Abstract
Neovascular age-related macular degeneration (exudative or wet AMD) is a prevalent, progressive retinal degenerative macular disease that is characterized by neovascularization of the choroid, mainly affecting the elderly population causing gradual vision impairment. Risk factors such as age, race, genetics, iris color, smoking, drinking, BMI, and diet all play a part in nvAMD’s progression, with anti-vascular endothelial growth factor (anti-VEGF) therapy being the mainstay of treatment. Current therapeutic advancements slow the progression of the disease but do not cure or reverse its course. Newer therapies such as gene therapies, Rho-kinase inhibitors, and levodopa offer potential new targets for treatment.
Keywords: age-related macular degeneration, neovascular age-related macular degeneration, etiopathogenesis, risk factors, gene-therapy, anti-VEGF, future advancements
1. Introduction
Age-related macular degeneration (AMD) is a degenerative disease of the retina and is the most prevalent retinal disease in the Western world, with the advanced form affecting 1–3% of its total population [1]. AMD accounts for 8.7% of all blindness worldwide [1] and is the most common cause of blindness in developed nations, with the highest prevalence among Europeans compared to African, Hispanic, and Asian populations. The estimated population of those suffering from AMD worldwide is 196 million in 2020, projected to increase to 288 million by 2040 due largely to increasing lifespan globally and Westernization of diet and lifestyle [1,2].
AMD is classified into two categories: a neovascular “wet” form and a non-neovascular “dry” form, or a mixture of both, all resulting in partial or complete loss of central vision [3]. While less common than dry AMD, wet or nvAMD accounts for almost 90% of blindness associated with AMD. Dry AMD currently has no treatment, and is characterized by thickening of Bruch’s membrane (BrM) due to lipid and protein accumulation, leading to the formation of sub-retinal pigment epithelium (RPE) deposits called drusen [4,5,6]. This deposition interferes with the fluid efflux from the RPE across Bruch’s membrane, chemically and mechanically separating the RPE from the choroid, further reducing perfusion to the RPE [7]. Additional strain is caused by oxidative stress, normal aging, inflammation, and lipofuscin/drusen accumulation, further reducing the flow of nutrients across the Bruch’s membrane [8]. RPE cell death then leads to the loss of photoreceptors, which ultimately leads to loss of vision. In this review, we will discuss etiology and pathophysiology common to AMD as a whole and then focus on those distinct to neovascular AMD plus highlight advances in research and varying treatment modalities for nvAMD to preserve ocular health.
2. Etiology of Neovascular AMD (nvAMD)
nvAMD is a multifactorial disease with numerous risk factors that are thought to play a role in its development. Associated non-modifiable risk factors include increased age, female sex, genetic factors, Caucasian race, and light iris color (Table 1). Modifiable risk factors include smoking, increased BMI, alcohol intake, and dietary habits [9,10,11,12].
Table 1.
Estimated prevalence according to age and neovascular age-related macular degeneration (nvAMD) in populations of European ancestry.
| Age (Years) | Predicted Prevalence % (nvAMD) | Range |
|---|---|---|
| 50 | 0.04 | 0.02–0.07 |
| 55 | 0.08 | 0.05–0.13 |
| 60 | 0.17 | 0.11–0.25 |
| 65 | 0.34 | 0.23–0.49 |
| 70 | 0.7 | 0.48–0.97 |
| 75 | 1.4 | 0.99–1.90 |
| 80 | 2.79 | 1.99–3.79 |
| 85 | 5.48 | 3.91–7.45 |
| 90 | 10.49 | 7.45–14.37 |
Prevalence estimates are based on using International Classification or Wisconsin Age-Related Maculopathy Grading system together with fundus photography/imaging. Data obtained from Rudnicka et al [13].
Increasing age is the strongest demographic and leading risk factor associated with AMD, associated with increasing acellular deposits between the RPE and Bruch’s membrane [14,15]. Patients > 75 years of age had higher 15-year incidences of large drusen, RPE abnormalities, nvAMD, and geographic atrophy (GA) compared to people aged 43 to 54 years, with a 15-year cumulative incidence of late AMD in patients > 75 years at 8% [13]. A pooled meta-analysis of AMD in Europeans (25 studies, n = 57,173) shows a linear increase in the predicted prevalence of AMD, especially in patients > 60 years of age [13]. Progressive failure of repair mechanisms over a lifetime may explain this increased prevalence. One such example is atherosclerosis, the abnormal accumulation of cholesterol plaque within the vascular intima, for which increasing age is the dominant risk factor [5,16]. Subsequently, atherosclerosis is thought to contribute to AMD by atherosclerotic plaques altering choroidal circulation and venous drainage, therefore increasing blood flow resistance and actively increasing lipid deposition into Bruch’s membrane [17]. In support of this hypothesis, the Rotterdam study reported that atherosclerotic plaques in the carotid bifurcation in subjects below 85 years of age were associated with 4.7 times increased odds of developing and being affected by AMD. In addition, plaques in the common carotid artery as well as lower extremity arterial disease (ankle-arm index less than 0.90 on one side) showed a 2.5× increased prevalence developing AMD [18]. Progression of atherosclerosis is associated with hypertension (which is both modifiable and a disease of senesce) [19], with studies reporting that neovascular AMD was more positively associated with a diastolic blood pressure of >95 mm Hg (Odds ratio (OR) = 4.4) [12].
In addition to age, several studies report that race may also play a significant role in AMD (Table 2), with variations that occur in ethnic and geographic populations. Numerous studies have reported that Caucasians are more likely to develop AMD than blacks, including the Baltimore Eye Study, which reported a four-fold higher risk in whites than in blacks [20,21,22,23]. While Hispanics have been reported to be less susceptible to developing AMD, the overall prevalence of late AMD is 0.5%, and early AMD is increased in the Hispanic population [24]. A meta-analysis of 129,664 individuals among 39 studies reports that the prevalence of early and any AMD in Europeans was 11.2% and 12.3%, respectively, while that in Asians were 6.8% and 7.4%, and 7.1% and 7.5% in Africans, respectively [1].
Table 2.
nvAMD prevalence between different races.
| Study | Participants (n=) | Population | nvAMD Prevalence |
|---|---|---|---|
| Beaver Dam Eye Study [25] | 4771 | White | 1.2% Total |
| National Health and Nutrition Examination Survey III [26] | 7888 | White | 0.30% |
| Black | 0.10% | ||
| Mexican-Americans | 0.10% | ||
| The Baltimore eye Study [20] | 4361 | White | 0.60% |
| Black | 0.10% | ||
| The Los Angles Latino Eye Study [27] | 5875 | Mexican-Americans | 0.29% |
| Proyecto [24] | 2776 | Mexican-Americans | 0.14% |
| The Salisbury Eye Evaluation Project [21] | 2008 | White | 1.70% |
| Black | 1.00% | ||
| Hisayama Study [28] | 1486 | Japanese | 0.67% |
Race and associated facial and pigment characteristics may alter the physiological and anatomical configuration, altering the likelihood of certain individuals developing nvAMD. Patients with darker irides have decreased nvAMD incidence, possibly due to increased melanin that absorbs greater light preventing light-induced ROS production and subsequent metabolic demand of the RPE. Caucasians with blue/hazel irides have a greater incidence of extensive macular disease and a greater visual field impairment than dark-colored irides in patients with unilateral nvAMD [29,30]. The Beaver Dam Eye Study found no correlation among Caucasians compared by hair color, skin sun sensitivity, iris color, and late AMD development at the 10-year follow-up [31]. Pigmentary characteristics on nvAMD incidence is controversial and hypothetical at this moment. In this hypothesis, Asians, who have light-colored skin should have a predisposition to developing nvAMD, however their incidence of nvAMD is reduced compared to Caucasians. This suggests that genetics may also play a vital part in nvAMD progression, with several single-nucleotide polymorphisms seen in nvAMD. The reader is asked to refer to Maguire et al. and DeAngelis et al. for a detailed review on the various genetic loci seen in nvAMD [32,33].
While a wide range of studies reveals the impact of intrinsic and genetic influences on nvAMD development, numerous environmental risk factors have been reported (Table 3). Pooled data from the Rotterdam, Beaver Dam Eye, and Blue Mountains studies report a positive correlation with a 2–4-fold likelihood of developing any type of AMD in smokers when compared to non-smokers [5,34,35,36,37,38], suggesting a pathological insult by increased choroidal vascular resistance [39] or oxidative damage to Bruch’s membrane [40,41]. To determine or measure the insult, a combined analysis of 14,752 participants from the Beaver Dam Eye Study, Rotterdam Study, and Blue Mountains Eye Study discovered that the development of nvAMD is greater (odds ratio 4.55) in smokers compared to non-smokers [42], with another study showing the time of onset of nvAMD occurs 5.5 years earlier in smokers than in never smokers [43]. It is hypothesized that smoke-induced damage in nvAMD is mediated through direct oxidation, depletion of anti-oxidants, activation of the immune system, as well as atherosclerotic vascular changes [44]. In mouse models, nicotine has been reported to induce pathologic angiogenesis, promoting choroidal neovascularization (CNV) [45]. This may be in part due to hypoxia that occurs after smoking, which stimulates the production of vascular endothelial growth factor VEGF, leading to neovascularization. Atherosclerosis or vasoconstriction secondary to smoking may further promote this cycle [41,46,47]. Studies exploring the effects of alcohol consumption and AMD development have shown that alcohol increases oxidative damage to the retina [48]. Numerous studies report an increased risk of nvAMD development due to alcohol consumption [49,50,51]. According to the 10-year follow up of the Beaver Dam Eye Study, a history of heavy drinking (four or more servings/day, one drink = 12-ounce beer, 4-ounce glass of wine, or 1.5 ounces of liquor) increased the likelihood of developing nvAMD, with a relative risk of 6.51 (adjusted for age and gender), than in non-heavy drinkers (0 servings/day) [37]. This suggests a dose-dependent relationship to the development of nvAMD based on the quantity or pattern of alcohol consumption. In contrast, the same researchers found on 15-year follow-up that there was no statistically significant association between heavy drinking and nvAMD development, but reported an increased risk of developing GA in heavy drinkers [52,53]. Like smoking, increased light exposure, and heavy drinking, body mass index also increases the body’s oxidative stress and is associated with early and late (nvAMD or GA) AMD [54]. For individuals in the obese group (≥30 kg/m2), studies report a 32% increase in the risk of developing late AMD. Additionally, the overweight group individuals (25–29.9 kg/m2) remained insignificant for its association with early and late AMD [55]. The Beaver Dam Eye Study reported increased BMI was associated with early AMD in female non-smokers (hazard ratio (HR) 1.10), and risk of late AMD among female non-smokers with increased with BMI (HR per 2.5 kg/m2 1.31), waist to hip ratio (HR per 0.1 cm/cm 1.95), waist circumference (HR per 5 cm 1.21), and waist to height ratio (HR per 0.1 cm/cm 1.74) [56].
Table 3.
Modifiable risk factors for AMD.
| Factor | Characteristics | Increased Risk | Suggested Method of Action |
|---|---|---|---|
| Smoking | Current smokers | 4.55 fold nvAMD * [57] | Direct oxidation of RPE [4] |
| Depletion of antioxidants [58] | |||
| Former smokers | 1.54 fold nvAMD * [57] | Activation of Complement [59] | |
| * compared to having never smoked | Atherosclerotic vascular changes [60] | ||
| Alcohol | Heavy drinker (daily alcoholic beverages >4) | RR 6.51 nvAMD * [37] | Oxidative stress [61] |
| * compared to subjects with 0 g Alcohol/day, adjusted for age and gender | |||
| BMI | Obesity (≥30 kg/m2) | 32% increase incidence of late AMD [55] | Oxidative stress [62] |
| Diet | Mediterranean diet | −26% lower risk of progression to advanced (nvAMD or GA) AMD [2] | Certain foods are retino-protective or rich in anti-oxidants [63,64] |
| Western diet | +56% increase in early AMD | ||
| +270% increase advanced AMD [2] |
Together with BMI and other lifestyle choices, diet and nutrition are also thought to be modifiable risk factors (Table 3 and Table 4). The Mediterranean/Oriental diet lowers risks of nvAMD, while the Western diet increases the risk of nvAMD [2]. The Mediterranean and Oriental diets are characterized by high consumption of fruits, vegetables, legumes, whole grains, and seafood. These two regional diets have increased intake of micronutrients and lean meats while having reduced processed food in their diet; the Western diet pattern has a higher intake of red meat, processed meat, high-fat dairy products, fried potatoes, refined grains, and eggs. High adherence to the Mediterranean/Oriental diet was associated with a lower risk of advanced and nvAMD, while a high adherence to the Western diet showed an increased incidence for advanced AMD [2,65,66]. Higher intake of zinc, vitamin D, α-tocopherol, vitamin C, omega-3 fatty acids, and β-carotene was associated with a reduction of nvAMD by 60–90% with the Mediterranean and Oriental diets containing more nutritionally significant micronutrients than Western diets [2,67,68,69]. Dietary habits may also have a confounding impact on other factors such as body mass index and ethnicity, potentially leading to treatment modalities that target lifestyle modifications [70,71].
Table 4.
Nutritional and dietary factors in AMD.
| Study Authors | Diet Rich vs. Poor in Nutrient | Dose Required for Reduction | Odds Ratio | Reduction in AMD |
|---|---|---|---|---|
| Aoki et al., (2016) [72] | Zinc | ≥10.2 (mg/d) | 0.10 1 | nvAMD = 60–90% [2] |
| Vitamin D | ≥27.5 (μgram/d) | 0.40 1 | ||
| α-tocopherol | ≥10.6 (mg/d) | 0.20 1 | ||
| Vitamin C | ≥184.9 (mg/d) | 0.40 1 | ||
| Omega−3 fatty acids | ≥3.9 (g/d) | 0.20 1 | ||
| β-carotene | ≥6.04 (mg/d) | 0.20 1 | ||
| Hogg et al., (2017) [73] | MDS * score | Quartile 4 (high) | 0.53 2 0.61 3 |
nvAMD = 47% [2] nvAMD = 39% [2] |
| SanGiovanni et al., (2007) [74] | Total fish intake | >2 servings/week | nvAMD = 39% [2] | |
| Chiu et al., (2014) [66] | Oriental vs. Western diet | Diet quintile | ||
| Oriental pattern score | Quintile 5 (high) | 0.38 4 | Advanced AMD = 62% [2] | |
| Western pattern score | Quintile 5 (high) | 3.7 5 | Advanced AMD = 270% increase [2] |
* MDS = Mediterranean diet score; 1 ORs compare the highest (Q5) intake of micronutrients to lowest (Q1); 2 ORs compare the highest (≥6) to lowest adherence (≤4) of the MDS; 3 ORs compare >2 medium servings/week with controls for levels (<1 medium serving/month) of fin fish and shellfish intake; 4 ORs compare the highest to lowest quintile of the Oriental pattern score; 5 ORs compare the highest to lowest quintile of the Western pattern score.
