Abstract
Diabetes is a global health problem, and the number of diabetic patients is in continuous rise. Conventional antidiabetic therapies are associated with high costs and limited efficiency. The use of traditional medicine and plant extracts to treat diabetes is gaining high popularity in many countries. Countries in the Middle East region have a long history of using herbal medicine to treat different diseases, including diabetes. In this review, we compiled and summarized all the in vivo and in vitro studies conducted for plants with potential antidiabetic activity in the Middle East region. Plants of the Asteraceae and Lamiaceae families are the most investigated. It is hoped that this review will contribute scientifically to evidence the ethnobotanical use of medicinal plants as antidiabetic agents. Work has to be done to define tagetes, mechanism of action and the compound responsible for activity. In addition, safety and pharmacokinetic parameters should be investigated.
Keywords: antidiabetic plants, natural products, hyperglycemia, plant extracts
1. Introduction
Diabetes is a major endocrine health problem that has a fast-developing rate around the world. Diabetic patients are anticipated to increase in numbers to 300 million by 2025 according to the World Health Organization (WHO). The Middle Eastern and North African regions have the second highest rates of increase in diabetes globally, with the number of people with diabetes projected to increase by 96.2% in 2035, increasing the social and economic burden of many countries [1,2,3].
The syndrome of diabetes describes a metabolic disorder with disturbances of carbohydrate, fat and protein metabolism, resulting from defects in insulin secretion, insulin action, or both, eventually leading to chronic hyperglycemia and a wide range of complications, including damage to the nervous system, kidneys, blood vessels, eyes, heart, feet and skin [2,4]. For centuries, traditional medicine from plants has been used to treat vast numbers of ailments, including diabetes, as they are considered available and safe [2].
It was documented that 656 flowering plant species are used traditionally for diabetes. Different plant parts were used, including flowers, fruits, seeds, leaves, berries, bark and roots. Plants are selected depending on affordability, stage of progression, comorbidities, availability and safety [5] (https://stateoftheworldsplants.org/2017/).
Recently, several in vitro and in vivo studies have been conducted to study numerous herbs that were claimed to reduce blood glucose level, but of an estimated 250,000 plants, less than 2500 have been studied for pharmacological efficacy against diabetes [5,6].
At present, metformin, obtained from Galega officinalis, and the oligosaccharide acarbose, produced by the fermentation of Actinoplanes utahensi, are antidiabetic drugs derived from natural origins [6].
Limited efficacy, narrow tolerability, increased side effects and complications, high cost and decreased adherence are the major drawbacks of conventional antidiabetic therapies that raise the necessity to discover new antidiabetic plants [6].
In the present review, an attempt has been made to compile and summarize the reported antidiabetic medicinal plants of the Middle East area. The review also covers the common name of the plant, the parts used, extract type, phytochemical constituents, the test model and suspected mechanism of action, and provides recommendations for future research.
2. Medicinal Plants with Potential Antidiabetic Activity in the Arabian Peninsula
The Arabian Peninsula is formulated from the Kingdom of Saudi Arabia, Kuwait, Bahrain, Yemen, Qatar, and the United Arab Emarat, and is located in the Asian southwest [7].
The Kingdom of Saudi Arabia occupies around four-fifths of the Arabian Peninsula, with a population of more than 33.3 million people. The prevalence of diabetes mellitus registered a 10-fold upsurge in the past three decades. In fact, it affects over 25% of the adult population [8]. Oman is ranked 8th in the top 10 countries of the Middle Eastern and North African (MENA) region for diabetes prevalence in 2010 (13.4%) and 2030 (14.9%) [9], while the prevalence of diabetes mellitus is 16.7% in the adult Qatari population [10].
In developing countries, the use of medicinal plants species goes back thousands of years, and forms an important part of the culture, as a large segment of the population still relies on it to treat serious diseases, including diabetes [11].
2.1. Oman
2.1.1. Ajuga iva (Lamiaceae)
The hypoglycemic effect of the aqueous extract of the whole plant of Ajuga was examined in normal and streptozocin diabetic rats. A single oral administration of 10 mg/kg of the extract significantly decreased plasma glucose level over 2–6 h either in normoglycaemic rats or in hyperglycemic rats. However, daily treatment with the same extract for 21 days decreased the plasma glucose level slightly only in the third week in normoglycaemic rats, while diabetic rats showed a significant decrease after the first week and continuously decreased thereafter, supporting its traditional use for diabetes mellitus treatment [12].
On the contrary, the continuous intravenous infusion of the aqueous extract of the whole plant caused significant hypoglycemia in diabetic rats only [13].
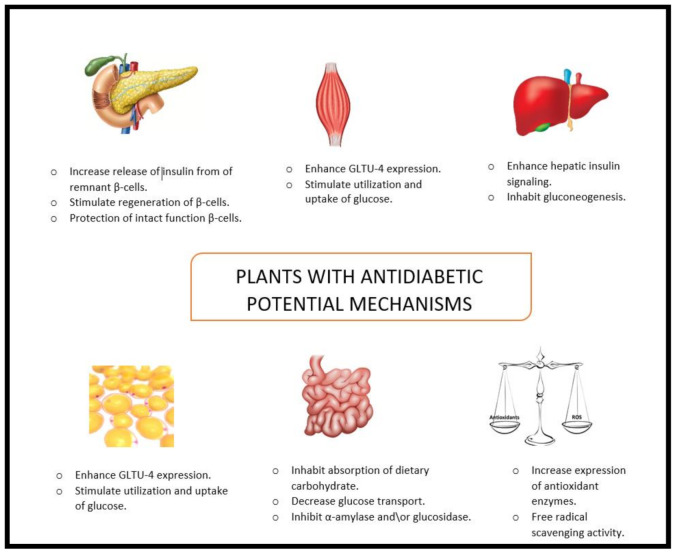
The observed pharmacological activity is thought to be related to flavonoids that can enhance insulin secretion, prevent β-cell apoptosis, and modulate proliferation. In particular, naringin and apigenin were able to lower blood glucose level significantly, thus showing great potential as antidiabetic agents [14].
Phytoecdysteroids were extracted from Ajuga iva and tested for their potential antidiabetic effect in alloxan diabetic rats. The glucose and insulin levels reduced significantly following the administration of phytoecdysteroids. Besides this, they attenuated the metabolic changes caused by diabetes [15]. Their effects may be explained by the stimulation of surviving β-cells of islets of Langerhans to release more insulin, controlling the β-cell potential [16].
2.1.2. Moringa pergrina (Moringaceae)
Previously, M. peregrina was reported to have hypoglycemic properties. The antidiabetic activity was reported for the hydroalcoholic extract fraction of M. peregrina seeds and aerial parts in streptozocin diabetic rats [17]. Both extracts significantly decreased blood glucose levels comparably to the oral antidiabetic reference drug gliclazide. On the other hand, the n-hexane fraction was the only one that showed a highly significant antihyperglycemic activity that was attributed to the lupeol acetate and β-sitosterol effect [18].
The ethanol and aqueous extracts of M. peregrina seeds at a dose of 150 mg/kg lowered the blood glucose level in diabetic rats and increased the activity of the enzymatic antioxidants, such as catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase and glutathione-S-transferase [19].
The hydroalcoholic extract of M. peregrina leaves showed inhibitory potential against three in vitro model enzyme assays: α-glucosidase, α-amylase, and dipeptidyl peptidase IV (DPP IV). The results for pancreatic α-amylase suggested that the enzyme responded to the extract when the concentration was increased, moderated intestinal α-glucosidase inhibitory potential, and caused a gradual inhibition of the activity of mammalian DPP IV enzyme [20].
The chloroform extract of M.peregrina leaves caused a significant decrease in the blood glucose levels in treated mice, probably by increasing peripheral utilization of glucose [21].
2.1.3. Rhazya stricta (Apocynaceae)
Recently, it was found that the oral administration of the leaf extract 0.5, 2, and 4 g/kg decreased the plasma glucose level and enhanced insulin level in streptozocin diabetic rats [22,23].
In another study, the effect of different doses of Rhazya aqueous extract on adiponectin protein and insulin resistance was analyzed. The data indicated a significant inverse correlation between adiponectin levels and insulin resistance, and a significant increase in adiponectin levels that is considered a promising therapeutic strategy in treating diabetes [23].
Crude methanolic leaf extract of R. stricta had the best antidiabetic effect compared to other methanolic extracts of different plant parts that were tested in vivo in both male and female albino mice for the reduction of blood glucose and other blood parameters [24]. The leaves extract was fractionated using n-hexane, ethyl acetate, chloroform, and water. All fractions were tested for the same activities. The ethyl acetate fraction was the most effective in fasting and random blood as regards the reduction in glucose level, and was comparable to metformin [24].
Later, three Omani traditional medicinal plants, i.e., Ajuga iva, Pteropyrum scoparium, and Rhazya stricta, were tested for their effect on reducing diabetic incidence. The results of the study revealed that the selected plants had reduced the blood sugar level of the treated mice; the extracts of R. stricta and A. iva had more pronounced effects than P. scoparium. The hypoglycemic effect of R. stricta extract may be due to its potentiating effect on the insulin-releasing mechanism; this suggests a mode of action resembling the mechanism of sulfonylureas [25].
2.2. Qatar
Cynomorium coccineum (Cynomoriaceae)
Pharmacological studies showed that the Cynomorium plants had many biological activities, including antioxidant, immunity-improving, antidiabetic, neuroprotective, and other bioactivities, some of which were already reported by traditional medicine [26].
α-glucosidase and α-amylase are the key enzymes for controlling the postprandial blood sugar level and managing hyperglycemia. The aqueous extract of C. coccineum demonstrated a relatively high α-glucosidase inhibitory activity and a moderate inhibition of α-amylase, indicating that the edible plant can be a diet-based solution for managing the early stages of diabetes when coupled with other pharmacological management strategies [27].
2.3. Saudi Arabia
2.3.1. Avicennia marina (Avicenniaceae)
The ethanolic leaf extract of A. marina at doses 250 and 500 mg/kg reduced blood glucose level significantly. It also reduced the level of serum urea that confirms the capacity to protect vital tissues, for example, kidney, and it also improved the biochemical parameters such as serum phosphorous, albumin, and globulin [28].
The methanolic extraction of the aerial roots of A. marina resulted in the isolation of stigmasterol-3-O-β-D-glucopyranoside, which may be responsible for the antihyperglycemic effect seen [29]
The oral supplementation of the ethanolic extracts from A. marina leaves 2 mg/gm exerted a significant hypoglycemic effect on streptozocin diabetic mice, similar to the effect of the aqueous and hydroalcoholic extract [30,31,32]. The possible mechanism underlying the antihyperglycaemic action of A. marina was attributed to inducing β-cells to release more insulin, and reducing oxidative stress by increasing the antioxidant activity of catalase, glutathione-S-transferase, and superoxide dismutase enzymes [28,30,31].
2.3.2. Caralluma sinaica (Asclepiadaceae)
The ethanolic extract of C. sinaica was evaluated in streptozocin diabetic rabbits. The blood glucose-lowering effect of alcoholic extract 100 mg/kg was more pronounced in diabetic animals than in the glibenclamide-treated group. In addition, it had the capacity to prevent an increase in hyperglycemia after oral glucose load [33]. As the dose increased from 150 to 200 mg/kg, there were no toxic or behavioral changes observed in the animals [33].
The plant extract could reverse weight loss and increase liver glycogen in diabetic rats. This could be consistent with the ability of the extract to restore an adequate insulin level, which prevents the metabolic changes associated with diabetes, lipogenesis and glycogenesis, and the breakdown of muscle proteins [33].
2.3.3. Ducrosia anethifolia (Apiaceae)
The ethanolic extract of D. anethifolia and its major isolated furanocoumarins demonstrated in vitro inhibitory effects against carbohydrate metabolizing enzymes, α-amylase, α-glucosidase, and β-galactosidase in a concentration-dependent manner [34]. The most potent inhibitors were imperatorin and 5-methoxypsoralen, while psoralen, oxypeucedanin hydrate, and isooxypeucedanin were moderated inhibitors [34]. The biological activity of the D. anethifolia ethanol extract showed a hypoglycemic effect that may be related to potentiating the pancreatic secretion of insulin from islet β-cells, or the transport of blood glucose to the peripheral tissue, or antioxidant activity in several areas [34].
The antihyperglycemic effect of different isolated furanocoumarin compounds was explained by their stimulatory action as regards glucose uptake by cells and the reduction of oxidative damage of the pancreas [34].
2.3.4. Jatropha curcas (Euphorbiaceae)
The chloroform extract of J. curcus leaves was reported at doses of 250 and 500 mg/kg for its antidiabetic potential in alloxan diabetic rats; it also caused a reversal in cholesterol, triglyceride, HDL, and LDL values when compared to untreated diabetic rats [35].
The hypoglycemic potentials of orally administered aqueous root extract of J. curcas 250 and 450 mg/kg were investigated in alloxan diabetic rats [36]. The extract produced a sustained significant reduction in blood glucose level, and elicited anti-infective activity and an ameliorative effect on alloxan-induced anemia [36].
The antidiabetic actions of different extracts of J. curcas leaves, petroleum ether, ethyl acetate, and methanol were evaluated in streptozocin diabetic rats. All caused significant improvements in the levels of glucose and α-amylase. The histopathological investigation revealed the regenerative and protective effect of extracts on β-cells and liver [37].
The antioxidant, antihyperglycemic and ameliorative properties of plant extracts may offer a potential therapeutic source for the treatment of diabetes attributed to the presence of flavonoids [37].
2.3.5. Loranthus acaciae (Loranthaceae)
The antidiabetic activity of the crude ethanolic extract and its n-hexane, chloroform, and n-butanol fractions was investigated in alloxan diabetic rats, and we also performed glucose-tolerance tests in normal rats [38].
The crude extract and the chloroform fraction at a dose of 500 mg/kg had the highest hypoglycemic effect in diabetic rats, with 33.6 and 47% reductions in blood sugar levels. This effect was statistically significant in comparison to the glibenclamide-treated group [38].
L.acaciae was thought to compact diabetes through its flavonoids, which are suggested to demonstrate powerful antioxidant activity, and act as inhibitors of biological targets, mostly enzymes such as α-glycosidase, α-amylase, and dipeptidyl peptidase IV (DPP-4). These results further support the claim for and use of L. acaciae in folklore medicine in Saudi Arabia as an antidiabetic drug [38].
2.3.6. Lyceum shawii (Solonaceae)
Experimentally, the traditional antidiabetic claim was assessed in normal and streptozocin diabetic rats by using the ethanolic extract of L. shawii’s aerial parts. There was a significant hypoglycemic potential in normal rats, as well as hyperglycemic rats that was comparable to the hypoglycemic drug glibenclamide, after both oral as well as intraperitoneal administration [39].
Phytochemical screening helps in explaining the antidiabetic activity of L. shawii. Diterpenoids were reported to inhibit α-glycosidase. Glycosides, polysaccharides, and saponins were reported to protect pancreatic islets and β-cells, and flavonoids were reported to possess glucosidase inhibitory effects and antioxidant activities [39].
The study also investigated the acute and chronic toxic potential of L. shawii treatment using mice as an experimental model. There were no alarming signs of acute toxicity, but the plant possessed a significant spermatotoxic potential with chronic use [39].
2.3.7. Marrubium vulgare (Lamiaceae)
After the administration of the M.vulgare’s aerial parts’ extract, the elevated plasma glucose levels in diabetic rats were lowered, similar to the effect of glibenclamide. The possible mechanism of the extract was attributed to phenolic compounds, which served as antioxidants, and was achieved through a stimulation of insulin release from the remnant pancreatic β-cells [40].
2.3.8. Moringa oleifera (Moringaceae)
It was reported that the administration of an aqueous extract of M. oleifera manifested potent antihyperglycemic and antihyperlipidemic effects in both insulin-resistant and insulin-deficient rat models [41].
The administration of Moringa seeds powder to streptozocin diabetic rats caused a drop in fasting blood glucose and increased the antioxidant activity of blood enzymes. However, the antidiabetic activity of the higher dose of the seeds powder (100 mg/kg) was more efficient than that of the lower dose (50 mg/kg) [42].
M. oleifera contains three classes of phytochemicals: glucomoringin, phenols, and flavonoids. All of these may contribute to antidiabetic activity through the stimulation of insulin secretion, and the protection of the intact functional β-cells from further deterioration or the generation of destroyed β-cells [41,42].
2.3.9. Morus nigra (Moraceae)
The leaves extract of M.nigra decreased glucose and increased insulin levels significantly in diabetic animals [43].
In alloxan diabetic rats, treatment with either M. alba or M. nigra fruit extracts decreased the hyperglycemia significantly, almost to the normal level. The same effect was reported earlier in streptozocin diabetic mice [44].
The antidiabetic effect can be explained by the inhibition of α-glucosidase, α-mannosidase, and β-galactosidase. N-containing pseudo-sugars were thought to be responsible for this activity. In addition, fagomine strengthened the glucose-induced insulin secretion similarly to the action of the sulfonylurea drug, and increased the tissue uptake of glucose [44].
2.3.10. Ocimum forskolei (Lamiaceae)
The O.forskolei extract from leaves and stem showed promising results in the α-amylase inhibition assay. They almost had the same potential for α-amylase inhibition (72.3 and 78.9 µg/mL, for leaves and corollas extracts, respectively), suggesting that O. forskolei might be effective in slowing down the hydrolysis of polysaccharides [45].
2.3.11. Plicosepalus curviflorus (Loranthaceae)
Traditionally, the stem of P. curviflorus has been used for the treatment of cancer in Yemen, and the treatment of diabetes in Saudi Arabia [46]. The petroleum ether, ethyl acetate and methanol soluble fractions of P. curviflorus were subjected to column chromatography. Two new flavane gallates were found to show significant hypoglycemic activities when tested in rats [46].
The antihyperglycemic activity of P. curviflorus and its nanoparticle suspension formulation was evaluated in high-fat diet/streptozocin diabetic rats. The total extracts of P. curviflorus, as well as the solid lipid nanoparticle (SLN) formulations, exhibited a significant lowering in blood glucose and insulin resistance, associated at least partly with antioxidant effects, in the diabetic rats and their SLN formulations [47].
2.3.12. Retama raetam (Fabaceae)
The administration of methanolic extract of R. raetam at 250 or 500 mg/kg daily for 4 weeks showed an appreciable antihyperglycemic effect in diabetic rats, explained based on the inhibition of the renal reabsorption of glucose, as evidenced by increased glycosuria [48].
The plant contains many quinolizidine alkaloids that have been reported to exhibit hypoglycemic activity due to their insulin-releasing properties and antioxidant activity, in addition to the inhibition of glucose absorption that was dose-related [48].
2.3.13. Rhizophora mucronata (Rhizosphoraceae)
Both aqueous and hydroalcoholic bark extracts of R. mucronata possessed significant hypoglycemic and antihyperglycemic activities attributed to their α-glucosidase inhibition potential [28].
The antidiabetic activity of the leaves extract of R. mucronata showed promising results in streptozocin diabetic rats. The extract reduced oxidative stress and increased antioxidants activity, and it had an insulin-mimetic effect. Also, flavonoids could play an important role in the prevention of β-cell apoptosis, and the promotion of β-cell propagation, beside the secretion and enhancement of insulin activity [31].
2.3.14. Salvadora persica (Salvadoraceae)
The administration of S.persica extract decreased blood glucose significantly in the first week of treatment, and this was more evident in the third week for both doses used [49]. Salvadora persica contains β-sitosterol with reported antioxidant effects, and many amides have been reported as having an insulin secretagogue effect and α-glucosidase-inhibition activity [49].
2.4. Yemen
Yemen is a small country that occupies an important location in the southwestern part of the Arabian Peninsula. The Yemeni highlands experience relatively high rainfall and have a temperate climate, giving them a high topographic diversity, which along with climatic factors has resulted in a diverse and rich flora [50].
2.4.1. Azadirachta indica (Meliaceae)
The water extract of neem leaves was tested in alloxan diabetic rabbits via administration daily for 25 days. The reduction in blood glucose level was significant with regard to control, in a dose- and time-dependent manner [51].
The hypoglycemic effect of neem root bark extract was tested in alloxan diabetic rats, and the reduction in glucose level was significant at high doses only [52].
The oily extract of neem had the potential to reduce the blood glucose levels within a short period time, and it also improved the glucose tolerance after a treatment period of 4 weeks, as suggested by oral glucose tolerance tests in alloxan diabetic rats [53].
Neem’s various parts have been actively used for the treatment of diabetes. Improving the expression of insulin signaling molecules, elevating insulin output, inhibiting epinephrine’s action on glucose metabolism, improving glucose tranporter-4 protein resulting in increased use of peripheral glucose, inhibiting the production of hepatic glucose, and free radical-scavenging properties were all attributed to the antidiabetic mechanism of Neem [54,55,56].
2.4.2. Boswellia carterii (Burseraceae)
Ursane, oleanane, and lupine of Olibanum were identified to be responsible for the observed activity in many cases [57]. The pharmacological testing for the water extract in streptozocin-nicotinamide diabetic rats showed a hypoglycemic effect resembling that of glibenclamide and metformin. The effect is possibly due to the stimulation of insulin secretion from the remaining β-cells, the antioxidant activity, and the increase in serum insulin [58]. It is thought that B. carterii exhibited antidiabetic action through liver glycogen, the inhibition of degenerative changes in the β-cells of the pancreas in an alloxan diabetic model, and the suppression of apoptosis of peri-insular cells, and these effects were attributed to β-Boswellic acid derivatives [59,60].
2.4.3. Cissus rotundifolia (Vitaceae)
The blood glucose data clearly indicated that the aqueous extract from C. rotundifolia produced significant hypoglycemic effects in streptozocin diabetic rats. The continuous treatment with 100 mg/kg of C. rotundifolia for a period of 28 days produced a significant decrease in the blood glucose levels of diabetic rats [58].
The antidiabetic activity of the plant was tested on healthy human subjects. The healthy volunteers received, in a random order, the control stew meal/control wheat bread and the test stew meals/wheat bread containing cissus flours. Compared with the controls, cissus meals elicited significant reductions in plasma glucose and insulin levels at various postprandial time points, possibly due to starch and water-soluble non-starch polysaccharides [61].
The plant was reported to have quercetin, which is known for its ability to inhibit the insulin-dependent activation of PI3K (Phosphoinositide 3-kinase) and to reduce intestinal glucose absorption by inhibiting glucose transporter [17]. Other species of the same genus, including C. verticillata and C. quadragualis, possess hypoglycemic and hypolipidemic activities [58,62].
2.4.4. Dracaena cinnabari (Dracaenaceae)
The resin of cinnabari trunk ethanol extract was tested against the MCF-7 cell line using different solvents for its antidiabetic activity, and the glucose uptake-inducing activity of ethylacetate extract was found to be higher than that of Metformin, which signifies the potential of this extract to be used as a source of antidiabetic drugs [63].
Furthermore, Al-Baoqai studied the antidiabetic potential of the ethanolic extract of cinnabari resin for alloxan diabetic rats. Both extract doses of 100 and 300 mg/kg resulted in a significant decrease in fasting blood glucose level, with a recovery in the destruction of pancreas cell [64].
The hypoglycemic activity can be related to flavonoids—the main chemical constituents of the Dracaena species—through the inhibition of α-glucosidase activity, the suppressing of intestinal carbohydrate absorption and the inducing of glucose uptake activity [64].
2.4.5. Opuntia ficus-indica (Cactaceae)
An earlier study compared the effects of an aqueous extract and stem/fruit skin blend prepared from O. indica on normoglycemic male Wistar rats. The blood glucose-lowering effect and plasma insulin-increasing effect of the aqueous extract was obvious and statistically significant, but less so than the effect of glyburide. After 15 and 30 min, the optimum effects of increasing plasma insulin and lowering blood glucose were observed, respectively, from a dose of 5.88 mg/kg. On the other hand, the proprietary blend did not affect basal glucose levels in a dose of 6 mg/kg over a period of 180 min, but exhibited a stimulation effect on basal plasma insulin secretion, pointing to its direct action on β-cells [65].
This result is supported by Al-Naqeb, who studied the effect of the oil seed in Streptozocin diabetic rats and its molecular mechanisms. Oil extracts exhibited strong antioxidant actives and caused a significant reduction in plasma glucose level in a dose-dependent manner. In addition, the extract elicited an increase in the expression level of the glucose transporter 2 (Slc2a2) gene that is present in the liver, the activity of which would be essential for both glucose secretion and for keeping the intracellular Glu-6-phosphate concentration low, avoiding the permanent activation of glycolytic and lipogenic genes [66].
2.4.6. Pulicaria inuloides (Asteraceae)
α-amylase and α-glucosidase inhibitory studies demonstrated that Pulicaria inuloides essential oil caused a concentration-dependent reduction in the percentage of inhibition of α-amylase and α-glucosidase. The highest concentration of Pulicaria inuloides (250 μg/mL) showed a maximum inhibition of nearly 85.50% and 86.52% of α-amylase and α-glucosidase, respectively [67].
To confirm the hypoglycemic effect, diabetic rats were treated with a 400 mg/kg (body weight) dose of essential oil orally for 21 days. The P. inuloides treatment given to the diabetic group was able to significantly lower the blood glucose level similarly to the standard glibenclamide [67].
The antihyperglycemic effect may be accounted for via several mechanisms, such as its ability to impair the absorption of glucose in the intestine through α-glucosidase inhibition, increase glucose uptake from the bloodstream and oxidation in the peripheral tissues, enhance insulin sensitivity, control lipid metabolism thereby fixing the putative inhibition of insulin signaling, and scavenge the free radicals, resulting in increases in the plasma membrane receptors or transporters necessary for the signaling and uptake of glucose from the bloodstream [67].
P. inuloids is a safe and effective intervention for diabetes, can correct the metabolic disturbances associated with diabetes, and can reinforce the healing of the liver according to the histopathological studies [68].
2.4.7. Solenostemma argel (Asclepiadaceae)
The blood glucose level of methylprednisolone-treated hyperglycemic rats was determined after daily oral administration of 1 g/kg of Argel extract for 10 days, or with glibenclamide at 6 mg/kg. The oral administration of the extract produced a significant reduction in fasting serum glucose in diabetic rats that was comparable to the glibenclamide group [69].
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were significantly lower in the Argel group compared to the control group, indicating a possible hepatoprotective effect caused by the extract. On the other hand, the administration of glibenclamide resulted in the significant elevation of serum ALT and AST levels, which indicates its side effects [69].
A similar effect was observed in diabetic albino rats treated with water extract of S. argel. The author reported the extract’s ability to affect α-amylase activity after 2 h [70] (Table 1, Table 2 and Table 3).
Table 1.
Medicinal plants of Arabian Peninsula ethnobotany.
| County | Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|---|
| Oman | Ajuga iva | Chendgoura | Lamiaceae | Anthelmintic, analgesia, diuretic agent, diabetes | Leaf, stem | [71,72] |
| Moringa peregrina | Shua | Moringaceae | Convulsions or infantile paralysis, diabetes | Pod oil, seed | [17,73] | |
| Rhayza stricta | Harmal | Apocynaceae | Bad breath, chest pain, conjunctivitis, constipation, diabetes, fever, skin rash, anthelmintic, increase lactation | Seed, whole plant | [74] | |
| Qatar | Cynomorium coccineum | Tarthuth | Cynomoriaceae | The roots are edible and were sold in earlier times as a vegetable (Qatar) used as an aphrodisiac in Bahrain | Flower, root | [75] |
| Saudi Arabia | Avicennia marina | Shoura | Avicenniaceae | Smallpox, sores, pruritic, induce women infertility, diabetes | Branches | [76] |
| Caralluma sinaica | DedElkalba | Asclepiadaceae | Anticancer, diabetes, leprosy, obesity, rheumatism | Leaf | [77,78,79] | |
| Ducrosia anethifolia | Not mentioned | Apiaceae | Analgesic, anxiety, backache, carminative, colic, cold, galactogogue, insomnia, pain reliever for headache, useful for irregularities of menstruation (Iranian folk medicine) | Aerial parts, seed, whole herb | [80] | |
| Jatropha curcas | Kharat | Euphorbiaceae | Ailments related to skin, cancer, digestive disease, diabetes, infectious disease, respiratory disease | Barkk, fruit, latex, leaf, root, seed | [79,81] | |
| Loranthus acaciae | Not mentioned | Loranthaceae | Antipyretic, cancer, colds, cosmetic, ear aches, headache, hypertension, mosquito Repellent, obesity, rheumatoid diseases, skin infections |
Leaf | [82,83] | |
| Lycium shawii | Awsaj | Solonaceae | Diabetes, hypotensive agent | Flower, shoot | [39,84] | |
| Marrubiumvulgare | Not mentioned | Lamiaceae | Return to reference | Leaves, flowers, stem | [85] | |
| Moringa oleifera | Getha | Moringaceae | Diabetes, ascites, splenic enlargement, inflammatory swellings, abdominal tumors, colic, dyspepsia, fever, ulcers, paralysis, lumbago, skin diseases | Fruit | [86] | |
| Ocimum forskolei | Basil | Lamiaceae | Cosmetic, digestive agent, spasm, mosquito’s repellent factor, relieves fever, skin infections | Leaf | [45,87] | |
| Saudi Arabia | Plicosepalus curviflorus | EnamElTalh | Loranthaceae | Cancer, diabetes | Stem | [46] |
| Retama raetam | Al-retem | Fabaceae | Diabetes | Fruit | [88] | |
| Rhizophora mucronata | Kindale | Rhizosphoraceae | Elephantiasis, hematoma, hepatitis, ulcers, diarrhea or gastric motility disorder, diabetes | Bark, branches, flower, fruit, leaf | [76,89] | |
| Salvadora persica | Miswak | Salvadoraceae | Oral and dental cleaning tool | Bark | [49] | |
| Yemen | Azadirachta indica | Neem | Meliaceae | Diabetes, GIT disorder, general health promoter, leprosy, respiratory disorders | Leaf, bark | [90] |
| Boswellia carterii | Olibanum | Burseraceae | Anti-inflammatory, bruises, infected sores psychoactive, reduce the loss of blood in the urine from schistosomiasis infestation, tranquilizer, wound healing, various non-pharmaceutical applications | Resin | [58,91] | |
| Cissus rotundifolia | Arabian wax | Vitaceae | Appetizer, antipyretic carbuncles, dengue fever, malaria, rheumatic pain, snake bites | Leaf, root, stem, | [58] | |
| Dracaena cinnabari | Damm Al-Khwain | Agavaceae | Burn, control of bleeding, diarrhea, fractures, fevers, healing of fractures, hemorrhage, pain, stimulation of circulation, sprains, ulcers, wounds | Resin | [63,92] | |
| Opuntia ficus-indica | Cactus | Cactaceae | Allergy, analgesic, anti-inflammatory, dyspnoea, fatigue, glaucoma, gastric ulcer, health supporting nutrient, healing wounds, hypoglycemic agent, liver diseases, prostate cancer, urological problems | Flower, fruit | [93] | |
| Yemen | Pulicaria inuloides | False fleabane | Asteraceae | Anthelmintic, carminative, cold, diuretic, fever, inflammation, insect repellent, pain, intestinal disorder, pyritic conditions in urogenetic organs | Flowers | [94,95] |
| Solenostemma argel | Argel | Asclepiadaceae | Diabetes, cardiovascular disorders, gastrointestinal problems, kidney and liver diseases, pain, respiratory tract infections, urinary tract infections | Bark, leaf, stem | [69,96] |
Table 2.
Medicinal plants of Arabian Peninsula reported constituents use.
| Country | Name | Chemical Constituents | Scientific Reports | Reference |
|---|---|---|---|---|
| Oman | Ajuga iva | Anthocyanins, ecdysteroids, flavones, glycosides, terpenes; diterpenes, triterpenes, tannins, withanolides | Antibacterial, antifungal, antihypertensive, antimycobacterial, antiplasmodial, hypoglycaemic, larvae and insect antifeedant activity |
[12,16,97,98] |
| Moringa | Flavonoid, isothiocyanate, glycosides, phytosterol, polyphenol, triterpene, volatile oil | Anticancer, antioxidant, antimicrobial, antidiabetic, anti-inflammatory, anti-spasmodic, hypertension, hepatotoxicity, lipid lowering activity, memory disorders | [17] | |
| Rhazya stricta | Indole alkaloid, flavonoids, carbolines, alkaloids with β-carboline nucleus (akuammidine, rhazinilam tetrahydrosecamine), triterpenes, tannins, volatile bases | Antioxidant, blood pressure, diabetes mellitus, immunomodulation effect | [22,99] | |
| Qatar | C. coccineum | Flavonoids, organic acids, scharrides, lipids | Anticancer, antidiabetic, antioxidant, anti-tyrosinase antimicrobial, cardioprotective, immunity-improving, neuroprotective, increase and folliculogenesis, testicular development, spermatogenesis | [100,101] |
| Saudi Arabia | Avicennia marina | Alkaloids, glycosides, flavonoids, phenols, saponins, tannins, terpenoids | Antidiabetic, anti-inflammatory, antimicrobial, antioxidant, antiviral | [102] |
| Caralluma sinaica | Coumarin, flavonoids, glycosides, phenolic alkaloids, steroids, tannins | Antidiabetic agent, antimicrobial | [78] | |
| Ducrosia anethifolia | Monoterpenes hydrocarbons(essential oil), coumarins | Anticancer, antidiabetic, anti-inflammatory, antimicrobial, anxiolytic, radical scavenging | [80,103] | |
| Saudi Arabia | Jatropha curcas | C yclic peptide alkaloids, flavonoid, lignans, saponins, terpenes, diterpenoids | Antioxidant, anticancer, anti-inflammatory, gastroprotective, anthelmintic, antidiarrhea activity, antiulcer | [103,104,105,106] |
| Loranthus acaciae | Alkaloid, cardiac glycosides, flavonoids saponins tannins, terpenoids, catechin | Analgesic, antidiabetic, anti-inflammatory, antimicrobial, antitumor, antioxidant, antihepatotoxic, antiviral | [38,107] | |
| Lycium shawii | Alkaloids, flavonoids, phenolics, tannins, saponins, glycosides, terpenoids, steroids, coumarins | Antioxidant, diuretic, laxative, anticancer, antimicrobial anti-inflammatory, tonic agent | [108] | |
| Marrubiumvulgare | Flavonoid, phenylpropanoids, terpenes, sesqui and diterpenes | Antihypertensive, analgesic, anti-inflammatory, antioxidant | [85] | |
| Moringa oleifera | Carotenoids, glucosinolates, flavonoids, phenolic acids, polyunsaturated fatty acids, tocopherols | Antidyslipidemic, anthelmintic, antihyperglycemic, anti-inflammatory, antimicrobial, antioxidant, apoptotic properties, antiproliferative, anti-ulcer, antiurolithiatic, hepatoprotective | [109] | |
| Morus nigra | Alkaloids, flavonoids, phenols | Antihyperlipidemic, antidepressant, antioxidant, neuroprotective | [110] | |
| Saudi Arabia | Ocimum forskolei | Alkaloids, essential oils, flavonoids, glycosides, phenylpropanoids, saponins, steroids, tannins | Activities against bacteria and dermatophytes, antihyperglycemic, nematicidal activity, weak antioxidant | [87,111] |
| Plicosepalus curviflorus | Flavonol rhamnosides, sesquiterpene lactones | Antihepatotoxic, antidiabetic, cytotoxic activities | [112] | |
| Retama raetam | Flavonoid, quinolizidine alkaloids | Antibacterial, antihyperlipidemic, antihypertensive, antioxidant, diuretic, hypoglycemic. | [48] | |
| Rhizophora mucronata | Alkaloids, anthocyanidins, anthraquinone, carotenoids, catechin, flavonoids, phenolics, saponin, steroids, triterpene | Antimicrobial, antioxidant, hepatoprotective | [89,102,113] | |
| Salvadora persica | Essential oil, organosulphur compounds, saponin, β-sitosterol | Anticonvulsant, antifertility, antimicrobial, analgesic, antiplaque, aphrodisiac, antipyretic, antiulcer, astringent, diuretic, hypolipidemic, stomachic activities. | [49,114] | |
| Yemen | Azadirachta indica | Alkaloid, aromatic compound, flavonoid, flavone, isoprenoidssesquiterpenes, terpenes;triterpenoids tetraanortriterpenoid, saponins, limonoids | Antibacterial, antidiabetic, antifertility, antihypercholesteremic. antimalarial, antioxidant, antiulcer, anti-tumor | [9,58] |
| Boswellia carterii | Pentacyclic triterpenoid, volatile oil | Anti-inflammatory, neuroprotective. | [115,116,117] | |
| Yemen | Cissus rotundifolia | Steroids, flavonoids, β-sitosterol | Analgesic, antidiabetic, anti-inflammatory, antiparasitic, antiulcerative, antioxidant, hepatoprotective activity |
[62,118,119] |
| Dracaena cinnabari | Flavone, chalcone | Antidiarrheal, anti-hemorrhagic, anti-inflammatory antimicrobial, antiviral activity, antitumor activity, antiulcer, immunomodulatory, antioxidant | [92] | |
| Opuntia ficus-indica | Flavonoid glycoside, oxygenated monoterpenes, sesquiterpene hydrocarbons, aldehydes with non-terpenic structure | Antioxidant, antiulcer, cardioprotective, hepatoprotective | [120,121] | |
| Pulicaria inuloides | Essential oil, monoterpenes, diterpenes, sesquiterpene lactones and caryophyllane derivatives | Antimicrobial, antifungal, antimalaria, insecticides properties | [94,95] | |
| Solenostemma argel | Phenolic acids, flavones, glycosylated flavonoids, polyphenols, β-carotene, β-sitosterol, terpenes, mono and triterpenes | Antibacterial, antifungal, antioxidant | [69,96,122,123] |
Table 3.
Medicinal plants with potential antidiabetic activity in Arabian Peninsula.
