Keywords: fibroblasts, heterogeneity, tumor microenvironment
Abstract
Efforts to develop anti-cancer therapies have largely focused on targeting the epithelial compartment, despite the presence of non-neoplastic stromal components that substantially contribute to the progression of the tumor. Indeed, cancer cell survival, growth, migration, and even dormancy are influenced by the surrounding tumor microenvironment (TME). Within the TME, cancer-associated fibroblasts (CAFs) have been shown to play several roles in the development of a tumor. They secrete growth factors, inflammatory ligands, and extracellular matrix proteins that promote cancer cell proliferation, therapy resistance, and immune exclusion. However, recent work indicates that CAFs may also restrain tumor progression in some circumstances. In this review, we summarize the body of work on CAFs, with a particular focus on the most recent discoveries about fibroblast heterogeneity, plasticity, and functions. We also highlight the commonalities of fibroblasts present across different cancer types, and in normal and inflammatory states. Finally, we present the latest advances regarding therapeutic strategies targeting CAFs that are undergoing preclinical and clinical evaluation.
This review summarizes the current knowledge on cancer-associated fibroblasts (CAFs) and focuses on the recent discoveries of CAF molecular and functional heterogeneity in several malignancies. The discovery of CAF heterogeneity provides a potential explanation for previous seeming conflicting studies that targeted CAFs. Indeed, CAFs have been demonstrated to play multiple tumor-promoting roles, but also to potentially have tumor-restraining functions, which highlights the need to design subtype-specific therapies. The characterization of CAF heterogeneity will be pivotal for the development of effective combinatorial approaches for cancer treatment.
I. INTRODUCTION
Fibroblasts play key roles in disease, tissue homeostasis, cancer progression, inflammatory and fibrotic conditions, and wound healing processes. In cancer, a better understanding of the complex nature of cancer-associated fibroblasts (CAFs) could have prognostic and therapeutic value, especially in the instance that a specific CAF population is associated with a particular cancer subtype, which could help stratify patients and tailor therapies.
CAFs are a major component of the stroma and secrete growth factors, inflammatory ligands, and extracellular matrix (ECM) proteins that promote tumor proliferation, therapy resistance, and immune exclusion (129). For these reasons, CAFs have been historically considered tumor-promoting components. However, studies mainly focused on targeting the Hedgehog (Hh) signaling pathway, which is activated in CAFs, indicate that, in some circumstances, CAFs could also have tumor-restraining functions (221, 248). Previous work has revealed the presence of various stromal transcriptional signatures in human cancer specimens (17, 193, 200, 292), and preinvasive or invasive malignancies have been associated with different stromal signatures (257). However, lack of single-cell resolution or limited cell numbers precluded the identification of distinct CAF subtypes. The development of new co-culture models to study CAF biology and the implementation of single-cell RNA-sequencing (scRNA-seq) techniques have recently revealed a previously unappreciated level of CAF heterogeneity in a number of cancer types. Importantly, the discovery of CAF heterogeneity offers a potential explanation for the seeming controversy of CAFs playing both tumor-restraining and tumor-promoting functions. Although these recent studies provide insights into the nature of CAF heterogeneity, the extent of this heterogeneity, the roles of distinct CAF subtypes, and how to selectively target these subtypes remain unclear. As distinct CAF populations could play different roles in cancer progression, targeting them individually may lead to disparate outcomes. A deep characterization of different CAF subtypes will therefore be pivotal for the design of effective combinatorial approaches. Importantly, the emerging understanding that CAF heterogeneity is common in solid tumors indicates that discoveries made in one cancer type may more broadly impact the field of oncology.
This review aims to summarize historic milestones of the research on CAF biology, highlighting the most recent discoveries on CAF molecular and functional heterogeneity and new potential therapeutic strategies.
II. FIBROBLAST HETEROGENEITY IN NORMAL TISSUES
Fibroblast activation protein (FAP) is expressed in most CAFs (146) and normal fibroblasts from different sites (252), which indicates a common cell lineage. With the increasing understanding that CAFs are heterogeneous, an immediate question to address is whether this fibroblast heterogeneity is restricted to the tumor microenvironment (TME) or whether precursors to distinct CAF subtypes are present in nonmalignant states. Although several studies have demonstrated the molecular and functional heterogeneity of fibroblasts in normal tissues (137, 175, 232) and during development (33), few comparisons have been made between these populations and CAF subtypes (64, 111). Highlighting commonalities between fibroblast phenotypes across normal and malignant states will advance our knowledge of CAF biology.
Recently, two large scRNA-seq data sets of normal cells from several murine tissues have been published (104, 287). These resources begin to define transcriptional signatures of known cell types, identify new cell subsets, and enable the comparison of the same cell population across different normal tissues and malignancies. Importantly, the analysis of normal and developmental states may help identify the cell of origin of distinct CAF populations and reconstruct their lineage relationships and dynamics. In support of this, recent studies indicate the presence of distinct normal fibroblast precursors that likely contribute to the different CAF subtypes in pancreatic ductal adenocarcinoma (PDAC) (64, 111).
III. FIBROBLAST HETEROGENEITY IN WOUND HEALING AND INFLAMMATORY STATES
Studying fibroblast heterogeneity in the context of inflammation and wound healing is highly relevant, as cancers share many features with these conditions, and local wound healing and inflammation have been shown to promote tumor growth (77, 96, 97, 145). Therefore, fibroblasts in different conditions could share common phenotypes, signaling pathways and cell of origin. For example, a number of studies have demonstrated key roles of nuclear factor (NF)-κB and transforming growth factor (TGF)-β signaling pathways in fibroblasts in both inflammatory and malignant states (24, 79, 116, 142, 222). Additionally, ECM deposition occurs both during wound healing and in cancer (27, 74), highlighting the presence of functions common to normal activated fibroblasts and CAFs. However, a rigorous comparison of fibroblast phenotypes in inflammation and cancer is lacking.
Flow cytometry and single-cell resolution studies have started to define the phenotypic and functional heterogeneity of fibroblasts during skin tissue repair (98, 271) and in inflammatory conditions, such as pulmonary fibrosis and colitis (21, 71, 137, 253, 315, 316) (TABLE 1). In rheumatoid arthritis, different fibroblast subtypes have nonoverlapping functions and distinct markers, and mediate either tissue inflammation through chemokine/cytokine secretion or tissue damage through elevated expression of metalloproteases (54, 199). Additionally, studies comparing fibroblasts in normal and inflammatory or wound healing states have shown similarities and differences between these populations (137, 271, 316).
Table 1.
Fibroblast heterogeneity in wound healing and inflammatory states
| State | Models/Methods | Fibroblast Subtypes | Fibroblast Subtype Defining Markers | Subtype Putative Functions | Additional Notes |
|---|---|---|---|---|---|
| Rheumatoid arthritis (54) | •Murine samples | •Immune effector fibroblasts (F1–F4) | •PDPN+ FAPα+ THY+ IL6+ LIF+ | •Inflammatory | •Located in the synovial sub-lining |
| •scRNA-seq by 10× genomics | •Destructive fibroblasts (F5) | •PDPN+ FAPα+ THY- MMP9+ RANKL+ | •Tissue (bone and cartilage) damaging | •Located in the synovial lining | |
| Rheumatoid arthritis (RA) and osteoarthritis (OA) (199) | •Human samples | •3 Subsets | •PDPN+ CDH11+ CD34- THY+ RANKL+ | •Inflammatory, migratory, promote osteoclast differentiation | •Located in the synovial perivascular zone, proliferative, more abundant in RA |
| •scRNA-seq by SMART-seq2 | •PDPN+ CDH11+ CD34- THY- | •Promote osteoclast differentiation | •More abundant in OA | ||
| •FACS followed by RNA-seq | •PDPN+ CDH11+ CD34+ IL6+ CXCL12+ CCL2+ | •Migratory, promote monocyte recruitment | •Located in the synovial sub-lining, proliferative | ||
| Pulmonary fibrosis (316) | •Murine samples | •Myofibroblasts | •αSMA+ TAGLN+ MYH11+ HHIPHigh PDGFRαLow | •Contractile signature | •HH activation? |
| •scRNA-seq by 10× genomics | •Col13a1 matrix fibroblasts | •αSMALow COL1A1High COL13A1High ITGA8+ | •ECM producing | ||
| >•Col14a1 matrix fibroblasts | •αSMALow COL1A1High COL14A1High Pi16+ MMP3+ | •ECM producing | |||
| •Lipofibroblasts | •ADRPHigh PPARGHigh FABP4+ CD9+ SLPI+ | •Inflammatory | •M2 macrophage-like signature | ||
| •Mesenchymal progenitors | •CD52+ | •Proliferative (Mki67High Ccnb2High Cks2High) | |||
| •Mesothelial cells | •UPK3B+ MSLN+ LRRN4+ NKAIN4+ | ||||
| •PDGFRβHigh fibroblasts | •αSMA+ PDGFRβHigh NOTCH3+ | •Pericytes? | |||
| Colitis (137) | •Human and murine samples | •Myofibroblasts | •αSMA+ MYH11+ | •Contractile signature | •No increase in inflammation |
| •scRNA-seq with Fluidgm C2 platform and by 10× genomics | •S1 | •COL14A1High FN1+ CXCL12+ ZEB2+ | •ECM producing | •High in nonfibrillar collagens, located in lamina propria | |
| •S2 | •COL4A5High WNT5AHigh POSTNHigh CXCL12+ SOX6+ | •Support epithelial stem cell maintenance | •High in sheet collagens, close to the epithelial monolayer | ||
| •S3 | •SMAD7+ | •ECM organization | •Murine S3: mesothelial cell origin? (WT1+) | ||
| •S4 | •PDPN+ TNFSF14+ IL33+ CD74+ FDCSPHigh CD24High | •Inflammatory, collagen cross-linking | •Prevalent in ulcerative colitis compared with healthy colon, source of oxidative stress | ||
| Skin wound (271) | •Murine samples | •3 Myofibroblastic subtypes | •CD34+ CD29+ SCA1+ PDGFRα+ CD26High CD9+ | •ECM producing, inflammatory, collagen cross-linking | •Activated by CD301b+ macrophage-derived PDGFC and IGF1, reduced in advanced age, adipocyte precursors (APs) |
| •FACS followed by RNA-seq | •CD29High SCA1- PDGFRα- CD26Low | •Increase in wound beds, in the most superficial outer wound bed edge | |||
| •CD29Low αSMALow SCA1- PDGFRα- |
List of recent scRNA-seq and multi-color flow cytometric studies that show differences and commonalities of fibroblast populations in inflammatory and fibrotic conditions, such as colitis, arthritis, and pulmonary fibrosis. ECM, extracellular matrix; IGF1, insulin-like growth factor 1; PDGF, platelet-derived growth factor; αSMA, α-smooth muscle actin.
The development and analysis of in vivo models of inflammatory and fibrotic conditions, such as rheumatoid arthritis, inflammatory bowel disease (IBD), hepatitis, and pancreatitis (77, 93, 99, 115, 143, 156, 170, 263), have given insights into the heterogeneity and biology of fibroblasts in these contexts. The historical model to study pancreatitis employs treatment with the cholecystokinin analogue cerulein (311, 323), although dosage regimens vary substantially across laboratories depending on the desired effect. More recently, genetically engineered mouse models (GEMMs) have been established to induce pancreatitis by expressing the glycan carbohydrate antigen 19–9 (CA19–9) (77) or activating mutations in trypsinogen (93, 99, 115). For inducing IBD as well as liver or pulmonary fibrosis, administration of dextran sodium sulfate (DSS) (156), carbon tetrachloride (CCl4) (263), or bleomycin (170) is the most commonly used mouse model, respectively.
IV. FIBROBLAST HETEROGENEITY IN CANCER
A. Models to Study CAFs
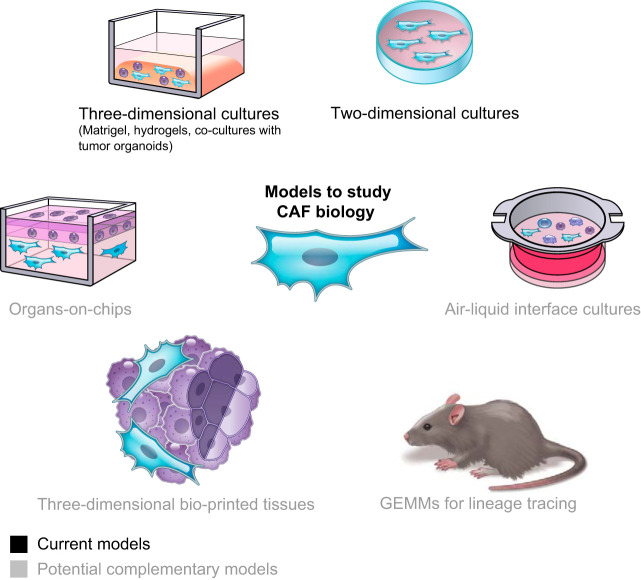
With the emerging understanding that CAFs are comprised of multiple subtypes, in vitro and in vivo models will facilitate the systematic characterization of CAF heterogeneity and biology (FIGURE 1).
FIGURE 1.
Models to study cancer-associated fibroblast (CAF) biology. Current models include two-dimensional and three-dimensional cultures. Potential complementary models that have not yet been applied to the study of CAF biology include air-liquid interface cultures, organs-on-chips, three-dimensional bio-printed tissues, and new genetically engineered mouse models (GEMMs) for lineage tracing of CAFs.
1. In vitro models
In vitro models enable to mechanistically investigate the crosstalk between different cell populations. Although monolayer two-dimensional cultures of fibroblasts can be useful to dissect some aspects of CAF biology, it has been shown that the transcriptome of CAFs cultured in this fashion does not recapitulate the heterogeneity of CAFs in vivo (240, 292, 305). Three-dimensional matrices, such as Matrigel and hydrogels, and tumor organoid/fibroblast co-cultures have significantly advanced our knowledge of fibroblast biology, as they more faithfully mirror the transcriptional profiles and phenotypes of CAF populations found in vivo (15, 24, 213). Nonetheless, there are limitations to using Matrigel or hydrogel scaffolds, as they are distinct from the composition of the tumor ECM, and co-cultures with these matrices may not entirely recapitulate the in vivo repertoire of fibroblasts (305). Implementation of models that use the ECM produced by the fibroblasts (87), short-term co-cultures that retain all cell populations found in tumors, such as liquid-air interface models (204, 217), multi-cell-type three-dimensional bio-printed tissues (155, 178) and microfluidic culture technologies, such as organs-on-chips (8, 78, 160, 275), are being implemented and optimized and could complement or validate other models (FIGURE 1).
2. In vivo models
GEMMs have been developed for lineage tracing of fibroblasts in wound healing and normal and inflammatory tissues (71, 196, 252–254, 271, 315) and have significantly advanced our knowledge of fibroblast origin, heterogeneity, plasticity, and roles. On the contrary, lineage tracing models of CAFs are lacking. These GEMMs are needed in particular as CAFs can originate from multiple cell populations, and this may partially determine their function. Cre recombinase-based models are present (333) and could be useful to dissect the role of fibroblasts in the TME in vivo. However, recent studies warn about the use of tamoxifen when studying the microenvironment (51, 52), highlighting the need for the generation and analysis of alternative fibroblast-specific mouse models to study CAF biology. Finally, intravital microscopy imaging techniques (80) applied to GEMMs for lineage tracing of CAFs could evaluate the origin and plasticity of dynamic CAF subtypes (24).
B. Molecular Heterogeneity: Markers of CAF Subtypes
A number of intracellular, extracellular, and cell surface proteins have been used to isolate or identify CAFs (FIGURE 2). However, no ubiquitous marker to study CAFs exists. CAF heterogeneity in primary tumors first emerged in immunofluorescence experiments, which showed that, among various fibroblast markers, none was all-inclusive and many were present in different combinations (206, 285). The development of new in vitro models (213), the optimization of multicolor flow cytometry and immunohistochemical methodologies (53, 123, 284), and the extensive use of scRNA-seq techniques enabled the redefinition of some classical markers as subtype-specific. For example, elevated expression of α-smooth muscle actin (αSMA) has been historically considered a distinctive marker in activated CAFs compared with normal fibroblasts (158). Nonetheless, we and others have recently demonstrated the presence of low-αSMA CAFs (19, 20, 24, 53, 73, 111, 166, 213), highlighting the need to consider multiple parameters for the analysis of CAF activation.
FIGURE 2.
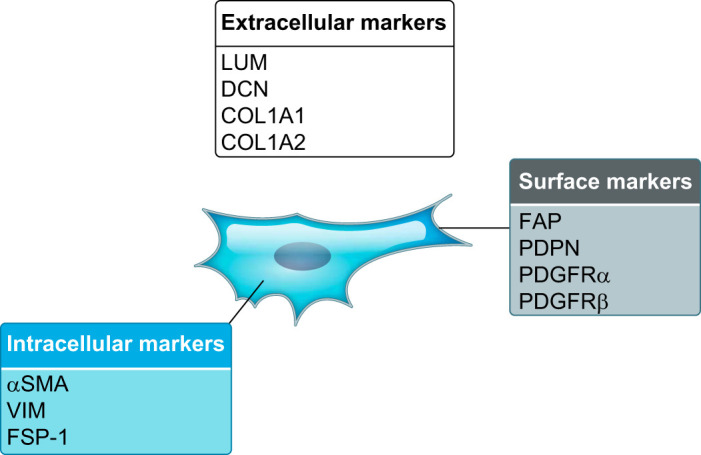
Common cancer-associated fibroblast (CAF) markers. Examples of extracellular, intracellular, and surface protein markers of CAFs. LUM, lumican; DCN, decorin; COL, collagen; FAP, fibroblast activation protein; PDPN, podoplanin; PDGFR, platelet-derived growth factor receptor; αSMA, α-smooth muscle actin; VIM, vimentin; FSP-1, fibroblast specific protein 1.
An additional technical challenge for the study of CAFs is the absence of a specific marker for their isolation. This is due to the fact that most markers are shared with at least one other cell population. For example, proteins broadly expressed across CAFs, such as αSMA, podoplanin (PDPN), and FAP, are highly expressed in pericytes, lymphatic endothelial cells, and fibroblastic reticular cells, respectively. Therefore, strategies that rely on the negative selection of other cell populations are preferable in contexts where fibroblast heterogeneity has yet to be dissected. However, when subtype-specific markers are known, a combination of these with broadly expressed fibroblast markers could be used to isolate specific CAF populations.
Whereas some markers are simply identifiers of specific CAF subtypes, some, such as caveolin 1 in breast cancer (94), have been shown to also have a functional role. Additionally, a number of transcription factors, including HSF1 (262), STAT3 (24), MYC (264), and YAP (36), are involved in driving specific CAF signatures and reprogramming. Adding to this complexity, pathways that define a CAF state, such as NF-κB signaling, can be either tumor-promoting or tumor-suppressive, depending on the cancer type and context (24, 79, 142, 222).
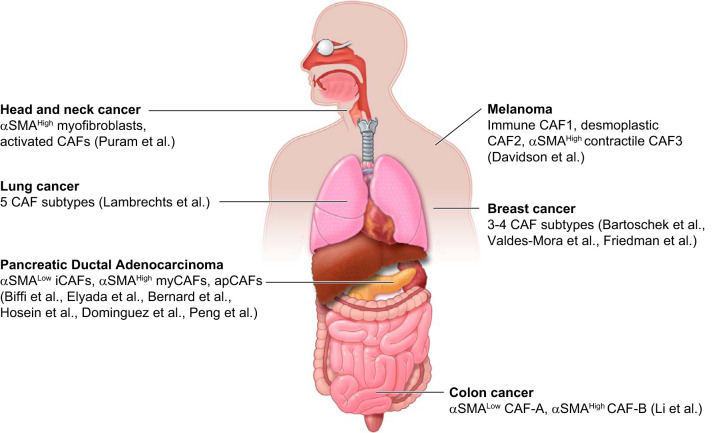
Importantly, emerging evidence suggests the presence of similar CAF populations across different cancer types. For example, whereas a tumor-promoting CD10+ GPR77+ CAF subtype has been recently characterized in breast cancer (284), CD10+ CAFs have been previously shown to promote PDAC progression (118). Additionally, scRNA-seq and multicolor flow cytometric data sets of several cancer types demonstrate the presence of transcriptionally distinct CAF populations, but also highlight commonalities of fibroblast signatures across different malignancies (19, 20, 24, 53, 57, 73, 111, 154, 166, 240, 284, 298) (FIGURE 3 and TABLE 2). Analyzing the similarities in fibroblast composition across various cancer types could reveal new therapeutic opportunities with a broad impact.
FIGURE 3.
Cancer-associated fibroblast (CAF) heterogeneity across malignancies. Schematic illustration summarizing the distinct CAF populations identified in different cancer types by single-cell RNA-sequencing (scRNA-seq). αSMA, α-smooth muscle actin.
Table 2.
