Abstract
Function and the independent performance of daily activities are of critical importance to older adults. Although function was once a domain of interest primarily limited to geriatricians, transdisciplinary research has demonstrated its value across the spectrum of medical and surgical care. Nonetheless, integrating a functional perspective into medical and surgical therapeutics has yet to be implemented consistently into clinical practice. This article summarizes the presentations and discussions from a workshop, “Embedding/Sustaining a Focus on Function in Specialty Research and Care,” held on January 31 to February 1, 2019. The third in a series supported by the National Institute on Aging and the John A. Hartford Foundation, the workshop aimed to identify scientific gaps and recommend research strategies to advance the implementation of function in care of older adults. Transdisciplinary leaders discussed implementation of mobility programs and functional assessments, including comprehensive geriatric assessment; integrating cognitive and sensory functional assessments; the role of culture, environment, and community in incorporating function into research; innovative methods to better identify functional limitations, techniques, and interventions to facilitate functional gains; and the role of the health system in fostering integration of function. Workshop participants emphasized the importance of aligning goals and assessments and adopting a team science approach that includes clinicians and frontline staff in the planning, development, testing, and implementation of tools and initiatives. This article summarizes those discussions.
Keywords: function, physical performance, cognition, sensory health, health system, implementation science
INTRODUCTION
A significant proportion of health care for older adults, defined as aged 65 years and older, is delivered by clinicians in internal medicine or surgery specialties.1,2 Although specialty care focuses primarily on disease-based episodes, function is a primary concern for older adults, and functional evaluations have proven valuable in guiding patient-centered care.3,4 Thus, function is clinically valuable not only in geriatrics, but across medical and surgical specialties.4 However, despite the emerging consensus, specialty research rarely captures functional outcomes, and specialty clinical guidelines and quality metrics rarely mention function.
A Transdisciplinary View of Function
Function, defined as the performance of routine activities in a person’s daily life, encompasses not only physical performance and mobility, but also cognitive and sensory performance.4 Function reflects the consequences of multimorbidity; affects an individual’s need for support from family, community, and institutions; engages with patient preferences, societal values, and costs; and informs shared decision-making in health care. Physical, cognitive, and sensory function comprise a wide range of abilities and are heterogeneous, particularly among older adults. Declining functional status and disability, defined as declines in individuals’ ability to perform activities related to basic needs and expected roles within their environment,4,5 can predict several adverse effects across medical and surgical specialties and care settings.
Barriers to Implementation of Functional Metrics and Interventions
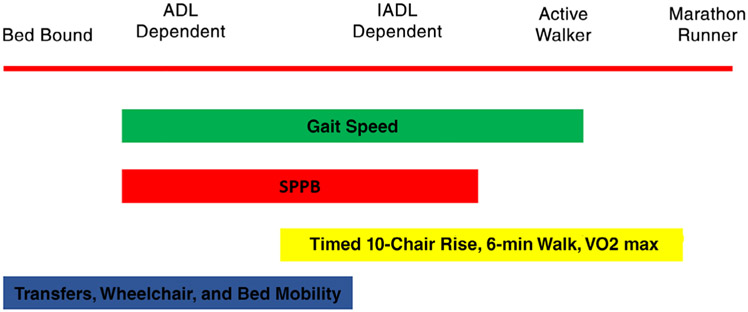
Interventions to prevent disability or functional decline, improve function, accelerate recovery, or prevent complications associated with chronic conditions have been widely tested in subpopulations among adults aged 65 years and older. To date, they have shown modest benefits.6 However, these studies and meta-analyses have been hindered by functional heterogeneity among older adults, difficulties in enrolling older participants, and challenges with study reproducibility. Moreover, functional assessment itself is challenged by heterogeneity among measures with respect to terminology, content, methods, and scoring.7 This lack of consensus represents an initial research gap. Collaborative research to standardize measures and definitions would facilitate not only data pooling and comparisons across studies, but also implementation of functional assessments in practice. Such research could promote a unified understanding of how ability is distributed across the population: the abilities measured by functional assessments are incremental and ordered (Figure 1), and several measures have floor and ceiling effects that require targeting with selected or sequential assessments in a given population.
Figure 1.
The broad spectrum of mobility function affects the choice of measures. Clinicians and researchers must determine where their patients fit on this spectrum. ADL, activity of daily living; IADL, instrumental ADL; SPPB, Short Performance Physical Battery, VO2 max, maximal oxygen uptake.
The implementation and maintenance of evidence-based functional assessments and interventions requires alignments and collaborations among researchers, clinicians, health systems, and payers to promote sustainable strategies and processes. The John A. Hartford Foundation and the Institute for Healthcare Improvement, along with the American Hospital Association and the Catholic Health Association, have partnered to lead the “Age-Friendly Health Systems” (AFHS)8 movement, focusing on the importance of the “4 Ms”: What Matters, Medications, and—most notably for a focus on function—Mobility and Mentation. Several health systems have already implemented AFHS initiatives, fostering collaboration and communication across the silos of geriatrics and specialty care.