3. Pathophysiology
nvAMD’s pathophysiology is not fully understood; however, many studies have shown a multifactorial cause among genetic factors, metabolic factors, and environmental factors (Figure 1) [75,76]. Dry AMD is the precursor to wet AMD, with retinal dysfunction causing atrophic changes and subsequently leading to wet AMD [77]. The human retina is composed of roughly 91 million dim, light-sensitive rods, while the number of cones is 4.5 million, all with extremely high metabolic demand. The fovea is populated by a dense array of cones (200× the density in the fovea compared to the rest of the retina) [78]. Photoreceptor cells shed ~10% of their volume, and the outer photoreceptor segments (OS) basally regenerate roughly the same volume of cellular material each day, which are phagocytosed by the RPE and metabolically processed into waste products that are then ideally secreted through Bruch’s membrane into the choroidal circulation. Certain byproducts are more taxing to break down, such as lipofuscin [79]. Lipofuscin is a fluorescent material with significant phototoxic potential that is recognized as one of the hallmarks of aging [80] with three defining characteristics: they consist of intracellular secondary lysosomes, they emit yellow fluorescence when excited by near-ultraviolet or blue light, and they accumulate during normal senescence [81].
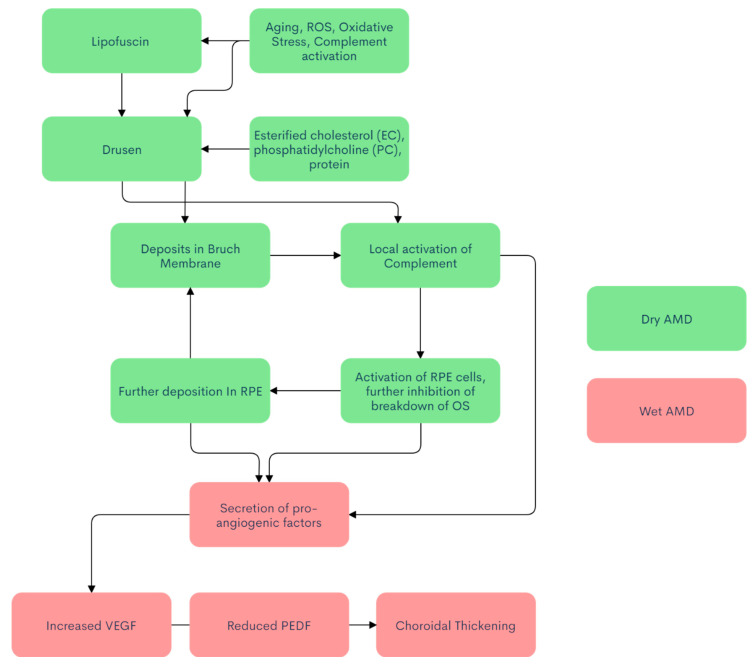
Figure 1.
Flowchart of AMD pathogenesis.
Lipofuscin in the retina is formed from the condensation of two molecules of all-trans-retinal and one molecule of phoshatidylethanolamine [82] and is believed to accumulate because of incomplete digestion of the shed OS in the RPE [83]. Once lipofuscin is in the RPE, it is converted to N-Retinylidene-N-Retinylethanolamine (A2E), a pyridinium bis-retinoid, which is toxic to RPE cells. RPE cells cannot dilute A2E by simple cellular division, so there is a progressive buildup of A2E within the cell, which inhibits phagolysosomal degradation of the OS by the RPE [84]. As the hallmark of AMD, drusen are amorphous, extra-cellular debris that accumulate with age in the area between RPE and the inner collagenous zone of Bruch’s membrane. They are categorized into two categories, “hard” and “soft,” depending on their size and shape. Few small hard drusen less than 63 µm can be found in at least 95% of the aged populations, but the presence of larger (≥125 µm) hard drusen, and more importantly, soft drusen (≥125–250 µm) in the macula is considered a major risk factor for developing advanced forms of AMD, including nvAMD [85].
Drusogenesis is a complex and multifactorial process that takes place over many years. The negative effect of drusen involves drusen formation between RPE and Bruch’s membrane, further decreasing perfusion from the choroid displacing the RPE and photoreceptor layer, effectively distorting vision. Drusen not only acts as a mechanical barrier but also activates the immune system, inducing local inflammation. Drusen is primarily composed of esterified cholesterol (EC), phosphatidylcholine (PC), and protein. Major proteins include vitronectin, complement component 9, apolipoprotein E, clusterin, ATP synthase subunit β, scavenger receptor B2, and retinol dehydrogenase 5 [86]. The immune-associated components include dendritic cell processes [87], major histocompatibility complex (MHC) class II antigens [88], immunoglobulins [89], and components of the complement cascade [90]. Recent studies support the hypothesis that deposits between Bruch’s membrane and the RPE may act as a stimulus for the complement system’s local activation. Due to the strong chemotactic activity of complement activation products, there is an influx of inflammatory cells, which may cause further growth of the deposits and subsequent RPE oxidative cell death. The extra-cellular deposits also contribute to local ischemia of RPE cells, which release angiogenic stimuli leading to choroidal neovascularization [91].
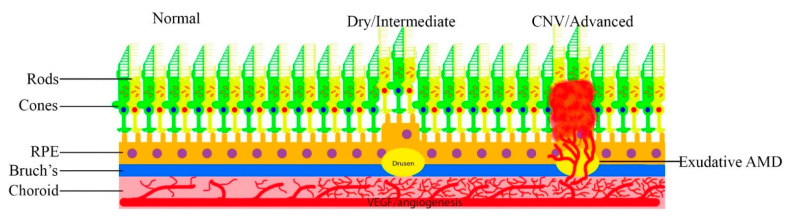
Neovascular AMD, wherein abnormal vessels break through Bruch’s membrane (Figure 2) is one of the leading causes of blindness in the West. It is mediated through several mechanisms. Oxidized low-density lipoproteins, which can exacerbate the deposits in the RPE, inhibit the breakdown of OS in the RPE by delaying the maturation of RPE phagosomes. This leads to the inefficient degradation of the OS and leads to a build-up of undigested lipids and proteins within the RPE, resulting in further production of compounds like A2E [92]. A2E-treated ARPE19 cells increase the expression of pro-angiogenic factors and decreases the expression of anti-angiogenic factors both in vitro and in vivo, with CNV activity greater in A2E-treated eyes [93]. The accumulation of photo-oxidized products in the RPE is believed to be an underlying cause of age-related macular degeneration [79].
Figure 2.
Progression of AMD.
With the retina being one of the highest oxygen-consuming tissues in the body, it is particularly susceptible to oxidative stress because of its high consumption of oxygen, a high proportion of polyunsaturated fatty acids, and its exposure to visible light [94]. The macular pigment acts as a natural barrier protecting the central retina against oxidative damage. The macular pigment (MP) contains three main dietary carotenoids; lutein, zeaxanthin, and meso-zeaxanthin, which forms a natural barrier protecting the retina against oxidative damage, as well as protecting the retina via acting as an optical filter that absorbs short-wave visible light [95,96,97]. Reduced intake of carotenoids and smoking is shown to reduce these macular pigments; however, the association with MP reduction and smoking is not yet clear [98,99]. Still, smoking is a key risk for nvAMD development.
While the MP protects the retina from light oxidation, the shed photoreceptor outer segment membranes are rich in PUFA and can be readily oxidized to induce a cytotoxic chain reaction. The OS segments are degraded in the RPE phagolysosomes, which are not completely digested and aggregate as lipofuscin, deposited in RPE cells’ lysosomes [97]. A2E-loaded RPE cells generated high levels of both hydrogen peroxide (H2O2) and superoxide anion (O2•−) when exposed to blue-violet light [100], mice deficient in superoxide dismutase develop features of AMD including CNV [101]. Factors like cigarette smoke increase oxidative stress by generating ROS and reducing anti-oxidant capacity. Cigarette smoke has been shown in a mouse model to form sub-RPE deposits, thickening of Bruch’s membrane, and accumulation of deposits within the Bruch’s membrane. Normally, oxidative damage is minimized by the presence of anti-oxidant damage and repair systems [102], but anti-oxidant systems are affected with smoking, with dietary anti-oxidants such as carotenoids, retinoids, and tocopherols reduced in smokers (after adjusting for dietary intake), but the mechanism behind this reduction is not yet clear [34,35,36,37,38,103,104,105]. Aging also reduces restorative/ROS coping systems, with increased oxidative damage, including mitochondrial DNA that is more susceptible and essential to the high metabolic demand of RPE [106]. Aging also increases the deposition of beta-amyloid proteins in the eyes. Amyloid proteins are a known constituent of drusen, which can further cause progression to nvAMD [107,108].
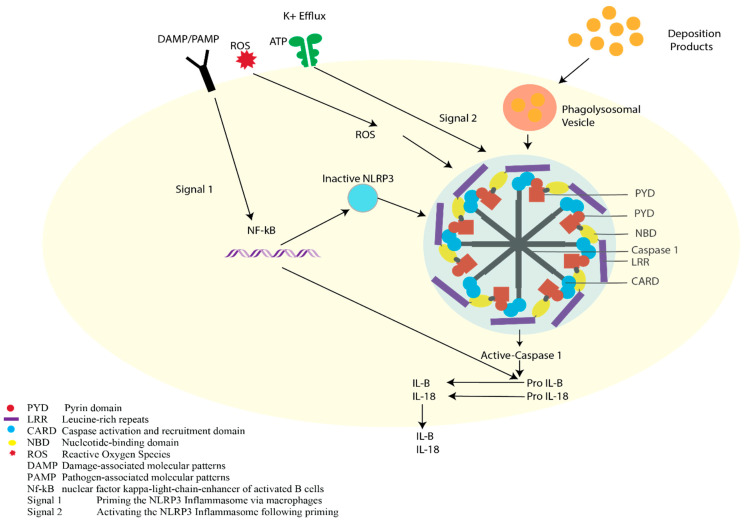
Another type of cell damage or death is induced by caspases, which are cysteine proteases that initiate cellular programs leading to inflammation or cell death [109]. They can either be pro-inflammatory or proapoptotic, depending on the domain architecture and the described functions [110]. Caspase-1 is produced as an inactive zymogen that is recruited by multi-protein complexes called inflammasomes [111], which act as molecular platforms activated by cellular stress. They function in the innate immune response for activation of caspase-1, and subsequent secretion of active interleukins (IL-18 and IL- 1β), which mediate the inflammatory response [112,113,114]. The NLRP3 inflammasome is a critical component of the innate immune system that is linked with AMD (Figure 3) [115].
Figure 3.
NLRP3 inflammasome [116].
IL-18 and IL-1β are synthesized as inactive precursors that require cleavage by caspase-1 to produce biologically active cytokines [117]. IL-1β is considered a classic activator and mediator of inflammation [118] and functions as a potent pro-angiogenic factor by stimulating VEGF production [119]. IL-18 can stimulate both Th1 and Th2 responses depending on the local microenvironment [120,121] and has been implicated in several inflammatory conditions. IL-18 function has been theorized to have a protective effect in other disease models [122]. IL-18 can play either a protective and/or a pro-inflammatory role, depending on the cell’s immunological status or the type and phase of the inflammatory process [123].
With drusen and lipofuscin being essential components of nvAMD development, it has been shown that drusen components, as well as A2E, could activate NLRP3 leading to the activation of the caspase-1 cascade [124,125]. It has been reported that NLRP3 activation can also be caused by oxidative stress and VEGF-A expression [126,127,128]. In addition, studies report that a decrease in extra-cellular osmolarity induced a K(+)-dependent conformational change of the preassembled NLRP3-inactive inflammasome during cell swelling, followed by activation of NLRP3 [129], leading to the cascade of caspase activation and resulting inflammatory response to the retina. Chronic inflammation also leads to P2 × 7R activation by ATP released from damaged retinal cells. P2X7R also directly activates the NLRP3 inflammasome causing further inflammation [130].
NLRP3 was also activated in GA in response to repetitive Alu RNA accumulated in RPE due to a loss of DICER1. Alu RNA was also required for activation of caspase-1 in the inflammasome complex [131,132]. Alu RNAs are short (approximately 300 base pairs) interspersed nuclear elements transcribed from Alu elements in retrotransposons and are the most abundant repetitive elements in the human genome [133]. DICER1 is an RNase that processes double-stranded and self-complementary RNAs, including a majority of premature micro-RNAs (miRNAs) into their bioactive forms [134,135]. DICER1 also metabolizes Alu RNAs in humans with DICER1 deficiency implicated in RPE cell death in nvAMD due to accumulation of unprocessed Alu RNAs, resulting in activation of the NLRP3 inflammasome cascade [132,136]. Wright et al. reported that genetic suppression of DICER1 in three independent mouse models manifested as focal RPE atrophy and aberrant CRNV (choroidal and retinal neovascularization) and that DICER1 expression is reduced in a mouse model of spontaneous CNV. DICER1 as a means of treatment has also been reported, with reports that Aden-associated virus (AAV)-enforced expression of a DICER1 construct successfully escaped miRNA negative feedback, reducing spontaneous CNV in mice [137]. The RPE is crucial to nvAMD development, and a pro-inflammatory retinal environment is promoted by RPE response to various stresses modulating CNV development and progression. The complement system here plays an important role in creating a pro-inflammatory retinal milieu with complement factors C3a and C5a inducing RPE secretion of VEGF and being potent chemotactic agents by recruiting leukocytes to the choroid [138].
Oxidative stress to the RPE by photo by-products such as lipofuscin activates complement, and the induced autoimmune reaction causes complement deposition in the retina [138,139].
