Abstract
Background:
The incidence of Neuroendocrine Tumors (NETs) are rapidly rising. There are very few studies investigating the role of sociodemographic factors on NETs. Our study aimed to examine how geographic and socio-demographic characteristics shape outcomes in the NET population.
Methods:
We conducted a retrospective analysis utilizing the Surveillance, Epidemiology, and End Results (SEER) database, and studied NETs patient population from 1973 to 2015. Univariate and multivariable analyses were performed to evaluate patients’ disease-specific survival (DSS) and overall survival (OS). Geographic and socio-demographic factors, including the location of residence: urban area (UA) versus rural area (RA), gender, race, insurance status, and marital status, were included in the analysis.
Results:
A total of 53,522 [RA: N=5,517; UA: N=47,517] patients were included in the analysis. The incidence of NETs was found to be rising in both RA and UA but more rapidly in RA (with the highest incidence in 2006–2015, 5.93 per 100,000 in RA vs. 4.10 in UA). Patients from RA presented at advanced stages when compared to UA (regional: 18% versus 16%, distant: 15% versus 13%, p<0.01. In the multivariable model, RA had a trend towards poorer DSS (HR:1.02, P=0.615) and OS (HR=1.05, P=0.053) compared to UA. Multivariable analysis showed significantly worse DSS and OS in uninsured, single, and male patients compared to insured, married, and female patients, respectively.
Conclusions:
Our study identified socio-demographic disparities on NET outcomes. Access to healthcare could be a potential contributing factor, although differences in environmental exposure, health behavior, and tumor biology could also be responsible.
Keywords: neuroendocrine, disparities, rural, urban, incidence, outcomes, carcinoid, NET
Introduction:
Neuroendocrine tumors (NET) are a heterogeneous group of tumors arising from neuroendocrine cells, mostly involving the bronchopulmonary system and the gastrointestinal tract 1,2. In the United States (US), the incidence of NETs has increased by almost 6.4 fold in the last four decades, incurring a substantial economic burden on the healthcare system. Due to such a dramatic rise in the incidence of this relatively indolent disease, the prevalence of NETs in the US has also increased from 0.006% in 1993 to 0.048% in 20123,4.
Very few studies have looked into the potential socio-demographic disparities in NETs patients worldwide. In a recently published Surveillance, Epidemiology, and End Results Program (SEER) database study on pancreatic NETs patients, black individuals had a significantly worse overall survival (OS) and tumor-specific survival compared to their non-black counterparts. Advanced stage at diagnosis and poor socioeconomic status (SES) were proposed as possible reasons for this observed racial disparity in the outcome, as evidenced by several other studies in different cancers 5,6. In a population-based study conducted in Canada, low SES was an independent predictor of worse OS in 5,619 patients with NET 7. The same group of investigators also noted that the NET patients living in rural areas experienced a higher incidence and significantly worse distant recurrence-free survival and OS, compared to those of urban regions 8,9. Another study from Australia confirmed these findings, where the remote residence of patients was thought to have a significant negative impact on OS 8. An analysis of SEER data (1973–2009) on patients with malignant carcinoid tumors showed that people from rural areas had a worse cause-specific survival 10.
The ever-expanding landscape of the management of NETs is increasingly getting more complex and dependent on multidisciplinary involvement. Access to specialized cancer centers and affordability of care are critical in ensuring timely diagnosis and optimal management, translating into improved outcome in patients. A recent report showed that cancer mortality had been steadily declining for the most common cancers in the US, which was attributed to early detection. It was also found that the racial gap in cancer death has been narrowing, but socioeconomic inequalities are getting wider 11. The study, however, did not specifically look at NET patient population. Therefore, to understand the effect of geographic and sociodemographic factors in the NET population, we conducted a large population-based study utilizing the SEER database.
Materials and Methods
Data Source
For our retrospective analysis, we utilized the SEER database and evaluated data from years 1973–2015. The SEER database is a program of the National Cancer Institute (NCI), that collects cancer-specific incidence data from population-based cancer registries, covering approximately 35% of US population 12. The SEER program collects incidence and other essential data ranging from patient’s demographics, the morphology of tumor, stage at diagnosis, treatment received, vital status of patients, and survival data. It obtains population data on cancer rates from Census Bureau, while mortality data is collected from the Nation Center for Health Statistics. Since SEER’s introduction in 1973 (SEER 9 registry), the program has undergone two major updates, SEER 13 in 1992 and SEER 18 in 2000, which includes almost 20 geographic areas in the US.
