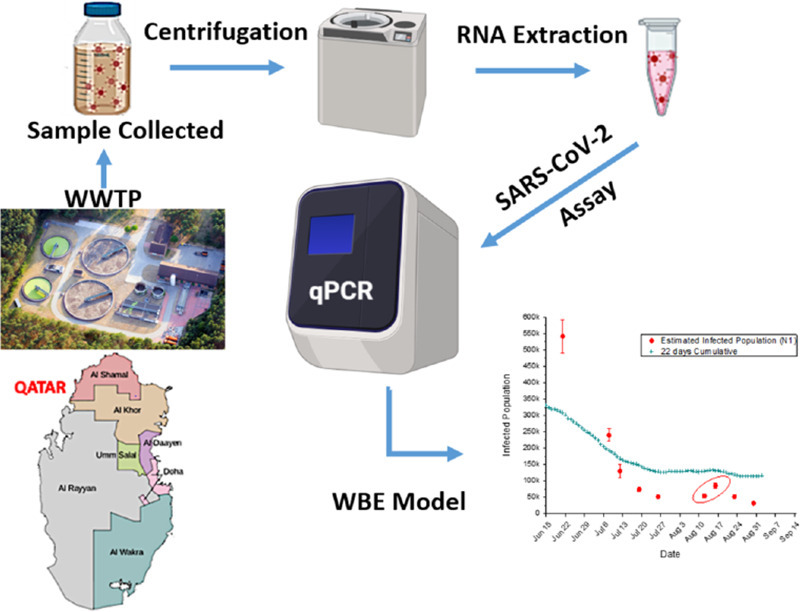
Abstract
Raw municipal wastewater from five wastewater treatment plants representing the vast majority of the Qatar population was sampled between the third week of June 2020 and the end of August 2020, during the period of declining cases after the peak of the first wave of infection in May 2020. The N1 region of the SARS-CoV-2 genome was used to quantify the viral load in the wastewater using RT-qPCR. The trend in Ct values in the wastewater samples mirrored the number of new daily positive cases officially reported for the country, confirmed by RT-qPCR testing of naso-pharyngeal swabs. SARS-CoV-2 RNA was detected in 100% of the influent wastewater samples (7889 ± 1421 copy/L – 542,056 ± 25,775 copy/L, based on the N1 assay). A mathematical model for wastewater-based epidemiology was developed and used to estimate the number of people in the population infected with COVID-19 from the N1 Ct values in the wastewater samples. The estimated number of infected population on any given day using the wastewater-based epidemiology approach declined from 542,313 ± 51,159 to 31,181 ± 3081 over the course of the sampling period, which was significantly higher than the officially reported numbers. However, seroprevalence data from Qatar indicates that diagnosed infections represented only about 10% of actual cases. The model estimates were lower than the corrected numbers based on application of a static diagnosis ratio of 10% to the RT-qPCR identified cases, which is assumed to be due to the difficulty in quantifying RNA losses as a model term. However, these results indicate that the presented WBE modeling approach allows for a realistic assessment of incidence trend in a given population, with a more reliable estimation of the number of infected people at any given point in time than can be achieved using human biomonitoring alone.
Keywords: SARS-CoV-2, COVID-19, Wastewater-based epidemiology (WBE), Outbreaks, Community, Health risks
Graphical abstract
1. Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has given rise to more than 91.6 million laboratory-confirmed infections and a large number of Coronavirus Disease (COVID-19) cases, in addition to over a million deaths globally as of January 2021 (World Health Organization, 2020). Qatar is an Arab country with an estimated population of 2.8 million people (Planning and Statistics Authority of Qatar 2020). The first SARS-CoV-2 community transmission case was recorded on March 6, 2020 with the source of infection being traced to a shared-accommodation setting of migrant workers (Abu-Raddad et al., 2020a; Kuwari et al., 2020). By October 29, 2020, 965,389 people had undergone RT-qPCR testing (34% of the population), with an infection rate that exceeded 50,000 laboratory-confirmed infections per million population, one of the highest worldwide (Abu-Raddad et al., 2020b; Jeremijenko et al., 2020). The positivity rate among those tested reached a high of 35.4% at the epidemic peak towards the end of May 2020 (Abu-Raddad et al., 2020a). Since then, the epidemic has declined rapidly at first before plateauing for several weeks at a constant incidence (Abu-Raddad et al., 2020a).
A national population-based survey was conducted in the first week of May 2020 and estimated the weighted SARS-CoV-2 RT-qPCR prevalence (for the total population of Qatar) at 14.9% (95% CI: 11.5–19.0%) (Abu-Raddad et al., 2020a). The survey found that 58.5% of those PCR positive did not report any symptoms in the preceding 14 days (Abu-Raddad et al., 2020a). A series of serological studies completed by October 2020 indicated that about half of the population has detectable SARS-CoV-2-specific antibodies. WBE may be of increased importance to identify the emergence of a second wave or surge in Qatar due to the young age profile of the population with only 2% being >60 years of age, the sociodemographic and other factors that contribute to asymptomatic and mild disease. During the first wave in Qatar, communities were identified where less than 15% of the antibody-positive subjects had a prior PCR positive diagnosis of SARS-CoV-2 infection. This indicates that there was a significant unidentified spread of SARS-CoV-2 in Qatar during the first wave.
Wastewater-Based Epidemiology (WBE) has been shown to have excellent potential for early detection of the outbreak of diseases and to be a useful tool for monitoring biomarkers of human health at the population level. Wastewater contains a diverse range of chemical and biological targets which gives incredibly detailed information about the population that contributes to it. However, the successful extraction from the matrix and subsequent analysis of specific targets can prove to be difficult. Many countries have taken appropriate steps in investing in WBE practice (Asghar et al., 2014; Feng et al., 2018). A working strategy aims for effective monitoring where WBE can help to detect and estimate the number of infected cases, followed by clinical testing to identify the affected people.
Many WBE studies have confirmed the detection of SAR-COV-2 in the influent of wastewater treatment plants and its significant correlation with the reported COVID-19 cases (Ahmed et al., 2020a; Medema et al., 2020). Viral loads ranging between 7.50E+02 and 3.40E+04 gene copies/L were detected in the influent of several wastewater treatment plants in the United Arab Emirates (Hasan et al., 2020). Another WBE study conducted in the region of Murcia (Spain) reported that 35 out of 42 wastewater influent samples were positive while all the tertiary water samples were tested negative (Randazzo et al., 2020). Analyses of wastewater samples collected from a wastewater plant in Ahmedabad, Gujarat, India indicated that all the samples were tested positive with an estimated maximum concentration of 3500 copies/L.
