Abstract
Background:
High levels of nitrate () in drinking water cause methemoglobinemia in infants; however, few studies have examined the potential effects of low-level exposure on fetal growth, and the results have been inconsistent.
Objectives:
We sought to assess the association between maternal exposure to nitrate in drinking water during pregnancy and offspring size at birth in a nationwide study of full-term ( gestation) live-born singletons.
Methods:
We estimated maternal nitrate exposure for 898,206 births in Denmark during 1991–2011 by linkage of individual home address(es) with nitrate data from the national monitoring database. Maternal address during pregnancy, infant size at birth [i.e., birth weight, low birth weight (LBW), body length, and birth head circumference] and covariates were compiled from the Danish Civil Registration System, the Danish Medical Birth Register, and The Integrated Database for Longitudinal Labor Market Research. Linear and logistic models with generalized estimating equations were used to account for multiple births to an individual. Nitrate exposure was modeled using five categories and as a log-transformed continuous variable.
Results:
There was evidence of a decreasing trend in models for term birth weight using categorical or continuous measures of exposure. Modeling exposure continuously, a difference of (95% confidence interval: , ) was predicted at (half the value of the European Union drinking water standard) compared with . Body length also decreased as nitrate concentrations increased in categorical and continuous models. There was little evidence of an association between and head circumference or LBW.
Discussion:
Although the estimated effects were small, our findings for live singleton births to Danish-born parents suggest that maternal intake of nitrate from drinking water may reduce term birth weight and length, which are markers of intrauterine growth. However, there was little evidence for an association between nitrate and head circumference or LBW. Future studies in other populations and with data on dietary sources of nitrate are encouraged to confirm or refute these findings. https://doi.org/10.1289/EHP7331
Introduction
Nitrate () is one of the most common contaminants in the world’s aquifers (Shukla and Saxena 2018; Spalding and Exner 1993) and of particular concern in agricultural countries, such as Denmark, that use nitrogen fertilizers and have intensive animal production (Hansen et al. 2017; Burow et al. 2010; Nolan et al. 1997). Excess nitrogen on agricultural fields may leach as water-soluble nitrate into groundwater depending on local soil and hydrogeological and geochemical conditions. Nitrate is stable in the upper oxic part of aquifers and may take decades before it reaches drinking water abstraction wells. This means that nitrogen mitigation measures at the agricultural fields can also take decades to reduce the nitrate concentrations in groundwater-based drinking water (Kim et al. 2020; Hansen et al. 2017; Dalgaard et al. 2014; NRC 1995). In Denmark, drinking water is entirely based on groundwater and in most cases only undergoes simple water treatment such as aeration and sand-filtration (Schullehner and Hansen 2014).
Since the mid-1980s, Denmark has implemented European policy initiatives including the European Union (EU) Nitrates Directive, the Water Framework Directive, and the Groundwater Directive into national legislation, in part, to protect groundwater from the impact of nitrogen from agriculture. So far, these nitrogen mitigation measures (e.g., maximum livestock density limit, prescriptions for manure handling, catch crops, maximum nitrogen allowance for crops, subsidies for afforestation, conversion to organic farming) have resulted in a reduction of approximately 45% in nitrogen runoff from the Danish agricultural sector (Dalgaard et al. 2014).
Although nitrate concentrations in Danish drinking water have fallen, in part due to national agricultural nitrogen regulations, local action plans, and infrastructural changes (Schullehner and Hansen 2014), 5.1% of the population is still exposed to drinking water with nitrate concentrations (Schullehner and Hansen 2014). Worldwide, regulatory limits for nitrate in public drinking water supplies (EU: ; United States: ) were originally set to protect infants from methemoglobinemia (blue baby syndrome). Only during the latest decades have other health effects, and lower-level exposures, been studied.
The general population is at an increased risk of adverse health effects resulting from nitrate ingestion (Ward et al. 2018). Several plausible mechanisms might contribute to effects of nitrate on fetal development. First, nitrate can be converted to nitrite () in the gastrointestinal tract (Tiso and Schechter 2015) and is readily absorbed by the circulatory system. Nitrite hinders the oxygen-carrying capacity of the blood by oxidation of hemoglobin (Hb) to methemoglobin (MetHb). Fetuses are believed to be at particularly high risk of adverse outcomes from nitrite exposure because they have low levels of MetHb reductase (cytochrome b5 reductase), which converts MetHb back to Hb (Lukens 1987; Gruener et al. 1973). Nitrate intake has also been shown to interfere with thyroid function in both animals (Eskiocak et al. 2005; Jahreis et al. 1986, 1987) and humans (Aschebrook-Kilfoy et al. 2012) and to be teratogenic (Kakavandi et al. 2018).
Health outcomes stemming from fetal growth restriction and reduced birth weight are costly to the health care system and to families because they may cause substantial morbidity and mortality to the affected child. Meta-analyses have shown a J-shaped relationship between birth weight and type 2 diabetes mellitus and cardiovascular disease risk (Knop et al. 2018), an inverse relationship with hypertension (Knop et al. 2018), and a positive association with lung function (Saad et al. 2017). In addition, low birth weight (LBW; at term) has been associated with increased cardiovascular disease risk (Mohseni et al. 2020) and loss in intelligence quotient in LBW individuals in comparison with those of normal birth weight (Gu et al. 2017; Kormos et al. 2014).
Given the ubiquity of nitrate-contaminated water, the biological plausibility, and the importance of fetal growth restriction on future health outcomes, there are surprisingly few epidemiological studies on the potential relationship between nitrate in drinking water and fetal growth restriction. Only four studies have been previously published (Stayner et al. 2017; Blake 2014; Migeot et al. 2013; Bukowski et al. 2001), and they share common limitations, including uncertain assessments of nitrate levels in drinking water; small sample size and correspondingly limited statistical power; and inability to control for potential confounders like parity, maternal stature, and lifestyle factors such as smoking. In addition, three of the four studies used an ecological design.
There is a need for larger studies with well-characterized individual-level estimates of exposures, outcomes, and covariates. Our study relies on extensive nitrate measures in drinking water samples collected across Denmark, detailed information on the very decentralized structure of household water supply, and largely complete residential and birth registries for the entire Danish population (Schullehner et al. 2017a) over the span of two decades. Furthermore, the study population is less diverse than in other countries (e.g., the United States), and residents have access to free health and prenatal care, reducing the possibility of confounding by these important factors. Although this homogeneity helps to avoid possible confounding by socioeconomic factors, it does limit the generalizability of the study in terms of income and ethnicity. The aim of this analysis is to examine the potential role of nitrate in drinking water on markers of fetal growth in a large nationwide population.
Methods
Study Design and Population
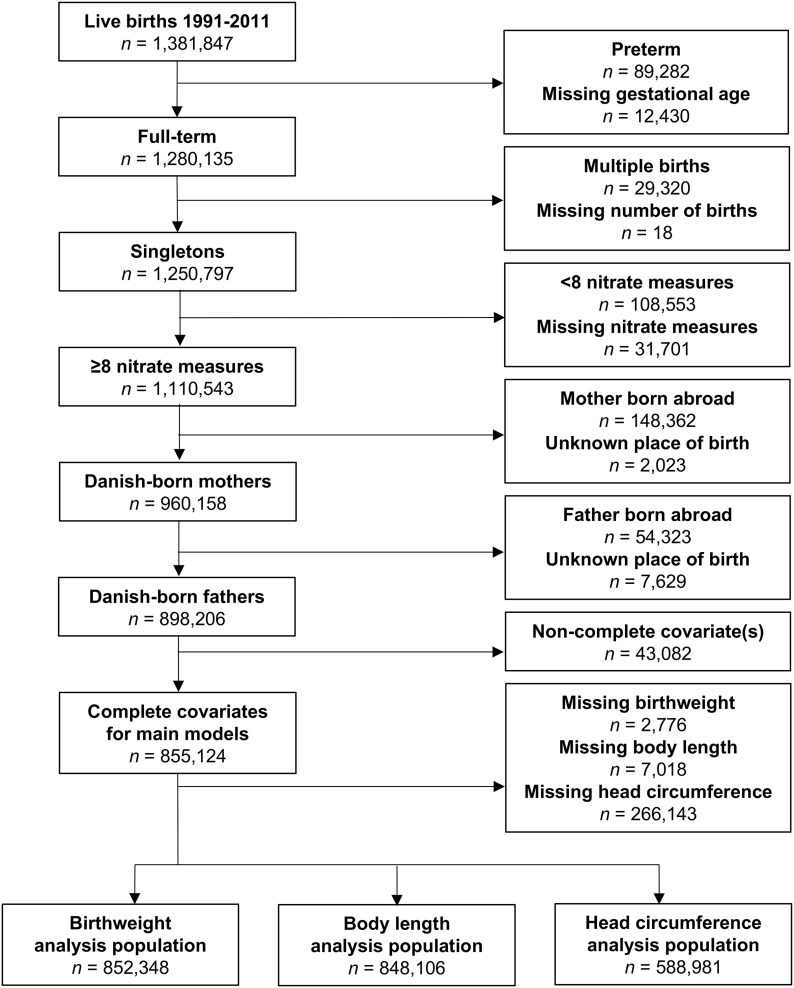
This prospective study links data on birth outcomes from the Danish Medical Birth Registry (DMBR; Knudsen and Olsen 1998) with estimates of household concentrations of nitrate in drinking water that were developed from the Danish national geodatabase known as Jupiter (Hansen and Pjetursson 2011). We restricted our population to live-born singletons born in Denmark with 37 or more weeks of completed gestation during the period 1991–2011 of Danish-born mothers and fathers (Figure 1). Data linkage was done using the unique personal identification number assigned to each resident in Denmark (Pedersen 2011).
