Abstract
Alkaloids are a class of secondary metabolites that can be derived from plants, fungi and marine sponges. They are widely known as a continuous source of medicine for the management of chronic disease including cancer, diabetes and neurodegenerative diseases. For example, galanthamine and huperzine A are alkaloid derivatives currently being used for the symptomatic management of neurodegenerative disease. The etiology of neurodegenerative diseases is polygenic and multifactorial including but not limited to inflammation, oxidative stress and protein aggregation. Therefore, natural-product-based alkaloids with polypharmacology modulation properties are potentially useful for further drug development or, to a lesser extent, as nutraceuticals to manage neurodegeneration. This review aims to discuss and summarise recent developments in relation to naturally derived alkaloids for neurodegenerative diseases.
Keywords: alkaloids, multi-targeted agent, cholinesterase, neuroprotective, neuroinflammation, neurogenesis, amyloid beta, tau protein, drug likeness
1. Introduction
Neurodegenerative diseases are classified as a group of chronic diseases that are mostly incurable and are characterised by a progressive memory loss and/or neuronal cell death in the central nervous system. Some examples include Alzheimer’s disease (AD) and Parkinson’s disease (PD) and the management plans for these diseases are merely symptomatic treatments that do not halt the disease progression [1,2]. Most of these diseases are closely related to age. Each neurodegenerative disease is associated with diverse clinical manifestations such as cognitive dysfunction and impaired daily functioning [3]. In 2016, there were around 43.8 million people suffering from dementia and 6.1 million people suffering from Parkinson’s disease and the numbers continue to increase annually [4,5]. AD accounts for approximately 75% of all cases of dementia, therefore it is deemed the most common form of dementia [6,7].
AD symptoms include memory loss, and it can progress to an advanced stage that manifests as agitation, apathy, aggression, hallucination, false beliefs and cognitive dysfunction; eventually severe AD patients die from loss of basic physiological functions and complications from infections [8,9,10]. The treatment options for AD are limited to cholinesterase inhibitors including donepezil, rivastigmine and galanthamine, and an N-methyl-D-aspartate receptor (NMDA) antagonist named memantine. Whilst cholinesterase inhibitors inhibiting the breakdown of acetylcholine to increase cholinergic neuronal activity in the central nervous system, memantine hindering neurotoxicity induced by excess glutamate [11]. PD is characterised by the presence of slowness of movement and at least one of the following symptoms including resting tremor, postural instability or muscle rigidity. PD is generally managed with medications that increase dopaminergic nerve activity including levodopa and dopamine agonists, as well as medications that suppress dopamine metabolism including catechol-O-methyltransferase (COMT) inhibitors and monoamine oxidase B (MAO-B) inhibitors [12]. Both conditions have no cure that either halts the disease progression or reverses the damage. AD is a multifactorial disease as its etiology is associated with accumulation of amyloid beta, hyperphosphorylation of tau protein, excitotoxicity, oxidative stress and neuroinflammation [7,13]. While, PD is associated with a buildup of intracellular aggregate-containing proteins such as ubiquitin and alpha-synuclein (α-Syn) that form Lewy bodies, dopaminergic neurodegeneration in the substantia nigra pars compacta (SNpc) and neuroinflammation [12,14]. Another similarity of AD and PD is that both diseases are related to neuroinflammation. Neuroinflammation induced by amyloid beta plaques in AD and alpha-synuclein aggregates in PD substantially worsens the loss of cholinergic and dopaminergic neurons, respectively [11,14].
Since the current treatment options solely provide symptomatic relief, ongoing research has been focusing on identifying multi-targeted therapeutic options for neurodegenerative diseases. Natural products are a prolific source of therapeutic leads. For instance, naturally derived alkaloids including huperzine A and galanthamine from medicinal plants have been discovered and explored for their potential in the management of AD [15]. To date, a paradigm shift towards a multi-targeted modulation approach in the management of neurodegenerative diseases is in line with the prevalent characteristic natural product derivatives with multi-targeting properties. This review provides an update on the recent literature on alkaloid-based cholinesterase inhibitors and their in vivo efficacy in animal models, highlighting their mechanisms in neurodegenerative-disorder-related experimental models including neuroprotection, neuroinflammation, neurogenesis, tau pathology and amyloid beta accumulation. One of the hurdles in drug development includes drug-like properties. Therefore, an analysis of the physicochemical properties of alkaloid-based compounds is also conducted.
2. Cholinesterase Inhibitory Potential of Natural-Product-Derived Alkaloids
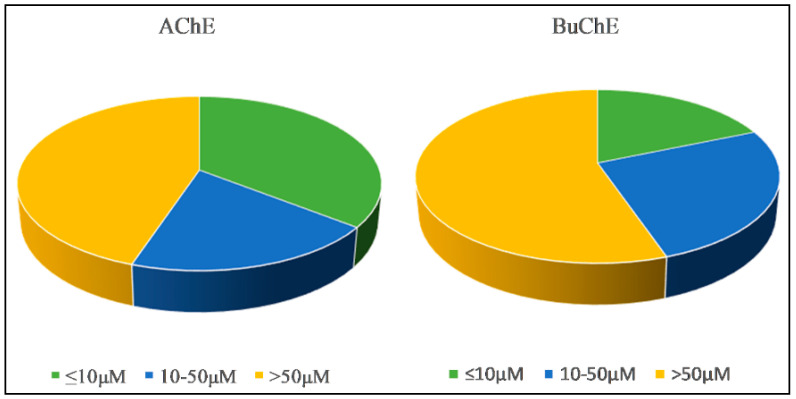
Acetylcholinesterase enzyme (AChE) is predominantly found in the cholinergic synapses while butyrylcholinesterase enzyme (BuChE) is a non-substrate specific enzyme that can be found throughout the body including in glial cells. Low levels of AChE and high levels of BuChE have been reported as AD progresses [16]. Acetylcholinesterase enzyme inhibitors (AChEi) including galanthamine (1) and donepezil are drugs that have been approved by the Food and Drug Agency (FDA) to manage AD. AChEi is used to enhance acetylcholine (ACh), a neurotransmitter responsible for cognition at a homeostatic level in the brain [17,18,19]. Therefore, chemical compounds able to inhibit AChE enzyme or both AChE and BuChE enzymes (dual inhibitor) are considered essential in the management of the progression of AD. In this review, all naturally occurring alkaloids are categorized by their inhibitory activities in IC50. By definition, IC50 is a measure of the potency that one chemical substance has to inhibit a specific biological or biochemical function [11]. The inhibition in three categories (IC50 ≤ 10 μM, 10–50 μM and >50 μM) is presented in Figure 1.
Figure 1.
Cholinesterase enzyme inhibition of alkaloids (2011–2020).
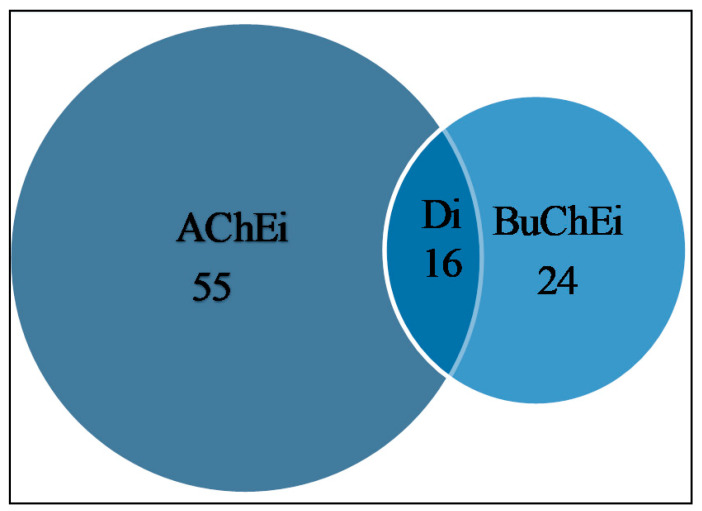
Figure 2 reveals that a total of 55 alkaloids were identified as AChEi, while 24 alkaloids were identified as BuChEi. Essentially, 16 alkaloids were shown to inhibit both enzymes at IC50 values equal to or less than 10 μM. Table 1 lists the bioactive alkaloids isolated from plants along with their cholinesterase inhibitory activities, while Figure 3 and Figure 4 display the chemical structures of alkaloids 1–61.
Figure 2.
Potential cholinesterase enzyme inhibitors with IC50 ≤ 10 μM. AChEi represents acetylcholinesterase inhibitors, Di represents dual cholinesterase inhibitors, BuChEi represents butyrylcholinesterase inhibitors.
Table 1.