CAF heterogeneity across malignancies
| Cancer Type | Models/Methods | CAF Subtypes | CAF Subtype Defining Markers | Subtype Putative Functions | Additional Notes |
|---|---|---|---|---|---|
| PDAC (24, 73) | •KPC tumors and patient samples (PDAC and adjacent normal) | •Inflammatory CAFs (overlaps with iCAFs) | •Ly6CHigh αSMALow CXCL12+ PDGFRαHigh C3+ IL6+ | •Immunosuppressive/tumor promoting | •IL-1 and JAK/STAT signatures; distally located; less proliferative; can convert into myCAFs in vivo |
| •scRNA-seq by 10× genomics | •Myofibroblastic CAFs (overlaps with myCAFs) | •αSMAHigh CTGF+ TNC+ TAGLN+ | •ECM producing | •TGF-β signature; tumor adjacent | |
| •Antigen-presenting CAFs (apCAFs) | •MHCII+ | •Immunomodulatory | •Mesothelial cell origin? | ||
| PDAC (20) | •Patient samples (pre-neoplastic IPMNs and PDAC) | •Inflammatory CAFs (iCAFs) | •αSMALow CXCL12+ C3+ IL6+ | •Immunosuppressive/tumor promoting | •Absent in IPMNs |
| •scRNA-seq by Drop-seq | •Myofibroblasts (myCAFs) | •αSMAHigh | •ECM producing | •Prevalent at later stage | |
| PDAC (111) | •KPC, KPfC and KIC tumors (early and late stage) | •FB1 (overlaps with iCAFs) | •αSMALow CXCL12+ PDGFRαHigh IL6+ | •Immunosuppressive/tumor promoting | •Express some apCAF/mesothelial markers (e.g., MSLN, CD74, H2-AA); prevalent at later stage |
| •scRNA-seq by 10× genomics | •FB3 (overlaps with myCAFs) | •αSMAHigh TAGLN+ CTGF+ | |||
| PDAC (64) | •KPP tumors (early and late stage) | •C8 (overlaps with iCAFs) | •αSMALow Ly6CHigh IL6+ | •Immunosuppressive | •IL-1 and NF-κB signature; has normal precursor (C3) |
| •scRNA-seq by 10× genomics | •C2 (overlaps with myCAFs) | •αSMAHigh TAGLN+ LRCC15+ | •ECM producing/immunosuppressive | •TGF-β signature; has normal precursor (C4); prevalent at later stage | |
| Lung cancer (154) | •Patient samples (tumors and matched nonmalignant lung) | •5 Clusters | •#2: αSMAHigh | •Angiogenesis; myogenesis | •Co-clusters with pericytes |
| •scRNA-seq by 10× genomics | •#1: EMT signature | •ECM producing | •Enriched in tumors compared with normal tissues; TGF-β signature | ||
| Breast/lung cancer (284) | •Patient samples | •CD10+ GPR77+ CAFs | •CD10+ GPR77+ IL6+ | •Promote cancer stemness and chemotherapy resistance | •NF-κB signature |
| •mRNA microarray analysis and flow cytometry | |||||
| Breast cancer (53) | •Patient samples (LumA, HER2, and TNBC subtypes) | •CAF-S1 | •FAPHigh αSMA+ CXCL12+ IL6+ | •Immunosuppressive/ECM producing | •Peri-tumoral location/mostly in TNBC |
| •Multicolor flow cytometry and immunohistochemistry | •CAF-S2 | •Negative for all markers | •Contractile signature | •Potentially not fibroblasts | |
| •CAF-S3 | •αSMALow PDGFRβ+ | •Prevalent in adjacent normal/mostly in TNBC | |||
| •CAF-S4 | •αSMA+ CD29High | •Pericytes? | |||
| Breast cancer (19) | •MMTV-PyMT tumors | •Vascular CAFs (vCAFs) | •αSMAHigh PDGFRβHigh | •Angiogenesis | •Proximal to the vasculature; Prevalent at later stage |
| •scRNA-seq by SMART-seq2 | •Matrix CAFs (mCAFs) | •αSMALow PDGFRαHigh | •ECM producing | •Prevalent at earlier stage | |
| •Cycling CAFs (cCAFs) | •PDGFRβHigh | •Angiogenesis | •Cycling vCAFs | ||
| •Developmental CAFs (dCAFs) | •PDGFRβ- SOX9+ SCRG1+ | •Cell differentiation | •EMT cells? | ||
| Breast cancer (298) | •MMTV-PyMT tumors | •ECM-CAFs | •TNC+ | •ECM producing | •Desmoplastic signature |
| •scRNA-seq by Drop-seq | •Inflammatory CAFs (iCAFs) | •Ly6CHigh, C3+, CXCL12+ PDGFRαHigh | •Immunomodulatory | •JAK/STAT signature | |
| •Myofibroblastic CAFs | •αSMAHigh, MYLK+ | •Contractile signature | |||
| Melanoma (57) | •Tumors from B16-F10 cell transplantation model | •Immune CAF1 | •CD34High CXCL12+ C3+ | •Immunosuppressive | •Prevalent at early stage; less proliferative |
| •scRNA-seq by 10× genomics | •Desmoplastic CAF2 | •CD34Low CTGF+ TNC+ PDGFRα+ | •ECM producing | •Prevalent at early stage; intermediate CAFs | |
| •Contractile CAF3 | •αSMAHigh RGS5+ | •Contractile signature | •Prevalent at late stage; pericytes? | ||
| Head and neck cancer (240) | •Patient samples (tumors and lymph node metastases) | •Myofibroblasts | •αSMAHigh, MYL9+, MYLK+ | •Contractile signature | •The prevalent subtype in lymph node metastases |
| •scRNA-seq by SMART-seq2 | •Activated CAFs | •PDGFRαHigh | •ECM producing | •Non-myofibroblastic; 2 subclusters | |
| Colon cancer (166) | •Patient samples (tumors and matched normal mucosa) | •CAF-A | •αSMALow TAGLNLow FAP+ | •ECM remodeling | •Intermediate state? |
| •scRNA-seq with Fluidigm C1 platform | •CAF-B | •αSMAHigh TAGLNHigh FAP- | •Activated myofibroblasts |
List of recent scRNA-seq and multi-color flow cytometric studies that reveal differences and commonalities in cancer-associated fibroblast (CAF) subtypes across pancreatic ductal adenocarcinoma (PDAC), breast cancer, lung cancer, melanoma, colon cancer, and head and neck cancer. ECM, extracellular matrix; FAP, fibroblast activation protein; IL, interleukin; PDGF, platelet-derived growth factor; αSMA, α-smooth muscle actin; TGF, transforming growth factor.
Overall, the existence of both myofibroblastic and non-myofibroblastic CAF populations appears to be the most consistent observation across different cancer types (19, 20, 24, 53, 73, 89, 111, 166, 231). We first described these two populations in a tumor organoid/fibroblast co-culture model of PDAC that revealed the presence of αSMA-high myofibroblastic CAFs (myCAFs) and αSMA-low inflammatory CAFs (iCAFs) (213). We and others have confirmed these findings in vivo by scRNA-seq and immunochemical analysis of murine and human PDAC specimens (20, 24, 73, 111, 231). Across various cancer types, myofibroblastic CAFs are associated with an ECM signature, whereas non-myofibroblastic CAFs are generally characterized by a secretory, inflammatory phenotype (TABLE 3). Notably, inflammatory CAFs share similar transcriptional profiles and signaling pathway activation with senescent fibroblasts, which are characterized by the senescence-associated secretory phenotype (SASP) (48, 109, 148, 149). Additionally, inflammatory CAFs have been shown to proliferate at a lower rate than myofibroblasts or to not proliferate at all (24, 57). Regardless, we have shown that a substantial fraction of iCAFs actively proliferates in PDAC (24, 213), indicating that iCAFs should not be equated with fibroblasts undergoing SASP. However, these similarities may indicate overlapping functions of iCAFs and senescent fibroblasts. In cancer, the SASP of stromal cells has been reported to play various roles (147, 256), highlighting the need to identify the effects of inflammatory CAF-secreted ligands on cancer progression.
Table 3.
CAF subtypes in PDAC
| PDAC CAF Subtype | Other Nomenclature | Subtype Defining Markers (in combination with pan-CAF markers, observed across data sets) | Potential Functions | Potential Cell of Origin | Potential Targeting Agents (nonselective) |
|---|---|---|---|---|---|
| myCAF (20, 24, 64, 73, 111, 273) | •Myofibroblastic CAFs | •αSMAHigh | •ECM producing: loss of αSMA+ CAFs has been associated with ECM depletion (24, 161, 215, 221, 248, 330) | •Can originate from PSCs (24, 213, 273) | •TGFBR inhibitors, TGF-β antibodies, losartan |
| •Myofibroblasts | •CTGF | •Immunosuppressive: express TGF-β (24, 64, 73) | •Pericytes (similar transcriptional profile) | •SMO inhibitors (IPI-925, Vismodegib, LDE225) | |
| •FB3 | •TAGLN | •Tumor restraining: loss of αSMA+ CAFs has been associated with reduced survival and increased vascularization and/or recruitment of immunosuppressive cell populations (161, 221, 248) | •Col4a1+ ENG+ C4 fibroblasts (64) | ||
| •C2 | •THY1 | ||||
| •LRRC15 (subset) | |||||
| iCAF (20, 24, 64, 73, 111, 273) | •Inflammatory CAFs | •αSMALow | •Immunosuppressive: express ligands (e.g., CXCL12, CXCL1, IL6, G-CSF, …) involved in T cell exclusion and neutrophil recruitment (24, 73, 213, 273) | •Can originate from PSCs (24, 213, 273) | •JAK/STAT inhibitors |
| •FB1 | •CXCL12 | •Tumor promoting: induce phospho-STAT3 in PDAC organoids (213); loss of iCAFs leads to smaller tumors (24) | •Fbn1+ LY6C+ DPP4+ C3 fibroblasts (64) | •IL1R antagonist | |
| •C8 | •C3 | •Overlapping functions with senescent fibroblasts: iCAF secretome ~ SASP (24, 73, 213, 273) | •NF-κB inhibitors | ||
| •IL6 | |||||
| •Ly6CHigh | |||||
| •PDGFRαHigh | |||||
| apCAF (73) | •MHCII (Cd74, H2-Aa, H2-Ab) | •Immunomodulatory (do not express costimulatory molecules, may act as decoy receptor to inhibit optimal T cell response) | •Cannot originate from PSCs? | ||
| •Tumor promoting: express SAA3, SLPI | •Mesothelial cells (64, 73) | ||||
| •MHCII expression induced by IFN-γ |
Summary of actual and potential features of inflammatory cancer-associated fibroblasts (iCAFs), myofibroblastic CAFs (myCAFs), and antigen-presenting CAFs (apCAFs) in pancreatic ductal adenocarcinoma (PDAC). ECM, extracellular matrix; G-CSF, granulocyte colony stimulating factor; IFN, interferon; IL, interleukin; PDGF, platelet-derived growth factor; PSCs, pluripotent stem cells; SASP, senescence-associated secretory phenotype; αSMA, α-smooth muscle actin; SMO, Smoothened; TGF, transforming growth factor.
New CAF markers are constantly found, and the implementation of scRNA-seq methods has been significantly contributing to this. CAF subtype-specific markers could guide the development of novel genetic and pharmacological approaches to target specific CAF populations.
C. Specification of CAF Subtypes
Although the genetic and epigenetic drivers of cancer cells have been extensively investigated, the mechanisms governing the recruitment and activation of CAFs are largely unknown. Whereas genetic alterations in CAFs are rare (4, 11, 112, 241), with only a few exceptions (201, 227, 297), we and others have shown that the genetic profile of cancer cells can affect the surrounding stroma (209, 237, 273, 302, 312). Therefore, CAF signatures defined across diverse cancer genetic profiles could be used to stratify patients, provide prognostic information, and tailor therapies.
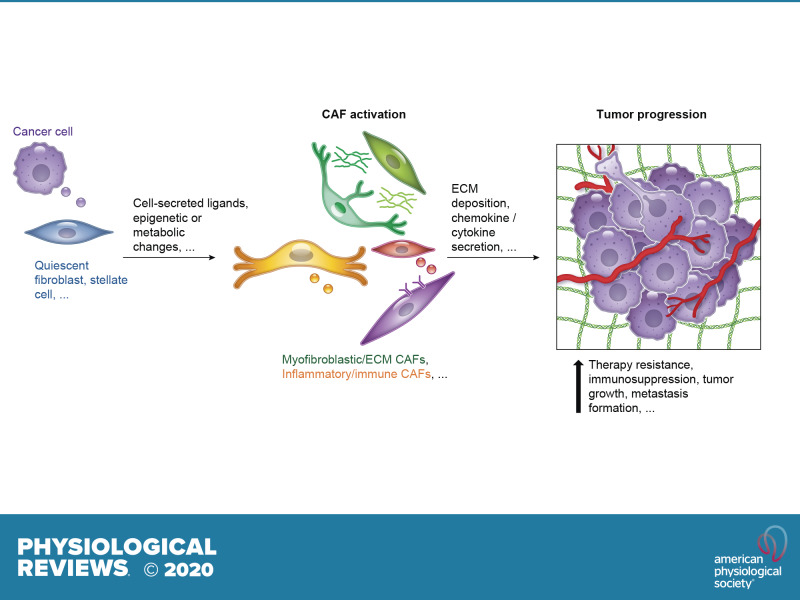
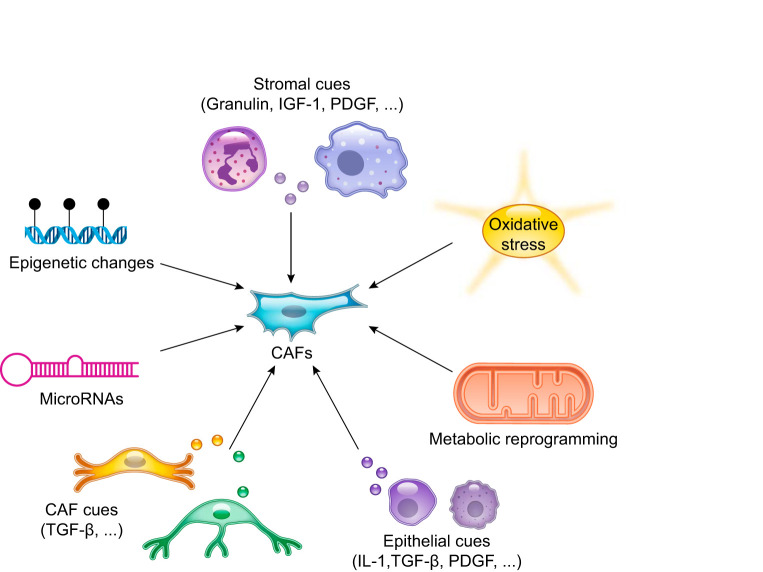
Several factors have been demonstrated to contribute to the reprogramming of CAFs or the activation of fibroblasts in inflammatory and fibrotic conditions (FIGURE 4), including 1) epithelial cues, such as interleukin-1 (IL-1), platelet-derived growth factor (PDGF), and TGF-β (6, 24, 35, 79, 162, 236, 242, 272, 317); 2) metabolic reprogramming (328); 3) oxidative stress (189, 294); 4) stromal cues (207, 271, 279); 5) microRNAs (83, 198, 319); 6) epigenetic changes (2, 22, 113, 230, 235, 314); and 7) other CAF-secreted ligands (141). In PDAC, we and others found that cancer-secreted IL-1 and TGF-β antagonize each other and define inflammatory iCAF and myofibroblastic myCAF formation, respectively (24, 64, 273, 330).
FIGURE 4.
Factors involved in fibroblast reprogramming in inflammation and cancer. Schematic illustration of multiple stimuli that have been shown to determine fibroblast activation. These factors include epithelial and stromal cues, metabolic reprogramming, epigenetic changes, microRNAs, and oxidative stress. CAF, cancer-associated fibroblast; IGF-1, insulin-like growth factor 1; IL-1, interleukin-1; PDGF, platelet-derived growth factor; TGF-β, transforming growth factor-β.
D. Lineage-Dependent Heterogeneity: Cell of Origin of CAF Subtypes
We and others have shown that CAF activation and reprogramming can occur within the TME. Nonetheless, the identification of the cells of origin of CAF subtypes is a central question, as it may partially determine the functions of distinct CAF populations and could indicate new therapeutic strategies.
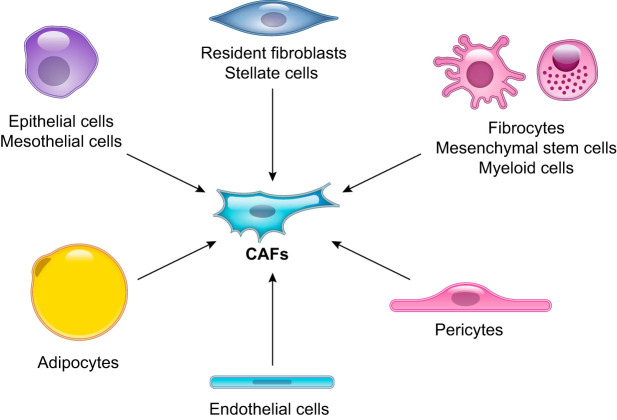
Whereas elegant lineage tracing studies have revealed the origin of fibroblasts in normal or injured fibrotic tissues (65, 68, 251, 271), little information exists about the origin of CAFs. Several cell types have been suggested as precursors of CAFs (FIGURE 5), including 1) pancreatic and hepatic stellate cells (10, 16, 322), 2) resident fibroblasts (12, 141, 218, 255, 303), 3) mesenchymal stem cells (MSCs) (128, 132, 135, 159, 197, 242), 4) adipocytes (26, 92, 135), 5) adipose-derived MSCs (282, 288), 6) mesothelial cells (261), 7) endothelial cells (326, 327), 8) myeloid cells (211), 9) pericytes (110), 10) epithelial cells (122), 11) hematopoietic stem cells (195), and 12) circulating bone marrow cells known as fibrocytes (62). However, the majority of the evidence that identifies these cell types as CAF precursors comes from in vitro experiments and bone marrow transplantation studies and has been reported only for one or a few cancer types. Even less information exists about the distinct cells of origin of specific CAF subtypes. We have shown that, at least in vitro, pancreatic stellate cells can differentiate into both inflammatory iCAFs and myofibroblastic myCAFs (213). On the contrary, the antigen-presenting CAF (apCAF) subtype, which we recently identified in PDAC by scRNA-seq and immunohistochemical analysis (73), shares an overlapping transcriptional signature with mesothelial cells (31, 64, 316) (TABLE 3). This observation suggests that apCAFs could derive from mesothelial cells, following their recruitment into the tumor, similarly to what observed in inflammatory conditions (168, 174, 250).
FIGURE 5.
Cell of origin of cancer-associated fibroblasts (CAFs). Schematic illustration of the potential cells of origin of CAFs that have been reported, including epithelial cells, mesothelial cells, resident fibroblasts, pancreatic and hepatic stellate cells, pericytes, adipocytes, mesenchymal stem cells, myeloid cells, fibrocytes, and endothelial cells.
CAF subtype-specific lineage tracing models coupled with in vivo imaging methods could reveal the cells of origin of distinct CAF populations.
E. Functional Heterogeneity of CAF Subtypes
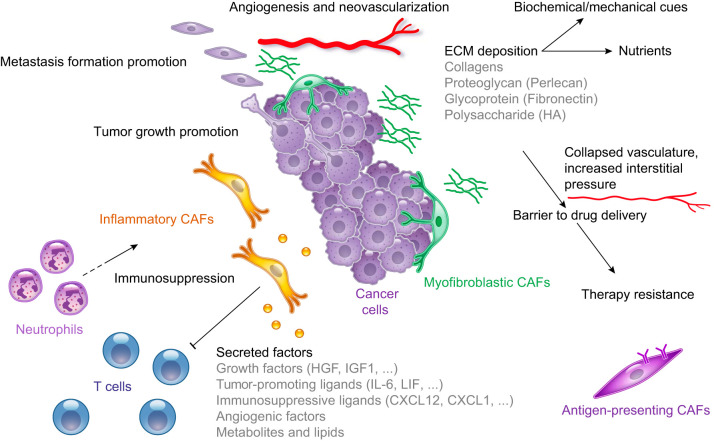
Whereas normal fibroblasts have been shown to inhibit cancer progression (3, 280, 296), several studies have demonstrated that CAFs can promote tumor growth in multiple ways (FIGURE 6), including 1) secreting ECM proteins (124, 215, 239); 2) inducing inflammation and neovascularization (79, 218); 3) increasing angiogenesis (44, 134); 4) increasing the incidence of tumor-initiating cells (157); 5) affecting the signaling of cancer cells (289); 6) changing the metabolism and epigenome of cancer cells (13, 70, 267, 332); 7) establishing an immunosuppressive TME (84, 146, 152); 8) conferring therapy resistance (106, 121, 134, 281, 302) and radioprotection (183); 9) promoting tumor invasion, metastasis formation, and epithelial-to-mesenchymal transition (9, 35, 63, 67, 91, 94, 95, 134, 151, 207, 304, 313, 329); 10) secreting pro-tumorigenic ligands (218, 269); 11) promoting the stemness of cancer cells (40, 45, 66, 163, 182, 247, 284, 286, 325); 12) contributing to systemic effects, such as cachexia, anemia, and immunosuppression (86, 252); and 13) fueling cancer growth by providing metabolites, such as amino acids (214, 276), fatty acids (13), and lactate (229).
