Abstract
Colorectal cancer (CRC) remains a leading cause of cancer-related deaths in the United States despite an array of available treatment options. Current standard-of-care interventions for this malignancy include surgical resection, chemotherapy, and targeted therapies depending on the disease stage. Specifically, infusion of anti-vascular endothelial growth factor agents in combination with chemotherapy was an important development in improving the survival of patients with advanced colorectal cancer, while also helping give rise to other forms of anti-angiogenic therapies. Yet, one approach by which tumor angiogenesis may be further disrupted is through the administration of a dendritic cell (DC) vaccine targeting tumor-derived blood vessels, leading to cytotoxic immune responses that decrease tumor growth and synergize with other systemic therapies. Early generations of such vaccines exhibited protection against various forms of cancer in pre-clinical models, but clinical results have historically been disappointing. Sipuleucel-T (Provenge®) was the first, and to-date, only dendritic cell-based therapy to receive FDA approval after significantly increasing overall survival in prostate cancer patients. The unparalleled success of Sipuleucel-T has helped revitalize the clinical development of dendritic cell vaccines, which will be examined in this review. We also highlight the promise of these vaccines to instill anti-angiogenic immunity for individuals with advanced colorectal cancer.
Keywords: tumor angiogenesis, immunotherapy, targeted cancer therapy, listeria monocytogenes, cytotoxic chemotherapy, Sipuleucel-T
Graphical abstract
INTRODUCTION
In the United States, over 100,000 new cases of colon cancer and 43,000 cases of rectal cancer will be diagnosed in 2020 [1]. There will also be approximately 53,200 deaths due to colorectal cancer (CRC), making the disease the third most common cause of cancer-related incidence and death in the country. Common risk factors for CRC include obesity, smoking, poor diet, genetic predisposition (e.g., Lynch syndrome, familial adenomatous polyposis), and other medical conditions such as chronic inflammatory bowel disorders. Yet, due in part to available screening methods (e.g., colonoscopy) that help detect/remove precancerous lesions, CRC incidence and mortality rates have seen dramatic declines in older adults[1, 2]. However, the incidence of this cancer type is increasing in individuals younger than the age of 55 [3].
CRC first develops in the mucosa of either the colon or rectum in the form of a noncancerous polyp (more commonly diagnosed as an adenoma) [4]. Adenomas typically grow slowly over time in the inner lining of the large intestine, but the risk of cancer increases as adenomas grow larger eventually penetrating the colon/rectal wall and accessing nearby tissues or underlying blood and lymphatic vessels that allows spread to the liver, lungs, or peritoneum [5]. Early-stage disease (Stage 0-II) that is confined to the bowel is usually treated with surgery only and results in an exceptional prognosis (90% 5-year survival rate) [6]. Unfortunately, a majority of patients present with either regional or metastatic disease (i.e., Stage III and beyond) that requires additional standard-of-care treatments such as chemotherapy and targeted drugs to help minimize cancer progression. Approximately 22% of CRC patients are found with metastases (designated mCRC) upon diagnosis, and 50–60% of individuals will develop metastases during the course of their disease [6, 7]. Despite rigorous medical interventions, 5-year survival rates for mCRC are only 14%, which clearly supports the need for new and more efficacious strategies once the cancer spreads beyond the large intestine [6].
Current standard-of-care treatments for stage IV colon cancer may include surgical resection of primary lesions and hepatic/pulmonary metastases (if possible) and systemic regimens that combine chemotherapy (e.g., fluorouracil-based) with targeted agents [8]. FDA-approved first-line targeted therapies include the more commonly used anti-epidermal growth factor receptor (EGFR) (cetuximab [Erbitux®], panitumumab [Vectibix®]) and anti-vascular endothelial growth factor (VEGF) (bevacizumab [Avastin®]) antibodies [9]. However, cancer cells harboring mutations downstream of EGFR signaling such as in KRAS, NRAS, BRAF, or PIK3CA, will be unresponsive to EGFR inhibition, and treatment options must be adjusted accordingly [10–12]. In fact, CRC patients that do not exhibit wild-type RAS, will demonstrate reduced progression-free survival and overall survival following cetuximab or panitumumab treatment [13, 14]. In cases where cancer progresses following first-line treatment, approved second-line targeted options include ramucirumab (Cyramza®) (anti-VEGFR2 antibody), regorafenib (Stivarga®) (kinase inhibitor), or ziv− aflibercept (Eylea® and Zaltrap®) (multiple angiogenic factor trap). The relative short-term successes of all of these treatment options for most advanced CRC patients have previously been reported [8, 15–17].
It is not currently clear whether VEGF abrogation is preferred to EGFR inhibition with first-line chemotherapy, but, historically, the addition of anti-VEGF agents to chemotherapy has provided increased survival to mCRC patients [18]. Bevacizumab, in particular, was the first angiogenic treatment to be approved by the FDA for the treatment of cancer and works by blocking VEGF interaction with cognate VEGF receptors (VEGFRs), helping prevent tumor growth by inhibiting tumor angiogenesis [19]. In one pivotal phase III trial, 813 previously untreated mCRC patients were randomly assigned to receive a fluorouracil-based chemotherapy regimen with/without bevacizumab [20]. The addition of VEGF blockade provided superior enhancements in median overall survival (20.3 months v. 15.6 months), median progression-free survival (10.6 months v. 6.2 months), and overall response rates (44.8% v. 34.8%). As a major development in the treatment of advanced CRC, bevacizumab has helped paved the way for expanding anti-angiogenic strategies against vascularized tumors such as colon cancer [21].
TUMOR ANGIOGENESIS
Primary (avascular) tumors will generally grow only to a stable size of 1–2 mm3 without a sufficient blood system to supply oxygen and nutrients and alleviate waste accumulation [22]. During the initial phases of cancer growth, there is a presumed balance between pro-angiogenic and anti-angiogenic factors, but when this balance is disrupted to favor angiogenesis (a threshold referred to as “the angiogenic switch”), the influence of pro-angiogenic factors is increased and tumor progression continues [23]. A defining trigger of the angiogenic switch is a lack of sufficient oxygen within the tumor microenvironment that results from unabated cancer cell growth [24, 25]. As a tumor expands, areas within the lesion too remote from blood vessel support (typically >100 μM) will experience hypoxia that disrupts (via hypoxia inducible factors [HIFs]) normal metabolic processes to instead drastically upregulate expression of key angiogenic molecules such as VEGF and platelet-derived growth factor [26]. Additionally, HIF-dependent angiogenesis can be prompted from genomic damage sustained by tumor cells. For example, mutations in tumor suppressor genes such as P53 and PTEN are directly linked to HIF accumulation that works to promote VEGF expression [27, 28].
The continued development of a tumor lesion’s vasculature is reliant on a wealth of other soluble mediators (e.g., cytokines, chemokines, extracellular matrix remodeling factors, and pro-angiogenic molecules) like fibroblast growth factor, angiopoietin-2, placenta growth factor, and matrix metalloproteases that are supplied by tumor cells and accessory cells such as cancer-associated fibroblasts and immune cells [29]. However, the continued overexpression of VEGF binds endothelial cell-derived VEGFR2 to initiate/sustain sprouting angiogenesis and vasculogenesis [30, 31]. Overall, the resultant vasculature within the tumor microenvironment is phenotypically distinct from blood vessels occurring in healthy tissues. Newly formed cancer-derived blood vessels may be deficient in a basement membrane and supportive cells such as pericytes and yield abnormalities in length/surface area that preclude archetypal blood vessel hierarchies in normal vascularized tissues [32, 33]. Functionally, this chaotic blood system demonstrates increased vascular permeability that sustains hypoxia and permits fluid leak into the surrounding tissue, exacerbating interstitial pressure [34]. Those tumor-retained cells that evolved during tumorigenesis will also continue to influence angiogenesis in order to further promote cancer progression and spread [35, 36].
Although passive infusions of anti-vascular immune agents (e.g., bevacizumab, ramucirumab) work to minimize such tumor-promoting networks and have provided mCRC patients short-term clinical relief, a conceptual therapeutic improvement relates to instituting sustained immune-related activity in the host through vaccination [37, 38] or adoptive cell therapy [39, 40]. Given their unique nature, tumor-derived blood vessels are immunogenic and can be specifically targeted by immune cells to induce tumor regression and institute immunologic memory to help prevent cancer recurrence [41, 42]. One such powerful immune cell inducer of anti-tumor immunity that also holds tremendous promise as an immunotherapeutic strategy is the dendritic cell (DC).
DENDRITIC CELLS
DCs are professional antigen presenting cells (APCs) that belong to the mononuclear phagocyte system and can activate naïve T cells against various host insults including cancer. Initially discovered in 1868 by Paul Langerhans, DCs received their name in 1973 upon the identification of cells in the mouse spleen displaying long cytoplasmic processes [43]. The various DC subsets first arise from a unique hematopoietic lineage in the bone marrow. Like other leukocytes, DCs develop from bone marrow-derived hematopoietic CD34+ stem cells and further differentiate from common myeloid progenitors, although a small fraction of DCs can arise from common lymphoid progenitor cells [44, 45]. Downstream of the common myeloid progenitors, macrophage/DC progenitor cells serve to provide the host a steady supply of monocytes, macrophages, and “classical” DCs. In particular to DC development, macrophage/DC progenitors give rise to common DC progenitor cells that expand into pre-DCs, which travel to lymphoid and non-lymphoid organs to further mature into functional DC subtypes based on intrinsic and/or external factors as clarified below [46, 47].
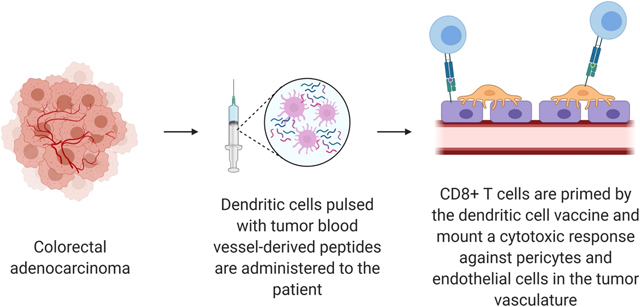
While residing in peripheral tissues, DCs sample the surrounding environment through receptor-mediated phagocytosis or macropinocytosis, and, following antigen uptake, migrate to draining lymph nodes by responding to the chemokines CCL19 (secreted by mature dendritic cells) or CCL21 (secreted by lymphatic vessel-derived endothelial cells) through upregulation of CCR7 [48–51]. During their trafficking, antigen-bearing DCs assume a mature APC phenotype, which is marked by increased expression of surface-molecules such as major histocompatibility complex (MHC) class II and CD80/CD86 [52]. DCs then engage/present processed antigen to T cells in the lymph node paracortex via the MHC and provide the necessary costimulation and cytokine support for T cell activation and proliferation [53, 54] (outlined in Figure 1).
