Abstract
Objectives
Quality assurance (QA) plays an integral role in Point of Care Testing (POCT) programs. Quality control (QC) is an important QA program component to ensure high quality results and enhanced patient care. The measurement of transcutaneous bilirubin (TcB) in the POCT setting is an essential part of newborn care in Alberta, Canada. However, there is currently no available commercial QC material for TcB meters. An in-house developed QC material has been in use within a single TcB POCT program within Alberta. The objective of this study was to determine the performance of this QC material by other POCT staff and clinical end-users to assess whether its use could be expanded.
Design and methods
Two levels of QC material were measured by POCT staff and clinical end-users across 12 different sites using the Dräger Jaundice Meter JM-103® and JM-105® meters.
Results
The performance of the QC material was acceptable when tested by POCT staff, was stable and reliable over time, and had an acceptable CV (≤8%). However, the data does not support use of the QC material by clinical end-users.
Conclusions
The use of the QC material could be expanded into other TcB settings for use by POCT staff. Additional training and experience with the QC material by end-users is needed to facilitate QC use in the clinical setting.
Keywords: Hyperbilirubinemia, POCT, Quality, Quality control, Transcutaneous bilirubin
Abbreviations: Quality Assurance, QA; Quality Control, QC; Point of Care Testing, POCT; Transcutaneous bilirubin, TcB; Total serum bilirubin, TSB; Out-of-QC Range, OQR
Highlights
-
•
Transcutaneous bilirubin (TcB) is often an essential part of newborn care.
-
•
A TcB QC material was developed due to a lack of commercial TcB QC material.
-
•
The QC material shows value when used by POCT staff.
-
•
The QC material can decrease reliance on total serum bilirubin (TSB) samples.
-
•
Use of the QC material by clinical staff is not yet supported.
1. Introduction
A laboratory quality management system enhances patient safety through the implementation of policies and procedures to ensure that laboratory results are of high quality [1]. A strong quality assurance (QA) program, including appropriate quality control (QC), plays an integral role in improving laboratory testing and this should be extended to Point of Care Testing (POCT) [2,3]. POCT QA practices should include prescribed measurement of QC material, participation in an external quality assessment program, and ongoing comparisons of the POCT device results with a central accredited laboratory [3]. A strong POCT quality management system can be achieved through several actions, such as central laboratory governance, oversight and collaboration, strong training and competency of end-users, and a robust QA program [3,4].
Transcutaneous bilirubin (TcB) measurement is a non-invasive, POCT-based approach for neonatal bilirubin screening [5]. For example, the Dräger Jaundice Meters measure the yellowness of subcutaneous tissue [6]. TcB measurement is used to identify newborns that are at risk of pathological increases in blood bilirubin. Neonatal hyperbilirubinemia occurs in up to ~80% of term and preterm newborns in the first week of life [[7], [8], [9], [10], [11]]. However, approximately 2% of infants have total serum bilirubin (TSB) that is considered to be sufficiently elevated for the free unconjugated bilirubin to cross the blood-brain barrier and deposit in the brain [7,8,12]. In rare cases kernicterus, a term often used to describe chronic and/or permanent neurological consequences of this deposition, may also occur without elevated TSB [13,14]. Short- and long-term consequences of this deposition can include acute bilirubin encephalopathy, cerebral and/or gaze palsy, delayed development of fine- and gross-motor abilities, mental retardation, and death [[7], [8], [9],12,15]. As such, prompt identification of newborns with a higher-than-normal concentration of bilirubin in the blood is critical to ensure appropriate initiation of treatment, such as phototherapy [5,16]. Notably, the terminology surrounding bilirubin-induced neurological dysfunction in the context of neonatal hyperbilirubinemia can be inconsistent, and Shapiro et al. suggest that the term “Kernicterus Spectrum Disorder” is better suited to describe the neurological sequelae of neonatal hyperbilirubinemia [15].
Though TSB measurement is required for the definitive diagnosis of pathological jaundice [17], timely TSB assessment can be challenging. For example, since the concentration of bilirubin in the blood changes over time, newborns should be assessed for hyperbilirubinemia at the time of hospital discharge, and between 24 and 72 h of life [17]. Notably, peak TSB concentration predominantly occurs when infants have been discharged [17] and follow-up 24–72 h after birth is challenging, especially since many infants are discharged before they are 24 h old or they may be born out-of-hospital [17]. POCT hand-held transcutaneous bilirubinometers measure TcB to estimate TSB and can help alleviate these testing limitations. TcB meters use spectral reflectance to determine the amount of bilirubin (a chromophore) in the skin and subcutaneous tissue, and this correlates with and therefore provides an estimate of TSB concentration [18]. The major advantage of TcB measurement is the reduction in the number of heel pokes performed in infants, especially in infants that do not require a TSB result in their clinical management [9,19]; other benefits include instantaneous results and its ease of use [18]. However, as several factors may affect TcB results, such as skin tone, measurement site (i.e. sternum or the forehead), treatment with phototherapy, and gestational age [6,20], TcB meter use is not appropriate in all clinical contexts.
