Abstract
Background
Disseminated intravascular coagulation (DIC) is noted in severe cases of coronavirus disease 2019 (COVID-19). Recently, a number of studies evaluating the diagnosis and treatment of DIC in COVID-19 patients have been reported.
Objective
The aim of this study is to identify existing gaps where further research is needed on the diagnosis and treatment of DIC complicated by COVID-19.
Methods
We used the PRISMA Extension for Scoping Reviews. MEDLINE, CENTRAL, WHO-ICTRP, ClinicalTrial.gov and PROSPERO were searched from their inception to 6 October 2020.
Results
Seven studies were selected; five were already published and two are ongoing. DIC was diagnosed using the International Society on Thrombosis and Hemostasis (ISTH) DIC score (n = 4) and the sepsis-induced coagulopathy (SIC) DIC score (n = 5). Seven studies examined the effectiveness of low molecular weight heparin (LMWH); of these, four studies used a prophylactic dose and five used a therapeutic dose of LMWH. A prophylactic dose of unfractionated heparin (UFH) was investigated in two studies.
Conclusion
Studies on DIC diagnostic criteria and anticoagulants were limited to the ISTH or SIC scores and heparinoids, particularly LMWH. Further studies are needed to compare these with other available DIC scoring systems and anticoagulants.
Keywords: COVID-19, Coagulopathy, Disseminated intravascular coagulation, Anticoagulants, Scoping review
Background
One of the major clinical manifestations noted in coronavirus disease 2019 (COVID-19) patients is coagulopathy, including disseminated intravascular coagulation (DIC) and thrombosis that is triggered, at least in part, by cytokines produced by inflammatory cells [1]. In addition, the endothelial cell derangement caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the pathogen that causes COVID-19, may contribute to the development of coagulopathy; SARS-CoV-2 exploits the angiotensin-converting enzyme 2 (ACE2) receptor expressed on the cell surface of vascular endothelial cells and induces diffuse endothelial inflammation, so-called endothelialitis [2], resulting in thrombotic microangiopathies [3]. Moreover, SARS-Cov-2 infected neutrophils via ACE2, leading to the release of neutrophil extracellular traps (NETs) which activates the coagulation pathways and platelets [4]. The NETs also cause endothelial cell derangements. This complex process plays a role in the development of the coagulopathy induced by SARS-CoV-2. A previous observational study found that the presence of DIC is associated with poor prognosis in COVID-19 patients [5]. Currently, many papers are focusing on the treatment of COVID-19-associated coagulopathy; however, there is no scoping review on this topic. The scoping review is a useful method to review evidence in rapidly emerging topics. The identification and analysis of knowledge gaps are valuable indications for conducting a scoping review [6]. The objective of this review is to conduct a scoping review to systematically map the DIC diagnosis criteria and anticoagulants used in this area and to identify the existing gaps where further research needs to be performed.
Methods
Protocol and registration
We conducted this scoping review after the a priori protocol was registered in Protocols.io. Link: https://dx.doi.org/10.17504/protocols.io.bgrpjv5n. Please refer to the protocol for detail information of the inclusion and exclusion criteria and the review process. The methodology to conduct scoping reviews by the Joanna Briggs Institute was followed [7], and the results are presented using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Reviews (PRISMA-ScR) [8].
Results
The results of the checklist [8] are described in Table 1.
Table 1.