Figure 1.
General overview of directing dendritic cell vaccines against colorectal cancer-derived blood vessels. Abbreviation used: dendritic cell (DC). Created with Biorender.com.
Mouse DCs have been well-classified based on the expression of defining markers such as CD4 and CD8α [55]. However, human DCs have only begun to reach a similar level of characterization in the last decade, with many details still missing pertaining to the phenotypic/functional similarities and differences between human and mouse DC populations. Although there may be uncertainty regarding the most appropriate way to taxonomically define DC lineages, several iterations of nomenclature have been proposed in recent years [56–58]. In particular, human DC subsets that have and continue to be extensively studied within the context of therapeutic DC vaccines are conventional DCs (cDCs), plasmacytoid DCs (pDCs), and monocyte-derived DCs (moDCs) (summarized in Table 1).
Table 1.
Human dendritic cell subsets
| DC type | Major function | Selective markers | Transcription factor dependence |
|---|---|---|---|
| cDC | |||
| cDC1 | Antigen cross-presentation to CD8+ T cells | CD11c, Clec9, CD141 | IRF8, BATF3, ID2, |
| cDC2A | CD4+ T cell polarization; antiinflammatory phenotype | CD1c, CLEC4A | IRF4, KLF4, Notch2 |
| cDC2B | CD4+ T cell polarization; pro-inflammatory phenotype | CD14, CD32, CD36, CD163, CLEC10A | RORγT, ZEB2 |
| pDC | |||
| Type I IFN production upon encountering pathogens | CD123, BDCA-2, BDCA-4 | Flt3L, E2–2, STAT3 | |
| moDC | |||
| T cell stimulation; inflammatory cytokine secretion | CD11c, CD1c, CD1a, CD1b, FcεRl, CD206, CD14, CD11b | MAFB, KLF4 | |
Abbreviations used: conventional DC (cDC), dendritic cell (DC), plasmacytoid DC (pDC), monocyte-derived DC (moDC), interferon (IFN)
Types of dendritic cells
cDCs specialize in antigen uptake and presentation to naïve T cells and are characterized by a CD11c+/CD123− phenotype [59]. cDCs in humans have been further divided into two larger groups (cDC1 and cDC2) based on mouse DC work and transcription factor dependence. Both subsets can be found in the blood, lymphoid, and non-lymphoid tissues [60] and are further defined by CD141+ that is analogous to mouse CD8a+/CD103+ DCs [61]. cDC1 development in the bone marrow is driven by IRF8, BATF3, ID2, and Flt3L [62]. cDC1s also have high expression of toll-like receptor (TLR) 3, TLR11, and TLR12 but lack TLR4 and TLR9 expression, which are observed in their mouse DC counterparts [63]. Functionally, cDC1s specialize in antigen cross-presentation to CD8+ T cells and production of IL-12 following migration to lymph nodes [64–67]. cDC2s are distinguished by CD1c+ expression and their development is dependent on IRF4, KLF4, and Notch2 [68]. The cDC2 subtype has recently been subdivided further into cDC2A and cDC2B cells as observed in mice. Initially, cDC2s were found to have varying levels of CD5 and were loosely divided into CD5hi and CD5lo populations [69], but cDC2As are more similar to cDC1s than their cDC2B counterparts, as evidenced by a higher expression of CD1c, HLA-DQ, and interferon regulatory factors. cDC2Bs more closely resemble monocytes, with higher expression of CD14, CD32, CD36, CD163, and MAFB [70]. In mice, cDC2As preferentially express TLR1, TLR5, and TLR7, whereas, cDC2Bs express TLR1, TLR2, TLR5, TLR6, and TLRs 7–9 [71]. Additionally, recent transcriptional analysis in mice has identified cDC2As as T-bet dependent while cDC2Bs rely on RORγt [71]. Generally, cDC2s are important for polarizing CD4+ T cells towards Th2, Th9, Th17, and Treg subsets [72]. An inflammatory cDC2 subset has also been identified in mice within the context of viral infections [73]. Inflammatory-cDC2s developed characteristics such as CD64 and IRF8 expression, which are usually associated with monocytes and cDC1s, respectively. It remains to be seen whether inflammatory-cDC2s are a distinct DC subset or an infection-driven variant of pro-inflammatory cDC2Bs. Since the cDC2A and cDC2B stratification has only recently been recognized, further work is needed to validate these findings in humans [74].
pDCs are generally characterized by substantial production of type I interferons (IFNs) upon encountering nucleic acids from pathogens and a morphology similar to B cell-differentiated plasma cells [75]. pDCs traffic through the blood and accumulate in peripheral tissues experiencing inflammation [76], and, upon sensing pathogenic RNA and DNA (through TLR7 and TLR9, respectively), secrete IFN-β, most types of IFN-α, and type III IFN (among other cytokines and chemokines) to further direct/activate locoregional immune responses [77]. pDCs will also upregulate MHC class I/class II expression to directly engage T cells during periods of pathogen infection [78]. Like cDCs, pDCs are heavily dependent on Flt3L for development [79]. Progenitor cells in the bone marrow are destined to the pDC lineage by the transcription factor E2–2, which directs their differentiation via STAT3 [80]. pDCs have also been shown to develop from common DC and lymphoid progenitor cells, but recent work suggests pDCs derive predominately from an IL-7R+ lymphoid progenitor that requires exposure to IRF8 [81].
Monocytes are circulating leukocytes that provide innate immune responses, help modulate adaptive immunity, and support the maintenance of tissue homeostasis [82]. Monocytes are developmental precursors to both macrophages and moDCs, but they also perform effector functions in the blood [83]. Monocytes have been further subdivided into classical, nonclassical, and intermediate categories, although heterogeneity has been described even within these subtypes (reviewed in detail elsewhere [84, 85]). Classical monocytes are CD14++ CD16− and are recruited to areas of inflammation by CCR2 to respond to lipopolysaccharide and produce TNF-α/IL-1. Nonclassical monocytes (CD14 low/CD16++) primarily survey cells via CX3CR1 to maintain overall homeostasis by performing endothelium repair and removing cell debris [86]. Intermediate monocytes (CD14+/CD16+) display an inflammatory phenotype similar to classical monocytes but also express CX3CR1 as seen with the nonclassical subset [87]. Importantly, classical monocytes are capable of differentiating into moDCs, especially following recruitment to sites of inflammation that is controlled by the transcription factors MAFB and KLF4 [70]. These inflammatory-stimulated moDCs are typified by expression of HLA-DR+/CD11c+ and a combination of DC and macrophage markers (i.e., CD1c, CD1a, CD1b, FcεR1, CD206, CD14, and CD11b) [88]. Like the previously discussed DC subsets, moDCs can develop dendrites upon differentiation and stimulate T cells [89]. Although much remains to be determined about this DC subset in humans, particularly from a functional standpoint, moDCs can secrete inflammatory cytokines and induce Th17 polarization in vitro [90]. Lastly, DCs can be derived from isolated monocytes in vitro with the cytokines GM-CSF and IL-4 [91] (summarized in Figure 2). The discovery of this directed tissue culturing approach has had a tremendous influence on the field by providing a suitable supply of DCs for vaccine purposes in patients with cancer [92].
Figure 2.
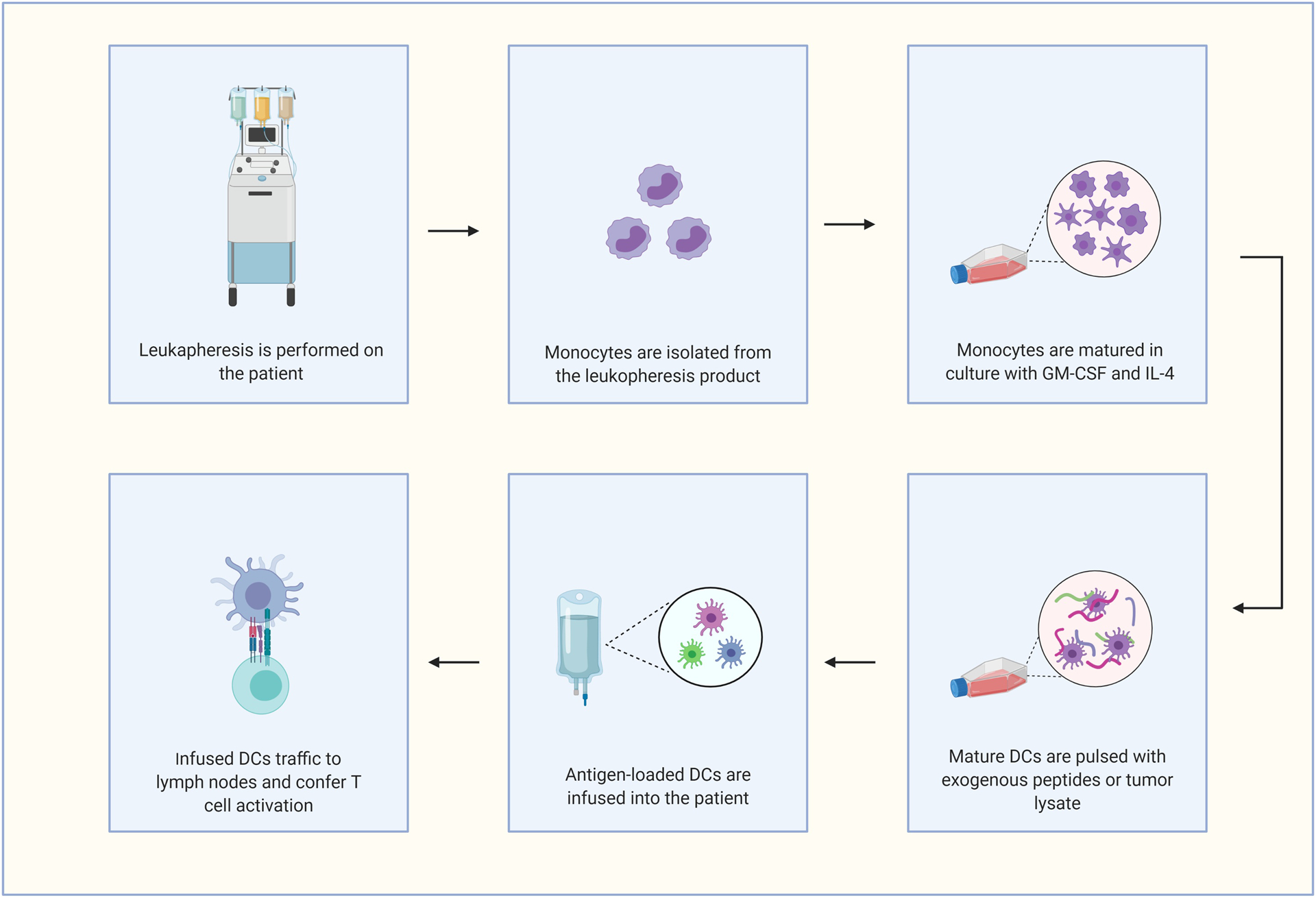
Standard ex vivo approach for generating dendritic cell vaccines for patient infusion. Abbreviation used: dendritic cell (DC). Created with Biorender.com.
