What is PCOS?
PCOS is a highly heritable complex genetic disorder 1. It is the most common disorder of reproductive-age women, affecting up to 15% of this population worldwide, depending on the diagnostic criteria applied 2, 3. PCOS is a leading cause of anovulatory infertility, obesity, and type 2 diabetes (T2D) 3, 4. It is a syndrome, a collection of signs and features, of unknown etiology. Despite its high prevalence and major morbidities, affected women remain remarkably underserved 1. Diagnosis is frequently delayed 5 and physicians are often poorly informed about PCOS 6.
Pathophysiology.
PCOS is characterized by enhanced luteinizing hormone (LH) relative to follicle stimulating hormone (FSH) release, increased LH-dependent ovarian testosterone (T) production, frequent adrenal androgen excess, profound insulin resistance, dysglycemia, and obesity 1, 3. Anti-Müllerian hormone (AMH) levels are increased in PCOS, and recent rodent 7 and human 8 studies suggest AMH plays a direct role in PCOS pathogenesis.
PCOM is characterized by an excessive number of antral follicles, which may be the result of accelerated follicle growth and/or prolonged survival of small follicles 9, 10. Additional hallmarks of PCOM are ovarian stromal hypertrophy, theca cell hyperplasia, and ovarian cortical thickening 11. Theca cells in women with polycystic ovaries (PCO) secrete more androgens, basally and in response to LH and insulin 12. Abnormalities of both theca and granulosa cells may contribute to the arrest of follicular development seen in PCOS 12–14.
Diagnostic Criteria
NIH Criteria.
Stein and Leventhal are generally credited with the original description in 1935 of what has come to be known as PCOS 15, although there are clear reports of the disorder dating back to Hippocrates in the 5th century BCE 16. However, it was not until 1990 that there was a formal effort to develop standard diagnostic criteria as part of a meeting of experts in medical and reproductive endocrinology sponsored by the NIH 17. The participants were asked to vote on the potential diagnostic features of PCOS; features receiving the most votes, clinical and/or biochemical evidence of hyperandrogenism (HA) and ovulatory dysfunction (OD) with the exclusion of secondary causes, became known as the NIH criteria 17. These criteria did not include PCOM because, even at that time, it was recognized that PCOM was present in 20–30% of women with regular menses and no hyperandrogenic symptoms 18. The prevalence of PCOS has been constant at 5–8% using the NIH criteria 19.
Rotterdam Criteria.
In 2003, the European Society for Human Reproduction and Embryology and the American Society for Reproductive Medicine (ASRM) sponsored another meeting of experts in Rotterdam, Netherlands, during which PCOM was added as a diagnostic criterion 20, 21. The Rotterdam criteria required two of the three features for the diagnosis of PCOS: (1) OD, (2) HA, and (3) PCOM. The intent of the Rotterdam criteria was to encompass the NIH criteria as well as to broaden the definition of PCOS. The result was two new phenotypes, HA+PCOM, and OD+PCOM (Figure 1). The prevalence of PCOS using the Rotterdam criteria is as high as ~15% of reproductive age women 22, 23.
