Abstract
Objective
To validate the previously derived Canadian TIA Score to stratify subsequent stroke risk in a new cohort of emergency department patients with transient ischaemic attack.
Design
Prospective cohort study.
Setting
13 Canadian emergency departments over five years.
Participants
7607 consecutively enrolled adult patients attending the emergency department with transient ischaemic attack or minor stroke.
Main outcome measures
The primary outcome was subsequent stroke or carotid endarterectomy/carotid artery stenting within seven days. The secondary outcome was subsequent stroke within seven days (with or without carotid endarterectomy/carotid artery stenting). Telephone follow-up used the validated Questionnaire for Verifying Stroke Free Status at seven and 90 days. All outcomes were adjudicated by panels of three stroke experts, blinded to the index emergency department visit.
Results
Of the 7607 patients, 108 (1.4%) had a subsequent stroke within seven days, 83 (1.1%) had carotid endarterectomy/carotid artery stenting within seven days, and nine had both. The Canadian TIA Score stratified the risk of stroke, carotid endarterectomy/carotid artery stenting, or both within seven days as low (risk ≤0.5%; interval likelihood ratio 0.20, 95% confidence interval 0.09 to 0.44), medium (risk 2.3%; interval likelihood ratio 0.94, 0.85 to 1.04), and high (risk 5.9% interval likelihood ratio 2.56, 2.02 to 3.25) more accurately (area under the curve 0.70, 95% confidence interval 0.66 to 0.73) than did the ABCD2 (0.60, 0.55 to 0.64) or ABCD2i (0.64, 0.59 to 0.68). Results were similar for subsequent stroke regardless of carotid endarterectomy/carotid artery stenting within seven days.
Conclusion
The Canadian TIA Score stratifies patients’ seven day risk for stroke, with or without carotid endarterectomy/carotid artery stenting, and is now ready for clinical use. Incorporating this validated risk estimate into management plans should improve early decision making at the index emergency visit regarding benefits of hospital admission, timing of investigations, and prioritisation of specialist referral.
Introduction
Patients who have a transient ischaemic attack are at high risk of a subsequent stroke, especially in the short term. Historically, studies have estimated the overall risk of stroke to be 4-10% within seven days of transient ischaemic attack, increasing to 8-12% by 90 days.1 2 3 4 5 6 7 8 9 However, the management of transient ischaemic attacks has changed markedly in the past decade,4 10 11 and much lower subsequent stroke rates are attainable.4 5 6 7 8 9 12 Importantly, most of the subsequent risk of stroke is in the first days after the index visit.4 9 Nevertheless, comprehensive investigation, aggressive treatment, and/or hospital admission for all patients who present to the emergency department with symptoms suggestive of a transient ischaemic attack is both inefficient and challenging for most health systems, so prioritising care to those most likely to benefit is essential. Likewise, the ability to identify patients at very low risk benefits both providers and patients.
Clinical decision rules or scores derived from original research help clinicians with diagnostic or therapeutic decisions at the bedside but need to be validated before implementation.13 14 15 The best known score for triage of transient ischaemic attack is the ABCD2 score. However, this instrument has not been able to discriminate particularly well between groups at low and high risk during prospective validation.16 17 18 19 Several variations of the ABCD2 score are now available, which include neuroimaging, recent previous transient ischaemic attack, and vascular imaging.20 These scores are typically used to dichotomise risk as low versus high. Our study team previously prospectively derived the Canadian TIA Score (table 1) from nearly 4000 patients prospectively enrolled at eight large Canadian hospital emergency departments.21 This score incorporates 13 predictive variables from history, physical examination, and testing routinely performed at the time of presentation to the emergency department. The assigned score, which ranges from −3 to 23, can be used to assign a graded probability of stroke in the subsequent week ranging from 0.01% to 28%. Alternatively, it can be grouped into three risk levels—namely, low, medium, and high risk—to prioritise investigations, admission, and follow-up in specialty clinics at the time of the index emergency department visit. Therefore, to ensure that this score can be safely introduced into clinical practice, our objective was to validate the previously derived Canadian TIA Score to stratify subsequent risk of stroke in a new cohort of emergency department patients and to compare it with existing risk stratification scores.
Table 1.
Canadian TIA Score
| Items | Points |
|---|---|
| Clinical findings: | |
| 1) First transient ischaemic attack (in lifetime) | 2 |
| 2) Symptoms ≥10 minutes | 2 |
| 3) Past history of carotid stenosis | 2 |
| 4) Already on antiplatelet therapy | 3 |
| 5) History of gait disturbance | 1 |
| 6) History of unilateral weakness | 1 |
| 7) History of vertigo | −3 |
| 8) Initial triage diastolic blood pressure ≥110 mm Hg | 3 |
| 9) Dysarthria or aphasia (history or examination) | 1 |
| Investigations in emergency department: | |
| 1) Atrial fibrillation on electrocardiogram | 2 |
| 2) Infarction (new or old) on computed tomography | 1 |
| 3) Platelet count ≥400×109/L | 2 |
| 4) Glucose ≥15 mmol/L | 3 |
| Total score (−3 to 23): | X |
Methods
Study population and setting
We conducted a prospective multicentre cohort study at 13 Canadian emergency departments (10 university affiliated tertiary care hospitals and three urban community hospitals), including six sites (two community, four university) not involved in the original derivation study. We enrolled patients over a five year period from 31 October 2012 to 30 May 2017.
We prospectively enrolled consecutive patients attending the emergency department seven days a week, 24 hours a day at all sites. Patients were aged 18 years or older, with transient ischaemic attack or minor stroke as their final emergency department diagnosis at the time of discharge or specialist consultation. We excluded patients who had neurological deficits for more than 24 hours (that is, a stroke according to the World Health Organization’s definition), had a decreased level of consciousness from their baseline (that is, Glasgow Coma Scale <15 in previously cognitively normal patients), had an alternative diagnosis (for example, hypoglycaemia, seizure, electrolyte imbalance, or migraine), presented more than seven days after onset of the neurological symptoms, or were treated with tissue plasminogen activator or embolectomy for an acute stroke.
Data collection
Attending emergency physicians, neurologists, or supervised resident physicians completed all assessments. Physicians were oriented to the study and the standardised data collection forms by means of a formal one hour lecture, as well as individual orientation of the study forms including definitions and procedures by local study staff. Physicians completed data forms to explicitly record each element of the Canadian TIA Score, the ABCD2 score, and the ABCD2i score (ABCD2 score plus 3 points for infarction on neuroimaging).