Dendritic cell vaccines for cancer
The initial success of DC vaccines to combat cancer became evident from pre-clinical studies in the late 1980s and early 1990s that demonstrated protective anti-tumor effects afforded by tumor lysate-pulsed DCs [93, 94]. The translation of these experiments then culminated into clinical trials where autologous DCs were isolated from patients, modified ex vivo with antigen +/− maturation signals, and re-administrated. Pivotal clinical observations published in 1996 revealed that a series of infusions of antigen-specific DCs (initially obtained by leukapheresis from peripheral blood) were capable of generating anti-tumor immune responses as well as tumor regressions in a small cohort of patients with B-cell lymphoma [95]. A similar study in patients with stage IV melanoma exhibited that a MAGE-1 peptide-pulsed moDC vaccine could induce antigen-specific cytotoxic T cells, although no observable therapeutic benefit was demonstrated [96]. Importantly, these early studies helped establish that autologous DC vaccines were overwhelmingly safe following infusion in patients [97].
To date, the only DC-associated therapeutic currently approved by the FDA is Sipuleucel-T (Provenge®). Approved in 2010, Sipuleucel-T is a preparation of autologous CD54+ APCs for the treatment of minimally symptomatic, hormone refractory prostate cancer [98]. Based on the preparation method, Sipuleucel-T is not strictly a DC vaccine, as other mononuclear cells are also present. However, following collection of peripheral blood mononuclear cells via leukapheresis, CD54+ cells are isolated by density gradient centrifugation and co-cultured with the recombinant protein PA2024, which consists of prostatic acid phosphatase fused with GM-CSF that provides APCs both antigen specificity and maturation potential [99]. Patients are provided at least 50 million antigen-pulsed CD54+ cells for each vaccine treatment that is provided up to 3 times over a period of 4 weeks. Preliminary characterizations of the vaccine product demonstrated enhanced APC and T cell activity through elaboration of activation-associated cytokines (e.g., IFN-γ, TNF-α) following autologous cell infusions [100] as well as antibody and T cell-specific responses against PA2024 [101]. In the pivotal stage III IMPACT trial, 512 men with metastatic castration-resistant prostate cancer were randomized to treatment with Sipuleucel-T or placebo and overall survival was assessed as the primary endpoint. IMPACT demonstrated a 4.1 month increase in the survival of patients treated with Sipuleucel-T over placebo as well as an increase in the long-term benefit of the therapy when assessing 3-year survival rates (31.7% for Sipuleucel-T versus 23.0% for placebo). Notably, the effects of Sipuleucel-T were consistent even in groups with adverse prognostic factors known to impact patient survival such as elevated PSA levels and bone metastases. As seen with earlier DC vaccine trials, few serious safety responses were observed, and the most common adverse events from Sipuleucel-T included elevated flu-like symptoms (i.e., chills and fever) within 1 day after infusion that was likely the result of cytokines released by activated immune cells [101]. It is important to note that there are puzzling aspects of the IMPACT trial such as the overall lack of effects on PSA level or time-to-tumor progression between the Sipuleucel-T and placebo groups. An independent review of internal FDA data (that did not become available until after Sipuleucel-T approval) explores these matters at length, but, briefly, post-hoc subgroup analyses revealed unexpected correlations between age and overall survival [102]. First, effects on median survival could only be seen in patients 65 years or older and seems to contrast with the long-standing principle that younger patients develop more robust immune responses following immunizations[103]. Second, patients over 65 in the placebo group saw shorter overall survival than expected when compared to placebo groups in trials with similar enrollment restrictions. Finally, several major disparities in the processing/reinfusion of cells appeared to occur between the Sipuleucel-T and placebo-treated patients and included: [i] only a fraction of bulk processed cells were eventually delivered to placebo patients, [ii] placebo cells were not incubated with GM-CSF, and [iii] placebo cells were first incubated at 2–8 °C for 36–44 hrs that could have facilitated cell death prior to infusion. In all, the survival benefits of the IMPACT trial could potentially be a result of study design issues adversely impacting older placebo-treated patients. Yet, these alternative analyses/explanations have also been the subject of intense refute [104–106].
On the whole, though, these aforementioned DC vaccine clinical attempts have demonstrated a proof-of-principle that the DC platform can unleash potentially protective anti-cancer immune responses in patients. These trials have also helped spur additional work to enhance the therapeutic index of the DC vaccine approach for various forms of malignancies such as CRC [97].
Dendritic cell vaccine experience in the treatment of colorectal cancer
Currently, the majority of ongoing clinical trials investigating DC vaccines for CRC involve administrating DCs pulsed with autologous tumor lysates since this methodology has historically induced tumor-specific immune responses in patients [107, 108] (detailed in Table 2). Although this strategy ensures patients receive a personalized vaccine (by way of presenting unique tumor antigens), vaccine development is dependent on retrieval of an adequate amount of resected tumor that contains immunogenic material. Alternatively, DCs may be pulsed with exogenous tumor-associated peptides such carcinoembryonic antigen (CEA), which is expressed broadly by most colon cancer specimens [109]. Results of several early-phase clinical trials demonstrated that a CEA DC vaccine for CRC is effective at safely generating anti-CEA specific responses; however, overall survival or progression-free survival benefit has not been realized for a majority of patients [110–112]. An ongoing Phase I/II clinical trial is utilizing a DC vaccine loaded with CEA and the frameshift neoantigens caspase-5 and TFG-βRII in treating patients with microsatellite instability (MSI) CRC or as a preventative measure for germline mismatch repair (MMR) mutation carriers (Clinicaltrials.gov Identifier: NCT01885702). As Sipuleucel-T demonstrated improved efficacy in patients with reduced tumor burden, a preventative vaccine course may be an acceptable application for individuals at high-risk for developing CRC [113]. Relatedly, two clinical trials are exploring the usefulness of a DC vaccine to prevent relapse in either surgically resected stage I/II hypermutated or stage IV CRC where curative resection had been performed (NCT03730948, NCT02919644). In both scenarios, DC vaccines would be expected to inspire immune surveillance against microlesions that escaped initial detection.
Table 2.
Representative colorectal cancer clinical trials utilizing dendritic cell vaccines
| Clinicaltrials.gov identifier (Estimated completion year) | Phase | CRC indication | Treatment |
|---|---|---|---|
| NCT01885702 (2020) | I/II | Adjuvant DC vaccine for MSI-positive CRC/preventative DC vaccine for germline MMR-gene mutation carriers | Autologous DC vaccine loaded with CEA, and frameshift-derived neoantigens |
| NCT02503150 (2020) | III | Metastatic CRC with no previous therapy for metastatic lesions | Autologous DC vaccine loaded with autologous tumor lysate in combination with modified FOLFOX-6 |
| NCT03152565 (2020) | I/II | MSS metastatic CRC treated with at least two forms of chemotherapy | Autologous DC vaccine in combination with avelumab |
| NCT03730948 (2021) | I | Surgically resected stage I and II hypermutated CRC | Autologous DC vaccine with mutated peptides |
| NCT02919644 (2024) | II | Curative resection of stage IV CRC | Autologous DC vaccine with autologous tumor lysate followed by IL-2 injection |
Abbreviations used: carcinoembryonic antigen (CEA), colorectal cancer (CRC), dendritic cell (DC), mismatch repair (MMR), microsatellite instability (MSI), microsatellite stable (MSS)
It is clear that immune responses to CRC targets can be generated following DC vaccination, but improved clinical parameters (such as overall survival) are typically not observed in most patients. Obviously, this immunotherapeutic strategy holds tremendous potential (given the importance of DCs to fuel cytotoxic immune responses), but further research is required to enhance the approach for malignancies such as CRC. Major areas of continued development include identifying suitable patient subsets where DC vaccine treatment would provide the most benefit, improving ex vivo culturing techniques and inoculation routes, determining synergistic therapies best suited for use alongside DC vaccination, and predicting/characterizing CRC-associated antigens (e.g., neoepitopes, vascular targets) for DC processing/presentation [114].
IMPROVING THE DC VACCINE PLATFORM
Dendritic cell subsets
Since blood-derived DC populations exist only in small numbers, moDCs have preferentially dominated the majority of DC vaccine clinical studies. There is evidence, though, that peripheral DCs (namely cDCs and pDCs) may be superior DC subsets for migration and antigen cross-presentation purposes, given their biological roles in the host (see the “Types of DCs” Section). A first-in-human trial of an allogeneic pDC line that was irradiated and loaded with melanoma antigens (MLANA, MAGEA3, PMEL, TYR) was able to significantly increase the number of antigen-specific T cells in 4 of 9 metastatic patients experiencing some period of stable disease up to 48 weeks [115]. While the small study size and prior treatment record obscure the seeming cause of clinical benefit, the pDC vaccine was safe and able to induce T cell expansion without causing neutralizing immune responses to the vaccine. A separate study comparing pDC and cDC2 vaccines in melanoma patients also revealed that pDCs produced higher levels of CXCR3/CCR5 ligands (promoting cytolytic immune cells) and attracted greater numbers of CD8+ T cells in skin biopsies while cDC2s expressed elevated levels of chemokines binding CXCR1/CXCR2 (yielding T cell priming properties) [116]. These findings suggest that a combined pDCs and cDC2 vaccine might effectively provide chemoattractive and anti-cancer properties to T cells.
Altogether, although underutilized for vaccine purposes, other peripheral DC subsets may provide alternative (or additional) strengths to the standard route of infusing patients moDCs for therapeutic use. However, based in part on current deficiencies in cell isolation and culturing techniques, major challenges to incorporating this method include purifying sufficient numbers of peripheral DCs for ex vivo maturation/expansion purposes, especially in patients who have previously received immunosuppressive chemotherapeutic regimens. For example, cDC1s hold great appeal for use as a DC vaccine due to an enhanced ability to cross-present exogenous antigen to CD8+ T cells. Unfortunately, the low percentage of cDC1s in circulation (approximately 0.03% of human peripheral blood mononuclear cells) and lack of an appropriate clinical-grade reagent for cell isolation have excluded their use in the clinic [117].
