Abstract
The polarization of monocytes into macrophages that possess anti-inflammatory and pro-angiogenic properties could provide a novel therapeutic strategy for patients who are at a high risk for developing heart failure following myocardial infarction (MI). Here in, we describe a novel method of “educating” monocytes into a distinct population of macrophages that exhibit anti-inflammatory and pro-angiogenic features through a 3-day culture on fibronectin-rich cardiac matrix (CX) manufactured using cultured human cardiac fibroblasts. Our data suggest that CX can educate monocytes into a unique macrophage population termed CX educated macrophages (CXMq) that secrete high levels of VEGF and IL-6. In vitro, CXMq also demonstrate the ability to recruit mesenchymal stromal cells (MSC) with known anti-inflammatory properties. Selective inhibition of fibronectin binding to αVβ3 surface integrins on CXMq prevented MSC recruitment. This suggests that insoluble fibronectin within CX is, at least in part, responsible for CXMq conversion.
Keywords: Cardiac fibroblast, Cell-derived matrix, Monocyte, Macrophage, Fibronectin, Integrin
Graphical Abstract
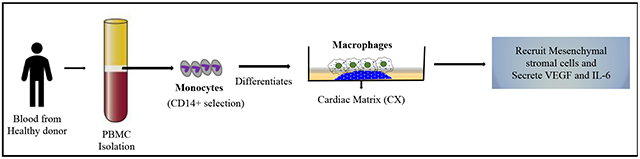
1. Introduction
Myocardial infarction (MI) due to coronary artery disease is a common, disabling and deadly disorder. The post MI inflammatory response causes extracellular matrix disruption, myonecrosis, fibrosis, infarct thinning, ventricular dilatation, heart failure and eventually, death. Standard therapy for post-MI heart failure patients involve medications that block maladaptive neurohormonal pathways, but these drugs are only partially effective and are not universally tolerated. Mechanical assist devices and heart transplant are expensive and limited by device failure, stroke, infection, and organ shortages. The American Heart Association estimates that >800,000 patients suffer from myocardial infarction (MI) annually in the United States.1 Of these, an estimated 30% of patients will develop infarct thinning, progressive ventricular enlargement and heart failure, which is associated with 50% mortality after 5 years.2 This growing problem has prompted investigations to find more effective and durable treatments to curb post infarct adverse ventricular remodeling. Recent discoveries have shed light on the innate immune system and its role in pathologic and reparative responses following MI, and it is an area of investigation that may yield novel therapeutic opportunities. 3
Macrophages and their precursor cells, monocytes, have dual roles as both effectors and inhibitors of inflammation.4, 5 In mouse MI models, early in the process, pro-inflammatory macrophages are recruited, followed by a second population of macrophages that have been linked to the resolution of inflammation and cardiac repair.6–8 These two macrophage subpopulations have demonstrated varying effects on left ventricular function following MI. The pro-inflammatory subpopulation, which has been referred to as “classically activated” or “M1” macrophages,9, 10 are negatively correlated with left ventricular function,7, 11 whereas the anti-inflammatory, “non-classically activated” or “M2” macrophages (reviewed in 9, 10, 12) are correlated with improved left ventricular function following MI.13 Furthermore, patients who develop circulating monocytosis following MI have worse clinical outcomes.14–16 It has been speculated that some patients develop an accentuated and prolonged inflammatory post MI response that may, in part, be due to the excessive recruitment of pro-inflammatory macrophages.3, 6, 9, 11, 17, 18 Therefore, supplementing ischemic myocardium with anti-inflammatory macrophages in the early post MI period may prevent infarct expansion, ventricular dilatation and heart failure. The ability to manufacture these protective M2 like macrophages using clinically acceptable methods, has thus far not been investigated.
Our group previously described a method to engineer a cardiac matrix (CX) derived from isolated human cardiac fibroblasts that were isolated and cultured to high density.19–23 Cardiac extracellular matrix derived from decellularized adult heart tissue consists primarily composed of collagen.24 In contrast, we discovered that engineered CX is highly abundant in fibronectin.19 In humans, fibronectin expression in the heart is relatively constant during fetal life but decreases after birth.25 Fibronectin assembly and deposition are driven through cell surface integrin binding26 and may be linked to monocyte differentiation. Herein, we describe a novel method to use CX to educate monocytes into a distinct population of macrophages (CXMq) secrete Vascular Endothelial Growth Factor (VEGF) and Interleukin-6 (IL-6). Additionally, we demonstrate CXMq signal mesenchymal stromal cell (MSC) migration, which are cells that are being investigated in numerous clinical trials for post MI healing.27, 28
2. Materials and Methods
2.1. Generation of Cardiac Matrix (CX) from culture expanded human cardiac fibroblasts
2.1.1. Human cardiac fibroblast isolation:
Normal human hearts that went unused for organ transplant were obtained from the University of Wisconsin Organ Procurement Organization, Madison, WI, with local Institutional Review Board (IRB) approval. At the time of harvest, hearts were aseptically excised, perfused and stored in cold cardioplegia solution, and transported on ice. Hearts were processed within 12 hours of explant. Epicardial fat was removed and approximately 30 grams of left ventricular free wall was minced and then transferred into 3-5 gentleMACS C tubes (Miltenyi Biotec, Bergisch Gladbach, Germany). 10 ml of digestion media containing DMEM (Corning, Corning, NY) and 1.25 mg Liberase TM (Roche, Basel, Switzerland) was added to each tube. The sample was homogenized with a gentleMACS Dissociator (Miltenyi Biotec) using a C-tube, incubated at 37°C for 30 minutes with constant agitation and the process repeated for 1 hour to induce tissue dissociation. Heart samples were sieved through a 200 µm filter and then centrifuged at 1000 x g for 20 minutes. The cell pellet was suspended in 20 ml complete media (MCDB 131 with 10% FBS, 1ng/mL basic fibroblast growth factor (bFGF), 5 µg/ml insulin, 10 µg/ml ciprofloxacin and 2.5 mg/ml amphotericin B) and plated into T75 flasks. Cells were permitted to attach for 2 hours. Non-adherent cells were removed by washing in phosphate-buffered saline (PBS). Finally, complete media was added and the cells were cultured at 37°C, 5% CO2 and 100% humidity, and passaged once 90% confluent. Six biological replicates were used for fibroblast isolation. All procedures were performed under aseptic conditions, using cGMP compliant manufacturing methods and protocols.
2.1.2. Cardiac matrix production:
Cardiac fibroblast passage 3-8 were suspended in high glucose DMEM with 10% FBS and 10 µg/ml ciprofloxacin and 2.5 mg/ml amphotericin B at 1.1 × 105 to 2.2 × 105 cells per cm2 and maintained under standard culture conditions with media replaced every 2-3 days for 7-10 days. The cells were lifted off the plate as a contiguous sheet by contact with 2mm EDTA at 37°C and gentle agitation. Matrix sheets were decellularized by four alternating washes of hypo/hypertonic PBS (15 minutes each) followed by incubation in 1% Triton X-100 and Tri-N-Butyl-phosphate for 48 to 72 hours at 4°C with constant agitation. All scaffolds (six different batches of scaffolds) were rinsed in PBS. Residual DNA was removed by incubation 100 U/ml Benzonase for 20 hours at 37 C then 100 % ethanol followed by a PBS rinse (3×15 min) and 2 hours in molecular grade water and stored in PBS at 4°C.