Study personnel collected data forms, verified data, confirmed eligibility, and recorded non-subjective data (for example, age, triage vital signs, laboratory results) from review of all medical records, including those from physicians, nurses, consultants, emergency medical services, follow-up neurological consultations, discharge summaries, and laboratory and radiology reports. We searched the study hospital’s electronic medical records to identify subsequent emergency department visits, stroke/neurology clinic visits, and diagnostic imaging. We conducted telephone follow-up calls at seven and 90 days for patients not in hospital at these time points, to assess for subsequent stroke or subsequent transient ischaemic attacks, using the previously validated Questionnaire for Verifying Stroke Free Status.22 23 In addition to this questionnaire, we asked patients whether they had been admitted for stroke subsequent to their initial emergency department visit and whether they experienced subsequent neurological deficits and, if so, the duration, the date of onset, and the side(s) affected. We have successfully used the same proxy outcome method in previous work.24
Study nurses reviewed all emergency department visits during the study period to identify any missed and potentially eligible patients. If such patients were not clearly ineligible on the basis of review of medical record, they were deemed to be missed eligible patients, and their characteristics were abstracted onto a standardised data collection form for missed eligible patients. Data management and study coordination were conducted at the Ottawa Hospital Research Institute.
Outcome measures
The primary outcome was the composite of subsequent stroke or carotid revascularisation (that is, carotid endarterectomy or carotid artery stenting) within seven days of the index emergency department visit. We defined subsequent stroke as new, rapidly developing clinical symptom(s) of focal (or occasionally global) disturbance of cerebral function lasting more than 24 hours or until death, with no apparent non-vascular cause.25 As a secondary outcome, we examined only subsequent stroke within seven days of the emergency department diagnosis of transient ischaemic attack, regardless of carotid revascularisation.
Outcome assessment
We assessed the outcomes for all patients, incorporating data from all possible sources including site hospital records for stroke occurrence, admission, or mortality; autopsy report at the site hospital; or patients answering “yes” to one or more of the telephone follow-up questions. Local adjudication committees, blinded to the initial emergency department visit (that is, study form and physician and nursing notes before a possible subsequent event), reviewed all possible outcomes. The adjudication committees were composed of two stroke neurologists and one experienced emergency physician (two sites used two experienced emergency physicians and one stroke neurologist). These assessors independently assessed each possible outcome, and every outcome event required majority agreement.
Data analyses
We calculated the classification performance of the Canadian TIA Score for each risk category (low, medium, and high) by using interval likelihood ratios with 95% confidence intervals. The interval likelihood ratio is the multilevel extension of the positive and negative likelihood ratios, which are applicable only when a test/risk score is dichotomised into two levels (positive versus negative or high risk versus low risk). We pre-specified risk thresholds (low risk <1%, medium risk 1-5%, and high risk >5%) on the basis of previous surveys of emergency physicians and neurologists to group discrete risk scores into these three risk levels.26 27 We calculated the sensitivity and specificity when the score was dichotomised at each integer value from −3 to 23. We also assessed the classification of the ABCD2 and ABCD2i scores for both the primary and secondary outcomes according to their ability to classify patients as being at low, medium, and high risk using cut-off points based on the same risk thresholds (low risk <1%, medium risk 1-5%, and high risk >5%). We also compared the C statistic (area under the curve) by using the discrete values for each score. We used the DeLong method to test for the significance of these differences. We also calculated the absolute net reclassification indices by using three levels (low, medium, and high risk) comparing the Canadian TIA Score with the ABCD2 and ABCD2i scores. Sample size was based on estimation of the precision of the classification performance of the risk scale. Our goal was to enrol enough subsequent patients with stroke to be able to evaluate the sensitivity with 95% confidence bands plus/minus 10%, corresponding to 90 subsequent stroke cases. From our previous study, in which 2.2% of eligible patients had a stroke within seven days of their diagnosis of transient ischaemic attack, we estimated that we would need a sample size of 5000 patients with transient ischaemic attack.
With the increasing use of prompt emergency carotid endarterectomy at study sites during the accrual phase of this study, and its potentially large effect on short term stroke (that is, an estimated number needed to treat to avert stroke or death of 3.328), our primary outcome was altered from our previous derivation study to become the composite of either subsequent stroke or carotid endarterectomy/carotid artery stenting within seven days. Although we expected this change to increase the primary outcome event rate, we maintained the original study sample size target of 90 stroke events (with or without carotid endarterectomy/carotid artery stenting), which we designated as our secondary outcome of interest.
Patient and public involvement
Neither patients nor the public were formally involved in the planning of the study. We plan to involve patients before assessing the effects of implementing this rule in clinical practice.
Results
We enrolled 7607 patients, representing 80.6% of all potentially eligible patients seen at the participating emergency departments during the study period. Follow-up by telephone, clinic assessment, or both was almost complete, with only 34 (0.4%) patients lost to follow-up by seven days (that is, not reached by telephone and no subsequent hospital encounters). One hundred and eight (1.4%) patients had a subsequent stroke and 83 (1.1%) had carotid revascularisation within seven days of their index visit (total of 182 outcomes, as nine patients had both). Missed patients who were not enrolled were similar to enrolled patients with regards to age, sex, and diagnostic testing but were admitted to hospital more often (18.4% v 5.8%) (appendix 1).
Table 2 shows the clinical features of our cohort. The patients had a mean age of 68.5 years, and 52.3% were women; three quarters reported this being their first transient ischaemic attack. The most common presenting symptoms were sensory deficits, weakness, and speech difficulties. More than a third of patients arrived by ambulance, and half had had symptoms for more than an hour. Almost all patients had computed tomography of the head (96.5%) and electrocardiography (91.0%). Most patients either continued or started taking aspirin, clopidogrel, or both. Very few patients (5.8%) were admitted to hospital from the emergency department at the time of their index visit.
Table 2.