As part of a robust QA program, QC is necessary to ensure the reliability of results [3]. Several factors specific to TcB testing have made standard QC testing a challenge, notably the lack of manufacturer-supplied QC material. When no QC material is available for a POCT device, patient samples are often used as a replacement and compared against a laboratory result [21]. In the context of TcB, this approach may require an additional heel poke(s) in the infant to allow comparison of the TcB measurement to a clinical laboratory TSB concentration. These TSB collections add stress and pain to the infant and its caregivers; it is not feasible nor acceptable to perform daily meter comparisons using these types of samples. An additional heel poke may also not be clinically acceptable in some cases, which limits the ability for assessment of TcB estimation of TSB. Since newborns identified as high-risk by TcB require a follow-up TSB measurement, it is possible to compare those two values. Limitations to this process are notable and include: 1) meter performance is only assessed in high-risk infants, 2) documented underestimation of TcB at high TSB (decision rules can help mitigate but not eliminate this) [22], and 3) a poorly functioning TcB meter may be in use for some time before it is used on a high-risk infant and these results are reviewed. Further, although the function of the TcB device is evaluated and/or calibrated using various manufacturer-specific approaches, such as the use of calibration tips [23] or wavelength checks [24], these assessments do not determine whether the measurement of bilirubin concentration in subcutaneous tissue is actually correct. Importantly, it is recommended that third-party QC be used whenever possible to independently check the POCT system, and comparison with TSB samples alone would not fulfil this [21].
Challenges in POCT TcB QC clearly exist. In order to improve the POCT TcB programs in Alberta to better support patient care, a unique approach to TcB QC was required. This included the development of an in-house TcB QC material to be used with our current transcutaneous bilirubinometers, the Dräger Jaundice Meter JM-103® and JM-105® meters. After many iterations, a final QC product was developed that consisted of two individual materials (i.e. levels) of QC corresponding to the low/medium risk (green/yellow zone) and the high risk (red zone) sections of the locally developed and validated TcB nomogram; a previous version of the nomogram has been published [19]. Current and previous versions of this QC material have been an integral part of the TcB QA program in the Calgary, Alberta area since 2009. For example, the material ensures TcB meters are assessed more frequently and tightly by having an available material to assess meters during site visits and to more rapidly troubleshoot a meter, and it aims to decrease the amount of newborn blood samples required for hyperbilirubinemia and meter assessments.
The use of the QC material has historically been limited to one local health region within Alberta and a single POCT staff member that has extensive experience with the product and the TcB program. This was valuable for product development and assessment, as it provided an opportunity for building a robust product to be used locally to help assess the performance of program meters, for example after calibration or when troubleshooting a TcB meter. However, how well the QC product would work in a less tightly controlled environment was unknown. The aim of this study was to assess the performance of the QC material when used by extra-regional POCT staff and by clinical end-users, such as nursing staff. Variables that were assessed included: type of end-user, level of QC and model of bilirubinometer.
2. Materials and methods
Throughout this manuscript, local staff refers to the staff working in the region where the QC product was originally developed, which was a single, experienced POCT staff member who was very familiar with the QC product and TcB meter use. Staff and sites outside of this health region are referred to as external staff and external sites, respectively. Two levels of QC material were developed using heat injection molding of a polymer (Fig. 1). To assess performance of the QC material when used by external users, 12 different sets (level 1 and level 2 in each set) of the same lot of QC material were tested across 12 sites (4 Acute Care sites, 6 Public Health sites, 2 POCT sites; 1 set for each site). Testing was performed using the Dräger Jaundice Meter JM-103® and JM-105® meters. All clinical end-users were nurses and considered competent users of TcB meters, as each user must complete specific training and demonstrate competency to be an approved user of the TcB meters; auditing of approved users’ technique may also occur as part of specific TcB programs. POCT lab staff had considerable expertise and involvement with their local POCT TcB programs. All users received written instruction on how to use the QC material. A total of 33 m were used by external staff and sites to measure the QC material during this study, and data was collected over a period of 16 weeks. One meter was used at each of the external POCT sites. A subset of the local data was derived as part of regular POCT staff visits to end-user sites and included 53 m.
Fig. 1.