2.2. Isolation and Culture of Circulating Human CD14+ Monocytes
2.2.1. Monocyte isolation:
Peripheral blood mononuclear cells were collected from healthy stem cell donors by density gradient separation using Ficoll-Paque Plus (endotoxin tested) (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) using a local IRB approved protocol 29. Red blood cells were lysed by incubating cells in ACK lysis buffer for 3-5 minutes and mononuclear cells were washed with phosphate-buffered saline (PBS) (Hyclone, Logan, UT, USA). To reduce platelet contamination, cell suspensions were centrifuged at 300-700 x g for 10 minutes. The CD14+ cells were then isolated using anti-human CD14 microbeads (Miltenyi Biotec, Auburn, CA, USA) performed according to the manufacturer. Cell pellets were re-suspended Miltenyi running buffer and incubated with beads for 20 minutes at 4°C. After washing to remove unbound antibody, cell separation was done using an autoMACS Pro Separator (Miltenyi Biotec). Purity of isolated CD14+ cells was >85%. The percent of viable CD14+ cells was determined using Trypan Blue dye exclusion test. Cells were diluted 1:1 in a 0.4% Trypan Blue solution then loaded in a Bright-line hemocytometer and examined immediately under a microscope at low magnification. The number of viable (unstained), blue staining cells (nonviable) and the number of total cells was measured.
Purified CD14+ monocytes were plated into six-well cell culture plates at a concentration of 0.5–1 × 106 per well for characterization studies (Greiner Bio-One, Monroe, NC, USA) in complete macrophage medium consisting of Iscove’s modified Dulbecco’s media (Gibco, Life Technologies, Grand Island, NY) supplemented with 10% human serum blood type AB (Mediatech, Herndon, VA, USA), 1× nonessential amino acids (Lonza, Walkersville, MD, USA), 1 x sodium pyruvate (Mediatech), and 4 µg/mL recombinant human insulin (Invitrogen, Carlsbad, CA).
2.2.2. Culturing Monocytes on cardiac matrix:
CD14+ monocytes were plated on to the surface of CX scaffolds in six-well culture plates (Eppendorf, Hamburg Germany). Tissue culture plastic (plastic), 1% gelatin-coating (ESGRO Complete Gelatin Solution, Millipore Sigma) and blood plasma derived fibronectin (Sigma, MO) were selected as comparison control surfaces because they are commonly used for cell culture. Monocytes were plated at a density of 0.5-1 × 106 cells per well in complete macrophage medium (Gibco, Grand Island, NY) and supplemented with 10% human type AB serum (Valley Biomedical, Winchester, VA, 200 mM L-glutamate (100x) (Mediatech, Manassas, VA), nonessential amino acids (100x) (Mediatech, Manassas, VA) 100 mM sodium pyruvate (100x) (Mediatech, Manassas, VA) and 4 mg/mL recombinant human insulin (1000x) (Gibco, Grand Island, NY). Cells were cultured in 5% CO2 for 3 days at 37 °C. The cardiac matrices used in these experiments were generated from proliferating cardiac fibroblasts, followed by a complete decellularization step. Monocytes, which are non-proliferative cells, are plated directly on the decellularized cardiac matrix. Attached macrophages were removed from plastic, gelatin and fibronectin coated plates by exposure to liberase and vigerous pipetting. CXMq were tightly adhered to CX. 5mg/mL Liberase (Roch, Basel, Switzerland) was used to release CXMq from CX by incubating at 37 °C for 5 minutes. Effects of Liberase on macrophage phenotype was tested by exposing plastic and gelatin adherent macrophages to Liberase. Liberase did not affect cell surface marker expression (data not shown).
2.3. Isolation and Culture of Mesenchymal Stem Cells:
We collected human bone marrow (BM) from filters left over from a BM harvest from normal healthy donors. All protocols were approved by the Health Sciences Institutional Review Board of University of Wisconsin-Madison School of Medicine and Public Health. Mesenchymal stem cells (MSCs) were isolated by washing BM cells trapped in filter30 with phosphate-buffered saline (PBS, Hyclone, Logan UT, USA) and BM mononuclear cells were separated using Ficoll-Hypaque plus 1.073 low-endotoxin (GE Healthcare Bio-sciences, Uppsala, Sweden) performed according to the manufacturer’ protocol. Residual red blood cells were lysed with 3-5 minute incubation in ACK lysis buffer (Lonza, Walkersville, MD, USA) washed with PBS and mononuclear cells were suspended in αMEM (Corning, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS, US origin, uncharacterized - Hyclone), 100X non-essential amino acid (NEAA, Corning) and 4mM L-Glutamine (GlutaGro, Corning). MSCs were confirmed by their characteristic morphology, adherence onto 75 cm2 plastic flasks (Greiner Bio-one, Monroe, NC) and flow cytometry.
2.4. Characterization of Cardiac Matrix-educated Macrophages
2.4.1. Cell Surface Marker Expression of Cardiac Matrix-educated Macrophages:
To assess the effects of culture substrate, macrophages were stained after a 3-day culture on i) CX sheet, ii) plastic (control), iii) gelatin-coated plastic (control) and iv) fibronectin. The flow cytometry surface marker antibodies are listed in supplementary table 1. Prior to staining, cells were incubated at 4 °C with an Fc receptor blocking agent (Human TruStain FcX, Biolegend, San Diego, CA). Surface antibodies were added for an additional 30 minutes at 4°C. Expression of cell surface markers was measured using an Attune NxT flow cytometry (Thermo Fisher Scientific, Waltham, MA). The collected data was analyzed using Flowjo™10 software and gating was done in a hierarchy relative to the CD14 population. The % expression based on fluorescent signals was plotted. Six independent experiments with 6 different batches of matrix were used in this study.
2.4.2. Expression VEGF and IL-6 in Cardiac Matrix-educated macrophages:
VEGF and IL-6 levels were measured from the spent media of human CD14+ cells cultured on different surfaces (plastic, gelatin and CX) for 72h using Human angiogenic LEGENDplex™ (Biolegend) panel kit and acquired by Attune NxT Flow Cytometer (Thermo Fisher scientific, Waltham, MA). Data was analyzed using Biolengend’s LegendPlex™ data analysis software version 8 (n=3, 3 independent experiments with 3 different batches of matrix were used in this study). VEGF was chosen to be studied as it is an angiogenic cytokine which is a member of the fibroblast growth factor (FGF) family and is known to stimulate angiogenesis in the ischemic heart.31 IL-6 is a pleiotropic cytokine that has been shown to have an important role in cardiac protection post MI.32
2.4.3. Effect of Cardiac Matrix-educated macrophages on mesenchymal stromal cell migration:
A transwell migration assay was performed using matrigel coated transwell chambers (BD Bioscience) as per the manufacturer protocol. We hypothesized that CXMq would signal MSC migration. MSC were chosen due to their anti-inflammatory role in post MI repair 33. Briefly, monocytes (Mo) were seeded on CX and plastic in a 24 well plate with serum containing media. 5 Χ 104 cells/ml MSC were seeded onto the transwell matrigel coated inserts. The plate was incubated for 22 h at 37°C and 5% CO2. The selective cell adhesion blocker, Integrin alpha v beta 3 (αVß3) inhibitor (Cyclo-RGDfk, Targetmol, MA) was added to block monocyte fibronectin binding domains. This was done to test the role of αVß3 integrin in the conversion of monocytes to CXMq. αVß3 integrin recognizes the RGD572-574 motif in the alpha chain of human fibrinogen34 and is linked to cell attachment to extracellular matrices.35 The total number of MSC that migrated to the lower chamber of the matrigel membrane after 22 h were extracted by adding 333 µL of stock Liberase (5 mg/mL) in 1mLof 1X warm PBS. The negative control was media alone in the lower chamber. Other controls used in this study were monocyte along with αVß3 inhibitor and CX + αVß3 inhibitor in the lower chamber. Additionally, MSC’s were incubated with the αVß3inhibitor for 1 hr, washed and plated on the upper chamber of the transwell having CX+monocytes in the lower chamber. Finally, flow markers (CD16, CD163, CD206, CD86, HLADR, PDL-1) were checked to better understand the role of αVß3 integrin inhibitor. This assay was performed in triplicates. The total number of harvested cells were stained with monocyte marker CD14, MSC marker CD73, and analyzed using Attune NxT flow cytometry (Thermo Fisher Scientific, Waltham, MA). Flow analysis was done using the Flow Jo version 10 software.