There are multiple pathways the RPE can regulate the retinal immune-landscape and, therefore, neovascularization in AMD [140]. In CNV, the macrophage is attracted to the retina with large numbers of retinal macrophages being a hallmark of CNV. However, it could be debated that the increase of macrophages may represent an exacerbation of disease vs. a compensatory vascular-dampening response [140]. In support of macrophages being pro-angiogenic, inhibition of monocyte migration to the retina reduced CNV in a laser-induced mouse model of the disease [141,142]. In contrast, in a non-injury mouse model of AMD, mice that possess defects in macrophage mobilization develop choroidal neovascularization, suggesting that macrophages somehow also protect against CNV [143]. Given the available evidence, macrophages’ most likely role in CNV is determined by local macrophage-polarizing factors. Undifferentiated M0 macrophages can differentiate into the classical (M1) or alternate (M2) pathway. M1 pathway is pro-inflammatory, and the M2 pathway is anti-inflammatory with stimulation of the M2 macrophages, showing increased angiogenic potential compared to M1 macrophages [144,145,146,147]. The uncertainty of macrophages’ role in CNV is also unclear following anti-vascular treatment, as bevacizumab increases the number of retinal macrophages within human neovascular membranes [148].Walshe et al. showed that blockage of VEGF-A increased leucocyte-endothelial adhesion (LEA), which could increase retinal macrophage following anti-VEGF treatments [149], as well as compensatory elevation in VEGF-A levels following anti-VEGF therapy [150]. This warrants further study on the impact and role of retinal macrophages in angiogenesis in AMD.
Another theory of neovascularization is angiopoietin vascular growth factors that activate pathways in the retinal vasculature. Tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2 (Tie2) is a transmembrane receptor that serves as a binding site for hormones angiopoietin 1 (Ang1) and angiopoietin 2 (Ang2) ligands [151]. Ang1 is a full endogenous agonist of Tie2 produced in pericytes surrounding the retinal vasculature. Once bound to Tie2, it phosphorylates the receptor, activating downstream pathways that suppress vascular permeability and maintain vascular stability [152]. Conversely, Ang2 is an endogenous weak, partial agonist of Tie2 that competes with Ang1 to suppress phosphorylation and leads to the activation of Tie2. Overexpression of Ang2 and Tie2 antagonism in animal models results in incorrect vessel organization and failure of vessels to mature [153]. Ang1 co-expressed with VEGF-A prevented retinal detachment and blocked neovascularization [154], while Ang2 is believed to play a role in angiogenesis, vessel destabilization, and inflammation [155,156]. Elevated Ang2 was also found in the aqueous humor of patients with nvAMD and in the vitreous of diabetic patients undergoing a vitrectomy, further adding proof of its pro-inflammatory and pro-angiogenic nature [157,158]. Farcizumab is a new anti-VEGF injection that blocks VEGF as well as Ang2, which is currently being tested [159].
Vascular endothelial growth factor is a potent proangiogenic factor first thought to be an endothelial cell specific mitogen [160], with physiological functions including bone formation [161], hematopoiesis [162], wound healing [163], and development of the vascular and lymphatic system [164,165]. VEGF is upregulated in many tumors and is one of the contributing factors of tumor angiogenesis [166]. VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E and placental growth factor (PGF) are members of the VEGF family [167,168], with VEGF-A strongly inducing vascular proliferation and migration of endothelial cells essential for both physiological and pathological angiogenesis [169]. The production of VEGF can be induced in hypoxic cells; when cells are in a low-oxygen environment, they produce the transcription of the hypoxia-inducible factor 1 alpha (HIF-1 alpha) inducing the release of VEGF-A [170]. VEGF-A acts via endothelial-specific receptor tyrosine kinases (VEGFR1, VEGFR2) located in endothelial cells, with VEGFR-2 being the most important for angiogenesis [171,172]. Oxidative stress triggers many other pathways, with the autocrine signaling and upregulation of VEGF being no exception. Oxidative stress and ROS synergize with other pathways (NLRP3, complement cascade, Ang 2, hypoxia), further promoting angiogenesis and progression of nvAMD [173,174]. Once VEGF-A binds to the receptor, several signaling pathways are activated: (1) the Mitogen-activated protein kinase- (MAPK-) p38 signaling pathway, where the effector heat shock protein (HSP27) reorganizes actin [175], (2) the phosphatidylinositol 3-kinase- (PI3K-) AKT protein kinase B pathway, promoting the formation of nitric oxide [176,177], and (3) the phospholipase C gamma (PLCγ) releasing intracellular calcium, promoting prostaglandin production, increasing vascular permeability [178,179]. Rearrangement of the cytoskeleton is crucial for various steps of angiogenesis, with actin being a key cytoskeletal element integral for cell division and motility; actin rearrangement leads to formation of protrusive structures, generating intracellular forces required for cell migration [180,181,182]. Nitric oxide is a known vasodilator that can lead to upregulation of VEGF by enhancing HIF-1 alpha activity under normoxic conditions [183,184].
Under healthy conditions, the production of VEGF upregulates the production of PEDF [185] (pigment epithelium-derived factor) via the RPE [186]. PEDF is a potent endogenous down-regulator of angiogenesis [187], via intracellular translocation of the transmembrane domain of VEGFR-1 [188]. With RPE dysregulation being a key feature of AMD [189], the protective role of PEDF is reduced, with studies reporting a decrease in PEDF in the choroid of AMD eyes, suggesting that decreased PEDF in the choroid-BrM-RPE [190] creates a non-restrictive environment for the progression to nvAMD [191].
In nvAMD, anti-VEGF therapy is currently the only treatment available, which only slows down the progression of the disease, however more knowledge about the downstream effects of factors such as VEGF may offer another treatment modality.
4. Treatment
The treatment modalities for nvAMD have greatly evolved over time. The earliest form of nvAMD treatment with laser photocoagulation showed reduced loss of visual acuity (VA) with severe VA loss in 60% of untreated eyes, compared to 25% of eyes treated with argon laser photocoagulation [192]. Krypton red laser photocoagulation for CNV slightly reduced VA loss of six or more lines at three years in 49% of treated eyes vs. 58% of untreated eyes [193]. Photodynamic therapy (PDT) with verteporfin was a predominant treatment for sub-retinal neovascular membrane (SNRVM). When irradiated with a 689 nm laser light, verteporfin releases radical oxygen species that damage the choroidal neovascular endothelium [194,195], circumventing the adjacent’s damage retina and resulting in short term cessation of fluorescein leakage from choroidal neovascularization [196,197]. Through extensive clinical trials (Table 5), anti-VEGF compounds have drastically changed treatment for nvAMD. The first anti-VEGF agent approved for use in nvAMD was Pegaptanib (Macugen) [198]. Since then, newer anti-VEGF therapies have emerged and widely replaced the use of Pegaptanib, such as brolucizumab (Beovu), ranibizumab (Lucentis), aflibercept (Eylea), and the off-label use of bevacizumab (Avastin) [199]. Anti-VEGF therapy is the mainstay of wet AMD treatment as it affects multiple key pathogenic pathways (Figure 4). Apart from nvAMD, anti-VEGF therapy has been used for other ocular (diabetic macular edema [200], vascular occlusions [201], vitreous hemorrhage [202], severe diabetic retinopathy [203], neovascular glaucoma [204], Eales disease [205], Coats disease [206], iris neovascularization [207], post-operative trabeculectomy [208], pterygium [209], retinopathy of prematurity [210], post-operative cystoid macular edema [211]) and oncological diseases (ovarian cancer [212], gastric cancer [213], prostate cancer [214], GIST(Gastrointestinal stromal tumor) [215], pancreatic cancer [216], non-small cell lung carcinoma [217]).
Table 5.
Anti-vascular endothelial growth factor (Anti-VEGF) clinical trials.
| Study Name | Drug | Control | Treatment | Follow up | Change in ETDRS | |
|---|---|---|---|---|---|---|
| Treatment 1 | Control 2 | |||||
| MARINA [218] | Ranibizumab | Sham injections q 4 weeks | Ranibizumab 0·5 mg q 4 weeks | 1 year | +7.2 | –10.4 |
| ANCHOR [219] | Ranibizumab | Sham injection q 4 weeks plus PDT | Ranibizumab 0·5 mg q 4 weeks+ sham PDT | 1 year | +11.3 | –9.5 |
| HARBOR [220] | Ranibizumab | Ranibizumab 0·5 q 4 weeks | Ranibizumab 0·5 mg q 4 weeks × 3 months then as required | 2 years | +7.9 | +9.1 |
| TREX [221] | Ranibizumab | Ranibizumab 0·5 q 4 weeks | Ranibizumab 0·5 mg q 4 weeks × 3 months until disease inactive then extension per protocol | 1 year | +10.5 | +9.2 |
| FOCUS [222] | Ranibizumab | Sham injections+PDT | Ranibizumab+PDT 0.5 mg q 4 weeks, PDT = day one + quarterly if needed | 2 years | 4.6 | −7.8 |
| BRAMD [223] | Bevacizumab | Ranibizumab 0·5 mg q 4 weeks | Bevacizumab 1·25 mg q 4 weeks | 1 year | +5.1 | +6.4 |
| LUCAS [224] | Bevacizumab | Ranibizumab 0·5 mg q 4 weeks until nvAMD is inactive, then extension by 2 weeks (maximum of 12 weeks) | Bevacizumab 1·25 mg q 4 weeks until nvAMD is inactive, then extension by 2 weeks (maximum of 12 weeks) | 1 year | +7.9 | +8.2 |
| CATT [225] | Bevacizumab | Ranibizumab 0·5 mg q 4 weeks | Bevacizumab 1·25 mg q 4 weeks | 1 year | +7.8 | +8.8 |
| GEFAL [226] | Bevacizumab | Ranibizumab 0·5 mg q 4 weeks × 3 months, then as needed | Bevacizumab 0·5 mg q 4 weeks × 3 months, then as needed | 1 year | +4.8 | +2.9 |
| IVAN [227] | Bevacizumab | Ranibizumab 0·5 mg q 4 weeks | Bevacizumab 1·25 mg q 4 weeks | 2 yearss | +4.1 | +4.9 |
| VIEW 1 [228] | Aflibercept | Ranibizumab 0·5 mg q 4 weeks | Aflibercept 2 mg every 2 months | 1 year | +7.9 | +8.1 |
| VIEW 2 [228] | Aflibercept | Ranibizumab 0·5 mg q 4 weeks | Aflibercept 2 mg every 2 months | 1 year | +8.9 | +9.4 |
| HAWK [229] | Brolucizumab | Aflibercept 2 mg q 8 weeks | Brolucizumab 3 mg q 12 weeks Or Brolucizumab 6 mg q12 weeks |
96 weeks | +5.6 +5.9 |
+5.3 |
| HARRIER [229] | Brolucizumab | Aflibercept 2 mg q 8 weeks | Brolucizumab 6 mg q12 weeks | 96 weeks | +6.1 | +6.6 |
| AURORA [230] | Conbercept | Conbercept 0·5 mg q 4 weeks × 3 months, then monthly or as required | Conbercept 2 mg q 4 weeks × 3 months, then monthly or as required | 1 year | +15.4 | +9.3 |
| PHOENIX [231] | Conbercept | Sham injections q 4 weeks × 3 months then conbercept 0.5 mg q 4 weeks × 3 months | Conbercept 0.5 mg q 4 weeks × 3 months, then quarterly | 1 year | +9.98 | +8.81 |
| STAIRWAY [232] | Farcizumab | 0.5 mg ranibizumab q 4 weeks × 52 weeks | Farcizumab 6 mg q 16 weeks | 52 weeks | +11.4 | +9.6 |
| Or | ||||||
| Farcizumab 6 mg q 12 weeks | +10.1 | |||||
1 = Letters gained with treatment; 2 = Letters gained/lost from baseline control.
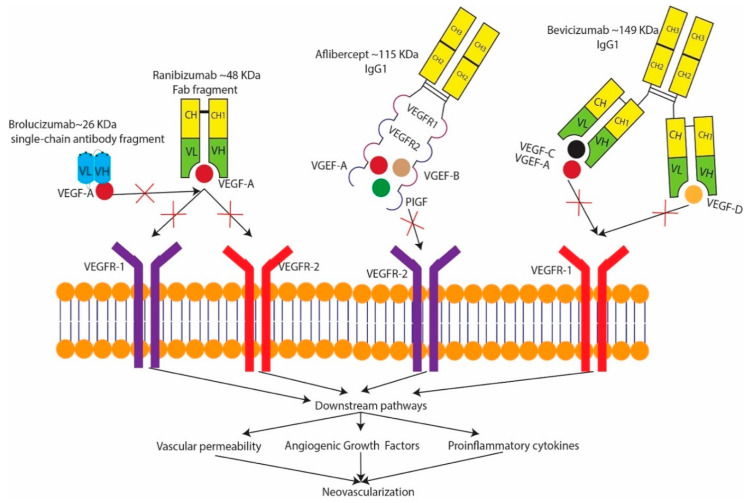
Figure 4.
Mechanism of anti-VEGF drugs.
Ranibizumab (Lucentis) is a 48-kD Fab fragment of the A4.6.1 antibody. The A4.6.1 antibody is one of the four antibodies of the IgG1 isotope that most effectively binds and neutralizes different isomers of VEGF-A [233,234]. Bevacizumab (Avastin) is a full-length monoclonal antibody (149 kDa) that binds to all isoforms of VEGF-A [235]. It was approved by the FDA in February 2004 for the treatment of metastatic colorectal cancer, and is currently the cheapest of the anti-VEGF drugs [236]. Aflibercept is a 115 kDa fully human, a recombinant fusion protein composed of immunoglobulin binding domain of VEGF receptor 1 and 2, fused to the Fc region of human IgG that binds to VEGF-A, VEGF-B, and placental growth factor (PlGF). In addition to its use as a nonirritating intravitreal injection, it is also used for the treatment of cancer. Aflibercept uniquely binds to PlGF, which is present in human CNV membranes and contributes to the development of experimental CNV [237,238]. The loss of PIGF signaling seen in aflibercept is also useful in treatment of diabetic retinopathy, as aflibercept leads to blockage of the ERK pathway and subsequent TNF-alpha suppression in high glucose states [239]. Brolucizumab (Beovu) is a humanized single-chain variable fragment (scFv) that is a potent inhibitor of VEGF-A. The scFv comprises the monoclonal antibody’s variable light and heavy chain domains tethered by a flexible linker, resulting in a small fragment weighing approximately 26 kDa, which increases bioavailability, reduces immunogenicity, and allows better ocular tissue penetration [229,240,241]. Brolucizumab is the newest anti-VEGF drug on the market, and aims to reduce the number of annual anti-VEGF injections. The HAWK and HARRIER trials compared 3 mg and 6 mg of brolucizumab with 2 mg of aflibercept spaced at 12 weeks (brolucizumab) and 8 weeks (aflibercept), with a reduction to q 8 weeks (brolucizumab) if necessary. In brolucizumab-treated eyes on the q12w regimen at the week 44 in HAWK, there was an 80.5% (3 mg) and 81.5% (6 mg) probability of remaining on an q12w interval until week 92, with a 75.4% for the brolucizumab 6 mg group in HARRIER. The probability of maintaining the q 12-week regimen from loading to week 92 was 39.7% and 45.4% for the brolucizumab 3 mg and 6 mg treatment groups in HAWK, and 38.6% for the 6 mg group in HARRIER [229]. Pluykhova et al. compared the safety profile of long-term use of ranibizumab, bevacizumab, and aflibercepts in the RCT (randomized clinical trial) meta-analysis, with the study hypothesizing the potential benefits of ranibizumab vs. bevacizumab in terms of a systemic safety profile. However, more direct RCT on ocular safety needs to be conducted to validate these findings further [242].
