Abstract
Aims
Disparities persist on the prevalence of undiagnosed type 2 diabetes in racial/ethnic minorities in the USA. This study evaluated the association between BMI and incident type 2 diabetes risk by racial/ethnic group, to determine whether BMI and presence of type 2 diabetes risk factors may help clinicians better target type 2 diabetes screening.
Methods
This prospective cohort analysis included 5659 adults free of type 2 diabetes at baseline from the Multi-Ethnic Study of Atherosclerosis (MESA), a population-based cohort (2000–2011). BMI was measured at baseline and time-updated at subsequent visits. Incident type 2 diabetes was defined as fasting glucose ≥ 7.0 mmol/l, or use of any diabetes medications.
Results
The mean (sd) age was 62 (10) years and 42% of participants were white, 26% African American, 20% Hispanic and 12% Chinese American. During follow-up, 696 (12%) new type 2 diabetes cases were observed. In age- and sex-adjusted models, in the presence of one or more type 2 diabetes risk factors (the most common scenario), a 10% risk of incident type 2 diabetes was observed at a BMI of 21.7 kg/m2 [95% confidence interval (CI) 20.1 to 22.8] in Chinese Americans, 23.8 kg/m2 (22.7 to 24.9) in Hispanics, 24.7 kg/m2 (23.7 to 25.6) in African Americans and 26.2 kg/m2 (25.1 to 26.9) in white participants.
Conclusions
This study supports including BMI and presence of type 2 diabetes risk factors as action points for clinicians to prioritize which adults aged ≥ 45 years should be screened. The application of race/ethnicity-specific BMI thresholds may reduce the disparity of undiagnosed type 2 diabetes observed in minority groups.
Introduction
In recent decades, the prevalence of type 2 diabetes has increased significantly [1]. Currently, 15% of US adults have type 2 diabetes [2], and if these trends continue, it is projected that the prevalence could rise to 33% by 2050 [3]. The burden also disproportionately affects racial/ethnic minorities; while the current prevalence is 12% among white populations, it is nearly double that among Asian Americans, African Americans and Hispanics [2]. Likewise, the prevalence of undiagnosed type 2 diabetes disproportionally affects minority groups; it is 6.4% in African Americans, 8.6% in Asian Americans and 8.9% in Hispanics, compared with 4.2% in white populations [2].
Screening high-risk asymptomatic persons is recommended because reliable tests are available, and may lead to earlier identification and treatment that can potentially reduce progression and improve health outcomes [4]. Several societies recommend screening including the US Preventive Services Task Force (USPSTF) and the American Diabetes Association (ADA). USPSTF recommends screening adults aged 40–70 years who are overweight/obese (BMI ≥ 25 kg/m2) [5]. They also recommend screening persons at a lower BMI if they have risk factors such as a family history of diabetes, history of gestational diabetes or polycystic ovarian syndrome, or are members of racial/ethnic minority groups. By comparison, the ADA recommends universal screening for adults aged ≥ 45 years [6]. Despite these recommendations, recent data from the National Health and Nutrition Examination Survey (NHANES) found that only half of adults aged ≥ 45 years reported having been screened for diabetes [7], likely explaining part of why a high prevalence of undiagnosed type 2 diabetes remains a problem in the USA.
Under-screening of minority populations may occur for many reasons. A recent study of primary care physicians found the least commonly identified risk factors were patient race/ethnicity, and lower BMI among Asian Americans [8]. Clinicians may associate diabetes with overt obesity and hence target screening to adults with BMIs > 30 kg/m2. In this study, we used longitudinal data from a multi-ethnic cohort of adults aged ≥ 45 years to evaluate how BMI predicted incident type 2 diabetes risk by racial/ethnic group and presence of risk factors. The overarching objective was to determine how BMI could be used to help clinicians better target screening among adults aged ≥ 45 years, who continue to be screened for type 2 diabetes at suboptimal rates.
