Abstract
Chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME) negatively impacts the quality of life for children with the condition. Although up to 2% of children have CFS/ME, the bulk of research investigates adults with CFS/ME. Using the PRISMA extension for a scoping review and the work of Arksey and O’Malley (2005), a scoping review was conducted of all relevant peer-reviewed research investigating nutrition, exercise, and psychosocial factors within a pediatric population diagnosed with CFS/ME. Key themes found were nutrition and dietary components, exercise therapy, psychosocial factors, and multifaceted treatment. Nutrition was explored on its own as a tool to decrease symptoms; however, there were very few studies found to examine nutritional deficiency or treatment with those under the age of 18. Graded exercise and resistance training improved fatigue severity and symptoms of depression in adolescents with CFS/ME. Research exploring psychosocial factors of CFS/ME presented attributes that could lead to being diagnosed as well as barriers to treatment. The multifaceted treatment undertaken typically consists of graded activities/exercise, cognitive behavioral therapy, nutritional advice, and family sessions. This has shown to increase school attendance and decrease the severity of the fatigue for adolescents. Minimal literature exploring CFS/ME within a prepubescent population presents the need for further research.
Keywords: Adolescent, children, chronic fatigue syndrome, myalgic encephalomyelitis, pediatric
Introduction
Chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME) is a serious and relatively common condition characterized by overwhelming fatigue and a decrease in physical and cognitive function (Mackenzie and Wray, 2013; Rowe et al., 2017). Pediatric CFS/ME has a prevalence of 0.4–2.4% (Chalder et al., 2003; Crawley et al., 2011; Crawley et al., 2012; Rimes et al., 2007) in population studies and .06–.1% (Haines et al., 2005; Nijhof et al., 2011) within a hospital setting. ME/CFS affects all ages, ethnicities, and socio-economic status (Rowe et al., 2017). For adults, it has been found that there is a higher prevalence in women (Nacul et al., 2011). Recognition of this disabling condition is increasing and incidence peaks at 10–19 years and 30–39 years (Bakken et al., 2014; Parslow et al., 2017a).
CFS/ME negatively impacts quality of life (QOL) for children (Collin et al., 2016; Mackenzie and Wray, 2013). Physical symptoms such as a sore throat, joint and muscle pain, nausea, heightened inactivity, and sleep dysfunction have been identified in children with CFS/ME (Collin et al., 2015). Other cognitive and psychosocial symptoms include cognitive dysfunction, social isolation, depression, and anxiety (Collin et al., 2015). It is a complex condition; the etiology is unknown, and currently, there is no known cure (Collin et al., 2015; Richards et al., 2006; Rowe et al., 2017).
Studies have shown that dietary, exercise, and psychosocial factors can reduce symptoms and improve QOL (Jenkins and Rayman, 2005; Lopez et al., 2011; Maes et al., 2006; Maric et al., 2014). Thus appropriate nutrition, exercise, and psychosocial management strategies may be beneficial in alleviating symptoms and improving QOL in children with CFS/ME. The objective of this article is to conduct a scoping review exploring current peer-reviewed research investigating these strategies to decrease symptoms of CFS/ME within a pediatric population (aged under 18 years). Therefore, the research question was: What has been shown in the literature investigating management of CFS/ME in a pediatric population within the key areas of nutrition, exercise, psychology and social factors?
Methods
Following PRISMA extension for scoping review guidelines (Tricco et al., 2018) and the work of Arksey and O’Malley (2005), a comprehensive electronic literature search of relevant peer-reviewed journal articles was conducted by the main researcher (SC). Subsequent hand searching of relevant articles and reports was conducted to identify additional literature. Inclusion criteria consisted of peer-reviewed research investigating clinically diagnosed individuals with CFS/ME who are under the age of 18; research exploring nutritional components, exercise and/or physical activity, and lifestyle and wellbeing for children with CFS/ME; and psychosocial factors that may influence the treatment and diagnosis of CFS/ME. Excluded literature investigated individuals with no medical diagnosis of CFS/ME; adults (over 18 years old) with CFS/ME; not peer-reviewed research or commentary/opinion pieces; and research prior to 1994 as this was the date of the published clinical definition of CFS/ME.
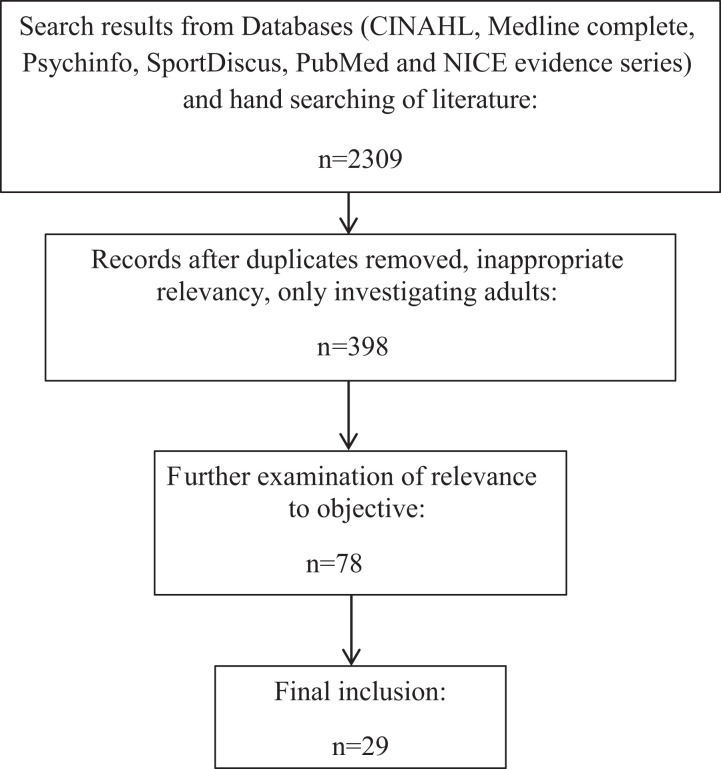
Databases used to search were CINAHL, Medline complete, Psychinfo, SportDiscus, PubMed and NICE evidence series. Multiple search terms were used to obtain findings. Such terms included child, adolescent, chronic fatigue syndrome, myalgic encephalomyelitis, CFS, ME, nutrition, trace elements (e.g., zinc, selenium, copper, aluminium, iron, magnesium), supplement, exercise, physical activity, lifestyle, wellbeing, family, treatment, therapy, environment, and psychology. Boolean operators and truncation of search terms were used to obtain a larger amount of the research results that were relevant. Following a comprehensive literature search by SC, the decisions of inclusion of literature were discussed with the second author (JM). As a result, 29 peer-reviewed research articles were used within this review (see Figure 1).