Figure 1.
Flow diagram of restriction of the cohort to the birth weight, body length at birth, and head circumference at birth analysis populations. Note: Main model covariates included: maternal age, calendar year, sex, gravidity, maternal smoking, maternal education, maternal income, maternal employment status, region, and urbanicity. Head circumference data were available only for births in and after 1997.
Exposure Assessment
The methodology for the estimation of household levels of nitrate in drinking water has been previously described (Schullehner et al. 2017a, 2017b). In brief, extensive data of measured nitrate concentrations in water systems are available from the Jupiter database. Nitrate was quantified via high-performance liquid chromatography by certified Danish laboratories. As analytic tests improved during the two decades this study spans, the limit of detection has decreased from 1 to . It is important to note that analyses of water samples at the exit of treatment plants and from home faucets supplied by treatment plants have demonstrated nearly identical nitrate levels (: 0.98) (Schullehner et al. 2017b). This consistency is due to a lack of removal or transformation of nitrate at the waterworks or in the drinking water distribution system (MoEFD 2019). Further, household nitrate concentrations in the tap water are stable over a 1-y period (Schullehner et al. 2017b).
Households supplied by private wells were identified based on their proximity to a private well in Jupiter or in municipal databases. The majority of households are connected to a public waterworks (97%); use their own private well. Of these 3%, nitrate water concentrations are available for only about half (53%) (Schullehner and Hansen 2014).
Residential address histories for each mother–child dyad were obtained from the Danish Civil Registration System (Pedersen 2006, 2011). Reports of nitrate concentrations from drinking water facilities were then linked to maternal address(es). By law, Danish residents have 5 d to inform the authorities of a change of address (Pedersen et al. 2006).
Maternal exposure, per month of pregnancy, was assigned by an average of the nitrate measurements from the waterworks supplying the residence during that year (Schullehner et al. 2018). The analyses presented here were restricted to individuals with at least 8 of the 10 months prior to birth with nitrate exposure measures. Missingness could arise if a mother moved to a residence without a linkable measurement or if a pregnancy spanned 2 calendar years and in 1 y a measurement was not recorded. Using these monthly exposure values, time-weighted averages of prenatal exposure were estimated for the entire pregnancy. Due to our use of yearly weighted average exposures, trimester-specific estimates were highly correlated with the pregnancy average estimates of exposure (: 0.97–0.99) and one another (: 0.91–0.96). Thus, analyses using trimester-specific exposures are not presented. Date of last menses corrected by two ultrasounds, if ultrasound data were available (mostly after 2004), was used to assess date of conception.
Outcome Definition
Birth weight was obtained from the DMBR (Knudsen and Olsen 1998) and then used to create indicator variables for LBW ( at term). Body length and head circumference measurements at birth were also obtained from the DMBR. Head circumference data were available only for those born in 1997 and onward.
Covariates
Established and suspected risk factors of fetal growth restriction were identified a priori. Data on potential confounders were obtained from The Integrated Database for Longitudinal Labor Market Research and the DMBR. Continuous covariates were modeled either as categorical variables or restricted cubic splines with two knots defined by R (Brenner and Blettner 1997). Covariates in the main models were: sex of the child, year of birth (in five year categories), gravidity (1, 2, or ), urbanicity (five categories), geopolitical region (five categories), maternal age (spline), maternal smoking during early pregnancy (yes/no), and markers of socioeconomic status including maternal reported income normalized for inflation using the Consumer Price Index (The World Bank 2019) (spline), maternal educational attainment (less than high school, high school, or higher; Jensen and Rasmussen 2011), and maternal employment status (employed, unemployed, not in the workforce). All socioeconomic status variables (i.e., income, education, and employment) were as recorded 2 y prior to the birth. For children born in the period before 1997, maternal smoking was recorded at the mother’s first visit with a midwife with no specifications as to the timing. For children born from 1997 onward, smoking is during pregnancy. Urbanicity of maternal address at birth was defined as rural areas (municipalities in Denmark where the largest town has inhabitants), provincial towns (municipalities where the largest town has between 10,000 and 100,000 inhabitants), provincial city (municipalities where the largest town has inhabitants), suburbs of Copenhagen, and Copenhagen.
Due to the small percentage of persons with missing birth data (), we did not use multiple imputation and conducted a complete case analysis.
Statistical Analyses
Multivariate linear regression models were used for outcomes on a continuous scale. Multivariate logistic regression models were fitted for LBW. We used generalized estimating equations (GEE) to account for the nonindependence of births from the same mother.
Nitrate concentrations were modeled as categorical or log-transformed continuous variables for each outcome. Five cut points for the categorical analysis were defined a priori based on the distribution of exposure in the population and their usefulness for assessing current regulatory standards. The referent category was defined as any weighted average less than or equal to the uppermost limit of detection (), whereas the highest category included only those “elevated” weighted averages (), a nonregulatory level in Denmark but one that indicates action must be taken so levels do not exceed a regulatory limit (Swiss Confederation Federal Office for the Environment and Federal Office of Public Health 2010; Mohaupt et al. 1996).
Interaction tests were conducted using likelihood ratio tests comparing models with and without the interactions. The models were fitted without GEE because a likelihood ratio test cannot be performed with GEE. We modeled product terms between ln-transformed and each covariate, such that categorical variables with n categories would be modeled using n-1 product terms. To ease interpretation of the interactions, we used untransformed continuous variables instead of splines in the cross-product term, though the remaining continuous variables in the model were still represented as splines. Trend tests were derived by modeling the categorical nitrate variable (values of 1, 2, 3 … n) as a continuous variable, which yields a score test.
All statistical analyses were conducted using R (version 3.6; R Development Core Team).
Sensitivity Analyses
We conducted several sensitivity analyses, the first of which explored the impact that potential exposure misclassification of well water data may have had on our findings, because private wells are sampled less frequently than the water from public supplies. In this analysis, we excluded those born to mothers who were on a private water source or with an unknown source at any point during pregnancy (a reduction of up to 4,032 births).
In an effort to assess the adequacy of the current regulatory standards, a second sensitivity analysis was restricted to births with each monthly average of nitrate contamination at or below the EU standard of (a loss of up to 6,649 births).
As noted above, the concentration of nitrate in drinking water has been falling over time, and head circumference data were only available from 1997 onward. We therefore conducted a third sensitivity analysis restricting the other outcomes to this potentially lower-level exposure time period consistent with head circumference (1997–2011), with a loss of up to 253,979 births from the original cohort.
In a fourth sensitivity analysis, we included variables for maternal height and weight, which may influence fetal growth. These data were only available from 2003 onward, resulting in a reduction of up to 554,595 births from the original cohort.
The fifth sensitivity analysis examined additional potential covariates not considered a priori confounders and thus not in our base model. These variables were added to the base model one at a time to assess their influence on the association. They included gestational age (spline with two knots), birth via cesarean section (binary), and season of birth (four categories), as well as paternal age, income, education, and employment status.