Figure 1: Comparing Components of Different Diagnostic Criteria.
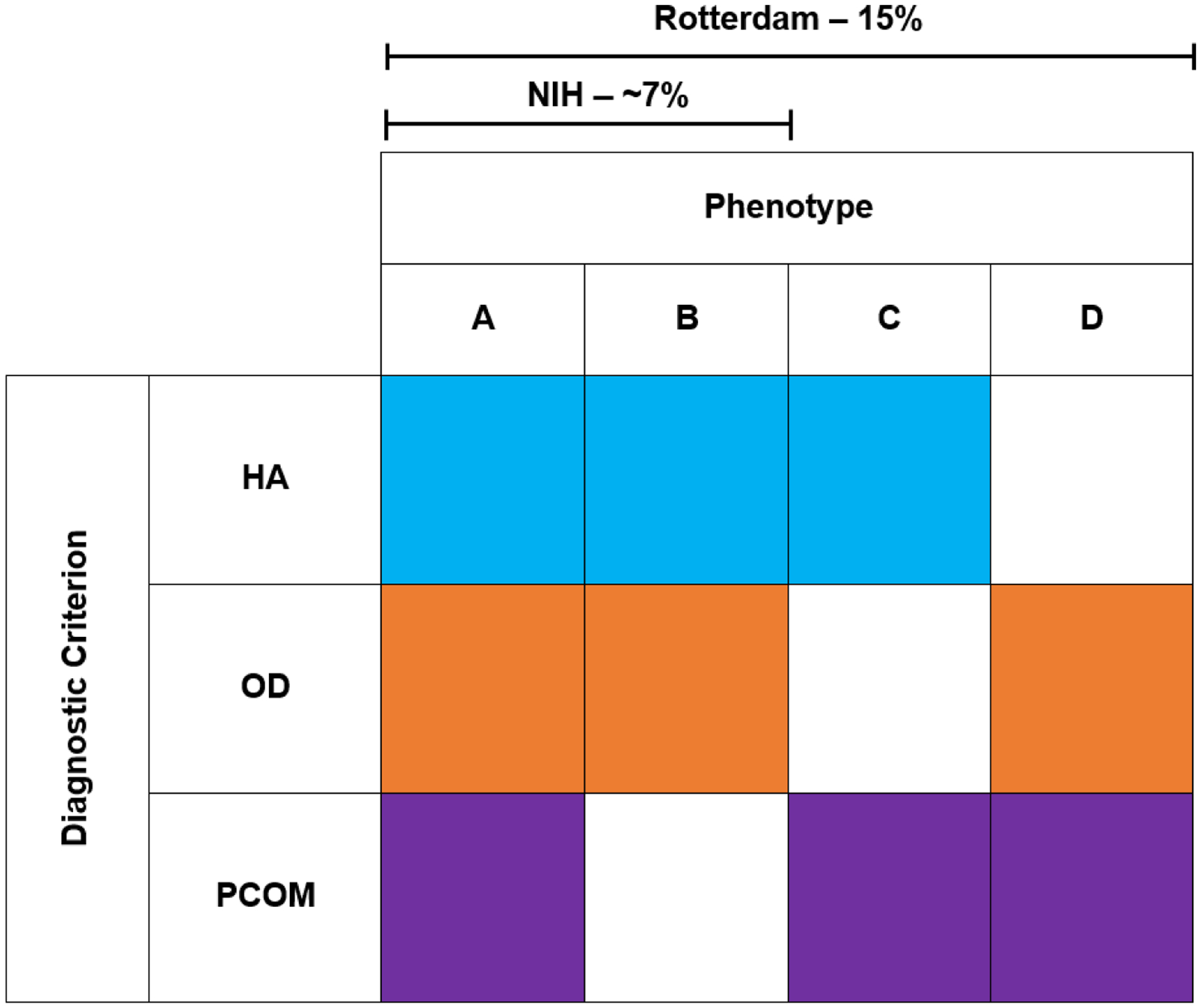
NIH PCOS must include both HA+OD, +/− PCOM. There are no endocrine or metabolic differences between phenotypes A and B; there is no need to assess PCOM in NIH PCOS. Rotterdam Criteria requires at least 2 out of the 3 criteria to be present, adding 2 new phenotypes HA+PCOM and OD+PCOM. The prevalence of PCOS increases from ~7% to 15% when including the two non-NIH Rotterdam phenotypes.
AES Criteria.
In 2006, the Androgen Excess Society (now known as the Androgen Excess-PCOS Society [AE-PCOS]) convened another task force of experts 24. This group recommended that HA should be a requirement for the diagnosis of PCOS, which limited the diagnosis of PCOS to three of the four Rotterdam phenotypes: HA+OD+PCOM, HA+OD, and HA+PCOM 24, 25. The AE-PCOS criteria have never been widely adopted.
NIH-sponsored Evidence-based Methodology Workshop on PCOS.
In 2012, the NIH sponsored an evidence-based methodology workshop on PCOS to review the current state-of-the-science in the field 26. This meeting differed from the previous PCOS meetings in that it followed a formal consensus process. Although it was not an official NIH Consensus Development Conference (https://consensus.nih.gov/), the meeting followed the same “court” model where the evidence is presented to a panel that functions as a jury. The panel consisted of individuals who are experts in their fields (Gynecology, Diabetes and Metabolism, Cardiology, and Primary Care) but are not engaged in PCOS research, allowing independent assessment of the scientific evidence by an unbiased panel. The panel’s final report noted that “the name ‘PCOS’ was a distraction and an impediment to progress,” and that the emphasis on PCOM created confusion because it was neither necessary nor sufficient for the diagnosis of PCOS 26. They recommended using the Rotterdam criteria with precise specification of phenotype (Figure 1) and proposed a comprehensive research agenda that included assessment of the epidemiology and long-term health outcomes of the PCOS phenotypes.
Evidence-Based Guidelines.
Consensus conferences have since been replaced by evidence-based guidelines for the diagnosis and management of PCOS, such as those published in 2013 by the Endocrine Society 27, and the 2018 International Evidence-Based Guideline 28–30. However, the quality of the evidence upon which these guidelines are based is predominantly low due to a paucity of randomized clinical trials (RCTs). Unfortunately, the key research initiatives recommended by the 2012 Evidence-based Methodology Workshop on PCOS to critically assess the utility of the PCOS diagnostic criteria as well as the optimal therapies and long-term health outcomes have not been undertaken, in large part due to underfunding of the field 31. Of the 34 recommendations in the Endocrine Society Clinical Practice Guideline 27, the evidence supporting 24 of these was rated as “low” or “very low”. There were almost 175 recommendations in the International Guideline 28–30, of which only 31 were ranked as evidence-based, the remainder were clinical consensus recommendations or clinical practice points.
Limitations of the Individual Diagnostic Criteria
Hyperandrogenism
Clinical HA.