Figure 1.
Search strategy and selection process.
Upon finalizing the search, SC developed a data charting form to collate the literature and extract the descriptive characteristics of the studies (e.g., authors, date, title, method, key findings). The finalized data chart was discussed with the co-author and approved (see Table 1). The data extracted for analysis consisted of the participants’ descriptions (e.g., age, male, female), main focus (e.g., nutrition, exercise, psychology therapy, etc.) and results. Once finalized, exploration of the relationships between studies (thematic analysis) was conducted and the strength of the summarized report was assessed (refining and organizing themes to provide an overall thematic summary). This was reviewed by JM and discussed with SC for the final article.
Table 1.
Summary of research included.
| Title/authors | Objective | Intervention | Participants | Country | Method | Key findings |
|---|---|---|---|---|---|---|
| Biofeedback and cognitive behavioral therapy for Egyptian adolescents suffering from chronic fatigue syndrome; Al-Haggar et al. (2006) | Evaluate the efficacy of CBT with biofeedback in adolescents with CFS | RCT | 92 adolescents (mean age 12.52 ± 3.32 years) | Egypt | CBT aided by biofeedback intervention group versus control group. Assessed post-intervention: fatigue, school attendance, CFS symptoms | Fatigue severity was significantly lower and school attendance significantly higher in intervention group compared to the control group. Self-reported decreased in intervention group |
| Chronic fatigue syndrome: an evaluation of a community based management programme for adolescents and their families; Ashby et al. (2006) | Assess community-based programme | N/A | 10 children and adolescents (8–16 years old) and their parents | United Kingdom | Semi-structured interviews | Positive feedback of the approach conducted to include the family within the programme |
| Chronic fatigue syndrome (CFS) or myalgic encephalomyelitis is different in children compared to in adults: a study of UK and Dutch clinical cohorts; Collin et al. (2015) | Examine differences between young children, adolescents and adults with CFS | N/A | United Kingdom (2004–2014) and the Netherlands (2008–2010) database; 1568 United Kingdom adolescents (12–18 years) and 210 (under 12); 135 Dutch adolescents | United Kingdom and the Netherlands | Investigation of clinical cohorts from the United Kingdom and RCT from the Netherlands; multiple outcome measures | Younger aged children had less of a gender imbalance. Differences in elevated symptoms and range of symptoms depending on the age |
| Chronic fatigue syndrome at age 16 years; Collin et al. (2015) | Estimate the prevalence of CFS at 16 years of age | N/A | 14,541 pregnancies and 13,978 children alive at 12 months of age (excluding triplets and quads) | United Kingdom | ALSPAC data to estimate the prevalence of CFS at age 16. Used parent report of unexplained disabling fatigue lasting ≥six months; ALSPAC Family Adversity Index; school absence data |
Family adversity created higher risk of diagnosis of CFS. Female gender posed higher risk at 16 years of age as well as other mental health concerns |
| Maternal and childhood psychological factors predict chronic disabling fatigue at age 13 years; Collin et al. (2015) | Investigate if premorbid maternal and childhood psychological problems are risk factors for CFS at age 13 | N/A | 110 children of 5657 by age 13; data from the Avon Longitudinal Study of Parents and Children | United Kingdom | Edinburgh Postnatal Depression Scale and the Crown-Crisp Experiential Index at multiple time points | Mental health of both child and mother is a risk factor for CFS |
| Comparing specialist medical care with specialist medical care plus the Lightning Process® for chronic fatigue syndrome or myalgic encephalomyelitis (CFS/ME): study protocol for a randomised control trial (SMILE Trial); Crawley et al. (2013) | Examine the effectiveness of Lightning Process to Standard medical care and details on implementation | RCT | 80 participants (12–18 years old) | United Kingdom | RCT: Standard medical care or Standard medical care plus Lightning Process; primary outcomes are physical function (SF-36 physical function short form) and fatigue (Chalder Fatigue Scale) | Ongoing |
| Clinical and cost-effectiveness of the Lightning Process in addition to specialist medical care for pediatric chronic fatigue syndrome: randomized control trial; Crawley et al. (2017) | Compare effectiveness and cost-effectiveness of Lightning Process plus specialist medical care to Standard medical care alone in children with CFS/ME | RCT | 100 participants (12–18 years old) | United Kingdom | Measured at multiple times (3, 6 and 12 months). Primary outcome measure: SF-36-PFS (six months). Secondary measures: pain, anxiety, depression, school attendance and cost-effectiveness from health service viewpoint (3, 6 and 12 months) |
Lightning Process plus Standard medical care improved physical function, fatigue, decreased anxiety and depression, and improved school attendance. This was after 6 and 12 months. Cost-effective for mild to moderately affected adolescents in relation to health-related quality of life. Not all children wanted to take part and the reasons were not known |
| A multidimensional treatment plan for chronic fatigue syndrome; Gibson and Gibson (1999) | Test validity of multi-therapeutic treatment for people with CFS | Treatment-dietary intervention | 64 participants completed (10–59 years old) | United Kingdom | Six-month treatment intervention; intervention-wheat-free diet with nutritional supplements; homeopathic treatment of allergies; homeopathic constitutional prescribing; psychotherapy | 70% benefited wheat-free diet and supplements most helpful |