PRISMA-ScR checklist item, PRISMA-ScR preferred reporting items for systematic reviews and meta-analyses extension for Scoping Reviews
| Section | Item | PRISMA-ScR checklist item | Reported on page # or section |
|---|---|---|---|
| Title | 1 | Identify the report as a scoping review | Front page |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary that includes (as applicable): background, objectives, eligibility criteria, sources of evidence, charting methods, results, and conclusions that relate to the review questions and objectives | Abstract |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. Explain why the review questions/objectives lend themselves to a scoping review approach | Introduction |
| Objectives | 4 | Provide an explicit statement of the questions and objectives being addressed with reference to their key elements (e.g., population or participants, concepts, and context) or other relevant key elements used to conceptualize the review questions and/or objectives | Introduction |
| Methods | |||
| Protocol and registration | 5 | Indicate whether a review protocol exists; state if and where it can be accessed (e.g., a Web address); and if available, provide registration information, including the registration number | Method |
| Eligibility criteria | 6 | Specify characteristics of the sources of evidence used as eligibility criteria (e.g., years considered, language, and publication status), and provide a rationale | p. 3 in protocol |
| Information sources | 7 | Describe all information sources in the search (e.g., databases with dates of coverage and contact with authors to identify additional sources), as well as the date the most recent search was executed | pp. 3–4 in protocol |
| Search | 8 | Present the full electronic search strategy for at least 1 database, including any limits used, such that it could be repeated | pp. 4–5 in protocol |
| Selection of sources of evidence | 9 | State the process for selecting sources of evidence (i.e., screening and eligibility) included in the scoping review | p. 6 in protocol |
| Data charting process | 10 | Describe the methods of charting data from the included sources of evidence (e.g., calibrated forms or forms that have been tested by the team before their use, and whether data charting was done independently or in duplicate) and any processes for obtaining and confirming data from investigators | p. 6 in protocol |
| Data items | 11 | List and define all variables for which data were sought and any assumptions and simplifications made | p. 7 in protocol |
| Critical appraisal of individual sources of evidence | 12 | If done, provide a rationale for conducting a critical appraisal of included sources of evidence; describe the methods used and how this information was used in any data synthesis (if appropriate) | Not applicable |
| Synthesis of results | 13 | Describe the methods of handling and summarizing the data that were charted | p. 7 in protocol |
| Results | |||
| Selection of sources of evidence | 14 | Give numbers of sources of evidence screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally using a flow diagram | Table 1 in review |
| Characteristics of sources of evidence | 15 | For each source of evidence, present characteristics for which data were charted and provide the citations | Fig. 2 in review |
