Abstract
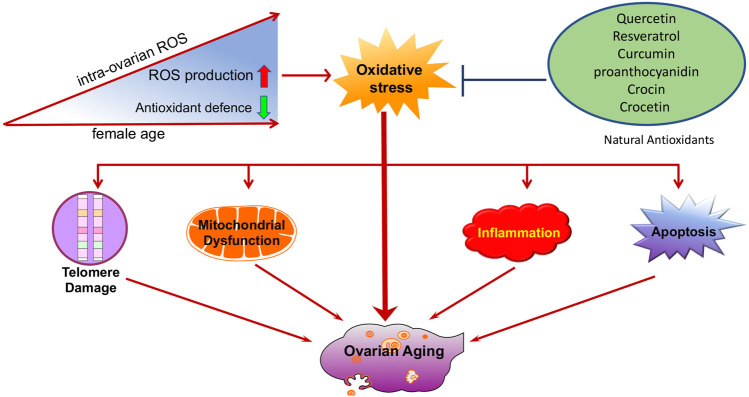
The ovarian system comprises vital organs in females and is of great significance for the maintenance of reproductive potential and endocrine stability. Although complex pathogenesis undoubtedly contributes to ovarian aging, increasing attention is being paid to the extensive influence of oxidative stress. However, the role of oxidative stress in ovarian aging is yet to be fully elucidated. Exploring oxidative stress-related processes might be a promising strategy against ovarian aging. In this review, compelling evidence is shown that oxidative stress plays a role in the etiology of ovarian aging and promotes the development of other ovarian aging-related etiologies, including telomere shortening, mitochondrial dysfunction, apoptosis, and inflammation. In addition, some natural antioxidants such as quercetin, resveratrol, and curcumin have a protective role in the ovaries through multiple mechanisms. These findings raise the prospect of oxidative stress modulator-natural antioxidants as therapeutic interventions for delaying ovarian aging.
Keywords: oxidative stress, natural antioxidants, anti-ovarian aging, reactive oxygen species, mitochondria, inflammation, apoptosis, telomeres
Introduction
Dreams of longevity are as old as humanity itself. Over the past decades, human longevity has increased substantially worldwide. It is predicted that approximately 22% of the global population will be older than 60 by 2050 (Bellantuono, 2018). Consequently, age-related diseases, such as cognitive impairment, cardiovascular diseases, and cancer are markedly increasing and are the leading cause of death and reduced quality of life (Franceschi et al., 2018a).
Ovarian state influence the health of all phases of life and are the main determinants of female life span (de Kat et al., 2016; Rajkovic and Pangas, 2017; Mason et al., 2018; Quinn and Cedars, 2018; Tsiligiannis et al., 2019). Ovarian aging, which occurs earlier than the aging of most other organs, is a continuous process starting from the oocyte death of the embryo at 20 weeks of gestation. (Amanvermez and Tosun, 2016). The most significant characteristic of ovarian aging is a diminished ovarian reserve (DOR), i.e., the decline in the quality and quantity of oocytes, which is also the main reason for infertility and failure in assisted reproductive technology (ART) (May-Panloup et al., 2016). Moreover, ovarian aging is associated with an increased risk for diabetes, heart disease, cancer, and other age-related conditions. The importance of the ovary in maintaining health and extending lifespan has also been well demonstrated in animal models. Transplanting the ovaries of young mice into old mice can prolong the life span of the old mice (Mason et al., 2018). Cardioprotective benefits, cognitive behavior, and immune and renal functions can be positively restored by re-establishment of active ovarian function in aged female mice (Mason et al., 2011; Parkinson et al., 2017; Peterson et al., 2017). Therefore, keeping the ovaries “young” is critical.
Several theories have been proposed to explain the mechanism underlying ovarian aging, including free radical theory, apoptosis, telomere shortening, mitochondrial dysfunction, and inflammation theory (Shi et al., 2016; Regan et al., 2018a; Huang Y et al., 2019; Kasapoğlu and Seli, 2020; Wang et al., 2020). Free radical theory, a classical theory of aging, proposes that oxidative stress caused by elevated intracellular levels of reactive oxygen species (ROS) is the most significant contributor to cellular senescence and aging in mammals (Liochev, 2013; Zhang H et al., 2015). Furthermore, numerous studies have documented that oxidative stress is a leading driver of the ovarian aging process and promotes the development of other ovarian aging-related etiologies, such as telomere shortening, mitochondrial dysfunction, apoptosis, and inflammation (Lim and Luderer, 2011; Arulselvan et al., 2016; Bauer et al., 2017; Prasad et al., 2017; Takeo et al., 2017; Huang ML et al., 2019; Sasaki et al., 2019; Pineda-Pampliega et al., 2020). The role of oxidative stress is also becoming increasingly evident in the pathogenesis of a diverse range of pathological conditions, including Alzheimer’s disease, cardiometabolic diseases, cancer, diabetes mellitus, and retinal dystrophies (Niemann et al., 2017; Saha et al., 2017; Butterfield and Boyd-Kimball, 2018; Cioffi et al., 2019; Domènech and Marfany, 2020; Yaribeygi et al., 2020).
Based on these theoretical considerations, alleviating oxidative stress in the ovaries is an important means of delaying ovarian aging. Effective natural antioxidants could provide novel and safe interventional strategies to delay or prevent ovarian aging and related diseases. Despite a series of reports regarding the antioxidative effects of natural products on ovarian aging, to date, there are no related systematic reviews in the literature. In this review, the underlying mechanism of oxidative stress during ovarian aging and the molecular protective mechanisms of natural antioxidants in anti-ovarian aging are comprehensively explored.
Overview of Oxidative Stress in Ovarian Aging
ROS, including both free radical and non-free radical, oxygenated molecules, such as superoxide radicals (O2•−), H2O2, hydroxyl radicals (•OH), and singlet oxygen (1O2), are mostly generated continuously as byproducts during common metabolic processes in eukaryotic cells (Liguori et al., 2018). They all contain oxygen atoms and have strong oxidizing abilities. A few reviews summarized the pathophysiological functions of ROS (Sugino, 2005; Dizdaroglu and Jaruga, 2012; Rizzo et al., 2012; Kamat et al., 2016; Lu et al., 2018). At low levels, ROS widely participate in cell signal transduction and promote cell survival, proliferation, and differentiation (Rizzo et al., 2012; Lu et al., 2018). ROS are also physiological regulators of ovarian processes and play a key role in follicular development and survival (Sugino, 2005). However, heightened levels of ROS, which overpower cellular antioxidant and repair capacities, can trigger oxidative stress in cells, directly causing oxidative damage to all biomolecules within the cellular environment (including proteins, lipids, and DNA), and thus contribute to the development of aging and related diseases (Dizdaroglu and Jaruga, 2012; Kamat et al., 2016).
Oxidative stress, caused by the imbalance between the production and destruction of ROS, directly damages the intraovarian environment, just as it does in many other cells. Moreover, all primary oocytes are formed by the fifth month of fetal life and remain dormant before complete meiosis I, a decades-long process rendering the oocyte susceptible to chronic oxidative insult (Peters et al., 2020). To date, several studies have shown that the accumulation of ROS in the ovaries deteriorates oocyte quality, induces granulosa cell (GC) apoptosis, and accelerates degeneration of the corpus luteum (Chaube et al., 2014; Tiwari et al., 2015; Prasad et al., 2016; Wang S. et al., 2017; Yang et al., 2017; Sohel et al., 2019). Furthermore, it decreases communication between oocytes and GCs, affecting preovulatory oocyte maturation (Cajas et al., 2020). Oxidative damage to the ovary is generally caused by the propagation of lipid peroxidation cascades, which seriously influences folliculogenesis, meiosis, and ovulation, and eventually leads to ovarian aging (Lim and Luderer, 2011; Edrey and Salmon, 2014; Maclaran and Nikolaou, 2019).
The level of intra-ovarian ROS has been confirmed to be positively correlated with female age, which also makes the female germline particularly vulnerable to the cumulative effects of chronic oxidative stress (Lim and Luderer, 2011; Takeo et al., 2017; Sasaki et al., 2019). Age-related increases in ROS have been found in the follicular fluid and oocytes of women undergoing ART. Among these elderly women with high ROS levels, the success rate of ART is relatively low (Oyawoye et al., 2003; Wiener-Megnazi et al., 2004). In contrast, the content of antioxidants in the intraovarian environment is reduced with age, which means there is a diminished ability to scavenge ROS. Superoxide dismutases (SODs) and catalase (CAT) are the major endogenous antioxidant defense systems against ROS (Racchi, 2013; Ighodaro and Akinloye, 2018). Clinical studies have shown that the mRNA and protein levels of the aforementioned antioxidants in GCs of elderly women undergoing in vitro fertilization (IVF) were significantly lower than those of younger women. The elderly group also showed abnormal mitochondrial morphology and fewer lipid droplets at the ultrastructural level (Tatone et al., 2006). In addition, Matos et al. presented evidence that SOD activity and SOD1 levels in cumulus cells surrounding ovulated oocytes decreased remarkably with female age, and low SOD activity is related to unsuccessful IVF outcomes (Matos et al., 2009). In conclusion, the imbalance between ROS and antioxidants leads to a decline in oocyte quality, which is an important factor affecting the success of ART and is implicated in the aging process of the ovaries.
In addition to direct damage to the ovaries, oxidative stress can also promote the development of other ovarian aging-related mechanisms, including telomere shortening, mitochondrial dysfunction, inflammation, and apoptosis.
Effects of Oxidative Stress on Telomeres in the Ovaries
Telomeres and Ovarian Aging
Telomeres, the dynamic nucleoprotein–DNA structures present at both ends of each chromosome, are responsible for maintaining genome integrity and chromosome stability (Zhang et al., 2018). The length of telomeres shortens progressively with each cell division (mitosis) and is strongly associated with lifespan (Heidinger et al., 2012; Turner et al., 2019). Excessive shortening triggers persistent DNA damage response or genomic instability, causing cellular senescence, and is also strongly linked to lifespan (Whittemore et al., 2019; Smith et al., 2020). Recently, the correlation between the telomere status of ovarian cells and the human female reproductive lifespan has drawn growing attention. A cross-sectional study indicated that the lack of human GC telomerase activity was associated with occult ovarian insufficiency (Butts et al., 2009). Additionally, shortened telomere length and diminished telomerase activity in GCs have also been associated with primary ovarian insufficiency (POI), thereby serving as potential molecular markers for the progression of ovarian function decline (Xu X et al., 2017). Telomere length in cumulus cells is positively correlated with oocyte and embryo quality (Cheng et al., 2013). Consistent with this, Keefe et al. found that in IVF, the pregnancy rate is higher in women with longer oocyte telomere length than in those with shorter oocyte telomere lengths (Keefe et al., 2007).
Oxidative Stress and Telomeres in the Ovaries
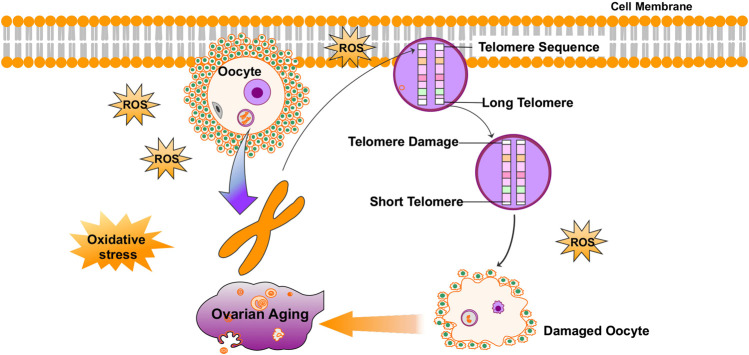
A few reviews summarized that telomeres intrinsically comprise hundreds of guanine bases, which is most easily oxidized by ROS because they have the lowest redox potential amongst the DNA bases (Tardat and Déjardin, 2018). Furthermore, telomere oxidative lesions are less efficiently repaired because of the telomere heterochromatin state, suggesting that telomeres are most vulnerable to oxidative damage (Smith, 2018; Coluzzi et al., 2019; Singh et al., 2019). Accordingly, oxidative stress is considered to be the main cause of telomere shortening (Erusalimsky, 2020). Cigarette smoke, a source of exogenous pro-oxidants, has been confirmed to be associated with increased oxidative stress in the ovary (Sobinoff et al., 2013; Mai et al., 2014; Kim et al., 2018; Li et al., 2020). Related studies have shown that mice exposed to chronic cigarette smoke condensate or smoke exhibited increased egg fragmentation, delayed fertilization, increased apoptosis in blastocysts, and shortened telomeres in embryos (Huang et al., 2009; Dechanet et al., 2011). Furthermore, another study found that the smoke component-induced oxidative damage is greatly reduced by the antioxidant, N-acetylcysteine (NAC) (Huang et al., 2010). It has also been confirmed that increased ROS levels in oocytes result in telomere shortening and reduced developmental competence of aged oocytes (Sasaki et al., 2019). ROS levels are lower, and telomeres are longer, in oocytes from young females (6–8 weeks of age) than in those from reproductively aged female mice (42–48 weeks of age) (Yamada-Fukunaga et al., 2013). In summary, as an important factor of aging, oxidative stress can also mediate telomere damage in the ovaries and cause ovarian aging (Figure 1).
FIGURE 1.
Oxidative stress mediates telomere damage in the ovaries and causes ovarian aging.