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref. |
|---|---|---|---|---|---|---|
| Ajuga iva | Root, stem, leaf | Ethanol, maceration | Streptozocin diabetic rats | 21 days | Hypoglycemic activity | [25] |
| Moringa pergrina | Leaf | Chloroform, soxhlet | Mice | 1 mL of 0.5%, 1%, 1.5%, or 2%, 21 days | Weight reduction, influenced the reproductive system | [21] |
| Pteropyrum scoparium | Leaf, root, stem | Ethanol, maceration | streptozocin diabetic rats | 21 days | Hypoglycemic activity | [25] |
| Rhazya stricta | Root, stem, leaf | Ethanol, maceration | Streptozocin diabetic rats | 21 days | Hypoglycemic activity | [25] |
| Cynomorium coccineum | Inner flesh of stem | Water, reflux | In vitro | Antioxidant activity associated with angiotensin converting enzyme | [27] | |
| Avicennia marina | Leaf | Ethanol, maceration |
Streptozocin diabetic rats | 2 mg/gm, 4 weeks | Protected liver, improved the neurobehavioral changes, decreased inflammatory cells aggregation, vacuolation, and hemorrhage. | [30] |
| Leaf | Water, decoction | Streptozocin diabetic rats | 400 mg/kg, 6 weeks | Significant increase in the muscle levels of CAT, SOD and glutathione | [31] | |
| Caralluma sinaica | Aerial parts, root | Ethanol (80%), maceration | Streptozocin diabetic rats | 100 mg/kg, 30 days | Reversed streptozocin effect on glycogen content | [33] |
| Ducrosia anethifolia | Aerial parts | Ethanol (80%),maceration | Streptozocin diabetic rats | 500 mg/kg, 45 days | Normalized liver enzymes, total protein, lipid, cholesterol levels, antioxidant markers, glycolytic, and elevated level of kidney biomarkers | [34] |
| Jatropha curcas | Aerial parts | Ethanol (96%), chloroform, hexane, maceration | Alloxan diabetic mice | 400 mg/kg, 1 day | Safe up to dose of 5 g/kg | [124] |
| Loranthus acaciae | Leaf, stem | Ethanol, soxhlet | Alloxan diabetic rats, normal rats | 500 mg/kg, 1 week | LD50 of the extract and its fractions was more than 5 g/kg, a potent anti-inflammatory and antioxidant effect was detected for the chloroform fraction | [38] |
| Lycium shawii | Aerial parts | Ethanol, decoction | Alloxan diabetic rats, normal rats | 500 mg/kg, o.p or i.p, 90 days | None of the mice died up to 3 g/kg dose. Prolonged L. shawii treatment is toxic. | [39] |
| Marrubium vulgare | Aerial parts | Methanol, maceration | Streptozocin diabetic rats | 500 mg/kg, 28 days | Significant reduction in plasma TC, TG, LDL, increased HDL | [40] |
| Moringa oleifera | Seed | Powder | Streptozocin diabetic rats | 50, 100 mg/kg, 4 weeks | Restoration of the normal histology of both kidney and pancreas | [42] |
| Morus nigra | Leaf | Ethylalcohol, maceration | Streptozocin diabetic rats | 500 mg/kg, 10 days | Significant increase in insulin level | [43] |
| Ocimum basilicum | Leaf | Water, infusion | In vitro | 20, 18.2, 16.3, 14.5 mg/mL | Significant dose-dependent inhibition of sucrase, maltase and pancreatic α-amylase | [125] |
| Ocimum forskolei | Leaf, stem | Methanol (70%), maceration | In vitro | 10, 20, 30 mg/mL | The inhibitory activity (IC50) of both leaf and stems methanol extracts are almost the same | [45] |
| Plicosepalus curviflorus | Leaf | Serial exhaustive extraction, maceration | Mice | Extract: 500 mg/kg, i.p Isolated compounds 50, 100 mg/kg, i.p |
The mixture of the isolated compounds synergized with each other. | [45] |
| Whole plant | Methanol, maceration | High-fat diet followed by injection of streptozocin diabetic rats | Extract or solid lipid nanoparticles: 250 mg/kg, 4 weeks | The SLN preparation with the highest lipid content gave the best result of reduction of hyperglycemia and insulin resistance | [45] | |
| Retama raetam | Fruit | Methanol, maceration | Streptozocin diabetic rats | 100, 250, 500 mg/kg, 4 weeks | The extract significantly inhibits glucose absorption by rat isolated intestine | [48] |
| Rhizophora mucronata | Leaf | Water | Streptozocin diabetic rats | 400 mg/kg, 6 weeks | Significant increase in the muscle levels of CAT, GSH and SOD | [31] |
| Salvadora persica | Root | Water, soxhlet |
Streptozocin diabetic rats | 250, 500 mg/kg, 21 days | Significant decrease in TC, TG, LDL, VLDL, increase in HDL | [49] |
| Azadirachta indica | Leaf | Water, percolation | Alloxan diabetic rabbits | 200, 400 mg/kg, 25 days | Decrease in serum TG, cholesterol, LDL, increase in HDL | [51] |
| Boswellia carterii | Resin | Water, decoction | Streptozocin/Nicotinamide-diabetic rats | 100 mg/kg, 4 weeks | Decrease in the serum cholesterol and TG | [58] |
| Cissus rotundifolia | Aerial parts | Water, maceration | Streptozocin/Nicotinamide-diabetic rats | 100 mg/kg, 4 weeks | Decrease in serum cholesterol and TG | [58] |
| Leaf | Water, maceration | Streptozocin/Nicotinamide-diabetic rats | 100 mg/kg, 4 weeks | Significant decrease in urea, ALT, AST | [62] | |
| Dracaena cinnabari | Resin | Ethanol 99%, maceration | Alloxan diabetic rats | 100, 300 mg/kg, 14 days | Antihyperlipidemic effect | [64] |
| Dracaena cinnabari | Resin | Serial exhaustive extraction, soxhlet | In vitro MCF-7 cell line | 100 μg/mL | Glucose uptake inducing activity of ethylacetate extract was found to be higher than Metformin | [63] |
| Opuntia ficus-indica | Seed | Hexane, p-ether, chloroform, maceration | Streptozocin-diabetic rats | 0.2, 0.4, 0.6 g/kg, twice daily, 21 days | Reducing the expression of the PCK1 gene while increasing the expression of Slc2a2 gene in the liver tissue. | [66] |
| Pulicaria inuloides | Leaf | Oil extract, stem distillation |
Streptozocin diabetic rats | 400 mg/kg, 21 days | Inhibitory effect on α-glycosidase and α-amylase | [67] |
| Solenostemma argel | Leaf | Methanol, maceration |
Methylprednisol-one diabetic rats | 1 gm/kg, 14 days | Antioxidant activity | [69] |
3. Medicinal Plants with Potential Antidiabetic Activity in Egypt
The International Diabetes Federation (IDF) listed Egypt among the world’s top 10 countries in the number of patients with diabetes. It is expected this number will jump up to 13.1 million by 2035 [126].
There are abundant and biodiverse medicinal and aromatic plant kinds in Egypt. An Egyptian large-scale bio-study was performed, which aimed to screen 264 plant extracts for their in vitro α-glucosidase inhibitory activity. Of all extracts, 63 achieved an inhibition of α-glucosidase of more than or equal to 70% at the tested concentration (25 ppm, and the most active plant extract is Pinusrox burghii (IC50 is 2.47 ppm) [127,128].
3.1. Cassia acutifolia (Fabaceae)
Several allied species had been shown to lower blood glucose level. The hydroethanolic extract of C. acutifolia leaves showed a high antihyperglycemic effect at dose levels 10 and 50 mg/kg. An anthraquinone, chrysophanol, was isolated from the leaves of C. acutifolia and showed mild antidiabetic properties in cell culture. Its activity was proposed to be mediated through affecting glucose transport and the tyrosine phosphorylation of the insulin receptor, improving insulin action or insulin-independent effects [129].
3.2. Centaurea alexanderina (Asteraceae)
A significant decrease in elevated blood glucose level was seen in normoglycemic and streptozocin diabetic rats. The methanol extract at a dose level of 600 and 300 mg/kg showed a significant reduction in plasma glucose level after thirty days of treatment, and the maximum effect was observed after 60 days of treatment [130].
3.3. Cyperus laevigatus (Cyperaceae)
The biochemical markers caused a decrease in glucose level and the promotion of serum insulin in the diabetic group treated with C. laevigatus extract, and this can be explained by flavonoid activity. In addition, the histological examination of the pancreas of the extract-treated rats indicated the normal architecture that was attributed to flavonoids’, flavonoid glycosides’ and phenolic acids’ abilities to regenerate β-cells [131].
3.4. Fraxinus ornus (Oleaceae)
The hydroalcoholic extract of F.ornus fruit showed a significantly antihyperglycemic effect. Improving insulin action or insulin-independent effects was postulated as a possible mechanism for the antidiabetic effect [129].
3.5. Phoneix dactylifera (Arecaceae)
It was reported that the oral administration of date seed extract combined with insulin had an antihyperglycemic effect as compared to seed extract alone in streptozocin diabetic rats, and could minimize the toxic effects of diabetes on the liver and kidney for diabetic rats [132].
Good glycemic control was achieved by the administration of an aqueous suspension of P. dactylifera seeds. It resulted in a significant reduction (by 51%) in the blood glucose level compared with the untreated diabetic group. In addition, it is reported to possess a protective effect against diabetic complications both for kidney and liver [133]. The hydroalcoholic extract of P. dactylifera leaves had a strong inhibitory effect against α-glucosidase, and a significant antidiabetic activity superior to the antidiabetic drug acarbose in alloxan diabetic rats [134].
3.6. Nepeta cataria (Lamiaceae)
All crude extracts of N. cataria, ethanol, petroleum ether, and chloroform extracts revealed significant amelioration in blood glucose and insulin levels, and an improvement in hepatocytes and pancreas β-cells, for diabetic rats. The hypoglycemic action of the extract was explained by the presence of antioxidants such as flavonoids and polyphenols, which may prevent the progressive impairment of pancreatic β-cell function, and may improve all carbohydrate brush border hydrolyzing enzymes [135].
3.7. Securigera securidaca (Fabaceae)
Previously, the aqueous extract of S. securidaca seeds showed a significant decrease in blood glucose level in glucose-loaded mice and alloxan diabetic rats. The hypoglycemic effect of the seed was estimated to be related to its flavonoid content [136].
The ethanolic extract of the flowers showed significant antidiabetic activity with a potency comparable to gliclazide in alloxan-induced diabetic rats [136].
The mechanism of hypoglycemia was attributed to several phenolic compounds; Vicenin-2 was reported to be an antioxidant that strongly inhibited α-glucosidase, isoquercetrin and astragalin were found to be glycation inhibitors, rutin was reported to enhance peripheral glucose utilization by skeletal muscle, the stimulation of β-cells, quercetin and kaempferol were found to improve insulin-stimulated glucose uptake in mature adipocytes, rutin and hesperidin were reported to increase hepatic glycolysis and glycogen concentration and lower hepatic gluconeogenesis, and catechin showed potential insulin-mimetic activity [136].
3.8. Trigonella stellate (Fabaceae)
The ethanol extract of T. stellate did not show any significant activation of PPARα and PPARγ (peroxisome proliferator-activated receptor α/γ), but the ethyl acetate fraction showed activation of both genes. On other hand, the isolated compounds, methoxyisoflavan and dimethoxyisoflavan derivatives, showed an increase in PPARα activity, while the other compound, glucopyranosyl isoflavan derivative, showed an ability to activate the PPARγ receptor. The other reported isolated compounds manifested a mild to moderate activation of PPARγ receptors [137].
3.9. Urtica pilulifera (Urticaceae)
The ethyl acetate and chloroform fractions of U. pilulifera extract decreased the glucose level significantly in diabetic rats. Previously, seeds lectin of U. pilulifera was reported to mimic insulin actions by interacting with the glycoprotein residues of the insulin receptor [138].
Other bioactive compounds may contribute to the antidiabetic activity, for example, β-sitosterol, β-amyrin and ursolic acid through increasing glucose utilization and metabolism in peripheral tissue [138].
3.10. Zizyphus spina-christi (Rhamnaceae)
The butanol extract from Z. spinachristi leaves, and its main saponins glycoside and christinin-A, improved the oral glucose tolerance and potentiated glucose-induced insulin release in type II diabetic rats. The sulfonylurea-like activity of the extract was reported to have a safe LD50 of 3820 mg/kg when compared to glibenclamide [139].
In another study, the oral administration of Z. spina-christi leaf extract, plain or formulated for 28 days, reduced blood glucose level along with a significant increase in serum insulin level and antioxidant capacity. A normalization of the percentage of glycated hemoglobin (HbA1C %) and a dose-dependent inhibitory activity of the extract against α-amylase was also reported. Z. spina-christi leaf extract improved the glucose level in diabetic rats by increasing insulin secretion, which may be due to both saponin and polyphenols contents; attenuating meal-derived glucose absorption, which might be attributed to the total polyphenols; and restoring liver and muscle glycogen content, together with significantly decreasing hepatic glucose-6-phosphatase and increasing glucose-6-phosphate dehydrogenase activities [140] (Table 4, Table 5 and Table 6).
Table 4.
Medicinal plants of Egypt ethnobotany.
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Cassia acutifolia | Not mentioned | Fabaceae | Constipation | Not mentioned | [141] |
| Centaurea alexanderina | Not mentioned | Asteraceae | Antimalarial, bitter tonic, diuretic, mild astringent, stomachic | Not mentioned | [130] |
| Fraxinus ornus | HabElderdar | Oleaceae | Diabetes | Not mentioned | [129] |
| Moringa peregrina | Hadendowa | Moringaceae | Edible, fever, headache, earache, burns, disinfectant, adenopathy, skin disorders, itching, soothe rash, purify water, wound, cancer, laxative, cathartic, malnutrients, ascites, leprosy, swellings | Flower, leaf, root, seed | [142,143] |
| Nepeta Cataria | Qatram, hashishat al-her (Arabic name) | Lamiaceae | Asthma, bronchitis, cough, diarrhea, gastrointestinal, respiratory hyperactivedisorders | Leaf | [135,144] |
| Phoneix dactylifera | Not mentioned | Arecaceae | Aphrodiasiac, tonic | Pollen, male flower | [145] |
| Securigera securidaca | Not mentioned | Fabaceae | Antidiabetic, antihyperlipidemic | Seed | [146] |
| Trigonella stellate | Not mentioned | Fabaceae | Dantidiabetic, antihyperlipidemic | Not mentioned | [137] |
| Urtica pilulifera | Nettle (Palestine) | Urticaceae | A diuretic, antiasthmatic, anti-inflammatory, hypoglycemic, hemostatic, antidandruff and astringent | Whole plant | [138,147] |
| Zizyphus spina-christi | Nabka, Seder (Palestine) | Rhamnaceae | Swellings, pain, and heat, eye inflammation, constipation, heartburn, diarrhea, wound, diuretic, liver problems, anus problems | Leaf, root, seed | [148] |
Table 5.
Medicinal Plants of Egypt reported constituents, use.
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Cassia acutifolia | Anthraquinone, phenolic glycoside | Laxative | [149,150] |
| Centaurea alexanderina | Sesquiterpene lactones, flavonoids | Hypoglycemia, cytotoxicity | [130] |
| Cyperus laevigatus | Quinones, flavonoids, sesquiterpenes, Steroids, essential oils |
Anti-inflammatory, hepatoprotective, gastroprotective, antimalarial, antidiabetic activities |
[131] |
| Fraxinus ornus | Alkaloid, coumarins, flavonoid, phenylethanoids, secoiridoid, tannin |
Antimicrobial, antioxidant, anti-inflammatory |
[129] |
| Moringa peregrina | Flavonoid | Antioxidant | [151] |
| Nepeta cataria | Essential oil, urosolic acid, β-sisoterol, campesterol, α-amyrin, β-amyrin, neptalactones, alkaloids, carenolides, tannins, saponins, coumarins |
Antibacterial, antifungal, analgesic | [135,152] |
| Phoneix dactylifera | Phenolic acid, sterol, carotenoid, flavonoid, procyanidins, anthocyanins | Immune system, nephroprotective, hepatoprotective, gastrointestinal protective, antioxidant, antihyperlipidemic activity, anticancer | [145] |
| Securigera securidaca | Alkaloid, cardiac glycosides, coumarins flavonoid, saponins, tannins | Antihyperlipidemic, chronotropic, diabetes, diuretic, gastroprotective | [153] |
| Trigonella stellate | Flavonoids; isoflavonoid, phenolic compounds | Antihyperlipidemic, antioxidant | [137] |
| Urtica pilulifera | Lectins, b-sitosterol, phenolic acids triterpene | Antitumor, astringent, asthma, antidandruff, diuretic, diabetes, deputative, galactogogue | [154] |
| Zizyphus spina-christi | Triterpenoid saponin glycosides, polyphenol, tannin, flavonoid, cyclopeptides, cardiac glycoside, essential oil, alkaloid | Antinociceptive, antioxidant, antifungal, antibacterial, antidiabetic | [139,140] |
Table 6.
Medicinal plants with potential antidiabetic activity in Egypt.
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref. |
|---|---|---|---|---|---|---|
| Cassia acutifolia | Leaf | Ethanol 80%, maceration | Nicotinamide-Streptozocin diabetic mice | 10, 50 mg/kg, 1 week | No effect on serum insulin level | [129] |
| Crataegus aronia | Whole plant | Water, maceration | High-fat diet with small dose of streptozocin diabetic rats | 500 mg/kg, 60 days | Lowered serum lipid levels, hepatic glycogen, hepatic lipid peroxidation, tumor necrosis factor α, interleukin, enhanced the level of reduced glutathione, superoxide dismutase, hepatic mRNA expression of the insulin receptor A isoform, glucose 6-phosphatase | [155] |
| Centaurea alexanderina | Leaf | Methanol 80%, reflux | Streptozocin diabetic rats | 600 mg/kg, 60 days | Anti-inflammatory activity was seen for the extract | [130] |
| Citrullus colocynthis | Seed | Ethanol | Alloxan diabetic rats | 50 mg/kg, 8 weeks | Decrease in lipid peroxidation, total cholesterol, triglyceride, total, direct bilirubin, increase on glutathione, lactate dehydrogenase, ALT, AST, ALP, total lipid were significantly increased in both normoglycemic and hyperglycemic rats | [156] |
| Cyperus laevigatus | Aerial parts | Methanol 70%, maceration | Streptozocin diabetic rats | 50mg/kg, 14 days | Decreasing levels of NO, glucagon, promoted paraoxonase activity | [131] |
| Fraxinus ornus | Fruit | Ethanol 80%, maceration | Nicotinamide-Streptozocin diabetic mice | 10, 50 mg/kg, 1 week | No effect on insulin serum level | [129] |
| Moringa oleifera | Leaf | Ethanol, maceration | Alloxan diabetic rats | 150 mg/kg, 21 days | Quercetin has the greatest potential antidiabetic activity in the extract, followed by chlorogenic acid and moringinine | [157] |
| Moringa peregrina | Aerial parts | Ethanol 95%, percolation | Streptozocin diabetic rats | Extracts: 25 mg/kg, fractions: 50 mg/kg, 1 day | The n-hexane fraction was the only fraction that showed a highly significant antihyperglycemic activity | [18] |
| Seed | ethanol 70%, soxhlet | Streptozocin diabetic rats | 150 mg/kg, 30 days | The extracts exerted protective effects against lipid peroxidation ethanolic extract > aqueous extract | [19] | |
| Nepeta cataria | Flowering aerial parts, seed | P-ether, ethyl acetate, ethanol 70%, soxhlet | Streptozocin diabetic rats | 50 g/kg, 30 days | All extracts had significant scavenging of free radicals abilities, normalized liver function and inhibited lipid synthesis | [135] |
| Phoenix dactylifera | Seed | Aqueous suspension | Streptozocin diabetic rats | 1 gm/kg, 4 weeks | Protective effects for kidney, liver | [133] |
| Epicarp | Isolated compounds | Alloxan diabetic rats | 20 mg/kg, 30 days | Significant decrease in ALT, AST, cholesterol, TG, increase in glutathione peroxidase and superoxide dismutase in liver | [158] | |
| Securigera securidaca | Flower | Ethanol 90%, maceration | Alloxan diabetic rats | 100, 200, 400 mg/kg | The extract was safe up to a dose of 2000 mg/kg (body weight), reduction in serum TG and total cholesterol levels | [136] |
| Trigonella stellate | Aerial parts, root | Ethanol 90%, maceration | Human hepatoma (HepG2) cell line | 12.5, 25, 50 μg/mL | Activation of PPARα and PPARγ (fractions and isolated comp) | [137] |
| Urtica pilulifera | Aerial parts | Methanol, maceration | High-fat, low-dose streptozocin diabetic rats | 250, 500 mg/kg | A significant antioxidant, anti-inflammatory effect | [138] |
4. Medicinal Plants with Potential Antidiabetic Activity in Iran
The burden of diabetes is growing in Iran. The prevalence of type 2 diabetes was 11.4% of the adult population, and it was estimated that the rate would increase to 12.8% (9.2 million) by 2030 [159,160]. Iran has a great diversity of medicinal plants due to the specific climate conditions [161].
It is now believed that α-amylase and α-glucosidase play an important role in controlling diabetes mellitus, especially in patients with type-2 diabetes. Several plants have been recommended in traditional Iranian medicine to treat diabetes [162].
Various extracts, such as n-hexane and ethyl acetate, and the methanol of various parts of Allium paradoxum, Buxus hyrcana, Convolvulus persicus, Pimpinella affinis, Parrotia persica, Primula heterochroma, Ruscus hyrcanus and Smilax excelsa, were examined for α-glucosidase and α-amylase inhibition. These plants mostly serve as food flavoring. Some extracts of S. excels, P. persica, and P. heterochroma exhibited significant antidiabetic activities in α-amylase and α-glucosidase assays, which were even more effective than acarbose. In addition, C. persicus and P. heterochroma showed strong antioxidant activity, compared with butylated hydroxytoluene [163].
Dichloromethane, n-hexane, chloroform, ethyl acetate, and methanol were used to prepare various extracts of the plants; Cinnamomum zeylanicum, Crataegus oxyacantha, Hibiscus sabdariffa, Morus alba, Portulaca oleracea, Rubus fruticosus, Syzygium aromaticum, Teucrium polium, Trigonella foenum-graecum and Vaccinium arctostaphylos were tested for their inhibition of α-glucosidase, α-amylase, and antioxidant activity [163]. S. aromaticum methanolic extract exerted the highest inhibitory effect against both α-glucosidase and α-amylase enzymes. Previously, the aqueous extracts were reported to have insulin-like effects, such as increasing glucose uptake into adipocytes. Among the other analyzed plants, C. zeylanicum, H. sabdariffa, R. fruticous, C. oxyacantha, V. arctostaphylos, and M. alba exhibited strong inhibitory activities against α-glucosidase in comparison with the reference drug, acarbose [164].
Other plant extracts were screened for α-amylase inhibitory activity, and these were Juglans regia, Olea europaea, Camellia sinensis, Coriandrum sativum, Trigonella foenum-graecum, Urtica dioica, Urtica pilulifera, Arctium lappa, Calendula officinalis, and Hibiscus gossypifolius [162].
The α-amylase-inhibitory activity varied among the tested plant extracts, but all of them demonstrated a significant dose-dependent reduction effect on the α-amylase enzyme. The most potent inhibitions appeared to correlate with the extracts of Camellia sinensis, Trigonella foenum-graecum and Urtica dioica leaves, and of Trigonella foenum-graecum seeds [162].
The genus Salvia generally comprises a variety of phenolic metabolites, especially flavonoids. Bioassay-guided fractionation of Salvia virgate extract led to the isolation and identification of an active flavone compound, chrysoeriol. The compound concentration-dependently inhibited the α-amylase activity with an IC50 value 1.27 mM [165] (Table 7).
Table 7.
Medicinal plants of Iran ethnobotany.
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Allium paradoxum | Alezi | Liliaceae | Acne, food flavoring | Raw vegetable | [161,163] |
| Allium ascalonicum | Not mentioned | Alliaceae | Diabetes | Not mentioned | [166] |
| Allium sativum | Sarimsaq, sir | Alliaceae | Diabetes, digestive system, blood fat, rabies | Bulb | [166,167,168] |
| Arctium lappa | Palvarg | Asteraceae | Antiscorbutic, antioxidant, blood purifiers, constipation, diuretic, disinfectant, gout | Berries, leaf, root | [169] |
| Buxus hyrcana | Shemshad | Buxaceae | Broken bone, toothache | Leaf | [167] |
| Calendula officinalis | Marigold, HamisheBahar | Asteraceae | Blood cleanser, eczema, dermal disorders, sudorific | Flower | [168,170] |
| Camellia sinensis | Chai Sabz | Theaceae | Anticancer, blood pressure, antihyperlipidemia, hepatitis, obesity | Leaf | [168,170] |
| Convolvulus persicus | Not mentioned | Convolvulaceae | Not mentioned | Not mentioned | [72] |
| Cinnamomum zeylanicum | Darchin | Lauraceae | Antibacterial, antioxidant, hypertension, diabetes | Bark | [164] |
| Coriandrum sativum | Geshniz | Apiaceae | Acne, antiseptic, appetizer, aphrodisiac, aromatic, calmative, flatulence, jaundice |
Fruit | [168,170] |
| Crataegus oxyacantha | Sorkhevalik | Rosaceae | Diabetes, hypertension | Leaf | [164] |
| Hibiscus sabdariffa | Chay-e-makki | Malvaceae | Antioxidant, diabetes, hypertension | Flower | [164] |
| Juglans regia | Gerdu | Juglandaceae | Antidiarrheal, eczema, hair color | Fruit, leaf | [168] |
| Morus alba | Toot | Moraceae | Constipation, diabetes blood lipid reduction | Leaf | [164] |
| Olea europaea | Zeytoun | Oleaceae | Anti-hemorrhoids, blood lipid, diabetes, dermal allergy, hypertension, kidney stone, laxative, renal problems | Fruit, leaf, seed | [167,168,171,172] |
| Parrotia persica | Not mentioned | Hamamelidaceae | Antifever, broken bone, food coloring and flavoring | Bark | [163,173] |
| Pimpinella affinis | Taretizakebaghi | Apiaceae | Asthma, antimicrobial, antispasmodic, carminative, cholera, diuretic, migraine, narcotic | Flowering shoot, Seed | [163,174] |
| Primula heterochroma | Not mentioned | Primulaceae | Food flavoring | Not mentioned | [163] |
| Portulaca oleracea | Khorfeh | Portulaceae | Antibacterial, antiviral, diabetes, enhancing immunity | Seed | [164] |
| Rubus fruticosus | Tameshk | Rosaceae | Anti-infection, anticramp, hypertension, food flavoring, narcotic | Leaf | [164] |
| Ruscus hyrcanus | Butcher’s broom | Asparagaceae | Appetizer, antibleeding, antilaxative, anti-nephritis, anti-infection, antivaricose, aperient, diuretic, jaundice, laxative, vasoconstrictor | Leaves, fruit | [163,175] |
| Smilax excelsa | Not mentioned | Smilacaceae | Antieczema, diuretic, food flavoring | Not mentioned | [163] |
| Syzygium aromaticum | Alezi | Liliaceae | Acne, digestive disorder food flavoring | Raw vegetable | [164] |
| Teucrium polium | Kalpoore | Buxaceae | Analgesic, aperient, antiepileptic, antihairloss, antiheadache, anti-infection, antimalaria, antipneumonia, antirheumatism, carminative, constipation, febrifuge, stomachache | Aerial part | [164,176] |
| Trigonella foenum-graecum | Sic lefuit fenugreek | Fabaceae | Not mentioned | Not mentioned | [164,170] |
| Urtica dioica | Nettle | Urticaceae | Diabetes | Aerial parts, seed | [177] |
| Urtica pilulifera | Kara Isırgan (Turkey) | Urticaceae | Antidandruff, anti-inflammatory, asthma, astringent, blood purifier, diuretic, diabetes, enhancement of hemoglobin concentration, hemostatic, lower urinary tract infection, stimulating tonic (Egypt) | Whole plant, leaf | [138,178] |
| Vaccinium arctostaphylos | Darchin | Lauraceae | Antibacterial, antioxidant, blood pressure, diabetes | Bark | [164] |
The genus allium contains more than 500 species, of which 93 are known from Iran and only a few of them are used as a foodstuff. The most important species existing in this genus include garlics, onions, and leek, which have long been used as spices and for medicinal purposes, including reducing the risk of cardiovascular disease and diabetes, stimulating the immune system, protecting against infections, and exhibiting anti-aging as well as anti-cancer properties. These biological effects of Allium vegetables are mainly associated with organosulphur compounds [179,180,181].
4.1. Allium ampeloprasum (Liliaceae)
The essential oils of the green parts of Egyptian A. ampeloprasum—which are not edible—were tested for their diabetic activity on streptozocin diabetic rats. The extract showed a significant decrease in glucose level and an improvement in lipid profile and oxidative stress parameters. Further studies are required to identify the active constituents [182].
Recently, alloxan diabetic rats were treated with a hydroalcoholic A. ampeloprasum extract that showed a treatment effect for hyperglycemia in rats and significantly decreased cholesterol and triglycerides, which is consistent with previous studies [183].
It is well known that the imbalance between reactive oxygen species production and metabolism ends in a range of disorders, including diabetes. The antioxidant power of A. ampeloprasum extract is more marked than that of other plants from this genus due to its significantly larger amounts of polyphenolic compounds, phenolic acids, flavonoids, tannins, and saponin than other plants from the same genus. Therefore, these compounds may play an essential role in maintaining the integrity of pancreatic β-cells [183].
Generally, plants from the genus Allium can cause a decrease in glucose level in an experimentally induced diabetes model, or after glucose loading through increasing the peripheral consumption of glucose, inhibiting the intestinal absorption of glucose, or intensifying the insulin secretion from residual β-cells [183].
4.2. Allium ascalonicum (Liliaceae)
Thiosulfinates volatile sulfur compounds are typical of the Allium species and are reported to cause many of the biological effects of garlic, and are also responsible for their characteristic pungent aroma and taste [166].
A.ascalonicum methanolic extract decreased blood glucose level in alloxan diabetic rats in a way that resembles the hypoglycemic action of glibenclamide. In the long-term period, the effect of the reduction in blood glucose was similar to that of metformin. The Allium genus is rich in flavonoids that can inhibit the enzymes responsible for the controlling of gluconeogenesis, glucokinase and glucose 6-phosphatase, and can increase the storage of glucose in the liver with a reduction of glycogen breakdown [166].
4.3. Allium sativum (Liliaceae)
Several experiments were performed to test the hypoglycemic activity of the plant. In most of the studies, garlic had been found to be effective in lowering the serum glucose level in streptozocin as well as alloxan-induced diabetic rats, mice, and rabbits [184].
In 1996, Augusti and Sheela showed that S-allyl cysteine sulphoxide and allicin had the potential to reduce the diabetic condition in rats almost to the same extent as did glibenclamide and insulin. In addition, both garlic oil and diallyl trisulphide were reported to improve glycemic control in streptozocin diabetic rats [185].
The aqueous extract for fresh bulbs and seeds of garlic administered orally or by injection showed a significant decrease in serum glucose levels in streptozocin diabetic rats in all the reported studies [184,186,187]
Furthermore, Allium sativum methanolic extract decreased blood glucose levels in alloxan diabetic rats similarly to glibenclamide. It reduced blood sugar similar to metformin for a long-term period [166].
The proposed mechanisms for the hypoglycemic effect of garlic were the stimulation of insulin secretion, the enhancement of glucose utilization, the inhibition of the intestinal absorption of glucose, and a sparing insulin effect [186].
4.4. Amygdalus lycioides (Rosaceae)
The effect of the hydroalcoholic A. lycioides extract was evaluated on diabetic rats. The glucose serum level and glucose tolerance test showed a decrease after treatment with plant extract (1000 mg/kg), and the total number and numerical density of β-cells were increased [188].
Flavonoids such as quercetin were reported to inhibit glucose absorption in the intestine, and stimulate the insulin secretion and regenerate the β-cells of the pancreas, a hypothesis that was confirmed by the stereological studies and the exhibition of strong antioxidant scavenging activity; all are possible mechanisms of the Amygdalus lycioides antidiabetic activity [188].
4.5. Amygdalus scoparia (Rosaceae)
The daily oral administration of 200 mg/kg of A. scoparia extract for 15 days decreased the blood glucose concentration to the normal range in streptozocin diabetic mice, slightly increased the size of pancreatic islets, and markedly regenerated β-cells [189].
4.6. Arctium lappa (Asteraceae)
The A. lappa root extract had an antidiabetic effect through its hypolipidemic and insulinotropic properties. Flavonoids that are known as bioactive antioxidant and antidiabetic agents, have an alkaloid content that can modulate insulin secretion, and have saponins that have blood glucose-lowering effects, and all are responsible for the reported activity [190].
According to the insulin-related biomarkers assay, it was indicated that the A. lappa root extract had improvement effects for type 2 diabetes complications through the enhancement of β-cells function, the induction of insulin sensitivity and insulin secretion, and a reduction in the insulin-resistance index [190].
4.7. Berberis integerrima (Berberidaceae)
The aqueous extract of B. integerrima root was tested on streptozocin diabetic rats. Different doses of the aqueous extract resulted in a significant decrease in blood glucose and lipid profile, while HDL-cholesterol was markedly increased [191]. On the contrary, the daily administration of aqueous fruit extract did not possess a hypoglycemic and hypolipidemic activity in streptozocin diabetic rats [192].
Moreover, the antihyperglycemic effect of the anthocyanin fraction of B. integerrima fruits in normal and streptozocin diabetic rats was investigated, and the synergic effect of this fraction with metformin or glibenclamide was evaluated [193]. The blood glucose level was significantly decreased in treated diabetic rats. Nevertheless, there was no synergistic effect [193].
Berberis species are rich in anthocyanins that can protect the pancreatic β-cells against oxidative stress through antioxidant properties, promote insulin release from the pancreatic β-cells, activate AMPK (5′-adenosine monophosphate-activated protein kinase), the main enzyme for enhancing glucose transport into skeletal muscles, and inhibit α-glucosidase in the small intestine; all of these are the recognized mechanisms of anthocyanins antidiabetic activity [193].
4.8. Brassica napus (Brassicaceae)
The administration of raw and cooked Brassica napus extract to alloxan diabetic rats significantly reduced blood glucose compared to diabetic control rats [194].
Brassica napus is rich in anthocyanins, which play a role in decreasing and/or inhibiting α-glucosidase and inducing insulin secretion via the stimulating of the pancreas β-cells [194]. It also contains sulfur-containing amino acids that have a role in glucose-lowering function, the enhancement of insulin’s effect on the body, and the increase in liver glycogen synthesis in diabetic rats [194].
4.9. Brassica rapa (Brassicaceae)
Turnip leaf extract significantly decreased serum glucose and prevented the elevation of plasma ALT in a dose-dependent manner. This activity may be due to the possession of high levels of polyphenolic compounds and the presence of flavonoids and tannins. Therefore, turnip leaf chemical components may have exerted a regenerative effect on β-cells and stimulated these cells to produce more insulin, or have contained some insulin-like substances [195].
4.10. Capparis spinosa (Capparaceae)
Several experimental studies have confirmed the antidiabetic properties of aqueous and hydroalcoholic extracts of C. spinosa. The antihyperglycemic effect was observed for different parts of the plant, including fruit, leaves, root, and seeds, and in a wide range of doses and treatment periods [196,197,198,199].
Both Jalali et al. and Eddouks et al. reported the glucose-lowering effect of the aqueous extract of the C. spinosa fruit in streptozocin diabetic rats after the oral administration of 20 mg/kg. The effect was undetectable in normoglycemic rats [200].
The antihyperglycemic effects of C. spinosa are due to its inhibition of α-amylase activity, the reduction in the mRNA expressions, and thew activities of PEPCK (Phosphoenolpyruvate carboxykinase) and G6Pase (Glucose 6-phosphatase) that cause a reduction in basal endogenous glucose production by the liver, the stimulation of intracellular insulin signaling pathways and the enhancing of glucose uptake in the liver, muscle, and adipose tissue, causing an improvement in insulin sensitivity in these tissues [196].
4.11. Centaurea bruguierana (Asteraceae)
The hypoglycemic effect of C. bruguierana was demonstrated using various extracts of the fruiting aerial parts [201].
All the extracts decreased blood glucose nearly equally to glibenclamide, and reached a steady state after 3 h. The aqueous extract’s hypoglycemic effect continued to increase significantly after 3 h. The plants worked through increasing hepatic glycogenolysis [201].
4.12. Cichorium intybus (Asteraceae)
The whole plant ethanolic extract significantly attenuated the serum glucose level by reducing hepatic glucose-6-phosphatase activity [202]. Ethanolic seed extract, fruit and leaf powder were all reported to improve glycemia in rats [202,203,204].
The aqueous seed extract of C. intybus’ effects on blood sugar and some blood parameters were investigated for detailed differences between early and late stages of diabetes in the diabetic male rats. The treatment with chicory extract over four weeks prevented weight loss in both early-stage and late-stage diabetic rats, and the levels of cholesterol, triglycerides and HbA1c were decreased [203].
The main observation in Chicory-treated diabetic animals was the resistance to excessive increases in fasting blood sugar. In addition, chicory treatment led to an increase in insulin level in the early stage of diabetes, pointing toward the insulin-sensitizing action of chicory [203].
Chicory may be useful as a natural dietary supplement for slowing down the pace of diabetes’ progress due to caffeic acid and chlorogenic acid presence. Both have the potential for increasing glucose uptake in muscle cells, and stimulating insulin secretion from an insulin-secreting cell line and islets of Langerhans [203].
A new potential antidiabetic agent present in the plant is Chicoric, which was reported to exhibit both insulin-sensitizing and insulin-secreting properties [203].
4.13. Citrullus colocynthis (Cucurbitaceae)
Various parts of the plant, such as the root, fruit, rind, and leaf, were used to prepare ethanolic, methanolic, or aqueous extracts at varying doses from 10 to 500 mg/kg, and all elaborated the plant’s antiglycemic activity [205].
Furthermore, the plant is widely used traditionally for the treatment of diabetes in Iran [206]. The extract of the plant seed was able to reduce blood glucose level significantly, and to enhance the regeneration of β-cells and increase the size of the pancreatic island in alloxan diabetic rats [207].
The administration of fruit powder significantly decreased lipid parameters and glucose level, and increased inulin level [204].
The antidiabetic effect of C. colocynthis extract could be linked to the plant’s ability to stimulate the β-cells, to activate the insulin receptors, to decrease gluconeogenesis, to inhibit the release of counter-regulatory hormones, to inhibit the effect of glucose absorption, to increase the incorporation of circulating glucose as hepatic glycogen, and to partially regenerate or preserve the pancreatic β-cell mass [204,207].
4.14. Cornus mas (Cornaceae)
The promising efficacy of C. mas in the modulation of blood glucose, as well as lipid parameters, in alloxan diabetic rats was reported. A plausible mechanism is the inhibition of α-glucosidase by the acylated anthocyanins found richly in the fruit. Oleanolic acid is a triterpenoid considered to be part of the observed effect, acting by reducing glucose absorption and enhancing the release of acetylcholine from nerve terminals at muscarinic M3 receptors in the pancreatic cells, and it also augments insulin release [208].
4.15. Cucumis sativus (Cucurbitaceae)
The oral administration of ethanol extract from fruit or the methanolic fruit pulp extract of C. sativus exhibited significant antidiabetic effects in streptozocin or alloxan diabetic rats [209,210,211].
In Asian traditional medicine, C. sativus seed has been used as a suitable functional food for medical purposes, such as in diabetes and hyperlipidemia, and as a diuretic for the treatment of hypertension, gall bladder stones, constipation, and dyspepsia [212].
The saponin- and steroid-rich fractions of butanolic extract and the hydroalcoholic total extract were investigated for their pharmacologic action in normal and diabetic rats. It was found that none of the fractions were able to cause hypoglycemia in normal groups, but both applied extracts were effective in lowering blood glucose in diabetic animals [212]. It was postulated that C. sativus seed extracts have biguanides-like or euglycemic effects in diabetic condition [212].