| Graduated exercise training and progressive training in adolescents with chronic fatigue syndrome: a randomized controlled pilot study; Gordon and Knapman (2010) | Effects of aerobic graded exercise and progressive resistance training on exercise intolerance, fatigue and quality of life | RCT | 22 participants (aged 13–18) | Australia | Measures: exercise tolerance, metabolic equivalents, quality of life, muscular strength and endurance. Evaluation of depressive symptoms and fatigue severity |
No significant difference between groups. Improvement in physical capacity and quality of life. Fatigue severity and depressive symptoms only improved in aerobic training group |
| Promising outcomes of an adolescent chronic fatigue syndrome inpatient programme; Gordon and Lubitz (2009) | Impact of graded exercise programme on physical outcomes, fatigue and mental state | Exercise programme assessment | 16 participants (mean age: 16.2 ± 1.28 years) | Australia | Outcome measures: quality of life, fatigue and depression; exercise assessment pre and post-treatment | GET significantly improves aerobic capacity. Improvement in depression scores. Fatigue severity improved |
| A qualitative investigation of eating difficulties in adolescents with chronic fatigue syndromes/myalgic encephaliomyelitis; Harris et al. (2017) | Exploring impact of eating difficulties in children with CFS | N/A | 11 participants (aged 12–17 years) | United Kingdom | Semi-structured interviews; thematic analysis | Difficulties caused by being too fatigued, low mood to eat and changes to their taste and smell. Variety of adaptations to ease difficulties |
| Early Adverse Experience and Risk for Chronic Fatigue Syndrome; Heim et al. (2006) | Investigate the relationship between early adverse experience and risk for CFS | N/A | 43 individuals with CFS and 60 healthy Control participants (73 total) (18–69 years old) | United States | Self-reported childhood trauma and psychopathology | Higher response of childhood trauma from those with CFS/ME. Childhood trauma increased severity of symptoms in those with CFS/ME |
| Interventions in pediatric chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review; Knight et al. (2013) | Systematic review of literature on interventions for paediatric CFS/ME | N/A | Children and/or adolescents (<18 years of age) | N/A | PRISMA guidelines for systematic review; databases searched: CINAHL, PsycINFO and Medline; 24 papers on 21 studies were included | Strongest evidence for CBT. Only one study examining exercise therapy in isolation. No evidence of detrimental impact of exercise as therapy. Lack of research in efficacy and safety of pharmaceutical or immunological treatment |
| A review of the predisposing, precipitating and perpetuating factors in chronic fatigue syndrome in children and adolescents; Lievesley et al. (2014) | Review of CFS in children and adolescents | N/A | N/A | N/A | Narrative synthesis; multiple databases searched; published articles from 1980 to 2013 | Psychiatric comorbidity higher in young people with CFS compared to healthy controls. Infection prior to diagnosis. Psychological and social risk factors (e.g., family history of CFS) had mixed results |
| Chronic fatigue syndrome: successful outcome of an intensive inpatient programme; Lim and Lubitz (2002) | Study the outcome of an intensive multidisciplinary inpatient programme | Multidisciplinary inpatient programme | 59 adolescents completed the programme (ages 10–19); 42 returned the questionnaire | Australia | Measured three months to five years after completion of the programme | Improvement in school attendance and physical activity. Symptoms improved overall |
| Telephone-based guided self-help for adolescents with chronic fatigue syndrome: a non-randomised cohort study; Lloyd et al. (2012) | Examine the efficacy of a telephone-based guided self-help intervention for adolescents with CFS | Preliminary evidence | 63 participants (11–18 years old) | United Kingdom | Outcome measures completed at baseline, pretreatment, end of treatment and at three and six months post-treatment. Primary outcomes: Fatigue (Chalder Fatigue Scale) and School attendance. Secondary outcomes: Impairment (Social Adjustment Scale), depression (Birleson Feelings Scale), adjustment (Strengths and Difficulties Questionnaire Total Difficulties Scale), anxiety (Spence Children’s Anxiety Scale), perfectionism (Child and Adolescent Perfectionism Questionnaire) and maternal mental wellbeing (General Health Questionnaire) |
Decrease in fatigue and significant increase in school attendance |
| Chronic fatigue syndrome in children and young people; Mackenzie and Wray (2013) | Reviews the best approach to assessment, diagnosis and management of CFS/ME in children and young people | N/A | N/A | N/A | Literature review | Early diagnosis and appropriate multidisciplinary intervention aid recovery. Further research needed to improve understanding and management of condition in young people |
| Internet-based therapy for adolescents with CFS: long-term follow-up; Nijhof et al. (2013) | Assessing long-term outcome of CFS for adolescents after FITNET | FITNET trial | 112 participants (aged 12–18 years) | Netherlands | Long-term follow-up (mean 2.7 years) of FITNET. Primary outcomes: fatigue severity (Checklist Individual Strength-20), physical functioning (87-iten Child Health Questionnaire) and school/work attendance |
Short-term effectiveness of FITNET is maintained at long-term follow-up |
| Children’s experiences of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a systematic review and meta-ethnography of qualitative studies; Parslow et al. (2017a) | Conduct a review of the qualitative studies presenting children’s experiences of CFS/ME | N/A | N/A | N/A | Systematic review and meta-ethnography | Biographical disruption; barriers and facilitators to coping; emotional aspects of recovery |
| Important factors to consider when treating children with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): perspectives of health professionals from specialist services; Parslow et al. (2017b) | To understand the perspectives of pediatric CFS/ME health professionals and identify outcomes that are clinically important | N/A | 15 health professionals | United Kingdom | Qualitative; focus groups and interviews | Children with CFS/ME are impacted across multiple aspects of health |
