Abstract
Cue reactivity is an important biomarker of cannabis use disorder (CUD). Despite high rates of cigarette and cannabis co‐use, its role in cannabis cue reactivity remains unclear. Using a visual functional magnetic resonance imaging cue reactivity paradigm, we investigated interactive effects of cannabis and cigarette use on cannabis cue relative to cigarette and neutral cue reactivity in a priori regions of interest—the amygdala, striatum, anterior cingulate cortex (ACC), ventral tegmental area (VTA), and orbitofrontal cortex—and a whole‐brain analysis. In our sample of cannabis users and controls closely matched on cigarette use, significant interactions between cannabis and cigarette use status emerged in the amygdala, striatum, ACC, frontal pole, and inferior frontal gyrus. Cannabis‐only users showed heightened cue reactivity in the amygdala compared with nonusing controls. Co‐users did not show heightened cue reactivity compared with cigarette smoking controls, although cue‐induced VTA activity was positively correlated with grams per week of cannabis. Cigarette smoking controls showed unexpectedly heightened cue reactivity compared to co‐users and nonsmoking controls. These findings and the high prevalence of cannabis and cigarette co‐use underscore the importance of considering cigarette smoking status when investigating the role of cue reactivity in heavy cannabis use.
Keywords: cannabis use, cigarette use, cue reactivity, fMRI
Heightened neural cue reactivity is considered a biomarker of substance use disorders. Cigarette co‐use is common among heavy cannabis users and it matters when investigating cannabis cue reactivity. Across several regions of interest, cannabis only users demonstrated significantly elevated heightened cue reactivity compared to non‐cigarette smoking controls, while cannabis and cigarette co‐users did not. Cigarette smokers in the control group showed unexpectedly heightened levels of cannabis cue reactivity compared to non‐smoking controls and heavy cannabis users.
1. INTRODUCTION
Cannabis users often use tobacco products, either as cigarettes or in combination with cannabis (e.g., spliffs), 1 and 37.5% of individuals with a cannabis use disorder (CUD) also meet criteria for nicotine dependence. 2 Neural hyper‐responsivity to cannabis‐related cues is believed to be an important biomarker of CUD. 3 Preliminary evidence suggests that cannabis and tobacco have interactive effects on the brain and cognition. 4 , 5 , 6 Even though cannabis and tobacco co‐use is more of a rule than exception, the role of cigarette co‐use in neural cue reactivity remains untested.
Paralleling the steady rise in cannabis use and CUD across the globe, 7 the past decade saw a surge in studies aiming to unravel the mechanisms underlying cannabis use and CUD. Cue reactivity is a heightened subjective (e.g., self‐reported craving) or physiological (e.g., heart rate, skin conductance, and neural activity) response to drug‐related cues that is suggested to play an important role in the development and maintenance of addictive behavior across substances. 8 , 9 , 10 Cue reactivity is a multi‐faceted phenomenon involving reward, learning, memory, attentional, and motor processes. 3 , 11 , 12 Across studies in near‐daily cannabis users compared to non‐users, cannabis users show higher levels of subjective craving, attention, and approach action tendencies in response to cannabis relative to neutral cues. 3 , 13 , 14 , 15 , 16 These heightened behavioral responses to cannabis cues are thought to arise from changes in the functioning of brain networks involved in reward processing, salience, and cognitive control. Indeed, neuroimaging studies have shown elevated cannabis cue‐induced activity in these brain networks, 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 which covaries with cannabis craving and the severity of use and problems. 17 , 20 , 24 This provides further evidence of the role of cue‐induced craving as an underlying mechanism of CUD. Importantly, the cannabis users in these studies commonly used more tobacco than the controls, making inferences about cannabis‐specific effects challenging. 20 , 24 Moreover, previous cannabis cue reactivity research often utilized visual stimuli that may have unintentionally triggered craving for tobacco products due to the visual similarities between joints and cigarettes. 24
Common co‐use of cannabis and tobacco is more than just a research confound, and the lack of studies addressing the effect of tobacco co‐use constitutes a significant gap in our current understanding of the neurocognitive mechanisms of heavy cannabis use and CUD. With the common use of spliffs, 2 high rates of cigarette use in cannabis users (e.g., 81.4%–90.9% in Europe and 77.2% in the United Kingdom), 1 and common comorbidity of CUD and nicotine dependence, 2 it is also necessary to address whether co‐users differ from heavy users of cannabis‐only in their response to cannabis cues. Two previous studies have shown differential effects of co‐use compared with single‐use on brain functioning 4 , 25 with co‐users showing differences in functional connectivity and dynamic functional connectivity compared with cannabis and tobacco only users.
Research on the effects of co‐use on cognitive functioning and craving is limited. In the domain of memory performance, smaller hippocampal volume has been associated with better memory performance in co‐users but not in cannabis‐only users. 5 Interestingly, the opposite pattern was observed in the nonusing controls. During acute intoxication in regular cannabis and tobacco users, combined cannabis and tobacco administration inhibited the impairing effects of cannabis alone on delayed but not immediate recall. 6 However, no effect of tobacco intoxication alone or in combination with cannabis was observed on cannabis craving or liking. 26 Animal studies suggest that nicotine may specifically interact with the endocannabinoid system—the neurotransmitter system that the psychoactive compounds in cannabis act on—in the mesolimbic reward circuitry in the brain. Nicotine triggers anandamide (an endocannabinoid neurotransmitter) release in the ventral tegmental area (VTA) and nucleus accumbens (NA) with the downstream effect of increasing dopamine levels. 27 Importantly, cannabis users show heightened cue reactivity in the same regions. 19 , 22 , 24 Together, these early findings suggest that cannabis and tobacco co‐use may impact cannabis cue reactivity. Aberrant behavioral and neural functioning may be more pronounced in cannabis‐only users relative to cannabis and tobacco co‐users. However, the evidence is very preliminary, and the neurobiological mechanisms underlying this potential interactive effect remain unclear.
The goal of the current study was to specifically isolate cannabis cue reactivity in heavy cannabis users compared with non‐using controls. Groups were closely matched on cigarette use in order to replicate previous associations between cannabis use and brain functioning as well as investigate potential interactive effects of cannabis and cigarette use. The design of the current study was kept highly similar to our previous cue reactivity study, 24 while adding a cigarette condition to the cannabis cue reactivity paradigm. Using an a priori region of interest (ROI) approach, we expected to see heightened activity in the amygdala, striatum, anterior cingulate cortex (ACC), VTA, and orbitofrontal cortex (OFC) in heavy cannabis users compared with controls, as these are core regions associated with cue‐induced craving in substance use disorders and have previously been identified in cannabis cue reactivity. 17 , 24 Furthermore, we expected activity in these areas to be associated with the severity of problems and quantity of cannabis use. In addition, we aimed to investigate potential differences in cannabis cue reactivity between co‐users and cannabis‐only users in the ROIs and in a whole brain explorative analysis. Based on previous results suggesting a potentially mitigating effect of tobacco on functional activity, 4 , 25 we speculated that cannabis‐only users would show higher neural cannabis cue reactivity than co‐users.