Inoculations
Even though Sipuleucel-T is administered intravenously other DC inoculation routes are being explored to potentially improve DC localization to lymph nodes for T cell activation. In mouse models, intradermally administered DC vaccines only result in the delivery of 2–4% injected material to tumor-draining lymph nodes intravenously administrated DCs largely traffic to vascularized organs such as the spleen, liver, and kidneys [118, 119]. A study comparing intranodal versus intradermal administrations in advanced melanoma patients also revealed that intranodal injections resulted in higher numbers of DCs migrating to local lymph nodes, but intradermal injection provided a superior induction of T cells responding to tumor associated-antigens [120]. Additionally, intratumoral injection seems relatively efficacious since patients with metastatic disease exhibited an increased infiltration of CD8+ T cells in 5 out of 12 resected kidney tumors [121]. Despite the uncertainty of whether one injection scheme is superior overall, there is likely room for further optimization in this area. For example, the total number of DCs and timing of doses are likely critical for achieving maximal anti-tumor benefits in patients [122].
Combinations
A major factor, thus far, in the disappointing clinical performance of DC vaccines is an inability to overcome immunosuppressive properties of the tumor-microenvironment. Therefore, treatment combinations of DC vaccines with other immunomodulatory therapies is likely a positive way forward to achieve durable anti-tumor responses in patients. DC vaccines have frequently been administered alongside cyclophosphamide in order to suppress regulatory T cells [123–125], but success (in terms of patient responses) has still been limited, as regulatory T cells are not the only barrier to DC function within the tumor [92, 124, 126]. Other promising treatments that may have synergistic effects with DC vaccines include immune checkpoint inhibitors (ICIs), immunogenic cell death (ICD) inducers, and anti-angiogenic therapeutics.
The unprecedented success of ICIs has heralded a new era of immunotherapeutic promise for cancer in general. While clinical successes have been demonstrated with ICIs in select patients with tumors such as metastatic melanoma and MSI-high CRC, therapeutic efficacy as a single agent is still limited on a broader scale [127]. In the context of mCRC, FDA approval has only been granted for anti-PD-1 antibodies (nivolumab and pembrolizumab) in patients with MSI-high or MMR CRC subtypes [128, 129]. Yet, even with approved indications for immune checkpoint blockade, individuals may not respond, exhibit resistance (developed or inherent), or experience hyperprogression as a result of treatment [130]. In recent years, a crucial connection between ICIs and DCs has become evident, with the potential for these antibodies to boost the downstream effects of DC immunization. Vaccination with DCs loaded with autologous tumor lysates in 16 patients with metastatic melanoma revealed that patients with a significant increase in tumor-infiltrating CD8+ T cells also experienced upregulation of tumoral PD-L1 expression, indicating that concurrent PD-1/PD-L1 inhibition may improve immune-driven effects against immunosuppressive tumors [131, 132]. Conversely, timing of ICI administration severely alters immune responses to DC vaccines. A recent study of patients who received an autologous melanoma-specific DC vaccine reported that individuals provided immune checkpoint blockade after vaccine administration had considerably increased numbers of melanoma-specific CD8+ T cells in circulation, whereas, ICIs given prior to DC vaccination did not translate into improved cytotoxic T cell responses (by way of increased IFNy expression) [133]. PD-L1 abrogation may also instigate direct effects on DC function. For example, a patient sample analysis suggests that DCs could be suitable targets for anti-PD-L1 treatment by blocking PD-L1/B7.1 cis interactions on DCs, thus, freeing B7.1 to ligate CD28 and co-stimulate anti-tumor T cells [134]. Maturation of DCs with pro-inflammatory cytokines and TLR ligands tends to upregulate PD-L1 surface expression, perhaps making DC vaccines themselves a prime target for ICIs during ex vivo manipulations [135, 136].
While certain anti-cancer cytotoxic therapies are known to be immunosuppressive, several chemotherapeutics (e.g., doxorubicin, fluorouracil, and oxaliplatin [137]) or physical interventions such as radiotherapy could be employed to promote ICD [138]. The discrete actions of ICD are generally engaged following target cell production of reactive oxygen species or endoplasmic reticulum stress that releases (usually hidden) internal components to the extracellular environment. These secreted or cell surface-expressed molecules then stimulate immune cell activity by interacting most prominently with pattern-recognition receptors on APCs [139, 140]. Some potential ICD pathways involve the surface appearance of calreticulin on dead/dying target cells, which encourages phagocytosis by APCs like DCs [141]. ATP may also be secreted from dying cells and serve as a “find me” signal for DC precursors [142]. Additionally, HMGB1 is released from cells in late stages of apoptosis and binds TLR4 on DCs [143, 144]. Ultimately, the ICD process can be harnessed (i.e., as an endogenous adjuvant) for DC vaccines since it creates an environment rich in inflammatory mediators that stimulates DC activation. As one example, in a preclinical study, mice received DCs loaded with doxorubicin-treated neuroblastoma tumor cell lysates, and, when given prophylactically, generated superior tumor protection versus DCs exposed to untreated neuroblastoma cell lysates [145]. Therapeutically, the DC/doxorubicin vaccine regimen was further augmented when a CXCR4 agonist was also delivered to mice.
Lastly, tumor-derived blood vessels may serve to inhibit the collective effects of DC vaccines. Sustained tumor-produced VEGF can mediate detrimental effects to DC function through mechanisms that include inducing PD-L1 expression on myeloid DCs, impairing mature DC mobility, and suppressing expression of MHC class II and other costimulatory molecules [146–150]. Although anti-angiogenic agents such as VEGF-specific antibodies (e.g., bevacizumab) and small molecule drugs (e.g., axitinib, dasatinib, sunitinib) have been approved for some time, resistance to these monotherapies develops quickly in patients due to tumor blood vessels adopting compensatory reliance on other growth factors [151]. Yet, VEGF blockade in combination with other drugs such as chemotherapy [152], ICIs [153], or mTOR inhibitors [154] have shown improved anti-tumor effects. One possible explanation for such synergy involves the “vascular normalization” hypothesis, which proposes that in addition to causing limited vascular destruction, anti-angiogenic drugs transform the chaotic tumor vasculature into a more normal arrangement that allows co-applied drugs to effectively distribute and function throughout the tumor [155]. Ultimately, while DC vaccines alone may have a limited ability to catalyze T cell infiltration and immune cell cytotoxicity within the tumor microenvironment based on the aberrant and immunosuppressive properties of the tumor lesion, vascular normalization strategies could help unleash the ability of DCs to induce superior anti-tumor immunity by restoring blood flow dynamics and minimizing immune-defeating properties like hypoxia, acidosis, and downregulation of leukocyte adhesion molecules (e.g., ICAM-1, VCAM-1, E-selectin, and CD34) [156–159]. To further support this concept, in mouse models of melanoma, DC vaccines were capable of instituting superior antigen-specific tumor protection when animals were first sensitized to anti-angiogenic drugs like axitinib or dasatinib [160, 161].
Vascular dendritic cell targets
The relative clinical success of bevacizumab (and other FDA approved anti-angiogenic agents) has helped attract attention to furthering the development of immunotherapeutic strategies such as vaccines that target the tumor vasculature. In many cases, DC-inspired immune responses against the underlying endothelium (by way of CD8+ T cell cytotoxicity, for example) would be favored to induce poly-specific immunity and immunological memory (Figure 1).
To maintain vaccine safety, the ideal vascular target is one that is overexpressed within the tumor but not found or only evident at low levels on healthy endothelium to avoid disruptions to normal physiological processes such as wound healing [162]. Abrogating tumor-derived VEGF/VEGFR dynamics has been extensively studied in the clinic, particularly from a standpoint of infusing CRC patients blocking antibodies such as bevacizumab (anti-VEGF) or ramucirumab (anti-VEGFR2). Patients may experience dose-dependent hypertension following antibody infusions but such issues can be clinically manageable [163]. Since VEGF maintains roles in physiological angiogenesis, a vaccine-inspired immune response against VEGF/VEGFR also raises concerns about adverse events occurring outside of the tumor microenvironment. In one scenario, a vaccine consisting of recombinant human VEGF in combination with an adjuvant derived from Neisseria meningitides was assessed in a multi-center phase I clinical trial (CENTAURO-2) [154]. Dosing schemes resulted in 75% seroconversion in patients exhibiting anti-VEGF blocking antibody responses while still maintaining an acceptable safety profile. The placental endothelial cell vaccine ValloVax™ has also been utilized as a whole cell vaccine to immunize patients against naturally occurring blood vessel components [164]. Early clinical experience indicates that all patients vaccinated elicited enhanced antibody responses against vascular antigens such as VEGFR1, VEGFR2, CD105, and FGFR without mediating abnormal safety responses [165]. Ultimately, such examples give some degree of assurance that immunologic responses can be generated in individuals against self-vascular targets without inspiring unmanageable off-target toxicities in healthy tissues.
In relation to engaging DC activity, DNA and peptide-based vaccines have also been formulated against tumor-derived angiogenic factors such as bFGF, FGFR-1, avB3, angiomotin, CD105, survivin, Robo4, Tie-2, EGFR, HP59, PDGFRβ, TEM1, and TEM8 [166]. The goal of a tumor associated peptide vaccine is to conform MHC class I or II binding requirements so that an APC like a DC will present the administered peptide and activate CD8+ T cells or CD4+ T cells, respectively, against target cells. DNA vaccines function in a similar manner by essentially directing the expression/presentation of targets of interest in cells such as DCs upon plasmid DNA uptake [167]. Unfortunately, peptide and DNA vaccines, overall, have notoriously fallen flat in clinical trials, despite preclinical successes [167–169]. However, one encouraging DC preparative approach utilizes the unique properties of the intracellular bacterium Listeria monocytogenes (Lm) to direct DC antigen-specificity. Lm is a gram-positive bacterium that readily infects APCs, through expression of phospholipases and cytolysins, and can escape phagolysomal destruction and gain entry to the cytosol where it replicates prior to infecting a nearby cell [170, 171]. Once in the cytosol, the bacterium facilitates proteasomal processing, MHC Class I presentation, and robust induction of cytotoxic T lymphocyte responses against protein antigens that it secretes [172, 173]. As such, Lm-based therapeutic vaccines have been designed to express and secrete tumor associated-antigens of interest, and, upon DC infection, induce DC maturation and antigen presentation for CD8+ T cell activation, even at mucosal surfaces [174–176]. Due to the ability to break immunologic tolerance to self-antigens, Lm-based vaccines are ideal for targeting both tumors directly and the tumor-associated vasculature [177–180]. In fact, Lm-based vaccines targeting vasculature-associated antigens such as VEGFR2, HMWMAA, and CD105 have been able to generate both protective and therapeutic cytotoxic T lymphocyte responses in pre-clinical models of melanoma and breast cancer [179, 180]. The general clinical outlook for Lm-based vaccines is promising with numerous studies demonstrating significant efficacy in addition to high tolerability and safety in patients [181]. While Lm-based vaccines have advanced to phase III clinical trials, recent publication of phase II clinical trial results demonstrated that cervical cancer patients receiving an Lm-based vaccine targeting HPV16 E7 had clinically relevant tumor responses and prolonged survival in a select population, outperforming historical standards [182]. Further, the anti-tumor efficacy of Lm-based vaccines is enhanced when administered in combination with other interventions such as ICIs and radiation, suggesting a promising future for this therapy on improving patient survival [183–185].