4.16. Cucurbita pepo (Cucurbitaceae)
The results obtained indicated that the administration of raw pumpkin fruit powder for four weeks in diabetic rats significantly reduced blood glucose level and had a pancreatic protective effect [213].
4.17. Eryngium caucasicum (Apiaceae)
Fresh leaves are used as a food additive, flavoring and cooked vegetable in the preparation of several local foods [214].
In one study the fresh leaves of E. caucasicum were investigated in streptozocin-nicotinamide diabetic rats. The result showed that E. caucasicum extract significantly decrease fasting blood sugar in high doses (200 and 300 mg/kg), and improved insulin secretion significantly [215].
4.18. Eucalyptus globulus (Myrtaceae)
In streptozocin diabetic mice, the incorporation of E. globulus aqueous leaf extract in the diet (62.5 gm/kg) and drinking water (2.5 g/L) reduced hyperglycemia in a dose-dependent manner, with the partial restoration of pancreatic β-cells. In addition, the hypoglycemic effect was seen in alloxan diabetic rats treated with the plant extract. The antihyperglycemic effect with the improvement in insulin level was reported in streptozocin rats fed with plant leaf powder [129,216,217].
E. globulus possessed an antioxidant property. It reduced oxidative stress mostly by reducing the plasma glucose level in diabetic rats, thereby preventing the excessive production of free radicals through glycation of the proteins. It affected the glucose metabolism in fat and muscle cells, and decreased blood sugar by increasing the glucose influx in the cells [217].
4.19. Falcaria vulgaris (Apiaceae)
Several doses of 200, 600, and 1800 μg/kg of the plant aqueous extract caused a significant decrease in blood glucose similarly to glibenclamide [218]. The antidiabetic effect was assumed to be due to the plant’s ability to improve the diameter of the β-islet of the pancreas and promote insulin secretion [219].
4.20. Ferula assafoetida (Apiaceae)
Asafoetida extract had an antidiabetic effect both on alloxan and streptozocin diabetic rats. The aqueous extract of the plant significantly reduced blood glucose and increased insulin level in alloxan diabetic rats. The possible mechanisms of its effect were potentiating insulin release at lower doses of the extract, preventing intestinal α-glucosidase, and activating glucokinase [220,221,222].
Ferulic acid is the main phytoconstituent in F. assafoetida. It can increase the activity of glucokinase, hinder glucosidase inside the gut, and inhibit oxidation response [220,221]. In addition, umbelliferone had an antihyperlipidemic potential. It decreased plasma glucose, accelerated insulin level, and decreased insulin resistance, all of which were better under diabetic conditions [221]. The alternative factor is flavonol quercetin, which may probably control hyperglycemia and growth hexokinase, reduce glucose-6-phosphatase and fructose-bis phosphatase sports, and possesses α-glucosidase interest [221].
4.21. Galega officinalis (Fabaceae)
The oral administration of G. officinalis leaf powder to diabetic rats decreased the blood glucose levels to normoglycaemic values only at the higher dose of 3 mg/kg [223].
G. officinalis hydroalcoholic extract was able to greatly control the blood sugar in diabetic rats. The reasons for its antihyperglycemic effects were explained by its increasing of the insulin level and its protection of β-cells against oxidative stress due to the presence of alkaloids, flavonoids, glycosides, resin, steroids, tannins, and phenols, which, according to the research conducted, have an antidiabetic trait [224].
4.22. Gundelia tournefortii (Asteraceae)
The aqueous root, shoot, and aerial part extracts all demonstrated a significant hypoglycemic effect in diabetic mice. The possible mechanisms of antidiabetic activity include improving the pancreas, increasing the number and average diameter of the islets of Langerhans, increasing the secretion of insulin from the pancreas, increasing the insulin sensitivity, activating the peroxisome proliferator-activated receptor (PPAR) that regulates the transcription of the gene involved in lipid and glucose homeostasis and metabolism within the cell, decreasing the glucose absorption from the intestine through decreasing the processes of glucose transport or the inhibition of α-amylase, and increasing antioxidant enzymes [225,226].
4.23. Hordeum vulgare (Poaceae)
None of the Barley extracts, hydroalcoholic extracts or protein-enriched fractions were able to cause hypoglycemia in normal rats, even after an extended period of treatment, but were able to reduce blood glucose level in diabetic rats after the subacute phase [227].
A possible mechanism for the antihyperglycemic effect of barely might be the antioxidant capacity of the minerals which are abundant in barley seeds, and the presence of small proteins and essential amino acids and their ability to induce insulin release or amplify its secretion, as induced by glucose [227].
4.24. Juglans regia (Juglandaceae)
The consumption of a hydroalcoholic dose-dependent extract of walnut leaves was reported to decrease the level of blood glucose in diabetic rats at a level comparable to metformin [228].
It was reported that the consumption of walnut oil for six weeks significantly reduced several biochemical parameters, including blood glucose, insulin, HbA1C, total cholesterol and triglyceride, and the size of Langerhans islets, in alloxan diabetic rats [229].
A hydroalcoholic extract of walnut leaves administered to diabetic rats also contributed to a rise in insulin levels through improvements in the Langerhans islets and β-cell restoration [230].
In another study, the hydroalcoholic extract of walnut leaves was reported to cause changes in streptozocin diabetic rat pancreatic tissue, leading to improved Langerhans islets [231].
The antidiabetic activities of walnuts were associated with the release of insulin from the remaining β-cells or replicated β-cells, the rise in insulin sensitivity, the interference with the absorption of dietary carbohydrates in the small intestine, and the peripheral tissue usage of glucose [228,232].
4.25. Mentha spicata (Lamiaceae)
The leaves aqueous extract of Mentha spicata caused a significant reduction in blood glucose level caused by the prevention of free radical formation induced by alloxan. The leaves extract of this plant increased the activity of serum non-enzymatic antioxidants and erythrocyte antioxidant enzymes, and markedly decreased lipid peroxidation in erythrocytes and plasma in diabetic rats [233].
4.26. Nasturtium officinale (Brassicaceae)
It has already been shown that the ethyl acetate extract of aerial parts of N. officinale decreased the blood glucose level in diabetic rats after two months of treatment. On the other hand, the aqueous and methanolic extracts of aerial parts of N. officinale did not affect blood glucose levels after a one-week treatment. The conflicting results could be due to the type of the extract and duration of the treatment [234].
In another study, the hydroalcoholic extract of watercress leaves significantly reduced serum glucose, total cholesterol, and LDL (low density lipoprotein) [234].
A feasible mechanism of the hypoglycemic effect is the stimulation of Langerhans islets, the development of peripheral sensitivity to remnant insulin, and the antioxidant houses of watercress. [234].
4.27. Otostegia persica (Lamiaceae)
Reductions in insulin resistance, glucose and triglycerides concentrations, and increases in β-cells regeneration after treatment with aqueous extract of O. persica, were reported [235].
Several experiments revealed that the methanol and ethanol extracts of O. persica possessed antihyperglycemic action against streptozocin diabetic rats. This was mediated through the stimulation of insulin release, the improvement of the pancreas tissue, the stimulation of glucose utilization in peripheral tissues, the modulation of hormones regulating carbohydrate metabolism, the inhibition of the proximal tubular reabsorption mechanism for glucose in the kidney, and the inhibition of the α-glucosidase enzyme [235].
Stereological methods were used to investigate the effects of O. persica extract on pancreatic β-cells in streptozocin diabetic rats. The study found a significant reduction in pancreatic weight and volume, and pancreatic islet volume, in diabetic and extract-treated groups, although these reductions were significantly less significant in treated groups, indicating the ability of the plant to prevent the remaining β-cells in the pancreatic islets from undergoing some pathologic changes, such as hypertrophy [236,237].
4.28. Pyrus boissieriana (Rosaceae)
Wild pear leaf extract was investigated for antihyperglycaemic, antihyperlipidemic and antioxidant effects in alloxan diabetic rats. Both high and low doses of the Pyrus leaf extract significantly limited the increase in serum glucose concentration and enhanced serum insulin levels compared to the control group [238,239].
The methanol extract of P. boissieriana leaves and all extracts of its stem (n-hexane, ethylacetate, and methanol) inhibited α-glucosidase more effectively than acarbose [163].
Another in vitro experiment showed that P. boissieriana aqueous methanolic leaf extract was able to inhibit both α-amylase and glucosidase significantly. Arbutin, a characteristic of the genus Pyrus and a major compound in the P. boissieriana leaves, also showed the strong inhibition of α-amylase and α-glucosidase, though less potently than the extract [240,241].
4.29. Phoenix dactylifera (Arecaceae)
Both diosmetin 7-O-b-L-arabinofuranosyl apiofuranoside and diosmetin apiofuranoside caused a marked improvement in the blood glucose homeostasis in diabetic rats. These compounds work through the regeneration of the endocrine pancreas, increasing insulin secretion and stimulating the enzyme glycogen synthetase [158].
Several studies determined the inhibiting capabilities of the glucose absorption of different plant parts. The parthenocarpic, pit, fruit, seed, and leaf extracts prevented glucose absorption by α-amylase and α-glucosidase inhibition [134,242,243,244].
The effectiveness of the hydroalcoholic extract of Phoenix dactylifera leaves was evaluated in an animal model of type II diabetes. The postprandial hyperglycemia level was decreased in plasma glucose after 60 min of administration of 20 mg/kg of the extract, which was similar to the acarbose 50 mg/kg effect, while the oral administration of the extract (20 mg/kg) for 28 days in alloxan diabetic mice showed a more significant antidiabetic activity than that of acarbose [134].
The aqueous seed extract in a dosage of 1 gm/day given to streptozocin diabetic rats brought about a significant reduction in blood glucose levels in comparison to control rats, and restored the function of the liver and kidney and the balance of the oxidative stress condition with prolonged treatment [245].
A significant growth improvement, the restoration of hyperlipidemia, the restoration of relative kidney weight, the amelioration of the elevations in serum urea and creatinine levels, the reverse in the elevation of hepatic markers (ALT and AST), the improvement in the hepatic and renal antioxidant enzymes activity, an increase in GSH (glutathinone) levels and a good glycemic control all were biological effects reported in the streptozocin diabetic rats which received an aqueous suspension of P. dactylifera seeds 1 gm/kg [133].
These findings were explained by the extract’s ability to decrease the glycation of enzymes and to scavenge the free radicals. It is worth noting that P. dactylifera seeds have a significant amount of antioxidants, and constitute one of the highest sources of polyphenols, surpassing grapes, flaxseed, and nut seeds [133].
The antihyperglycemic effect of the oral administration of date seed extract combined with insulin was comparable to the seed extract administered alone in streptozocin diabetic rats [132].
Finally, P. dactylifera leaf ethanolic extract was investigated for its antidiabetic effects in alloxan diabetic rats. Their blood glucose level was checked after receiving different doses of 100, 200, and 400 mg/kg. The plasma insulin level was increased, and a significant reduction in blood glucose, serum triacylglycerol, and cholesterol were reported when compared with the control group [246].
4.30. Punica granatum (Punicaceae)
The treatment of streptozocin diabetic guinea pig or rats with fruit extract from pomegranate showed a marked hypoglycemic effect. It improved glucose homeostasis by restoring the level of glycogen, elevated hepatic glucose-6-phosphate dehydrogenase activity, improved total protein level, improved blood urea nitrogen and creatinine levels, reduced serum levels of total cholesterol and triglycerides, and enhanced the antioxidant activity in treated animals [247].
The mechanism for such effects was attributed to the active principles present in these extracts, such as polyphenols and flavonoids [247].
4.31. Rhus coriaria (Anacardiaceae)
Blood glucose and MDA (malondialdehyde) levels of the liver and kidney were lower in diabetic animals treated with aqueous extracts of R. coriaria, a source of antioxidant and free radical-scavenging activities [248].
Dogan et al. reported an antidiabetic property, with beneficial effects on lipid profile, levels of serum enzymes, renal function markers, α-glucosidase activity, and lipid peroxidation for lyophilized hydrophilic extract, obtained from the fruit in streptozocin diabetic rats, all without toxic effects [249].
4.32. Rheum turkestanicum (Polygonaceae)
The administration of the macerated extract significantly reduced the levels of fasting blood glucose and HbA1c, while the decoded extract did not change serum glucose over a three-week period, and these conflicting results can be explained by the differences between the type of extract and the duration of the study [250,251].
Generally, the antidiabetic effects of the Rheum genus may be related to the presence of high amounts of anthraquinones and flavonoids. They work through the regulating genes involved in the control of metabolism, enhancing the effect of insulin on glucose uptake, decreasing glucose absorption from the small intestine, enhancing glucose uptake in tissues, reducing gluconeogenesis, stimulating insulin release from β-cells, and preserving β-cell mass [250].
4.33. Salvia hypoleuca (Lamiaceae)
The effect of S. hypoleuca ethanolic extract was elucidated in normal and alloxan diabetic rats. In diabetic rats, blood glucose levels were significantly reduced after consumption of the extract, and the histological studies showed a restoration effect of the pancreatic islet cells [252].
α- amylase activity was reduced by S. hydrangea and various other species of the Salvia genus [253].
4.34. Salvia officinalis (Lamiaceae)
The methanolic extract of S. officinalis decreased the blood glucose level in alloxan diabetic rats in a dose-dependent manner, similarly to acarbose [166].
The administration of S. officinalis to diabetic rats lowered glucose, increased insulin level, and decreased lipid parameters [204].
The essential oil from sage increased hepatocyte sensitivity to insulin and inhibited gluconeogenesis. Rosmarinic acid and other phenolic components of the plant inhibited α-glucosidase. Altogether, sage may act by similar hypoglycemic mechanisms to metformin and acarbose [166].
4.35. Securigera securidaca (Fabaceae)
The oral administration of seed suspension significantly reduced elevated serum glucose concentration and increased antioxidant power in alloxan diabetic rats [254].
The capability of lowering serum glucose and lipid profile level using different organic solvents, carbon tetrachloride, 70% ethanol–water, and dichloromethane were investigated in an animal model [255].
Carbon tetrachloride solvent had the best and most significant activity for reducing glucose and lipid levels in comparison to other solvents, probably because of the higher content of sterols and fatty acids [255].
The hypoglycemic action of S. securidaca extract may be due to either enhanced insulin secretion, the inhibition of gluconeogenesis and direct stimulation of glycolysis, the reduction in serum glucagon level, or an extra-hepatic effect [176,255].
The hypoglycemic activity of methanol and chloroform fractions of S. securidaca seeds were comparable to glibenclamide. The higher doses of both extracts showed equal hypoglycemic responses to insulin. In addition, three cardiac glycosides were isolated as active constituents responsible for its hypoglycemic activity, and were found to act via an increase in insulin levels in a diabetic mouse model [256].
4.36. Solanum nigrum (Solanaceae)
The antidiabetic activity was reported for extracts of different parts of S. nigrum [257]. The administration of 1 g/L of S. nigrum fruit extract with the drinking water of diabetic animals decreased blood glucose levels. It was suggested that the plant can repair pancreatic β-cells, and enhance insulin secretion or increase glucose transporter translocation in the cell membrane, which then decreases blood glucose levels [257].
4.37. Teucrium polium (Lamiaceae)
The aqueous extract of T. polium showed a marked antihyperglycemic effect in diabetic rats. Previously, several studies reported hypoglycemic activity using different plant extracts, parts, and doses, and the ability to produce a dose-dependent stimulation of basal insulin release [258].
The plant had the ability to protect and in part restore the secretory function of β-cells in pancreatic tissue. Some flavonoids of the plant could inhibit hepatic gluconeogenesis, could exert an insulin-mimetic effect, and can attenuate oxidative stress and lipid peroxidation [259].
The added T. polium extract on the pancreatic MIN6 cell line caused changes in the β-cell cytosolic calcium ions concentration, triggered by the initiation of insulin release. Altogether, T. polium was thought of as a potential candidate with diabetic pharmacological modulation qualities [260].
4.38. Trigonella foenum-graecum (Fabaceae)
The antidiabetic activity of fenugreek has been studied the most, and has also been utilized by diabetic patients [261].
The daily oral administration of Trigonella seed-derived extract for type-2 diabetic rats decreased serum glucose, increased liver glycogen content, and enhanced total antioxidant status. In addition, in cultured 3T3-L1 adipocytes, glucose transport and insulin action were increased by Trigonella [262].
Fenugreek seed-derived polyphenols were observed to cause insulin-sensitizing actions, and were found to be comparable to metformin in the rat model [263]. A significant decrease in plasma glucose and insulin levels was seen in animals receiving a fructose-rich diet and aqueous extract of Trigonella [264].
Taking all this together, these studies suggest that the antidiabetic effect of fenugreek is mediated through the inhibition of carbohydrate digestion and the absorption and enhancement of peripheral insulin action [261].
4.39. Vaccinium arctostaphylos (Ericaceae)
The extract obtained from V. arctostaphylos berries produced a dose-dependent reduction in the α-amylase activity, and the compound responsible was Malvidin3-O-β-glucoside [265].
A dose-dependent antihyperglycemic effect of V. arctostaphylos fruit ethanolic extract was reported in alloxan diabetic rats. The effect was observed upon long-term treatment, and it was comparable to that of metformin. Besides that, the extract can improve the antioxidant and lipid profiles [266].
4.40. Vitex agnus-castus (Lamiaceae)
The hypoglycemic activity of V. agnus-castus was confirmed in streptozocin diabetic rats. The hypoglycemic and pancreatic protective effects were observed in aging animal models after treatment with fruit extract. Aging, insulin resistance, and β-cells destruction were all improved after treatment with the extract [267]. Glycation occurs due to high blood glucose levels for various structural and functional proteins. The fruit extract of V. agnus-castus showed the strongest antiglycation inhibitory activity, which may lead to a protective effect [268].
4.41. Urtica dioica (Utricaceae)
The antidiabetic activity of U. dioica extract was attributed to the insulin secretagogues effect and α-amylase and/or α-glucosidase inhibitory activity [269,270]. The hydro-distillate of the plant reduced fasting blood glucose and recovered the insulin levels in streptozocin diabetic rats—an effect not seen using glibenclamide. This effect can be attributed to the recovery of the pancreatic damage [269,270].
Ranjbari et al. reported a significant improvement in diabetic markers, increased insulin sensitivity, decreased insulin resistance, and improved the function of β-cells with the administration of U. dioica aqueous extract and swimming activity in diabetic rats [271]. Exposing the L6-GLUT4myc cell line to 125 and 250 μg/mL of nettle leaf and stem extracts doubled glucose transporter translocation to the plasma membrane, and increased the insulin-stimulated state 1.6-fold [272].
4.42. Zataria multiflora (Lamiaceae)
The essential oil of Z. multiflora reduced plasma glucose levels in experimental rats [273]. Antioxidant therapy is considered a major strategy for diabetes treatment. Z. moltiflora is an antioxidant source, with increased insulin level and reduced plasma glucose level. Recently, α-amylase inhibitory activity was reported for the plant [273,274].
4.43. Ziziphus vulgaris (Rhamnaceae)
The water extract of Z. vulgaris significantly decreased fasting blood glucose, cholesterol, and triglyceride levels after 14 days of treatment. The levels of HDL-cholesterol and insulin, and the activities of liver enzymes were not changed significantly in the extract-supplemented group compared to the control group [275].
Both the extract of Z. spina-christi leaves and its major saponin glycoside, christinin-A, improved the oral glucose tolerance and potentiated glucose-induced insulin release [276] (Table 8, Table 9 and Table 10).
Table 8.
Ethnobotany of plants form Iran.
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Allium ampeloprasum | Tarreh (Iran) Kurrat (Eygept) |
Liliaceae | Asthma, antiseptic, diuretic, goat, vasodilatory, expectorant, constipation, hemoptysis, obesity, hemorrhoids, headache and as a diuretic, emmenagoug | Leaf, bulb | [182,183,277,278] |
| Allium sativum | Som, sir | Liliaceae | Antifungal, antiprotozoal, anthelminthic, antiviral, disinfectant, antitumor, gastric hepatic disorders, diabetes, hypertension, hypercholesterolemia, immunodeficiency syndromes | Bulb | [279,280,281] |
| Amygdalus lycioides | BadamTalkhkuhi | Rosacese | Antimicrobial, anti-inflammatory, diabetes | Aerial parts, root | [188] |
| Amygdalus scoparia | Badam-Koohi-Arzhan | Rosaceae | Appetizer, cardiovascular and respiratory diseases, headache, rheumatism, wound healing | Leaf | [281] |
| Arctium lappa | Baba adam | Asteraceae | Diabetes | Root, leaf | [190] |
| Berberis integerrima | Zarch | Berberidaceae | Abdominal ache, depurative, diabetes, hypertension | Fruit, root, leaf, stem | [176,282,283] |
| Brassica napus | Colza | Brassicaceae | anti-goat, anti-inflammatory, anti-scurvy, diuretic, bladder and hepatic and kidney colic | Root, seed | [284] |
| Brassica rapa | Kolza | Brassicaceae | Diabetes | Root, seed, leaf | [285] |
| Capparis spinosa | Shomisheytoni | Capparaceae | Diabetes, diuretic, gout arthritis, liver disorders, neurological conditions, rheumatoid, paralysis | Fruit, leaf | [283,286] |
| Centaurea bruguierana | Baad-Avard | Asteraceae | Hypoglycemic | Aerial fruiting parts | [201] |
| Cichorium intybus | Chicory or Kasni | Asteraceae | Antipyretic, depurative, diabetes, choleretic, eupeptic, hypotension, laxative, stomachic, tonic | Whole plant | [202,203] |
| Citrullus colocynthis | Hanzal | Cucurbitaceae | Abortifacient, antiepileptic, analgesic, anti-inflammatory, diabetes, hair growth-promoting | Seeds, root, pulp, fruits | [279,287] |
| Cornus mas | Not mentioned | Cornaceae | Anemia, chickenpox, diarrhea, diabetes, digestion problems, hepatitis A, heal wounds inflammatory bowel disease, fever, kidney stones, malaria, measles, pyelonephritis, rickets, sore throat, sunstroke, urinary tract infections, ulcer | Leaf, flowers, fruits | [208,288] |
| Cucumis sativus | Tokhm-e-khiyar | Cucurbitaceae | Anti-fever, demulcent, diabetes | Seed | [212] |
| Cucurbita pepo | Kadohalvai | Cucurbitaceae | Diabetes | Seed, fruit | [285] |
| Eryngium caucasicum | Aweiyeh | Apiaceae | Flavoring | Leaf | [289] |
| Eucalyptus globules | Okaliptus | Myrtaceae | Diabetes | Leaf | [290] |
| Falcaria vulgaris | Paghazou | Apiaceae | Diabetes, gastric and duodenal ulcers, wound healing | Stem, leaf | [291] |
| Ferula assafoetida | “Anghouzeh”, “Khorakoma” “Anguzakoma” | Apiaceae | Asthma, epilepsy, flatulence, influenza, intestinal parasites, stomach ache, weak digestion | Oleo-gum resin | [221] |
| Galega officinalis | Galega | Fabaceae | Diabetes | Flower | [290] |
| Gundelia tournefortii | Kangar | Asteraceae | Antiparasite, diabetes | Leaf, root | [281,292] |
| Heracleum persicum | Glopar | Apiaceae | Carminative | Fruit | [293] |
| Hordeum vulgare | Jo dosar | Poaceae | Diabetes | Seed, bran | [285] |
| Juglan regia | Gerdoo | Juglandaceae | Antidiarrhea, antiparasitic, blood purifier, diabetes, hemorroides, venous insufficiency | Leaf | [228,281] |
| Mentha spicata | Nana, pooneh | Lamiaceae | Antispasmodic, carminative, diabetes, digestive, stomach pain-relieving agent, | Aerial parts, leaves, essence | [233] |
| Nasturtium officinale | Bakalu | Brassicaceae | Jaundice in children, abortion |
Aerial parts, leaf | [292] |
| Otostegia persica | ShekarShafa | Lamiaceae | Antipyretic, antihyperlipidemia, analgesic, arthritis, cardiac distress, carminative, cough, diabetes, headache, laxative, parasite repellent, reducing palpitation, regulating blood pressure, rheumatoid toothache, stomachache, morphine withdrawal | Aerial Parts | [285,294] |
| Phoenix dactylifera | Khorma, Tarooneh | Arecaceae | Aphthous, antidepressant, bladder and nerve tonic, diabetes, pertussis, rheumatoid arthritis sedative, tranquilizer, wound healer | Spathe, pollen, leaf | [145,295,296,297] |
| Pistacia atlantica | Zhevi | Anacardiaceae | Diabetic ulcers, hyperglycemia | Juice | [283] |
| Punica granatum | Anar | Punicaceae | Astringent, burns, diarrhea, hematopoiesis, gastrointestinal disorders inflammation, pain, oral aphthous, sore throat | Fruit | [298,299] |
| Pyrus boissieriana | Not mentioned | Rosaceae | Anticramp, antihypertensive, food flavoring, anti-infection, narcotic, | Not mentioned | [163] |
| Rheum turkestanicum | Eshghan | Polygonaceae | Depurative, diabetes, hypertension jaundice | Root | [300] |
| Rhus coriaria | Sumac | Anacardiaceae | Adjustment blood lipid, diabetes, diarrhea, dysentery, hemorrhoids, gout, reduction of uric acid, wound healing table spice | Not mentioned | [248,249] |
| Salvia hydrangea | Gol-e Arooneh | Anti-inflammatory, antispasmodic, analgesic, cold | Leaf | [253] | |
| Salvia hypoleuca | Lamiaceae | diabetes, hemorrhoids, insecticide, purgative | [301] | ||
| Salvia officinalis | Maryam | Lamiaceae | Antiseptic diabetes, diuretic, dyspepsia, emmenagogue, fever | Leaf, flower | [302] |
| Securigera securidaca | GandehTalkheh | Fabaceae | Diabetes, hypertension, hyperlipidemia | Seed | [256] |
| Solanum nigrum | Hava | Solonaceae | Antibacterial, anticonvulsant, antipyretic, antioxidant, anti-inflammatory, antitumorigenic, cytotoxicity, diabetes, diuretic, enteric diseases, fever, hepatoprotective, inflammation, mycotic infection, pain, ulcer, sexually transmitted diseases | Aerial parts, fruit | [257,285] |
| Teucrium polium | Kalpooreh | Lamiaceae | Abdominal pain, cold, diabetes, indigestion, urogenital diseases | Aerial parts | [259] |
| Trigonella foenum-graecum | Shanbalileh | Fabaceae | Backache, bladder cooling reflex, cold, cough, diabetes, emollient, hepatitis, gastrointestinal disorders, loss of appetite, mouth odor, splenomegaly, undesired odor of body and sweat, skin disease, red spot of eye, tonic | Leaf, seed, aerial part | [285,303] |
| Urtica dioica | Gazane, chitchitiodghin, Gazgazuk, Aragh | Urticaceae | Allergic rhinitis, diabetes, digestive, diuretic, hypertension, galactogogue, inflammation, prostatic hyperplasia, rheumatoid arthritis, tonic | Root, aerial parts, leaf | [268,284] |
| Vaccinium arctostaphylos | Qaraqat, Cyah-gileh | Ericaceae | Diabetes, hypertension | Berries | [265] |
| Zataria multiflora | Avishan | Lamiaceae | Anesthetic, antinociceptive, antispasmodic, flavoring-preserving foods/drinks, irritable bowel syndrome, respiratory tract infections | Not mentioned | [304] |
| Zizyphus spina-christi | Sedr | Rhamnaceae | Antiseptic, antifungal, anti-inflammatory, bronchitis, cough, dysentery, nausea, sedative, skin diseases, wound healing, tuberculosis, vomiting, abdominal pains associated with pregnancy | Leaf, fruit, seed | [305] |
Table 9.
Medicinal plants of Iran constituents, use.
| Name | Chemical Constituents | Biological and Pharmacological Activities | Reference |
|---|---|---|---|
| Allium ampeloprasum | Cinnamic acid derivatives, nitrates, flavonoids, polysaccharides, glucosinolates, organosulphur compounds | Antibacterial, antioxidant, antifungal, protect skin against damage due to pathogenic agents, alleviate gastrointestinal diseases, inflammatory, hepatotoxicity | [183,277,306] |
| Allium ascalonicum | Volatile sulfur compounds, saponins, flavonoids, furostanol saponin | Antibacterial, anti-fungal, antihelicobacter pylori, beneficial hematological influences, antioxidant, peroxynitrite-scavenging capacity | [307,308] |
| Allium sativum | Organo-sulphur compounds, volatile sulphur compounds (diallyl sulphide), polyphenols | Hypoglycemic, cardiac diseases, antiseptic, toothache, antihyperlipidemia, anthelmintic, antihypertensive | [166,168,309] |
| Amygdalus lycioides | Alkaloids, amygdalin, flavonoid, phenolic compound, terpenoids, | Antioxidant, anticancer, anti-inflammatory, antiaging, antimicrobial, decrease the risk of colonic cancer, increase HDL cholesterol, reduce LDL cholesterol, cardioprotective | [189,310] |
| Amygdalus scoparia | Amygdalin, flavonoids, amino acids, linoleic acid | Antioxidant | [311] |
| Arctium lappa | Flavonoids, lignans, phenols, saponins, tannin | Anti-inflammatory, hepatoprotective, free radical scavenging activities | [190] |
| Berberis integerrima | Anthocyanins, alkaloids, berberine | Anticonvulsant, bleeding, diarrhea, fever, gastrointestinal disease, hepatitis, malaria, swollen gums teeth, sore throat, bile inflammation, reducing blood cholesterol | [191,312] |
| Brassica napus | Anthocyanin, cinnamic acid hydroxyl derivatives, flavonoids, erucic acid | Antioxidant | [194,284] |
| Brassica rapa | Flavonoids, phenylpropanoid derivatives, indole alkaloids, sterol glucosides, glucosinolates and isothiocyanate | Analgesic, anticancer, anti-inflammatory, antimicrobial, antioxidant, cardiovascular and hypolipidemic effect, diabetes, hepatoprotective, metabolic syndrome neuroprotective, obesity | [195,313] |
| Capparis spinosa | alkaloids, lipids, polyphenols, flavonoids, Tocopherols, carotenoids, glycosides, capparic acids, tannins |
Antioxidant, antimicrobial, anticancer, antiallergic, hepatoprotective effects | [199,286] |
| Centaurea bruguierana | Sesquiterpene lactone, flavonoid (kaempferol, rutin, quercetin) | Antiplasmodial, antipeptic ulcer | [201] |
| Cichorium intybus | Aliphatic compounds, terpenoids, saccharides, methoxycoumarin cichorine, flavonoids, essential oils and anthocyanins | Antimicrobial, anthelmintic, antimalarial, diabetes, hepatoprotective, gastroprotective, anti-inflammatory, analgesic, antioxidant, tumor inhibitory, antiallergic | [202] |
| Citrullus colocynthis | a bitter compound (Cucurbitacins), colocynthein, colocynthetin, pectin, gum, flavonoids, alkaloid (choline), volatile terpenoids, fixed oil albuminoids, tocopherols and carotenes | Antidiabetic, hypolipidemic, antimicrobial, anti-inflammatory, antioxidant, cytotoxic, insecticidal, antiallergic | [206,287] |
| Cornus mas | Sugar, organic acids, tannins, anthocyanins, phenolic acid, tannin, vitamin C, flavonoid, iridoids, terpene (mono, tri), Carotenoids | Antimicrobial, antidiabetic, antiobesity, hypolipidemic and anti-atherosclerotic, cytotoxicity, hepatoprotective, renalprotective, neuroprotective, anti-inflammatory, antioxidant, antiplatelet, cardioprotective, antiglaucomic, reproductive organ -protective, radioprotective, aldose redustase inhibitory |
[231,288,314] |
| Cucurbita pepo | Tetra cyclic triterpens, saponins, proteins, fibers, polysaccharides, miberal, carotenoid, tocopherol | Antioxidant, lipid-lowering, hepatoprotective, anticarcinogenic, antimicrobial, antidiabetic | [213] |
| Cucumis sativus | Flavonoids, saponin, tannin, steroid | Antimicrobial, antitumor, antacid, carminative, wound healing, against ulcerative colitis, skin irritation, hypoglycemic and hypolipidemic | [315] |
| Eucalyptus globulus | Euglobals, essential oils, hydrocarbons | Antidiabetic, anti-bacterial, antiplaque, antitumor, antiviral, antifungal, antihistaminic, anti-inflammatory, antioxidant, antimalarial |
[217] |
| Falcaria vulgaris | Volatile oil, phenolics, flavonoid, | Anti-inflammatory, antioxidant, antibacterial, antifungal, antiviral, and bleeding inhibitor activities | [316,317] |
| Ferula assafoetida | Coumarine, flavonoids, gum, phenolic acids terpene, volatile oil | Antioxidant, antiviral, antifungal, cancer chemopreventive, antidiabetic, antispasmodic, hypotensive, molluscicide | [220,221] |
| Galega officinalis | Alkaloid, flavonoid | Antioxidant | [318] |
| Gundelia tournefortii | Coumarin, terpene, sterol, essential oil, phenolic compounds, | Antibacterial, anti-inflammatory, hypolipemic activity, hepatoprotective, antiplatelet, antioxidant | [319] |
| Heracleum persicum | Alkaloids, flavonoids, furanocoumarin, terpenoids; triterpenes, volatile substances | Anti-inflammatory, immunomodulatory, growth enhancer, antioxidant, anticonvulsant, analgesic, hypocholesterolaemic agent | [320,321] |
| Hordeum vulgare | Soluable fiber components especially β-glucans | Anti-inflammatory, antilactagogue, antimutagenic, antiviral, astringent, antioxidant, antiprotozoal, aphrodisiac, demulcent, digestive, diuretic, expectorant, febrifuge, hypocholesterolemic, refrigerant, sedative, stomachic, tonic, poultice for burns and wounds | [322] |
| Juglans regia | Flavonoids, phenolics, tannins, flavonoids, phytosterols, tocopherol | Antioxidant, antidiabetic, antihypertensive, antimicrobial, anticancer, liver and kidney protection, lipid-lowering effect | [228] |
| Mentha spicata | Flavonoids, terpenoids, monoterpenes phenols | Antimicrobial, antispasmodic, antiplatelet, insecticidal, neutraceutical and cosmetic industries | [233] |
| Nasturtium officinale | carotenoids, polyphenols, vitamin C, vitamin A and α-tocopherol | Antimicrobial, antioxidant, antiestrogenic, anticarcinogenic, for the prevention of cancer | [234] |
| Otostegia persica | Alkaloids, essential oil, flavonoids, tannin, terpenes, mono, di and sesquiterpene, steroids | Antimicrobial, antioxidant, antidiabetic, antiglycation, anti-aphids, hepatoprotective | [235,294] |
| Olea europaea | Lignan like compounds, lignan glycoside, coumarin, flavonoid, oleuropein, phenolics, hydroxytyrosol derivatives, secoiridoid, secoiridoid glycosides, triterpene | Antidiabetic, anticancer, antimicrobial, antioxidant, antihypertensive, enzyme inhibitory activities, anti-inflammatory, antinociceptive activities, gastroprotective, neuroprotective | [323] |
| Phoenix dactylifera | Alkaloids, anthocyanins, carotenoids, flavonoids, phenolics, procyanidins, sterols, vitamins, tannins. | Anticancer, antioxidant, antimutagenic, antihemolytic, antiviral, antifungal, anti-inflammatory, hepatoprotective, gonadotropic activity immunostimulant, nephroprotective, | [145] |
| Punica granatum | Alkaloid, anthocyanin, catechin, ellagitannins, flavonoid, sterol, tannins | anticancer, antidiabetic, anti-inflammatory, antimicrobial, healing activity | [324] |
| Pyrus boissieriana | Arbutin, glycosides, flavonoids, phenols | Antioxidant, antihyperlipidemic, bactericidal and antifungal effects, diabetes | [163,240] |
| Rheum turkestanicum | Anthraquinone, flavonoid, phytosterol, phenolic glycosides | Anticancer, cardioprotective, diabetes, nephroprotective, hepatoprotective, neuroprotective | [250] |
| Rhus coriaria | tannins, anthocyanins, various organic acids such as malic and citric acids, fatty acids, vitamins, flavonoids and terpenoids, essential oil | Antimicrobial, antifungal, antiviral, antioxidant, anti-inflammatory, hepatoprotective, xanthine oxidase inhibition, hypoglycemic, cardiovascular protective activities | [248] |
| Salvia hydrangea | Flavonoids, phenolic acid, terpenoids | Antioxidant | [253] |
| Salvia hypoleuca | Essential oil, lactones, isomeric epoxides, monolactone and hypoleuenoic acid, sterols, terpenes, sesqui, di and triterpenes | Antioxidant | [325,326] |
| Salvia officinalis | Flavonoids, carnosic acid, rosmarinic acid | Antioxidant | [302] |
| Securigera securidaca | Cardenolides, coumarins, dihydrobenzofuran derivatives, flavonoids, steroidal and pentacyclic triterpenoid-type saponins | Antiulcerogenic, antiepileptic, chronotropic, diuretic, hypokalemic | [136,256] |
| Solanum nigrum | Catechin, caffeic acid, epicatechin, flavonoids, glycoalkaloids, glycoproteins, polysaccharides, polyphenolic compounds, protocatechuic acid | Antimicrobial, anti-HCV, anti-ulcer, analgesic, anti-inflammatory, antidiarrheal, anticancer, antiseizure, cardioprotective, cytotoxic activity diabetes, immunostimulant, hepatoprotective, larvicidal activity | [257] |
| Teucrium polium | Terpenoids, mono, di and sesquiterpene, flavonoids, neoclerodane, polyphenols | Antioxidant, antimutagenic, anticancer, anti-inflammatory, antinociceptive, antispasmodic, antiulcer, antimicrobial, diabetes, hepatoprotective, hypolipidemic | [258,259] |
| Trigonella foenum-graecum | Flavonoids, alkaloids, coumarins, vitamins, steroidal saponins | Anti-inflammatory, antilipidemic, antioxidant, antimicrobial, antiulcer, anticarcinogenic, carminative, diabetes, hypocholesterolemic, hepatoprotective, galactogogue | [261,285,327] |
| Vaccinium arctostaphylos | Anthocyanins, flavonoids, phenolic acids | Antioxidant, antimicrobial | [265,328] |
| Urtica dioica | Alkaloids, agglutinin, lecithin, flavonoid, phenolic acids, stigmasterol, terpenes, coproporphyrin, lignan, and violaxanthin, coxamarins | Antiproliferative, antioxidant, antidandruff, anti-inflammatory, antimicrobial, cancer, coronary heart disease, diabetes, joint pain reduction, urinary tract infection, psychotic disorders, viral and parasitic diseases | [271] |
| Zataria multiflora | Volatile oil | Antioxidant, antinociceptive, anti-inflammatory, antibacterial, antiviral, antifungal, immunostimulant, pain-relieving | [273,329] |
| Zizyphus spina-christi | Flavonoid, isoquinoline alkaloid, cyclopeptide, saponin, triterpenes | Analgesic effect, antioxidant, antidiabetic, antifungal, antibacterial, antinociceptive | [275,305] |
Table 10.