Effects of Oxidative Stress on Mitochondria in the Ovaries
Mitochondria and Ovarian Aging
Growing evidence suggests that there is a close relationship between mitochondria and ovarian aging (May-Panloup et al., 2016; Wang T et al., 2017; Kasapoğlu and Seli, 2020). Mitochondria act as energy factories for cells, providing energy and regulating cellular signaling pathways for oocyte maturation, fertilization, and embryogenesis through aerobic respiration (Zhang et al., 2017; Harvey, 2019). Studies have shown that blocking adenosine triphosphate (ATP) synthase prevents germ stem cells from developing into oocytes (Teixeira et al., 2015). Unlike Mendelian inheritance, mitochondrial DNA (mtDNA) is derived exclusively from maternal egg cells (Lutz-Bonengel and Parson, 2019). The mtDNA copy number of oocytes, GCs, and cumulus cells are being explored as predictors of oocyte quality and embryo viability (Wells, 2017; Cecchino and Garcia-Velasco, 2019). Ovarian aging is related to reduced oocyte mtDNA content, and studies have shown that the mtDNA copy number per oocyte of young women is significantly higher than that of aged women or those of the same age with DOR (Pasquariello et al., 2019). In addition, the accumulation of female mouse germline mtDNA mutations exacerbates ovarian aging and reduces lifespan (Ross et al., 2014; Yang et al., 2020). Evidence for this is that the mitochondrial content of GCs and oocytes was significantly decreased in a mouse model of fragile X primary ovarian insufficiency (Conca Dioguardi et al., 2016). Therefore, reproductive experts proposed that mitochondrial transplantation might be a novel possibility for rejuvenating oocyte quality and overcoming age-related infertility or recurrent IVF failure (Kristensen et al., 2017; Labarta et al., 2019). In humans as well as in other animal species, the transfer of autologous or heterologous mitochondria has been proven to improve oocyte quality and IVF outcomes (Hua et al., 2007; Oktay et al., 2015; Srirattana and St John, 2018). Therefore, mitochondria have multiple effects on ovarian aging.
Oxidative Stress and Mitochondria in the Ovaries
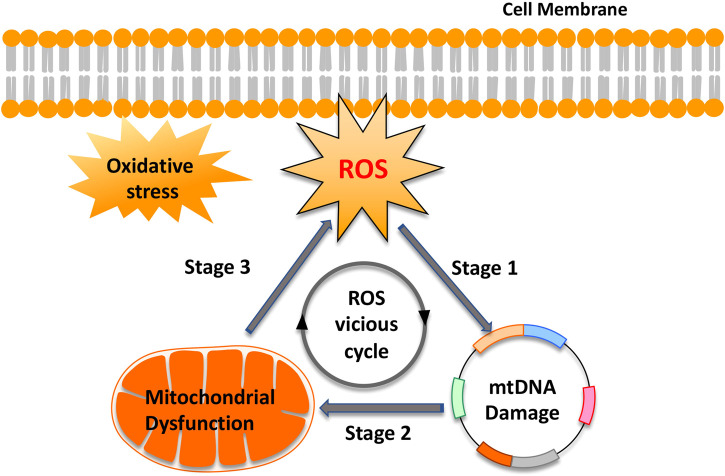
Mitochondria produce energy to drive the cell’s biochemical reactions. In addition, they are also the main source of ROS based on electron leakage from the respiratory chain (Scialò et al., 2017). However, paradoxically, mtDNA, because of the lack of protection by histones or DNA-binding proteins, is particularly vulnerable to ROS-mediated damage (Aryaman et al., 2017; Kauppila et al., 2017; Kaarniranta et al., 2019). Moreover, the ROS generation sites overlap with mtDNA positions, which are mainly attached to the matrix side of the inner mitochondrial membrane (Murphy, 2012). This condition creates an opportunity to form mtDNA-protein crosslinks mediated by ROS, which increases mitochondrial fission and exacerbates mtDNA damage (Caston and Demple, 2017; Yang S.-G. et al., 2018). In turn, mtDNA damage and mutagenesis are directly responsible for a gradual impairment of the respiratory chain function and thus increase electron leakage and ROS production in the mitochondria. This “ROS vicious cycle” (Figure 2), present in different tissues and cells, causes exponentially accelerating oxidative stress with age (Pinto and Moraes, 2015). Consistent with this theory, Aitken et al. confirmed that in MII mouse oocytes, oxidative stress-catalyzed lipid peroxidation could initiate cyclic ROS transmission by directly destroying mitochondrial components (Lord et al., 2015). Studies also confirmed that exposure of mouse MII oocytes to exogenous H2O2 resulted in the dissipation of mitochondrial membrane potential, a decrease in cytoplasmic ATP levels, and the disruption of meiotic spindles (Zhang et al., 2006). However, H2O2-induced damage to mouse oocytes was ameliorated by supplementation with the antioxidant, NAC (Zhang et al., 2006). In addition, oxidative stress-induced cytochrome c (Cyt c) release from mitochondria, by changing the mitochondrial membrane potential, activates the apoptosis cascade (Redza-Dutordoir and Averill-Bates, 2016).
FIGURE 2.
“Reactive oxygen species (ROS) vicious cycle” of ROS production, mtDNA damage, mitochondrial dysfunction, and further ROS production. The cycle implies an exponential growth of ROS production and mtDNA mutagenesis.
Effects of Oxidative Stress on Inflammation in the Ovaries
Inflammation and Ovarian Aging
Inflammation is associated with the pathogenesis of human aging (López-Otín et al., 2013; Franceschi et al., 2018b; Chung et al., 2019). This is also the case in the ovaries. Recent studies have shown that inflammation is a key marker of the aging ovarian stroma and is considered a new mechanism of POI (Huang et al., 2019b; Navarro-Pando et al., 2020). Clinical studies in humans have shown that inflammatory marker levels are associated with the risk of POI or early menopause. Ylmaz et al. detected the levels of serum inflammatory marker levels in patients with POI and normal fertile women, and found that the neutrophil-to-lymphocyte ratio (NLR) was significantly lower in the POI group than in the normal fertility group, and multivariate logistic regression analysis showed that NLR ≤ 1.5 was an independent risk factor for POI (Yldrm et al., 2015). Yue et al. also found that compared to the control group, the serum levels of interleukin (IL)-6 and IL-21 in the primary ovarian failure (POF) group were significantly higher (Sun et al., 2018). Chronic inflammation triggered by obesity might impair oocyte meiosis and oocyte quality (Snider and Wood, 2019). Consistent with these results, Whitcomb et al. found that early menopause cases had higher tumor necrosis factor receptor 2 (TNFR2) levels than controls (Bertone-Johnson et al., 2019). The concentration of TNFR2 was strongly related to TNFα and had the function of regulating TNFα activity (Yang S et al., 2018). Studies in animal models demonstrated that TNFα knockout mice had higher levels of GC proliferation, less incidence of follicle atresia, and higher fertility than wild-type mice (Cui et al., 2011). Therefore, inflammation also plays an important role in ovarian aging.
Oxidative Stress and Inflammation in the Ovaries
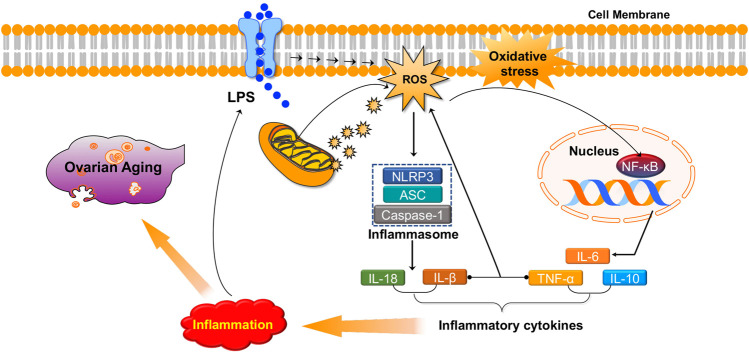
Numerous studies have revealed that there is a strong association between inflammation and oxidative stress. They seem to accompany one another and promote each other in many chronic diseases (Li S et al., 2016; Biswas, 2016; Steven et al., 2019; Zuo et al., 2019). ROS may serve as a “kindling” to activate NLRP3 inflammasomes, leading to pro-inflammatory cytokine secretion (IL-1β and IL-18) (Abais et al., 2015; Long et al., 2020). ROS may also induce the activation of nuclear factor kappa B (NF-κB), a crucial mediator of inflammatory responses, and is associated with the pathogenesis of many disorders (Liu T et al., 2017; Liang et al., 2017; Qin et al., 2017). In addition, the inflammatory agents interferon (IFN)-γ and lipopolysaccharides (LPS) synergistically increase both extracellular and intracellular ROS production in human pancreatic cancer cells (Wu et al., 2013). Furthermore, studies have demonstrated that TNFα, IL-1β, and IFN-γ induce ROS production in multiple types of human cells (Yang et al., 2007; Agharazii et al., 2014). Similarly, increased levels of pro-inflammatory factors and oxidative products have been observed in animal POI models induced by chemotherapy drugs or radiotherapy, and antioxidats can simultaneously reduce pro-inflammatory factors (TNF α, IL-6, and IL-10) and oxidative product (malondialdehyde (MDA), H2O2, and ROS) levels by activating PI3K/AKT/mTOR or inhibiting NF-κB signaling pathways, thereby improving ovarian function (He et al., 2017; Liu C et al., 2018; Li and Liu, 2018; Jiang et al., 2019). In summary, oxidative stress can promote inflammation in the ovaries and cause ovarian aging (Figure 3).
FIGURE 3.
Oxidative stress promotes inflammation in the ovaries and causes ovarian aging.
Effects of Oxidative Stress on Apoptosis in the Ovaries
Apoptosis and Ovarian Aging
Apoptosis plays an important role in germ cell depletion in the mammalian ovaries (Tiwari et al., 2015). Follicular atresia caused by GC apoptosis is the primary process responsible for the loss of follicles and oocytes in the mammalian ovary and is one of the pathogenic mechanisms of premature ovarian failure (Krysko et al., 2008; Regan et al., 2018a; Regan et al., 2018b). Excessive GC apoptosis may deprive follicular oocytes of nutrients, maturation-enabling factors, and survival factors, and thus directly lead to follicular death (Regan et al., 2018a). Clinic studies have shown that higher mural GC apoptosis is correlated with decreased ovarian reserves, fewer oocytes retrieved, and a lower rate of high-quality embryos as well as with age (Sadraie et al., 2000; Fan et al., 2019). Consistent with this, Ebner et al. reported that the apoptotic GCs in younger patients (≤35 years) were significantly fewer than in older ones, and the processes of apoptosis seems to impair oocyte and gamete maturation (Corn et al., 2005). Animal experiments also provide strong evidence that the ovarian phenotype, including reduced ovulation rate and a dramatic decline in fertility, observed in hyh mutant (Napahyh/hyh) mice is based on an increased rate of apoptosis in GCs and follicular atresia (Arcos et al., 2017).
Oxidative Stress and Apoptosis in the Ovaries
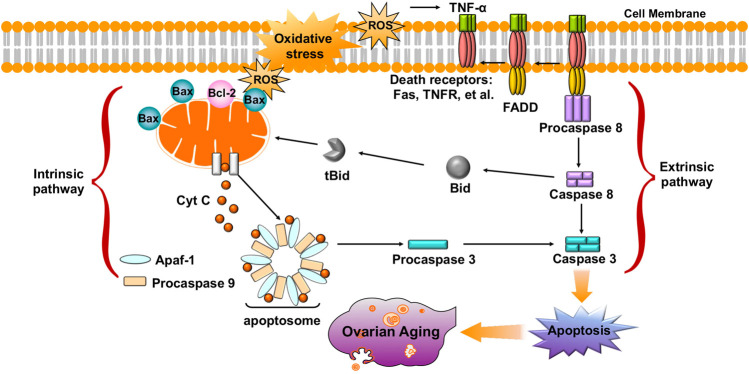
Accumulating evidence shows that oxidative stress is one of the key factors that induces oocyte and GC apoptosis in mammals (Prasad et al., 2016; Tiwari et al., 2016). To date, research indicates that there are two main pathways of oocyte and GC apoptosis caused by oxidative stress: the extrinsic (death receptor) pathway and the intrinsic (mitochondrial) pathway (Figure 4) (Regan et al., 2018a; Yadav et al., 2018). First, oxidative stress can induce the activation of mitochondrial pathways. Excessive ROS may change the mitochondrial membrane potential (MMP) by regulating the ratio of pro-/anti-apoptosis factors, which leads to the release of Cyt c from the mitochondria into the cytosol (Circu and Aw, 2010; Tripathi et al., 2013; Premkumar and Chaube, 2015). Cyt c further binds to apoptotic protease-activating factor 1 (APAF1), causing activation of caspase-9 (Monian and Jiang, 2012; Xiong et al., 2014). The effector, caspase-3, executes the final steps of apoptosis and cleaves various structural and regulatory proteins in female germ cells (Xiong et al., 2014). Li et al. demonstrated that oxidative stress induced bovine GC apoptosis by increasing the expression of cleaved caspase-3 and the Bax/Bcl-2 ratio as well as decreasing the expression of antioxidant enzymes (SOD2, glutathione peroxidase (GSH-Px)) (Wang Y et al., 2019). In addition, excessive ROS in oocytes and GCs can induce the release of TNFα (Kong et al., 2018; Miao et al., 2018). TNF-α binds to its death receptors (such as Fas and TNFR) and then activates the death receptor (extrinsic) pathway of apoptosis through Fas-associated death domain-dependent activation of caspase-8 (Morgan et al., 2008; Guicciardi and Gores, 2009; Redza-Dutordoir and Averill-Bates, 2016). Moreover, activated caspase-8 can also cleave Bid, which then causes the release of Cyt c from mitochondria and activates a crosstalk pathway between death receptors and mitochondria (Huang et al., 2016). Another study found that NAC counteracted H2O2-induced GC apoptosis via the ROS-JNK-p53 pathway, suggesting a functional role of ROS during this process (Yang et al., 2017). Therefore, protecting female germ cells against oxidative stress-induced apoptosis might be of great therapeutic value in the treatment of ovarian aging.
FIGURE 4.
Oxidative stress leads to oocyte and GC apoptosis, and causes ovarian aging via extrinsic (death receptor) pathway and intrinsic (mitochondrial) pathway.
