Abstract
Background
A large proportion of our older adults live with Alzheimer’s Disease and Related Dementias and the number of those diagnosed in the future is expected to increase dramatically as the population ages. Persons with dementia bring unique healthcare challenges due to the manifestation of behavioral and psychological symptoms associated with the disease. The lack of geriatric clinicians as well as a properly trained non-geriatric specialist workforce capable of addressing the symptoms persons with dementia exacerbate the challenge of providing effective care. Pharmacological interventions are contraindicated for treatment of most behavioral psychological symptoms of dementia (BPSD). The Centers for Medicare and Medicaid Services now requires that nonpharmacological interventions be used as a first-line treatment. It has not been determined what nonpharmacological intervention for BPSD are most effective and what the infrastructure would entail for such interventions for PWD living at home.
Purpose of Review
The purpose of this study is to examine the literature focusing on interventions aimed towards managing persons’ symptoms of dementia living in home-based settings. A scoping review examining the literature published on this topic over the last three years was conducted.
Recent Findings
One thousand twenty four articles were found, of which nine met inclusion criteria. Five articles used occupational based therapy, two used exercise therapy and one article was found utilizing aromatherapy and music therapy.
Keywords: Dementia, non-pharmacological, symptom management, intervention, home-based care
Summary
The majority of articles used occupational based therapy as their intervention for BPSD. Overall, research showed non-pharmacological interventions can be effective in helping mange BPSD in persons living in home-based settings, although maintenance effects of interventions should be further explored in future research as well as how to ensure these interventions are more widely utilized by caregivers in this setting.
Introduction
Due to the drastic aging of the population, Alzheimer’s Disease and related disorders (ADRD) are becoming more prevalent both in the United States and globally[1, 2]. In the United States 17% of adults 75–84 years old have ADRD and this rises to 32% in those over 85 [3]. Symptoms experienced by persons with dementia (PWD) are complex and widespread including behavioral (e.g. agitation and aggression), psychological (e.g. anxiety and depression) and somatic (e.g. pain). Behavioral and psychological symptoms of dementia (BPSD) are shown to be present in the vast majority of PWD [4]. In fact, estimates suggest 90% of individuals will experience at least one while living with ADRD [5]. Therefore, dementia symptom management is currently a complex problem exasperated by an increasing aging population and consequential rise in rates of ADRD that can have serious detrimental effects.
While BPSD are highly prevalent and an important factor in providing care for PWD, the symptoms are not classified in standard classification systems including the DSM-V [4, 5]. As a result Cerejeira et al. [5] created a classification system, dividing BPSD into five categories: psychopathological features, perceptual disturbances, disturbances in motor function, circadian rhythms, and appetite and eating disorders, which are the categories seen in the following review. Due to the chronic nature of ADRD, these symptoms frequently get worse over time resulting in worsening caregiver burden [6–8]. This may lead to hospitalization [9] and institutionalization [10, 11], which result in large financial repercussions [12, 13]. Moreover, BPSD decreases quality of life of PWD and their caregivers and can lead to burnout and turnover [14], affecting care quality [15].
Most pharmacologic interventions, including antipsychotics and other sedative-hypnotics have been shown to be largely ineffective, with significant side effects that can often worsen quality of life for PWD [16, 17] These side effects include: sedation, weight loss, debility, syncope and more [16]. Antipsychotic use has also been linked with an increased risk of mortality, leading to a black box warning being issued from the U.S. Food and Drug Administration [17–19]. The only pharmacologic intervention to have shown benefit in reducing BPSD, particularly agitation and aggression, is SSRIs [20, 21]. However, SSRIs also increase the risk for falls [22] and can lead to hyponatremia. Overall, with the exception of SSRIs, in most cases the benefits of using medication to treat symptoms of dementia fail to outweigh the risks associated with antipsychotic medications.
Non-pharmacological interventions represent the recommended therapy for the management of most BPSD [23, 24]. Examples of non-pharmacological interventions used to treat BPSD include occupational activities, music therapy, acupuncture, aromatherapy, physical exercise, bright light therapy, touch therapy, cognitive rehabilitation, validation therapy, reminiscence therapy, personalized pleasant activities, person-centered care training and practice development [25, 26].
Little is known regarding the implementation of non-pharmacological interventions targeting BPSD of PWD living in home-based settings. Most research has focused in nursing homes, despite the fact that the overwhelming majority of PWD live in a home-based setting [27]. Consideration of the use of non-pharmacological interventions in home-based settings therefore requires further research. This scoping review will examine non-pharmacological interventions or management of BPSD in home-based settings with the goals of:
Discovering what dementia symptom management programs exist settings and what their infrastructure entails;
examining the strengths and weaknesses of dementia symptom management programs or interventions;
identifying ways science can improve in taking care of PWD and their symptomology.
Methods
Purpose of the Study
Scoping reviews follow a specific process to explore evidence addressing the identified research question. [28]. Scoping reviews support recognition of gaps existing in literature. Identification of these gaps support development of future research. The following scoping review examined literature focusing on management of BPSD. The purpose of the study was to conduct a scoping review of the current research evidence on non-pharmacological interventions or symptom management programs for BPSD in PWD living in home-based settings.
Identifying Studies
Four databases were searched including CINHAL, PubMED, PsychINFO and Cochrane. A professional librarian facilitated the search process. Per journal guidelines, we searched literature within the past 3 years. Previous reviews on this topic exist but fall outside of the three-year period used in this search or did not focus on PWD living in a home-based setting [5, 25, 20]. Additionally, multiple reviews and research on the topic focus on interventions targeting dementia symptom management programs implementing education programs for formal and informal caregivers rather than programs directly targeting the person with dementia, which is what this review focused on [29, 26].