Viral RNA is found in nasal discharge, sputum, blood or feces (Huang et al., 2020) or urine (Kim et al., 2020). There are many reports of SARS-CoV-2 shedding in the human stool that have been documented in recent literature (Gao et al., 2020; Holshue et al., 2020). Using a WBE modeling approach, the number of people in the population infected with SARS-CoV-2 can be estimated based on: (i) concentration of SARS-CoV-2 RNA at the inlet of the wastewater treatment plant, (ii) volumetric flow rate of the wastewater treatment plant, (iii) fecal load, (iv) RNA shedding in the stool, and (v) RNA losses in the sewer pipe. The Fecal Load is the amount of feces generated per person. The reported range in the literature is 51–796 g feces/day/person at an average density of 1.06 g/mL (Hart and Halden, 2020; Rose et al., 2015). The RNA fecal shedding represents the number of RNA copies found in one gram of feces. The amount of RNA found in stool varies from patient to patient and varies within a single patient as the illness progresses. It has been reported that range (1000−10,000,000) RNA copies of SARS-CoV-2 can be found in a gram of feces, with a peak of more than 107 during the first week of the signs and symptoms of COVID-19 (Wölfel et al., 2020). Another study reported a median viral load of 105.1 and 103.9 copies/mL in COVID-19 patients with and without diarrhea respectively (Cheung et al., 2020). The RNA loss factor accounts for losses due to the degradation of the viral RNA over time, adsorption onto solids, and other factors related to losses during transit. Hart and Halden (2020) stressed the importance of incorporating temperature effect and sewer transit time in WBE models, as these factors govern the decay kinetics of SARS-CoV-2.
In order for COVID-19 WBE to provide a reliable early warning system that is fit for purpose, RNA recovery and detection methods must also be sensitive enough to quantify low viral concentrations in a wastewater sample. Several extraction methods have been reported in the literature such as centrifugal ultrafiltration (Medema et al., 2020), adsorption-extraction method using electronegative membranes (Ahmed et al., 2020b; Sherchan et al., 2020), aluminum hydroxide adsorption-precipitation method (Randazzo et al., 2020), and PEG concentration method with or without ultrafiltration (Ahmed et al., 2020a; Alpaslan Kocamemi et al., 2020; Kumar et al., 2020; Wu et al., 2020); the glycine buffered with PEG extraction concentration protocol was applied in this study. Murine hepatitis virus (MHV) enveloped virus was examined in raw wastewater samples, with a recovery rate ranging between 26.7 and 65.7% (Ahmed et al., 2020b). However, the recovery efficiency of enveloped SARS-CoV-2 may be different from that of other enveloped and non-enveloped enteric viruses (Kitajima et al., 2020). The total population served by the wastewater treatment plants (WWTPs) can be estimated by using published census data, WWTP design capacity, biomarkers, and/or the water quality parameters of wastewater. In this study, ammonium‑nitrogen (NH4 +-N), an indirect biomarker of urine, is used to estimate the total population served by each WWTP. Despite these challenges, this WBE approach coupled with the data generated can be broadly applied globally is significantly less expensive than large scale human biomonitoring campaigns (Hart and Halden, 2020). This would help in managing the pandemic and accelerating the global economic recovery.
Here, we report, for the first time, the presence of SARS-CoV-2 RNA fragments in municipal wastewater in the State of Qatar and we present an estimation of the number of infected people in the population by applying a WBE model. The WBE estimated numbers were found higher than the reported daily cases identified by clinical testing and indicated reasonable agreement with estimated numbers using a seroprevalence calculated clinical diagnosis ratio.
2. Material and methods
2.1. Sample collection and pretreatment step
Wastewater samples (n = 43) were collected from five WWTPs situated across the greater Doha area between 21st June and 30th August 2020. The locations of these plants are shown in Fig. S1. Twenty-four h refrigerated composite wastewater samples were collected in sterile 1 L glass bottles. The collected samples were heat-treated on-site at 56 °C for 30 min to minimize the risk of laboratory contamination and human infections while maintaining RNA integrity. To examine the effect of heat treatment duration on SARS-CoV-2 RNA integrity, two raw composite samples (WWTP2, WWTP3) were heated in a water bath at 56C° for 30 and 60 min. The heat-treated samples were transported on ice to the laboratory, arriving within 2 h, and processed within 24 h of sample collection (either RNA extracted and/or stored at −80 °C). Necessary precautions were taken and standard personal protective equipment (PPE) was used during sample collection and processing.
2.2. SARS-CoV-2 concentration
Samples were processed using the PEG extraction protocol as described previously (Ahmed et al., 2020b; Bibby and Peccia, 2013). Briefly, 25 mL of glycine buffer (0.05 M glycine, 3% Bovine Serum Albumin (BSA)) was added to 200 mL of wastewater to detach the virions particles from the organic matter, which binds to organic material. The mixture was incubated at 4 °C for 2 h with shaking at 200 rpm. The samples were then centrifuged at 4500 ×g for 30 min at 4 °C (without brake) to remove large debris and bacterial cells. After centrifugation, the supernatant was carefully transferred into a sterilized container. PEG 8000 (100 g/L) and NaCl (22.5 g/L) was added to the supernatant and dissolved by gentle shaking to avoid warming up the solution. The dissolved mixture was incubated overnight at 4 °C and 100 rpm agitation followed by centrifugation for 90 min (13,000 × g and 4 °C) without braking. After centrifugation, the sample was decanted carefully and the sample was centrifuged again for 10 min at 13,000 × g 4 °C with brake set to 3 (of 9). The resulting viral-containing pellet was eluted in 1 mL DNA/RNA Shield™ (Zymo Research, Irvine CA, USA Cat. No. R1100-250) and stored at −80 °C until further processing. All of the samples were processed in duplicate except for the July 19, 2020 batch which was processed in triplicate.
2.3. Extraction of viral RNA
Viral RNA was extracted from 200 μL of the concentrated wastewater sample using Quick-RNA Viral Kits (Zymo Research, Irvine CA, USA Cat. No. R1041) according to the manufacturer's protocol. The viral RNA was eluted in 30 μL of nuclease-free water and stored at −80 °C until the next step. All samples were extracted in duplicate.