Natural Antioxidant-Based Interventions to Reduce Oxidative Stress-Related Effects on the Ovaries
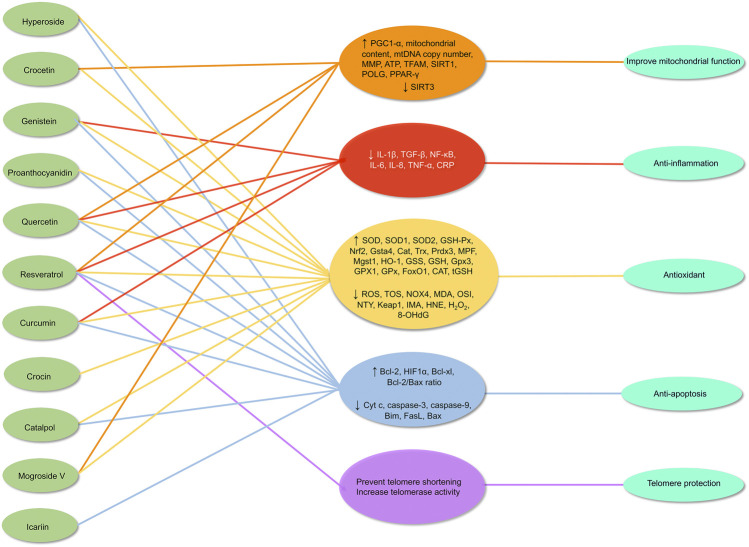
Although aging has historically been viewed as an inevitable and unstoppable process, it is entirely possible to slow down the aging rate. Owing to the important role of oxidative stress in ovarian aging, an enormous amount of related research has focused on antioxidants. Medicinal plants have been used for thousands of years and represent an extraordinary inventory of high-diversity structural scaffolds in addition to being the most important source of antioxidants (Xu DP et al., 2017). Moreover, antioxidants from medicinal plants seem to act on various diseases, including ovarian aging, with their excellent anti-oxidative stress capacities. Some natural compounds with antioxidant properties that are isolated from medicinal plants have also been shown to protect against ovarian aging through multiple mechanisms (Xu X et al., 2017). A summary of these findings and the main efficacy indicators and mechanisms are summarized in Table 1 and Figure 5.
TABLE 1.
Efficacy of natural antioxidants in protecting against ovarian aging.
| Active ingredients | Experimental model | Efficacy | References |
|---|---|---|---|
| Resveratrol | MTX-induced ovarian-damaged rats | Counteracts MTX-induced cytotoxicity in rat ovary | Ata et al. (2019) |
| Cisplatin-induced ovarian-damaged rats | Prevents the loss of the GCs | Said et al. (2019) | |
| Radiotherapy-induced POF rats | Restores ovarian function and diminishes ovarian inflammation | Said et al. (2016) | |
| Busulfan/cyclophosphamide-induced ovarian-aging mice | Improves ovarian aging and renewal capacity of oogonial stem cell | Wu et al. (2019) | |
| Rats exposed to chromium | Mitigates chromium-induced follicle atresia; rescues oocytes and GCs from apoptosis | Banu et al. (2016) | |
| VCD-induced diminished ovarian follicle rats | Improves VCD-induced DOR; increases the number of primary, primordial, and growing follicles | Ozatik et al. (2020) | |
| Mice exposed to Mancozeb | Alleviates Mancozeb induced infertility; increases ovary weight and primary follicles | Liu Y et al. (2017) | |
| C57/BL6 female mice under long-term oral administration of resveratrol | Increases primary and growing follicles; enhances oocyte quantity and quality in aged mice; counteracts age-related fertility decline | Liu et al. (2013) | |
| Aging oocytes of mice | Increases the rates of fertilization and blastocyst in POA oocytes; reduces the loss of sperm-binding sites; maintains the normal morphology of spindle and mitochondrion distribution; alleviates early apoptosis | Sun et al. (2019) | |
| Aged bovine oocytes and GCs | Improves the developmental progress of oocytes to the blastocyst stage; increases the mtDNA copy numbers and ATP content of oocytes | Sugiyama et al. (2015) | |
| Vitrified-warmed porcine oocytes | Improves survival, maturation, and MMP of vitrified-warmed oocytes | Ito et al. (2020) | |
| Quercetin | CP-induced POF mice | Increases primordial follicles number and AMH level; decreases atretic follicles number | Elkady et al. (2019) |
| Streptozotocin-induced diabetic mice | Increases the volume of the ovaries and growing follicles, significantly decreases the number of atretic follicles | Bolouki et al. (2019) | |
| Experimental ovarian ischemia-reperfusion injury rats | Prevents follicular cell degeneration, hemorrhage, vascular congestion, and edema | Gencer et al. (2014) | |
| Cadmium chloride-induced reproductive toxicity rats | Increases the number of follicles; decreases apoptosis of follicular cells | Nna et al. (2017) | |
| Goat oocytes | Improves mitochondrial activity and the percentage of MII oocytes; decreases the rate of apoptosis of MII oocytes | Silva et al. (2018) | |
| H2O2-induced oxidatively stressed human GCs | Significantly improves viability of human GCs; reduces the percentage of early apoptotic cells | Rashidi et al. (2019) | |
| H2O2-induced oxidatively stressed bovine GCs | Increases GC proliferation and mitochondrial activity; reduces intracellular ROS levels | Khadrawy et al. (2019) | |
| Oocytes of aging mice | Relieves deterioration in oocyte quality and improves subsequent embryo development | Wang H et al. (2017) | |
| Curcumin | D-galactose-induced POF model mice | Increases the follicles at different developmental stages | Yan et al. (2018) |
| Sodium arsenite-induced ovarian oxidative injury mice | Markedly reduces atretic follicles; increases the number of GCs | Wang XN et al. (2017) | |
| Exposed-to-whole-body ionizing radiation mice | Improves histological appearance of oocytes; reduces follicular atresia and GC apoptosis | Aktas et al. (2012) | |
| Zearalenone-induced oxidative stress porcine GCs | Rescues oxidative stress induced by Zearalenone | Qin et al. (2015) | |
| Estradiol valerate-induced PCOS rats | Significantly increases the number of primordial follicles, preantral, and corpus luteum; reduces the number of cysts and antral follicles | Mohammadi et al. (2017) | |
| CPM-induced POF rats | Significantly reduces atretic follicle, lipid peroxidation, hemorrhage around the corpus luteum, and vascular congestion in the ovarian stroma; improves histological parameters | Melekoglu et al. (2018) | |
| Rat ovaries with ischemia-reperfusion injury | Reverses tissue damage induced by ischemia-reperfusion injury in ovarian torsion | Sak et al. (2013) | |
| Proanthocyanidin | D-gal-induced aging hens | Decreases the oxidative stress, alleviates the inhibition of aging on ovarian somatic cell proliferation, and decreases cell apoptosis | Liu X et al. (2018) |
| 3-NPA-induced ovarian oxidatively damaged mice | Significantly lower the percentage of GCs apoptosis and atretic follicles | Qin et al. (2015) | |
| Human GCs | Decreases oxidative stress and increases steroidogenesis | Barbe et al. (2019) | |
| Diquat-induced ovarian oxidatively damaged mice | Improves GCs viability, reduces GCs apoptosis rate, and induces autophagy process | Zhang et al. (2016) | |
| Crocin | Mice oocytes | Improves IVM outcomes | Mokhber Maleki et al. (2014) |
| Mouse oocytes | Improves nuclear maturation rates and subsequent developmental potential of mouse oocytes | Mokhber Maleki et al. (2014) | |
| Crocetin | CPM-induced ovary injury mice | Reduces follicle loss; rescues fertility in CPM-treated mice | Di Emidio et al. (2017) |
| Mogroside V | Porcine oocytes | Improves IVM outcomes and subsequent embryonic development | Nie et al. (2020) |
| Porcine oocytes | Protects porcine oocytes against in vitro aging | Nie et al. (2019) | |
| Catalpol | Aged female rats | Nourishes ovarian tissues and improves both the quality and quantity of follicles | Wei et al. (2014) |
| H2O2-induced oxidatively damaged rat ovarian GCs | Improves GCs viability | Yan et al. (2020) | |
| Genistein | Pre-menopausal rats | Increases the number of surviving follicles and reserves; prolongs ovarian reproductive life | Zhuang et al. (2010) |
| Ovarian toxicity rats induced by CPM | Decreases recruitment of primordial cells; increases the number of mature follicles and corpora lutea | Saleh and Mansour, (2016) | |
| ϒ-radiation-induced POF rats | Preserves all stages of healthy follicles; diminishes the atretic follicle population; enhances GC proliferation | Haddad et al. (2020) | |
| Hyperoside | H2O2-stimulated Sprague-Dawley rats GCs | Improves GCs viability; protects GCs from H2O2-induced cell apoptosis and oxidative stress | Wang X et al. (2019) |
| Icariin | D-galactose-induced ovarian aging mice | Improves ovarian follicular development; inhibits follicular atresia; restores ovarian function of aging mice; enhances mice fertility | Wang J-L et al. (2019) |
FIGURE 5.
Chemical structures of the natural antioxidants summarized in this review.
Resveratrol
Resveratrol is a natural antioxidant polyphenol that is rich in the roots of Reynoutria japonica Houtt., wine, and grapes (Meng et al., 2020). Resveratrol has received extensive attention in recent years owing to its wide range of biological properties, including anti-aging, anti-oxidation, anti-inflammatory, and anti-cancer activities (Wang et al., 2018; Tong et al., 2020; Yu et al., 2020). Growing evidence indicates that resveratrol has potential effects in stimulating ovarian function and retarding ovarian aging. In POF animal models, induced by drugs, radiotherapy, or heavy metals, resveratrol significantly promotes primordial follicle activation, increases the number of primary, primordial, and growing follicles, stimulates GC proliferation, diminishes ovarian inflammation, maintains ovarian architecture, and rescues oocyte and GC apoptosis (Banu et al., 2016; Said et al., 2016; Liu Y et al., 2017; Chinwe et al., 2018; Li and Liu, 2018; Ata et al., 2019; Said et al., 2019; Wu et al., 2019; Ozatik et al., 2020). Besides these features, long-term (12 months) oral administration of resveratrol can prevent telomere shortening, increase telomerase activity to increase the primary and growing follicles, increase the oocyte quantity and quality, and enhance embryonic development in aging mice to achieve anti-ovarian aging (Liu et al., 2013). Treatment with resveratrol during in vitro maturation (IVM) has been shown to reverse the adverse effects of oxidative stress on oocytes, improve blastocyst production from oocytes, increase mtDNA copy numbers, and elevate ATP content in oocytes and subsequent embryo development ability as well as raise the rates of fertilization in postovulatory aging (POA) oocytes, maintain the normal morphology of spindle and mitochondrion distribution, and alleviate early apoptosis (Itami et al., 2015; Sugiyama et al., 2015; Piras et al., 2019; Sun et al., 2019; Ito et al., 2020). These studies suggest that resveratrol might serve as an effective approach to improve ovarian functions and delay ovarian aging, and its specific effects and mechanisms are as follows: 1) increases nuclear factor erythroid 2-related factor 2 (Nrf2), a redox-sensitive transcription factor that regulates the expression of antioxidant genes (Espinosa-Diez et al., 2015), total glutathione (tGSH), SOD, and SOD2; reduces ROS production and MDA levels; and reverses the adverse effects of oxidative stress; 2) promotes mitochondrial synthesis, increases transcription factor a (TFAM), polymerase subunit gamma (POLG), and peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1α) levels; 3) regulates the mitochondrial apoptotic pathway, reduces the release of Cyt c and cleaved caspase-3, increases Bcl-2 and hypoxia-inducible factor 1-alpha (HIF1α), and inhibits oocytes and GC apoptosis; 4) acts as an anti-inflammation agent by inhibiting NF-κB-provoked inflammatory cytokines (IL-6, IL-8); 5) prevents telomere shortening and increases telomerase activity; and 6) regulates the expression of the SIRT1 gene while activating the PI3K/Akt signaling pathway.
Quercetin
Quercetin, a bioactive flavonoid, is widely found in medicinal plants and foods, such as Ginkgo biloba L., Hypericum perforatum L., apples, berries (Li Y et al., 2016). It has extensive biological properties, including antioxidant, anti-inflammatory, and anti-apoptosis effects, and stimulates mitochondrial biogenesis (Li Y et al., 2016; Xu et al., 2019; Zeng et al., 2020). Recently, the use of quercetin in anti-ovarian aging treatment has been increasingly considered.
In laboratory animals, quercetin has been shown to increase the volume of the ovary and the primordial follicle number, the number of growing follicles along with the corpus luteum, and prevent follicular cell degeneration, hemorrhage, vascular congestion, and edema while decreasing apoptosis of follicular cells (Gencer et al., 2014; Nna et al., 2017; Bolouki et al., 2019; Elkady et al., 2019). In addition, in multiple in vitro studies using animal and human GCs, quercetin treatment enhanced viability of GCs, reduced the percentage of early apoptotic cells, relieved deterioration in oocyte quality, and improved subsequent embryo development (Wang H et al., 2017; Silva et al., 2018; Khadrawy et al., 2019; Rashidi et al., 2019). Accordingly, quercetin has a protective role in the ovaries,and its specific effects and mechanisms are as follows: 1) enhances Nrf2, SOD1, CAT, glutathione synthetase (GSS), GSH, and glutathione peroxidase (GPx) activity; reduces ROS production and MDA levels; and inhibits oxidative stress; 2) decreases anti-apoptotic caspase levels; 3) inhibits the TLR/NF-κB inflammation signaling pathway; and 4) enhances mitochondrial activity.
Curcumin
Curcumin is the main natural polyphenol extracted from the rhizome of Curcuma longa L., which has been traditionally used in Asian countries as a medical herb for thousands of years (Kotha and Luthria, 2019). Curcumin is a widely studied nutraceutical and is known recently to have antioxidant, anti-aging, anti-inflammatory, anti-apoptosis, and anti-cancer functions (Kocaadam and Şanlier, 2017; Bielak-Zmijewska et al., 2019; Boarescu et al., 2019). In laboratory animals, curcumin has been shown to play a stimulatory role in ovarian functions and prevents the compromise of ovarian functions caused by cyclophosphamide (CPM), ionizing radiation, and ischemia (Alekseyeva et al., 2011; Aktas et al., 2012; Sak et al., 2013; Qin et al., 2015; Wang XN et al., 2017; Mohammadi et al., 2017; Melekoglu et al., 2018; Yan et al., 2018). Treatment with curcumin has been shown to increase the number of follicles at different developmental stages and in GCs, improve histological appearance of oocytes, and markedly reduce atretic follicles, lipid peroxidation, hemorrhage around the corpus luteum, and vascular congestion in the ovarian stroma (Alekseyeva et al., 2011; Aktas et al., 2012; Sak et al., 2013; Qin et al., 2015; Wang XN et al., 2017; Mohammadi et al., 2017; Melekoglu et al., 2018; Yan et al., 2018). The specific effects and mechanisms involved are as follows: 1) alleviates ovarian oxidative injury, increases the levels of Nrf2, heme oxygenase-1(HO-1), SOD, and SOD1 while reducing ROS production and MDA levels; 2) decreases anti-apoptotic levels of caspase-3 and -9; and 3) as an anti-inflammation agent, reduces the levels of TNF-α, IL-6, and CRP.