Signs of HA in women include hirsutism, acne, and alopecia. Male pattern terminal hair growth is a fairly reliable indicator of androgen action 32. The development of hirsutism is determined by number of pilosebaceous units and their sensitivity to androgen action, which are genetically determined and vary by race and ethnicity 32, 33. While increased male-pattern terminal hair growth assessed by ethnicity specific Ferriman-Gallwey scores is pathognomonic for HA, as many as 50% of HA women do not have clinically significant terminal hair growth 34. Furthermore, women often remove unwanted hair using mechanical methods. Acne and alopecia can reflect HA but are not reliable enough to use as surrogate markers for androgen excess 35, 36.
Biochemical HA.
Hyperandrogenemia is characterized by elevated circulating endogenous androgen levels. Testosterone (T) circulates specifically bound to sex hormone binding globulin (SHBG) and loosely associated with albumin; only ~1% of circulating T is free T (FT) 37. Both FT and albumin-associated T are biologically available 37 and are referred to collectively as non-SHBG bound T (NSB-T). FT is elevated in ~70% of women with PCOS 25. However, NSB-T provides a better index of T that is bioavailable 38.
The main limitation of biochemical HA as a diagnostic criterion is that it can be difficult to detect at the lower circulating levels present in women and children 39. Measuring T is challenging because steroids are structurally similar, and antibodies used in immunoassays can cross-react with other steroids 39. The gold standard for measuring TT is liquid chromatography followed by tandem mass spectrometry (LC/MS-MS) 39; this is the method recommended by the Endocrine Society 39 and the Centers for Disease Control 40. Unfortunately, some clinical laboratories still use inaccurate TT assays, such as electrochemiluminescence immunoassays (ECLIA), performed directly on serum or plasma (direct assays) 39. These methods lack sensitivity and specificity and should not be used 39, especially in women and children.
The gold standard for measuring FT is equilibrium dialysis; However, it is expensive, cumbersome, and subject to variability if not correctly performed 39. Direct FT assays are unreliable and should not be used 39. Therefore, guidelines 25, 39 recommend calculating FT and NSB-T based on the binding affinity of T to SHBG and albumin utilizing the law of mass action 41. It is possible to order calculated FT and NSB-T as part of T profiles offered by clinical laboratories. It is also easy to perform these calculations using measured TT and SHBG values (e.g. http://www.issam.ch/freetesto.htm); albumin levels can be assumed to be normal 41. Obviously, the accuracy of these calculations will depend on the quality of the TT assay 39. Unreliable TT assays may lead to a failure to detect biochemical HA and result in misclassification of NIH PCOS as the non-NIH Rotterdam OD+PCOM phenotype (Table 1).
Table 1:
Misclassification of Rotterdam PCOS Phenotypes
| HA | OD | PCOM | Interpretation | |
|---|---|---|---|---|
| Each criterion appropriately assessed | NIH phenotype | |||
| HA not detected due to use of incorrect TT or FT assay | Non-NIH Rotterdam phenotype | |||
| Anovulation not detected due to reported regular menses | Non-NIH Rotterdam phenotype | |||
| Ovarian morphology not detected due to technology, operator, or patient factors | NIH phenotype |
Assessment of HA should include measurement of dehydroepiandrostenedione sulfate (DHEAS) because a substantial minority of women with HA have isolated DHEAS elevations 25. DHEAS is also a marker of adrenal androgen-secreting neoplasms. Measurement of androstenedione does not increase the detection of HA in most cases 42. Recently, 11-oxygenated C19 steroids, such as 11β-hydroxyandrostenedione, have been recognized as an important pool of circulating adrenal androgens 43, including in women with PCOS 44. Nevertheless, there is no current evidence to suggest that these steroids should be included in the clinical assessment of HA 45.
Ovulatory Dysfunction.
Twenty to thirty percent of women with PCOS who report regular menstrual cycles have been found to have anovulatory cycles with biochemical assessment of ovulation 24, 25. Oligomenorrhea (defined as <6–8 menstrual cycles per year) is virtually pathognomonic for anovulation, but more frequent cycles do not necessarily indicate ovulation. A recent study of >600,000 menstrual cycles revealed that there is more variation within normal menstrual cycles than previously recognized 46, suggesting that it may be difficult to determine ovulatory status based solely on self-report of 21–35 day cycles. To increase detection of anovulatory cycles, the ASRM recommends that women with other signs of PCOS who report regular menstrual cycles undergo ovulatory monitoring using basal body temperatures or measurement of a serum progesterone level in the luteal phase 47. Lack of confirmation of ovulation can lead to misclassification of NIH PCOS as the ovulatory non-NIH Rotterdam HA+PCOM phenotype (Table 1).
PCOM.
Despite the intrinsic abnormalities seen in PCO 9–11, the presence of PCOM is neither necessary nor sufficient for diagnosis of PCOS 18, 48. There is no evidence that the presence of PCOM has any implications with regard to the endocrine or metabolic features of PCOS 27, 49. There is also no evidence to support the addition of assessment of PCOM to the diagnosis of NIH PCOS. Indeed, the International Evidence-Based Guidelines state that ultrasound is not needed for the diagnosis of PCOS in women with HA+OD 28–30. Similarly, the Endocrine Society Guideline for the management of hirsutism states that demonstrating PCOM to diagnose ovulatory PCOS (HA+PCOM) is unlikely to affect management of hirsutism 50. Further, PCOM is so common in adolescents that ultrasound is not recommended for the diagnosis of PCOS in this age range 28–30, 42, 51.