Plants with antidiabetic potential from Iran.
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref. |
|---|---|---|---|---|---|---|
| Allium ampeloprasum | Bulb | Ethanol 70%, maceration | Alloxan diabetic rats | 400, 800 mg/kg, 8 weeks |
Prevent diabetes associated complications; decreased the levels of serum lipids and MDA, significantly increased the activity of antioxidant enzymes | [183] |
| Allium ascalonicum | Bulb | Methanol 80%, soxhlet | Alloxan diabetic rats | 250, 500 mg/kg, 3 weeks |
Elevated expression of both insulin and glucose transporters transcripts | [166] |
| Allium sativum | Bulb | Methanol 80%, soxhlet | Alloxan diabetic rats | 250, 500 mg/kg, 3 weeks |
Elevated expression of both insulin and glucose transporters transcripts | [166] |
| Arctium lappa | Root | Methanol 40%, maceration | nicotinamide-Streptozocin diabetic rats | 200, 300 mg/kg, 28 days |
Decreased level of triglyceride, vLDL, and alkaline phosphatase | [190] |
| Amygdalus lycioides | Spach branches | Methanol 50%, maceration | Streptozocin diabetic rat | 125, 250, 500, 1000 mg/kg, 2 weeks | Decreased total cholesterol, LDL, TG, Cr, and alkaline phosphatase levels, increased ALT, ASTT, total number and numerical density of β-cells increased | [188] |
| Amygdalus scoparia | Fruit | Hexane, maceration | Streptozocin diabetic rat | 1 mL/kg 15 days |
Improve dregeneration of B cells | [189] |
| Leaf | Aqueous, hydroalcoholic solvent | Streptozocin diabetic rats | 30, 60, 120 mg/kg, 3 days | Increase in the serum insulin level | [330] | |
| Avicennia marina | Leaf | Water, soxhlet | Streptozocin diabetic rats | 100 and 200 mg/kg, i.p., one month, alternate day | Decreased the serum levels of the liver enzymes and tissue level of MDA, and the activity of the liver tissue’s antioxidant enzymes was increased | [32] |
| Berberis integerrima | Root | Water, maceration | Streptozocin diabetic rat | 250 and 500 mg/kg, 6 weeks | Significant decrease in TG, cholesterol, LDL, ALT, AST, ALP, total bilirubin, Cr and urea, increase in HDL-cholesterol and total protein | [191] |
| Fruit | Water, maceration | Streptozocin diabetic rats | 250 and 500 mg/kg, 6 weeks |
Extract did not possess the hypoglycemic and hypolipidemic activity. | [192] | |
| Fruit | Anthocyanin fraction | Streptozocin diabetic rats | 200, 400 and 1000 mg/kg | Significantly increased liver glycogen and body weight | [193] | |
| Brassica napus | Juice | Water, decoction | Alloxan diabetic rats | 4 weeks | Decreased TG, cholesterol and LDL | [194] |
| Brassica rapa | Leaf | Water, maceration | Alloxan diabetic rats | 200, 400 mg/kg | Decreased ALT, cholesterol, LDL, HDL, increased TG, AST | [195] |
| Capparis spinosa | Fruit | Ethanol 80%, soxhlet | Alloxan diabetic rats | 300 mg/kg, 12 days | There was no evidence of regeneration in the liver of diabetic rats, brings the blood glucose significantly toward normal values from day 2 onwards | [198] |
| Fruit | Ethanol 70%, maceration, soxhlet |
Streptozocin diabetic rats | 5 mg/kg | Dose-dependent decrease in blood sugar, decrease in triglycerides | [197] | |
| Root | Ethanol 70%, maceration | Streptozocin diabetic rats | 0.2, 0.4 g/kg | Decreased LDL, AL, ALP, insulin levels did not increase, increased HDL | [331] | |
| Fruit | Water, decoction | Streptozocin diabetic rats | 20 mg/kg, 28 days | No significant influence on the insulin level, decreased blood TG, cholesterol content, reduced the mRNA expression, enzyme activities of glucose-6- phosphatase and phosphoenolpyruvate carboxykinase in liver | [200] | |
| Centaurea bruguierana | Aerial fruiting parts | Water, dichloromethane, ethylacetate, methanol, percolation | Streptozocin, alloxan diabetic rats | 200, 400 mg/kg | Aqueous extract showed best effect | [201] |
| Cichorium intybus | Seed | Water, maceration | Streptozocin, Streptozocin and niacinamide diabetic rats | ------- | Normalization of blood ALT, TG, total cholesterol, HB1C | [203] |
| Citrullus colocynthis | Seed | Ethanol 80%, maceration | Alloxan diabetic rat | 300 mg/kg, 12 days | Enhanced regeneration of B-cells, increased size of pancreatic islet, improvement of hepatic tissue | [207] |
| Cornus mas | Fruit | Ethanol 70%, maceration | Alloxan diabetic rat | 2 gm/kg, 1 month | Less severe hepatic portal inflammation, antidyslipidemia effects | [208] |
| Cucurbita pepo | Fruit | Powder | Alloxan diabetic rat | 1, 2 gm/kg 4 weeks |
Reduced cholesterol, TG, LDL and CRP levels | [213] |
| Cucumis sativus | Seed | Ethanol 75%, percolation buthanol, maceration | Normal and streptozocin diabetic rat | 0.2, 0.4, 0.8 g/kg, 9 days |
The extracts were not effective in reducing blood glucose levels in normal and diabetic rats | [212] |
| Eucalyptus globulus | Leaf | Water, decoction | Streptozocin diabetic mice | 20, 62.5 g/kg eucalyptus in the diet, and 2.5 g/L extract in drinking water, 4 weeks |
Dose-dependent amelioration of diabetic states by partial restoration of pancreatic β-cells | [216] |
| Leaf | Water, hot maceration | Streptozocin diabetic rats | 2.5 mg/mL, 4 weeks | Increase ALT, AST and ALP activity | [332] | |
| Falcaria vulgaris | Aerial parts | Water, maceration | Streptozocin diabetic rat | 200, 600, 1800 μg/kg | Hematoprotective and nephroprotective activity | [218] |
| Ferula assa-foetida | Oleo-gum resin | Ethanol 70%, maceration | Streptozocin diabetic rat | 150, 250 mg/kg, 6 weeks |
Protective effect of liver and kidney damage | [221] |
| Oleo-gum resin | Water, maceration | Streptozocin diabetic rat | 50, 100, 300 mg/kg, 4weeks |
50 mg/kg significantly lowered the serum glucose concentration | [219] | |
| Galega officinalis | Leaf | Powder | Streptozocin diabetic rats | 1.5, 3 g/kg | Body weight-reducing properties | [223] |
| Root | Ethanol, maceration | Streptozocin diabetic rats | 150 mg/kg, o.p, i.p, 6 weeks | Decreased LDL, leptin, increased TG, VLDL, AST | [333] | |
| Not mentioned | Hydroalcoholic solvent | Streptozocin diabetic rats | 50 mg/kg, i.p, 20 days | Decreased urea and creatinine | [224] | |
| Gundelia tournefortii | Aerial parts | Water, maceration | Alloxan diabetic rats | 5, 10, 20, 40 mg/kg, 20 days |
Alleviation of diabetic complications such as nephropathy | [225] |
| Shoots | Water | Streptozocin diabetic rats | 400 mg/kg, 21 days | Recovery of pancreas tissue | [226] | |
| Horddeum vulgare | Seed | Ethanol 75%, percolation and alkaline extract | Streptozocin diabetic rats | 0.1, 0.25, 0.5 g/kg, 11 days | Restored body weight, long term benefits | [227] |
| Not mentioned | Hexane oil extraction | Alloxan diabetic rats | i.p administration of oil, 6 weeks | Reduced insulin, HbA1C, total cholesterol, LDL, VLDL, HDL and TG | [229] | |
| Juglans regia | Leaf | Ethanol 90%, maceration | Streptozocin-nicotinamide diabetic rats | 200 mg/kg, 1 month | Reduced HbA1C, total cholesterol, LDL and TG | [230] |
| Leaf | Methanol 70%, maceration | Streptozocin diabetic rats | 200, 400 mg/kg, 4 weeks | Significant increase in diameter and number of β-cells compared to diabetic control group | [231] | |
| Leaf | Methanol 70%, maceration | Alloxan diabetic rats | 250, 500 mg/kg, 3 weeks | Significant inhibition of α-glucosidase, maltaseand sucrase enzymes | [232] | |
| Mentha spicata | Leaf | Water, soxhlet | Alloxan diabetic rats | 300 mg/kg, 21 days | LD50 ˃ 1500 mg/kg (body weight), decreasedcholesterol | [233] |
| Nasturtium officinale | Aerial parts | Water, maceration | Streptozocin diabetic rats | 100, 200 mg/kg, 4 weeks | Decrease in total cholesterol and LDL | [234] |
| Otostegia persica | Aerial parts | Water, decoction | Streptozocin diabetic rats | 100, 200, 400 mg/kg, 1 month | Reduced TG | [235] |
| Aerial parts | Methanol, soxhlet |
Streptozocin diabetic rats, C187 β-cell line |
200, 300, 400 mg/kg | Decreased MDA and increased GSH levels in the liver | [236] | |
| Otostegia persica | Aerial parts | Ethanol 50%, percolation | Streptozocin diabetic rat | 500 mg/kg | Helped prevent the entering of the remaining β-cells into some pathologic changes, such as hypertrophy | [237] |
| Phoenix dactylifera | Leaf | Ethanol extract, maceration | Alloxan diabetic rat | Extract: 100, 200, 400 mg/kg, 14 days Fractions: 50, 100, 200 mg/kg, 14 days |
Decrease in water intake, serum TG and cholesterol, increase in plasma insulin level | [246] |
| Pistacia atlantica | Fruit | Hexane extract, maceration | Streptozocin diabetic rat | 1 mL/kg, 15 days | Improvedregeneration of β cells | [189] |
| Punica granatum | Fruit | Methanol extract, maceration | Streptozocin diabetic guinea pigs | 500 mg/kg, 4 weeks | Enhanced glutathione content as well as the activity of catalase, decrease in total cholesterol and TG | [247] |
| Pyrus boissieriana | Leaf | Methanol, maceration | Alloxan diabetic rat | 500, 1000 mg/kg, 4 days | Decreased serum TG cholesterol, increased antioxidant status | [239,240] |
| Rheum turkestanicum | Rhizome | Water, decoction | Streptozocin diabetic rats | 200, 400, 600 mg/kg, 3 weeks |
Decreased triglycerides, no hypoglycemic or hepatoprotective effect in diabetic rats | [251] |
| Rhus coriaria | Fruit | Ethanol, maceration | Alloxan diabetic rats | 200, 400 mg/kg, 21 days | Increased serum HDL, superoxide dismutase and catalase activities, reduced LDL, inhibited maltase and sucrase activities | [249] |
| Not mentioned | Water | Alloxan diabetic rats | 50, 100, 250 mg/kg, 28 days | Increase catalase activities in liver and kidney | [248] | |
| Salvia hydrangea | Aerial parts | Ethanol 90%, maceration | Streptozocin diabetic rats | 100, 200 mg/kg, 21 days | Reduce blood fat | [253] |
| Salvia hypoleuca | Whole plant | Ethanol, maceration | Alloxan and normal diabetic rat | 250, 450 mg/kg, 14 days |
Non-significant reductions for the non-diabetic groups that received extracts. | [252] |
| Salvia officinalis | Leaves | Methanol, soxhlet | Alloxan diabetic rat | 250, 500 mg/kg, 21 days | Elevated expression of both Ins and Glut-4 transcripts | [166] |
| Securigera securidaca | Seed | Aqueous suspension | Alloxan diabetic rat | 2, 4 g/kg, 3 days, intra-gastric gavage | Protective effect against oxidative stress | [254] |
| Seed | CCl4, ethanol70%, dichloromethane, maceration | Streptozocin diabetic rat | 200 mg/kg, 16 days | Carbon tetrachloride extract showed the best and most significant hypoglycemic and hypolipidemic activities with a β-cells-protecting effect from high glucose-induced apoptosis, and also increased insulin level and sensitivity | [255] | |
| Seed | Ethanol 70%, maceration | Streptozocin diabetic rat | 100, 200 mg/kg, 4 weeks | Decreased serum total cholesterol, LDL, increased HDL | [334] | |
| Seed | Methanol 80%, chloroform fraction, maceration | Streptozocin diabetic rat | Methanol: 10, 400 mg/kg Fraction: 400, 600 mg/kg |
Securigenin glycosides (isolated) reduced blood glucose equivalent to glibenclamide and elevated insulin level to normal |
[256] | |
| Solanum nigrum | Fruit | Water, decoction | Streptozocin diabetic rat | 1 gm/L, 8 weeks | Improved lipid profile, decreased Ca/Mg ratio, decrease vessel atherosclerosis and prevented diabetic vesselcomplications | [306] |
| Teucrium polium | Leaf | Water, decoction | Streptozocin diabetic rats | 100 mg/kg, 3 weeks | No decrease in body weight for diabetic rats | [259] |
| Trigonella foenum-graecum | Whole plant | Water, decoction | High-fructose diet diabetic rat | 10% of extract 8 weeks |
The extract improve insulin resistance | [264] |
| Seed | Methanol, maceration | Streptozocin diabetic guinea pigs | 500 mg/kg, 4 weeks | Enhanced GSH content as well as the activity of catalase, decrease in total cholesterol and TG | [247] | |
| Urtica dioica | Whole plant | Aqueous distillate, distillation | Streptozocin diabetic rat | 12.5 mL/kg 1 month |
Prevents islet atrophy and/or regenerate pancreatic β-cells | [269] |
| Leaves | Ethanol 70%, maceration | Fructose induced diabetes | 50, 100, 200 mg/kg, 2 weeks |
Decreased LDL, leptin, LDL/HDL ratio, FIRI (fasting insulin resistance index), increased serum TG, VLDL, and AST | [335] | |
| Urtica dioica | Whole plant | Water, decoction | High fructose diet diabetic rat | 10% extract, 8 weeks | Urine glucose decreased significantly | [264] |
| Leaves | Water, infusion | Streptozocin diabetic rats, RIN-5F and L6 myotubes cell lines | 6.25 mg/kg, 1.25 g/kg 1 month |
Regeneration and less β cell damage, increased insulin secretion in the RIN-5F cells and glucose uptake in the L6 myotubes cells, lower TG and cholesterol |
[271] | |
| Vaccinium arctostaphylos | Fruit | Ethanol 95%, maceration | Alloxan diabetic rat | 200, 400 mg/kg, 21 days | Decreased total cholesterol and TG | [266] |
| Vitex agnus-castus | Fruit | Ethanol 70%, maceration | D-galactose-induced aging mouse | 500, 600 mg/kg, twice daily, 7 days | Pancreatic protective effects in natural aged and aging model mice | [267] |
| Zataria multiflora | Leaf | Hydrodistillation | Streptozocin diabetic rats | 50 μL/kg 28 days |
Decrease in in plasma ALP, AST, ALT, and significant increase in total protein and insulin | [273] |
| Ziziphus vulgaris | Fruit | Water, maceration | Onreptozocin diabetic rats | 0.25, 0.5, 1, 1.5, 2 g/kg 14 days | Decrease in LDL and TG | [275] |
5. Medicinal Plants with Potential Antidiabetic Activity in Iraq
The prevalence of type 2 diabetes in Iraq reached epidemic levels in 2007. Nowadays, around 1.4 million of Iraqis have diabetes [336,337].
5.1. Bauhinia variegate (Caesalpiniaceae)
The antihyperglycemic activity of the B. variegate was investigated in diabetic mice. The treatment resulted in a significant reduction effect for the blood glucose level compared to glibenclamide. B. variegate is rich in tannins, polyphenols and flavonoids, constituents that are reported to have antidiabetic effects [338].
5.2. Momordica charantia (Cucurbitaceae)
Over 140 studies worldwide investigated different extracts and ingredients of the plant for its antidiabetic activity and its mechanisms of action in both human and animal models [339].
The hypoglycemic effect was thought to be mediated through the stimulation of peripheral and skeletal muscle glucose utilization, the inhibition of intestinal glucose uptake, the inhibition of adipocyte differentiation, the suppression of key gluconeogenic enzymes, and the stimulation of key enzymes of the pentose phosphate pathway, and the preservation of islet β-cells and their functions [339,340].
5.3. Rheum ribes (Polygonaceae)
Previously, the hypoglycemic effect of different R. ribes extracts was reported. The aqueous root extract showed a significant hypoglycemic effect. In vitro, the extract stimulated insulin release from INS-1E cells. The hypoglycemic-active fraction contained anthraquinone glycosides of aloe-emodin, physcion, and chrysophanol derivatives [341].
Additionally, the aqueous root extract of R. ribes showed a hypoglycemic effect in a dose-dependent manner in alloxan diabetic rats [342].
R. ribes evoked an inhibitory effect for both α-amylase and α-glucosidase that showed a striking similarity to acarbose [343]. In another study, a significant increase in the activity of serum amylase in diabetic rats was observed after treatment with R. ribes [344].
The hypoglycemic effect of R. ribes may be due to the presence of insulin-like substances in plants that stimulate the regeneration and reactivation of β-cells to produce more insulin. Flavonoids may lead to the prevention or delaying of the progression of microvascular changes in the islet of Langerhans. However, these improvements did not lead to 100% recovery [344] (Table 11, Table 12 and Table 13).
Table 11.
Medicinal plants of Iraq ethnobotany.
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Bauhinia variegate | Mountain ebony, orchid-tree, poor-man’s orchid, camel’s foot, Napoleon’s hat | Fabaceae | Anthelmintic, astringent, bronchitis, diabetes, diarrhea, laxative, leprosy, piles, tumors, tonic, worm infestations | Stem bark, leaves, buds | [18] |
| Momordica charantia | Kerela | Cucurbitaceae | Anthelmintic, hemmoroid, gout, stomachic | Fruit | [345] |
| Rheum ribes | Rawand, Rewas | Polygonaceae | Anthelmintic, diabetes, diarrhea, expectorant, hypertension, obesity, ulcer | Root | [346] |
Table 12.
Medicinal plants of Iraq constituents, use.
| Scientific Name | Phytochemcal Constituent | Pharmacological Use | Refernce |
|---|---|---|---|
| Bauhinia variegate | Cardiac glycosides, flavonoids, reducing sugars, saponins, steroids terpenoids, tannins | Anticancer, antioxidant, antimicrobial, anti-inflammatory, antiulcer, hepatoprotective, hypolipidemic, immunomodulating, nephroprotective, molluscicidal and wound healing effects | [347] |
| Momordica charantia | Alkaloid, lipid, phenolics, triterpene, saponin, steroid | Diabetes | [339] |
| Rheum ribes | Anthraquinone, flavonoid, phenolic compounds, stilbene, | Antidiabetic, antioxidant | [341,348] |
Table 13.
Plants with antidiabetic potential form Iraq.
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Achillea santolina | Leaf | Water, infusion | Streptozocin diabetic rats | 150, 250 mg/kg, 28 days | Dose-dependent hypoglycemic response | [349] |
| Bauhinia variegate | Leaf | Ethanol 70%, maceration | Dexamethasone diabetic rats | 200 mg/kg, 28 days | Decrease in TC and TG, increase in HDL | [338] |
| Momordica charantia | Seed | Water, maceration | Alloxan diabetic rats | 150 mg/kg, 30 days | Decrease in blood glucose level more than glibenclamide | [340] |
| Rheum ribes | Root | Water, decoction | Alloxan diabetic rats | 200 mg/kg, 30 days | Increased activity of β-cells | [344] |
6. Medicinal Plants with Potential Antidiabetic Activity in Jordan
Over the past few decades, diabetes prevalence has increased to 13.1% according to the WHO.
6.1. Achillea santolina (Asteraceae)
The acute and chronic administration of aqueous extracts of A. santolina resulted in significant and marked reductions in serum glucose level in streptozocin diabetic rats [349].
An acute antihyperglycemic trend was observed for A. santolina bolus in starch-fed rats, explained by the intestinal luminal activation of effective entities. In addition, the treatment of MIN6 cells with the plant extract promoted dose-dependent pancreatic β-cell proliferation and monolayers expansion [343,350].
These effects may partly result from the inhibition of carbohydrates absorption. Moreover, A. santolina influenced insulin receptors or β-cells due to antioxidant activity [349].
6.2. Artemisia herba alba (Asteraceae)
The plant is a popular folk remedy for diabetes. The obtained data of several studies indicated that the aqueous, alcoholic, and organic solvents extracts of several parts of A. herba alba produced significant hypoglycemic effects in diabetic animals. The antidiabetic effect was comparable to that of the usual hypoglycemic drugs repaglinide, insulin, metformin, and glibenclamide [351,352].
It was hypothesized that the hypoglycemic effect is due to thujone, which can increase glucose transporter through the activation of adenosine monophosphate-activated protein kinase [352].
6.3. Artemisia sieberi (Asteracea)
The essential oil obtained from A. sieberi exhibited a significant reduction in blood glucose level in alloxan diabetic rats, which was comparable to metformin. The major constituents of the Artemisia species exert a potent antioxidant activity that provides a protective effect against diabetes [353].
A similar hypoglycemic effect was obtained in alloxan diabetic rabbits after treatment with aqueous extract from A. sieberi. The claimed mechanism could possibly be due to the increased peripheral glucose utilization, and the inhibition of the proximal tubular reabsorption mechanism for glucose in the kidneys [354].
6.4. Arum dioscoridis and palaestinum (Araceae)
A dual α-amylase- and α-glucosidase-inhibitory effect was observed for both Arum species as compared to acarbose. Apigenin, luteolin, and vitexin were identified in both species. All were strongly associated with the starch-digestive enzymes inhibitory effect [355]. The aqueous extract of A. dioscoridis significantly decreased the acute postprandial hyperglycemia induced in overnight fasting rats [355].
6.5. Crataegus aronia (Rosaceae)
The leaf extract of unripe fruits of C. aronia normalized plasma lipid peroxide levels and lowered blood glucose levels in diabetic animals [356]. The aqueous extracts of C. aronia at doses of 100, 200, and 400 mg/kg significantly decreased acute postprandial hyperglycemia and glycemic excursions in normoglycemic rats comparably to acarbose [357].
In addition, the C. aronia aerial parts’ and fruits’ aqueous extracts (0.1–10 mg/mL) gave rise to dual inhibitors of α-amylase and α-glucosidase [357].
A synergistic effect of antidiabetic and antioxidant activity was reported for the C. aronia extract of the whole plant. It was thought that the plant works through the inhibition of hepatic insulin resistance without affecting blood insulin level [155].
6.6. Cichorium pumilum (Asteraceae)
The ethanolic extract of C. pumilum leaves showed a significant antihyperglycemic activity [358].
6.7. Eryngium creticum (Apiaceae)
Leaf decoction was traditionally used as an antidiabetic therapy in Palestine and Jordan [359]. The aqueous extract of aerial parts decreased glucose levels in both diabetic and non-diabetic animals, but the effect was significant for the hyperglycemic rats only [358].
Although an acute antihyperglycemic property was observed after E. creticum bolus treatment in starch-fed rats, the in vitro inhibitory activity did not relate to the in vivo activity [360].
In another study, the MIN6 cell line was treated with an aqueous extract of E. creticum. It significantly potentiated glucose-stimulated insulin secretion, and augmented the β-cell proliferation in a dose-dependent manner (0.01 mg/mL) [350].
6.8. Geranium graveolens (Geraniaceae)
G. graveolens can improve glycemic control via α-amylase and α-glucosidase enzyme inhibition, and this was confirmed through acute and potent antihyperglycemic trends in starch-fed normal rats [360].
After treatment with the extract, the pancreatic mass increased maximally. This effect can provide a promising avenue for β-cell death in diabetes care [361].
Additionally, the administration of essential oil from G. graveolens significantly decreased blood glucose level and restored perturbed antioxidant activity comparably to glibenclamide activity in alloxan diabetic rats [362].
6.9. Phaseolus vulgaris (Fabaceae)
The aqueous seed extract and the alcoholic fresh pod extract of P. vulgaris were active for hypoglycemic potential. The antihyperglycemic activity might involve the inhibition of α-amylase activity and the enhancement of the activity of insulin-producing β-cells [358,363].
6.10. Pistacia atlantica (Anacardiaceae)
The administration of hexane seed extract decreased the blood glucose level to normal in diabetic rats. In addition, the aqueous leaf extract inhibited α-amylase and α-glucosidase enzymes comparably to metformin and glipizide in starch-fed rats [343].
Previously, it was found that P. atlantica can improve glucose homeostasis via insulin secretagogue and glucose absorption restrictive activity [350].
6.11. Tecoma stans (Bignoniaceae)
The ethanolic leaf extract of T. stans showed an antihyperglycemic action in alloxan diabetic rats, and this result was in agreement with previous studies that examined such effects in insulin-sensitive and insulin-resistant mice, cultured human adipocytes, and streptozocin diabetic rats, an effect attributed to a denominated alkaloid tecomine and tecostatine [358].
6.12. Teucrium polium (Lamiaceae)
In 1987 the hypoglycemic activity of T. polium was reported. The aqueous, hydroalcoholic extracts of aerial parts and leaves caused a significant reduction in blood glucose concentration in normoglycemic and hyperglycemic rats [258,358].
It seemed that the T. polium extract had an insulinotropic property, was able to enhance insulin secretion, and was capable of regenerating the islets of Langerhans [258,364].
6.13. Varthemia iphionoides (Asteraceae)
Since 1997, the aqueous extract of V. iphionoides has been reported to exhibit a hypoglycemic activity in both normal and diabetic rats [365].
Recently, a dose-dependent dual α-amylase and α-glucosidase inhibitory activity was demonstrated. In addition, a pancreatic proliferative capacity of V. iphionoides extracts in chronic treatments was identified [361] (Table 14, Table 15 and Table 16).
Table 14.
Ethnobotany of plants from Jordan.
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Achillea santolina | Kaisoom, jeaidatelsabian | Asteraceae | Colic, cold, depurative, diabetes, kidney stones, | Aerial parts | [351,366] |
| Artemisia herba alba | Shaih | Asteraceae | Fever, menstrual and nervous problem | Aerial parts, root | [367] |
| Artemisia sieberi | Shaih | Asteraceae | Antispasmodic, antiarthritis, diabetes, pectoral | Foliage | [368] |
| Arum dioscoridis | Louf | Araceae | Anticancer | Leaf | [369] |
| Arum palaestinum | Louf | Araceae | Anticancer, internal bacterial infections, poisoning, disturbances of the circulatorysystem, cooking | Leaf | [369] |
| Crataegus aronia | Zaeroor | Rosaceae | Cardiovascular diseases, diuretic, hypertension, hyperlipidemia, kidney stone, laxative | Leaf | [370,371] |
| Cichorium pumilum | Hendba | Asteraceae | Antiseptic, antidiabetic, eczema | Flower, root | [372] |
| Eryngium reticum | Gersaana | Apiaceae | Scorpion and snake bite | Root | [371] |
| Geranium graveolens | Utryye | Geraniaceae | Diuretic, diabetes, stomachic | Leaf | [351] |
| Pistacia atlantica | Botom | Anacardiacae | Asthma, cough, diabetes, stomach ache, | Fruit, leaf, resin | [373,374] |
| Rheum ribes | Rabbas | Polygonaceae | Diabetes, hypertension, kidney sand and stones, obesity, | Root | [351] |
| Teucrium polium | Ja’deh | Lamiaceae | Abdominal pain, diabetes, urinary tract infection, | Aerial parts, shoot, leaves | [351] |
| Varthemia iphionoides | Qtteileh | Asteraceae | Abdominal pain, diabetes | Shoot, leaf | [351] |
Table 15.
Medicinal plants of Jordan constituents, use.
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Achillea santolina | Flavonoids, terpenoids, essential oils | Anti-inflammatory, immunomodulatory, antibacterial | [375] |
| Artemisia herba alba | Sesquiterpene lactones, phenolic compounds, essential oil, flavonoid | Antioxidant, antimicrobial, antivenom, antispasmodic, anthelmintic, diabetes, nematicidal, neurological activity | [376] |
| Artemisia sieberi | Essential oil, flavonoid, polyphenolic compounds | Antioxidant | [353] |
| Arum dioscoridis | Flavonoids, phenolic acids | Antioxidant, antimicrobial | [355,369] |
| Arum palaestinum | Pyrrole alkaloid | Antioxidant, anticancer, antidiabetic | [369,377] |
| Cichorium pumilum | Flavonoids, coumarins, caffeic acid derivatives, sesquiterpene lactones | Antitumor | [378] |
| Eryngium reticum | Coumarine, essential oils, terpenes, sesqui and monoterpenes, sitosterols, tannins, resins | Anti-snake and anti-scorpion venom, antibacterial, antifungal, antimalaria, antileshmania, antioxidant, antimutagenic, antihyperglycemic, cytotoxic | [359] |
| Geranium graveolens | Essential oil | Antiemetic activity, antioxidant, fumigant repellent | [374] |
| Phaseolus vulgaris | Alkaloids, anthocyanin, catechin flavonoids, saponins, tannins, terpenoids | Antioxidant, anticancer | [363] |
| Pistacia atlantica | Flavonoids, phenolic compounds, terpenoids, mono and sesquiterpenoids, volatile oil | Antimicrobial, antiviral, antidiabetic, antitumor, anticholinesterase activity, antioxidant | [374] |
| Tecoma stans | Alkaloid, chlorogenic acid | Diabetes, lower cholesterol, | [358,379] |
| Teucrium polium | Essential oil, flavonoid, Iridoids, steroidal compounds | Antioxidant, anti-inflammatory, anticancer antihyperlipidemic, antimicrobial, antimutagenic, antiulcer, antispasmodic, antinociceptive, hepatoprotective, diabetes | [258] |
| Varthemia iphionoides | Sesquiterpene, essential oil Flavonoids | Antiplatelet, antioxidant, antitumor, antibacterial, antifungal | [375] |
Table 16.
Plants with potential antidiabetic activity form Jordan.
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Achillea santolina | Aerial parts | Water, reflux | Starch loaded rats | 125, 500 mg/kg | Enhanced oral glucose tolerance | [343] |
| Aerial parts | Water, reflux | MIN6 β-cell line | 0.05–1 mg/mL | Dose dependent pancreatic β-cell proliferation | [350] | |
| Artemisia herba alba | Aerial parts | Water, maceration | Alloxan diabetic rabbits | 1 day | Significant hypoglycemic effect | [380] |
| Artemisia sieberi | Aerial parts | Essential oil, hydrodistillation | Alloxan diabetic rats | 80 mg/kg, 30 days | LD50 800 mg/kg (body weight) | [353] |
| Arum dioscoridis & Arum palaestinum | Aerial parts | Water, ethanol, reflux | Fasted rats | 125, 250, 500 mg/kg | Concentration-dependent pancreatic lipase inhibition | [355] |
| Crataegus aronia | Flower, leaf | Water, reflux | Highcholesterol diet fed rats | 100, 200, 400 mg/kg, 10 weeks | Potent antiobesity, marked triacylglycerol-reducing efficacy | [357] |
| Cichorium pumilum | Leaf | Ethanol, percolation | Alloxan diabetic rats | 1 gm/kg | Reduced bloodglucose after 3 h of administration | [358] |
| Eryngium creticum | Aerial parts | Water, reflux | MIN6 β-cell line | 0.1, 0.5, 1 mg/mL | Dose-dependent highly significant pancreatic β-cell proliferation | [350] |
| Aerial parts | Water, reflux | Starch treated rats | 125, 250, 500 mg/kg | Lack of effect on enzymatic starch digestion, no overall glycemicexcursion, no improvement in OGTT | [360] | |
| Geranium graveolens | Leaf | Water reflux | Pancreatic β-cells MIN6 | 0.01–1.0 mg/mL | Augmented β-cell mass expansion, dose-dependent dual inhibition of α-amylase and α-glucosidase | [360,361] |
| Phaseolus vulgaris | Fresh pods | Ethanol, percolation | Alloxan diabetic rats | 1 gm/kg | Significantly reduced blood glucose after 3 h of administration | [358] |
| Pistacia atlantica | Aerial parts | Water, reflux | Starch-loaded rat | 125 mg/kg | Hypoglycaemic effect reversed at higher doses for the extract | [343] |
| Aerial parts | Water, reflux | MIN6 β-cell line | 0.01, 0.1, 0.5 mg/mL | Augmented acute β-cell insulin secretory efficacy, preserved β-cell integrity | [350] | |
| Rheum ribes | Root, rhizome | Water, reflux | Pancreatic β-cells MIN6 | 0.01, 0.05, 0.1 and 0.5 mg/mL | Acute insulin secretion, augmented β-cell mass expansion | [260] |
| Sarcopoterium spinosum | Aerial parts | Water, reflux | Pancreatic β-cells MIN6 | 0.01–1.0 mg/mL | Insulinotropic and proliferative effects in the pancreas, dose-dependent dual inhibition of α-amylase and α-glucosidase | [343,361] |
| Tecoma stans | Leaf | Ethanol, percolation | Alloxan diabetic rats | 1gm/kg | Significantly reduced bloodglucose after 3 h of administration | [358] |
| Teucrium polium | Aerial parts | Ethanol, percolation | Alloxan diabetic rats | 1gm/kg | Reduced blood glucose after 3 h of administration | [358] |
| Varthemia iphionoides | Aerial parts | Water, reflux | Pancreatic β-cells MIN6 | 0.01–1.0 mg/mL | Augmented β-cell mass expansion | [361] |
7. Medicinal Plants with Potential Antidiabetic Activity in Lebanon
7.1. Centaurea horrida (Asteraceae)
The root extract of C. horrida showed a significant drop in blood glucose level using several doses; therefore, the dose of 100 mg/kg was the most effective the for acute and sub-acute blood glucose of hyperglycemic mice [381].
The extract might inhibit endogenous glucose production or intestinal glucose absorption [381].
7.2. Hordeum spontaneum (Poaceae)
Barley is one of the most ancient crops cultivated for food. The importance of barley as a nutraceutical ingredient has increased since the high content of β-glucan affects glycemic control [381,382].
Hordeum spontaneum grain ethanolic extract reduced the blood glucose level in hyperglycemic mice, and was more potent than glibenclamide [381].
7.3. Inula viscosa and Inula vulgaris (Asteraceae)
The isolated flavanone 7-O-methylaromadendrin stimulated glucose uptake and improved insulin resistance [383].
Remarkable acute and subacute effects on blood glucose levels were reported for I. viscosa and I. vulgaris alcoholic extracts used in alloxan diabetic rats. The effect of I. viscosa was higher in comparison to I. vulgaris [384].
The diabetic mice treated with 25 mg/kg of each extract exhibited a significant rise in serum catalase activity—an enzyme of the antioxidant defense mechanisms that decreases oxidative stress. It was reported that the long-term treatment of diabetes with the two plant extracts reversed the activities of the free radicals [384].
7.4. Psoralea bituminosa (Fabaceae)
The aqueous extract of P. bituminosa leaves lowered blood glucose levels in streptozocin diabetic rats [385].
The acute effect of ethyl acetate, hexane, and ethanol in alloxan diabetic rats was investigated. At all doses of 25, 50, and 100 mg/kg, a significant decrease in blood glucose level and a significant antioxidant activity was reported [386].
7.5. Salvia libanotica (Lamiaceae)
The Salvia fruticosa leaves extract caused a statistically significant reduction in blood glucose level in alloxan hyperglycemic rabbits [381].
Salvia libanotica fruticosa root extract at several doses showed a significant drop in blood glucose level in hyperglycemic mice. The mechanism of the hypoglycemic activity may be due to the inhibition of the endogenous glucose production, or the inhibition of intestinal glucose absorption [381] (Table 17, Table 18 and Table 19).
Table 17.
Ethnobotany of Lebanon.
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Centaurea horrida | Not mentioned | Asteraceae | Diarrhea, diabetes, hypertension | Not mentioned | [387] |
| Inula viscosa, Inula vulgaris | Tayyoun, ‘Ergel-tayyoun | Asteraceae | Rheumatoisim | Whole plant | [388] |
| Psoralea bituminosa | Homan Homri | Fabaceae | Iintestinal ailments, gastric ulcers | Leaf, fruit | [389] |
| Salvia libanotica | Aiza’an Kassiin ‘Ouaiss’e Maryamiyy’e | Lamiaceae | Asthma, arthritis, antiseptic, aphrodisiac, antimicrobial, carminative, constipation carminative, cough, diabetes, expectorant, influenza, hypertension, gastralgia, hepatitis, febrifuge, nephropathy, rheumatism, spasmolytic, stomachic, stimulating memory | Flower, leaf | [390] |
Table 18.
Medicinal plants of Lebanon reported constituents, use.
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Centaurea horrida | Flavonoids, lactones, phenolic acids | Antioxidant | [387] |
| Inula viscosa, Inula vulgaris | Costic acid, flavonoid, guaianolide, phenolic compounds, terpenoid | Abortifacient, antibacetraial, anti-implantation, antihypertensive, cytotoxic, hypoglycemic | [383] |
| Psoralea bituminosa | Furanocoumarins, isoflavonoid, meroterpenoids, sesquiterpene, volatile oil | Antioxidant, cytotoxicity | [386] |
| Saliva libanotica | Essential oil | Antioxidant activity, anticholinesterase activity, anticancer | [391] |
Table 19.
Plants with antidiabetic potential from Lebanon.
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Centaurea horrida | Root | Ethanol 80%, maceration | Alloxan diabetic rats | 25, 50, 100 mg/kg, 8 days | Improve peripheral nerve function | [381] |
| Hordeum spontaneum | Root | Ethanol 80%, maceration | Alloxan diabetic rats | 25, 50, 100 mg/kg, i.p, 8 days | Improve peripheral nerve function | [381] |
| Inula viscosa, Inula vulgaris | Aerial parts | Ethanol, maceration | Alloxan diabetic rats | 12.5, 25 and 50 mg/kg, 8 days | Long treatment led to reverse free radicals activities | [384] |
| Psoralea bituminosa | Aerial parts | Ethanol (80%), ethyl acetate, hexane, maceration | Alloxan diabetic rats | 25, 50, 100 mg/kg, 8 and 21 days | Significant management of neuropathic pain | [386] |
| Rheum ribes | Rhizome | Water, maceration | Alloxan diabetic rats | 12.5, 25, 50 mg/kg, 8 days | Had a protective effect against diabetes and diabetic neuropathy | [381] |
| Salvia libanotica | Root | Ethanol 80%, maceration | Alloxan diabetic rats | 12.5, 25, 50 mg/kg, 8 days | Improve peripheral nerve function | [381] |
8. Medicinal Plants with Potential Antidiabetic Activity in Palestine
8.1. Atriplex halimus (Chenopodiaceae)
Saltbush was an extremely effective antidiabetic plant with an insulin-potentiating property in an animal model for diabetogenesis [392].
The aqueous extract of A. halium was beneficial in reducing the elevated blood glucose level and hepatic levels in streptozocin diabetic rats with a toxic effect at a dose of 200 mg/kg [392].
