Abstract
Neuroscientific research on pleasant touch has focused on the C-tactile pathway for gentle stroking and has successfully explained how these sensory fibers transmit information about affective social touch to the brain and induce sensations of pleasantness. The C-tactile social/affective touch hypothesis even proposes that C-tactile fibers form a privileged pathway underlying social touch. However, deep pressure is a type of touch commonly considered pleasant and calming, occurring in hugs, cuddling, and massage. In this paper we introduce a paradigm for studying pleasant deep pressure and propose that it constitutes another important form of social touch. We describe development of the oscillating compression sleeve (OCS) as one approach to administering deep pressure and demonstrate that this touch is perceived as pleasant and calming. Further, we show that deep pressure can be imaged with functional magnetic resonance imaging (MRI) using the air-pressure-driven OCS and that deep pressure activates brain regions highly similar to those that respond to C-tactile stroking, as well as regions not activated by stroking. We propose that deep pressure constitutes another social touch pathway of evolutionary importance signaling the close proximity of conspecifics.
Keywords: C-tactile, pressure, somatosensation, social touch, fMRI
Introduction
In recent decades neuroscientists have begun to investigate the physiological basis for the perception of pleasant touch. In particular, the C-tactile pleasant touch pathway has been the luminary of this pursuit. C-tactile sensory afferents are unmyelinated, low-threshold mechanosensory afferents activated most strongly by slow, gentle stroking touch--the type of touch with which one might comfort a loved one or pet a dog. This afferent pathway has been rigorously characterized through microneurography, psychophysics, patient studies, and brain imaging (Loken et al., 2009; Olausson et al., 2008; Olausson et al., 2002; Olausson et al., 2010), weaving a compelling story by which gentle social touch activates a subset of specialized affective sensory nerves and is processed in areas of the brain involved with the affective, rather than discriminatory, dimension of touch (Gordon et al., 2013; McGlone et al., 2007; Morrison et al., 2010; Olausson et al., 2008). C-tactile afferents are present only in hairy skin (Morrison et al., 2010; Vallbo et al., 1999). The C-tactile social/affective touch hypothesis (Morrison et al., 2010; Vallbo et al., 1999) has proposed that C-tactile afferents constitute a specialized pathway signaling “close, affiliative body contact with others” (Morrison et al., 2010). While C-tactile touch has basked in well-deserved limelight, other types of pleasurable touch have been eclipsed. Sexual touch is one obvious example but is challenging to study in the laboratory (though see (Allen et al., 2020; Komisaruk and Whipple, 2005; Komisaruk et al., 2004)). However, deep pressure is another form of pleasant touch that can be readily studied in the laboratory.
Deep pressure is embedded in several nearly universal forms of social touch including hugs, cuddling, and the swaddling and carrying of infants. Many species of mammals huddle to minimize body surface area for warmth and protection (Gilbert et al., 2010), leading to deep body-to-body pressure. Deep pressure touch is also a component of numerous manual therapies, in particular massage therapy, which is commonly regarded as a pleasurable experience. Massage therapy is known to significantly reduce depression, stress and pain as well as improve immune responses in adults and promote weight gain in infants (Field et al., 2007; Perlman et al., 2012). Deep pressure touch is also practiced by occupational therapists and has been found to reduce anxiety and increase calm (Grandin 1992; Krauss 1987). Further, moderate (versus light) pressure has been found to be necessary for the beneficial effects of massage therapy (Field et al., 2010), and leads to greater stress and anxiety reduction as well as higher ratings of pleasantness (Diego et al., 2004). These effects, however, involve interpersonal touch. Are there affective effects of deep pressure that are retained when removed from the social setting?
To study the effects of deep pressure touch without the confound of interpersonal touch, we set out to develop an MRI-compatible mechanical massage-like compression device and determine whether it would be perceived as pleasant. In Study 1 we used a compression sleeve to test a range of pressure levels across different body sites to identify the pressure level and location considered most pleasant. In Study 2, learning from the preferred location and pressure level determined in Study 1, we developed the Oscillating Compression Sleeve (OCS) to better resemble the movement of a manual massage. We compared the pleasantness of this novel massage-like compression to that of the well-established gentle stroking stimulus paradigm known to preferentially activate C-tactile afferents in the skin. With a successful model for delivering a pleasant, massage-like compression removed from the social context of interpersonal touch, we studied its effects on mood and calm/anxiety. In Study 3 we used functional magnetic resonance imaging (fMRI) to investigate the cortical correlates involved in processing pleasant deep touch from the OCS. An increased understanding of the underlying mechanisms involved in pleasant deep pressure touch may help to determine its role in social touch and determine its affective and therapeutic effects.
Experimental Procedures
The following studies were approved by the NIH CNS Institutional Review Board (IRB). All participants provided informed consent and were monetarily compensated for their time in accordance with approval from the IRB.
Study 1 – Inducing Pleasant Deep Pressure
Participants
15 healthy adult participants (age M = 32.5, SD = 9.2, 10 female) were recruited after their participation in previous National Center for Complementary and Integrative Health (NCCIH) protocols. Participants were screened to exclude those with major medical conditions, depression or anxiety, current drug use or dependency, or dermatological conditions that could interfere with sensory testing on the skin.