Similar to Sipuleucel-T, directly infusing ex vivo matured DCs into patients likely holds the best route for securing immunity against tumor-derived targets but requires the necessary infrastructure to deal with cell isolation, maturation/expansion, and infusion. In pre-clinical models, adoptive therapy of DC vaccines encoding the tumor blood vessel antigens DLK1, EphA2, HBB, NRP1, PDGFRB, RGS5, or TEM1 resulted in the regression of MC38 or B16 subcutaneous tumors in HLA-A2 transgenic mice. CD8+ T cells were specifically invoked against tumor-derived blood vessel antigens that resulted in long-term inhibition of tumor growth. Importantly, no adverse immune responses were observed against healthy vascularized tissues or impairment to wound healing [186]. This strategy has also translated to the clinical for treatment of patients with melanoma (NCT01876212) or breast (NCT02479230) cancer and is awaiting further action.
CONCLUSIONS
Despite the number of mCRC treatments coming to market over the last two decades, the disease still remains deadly in its later stages. DC vaccines have historically performed poorly in clinical trials for cancer, but renewed interest in this immunotherapeutic strategy has been sparked by the relative success of Sipuleucel-T for prostate cancer and advent of immunomodulatory agents that may synergistically improve DC function. However, further research advancements are required in order to establish DC vaccines as a clinically efficacious approach for advanced CRC. Typical vaccine characteristics such as DC subtype, administration, timing, and dosage still require heavy research investment. Additionally, reasonable improvements in DC-elicited immune responses could be expected through rational combinations that might include immune checkpoint blockade and/or anti-angiogenic therapies. Lastly, tumor blood vessel-derived antigens represent an exciting area for DC vaccination purposes in order to trigger cytotoxic CD8+ T cell responses against the underlying blood vessel network of CRC. As bevacizumab has helped pave the way for anti-angiogenic treatments against the disease, further success is possible by instilling durable and broad T cell responses against the tumor vasculature.
ACKNOWLEDGMENTS
DBL is supported in part by funds from the NIH (R15 CA215874), DOD (W81XWH-18-1-0293), and Dodge Jones Foundation-Abilene.
Abbreviations used:
- APC
antigen presenting cell
- CEA
carcinoembryonic antigen
- CRC
colorectal cancer
- cDC
conventional DC
- DC
dendritic cell
- EGFR
epidermal growth factor receptor
- HIF
hypoxia inducible factor
- ICI
immune checkpoint inhibitor
- ICD
immunogenic cell death
- IFN
interferon
- Lm
Listeria monocytogenes
- MHC
major histocompatibility complex
- mCRC
metastatic CRC
- MSI
microsatellite instability
- MMR
mismatch repair
- moDC
monocyte-derived DC
- pDC
plasmacytoid DC
- TLR
toll-like receptor
- VEGF
vascular endothelial growth factor
- VEGFR
VEGFR receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors have no competing interests to declare that would negatively influence the work.
References
- 1.American Cancer Society, Cancer Facts & Figures, 2020 2020, American Cancer Society: Atlanta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welch HG and Robertson DJ, Colorectal Cancer on the Decline--Why Screening Can’t Explain It All. N Engl J Med, 2016. 374(17): p. 1605–7. [DOI] [PubMed] [Google Scholar]
- 3.Mauri G, Sartore-Bianchi A, Russo AG, Marsoni S, Bardelli A, and Siena S, Early-onset colorectal cancer in young individuals. Mol Oncol, 2019. 13(2): p. 109–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shussman N and Wexner SD, Colorectal polyps and polyposis syndromes. Gastroenterol Rep (Oxf), 2014. 2(1): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart SL, Wike JM, Kato I, Lewis DR, and Michaud F, A population-based study of colorectal cancer histology in the United States, 1998–2001. Cancer, 2006. 107(5 Suppl): p. 1128–41. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, N. A, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Reiew, 1975–2016 based on November 2018 SEER data submission, posted to the SEER website, April 2019, National Cancer Institute: Bethesda, MD. [Google Scholar]
- 7.Van Cutsem E, Nordlinger B, Adam R, Kohne CH, Pozzo C, Poston G, et al. , Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer, 2006. 42(14): p. 2212–21. [DOI] [PubMed] [Google Scholar]
- 8.Network, N.C.C. Colon Cancer (Version 1.2020). 2/3/2020].
- 9.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. , Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med, 2009. 360(14): p. 1408–17. [DOI] [PubMed] [Google Scholar]
- 10.Hsu HC, Thiam TK, Lu YJ, Yeh CY, Tsai WS, You JF, et al. , Mutations of KRAS/NRAS/BRAF predict cetuximab resistance in metastatic colorectal cancer patients. Oncotarget, 2016. 7(16): p. 22257–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. , Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol, 2010. 11(8): p. 753–62. [DOI] [PubMed] [Google Scholar]
- 12.Zhao B, Wang L, Qiu H, Zhang M, Sun L, Peng P, et al. , Mechanisms of resistance to anti-EGFR therapy in colorectal cancer. Oncotarget, 2017. 8(3): p. 3980–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. , Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol, 2009. 27(5): p. 663–71. [DOI] [PubMed] [Google Scholar]
- 14.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. , Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol, 2008. 26(10): p. 1626–34. [DOI] [PubMed] [Google Scholar]
- 15.Verdaguer H, Tabernero J, and Macarulla T, Ramucirumab in metastatic colorectal cancer: evidence to date and place in therapy. Ther Adv Med Oncol, 2016. 8(3): p. 230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aljubran A, Elshenawy MA, Kandil M, Zahir MN, Shaheen A, Gad A, et al. , Efficacy of Regorafenib in Metastatic Colorectal Cancer: A Multi-institutional Retrospective Study. Clin Med Insights Oncol, 2019. 13: p. 1179554918825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Deiry WS, Winer A, Slifker M, Taylor S, Adamson BJS, Meropol NJ, et al. , Disease Control With FOLFIRI Plus Ziv-aflibercept (zFOLFIRI) Beyond FOLFIRI Plus Bevacizumab: Case Series in Metastatic Colorectal Cancer (mCRC). Front Oncol, 2019. 9: p. 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He K, Cui B, Li G, Wang H, Jin K, and Teng L, The effect of anti-VEGF drugs (bevacizumab and aflibercept) on the survival of patients with metastatic colorectal cancer (mCRC). Onco Targets Ther, 2012. 5: p. 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrara N, Hillan KJ, Gerber HP, and Novotny W, Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov, 2004. 3(5): p. 391–400. [DOI] [PubMed] [Google Scholar]
- 20.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. , Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med, 2004. 350(23): p. 2335–42. [DOI] [PubMed] [Google Scholar]
- 21.Jayson GC, Kerbel R, Ellis LM, and Harris AL, Antiangiogenic therapy in oncology: current status and future directions. Lancet, 2016. 388(10043): p. 518–29. [DOI] [PubMed] [Google Scholar]
- 22.Gimbrone MA Jr., Leapman SB, Cotran RS, and Folkman J, Tumor dormancy in vivo by prevention of neovascularization. J Exp Med, 1972. 136(2): p. 261–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naumov GN, Akslen LA, and Folkman J, Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle, 2006. 5(16): p. 1779–87. [DOI] [PubMed] [Google Scholar]
- 24.Fitzgerald G, Soro-Arnaiz I, and De Bock K, The Warburg Effect in Endothelial Cells and its Potential as an Anti-angiogenic Target in Cancer. Front Cell Dev Biol, 2018. 6: p. 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lv X, Li J, Zhang C, Hu T, Li S, He S, et al. , The role of hypoxia-inducible factors in tumor angiogenesis and cell metabolism. Genes Dis, 2017. 4(1): p. 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helmlinger G, Yuan F, Dellian M, and Jain RK, Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med, 1997. 3(2): p. 177–82. [DOI] [PubMed] [Google Scholar]
- 27.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, et al. , Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1α. Genes Dev, 2000. 14(1): p. 34–44. [PMC free article] [PubMed] [Google Scholar]
- 28.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, et al. , Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev, 2000. 14(4): p. 391–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Krock BL, Skuli N, and Simon MC, Hypoxia-induced angiogenesis: good and evil. Genes Cancer, 2011. 2(12): p. 1117–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eelen* Guy, T. L, Li Xuri, and Carmeliet Peter, Basic and Therapeutic Aspects of Angiogenesis Updated. Circulation Research, 2020. 127(2): p. 310–329. [DOI] [PubMed] [Google Scholar]
- 31.Abhinand CS, Raju R, Soumya SJ, Arya PS, and Sudhakaran PR, VEGF-A/VEGFR2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal, 2016. 10(4): p. 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang SH, Kanasaki K, Gocheva V, Blum G, Harper J, Moses MA, et al. , VEGF-A induces angiogenesis by perturbing the cathepsin-cysteine protease inhibitor balance in venules, causing basement membrane degradation and mother vessel formation. Cancer Res, 2009. 69(10): p. 4537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siemann DW, The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat Rev, 2011. 37(1): p. 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baish JW and Jain RK, Fractals and cancer. Cancer Res, 2000. 60(14): p. 3683–8. [PubMed] [Google Scholar]
- 35.Zuazo-Gaztelu I and Casanovas O, Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front Oncol, 2018. 8: p. 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dvorak HF, Tumor Stroma, Tumor Blood Vessels, and Antiangiogenesis Therapy. Cancer J, 2015. 21(4): p. 237–43. [DOI] [PubMed] [Google Scholar]
- 37.Schulze T, Kemmner W, Weitz J, Wernecke KD, Schirrmacher V, and Schlag PM, Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother, 2009. 58(1): p. 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caballero-Banos M, Benitez-Ribas D, Tabera J, Varea S, Vilana R, Bianchi L, et al. , Phase II randomised trial of autologous tumour lysate dendritic cell plus best supportive care compared with best supportive care in pre-treated advanced colorectal cancer patients. Eur J Cancer, 2016. 64: p. 167–74. [DOI] [PubMed] [Google Scholar]
- 39.Turin I, Delfanti S, Ferulli F, Brugnatelli S, Tanzi M, Maestri M, et al. , In Vitro Killing of Colorectal Carcinoma Cells by Autologous Activated NK Cells is Boosted by Anti-Epidermal Growth Factor Receptor-induced ADCC Regardless of RAS Mutation Status. J Immunother, 2018. 41(4): p. 190–200. [DOI] [PubMed] [Google Scholar]