| The course of severe chronic fatigue syndrome in childhood; Rangel et al. (2000) | Follow-up of children with CFS after diagnosis | N/A | 25 children and adolescents (12–19 years old) and parents | United Kingdom | Semi-structured interviews | Mixture of recovery and still experiencing debilitating symptoms; Factors were found to predict a quicker or more likely recovery |
| Illness beliefs in CFS: a study involving affected adolescents and their parents; Richards et al. (2006) | Investigate the beliefs of the causes and management of young people with CFS | N/A | 21 participants (each with one parent) | United Kingdom | Qualitative; open-ended interviews; content analysis | Virus infection most common cause, psychological problems as a cause was rarely reported. Resting and reducing activity managed symptoms. Positive and negative experiences of psychological treatment |
| Cortisol output in adolescents with chronic fatigue syndrome: pilot study on the comparison with healthy adolescents and change after cognitive behavioral guided self-treatment; Rimes et al. (2014) | Investigating cortisol outputs and psychological variables for adolescents with CFS after CBT | CBT through telephone-based–guided self-help | 49 adolescents with CFS and 36 healthy adolescents | United Kingdom | Saliva collection (multiple samples). Cortisol measured six months post-treatment. Multiple measures |
Daily cortisol output increased significantly after CBT |
| Cow’s milk protein intolerance in adolescents and young adults with chronic fatigue syndrome; Rowe et al. (2016) | Examine illness severity of cow milk’s protein intolerance in young people with CFS | Two-year prospective study: pre and post | 55 participants (10–23 years old) | United States | Outcome measures at baseline and six months-QOL, Multidimensional Fatigue Scale, Functional Disability Inventory | Thirty-one percentage prevalence of intolerance. Improvement after milk-free diet. Milk-sensitive participants had worse HRQOL at baseline |
| Myalgic encephalmyelitis/chronic fatigue syndrome diagnosis and management in young people: a primer; Rowe et al. (2017) | Literature review | N/A | N/A | N/A | Literature review | Overall review of literature in relation to symptoms, possible causes, prevalence and treatment strategies in pediatric population |
| Childhood predictors of self reported chronic fatigue syndrome/myalgic encephalomyelitis in adults: national birth cohort; Viner and Hotopf (2004) | Childhood risk factors for CFS in adults | N/A | 16,567 babies born 5–11 April 1970; followed up at 5, 10, 16 and 29–30 years old. | United Kingdom | Childhood data taken from parents and teachers. Maternal mental health examined through malaise inventory |
Higher risk of CFS/ME associated with having a chronic condition in childhood, female gender and high social status in childhood. Higher levels of exercise linked to lower risk |
| Outpatient rehabilitative treatment of chronic fatigue syndrome (CFS/ME); Viner et al. (2004) | To assess the outcome of outpatient multidisciplinary rehabilitative treatment (graded activities/exercise programme, family sessions and supportive care) compared with supportive care alone for children and adolescents with CFS/ME | Multidisciplinary programme (graded activities/exercise programme, family sessions and supportive care) | 56 young people (aged 9–17 years) with diagnosed CFS/ME | United Kingdom | After treatment, participants were followed up for 3–24 months. Primary outcome measures of Global Wellness and school attendance |
Significantly higher Wellness scores and school attendance than supportive care alone. Reduction in the overall severity of the illness |
| What stops children with a chronic illness accessing health care: a mixed methods study in children with CFS/ME; Webb et al. (2011) | Examine factors associated with amount of time it took to access specialist care | N/A | 405 children (under 18 years of age) | United Kingdom | Semi-structured interviews; thematic analysis | Inadequate time to assessment/treatment; medical practioners’ lack of knowledge; parents’ struggled with communicating CFS for child |
| A feasibility study comparing two treatment approaches for chronic fatigue syndrome in adolescents; Wright et al. (2005) | Feasibility of a larger treatment trial comparing the effectiveness of ‘Pacing’ versus ‘The STAIRway to Health’ in adolescents with CFS | Feasibility study | 13 participants (age range 8.9–16.9 years) | United Kingdom | Two treatments: ‘Pacing’ and ‘The STAIRway to Health’. Multiple outcome measures: global health; activity, school attendance, fatigue and emotional symptoms | Global health improved in both treatments. STAIRway to Health had a higher rate of improvement |
Note: CBT: cognitive behavioral therapy; CFS/ME: chronic fatigue syndrome/myalgic encephalomyelitis; RCT: randomized control trial; ALSPAC: Avon Longitudinal Study of Parents and Children; GET: graded exercise therapy; FITNET: Fatigue in Teenagers on the Internet; HRQOL: Health-related Quality of Life; QOL: quality of life.
Results
The initial literature search found 2309 records. Upon removing duplicates, studies only investigating adults, and those that were not relevant to this review, 398 papers remained. Further exclusions in relation to the focus and age of participants initially reduced the total to 78 articles, followed by further scrutiny of age of participants and focus of objective which resulted in 29 papers being included (see Figure 1).
Key themes emerging from the search were nutrition and dietary components, exercise therapy, psychosocial factors, and multifaceted treatment strategies. The research findings (see Table 1) within each key theme are discussed in the following.