Future Advancements
Conbercept (143 kDa) is an anti-VEGF agent that consists of the extra-cellular domain 2 of VEGFR1 and extra-cellular domains 3 and 4 of VEGFR2 fused to the Fc portion of human IgG1. Similar to aflibercept, conbercept targets VEGF-A, VEGF-B, and PIGF [243,244]. Farcizumab is the first bispecific antibody for both VEGF-A and angiopoietin 2 (Ang-2) specifically designed for intraocular use assembled using Roche’s CrossMAb technology, which binds to both VEGF-A and Ang-2 with high affinity and specificity. It has been engineered to abolish certain binding interactions to Fc ϒR and Fc Rn, which increased systemic but not the ocular clearance [245]. Patients treated with farcizumab had a greater mean BCVA increase that was maintained at week 52, with corresponding anatomic improvements compared to ranibizumab [232].
Ripasudil is a Rho-associated kinase inhibitor developed originally for the treatment of glaucoma and ocular hypertension [246]. Rho-associated protein kinase (ROCK 1 and ROCK 2) determine the macrophage polarization into the M1 and M2 subtypes. Aging increases ROCK2 signaling, resulting in over-expression of the pro-angiogenic macular degeneration associated macrophages, which increases IL-4 leading to angiogenesis [145,146]. A recent study showed that ripasudil suppressed expression levels of ROCK1, ROCK2, and miR-136-5p, resulting in inhibition of NLRP3, ASC, caspase1, IL-1β, and IL-18 [247].
Gene therapies targeting nvAMD have been performed on animal models, and small-scale clinical trials have been performed on human subjects. Soluble fms-like tyrosine kinase-1(sFlt-1) is an anti-angiogenic receptor for VEGF that binds and causes VEGF sequestration [248]. Animal models of rAAV.sFLT-1 injected mice caused upregulation of sFlt-1, reduced retinal neovascularization [249]. In human studies, small scale r.AAV.sFLT-1 have been conducted with inconclusive results [250,251]. There are many other vectors/targets being used in gene therapy (Table 6), but gene therapy is currently in its infancy [252,253,254,255,256]. With the immense potential of gene therapy to treat multiple diseases, more research into the vectors and their targets is needed to further validate possible therapeutic use in nvAMD.
Table 6.
Ongoing gene therapy clinical trials for nvAMD.
| Trial Name | Targeted Gene | Vector | Sponsor |
|---|---|---|---|
| NCT01494805 [257] | sFLT01 | AAV-2 | Adverum Biotechnologies, Inc. (Redwoodcity, CA, USA) |
| NCT03585556 [258] | sCD59 | AAV-2 | Hemera Biosciences (Waltham, MA, USA) |
| NCT01024998 [254] | sFLT-1 | AAV-2 | Sanofi Genzyme (Cambridge, MA, USA) |
| NCT03748784 [259] | Aflibercept | AAV-2 | Adverum Biotechnologies, Inc. (Redwoodcity, CA, USA) |
| NCT03066258 [253] | Anti-VEGF Fab | AAV-8 | Regenxbio Inc.(Rockville, MD, USA) |
| NCT01678872 [256] | Endostatin/Angiostatin | EIAV | Oxford BioMedica (Oxford, UK) |
| NCT00109499 [255] | PEDF | AAV5 | GenVec (Gaithersburg, MD, USA) |
| NCT03999801 [260] | Monoclonal antibody fragment | AAV-8 | Regenxbio Inc. (Rockville, MD, USA) |
Levodopa’s effects on nvAMD have been studied on the basis that the RPE has a G protein-coupled receptor (GPR143) activated by levodopa. As part of normal aging, melanin, as well as pigment epithelium-derived factor (PEDF), are reduced, and GPR413 activation via signaling reduces VEGF secretion and simultaneously increases PEDF. Studies show that patients using levodopa have a lower risk and a greater age of onset of nvAMD, with Figueroa et al., reporting that levodopa delayed anti-VEGF therapy while improving visual outcomes. In the first month, retinal fluid decreased by 29% without anti-VEGF treatment, with the retinal fluid sustaining for six months. Mean visual acuity improved by 4.7 and 4.8 letters in cohort-1 (levodopa, newly diagnosed, naïve anti-VEGF) and cohort-2 (levodopa, previous anti-VEGF) with a 52% reduction in the need for anti-VEGF injections in Cohort-2 [261].
5. Conclusions
AMD is one of the most common retinal disorders that affect millions, predominantly in the Western world. It is a disease of the developed world with worldwide implications. nvAMD is one subtype that causes 90% of blindness associated with AMD. With the abundant research being conducted, our understanding of nvAMD and the pathologic mechanisms has progressed along with a wide array of treatment modalities, with newer agents being investigated. Anti-VEGF therapy remains the mainstream treatment for slowing the disease’s progression, but is expensive, requires frequent treatments and ultimately does not treat the disease entirely. Due to the success of anti-VEGF therapy, newer anti-VEGF drugs are being research to further increase the treatment efficacy and to reduce cost by prolonging the treatment interval. Due to its multifactorial nature, patients must be educated about modifiable risk factors and the lifestyle changes that can prevent or delay the development of the disease in susceptible individuals. Treatment modalities are evolving that target inflammatory processes, signaling proteins, gene therapies, and newer drugs are currently under trial to treat/delay the disease or prolong the treatment interval.
Abbreviations
| AMD | Age-related macular degeneration |
| nvAMD | Neovascular age-related macular degeneration |
| CNV | Choroidal neovascularization |
| BrM | Bruch’s Membrane |
| GA | Geographic atrophy |
| OS | Outer photoreceptor segments |
| RPE | Retinal pigment epithelium |
| MHC | Major histocompatibility complex |
| IL | Interleukin |
| A2E | N-Retinylidene-N-Retinylethanolamine |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| VEGF | Vascular endothelial growth factor |
| PIGF | Placental growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
| Th2 | Helper T-cells type 1 |
| Th2 | Helper T-cells type 2 |
| Tie2 | Tyrosine kinase with immunoglobulin-like and epidermal growth factor-like domains 2 |
| Ang | Angiopoietin |
| PDT | Photodynamic therapy |
| SNRVM | Sub-retinal neovascular membrane |
| Anti-VEGF | Anti-vascular endothelial growth factor |
| AAV | Aden-associated virus |
| Rock | Rho-associated protein kinase |
| OR | Odds ratio |
| HR | Hazard ratio |
Author Contributions
Conceptualization, B.A., A.P., M.H., and P.J.; formal analysis, B.A., A.P., M.H., and P.J.; resources, B.A., A.P., M.H., and P.J.; writing—original draft preparation, B.A., A.P., M.H., and P.J.; writing—review and editing, B.A., A.P., M.H., and P.J.; visualization, B.A., A.P., M.H., and P.J.; supervision, B.A.; project administration, B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 2.Chapman N.A., Jacobs R.J., Braakhuis A.J. Role of diet and food intake in age-related macular degeneration: A systematic review. Clin. Exp. Ophthalmol. 2019;47:106–127. doi: 10.1111/ceo.13343. [DOI] [PubMed] [Google Scholar]
- 3.Kokotas H., Grigoriadou M., Petersen M.B. Age-related macular degeneration: Genetic and clinical findings. Clin. Chem. Lab. Med. 2011;49:601–616. doi: 10.1515/CCLM.2011.091. [DOI] [PubMed] [Google Scholar]
- 4.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research Group Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-Related Eye Disease Study Report Number 3. Ophthalmology. 2000;107:2224–2232. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis M.D., Gangnon R.E., Lee L.-Y., Hubbard L.D., Klein B.E.K., Klein R., Ferris F.L., Bressler S.B., Milton R.C., Age-Related Eye Disease Study G. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch. Ophthalmol. 2005;123:1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman H.R., Chan C.-C., Ferris F.L., 3rd, Chew E.Y. Age-related macular degeneration. Lancet. 2008;372:1835–1845. doi: 10.1016/S0140-6736(08)61759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: The Age-Related Eye Disease Study Report Number 6. Am. J. Ophthalmol. 2001;132:668–681. doi: 10.1016/S0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 9.Hyman L., Neborsky R. Risk factors for age-related macular degeneration: An update. Curr. Opin. Ophthalmol. 2002;13:171–175. doi: 10.1097/00055735-200206000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Ristau T., Ersoy L., Hahn M., den Hollander A.I., Kirchhof B., Liakopoulos S., Fauser S. Nongenetic Risk Factors for Neovascular Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2014;55:5228–5232. doi: 10.1167/iovs.14-14299. [DOI] [PubMed] [Google Scholar]
- 11.Velilla S., García-Medina J.J., García-Layana A., Dolz-Marco R., Pons-Vázquez S., Pinazo-Durán M.D., Gómez-Ulla F., Arévalo J.F., Díaz-Llopis M., Gallego-Pinazo R. Smoking and age-related macular degeneration: Review and update. J. Ophthalmol. 2013;2013:895147. doi: 10.1155/2013/895147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyman L., Schachat A.P., He Q., Leske M.C. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch. Ophthalmol. 2000;118:351–358. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 13.Rudnicka A.R., Jarrar Z., Wormald R., Cook D.G., Fletcher A., Owen C.G. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: A meta-analysis. Ophthalmology. 2012;119:571–580. doi: 10.1016/j.ophtha.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Huang J.-D., Presley J.B., Chimento M.F., Curcio C.A., Johnson M. Age-related changes in human macular Bruch’s membrane as seen by quick-freeze/deep-etch. Exp. Eye Res. 2007;85:202–218. doi: 10.1016/j.exer.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buch H., Vinding T., la Cour M., Jensen G.B., Prause J.U., Nielsen N.V. Risk factors for age-related maculopathy in a 14-year follow-up study: The Copenhagen City Eye Study. Acta Ophthalmol. Scand. 2005;83:409–418. doi: 10.1111/j.1600-0420.2005.00492.x. [DOI] [PubMed] [Google Scholar]
- 16.Head T., Daunert S., Goldschmidt-Clermont P.J. The Aging Risk and Atherosclerosis: A Fresh Look at Arterial Homeostasis. Front. Genet. 2017;8:216. doi: 10.3389/fgene.2017.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kur J., Newman E.A., Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog. Retin. Eye Res. 2012;31:377–406. doi: 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vingerling J.R., Dielemans I., Bots M.L., Hofman A., Grobbee D.E., de Jong P.T.V.M. Age-related Macular Degeneration Is Associated with Atherosclerosis: The Rotterdam Study. Am. J. Epidemiol. 1995;142:404–409. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- 19.Buford T.W. Hypertension and aging. Ageing Res. Rev. 2016;26:96–111. doi: 10.1016/j.arr.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman D.S., Katz J., Bressler N.M., Rahmani B., Tielsch J.M. Racial differences in the prevalence of age-related macular degeneration: The Baltimore eye survey. Ophthalmology. 1999;106:1049–1055. doi: 10.1016/S0161-6420(99)90267-1. [DOI] [PubMed] [Google Scholar]
- 21.Bressler S.B., Muñoz B., Solomon S.D., West S.K. The Salisbury Eye Evaluation (SEE) Study Team. Racial Differences in the Prevalence of Age-Related Macular Degeneration: The Salisbury Eye Evaluation (SEE) Project. Arch. Ophthalmol. 2008;126:241–245. doi: 10.1001/archophthalmol.2007.53. [DOI] [PubMed] [Google Scholar]
- 22.Clemons T.E., Milton R.C., Klein R., Seddon J.M., Ferris F.L., 3rd Risk factors for the incidence of Advanced Age-Related Macular Degeneration in the Age-Related Eye Disease Study (AREDS) AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein R., Klein B.E., Knudtson M.D., Wong T.Y., Cotch M.F., Liu K., Burke G., Saad M.F., Jacobs D.R., Jr. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113:373–380. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Muñoz B., Klein R., Rodriguez J., Snyder R., West S.K. Prevalence of age-related macular degeneration in a population-based sample of Hispanic people in Arizona: Proyecto VER. Arch. Ophthalmol. 2005;123:1575–1580. doi: 10.1001/archopht.123.11.1575. [DOI] [PubMed] [Google Scholar]
- 25.Klein R., Klein B.E., Linton K.L. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/S0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 26.Klein R., Klein B.E., Jensen S.C., Mares-Perlman J.A., Cruickshanks K.J., Palta M. Age-related maculopathy in a multiracial United States population: The National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–1065. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 27.Varma R., Fraser-Bell S., Tan S., Klein R., Azen S.P. Prevalence of age-related macular degeneration in Latinos: The Los Angeles Latino eye study. Ophthalmology. 2004;111:1288–1297. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Oshima Y., Ishibashi T., Murata T., Tahara Y., Kiyohara Y., Kubota T. Prevalence of age related maculopathy in a representative Japanese population: The Hisayama study. Br. J. Ophthalmol. 2001;85:1153–1157. doi: 10.1136/bjo.85.10.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandberg M.A., Gaudio A.R., Miller S., Weiner A. Iris pigmentation and extent of disease in patients with neovascular age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1994;35:2734–2740. [PubMed] [Google Scholar]
- 30.Nicolas C.M., Robman L.D., Tikellis G., Dimitrov P.N., Dowrick A., Guymer R.H., McCarty C.A. Iris colour, ethnic origin and progression of age-related macular degeneration. Clin. Exp. Ophthalmol. 2003;31:465–469. doi: 10.1046/j.1442-9071.2003.00711.x. [DOI] [PubMed] [Google Scholar]
- 31.Tomany S.C., Klein R., Klein B.E. The relationship between iris color, hair color, and skin sun sensitivity and the 10-year incidence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology. 2003;110:1526–1533. doi: 10.1016/S0161-6420(03)00539-6. [DOI] [PubMed] [Google Scholar]