A Workshop Series on Function
The National Institute on Aging and the John A. Hartford Foundation have sponsored three workshops to engage leaders, innovators, clinicians, and researchers in geriatrics, gerontology, and the medical and surgical subspecialties and identify priority areas for research on and translation of function into specialty research and care. The first characterized mechanisms and pathways of function distinct from illness.3 The second addressed the integration of function into clinical research and the validity of function as both a predictor and an outcome in defining therapeutic goals and treatment.4 This article summarizes the third workshop, “Embedding/Sustaining a Focus on Function in Specialty Research and Care,” which was held January 31 to February 1, 2019, in Pentagon City, Virginia. The aims of this workshop, which was divided into four part areas, were to discuss the current evidence base in implementing functional assessments and interventions, identify best practices and barriers in implementing these assessments to guide specialty care, and identify next steps in clinical and implementation research. This summary and the accompanying recommendations aim to inform future collaborative research and implementation efforts among clinical leaders and researchers.
PART I: PHYSICAL FUNCTION: ASSESSING AND PROMOTING MOBILITY
Low mobility, defined as bed rest or bed-to-chair activity, is common among hospitalized adults.9-12 It has been associated with increased length of stay (LOS) and declines in activities of daily living (ADLs).13-15 Early mobilization improves muscle strength, hospital LOS, functional outcomes, and quality of life, with few adverse effects.16 Despite recognition of benefits, implementation of in-hospital mobility programs has faced patient-level barriers, such as illness, comorbidity, or older adults’ perception of risk; treatment-related barriers, such as hospital devices and activity orders; and institutional barriers, such as staffing patterns, environments encouraging bed rest, and an emphasis on falls prevention.17,18
Further barriers to implementation may arise in settings such as intensive care units (ICUs) or after surgery, where functional goals compete with acute-care needs. The need for functional intervention in these settings has been well described; more than half of older adults surviving an ICU stay have poorer functional status or die within 30 days.19 Following discharge, intervening events, such as hospitalization, increase the risk for long-term disability.20
Early mobilization programs in the ICU use multidisciplinary teams to mobilize patients, regardless of age, devices, or level of consciousness, and can improve muscle strength and mobility status at discharge, as well as number of days alive and out of the hospital following discharge.21,22 One such program is the STEPS-ICU program at Yale-New Haven Hospital, which incorporates an assessment of mobility and functional limitations into a program of progressive mobility for medical ICU (MICU) patients. Program implementation has set “activity as tolerated” as a default within the electronic health record (EHR), tracks outcomes with mobility dashboards, and promotes a mobility culture that includes all members of the multidisciplinary team in the MICU. Among surgical patients aged 70 years and older, the level and speed of functional improvement following surgery depends on the presence or absence of disability before surgery.23 On the basis of findings from previous trials of nonsurgical older patients,24 “prehabilitation” before surgery can improve postsurgical functional outcomes among vulnerable, older patients.25 Likewise, early, goal-directed mobilization in the surgical ICU can shorten LOS and improve functional mobility at the time of discharge.26 Mobility has typically not been encouraged in these settings; however, data from these and related studies27-29 serve as a strong evidence base for future studies of implementation strategies for physical function and mobility in hospital settings.
Among outpatients, mobility is often low and declines further with age among patients with specific advanced comorbid conditions, such as end-stage renal disease (ESRD)30; most patients initiating dialysis are inactive.31 Walking disability affects a substantial proportion of these patients32 and has been associated with hospitalizations and loss of ADLs.33 As early as 1980, a randomized controlled trial showed that patients on dialysis could benefit from exercise.34 Yet, few interventions focus on improving functional outcomes in patients with ESRD. Functional declines are similarly predictable among older adults with cardiovascular disease (CVD), with serious prognostic implications amidst a high incidence of age-related cardiac hospitalizations for acute myocardial infarction (particularly as many develop posthospitalization syndrome)35,36 and progressive, chronic conditions, such as heart failure, valvular disease, atrial fibrillation, and peripheral arterial disease. Although cardiac rehabilitation may address functional recovery among vulnerable older adults with CVD, enrollment tends to be low, particularly for those who have become deconditioned and disabled as a result of their disease and cardiovascular treatments.37 Cardiac rehabilitation precepts do not include comprehensive geriatric assessment (CGA) and may not engage with geriatric complexities, such as multimorbidity, frailty, and cognitive decline. Similarly, although cardiac rehabilitation aims to improve cardiorespiratory fitness (CRF). older and/or bedbound patients may first need to address more rudimentary functional capacities, such as balance and strength, to initiate CRF goals. Trials such as Modified Application of Cardiac Rehabilitation for Older Adults (ClinicalTrials.gov identifier NCT03922529), which aims to infuse geriatric concepts into cardiac rehabilitation, will test best implementation practices, including options for rehabilitation site.
As noted in the second workshop, gaps in the clinical trials literature still exist. The degree to which low mobility reflects disease severity or multimorbidity is unclear. In addition, with the notable exception of the LIFE Study,38 few interventions to promote mobility have been tested in randomized clinical trials. Heterogeneity among tools further complicates testing and implementation of both assessments and interventions. A critical next step in the research pathway will be to test which elements of function, such as gait speed, would prove most feasible and acceptable for implementation. Recommendations for next steps in research for implementation of physical function assessment and intervention in both inpatient and outpatient environments are listed in Table 1.
Table 1.
Recommendations for Implementation Science to Improve Functional Assessments and Interventions in Specialty Clinical Settings
| Physical function and mobility |
|
| Cognitive and sensory function |
|
| Across functional domains |
|
| Innovation and implementation science: engaging the healthcare system |
|
Abbreviations: CAD, coronary artery disease; ESRD, end-stage renal disease.