2.5. Statistical Analysis
All quantitative analyses were performed using GraphPad PRISM 8.0 software (GraphPad Software, Inc., La Jolla, CA). Data was analyzed by one-way ANOVA, followed by Tukey’s post-hoc analysis and shown as mean ± standard error. Unless otherwise specified, a p-value < 0.05 was considered significant.
3. Results
3.1. Characterization of monocytes cultured on different coated surfaces
Monocytes were plated on plastic, gelatin, fibronectin and CX as in figure 1. At baseline, monocytes showed the following flow cytometry phenotype expression: CD14+ 99.4% ± 0.2%; CD16+ 17.6% ± 2.3%; CD163+ 70.5% ± 4.4%; CD206+ 32.9% ± 3.6%; and PDL1+ 22.8% ± 3.0% shown in figure 2. Monocytes had increased expression of CD86+ (71.1% ± 4.4) and HLA-DR+ (92.8% ± 1.4%) (Figure 2) on Day 0 before monocyte culture compared to monocytes cultured on cardiac matrix for three days. The viability of selected CD14+ monocytes was greater than 95%. Representative Pseudo plots of gating hierarchy and FMO controls for flow cytometry analysis are provided in supplementary figure 1–5. All gating was done in a hierarchy relative to the CD14 population.
Fig. 1. Schematic representation of monocyte education.
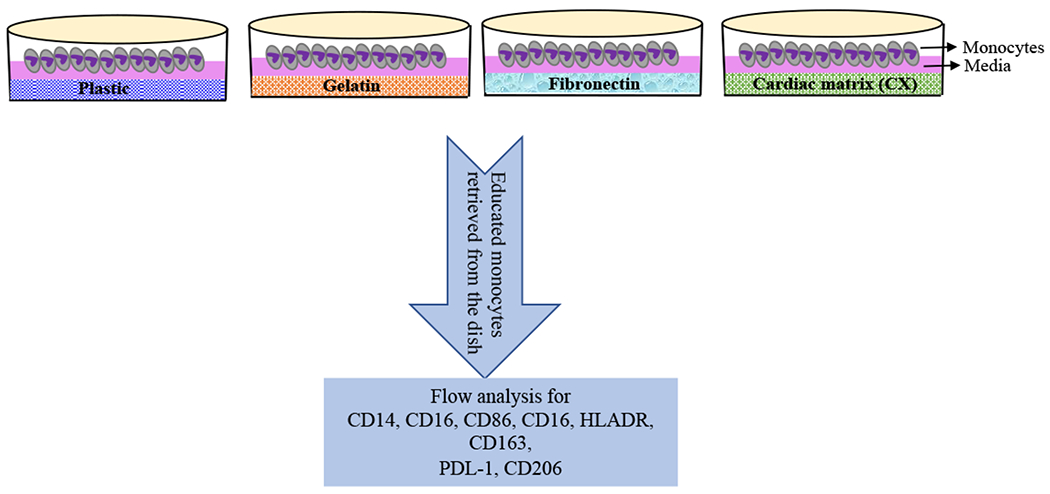
Monocytes were plated on plastic, gelatin and cardiac matrix (CX) for 3 days followed by cell harvest and flow cytometric analysis of the macrophage specific markers.
Fig. 2. Expression of various macrophage markers on educated macrophages.
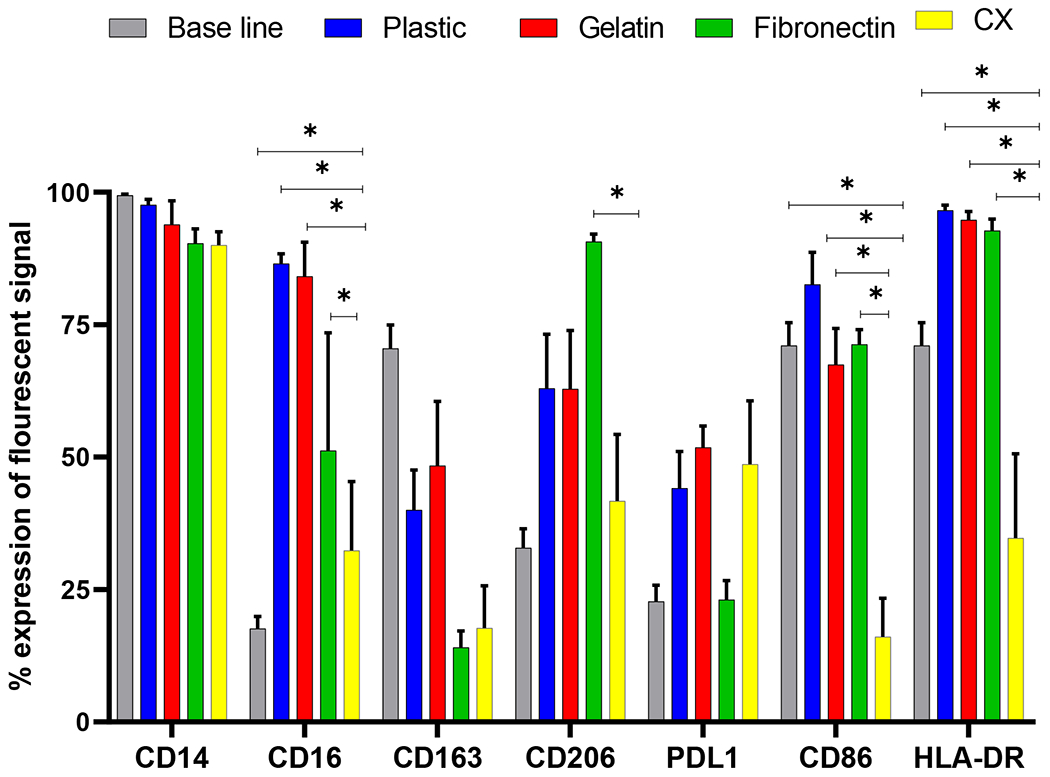
Monocytes were cultured on plastic, gelatin, fibronectin and CX for 3 days and flow cytometric analysis of M1 and M2 like macrophage markers was performed. Monocytes cultured on CX showed a significant reduction in inflammatory cell surface markers compared to plastic, gelatin, fibronectin and base line (pre-culture). * p < 0.05, (n=6).
After 3 days, expression of CD16+, CD86+, and HLA-DR+ was significantly reduced on CXMq compared to monocytes at baseline, and monocytes cultured on plastic or gelatin or blood plasma fibronectin coated plastic controls : CD16+ (CX: 32.4% ± 13.0%; plastic: 86.5% ± 1.9%; gelatin coated plastic: 84.2% ± 6.4%, p<0.05, fibronectin: 71.6± 1.95%, p<0.05); CD86+ (CX: 16.1% ± 7.2%; plastic: 82.6% ± 6.0%; gelatin: 67.5% ± 6.9%, p<0.05, fibronectin: 71.2± 2.80%, p<0.05); HLA-DR+ (CX: 34.8% ± 15.9%; plastic: 96.6% ± 1.0%; gelatin: 94.8% ± 1.5%, p<0.05, fibronectin: 92.7± 2.16%, p<0.05). Day 0 to Day 3 comparisons in Figure 3 shows changes from baseline cell surface marker expression to Day 3 post culture cell surface marker expression. There were no significant differences in surface marker expression between baseline and plastic compared to gelatin coated plastic.