- 32.Deangelis M.M., Silveira A.C., Carr E.A., Kim I.K. Genetics of age-related macular degeneration: Current concepts, future directions. Semin. Ophthalmol. 2011;26:77–93. doi: 10.3109/08820538.2011.577129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maguire M.G., Ying G.-S., Jaffe G.J., Toth C.A., Daniel E., Grunwald J., Martin D.F., Hagstrom S.A., Group C.R. Single-Nucleotide Polymorphisms Associated With Age-Related Macular Degeneration and Lesion Phenotypes in the Comparison of Age-Related Macular Degeneration Treatments Trials. JAMA Ophthalmol. 2016;134:674–681. doi: 10.1001/jamaophthalmol.2016.0669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell P., Wang J.J., Smith W., Leeder S.R. Smoking and the 5-Year Incidence of Age-Related Maculopathy: The Blue Mountains Eye Study. Arch. Ophthalmol. 2002;120:1357–1363. doi: 10.1001/archopht.120.10.1357. [DOI] [PubMed] [Google Scholar]
- 35.Klein R., Klein B.E., Knudtson M.D., Meuer S.M., Swift M., Gangnon R.E. Fifteen-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 36.Klein R., Klein B.E.K., Moss S.E. Relation of Smoking to the Incidence of Age-related Maculopathy: The Beaver Dam Eye Study. Am. J. Epidemiol. 1998;147:103–110. doi: 10.1093/oxfordjournals.aje.a009421. [DOI] [PubMed] [Google Scholar]
- 37.Klein R., Klein B.E.K., Tomany S.C., Moss S.E. Ten-Year Incidence of Age-related Maculopathy and Smoking and Drinking: The Beaver Dam Eye Study. Am. J. Epidemiol. 2002;156:589–598. doi: 10.1093/aje/kwf092. [DOI] [PubMed] [Google Scholar]
- 38.Thornton J., Edwards R., Mitchell P., Harrison R.A., Buchan I., Kelly S.P. Smoking and age-related macular degeneration: A review of association. Eye. 2005;19:935–944. doi: 10.1038/sj.eye.6701978. [DOI] [PubMed] [Google Scholar]
- 39.Hara K. Effects of cigarette smoking on ocular circulation chronic effect on choroidal circulation. Nippon Ganka Gakkai Zasshi. 1991;95:939–943. [PubMed] [Google Scholar]
- 40.Fields M.A., Bowrey H.E., Gong J., Moreira E.F., Cai H., Del Priore L.V. Extracellular matrix nitration alters growth factor release and activates bioactive complement in human retinal pigment epithelial cells. PLoS ONE. 2017;12:e0177763. doi: 10.1371/journal.pone.0177763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fujihara M., Nagai N., Sussan T.E., Biswal S., Handa J.T. Chronic cigarette smoke causes oxidative damage and apoptosis to retinal pigmented epithelial cells in mice. PLoS ONE. 2008;3:e3119. doi: 10.1371/journal.pone.0003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith W., Assink J., Klein R., Mitchell P., Klaver C.C.W., Klein B.E.K., Hofman A., Jensen S., Wang J.J., de Jong P.T.V.M. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001;108:697–704. doi: 10.1016/S0161-6420(00)00580-7. [DOI] [PubMed] [Google Scholar]
- 43.Khan J.C., Thurlby D.A., Shahid H., Clayton D.G., Yates J.R.W., Bradley M., Moore A.T., Bird A.C. Smoking and age related macular degeneration: The number of pack years of cigarette smoking is a major determinant of risk for both geographic atrophy and choroidal neovascularisation. Br. J. Ophthalmol. 2006;90:75. doi: 10.1136/bjo.2005.073643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suner I.J., Espinosa–Heidmann D.G., Pereira–Simon S., Piña Y., Cousins S.W. Cigarette Smoke Increases Severity of Experimental Choroidal Neovascularization (CNV): Role of Inflammation. Investig. Ophthalmol. Vis. Sci. 2005;46:3507. [Google Scholar]
- 45.Lee J., Cooke J.P. Nicotine and pathological angiogenesis. Life Sci. 2012;91:1058–1064. doi: 10.1016/j.lfs.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang M.A., Bressler S.B., Munoz B., West S.K. Racial Differences and Other Risk Factors for Incidence and Progression of Age-Related Macular Degeneration: Salisbury Eye Evaluation (SEE) Project. Investig. Ophthalmol. Vis. Sci. 2008;49:2395–2402. doi: 10.1167/iovs.07-1584. [DOI] [PubMed] [Google Scholar]
- 47.Akishima S., Matsushita S., Sato F., Hyodo K., Imazuru T., Enomoto Y., Noma M., Hiramatsu Y., Shigeta O., Sakakibara Y. Cigarette-smoke-induced vasoconstriction of peripheral arteries: Evaluation by synchrotron radiation microangiography. Circ. J. 2007;71:418–422. doi: 10.1253/circj.71.418. [DOI] [PubMed] [Google Scholar]
- 48.Cederbaum A.I. Role of lipid peroxidation and oxidative stress in alcohol toxicity. Free Radic. Biol. Med. 1989;7:537–539. doi: 10.1016/0891-5849(89)90029-4. [DOI] [PubMed] [Google Scholar]
- 49.Chong E.W., Kreis A.J., Wong T.Y., Simpson J.A., Guymer R.H. Alcohol consumption and the risk of age-related macular degeneration: A systematic review and meta-analysis. Am. J. Ophthalmol. 2008;145:707–715. doi: 10.1016/j.ajo.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Arnarsson A., Sverrisson T., Stefánsson E., Sigurdsson H., Sasaki H., Sasaki K., Jonasson F. Risk Factors for Five-Year Incident Age-related Macular Degeneration: The Reykjavik Eye Study. Am. J. Ophthalmol. 2006;142:419–428.e411. doi: 10.1016/j.ajo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 51.Coleman A.L., Seitzman R.L., Cummings S.R., Yu F., Cauley J.A., Ensrud K.E., Stone K.L., Hochberg M.C., Pedula K.L., Thomas E.L., et al. The Association of Smoking and Alcohol Use With Age-related Macular Degeneration in the Oldest Old: The Study of Osteoporotic Fractures. Am. J. Ophthalmol. 2010;149:160–169. doi: 10.1016/j.ajo.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knudtson M.D., Klein R., Klein B.E.K. Alcohol consumption and the 15-year cumulative incidence of age-related macular degeneration. Am. J. Ophthalmol. 2007;143:1026–1029. doi: 10.1016/j.ajo.2007.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adams M.K.M., Chong E.W., Williamson E., Aung K.Z., Makeyeva G.A., Giles G.G., English D.R., Hopper J., Guymer R.H., Baird P.N., et al. 20/20—Alcohol and Age-related Macular Degeneration: The Melbourne Collaborative Cohort Study. Am. J. Epidemiol. 2012;176:289–298. doi: 10.1093/aje/kws004. [DOI] [PubMed] [Google Scholar]
- 54.Manna P., Jain S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015;13:423–444. doi: 10.1089/met.2015.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q.Y., Tie L.J., Wu S.S., Lv P.L., Huang H.W., Wang W.Q., Wang H., Ma L. Overweight, Obesity, and Risk of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016;57:1276–1283. doi: 10.1167/iovs.15-18637. [DOI] [PubMed] [Google Scholar]
- 56.Howard K.P., Klein B.E.K., Lee K.E., Klein R. Measures of body shape and adiposity as related to incidence of age-related eye diseases: Observations from the Beaver Dam Eye Study. Investig. Ophthalmol. Vis. Sci. 2014;55:2592–2598. doi: 10.1167/iovs.13-13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vingerling J.R., Hofman A., Grobbee D.E., de Jong P.T.V.M. Age-Related Macular Degeneration and Smoking: The Rotterdam Study. Arch. Ophthalmol. 1996;114:1193–1196. doi: 10.1001/archopht.1996.01100140393005. [DOI] [PubMed] [Google Scholar]
- 58.Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180:121–137. doi: 10.1016/S0300-483X(02)00386-4. [DOI] [PubMed] [Google Scholar]
- 59.Rohrer B., Frazer-Abel A., Leonard A., Ratnapriya R., Ward T., Pietraszkiewicz A., O’Quinn E., Adams K., Swaroop A., Wolf B.J. Association of age-related macular degeneration with complement activation products, smoking, and single nucleotide polymorphisms in South Carolinians of European and African descent. Mol. Vis. 2019;25:79–92. [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J., Cooke J.P. The role of nicotine in the pathogenesis of atherosclerosis. Atherosclerosis. 2011;215:281–283. doi: 10.1016/j.atherosclerosis.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsukamoto H., Lu S.C. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- 62.Marseglia L., Manti S., D’Angelo G., Nicotera A., Parisi E., Di Rosa G., Gitto E., Arrigo T. Oxidative stress in obesity: A critical component in human diseases. Int. J. Mol. Sci. 2014;16:378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dai J., Jones D.P., Goldberg J., Ziegler T.R., Bostick R.M., Wilson P.W., Manatunga A.K., Shallenberger L., Jones L., Vaccarino V. Association between adherence to the Mediterranean diet and oxidative stress. Am. J. Clin. Nutr. 2008;88:1364–1370. doi: 10.3945/ajcn.2008.26528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cougnard-Grégoire A., Merle B.M., Korobelnik J.F., Rougier M.B., Delyfer M.N., Le Goff M., Samieri C., Dartigues J.F., Delcourt C. Olive Oil Consumption and Age-Related Macular Degeneration: The Alienor Study. PLoS ONE. 2016;11:e0160240. doi: 10.1371/journal.pone.0160240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Merle B.M.J., Silver R.E., Rosner B., Seddon J.M. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: A prospective cohort study. Am. J. Clin. Nutr. 2015;102:1196–1206. doi: 10.3945/ajcn.115.111047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chiu C.-J., Chang M.-L., Zhang F.F., Li T., Gensler G., Schleicher M., Taylor A. The relationship of major American dietary patterns to age-related macular degeneration. Am. J. Ophthalmol. 2014;158:118–127.e111. doi: 10.1016/j.ajo.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romagnolo D.F., Selmin O.I. Mediterranean Diet and Prevention of Chronic Diseases. Nutr. Today. 2017;52:208–222. doi: 10.1097/NT.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Castro-Quezada I., Román-Viñas B., Serra-Majem L. The Mediterranean diet and nutritional adequacy: A review. Nutrients. 2014;6:231–248. doi: 10.3390/nu6010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Serra-Majem L., Bes-Rastrollo M., Román-Viñas B., Pfrimer K., Sánchez-Villegas A., Martínez-González M.A. Dietary patterns and nutritional adequacy in a Mediterranean country. Br. J. Nutr. 2009;101:S21–S28. doi: 10.1017/S0007114509990559. [DOI] [PubMed] [Google Scholar]
- 70.Stevens G.A., Singh G.M., Lu Y., Danaei G., Lin J.K., Finucane M.M., Bahalim A.N., McIntire R.K., Gutierrez H.R., Cowan M., et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul. Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gunes F.E., Bekiroglu N., Imeryuz N., Agirbasli M. Relation between eating habits and a high body mass index among freshman students: A cross-sectional study. J. Am. Coll. Nutr. 2012;31:167–174. doi: 10.1080/07315724.2012.10720024. [DOI] [PubMed] [Google Scholar]
- 72.Aoki A., Inoue M., Nguyen E., Obata R., Kadonosono K., Shinkai S., Hashimoto H., Sasaki S., Yanagi Y. Dietary n-3 Fatty Acid, α-Tocopherol, Zinc, vitamin D, vitamin C and β-carotene are Associated with Age-Related Macular Degeneration in Japan. Sci. Rep. 2016;6:20723. doi: 10.1038/srep20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hogg R.E., Woodside J.V., McGrath A., Young I.S., Vioque J.L., Chakravarthy U., de Jong P.T., Rahu M., Seland J., Soubrane G., et al. Mediterranean Diet Score and Its Association with Age-Related Macular Degeneration: The European Eye Study. Ophthalmology. 2017;124:82–89. doi: 10.1016/j.ophtha.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 74.SanGiovanni J.P., Chew E.Y., Clemons T.E., Davis M.D., Ferris F.L., 3rd, Gensler G.R., Kurinij N., Lindblad A.S., Milton R.C., Seddon J.M., et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 2007;125:671–679. doi: 10.1001/archopht.125.5.671. [DOI] [PubMed] [Google Scholar]
- 75.Okubo A., Rosa R.H., Jr., Bunce C.V., Alexander R.A., Fan J.T., Bird A.C., Luthert P.J. The relationships of age changes in retinal pigment epithelium and Bruch’s membrane. Investig. Ophthalmol. Vis. Sci. 1999;40:443–449. [PubMed] [Google Scholar]
- 76.Al-Zamil W.M., Yassin S.A. Recent developments in age-related macular degeneration: A review. Clin. Interv. Aging. 2017;12:1313–1330. doi: 10.2147/CIA.S143508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hernández-Zimbrón L.F., Zamora-Alvarado R., Ochoa-De la Paz L., Velez-Montoya R., Zenteno E., Gulias-Cañizo R., Quiroz-Mercado H., Gonzalez-Salinas R. Age-Related Macular Degeneration: New Paradigms for Treatment and Management of AMD. Oxid. Med. Cell. Longev. 2018;2018:8374647. doi: 10.1155/2018/8374647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Purves D., Augustine G.J., Fitzpatrick D., Katz L.C., LaMantia A.-S., McNamara J.-O., Williams M., editors. Neuroscience. 2th ed. Sinauer Associates; Sunderland, MA, USA: 2001. Anatomical Distribution of Rods and Cones. [Google Scholar]
- 79.Kevany B.M., Palczewski K. Phagocytosis of retinal rod and cone photoreceptors. Physiology (Bethesda) 2010;25:8–15. doi: 10.1152/physiol.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brunk U.T., Terman A. Lipofuscin: Mechanisms of age-related accumulation and influence on cell. Free Radic. Biol. Med. 2002;33:611–619. doi: 10.1016/S0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 81.Katz M.L., Robison W.G., Jr. What is lipofuscin? Defining characteristics and differentiation from other autofluorescent lysosomal storage bodies. Arch. Gerontol. Geriatr. 2002;34:169–184. doi: 10.1016/S0167-4943(02)00005-5. [DOI] [PubMed] [Google Scholar]
- 82.Sparrow J.R., Wu Y., Kim C.Y., Zhou J. Phospholipid meets all-trans-retinal: The making of RPE bisretinoids. J. Lipid. Res. 2010;51:247–261. doi: 10.1194/jlr.R000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kennedy C.J., Rakoczy P.E., Constable I.J. Lipofuscin of the retinal pigment epithelium: A review. Eye (Lond) 1995;9:763–771. doi: 10.1038/eye.1995.192. [DOI] [PubMed] [Google Scholar]
- 84.Finnemann S., Leung L., Rodriguez-Boulan E. The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA. 2002;99:3842–3847. doi: 10.1073/pnas.052025899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nowak J.Z. Age-related macular degeneration (AMD): Pathogenesis and therapy. Pharm. Rep. 2006;58:353–363. [PubMed] [Google Scholar]