FIGURE 6.
Tumor-promoting cancer-associated fibroblast (CAF) functions. Schematic illustration of the functional heterogeneity of CAFs in the tumor microenvironment. CAFs have been demonstrated to play several roles, including promoting tumor growth and metastasis formation, depositing extracellular matrix (ECM), and establishing an immunosuppressive microenvironment. HA, hyaluronan; HGF, hepatic growth factor; IGF1, insulin growth factor 1; IL-6, interleukin-6; LIF, leukemia inhibitory factor.
1. ECM deposition
High mammographic density, which indicates greater abundance of connective tissue compared with fat, is an important risk factor in breast cancer (32). Far from simply being a read-out of the increased incidence in breast cancer, it has been shown that gene expression programs associated with this condition, such as downregulation of the membrane protein CD36, correlate with clinical outcomes (58). Importantly, high mammographic density shares features with the TME, in particular relative to the elevated ECM deposition and stromal content. Indeed, ECM remodeling and deposition are not restricted to cancer, but are also present during wound healing and in fibrotic and inflammatory states (27). However, although following tissue repair and inflammation the formation of desmoplasia is typically reversible (139), ECM levels continue to increase with cancer progression.
ECM deposition plays several roles within the TME, including 1) acting as a barrier to drug delivery (124, 215, 239); 2) leading to hypoperfusion and elevated interstitial fluid pressure by compressing blood vessels and lymphatic vessels, respectively (42, 188, 283); 3) providing nutrients (214); 4) contributing to the establishment of an immunosuppressive TME (105, 126, 259); and 5) supporting tumor growth with biochemical and mechanical cues (55, 233) (FIGURE 6). Indeed, the high tissue tension and stiffness caused by the ECM has been shown to promote cancer progression by increasing cell invasion and cancer spreading (126, 153, 165, 208).
More than any other cancer type, PDAC is characterized by an extensive ECM deposition, thus representing a potential paradigm for its composition and function. A recent study that looked at murine and human normal pancreas, pancreatitis, and PDAC samples found that the matrisome (i.e., ECM proteins, growth factors, and ECM-associated proteins) of pancreatitis almost entirely contributes to that of PDAC (291). This big overlap of ECM components shared by both the inflammatory and malignant states suggests that similar fibroblast populations could also be conserved. Importantly, several ECM components shared between the premalignant and malignant states are absent in normal tissue, suggesting that they could represent biomarkers and therapeutic targets (291). This study also confirmed that fibroblasts are the major producers of the ECM, in particular of fibrillar collagens (e.g., COL1A1, COL1A2), glycoproteins (e.g., fibronectin), and proteoglycans (e.g., perlecan). However, whereas the small percentage of cancer-derived ECM proteins was only found associated with worst prognosis (291), stroma-derived ECM components correlated with both poor and good survival. These results indicate that the ECM is functionally heterogeneous, and scRNA-seq studies could assess whether different ECM components are secreted by distinct CAF subtypes. Alterations of the ECM composition during cancer treatment (155, 293) could, thus, indicate additional changes in CAF subtypes and function.
Several ECM components have been therapeutically targeted to increase the efficacy of immunotherapy and chemotherapy (43, 61, 124, 205, 215, 239) or have been leveraged for noninvasive imaging of fibrotic conditions, premalignant lesions, primary tumors, and metastases (125). Indeed, the ECM is also present in metastatic tumors (182, 219, 220), although differences between the ECM composition and response to chemotherapy at primary and secondary sites have been reported (1, 310). Whether these differences are dependent on the presence of distinct CAF subtypes between these sites remains to be assessed.
2. Immunosuppression
Several lines of evidence have identified a major role of CAFs in the establishment of an immunosuppressive TME (FIGURE 6). CAFs have been shown to prevent cytotoxic T cell activity and recruitment within tumors (84, 152), in part by secreting immunosuppressive ligands, such as TGF-β (85, 101, 184, 290) and CXCL12 (75, 84, 218). Additionally, CAFs can recruit immunosuppressive populations, such as myeloid-derived suppressor cells (MDSCs) and neutrophils (150, 273, 321), which in turn can further activate CAFs (279). Furthermore, CAFs have been involved in monocyte recruitment and in macrophage differentiation and polarization (14, 49, 190). Although these studies highlight the several ways through which CAFs establish an immunosuppressive TME and limit the efficacy of immunotherapy strategies, it remains to be assessed which CAF subtypes are responsible for this. In PDAC, we have identified inflammatory iCAFs as the major producers of CXCL12 (73) and other immunosuppressive ligands (24), such as IL-6 (86), CXCL1 (167), and granulocyte colony stimulating factor (G-CSF) (234). These observations suggest that iCAFs may play a central immunosuppressive role in the PDAC TME (TABLE 3). Additionally, apCAFs, which express MHC class II (MHCII) proteins, may play an immunomodulatory role in PDAC and breast cancer (73, 89). Whereas MHCII molecules are constitutively present in professional antigen presenting cells (APCs), in other cell types, including fibroblasts, the expression of MHCII can be induced by stimuli such as interferon-γ (28, 119, 306). Notably, MHCII-expressing CAFs do not express co-stimulatory molecules (73, 89), which are typically present in professional APCs and are necessary for the induction of CD4+ T cell clonal proliferation (320). This observation indicates that apCAFs may act as a decoy receptor to inhibit optimal T cell response (TABLE 3). Although this is an intriguing hypothesis, a role of MHCII-expressing CAFs in cancer progression remains to be demonstrated.
3. Potential tumor-restraining roles of CAFs
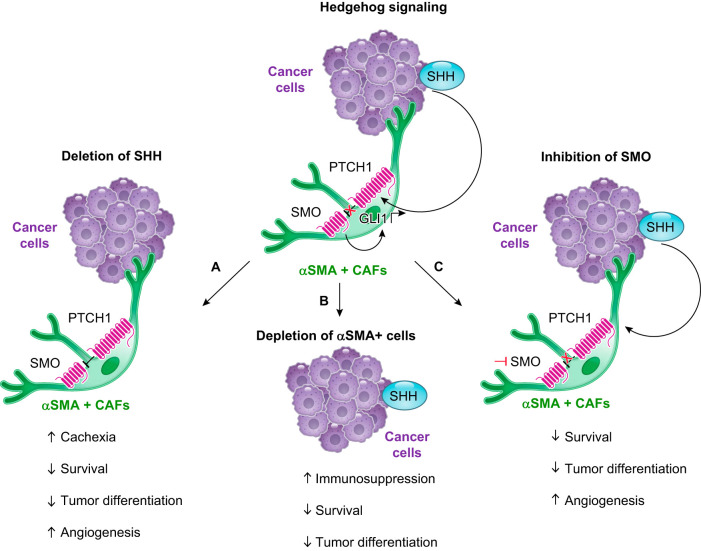
For the reasons outlined above, CAFs have been historically considered tumor-promoting components. However, recent work mostly carried out in PDAC indicates that CAFs may also have tumor-restraining functions (5, 136, 161, 221, 228, 248, 330). These studies largely focused on the genetic depletion of αSMA-positive cells and on the genetic deletion of sonic hedgehog (SHH) or the pharmacological inhibition of smoothened (SMO), which are key components of the Hedgehog (Hh) pathway (5, 136, 161, 221, 228, 248, 270) (FIGURE 7). Targeting CAFs with these approaches led to reduced survival in both preclinical and clinical studies, indicating a potential tumor-restraining role of these cells. Of note, these strategies were thought to be designed to target CAFs as a whole rather than specific CAF subtypes, as CAF heterogeneity had not yet been described and αSMA was still considered a ubiquitous marker of activated CAFs. However, with the current knowledge, we can speculate that selective targeting of αSMA-expressing cells preferentially depleted the myofibroblastic myCAF population (221). Moreover, this genetic approach would also affect other cell populations that express αSMA, such as pericytes, and therefore, it does not necessarily indicate a role of CAFs in restraining tumor progression. It also remains to be determined which CAF subtypes are affected following abrogation of Hh signaling, although the observation that inhibition of this pathway led to a reduction in ECM deposition and αSMA+ cells (215, 248) suggests that ECM-producing myCAFs are targeted.
FIGURE 7.
Potential tumor-restraining cancer-associated fibroblast (CAF) functions. Schematic illustration of the Hedgehog (Hh) signaling pathway (top) and of the genetic and therapeutic approaches that indicated the presence of tumor-restraining functions of pancreatic ductal adenocarcinoma (PDAC) CAFs (bottom). A: genetic deletion of the Hh ligand sonic hedgehog (SHH) in a mouse model of PDAC led to a reduction in survival and tumor differentiation and to an increase in angiogenesis and cachexia, a highly debilitating muscle-wasting condition (248). GLI1, GLI family zinc finger 1; PTCH1, protein patched homolog 1. αSMA, α-smooth muscle actin. B: genetic depletion of αSMA+ cells in a mouse model of PDAC led to a reduction in survival and tumor differentiation and to an increase in the infiltration of immunosuppressive CD4+ Foxp3+ regulatory T cells (221). C: prolonged pharmacological inhibition of the Smoothened (SMO) receptor in a mouse model of PDAC led to reduced survival and tumor differentiation, and to an increase in angiogenesis and cachexia (248).
Overall, although some progress has been made to determine the functions of phenotypically distinct CAF subtypes (24, 73, 284), more work needs to be undertaken to elucidate whether CAF heterogeneity is clinically relevant, rather than simply descriptive.
F. Stage-Dependent Fibroblast Heterogeneity
1. During cancer progression
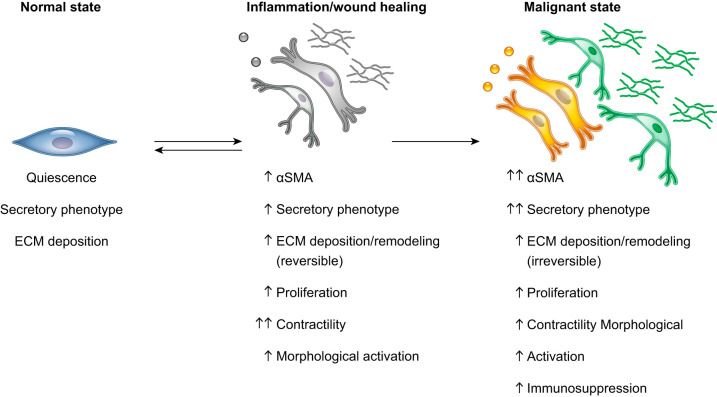
Although we now have a more complete picture of the CAF composition at primary tumors in various cancer types, it has to be contextualized during cancer progression. Indeed, the identity and proportion of distinct CAF subtypes could differ across normal, inflammatory, premalignant, and malignant states (FIGURE 8). A better understanding of the populations present during cancer progression is crucial to decipher the evolution of CAF heterogeneity and its effects on other cell populations of the TME.
FIGURE 8.
Fibroblast activation in inflammation and cancer. Schematic illustration showing the phenotype changes associated with fibroblast activation during inflammation and cancer progression. Compared with normal quiescent fibroblasts, fibroblasts in inflammatory and malignant states have various degrees of increased α-smooth muscle actin (αSMA) expression, secretory phenotype, extracellular matrix (ECM) deposition, proliferation, contractility, and morphological activation. Cancer-associated fibroblasts can also be immunosuppressive.
An increasing number of scRNA-seq studies currently provide some information about the fibroblast composition in early- or late-stage primary tumors, compared with adjacent normal or metastatic tissues (20, 57, 64, 73, 89, 111, 154, 166, 240). Single-cell pseudo-time trajectory analysis (295) of these and other data sets could predict the evolution of fibroblasts during malignant progression (64). Additionally, longitudinal studies are needed to dissect fibroblast heterogeneity over time and at distinct sites. Since for certain cancer types, such as PDAC, patient tissues will largely represent early cancer stages due to tissue sampling limitations, the use of mouse models will complement the analysis of human fibroblasts during cancer progression (298).
We currently have very little information about the CAF composition in metastases. scRNA-seq studies of metastatic cancers showed that neoplastic cells from different patients clustered separately both at the primary and secondary site, indicating the high degree of inter-individual heterogeneity in the epithelial cancer compartment (176, 226, 240, 292). On the contrary, although different in proportions, fibroblasts and other stromal cells clustered by cell type independent of patients, stages, and tissues of origin, indicating the presence of conserved transcriptional programs across different tumors and during cancer progression (176, 226, 240, 292). Nonetheless, diverse immune microenvironments, which respond differently to the same therapeutic regime, have been identified in distinct metastatic sites of the same ovarian cancer patient (127). A similar scenario could occur for CAFs and may influence the immune representation and therapy response at different sites as well. Accordingly, differences in fibroblast signatures have been identified between micrometastases and macrometastases (264). Furthermore, fibroblasts in metastatic sites can be activated differently than CAFs in primary tumors (207). Additionally, the ECM composition and response to chemotherapy can differ between primary and secondary sites (1, 310), further supporting the presence of functionally and phenotypically distinct CAF populations. Moreover, although it has been shown that CAFs can migrate with cancer cells from the primary site (67), resident fibroblasts have also been involved in the formation of a premetastatic niche (131, 210). Additional comparisons of primary and secondary sites at single cell resolution will help assess the contribution of both resident and recruited cell populations in defining the CAF composition.
2. During aging and senescence
Fibroblast abundance and ECM integrity have been shown to decrease with age (7, 100, 186, 224, 300). Previous reports have highlighted that aging in dermal fibroblasts is associated with accumulated genetic damage (169, 202), telomere attrition (181), increased secretion of inflammatory cytokines (180, 335), loss of identity, and gain of adipogenic traits (260). Transcriptomic, epigenomic, and metabolomic analyses of fibroblasts from young and old mice have revealed the presence of age-dependent heterogeneity in the rate of wound healing and in the efficiency of reprogramming to induced pluripotent stem cells (iPSCs) (180). In particular, the presence of a fibroblast activated signature typical of myofibroblasts involved in tissue repair (74) was associated with efficient reprogramming of aged fibroblasts to iPSCs (180).
Importantly, in melanoma, aged fibroblasts have an altered ECM-deposition compared with young fibroblasts, which can increase tumor motility, metastasis formation, and immunosuppression (133). However, despite the fact that cancer incidence increases with age, our knowledge of how distinct CAF subtypes of an aged microenvironment contribute to progression and therapy response in other cancer types remains limited. Indeed, most in vivo preclinical studies employ mice that have not yet reached the geriatric phenotype (∼18–24 mo of age, corresponding to ∼60 human years) (69). Therefore, such models may not fully recapitulate the stromal composition and molecular interactions between CAFs and cancer cells. New in vitro and in vivo models are needed to understand the impact of an aged TME in cancer progression.
Additionally, as tissues age, they accumulate cells that undergo a persistent arrest of the cell cycle, a process known as senescence, in response to multiple stimuli, such as chromatin remodeling, telomere attrition, and environmental stress (37, 76, 82, 88, 117, 164, 173, 216, 230, 324). Importantly, the accumulation of senescent cells has been associated with inflammation and tissue dysfunction (34, 88). Although senescence has been used as a model to study fibroblasts in aging, aged and senescent fibroblasts only have a few overlapping features (38, 133, 307, 308). In particular, senescent cells are characterized by the SASP, which includes cytokines, chemokines, and ECM-remodeling enzymes (50), and has been shown to play various roles in cancer progression (81, 147, 256). For example, senescent fibroblasts have been associated with inflammatory phenotypes that promoted pancreatic cancer progression (265, 309).
V. FIBROBLAST PLASTICITY
Recent evidence indicates that CAF subtypes are dynamic and able to interconvert depending on tumor cues, culture conditions, and therapeutic regimens (24, 73, 89, 213). This observation suggests that CAF subtypes could be cell states rather than end-points in differentiation. This plasticity represents a technical challenge as CAF populations may lose their subtype-defining phenotype when isolated from tumors, but it also represents a therapeutic opportunity. Indeed, rather than ablating a tumor-promoting CAF subtype, it could be more beneficial shifting it towards a tumor-restraining or quiescent population.
We recently showed that CAF subtype reprogramming in vivo is possible with the JAK inhibitor AZD1480, which shifted iCAFs to myCAFs and led to increased ECM deposition in a mouse model of PDAC (24) (FIGURE 9). These results raise the possibility that, in some circumstances, drug delivery could be decreased following CAF subtype switching. These observations thus indicate that the efficacy of standard of care drugs could be improved by understanding their effects on the stroma. Similarly, drugs that failed in clinical trials could be re-evaluated in combinatorial strategies with treatments that would mitigate their negative effect on the TME.
FIGURE 9.
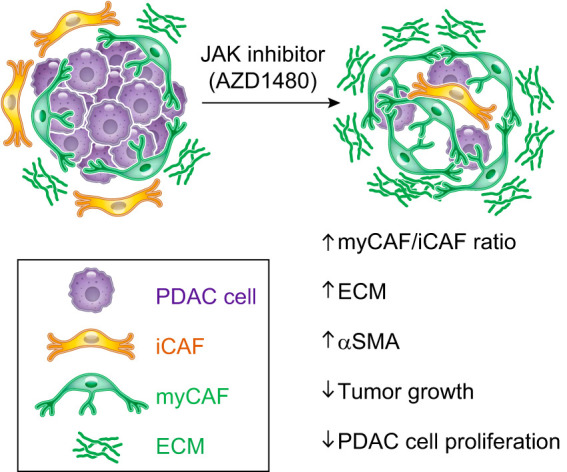
Cancer-associated fibroblast (CAF) plasticity. Schematic illustration showing the effects of the JAK inhibitor AZD1480 on reprogramming inflammatory CAFs (iCAFs) in a mouse model of pancreatic ductal adenocarcinoma (PDAC) (24). Treatment with the JAK inhibitor shifts the iCAF subtype towards an extracellular matrix (ECM)-producing myofibroblastic CAF (myCAF) population, leading to an increase in the myCAF/iCAF ratio and ECM deposition and to a reduction in tumor cell proliferation and tumor growth.
Fibroblast plasticity is not unique to malignant states, and scRNA-seq techniques have determined the molecular basis of fibroblast conversion to various cell states and types (171). For example, behavior switching between different states has been observed in the skin, where fibroblast populations fluctuate between ECM deposition and cell proliferation (254) and in pulmonary fibrosis (71). This plasticity also occurs during aging, as dermal fibroblasts acquire adipogenic markers (260).
VI. CAFs AND THERAPEUTICS
Although epithelial cells have been at the center of the efforts for the development of new anti-cancer therapies, a number of CAF-targeting strategies have been tested in preclinical and clinical trials (46) (FIGURE 10). The historic rationale for therapeutically targeting CAFs has been to decrease the ECM and the production of tumor-promoting and immunosuppressive ligands, to increase the efficacy of chemotherapies and immunotherapies. Moreover, long-range effects of CAFs in promoting metastasis formation and systemic conditions, such as cachexia and anemia, recently emerged as additional reasons to target CAFs (129, 212).
FIGURE 10.
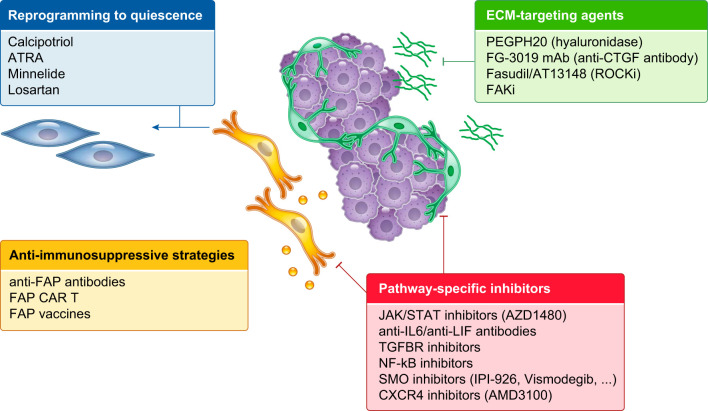
Stroma-targeting therapies. Schematic illustration of the stroma-targeting strategies that have been tested in preclinical and clinical studies, including agents targeting components of the ECM (e.g., PEGPH20 and FG-3019), anti-immunosuppressive strategies, pathway-specific inhibitors (e.g., SMO inhibitors) and drugs that reprogram CAFs to a quiescent state (e.g., calcipotriol). ATRA, all-trans-retinoic acid; FAK, focal adhesion kinase; FAP, fibroblast activation protein; IL6, interleukin-6; LIF, leukemia inhibitory factor; SMO, Smoothened.