Study Population
NET patients of all ages, with any analytical stage (stage I, II, III, IV) were included. To obtain relevant data about NET patients from the SEER database, we used diagnostic codes from the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3). Patients with following ICD histologic codes were included: well-differentiated NETs, carcinoid tumor and of uncertain origin, enterochromaffin, tubular carcinoid, adenocarcinoid tumor, atypical carcinoid tumor, pancreatic endocrine tumor origin, insulinoma, glucagonoma, gastrinoma, VIPoma, somatostatinoma (ICD-O codes 8240, 8241, 8242, 8243, 8245, 8249, 8150, 8151, 8152, 8153, 8155, 8156). Tumors that necessarily are different biologically and often are aggressive in nature were excluded: small cell and large cell lung carcinoma, pheochromocytoma, paraganglioma, mixed adenoneuroendocrine carcinoma, neuroendocrine carcinoma, Merkel cell carcinoma, mixed pancreatic exocrine and endocrine tumor (ICD-O codes 8002, 8041, 8042, 8043, 8045, 8013, 8700, 8680, 8693, 8244, 8246, 8247, 8154).
Age-adjusted incidence rates were classified into urban and rural population categories utilizing the Rural-Urban Continuum Code (RUCC), a variable available in the SEER database. The Office of Management and Budget (OMB) classified RUCC into two categories, metropolitan and non-metropolitan areas. A metropolitan was defined to be the area that contained a large urbanized population of at least 50,000 individuals. On the other hand, a non-metropolitan statistical area is comprised of less than 50,000 individuals. To be consistent with other studies, counties classified as metropolitan (RUCCs 1–3) were classified as urban, and counties classified as non-metropolitan (RUCCs 4–9) were classified as rural 13,14.
Statistical Analysis:
De-identified data were analyzed based on various socio-demographic factors, including age, sex, race, marital status, insurance status, residence (rural or urban), and also tumor-related factors (stage, grade, histology, and primary site). Associations between the area of residence and various socio-demographic and tumor-specific characteristics were assessed using the Kruskal-Wallis test (for ordinal responses) or the Pearson Chi-square test (for categorical responses). The primary endpoint of the study was to estimate overall survival (OS), defined by the time from diagnosis of NET to death due to any cause. Disease-specific survival (DSS), represented by the time from the diagnosis to death, precisely due to cancer itself, was considered as the secondary endpoint. Univariate and multivariable Cox proportional hazard models (adjusting for age, sex, stage, grade, year of diagnosis, insurance status, marital status, race, and area of residence) were used to estimate the effects of different covariates on both OS and DSS in study participants. Result estimates were expressed as hazard ratios (HR) with 95% confidence intervals (CI). Kaplan-Meier method was used to calculate OS and DSS. Associations were considered statistically significant if the p-value was ≤ 0.05. All statistical analyses were performed using Statistical Analysis Software (SAS version 9.4).
Results
A total of 53,522 patients with NET were included in the analysis, of whom 5,517 (10%) patients had a residence in rural areas (RA), and 47,517 (90%) individuals resided in urban areas (UA). Median age at diagnosis was 58 years. Individuals in RA had a slightly older age at presentation compared to their urban counterparts (median 60 years versus 58 years, p<0.001). Rural population had a larger proportion of non-Hispanic whites as compared to the urban population (83% versus 64%, p<0.001). Patients from RA presented at more advanced stages (regional 18% versus 16%, distant: 15% versus 13%, p<0.001), as compared to those from UA. Further comparison of all the baseline characteristics between RA and UA population is summarized in table 1 below.