2. MATERIALS AND METHODS
2.1. Participants
Thirty‐eight non‐treatment‐seeking heavy cannabis users (18 cigarette smokers) and 34 non‐cannabis users (15 cigarette smokers) aged 18–25 were recruited through advertisements in the local media and cannabis dispensaries in the Netherlands. The heavy cannabis users were required to use cannabis >10 times per month for the past 2 years and have no history of treatment for CUD. This criterion is based on our previous study 24 and was chosen to maximize comparability of findings across studies. Controls were allowed to have used cannabis up to 50 times in their life, but not during the past year. The heavy cannabis users and controls were matched on biological sex, age, estimated IQ, alcohol use and problems, other substance use, and symptoms of anxiety, depression, and attention deficit hyperactivity disorder (ADHD; disorders highly comorbid with cannabis use). This resulted in four closely matched subgroups of cannabis (CAN) and non‐cannabis using controls (CON) with and without co‐morbid cigarette use (+, −) in the sample: cannabis‐only users (CAN−), cigarette only users (CON+), co‐users of cannabis and cigarettes (CAN+), and non‐users of either (CON‐).
To control for confounding effects of other substance use, the following substance‐related exclusion criteria were used for participant selection: (1) Alcohol Use Disorder Identification Test (AUDIT) score over 12, 28 (2) smoking >20 tobacco cigarettes per day, (3) use of non‐cannabinoid drugs more than 100 times in their lifetime, 24 and (4) current use of prescribed or illicit psychoactive drugs. All participants were required to have no magnetic resonance imaging (MRI) contraindications, no history of major axis I psychiatric disorders (assessed by the Mini‐International Neuropsychiatric Interview, MINI), 29 and completed required education up to at least 16 years of age.
Six participants were excluded from analyses for the following reasons: (1) two for recent other drug use (cocaine and XTC) based on urine screen, (2) one for excessive head movement during scanning (>3 mm), (3) one for missing brain volumes from the cue reactivity task, and (4) two for fMRIPrep 30 preprocessing errors that could not be fixed. Three participants did not complete the urine drug screen but were retained in the sample. This resulted in a final sample consisting of 34 heavy cannabis users (16 cigarette smokers) and 32 matched non‐using controls (14 cigarette smokers).
3. QUESTIONNAIRES
To assess cannabis use problems and severity, the Cannabis Use Disorder Identification Test (CUDIT‐R) 31 was administered. Severity of nicotine and alcohol use was assessed with the Fagerstrom Test for Nicotine Dependence (FTND) 32 and the AUDIT, 28 respectively. To obtain a detailed overview of recent cannabis, nicotine, and alcohol use, a 14‐day Timeline Followback questionnaire (TLFB) 33 was administered in which participants reported their drug use over the previous 14 days before the test session. A custom substance use history questionnaire was used to obtain an overview of lifetime drug use. At the beginning and end of the testing session, craving was assessed with the short version of the Marijuana Craving Questionnaire (MCQ). 34 IQ was estimated with the similarities and matrix reasoning subscales of the Wechsler Adult Intelligence Scale (WAIS‐IV‐NL). 35 Depression, anxiety, and ADHD symptom severity were assessed with the Beck Depression Inventory (BDI), 36 State–Trait Anxiety Inventory for Adults (STAI), 37 and Conners' Adult ADHD Rating Scales (CAARS). 38
4. CANNABIS‐CIGARETTE FMRI CUE REACTIVITY TASK
The cue reactivity task was adapted from our previously validated cannabis cue reactivity task. 24 Adding a cigarette condition, the currently employed task used an event‐related design and consisted of four conditions: cannabis, cigarette, neutral, and animal. Ten visual stimuli from each condition were presented twice for 4s each. In between trials, a fixation cross was presented for 2 to 6s. The cannabis images were flower nuggets, joints, and individuals smoking cannabis. The cigarette images were cigarettes, individuals smoking cigarettes, and cigarette packs. The neutral images were office supplies visually matched to the cannabis and cigarette images for color and composition (e.g., individuals holding pens). The same male and female actors were displayed for each category. Cigarette filters were clearly visible, and cannabis joints were all cone shaped to ensure a clear distinction between cigarette and cannabis images. Participants were instructed to fixate on the images and press a button when they saw an animal image to maintain attention during the task.
5. PROCEDURE
The University of Amsterdam Faculty of Social and Behavioral Sciences ethics committee approved (2015‐DP‐6387) the study, and all participants gave informed consent prior to participating. Potential participants were contacted by phone to verify inclusion and exclusion criteria and to schedule an appointment. Twenty‐four hours before testing, all participants were asked to abstain from any alcohol or drug use (excluding caffeine and tobacco products to avoid acute withdrawal effects). On the test day, a multi‐panel urine drug screen was used to verify abstinence from amphetamine, benzodiazepine, cocaine, and opioids. Urine analysis of THC metabolites are sensitive to the presence of THC metabolites for longer than the requested 24‐h abstinence period; therefore, we cannot objectively verify 24‐h abstinence preceding the testing session. The drug screen was still conducted because it has been shown to increase compliance with abstinence periods. 39 At the beginning of the session, participants immediately filled out the MCQ followed by the WAIS‐IV subtests. After a verbal explanation of the cue reactivity task, participants completed the MRI session. Upon exiting the scanner, mental health and substance use‐related questionnaires were completed. At the end of the test session, participants again completed the MCQ. Test sessions were always conducted in the afternoon, and participants were financially compensated.