Design
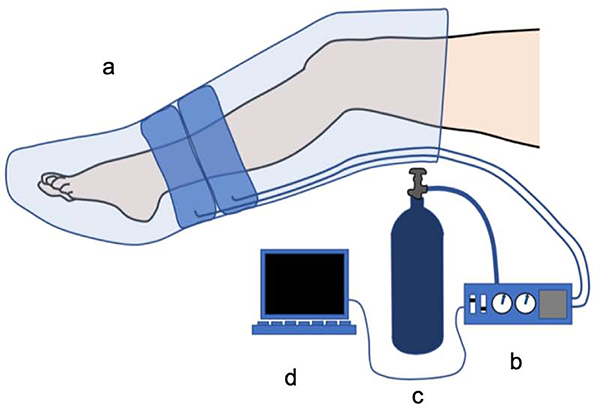
The primary goal of this study was to determine whether we could administer a single pulse (up and down ramp) of sleeve pressure that participants would perceive as pleasant. The secondary goal was to determine whether the pleasantness perception would vary across pressure levels and across different body locations. Pressure was administered using a commercially available, automated, upper and lower limb lymphedema pump (Chattanooga PresSsion 652–8 Chamber Lymphedema Pump with 8-Chamber Garment sleeves). This device is typically used to reduce edema by sequentially inflating air chambers along sections of a compression sleeve. See Figure 1(a).
Figure 1. Design of Oscillating Compression Sleeve (OCS).
a). Commercially available lower limb lymphedema compression sleeve. Darker area shows the two chambers that were inflated in Study 2.
b.) Custom built pressure regulation device. Device converts USB signaling into electric current to drive the regulator and delivers information from the sleeve back to the USB.
c.) Freestanding compressed air tank to supply air to the sleeve.
d.) Matlab computer connected via USB to the flow regulator and used to regulate pressure level and flow rate.
Participants were seated comfortably in a reclining exam chair, with the test leg resting on a stool in front of the chair and the test arm resting on the chair armrest. Participants were outfitted with an 8-chamber arm sleeve and an 8-chamber leg sleeve on the non-dominant side of their body. The sleeves enveloped the arm from the wrist to the shoulder and the leg from the foot to the upper thigh. Participants wore their own clothes underneath the sleeve, but shoes and accessories were removed. Participants were asked to close their eyes during the periods of stimulation. Pressure pulses were applied to the ankle, calf, wrist, forearm and upper arm at 30, 50, 70, and 90 mm Hg. An air chamber surrounding each of these areas inflated sequentially for 5 seconds, held for 12 seconds, and then deflated for 8 seconds before cycling to the next location. Once all five locations (in a randomized order) had received one compression, the amount of applied pressure was increased by 20 mm Hg. This process began at an initial pressure level of 30 mm Hg and continued up until 90 mm Hg of pressure. The ascending sequence order was chosen to avoid any sensitization from high pressure levels that might alter perception of lower levels.
Ratings
Subjects were asked to provide ratings for each combination of location and pressure during the 8 second deflation period following each stimulus. Participants used numeric rating scales to report how subjectively intense, painful, pleasant, unpleasant, liked, or disliked each sensation was. The intensity scale ranged from 0 (no sensation) to 100 (most extreme sensation imaginable) and the pain scale from 0 (no pain) to 100 (most extreme pain imaginable). Likewise, the pleasantness scale ranged from 0 (no pleasantness) to 100 (most extreme pleasantness imaginable) and the unpleasantness scale from 0 (no unpleasantness) to 100 (most extreme unpleasantness imaginable). For the last two scales, subjects first indicated whether they liked or disliked each sensation and then provided one rating of 0 (neutral) to 100 (most liked/disliked sensation imaginable).
In addition, participants completed the Touch Perception Task (TPT) (Guest et al., 2011). The TPT is a list of 14 affective word descriptors that can be used to further characterize the subjective sensations associated with sensory stimuli. Each endorsed word is assigned a value from 1–5 to indicate how well that particular affective word describes the sensation experienced.
Data Analysis
The mean pleasantness rating was the outcome measure of greatest interest and only this data was plotted for visualization using GraphPad Prism 7. JMP (1989–2007) was used to compute a mixed effects model examining the effect of pressure level and body location (fixed effects) and subject (random effect) on pressure pleasantness.
Study 2 – Design and Validation of the Oscillating Compression Sleeve
Participants
39 healthy adults (20 female), mean age M = 27.6, SD = 7.4 were recruited after their participation in previous NCCIH protocols. Participants were screened for the same exclusion criteria as in Study 1.
Design
Based on piloting and feedback from Study 1, we determined that an oscillating pressure stimulus might be more pleasant than a static one. We therefore designed a custom-made device allowing for more precise control of pressure administration. We call our device the Oscillating Compression Sleeve (OCS). The stimulus pattern of the OCS is more similar to that of a manual massage i.e. with an oscillatory compression level. We cut the plastic tubing connecting the garment sleeves to the PresSsion pump and connected them to a custom-built pressure regulation device. Airflow to two chambers of the compression sleeve (Chattanooga Group PresSsion 8 Chamber Garment) was supplied by freestanding compressed air tanks and regulated by the custom device. The device converted USB signaling into electrical current to drive a flow regulator, and also included a sleeve pressure sensor for digitizing and delivering a signal back to the USB. Sleeve inflation flow rate was controlled via Matlab (2016) programming, causing pressure to reach the target peak and then to passively drop to the target baseline. See Figure 1.
We compared ratings of OCS pressure and superficial skin brushing, in randomized order. Based on our previous findings in Study 1 we administered stimuli to the lower limb. All stimuli were introduced to the participant before the test phase. The participant lay in a mock magnetic resonance imaging (MRI) scanner (Psychology Software Tools, Inc) for the purpose of later reproducibility during future MRI studies. The participants legs were elevated to approximately 22 cm onto a pillow for comfort. All participants were asked to change into synthetic fiber scrubs. The lower left leg sleeve of the scrubs was cut or loosely rolled up to expose the lower leg. A cotton pillowcase was worn over the foot and lower leg beneath the OCS sleeve to standardize the sensation of the OCS. The OCS was worn at all times but was unzipped, with the pillowcase rolled down, during the brush stroking. Participants were instructed to keep their eyes on a fixation cross during each task. Participants received one block of fast brushing, one of slow brushing, one of higher pressure, and one of low pressure, in counterbalanced order. At the end of each block participants rated the sensation using visual analog scales (VASs) (described below). Mood ratings were collected before and after each stimulus type.