Table 1:
Demographic and clinical characteristics of patients with NETs residing in rural and urban areas
| Rural | Urban | p-Value | ||
|---|---|---|---|---|
| Count, N (%) | 5,517 (10) | 47,517 (90) | ||
| Age, median (range in years) | 60 (8–97) | 58 (2–100) | <0.001 | |
| Race | NH White (%) | 4,574 (83) | 30,389 (64) | <0.001 |
| NH Black (%) | 486 (9) | 7,620 (16) | ||
| Hispanic (%) | 248 (4) | 5,282 (11) | ||
| Other (%) | 209 (4) | 4,226 (9) | ||
| Sex | Male (%) | 2,541 (46) | 21,578 (45) | 0.361 |
| Female (%) | 2,976 (54) | 25,939 (55) | ||
| Marital Status | Single (%) | 2,230 (40) | 21,065 (44) | <0.001 |
| Married (%) | 3,287 (60) | 26,452 (56) | ||
| Insurance Status | Uninsured (%) | 89 (2) | 650 (1) | 0.303 |
| Insured (%) | 2,759 (50) | 23,980 (50) | ||
| Staging | Localized (%) | 3,064 (55) | 27,841 (59) | <0.001 |
| Regional (%) | 1,017 (18) | 7,741 (16) | ||
| Distant (%) | 816 (15) | 5,999 (13) | ||
| NET Type | Pancreatic (%) | 340 (10) | 3,060 (90) | <0.001 |
| Pulmonary (%) | 956 (10) | 8,220 (90) | ||
| Gastrointestinal (%) | 3,632 (10) | 31,376 (90) | ||
NH – non-Hispanics
We found that the incidence rates of NETs have been slowly increasing over decades, in both RA and UA, with the highest incidence (5.93 per 100,000 in RA vs. 4.10 per 100,000 in UA) observed in the period 2006–2015. The cause-specific mortality rate in these patients with NET has shown a steady downward trend over the years. However, the mortality rate remained higher in patients from RA throughout the study period. Findings showed that in recent years, NET patients are getting diagnosed at an earlier stage (62.8% in 2006–2015 versus 23.5% in 1976–1985, p<0.001), with a significant decrease in the proportion of individuals with distant disease (12.9% in years 2006–2015 versus 32.7% in 1976–1985, p<0.001) at presentation. Stages at the presentation in different regions, UA versus RA, are depicted in table 2 below. On Kaplan-Meier analysis of survival estimates, unadjusted median OS for patients from RA