2.4. SARS-CoV-2 detection, quantification, and normalization using RT-qPCR
The detection of SARS-CoV-2 in the extracted viral RNA was performed using SARS-CoV-2 (2019-nCoV) CDC qPCR Probe Assay Research Use Only (RUO) kit (Integrated DNA Technologies, IDT, Coralville, IA, USA Cat number 10006713) (Centers for Disease Control and Prevention, 2020; US Food and Drug Administration, 2020). Table 1 depicts the sequences of primers and probes used in the assay. SARS-CoV-2 (2019-nCoV) CDC RUO Plasmid Controls was used as the positive control (Integrated DNA Technologies, IDT, Coralville, IA, USA Cat number 10006625) and nuclease-free water as a negative control for RT-qPCR. Both the positive and negative controls were used in each run along with samples to ensure that the master mix, primers, probes and the machine were functioning well and the RNA elution water was free of N-gene amplicon contamination.
Table 1.
Oligonucleotide sequences of primers and probes used in this study (Centers for Disease Control and Prevention, 2020).
| Assay | Target gene | Primer/probe | Sequence (5′–3′) |
|---|---|---|---|
| CDC N1 | Nucleocapsid (N) | 2019-nCoV_N1-F | GACCCCAAAATCAGCGAAAT |
| 2019-nCoV_N1-R | TCTGGTTACTGCCAGTTGAATCTG | ||
| 2019-nCoV_N1-P | FAM-ACCCCGCATTACGTTTGGTGGACCa | ||
| CDC N2 | Nucleocapsid (N) | 2019-nCoV_N2-F | TTACAAACATTGGCCGCAAA |
| 2019-nCoV_N2-R | GCGCGACATTCCGAAGAA | ||
| 2019-nCoV_N2-P | FAM-ACAATTTGCCCCCAGCGCTTCAGa | ||
| CDC RP | Human RNase P | RNAse P-F | AGATTTGGACCTGCGAGCG |
| RNAse P-R | GAGCGGCTGTCTCCACAAGT | ||
| RNAse P-P | FAM–TTCTGACCTGAAGGCTCTGCGCGa |
FAM, 6-carboxyfluorescein;
All RT-qPCR amplifications were performed in 20 μL reaction mixtures using Luna Universal Probe One-Step RT-qPCR Kit (New England BioLabs, Ipswich, MA, USA Cat number E3006E). RT-qPCR assays were performed in a 96-well plate on an Applied Biosystems 7500 Fast Real-Time PCR Instrument (Applied Biosystems, CA, USA). The reactions were performed in 20 μL reaction mixtures each containing: 2.5 μL nuclease-free water, 1.5 μL of each combined primers and probe mix, 1 μL Luna WarmStart RT Enzyme Mix (20×), 10 μL Luna Universal Probe One-Step Reaction Mix and 5 μL extracted viral RNA. Cycling conditions: reverse transcription at 55 °C for 10 min, initial denaturation 95 °C for 1 min, denaturation & extension (10 s & 60 s) for 45 cycles. Instrument setting: detector (FAM) and Quencher (none). All RT-qPCR assays were duplicated.
Positive controls were used for RNA extraction. The controls for RNA extractions were conducted on 4 batches collected in July 2020 by running RT-qPCR using the human RNAP gene assay from the CDC set of assays (Table S1). This control is based on the assumption that wastewater will contain a certain amount of human RNA and allow us to ensure the full process did not regularly introduce inhibitors of RNA extraction or RT-qPCR. Any human RNA present in the wastewater goes through the full extraction process and positive results ensure that RNA extraction occurred sufficiently to allow viral RNA detection.
The amplification efficiencies (E) were calculated based on the equation: E = 10(−1/slope) − 1. Negative and positive controls were included in each qPCR run and all qPCR assays were performed in duplicate according to the guideline. The number of viral RNA copies/L in the samples was calculated based on the calibration curve (y = m(log10x) + c), as measured in wastewater using RT-qPCR assay. All of the extracted RNA samples were completely free from PCR inhibitors as determined by Sketa22 qPCR Assay and consequently used for downstream SARS-CoV-2 RT-qPCR analysis. The amplification efficiency percent (E%) of CDC N1 (2019-nCOV_N) was within the prescribed range (90–110%) of MIQE guidelines (Bustin et al., 2009), but the amplification (E %) of CDC N2 (2019-nCOV_N) assays was outside the recommended range. The correlation coefficient (R2) values for all assays were 1. The slope of the standard curves and Y-intercepts are shown in Table 2 .
Table 2.
RT-qPCR performance characteristics.
| Assay | RT-qPCR characteristics |
|||
|---|---|---|---|---|
| Efficiency (E) (%) | Linearity (R2) | Slope | Y-intercept | |
| CDC N1 | 95.70 | 1 | −3.4295 | 39.102 |
| CDC N2 | 81.26 | 1 | −3.8716 | 41.041 |
Viral genome sequencing library preparation was conducted with Paragon Genomics Cleanplex SARS-CoV-2 panel according to the manufacturers recommended protocol. Samples were sequenced on an Illumina MiSeq according to the manufacturer's recommended protocol with 2 × 150 bp read lengths and at least 10,000 reads per sample. FASTQ files were trimmed for quality and Paragon Genomics provided BED file of Primer information used to annotate primer regions. Sequences were aligned with BWA-MEM and at least 10× sequence coverage was required to call a region sufficiently covered.
2.5. Modeling methodology
The infected population (N) within the total population served by a WWTP can be estimated based on the measured RNA concentration and other parameters introduced in Section 1. If there were no person-to-person or day-to-day variations in the parameters, the infected population would be given by the equation:
| (1) |
where
CRNA = measured SARS-CoV-2 RNA concentration at the inlet of the WWTP (RNA copies/L)
F = Volumetric flow rate of the WWTP (L/day)
α = Fecal load (g/day/person)
β = Fecal shedding (viral copies/g)
γ= losses in the sewer
The RNA concentrations used in the present study were obtained from the RT-qPCR analysis, while volumetric flow rates of the wastewater plants were provided by the plant operators.
The total population (P) served by the WWTP can be estimated using the equation:
| (2) |
Here,
CNH4 = Measured ammonium concentration in the inlet of the WWTP (mg/L)
MNH4−N = Estimated average amount of daily NH4-N production per person (mg/day/person)
Parameters such as α and β have large variations as mentioned in Section 1 and Table 3 . This complicates the applicability and interpretation of Eqs. (1), (2). One can use Eq. (1) with the mean values of the parameters in the denominator to obtain an estimate of the infected population. However, because of the unavoidable variations, it is impossible to calculate the exact value of N; there will be a range of N values that can all lead to the measured RNA concentration. This range can be calculated using a statistical Monte-Carlo-Bayesian approach that is explained in detail in the supporting information. Importantly, the central limit theorem of probability theory allows us to avoid performing lengthy Monte-Carlo calculations and instead apply a simple formula to calculate the standard deviation in the infected population δN. The calculated value is expected to be accurate whenever the formula gives a value for δN that is significantly smaller than N.