Clinical characteristics of 7607 patients with transient ischaemic attack (TIA) admitted to the emergency department. Values are numbers (percentages) unless stated otherwise
| Characteristics | All patients (n=7607) | Low risk (n=1242) | Medium risk (n=5484) | High risk (n=881) |
|---|---|---|---|---|
| Demographics | ||||
| Mean (SD) age, years | 68.5 (14.7) | 65.3 (14.5) | 68.1 (14.8) | 75 (11.7) |
| Median (interquartile range) age, years | 70 (58-80) | 66 (54-77) | 69 (58-80) | 76 (68-84) |
| Age range, years | 18-103 | 18-98 | 18-103 | 36-99 |
| Female sex | 3982 (52.3) | 695 (56.0) | 2936 (53.5) | 351 (39.8) |
| Clinical features: history | ||||
| Arrival by ambulance | 2765/7605 (36.4) | 365/1242 (29.4) | 1962/5482 (35.8) | 438/881 (49.7) |
| First ever TIA | 5706/7589 (75.2) | 765/1237 (61.8) | 4208/5474 (76.9) | 733/878 (83.5) |
| Duration of symptoms: | (n=7543) | (n=1222) | (n=5445) | (n=876) |
| <1 minute | 221 (2.9) | 132 (10.8) | 86 (1.6) | 3 (0.3) |
| 1-4 minutes | 468 (6.2) | 232 (19.0) | 226 (4.2) | 10 (1.1) |
| 5-9 minutes | 486 (6.4) | 216 (17.7) | 247 (4.5) | 23 (2.6) |
| 10-29 minutes | 1383 (18.3) | 130 (10.6) | 1076 (19.8) | 177 (20.2) |
| 30-59 minutes | 1120 (14.8) | 126 (10.3) | 844 (15.5) | 150 (17.1) |
| ≥60 minutes | 3865 (51.2) | 386 (31.6) | 2966 (54.5) | 513 (58.6) |
| History of altered sensation | 3269/7521 (43.5) | 470/1231 (38.2) | 2465/5419 (45.5) | 334/871 (38.3) |
| History of weakness | 3019/7544 (40.0) | 273/1234 (22.1) | 2164/5435 (39.8) | 582/875 (66.5) |
| Language disturbance | 2943/7442 (39.5) | 327/1208 (27.1) | 2109/5370 (39.3) | 507/864 (58.7) |
| Light-headedness | 1297/7281 (17.8) | 302/1201 (25.1) | 871/5246 (16.6) | 124/834 (14.9) |
| Vertigo | 779/7338 (10.6) | 474/1222 (38.8) | 281/5278 (5.3) | 24/838 (2.9) |
| Gait disturbance | 1571/7356 (21.4) | 226/1203 (18.8) | 1027/5311 (19.3) | 318/842 (37.8) |
| Visual loss | 1069/7113 (15.0) | 254/1158 (21.9) | 740/5139 (14.4) | 75/816 (9.2) |
| Clinical features: examination | ||||
| Mean (SD) initial systolic blood pressure, mm Hg (n=7597) | 151.9 (26.1) | 150.6 (24.1) | 151.7 (25.7) | 155.4 (30.4) |
| Mean (SD) initial diastolic blood pressure, mm Hg (n=7594) | 82.9 (13.7) | 82.9 (11.8) | 82.7 (13.3) | 84.3 (18.3) |
| Mean (SD) initial heart rate, bpm (n=7600) | 77.6 (15.0) | 77.2 (14.2) | 77.7 (14.9) | 77.6 (16.3) |
| Weakness | 997/7547 (13.2) | 57/1234 (4.6) | 720/5440 (13.2) | 220/873 (25.2) |
| Altered sensation | 868/7489 (11.6) | 105/1229 (8.5) | 675/5395 (12.5) | 88/865 (10.2) |
| Any speech difficulty | 850/7523 (11.3) | 51/1226 (4.2) | 617/5421 (11.4) | 182/873 (20.8) |
| Gait abnormality | 617/7397 (8.3) | 105/1222 (8.6) | 383/5338 (7.2) | 129/837 (15.4) |
| Dysarthria | 564/7486 (7.5) | 30/1229 (2.4) | 397/5392 (7.4) | 137/868 (15.8) |
| Pronator drift | 405/7053 (5.7) | 33/1157 (2.9) | 278/5095 (5.5) | 94/801 (11.7) |
| Aphasia | 268/7486 (3.6) | 18/1226 (1.5) | 205/5392 (3.8) | 45/868 (5.2) |
| Abnormal finger-nose test | 262/7312 (3.6) | 30/1213 (2.5) | 182/5268 (3.5) | 50/831 (6.0) |
| Clinical features: past medical history | (n=7592) | (n=1238) | (n=5474) | (n=880) |
| Hypertension | 4505 (59.3) | 599 (48.4) | 3191 (58.3) | 715 (81.3) |
| High cholesterol | 2772 (36.5) | 379 (30.6) | 1973 (36.0) | 420 (47.7) |
| Diabetes mellitus | 1448 (19.1) | 138 (11.1) | ,002 (18.3) | 308 (35.0) |
| Coronary artery disease | 1289 (17.0) | 101 (8.2) | 866 (15.8) | 322 (36.6) |
| Known previous stroke | 976 (12.9) | 119 (9.6) | 672 (12.3) | 185 (21.0) |
| Current smoker | 840 (11.1) | 128 (10.3) | 616 (11.3) | 96 (10.9) |
| Atrial fibrillation | 806 (10.6) | 96 (7.8) | 535 (9.8) | 175 (19.9) |
| Peripheral vascular disease | 269 (3.5) | 17 (1.4) | 176 (3.2) | 76 (8.6) |
| Carotid stenosis | 251 (3.3) | 9 (0.7) | 138 (2.5) | 104 (11.8) |
| Diagnostic tests in emergency department | ||||
| Computed tomography of head | 7337 (96.5) | 1191 (95.9) | 5287 (96.4) | 859 (97.5) |
| Evidence of acute or old infarction | 2080 (27.3) | 172 (13.8) | 1413 (25.8) | 495 (56.2) |
| Electrocardiography | 6923 (91.0) | 1114 (89.7) | 4993 (91.0) | 816 (92.6) |
| Evidence of atrial fibrillation | 425 (5.6) | 22 (1.8) | 255 (4.7) | 148 (16.8) |
| Magnetic resonance imaging of head | 323 (4.2) | 37 (3.0) | 244 (4.4) | 42 (4.8) |
| Carotid Doppler | 4382 (57.6) | 684 (55.1) | 3225 (58.8) | 473 (53.7) |
| Computed tomography angiography of neck | 2085 (27.4) | 309 (24.9) | 1493 (27.2) | 283 (32.1) |
| Routine drugs at time of index TIA | ||||
| Antihypertensive | 3579 (47.0) | 461 (37.1) | 2522 (46.0) | 596 (67.7) |
| Any antithrombotic | 3274 (43.0) | 231 (18.6) | 2328 (42.5) | 715 (81.2) |
| Aspirin | 2274 (29.9) | 101 (8.1) | 1593 (29.0) | 580 (65.8) |
| Clopidogrel | 588 (7.7) | 31 (2.5) | 423 (7.7) | 134 (15.2) |
| Warfarin | 348 (4.6) | 66 (5.3) | 224 (4.1) | 58 (6.6) |
| Dipyridamole/aspirin | 55 (0.7) | 3 (0.2) | 41 (0.7) | 11 (1.2) |
| Ticlopidine | 6 (0.1) | 2 (0.2) | 3 (0.1) | 1 (0.1) |
| Other anticoagulant | 367 (4.8) | 49 (3.9) | 269 (4.9) | 49 (5.6) |
| Statin | 2772 (36.4) | 342 (27.5) | 1946 (35.5) | 484 (54.9) |