was 13.5 years, and for those from UA was 16.5 years. Survival rates, both OS and DSS (3-year, 5-year, and median) are summarized in figures 1 and 2. Looking individually pancreatic, pulmonary and gastrointestinal NETs, the three most common subtypes of NETs, similar trends were observed for OS and DSS (supplementary figure 1–6).
Table 2:
Rural versus urban NETs incidence from years 1973–2015, differentiating based on the disease spread pattern
| Years | 1973–1975 | 1976–1985 | 1986–1995 | 1996–2005 | 2006–2015 | |
|---|---|---|---|---|---|---|
| Rural | Localized | 9 (23%) | 54 (25%) | 204 (49%) | 879 (63%) | 1,918 (68%) |
| Regional | 16 (41%) | 81 (38%) | 107 (25%) | 295 (21%) | 518 (18%) | |
| Distant | 14 (36%) | 79 (37%) | 108(26%) | 231 (16%) | 384 (14%) | |
| Urban | Localized | 112 (36%) | 356 (28%) | 2,183 (59%) | 8,244 (66%) | 16,946 (71%) |
| Regional | 84 (27%) | 441 (34%) | 795 (21%) | 2,325 (19%) | 4,096 (17%) | |
| Distant | 116(37%) | 490(38%) | 746 (20%) | 1,865 (15%) | 2,782 (12%) | |
Figure 1:
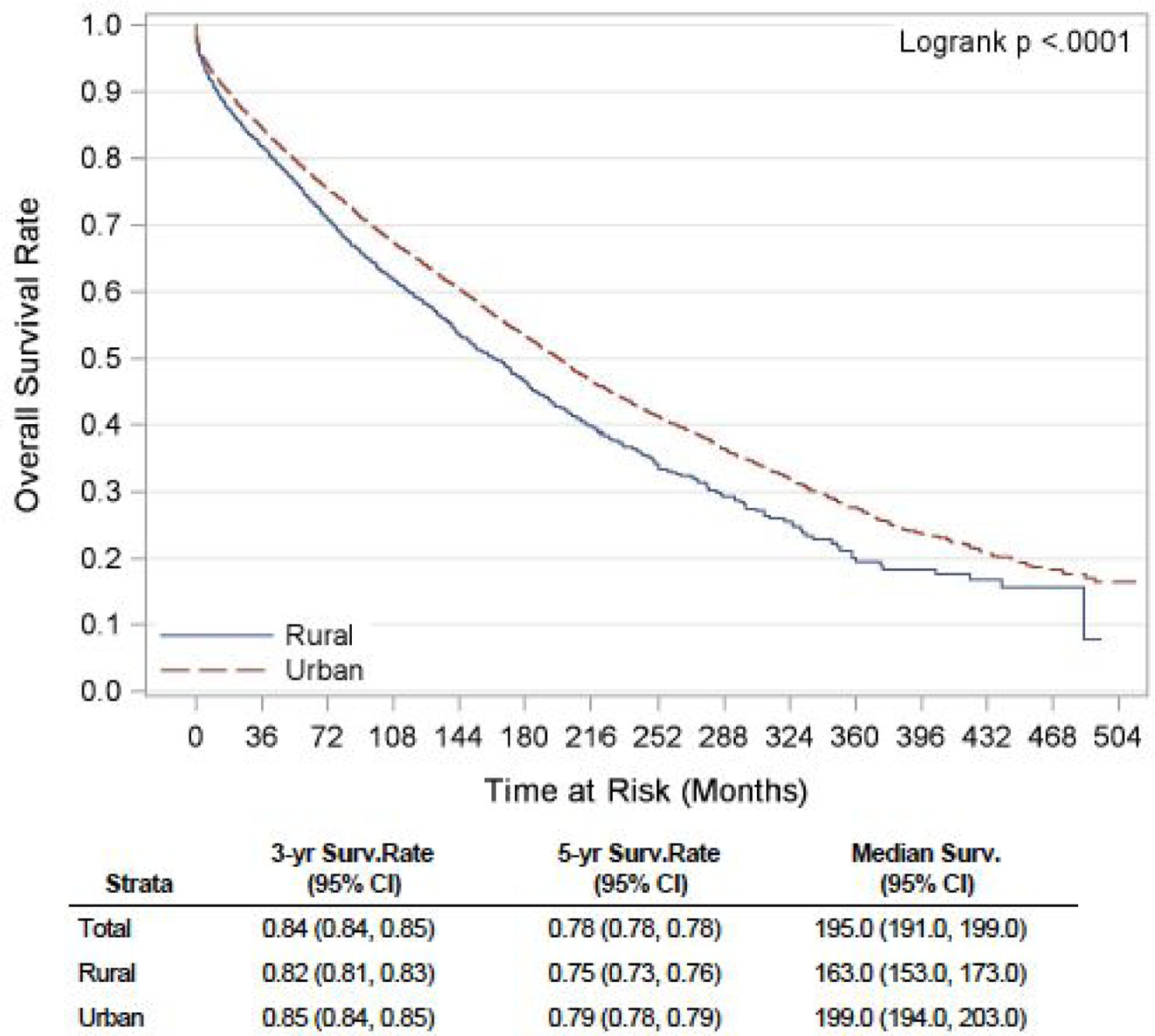
Unadjusted Kaplan-Meier OS curve for NET patients in rural versus urban region
Figure 2:
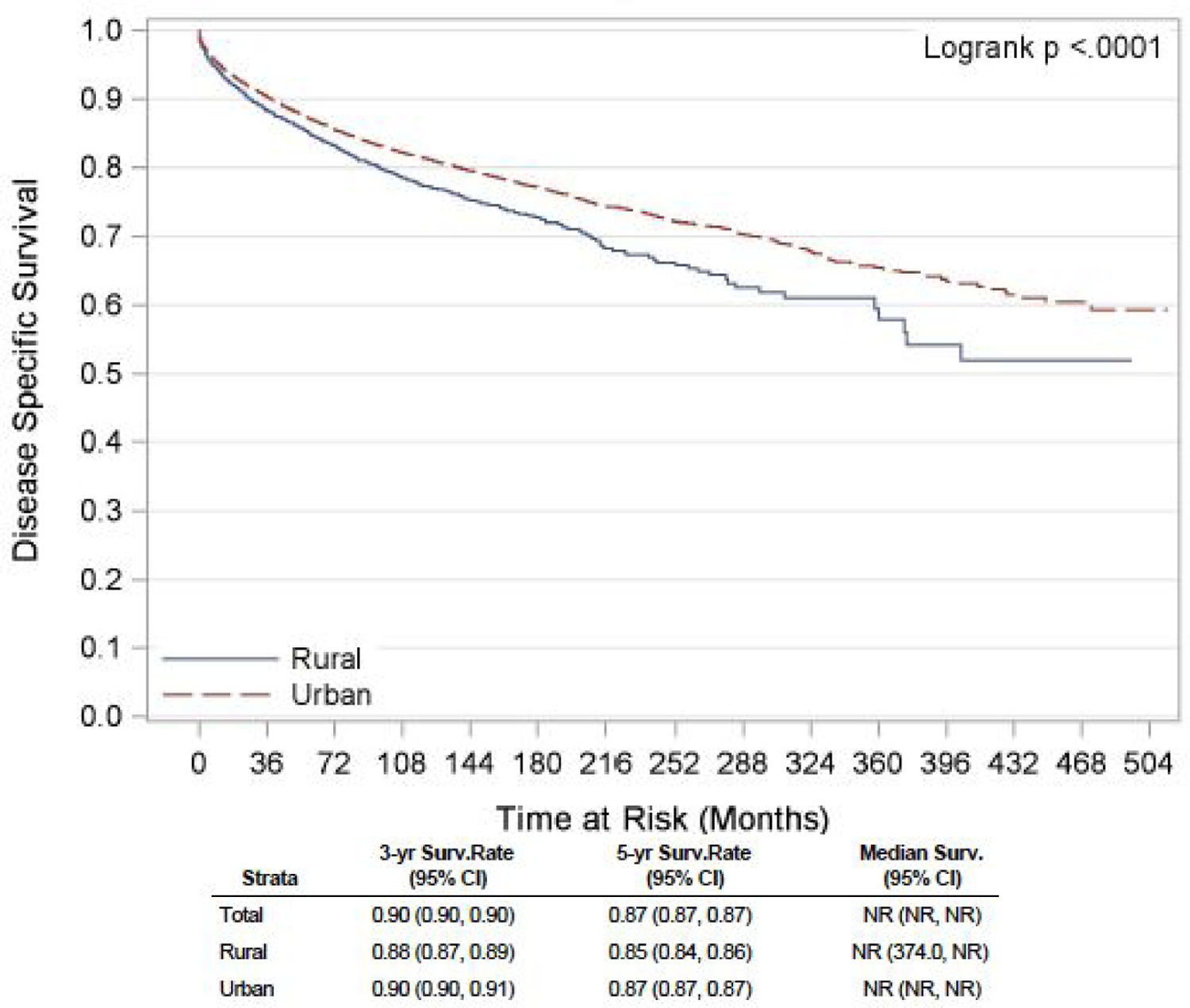
Unadjusted Kaplan-Meier DSS curve for NET patients in rural versus urban region
On the univariate analyses, patients residing in RA, those with high grade (III/IV) disease, advanced stage NET, male gender and single individuals, had a significantly worse OS and DSS, when compared to UA patients, low grade (I/II), localized disease, female gender and married couples respectively. These findings are summarized in supplementary tables 1 and 2. The multivariable Cox proportional hazards regression models showed that high grade (III/IV), uninsured patients, male gender, advanced disease had a significantly worse OS and DSS when compared to patients with low-grade tumors (I/II), insured patients, female gender, and localized disease respectively. RA patients showed a trend towards worse OS and DSS, though the results were not significant in the multivariable model. These results are further summarized in tables 3 and 4 below.