| Critical appraisal within sources of evidence | 16 | If done, present data on critical appraisal of included sources of evidence (see item 12) | Not applicable |
| Results of individual sources of evidence | 17 | For each included source of evidence, present the relevant data that were charted that relate to the review questions and objectives | Result in review |
| Synthesis of results | 18 | Summarize and/or present the charting results as they relate to the review questions and objectives | Mapping studies in review |
| Discussion | |||
| Summary of evidence | 19 | Summarize the main results (including an overview of concepts, themes, and types of evidence available), link to the review questions and objectives, and consider the relevance to key groups | Discussion in review |
| Limitations | 20 | Discuss the limitations of the scoping review process | Discussion in review |
| Conclusions | 21 | Provide a general interpretation of the results with respect to the review questions and objectives, as well as potential implications and/or next steps | Conclusion in review |
| Funding | 22 | Describe sources of funding for the included sources of evidence, as well as sources of funding for the scoping review. Describe the role of the funders of the scoping review | p. 8 in protocol |
Selection of sources of evidence
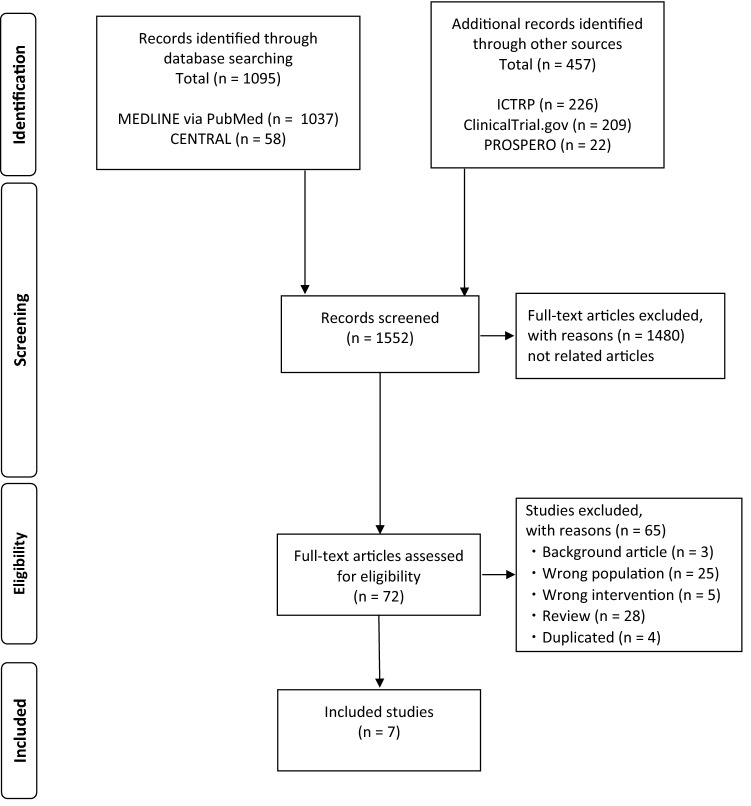
A total of 1552 articles from their inception to 6 October 2020 (MEDLINE, 1037; CENTRAL, 58; ClinicalTrial.gov, 209; WHO-ICTRP, 226; PROSPERO, 22) regarding the diagnosis and treatment of DIC in COVID-19 patients were identified, and 72 studies were selected according to the title and abstract for further full-text review (Fig. 1). Among them, seven articles fulfilled the inclusion criteria were analyzed in the scoping review [9–15]. The results of the literature search strategy are described in Table 2. There was no previous scoping review on this topic.
Fig. 1.
PRISMA flow diagram. PRISMA preferred reporting items for systematic reviews and meta-analyses
Table 2.
Results of search strategy
| No. | |||
|---|---|---|---|
| MEDLINE | |||
|
#1 “disseminated intravascular coagulation” [MeSH Terms] #2 “disseminated intravascular coagulation” [Title/Abstract] #3 “disseminated intravascular coagulations” [Title/Abstract] #4 “coagulopathy” [Title/Abstract] #5 “coagulopathies” [Title/Abstract] #6 “Anticoagulants” [MeSH Terms] #7 “anticoagulants”[Title/Abstract] #8 “anticoagulant” [Title/Abstract] #9 “anticoagulation” [Title/Abstract] #10 “anticoagulations” [Title/Abstract] #11 #1–#10/OR #12 “Coronavirus” [MeSH Terms] #13 “coronavirus” [Title/Abstract] #14 “Coronavirus Infections” [MeSH Terms] #15 “COVID” [Title/Abstract] #16 “cov 2” [Title/Abstract] #17 #7–#11/OR #18 #10 AND #17 |
11,061 10,118 9 13,820 1576 83,353 28,529 47,168 42,726 14 160,193 36,095 34,850 38,656 53,448 18,457 78,193 1037 |
||
| Cochrane CENTRAL | |||
|
#1 MeSH descriptor: [disseminated intravascular coagulation] explode all trees #2 "disseminated intravascular coagulation":ti,ab #3 "disseminated intravascular coagulations":ti,ab #4 coagulopathy:ti,ab #5 coagulopaties:ti,ab #6 MeSH descriptor [Anticoagulants] explode all trees #7 anticoagulants:ti,ab #8 anticoagulant:ti,ab #9 anticoagulations:ti,ab #10 anticoagulation:ti,ab #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor [Coronavirus] explode all trees #13 coronavirus:ti,ab #14 MeSH descriptor [Coronavirus Infections] explode all trees #15 COVID:ti,ab #16 "cov 2":ti,ab #17 #12–#16 #18 #11 AND #17 |