- 40.Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, et al. , Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin Cancer Res, 2015. 21(14): p. 3149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johansson-Percival A, He B, and Ganss R, Immunomodulation of Tumor Vessels: It Takes Two to Tango. Trends Immunol, 2018. 39(10): p. 801–814. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Wang Y, Huang Y, Kim BYS, Shan H, Wu D, et al. , Tumor Vasculatures: A New Target for Cancer Immunotherapy. Trends Pharmacol Sci, 2019. 40(9): p. 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinman RM and Cohn ZA, Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med, 1973. 137(5): p. 1142–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Laar L, Buitenhuis M, Wensveen FM, Janssen HL, Coffer PJ, and Woltman AM, Human CD34-derived myeloid dendritic cell development requires intact phosphatidylinositol 3-kinase-protein kinase B-mammalian target of rapamycin signaling. J Immunol, 2010. 184(12): p. 6600–11. [DOI] [PubMed] [Google Scholar]
- 45.Naik SH, Demystifying the development of dendritic cell subtypes, a little. Immunol Cell Biol, 2008. 86(5): p. 439–52. [DOI] [PubMed] [Google Scholar]
- 46.Lee J, Breton G, Oliveira TY, Zhou YJ, Aljoufi A, Puhr S, et al. , Restricted dendritic cell and monocyte progenitors in human cord blood and bone marrow. J Exp Med, 2015. 212(3): p. 385–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, et al. , Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol, 2007. 8(11): p. 1217–26. [DOI] [PubMed] [Google Scholar]
- 48.O’Keeffe M, Mok WH, and Radford KJ, Human dendritic cell subsets and function in health and disease. Cell Mol Life Sci, 2015. 72(22): p. 4309–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fyrstenberg Laursen M, Kofod-Olsen E, and Agger R, Activation of dendritic cells by targeted DNA: a potential addition to the armamentarium for anti-cancer immunotherapy. Cancer Immunol Immunother, 2019. 68(11): p. 1875–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saban DR, The chemokine receptor CCR7 expressed by dendritic cells: a key player in corneal and ocular surface inflammation. Ocul Surf, 2014. 12(2): p. 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan Y, Chen R, Wang X, Hu K, Huang L, Lu M, et al. , CCL19 and CCR7 Expression, Signaling Pathways, and Adjuvant Functions in Viral Infection and Prevention. Front Cell Dev Biol, 2019. 7: p. 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudek AM, Martin S, Garg AD, and Agostinis P, Immature, Semi-Mature, and Fully Mature Dendritic Cells: Toward a DC-Cancer Cells Interface That Augments Anticancer Immunity. Front Immunol, 2013. 4: p. 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chudnovskiy A, Pasqual G, and Victora GD, Studying interactions between dendritic cells and T cells in vivo. Curr Opin Immunol, 2019. 58: p. 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steinman RM and Cohn ZA, Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med, 1974. 139(2): p. 380–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sichien D, Lambrecht BN, Guilliams M, and Scott CL, Development of conventional dendritic cells: from common bone marrow progenitors to multiple subsets in peripheral tissues. Mucosal Immunol, 2017. 10(4): p. 831–844. [DOI] [PubMed] [Google Scholar]
- 56.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. , Nomenclature of monocytes and dendritic cells in blood. Blood, 2010. 116(16): p. e74–80. [DOI] [PubMed] [Google Scholar]
- 57.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, et al. , Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol, 2014. 14(8): p. 571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Collin M and Bigley V, Monocyte, Macrophage, and Dendritic Cell Development: the Human Perspective. Microbiol Spectr, 2016. 4(5). [DOI] [PubMed] [Google Scholar]
- 59.Merad M, Sathe P, Helft J, Miller J, and Mortha A, The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol, 2013. 31: p. 563–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Patente TA, Pinho MP, Oliveira AA, Evangelista GCM, Bergami-Santos PC, and Barbuto JAM, Human Dendritic Cells: Their Heterogeneity and Clinical Application Potential in Cancer Immunotherapy. Front Immunol, 2018. 9: p. 3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Minoda Y, Virshup I, Leal Rojas I, Haigh O, Wong Y, Miles JJ, et al. , Human CD141(+) Dendritic Cell and CD1c(+) Dendritic Cell Undergo Concordant Early Genetic Programming after Activation in Humanized Mice In Vivo. Front Immunol, 2017. 8: p. 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma W, Lee J, Backenroth D, Zhou YJ, Bush E, Sims P, et al. , Single cell RNA-Seq reveals pre-cDCs fate determined by transcription factor combinatorial dose. BMC Mol Cell Biol, 2019. 20(1): p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macri C, Pang ES, Patton T, and O’Keeffe M, Dendritic cell subsets. Semin Cell Dev Biol, 2018. 84: p. 11–21. [DOI] [PubMed] [Google Scholar]
- 64.Tussiwand R and Gautier EL, Transcriptional Regulation of Mononuclear Phagocyte Development. Front Immunol, 2015. 6: p. 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. , Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity, 2006. 25(1): p. 153–62. [DOI] [PubMed] [Google Scholar]
- 66.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, et al. , Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J Exp Med, 2010. 207(4): p. 823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noubade R, Majri-Morrison S, and Tarbell KV, Beyond cDC1: Emerging Roles of DC Crosstalk in Cancer Immunity. Front Immunol, 2019. 10: p. 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curato C, Bernshtein B, Zupancic E, Dufner A, Jaitin D, Giladi A, et al. , DC Respond to Cognate T Cell Interaction in the Antigen-Challenged Lymph Node. Front Immunol, 2019. 10: p. 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yin X, Yu H, Jin X, Li J, Guo H, Shi Q, et al. , Human Blood CD1c+ Dendritic Cells Encompass CD5high and CD5low Subsets That Differ Significantly in Phenotype, Gene Expression, and Functions. J Immunol, 2017. 198(4): p. 1553–1564. [DOI] [PubMed] [Google Scholar]
- 70.Collin M and Bigley V, Human dendritic cell subsets: an update. Immunology, 2018. 154(1): p. 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brown CC, Gudjonson H, Pritykin Y, Deep D, Lavallee VP, Mendoza A, et al. , Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell, 2019. 179(4): p. 846–863 e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar S, Jeong Y, Ashraf MU, and Bae YS, Dendritic Cell-Mediated Th2 Immunity and Immune Disorders. Int J Mol Sci, 2019. 20(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bosteels C, Neyt K, Vanheerswynghels M, van Helden MJ, Sichien D, Debeuf N, et al. , Inflammatory Type 2 cDCs Acquire Features of cDC1s and Macrophages to Orchestrate Immunity to Respiratory Virus Infection. Immunity, 2020. 52(6): p. 1039–1056 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shin JY, Wang CY, Lin CC, and Chu CL, A recently described type 2 conventional dendritic cell (cDC2) subset mediates inflammation. Cell Mol Immunol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Colonna M, Trinchieri G, and Liu YJ, Plasmacytoid dendritic cells in immunity. Nat Immunol, 2004. 5(12): p. 1219–26. [DOI] [PubMed] [Google Scholar]
- 76.Jegalian AG, Facchetti F, and Jaffe ES, Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol, 2009. 16(6): p. 392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reizis B, Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity, 2019. 50(1): p. 37–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tel J, Schreibelt G, Sittig SP, Mathan TS, Buschow SI, Cruz LJ, et al. , Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T cells despite lower Ag uptake than myeloid dendritic cell subsets. Blood, 2013. 121(3): p. 459–67. [DOI] [PubMed] [Google Scholar]
- 79.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, et al. , The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol, 2008. 9(6): p. 676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Musumeci A, Lutz K, Winheim E, and Krug AB, What Makes a pDC: Recent Advances in Understanding Plasmacytoid DC Development and Heterogeneity. Frontiers in Immunology, 2019. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Manz MG, Plasmacytoid dendritic cells: origin matters. Nat Immunol, 2018. 19(7): p. 652–654. [DOI] [PubMed] [Google Scholar]
- 82.Lauvau G, Chorro L, Spaulding E, and Soudja SM, Inflammatory monocyte effector mechanisms. Cell Immunol, 2014. 291(1–2): p. 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chow KV, Sutherland RM, Zhan Y, and Lew AM, Heterogeneity, functional specialization and differentiation of monocyte-derived dendritic cells. Immunol Cell Biol, 2017. 95(3): p. 244–251. [DOI] [PubMed] [Google Scholar]
- 84.Wolf AA, Yanez A, Barman PK, and Goodridge HS, The Ontogeny of Monocyte Subsets. Front Immunol, 2019. 10: p. 1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guilliams M, Mildner A, and Yona S, Developmental and Functional Heterogeneity of Monocytes. Immunity, 2018. 49(4): p. 595–613. [DOI] [PubMed] [Google Scholar]
- 86.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. , Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science, 2007. 317(5838): p. 666–70. [DOI] [PubMed] [Google Scholar]
- 87.Zhu YP, Thomas GD, and Hedrick CC, 2014 Jeffrey M. Hoeg Award Lecture: Transcriptional Control of Monocyte Development. Arterioscler Thromb Vasc Biol, 2016. 36(9): p. 1722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tang-Huau TL and Segura E, Human in vivo-differentiated monocyte-derived dendritic cells. Semin Cell Dev Biol, 2019. 86: p. 44–49. [DOI] [PubMed] [Google Scholar]
- 89.Segura E, Touzot M, Bohineust A, Cappuccio A, Chiocchia G, Hosmalin A, et al. , Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity, 2013. 38(2): p. 336–48. [DOI] [PubMed] [Google Scholar]
- 90.Zaba LC, Fuentes-Duculan J, Eungdamrong NJ, Abello MV, Novitskaya I, Pierson KC, et al. , Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol, 2009. 129(1): p. 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sallusto F and Lanzavecchia A, Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med, 1994. 179(4): p. 1109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cannon MJ, Block MS, Morehead LC, and Knutson KL, The evolving clinical landscape for dendritic cell vaccines and cancer immunotherapy. Immunotherapy, 2019. 11(2): p. 75–79. [DOI] [PubMed] [Google Scholar]
