Abstract
Proteasome inhibitor–based strategies hold promise in transplant but have yielded varying results. Carfilzomib, a second-generation proteasome inhibitor, may possess advantages over bortezomib, the first-generation proteasome inhibitors. The purpose of this study was to evaluate the safety, toxicity, and preliminary efficacy of carfilzomib in highly HLA-sensitized kidney transplant candidates. Renal transplant candidates received escalating doses of carfilzomib followed by plasmapheresis (group A) or an identical regimen with additional plasmapheresis once weekly before carfilzomib dosing. Thirteen participants received carfilzomib, which was well tolerated with most adverse events classified as low grade. The safety profile was similar to bortezomib desensitization; however, neurotoxicity was not observed with carfilzomib. Toxicity resulted in permanent dose reduction in 1 participant but caused no withdrawals or deaths. HLA antibodies were substantially reduced with carfilzomib alone, and median maximal immunodominant antibody reduction was 72.8% (69.8% for group A, P = .031, 80.1% for group B, P = .938). After depletion, rebound occurred rapidly and antibody levels returned to baseline between days 81 and 141. Bone marrow studies revealed that approximately 69.2% of plasma cells were depleted after carfilzomib monotherapy. Carfilzomib monotherapy–based desensitization provides an acceptable safety and toxicity profile while leading to significant bone marrow plasma cell depletion and anti-HLA antibody reduction.
Keywords: alloantibody, clinical research/practice, clinical trial, desensitization, histocompatibility, immunosuppression/immune modulation, kidney transplantation/nephrology, panel reactive antibody (PRA), plasma cells, translational research/science
1 |. BACKGROUND
Sensitization to HLA through pregnancy, blood transfusions, or transplant remains one of the most significant barriers to transplant. This sensitization excludes many potential donors, thereby increasing waiting times and, in those patients who are transplanted, driving a significantly increased risk of antibody-mediation rejection (AMR).1,2 Approximately 40% of kidney transplant recipients in the United States are considered sensitized to HLA.3 However, commonly used pretransplant therapies (intravenous immune globulin [IVIG], plasmapheresis, and rituximab) have limited and transient effects on HLA antibody levels and are associated with significant AMR rates. Importantly, these therapies do not deplete the cellular source of HLA antibody production—plasma cells (PCs).4-7 More than 13 years ago, we hypothesized that HLA antibody elimination could be achieved via PC targeting using proteasome inhibitors (PIs), drugs that are highly effective at depleting malignant PCs in multiple myeloma.8 Our initial experience with PI-based PC therapy targeted AMR that was refractory to traditional therapies with IVIG and plasmapheresis.9,10 With increasing experience, we found that late and early AMR rejection therapy differed in response to PI therapy, which indicated that therapeutic resistance in late AMR was conferred by long-lived niche-resident PC.11 In addition, our experience with bortezomib-based desensitization indicated that, despite significant reductions in HLA antibodies, rebound was commonly observed and treatment was particularly limited by peripheral neuropathy.12 Therefore, we sought to evaluate the safety, toxicity, and effectiveness of second-generation PIs.
Carfilzomib is a second-generation irreversible PI that is an expoxyketone nonboronated agent.13 This irreversible nature has led to more profound and long-lasting proteasome inhibition in multiple myeloma cells, whereas the avoidance of boronation has substantially improved the toxicity profile of this PI and reduced off-target effects.13 We hypothesized that carfilzomib would lead to significant bone marrow (BM) PC depletion and reductions in circulating HLA antibody levels in highly sensitized kidney transplant candidates, with a potentially improved safety and toxicity profile.
2 |. MATERIAL S AND METHODS
2.1 |. Study design
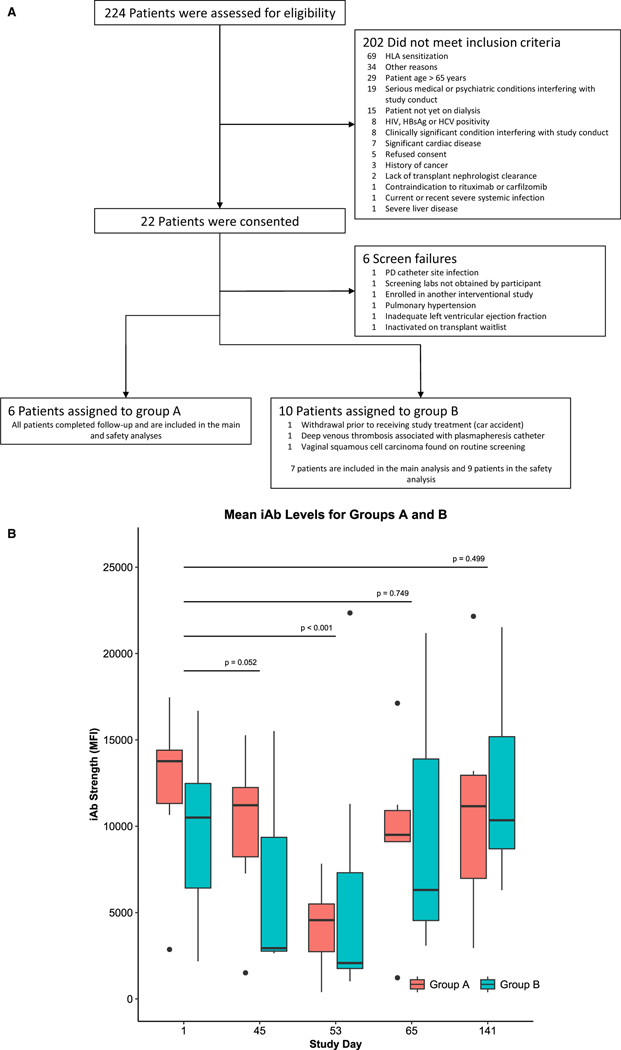
This study is a prospective, nonrandomized, iterative trial with adaptive enrollment and up to 196 days of follow-up. It is registered at ClinicalTrials.gov as NCT02442648, has been approved by the University of Cincinnati Institutional Review Board (approval number 2014-0577), and was conducted in accordance with the Declaration of Helsinki. Once participants provided informed consent, planned enrollment was consecutive (ie, enrollment was to be completed in each treatment group before initiating enrollment in the subsequent treatment group) in 4 predefined treatment groups, with a target total enrollment of 32 patients. Total enrollment in a particular group was not predefined, but rather adaptive, with a minimum of 5 patients and a