Methods
Participant population
We analysed follow-up data from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, a well-characterized cohort of 6814 participants aged 45–84 years, free of known cardiovascular disease (CVD). MESA objectives and design have been described in detail elsewhere [9]. Briefly, MESA recruited adults from six university clinics in the USA (Columbia, New York; Johns Hopkins, Baltimore; Northwestern, Chicago; University of California, Los Angeles; University of Minnesota, Twin Cities; and Wake Forest, Winston-Salem). Recruitment into MESA began in 2000 and participants are still being followed. Approximately 38% of the cohort was white, 28% African American, 22% Hispanic and 12% Chinese American. For our analysis, we excluded participants with prevalent type 2 diabetes at baseline (n = 829), or with type 1 diabetes (n = 10) and participants with missing covariates (n = 316). Our final sample size was 5659 who had complete data on at least one visit.
Ethics approval
Informed consent was obtained from all study participants and institutional review board (IRB) approval at the sites conducting MESA was obtained. Ethics approval for the use of anonymized data was obtained from the University of California San Francisco IRB on 2 January 2018 (16–21085). This study adhered to the principles detailed in the US Federal Policy for the Protection of Human Subjects.
Exposure assessment
Anthropometric measures were taken in light clothing and no shoes, and were measured twice and averaged at all study visits using standardized procedures [9]. BMI (kg/m2) was calculated from weight (kg) divided by height squared (m2).
Outcome assessment
Individuals were followed for incident type 2 diabetes until examination 5. Diabetes status was defined by fasting glucose ≥ 7.0 mmol/l and/or diabetes treatment, ascertained at each of the five clinic examinations (2000–2002, 2002–2004, 2004–2005, 2005–2007 and 2010–2012). HbA1c was measured in examinations 2 and 5 only, consequently we were unable to use this diagnostic criterion to identify and exclude prevalent cases at baseline or consistently capture incident cases over time.
Diabetes risk factors
We considered the following risk factors: first-degree relative with diabetes, high-risk race/ethnic group (i.e. non-white participants), hypertension (≥ 140/90 mmHg or on therapy), HDL-cholesterol level < 0.90 mmol/l, fasting triglyceride levels > 2.82 mmol/l, and physical inactivity [5,10]. Family history of diabetes was ascertained at the second examination visit for 5382 participants; this information was backdated to the baseline visit and included as a risk factor in sensitivity analysis. Race/ethnicity was self-reported. Resting blood pressure was measured three times in a seated position, and the average of the last two measurements were used. Blood was drawn in the fasted state from which serum HDL-cholesterol and triglycerides were measured [9]. Duration and frequency of various physical activities during a typical week in the past month were assessed using a detailed, semi-quantitative questionnaire adapted from the Cross-cultural Activity Participation Study [9,11]. Metabolic equivalent minutes of physical activity was calculated from the duration and intensity of total intentional exercises. Sex was self-reported.
Statistical analysis
Baseline characteristics between the four racial/ethnic groups were presented as means for continuous variables or percentages for categorical variables. Because observations of time to diagnosis of type 2 diabetes may be interval-censored in MESA, we initially fit appropriate Weibull proportional hazards models with robust standard errors [12]. However, treating the time of incident cases of type 2 diabetes as the mid-point between two examination visits using either Weibull or Cox models resulted in essentially the same point estimates and similar standard errors as the interval censored Weibull model; accordingly, we present our results based on the Weibull model with the midpoint time. In addition, race/ethnic-specific differences in the association between BMI and type 2 diabetes risk were evaluated using interaction terms, but because these were not significant at the 10% significance level, and inclusion of these did not meaningfully alter our results, we did not include interaction in our final models.
Using the Weibull model described above, we estimated the 10-year probability of developing diabetes as a function of BMI using a three-knot restricted cubic spline, and adjusting for race/ethnicity, age (continuous), sex, and included an indicator variable for the presence of one or more risk factors (low HDL-cholesterol, hypertriglyceridaemia, hypertension or physical inactivity) [5,10]. BMI and risk factors were time-updated at each examination visit, with missing values imputed by carrying forward the last complete value; < 3% of data was missing and carried forward. As a sensitivity analysis, we included family history of diabetes as a risk factor. Marginal estimates of the expected 10-year probability of developing type 2 diabetes as a function of BMI for each of the four racial/ethnic groups in the presence or absence of diabetes risk factors, or not accounting for risk factors were then calculated by regression standardization, averaging over the remaining covariates included in the model, as evaluated at baseline. Lastly, we used a simple line search, with step size of 0.1 kg/m2, to find the critical BMI values for each of the four groups corresponding to an estimated 10-year risk of type 2 diabetes of ~ 10%. Confidence intervals (CI) for the estimated BMI critical values were obtained by using bootstrap resampling with 1000 repetitions. All statistical analyses were done in SAS v.9.4 (SAS Institute, Cary, NC, USA) using the PARM_ICE macro to fit the interval-censored Weibull models, and in Stata v.15 (StataCorp, College Station, TX, USA) using the streg command to fit the Weibull models, the margins command for regression standardization, and the bootstrap command to obtain confidence intervals.