PART II: COGNITIVE AND SENSORY FUNCTION: COGNITION, VISION, AND HEARING
Sensory loss, multimorbidity, and cognitive impairment all increase with older age and are interconnected. Age and multimorbidity jointly contribute to a higher risk for mild cognitive impairment or dementia among individuals with two or more chronic conditions.39
Among Americans, many vision impairments are easily correctable; millions live with uncorrected refractive errors or operable cataracts.40 Yet, limited research exists on testing implementation of visual assessments in clinical practice. Distance visual acuity is the most common measure used in screening for vision loss, but vision comprises several domains, and an individual with normal visual acuity measures might have function-limiting impairments in other domains. This presents a considerable challenge for incorporating vision assessment into research protocols, and for assessing best strategies for implementation. Few standard vision assessment protocols have been implemented, and those designed for individuals with cognitive impairment are rare. Vision loss has also been associated with cognitive decline and risk for dementia.41-45
Hearing loss can lead to cognitive fatigue and brain remodeling45 and has been associated with nearly a twofold risk of dementia (risk ratio = 1.94).46,47 Each 25-dB increase (worsening) of the hearing threshold equates to a 6- or 7-year increase in cognitive age.48 Thus, hearing loss is considered a potentially modifiable risk factor for dementia.47 Few studies over the past 20 years have assessed associations between hearing acuity and communication quality.49 Similarly to vision, although many hearing impairments can be improved, hearing loss is undertreated. As little as 30% of distributed hearing aids are used regularly; barriers include stigma and difficulty with use.50 Assessments, such as the Hearing Handicap Inventory for the Elderly,51,52 can predict whether patients will use a hearing aid,52-54 but the instrument may lack broad sociocultural relevance. Studies are assessing sensory impairments as modifiable aspects of cognitive impairment to elevate sensory health within patients’ own and national health priorities.40,54,55 A key next step for researchers will include assessing barriers, facilitators, and processes for integrating sensory assessments and aides within clinical practice overall, and cognitive assessments in particular.
As with vision and hearing, cognition is not a single entity, but a multistage, multidetermined set of processes reflecting relatively independent but interacting cognitive domains.56 Thus, assessing cognition itself is complex, which factors into impediments to routine implementation of cognitive testing.57-59 Cognitive screening measures differ from cognitive assessments. Cognitive screening tests inform referral, management, counseling, and therapeutic interventions.60 As a screening test, the focus is on reliability, sensitivity, specificity, brevity, and ease of administration and scoring. In contrast, cognitive assessments focus on multiple specific cognitive domains; they are sensitive, with fewer ceiling effects. Their sensitivity in monitoring disease progression or treatment effects depends on the number of cognitive domains assessed and the relationship of these domains to the underlying pathophysiology. Although cognitive screens aim to detect cognitive impairments quickly, cognitive assessments can distinguish among the different cognitive profiles of various dementias. Despite a plethora of testing options, health system, patient-level, and community barriers have prevented a sophisticated standard-of-care approach impractical beyond specialty dementia care.58,61
Adding to the complexity of cognitive assessment is its reliance on sensory inputs. Removing visual and auditory items from the Montreal Cognitive Assessment substantially reduces its sensitivity.62,63 Further, sensory adjustments lead to improved performance in auditory verbal memory.64 The number of chronic conditions a person has also increases the complexity of cognitive assessment, making it difficult to diagnose and treat cognitive impairment in patients with multimorbidities. Cognitive evaluations of older adults with multimorbidities therefore may benefit from a detailed assessment, drawing from the principles of the CGA, and include not only traditional cognitive, laboratory, and imaging assessments, but also a detailed medical history, medication history, and understanding of the person’s environment. Facilitators to implementation may include innovative approaches, such as telemedicine, community health partnerships, and health system navigators, to address sensory and cognitive health and facilitate their integration into chronic care. In addition, cognitive and physical performance assessments that account for sensory status will provide a deeper understanding of each interrelated construct. Implementation of cognitive assessments that are not confounded by sensory impairments may be balanced with the recognition that a sensory impairment will interact with cognitive function in real-world tasks; therefore, the choice of cognitive or sensory assessments will depend on the target population, study question, and study protocol. Interdisciplinary collaboration with experts in sensory assessments can guide optimal measurements and testing environments for a target population. Recommendations for next research steps in cognitive and sensory function are listed in Table 1.
PART III: FUNCTION IS PERSON CENTERED: THE IMPORTANCE OF CULTURAL, ENVIRONMENTAL, AND COMMUNITY CONTEXT
The interpretation of functional “norms” is heterogeneous across cultures, environments, and communities. For example, differences in preferences and perceptions of ideal body shape between White individuals and Black or Hispanic individuals arise partly from cultural differences. These differences can influence engagement in behaviors like physical activity and may contribute to differences in the prevalence of obesity and diabetes mellitus.65,66 Likewise, assessments, responses to interventions, and internal motivations can vary based on environment, culture, and community. Individuals from racial and ethnic minority populations might be more skeptical of behavioral interventions because of perceptions about weakness or a lack of faith. Even when patients are motivated to engage in functional interventions, their ability to do so depends on their environment. Such heterogeneity can interfere with the standardization of assessments and interventions. Thus, researchers should consider how functional and behavioral health interventions can intersect with cultural and community norms and the environment in which an individual lives.67,68 Recommendations for enhancing cultural and community contributions in research are in Table 1.
