Abstract
BACKGROUND
Cardiovascular disease (CVD) has become an increasingly common limitation to effective anticancer therapy. Yet, whether CVD events were consistently reported in pivotal trials supporting contemporary anticancer drugs is unknown.
OBJECTIVES
The authors sought to evaluate the incidence, consistency, and nature of CVD event reporting in cancer drug trials.
METHODS
From the Drugs@FDA, clinicaltrials.gov, MEDLINE, and publicly available U.S. Food and Drug Administration (FDA) drug reviews, all reported CVD events across latter-phase (II and III) trials supporting FDA approval of anticancer drugs from 1998 to 2018 were evaluated. The primary outcome was the report of major adverse cardiovascular events (MACE), defined as incident myocardial infarction, stroke, heart failure, coronary revascularization, atrial fibrillation, or CVD death, irrespective of treatment arm. The secondary outcome was report of any CVD event. Pooled reported annualized incidence rates of MACE in those without baseline CVD were compared with reported large contemporary population rates using relative risks. Population risk differences for MACE were estimated. Differences in drug efficacy using pooled binary endpoint hazard ratios on the basis of the presence or absence of reported CVD were also assessed.
RESULTS
Overall, there were 189 trials, evaluating 123 drugs, enrolling 97,365 participants (58.5 5 years, 46.0% female, 72.5% on biologic, targeted, or immune-based therapies) with 148,138 person-years of follow-up. Over a median follow-up of 30 months, 1,148 incidents of MACE (375 heart failure, 253 myocardial infarction, 180 strokes, 65 atrial fibrillation, 29 revascularizations, and 246 CVD deaths; 792 in the intervention vs. 356 in the control arm; p < 0.01) were reported from the 62.4% of trials noting any CVD. The overall weighted-average incidence was 542 events per 100,000 person-years (716 per 100,000 in the intervention arm), compared with 1,408 among similar-aged non-cancer trial subjects (relative risk: 0.38; p < 0.01), translating into a risk difference of 866. There was no association between reporting CVD events and drug efficacy (hazard ratio: 0.68 vs. 0.67; p = 0.22).
CONCLUSIONS
Among pivotal clinical trials linked to contemporary FDA-approved cancer drugs, reported CVD event rates trail expected population rates.
Keywords: cancer clinical trials, cardiovascular disease, U.S. Food and Drug Administration, cardio-oncology, reporting of adverse events
Cardiotoxicity is an increasingly common, but important, limitation of effective cancer therapy (1). Over the past 2 decades, there has been a rapid uptake in the number of novel anticancer therapies, with >120 new U.S. Food and Drug Administration (FDA) drug approvals since 2000 alone (1). Many of these drugs have been associated with dramatic improvements in survival (1–7). However, concurrently, cardiovascular disease (CVD) has become increasingly prevalent among cancer patients receiving novel cancer therapies, with a reported incidence of up to 20% (8–13). Unfortunately, many of these events are not well explained by traditional CVD risk factors, and both their frequency and severity often differ from those reported in isolated supportive clinical trials (8–10,13).
In the United States, novel anticancer therapies are reviewed by the FDA for safety, with particular emphasis on supporting latter-phase clinical trials, before drug approval and release (14). Incumbent on these (pivotal) clinical trials is the rigorous reporting of potentially limiting or impactful adverse events to allow informed assessment (15). Among cancer patients, the burden of CVD risk (e.g., age or hypertenion) is generally elevated at the time of treatment initiation (16). In addition, available retrospective data have increasingly linked anticancer therapy initiation with a disproportionate rise in general CVD risk (10). As such, it may be reasonable to postulate that the observance of these events would not be uncommon. Yet, whether CVD events are routinely and consistently reported within these pivotal anticancer clinical trials is unknown.
METHODS
STUDY POPULATION.
Leveraging the Drugs@FDA database, we performed a manual search of all anticancer therapies given new drug application (NDA) approvals from January 1, 1998, to June 30, 2018 (14). Drugs or biologics approved for patients $18 years old were considered eligible for final consideration.
Non-cancer treatment therapies were excluded. We accessed publicly available FDA drug labeling and reporting sites, as well as medical and statistical reviews available at Drugs@FDA to comprehensively capture all drugs approved for anticancer treatment. Latter-phase (II and III) clinical trials tied to drug approval, including those identified through MEDLINE, published abstracts, trial supplements, clinicaltrial.gov registration, or reported on any other publicly available FDA drug reviews, were identified and extracted. Corresponding authors were contacted in cases of ambiguity regarding reporting. All drug trial data were procured and reviewed by 2 independent reviewers before entry. Following validation, all additional potentially relevant cancer and cardiovascular variables within the trials were collected, including study duration, funding source, number of participants, therapy class/type (e.g., biologic or immunotherapy), and preceding reports of cardiotoxicity within therapeutic-class (17). Cardiovascular events were defined as incident myocardial infarction, stroke, heart failure (HF) development, any coronary or peripheral revascularization, acute thromboembolism, myocarditis, cardiac tamponade, atrial fibrillation (AF) (or any other arrhythmia), valvular heart disease, significant ($grade 3 common terminology criteria for adverse event [CTCAE]) hypertension including hypertensive emergency, cardiovascular death, or any other mention of CVD (18).