6. IMAGING PARAMETERS AND PREPROCESSING
Imaging was conducted with a Phillips 3T Intera MR scanner using a 32‐channel SENSE head coil at the Spinoza Centre for Neuroimaging at the Amsterdam University Medical Center. A high‐resolution T1‐weighted structural scan was acquired first for each participant (T1 turbo field echo, TR 8.2 s, TE 3.8 ms, 220 slices, slice thickness 1 mm, voxel size 1 × 1 × 1 mm, field of view [FOV] 240 × 188 mm, flip angle 8°). During the cue reactivity task, the blood oxygen dependent (BOLD) signal was measured with a T2*single shot echo planar imaging (EPI) sequence (TR 2.0 s, TE 27.63 ms, 37 slices, slice thickness 3 mm, voxel size 3 × 3 × 3 mm, interslice gap 3 mm, FOV 240 × 240 mm, flip angle 76.1°). Neuroimaging preprocessing was performed using fMRIPrep 1.3.2 30 that is based on Nipype 1.1.9. 40 See Data S1 for more detailed preprocessing information.
6.1. Statistical analysis
6.1.1. Behavioral analyses
Two‐way analysis of variances (ANOVAs) using cannabis and cigarette use status as the between group factors were conducted to test for differences in sample characteristics. Chi‐squared tests were used to test for differences in biological sex composition of the groups. For variables that did not have values for all groups, such as cigarette and cannabis use, independent sample t tests were conducted. To test for differences between heavy cannabis users and controls in self‐reported session‐induced craving, a two‐way repeated measures ANOVA was conducted with cannabis and cigarette use status as between group factors and MCQ score (measured before and after the session) as the dependent variable. Missing values for scale items were imputed using the mean score within individuals, and missing cannabis use data points were imputed using the group mean. 41
6.1.2. fMRI analyses
Functional MRI (fMRI) analyses were conducted using FMRIB Software Library (FSL). 42 The preprocessed imaging data were registered to the FSL MNI 152 template. A standard general linear model (GLM, ordinary least squares) with separate regressors for each condition (cannabis, cigarette, neutral, and animal) and the ITI fixation cross was used. A double gamma hemodynamic response function was convolved with each regressor. To improve the fit, temporal derivatives were added to the model as regressors of no interest. In order to examine cannabis‐specific cue reactivity, two subtraction contrasts were created: Can > Neutral and Can > Neutral + Cigarette.
To increase comparability with our previous study of cannabis cue reactivity, 24 the same anatomical masks were used for ROI analyses. The OFC, ACC, striatum, and amygdala masks were based on Nielson and Hansen's volume of interest database. 43 The VTA mask was manually created by Cousijn et al 24 using the Talairach Daemon in FSL with coordinates based on the laboratory of neuro imaging (LONI) probability atlas (coordinates: x = −20 to 20, y = −10 to 24, and z = −6 to −22). 44 The mean activity in each ROI for both contrasts was extracted for each participant. To test for the hypothesized main effect of cannabis use and exploratory interactions between cannabis and cigarette use, two‐way ANOVAs were conducted in each ROI for both contrasts with cannabis use status and tobacco use status as between group factors. To control for the use of five ROIs, a Bonferroni correction was used for each contrast such that p values were considered significant when less than .05/5 = .01. When significant interactions were observed, post hoc simple main effects were examined by pairwise comparisons using the Sidak adjustment for multiple comparisons with a significance threshold of p < .05.
The relationship between brain activity in the ROIs and cannabis use and related problems was examined with bivariate correlations with grams of cannabis used per week and CUDIT‐R scores for each contrast in the CAN− and CAN+ groups separately. Pearson and Spearman correlations were performed as necessary based on Shapiro–Wilk tests of normality. A Bonferroni correction was used to control for multiple comparisons with a p value of .05/2 = .025.
Finally, exploratory whole‐brain voxel‐wise group analyses were conducted with FEAT to test for main and interactive effects of cannabis and cigarette use on cannabis cue reactivity using a 2 × 2 ANOVA with random effects. F‐tests for the main effects were calculated using FSL command fslmaths and manually thresholded with command easythresh. The cluster‐wise multiple comparison correction was set at the default Z‐threshold of 2.3 and a cluster‐p significance threshold of .05.
7. RESULTS
7.1. Sample characteristics
No group differences were observed for age, biological sex, IQ, BDI, STAI, or CAARS. There was a main effect of cigarette use on alcohol use and related problems (F(1, 65) = 6.6, p = .013, ηp 2 = .096). The CAN+ and CON+ groups (cigarette users) had significantly higher AUDIT scores than the CAN− and CON− groups (non‐cigarette users). The CAN+ and CAN− subgroups did not differ on any of the cannabis use measures, and the CAN+ and CON+ subgroups did not differ on cigarette use measures. See Table 1 for a full overview of the sample characteristics.
TABLE 1.
Sample characteristics
| Characteristic | Cannabis users | Controls | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | CAN+ | CAN− | Overall | CON+ | CON− | |||||||
| n (% female) | 34 (47%) | 16 (37.5%) | 18 (55.5%) | 32 (56%) | 14 (57%) | 18 (55.5%) | ||||||
| Age, mean (SD) | 21.0 (2.1) | 21.3(2.0) | 20.7(2.1) | 21.2 (2.4) | 20.7 (2.5) | 21.6 (2.4) | ||||||
| IQ (Total of WAIS verbal and matrix reasoning) | 21.3 (4.4) | 20.7 (3.4) | 21.8 (5.1) | 22.1(4.4) | 21.6 (5.5) | 22.4 (3.4) | ||||||
| Alcohol use and related problems (AUDIT), mean (SD) | 6.8 (4.5) | 8.9 (4.1) * | 4.9 (4.1) * | 5.8 (3.2) | 6.2 (3.4) * | 5.4 (3.2) * | ||||||
| Nicotine dependence (FTND), mean (SD) | — | 3.0 (2.0) | — | — | 2.3 (1.6) | — | ||||||
| Duration cigarette smoking (year), mean (SD) | — | 5.2 (2.4) | — | — | 4.9 (2.6) | — | ||||||
| Cigarettes per day, mean (SD) | — | 10.1 (4.1) | — | — | 9.8 (5.6) | — | ||||||
| Lifetime cannabis use (number of uses), mean (SD) | 1301.0 (1819.9) ** | 1355.0 (1700.1) | 1253.0 (1966.0) | 14.7 (36.0) ** | 27.57 (52.0) | 4.6 (7.5) | ||||||
| Problem severity cannabis use (CUDIT‐R), mean (SD) | 13.5 (4.5) ** | 13.9 (3.5) | 13.1 (5.3) | 0.5 (1.0) ** | 1.0 (1.2) | 0.1 (0.2) | ||||||
| Duration frequent cannabis use (year), mean (SD) | 3.9 (2.1) | 4.3 (2.2) | 3.5 (2.0) | — | — | — | ||||||
| Cannabis use days/week, mean (SD) | 4.8 (1.7) | 5.3 (1.7) | 4.4 (1.7) | — | — | — | ||||||
| Cannabis use gram/week, mean (SD) | 2.9 (2.5) | 3.2 (2.8) | 2.6 (2.2) | — | — | — | ||||||
| Depression (BDI), mean (SD) | 5.2 (4.3) | 5.9 (5.0) | 4.6 (3.6) | 3.5 (3.1) | 4.0 (3.2) | 3.1 (3.1) | ||||||
| Anxiety (STAI‐trait), mean (SD) | 34.5 (8.2) | 33.4 (7.2) | 35.4 (9.1) | 33.7 (5.7) | 36.4 (4.8) | 31.6 (5.5) | ||||||
| ADHD (CAARS), mean (SD) | 16.6 (8.7) | 16.5 (7.5) | 16.6 (19.9) | 16.5 (7.7) | 19.3 (7.2) | 14.2 (7.4) | ||||||
| Pre‐test | Post‐test | Pre‐test | Post‐test | Pre‐test | Post‐test | Pre‐test | Post‐test | Pre‐test | Post‐test | Pre‐test | Post‐test | |
| Craving (MCQ score), mean (SD) | 27.2 (8.3) ## | 31.2 (9.5) ## | 26.0 (8.8) | 29.2 (10.5) | 28.2 (7.9) | 32.3 (8.4) | 13.5 (5.3) ## | 13.1 (4.2) ## | 15.1 (7.9) | 14.4 (6.1) | 12.3 (0.8) | 12.1 (0.5) |
Abbreviations: AUDIT, Alcohol Use Disorder Identification test; CAN+, co‐users of cannabis and cigarettes; CAN−, cannabis‐only users; CON+, cigarette users; CON−, nonusers of cannabis or cigarettes; CUDIT‐R, Cannabis Use Disorder Identification Test—Revised; FTND, Fagerström Test for Nicotine Dependence; MCQ, Marijuana Craving Questionnaire; SD, standard deviation.
