatsu Y., Breyer R.M., Fukuda A., Seno H. Epithelial EP4 plays an essential role in maintaining homeostasis in colon. Sci. Rep. 2019;9:15244. doi: 10.1038/s41598-019-51639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem M.N., Saleemi M.K., Abbas R.Z., Khan A., Khatoon A., Gul S.T., Imran M., Sindhu Z.-u.-D., Sultan A. Hematological and serum biochemical effects of aflatoxin B1 intoxication in broilers experimentally infected with Fowl Adenovirus-4 (FAdV-4) Pak. Vet. J. 2018;38:209–213. [Google Scholar]
- Niu L., Qiao W., Hu Z., Li N., Huang Q., Gong J., Li Q., Zhu W., Li J. Berberine attenuates lipopolysaccharide-induced impairments of intestinal glutamine transport and glutaminase activity in rat. Fitoterapia. 2011;82:323–330. doi: 10.1016/j.fitote.2010.11.007. [DOI] [PubMed] [Google Scholar]
- NRC . 9th rev. ed. Natl. Acad. Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- Ortatatli M., Oğuz H., Hatipoglu F., Karaman M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res. Vet. Sci. 2005;78:61–68. doi: 10.1016/j.rvsc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Piotrowska M., Masek A. Saccharomyces cerevisiae cell wall components as tools for ochratoxin a decontamination. Toxins (Basel) 2015;7:1151–1162. doi: 10.3390/toxins7041151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J.L., Pier A.C., Stubblefield R.D., Shotwell O.L., Lyon R.L., Cutlip R.C. Effect of feeding corn naturally contaminated with aflatoxin on feed efficiency, on physiologic, immunologic, and pathologic changes, and on tissue residues in steers. Am. J. Vet. Res. 1983;44:1294–1299. [PubMed] [Google Scholar]
- Ruan D., Wang W.C., Lin C.X., Fouad A.M., Chen W., Xia W.G., Wang S., Luo X., Zhang W.H., Yan S.J., Zheng C.T., Yang L. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal. 2019;13:42–52. doi: 10.1017/S1751731118000678. [DOI] [PubMed] [Google Scholar]
- Sakamoto K., Hirose H., Onizuka A., Hayashi M., Futamura N., Kawamura Y., Ezaki T. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 2000;94:99–106. doi: 10.1006/jsre.2000.5937. [DOI] [PubMed] [Google Scholar]
- Schulte-Hermann R., Bursch W., Löw-Baselli A., Wagner A., Grasl-Kraupp B. Apoptosis in the liver and its role in hepatocarcinogenesis. Cell Biol. Toxicol. 1997;13:339–348. doi: 10.1023/a:1007495626864. [DOI] [PubMed] [Google Scholar]
- Shi Y.H., Xu Z.R., Feng J.L., Wang C.Z. Efficacy of modified montmorillonite nanocomposite to reduce the toxicity of aflatoxin in broiler chicks. Anim. Feed Sci. Technol. 2006;129:138–148. [Google Scholar]
- Shotwell O.L., Hesseltine C.W., Stubblefield R.D., Sorenson W.G. Production of aflatoxin on rice. Appl. Microbiol. 1966;14:425–428. doi: 10.1128/am.14.3.425-428.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solcan C., Pavel G., Floristean V., Chiriac I., Şlencu B., Solcan G. Effect of ochratoxin A on the intestinal mucosa and mucosa-associated lymphoid tissues in broiler chickens. Acta Vet. Hung. 2015;63:30–48. doi: 10.1556/AVet.2015.004. [DOI] [PubMed] [Google Scholar]
- Sun Y., Su J., Yang S., Liu Z., Liu D., Gan F., Chen X., Huang K. Mannan oligosaccharide protects against the aflatoxin-B1-Promoted Influenza replication and tissue damages in a Toll-like-receptor-4-dependent manner. J. Agric. Food Chem. 2019;67:735–745. doi: 10.1021/acs.jafc.8b05829. [DOI] [PubMed] [Google Scholar]
- Tang J., Feng Y., Tsao S., Wang N., Curtain R., Wang Y. Berberine and Coptidis Rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations. J. Ethnopharmacol. 2009;126:5–17. doi: 10.1016/j.jep.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Tedesco D., Steidler S., Galletti S., Tameni M., Sonzogni O., Ravarotto L. Efficacy of silymarin-phospholipid complex in reducing the toxicity of aflatoxin B1 in broiler chicks. Poult. Sci. 2004;83:1839–1843. doi: 10.1093/ps/83.11.1839. [DOI] [PubMed] [Google Scholar]