OUTCOMES.
Our primary outcome was the reported rate of incident major adverse cardiovascular events (MACE), including myocardial infarction, stroke, congestive heart failure, AF, coronary revascularization, or cardiovascular death, among anticancer therapy trial participants. The secondary outcome was the report of any CVD event among all trial participants. Follow-up began from the time of drug initiation to the trial closure date for each approved drug, respectively.
STATISTICAL ANALYSIS.
Descriptive statistics were used to summarize patient characteristics, using mean ± SD or median (interquartile range) for continuous variables, and frequency counts with percentages for categorical variables. Depending on the expected cell counts of the corresponding contingency tables, chi-square or Fisher exact tests were used to explore the association between groups and other categorical variables. Multivariable stepwise logistic regression was used to assess for trial characteristics associated with CVD nonreporting. Specifically, all variables were initially considered for the multivariable model. Those variables meeting an entry significance level of p = 0.3 were allowed into the model, after which those above an exit significance level of p = 0.2 were sequentially removed from the final model. For this analysis, the covariates considered included: cancer type, therapeutic class, trial size, trial funding source, trial start of enrollment, year of approval, trial duration (months), trial setting, exclusion of patients with baseline CVD, prior cardiotoxicity within (same) therapeutic class, and excess CVD risk in preceding (safety) trials. Additionally, to further evaluate relationships considered, the Hosmer-Lemeshow test was used to assess parsimony of the model.
Person-years incidence rates for CVD events among trial participants were calculated to compare the annualized incidence of CVD events reported within the study trials and epidemiological rates from a contemporary similar-aged large-population, using the z-test of proportions. The prospective MESA (Multi-Ethnic Study of Atherosclerosis) observational cohort study measured contemporary incidence rates of CVD and served as our comparison population (19). This population was composed of middle-aged patients, free of clinical CVD, in whom the frequency of subsequent incident CVD events were tracked and captured. The proportion of CVD events reported was estimated by dividing the incidence of the CVD among trial participants by the total incidence among MESA participants. A calculated ratio of 1 indicates that the trial event rates approximates that of the epidemiological data. A ratio <0.8 or >1.2 indicates that CVD events were underreported or overreported, respectively, relative to the control population. Where available, reported CVD-specific event rates were compared with similar-aged contemporary (nontrial) population rates (20–27). Risk differences (RD) were calculated as: ([number of cases from our selected trials] [number of expected cases per the epidemiological data] 100,000)/person-years at risk (28). For example, consider a trial where the expected number of CVD events was 35 per 1,000 person-years of follow-up. If a clinical trial were to report 14 events per 1,000 person-years, the reported-to-expected ratio would be: 14 (events reported) / 35 (events expected) = 0.4.
To describe the differences in the magnitude of drug efficacy among trials that reported or did not report CVD events, we used pooled binary endpoint hazard ratios (HRs). More specifically, to correlate the relationship between the reporting of CVD events and trial therapy efficacy, we used reported HRs and 95% confidence intervals for trials that reported binary endpoints (progression-free survival, disease response, or mortality). These values accounted for individual trial size within subgroups. The results from these trials were expressed by stratification according to trial reporting of CVD events in a single forest plot, for visual assessment. Drug approvals by both primary cancer type (e.g., breast cancer) and therapeutic-class (e.g., immunotherapy) were considered. These drugs were primarily approved on the basis of efficacy data from a single trial. Due to the significant heterogeneity in continuous endpoints among trials and lack of consistent controls we focused on binary endpoints to describe efficacy. Percentages and mean ± SD values were reported to describe this distribution of categorical and continuous variables, respectively. All analyses were performed with SAS software version 9.4 (SAS Institute, Cary, North Carolina), and the statistical tests were 2sided with statistical significance evaluated at the α = 0.05 significance level.
RESULTS
Overall, there were 240 drugs given new drug application status, of which 123 met the pre-specified inclusion criteria, enrolling a total of 97,365 patients, including 57,978 in the intervention arm(s) (Online Figure 1). The median age of study participants was 61.0 years (interquartile range: 54.5 to 63.8 years), including 46.0% female, with a median trial size of 100 to 499 participants, whereas biologic, targeted, or immune-based therapies accounted for the majority (72.5%) of drug approvals (Table 1); threshold definiions for CVD exclusion were previously described (18). CTCAE was most commonly (81.0%) used for CVD threshold definitions. In total, 51.3% of trials did not report MACE, including 37.6% not reporting any CVD events in follow-up (Figure 1). In multivariable analysis, outside of trial duration, and time of trial initiation, no specific trial characteristics were associated with the presence or absence of reported CVD events (Table 1). There was no association between prior reported in-class cardiotoxicity and the subsequent reporting of CVD during trial follow-up (odds ratio: 2.03 [95% confidence interval: 0.92 to 4.50]).