Gentle brush stroking
Gentle brushing was administered manually using a soft goat-hair brush (7.5 cm width) over a distance of 6 cm that was marked on the anterior side of the lower leg. Gentle brushing was performed at two speeds, with one block containing slow ~3cm/s brushing (optimal for C-tactile fibers) and the other containing fast ~30 cm/s brushing (suboptimal for C-tactile fibers). In each brushing block there were eight trials lasting 15 seconds each, with a 20-second inter-stimulus-interval. Brushing was administered by a trained experimenter who stood by the scanner bed and was not visible to the participant lying in the scanner bore. The experimenter received audio cues over headphones to cue speed and timing of the stimuli.
Deep pressure
Deep pressure was administered using the OCS on the lower left leg. A single chamber of the OCS sleeve was inflated over the most distal 14 cm of the lower leg starting just above the ankle joint. Deep pressure was administered at two levels of intensity (a peak of 30mmHg and a peak of 65mmHg). In each compression block, there were eight trials lasting between 15–30 seconds each, with a 20-second inter-stimulus-interval. During each trial, the pressure rose to the peak, dropped to a baseline (10–15mmHg), then immediately rose again for a total of five oscillations.
Ratings
After each stimulus block participants used VASs to indicate how (un)pleasant each sensation was to them, with anchors “extremely unpleasant” (far left) to “neutral” (middle) to “extremely pleasant” (far right). Participants also rated their mood with anchors “extremely bad” (far left) to “neutral” (middle) to “extremely good” (far right) and their level of anxiety or calm (“extremely anxious” (far left) to “neutral” (middle) to “extremely calm” (far right). Ratings were converted to numbers ranging from −100 to 100 with 0 as neutral. Ratings of intensity, liking, and wanting were also collected (data not presented here). We used E-prime (version 2.0) as our stimulus presentation software for recording of ratings. The VASs were displayed on a computer screen placed at one end of the MRI scanner, which participants viewed using a mirror placed in the head coil. Participants held a response box that was used to move a cursor along the VAS. Finally, at the end of the test, outside of the mock scanner, participants manually completed the TPT (see Study 1).
Data Analysis
Ratings were converted to correspond to the same numeric scales used in Study 1 (eg −100 for “no sensation” was rescaled to 0). The pleasantness of slow and fast brushing and low- and high-pressure stimuli were compared in JMP using a mixed effects model with fixed factor of stimulus (four stimulus types) and random effects of subjects and subject*stimuli. We compared ratings of pleasantness for static (Study 1) and oscillating (Study 2) compression using paired t-tests. Mood and anxiety ratings were compared from before to after each stimulus block using paired t-tests and corrected for multiple comparisons using the Bonferroni method. Data were checked for outliers; there were no data points more than three standard deviations from the mean. Pearson’s bivariate correlations were used to determine the correlation between subjects’ ratings of touch pleasantness and mood or anxiety.
Study 3: fMRI of cortical response to deep pressure and brush stroking
Participants
Twenty-four right-handed healthy adults (mean age 26.7 ± 7.3 years; range 18–46 years; 12 female) completed the study. All participants had been previously screened through participation in Study 2. Participants were screened for the same exclusion criteria as in Study 1 and 2 with the addition of or inability to tolerate the sensory stimuli or scanner environment. Participants were screened for drug use and pregnancy using a urine sample collected at the beginning of each experimental session.
Design
As part of a larger study, participants completed two fMRI scans on separate days. The beginning of each session contained one block of fast brushing, one block of slow brushing, one block of high compression, and one block of low compression, in randomized order. All sensory testing occurred on the lower left leg following the same sequence as in Study 2. Perceptual ratings were collected at the end of each sensory block as in Study 2.
fMRI acquisition
All brain images were acquired using a 3 Tesla Siemens Skyra MRI scanner (Siemens, Erlangen, Germany) with a 20-channel head and neck coil. A whole-brain T1-weighted anatomical scan was acquired using a 3D MP-RAGE (Magnetization Prepared Rapid Acquisition by Gradient Echo) sequence with parameters: TR = 1900ms, TE = 2.07ms, flip angle = 9°, resolution 1 × 1 × 1 mm, image matrix = 256 × 256 with 192 slices. Whole-brain functional images were acquired using a blood oxygenation level-dependent (BOLD) protocol with a T2*-weighted gradient echo planar imaging (EPI) sequence (TR = 2000ms, TE = 29ms, flip angle = 70°, resolution 3.5 × 3.5 × 3.5 mm, image matrix = 64 × 64, 38 slices).
Data Analysis
Whole-brain analyses were conducted to determine the main effects of brushing and compression. First-level mass univariate GLM analyses were carried out in FEAT (FMRI Expert Analysis Tool) Version 6.00, a part of FSL (Jenkinson et al., 2012). The first and second half of sensory stimulation periods were modeled separately using boxcar functions convolved with the double-gamma hemodynamic response function. Twelve additional parameters of no interest were included to model rigid body translation and rotation during the alignment to standardized space (MNI 2mm brain). MCFLIRT was applied for motion correction and BET was used to mask activation outside the brain. A high pass filter was applied with a cutoff of 45s (brushing) or 60s (pressure) based on the average duration of each stimulus type. Spatial smoothing was set to 2mm FWHM. Fixed effect models were then conducted to combine activations across the two separate fMRI sessions and across the levels (slow/fast; high/low) of stimuli.