Results
Among the 5659 study participants free of diabetes at baseline, 42% were white, 26% African American, 20% Hispanic and 12% Chinese American (Table 1). Mean age was 62 years. White participants had higher family incomes and education levels, particularly compared with Hispanics. Chinese Americans had a lower mean BMI compared with the other groups, while African Americans and Hispanics had a higher mean BMI than white participants. Racial/ethnic minority groups were more likely to have at least one diabetes risk factor compared with white participants. In particular, African Americans were more likely to have hypertension, less likely to have hypertriglyceridaemia, and Hispanics and Chinese Americans were more likely to be physically inactive, compared with white participants.
Table 1.
Baseline characteristics of study population by race/ethnicity group, the multi-ethnic study of atherosclerosis, 2000–2002
| Characteristics | All (N = 5659) |
White (n = 2383, 42%) |
African American (n = 1462, 26%) |
Hispanic (n = 1161, 20%) |
Chinese American (n = 653, 12%) |
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age, years* | 62 (10) | 62 (10) | 62 (10) | 61 (10) | 61 (10) |
| Women | 3017 (53) | 1250 (52) | 818 (57) | 611 (53) | 338 (52) |
| Family income ≥ $75 000/year | 1349 (24) | 871 (37) | 260 (18) | 98 (8) | 120 (18) |
| Education ≥ Bachelor’s degree | 2137 (38) | 1213 (51) | 528 (36) | 129 (11) | 267 (41) |
| Diabetes risk factors | |||||
| BMI, kg/m2* | 28.0 (5.3) | 27.5 (4.9) | 29.8 (5.8) | 29.1 (4.9) | 23.9 (3.3) |
| Family history of diabetes† | 1882 (35) | 673 (29) | 588 (43) | 458 (42) | 163 (26) |
| Hypertension ≥ 140/90 mmHg, or on therapy | 2677 (47) | 1034 (42) | 896 (57) | 483 (39) | 264 (38) |
| HDL cholesterol < 0.90 mmol/l | 494 (8.7) | 206 (8.6) | 112 (7.7) | 132 (11) | 44 (6.7) |
| Triglyceride level > 2.82 mmol/l | 355 (6.3) | 157 (6.6) | 24 (1.6) | 125 (11) | 49 (7.5) |
| Physical inactivity‡ | 1323 (23) | 414 (17) | 360 (25) | 370 (32) | 179 (27) |
| One or more diabetes risk factors§ | 3677 (65) | 1377 (58) | 1082 (74) | 793 (68) | 425 (65) |
| One or more diabetes risk factors¶ | 3983 (74) | 1554 (68) | 1124 (82) | 872 (79) | 433 (70) |
Values are given as n (%) except *mean (sd).
Family history of diabetes at examination visit 2. n = 5382; white participants, 2289; African American, 1367; Latino, 1104; Chinese American, 622.
Physical inactivity was defined as engaging in no intentional exercise during a typical week in the past month.
Risk factors include hypertension, low HDL-cholesterol, high triglyceride or physical inactivity.
Risk factors include family history of diabetes, hypertension, low-HDL cholesterol, high triglyceride or physical inactivity. Including family history of diabetes, total n = 5382.
Over 42 686 person-years of follow-up, 696 (12%) new cases of type 2 diabetes were observed. Crude type 2 diabetes incidence rate was 1.1 cases (95% CI 1.0 to 1.3), 2.0 cases (1.8 to 2.3), 2.2 cases (1.9 to 2.5) and 1.6 cases (1.3 to 2.0) per 100 person-years among white participants, African Americans, Hispanics and Chinese Americans, respectively. Compared with white participants, Chinese Americans had more than twice the risk of type 2 diabetes [hazard ratio (HR) 2.6; 95% CI 2.0 to 3.4), while African Americans had a 30% higher risk (HR 1.3; 95% CI 1.1 to 1.6) and Hispanics had a 60% higher risk (HR 1.6; 95% CI 1.3 to 2.0), upon accounting for age, sex and the presence of one or more diabetes risk factors. Greater BMI was associated with a higher 10-year type 2 diabetes risk across all four racial/ethnic groups, though a similar probability of type 2 diabetes risk was observed at lower BMI levels for non-white participants compared with white participants.