Alkaloids from different species and respective IC50 towards AChE and BuChE.
| Species | Part | Alkaloid Class | Chemical Constituent | IC50 (μM) | References | |
|---|---|---|---|---|---|---|
| AChE | BuChE | |||||
| Rauvolfia reflexa | Bark | Indole | Rescinnamine (3) | >10 | 8.06 | [20] |
| Nauclea officinalis | Bark | Indole β-carboline |
Angustidine (4) | >10 | 1.03 | [21] |
| Nauclefine (5) | - | 7.70 | ||||
| Angustine (6) | - | 4.98 | ||||
| Peganum harmala | Seed | Indole β-carboline |
Harmane (7) | 3.64 | 1.04 | [22] |
| Harmol (8) | 1.90 | 0.35 | ||||
| Harmine (9) | 1.21 | 2.79 | ||||
| 3-Hydroxylated harmine (10) | >10 | 3.25 | ||||
| 1-Hydroxy-7-methoxy-β-carboline (11) | 7.19 | 5.15 | ||||
| Harmaline (12) | 1.95 | 5.38 | ||||
| Harmalol (13) | 3.45 | 0.66 | ||||
| Harmalanine (14) | >10 | 3.24 | ||||
| β-carboline | Deoxyvasicine (15) | 2.37 | 0.04 | |||
| Vasicine (16) | 3.38 | 0.10 | ||||
| Picrasma quassioide | Stem | β-carboline | 3-Ethyl-12-methoxy-β-carboline (17) | 6.37 | - | [23] |
| 6,12-Dimethoxy-3-ethyl-β-carboline (18) | 9.01 | - | ||||
| Zanthoxylum rigidum | Root | Isoquinoline | Avicine (19) | 0.15 | 0.88 | [24] |
| Nitidine (20) | 0.65 | 5.73 | ||||
| Coptis chinensis | Rhizome | Isoquinoline | Berberine chloride (21) | 1.1 | > 10 | [25] |
| 13-alkylberberine (22) | 5.6 | > 10 | ||||
| Stephania epigaea | Root | Proaporphine Aporphine |
Epigasine B (23) | 4.36 | - | [26] |
| Dehydrodicentrine (24) | 2.98 | - | ||||
| Romerine (25) | 8.32 | - | ||||
| Dicentrine (26) | 6.6 | - | ||||
|
Beilschmiedia alloiophylla
Beilschmiedia kunstleri |
Bark | Aporphine | 2-Hydroxy-9-methoxyaporphine (27) | 2.0 | - | [27] |
| Laurotetanine (28) | 3.2 | - | ||||
| Oxoaporphine | Liriodenine (29) | 3.5 | - | |||
| Morphinan (isoquinoline) |
Oreobeiline (30) | 5.0 | - | |||
| Aporphine | Boldine (31) | 8.5 | - | |||
| Secoboldine (32) | 10.0 | - | ||||
| Asimilobine (33) | 8.7 | - | ||||
| (S)-3-Methoxynordomesticine (34) | 10.0 | - | ||||
| Isoboldine (35) | 9.4 | - | ||||
| Zephyranthes carinata | Whole plant |
Lycorine-type | Galanthine (36) | 6.10 | - | [28] |
| Galanthamine (1) | 1.27 | - | ||||
| Holarrhena pubescens | Bark | Steroid | Mokluangin C (37) | 1.44 | - | [29] |
| Mokluangin A (38) | 2.12 | - | ||||
| Antidysentericine (39) | 4.09 | - | ||||
| Fritillaria walujewii | Bulb | Isosteroid | Tortifoline (40) | 5.8 | 2.08 | [30] |
| Walujewine C (41) | 7.2 | 2.58 | ||||
| Sinpeinine A (42) | 8.3 | 3.05 | ||||
| Walujewine B (43) | >10 | 3.89 | ||||
| Walujewine E (44) | 9.8 | 5.71 | ||||
| Hepehenizioiside (45) | >10 | 6.80 | ||||
| Walujewine A (46) | 7.6 | >10 | ||||
| Antarctic Latrunculia spp. | Sponge | Pyrroloiminoquinone | (+)-Discorhabdin G (47) | 1.3 | - | [31] |
| (+)-Discorhabdin B (48) | 5.7 | - | ||||
| Huperzia squarrosa | Aerial | Lycopodium | Squarrosine A (49) | 7.3 | - | [32] |
| Pyrrolhuperzine A (50) | 8.91 | - | ||||
| 12-epilycodoline N-oxide (51) | 0.59 | - | ||||
| Huperzine A (2) | 0.01 | - | ||||
| Huperzia squarrosa | Whole plant | Lycopodium | Squarrosinoxide (52) | 3.12 | - | [33] |
| Huperzine A (2) | 0.034 | - | ||||
| Lycopodiastrum casuarinoides | Aerial part | Lycodine-type | Lycocasuarinine D (53) | 0.22 | >10 | [34] |
| Lycocasuarinine A (54) | 4.74 | >10 | ||||
| N-demethylhuperzinine (55) | 0.89 | 1.86 | ||||
| Huperzine C (56) | 0.37 | 7.33 | ||||
| Camellia sinensisvar | Leaf | Flavoalkaloid | 3-O-Cinnamoylepicatechin (57) | 1.0 | - | [35] |
| (−)-6-(5‴S)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (58) | 0.14 | - | ||||
| (−)-6-(5‴R)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (59) | 0.13 | - | ||||
| (−)-8-(5‴S)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (60) | 0.18 | - | ||||
| (−)-8-(5‴R)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (61) | 0.21 | - | ||||
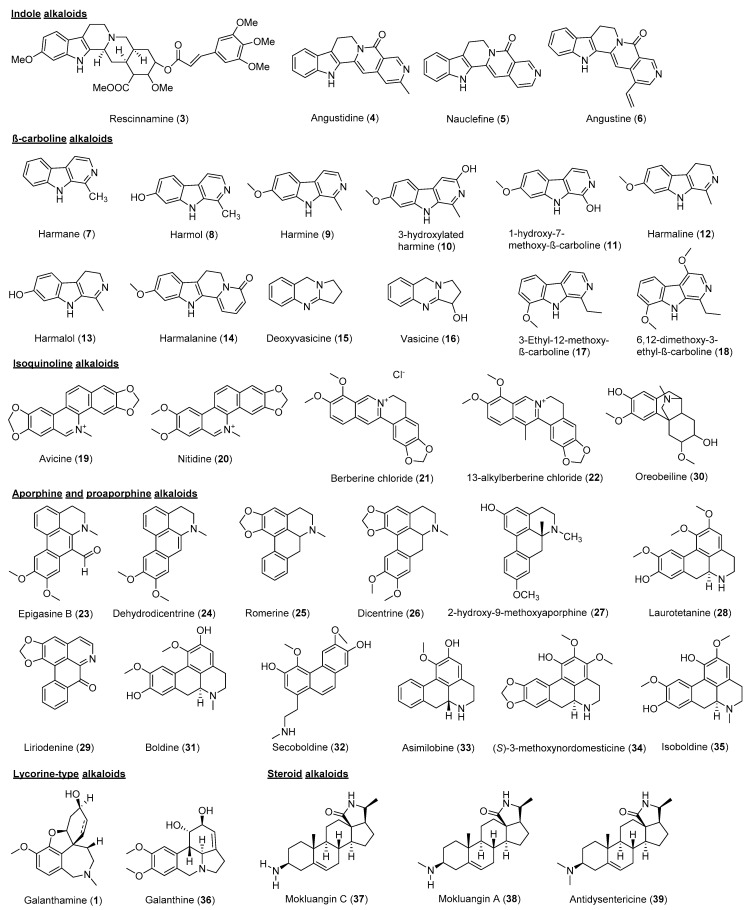
Figure 3.
Chemical structures of alkaloids 1, 3–39.
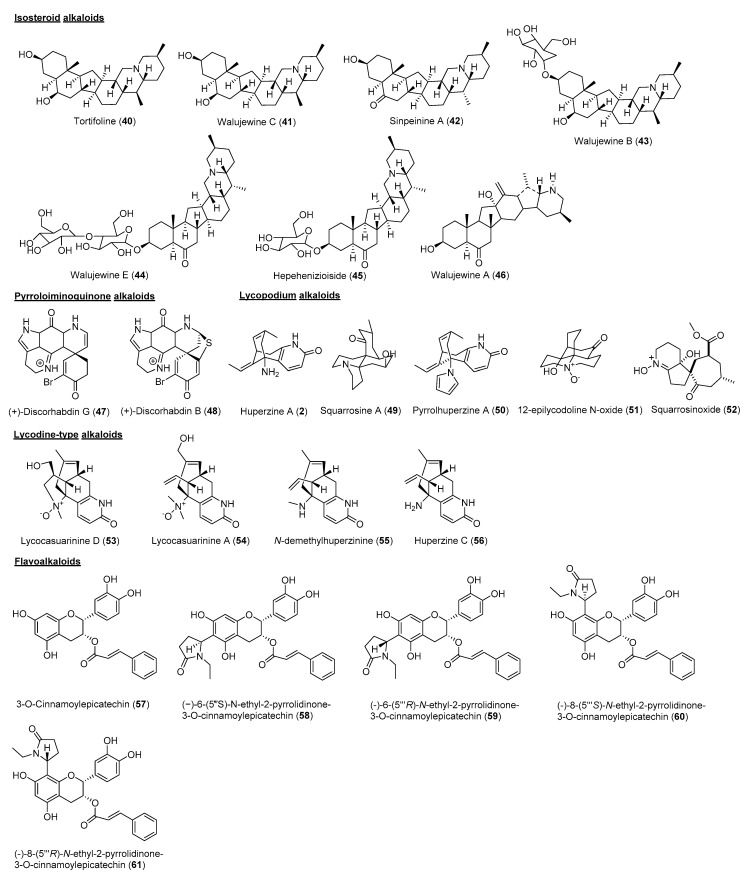
Figure 4.
Chemical structures of alkaloid 2, 40–61.
Indole alkaloids are among the largest group of heterogeneous secondary metabolites, comprising a six-membered aromatic ring fused to a five-membered nitrogen-containing pyrrole ring [36]. Rescinnamine (3) from Rauvolfia reflexa was reported to behave as a dual cholinesterase inhibitor (IC50 AChE 11.01 μM; BuChE 8.06 μM) [20]. Monoterpene indole alkaloids are recognised as molecules containing a monoterpenoid unit fused to an indole moiety. Monoterpene indole alkaloids (4–6) isolated from the Nauclea officinalis possess selective BuChE inhibitory activity. Angustidine (4), nauclefine (5) and angustine (6) were proven to inhibit BuChE with an IC50 in the range 1.03 to 7.70 μM. A kinetic study showed that 4 was a mixed-mode inhibitor with a Ki value of 6.12 μM [21].