108 | ||
| 303 | |||
| 0 | |||
| 1243 | |||
| 139 | |||
| 4621 | |||
| 2489 | |||
| 4610 | |||
| 6 | |||
| 5525 | |||
| 13,881 | |||
|
72 611 395 1544 109 1683 58 | |||
| ClinicalTrial.gov | |||
| COVID-19 | 3662 | ||
| Other terms: coagulation OR DIC OR coagulopathy OR anticoagulants OR anticoagulation | |||
| Total after duplication elimination | 209 | ||
| PROSPERO | |||
| #1 (((coronavirus or corona-virus) AND (wuhan or beijing or shanghai or Italy or South-Korea or korea or China or Chinese or 2019-nCoV or nCoV or COVID-19 or Covid19 or SARS-CoV* or SARSCov2 or ncov)) OR (pneumonia AND Wuhan) or "COVID-19" or "2019-nCoV" or "SARS-CoV" or SARSCOV2 or 2019-nCov or "2019 coronavirus" or "2019 corona virus" or covid19 or ncov OR "novel corona virus" or "new corona virus" or "nouveau corona virus" or "2019 corona virus" OR "novel coronavirus" or "new coronavirus" or "nouveau coronavirus" or "2019 coronavirus" OR (MeSH DESCRIPTOR Coronavirus Infections EXPLODE ALL TREES) OR ((MeSH DESCRIPTOR Coronavirus EXPLODE ALL TREES) AND (wuhan or beijing or shanghai or Italy or South-Korea or korea or China or Chinese or 2019-nCoV or nCoV or COVID-19 or Covid19 or SARS-CoV* or SARSCov2 or ncov))) | 2319 | ||
| #2 disseminated intravascular coagulation” or “coagulation” or “DIC” or “coagulopathy” or “coagulopathy” or “anticoagulant” | |||
| #3 #1 AND #3 | 22 | ||
| WHO-ICTRP | |||
| We used the WHO-ICTRP providing COVID-19 trials | |||
| Other terms: coagulation OR coagulations OR coagulopathy OR coagulopathy OR coagulants OR anticoagulation | |||
| Total | 266 | ||
Characteristics of the sources of evidence
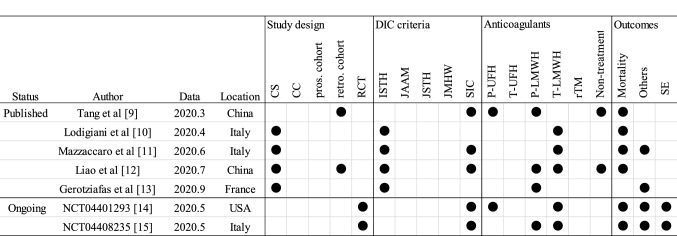
The author, status, publication or registration data, location, study design, diagnosis criteria of DIC, anticoagulants, and outcomes of all the included studies are summarized in Fig. 2.
Fig. 2.
Characteristics of the sources of evidence. CR case report, CS case series, CC case control, pros prospective cohort study, retro retrospective cohort study, ISTH International Society on Thrombosis and Haemostasis, JAAM Japanese Association for acute medicine, JMHW Japanese Ministry of Health and Welfare, SIC sepsis-induced coagulopathy, P-UFH prophylactic dose of unfractionated heparin, T-UFH therapeutic dose of unfractionated heparin, P-LMWH prophylactic dose of low molecular weight heparin, T-LMWH therapeutic dose of low molecular weight heparin, rTM recombinant thrombomodulin, SE side effects, No. number
Status, publication or registration data, and location
Regarding the status of the studies, five studies have been published and two clinical studies are ongoing. The first study was published in March 2020 [9], followed by a study in April (n = 1) [10], June (n = 1) [11], July (n = 1) [12] and September (n = 1) [13]. The ongoing studies were registered in May (n = 2) [14, 15]. The studies or protocols were reported from Italy (n = 3) [10, 11, 15], China (n = 2) [9, 12], USA (n = 1) [14] and France (n = 1) [13].
Study design
The study design included case series (n = 4) [10–13], retrospective cohort studies (n = 2) [9, 12], and randomized control trial (RCT) (n = 2) [14, 15]. None of the studies followed case–control or prospective cohort studies.