Diabetes risk in the absence of diabetes risk factors
In a low-risk scenario (assuming no one had additional risk factors), in age- and sex-adjusted models, among white participants a 10% risk of type 2 diabetes over 10 years was observed in the obese category at a BMI of 30.5 kg/m2 (95% CI 28.9 to 33.0). By comparison, this same level of risk was observed in the overweight category at a BMI of 24.3 kg/m2 (23.2 to 25.7) in Chinese Americans, 26.8 kg/m2 (25.8 to 28.3) in Hispanics and 27.9 kg/m2 (26.5 to 29.9) in African Americans (Fig. 1).
FIGURE 1.
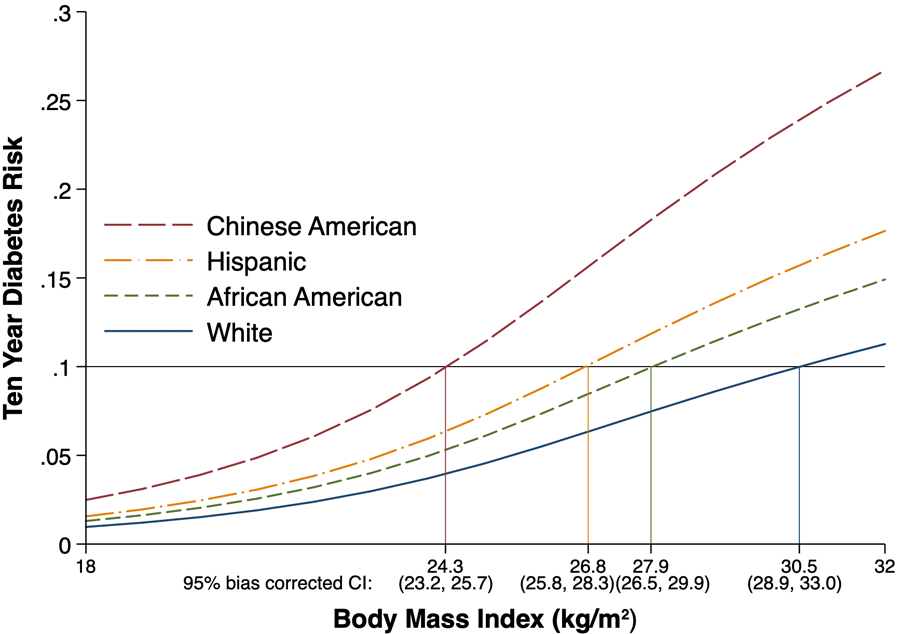
Ten-year risk of developing type 2 diabetes as a function of BMI, by race/ethnicity, in the absence of traditional risk factors (Multi-Ethnic Study of Atherosclerosis 2000–2012).
Diabetes risk in the presence of one or more diabetes risk factors
In a clinical screening scenario, assuming that everyone had at least one risk factor, in age- and sex-adjusted models, a 10% risk of developing diabetes over 10 years occurred at a BMI of 21.7 kg/m2 (95% CI 20.1 to 22.8) in Chinese Americans, 23.8 kg/m2 (22.7 to 24.9) in Hispanics, 24.7 kg/m2 (23.7 to 25.6) in African Americans and 26.2 kg/m2 (25.1 to 26.9) in white participants (Fig. 2).
FIGURE 2.