TABLE 1.
Report of CVD in Follow-Up, by Cancer Trial Characteristic
| Trials | Patients | CVD Not Reported* | p Value† | ||
|---|---|---|---|---|---|
| Overall | 189 | 97,566 | 71 (37.6) | Univariate | Multivariable |
| Cancer type | |||||
| Breast | 22 (11.3) | 16,536 | 10 (45.4) | 0.078 | 0.070 |
| Colorectal/GI | 24 (12.7) | 13,441 | 6 (25.0) | ||
| Genitourinary | 33 (17.5) | 27,461 | 14 (42.4) | ||
| Leukemia | 24 (11.8) | 8,469 | 7 (29.2) | ||
| Lung | 19 (10.1) | 9,666 | 7 (36.8) | ||
| Lymphoma | 25 (12.8) | 4,402 | 5 (20.0) | ||
| Skin | 11 (5.8) | 7,048 | 6 (54.5) | ||
| Other | 31 (15.9) | 10,543 | 17 (54.8) | ||
| Therapeutic class | |||||
| Chemotherapy | 31 (16.4) | 14,014 | 19 (61.3) | <0.001 | 0.157 |
| Biologic or immunotherapy | 67 (35.5) | 33,747 | 25 (37.3) | ||
| Hormonal | 14 (7.4) | 17,845 | 7 (50.0) | ||
| Targeted | 70 (37.0) | 30,111 | 17 (24.3) | ||
| Other therapies | 7 (3.7) | 1,849 | 3 (42.9) | ||
| Trial size | |||||
| <100 | 21 (11.3) | 1,257 | 9 (42.9) | 0.889 | - |
| 100–499 | 97 (51.3) | 26,853 | 37 (38.1) | ||
| 500–999 | 54 (28.6) | 37,051 | 20 (37.0) | ||
| >1,000 | 17 (9.0) | 32,405 | 5 (29.4) | ||
| Trial phase‡ | |||||
| Phase 2 | 52 (27.5) | 7,569 | 17 (32.7) | 0.394 | - |
| Phase 3 | 137 (72.5) | 89,997 | 54 (39.4) | ||
| Funding source | |||||
| Industry 160 (84.7) | 87,469 | 59 (36.9) | 0.975 | - | |
| Government/nonprofit | 4 (2.1) | 1,325 | 2 (50.0) | ||
| Both | 25 (13.2) | 8,772 | 9 (36.0) | ||
| Start of enrollment | |||||
| Before 1998 | 18 (9.5) | 14,083 | 7 (43.7) | <0.001 | 0.003 |
| 1998–2002 | 30 (15.9) | 15,039 | 9 (28.1) | ||
| 2003–2007 | 39 (20.6) | 20,567 | 15 (38.5) | ||
| 2008–2012 | 67 (35.5) | 34,164 | 27 (40.3) | ||
| 2013–2018 | 35 (18.5) | 13,713 | 6 (17.1) | ||
| Year of approval | |||||
| 1998–2002 | 28 (14.4) | 10,732 | 14 (50.0) | 0.178 | 0.199 |
| 2003–2007 | 27 (14.3) | 14,258 | 13 (48.2) | ||
| 2008–2012 | 52 (27.5) | 35,801 | 19 (36.6) | ||
| 2013–2018 | 82 (43.4) | 36,775 | 25 (30.6) | ||
| Trial duration, months | |||||
| <24 | 55 (29.1) | 19,019 | 14 (25.5) | 0.013 | 0.001 |
| 25–36 | 63 (33.3) | 30,569 | 21 (33.3) | ||
| 37–48 | 22 (11.6) | 15,569 | 9 (40.9) | ||
| >48 | 37 (19.6) | 28,386 | 16 (43.2) | ||
| Not available | 12 (6.3) | 4,023 | 4 (33.3) | ||
| Trial setting | |||||
| International§ | 181 (95.8) | 95,885 | 65 (35.9) | 0.054|| | - |
| United States only | 8 (4.1) | 1,681 | 6 (75.0) | ||
| Cardiotoxicity within therapeutic class# | |||||
| Yes | 86 (45.5) | 39,531 | 27 (31.4) | 0.109 | 0.149 |
| No | 103 (54.5) | 58,035 | 44 (42.7) | ||
| Excess CVD risk in preceding (safety) trials | |||||
| Yes | 60 (31.8) | 25,911 | 20 (33.3) | 0.412 | - |
| No | 129 (68.3) | 71,655 | 51 (39.5) | ||
Values are n or n (%).
The percentages reported reflect a denominator of the number of trials within the subgroup considered.