maximum of 8 patients per group. Adaptive enrollment was based on a predefined Bayesian statistical product (BSP) that considered both absolute treatment effect and interparticipant variability. Treatment effect was defined as the reduction in immunodominant antibody (iAb). The BSP was defined as the percent change in mean fractional iAb reduction from participant n to participant n + 1 multiplied by the range of the 90% confidence interval (CI) in the fractional iAb (FiR) reduction for all participants enrolled within the treatment group [(%Δ mean FiRn->n + 1) × (range CI 90% FiR n -> n + 1)]. We arbitrarily determined that a BSP ≤ 0.02 (the mean change in iAb reduction multiplied by the range of the 90% CI resulting in <2% variation) would provide adequate confidence of the characterization of treatment effect and variability in this population. The BSP was calculated at the time of primary efficacy endpoint assessment, which was 48 hours after the last session of plasmapheresis, after 2 cycles of carfilzomib therapy (day 53, see Figure 1). The BSP was first calculated after the enrollment of 5 participants and, if attained, enrollment was stopped. In the event that the BSP was not reached, an additional participant was enrolled and the BSP was recalculated at day 53. Enrollment continued until the BSP was reached or 8 participants were enrolled in a particular group. Data from the first 2 study groups are presented, as more patients are being recruited in the subsequent groups.
FIGURE 1.
Prospective, iterative trial of carfilzomib-based desensitization. A, Treatment regimen for group A. Carfilzomib was administered in a within-patient dose-escalation fashion (20 to 36 mg/m2). Bone marrow biopsies and aspirates were performed before treatment and after 1 cycle of carfilzomib therapy. B, Treatment regimen for group B. Treatment was identical to group A, but plasmapheresis was added once weekly prior to carfilzomib administration. Bone marrow biopsies and aspirates were optional in both groups
2.2 |. Eligibility criteria
Highly sensitized kidney transplant candidates at the University of Cincinnati Medical Center and The Christ Hospital, Cincinnati, OH, who were 18 to 65 years old and receiving dialysis were eligible for participation. To be considered highly sensitized, participants had to have 1 or the following: a potential living donor with a positive cytotoxic crossmatch, a moderately to strongly (≥75 median channel shifts on a 1024 scale) positive T or B cell flow cytometry crossmatch with a confirmed donor-specific antibody (DSA), >2 low to moderate (1500 to 8000 mean fluorescence intensity units [MFI]) DSAs, or be on the kidney transplant waiting list with a current or peak cytotoxic PRA of >30%. Participants were excluded if they had baseline painful or grade 3 or 4 neuropathy as defined by the common terminology criteria for adverse events (CTCAE), recent or significant cardiac events, hemoglobin <8 g/dL, absolute neutrophil count <1000/mm3, platelet count <75 000/mm3 within 14 days of consent, positivity for HIV or hepatitis B surface antigen, or current or recent severe systemic infections; were pregnant or breastfeeding; or were not receiving dialysis. A full list of inclusion and exclusion criteria are available in Table S1. Participants were randomly selected by screening the kidney transplant waiting list for potentially eligible participants and were recruited from a dedicated sensitized participant education and evaluation clinic at both the University of Cincinnati and The Christ Hospital after a medical evaluation.
2.3 |. Study endpoints
The primary efficacy endpoint was the percent reduction in iAb from pretreatment to 48 hours after the last plasmapheresis. The primary safety endpoint was overall safety, defined as the incidence of grade ≥3 nonhematologic or grade 4 hematologic toxicities, and incidence of all grades of peripheral neuropathy. Secondary efficacy outcomes included the proportion of participants with a living donor achieving a negative T cell flow crossmatch (FXM) or in whom the FXM decreased by 50 and 100 channels; change in calculated panel reactive antibody (cPRA) from pretransplant to the last antibody testing; percent change in iAb at 7, 30, and 90 days postplasmapheresis; and percent rebound in iAb at 14 and 30 days after the last plasmapheresis (compared with 48 hours after the last plasmapheresis treatment). During the conduct of the trial, we developed novel analysis techniques and added a post hoc endpoint that included the change in number of donors required to match (DRTM) and the corresponding fold change in DRTM from baseline to 48 hours after the last session of plasmapheresis. All participants who received at least 1 dose of study drug were included in the safety analysis, and all participants who reached the time point of efficacy endpoint assessment were included in the efficacy analysis.