A. halimus’ antidiabetic activity was mediated at least through increasing glucose uptake to the muscle, liver, and fat cells [272].
8.2. Ocimum basilicum (Lamiaceae)
A significant dose-dependent inhibition against intestinal sucrose, maltose, and pancreatic α- amylase was reported [125].
The extract of aerial parts of O.basilicum showed a higher hypoglycemic effect in treated rats than in metformin-treated rats. It improved oral glucose tolerance, increased the liver glycogen content in a dose-dependent manner, and enhanced glucose mobilization by stimulating hepatic glycogen synthesis [393].
Methanol, hexane, and dichloromethane extracts of the aerial parts of O. basilicum were evaluated for their antidiabetic properties in vitro. They increased glucose transporter translocation to the plasma membrane, especially the methanol and hexane extracts [394].
8.3. Sarcopoterium spinosum (Rosaceae)
An aqueous extract of S. spinosum was used to treat multiple cell lines (RINm pancreatic β-cells, L6 myotubes, 3T3-L1 adipocytes, and AML-12 hepatocytes). Skeletal muscle, adipose tissue, and hepatocytes have been reported to have insulin-like effects [395].
The plant extract decreased insulin resistance and increased insulin sensitivity in diabetic mice in another study [396].
The extract of the aerial parts inhibited α-amylase and α-glucosidase, also enhancing glucose cell uptake by activating the insulin-signaling cascade [397].
Glucose tolerance and insulin sensitivity were improved in high-fat diet mice and diabetic mice—two models of insulin resistance—and insulin resistance was decreased after treatment with S. spinosum [398].
8.4. Trigonella foenum-graecum (Fabaceae)
Fenugreek is well-known as an antidiabetic remedy. Preliminary animal and human trials proposed the possible hypoglycemic and antihyperlipidemic properties of fenugreek powder, seed, leaf and their extracts [399,400].
Fenugreek contains galactomannan-rich soluble fiber, which combines with bile acid and lowers triglyceride and LDL cholesterol levels, and may be responsible for the antidiabetic activity of the seeds. In addition, it contains nicotinic acid, alkaloid trigonelline, and coumarin, which were proven to be responsible for its antidiabetic properties [272,399].
The extract of fenugreek was able to inhibit α-amylase activity in a dose-dependent manner, suggesting that the hypoglycemic effect of the used plant extract was mediated through insulin mimetic properties [400].
The seed extract of T. foenum-graecum doubled the translocation of glucose transporter to plasma membrane, and at non-cytotoxic concentrations [272].
Finally, studies showed that the treatment of severely diabetic rabbits with a fenugreek seeds isolated compound corrected the altered serum lipids, tissue lipids, glycogen, enzymes of glycolysis, gluconeogenesis, glycogen metabolism, polyol pathway and antioxidant enzymes, and corrected all the histopathological abnormalities seen in the pancreas, liver, heart and kidneys [401].
8.5. Withania somnifera (Solanaceae)
The aqueous extract of W. somnifera significantly improved insulin sensitivity index in streptozocin diabetic rats [402]. The leaf and root extracts of W. somnifera increased glucose uptake in myotubes and adipocytes in a dose-dependent manner, and increased insulin secretion. Withaferin was found to increase glucose cell uptake [403].
The extract also decreased blood glucose levels in alloxan and streptozocin diabetic rats [402,404]. The antidiabetic activity may be due to an increase in hepatic metabolism, increased insulin release from pancreatic β-cells, or the insulin-sparing effect [405] (Table 20, Table 21 and Table 22).
Table 20.
Medicinal plants of Palestine ethnobotany.
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Atriplex halimus | Katf | Chenopodiaceae | Diabetes, heart disease | Leaf | [406] |
| Ocimum basilicum | Rehan | Lamiaceae | Diarrhea, kidney stone, carminative, diuretic, dysentery, skin disease, anti-colic. | Whole palnt | [407,408] |
| Sarcopoterium spinosum | Bullan, Natesh | Rosaceae | Anti-inflammatory, toothache, analgesic, for hemorrhoids, antidiabetic, stomach pain, diuretic, renal calculi, stimulant for circulation | Root, fruit, seed | [409] |
| Trigonella foenum-graecum | Hilbeh | Fabaceae | Diabtes, cancer | Seed | [410,411] |
| Withania somnifera | Samoh | Solanaceae | Skin disease, kidney stone, wound healing | Leaves, root, young branches | [412,413] |
Table 21.
Medicinal plants of Palestine reported constituents, use.
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Atriplex halimus | Alkaloid, flavonoid, saponin, sterol, tannin | Antidiabetic, antimicrobial, antioxidant, insecticidal | [392] |
| Ocimum basilicum | Cardiac glycosides, caffeic acid derivatives, essential oil, flavonoid, phenolic acids, saponins, tannins | Antimicrobial, hepatoprotective, hypertension nematicidal | [393,394] |
| Sarcopoterium spinosum | Catechin, epicatechin, essential oil | Antidiabetic | [414] |
| Trigonella foenum-graecum | Alkaloid, flavonoid, polyphenols, saponin, volatile oil | Antidiabetic, antilipidemic, anticarcinogenic, anticataract, antioxidant, immunomodulatory | [399,400,415] |
| Withania somnifera | steroidal alkaloids and lactones, withanolides | Anticancer, immunomodulatory, anti-inflammatory, antistress, adaptogenic, CNS activities, cardiovascular activities | [416] |
Table 22.
Plants with antidiabetic potential from Palestine.
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Atriplex halimus | Leaf, stem | Ethanol (50%) extract, maceration | HepG2, L6myc cell line | 0–2 mg/mL | Increased GLUT4 translocation, EC50 was about 2 mg/mL | [272] |
| Gundelia tournefortii | Aerial parts | Methanol, hexane extract, maceration | Rat L6 muscle cell lines | 0–1 mg/mL | Methanol extract was the most efficient in GLUT4 translocation enhancement in skeletal muscle | [417] |
| Ocimum basilicum | Aerial part | Methanol, hexane, dichloromethane extracts, reflux | L6 muscle cell line | 0–2 mg/mL | The extracts increased GLUT4 translocation up to 7 times | [394] |
| Olea europaea | Leaf | Ethanol(80%) extract, soxhlet | Streptozocin diabetic rats | 10, 20, 40 mg | The extract inhibited both digestion and absorption (concentration-dependent) |
[418] |
| Sarcopoterium spinosum | Root | Aqueous extract, decoction | RIN β-cells, L6 myotubes, 3T3-L1 adipocytes, AML-12 hepatocytes | 0.001–10 mg/mL | Preventive effect for progression in diabetes | [395] |
| Root | Aqueous extract, decoction | KK-a/y mice | 600 mg/kg, 6 weeks | Improved insulin sensitivity, increased glucose uptake | [396] | |
| Root, leaf, fruit | Aqueous extract, decoction | 3 T3-L1 adipocytes cell line | Root: 1 mg/mLFruit: 2 mg/mL | Inhibited α-glucosidase and amylase, induced insulin secretion | [397] | |
| Root | Aqueous extract, decoction | High-fat diet mice, KK-Ay male mice | 70 mg/day, 6 weeks | Improved glucose tolerance and sensitivity | [398] | |
| Trigonella foenum-graecum | Seed | Ethanol (50%) extract, maceration | HepG2, L6myc cell line | 0–2 mg/mL | Significant translocation of GLUT4 | [272] |
| Urtica dioica | Leaf, stem | Ethanol (50%) extract, maceration | HepG2, L6myc cell line | 0–2 mg/mL | Extract almost doubled GLUT4 translocation | [272] |
| Withania somnifera | Leaf, root | Methanol extract, maceration, isolated compound | Pancreatic RIN-5F | 50 mg/mL | Extracts increased glucose uptake in myotubes and adipocytes (dose-dependent). Leaf extract increased insulin secretion, Withaferin A increased glucose uptake. | [403] |
9. Medicinal Plants with Potential Antidiabetic Activity in Turkey
Turkey is considered as one of the richest countries in the temperate world with regards to the floristic diversity [419].
Phlomis armeniaca, Salvia limbata and Plantago lanceolata exhibited weak inhibitory activity against α-amylase and pronounced inhibitory activity against α-glucosidase [419].
Methanolic and aqueous extracts of Hedysarum varium and Onobrychis hypargyrea showed significant inhibition against α-glucosidase when compared to acarbose [420].
In another study, Hedysarum varium, Onobrychis hypargyrea and Salvia limbata extracts showed inhibition against α-amylase and potent inhibition against α-glucosidase compared to acarbose [420].
The hydroalcoholic extract of Helichrysum graveolens inhibited the α-amylase enzyme similarly to acarbose at a dose of 3000 μg/mL. Several secondary metabolites were reported in this genus, and chlorogenic acid was found to be the major phenolic in the extract [421].
The lyophilized hydrophilic extract obtained from leaves of Cichorium intybus effectively suppressed the activity of α-glucosidase, but the inhibitory activity towards α-amylase was limited. It is well known that plant extracts which selectively inhibit α-glucosidase are the preferred agents in controlling the uptake of glucose in diabetes. The inhibition of both enzymes would result in abnormal bacterial fermentation in the colon due to the presence of undigested carbohydrates [422].
Cinnamomum verum aqueous and ethanolic extracts of the bark showed more affinity for α-amylase than for α-glycosidase enzyme. It was reported that cinnamon might improve anthropometric parameters, and can also activate the glycogen synthase through stimulating glucose uptake, and inhibiting glycogen synthase kinase [423].
Another investigation revealed that the methanol extract of Origanum onites exerted a higher inhibition against α-amylase and α-glucosidase compared to the aqueous extract [424].
Additionally, the aqueous and methanol extracts of Mentha pulegium had more affinity for α-amylase than for the α-glycosidase enzyme. Both caused greater inhibition of α-glycosidase than acarbose [425].
Both ethyl acetate and petrolum ether extracts of Haplophyllum myrtifolium showed a similar, significant, α-amylase inhibition rate that was higher than that of the methanol and water extracts. For the α-glucosidase-inhibitory effect, the ethyl acetate extract exhibited the highest activity followed, by the petroleum ether and methanol extracts. However, the water extract tested did not have any effect on the α-glucosidase activity [426].
Previous studies showed that the extracts rich in phenolics possessed good inhibitory activity for α-glucosidase and α-amylase. Interestingly, the ethyl acetate extract had the highest level of phenolics [426].
Laurus nobilis essential oil and its major component, 1,8-cineole, inhibited α-glucosidase by competitive inhibition, but 1-(S)-α-pinene and R-(+)-limonene were uncompetitive inhibitors. All possessed an in vitro antioxidant property [427] (Table 23).
Table 23.
Ethnobotany of plants from Turkey.
| Scientific name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Cichorium intybus | Sakızotu, hindiba, sakızçiçeği | Asteraceae | Cancer, kidney stone, hemorrhoids, urinary disorders, wound keeling | Aerial parts, leaf, root | [202,428] |
| Cinnamomum verum | Tarçın | Lauraceae | Not mentioned | Not mentioned | [429] |
| Haplophyllummyrtifolium | Not mentioned | Rutaceae | Sudanese and Mongolian folk medicin: antipyretic, diarrhea | Not mentioned | [430] |
| Helichrysum graveolens | Olmezcicek or altinotu | Asteraceae | diuretics, as lithagogues, for stomachache, for anti-asthmatic properties, against kidney stones | Not mentioned | [431,432] |
| Hedysarum varium | Fabaceae | [420] | |||
| Laurus nobilis | Define | Lauraceae | Antiseptic, against rheumatic pain | Fruit, seed | [433,434] |
| Mentha pulegium | Filiskin, yarpuz | Lamiaceae | Bronchitis, cold, flatulent dyspepsia, intestinal colic, chlorera, food posioning, sinusitis, tuberculosis | Flowering aerial parts | [425,435,436] |
| Phlomis armeniaca | Calba | Lamiaceae | Colds, stomachache | Not mentioned | [419] |
| plantago lanceolata | Giyamambel | Plantaginaceae | Diabetes, stomach ache | Not mentioned | [419] |
| Onobrychis hypargyrea | Not mentioned | Fabaceae | Cold and flu | Not mentioned | [420] |
| Origanum onites | Kekik’ | Lamiaceae | Abdominal ache, bronchitis, cold, diabetes, dizziness, headache, hypertension, high cholesterol, itching, gastralgia, leukemia, toothache, stomach disorders |
Aerial parts flowers, leaves | [424] |
| Salvia limbata | Baresaspi | Lamiaceae | Colds, stomach ache, wounds | Not mentioned | [419] |
9.1. Cistus laurifolius (Cistaceae)
The ethanolic extract of the leaves was found to have a promising antidiabetic activity in streptozocin diabetic rats and significant inhibitory activity for α-amylase and glucosidase enzymes [437].
Twelve major flavonoids and other phenolic compounds were isolated from the ethanolic extract of the leaves. Some were reported to inhibit aldose reductases, enhance both basal and insulin-stimulated glucose uptake, prevent β-cell apoptosis and modulate their proliferation, and inhibit α-glucosidase and amylase enzymes [437].
9.2. Juniperus oxycedrus (Cupressaceae)
Infusions and decoctions of Juniperus fruits and leaves were used for diabetes in Turkey as folk remedy. According to the literature, different species of Juniper possess hypoglycemic effects [438,439].
Aqueous and ethanolic extracts of J. oxycedrus were tested in streptozocin diabetic rats. The aqueous extract exhibited a mild hypoglycemic effect, wherein the ethanolic extract showed a higher and more continuous hypoglycemic effect. In the same study, isolated shikimic acid possessed a promising antidiabetic activity at a dose of 30 mg/kg [438].
Additionally, the effects of J. oxycedrus leaf extracts were investigated in diabetic animals. The study indicated that the treatment of diabetic rats with the aqueous extract showed a moderate hypoglycemic effect, while the antihyperglycemic effect of ethanol extract was significant and continuous at a dose of 1000 mg/kg [440].
Leaf and fruit hydroethanolic extracts decreased blood glucose level and lipid peroxidation in tissues remarkably [441].
Phytochemical analysis showed that J. oxycedrus was rich in secondary metabolites that were responsible for its antidiabetic effect [441].
The increase in insulin stimulation, insulin sensitivity and release were related to the unsaturated fatty acids-rich fraction of leaves extract [440].
Shikimic acid, a major molecule found in berry extract, has a well-known antidiabetic activity through its antioxidant activity and ability to increase the insulin-like growth factor-1 [438].
Augmenting Zn concentration in the liver is another possible mechanism. Zn plays a key role in the synthesis and action of insulin both physiologically and in the pathologic state of diabetes [441].
9.3. Heracleum persicum (Apiaceae)
The increased levels of plasma glucose in diabetic rats were lowered by extract supplementations in all groups after 21 days. The antihyperglycemic action resulted from the potentiation of insulin release from existing β-cells, and regenerating the functions of islet cells over time [320].
Among the isolated compounds taken from the roots of H. persicum, pimpinellin-dimer was identified as a potent α-glucosidase inhibitor, more effective than acarbose. In addition, it exhibited significant antioxidant activity [442].
It is well known that oxidative stress is caused by reactive oxygen species (ROS) and plays an important role in the pathogenesis of various degenerative diseases, such as diabetes; similarly, postprandial oxidative stress is associated with a higher risk for diabetes, and therefore, H. persicum can be used as a potent antioxidant and as a strong inhibitor of α-amylase in the improvement of type 2 diabetes patients [443].
9.4. Juniperus foetidissima and Juniperus sabina (Cupressaceae)
All extracts of J. foetidissima and Sabina had promising α-glucosidase inhibitory activities. The ethanolic leaf extract of J. foetidissima had the highest activity. On the other hand, the α-amylase inhibitory activity of Juniperus extracts was moderate [439].
In the same study, the ethanolic fruit extract of J. foetidissima at a dose of 500 mg/kg significantly lowered the blood glucose level for diabetic animals, similarly to glipizide [439].
The antidiabetic activity was attributed to amentoflavone, which showed significant inhibitory activity for carbohydrate digestive enzymes, as well as positive effects on insulin resistance [439].
9.5. Origanum minutiflorum (Labmiaceae)
A significant time- and dose-dependent antihyperglycemic activity of aqueous O. minutiflorum extract was observed in diabetic rats. This effect was attributed to its flavonoid-rich contents, well-known compounds for their antidiabetic effects [444].
9.6. Salvia triloba (Lamiaceae)
The methanolic extract of S. triloba decreased blood glucose level in a non-dose-dependent manner in streptozocin/nicotinamide diabetic rats. It performed its action by improving insulin sensitivity [445].
9.7. Thymus praecox (Lamiaceae)
The methanolic extract of T. praecox decreased blood glucose level in Streptozocin/nicotinamide diabetic rats. It performed its action by improving insulin sensitivity [445] (Table 24, Table 25 and Table 26).
Table 24.
Ethnobotany of plants from Turkey.
| Scientific Name | Common Name | Family | Traditional Use | Part Used | Reference |
|---|---|---|---|---|---|
| Cistus laurifolius | Defne yapraklı | Cistaceae | Diabetes, fever in common cold, rheumatic and inflammatory diseases, peptic ulcer, applied externally in a line of the kidneys for urinary inflammations | Leaf, flower, bud, branch | [437,446] |
| Juniperus oxycedrus | KatranArdıcı | Cupressaceae | Abdominal pain bronchitis, calcinosis, common cold, cough, diabetes, gynecological diseases, fungal infections, hemorrhoids, kidney stone, stomachic disorders, kidney inflammation, wounds | Tar, leaf, fruit, berry | [438,447] |
| Origanum minutiflorum | Toga kekik, mountain kekik, | Lamiaceae | Herbal tea | Leaf | [444,448] |
| Rhus coriaria | Sumac | Anacardiacea | Diabetes, toothache, eye diseses | Not mentioned | [449,450] |
| Salvia triloba | AnadoluAdacay | Lamiaceae | Blood, skin andinfectious, ailments of the digestive, circulatory and respiratory systems | Leaf | [445] |
| Thymus praecox | Kekik | Lamiaceae | Herbal tea, condiment | Herbal parts | [451,452] |
Table 25.
Medicinal plants of Turkey reported constituents, use.
| Scientific Name | Phytochemical Constituent | Pharmacological Use | Reference |
|---|---|---|---|
| Cistus laurifolius | Flavonoids, phenolic acids | Antiulcerogenic, analgesic, antioxidant, hepatoprotective | [437] |
| Juniperus oxycedrus | Lignans, abietane, sesquiterpenes, diterpenes, biflavonols, tannins, coumarins, flavonoids, sterols, terpenes, mono, sesqui and diterpenes, alkanes, fatty acids, waxes | Antioxidant, antiseptic, antiviral, anti-inflammatory, analgesic, anticancer, antidiabetic, neuroprotective | [438,447,453] |
| Origanum minutiflorum | Essential oils | Antioxidant, antibacterial, antiviral, antifungal | [444] |
| Rhus coriaria | Anthocyanins, flavonoids, isoflavonoids, tannins, terpenoids | Antioxidant, antiseptic, antifungal, antibacterial, anti-ischemic, antifibrogenic, antitumourigenic activities, hypoglycemic, non mutagenic, fever, DNA protective, hypouricaemic, hepatoprotective properties |
[449,454] |
| Salvia triloba | Essential oil, flavonoids, flavone, terpene, sesqui, di and triterpenes, steroidal compounds | Antimicrobial | [452,455] |
| Thymus praecox | Essential oil | Anti-inflammatory, antifungal, antiviral, antioxidant, anticancer, antidiabetic effects | [445,452] |
Table 26.
Plants with antidiabetic potential from Turkey.
| Scientific Name | Part Used | Extraction Method, Solvent | Target | Intervention and Duration | Observations | Ref |
|---|---|---|---|---|---|---|
| Cistus laurifolius | Leaf | Water, ethanol, maceration | Streptozocin diabetic rats | 250, 500 mg/kg | Inhibited of α-glucosidaseand α-amylase | [437] |
| Heracleum persicum | Whole plant | Water, maceration | Streptozocin diabetic rats | 100, 200, 400 mg/kg 21 days |
Decreased HbA1c, restored diabetic complications parameters towards normal, increased AST and ALT significantly | [320] |
| Juniperus oxycedrus | Berries | Water, maceration isolated compound |
Streptozocin diabetic rats | 500, 1000 mg/kg Shikimic acid: 15 and 30 mg/kg, 8 days |
The effect of shikimic acid was more effective than the reference antidiabetic drug glipizide, TG and enzyme levels were reduced significantly | [438] |
| Leaf | Water, ethanol, maceration | Streptozocin diabetic rats | 500, 1000 mg/kg | Fatty acids were found as the major compounds | [440] | |
| Fruit, leaf | Ethanol 80%, maceration | Streptozocin diabetic rats | 500, 1000 mg/kg, 10 days | Augment Zn level in liver | [441] | |
| Juniperus foetidissima & Juniperus sabina | Leaf, fruit | Ethanol 80%, maceration | Streptozocin diabetic rats | 500, 1000 mg/kg, 8 days | Leaf extract caused death at both doses | [439] |
| Origanum minutiflorum | Whole plant | Water, decoction | Streptozocin diabetic rats | 150 mg/kg, 30 days | Decreased ALT and AST levels | [444] |
| Salvia triloba | Aerial parts | Methanol 80%, soxhlet | Streptozocin-nicotinamide diabetic rats | 100, 200 mg/kg, 21 days | Little weight loss | [445] |
| Thymus praecox | Aerial parts | Methanol, maceration | Streptozocin-nicotinamide diabetic rats | 100, 200 mg/kg, 21 days | Little weight loss | [445] |
10. Middle East Statistics
The flora in the Middle East area provides diverse useful species. A total of 140 medicinal plant species were studied scientifically for their potential antidiabetic activities. These species were classified among 59 families (Figure 1). Lamiaceae (20 species, 14%) and Asteraceae (16 species, 11%) have a greater number of medicinal plants with antidiabetic activities in Middle East countries.
Figure 1.
Botanical families studied.
Scientific investigations try to validate the antidiabetic potential of several Middle East medicinal plants using several methods of extraction, plant organs, and solvents. The majority of the studies used aqueous maceration as an extraction method (Figure 2 and Figure 3) Water was mostly used as a solvent as the researchers simulated the ethnobotanical use by people. Among plant parts, leaves were the most frequently used part (28%) (Figure 4.)
Figure 2.
The percentage of extraction methods.
Figure 3.
The percentage of solvents used for extraction.
Figure 4.
The percentage of different plant parts used.
The prevalence of type 2 diabetes has risen dramatically. About 422 million people worldwide have diabetes, particularly in low- and middle-income countries, and 1.6 million deaths are directly attributed to diabetes each year (World Health Organization, 2020).
There are numerous plant species in the Middle East. They were estimated to exceed 13,500 species, mainly found in Turkey and Iran [456]. To exert their effects, plants with potential antidiabetic properties have many targets and diverse mechanisms of actions, (Figure 5).
Figure 5.
Targets of medicinal plant with antidiabetic activity.
11. Conclusions
Above is the collected information regarding plants used in the treatment of diabetes mellitus in Middle East countries. In recent years, the ethnobotanical and traditional use of natural compounds, especially those of plant origin, has received much attention, as they are well tested for their efficacy and are generally believed to be safe for the treatment of human ailments. It is the best classical approach in the search for new molecules for the management of various diseases.
Many new bioactive drugs isolated from plants showed antidiabetic activity that was equally as potent as, and sometimes even more potent than, known oral hypoglycemic agents, such as metformin and glibenclamide. However, many other active agents obtained from plants have not been well characterized. More investigations must be carried out to elucidate the mechanisms of action and the toxicity of medicinal plants with antidiabetic effects.
This review contributes scientifically to evidence for the ethnobotanical use of medicinal plants as antidiabetic agents. Work has to be done to define the target, mechanism of action and the responsible compound for activity. In addition, safety and pharmacokinetic parameters should be investigated.
Funding
This research received no external funding.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
Authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mannan A., Rupa B.A., Azam N.K., Ahmed N., Hasan N. A quick review on anti-diabetic plants and action of phytochemicals. Int. J. Adv. Res. 2014;2:227. [Google Scholar]
- 2.Sathasivampillai S.V., Rajamanoharan P.R., Munday M., Heinrich M. Plants used to treat diabetes in Sri Lankan Siddha Medicine–An ethnopharmacological review of historical and modern sources. J. Ethnopharmacol. 2017;198:531–599. doi: 10.1016/j.jep.2016.07.053. [DOI] [PubMed] [Google Scholar]
- 3.Abuyassin B., Laher I. Diabetes epidemic sweeping the Arab world. World J. Diabetes. 2016;7:165. doi: 10.4239/wjd.v7.i8.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade-Cetto A., Heinrich M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005;99:325–348. doi: 10.1016/j.jep.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 5.Verma S., Gupta M., Popli H., Aggarwal G. Diabetes mellitus treatment using herbal drugs. Int. J. Phytomedicine. 2018;10:1–10. doi: 10.5138/09750185.2181. [DOI] [Google Scholar]
- 6.Chinsembu K.C. Diabetes mellitus and nature’s pharmacy of putative antidiabetic plants. J. Herb. Med. 2019;15:100230. doi: 10.1016/j.hermed.2018.09.001. [DOI] [Google Scholar]
- 7.Aati H., El-Gamal A., Shaheen H., Kayser O. Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. J. Ethnobiol. Ethnomedicine. 2019;15:2. doi: 10.1186/s13002-018-0263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alwin Robert A., Al Dawish M.A. Microvascular complications among patients with diabetes: An emerging health problem in Saudi Arabia. Diabetes Vasc. Dis. Res. 2019;16:227–235. doi: 10.1177/1479164118820714. [DOI] [PubMed] [Google Scholar]
- 9.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Bener A., Zirie M., Janahi I.M., Al-Hamaq A.O., Musallam M., Wareham N.J. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res. Clin. Pract. 2009;84:99–106. doi: 10.1016/j.diabres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Yaser A.J., Muneer A., Abdelhafid B., Dauodi C., Hammadi L. Chemical and phytochemical analysis of some antidiabetic plants in Yemen. Int. J. Res. Pharm. 2013;4:72–76. doi: 10.7897/2230-8407.04915. [DOI] [Google Scholar]
- 12.El Hilaly J., Lyoussi B. Hypoglycaemic effect of the lyophilised aqueous extract of Ajuga iva in normal and Streptozocin diabetic rats. J. Ethnopharmacol. 2002;80:109–113. doi: 10.1016/S0378-8741(01)00407-X. [DOI] [PubMed] [Google Scholar]
- 13.El-Hilaly J., Tahraoui A., Israili Z.H., Lyoussi B. Acute hypoglycemic, hypocholesterolemic and hypotriglyceridemic effects of continuous intravenous infusion of a lyophilised aqueous extract of Ajuga iva L. Schreber whole plant in Streptozocin-induced diabetic rats. Pak. J. Pharm. Sci. 2007;20:261–268. [PubMed] [Google Scholar]
- 14.Boudjelal A., Siracusa L., Henchiri C., Sarri M., Abderrahim B., Baali F., Ruberto G. Antidiabetic effects of aqueous infusions of Artemisia herba-alba and Ajuga iva in alloxan-induced diabetic rats. Planta Med. 2015;81:696–704. doi: 10.1055/s-0035-1546006. [DOI] [PubMed] [Google Scholar]
- 15.Wang J.-J., Jin H., Zheng S.-L., Xia P., Cai Y., Ni X.-J. Phytoecdysteroids from Ajuga iva act as potential antidiabetic agent against alloxan-induced diabetic male albino rats. Biomed. Pharmacother. 2017;96:480–488. doi: 10.1016/j.biopha.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Hamden K., Ayadi F., Jamoussi K., Masmoudi H., Elfeki A. Therapeutic effect of phytoecdysteroids rich extract from Ajuga iva on alloxan induced diabetic rats liver, kidney and pancreas. Biofactors. 2008;33:165–175. doi: 10.1002/biof.5520330302. [DOI] [PubMed] [Google Scholar]
- 17.Senthilkumar A., Karuvantevida N., Rastrelli L., Kurup S.S., Cheruth A.J. Traditional Uses, Pharmacological Efficacy, and Phytochemistry of Moringa peregrina (Forssk.) Fiori.—A Review. Front. Pharmacol. 2018;9:465. doi: 10.3389/fphar.2018.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Alfy T.S., Ezzat S.M., Hegazy A.K., Amer A.M., Kamel G.M. Isolation of biologically active constituents from Moringa peregrina (Forssk.) Fiori.(family: Moringaceae) growing in Egypt. Pharmacogn. Mag. 2011;7:109. doi: 10.4103/0973-1296.80667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koheil M.A., Hussein M.A., Othman S.M., El-Haddad A. In-vivo antioxidant activity of Moringa peregrina against STZ–induced oxidative stress in type 2 diabetic rats. Mol. Clin. Pharm. 2013;4:65–75. [Google Scholar]
- 20.Ullah M.F., Bhat S.H., Abuduhier F.M. Antidiabetic Potential of Hydro-Alcoholic Extract of M oringa Peregrina Leaves: Implication as Functional Food for Prophylactic Intervention in Prediabetic Stage. J. Food Biochem. 2015;39:360–367. doi: 10.1111/jfbc.12140. [DOI] [Google Scholar]
- 21.Reddy S.H., Al-Neeri I.S., Al-Issaei H.K., Al-Jabri S.M. Effect of selective medicinal plant extract on blood glucose, sperm shape and various physiological parameters. Am. J. Plant Sci. 2015;6:1109. doi: 10.4236/ajps.2015.68115. [DOI] [Google Scholar]
- 22.Al Maharooqi A., Al Hilali M., Al Hinai Z., Unnikrishnan D. A Review on Medicinal Plant Decne Rhazya Stricta. Adv. Pharm. J. 2016;1:119–125. [Google Scholar]
- 23.Baeshen M., Khan R., Bora R., Baeshen N. Therapeutic potential of the folkloric medicinal plant Rhazya stricta. Biol. Syst. Open Access. 2015;5:1000151. doi: 10.4172/2329-6577.1000151. [DOI] [Google Scholar]
- 24.Ahmed A., Asad M.J., Ahmad M.S., Qureshi R., Shah S.I., Gul H., Gulfraz M. Antidiabetic and hypolipidemic potential of Rhazya stricta Decne extract and its fractions. Int. Curr. Pharm. J. 2015;4:353–361. doi: 10.3329/icpj.v4i2.21484. [DOI] [Google Scholar]
- 25.Reddy S.H., Al-Hinai A.K., AL-Yaqoobi H.H., Al-Ajmi F.J. Phytochemical analysis, antimicrobial screening and hypoglycemic effect of some selected medicinal plant extract from Oman. J. Exp. Biol. 2016;4:2. [Google Scholar]
- 26.Hao-Cong M., Shuo W., Ying L., KUANG Y.-Y., Chao-Mei M. Chemical constituents and pharmacologic actions of Cynomorium plants. Chin. J. Nat. Med. 2013;11:321–329. doi: 10.1016/S1875-5364(13)60049-7. [DOI] [PubMed] [Google Scholar]
- 27.Phoboo S., Shetty K., ElObeid T. In Vitro assays of anti-diabetic and anti-hypertensive potential of some traditional edible plants of qatar. J. Med. Act. Plants. 2015;4:22–29. [Google Scholar]
- 28.Das S.K., Samantaray D., Patra J.K., Samanta L., Thatoi H. Antidiabetic potential of mangrove plants: A review. Front. Life Sci. 2016;9:75–88. doi: 10.1080/21553769.2015.1091386. [DOI] [Google Scholar]
- 29.Mahera S., Saifullah S., Ahmad V., Mohammad F. Phytochemical studies on mangrove Avicennia marina. Pak. J. Bot. 2013;45:2093–2094. [Google Scholar]
- 30.Okla M.K., Alamri S.A., Alatar A.A., Hegazy A.K., Al-Ghamdi A.A., Ajarem J.S., Faisal M., Abdel-Salam E.M., Ali H.M., Salem M.Z. Antioxidant, Hypoglycemic, and Neurobehavioral Effects of a Leaf Extract of Avicennia marina on Autoimmune Diabetic Mice. Evid. Based Complementary Altern. Med. 2019;2019:1750368. doi: 10.1155/2019/1263260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeid I.E.M.E.A., Al-Jaghthmi O.H.A., Heba H.M. Augmentation of Insulin Secretion Induced by Rhizophora Mucronata and Avicennia Marina Extracts in Streptozocin-Induced Diabetic Rats. Int. J. Pharm. Res. Allied Sci. 2019;8:14–22. [Google Scholar]
- 32.Hamzevi A., Sadoughi S.D., Rahbarian R. The effect of aqueous extract of Avicennia marina (Forsk.) Vierh. leaves on liver enzymes’ activity, oxidative stress parameters and liver histopathology in male diabetic rat. Feyz J. Kashan Univ. Med. Sci. 2017;21:305–316. [Google Scholar]
- 33.Habibuddin M., Daghriri H.A., Humaira T., Al Qahtani M.S., Hefzi A.A.H. Antidiabetic effect of alcoholic extract of Caralluma sinaica L. on Streptozocin-induced diabetic rabbits. J. Ethnopharmacol. 2008;117:215–220. doi: 10.1016/j.jep.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Shalaby N.M., Abd-Alla H.I., Aly H.F., Albalawy M.A., Shaker K.H., Bouajila J. Preliminary in vitro and in vivo evaluation of antidiabetic activity of Ducrosia anethifolia Boiss. and its linear furanocoumarins. Biomed Res. Int. 2014;2014:1–13. doi: 10.1155/2014/480545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil R.N., Patil R.Y., Ahirwar B., Ahirwar D. Evaluation of antidiabetic and related actions of some Indian medicinal plants in diabetic rats. Asian Pac. J. Trop. Med. 2011;4:20–23. doi: 10.1016/S1995-7645(11)60025-4. [DOI] [PubMed] [Google Scholar]
- 36.Aladodo R. Effects of aqueous root extract of Jatropha curcas on hyperglycaemic and haematological indices in alloxan-induced diabetic rats. Fountain J. Nat. Appl. Sci. 2013;2:52–58. [Google Scholar]
- 37.El-Baz F.K., Aly H.F., Abd-Alla H.I., Saad S.A. Bioactive flavonoid glycosides and antidiabetic activity of Jatropha curcas on Streptozocin-induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2014;29:143–156. [Google Scholar]
- 38.Noman O.M., Mothana R.A., Al-Rehaily A.J., Nasr F.A., Khaled J.M., Alajmi M.F., Al-Said M.S. Phytochemical analysis and anti-diabetic, anti-inflammatory and antioxidant activities of Loranthus acaciae Zucc. Grown in Saudi Arabia. Saudi Pharm. J. 2019;24:724–730. doi: 10.1016/j.jsps.2019.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sher H., Alyemeni M.N. Evaluation of anti-diabetic activity and toxic potential of Lycium shawii in animal models. J. Med. Plants Res. 2011;5:3387–3395. [Google Scholar]
- 40.Elberry A.A., Harraz F.M., Ghareib S.A., Gabr S.A., Nagy A.A., Abdel-Sattar E. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in Streptozocin-induced diabetic rats. Int. J. Diabetes Mellit. 2015;3:37–44. doi: 10.1016/j.ijdm.2011.01.004. [DOI] [Google Scholar]
- 41.Divi S.M., Bellamkonda R., Dasireddy S.K. Evaluation of antidiabetic and antihyperlipedemic potential of aqueous extract of Moringa oleifera in fructose fed insulin resistant and STZ induced diabetic wistar rats: A comparative study. Asian J. Pharm. Clin. Res. 2012;5:67–72. [Google Scholar]