The following search terms were used: dementia, behavioral or psychological, symptom, non-pharmacological, symptom management and intervention. Articles were filtered by age, 65 years of age and older and by date of publication.
Inclusion Criteria
Inclusion criteria were as follows: a) peer reviewed research studies, b) human subjects, participant 65 years of age or older; c) diagnosis of dementia; d) BPSD symptoms of dementia reported as an outcome measure; e) a management or intervention program for BPSD; and f) PWD living in home-based settings.
Several different types of interventions for BPSD were examined in the included studies. Home-based settings were defined as either home-care, group home (sometimes referred to as board and care) or assisted living facilities. English language studies completed in the years 2016 and 2019 were included.
The review excluded gray literature, unpublished papers, review articles, qualitative studies, dissertations or protocol papers. During the screening of articles, guidelines adapted from Sackett et al. [30] were used to evaluate level of evidence. The level of evidence guidelines included:
Level I: Systematic reviews, meta-analysis, randomized control trials
Level II: Two groups, nonrandomized studies (i.e., cohort, case control)
Level III: One group, nonrandomized (i.e., pretest and posttest)
Level IV: Descriptive studies that include analysis of outcomes (i.e., single-subject design, case series)
Level V: Case reports and expert opinion that included, narrative literature reviews and consensus statements.
Exclusion Criteria
Articles were excluded if any of the following were met: a) used animal models; b) did not have a primary outcome of BPSD symptom management; and c) focused pharmacological interventions. Furthermore, interventions and BPSD management programs were excluded if they took place in hospital, nursing homes or other institutional settings, as the study only looked at home-based care of persons with dementia. Interventions targeting PTSD, TBI or MCI were not included. Further, interventions targeting caregivers alone were not included in the study, as the review focused on programs and interventions directly targeted at the PWD’s symptom expression from dementia. Interventions examining outcomes measures such as quality of life or symptoms that are not BPSD were excluded, examples of such symptoms include pain, physical impairment and incontinence.
Data Abstraction
The list of articles generated by New York University library were uploaded onto Endnote and then Covidence for management and analysis. They were then extracted and put into an Evidence Table (Table 1). Data collected included the authors, year of publication, level of evidence of the study, study design and participants, interventions and results. The primary and secondary authors reviewed the studies and collaborated on decisions of inclusion and exclusion as well as on synthesizing data into themes.
Table 1.
Evidence Table: Non-pharmacological Dementia Symptom Management.
| Author/year | Level of Evidence/Study design | Participants/Incl usion Criteria | Residenc e of Participants | Intervention Location | Non-pharmacological Intervention | Results/Interve ntion effectiveness |
|---|---|---|---|---|---|---|
| Lamb et al. 2018 | Level I Multicenter, pragmatic, investigator masked RCT |
494 PWD (329 intervention group, 165 usual
care) >70 years of age (mean age 77 years) MMSE > 10, able to sit in a chair and walk 10 feet w/out assistance |
Home Dwelling | Gym facilities and outpatient facility premises | 4 months of supervised exercise (cycling, weight training, walking) and support for physical activity | No differences found in neuropsychiatric symptoms (95% CI −2.1 to 0.14) |
| De Oliveira et al., 2017 | Level I RCT, Double-blind pilot study |
21 PWD, 70–91 years of age (mean age
78.7 years) MMSE < 24, Presence of at least 3 types of NPS, stable dose of psychotropic medication for 60 days |
Home-Based Dwelling | Outpatient clinic | TAP-O: Tailored activity Program-outpatient version using occupational therapists | Intervention group had significant decrease in hallucination (P = 0.04), agitation (P = 0.03), anxiety (P = 0.02), aggression (P = 0.01), sleep disorder (P = 0.02), aberrant motor behavior (P = 0.02), and in caregiver burden (P = 0.003) compared with controls |
| Gitlin et al., 2018 | Level I Single-blind, parallel, RCT |
80 PWD, mean age 80.4 years English speaking Veterans with dementia, MMSE < 23, able to participate in 2 or more self-care activities, not involved in another study |