β-carboline alkaloids constitute an indole moiety fused to C-3 and C-4 of a pyridine at its ortho-position [37]. Ten β-carboline alkaloids (7–16) of Peganum harmala were reported to possess cholinesterase inhibitory activities with IC50 values <10 μM. Harmol (8), harmalol (13), deoxyvasicine (15) and vasicine (16) were potent inhibitors of BuChE with IC50 values in the range 0.04 to 0.66 μM. However, harmine was the most potent AChE inhibitor with an IC50 of 1.21 μM among the indole alkaloids. A preliminary structure-activity relationship study showed that multiple substitutions at the indole ring and saturation of the pyridine ring were essential for the cholinesterase inhibition effects [22]. 3-Ethyl-12-methoxy-β-carboline (17) and 6,12-dimethoxy-3-ethyl-β-carboline (18) from Picrasma quassioides were reported as possessing AChE inhibitory properties [23].
Isoquinoline alkaloids are among the various classes of alkaloids obtained from natural sources. They consist of an isoquinoline or a tetrahydroisoquinoline ring as their basic skeleton [38]. Avicine (19) was the most potent dual cholinesterase inhibitor with IC50 values of 0.15 and 0.88 μM for both AChE and BuChE. Nitidine (20) had weaker inhibitory activity towards both AChE and BuChE than 19 with IC50 values of 0.65 μM and 5.73 μM, respectively [24]. By comparison of the molecular structures, a tertiary amine is present at position 7 of 19 and 20 and is responsible for the high binding affinity towards both AChE and BuChE. Berberine chloride (21) and 13-alkylberberine (22) are another two isoquinoline alkaloids derived from rhizomes of Coptis chinensis that possess slightly different molecular structures from 19 and 20. Although both 21 and 22 had IC50 > 10 μM towards BuChE, 21 displayed a stronger inhibitory activity towards AChE with an IC50 of 1.1 μM than 22 with an IC50 of 5.6 μM [25]. By comparing the molecular structures of both alkaloids, it is seen that the presence of a methyl group at position 13 in the structure of 22 is responsible for the reduced inhibitory activity against both AChE and BuChE.
The aporphine and proaporphine alkaloids are naturally derived from isoquinoline. Generally, they are distributed in the families of Annonaceae, Lauraceae, Magnoliaceae and Menispermaceae [39]. Examination of the extracts from Stephania epigaea [26], Illigera aromatica [40], Beilschmiedia sp. [27], Monimiaceae and Magnoliacea [41] resulted in the isolation of a series of aporphine and proaporphine-type alkaloids. Among them, epigasine B (23), dehydrodicentrine (24), romerine (25), dicentrine (26), 2-hydroxy-9-methoxyaporphine (27), laurotetanine (28), liriodenine (29), oreobeiline (30), boldine (31), secoboldine (32), asimilobine (33), (S)-3-methoxynordomesticine (34) and isoboldine (35) were reported to exhibit significant AChE inhibitory activities [26,27].
Lycorine-type alkaloids, also known as Amaryllidaceae alkaloids, belong to the large group of isoquinoline alkaloids. They can be found in plants of the family Amaryllidaceae. Many lycorine-type alkaloids have been isolated, mostly concentrated in bulbs and leaves [42]. Lycorine-type alkaloids including galanthine (36) and 1 from Zephyranthes carinata showed AChE inhibitory activity against AChE [28].
Steroidal alkaloids are one of the important classes of alkaloids derived from plants. They have a basic steroidal backbone with a nitrogen atom present in the ring or side chain [43]. Three new steroidal alkaloids Mokluangins A–C (37–39) from Holarrhena pubescens were reported to possess AChE inhibitory activity in the range 1.44 to 4.09 µM, in which substitution at C-3 serves as key in the modulation of AChE inhibitory activity [29].
Isosteroidal alkaloids are one of the representative steroidal alkaloids belonging to the C-27 skeleton type [43]. Seven isosteroidal alkaloids (40–46) from Fritillaria walujewii were reported for their potential cholinesterase inhibitory activities. Tortifoline (40), Walujewine C (41), Sinpeinine A (42), Walujewine A (46), and Walujewine E (44) were shown to inhibit AChE with IC50 values of 5.8 to 9.8 µM, while all compounds were proven to inhibit BuChE less than 10 µM except 46. It can be deduced that all compounds are dual cholinesterase inhibitors except 43, 45 and 46 [30].
Pyrroloiminoquinone alkaloids are mainly isolated from marine organisms. Important pyrroloiminoquinone alkaloids include discorhabdins, prianosins, batzellins, wakayins and damirones [44]. Discorhabdins G (47) and B (48) from Antarctic Latrunculia sp. sponges exhibited AChE inhibitory activities with IC50 values of 1.3 and 5.7 µM, respectively [31].
Lycopodium alkaloids are an interesting class of alkaloids, commonly found in the plants of Lycopodiaceae. They consist of quinolizine, pyridine and α-pyridone type alkaloids [45]. Generally, lycopodium alkaloids are composed of C16 skeletons and occasionally have C32 skeletons when they exist as dimers [46]. Among the isolated lycopodium alkaloids, huperzine A (2) has appeared as a well-known AChE inhibitor in the treatment of AD [45]. Squarrosine A (49) and pyrrolhuperzine A (50) were isolated as new Lycopodium alkaloids from Huperzia squarrosa, along with known 2 and 12-epilycodoline N-oxide (51). Based on the findings, huperzine A was the most potent for AChE inhibition, followed by 49, 50 and 51 [32]. In a continuation study on the same plant, H. squarrosa yielded 52 as a new lycopodium alkaloid with AChE inhibitory potential [33].
Lycodine-type alkaloids belong to the class of lycopodium alkaloids. Generally, they consist of four rings, including one pyridine or pyridone ring. Lycodine-type alkaloids have been known to show AChE inhibitory activity [46]. In a study conducted on lycodine-type alkaloids including Lycocasuarinine D (53), Lycocasuarinine A (54), N-demethylhuperzinine (55), and huperzine C (56) from Lycopodiastrum casuarinoides, they were reported for their cholinesterase inhibition potential. 50 and 51 showed AChE inhibition with IC50 values of 0.22 and 4.74 μM, while 55 and 56 were dual cholinesterase inhibitors [34].
Flavoalkaloids are a unique subclass of alkaloids consisting of a basic skeleton of flavonoid fused with a nitrogen containing ring, such as pyrrolidinone, pyrrolidine, indole, piperidine, piperidinone and aminoglycoside [47]. To date, less than 100 naturally occurring flavoalkaloids have been reported, although they have been found to show a wide range of bioactivities [35,47]. In a recent study, five new cinnamoylated flavoalkaloids were isolated from Camellia sinensis. The compounds were known as 3-O-cinnamoylepicatechin (57), (−)-6-(5‴S)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (58), (−)-6-(5‴R)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (59), (−)-8-(5‴S)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (60) and (−)-8-(5‴R)-N-ethyl-2-pyrrolidinone-3-O-cinnamoylepicatechin (61). All flavoalkaloids showed significant AChE inhibitory activity with IC50 ranging from 0.126 to 1.040 μM. The study revealed that the C-6 position was crucial in AChE inhibitory activities [35].
3. Multi-Target Modulation Potential of Alkaloids in Neurodegenerative Diseases
For decades, researchers have been heavily focused on discovering drugs that can potentially modify the progression of neurodegenerative diseases, for example, the discovery of secretase inhibitors based on the amyloid hypothesis for Alzheimer’s disease. Unfortunately, none of the candidates have shown promising results improving the progressing of the disease in the final phase of clinical trials. Cholinesterase inhibitors have remained the drugs for the management of the disease and further studies are prompted to explore the potential of alkaloids for the management of neurodegenerative conditions in addition to their cholinesterase activity [48]. Despite having potent anti-cholinesterase properties that help to improve cognitive function, we have conducted a search on the alkaloids in Table 1 that have potential cholinesterase inhibitory activities and compiled several studies that have reported the potential of these alkaloids to act on multiple other targets, to provide an overview of the multi-target modulation potential of these compounds. However, it cannot be ruled out that compounds with lesser cholinesterase inhibitory activities are not potentially useful for some other targets in neurodegenerative diseases. This section focuses mainly on Alzheimer’s disease (AD) and Parkinson’s disease (PD). The collection of mechanisms that these alkaloids act upon include neuroprotection, neuroinflammation, neurogenesis, amyloid beta aggregation (Aβ) and tau hyperphosphorylation.
3.1. Neuroprotection
Neuroprotection slows down loss of neurons and subsequently progression of neurodegenerative diseases via various pathways, including reduction in oxidative stress, mitochondrial dysfunction, protein aggregation, inflammation, excitotoxicity and cell apoptosis [49].
Several studies have shown that harmane (7), harmol (8), harmine (9), harmaline (12), and harmalol (13) which belong to the indole β-carboline class (Table 1), exhibit neuroprotective potential against neuronal damage. An In Vitro study showed that 7 protects against H2O2-induced toxicity in neuroblastoma cells by attenuating the decreased cell viability [50]. This model is associated with neuronal injuries caused by oxidative stress that is implicated in neurodegenerative disease development.
12 is widely used to induce tremor in rodents, no negative impact was found on dopaminergic PC12 cells alone at concentration of 50 µM, same for its metabolite harmalol 13 [51]. The result coincides with two other studies that showed no toxicity imposed on PC12 cells by 12 and 13 alone [52,53]. MPP+ is a neurotoxin to the dopaminergic neurons that implicated in Parkinson’s disease (PD). The role of 7 in suppressing 1-Methyl-4-phenylpyridinium (MPP+) effects is achieved by suppressed mitochondrial transmembrane potential (MMP), cytochrome c release, caspase-3 activation, reactive oxygen species (ROS) and GSH levels In Vitro [51,52,53]. Dopamine oxidation initiates different cascades to form endogenous neurotoxins that contribute to neurodegeneration [54,55]. In the case of 12, it was shown to offset the toxic effects of dopamine oxidation imposed on brain mitochondria Ex Vivo. This is attributed to the antioxidative properties of 12 via maintaining thioredoxin reductase activity and inhibiting thiol oxidation and therefore dopamine oxidation product formation [56]. In addition, 9 and 12 upregulated antioxidant enzymes such as superoxide dismutase [57] and glutathione peroxidase (GPx) In Vitro, while in other studies, they reduced ROS elevation and thiol oxidation, leading to an enhanced antioxidant defence mechanism to produce a neuroprotective effect [52,53,58,59].