Fig. 3. Cardiac matrix educated monocytes recruit MSC.
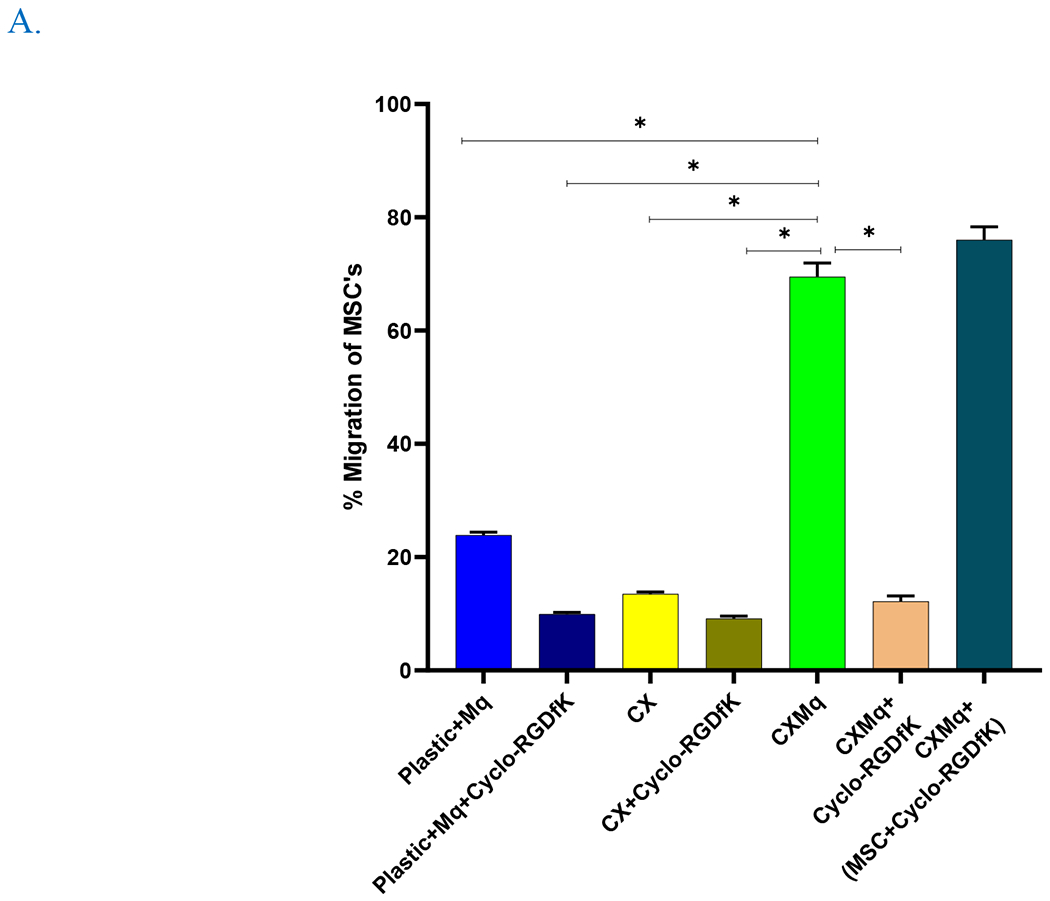
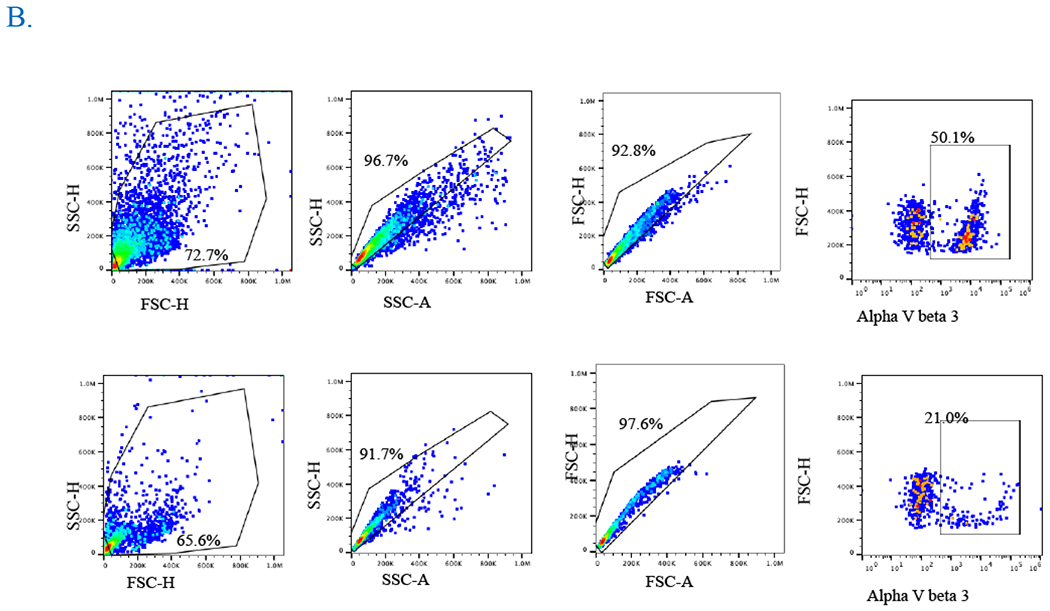
A) CXMq (untreated MSC) and CXMq (inhibitor treated MSC) caused significantly greater MSC migration compared to matrix alone without macrophages (CX) and uneducated macrophages (plastic+Mq). Adding fibronectin αVβ3 surface integrin inhibitor to CXMq prevented MSC migration. This suggests that matrix-assembled (insoluble) fibronectin may be linked to monocyte education. (B) Pseudoplot showing decrease in the αVβ3 surface integrin upon treatment with its inhibitor Cyclo-RGDfk (TargetMol, MA). (* p < 0.05), (n=3).
3.2. Cardiac matrix-educated macrophages recruit MSC
CXMq recruited MSC to a significantly greater (70.0% ± 2.4%, p<0.05) degree than monocytes plated on plastic (23.9% ±0.49%, p<0.05) and matrix alone without monocytes (13.5% ±0.35%, p<0.05) (Figure 3 A). The gating strategy is shown in supplementary figure 6. The aVß3 fibrinogen surface integrin inhibitor added to CXMq prevented MSC migration, (12.2 % ± 0.96%, p<0.05) which suggests that insoluble fibronectin in the matrix contributes to monocyte education (Figure 3 B). Additionally, when αVß3 fibrinogen surface integrin inhibitor was added to the monocytes, there was no difference in migration potential when compared without the inhibitor. Furthermore, when MSC’s were incubated with αVß3 fibrinogen inhibitor for 1 hour, washed and plated into the upper chamber of the transwell with CX + monocytes in the lower chamber, there was no difference in the MSC migration potential compared to the MSC with no inhibitor treatment. This confirms that inhibition of αVß3 fibrinogen surface integrin on MSCs does not account for the reduction in MSC migration observe in the CXMq+CycloRDGfK group. Finally, the expression of CD16, CD163, CD206, CD86, HLADR, PDL-1 were checked after 22 hours of culture (supplementary figure 7). Schematic representation of transwell migration assay with controls is shown in supplementary Figure 8.