Ovarian ultrasound is a critical component of assisted reproductive technology (ART), as assessment of antral follicle count predicts ovarian responses to medications 52 as well as risk of ovarian hyperstimulation syndrome 53. All women undergoing infertility evaluation and treatment routinely have pelvic ultrasounds at baseline and throughout their treatment to monitor follicular growth. It is important to note that the detection of PCOM is dependent on the sensitivity of the ultrasound equipment, the skill of the operator, the approach (vaginal vs abdominal) and the weight of the patient 28–30. Further, ovarian ultrasound examinations performed by clinical radiologists frequently do not use the Rotterdam Criteria to interpret their findings. Therefore, it is challenging for medical endocrinologists to obtain an accurate ovarian ultrasound assessment of PCOM. In summary, assessment of PCOM is not needed for the endocrine management of PCOS or hirsutism; access to accurate ovarian sonography is limited outside of reproductive endocrinology.
PCOS Phenotypes
Application of the Rotterdam Criteria results in four PCOS phenotypes: A) HA+OD+PCOM; B) HA+OD, C) HA+PCOM, and D) OD+PCOM (Figure 1). Phenotypes A and B are also known as NIH PCOS; phenotypes C and D are also known as non-NIH Rotterdam PCOS. However, there is no evidence that there are endocrine or metabolic differences between HA+OD and HA+OD+PCOM 27, 49. Thus, there is no rationale for stratifying HA+OD cases by the presence of absence of PCOM. Stratification by the presence or absence of hirsutism has been proposed in the Androgen Excess Society Guidelines 24 adding 12 potential phenotypes. However, hirsutism is a distinct biologic process related to androgen action and there are insufficient data to support using it to further subgroup PCOS 50.
Towards a Biological Basis for PCOS Classification
Current PCOS Classification.
Michael Crichton stated “the work of science has nothing whatever to do with consensus. Consensus is the business of politics. Science, on the contrary, requires only one investigator who happens to be right…consensus is evoked only in situations where the science is not solid enough” 54. Despite being labeled as consensus criteria, all of the PCOS diagnostic criteria are based on expert opinion—the lowest level of evidence 55. As better scientific evidence accumulates, consensus is no longer necessary. Unfortunately, the newer so-called evidence-based PCOS guidelines 27–30 are still largely based on expert opinion because of the paucity of high quality RCTs addressing most features of PCOS 31.
Validation of Diagnostic Criteria.
Diagnostic criteria should identify discrete biologically meaningful subgroups that are stratified by associated disease risk, biomarker profiles, responses to treatment, and/or genetic architecture. The consistent difference among the PCOS phenotypes is the severity of insulin resistance. It has been well established that the NIH PCOS phenotype is at higher risk for insulin resistance and associated metabolic abnormalities as compared to non-NIH Rotterdam PCOS phenotypes 3, 56, 57. Ovulatory PCOS (HA+PCOM) tend to have lower BMI than the NIH phenotype 58 and have either mild or no metabolic abnormalities 59.
While current criteria have identified two major subgroups (NIH vs. non-NIH Rotterdam) with differing metabolic risk, a recent well-powered meta-analysis of PCOS genomewide association studies (GWAS) has suggested that current criteria do not identify genetically distinct subgroups 60. GWAS permit the agnostic interrogation of the entire genome for regions that are associated with a given trait or disease and provide insight into causal pathways 61. GWAS in PCOS case-control cohorts of Han Chinese and European ancestry have implicated pathways regulating gonadotropin secretion and action, androgen biosynthesis, insulin resistance, and ovarian aging in the pathogenesis of PCOS 1, 62, 63. The largest genetic analysis of PCOS to date—a GWAS meta-analysis that contained >10,000 PCOS cases and 100,000 controls—compared NIH, non-NIH Rotterdam and self-reported PCOS, and found no significant differences in genetic architecture for 13 of 14 identified loci 60. There was one locus that was significantly more strongly associated with NIH PCOS than with non-NIH Rotterdam and self-reported PCOS 60, which suggests that this locus is involved in pathways regulating insulin action since NIH PCOS are more insulin resistant than the other PCOS phenotypes. This metanalysis suggests that, overall, the genetic architecture of NIH PCOS, non-NIH Rotterdam PCOS and self-reported PCOS is similar.
Objective Approaches to PCOS Classification.