Group-level analyses were conducted using mixed effects models in FLAME1+2 (FMRIB’s local analysis of mixed effects (Smith SM et al., 2004)) to identify the main effects of each stimulus and the contrast between them. All analyses were thresholded at Z = 3.1 and cluster-corrected using Gaussian Random Field (GRF) theory to identify clusters significant at the p < 0.001 level. Additional re-clustering at a reduced cluster-correction threshold of p < 0.01 was conducted to check for clusters near the cutoff threshold. A mask was created for regions responsive to brushing and/or compression and contrasts between brushing and compression were computed within this mask.
Results
Study 1
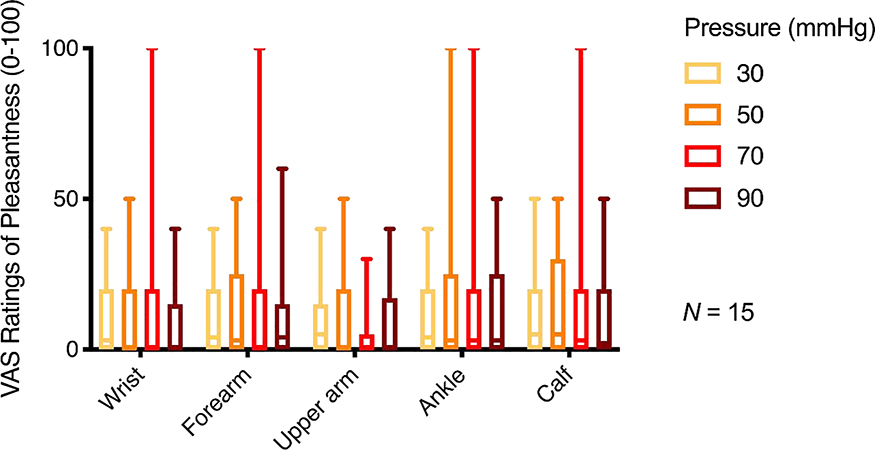
The compression sleeves evoked ratings of mild pleasantness (see Figure 2). Numerically, pleasantness ratings were highest for 70mmHg, but the effect was not statistically significant in our sample (F(3,39) = 0.42, p = 0.74). Numerically, ratings were highest on the ankle and calf, but differences between limb locations did not reach significance (F(4, 52) = 2.02, p = 0.10).
Figure 2. Pleasantness ratings for pressure on five body locations.
Box plot of pleasantness ratings for static pressure pulses showing the third quartile and first quartile range of the data. Lower and upper error lines display the 5th and 95th percentiles. Pressure pulses of 30, 50, 70, and 90 mmHg were applied to the wrist, forearm, bicep, ankle, and calf on the non-dominant side using commercially available lymphedema compression sleeves. Healthy adult participants provided pleasantness ratings for each body location/intensity combination. The VAS scale ranged from 0 (no pleasantness) to 100 (most extreme pleasantness imaginable).
Study 2
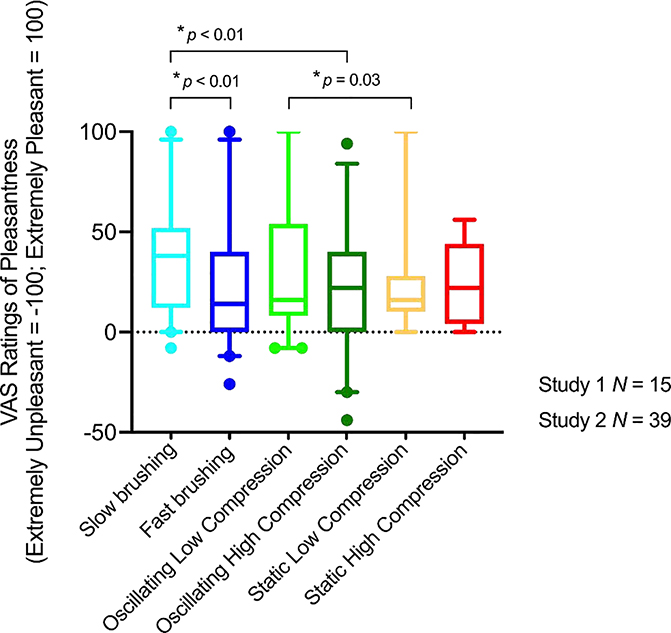
Brushing and pressure stimuli were rated as pleasant (see Figure 3). The stimuli differed significantly in ratings of pleasantness (F(3.114) = 5.32, p = 0.002). Posthoc comparisons using the Tukey Honestly Significant Difference procedure indicated that the mean score for slow brushing (M = 37.6) was significantly different than the mean score for fast brushing (M = 22.0), with a 95% confidence interval of the difference between means from 3.7 to 27.5 points on the VAS scale, and from high compression (M = 21.8), with a 95% confidence interval of the difference between means from 4.0 to 27.7 points on the VAS scale, but did not differ significantly from low compression (28.1).
Figure 3. Pleasantness ratings for brushing and pressure.
Box plot of pleasantness ratings of gentle brushing (Study 2), oscillating compression (Study 2), and static compression (Study 1) on the left calf. Lower and upper error lines display the 5th and 95th percentiles, and filled circles display data falling outside these percentiles. Gentle brushing was applied at two velocities (~3cm/s and ~30cm/s) using a soft goat-hair brush. Pressure was applied at two intensities. Oscillating pressure rose from a baseline of 10–15mmHg to a peak of 30mmHg (low) or 65mmHg (high). Static pressure rose to hold at 30mmHg (low) or 70mmHg (high). Trials of each stimulus lasted 15–30 seconds. Participants rated the pleasantness of each sensation on a VAS scale that ranged from 0 (no pleasantness) to 100 (most extreme pleasantness imaginable).