| Drugs on discharge | ||||
| Antihypertensive | 3728 (49.0) | 473 (38.1) | 2634 (48.0) | 621 (70.5) |
| Any antithrombotic | 6667 (87.6) | 1002 (80.7) | 4807 (87.7) | 858 (97.4) |
| Aspirin | 5477 (72.0) | 866 (69.7) | 3943 (71.9) | 668 (75.8) |
| Clopidogrel | 1251 (16.4) | 65 (5.2) | 898 (16.4) | 288 (32.7) |
| Warfarin | 362 (4.8) | 66 (5.3) | 233 (4.2) | 63 (7.2) |
| Dipyridamole/aspirin | 136 (1.8) | 8 (0.6) | 97 (1.8) | 31 (3.5) |
| Ticlopidine | 6 (0.1) | 2 (0.2) | 3 (0.1) | 1 (0.1) |
| Other anticoagulant | 413 (5.4) | 51 (4.1) | 296 (5.4) | 66 (7.5) |
| Statin | 3109 (40.9) | 401 (32.3) | 2195 (40.0) | 513 (58.2) |
| Primary outcome | ||||
| Stroke or carotid revascularisation ≤7 days | 183 (2.4) | 6 (0.5) | 124 (2.3) | 53 (6.0) |
| Secondary outcomes | ||||
| Carotid revascularisation ≤7 days from index visit | 84 (1.1) | 3 (0.2) | 52 (0.9) | 29 (3.3) |
| Carotid revascularisation ≤90 days from index visit | 156 (2.1) | 12 (1.0) | 95 (1.7) | 49 (5.6) |
| Carotid endarterectomy ≤7 days from index visit | 69 (0.9) | 3 (0.2) | 42 (0.8) | 24 (2.7) |
| Carotid endarterectomy ≤90 days from index visit | 130 (1.7) | 12 (1.0) | 80 (1.5) | 38 (4.3) |
| Carotid stent ≤7 days from index visit | 16 (0.2) | 0 (0) | 11 (0.2) | 5 (0.6) |
| Carotid stent ≤90 days from index visit | 29 (0.4) | 0 (0) | 17 (0.3) | 12 (1.4) |
| Cumulative stroke ≤2 days from index visit | 70 (0.9) | 1 (0.1) | 51 (0.9) | 18 (2.0) |
| Cumulative stroke ≤7 days from index visit | 108 (1.4) | 3 (0.2) | 81 (1.5) | 24 (2.7) |
| Cumulative stroke ≤30 days from index visit | 153 (2.0) | 4 (0.3) | 116 (2.1) | 33 (3.7) |
| Cumulative stroke ≤90 days from index visit | 192 (2.5) | 5 (0.4) | 141 (2.6) | 46 (5.2) |
| Cumulative recurrent TIA ≤2 days from index visit | 81 (1.1) | 5 (0.4) | 56 (1.0) | 20 (2.3) |
| Cumulative recurrent TIA ≤7 days from index visit | 154 (2.0) | 15 (1.2) | 108 (2.0) | 31 (3.5) |
| Cumulative recurrent TIA ≤30 days from index visit | 261 (3.4) | 30 (2.4) | 180 (3.3) | 51 (5.8) |
| Cumulative recurrent TIA ≤90 days from index visit | 357 (4.7) | 44 (3.5) | 244 (4.4) | 69 (7.8) |
| Myocardial infarction ≤90 days from index visit | 26 (0.3) | 2 (0.2) | 16 (0.3) | 8 (0.9) |
| Admitted to hospital during index visit | 441 (5.8) | 40 (3.2) | 298 (5.4) | 103 (11.7) |
The Canadian TIA score was able to risk stratify patients into the three risk groups efficiently (table 3), with about one in six patients found to be at low risk (<1% risk for the primary outcome; interval likelihood ratio 0.20, 95% confidence interval 0.09 to 0.44), and one in eight at high risk (>5% risk; interval likelihood ratio 2.56, 2.02 to 3.25). The remainder were at medium risk, with a subsequent seven day event rate of 2.3% and an interval likelihood ratio of 0.94 (0.85 to 1.04). These risk strata were similar for the secondary outcome of subsequent stroke (table 4) and for risk stratification at both two and 90 days (appendix 2).
Table 3.
Classification performance, sensitivity, and specificity by score and risk category of Canadian TIA Score for subsequent stroke or carotid revascularisation within 7 days of index emergency department visit for transient ischaemic attack (n=7607)
| Score | No (%) total patients | No (%) estimated stroke or carotid revascularisation ≤7 days | No (%) observed stroke or carotid revascularization ≤7 days | Risk category | Proportion of patients (%) | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|---|
| –3 | 4 (0.1) | 0 (0.2) | 0 (0) | Low | 16.3 | 1.00 (0.98 to 1.00) | 0.00 (0.00 to 0.00) |
| –2 | 2 (0.0) | 0 (0.2) | 0 (0) | 1.00 (0.98 to 1.00) | 0.00 (0.00 to 0.00) | ||
| –1 | 35 (0.5) | 0 (0.3) | 0 (0) | 1.00 (0.98 to 1.00) | 0.00 (0.00 to 0.00) | ||
| 0 | 80 (1.1) | 0 (0.4) | 0 (0) | 1.00 (0.98 to 1.00) | 0.01 (0.00 to 0.01) | ||
| 1 | 148 (1.9) | 1 (0.5) | 1 (0.7) | 1.00 (0.98 to 1.00) | 0.02 (0.01 to 0.02) | ||
| 2 | 432 (5.7) | 3 (0.7) | 3 (0.7) | 0.99 (0.97 to 1.00) | 0.04 (0.03 to 0.04) | ||
| 3 | 541 (7.1) | 5 (0.9) | 2 (0.4) | 0.98 (0.94 to 0.99) | 0.09 (0.09 to 0.10) | ||
| 4 | 1245 (16.4) | 15 (1.2) | 15 (1.2) | Medium | 72.1 | 0.97 (0.93 to 0.99) | 0.17 (0.16 to 0.18) |
| 5 | 1499 (19.7) | 24 (1.6) | 22 (1.5) | 0.88 (0.83 to 0.93) | 0.33 (0.32 to 0.34) | ||
| 6 | 1042 (13.7) | 23 (2.2) | 28 (2.7) | 0.76 (0.70 to 0.82) | 0.53 (0.52 to 0.54) | ||
| 7 | 920 (12.1) | 26 (2.8) | 25 (2.7) | 0.61 (0.54 to 0.68) | 0.67 (0.66 to 0.68) | ||
| 8 | 778 (10.2) | 29 (3.7) | 34 (4.4) | 0.47 (0.40 to 0.55) | 0.79 (0.78 to 0.80) | ||
| 9 | 467 (6.1) | 23 (4.9) | 27 (5.8) | High | 11.6 | 0.29 (0.22 to 0.36) | 0.89 (0.88 to 0.90) |
| 10 | 228 (3.0) | 15 (6.4) | 11 (4.8) | 0.14 (0.09 to 0.20) | 0.95 (0.94 to 0.95) | ||
| 11 | 117 (1.5) | 10 (8.3) | 10 (8.5) | 0.08 (0.04 to 0.13) | 0.98 (0.97 to 0.98) | ||
| 12 | 54 (0.7) | 6 (10.7) | 2 (3.7) | 0.02 (0.01 to 0.06) | 0.99 (0.99 to 0.99) | ||
| 13 | 13 (0.2) | 2 (13.7) | 1 (7.7) | 0.01 (0.00 to 0.04) | 1.00 (1.00 to 1.00) | ||
| 14 | 2 (0.0) | 0 (17.4) | 1 (50.0) | 0.01 (0.00 to 0.03) | 1.00 (1.00 to 1.00) |
Table 4.