Cluster analysis is a well-established mathematical approach to aggregate traits into clusters of similar data. Cluster analysis is being increasingly applied to large biomedical datasets to identify subtypes of disorders such as T2D 64. We performed unsupervised hierarchical cluster analysis in women of European ancestry with PCOS diagnosed by NIH criteria using quantitative anthropometric, reproductive, and metabolic traits and identified two distinct clusters, which we designated “reproductive” and “metabolic” (Figure 2) 65. The “reproductive” subtype had higher LH and SHBG levels with relatively low body mass index (BMI) and insulin levels, whereas the “metabolic” subtype had higher BMI, glucose, and insulin levels with lower SHBG and LH levels 65. We replicated these clusters in an independent cohort of European ancestry NIH PCOS cases. GWAS was performed limiting the cases to the “reproductive” or “metabolic” subtypes. Five novel susceptibility loci were discovered, four associated with the “reproductive” subtype and one associated with the “metabolic” subtype 65. Our findings suggest that these subtypes are biologically relevant since they appear to have distinct genetic architectures. This study presents an objective, unbiased mathematical approach to PCOS classification and validates this approach by demonstrating that the reproductive and metabolic subtypes thus identified are associated with novel and distinct gene regions. These PCOS subtypes need to be replicated in PCOS diagnosed by Rotterdam criteria as well as in PCOS cohorts of non-European ancestry.
Figure 2: Principal component analysis plot of quantitative traits for a genotyped PCOS clustering cohort showing a biologically driven method of PCOS classification.
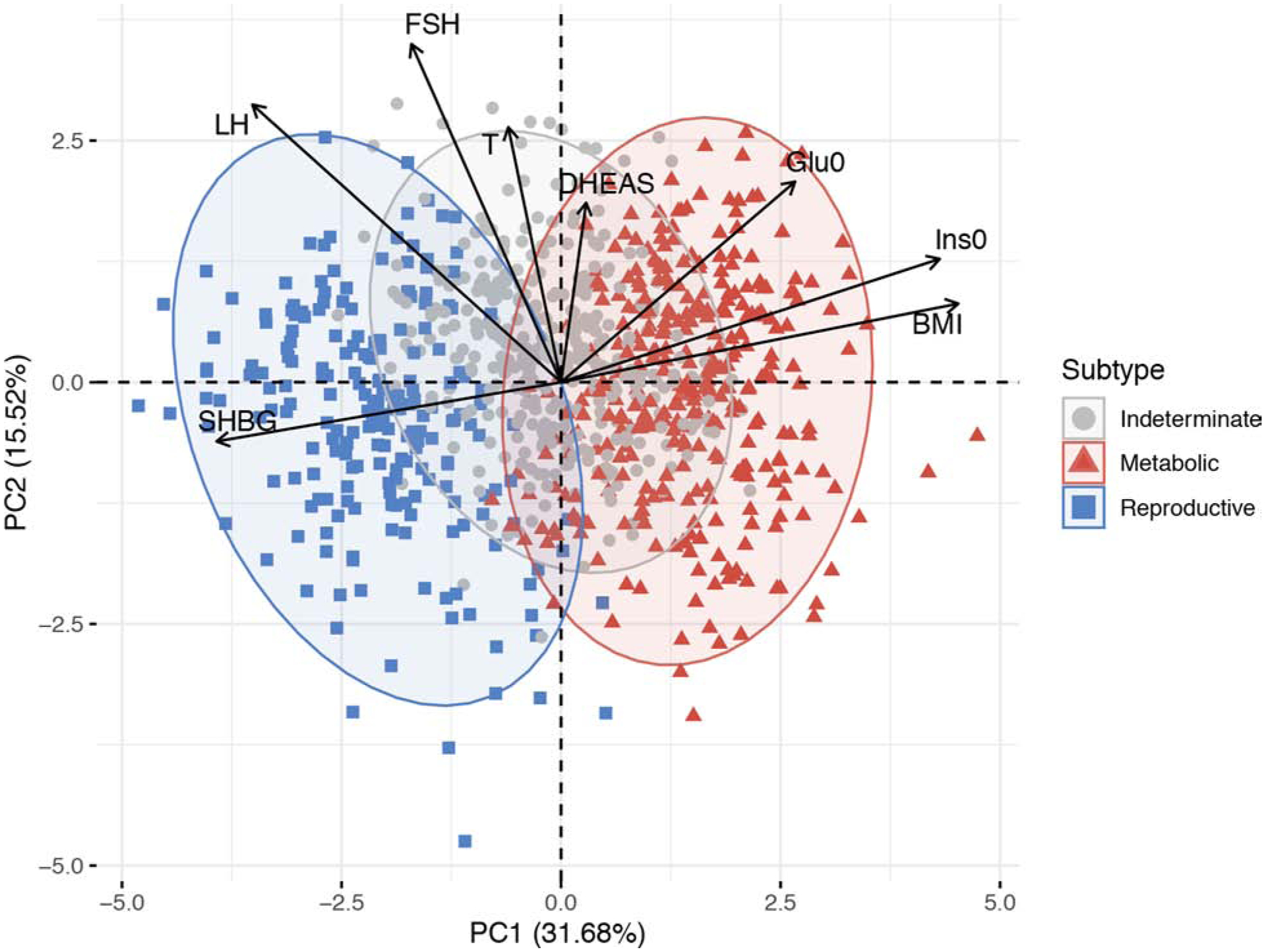
Women with PCOS were clustered into distinct groups—metabolic, reproductive, and indeterminate—based on BMI, fasting insulin, fasting glucose, DHEAS, T, FSH, LH, SHBG, and genotype data. The relative magnitude and direction of trait correlations with the principal components are shown with black arrows. PC=principal component; Ins0= fasting insulin; Glu0= fasting glucose.