Table 3:
Multivariable Cox proportional hazards regression model highlighting OS in NET patient population
| Variable | Hazard ratio | 95% Confidence Interval (CI) | p-value | |
|---|---|---|---|---|
| Grade (III/IV) vs. grade (I/II) | 2.60 | 2.35 – 2.87 | <0.001 | |
| Staging | Regional vs. localized | 1.68 | 1.61 – 1.76 | <0.001 |
| Distant vs. localized | 4.44 | 4.27 – 4.63 | <0.001 | |
| Female vs. male | 0.77 | 0.75 – 0.80 | <0.001 | |
| Uninsured vs. insured | 1.77 | 1.47 – 2.14 | <0.001 | |
| Marital status (single vs. married) | 1.22 | 1.18 – 1.26 | <0.001 | |
| Rural vs. urban | 1.05 | 1.00 – 1.10 | 0.053 | |
Table 4:
Multivariable Cox proportional hazards regression model highlighting DSS in NET patient population
| Variable | Hazard ratio | 95% Confidence Interval (CI) | p-value | |
|---|---|---|---|---|
| Grade (III/IV) vs. grade (I/II) | 3.54 | 3.14 – 3.98 | <0.001 | |
| Staging | Regional vs. localized | 4.24 | 3.93 – 4.58 | <0.001 |
| Distant vs. localized | 16.25 | 15.18 – 17.39 | <0.001 | |
| Female vs. male | 0.85 | 0.81 – 0.89 | <0.001 | |
| Uninsured vs. insured | 1.83 | 1.45 – 2.32 | <0.001 | |
| Marital status (single vs. married) | 1.11 | 1.06 – 1.16 | <0.001 | |
| Rural | 1.02 | 0.95 – 1.09 | 0.615 | |
Discussion
Neuroendocrine tumors are a group of rare malignancies, often characterized by hormonal syndromes due to the production of active peptides by tumor cells 1. The incidence and prevalence of NET in the US have steadily increased over the last few decades. In a recently published large population-based study, the median OS for patients with NET was 9.3 years, with better survival for individuals with localized or lower grade tumors 3. In our study, the steady increase in incidence rates of NET over time matches the trends observed in other population-based studies. A decline in cause-specific mortality rate over the years is also consistent with previous reports 1,3,15.