CAFs promote chemotherapy resistance in part through the secretion of ECM proteins, which limit access to drug delivery by forming a thick barrier (61, 124, 178, 215, 239) and compressing blood vessels and lymphatic vessels, leading to hypoperfusion and elevated interstitial fluid pressure (42, 188, 283). Among strategies that deplete the ECM, PEGPH20 targets the ECM component hyaluronan (HA) (124, 239). Treatment with hyaluronidase PEGPH20 and chemotherapy initially appeared promising in clinical trials for PDAC (107, 108), with the exception of a combinatorial approach with mFOLFIRINOX (243). However, a phase III clinical trial targeted at HA-high patients (HALO 301) recently failed, and PEGPH20 development has been discontinued. Similarly, SMO inhibitors, which lead to ECM depletion by blocking the Hh signaling pathway and targeting ECM-producing αSMA+ CAFs (40, 161, 215, 248), have shown adverse or no effects in clinical trials for PDAC (39, 136, 140, 161, 248). These results could be due to the role of Hh dosage and the diverse consequences of Hh inhibition on PDAC progression and pancreatic tissue remodeling (191, 192, 215, 248). Other strategies to target the ECM include the use of Rho-associated protein kinase inhibitors, such as Fasudil and AT13148 (244, 245, 301), and antibodies against ECM proteins, such as connective tissue growth factor (CTGF), fibronectin, and tenascin C (205, 210, 246). Interestingly, blocking CTGF in PDAC led to enhanced chemotherapy response by modulating survival cues in cancer cells without affecting drug delivery (205), indicating that targeting of ECM components can be therapeutically effective independently from the reduction of desmoplasia.
CAF deactivation with loss of myofibroblastic features has been observed during chronic hypoxia (179), arguing that conversion of CAFs to a less activated state is possible. Therapeutic strategies that reverse activated CAFs to quiescence include treatments with all-trans-retinoic acid (ATRA) (75, 90, 103), minnelide (18, 56), and the vitamin D receptor agonist calcipotriol, which ameliorated liver and pancreas fibrosis and enhanced PDAC therapy (60, 266). Moreover, treatment with the angiotensin receptor II antagonist losartan has been shown to decrease TGF-β activation in αSMA+ CAFs, leading to a reduction in desmoplasia and an increase in drug delivery (42, 61) and immunotherapy efficacy (41). Clinical trials with losartan in combination with standard of care regimens for PDAC are ongoing and appear promising for the treatment of local-advanced disease (185, 203).
Some markers, such as FAP (84, 146, 172), fibroblast specific protein (FSP-1) (23, 47), and αSMA (221), have also been used to genetically or pharmacologically deplete CAFs. In particular, FAP has been targeted in preclinical therapeutic strategies with vaccines, antibodies, and chimeric antigen receptor (CAR) T cells. Similar to the genetic depletion of FAP+ CAFs, inhibition of CXCR4 by AMD3100 has been shown to increase T cell infiltration and ameliorate the efficacy of checkpoint inhibitors in PDAC (84). The CXCL12/CXCR4 axis has been also demonstrated to promote disease progression and immunosuppression in breast cancer (53, 218). In this context, abrogation of CXCR4 signaling in the CAFs has been shown to reduce the levels of fibrosis and αSMA+ cells, leading to vasculature normalization, a decrease in immunosuppressive cell populations, and an increase in immunotherapy efficacy (43).
Alternative anti-fibrotic strategies include the anti-histamine drug tranilast (223, 225), the glucocorticoid steroid dexamethasone (187, 268), the hormone relaxin (25, 29, 130), halofuginone (30, 72, 277, 278, 336), and metformin, which has been shown to reduce the levels of fibrosis in both aging and cancer (120, 194, 318). Additional clinically approved drugs for fibrotic and inflammatory conditions, such as idiopathic pulmonary fibrosis (138, 144, 238, 249), could also be repurposed for targeting CAFs and reducing the ECM.
The recent advances on CAF molecular and functional heterogeneity open new avenues for cancer treatment. Potential strategies to design new therapies would be either by targeting CAF-secreted tumor-promoting and immunosuppressive ligands, such as IL-6 (177, 331), LIF (269), and TGF-β (184, 290), or by inhibiting subtype-specific signaling pathways that would ablate a particular CAF population. Another strategy would be to design therapies that leverage the plasticity of CAFs and shift tumor-promoting CAF subtypes towards a quiescent or tumor-restraining phenotype. Finally, only combinatorial approaches that target CAFs, cancer cells, immune cells, and drug delivery may be successful as effective treatments.
The emerging evidence that cancer cells and CAFs share certain signaling pathways will guide the design of therapeutic approaches that target cancer cells without affecting the stroma in ways that could reduce drug efficacy. Moreover, new combinatorial therapies could be designed to target specific pro-tumorigenic functions that are common to both the epithelial and CAF compartment. For example, the IL-1 receptor antagonist anakinra, which has been shown to target PDAC cancer cells (334), could also target potentially tumor-promoting, immunosuppressive iCAFs, whose phenotype is driven by IL-1 signaling (24, 64, 273). However, the presence of overlapping signaling pathways across different cell types also represents a potential issue that should be considered in the design of combinatorial therapeutic approaches. For example, the use of JAK inhibitors, although effective in targeting cancer cells and inflammatory CAFs (24), could also target the proliferation and/or activity of cytotoxic T cells, which would be problematic in combination with immunotherapy strategies. Therefore, imaging, genetic, and immunohistochemical analyses will need to be incorporated in the design of clinical trials to determine the effects of chemotherapy and targeted drugs on the CAF subtypes and on the immune tumor microenvironment. Likely, only combinatorial therapeutic strategies addressing these issues and designed to target multiple tumor-promoting components of the TME will be successful.
VII. CONCLUSIONS: PRESENT CHALLENGES AND FUTURE DIRECTIONS
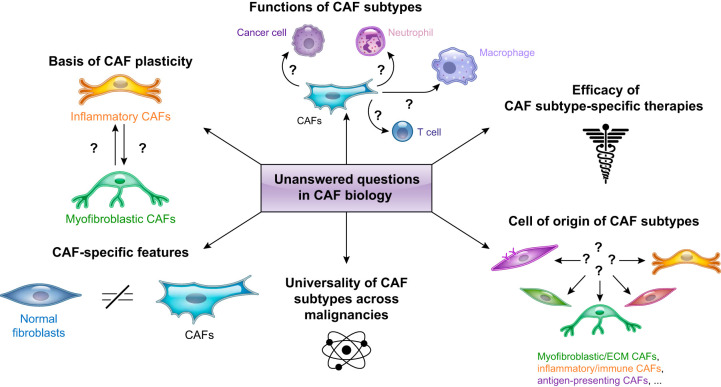
Throughout this review, we have highlighted the multiple levels of CAF molecular and functional heterogeneity and the outstanding questions that remain to be addressed (FIGURE 11). Valuable information will come from studies that focus on 1) comparing fibroblasts across different tissues of origin and normal, inflammatory, and malignant states; 2) optimizing in vitro co-culture models and generating lineage-tracing in vivo models to study the cell of origin and functions of CAFs; and 3) going beyond the description of CAF heterogeneity and identifying the dependencies of each CAF population for the development of subtype-specific therapies. Additionally, the refinement of CAF subtype-specific transcriptional signatures at single-cell resolution and the identification of these profiles in bulk RNA-seq analyses could represent a powerful tool to predict the course of the disease and instruct patient treatment.
FIGURE 11.
Unanswered questions in cancer-associated fibroblast (CAF) biology. Schematic illustration of key areas in CAF biology that remain underexplored.
A more methodical standardization of the study of CAFs is also needed. One of the challenges in defining CAF heterogeneity by scRNA-seq analysis is that population subclustering can be arbitrarily defined and is limited by the number of samples and cells analyzed. An additional issue is the fact that CAFs are difficult to isolate, as they are embedded in the ECM. Therefore, CAFs are usually under-represented in scRNA-seq data sets, and tissue-specific protocols for CAF isolation should be developed (305). Although many dissociation protocols have been described, little justification has been given for the choice of enzymes and conditions employed. This variety of protocols further complicates the analysis of scRNA-seq data sets, since artefactual disaggregation-associated signatures (i.e., transcriptomic changes associated with a specific dissociation protocol) have been identified and can further confound the data interpretation (299). More gentle protocols may limit this problem, but likely to the detriment of the number of cells retrieved. The comparison of multiple dissociation protocols and sequencing platforms would allow the identification of transcriptional changes associated with the duration, temperature, and digestion conditions of a specific method (59). As an alternative approach, single-nucleus sequencing strategies (102, 114) could assist in the analysis of CAFs.
Additionally, the presence of similar CAF populations across different cancer types (FIGURE 3 and TABLE 2) suggests the need to define a common nomenclature of CAFs. Reaching this consensus is particularly important when multiple scRNA-seq data sets exist for the same cancer type (20, 24, 64, 73, 111, 258). Moreover, the presence of similarities between fibroblasts in normal, inflammatory, and malignant states highlights the need to distinguish between transcriptional signatures that are unique to CAFs or common to fibroblast populations in multiple contexts.
A paradigm-shifting approach that expands our therapeutic target selection is necessary to tackle malignancies with dismal prognosis, such as PDAC. Accordingly, gaining a comprehensive view of the dynamics and heterogeneity of CAFs could identify new therapeutic vulnerabilities. The progress that will be made in the next few years towards the functional characterization of distinct CAF subtypes will determine whether combinatorial strategies targeting cancer cells and tumor-promoting fibroblast populations could provide a clinically actionable approach for cancer treatment.
GRANTS
The authors are supported by National Institutes of Health (NIH) Cancer Center Support Grant 5P30CA045508 and the Lustgarten Foundation, where D. A. Tuveson is a distinguished scholar and Director of the Lustgarten Foundation–designated Laboratory of Pancreatic Cancer Research. D. A. Tuveson is also supported by the Cold Spring Harbor Laboratory and Northwell Health Affiliation, the Cold Spring Harbor Laboratory Association, and NIH Grants 5P30CA45508, U01CA210240, R01CA229699, U01CA224013, 1R01CA188134, and 1R01CA190092. G. Biffi was a fellow of the Human Frontiers Science Program (LT000195/2015-L) and EMBO (ALTF 1203-2014) and is supported by Cancer Research UK core funding (A27463).
DISCLOSURES
D. A. Tuveson reports receiving commercial research grants from Fibrogen and ONO, has ownership interest (including stock, patents, etc.) in Leap Therapeutics and Surface Oncology, and is a consultant/advisory board member for Leap Oncology and Surface Oncology, Cygnal, and Merck. G. Biffi has no conflicts of interest, financial or otherwise.
ACKNOWLEDGMENTS
Correspondence: G. Biffi (e-mail: Giulia.Biffi@cruk.cam.ac.uk) or D. A. Tuveson (dtuveson@cshl.edu).
REFERENCES
- 1.Aiello NM, Bajor DL, Norgard RJ, Sahmoud A, Bhagwat N, Pham MN, Cornish TC, Iacobuzio-Donahue CA, Vonderheide RH, Stanger BZ. Metastatic progression is associated with dynamic changes in the local microenvironment. Nat Commun 7: 12819, 2016. doi: 10.1038/ncomms12819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrengues J, Bertero T, Grasset E, Bonan S, Maiel M, Bourget I, Philippe C, Herraiz Serrano C, Benamar S, Croce O, Sanz-Moreno V, Meneguzzi G, Feral CC, Cristofari G, Gaggioli C. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat Commun 6: 10204, 2015. doi: 10.1038/ncomms10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkasalias T, Flaberg E, Kashuba V, Alexeyenko A, Pavlova T, Savchenko A, Szekely L, Klein G, Guven H. Inhibition of tumor cell proliferation and motility by fibroblasts is both contact and soluble factor dependent. Proc Natl Acad Sci USA 111: 17188–17193, 2014. doi: 10.1073/pnas.1419554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, Schnitt S, Sellers WR, Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 6: 17–32, 2004. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med 19: 1410–1422, 2013. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 6.Anderberg C, Li H, Fredriksson L, Andrae J, Betsholtz C, Li X, Eriksson U, Pietras K. Paracrine signaling by platelet-derived growth factor-CC promotes tumor growth by recruitment of cancer-associated fibroblasts. Cancer Res 69: 369–378, 2009. doi: 10.1158/0008-5472.CAN-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrew W, Behnke RH, Sato T. Changes with advancing age in the cell population of human dermis. Gerontologia 10: 1–19, 1964. doi: 10.1159/000211369. [DOI] [PubMed] [Google Scholar]
- 8.Ao M, Brewer BM, Yang L, Franco Coronel OE, Hayward SW, Webb DJ, Li D. Stretching fibroblasts remodels fibronectin and alters cancer cell migration. Sci Rep 5: 8334, 2015. doi: 10.1038/srep08334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ao Z, Shah SH, Machlin LM, Parajuli R, Miller PC, Rawal S, Williams AJ, Cote RJ, Lippman ME, Datar RH, El-Ashry D. Identification of cancer-associated fibroblasts in circulating blood from patients with metastatic breast cancer. Cancer Res 75: 4681–4687, 2015. doi: 10.1158/0008-5472.CAN-15-1633. [DOI] [PubMed] [Google Scholar]
- 10.Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut 44: 534–541, 1999. doi: 10.1136/gut.44.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arandkar S, Furth N, Elisha Y, Nataraj NB, van der Kuip H, Yarden Y, Aulitzky W, Ulitsky I, Geiger B, Oren M. Altered p53 functionality in cancer-associated fibroblasts contributes to their cancer-supporting features. Proc Natl Acad Sci USA 115: 6410–6415, 2018. doi: 10.1073/pnas.1719076115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arina A, Idel C, Hyjek EM, Alegre ML, Wang Y, Bindokas VP, Weichselbaum RR, Schreiber H. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc Natl Acad Sci USA 113: 7551–7556, 2016. doi: 10.1073/pnas.1600363113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auciello FR, Bulusu V, Oon C, Tait-Mulder J, Berry M, Bhattacharyya S, Tumanov S, Allen-Petersen BL, Link J, Kendsersky ND, Vringer E, Schug M, Novo D, Hwang RF, Evans RM, Nixon C, Dorrell C, Morton JP, Norman JC, Sears RC, Kamphorst JJ, Sherman MH. A stromal lysolipid-autotaxin signaling axis promotes pancreatic tumor progression. Cancer Discov 9: 617–627, 2019. doi: 10.1158/2159-8290.CD-18-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Augsten M, Sjöberg E, Frings O, Vorrink SU, Frijhoff J, Olsson E, Borg Å, Östman A. Cancer-associated fibroblasts expressing CXCL14 rely upon NOS1-derived nitric oxide signaling for their tumor-supporting properties. Cancer Res 74: 2999–3010, 2014. doi: 10.1158/0008-5472.CAN-13-2740. [DOI] [PubMed] [Google Scholar]
- 15.Avery D, Govindaraju P, Jacob M, Todd L, Monslow J, Puré E. Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol 67: 90–106, 2018. doi: 10.1016/j.matbio.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 115: 421–432, 1998. doi: 10.1016/S0016-5085(98)70209-4. [DOI] [PubMed] [Google Scholar]
- 17.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grützmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM; Australian Pancreatic Cancer Genome Initiative . Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531: 47–52, 2016. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee S, Modi S, McGinn O, Zhao X, Dudeja V, Ramakrishnan S, Saluja AK. Impaired synthesis of stromal components in response to minnelide improves vascular function, drug delivery, and survival in pancreatic cancer. Clin Cancer Res 22: 415–425, 2016. doi: 10.1158/1078-0432.CCR-15-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartoschek M, Oskolkov N, Bocci M, Lövrot J, Larsson C, Sommarin M, Madsen CD, Lindgren D, Pekar G, Karlsson G, Ringnér M, Bergh J, Björklund Å, Pietras K. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun 9: 5150, 2018. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard V, Semaan A, Huang J, San Lucas FA, Mulu FC, Stephens BM, Guerrero PA, Huang Y, Zhao J, Kamyabi N, Sen S, Scheet PA, Taniguchi CM, Kim MP, Tzeng CW, Katz MH, Singhi AD, Maitra A, Alvarez HA. Single-cell transcriptomics of pancreatic cancer precursors demonstrates epithelial and microenvironmental heterogeneity as an early event in neoplastic progression. Clin Cancer Res 25: 2194–2205, 2019. doi: 10.1158/1078-0432.CCR-18-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13: 392–402, 2013. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Bhagat TD, Von Ahrens D, Dawlaty M, Zou Y, Baddour J, Achreja A, Zhao H, Yang L, Patel B, Kwak C, Choudhary GS, Gordon-Mitchell S, Aluri S, Bhattacharyya S, Sahu S, Bhagat P, Yu Y, Bartenstein M, Giricz O, Suzuki M, Sohal D, Gupta S, Guerrero PA, Batra S, Goggins M, Steidl U, Greally J, Agarwal B, Pradhan K, Banerjee D, Nagrath D, Maitra A, Verma A. Lactate-mediated epigenetic reprogramming regulates formation of human pancreatic cancer-associated fibroblasts. eLife 8: e50663, 2019. doi: 10.7554/eLife.50663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303: 848–851, 2004. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 24.Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J, Tuveson DA. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov 9: 282–301, 2019. doi: 10.1158/2159-8290.CD-18-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blessing WA, Okajima SM, Cubria MB, Villa-Camacho JC, Perez-Viloria M, Williamson PM, Sabogal AN, Suarez S, Ang LH, White S, Flynn E, Rodriguez EK, Grinstaff MW, Nazarian A. Intraarticular injection of relaxin-2 alleviates shoulder arthrofibrosis. Proc Natl Acad Sci USA 116: 12183–12192, 2019. doi: 10.1073/pnas.1900355116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bochet L, Lehuédé C, Dauvillier S, Wang YY, Dirat B, Laurent V, Dray C, Guiet R, Maridonneau-Parini I, Le Gonidec S, Couderc B, Escourrou G, Valet P, Muller C. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res 73: 5657–5668, 2013. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 27.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15: 786–801, 2014. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boots AM, Wimmers-Bertens AJ, Rijnders AW. Antigen-presenting capacity of rheumatoid synovial fibroblasts. Immunology 82: 268–274, 1994. [PMC free article] [PubMed] [Google Scholar]
- 29.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med 9: 796–800, 2003. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 30.Bruck R, Genina O, Aeed H, Alexiev R, Nagler A, Avni Y, Pines M. Halofuginone to prevent and treat thioacetamide-induced liver fibrosis in rats. Hepatology 33: 379–386, 2001. doi: 10.1053/jhep.2001.21408. [DOI] [PubMed] [Google Scholar]
- 31.Buechler MB, Kim KW, Onufer EJ, Williams JW, Little CC, Dominguez CX, Li Q, Sandoval W, Cooper JE, Harris CA, Junttila MR, Randolph GJ, Turley SJ. A stromal niche defined by expression of the transcription factor WT1 mediates programming and homeostasis of cavity-resident macrophages. Immunity 51: 119–130.e5, 2019. doi: 10.1016/j.immuni.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byrne C, Schairer C, Wolfe J, Parekh N, Salane M, Brinton LA, Hoover R, Haile R. Mammographic features and breast cancer risk: effects with time, age, and menopause status. J Natl Cancer Inst 87: 1622–1629, 1995. doi: 10.1093/jnci/87.21.1622. [DOI] [PubMed] [Google Scholar]