Canadian TIA Score: interval likelihood ratios and risk of outcome within 7 days (n=7607)
| Outcome | Interval likelihood ratio (95%CI) | Observed risk (%) | Estimated risk (%) | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Subsequent stroke/carotid revascularisation | |||||
| Low risk (−3 to 3) | 6 | 1236 | 0.20 (0.09 to 0.44) | 0.5 | 0.7 |
| Medium risk (4 to 8) | 124 | 5360 | 0.94 (0.85 to 1.04) | 2.3 | 2.1 |
| High risk (≥9) | 52 | 829 | 2.56 (2.02 to 3.25) | 5.9 | 6.3 |
| Subsequent stroke | |||||
| Low risk (−3 to 3) | 3 | 1239 | 0.17 (0.06 to 0.51) | 0.2 | 0.5 |
| Medium risk (4 to 8) | 81 | 5403 | 1.04 (0.93 to 1.16) | 1.5 | 1.3 |
| High risk (≥9) | 24 | 857 | 1.94 (1.36 to 2.78) | 2.7 | 3.3 |
Neither the ABCD2 nor the ABCD2i score was able to classify any patients as being low risk (<1% for subsequent stroke or carotid revascularisation within seven days). Moreover, these rules classified all but 3-7% of patients into a single, medium risk stratum (appendix 3 and appendix 4). The area under the curve for the Canadian TIA Score was higher than that of the ABCD2 or ABCD2i score (0.70 (95% confidence interval 0.66 to 0.73) versus 0.60 (0.55 to 0.64) or 0.64 (0.59 to 0.68)), indicating improved classification. The DeLong comparison of the C statistics for stroke or carotid revascularisation within seven days found a difference in the C statistic of 0.10 (95% confidence interval 0.05 to 0.15; P<0.001) between the Canadian TIA Score and the ABCD2 score and a difference in the C statistic of 0.06 (0.01 to 0.11; P=0.01) for the Canadian TIA Score versus the ABCD2i score (table 5 and fig 1). The ABCD2i score, but not the ABCD2 score, classified nearly half of patients as being at low risk for the secondary outcome restricted to subsequent stroke only, including about one third (25/74) of the patients who had carotid revascularisation but remained stroke-free at seven days, to this risk stratum.
Table 5.
DeLong method for comparing Canadian TIA Score with ABCD2 and ABCD2i scores for subsequent stroke or carotid revascularisation within 7 days (n=7607)
| Score or comparison | AUC (95% CI) | Difference in AUC (95% CI) | P value |
|---|---|---|---|
| Canadian TIA Score | 0.70 (0.66 to 0.73) | - | - |
| ABCD2 | 0.60 (0.56 to 0.64) | - | - |
| ABCD2i | 0.64 (0.59 to 0.68) | - | - |
| Canadian TIA Score v ABCD2 | - | 0.10 (0.05 to 0.15) | <0.001 |
| Canadian TIA Score v ABCD2i | - | 0.06 (0.01 to 0.11) | 0.01 |
AUC=area under curve; TIA=transient ischaemic attack.
Fig 1.
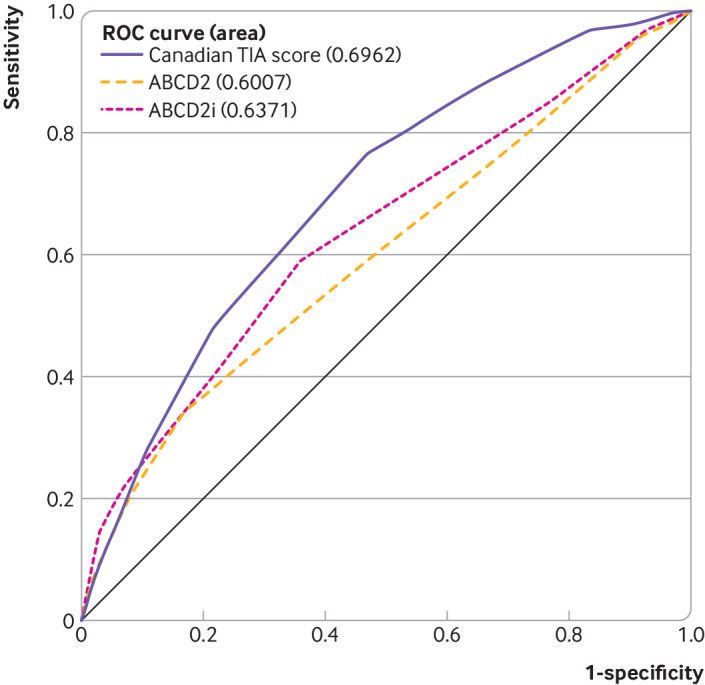
Receiver operating characteristic (ROC) curve for comparison of Canadian TIA Score with ABCD2 and ABCD2i scores for subsequent stroke or carotid revascularisation within 7 days (n=7607)
Figure 2 shows that the absolute net reclassification index between the Canadian TIA Score and the ABCD2 score for stroke or carotid revascularisation within seven days was 12.0%. The absolute net reclassification index between the Canadian TIA Score and the ABCD2i score was 8.5%.
Fig 2.