Proanthocyanidin
Proanthocyanidin (PA) is an important class of polyphenols abundant in grape seeds, Lycium ruthenicum Murry., and blueberries, possessing antioxidant, anti-inflammatory, and anti-cancer activities (Shi et al., 2003; He et al., 2018). In hens, PA treatment significantly alleviated the inhibition of ovarian somatic cell proliferation and decreased cell apoptosis in D-gal-induced and natural aging ovarian tissues by reducing oxidative stress (Liu X et al., 2018). Similarly, in 3-nitropropionic acid (3-NPA)-induced oxidative ovarian damaged mice, PA significantly reduced the percentage of GC apoptosis and atretic follicles in ovarian tissues, increased the expression of antioxidant genes, and inhibited the expression of pro-apoptotic genes (Zhang JQ et al., 2015). In addition, in multiple in vitro studies using animal and human GCs, PA and procyanidin B2 (B type of PA) exert a potent effect in terms of diminishing GC apoptosis and intracellular ROS production (Zhang et al., 2016; Barbe et al., 2019). Taken together, the administration of PA has the potential to alleviate ovarian oxidative injury and delay ovarian aging mainly through antioxidant and anti-apoptotic activities.
Crocin and Crocetin
Crocin and crocetin are both carotenoid chemical compounds of Crocus sativus L. and are well-known in traditional medicine (Leone et al., 2018). They show high radical-scavenging activity as well as anti-inflammatory, anti-apoptosis, and probably anti-aging activity (Assimopoulou et al., 2005; Bukhari et al., 2018). Abedi et al. demonstrated that supplementation of IVM media with crocin significantly reduces the adverse effects of oxidative stress, thereby improving nuclear maturation rates and subsequent developmental potential of mouse oocytes (Mokhber Maleki et al., 2014; Mokhber Maleki et al., 2016). In addition, crocetin administration reduces follicle loss and rescues fertility in CPM-treated mice. Mechanistically, crocetin protects the ovary against CPM by modulating redox balance, decreasing SIRT3, and increasing the antioxidant enzyme, SOD2, as well as the mitochondrial biogenesis activator, PGC1α (Di Emidio et al., 2017).
Mogroside V
Mogroside V (MV) is the most abundant form of triterpenoid compound isolated from Siraitia grosvenorii (Swingle) C.Jeffrey ex A.M.Lu & Zhi Y.Zhang (Liu H et al., 2018; Gong et al., 2019). Numerous studies have demonstrated that MV possesses broad pharmacological characteristics and properties, including antioxidant, anti-tussive, immunoregulatory, and anti-inflammatory effects (Di et al., 2011; Itkin et al., 2016; Liu C et al., 2018). Liang et al. reported that supplementation of IVM media with MV significantly increased the IVM rate and subsequent embryonic development. Furthermore, MV reduced intracellular ROS and increased the mRNA expression of oxidative stress-related genes (SOD, CAT, and SIRT1) while enhancing mitochondrial function (Nie et al., 2020). MV markedly reduces the decline in porcine oocyte quality during in vitro aging, possibly by reducing oxidative stress and early apoptosis in aged oocytes while improving mitochondrial contents and function (Nie et al., 2019).
Catalpol
Catalpol is an iridoid glycoside abundant in the roots of Rehmannia glutinosa (Gaertn.) DC. and has been shown to possess a broad range of bioactivities, especially antioxidative effects, and is considered a potential candidate for treating oxidative stress-induced neurodegenerative disease (Jiang et al., 2015; Tong et al., 2015; Zheng et al., 2017). Recently, the protective role of catalpol in the ovaries has also been confirmed. Studies have shown that catalpol can nourish ovarian tissue and improve the quality and quantity of follicles and has a direct anti-aging effect on the rat ovarian system (Wei et al., 2014). Catalpol also improved GC viability and protected GCs from H2O2-induced oxidative injury and apoptosis (Yan et al., 2020). The mechanism likely involved is that catalpol inhibits H2O2-induced ROS, MDA, Bax, and caspase-9 production while activating SOD, GSH-Px, and Bcl-2 expression (Yan et al., 2020).
Genistein
Genistein, one of the most important isoflavones, is found in soybean products, and in the traditional medicinal plants, including Pueraria montana (Lour.) Merr., Cistanche deserticola Ma, and Eucommia ulmoides Oliv. (Park et al., 2019; Tang et al., 2019). It shows estrogenic properties via binding to different estrogen receptors and has various biological effects in antioxidation and anti-aging (Spagnuolo et al., 2015; Liu et al., 2020). Indeed, in vitro studies have shown that genistein increased the number of follicles surviving as reserves and prolonged ovarian reproductive life (Zhuang et al., 2010). In addition, genistein alleviated the oxidative stress and inflammation in CMP-induced ovarian toxicity rats (Saleh and Mansour, 2016). Specifically, genistein decreased the recruitment of primordial cells and increased the number of mature follicles and corpora lutea by upregulating ovarian SOD and GSH levels, and downregulating IL-1β and MAD levels, which means preservation of ovarian function and follicular reservoirs (Saleh and Mansour, 2016). Moreover, compared with estradiol, genistein was superior in preserving all stages of healthy follicles, enhancing GCs proliferation, and reducing the population of atretic follicles in ϒ-radiation-induced POF rats (Haddad et al., 2020).
There are several other natural compounds with antioxidant, anti-inflammatory, anti-apoptosis, and anti-aging properties, such as hyperoside and icariin, which may also exert beneficial effects on ovarian aging (Wang J-L et al., 2019; Wang X et al., 2019). The chemical structures of the natural antioxidants summarized in this review are presented in Figure 6.
FIGURE 6.
Schematic illustration of oxidative stress during ovarian aging. Oxidative stress promotes the development of other ovarian aging-related etiologies, including telomere shortening, mitochondrial dysfunction, apoptosis, and inflammation. Natural antioxidants supplements, such as resveratrol, quercetin, and curcumin might be beneficial in preventing ovarian aging.
Apart from natural compounds, various diets rich in antioxidants are also sources of natural antioxidants. For example, kiwifruit, nuts, tomatoes are rich in vitamins C, D, and E; walnuts, peanuts, cherries, corn are high in melatonin. A prospective study showed that there is a highly significant correlation between vitamin D deficiency and DOR (Arefi et al., 2018). The combined use of vitamin E and C can prevent apoptosis of ovarian tissue following mancozeb exposure in the first generation of mouse pups (Mahdi et al., 2019). In addition, melatonin may act as a free radical scavenger and has antioxidant properties. Its role in anti-ovarian aging has been widely reported. Oral melatonin can ameliorate intrafollicular oxidative stress, improve the quantity and quality of oocytes and IVF outcomes (Jahromi et al., 2017; Espino et al., 2019; Hu et al., 2020). In contrast, night shift work may lead to impaired pineal function and inhibit melatonin production (James et al., 2017). Teixeira et al. reported that night shift workers have a higher level of oxidative stress damage and a lower level of antioxidant defenses (Teixeira et al., 2019). Women who rotate night shift work have an increased risk of early menopause and ovarian failure (Stock et al., 2019). Therefore, healthy sleep also contributes to antioxidant stress and anti-ovarian aging.
Conclusion
Driven by societal trends, many young women choose to postpone marriage and/or childbirth. Age-related fertility issues have become serious challenges in reproductive medicine because aging causes a reduction in both oocyte quality and quantity. Oxidative stress is a crucial factor in ovarian functional decline with age. It acts as a driver of the etiology of ovarian aging. Compelling evidence has shown that oxidative stress promotes the development of other ovarian aging-related etiologies, including telomere shortening, mitochondrial dysfunction, apoptosis, and inflammation, which provides new insights into our understanding of the mechanisms of ovarian aging.
Therefore, alleviating oxidative stress in the ovaries is an important entry point for delaying ovarian aging. Compared with synthetic antioxidants, antioxidants from natural products have a high anti-oxidative stress capacity and are safe and acceptable. In our review, certain natural antioxidants have been tested in vivo and in vitro with promising results and multiple mechanisms. These findings raise the prospect of oxidative stress modulatory natural antioxidants as therapeutic interventions for delaying ovarian aging (Figure 7). While substantial research supports these strategies, further investigation is warranted, particularly through clinical trials.
FIGURE 7.
Mechanism of natural antioxidants in protecting against ovarian aging through multiple mechanisms. ↑ = up-regulates, ↓ = down-regulates.
Author Contributions
QZ conceptualized the manuscript. LY, XC, YC,YL, LY, YX, CM, and YZ collected the literature, wrote the manuscript, and prepared figures. QZ edited and made significant revisions to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
QZ got the Programs: Zhejiang Provincial TCM Sci-tech Plan (2020ZA078) and Zhejiang Zhangqin famous Traditional Chinese Medicine expert inheritance studio project (GZS2012014). YC got the Program: Medical and health science and technology plan of Zhejiang Province (2021KY920).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Editage (http://www.editage.cn) for English language editing of the manuscript.
Glossary
- ART
assisted reproductive technology
- ATP
adenosine triphosphate
- CAT
catalase
- CPM
cyclophosphamide
- Cyt c
cytochrome c
- DOR
diminished ovarian reserve
- FADD
fas-associated death domain
- GCs
granulosa cells
- GPx
glutathione peroxidase
- GPx3
glutathione peroxidase 3
- GSH
glutathione
- GSH-Px
glutathione peroxidase
- GSS
glutathione synthetase
- Gsta4
glutathione transferase α4
- H2O2
hydrogen peroxide
- HIF1α
hypoxia-inducible factor 1-alpha
- HO-1
heme oxygenase-1
- IFN-γ
interferon gamma
- IL-18
interleukin 18
- IL-1β
interleukin 1 beta
- iNOS
inducible nitric oxide synthase
- IVM
in vitro maturation
- LPS
lipopolysaccharides
- MDA
malondialdehyde
- Mgst1
microsomal glutathione S-transferase 1
- MMP
mitochondrial membrane potential
- MPF
maturation promoting factor
- MTX
methotrexate
- MV
Mogroside V
- NAC
N-acetylcysteine
- NF-κB
nuclear transcription factor B
- 3-NPA
3-nitropropionic acid
- Nrf2
nuclear factor erythroid 2-related factor 2
- PA
Proanthocyanidin
- PCOS
polycystic ovary syndrome
- PGC1α
peroxisome proliferator-activated receptor gamma coactivator 1-α
- POA
postovulatory aging
- POF
primary ovarian failure
- POI
primary ovarian insufficiency
- POLG
polymerase subunit gamma
- Prdx3
peroxiredoxin 3
- ROS
reactive oxygen species
- SIRT1
nicotinamide adenosine dinucleotide-dependent deacetylase sirtuin-1
- SODs
auperoxide dismutases
- TAS
total antioxidant status
- TFAM
transcription factor a
- tGSH
total glutathione
- TNF α
tumor necrosis factor-alpha
- TNFR2
tumor necrosis factor receptor 2
- TOS
total oxidant status
References
- Abais J. M., Xia M., Zhang Y., Boini K. M., Li P. L. (2015). Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector?. Antioxid Redox Signal. 22 (13), 1111–1129. 10.1089/ars.2014.5994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agharazii M., St-Louis R., Gautier-Bastien A., Ung R.-V., Mokas S., Larivière R., et al. (2014). Inflammatory cytokines and reactive oxygen species as mediators of chronic kidney disease-related vascular calcification. Am. J. Hypertens. 28 (6), 746–755. 10.1093/ajh/hpu225 [DOI] [PubMed] [Google Scholar]
- Aktas C., Kanter M., Kocak Z. (2012). Antiapoptotic and proliferative activity of curcumin on ovarian follicles in mice exposed to whole body ionizing radiation. Toxicol. Ind. Health. 28 (9), 852–863. 10.1177/0748233711425080 [DOI] [PubMed] [Google Scholar]
- Alekseyeva I. N., Makogon N. V., Bryzgina T. M., Voznesenskaya T. Y., Sukhina V. S. (2011). Effects of NF-κB blocker curcumin on oogenesis and immunocompetent organ cells in immune ovarian injury in mice. Bull. Exp. Biol. Med. 151 (4), 432–435. 10.1007/s10517-011-1349-1 [DOI] [PubMed] [Google Scholar]
- Amanvermez R., Tosun M. (2016). An update on ovarian aging and ovarian reserve tests. Int. J. Fertil. Steril. 9 (4), 411–415. 10.22074/ijfs.2015.4591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcos A., de Paola M., Gianetti D., Acuña D., Velásquez Z. D., Miró M. P., et al. (2017). α-SNAP is expressed in mouse ovarian granulosa cells and plays a key role in folliculogenesis and female fertility. Sci. Rep. 7 (1), 11765 10.1038/s41598-017-12292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arefi S., Khalili G., Iranmanesh H., Farifteh F., Hosseini A., Fatemi H. M., et al. (2018). Is the ovarian reserve influenced by vitamin D deficiency and the dress code in an infertile Iranian population?. J. Ovarian Res. 11 (1), 62 10.1186/s13048-018-0435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arulselvan P., Fard M. T., Tan W. S., Gothai S., Fakurazi S., Norhaizan M. E., et al. (2016). Role of antioxidants and natural products in inflammation. Oxid. Med. Cell Longev. 2016, 5276130 10.1155/2016/5276130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryaman J., Hoitzing H., Burgstaller J. P., Johnston I. G., Jones N. S. (2017). Mitochondrial heterogeneity, metabolic scaling and cell death. Bioessays. 39 (7). 10.1002/bies.201700001 [DOI] [PubMed] [Google Scholar]
- Assimopoulou A. N., Sinakos Z., Papageorgiou V. P. (2005). Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 19 (11), 997–1000. 10.1002/ptr.1749 [DOI] [PubMed] [Google Scholar]