Socioeconomic and demographic disparities have been noted to influence the outcome of different cancers 11. In a recently published study on melanoma patients in the Nationwide Inpatient Sample database, people with low income and Medicaid coverage were more likely to have worse outcome 16. In the field of NETs, black patients with pancreatic NETs had worse survival due to a more advanced stage at presentation and relatively limited access to surgery 7. However, recent evidence indicates that the racial disparities are going down, and the differences in economic status, insurance coverage, and ease of healthcare access are becoming more prominent drivers in determining the outcome of cancer patients 5,17,18. With the recent Affordable Care Act (ACA) Medicaid Expansion program, Adamson et al. reported improvement in African American (AA) cancer patients receiving timely treatment. Before the ACA Medicaid Expansion, it was reported that AA patients were 4.9% less likely to receive timely treatment in comparison to white patients, while post-expansion the adjusted disparity decreased to 0.2%19. Disparities related to the area of living (rural vs. urban) have been identified as essential causes resulting in differences in outcome among patients with several common cancers 20,21. In a Canadian retrospective cohort study, rural residence and low socioeconomic status were found to be independent predictors of survival in adult patients with NET 7.
In our study population, incidence rates were higher in RA than UA. The higher incidence rate in RA could be related to environmental or lifestyle associated factors. One probable hypothesis might be oncogenic genetic modulation due to more exposure to pesticides (e.g., organochlorine) in patients in RA 22, although differences at the genomic level may also contribute. The recent increase in the incidence of NETs has been attributed to the diagnosis of early stage, asymptomatic tumors that are often diagnosed incidentally 3. In our study, however, RA not only had a higher incidence than UA but also presented at more advanced stages, suggesting that our recent advancements on diagnostic techniques possibly did not contribute to the higher incidences in RA.
In the univariate analysis, patients residing in RA had a significantly worse OS (HR 1.22, 95% CI: 1.16 to 1.28, p<0.001) and DSS (HR 1.23, 95% CI: 1.15 to 1.31, p<0.001) compared to the urban population. On multivariable analysis, the association between the rural residence and poor OS showed a trend towards statistical significance (HR 1.05, 95% CI: 1.00 to 1.10, p= 0.053). Similar findings were reported in the Canadian cohort study, with a worse 10-year distant recurrence-free survival (62.8% versus 65.9%, p = 0.03) and OS (44.6% versus 48.8%, p = 0.004) in patients with NET in RA in comparison with UA 9. A recent single-center retrospective study done in Australia revealed a significantly more inferior OS in patients with NET in RA compared to those in regional areas 8.
Management of NETs has revolutionized in the last decade, with the advent of better diagnostic techniques for prompt and accurate diagnosis and the improvement of survival with the success of newer treatment modalities 23. Patients in rural areas are often away from well-equipped specialized tertiary care centers having all these facilities. In our analysis, patients from RA were more likely to present at an advanced stage of disease compared to their urban counterparts, in contrast to the findings in a similar earlier study 9. This difference, a crucial possible determinant in the overall outcome or survival, may be a result of lack of education and awareness about NET among patients and treating physicians in RA and non-availability of improved diagnostics in rural areas. Lack of insurance coverage was also more prevalent in the rural population in our study, further compromising the access of rural patients to high-quality care. When we adjusted for these factors in the multivariable model, the difference in survival between RA and UA became marginal, suggesting their potential role in the survival of NET patients.