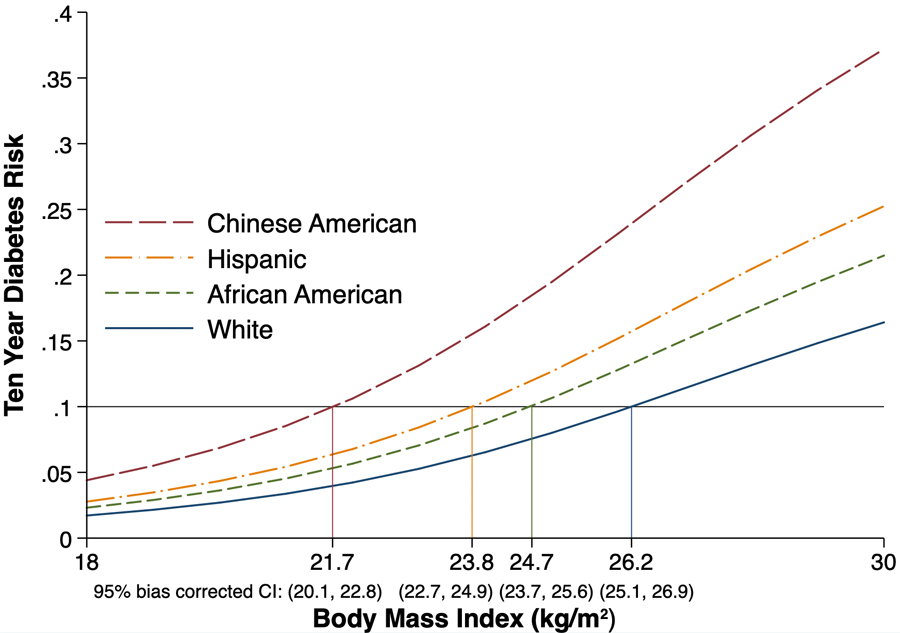
Ten-year risk of developing type 2 diabetes as a function of BMI, by race/ethnicity, in the presence of one or more traditional risk factors (Multi-Ethnic Study of Atherosclerosis 2000–2012).
Diabetes risk not considering other diabetes risk factors
To represent a public health screening scenario in which other diabetes risk factors are not considered, analogous estimates were made adjusting for age and sex but not accounting for any other diabetes risk factors. This resulted in estimated BMI levels associated with a 10% risk of developing type 2 diabetes over 10 years of 22.5 kg/m2 (95% CI 21.3 to 23.3) in Chinese Americans, 24.7 kg/m2 (23.9 to 25.6) in Hispanics, 25.6 kg/m2 (24.8 to 26.6) in African Americans and 27.3 kg/m2 (26.5 to 28.3) in white participants (Fig. 3).
FIGURE 3.
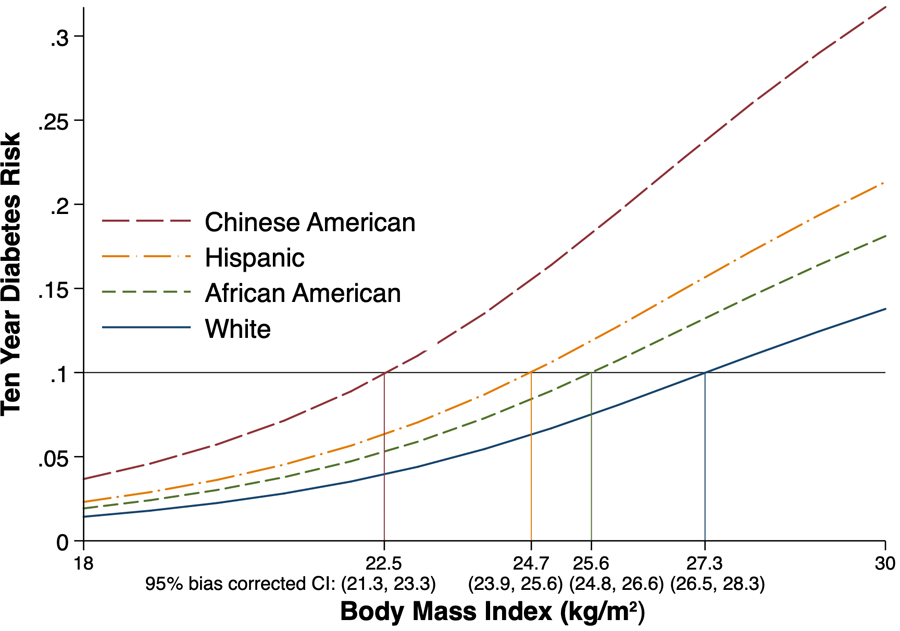
Ten-year risk of developing type 2 diabetes as a function of BMI, by race/ethnicity, not considering other traditional diabetes risk factors (Multi-Ethnic Study of Atherosclerosis 2000–2012).