- 93.Shimizu J, Suda T, Yoshioka T, Kosugi A, Fujiwara H, and Hamaoka T, Induction of tumor-specific in vivo protective immunity by immunization with tumor antigen-pulsed antigen-presenting cells. J Immunol, 1989. 142(3): p. 1053–9. [PubMed] [Google Scholar]
- 94.Grabbe S, Bruvers S, Gallo RL, Knisely TL, Nazareno R, and Granstein RD, Tumor antigen presentation by murine epidermal cells. J Immunol, 1991. 146(10): p. 3656–61. [PubMed] [Google Scholar]
- 95.Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. , Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med, 1996. 2(1): p. 52–8. [DOI] [PubMed] [Google Scholar]
- 96.Mukherji B, Chakraborty NG, Yamasaki S, Okino T, Yamase H, Sporn JR, et al. , Induction of antigen-specific cytolytic T cells in situ in human melanoma by immunization with synthetic peptide-pulsed autologous antigen presenting cells. Proc Natl Acad Sci U S A, 1995. 92(17): p. 8078–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ridgway D, The first 1000 dendritic cell vaccinees. Cancer Invest, 2003. 21(6): p. 873–86. [DOI] [PubMed] [Google Scholar]
- 98.Gardner TA, Elzey BD, and Hahn NM, Sipuleucel-T (Provenge) autologous vaccine approved for treatment of men with asymptomatic or minimally symptomatic castrate-resistant metastatic prostate cancer. Hum Vaccin Immunother, 2012. 8(4): p. 534–9. [DOI] [PubMed] [Google Scholar]
- 99.So-Rosillo R and Small EJ, Sipuleucel-T (APC8015) for prostate cancer. Expert Rev Anticancer Ther, 2006. 6(9): p. 1163–7. [DOI] [PubMed] [Google Scholar]
- 100.Sheikh Nadeem A., R. C.P.d., Frohlich Mark W., Urdal David L. and Provost Nicole M.. Sipuleucel-T treatment results in sequential ex vivo activation of APCs and T cells during the culture step - evidence for in vivo immunological priming [abstract] in Proceedings of the 101st Annual Meeting of the American Association for Cancer Research;. 2010. Washington, DC. Philadelphia (PA):AACR: Cancer Res; 2010;70(8 Suppl):Abstract nr 5608. [Google Scholar]
- 101.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. , Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med, 2010. 363(5): p. 411–22. [DOI] [PubMed] [Google Scholar]
- 102.Huber ML, Haynes L, Parker C, and Iversen P, Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst, 2012. 104(4): p. 273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marrone KA and Forde PM, Cancer Immunotherapy in Older Patients. Cancer J, 2017. 23(4): p. 219–222. [DOI] [PubMed] [Google Scholar]
- 104.Gulley JL, Leitman SF, Dahut W, and Schlom J, Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst, 2012. 104(14): p. 1106; author reply 1109–12. [DOI] [PubMed] [Google Scholar]
- 105.Kantoff PW, Higano CS, Small EJ, Whitmore JB, Frohlich MW, and Schellhammer PF, Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst, 2012. 104(14): p. 1107–9; author reply 1109–12. [DOI] [PubMed] [Google Scholar]
- 106.Drake CG, Re: interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst, 2012. 104(18): p. 1422; author reply 1422–3. [DOI] [PubMed] [Google Scholar]
- 107.Barth RJ Jr., Fisher DA, Wallace PK, Channon JY, Noelle RJ, Gui J, et al. , A randomized trial of ex vivo CD40L activation of a dendritic cell vaccine in colorectal cancer patients: tumor-specific immune responses are associated with improved survival. Clin Cancer Res, 2010. 16(22): p. 5548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maurel J, Caballero-Baños M, Mila J, Tabera J, Varea S, Vilana R, et al. , Phase II randomized trial of autologous tumor lysate dendritic cell vaccine (ADC) plus best supportive care (BSC) compared with BSC, in pre-treated advanced colorectal cancer patients. Journal of Clinical Oncology, 2015. 33(15_suppl): p. 3048–3048. [Google Scholar]
- 109.Tong G, Xu W, Zhang G, Liu J, Zheng Z, Chen Y, et al. , The role of tissue and serum carcinoembryonic antigen in stages I to III of colorectal cancer-A retrospective cohort study. Cancer Med, 2018. 7(11): p. 5327–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, et al. , Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci U S A, 2001. 98(15): p. 8809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Itoh T, Ueda Y, Kawashima I, Nukaya I, Fujiwara H, Fuji N, et al. , Immunotherapy of solid cancer using dendritic cells pulsed with the HLA-A24-restricted peptide of carcinoembryonic antigen. Cancer Immunol Immunother, 2002. 51(2): p. 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morse MA, Deng Y, Coleman D, Hull S, Kitrell-Fisher E, Nair S, et al. , A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)-pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clin Cancer Res, 1999. 5(6): p. 1331–8. [PubMed] [Google Scholar]
- 113.Schellhammer PF, Chodak G, Whitmore JB, Sims R, Frohlich MW, and Kantoff PW, Lower baseline prostate-specific antigen is associated with a greater overall survival benefit from sipuleucel-T in the Immunotherapy for Prostate Adenocarcinoma Treatment (IMPACT) trial. Urology, 2013. 81(6): p. 1297–302. [DOI] [PubMed] [Google Scholar]
- 114.Rus Bakarurraini NAA, Ab Mutalib NS, Jamal R, and Abu N, The Landscape of Tumor-Specific Antigens in Colorectal Cancer. Vaccines (Basel), 2020. 8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Charles J, Chaperot L, Hannani D, Bruder Costa J, Templier I, Trabelsi S, et al. , An innovative plasmacytoid dendritic cell line-based cancer vaccine primes and expands antitumor T-cells in melanoma patients in a first-in-human trial. Oncoimmunology, 2020. 9(1): p. 1738812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.van Beek JJP, Florez-Grau G, Gorris MAJ, Mathan TSM, Schreibelt G, Bol KF, et al. , Human pDCs Are Superior to cDC2s in Attracting Cytolytic Lymphocytes in Melanoma Patients Receiving DC Vaccination. Cell Rep, 2020. 30(4): p. 1027–1038 e4. [DOI] [PubMed] [Google Scholar]
- 117.Calmeiro J, Carrascal MA, Tavares AR, Ferreira DA, Gomes C, Falcao A, et al. , Dendritic Cell Vaccines for Cancer Immunotherapy: The Role of Human Conventional Type 1 Dendritic Cells. Pharmaceutics, 2020. 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Verdijk P, Aarntzen EH, Lesterhuis WJ, Boullart AC, Kok E, van Rossum MM, et al. , Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin Cancer Res, 2009. 15(7): p. 2531–40. [DOI] [PubMed] [Google Scholar]
- 119.Dekaban GA, Hamilton AM, Fink CA, Au B, de Chickera SN, Ribot EJ, et al. , Tracking and evaluation of dendritic cell migration by cellular magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 2013. 5(5): p. 469–83. [DOI] [PubMed] [Google Scholar]
- 120.Lesterhuis WJ, de Vries IJ, Schreibelt G, Lambeck AJ, Aarntzen EH, Jacobs JF, et al. , Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res, 2011. 17(17): p. 5725–35. [DOI] [PubMed] [Google Scholar]
- 121.Laurell A, Lonnemark M, Brekkan E, Magnusson A, Tolf A, Wallgren AC, et al. , Intratumorally injected pro-inflammatory allogeneic dendritic cells as immune enhancers: a first-in-human study in unfavourable risk patients with metastatic renal cell carcinoma. J Immunother Cancer, 2017. 5: p. 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aarntzen EH, Srinivas M, Schreibelt G, Heerschap A, Punt CJ, Figdor CG, et al. , Reducing cell number improves the homing of dendritic cells to lymph nodes upon intradermal vaccination. Oncoimmunology, 2013. 2(7): p. e24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, et al. , Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother, 2007. 56(10): p. 1597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cornelissen R, Hegmans JP, Maat AP, Kaijen-Lambers ME, Bezemer K, Hendriks RW, et al. , Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am J Respir Crit Care Med, 2016. 193(9): p. 1023–31. [DOI] [PubMed] [Google Scholar]
- 125.Tanyi JL, Bobisse S, Ophir E, Tuyaerts S, Roberti A, Genolet R, et al. , Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci Transl Med, 2018. 10(436). [DOI] [PubMed] [Google Scholar]
- 126.Belderbos RA, Aerts J, and Vroman H, Enhancing Dendritic Cell Therapy in Solid Tumors with Immunomodulating Conventional Treatment. Mol Ther Oncolytics, 2019. 13: p. 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Decker WK, da Silva RF, Sanabria MH, Angelo LS, Guimaraes F, Burt BM, et al. , Cancer Immunotherapy: Historical Perspective of a Clinical Revolution and Emerging Preclinical Animal Models. Front Immunol, 2017. 8: p. 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yaghoubi N, Soltani A, Ghazvini K, Hassanian SM, and Hashemy SI, PD-1/ PD-L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother, 2019. 110: p. 312–318. [DOI] [PubMed] [Google Scholar]
- 129.Thomas J, Leal A, and Overman MJ, Clinical Development of Immunotherapy for Deficient Mismatch Repair Colorectal Cancer. Clin Colorectal Cancer, 2020. 19(2): p. 73–81. [DOI] [PubMed] [Google Scholar]
- 130.Arasanz H, Zuazo M, Bocanegra A, Gato M, Martinez-Aguillo M, Morilla I, et al. , Early Detection of Hyperprogressive Disease in Non-Small Cell Lung Cancer by Monitoring of Systemic T Cell Dynamics. Cancers (Basel), 2020. 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bulgarelli J, Tazzari M, Granato AM, Ridolfi L, Maiocchi S, de Rosa F, et al. , Dendritic Cell Vaccination in Metastatic Melanoma Turns “Non-T Cell Inflamed” Into “T-Cell Inflamed” Tumors. Front Immunol, 2019. 10: p. 2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L, et al. , Application of PD-1 Blockade in Cancer Immunotherapy. Comput Struct Biotechnol J, 2019. 17: p. 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Santos PM, Adamik J, Howes TR, Du S, Vujanovic L, Warren S, et al. , Impact of checkpoint blockade on cancer vaccine-activated CD8+ T cell responses. J Exp Med, 2020. 217(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, et al. , Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med, 2020. 12(534). [DOI] [PubMed] [Google Scholar]
- 135.Pulko V, Liu X, Krco CJ, Harris KJ, Frigola X, Kwon ED, et al. , TLR3-stimulated dendritic cells up-regulate B7-H1 expression and influence the magnitude of CD8 T cell responses to tumor vaccination. J Immunol, 2009. 183(6): p. 3634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hobo W, Novobrantseva TI, Fredrix H, Wong J, Milstein S, Epstein-Barash H, et al. , Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol Immunother, 2013. 62(2): p. 285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang YJ, Fletcher R, Yu J, and Zhang L, Immunogenic effects of chemotherapy-induced tumor cell death. Genes Dis, 2018. 5(3): p. 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adkins I, Fucikova J, Garg AD, Agostinis P, and Spisek R, Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology, 2014. 3(12): p. e968434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, et al. , Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology, 2014. 3(9): p. e955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rufo N, Garg AD, and Agostinis P, The Unfolded Protein Response in Immunogenic Cell Death and Cancer Immunotherapy. Trends Cancer, 2017. 3(9): p. 643–658. [DOI] [PubMed] [Google Scholar]