p < .05.
p < .001 for group comparisons.
p < .001 for pre‐test–post‐test comparison.
7.2. Session‐induced craving
As expected, a mixed repeated measures ANOVA revealed a significant interaction between time and cannabis use (F(1, 62) = 14.25, p < .001, ηp 2 = .187). Post hoc pairwise comparisons indicate that the CAN− and CAN+ groups reported an increase in craving level after the session, whereas the CON+ and CON− groups did not. No main effect of cigarette use was observed on cannabis craving.
7.3. ROI analyses
Two‐way ANOVAs were conducted for each ROI for the Can > Neu and Can > Neutral + Cig contrasts. No main effects of cannabis or cigarette use emerged in any of the ROIs; however, significant interactions were observed in the striatum, amygdala, and ACC (see Figure 1). Post hoc simple main effects were examined with pairwise comparisons using a Sidak adjustment for family‐wise multiple comparisons with a significance threshold of p < .05. For the Can > Neu contrast, the CAN− group showed significantly higher cannabis cue‐induced brain activity in the amygdala than the CON− group, but not relative to the CON+ group. The CAN+ group did not show significantly heightened activity compared with the cigarette‐matched CON+ group. Unexpectedly, the CON+ group showed significantly higher cannabis cue‐induced activity in the amygdala and striatum than the CAN+ group and the CON− group. In the ACC, the CON+ group again showed significantly higher activity than the CAN+ but did not significantly differ from the CAN− or CON− groups in this area. The pattern of results was similar for the stricter Can > Neu + Cig contrast. The CON+ group consistently showed significantly higher cannabis cue reactivity than both the CON− and CAN+ groups in the striatum, amygdala, and ACC. Neither of the heavy cannabis‐using groups (CAN+ and CAN−) showed significantly increased activity compared with the control groups in this contrast. See Table 2 for results of the post hoc pairwise comparisons. Analysis of covariance (ANCOVA) analyses were also conducted to control for group differences in AUDIT score, and results were similar.
FIGURE 1.
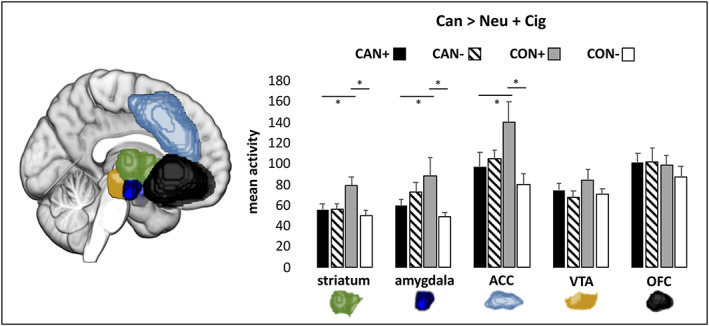
Mean cannabis cue‐induced brain activity for each region of interest (ROI). Results of the two‐way ANOVA analysis of the cannabis cue > cigarette + neutral cue contrast are depicted, Bonferroni corrected at * p < .01. CAN+, co‐users of cannabis and cigarettes; CAN−, cannabis‐only users; CON+, cigarette only users; CON−, nonusers of cannabis or cigarettes
TABLE 2.
Post hoc pairwise comparisons in region of interests (ROIs)
| Region of interest | Pairwise comparison | Can > Neu | Can > Neu + Cig | |||||
|---|---|---|---|---|---|---|---|---|
| Mean difference | SE | Sig. | Mean difference | SE | Sig. | |||
| Amygdala | CAN+ | CAN− | −11.549 | 7.368 | .122 | −13.165 | 13.469 | .332 |
| CAN− | CON− | 17.77 | 7.148 | .016 * | 24.004 | 13.067 | .071 | |
| CON+ | CON− | 24.403 | 7.641 | .002 * | 39.589 | 13.969 | .006 * | |
| CON+ | CAN+ | 18.187 | 7.848 | .024 * | 28.75 | 14.346 | .049 * | |
| Striatum | CAN+ | CAN− | −2.701 | 4.944 | .587 | −0.643 | 7.707 | .934 |
| CAN− | CON− | 2.062 | 4.796 | .669 | 6.108 | 7.477 | .417 | |
| CON+ | CON− | 15.999 | 5.127 | .003 * | 28.669 | 7.993 | .001 * | |
| CON+ | CAN+ | 16.639 | 5.265 | .002 * | 23.204 | 8.209 | .006 * | |
| ACC | CAN+ | CAN− | −13.75 | 10.586 | .199 | −7.836 | 18.726 | .677 |
| CAN− | CON− | 15.244 | 10.42 | .149 | 24.034 | 18.182 | .191 | |
| CON+ | CON− | 28.251 | 11.12 | .014 * | 59.398 | 19.158 | .003 * | |
| CON+ | CAN+ | 26.757 | 11.276 | .021 * | 43.2 | 19.675 | .032 * | |
Note: Results of the post hoc pairwise comparisons conducted using a simple main effect analysis with a Sidak adjustment for multiple comparisons.