Sensitivity analysis
In a sensitivity analysis, we included information about family history of type 2 diabetes as a risk factor, captured in examination visit 2 and backdated to the baseline exam visit and our results were essentially unchanged from our primary findings (results not shown).
Discussion
In this large multi-ethnic population-based prospective study of adults aged ≥ 45 years free of type 2 diabetes at baseline, we found that BMI was a practical and useful predictor of type 2 diabetes risk. The BMI levels associated with a 10% risk over 10 years varied according to race/ethnicity and presence or absence of other traditional risk factors. Our primary finding showed that in high-risk individuals, who were a majority, with at least one risk factor, screening should be considered at a BMI ≥ 22 kg/m2 for Chinese Americans, ≥ 24 kg/m2 for Hispanics, ≥ 25 kg/m2 for African Americans and ≥ 26 kg/m2 for white participants.
This study adds to the body of literature that underscores the value of using different BMI cut-off points for different racial/ethnic groups, and presents new evidence that the cut-off points may also differ between these groups based on the presence of risk factors. Although current ADA guidelines recommend screening everyone aged ≥ 45 years, findings from NHANES (2005–2012) showed that only half of adults aged ≥ 45 reported having been screened for diabetes [7]. Our study therefore adds evidence to support including BMI and traditional risk factors as action points to prioritize and identify who among adults aged ≥ 45 should be screened for type 2 diabetes. Further, our study confirms previous findings from cross-sectional studies [13,14], which were later used by ADA to modify its recommendations, that lower BMI cut-off points should be used to screen undiagnosed type 2 diabetes among Asian Americans, including Chinese Americans. Lastly, although we selected a 10% risk of developing type 2 diabetes over 10 years as the threshold at which screening should be considered, our findings show robust results across different risk thresholds. Regardless of the 10-year risk selected, we found that across the BMI distribution, Chinese Americans have a similar type 2 diabetes risk at ~ 5 BMI points lower compared with white participants, and Hispanics and African Americans ~ 2–2.5 BMI points lower compared with white participants.
Our findings are consistent with cross-sectional and follow-up studies in Canada [15] and the USA [16–19]. In a large multi-ethnic cohort in Canada, Chiu et al. [15] found that equivalent incidence rates of type 2 diabetes occurred at ~ 4 BMI points lower in black adults, 5 BMI points lower in Chinese adults and 6 BMI points lower in South Asian adults, compared with white adults with a BMI of 30 kg/m2. In the USA, using MESA follow-up data, Lutsey et al. [17] found that similar type 2 diabetes risk occurred at lower waist circumference points among Chinese American, African American and Hispanic participants compared with white participants. In another study using data from the Women’s Health Initiative, Luo et al. [18] found higher rates of incident type 2 diabetes among Hispanic and Asian women compared with white women in the same BMI categories. In the Nurses’ Health Study, in which 4% of nurses belonged to minority groups, Shai et al. found the risk of diabetes higher among Asian, Hispanic and African American participants compared with white participants [19]. In the Multi-Ethnic Cohort, the prevalence of self-reported type 2 diabetes at baseline by traditional BMI categories were two- to threefold greater for African Americans, Latinos, Japanese and Hawaiians compared with white participants [16]. And lastly, in a consortium of three integrated healthcare systems in the USA, which included nearly 5 million adults, Asians, Hawaiians/Pacific Islanders, Hispanics, African Americans and American Indians/Alaskan Natives had a higher burden of type 2 diabetes and prediabetes at lower BMIs compared with white participants [20].
The relationship between increased adiposity and type 2 diabetes is more strongly linked with the distribution of body fat than overall obesity as measured by BMI. In general, greater amounts of visceral adiposity or hepatic steatosis are associated with higher risk for insulin resistance, metabolic abnormalities and type 2 diabetes than higher levels of subcutaneous fat [21,22]. The distribution of these fat depots as well as the observed association with cardiometabolic disease vary significantly by racial/ethnic background [23–27], which may explain part of the reason why the observed association between BMI and incident type 2 diabetes is modified by race/ethnicity.