- 141.Kroemer G, Galluzzi L, Kepp O, and Zitvogel L, Immunogenic cell death in cancer therapy. Annu Rev Immunol, 2013. 31: p. 51–72. [DOI] [PubMed] [Google Scholar]
- 142.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, et al. , Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science, 2011. 334(6062): p. 1573–7. [DOI] [PubMed] [Google Scholar]
- 143.Bell CW, Jiang W, Reich CF 3rd, and Pisetsky DS, The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol, 2006. 291(6): p. C1318–25. [DOI] [PubMed] [Google Scholar]
- 144.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. , Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med, 2007. 13(9): p. 1050–9. [DOI] [PubMed] [Google Scholar]
- 145.Komorowski M, Tisonczyk J, Kolakowska A, Drozdz R, and Kozbor D, Modulation of the Tumor Microenvironment by CXCR4 Antagonist-Armed Viral Oncotherapy Enhances the Antitumor Efficacy of Dendritic Cell Vaccines against Neuroblastoma in Syngeneic Mice. Viruses, 2018. 10(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. , Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med, 2003. 9(5): p. 562–7. [DOI] [PubMed] [Google Scholar]
- 147.Laxmanan S, Robertson SW, Wang E, Lau JS, Briscoe DM, and Mukhopadhyay D, Vascular endothelial growth factor impairs the functional ability of dendritic cells through Id pathways. Biochem Biophys Res Commun, 2005. 334(1): p. 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bai WK, Zhang W, and Hu B, Vascular endothelial growth factor suppresses dendritic cells function of human prostate cancer. Onco Targets Ther, 2018. 11: p. 1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Long J, Hu Z, Xue H, Wang Y, Chen J, Tang F, et al. , Vascular endothelial growth factor (VEGF) impairs the motility and immune function of human mature dendritic cells through the VEGF receptor 2-RhoA-cofilin1 pathway. Cancer Sci, 2019. 110(8): p. 2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oussa NA, Dahmani A, Gomis M, Richaud M, Andreev E, Navab-Daneshmand AR, et al. , VEGF Requires the Receptor NRP-1 To Inhibit Lipopolysaccharide-Dependent Dendritic Cell Maturation. J Immunol, 2016. 197(10): p. 3927–3935. [DOI] [PubMed] [Google Scholar]
- 151.Gacche RN and Assaraf YG, Redundant angiogenic signaling and tumor drug resistance. Drug Resist Updat, 2018. 36: p. 47–76. [DOI] [PubMed] [Google Scholar]
- 152.Fuso Nerini I, Cesca M, Bizzaro F, and Giavazzi R, Combination therapy in cancer: effects of angiogenesis inhibitors on drug pharmacokinetics and pharmacodynamics. Chin J Cancer, 2016. 35(1): p. 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gao F and Yang C, Anti-VEGF/VEGFR2 Monoclonal Antibodies and their Combinations with PD-1/PD-L1 Inhibitors in Clinic. Curr Cancer Drug Targets, 2020. 20(1): p. 3–18. [DOI] [PubMed] [Google Scholar]
- 154.Pal K, Madamsetty VS, Dutta SK, Wang E, Angom RS, and Mukhopadhyay D, Synchronous inhibition of mTOR and VEGF/NRP1 axis impedes tumor growth and metastasis in renal cancer. NPJ Precis Oncol, 2019. 3: p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Goel S, Wong AH, and Jain RK, Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med, 2012. 2(3): p. a006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mpekris F, Voutouri C, Baish JW, Duda DG, Munn LL, Stylianopoulos T, et al. , Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci U S A, 2020. 117(7): p. 3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schaaf MB, Garg AD, and Agostinis P, Defining the role of the tumor vasculature in antitumor immunity and immunotherapy. Cell Death Dis, 2018. 9(2): p. 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Griffioen AW, Anti-angiogenesis: making the tumor vulnerable to the immune system. Cancer Immunol Immunother, 2008. 57(10): p. 1553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Griffioen AW, Damen CA, Blijham GH, and Groenewegen G, Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood, 1996. 88(2): p. 667–73. [PubMed] [Google Scholar]
- 160.Lowe DB, Bose A, Taylor JL, Tawbi H, Lin Y, Kirkwood JM, et al. , Dasatinib promotes the expansion of a therapeutically superior T-cell repertoire in response to dendritic cell vaccination against melanoma. Oncoimmunology, 2014. 3(1): p. e27589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bose A, Lowe DB, Rao A, and Storkus WJ, Combined vaccine+axitinib therapy yields superior antitumor efficacy in a murine melanoma model. Melanoma Res, 2012. 22(3): p. 236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, and St Croix B, Genes that distinguish physiological and pathological angiogenesis. Cancer Cell, 2007. 11(6): p. 539–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. , Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev, 2020. 86: p. 102017. [DOI] [PubMed] [Google Scholar]
- 164.Wagner SC, Ichim TE, Bogin V, Min WP, Silva F, Patel AN, et al. , Induction and characterization of anti-tumor endothelium immunity elicited by ValloVax therapeutic cancer vaccine. Oncotarget, 2017. 8(17): p. 28595–28613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wagner SC, Riordan NH, Ichim TE, Szymanski J, Ma H, Perez JA, et al. , Safety of targeting tumor endothelial cell antigens. J Transl Med, 2016. 14: p. 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wagner SC, Ichim TE, Ma H, Szymanski J, Perez JA, Lopez J, et al. , Cancer anti-angiogenesis vaccines: Is the tumor vasculature antigenically unique? J Transl Med, 2015. 13: p. 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lopes A, Vandermeulen G, and Preat V, Cancer DNA vaccines: current preclinical and clinical developments and future perspectives. J Exp Clin Cancer Res, 2019. 38(1): p. 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kumai T, Kobayashi H, Harabuchi Y, and Celis E, Peptide vaccines in cancer-old concept revisited. Curr Opin Immunol, 2017. 45: p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Slingluff CL Jr., The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J, 2011. 17(5): p. 343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Smith GA, Marquis H, Jones S, Johnston NC, Portnoy DA, and Goldfine H, The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun, 1995. 63(11): p. 4231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gedde MM, Higgins DE, Tilney LG, and Portnoy DA, Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes. Infect Immun, 2000. 68(2): p. 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Finelli A, Kerksiek KM, Allen SE, Marshall N, Mercado R, Pilip I, et al. , MHC class I restricted T cell responses to Listeria monocytogenes, an intracellular bacterial pathogen. Immunol Res, 1999. 19(2–3): p. 211–23. [DOI] [PubMed] [Google Scholar]
- 173.Ladel CH, Flesch IE, Arnoldi J, and Kaufmann SH, Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol, 1994. 153(7): p. 3116–22. [PubMed] [Google Scholar]
- 174.Singh R and Paterson Y, Vaccination strategy determines the emergence and dominance of CD8+ T-cell epitopes in a FVB/N rat HER-2/neu mouse model of breast cancer. Cancer Res, 2006. 66(15): p. 7748–57. [DOI] [PubMed] [Google Scholar]
- 175.Peters C, Peng X, Douven D, Pan ZK, and Paterson Y, The induction of HIV Gag-specific CD8+ T cells in the spleen and gut-associated lymphoid tissue by parenteral or mucosal immunization with recombinant Listeria monocytogenes HIV Gag. J Immunol, 2003. 170(10): p. 5176–87. [DOI] [PubMed] [Google Scholar]
- 176.Mustafa W, Maciag PC, Pan ZK, Weaver JR, Xiao Y, Isaacs SN, et al. , Listeria monocytogenes delivery of HPV-16 major capsid protein L1 induces systemic and mucosal cell-mediated CD4+ and CD8+ T-cell responses after oral immunization. Viral Immunol, 2009. 22(3): p. 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Souders NC, Sewell DA, Pan ZK, Hussain SF, Rodriguez A, Wallecha A, et al. , Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun, 2007. 7: p. 2. [PMC free article] [PubMed] [Google Scholar]
- 178.Seavey MM, Maciag PC, Al-Rawi N, Sewell D, and Paterson Y, An anti-vascular endothelial growth factor receptor 2/fetal liver kinase-1 Listeria monocytogenes anti-angiogenesis cancer vaccine for the treatment of primary and metastatic Her-2/neu+ breast tumors in a mouse model. J Immunol, 2009. 182(9): p. 5537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wood LM, Pan ZK, Guirnalda P, Tsai P, Seavey M, and Paterson Y, Targeting tumor vasculature with novel Listeria-based vaccines directed against CD105. Cancer Immunol Immunother, 2011. 60(7): p. 931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maciag PC, Seavey MM, Pan ZK, Ferrone S, and Paterson Y, Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res, 2008. 68(19): p. 8066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Maciag PC, Radulovic S, and Rothman J, The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine, 2009. 27(30): p. 3975–83. [DOI] [PubMed] [Google Scholar]
- 182.Huh WK, Brady WE, Fracasso PM, Dizon DS, Powell MA, Monk BJ, et al. , Phase II study of axalimogene filolisbac (ADXS-HPV) for platinum-refractory cervical carcinoma: An NRG oncology/gynecologic oncology group study. Gynecol Oncol, 2020. 158(3): p. 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mkrtichyan M, Chong N, Abu Eid R, Wallecha A, Singh R, Rothman J, et al. , Anti-PD-1 antibody significantly increases therapeutic efficacy of Listeria monocytogenes (Lm)-LLO immunotherapy. J Immunother Cancer, 2013. 1: p. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shrimali R, Ahmad S, Berrong Z, Okoev G, Matevosyan A, Razavi GSE, et al. , Agonist anti-GITR antibody significantly enhances the therapeutic efficacy of Listeria monocytogenes-based immunotherapy. J Immunother Cancer, 2017. 5(1): p. 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lim JY, Brockstedt DG, Lord EM, and Gerber SA, Radiation therapy combined with Listeria monocytogenes-based cancer vaccine synergize to enhance tumor control in the B16 melanoma model. Oncoimmunology, 2014. 3: p. e29028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao X, Bose A, Komita H, Taylor JL, Chi N, Lowe DB, et al. , Vaccines targeting tumor blood vessel antigens promote CD8(+) T cell-dependent tumor eradication or dormancy in HLA-A2 transgenic mice. J Immunol, 2012. 188(4): p. 1782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