Diagnosis criteria of DIC
DIC was diagnosed using several scales, such as the International Society on Thrombosis and Hemostasis (ISTH) DIC score [16] (n = 4) [10–13] and sepsis-induced coagulopathy (SIC) DIC score [17] (n = 5) [9, 11, 12, 14, 15]. The DIC diagnostic criteria released from the Japanese Association for Acute Medicine (JAAM) DIC score [18], Japanese Society on Thrombosis and Hemostasis (JSTH) DIC score [19] or Japanese Ministry of Health and Welfare (JMHW) DIC score [20] were not used.
Anticoagulants
The anticoagulants therapies used in the studies were a prophylactic dose of unfractionated heparin (UFH) (n = 2) [9, 14], prophylactic dose of low molecular weight heparin (LMWH) (n = 4) [9, 12, 13, 15], therapeutic dose of LMWH (n = 5) [10–12, 14, 15], and non-treatment (n = 2) [9, 12]. The therapeutic dose of UFH or rTM was not reported.
Outcomes
The survival of patients was assessed in six studies [9–12, 14, 15]. The outcomes of clinical practices other than death, such as requiring ICU admission, non-invasive ventilation (NIV), or DIC scores were assessed in four studies [11, 13–15]. The side effects caused by the anticoagulant therapy will be reported in two studies [14, 15].
Results of the individual sources of evidence
Tang et al. [9]
Publication data and location: March 2020 from China.
Study design: Retrospective cohort study.
Patients: Ninety-seven out of 449 patients (21.6%) met the SIC criteria (total score ≥ 4).
Intervention: Enoxaparin 40—60 mg daily or UFH 10,000—15,000 IU daily for at least 7 days.
Comparisons: No anticoagulants or heparin treatment for less than 7 days.
Outcome: The 28-day mortality of heparin users was lower than that of nonusers in patients with an SIC score ≥ 4 (40.0 vs. 64.2%, p = 0.029).
Notes: The number of heparin users and nonusers with SIC scores ≥ 4 was not reported but 94 patients received LMWH and 5 patients received UFH. Neither therapy-related side effects nor the study design was reported.
Lodigiani et al. [10]
Publication data and location: April 2020 from Italy.
Study design: Case series.
Patients: The ISTH DIC score of two patients was 5 or greater.
Interventions: A Patient was treated with nadroparin 5700 IU twice daily and the other patient nadroparin 5700 IU once daily.
Outcome: Two patients were dead.
Note: Eight patients met the ISTH DIC criteria and seven patients died during hospitalization but the dose prescribed to the six patients except the two patients was not described.
Mozzaccaro et al. [11]
Publication data and location: June 2020 from Italy.
Study design: Case series in ISTH DIC and retrospective cohort in non-overt DIC.
Patients: Two patients were confirmed to have SIC score ≧ 4 and ISTH-DIC score ≧ 5.
Interventions: Enoxaparin 1 mg/kg twice daily adjusted for glomerular filtration rate.
Outcome: One patient required NIV continuous positive airway pressure and died for multiorgan failure. The other patient needed NIV and then admission to ICU for mechanical ventilation.
Note: The study design was not reported as a case series. Reviewers judged the study design based on how the results are reported.
Liao et al. [12]
Publication data and location: July 2020 from China.
Study design: Case series and retrospective cohort study.
Case series
Patients: Two patients were diagnosed as SIC.
Interventions: LMWH 40 mg once daily.
Outcome: Two patients were discharged.
Cohort study
Patients: Eight patients were diagnosed as ISTH DIC.
Interventions and comparisons: Five patients were treated with LMWH 40 mg once daily, two LMWH 40 mg twice daily and one without any anticoagulants.
Outcome: All patients died.
Note: The original article reported 8 non-survivors and 12 survivors diagnosed with SIC but the data that could be extracted was limited.
Gerotziafas et al. [13]
Publication data and location: September 2020 from France.
Study design: Case series.