- Ata N., Kulhan N. G., Kulhan M., Turkler C., Kiremitli T., Kiremitli S., et al. (2019). The effect of resveratrol on oxidative ovary-damage induced by methotrexate in rats (Resveratrol oxidative ovary-damage). Gen. Physiol. Biophys. 38 (6), 519–524. 10.4149/gpb_20190281 [DOI] [PubMed] [Google Scholar]
- Banu S. K., Stanley J. A., Sivakumar K. K., Arosh J. A., Burghardt R. C. (2016). Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol. Appl. Pharmacol. 303, 65–78. 10.1016/j.taap.2016.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe A., Ramé C., Mellouk N., Estienne A., Bongrani A., Brossaud A., et al. (2019). Effects of grape seed extract and proanthocyanidin B2 on in vitro proliferation, viability, steroidogenesis, oxidative stress, and cell signaling in human granulosa cells. Int. J. Mol. Sci. 20 (17), 4215 10.3390/ijms20174215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D., Werth F., Nguyen H. A., Kiecker F., Eberle J. (2017). Critical role of reactive oxygen species (ROS) for synergistic enhancement of apoptosis by vemurafenib and the potassium channel inhibitor TRAM-34 in melanoma cells. Cell Death Dis. 8 (2), e2594 10.1038/cddis.2017.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellantuono I. (2018). Find drugs that delay many diseases of old age. Nature 554 (7692), 293–295. 10.1038/d41586-018-01668-0 [DOI] [PubMed] [Google Scholar]
- Bertone-Johnson E. R., Manson J. E., Purdue-Smithe A. C., Hankinson S. E., Rosner B. A., Whitcomb B. W. (2019). A prospective study of inflammatory biomarker levels and risk of early menopause. Menopause 26 (1), 32–38. 10.1097/gme.0000000000001162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielak-Zmijewska A., Grabowska W., Ciolko A., Bojko A., Mosieniak G., Bijoch Ł., et al. (2019). The role of curcumin in the modulation of ageing. Int. J. Mol. Sci. 20 (5), 1239 10.3390/ijms20051239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S. K. (2016). Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell Longev. 2016, 5698931 10.1155/2016/5698931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boarescu P. M., Boarescu I., Boarescu I. C., Gheban D., Bulboacă A. E., Nicula C., et al. (2019). Antioxidant and anti-inflammatory effects of curcumin nanoparticles on drug-induced acute myocardial infarction in diabetic rats. Antioxidants 8 (10), 504 10.3390/antiox8100504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouki A., Zal F., Bordbar H. (2019). Ameliorative effects of quercetin on folliculogenesis in diabetic mice: a stereological study. Gynecol. Endocrinol. 36, 864–868. 10.1080/09513590.2019.1707796 [DOI] [PubMed] [Google Scholar]
- Bukhari S. I., Manzoor M., Dhar M. K. (2018). A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 98, 733–745. 10.1016/j.biopha.2017.12.090 [DOI] [PubMed] [Google Scholar]
- Butterfield D. A., Boyd-Kimball D. (2018). Oxidative stress, amyloid-β peptide, and altered key molecular pathways in the pathogenesis and progression of alzheimer’s disease. J. Alzheimers Dis. 62 (3), 1345–1367. 10.3233/jad-170543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts S., Riethman H., Ratcliffe S., Shaunik A., Coutifaris C., Barnhart K. (2009). Correlation of telomere length and telomerase activity with occult ovarian insufficiency. J. Clin. Endocrinol. Metab. 94 (12), 4835–4843. 10.1210/jc.2008-2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajas Y. N., Cañón-Beltrán K., Ladrón de Guevara M., Millán de la Blanca M. G., Ramos-Ibeas P., Gutiérrez-Adán A., et al. (2020). Antioxidant nobiletin enhances oocyte maturation and subsequent embryo development and quality. Int. J. Mol. Sci. 21 (15), 5340 10.3390/ijms21155340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caston R. A., Demple B. (2017). Risky repair: DNA-protein crosslinks formed by mitochondrial base excision DNA repair enzymes acting on free radical lesions. Free Radic. Biol. Med. 107, 146–150. 10.1016/j.freeradbiomed.2016.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchino G. N., Garcia-Velasco J. A. (2019). Mitochondrial DNA copy number as a predictor of embryo viability. Fertil. Steril. 111 (2), 205–211. 10.1016/j.fertnstert.2018.11.021 [DOI] [PubMed] [Google Scholar]
- Chaube S. K., Shrivastav T. G., Tiwari M., Prasad S., Tripathi A., Pandey A. K. (2014). Neem (Azadirachta indica L.) leaf extract deteriorates oocyte quality by inducing ROS-mediated apoptosis in mammals. Springerplus. 3, 464 10.1186/2193-1801-3-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng E. H., Chen S. U., Lee T. H., Pai Y. P., Huang L. S., Huang C. C., et al. (2013). Evaluation of telomere length in cumulus cells as a potential biomarker of oocyte and embryo quality. Hum. Reprod. 28 (4), 929–936. 10.1093/humrep/det004 [DOI] [PubMed] [Google Scholar]
- Chinwe G. S., Azuka O. I., Adaeze N. C. (2018). Resveratrol supplementation rescues pool of growing follicles and ovarian stroma from Cisplatin-induced toxicity on the ovary in Sprague-Dawley rats: an experimental study. Int. J. Reprod. Biomed. 16 (1), 19–30. 10.29252/ijrm.16.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H. Y., Kim D. H., Lee E. K., Chung K. W., Chung S., Lee B., et al. (2019). Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis. 10 (2), 367–382. 10.14336/ad.2018.0324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioffi F., Adam R. H. I., Broersen K. (2019). Molecular mechanisms and genetics of oxidative stress in alzheimer’s disease. J. Alzheimers Dis. 72 (4), 981–1017. 10.3233/jad-190863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu M. L., Aw T. Y. (2010). Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 48 (6), 749–762. 10.1016/j.freeradbiomed.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluzzi E., Leone S., Sgura A. (2019). Oxidative stress induces telomere dysfunction and senescence by replication fork arrest. Cells 8 (1), 19 10.3390/cells8010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conca Dioguardi C., Uslu B., Haynes M., Kurus M., Gul M., Miao D. Q., et al. (2016). Granulosa cell and oocyte mitochondrial abnormalities in a mouse model of fragile X primary ovarian insufficiency. Mol. Hum. Reprod. 22 (6), 384–396. 10.1093/molehr/gaw023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn C. M., Hauser-Kronberger C., Moser M., Tews G., Ebner T. (2005). Predictive value of cumulus cell apoptosis with regard to blastocyst development of corresponding gametes. Fertil. Steril. 84 (3), 627–633. 10.1016/j.fertnstert.2005.03.061 [DOI] [PubMed] [Google Scholar]
- Cui L. L., Yang G., Pan J., Zhang C. (2011). Tumor necrosis factor α knockout increases fertility of mice. Theriogenology 75 (5), 867–876. 10.1016/j.theriogenology.2010.10.029 [DOI] [PubMed] [Google Scholar]
- de Kat A. C., Verschuren W. M., Eijkemans M. J., van der Schouw Y. T., Broekmans F. J. (2016). The association of low ovarian reserve with cardiovascular disease risk: a cross-sectional population-based study. Hum. Reprod. 31 (8), 1866–1874. 10.1093/humrep/dew159 [DOI] [PubMed] [Google Scholar]
- Dechanet C., Anahory T., Mathieu Daude J. C., Quantin X., Reyftmann L., Hamamah S., et al. (2011). Effects of cigarette smoking on reproduction. Hum. Reprod. Update 17 (1), 76–95. 10.1093/humupd/dmq033 [DOI] [PubMed] [Google Scholar]
- Di Emidio G., Rossi G., Bonomo I., Alonso G. L., Sferra R., Vetuschi A., et al. (2017). The natural carotenoid crocetin and the synthetic tellurium compound AS101 protect the ovary against cyclophosphamide by modulating SIRT1 and mitochondrial markers. Oxid. Med. Cell Longev. 2017, 1–14. 10.1155/2017/8928604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di R., Huang M. T., Ho C. T. (2011). Anti-inflammatory activities of mogrosides from Momordica grosvenori in murine macrophages and a murine ear edema model. J. Agric. Food Chem. 59 (13), 7474–7481. 10.1021/jf201207m [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M., Jaruga P. (2012). Mechanisms of free radical-induced damage to DNA. Free Radic. Res. 46 (4), 382–419. 10.3109/10715762.2011.653969 [DOI] [PubMed] [Google Scholar]
- Domènech E. B., Marfany G. (2020). The relevance of oxidative stress in the pathogenesis and therapy of retinal dystrophies. Antioxidants 9 (4), 347 10.3390/antiox9040347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrey Y. H., Salmon A. B. (2014). Revisiting an age-old question regarding oxidative stress. Free Radic. Biol. Med. 71, 368–378. 10.1016/j.freeradbiomed.2014.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkady M. A., Shalaby S., Fathi F., El-Mandouh S. (2019). Effects of quercetin and rosuvastatin each alone or in combination on cyclophosphamide-induced premature ovarian failure in female albino mice. Hum. Exp. Toxicol. 38 (11), 1283–1295. 10.1177/0960327119865588 [DOI] [PubMed] [Google Scholar]
- Erusalimsky J. D. (2020). Oxidative stress, telomeres and cellular senescence: what non-drug interventions might break the link? Free Radic. Biol. Med. 150, 87–95. 10.1016/j.freeradbiomed.2020.02.008 [DOI] [PubMed] [Google Scholar]
- Espino J., Macedo M., Lozano G., Ortiz Á., Rodríguez C., Rodríguez A. B., et al. (2019). Impact of melatonin supplementation in women with unexplained infertility undergoing fertility treatment. Antioxidants 8 (9), 338 10.3390/antiox8090338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sánchez-Pérez P., Cadenas S., et al. (2015). Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 6, 183–197. 10.1016/j.redox.2015.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Chang Y., Wei L., Chen J., Li J., Goldsmith S., et al. (2019). Apoptosis of mural granulosa cells is increased in women with diminished ovarian reserve. J. Assist. Reprod. Genet. 36 (6), 1225–1235. 10.1007/s10815-019-01446-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Garagnani P., Morsiani C., Conte M., Santoro A., Grignolio A., et al. (2018a). The continuum of aging and age-related diseases: common mechanisms but different rates. Front. Med. 5, 61 10.3389/fmed.2018.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Garagnani P., Parini P., Giuliani C., Santoro A. (2018b). Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 14 (10), 576–590. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- Gencer M., Karaca T., Gungor A., Hacıvelioğlu S., demirtaş S., Türkön H., et al. (2014). The protective effect of quercetin on IMA levels and apoptosis in experimental ovarian ischemia-reperfusion injury. Eur. J. Obstet. Gynecol. Reprod. Biol. 177, 135–140. 10.1016/j.ejogrb.2014.03.036 [DOI] [PubMed] [Google Scholar]
- Gong X., Chen N., Ren K., Jia J., Wei K., Zhang L., et al. (2019). The fruits of Siraitia grosvenorii: a review of a Chinese food-medicine. Front. Pharmacol. 10, 1400 10.3389/fphar.2019.01400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicciardi M. E., Gores G. J. (2009). Life and death by death receptors. Faseb. J. 23 (6), 1625–1637. 10.1096/fj.08-111005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad Y. H., Said R. S., Kamel R., Morsy E. M. E., El-Demerdash E. (2020). Phytoestrogen genistein hinders ovarian oxidative damage and apoptotic cell death-induced by ionizing radiation: co-operative role of ER-β, TGF-β, and FOXL-2. Sci. Rep. 10 (1), 13551 10.1038/s41598-020-70309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. J. (2019). Mitochondria in early development: linking the microenvironment, metabolism and the epigenome. Reproduction. 157 (5), R159–R179. 10.1530/rep-18-0431 [DOI] [PubMed] [Google Scholar]
- He L., Ling L., Wei T., Wang Y., Xiong Z. (2017). Ginsenoside Rg1 improves fertility and reduces ovarian pathological damages in premature ovarian failure model of mice. Exp. Biol. Med. 242 (7), 683–691. 10.1177/1535370217693323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Du B., Xu B. (2018). Extraction optimization of phenolics and antioxidants from black goji berry by accelerated solvent extractor using response surface methodology. Appl. Sci. 8, 1905 10.3390/app8101905 [DOI] [Google Scholar]
- Heidinger B. J., Blount J. D., Boner W., Griffiths K., Metcalfe N. B., Monaghan P. (2012). Telomere length in early life predicts lifespan. Proc. Natl. Acad. Sci. U.S.A. 109 (5), 1743–1748. 10.1073/pnas.1113306109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K. L., Ye X., Wang S., Zhang D. (2020). Melatonin application in assisted reproductive technology: a systematic review and meta-analysis of randomized trials. Front. Endocrinol. 11, 160 10.3389/fendo.2020.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S., Zhang Y., Li X. C., Ma L. B., Cao J. W., Dai J. P., et al. (2007). Effects of granulosa cell mitochondria transfer on the early development of bovine embryos in vitro . Clon. Stem Cell 9 (2), 237–246. 10.1089/clo.2006.0020 [DOI] [PubMed] [Google Scholar]
- Huang J., Okuka M., McLean M., Keefe D. L., Liu L. (2009). Effects of cigarette smoke on fertilization and embryo development in vivo . Fertil. Steril. 92 (4), 1456–1465. 10.1016/j.fertnstert.2008.07.1781 [DOI] [PubMed] [Google Scholar]
- Huang J., Okuka M., McLean M., Keefe D. L., Liu L. (2010). Telomere susceptibility to cigarette smoke-induced oxidative damage and chromosomal instability of mouse embryos in vitro . Free Radic. Biol. Med. 48 (12), 1663–1676. 10.1016/j.freeradbiomed.2010.03.026 [DOI] [PubMed] [Google Scholar]