- 33.Byrnes LE, Wong DM, Subramaniam M, Meyer NP, Gilchrist CL, Knox SM, Tward AD, Ye CJ, Sneddon JB. Lineage dynamics of murine pancreatic development at single-cell resolution. Nat Commun 9: 3922, 2018. doi: 10.1038/s41467-018-06176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev 99: 1047–1078, 2019. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 35.Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Céspedes MV, Sevillano M, Nadal C, Jung P, Zhang XH, Byrom D, Riera A, Rossell D, Mangues R, Massagué J, Sancho E, Batlle E. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 22: 571–584, 2012. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, Sahai E. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15: 637–646, 2013. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao K, Blair CD, Faddah DA, Kieckhaefer JE, Olive M, Erdos MR, Nabel EG, Collins FS. Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J Clin Invest 121: 2833–2844, 2011. doi: 10.1172/JCI43578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casella G, Munk R, Kim KM, Piao Y, De S, Abdelmohsen K, Gorospe M. Transcriptome signature of cellular senescence. Nucleic Acids Res 47: 11476, 2019. doi: 10.1093/nar/gkz879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol 33: 4284–4292, 2015. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cazet AS, Hui MN, Elsworth BL, Wu SZ, Roden D, Chan CL, Skhinas JN, Collot R, Yang J, Harvey K, Johan MZ, Cooper C, Nair R, Herrmann D, McFarland A, Deng N, Ruiz-Borrego M, Rojo F, Trigo JM, Bezares S, Caballero R, Lim E, Timpson P, O’Toole S, Watkins DN, Cox TR, Samuel MS, Martín M, Swarbrick A. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat Commun 9: 2897, 2018. doi: 10.1038/s41467-018-05220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chauhan VP, Chen IX, Tong R, Ng MR, Martin JD, Naxerova K, Wu MW, Huang P, Boucher Y, Kohane DS, Langer R, Jain RK. Reprogramming the microenvironment with tumor-selective angiotensin blockers enhances cancer immunotherapy. Proc Natl Acad Sci USA 116: 10674–10680, 2019. doi: 10.1073/pnas.1819889116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popović Z, Huang P, Bawendi MG, Boucher Y, Jain RK. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 4: 2516, 2013. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, Huang P, Lindeman N, Langer R, Jain RK. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci USA 116: 4558–4566, 2019. doi: 10.1073/pnas.1815515116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, Tang T, Eastham-Anderson J, Dunlap D, Alicke B, Nannini M, Gould S, Yauch R, Modrusan Z, DuPree KJ, Darbonne WC, Plowman G, de Sauvage FJ, Callahan CA. Canonical hedgehog signaling augments tumor angiogenesis by induction of VEGF-A in stromal perivascular cells. Proc Natl Acad Sci USA 108: 9589–9594, 2011. doi: 10.1073/pnas.1017945108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen WJ, Ho CC, Chang YL, Chen HY, Lin CA, Ling TY, Yu SL, Yuan SS, Chen YJ, Lin CY, Pan SH, Chou HY, Chen YJ, Chang GC, Chu WC, Lee YM, Lee JY, Lee PJ, Li KC, Chen HW, Yang PC. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat Commun 5: 3472, 2014. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 46.Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov 18: 99–115, 2019. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, LeBleu VS, Carstens JL, Sugimoto H, Zheng X, Malasi S, Saur D, Kalluri R. Dual reporter genetic mouse models of pancreatic cancer identify an epithelial-to-mesenchymal transition-independent metastasis program. EMBO Mol Med 10: e9085, 2018. doi: 10.15252/emmm.201809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chien Y, Scuoppo C, Wang X, Fang X, Balgley B, Bolden JE, Premsrirut P, Luo W, Chicas A, Lee CS, Kogan SC, Lowe SW. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev 25: 2125–2136, 2011. doi: 10.1101/gad.17276711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 33: 2423–2431, 2014. doi: 10.1038/onc.2013.191. [DOI] [PubMed] [Google Scholar]
- 50.Coppé JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118, 2010. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cortes E, Lachowski D, Robinson B, Sarper M, Teppo JS, Thorpe SD, Lieberthal TJ, Iwamoto K, Lee DA, Okada-Hatakeyama M, Varjosalo MT, Del Río Hernández AE. Tamoxifen mechanically reprograms the tumor microenvironment via HIF-1A and reduces cancer cell survival. EMBO Rep 20: 20, 2019. doi: 10.15252/embr.201846557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cortes E, Sarper M, Robinson B, Lachowski D, Chronopoulos A, Thorpe SD, Lee DA, Del Río Hernández AE. GPER is a mechanoregulator of pancreatic stellate cells and the tumor microenvironment. EMBO Rep 20: e46556, 2019. doi: 10.15252/embr.201846556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, Bonneau C, Kondratova M, Kuperstein I, Zinovyev A, Givel AM, Parrini MC, Soumelis V, Vincent-Salomon A, Mechta-Grigoriou F. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 33: 463–479.e10, 2018. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Croft AP, Campos J, Jansen K, Turner JD, Marshall J, Attar M, Savary L, Wehmeyer C, Naylor AJ, Kemble S, Begum J, Dürholz K, Perlman H, Barone F, McGettrick HM, Fearon DT, Wei K, Raychaudhuri S, Korsunsky I, Brenner MB, Coles M, Sansom SN, Filer A, Buckley CD. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature 570: 246–251, 2019. doi: 10.1038/s41586-019-1263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Semin Cancer Biol 20: 139–145, 2010. doi: 10.1016/j.semcancer.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dauer P, Zhao X, Gupta VK, Sharma N, Kesh K, Gnamlin P, Dudeja V, Vickers SM, Banerjee S, Saluja A. Inactivation of cancer-associated-fibroblasts disrupts oncogenic signaling in pancreatic cancer cells and promotes its regression. Cancer Res 78: 1321–1333, 2018. doi: 10.1158/0008-5472.CAN-17-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davidson S, Efremova M, Riedel A, Mahata B, Pramanik J, Huuhtanen J, Kar G, Vento-Tormo R, Hagai T, Chen X, Haniffa MA, Shields JD, Teichmann SA. Single-cell RNA sequencing reveals a dynamic stromal niche within the evolving tumour microenvironment (Preprint). bioRxiv 467225, 2018. doi: 10.1101/467225. [DOI] [PMC free article] [PubMed]
- 58.DeFilippis RA, Chang H, Dumont N, Rabban JT, Chen YY, Fontenay GV, Berman HK, Gauthier ML, Zhao J, Hu D, Marx JJ, Tjoe JA, Ziv E, Febbraio M, Kerlikowske K, Parvin B, Tlsty TD. CD36 repression activates a multicellular stromal program shared by high mammographic density and tumor tissues. Cancer Discov 2: 826–839, 2012. doi: 10.1158/2159-8290.CD-12-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denisenko E, Guo BB, Jones M, Hou R, de Kock L, Lassmann T, Poppe D, Clement O, Simmons RK, Lister R, Forrest ARR. Systematic bias assessment in solid tissue 10x scRNA-seq workflows (Preprint). bioRxiv 832444, 2019. doi: 10.1101/832444. [DOI] [PMC free article] [PubMed]
- 60.Ding N, Yu RT, Subramaniam N, Sherman MH, Wilson C, Rao R, Leblanc M, Coulter S, He M, Scott C, Lau SL, Atkins AR, Barish GD, Gunton JE, Liddle C, Downes M, Evans RM. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 153: 601–613, 2013. doi: 10.1016/j.cell.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci USA 108: 2909–2914, 2011. doi: 10.1073/pnas.1018892108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells 21: 514–520, 2003. doi: 10.1634/stemcells.21-5-514. [DOI] [PubMed] [Google Scholar]
- 63.Djurec M, Graña O, Lee A, Troulé K, Espinet E, Cabras L, Navas C, Blasco MT, Martín-Díaz L, Burdiel M, Li J, Liu Z, Vallespinós M, Sanchez-Bueno F, Sprick MR, Trumpp A, Sainz B Jr, Al-Shahrour F, Rabadan R, Guerra C, Barbacid M. Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors. Proc Natl Acad Sci USA 115: E1147–E1156, 2018. doi: 10.1073/pnas.1717802115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dominguez CX, Müller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, Breart B, Foreman O, Bainbridge TW, Castiglioni A, Senbabaoglu Y, Modrusan Z, Liang Y, Junttila MR, Klijn C, Bourgon R, Turley SJ. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov 10: 232–253, 2020. doi: 10.1158/2159-8290.CD-19-0644. [DOI] [PubMed] [Google Scholar]
- 65.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504: 277–281, 2013. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du Y, Shao H, Moller M, Prokupets R, Tse YT, Liu ZJ. Intracellular Notch1 signaling in cancer-associated fibroblasts dictates the plasticity and stemness of melanoma stem/initiating cells. Stem Cells 37: 865–875, 2019. doi: 10.1002/stem.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duda DG, Duyverman AM, Kohno M, Snuderl M, Steller EJ, Fukumura D, Jain RK. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci USA 107: 21677–21682, 2010. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 18: 1262–1270, 2012. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 69.Dutta S, Sengupta P. Men and mice: relating their ages. Life Sci 152: 244–248, 2016. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 70.Eckert MA, Coscia F, Chryplewicz A, Chang JW, Hernandez KM, Pan S, Tienda SM, Nahotko DA, Li G, Blaženović I, Lastra RR, Curtis M, Yamada SD, Perets R, McGregor SM, Andrade J, Fiehn O, Moellering RE, Mann M, Lengyel E. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 569: 723–728, 2019. doi: 10.1038/s41586-019-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.El Agha E, Moiseenko A, Kheirollahi V, De Langhe S, Crnkovic S, Kwapiszewska G, Szibor M, Kosanovic D, Schwind F, Schermuly RT, Henneke I, MacKenzie B, Quantius J, Herold S, Ntokou A, Ahlbrecht K, Braun T, Morty RE, Günther A, Seeger W, Bellusci S. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell 20: 571, 2017. doi: 10.1016/j.stem.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Elahi-Gedwillo KY, Carlson M, Zettervall J, Provenzano PP. Antifibrotic therapy disrupts stromal barriers and modulates the immune landscape in pancreatic ductal adenocarcinoma. Cancer Res 79: 372–386, 2019. doi: 10.1158/0008-5472.CAN-18-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS, Sivajothi S, Armstrong TD, Engle DD, Yu KH, Hao Y, Wolfgang CL, Park Y, Preall J, Jaffee EM, Califano A, Robson P, Tuveson DA. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov 9: 1102–1123, 2019. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6: 265sr6, 2014. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG, Ramsay AG, Kocher HM. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 145: 1121–1132, 2013. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Enge M, Arda HE, Mignardi M, Beausang J, Bottino R, Kim SK, Quake SR. Single-cell analysis of human pancreas reveals transcriptional signatures of aging and somatic mutation patterns. Cell 171: 321–330.e14, 2017. doi: 10.1016/j.cell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engle DD, Tiriac H, Rivera KD, Pommier A, Whalen S, Oni TE, Alagesan B, Lee EJ, Yao MA, Lucito MS, Spielman B, Da Silva B, Schoepfer C, Wright K, Creighton B, Afinowicz L, Yu KH, Grützmann R, Aust D, Gimotty PA, Pollard KS, Hruban RH, Goggins MG, Pilarsky C, Park Y, Pappin DJ, Hollingsworth MA, Tuveson DA. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science 364: 1156–1162, 2019. doi: 10.1126/science.aaw3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, Washington MK, Shi C, Franco OE, Weaver AM, Hayward SW, Li D, Webb DJ. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol 216: 3799–3816, 2017. doi: 10.1083/jcb.201704053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erez N, Truitt M, Olson P, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. [Correction in Cancer Cell 17: 523, 2010.] Cancer Cell 17: 135–147, 2010. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 80.Ewald AJ, Werb Z, Egeblad M. Dynamic, long-term in vivo imaging of tumor-stroma interactions in mouse models of breast cancer using spinning-disk confocal microscopy. Cold Spring Harb Protoc 2011: pdb.top97, 2011. doi: 10.1101/pdb.top97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faget DV, Ren Q, Stewart SA. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer 19: 439–453, 2019. doi: 10.1038/s41568-019-0156-2. [DOI] [PubMed] [Google Scholar]
- 82.Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer 20: 89–106, 2020. doi: 10.1038/s41568-019-0222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, Cao D, Tang L, Tang S, Wu M, Yang W, Wang H. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun 9: 191, 2018. doi: 10.1038/s41467-017-02583-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL, Teichmann SA, Janowitz T, Jodrell DI, Tuveson DA, Fearon DT. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci USA 110: 20212–20217, 2013. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 10: 554–567, 2010. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flint TR, Janowitz T, Connell CM, Roberts EW, Denton AE, Coll AP, Jodrell DI, Fearon DT. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab 24: 672–684, 2016. doi: 10.1016/j.cmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franco-Barraza J, Beacham DA, Amatangelo MD, Cukierman E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr Protoc Cell Biol 71: 10.9.1–10.9.34, 2016. doi: 10.1002/cpcb.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frey N, Venturelli S, Zender L, Bitzer M. Cellular senescence in gastrointestinal diseases: from pathogenesis to therapeutics. Nat Rev Gastroenterol Hepatol 15: 81–95, 2018. doi: 10.1038/nrgastro.2017.146. [DOI] [PubMed] [Google Scholar]
- 89.Friedman G, Levi-Galibov O, David E, Bornstein C, Giladi A, Dadiani M, Mayo A, Halperin C, Pevsner-Fischer M, Lavon H, Nevo R, Stein Y, Ali RH, Caldas C, Nili-Gal-Yam E, Alon U, Amit I, Scherz-Shouval R. Cancer-associated fibroblast compositions change with breast-cancer progression linking S100A4 and PDPN ratios with clinical outcome (Preprint). bioRxiv 903039, 2020. doi: 10.1101/2020.01.12.903039. [DOI] [PMC free article] [PubMed]
- 90.Froeling FE, Feig C, Chelala C, Dobson R, Mein CE, Tuveson DA, Clevers H, Hart IR, Kocher HM. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 141: 1486–1497.e14, 2011. doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 91.Gaggioli C, Hooper S, Hidalgo-Carcedo C, Grosse R, Marshall JF, Harrington K, Sahai E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol 9: 1392–1400, 2007. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 92.Gao Z, Daquinag AC, Su F, Snyder B, Kolonin MG. PDGFRα/PDGFRβ signaling balance modulates progenitor cell differentiation into white and beige adipocytes. Development 145: dev155861, 2018. doi: 10.1242/dev.155861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geisz A, Sahin-Tóth M. A preclinical model of chronic pancreatitis driven by trypsinogen autoactivation. Nat Commun 9: 5033, 2018. doi: 10.1038/s41467-018-07347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goetz JG, Minguet S, Navarro-Lérida I, Lazcano JJ, Samaniego R, Calvo E, Tello M, Osteso-Ibáñez T, Pellinen T, Echarri A, Cerezo A, Klein-Szanto AJ, Garcia R, Keely PJ, Sánchez-Mateos P, Cukierman E, Del Pozo MA. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 146: 148–163, 2011. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grugan KD, Miller CG, Yao Y, Michaylira CZ, Ohashi S, Klein-Szanto AJ, Diehl JA, Herlyn M, Han M, Nakagawa H, Rustgi AK. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci USA 107: 11026–11031, 2010. doi: 10.1073/pnas.0914295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guerra C, Collado M, Navas C, Schuhmacher AJ, Hernández-Porras I, Cañamero M, Rodriguez-Justo M, Serrano M, Barbacid M. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell 19: 728–739, 2011. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11: 291–302, 2007. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 98.Guerrero-Juarez CF, Dedhia PH, Jin S, Ruiz-Vega R, Ma D, Liu Y, Yamaga K, Shestova O, Gay DL, Yang Z, Kessenbrock K, Nie Q, Pear WS, Cotsarelis G, Plikus MV. Single-cell analysis reveals fibroblast heterogeneity and myeloid-derived adipocyte progenitors in murine skin wounds. Nat Commun 10: 650, 2019. doi: 10.1038/s41467-018-08247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gui F, Zhang Y, Wan J, Zhan X, Yao Y, Li Y, Haddock AN, Shi J, Guo J, Chen J, Zhu X, Edenfield BH, Zhuang L, Hu C, Wang Y, Mukhopadhyay D, Radisky ES, Zhang L, Lugea A, Pandol SJ, Bi Y, Ji B. Trypsin activity governs increased susceptibility to pancreatitis in mice expressing human PRSS1R122H. J Clin Invest 130: 189–202, 2020. doi: 10.1172/JCI130172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gunin AG, Kornilova NK, Petrov VV, Vasil’eva OV. [Age-related changes in the number and proliferation of fibroblasts in the human skin]. Adv Gerontol 24: 43–47, 2011. [PubMed] [Google Scholar]
- 101.Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity 34: 396–408, 2011. doi: 10.1016/j.immuni.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Habib N, Avraham-Davidi I, Basu A, Burks T, Shekhar K, Hofree M, Choudhury SR, Aguet F, Gelfand E, Ardlie K, Weitz DA, Rozenblatt-Rosen O, Zhang F, Regev A. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat Methods 14: 955–958, 2017. doi: 10.1038/nmeth.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han X, Li Y, Xu Y, Zhao X, Zhang Y, Yang X, Wang Y, Zhao R, Anderson GJ, Zhao Y, Nie G. Reversal of pancreatic desmoplasia by re-educating stellate cells with a tumour microenvironment-activated nanosystem. Nat Commun 9: 3390, 2018. doi: 10.1038/s41467-018-05906-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, Saadatpour A, Zhou Z, Chen H, Ye F, Huang D, Xu Y, Huang W, Jiang M, Jiang X, Mao J, Chen Y, Lu C, Xie J, Fang Q, Wang Y, Yue R, Li T, Huang H, Orkin SH, Yuan GC, Chen M, Guo G. Mapping the mouse cell atlas by Microwell-Seq. Cell 173: 1307, 2018. doi: 10.1016/j.cell.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 105.Hartmann N, Giese NA, Giese T, Poschke I, Offringa R, Werner J, Ryschich E. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res 20: 3422–3433, 2014. doi: 10.1158/1078-0432.CCR-13-2972. [DOI] [PubMed] [Google Scholar]
- 106.Hessmann E, Patzak MS, Klein L, Chen N, Kari V, Ramu I, Bapiro TE, Frese KK, Gopinathan A, Richards FM, Jodrell DI, Verbeke C, Li X, Heuchel R, Löhr JM, Johnsen SA, Gress TM, Ellenrieder V, Neesse A. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut 67: 497–507, 2018. doi: 10.1136/gutjnl-2016-311954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, Tjulandin SA, Gladkov OA, Holcombe RF, Korn R, Raghunand N, Dychter S, Jiang P, Shepard HM, Devoe CE. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res 22: 2848–2854, 2016. doi: 10.1158/1078-0432.CCR-15-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: randomized phase II study of PEGPH20 plus Nab-Paclitaxel/Gemcitabine versus Nab-Paclitaxel/Gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol 36: 359–366, 2018. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 109.Hoare M, Ito Y, Kang TW, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R, Antrobus R, Tomimatsu K, Howat W, Lehner PJ, Zender L, Narita M. NOTCH1 mediates a switch between two distinct secretomes during senescence. Nat Cell Biol 18: 979–992, 2016. doi: 10.1038/ncb3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, Hartman J, Religa P, Morikawa H, Ishii Y, Sasahara M, Larsson O, Cossu G, Cao R, Lim S, Cao Y. Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci USA 113: E5618–E5627, 2016. doi: 10.1073/pnas.1608384113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hosein AN, Huang H, Wang Z, Parmar K, Du W, Huang J, Maitra A, Olson E, Verma U, Brekken RA. Cellular heterogeneity during mouse pancreatic ductal adenocarcinoma progression at single-cell resolution. JCI Insight 5: e129212, 2019. doi: 10.1172/jci.insight.129212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hosein AN, Wu M, Arcand SL, Lavallée S, Hébert J, Tonin PN, Basik M. Breast carcinoma-associated fibroblasts rarely contain p53 mutations or chromosomal aberrations. Cancer Res 70: 5770–5777, 2010. doi: 10.1158/0008-5472.CAN-10-0673. [DOI] [PubMed] [Google Scholar]