The cPRAs were calculated according to the United Network for Organ Sharing cPRA calculator.14,15 Precision PRA (pPRA) was calculated using an internally developed computation based on the 2013 HLA distributions and most recent policies from the United Network for Organ Sharing Histocompatibility Committee.16 DRTM were defined as 1/(1 – pPRA).
2.4 |. Histocompatibility testing
Anti-HLA antibodies were identified and measured in participant serum using single antigen bead (SAB; OneLambda, Canoga Park, CA) on a Luminex (Luminex, Austin, TX) platform and reported as MFI. Participant samples were robotically processed (LABXpress pipettor; OneLambda, Canoga Park, CA) and analyzed in single batch runs as previously described for bortezomib-based desensitization.12 For samples in which any SAB readout was saturated (MFI > 15 000 MFI), working titers were established. These were obtained by diluting serum samples until 3 consecutive SAB tests that resulted in the highest MFI SAB readout between 500 and 15 000 MFI, representing the linear measurement range of the assay. The working titer was defined as the second consecutive dilution resulting in a linear reading. All further samples for a given participant were analyzed at that working titer. In order to guide the adaptive enrollment and calculate the BSP, the iAb was determined at day 0 and at day 53. After full enrollment in a group, all samples were then diluted at the working titer if necessary and batched for analysis. In the event that up to 2 additional SABs shared an epitope/specificity within 20% MFI of the strongest SAB, they were averaged with the strongest SAB and considered the iAb. These beads were then used for response analysis.
2.5 |. Intervention
After informed consent and medical clearance, participants were sequentially assigned to 1 of 2 study groups (A and B). Both groups included 12 doses of carfilzomib (escalating from 20 mg/m2 to 36 mg/m2) preceded by 50 to 100 mg of methylprednisolone (Figure 1). After the last dose of carfilzomib, participants underwent 3 sessions of plasmapheresis (1.5 plasma volume exchange with volume replacement per investigator’s discretion) on days 47, 49, and 51 and serial HLA SAB testing. In addition, group B received plasmapheresis once weekly before carfilzomib dosing (Figure 1). All participants received antiviral prophylaxis with acyclovir or valacyclovir from the start of the treatment to 2 weeks after the last dose of carfilzomib. Fungal and Pneumocystis jiroveci prophylaxis were allowed per investigator discretion. In participants who provided additional consent, BM biopsies and aspirates were performed before the start of study treatment and on day 28 in both groups.
2.6 |. BM aspiration, cell viability assay, and flow cytometry analysis
BM aspirates were obtained under sterile conditions from the iliac crest, and up to 20 mL of unfractionated BM was obtained. BM aspirates were processed through a Ficoll gradient, and CD138+ cells were isolated by using magnetic bead positive selection (Miltenyi Biotec, Auburn, CA). Cell viability assays were conducted by using Trypan blue exclusion test of cell viability.17 The percentage of live cells was defined as the proportion of CD138+ cells not stained with Trypan blue divided by the total amount of CD138+ cells and evaluated before (days −7 to 0) and after 1 cycle of carfilzomib (day 28 for all participants). In addition, markers of apoptosis following incubation of BM CD138+ cells with control media, bortezomib, or carfilzomib were identified by annexin-V staining and characterized using a BD FACSCalibur (BD Biosciences, Franklin Lakes, NJ) flow cytometer.
2.7 |. Toxicity assessments
Before each study procedure, a complete blood count, differential, and liver function tests were obtained, and participants were screened for neurotoxicity using the Functional Assessment of Cancer Therapy Scale/Gynecologic Oncology Group-Neurotoxicity questionnaire.18,19 All toxicities were graded by using the CTCAE terminology, version 4.0.20
2.8 |. Statistical analyses
No formal sample size calculation was performed because this was an iterative pilot study. Continuous data are presented as means with standard deviation or medians with IQRs and were compared by using t-tests or nonparametric alternatives as appropriate. Categorical data are reported as proportions and percentages and compared by using the χ2 or Fisher’s exact test as appropriate. Repeated-measures analysis using mixed-effects models (with treatment day as fixed effect and participant as random effect) was performed on iAB MFI levels and cPRA measurements. A P-value of <.05 was considered statistically significant. All analyses were performed with R version 3.4.3 or higher (R Foundation for Statistical Computing, Vienna, Austria).21
3 |. RESULTS
Between January 2015 and November 2017, 16 participants were enrolled at The Christ Hospital and the University of Cincinnati, both in Cincinnati, OH. One participant was withdrawn before and 2 were withdrawn after starting treatment. Two of these withdrawals were unrelated to study participation (Figure 2A). Demographic data are presented in Table 1. Mean age at consent was approximately 43 years, and most participants were white women with end-stage renal disease (ESRD), most often the result of hypertension, lupus, and/or congenital abnormalities. All participants were receiving dialysis, with 15.4% receiving peritoneal dialysis. Mean cPRA1500 was 92.90% before study treatment, and sensitization was the result of prior transplant (76.9%), pregnancy (66.7%), and blood transfusion (46.2%). None of the participants had living kidney donors at the time of enrollment. There were no statistically significant differences between groups A and B.