(From Dapas M, Lin FTJ, Nadkarni GN, et al. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. 2020;17(6):e1003132; with permission.)
Which PCOS Diagnostic Criteria to Use When.
The NIH criteria of HA+OD are sufficient to detect women at high risk of insulin resistance and associated metabolic abnormalities 3. The presence of PCOM has no impact on the diagnosis or endocrine management of NIH PCOS. In contrast, ovulatory women with HA are at low risk for insulin resistance and associated metabolic abnormalities, whether or not they have PCOM 3, 56. Therefore, medical endocrinologists should focus on determining whether an HA patient is ovulatory. Usually, OD is not a subtle finding as it is accompanied by oligomenorrhea (<6–8 menses per year). However, since a substantial minority of women reporting regular menstrual cycles may be anovulatory, it is prudent to confirm ovulation in this subgroup of HA patients 47. Glucose tolerance and lipid levels should be evaluated in NIH PCOS 3, 27, 66.
Symptom-based management for hirsutism, or for acne and alopecia with biochemically documented HA, can be undertaken in ovulatory women without additional metabolic screening 3. As highlighted in the Endocrine Society Guideline for the management of hirsutism, establishing a diagnosis of Rotterdam PCOS by assessing ovarian morphology will not affect management 50. Indeed, there is no evidence to support the use of the Rotterdam criteria for the diagnosis of PCOS, i.e. the addition of PCOM, for the management of endocrine or metabolic features of PCOS. In contrast, treatment of anovulatory infertility associated with PCOS using ART requires monitoring of ovarian morphology. Assessment of HA is important for establishing a diagnosis of NIH PCOS since these patients require monitoring for associated metabolic abnormalities. However, the presence or absence of HA does not affect short-term reproductive management.
Summary and Conclusions
The diagnostic criteria for PCOS are based upon expert opinion and do not identify genetically distinct subgroups of women. Further, each individual diagnostic criterion has limitations that may lead to misclassification between PCOS phenotypes. The endocrine management of PCOS does not require assessment of ovarian morphology. Unsupervised cluster analysis of clinical and biochemical traits has shown promise in identifying genetically distinct metabolic and reproductive PCOS subtypes. Such objective approaches will enable the transition from PCOS classification based on expert opinion, which is subjective, to classification based on demonstrable biologic differences.
Synopsis:
Current diagnostic criteria for PCOS are based on expert opinion. We will review the rationale for and the limitations of these criteria, as well as which criteria to use when. The insights provided into PCOS pathogenesis by modern genetic analyses and the promise of objective data mining approaches for biologically-relevant disease classification will be discussed.
Key Points.
Current diagnostic criteria for polycystic ovary syndrome (PCOS) are based on expert opinion, the lowest level of evidence. There has never been a formal consensus process to determine criteria for the diagnosis of PCOS.
Individual diagnostic criteria have limitations that may result in misclassification of National Institutes of Health (NIH) and non-NIH Rotterdam PCOS phenotypes.
While current criteria have identified two major groups (NIH and non-NIH Rotterdam) that have different metabolic risks, recent genetic analyses have suggested that these criteria do not identify biologically distinct PCOS subtypes.
Diagnosis of PCOS should therefore depend on management goals. The assessment of polycystic ovarian morphology (PCOM) is not needed to manage the endocrine and metabolic features of PCOS but is critical for reproductive endocrinologists managing infertility associated with PCOS.
Agnostic data mining approaches have identified PCOS subtypes (metabolic and reproductive) that appear to be genetically distinct, potentially providing a biologically meaningful way to establish criteria for the diagnosis of PCOS.
Clinics Care Points.
Hirsutism is sufficient to diagnose HA but not all HA women are hirsute.
Acne and alopecia are not reliable markers of HA.
LC/MS-MS assay is needed for accurate TT measurement. FT and NSB-T should be calculated based on TT and SHBG.
Women presenting with HA who report regular menses should undergo testing to confirm ovulation.