- Huang K., Zhang J., O’Neill K. L., Gurumurthy C. B., Quadros R. M., Tu Y., et al. (2016). Cleavage by caspase 8 and mitochondrial membrane association activate the BH3-only protein Bid during TRAIL-induced apoptosis. J. Biol. Chem. 291 (22), 11843–11851. 10.1074/jbc.M115.711051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. L., Chiang S., Kalinowski D. S., Bae D. H., Sahni S., Richardson D. R. (2019). The role of the antioxidant response in mitochondrial dysfunction in degenerative diseases: cross-talk between antioxidant defense, autophagy, and apoptosis. Oxid. Med. Cell Longev. 2019, 6392763 10.1155/2019/6392763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Hu C., Ye H., Luo R., Fu X., Li X., et al. (2019). Inflamm-aging: a new mechanism affecting premature ovarian insufficiency. J. Immunol. Res. 2019, 8069898 10.1155/2019/8069898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ighodaro O. M., Akinloye O. A. (2018). First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 54 (4), 287–293. 10.1016/j.ajme.2017.09.001 [DOI] [Google Scholar]
- Itami N., Shirasuna K., Kuwayama T., Iwata H. (2015). Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology 83 (8), 1360–1367. 10.1016/j.theriogenology.2015.01.029 [DOI] [PubMed] [Google Scholar]
- Itkin M., Davidovich-Rikanati R., Cohen S., Portnoy V., Doron-Faigenboim A., Oren E., et al. (2016). The biosynthetic pathway of the nonsugar, high-intensity sweetener mogroside V from Siraitia grosvenorii. Proc. Natl. Acad. Sci. U.S.A. 113 (47), E7619–E7628. 10.1073/pnas.1604828113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito J., Shirasuna K., Kuwayama T., Iwata H. (2020). Resveratrol treatment increases mitochondrial biogenesis and improves viability of porcine germinal-vesicle stage vitrified-warmed oocytes. Cryobiology 93, 37–43. 10.1016/j.cryobiol.2020.02.014 [DOI] [PubMed] [Google Scholar]
- Jahromi B. N., Sadeghi S., Alipour S., Parsanezhad M. E., Alamdarloo S. M. (2017). Effect of melatonin on the outcome of assisted reproductive technique cycles in women with diminished ovarian reserve: a double-blinded randomized clinical trial. Iran. J. Med. Sci. 42 (1), 73–78. [PMC free article] [PubMed] [Google Scholar]
- James S. M., Honn K. A., Gaddameedhi S., Van Dongen H. P. A. (2017). Shift work: disrupted circadian rhythms and sleep-implications for health and well-being. Curr. Sleep Med. Rep. 3 (2), 104–112. 10.1007/s40675-017-0071-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Shen R. F., Bi J., Tian X. S., Hinchliffe T., Xia Y. (2015). Catalpol: a potential therapeutic for neurodegenerative diseases. Curr. Med. Chem. 22 (10), 1278–1291. 10.2174/0929867322666150114151720 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhang Z., Cha L., Li L., Zhu D., Fang Z., et al. (2019). Resveratrol plays a protective role against premature ovarian failure and prompts female germline stem cell survival. Int. J. Mol. Sci. 20 (14), 3605 10.3390/ijms20143605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarniranta K., Pawlowska E., Szczepanska J., Jablkowska A., Blasiak J. (2019). Role of mitochondrial DNA damage in ROS-mediated pathogenesis of age-related macular degeneration (AMD). Int. J. Mol. Sci. 20 (10), 2374 10.3390/ijms20102374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat P. K., Kalani A., Rai S., Swarnkar S., Tota S., Nath C., et al. (2016). Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of alzheimer’s disease: understanding the therapeutics strategies. Mol. Neurobiol. 53 (1), 648–661. 10.1007/s12035-014-9053-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasapoğlu I., Seli E. (2020). Mitochondrial dysfunction and ovarian aging. Endocrinology 161 (2), bqaa001 10.1210/endocr/bqaa001 [DOI] [PubMed] [Google Scholar]
- Kauppila T. E. S., Kauppila J. H. K., Larsson N. G. (2017). Mammalian mitochondria and aging: an update Cell Metabol. 25 (1), 57–71. 10.1016/j.cmet.2016.09.017 [DOI] [PubMed] [Google Scholar]
- Keefe D. L., Liu L., Marquard K. (2007). Telomeres and aging-related meiotic dysfunction in women. Cell. Mol. Life Sci. 64 (2), 139–143. 10.1007/s00018-006-6466-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadrawy O., Gebremedhn S., Salilew-Wondim D., Taqi M. O., Neuhoff C., Tholen E., et al. (2019). Endogenous and exogenous modulation of Nrf2 mediated oxidative stress response in bovine granulosa cells: potential implication for ovarian function. Int. J. Mol. Sci. 20 (7), 1635 10.3390/ijms20071635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. W., Go R. E., Hwang K. A., Bae O. N., Lee K., Choi K. C. (2018). Effects of cigarette smoke extracts on apoptosis and oxidative stress in two models of ovarian cancer in vitro . Toxicol. Vitro. 52, 161–169. 10.1016/j.tiv.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Kocaadam B., Şanlier N. (2017). Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 57 (13), 2889–2895. 10.1080/10408398.2015.1077195 [DOI] [PubMed] [Google Scholar]
- Kong Q. Q., Wang J., Xiao B., Lin F. H., Zhu J., Sun G. Y., et al. (2018). Cumulus cell-released tumor necrosis factor (TNF)-α promotes post-ovulatory aging of mouse oocytes. Aging 10 (7), 1745–1757. 10.18632/aging.101507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotha R. R., Luthria D. L. (2019). Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. 24 (16), 2930 10.3390/molecules24162930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen S. G., Pors S. E., Andersen C. Y. (2017). Improving oocyte quality by transfer of autologous mitochondria from fully grown oocytes. Hum. Reprod. 32 (4), 725–732. 10.1093/humrep/dex043 [DOI] [PubMed] [Google Scholar]
- Krysko D. V., Diez-Fraile A., Criel G., Svistunov A. A., Vandenabeele P., D’Herde K. (2008). Life and death of female gametes during oogenesis and folliculogenesis. Apoptosis 13 (9), 1065–1087. 10.1007/s10495-008-0238-1 [DOI] [PubMed] [Google Scholar]
- Labarta E., de Los Santos M. J., Escribá M. J., Pellicer A., Herraiz S. (2019). Mitochondria as a tool for oocyte rejuvenation. Fertil. Steril. 111 (2), 219–226. 10.1016/j.fertnstert.2018.10.036 [DOI] [PubMed] [Google Scholar]
- Leone S., Recinella L., Chiavaroli A., Orlando G., Ferrante C., Leporini L., et al. (2018). Phytotherapic use of the Crocus sativus L. (Saffron) and its potential applications: a brief overview. Phytother Res. 32 (12), 2364–2375. 10.1002/ptr.6181 [DOI] [PubMed] [Google Scholar]
- Li N., Liu L. (2018). Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. J. Obstet. Gynaecol. Res. 44 (8), 1431–1438. 10.1111/jog.13680 [DOI] [PubMed] [Google Scholar]
- Li F., Ding J., Cong Y., Liu B., Miao J., Wu D., et al. (2020). Trichostatin A alleviated ovarian tissue damage caused by cigarette smoke exposure. Reprod. Toxicol. 93, 89–98. 10.1016/j.reprotox.2020.01.006 [DOI] [PubMed] [Google Scholar]
- Li S., Hong M., Tan H. Y., Wang N., Feng Y. (2016). Insights into the role and interdependence of oxidative stress and inflammation in liver diseases. Oxid. Med. Cell Longev. 2016, 4234061 10.1155/2016/4234061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yao J., Han C., Yang J., Chaudhry M. T., Wang S., et al. (2016). Quercetin, inflammation and immunity. Nutrients 8 (3), 167 10.3390/nu8030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zhang D., Liu W., Yan Y., Zhou F., Wu W., et al. (2017). Reactive oxygen species trigger NF-κB-mediated NLRP3 inflammasome activation induced by zinc oxide nanoparticles in A549 cells. Toxicol. Ind. Health. 33 (10), 737–745. 10.1177/0748233717712409 [DOI] [PubMed] [Google Scholar]
- Liguori I., Russo G., Curcio F., Bulli G., Aran L., Della-Morte D., et al. (2018). Oxidative stress, aging, and diseases. Clin. Interv. Aging 13, 757–772. 10.2147/cia.S158513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Luderer U. (2011). Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod. 84 (4), 775–782. 10.1095/biolreprod.110.088583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev S. I. (2013). Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 60, 1–4. 10.1016/j.freeradbiomed.2013.02.011 [DOI] [PubMed] [Google Scholar]
- Liu C., Dai L., Liu Y., Dou D., Sun Y., Ma L. (2018). Pharmacological activities of mogrosides. Future Med. Chem. 10 (8), 845–850. 10.4155/fmc-2017-0255 [DOI] [PubMed] [Google Scholar]
- Liu H., Wang C., Qi X., Zou J., Sun Z. (2018). Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 55 (5), 1880–1888. 10.1007/s13197-018-3105-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Yin Y., Ye X., Zeng M., Zhao Q., Keefe D. L., et al. (2013). Resveratrol protects against age-associated infertility in mice. Hum. Reprod. 28 (3), 707–717. 10.1093/humrep/des437 [DOI] [PubMed] [Google Scholar]
- Liu T., Li N., Yan Y. Q., Liu Y., Xiong K., Liu Y., et al. (2020). Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytother. Res. 34 (3), 435–447. 10.1002/ptr.6538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Zhang L., Joo D., Sun S. C. (2017). NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2, 17023 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lin X., Mi Y., Li J., Zhang C. (2018). Grape seed proanthocyanidin extract prevents ovarian aging by inhibiting oxidative stress in the hens. Oxid. Med. Cell Longev. 2018, 9390810 10.1155/2018/9390810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wang Y. L., He S. W., Chen M. H., Zhang Z., Fu X. P., et al. (2017). Protective effects of resveratrol against mancozeb induced apoptosis damage in mouse oocytes. Oncotarget 8 (4), 6233–6245. 10.18632/oncotarget.14056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Y., Liu X., Tan X. Z., Jiang C. X., Chen S. W., Liang G. N., et al. (2020). ROS-induced NLRP3 inflammasome priming and activation mediate PCB 118- induced pyroptosis in endothelial cells. Ecotoxicol. Environ. Saf. 189, 109937 10.1016/j.ecoenv.2019.109937 [DOI] [PubMed] [Google Scholar]
- López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. (2013). The hallmarks of aging. Cell 153 (6), 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord T., Martin J. H., Aitken R. J. (2015). Accumulation of electrophilic aldehydes during postovulatory aging of mouse oocytes causes reduced fertility, oxidative stress, and apoptosis. Biol. Reprod. 92 (2), 33 10.1095/biolreprod.114.122820 [DOI] [PubMed] [Google Scholar]
- Lu J., Wang Z., Cao J., Chen Y., Dong Y. (2018). A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 16 (1), 80 10.1186/s12958-018-0391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz-Bonengel S., Parson W. (2019). No further evidence for paternal leakage of mitochondrial DNA in humans yet. Proc. Natl. Acad. Sci. U.S.A. 116 (6), 1821–1822. 10.1073/pnas.1820533116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclaran K., Nikolaou D. (2019). Early ovarian ageing. Obstet. Gynaecol. 21 (2), 107–116. 10.1111/tog.12558 [DOI] [Google Scholar]
- Mahdi H., Tahereh H., Esmaiel S., Massood E. (2019). Vitamins E and C prevent apoptosis of testicular and ovarian tissues following mancozeb exposure in the first-generation mouse pups. Toxicol. Ind. Health. 35 (2), 136–144. 10.1177/0748233718818692 [DOI] [PubMed] [Google Scholar]
- Mai Z., Lei M., Yu B., Du H., Liu J. (2014). The effects of cigarette smoke extract on ovulation, oocyte morphology and ovarian gene expression in mice. PLoS One 9 (4), e95945 10.1371/journal.pone.0095945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. B., Cargill S. L., Griffey S. M., Reader J. R., Anderson G. B., Carey J. R. (2011). Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell 10 (3), 448–456. 10.1111/j.1474-9726.2011.00691.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. B., Parkinson K. C., Habermehl T. L. (2018). Orthotopic ovarian transplantation procedures to investigate the life- and health-span influence of ovarian senescence in female mice. J. Vis. Exp. 132, 56638 10.3791/56638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos L., Stevenson D., Gomes F., Silva-Carvalho J. L., Almeida H. (2009). Superoxide dismutase expression in human cumulus oophorus cells. Mol. Hum. Reprod. 15 (7), 411–419. 10.1093/molehr/gap034 [DOI] [PubMed] [Google Scholar]
- May-Panloup P., Boucret L., Chao de la Barca J.-M., Desquiret-Dumas V., Ferré-L’Hotellier V., Morinière C., et al. (2016). Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum. Reprod. Update 22 (6), 725–743. 10.1093/humupd/dmw028 [DOI] [PubMed] [Google Scholar]
- Melekoglu R., Ciftci O., Eraslan S., Cetin A., Basak N. (2018). Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. J. Ovarian Res. 11 (1), 33 10.1186/s13048-018-0409-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X., Zhou J., Zhao C. N., Gan R. Y., Li H. B. (2020). Health benefits and molecular mechanisms of resveratrol: a narrative review. Foods 9 (3), 340 10.3390/foods9030340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y., Zhou C., Cui Z., Zhang M., ShiYang X., Lu Y., et al. (2018). Postovulatory aging causes the deterioration of porcine oocytes via induction of oxidative stress. Faseb. J. 32 (3), 1328–1337. 10.1096/fj.201700908R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi S., Kayedpoor P., Karimzadeh-Bardei L., Nabiuni M. (2017). The effect of curcumin on TNF-α, IL-6 and CRP expression in a model of polycystic ovary syndrome as an inflammation state. J. Reprod. Infertil. 18 (4), 352–360. [PMC free article] [PubMed] [Google Scholar]
- Mokhber Maleki E., Eimani H., Bigdeli M. R., Ebrahimi B., Shahverdi A. H., Golkar Narenji A., et al. (2014). A comparative study of saffron aqueous extract and its active ingredient, crocin on the in vitro maturation, in vitro fertilization, and in vitro culture of mouse oocytes. Taiwan. J. Obstet. Gynecol. 53 (1), 21–25. 10.1016/j.tjog.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Mokhber Maleki E., Eimani H., Bigdeli M. R., Golkar Narenji A., Abedi R. (2016). Effects of crocin supplementation during in vitro maturation of mouse oocytes on glutathione synthesis and cytoplasmic maturation. Int. J. Fertil. Steril. 10 (1), 53–61. 10.22074/ijfs.2016.4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monian P., Jiang X. (2012). Clearing the final hurdles to mitochondrial apoptosis: regulation post cytochrome C release. Exp. Oncol. 34 (3), 185–191. [PubMed] [Google Scholar]