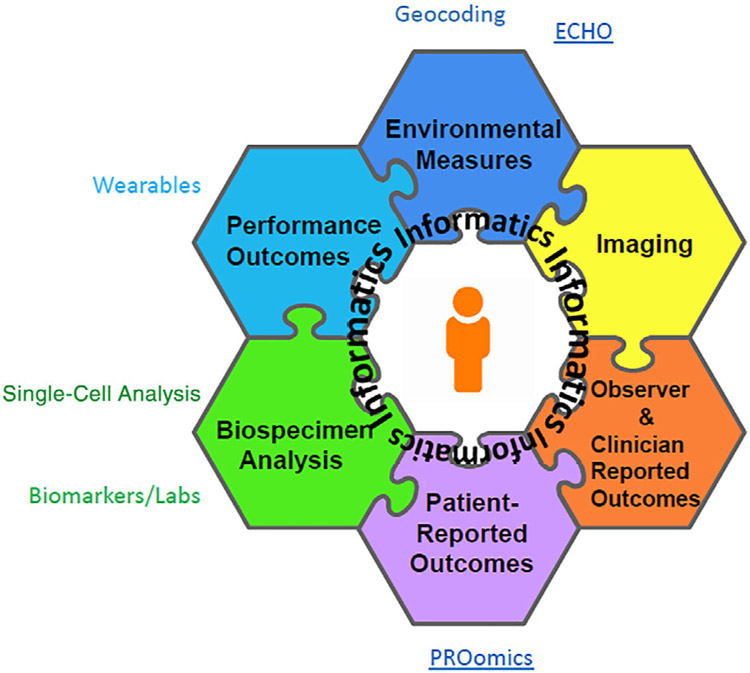
Because function is specific to an individual person, research into implementation would benefit from incorporating person-centered perspectives. Health systems and implementation research can include patient-reported outcomes (PROs) as well as outcomes measured in the clinic; further research can integrate PROs with biological, genetic, and clinical data points (Figure 2). Clinical care and industry are incorporating measures from the National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS®); these may prove a facilitator to collecting additional functional data.
Figure 2.
Patient-reported outcomes (PROs) are as important to include in informatics as other measures. The Patient-Reported Outcomes Measurement Information System (PROMIS®), which enables computer-adaptive testing, facilitates the integration of PROs with -omics approaches in research.69 Figure developed by Dr James Witter and shared with his permission. ECHO refers to the Environmental influences on Child Health Outcomes (ECHO) Program; Labs=laboratory studies.
Such measures can be tracked through the EHR or a custom platform. For example, the University of Rochester’s Validated Outcomes in Clinical Experience system leverages PROMIS measures in computer-adaptive testing to assess physical, psychological, and social health. The orthopedics department at the University of Rochester has shown that PROMIS physical function scores correlate with GAITRite velocity scores among orthopedic surgical patients70; that PROMIS can track improvements in depression, pain interference, and physical function among patients with musculoskeletal conditions71; and that preoperative PROMIS scores can predict postoperative success in foot and ankle patients.71,72
PART IV: DISRUPTIVE INNOVATION IN THE HEALTHCARE SYSTEM: LEADING SYSTEMS CONDUCIVE TO GERIATRIC CARE
Sustained integration of physical, cognitive, and sensory assessments and interventions into specialty research and practice will require implementation and dissemination evidence that incorporate both person-centered and health system priorities. Despite the rapid advancement of medical knowledge, less than 1% of new knowledge is translated into practice, and translation of that knowledge takes at least 17 years with excessive costs.73 The success rate for implementation is only 15%, and the current implementation paradigm often ignores a significant step in the discovery-to-delivery translational cycle: assuring the existence of market demand for new interventions.74
Thus, in addition to engaging with strong evidence, feasibility, and acceptability, researchers seeking to assess implementation best practices would do well to consider the value proposition for preventing declines in patients’ function and mobility to stakeholders within health systems, payers, and other entities, as a means of creating sustainable change. For example, a business case to include functional assessments in specialty care would note Centers for Medicare & Medicaid Services payment codes relevant to those assessments. The AFHS movement, with support from the John A. Hartford Foundation and the Institute for Healthcare Improvement, has provided guidance for developing business case for clinical implementation of a function focus when communicating with health system leaders.8 Further research can extrapolate on cost savings, healthcare utilization rates, and most effective implementation strategies at a policy, health system, local environment, and personal level.
Several models have successfully incorporated function into specialty care by aligning goals and motivations with those of the health system. Yale-New Haven Hospital implemented STEPS-ICU as a quality-improvement program, rather than a research study, and has created a sustainable change in care processes by obtaining buy-in from the multidisciplinary team, empowering key stakeholders to serve as equal partners in the program’s leadership, and leveraging the EHR to change activity defaults and track outcomes. Likewise, the University of Alabama at Birmingham (UAB) is embedding its safe-mobility protocol into its hospital unit microsystems and workflows as a quality-improvement program to promote function and mobility. In the first pilot test on an orthopedic surgery unit, this initiative increased the proportion of patients mobilized out of bed without the need for additional personnel or costs.75 The development of tools that incorporate functional assessments into the EHR and clinical care can also help to embed and sustain a focus on function. Further study on best methods for implementation is needed to expand these beyond single success stories to a sustained integration into clinical care; recommendations are listed in Table 1.
Pragmatic clinical trials, embedded research, implementation science, and multidisciplinary team approaches will also promote the incorporation of function into specialty care. In the Learning Health System concept,76 evidence is implemented rapidly into practice and practice generates new evidence for continuous improvement and research dissemination. Kaiser Permanente Washington Health Research Institute, which participates in the NIH Common Fund Pragmatic Trials Collaboratory, exemplifies this concept and emphasizes testing implementation of functional components.