Interestingly, marriage seemed to be protective against the poor outcome in NET patients in our analysis. A similar trend was observed by Zhou et al. in their study of pancreatic NET patients 24. This difference may be attributed to different psychosocial and socioeconomic factors. Black patients had a worse OS but a similar DSS compared to the non-Hispanic white population suggesting possible poorer overall health-related parameters rather than NET specific elements in this minority population.
To our knowledge, this is the first population-based study investigating the rural-urban disparities in the care of patients with NET in the US. We evaluated the impact of different socio-demographic factors on both the overall and disease-specific mortality. However, our study is not without limitations. Firstly, we had limited information on the stage and grade of tumors in the SEER database. Secondly, treatment data like the use of different systemic therapies, type, and quality of surgeries and liver-directed therapies are not available in the SEER database. Lastly, although we were able to account for some socioeconomic and health care delivery factors, it is essential to note that variation in the genetic profile of patients might also be a vital determinant in the evolution and progression of NET, as suggested in several recent studies 25,26.
Conclusions
Our SEER-based population study showed that there are significant disparities in the care of patients with NET in the US. Rural residence was associated with marginally worse survival compared to their urban counterparts. Uninsured and single patients had poorer survival compared to the insured and married patients, respectively. Health policy should be tailored towards directed and equitable allocation of resources to adequately address these disparities and improve access to quality care among all patients with NET across the US.
Supplementary Material
Footnotes
Conflict of Interest:
Rohit Gosain – None
Somedeb Ball – None
Navpreet Rana – None
Adrienne Groman – None
Elizabeth Gage-Bouchard – None
Arvind Dasari – None
Sarbajit Mukherjee – Board of Directors: Esophageal Cancer Action Network (voluntary), Research funding: National Comprehensive Cancer Network (paid to the institute), Panel member: National Comprehensive Cancer Network.
References:
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 2.Gosain R, Mukherjee S, Yendamuri SS, Iyer R. Management of Typical and Atypical Pulmonary Carcinoids Based on Different Established Guidelines. Cancers (Basel). 2018;10(12). doi: 10.3390/cancers10120510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed]
- 4.Hallet J, Law CHL, Cheung M, et al. Patterns and Drivers of Costs for Neuroendocrine Tumor Care: A Comparative Population-Based Analysis. Ann Surg Oncol. 2017. doi: 10.1245/s10434-017-5986-0. [DOI] [PubMed]
- 5.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for African Americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016. doi: 10.3322/caac.21340. [DOI] [PubMed]
- 6.Zhou H, Zhang Y, Wei X, et al. Racial disparities in pancreatic neuroendocrine tumors survival: a SEER study. Cancer Med. 2017. doi: 10.1002/cam4.1220. [DOI] [PMC free article] [PubMed]
- 7.Hallet J, Law CHL, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015. doi: 10.1002/cncr.29099. [DOI] [PubMed]
- 8.Hafeez U, Joshi A, Bhatt M, Kelly J, Sabesan S, Vangaveti V. Clinical profile and treatment outcomes of advanced neuroendocrine tumours in rural and regional patients: a retrospective study from a regional cancer centre in North Queensland, Australia. Intern Med J. 2017. doi: 10.1111/imj.13333. [DOI] [PubMed]
- 9.Hallet J, Law CHL, Karanicolas PJ, Saskin R, Liu N, Singh S. Rural-urban disparities in incidence and outcomes of neuroendocrine tumors: A population-based analysis of 6271 cases. Cancer. 2015. doi: 10.1002/cncr.29338. [DOI] [PubMed]
- 10.Cheung R. Racial and Socioeconomic Disparities in Malignant Carcinoid Cancer Cause Specific Survival: Analysis of the Surveillance, Epidemiology and End Results National Cancer Registry. Asian Pacific J Cancer Prev. 2014. doi: 10.7314/apjcp.2013.14.12.7117. [DOI] [PubMed]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019. doi: 10.3322/caac.21551. [DOI] [PubMed]
- 12.National Institutes of Health, National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Incidence Data, 1973–2015. https://seer.cancer.gov/data/ 2019. https://seer.cancer.gov/data/.