A final sensitivity analysis examined our categorization of exposure, with the first and second of five exposure categories collapsed into the referent group. This collapsing was done because nitrate concentrations below are close to the limit of detection, but this collapsing also makes this category much larger than the others.
For each outcome, we examined statistical interactions between ln-transformed nitrate and each main model covariate and those in the sensitivity analyses of unrestricted models. Because we had no prior hypotheses regarding effect modification, we decided a priori to report results only for covariates with interaction for all continuous outcomes.
Ethical Considerations
In keeping with Danish legislation, the Danish Data Protection Agency, the Danish Health Data Authority, and Statistics Denmark approved this registry-based study. In accordance with Danish legislation, informed consent was not necessary. This study has also been approved by the University of Illinois at Chicago’s institutional review board.
Results
Main Analyses
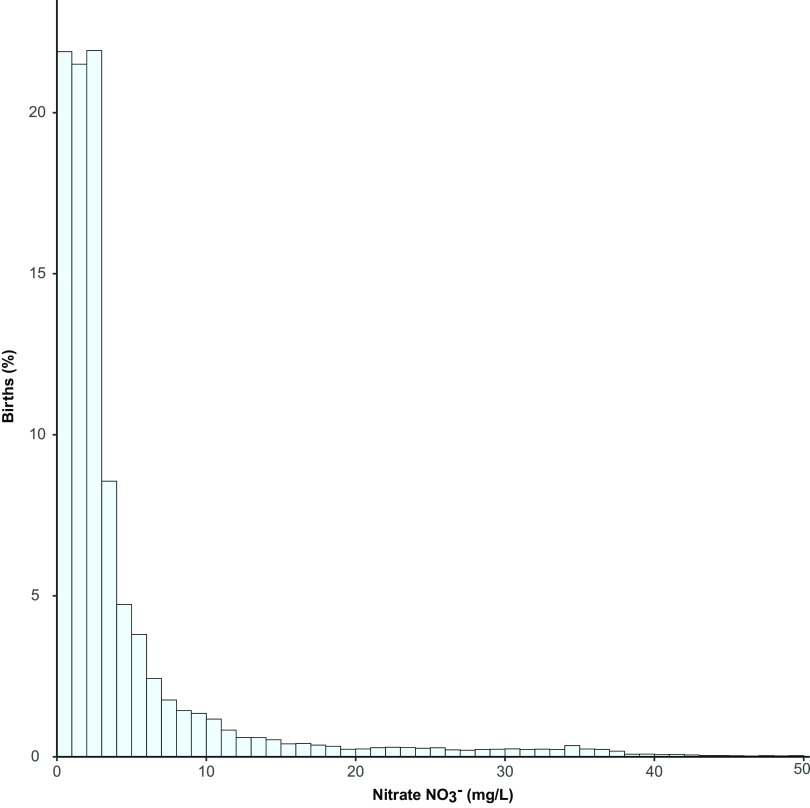
A total of 852,348 births met the inclusion criteria for the main study (i.e., had reported birth weight; were born 1991–2011 to Danish-born parents; were live-born, full-term, singletons; and had at least eight monthly measurements linked to the maternal home address) (Figure 1). In the study, the median nitrate exposure, averaged over the entire pregnancy, was 2.2 [interquartile range (IQR): 1.1–4.3] (Figure 2), and 33,809 (4.0%) experienced drinking water with elevated nitrate contamination () (Table 1). As also noted in Table 1, the distribution of characteristics differed significantly among the five nitrate exposure categories, where all tests were significant at except for sex (); however, these differences are small, with the exception of region, with North Jutland having the highest exposures, and for urbanicity, with the majority of exposures nitrate occurring in rural areas and provincial cities. The study included 10,028 (1.2%) LBW infants. Characteristics of the study population stratified by LBW are presented in Table S1. Their distribution differed significantly between LBW cases and noncases (), with the exception of the type of water supply () and the estimated nitrate concentration during pregnancy ().
Figure 2.
The distribution of the pregnancy average nitrate exposure in the birth weight study population, truncated to those with average nitrate exposure (). Note: 10th percentile: ; 90th percentile: . Main birth weight model covariates included: maternal age, calendar year, sex, gravidity, maternal smoking, maternal education, maternal income, maternal employment status, region, and urbanicity.
Table 1.
Characteristics of the study population by nitrate in home drinking water.
| Characteristic | Household concentration (mg/L) | ||||
|---|---|---|---|---|---|
| Total populationa [ (%)] | 186,182 (22) | 182,870 (21) | 299,468 (35) | 150,019 (18) | 33,809 (4) |
| Birth weightb [g ()] | |||||
| Body length at birthc [cm ()] | |||||
| Head circumference at birthd [cm ()] | |||||
| Low birth weightb [ (%)] | 2,026 (20) | 2,057 (21) | 3,573 (36) | 1,972 (20) | 400 (4) |
| Gestational age [wk ()] | |||||
| Maternal age [y ()] | |||||
| Maternal incomee [DKK ()] | |||||
| Paternal age [y ()] | |||||
| Paternal incomee [DKK ()] | |||||
| Missing [ (%)] | 71 (16) | 110 (26) | 195 (45) | 55 (13) | —f |
| Maternal heightg [cm ()] | |||||
| Maternal prepregnancy weightg [kg ()] | |||||
| Sex [ (%)] | |||||
| Female | 91,440 (22) | 89,634 (22) | 146,011 (35) | 73,087 (18) | 16,389 (4) |
| Male | 94,742 (22) | 93,236 (21) | 153,457 (35) | 76,932 (18) | 17,420 (4) |
| Gravidity [ (%)] | |||||
| 1 | 70,979 (19) | 80,780 (22) | 144,448 (39) | 62,614 (17) | 15,151 (4) |
| 2 | 75,704 (23) | 71,682 (22) | 112,241 (34) | 58,973 (18) | 12,620 (4) |
| 39,499 (27) | 30,408 (21) | 42,779 (29) | 28,432 (19) | 6,038 (4) | |
| Maternal smokingh [ (%)] | |||||
| No | 148,461 (22) | 144,378 (22) | 234,951 (35) | 108,978 (16) | 25,787 (4) |
| Yes | 37,721 (20) | 38,492 (20) | 64,517 (34) | 41,041 (22) | 8,022 (4) |
| Maternal educationi [ (%)] | |||||
| Compulsory | 41,635 (21) | 40,031 (20) | 64,855 (33) | 43,461 (22) | 8,546 (4) |
| Secondary | 93,282 (23) | 86,778 (21) | 137,403 (34) | 74,044 (18) | 16,813 (4) |
| Post-secondary | 51,265 (21) | 56,061 (23) | 97,210 (40) | 32,514 (13) | 8,450 (3) |
| Maternal employment statusi [n (%)] | |||||
| Employed | 153,878 (22) | 149,709 (22) | 246,612 (35) | 118,908 (17) | 26,940 (4) |
| Unemployed | 10,571 (20) | 10,874 (20) | 16,803 (31) | 13,080 (24) | 2,610 (5) |
| Not seeking work | 21,733 (21) | 22,287 (22) | 36,053 (35) | 18,031 (18) | 4,259 (4) |
| Paternal educationi [ (%)] | |||||
| Compulsory | 39,882 (21) | 37,268 (20) | 61,960 (33) | 39,013 (21) | 7,932 (4) |
| Secondary | 102,348 (23) | 93,390 (21) | 146,788 (33) | 82,173 (19) | 18,400 (4) |
| Postsecondary | 42,382 (20) | 50,190 (23) | 86,896 (41) | 27,072 (13) | 7,184 (3) |
| Missing | 1,570 (17) | 2,022 (21) | 3,824 (40) | 1,761 (19) | 293 (3) |
| Paternal employment statusi [ (%)] | |||||
| Employed | 172,342 (22) | 164,277 (21) | 267,848 (35) | 134,587 (17) | 30,152 (4) |
| Unemployed | 5,545 (16) | 7,116 (21) | 12,217 (36) | 7,693 (22) | 1,644 (5) |
| Not seeking work | 7,916 (17) | 10,869 (24) | 18,078 (39) | 7,321 (16) | 1,957 (4) |
| Missing | 379 (14) | 608 (22) | 1,325 (48) | 418 (15) | 56 (2) |
| Urbanicity of maternal address at birth [ (%)] | |||||