- 86.Wang L., Clark M.E., Crossman D.K., Kojima K., Messinger J.D., Mobley J.A., Curcio C.A. Abundant Lipid and Protein Components of Drusen. PLoS ONE. 2010;5:e10329. doi: 10.1371/journal.pone.0010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ambati J., Atkinson J.P., Gelfand B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jorgenson E., Melles R.B., Hoffmann T.J., Jia X., Sakoda L.C., Kvale M.N., Banda Y., Schaefer C., Risch N., Shen L. Common coding variants in the HLA-DQB1 region confer susceptibility to age-related macular degeneration. Eur. J. Hum. Genet. 2016;24:1049–1055. doi: 10.1038/ejhg.2015.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nita M., Strzałka-Mrozik B., Grzybowski A., Mazurek U., Romaniuk W. Age-related macular degeneration and changes in the extracellular matrix. Med. Sci. Monit. 2014;20:1003–1016. doi: 10.12659/MSM.889887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mullins R.F., Russell S.R., Anderson D.H., Hageman G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. doi: 10.1096/fasebj.14.7.835. [DOI] [PubMed] [Google Scholar]
- 91.Kijlstra A., La Heij E., Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul. Immunol. Inflamm. 2005;13:3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 92.Hoppe G., O’Neil J., Hoff H.F., Sears J. Accumulation of oxidized lipid-protein complexes alters phagosome maturation in retinal pigment epithelium. Cell. Mol. Life. Sci. 2004;61:1664–1674. doi: 10.1007/s00018-004-4080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iriyama A., Yanagi Y., Inoue Y., Takahashi H., Tamaki Y. Role of A2E in the Pathogenesis of CNV. Investig. Ophthalmol. Vis. Sci. 2006;47:4146. [Google Scholar]
- 94.Beatty S., Koh H., Phil M., Henson D., Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45:115–134. doi: 10.1016/S0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 95.Loskutova E., Nolan J., Howard A., Beatty S. Macular pigment and its contribution to vision. Nutrients. 2013;5:1962–1969. doi: 10.3390/nu5061962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Drobek-Słowik M., Karczewicz D., Safranow K. The potential role of oxidative stress in the pathogenesis of the age-related macular degeneration (AMD) Postepy Hig. Med. Dosw. (Online) 2007;61:28–37. [PubMed] [Google Scholar]
- 97.Wiktorowska-Owczarek A., Nowak J.Z. Pathogenesis and prophylaxis of AMD: Focus on oxidative stress and antioxidants. Postepy Hig. Med. Dosw. (Online) 2010;64:333–343. [PubMed] [Google Scholar]
- 98.Hammond B.R., Jr., Wooten B.R., Snodderly D.M. Cigarette smoking and retinal carotenoids: Implications for age-related macular degeneration. Vis. Res. 1996;36:3003–3009. doi: 10.1016/0042-6989(96)00008-9. [DOI] [PubMed] [Google Scholar]
- 99.Wu J., Cho E., Willett W.C., Sastry S.M., Schaumberg D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015;133:1415–1424. doi: 10.1001/jamaophthalmol.2015.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marie M., Bigot K., Angebault C., Barrau C., Gondouin P., Pagan D., Fouquet S., Villette T., Sahel J.-A., Lenaers G., et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018;9:287. doi: 10.1038/s41419-018-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dong A., Xie B., Shen J., Yoshida T., Yokoi K., Hackett S.F., Campochiaro P.A. Oxidative stress promotes ocular neovascularization. J. Cell Physiol. 2009;219:544–552. doi: 10.1002/jcp.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davies K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–289. doi: 10.1080/15216540051081010. [DOI] [PubMed] [Google Scholar]
- 103.Espinosa-Heidmann D.G., Suner I.J., Catanuto P., Hernandez E.P., Marin-Castano M.E., Cousins S.W. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Investig. Ophthalmol. Vis. Sci. 2006;47:729–737. doi: 10.1167/iovs.05-0719. [DOI] [PubMed] [Google Scholar]
- 104.Gabriel H.E., Liu Z., Crott J.W., Choi S.-W., Song B.C., Mason J.B., Johnson E.J. A Comparison of Carotenoids, Retinoids, and Tocopherols in the Serum and Buccal Mucosa of Chronic Cigarette Smokers versus Nonsmokers. Cancer Epidemiol. Biomark. Amplif. Amplif. Prev. 2006;15:993. doi: 10.1158/1055-9965.EPI-05-0664. [DOI] [PubMed] [Google Scholar]
- 105.Rimm E., Colditz G. Smoking, Alcohol, and Plasma Levels of Carotenes and Vitamin Ea. Ann. N. Y. Acad. Sci. 1993;686:323–333. doi: 10.1111/j.1749-6632.1993.tb39195.x. [DOI] [PubMed] [Google Scholar]
- 106.Shokolenko I., Venediktova N., Bochkareva A., Wilson G.L., Alexeyev M.F. Oxidative stress induces degradation of mitochondrial DNA. Nucleic. Acids Res. 2009;37:2539–2548. doi: 10.1093/nar/gkp100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoh Kam J., Lenassi E., Jeffery G. Viewing ageing eyes: Diverse sites of amyloid Beta accumulation in the ageing mouse retina and the up-regulation of macrophages. PLoS ONE. 2010;5:e13127. doi: 10.1371/journal.pone.0013127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fisichella V., Giurdanella G., Platania C.B., Romano G.L., Leggio G.M., Salomone S., Drago F., Caraci F., Bucolo C. TGF-β1 prevents rat retinal insult induced by amyloid-β (1-42) oligomers. Eur. J. Pharm. 2016;787:72–77. doi: 10.1016/j.ejphar.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Julien O., Wells J.A. Caspases and their substrates. Cell Death Differ. 2017;24:1380–1389. doi: 10.1038/cdd.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van Opdenbosch N., Lamkanfi M. Caspases in Cell Death, Inflammation, and Disease. Immunity. 2019;50:1352–1364. doi: 10.1016/j.immuni.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lamkanfi M., Dixit V.M. The Inflammasomes. PLoS Pathog. 2009;5:e1000510. doi: 10.1371/journal.ppat.1000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martinon F., Burns K., Tschopp J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β. Mol. Cell. 2002;10:417–426. doi: 10.1016/S1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 113.Kosmidou C., Efstathiou N.E., Hoang M.V., Notomi S., Konstantinou E.K., Hirano M., Takahashi K., Maidana D.E., Tsoka P., Young L., et al. Issues with the Specificity of Immunological Reagents for NLRP3: Implications for Age-related Macular Degeneration. Sci. Rep. 2018;8:461. doi: 10.1038/s41598-017-17634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dinarello C.A. Interleukin 1 and interleukin 18 as mediators of inflammation and the aging process. Am. J. Clin. Nutr. 2006;83:447s–455s. doi: 10.1093/ajcn/83.2.447S. [DOI] [PubMed] [Google Scholar]
- 115.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jha S., Ting J.P.Y. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J. Immunol. 2009;183:7623–7629. doi: 10.4049/jimmunol.0902425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Voronov E., Carmi Y., Apte R.N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol. 2014;5:114. doi: 10.3389/fphys.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krishnan S.M., Sobey C.G., Latz E., Mansell A., Drummond G.R. IL-1β and IL-18: Inflammatory markers or mediators of hypertension? Br. J. Pharm. 2014;171:5589–5602. doi: 10.1111/bph.12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fahey E., Doyle S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019;10:1426. doi: 10.3389/fimmu.2019.01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim E.J., Cho D., Kim T.S. Efficient induction of T helper type 1-mediated immune responses in antigen-primed mice by anti-CD3 single-chain Fv/interleukin-18 fusion DNA. Immunology. 2004;111:27–34. doi: 10.1111/j.1365-2567.2004.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakanishi K., Yoshimoto T., Tsutsui H., Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu. Rev. Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 122.Doyle S.L., Campbell M., Ozaki E., Salomon R.G., Mori A., Kenna P.F., Farrar G.J., Kiang A.-S., Humphries M.M., Lavelle E.C., et al. NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat. Med. 2012;18:791–798. doi: 10.1038/nm.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bamias G., Corridoni D., Pizarro T.T., Cominelli F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine. 2012;59:451–459. doi: 10.1016/j.cyto.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anderson O.A., Finkelstein A., Shima D.T. A2E induces IL-1ß production in retinal pigment epithelial cells via the NLRP3 inflammasome. PLoS ONE. 2013;8:e67263. doi: 10.1371/journal.pone.0067263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu R.T., Gao J., Cao S., Sandhu N., Cui J.Z., Chou C.L., Fang E., Matsubara J.A. Inflammatory mediators induced by amyloid-beta in the retina and RPE in vivo: Implications for inflammasome activation in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013;54:2225–2237. doi: 10.1167/iovs.12-10849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kauppinen A., Niskanen H., Suuronen T., Kinnunen K., Salminen A., Kaarniranta K. Oxidative stress activates NLRP3 inflammasomes in ARPE-19 cells--implications for age-related macular degeneration (AMD) Immunol. Lett. 2012;147:29–33. doi: 10.1016/j.imlet.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 128.Marneros A.G. NLRP3 inflammasome blockade inhibits VEGF-A-induced age-related macular degeneration. Cell Rep. 2013;4:945–958. doi: 10.1016/j.celrep.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Compan V., Baroja-Mazo A., López-Castejón G., Gomez A.I., Martínez C.M., Angosto D., Montero M.T., Herranz A.S., Bazán E., Reimers D., et al. Cell volume regulation modulates NLRP3 inflammasome activation. Immunity. 2012;37:487–500. doi: 10.1016/j.immuni.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 130.Platania C.B.M., Giurdanella G., Di Paola L., Leggio G.M., Drago F., Salomone S., Bucolo C. P2X7 receptor antagonism: Implications in diabetic retinopathy. Biochem. Pharm. 2017;138:130–139. doi: 10.1016/j.bcp.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 131.Kim Y., Tarallo V., Kerur N., Yasuma T., Gelfand B.D., Bastos-Carvalho A., Hirano Y., Yasuma R., Mizutani T., Fowler B.J., et al. DICER1/Alu RNA dysmetabolism induces Caspase-8–mediated cell death in age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 2014;111:16082. doi: 10.1073/pnas.1403814111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tarallo V., Hirano Y., Gelfand B.D., Dridi S., Kerur N., Kim Y., Cho W.G., Kaneko H., Fowler B.J., Bogdanovich S., et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Häsler J., Strub K. Alu elements as regulators of gene expression. Nucleic. Acids Res. 2006;34:5491–5497. doi: 10.1093/nar/gkl706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bernstein E., Caudy A.A., Hammond S.M., Hannon G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 135.Du Z., Lee J.K., Tjhen R., Stroud R.M., James T.L. Structural and biochemical insights into the dicing mechanism of mouse Dicer: A conserved lysine is critical for dsRNA cleavage. Proc. Natl. Acad. Sci. USA. 2008;105:2391–2396. doi: 10.1073/pnas.0711506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kaneko H., Dridi S., Tarallo V., Gelfand B., Fowler B., Cho W., Kleinman M., Ponicsan S., Hauswirth W., Chiodo V., et al. DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature. 2011;471:325–330. doi: 10.1038/nature09830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wright C.B., Uehara H., Kim Y., Yasuma T., Yasuma R., Hirahara S., Makin R.D., Apicella I., Pereira F., Nagasaka Y., et al. Chronic Dicer1 deficiency promotes atrophic and neovascular outer retinal pathologies in mice. Proc. Natl. Acad. Sci. USA. 2020;117:2579–2587. doi: 10.1073/pnas.1909761117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nozaki M., Raisler B.J., Sakurai E., Sarma J.V., Barnum S.R., Lambris J.D., Chen Y., Zhang K., Ambati B.K., Baffi J.Z., et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA. 2006;103:2328–2333. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou J., Jang Y.P., Kim S.R., Sparrow J.R. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ambati J., Fowler B.J. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Espinosa-Heidmann D.G., Suner I.J., Hernandez E.P., Monroy D., Csaky K.G., Cousins S.W. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2003;44:3586–3592. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 142.Sakurai E., Anand A., Ambati B.K., van Rooijen N., Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2003;44:3578–3585. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 143.Ambati J., Anand A., Fernandez S., Sakurai E., Lynn B.C., Kuziel W.A., Rollins B.J., Ambati B.K. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat. Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 144.Tsutsui H., Yoshimoto T., Hayashi N., Mizutani H., Nakanishi K. Induction of allergic inflammation by interleukin-18 in experimental animal models. Immunol. Rev. 2004;202:115–138. doi: 10.1111/j.0105-2896.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 145.Sica A., Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zandi S., Nakao S., Chun K.-H., Fiorina P., Sun D., Arita R., Zhao M., Kim E., Schueller O., Campbell S., et al. ROCK-isoform-specific polarization of macrophages associated with age-related macular degeneration. Cell Rep. 2015;10:1173–1186. doi: 10.1016/j.celrep.2015.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jetten N., Verbruggen S., Gijbels M.J., Post M.J., De Winther M.P., Donners M.M. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17:109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 148.Tatar O., Yoeruek E., Szurman P., Bartz-Schmidt K.U., Adam A., Shinoda K., Eckardt C., Boeyden V., Claes C., Pertile G., et al. Effect of Bevacizumab on Inflammation and Proliferation in Human Choroidal Neovascularization. Arch. Ophthalmol. 2008;126:782–790. doi: 10.1001/archopht.126.6.782. [DOI] [PubMed] [Google Scholar]