The univariate p values presented describe associations estimated using Cochran-Armitage test for trend, chi-square, or Fisher exact tests, as appropriate. Use of the Cochran-Armitage trend test for the association of time (start of enrollment or year of approval), trial size, and duration with CVD reporting yielded p values of <0.001, 0.031, 0.039, and 0.513, respectively. For the final model, all variables were considered and those with p values <0.30 (i.e., cancer type, therapeutic class, start of trial enrollment, year of approval, trial duration, and prior cardiotoxicity within therapeutic class) were included in the multivariable stepwise regression model, where a p = 0.20 significance level was used to determine variables that remained in the final multivariable model.
Several therapies were approved on the basis of breakthrough phase II data, with ongoing or as yet to be initiated phase III trials.
Most international trials, included sites in the United States as well.
Not included in multivariable model due to low (United States only) cell count.
Prior established cardiotoxicity among drugs within the same therapeutic class.
CVD = cardiovascular disease; GI = gastrointestinal
FIGURE 1. Reported Cardiovascular Events During Follow-Up.
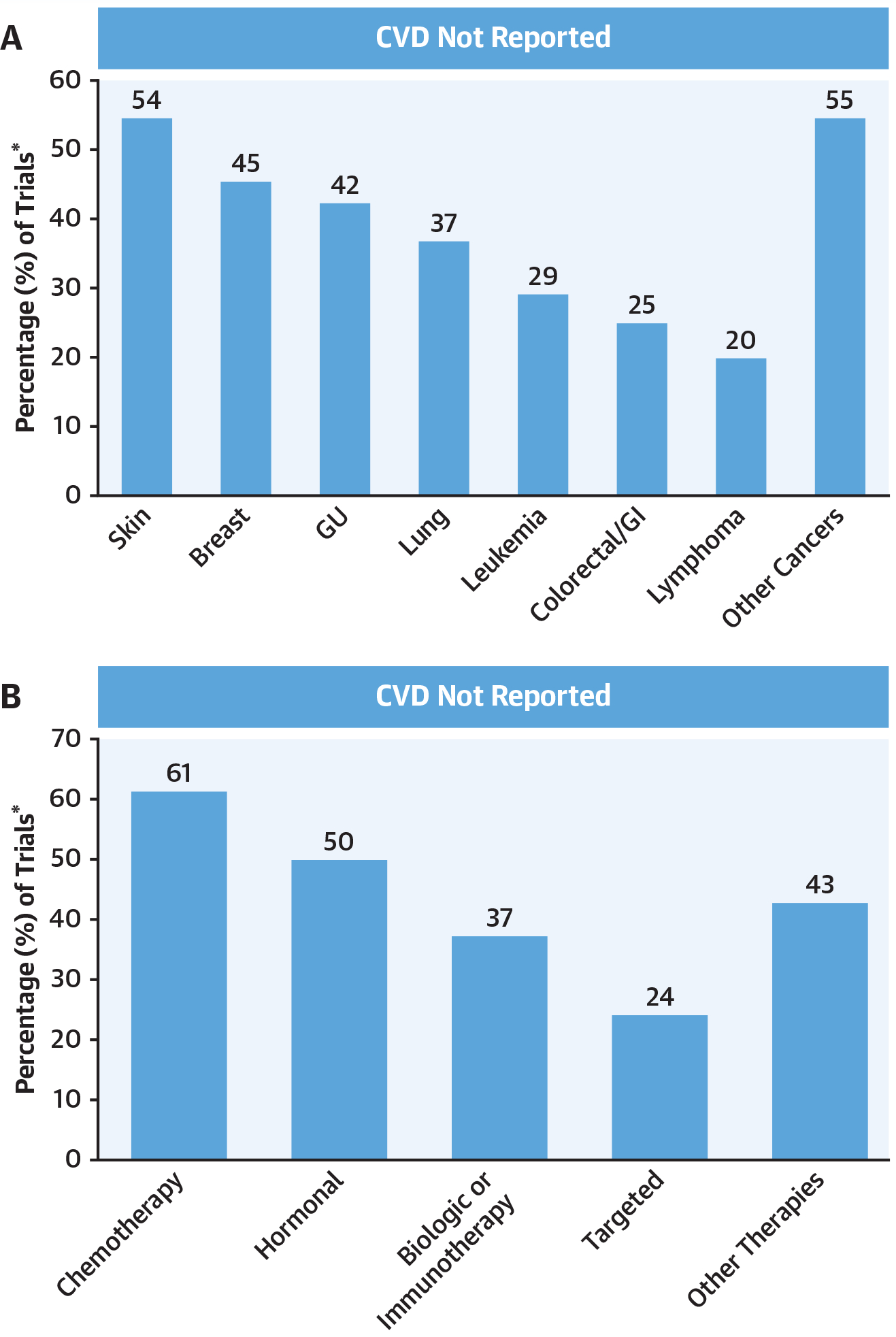
Events are stratified by cancer type (A), and therapeutic class (B), respectively. *The percentages reported reflect a denominator of the total number of trials within the subgroup considered. CVD = cardiovascular disease; GI = gastrointestinal; GU = genitourinary.