FIGURE 2.
Study results. A, Patient disposition diagram. B, Reduction in iAb for groups A and B. Comparisons are for overall pairwise differences between days 1, 45, and 53
TABLE 1.
Demographics
| Group | Overall | A | B | P |
|---|---|---|---|---|
| n | 13 | 6 | 7 | |
| Demographics | ||||
| Age, mean y (SD) | 43.07 (11.60) | 36.18 (7.32) | 48.97 (11.68) | .041 |
| Female, n (%) | 9 (69.2) | 3 (50.0) | 6 (85.7) | .431 |
| Black/African American, n (%) | 4 (30.8) | 1 (16.7) | 3 (42.9) | .676 |
| Etiology of ESRD, n (%) | ||||
| Hypertension, n (%) | 2 (15.4) | 0 (0.0) | 2 (28.6) | .439 |
| Glomerulonephritis/glomerular disease, n (%) | 1 (7.7) | 0 (0.0) | 1 (14.3) | |
| Systemic lupus erythematosus, n (%) | 2 (15.4) | 1 (16.7) | 1 (14.3) | |
| Obstructive disorder/reflux, n (%) | 1 (7.7) | 1 (16.7) | 0 (0.0) | |
| Renal hyperplasia/dysplasia, n (%) | 1 (7.7) | 1 (16.7) | 0 (0.0) | |
| Alport syndrome, n (%) | 1 (7.7) | 1 (16.7) | 0 (0.0) | |
| Dysplastic kidneys, n (%) | 2 (15.4) | 1 (16.7) | 1 (14.3) | |
| Lupus nephritis, n (%) | 1 (7.7) | 0 (0.0) | 1 (14.3) | |
| Unknown, n (%) | 1 (7.7) | 1 (16.7) | 0 (0.0) | |
| Other, n (%) | 1 (7.7) | 0 (0.0) | 1 (14.3) | |
| Coronary artery disease, n (%) | 1 (7.7) | 1 (16.7) | 0 (0.0) | .936 |
| Hypertension, n (%) | 11 (84.6) | 4 (66.7) | 7 (100.0) | .374 |
| Hyperlipidemia, n (%) | 4 (30.8) | 2 (33.3) | 2 (28.6) | >.999 |
| Diabetes, n (%) | 2 (15.4) | 1 (16.7) | 1 (14.3) | >.999 |
| Receiving dialysis, n (%) | 13 (100.0) | 6 (100.0) | 7 (100.0) | NA |
| Peritoneal dialysis, n (%) | 2 (15.4) | 0 (0.0) | 2 (28.6) | .514 |
| Time on dialysis, y, mean (SD) | 6.16 (3.82) | 4.66 (3.05) | 7.44 (4.15) | .204 |
| Sensitization history | ||||
| Prior transplant, n (%) | 10 (76.9) | 5 (83.3) | 5 (71.4) | >.999 |
| Number of prior transplants, median [IQR] | 1.00 [1.00, 1.00] | 1.00 [1.00, 1.00] | 1.00 [1.00, 1.00] | .317 |
| Type of prior transplant, n (%) | ||||
| Deceased donor kidney transplant, n (%) | 5 (50.0) | 2 (40.0) | 3 (60.0) | .549 |
| Living-unrelated kidney transplant, n (%) | 1 (10.0) | 1 (20.0) | 0 (0.0) | |
| Living-related kidney transplant, n (%) | 4 (40.0) | 2 (40.0) | 2 (40.0) | |
| Etiology of transplant failure, n (%) | ||||
| Acute AMR | 1 (10.0) | 1 (20.0) | 0 (0.0) | .549 |
| Chronic rejection | 4 (40.0) | 2 (40.0) | 2 (40.0) | |
| Transplant glomerulopathy | 1 (10.0) | 1 (20.0) | 0 (0.0) | |
| Recurrent disease | 1 (10.0) | 0 (0.0) | 1 (20.0) | |
| Polyoma (BK) nephropathy | 1 (10.0) | 0 (0.0) | 1 (20.0) | |
| Other | 2 (20.0) | 1 (20.0) | 1 (20.0) | |
| Transplant nephrectomy, n (%) | 3 (23.1) | 0 (0.0) | 3 (42.9) | .243 |
| Pregnancy, n (%) | 6 (66.7) | 2 (66.7) | 4 (66.7) | >.999 |
| Blood transfusion, n (%) | 6 (46.2) | 1 (16.7) | 5 (71.4) | .157 |
| cPRA1500, MFI, mean (SD) | 92.90 (19.47) | 87.89 (28.74) | 97.19 (4.83) | .414 |
| cPRA1500, median [IQR] | 99.89 [98.53, 99.99] | 99.80 [98.82, 99.94] | 99.97 [96.23, 99.99] | .616 |
| cPRA4000, MFI, mean (SD) | 84.52 (29.56) | 80.62 (39.67) | 87.86 (20.20) | .679 |
| cPRA4000, median [IQR] | 97.50 [89.80, 99.94] | 97.10 [91.53, 99.17] | 99.88 [84.05, 99.96] | .568 |
| cPRA8000, MFI, mean (SD) | 74.89 (34.70) | 69.81 (40.63) | 79.24 (31.37) | .646 |
| cPRA8000, median [IQR] | 93.03 [59.39, 99.23] | 89.90 [52.35, 96.91] | 98.90 [69.83, 99.56] | .475 |
cPRA, calculated panel reactive antibody; ESRD, end-stage renal disease; MFI, mean fluorescence intensity.