Nutritional components and therapy
Few studies examined the influence of diet or dietary modifications on alleviating symptoms (Gibson and Gibson, 1999; Rowe et al., 2016). While included as an integral part of a multifaceted treatment, nutritional interventions were also explored separately as an independent factor which could decrease symptoms. Rowe et al. (2016) investigated the impact of cow’s milk intolerance in adolescents and young adults (10–23 years old) with CFS/ME as a contributor to gastrointestinal symptoms using mixed methods. Adolescents and young adults with CFS/ME had a higher rate of treatable milk intolerance (31%), compared to recent research presenting 6–7% milk intolerance within children of a healthy population (Boyce et al., 2010). Upon implementation of a six-month milk-free diet in the milk intolerant group, the participants’ Health-related Quality of Life no longer significantly differed to the CFS/ME participants who were milk tolerant.
Gibson and Gibson (1999)’s research of a nutritional intervention (wheat-free diet and nutritional supplements (e.g., Co-enzyme Q10, evening primrose oil, magnesium and fluoride) as part of a multifaceted treatment plan) showed a positive impact on improving symptoms after a six-month intervention period. This pilot study presented the first indication that such inclusions of nutritional components could potentially result in a positive impact on the reduction of symptoms for people with CFS/ME.
Harris et al. (2017) used qualitative methods to explore eating difficulties in adolescents (12–17 years old) with CFS/ME. This study found that the eating difficulties commonly experienced by adolescents with CFS/ME were mainly caused by abdominal symptoms, being too tired to eat, and changes in their ability to taste and smell. Psychological symptoms of low mood and anxiety exacerbated these difficulties. Participants expressed interest for interventions through medication and modifying diet, as well as interventions to include families in relation to education in caring for and living with an adolescent with CFS/ME. Furthermore, education and support for those experiencing eating difficulties were one method to ease this experience and thus also decrease the subsequent psychological co-morbidities.
Exercise therapy
Exercise to decrease symptoms of CFS/ME in children under 18 years of age has been explored through various means, for example, graded exercise therapy and progressive resistance training (Gordon and Knapman, 2010; Gordon and Lubitz, 2009). Although exercise has provided both positive and negative results as an effective treatment of CFS/ME in adults (Clark et al., 2017; Larun and Malterud, 2011; Loy et al., 2016; Yoshiuchi et al., 2007), research has shown that cardiovascular exercise and resistance training improve fatigue severity and symptoms of depression in adolescents with CFS/ME (Gordon and Knapman, 2010; Gordon and Lubitz, 2009). Gordon and Lubitz (2009) examined a graded exercise training program for adolescents (mean age: 16 ± 1.25 years) with CFS/ME as part of a four-week inpatient program. Results showed a decrease in fatigue, depression, and mental outlook as a result of the program. Additionally, positive physiological effects (e.g., upper body strength and function improvement) were shown. These results were similar to graded exercise training results reported in adult studies (Gordon and Lubitz, 2009).
Gordon and Knapman (2010) conducted a randomized control trial (RCT) examining the difference between aerobic graded exercise and progressive resistance training for adolescents (13–18 years old) diagnosed with CFS/ME. Compared to baseline measures, results showed no significant difference between the two intervention types in relation to physical capacity and QOL. However, fatigue severity and symptoms of depression improved for those in the aerobic graded exercise group.
Psychosocial factors
Psychosocial factors were also found to impact diagnosis and increase symptoms of CFS/ME (Lievesley et al., 2014; Parslow et al. 2017a; Rangel et al., 2000; Webb et al., 2011). Psychosocial factors are grounded in a psychological and social context. For example, the psychological impact upon the individual of the disbelief about the condition by healthcare professionals and the social impact of being treated as different and abnormal. Webb et al. (2011) found that living with the condition as a child can prevent access to appropriate treatment as the lack of knowledge of the condition (both carers and doctors) was one barrier to access. In addition, negative attitudes and beliefs concerning the condition in children have shown to reduce timely access to treatment and care for children with CFS/ME (Parslow et al., 2017a; Webb et al., 2011). Recent research on healthcare practitioners’ views of the complex nature of the condition and particular aspects of treating/diagnosing children with the condition show the impact having a child with CFS/ME has upon the family, the difficulty in treating symptoms where the child has difficulty in expressing the symptoms and a lack of support from schools in providing a nurturing return to education or environment where the child can work at his/her own pace (Parslow et al., 2017b).
Multiple studies have examined the topic of negative psychological experiences in childhood that could lead to a diagnosis of CFS/ME (Collin et al., 2015; Heim et al., 2006; Viner and Hotopf, 2004). Exploring childhood trauma in relation to an adult diagnosis of CFS/ME, it was shown that through a population study in the United States, childhood trauma was a risk factor for developing CFS/ME in adulthood (Crawley et al., 2013). Furthermore, Collin et al. (2015) explored the risk factor of maternal and childhood mental health in relation to a diagnosis of CFS/ME by 13 years of age. Through parent-completed questionnaires during the antenatal period and regular intervals after birth, this study found that maternal mood (e.g., anxiety, depression) could also be a potential risk factor for childhood diagnosis of CFS/ME (Rowe et al., 2017). However, this was not a clear and direct correlation as diagnosis of CFS/ME could also be a consequence of maternal anxiety and depression altering childhood behaviour, thus creating a risk of developing CFS/ME (Collin et al., 2015).
Rangel et al. (2000) showed no link between gender, age of onset of CFS/ME or symptoms during the worst of their episodes in children with severe CFS/ME and his/her parents over time. However, there was a positive association between low socio-economic status and poor recovery outcome (Rangel et al., 2000). One reason provided was the decreased access to care that could result from having a lower socio-economic status. Other links to poor recovery depended on the timing of the beginning of his/her symptoms (e.g., outside the autumn term) and if it was or was not preceded by a flu-type illness (Rangel et al., 2000).