Patients: 49 patients with the ISTH DIC score of 5 points or higher.
Interventions: All patients routinely received thromboprophylaxis with body-weight-adapted enoxaparin.
Outcome: 30 patients required ICU admission.
Note: The dose was not described.
NCT04401293 [14]
Publication data and location: May 2020 from USA.
study design: RCT.
Patients: Patients admitted to hospitals with SIC score of ≥ 4 or D-dimer > 4 times the upper level of the normal reference range.
Interventions: Therapeutic doses of enoxaparin 1 mg/kg subcutaneously twice daily adjusted for creatinine clearance during the course of their hospitalization.
Comparison: Prophylactic or intermediate dose of UFH 5000–7500 IU subcutaneously twice or three times daily, enoxaparin 30–40 mg subcutaneously twice or three times daily, dalteparin 2500–5000 IU four times daily.
Outcome: All-cause mortality, SIC score, requiring intubation and major bleeding will be measured.
Note: Whether outcomes for patients with the SIC score ≥ 4 would be reported was not stated.
NCT04408235 [15]
Publication data and location: May 2020 from Italy.
study design: RCT.
Patients: Inpatients with SIC score > 4 or D-dimer > 4 times the upper level of the normal reference range.
Interventions: High dose of enoxaparin 0.7 mg/kg twice daily.
Comparison: Standard prophylactic dose of enoxaparin 40 mg once a day.
Outcome: All-cause mortality, requiring NIV and major bleeding will be measured.
Note: Whether outcomes for patients with the SIC score > 4 would be reported was not stated.
Protocol versus overview
Our planned search strategy registered in protocol.io was compared with the final reported review methods. The search was updated up to October 6, 2020. The inclusion criteria were specifically defined for patients with DIC or SIC diagnosed by scoring systems. There was a difference between the study designs reported by original articles and those judged by our reviews. We reported the study design based on our judgment.
Discussion
This study is the first scoping review that maps the available evidence on the diagnosis and treatment of DIC induced by COVID-19 and identified some gaps.
Gaps pertaining to assessment of DIC
Although multiple scoring systems exist to assess DIC [19], COVID-19 patients treated with anticoagulants have been assessed primarily by the ISTH DIC scores and SIC scores. Unfortunately, none of the studies utilized JAAM, JSTH, and JMHW. Certainly, the accumulation of evidence from a uniform assessment criterion would lead to quantitatively synthesized and reliable conclusions from a meta-analysis. On the other hand, more studies evaluating DIC with more available scoring are needed to elucidate the overall clinical coagulopathy in patients with COID-19.
Gaps pertaining to anticoagulants
Our results provide insights into the gap that exists among anticoagulants recommended by current therapeutic strategies. Most reviewed studies were related to heparin, especially the prophylactic dose and therapeutic dose of LMWH. A prophylactic dose of UFH or LMWH is not one of the therapeutic strategies to treat DIC caused by severe underlying diseases [21]. However, the prophylactic dose of those anticoagulants was used in five studies. A randomized control trial to compare the prophylactic dose to the therapeutic dose of LMWH is ongoing [14, 15].
Another gap pointed out was that no published research evaluating the efficacy of recombinant human soluble thrombomodulin (rTM) was identified. rTM is approved only in Japan for use in daily clinical practice, which could explain why, currently, no study has reported the rTM treatment of COVID-19-induced coagulopathy. However, an international randomized trial to assess the effect of rTM on sepsis-associated coagulopathy was conducted [22]. Compared with UFH, the use of rTM improved the DIC resolution rate in patients with DIC caused by hematological malignancies or infectious diseases [23], and it is the rationale to register a clinical trial comparing rTM with other anticoagulants.
Heparin may possess some anti-inflammatory function as a systematic review showed that the use of heparin decreased the serum levels of inflammatory-related biomarkers in patients with inflammatory diseases [24]. In addition, a high dose of heparin was shown to inhibit the interaction of histone, a major component of NETs, with platelets [25]. Given that the lectin-like domain of rTM possesses anti-inflammatory function [26] and rTM degrades histones via activated protein C [27], we assume that the use of rTM is the reasonable option to cope with DIC in CVID-19 patients.