Over the available 148,138 person-years of follow-up (median trial duration 30 months, range of 12 to 92 months), there were 1,148 incident MACE events reported (375 HF, 253 ACS, 180 strokes, 65 AF, 29 coronary revascularizations, and 246 CVD deaths) (Table 2). Among trials that reported MACE, 792 were noted within the intervention arms compared with 356 in the control (p < 0.01). Furthermore, there were 4,739 total reported CVD events reported, with 3,142 noted in the intervention arms and 1,597 within the control arms (p < 0.01) of trials reporting CVD.
TABLE 2.
Types of CVD Events Reported
| Type of CVD Reported* | Trials† |
|---|---|
| Heart failure | 30 (15.9) |
| ACS | 13 (6.9) |
| Atrial fibrillation | 9 (4.8) |
| Uncontrolled HTN‡ | 30 (15.9) |
| Thromboembolic disease | 34 (18.0) |
| CVA | 26 (13.8) |
| Coronary revascularization§ | 1 (0.5) |
| Myocarditis | 0 (0.0) |
| Cardiovascular death|| | 56 (29.6) |
| Other CVD¶ | 17 (9.0) |
| Multiple CVD events# | 39 (20.6) |
Values are n (%).
The sum of the trial excluded is >100% and greater than the overall number of excluded trials because several studies used multiple exclusion criteria.
The percentages reported reflect a denominator of 189.
Usually defined as >150/90 mm Hg, consistent with mild (i.e., stage 1) hypertension by the available contemporary guidelines.
No trials reported peripheral revascularizations.
Includes those with sudden cardiac death or coronary artery disease–related death or any other mention of cardiovascular death.
Other CVD included valvular disease, myocarditis, or other mention of cardiovascular disease
Trials where multiple types of CVD events (e.g., ACS, and heart failure) were reported. ACS = acute coronary syndrome; CVA = cerebrovascular accident;
ACS = acute coronary syndrome; CVA = cerebrovascular accident; CVD = cardiovascular disease; HTN = hypertension.
There were 63 drugs, supported by 99 trials, approved at least in part on the basis of a binary efficacy endpoint. Among these trials, there was no difference in efficacy on the basis of CVD event reporting (HR: 0.68 vs. 0.67; p = 0.22) (Online Figure 2).
Moreover, among the 64 trials (34%) where patients with baseline CVD were excluded, 24 (37.5%) did not report any MACE. There were 269 MACE reported (157 HF, 18 ACS, 16 stroke, 13 AF, 52 CVD deaths, and no coronary revascularizations) from a total of 2,590 CVD events during a combined 49,660 years of trial patient follow-up. This translated into an overall weighted average reported incidence rate of 542 per 100,000 person-years (716 per 100,000 in the intervention arm) for MACE events. Compared with the reported incidence rate of 1,408 per 100,000 person-years among a similar aged noncancer trial population, this represented a reported-to-expected ratio of 0.385 (or 2.60-fold lower rate of reported events; p < 0.001) and a RD of 866 (Central Illustration). Similarly, CVD also appeared to be reported at lower rates compared with non-CVD events (Online Figure 3) (29). In those trials allowing persons with baseline CVD (125 trials), 57.6% did not report MACE. In these trials, there were 892 events (218 HF, 235 ACS, 164 strokes, 52 AF, 29 coronary revascularizations, and 194 CVD deaths) with 600 in the intervention versus 292 in the control arm (p < 0.01).
CENTRAL ILLUSTRATION. Cardiovascular Events in Pivotal Cancer Trials.