In addition, 9 exerted neuroprotective effects by upregulating glutamate transporter-1 (GLT-1) protein levels and GLT-1 and glutamate aspartate transporter (GLAST)-dependent glutamate uptake in astroglial cells and in the cortical tissue of SOD1G93A mice, a transgenic mouse model of amyotrophic lateral sclerosis [60]. These glutamate transporters maintain low extracellular concentration of glutamate, which is an excitatory neurotransmitter, whereby its accumulation contributes to excitotoxicity [61]. Moreover, a recent systematic review showed that 9 improved memory and learning and demonstrated neuroprotective effects on the hippocampus in preclinical experimental models [62]. It was purportedly involved in GLT-1 upregulation, ROS decrement, brain-derived neurotrophic factor (BDNF) elevation and had anti-inflammatory effects. In the context of PD, 9 was recently investigated for its degradation effect on α-synuclein In Vitro. Ubiquitin-proteasome system (UPS) is one of the systems that removes α- synuclein via proteasome proteolytic activity and it was shown that 9 increased the proteolytic activity via protein kinase A (PKA) phosphorylation and hence enhanced UPS for α-synuclein clearance [63,64].
13, a metabolite of 9, was included in the results from previously mentioned studies as a compound of interest and it was found that 13 provided neuroprotection via modulating oxidative stress, MMP and apoptosis In Vitro [51,52,53]. These findings showed a decrease in ROS and thiol oxidation, an increase in GSH levels and attenuation in MMP loss [51,52,53,65]. The effect of 13 on two of the processes involved in mediating cell death, which are release of cytochrome c and caspase-3 activation, were investigated as well, and 13 decreased both [51,66].
Research on the protective effects of berberine chloride (21), which is an isoquinoline alkaloid (Table 1), on neurodegeneration has been gaining popularity in recent years. In an in vivo study, pretreatment of 21 on 6-hydroxydopamine (6-OHDA)-stimulated neurotoxicity that models PD in rats significantly reduced apomorphine-induced rotations and the loss of Nissl-stained substantia nigra pars compacta (SNpc) neurons, and attenuated the reduction of tyrosine hydroxylase immunoreactivity in SNpc dopaminergic neurons [67]. An In Vitro study showed that 21 exerted neuroprotective properties against oxidative-stress-mediated neurodegeneration through activating Akt/GSK, Akt/GSK3β/Nrf-2 and PI3K/Akt/Nrf2 signalling cascades, increasing p-CREB, stimulating NGF and BDNF release, decreasing NF-κB nuclear translocation, suppressing TNFα, COX-2, IL-1B, NF-κB, and downregulating caspase-1, caspase-3, Bax, Bcl-2 elevation, cyclin D1, and p53 [68]. Aside from the results on par with previous studies, Deng and the team reported 21 decreased ROS production and reversed MMP reduction In Vitro, and suggested 21 exerted neuroprotective effects by PI3K/Akt pathway activation in rotenone-induced neurotoxicity [69]. 21 was also proven to protect PC-12 cells from oxidative damage via PI3K/AKT/mTOR-mediated mitophagy [70]. Wallerian-like degeneration (WLD) is an axonal degeneration, extending from the axon distal to the site of injury and occurs in neurodegenerative diseases [71]. Interestingly, 21 was found to be a non-competitive inhibitor of Sterile Alpha and Toll Interleukin Receptor Motif–containing protein 1 (SARM1), which is a key mediator for WLD, yielding a percentage inhibition from a primary screen of 70% [72]. The same study further tested its inhibition activity on the SAM1−2TIR domain of bacterial and Expi293 cells to confirm the findings and obtained IC50 values of 110 ± 10 μM and 77 ± 5 μM, respectively. In addition, 21 increased CYP2J2, a protein that was found to be protective against a PD model, via stimulation of Peroxisome proliferator-activated receptor alpha (PPAR-α) In Vitro [73]. An in vivo study showed that 21 prevented aminolevulinic acid dehydratase (δ-ALA-D) inhibition and prevented damage on purinergic transmission by attenuating NTPDase, ADP, 5′-nucleotidase and ADA activity loss in streptozotocin-induced dementia, in which the regulation of this transmission plays a role in memory processing [74,75]. However, δ-ALA-D activity in specific neuronal cells warrants further investigation due to the limited studies available.
The stimulation of glial hemichannel activity enhances ATP and glutamate release that subsequently induces neuronal death, whereas Reticulon-3 (RTN3) aggregates in the AD brain and facilitates development of dystrophic neurites [76,77]. Boldine (31), an aporphine alkaloid (Table 1), was reported to decrease astroglial hemichannel activity without affecting gap junctional communication, reducing ATP and glutamate release. An in vivo study showed 31 protected against neuronal oxidative stress and neuritic dystrophies surrounding amyloid beta (Aβ) plaques, where smaller and fewer areas of RTN3 immunoreactive dystrophic neurites (RIDNs) were observed in APP/PS1 mice [78]. In another study, 31 decomposed H2O2, decreased iron and EDTA-mediated deoxyribose degradation and formation of melanin by dopamine oxidation, attenuated loss of MMP, cytochrome C elevation, loss of thioredoxin reductase activity, inhibited thiol oxidation induced by dopamine and 6-OHDA and PC-12 cell viability loss, and caspase-3 activation induced by dopamine [79].
An in vivo study showed that deoxyvasicine (15), which is a β-carboline alkaloid (Table 1), protected the neurons from oxidative-stress-induced damage at a dose of 15 mg kg−1. The study demonstrated that the amount of glutathione peroxidase (GPx) was significantly elevated by administration of 15, subsequently enhancing the antioxidant defence mechanism in the brain. In addition, 15 was capable of enhancing the survival of nerves by elevating brain-derived neurotrophic factor (BDNF) at 45 mg kg−1 [80].
3.2. Neuroinflammation
Over the years, studies have been carried out to investigate the pathology of neurodegenerative conditions and neuroinflammation has been found to be one of the underlying mechanisms. A defect in immune cell function can initiate inflammation in the central nervous system (CNS) and eventually cause nerve injury. In particular, acute inflammation can be beneficial to the brain. Conversely, chronic inflammation can harm the brain by promoting hyperphosphorylation of tau protein and amyloid beta (Aβ) aggregation. These mechanisms can be activated by proinflammatory mediators such as interleukin 1 (IL-1) and interleukin 6 (IL-6). Since inflammation is closely related to the pathology of neurodegenerative conditions such as AD and PD, alkaloids that are able to prevent neuroinflammation might be able to ameliorate neuroinflammation as well as helping to manage the conditions [81,82,83].
9 and 12 were highlighted to be capable of counteracting neuroinflammation. It was suggested that they were able to decrease the production of inflammatory cytokines such as tumour necrosis factor alpha (TNF-α) and myeloperoxidase (MPO) and mediators such as nitric oxide (NO). In particular, 7 and 12 showed IC50 of 0.08 μM and 0.26 μM towards MPO, respectively, therefore proving their potential to be incorporated as an agent to counteract neuroinflammation [58,59]. In addition, an in vivo study suggested that 9 significantly improved cognitive deficits in chemically induced diabetic rats at 20 mg kg−1. The study proposed that diabetes mellitus was closely related to cognitive dysfunction and both were related to inflammation. When 9 was administered to the diabetic rats, NLRP3 inflammasome activity was supressed, leading to an increase in the expression of brain-derived neurotrophic factor (BDNF) and subsequently improved cognitive ability [84]. In addition, an in vivo study reported that 9 was also capable of crossing the blood–brain barrier (BBB) soon after oral intake [85].
Another β-carboline alkaloid 15 was able to inhibit neuroinflammation via several pathways. An in vivo study had proved that 15 was capable of increasing the amount of γ-GABA and decreasing the amount of glutamate in the brain at concentrations 5 1, 15 and 45 mg kg−1. γ-GABA is a vital neurotransmitter that acts to inhibit neuroinflammation initiated by astrocytes and microglia by hindering the release of TNF-α. Besides, production of TNF-α, which is a cytokine that promotes inflammation, was also significantly downregulated in a concentration-dependent pattern when treated with 15 at concentrations of 5–45 mg kg−1 [80].
21 acts on multiple targets to improve AD conditions. One of the mechanisms of action of 21 is to attenuate neuroinflammation [86]. For example, an in vivo study reported a decrease in pro-inflammatory mediators including COX-2, IL-12, IL-6, IL-1β and TNF-α resulting from the administration of 21 in an AD rat brain [87]. Furthermore, an in vivo study reported that 21 hindered the p65 subpart expression and phosphorylation at 50 mg kg−1. p65 is a subpart of the NF-κB heterodimer that is vital in modulating inflammatory protein production [88]. In addition, an in vivo study also reported that 21 was able to counteract neuroinflammation by suppressing IL-6, TNF and p38 MAPK signalling cascades [89].
3.3. Neurogenesis
Adult neurogenesis is a process that occurs in the subgranular zone (SGZ) and subventricular zone (SVZ) of the hippocampus to produce neurons throughout a human’s lifetime. A defect in adult neurogenesis can potentially result in neurodegenerative conditions such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease (HD) [90]. For instance, an in vivo study reported that adult neurogenesis impairment may have started in the early AD disease stage even before the formation of neurotoxic neurofibrillary tangles and Aβ plaques [91,92].
An In Vitro study suggested that 8, 9, and 12 successfully induced neurogenesis when tested on progenitor cells cultured from SGZ and SVZ. Furthermore, the metabolism of 9 in the human body produced 8 as the main product. These β-carboline alkaloids were also capable of inducing neuronal cell specialization and this was proven by the presence of MAP-2 and Tuj-1 expression. The study proposed that the neurogenesis potential of these β-carboline alkaloids was attributed to their inhibitory activity towards monoamine oxidase [70] and DYRK1A [93].