Home Dwelling | Home | TAP-VA: Tailored activity program for veterans, 8 in-home sessions delivered by occupational therapists | TAP-VA group showed reduction in number (−0.68, 95% CI = −1.23 to −0.13) and frequency by severity (−24.3, 955 CI =−45.6 TO −3.1) of behavioral symptoms compared to controls |
| Ikemata et al., 2017 | Level I RCT, double blind |
44 PWD >65 years of age (mean age 84.9 years), diagnosis of dementia; MMSE between >11 and <23 points, can participate in recreational activities and sit during vial signs and muscle relaxation; presence of BPSD |
Group Home | Home | Progressive muscle relaxation for 15 min. each day for 90 days in group environment | No difference between intervention group and
control group in total NPI-NH score Total NPI-NH score significantly improved in intervention group following progressive muscle relaxation (4.78 +_or – 5.07). |
| Liang et al., 2017 | Level I Pilot Block RCT |
30 PWD (15 intervention group, 15 control
group), 67–98 years of age Inclusion criteria not reported |
Home Dwelling | Adult Day Health Center and Home | 30 min. group sessions with a companion robot named Paro at dementia day care centers 2–3 times a week for 6 weeks | No differences in behavioral and physiological measure between intervention and control condition were found. |
| Pimougu et et al., 2017 | Level III Observation al Study, pre and post test design |
421 PWD (mean age 82.2 years)
Community dwelling, MMSE > 18, restriction in daily functioning |
Home Dwelling | Home | 12–15 occupational therapy session by a therapist over a 3month period | Behavioral troubles were significantly reduced over the intervention period and remained stable thereafter (p < 0.01). |
| Raglio et al., 2016 | Level III Pre-post Test Observation al Design |
Four PWD, mean age 78.25 years
Inclusion criteria not reported |
Home Dwelling | Not specified | 12 Active Music Therapy sessions, 40 min. each, twice a week by a music therapist | BPSD improved based on NPI global scores (T0 = 30.75; TI = 28.50; T2 = 27.25) and Geriatric Depression Scale (T0 = 14.5; T1 = 11; T2 = 11.75). |
| Straubmeier et al., 2017 | Level I Cluster-randomized, multicenter, prospective study |
362 Cognitively Impaired Persons, (208
intervention group, 154 control group) mean age 81.3 years
Inclusion criteria not reported |
Home Dwelling | Adult Day Health Center | MAKS therapy: group therapy consisting of social warm-up sessions, sensorimotor activity, cognitive activation, activation of ADLs | Neuropsychiatric symptoms evolved more favorably in the intervention group (Cohen’s d = 0.23, p = 0.055). |
| Kaymaz et al., 2016 | Level I Pilot RCT |
28 Participants (14 intervention group, 14
control group) ≥65 years of age (mean age 78.11 years); diagnosis of severe or moderate dementia by a physician, a planned consistent drug regimen, agitation-related behaviors, residence at home, no change in caregiver over last 3 months |
Home Dwelling | Home | Aromatherapy via massage and inhalation at home for 4 weeks | NPI scores were significantly lower in the intervention group (p < 0.05). At 4-weeks, the CMAI and ZBI scores were significantly lower in the intervention group (p < 0.05). |
Note. AD, Alzheimer’s Disease; ADLs, Activities of Daily Living; BPSD, Behavioral and Psychological Symptoms of Dementia; Et al., And Colleagues; NH, Nursing Home; RCT, Randomized Control Trial; CI, Confidence Interval; MMSE, Mini-Mental State Examination; PWD, Persons with Dementia; NPI-Q, Neuropsychiatric Inventory Questionnaire; T0 – T2, Time Point; CMAI, Cohen-Mansfield Agitation Inventory; ZBI, Zarit Burden Interview **Table reported outcomes for persons with dementia only, did not include caregiver reported outcomes and results.
Results
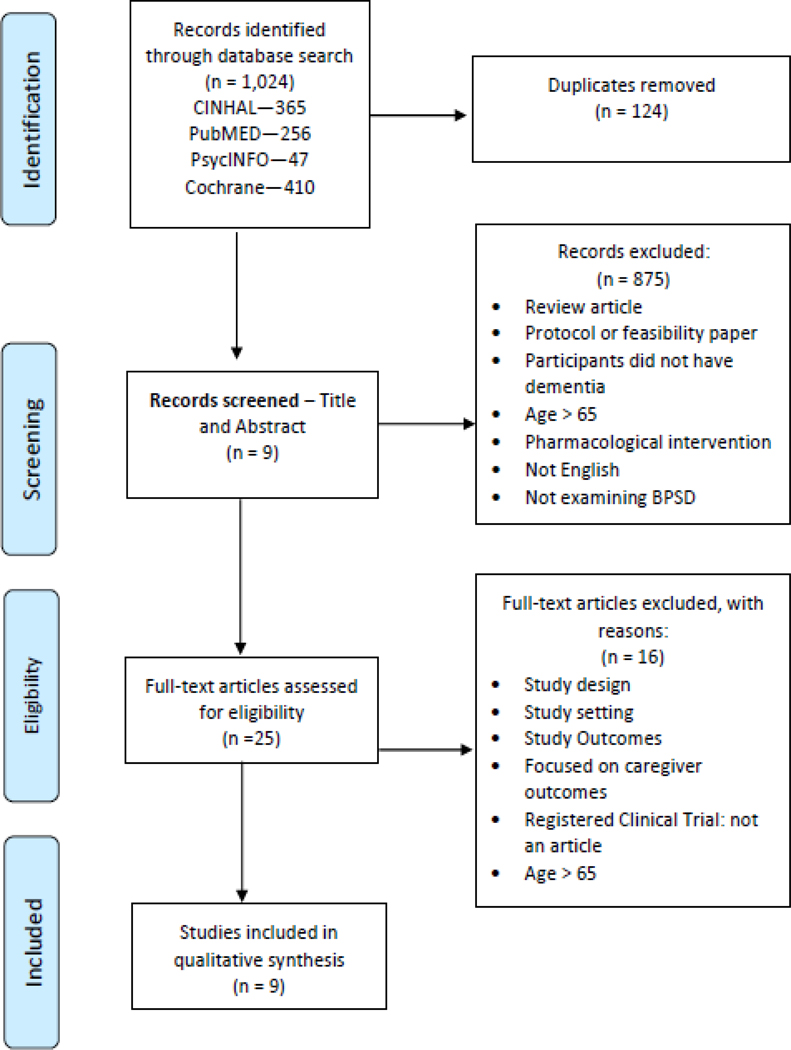
This scoping review identified a total of 1069 articles from the electronic database search. Nine articles met inclusion criteria and were included. Overall, the majority of articles used a randomized control design demonstrating Level I evidence, with the rest representing Level III evidence (Table 1). Although results were positive from the dementia symptom management interventions, most studies had a small sample size [*31–36] ranging from 4–80 participants in size, which may limit generalizability, therefore larger trials are needed to validate these results on a larger scale.
All included articles assessed BPSD using a version of the neuropsychiatric inventory (NPI) questionnaire. The NPI provides assessment of neuropsychiatric disturbances such as delusions, agitation, dysphoria, hallucinations, anxiety, apathy, euphoria, disinhibition, irritability, night-time behavior disturbances, aberrant motor behavior, and appetite and eating abnormalities [37]. Different versions of the NPI were used across the studies included in this review (Table 2).