Image of the in-house developed TcB QC material being measured using the Dräger Jaundice Meter 105®. The yellow material on the left is a Level 1 (L1) QC and its measurement corresponds to the low/medium risk (green/yellow zones) section of the locally validated TcB nomogram; yellow material on the right is a Level 2 (L2) QC and corresponds to the high risk (red zone) of the locally validated TcB nomogram. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Acceptable QC ranges for the two levels of QC material were as follows: Level 1 (L1) range of 159–199 umol/L; Level 2 (L2) range of 242–278 umol/L. These ranges were established following extensive local testing that included five different meters (three JM-105® and two JM-103® meters) and 10 tests per meter, and the acceptable range encompasses the mean ± 2 standard deviations (SDs) with a coefficient of variation (CV) of <10%. Data from QC material measured by JM-105® meters post-calibration was used to represent ideal performance of the QC material. Both POCT lab staff and clinical end-users were involved in the testing. Results from the TcB meters were based on the average of three individual measurements (value calculated by TcB meter). The provided directions for QC product use included instructions to repeat testing when the meter returned an ‘error’. However, it was not always recorded if repeat testing was performed, therefore a proportion of the results may reflect either repeat measurements or a single error with no repeat measurement. Additionally, some users may have repeated the TcB measurement if the reading was outside of the device measuring range, but this data was not recorded. For this study, TcB result errors were considered to be either a) results outside the meter range, or b) an instrument ‘error’ measurement code. The three main considerations when comparing performance of the QC material were: 1) the percent of values that fell outside the acceptable QC range (out-of-QC range, OQR), 2) the dispersion of the TcB measurements around the mean, represented by the CV (%), and 3) the number of result errors that occurred. POCT lab staff, but not clinical end-users, were aware of the QC material target ranges during testing. All results were from measurements using the JM-105® meters unless otherwise stated.
3. Results
3.1. Measurement of QC material by a local POCT staff member with extensive QC material experience
There were ≥90% of results within the acceptable range for L1 and L2 (CV <5%). This occurred when the QC material was tested by an experienced POCT staff within a laboratory setting following factory calibration and involved 10 measurements of the QC material per meter (Table 1).
Table 1.
TcB QC results over a four-month period when measured by an experienced POCT staff familiar with the meter and QC material.
| QC Level | Level 1 | Level 2 |
|---|---|---|
| Number of JM-105® TcB Metersa | 33 | 33 |
| Number of TcB Resultsb | 330 | 330 |
| Number of Errors | 0 | 0 |
| Mean TcB Result, umol/L (Acceptable Range) | 167 (159–199) | 250 (242–278) |
| SD (umol/L) | 7 | 10 |
| CV (%) | 4 | 4 |
| Percent OQRc (n) | 2 (6) | 9 (31) |
Meters were post-factory calibration and used in a controlled environment.
Each meter tested 10 times per level, therefore each meter contributes 10 individual results.
OQR = Out-of-QC Range.
The addition of measurements from three JM-103® meters used in a laboratory environment had no impact on the CVs or the means obtained by JM-105® meters, however there were two additional L2 measurements that were considered OQR. The QC material performed similarly when the QC material was tested by an experienced POCT staff as part of standard visits to end-user sites, with only slightly higher CVs (Table 2).
Table 2.
TcB QC results when measured by an experienced POCT staff familiar with the meter and QC material during clinical site as part of standard meter assessments.
| Meter |
JM-103® and 105® |
JM-103® |
JM-105® |
|||
|---|---|---|---|---|---|---|
| QC Level | Level 1 | Level 2 | Level 1 | Level 2 | Level 1 | Level 2 |
| Number of TcB Resultsa | 74 | 70 | 20 | 16 | 54 | 54 |
| Number of Errors | 0 | 4 | 0 | 4 | 0 | 0 |
| Mean TcB Result, umol/L (Acceptable Range) | 176 (159–199) | 260 (242–278) | 183 (159–199) | 267 (242–278) | 173 (159–199) | 258 (242–278) |
| SD (umol/L) | 12 | 12 | 15 | 14 | 9 | 12 |
| CV (%) | 7 | 5 | 8 | 5 | 5 | 5 |
| Percent OQRb (n) | 5 (1) | 6 (4) | 5 (1) | 12 (2) | 0 | 4 (2) |
53 m total.
OQR = Out-of-QC Range.
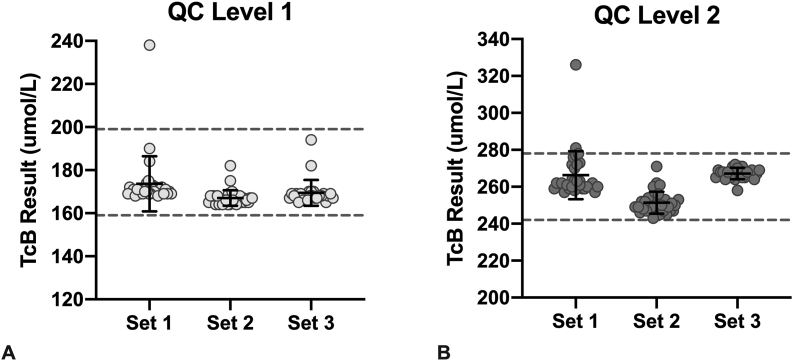
In total, 30% of all measurements on the L2 material underwent repeat testing, and 5% (n = 1) of L1 results were repeated (Table 2). One L1 and two L2 measurements were OQR, but these measurements were not repeated for unknown reasons. Data in Table 1, Table 2 exclude the initial value, and only include the repeat value when available. If the initial measurements were used in the analysis, the L2 CVs, means, and percent of values that were OQR increased (both meter types: mean = 266 umol/L, CV = 7%, percent OQR = 26%; JM-103®: mean = 270 umol/L, CV = 7%, percent OQR = 40%; JM-105®: mean = 265 umol/L, CV = 7%, percent OQR = 23%). When comparing the performance of the two models of TcB meter, the JM-105® meters performed slightly better, as more results fell within the target range and it had smaller CVs (Table 2). Performance of the L2 material was less consistent than that of L1, as it required more repeat measurements and had more errors. All QC means fell within the acceptable range. Importantly, different sets of QC performed similarly when used by the local, experienced POCT staff (Fig. 2).