- Morgan M. J., Kim Y.-S., Liu Z.-g. (2008). TNFα and reactive oxygen species in necrotic cell death. Cell Res. 18 (3), 343–349. 10.1038/cr.2008.31 [DOI] [PubMed] [Google Scholar]
- Murphy M. P. (2012). Modulating mitochondrial intracellular location as a redox signal. Sci. Signal. 5 (242), pe39 10.1126/scisignal.2003386 [DOI] [PubMed] [Google Scholar]
- Navarro-Pando J. M., Alcocer-Gómez E., Castejon Vega B., Muntané J., Bullon P., Wang C., et al. (2020). Inhibition of the NLRP3 inflammasome prevents ovarian aging. bioRxiv. 10.1101/2020.04.26.062646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Sui L., Zhang H., Zhang H., Yan K., Yang X., et al. (2019). Mogroside V protects porcine oocytes from in vitro ageing by reducing oxidative stress through SIRT1 upregulation. Aging (Albany NY). 11 (19), 8362–8373. 10.18632/aging.102324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie J., Yan K., Sui L., Zhang H., Zhang H., Yang X., et al. (2020). Mogroside V improves porcine oocyte in vitro maturation and subsequent embryonic development. Theriogenology 141, 35–40. 10.1016/j.theriogenology.2019.09.010 [DOI] [PubMed] [Google Scholar]
- Niemann B., Rohrbach S., Miller M. R., Newby D. E., Fuster V., Kovacic J. C. (2017). Oxidative stress and cardiovascular risk: obesity, diabetes, smoking, and pollution: Part 3 of a 3-Part Series. J. Am. Coll. Cardiol. 70 (2), 230–251. 10.1016/j.jacc.2017.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nna V. U., Usman U. Z., Ofutet E. O., Owu D. U. (2017). Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride – induced oxidative stress in the uterus and ovaries of female Wistar rats. Food Chem. Toxicol. 102, 143–155. 10.1016/j.fct.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Oktay K., Baltaci V., Sonmezer M., Turan V., Unsal E., Baltaci A., et al. (2015). Oogonial precursor cell-derived autologous mitochondria injection to improve outcomes in women with multiple IVF failures due to low oocyte quality: a clinical translation. Reprod. Sci. 22 (12), 1612–1617. 10.1177/1933719115612137 [DOI] [PubMed] [Google Scholar]
- Oyawoye O., Abdel Gadir A., Garner A., Constantinovici N., Perrett C., Hardiman P. (2003). Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum. Reprod. 18 (11), 2270–2274. 10.1093/humrep/deg450 [DOI] [PubMed] [Google Scholar]
- Ozatik F. Y., Ozatik O., Yigitaslan S., Kaygısız B., Erol K. (2020). Do resveratrol and dehydroepiandrosterone increase diminished ovarian reserve?. Eurasian J. Med. 52 (1), 6–11. 10.5152/eurasianjmed.2019.19044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Cha H. J., Lee H., Hwang-Bo H., Ji S. Y., Kim M. Y., et al. (2019). Induction of G2/M cell cycle arrest and apoptosis by genistein in human bladder cancer T24 cells through inhibition of the ROS-dependent PI3k/akt signal transduction pathway. Antioxidants 8 (9), 327 10.3390/antiox8090327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson K. C., Peterson R. L., Mason J. B. (2017). Cognitive behavior and sensory function were significantly influenced by restoration of active ovarian function in postreproductive mice. Exp. Gerontol. 92, 28–33. 10.1016/j.exger.2017.03.002 [DOI] [PubMed] [Google Scholar]
- Pasquariello R., Ermisch A. F., Silva E., McCormick S., Logsdon D., Barfield J. P., et al. (2019). Alterations in oocyte mitochondrial number and function are related to spindle defects and occur with maternal aging in mice and humans†. Biol. Reprod. 100 (4), 971–981. 10.1093/biolre/ioy248 [DOI] [PubMed] [Google Scholar]
- Peters A. E., Mihalas B. P., Bromfield E. G., Roman S. D., Nixon B., Sutherland J. M. (2020). Autophagy in female fertility: a role in oxidative stress and aging. Antioxid Redox Signal. 32 (8), 550–568. 10.1089/ars.2019.7986 [DOI] [PubMed] [Google Scholar]
- Peterson R. L., Parkinson K. C., Mason J. B. (2017). Restoration of immune and renal function in aged females by re-establishment of active ovarian function. Reprod. Fertil. Dev. 29 (10), 2052–2059. 10.1071/rd16333 [DOI] [PubMed] [Google Scholar]
- Pineda-Pampliega J., Herrera-Dueñas A., Mulder E., Aguirre J. I., Höfle U., Verhulst S. (2020). Antioxidant supplementation slows telomere shortening in free-living white stork chicks. Proc. Biol. Sci. 287 (1918), 20191917 10.1098/rspb.2019.1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M., Moraes C. T. (2015). Mechanisms linking mtDNA damage and aging. Free Radic. Biol. Med. 85, 250–258. 10.1016/j.freeradbiomed.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras A. R., Menéndez-Blanco I., Soto-Heras S., Catalá M. G., Izquierdo D., Bogliolo L., et al. (2019). Resveratrol supplementation during in vitro maturation improves embryo development of prepubertal goat oocytes selected by brilliant cresyl blue staining. J. Reprod. Dev. 65 (2), 113–120. 10.1262/jrd.2018-077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad K. N., Wu M., Bondy S. C. (2017). Telomere shortening during aging: attenuation by antioxidants and anti-inflammatory agents. Mech. Ageing Develop. 164, 61–66. 10.1016/j.mad.2017.04.004 [DOI] [PubMed] [Google Scholar]
- Prasad S., Tiwari M., Pandey A. N., Shrivastav T. G., Chaube S. K. (2016). Impact of stress on oocyte quality and reproductive outcome. J. Biomed. Sci. 23, 36 10.1186/s12929-016-0253-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar K. V., Chaube S. K. (2015). Nitric oxide signals postovulatory aging-induced abortive spontaneous egg activation in rats. Redox Rep. 20 (4), 184–192. 10.1179/1351000215y.0000000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J., Wang W., Zhang R. (2017). Novel natural product therapeutics targeting both inflammation and cancer. Chin. J. Nat. Med. 15 (6), 401–416. 10.1016/s1875-5364(17)30062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X., Cao M., Lai F., Yang F., Ge W., Zhang X., et al. (2015). Oxidative stress induced by zearalenone in porcine granulosa cells and its rescue by curcumin in vitro . PLoS One 10 (6), e0127551 10.1371/journal.pone.0127551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. M., Cedars M. I. (2018). Cardiovascular health and ovarian aging. Fertil. Steril. 110 (5), 790–793. 10.1016/j.fertnstert.2018.07.1152 [DOI] [PubMed] [Google Scholar]
- Racchi M. L. (2013). Antioxidant defenses in plants with attention to prunus and citrus spp. Antioxidants 2 (4), 340–369. 10.3390/antiox2040340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkovic A., Pangas S. (2017). Ovary as a biomarker of health and longevity: insights from genetics. Semin. Reprod. Med. 35 (3), 231–240. 10.1055/s-0037-1603571 [DOI] [PubMed] [Google Scholar]
- Rashidi Z., Aleyasin A., Eslami M., Nekoonam S., Zendedel A., Bahramrezaie M., et al. (2019). Quercetin protects human granulosa cells against oxidative stress via thioredoxin system. Reprod. Biol. 19 (3), 245–254. 10.1016/j.repbio.2019.07.002 [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M., Averill-Bates D. A. (2016). Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta Mol. Cell Res. 1863 (12), 2977–2992. 10.1016/j.bbamcr.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Regan S. L. P., Knight P. G., Yovich J. L., Leung Y., Arfuso F., Dharmarajan A. (2018a). Granulosa cell apoptosis in the ovarian follicle-A changing view. Front. Endocrinol. 9, 61 10.3389/fendo.2018.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan S. L. P., Knight P. G., Yovich J. L., Stanger J. D., Leung Y., Arfuso F., et al. (2018b). The effect of ovarian reserve and receptor signalling on granulosa cell apoptosis during human follicle development. Mol. Cell. Endocrinol. 470, 219–227. 10.1016/j.mce.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Rizzo A., Roscino M. T., Binetti F., Sciorsci R. L. (2012). Roles of reactive oxygen species in female reproduction. Reprod. Domest. Anim. 47 (2), 344–352. 10.1111/j.1439-0531.2011.01891.x [DOI] [PubMed] [Google Scholar]
- Ross J. M., Coppotelli G., Hoffer B. J., Olson L. (2014). Maternally transmitted mitochondrial DNA mutations can reduce lifespan. Sci. Rep. 4 (1), 6569 10.1038/srep06569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadraie S. H., Saito H., Kaneko T., Saito T., Hiroi M. (2000). Effects of aging on ovarian fecundity in terms of the incidence of apoptotic granulosa cells. J. Assist. Reprod. Genet. 17 (3), 168–173. 10.1023/a:1009422323306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S. K., Lee S. B., Won J., Choi H. Y., Kim K., Yang G. M., et al. (2017). Correlation between oxidative stress, nutrition, and cancer initiation. Int. J. Mol. Sci. 18 (7), 1544 10.3390/ijms18071544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said R. S., El-Demerdash E., Nada A. S., Kamal M. M. (2016). Resveratrol inhibits inflammatory signaling implicated in ionizing radiation-induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly(ADP-ribose) polymerase 1 (PARP-1). Biochem. Pharmacol. 103, 140–150. 10.1016/j.bcp.2016.01.019 [DOI] [PubMed] [Google Scholar]
- Said R. S., Mantawy E. M., El-Demerdash E. (2019). Mechanistic perspective of protective effects of resveratrol against cisplatin-induced ovarian injury in rats: emphasis on anti-inflammatory and anti-apoptotic effects. Naunyn-Schmiedeberg’s Arch. Pharmacol. 392 (10), 1225–1238. 10.1007/s00210-019-01662-x [DOI] [PubMed] [Google Scholar]
- Sak M. E., Soydinc H. E., Sak S., Evsen M. S., Alabalik U., Akdemir F., et al. (2013). The protective effect of curcumin on ischemia-reperfusion injury in rat ovary. Int. J. Surg. 11 (9), 967–970. 10.1016/j.ijsu.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Saleh D. O., Mansour D. F. (2016). Ovario-protective effects of genistein against cyclophosphamide toxicity in rats: role of anti-müllerian hormone and oestradiol. Eur. J. Pharmacol. 789, 163–171. 10.1016/j.ejphar.2016.07.026 [DOI] [PubMed] [Google Scholar]
- Sasaki H., Hamatani T., Kamijo S., Iwai M., Kobanawa M., Ogawa S., et al. (2019). Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front. Endocrinol. 10, 811 10.3389/fendo.2019.00811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scialò F., Fernández-Ayala D. J., Sanz A. (2017). Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front. Physiol. 8, 428 10.3389/fphys.2017.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Yu J., Pohorly J. E., Kakuda Y. (2003). Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 6 (4), 291–299. 10.1089/109662003772519831 [DOI] [PubMed] [Google Scholar]
- Shi L., Zhang J., Lai Z., Tian Y., Fang L., Wu M., et al. (2016). Long-term moderate oxidative stress decreased ovarian reproductive function by reducing follicle quality and progesterone production. PLoS One 11 (9), e0162194 10.1371/journal.pone.0162194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A. A. A., Silva M. N. P., Figueiredo L. B. F., Gonçalves J. D., Silva M. J. S., Loiola M. L. G., et al. (2018). Quercetin influences in vitro maturation, apoptosis and metabolically active mitochondria of goat oocytes. Zygote 26 (6), 465–470. 10.1017/s0967199418000485 [DOI] [PubMed] [Google Scholar]
- Singh A., Kukreti R., Saso L., Kukreti S. (2019). Oxidative stress: role and response of short guanine tracts at genomic locations. Int. J. Mol. Sci. 20 (17), 4258 10.3390/ijms20174258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. (2018). Telomerase can’t handle the stress. Genes Dev. 32 (9–10), 597–599. 10.1101/gad.316042.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Pendlebury D. F., Nandakumar J. (2020). Structural biology of telomeres and telomerase. Cell. Mol. Life Sci. 77 (1), 61–79. 10.1007/s00018-019-03369-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider A. P., Wood J. R. (2019). Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. 158 (3), R79–R90. 10.1530/rep-18-0583 [DOI] [PubMed] [Google Scholar]
- Sobinoff A. P., Beckett E. L., Jarnicki A. G., Sutherland J. M., McCluskey A., Hansbro P. M., et al. (2013). Scrambled and fried: cigarette smoke exposure causes antral follicle destruction and oocyte dysfunction through oxidative stress. Toxicol. Appl. Pharmacol. 271 (2), 156–167. 10.1016/j.taap.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Sohel M. M. H., Akyuz B., Konca Y., Arslan K., Sariozkan S., Cinar M. U. (2019). Oxidative stress modulates the expression of apoptosis-associated microRNAs in bovine granulosa cells in vitro . Cell Tissue Res. 376 (2), 295–308. 10.1007/s00441-019-02990-3 [DOI] [PubMed] [Google Scholar]
- Spagnuolo C., Russo G. L., Orhan I. E., Habtemariam S., Daglia M., Sureda A., et al. (2015). Genistein and cancer: current status, challenges, and future directions. Adv. Nutr. 6 (4), 408–419. 10.3945/an.114.008052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srirattana K., St John J. C. (2018). Additional mitochondrial DNA influences the interactions between the nuclear and mitochondrial genomes in a bovine embryo model of nuclear transfer. Sci. Rep. 8 (1), 7246 10.1038/s41598-018-25516-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Bayo Jimenez M. T., et al. (2019). Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid. Med. Cell Longev. 2019, 7092151 10.1155/2019/7092151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock D., Knight J. A., Raboud J., Cotterchio M., Strohmaier S., Willett W., et al. (2019). Rotating night shift work and menopausal age. Hum. Reprod. 34 (3), 539–548. 10.1093/humrep/dey390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino N. (2005). Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 4 (1), 31–44. 10.1007/bf03016135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama M., Kawahara-Miki R., Kawana H., Shirasuna K., Kuwayama T., Iwata H. (2015). Resveratrol-induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J. Reprod. Dev. 61 (4), 251–259. 10.1262/jrd.2015-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S., Chen H., Zheng X., Ma C., Yue R. (2018). Analysis on the level of IL-6, IL-21, AMH in patients with auto-immunity premature ovarian failure and study of correlation. Exp. Ther. Med. 16 (4), 3395–3398. 10.3892/etm.2018.6592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. L., Tang S. B., Shen W., Yin S., Sun Q. Y. (2019). Roles of resveratrol in improving the quality of postovulatory aging oocytes in vitro. Cells 8 (10), 1132 10.3390/cells8101132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeo S., Kimura K., Shirasuna K., Kuwayama T., Iwata H. (2017). Age-associated deterioration in follicular fluid induces a decline in bovine oocyte quality. Reprod. Fertil. Dev. 29 (4), 759–767. 10.1071/rd15228 [DOI] [PubMed] [Google Scholar]