Table 2.
Measurements Table: Confirmation of Dementia and Screening of BPSD
| Study | Intervention | Confirmation of Dementia | Measurement of BPSD |
|---|---|---|---|
| Lamb et al., 2018 | Exercise | Confirmed diagnosis of dementia according to DSM-IV | NPI-Q—Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia |
| De Oliveira, 2018 | TAP-O (OT Based) | Physician diagnosis of dementia | Brazilian version of the NPI-C: Neuropsychiatric inventory-Clinician. |
| Gitlin et al., 2018 | TAP-VA (OT Based) | Physician diagnosis of dementia | NPI-C |
| Ikemata et a., 2017 | Progressive Muscle Relaxation | Diagnosis of dementia | NPI-NH: Neuropsychiatric Inventory Nursing Home Version |
| Liang et al., 2017 | Companion Robot | Diagnosis of dementia | Caregiver proxy reports assessed agitation,
neuropsychiatric and depressive symptoms, Cohen Mansfield Agitation
Inventory—Short Form, NPI-Q, Cornell Scale for Depression in Dementia |
| Pimouguet et al., | OT Based | Diagnosis of dementia | NPI |
| Raglio et al., 2016 | Music Therapy | Not specified | NPI and Geriatric Depression Scale |
| Straubmeier et al., 2017 | MAKS Therapy (OT Based) |
MMSE and MoCA | NPI-Q |
| Kaymaz et al., 2016 | Aromatherapy | Physician diagnosis of dementia | NPI, CMAI, ZBI |
Note. DSM, Dementia Symptom Management; NPI-Q, Neuropsychiatric Inventory Questionnaire; CMAI, Cohen-Mansfield Agitation Inventory; ZBI, Zarit Burden Interview; TAP-O, Tailored Activity Program-Outpatient Version; TAP-VA, Tailored Activity Program-For Veterans; OT, Occupational Therapy; MAKS, motor stimulation, activities of daily living, cognitive stimulation, spiritual element; MoCA, Montreal Cognitive Assessment
In this review, articles varied in the process adopted to confirm the inclusion criteria of dementia. The majority of articles did not specify any criteria used to assess for the presence of dementia and appeared to rely upon documented physician diagnosis of dementia. One article utilized criteria established by the DSM-IV (Table 2). Interventions that were effective based on symptom type can be an essential use of this research for practical application in the field (Table 3).
Table 3.
Symptoms and Selected Effective Interventions
| Author | Symptoms Addressed | Intervention Used |
|---|---|---|
| De Oliveira et al., 2017 | Hallucination, agitation, anxiety, aggression, sleep disorder and aberrant motor behavior | Tailored Occupational Therapy |
| Gitlin et al., 2018 | Neuropsychiatric and Behavioral Symptoms | Tailored Occupational Therapy |
| Ikemata et al., 2017 | Anxiety, agitation, apathy and irritability | Progressive Muscle Relaxation |
| Pimouguet et al., 2017 | Behavioral troubles, neuropsychiatric symptoms | Occupational Therapy |
| Raglio et al., 2016 | Behavioral and psychological symptoms, neuropsychological symptoms, depression and anxiety | Active Music Therapy |
| Straubmeier et al., 2017 | Nonspecific Neuropsychiatric symptoms | MAKS Therapy |
| Kaymaz et al. 2016 | Nonspecific Neuropsychiatric Symptoms | Aromatherapy |
Note. Seven of nine interventions are noted to have significantly treated dementia symptoms
Physical Activity/Exercise Based Interventions
Two articles focused on addressing exercise or muscle relaxation to reduce BPSD in PWD. Specifically, one article addressed traditional elements of physical activity based in a gym type setting and using equipment such as a treadmill [38]. The study utilized therapists to supervise exercise ranging from moderate to high intensity levels. It required individuals to attend group sessions twice a week lasting 60–90 minutes as well as home exercises (Lamb et al.). Lamb et al. demonstrated no differences in BPSD.
In contrast to direct physical activity, one study considered the effectiveness of progressive muscle relaxation. In a group setting, PWD participated in 15 minutes of daily progressive muscle relaxation for a period of 90 days. PWD participating in the intervention group demonstrated decreased NPI scores as well as apathy and irritability scores. Improvement was seen in the interest, volition, and social relationship scores on the Mental State Scale [33].
Occupational Therapy Based Interventions
Several articles addressed overall activity-based interventions, which targeted several components of activities of daily living (ADLs). Often these interventions were led or developed by occupational therapists. For example, Pimouguet et al. [39] developed an intervention, which addressed performance of functional activities and social participation. Occupational therapists worked within the home setting with both PWD and caregivers to address compensatory and environmental strategies targeting activity levels. The intervention demonstrated a decrease in BPSD following the intervention period as well as over a 6-month period.
Two studies utilized the tailored activity programs (TAP& TAP) interventions in an outpatient (TAP-O) and home (TAP-VA) setting [*31, **32]. In both studies, OTs assessed PWD’s abilities and created tailored activity interventions. A reduction in BPSD symptoms was apparent in both studies. One demonstrated a decrease in hallucination, agitation, anxiety, aggression, and sleep disorder [*31]. Gitlin et al. [**32] showed a reduction in BPSD as well as function dependence level and pain.
One study introduced the use of a companion robot, Paro, to support decreased BPSD. The intervention group received 30-minute group sessions with Paro at an adult day health center two to three times a week for six weeks; participants also had Paro at home for six weeks. The use of Paro demonstrated a significant difference in affective and social outcomes between care recipients and the control group. Observations showed that Paro the robot improved care recipients’ facial expressions (affect) and created a positive effect on mood and behavior. PWD communication with staff and social interaction also increased at the adult day health center. Care recipients with less cognitive impairment responded significantly better to Paro. However, there were no significant differences in care recipient BPSD between the intervention and control group [34].