Fig. 2.
Performance of three different sets of QC material measured by an experienced POCT staff using the JM-105® meter. Each result (individual point) is the average of three measurements (calculated by the TcB meter). Solid black line and corresponding error bars represent the mean ± SD. Dashed lines represent the lower and upper acceptable limits of the QC range. At least two TcB meters were used to measure each set of QC.
3.2. Measurement of QC material by external POCT staff
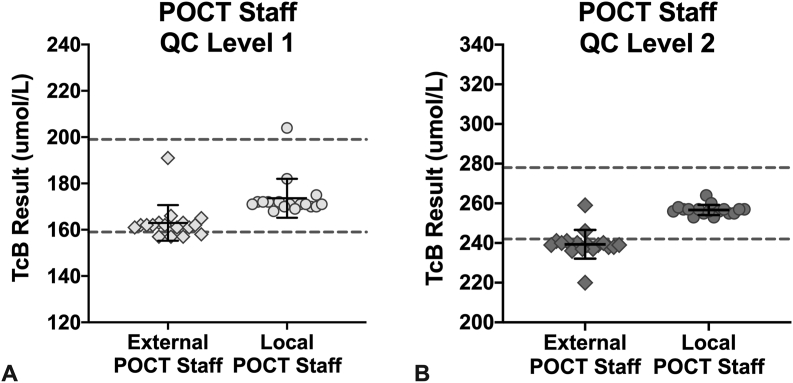
Testing by two external POCT staff that were competent TcB meter users, but unfamiliar with the QC material, was carried out at two different sites (one user per site). The mean of both levels of QC fell within target range, showed similar CVs to when the material was tested by the experienced user, and displayed no errors (Table 3). Accordingly, QC material performance was considered to be excellent. However, one of the two users had nearly all L2 QC values, and several L1 QC, as OQR (Fig. 3). This resulted in 40% of the L2 values falling OQR for these external POCT staff. Repeat testing by the same staff member did not change the data (data not shown). When this same set of QC material was re-tested by the local POCT staff using a different meter, all except one value were within range (Fig. 3). In practice, repeated QC measurements outside of the acceptable QC range would prompt troubleshooting of the measurement procedure, such as investigation of the clinical performance of the meter, evaluation of the measurement technique of the user, and/or re-testing of the QC product on a different meter.
Table 3.
TcB QC results when measured at two external sites by POCT staff using a single JM-105® meter at each site.
| QC Level | Level 1 | Level 2 |
|---|---|---|
| Number of TcB Resultsa | 42 | 42 |
| Number of Errors | 0 | 0 |
| Mean TcB Result, umol/L (Acceptable Range) | 163 (159–199) | 255 (242–278) |
| SD (umol/L) | 5 | 14 |
| CV (%) | 3 | 6 |
| Percent OQRb (n) | 5 (2) | 40 (16) |
One meter and one user per site.
OQR = Out-of-QC Range.
Fig. 3.
Comparison of the performance of one set of QC material by external POCT staff (diamonds, 1 user per site) and one experienced POCT staff familiar with the material (circles) using the JM-105®. Each result (individual point) is the average of three measurements as calculated by the TcB meter. Solid black line and corresponding error bars represent the mean ± SD. Dashed lines represent the lower and upper acceptable limits of the QC range.