Ovarian morphology is not needed for the endocrine management of PCOS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
References
- 1.Dunaif A Perspectives in Polycystic Ovary Syndrome: From Hair to Eternity. J Clin Endocrinol Metab. March 2016;101(3):759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab. June 1999;84(6):1897–9. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. December 2012;33(6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubin KH, Glintborg D, Nybo M, Abrahamsen B, Andersen M. Development and Risk Factors of Type 2 Diabetes in a Nationwide Population of Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 10 2017;102(10):3848–3857. [DOI] [PubMed] [Google Scholar]
- 5.Gibson-Helm M, Teede H, Dunaif A, Dokras A. Delayed Diagnosis and a Lack of Information Associated With Dissatisfaction in Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 02 2017;102(2):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin AW, Bergomi EJ, Dollahite JS, Sobal J, Hoeger KM, Lujan ME. Trust in Physicians and Medical Experience Beliefs Differ Between Women With and Without Polycystic Ovary Syndrome. J Endocr Soc. September 2018;2(9):1001–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tata B, Mimouni NEH, Barbotin AL, et al. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 06 2018;24(6):834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gorsic LK, Kosova G, Werstein B, et al. Pathogenic Anti-Müllerian Hormone Variants in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 08 2017;102(8):2862–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webber LJ, Stubbs S, Stark J, et al. Formation and early development of follicles in the polycystic ovary. Lancet. September 2003;362(9389):1017–21. [DOI] [PubMed] [Google Scholar]
- 10.Webber LJ, Stubbs SA, Stark J, et al. Prolonged survival in culture of preantral follicles from polycystic ovaries. J Clin Endocrinol Metab. May 2007;92(5):1975–8. [DOI] [PubMed] [Google Scholar]
- 11.Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. February 1982;37(2):59–77. [DOI] [PubMed] [Google Scholar]
- 12.Gilling-Smith C, Willis DS, Beard RW, Franks S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J Clin Endocrinol Metab. October 1994;79(4):1158–65. [DOI] [PubMed] [Google Scholar]
- 13.Mason HD, Willis DS, Beard RW, Winston RM, Margara R, Franks S. Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J Clin Endocrinol Metab. November 1994;79(5):1355–60. [DOI] [PubMed] [Google Scholar]
- 14.Willis DS, Watson H, Mason HD, Galea R, Brincat M, Franks S. Premature response to luteinizing hormone of granulosa cells from anovulatory women with polycystic ovary syndrome: relevance to mechanism of anovulation. J Clin Endocrinol Metab. November 1998;83(11):3984–91. [DOI] [PubMed] [Google Scholar]
- 15.Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–191. [Google Scholar]
- 16.Azziz R, Dumesic DA, Goodarzi MO. Polycystic ovary syndrome: an ancient disorder? Fertil Steril. April 2011;95(5):1544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: Towards a rational approach In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, eds. Polycystic ovary syndrome. Polycystic ovary syndrome; 1992:377–384. [Google Scholar]
- 18.Polson DW, Adams J, Wadsworth J, Franks S. Polycystic ovaries--a common finding in normal women. Lancet. April 1988;1(8590):870–2. [DOI] [PubMed] [Google Scholar]
- 19.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 12 2016;31(12):2841–2855. [DOI] [PubMed] [Google Scholar]
- 20.group REA-SPcw. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. January 2004;19(1):41–7. [DOI] [PubMed] [Google Scholar]
- 21.Group REA-SPCW. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. January 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 22.Fauser BC, Tarlatzis BC, Rebar RW, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. January 2012;97(1):28–38.e25. [DOI] [PubMed] [Google Scholar]
- 23.March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. February 2010;25(2):544–51. [DOI] [PubMed] [Google Scholar]
- 24.Azziz R, Carmina E, Dewailly D, et al. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. November 2006;91(11):4237–45. [DOI] [PubMed] [Google Scholar]
- 25.Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. February 2009;91(2):456–88. [DOI] [PubMed] [Google Scholar]
- 26.NIH. Evidence-based Methodology Workshop on Polycystic Ovary Syndrome. presented at: 2012-EXECUTIVE SUMMARY (Final Report); December 3–5 2012; Accessed March 10, 2020 https://prevention-archive.od.nih.gov/docs/programs/pcos/FinalReport.pdf
- 27.Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. December 2013;98(12):4565–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 08 2018;110(3):364–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Clin Endocrinol (Oxf). 09 2018;89(3):251–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 09 2018;33(9):1602–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brakta S, Lizneva D, Mykhalchenko K, et al. Perspectives on Polycystic Ovary Syndrome: Is Polycystic Ovary Syndrome Research Underfunded? J Clin Endocrinol Metab. 12 2017;102(12):4421–4427. [DOI] [PubMed] [Google Scholar]
- 32.Paparodis R, Dunaif A. The Hirsute woman: challenges in evaluation and management. Endocr Pract. September 1 2011;17(5):807–18. [DOI] [PubMed] [Google Scholar]
- 33.Lee HJ, Ha SJ, Lee JH, Kim JW, Kim HO, Whiting DA. Hair counts from scalp biopsy specimens in Asians. J Am Acad Dermatol. February 2002;46(2):218–21. [DOI] [PubMed] [Google Scholar]
- 34.Azziz R, Ehrmann D, Legro RS, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. April 2001;86(4):1626–32. [DOI] [PubMed] [Google Scholar]