Interdisciplinary and interprofessional relationships also have been established to develop and test novel assessment tools and processes that include function. Such collaborations include frontline staff who will use these processes, emphasize listening and communication, increase trust, and clarify roles, with an awareness of interprofessional involvement in integration and implementation. In developing its protocol, UAB emphasized an interdisciplinary, interprofessional team approach to hardwire care processes promoting function and mobility at the system level. Strategies included a geriatric professional development and quality improvement program that promotes mobility and is available to all hospital employees, the use of frontline staff as experts in eliminating unnecessary tasks, and an accessible and transparent mobility process and outcome measures dashboard that hospital unit teams can use to monitor performance. Mobility outcomes are also reported to hospital leaders in a format that aligns this work with system strategic priorities, such as LOS and fall rates. Likewise, the Aurora Health System communicated with patient and family advisory teams, as well as with various clinicians and paramedics, to develop a functional status checklist and tracker that compiles information into a simple, brief, and real-time quality report teams can use when discussing the needs of older patients in medical and surgical units.77 Characterizing these maintenance efforts may build the evidence base for best practices for implementation on a broader level.
More hospitals are adopting geriatric emergency departments, which feature age-friendly structural modifications and interventions to address function. These include nonslip, no-glare floors; thicker mattress pads to reduce the risk for pressure ulcers; vision and hearing enhancements; and the incorporation of geriatric care processes and protocols into usual emergency department care.78,79 Clinicians in the Mount Sinai geriatric emergency department worked with programmers to leverage the EHR to create templates, track boards, and order sets, and they have incorporated geriatric assessments, such as Mount Sinai’s Identification of Seniors at Risk protocol and mobility assessments, into routine workflows.
All these efforts are consistent with more widespread initiatives to incorporate function into care. Implementation research to incorporate function into specialty care should also emphasize scalability and sustainability from the start. For example, agile implementation74 facilitates the rapid, efficient, scalable, and sustainable implementation of evidence-based services in the real world, drawing from behavioral economics, business, engineering, and computer science.
FUTURE DIRECTIONS
Researchers and clinicians considering next steps for studying implementation may benefit from reflecting on their target population’s functional range fits within the broad spectrum of function (Figure 1); consistent, explicit engagement with these considerations will both guide their choice of accepted, standardized measures and build an evidence base for the interrelationship among functional assessments. Using existing measures, such as gait speed, that are easily performed and interpreted when focusing on implementation at the point of care may facilitate the measure of function as an outcome or quality measure. Broad incorporation of widely accepted tools, such as gait speed, could fuel research into both the impact of various health states and stressors on gait speed, and the functional outcomes of pragmatic interventions.
Embedding and sustaining a focus on function in specialty care will require an exploration of the policy, environments, and outer and inner setting of health systems,74,80 engaging systematic approaches to implementation and dissemination science. Although several presentations at this workshop highlighted a team approach to streamline workflows and accommodate functional assessments, the possibility of overburdening clinicians and frontline staff continues to be a concern and significant target for assessment before implementation. In addition, the scalability and sustainability of functional measurements or interventions may be addressed from the start,81 a feature common in behavioral interventions that would translate well to research in function. Specific research questions and recommendations to promote the integration of function into specialty care are listed in Table 1.
Incorporating a focus on function into specialty care will also require assessments and interventions engaging patient and caregiver perspectives. Patient education materials about function and disability can encourage mobility. Tailored interventions can improve motivation based on what matters most to patients, including their cultural perspectives and attitudes. Interdisciplinary teams, including physical and occupational therapists, can serve as resources and potential collaborators. The rise of implementation and team science uniquely enables geriatrics researchers and clinical leaders to collaborate with interprofessional, interdisciplinary teams to establish sustainable methods for testing and implementing functional assessments and interventions. These efforts will highlight function as “What Matters” for older adults.
ACKNOWLEDGMENTS
We thank National Institute on Aging (NIA) staff Susan Zieman, MD, PhD, and Molly V. Wagster, PhD, for their significant and meaningful contributions to planning and running this workshop and to the overall grant planning; these efforts were critical to the workshop’s success. The participation of these individuals or the materials should not be interpreted as representing the official viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health, or the NIA, except where noted. The authors, serving as the writing group for the contributors to and participants of the “Workshop on Embedding/Sustaining a Focus on Function in Specialty Research and Care,” wish to thank the following speakers and moderators:
Jamy Ard, Wake Forest Baptist Health.
Cynthia J. Brown, University of Alabama at Birmingham; Birmingham/Atlanta Veterans Affairs Geriatric Research, Education, and Clinical Center.
Joshua Chodosh, New York University; Freedman Center for Aging, Technology, and Cognitive Health; VA Harbor Healthcare System.
Janice D. Crist, University of Arizona.
Lauren Ferrante, Yale School of Medicine.
Kellie Flood, University of Alabama at Birmingham.
Terry Fulmer, John A. Hartford Foundation.
Thomas M. Gill, Yale School of Medicine.
Ula Hwang, Mount Sinai and James J. Peters Veterans Affairs Medical Center.
Lyndon Joseph, National Institute on Aging.
Nancy Kutner, Emory University School of Medicine.
Eric B. Larson, Kaiser Foundation Health Plan of Washington; Kaiser Permanente Washington Health Research Institute.
Shari Ling, Centers for Medicare & Medicaid Services.