3.3. Measurement of QC material by clinical end-users
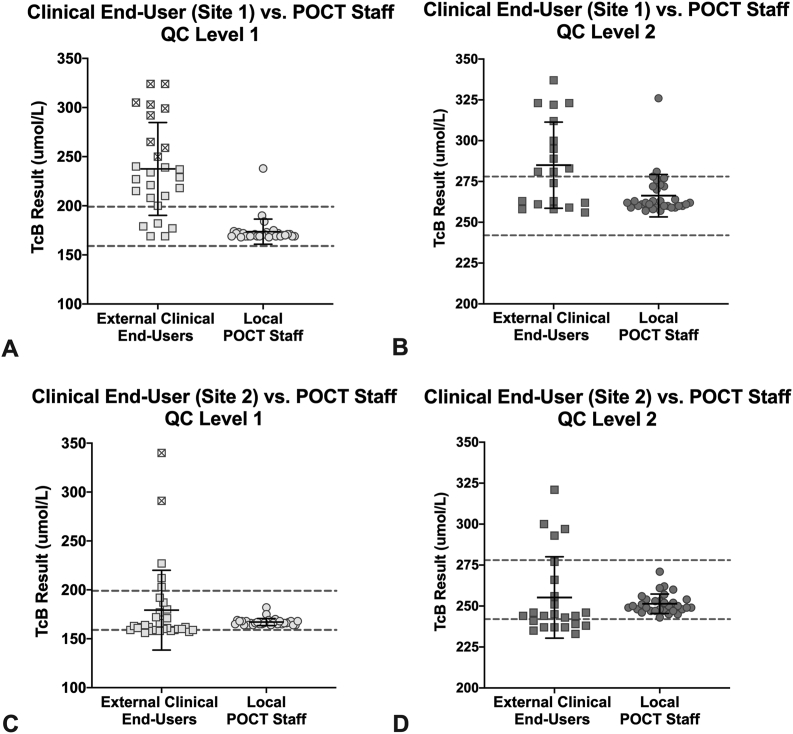
The performance of the QC material was poor when measured by clinical end-users, with 26% of all the L1 values and almost 30% of all the L2 values falling OQR (Table 4). While the L1 mean was within the target range when measured by clinical end-users, it had a much higher CV compared to when the material was measured by POCT staff. The CV of the L2 QC was 9% and this could suggest acceptable performance, however >40% of TcB measurements resulted in errors. In practice, an error means there is no result, and repeat testing or other troubleshooting would be required should it be used clinically. Therefore, performance of the L2 material was less favorable than the L1 material, similar to what was found with POCT staff. An example of this poorer performance by clinical end-users is shown in Fig. 4, where one single set of QC was measured both by clinical end-users and the experienced POCT staff member. Of note, nine L1 measurements from clinical end-users at external site 1 and two L1 measurements from clinical end-users at external site 2 were at or above the L2 target range. In addition, the means for both L1 and L2 were OQR when measured by clinical end-users at external site 1. Clinical users also showed that the performance of the JM-103® model was poorer than the JM-105®, as it had a higher proportion of errors and a higher percentage of values that fell OQR.
Table 4.
TcB QC results when measured by clinical end-users at 10 different sites.
| Meter |
JM-103® and 105® |
JM-103® |
JM-105® |
|||
|---|---|---|---|---|---|---|
| QC Level | Level 1 | Level 2 | Level 1 | Level 2 | Level 1 | Level 2 |
| Number of TcB Resultsa | 354 | 265 | 87 | 49 | 267 | 216 |
| Number of Errors | 26 | 115 | 4 | 42 | 23 | 74 |
| Mean TcB Result, umol/L (Acceptable Range) | 185 (159–199) | 267 (242–278) | 177 (159–199) | 264 (242–278) | 187 (159–199) | 268 (242–278) |
| SD (umol/L) | 36.79 | 23.71 | 24.54 | 24.00 | 39.71 | 23.64 |
| CV (%) | 20 | 9 | 14 | 9 | 21 | 9 |
| Percent OQRb (n) | 26 (93) | 28 (75) | 24 (21) | 33 (16) | 27 (72) | 28 (60) |
Approximately 50 different clinical end-users.
OQR = Out-of-QC Range.
Fig. 4.
Performance of the same set of QC material using JM-105® meters when measured by clinical end-users (squares) at two different sites and an experienced local POCT staff familiar with the QC material (circles). A,B: Results from external site 1. C,D: Results from external site 2. At the external sites, each measurement was performed by a single user operating a single meter at each site. For local POCT staff, at least two TcB meters were used. Each result (individual point) is the average of three measurements as calculated by the TcB meter. The initial result from each measurement was included. L1 TcB measurements that fell above the lower acceptable limit of the L2 target range are marked with an ‘x’ inside their square. Solid black line and corresponding error bars represent the mean ± SD. Dashed lines represent the lower and upper acceptable limits of the QC range. Local POCT staff data is also included in Fig. 2, and clinical end-user data is also included in Table 3.
4. Discussion
High-quality TcB results are critically important to patient care, and the use of QC can provide significant benefit to a TcB POCT program. A locally developed QC product has improved the TcB POCT program in Alberta, however its use previously remained restricted to a single region while it was being developed and evaluated. This study shows that when the QC material is used by POCT staff familiar with the TcB program and the product itself, the material is stable and produces consistent results over time and over different sets of QC. The similar performance of QC material in newly validated meters and those currently in use also provides supporting evidence to the reliability of the QC material and of the TcB meters. The two levels of QC consistently fell within their respective target ranges when measured by POCT staff; these ranges align to the clinically relevant low/moderate risk and high risk regions of the locally validated TcB nomogram. Overall, this demonstrates that the QC material can successfully be used by experienced POCT staff as a tool to assess the accuracy of the TcB meter measurements as part of a comprehensive, high quality POCT TcB program.