- 42.Al-Malki A.L., El Rabey H.A. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on Streptozocin induced diabetes and diabetic nephropathy in male rats. Biomed Res. Int. 2015;2015:1–13. doi: 10.1155/2015/381040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.ABD EL-MAWLA A., Mohamed K.M., Mostafa A.M. Induction of biologically active flavonoids in cell cultures of Morus nigra and testing their hypoglycemic efficacy. Sci. Pharm. 2011;79:951–962. doi: 10.3797/scipharm.1101-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahmoud H.I., ElRab S.M.G., Khalil A.F., Ismael S.M. Hypoglycemic effect of white (Morus alba L.) and black (Morus nigra L.) mulberry fruits in diabetic rat. Eur. J. Chem. 2014;5:65–72. doi: 10.5155/eurjchem.5.1.65-72.903. [DOI] [Google Scholar]
- 45.Khalil H.E., Alharbi A.G.A., Ibrahim I.M. In vitro antidiabetic assessment of Ocimum forskolei L. growing in Saudi Arabia. J. Pharmacogn. Phytochem. 2019;8:355–357. [Google Scholar]
- 46.Al-Taweel A.M., Perveen S., Fawzy G.A., Alqasoumi S.I., El Tahir K.E. New flavane gallates isolated from the leaves of Plicosepalus curviflorus and their hypoglycemic activity. Fitoterapia. 2012;83:1610–1615. doi: 10.1016/j.fitote.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 47.Aldawsari H.M., Hanafy A., Labib G.S., Badr J.M. Antihyperglycemic activities of extracts of the mistletoes Plicosepalus acaciae and P. curviflorus in comparison to their solid lipid nanoparticle suspension formulations. Z. Für Nat. C. 2014;69:391–398. doi: 10.5560/znc.2014-0047. [DOI] [PubMed] [Google Scholar]
- 48.Algandaby M.M., Alghamdi H.A., Ashour O.M., Abdel-Naim A.B., Ghareib S.A., Abdel-Sattar E.A., Hajar A.S. Mechanisms of the antihyperglycemic activity of Retama raetam in Streptozocin-induced diabetic rats. Food Chem. Toxicol. 2010;48:2448–2453. doi: 10.1016/j.fct.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Khan M., Ali M., Ali A., Mir S. Hypoglycemic and hypolipidemic activities of Arabic and Indian origin Salvadora persica root extract on diabetic rats with histopathology of their pancreas. Int. J. Health Sci. 2014;8:45. doi: 10.12816/0006071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chhetri B.K., Ali N.A.A., Setzer W.N. A survey of chemical compositions and biological activities of Yemeni aromatic medicinal plants. Medicines. 2015;2:67–92. doi: 10.3390/medicines2020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohammed S.A., Al-Awar A.A.A.M., Elias M.A. Antihyperglycemic and Hypolipidemic Effect of Azadirachta indica Leaves Aqueous Extract in Alloxan-Induced Diabetic Male Rabbits. Int. J. Pharm. Biol. Arch. 2018;9:47–51.5. [Google Scholar]
- 52.Patil P., Patil S., Mane A., Verma S. Antidiabetic activity of alcoholic extract of neem (Azadirachta indica) root bark. Natl. J. Physiol. Pharm. Pharmacol. 2013;3:142. doi: 10.5455/njppp.2013.3.134-138. [DOI] [Google Scholar]
- 53.Nagashayana G., Jagadeesh K., Shreenivas P.R. Evaluation of hypoglycemic activity of neem (Azadirachta indica) in albino rats. Iosr J. Dent. Med. Sci. (Iosr-Jdms) 2014;13:0411. [Google Scholar]
- 54.Alzohairy M.A. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid. Based Complement. Altern. Med. 2016;2016:7382506. doi: 10.1155/2016/7382506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saleem S., Muhammad G., Hussain M.A., Bukhari S.N.A. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother. Res. 2018;32:1241–1272. doi: 10.1002/ptr.6076. [DOI] [PubMed] [Google Scholar]
- 56.Satyanarayana K., Sravanthi K., Shaker I.A., Ponnulakshmi R. Molecular approach to identify antidiabetic potential of Azadirachta indica. J. Ayurveda Integr. Med. 2015;6:165. doi: 10.4103/0975-9476.157950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mothana R.A., Hasson S.S., Schultze W., Mowitz A., Lindequist U. Phytochemical composition and in vitro antimicrobial and antioxidant activities of essential oils of three endemic Soqotraen Boswellia species. Food Chem. 2011;126:1149–1154. doi: 10.1016/j.foodchem.2010.11.150. [DOI] [Google Scholar]
- 58.Al-Mehdar A.A., Al-Battah A.M. Evaluation of Hypoglycemic Activity of Boswellia carterii and Cissus rotundifolia in Streptozocin/Nicotinamide-Induced Diabetic Rats. Yemeni J. Med. Sci. 2016;10:30–38. doi: 10.20428/YJMS.10.1.A4. [DOI] [Google Scholar]
- 59.Farzaei F., Morovati M.R., Farjadmand F., Farzaei M.H. A mechanistic review on medicinal plants used for diabetes mellitus in traditional Persian medicine. J. Evid.-Based Complement. Altern. Med. 2017;22:944–955. doi: 10.1177/2156587216686461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hamidpour R., Hamidpour S., Hamidpour M., Shahlari M. Frankincense (Boswellia species): From the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J. Tradit. Complementary Med. 2013;3:221. doi: 10.4103/2225-4110.119723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onyechi U.A., Judd P.A., Ellis P.R. African plant foods rich in non-starch polysaccharides reduce postprandial blood glucose and insulin concentrations in healthy human subjects. Br. J. Nutr. 1998;80:419–428. doi: 10.1017/S0007114598001482. [DOI] [PubMed] [Google Scholar]
- 62.AliMohammed W.M., Abbas A.A., Mohmmed H., Qasem M.A., Saleem H.A.M., Shikoo E.Y. Antidiabetic activity of cissus routndifolia leaves. World J. Pharm. Res. 2018;8:47–55. [Google Scholar]
- 63.Mohammed Y.H.E., Khanum S.A. Anti-Diabetic Activity of Dracaen cinnabari Balf. f Extracts from Resin in Socotra Island-Yemen. J. Plant Biochem. Physiol. 2016;4:100162. [Google Scholar]
- 64.Al-Baoqai N., Al-Mahbashi H., Al-Adhal A. antidiabetic and antihyperlipidemic activity of dracaena cinnabari balf. resin ethanolic extract of soqatra island in experimental animals. J. Pharm. Res. 2018;3:1–11. doi: 10.22270/ujpr.v3i5.194. [DOI] [Google Scholar]
- 65.Butterweck V., Semlin L., Feistel B., Pischel I., Bauer K., Verspohl E.J. Comparative evaluation of two different Opuntia ficus-indica extracts for blood sugar lowering effects in rats. Phytother. Res. 2011;25:370–375. doi: 10.1002/ptr.3271. [DOI] [PubMed] [Google Scholar]
- 66.Al-Naqeb G. Effect of prickly pear cactus seeds oil on the blood glucose level of Streptozocin-induced diabetic rats and its molecular mechanisms. Int. J. Herb. Med. 2015;3:29–34. [Google Scholar]
- 67.Al-Hajj N.Q.M., Sharif H.R., Aboshora W., Wang H. In Vitro and in Vivo Evaluation of Antidiabetic Activity of Leaf Essential Oil of Pulicaria inuloides-Asteraceae. J. Food Nutr. Res. 2016;4:461–470. [Google Scholar]
- 68.Galala A.A., Sallam A., Abdel-Halim O.B., Gedara S.R. New ent-kaurane diterpenoid dimer from Pulicaria inuloides. Nat. Prod. Res. 2016;30:2468–2475. doi: 10.1080/14786419.2016.1201671. [DOI] [PubMed] [Google Scholar]
- 69.Al-Deen A.T., Al-Naqeb G. Hypoglycemic effect and in vitro antioxidant activity of methanolic extract from Argel (Solenostemma Argel) plant. Int. J. Herb. Med. 2014;2:128–131. [Google Scholar]
- 70.Taha L.E., Bakhit S.M., Al-Sa’aidi J.A., Uro A.B.O. The anti-hyperglycemic effect of Solenostemma argel compared with Glibenclamide. Al-Qadisiyah J. Vet. Med. Sci. 2014;13:113–117. [Google Scholar]
- 71.Toiu A., Mocan A., Vlase L., Pârvu A.E., Vodnar D.C., Gheldiu A.-M., Moldovan C., Oniga I. Phytochemical Composition, Antioxidant, Antimicrobial and in Vivo Anti-inflammatory Activity of Traditionally Used Romanian Ajuga laxmannii (Murray) Benth.(“Nobleman’s Beard”–Barba Împăratului) Front. Pharmacol. 2018;9:7. doi: 10.3389/fphar.2018.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tahraoui A., El-Hilaly J., Israili Z., Lyoussi B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province) J. Ethnopharmacol. 2007;110:105–117. doi: 10.1016/j.jep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Hanif M.A., Al-Maskri A.Y., Al-Mahruqi Z.M.H., Al-Sabahi J.N., Al-Azkawi A., Al-Maskari M.Y. Analytical evaluation of three wild growing Omani medicinal plants. Nat. Prod. Commun. 2011;6:1934578X1100601010. doi: 10.1177/1934578X1100601010. [DOI] [PubMed] [Google Scholar]
- 74.Ghazanfar S.A., Al-Al-Sabahi A.M. Medicinal plants of northern and central Oman (Arabia) Econ. Bot. 1993;47:89–98. doi: 10.1007/BF02862209. [DOI] [Google Scholar]
- 75.Norton J., Majid S.A., Allan D., Al Safran M., Böer B., Richer R. An Illustrated Checklist of the Flora of Qatar. Browndown Publications; Gosport, UK: 2009. [Google Scholar]
- 76.Tounekti T., Mahdhi M., Khemira H. Ethnobotanical Study of Indigenous Medicinal Plants of Jazan Region, Saudi Arabia. Evid. Based Complementary Altern. Med. 2019;2019:3190670. doi: 10.1155/2019/3190670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Albalawi M.A.D., Bashir N.A.O., Tawfik A. Anticancer and antifolate activities of extracts of six Saudi Arabian wild plants used in folk medicine. J. Life Sci. 2015;9:334–340. [Google Scholar]
- 78.Adnan M., Jan S., Mussarat S., Tariq A., Begum S., Afroz A., Shinwari Z.K. A review on ethnobotany, phytochemistry and pharmacology of plant genus C aralluma R. Br. J. Pharm. Pharmacol. 2014;66:1351–1368. doi: 10.1111/jphp.12265. [DOI] [PubMed] [Google Scholar]
- 79.Rahman M.A., Al-Said M.S., Mossa J.S., Al-Yahya M.A., Al-Hemaid M.F. A check list of angiosperm flora of Farasan Islands, Kingdom of Saudi Arabia. Pak. J. Biol. Sci. 2002;5:1162–1166. [Google Scholar]
- 80.Mottaghipisheh J., Nové M., Spengler G., Kúsz N., Hohmann J., Csupor D. Antiproliferative and cytotoxic activities of furocoumarins of Ducrosia anethifolia. Pharm. Biol. 2018;56:658–664. doi: 10.1080/13880209.2018.1548625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdelgadir H.A., Staden J. Ethnobotany, ethnopharmacology and toxicity of Jatropha curcas L. (Euphorbiaceae): A review. S. Afr. J. Bot. 2013;88:204–208. doi: 10.1016/j.sajb.2013.07.021. [DOI] [Google Scholar]
- 82.Kashyap C., Ranjeet K., Vikrant A., Vipin K. Therapeutic Potency of Ocimum KilimandscharicumGuerke-A Review. Glob. J. Pharmacol. 2011;5:191–200. [Google Scholar]
- 83.Zahran E.M., Desoukey S.Y., Fouad M.A., Kamel M.S. The antiinflammatory activity and LD50 of Ocimum forskolei Benth. family Lamiaceae. J. Adv. Biomed. Pharm. Sci. 2019;2:116–120. doi: 10.21608/jabps.2019.12973.1048. [DOI] [Google Scholar]
- 84.Ahmed T.A., Al Naemi H. Biological activities of Lycium shawii Leaves Extract. Int. J. Pharm. Biol. Arch. 2010;3:697–700. [Google Scholar]
- 85.Lodhi S., Vadnere G.P., Sharma V.K., Usman M.R. Marrubium vulgare L.: A review on phytochemical and pharmacological aspects. J. Complementary Med. Res. 2017;6:429–452. doi: 10.5455/jice.20170713060840. [DOI] [Google Scholar]
- 86.Mossa J. A study on the crude antidiabetic drugs used in Arabian folk medicine. Int. J. Crude Drug Res. 1985;23:137–145. doi: 10.3109/13880208509069018. [DOI] [Google Scholar]
- 87.Ali N., Chhetri B., Dosoky N., Shari K., Al-Fahad A., Wessjohann L., Setzer W. Antimicrobial, antioxidant, and cytotoxic activities of Ocimum forskolei and Teucrium yemense (Lamiaceae) essential oils. Medicines. 2017;4:17. doi: 10.3390/medicines4020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ali N.A.A., Al Sokari S.S., Gushash A., Anwar S., Al-Karani K., Al-Khulaidi A. Ethnopharmacological survey of medicinal plants in Albaha Region, Saudi Arabia. Pharmacogn. Res. 2017;9:401. doi: 10.4103/pr.pr_11_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Batool N., Ilyas N., Shahzad A. Asiatic Mangrove (Rhizophora mucronata)–An overview. Eur. Acad. Res. 2014;2:3348–3363. [Google Scholar]
- 90.Dholi S.K., Raparla R., Mankala S.K., Nagappan K. Invivo Antidiabetic evaluation of Neem leaf extract in alloxan induced rats. J. Appl. Pharm. Sci. 2011;1:100–105. [Google Scholar]
- 91.Moussaieff A., Mechoulam R. Boswellia resin: From religious ceremonies to medical uses; a review of in-vitro, in-vivo and clinical trials. J. Pharm. Pharmacol. 2009;61:1281–1293. doi: 10.1211/jpp/61.10.0003. [DOI] [PubMed] [Google Scholar]
- 92.Gupta D., Bleakley B., Gupta R.K. Dragon’s blood: Botany, chemistry and therapeutic uses. J. Ethnopharmacol. 2008;115:361–380. doi: 10.1016/j.jep.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 93.Shetty A.A., Rana M., Preetham S. Cactus: A medicinal food. J. Food Sci. Technol. 2012;49:530–536. doi: 10.1007/s13197-011-0462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al-Hajj N.Q.M., Wang H., Gasmalla M.A., Ma C., Thabit R., Rahman M.R.T., Tang Y. Chemical composition and antioxidant activity of the essential oil of Pulicaria inuloides. J. Food Nutr. Res. 2014;2:221–227. doi: 10.12691/jfnr-2-5-3. [DOI] [Google Scholar]
- 95.Al-Hajj N.Q.M., Rashid H., Al-Hashedi S., Thabit R., Wang H.X. Total Phenolic Content and Antioxidant, antimicrobial Activity from Some Yemani Plants. Eur. Acad. Res. II. 2014;8:10196–10215. [Google Scholar]
- 96.Al-Juhaimi F.Y., Shahzad S.A., Ahmed A.S., Adiamo O.Q., Ahmed I.A.M., Alsawmahi O.N., Ghafoor K., Babiker E.E. Effect of Argel (Solenostemma argel) leaf extract on quality attributes of chicken meatballs during cold storage. J. Food Sci. Technol. 2018;55:1797–1805. doi: 10.1007/s13197-018-3094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.El Hilaly J., Israili Z.H., Lyoussi B. Acute and chronic toxicological studies of Ajuga iva in experimental animals. J. Ethnopharmacol. 2004;91:43–50. doi: 10.1016/j.jep.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 98.Chenni A., Yahia D.A., Boukortt F., Prost J., Lacaille-Dubois M., Bouchenak M. Effect of aqueous extract of Ajuga iva supplementation on plasma lipid profile and tissue antioxidant status in rats fed a high-cholesterol diet. J. Ethnopharmacol. 2007;109:207–213. doi: 10.1016/j.jep.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 99.Ali B.H., Al-Qarawi A.A., Bashir A.K., Tanira M.O. Phytochemistry, pharmacology and toxicity of Rhazya stricta Decne: A review. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2000;14:229–234. doi: 10.1002/1099-1573(200006)14:4<229::aid-ptr673>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 100.Sdiri M., Li X., Du W., El-Bok S., Xie Y.-Z., Ben-Attia M., Yang B. Anticancer activity of Cynomorium coccineum. Cancers. 2018;10:354. doi: 10.3390/cancers10100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zucca P., Bellot S., Rescigno A. The modern use of an ancient plant: Exploring the antioxidant and nutraceutical potential of the maltese mushroom (Cynomorium coccineum L.) Antioxidants. 2019;8:289. doi: 10.3390/antiox8080289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aljaghthmi O., Heba H., Zeid I.A. Antihyperglycemic properties of mangrove plants (Rhizophora mucronata and Avicennia marina): An overview. Adv. Biol. Res. 2017;11:161–170. [Google Scholar]
- 103.Elsharkawy E.R., Abdallah E.M., Shiboob M.H., Alghanem S. Phytochemical, Antioxidant and Antibacterial Potential of Ducrosia anethifolia in Northern Border Region of Saudi Arabia. J. Pharm. Res. Int. 2019;31:1–8. doi: 10.9734/jpri/2019/v31i630361. [DOI] [Google Scholar]
- 104.Oskoueian E., Abdullah N., Ahmad S., Saad W.Z., Omar A.R., Ho Y.W. Bioactive compounds and biological activities of Jatropha curcas L. kernel meal extract. Int. J. Mol. Sci. 2011;12:5955–5970. doi: 10.3390/ijms12095955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laxane S.N., Swarnkar S., Zanwar S.B., Setty M.M. Jatropha curcas: A systemic review on pharmacological, phytochemical, toxicological profiles and commercial applications. Res. J. Pharm. Biol. Chem. Sci. 2013;4:989–1010. [Google Scholar]
- 106.Carels N. Jatropha curcas: A review. Adv. Bot. Res. 2009;50:39–86. [Google Scholar]
- 107.Mariita R., Ogol C., Oguge N., Okemo P. Antitubercular and phytochemical investigation of methanol extracts of medicinal plants used by the Samburu community in Kenya. Trop. J. Pharm. Res. 2010;9:379–385. doi: 10.4314/tjpr.v9i4.58935. [DOI] [Google Scholar]
- 108.Ali S.S., El-Zawawy N.A., Al-Tohamy R., El-Sapagh S., Mustafa A.M., Sun J. Lycium shawii Roem. & Schult.: A new bioactive antimicrobial and antioxidant agent to combat multi-drug/pan-drug resistant pathogens of wound burn infections. J. Tradit. Complementary Med. 2019;10:13–25. doi: 10.1016/j.jtcme.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maizuwo A.I., Hassan A.S., Momoh H., Muhammad J.A. Phytochemical constituents, biological activities, therapeutic potentials and nutritional values of Moringa oleifera (Zogale): A review. J. Drug Des. Med. Chem. 2017;3:60. [Google Scholar]
- 110.Dalmagro A.P., Camargo A., da Silva Filho H.H., Valcanaia M.M., de Jesus P.C., Zeni A.L.B. Seasonal variation in the antioxidant phytocompounds production from the Morus nigra leaves. Ind. Crop. Prod. 2018;123:323–330. doi: 10.1016/j.indcrop.2018.06.085. [DOI] [Google Scholar]
- 111.Al-Hajj N.Q.M., Rashid H., Wang H., Thabit R., Rashed M. Antioxident, antimicrobial and pulicaria inuloides and ocimum froskolei: A review. Am. Res. Thoughts. 2014;1:973–1000. [Google Scholar]
- 112.Fawzy G.A., Al-Taweel A.M., Perveen S. Anticancer activity of flavane gallates isolated from Plicosepalus curviflorus. Pharmacogn. Mag. 2014;10(Suppl. 3):S519. doi: 10.4103/0973-1296.139787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Balasubramanian V., Rajesh P., Rajaram R., Kannan V.R. A review on Rhizophora genus: Therapeutically important perspective phytochemical constituents. In: Gupta V.K., editor. Bioactive Phytochemicals: Perspectives for Modern Medicine. Volume 3. Daya Publishing House; New Delhi, India: 2015. pp. 211–234. [Google Scholar]
- 114.Khatak M., Khatak S., Siddqui A., Vasudeva N., Aggarwal A., Aggarwal P. Salvadora persica. Pharmacogn. Rev. 2010;4:209. doi: 10.4103/0973-7847.70920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hussain H., Al-Harrasi A., Al-Rawahi A., Hussain J. Chemistry and biology of essential oils of genus boswellia. Evid. Based Complementary Altern. Med. 2013;2013:140509. doi: 10.1155/2013/140509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Zhou J., Cui R. Chemical components of Boswellia carterii. Yao Xue Xue Bao= Acta Pharm. Sin. 2002;37:633–635. [PubMed] [Google Scholar]
- 117.Basar S., Koch A., König W.A. A verticillane-type diterpene from Boswellia carterii essential oil. Flavour Fragr. J. 2001;16:315–318. doi: 10.1002/ffj.992. [DOI] [Google Scholar]
- 118.Said A.A., Aboutabl E.A., El Awdan S.A., Raslan M.A. Proximate analysis, phytochemical screening, and bioactivities evaluation of Cissus rotundifolia (Forssk.) Vahl.(Fam. Vitaceae) and Sansevieria cylindrica Bojer ex Hook.(Fam. Dracaenaceae) growing in Egypt. Egypt. Pharm. J. 2015;14:180. [Google Scholar]
- 119.Said A., Aboutabl E.A., Melek F.R., Abdel Jaleel Raheem Abdel Jaleel G., Raslan M. Phytoconstituents profiling of Cissus rotundifolia (Forssk.) Vahl. by HPLC-MS/MS, and evaluation of its free radical scavenging activity (DPPH) and cytotoxicity. Trends Phytochem. Res. 2018;2:65–74. [Google Scholar]
- 120.De Leo M., De Abreu M.B., Pawlowska A., Cioni P., Braca A. Profiling the chemical content of Opuntia ficus-indica flowers by HPLC–PDA-ESI-MS and GC/EIMS analyses. Phytochem. Lett. 2010;3:48–52. doi: 10.1016/j.phytol.2009.11.004. [DOI] [Google Scholar]
- 121.Livrea M.A., Tesoriere L. Health benefits and bioactive components of the fruits from Opuntia ficus-indica [L.] Mill. J. Prof. Assoc. Cactus Dev. 2006;8:73–90. [Google Scholar]
- 122.Mohamed S.E., Azhari H.E.E., Rasha M., Mona A.A. Adverse reactions of solenostemma argel leaves, extracts and alkaloids tablets administered to patients. Glob. J. Tradit. Med. Syst. 2013;2:14–18. [Google Scholar]
- 123.Teia F.K.F. A review of Solennostemma argel: Phytochemical, pharmacological activities and agricultural applications. J. Ayurvedic Herb. Med. 2018;4:99–101. [Google Scholar]
- 124.Farag M., Al-Rehaily A., Ahmad M.S., Mothana R.A. Detection of hypoglycemic and antidiabetic fraction in ethanol extract of Jatropha curcas aerial parts. Pharmacol. Pharm. 2014;5:663. doi: 10.4236/pp.2014.57076. [DOI] [Google Scholar]
- 125.El-Beshbishy H., Bahashwan S. Hypoglycemic effect of basil (Ocimum basilicum) aqueous extract is mediated through inhibition of α-glucosidase and α-amylase activities: An in vitro study. Toxicol. Ind. Health. 2012;28:42–50. doi: 10.1177/0748233711403193. [DOI] [PubMed] [Google Scholar]
- 126.Hegazi R., El-Gamal M., Abdel-Hady N., Hamdy O. Epidemiology of and risk factors for type 2 diabetes in Egypt. Ann. Glob. Health. 2015;81:814–820. doi: 10.1016/j.aogh.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 127.EL-Manawaty M., Gohar L. In vitro α-glucosidase inhibitory activity of Egyptian plant extracts as an indication for their antidiabetic activity. Vitro. 2018;11:360–367. doi: 10.22159/ajpcr.2018.v11i7.25856. [DOI] [Google Scholar]
- 128.Mostafa N., Singab A. Prospective of Herbal Medicine in Egypt. Med. Chem. (Los Angeles) 2018;8:116–117. doi: 10.4172/2161-0444.1000502. [DOI] [Google Scholar]
- 129.AbouZid S.F., Ahmed O.M., Ahmed R.R., Mahmoud A., Abdella E., Ashour M.B. Antihyperglycemic effect of crude extracts of some Egyptian plants and algae. J. Med. Food. 2014;17:400–406. doi: 10.1089/jmf.2013.0068. [DOI] [PubMed] [Google Scholar]
- 130.Kubacey T.M., Haggag E.G., El-Toumy S.A., Ahmed A.A., El-Ashmawy I.M., Youns M.M. Biological activity and flavonoids from Centaurea alexanderina leaf extract. J. Pharm. Res. 2012;5:3352–3361. [Google Scholar]
- 131.Elshamy A.I., El-Shazly M., Yassine Y.M., El-Bana M.A., Farrag A.-R., Nassar M.I., Singab A.N., Noji M., Umeyama A. Phenolic Constituents, Anti-Inflammatory and Antidiabetic Activities of Cyperus laevigatus L. Pharmacogn. J. 2017;9:828–833. doi: 10.5530/pj.2017.6.129. [DOI] [Google Scholar]
- 132.Khaliq T., Sarfraz M., Ashraf M. Recent progress for the utilization of curcuma longa, Piper nigrum and Phoenix dactylifera seeds against type 2 diabetes. West Indian Med. J. 2015;64:527. doi: 10.7727/wimj.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abdelaziz D.H., Ali S.A., Mostafa M.M. Phoenix dactylifera seeds ameliorate early diabetic complications in streptozotocin-induced diabetic rats. Pharm. Biol. 2015;53:792–799. doi: 10.3109/13880209.2014.942790. [DOI] [PubMed] [Google Scholar]
- 134.Chakroun M., Khemakhem B., Mabrouk H.B., El Abed H., Makni M., Bouaziz M., Drira N., Marrakchi N., Mejdoub H. Evaluation of anti-diabetic and anti-tumoral activities of bioactive compounds from Phoenix dactylifera L’s leaf: In vitro and in vivo approach. Biomed. Pharmacother. 2016;84:415–422. doi: 10.1016/j.biopha.2016.09.062. [DOI] [PubMed] [Google Scholar]
- 135.Aly H.F., Ebrahim M.E., Metawaa H.M., Hosni E.A.-M.A., Ebrahim F.M. In vitro and in vivo evaluation of the antidiabetic effect of different extracts of Nepeta cataria in Streptozocin induced diabetic rats. J. Am. Sci. 2010;6:364–386. [Google Scholar]
- 136.Ibrahim R.M., El-Halawany A.M., Saleh D.O., El Naggar E.M.B., El-Shabrawy A.E.-R.O., El-Hawary S.S. HPLC-DAD-MS/MS profiling of phenolics from Securigera securidaca flowers and its anti-hyperglycemic and anti-hyperlipidemic activities. Rev. Bras. De Farmacogn. 2015;25:134–141. [Google Scholar]
- 137.Shams Eldin S.M., Radwan M.M., Wanas A.S., Habib A.-A.M., Kassem F.F., Hammoda H.M., Khan S.I., Klein M.L., Elokely K.M., ElSohly M.A. Bioactivity-Guided Isolation of Potential Antidiabetic and Antihyperlipidemic Compounds from Trigonella stellata. J. Nat. Prod. 2018;81:1154–1161. doi: 10.1021/acs.jnatprod.7b00707. [DOI] [PubMed] [Google Scholar]
- 138.Abo-elmatty D.M., Essawy S.S., Badr J.M., Sterner O. Antioxidant and anti-inflammatory effects of Urtica pilulifera extracts in type2 diabetic rats. J. Ethnopharmacol. 2013;145:269–277. doi: 10.1016/j.jep.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 139.Asgarpanah J., Haghighat E. Phytochemistry and pharmacologic properties of Ziziphus spina christi (L.) Willd. Afr. J. Pharm. Pharmacol. 2012;6:2332–2339. doi: 10.5897/AJPP12.509. [DOI] [Google Scholar]
- 140.Michel C.G., Nesseem D.I., Ismail M.F. Anti-diabetic activity and stability study of the formulated leaf extract of Zizyphus spina-christi (L.) Willd with the influence of seasonal variation. J. Ethnopharmacol. 2011;133:53–62. doi: 10.1016/j.jep.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 141.AbouZid S.F., Mohamed A.A. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomedicine. 2011;7:18. doi: 10.1186/1746-4269-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Soltan M.M., Zaki A.K. Antiviral screening of forty-two Egyptian medicinal plants. J. Ethnopharmacol. 2009;126:102–107. doi: 10.1016/j.jep.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 143.Eissa T., Palomino O., Carretero M., Gómez-Serranillos M. Ethnopharmacological study of medicinal plants used in the treatment of CNS disorders in Sinai Peninsula, Egypt. J. Ethnopharmacol. 2014;151:317–332. doi: 10.1016/j.jep.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 144.Abu-Rabia A. Ethno-botanic treatments for paralysis (falij) in the Middle East. Chin. Med. 2012;3:25949. doi: 10.4236/cm.2012.34025. [DOI] [Google Scholar]
- 145.Baliga M.S., Baliga B.R.V., Kandathil S.M., Bhat H.P., Vayalil P.K. A review of the chemistry and pharmacology of the date fruits (Phoenix dactylifera L.) Food Res. Int. 2011;44:1812–1822. doi: 10.1016/j.foodres.2010.07.004. [DOI] [Google Scholar]
- 146.Minaiyana M., Moattar F., Vali A. Effect of Securigera securidaca seeds on blood glucose level of normal and diabetic rats. Iran. J. Pharm. Sci. 2006;2:151–156. [Google Scholar]
- 147.Ali-Shtayeh M.S., Yaniv Z., Mahajna J. Ethnobotanical survey in the Palestinian area: A classification of the healing potential of medicinal plants. J. Ethnopharmacol. 2000;73:221–232. doi: 10.1016/S0378-8741(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 148.Ali-Shtayeh M.S., Jamous R., Jamous R. Complementary and alternative medicine use amongst Palestinian diabetic patients. Complementary Ther. Clin. Pract. 2012;18:12–21. doi: 10.1016/j.ctcp.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 149.Singh S., Singh S.K., Yadav A. A review on Cassia species: Pharmacological, traditional and medicinal aspects in various countries. Am. J. Phytomedicine Clin. Ther. 2013;1:291–312. [Google Scholar]
- 150.Selvaraj Y., Chander M.S. Senna-its chemistry, distribution and pharmaceutical value. J. Indian Inst. Sci. 2013;60:179. [Google Scholar]
- 151.Al-Dabbas M.M. Antioxidant activity of different extracts from the aerial part of Moringa peregrina (Forssk.) Fiori, from Jordan. Pak. J. Pharm. Sci. 2017;30:2151–2157. [PubMed] [Google Scholar]
- 152.Said-Al Ahl H., Naguib N.Y., Hussein M.S. Evaluation growth and essential oil content of catmint and lemon catnip plants as new cultivated medicinal plants in Egypt. Ann. Agric. Sci. 2018;63:201–205. doi: 10.1016/j.aoas.2018.11.005. [DOI] [Google Scholar]
- 153.Tofighi Z., Asgharian P., Goodarzi S., Hadjiakhoondi A., Ostad S.N., Yassa N. Potent cytotoxic flavonoids from Iranian Securigera securidaca. Med. Chem. Res. 2014;23:1718–1724. doi: 10.1007/s00044-013-0773-3. [DOI] [Google Scholar]
- 154.Irshaid F., Mansi K. The effects of methanol extract derived from Urtica pilulifera leaves on some hematological and biochemical parameters of diabetic rats. Res. J. Biol. Sci. 2009;4:675–681. [Google Scholar]
- 155.Mostafa D.G., Khaleel E.F., Abdel-Aleem G.A. Inhibition of the hepatic glucose output is responsible for the hypoglycemic effect of Crataegus aronia against type 2 diabetes mellitus in rats. Arch. Biol. Sci. 2018;70:277–287. [Google Scholar]
- 156.Abdullah A., Bakry S., ABD EL-BAKY A., Mansour A. Evaluation of the antioxidative, antidiabetic and antilipidemic effect of bitter mel on seeds (citrullus colocynthis) alcoholic extract on female rats. Al-Azhar Bull. Sci. 2010;21:13–26. [Google Scholar]
- 157.Ali F.T., Hassan N.S., Abdrabou R.R. Potential activity of Moringa oleifera leaf extract and some active ingredients against diabetes in rats. Int. J. Sci. Eng. Res. 2015;6:1490. [Google Scholar]
- 158.Michael H.N., Salib J.Y., Eskander E.F. Bioactivity of diosmetin glycosides isolated from the epicarp of date fruits, Phoenix dactylifera, on the biochemical profile of alloxan diabetic male rats. Phytother. Res. 2013;27:699–704. doi: 10.1002/ptr.4777. [DOI] [PubMed] [Google Scholar]
- 159.Niknami M., Mirbalouchzehi A., Zareban I., Kalkalinia E., Rikhtgarha G., Hosseinzadeh H. Association of health literacy with type 2 diabetes mellitus self-management and clinical outcomes within the primary care setting of Iran. Aust. J. Prim. Health. 2018;24:162–170. doi: 10.1071/PY17064. [DOI] [PubMed] [Google Scholar]
- 160.Esteghamati A., Larijani B., Aghajani M.H., Ghaemi F., Kermanchi J., Shahrami A., Saadat M., Esfahani E.N., Ganji M., Noshad S. Diabetes in Iran: Prospective analysis from first nationwide diabetes report of national program for prevention and control of diabetes (NPPCD-2016) Sci. Rep. 2017;7:13461. doi: 10.1038/s41598-017-13379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ghavam-Haghi F., Sadeghi Dinani M. Isolation and identification of Astragalin and 2-methoxy tyrosol from the bulbs of Allium paradoxum. J. Herbmed. Pharmacol. 2017;6:114–118. [Google Scholar]
- 162.Nickavar B., Yousefian N. Evaluation of α-amylase inhibitory activities of selected antidiabetic medicinal plants. J. Für Verbrauch. Und Lebensm. 2011;6:191–195. doi: 10.1007/s00003-010-0627-6. [DOI] [Google Scholar]
- 163.Dehghan H., Sarrafi Y., Salehi P. Antioxidant and antidiabetic activities of 11 herbal plants from Hyrcania region, Iran. J. Food Drug Anal. 2016;24:179–188. doi: 10.1016/j.jfda.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Salehi P., Asghari B., Esmaeili M.A., Dehghan H., Ghazi I. α-Glucosidase and α-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J. Med. Plants Res. 2013;7:257–266. [Google Scholar]
- 165.Nickavar B., Abolhasani L. Bioactivity-guided separation of an α-amylase inhibitor flavonoid from Salvia virgata. Iran. J. Pharm. Res. Ijpr. 2013;12:57. [PMC free article] [PubMed] [Google Scholar]
- 166.Moradabadi L., Kouhsari S.M., Sani M.F. Hypoglycemic effects of three medicinal plants in experimental diabetes: Inhibition of rat intestinal α-glucosidase and enhanced pancreatic insulin and cardiac glut-4 mrnas expression. Iran. J. Pharm. Res. Ijpr. 2013;12:387. [PMC free article] [PubMed] [Google Scholar]
- 167.Ghorbani A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran:(Part 1): General results. J. Ethnopharmacol. 2005;102:58–68. doi: 10.1016/j.jep.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 168.Amiri M.S., Joharchi M.R. Ethnobotanical investigation of traditional medicinal plants commercialized in the markets of Mashhad, Iran. Avicenna J. Phytomed. 2013;3:254. [PMC free article] [PubMed] [Google Scholar]
- 169.Nejad A.M., Kamkar A., Giri A., Pourmahmoudi A.A. Ethnobotany and folk medicinal uses of major trees and shrubs in Northern Iran. J. Med. Plants Res. 2013;7:284–289. [Google Scholar]
- 170.Bonjar G.S. Screening for antibacterial properties of some Iranian plants against two strains of Escherichia coli. Asian J. Plant Sci. 2004;3:310–314. [Google Scholar]
- 171.Baharvand-Ahmadi B., Asadi-Samani M. A mini-review on the most important effective medicinal plants to treat hypertension in ethnobotanical evidence of Iran. J. Nephropharmacology. 2017;6:3. [PMC free article] [PubMed] [Google Scholar]
- 172.Delfan B., Bahmani M., Kazemeini H., Zargaran A., Rafieian-Kopaei M., Asadi-Samani M., Shahsavari S. Identification of Effective Medicinal Plants for Hyperlipidemia: An Ethnobotanical Study in Lorestan Province, West of Iran. Tradit. Integr. Med. 2016;1:28–34. [Google Scholar]
- 173.Sahranavard S., Naghibi F., Mosaddegh M., Esmaeili S., Sarkhail P., Taghvaei M., Ghafari S. Cytotoxic activities of selected medicinal plants from Iran and phytochemical evaluation of the most potent extract. Res. Pharm. Sci. 2009;4:133. [PMC free article] [PubMed] [Google Scholar]
- 174.Saki K., Bahmani M., Rafieian-Kopaei M., Hassanzadazar H., Dehghan K., Bahmani F., Asadzadeh J. The most common native medicinal plants used for psychiatric and neurological disorders in Urmia city, northwest of Iran. Asian Pac. J. Trop. Dis. 2014;4:S895–S901. doi: 10.1016/S2222-1808(14)60754-4. [DOI] [Google Scholar]
- 175.Bakhshi Jouybari H., Hosseini A.S., Davoodi A., Mirzaee F. Materia medica used in jaundice based on Persian medicine. Res. J. Pharmacogn. 2018;5:83–93. [Google Scholar]
- 176.Rajaei P., Mohamadi N. Ethnobotanical study of medicinal plants of Hezar mountain allocated in south east of Iran. Iran. J. Pharm. Res. Ijpr. 2012;11:1153. [PMC free article] [PubMed] [Google Scholar]
- 177.Bahmani M., Zargaran A., Rafieian-Kopaei M., Saki K. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac. J. Trop. Med. 2014;7:S348–S354. doi: 10.1016/S1995-7645(14)60257-1. [DOI] [PubMed] [Google Scholar]
- 178.Özen T., Çöllü Z., Korkmaz H. Antioxidant properties of Urtica pilulifera root, seed, flower, and leaf extract. J. Med. Food. 2010;13:1224–1231. doi: 10.1089/jmf.2009.1303. [DOI] [PubMed] [Google Scholar]
- 179.Ehsani A., Mahmoudi R. Phytochemical properties and hygienic effects of Allium ascalonicum and Pimpinella anisum essential oils in Iranian white brined cheese. J. Essent. Oil Bear. Plants. 2012;15:1013–1020. doi: 10.1080/0972060X.2012.10662606. [DOI] [Google Scholar]
- 180.Karamian R., Hosseini D.A. Screening of total phenol and flavonoid contents, antioxidant and antibacterial activities of the methanolic extract of Allium ampeloprasum L.(Alliaceae) from Iran. Q. J. Sci. Kharazmi Univ. 2014;14:225–238. [Google Scholar]
- 181.Kim S., Kim D.-B., Jin W., Park J., Yoon W., Lee Y., Kim S., Lee S., Kim S., Lee O.-H. Comparative studies of bioactive organosulphur compounds and antioxidant activities in garlic (Allium sativum L.), elephant garlic (Allium ampeloprasum L.) and onion (Allium cepa L.) Nat. Prod. Res. 2018;32:1193–1197. doi: 10.1080/14786419.2017.1323211. [DOI] [PubMed] [Google Scholar]
- 182.Selim Y.A., Sakeran M.I. Effect of Time Distillation on Chemical Constituents and Anti-Diabetic Activity of the Essential Oil from Dark Green Parts of Egyptian Allium ampeloprasum L. J. Essent. Oil Bear. Plants. 2014;17:838–846. doi: 10.1080/0972060X.2014.935064. [DOI] [Google Scholar]