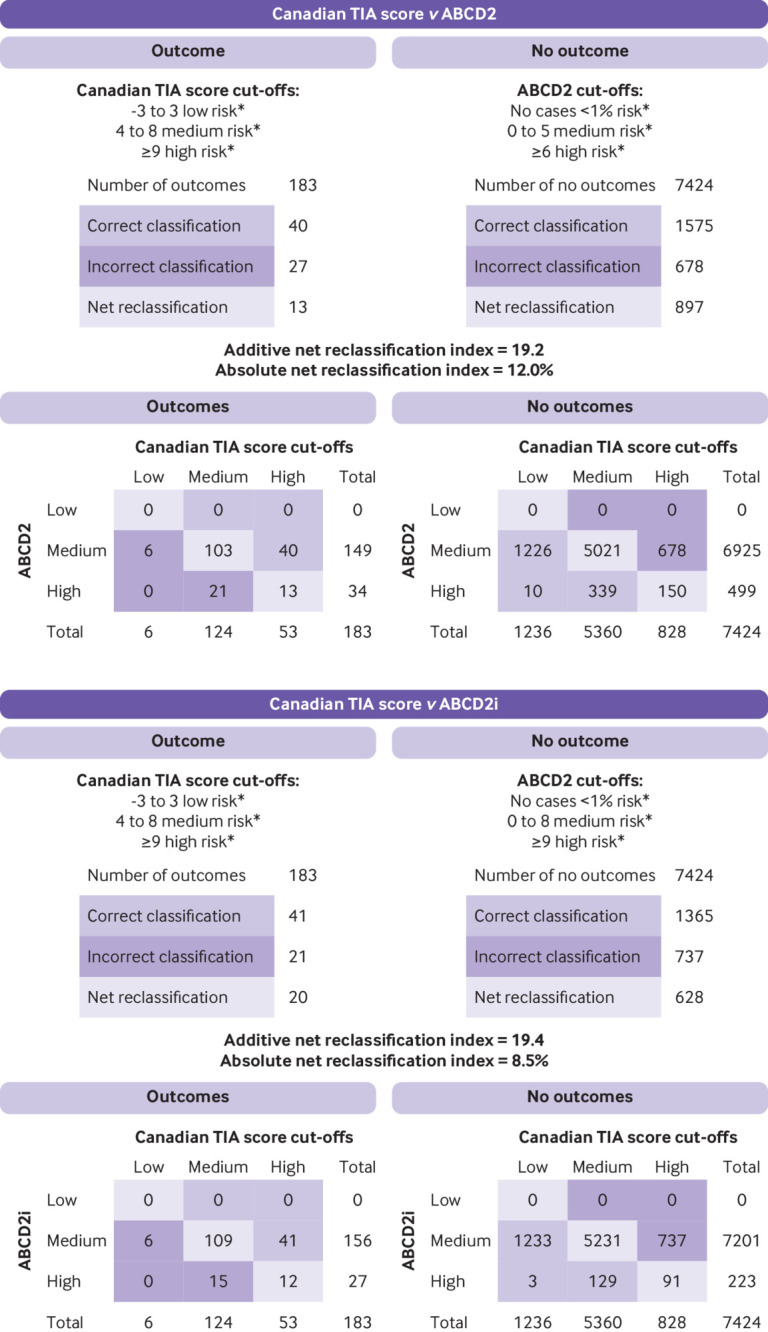
Net reclassification index comparing Canadian TIA Score with ABCD2 and ABCD2i for subsequent stroke or carotid revascularisation within 7 days (n=7607). *Low risk defined by risk of outcome of <1%, medium risk 1-5%, and high risk >5%
Discussion
Our study validates the predictive performance of the Canadian TIA Score in a broad sample of patients prospectively enrolled in the emergency department with a diagnosis of transient ischaemic attack or minor stroke. To improve the generalisability of the score, we included both community and academic centres, including six new sites that were not involved in the derivation study. The score was able to correctly stratify many more patients into pre-specified risk zones than were other scores based on the ABCD paradigm. Having withstood prospective validation in a newly assembled, contemporaneous cohort, and satisfying stringent criteria for the development of a clinical decision rule/score, this tool can now be adopted into clinical practice.
The performance of the score was consistent irrespective of whether we included immediate carotid revascularisation (that is, a potentially averted early stroke) as an outcome of interest. The ability of the score to stratify patients according to risk remained robust when we used only the outcome of subsequent stroke. Among the various changes in the management of transient ischaemic attack in the decade since we began developing this decision tool, carotid revascularisation for patients with high grades of carotid disease has been among the most notable, and its widespread adoption in Canadian stroke centres necessitated a change to the original study outcome of interest. We also increased our target sample size by half to ensure that we had sufficient precision for the original outcome of subsequent stroke alone, without considering carotid revascularisation.
The Canadian TIA Score performed significantly better than the ABCD2 scores. The ABCD2 based scores classified almost all patients as being at medium risk when using our pre-specified risk thresholds (low risk <1%, medium risk 1-5%, high risk >5%). This resulted in more patients with subsequent events being classified as at high risk by the Canadian TIA Score than by either of the ABCD2 scores. It also resulted in more patients without subsequent events being classified as at low risk. However, more patients were deemed to be at high risk by the Canadian TIA Score than both the ABCD2 scores (using thresholds of 6 for ABCD2 and 9 for ABCD2i). Both the ABCD2 and ABCD2i were designed as dichotomous scores. Therefore, in practice, many of the patients at medium risk would be deemed to be at high risk by the respective ABCD2 score. This dichotomy is limiting for practising physicians, and we believe that having three levels of risk provides clinicians with more options for management. When we compared the two ABCD2 scores, the ABCD2i score was better than the ABCD2 score; it identified many patients at low risk for the secondary outcome of subsequent stroke, but at the expense of missing many patients who underwent early carotid revascularisation. Although our score is more complex and is not intended to be memorised, it requires only routinely available information from the history, bedside assessment, and test results to stratify patients. It can be readily used and applied by physicians in the emergency department, as it does not require advanced neuroimaging, which is often unavailable. It allows one to customise the urgency of, for example, advanced neuroimaging or to inform the decision surrounding inpatient admission versus outpatient specialty consultation according to local preferences or to incorporate patients’ preferences. Many hospitals are unable to offer 24/7 access to magnetic resonance imaging and/or need to transfer patients for specialty consultation. Stratifying the risk for patients allows for more standardised care, more equitable deployment of constrained resources, and probably better outcomes.29 30
Comparisons with other studies
The definition of transient ischaemic attack continues to evolve and requires absence of infarction on magnetic resonance imaging.31 Although this definition provides greater diagnostic accuracy and excludes many non-ischaemic aetiologies that mimic transient ischaemic attack or stroke than the World Health Organization’s time based definition, it is not practical in emergency departments in much of the US, most of Canada, and most of the world, given the requirement for immediate magnetic resonance imaging. Hence, our work has emphasised a working emergency department diagnosis of transient ischaemic attack or minor stroke as the target population for the Canadian TIA Score.9 Conversely, an abnormal magnetic resonance imaging scan alone confers only a modest increase in subsequent risk of stroke, as shown in a recent study of patients diagnosed as having a possible transient ischaemic attack or minor stroke: very few had a subsequent stroke at one year despite 13.5% having abnormalities on imaging. Patients high risk features with or without positive diffusion weighted magnetic resonance imaging scans had a combined subsequent stroke rate of 0.7%.32 Five high risk features were identified for the composite outcome of subsequent stroke, subsequent transient ischaemic attack, death, or myocardial infarction, but the authors concluded that they were not sensitive enough in identifying patients with subsequent events to be used clinically.
We had previously surveyed both neurologists and emergency physicians to identify thresholds of stroke risk that would alter clinical decisions.26 27 In these studies, respondents indicated that patients with a subsequent risk of stroke below 1% were most appropriate for outpatient investigation, whereas patients with a subsequent risk of stroke above 5% constitute a high risk group that might benefit from comprehensive investigation, more intensive therapy, and possible admission at the time of the initial emergency department visit. These opinions reflect contemporary thinking in Canada but can serve as a starting point for important discussions on the allocation of resources in other settings, as well as for planning a future implementation study.
Strengths and limitations of study
Our study included a new cohort of patients (temporal validation) from new study sites (geographical validation) and included both academic and community hospitals. This large multicentre cohort study of patients with transient ischaemic attack prospectively assessed findings from the history, examination, and investigations to identify patients at highest risk for an impending stroke. We followed the methodological standards recommended for validation studies.13 14 15 Our study enrolled patients diagnosed, mostly by front line emergency physicians, as having had a transient ischaemic attack or minor stroke. Although some patients with mimics of transient ischaemic attack (that is, neurological symptoms not due to a transient ischaemic attack or stroke) were necessarily enrolled, these very patients are nevertheless part of the intended target population for risk stratification in the emergency department and comparable settings. Our use of blinded adjudication committees to assess subsequent strokes provided rigorous outcome classification. These committees were blinded to the initial emergency department visit documentation but used all sources of subsequent information available (telephone follow-up, clinic visits, testing, admissions). Our study also compared, prospectively, the ABCD2 and ABCD2i scores.