Table 3.
Input parameters used in the modeling study.
| Symbol | Unit | Probability distribution | Mean | Other Details | Ref | |
|---|---|---|---|---|---|---|
| Estimated average amount of daily NH4-N production of each person | MNH4−N | mg/d/capita | Triangular | 6000 | Min:4000 Max:8000 |
(Zheng et al., 2017) |
| Flow rate of the wastewater plant | F | L/day | Normal | Table S2 | σ = 5% of the reading | |
| Measured ammonium concentration in the inlet of the wastewater plant | CNH4−N | mg/L | Normal | Table S2 | σ = 10% of the reading | |
| RNA copies at the inlet of the wastewater plant | CRNA | copies/L | – | Table S2 | ||
| Estimated Fecal Load | α | g/day/capita | Lognormal | 149 | Min = 51 g Max = 796 g σ = 95.0 |
(Rose et al., 2015) |
| Estimated RNA Fecal shedding | β | copies/g | Lognormal | 3.634 ∗ 106a | σ = 2.946 ∗ 107a Range: 103–109 |
(Zheng et al., 2020) |
Calculated based on the data reported by Zheng et al. (2020).
In our study, the sewer transit time is highly variable as some of the WWTPs are also served by tankers that transport wastewater from the districts and areas that are not connected to the sewage network. The determination of RNA losses in wastewater samples due to the myriad of factors listed earlier (e.g., adsorption, temperature, half-life, etc.) is difficult to estimate. Due to a lack of sufficient information, the RNA losses in the sewer were ignored (γ = 0) in this study, with the understanding that this approach can lead to an underestimation of the infected population. The total population served by the WWTPs was calculated based on Eq. (2) using NH4 +-N as a biomarker.
As the infected population excretes SARS-CoV-2 virus over a period of time which varies from person to person, the measured CRNA at the inlet a WWTP represents a cumulative concentration shed by patients that were infected several days or weeks before each sampling day. In this study, a cumulative estimate of 22 days is used as a reference based on a clinical study that reported the median duration of SARS-CoV-2 virus in the stool of infected patients as 22 days with an interquartile range between 17 and 31 days (Zheng et al., 2020). Assuming a typical delay in diagnosis of approximately 10 days, the number of people infected in the population on any given date was estimated by taking the sum of patients diagnosed on the day of sampling, 10 days prior to the sampling date, and 11 days after each sampling date. This allows the model to account for the patients already diagnosed and those not yet diagnosed but already positive within the population. The 22 days cumulative positive cases in Qatar were then corrected using a diagnosis ratio of 10% to include the infected population that was not captured as part of the reported daily new cases. The estimated infected population from the WBE mathematical model on any given sampling day was then compared with the corrected 22 days of cumulative SARS-CoV-2 positive cases. Statistical analysis was performed using GraphPad Prism and Origin Pro while mathematical modeling codes were written using Matlab.
3. Results and discussion
3.1. Characteristics and optimization of RT-qPCR assays
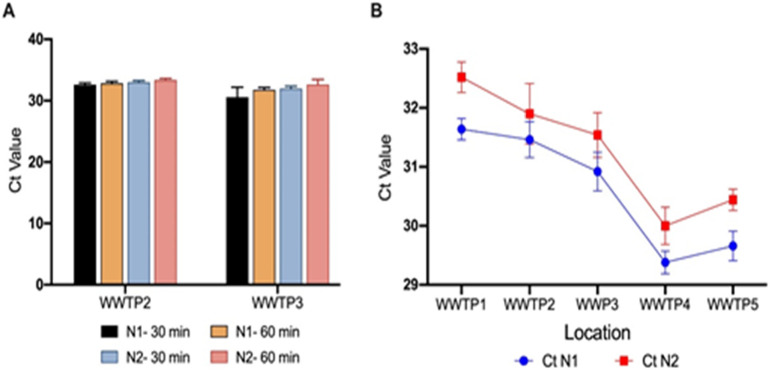
The SARS-CoV-2 RNA Nucleocapsid (N) genes were successfully amplified in the raw wastewater composite samples, where all the samples collected from the five major WWTPs in Qatar were SARS-CoV-2 positive with RT-qPCR assays using the Nucleocapsid (N) target gene (N1 and N2) assays. To minimize the human health risk during sample processing inside the laboratory, the collected samples were inactivated by heat exposure at the collection site. Heat pasteurization of two composite samples for 30 and 60 min showed comparable average Ct values for N1 and N2, indicating minimal variation in the viral RNA integrity between the two treatment times (Fig. 1A). WHO has recommended the heat treatment of 56 °C for SARS-CoV-1 virus inactivation (World Health Organization, 2003), and a 30-min heat inactivation at 60 °C was sufficient for a greater than 6 log decrease in SARS-CoV-1 viral infectivity in wastewater samples (Rabenau et al., 2005). At 56 °C, no infectious virus was detected within 30 min in nasopharyngeal and sera samples containing SARS-CoV-2 (Batéjat et al., 2020). Pastorino et al. (2020) reported that 56 °C for 30 min and 60 °C for 60 min did not affect significantly the number of detectable RNA copies in the clinical samples. Based on these results we identified that 30 min is sufficient for safety purposes without a significant loss in the viral RNA integrity.
Fig. 1.
A) Effects of heat treatment at 56 °C at 30 min and 60 min on the SARS-CoV-2 RNA integrity; B) Detection of SARS-CoV-2 RNA in wastewater samples (19th July 2020). Standard deviations shown in this figure were based on duplicate measurements.
The results of the RT-qPCR assay are consistent for both 2019-nCOV_N1 and 2019-nCOV_N2 assays. All RT-qPCR samples were positive and within the quantification level (Ct values below 35 cycles). As shown in Fig. 1B, the PEG concentration method used in this study produced consistent results with a low standard deviation. The Ct values of the 2019-nCoV marker (N1) of the wastewater samples collected from the five WWTPs on the 19th July 2020 were between 31.64 ± 0.18 and 29.38 ± 0.19 which corresponds to a range of 22,620–103,239 RNA copies/L. Ct values for the N2 marker were between 32.62 ± 0.26 and 30.00 ± 0.32 (24,052–108,080 RNA copies/L).