An In Vitro study investigating the neurogenesis potential of aporphine alkaloids (Table 1) on PC-12 cells showed that asimilobine (33) significantly stimulated the outgrowth of neurite in PC-12 cells but with no obvious effect on the mRNA expression coding for proteins essential for cell division and specialisation. In addition, the study also reported that 33 was highly penetrable across the blood–brain barrier (BBB), making it a potential drug candidate for the management of neurodegenerative conditions [94].
3.4. Aggregation of Amyloid Beta
Amyloid beta (Aβ) is a product resulting from the enzymatic breakdown of amyloid precursor protein (APP) by γ-secretase and β-secretase and it is suggested that Aβ plays a role in the development of AD. Aβ is a peptide that mostly consists of 40–42 amino acids and is susceptible to aggregation to form neurotoxic Aβ plaques. Once Aβ plaques accumulate in the brain, abnormal synaptic and neuronal activities will develop, causing damage to the brain and producing symptoms such as cognitive deficits and gradual loss of memory [95,96]. Therefore, preventing the aggregation of Aβ may help to delay the progression of AD. Aside from the anti-cholinesterase activity displayed by plant-derived alkaloids, some of them are capable of inhibiting Aβ aggregation.
Avicine (19) and nitidine (20) are isoquinoline alkaloids (Table 1) and they possess the ability to inhibit oligomerization of Aβ. An In Vitro study reported that 19 and 20 demonstrated moderate intensity suppression towards Aβ aggregation as seen from their IC50 values of 5.56 and 1.89 μM towards Aβ, respectively. In fact, it was also suggested that both 19 and 20 possessed the most significant inhibition activity towards multiple targets involved in neurodegenerative conditions and they had similar molecular structures when compared to other isoquinoline alkaloids extracted from Zanthoxylum rigidum. Therefore, it is encouraged to investigate the molecular scaffold associated with their multi-targeted activity [24].
In addition, 21 has been studied for years and a review reported that it hindered ERK1/2 and mitogen-activated protein kinase (MAPK) signalling cascades, consequently deactivating the β-secretase-1 (BACE-1) and diminishing Aβ generation [86]. In fact, 21 is marketed as an over-the-counter product that is consumed orally in China, as studies investigating its efficacy and safety after oral consumption reported acceptable results. Apart from its anti-cholinesterase activity, which is comparable to 1 and 21 also possessed the ability to decrease Aβ aggregation by decreasing the formation of Aβ. An in vivo study reported that 21 was able to significantly inhibit β-secretase activity in an AD brain, consequently reducing the formation of Aβ by up to 40% and improving the symptoms of AD. Aside from suppressive action towards β-secretase, another in vivo study reported that 21 enhanced Aβ40 activity that lowered the neurotoxic potential of Aβ42 by modifying the stability, morphology and solubility of Aβ42 to impair Aβ42 aggregation [87,97]. This statement is further supported by an in vivo study that claimed that 21 at concentrations of 50 and 100 mg/kg/day was able to downregulate the Pen-2, Aph-1α and PS1 parts of γ-secretase and β-secretase, consequently suppressing the production of Aβ. In addition, 21 was shown to significantly increase α-secretase activity at identical concentrations [98]. An In Vitro study further suggested that 21 was able to inhibit oligomerization of Aβ and fibril formation [99].
3.5. Tau Hyperphosphorylation
Tau protein (τ) plays a vital role in stabilizing the neuronal microtubules and hence supporting the structure of a neuron and the transport of nutrients intracellularly. Kinases that include dual specificity tyrosine phosphorylation regulated kinase 1A (DYRK1A), glycogen synthase kinase-3β (GSK-3β), Ca2+/calmodulin activated protein kinase II and cyclin-dependent kinase-5 (Cdk5) can induce tau hyperphosphorylation. Hyperphosphorylation of tau protein leads to the clumping of phosphorylated tau proteins to form neurofibrillary tangles or paired helical filament tau. Consequently, they no longer support the microtubules within a neuron, eventually leading to neuronal apoptosis and hence neurodegeneration [100].
9 was found to possess inhibitory activities towards DYRK1A that in turn hindered tau protein hyperphosphorylation. It was proven by an In Vitro study that 9 was a strong inhibitor of DYRK1A with an IC50 of approximately 80 nM. 9 deactivated DYRK1A, hence suppressing tau phosphorylation on serine 396, subsequently reducing all three types of phosphorylated forms of tau protein. By reducing the amount of phosphorylated tau, harmine (9) preserved the function of tau to support the microtubules in a neuron, preventing neuronal death. However, it was reported that 9 could be neurotoxic at concentrations beyond 8 μM due to excessive tau protein depletion [100].
Aside from 9, 21 was also able to impede hyperphosphorylation of tau protein. Although more research needs to be carried out on the actual mechanism of 21 to decrease tau hyperphosphorylation, two In Vitro studies have shown that 21 is capable of preventing the hyperphosphorylation of tau protein stimulated by calyculin A at concentrations of 20 and 25 μg mL−1 by diminishing GSK-3β activity and upregulating the activity of protein phosphatase 2A. In addition, reversal of tau phosphorylation was induced by 21 at Ser262 [86,101,102]. An in vivo study also reported that the antioxidant properties of 21 could help to inhibit overexpression of tau protein and tau hyperphosphorylation in an AD brain [87].
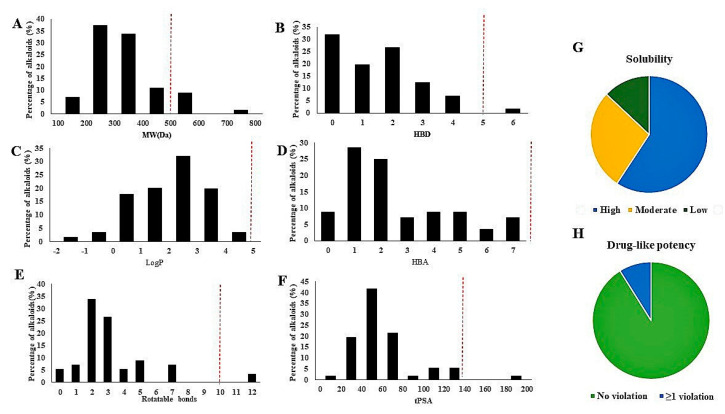
4. Physicochemical Analysis of the Alkaloids
Figure 5 shows the analysis of the physicochemical properties of the alkaloids included in this review. The molecular weight of the majority compounds was below 500 Da. The higher molecular weight compounds included 44, 45, and flavoalkaloids (58–61). Only one compound violates the Lipinski rule for hydrogen bond donating, rotatable bonds and topological polar surface area (TPSA). Interestingly, all the compounds included in this review followed the Lipinski rule for Log P and hydrogen bond acceptor (HBA). 32 compounds were classified as highly soluble, 15 compounds had moderate solubility and 7 compounds had low solubility.
Figure 5.
Analysis of physicochemical properties of alkaloids: (A) molecular weights (MW(Da)); (B) hydrogen bond donors (HBDs); (C) calculated LogP; (D) hydrogen bond acceptors (HBAs); (E) rotatable bonds; (F) topological polar surface area (TPSA); (G) solubility; (H) Drug-like potency.
5. Method
All naturally derived alkaloids reported for their cholinesterase inhibitory activity in the literature between 2011–2020 were included in this study. Synthetic alkaloids, alkaloids tentatively identified by Gas chromatography-mass spectrometry (GC-MS) and Liquid chromatography-mass spectrometry LCMS reported for their cholinesterase inhibitory were excluded in this study. Figure 1 shows that 38% and 18.7% of the AChE and BuChE inhibitors with IC50 ≤ 10 μM were included in this review. As a result, 61 compounds with IC50 less than or equal to 10 μM except 1 and 2 were included in the text and review for the disease-modifying potential including amyloid beta inhibitory, tau hyperphosphorylation inhibitory, neuro-inflammation, neurogenesis and neuroprotective effects. The physicochemical properties of the 59 alkaloids (except galanthamine and huperzine A) were predicted (Instant JChem 17.10.0, 2020 ChemAxon Ltd. (http://www.chemaxon.com)).
6. Conclusions
A growing body of evidence has shown the importance of naturally derived alkaloids as neurodegenerative disease modulators. Although alkaloids are the major source of AChE inhibitors, a significant number of dual cholinesterase inhibitors and BuChE inhibitors are being discovered. It is interesting to note that several alkaloids documented in this review protect neurons against mechanisms which are deleterious such as neuroinflammation, oxidative stress, excitotoxicity, apoptosis, Aβ accumulation and tau phosphorylation, and could be developed for the management of Alzheimer’s and Parkinson’s diseases. Specifically, 9 and 21 are potential modulators in the management of the progression of these diseases. Nevertheless, dosage of these alkaloids to be used in neurodegenerative diseases remained inconclusive. Physicochemical analysis revealed that the majority of alkaloids follow the Lipinski rules of drug likeliness. The blood–brain barrier is an important factor guarding the penetration of compounds into the brain, and requires further attention to be paid to it. Understandably, the setbacks of naturally derived compounds such as low extraction yield have halted the development of potential candidates into therapeutic leads. Further development is needed to improve their usefulness as viable therapeutics.