3.3. CXMq secrete VEGF and IL-6
CXMq secreted higher levels of pro-angiogenic factor VEGF (40.0 pg/ mL ± 2.36 pg/ mL, p<0.05) and pleiotropic cytokine IL-6 (46.18pg/ mL ± 4.39 pg/ mL, p<0.05), in comparison to plastic (VEGF: 1.51 pg/ mL ± 0.42 pg/ mL, IL-6: 1.50 pg/ mL ± 0.42 pg/ mL, p<0.05) and gelatin (VEGF: 10.11 pg/ mL ± 1.75 pg/ mL, IL-6: 12.17 pg/ mL ± 6.49 pg/ mL, p<0.05) control groups
4. Discussion
In this study, we found that cardiac fibroblast-derived matrix educates monocytes into macrophages (CXMq) with a distinct flow marker phenotype characterized by CD16low, CD163low, MHC class II HLA-DRlow and CD86low which is distinctly different from that observed in mouse36. Although macrophages have traditionally been described in terms of M1 and M2 phenotypes37 in mice with pro-inflammatory and anti-inflammatory properties respectively, it is now recognized that human macrophages exist on a phenotypic spectrum with, at times, overlapping surface marker expression9. Thus, using surface markers alone to describe macrophages as either pro-inflammatory or pro-regenerative may be misleading. For example, low expression of CD86 has been associated with both a pro-inflammatory phenotype38 and a pro-regenerative phenotype39 using mouse models. IL6 secretion has likewise been associated with both pro- and anti-inflammatory phenotypes38, 40. In this paper, we have taken an unbiased approach to describe flow marker expression, while highlighting markers that have previously been “associated” with anti-inflammatory properties, rather than specifically categorize them within the M1/M2 paradigm which was established in mice, and not in humans. CXMq secreted high levels of VEGF and IL-6 and participated in paracrine mediated MSC migration. We chose MSC as they are cells with known anti-inflammatory41 and pro-angiogenic42 properties that can augment post MI repair.43 To our knowledge, this is the first report of a cardiac cell-derived matrix capable of stimulating monocytes into a distinct population of macrophages secreting VEGF and IL-6 which are known to play a role in angiogenesis and inflammation, respectively.
Previously, our lab developed a novel methodology to manufacture a matrix using cardiac fibroblasts obtained from cadaveric donors.19 In contrast to decellularized adult heart, which is primarily composed of collagen24, cardiac fibroblast derived matrix is primarily composed of fibronectin (>80%).19 Fibronectin orchestrates important cell adhesion, alignment, differentiation, migration and specific gene expression during developmental cardiac and blood vessel morphogenesis.44–52 In humans, fibronectin expression in the heart is relatively constant during fetal life but decreases after birth.25 Furthermore, fibronectin assembly and deposition are driven through cell surface integrin binding,26 which in our case, may be linked to monocyte differentiation. Insoluble fibronectin assembled within tissue matrix has a substantially different three-dimensional structural assembly and surface integrin exposure compared to soluble blood plasma derived fibronectin53–56. This may explain the differences we observed in macrophage expression. In this study, selective αVβ3 integrin inhibition on CXMq prevented MSC migration. Moreover, αVβ3 integrin inhibitor combined directly with MSC did not prevent MSC migration. These observations points to a link between insoluble fibronectin in the cardiac matrix, αVβ3 integrin binding, CXMq education, secretome modulation and cell-cell signaling.
Compared to the studies discussed above, education of macrophages with cardiac fibroblast derived matrix results in a unique phenotypic population of macrophage leading to reduction in inflammatory cell surface markers such as CD86 and HLA-DR. More interestingly, we demonstrated that CXMq increased VEGF and IL-6 release when compared to monocytes plated on plastic and gelatin. VEGF is known to play an important role in vascular hyper-permeability57 and interacts with numerous receptors and activates multiple signaling pathways. In ischemia animal models, VEGF treatment was shown to improve healing after ischemic myocardial injury.58 In clinical trials, VEGF gene therapy for myocardial ischemia and coronary artery disease has yielded variable efficacy.59, 60 Further, CXMq also secreted IL-6 at high levels compared to controls. IL-6 is a pleiotropic cytokine that regulates the immune and inflammatory response in a context dependent manner.61 In 2009, Kim and Hematti have shown that a unique feature of macrophages educated with mesenchymal stromal cells or their exosomes express a high level of IL-6.62 Furthermore, these findings are consistent with the recent understanding of the role of IL-6 in cardiac repair and regeneration.63, 64 Overall, we surmise that CXMq, which secrete VEGF and IL-6 and recruits MSC, could potentially be cardio-reparative.
Several groups have investigated macrophage differentiation and variable phenotypic expression in association with biomaterials18, 50, 65–67. For example, culture of human macrophages on polypropylene-polyglactin meshes resulted in macrophages characterized by high expression of IL-6, and IL-10.68They were shown to differentiate macrophages with low expression of inflammatory mediators.69 Keratin-based biomaterials polarized macrophages to an anti-inflammatory phenotype.70 Hussey et al. demonstrated that matrix bound nanovesicle associated IL-33 induced macrophages to a pro-remodeling and potentially reparative phenotype.71 Brown et al. showed that macrophages exhibit pleotropic responses, including constructive remodeling, to surgically implanted synthetic materials.72 Moreover, in a recent study, Bloise et al. used biomaterial with alginate hydrogels as an injectable carrier for anti-inflammatory interleukins (IL-4 or a combination of IL-4/IL-6/IL-13) and showed increased expression of CD163 and CD206 macrophage markers, thereby increasing monocytes polarization to a reparative macrophage phenotype.73. In comparison, our cardiac matrix educates monocytes into a distinct population of macrophages that exhibit anti-inflammatory and pro-angiogenic features through a 3-day culture on fibronectin-rich cardiac matrix (CX) sharing similar expression of CD206, IL-6, VEGF but also uniquely demonstrates decreased expression of CD16low, CD163low, MHC class II HLA-DRlow and CD86low. These observations suggest that biomaterials influence macrophage polarization uniquely which may be exploited for potential therapeutic purposes.
This study has several limitations. We chose to investigate the migratory responses of MSC because these cells are widely studied and are known to have anti-inflammatory, cardio-reparative effects. Whether other cell types, such as endothelial progenitor cells and cardiac fibroblasts are similarly recruited remains unknown. The increased MSC counts in the lower chamber of the transwell were presumed due to migration from the upper chamber given the relatively short time period; however, rapid MSC division is also possible, though unlikely. A limited secretome analysis for VEGF and IL-6 was performed as an initial observation due to cost and availability of antibodies. A more complete secretome profile analyses is planned pending additional research funding. Third, plastic and gelatin coating were chosen as control materials due to availability. How CXMq compare to macrophages that attach to other natural and synthetic materials, such as decellularized adult cardiac extracellular matrix, is unknown.
5. Conclusion
We discovered that cardiac fibroblast-derived matrix educates CD14+ monocytes into a distinct population of macrophages with a unique immunophenotype with potential immunomodulatory and pro-angiogenic potential that are capable of MSC recruitment. These findings could eventually lead to a potential novel therapeutic approach to prevent post MI heart failure in patients.
Supplementary Material
Fig. 4. CXMq secretes VEGF and IL-6.