We have normalized the wastewater results across disparate conditions utilizing the CDC Human RNAP gene assay included in the CDC SARS-CoV-2 assays as described in the methodology section. The Human RNAP gene assay is used in control samples for the RNA extraction process, assuming a swab collects human cells and virus at the same time. In the case of wastewater, the assay could be used to detect the amount of human RNA in the wastewater and utilize that for normalization across conditions and extraction processes. The assumption would be that human RNA levels in wastewater correlate well to the number of people contributing to the volume of the wastewater samples collected. Initial results (Table S1) revealed that the RNAP Ct values were relatively constant in the samples tested here and so may serve well for comparing results across seasons, population sizes contributing to a collection, and different countries.
3.2. Concentration of SARS-CoV-2 RNA in wastewater samples
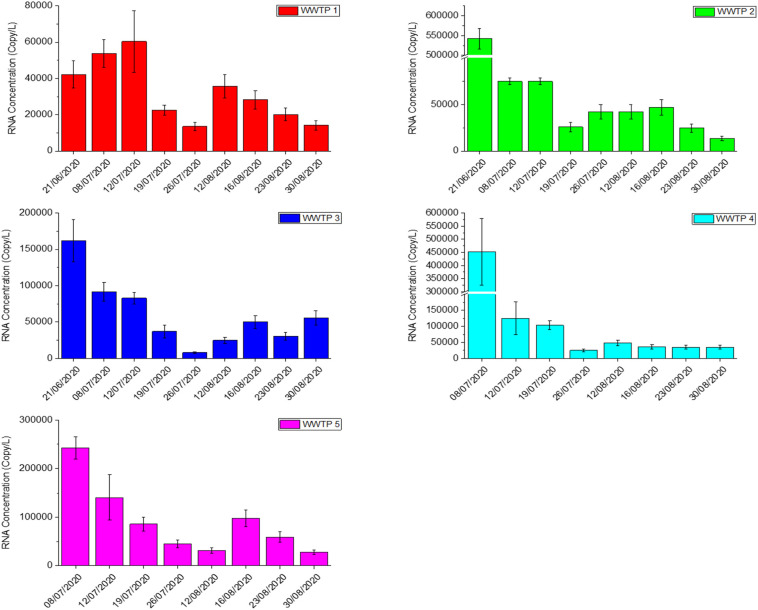
SARS-CoV-2 RNA (N1 and N2 assays) was detected in all influent wastewater samples (n = 43) collected from the five major WWTPs in Qatar (cumulative influent loading >760,000 m3 of wastewater per day) over the sampling period, between 21st June 2020 and 30th August 2020. The viral loads in all samples collected from 5 WWTPs during this period can be seen in Table S2. As shown in Fig. 2 , except for WWTP 1 all locations started to witness a decrease in SARS-CoV-2 viral load from late June. In spite of the initial increase of viral load for WWTP1 area until 12th July 2020, CRNA values based on the N1 assay decreased from 42,268 ± 7608 copies/L to 14,199 ± 2556 copies/L by the end of August (66.4% drop). As for WWTP2, a sharper decline can be observed where CRNA (N1) of WWTP2 reduced from 542,056 ± 25,775 copies/L to 13,313 ± 2396 copies/L which represents a fall of 97.6%. The lowest CRNA (N1) of WWTP3 was registered at the end of July 2020 where it dropped 161,877 ± 29,138 copies/L to 7880 ± 1420 copies/L. CRNA (N1) profile in WWTP4 dropped sharply after 8th July 2020 and reported an overall decrease of 92.4% by the end of August 2020. WWTP4 serves an industrial zone which was identified as one of the COVID-19 hotspots in Qatar. Proactive testing on the labor communities and other safety measures taken by local authorities were successful in controlling the rapid spread of COVID-19 in that particular area. This successful effort in containing COVID-19 can be correlated with the CRNA (N1) profile of WWT4 which became relatively constant towards the end of the study period. A declining trend was also observed in WWTP5 where CRNA (N1) decreased to 27,573 ± 4963 copies/L by the end of August. However, due to the variation of the flow-through between each sampled location over 24 h, comparing the viral load in gene copies/L should be avoided.
Fig. 2.
SARS-CoV-2 RNA concentration in the influent of five major WWTPs in Qatar. Standard deviations shown in the bar charts were based on duplicate measurements.
These sampling days coincide with the period where the number of positive cases in Qatar was declining after reaching a peak in late May 2020 (Abu-Raddad et al., 2020a). As the daily reported SARS-CoV-2 positive cases in Qatar decreased by over 66% between 21st June and the end of August 2020, declining CRNA trends were also observed in all the WWTPs by the end of the study period. For the CRNA counts based on the N2 assay, overall decreasing patterns were also observed albeit with a larger fluctuation between the readings. It is interesting to note that a momentary surge in the CRNA profile can be observed in almost all the WWTPs during the first two weeks of August 2020 which coincided with the rise in the daily reported SARS-CoV-2 positive cases in Qatar (circled in Fig. 3 ). This upswing was attributed due to the increase of social and family gatherings as well as reduced caution against COVID-19 transmission risks during national holidays that were declared in the first week of August 2020 in conjunction with the muted Eid Al Adha celebration (Qatar Tribune, 2020).
Fig. 3.
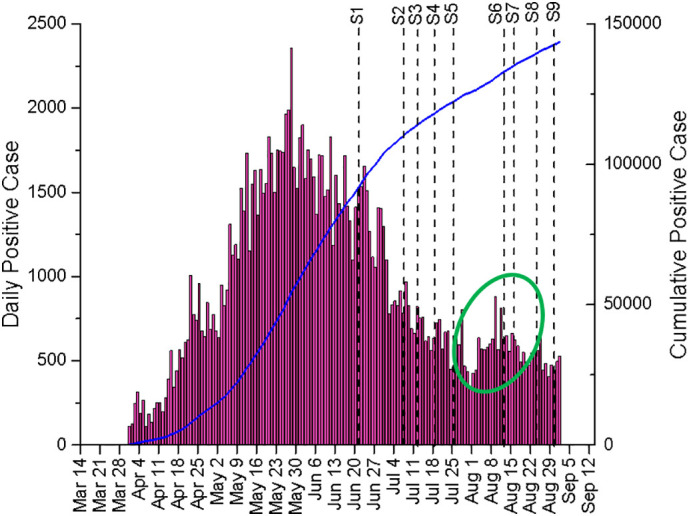
Daily and Cumulative SARS-CoV-2 positive cases as reported in Qatar from late March to August 2020. S1–9 represents the sampling days conducted between 21st June to 30th August 2020.