Oscillating low pressure was rated as more pleasant than the static low pressure in Study 1 (applied on the leg at 30mmHg, t(52) = 2.23, p = 0.03) but there was no difference in ratings of oscillating and static high pressure (applied on the leg at 70mmHg, t(52) = 1.3, p = 0.20) (see Figure 3).
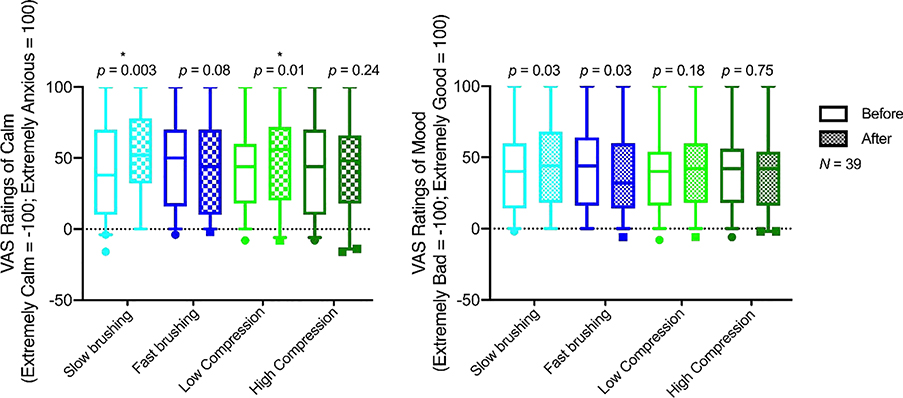
Using a Bonferroni adjusted alpha of p = 0.0125, slow brushing significantly increased ratings of calm (t(38) = 3.19, p = 0.003), as did low compression (t(38) = 2.64, p = 0.01). Fast brushing and high compression did not influence ratings of calm (fast brushing t(38) = 1.83, p = 0.08; high compression t(38) = 1.18, p = 0.24).
Ratings of touch pleasantness and calm were positively correlated across subjects for every type of touch (slow brushing r = 0.53, p = 0.0006, fast brushing r = 0.43, p = 0.006, low compression r = 0.61, p < 0.0001, high compression r = 0.56, p = 0.0002).
The effects of brushing on mood were not significant using a Bonferroni adjusted alpha of p = 0.0125 (slow brushing increased good mood (t(38) = 2.31, p = 0.03), while fast brushing decreased mood (t(38) = 2.30, p = 0.03)). Compression did not alter mood (low compression t(38) = 1.36, p = 0.18; high compression t(38) = 0.32, p = 0.75)). See Figure 4.
Figure 4. Effects of brushing and pressure and mood on anxiety/calm.
Box plot of ratings of anxiety/calm and mood before and after affective stimuli. Lower and upper error lines display the 5th and 95th percentiles, and filled circles display data falling outside these percentiles. Gentle brushing was applied to the left calf at two speeds (~3cm/s and ~30cm/s) using a soft goat hair brush. Oscillating pressure was applied to the same area at two intensities (rising from a baseline of 10–15mmHg to a peak of 30 or 65mmHg) using the Oscillating Compression Sleeve. Trials of each stimulus lasted 15–30 seconds, with a 20 second inter-stimulus-interval. At baseline and after each trial, participants rated their current mood and level of anxiety on a VAS scale with anchors from −100 (Extremely bad mood/Extremely anxious) to 0 (neutral) to 100 (Extremely good mood/Extremely calm).
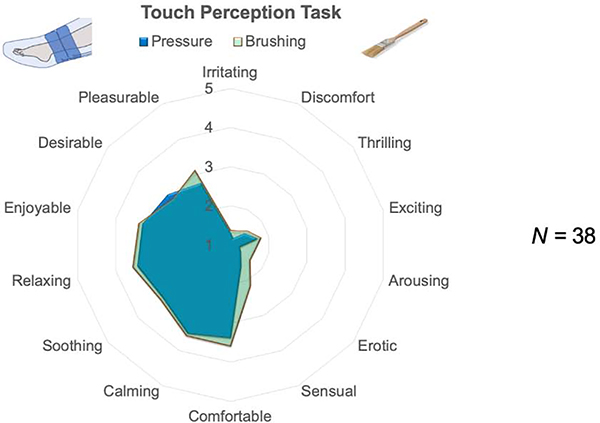
Adjectives endorsed on the TPT were highly similar to those endorsed for gentle skin stroking (see Figure 5).
Figure 5. Affective ratings of brushing and pressure.
Participants completed the Touch Perception Task (17), rating their affective experience of brushing (green) and pressure (blue). Participants were not asked to distinguish between slow/fast brushing or high/low pressure but to rate the most strongly they experienced each adjective.