It is expected that the use of TcB meters in the POCT setting will increase, as several health jurisdictions have implemented universal screening of hyperbilirubinemia using TcB or TSB measurement [9]. This will necessitate the implementation of strong QA practices. QC testing processes within a specific POCT QA program can depend on several factors. Holt and Freedman (2016) describe five main aspects of internal QC that help inform POCT QC requirements, and consideration of them within a POCT TcB program supports the utility of a robust QC material [21]. Although the Dräger Jaundice Meter has a built-in wavelength (light) check that assesses the light output of the device [24] to help reduce the reliance on heel pokes in a QA program, this process does not assess meter accuracy. The frequency of QC measurement will be influenced by multiple factors, and increased QC frequency may occur with high patient test volumes, when the TcB devices are used by multiple end-users, and if the assessed risk associated with an incorrect test result is high. QC material should also include clinically relevant concentrations of the analyte of interest [21]. However, this property is absent from most POCT TcB programs, as they may only rely on comparisons of TcB versus TSB values in samples from newborns identified as being high-risk.
This QC material was successfully manufactured in-house. It has the ability to test meter function at two clinically relevant concentrations and it is a ready-to-use material that it is stable at room temperature; these are favorable characteristics of QC material [21]. A previous iteration of the QC material was an important part of the meter accuracy check within the local POCT TcB program that has shown to contribute to improved resource utilization for neonatal hyperbilirubinemia assessment [19], and the use of the current QC material in the local POCT TcB program continues to help minimize the number and/or volume of blood samples from infants that are performed for QA purposes (unpublished). These factors make it an attractive option for other healthcare regions to consider within their TcB program.
Several findings in this study highlight challenges inherent to QC implementation in the POCT setting. Notably, the QC material did not have consistent performance in the hands of all users. Although there was excellent performance in the hands of POCT staff, a large proportion of measurements by clinical end-users were either OQR or gave meter errors. For example, only ~50% of total measurements of the L2 QC performed by clinical end-users returned values that were within the target range. Though repeat testing of errors or OQR results could improve the QC performance, this high rate of extra QC testing would increase the complexity of a QA program. This may also be viewed as unmanageable workload by clinical end-users [21]. A second option for overcoming the high rate of OQR results could be to expand the range of acceptable results [21]. However, it is important to first consider the clinical setting and device application before widening QC ranges. For example, several QC L1 results were very high and fell within the acceptable range of the L2 material when measured by clinical end-users, which was reflected in the high CV. If the QC range was expanded to ensure, for example, <10% CV by the clinical end-users, acceptable results could overlap between two distinct, clinically relevant ranges. Widening the QC range would also not help reduce the number of meter errors, which were more common in clinical end-users compared to POCT staff. The difference in performance across users may in part be due to the material of the QC product. It was designed to enable a practical and relatively consistent assessment of meter accuracy, however it is made of hard plastic. As this is very different from newborn skin, it could contribute to its poorer performance by the clinical end-users whom were proficient TcB meter users. This material difference does not preclude the eventual use of the QC material by both POCT and clinical staff, since the study did show that with QC experience, the material performs very well. Preliminary data also supports that the performance of the QC material improves over time in clinical end-users (data not shown); further evaluation of the QC material for incorporation into TcB meter validations by POCT staff is underway. Overall, implementation of the QC to clinical end-users may not be preferred and it may be more appropriate to limit QC use and incorporate it solely for POCT staff.
Use of the QC material in the local Alberta program prior to this study has focused on meter validation. It has also been used for monthly assessment of TcB meters to proactively identify meters in clinical use that may not be functioning. QC material that will be formally incorporated into a POCT program should be able to trigger appropriate troubleshooting [25]. An OQR result obtained by an external POCT staff could be due to several factors, including minimal familiarity with the QC material, a TcB meter issue, or a QC material issue. Regardless of the underlying reason, OQR results show how measurement of the QC material by a POCT staff would prompt further meter investigation that could confirm meter malfunction, identify staff that may require additional training, or identify the need to reassess the acceptable QC range(s). Importantly, the QC product cannot be used in isolation. Strong clinical laboratory oversight and supportive POCT staff are required to best incorporate the QC material to improve the overall quality of the POCT TcB program.
This study was predominantly performed on JM-105 m, but where possible, comparison was made between JM-103® and JM-105® meters. The QC material did not perform as well on the JM-103® meters. This demonstrates that implementation of POCT QC can be a challenge if different models of a device are in use. Interestingly, standardization of POCT devices and models has been previously suggested as a way to make POCT programs more successful [26]. As JM-105® meters replace JM-103® meters, the potential impact of meter model on QC performance is expected to decrease within the TcB program.