The dynamic CRNA profile of each WWTP could provide useful information in managing COVID-19 spread. For instance, while all other WWTPs displayed a decreasing trend from June to the end of July 2020, CRNA profile in WWTP1 was ascending prior to decline after mid-June. On the other hand, the CRNA profile of WWTP4 remained fairly constant after the initial decrease in mid-July 2020. These different profiles could potentially assist local authorities to deploy targeted approach and manage COVID-19 hot zones in the country by monitoring the CRNA profiles of each wastewater treatment plant. It is worth mentioning that identification of hot zone based on individual WWTP might not be straightforward in Qatar as some of the WWTPs were also served by sewage tankers that operate from various locations.
The CRNA values reported in this study are in a similar range to those reported by Sherchan et al. (2020) but are three to four orders higher than the concentration reported by (Ahmed et al., 2020a). Differences between studies in different regions can be attributed to many varied factors, such as the efficiency of recovery of RNA from the wastewater samples, the variability in the severity and shedding of virus from positive cases, losses in the wastewater samples, environmental conditions such as temperature, dry or wet season, and possibly other unknown parameters.
3.3. Prevalence estimate of SARS-CoV-2
Due to the small geographical area of the country, the number of daily new positive cases reported by the Ministry of Public Health, Qatar, represented the entire country and were not segregated according to the district. WWTPs in Qatar serve multiple districts, hence it is only possible to compare the daily positive cases against the total estimated infected population of the five WWTPs. The total population served by the WWTPs was estimated using ammonium as a biomarker (Eq. (2)). This calculation confirmed that these five WWTPs served the majority of the population in Qatar which is approximately 2.8 million (Planning and Statistics Authority of Qatar, 2020). The estimated total infected population calculated using the mathematical model (Table S3) is much higher than the daily reported positive cases, as has been the case in other studies (Wu et al., 2020). This is expected as the CRNA values capture both the diagnosed and undiagnosed cases, (a substantial number of the population are either asymptomatic or paucisymptomatic and may not be documented) (La Rosa et al., 2020). Indeed, a population-based survey in Qatar found that 58.5% of those PCR positive did not report any symptoms in the preceding 14 days.
A nation-wide seroprevalence study of craft and manual workers in Qatar has found that only 9.3% of those antibody-positive had a prior PCR laboratory-confirmed infection. This is still a higher diagnosis ratio than that reported for 15 other countries at 5.5% (Phipps et al., 2020), possibly because of the high testing rate in Qatar at 573,000 tests per million population, as of October 29, 2020, one of the highest worldwide (Abu-Raddad et al., 2020a, Abu-Raddad et al., 2020b; Jeremijenko et al., 2020). An epidemiologic mathematical modeling study of the Qatar epidemic estimated that 11.5% of infections have been diagnosed (Abu-Raddad et al., 2020a). Thus a diagnosis ratio of 10%, as informed by seroprevalence data and epidemiological modeling, is an appropriate comparator to determine the efficacy of WBE for monitoring of SARS-CoV-2 infection in the population (Abu-Raddad et al., 2020a).
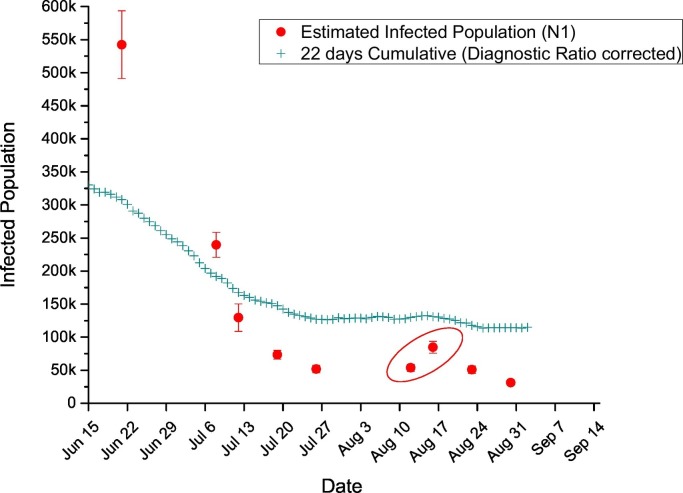
Fig. 4 compares the estimated infected population from the WBE model against the diagnosis ratio corrected 22 days of cumulative SARS-CoV-2 positive cases. There was a steady decline in the numbers until the first week of August 2020 where the estimated infected population dropped from 542,313 ± 51,159 to 51,752 ± 5594. Thereafter, a rise in the estimated infected population can be observed (circled in Fig. 4) where it coincided with the reported increase in the daily reported positive cases (circled in Fig. 3). As explained earlier, this increase could be attributed to the national Eid Al Adha celebration. As the reported positive cases started to fall after this momentary increase, the estimated infected population continued to decline until the end of the study period. Overall, the estimated infected population dropped approximately 17-fold from 542,313 ± 51,159 to 31,181 ± 3081 as the numbers of daily positive cases in Qatar fell by 66.4% between the third week of June and the end of August 2020.
Fig. 4.
Estimated infected population against the corrected 22 days of cumulative positive cases.
Our results did not indicate that SARS-CoV-2 had appeared earlier in the wastewater than the confirmed virological laboratory testing as reported by others (Medema et al., 2020; Trottier et al., 2020). Nemudryi et al. (2020) opined that the detection of SARS-CoV-2 RNA in wastewater could closely overlap with the virological laboratory test dates, with SARS-CoV-2 RNA detectable in the wastewater 5–8 days after infection. As hospitalization/clinical testing generally occurs between 3 and 9 days after the onset of the symptoms, there is a possibility for these events to coincide.
It was observed that the model tends to be underpredicting when the number of infected cases was lower in Qatar, when compared to the corrected 22 days of cumulative cases. During the period of this study, the number of SARS-CoV-2 cases in Qatar had passed the peak of the first wave and was in the decline phase. Losses in the RNA during in-sewer transport, sample processing and extraction are inevitable, and quantification of losses is difficult. A sizable portion of RNA can be lost in the primary sludge removed during influent pre-processing, with higher RNA concentrations found in the primary sludge than in the aqueous influent phase (Alpaslan Kocamemi et al., 2020; Westhaus et al., 2021). Less than ideal recovery of RNA during the sample processing can also be exacerbated when lower amounts of RNA are present. The huge variability in the viral shedding in the stool is another crucial factor that governs the accuracy of the estimation of the infected population (Wu et al., 2020). We applied a constant diagnosis rate throughout the sampling period, however the actual diagnosis rate may have varied due to testing capacity, conducting of wide-scale surveillance testing, and demographics of the population affected at any given time. Epidemiological modeling in Qatar indicated that the diagnosis rate increased steadily with time, explaining in part the differences seen in the trends of the 22-day cumulative cases and the estimated number of infections (Fig. 4).