| Rural areasj | 89,144 (32) | 53,625 (19) | 64,527 (23) | 55,015 (20) | 13,349 (5) |
| Provincial townk | 57,560 (24) | 38,554 (16) | 76,027 (31) | 66,924 (27) | 5,435 (2) |
| Provincial cityl | 30,993 (29) | 44,735 (41) | 6,015 (6) | 11,442 (11) | 14,778 (14) |
| Suburb of capital | 8,420 (8) | 34,265 (33) | 48,728 (47) | 12,165 (12) | 222 (0) |
| Capital | 65 (0) | 11,691 (10) | 104,171 (87) | 4,473 (4) | 25 (0) |
| Region of maternal address at birth [ (%)] | |||||
| North Jutland | 11,695 (13) | 4,853 (5) | 9,836 (11) | 38,892 (43) | 24,757 (27) |
| Middle Jutland | 96,765 (46) | 47,672 (23) | 20,673 (10) | 40,023 (19) | 5,342 (3) |
| Southern Jutland | 54,000 (30) | 53,983 (30) | 33,757 (19) | 34,866 (20) | 1,625 (1) |
| Capital area | 16,164 (6) | 50,880 (20) | 165,287 (65) | 22,750 (9) | 926 (0) |
| Zealand | 7,558 (6) | 25,482 (22) | 69,915 (59) | 13,488 (11) | 1,159 (1) |
| Year of birth [ (%)] | |||||
| 1991–1995 | 35,564 (17) | 43,235 (21) | 65,475 (31) | 57,190 (27) | 9,290 (4) |
| 1996–2000 | 46,252 (22) | 44,181 (21) | 67,590 (33) | 41,022 (20) | 8,850 (4) |
| 2001–2005 | 49,922 (25) | 39,440 (20) | 74,761 (37) | 28,053 (14) | 7,957 (4) |
| 2006–2011 | 54,444 (23) | 56,014 (24) | 91,642 (39) | 23,754 (10) | 7,712 (3) |
| Season of birth [ (%)] | |||||
| January–March | 44,765 (22) | 43,611 (21) | 72,398 (35) | 37,353 (18) | 8,350 (4) |
| April–June | 45,716 (21) | 45,005 (21) | 76,384 (36) | 38,749 (18) | 8,109 (4) |
| July–September | 49,660 (22) | 50,369 (22) | 80,856 (35) | 39,742 (17) | 9,148 (4) |
| October–December | 46,041 (23) | 43,885 (22) | 69,830 (35) | 34,175 (17) | 8,202 (4) |
| Water supply [ (%)] | |||||
| Publicm | 185,339 (22) | 182,322 (21) | 298,381 (35) | 148,999 (18) | 33,275 (4) |
| Privaten | 710 (23) | 340 (11) | 632 (21) | 843 (28) | 506 (17) |
| Mixture of public and an unknown sourceo | 133 (13) | 208 (21) | 455 (45) | 177 (18) | 28 (3) |
| Cesarean deliveryp [ (%)] | |||||
| No | 119,432 (24) | 106,932 (21) | 183,803 (37) | 69,121 (14) | 19,216 (4) |
| Yes | 23,259 (23) | 21,913 (22) | 37,953 (38) | 13,325 (13) | 3,400 (3) |
Note: All tests for difference between strata were significant at , except for sex (). Main model covariates included: maternal age, calendar year, sex, gravidity, maternal smoking, maternal education, maternal income, maternal employment status, region, and urbanicity.
The study population: full-term singleton live births in Denmark from 1 January 1991 to 31 December 2011 to Danish-born parents who have at least eight address-linked measurements, with a birth weight measurement, and with non-missing covariates in the base model.
.
.
.
As reported 2 y prior to birth and standardized to 2009 values.
In accordance with Danish data privacy regulations, data from cells with persons cannot be reported. Thus, this cell was not used in the calculation of the row percentages for missing paternal income.
Available from 2003 onward only, which reduces the sample size to 297,753.
For children born in the period before 1997 smoking was recorded at the first visit with the midwife with no specifications as to the timing. For children born from 1997 onward, smoking is during pregnancy.
As reported 2 y before birth.
Municipalities in Denmark where the largest town has inhabitants.
Municipalities having a town with between 10,000 and 100,000 inhabitants.
Municipalities having a town with inhabitants.
Public water for 10 out of 10 months of pregnancy.
Private well for at least 1 month of pregnancy.
Public water supply for at least 8 out of the 10 months during pregnancy and unknown water supply for the remaining months.
Available from 1997 onward only, which reduces the sample size to 598,354.
Birth weight.
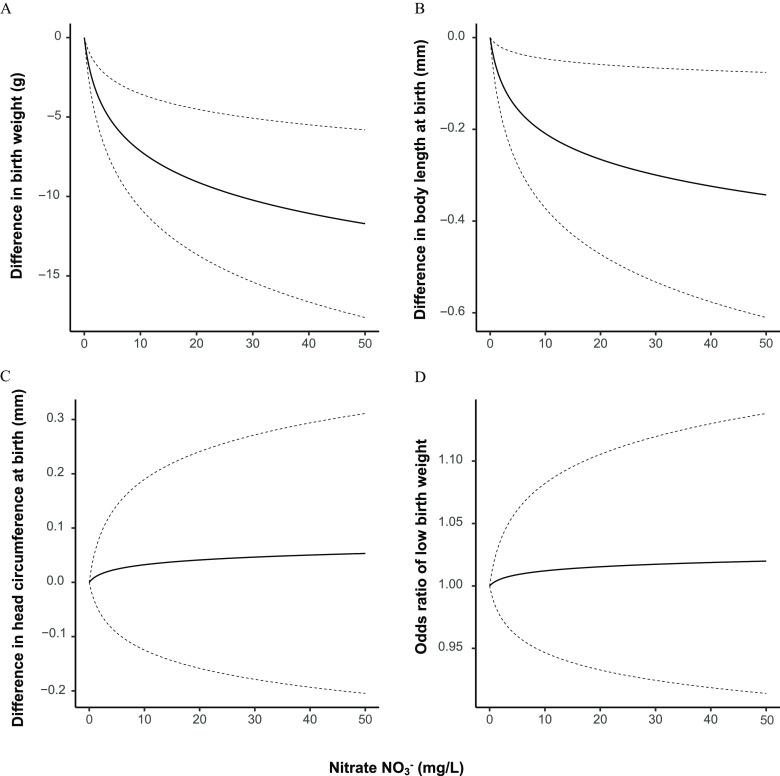
Relative to the lowest category (), mean birth weight decreased with increasing exposure up to { [95% confidence interval (CI): , , and remained inversely associated with the highest () vs. lowest exposure group [ (95% CI: , )] (Table 2). A model of ln-transformed pregnancy-average concentration also indicated an inverse association (Figure 3), with an estimated mean difference in birth weight of (95% CI: , ) for an average exposure of compared with (Table 2).
Table 2.
Difference in the mean birth weight, body length at birth, and head circumference at birth using categorical and continuous variables for concentrations in household drinking water.
| Birth weight (g) | Body length (mm) | Head circumference (mm)a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (mg/L) | (95% CI) | -Value | (95% CI) | -Value | (95% CI) | -value | |||
| Categorical | |||||||||
| 186,182 | Ref (0) | 185,379 | Ref (0) | 140,486 | Ref (0) | ||||
| 182,870 | (,) | 0.02 | 182,001 | (, 0.1) | 0.24 | 126,561 | 0.02 (, 0.2) | 0.79 | |
| 299,468 | (, ) | 297,885 | (, ) | 0.03 | 218,398 | (, ) | 0.001 | ||
| 150,019 | (, ) | 149,114 | (, ) | 81,085 | 0.1 (, 0.2) | 0.52 | |||
| 33,809 | (, ) | 0.03 | 33,727 | (, 0.1) | 0.27 | 22,451 | 0.1 (, 0.3) | 0.62 | |
| Trend | —c | —c | —c | —c | —c | —c | 0.52 | ||
| Continuousa | 852,348 | (, ) | 848,106 | (, ) | 0.01 | 588,981 | 0.04 (, 0.3) | 0.69 | |
Note: Models were fitted using linear regression with generalized estimating equations to control for the non-independence of births from the same mother and were controlled for maternal age, calendar year, sex, gravidity, maternal smoking, maternal education, maternal income, maternal employment status, region, and urbanicity. CI, confidence interval; Ref, reference.