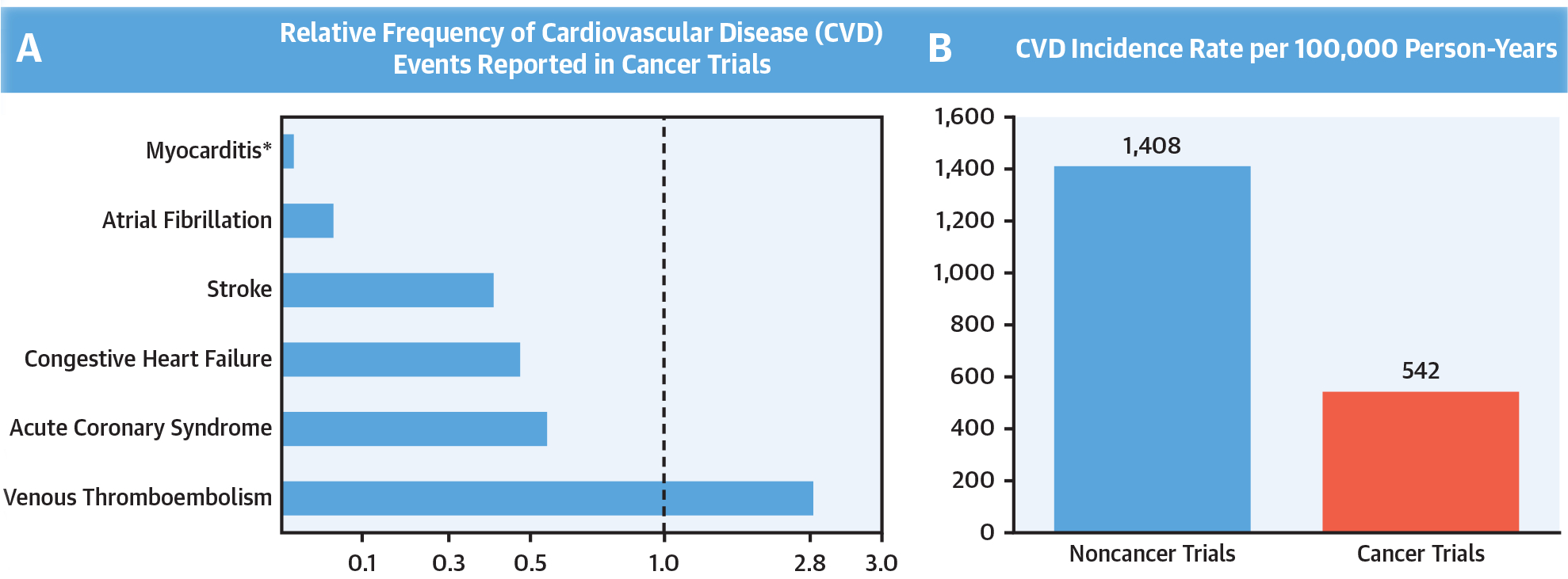
(A) Proportional frequency of incident reported cardiovascular disease(CVD)events during cancer trial follow-up, compared with reported contemporary nontribal population estimates (19–27) Plots stratified by CVD event types (hypertension not shown to due to high variation in rates based on drug type). (B) Reported versus observed cumulative CVD incidence rates, depicted in events per 100,000 person-years of available follow-up. Estimates reflect major adverse cardiovascular events from supporting anticancer trials compared with observed rates in a similar-aged contemporary population. *There were zero events reported for myocarditis
Stratification by all available CVD event types showed lowest relative reporting rates for AF and myocarditis, respectively, with higher rates of venous thromboemboli (Central Illustration, panel A). Further, when considering all anticancer trial subjects irrespective of baseline CVD status (97,365 participants), the overall reported-to-expected ratio remained low, at 0.564 (or 1.77-fold lower rate of reported events; p < 0.001) and a RD of 613.
DISCUSSION
In this evaluation of pivotal clinical trials supporting the FDA approval of contemporary anticancer therapies, nearly 40% of trials did not report any CVD events in follow-up. In those trials reporting CVD events, the noted rates of events were markedly lower than that observed among general, nontrial populations. This pattern remained, even after accounting for the presence of preceding reports of excess therapeutic-class CVD, and the inclusion (or exclusion) of patients with baseline CVD. These findings suggest a general underreporting and/or appreciation of CVD events among cancer clinical trial participants. This is concerning, particularly given the growing prevalence of limiting CVD among patients treated with novel anticancer therapies (8–13). Moreover, these discrepancies may in part underlie the discordance between perceived CVD risk and that observed in actual clinical care (1,8,11,13).
Clinicians and patients faced with challenging decisions regarding the use of potentially life-saving anticancer therapies rely on the consistency of reporting within supporting clinical trials (30,31). Often within this review, significant heterogeneity in the frequency and interpretation of CVD events was observed. This was exemplified by the absence of reported events with hormonal therapies in early breast cancer trials, and by low rates of CVD in early immune checkpoint therapy trials among lung cancer treatment trials, populations known to be at elevated risk for incident CVD events and where CVD has emerged as a limiting factor (3,32–36). And although some uptake was seen in reporting among more recent studies, well after 2006, a time when more uniform federal regulations regarding adverse event reporting were announced, many of the more recent trials evaluating therapies with apparent cardiotoxicities did not report a signal of CVD (2,3,8,11,12,36).
Furthermore, threshold definitions of CVD varied between studies (e.g., cardiomyopathy was defined as clinical HF in some trials, whereas objective decline in ejection fraction to <50% was used in others) (17,36). Observance of these discrepancies, coupled with increasingly appreciated real-world events, suggest enhanced emphasis on more objective thresholds for CVD identification may be of benefit (8,13).
A careful balance between anticancer drug efficacy and toxicity is necessary for the accurate interpretation of benefit and risk. Due to the often debilitating nature of CVD, significant priority to its reporting, even where final attribution is not known, is crucial for the accurate determination of risk (8–10). Within cancer clinical trials, the adoption and adherence to systemic reporting of adverse events is standard for the evaluation of clinical data (30,31). However, many trials focused primarily on anticancer efficacy, with likely limited awareness of the impacts of CVD, may still have underreported CVD events, particularly given their prevalence in the general population (37,38). In addition, significant variability in the interpretation of the signs and symptoms of CVD among more focused providers may limit the reporting of potentially relevant events (37). Furthermore, although mortality and anticancer drug discontinuation are frequently considered, attribution as to the exact factors relating to these events are often underreported (38). Despite these challenges, reporting of these potentially major or lifethreatening events is inadequate. Given the high morbidity and mortality associated with CVD, understanding these patterns may bear insight into the factors relating to the effects of CVD in this growing population.