Author Contributions
Conceptualization, K.Y.K.; data collection; methodology Y.X.S., C.K.W., K.Y.K.; software, K.Y.K., Y.R.K., K.C.T.; writing—original draft preparation, Y.R.K., K.C.T., W.N.T., K.Y.K.; writing—review and editing, Y.R.K., K.C.T., W.N.T., K.Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge Universiti Sains Malaysia for the funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh S., Srivastava A., Srivastava P., Dhuriya Y.K., Pandey A., Kumar D., Rajpurohit C.S. Advances in Stem Cell Research- A Ray of Hope in Better Diagnosis and Prognosis in Neurodegenerative Diseases. Front. Mol. Biosci. 2016;3:72. doi: 10.3389/fmolb.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayeux R. EPIDEMIOLOGY OFNEURODEGENERATION. Annu. Rev. Neurosci. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- 3.Gitler A.D., Dhillon P., Shorter J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017;10:499–502. doi: 10.1242/dmm.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dorsey E.R., Elbaz A., Nichols E., Abd-Allah F., Abdelalim A., Adsuar J.C., Ansha M.G., Brayne C., Choi J.-Y.J., Collado-Mateo D., et al. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nichols E., E I Szoeke C., Vollset S.E., Abbasi N., Abd-Allah F., Abdela J., Aichour M.T.E., O Akinyemi R., Alahdab F., Asgedom S.W., et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bondi M.W., Edmonds E.C., Salmon D.P. Alzheimer’s Disease: Past, Present, and Future. J. Int. Neuropsychol. Soc. 2017;23:818–831. doi: 10.1017/S135561771700100X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs R., Kennelly S.P., O’Neill D. Drug treatments in Alzheimer’s disease. Clin. Med. 2016;16:247–253. doi: 10.7861/clinmedicine.16-3-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jahn H. Memory loss in Alzheimer’s disease. Dialog Clin. Neurosci. 2013;15:445–454. doi: 10.31887/dcns.2013.15.4/hjahn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyketsos C.G., Carrillo M.C., Ryan J.M., Khachaturian A.S., Trzepacz P., Amatniek J., Cedarbaum J., Brashear R., Miller D.S. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimer Dement. 2011;7:532–539. doi: 10.1016/j.jalz.2011.05.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarawneh R., Holtzman D.M. The Clinical Problem of Symptomatic Alzheimer Disease and Mild Cognitive Impairment. Cold Spring Harb. Perspect. Med. 2012;2:a006148. doi: 10.1101/cshperspect.a006148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moneim A.E.A. Oxidant/Antioxidant Imbalance and the Risk of Alzheimer’s Disease. Curr. Alzheimer Res. 2015;12:335–349. doi: 10.2174/1567205012666150325182702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beitz J.M. Parkinson s disease: A review. Front. Biosci. 2014;S6:65–74. doi: 10.2741/S415. [DOI] [PubMed] [Google Scholar]
- 13.Farlow M.R. Etiology and pathogenesis of Alzheimer’s disease. Am. J. Heal. Pharm. 1998;55:S5–S10. doi: 10.1093/ajhp/55.suppl_2.S5. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q., Liu Y., Zhou J.-W. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015;4:1–9. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sahoo A.K., Dandapat J., Dash U.C., Kanhar S. Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer’s disease. J. Ethnopharmacol. 2018;215:42–73. doi: 10.1016/j.jep.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Grieg N.H., Kamal M.A., Jabir N.R., Tabrez S., Nasim F.H., Abuzenadah A.M., Aliev G. Chapter 6—Specific Cholinesterase Inhibitors: A Potential Tool to Assist in Management of Alzheimer Disease. In: Atta ur R., Choudhary M.I., editors. Drug Design and Discovery in Alzheimer’s Disease. Elsevier; Amsterdam, The Netherlands: 2014. pp. 366–386. [DOI] [Google Scholar]
- 17.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016;14:101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perry R.J. Attention and executive deficits in Alzheimer’s disease: A critical review. Brain. 1999;122:383–404. doi: 10.1093/brain/122.3.383. [DOI] [PubMed] [Google Scholar]
- 19.Martorana A., Esposito Z., Koch G. Beyond the Cholinergic Hypothesis: Do Current Drugs Work in Alzheimer’s Disease? CNS Neurosci. Ther. 2010;16:235–245. doi: 10.1111/j.1755-5949.2010.00175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fadaeinasab M., Basiri A., Kia Y., Karimian H., Ali H.M., Murugaiyah V. New Indole Alkaloids from the Bark of Rauvolfia Reflexa and their Cholinesterase Inhibitory Activity. Cell. Physiol. Biochem. 2015;37:1997–2011. doi: 10.1159/000438560. [DOI] [PubMed] [Google Scholar]
- 21.Liew S.Y., Khaw K., Murugaiyah V., Looi C.Y., Wong Y.L., Mustafa M.R., Litaudon M., Awang K. Natural indole butyrylcholinesterase inhibitors from Nauclea officinalis. Phytomedicine. 2015;22:45–48. doi: 10.1016/j.phymed.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 22.Yang Y., Cheng X., Liu W., Chou G., Wang Z., Wang C. Potent AChE and BChE inhibitors isolated from seeds of Peganum harmala Linn by a bioassay-guided fractionation. J. Ethnopharmacol. 2015;168:279–286. doi: 10.1016/j.jep.2015.03.070. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Wang C.-X., Song X.-J., Li S.-, Fan C.-L., Chen G.-D., Hu D., Yao X.-S., Gao H. A new cinnamamide derivative and two new β-carboline alkaloids from the stems of Picrasma quassioides. Fitoterapia. 2019;139:104375. doi: 10.1016/j.fitote.2019.104375. [DOI] [PubMed] [Google Scholar]
- 24.Plazas E., Hagenow S., Murillo M.A., Stark H., Cuca L.E., Plazas E., Suarez L.C. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ1-42 aggregation. Bioorganic Chem. 2020;98:103722. doi: 10.1016/j.bioorg.2020.103722. [DOI] [PubMed] [Google Scholar]
- 25.Cao T.Q., Ngo Q.-M.T., Seong S.H., Youn U.J., Kim J.A., Kim J., Kim J.-C., Woo M.H., Choi J.S., Min B.S. Cholinesterase inhibitory alkaloids from the rhizomes of Coptis chinensis. Bioorganic Chem. 2018;77:625–632. doi: 10.1016/j.bioorg.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Dong J.-W., Cai L., Fang Y.-S., Xiao H., Li Z.-J., Ding Z. Proaporphine and aporphine alkaloids with acetylcholinesterase inhibitory activity from Stephania epigaea. Fitoterapia. 2015;104:102–107. doi: 10.1016/j.fitote.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Mollataghi A., Coudiere E., Hadi A.H.A., Mukhtar M.R., Awang K., Litaudon M., Ata A. Anti-acetylcholinesterase, anti-α-glucosidase, anti-leishmanial and anti-fungal activities of chemical constituents of Beilschmiedia species. Fitoterapia. 2012;83:298–302. doi: 10.1016/j.fitote.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Zhan G., Zhou J., Liu J., Huang J., Zhang H., Liu R., Yao G. Acetylcholinesterase Inhibitory Alkaloids from the Whole Plants of Zephyranthes carinata. J. Nat. Prod. 2017;80:2462–2471. doi: 10.1021/acs.jnatprod.7b00301. [DOI] [PubMed] [Google Scholar]
- 29.Cheenpracha S., Jitonnom J., Komek M., Ritthiwigrom T., Laphookhieo S. Acetylcholinesterase inhibitory activity and molecular docking study of steroidal alkaloids from Holarrhena pubescens barks. Steroids. 2016;108:92–98. doi: 10.1016/j.steroids.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y.-M., Feng Y.-D., Lu X., Nie J.-B., Li W., Wang L.-N., Tian L.-J., Liu Q.-H. Isosteroidal alkaloids as potent dual-binding site inhibitors of both acetylcholinesterase and butyrylcholinesterase from the bulbs of Fritillaria walujewii. Eur. J. Med. Chem. 2017;137:280–291. doi: 10.1016/j.ejmech.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Botić T., Defant A., Zanini P., Žužek M.C., Frangež R., Janussen D., Kersken D., Knez Ž., Mancini I., Sepčić K. Discorhabdin alkaloids from Antarctic Latrunculia spp. sponges as a new class of cholinesterase inhibitors. Eur. J. Med. Chem. 2017;136:294–304. doi: 10.1016/j.ejmech.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Nilsu T., Thorroad S., Ruchirawat S., Thasana N. Squarrosine A and Pyrrolhuperzine A, New Lycopodium Alkaloids from Thai and Philippine Huperzia squarrosa. Planta Med. 2016;82:1046–1050. doi: 10.1055/s-0042-106904. [DOI] [PubMed] [Google Scholar]
- 33.Nilsu T., Thaisaeng W., Thamnarak W., Eurtivong C., Jumraksa A., Thorroad S., Khunnawutmanotham N., Ruchirawat S., Thasana N. Three Lycopodium alkaloids from Thai club mosses. Phytochemistry. 2018;156:83–88. doi: 10.1016/j.phytochem.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Xu P.-S., Ren Q., Chen X., Zhou G., Li D., Li X.-M., Xu K.-P., Yu X., Tan G.-S. Lycodine-type alkaloids from Lycopodiastrum casuarinoides and their cholinesterase inhibitory activities. Fitoterapia. 2018;130:203–209. doi: 10.1016/j.fitote.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Gaur R., Ke J.-P., Zhang P., Yang Z., Bao G.-H. Novel Cinnamoylated Flavoalkaloids Identified in Tea with Acetylcholinesterase Inhibition Effect. J. Agric. Food Chem. 2020;68:3140–3148. doi: 10.1021/acs.jafc.9b08285. [DOI] [PubMed] [Google Scholar]