Multifaceted treatment strategies
A key focus of the research involving children and adolescents with CFS/ME is the examination of interventions to decrease symptoms and improve overall QOL. The interventions reported were cognitive behavioral therapy (CBT), exercise therapy (e.g., graded exercise therapy), dietary amendments and education, and multifaceted therapy (typically included more than one of the above-mentioned intervention types) (Al-Haggar et al., 2006; Ashby et al., 2006; Crawley et al., 2013; Crawley et al., 2017; Gordon and Knapman, 2010; Gordon and Lubitz, 2009; Knight et al., 2013; Lim and Lubitz, 2002; Lloyd et al., 2012; Rimes et al., 2014; Viner et al., 2004; Wright et al., 2005). Key findings presented the positive impact that a multifaceted treatment strategy can have for children with CFS/ME (Al-Haggar et al., 2006; Crawley et al., 2013; Crawley et al., 2017; Gibson and Gibson, 1999; Rimes et al., 2014; Viner and Hotopf, 2004; Viner et al., 2004; Wright et al., 2005).
The multifaceted treatment typically consisted of graded activities and/or exercise programme, CBT, nutritional advice, and in some cases, family sessions (e.g., counselling, education). Research revealed that through comparing multifaceted treatment to supportive care alone, there was a reduction in the severity of the illness, improved school attendance, and higher Wellness scores (Viner et al., 2004). This treatment strategy has shown to increase school attendance and decrease the severity of fatigue for adolescents (Al-Haggar et al., 2006; Ashby et al., 2006; Gibson and Gibson, 1999; Rimes et al., 2014; Viner et al., 2004; Wright et al., 2005). Although there was a positive impact on symptom relief, only two studies were found that examined these treatment programs for those under the age of 10 (Viner et al., 2004; Wright et al., 2005).
CBT was one of the most commonly prescribed psychological therapies used to manage adolescents with CFS/ME (Al-Haggar et al., 2006; Lloyd et al., 2012; Rimes et al., 2014) and deemed the most successful because of its consistent positive impact on overall QOL, school attendance, mood, and symptoms of CFS/ME (Lloyd et al., 2012). CBT was often combined with another therapy tool, for example, the Lightning Process (LP) or biofeedback (Al-Haggar et al., 2006; Crawley et al., 2013; Crawley et al., 2017; Viner and Hotopf, 2004). Crawley et al. (2013, 2017) conducted an RCT of children and adolescents (aged 12–18 years) investigating standard medical care (SMC) which included CBT, compared to SMC plus the LP. Findings showed a decrease in symptoms (e.g., fatigue, physical function, anxiety) over time (6 and 12 months) in the LP plus SMC treatment. As seen in other intervention research, there was also an increase in school attendance by 12 months.
Family sessions were one other inclusion within multifaceted treatment interventions. Viner et al. (2004) showed that including the family sessions within multifaceted treatment (e.g., family sessions, graded-exercise) created a learning tool for the family as well as decreased negative psychosocial influences.
As the positive benefits of a cognitive-behavioral approach have been shown (Al-Haggar et al., 2006; Crawley et al., 2013; Crawley et al., 2017; Knight et al., 2013; Rimes et al., 2014), research has also discussed methods of adapting the treatment through the use of technology. In order to allow increased access to treatment compared to solely face-to-face therapy, telephone-based therapy was examined and shown to be successful in decreasing fatigue and improving school attendance after a six-month follow-up (Lloyd et al., 2012). To increase the uptake and outreach of these programs, the adaption of multifaceted treatment has also led to the development of an Internet-based therapy for adolescents with CFS/ME, which was shown to maintain a similar recovery rate when compared to usual care after a long-term follow-up (1.7– 3.8 years) (Nijhof et al., 2013). Through these multifaceted interventions, there are common threads of nutrition, exercise, and cognitive-behavioral aspects that are embedded in these approaches.
Discussion
This scoping review has shown the range of peer-reviewed literature exploring nutrition, exercise, and psychosocial factors in managing symptoms of CFS/ME, particularly in adolescents. However, there is a lack of research within these areas investigating younger children, specifically those under 12 years of age. The literature included in this review investigated those under 18 years of age, but this also included studies investigating both adolescents and adults. Only two studies were found to use a multifaceted treatment approach with those of a younger age; however, there were fewer numbers of those of a younger age compared to the adolescents that participated. This may be attributed to a lack of recognition of this condition in children as well as the impact of the social support network prior to and upon diagnosis (e.g., children not being believed or the inability to express their symptoms). There are only a few studies exploring CFS/ME within a prepubescent population and this highlights the need for more research within this age group. In this review, a range of instruments, some validated and non-validated, were used to evaluate outcome measures in the included studies. This highlights the lack of agreement of the best instrument to measure CFS/ME symptoms.
To improve CFS/ME treatment in a pediatric population, there are multiple avenues to further explore. There is insufficient evidence for the use of dietary modifications or the use of nutritional supplements to relieve CFS/ME symptoms in prepubescent children. For instance, there have been few studies, small sample sizes, mostly pilot studies, and the nutritional intervention has more commonly been part of a multifaceted intervention. Further research is warranted in children aged under 18 years through appropriately designed dietary modifications or controlled interventions. Studies with larger and younger populations should be conducted to determine if CFS/ME was the direct cause of dietary intolerance (e.g., the development of milk intolerance). Further research in larger and younger (e.g., prepubescent) samples would provide valuable information to determine if there is and/or should be a difference in nutritional treatment strategies for prepubescent children compared to adolescents. Subsequently, this new knowledge could support the development of new approaches to help reduce symptoms in children with CFS/ME.
In relation to exercise therapy, this review provided positive findings for the benefits of exercise for adolescents with CFS/ME in reducing physical and cognitive symptoms and subsequently improving QOL. There remains the need for further research in those diagnosed with CFS/ME at a younger age and subsequently, the impact of such exercise therapy on their symptoms and QOL. Lastly, the combination of possible psychosocial factors presents a need to conduct more exploratory research to support or disprove developing theories as well as add new possibilities to improve recovery and/or prevent the diagnosis of CFS/ME.