- 13.Glynn ME, Keeton KA, Gaffney SH, Sahmel J. Ambient Asbestos Fiber Concentrations and Long-Term Trends in Pleural Mesothelioma Incidence between Urban and Rural Areas in the United States (1973–2012). Risk Anal. 2018. doi: 10.1111/risa.12887. [DOI] [PubMed]
- 14.Paquette I, Finlayson SRG. Rural Versus Urban Colorectal and Lung Cancer Patients: Differences in Stage at Presentation. J Am Coll Surg. 2007. doi: 10.1016/j.jamcollsurg.2007.04.043. [DOI] [PubMed]
- 15.Cetinkaya RB, Aagnes B, Thiis-Evensen E, Tretli S, Bergestuen DS, Hansen S. Trends in incidence of neuroendocrine neoplasms in Norway: A report of 16,075 cases from 1993 through 2010. Neuroendocrinology. 2016. doi: 10.1159/000442207. [DOI] [PubMed]
- 16.Z. A-Q, S. S, A. W, et al. Disparities in the presentation and management of cutaneous melanoma that required admission. Oncol. 2018. doi:10.1159/000468152 LK - 10.1159/000468152http://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=14230232&id=doi:10.1159%2F000468152&atitle=Disparities+in+the+presentation+and+management+of+cutaneous+melanoma+that+required+admission&stitle=Oncology&title=Oncology+%28Switzerland%29&volume=95&issue=2&spage=69&epage=80&aulast=Al-Qurayshi&aufirst=Zaid&auinit=Z.&aufull=Al-Qurayshi+Z.&coden=ONCOB&isbn=&pages=69-80&date=2018&auinit1=Z&auinitm= LK - http://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=14230232&id=doi:10.1159%2F000468152&atitle=Disparities+in+the+presentation+and+management+of+cutaneous+melanoma+that+required+admission&stitle=Oncology&title=Oncology+%28Switzerland%29&volume=95&issue=2&spage=69&epage=80&aulast=Al-Qurayshi&aufirst=Zaid&auinit=Z.&aufull=Al-Qurayshi+Z.&coden=ONCOB&isbn=&pages=69-80&date=2018&auinit1=Z&auinitm=. [DOI] [PubMed]
- 17.Pulte D, Jansen L, Brenner H. Disparities in colon cancer survival by insurance type: A population-based analysis. Dis Colon Rectum. 2018. doi: 10.1097/DCR.0000000000001068. [DOI] [PubMed]
- 18.Osuoha CA, Callahan KE, Ponce CP, Pinheiro PS. Disparities in lung cancer survival and receipt of surgical treatment. Lung Cancer. 2018. doi: 10.1016/j.lungcan.2018.05.022. [DOI] [PubMed]
- 19.Adamson B, Cohen A, Estevez M, et al. Affordable Care Act (ACA) Medicaid expansion impact on racial disparities in time to cancer treatment. Plenary Sess ASCO Annu Meet. 2019.
- 20.Leung J, McKenzie S, Martin J, McLaughlin D. Effect of rurality on screening for breast cancer: a systematic review and meta-analysis comparing mammography. Rural Remote Health. 2014. [PubMed]
- 21.Obertova Z, Brown C, Holmes M, Lawrenson R. Prostate cancer incidence and mortality in rural men - A systematic review of the literature. Rural Remote Health. 2012. [PubMed]
- 22.Vakonaki E, Androutsopoulos VP, Liesivuori J, Tsatsakis AM, Spandidos DA. Pesticides and oncogenic modulation. Toxicology. 2013. doi: 10.1016/j.tox.2013.01.008. [DOI] [PubMed]
- 23.Tsoli M, Chatzellis E, Koumarianou A, Kolomodi D, Kaltsas G. Current best practice in the management of neuroendocrine tumors. Ther Adv Endocrinol Metab. 2018. doi: 10.1177/2042018818804698. [DOI] [PMC free article] [PubMed]
- 24.Zhou H, Zhang Y, Song Y, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Clin Res Hepatol Gastroenterol. 2017. doi: 10.1016/j.clinre.2017.02.008. [DOI] [PubMed]
- 25.Yao J, Garg A, Chen D, et al. Genomic profiling of NETs: A comprehensive analysis of the RADIANT trials. Endocr Relat Cancer. 2018. doi: 10.1530/ERC-18-0332. [DOI] [PubMed]
- 26.Scarpa A, Chang DK, Nones K, et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017. doi: 10.1038/nature21063. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