Another study administered therapy at adult day health centers targeting social interactions, sensorimotor activity, activities of daily living, and cognitive activation. After 6 months, PWD receiving the therapy interventions demonstrated improved BPSD [40].
Overall, activity-based interventions often required tailoring to meet individual needs. Tailoring of interventions often required input from trained therapists, increasing the difficulty of standardizing interventions across home-based settings.
Active Music and Aromatherapy Interventions
Music and aromatherapy represent important non-pharmacological interventions in the management of BPSD. Nonetheless, this review identified only two articles addressing their use in persons living in a home-based setting. Raglio et al. [35] utilized an active music intervention conducted by a music therapist. The music therapist was able to support communication between the PWD and caregiver, reducing BPSD. An overall reduction in BPSD was demonstrated.
Aromatherapy also demonstrated a reduction in BPSD. Kaymaz, Ozdemir [*41] utilized hand massage and aromatherapy inhalation at home. At 2 and 4 weeks, BPSD were significantly lower in the intervention group. At 4 weeks, the agitation and caregiver burden scores were lower in the intervention group as well.
Discussion
Overwhelmingly, articles in this review demonstrated the important role of occupational therapy in the non-pharmacological management of BPSDs in PWD living in home-based settings. Occupational therapy can be defined as addressing at least one or more of the following components: training of cognitive functions, training of skills, training of sensory-motor functions, advice and instruction regarding use of assistive devices, and counseling of the caregiver [42, 24]. Most articles included in this review addressed occupational components such as ADLs, cognitive activation, caregiver education, and social interactions. Additionally, several studies included caregiver education and participation during the intervention.
This review demonstrated an important relationship between BPSD and occupational therapy-based interventions. For example, the TAP-O and TAP-VA programs demonstrated significant reductions in BPSD scores [*31, **32]. Another intervention led by occupational therapists demonstrated reduced BPSD scores that remained stable over a three-month period [39]. Intervention programs aimed at addressing key activity factors, such as socialization, cognitive activation, and ADLs, and tailored based upon individual needs, supports reduction in BPSD scores. The importance in addressing the multiple components of activity for PWD supports reduction in the presence of BPSD.
The important role of the occupational therapists in PWD living in home-based settings may be under-recognized and/or under-utilized. Previous research has demonstrated caregivers and PWD often fail to understand or recognize the role of occupational therapy in the management of dementia [43]. Caregivers may recognize specific challenges facing PWD that would benefit from structured occupational therapy interventions and support a reduction in BPSD. Nonetheless, previous reviews have demonstrated that interventions targeting the functional ability of PWD should be used as first line therapy in the management of BPSD [23]. Interventions addressing functional ability carry little risk of negative side effects [23]. Increased awareness of occupational therapy-based interventions represents a critical finding from this review.
Physical activity, such as structured exercise programs, also demonstrated positive reductions in the presence or severity of BPSDs [38]. However, physical activity-based interventions required transportation to an outpatient setting [38]. Caregivers may negatively view interventions or treatments requiring frequent weekly trips to outpatient settings. Implementation of rigorous physical activity may not be feasible based upon location of the PWD in a home-based setting while the intervention is offered at another facility or requires special equipment. Consideration of how to encourage physical activity within the home-based setting without requiring specialized equipment or therapists represents a key aspect in tailoring future exercise-based interventions for PWD living in the home-based setting.
While not heavily featured in this review, music and aromatherapy also demonstrated reductions in the presence and/or severity of BPSD [35, *41]. Music and aromatherapy represent widely recognized and adopted strategies in the management of pain and other BPSD. Aromatherapy has been used in hospital and community settings to address pain and BPSD [44, 45]. Previous research has demonstrated conflicting results in the management of BPSD [45]. However, use of music and aromatherapy interventions may be easily adapted for PWD living in the home-based setting and employed by caregivers.
Throughout this review, most of the reported interventions required the presence of a trained specialist, such as a music therapist, physiotherapist, or occupational therapists. Trained individuals were necessary to conduct assessments as well as develop and tailor interventions. The reliance upon therapists in the delivery or guidance of PWD and/or caregivers through interventions targeting BPSD may limit the generalizability of the interventions across home-based settings, as these types of therapists either may not be accessible or may lack effective training in caring for PWD. Further work is therefore needed to disseminate these interventions and integrate into standard care for physical and occupational therapists.
Nonetheless, the connection between physical activity and reduction in BPSD highlights the importance in considering how to improve activity levels for PWD. Consideration of how to tailor physical activities for home-based settings represents a critical need. Training caregivers regarding the delivery of appropriate home-based activity interventions may represent a key method for improving activity levels and decreasing BPSD.
The articles included in this review presented several limitations. These included demonstrated inconsistency in the criteria used to determine a diagnosis of dementia. Moreover, the stage or type of dementia was not indicated. Dementia represents a growing challenge in primary care settings [46, 47]. Barriers to diagnosis have been found to relate more to a lack of adequate time with each patient instead of provider ability to diagnosis dementia [48]. Previous reviews have demonstrated primary care providers are able to support recognition and diagnosis of dementia in community settings [49, 48]. Nonetheless, it is necessary to consider the criteria used by providers during the diagnosis process. For example, the majority of studies relied upon the presence of a documented diagnosis of dementia from a patient’s medical record [*31]. Discussion of how the dementia diagnosis occurred was missing or not addressed. Only one article identified use of the diagnostic criteria from the DSM-IV [38]. Another article in this review utilized other non-diagnostic tools, such as the Mini Mental State Exam (MMSE) [50] and the Montreal Cognitive Assessment (MoCA) to determine the presence of dementia [51]. The MMSE and MoCA were designed to assess for the presence of cognitive changes, however, they fail to represent stand-alone diagnostic tools. Previous reviews have established the MMSE may support a diagnosis of dementia but should not be used as the primary assessment instrument [52]. Recognition of the diagnostic tools used by primary care providers to diagnose the presence, type, and stage of dementia is necessary to support understanding of the effectiveness of specific non-pharmacological interventions on BPSD.