Abbreviations: ACC, anterior cingulate cortex; CAN+, co‐users of cannabis and cigarettes; CAN−, cannabis‐only users; CON+, cigarette users; CON−, nonusers of cannabis or cigarettes; SE, standard error.
p < .05 significance threshold.
7.4. Association between brain activity and cannabis use
A significant correlation was observed between VTA activity (Can > Neu contrast) and weekly cannabis use (grams) in the CAN+ group (r s(14) = .603, p = .013), indicating that higher consumption was associated with increased VTA activity to cannabis cues. No significant correlations were observed in CAN− users for either contrast.
7.5. Exploratory whole brain analyses
Exploratory higher level whole‐brain two‐way ANOVAs with random effects were conducted for both contrasts of interest to identify potential interactive effects between cannabis and cigarette use status on cannabis cue reactivity in other regions of the brain. Consistent with the ROI analyses, no main effects of cigarette or cannabis use emerged for either contrast, but significant interactive effects emerged in a cluster of voxels in a frontal cortical area for the Can > Neu + Cig contrast. For the Can > Neu contrast, no significant interactions were observed. The contrast parameter estimates at the activation peak in the significant cluster were extracted to aid in interpretation of the interaction (see Figure 2), revealing a similar pattern as with the ROI analyses: the CON+ group showed the highest mean activation followed by the CAN− group, with the CAN+ group showing similar levels of activity as the CON− group. An analysis was also conducted to control for group differences in AUDIT scores, and results were similar.
FIGURE 2.
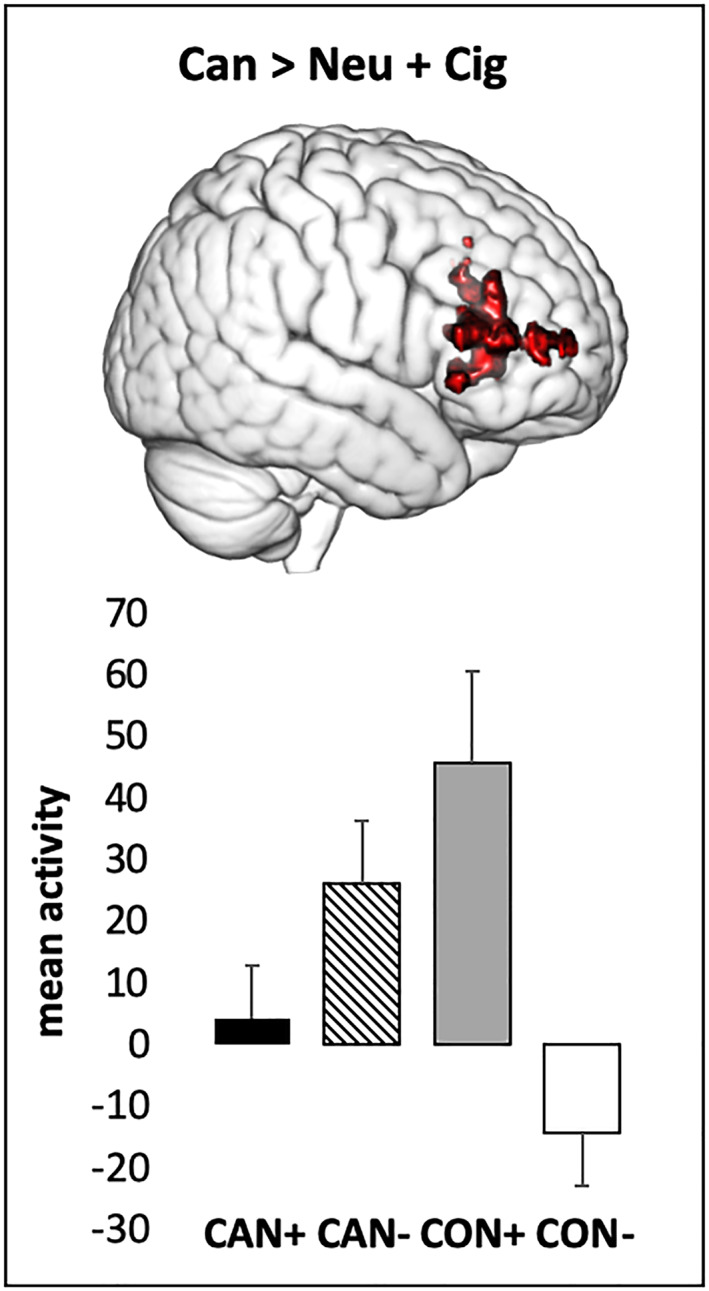
Significant interaction between cannabis and cigarette use status on cannabis cue‐induced brain activity in the frontal pole and inferior frontal gyrus (Z > 2.3, cluster‐corrected at p > .05). Mean activity extracted from the peak voxel (MNI coordinates: x = 52, y = 38, and z = 14) for the cannabis cue > cigarette + neutral cue contrast is depicted. CAN+, co‐users of cannabis and cigarettes; CAN−, cannabis‐only users; CON+, cigarette only users; CON−, nonusers of cannabis or cigarettes
8. DISCUSSION
Despite the prevalence of cannabis and cigarette co‐use and emerging evidence of interactive effects of these substances on the brain, the impact of cigarette co‐use on cannabis cue reactivity—an important biomarker of CUD—has not previously been investigated. As such, the goal of the current study was twofold: first, to replicate previous findings showing heightened neural cannabis cue reactivity in heavy users compared with controls using groups closely matched on cigarette use; second, to investigate potential differences in neural cannabis cue reactivity between co‐users and cannabis‐only users. In contrast to our expectations, we did not find elevated cannabis cue‐specific brain activity in our five ROIs or in the exploratory whole brain analysis when we combined cigarette and non‐cigarette smoking cannabis users compared with a sample of matched noncannabis using controls. This differs from previous findings of heightened activity to cannabis cues in frequent (at least 10 uses per month), daily, and dependent (meet DSM‐IV criteria for CUD) cannabis users regardless of cigarette use status in the VTA, ACC, amygdala, striatum, and frontal cortical regions. 19 , 22 , 24 When cannabis users and controls were split based on cigarette use, we observed heightened cannabis cue‐induced activity in the amygdala in the cannabis‐only users compared with non‐cigarette smoking controls, but not in co‐users. In co‐users, cannabis cue reactivity in the VTA was associated with severity of use, but not in the other ROIs. Unexpectedly, no associations between cannabis use measures and brain activity were observed in cannabis‐only users. These results show that cigarette use matters when studying cannabis cue reactivity and should be considered in future research and interpretations of previous studies.