Gaps pertaining to the study design.
There is also a gap in the study design. Most of the reviewed studies were observational, starting with low-quality evidence, according to the GRADE system for grading the quality of evidence [28], which likely limits the validity and reproducibility of the results. In addition to this gap, three studies did not report the study design, and one reported the design incorrectly and was judged by our reviewers to be a case series because of not comparing the results of anticoagulants. Therefore, future studies that could apply to clinical practice should focus on improving study quality.
Strengths and limitations
Our review was conducted systematically according to the methodology after registration of a prior protocol. Performance was based on the recommendations from the PRISMA Extension for Scoping Reviews. We believe that this review followed a robust method.
We acknowledge several limitations of our study. Due to the urgent need for evidence on this topic and limited time, we did not contact authors to clarify the details of primary data when necessary. Because studies on this topic are rapidly being conducted, there may be other studies that were not examined in this review when the results are published. Another limitation is that this scoping review only identified future needed studies to clarify the prognosis of patients who received anticoagulants for part of the COVID-19-related coagulopathy. A previous study showed that a significant portion of COVID-19-related coagulopathy was a specific phenotype of coagulopathy which was far from SIC in terms of hemostatic parameters [29]. The authors concluded that the coagulopathy noted in COVID-19 patients might be promoted by the local thrombus [29]. The population covered by this review was limited to patients diagnosed with DIC or SIC and treated with anticoagulants. However, this limitation of the population did not significantly undermine the generalizability of the results in this study. As the previous study also argued that there were no significant differences in the incidence of JAAM DIC and ISTH DIC during the first seven days between the COVID and non-COVID groups. To the best of our knowledge, there was no scoping review that examined the concept of COVID-19-related coagulopathy which is neither DIC nor SIC, and identified the overall COVID-19-related coagulopathy including the specific phenotype of coagulopathy and typical DIC and SIC. Therefore, to identify gaps in comparison with existing evidence on anticoagulants [30], it was necessary and appropriate to limit the population of this review to DIC patients for whom definitions have been established. Future scoping reviews should be conducted to examine the definition of the overall COVID-19-related coagulopathy and to investigate the prognosis of anticoagulants for COVID-19-related coagulopathy.
Conclusion
Evidence on the treatment of DIC in COVID-19 patients is accumulating but is still mainly focused on the assessment of DIC by the score of ISTH DIC or SIC, the use of heparinoids, especially LMWH and observational study designs. The evidence found on this topic was not qualitatively or quantitatively sufficient to conduct a systematic review that would provide practical guidance. Further clinical trials are needed to compare other available DIC scoring systems and anticoagulants and may provide more accurate results by improving their study design.
Recently, ISTH has released the interim guidance on recognition and management of coagulopathy in COVID-19; it recommends the physicians to measure D-dimer, PT, and the platelet count to determine the patients requiring admission. Based on the reports by Tang et al. [8], the prophylactic dose of LMWH is recommended for all admitted patients [29]. This guidance should be modified after the accumulation of more solid evidences.
Acknowledgements
This study was supported by KAKENHI (18H02844) to T. Ikezoe.
Author contributions
HM conceived this study and designed the protocol. HM and HO developed and conducted the literature search strategy. HM, HO, RT, MR, YS, and TI drafted and revised the manuscript.