Differences in the type and stage of dementia limit understanding regarding the effectiveness of the interventions targeting BPSD. Most articles in this review failed to address the potential impact of differing levels of cognitive impairment on the effectiveness of the intervention. Of note, articles addressing occupational-based interventions highlighted the importance of assessing and tailoring interventions based upon cognitive and functional abilities [*31, **32, 39]. Only one study included a subgroup analyses to assess the different effectiveness based upon cognitive impairment [40]. The authors found a stronger effect for individuals with mild cognitive impairment and moderate dementia verses individuals with a mild stage of dementia [40]. Previous research has also demonstrated the need to consider matching non-pharmacologic interventions with the specific types of BPSD associated with the different types of dementia [53]. In this review, it is difficult to assess the potential influence of the intervention on PWD or evaluate effectiveness based upon stage or type of dementia. The effectiveness of non-pharmacological interventions on BPSD may be limited based upon the level of cognitive impairment, requiring further exploration of the effectiveness of interventions.
During the assessment of BPSD, all articles utilized a version of the NPI. Most articles included the NPI-Q or NPI-Clinician. One study used the NPI: Nursing Home version. Two studies also included either caregiver proxy reports to determine changes in agitation, and neuropsychiatric and depressive symptoms [34].or various items from the Agitated Behaviors in Dementia Scale [54] and the Revised Memory and Behavior Problem Checklist. Differences in the assessment of BPSD presents challenges regarding the evaluation of effectiveness across articles in this review. As this review sought to consider the effectiveness of non-pharmacologic interventions on BPSD, a standardized approach to measuring the level of BPSD represents a key concern.
During identification of articles, difficulty arose regarding the lack of standardize language regarding the concepts of home-based care. The terms long-term care, community-dwelling, group home and assisted living were used interchangeably across articles. However, the meaning and actual location of the services often did not seem to match the terms used to describe the setting. For example, long-term care may often refer to a skilled nursing facility; however, the authors discovered it was also used to describe care provided across a continuum, making it difficult to determine the location of where an individual was receiving the intervention. Similarly, the term ‘assisted living’ reflected a wide-variation in settings described. Therefore, for this review it was necessary to describe home-dwelling as residing in a non-institutional, community-based setting.
Limitations
During the process of searching for articles, several registered clinical trials were discovered with the World Health Organization as well as the National Institute of Health for which data were not yet published. Therefore, more literature on non-pharmacological interventions for dementia symptom management may be discoverable in the near future. Additionally, articles included in this review did not always address BPSDs as primary outcomes. Often BPSDs were examined as secondary outcomes. Finally, given the short sampling window, this article did not take into account other studies prior to 2016 that may have helped to further generalize the work presented. However, we do refer to several impactful prior studies within the discussion and how they relate to the current findings from this review.
Conclusions
Non-pharmacological interventions can significantly reduce the presence or severity of BPSD for PWD living in home-based settings. This review demonstrated the important role in particular of occupational therapy-based interventions, which supported a reduction in the presence of BPSD. Additionally, physical activity-based interventions supported a reduction in BPSD. While other interventions in the home, including music therapy and aromatherapy show promise, there is not yet the evidence in this setting for their usage. Further, while efficacy has been found, the follow-up periods have often been of short duration and the interventions have not been disseminated widely within the professions outside of the research as of yet. Finally, further evidence is needed as to what stage of the illness various interventions are effective, as that was largely lacking within the studies reviewed.
Figure 1.
PRISMA flow diagram.
Acknowledgement:
We want to acknowledge Ann Yoo who helped with formatting and data extraction on this article as a research assistant.
Funding: This article was partially supported through funding from NIH grants R01AG056610 and R61AG06190
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Declaration of Conflicting Interest
Catherine E. Schneider, Alycia A. Bristol, and Ab Brody each declare no potential conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Contributor Information
Catherine E. Schneider, Hartford Institute for Geriatric Nursing, Rory Meyers College of Nursing, 433 First Avenue, New York, NY, 10010.
Alycia A. Bristol, Hartford Institute for Geriatric Nursing, Rory Meyers College of Nursing, 433 First Avenue, New York, NY, 10010, P: 212-992-7170.
Abraham A. Brody, Hartford Institute for Geriatric Nursing, Rory Meyers College of Nursing, 433 First Avenue, New York, NY, 10010, P: 212-992-7341.