Study 3
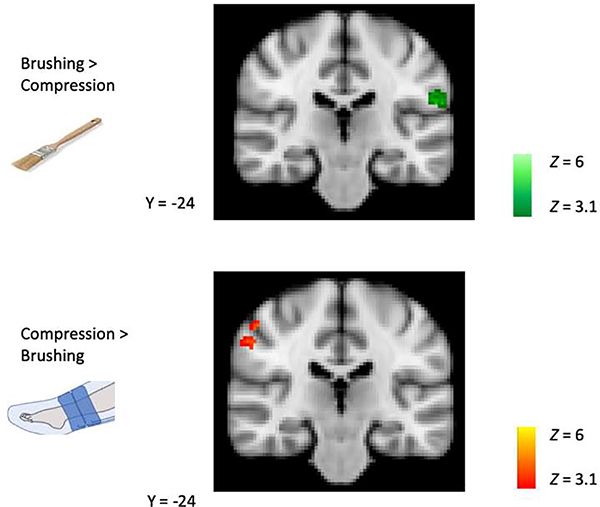
Significant clusters for brushing > rest were identified in contralateral primary somatosensory cortex (S1), bilateral secondary somatosensory cortex (S2) and supramarginal gyrus (SMG), and contralateral posterior insula. Significant clusters for compression > rest were identified in contralateral S2 and contralateral mid insula. When the cluster correction threshold was reduced from p < 0.001 to p < 0.01, a significant cluster was observed in contralateral S1. See Figure 6 and Table 1. Within the areas that responded to brushing or compression (masked analysis), significantly greater response was observed to brushing in ipsilateral S1/SMG and significantly greater response to compression was observed in contralateral SMG. See Figure 7 and Table 2.
Figure 6. BOLD response to brushing and compression.
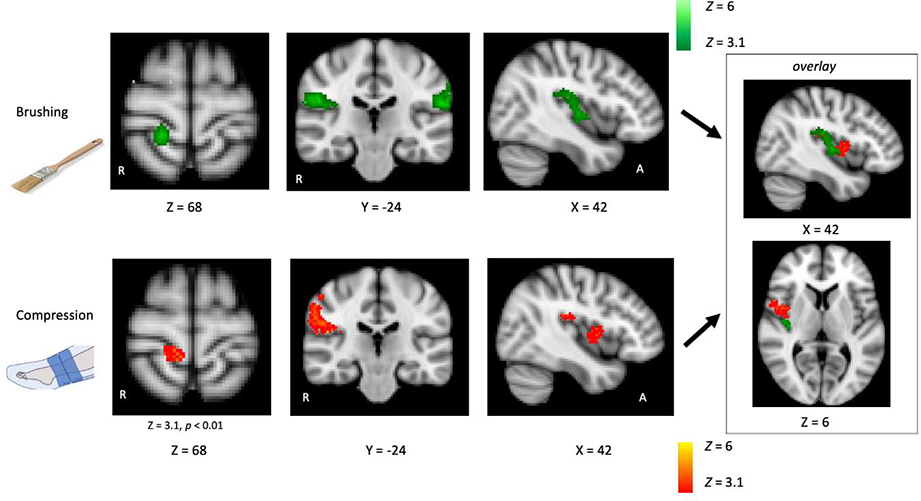
BOLD images were acquired using a 3T MRI scanner while participants received brushing at two speeds (~3cm/s and ~30cm/s) and oscillating pressure at two levels (a peak of 30mmHg or 65mmHg) in separate blocks on their lower left calf. Contrasts were conducted to identify the brain regions activated by brushing > rest (fast and slow brushing combined) and compression > rest (low and high pressure combined). Activation maps were thresholded at Z = 3.1 and cluster corrected at p < 0.001 unless noted otherwise. Response to brushing was observed in contralateral S1, bilateral S2 and SMG, and contralateral insula. Response to compression was observed in contralateral S2 and insula, and subthreshold in contralateral S1. Insert on far right displays brushing and compression representation overlaid in the insula. Coordinates are in the MNI 152 space.
Table 1.
Activation evoked by brushing or compression (> rest) on the lower left leg. The peak is quantified as the voxel in each cluster with the highest Z score. Clusters displayed surpass a whole-brain statistical threshold of Z > 3.1, corrected for multiple comparisons (p < 0.001 or p < 0.01 for compression S1 cluster). X, Y and Z MNI coordinates correspond to the left–right, anterior–posterior and inferior–superior axes, respectively.
| Brushing | ||||
| Region | # of voxels | Cluster p value | Peak Z score | Peak MNI coordinates (X, Y, Z) |
| right S2 and posterior insula | 972 | p < 0.00001 | 7.56 | 62, −24, 22 |
| left S2/SMG | 492 | p < 0.00001 | 6.69 | −54, −32, 20 |
| right S1 | 146 | 0.000287 | 8.98 | 18, −38, 64 |
| Compression | ||||
| Region | # of voxels | Cluster p value | Peak Z score | Peak MNI coordinates (X, Y, Z) |
| right S2/SMG | 811 | p < 0.00001 | 5.64 | 48, −24, 18 |
| right mid insula | 312 | p < 0.00001 | 4.65 | 46, −2, 8 |
| right S1 | 108 | 0.00326 significant only at cluster threshold p < 0.01 |
4.94 | 16, −40, 66 |
Figure 7. BOLD response to brushing > compression and compression > brushing.
A masked analysis was conducted to identify significantly greater responses to brushing or compression, within areas significantly activated by brushing or compression. Greater response to brushing was found in ipsilateral S1/SMG and greater response to compression was observed in contralateral SMG. Coordinates are in the MNI 152 space.
Table 2.