- 35.Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC. Acne vulgaris. Nat Rev Dis Primers. 09 2015;1:15029. [DOI] [PubMed] [Google Scholar]
- 36.Lin RL, Garibyan L, Kimball AB, Drake LA. Systemic causes of hair loss. Ann Med. 09 2016;48(6):393–402. [DOI] [PubMed] [Google Scholar]
- 37.Rosenfield R, Moll G. The role of proteins in the distribution of plasma androgens and estradiol In: G M, L M, V J, eds. Androgenization in Women. Raven Press; 1983:25–45. [Google Scholar]
- 38.Manni A, Pardridge WM, Cefalu W, et al. Bioavailability of albumin-bound testosterone. J Clin Endocrinol Metab. October 1985;61(4):705–10. [DOI] [PubMed] [Google Scholar]
- 39.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. February 2007;92(2):405–13. [DOI] [PubMed] [Google Scholar]
- 40.CDC. CDC Hormone Standardization Program (CDC HoSt). Accessed July 29, 2020 https://www.cdc.gov/labstandards/pdf/hs/CDC_Certified_Testosterone_Assays-508.pdf
- 41.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. October 1999;84(10):3666–72. [DOI] [PubMed] [Google Scholar]
- 42.Goodman NF, Cobin RH, Futterweit W, et al. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME--PART 1. Endocr Pract. November 2015;21(11):1291–300. [DOI] [PubMed] [Google Scholar]
- 43.Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. March 2013;98(3):1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Reilly MW, Kempegowda P, Jenkinson C, et al. 11-Oxygenated C19 Steroids Are the Predominant Androgens in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 03 2017;102(3):840–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pretorius E, Arlt W, Storbeck KH. A new dawn for androgens: Novel lessons from 11-oxygenated C19 steroids. Mol Cell Endocrinol. 02 2017;441:76–85. [DOI] [PubMed] [Google Scholar]
- 46.Bull JR, Rowland SP, Scherwitzl EB, Scherwitzl R, Danielsson KG, Harper J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med. 2019;2:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medicine PCotASfR. The evaluation and treatment of androgen excess. Fertil Steril. November 2006;86(5 Suppl 1):S241–7. [DOI] [PubMed] [Google Scholar]
- 48.Murphy MK, Hall JE, Adams JM, Lee H, Welt CK. Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J Clin Endocrinol Metab. October 2006;91(10):3878–84. [DOI] [PubMed] [Google Scholar]
- 49.Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-rotterdam: a common, age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. November 2010;95(11):4965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin KA, Anderson RR, Chang RJ, et al. Evaluation and Treatment of Hirsutism in Premenopausal Women: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 04 2018;103(4):1233–1257. [DOI] [PubMed] [Google Scholar]
- 51.Screening and Management of the Hyperandrogenic Adolescent: ACOG Committee Opinion, Number 789. Obstet Gynecol. 10 2019;134(4):e106–e114. [DOI] [PubMed] [Google Scholar]
- 52.Ng EH, Tang OS, Ho PC. The significance of the number of antral follicles prior to stimulation in predicting ovarian responses in an IVF programme. Hum Reprod. September 2000;15(9):1937–42. [DOI] [PubMed] [Google Scholar]
- 53.Steward RG, Lan L, Shah AA, et al. Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril. April 2014;101(4):967–73. [DOI] [PubMed] [Google Scholar]
- 54.Crichton M Aliens Cause Global Warming. The Caltech Michelin Lecture, January 17, 2003. 2003. [Google Scholar]
- 55.Force USPST. Guide to Clinical Preventive Services: Report of the U.S. Preventive Services Task Force. Task Force; 1989.
- 56.Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. September 1987;65(3):499–507. [DOI] [PubMed] [Google Scholar]
- 57.Moghetti P, Tosi F, Bonin C, et al. Divergences in insulin resistance between the different phenotypes of the polycystic ovary syndrome. J Clin Endocrinol Metab. April 2013;98(4):E628–37. [DOI] [PubMed] [Google Scholar]
- 58.Wild RA, Carmina E, Diamanti-Kandarakis E, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. May 2010;95(5):2038–49. [DOI] [PubMed] [Google Scholar]
- 59.Carmina E, Chu MC, Longo RA, Rini GB, Lobo RA. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab. May 2005;90(5):2545–9. [DOI] [PubMed] [Google Scholar]
- 60.Day F, Karaderi T, Jones MR, et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLoS Genet. 12 2018;14(12):e1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo X, Rotter JI. Genome-Wide Association Studies. JAMA. 11 2019;322(17):1705–1706. [DOI] [PubMed] [Google Scholar]
- 62.Day FR, Hinds DA, Tung JY, et al. Causal mechanisms and balancing selection inferred from genetic associations with polycystic ovary syndrome. Nat Commun. September 2015;6:8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hayes MG, Urbanek M, Ehrmann DA, et al. Genome-wide association of polycystic ovary syndrome implicates alterations in gonadotropin secretion in European ancestry populations. Nat Commun. August 2015;6:7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahlqvist E, Storm P, Käräjämäki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 05 2018;6(5):361–369. [DOI] [PubMed] [Google Scholar]
- 65.Dapas M, Lin FTJ, Nadkarni GN, et al. Distinct subtypes of polycystic ovary syndrome with novel genetic associations: An unsupervised, phenotypic clustering analysis. PLoS Med. June 2020;17(6):e1003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goodman NF, Cobin RH, Futterweit W, et al. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS, AMERICAN COLLEGE OF ENDOCRINOLOGY, AND ANDROGEN EXCESS AND PCOS SOCIETY DISEASE STATE CLINICAL REVIEW: GUIDE TO THE BEST PRACTICES IN THE EVALUATION AND TREATMENT OF POLYCYSTIC OVARY SYNDROME - PART 2. Endocr Pract. December 2015;21(12):1415–26. [DOI] [PubMed] [Google Scholar]