- 149.Walshe T.E., Dole V.S., Maharaj A.S.R., Patten I.S., Wagner D.D., D’Amore P.A. Inhibition of VEGF or TGF-{beta} signaling activates endothelium and increases leukocyte rolling. Arter. Thromb. Vasc. Biol. 2009;29:1185–1192. doi: 10.1161/ATVBAHA.109.186742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Willett C.G., Boucher Y., di Tomaso E., Duda D.G., Munn L.L., Tong R.T., Chung D.C., Sahani D.V., Kalva S.P., Kozin S.V., et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Leppänen V.-M., Saharinen P., Alitalo K. Structural basis of Tie2 activation and Tie2/Tie1 heterodimerization. Proc. Natl. Acad. Sci. USA. 2017;114:4376. doi: 10.1073/pnas.1616166114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Davis S., Aldrich T.H., Jones P.F., Acheson A., Compton D.L., Jain V., Ryan T.E., Bruno J., Radziejewski C., Maisonpierre P.C., et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/S0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 153.Maisonpierre P.C., Suri C., Jones P.F., Bartunkova S., Wiegand S.J., Radziejewski C., Compton D., McClain J., Aldrich T.H., Papadopoulos N., et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 154.Nambu H., Umeda N., Kachi S., Oshima Y., Akiyama H., Nambu R., Campochiaro P.A. Angiopoietin 1 prevents retinal detachment in an aggressive model of proliferative retinopathy, but has no effect on established neovascularization. J. Cell Physiol. 2005;204:227–235. doi: 10.1002/jcp.20292. [DOI] [PubMed] [Google Scholar]
- 155.Fiedler U., Reiss Y., Scharpfenecker M., Grunow V., Koidl S., Thurston G., Gale N.W., Witzenrath M., Rosseau S., Suttorp N., et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat. Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 156.Thurston G., Daly C. The complex role of angiopoietin-2 in the angiopoietin-tie signaling pathway. Cold Spring Harb. Perspect. Med. 2012;2:a006550. doi: 10.1101/cshperspect.a006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ng D.S., Yip Y.W., Bakthavatsalam M., Chen L.J., Ng T.K., Lai T.Y., Pang C.P., Brelén M.E. Elevated angiopoietin 2 in aqueous of patients with neovascular age related macular degeneration correlates with disease severity at presentation. Sci. Rep. 2017;7:45081. doi: 10.1038/srep45081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Watanabe D., Suzuma K., Suzuma I., Ohashi H., Ojima T., Kurimoto M., Murakami T., Kimura T., Takagi H. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am. J. Ophthalmol. 2005;139:476–481. doi: 10.1016/j.ajo.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 159.Sahni J., Patel S.S., Dugel P.U., Khanani A.M., Jhaveri C.D., Wykoff C.C., Hershberger V.S., Pauly-Evers M., Sadikhov S., Szczesny P., et al. Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial. Ophthalmology. 2019;126:1155–1170. doi: 10.1016/j.ophtha.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 160.Ferrara N., Henzel W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;161:851–858. doi: 10.1016/0006-291X(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 161.Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 162.Gerber H.P., Ferrara N. The role of VEGF in normal and neoplastic hematopoiesis. J. Mol. Med. (Berl.) 2003;81:20–31. doi: 10.1007/s00109-002-0397-4. [DOI] [PubMed] [Google Scholar]
- 163.Johnson K.E., Wilgus T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care (New Rochelle) 2014;3:647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Joukov V., Pajusola K., Kaipainen A., Chilov D., Lahtinen I., Kukk E., Saksela O., Kalkkinen N., Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. doi: 10.1002/j.1460-2075.1996.tb00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Coultas L., Chawengsaksophak K., Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 166.Verheul H.M., Pinedo H.M. The role of vascular endothelial growth factor (VEGF) in tumor angiogenesis and early clinical development of VEGF-receptor kinase inhibitors. Clin. Breast Cancer. 2000;1(Suppl. 1):S80–S84. doi: 10.3816/CBC.2000.s.015. [DOI] [PubMed] [Google Scholar]
- 167.Li X., Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int. J. Biochem. Cell Biol. 2001;33:421–426. doi: 10.1016/S1357-2725(01)00027-9. [DOI] [PubMed] [Google Scholar]
- 168.Leung D.W., Cachianes G., Kuang W.J., Goeddel D.V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 169.Oommen S., Gupta S.K., Vlahakis N.E. Vascular endothelial growth factor A (VEGF-A) induces endothelial and cancer cell migration through direct binding to integrin {alpha}9{beta}1: Identification of a specific {alpha}9{beta}1 binding site. J. Biol. Chem. 2011;286:1083–1092. doi: 10.1074/jbc.M110.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Forsythe J.A., Jiang B.H., Iyer N.V., Agani F., Leung S.W., Koos R.D., Semenza G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996;16:4604–4613. doi: 10.1128/MCB.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lohela M., Bry M., Tammela T., Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 172.Neufeld G., Cohen T., Gengrinovitch S., Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. doi: 10.1096/fasebj.13.1.9. [DOI] [PubMed] [Google Scholar]
- 173.Monaghan-Benson E., Hartmann J., Vendrov A.E., Budd S., Byfield G., Parker A., Ahmad F., Huang W., Runge M., Burridge K., et al. The role of vascular endothelial growth factor-induced activation of NADPH oxidase in choroidal endothelial cells and choroidal neovascularization. Am. J. Pathol. 2010;177:2091–2102. doi: 10.2353/ajpath.2010.090878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rossino M.G., Lulli M., Amato R., Cammalleri M., Monte M.D., Casini G. Oxidative Stress Induces a VEGF Autocrine Loop in the Retina: Relevance for Diabetic Retinopathy. Cells. 2020;9:1452. doi: 10.3390/cells9061452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rousseau S., Houle F., Landry J., Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 176.Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C.-O., Buerk D.G., Huang P.L., Jain R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. USA. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Feliers D., Chen X., Akis N., Choudhury G.G., Madaio M., Kasinath B.S. VEGF regulation of endothelial nitric oxide synthase in glomerular endothelial cells. Kidney Int. 2005;68:1648–1659. doi: 10.1111/j.1523-1755.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 178.McLaughlin A.P., De Vries G.W. Role of PLCgamma and Ca(2+) in VEGF- and FGF-induced choroidal endothelial cell proliferation. Am. J. Physiol. Cell Physiol. 2001;281:C1448–C1456. doi: 10.1152/ajpcell.2001.281.5.C1448. [DOI] [PubMed] [Google Scholar]
- 179.Murohara T., Horowitz J.R., Silver M., Tsurumi Y., Chen D., Sullivan A., Isner J.M. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.CIR.97.1.99. [DOI] [PubMed] [Google Scholar]
- 180.Bayless K.J., Johnson G.A. Role of the cytoskeleton in formation and maintenance of angiogenic sprouts. J. Vasc. Res. 2011;48:369–385. doi: 10.1159/000324751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pollard T.D., Borisy G.G. Cellular Motility Driven by Assembly and Disassembly of Actin Filaments. Cell. 2003;112:453–465. doi: 10.1016/S0092-8674(03)00120-X. [DOI] [PubMed] [Google Scholar]
- 182.Zhou Q., Anderson C., Zhang H., Li X., Inglis F., Jayagopal A., Wang S. Repression of Choroidal Neovascularization Through Actin Cytoskeleton Pathways by MicroRNA-24. Mol. Ther. 2014;22:378–389. doi: 10.1038/mt.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dulak J., Józkowicz A., Dembinska-Kiec A., Guevara I., Zdzienicka A., Zmudzinska-Grochot D., Florek I., Wójtowicz A., Szuba A., Cooke J.P. Nitric oxide induces the synthesis of vascular endothelial growth factor by rat vascular smooth muscle cells. Arter. Thromb. Vasc. Biol. 2000;20:659–666. doi: 10.1161/01.ATV.20.3.659. [DOI] [PubMed] [Google Scholar]
- 184.Kuwabara M., Kakinuma Y., Ando M., Katare R.G., Yamasaki F., Doi Y., Sato T. Nitric oxide stimulates vascular endothelial growth factor production in cardiomyocytes involved in angiogenesis. J. Physiol. Sci. 2006;56:95–101. doi: 10.2170/physiolsci.RP002305. [DOI] [PubMed] [Google Scholar]
- 185.Ohno-Matsui K., Morita I., Tombran-Tink J., Mrazek D., Onodera M., Uetama T., Hayano M., Murota S.I., Mochizuki M. Novel mechanism for age-related macular degeneration: An equilibrium shift between the angiogenesis factors VEGF and PEDF. J. Cell Physiol. 2001;189:323–333. doi: 10.1002/jcp.10026. [DOI] [PubMed] [Google Scholar]
- 186.Adamis A.P., Shima D.T., Yeo K.T., Yeo T.K., Brown L.F., Berse B., D’Amore P.A., Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem. Biophys. Res. Commun. 1993;193:631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- 187.Dawson D.W., Volpert O.V., Gillis P., Crawford S.E., Xu H., Benedict W., Bouck N.P. Pigment epithelium-derived factor: A potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245. [DOI] [PubMed] [Google Scholar]
- 188.Cai J., Jiang W.G., Grant M.B., Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J. Biol. Chem. 2006;281:3604–3613. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 189.Mitter S.K., Song C., Qi X., Mao H., Rao H., Akin D., Lewin A., Grant M., Dunn W., Jr., Ding J., et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy. 2014;10:1989–2005. doi: 10.4161/auto.36184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Holekamp N.M., Bouck N., Volpert O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am. J. Ophthalmol. 2002;134:220–227. doi: 10.1016/S0002-9394(02)01549-0. [DOI] [PubMed] [Google Scholar]
- 191.Duh E.J., Yang H.S., Suzuma I., Miyagi M., Youngman E., Mori K., Katai M., Yan L., Suzuma K., West K., et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Investig. Ophthalmol. Vis. Sci. 2002;43:821–829. [PubMed] [Google Scholar]
- 192.Macular Photocoagulation Study Group Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch. Ophthalmol. 1982;100:912–918. doi: 10.1001/archopht.1982.01030030920003. [DOI] [PubMed] [Google Scholar]
- 193.Macular Photocoagulation Study Group Krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Results of a randomized clinical trial. Macular Photocoagulation Study Group. Arch. Ophthalmol. 1990;108:816–824. doi: 10.1001/archopht.1990.01070080058036. [DOI] [PubMed] [Google Scholar]
- 194.Schlötzer-Schrehardt U., Viestenz A., Naumann G.O., Laqua H., Michels S., Schmidt-Erfurth U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefe‘s Arch. Clin. Exp. Ophthalmol. 2002;240:748–757. doi: 10.1007/s00417-002-0517-4. [DOI] [PubMed] [Google Scholar]
- 195.Donati G., Kapetanios A.D., Pournaras C.J. Principles of treatment of choroidal neovascularization with photodynamic therapy in age-related macular degeneration. Semin. Ophthalmol. 1999;14:2–10. doi: 10.3109/08820539909056057. [DOI] [PubMed] [Google Scholar]
- 196.Miller J.W., Schmidt-Erfurth U., Sickenberg M., Pournaras C.J., Laqua H., Barbazetto I., Zografos L., Piguet B., Donati G., Lane A.M., et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: Results of a single treatment in a phase 1 and 2 study. Arch. Ophthalmol. 1999;117:1161–1173. doi: 10.1001/archopht.117.9.1161. [DOI] [PubMed] [Google Scholar]
- 197.Schmidt-Erfurth U., Miller J.W., Sickenberg M., Laqua H., Barbazetto I., Gragoudas E.S., Zografos L., Piguet B., Pournaras C.J., Donati G., et al. Photodynamic therapy with verteporfin for choroidal neovascularization caused by age-related macular degeneration: Results of retreatments in a phase 1 and 2 study. Arch. Ophthalmol. 1999;117:1177–1187. doi: 10.1001/archopht.117.9.1177. [DOI] [PubMed] [Google Scholar]
- 198.Chakravarthy U., Adamis A.P., Cunningham E.T., Jr., Goldbaum M., Guyer D.R., Katz B., Patel M. Year 2 efficacy results of 2 randomized controlled clinical trials of pegaptanib for neovascular age-related macular degeneration. Ophthalmology. 2006;113:1508.e1-25. doi: 10.1016/j.ophtha.2006.02.064. [DOI] [PubMed] [Google Scholar]
- 199.Clearkin L., Ramasamy B., Wason J., Tiew S. Anti-VEGF intervention in neovascular AMD: Benefits and risks restated as natural frequencies. BMJ Open Ophthalmol. 2019;4:e000257. doi: 10.1136/bmjophth-2018-000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stefanini F.R., Badaró E., Falabella P., Koss M., Farah M.E., Maia M. Anti-VEGF for the management of diabetic macular edema. J. Immunol. Res. 2014;2014:632307. doi: 10.1155/2014/632307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Campa C., Alivernini G., Bolletta E., Parodi M.B., Perri P. Anti-VEGF Therapy for Retinal Vein Occlusions. Curr. Drug Targets. 2016;17:328–336. doi: 10.2174/1573399811666150615151324. [DOI] [PubMed] [Google Scholar]
- 202.El Annan J., Carvounis P.E. Current management of vitreous hemorrhage due to proliferative diabetic retinopathy. Int. Ophthalmol. Clin. 2014;54:141–153. doi: 10.1097/IIO.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhao Y., Singh R.P. The role of anti-vascular endothelial growth factor (anti-VEGF) in the management of proliferative diabetic retinopathy. Drugs Context. 2018;7:212532. doi: 10.7573/dic.212532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Simha A., Aziz K., Braganza A., Abraham L., Samuel P., Lindsley K.B. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst. Rev. 2020;2:Cd007920. doi: 10.1002/14651858.CD007920.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cp J., Al G., Jd L. Combination of intravitreal bevacizumab and peripheral photocoagulation: An alternative treatment in eales disease. Med. Hypothesis Discov. Innov. Ophthalmol. 2013;2:30–34. [PMC free article] [PubMed] [Google Scholar]