- 183.Rahimi-Madiseh M., Heidarian E., Kheiri S., Rafieian-Kopaei M. Effect of hydroalcoholic Allium ampeloprasum extract on oxidative stress, diabetes mellitus and dyslipidemia in alloxan-induced diabetic rats. Biomed. Pharmacother. 2017;86:363–367. doi: 10.1016/j.biopha.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 184.Thomson M., Al-Amin Z.M., Al-Qattan K.K., Shaban L.H., Ali M. Anti-diabetic and hypolipidaemic properties of garlic (Allium sativum) in Streptozocin-induced diabetic rats. Int. J. Diabetes Metab. 2007;15:108–115. [Google Scholar]
- 185.Liu C.-T., Hse H., Lii C.-K., Chen P.-S., Sheen L.-Y. Effects of garlic oil and diallyl trisulfide on glycemic control in diabetic rats. Eur. J. Pharmacol. 2005;516:165–173. doi: 10.1016/j.ejphar.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 186.Eidi A., Eidi M., Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and Streptozocin-induced diabetic rats. Phytomedicine. 2006;13:624–629. doi: 10.1016/j.phymed.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 187.Mostofa M., Choudhury M., Hossain M., Islam M., Islam M., Sumon M. Antidiabetic effects of Catharanthus roseus, Azadirachta indica, Allium sativum and glimepride in experimentally diabetic induced rat. Bangladesh J. Vet. Med. 2007;5:99–102. doi: 10.3329/bjvm.v5i1.1324. [DOI] [Google Scholar]
- 188.Moezi L., Arshadi S.S., Motazedian T., Seradj S.H., Dehghani F. Anti-diabetic effects of amygdalus lycioides spach in streptozocin-induced diabetic rats. Iran. J. Pharm. Res. Ijpr. 2018;17:353. [PMC free article] [PubMed] [Google Scholar]
- 189.Hashemnia M., Nikousefat Z., Yazdani-Rostam M. Antidiabetic effect of Pistacia atlantica and Amygdalus scoparia in Streptozocin-induced diabetic mice. Comp. Clin. Pathol. 2015;24:1301–1306. doi: 10.1007/s00580-015-2068-1. [DOI] [Google Scholar]
- 190.Ahangarpour A., Heidari H., Oroojan A.A., Mirzavandi F., Esfehani K.N., Mohammadi Z.D. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-Streptozocin induced type 2 model of diabetes in male mice. Avicenna J. Phytomed. 2017;7:169. [PMC free article] [PubMed] [Google Scholar]
- 191.Ashraf H., Heidari R., Nejati V., Ilkhanipoor M. Effects of aqueous extract of Berberis integerrima root on some physiological parameters in Streptozocin-induced diabetic rats. Iran. J. Pharm. Res. Ijpr. 2013;12:425. [PMC free article] [PubMed] [Google Scholar]
- 192.Ashraf H., Heidari R., Nejati V. Antihyperglycemic and antihyperlipidemic effects of fruit aqueous extract of Berberis integerrima Bge. in Streptozocin-induced diabetic rats. Iran. J. Pharm. Res. Ijpr. 2014;13:1313. [PMC free article] [PubMed] [Google Scholar]
- 193.Sabahi Z., Khoshnood-Mansoorkhani M.J., Rahmani Namadi S., Moein M. Antidiabetic and synergistic effects study of anthocyanin fraction from berberis integerrima fruit on Streptozocin-induced diabetic rats model. Trends Pharm. Sci. 2016;2:43–50. [Google Scholar]
- 194.Akbari F., Khodadadi S., Asgari S., Shirzad H., Mirhoseini M., Shahinfard N., Rafieian-Kopaei M. A comparative study on hypoglycemic properties, lipid profile and bioactive components of hydro-alcoholic extracts of cooked and raw Brassica napus. J. Nephropharmacol. 2016;5:86. [PMC free article] [PubMed] [Google Scholar]
- 195.Fard M.H., Naseh G., Lotfi N., Hosseini S.M., Hosseini M. Effects of aqueous extract of turnip leaf (Brassica rapa) in alloxan-induced diabetic rats. Avicenna J. Phytomed. 2015;5:148. [PMC free article] [PubMed] [Google Scholar]
- 196.Vahid H., Rakhshandeh H., Ghorbani A. Antidiabetic properties of Capparis spinosa L. and its components. Biomed. Pharmacother. 2017;92:293–302. doi: 10.1016/j.biopha.2017.05.082. [DOI] [PubMed] [Google Scholar]
- 197.Rahmani R., Mahmoodi M., Karimi M., Hoseini F., Heydari R., Salehi M., Yousefi A. Effect of hydroalcoholic extract of Capparis spinosa fruit on blood sugar and lipid profile of diabetic and normal rats. Zahedan J. Res. Med. Sci. 2013;15:34–38. [Google Scholar]
- 198.Hashemnia M., Oryan A., Hamidi A.-R., Mohammadalipour A. Blood glucose levels and pathology of organs in alloxan-induced diabetic rats treated with hydro-ethanol extracts of Allium sativum and Capparis spinosa. Afr. J. Pharm. Pharmacol. 2012;6:1559–1564. [Google Scholar]
- 199.Mishra P.R., Panda P.K., Chowdary K.A., Panigrahi S. Antidiabetic and antihyperlipidemic activity of Capparis spinosa extract. Int. J. Pharm. Sci. Rev. Res. 2012;14:38–43. [Google Scholar]
- 200.Jalali M.T., Mohammadtaghvaei N., Larky D.A. Investigating the effects of Capparis spinosa on hepatic gluconeogenesis and lipid content in Streptozocin-induced diabetic rats. Biomed. Pharmacother. 2016;84:1243–1248. doi: 10.1016/j.biopha.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 201.Khanavi M., Taheri M., Rajabi A., Fallah-Bonekohal S., Baeeri M., Mohammadirad A., Amin G., Abdollahi M. Stimulation of hepatic glycogenolysis and inhibition of gluconeogenesis are the mechanisms of antidiabetic effect of Centaurea bruguierana ssp. belangerana. Asian J. Anim. Vet. Adv. 2012;7:1166–1174. doi: 10.3923/ajava.2012.1166.1174. [DOI] [Google Scholar]
- 202.Street R.A., Sidana J., Prinsloo G. Cichorium intybus: Traditional uses, phytochemistry, pharmacology, and toxicology. Evid. Based Complementary Altern. Med. 2013;2013:579319. doi: 10.1155/2013/579319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ghamarian A., Abdollahi M., Su X., Amiri A., Ahadi A., Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. Daru J. Pharm. Sci. 2012;20:56. doi: 10.1186/2008-2231-20-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.El-Ghany M., Nagib R.M., Mamdouh S.M. Anti-diabetic effect of some herbs and fruit against Streptozocin induced diabetic rats. Glob. Vet. 2014;12:541–549. [Google Scholar]
- 205.Shi C., Karim S., Wang C., Zhao M., Murtaza G. A review on antidiabetic activity of Citrullus colocynthis Schrad. Acta Pol. Pharm. 2014;71:363–367. [PubMed] [Google Scholar]
- 206.Hussain A.I., Rathore H.A., Sattar M.Z., Chatha S.A., Sarker S.D., Gilani A.H. Citrullus colocynthis (L.) Schrad (bitter apple fruit): A review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J. Ethnopharmacol. 2014;155:54–66. doi: 10.1016/j.jep.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 207.Oryan A., Hashemnia M., Hamidi A.-R., Mohammadalipour A. Effects of hydro-ethanol extract of Citrullus colocynthis on blood glucose levels and pathology of organs in alloxan-induced diabetic rats. Asian Pac. J. Trop. Dis. 2014;4:125–130. doi: 10.1016/S2222-1808(14)60328-5. [DOI] [Google Scholar]
- 208.Asgary S., Rafieian-Kopaei M., Shamsi F., Najafi S., Sahebkar A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J. Complement. Integr. Med. 2014;11:63–69. doi: 10.1515/jcim-2013-0022. [DOI] [PubMed] [Google Scholar]
- 209.Saidu A.N., Oibiokpa F.I., Olukotun I.O. Phytochemical screening and hypoglycemic effect of methanolic fruit pulp extract of Cucumis sativus in alloxan induced diabetic rats. J. Med. Plants Res. 2014;8:1173–1178. [Google Scholar]
- 210.Karthiyayini T., Kumar R., Kumar K.S., Sahu R.K., Roy A. Evaluation of antidiabetic and hypolipidemic effect of Cucumis sativus fruit in Streptozocin-induced-diabetic rats. Biomed. Pharmacol. J. 2015;2:351–355. [Google Scholar]
- 211.Antido J.W.A., Gatil Y.L.B., Rabajante N.A.L. Hypoglycemic activity of cucumis sativus extract on alloxan-induced diabetic sprague-dawley rats: A pilot study. Lyceum Philipp. St. Cabrini Coll. Allied Med. Res. 2017;2:12–28. [Google Scholar]
- 212.Minaiyan M., Zolfaghari B., Kamal A. Effect of hydroalcoholic and buthanolic extract of Cucumis sativus seeds on blood glucose level of normal and Streptozocin-induced diabetic rats. Iran. J. Basic Med. Sci. 2011;14:436. [PMC free article] [PubMed] [Google Scholar]
- 213.Asgary S., Moshtaghian S.J., Setorki M., Kazemi S., Rafieian-Kopaei M., Adelnia A., Shamsi F. Hypoglycaemic and hypolipidemic effects of pumpkin (Cucurbita pepo L.) on alloxan-induced diabetic rats. Afr. J. Pharm. Pharmacol. 2011;5:2620–2626. [Google Scholar]
- 214.Eslami S., Ebrahimzadeh M., Moghaddam A.H., Nabavi S., Jafari N., Nabavi S. Renoprotective effect of Eryngium caucasicum in gentamicin-induced nephrotoxic mice. Arch. Biol. Sci. 2011;63:157–160. doi: 10.2298/ABS1101157E. [DOI] [Google Scholar]
- 215.Afshari M., Mohammadshahi M., Malayeri A.R., Zaheri L. Antidiabetic, Hepato-Protective and Hypolipidemic Effects of Eryngium Caucasicum Extract in Streptozocin-Nicotinamide Induced Type 2 Diabetes in Male Rats. Iraq Med. J. 2019;3:11–16. [Google Scholar]
- 216.Mahmoudzadeh-Sagheb H., Heidari Z., Bokaeian M., Moudi B. Antidiabetic effects of Eucalyptus globulus on pancreatic islets: A stereological study. Folia Morphol. 2010;69:112–118. [PubMed] [Google Scholar]
- 217.Dey B., Mitra A. Chemo-profiling of eucalyptus and study of its hypoglycemic potential. World J. Diabetes. 2013;4:170. doi: 10.4239/wjd.v4.i5.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zangeneh M.M., Zangeneh A., Tahvilian R., Moradi R. Antidiabetic, hematoprotective and nephroprotective effects of the aqueous extract of Falcaria vulgaris in diabetic male mice. Arch. Biol. Sci. 2018;70:655–664. doi: 10.2298/ABS180222027Z. [DOI] [Google Scholar]
- 219.Rafiey Z., Jalili F., Sohrabi M., Jalili C. Effects of hydro-alcoholic extract of Falcaria vulgaris on pancreas tissue in Streptozocin-induced diabetic rats. Iran. J. Endocrinol. Metab. 2017;19:91–98. [Google Scholar]
- 220.Iranshahi M., Alizadeh M. Antihyperglycemic effect of Asafoetida (Ferula assafoetida Oleo-Gum-Resin) in Streptozocin-induced diabetic rats. World Appl. Sci. J. 2012;17:157–162. [Google Scholar]
- 221.Latifi E., Mohammadpour A.A., Fathi B., Nourani H. Antidiabetic and antihyperlipidemic effects of ethanolic Ferula assa-foetida oleo-gum-resin extract in Streptozocin-induced diabetic wistar rats. Biomed. Pharmacother. 2019;110:197–202. doi: 10.1016/j.biopha.2018.10.152. [DOI] [PubMed] [Google Scholar]
- 222.Abu-Zaiton A.S. Anti-diabetic activity of Ferula assafoetida extract in normal and alloxan-induced diabetic rats. Pak. J. Biol. Sci. 2010;13:97. doi: 10.3923/pjbs.2010.97.100. [DOI] [PubMed] [Google Scholar]
- 223.Shojaee S.S., Vahdati A., Assaei R., Sepehrimanesh M. Effect of Galega officinalis leaf powder and Trigonella foenum-graecum seed powder on blood glucose levels and weight gain in a diabetes mellitus rat model. Comp. Clin. Pathol. 2015;24:145–148. doi: 10.1007/s00580-013-1873-7. [DOI] [Google Scholar]
- 224.Abtahi-Evari S.-H., Shokoohi M., Abbasi A., Rajabzade A., Shoorei H., Kalarestaghi H. Protective Effect of Galega officinalis extract on Streptozocin-induced kidney damage and biochemical factor in diabetic rats. Crescent J. Med. Biol. Sci. 2017;4:108–114. [Google Scholar]
- 225.Mohammadi G., Zangeneh M.M., Rashidi K., Zangeneh A. Evaluation of nephroprotective and antidiabetic effects of Gundelia tournefortii aqueous extract on diabetic nephropathy in male mice. Res. J. Pharmacogn. 2018;5:65–73. [Google Scholar]
- 226.Alimoradi M., Jalili C., Kakeh-Baraei S., Tajehmiri A., Khodarahmi R. Effects of Aqueous Extract of Gunnera (Gundelia tournefortii L.) on the Blood Serum Sugar Levels and Changes in the Streptozocin-induced Diabetic Pancreatic Tissue of Rat. Int. J. Sci. Study. 2017;5:186–191. [Google Scholar]
- 227.Minaiyan M., Ghannadi A., Movahedian A., Hakim-Elahi I. Effect of Hordeum vulgare L.(Barley) on blood glucose levels of normal and STZ-induced diabetic rats. Res. Pharm. Sci. 2014;9:173. [PMC free article] [PubMed] [Google Scholar]
- 228.Delaviz H., Mohammadi J., Ghalamfarsa G., Mohammadi B., Farhadi N. A review study on phytochemistry and pharmacology applications of Juglans regia plant. Pharmacogn. Rev. 2017;11:145. doi: 10.4103/phrev.phrev_10_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rahimi P., Kabiri N., Asgary S., Setorki M. Anti-diabetic effects of walnut oil on alloxan-induced diabetic rats. Afr J. Pharm. Pharm. 2011;5:2655–2661. [Google Scholar]
- 230.Mohammadi J., Delaviz H., Malekzadeh J.M., Roozbehi A. The effect of hydro alcoholic extract of Juglans regia leaves in Streptozocin-nicotinamide induced diabetic rats. Pak. J. Pharm. Sci. 2012;25:407–411. [PubMed] [Google Scholar]
- 231.Mohammadi J., Mirzaie A., Azizi A., Roozbehi A., Delaviz H. The effects of hydroalcoholic extract of Juglans regia leaf on histological changes of Langerhans islet in diabetic rats model. ISMJ. 2012;15:293–302. [Google Scholar]
- 232.Teymouri M., Montaser K.S., Ghafarzadegan R., Haji A.R. Study of hypoglycemic effect of Juglans regia leaves and its mechanism. J. Med. Plants. 2010;9:57–65. [Google Scholar]
- 233.Bayani M., Ahmadi-hamedani M., Javan A.J. Study of hypoglycemic, hypocholesterolemic and antioxidant activities of Iranian Mentha spicata leaves aqueous extract in diabetic rats. Iran. J. Pharm. Res. Ijpr. 2017;16:75. [PMC free article] [PubMed] [Google Scholar]
- 234.Mousa-Al-Reza Hadjzadeh Z.R., Moradi R., Ghorbani A. Effects of hydroalcoholic extract of watercress (Nasturtium officinale) leaves on serum glucose and lipid levels in diabetic rats. Indian J. Physiol. Pharm. 2015;59:223–230. [PubMed] [Google Scholar]
- 235.Akbarzadeh S., Bazzi P., Daneshi A., Nabipour I., Pourkhalili Kh M.G., Sartavi K., Abdi M., Mirzaei M., Bargahi A. Anti-diabetic effect of Otostegia persica extract on diabetic rats. J. Med. Plant. Res. 2012;6:3176–3180. doi: 10.5897/JMPR11.1661. [DOI] [Google Scholar]
- 236.Manzari-Tavakoli A., Pouraboli I., Yaghoobi M.-M., Mehrabani M., Mirtadzadini S.-M. Antihyperglycemic, antilipid peroxidation, and insulin secretory activities of Otostegia persica shoot extract in Streptozocin-induced diabetic rats and in vitro C187 pancreatic β-cells. Pharm. Biol. 2013;51:253–259. doi: 10.3109/13880209.2012.718351. [DOI] [PubMed] [Google Scholar]
- 237.Ebrahimpoor-Mashhadi M.R., Khaksar Z., Noorafshan A., Mogheisi B. Stereological study of the effects of orally administrated Otostegia persica extract on pancreatic beta cells in male diabetic rats. Comp. Clin. Pathol. 2014;23:761–767. doi: 10.1007/s00580-013-1682-z. [DOI] [Google Scholar]
- 238.Heidari P., Rezaei M., Sahebi M., Khadivi A. Phenotypic variability of Pyrus boissieriana Buhse: Implications for conservation and breeding. Sci. Hortic. 2019;247:1–8. doi: 10.1016/j.scienta.2018.11.075. [DOI] [Google Scholar]
- 239.Shahaboddin M.-E., Pouramir M., Moghadamnia A.-A., Parsian H., Lakzaei M., Mir H. Pyrus biossieriana Buhse leaf extract: An antioxidant, antihyperglycaemic and antihyperlipidemic agent. Food Chem. 2011;126:1730–1733. doi: 10.1016/j.foodchem.2010.12.069. [DOI] [PubMed] [Google Scholar]
- 240.Yousefi F., Mahjoub S., Pouramir M., Khadir F. Hypoglycemic activity of Pyrus biossieriana Buhse leaf extract and arbutin: Inhibitory effects on α amylase and α glucosidase. Casp. J. Intern. Med. 2013;4:763. [PMC free article] [PubMed] [Google Scholar]
- 241.Kundakovic T., Ciric A., Stanojkovic T., Sokovic M., Kovacevic N. Cytotoxicity and antimicrobial activity of Pyrus pyraster Burgsd. and Pyrus spinosa Forssk.(Rosaceae) Afr. J. Microbiol. Res. 2014;8:511–518. [Google Scholar]
- 242.Khan S.A., Al Kiyumi A.R., Al Sheidi M.S., Al Khusaibi T.S., Al Shehhi N.M., Alam T. In vitro inhibitory effects on α-glucosidase and α-amylase level and antioxidant potential of seeds of Phoenix dactylifera L. Asian Pac. J. Trop. Biomed. 2016;6:322–329. doi: 10.1016/j.apjtb.2015.11.008. [DOI] [Google Scholar]
- 243.Masmoudi-Allouche F., Touati S., Mnafgui K., Gharsallah N., El Feki A., Allouche N. Phytochemical profile, antioxidant, antibacterial, antidiabetic and anti-obesity activities of fruits and pits from date palm (Phoenix dactylifera L.) grown in south of Tunisia. J. Pharmacogn. Phytochem. 2016;5:15. [Google Scholar]
- 244.El Abed H., Chakroun M., Fendri I., Makni M., Bouaziz M., Drira N., Mejdoub H., Khemakhem B. Extraction optimization and in vitro and in vivo anti-postprandial hyperglycemia effects of inhibitor from Phoenix dactylifera L. parthenocarpic fruit. Biomed. Pharmacother. 2017;88:835–843. doi: 10.1016/j.biopha.2017.01.129. [DOI] [PubMed] [Google Scholar]
- 245.Hasan M., Mohieldein A. In vivo evaluation of anti diabetic, hypolipidemic, antioxidative activities of Saudi date seed extract on Streptozocin induced diabetic rats. J. Clin. Diagn. Res. Jcdr. 2016;10:FF06. doi: 10.7860/JCDR/2016/16879.7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mard S.A., Jalalvand K., Jafarinejad M., Balochi H., Naseri M.K.G. Evaluation of the antidiabetic and antilipaemic activities of the hydroalcoholic extract of Phoenix dactylifera palm leaves and its fractions in alloxan-induced diabetic rats. Malays. J. Med. Sci. Mjms. 2010;17:4. [PMC free article] [PubMed] [Google Scholar]
- 247.Hasona N.A.S.A., Qumani M.A., Alghassab T.A., Alghassab M.A., Alghabban A.A. Ameliorative properties of Iranian Trigonella foenum-graecum L. seeds and Punica granatum L. peel extracts in Streptozocin-induced experimental diabetic guinea pigs. Asian Pac. J. Trop. Biomed. 2017;7:234–239. doi: 10.1016/j.apjtb.2016.12.004. [DOI] [Google Scholar]
- 248.Morshedloo M.R., Maggi F., Neko H.T., Aghdam M.S. Sumac (Rhus coriaria L.) fruit: Essential oil variability in Iranian populations. Ind. Crop. Prod. 2018;111:1–7. doi: 10.1016/j.indcrop.2017.10.002. [DOI] [Google Scholar]
- 249.Mohammadi S., Kouhsari S.M., Feshani A.M. Antidiabetic properties of the ethanolic extract of Rhus coriaria fruits in rats. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2010;18:270. [PMC free article] [PubMed] [Google Scholar]
- 250.Ghorbani A., Amiri M.S., Hosseini A. Pharmacological properties of Rheum turkestanicum Janisch. Heliyon. 2019;5:e01986. doi: 10.1016/j.heliyon.2019.e01986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Mousa-Al-Reza Hadjzadeh Z.R., Khodaei E., Malek M., Ghanbari H. Rheum turkestanicum rhizomes possess anti-hypertriglyceridemic, but not hypoglycemic or hepatoprotective effect in experimental diabetes. Avicenna J. Phytomed. 2017;7:1. [PMC free article] [PubMed] [Google Scholar]
- 252.Estakhr J., Javdan N. Hypoglycemic properties of ethanolic extracts of Salvia hypoleuca in rats. Pharmacologyonline. 2011;3:354–360. [Google Scholar]
- 253.Zarei A., Vaezi G., Malekirad A.A., Abdollahi M. Hypoglycemic and hypolipidemic activities of Salvia hydrangea in Streptozocin-induced diabetes in rats. Iran. J. Basic Med. Sci. 2015;18:417. [PMC free article] [PubMed] [Google Scholar]
- 254.Pouramir M., Shahaboddin M.E., Moghadamnia A.-A., Parastouei K. To study the effects of Securigera securidaca (L.) seed against alloxan-induced hyperglycemia. J. Med. Plants Res. 2011;5:3188–3191. [Google Scholar]
- 255.Ahmadi A., Khalili M., Margedari S., Nahri-Niknafs B. The effects of solvent polarity on hypoglycemic and hypolipidemic activities of Securigera securidaca (L.) seeds. Drug Res. 2016;66:130–135. doi: 10.1055/s-0035-1555773. [DOI] [PubMed] [Google Scholar]
- 256.Tofighi Z., Moradi-Afrapoli F., Ebrahimi S.N., Goodarzi S., Hadjiakhoondi A., Neuburger M., Hamburger M., Abdollahi M., Yassa N. Securigenin glycosides as hypoglycemic principles of Securigera securidaca seeds. J. Nat. Med. 2017;71:272–280. doi: 10.1007/s11418-016-1060-7. [DOI] [PubMed] [Google Scholar]
- 257.Rani Y.S., Reddy V.J., Basha S.J., Koshma M., Hanumanthu G., Swaroopa P. A review on Solanum nigrum. World J. Pharm. Pharm. Sci. 2017;6:293–303. [Google Scholar]
- 258.Bahramikia S., Yazdanparast R. Phytochemistry and medicinal properties of Teucrium polium L.(Lamiaceae) Phytother. Res. 2012;26:1581–1593. doi: 10.1002/ptr.4617. [DOI] [PubMed] [Google Scholar]
- 259.Sabet Z., Roghani M., Najafi M., Maghsoudi Z. Antidiabetic effect of Teucrium polium aqueous extract in multiple low-dose Streptozocin-induced model of type 1 diabetes in rat. J. Basic Clin. Pathophysiol. 2013;1:34–38. [Google Scholar]
- 260.Afifi F.U., Abu-Dahab R., Kasabri V. In vitro modulation of pancreatic MIN6 insulin secretion and proliferation and extrapancreatic glucose absorption by Paronychia argentea, Rheum ribes and Teucrium polium extracts. Jordan J. Pharm. Sci. 2012;108:1–34. [Google Scholar]
- 261.Yadav U.C., Baquer N.Z. Pharmacological effects of Trigonella foenum-graecum L. in health and disease. Pharm. Biol. 2014;52:243–254. doi: 10.3109/13880209.2013.826247. [DOI] [PubMed] [Google Scholar]
- 262.Hannan J.M., Ali L., Rokeya B., Khaleque J., Akhter M., Flatt P., Abdel-Wahab Y.H. Soluble dietary fibre fraction of Trigonella foenum-graecum (fenugreek) seed improves glucose homeostasis in animal models of type 1 and type 2 diabetes by delaying carbohydrate digestion and absorption, and enhancing insulin action. Br. J. Nutr. 2007;97:514–521. doi: 10.1017/S0007114507657869. [DOI] [PubMed] [Google Scholar]
- 263.Kannappan S., Anuradha C. Insulin sensitizing actions of fenugreek seed polyphenols, quercetin & metformin in a rat model. Indian J. Med. Res. 2009;129:401. [PubMed] [Google Scholar]
- 264.Goodarzi M.T., Tootoonchi A.S., Karimi J., Abbasi Oshaghi E. Anti-diabetic effects of aqueous extracts of three Iranian medicinal plants in type 2 diabetic rats induced by high fructose diet. Avi. J. Med. Biochem. 2013;1:7–13. [Google Scholar]
- 265.Nickavar B., Amin G. Bioassay-guided separation of an α-amylase inhibitor anthocyanin from Vaccinium arctostaphylos berries. Z. Für Nat. C. 2010;65:567–570. doi: 10.1515/znc-2010-9-1006. [DOI] [PubMed] [Google Scholar]
- 266.Feshani A.M., Kouhsari S.M., Mohammadi S. Vaccinium arctostaphylos, a common herbal medicine in Iran: Molecular and biochemical study of its antidiabetic effects on alloxan-diabetic Wistar rats. J. Ethnopharmacol. 2011;133:67–74. doi: 10.1016/j.jep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 267.Ahangarpour A., Oroojan A.A., Khorsandi L., Najimi S.A. Pancreatic protective and hypoglycemic effects of Vitex agnus-castus L. fruit hydroalcoholic extract in D-galactose-induced aging mouse model. Res. Pharm. Sci. 2017;12:137. doi: 10.4103/1735-5362.202452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yarizade A., Niazi A., Kumleh H.H. Investigation of antiglycation and antioxidant potential of some antidiabetic medicinal plants. J. Pharm. Sci. Res. 2017;9:2382–2387. [Google Scholar]
- 269.Gohari A., Noorafshan A., Akmali M., Zamani-Garmsiri F., Seghatoleslam A. Urtica Dioica Distillate Regenerates Pancreatic Beta Cells in Streptozocin-Induced Diabetic Rats. Iran. J. Med. Sci. 2018;43:174. [PMC free article] [PubMed] [Google Scholar]
- 270.Rahimzadeh M., Jahanshahi S., Moein S., Moein M.R. Evaluation of α-amylase inhibition by Urtica dioica and Juglans regia extracts. Iran. J. Basic Med. Sci. 2014;17:465. [PMC free article] [PubMed] [Google Scholar]
- 271.Ranjbari A., Azarbayjani M.A., Yusof A., Mokhtar A.H., Akbarzadeh S., Ibrahim M.Y., Tarverdizadeh B., Farzadinia P., Hajiaghaee R., Dehghan F. In vivo and in vitro evaluation of the effects of Urtica dioica and swimming activity on diabetic factors and pancreatic beta cells. BMC Complement. Altern. Med. 2016;16:101. doi: 10.1186/s12906-016-1064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Kadan S., Saad B., Sasson Y., Zaid H. In vitro evaluations of cytotoxicity of eight antidiabetic medicinal plants and their effect on GLUT4 translocation. Evid. Based Complement. Altern. Med. 2013;2013:549345. doi: 10.1155/2013/549345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Kavoosi G. Zataria multiflora essential oil reduces diabetic damages in Streptozocin-induced diabetic rats. Afr. J. Biotechnol. 2011;10:17632–17639. [Google Scholar]
- 274.Moein S., Pimoradloo E., Moein M., Vessal M. Evaluation of antioxidant potentials and α-amylase inhibition of different fractions of Labiatae plants extracts: As a model of antidiabetic compounds properties. Biomed. Res. Int. 2017;2017:7319504. doi: 10.1155/2017/7319504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Solati J., Soleimani N. Antihyperglycemic and antihyperlipidemic effects of Ziziphus vulgaris L. onreptozocin-induced diabetic adult male Wistar rats. Acta Diabetol. 2010;47:219–223. doi: 10.1007/s00592-009-0166-8. [DOI] [PubMed] [Google Scholar]
- 276.Abdel-Zaher A.O., Salim S.Y., Assaf M.H., Abdel-Hady R.H. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J. Ethnopharmacol. 2005;101:129–138. doi: 10.1016/j.jep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 277.Sadeghi M., Zolfaghari B., Senatore M., Lanzotti V. Antifungal cinnamic acid derivatives from Persian leek (Allium ampeloprasum Subsp. Persicum) Phytochem. Lett. 2013;6:360–363. doi: 10.1016/j.phytol.2013.04.007. [DOI] [Google Scholar]
- 278.Ghasemi P.A., Momeni M., Bahmani M. Ethnobotanical study of medicinal plants used by Kurd tribe in Dehloran and Abdanan districts, Ilam province, Iran. Afr. J. Tradit. Complement. Altern. Med. 2013;10:368–385. doi: 10.4314/ajtcam.v10i2.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Niroumand M.C., Farzaei M.H., Razkenari E.K., Amin G., Khanavi M., Akbarzadeh T., Shams-Ardekani M.R. An evidence-based review on medicinal plants used as insecticide and insect repellent in traditional Iranian medicine. Iran. Red Crescent Med. J. 2016;18:e22361. doi: 10.5812/ircmj.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shams-Ghahfarokhi M., Shokoohamiri M.-R., Amirrajab N., Moghadasi B., Ghajari A., Zeini F., Sadeghi G., Razzaghi-Abyaneh M. In vitro antifungal activities of Allium cepa, Allium sativum and ketoconazole against some pathogenic yeasts and dermatophytes. Fitoterapia. 2006;77:321–323. doi: 10.1016/j.fitote.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 281.Baharvand-Ahmadi B., Bahmani M., Tajeddini P., Naghdi N., Rafieian-Kopaei M. An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J. Nephropathol. 2016;5:44. doi: 10.15171/jnp.2016.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nasab F.K., Khosravi A.R. Ethnobotanical study of medicinal plants of Sirjan in Kerman Province, Iran. J. Ethnopharmacol. 2014;154:190–197. doi: 10.1016/j.jep.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 283.Delfan B., Saki K., Bahmani M., Rangsaz N., Delfan M., Mohseni N., Babaeian Z. A study on anti-diabetic and anti-hypertension herbs used in Lorestan province, Iran. J. Herbmed. Pharmacol. 2014;3:71–76. [Google Scholar]
- 284.Saeidnia S., Gohari A.R. Importance of Brassica napus as a medicinal food plant. J. Med. Plants Res. 2012;6:2700–2703. doi: 10.5897/JMPR11.1103. [DOI] [Google Scholar]
- 285.Asadi-Samani M., Moradi M.-T., Mahmoodnia L., Alaei S., Asadi-Samani F., Luther T. Traditional uses of medicinal plants to prevent and treat diabetes; an updated review of ethnobotanical studies in Iran. J. Nephropathol. 2017;6:118. doi: 10.15171/jnp.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Taghavi M., Nazari M., Rahmani R., Sayadi A., Hajizadeh M., Mirzaei M., Ziaaddini H., Hosseini-Zijoud S., Mahmoodi M. Outcome of Capparis spinosa fruit extracts treatment on liver, kidney, pancreas and stomach tissues in normal and diabetic rats. Med. Chem. 2014;4:717–721. doi: 10.4172/2161-0444.1000218. [DOI] [Google Scholar]
- 287.Rahimi R., Amin G., Ardekani M.R.S. A review on Citrullus colocynthis Schrad.: From traditional Iranian medicine to modern phytotherapy. J. Altern. Complementary Med. 2012;18:551–554. doi: 10.1089/acm.2011.0297. [DOI] [PubMed] [Google Scholar]
- 288.Abdollahi B., Abbasi M.M., Milani P.Z., Nourdadgar A.S., Khojasteh S.M.B., Nejati V. Hydro-methanolic extract of Cornus mas L. and blood glucose, lipid profile and hematological parameters of male rats. Iran. Red Crescent Med. J. 2014;16:e17784. doi: 10.5812/ircmj.17784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Khoshbakht K., Hammer K., Pistrick K. Eryngium caucasicum Trautv. cultivated as a vegetable in the Elburz Mountains (Northern Iran) Genet. Resour. Crop Evol. 2007;54:445–448. doi: 10.1007/s10722-006-9121-5. [DOI] [Google Scholar]
- 290.Nowbandegani A.S., Kiumarcy S., Rahmani F., Dokouhaki M., Khademian S., Zarshenas M.M., Faridi P. Ethnopharmacological knowledge of Shiraz and Fasa in Fars region of Iran for diabetes mellitus. J. Ethnopharmacol. 2015;172:281–287. doi: 10.1016/j.jep.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 291.Choobkar N., Kakoolaki S., Mohammadi F. The biological effects of herbal medicine, Falcaria vulgaris: An article review. Iran. J. Aquat. Anim. Health. 2017;3:74–81. doi: 10.18869/acadpub.ijaah.3.1.74. [DOI] [Google Scholar]
- 292.Mosaddegh M., Naghibi F., Moazzeni H., Pirani A., Esmaeili S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J. Ethnopharmacol. 2012;141:80–95. doi: 10.1016/j.jep.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 293.Asgarpanah J., Dadashzadeh Mehrabani G., Ahmadi M., Ranjbar R., Safi-AldinArdebily M. Chemistry, pharmacology and medicinal properties of Heracleum persicum Desf. Ex Fischer: A review. J. Med. Plants Res. 2012;6:1813–1820. [Google Scholar]
- 294.Sadeghi Z., Akaberi M., Valizadeh J. Otostegia persica (Lamiaceae): A review on its ethnopharmacology, phytochemistry, and pharmacology. Avicenna J. Phytomed. 2014;4:79. [PMC free article] [PubMed] [Google Scholar]
- 295.Farzaei M.H., Shams-Ardekani M.R., Abbasabadi Z., Rahimi R. Scientific evaluation of edible fruits and spices used for the treatment of peptic ulcer in traditional Iranian medicine. Isrn Gastroenterol. 2013;2013:136932. doi: 10.1155/2013/136932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shakiba M., Kariminik A., Parsia P. Antimicrobial activity of different parts of Phoenix dactylifera. Int. J. Mol. Clin. Microbiol. 2011;1:107–111. [Google Scholar]
- 297.Abedi A., Parviz M., Karimian S.M., Rodsari H.R.S. Aphrodisiac activity of aqueous extract of Phoenix dactylifera pollen in male rats. Adv. Sex. Med. 2013;3:28. doi: 10.4236/asm.2013.31006. [DOI] [Google Scholar]
- 298.Amirghofran Z. Medicinal plants as immunosuppressive agents in traditional Iranian medicine. Iran J. Immunol. 2010;7:65–73. [PubMed] [Google Scholar]
- 299.Jazayeri S.B., Amanlou A., Ghanadian N., Pasalar P., Amanlou M. A preliminary investigation of anticholinesterase activity of some Iranian medicinal plants commonly used in traditional medicine. Daru J. Pharm. Sci. 2014;22:17. doi: 10.1186/2008-2231-22-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Amiri M.S., Joharchi M.R., TaghavizadehYazdi M.E. Ethno-medicinal plants used to cure jaundice by traditional healers of Mashhad, Iran. Iran. J. Pharm. Res. IJPR. 2014;13:157. [PMC free article] [PubMed] [Google Scholar]
- 301.Javdan N., Estakhr J. Evaluation of the effects of Salvia hypoleuca on the expression of cytokines: IL-6, IL-10 and TNF-α in high fat diet-fed mice towards a cure for diabetes mellitus. Pharmacologyonline. 2011;2:842–852. [Google Scholar]
- 302.Naghibi F., Mosadegh M., Mohammadi M.S., Ghorbani A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran. J. Pharm. Res. 2005;4:63–79. [Google Scholar]
- 303.Bahmani M., Shirzad H., Mirhosseini M., Mesripour A., Rafieian-Kopaei M. A review on ethnobotanical and therapeutic uses of fenugreek (Trigonella foenum-graceum L) J. Evid. Based Complement. Altern. Med. 2016;21:53–62. doi: 10.1177/2156587215583405. [DOI] [PubMed] [Google Scholar]
- 304.Saei-Dehkordi S.S., Tajik H., Moradi M., Khalighi-Sigaroodi F. Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol. 2010;48:1562–1567. doi: 10.1016/j.fct.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 305.Avizeh R., Najafzadeh H., Pourmahdi M., Mirzaee M. Effect of glibenclamide and fruit extract of Zizyphus spina-christi on alloxan-induced diabetic dogs. J. Appl. Res. Vet. Med. 2010;8:109. [Google Scholar]
- 306.Abd F.A.E.-R.A., Ali R.F.M. Proximate compositions, phytochemical constituents, antioxidant activities and phenolic contents of seed and leaves extracts of Egyptian leek (Allium ampeloprasum var. kurrat) Eur. J. Chem. 2013;4:185–190. [Google Scholar]
- 307.Fattorusso E., Iorizzi M., Lanzotti V., Taglialatela-Scafati O. Chemical composition of shallot (Allium ascalonicum Hort.) J. Agric. Food Chem. 2002;50:5686–5690. doi: 10.1021/jf020396t. [DOI] [PubMed] [Google Scholar]
- 308.Mohammadi-Motlagh H.-R., Mostafaie A., Mansouri K. Anticancer and anti-inflammatory activities of shallot (Allium ascalonicum) extract. Arch. Med. Sci. Ams. 2011;7:38. doi: 10.5114/aoms.2011.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Fratianni F., Ombra M.N., Cozzolino A., Riccardi R., Spigno P., Tremonte P., Coppola R., Nazzaro F. Phenolic constituents, antioxidant, antimicrobial and anti-proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.) J. Funct. Foods. 2016;21:240–248. doi: 10.1016/j.jff.2015.12.019. [DOI] [Google Scholar]
- 310.Babaei H., Sadeghpour O., Nahar L., Delazar A., Nazemiyeh H., Mansouri M.R., Poursaeid N., Asnaashari S., Moghadam S.B., Sarker S.D. Antioxidant and vasorelaxant activities of flavonoids from Amygdalus lycioides var. horrida. Turk. J. Biol. 2008;32:203–208. [Google Scholar]
- 311.Takallozadeh M., Bashtani M., Farhangfar H. Evaluation of the nutritive value of wild almond seed (Amygdalus scoparia) and its effect on performance, milk fatty acid composition and antioxidant activity in lactating goats. J. Livest. Sci. Technol. 2019;7:21–31. [Google Scholar]
- 312.Alemardan A., Asadi W., Rezaei M., Tabrizi L., Mohammadi S. Cultivation of Iranian seedless barberry (Berberis integerrima ‘Bidaneh’): A medicinal shrub. Ind. Crop. Prod. 2013;50:276–287. doi: 10.1016/j.indcrop.2013.07.061. [DOI] [Google Scholar]
- 313.Paul S., Geng C.A., Yang T.H., Yang Y.P., Chen J.J. Phytochemical and Health-Beneficial Progress of Turnip (Brassica rapa) J. Food Sci. 2019;84:19–30. doi: 10.1111/1750-3841.14417. [DOI] [PubMed] [Google Scholar]
- 314.Dinda B., Kyriakopoulos A.M., Dinda S., Zoumpourlis V., Thomaidis N.S., Velegraki A., Markopoulos C., Dinda M. Cornus mas L.(cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016;193:670–690. doi: 10.1016/j.jep.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 315.Sahu T., Sahu J. Cucumis sativus (cucumber): A review on its pharmacological activity. J. Appl. Pharm. Res. 2015;3:4–9. [Google Scholar]
- 316.Zengin G., Mahomoodally M.F., Paksoy M.Y., Picot-Allain C., Glamocilja J., Sokovic M., Diuzheva A., Jekő J., Cziáky Z., Rodrigues M.J. Phytochemical characterization and bioactivities of five Apiaceae species: Natural sources for novel ingredients. Ind. Crop. Prod. 2019;135:107–121. doi: 10.1016/j.indcrop.2019.04.033. [DOI] [Google Scholar]