Response to brushing > compression or brushing > compression within the mask of areas activated by brushing or compression. The peak is quantified as the voxel in each cluster with the highest Z score. Clusters displayed surpass a whole-brain statistical threshold of Z > 3.1, corrected for multiple comparisons (p < 0.001). X, Y and Z MNI coordinates correspond to the left–right, anterior–posterior and inferior–superior axes, respectively.
| Brushing > Compression | ||||
| Region | # of voxels | Cluster p value | Peak Z score | Peak MNI coordinates (X, Y, Z) |
| left S1/SMG | 109 | p = 0.00003 | 4.77 | −58, −24, 20 |
| Compression > Brushing | ||||
| Region | # of voxels | Cluster p value | Peak Z score | Peak MNI coordinates (X, Y, Z) |
| right SMG | 73 | p = 0.00028 | 4.67 | 54, −22, 42 |
Discussion
These investigations demonstrate that certain patterns of deep pressure remain pleasant even when applied mechanically, in the absence of a social interaction. We demonstrate that oscillating deep pressure has similar affective effects to that of C-tactile gentle stroking, including similar ratings of touch pleasantness and increased ratings of calm. Further, we demonstrate that our paradigm for administering deep pressure touch can be applied during fMRI. The brain activations are similar in S1 (and similar to recent findings for lower leg representation (Akselrod et al., 2017; Bao et al., 2012; Dietrich et al., 2017; Nakagoshi et al., 2005)), but show distinct representations in S2 and the insula: tactile gentle stroking activates bilateral S2 and posterior insula, while deep pressure predominantly activates contralateral S2 and mid insula. Deep pressure also obtained a stronger response in contralateral SMG than gentle touch, similar to recent findings for firm, visceral versus light, somatic stimulation of the penile shaft (Allen et al., 2020). These findings lead us to propose that oscillating deep pressure activates a novel pleasant touch pathway, whose underlying physiology may be probed using nonsocial compression devices that are MRI-compatible.
We theorize that as for the C-tactile system, pleasant and calming effects of affective deep pressure may play important roles in social bonding. Infants are often held tightly to the body, and the rhythmic breathing of the parent would create an oscillating pressure stimulus. Similarly, humans or animals huddling for warmth or sleeping in close contact for protection would experience deep pressure with an oscillatory pattern due to the respiratory cycle (Holsti et al., 2019). We speculate that the relaxing effects of deep pressure may relate to the protection and safety conferred by being held by others or sleeping body-to-body. We therefore propose that the C-tactile social/affective touch hypothesis be extended, to encompass the deep pressure sensory pathway along with the C-tactile stroking pathway as critical components of social touch.
Little is understood about the physiological basis of the affective benefits of deep pressure touch, and how they relate to the pleasant sensation deep pressure can evoke. We suggest, however, that pleasant deep pressure is not conveyed by C-tactile afferents. Sensations of deep pressure remain strong after anesthetization of the skin (Graven-Nielsen et al., 2004). This is likely due to the presence of pressure-sensitive afferents in muscle and connective tissues. Indeed, pressure-sensitive afferents that respond to innocuous levels of pressure have previously been identified in the skeletal muscle and tendon of cat (Mense and Meyer, 1985)) and rat (Corey et al., 2011; Hoheisel et al., 2005). We hypothesize that some subset of deep tissue afferents convey the pleasant effects of deep touch. Such a system would parallel the more superficial C-tactile afferent system of the skin that has been shown to mediate the pleasantness perception of gentle stroking touch (Loken et al., 2011; Loken et al., 2009; McGlone et al., 2012; Olausson et al., 2002; Olausson et al., 2008). We are currently conducting studies to test this hypothesis.
In sum, deep pressure can be studied in the lab and can be removed from the effects of interpersonal touch. Deep touch elicits similar affective ratings as C-tactile stroking and exerts a similar calming effect as C-tactile touch. Touch perception and cortical activation patterns for these types of pleasant touch are similar but distinct, and the peripheral sensory afferents that transduce these sensations are likely different. We propose an expanded Social-Affective Touch Hypothesis proposing that gentle stroking and deep pressure are two primary sensory inputs for pleasant, rewarding social touch. Our novel paradigm for the study of deep pressure independent of social context is ideal for studying the affective effects of deep pressure and their physiologic basis. Using this basic paradigm, social interaction can then be added back into the equation to lend further variations to the future study of deep pressure and its role in social touch.
Highlights.
The C-tactile social touch hypothesis proposes that C-tactile fibers form a privileged pathway underlying social touch
We propose that deep pressure, as found in hugs and massage, constitutes another important form of social touch
We develop an oscillating compression sleeve to administer deep pressure and find it is perceived as pleasant and calming
Brain activations to deep pressure are highly similar to C-tactile stroking, with differences in S2, SMG, and insula
Deep pressure may constitute another social touch pathway signaling the close proximity of conspecifics
Acknowledgments
The authors would like to thank Bruce Pritchard and Tom Talbot from NIH Machine Shop for their contributions to designing and constructing the OCS, and Mark Pitcher for the illustration of the OCS.