References
- 1.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA internal medicine. 2017;177(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pérez AR, González SG. The importance of behavioural and pyschological symptoms in Alzheimer’s disease. Neurologia (Barcelona, Spain). 2016. [DOI] [PubMed] [Google Scholar]
- 5.Cerejeira J, Lagarto L, Mukaetova-Ladinska E. Behavioral and psychological symptoms of dementia. Frontiers in neurology. 2012;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armstrong NM, Gitlin LN, Parisi JM, Roth DL, Gross AL. Association of physical functioning of persons with dementia with caregiver burden and depression in dementia caregivers: an integrative data analysis. Aging & mental health. 2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arthur PB, Gitlin LN, Kairalla JA, Mann WC. Relationship between the number of behavioral symptoms in dementia and caregiver distress: what is the tipping point? International psychogeriatrics. 2018;30(8):1099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amo‐Korankye S, Gul S, Ninan S, Patel‐Palfreman M. Alzheimer’s dementia, posterior cortical atrophy variant with BPSD. Progress in Neurology and Psychiatry. 2019;23(1):19–22. [Google Scholar]
- 9.Matsuoka T, Manabe T, Akatsu H, Hashizume Y, Yamamoto S, Ogawa N et al. Factors influencing hospital admission among patients with autopsy‐confirmed dementia. Psychogeriatrics. 2019. [DOI] [PubMed] [Google Scholar]
- 10.Szalontay A, Burtea V, Ifteni P. Predictors of institutionalization in dementia. Revista de Cercetare si Interventie Sociala. 2015;49:249. [Google Scholar]
- 11.Toot S, Swinson T, Devine M, Challis D, Orrell M. Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. International Psychogeriatrics. 2017;29(2):195–208. [DOI] [PubMed] [Google Scholar]
- 12.Jutkowitz E, Kane RL, Gaugler JE, MacLehose RF, Dowd B, Kuntz KM. Societal and family lifetime cost of dementia: implications for policy. Journal of the American Geriatrics Society. 2017;65(10):2169–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaller S, Mauskopf J, Kriza C, Wahlster P, Kolominsky-Rabas PL. The main cost drivers in dementia: a systematic review. Int J Geriatr Psychiatry. 2015;30(2):111–29. doi: 10.1002/gps.4198. [DOI] [PubMed] [Google Scholar]
- 14.Song J-A, Oh Y. The association between the burden on formal caregivers and behavioral and psychological symptoms of dementia (BPSD) in Korean elderly in nursing homes. Archives of Psychiatric Nursing. 2015;29(5):346–54. [DOI] [PubMed] [Google Scholar]
- 15.Passalacqua SA, Harwood J. VIPS communication skills training for paraprofessional dementia caregivers: an intervention to increase person-centered dementia care. Clinical Gerontologist. 2012;35(5):425–45. [Google Scholar]
- 16.Buckley JS, Salpeter SR. A risk-benefit assessment of dementia medications: systematic review of the evidence. Drugs & aging. 2015;32(6):453–67. [DOI] [PubMed] [Google Scholar]
- 17.Schwertner E, Secnik J, Garcia-Ptacek S, Johansson B, Nagga K, Eriksdotter M et al. Antipsychotic Treatment Associated With Increased Mortality Risk in Patients With Dementia. A Registry-Based Observational Cohort Study. J Am Med Dir Assoc. 2019;20(3):323–9 e2. doi: 10.1016/j.jamda.2018.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–43. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 19.Maust DT, Kim HM, Seyfried LS, Chiang C, Kavanagh J, Schneider LS et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438–45. doi: 10.1001/jamapsychiatry.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. Bmj. 2015;350:h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porsteinsson AP, Drye LT, Pollock BG, Devanand D, Frangakis C, Ismail Z et al. Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. Jama. 2014;311(7):682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcum ZA, Perera S, Thorpe JM, Switzer GE, Castle NG, Strotmeyer ES et al. Antidepressant use and recurrent falls in community-dwelling older adults: findings from the Health ABC Study. Annals of pharmacotherapy. 2016;50(7):525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyer SM, Harrison SL, Laver K, Whitehead C, Crotty M. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. International psychogeriatrics. 2018;30(3):295–309. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health adn Clinical Excellence/Social Care Institute for Excellene. Dementia: Supporting people with dementia nard their carers in health and social care. NICE Clinical Guidance 42. London: National Institute for Health and Clinical Excellence; 2007. [Google Scholar]
- 25.Oliveira AMd, Radanovic M, Mello PCHd, Buchain PC, Vizzotto ADB, Celestino DL et al. Nonpharmacological interventions to reduce behavioral and psychological symptoms of dementia: a systematic review. BioMed research international. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Testad I, Corbett A, Aarsland D, Lexow KO, Fossey J, Woods B et al. The value of personalized psychosocial interventions to address behavioral and psychological symptoms in people with dementia living in care home settings: a systematic review. International psychogeriatrics. 2014;26(7):1083–98. [DOI] [PubMed] [Google Scholar]
- 27.Khillan R, Lepore M, Ferrell A, Wiener JM. Living Arrangements of People with Alzheimer’s Disease and Related Dementias: Implications for Services and Supports. 2017. [Google Scholar]
- 28.Brien SE, Lorenzetti DL, Lewis S, Kennedy J, Ghali WA. Overview of a formal scoping review on health system report cards. Implementation Science. 2010;5(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton C, Miller B, Yaffe K. Improved evaluation and management of cognitive impairment: results of a comprehensive intervention in long-term care. J Am Med Dir Assoc. 2006;7(2):84–9. doi: 10.1016/j.jamda.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Sackett DL, Rosenberg WM, Gray JM, Haynes RB, Richardson WS. Evidence based medicine. BMJ: British Medical Journal. 1996;313(7050):170. [Google Scholar]
- 31.de Oliveira AM, Radanovic M, Homem de Mello PC, Buchain PC, Dias Vizzotto A, Harder J et al. An intervention to reduce neuropsychiatric symptoms and caregiver burden in dementia: P reliminary results from a randomized trial of the tailored activity program–outpatient version. International journal of geriatric psychiatry. 2017.* This article is “of importance” because it was one of the articles we found in the scoping review of literature that showed positive results from their interveniton using occupaiton therapy at reducing neuropsychiatric symptoms of dementia. It was a rigorous study with high (Level I) levels of evidence through an RCT and the study was conducted within the past two years (2017).