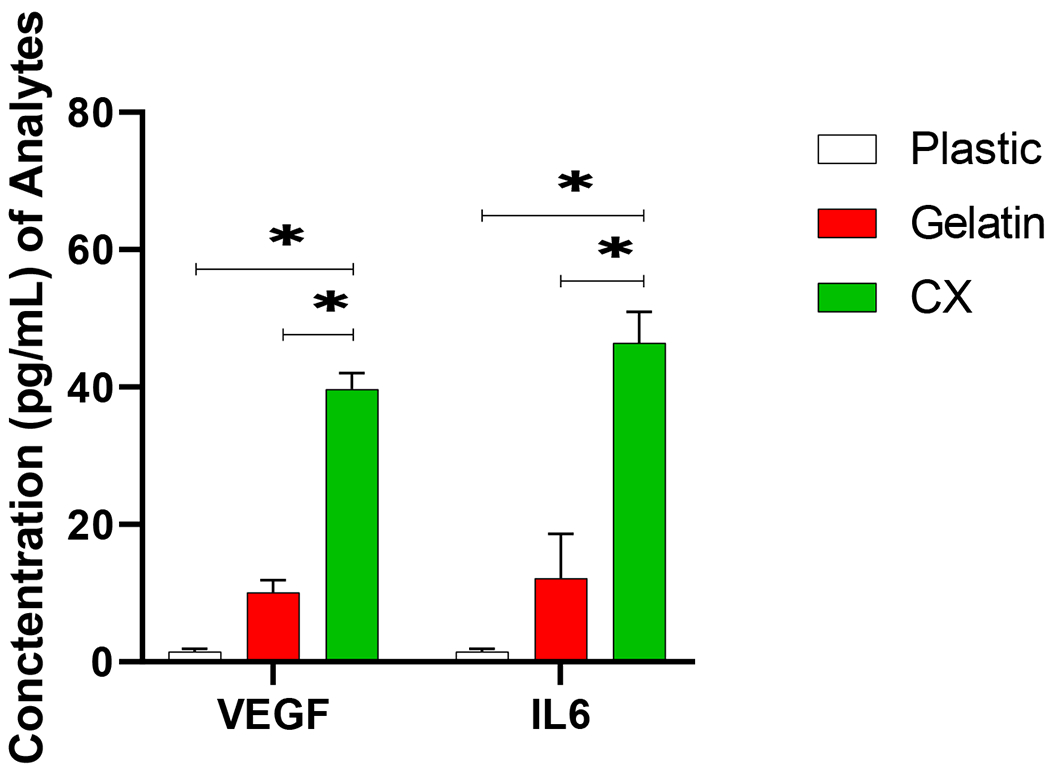
Macrophages (Mq) co-cultured on Cardiac fibroblast derived matrix (CX) prompts VEGF and IL-6 release compared to monocytes cultured on plastic and gelatin surfaces. (* p < 0.05), (n=3).
Highlights.
Cardiac fibroblast derived matrix (CX) is a novel fibronectin rich biomaterial which educates monocytes to a distinct population of macrophage (CXMq).
CXMq recruit mesenchymal stromal cells (MSC).
Blocking fibronectin binding to αVβ3 surface integrins on monocytes prevents MSC recruitment.
CXMq secrete high levels of VEGF and IL-6.
Acknowledgments
Support for this research was provided by the Wisconsin Organ Procurement Organization, University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation and the State of Wisconsin SEED fund. This work was supported in part by University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520.
Disclosures
EGS, PXH are consultants and have equity ownership in Cellular Logistics Inc.
ANR: Research or educational grants from Fujifilm Cellular Dynamics, Siemens Healthcare, BioCardia, Consultant Blue Rock Therapeutics, co-founder with equity interest: Cellular Logistics Inc.
References
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on E, Prevention Statistics C and Stroke Statistics S, Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation, 2020: p. CIR0000000000000757 DOI: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Gerber Y, Weston SA, Enriquez-Sarano M, Manemann SM, Chamberlain AM, Jiang R and Roger VL, Atherosclerotic Burden and Heart Failure After Myocardial Infarction. JAMA Cardiol, 2016. 1(2): p. 156–62 DOI: 10.1001/jamacardio.2016.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhu SD and Frangogiannis NG, The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ Res, 2016. 119(1): p. 91–112 DOI: 10.1161/CIRCRESAHA.116.303577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, Rosenthal N and Kovacic JC, The Macrophage in Cardiac Homeostasis and Disease: JACC Macrophage in CVD Series (Part 4). J Am Coll Cardiol, 2018. 72(18): p. 2213–2230 DOI: 10.1016/j.jacc.2018.08.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams JW, Giannarelli C, Rahman A, Randolph GJ and Kovacic JC, Macrophage Biology, Classification, and Phenotype in Cardiovascular Disease: JACC Macrophage in CVD Series (Part 1). J Am Coll Cardiol, 2018. 72(18): p. 2166–2180 DOI: 10.1016/j.jacc.2018.08.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R and Pittet MJ, The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of experimental medicine, 2007. 204(12): p. 3037–47 DOI: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, Kitabata H, Okochi K, Arita Y, Ishibashi K, Komukai K, Kataiwa H, Nakamura N, Hirata K, Tanaka A and Akasaka T, Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol, 2009. 54(2): p. 130–8 DOI: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Panizzi P, Swirski FK, Figueiredo JL, Waterman P, Sosnovik DE, Aikawa E, Libby P, Pittet M, Weissleder R and Nahrendorf M, Impaired infarct healing in atherosclerotic mice with Ly-6C(hi) monocytosis. J Am Coll Cardiol, 2010. 55(15): p. 1629–38 DOI: 10.1016/j.jacc.2009.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray PJ and Wynn TA, Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology, 2011. 11(11): p. 723–37 DOI: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBerge M, Shah SJ, Wilsbacher L and Thorp EB, Macrophages in Heart Failure with Reduced versus Preserved Ejection Fraction. Trends in molecular medicine, 2019. 25(4): p. 328–340 DOI: 10.1016/j.molmed.2019.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG and Lavine KJ, The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med, 2018. 24(8): p. 1234–1245 DOI: 10.1038/s41591-018-0059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN and Wynn TA, Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity, 2014. 41(1): p. 14–20 DOI: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leor J, Rozen L, Zuloff-Shani A, Feinberg MS, Amsalem Y, Barbash IM, Kachel E, Holbova R, Mardor Y, Daniels D, Ocherashvilli A, Orenstein A and Danon D, Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation, 2006. 114(1 Suppl): p. I94–100 DOI: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
- 14.Maekawa Y, Anzai T, Yoshikawa T, Asakura Y, Takahashi T, Ishikawa S, Mitamura H and Ogawa S, Prognostic significance of peripheral monocytosis after reperfused acute myocardial infarction:a possible role for left ventricular remodeling. J Am Coll Cardiol, 2002. 39(2): p. 241–6 DOI: 10.1016/s0735-1097(01)01721-1. [DOI] [PubMed] [Google Scholar]
- 15.Sabatine MS, Morrow DA, Cannon CP, Murphy SA, Demopoulos LA, DiBattiste PM, McCabe CH, Braunwald E and Gibson CM, Relationship between baseline white blood cell count and degree of coronary artery disease and mortality in patients with acute coronary syndromes: a TACTICS-TIMI 18 (Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy- Thrombolysis in Myocardial Infarction 18 trial)substudy. J Am Coll Cardiol, 2002. 40(10): p. 1761–8 DOI: 10.1016/s0735-1097(02)02484-1. [DOI] [PubMed] [Google Scholar]
- 16.Mariani M, Fetiveau R, Rossetti E, Poli A, Poletti F, Vandoni P, D’Urbano M, Cafiero F, Mariani G, Klersy C and De Servi S, Significance of total and differential leucocyte count in patients with acute myocardial infarction treated with primary coronary angioplasty. Eur Heart J, 2006. 27(21): p. 2511–5 DOI: 10.1093/eurheartj/ehl191. [DOI] [PubMed] [Google Scholar]
- 17.Nahrendorf M, Pittet MJ and Swirski FK, Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation, 2010. 121(22): p. 2437–45 DOI: 10.1161/CIRCULATIONAHA.109.916346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frangogiannis NG, Regulation of the inflammatory response in cardiac repair. Circ Res, 2012. 110(1): p. 159–73 DOI: 10.1161/CIRCRESAHA.111.243162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmuck EG, Mulligan JD, Ertel RL, Kouris NA, Ogle BM, Raval AN and Saupe KW, Cardiac fibroblast-derived 3D extracellular matrix seeded with mesenchymal stem cells as a novel device to transfer cells to the ischemic myocardium. Cardiovasc Eng Technol, 2014. 5(1): p. 119–131 DOI: 10.1007/s13239-013-0167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmuck EG, Hacker TA, Schreier DA, Chesler NC and Wang Z, Beneficial effects of mesenchymal stem cell delivery via a novel cardiac bioscaffold on right ventricles of pulmonary arterial hypertensive rats. Am J Physiol Heart Circ Physiol, 2019. 316(5): p. H1005–H1013 DOI: 10.1152/ajpheart.00091.