Data were available only for births in and after 1997.
Continuous exposure estimates were log transformed, ln() and (95% CI) shown for exposures compared with .
The empty cells correspond to the p-for-trend analyses and so there is no point estimate or 95% CI.
Figure 3.
The difference in (A) birth weight, (B) body length, and (C) head circumference at birth, and (D) odds of low birth weight at nitrate values from to within the cohort, with the dotted lines representing the 95% confidence intervals of each estimate. Note: Models were fitted using linear regression with generalized estimating equations to control for the nonindependence of births from the same mother and were controlled for maternal age, calendar year, sex, gravidity, maternal smoking, maternal education, maternal income, maternal employment status, region, and urbanicity. Head circumference data were available only for births in or after 1997.
Body length at birth.
Similar to term birth weight, mean body length decreased with increasing exposure up to (; 95% CI: , compared with ), but the inverse association was weaker for the highest () exposure group [; (95% CI: , 0.1)] (Table 2). When was modeled as a ln-transformed continuous variable (Figure 3), the estimated difference in body length associated with a mean exposure of vs. was (95% CI: , ).
Head circumference at birth.
There was little evidence of an association between mean pregnancy and head circumference at birth. Associations for modeled as a continuous variable (Figure 3) and a categorical variable were null (trend ), with the exception of an inverse association with vs. (; 95% CI: , ) (Table 2).
Low birth weight.
In contrast with continuous term birth weight, mean pregnancy was not associated with term LBW (Table 3).
Table 3.
Adjusted odds ratios (aOR) for the association between term low birth weight and average household concentrations over the pregnancy.
| Low birth weight | |||
|---|---|---|---|
| (mg/L) | aOR (95% CI) | -Value | |
| Categorical | |||
| 186,182 | Ref (1) | ||
| 182,870 | 0.98 (0.92, 1.05) | 0.52 | |
| 299,468 | 1.01 (0.94, 1.08) | 0.86 | |
| 150,019 | 1.02 (0.95, 1.09) | 0.55 | |
| 33,809 | 0.99 (0.88, 1.12) | 0.91 | |
| Trend | —b | —b | 0.51 |
| Continuousa | 852,348 | 1.02 (0.93, 1.11) | 0.73 |
Note: Models were fitted using logistic regression with generalized estimating equations to control for the nonindependence of births from the same mother and were controlled for maternal age, calendar year, sex, gravidity, maternal smoking, maternal education, maternal income, maternal employment status, region, and urbanicity. aOR, adjusted odds ratio; CI, confidence interval; Ref, reference.
Continuous exposure was log transformed, ln() and aOR (95% CI) shown for exposures compared with .
The empty cells correspond to the p-for-trend analyses and so there is no point estimate or 95% CI.
Interactions.
Urbanicity was the only variable that met our a priori criterion for a notable interaction ( for all continuous outcomes) (Table 4). In models that did not account for nonindependence of births to mothers with multiple births in the cohort, stratum-specific estimates for birth weight varied with regard to direction and magnitude, with a positive association between and birth weight in Copenhagen (the capital), a weak positive association for provincial cities, and inverse associations in the other regions. The pattern was similar for body length, with a positive association in the capital, a null association in provincial cities, and inverse associations elsewhere. In contrast, head circumference was positively associated with in provincial towns, but inversely associated with exposure in the capital region as well as the other urbanicity groups.
Table 4.
Effect modification of the associations between urbanicity and nitrate exposure on birth weight, body length at birth, head circumference at birth, and low birth weight.
| Birth weight (g) | Body length (mm) | Head circumference (mm)a | Low birth weight | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (95% CI) | -Valueb | (95% CI) | -Valueb | (95% CI) | -Valueb | OR (95% CI) | -Valueb | |||||
| Rural areasc | 275,660 | (, ) | 274,406 | (, 0.002) | 188,896 | (, 0.1) | 275,660 | 0.93 (0.82, 1.06) | ||||
| Provincial townd | 244,500 | (, ) | 243,189 | (, ) | 166,647 | 0.5 (0.2, 0.9) | 244,500 | 1.15 (0.99, 1.34) | ||||
| Provincial citye | 107,963 | 3.8 (, 13.5) | 107,405 | (, 0.4) | 74,980 | (, 0.3) | 107,963 | 0.95 (0.78, 1.16) | ||||
| Suburb of capital | 103,800 | (, ) | 103,286 | (, ) | 69,331 | (, 0.5) | 103,800 | 1.20 (0.81, 1.77) | ||||
| Capital | 120,425 | 17.6 (, 58.6) | 119,820 | 4.2 (2.3, 6.1) | 89,127 | (,) | 120,425 | 1.14 (0.51, 2.55) | ||||
| 0.002 | 0.001 | 0.20 | ||||||||||
Note: Models were fitted using linear regression with GLM and were controlled for maternal age, calendar year, sex, gravidity, maternal smoking, maternal education, maternal income, maternal employment status, region, and urbanicity. Models did not account for non-independence of observations for mothers with multiple births in the cohort. (95% CI) shown for exposures compared to . CI, confidence interval; GLM, generalized linear models; OR, odds ratio.
Data were available only for births in or after 1997.
p-Value from likelihood ratio tests comparing models with and without product interaction terms.
Municipalities in Denmark where the largest town has inhabitants.
Municipalities where the largest town has between 10,000 and 100,000 inhabitants.
Municipalities where the largest town has inhabitants.
Sensitivity Analyses
Overall, results of the sensitivity analyses were similar to those from the main analyses when the study was restricted to mothers who used water from public water systems or to mothers who always had water concentrations lower than the current European standard () (Tables S2–S5) and when data were restricted to the time period (1997 and later) when head circumference data were available (Table S6). Restricting the population to only those with maternal height and weight measurements and controlling for these covariates weakened the results for birth weight but did not change effect estimates for the other outcomes (Table S7). For example, in the main continuous birth weight model, we estimated a (95% CI: , ) difference per milligram per liter increase in , but with the restriction to dyads with maternal height and weight measurements we estimated a (95% CI: , 6.0) difference and a (95% CI: , 6.4) difference when maternal height and weight were controlled for. Controlling for additional covariates in the main models did not result in any important changes in the results (Table S8–S9). In addition, our inference did not change when exposure was modeled in four categories vs. five (Table S10).
Discussion
To our knowledge, this nationwide study, with nearly 1 million full-term births is the largest published analysis of the association between prenatal exposure to nitrate from drinking water and markers of fetal growth restriction. It utilized both individual-level exposure and outcome data in contrast to past studies which were mostly ecologic. Our findings provide evidence of reduced term birth weight and body length in association with mean pregnancy exposure. Associations were strongest for the second highest exposure group, which may reflect instability in the estimates for the highest exposure group () due to its relatively small size (; 4.0% of the study population) but also might indicate potential bias related to uncontrolled confounding, exposure misclassification, selection bias, or other factors. Exposure categories were defined a priori, based on advisory and regulatory limits and in an attempt to balance the number of births, but this approach left the highest category of exposure with by far the fewest individuals. Due to the large number of births in the cohort and the accuracy of the nitrate estimates, we were adequately powered to observe small differences in birth size. However, the relatively large differences in birth weight with small differences in exposure is surprising, particularly between the lowest categories ( and to ), which are barely above the limit of detection. This result might be real, or alternatively, may reflect residual confounding or other biases.
We estimated a reduction in mean birth weight of approximately (95% CI: , ) for an average exposure during pregnancy of (half of the European drinking water standard) compared with (Table 2). While this reduction may not be clinically relevant at individual level, it may be important from a population perspective given the widespread exposure to in drinking water in Denmark and other countries. It is also noteworthy that our findings were robust to the exclusion of those exposed to estimated nitrate concentrations above current allowable limits (EU: ; US: ), suggesting that the current drinking water standard is not protective for all health outcomes.