Michael Malone, Aurora Health Care.
Esther Oh, Johns Hopkins University School of Medicine.
Natalie Phillips, Concordia University; Center for Research on Language, Mind, and Brain; Lady Davis Institute for Medical Research.
Heather Whitson, Duke School of Medicine and Durham VA GRECC.
James Witter, National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Financial Disclosure: The workshop and this article were supported by a grant from the National Institute on Aging (NIA) at the National Institutes of Health (NIH) (grant U13AG040938), with matching funds from the John A. Hartford Foundation (grant 2016-0048). K.C. receives funding from the NIA (K76 AG059986), Implementing Frailty in Primary Care: Implementation of an EMR-Based Frailty Index. D.L.F. receives funding from NIA (R01 AG060499-01) for the trial Modified Application of Cardiac Rehabilitation for Older Adults. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the NIH, NIA, or the John A. Hartford Foundation.
Footnotes
Conflict of Interest: K.E.C.: none.
M.B.: none.
L.F.: none.
D.L.F.: none.
J.G. serves as a member of the Pharmacy & Therapeutics Committee of United Healthcare.
K.P.H.: none.
F.M.: none.
T.R.: none.
S.S.: none.
M.Y.: none.
K.E.S.: none.
Sponsor’s Role: The content is solely the responsibility of the authors. Sponsors had no role in the design or preparation of the article. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the NIH, NIA, or the John A. Hartford Foundation.
REFERENCES
- 1.Lee AG, Burton JA, Lundebjerg NE. Geriatrics-for-specialists initiative: an eleven-specialty collaboration to improve care of older adults. J Am Geriatr Soc. 2017;65(10):2140–2145. [DOI] [PubMed] [Google Scholar]
- 2.Committee on the Future Health Care Workforce for Older Americans Board on Health Care Services. Retooling for an Aging America: Building the Health Care Workforce Report of the Institute of Medicine of the National Academies. Washington, DC: The National Academies Press; 2008. [PubMed] [Google Scholar]
- 3.Kritchevsky SB, Forman DE, Callahan K, et al. Pathways, contributors, and correlates of functional limitation across specialties: workshop summary. J Gerontol A Biol Sci Med Sci. 2019;74(4):534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.High KP, Zieman S, Gurwitz J, et al. Use of functional assessment to define therapeutic goals and treatment. J Am Geriatr Soc. 2019;67(9):1782–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ustun TB, Chatterji S, Kostanjsek N, et al. Developing the World Health Organization disability assessment schedule 2.0. Bull World Health Organ. 2010;88(11):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cherubini A, Bernabei R, Ferrucci L, Marchionni N, Studenski S, Vellas B. Clinical Trials in Older Adults. West Sussex, England: John Wiley & Sons; 2016. [Google Scholar]
- 7.Walston J, Robinson TN, Zieman S, et al. Integrating frailty research into the medical specialties-report from a U13 conference. J Am Geriatr Soc. 2017;65(10):2134–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fulmer T, Mate KS, Berman A. The age-friendly health system imperative. J Am Geriatr Soc. 2018;66(1):22–24. [DOI] [PubMed] [Google Scholar]
- 9.Zisberg A, Syn-Hershko A. Factors related to the mobility of hospitalized older adults: a prospective cohort study. Geriatr Nurs. 2016;37(2):96–100. [DOI] [PubMed] [Google Scholar]
- 10.Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013;68(3):331–337. [DOI] [PubMed] [Google Scholar]
- 11.Fisher SR, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Early ambulation and length of stay in older adults hospitalized for acute illness. Arch Intern Med. 2010;170(21):1942–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57(9):1660–1665. [DOI] [PubMed] [Google Scholar]
- 13.Kalisch BJ, Lee S, Dabney BW. Outcomes of inpatient mobilization: a literature review. J Clin Nurs. 2014;23(11–12):1486–1501. [DOI] [PubMed] [Google Scholar]
- 14.Brown CJ, Foley KT, Lowman JD Jr, et al. Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: a randomized clinical trial. JAMA Intern Med. 2016;176(7):921–927. [DOI] [PubMed] [Google Scholar]
- 15.Hastings SN, Sloane R, Morey MC, Pavon JM, Hoenig H. Assisted early mobility for hospitalized older veterans: preliminary data from the STRIDE program. J Am Geriatr Soc. 2014;62(11):2180–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mundy LM, Leet TL, Darst K, Schnitzler MA, Dunagan WC. Early mobilization of patients hospitalized with community-acquired pneumonia. Chest. 2003;124(3):883–889. [DOI] [PubMed] [Google Scholar]
- 17.Hoyer EH, Brotman DJ, Chan KS, Needham DM. Barriers to early mobility of hospitalized general medicine patients: survey development and results. Am J Phys Med Rehabil. 2015;94(4):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown CJ, Williams BR, Woodby LL, Davis LL, Allman RM. Barriers to mobility during hospitalization from the perspectives of older patients and their nurses and physicians. J Hosp Med. 2007;2(5):305–313. [DOI] [PubMed] [Google Scholar]
- 19.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175(4):523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill TM, Gahbauer EA, Murphy TE, Han L, Allore HG. Risk factors and precipitants of long-term disability in community mobility: a cohort study of older persons. Ann Intern Med. 2012;156(2):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43(2):171–183. [DOI] [PubMed] [Google Scholar]