Funding: This research was funded by the Intramural Research program of the National Center for Complementary and Integrative Health–National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- JMP®, SAS Institute Inc., Cary, NC, 1989–2007. [Google Scholar]
- MATLAB and Statistics Toolbox, The MathWorks, Inc., Natick, MA, United States, 2016. [Google Scholar]
- Akselrod M, Martuzzi R, Serino A, Van der Zwaag W, Gassert R, Blanke O (2017), Anatomical and functional properties of the foot and leg representation in areas 3b, 1 and 2 of primary somatosensory cortex in humans: a 7T fMRI study. NeuroImage 159:473–487. [DOI] [PubMed] [Google Scholar]
- Allen K, Wise N, Frangos E, Komisaruk B (2020), Male Urogenital System Mapped Onto the Sensory Cortex: Functional Magnetic Resonance Imaging Evidence. J Sex Med. [DOI] [PubMed] [Google Scholar]
- Bao R, Wei P, Li K, Lu J, Zhao C, Wang Y, Zhang T (2012), Within-limb somatotopic organization in human SI and parietal operculum for the leg: an fMRI study. Brain Res 1445:30–39. [DOI] [PubMed] [Google Scholar]
- Corey SM, Vizzard MA, Badger GJ, Langevin HM (2011), Sensory innervation of the nonspecialized connective tissues in the low back of the rat. Cells Tissues Organs 194:521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego MA, Field T, Sanders C, Hernandez-Reif M (2004), Massage therapy of moderate and light pressure and vibrator effects on EEG and heart rate. Int J Neurosci 114:31–44. [DOI] [PubMed] [Google Scholar]
- Dietrich C, Blume KR, Franz M, Huonker R, Carl M, Preiβler S, Hofmann GO, Miltner WH, et al. (2017), Dermatomal organization of SI leg representation in humans: revising the somatosensory homunculus. Cereb Cortex 27:4564–4569. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M (2010), Moderate pressure is essential for massage therapy effects. Int J Neurosci 120:381–385. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, and Hernandez-Reif M (2007), Massage therapy research. Dev Rev 27:75–89. [Google Scholar]
- Gilbert C, McCafferty D, Le Maho Y, Martrette JM, Giroud S, Blanc S, Ancel A (2010), One for all and all for one: the energetic benefits of huddling in endotherms. Biol Rev 85:545–569. [DOI] [PubMed] [Google Scholar]
- Gordon I, Voos AC, Bennett RH, Bolling DZ, Pelphrey KA, Kaiser MD (2013), Brain mechanisms for processing affective touch. Human Brain Mapping 34:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandin T (1992), Calming effects of deep touch pressure in patients with autistic disorder, college students, and animals. J Child Adolesc Psychopharmacol 2:63–72. [DOI] [PubMed] [Google Scholar]
- Graven-Nielsen T, Mense S, Arendt-Nielsen L (2004), Painful and non-painful pressure sensations from human skeletal muscle. Exp Brain Res 159:273–283. [DOI] [PubMed] [Google Scholar]
- Guest S, Dessirier JM, Mehrabyan A, McGlone F, Essick G, Gescheider G, Fontana A, Xiong R, et al. (2011), The development and validation of sensory and emotional scales of touch perception. Atten Percept Psychophys 73:531–550. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Unger T, Mense S (2005), Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain 114:168–176. [DOI] [PubMed] [Google Scholar]
- Holsti L, MacLean K, Oberlander T, Synnes A, Brant R (2019), Calmer. Pain Reports 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann C, Behrens T, Woolrich M (2012), Smith SM. Fsl Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Whipple B (2005), Functional MRI of the brain during orgasm in women. Annu Rev Sex Res 16:62–86. [PubMed] [Google Scholar]
- Komisaruk BR, Whipple B, Crawford A, Grimes S, Liu W-C, Kalnin A, Mosier K (2004), Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res 1024:77–88. [DOI] [PubMed] [Google Scholar]
- Krauss KE (1987), The effects of deep pressure touch on anxiety. Am J Occup Ther 41:366–373. [DOI] [PubMed] [Google Scholar]
- Loken LS, Evert M, Wessberg J (2011), Pleasantness of touch in human glabrous and hairy skin: order effects on affective ratings. Brain Res 1417:9–15. [DOI] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H (2009), Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 12:547–548. [DOI] [PubMed] [Google Scholar]
- McGlone F, Olausson H, Boyle JA, Jones-Gotman M, Dancer C, Guest S, Essick G (2012), Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. Eur J Neurosci 35:1782–1788. [DOI] [PubMed] [Google Scholar]
- McGlone F, Vallbo AB, Olausson H, Loken L, Wessberg J (2007), Discriminative touch and emotional touch. Can J Exp Psychol 61:173–183. [DOI] [PubMed] [Google Scholar]
- Mense S, Meyer H (1985), Different types of slowly conducting afferent units in cat skeletal muscle and tendon. The Journal of physiology 363:403–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I, Loken LS, Olausson H (2010), The skin as a social organ. Exp Brain Res 204:305–314. [DOI] [PubMed] [Google Scholar]
- Nakagoshi A, Fukunaga M, Umeda M, Mori Y, Higuchi T, Tanaka C (2005), Somatotopic representation of acupoints in human primary somatosensory cortex: an FMRI study. Magn Reson Med Sci 4:187–189. [DOI] [PubMed] [Google Scholar]
- Olausson H, Cole J, Rylander K, McGlone F, Lamarre Y, Wallin BG, Krämer H, Wessberg J, et al. (2008), Functional role of unmyelinated tactile afferents in human hairy skin: sympathetic response and perceptual localization. Exp Brain Res 184:135–140. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin B, Starck G, Ekholm S, Strigo I, et al. (2002), Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci 5:900–904. [DOI] [PubMed] [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, Ekholm S, Strigo I, et al. (2002), Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci 5:900–904. [DOI] [PubMed] [Google Scholar]
- Olausson H, Wessberg J, McGlone F, Vallbo Å (2010), The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev 34:185–191. [DOI] [PubMed] [Google Scholar]
- Olausson HW, Cole J, Vallbo A, McGlone F, Elam M, Kramer HH, Rylander K, Wessberg J, et al. (2008), Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci Lett 436:128–132. [DOI] [PubMed] [Google Scholar]
- Olausson HW, Cole J, Vallbo Å, McGlone F, Elam M, Krämer HH, Rylander K, Wessberg J, et al. (2008), Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci Lett 436:128–132. [DOI] [PubMed] [Google Scholar]
- Perlman AI, Ali A, Njike VY, Hom D, Davidi A, Gould-Fogerite S, Milak C, Katz DL (2012), Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PloS One 7:e30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, et al. (2004), Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Vallbo AB, Olausson H, Wessberg J (1999), Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Neurophysiol 81:2753–2763. [DOI] [PubMed] [Google Scholar]