Thirteen participants reached the primary endpoint evaluation and are included in the efficacy analysis: 6 in group A and 7 in group B. The BSP was reached after the enrollment of six participants in group A and has not yet been reached in group B given the variability in iAb responses in group B. Progression of the BSP after successive enrollment is presented in Figure S1.
Treatment was well tolerated in this ESRD population (Table 2 and Table S1). Most reported adverse events were mild and graded as CTCAE grade 1 or 2. The most commonly reported adverse events were nausea (61.5%), anemia (23.1%), fatigue, headache, paresthesia, thrombocytopenia, and vomiting (23.1%). Three participants experienced grade 3 toxicities (neutropenia, thromboembolic event/abdominal pain, and squamous cell carcinoma), and 1 of these 3 experienced a grade 4 toxicity (small bowel obstruction requiring surgical management). Grade 3 or 4 toxicities represented 6.4% of all reported adverse events. No grade 4 hematologic toxicity was reported, and peripheral neuropathy was not observed. One participant was unable to receive the 36 mg/m2 dose because of adverse events. All other participants were able to receive the maximal dose per protocol. Two participants experienced serious adverse events during the course of the study: 1 with an episode of small bowel obstruction resulting in sepsis and 1 with a plasmapheresis catheter-related deep venous thrombosis. A single participant required a dose reduction because of an adverse event at the 36 mg/m2 level. No participant died during the study, and no participants required study discontinuation for drug-related toxicities.
TABLE 2.
Adverse events reported in at least 2 participants
| Total | Group A | Group B | |
|---|---|---|---|
| Adverse event, n (%) | n = 13 | n = 6 | n = 7 |
| Nausea | 8 (61.5) | 4 (66.7) | 4 (57.1) |
| Anemia | 3 (23.1) | 1 (16.7) | 2 (28.6) |
| Fatigue | 3 (23.1) | 3 (50) | 0 (0) |
| Headache | 3 (23.1) | 2 (33.3) | 1 (14.3) |
| Paresthesia | 3 (23.1) | 2 (33.3) | 1 (14.3) |
| Platelet count decreased | 3 (23.1) | 0 (0) | 3 (42.9) |
| Cough | 2 (15.4) | 1 (16.7) | 1 (14.3) |
| Diarrhea | 2 (15.4) | 1 (16.7) | 1 (14.3) |
| Dry mouth | 2 (15.4) | 1 (16.7) | 1 (14.3) |
| Gastroesophageal reflux disease |
2 (15.4) | 1 (16.7) | 1 (14.3) |
| Myalgia | 2 (15.4) | 0 (0) | 2 (28.6) |
| Neutrophil count decreased | 2 (15.4) | 1 (16.7) | 1 (14.3) |
| Pain | 2 (15.4) | 1 (16.7) | 1 (14.3) |
| Thromboembolic event | 2 (15.4) | 0 (0) | 2 (28.6) |
| Upper respiratory infection | 2 (15.4) | 1 (16.7) | 1 (14.3) |
| Vomiting | 2 (15.4) | 0 (0) | 2 (28.6) |
To perform HLA antibody analysis measurement, 76.9% of participants’ sera required dilutional analyses to assess immunodominant HLA antibody reductions, with dilutions ranging from 1:8 to 1:2048 (Table 2). Group A iAbs were mostly class I, whereas group B mostly had class II. Median iAb reduction at day 53 was 69.84% (IQR 66.14% to 82.13%, P = .031, 95% CI 26.53% to 86.14%) and 88.20% (IQR −168.69% to 85.63%, P = .937, 95% CI −471.17% to 87.95%) for group A and group B, respectively. A median reduction in iAb strength of 34.2% (IQR −72.81% to 0.043%, P = .244, 95% CI −172.28% to 62.24%) was observed by day 45, before plasmapheresis. Absolute iAb strength was also reduced at days 45 (before plasmapheresis, P = .052) and 53 (P < .001, Figure 2B and Figure S2), and group assignment had no statistically significant impact on treatment effect. Eighty-three percent and 71.4% of participants had an iAb decrease by >50% in group A and group B, respectively, and were considered responders to therapy. Two participants in group B had increases in iAb level, which were associated with upper respiratory tract infections. In addition, both class I and class II iAbs were significantly reduced by treatment. Furthermore, iAb reduction was observed with the use of carfilzomib alone up to day 45 (Figure 2B and Figure S2). Rebound occurred rapidly and iAb levels returned to baseline between days 81 and 141.