The use of a composite outcome could be criticised, given that a subsequent stroke typically has greater morbidity than a procedure to revascularise a carotid artery. However, we fully validated the score using the original outcome restricted to subsequent stroke (n=108). Some potentially eligible patients were missed, but we enrolled more than 80% of eligible patients, and missed patients seemed to be similar to enrolled patients. In addition, a small number of patients were lost to follow-up. Given that the rate of loss to follow-up was less than 1%, these cases are not likely to have a significant effect on our results.
The Canadian TIA Score includes 13 variables, so clinicians will probably need to use an online calculator or smartphone application to calculate the risk of their patients. Given that physicians already use these tools for many patients, this is likely a minor limitation and represents the heterogeneity of risk assessment for cerebral ischaemia.
Policy implications
Clinicians may now use the Canadian TIA Score to stratify patients as being at low, medium, or high risk for subsequent early stroke (with or without early carotid revascularisation). The optimal management pathway at the local or regional level can be determined on the basis of the expected risk at a given risk category (for example, same day computed tomography with routine follow-up for patients at low risk, computed tomography angiography and rapid follow-up for those at medium risk, and neurology consultation in the emergency department for those at high risk).
Research implications
A prospective multicentre implementation study following the established guidelines to implement a clinical prediction score is now needed to assess the impact of the Canadian TIA Score when applied in clinical practice. Additional research can further identify specific cut-off points for any given intervention and inform efforts to optimise stroke prevention. Further refinements and simplification of the rule are also important, especially as changes in diagnostic specificity and the intensity of initial investigation and treatment of transient ischaemic attack continue to evolve.
Conclusion
The Canadian TIA Score identifies the risk of patients with transient ischaemic attack for subsequent stroke or carotid artery revascularisation within seven days. Incorporating this validated risk estimate into management plans should improve decisions on the benefits of hospital admission at the index visit, urgency of testing and interventions, and prioritisation of specialist follow-up for patients discharged from the emergency department.
What is already known on this topic
Patients with transient ischaemic attack are at heightened risk for a subsequent major stroke or death, especially within the first few days
Optimising stroke prevention requires more precise risk stratification than existing tools can offer, to minimise both under-treatment and over-treatment
What this study adds
After transient ischaemic attack, the Canadian TIA Score outperformed other tools to stratify seven day risk of stroke, with or without carotid interventions
Incorporating this now validated core into management plans at the index emergency visit should improve early decision making on hospital admission, timing of investigations, and specialist referral
Acknowledgments
We thank the hundreds of physicians who completed our data collection forms and all the emergency department nurses and clerks at the 13 study sites for their cooperation with the study. We also thank the following research personnel at the study hospitals: Ottawa Hospital (Civic Campus and General Campus), Ottawa, Ontario (Rebecca Briscoe, Renée Labreche, Natalie Bilodeau, Tara Leach, Sarai Cohn-Kalter, Jane Sutherland, Juanita Wilzer, Ruth Glenwright, Carly O’Brien, Kathryn Madill, Alana Mistry, Kelly Smith, Connor Sheehan); Kingston General Hospital and Hotel Dieu Hospital, Kingston, Ontario (Jane Reid, Vlad Latiu, Jessica Montagner, Nicole O’Callaghan); Hôpital de L’Enfant-Jésus, Quebec City, Quebec (Suzy Lavoie); Hamilton Health Sciences Centre (General and Henderson Sites), Hamilton, Ontario (Natasha Clayton); Montfort Hospital, Ottawa, Ontario (Christine-Nadia Compas); Vancouver General Hospital, Vancouver, British Columbia (Vi Ho); Sacré Coeur Hospital, Montreal, Quebec (Chantal Lanthier); Sunnybrook Health Sciences Centre, Toronto, Ontario (Joanna Yeung); Queensway Carleton Hospital, Ottawa, Ontario (Karen Lemay, Katie Girimonte). We thank our colleagues at the Ottawa Hospital Research Institute (Sheryl Domingo, My-Linh Tran, and Angela Marcantonio) for their assistance with this project.
Web extra.
Extra material supplied by authors
Web appendix: Supplementary appendices
Contributors: JJP had the idea for the study and prepared the manuscript. GAW provided statistical assistance and revised the manuscript. MS and IGS provided input into the study design and revision of the manuscript. MJN assisted with the statistical analysis. MLAS, MÉ, AW, GS, JM, JL, AYJ, WJO, KWC, DJS, HEM, AM, SV, TS, JT, PT, SY, MCC, DJG, MIB, KA, NC, ES, and CA assisted with study design and revised the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. JJP is the guarantor.
Funding: The study was funded by the Canadian Institutes of Health Research. The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. Researchers are independent from funders, and all authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: the study was funded by the Canadian Institutes of Health Research; JJP is supported by a mid-career award from the Heart and Stroke Foundation of Ontario; JL is supported by the Schwartz/Reisman Emergency Medicine Institute inaugural research chair in geriatric emergency medicine; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study was approved by the research ethics board at each site as meeting the requirements for a waiver of written informed consent. Verbal consent was obtained at the time of each telephone call for patients contacted for follow-up.