Strengths and limitations
Our study had the following strengths. First, MESA included data from a follow-up of 11 years among a large multi-ethnic population, which allowed us to compare findings between four large US racial/ethnic groups, overcoming limitations of prior studies that used cross-sectional data [13,14]. Second, BMI was calculated from repeated measures of standing height and weight, improving upon prior studies that used self-report measures [15].
Although our study has notable strengths, there are a few limitations. First, HbA1c was available at examinations 2 and 5 only and we were therefore unable to use this diagnostic criterion to identify and exclude prevalent cases at baseline or consistently capture incident cases over time. Our diagnostic method, fasting glucose and/or diabetes treatment, may have led to measurement error, as this test is less sensitive than the gold-standard 2-h glucose tolerance test, particularly for African Americans and Chinese Americans [2,28]. Future studies should augment the diagnostic method by including HbA1c and 2-h glucose tolerance tests. Second, MESA did not examine participants for the presence of acanthosis nigricans, nor collected data on polycystic ovarian syndrome, which may have resulted in misclassification of exposure. Nevertheless, given the overlap between these conditions and the other risk factors that were included in our analyses, it is likely that misclassification of exposure was minimal, thus we would not expect our primary conclusions to be qualitatively different. Third, MESA excluded participants with known cardiovascular disease, thus our sample represents a healthier subpopulation, nevertheless since this exclusion was consistent across racial/ethnic groups, we would not expect this selection bias to qualitatively shape our inferences. Fourth, because of important between-group differences in the pathophysiological backgrounds of diabetes onset among different Asian ethnic groups [29,30], our inferences are limited to Chinese Americans. Future research should include additional Asian subgroups, including Pacific Islanders and South Asians.
Conclusion
Given that adults aged ≥ 45 are currently under-screened for type 2 diabetes, this study adds evidence to support including BMI and the presence of traditional type 2 diabetes risk factors as action points for clinicians to prioritize and identify those among older adults (aged ≥ 45 years) should be screened for type 2 diabetes. Future studies should evaluate if the application of race/ethnicity-specific BMI thresholds may help reduce the high prevalence of undiagnosed type 2 diabetes, as well as the disparity observed in minority groups.
What’s new?
The American Diabetes Association recommends universal type 2 diabetes screening for adults aged > 45 years; however, currently in the US only 50% are screened.
BMI is a useful predictor of type 2 diabetes risk.
Racial/ethnic minorities have similar type 2 diabetes risks at lower BMI values compared with white participants.
BMI can be used to prioritize adults aged > 45 years for type 2 diabetes screening.
Racial/ethnic minority populations should be screened for type 2 diabetes at lower BMI cut-off points compared with white participants.
Acknowledgments
The authors thank the other investigators, staff, and the MESA participants for their valuable contributions. A full list of participating MESA investigators and institutions may be found at www.mesa-nhlbi.org. MESA was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). L.A.R. was supported by the National Institute Of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health (NIH) under Award Number F31DK115029, and by a University of California Dissertation-Year Fellowship Award. A.F. was supported by NIH grant K24DK102057. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or of the University of California.
Footnotes
Competing interests
None declared.