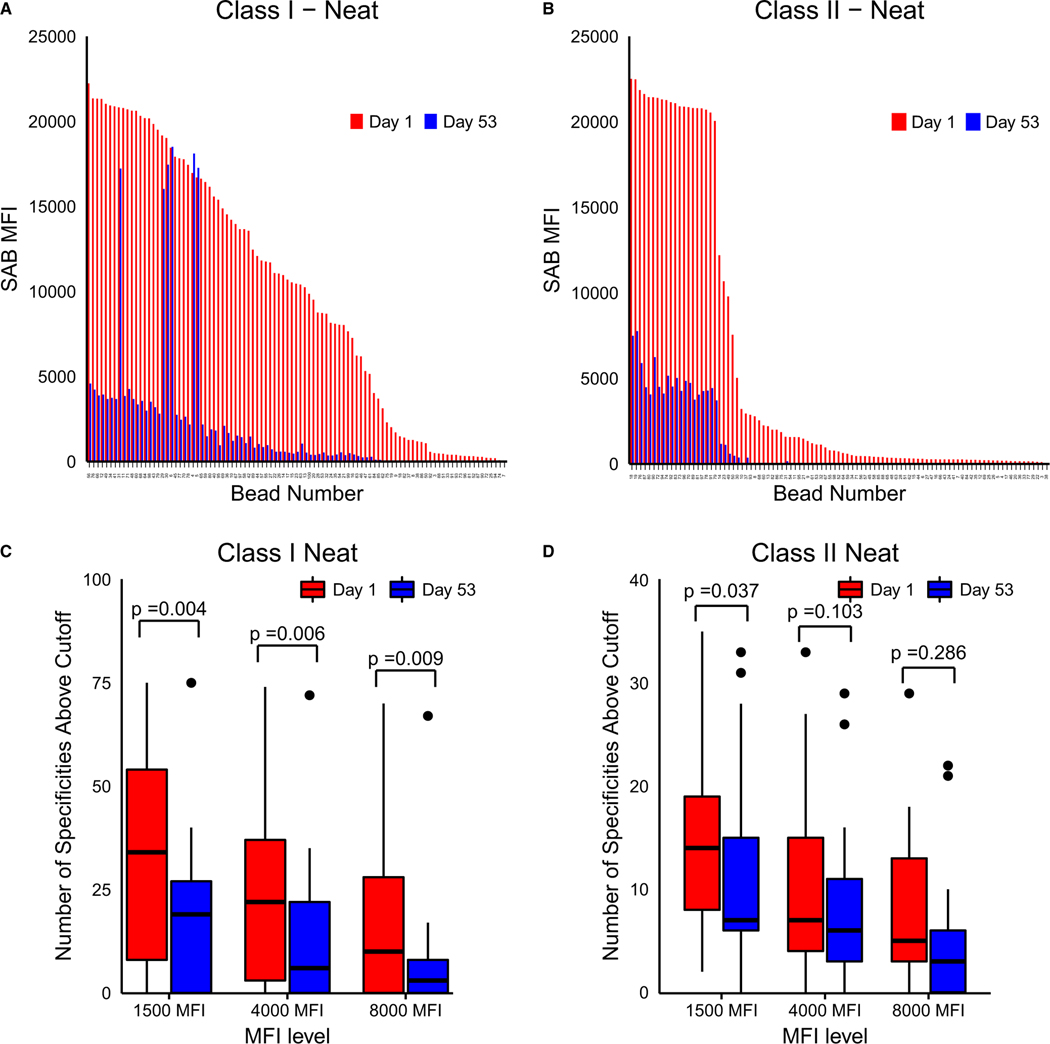
In addition to analysis of the effects on iAb, more global effects on HLA antibodies were analyzed by using traditional population-based HLA antibody reactivity by assessing the overall number of positive HLA antibody specificities and cPRA (and pPRA/#DRTM analysis). Carfilzomib therapy led to a significant reduction in the number of positive class I and class II specificities as depicted in Figure 3A,B. The total number of specificities was reduced at all MFI levels for class I but only at the 1500 MFI level for class II anti-HLA antibodies (Figure 3C,D). Participants had a baseline cPRA1500 of 92.90% ± 19.47% (87.89% ± 28.74% and 97.19% ± 4.83% for groups A and B, respectively). Reductions at day 53 were modest at 1500 (6.17% ± 11.44%, P = .031 and 12.79% ± 33.41%, P = .469), 4000 (6.80% ± 14.36%, P = .100 and 8.64% ± 24.10%, P = .156), and 8000 MFI (8.93% ± 15.26%, P = .584 and 11.87% ± 31.06%, P = .297) cutoffs for groups A and B, respectively (Figure S3A,B). In mixed-effects models, the group did not have a significant impact on the observed reductions in iAb (P = .698).
FIGURE 3.
Carfilzomib reduces the breadth of HLA sensitization for both class I and class II antigens. A, Representative bar graph of reduction in a wide range of HLA class I antibodies before and after desensitization treatment. B, Representative bar graph of reduction in a wide range of HLA class II antibodies before and after desensitization treatment. C, Boxplot depicting reduction in overall class I HLA specificities for the entire study group. D, Boxplot depicting reduction in overall class II HLA specificities for the entire study group
Our previous observations have indicated that the #DRTM may provide a more sensitive approach toward global assessment of therapeutic effects on HLA antibody populations induced by desensitization therapy.22 For cPRA1500, the mean #DRTM was 6975 ± 15 470 and 7645 ± 10 287 for groups A and B, respectively. At day 53, a significant reduction occurred in #DRTM, which decreased 5.13- ± 4.55- fold (P = .003) and 2.71- ± 4.04-fold (P = .295) from baseline for group A at 1500 and 8000 MFI, respectively, and 205.22- ± 573.81-fold at 1500 MFI and 126.44- ± 187.40-fold at 8000 MFI for group B (Figure S3C,D). This difference in #DRTM persisted from days 43 to 81, even after the cessation of study treatments.