- 36.Hamid H.A., Ramli A.N.M., Yusoff M. Indole Alkaloids from Plants as Potential Leads for Antidepressant Drugs: A Mini Review. Front. Pharmacol. 2017;8:96. doi: 10.3389/fphar.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passos C.D.S., Simoes-Pires C., Henriques A., Cuendet M., Carrupt P.-A., Christen P. Chapter 4—Alkaloids as Inhibitors of Monoamine Oxidases and Their Role in the Central Nervous System. In: Atta ur R., editor. Studies in Natural Products Chemistry. Volume 43. Elsevier; Amsterdam, The Netherlands: 2014. pp. 123–144. [Google Scholar]
- 38.Kukula-Koch W., Widelski J. Chapter 9—Alkaloids. Pharmacognosy. 2017:163–198. doi: 10.1016/B978-0-12-802104-0.00009-3. [DOI] [Google Scholar]
- 39.Shamma M., Slusarchyk W.A. The Aporphine Alkaloids. Chem. Rev. 1964;64:59–79. doi: 10.1021/cr60227a004. [DOI] [Google Scholar]
- 40.Dong J.-W., Cai L., Li X.-J., Wang J.-P., Mei R.-F., Ding Z. Monoterpene esters and aporphine alkaloids from Illigera aromatica with inhibitory effects against cholinesterase and NO production in LPS-stimulated RAW264.7 macrophages. Arch. Pharmacal Res. 2017;40:1394–1402. doi: 10.1007/s12272-016-0860-3. [DOI] [PubMed] [Google Scholar]
- 41.Kostelnik A., Pohanka M. Inhibition of Acetylcholinesterase and Butyrylcholinesterase by a Plant Secondary Metabolite Boldine. BioMed Res. Int. 2018;2018:1–5. doi: 10.1155/2018/9634349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin Z. Amaryllidaceae and Sceletium alkaloids. Nat. Prod. Rep. 2013;30:849–868. doi: 10.1039/c3np70005d. [DOI] [PubMed] [Google Scholar]
- 43.Jiang Q., Chen M.-W., Cheng K.-J., Yu P.-Z., Wei X., Shi Z. Therapeutic Potential of Steroidal Alkaloids in Cancer and Other Diseases. Med. Res. Rev. 2015;36:119–143. doi: 10.1002/med.21346. [DOI] [PubMed] [Google Scholar]
- 44.Fujioka H., Kita Y. Marine Pyrroloiminoquinone Alkaloids, Makaluvamines and Discorhabdins, and Marine Pyrrole-Imidazole Alkaloids. In: Ramawat K.G., Mérillon J.-M., editors. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Springer Berlin Heidelberg; Berlin/Heidelberg, Germany: 2013. pp. 251–283. [DOI] [Google Scholar]
- 45.Kobayashi J., Morita H. The Lycopodium Alkaloids. Alkaloids Chem. Biol. 2005;61:1–57. doi: 10.1016/s1099-4831(05)61001-2. [DOI] [PubMed] [Google Scholar]
- 46.Ma X., Gang D.R. The Lycopodium alkaloids. Nat. Prod. Rep. 2004;21:752–772. doi: 10.1039/b409720n. [DOI] [PubMed] [Google Scholar]
- 47.Blair L.M., Calvert M.B., Sperry J. Flavoalkaloids—Isolation, Biological Activity, and Total Synthesis. Alkaloids Chem. Biol. 2017;77:85–115. doi: 10.1016/bs.alkal.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Hussain G., Rasul A., Anwar H., Aziz N., Razzaq A., Wei W., Ali M., Li J., Li X. Role of Plant Derived Alkaloids and Their Mechanism in Neurodegenerative Disorders. Int. J. Biol. Sci. 2018;14:341–357. doi: 10.7150/ijbs.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seidl S.E., Potashkin J.A. The Promise of Neuroprotective Agents in Parkinson’s Disease. Front. Neurol. 2011;2:68. doi: 10.3389/fneur.2011.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Y.-Y., Li X., Yu H.-Y., Xiong Y.-F., Zhang P., Pi H.-F., Ruan H. Alkaloids from the bulbs of Lycoris longituba and their neuroprotective and acetylcholinesterase inhibitory activities. Arch. Pharmacal. Res. 2015;38:604–613. doi: 10.1007/s12272-014-0397-2. [DOI] [PubMed] [Google Scholar]
- 51.Park T.H., Kwon O., Park S.Y., Han E.S., Lee C.S. N-methylated β-carbolines protect PC12 cells from cytotoxic effect of MPP+ by attenuation of mitochondrial membrane permeability change. Neurosci. Res. 2003;46:349–358. doi: 10.1016/S0168-0102(03)00097-X. [DOI] [PubMed] [Google Scholar]
- 52.Kim D.H., Jang Y.Y., Han E.S., Lee C.S. Protective effect of harmaline and harmalol against dopamine- and 6-hydroxydopamine-induced oxidative damage of brain mitochondria and synaptosomes, and viability loss of PC12 cells. Eur. J. Neurosci. 2001;13:1861–1872. doi: 10.1046/j.0953-816x.2001.01563.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee C.S., Han E.S., Jang Y.Y., Han J.H., Ha H.W., Kim D.E. Protective Effect of Harmalol and Harmaline on MPTP Neurotoxicity in the Mouse and Dopamine-Induced Damage of Brain Mitochondria and PC12 Cells. J. Neurochem. 2002;75:521–531. doi: 10.1046/j.1471-4159.2000.0750521.x. [DOI] [PubMed] [Google Scholar]
- 54.Meiser J., Weindl D., Hiller K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013;11:1–34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Napolitano A., Pezzella A., Prota G. New Reaction Pathways of Dopamine under Oxidative Stress Conditions: Nonenzymatic Iron-Assisted Conversion to Norepinephrine and the Neurotoxins 6-Hydroxydopamine and 6,7-Dihydroxytetrahydroisoquinoline. Chem. Res. Toxicol. 1999;12:1090–1097. doi: 10.1021/tx990079p. [DOI] [PubMed] [Google Scholar]
- 56.Lee C.S., Lee C.S., Ko H.H., Song J.H., Han E.S. Effect of R-(-)-deprenyl and harmaline on dopamine- and peroxynitrite-induced membrane permeability transition in brain mitochondria. Neurochem. Res. 2002;27:215–224. doi: 10.1023/A:1014832520809. [DOI] [PubMed] [Google Scholar]
- 57.Othman W.N.N.W., Sivasothy Y., Liew S.Y., Bin Mohamad J., Nafiah M.A., Ahmad K., Litaudon M., Awang K. Alkaloids from Cryptocarya densiflora Blume (Lauraceae) and their cholinesterase inhibitory activity. Phytochem. Lett. 2017;21:230–236. doi: 10.1016/j.phytol.2017.07.002. [DOI] [Google Scholar]
- 58.Hussain T., Tan B., Yin Y., Blachier F., Tossou M.C.B., Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016;2016:1–9. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y., Wu J., Yu X., Na S., Li K., Yang Z., Xie X., Yang J., Yue J. The Protective Role of Brain CYP2J in Parkinson’s Disease Models. Oxidative Med. Cell. Longev. 2018;2018:1–12. doi: 10.1155/2018/2917981. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Li Y., Sattler R., Yang E.J., Nunes A., Ayukawa Y., Akhtar S., Ji G., Zhang P.-W., Rothstein J.D. Harmine, a natural beta-carboline alkaloid, upregulates astroglial glutamate transporter expression. Neuropharmacology. 2011;60:1168–1175. doi: 10.1016/j.neuropharm.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ouyang Y.-B., Xu L., Liu S., Giffard R.G. Role of Astrocytes in Delayed Neuronal Death: GLT-1 and its Novel Regulation by MicroRNAs. In: Parpura V., Schousboe A., Verkhratsky A., editors. Glutamate and ATP at the Interface of Metabolism and Signaling in the Brain. Springer International Publishing; Cham, Switzerland: 2014. pp. 171–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dos Santos R.G., Hallak J.E.C. Effects of the Naturalβ-Carboline Alkaloid Harmine, a Main Constituent of Ayahuasca, in Memory and in the Hippocampus: A Systematic Literature Review of Preclinical Studies. J. Psychoact. Drugs. 2017;49:1–10. doi: 10.1080/02791072.2016.1260189. [DOI] [PubMed] [Google Scholar]
- 63.Cai C.-Z., Zhou H.-F., Yuan N.-N., Wu M.-Y., Lee S.M.-Y., Ren J.-Y., Su H.-X., Lu J.-J., Chen X., Li M., et al. Natural alkaloid harmine promotes degradation of alpha-synuclein via PKA-mediated ubiquitin-proteasome system activation. Phytomedicine. 2019;61:152842. doi: 10.1016/j.phymed.2019.152842. [DOI] [PubMed] [Google Scholar]
- 64.Ciechanover A., Kwon Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015;47:e147. doi: 10.1038/emm.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han Y.-S., Kim J.-M., Cho J.-S., Lee C.S., Kim D.-E. Comparison of the Protective Effect of Indole β-carbolines and R-(-)-deprenyl Against Nitrogen Species-Induced Cell Death in Experimental Culture Model of Parkinson’s Disease. J. Clin. Neurol. 2005;1:81–91. doi: 10.3988/jcn.2005.1.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kluck R.M., Bossy-Wetzel E., Green D.R., Newmeyer D.D. The Release of Cytochrome c from Mitochondria: A Primary Site for Bcl-2 Regulation of Apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 67.Negahdar F., Mehdizadeh M., Joghataei M.T., Roghani M., Mehraeen F., Poorghayoomi E. Berberine chloride pretreatment exhibits neuroprotective effect against 6-hydroxydopamine-induced neuronal insult in rat. Iran. J. Pharm. Res. IJPR. 2015;14:1145–1152. [PMC free article] [PubMed] [Google Scholar]
- 68.Azam S., Al Mamun A., Kabir T., Ahmad J., Jeandet P., Sarwar S., Ashraf G.M., Aleya L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020;886:173412. doi: 10.1016/j.ejphar.2020.173412. [DOI] [PubMed] [Google Scholar]
- 69.Deng H., Jia Y., Pan D., Ma Z. Berberine alleviates rotenone-induced cytotoxicity by antioxidation and activa-tion of PI3K/Akt signaling pathway in SH-SY5Y cells. Neuroreport. 2020;31:41–47. doi: 10.1097/WNR.0000000000001365. [DOI] [PubMed] [Google Scholar]