There were some limitations to this study. First, the data that represented ideal use of the QC material (Table 1) was derived from TcB measurements using only the JM-105® meters, therefore it is biased towards one model of a TcB meter. Nevertheless, since including JM-103® measurement data had little impact on the QC means and CVs (Table 2), it is still a good representation of ideal performance of the QC material. In addition, QC measurements were only repeated if a QC result was OQR when the meter function was being assessed by an experienced POCT staff during site visits. This reduced the CVs and percent of values that were OQR. Although repeat testing for all OQR would have been beneficial, repeating only during site visits more accurately represents how the QC would be used in practice and this data can be used when considering QC performance benchmarks. Third, most data outside of the local POCT staff was obtained from clinical end-users, as opposed to external POCT staff. As such, the smaller CVs obtained by external POCT staff could have been due, in part, to the use of only two staff and two TcB meters in this data set. These differences are a result of study design, where the clinical end-users were the primary target for QC product assessment. Although this study aimed to show the value of the QC product in the hands of clinical staff, data review supports that greater education and experience with the QC product must occur before implementation with clinical staff occurs.
5. Conclusions
TcB measurement helps identify newborns at risk of neonatal hyperbilirubinemia, while minimizing the number of blood samples obtained for serum bilirubin measurement. QC is an important aspect of a POCT QA program, however there currently exists no commercial QC material for TcB testing. This study supports that the in-house developed QC material performs well when tested by POCT staff and should be incorporated into their QA processes (e.g. meter validation, site visits, meter troubleshooting), but its use by clinical end users is not yet supported. Since its performance appears to improve with greater product experience, it is possible that the QC material could be used by clinical users as an additional QA practice for meter support after more familiarity and training occurred. A QC material for POCT TcB meters may help reduce the requirement for infant blood samples, and ultimately improve infant care and the quality of the POCT program.
Author Statement - “Improving quality of transcutaneous bilirubin measurements: Value of in-house developed quality control”
HAP conducted data analysis and interpreted the data, wrote the manuscript, and revised the manuscript. BA conceived the study, collected data, analyzed and interpreted the data, and revised the manuscript. AAV conceived the study, assisted with data analysis and interpretation, and revised the manuscript. All authors approved the draft and provided critical feedback.
Funding
Development of the QC product was funded by a Calgary Laboratory Services Research Competition Grant.
Declaration of competing interest
The authors declare no conflicts of interest. Development of the QC product was funded by a Calgary Laboratory Services Research Competition Grant.
Acknowledgments
The authors would like to thank the Alberta Precision Laboratories POCT Department, especially POCT staff in both Southern and Central Alberta, including Drs. Michael O’Connor and Yury Butorin, for their support and contributions to this study. HAP conducted data analysis and interpreted the data, wrote the manuscript, and revised the manuscript. BA conceived the study, collected data, analyzed and interpreted the data, and revised the manuscript. AAV conceived the study, assisted with data analysis and interpretation, and revised the manuscript. All authors approved the draft and provided critical feedback.
Contributor Information
Heather Anne Paul, Email: Heather.Paul@albertaprecisionlabs.ca.
Brenda Joanne Adams, Email: brenda.adams@albertaprecisionlabs.ca.
Allison Anne Venner, Email: Allison.Venner@albertaprecisionlabs.ca.
References
- 1.Allen L.C. Role of a quality management system in improving patient safety - laboratory aspects. Clin. Biochem. 2013;46:1187–1193. doi: 10.1016/j.clinbiochem.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Homolka S., Zallet J., Albert H., Witt A.K., Kranzer K. Introduction of quality management in a national reference laboratory in Germany. PloS One. 2019;14 doi: 10.1371/journal.pone.0222925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yip P.M., Venner A.A., Shea J., Fuezery A., Huang Y., Massicotte L., Tetreault N., Tomalty C., Shaw J.L.V. Point-of-care testing: a position statement from the Canadian society of clinical chemists. Clin. Biochem. 2018;53:156–159. doi: 10.1016/j.clinbiochem.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Makic M.B.F., Barton A.J. Point of care testing: ensuring accuracy. Clin. Nurse Spec. 2015;29:306–307. doi: 10.1097/NUR.0000000000000165. [DOI] [PubMed] [Google Scholar]