In contrast to our findings for birth weight and body length at birth, and counter to our study hypothesis, our analyses did not provide evidence of an association between and either LBW or head circumference at birth. Although LBW is commonly used for public health surveillance and medical decision-making, its use in epidemiology is problematic (Wilcox 2001) and it is a statistically less powerful outcome than birth weight. Assuming a linear relation between exposure and birth weight, LBW captures the tail of the continuous distribution of birth weight (i.e., ). There is a clear loss of information in making this dichotomization. Modeling birth weight as a continuous variable takes full advantage of the data and is thus a more statistically powerful analysis than modeling birth weight as a binary variable (i.e., LBW/normal birth weight). This loss of information might explain why we see evidence of an association with birth weight but not with LBW. Body length has been shown to be a less accurate measure than birth weight and head circumference at birth (Johnson et al. 1997). However, head circumference was recorded in Danish databases from 1997, reducing the sample size (a 31% decrease) and the precision of effect estimates.
If nitrate contributes to fetal growth restriction, our estimates suggest it would have a weak effect compared with established risk factors, such as maternal active smoking during pregnancy (Wollmann 1998; Horta et al. 1997; Floyd et al. 1993; Sexton and Hebel 1984; Butler et al. 1972). For example, in our study population, with adjustment for the same covariates included in our main model, maternal active smoking was associated with mean reductions of (95% CI: , ) and (95% CI: , ) in term birth weight and length, respectively, compared with estimated mean reductions of (95% CI: , ) and (95% CI: , ) in term birth weight and length with a mean exposure during pregnancy of of nitrate vs. . However, given the widespread and largely unavoidable nature of exposure, nitrate might still make an important population-level contribution to fetal growth restriction worldwide by shifting the distribution and increasing the number of births with clinically relevant reductions in birth size.
Comparison with Other Studies
Bukowski et al. conducted a population-based case–control study that included 210 cases of intrauterine growth restriction (IUGR) on Prince Edward Island, Canada, an agricultural area with considerable groundwater contamination by nitrate (Bukowski et al. 2001). Their study used an ecological exposure metric based on postal codes, with IUGR defined as any birth with an International Classification of Diseases, 9th Revision–Clinical Modification (ICD-9CM) diagnosis of 764–764.19 or birth weight at term, resembling our LBW definition. As in our study, the cases were predominantly exposed to levels below the regulatory limit (only three were estimated to be ). At an exposure roughly equal to , they reported an OR for IUGR of 2.2 (95% CI: 1.4, 3.4) and at an OR of 2.3 (95% CI: 1.3, 4.0), whereas we observed a null association for nitrate and LBW.
Stayner et al. (2017) saw an association between total nitrogen (nitrate and nitrite combined) and very LBW (risk ratio : 1.17; 95% CI: 1.08, 1.25) in the Midwestern United States. This study, which included 134,258 births, was the largest study prior to ours, but it was based on an ecological design, with births and analyzed at the county level. Their study was also not limited to term births and thus very LBW was related to very preterm births. Owing to the small number of very LBW cases in our data set (), we were unable to examine this outcome.
Blake conducted a study of nitrate in drinking water and LBW from publicly available data at the postal code level in an agricultural area of California (USA), with no evidence of a statistical association as reported by mapping hot spots of high nitrate overlayed with LBW (Blake 2014). This exploratory study did not adjust for any confounders.
In the only study other than ours to use individual-level exposure estimates and outcome data, Migeot et al. conducted a cohort study of 11,446 births in western France examining the impact of nitrate and atrazine (a herbicide banned in Denmark in the 1990s but possibly still present in groundwater) in drinking water. In those exposed to nitrate during the second trimester of pregnancy but unexposed to atrazine, they found an increased risk of small-for-gestational age [OR: 1.74 (95% CI: 1.10, 2.75) for second tertile of exposure vs. the first] (Migeot et al. 2013). As in many of our analyses, they also observed a drop in estimated effects in the upper category of exposure [OR for third tertile of exposure () vs. the first (): 1.51 (95% CI: 0.96, 2.40)].
Design Considerations
We were unable to account for differences in individual dietary sources of nitrate and nitrite, vitamin C, or other antioxidant supplementation (Ward et al. 2005; Super et al. 1981). It is known that dietary factors differ by socioeconomic status in Denmark (Hare-Bruun et al. 2011), and they may also differ by region or urbanicity, a reason for including these covariates in our main models; however, bias due to residual confounding by dietary factors may still exist. In addition, we were also unable to account for nitrosatable drug use (Brender et al. 2004), or the maternal oral microbiome, which contributes to transformation of nitrate in the body (Ward et al. 2018).
We were also unable to adjust for pesticides and other compounds found in Danish drinking water which might be correlated with nitrate. However, tap water production in Denmark is based on the key principle of abstraction of pure groundwater with very low impact from anthropogenic pressures (Jørgensen and Stockmarr 2009).
We were also unable to quantify the amount of water a woman consumed, so we assumed equivalent consumption for all pregnancies. Exposure misclassification due to consumption of bottled water or water not from the home tap (e.g., at work) is possible, which is why we have chosen to describe the exposure as being at the household level. We do not consider the lack of data on bottle water consumption to be a large source of bias, because the use of prepackaged bottled water in Denmark is minimal, with only consumed per capita annually (2012) (UNESDA 2018) and much less for earlier years.
Exposure misclassification could also arise from differences in sizes of waterworks. Nitrate monitoring of drinking water depends on the abstraction volume of the specific waterworks, with data reported to Jupiter at minimum every other year and up to 37 times per year (MoE 2014). This may lead to more exposure misclassification of those being serviced by smaller waterworks than larger. It also is possible that even though a resident lies within the boundary of a public waterworks the home may be supplied by an unregistered private well; however, private wells typically have higher nitrate concentrations than public waterworks.
Furthermore, 22% of water supply areas have more than one waterworks supplying the area (Schullehner and Hansen 2014). We used maps of waterworks, engineering records, and weighting to account for these supply areas, but the possibility of residual misclassification exists. We are currently unable to model from which waterworks a household predominantly gets their water from, as this can change during a day depending on production and consumption patterns and often not even the water suppliers know the exact distribution of the water in their network. To address this issue, when calculating average values for the water supply area, we weighed nitrate concentrations by drinking water production volume to give smaller weight to smaller production facilities within the supply area.
We were unable to examine differences between trimesters because we found little difference between the overall pregnancy mean and the trimester-specific means due to the lack of variation in water contamination during pregnancy (). Danish drinking water is exclusively from groundwater sources, which are typically 10–60 y old and lack seasonal variation in nitrate or nitrite concentrations (Schullehner et al. 2017b).
Exclusion of preterm births from our analyses slightly reduced the sample size ( observations lost) and might have resulted in a reduced variability in birth weight, body length, and head circumference at birth compared with the entire population. Thus, our study population is generalizable only to full-term, live births.
Of the four outcomes we examined, body length at birth is the measure most prone to mismeasurement, followed by head circumference, with birth weight being the most accurate in intra- and interrater studies (Johnson et al. 1997; Johnson and Engstrom 2002). However, these studies of full-term neonates were carried out in the United States and were under ideal conditions where participants knew they were being watched and their measurements scored against themselves and one another. The outcome data used in the present study was not collected for research purposes and especially body length and head circumference may suffer from measurement error.
Further, we did not have an a priori reason for testing interactions except to understand whether there was as a lack of homogeneity in our findings. Thus, the finding of an interaction between exposure and urbanicity was exploratory and should be evaluated with caution.
Strengths
Most prior studies relied on ecological estimates of exposure, but we were able to make use of individual-level measures of exposures, outcomes, and covariates in this population-based national study. Our data, based on reliable and accurate measurements performed by certified laboratories in both public and private water systems, has been shown to be a useful proxy for measurements taken in homes at the faucet (Schullehner et al. 2017b). The validity of the Danish Medical Birth Registry (DMBR) with children born at hospital or at home in Denmark is also considered very high and includes information on maternal smoking (Kristensen et al. 1996).
Finally, a strength of our study is its location. Economically and culturally, Denmark is a relatively homogenous population in which all individuals have free access to universal health care, including prenatal health care. Appreciable confounding by socioeconomic factors such as substantial inequalities in income and access to health care, as in the United States (Schaider et al. 2019), are far less likely; however, this may limit generalizability outside of high-income countries with national health care. Danes predominantly drink tap water that is not chlorinated, based on groundwater, and relatively free of other pollutants, further reducing the potential of confounding by water contaminants found in other countries (Zogorski et al. 2006).