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warrick JW, Young EW, Schmuck EG, Saupe KW and Beebe DJ, High-content adhesion assay to address limited cell samples. Integrative biology : quantitative biosciences from nano to macro, 2013. 5(4): p. 720–7 DOI: 10.1039/c3ib20224k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao X, Lian X, Hacker TA, Schmuck EG, Qian T, Bhute VJ, Han T, Shi M, Drowley L, Plowright A, Wang QD, Goumans MJ and Palecek SP, Long-term self-renewing human epicardial cells generated from pluripotent stem cells under defined xeno-free conditions. Nat Biomed Eng, 2016. 1 DOI: 10.1038/s41551-016-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Tao R, Campbell KF, Carvalho JL, Ruiz EC, Kim GC, Schmuck EG, Raval AN, da Rocha AM, Herron TJ, Jalife J, Thomson JA and Kamp TJ, Functional cardiac fibroblasts derived from human pluripotent stem cells via second heart field progenitors. Nat Commun, 2019. 10(1): p. 2238 DOI: 10.1038/s41467-019-09831-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weber KT, Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol, 1989. 13(7): p. 1637–52 DOI: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim H, Yoon CS, Kim H and Rah B, Expression of extracellular matrix components fibronectin and laminin in the human fetal heart. Cell Struct Funct, 1999. 24(1): p. 19–26 DOI: 10.1247/csf.24.19. [DOI] [PubMed] [Google Scholar]
- 26.Andries LJ, Sys SU and Brutsaert DL, Morphoregulatory interactions of endocardial endothelium and extracellular material in the heart. Herz, 1995. 20(2): p. 135–45. [PubMed] [Google Scholar]
- 27.Copland IB, Mesenchymal stromal cells for cardiovascular disease. J Cardiovasc Dis Res, 2011. 2(1): p. 3–13 DOI: 10.4103/0975-3583.78581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM and Pinteaux E, Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther, 2017. 8(1): p. 79 DOI: 10.1186/s13287-017-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kink JA, Forsberg MH, Reshetylo S, Besharat S, Childs CJ, Pederson JD, Gendron-Fitzpatrick A, Graham M, Bates PD, Schmuck EG, Raval A, Hematti P and Capitini CM, Macrophages Educated with Exosomes from Primed Mesenchymal Stem Cells Treat Acute Radiation Syndrome by Promoting Hematopoietic Recovery. Biol Blood Marrow Transplant, 2019. 25(11): p. 2124–2133 DOI: 10.1016/j.bbmt.2019.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundin M, Remberger M, Lonnies H, Sundberg B, Ringden O and Le Blanc K, No increased trapping of multipotent mesenchymal stromal cells in bone marrow filters compared with other bone marrow cells. Cytotherapy, 2008. 10(3): p. 238–42 DOI: 10.1080/14653240801965164. [DOI] [PubMed] [Google Scholar]
- 31.Simons M and Ware JA, Therapeutic angiogenesis in cardiovascular disease. Nat Rev Drug Discov, 2003. 2(11): p. 863–71. [DOI] [PubMed] [Google Scholar]
- 32.Fontes JA, Rose NR and Cihakova D, The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine, 2015. 74(1): p. 62–8 DOI: 10.1016/j.cyto.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Akker F, de Jager SC and Sluijter JP, Mesenchymal stem cell therapy for cardiac inflammation: immunomodulatory properties and the influence of toll-like receptors. Mediators Inflamm, 2013. 2013: p. 181020 DOI: 10.1155/2013/181020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokoyama K, Zhang XP, Medved L and Takada Y, Specific binding of integrin alpha v beta 3 to the fibrinogen gamma and alpha E chain C-terminal domains. Biochemistry, 1999. 38(18): p. 5872–7 DOI: 10.1021/bi9827619. [DOI] [PubMed] [Google Scholar]
- 35.Wayner EA, Orlando RA and Cheresh DA, Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol, 1991. 113(4): p. 919–29 DOI: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou Y, Yoshida S, Kubo Y, Yoshimura T, Kobayashi Y, Nakama T, Yamaguchi M, Ishikawa K, Oshima Y and Ishibashi T, Different distributions of M1 and M2 macrophages in a mouse model of laser-induced choroidal neovascularization. Molecular medicine reports, 2017. 15(6): p. 3949–3956 DOI: 10.3892/mmr.2017.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wasnik S, Rundle CH, Baylink DJ, Yazdi MS, Carreon EE, Xu Y, Qin X, Lau KW and Tang X, 1,25-Dihydroxyvitamin D suppresses M1 macrophages and promotes M2 differentiation at bone injury sites. JCI Insight, 2018. 3(17) DOI: 10.1172/jci.insight.98773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan X, Anzai A, Katsumata Y, Matsuhashi T, Ito K, Endo J, Yamamoto T, Takeshima A, Shinmura K, Shen W, Fukuda K and Sano M, Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol, 2013. 62: p. 24–35 DOI: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 39.Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA and Olson EN, Macrophages are required for neonatal heart regeneration. The Journal of clinical investigation, 2014. 124(3): p. 1382–92 DOI: 10.1172/JCI72181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jian ZJ, Yang Z, Miller MS, Carter CD, Slauson DO and Bochsler PN, Interleukin-6 secretion by bacterial lipopolysaccharide-stimulated bovine alveolar macrophages in vitro. Veterinary immunology and immunopathology, 1995. 49(1-2): p. 51–60 DOI: 10.1016/0165-2427(95)05447-e. [DOI] [PubMed] [Google Scholar]
- 41.Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK and Roy S, Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol, 2015. 185(10): p. 2596–606 DOI: 10.1016/j.ajpath.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferraro B, Leoni G, Hinkel R, Ormanns S, Paulin N, Ortega-Gomez A, Viola JR, de Jong R, Bongiovanni D, Bozoglu T, Maas SL, D’Amico M, Kessler T, Zeller T, Hristov M, Reutelingsperger C, Sager HB, Doring Y, Nahrendorf M, Kupatt C and Soehnlein O, Pro-Angiogenic Macrophage Phenotype to Promote Myocardial Repair. J Am Coll Cardiol, 2019. 73(23): p. 2990–3002 DOI: 10.1016/j.jacc.2019.03.503. [DOI] [PubMed] [Google Scholar]
- 43.Sager HB, Kessler T and Schunkert H, Monocytes and macrophages in cardiac injury and repair. J Thorac Dis, 2017. 9(Suppl 1): p. S30–S35 DOI: 10.21037/jtd.2016.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterman RE and Balian G, Indirect immunofluorescent staining of fibronectin associated with the floor of the foregut during formation and rupture of the oral membrane in the chick embryo. Anat Rec, 1980. 198(4): p. 619–35 DOI: 10.1002/ar.1091980407. [DOI] [PubMed] [Google Scholar]
- 45.George EL, Baldwin HS and Hynes RO, Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood, 1997. 90(8): p. 3073–81. [PubMed] [Google Scholar]