Reductions in HLA antibody levels and cPRA/pPRA/#DRTM were supported by assessment of effects on BMPCs. Six participants (3 in each group) consented to additional BM studies. Data for BM CD138+ cell survival before and after carfilzomib were available for 5 patients and are presented in Figure 4A. Carfilzomib led to a 69.2% ± 4.3% decrease in CD138+ BMPCs 13 days after carfilzomib monotherapy (Figure 4A and Figure S4). This effect was consistent across all studied participants. To further confirm that carfilzomib therapy induced BMPC death, CD138+ BM cells were isolated from patients before PI therapy, incubated with bortezomib and carfilzomib, and stained 24 hours later with annexin-V. Flow cytometric analysis revealed that culture with PIs drove a >10-fold increase in the percentage of annexin-V+ PCs compared with untreated cells (Figure 4B).
FIGURE 4.
Carfilzomib induces bone marrow (BM) CD138+ cell death. A, Reduction in live BM CD138+ cells as a function of total BM nucleated cells after 1 cycle of carfilzomib therapy. B, BM CD138+ cells, unexposed to proteasome inhibitors, were incubated with control media, bortezomib, and carfilzomib. After incubation, expression of annexin-V, a marker of apoptosis, was increased in cells incubated with proteasome inhibitors compared with control media
Despite this profound depletion of BMPCs and reductions in HLA antibody levels, HLA antibody rebound was observed rapidly after carfilzomib therapy, with iAb and cPRA levels returning to baseline within approximately 30 days from the end of desensitization therapy.
4 |. DISCUSSION
We report the first prospectively conducted trial that evaluated the safety, toxicity, and efficacy of carfilzomib in kidney transplant candidates. Importantly, the early phases of this trial were aimed at establishing safety of carfilzomib in this novel participant population. Treatment was well tolerated, with a low incidence of grade 3 or 4 toxicities, and a reduction in the incidence of peripheral neuropathy compared with bortezomib-based regimens. No participants were withdrawn due to drug-related adverse events and no unexpected adverse events were reported.
The safety profile of carfilzomib in kidney transplant candidates compared favorably to the carfilzomib approval trials and trials of other PIs in transplant candidates and recipients. In the ASPIRE trial, a phase 3 randomized controlled trial of carfilzomib in combination with dexamethasone and lenalidomide for the treatment of relapsed multiple myeloma, the most commonly reported adverse events were diarrhea, fatigue, and upper respiratory tract infection in >25% of participants.23 In the current trial, the only adverse event occurring in >25% of participants was nausea. Diarrhea, fatigue, cough, and upper respiratory tract infections were also reported much less frequently than in the oncology population. Importantly, dyspnea and cardiac complications were not observed. There are important differences between the ASPIRE trial and the one we conducted, as participants in the ASPIRE trial were older (49.5% were aged 65 or older) and all participants had received at least 1 prior treatment for multiple myeloma, therefore predisposing them to additional adverse events. Participants in our trial were younger and dialysis dependent and were carefully screened for cardiopulmonary disease before enrollment.
An assessment of PI safety in participants with ESRD has previously been published.24 In a bortezomib-based desensitization study, grade 3 and 4 toxicities occurred in approximately 14% of participants, which is similar to what has been observed in this trial.12 In addition, new-onset neuropathy was observed in 37% of participants, a phenomenon that was not observed with carfilzomib. Interestingly, grade 3 or 4 hematologic toxicity was not observed with carfilzomib with the exception of 1 case of neutropenia, whereas these toxicities were more common with bortezomib (ranging from 0% to 20% in incidence).
Treatment efficacy of carfilzomib was measured 2-fold: clinically and mechanistically. From a clinical standpoint, iAb reduction was impressive in both groups A and B, which reached statistical significance in group A. More than 70% of participants in either group had a ≥50% reduction in iAb, which is significantly more than with bortezomib therapy despite a conservative treatment regimen.12 These reductions were measured after 3 sessions of plasmapheresis, which may have enhanced the observed treatment effect. However, given the long half-life of human IgG, plasmapheresis allowed for the washout of antibody produced before the start of treatment. In addition, reductions were observed before the initiation of plasmapheresis, further supporting the observed reductions as a result of treatment.
Two participants had marked increase in their iAb associated with upper respiratory tract infections that, in our experience, have contributed to the iAb increases in desensitization therapy. Despite profound iAb reductions, rebound was observed early, and by day 141, average iAb reduction was 4.2% and 26.2% for groups A and B respectively, when eliminating participants with iAb increases at day 53. Rebound in HLA antibody levels has also been frequently described with plasmapheresis with or without IVIG or rituximab.12,25,26 Potential explanations for this rebound may include lack of B cell depletion and PC repopulation, antibody redistribution from the interstitial fluid to the central compartment, and/or alterations in resistant BMPCs surviving treatment.27-29 Future phases of this trial will use rituximab to deplete B cells and address this question.
cPRA was modestly decreased at day 53, a phenomenon that can be explained by the fact that participants had high levels of antibody, as 83% of participants in group A and 86% of participants in group B required dilutional analyses (up to a 1:2048 titer). Despite these elevated titers, 2 participants reached a cPRA of 0% by day 53, further highlighting the broad effect of repeated carfilzomib doses on desensitization. These reductions can be explained by the depletion of BMPCs, a phenomenon that is not observed with IVIg.