- 70.Li Z., Jiang T., Lu Q., Xu K., He J., Xie L., Chen Z., Zheng Z., Ye L., Xu K., et al. Berberine attenuated the cytotoxicity induced by t-BHP via inhibiting oxidative stress and mitochondria dysfunction in PC-12 cells. Cell. Mol. Neurobiol. 2019;40:587–602. doi: 10.1007/s10571-019-00756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krauss R., Bosanac T., Devraj R., Engber T., Hughes R.O. Axons Matter: The Promise of Treating Neurodegenerative Disorders by Targeting SARM1-Mediated Axonal Degeneration. Trends Pharmacol. Sci. 2020;41:281–293. doi: 10.1016/j.tips.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Loring H.S., Parelkar S.S., Mondal S., Thompson P.R. Identification of the first noncompetitive SARM1 inhibitors. Bioorganic Med. Chem. 2020;28:115644. doi: 10.1016/j.bmc.2020.115644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu X., Wang S., Wang J., Gong J., Shi J., Yu S. Berberine Induces CYP2J2 Expression in Human U251 Glioma Cells via Regulation of Peroxisome Proliferator-Activated Receptor Alpha. Pharmacology. 2019;105:360–368. doi: 10.1159/000503884. [DOI] [PubMed] [Google Scholar]
- 74.Bonan C. Ectonucleotidases and nucleotide/nucleoside transporters as pharmacological targets for neurological disorders. CNS Neurol Disord Drug Targets. 2012;11:739–750. doi: 10.2174/187152712803581092. [DOI] [PubMed] [Google Scholar]
- 75.De Oliveira J.S., Abdalla F.H., Dornelles G.L., Palma T.V., Signor C., Bernardi J.D.S., Baldissarelli J., Lenz L.S., De Oliveira V.A., Schetinger M.R.C., et al. Neuroprotective effects of berberine on recognition memory impairment, oxidative stress, and damage to the purinergic system in rats submitted to intracerebroventricular injection of streptozotocin. Psychopharmacology. 2019;236:641–655. doi: 10.1007/s00213-018-5090-6. [DOI] [PubMed] [Google Scholar]
- 76.Hu X., Shi Q., Zhou X., He W., Yi H., Yin X., Gearing M., Levey A., Yan R. Transgenic mice overexpressing reticulon 3 develop neuritic abnormalities. EMBO J. 2007;26:2755–2767. doi: 10.1038/sj.emboj.7601707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Orellana J.A., Von Bernhardi R., Giaume C., Sáez J.C. Glial hemichannels and their involvement in aging and neurodegenerative diseases. Rev. Neurosci. 2012;23:163–177. doi: 10.1515/revneuro-2011-0065. [DOI] [PubMed] [Google Scholar]
- 78.Yi C., Ezan P., Fernández P., Schmitt J., Sáez J.C., Giaume C., Koulakoff A. Inhibition of glial hemichannels by boldine treatment reduces neuronal suffering in a murine model of Alzheimer’s disease. Glia. 2017;65:1607–1625. doi: 10.1002/glia.23182. [DOI] [PubMed] [Google Scholar]
- 79.Youn Y.C., Kwon O., Han E.S., Song J.H., Shin Y.K., Lee C.S. Protective effect of boldine on dopamine-induced membrane permeability transition in brain mitochondria and viability loss in PC12 cells. Biochem. Pharmacol. 2002;63:495–505. doi: 10.1016/S0006-2952(01)00852-8. [DOI] [PubMed] [Google Scholar]
- 80.Deng G., Wu C., Rong X., Li S., Ju Z., Wang Y., Ma C., Ding W., Guan H., Cheng X., et al. Ameliorative effect of deoxyvasicine on scopolamine-induced cognitive dysfunction by restoration of cholinergic function in mice. Phytomedicine. 2019;63:153007. doi: 10.1016/j.phymed.2019.153007. [DOI] [PubMed] [Google Scholar]
- 81.Chitnis T., Weiner H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017;127:3577–3587. doi: 10.1172/JCI90609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guzman-Martinez L., Maccioni R.B., Andrade V., Navarrete L.P., Pastor M.G., Ramos-Escobar N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019;10:1008. doi: 10.3389/fphar.2019.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kinney J.W., BeMiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu P., Li H., Wang Y., Su X., Li Y., Yan M., Ma L., Che H. Harmine Ameliorates Cognitive Impairment by Inhibiting NLRP3 Inflammasome Activation and Enhancing the BDNF/TrkB Signaling Pathway in STZ-Induced Diabetic Rats. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.He D., Wu H., Wei Y., Liu W., Huang F., Shi H., Zhang B., Wu X., Wang C. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur. J. Pharmacol. 2015;768:96–107. doi: 10.1016/j.ejphar.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 86.Singh A.K., Singh S.K., Nandi M.K., Mishra G., Maurya A., Rai A., Rai G.K., Awasthi R., Sharma B., Kulkarni G.T. Berberine: A Plant-derived Alkaloid with Therapeutic Potential to Combat Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2019;19:154–170. doi: 10.2174/1871524919666190820160053. [DOI] [PubMed] [Google Scholar]
- 87.Hussien H.M., Abd E.-M.N., Ghareeb D.A., Hafez H.S., Ahmed H.E., El-Moneam N.A. Neuroprotective effect of berberine against environmental heavy metals-induced neurotoxicity and Alzheimer’s-like disease in rats. Food Chem. Toxicol. 2018;111:432–444. doi: 10.1016/j.fct.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 88.Seo E.-J., Fischer N., Efferth T. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Pharmacol. Res. 2018;129:262–273. doi: 10.1016/j.phrs.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 89.Sadraie S., Kiasalari Z., Razavian M., Azimi S., SedighNejad L., Afshin-Majd S., Baluchnejadmojarad T., Roghani M. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: Insights into underlying molecular mechanisms. Metab. Brain Dis. 2019;34:245–255. doi: 10.1007/s11011-018-0349-5. [DOI] [PubMed] [Google Scholar]
- 90.Winner B., Winkler J. Adult Neurogenesis in Neurodegenerative Diseases: Figure 1. Cold Spring Harb. Perspect. Biol. 2015;7:a021287. doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moreno-Jiménez E.P., Flor-García M., Terreros-Roncal J., Rábano A., Cafini F., Pallas-Bazarra N., Ávila J., Llorens-Martin M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019;25:554–560. doi: 10.1038/s41591-019-0375-9. [DOI] [PubMed] [Google Scholar]
- 92.Tobin M.K., Musaraca K., Disouky A., Shetti A., Bheri A., Honer W.G., Kim N., Dawe R.J., Bennett D.A., Arfanakis K., et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell. 2019;24:974–982.e3. doi: 10.1016/j.stem.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Morales-Garcia J.A., Revenga M.D.L.F., Alonso-Gil S., Rodríguez-Franco M.I., Feilding A., Perez-Castillo A., Riba J. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis In Vitro. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-05407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yano M., Nakashima S., Oda Y., Nakamura S., Matsuda H. BBB-permeable aporphine-type alkaloids in Nelumbo nucifera flowers with accelerative effects on neurite outgrowth in PC-12 cells. J. Nat. Med. 2020;74:212–218. doi: 10.1007/s11418-019-01368-7. [DOI] [PubMed] [Google Scholar]
- 95.Prasansuklab A., Tencomnao T. Amyloidosis in Alzheimer’s Disease: The Toxicity of Amyloid Beta (Aβ), Mechanisms of Its Accumulation and Implications of Medicinal Plants for Therapy. Evid. Based Complement. Altern. Med. 2013;2013:1–10. doi: 10.1155/2013/413808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X., Fu Z., Meng L., He M., Zhang Z. The Early Events That Initiate β-Amyloid Aggregation in Alzheimer’s Disease. Front. Aging Neurosci. 2018;10:359. doi: 10.3389/fnagi.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panahi N., Mahmoudian M., Mortazavi P., Hashjin G.S. Experimental research Effects of berberine on β-secretase activity in a rabbit model of Alzheimer’s disease. Arch. Med Sci. 2013;1:146–150. doi: 10.5114/aoms.2013.33354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cai Z., Wang C., He W., Chen Y. Berberine Alleviates Amyloid-Beta Pathology in the Brain of APP/PS1 Transgenic Mice via Inhibiting β/γ-Secretases Activity and Enhancing α-Secretases. Curr. Alzheimer Res. 2018;15:1045–1052. doi: 10.2174/1567205015666180702105740. [DOI] [PubMed] [Google Scholar]
- 99.Fawver J.N., Duong K.T., Wise-Scira O., Chapa R.P., Schall H.E., Coskuner O., Zhu X., Colom L.V., Murray I.V., Coskuner-Weber O. Probing and Trapping a Sensitive Conformation: Amyloid-β Fibrils, Oligomers, and Dimers. J. Alzheimer Dis. 2012;32:197–215. doi: 10.3233/JAD-2012-120880. [DOI] [PubMed] [Google Scholar]
- 100.Patil P., Thakur A., Sharma A., Flora S.J.S. Natural products and their derivatives as multifunctional ligands against Alzheimer’s disease. Drug Dev. Res. 2020;81:165–183. doi: 10.1002/ddr.21587. [DOI] [PubMed] [Google Scholar]
- 101.Liu X., Zhou J., Abid M.D.N., Yan H., Huang H., Wan L., Feng Z., Chen J. Berberine Attenuates Axonal Transport Impairment and Axonopathy Induced by Calyculin A in N2a Cells. PLoS ONE. 2014;9:e93974. doi: 10.1371/journal.pone.0093974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu G., Li Y., Tian Q., Liu R., Wang Q., Wang J.-Z., Wang X. Berberine Attenuates Calyculin A-Induced Cytotoxicity and Tau Hyperphosphorylation in HEK293 Cells. J. Alzheimer Dis. 2011;24:525–535. doi: 10.3233/JAD-2011-101779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.