- 46.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM and Srivastava D, Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Developmental cell, 2009. 16(2): p. 233–44 DOI: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P and Leri A, Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A, 2006. 103(24): p. 9226–31 DOI: 10.1073/pnas.0600635103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bentzinger CF, Wang YX, von Maltzahn J, Soleimani VD, Yin H and Rudnicki MA, Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell stem cell, 2013. 12(1): p. 75–87 DOI: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konstandin MH, Toko H, Gastelum GM, Quijada PJ, De La Torre A, Quintana M, Collins B, Din S, Avitabile D, Volkers MJ, Gude NA, Fassler R and Sussman MA, Fibronectin is Essential for Reparative Cardiac Progenitor Cell Response Following Myocardial Infarction. Circ Res, 2013. DOI: 10.1161/CIRCRESAHA.113.301152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobaczewski M, Gonzalez-Quesada C and Frangogiannis NG, The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol, 2010. 48(3): p. 504–11 DOI: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS and Brecher P, Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. The Journal of clinical investigation, 1992. 89(4): p. 1060–8 DOI: 10.1172/JCI115685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ambesi A and McKeown-Longo PJ, Conformational remodeling of the fibronectin matrix selectively regulates VEGF signaling. Journal of cell science, 2014. 127(Pt 17): p. 3805–16 DOI: 10.1242/jcs.150458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K and Jichi Medical School Cohort Study G, Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School cohort study. J Epidemiol, 2011. 21(3): p. 169–75 DOI: 10.2188/jea.je20100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters DM, Portz LM, Fullenwider J and Mosher DF, Co-assembly of plasma and cellular fibronectins into fibrils in human fibroblast cultures. J Cell Biol, 1990. 111(1): p. 249–56 DOI: 10.1083/jcb.111.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maurer LM, Ma W and Mosher DF, Dynamic structure of plasma fibronectin. Crit Rev Biochem Mol Biol, 2015. 51(4): p. 213–27 DOI: 10.1080/10409238.2016.1184224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mosher DF, Fogerty FJ, Chernousov MA and Barry EL, Assembly of fibronectin into extracellular matrix. Ann N Y Acad Sci, 1991. 614: p. 167–80 DOI: 10.1111/j.1749-6632.1991.tb43701.x. [DOI] [PubMed] [Google Scholar]
- 57.Crafts TD, Jensen AR, Blocher-Smith EC and Markel TA, Vascular endothelial growth factor: therapeutic possibilities and challenges for the treatment of ischemia. Cytokine, 2015. 71(2): p. 385–93 DOI: 10.1016/j.cyto.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 58.Luo Z, Diaco M, Murohara T, Ferrara N, Isner JM and Symes JF, Vascular endothelial growth factor attenuates myocardial ischemia-reperfusion injury. Ann Thorac Surg, 1997. 64(4): p. 993–8 DOI: 10.1016/s0003-4975(97)00715-7. [DOI] [PubMed] [Google Scholar]
- 59.Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, Ashare AB, Lathi K and Isner JM, Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation, 1998. 98(25): p. 2800–4 DOI: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- 60.Rosengart TK, Lee LY, Patel SR, Kligfield PD, Okin PM, Hackett NR, Isom OW and Crystal RG, Six-month assessment of a phase I trial of angiogenic gene therapy for the treatment of coronary artery disease using direct intramyocardial administration of an adenovirus vector expressing the VEGF121 cDNA. Annals of surgery, 1999. 230(4): p. 466–70; discussion 470-2 DOI: 10.1097/00000658-199910000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, Kashiwamura S, Nakajima K, Koyama K, Iwamatsu A and et al. , Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature, 1986. 324(6092): p. 73–6 DOI: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 62.Kim J and Hematti P, Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Experimental hematology, 2009. 37(12): p. 1445–53 DOI: 10.1016/j.exphem.2009.09.004 S0301-472X(09)00365-8 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayfield AE, Kanda P, Nantsios A, Parent S, Mount S, Dixit S, Ye B, Seymour R, Stewart DJ and Davis DR, Interleukin-6 Mediates Post-Infarct Repair by Cardiac Explant-Derived Stem Cells. Theranostics, 2017. 7(19): p. 4850–4861 DOI: 10.7150/thno.19435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang P, Ma S, Dong M, Wang J, Chai S, Liu T and Li J, Effect of interleukin-6 on myocardial regeneration in mice after cardiac injury. Biomed Pharmacother, 2018. 106: p. 303–308 DOI: 10.1016/j.biopha.2018.06.090. [DOI] [PubMed] [Google Scholar]
- 65.Dziki JL and Badylak SF, Extracellular Matrix for Myocardial Repair. Adv Exp Med Biol, 2018. 1098: p. 151–171 DOI: 10.1007/978-3-319-97421-7_8. [DOI] [PubMed] [Google Scholar]
- 66.Hanson SE, King SN, Kim J, Chen X, Thibeault SL and Hematti P, The effect of mesenchymal stromal cell-hyaluronic acid hydrogel constructs on immunophenotype of macrophages. Tissue Eng Part A, 2011. 17(19-20): p. 2463–71 DOI: 10.1089/ten.TEA.2010.0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW and Vunjak-Novakovic G, The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials, 2014. 35(15): p. 4477–88 DOI: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schachtrupp A, Klinge U, Junge K, Rosch R, Bhardwaj RS and Schumpelick V, Individual inflammatory response of human blood monocytes to mesh biomaterials. Br J Surg, 2003. 90(1): p. 114–20 DOI: 10.1002/bjs.4023. [DOI] [PubMed] [Google Scholar]
- 69.McBane JE, Battiston KG, Wadhwani A, Sharifpoor S, Labow RS and Santerre JP, The effect of degradable polymer surfaces on co-cultures of monocytes and smooth muscle cells. Biomaterials, 2011. 32(14): p. 3584–95 DOI: 10.1016/j.biomaterials.2011.01.069. [DOI] [PubMed] [Google Scholar]
- 70.Waters M, VandeVord P and Van Dyke M, Keratin biomaterials augment anti-inflammatory macrophage phenotype in vitro. Acta Biomater, 2018. 66: p. 213–223 DOI: 10.1016/j.actbio.2017.10.042. [DOI] [PubMed] [Google Scholar]
- 71.Hussey GS, Dziki JL, Lee YC, Bartolacci JG, Behun M, Turnquist HR and Badylak SF, Matrix bound nanovesicle-associated IL-33 activates a pro-remodeling macrophage phenotype via a non-canonical, ST2-independent pathway. J Immunol Regen Med, 2019. 3: p. 26–35 DOI: 10.1016/j.regen.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE and Badylak SF, Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater, 2012. 8(3): p. 978–87 DOI: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bloise N, Rountree I, Polucha C, Montagna G, Visai L, Coulombe KLK and Munarin F, Engineering Immunomodulatory Biomaterials for Regenerating the Infarcted Myocardium. Front Bioeng Biotechnol, 2020. 8: p. 292 DOI: 10.3389/fbioe.2020.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