References
- 1.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA 2015; 314: 1021–1029. [DOI] [PubMed] [Google Scholar]
- 2.Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW et al. Prevalence of diabetes by race and ethnicity in the United States, 2011–2016. JAMA 2019; 322: 2389–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010; 8: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selph S, Dana T, Blazina I, Bougatsos C, Patel H, Chou R. Screening for type 2 diabetes mellitus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2015; 162: 765–776. [DOI] [PubMed] [Google Scholar]
- 5.Siu AL, on behalf of the U.S. Preventive Services Task Force. Screening for abnormal blood glucose and type 2 diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2015; 163: 861–868. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association. 5. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018; 41: S51–S54. [DOI] [PubMed] [Google Scholar]
- 7.Kiefer MM, Silverman JB, Young BA, Nelson KM. National patterns in diabetes screening: data from the National Health and Nutrition Examination Survey (NHANES) 2005–2012. J Gen Intern Med 2015; 30: 612–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng E, Greer RC, O’Rourke P, Yeh H-C, McGuire MM, Albright AL et al. National survey of primary care physicians’ knowledge, practices, and perceptions of prediabetes. J Gen Intern Med 2019; 34: 2475–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AVD, Folsom AR et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002; 156: 871–881. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of Medical Care in Diabetes—2019 abridged for primary care providers. Clin Diabetes 2019; 37: 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAuley PA, Hsu F-C, Loman KK, Carr JJ, Budoff MJ, Szklo M et al. Liver attenuation, pericardial adipose tissue, obesity, and insulin resistance: the Multi-Ethnic Study of Atherosclerosis (MESA). Obesity 2011; 19: 1855–1860. [DOI] [PubMed] [Google Scholar]
- 12.Sparling YH. Parametric survival models for interval-censored data with time-dependent covariates. Biostatistics 2006; 7: 599–614. [DOI] [PubMed] [Google Scholar]
- 13.Hsu WC, Araneta MRG, Kanaya AM, Chiang JL, Fujimoto W. BMI cut points to identify at-risk Asian Americans for type 2 diabetes screening. Diabetes Care 2015; 38: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araneta MRG, Kanaya AM, Hsu WC, Chang HK, Grandinetti A, Boyko EJ et al. Optimum BMI cut points to screen Asian Americans for type 2 diabetes. Diabetes Care 2015; 38: 814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV. Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care 2011; 34: 1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maskarinec G, Grandinetti A, Matsuura G, Mau M, Henderson BE, Kolonel LN. Diabetes prevalence and body mass index differ by ethnicity: the multiethnic cohort. Ethn Dis 2009; 19: 49–60. [PMC free article] [PubMed] [Google Scholar]
- 17.Lutsey PL, Pereira MA, Bertoni AG, Kandula NR, Jacobs DR. Interactions between race/ethnicity and anthropometry in risk of incident diabetes. Am J Epidemiol 2010; 172: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J, Hendryx M, Laddu D, Phillips LS, Chlebowski R, LeBlanc ES et al. Racial and ethnic differences in anthropometric measures as risk factors for diabetes. Diabetes Care 2019; 42: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shai I, Jiang R, Manson JE, Stampfer MJ, Willett WC, Colditz GA et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care 2006; 29: 1585–1590. [DOI] [PubMed] [Google Scholar]
- 20.Zhu Y, Sidell MA, Arterburn D, Daley MF, Desai J, Fitzpatrick SL et al. Racial/ethnic disparities in the prevalence of diabetes and prediabetes by BMI: Patient Outcomes Research To Advance Learning (PORTAL) multisite cohort of adults in the U.S. Diabetes Care 2019; 42: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araneta MRG, Barrett-Connor E. Ethnic differences in visceral adipose tissue and type 2 diabetes: Filipino, African-American, and White Women. Obes Res 2005; 13: 1458–1465. [DOI] [PubMed] [Google Scholar]
- 22.Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB et al. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging 2014; 7: 1221–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahima RS, Lazar MA. The health risk of obesity—better metrics imperative. Science 2013; 341: 856–858. [DOI] [PubMed] [Google Scholar]
- 24.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB et al. Heart disease and stroke statistics—2012 update. Circulation 2012; 125: e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wildman RP. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004). Arch Intern Med 2008; 168: 1617–1624. [DOI] [PubMed] [Google Scholar]
- 26.Graham RC, Burke A, Stettler N. Ethnic and sex differences in the association between metabolic syndrome and suspected nonalcoholic fatty liver disease in a nationally representative sample of US adolescents. J Pediatr Gastroenterol Nutr 2009; 49: 442–449. [DOI] [PubMed] [Google Scholar]
- 27.Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/latino individuals of diverse backgrounds in the United States. JAMA 2012; 308: 1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiao Q, Nakagami T, Tuomilehto J, Borch-Johnsen K, Balkau B, Iwamoto Y et al. Comparison of the fasting and the 2-h glucose criteria for diabetes in different Asian cohorts. Diabetologia 2000; 43: 1470–1475. [DOI] [PubMed] [Google Scholar]
- 29.Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJ et al. Understanding the high prevalence of diabetes in U.S. South Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes Care 2014; 37: 1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karter AJ, Schillinger D, Adams AS, Moffet HH, Liu J, Adler NE et al. Elevated rates of diabetes in Pacific Islanders and Asian subgroups: the Diabetes Study of Northern California (DISTANCE). Diabetes Care 2013; 36: 574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]