One explanation for the limited cannabis cue‐specific activity observed in cannabis‐only users—and not in co‐users—may be the inclusion of a community sample of heavy users with varying degrees of problematic cannabis use, as cannabis cue reactivity increases with severity of use. 19 The average score on the CUDIT‐R in this sample was 13.5, which is above the suggested cutoff of 13 indicative of problematic and potentially dependent use. 31 However, scores ranged from 3 to 24 and 41% of the sample fell below the cutoff point, which may explain the limited cannabis cue reactivity observed when averaged across the group. The consistent cannabis–cigarette use interactions we observed across multiple ROIs indicate that cigarette use in both cannabis users and controls matters when assessing cannabis cue reactivity. These findings suggest that combining co‐users and cannabis‐only users into a single group may diminish the ability to detect cannabis‐specific cue reactivity in the brain. In support of this hypothesis, in previous research, the most widespread cannabis cue‐induced activation patterns emerged across the frontal, cingulate, and midbrain (e.g., VTA) areas when the proportion of co‐users in the sample was low (i.e., Filbey et al 22 ; average of 1.5 cigarette smoking days in past 60 days). In studies that had high proportions of co‐use in nondependent heavy users (58% in Zhou et al 19 and 68% in Cousijn et al 24 ), cannabis cue‐induced activity was observed in much narrower circuits including the VTA, medial prefrontal cortex, and superior parietal areas.
The mechanism underlying a potential reduced or lack of cannabis neural cue reactivity in co‐users remains unclear. Preliminary evidence suggests that co‐occurring use of tobacco products may have a “masking” effect on cannabis‐induced alterations in cognition and brain function. However, very little research has been done in this area, with the only evidence in humans coming from studies of functional connectivity and memory performance. In the studies of functional connectivity, co‐users differed from cannabis and tobacco only users, but these differences were not clearly additive, making interpretation challenging. 4 , 25 The clearest evidence for a masking effect of tobacco use is from memory performance during acute intoxication, where co‐administration inhibited the impairment of cannabis administration alone on delayed memory recall. 6 Regardless of the underlying mechanism, an absence of cue reactivity in co‐users has potentially important methodological implications for cannabis cue exposure research in terms of study design and user group characteristics. It is common for studies investigating heavy use and CUD to match users and controls on cigarettes use. Although this theoretically prevents cigarette use from confounding results, our results suggest that large proportions of cigarette smokers in cannabis users and control groups may in fact obscure cannabis‐specific reactivity. Given the limited evidence base on the effects of co‐use, our findings provide preliminary evidence for differences between cannabis‐only and co‐users on an important biomarker of CUD. It is necessary to replicate these findings in a clinical sample with CUD, as cannabis cue reactivity increases with severity of use‐related problems and individuals who seek treatment may differ from those who do not. 19 Future studies should aim to determine the potential clinical implications of these findings. Based on our results, it may be that cue reactivity is a more important factor in the development and maintenance of CUD in cannabis‐only users. If this is the case, therapies that involve cue exposure may be less effective in individuals with CUD who also regularly smoke cigarettes. Furthermore, a next step would be to investigate whether specific patterns of co‐use of cannabis and cigarettes (or other tobacco products) differentially influence cannabis cue reactivity. For instance, does it matter whether the drug effects overlap? How important is the route of administration in the potential masking effect of cigarette use? Would we see reduced cannabis cue reactivity in individuals who smoke joints but vape nicotine products? Ecological momentary assessment may be a useful tool to clarify whether daily patterns of use (e.g., used in conjunction in the case of a spliff or used separately in the day) relate to alterations in cannabis cue reactivity and what mechanisms may underlie these alterations.
Interestingly, our results also revealed a consistent pattern of cannabis–cigarette use interactions driven by unexpectedly elevated cannabis cue‐specific activity in cigarette smoking controls. That is, relative to the cannabis users, cigarette using controls showed elevated cannabis cue‐specific activity in bilateral amygdala, striatum and ACC, and in the left frontal pole and inferior frontal gyrus (IFG). But why would cigarette smokers show elevated cannabis cue reactivity? A tempting but tentative explanation could lie in a “tobacco gateway effect.” Previous research indicates that adolescents who currently or formerly use tobacco are more likely to initiate cannabis use and are at higher risk of developing cannabis dependence. 45 , 46 , 47 Post hoc correlational analysis suggests that our effects are independent of the severity of cigarette use (nonsignificant Pearson correlation coefficients ranging from r = −.159 to .233 in the ROIs). Tobacco use may sensitize individuals to cannabis cues regardless of severity of use, which might give insight into a potential neurocognitive mechanism of the gateway from cigarette to cannabis use. If this hypothesis is true, cannabis cue reactivity should predict future cannabis use in tobacco users. Future research should aim to replicate our coincidental finding of heightened cannabis cue reactivity in cigarette smokers in light of the gateway hypothesis with a larger sample and a longitudinal design to test the predictive value of neural cannabis cue reactivity in the initiation of cannabis use in smokers. Alternatively, it is possible that the incorporation of cigarette cues in the cue reactivity task may have caused diffused cigarette craving across conditions. However, in the amygdala, striatum, and ACC, we consistently found that cigarette smoking controls had higher activity that co‐users. If the cigarette cues were causing diffuse cigarette craving, we would expect co‐users to also show this effect.
A strength of the current study is the use of closely matched groups on key demographic characteristics, drug use, and mental health variables, which allowed us to examine cannabis‐ and cigarette‐specific effects. AUDIT score was the only matching variable in which any group difference was observed; cigarette smokers had significantly higher alcohol use and related problems than nonsmokers. Because we did not observe main effects of cigarette use on our outcome variables of interest, it is unlikely that this difference confounded our results although it cannot be ruled out entirely. A further strength is the systematic assessment of the effect of cigarette use on cannabis cue reactivity. First, by recruiting closely matched subgroups of cigarette smokers and nonsmokers in both the cannabis users and nonusing controls, we were able to examine interactive effects of cigarette and cannabis use. Second, by incorporating cigarette cues in the cue reactivity paradigm, we were able to isolate cannabis‐induced activity using a strict (Cannabis > Neutral + Cigarette) contrast. Given the similarity in the route of administration, cannabis cues (e.g., someone smoking a joint) may unintentionally activate cigarette‐related activity in the brain. By subtracting out all activity observed during the presentation of cigarette cues, we were able to isolate cannabis‐specific activity.