- Tang H., Wang S., Li X., Zou T., Huang X., Zhang W., et al. (2019). Molecular mechanisms of action of genistein in cancer: recent advances. Front. Pharmacol. 27 (149), 177–188. [Google Scholar]
- Tardat M., Déjardin J. (2018). Telomere chromatin establishment and its maintenance during mammalian development. Chromosoma. 127 (1), 3–18. 10.1007/s00412-017-0656-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatone C., Carbone M. C., Falone S., Aimola P., Giardinelli A., Caserta D., et al. (2006). Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol. Hum. Reprod. 12 (11), 655–660. 10.1093/molehr/gal080 [DOI] [PubMed] [Google Scholar]
- Teixeira F. K., Sanchez C. G., Hurd T. R., Seifert J. R., Czech B., Preall J. B., et al. (2015). ATP synthase promotes germ cell differentiation independent of oxidative phosphorylation. Nat. Cell Biol. 17 (5), 689–696. 10.1038/ncb3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira K. R. C., dos Santos C. P., de Medeiros L. A., Mendes J. A., Cunha T. M., De Angelis K., et al. (2019). Night workers have lower levels of antioxidant defenses and higher levels of oxidative stress damage when compared to day workers. Sci. Rep. 9 (1), 4455 10.1038/s41598-019-40989-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari M., Prasad S., Tripathi A., Pandey A. N., Ali I., Singh A. K., et al. (2015). Apoptosis in mammalian oocytes: a review. Apoptosis. 20 (8), 1019–1025. 10.1007/s10495-015-1136-y [DOI] [PubMed] [Google Scholar]
- Tiwari M., Prasad S., Tripathi A., Pandey A. N., Singh A., Shrivastav T., et al. (2016). Involvement of reactive oxygen species in meiotic cell cycle regulation and apoptosis in mammalian oocytes. React. Oxygen Species 1, 99–106. 10.20455/ros.2016.817 [DOI] [Google Scholar]
- Tong J., Gao J., Liu Q., He C., Zhao X., Qi Y., et al. (2020). Resveratrol derivative excited postsynaptic potentiation specifically via PKCβ-NMDA receptor mediation. Pharmacol. Res. 152, 104618 10.1016/j.phrs.2019.104618 [DOI] [PubMed] [Google Scholar]
- Tong S., Chen L., Zhang Q., Liu J., Yan J., Ito Y. (2015). Separation of catalpol from Rehmannia glutinosa Libosch. by high-speed countercurrent chromatography. J. Chromatogr. Sci. 53 (5), 725–729. 10.1093/chromsci/bmu114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A., Shrivastav T. G., Chaube S. K. (2013). An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. Int. J. Appl. Basic Med. Res. 3 (1), 27–36. 10.4103/2229-516x.112238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiligiannis S., Panay N., Stevenson J. C. (2019). Premature ovarian insufficiency and long-term health consequences. Curr. Vasc. Pharmacol. 17 (6), 604–609. 10.2174/1570161117666190122101611 [DOI] [PubMed] [Google Scholar]
- Turner K. J., Vasu V., Griffin D. K. (2019). Telomere biology and human phenotype. Cells 8 (1), 73 10.3390/cells8010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Jo Y. J., Oh J. S., Kim N. H. (2017). Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity. Oncotarget 8 (24), 38631–38641. 10.18632/oncotarget.16219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-L., Liu B., Zhang C., Wang X.-M., Zhen D., Huang X.-M., et al. (2019). Effects of icariin on ovarian function in d-galactose-induced aging mice. Theriogenology 125, 157–167. 10.1016/j.theriogenology.2018.10.028 [DOI] [PubMed] [Google Scholar]
- Wang S., He G., Chen M., Zuo T., Xu W., Liu X. (2017). The role of antioxidant enzymes in the ovaries. Oxid. Med. Cell Longev. 2017, 4371714 10.1155/2017/4371714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Zheng Y., Li J., Yu Y., Zhang W., Song M., et al. (2020). Single-cell transcriptomic atlas of primate ovarian aging. Cell 180 (3), 585–600.e519. 10.1016/j.cell.2020.01.009 [DOI] [PubMed] [Google Scholar]
- Wang T., Zhang M., Jiang Z., Seli E. (2017). Mitochondrial dysfunction and ovarian aging. Am. J. Reprod. Immunol. 77 (5). 10.1111/aji.12651 [DOI] [PubMed] [Google Scholar]
- Wang W., Qin J. J., Li X., Tao G., Wang Q., Wu X., et al. (2018). Prevention of prostate cancer by natural product MDM2 inhibitor GS25: in vitro and in vivo activities and molecular mechanisms. Carcinogenesis 39 (8), 1026–1036. 10.1093/carcin/bgy063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Fan G., Wei F., Bu Y., Huang W. (2019). Hyperoside protects rat ovarian granulosa cells against hydrogen peroxide-induced injury by sonic hedgehog signaling pathway. Chem. Biol. Interact. 310, 108759 10.1016/j.cbi.2019.108759 [DOI] [PubMed] [Google Scholar]
- Wang X. N., Zhang C. J., Diao H. L., Zhang Y. (2017). Protective effects of curcumin against sodium arsenite-induced ovarian oxidative injury in a mouse model. Chin Med. J. (Engl). 130 (9), 1026–1032. 10.4103/0366-6999.204927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yang C., Elsheikh N. A. H., Li C., Yang F., Wang G., et al. (2019). HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging (Albany NY). 11 (15), 5535–5547. 10.18632/aging.102136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M., Lu Y., Liu D., Ru W. (2014). Ovarian failure-resistant effects of catalpol in aged female rats. Biol. Pharm. Bull. 37 (9), 1444–1449. 10.1248/bpb.b14-00064 [DOI] [PubMed] [Google Scholar]
- Wells D. (2017). Mitochondrial DNA quantity as a biomarker for blastocyst implantation potential. Fertil. Steril. 108 (5), 742–747. 10.1016/j.fertnstert.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Whittemore K., Vera E., Martínez-Nevado E., Sanpera C., Blasco M. A. (2019). Telomere shortening rate predicts species life span. Proc. Natl. Acad. Sci. U. S. A. 116 (30), 15122–15127. 10.1073/pnas.1902452116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener-Megnazi Z., Vardi L., Lissak A., Shnizer S., Reznick A. Z., Ishai D., et al. (2004). Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil. Steril. 82 (Suppl. 3), 1171–1176. 10.1016/j.fertnstert.2004.06.013 [DOI] [PubMed] [Google Scholar]
- Wu M., Ma L., Xue L., Ye W., Lu Z., Li X., et al. (2019). Resveratrol alleviates chemotherapy-induced oogonial stem cell apoptosis and ovarian aging in mice. Aging (Albany NY). 11 (3), 1030–1044. 10.18632/aging.101808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Lu J., Antony S., Juhasz A., Liu H., Jiang G., et al. (2013). Activation of TLR4 is required for the synergistic induction of dual oxidase 2 and dual oxidase A2 by IFN-γ and lipopolysaccharide in human pancreatic cancer cell lines. J. Immunol. 190 (4), 1859–1872. 10.4049/jimmunol.1201725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S., Mu T., Wang G., Jiang X. (2014). Mitochondria-mediated apoptosis in mammals. Protein Cell 5 (10), 737–749. 10.1007/s13238-014-0089-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Hu M. J., Wang Y. Q., Cui Y. L. (2019). Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 24 (6), 1123 10.3390/molecules24061123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. P., Li Y., Meng X., Zhou T., Zhou Y., Zheng J., et al. (2017). Natural antioxidants in foods and medicinal plants: extraction, assessment and resources. Int. J. Mol. Sci. 18 (1), 96 10.3390/ijms18010096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Chen X., Zhang X., Liu Y., Wang Z., Wang P., et al. (2017). Impaired telomere length and telomerase activity in peripheral blood leukocytes and granulosa cells in patients with biochemical primary ovarian insufficiency. Hum. Reprod. 32 (1), 201–207. 10.1093/humrep/dew283 [DOI] [PubMed] [Google Scholar]
- Yadav P. K., Tiwari M., Gupta A., Sharma A., Prasad S., Pandey A. N., et al. (2018). Germ cell depletion from mammalian ovary: possible involvement of apoptosis and autophagy. J. Biomed. Sci. 25 (1), 36 10.1186/s12929-018-0438-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Fukunaga T., Yamada M., Hamatani T., Chikazawa N., Ogawa S., Akutsu H., et al. (2013). Age-associated telomere shortening in mouse oocytes. Reprod. Biol. Endocrinol. 11, 108 10.1186/1477-7827-11-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Deng D., Wu Y., Wu K., Qu J., Li F. (2020). Catalpol protects rat ovarian granulosa cells against oxidative stress and apoptosis through modulating the PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 40 (4), BSR20194032 10.1042/bsr20194032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z., Dai Y., Fu H., Zheng Y., Bao D., Yin Y., et al. (2018). Curcumin exerts a protective effect against premature ovarian failure in mice. J. Mol. Endocrinol. 60 (3), 261–271. 10.1530/jme-17-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Elner S. G., Bian Z. M., Till G. O., Petty H. R., Elner V. M. (2007). Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 85 (4), 462–472. 10.1016/j.exer.2007.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Xie Y., Yang D., Ren D. (2017). Oxidative stress-induced apoptosis in granulosa cells involves JNK, p53 and Puma. Oncotarget 8 (15), 25310–25322. 10.18632/oncotarget.15813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Lin X., Tang H., Fan Y., Zeng S., Jia L., et al. (2020). Mitochondrial DNA mutation exacerbates female reproductive aging via impairment of the NADH/NAD(+) redox. Aging Cell 19, e13206 10.1111/acel.13206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.-G., Park H.-J., Kim J.-W., Jung J.-M., Kim M.-J., Jegal H.-G., et al. (2018). Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci. Rep. 8 (1), 10130 10.1038/s41598-018-28497-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Wang J., Brand D. D., Zheng S. G. (2018). Role of TNF-TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front. Immunol. 9, 784 10.3389/fimmu.2018.00784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaribeygi H., Sathyapalan T., Atkin S. L., Sahebkar A. (2020). Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell Longev. 2020, 8609213 10.1155/2020/8609213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yldrm G., Tokmak A., Kokanal M. K., Sarkaya E., Züngün C., İnal H. A., et al. (2015). Association between some inflammatory markers and primary ovarian insufficiency. Menopause 22 (9), 1000–1005. 10.1097/gme.0000000000000423 [DOI] [PubMed] [Google Scholar]
- Yu W. K., Xu Z. Y., Yuan L., Mo S., Xu B., Cheng X. D., et al. (2020). Targeting β-catenin signaling by natural products for cancer prevention and therapy. Front. Pharmacol. 11, 984 10.3389/fphar.2020.00984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Song J., Zhang M., Wang H., Zhang Y., Suo H. (2020). Comparison of in vitro and in vivo antioxidant activities of six flavonoids with similar structures. Antioxidants 9 (8), 732 10.3390/antiox9080732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Keilty D., Zhang Z. F., Chian R. C. (2017). Mitochondria in oocyte aging: current understanding. Facts Views Vis. Obgyn. 9 (1), 29–38. [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Davies K. J. A., Forman H. J. (2015). Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 88, 314–336. 10.1016/j.freeradbiomed.2015.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Q., Gao B. W., Wang J., Ren Q. L., Chen J. F., Ma Q., et al. (2016). Critical role of FoxO1 in granulosa cell apoptosis caused by oxidative stress and protective effects of grape seed procyanidin B2. Oxid. Med. Cell Longev. 2016, 6147345 10.1155/2016/6147345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Q., Xing B. S., Zhu C. C., Shen M., Yu F. X., Liu H. L. (2015). Protective effect of proanthocyanidin against oxidative ovarian damage induced by 3-nitropropionic acid in mice. Genet. Mol. Res. 14 (1), 2484–2494. 10.4238/2015.March.30.6 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wu X. Q., Lu S., Guo Y. L., Ma X. (2006). Deficit of mitochondria-derived ATP during oxidative stress impairs mouse MII oocyte spindles. Cell Res. 16 (10), 841–850. 10.1038/sj.cr.7310095 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang C., Jin Y., Yang Q., Meng Q., Liu Q., et al. (2018). Activating the PGC-1α/TERT pathway by catalpol ameliorates atherosclerosis via modulating ROS production, DNA damage, and telomere function: implications on mitochondria and telomere link. Oxid. Med. Cell Longev. 2018, 2876350 10.1155/2018/2876350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X. W., Yang W. T., Chen S., Xu Q. Q., Shan C. S., Zheng G. Q., et al. (2017). Neuroprotection of catalpol for experimental acute focal ischemic stroke: preclinical evidence and possible mechanisms of antioxidation, anti-inflammation, and antiapoptosis. Oxid. Med. Cell Longev. 2017, 5058609 10.1155/2017/5058609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X. L., Fu Y. C., Xu J. J., Kong X. X., Chen Z. G., Luo L. L. (2010). Effects of genistein on ovarian follicular development and ovarian life span in rats. Fitoterapia. 81 (8), 998–1002. 10.1016/j.fitote.2010.06.018 [DOI] [PubMed] [Google Scholar]
- Zuo L., Prather E. R., Stetskiv M., Garrison D. E., Meade J. R., Peace T. I., et al. (2019). Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int. J. Mol. Sci. 20 (18), 4472 10.3390/ijms20184472 [DOI] [PMC free article] [PubMed] [Google Scholar]