- 32.Gitlin LN, Arthur P, Piersol C, Hessels V, Wu SS, Dai Y et al. Targeting behavioral symptoms and functional decline in dementia: a randomized clinical trial. Journal of the American Geriatrics Society. 2018;66(2):339–45.** This article is “of outstanding importance” as it was published last year in 2018. They used occupational therapy as an intervention, which showed not only reductions in number of behavioral symptoms of dementia patients, but also reductions in the severity of the behavioral symptoms. These results were found while conducting rigorous research by conduting an RCT, indicating the highest level of evidence.
- 33.Ikemata S, Momose Y. Effects of a progressive muscle relaxation intervention on dementia symptoms, activities of daily living, and immune function in group home residents with dementia in J apan. Japan Journal of Nursing Science. 2017;14(2):135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang A, Piroth I, Robinson H, MacDonald B, Fisher M, Nater UM et al. A pilot randomized trial of a companion robot for people with dementia living in the community. Journal of the American Medical Directors Association. 2017;18(10):871–8. [DOI] [PubMed] [Google Scholar]
- 35.Raglio A, Fonte C, Reani P, Varalta V, Bellandi D, Smania N. Active music therapy for persons with dementia and their family caregivers. International journal of geriatric psychiatry. 2016;31(9):1085–7. [DOI] [PubMed] [Google Scholar]
- 36.Kaymaz TT, Ozdemir L. Effects of aromatherapy on agitation and related caregiver burden in patients with moderate to severe dementia: A pilot study. Geriatric Nursing. 2016;20:1e7. [DOI] [PubMed] [Google Scholar]
- 37.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48(5 Suppl 6):10S-6S. [DOI] [PubMed] [Google Scholar]
- 38.Lamb SE, Sheehan B, Atherton N, Nichols V, Collins H, Mistry D et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. bmj. 2018;361:k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pimouguet C, Le Goff M, Wittwer J, Dartigues J-F, Helmer C. Benefits of occupational therapy in dementia patients: findings from a real-world observational study. Journal of Alzheimer’s Disease. 2017;56(2):509–17. [DOI] [PubMed] [Google Scholar]
- 40.Straubmeier M, Behrndt E-M, Seidl H, Özbe D, Luttenberger K, Graessel E. Non-pharmacological treatment in people with cognitive impairment: Results from the randomized controlled german day care study. Deutsches Ärzteblatt International. 2017;114(48):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaymaz TT, Ozdemir L. Effects of aromatherapy on agitation and related caregiver burden in patients with moderate to severe dementia: A pilot study. Geriatric Nursing. 2017;38(3):231–7.* This study is “of importance” because it was conducted with the highest level of evidence (level 1) and showed reductions in MPI scores in the intervention group using aromatherapy. Even though this study is a pilot RCT, it is crucial as it informs future research on what to potentially focus on while developing interventions to improve dementia symptom mangement for patients in the future.
- 42.Laver K, Cumming R, Dyer S, Agar M, Anstey KJ, Beattie E et al. Evidence‐based occupational therapy for people with dementia and their families: What clinical practice guidelines tell us and implications for practice. Australian occupational therapy journal. 2017;64(1):3–10. [DOI] [PubMed] [Google Scholar]
- 43.Nissen RM, Hersch G, Tietze M, Chang P-FJ. Persons With Dementia and Their Caregivers’ Perceptions About Occupational Therapy and Telehealth: A Qualitative Descriptive Study. Home healthcare now. 2018;36(6):369–78. [DOI] [PubMed] [Google Scholar]
- 44.Fung JKK, Tsang HW, Chung RC. A systematic review of the use of aromatherapy in treatment ofbehavioral problems in dementia. Geriatrics & gerontology international. 2012;12(3):372–82. [DOI] [PubMed] [Google Scholar]
- 45.Anderson AR, Deng J, Anthony RS, Atalla SA, Monroe TB. Using complementary and alternative medicine to treat pain and agitation in dementia: a review of randomized controlled trials from long-term care with potential use in critical care. Critical Care Nursing Clinics. 2017;29(4):519–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lerner BS, Kalish V, Ledford CJ. Exploring Residents’ Skills in Diagnosing Dementia: The Unexpected Dissonance Between Ability and Confidence. Family medicine. 2017;49(6):460–3. [PubMed] [Google Scholar]
- 47.Galvin JE, Sadowsky CH. Practical guidelines for the recognition and diagnosis of dementia. J Am Board Fam Med. 2012;25(3):367–82. [DOI] [PubMed] [Google Scholar]
- 48.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer disease and associated disorders. 2009;23(4):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aminzadeh F, Molnar FJ, Dalziel WB, Ayotte D. A review of barriers and enablers to diagnosis and management of persons with dementia in primary care. Canadian Geriatrics Journal. 2012;15(3):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braekhus A, Laake K, Engedal K. The Mini‐Mental State Examination: identifying the most efficient variables for detecting cognitive impairment in the elderly. Journal of the American Geriatrics Society. 1992;40(11):1139–45. [DOI] [PubMed] [Google Scholar]
- 51.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society. 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 52.Creavin ST, Wisniewski S, Noel‐Storr AH, Trevelyan CM, Hampton T, Rayment D et al. Mini‐MentalState Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database of Systematic Reviews. 2016(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chiu MJ, Chen TF, Yip PK, Hua MS, Tang LY. Behavioral and psychologic symptoms in different types of dementia. J Formos Med Assoc. 2006;105(7):556–62. doi: 10.1016/S0929-6646(09)60150-9. [DOI] [PubMed] [Google Scholar]
- 54. Logsdon RG, Teri L, Weiner MF, Gibbons LE, Raskind M, Peskind E et al. Assessment of agitation in Alzheimer’s disease: the agitated behavior in dementia scale. Journal of the American Geriatrics Society. 1999;47(11):1354–8. [DOI] [PubMed] [Google Scholar]