A limitation of the current study is the small sample size once the cannabis and control groups were split on cigarette use. Given the general pattern of reduced neural cue reactivity in co‐users compared with cannabis‐only users, future studies investigating cannabis–cigarette interactions on cue reactivity and cannabis‐specific effects in general should aim to use larger samples in order to be sufficiently powered to detect small to medium effect sizes. In addition, a limitation of the current cue exposure task was the lack of “online” assessments of craving during the task. It is therefore advised to incorporate in‐task assessments of craving after cue presentation (as in Filbey et al 17 , 22 cue reactivity paradigm) in order to further delineate between cannabis and cigarette craving. Furthermore, the influence of subacute intoxication on neurocognitive responses is a significant issue cutting across research on current heavy cannabis users. We were limited in our ability to control for potential residual subacute effects of intoxication, a salient issue given evidence that acute THC intoxication is associated with reduced striatal responding. 48 Investigating the associations between residual concentrations of drug metabolites and neural responses could garner important insight into potential mechanisms underlying differences between cannabis‐only and co‐users.
In conclusion, our findings offer preliminary evidence that cigarette use matters when measuring neural cannabis cue reactivity. Cannabis‐only users showed heightened cannabis cue reactivity in the amygdala, but co‐users did not in any ROI. These findings underscore the importance of considering cigarette smoking status when investigating the role of cue reactivity in heavy cannabis use, especially in the context of sample composition. Even when user and control groups are matched on cigarette use, co‐use may obscure specific effects of cannabis in single‐substance users. Furthermore, given the high prevalence of co‐use, 2 it is crucial for future studies to specifically investigate differences between cannabis‐only and co‐users in the underlying mechanisms of CUD.
AUTHORS CONTRIBUTION
LK and JC were responsible for the study concept, design, and analytic approach. LK performed the analyses and drafted the manuscript. EK, FF, and JC aided in the interpretation of the results and provided critical input during the revision of the content in the manuscript. All authors critically reviewed the content and approved the final version for publication.
Supporting information
Data S1 Supporting Information
ACKNOWLEDGEMENTS
The authors would like to thank Sabrina Okx, Fabio Melis, Anne Kramer, and Scarlett Slagter for their help with data collection. This research was supported by grant 1R01 DA042490‐01A1 awarded to J.C. and F.F. from the National Institute on Drug Abuse/National Institutes of Health. Open access funding enabled and organized by Projekt DEAL.
Kuhns L, Kroon E, Filbey F, Cousijn J. Unraveling the role of cigarette use in neural cannabis cue reactivity in heavy cannabis users. Addiction Biology. 2021;26:e12941. 10.1111/adb.12941
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hindocha C, Freeman TP, Ferris JA, Lynskey MT, Winstock AR. No smoke without tobacco: a global overview of cannabis and tobacco routes of administration and their association with intention to quit. Front Psych. 2016;7:1‐9. 10.3389/fpsyt.2016.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agrawal A, Budney AJ, Lynskey MT. The co‐occurring use and misuse of cannabis and tobacco: a review. Addiction. 2012;107(7):1221‐1233. 10.1111/j.1360-0443.2012.03837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filbey FM, DeWitt SJ. Cannabis cue‐elicited craving and the reward neurocircuitry. Prog Neuro‐Psychopharmacology Biol Psychiatry. 2012;38(1):30‐35. 10.1016/J.PNPBP.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vergara VM, Weiland BJ, Hutchison KE, Calhoun VD. The impact of combinations of alcohol, nicotine, and cannabis on dynamic brain connectivity. Neuropsychopharmacology. 2018;43(4):877‐890. 10.1038/npp.2017.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Filbey FM, McQueeny T, Kadamangudi S, Bice C, Ketcherside A. Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav Brain Res. 2015;293:46‐53. 10.1016/J.BBR.2015.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hindocha C, Freeman TP, Xia JX, Shaban NDC, Curran HV. Acute memory and psychotomimetic effects of cannabis and tobacco both “joint” and individually: a placebo‐controlled trial. Psychol Med. 2017;47:2708‐2719. 10.1017/S0033291717001222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. United Nations Office of Drugs and Crime . Word Drug Report 2018; 2018. 10.18356/bdc264f4-en [DOI]
- 8. Courtney KE, Schacht JP, Hutchison K, Roche DJO, Ray LA. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. 2015;21:3‐22. 10.1111/adb.12314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dager AD, Anderson BM, Rosen R, et al. Functional magnetic resonance imaging (fMRI) response to alcohol pictures predicts subsequent transition to heavy drinking in college students. Addiction. 2014;109(4):585‐595. 10.1111/add.12437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garland EL, Howard MO. Opioid attentional bias and cue‐elicited craving predict future risk of prescription opioid misuse among chronic pain patients. Drug Alcohol Depend. 2014;144:283‐287. 10.1016/J.DRUGALCDEP.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760‐773. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs—a quantitative meta‐analysis of cue‐reactivity brain response. Eur J Neurosci. 2011;33(7):1318‐1326. 10.1111/j.1460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- 13. Field M. Cannabis “dependence” and attentional bias for cannabis‐related words. Behav Pharmacol. 2005;16(5–6):473‐476. Accessed May 2, 2019 [DOI] [PubMed] [Google Scholar]
- 14. Field M, Eastwood B, Bradley BP, Mogg K. Selective processing of cannabis cues in regular cannabis users. Drug Alcohol Depend. 2006;85(1):75‐82. 10.1016/J.DRUGALCDEP.2006.03.018 [DOI] [PubMed] [Google Scholar]