- 113.Hu M, Yao J, Cai L, Bachman KE, van den Brûle F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet 37: 899–905, 2005. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 114.Hu P, Fabyanic E, Kwon DY, Tang S, Zhou Z, Wu H. Dissecting cell-type composition and activity-dependent transcriptional state in mammalian brains by massively parallel single-nucleus RNA-Seq. Mol Cell 68: 1006–1015.e7, 2017. doi: 10.1016/j.molcel.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang H, Swidnicka-Siergiejko AK, Daniluk J, Gaiser S, Yao Y, Peng L, Zhang Y, Liu Y, Dong M, Zhan X, Wang H, Bi Y, Li Z, Ji B, Logsdon CD. Transgenic expression of PRSS1R122H sensitizes mice to pancreatitis. Gastroenterology 158: 1072–1082.e7, 2020. doi: 10.1053/j.gastro.2019.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang H, Zhang Y, Gallegos V, Sorrelle N, Zaid MM, Toombs J, Du W, Wright S, Hagopian M, Wang Z, Hosein AN, Sathe AA, Xing C, Koay EJ, Driscoll KE, Brekken RA. Targeting TGFβR2-mutant tumors exposes vulnerabilities to stromal TGFβ blockade in pancreatic cancer. EMBO Mol Med 11: e10515, 2019. doi: 10.15252/emmm.201910515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Idda ML, McClusky WG, Lodde V, Munk R, Abdelmohsen K, Rossi M, Gorospe M. Survey of senescent cell markers with age in human tissues. Aging (Albany NY) 12: 4052–4066, 2020. doi: 10.18632/aging.102903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ikenaga N, Ohuchida K, Mizumoto K, Cui L, Kayashima T, Morimatsu K, Moriyama T, Nakata K, Fujita H, Tanaka M. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology 139: 1041–1051.e8, 2010. doi: 10.1053/j.gastro.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 119.Ilangumaran S, Finan D, La Rose J, Raine J, Silverstein A, De Sepulveda P, Rottapel R. A positive regulatory role for suppressor of cytokine signaling 1 in IFN-gamma-induced MHC class II expression in fibroblasts. J Immunol 169: 5010–5020, 2002. doi: 10.4049/jimmunol.169.9.5010. [DOI] [PubMed] [Google Scholar]
- 120.Incio J, Suboj P, Chin SM, Vardam-Kaur T, Liu H, Hato T, Babykutty S, Chen I, Deshpande V, Jain RK, Fukumura D. Metformin reduces desmoplasia in pancreatic cancer by reprogramming stellate cells and tumor-associated macrophages. PLoS One 10: e0141392, 2015. doi: 10.1371/journal.pone.0141392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ireland L, Santos A, Ahmed MS, Rainer C, Nielsen SR, Quaranta V, Weyer-Czernilofsky U, Engle DD, Perez-Mancera PA, Coupland SE, Taktak A, Bogenrieder T, Tuveson DA, Campbell F, Schmid MC, Mielgo A. Chemoresistance in pancreatic cancer is driven by stroma-derived insulin-like growth factors. Cancer Res 76: 6851–6863, 2016. doi: 10.1158/0008-5472.CAN-16-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002. doi: 10.1172/JCI0215518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jackson HW, Fischer JR, Zanotelli VRT, Ali HR, Mechera R, Soysal SD, Moch H, Muenst S, Varga Z, Weber WP, Bodenmiller B. The single-cell pathology landscape of breast cancer. Nature 578: 615–620, 2020. doi: 10.1038/s41586-019-1876-x. [DOI] [PubMed] [Google Scholar]
- 124.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62: 112–120, 2013. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jailkhani N, Ingram JR, Rashidian M, Rickelt S, Tian C, Mak H, Jiang Z, Ploegh HL, Hynes RO. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. [Correction in Proc Natl Acad Sci USA 116: 18745, 2019.] Proc Natl Acad Sci USA 116: 14181–14190, 2019. doi: 10.1073/pnas.1817442116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, Nywening TM, Hawkins WG, Shapiro IM, Weaver DT, Pachter JA, Wang-Gillam A, DeNardo DG. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med 22: 851–860, 2016. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jiménez-Sánchez A, Memon D, Pourpe S, Veeraraghavan H, Li Y, Vargas HA, Gill MB, Park KJ, Zivanovic O, Konner J, Ricca J, Zamarin D, Walther T, Aghajanian C, Wolchok JD, Sala E, Merghoub T, Snyder A, Miller ML. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 170: 927–938.e20, 2017. doi: 10.1016/j.cell.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Wang J, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 4: 1795, 2013. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kalluri R The biology and function of fibroblasts in cancer. Nat Rev Cancer 16: 582–598, 2016. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 130.Kanai AJ, Konieczko EM, Bennett RG, Samuel CS, Royce SG. Relaxin and fibrosis: emerging targets, challenges, and future directions. Mol Cell Endocrinol 487: 66–74, 2019. doi: 10.1016/j.mce.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827, 2005. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449: 557–563, 2007. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 133.Kaur A, Ecker BL, Douglass SM, Kugel CH III, Webster MR, Almeida FV, Somasundaram R, Hayden J, Ban E, Ahmadzadeh H, Franco-Barraza J, Shah N, Mellis IA, Keeney F, Kossenkov A, Tang HY, Yin X, Liu Q, Xu X, Fane M, Brafford P, Herlyn M, Speicher DW, Wargo JA, Tetzlaff MT, Haydu LE, Raj A, Shenoy V, Cukierman E, Weeraratna AT. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov 9: 64–81, 2019. doi: 10.1158/2159-8290.CD-18-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH III, Dang VM, Appleton J, O’Connell MP, Cheng P, Valiga AA, Morissette R, McDonnell NB, Ferrucci L, Kossenkov AV, Meeth K, Tang HY, Yin X, Wood WH III, Lehrmann E, Becker KG, Flaherty KT, Frederick DT, Wargo JA, Cooper ZA, Tetzlaff MT, Hudgens C, Aird KM, Zhang R, Xu X, Liu Q, Bartlett E, Karakousis G, Eroglu Z, Lo RS, Chan M, Menzies AM, Long GV, Johnson DB, Sosman J, Schilling B, Schadendorf D, Speicher DW, Bosenberg M, Ribas A, Weeraratna AT. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. [Correction in Nature 537: 254, 2016.] Nature 532: 250–254, 2016. doi: 10.1038/nature17392. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 135.Kidd S, Spaeth E, Watson K, Burks J, Lu H, Klopp A, Andreeff M, Marini FC. Origins of the tumor microenvironment: quantitative assessment of adipose-derived and bone marrow-derived stroma. PLoS One 7: e30563, 2012. doi: 10.1371/journal.pone.0030563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, Zalupski MM, Simeone DM. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res 20: 5937–5945, 2014. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kinchen J, Chen HH, Parikh K, Antanaviciute A, Jagielowicz M, Fawkner-Corbett D, Ashley N, Cubitt L, Mellado-Gomez E, Attar M, Sharma E, Wills Q, Bowden R, Richter FC, Ahern D, Puri KD, Henault J, Gervais F, Koohy H, Simmons A. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175: 372–386.e17, 2018. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, Lederer DJ, Nathan SD, Pereira CA, Sahn SA, Sussman R, Swigris JJ, Noble PW; ASCEND Study Group . A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 370: 2083–2092, 2014. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 139.Kisseleva T, Cong M, Paik Y, Scholten D, Jiang C, Benner C, Iwaisako K, Moore-Morris T, Scott B, Tsukamoto H, Evans SM, Dillmann W, Glass CK, Brenner DA. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci USA 109: 9448–9453, 2012. doi: 10.1073/pnas.1201840109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ko AH, LoConte N, Tempero MA, Walker EJ, Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci DV, Venook AP, Kindler HL. A phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 45: 370–375, 2016. doi: 10.1097/MPA.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci USA 107: 20009–20014, 2010. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Koliaraki V, Pasparakis M, Kollias G. IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer. J Exp Med 212: 2235–2251, 2015. doi: 10.1084/jem.20150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kollias G, Papadaki P, Apparailly F, Vervoordeldonk MJ, Holmdahl R, Baumans V, Desaintes C, Di Santo J, Distler J, Garside P, Hegen M, Huizinga TW, Jüngel A, Klareskog L, McInnes I, Ragoussis I, Schett G, Hart B, Tak PP, Toes R, van den Berg W, Wurst W, Gay S. Animal models for arthritis: innovative tools for prevention and treatment. Ann Rheum Dis 70: 1357–1362, 2011. doi: 10.1136/ard.2010.148551. [DOI] [PubMed] [Google Scholar]
- 144.Kozono S, Ohuchida K, Eguchi D, Ikenaga N, Fujiwara K, Cui L, Mizumoto K, Tanaka M. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res 73: 2345–2356, 2013. doi: 10.1158/0008-5472.CAN-12-3180. [DOI] [PubMed] [Google Scholar]
- 145.Krall JA, Reinhardt F, Mercury OA, Pattabiraman DR, Brooks MW, Dougan M, Lambert AW, Bierie B, Ploegh HL, Dougan SK, Weinberg RA. The systemic response to surgery triggers the outgrowth of distant immune-controlled tumors in mouse models of dormancy. Sci Transl Med 10: eaan3464, 2018. doi: 10.1126/scitranslmed.aan3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 330: 827–830, 2010. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 147.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA 98: 12072–12077, 2001. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031, 2008. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 149.Kuilman T, Peeper DS. Senescence-messaging secretome: SMS-ing cellular stress. Nat Rev Cancer 9: 81–94, 2009. doi: 10.1038/nrc2560. [DOI] [PubMed] [Google Scholar]
- 150.Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA, Mulligan C, Nam B, Hockstein N, Denstman F, Shakamuri S, Speicher DW, Weeraratna AT, Chao T, Vonderheide RH, Languino LR, Ordentlich P, Liu Q, Xu X, Lo A, Puré E, Zhang C, Loboda A, Sepulveda MA, Snyder LA, Gabrilovich DI. Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 32: 654–668.e5, 2017. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Labernadie A, Kato T, Brugués A, Serra-Picamal X, Derzsi S, Arwert E, Weston A, González-Tarragó V, Elosegui-Artola A, Albertazzi L, Alcaraz J, Roca-Cusachs P, Sahai E, Trepat X. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat Cell Biol 19: 224–237, 2017. doi: 10.1038/ncb3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 + T Cells to protect tumour cells. Nat Commun 9: 948, 2018. doi: 10.1038/s41467-018-03347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Laklai H, Miroshnikova YA, Pickup MW, Collisson EA, Kim GE, Barrett AS, Hill RC, Lakins JN, Schlaepfer DD, Mouw JK, LeBleu VS, Roy N, Novitskiy SV, Johansen JS, Poli V, Kalluri R, Iacobuzio-Donahue CA, Wood LD, Hebrok M, Hansen K, Moses HL, Weaver VM. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat Med 22: 497–505, 2016. doi: 10.1038/nm.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, Bassez A, Decaluwé H, Pircher A, Van den Eynde K, Weynand B, Verbeken E, De Leyn P, Liston A, Vansteenkiste J, Carmeliet P, Aerts S, Thienpont B. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 24: 1277–1289, 2018. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 155.Langer EM, Allen-Petersen BL, King SM, Kendsersky ND, Turnidge MA, Kuziel GM, Riggers R, Samatham R, Amery TS, Jacques SL, Sheppard BC, Korkola JE, Muschler JL, Thibault G, Chang YH, Gray JW, Presnell SC, Nguyen DG, Sears RC. Modeling tumor phenotypes in vitro with three-dimensional bioprinting. Cell Rep 26: 608–623.e6, 2019. doi: 10.1016/j.celrep.2018.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Laroui H, Ingersoll SA, Liu HC, Baker MT, Ayyadurai S, Charania MA, Laroui F, Yan Y, Sitaraman SV, Merlin D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS One 7: e32084, 2012. doi: 10.1371/journal.pone.0032084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lau EY, Lo J, Cheng BY, Ma MK, Lee JM, Ng JK, Chai S, Lin CH, Tsang SY, Ma S, Ng IO, Lee TK. Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/HEY1 signaling. Cell Rep 15: 1175–1189, 2016. doi: 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 158.Lazard D, Sastre X, Frid MG, Glukhova MA, Thiery JP, Koteliansky VE. Expression of smooth muscle-specific proteins in myoepithelium and stromal myofibroblasts of normal and malignant human breast tissue. Proc Natl Acad Sci USA 90: 999–1003, 1993. doi: 10.1073/pnas.90.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest 120: 3340–3349, 2010. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lee JH, Kim SK, Khawar IA, Jeong SY, Chung S, Kuh HJ. Microfluidic co-culture of pancreatic tumor spheroids with stellate cells as a novel 3D model for investigation of stroma-mediated cell motility and drug resistance. J Exp Clin Cancer Res 37: 4, 2018. doi: 10.1186/s13046-017-0654-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee JJ, Perera RM, Wang H, Wu DC, Liu XS, Han S, Fitamant J, Jones PD, Ghanta KS, Kawano S, Nagle JM, Deshpande V, Boucher Y, Kato T, Chen JK, Willmann JK, Bardeesy N, Beachy PA. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc Natl Acad Sci USA 111: E3091–E3100, 2014. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lee JW, Stone ML, Porrett PM, Thomas SK, Komar CA, Li JH, Delman D, Graham K, Gladney WL, Hua X, Black TA, Chien AL, Majmundar KS, Thompson JC, Yee SS, O’Hara MH, Aggarwal C, Xin D, Shaked A, Gao M, Liu D, Borad MJ, Ramanathan RK, Carpenter EL, Ji A, de Beer MC, de Beer FC, Webb NR, Beatty GL. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567: 249–252, 2019. doi: 10.1038/s41586-019-1004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lenos KJ, Miedema DM, Lodestijn SC, Nijman LE, van den Bosch T, Romero Ros X, Lourenço FC, Lecca MC, van der Heijden M, van Neerven SM, van Oort A, Leveille N, Adam RS, de Sousa E Melo F, Otten J, Veerman P, Hypolite G, Koens L, Lyons SK, Stassi G, Winton DJ, Medema JP, Morrissey E, Bijlsma MF, Vermeulen L. Stem cell functionality is microenvironmentally defined during tumour expansion and therapy response in colon cancer. Nat Cell Biol 20: 1193–1202, 2018. doi: 10.1038/s41556-018-0179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Leonardi GC, Accardi G, Monastero R, Nicoletti F, Libra M. Ageing: from inflammation to cancer. Immun Ageing 15: 1, 2018. doi: 10.1186/s12979-017-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139: 891–906, 2009. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, Kong SL, Chua C, Hon LK, Tan WS, Wong M, Choi PJ, Wee LJK, Hillmer AM, Tan IB, Robson P, Prabhakar S. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. [Correction in Nat Genet 50: 1754, 2018.] Nat Genet 49: 708–718, 2017. doi: 10.1038/ng.3818. [DOI] [PubMed] [Google Scholar]
- 167.Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T, Richman LP, Lin JH, Sun YH, Rech AJ, Balli D, Hay CA, Sela Y, Merrell AJ, Liudahl SM, Gordon N, Norgard RJ, Yuan S, Yu S, Chao T, Ye S, Eisinger-Mathason TSK, Faryabi RB, Tobias JW, Lowe SW, Coussens LM, Wherry EJ, Vonderheide RH, Stanger BZ. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity 49: 178–193.e7, 2018. doi: 10.1016/j.immuni.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial-mesenchymal transition in liver injury. Proc Natl Acad Sci USA 110: 2324–2329, 2013. doi: 10.1073/pnas.1214136110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Z, Zhang W, Chen Y, Guo W, Zhang J, Tang H, Xu Z, Zhang H, Tao Y, Wang F, Jiang Y, Sun FL, Mao Z. Impaired DNA double-strand break repair contributes to the age-associated rise of genomic instability in humans. Cell Death Differ 23: 1765–1777, 2016. doi: 10.1038/cdd.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Liu T, De Los Santos FG, Phan SH. The bleomycin model of pulmonary fibrosis. Methods Mol Biol 1627: 27–42, 2017. doi: 10.1007/978-1-4939-7113-8_2. [DOI] [PubMed] [Google Scholar]
- 171.Liu Z, Wang L, Welch JD, Ma H, Zhou Y, Vaseghi HR, Yu S, Wall JB, Alimohamadi S, Zheng M, Yin C, Shen W, Prins JF, Liu J, Qian L. Single-cell transcriptomics reconstructs fate conversion from fibroblast to cardiomyocyte. Nature 551: 100–104, 2017. doi: 10.1038/nature24454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lo A, Li CP, Buza EL, Blomberg R, Govindaraju P, Avery D, Monslow J, Hsiao M, Puré E. Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. JCI Insight 2: e92232, 2017. doi: 10.1172/jci.insight.92232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 153: 1194–1217, 2013. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lua I, Li Y, Pappoe LS, Asahina K. Myofibroblastic conversion and regeneration of mesothelial cells in peritoneal and liver fibrosis. Am J Pathol 185: 3258–3273, 2015. doi: 10.1016/j.ajpath.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lynch MD, Watt FM. Fibroblast heterogeneity: implications for human disease. J Clin Invest 128: 26–35, 2018. doi: 10.1172/JCI93555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, Rae Z, Hernandez JM, Davis JL, Martin SP, Kleiner DE, Hewitt SM, Ylaya K, Wood BJ, Greten TF, Wang XW. Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell 36: 418–430.e6, 2019. doi: 10.1016/j.ccell.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mace TA, Shakya R, Pitarresi JR, Swanson B, McQuinn CW, Loftus S, Nordquist E, Cruz-Monserrate Z, Yu L, Young G, Zhong X, Zimmers TA, Ostrowski MC, Ludwig T, Bloomston M, Bekaii-Saab T, Lesinski GB. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 67: 320–332, 2018. doi: 10.1136/gutjnl-2016-311585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Madden LR, Nguyen TV, Garcia-Mojica S, Shah V, Le AV, Peier A, Visconti R, Parker EM, Presnell SC, Nguyen DG, Retting KN. Bioprinted 3D primary human intestinal tissues model aspects of native physiology and ADME/Tox functions. iScience 2: 156–167, 2018. doi: 10.1016/j.isci.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Madsen CD, Pedersen JT, Venning FA, Singh LB, Moeendarbary E, Charras G, Cox TR, Sahai E, Erler JT. Hypoxia and loss of PHD2 inactivate stromal fibroblasts to decrease tumour stiffness and metastasis. EMBO Rep 16: 1394–1408, 2015. doi: 10.15252/embr.201540107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Mahmoudi S, Mancini E, Xu L, Moore A, Jahanbani F, Hebestreit K, Srinivasan R, Li X, Devarajan K, Prélot L, Ang CE, Shibuya Y, Benayoun BA, Chang ALS, Wernig M, Wysocka J, Longaker MT, Snyder MP, Brunet A. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 574: 553–558, 2019. doi: 10.1038/s41586-019-1658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Makpol S, Abidin AZ, Sairin K, Mazlan M, Top GM, Ngah WZ. γ-Tocotrienol prevents oxidative stress-induced telomere shortening in human fibroblasts derived from different aged individuals. Oxid Med Cell Longev 3: 35–43, 2010. doi: 10.4161/oxim.3.1.9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Malanchi I, Santamaria-Martínez A, Susanto E, Peng H, Lehr HA, Delaloye JF, Huelsken J. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481: 85–89, 2012. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 183.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res 71: 3453–3458, 2011. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 554: 544–548, 2018. doi: 10.1038/nature25501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Markham MJ, Wachter K, Agarwal N, Bertagnolli MM, Chang SM, Dale W, Diefenbach CSM, Rodriguez-Galindo C, George DJ, Gilligan TD, Harvey RD, Johnson ML, Kimple RJ, Knoll MA, LoConte N, Maki RG, Meisel JL, Meyerhardt JA, Pennell NA, Rocque GB, Sabel MS, Schilsky RL, Schneider BJ, Tap WD, Uzzo RG, Westin SN. Clinical Cancer Advances 2020: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol 38: 1081, 2020. doi: 10.1200/JCO.19.03141. [DOI] [PubMed] [Google Scholar]
- 186.Marsh E, Gonzalez DG, Lathrop EA, Boucher J, Greco V. Positional stability and membrane occupancy define skin fibroblast homeostasis in vivo. Cell 175: 1620–1633.e13, 2018. doi: 10.1016/j.cell.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Martin JD, Panagi M, Wang C, Khan TT, Martin MR, Voutouri C, Toh K, Papageorgis P, Mpekris F, Polydorou C, Ishii G, Takahashi S, Gotohda N, Suzuki T, Wilhelm ME, Melo VA, Quader S, Norimatsu J, Lanning RM, Kojima M, Stuber MD, Stylianopoulos T, Kataoka K, Cabral H. Dexamethasone increases cisplatin-loaded nanocarrier delivery and efficacy in metastatic breast cancer by normalizing the tumor microenvironment. ACS Nano 13: 6396–6408, 2019. doi: 10.1021/acsnano.8b07865. [DOI] [PubMed] [Google Scholar]
- 188.Martin JD, Seano G, Jain RK. Normalizing function of tumor vessels: progress, opportunities, and challenges. Annu Rev Physiol 81: 505–534, 2019. doi: 10.1146/annurev-physiol-020518-114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, Lisanti MP. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 9: 3256–3276, 2010. doi: 10.4161/cc.9.16.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mathew E, Brannon AL, Del Vecchio A, Garcia PE, Penny MK, Kane KT, Vinta A, Buckanovich RJ, di Magliano MP. Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia 18: 142–151, 2016. doi: 10.1016/j.neo.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Mathew E, Collins MA, Fernandez-Barrena MG, Holtz AM, Yan W, Hogan JO, Tata Z, Allen BL, Fernandez-Zapico ME, di Magliano MP. The transcription factor GLI1 modulates the inflammatory response during pancreatic tissue remodeling. J Biol Chem 289: 27727–27743, 2014. doi: 10.1074/jbc.M114.556563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mathew E, Zhang Y, Holtz AM, Kane KT, Song JY, Allen BL, Pasca di Magliano M. Dosage-dependent regulation of pancreatic cancer growth and angiogenesis by hedgehog signaling. Cell Rep 9: 484–494, 2014. doi: 10.1016/j.celrep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Maurer C, Holmstrom SR, He J, Laise P, Su T, Ahmed A, Hibshoosh H, Chabot JA, Oberstein PE, Sepulveda AR, Genkinger JM, Zhang J, Iuga AC, Bansal M, Califano A, Olive KP. Experimental microdissection enables functional harmonisation of pancreatic cancer subtypes. Gut 68: 1034–1043, 2019. doi: 10.1136/gutjnl-2018-317706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.McCloskey CW, Cook DP, Kelly BS, Azzi F, Allen CH, Forsyth A, Upham J, Rayner KJ, Gray DA, Boyd RW, Murugkar S, Lo B, Trudel D, Senterman MK, Vanderhyden BC. Metformin abrogates age-associated ovarian fibrosis. Clin Cancer Res 26: 632–642, 2020. doi: 10.1158/1078-0432.CCR-19-0603. [DOI] [PubMed] [Google Scholar]