- 22.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stabenau HF, Becher RD, Gahbauer EA, Leo-Summers L, Allore HG, Gill TM. Functional trajectories before and after major surgery in older adults. Ann Surg. 2018;268(6):911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347(14):1068–1074. [DOI] [PubMed] [Google Scholar]
- 25.Wynter-Blyth V, Moorthy K. Prehabilitation: preparing patients for surgery. BMJ. 2017;358:j3702. [DOI] [PubMed] [Google Scholar]
- 26.Schaller SJ, Anstey M, Blobner M, et al. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016; 388(10052):1377–1388. [DOI] [PubMed] [Google Scholar]
- 27.Growdon ME, Shorr RI, Inouye SK. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med. 2017;177(6): 759–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent non-pharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mudge AM, Banks MD, Barnett AG, et al. CHERISH (collaboration for hospitalised elders reducing the impact of stays in hospital): protocol for a multi-site improvement program to reduce geriatric syndromes in older inpatients. BMC Geriatr. 2017;17(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Loon I, Hamaker ME, Boereboom FTJ, et al. A closer look at the trajectory of physical functioning in chronic hemodialysis. Age Ageing. 2017;46 (4):594–599. [DOI] [PubMed] [Google Scholar]
- 31.Johansen KL, Chertow GM, Kutner NG, Dalrymple LS, Grimes BA, Kaysen GA. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78(11):1164–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray A, Solid C. The burden of walking disability in U.S. dialysis and CKD patients. https://www.usrds.org/2008/pres/15U_asn08_walking_disability.pdf. Accessed February 12, 2019. [Google Scholar]
- 33.Kutner NG, Zhang R, Huang Y, Painter P. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: a US renal data system special study. Am J Kidney Dis. 2015;66(2):297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldberg AP, Hagberg J, Delmez JA, et al. The metabolic and psychological effects of exercise training in hemodialysis patients. Am J Clin Nutr. 1980;33 (7):1620–1628. [DOI] [PubMed] [Google Scholar]
- 35.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandesara PB, Lambert CT, Gordon NF, et al. Cardiac rehabilitation and risk reduction: time to "rebrand and reinvigorate.". J Am Coll Cardiol. 2015;65(4):389–395. [DOI] [PubMed] [Google Scholar]
- 37.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653–1662. [DOI] [PubMed] [Google Scholar]
- 38.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vassilaki M, Aakre JA, Cha RH, et al. Multimorbidity and risk of mild cognitive impairment. J Am Geriatr Soc. 2015;63(9):1783–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Academies of Sciences Engineering and Medicine. Making Eye Health a Population Health Imperative. Washington, DC: The National Academies Press; 2016. [PubMed] [Google Scholar]
- 41.Chen SP, Bhattacharya J, Pershing S. Association of vision loss with cognition in older adults. JAMA Ophthalmol. 2017;135(9):963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swenor BK, Wang J, Varadaraj V, et al. Vision impairment and cognitive outcomes in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2019;74(9):1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng DD, Swenor BK, Christ SL, West SK, Lam BL, Lee DJ. Longitudinal associations between visual impairment and cognitive functioning: the Salisbury Eye Evaluation Study. JAMA Ophthalmol. 2018;136(9): 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies-Kershaw HR, Hackett RA, Cadar D, Herbert A, Orrell M, Steptoe A. Vision impairment and risk of dementia: findings from the English longitudinal study of ageing. J Am Geriatr Soc. 2018;66(9):1823–1829. [DOI] [PubMed] [Google Scholar]
- 45.Fischer ME, Cruickshanks KJ, Schubert CR, et al. Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc. 2016;64 (10):1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peelle JE. Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. 2018;39(2):204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734. [DOI] [PubMed] [Google Scholar]
- 48.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore longitudinal study of aging. Neuropsychology. 2011;25(6):763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen JM, Blustein J, Weinstein BE, et al. Studies of physician-patient communication with older patients: how often is hearing loss considered? a systematic literature review. J Am Geriatr Soc. 2017;65(8):1642–1649. [DOI] [PubMed] [Google Scholar]
- 50.Yueh B, Collins MP, Souza PE, et al. Long-term effectiveness of screening for hearing loss: the screening for auditory impairment—which hearing assessment test (SAI-WHAT) randomized trial. J Am Geriatr Soc. 2010;58 (3):427–434. [DOI] [PubMed] [Google Scholar]
- 51.Ventry IM, Weinstein BE. The hearing handicap inventory for the elderly: a new tool. Ear Hear. 1982;3(3):128–134. [DOI] [PubMed] [Google Scholar]
- 52.Knudsen LV, Oberg M, Nielsen C, Naylor G, Kramer SE. Factors influencing help seeking, hearing aid uptake, hearing aid use and satisfaction with hearing aids: a review of the literature. Trends Amplif. 2010;14 (3):127–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng JH, Loke AY. Determinants of hearing-aid adoption and use among the elderly: a systematic review. Int J Audiol. 2015;54(5):291–300. [DOI] [PubMed] [Google Scholar]
- 54.Goman AM, Lin FR. Hearing loss in older adults - from epidemiological insights to national initiatives. Hear Res. 2018;369:29–32. [DOI] [PubMed] [Google Scholar]
- 55.Todd J, Whitson HE, Marshall EC. Eye and vision health for tomorrow: from recommendations to coordinated action. JAMA Ophthalmol. 2019; 137:208–211. [DOI] [PubMed] [Google Scholar]