- 15. Cousijn J, Goudriaan AE, Wiers RW. Reaching out towards cannabis: approach‐bias in heavy cannabis users predicts changes in cannabis use. Addiction. 2011;106(9):1667‐1674. 10.1111/j.1360-0443.2011.03475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cousijn J, Watson P, Koenders L, Vingerhoets WAM, Goudriaan AE, Wiers RW. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict Behav. 2013;38(12):2825‐2832. 10.1016/J.ADDBEH.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 17. Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106(31):13016‐13021. www.pnas.orgcgidoi10.1073pnas.0903863106 Accessed May 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karoly HC, Schacht JP, Meredith LR, et al. Investigating a novel fMRI cannabis cue reactivity task in youth. Addict Behav. 2019;89:20‐28. 10.1016/j.addbeh.2018.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou X, Zimmermann K, Xin F, et al. Cue‐reactivity in the ventral striatum characterizes heavy cannabis use, whereas reactivity in the dorsal striatum mediates dependent use. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(8):751‐762. 10.1016/j.bpsc.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 20. Wetherill RR, Childress AR, Jagannathan K, et al. Neural responses to subliminally presented cannabis and other emotionally evocative cues in cannabis‐dependent individuals. Psychopharmacology (Berl). 2014;231(7):1397‐1407. 10.1007/s00213-013-3,342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldman M, Szucs‐Reed RP, Jagannathan K, et al. Reward‐related brain response and craving correlates of marijuana cue exposure: a preliminary study in treatment‐seeking marijuana‐dependent subjects. J Addict Med. 2013;7(1):8‐16. 10.1097/ADM.0b013e318273863a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Filbey FM, Dunlop J, Ketcherside A, et al. fMRI study of neural sensitization to hedonic stimuli in long‐term, daily cannabis users. Hum Brain Mapp. 2016;37(10):3431‐3443. 10.1002/hbm.23250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zilverstand A, Huang AS, Alia‐Klein N, Goldstein RZ. Neuroimaging impaired response inhibition and salience attribution in human drug addiction: a systematic review. Neuron. 2018;98(5):886‐903. 10.1016/j.neuron.2018.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue‐reactivity in frequent cannabis users. Addict Biol. 2013;18(3):570‐580. 10.1111/j.1369-1600.2011.00417.x [DOI] [PubMed] [Google Scholar]
- 25. Filbey FM, Gohel S, Prashad S, Biswal BB. Differential associations of combined vs. isolated cannabis and nicotine on brain resting state networks. Brain Struct Funct. 2018;223(7):3317‐3326. 10.1007/s00429-018-1,690-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hindocha C, Lawn W, Freeman TP, Curran HV. Individual and combined effects of cannabis and tobacco on drug reward processing in non‐dependent users. Psychopharmacology (Berl). 2017;234(21):3153‐3163. 10.1007/s00213-017-4,698-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Scherma M, Fadda P, Le Foll B, et al. The endocannabinoid system: a new molecular target for the treatment of tobacco addiction. CNS Neurol Disord Drug Targets. 2008;7(5):468‐481. http://www.ncbi.nlm.nih.gov/pubmed/19128204 Accessed May 2, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saunders JB, Babor TF. AUDIT questionnaire: screen for. Addiction. 1993;88(6):791‐804. [DOI] [PubMed] [Google Scholar]
- 29. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini‐International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry. 1998;59:22‐33. [PubMed] [Google Scholar]
- 30. Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16(1):111‐116. 10.1038/s41592-018-0235-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Adamson SJ, Kay‐Lambkin FJ, Baker AL, et al. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test‐Revised (CUDIT‐R). Drug Alcohol Depend. 2010;110:137‐143. [DOI] [PubMed] [Google Scholar]
- 32. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström K‐O. The Fagerström test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119‐1127. 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- 33. Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the timeline followback for cocaine, cannabis, and cigarette use. 2012. 10.1037/a0030992 [DOI] [PubMed]
- 34. Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009;102(1–3):35‐40. 10.1016/j.drugalcdep.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wechsler D. WAIS‐IV‐NL: Wechsler Adult Intelligence Scale‐Nederlandstalige Bewerking. No Title. Pearson; 2012. [Google Scholar]
- 36. Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck depression inventories ‐IA and ‐II in psychiatric outpatients. J Pers Assess. 1996;67(3):588‐597. 10.1207/s15327752jpa6703_13 [DOI] [PubMed] [Google Scholar]
- 37. Spielberger CD. State–trait anxiety inventory. In: The Corsini Encyclopedia of Psychology. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2010. 10.1002/9780470479216.corpsy0943 [DOI] [Google Scholar]
- 38. Erhardt D, Epstein JN, Conners CK, Parker JDA, Sitarenios G. Self‐ratings of ADHD symptomas in auts II: reliability, validity, and diagnostic sensitivity. J Atten Disord. 1999;3(3):153‐158. 10.1177/108705479900300304 [DOI] [Google Scholar]
- 39. Roese NJ, Jamieson DW. . Twenty years of bogus pipeline research: A critical review and meta‐analysis.. Psychological Bulletin. 1993;114 (2):363–375. 10.1037/0033-2909.114.2.363. [DOI] [Google Scholar]
- 40. Gorgolewski K, Burns CD, Madison C, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;5:1–15. . 10.3389/fninf.2011.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siddiqui OI. Methods for computing missing item response in psychometric scale construction. Am J Biostat. 2015;5(1):1‐6. 10.3844/amjbsp.2015.1.6 [DOI] [Google Scholar]
- 42. Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173‐S186. 10.1016/j.neuroimage.2008.10.055 [DOI] [PubMed] [Google Scholar]
- 43. Nielsen F, Hansen LK. Automatic anatomical labeling of Talairach coordinates and generation of volumes of interest via the BrainMap database. Neuroimage. 2002;16(2):2‐6. http://hendrix.imm.dtu.dk/services/jerne/ Accessed March 6, 2020 [Google Scholar]
- 44. Shattuck DW, Mirza M, Adisetiyo V, et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage. 2008;39(3):1064‐1080. 10.1016/j.neuroimage.2007.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu J, Williford WR. The age of alcohol onset and alcohol, cigarette, and marijuana use patterns: an analysis of drug use progression of young adults in New York state. Subst Use Misuse. 1992;27(11):1313‐1323. 10.3109/10826089209047353 [DOI] [PubMed] [Google Scholar]
- 46. Patton GC, Coffey C, Carlin JB, Sawyer SM, Wakefield M. Teen smokers reach their mid twenties. J Adolesc Health. 2006;39(2):214‐220. 10.1016/j.jadohealth.2005.11.027 [DOI] [PubMed] [Google Scholar]
- 47. Korhonen T, Levälahti E, Dick DM, et al. Externalizing behaviors and cigarette smoking as predictors for use of illicit drugs: a longitudinal study among finnish adolescent twins. Twin Res Hum Genet. 2010;13(6):550‐558. 10.1375/twin.13.6.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Sousa Fernandes Perna EB, Theunissen EL, Kuypers KPC, et al. Brain reactivity to alcohol and cannabis marketing during sobriety and intoxication. Addict Biol. 2017;22(3):823‐832. 10.1111/adb.12351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