Conclusions
Our study leveraged comprehensive individual-level data on nitrate concentrations in drinking water and markers of fetal growth. We observed evidence of a small decrease in term birth weight and body length at birth but little evidence of an association with head circumference at birth or LBW. These mixed findings may be explained by lack of data for head circumference prior to 1997 and the dichotomization of birth weight to LBW. Although the estimated effects were relatively small, our findings may have serious public health implications given the ubiquity of nitrate in drinking water and the severity of consequences from fetal growth restriction. Our findings were unchanged when mothers who were ever exposed to nitrate levels above the current EU drinking water standard (; 0.8%) were excluded. This might suggest that the current standard may be inadequate to protect children from fetal growth restriction; however, these findings need to be confirmed in other populations and geographic locations to increase generalizability and reduce the likelihood of a spurious association.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (NIH)/National Institute for Environmental Health Sciences (NIEHS) grant R01 ES027823-01A1.
References
- Aschebrook-Kilfoy B, Heltshe SL, Nuckols JR, Sabra MM, Shuldiner AR, Mitchell BD, et al. 2012. Modeled nitrate levels in well water supplies and prevalence of abnormal thyroid conditions among the old order Amish in Pennsylvania. Environ Health 11(1):6, PMID: 22339761, 10.1186/1476-069X-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake SB 2014. Spatial relationships among dairy farms, drinking water quality, and maternal‐child health outcomes in the San Joaquin Valley. Public Health Nurs 31(6):492–499, PMID: 25412694, 10.1111/phn.12166. [DOI] [PubMed] [Google Scholar]
- Brender JD, Olive JM, Felkner M, Suarez L, Marckwardt W, Hendricks KA. 2004. Dietary nitrites and nitrates, nitrosatable drugs, and neural tube defects. Epidemiology 15(3):330–336, PMID: 15097014, 10.1097/01.ede.0000121381.79831.7b. [DOI] [PubMed] [Google Scholar]
- Brenner H, Blettner M. 1997. Controlling for continuous confounders in epidemiologic research. Epidemiology 429–434, PMID: 9209859. [PubMed] [Google Scholar]
- Bukowski J, Somers G, Bryanton J. 2001. Agricultural contamination of groundwater as a possible risk factor for growth restriction or prematurity. J Occup Environ Med 43(4):377–383, PMID: 11322099, 10.1097/00043764-200104000-00016. [DOI] [PubMed] [Google Scholar]
- Burow KR, Nolan BT, Rupert MG, Dubrovsky NM. 2010. Nitrate in groundwater of the United States, 1991–2003. Environ Sci Technol 44(13):4988–4997, PMID: 20540531, 10.1021/es100546y. [DOI] [PubMed] [Google Scholar]
- Butler NR, Goldstein H, Ross EM. 1972. Cigarette smoking in pregnancy: its influence on birth weight and perinatal mortality. Br Med J 2(5806):127–130, PMID: 5017304, 10.1136/bmj.2.5806.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard T, Hansen B, Hasler B, Hertel O, Hutchings NJ, Jacobsen BH, et al. 2014. Policies for agricultural nitrogen management—trends, challenges and prospects for improved efficiency in Denmark. Environ Res Lett 9(11):115002, 10.1088/1748-9326/9/11/115002. [DOI] [Google Scholar]
- Eskiocak S, Dundar C, Basoglu T, Altaner S. 2005. The effects of taking chronic nitrate by drinking water on thyroid functions and morphology. Clin Exp Med 5(2):66–71, PMID: 16096856, 10.1007/s10238-005-0068-1. [DOI] [PubMed] [Google Scholar]
- Floyd RL, Barbara KR, Gary AG, Patricia DM, Susan ES. 1993. A review of smoking in pregnancy: effects on pregnancy outcomes and cessation efforts. Annual Review of Public Health 14(1):379–411, PMID: 8323595, 10.1146/annurev.pu.14.050193.002115. [DOI] [PubMed] [Google Scholar]
- Gruener N, Shuval HI, Behroozi K, Cohen S, Shechter H. 1973. Methemoglobinemia induced by transplacental passage of nitrites in rats. Bull Environ Contam Toxicol 9(1):44–48, PMID: 4780432, 10.1007/BF01856770. [DOI] [PubMed] [Google Scholar]
- Gu H, Wang L, Liu L, Luo X, Wang J, Hou F, et al. 2017. A gradient relationship between low birth weight and IQ: a meta-analysis. Sci Rep 7(1):1–13, PMID: 29269836, 10.1038/s41598-017-18234-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Pjetursson B. 2011. Free, online Danish shallow geological data. Geus Bulletin 23(1):53–56, 10.34194/geusb.v23.4842. [DOI] [Google Scholar]
- Hansen B, Thorling L, Schullehner J, Termansen M, Dalgaard T. 2017. Groundwater nitrate response to sustainable nitrogen management. Sci Rep 7(1):1–12, PMID: 28819258, 10.1038/s41598-017-07147-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare-Bruun H, Togo P, Andersen LB, Heitmann BL. 2011. Adult food intake patterns are related to adult and childhood socioeconomic status. J Nutr 141(5):928–934, PMID: 21451129, 10.3945/jn.110.133413. [DOI] [PubMed] [Google Scholar]
- Horta BL, Victora CG, Menezes AM, Halpern R, Barros FC. 1997. Low birthweight, preterm births and intrauterine growth retardation in relation to maternal smoking. Paediatr Perinat Epidemiol 11(2):140–151, PMID: 9131707, 10.1046/j.1365-3016.1997.d01-17.x. [DOI] [PubMed] [Google Scholar]
- Jahreis G, Hesse V, Schöne F, Hennig A, Gruhn K. 1986. Effect of chronic dietary nitrate and different iodine supply on porcine thyroid function, somatomedin-C-level and growth. Exp Clin Endocrinol Diabetes 88(5):242–248, PMID: 3556413, 10.1055/s-0029-1210603. [DOI] [PubMed] [Google Scholar]
- Jahreis G, Schöne F, Lüdke H, Hesse V. 1987. Growth impairment caused by dietary nitrate intake regulated via hypothyroidism and decreased somatomedin. Endocrinol Exp 21(3):171–180, PMID: 3499306. [PubMed] [Google Scholar]
- Jensen VM, Rasmussen AW. 2011. Danish education registers. Scand J Public Health 39(7 Suppl):91–94, PMID: 21775362, 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- Johnson TS, Engstrom JL. 2002. State of the science in measurement of infant size at birth. Newborn Infant Nurs Rev 2(3):150–158, 10.1053/nbin.2002.35122. [DOI] [Google Scholar]
- Johnson TS, Engstrom JL, Gelhar DK. 1997. Intra-and interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr 24(5):497–505, PMID: 9161941, 10.1097/00005176-199705000-00001. [DOI] [PubMed] [Google Scholar]
- Jørgensen LF, Stockmarr J. 2009. Groundwater monitoring in Denmark: characteristics, perspectives and comparison with other countries. Hydrogeol J 17(4):827–842, 10.1007/s10040-008-0398-7. [DOI] [Google Scholar]
- Kakavandi NR, Hasanvand A, Ghazi-Khansari M, Sezavar AH, Nabizadeh H, Parohan M. 2018. Maternal dietary nitrate intake and risk of neural tube defects: a systematic review and dose–response meta-analysis. Food Chem Toxicol 118:287–293, PMID: 29763679, 10.1016/j.fct.2018.05.033. [DOI] [PubMed] [Google Scholar]
- Kim H, Surdyk N, Møller I, Graversgaard M, Blicher-Mathiesen G, Henriot A, et al. 2020. Lag time as an indicator of the link between agricultural pressure and drinking water quality state. Water 12(9):2385, 10.3390/w12092385. [DOI] [Google Scholar]
- Knop MR, Geng T-T, Gorny AW, Ding R, Li C, Ley SH, et al. 2018. Birth weight and risk of type 2 diabetes mellitus, cardiovascular disease, and hypertension in adults: a meta‐analysis of 7,646,267 participants from 135 studies. J Am Heart Assoc 7(23):e008870, PMID: 30486715, 10.1161/JAHA.118.008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen LB, Olsen J. 1998. The Danish Medical Birth Registry. Dan Med Bull 45(3):320–323, PMID: 9675544. [PubMed] [Google Scholar]
- Kormos CE, Wilkinson AJ, Davey CJ, Cunningham AJ. 2014. Low birth weight and intelligence in adolescence and early adulthood: a meta-analysis. J Public Health (Oxf) 36(2):213–224, PMID: 23896861, 10.1093/pubmed/fdt071. [DOI] [PubMed] [Google Scholar]
- Kristensen J, Langhoff-Roos J, Skovgaard LT, Kristensen FB. 1996. Validation of the Danish birth registration. J Clin Epidemiol 49(8):893–897, PMID: 8699210, 10.1016/0895-4356(96)00018-2. [DOI] [PubMed] [Google Scholar]
- Lukens JN 1987. The legacy of well-water methemoglobinemia. JAMA 257(20):2793–2795, PMID: 3553638, 10.1001/jama.1987.03390200133028. [DOI] [PubMed] [Google Scholar]
- Migeot V, Albouy-Llaty M, Carles C, Limousi F, Strezlec S, Dupuis A, et al. 2013. Drinking-water exposure to a mixture of nitrate and low-dose atrazine metabolites and small-for-gestational age (SGA) babies: a historic cohort study. Environ Res 122:58–64, PMID: 23340115, 10.1016/j.envres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- MoE [Ministry of the Environment (Miljøministeriet)]. 2014. Ministerial order on water quality and monitoring of water supply facilities. www.retsinformation.dk/Forms/R0710.aspx?id=160400 [accessed 30 December 2019].