- Trenk H.L., Butz M.E., Chu F.S. Production of ochratoxins in different cereal products by Aspergillus ochraceus. Appl. Microbiol. 1971;21:1032–1035. doi: 10.1128/am.21.6.1032-1035.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira S.L. Nutritional implications of mould development in feedstuffs and alternatives to reduce the mycotoxin problem in poultry feeds. Worlds Poult. Sci. J. 2003;59:111–122. [Google Scholar]
- Vuddanda P.R., Chakraborty S., Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs. 2010;19:1297–1307. doi: 10.1517/13543784.2010.517745. [DOI] [PubMed] [Google Scholar]
- Wang X.-H., Li W., Wang X.-H., Han M.-Y., Muhammad I., Zhang X.-Y., Sun X.-Q., Cui X.-X. Water-soluble substances of wheat: a potential preventer of aflatoxin B1-induced liver damage in broilers. Poult. Sci. 2019;98:136–149. doi: 10.3382/ps/pey358. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhao G., Cheng L., Zhou H.-Y., Fu L.-Y., Yao W.-X. Effects of berberine on potassium currents in acutely isolated CA1 pyramidal neurons of rat hippocampus. Brain Res. 2004;999:91–97. doi: 10.1016/j.brainres.2003.11.036. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO) Aflatoxins . 2018. Food Safety Digest, Department of Food Safety and Zoonoses. REF. No.: WHO/NHM/FOS/RAM/18.1. Accessed Nov. 2020. https://www.who.int/foodsafety/FSDigest_Aflatoxins_EN.pdf. [Google Scholar]
- Wild C.P., Gong Y.Y. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E.D. Mechanisms of lipid peroxide formation in animal tissues. Biochem. J. 1966;99:667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Liao P., He L., Ren W., Yin J., Duan J., Li T. Growth performance, serum biochemical profile, jejunal morphology, and the expression of nutrients transporter genes in deoxynivalenol (DON)- challenged growing pigs. BMC Vet. Res. 2015;11:144. doi: 10.1186/s12917-015-0449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.-J., Lu H.-Y., Li Z.-Y., Bian Q., Qiu L.-L., Li Z., Liu Q., Li J., Wang X., Wang S.-L. Cytochrome P450 2A13 mediates aflatoxin B1-induced cytotoxicity and apoptosis in human bronchial epithelial cells. Toxicology. 2012;300:138–148. doi: 10.1016/j.tox.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Yang Z., Wang W.E., Zhang Q. CIAPIN1 siRNA inhibits proliferation, migration and promotes apoptosis of VSMCs by regulating Bcl-2 and Bax. Curr. Neurovasc. Res. 2013;10:4–10. doi: 10.2174/156720213804805909. [DOI] [PubMed] [Google Scholar]
- Yunus A.W., Razzazi-Fazeli E., Bohm J. Aflatoxin B1 in affecting broiler's performance, immunity, and gastrointestinal tract: a review of history and contemporary issues. Toxins (Basel) 2011;3:566–590. doi: 10.3390/toxins3060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala-Franco A., Hernandez-Patlan D., Solis-Cruz B., Lopez-Arellano R., Tellez-Isaias G., Vázquez-Duran A., Méndez-Albores A. Assessing the aflatoxin B₁ Adsorption capacity between Biosorbents using an in vitro Multicompartmental model Simulating the Dynamic conditions in the gastrointestinal tract of poultry. Toxins (Basel) 2018;10:484. doi: 10.3390/toxins10110484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Jiang L., Wang M., Jin M., Zhang X., Liu D., Wang Z., Yang L., Xu X. Berberine inhibits intestinal epithelial barrier dysfunction in colon caused by peritoneal dialysis fluid by improving cell migration. J. Ethnopharmacol. 2021;264 doi: 10.1016/j.jep.2020.113206. 113206. [DOI] [PubMed] [Google Scholar]
- Zhang N.-Y., Qi M., Zhao L., Zhu M.-K., Guo J., Liu J., Gu C.-Q., Rajput S.A., Krumm C.S., Qi D.-S., Sun L.-H. Curcumin prevents aflatoxin B₁ Hepatoxicity by inhibition of cytochrome P450 Isozymes in chick liver. Toxins (Basel) 2016;8:327. doi: 10.3390/toxins8110327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zhou S., Tang J., Zhang K., Guang L., Huang Y., Xu Y., Ying Y., Zhang L., Li D. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur. J. Pharmacol. 2009;606:262–268. doi: 10.1016/j.ejphar.2008.12.056. [DOI] [PubMed] [Google Scholar]