- 195.McDonald LT, Russell DL, Kelly RR, Xiong Y, Motamarry A, Patel RK, Jones JA, Watson PM, Turner DP, Watson DK, Soloff AC, Findlay VJ, LaRue AC. Hematopoietic stem cell-derived cancer-associated fibroblasts are novel contributors to the pro-tumorigenic microenvironment. Neoplasia 17: 434–448, 2015. doi: 10.1016/j.neo.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, Pradere JP, Schwabe RF. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun 4: 2823, 2013. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res 68: 4331–4339, 2008. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME, Lengyel E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov 2: 1100–1108, 2012. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mizoguchi F, Slowikowski K, Wei K, Marshall JL, Rao DA, Chang SK, Nguyen HN, Noss EH, Turner JD, Earp BE, Blazar PE, Wright J, Simmons BP, Donlin LT, Kalliolias GD, Goodman SM, Bykerk VP, Ivashkiv LB, Lederer JA, Hacohen N, Nigrovic PA, Filer A, Buckley CD, Raychaudhuri S, Brenner MB. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun 9: 789, 2018. doi: 10.1038/s41467-018-02892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Moffitt RA, Marayati R, Flate EL, Volmar KE, Loeza SG, Hoadley KA, Rashid NU, Williams LA, Eaton SC, Chung AH, Smyla JK, Anderson JM, Kim HJ, Bentrem DJ, Talamonti MS, Iacobuzio-Donahue CA, Hollingsworth MA, Yeh JJ. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet 47: 1168–1178, 2015. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Moinfar F, Man YG, Arnould L, Bratthauer GL, Ratschek M, Tavassoli FA. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res 60: 2562–2566, 2000. [PubMed] [Google Scholar]
- 202.Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev 12: 661–684, 2013. doi: 10.1016/j.arr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 203.Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, Drapek LC, Ly L, Baglini CV, Blaszkowsky LS, Ferrone CR, Parikh AR, Weekes CD, Nipp RD, Kwak EL, Allen JN, Corcoran RB, Ting DT, Faris JE, Zhu AX, Goyal L, Berger DL, Qadan M, Lillemoe KD, Talele N, Jain RK, DeLaney TF, Duda DG, Boucher Y, Fernández-Del Castillo C, Hong TS. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol 5: 1020–1027, 2019. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, Liu IH, Chiou SH, Salahudeen AA, Smith AR, Deutsch BC, Liao L, Zemek AJ, Zhao F, Karlsson K, Schultz LM, Metzner TJ, Nadauld LD, Tseng YY, Alkhairy S, Oh C, Keskula P, Mendoza-Villanueva D, De La Vega FM, Kunz PL, Liao JC, Leppert JT, Sunwoo JB, Sabatti C, Boehm JS, Hahn WC, Zheng GXY, Davis MM, Kuo CJ. Organoid modeling of the tumor immune microenvironment. Cell 175: 1972–1988.e16, 2018. doi: 10.1016/j.cell.2018.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci USA 110: 12325–12330, 2013. doi: 10.1073/pnas.1300415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, Erkan M, Kleeff J, Wilson J, Apte M, Tosolini M, Wilson AS, Delvecchio FR, Bousquet C, Paradis V, Hammel P, Sadanandam A, Kocher HM. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol 248: 51–65, 2019. doi: 10.1002/path.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, Ireland L, Sakai T, Sakai K, Kim YS, Engle D, Campbell F, Palmer D, Ko JH, Tuveson DA, Hirsch E, Mielgo A, Schmid MC. Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. [Correction in Nat Cell Biol 18: 822, 2016.] Nat Cell Biol 18: 549–560, 2016. doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Northey JJ, Przybyla L, Weaver VM. Tissue force programs cell fate and tumor aggression. Cancer Discov 7: 1224–1237, 2017. doi: 10.1158/2159-8290.CD-16-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Novo D, Heath N, Mitchell L, Caligiuri G, MacFarlane A, Reijmer D, Charlton L, Knight J, Calka M, McGhee E, Dornier E, Sumpton D, Mason S, Echard A, Klinkert K, Secklehner J, Kruiswijk F, Vousden K, Macpherson IR, Blyth K, Bailey P, Yin H, Carlin LM, Morton J, Zanivan S, Norman JC. Mutant p53s generate pro-invasive niches by influencing exosome podocalyxin levels. Nat Commun 9: 5069, 2018. doi: 10.1038/s41467-018-07339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR, Resnick MB, Neilson EG, Zeisberg M, Kalluri R. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci USA 108: 16002–16007, 2011. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: its pathophysiologic implications. Blood 108: 2893–2896, 2006. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 212.Öhlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med 211: 1503–1523, 2014. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio II, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 214: 579–596, 2017. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Olivares O, Mayers JR, Gouirand V, Torrence ME, Gicquel T, Borge L, Lac S, Roques J, Lavaut MN, Berthezène P, Rubis M, Secq V, Garcia S, Moutardier V, Lombardo D, Iovanna JL, Tomasini R, Guillaumond F, Vander Heiden MG, Vasseur S. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat Commun 8: 16031, 2017. doi: 10.1038/ncomms16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324: 1457–1461, 2009. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Olivieri F, Prattichizzo F, Grillari J, Balistreri CR. Cellular senescence and inflammaging in age-related diseases. Mediators Inflamm 2018: 9076485, 2018. doi: 10.1155/2018/9076485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR, Kuo CJ. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15: 701–706, 2009. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 121: 335–348, 2005. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 219.Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, Downey RJ, Manova-Todorova K, Brogi E, Massagué J. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med 17: 867–874, 2011. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Oskarsson T, Massagué J. Extracellular matrix players in metastatic niches. EMBO J 31: 254–256, 2012. doi: 10.1038/emboj.2011.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. [Correction in Cancer Cell 28: 831–833, 2015.] Cancer Cell 25: 719–734, 2014. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pallangyo CK, Ziegler PK, Greten FR. IKKβ acts as a tumor suppressor in cancer-associated fibroblasts during intestinal tumorigenesis. J Exp Med 212: 2253–2266, 2015. doi: 10.1084/jem.20150576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Panagi M, Voutouri C, Mpekris F, Papageorgis P, Martin MR, Martin JD, Demetriou P, Pierides C, Polydorou C, Stylianou A, Louca M, Koumas L, Costeas P, Kataoka K, Cabral H, Stylianopoulos T. TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity. Theranostics 10: 1910–1922, 2020. doi: 10.7150/thno.36936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Panwar P, Lamour G, Mackenzie NC, Yang H, Ko F, Li H, Brömme D. Changes in structural-mechanical properties and degradability of collagen during aging-associated modifications. J Biol Chem 290: 23291–23306, 2015. doi: 10.1074/jbc.M115.644310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Papageorgis P, Polydorou C, Mpekris F, Voutouri C, Agathokleous E, Kapnissi-Christodoulou CP, Stylianopoulos T. Tranilast-induced stress alleviation in solid tumors improves the efficacy of chemo- and nanotherapeutics in a size-independent manner. Sci Rep 7: 46140, 2017. doi: 10.1038/srep46140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Patel AP, Tirosh I, Trombetta JJ, Shalek AK, Gillespie SM, Wakimoto H, Cahill DP, Nahed BV, Curry WT, Martuza RL, Louis DN, Rozenblatt-Rosen O, Suvà ML, Regev A, Bernstein BE. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344: 1396–1401, 2014. doi: 10.1126/science.1254257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL, Platzer P, Eng C. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med 357: 2543–2551, 2007. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 228.Paulsson J, Micke P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin Cancer Biol 25: 61–68, 2014. doi: 10.1016/j.semcancer.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 229.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle 8: 3984–4001, 2009. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 230.Pazolli E, Alspach E, Milczarek A, Prior J, Piwnica-Worms D, Stewart SA. Chromatin remodeling underlies the senescence-associated secretory phenotype of tumor stromal fibroblasts that supports cancer progression. Cancer Res 72: 2251–2261, 2012. doi: 10.1158/0008-5472.CAN-11-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Peng J, Sun BF, Chen CY, Zhou JY, Chen YS, Chen H, Liu L, Huang D, Jiang J, Cui GS, Yang Y, Wang W, Guo D, Dai M, Guo J, Zhang T, Liao Q, Liu Y, Zhao YL, Han DL, Zhao Y, Yang YG, Wu W. Single-cell RNA-seq highlights intra-tumoral heterogeneity and malignant progression in pancreatic ductal adenocarcinoma. [Correction in Cell Res 29: 777, 2019.] Cell Res 29: 725–738, 2019. doi: 10.1038/s41422-019-0195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Philippeos C, Telerman SB, Oulès B, Pisco AO, Shaw TJ, Elgueta R, Lombardi G, Driskell RR, Soldin M, Lynch MD, Watt FM. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J Invest Dermatol 138: 811–825, 2018. doi: 10.1016/j.jid.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15: 1243–1253, 2014. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pickup MW, Owens P, Gorska AE, Chytil A, Ye F, Shi C, Weaver VM, Kalluri R, Moses HL, Novitskiy SV. Development of aggressive pancreatic ductal adenocarcinomas depends on granulocyte colony stimulating factor secretion in carcinoma cells. Cancer Immunol Res 5: 718–729, 2017. doi: 10.1158/2326-6066.CIR-16-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pidsley R, Lawrence MG, Zotenko E, Niranjan B, Statham A, Song J, Chabanon RM, Qu W, Wang H, Richards M, Nair SS, Armstrong NJ, Nim HT, Papargiris M, Balanathan P, French H, Peters T, Norden S, Ryan A, Pedersen J, Kench J, Daly RJ, Horvath LG, Stricker P, Frydenberg M, Taylor RA, Stirzaker C, Risbridger GP, Clark SJ. Enduring epigenetic landmarks define the cancer microenvironment. Genome Res 28: 625–638, 2018. doi: 10.1101/gr.229070.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Pietras K, Pahler J, Bergers G, Hanahan D. Functions of paracrine PDGF signaling in the proangiogenic tumor stroma revealed by pharmacological targeting. PLoS Med 5: e19, 2008. doi: 10.1371/journal.pmed.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Pinho AV, Van Bulck M, Chantrill L, Arshi M, Sklyarova T, Herrmann D, Vennin C, Gallego-Ortega D, Mawson A, Giry-Laterriere M, Magenau A, Leuckx G, Baeyens L, Gill AJ, Phillips P, Timpson P, Biankin AV, Wu J, Rooman I. ROBO2 is a stroma suppressor gene in the pancreas and acts via TGF-β signalling. Nat Commun 9: 5083, 2018. doi: 10.1038/s41467-018-07497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Polydorou C, Mpekris F, Papageorgis P, Voutouri C, Stylianopoulos T. Pirfenidone normalizes the tumor microenvironment to improve chemotherapy. Oncotarget 8: 24506–24517, 2017. doi: 10.18632/oncotarget.15534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21: 418–429, 2012. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, Deschler DG, Varvares MA, Mylvaganam R, Rozenblatt-Rosen O, Rocco JW, Faquin WC, Lin DT, Regev A, Bernstein BE. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171: 1611–1624.e24, 2017. doi: 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Qiu W, Hu M, Sridhar A, Opeskin K, Fox S, Shipitsin M, Trivett M, Thompson ER, Ramakrishna M, Gorringe KL, Polyak K, Haviv I, Campbell IG. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat Genet 40: 650–655, 2008. doi: 10.1038/ng.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 19: 257–272, 2011. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ramanathan RK, McDonough SL, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, Thumar J, Chiorean EG, Shields AF, Behl D, Mehan PT, Gaur R, Seery T, Guthrie KA, Hochster HS. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J Clin Oncol 37: 1062–1069, 2019. doi: 10.1200/JCO.18.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Rath N, Morton JP, Julian L, Helbig L, Kadir S, McGhee EJ, Anderson KI, Kalna G, Mullin M, Pinho AV, Rooman I, Samuel MS, Olson MF. ROCK signaling promotes collagen remodeling to facilitate invasive pancreatic ductal adenocarcinoma tumor cell growth. EMBO Mol Med 9: 198–218, 2017. doi: 10.15252/emmm.201606743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rath N, Munro J, Cutiongco MF, Jagiełło A, Gadegaard N, McGarry L, Unbekandt M, Michalopoulou E, Kamphorst JJ, Sumpton D, Mackay G, Vennin C, Pajic M, Timpson P, Olson MF. Rho kinase inhibition by AT13148 blocks pancreatic ductal adenocarcinoma invasion and tumor growth. Cancer Res 78: 3321–3336, 2018. doi: 10.1158/0008-5472.CAN-17-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Reardon DA, Akabani G, Coleman RE, Friedman AH, Friedman HS, Herndon JE II, McLendon RE, Pegram CN, Provenzale JM, Quinn JA, Rich JN, Vredenburgh JJ, Desjardins A, Guruangan S, Badruddoja M, Dowell JM, Wong TZ, Zhao XG, Zalutsky MR, Bigner DD. Salvage radioimmunotherapy with murine iodine-131-labeled antitenascin monoclonal antibody 81C6 for patients with recurrent primary and metastatic malignant brain tumors: phase II study results. [Correction in J Clin Oncol 24: 1484, 2006.] J Clin Oncol 24: 115–122, 2006. doi: 10.1200/JCO.2005.03.4082. [DOI] [PubMed] [Google Scholar]
- 247.Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, Wang Y, Wang T, Hou Y. Carcinoma-associated fibroblasts promote the stemness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 8: 3932–3948, 2018. doi: 10.7150/thno.25541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW, Westphalen CB, Kitajewski J, Fernandez-Barrena MG, Fernandez-Zapico ME, Iacobuzio-Donahue C, Olive KP, Stanger BZ. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25: 735–747, 2014. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, Kim DS, Kolb M, Nicholson AG, Noble PW, Selman M, Taniguchi H, Brun M, Le Maulf F, Girard M, Stowasser S, Schlenker-Herceg R, Disse B, Collard HR; INPULSIS Trial Investigators . Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 370: 2071–2082, 2014. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 250.Rinkevich Y, Mori T, Sahoo D, Xu PX, Bermingham JR Jr, Weissman IL. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nat Cell Biol 14: 1251–1260, 2012. doi: 10.1038/ncb2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science 348: aaa2151, 2015. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Roberts EW, Deonarine A, Jones JO, Denton AE, Feig C, Lyons SK, Espeli M, Kraman M, McKenna B, Wells RJ, Zhao Q, Caballero OL, Larder R, Coll AP, O’Rahilly S, Brindle KM, Teichmann SA, Tuveson DA, Fearon DT. Depletion of stromal cells expressing fibroblast activation protein-α from skeletal muscle and bone marrow results in cachexia and anemia. J Exp Med 210: 1137–1151, 2013. doi: 10.1084/jem.20122344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA 108: E1475–E1483, 2011. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rognoni E, Pisco AO, Hiratsuka T, Sipilä KH, Belmonte JM, Mobasseri SA, Philippeos C, Dilão R, Watt FM. Fibroblast state switching orchestrates dermal maturation and wound healing. Mol Syst Biol 14: e8174, 2018. doi: 10.15252/msb.20178174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Rønnov-Jessen L, Petersen OW. Induction of alpha-smooth muscle actin by transforming growth factor-beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 68: 696–707, 1993. [PubMed] [Google Scholar]
- 256.Ruhland MK, Loza AJ, Capietto AH, Luo X, Knolhoff BL, Flanagan KC, Belt BA, Alspach E, Leahy K, Luo J, Schaffer A, Edwards JR, Longmore G, Faccio R, DeNardo DG, Stewart SA. Stromal senescence establishes an immunosuppressive microenvironment that drives tumorigenesis. Nat Commun 7: 11762, 2016. doi: 10.1038/ncomms11762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Saadi A, Shannon NB, Lao-Sirieix P, O’Donovan M, Walker E, Clemons NJ, Hardwick JS, Zhang C, Das M, Save V, Novelli M, Balkwill F, Fitzgerald RC. Stromal genes discriminate preinvasive from invasive disease, predict outcome, and highlight inflammatory pathways in digestive cancers. Proc Natl Acad Sci USA 107: 2177–2182, 2010. doi: 10.1073/pnas.0909797107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR, Hunter T, Hynes RO, Jain RK, Janowitz T, Jorgensen C, Kimmelman AC, Kolonin MG, Maki RG, Powers RS, Puré E, Ramirez DC, Scherz-Shouval R, Sherman MH, Stewart S, Tlsty TD, Tuveson DA, Watt FM, Weaver V, Weeraratna AT, Werb Z. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 20: 174–186, 2020. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Salmon H, Franciszkiewicz K, Damotte D, Dieu-Nosjean MC, Validire P, Trautmann A, Mami-Chouaib F, Donnadieu E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest 122: 899–910, 2012. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Salzer MC, Lafzi A, Berenguer-Llergo A, Youssif C, Castellanos A, Solanas G, Peixoto FO, Stephan-Otto Attolini C, Prats N, Aguilera M, Martín-Caballero J, Heyn H, Benitah SA. Identity noise and adipogenic traits characterize dermal fibroblast aging. Cell 175: 1575–1590.e22, 2018. doi: 10.1016/j.cell.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 261.Sandoval P, Jiménez-Heffernan JA, Rynne-Vidal Á, Pérez-Lozano ML, Gilsanz Á, Ruiz-Carpio V, Reyes R, García-Bordas J, Stamatakis K, Dotor J, Majano PL, Fresno M, Cabañas C, López-Cabrera M. Carcinoma-associated fibroblasts derive from mesothelial cells via mesothelial-to-mesenchymal transition in peritoneal metastasis. J Pathol 231: 517–531, 2013. doi: 10.1002/path.4281. [DOI] [PubMed] [Google Scholar]
- 262.Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M, Stemmer SM, Whitesell L, Lindquist S. The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell 158: 564–578, 2014. doi: 10.1016/j.cell.2014.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Scholten D, Trebicka J, Liedtke C, Weiskirchen R. The carbon tetrachloride model in mice. Lab Anim 49, Suppl: 4–11, 2015. doi: 10.1177/0023677215571192. [DOI] [PubMed] [Google Scholar]
- 264.Shani O, Raz Y, Megides O, Shacham H, Cohen N, Silverbush D, Monteran L, Sharan R, Tsarfaty I, Erez N. Evolution of metastases-associated fibroblasts in the lung microenvironment is driven by stage-specific transcriptional plasticity (Preprint). bioRxiv 778936, 2019. doi: 10.1101/778936. [DOI] [PMC free article] [PubMed]
- 265.Shao C, Tu C, Cheng X, Xu Z, Wang X, Shen J, Chai K, Chen W. Inflammatory and senescent phenotype of pancreatic stellate cells induced by Sqstm1 downregulation facilitates pancreatic cancer progression. Int J Biol Sci 15: 1020–1029, 2019. doi: 10.7150/ijbs.27825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S, Martin P, Tseng TW, Dawson DW, Donahue TR, Masamune A, Shimosegawa T, Apte MV, Wilson JS, Ng B, Lau SL, Gunton JE, Wahl GM, Hunter T, Drebin JA, O’Dwyer PJ, Liddle C, Tuveson DA, Downes M, Evans RM. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159: 80–93, 2014. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sherman MH, Yu RT, Tseng TW, Sousa CM, Liu S, Truitt ML, He N, Ding N, Liddle C, Atkins AR, Leblanc M, Collisson EA, Asara JM, Kimmelman AC, Downes M, Evans RM. Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci USA 114: 1129–1134, 2017. doi: 10.1073/pnas.1620164114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shi K, Jiang J, Ma T, Xie J, Duan L, Chen R, Song P, Yu Z, Liu C, Zhu Q, Zheng J. Dexamethasone attenuates bleomycin-induced lung fibrosis in mice through TGF-β, Smad3 and JAK-STAT pathway. Int J Clin Exp Med 7: 2645–2650, 2014. [PMC free article] [PubMed] [Google Scholar]