- 56.Perry W, Lacritz L, Roebuck-Spencer T, et al. Population health solutions for assessing cognitive impairment in geriatric patients. Arch Clin Neuropsychol. 2018;33(6):655–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boustani M, Peterson B, Hanson L, Harris R, Lohr KN, U.S. Preventive Services Task Force. Screening for dementia in primary care: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003; 138(11):927–937. [DOI] [PubMed] [Google Scholar]
- 58.Boustani M, Callahan CM, Unverzagt FW, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20(7):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patnode C, Perdue L, Rossom R, et al. Screening for cognitive impairment in older adults: an evidence update for the U.S. Preventive Services Task Force. JAMA. 2020;328:764–785. 10.1001/jama.2019.22258. [DOI] [PubMed] [Google Scholar]
- 60.Janssen J, Koekkoek PS, Moll van Charante EP, Jaap Kappelle L, Biessels GJ, Rutten G. How to choose the most appropriate cognitive test to evaluate cognitive complaints in primary care. BMC Fam Pract. 2017;18(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fowler NR, Campbell NL, Pohl GM, et al. One-year effect of the Medicare annual wellness visit on detection of cognitive impairment: a cohort study. J Am Geriatr Soc. 2018;66(5):969–975. [DOI] [PubMed] [Google Scholar]
- 62.Wittich W, Phillips N, Nasreddine ZS, Chertkow H. Sensitivity and specificity of the Montreal Cognitive Assessment modified for individuals who are visually impaired. J Vis Impair Blind. 2010;104:360–368. [Google Scholar]
- 63.Al-Yawer F, Pichora-Fuller MK, Phillips NA. The Montreal Cognitive Assessment after omission of hearing-dependent subtests: psychometrics and clinical recommendations. J Am Geriatr Soc. 2019;67:1689–1694. [DOI] [PubMed] [Google Scholar]
- 64.Wong CG, Rapport LJ, Billings BA, Ramachandran V, Stach BA. Hearing loss and verbal memory assessment among older adults. Neuropsychology. 2019;33(1):47–59. [DOI] [PubMed] [Google Scholar]
- 65.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016; 315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang M Trends in the prevalence of diabetes among U.S. adults: 1999-2016. Am J Prev Med. 2018;55(4):497–505. [DOI] [PubMed] [Google Scholar]
- 67.Crist JD, Escandon-Dominguez S. Identifying and recruiting Mexican American partners and sustaining community partnerships. J Transcult Nurs. 2003;14(3):266–271. [DOI] [PubMed] [Google Scholar]
- 68.Crist JD, Kim SS, Pasvogel A, Velazquez JH. Mexican American elders’ use of home care services. Appl Nurs Res. 2009;22(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witter JP. The promise of patient-reported outcomes measurement information system-turning theory into reality: a uniform approach to patient-reported outcomes across rheumatic diseases. Rheum Dis Clin North Am. 2016;42(2):377–394. [DOI] [PubMed] [Google Scholar]
- 70.Papuga MO, Beck CA, Kates SL, Schwarz EM, Maloney MD. Validation of GAITRite and PROMIS as high-throughput physical function outcome measures following ACL reconstruction. J Orthop Res. 2014;32(6):793–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Papuga MO, Dasilva C, McIntyre A, Mitten D, Kates S, Baumhauer JF. Large-scale clinical implementation of PROMIS computer adaptive testing with direct incorporation into the electronic medical record. Health Syst (Basingstoke). 2018;7(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho B, Houck JR, Flemister AS, et al. Preoperative PROMIS scores predict postoperative success in foot and ankle patients. Foot Ankle Int. 2016;37(9): 911–918. [DOI] [PubMed] [Google Scholar]
- 73.Brownson RC, Kreuter MW, Arrington BA, True WR. Translating scientific discoveries into public health action: how can schools of public health move us forward? Public Health Rep. 2006;121(1):97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boustani M, van der Marck M, Adams N, et al. Developing the agile implementation playbook for integrating evidence-based health care services into clinical practice. Acad Med. 2019;94(4):556–561. [Google Scholar]
- 75.Booth KA, Simmons EE, Viles AF, et al. Improving geriatric care processes on two medical-surgical acute care units: a pilot study. J Healthc Qual. 2019;41(1):23–31. [DOI] [PubMed] [Google Scholar]
- 76.Greene SM, Reid RJ, Larson EB. Implementing the learning health system: from concept to action. Ann Intern Med. 2012;157(3):207–210. [DOI] [PubMed] [Google Scholar]
- 77.Malone ML, Vollbrecht M, Stephenson J, Burke L, Pagel P, Goodwin JS. AcuteCare for Elders (ACE) tracker and e-Geriatrician: methods to disseminate ACE concepts to hospitals with no geriatricians on staff. J Am Geriatr Soc. 2010;58(1):161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hartocollis A For the elderly, emergency rooms of their own. New York Times; 2012. http://www.nytimes.com/2012/04/10/nyregion/geriatric-emergency-units-opening-at-us-hospitals.html. Accessed February 25, 2019. [Google Scholar]
- 79.Hwang U, Morrison RS. The geriatric emergency department. J Am Geriatr Soc. 2007;55(11):1873–1876. [DOI] [PubMed] [Google Scholar]
- 80.Onken L, Carroll K, Shoham V, Cuthbert B, Riddle M. Reenvisioning clinical science: Unifying the discipline to improve the public health. Clinical Psychological Science. 2014;2:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.NIH stage model for behavioral intervention. https://www.nia.nih.gov/research/dbsr/nih-stage-model-behavioral-intervention-development.