These findings reveal the need for further research to understand the effect that dietary modifications or nutritional interventions, exercise therapy, psychosocial factors and multifaceted treatment could have upon the symptoms of CFS/ME in a prepubescent child. As a result, this could lead to a better understanding of how management strategies within these key areas could inform practice to reduce symptoms and improve QOL of children with CFS/ME.
Acknowledgements
The authors are grateful to The Silk Trust for providing the support for this scoping review.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Silk Trust.
ORCID iD
Sarah S Collard https://orcid.org/0000-0003-3813-957X
References
- Al-Haggar MS, Al-Naggar ZA, Abdel-Salam MA. (2006) Biofeedback and cognitive behavioral therapy for Egyptian adolescents suffering from chronic fatigue syndrome. Journal of Pediatric Neurology 4(3): 161–169. [Google Scholar]
- Arksey H, O’Malley L. (2005) Scoping studies: towards a methodological framework. International Journal of Social Research Methodology: Theory and Practice 8(1): 19–32. [Google Scholar]
- Ashby B, Wright B, Jordan J. (2006) Chronic fatigue syndrome: an evaluation of a community based management programme for adolescents and their families. Child and Adolescent Mental Health 11(1): 13–18. [DOI] [PubMed] [Google Scholar]
- Bakken IJ, Tveito K, Gunnes N. et al. (2014) Two age peaks in the incidence of chronic fatigue syndrome/myalgic encephalomyelitis: a population-based registry study from Norway 2008-2012. BMC Medicine 12(1): 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce JA, Assa’ad A, Burks AW. et al. (2010) Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID sponsored expert panel. Journal of Allergy and Clinical Immunology 126: S1–S58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalder T, Goodman R, Wessely S. et al. (2003) Epidemiology of chronic fatigue syndrome and self reported myalgic encephalomyelitis in 5–15 year olds: cross sectional study. British Medical Journal 327: 654–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LV, Pesola F, Thomas JM. et al. (2017) Guided graded exercise self-help plus specialist medical care versus specialist medical care alone for chronic fatigue syndrome (GETSET): a pragmatic randomised controlled trial. Lancet 390(10092): 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin SM, Norris T, Nuevo R. et al. (2016). Chronic fatigue syndrome at age 16 years. Pediatrics 137(2): e20153434–e20153434. [DOI] [PubMed] [Google Scholar]
- Collin SM, Nuevo R, van de Putte EM. et al. (2015) Chronic fatigue syndrome (CFS) or myalgic encephalomyelitis (ME) is different in children compared to in adults: a study of UK and Dutch clinical cohorts. British Medical Journal Open 5(10): e008830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin SM, Tilling K, Joinson C. et al. (2015) Maternal and childhood psychological factors predict chronic disabling fatigue at age 13 years. Journal of Adolescent Health 56(2): 181–187. [DOI] [PubMed] [Google Scholar]
- Crawley E, Hughes R, Northstone K. et al. (2012) Chronic disabling fatigue at age 13 and association with family adversity. Pediatrics 130(1): e71–e79. [DOI] [PubMed] [Google Scholar]
- Crawley E, Mills N, Hollingworth W. et al. (2013) Comparing specialist medical care with specialist medical care plus the Lightning Process® for chronic fatigue syndrome or myalgic encephalomyelitis (CFS/ME): study protocol for a randomised controlled trial (SMILE Trial). Trials 14(444): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley EM, Emond AM, Sterne JA. (2011) Unidentified Chronic Fatigue Syndrome/myalgic encephalomyelitis (CFS/ME) is a major cause of school absence: surveillance outcomes from school-based clinics. British Medical Journal Open 1(2): e000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley EM, Gaunt DM, Garfield K. et al. (2017) Clinical and cost-effectiveness of the lightning process in addition to specialist medical care for paediatric chronic fatigue syndrome: randomized control trial. Archives of Disease in Childhood 0: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SLM, Gibson RG. (1999) A multidimensional treatment plan for chronic fatigue syndrome. Journal of Nutritional and Environmental Medicine 9: 47–54. [Google Scholar]
- Gordon BA, Knapman M. (2010) Graduated exercise training and progressive resistance training in adolescents with chronic fatigue syndrome: a randomized controlled pilot study. Clinical Rehabilitation 24: 1072–1079. [DOI] [PubMed] [Google Scholar]
- Gordon B, Lubitz L. (2009) Promising outcomes of an adolescent chronic fatigue syndrome inpatient programme. Journal of Paediatrics and Child Health 45(5): 286–290. [DOI] [PubMed] [Google Scholar]
- Haines LC, Saidi G, Cooke RW. (2005) Prevalence of severe fatigue in primary care. Archives of Disease in Childhood 90(4): 367–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Gilbert M, Beasant L. et al. (2017) A qualitative investigation of eating difficulties in adolescents with chronic fatigue syndrome/myalgic encephalomyelitis. Clinical Child Psychology and Psychiatry 22(1): 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Wagner D, Maloney E. et al. (2006) Early adverse experience and risk for chronic fatigue syndrome. Archives of General Psychiatry 63(11): 1258. [DOI] [PubMed] [Google Scholar]
- Jenkins M, Rayman M. (2005) Nutrient intake is unrelated to nutrient status in patients with chronic fatigue syndrome. Journal of Nutritional and Environmental Medicine 15(4): 177–189. [Google Scholar]
- Knight SJ, Scheinberg A, Harvey AR. (2013) Interventions in pediatric chronic fatigue syndrome/myalgic encephalomyelitis: a systematic review. Journal of Adolescent Health 53: 154–165. [DOI] [PubMed] [Google Scholar]