- 206.Li S., Deng G., Liu J., Ma Y., Lu H. The effects of a treatment combination of anti-VEGF injections, laser coagulation and cryotherapy on patients with type 3 Coat’s disease. BMC Ophthalmol. 2017;17:76. doi: 10.1186/s12886-017-0469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xia T., Zarbin M.A., Bhagat N. Anti-VEGF for Management of Neovascularization of Iris and Neovascular Glaucoma. J. Vitreoretinal Dis. 2018;2:194–199. doi: 10.1177/2474126418782067. [DOI] [Google Scholar]
- 208.Slabaugh M., Salim S. Use of Anti-VEGF Agents in Glaucoma Surgery. J. Ophthalmol. 2017;2017:1645269. doi: 10.1155/2017/1645269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mak R.K., Chan T.C., Marcet M.M., Choy B.N., Shum J.W., Shih K.C., Wong I.Y., Ng A.L. Use of anti-vascular endothelial growth factor in the management of pterygium. Acta Ophthalmol. 2017;95:20–27. doi: 10.1111/aos.13178. [DOI] [PubMed] [Google Scholar]
- 210.Tran K.D., Cernichiaro-Espinosa L.A., Berrocal A.M. Management of Retinopathy of Prematurity--Use of Anti-VEGF Therapy. Asia Pac. J. Ophthalmol. (Phila) 2018;7:56–62. doi: 10.22608/APO.2017436. [DOI] [PubMed] [Google Scholar]
- 211.Ghasemi Falavarjani K., Parvaresh M.-M., Modarres M., Hashemi M., Samiy N. Intravitreal bevacizumab for pseudophakic cystoid macular edema; a systematic review. J. Ophthalmic. Vis. Res. 2012;7:235–239. [PMC free article] [PubMed] [Google Scholar]
- 212.Monk B.J., Minion L.E., Coleman R.L. Anti-angiogenic agents in ovarian cancer: Past, present, and future. Ann. Oncol. 2016;27(Suppl. 1):i33–i39. doi: 10.1093/annonc/mdw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Park D.J., Thomas N.J., Yoon C., Yoon S.S. Vascular endothelial growth factor a inhibition in gastric cancer. Gastric. Cancer. 2015;18:33–42. doi: 10.1007/s10120-014-0397-4. [DOI] [PubMed] [Google Scholar]
- 214.Melegh Z., Oltean S. Targeting Angiogenesis in Prostate Cancer. Int. J. Mol. Sci. 2019;20:2676. doi: 10.3390/ijms20112676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Blanke C.D., Rankin C., Corless C., Eary J.F., Mulder K., Okuno S.H., George S., Heinrich M. S0502: A SWOG Phase III Randomized Study of Imatinib, With or Without Bevacizumab, in Patients With Untreated Metastatic or Unresectable Gastrointestinal Stromal Tumors. Oncologist. 2015;20:1353–1354. doi: 10.1634/theoncologist.2015-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Van Cutsem E., Vervenne W.L., Bennouna J., Humblet Y., Gill S., Van Laethem J.L., Verslype C., Scheithauer W., Shang A., Cosaert J., et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J. Clin. Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 217.Korpanty G., Smyth E., Carney D.N. Update on anti-angiogenic therapy in non-small cell lung cancer: Are we making progress? J. Thorac. Dis. 2011;3:19–29. doi: 10.3978/j.issn.2072-1439.2010.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., Kim R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 219.Brown D.M., Kaiser P.K., Michels M., Soubrane G., Heier J.S., Kim R.Y., Sy J.P., Schneider S. Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 220.Ho A.C., Busbee B.G., Regillo C.D., Wieland M.R., Van Everen S.A., Li Z., Rubio R.G., Lai P. Twenty-four-Month Efficacy and Safety of 0.5 mg or 2.0 mg Ranibizumab in Patients with Subfoveal Neovascular Age-Related Macular Degeneration. Ophthalmology. 2014;121:2181–2192. doi: 10.1016/j.ophtha.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 221.Wykoff C.C., Croft D.E., Brown D.M., Wang R., Payne J.F., Clark L., Abdelfattah N.S., Sadda S.R. Prospective Trial of Treat-and-Extend versus Monthly Dosing for Neovascular Age-Related Macular Degeneration: TREX-AMD 1-Year Results. Ophthalmology. 2015;122:2514–2522. doi: 10.1016/j.ophtha.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 222.Antoszyk A.N., Tuomi L., Chung C.Y., Singh A. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): Year 2 results. Am. J. Ophthalmol. 2008;145:862–874. doi: 10.1016/j.ajo.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 223.Schauwvlieghe A.M., Dijkman G., Hooymans J.M., Verbraak F.D., Hoyng C.B., Dijkgraaf M.G., Peto T., Vingerling J.R., Schlingemann R.O. Comparing the Effectiveness of Bevacizumab to Ranibizumab in Patients with Exudative Age-Related Macular Degeneration. The BRAMD Study. PLoS ONE. 2016;11:e0153052. doi: 10.1371/journal.pone.0153052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Berg K., Pedersen T.R., Sandvik L., Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122:146–152. doi: 10.1016/j.ophtha.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 225.Martin D.F., Maguire M.G., Fine S.L., Ying G.S., Jaffe G.J., Grunwald J.E., Toth C., Redford M., Ferris F.L., 3rd Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kodjikian L., Souied E.H., Mimoun G., Mauget-Faÿsse M., Behar-Cohen F., Decullier E., Huot L., Aulagner G. Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 2013;120:2300–2309. doi: 10.1016/j.ophtha.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 227.Chakravarthy U., Harding S.P., Rogers C.A., Downes S.M., Lotery A.J., Culliford L.A., Reeves B.C. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382:1258–1267. doi: 10.1016/S0140-6736(13)61501-9. [DOI] [PubMed] [Google Scholar]
- 228.Heier J.S., Brown D.M., Chong V., Korobelnik J.F., Kaiser P.K., Nguyen Q.D., Kirchhof B., Ho A., Ogura Y., Yancopoulos G.D., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 229.Dugel P.U., Koh A., Ogura Y., Jaffe G.J., Schmidt-Erfurth U., Brown D.M., Gomes A.V., Warburton J., Weichselberger A., Holz F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 230.Li X., Xu G., Wang Y., Xu X., Liu X., Tang S., Zhang F., Zhang J., Tang L., Wu Q., et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: Results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121:1740–1747. doi: 10.1016/j.ophtha.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 231.Liu K., Song Y., Xu G., Ye J., Wu Z., Liu X., Dong X., Zhang M., Xing Y., Zhu S., et al. Conbercept for Treatment of Neovascular Age-related Macular Degeneration: Results of the Randomized Phase 3 PHOENIX Study. Am. J. Ophthalmol. 2019;197:156–167. doi: 10.1016/j.ajo.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 232.Danzig C., Quezada C., Basu K., Grzeschik S., Sahni J., Silverman D., Osborne A. Efficacy and safety of faricimab every 16 or 12 weeks for neovascular age-related macular degeneration: STAIRWAY phase 2 results. Investig. Ophthalmol. Vis. Sci. 2019;60:1212. [Google Scholar]
- 233.Lien S., Lowman H.B. Therapeutic anti-VEGF antibodies. Handb. Exp. Pharm. 2008:131–150. doi: 10.1007/978-3-540-73259-4_6. [DOI] [PubMed] [Google Scholar]
- 234.Blick S.K., Keating G.M., Wagstaff A.J. Ranibizumab. Drugs. 2007;67:1199–1206. doi: 10.2165/00003495-200767080-00007. [DOI] [PubMed] [Google Scholar]
- 235.Ferrara N., Hillan K.J., Gerber H.P., Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 236.van Asten F., Michels C.T.J., Hoyng C.B., van der Wilt G.J., Klevering B.J., Rovers M.M., Grutters J.P.C. The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration-A cost-effectiveness analysis from a societal perspective. PLoS ONE. 2018;13:e0197670. doi: 10.1371/journal.pone.0197670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Carmeliet P., Moons L., Luttun A., Vincenti V., Compernolle V., De Mol M., Wu Y., Bono F., Devy L., Beck H., et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 238.Stewart M.W., Grippon S., Kirkpatrick P. Aflibercept. Nat. Rev. Drug Discov. 2012;11:269–270. doi: 10.1038/nrd3700. [DOI] [PubMed] [Google Scholar]
- 239.Lazzara F., Fidilio A., Platania C.B.M., Giurdanella G., Salomone S., Leggio G.M., Tarallo V., Cicatiello V., De Falco S., Eandi C.M., et al. Aflibercept regulates retinal inflammation elicited by high glucose via the PlGF/ERK pathway. Biochem. Pharm. 2019;168:341–351. doi: 10.1016/j.bcp.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 240.Fernandes C.F.C., Pereira S.D.S., Luiz M.B., Zuliani J.P., Furtado G.P., Stabeli R.G. Camelid Single-Domain Antibodies As an Alternative to Overcome Challenges Related to the Prevention, Detection, and Control of Neglected Tropical Diseases. Front. Immunol. 2017;8:653. doi: 10.3389/fimmu.2017.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gaudreault J., Gunde T., Floyd H.S., Ellis J., Tietz J., Binggeli D., Keller B., Schmidt A., Escher D. Preclinical Pharmacology and Safety of ESBA1008, a Single-chain Antibody Fragment, Investigated as Potential Treatment for Age Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2012;53:3025. [Google Scholar]
- 242.Plyukhova A.A., Budzinskaya M.V., Starostin K.M., Rejdak R., Bucolo C., Reibaldi M., Toro M.D. Comparative Safety of Bevacizumab, Ranibizumab, and Aflibercept for Treatment of Neovascular Age-Related Macular Degeneration (AMD): A Systematic Review and Network Meta-Analysis of Direct Comparative Studies. J. Clin. Med. 2020;9:1522. doi: 10.3390/jcm9051522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang M., Yu D., Yang C., Xia Q., Li W., Liu B., Li H. The Pharmacology Study of a New Recombinant Human VEGF Receptor-Fc Fusion Protein on Experimental Choroidal Neovascularization. Pharm. Res. 2009;26:204–210. doi: 10.1007/s11095-008-9718-9. [DOI] [PubMed] [Google Scholar]
- 244.Zhang X., Wu J., Wu C., Bian A.-l., Geng S., Dai R.-p. Comparison of aqueous humor levels of PlGF and VEGF in proliferative diabetic retinopathy before and after intravitreal conbercept injection. Diabetes Res. Clin. Pract. 2020;162:108083. doi: 10.1016/j.diabres.2020.108083. [DOI] [PubMed] [Google Scholar]
- 245.Regula J.T., Lundh von Leithner P., Foxton R., Barathi V.A., Cheung C.M.G., Bo Tun S.B., Wey Y.S., Iwata D., Dostalek M., Moelleken J., et al. Targeting key angiogenic pathways with a bispecific CrossMAb optimized for neovascular eye diseases. EMBO Mol. Med. 2016;8:1265–1288. doi: 10.15252/emmm.201505889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Garnock-Jones K.P. Ripasudil: First global approval. Drugs. 2014;74:2211–2215. doi: 10.1007/s40265-014-0333-2. [DOI] [PubMed] [Google Scholar]
- 247.Gao Z., Li Q., Zhang Y., Gao X., Li H., Yuan Z. Ripasudil alleviated the inflammation of RPE cells by targeting the miR-136-5p/ROCK/NLRP3 pathway. BMC Ophthalmol. 2020;20:134. doi: 10.1186/s12886-020-01400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Ambati B.K., Nozaki M., Singh N., Takeda A., Jani P.D., Suthar T., Albuquerque R.J., Richter E., Sakurai E., Newcomb M.T., et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lai C.-M., Estcourt M.J., Wikstrom M., Himbeck R.P., Barnett N.L., Brankov M., Tee L.B.G., Dunlop S.A., Degli-Esposti M.A., Rakoczy E.P. rAAV.sFlt-1 Gene Therapy Achieves Lasting Reversal of Retinal Neovascularization in the Absence of a Strong Immune Response to the Viral Vector. Investig. Ophthalmol. Vis. Sci. 2009;50:4279–4287. doi: 10.1167/iovs.08-3253. [DOI] [PubMed] [Google Scholar]
- 250.Rakoczy E.P., Magno A.L., Lai C.M., Pierce C.M., Degli-Esposti M.A., Blumenkranz M.S., Constable I.J. Three-Year Follow-Up of Phase 1 and 2a rAAV.sFLT-1 Subretinal Gene Therapy Trials for Exudative Age-Related Macular Degeneration. Am. J. Ophthalmol. 2019;204:113–123. doi: 10.1016/j.ajo.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 251.Constable I.J., Pierce C.M., Lai C.-M., Magno A.L., Degli-Esposti M.A., French M.A., McAllister I.L., Butler S., Barone S.B., Schwartz S.D., et al. Phase 2a Randomized Clinical Trial: Safety and Post Hoc Analysis of Subretinal rAAV.sFLT-1 for Wet Age-related Macular Degeneration. EBioMedicine. 2016;14:168–175. doi: 10.1016/j.ebiom.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.EXPLORE: A Phase II Study to Evaluate the Safety and Efficacy of Two Doses of GT005. [(accessed on 15 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04437368.
- 253.RGX-314 Gene Therapy for Neovascular AMD Trial. [(accessed on 15 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03066258.
- 254.Safety and Tolerability Study of AAV2-sFLT01 in Patients with Neovascular Age-Related Macular Degeneration (AMD) [(accessed on 15 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01024998.
- 255.Study of AdGVPEDF.11D in Neovascular Age-Related Macular Degeneration (AMD) [(accessed on 15 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00109499.
- 256.A Follow-up Study to Evaluate the Safety of RetinoStat® in Patients with Age-Related Macular Degeneration. [(accessed on 15 October 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01678872.
- 257.Safety and Efficacy Study of rAAV.sFlt-1 in Patients with Exudative Age-Related Macular Degeneration. [(accessed on 15 October 2020)]; Available online: https://ClinicalTrials.gov/show/NCT01494805.
- 258.AAVCAGsCD59 for the Treatment of Wet AMD. [(accessed on 15 October 2020)]; Available online: https://ClinicalTrials.gov/show/NCT03585556.
- 259.ADVM-022 Intravitreal Gene Therapy for Wet AMD. [(accessed on 15 October 2020)]; Available online: https://ClinicalTrials.gov/show/NCT03748784.
- 260.Regenxbio Inc. Long-Term Follow-Up Study of RGX-314. [(accessed on 15 October 2020)]; Available online: https://ClinicalTrials.gov/show/NCT03999801: 2019.
- 261.Figueroa A.G., Boyd B.M., Christensen C.A., Javid C.G., McKay B.S., Fagan T.C., Snyder R.W. Levodopa Positively Affects Neovascular Age-Related Macular Degeneration. Am. J. Med. 2020 doi: 10.1016/j.amjmed.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.