Our results provide significant insights into the nature of CVD reporting within pivotal cancer trials. Prior, more isolated or disease-specific studies have suggested potential discrepancies in the reporting patterns of CVD events within cancer-focused trials (13,38,39). This is exemplified by trastuzumab, a monoclonoal antibody against erb-b2, where the trial-reported incidence of HF was 2.5% even in combination with anthracyclines, but later in practice, was observed to approach 20.1% upon focused CVD evaluation (38,39). Similarly, initial rates of myocarditis and incident CVD following initiation of immune checkpoint therapy were <0.05% (3,4). However, although the exact incidence is unclear, the rates appear to approach nearly 2%, depending on the population studied (35,40). Despite our observation of some uptake in reporting among more recent studies, solutions for these discrepancies will require more systematic and prospective evaluation. Yet, consideration for the implementation of routine centralized event adjudication committees, review of post-trial patient-specific international classification of diseases code event entries, cost-effective CVD screening, or the use of patient-reported outcomes may improve capture of potentially relevant events (30,37,41,42).
STUDY LIMITATIONS.
This study focused on latter phase pivotal clinical trials, and did not include data from early stage, palliative, or nonefficacious drugrelated trials. However, given the desire to best reflect drugs commonly used in contemporary clinical practice, we focused on these high-profile and more well-controlled studies. Also, CVD thresholds and definitions were not consistently specified beyond use of CTCAE cardiac grades, which often rely heavily on clinical interpretation (e.g., myocarditis) (18). The number of U.S.-only trials was relatively small. Due to absence of uniform primary source data across trials, competing risk analysis could not be performed. Available contemporary noncancer comparison CVD reporting was not readily available. Further, the methods of CVD event collection and adjudication varied across trials. CVD (non)-reporting rates among recent non-cancer trial populations were not readily available for comparison. Broad, controlled cancerspecific populations with robust CVD reports over time were not readily available (36). As such, the use of a contemporaneous, but general middle-aged population for comparison of CVD event rates may not reflect or may potentially even underestimate event rates among cancer patients (43). Moreover, clinical trial participants are often healthier than real-world patients. Yet, even when allowing for consideration of trials inclusive of patients with baseline CVD, low reporting patterns remained. Also, use of a similar population free of CVD for comparison would at least in part reduce this bias. Additionally, the use of potential CVD preventative agents, such as statins, was not routinely reported despite extensive search.
CONCLUSIONS
CVD is an increasingly important limitation of effective cancer therapy. Among clinical trials supporting contemporary anticancer therapies, cardiovascular events were frequently not reported. Enhanced focus on the consistency and systematic nature of CVD reporting following cancer treatment initiation is needed.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN SYSTEMS-BASED PRACTICE:
The frequency of cardiovascular events in clinical trials supportingcurrent cancer therapies is lower than expected population rates. Careful interpretation should be used when assessing the cardiotoxicity risk from these trials.
TRANSLATIONAL OUTLOOK:
Additional studies are neededto determine whether incorporating patient-reported outcomesor more rigorous event adjudication enhances the consistency of cardiovascular event reporting in clinical trials of cancer therapeutics.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grant P30 CA016058, and by NIH grants KL2-TR002734 (Dr. Brammer), R01-CA238946 (Dr. Lustberg), and K12-CA133250 (Dr. Addison). Ms. Baker was supported by the Ohio State University Comprehensive Cancer Center’s Pelotonia grant funds. Dr. Carter is supported in part by charitable contributions from the Mary H. and J. Churchill Hodges Clinical Prevention Program Fund at the Ohio State University Wexner Medical Center. Dr. Awan has received research funding from Innate Pharma, and Pharmacyclics; has provided consulting services to Gilead Sciences, Pharmacyclics, Janssen, Abbvie, Sunesis, AstraZeneca, Genentech, and Novartis Oncology; and has served on the Speakers Bureau of Abbvie and AstraZeneca. Dr. Paskett has received research funding from Merck, Foxconn, Pfizer, and The American Cancer Society; and has been a stockholder in Pfizer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- CVD
cardiovascular disease
- CTCAE
common terminology criteria for adverse event
- FDA
U.S. Food and Drug Administration
- HF
heart failure
- HR
hazard ratios
- MACE
major adverse cardiovascular events
- RD
risk differences
Footnotes
APPENDIX For supplemental figures, please see the online version of this paper.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363: 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373:1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid P, Adams S, Rugo HS, et al. , IMpassion130 Trial Investigators. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–21. [DOI] [PubMed] [Google Scholar]
- 5.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364: 501–13. [DOI] [PubMed] [Google Scholar]
- 6.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369: 507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dizon DS, Krilov L, Cohen E, et al. Clinical Cancer Advances 2016: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 2016;34:987–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moslehi JJ, Salem JE, Sosman JA, LebrunVignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guha A, Derbala MH, Zhao Q, et al. Ventricular arrhythmias following ibrutinib initiation for lymphoid malignancies. J Am Coll Cardiol 2018;72:697–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navi BB, Reiner AS, Kamel H, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol 2017;70:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santoni M, Guerra F, Conti A, et al. Incidence and risk of cardiotoxicity in cancer patients treated with targeted therapies. Cancer Treat Rev 2017; 59:123–31. [DOI] [PubMed] [Google Scholar]
- 12.Waxman AJ, Clasen S, Hwang WT, et al. Carfilzomib-associated cardiovascular adverse events: a systematic review and meta-analysis. JAMA Oncol 2018;4:e174519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2008;26:5204–12. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. Drugs@FDA. Available at: https://www.accessdata.fda.gov/scripts/cder/daf/. Accessed February 28, 2019.