- 5.Bosschaart N., Kok J.H., Newsum A.M., Ouweneel D.M., Mentink R., Van Leeuwen T.G., Aalders M.C.G. Limitations and opportunities of transcutaneous bilirubin measurements. Pediatrics. 2012;129:689–694. doi: 10.1542/peds.2011-2586. [DOI] [PubMed] [Google Scholar]
- 6.El-Beshbishi S.N., Shattuck K.E., Mohammad A.A., Petersen J.R. Hyperbilirubinemia and transcutaneous bilirubinometry. Clin. Chem. 2009;55:1280–1287. doi: 10.1373/clinchem.2008.121889. [DOI] [PubMed] [Google Scholar]
- 7.Okwundu C.I., Uthman O.A., Suresh G., Smith J., Wiysonge C.S., Bhutani V.K. Transcutaneous bilirubinometry versus total serum bilirubin measurement for newborns. Cochrane Database Syst. Rev. 2017 doi: 10.1002/14651858.CD012660. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauer B.J., Spector N.D. Hyperbilirubinemia in the newborn. Pediatr. Rev. 2011;32:341–349. doi: 10.1542/pir.32-8-341. [DOI] [PubMed] [Google Scholar]
- 9.McClean S., Baerg K., Smith-Fehr J., Szafron M. Cost savings with transcutaneous screening versus total serum bilirubin measurement for newborn jaundice in hospital and community settings: a cost-minimization analysis. C. Open. 2018;6:E285–E291. doi: 10.9778/cmajo.20170158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhutani V.K., Stark A.R., Lazzeroni L.C., Poland R., Gourley G.R., Kazmierczak S., Meloy L., Burgos A.E., Hall J.Y., Stevenson D.K. Predischarge screening for severe neonatal hyperbilirubinemia identifies infants who need phototherapy. J. Pediatr. 2013;162 doi: 10.1016/j.jpeds.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Keren R., Tremont K., Luan X., Cnaan A. Visual assessment of jaundice in term and late preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 2009;94 doi: 10.1136/adc.2008.150714. [DOI] [PubMed] [Google Scholar]
- 12.Porter M.L., Dennis B.L. Hyperbilirubinemia in the term newborn. Am. Fam. Physician. 2002;65 [PubMed] [Google Scholar]
- 13.Odutolu Y., Emmerson A.J. Low bilirubin kernicterus with sepsis and hypoalbuminaemia. BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-008042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watchko J.F., Jeffrey Maisels M. The enigma of low bilirubin kernicterus in premature infants: why does it still occur, and is it preventable? Semin. Perinatol. 2014;38:397–406. doi: 10.1053/j.semperi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro S., Le Pichon J.B., Riordan S.M., Watchkoe J. The neurological sequelae of neonatal hyperbilirubinemia: definitions, diagnosis and treatment of the kernicterus Spectrum disorders (KSDs) Curr. Pediatr. Rev. 2017;13 doi: 10.2174/1573396313666170815100214. [DOI] [PubMed] [Google Scholar]
- 16.Dai J., Parry D.M., Krahn J. Transcutaneous bilirubinometry: its role in the assessment of neonatal jaundice. Clin. Biochem. 1997;30:1–9. doi: 10.1016/S0009-9120(96)00131-2. [DOI] [PubMed] [Google Scholar]
- 17.Barrington K., Sankaran K. Guidelines for detection, management and prevention of hyperbilirubinemia in term and late preterm newborn infants. Paediatr. Child Health. 2007;12:1B–12B. doi: 10.1093/pch/12.suppl_b.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engle W.D., Jackson G.L., Engle N.G. Transcutaneous bilirubinometry. Semin. Perinatol. 2014;38:438–451. doi: 10.1053/j.semperi.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Wainer S., Parmar S.M., Allegro D., Rabi Y., Lyon M.E. Impact of a transcutaneous bilirubinometry program on resource utilization and severe hyperbilirubinemia. Pediatrics. 2012;129:77–86. doi: 10.1542/peds.2011-0599. [DOI] [PubMed] [Google Scholar]
- 20.Carceller-Blanchard A., Cousineau J., Delvin E.E. Point of care testing: transcutaneous bilirubinometry in neonates. Clin. Biochem. 2009;42:143–149. doi: 10.1016/j.clinbiochem.2008.09.106. [DOI] [PubMed] [Google Scholar]
- 21.Holt H., Freedman D.B. Internal quality control in point-of-care testing: where’s the evidence? Ann. Clin. Biochem. 2016;53:233–239. doi: 10.1177/0004563215615148. [DOI] [PubMed] [Google Scholar]
- 22.Maisels M.J., Bhutani V.K., Bogen D., Newman T.B., Stark A.R., Watchko J.F. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124:1193–1198. doi: 10.1542/peds.2009-0329. [DOI] [PubMed] [Google Scholar]
- 23.Raimondi F., Lama S., Landolfo F., Sellitto M., Borrelli A.C., Maffucci R., Milite P., Capasso L. Measuring transcutaneous bilirubin: a comparative analysis of three devices on a multiracial population. BMC Pediatr. 2012;12 doi: 10.1186/1471-2431-12-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dräger Medical Systems . 2018. Assessing Jaundice Risk. [Google Scholar]
- 25.Kinns H., Pitkin S., Housley D., Freedman D.B. Internal quality control: best practice. J. Clin. Pathol. 2013;66:1027–1032. doi: 10.1136/jclinpath-2013-201661. [DOI] [PubMed] [Google Scholar]
- 26.Nichols J.H. Reducing medical errors at the point of care. Lab. Med. 2005;36:275–277. doi: 10.1309/nxxwj31pwfht7l1q. [DOI] [Google Scholar]