Data sharing: Requests for sharing of the data will be considered and reviewed by the study’s steering committee. Requests can be made to the corresponding author.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: There is no formal plan to disseminate these results to study participants. All study sites have received study results. The results of this work will be disseminated through social media, conferences, and creation of an infographic. The results will be incorporated in the Ottawa Rules App, which will assist clinicians using the score.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Hill MD, Yiannakoulias N, Jeerakathil T, Tu JV, Svenson LW, Schopflocher DP. The high risk of stroke immediately after transient ischemic attack: a population-based study. Neurology 2004;62:2015-20. 10.1212/01.WNL.0000129482.70315.2F [DOI] [PubMed] [Google Scholar]
- 2. Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta-analysis. Arch Intern Med 2007;167:2417-22. 10.1001/archinte.167.22.2417 [DOI] [PubMed] [Google Scholar]
- 3. Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2007;6:1063-72. 10.1016/S1474-4422(07)70274-0 [DOI] [PubMed] [Google Scholar]
- 4. Gladstone DJ, Kapral MK, Fang J, Laupacis A, Tu JV. Management and outcomes of transient ischemic attacks in Ontario. CMAJ 2004;170:1099-104. 10.1503/cmaj.1031349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rothwell PM, Giles MF, Flossmann E, et al. A simple score (ABCD) to identify individuals at high early risk of stroke after transient ischaemic attack. Lancet 2005;366:29-36. 10.1016/S0140-6736(05)66702-5 [DOI] [PubMed] [Google Scholar]
- 6. Lovett JK, Dennis MS, Sandercock PAG, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke 2003;34:e138-40. 10.1161/01.STR.0000080935.01264.91 [DOI] [PubMed] [Google Scholar]
- 7. Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 2005;36:720-3. 10.1161/01.STR.0000158917.59233.b7 [DOI] [PubMed] [Google Scholar]
- 8. Johnston SC. Editorial comment--transient ischemic attacks are emergencies. Stroke 2005;36:724. 10.1161/str.36.4.724 [DOI] [PubMed] [Google Scholar]
- 9. Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA 2000;284:2901-6. 10.1001/jama.284.22.2901 [DOI] [PubMed] [Google Scholar]
- 10. Edlow JA, Kim S, Emond JA, Camargo CA. US Emergency Department visits for transient ischemic attack, 1992-2000. Acad Emerg Med 2003;10:432 10.1197/aemj.10.5.432 [DOI] [PubMed] [Google Scholar]
- 11. Goldstein LB, Bian J, Samsa GP, Bonito AJ, Lux LJ, Matchar DB. New transient ischemic attack and stroke: outpatient management by primary care physicians. Arch Intern Med 2000;160:2941-6. 10.1001/archinte.160.19.2941 [DOI] [PubMed] [Google Scholar]
- 12. Amarenco P, Lavallée PC, Labreuche J, et al. TIAregistry.org Investigators One-Year Risk of Stroke after Transient Ischemic Attack or Minor Stroke. N Engl J Med 2016;374:1533-42. 10.1056/NEJMoa1412981 [DOI] [PubMed] [Google Scholar]
- 13. Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA 1997;277:488-94. 10.1001/jama.1997.03540300056034 [DOI] [PubMed] [Google Scholar]
- 14. McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS, Evidence-Based Medicine Working Group Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. JAMA 2000;284:79-84. 10.1001/jama.284.1.79 [DOI] [PubMed] [Google Scholar]
- 15. Stiell IG, Wells GA. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med 1999;33:437-47. 10.1016/S0196-0644(99)70309-4 [DOI] [PubMed] [Google Scholar]
- 16. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283-92. 10.1016/S0140-6736(07)60150-0 [DOI] [PubMed] [Google Scholar]
- 17. Giles MF, Rothwell PM. Systematic review and pooled analysis of published and unpublished validations of the ABCD and ABCD2 transient ischemic attack risk scores. Stroke 2010;41:667-73. 10.1161/STROKEAHA.109.571174 [DOI] [PubMed] [Google Scholar]
- 18. Perry JJ, Sharma M, Sivilotti ML, et al. Prospective validation of the ABCD2 score for patients in the emergency department with transient ischemic attack. CMAJ 2011;183:1137-45. 10.1503/cmaj.101668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sheehan OC, Kyne L, Kelly LA, et al. Population-based study of ABCD2 score, carotid stenosis, and atrial fibrillation for early stroke prediction after transient ischemic attack: the North Dublin TIA study. Stroke 2010;41:844-50. 10.1161/STROKEAHA.109.571844 [DOI] [PubMed] [Google Scholar]
- 20. Giles MF, Albers GW, Amarenco P, et al. Addition of brain infarction to the ABCD2 Score (ABCD2I): a collaborative analysis of unpublished data on 4574 patients. Stroke 2010;41:1907-13. 10.1161/STROKEAHA.110.578971 [DOI] [PubMed] [Google Scholar]
- 21. Perry JJ, Sharma M, Sivilotti ML, et al. A prospective cohort study of patients with transient ischemic attack to identify high-risk clinical characteristics. Stroke 2014;45:92-100. 10.1161/STROKEAHA.113.003085 [DOI] [PubMed] [Google Scholar]
- 22. Meschia JF, Brott TG, Chukwudelunzu FE, et al. Verifying the stroke-free phenotype by structured telephone interview. Stroke 2000;31:1076-80. 10.1161/01.STR.31.5.1076 [DOI] [PubMed] [Google Scholar]
- 23. Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke-Free Status (QVSFS) by neurological history and examination. Stroke 2001;32:2232-6. 10.1161/hs1001.096191 [DOI] [PubMed] [Google Scholar]
- 24. Perry JJ, Stiell IG, Sivilotti ML, et al. High risk clinical characteristics for subarachnoid haemorrhage in patients with acute headache: prospective cohort study. BMJ 2010;341:c5204. 10.1136/bmj.c5204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. WHO MONICA Project Principal Investigators The World Health Organization MONICA Project (monitoring trends and determinants in cardiovascular disease): a major international collaboration. J Clin Epidemiol 1988;41:105-14. 10.1016/0895-4356(88)90084-4 [DOI] [PubMed] [Google Scholar]
- 26. Perry JJ, Losier JH, Stiell IG, Sharma M, Abdulaziz K. National Survey of Neurologists for Transient Ischemic Attack Risk Stratification Consensus and Appropriate Treatment for a Given Level of Risks. J Stroke Cerebrovasc Dis 2015;24:2514-20. 10.1016/j.jstrokecerebrovasdis.2015.06.034 [DOI] [PubMed] [Google Scholar]
- 27. Perry JJ, Losier JH, Stiell IG, Sharma M, Abdulaziz K. National survey of emergency physicians for transient ischemic attack (TIA) risk stratification consensus and appropriate treatment for a given level of risk. CJEM 2016;18:10-8. 10.1017/cem.2015.57 [DOI] [PubMed] [Google Scholar]
- 28. Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJM, Carotid Endarterectomy Trialists Collaboration Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915-24. 10.1016/S0140-6736(04)15785-1 [DOI] [PubMed] [Google Scholar]
- 29. Boulanger JM, Lindsay MP, Gubitz G, et al. Canadian Stroke Best Practice Recommendations for Acute Stroke Management: Prehospital, Emergency Department, and Acute Inpatient Stroke Care, 6th Edition, Update 2018. Int J Stroke 2018;13:949-84. 10.1177/1747493018786616 [DOI] [PubMed] [Google Scholar]
- 30. Wein T, Lindsay MP, Côté R, et al. Heart and Stroke Foundation Canadian Stroke Best Practice Committees Canadian stroke best practice recommendations: Secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke 2018;13:420-43. 10.1177/1747493017743062 [DOI] [PubMed] [Google Scholar]
- 31. Easton JD, Saver JL, Albers GW, et al. American Heart Association. American Stroke Association Stroke Council. Council on Cardiovascular Surgery and Anesthesia. Council on Cardiovascular Radiology and Intervention. Council on Cardiovascular Nursing. Interdisciplinary Council on Peripheral Vascular Disease Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276-93. 10.1161/STROKEAHA.108.192218 [DOI] [PubMed] [Google Scholar]
- 32. Coutts SB, Moreau F, Asdaghi N, et al. Diagnosis of Uncertain-Origin Benign Transient Neurological Symptoms (DOUBT) Study Group Rate and Prognosis of Brain Ischemia in Patients With Lower-Risk Transient or Persistent Minor Neurologic Events. JAMA Neurol 2019;76:1439-45. 10.1001/jamaneurol.2019.3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary appendices