- MoEFD (Ministry of Environment and Food of Denmark). 2019. Water Supply in Denmark. https://eng.ecoinnovation.dk/media/mst/8051461/Vandforsyning_artikel.pdf [accessed 5 December 2019.
- Mohaupt V, Behrendt H, Feldwisch N. 1996. Die aktuelle Nährstoffbelastung der Gewässer in Deutschland und der Stand der Belastungsvermeidung in den Kommunen und der Landwirtschaft In: Deutsche Gesellschaft für Limnologie (DGL), Tagungsbericht 1995. Berlin, Germany: Deutsche Gesellschaft für Limnologie, 376–383. [Google Scholar]
- Mohseni R, Mohammed SH, Safabakhsh M, Mohseni F, Monfared ZS, Seyyedi J, et al. 2020. Birth weight and risk of cardiovascular disease incidence in adulthood: a dose–response meta-analysis. Curr Atheroscler Rep 22(3):1–13, PMID: 32328820, 10.1007/s11883-020-0829-z. [DOI] [PubMed] [Google Scholar]
- Nolan BT, Ruddy BC, Hitt KJ, Helsel DR. 1997. Risk of nitrate in groundwaters of the United States–a national perspective. Environ Sci Technol 31(8):2229–2236, 10.1021/es960818d. [DOI] [Google Scholar]
- NRC (National Research Council). 1995. Nitrate and Nitrite in Drinking Water. Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- Pedersen CB 2011. The Danish Civil Registration System. Scand J Public Health 39(7 suppl):22–25, PMID: 21775345, 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Pedersen C, Bøcker H, Gøtzsche JØ, Mortensen Møller PB.. 2006. The Danish Civil Registration System: a cohort of eight million persons. Dan Med Bull 53(4):441–449, PMID: 17150149. [PubMed] [Google Scholar]
- Saad NJ, Patel J, Burney P, Minelli C. 2017. Birth weight and lung function in adulthood: a systematic review and meta-analysis. Ann Am Thorac Soc 14(6):994–1004, PMID: 28362513, 10.1513/AnnalsATS.201609-746SR. [DOI] [PubMed] [Google Scholar]
- Schaider LA, Swetschinski L, Campbell C, Rudel RA. 2019. Environmental justice and drinking water quality: are there socioeconomic disparities in nitrate levels in US drinking water? Environ Health 18(1):3, PMID: 30651108, 10.1186/s12940-018-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schullehner J, Hansen B. 2014. Nitrate exposure from drinking water in Denmark over the last 35 years. Environ Res Lett 9(9):095001, 10.1088/1748-9326/9/9/095001. [DOI] [Google Scholar]
- Schullehner J, Hansen B, Thygesen M, Pedersen CB, Sigsgaard T. 2018. Nitrate in drinking water and colorectal cancer risk: a nationwide population‐based cohort study. Int J Cancer 143(1):73–79, PMID: 29435982, 10.1002/ijc.31306. [DOI] [PubMed] [Google Scholar]
- Schullehner J, Linn Jensen N, Thygesen M, Hansen B, Sigsgaard T. 2017a. Drinking water nitrate estimation at household-level in Danish population-based long-term epidemiologic studies. J Geochem Exploration 183:178–186, 10.1016/j.gexplo.2017.03.006. [DOI] [Google Scholar]
- Schullehner J, Stayner L, Hansen B. 2017b. Nitrate, nitrite, and ammonium variability in drinking water distribution systems. Int J Environ Res Public Health 14(3):276, PMID: 28282914, 10.3390/ijerph14030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Hebel JR. 1984. A clinical trial of change in maternal smoking and its effect on birth weight. JAMA 251(7):911–915, PMID: 6363731, 10.1001/jama.1984.03340310025013. [DOI] [PubMed] [Google Scholar]
- Shukla S, Saxena A. 2018. Global status of nitrate contamination in groundwater: its occurrence, health impacts, and mitigation measures In: Handbook of Environmental Materials Management. Hussain C, ed. Cham, Switzerland: Springer, 869–888. 10.1007/978-3-319-58538-3_20-1. [DOI] [Google Scholar]
- Spalding RF, Exner ME. 1993. Occurrence of nitrate in groundwater—a review. J Environ Qual 22(3):392–402, 10.2134/jeq1993.00472425002200030002x. [DOI] [Google Scholar]
- Stayner LT, Almberg K, Jones R, Graber J, Pedersen M, Turyk M. 2017. Atrazine and nitrate in drinking water and the risk of preterm delivery and low birth weight in four Midwestern states. Environ Res 152:294–303, PMID: 27816866, 10.1016/j.envres.2016.10.022. [DOI] [PubMed] [Google Scholar]
- Super M, de H, Heese V, MacKenzie D, Dempster WS, Du Plessis J, Ferreira JJ. 1981. An epidemiological study of well-water nitrates in a group of South West African/Namibian infants. Water Res 15(11):1265–1270, 10.1016/0043-1354(81)90103-2. [DOI] [Google Scholar]
- Swiss Confederation Federal Office for the Environment and Federal Office of Public Health. 2010. Reporting for Switzerland under the Protocol on Water and Health. https://www.unece.org/fileadmin/DAM/env/water/Protocol_reports/reports_pdf_web/Switzerland_summary_report_en.pdf [accessed 4 December 2019.
- The World Bank. 2019. Consumer Price Index (2010 = 100) – Denmark. https://data.worldbank.org/indicator/FP.CPI.TOTL?locations=DK [accessed 30 December 2019].
- Tiso M, Schechter AN. 2015. Nitrate reduction to nitrite, nitric oxide and ammonia by gut bacteria under physiological conditions. PLoS One 10(3):e0119712, PMID: 25803049, 10.1371/journal.pone.0119712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNESDA (Union of European Soft Drinks Associations). 2018. Industry Volume Data: Denmark. https://www.unesda.eu/consumption/ [accessed 4 December 2019].
- Ward MH, DeKok TM, Levallois P, Brender J, Gulis G, Nolan BT, et al. 2005. Workgroup report: drinking-water nitrate and health—recent findings and research needs. Environ Health Perspect 113(11):1607–1614, PMID: 16263519, 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH, Jones RR, Brender JD, De Kok TM, Weyer PJ, Nolan BT, et al. 2018. Drinking water nitrate and human health: an updated review. Int J Environ Res Public Health 15(7):1557, PMID: 30041450, 10.3390/ijerph15071557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox AJ 2001. On the importance and the unimportance of birth weight. Int J Epidemiol 30(6):1233–1241, PMID: 11821313, 10.1093/ije/30.6.1233. [DOI] [PubMed] [Google Scholar]
- Wollmann HA 1998. Intrauterine growth restriction: definition and etiology. Horm Res 49(suppl 2):1–6, PMID: 9730664, 10.1159/000053079. [DOI] [PubMed] [Google Scholar]
- Zogorski, John S, Carter JM, Ivahnenko T, Lapham WW, Moran MJ, Rowe BL, et al. 2006. The quality of our Nation’s waters—Volatile organic compounds in the Nation’s ground water and drinking-water supply wells: U.S. Geological Survey Circular 1292, 101 10.3133/cir1292. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.