4. Conclusion
The economic implications and practical limits of medical screening for the SARS-CoV-2 (COVID-19) have rapidly come into the spotlight on a global scale. Following promising results in early 2020, public health authorities in many countries have effectively implemented the use of wastewater-based epidemiology (WBE) as a potential tool for assessing and monitoring the spread of SARS-CoV-2. Based on the findings of this study, it can be concluded that the WBE approach is suitable for monitoring the trend in the numbers of infected people in Qatar. This approach was shown to be more reliable in identifying the true magnitude of infection circulating within the population than the RT-qPCR diagnostic testing using predominantly symptom presentation and contact tracing. An approach that relies on clinical swabbing only would have missed the vast majority of infections, as confirmed by antibody testing. This indicates that the WBE model developed allows for a realistic estimation of the number of infected people in a given population at a point in time and confirms for the first time in Qatar the capability of the wastewater-based modeling approach in monitoring trends in the spread of SARS-CoV-2 infection. With proper calibration, such as to account for the RNA losses in the sewer parameterized by the parameter γ, estimates of the number of infected population can be further improved. In short, WBE can assist and guide public health officials in a future SARS-CoV-2 outbreak.
CRediT authorship contribution statement
Jayaprakash Saththasivam: Methodology, Visualization, Formal analysis, Writing – original draft. Shimaa S. El-Malah: Methodology, Visualization, Formal analysis, Writing – original draft. Tricia A. Gomez: Methodology. Khadeeja Abdul Jabbar: Methodology. Reshma Remanan: Methodology. Arun K. Krishnankutty: Resources. Oluwaseun Ogunbiyi: Writing – original draft. Kashif Rasool: Methodology, Resources. Sahel Ashhab: Methodology, Writing – review & editing. Sergey Rashkeev: Methodology, Writing – review & editing. Meryem Bensaad: Resources. Ayeda A. Ahmed: Resources. Yasmin A. Mohamoud: Resources, Formal analysis. Joel A. Malek: Methodology, Formal analysis, Writing – review & editing. Laith J. Abu Raddad: Methodology, Writing – review & editing. Andrew Jeremijenko: Resources. Hussein A. Abu Halaweh: Resources. Jenny Lawler: Conceptualization, Resources, Methodology, Writing - review &editing, Supervision. Khaled A. Mahmoud: Conceptualization, Resources, Methodology, Writing - review &editing, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We acknowledge the financial support received from QEERI, HBKU, QF. We are grateful to the Public Works Authority in Qatar (ASHGHAL) and their sub-entities for facilitating sample collection & site treatment. The research forms part of the environmental testing pilot program launched by the Scientific Reference and Research Task Force (SSRTF) of the Ministry of Public Health (MoPH), Qatar. We are grateful to K. Machaca of WCM-Q, L. Dalton and R. Bertollini of MoPH for their support. We also thank QEERI HSSE, HBKU facility teams for ensuring the work is safely conducted. Open Access funding is provided by the Qatar National Library.
Editor: Damia Barcelo
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2021.145608.
Appendix A. Supplementary data
Supplementary material
References
- Abu-Raddad L.J., Chemaitelly H., Ayoub H.H., Kanaani Z.A., Khal A.A., Kuwari E.A., Butt A.A., Coyle P., Jeremijenko A., Kaleeckal A.H., Latif A.N., Owen R.C., Rahim H.F.A., Abdulla S.A.A., Kuwari M.G.A., Kandy M.C., Saeb H., Ahmed S.N.N., Romaihi H.E.A., Bansal D., Dalton L., Thani S.M.A., Bertollini R. Characterizing the Qatar advanced-phase SARS-CoV-2 epidemic. medRxiv. 2020;(2020) doi: 10.1101/2020.07.16.20155317. 07.16.20155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Raddad L.J., Chemaitelly H., Malek J.A., Ahmed A.A., Mohamoud Y.A., Younuskunju S., Ayoub H.H., Kanaani Z.A., Khal A.A., Kuwari E.A., Butt A.A., Coyle P., Jeremijenko A., Kaleeckal A.H., Latif A.N., Shaik R.M., Rahim H.F.A., Yassine H.M., Kuwari M.G.A., Romaihi H.E.A., Thani S.M.A., Bertollini R. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. medRxiv. 2020;(2020) doi: 10.1101/2020.08.24.20179457. 08.24.20179457. [DOI] [Google Scholar]
- Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O’Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpaslan Kocamemi B., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. 2020. SARS-CoV-2 Detection in Istanbul Wastewater Treatment Plant Sludges. [DOI] [Google Scholar]
- Asghar H., Diop O.M., Weldegebriel G., Malik F., Shetty S., El Bassioni L., Akande A.O., Al Maamoun E., Zaidi S., Adeniji A.J., Burns C.C., Deshpande J., Oberste M.S., Lowther S.A. Environmental surveillance for polioviruses in the global polio eradication initiative. J. Infect. Dis. 2014;210:S294–S303. doi: 10.1093/infdis/jiu384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batéjat C., Grassin Q., Manuguerra J.C., Leclercq I. Heat inactivation of the severe acute respiratory syndrome coronavirus 2. Journal of biosafety and biosecurity. 2021;3(1):1–3. doi: 10.1016/j.jobb.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibby K., Peccia J. Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environmental science & technology. 2013;47:1945–1951. doi: 10.1021/es305181x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L. 2009. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2020. CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel.
- Cheung K.S., Hung I.F.N., Chan P.P.Y., Lung K.C., Tso E., Liu R., Ng Y.Y., Chu M.Y., Chung T.W.H., Tam A.R., Yip C.C.Y., Leung K.-H., Fung A.Y.-F., Zhang R.R., Lin Y., Cheng H.M., Zhang A.J.X., To, K.K.W, Chan K.-H., Yuen K.-Y., Leung W.K. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 infections during Eid Al Adha more than those during Eid Al Fitr [WWW Document] Qatar Tribune. 2020. https://www.qatar-tribune.com/Latest-News/ArtMID/423/ArticleID/32364/covid-19-infections-during-eid-al-adha-more-than-those-during-eid-al-fitr accessed 10.2.20.