- 15.U.S. Food and Drug Administration. CFRCode of Federal Regulations Title 21. April 2019. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=312.32. Accessed April 29, 2019.
- 16.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation 2016;133:1104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonsu J, Charles L, Guha A, et al. Representation of patients with cardiovascular disease in pivotal cancer clinical trials. Circulation 2019;139: 2594–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institute, Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0. Bethesda, MD: National Cancer Institute, 2017. [Google Scholar]
- 19.de Lemos JA, Ayers CR, Levine BD, et al. Multimodality strategy for cardiovascular risk assessment: performance in 2 population-based cohorts. Circulation 2017;135:2119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benjamin EJ, Virani SS, Callaway CW, et al. , American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137: e67–492. [DOI] [PubMed] [Google Scholar]
- 21.Cooper LT Jr., Keren A, Sliwa K, Matsumori A, Mensah GA. The global burden of myocarditis: part 1: a systematic literature review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 study. Glob Heart 2014;9:121–9. [DOI] [PubMed] [Google Scholar]
- 22.Kytö V, Sipilä J, Rautava P. Clinical profile and influences on outcomes in patients hospitalized for acute pericarditis. Circulation 2014;130: 1601–6. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez CJ, Soliman EZ, Alonso A, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the Multi-Ethnic Study of Atherosclerosis. Ann Epidemiol 2015;25:71–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flueckiger P, Longstreth W, Herrington D, Yeboah J. Revised Framingham stroke risk score, nontraditional risk markers, and incident stroke in a multiethnic cohort. Stroke 2018;49:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chahal H, Bluemke DA, Wu CO, et al. Heart failure risk prediction in the Multi-Ethnic Study of Atherosclerosis. Heart 2015;101:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budoff MJ, Young R, Lopez VA, et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2013;61: 1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell EJ, Lutsey PL, Basu S, et al. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med 2016;129:339.e19–339.e3.39E26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott PE, Unger EF, Jenkins MR, et al. Participation of women in clinical trials supporting FDA approval of cardiovascular drugs. J Am Coll Cardiol 2018;71:1960–9. [DOI] [PubMed] [Google Scholar]
- 29.Scharf O, Colevas AD. Adverse event reporting in publications compared with sponsor database for cancer clinical trials. J Clin Oncol 2006;24: 3933–8. [DOI] [PubMed] [Google Scholar]
- 30.Calvert M, Kyte D, Mercieca-Bebber R, et al. , the SPIRIT-PRO Group. Guidelines for inclusion of patient-reported outcomes in clinical trial protocols: the SPIRIT-PRO extension. JAMA 2018;319: 483–94. [DOI] [PubMed] [Google Scholar]
- 31.American Society of Clinical Oncology. The State of Cancer Care in America, 2017: a report by the American Society of Clinical Oncology. J Oncol Pract 2017;13:e353–94. [DOI] [PubMed] [Google Scholar]
- 32.Breast International Group (BIG) 1–98 Collaborative Group, Thürlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med 2005;353:2747–57. [DOI] [PubMed] [Google Scholar]
- 33.Garon EB, Rizvi NA, Hui R, et al. , KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018–28. [DOI] [PubMed] [Google Scholar]
- 34.Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 2018;19:1579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375: 1457–67. [DOI] [PubMed] [Google Scholar]
- 37.Seruga B, Templeton AJ, Badillo FE, et al. Under-reporting of harm in clinical trials. Lancet Oncol 2016;17:e209–19. [DOI] [PubMed] [Google Scholar]
- 38.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012:CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowles EJ, Wellman R, Feigelson HS, et al. Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 2012;104:1293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71: 1755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 2015;1:1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol 2007;25:5121–7. [DOI] [PubMed] [Google Scholar]
- 43.Barac A, Murtagh G, Carver JR, et al. Cardiovascular health of patients with cancer and cancer survivors: a roadmap to the next level. J Am Coll Cardiol 2015;65:2739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