- 317.Goorani S., Zangeneh M.M., Koohi M.K., Seydi N., Zangeneh A., Souri N., Hosseini M.-S. Assessment of antioxidant and cutaneous wound healing effects of Falcaria vulgaris aqueous extract in Wistar male rats. Comp. Clin. Pathol. 2019;28:435–445. doi: 10.1007/s00580-018-2866-3. [DOI] [Google Scholar]
- 318.Lupak M., Khokhla M., Hachkova G.Y., Kanyuka O., Klymyshyn N., Chajka Y.P., Skybitska M., Sybirna N. The alkaloid-free fraction from Galega officinalis extract prevents oxidative stress under experimental diabetes mellitus. Ukr. Biochem. J. 2015;87:78–86. doi: 10.15407/ubj87.04.078. [DOI] [PubMed] [Google Scholar]
- 319.Asadi-Samani M., Rafieian-Kopaei M., Azimi N. Gundelia: A systematic review of medicinal and molecular perspective. Pak. J. Biol. Sci. 2013;16:1238–1247. doi: 10.3923/pjbs.2013.1238.1247. [DOI] [PubMed] [Google Scholar]
- 320.Alkan E.E., Celik I. The therapeutics effects and toxic risk of Heracleum persicum Desf. extract on Streptozocin-induced diabetic rats. Toxicol. Rep. 2018;5:919–926. doi: 10.1016/j.toxrep.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Changxing L., Dongfang D., Lixue Z., Saeed M., Alagawany M., Farag M., Chenling M., Jianhua L. Heracleum persicum: Chemical composition, biological activities and potential uses in poultry nutrition. World’s Poult. Sci. J. 2019;75:207–218. doi: 10.1017/S0043933919000205. [DOI] [Google Scholar]
- 322.Sinha A., Meena A., Panda P., Srivastava B., Gupta M., Padhi M. Phytochemical, pharmacological and therapeutic potential of Hordeum vulgare Linn.-a review. Asian J. Res. Chem. 2012;5:1303–1308. [Google Scholar]
- 323.Hashmi M.A., Khan A., Hanif M., Farooq U., Perveen S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (olive) Evid. Based Complement. Altern. Med. 2015;2015:541591. doi: 10.1155/2015/541591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Arun N., Singh D. Punica granatum: A review on pharmacological and therapeutic properties. Int. J. Pharm. Sci. Res. 2012;3:1240. [Google Scholar]
- 325.Saeidnia S., Ghamarinia M., Gohari A.R., Shakeri A. Terpenes from the root of Salvia hypoleuca Benth. Daru J. Pharm. Sci. 2012;20:66. doi: 10.1186/2008-2231-20-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Firuzi O., Miri R., Asadollahi M., Eslami S., Jassbi A.R. Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iran. J. Pharm. Res. Ijpr. 2013;12:801. [PMC free article] [PubMed] [Google Scholar]
- 327.Zameer S., Najmi A.K., Vohora D., Akhtar M. A review on therapeutic potentials of Trigonella foenum graecum (fenugreek) and its chemical constituents in neurological disorders: Complementary roles to its hypolipidemic, hypoglycemic, and antioxidant potential. Nutr. Neurosci. 2018;21:539–545. doi: 10.1080/1028415X.2017.1327200. [DOI] [PubMed] [Google Scholar]
- 328.Mahboubi M., Kazempour N., Taghizadeh M. In vitro antimicrobial and antioxidant activity of Vaccinium arctostaphylos L. extracts. J. Biol. Act. Prod. Nat. 2013;3:241–247. [Google Scholar]
- 329.Saedi Dezaki E., Mahmoudvand H., Sharififar F., Fallahi S., Monzote L., Ezatkhah F. Chemical composition along with anti-leishmanial and cytotoxic activity of Zataria multiflora. Pharm. Biol. 2016;54:752–758. doi: 10.3109/13880209.2015.1079223. [DOI] [PubMed] [Google Scholar]
- 330.FathiMoghaddam H., Mokhtari M., Kamaei L., Ahangarpour A. Effects of Avicennia Marina leaves aqueous and hydro alcoholic extract on Streptozocin-induced diabetic male rats. J. Rafsanjan Univ. Med. Sci. 2011;10:245–254. [Google Scholar]
- 331.Kazemian M., Abad M., reza Haeri M., Ebrahimi M., Heidari R. Anti-diabetic effect of Capparis spinosa L. root extract in diabetic rats. Avicenna J. Phytomed. 2015;5:325. [PMC free article] [PubMed] [Google Scholar]
- 332.Shahraki A., Shahraki M. The comparison of eucalyptus aqueous extract and insulin on blood sugar and liver enzymes in diabetic male rats. Zahedan J. Res. Med. Sci. 2013;15:25–28. [Google Scholar]
- 333.Pashazadeh M., Mirzazadeh J., Alizadeh A. Effect of Fabaceae (Galega Officinalis L.) consumption on levels of blood glucose, lipids and lipoproteins in Streptozocin-induced diabetic rats. Bull. Env. Pharm. Life Sci. 2015;4:127–132. [Google Scholar]
- 334.Rajaei Z., Mousa-Al-Reza Hadjzadeh R.M., Ghorbani A., Saghebi A. Antihyperglycemic and antihyperlipidemic effects of hydroalcoholic extract of Securigera securidaca seeds in Streptozocin-induced diabetic rats. Adv. Biomed. Res. 2015;4:33. doi: 10.4103/2277-9175.150427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ahangarpour A., Mohammadian M., Dianat M. Antidiabetic effect of hydroalcholic Urtica dioica leaf extract in male rats with fructose-induced insulin resistance. Iran. J. Med. Sci. 2012;37:181. [PMC free article] [PubMed] [Google Scholar]
- 336.Abusaib M., Ahmed M., Nwayyir H.A., Alidrisi H.A., Al-Abbood M., Al-Bayati A., Al-Ibrahimi S., Al-Kharasani A., Al-Rubaye H., Mahwi T. Iraqi Experts Consensus on the Management of Type 2 Diabetes/Prediabetes in Adults. Clin. Med. Insights Endocrinol. Diabetes. 2020;13:1179551420942232. doi: 10.1177/1179551420942232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ali N.S.M., Allela O.Q., Salih H.M., Ahmed I.H. Prevalence of Type 2 Diabetes Associated Complications in Kurdistan Region Iraq. J. Basic Clin. Pharm. 2019;10:1–6. [Google Scholar]
- 338.Aljobouri A.M., Rashid K.I., Ibrahim S.A., Zyer A., Abbas Z.N. Study the effect of Bauhinia variegate Linn. ethanolic extract on reducing glucose and lipid levels of white albino mice. Int. J. Curr. Microbiol. App. Sci. 2015;4:652–658. [Google Scholar]
- 339.Joseph B., Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pac. J. Trop. Dis. 2013;3:93–102. doi: 10.1016/S2222-1808(13)60052-3. [DOI] [Google Scholar]
- 340.Al-Abassi N.N., Ibrahem A.M. Study Antidiabetic Effect of Momordica Charantia (bitter gourd) seeds on Alloxan Induced Diabetic Rats. Iraqi J. Vet. Med. 2010;34:165–170. [Google Scholar]
- 341.Naqishbandi A.M., Josefsen K., Pedersen M.E., Jäger A.K. Hypoglycemic activity of Iraqi Rheum ribes root extract. Pharm. Biol. 2009;47:380–383. doi: 10.1080/13880200902748478. [DOI] [Google Scholar]
- 342.Raafat K., Aboul-Ela M., El-Lakany A. Alloxan-induced diabetic thermal hyperalgesia, prophylaxis and phytotherapeutic effects of Rheum ribes L. in mouse model. Arch. Pharmacal Res. 2014:1–10. doi: 10.1007/s12272-014-0372-y. [DOI] [PubMed] [Google Scholar]
- 343.Kasabri V., Afifi F.U., Hamdan I. In vitro and in vivo acute antihyperglycemic effects of five selected indigenous plants from Jordan used in traditional medicine. J. Ethnopharmacol. 2011;133:888–896. doi: 10.1016/j.jep.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 344.Dizaye K.F., Sultan A.H., Banna H.B. Immunohistochemical and biochemical study of the effect of Rheum ribes on the pancreas of diabetic rats. Zanco J. Med. Sci. 2019;23:394–402. doi: 10.15218/zjms.2019.049. [DOI] [Google Scholar]
- 345.Al-douri N.A. A survey of medicinal plants and their traditional uses in Iraq. Pharm. Biol. 2000;38:74–79. doi: 10.1076/1388-0209(200001)3811-BFT074. [DOI] [PubMed] [Google Scholar]
- 346.Öztürk M., Aydoğmuş-Öztürk F., Duru M.E., Topçu G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): An edible medicinal plant. Food Chem. 2007;103:623–630. doi: 10.1016/j.foodchem.2006.09.005. [DOI] [Google Scholar]
- 347.Al-Sanafi A. The Pharmacological Importance of Bauhinia variegata. A Review. Int. J. Pharma Sci. Res. (Ijsr) 2013;12:160–164. [Google Scholar]
- 348.Tartik M., Darendelioglu E., Aykutoglu G., Baydas G. The various biological activities of Rheum ribes extract on different types of cell. Türk Doğa Ve Fen Dergİsİ. 2015;1:7–12. [Google Scholar]
- 349.Al-Awwadi N.A.J. Anti diabetics effect of Achillea santolina aqueous leaves extract. Int. J. Med. Plants Res. 2013;4:151–156. [Google Scholar]
- 350.Kasabri V., Abu-Dahab R., Afifi F.U., Naffa R., Majdalawi L. Modulation of pancreatic MIN6 insulin secretion and proliferation and extrapancreatic glucose absorption with Achillea santolina, Eryngium creticum and Pistacia atlantica extracts: In vitro evaluation. J. Exp. Integr. Med. 2012;2:245–254. doi: 10.5455/jeim.120612.or.036. [DOI] [Google Scholar]
- 351.Al-Aboudi A., Afifi F.U. Plants used for the treatment of diabetes in Jordan: A review of scientific evidence. Pharm. Biol. 2011;49:221–239. doi: 10.3109/13880209.2010.501802. [DOI] [PubMed] [Google Scholar]
- 352.Dabe N.E., Kefale A.T. Antidiabetic effects of Artemisia species: A systematic review. Anc. Sci. Life. 2017;36:175. doi: 10.4103/asl.ASL_87_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Irshaid F., Mansi K., Aburjai T. Antidiabetic effect of essential oil from Artemisia sieberi growing in Jordan in normal and alloxan induced diabetic rats. Pak. J. Biol. Sci. 2010;13:423. doi: 10.3923/pjbs.2010.423.430. [DOI] [PubMed] [Google Scholar]
- 354.Khayoon H.A., Ali A.H., Kadhim T.A., Abdulhadi H.A. Antidiabetic effect of Artemisia sieberi in rabbits that induced diabetic by alloxan. Health Perspect. 2001;109:69. [Google Scholar]
- 355.Afifi F.U., Kasabri V., Litescu S.C., Abaza I.M. In vitro and in vivo comparison of the biological activities of two traditionally and widely used Arum species from Jordan: Arum dioscoridis Sibth & Sm. and Arum palaestinum Boiss. Nat. Prod. Res. 2016;30:1777–1786. doi: 10.1080/14786419.2015.1072713. [DOI] [PubMed] [Google Scholar]
- 356.Kumar D., Arya V., Bhat Z.A., Khan N.A., Prasad D.N. The genus Crataegus: Chemical and pharmacological perspectives. Rev. Bras. De Farmacogn. 2012;22:1187–1200. doi: 10.1590/S0102-695X2012005000094. [DOI] [Google Scholar]
- 357.Al-Hallaq E.K., Kasabri V., Abdalla S.S., Bustanji Y.K., Afifi F.U. Anti-obesity and antihyperglycemic effects of Crataegus aronia extracts: In vitro and in vivo evaluations. Food Nutr. Sci. 2013;4:972–983. [Google Scholar]
- 358.Alkofahi A.S., Abdul-Razzak K.K., Alzoubi K.H., Khabour O.F. Screening of the Anti-hyperglycemic activity of some medicinal plants of Jordan. Pak. J. Pharm. Sci. 2017;30:907–912. [PubMed] [Google Scholar]
- 359.Kikowska M., Dworacka M., Kędziora I., Thiem B. Eryngium creticum–ethnopharmacology, phytochemistry and pharmacological activity. A review. Rev. Bras. De Farmacogn. 2016;26:392–399. doi: 10.1016/j.bjp.2016.01.008. [DOI] [Google Scholar]
- 360.Kasabri V., Afifi F.U., Hamdan I. Evaluation of the acute antihyperglycemic effects of four selected indigenous plants from Jordan used in traditional medicine. Pharm. Biol. 2011;49:687–695. doi: 10.3109/13880209.2010.539619. [DOI] [PubMed] [Google Scholar]
- 361.Kasabri V., Abu-Dahab R., Afifi F.U., Naffa R., Majdalawi L., Shawash H. In vitro effects of Geranium graveolens, Sarcopoterium spinosum and Varthemia iphionoides extracts on pancreatic MIN6 proliferation and insulin secretion and on extrapancreatic glucose diffusion. Int. J. Diabetes Dev. Ctries. 2013;33:170–177. doi: 10.1007/s13410-013-0131-5. [DOI] [Google Scholar]
- 362.Boukhris M., Bouaziz M., Feki I., Jemai H., El Feki A., Sayadi S. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induced diabetic rats. Lipids Health Dis. 2012;11:81. doi: 10.1186/1476-511X-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Atchibri A., Brou K., Kouakou T., Kouadio Y., Gnakri D. Screening for antidiabetic activity and phytochemical constituents of common bean (Phaseolus vulgaris L.) seeds. J. Med. Plants Res. 2010;4:1757–1761. [Google Scholar]
- 364.Yazdan P.R., Esmaeili M.A., Ashrafi H.J. Teucrium polium extract effects pancreatic function of Streptozocin diabetic rats: A histopathological examination. Iran. Biomed. J. 2005;9:81–85. [Google Scholar]
- 365.Afifi F., Saket M., Jaghabir M., Al-Eisawi D. Effect of Varthemia iphionoides on blood glucose level of normal rats and rats with streptozocin-induced diabetes mellitus. Curr. Ther. Res. 1997;58:888–892. doi: 10.1016/S0011-393X(97)80055-0. [DOI] [Google Scholar]
- 366.Al-Qura’n S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. J. Ethnopharmacol. 2009;123:45–50. doi: 10.1016/j.jep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 367.Nawash O., Shudiefat M., Al-Tabini R., Al-Khalidi K. Ethnobotanical study of medicinal plants commonly used by local bedouins in the badia region of Jordan. J. Ethnopharmacol. 2013;148:921–925. doi: 10.1016/j.jep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 368.Hudaib M., Mohammad M., Bustanji Y., Tayyem R., Yousef M., Abuirjeie M., Aburjai T. Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J. Ethnopharmacol. 2008;120:63–71. doi: 10.1016/j.jep.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 369.Azab A. Arum: A plant genus with great medicinal potential. Eur. Chem. Bull. 2017;6:59–68. doi: 10.17628/ecb.2017.6.59-68. [DOI] [Google Scholar]
- 370.Al-Hallaq E.K., Afifi F.U., Abdalla S.S. Evaluation of the hypocholesterolemic effect and phytochemical screening of the hydroethanolic extract of Crataegus aronia from Jordan. Nat. Prod. Commun. 2012;7:1934578. doi: 10.1177/1934578X1200700113. [DOI] [PubMed] [Google Scholar]
- 371.Aburjai T., Hudaib M., Tayyem R., Yousef M., Qishawi M. Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J. Ethnopharmacol. 2007;110:294–304. doi: 10.1016/j.jep.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 372.Oran S.A., Al-Eisawi D. Ethnobotanical survey of the medicinal plants in the central mountains (North-South) in Jordan. J. Biodivers. Environ. Sci. 2015;6:381–400. [Google Scholar]
- 373.Awwad O., Abu-Dahab R., Abaza I.F., Alabbassi R., Majdalawi L., Afifi F.U. Effect of the galling aphid of Baizongia pistaciae L. on composition and biological activities of essential oils of Pistacia atlantica Desf. growing wild in Jordan. J. Essent. Oil Bear. Plants. 2017;20:791–800. doi: 10.1080/0972060X.2017.1341343. [DOI] [Google Scholar]
- 374.Bozorgi M., Memariani Z., Mobli M., Salehi Surmaghi M.H., Shams-Ardekani M.R., Rahimi R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World J. 2013;2013:219815. doi: 10.1155/2013/219815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Afifi F.U., Kasabri V. Pharmacological and phytochemical appraisal of selected medicinal plants from Jordan with claimed antidiabetic activities. Sci. Pharm. 2013;81:889–932. doi: 10.3797/scipharm.1212-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Mohamed A.E.-H.H., El-Sayed M., Hegazy M.E., Helaly S.E., Esmail A.M., Mohamed N.S. Chemical constituents and biological activities of Artemisia herba-alba. Rec. Nat. Prod. 2010;4:1–25. [Google Scholar]
- 377.Afifi-Yazar F.U., Kasabri V., Abu-Dahab R. Medicinal plants from Jordan in the treatment of cancer: Traditional uses vs. in vitro and in vivo evaluations–Part 1. Planta Med. 2011;77:1203–1209. doi: 10.1055/s-0030-1270832. [DOI] [PubMed] [Google Scholar]
- 378.Al Khateeb W., Hussein E., Qouta L., Alu’datt M., Al-Shara B., Abu-Zaiton A. In vitro propagation and characterization of phenolic content along with antioxidant and antimicrobial activities of Cichorium pumilum Jacq. Plant Cell Tissue Organ Cult. (Pctoc) 2012;110:103–110. doi: 10.1007/s11240-012-0134-9. [DOI] [Google Scholar]
- 379.Aguilar-Santamaría L., Ramírez G., Nicasio P., Alegría-Reyes C., Herrera-Arellano A. Antidiabetic activities of Tecoma stans (L.) Juss. ex Kunth. J. Ethnopharmacol. 2009;124:284–288. doi: 10.1016/j.jep.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 380.Twaij H.A., Al-Dujaili E.A. Evaluation of the anti-diabetic and anti-ulcer properties of some Jordanian and Iraqi medicinal plants; a screening study. JMED Res. 2014;2014:539605. doi: 10.5171/2014.539605. [DOI] [Google Scholar]
- 381.Raafat K., Boukhary R., Aboul-Ela M., El-Lakany A. Endogenous Lebanese plants treating diabetes and related complications. Nat. Prod. Chem. Res. 2013;1:112–120. [Google Scholar]
- 382.Rashid K., Kumar C.S., Haleel P. Healthcare Benefits of Hordeumvulgare L (Barley): A Phyto-Pharmacological Review. Res. J. Pharmacol. Pharmacodyn. 2017;9:207–210. doi: 10.5958/2321-5836.2017.00037.4. [DOI] [Google Scholar]
- 383.Seca A.M., Grigore A., Pinto D.C., Silva A.M. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014;154:286–310. doi: 10.1016/j.jep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 384.Assi M., Ela M., Raafat K., El-Lakany A. Role of antioxidants in the antidiabetic potential of two indigenous Lebanese Inula species. Med. Aromat. Plants S. 2015;2:2167-0412. [Google Scholar]
- 385.Lemouchi R., Selles C., Medjdoub H., Tabti B. Assessment of possible efficacy of aqueous leaves extract of Psoralea bituminosa L. for anti-hyperglycaemic activity. Asian Pac. J. Trop. Dis. 2015;5:575–578. doi: 10.1016/S2222-1808(15)60839-8. [DOI] [Google Scholar]
- 386.Al Ayoubi S., Raafat K., El-Lakany A., Aboul-Ela M. Phytochemical Investigation of Psoralea bituminosa L. and its Anti-Diabetic Potentials. Pharmacogn. J. 2018;10:841–853. doi: 10.5530/pj.2018.5.143. [DOI] [Google Scholar]
- 387.Boukhary R., Aboul-ElA M., Al-Hanbali O., El-Lakany A. Phenolic compounds from Centaurea horrida L growing in Lebanon. Phyto. 2017;9:1–4. doi: 10.25258/ijpapr.v9i1.8030. [DOI] [Google Scholar]
- 388.Nelly A., Annick D.-D., Frederic D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008;120:315–334. doi: 10.1016/j.jep.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 389.Arnold N., Baydoun S., Chalak L., Raus T. A contribution to the flora and ethnobotanical knowledge of Mount Hermon, Lebanon. Fl Medit. 2015;25:13–55. [Google Scholar]
- 390.Khoury M., Stien D., Eparvier V., Ouaini N., El Beyrouthy M. Report on the medicinal use of eleven Lamiaceae species in Lebanon and rationalization of their antimicrobial potential by examination of the chemical composition and antimicrobial activity of their essential oils. Evid. Based Complement. Altern. Med. 2016;2016:2547169. doi: 10.1155/2016/2547169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Fu Z., Wang H., Hu X., Sun Z., Han C. The pharmacological properties of Salvia essential oils. J. Appl. Pharm. Sci. 2013;3:122. [Google Scholar]
- 392.Mohammedi Z. Resistance, pharmacology properties and nutritional value of a shrub from arid environments Atriplex halimus. Res. J. Med. Plant. 2016;10:10–18. doi: 10.3923/rjmp.2016.10.18. [DOI] [Google Scholar]
- 393.Ezeani C., Ezenyi I., Okoye T., Okoli C. Ocimum basilicum extract exhibits antidiabetic effects via inhibition of hepatic glucose mobilization and carbohydrate metabolizing enzymes. J. Intercult. Ethnopharmacol. 2017;6:22. doi: 10.5455/jice.20161229054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Kadan S., Saad B., Sasson Y., Zaid H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016;196:1066–1074. doi: 10.1016/j.foodchem.2015.10.044. [DOI] [PubMed] [Google Scholar]
- 395.Smirin P., Taler D., Abitbol G., Brutman-Barazani T., Kerem Z., Sampson S.R., Rosenzweig T. Sarcopoterium spinosum extract as an antidiabetic agent: In vitro and in vivo study. J. Ethnopharmacol. 2010;129:10–17. doi: 10.1016/j.jep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 396.Rozenberg K., Smirin P., Sampson S.R., Rosenzweig T. Insulin-sensitizing and insulin-mimetic activities of Sarcopoterium spinosum extract. J. Ethnopharmacol. 2014;155:362–372. doi: 10.1016/j.jep.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 397.Elyasiyan U., Nudel A., Skalka N., Rozenberg K., Drori E., Oppenheimer R., Kerem Z., Rosenzweig T. Anti-diabetic activity of aerial parts of Sarcopoterium spinosum. Bmc Complement. Altern. Med. 2017;17:356. doi: 10.1186/s12906-017-1860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Rozenberg K., Rosenzweig T. Sarcopoterium spinosum extract improved insulin sensitivity in mice models of glucose intolerance and diabetes. PLoS ONE. 2018;13:e0196736. doi: 10.1371/journal.pone.0196736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Olaiya C.O., Soetan K.O. A review of the health benefits of fenugreek (Trigonella foenum-graecum L.): Nutritional, Biochemical and pharmaceutical perspectives. Am. J. Soc. Issues Hum. 2014;1:3–12. [Google Scholar]
- 400.Goyal S., Gupta N., Chatterjee S. Investigating therapeutic potential of Trigonella foenum-graecum L. as our defense mechanism against several human diseases. J. Toxicol. 2016;2016:1250387. doi: 10.1155/2016/1250387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Puri D., Prabhu K., Dev G., Agarwal S., Murthy P. Mechanism of antidiabetic action of compound GII purified from fenugreek (Trigonella foenum graecum) seeds. Indian J. Clin. Biochem. 2011;26:335–346. doi: 10.1007/s12291-011-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Anwer T., Sharma M., Pillai K.K., Iqbal M. Effect of Withania somnifera on Insulin Sensitivity in Non-Insulin-Dependent Diabetes Mellitus Rats. Basic Clin. Pharmacol. Toxicol. 2008;102:498–503. doi: 10.1111/j.1742-7843.2008.00223.x. [DOI] [PubMed] [Google Scholar]
- 403.Gorelick J., Rosenberg R., Smotrich A., Hanuš L., Bernstein N. Hypoglycemic activity of withanolides and elicitated Withania somnifera. Phytochemistry. 2015;116:283–289. doi: 10.1016/j.phytochem.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 404.Udayakumar R., Kasthurirengan S., Mariashibu T.S., Rajesh M., Anbazhagan V.R., Kim S.C., Ganapathi A., Choi C.W. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-induced diabetic rats. Int. J. Mol. Sci. 2009;10:2367–2382. doi: 10.3390/ijms10052367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Bano A., Sharma N., Dhaliwal H.S., Sharma V. A systematic and comprehensive review on Withania somnifera (L.) dunal-an indian ginseng. J. Pharm. Res. Int. 2015;7:63–75. doi: 10.9734/BJPR/2015/17102. [DOI] [Google Scholar]
- 406.Said O., Khalil K., Fulder S., Azaizeh H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2002;83:251–265. doi: 10.1016/S0378-8741(02)00253-2. [DOI] [PubMed] [Google Scholar]
- 407.Jaradat N.A., Ayesh O.I., Anderson C. Ethnopharmacological survey about medicinal plants utilized by herbalists and traditional practitioner healers for treatments of diarrhea in the West Bank/Palestine. J. Ethnopharmacol. 2016;182:57–66. doi: 10.1016/j.jep.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 408.Jaradat N. Ethnopharmacological survey of natural products in palestine. An-Najah Univ. J. Res. 2005;19:1880. [Google Scholar]
- 409.Ali-Shtayeh M., Yaghmour R.M.-R., Faidi Y., Salem K., Al-Nuri M. Antimicrobial activity of 20 plants used in folkloric medicine in the Palestinian area. J. Ethnopharmacol. 1998;60:265–271. doi: 10.1016/S0378-8741(97)00153-0. [DOI] [PubMed] [Google Scholar]
- 410.Ali-Shtayeh M.S., Jamous R.M. Herbal medicines in cancer care in the Palestinian authority. Eur. J. Integr. Med. 2011;3:125–131. doi: 10.1016/j.eujim.2011.05.049. [DOI] [Google Scholar]
- 411.Jaradat N.A., Shawahna R., Eid A.M., Al-Ramahi R., Asma M.K., Zaid A.N. Herbal remedies use by breast cancer patients in the West Bank of Palestine. J. Ethnopharmacol. 2016;178:1–8. doi: 10.1016/j.jep.2015.11.050. [DOI] [PubMed] [Google Scholar]
- 412.Kaileh M., Berghe W.V., Boone E., Essawi T., Haegeman G. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. J. Ethnopharmacol. 2007;113:510–516. doi: 10.1016/j.jep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 413.Jaradat N.A., Zaid A.N., Al-Ramahi R., Alqub M.A., Hussein F., Hamdan Z., Mustafa M., Qneibi M., Ali I. Ethnopharmacological survey of medicinal plants practiced by traditional healers and herbalists for treatment of some urological diseases in the West Bank/Palestine. BMC Complement. Altern. Med. 2017;17:255. doi: 10.1186/s12906-017-1758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Rao M.U., Sreenivasulu M., Chengaiah B., Reddy K.J., Chetty C.M. Herbal medicines for diabetes mellitus: A review. Int. J. Pharmtech. Res. 2010;2:1883–1892. [Google Scholar]
- 415.Nathiya S., Durga M., Devasena T. Therapeutic role of Trigonella foenum-graecum [Fenugreek]–a review. Int. J. Pharm. Sci. Rev. Res. 2014;27:74–80. [Google Scholar]
- 416.Kulkarni S., Dhir A. Withania somnifera: An Indian ginseng. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32:1093–1105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 417.Kadan S., Sasson Y., Saad B., Zaid H. Gundelia tournefortii antidiabetic efficacy: Chemical composition and GLUT4 translocation. Evid. Based Complement. Altern. Med. 2018;2018:8294320. doi: 10.1155/2018/8294320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Wainstein J., Ganz T., Boaz M., Bar Dayan Y., Dolev E., Kerem Z., Madar Z. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. J. Med. Food. 2012;15:605–610. doi: 10.1089/jmf.2011.0243. [DOI] [PubMed] [Google Scholar]
- 419.Dalar A., Konczak I. Phenolic contents, antioxidant capacities and inhibitory activities against key metabolic syndrome relevant enzymes of herbal teas from Eastern Anatolia. Ind. Crop. Prod. 2013;44:383–390. doi: 10.1016/j.indcrop.2012.11.037. [DOI] [Google Scholar]
- 420.Zengin G., Guler G.O., Aktumsek A., Ceylan R., Picot C.M.N., Mahomoodally M.F. Enzyme inhibitory properties, antioxidant activities, and phytochemical profile of three medicinal plants from Turkey. Adv. Pharmacol. Sci. 2015;2015:410675. doi: 10.1155/2015/410675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Orhan N., Hoçbaç S., Orhan D.D., Asian M., Ergun F. Enzyme inhibitory and radical scavenging effects of some antidiabetic plants of Turkey. Iran. J. Basic Med. Sci. 2014;17:426. [PMC free article] [PubMed] [Google Scholar]
- 422.Dalar A., Konczak I. Cichorium intybus from Eastern Anatolia: Phenolic composition, antioxidant and enzyme inhibitory activities. Ind. Crop. Prod. 2014;60:79–85. doi: 10.1016/j.indcrop.2014.05.043. [DOI] [Google Scholar]
- 423.Gulcin I., Kaya R., Goren A.C., Akincioglu H., Topal M., Bingol Z., Cetin Çakmak K., Ozturk Sarikaya S.B., Durmaz L., Alwasel S. Anticholinergic, antidiabetic and antioxidant activities of cinnamon (cinnamomum verum) bark extracts: Polyphenol contents analysis by LC-MS/MS. Int. J. Food Prop. 2019;22:1511–1526. doi: 10.1080/10942912.2019.1656232. [DOI] [Google Scholar]
- 424.Mahomoodally M.F., Zengin G., Aladag M.O., Ozparlak H., Diuzheva A., Jekő J., Cziáky Z., Aumeeruddy M.Z. HPLC-MS/MS chemical characterization and biological properties of Origanum onites extracts: A recent insight. Int. J. Environ. Health Res. 2019;29:607–621. doi: 10.1080/09603123.2018.1558184. [DOI] [PubMed] [Google Scholar]
- 425.Gülçin İ., Gören A.C., Taslimi P., Alwasel S.H., Kılıc O., Bursal E. Anticholinergic, antidiabetic and antioxidant activities of Anatolian pennyroyal (Mentha pulegium)-analysis of its polyphenol contents by LC-MS/MS. Biocatal. Agric. Biotechnol. 2020;23:101441. doi: 10.1016/j.bcab.2019.101441. [DOI] [Google Scholar]
- 426.Zengin G., Sarikurkcu C., Aktumsek A., Ceylan R., Ceylan O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crop. Prod. 2014;53:244–251. doi: 10.1016/j.indcrop.2013.12.043. [DOI] [Google Scholar]
- 427.Basak S.S., Candan F. Effect of Laurus nobilis L. essential oil and its main components on α-glucosidase and reactive oxygen species scavenging activity. Iran. J. Pharm. Res. Ijpr. 2013;12:367. [PMC free article] [PubMed] [Google Scholar]
- 428.Kültür Ş. An ethnobotanical study of Kırklareli (Turkey) Phytol. Balc. 2008;14:279–289. [Google Scholar]
- 429.Yücel E., Özel A.N. The plants consumed as food in Kemaliye (Erzincan/Turkey) district and other typical foods in this region. 2013. Biol. Divers. Conserv. 2013 [Google Scholar]
- 430.Özbel Y., Özbilgin A. In vitro and in vivo activities of Haplophyllum myrtifolium against Leishmania tropica. New Microbiol. 2007;30:439–445. [PubMed] [Google Scholar]
- 431.Bagci E., Elkiran O., Evren H. Constituents of the essential oils of Helichrysum graveolens (Bieb.) Sweet from Turkey. Asian J. Chem. 2013;25:7254. doi: 10.14233/ajchem.2013.14532. [DOI] [Google Scholar]
- 432.Aslan M., Orhan D.D., Orhan N., Sezik E., Yeşilada E. A study of antidiabetic and antioxidant effects of Helichrysum graveolens capitulums in Streptozocin-induced diabetic rats. J. Med. Food. 2007;10:396–400. doi: 10.1089/jmf.2006.293. [DOI] [PubMed] [Google Scholar]
- 433.Ugulu I. Traditional ethnobotanical knowledge about medicinal plants used for external therapies in Alasehir, Turkey. Int. J. Med. Arom. Plants. 2011;1:101–106. [Google Scholar]
- 434.Kupeli E., Orhan I., Yesilada E. Evaluation of some plants used in Turkish folk medicine for their anti-inflammatory and antinociceptive activities. Pharm. Biol. 2007;45:547–555. doi: 10.1080/13880200701498895. [DOI] [Google Scholar]
- 435.Baser K., Kürkçüoglu M., Tarimcilar G., Kaynak G. Essential oils of Mentha species from Northern Turkey. J. Essent. Oil Res. 1999;11:579–588. doi: 10.1080/10412905.1999.9701218. [DOI] [Google Scholar]
- 436.Sarikurkcu C., Eryigit F., Cengiz M., Tepe B., Cakir A., Mete E. Screening of the antioxidant activity of the essential oil and methanol extract of Mentha pulegium L. from Turkey. Spectrosc. Lett. 2012;45:352–358. doi: 10.1080/00387010.2012.666701. [DOI] [Google Scholar]
- 437.Orhan N., Aslan M., Şüküroğlu M., Orhan D.D. In vivo and in vitro antidiabetic effect of Cistus laurifolius L. and detection of major phenolic compounds by UPLC–TOF-MS analysis. J. Ethnopharmacol. 2013;146:859–865. doi: 10.1016/j.jep.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 438.Orhan N., Aslan M., Pekcan M., Orhan D.D., Bedir E., Ergun F. Identification of hypoglycaemic compounds from berries of Juniperus oxycedrus subsp. oxycedrus through bioactivity guided isolation technique. J. Ethnopharmacol. 2012;139:110–118. doi: 10.1016/j.jep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 439.Orhan N., Orhan D.D., Gökbulut A., Aslan M., Ergun F. Comparative Analysis of Chemical Profile, Antioxidant, In-vitro and In-vivo Antidiabetic Activities of Juniperus foetidissima Willd. and Juniperus sabina L. Iran. J. Pharm. Res. Ijpr. 2017;16:64. [PMC free article] [PubMed] [Google Scholar]
- 440.Orhan N., Aslan M., Demirci B., Ergun F. A bioactivity guided study on the antidiabetic activity of Juniperus oxycedrus subsp. oxycedrus L. leaves. J. Ethnopharmacol. 2012;140:409–415. doi: 10.1016/j.jep.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 441.Orhan N., Berkkan A., Orhan D.D., Aslan M., Ergun F. Effects of Juniperus oxycedrus ssp. oxycedrus on tissue lipid peroxidation, trace elements (Cu, Zn, Fe) and blood glucose levels in experimental diabetes. J. Ethnopharmacol. 2011;133:759–764. doi: 10.1016/j.jep.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 442.Dehghan H., Sarrafi Y., Salehi P., Ebrahimi S.N. α-Glucosidase inhibitory and antioxidant activity of furanocoumarins from Heracleum persicum. Med. Chem. Res. 2017;26:849–855. doi: 10.1007/s00044-017-1796-y. [DOI] [Google Scholar]
- 443.Afrisham R., Aberomand M., Ghaffari M.A., Siahpoosh A., Jamalan M. Inhibitory effect of Heracleum persicum and Ziziphus jujuba on activity of α-amylase. J. Bot. 2015;2015:824683. [Google Scholar]
- 444.Kiliçgün H., Korkmaz M., Kılıçgün H. Hepatoprotective and antidiabetic activity of origanum minutiflorum grown wild in Turkey. Bothalia J. 2014;44:3. [Google Scholar]
- 445.Cam M.E., YILDIZ S., ERTAŞ B., ACAR A.E., TAŞKIN T., Kabasakal L. Antidiabetic effects of Salvia triloba and Thymus praecox subsp. skorpilii var. skorpilii in a rat model of Streptozocin/nicotinamide-induced diabetes. Marmara Pharm. J. 2017;21:818–827. doi: 10.12991/mpj.2017.8. [DOI] [Google Scholar]
- 446.Durmuşkahya C., Öztürk M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes in Manisa, Turkey. Sains Malays. 2013;42:1431–1438. doi: 10.2174/2210289201304010288. [DOI] [Google Scholar]
- 447.Tumen I., Süntar I., Keleş H., Küpeli Akkol E. A therapeutic approach for wound healing by using essential oils of Cupressus and Juniperus species growing in Turkey. Evid. Based Complement. Altern. Med. 2012;2012:728281. doi: 10.1155/2012/728281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Cetin H., Yanikoglu A. A study of the larvicidal activity of Origanum (Labiatae) species from southwest Turkey. J. Vector Ecol. 2006;31:118–122. doi: 10.3376/1081-1710(2006)31[118:ASOTLA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 449.Doğan A., Çelik İ. Healing effects of sumac (Rhus coriaria) in Streptozocin-induced diabetic rats. Pharm. Biol. 2016;54:2092–2102. doi: 10.3109/13880209.2016.1145702. [DOI] [PubMed] [Google Scholar]
- 450.Sezik E., Tabata M., Yesilada E., Honda G., Goto K. Traditional medicine in Turkey. I: Folk medicine in Northeast anatolia. J. Ethnopharmacol. 1991;35:191–196. doi: 10.1016/0378-8741(91)90072-L. [DOI] [PubMed] [Google Scholar]
- 451.Ozen T., Demirtas I., Aksit H. Determination of antioxidant activities of various extracts and essential oil compositions of Thymus praecox subsp. skorpilii var. skorpilii. Food Chem. 2011;124:58–64. doi: 10.1016/j.foodchem.2010.05.103. [DOI] [Google Scholar]
- 452.Azaz A.D., Irtem H.A., Kurkcuoǧlu M., Baser K.H.C. Composition and the in vitro antimicrobial activities of the essential oils of some Thymus species. Z. Für Nat. C. 2004;59:75–80. doi: 10.1515/znc-2004-1-216. [DOI] [PubMed] [Google Scholar]
- 453.Chaouche T.M., Haddouchi F., Atik-Bekara F., Ksouri R., Azzi R., Boucherit Z., Tefiani C., Larbat R. Antioxidant, haemolytic activities and HPLC–DAD–ESI–MSn characterization of phenolic compounds from root bark of Juniperus oxycedrus subsp. oxycedrus. Ind. Crop. Prod. 2015;64:182–187. doi: 10.1016/j.indcrop.2014.10.051. [DOI] [Google Scholar]
- 454.Abu-Reidah I.M., Ali-Shtayeh M.S., Jamous R.M., Arráez-Román D., Segura-Carretero A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015;166:179–191. doi: 10.1016/j.foodchem.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 455.El-Sayed N., El-Eraky W., Ibrahim M., Mabry T. Antiinflammatory and ulcerogenic activities of Salvia triloba extracts. Fitoterapia. 2006;77:333–335. doi: 10.1016/j.fitote.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 456.Ghazanfar S., McDaniel T. Floras of the Middle East: A quantitative analysis and biogeography of the flora of Iraq. Edinb. J. Bot. 2016;73:1–24. doi: 10.1017/S0960428615000244. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available in a publicly accessible repository.