- Feng L., Zhang W., Li X. Monitoring of regional drug abuse through wastewater-based epidemiology—a critical review. Sci. China Earth Sci. 2018;61:239–255. doi: 10.1007/s11430-017-9129-x. [DOI] [Google Scholar]
- Gao Q.Y., Chen Y.X., Fang J.Y. 2019 novel coronavirus infection and gastrointestinal tract. J. Dig. Dis. 2020;21:125–126. doi: 10.1111/1751-2980.12851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart O.E., Halden R.U. Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci. Total Environ. 2020;730:138875. doi: 10.1016/j.scitotenv.2020.138875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S.W., Ibrahim Y., Daou M., Kannout H., Jan N., Lopes A., Alsafar H., Yousef A.F. Detection and quantification of SARS-CoV-2 RNA in wastewater and treated effluents: surveillance of COVID-19 epidemic in the United Arab Emirates. Science of The Total Environment. 2020:142929. doi: 10.1016/j.scitotenv.2020.142929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremijenko A., Chemaitelly H., Ayoub H.H., Abdulla M.A.H., Abou-Samra A.B., Ajmi J.A.A.A.A., Al-Ansari N.A.A., Kanaani Z.A., Khal A.A., Kuwari E.A., Al-Mohammed A., Molawi N.H.A.A., Naomi H.M.A., Butt A.A., Coyle P., Kahlout R.A.E., Gillani I., Kaleeckal A.H., Masoodi N.A., Thomas A.G., Nafady-Hego H., Latif A.N., Shaik R.M., Younes N.B.M., Rahim H.F.A., Yassine H.M., Kuwari M.G.A., Romaihi H.E.A., Thani S.M.A., Bertollini R., Abu-Raddad L.J. Evidence for and level of herd immunity against SARS-CoV-2 infection: the ten-community study. medRxiv. 2020;(2020) doi: 10.1101/2020.09.24.20200543. 09.24.20200543. [DOI] [Google Scholar]
- Kim J.-M., Kim H.M., Lee E.J., Jo H.J., Yoon Y., Lee N.-J., Son J., Lee Y.-J., Kim M.S., Lee Y.-P. Detection and isolation of SARS-CoV-2 in serum, urine, and stool specimens of COVID-19 patients from the Republic of Korea. Osong Public Health and Research Perspectives. 2020;11:112. doi: 10.24171/j.phrp.2020.11.3.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;139076 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwari H.M.A., Rahim H.F.A., Abu-Raddad L.J., Abou-Samra A.-B., Kanaani Z.A., Khal A.A., Kuwari E.A., Marri S.A., Masalmani M.A., Romaihi H.E.A., Thani M.H.A., Coyle P.V., Latif A.N., Owen R., Bertollini R., Butt A.A. Epidemiological investigation of the first 5685 cases of SARS-CoV-2 infection in Qatar, 28 February–18 April 2020. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-040428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Iaconelli M., Mancini P., Bonanno Ferraro G., Veneri C., Bonadonna L., Lucentini L., Suffredini E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020;736:139652. doi: 10.1016/j.scitotenv.2020.139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Monthly Figures on Total Population [WWW Document], 2020. URL https://www.psa.gov.qa/en/statistics1/StatisticsSite/pages/population.aspx (accessed 10.2.20).
- Nemudryi A., Nemudraia A., Wiegand T., Surya K., Buyukyoruk M., Cicha C., Vanderwood K.K., Wilkinson R., Wiedenheft B. Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. Cell Reports Medicine. 2020;1:100098. doi: 10.1016/j.xcrm.2020.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino B., Touret F., Gilles M., de Lamballerie X., Charrel R.N. Evaluation of heating and chemical protocols for inactivating SARS-CoV-2 [Preprint] bioRxiv. 2020;10:11.036855. doi: 10.3390/v12060624. 2020 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps S.J., Grafton R.Q., Kompas T. Estimating the true (population) infection rate for COVID-19: a backcasting approach with Monte Carlo methods (preprint) Epidemiology. 2020 doi: 10.1101/2020.05.12.20098889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabenau H., Cinatl J., Morgenstern B., Bauer G., Preiser W., Doerr H. Stability and inactivation of SARS coronavirus. Med. Microbiol. Immunol. 2005;194:1–6. doi: 10.1007/s00430-004-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. 2020;181:115942. doi: 10.1016/j.watres.2020.115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose C., Parker A., Jefferson B., Cartmell E. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015;45:1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L.M., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743:140621. doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier J., Darques R., Ait Mouheb N., Partiot E., Bakhache W., Deffieu M.S., Gaudin R. Post-lockdown detection of SARS-CoV-2 RNA in the wastewater of Montpellier, France. One Health. 2020;10:100157. doi: 10.1016/j.onehlt.2020.100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Food and Drug Administration Emergency Use Authorization [WWW Document]. Emergency Use Authorization. 2020. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
- Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751:141750. doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Müller M.A., Niemeyer D., Jones T.C., Vollmar P., Rothe C., Hoelscher M., Bleicker T., Brünink S., Schneider J., Ehmann R., Zwirglmaier K., Drosten C., Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2003. First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network. World Health Organization, Geneva, Switzerland. http://www. who. int/csr/sars/survival_2003_05_04/en/index. html.
- World Health Organization, 2020. Coronavirus disease (COVID-19): situation report, 209 [WWW Document]. Coronavirus disease (COVID-19): situation report, 209. URL https://pesquisa.bvsalud.org/portal/resource/pt/who-333897 (accessed 10.5.20).
- Wu F., Zhang J., Xiao A., Gu X., Lee W.L., Armas F., Kauffman K., Hanage W., Matus M., Ghaeli N., Endo N., Duvallet C., Poyet M., Moniz K., Washburne A.D., Erickson T.B., Chai P.R., Thompson J., Alm E.J. SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. mSystems. 2020;5 doi: 10.1128/mSystems.00614-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Q.-D., Lin J.-G., Pei W., Guo M.-X., Wang Z., Wang D.-G. Estimating nicotine consumption in eight cities using sewage epidemiology based on ammonia nitrogen equivalent population. Sci. Total Environ. 2017;590–591:226–232. doi: 10.1016/j.scitotenv.2017.02.214. [DOI] [PubMed] [Google Scholar]
- Zheng S., Fan J., Yu F., Feng B., Lou B., Zou Q., Xie G., Lin S., Wang R., Yang X., Chen W., Wang Q., Zhang D., Liu Y., Gong R., Ma Z., Lu S., Xiao Y., Gu Y., Zhang J., Yao H., Xu K., Lu X., Wei G., Zhou J., Fang Q., Cai H., Qiu Y., Sheng J., Chen Y., Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: retrospective cohort study. BMJ. 2020:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material