Compliance with ethical standards
Conflict of interest
All of the authors declare that they have no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Walborn A, Hoppensteadt D, Syed D, Mosier M, Fareed J. Biomarker profile of sepsis-associated coagulopathy using biochip assay for inflammatory cytokines. Clin Appl Thromb Hemost. 2018;24:625–632. doi: 10.1177/1076029617709084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gavriilaki E, Brodsky RA. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br J Haematol. 2020;189:e227–e230. doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- 4.Veras FP, Pontelli MC, Silva CM, Toller-Kawahisa JE, de Lima M, Nascimento DC, et al. SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med. 2020;217:e20201129. doi: 10.1084/jem.20201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143. doi: 10.1186/s12874-018-0611-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters MDJ GC, Mcinerney P, Soares CB, Khalil H, Parker D. The Joanna Briggs Institute reviewers’ manual 2015: Methodology for JBI scoping reviews. https://nursing.lsuhsc.edu/JBI/docs/ReviewersManuals/Scoping-.pdf
- 8.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 9.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzaccaro D, Giacomazzi F, Giannetta M, Varriale A, Scaramuzzo R, Modafferi A, et al. Non-overt coagulopathy in non-ICU Patients with mild to moderate COVID-19 pneumonia. J Clin Med. 2020;9:1781. doi: 10.3390/jcm9061781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7:e671–e678. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerotziafas GT, Sergentanis TN, Voiriot G, Lassel L, Papageorgiou C, Elabbadi A, et al. Derivation and validation of a predictive score for disease worsening in patients with COVID-19. Thromb Haemost. 2020;120:1680–1690. doi: 10.1055/s-0040-1716544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NCT04401293. Full dose heparin vs. prophylactic or intermediate dose heparin in high risk COVID-19 patients. http://clinicaltrials.gov/show/NCT04401293. 2020.
- 15.NCT04408235. Randomised controlled trial comparing efficacy and safety of high versus low low-molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. 2020;21:574. http://clinicaltrials.gov/ct2/show/NCT04408235. [DOI] [PMC free article] [PubMed]
- 16.Taylor FB, Jr, Toh CH, Hoots WK, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86:1327–1330. doi: 10.1055/s-0037-1616068. [DOI] [PubMed] [Google Scholar]
- 17.Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7:e017046. doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gando S, Wada H, Asakura H, Iba T, Eguchi Y, Okamoto K, et al. Evaluation of new Japanese diagnostic criteria for disseminated intravascular coagulation in critically ill patients. Clin Appl Thromb Hemost. 2005;11:71–76. doi: 10.1177/107602960501100108. [DOI] [PubMed] [Google Scholar]
- 19.Asakura H, Takahashi H, Uchiyama T, Eguchi Y, Okamoto K, Kawasugi K, et al. Proposal for new diagnostic criteria for DIC from the Japanese Society on Thrombosis and Hemostasis. Thromb J. 2016;14:42. doi: 10.1186/s12959-016-0117-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi N, Maekawa T, Takada M, Tanaka H, Gonmori H. Criteria for diagnosis of DIC based on the analysis of clinical and laboratory findings in 345 DIC patients collected by the Research Committee on DIC in Japan. Bibl Haematol. 1983:265–75. [DOI] [PubMed]
- 21.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 22.Vincent JL, Francois B, Zabolotskikh I, Daga MK, Lascarrou JB, Kirov MY, et al. Effect of a recombinant human soluble thrombomodulin on mortality in patients with sepsis-associated coagulopathy: the SCARLET randomized clinical trial. JAMA. 2019;321:1993–2002. doi: 10.1001/jama.2019.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5:31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- 24.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015:507151. doi: 10.1155/2015/507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikezoe T. Advances in the diagnosis and treatment of disseminated intravascular coagulation in haematological malignancies. Int J Hematol. 2021;113:34–44. doi: 10.1007/s12185-020-02992-w. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umemura Y, Yamakawa K, Kiguchi T, Nishida T, Kawada M, Fujimi S. Hematological phenotype of COVID-19-induced coagulopathy: far from typical sepsis-induced coagulopathy. J Clin Med. 2020;9:2875. doi: 10.3390/jcm9092875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murao S, Yamakawa K. A systematic summary of systematic reviews on anticoagulant therapy in sepsis. J Clin Med. 2019;8:1869. doi: 10.3390/jcm8111869. [DOI] [PMC free article] [PubMed] [Google Scholar]