- 269.Shi Y, Gao W, Lytle NK, Huang P, Yuan X, Dann AM, Ridinger-Saison M, DelGiorno KE, Antal CE, Liang G, Atkins AR, Erikson G, Sun H, Meisenhelder J, Terenziani E, Woo G, Fang L, Santisakultarm TP, Manor U, Xu R, Becerra CR, Borazanci E, Von Hoff DD, Grandgenett PM, Hollingsworth MA, Leblanc M, Umetsu SE, Collisson EA, Scadeng M, Lowy AM, Donahue TR, Reya T, Downes M, Evans RM, Wahl GM, Pawson T, Tian R, Hunter T. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569: 131–135, 2019. doi: 10.1038/s41586-019-1130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shin K, Lim A, Zhao C, Sahoo D, Pan Y, Spiekerkoetter E, Liao JC, Beachy PA. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell 26: 521–533, 2014. doi: 10.1016/j.ccell.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Shook BA, Wasko RR, Rivera-Gonzalez GC, Salazar-Gatzimas E, López-Giráldez F, Dash BC, Muñoz-Rojas AR, Aultman KD, Zwick RK, Lei V, Arbiser JL, Miller-Jensen K, Clark DA, Hsia HC, Horsley V. Myofibroblast proliferation and heterogeneity are supported by macrophages during skin repair. Science 362: eaar2971, 2018. doi: 10.1126/science.aar2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sodir N, Kortlever R, Barthet V, Pellegrinet L, Campos T, Kupczak S, Swigart L, Soucek K, Arends M, Littlewood T, Evan G. Myc instructs and maintains pancreatic adenocarcinoma phenotype (Preprint). bioRxiv 556399, 2019. doi: 10.1101/556399. [DOI] [PubMed]
- 273.Somerville TD, Biffi G, Daßler-Plenker J, Hur SK, He XY, Vance KE, Miyabayashi K, Xu Y, Maia-Silva D, Klingbeil O, Demerdash OE, Preall JB, Hollingsworth MA, Egeblad M, Tuveson DA, Vakoc CR. Squamous trans-differentiation of pancreatic cancer cells promotes stromal inflammation. eLife 9: e53381, 2020. doi: 10.7554/eLife.53381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sontheimer-Phelps A, Hassell BA, Ingber DE. Modelling cancer in microfluidic human organs-on-chips. Nat Rev Cancer 19: 65–81, 2019. doi: 10.1038/s41568-018-0104-6. [DOI] [PubMed] [Google Scholar]
- 276.Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. [Correction in Nature 540: 150, 2016.] Nature 536: 479–483, 2016. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Spector I, Honig H, Kawada N, Nagler A, Genin O, Pines M. Inhibition of pancreatic stellate cell activation by halofuginone prevents pancreatic xenograft tumor development. Pancreas 39: 1008–1015, 2010. doi: 10.1097/MPA.0b013e3181da8aa3. [DOI] [PubMed] [Google Scholar]
- 278.Spector I, Zilberstein Y, Lavy A, Nagler A, Genin O, Pines M. Involvement of host stroma cells and tissue fibrosis in pancreatic tumor development in transgenic mice. PLoS One 7: e41833, 2012. doi: 10.1371/journal.pone.0041833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Stairs DB, Bayne LJ, Rhoades B, Vega ME, Waldron TJ, Kalabis J, Klein-Szanto A, Lee JS, Katz JP, Diehl JA, Reynolds AB, Vonderheide RH, Rustgi AK. Deletion of p120-catenin results in a tumor microenvironment with inflammation and cancer that establishes it as a tumor suppressor gene. Cancer Cell 19: 470–483, 2011. doi: 10.1016/j.ccr.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Stoker MG, Shearer M, O’Neill C. Growth inhibition of polyoma-transformed cells by contact with static normal fibroblasts. J Cell Sci 1: 297–310, 1966. [DOI] [PubMed] [Google Scholar]
- 281.Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487: 500–504, 2012. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Strong AL, Pei DT, Hurst CG, Gimble JM, Burow ME, Bunnell BA. Obesity enhances the conversion of adipose-derived stromal/stem cells into carcinoma-associated fibroblast leading to cancer cell proliferation and progression to an invasive phenotype. Stem Cells Int 2017: 9216502, 2017. doi: 10.1155/2017/9216502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y, Munn LL, Jain RK. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc Natl Acad Sci USA 109: 15101–15108, 2012. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Su S, Chen J, Yao H, Liu J, Yu S, Lao L, Wang M, Luo M, Xing Y, Chen F, Huang D, Zhao J, Yang L, Liao D, Su F, Li M, Liu Q, Song E. CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172: 841–856.e16, 2018. doi: 10.1016/j.cell.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 285.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 5: 1640–1646, 2006. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 286.Sung PJ, Rama N, Imbach J, Fiore S, Ducarouge B, Neves D, Chen HW, Bernard D, Yang PC, Bernet A, Depil S, Mehlen P. Cancer-associated fibroblasts produce netrin-1 to control cancer cell plasticity. Cancer Res 79: 3651–3661, 2019. doi: 10.1158/0008-5472.CAN-18-2952. [DOI] [PubMed] [Google Scholar]
- 287.Tabula Muris Consortium Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562: 367–372, 2018. doi: 10.1038/s41586-018-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Tang H, Chu Y, Huang Z, Cai J, Wang Z. The metastatic phenotype shift towards myofibroblast of adipose-derived mesenchymal stem cells promotes ovarian cancer progression. Carcinogenesis 41: 182–193, 2020. doi: 10.1093/carcin/bgz083. [DOI] [PubMed] [Google Scholar]
- 289.Tape CJ, Ling S, Dimitriadi M, McMahon KM, Worboys JD, Leong HS, Norrie IC, Miller CJ, Poulogiannis G, Lauffenburger DA, Jørgensen C. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell 165: 1818, 2016. doi: 10.1016/j.cell.2016.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 554: 538–543, 2018. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 291.Tian C, Clauser KR, Öhlund D, Rickelt S, Huang Y, Gupta M, Mani DR, Carr SA, Tuveson DA, Hynes RO. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci USA 116: 19609–19618, 2019. doi: 10.1073/pnas.1908626116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tirosh I, Izar B, Prakadan SM, Wadsworth MH II, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, Fallahi-Sichani M, Dutton-Regester K, Lin JR, Cohen O, Shah P, Lu D, Genshaft AS, Hughes TK, Ziegler CG, Kazer SW, Gaillard A, Kolb KE, Villani AC, Johannessen CM, Andreev AY, Van Allen EM, Bertagnolli M, Sorger PK, Sullivan RJ, Flaherty KT, Frederick DT, Jané-Valbuena J, Yoon CH, Rozenblatt-Rosen O, Shalek AK, Regev A, Garraway LA. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352: 189–196, 2016. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Tokes AM, Szasz AM, Farkas A, Toth AI, Dank M, Harsanyi L, Molnar BA, Molnar IA, Laszlo Z, Rusz Z, Kulka J. Stromal matrix protein expression following preoperative systemic therapy in breast cancer. Clin Cancer Res 15: 731–739, 2009. doi: 10.1158/1078-0432.CCR-08-1523. [DOI] [PubMed] [Google Scholar]
- 294.Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, Richardson M, Rigaill G, Parrini MC, Lucchesi C, Bellanger D, Stern MH, Dubois T, Sastre-Garau X, Delattre O, Vincent-Salomon A, Mechta-Grigoriou F. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med 2: 211–230, 2010. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Trapnell C, Cacchiarelli D, Grimsby J, Pokharel P, Li S, Morse M, Lennon NJ, Livak KJ, Mikkelsen TS, Rinn JL. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat Biotechnol 32: 381–386, 2014. doi: 10.1038/nbt.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Trimboli AJ, Cantemir-Stone CZ, Li F, Wallace JA, Merchant A, Creasap N, Thompson JC, Caserta E, Wang H, Chong JL, Naidu S, Wei G, Sharma SM, Stephens JA, Fernandez SA, Gurcan MN, Weinstein MB, Barsky SH, Yee L, Rosol TJ, Stromberg PC, Robinson ML, Pepin F, Hallett M, Park M, Ostrowski MC, Leone G. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature 461: 1084–1091, 2009. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Tuhkanen H, Anttila M, Kosma VM, Heinonen S, Juhola M, Helisalmi S, Kataja V, Mannermaa A. Frequent gene dosage alterations in stromal cells of epithelial ovarian carcinomas. Int J Cancer 119: 1345–1353, 2006. doi: 10.1002/ijc.21785. [DOI] [PubMed] [Google Scholar]
- 298.Valdes-Mora F, Salomon R, Gloss B, Law AMK, Roden DL, Castillo L, Colino-Sanguino Y, Kikhtyak Z, Farbehi N, Conway JRW, Oakes SR, Cox T, Timpson P,Ormandy CJ, Gallego-Ortega D. Single-cell RNAseq uncovers involution mimicry as an aberrant development pathway during breast cancer metastasis (Preprint). bioRxiv 624890, 2019. doi: 10.1101/624890. [DOI]
- 299.Van den Brink SC, Sage F, Vértesy Á, Spanjaard B, Peterson-Maduro J, Baron CS, Robin C, van Oudenaarden A. Single-cell sequencing reveals dissociation-induced gene expression in tissue subpopulations. Nat Methods 14: 935–936, 2017. doi: 10.1038/nmeth.4437. [DOI] [PubMed] [Google Scholar]
- 300.Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am J Pathol 168: 1861–1868, 2006. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Vennin C, Chin VT, Warren SC, Lucas MC, Herrmann D, Magenau A, Melenec P, Walters SN, Del Monte-Nieto G, Conway JR, Nobis M, Allam AH, McCloy RA, Currey N, Pinese M, Boulghourjian A, Zaratzian A, Adam AA, Heu C, Nagrial AM, Chou A, Steinmann A, Drury A, Froio D, Giry-Laterriere M, Harris NL, Phan T, Jain R, Weninger W, McGhee EJ, Whan R, Johns AL, Samra JS, Chantrill L, Gill AJ, Kohonen-Corish M, Harvey RP, Biankin AV, Australian Pancreatic Cancer Genome Initiative, Evans TRJ, Anderson KI, Grey ST, Ormandy CJ, Gallego-Ortega D, Wang Y, Samuel MS, Sansom OJ, Burgess A, Cox TR, Morton JP, Pajic M, Timpson P. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci Transl Med 9: eaai8504, 2017. doi: 10.1126/scitranslmed.aai8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Vennin C, Mélénec P, Rouet R, Nobis M, Cazet AS, Murphy KJ, Herrmann D, Reed DA, Lucas MC, Warren SC, Elgundi Z, Pinese M, Kalna G, Roden D, Samuel M, Zaratzian A, Grey ST, Da Silva A, Leung W, Mathivanan S, Wang Y, Braithwaite AW, Christ D, Benda A, Parkin A, Phillips PA, Whitelock JM, Gill AJ, Sansom OJ, Croucher DR, Parker BL, Pajic M, Morton JP, Cox TR, Timpson P; Australian Pancreatic Genome Initiative (APGI) . CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat Commun 10: 3637, 2019. doi: 10.1038/s41467-019-10968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Vicent S, Sayles LC, Vaka D, Khatri P, Gevaert O, Chen R, Zheng Y, Gillespie AK, Clarke N, Xu Y, Shrager J, Hoang CD, Plevritis S, Butte AJ, Sweet-Cordero EA. Cross-species functional analysis of cancer-associated fibroblasts identifies a critical role for CLCF1 and IL-6 in non-small cell lung cancer in vivo. Cancer Res 72: 5744–5756, 2012. doi: 10.1158/0008-5472.CAN-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Waghray M, Yalamanchili M, Dziubinski M, Zeinali M, Erkkinen M, Yang H, Schradle KA, Urs S, Pasca Di Magliano M, Welling TH, Palmbos PL, Abel EV, Sahai V, Nagrath S, Wang L, Simeone DM. GM-CSF mediates mesenchymal-epithelial cross-talk in pancreatic cancer. Cancer Discov 6: 886–899, 2016. doi: 10.1158/2159-8290.CD-15-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Waise S, Parker R, Rose-Zerilli MJJ, Layfield DM, Wood O, West J, Ottensmeier CH, Thomas GJ, Hanley CJ. An optimised tissue disaggregation and data processing pipeline for characterising fibroblast phenotypes using single-cell RNA sequencing. Sci Rep 9: 9580, 2019. doi: 10.1038/s41598-019-45842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Waldburger JM, Suter T, Fontana A, Acha-Orbea H, Reith W. Selective abrogation of major histocompatibility complex class II expression on extrahematopoietic cells in mice lacking promoter IV of the class II transactivator gene. J Exp Med 194: 393–406, 2001. doi: 10.1084/jem.194.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Waldera Lupa DM, Kalfalah F, Safferling K, Boukamp P, Poschmann G, Volpi E, Götz-Rösch C, Bernerd F, Haag L, Huebenthal U, Fritsche E, Boege F, Grabe N, Tigges J, Stühler K, Krutmann J. Characterization of skin aging-associated secreted proteins (SAASP) produced by dermal fibroblasts isolated from intrinsically aged human skin. J Invest Dermatol 135: 1954–1968, 2015. doi: 10.1038/jid.2015.120. [DOI] [PubMed] [Google Scholar]
- 308.Waldera-Lupa DM, Kalfalah F, Florea AM, Sass S, Kruse F, Rieder V, Tigges J, Fritsche E, Krutmann J, Busch H, Boerries M, Meyer HE, Boege F, Theis F, Reifenberger G, Stühler K. Proteome-wide analysis reveals an age-associated cellular phenotype of in situ aged human fibroblasts. Aging (Albany NY) 6: 856–872, 2014. doi: 10.18632/aging.100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Wang T, Notta F, Navab R, Joseph J, Ibrahimov E, Xu J, Zhu CQ, Borgida A, Gallinger S, Tsao MS. Senescent carcinoma-associated fibroblasts upregulate IL8 to enhance prometastatic phenotypes. Mol Cancer Res 15: 3–14, 2017. doi: 10.1158/1541-7786.MCR-16-0192. [DOI] [PubMed] [Google Scholar]
- 310.Whatcott CJ, Diep CH, Jiang P, Watanabe A, LoBello J, Sima C, Hostetter G, Shepard HM, Von Hoff DD, Han H. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res 21: 3561–3568, 2015. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Willemer S, Elsässer HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res 24, Suppl 1: 29–39, 1992. doi: 10.1159/000129237. [DOI] [PubMed] [Google Scholar]
- 312.Wörmann SM, Song L, Ai J, Diakopoulos KN, Kurkowski MU, Görgülü K, Ruess D, Campbell A, Doglioni C, Jodrell D, Neesse A, Demir IE, Karpathaki AP, Barenboim M, Hagemann T, Rose-John S, Sansom O, Schmid RM, Protti MP, Lesina M, Algül H. Loss of P53 function activates JAK2-STAT3 signaling to promote pancreatic tumor growth, stroma modification, and gemcitabine resistance in mice and is associated with patient survival. Gastroenterology 151: 180–193.e12, 2016. doi: 10.1053/j.gastro.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 313.Wu MH, Hong HC, Hong TM, Chiang WF, Jin YT, Chen YL. Targeting galectin-1 in carcinoma-associated fibroblasts inhibits oral squamous cell carcinoma metastasis by downregulating MCP-1/CCL2 expression. Clin Cancer Res 17: 1306–1316, 2011. doi: 10.1158/1078-0432.CCR-10-1824. [DOI] [PubMed] [Google Scholar]
- 314.Xiao Q, Zhou D, Rucki AA, Williams J, Zhou J, Mo G, Murphy A, Fujiwara K, Kleponis J, Salman B, Wolfgang CL, Anders RA, Zheng S, Jaffee EM, Zheng L. Cancer-associated fibroblasts in pancreatic cancer are reprogrammed by tumor-induced alterations in genomic DNA methylation. Cancer Res 76: 5395–5404, 2016. doi: 10.1158/0008-5472.CAN-15-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Xie T, Liang J, Liu N, Huan C, Zhang Y, Liu W, Kumar M, Xiao R, D’Armiento J, Metzger D, Chambon P, Papaioannou VE, Stripp BR, Jiang D, Noble PW. Transcription factor TBX4 regulates myofibroblast accumulation and lung fibrosis. J Clin Invest 126: 3626, 2016. doi: 10.1172/JCI89968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Xie T, Wang Y, Deng N, Huang G, Taghavifar F, Geng Y, Liu N, Kulur V, Yao C, Chen P, Liu Z, Stripp B, Tang J, Liang J, Noble PW, Jiang D. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep 22: 3625–3640, 2018. doi: 10.1016/j.celrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Xu M, Xu HH, Lin Y, Sun X, Wang LJ, Fang ZP, Su XH, Liang XJ, Hu Y, Liu ZM, Cheng Y, Wei Y, Li J, Li L, Liu HJ, Cheng Z, Tang N, Peng C, Li T, Liu T, Qiao L, Wu D, Ding YQ, Zhou WJ. LECT2, a ligand for Tie1, plays a crucial role in liver fibrogenesis. Cell 178: 1478–1492.e20, 2019. doi: 10.1016/j.cell.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 318.Xu S, Yang Z, Jin P, Yang X, Li X, Wei X, Wang Y, Long S, Zhang T, Chen G, Sun C, Ma D, Gao Q. Metformin suppresses tumor progression by inactivating stromal fibroblasts in ovarian cancer. Mol Cancer Ther 17: 1291–1302, 2018. doi: 10.1158/1535-7163.MCT-17-0927. [DOI] [PubMed] [Google Scholar]
- 319.Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, Liu X, Chen CH, Fadare O, Pizzo DP, Wu J, Liu L, Liu X, Chin AR, Ren X, Chen Y, Locasale JW, Wang SE. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol 20: 597–609, 2018. doi: 10.1038/s41556-018-0083-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yang SY, Denning SM, Mizuno S, Dupont B, Haynes BF. A novel activation pathway for mature thymocytes. Costimulation of CD2 (T,p50) and CD28 (T,p44) induces autocrine interleukin 2/interleukin 2 receptor-mediated cell proliferation. J Exp Med 168: 1457–1468, 1988. doi: 10.1084/jem.168.4.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, Dang Y, Chu Y, Fan J, He R. FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res 76: 4124–4135, 2016. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 322.Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest 123: 1902–1910, 2013. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Yoo BM, Oh TY, Kim YB, Yeo M, Lee JS, Surh YJ, Ahn BO, Kim WH, Sohn S, Kim JH, Hahm KB. Novel antioxidant ameliorates the fibrosis and inflammation of cerulein-induced chronic pancreatitis in a mouse model. Pancreatology 5: 165–176, 2005. doi: 10.1159/000085268. [DOI] [PubMed] [Google Scholar]
- 324.Yousefzadeh MJ, Zhao J, Bukata C, Wade EA, McGowan SJ, Angelini LA, Bank MP, Gurkar AU, McGuckian CA, Calubag MF, Kato JI, Burd CE, Robbins PD, Niedernhofer LJ. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19: e13094, 2020. doi: 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yu B, Wu K, Wang X, Zhang J, Wang L, Jiang Y, Zhu X, Chen W, Yan M. Periostin secreted by cancer-associated fibroblasts promotes cancer stemness in head and neck cancer by activating protein tyrosine kinase 7. Cell Death Dis 9: 1082, 2018. doi: 10.1038/s41419-018-1116-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 326.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 67: 10123–10128, 2007. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 327.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13: 952–961, 2007. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 328.Zhang D, Wang Y, Shi Z, Liu J, Sun P, Hou X, Zhang J, Zhao S, Zhou BP, Mi J. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep 10: 1335–1348, 2015. doi: 10.1016/j.celrep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 329.Zhang XH, Jin X, Malladi S, Zou Y, Wen YH, Brogi E, Smid M, Foekens JA, Massagué J. Selection of bone metastasis seeds by mesenchymal signals in the primary tumor stroma. Cell 154: 1060–1073, 2013. doi: 10.1016/j.cell.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang Y, Lazarus J, Steele NG, Yan W, Lee HJ, Nwosu ZC, Halbrook CJ, Menjivar RE, Kemp SB, Sirihorachai VR, Velez-Delgado A, Donahue K, Carpenter ES, Brown KL, Irizarry-Negron V, Nevison AC, Vinta A, Anderson MA, Crawford HC, Lyssiotis CA, Frankel TL, Bednar F, di Magliano MP. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov 10: 422–439, 2020. doi: 10.1158/2159-8290.CD-19-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, Stanger BZ, Chung I, Rhim AD, di Magliano MP. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res 73: 6359–6374, 2013. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Zhao H, Yang L, Baddour J, Achreja A, Bernard V, Moss T, Marini JC, Tudawe T, Seviour EG, San Lucas FA, Alvarez H, Gupta S, Maiti SN, Cooper L, Peehl D, Ram PT, Maitra A, Nagrath D. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 5: e10250, 2016. doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts: a potentially powerful technique for investigating gene function in fibrosis. Am J Pathol 160: 1609–1617, 2002. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zhuang Z, Ju HQ, Aguilar M, Gocho T, Li H, Iida T, Lee H, Fan X, Zhou H, Ling J, Li Z, Fu J, Wu M, Li M, Melisi D, Iwakura Y, Xu K, Fleming JB, Chiao PJ. IL1 receptor antagonist inhibits pancreatic cancer growth by abrogating NF-κB activation. Clin Cancer Res 22: 1432–1444, 2016. doi: 10.1158/1078-0432.CCR-14-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Zinger A, Cho WC, Ben-Yehuda A. Cancer and aging - the inflammatory connection. Aging Dis 8: 611–627, 2017. doi: 10.14336/AD.2016.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zion O, Genin O, Kawada N, Yoshizato K, Roffe S, Nagler A, Iovanna JL, Halevy O, Pines M. Inhibition of transforming growth factor beta signaling by halofuginone as a modality for pancreas fibrosis prevention. Pancreas 38: 427–435, 2009. doi: 10.1097/MPA.0b013e3181967670. [DOI] [PubMed] [Google Scholar]