- Larun L, Malterud K. (2011) Finding the right balance of physical activity: a focus group study about experiences among patients with chronic fatigue syndrome. Patient and Education Counseling 83(2): 222–226. [DOI] [PubMed] [Google Scholar]
- Lievesley K, Rimes KA, Chalder T. (2014) A review of the predisposing, precipitating and perpetuating factors in chronic fatigue syndrome in children and adolescents. Clinical Psychology Review 34: 233–248. [DOI] [PubMed] [Google Scholar]
- Lim A, Lubitz L. (2002) Chronic fatigue syndrome: successful outcome of an intensive inpatient programme. Journal of Paediatrics and Child Health 38: 295–299. [DOI] [PubMed] [Google Scholar]
- Lloyd S, Chalder T, Sallis HM. et al. (2012) Telephone-based guided self-help for adolescents with chronic fatigue syndrome: a non-randomised cohort study. Behaviour Research and Therapy 50(5): 304–312. [DOI] [PubMed] [Google Scholar]
- Lopez C, Antoni M, Penedo F. et al. (2011) A pilot study of cognitive behavioral stress management effects on stress, quality of life, and symptoms in persons with chronic fatigue syndrome. Journal of Psychosomatic Research 70(4): 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy BD, O’Connor PJ, Dishman RK. (2016) Effect of acute exercise on fatigue in people with ME/CFS/SEID: a meta-analysis. Medicine and Science in Sports and Exercise 48(10): 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie C, Wray A. (2013) Chronic fatigue syndrome in children and young people. Paediatrics and Child Health 23(1): 35–39. [Google Scholar]
- Maes M, Mihaylova I, De Ruyter M. (2006) Lower serum zinc in Chronic Fatigue Syndrome (CFS): relationships to immune dysfunctions and relevance for the oxidative stress status in CFS. Journal of Affective Disorders 90: 141–147. [DOI] [PubMed] [Google Scholar]
- Maric D, Brkic S, Mikic AN. et al. (2014) Multivitamin mineral supplementation in patients with chronic fatigue syndrome. Medical Science Monitor 20: 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacul LC, Lacerda EM, Pheby D. et al. (2011) Prevalence of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) in three regions of England: a repeated cross-sectional study in primary care. BMC Medicine 9: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhof SL, Maijer K, Bleijenberg G. et al. (2011) Adolescent chronic fatigue syndrome: prevalence, incidence, and morbidity. Pediatrics 127(5): 1169–1175. [DOI] [PubMed] [Google Scholar]
- Nijhof SL, Priesterbach LP, Uiterwaal CSPM. et al. (2013) Internet-based therapy for adolescents with chronic fatigue syndrome: long-term follow-up. Pediatrics 131(6): e1788–e1795. [DOI] [PubMed] [Google Scholar]
- Parslow RM, Harris S, Broughton J. et al. (2017. a) Children’s experiences of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME): a systematic review and meta-ethnography of qualitative studies. British Medical Journal Open 7(1): e012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parslow RM, Shaw A, Haywood KL. et al. (2017. b) Important factors to consider when treating children with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME): perspectives of health professionals from specialist services. BMC Pediatric 17(1): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel L, Garralda M, Levin M. et al. (2000) The course of severe chronic fatigue syndrome in childhood. Journal of the Royal Society of Medicine 93: 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J, Chaplin R, Starkey C. et al. (2006) Illness beliefs in chronic fatigue syndrome: a study involving affected adolescents and their parents. Child and Adolescent Mental Health 11(4): 198–203. [DOI] [PubMed] [Google Scholar]
- Rimes KA, Goodman R, Hotopf M. et al. (2007) Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: a prospective community study. Pediatrics 119(3): 603–609. [DOI] [PubMed] [Google Scholar]
- Rimes KA, Papadopoulos AS, Cleare AJ. et al. (2014) Cortisol output in adolescents with chronic fatigue syndrome: pilot study on the comparison with healthy adolescents and change after cognitive behavioural guided self-help treatment. Journal of Psychosomatic Research 77(5): 409–414. [DOI] [PubMed] [Google Scholar]
- Rowe PC, Marden CL, Jasion SE. et al. (2016) Cow’s milk protein intolerance in adolescents and young adults with chronic fatigue syndrome. Acta Paediatrica 105(9): e412–e418. [DOI] [PubMed] [Google Scholar]
- Rowe PC, Underhill RA, Friedman KJ. et al. (2017) Myalgic encephalomyelitis/chronic fatigue syndrome diagnosis and management in young people: a primer. Frontiers in Pediatrics 5: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco AC, Lillie E, Zarin W. et al. (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Annals of Internal Medicine 169(7): 467–473. [DOI] [PubMed] [Google Scholar]
- Viner R, Hotopf M. (2004) Childhood predictors of self reported chronic fatigue syndrome/myalgic encephalomyelitis in adults: national birth cohort study. British Medical Journal 329(7472): 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner R, Gregorowski A, Wine C. et al. (2004) Outpatient rehabilitative treatment of Chronic Fatigue Syndrome (CFS/ME). Archives of Disease in Childhood 89: 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb CM, Collin SM, Deave T. et al. (2011) What stops children with a chronic illness accessing health care: a mixed methods study in children with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (CFS/ME). BMC Health Service Research 11(1): 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright B, Ashby B, Beverley D. et al. (2005) A feasibility study comparing two treatment approaches for chronic fatigue syndrome in adolescents. Archives of Disease in Childhood 90(4): 369–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiuchi K, Cook DB, Ohashi K. et al. (2007) A real-time assessment of the effect of exercise in chronic fatigue syndrome. Physiology and Behavior 92(5): 963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]