Our findings are compatible with nonhuman primate models in which carfilzomib depleted BMPCs while reducing anti-MHC antibodies.30 The authors also suggested that germinal center expansion following bortezomib therapy represents a mechanism of antibody/PC rebound in vivo.31 B cell depletion may help abrogate this expansion, reducing rebound and BMPC repopulation. However, despite this reduction in BMPCs, surviving PCs appear to be less sensitive to proteasome inhibition and alternative pathways will require testing to further enhance therapeutic response.
This initial experience indicates that carfilzomib is well tolerated and effective as desensitization monotherapy in depleting PCs and reducing HLA antibody levels in combination with plasmapheresis. Further experience will be gained in future treatment groups designed to assess the effects of plasmapheresis, combinatorial therapies, and increasing carfilzomib exposure as approaches for optimizing therapeutic results.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank all the patients who participated in this study. This study was funded by a grant from Onyx Pharmaceuticals to Dr Woodle.
Funding information
Onyx Pharmaceuticals
Abbreviations:
- AMR
antibody-mediated rejection
- ASPIRE
Carfilzomib, Lenalidomide, and Dexamethasone versus Lenalidomide and Dexamethasone for the Treatment of Patients with Relapsed Multiple Myeloma
- BM
bone marrow
- BMPC
bone marrow plasma cell
- BSP
Bayesian statistical product
- CI
confidence interval
- cPRA
calculated panel reactive antibody
- CTCAE
common terminology criteria for adverse events
- DRTM
donors required to match
- DSA
donor-specific antibody
- ESRD
end-stage renal disease
- FiR
fractional iAb reduction
- FXM
flow crossmatch
- iAb
immunodominant antibody
- IVIG
intravenous immune globulin
- MFI
mean fluorescence intensity
- PC
plasma cell
- PI
proteasome inhibitor
- pPRA
precision PRA
- SAB
single antigen bead
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request but may be restricted by third party authorizations and/or HIPAA regulations.
REFERENCES
- 1.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol. 2012;8:348–357. [DOI] [PubMed] [Google Scholar]
- 2.Bostock IC, Alberú J, Arvizu A, et al. Probability of deceased donor kidney transplantation based on % PRA. Transpl Immunol. 2013;28:154–158. [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2016 annual data report: kidney. Am J Transplant. 2018;18(Suppl 1):18–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alachkar N, Lonze BE, Zachary AA, et al. Infusion of high-dose intravenous immunoglobulin fails to lower the strength of human leukocyte antigen antibodies in highly sensitized patients. Transplantation. 2012;94:165–171. [DOI] [PubMed] [Google Scholar]
- 5.Marfo K, Ling M, Bao YI, et al. Lack of effect in desensitization with intravenous immunoglobulin and rituximab in highly sensitized patients. Transplantation. 2012;94:345–351. [DOI] [PubMed] [Google Scholar]
- 6.Jordan SC, Tyan D, Stablein D, et al. Evaluation of intravenous immunoglobulin as an agent to lower allosensitization and improve transplantation in highly sensitized adult patients with end-stage renal disease: report of the NIH IG02 trial. J Am Soc Nephrol. 2004;15:3256–3262. [DOI] [PubMed] [Google Scholar]
- 7.Patil V, Kaveri SV. The mechanisms of action of IVIG in autoimmune and inflammatory diseases. ISBT Sci Ser. 2013;8:185–188. [Google Scholar]
- 8.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. [DOI] [PubMed] [Google Scholar]
- 9.Everly MJ, Everly JJ, Susskind B, et al. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754–1761. [DOI] [PubMed] [Google Scholar]
- 10.Walsh RC, Everly JJ, Brailey P, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010;89:277–284. [DOI] [PubMed] [Google Scholar]
- 11.Walsh RC, Brailey P, Girnita A, et al. Early and late acute antibody-mediated rejection differ immunologically and in response to proteasome inhibition. Transplantation. 2011;91:1218–1226. [DOI] [PubMed] [Google Scholar]
- 12.Woodle ES, Shields AR, Ejaz NS, et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant. 2015;15:101–118. [DOI] [PubMed] [Google Scholar]
- 13.Gandolfi S, Laubach JP, Hideshima T, Chauhan D, Anderson KC, Richardson PG. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017;36:561–584. [DOI] [PubMed] [Google Scholar]
- 14.Cecka JM, Calculated P. (CPRA): the new measure of sensitization for transplant candidates. Am J Transplant. 2010;10:26–29. [DOI] [PubMed] [Google Scholar]
- 15.Allocation Calculators - cPRA. U.S. Department of Health and Human Services, 2013. http://optn.transplant.hrsa.gov/converge/resources/allocationcalculators.asp?index=78. Accessed April 2, 2015.
- 16.Organ Procurment and Transplantation Network. 2018. https://optn.transplant.hrsa.gov/governance/policies. Accessed August 27, 2018. [Google Scholar]
- 17.Strober W. Trypan blue exclusion test ofcell viability. Curr Protoc Immunol. 2001;A3.B.1-A3.B.3. 10.1002/0471142735.ima03bs111 [DOI] [PubMed] [Google Scholar]
- 18.Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System. Version 4. Evanston, IL: Center on Outcomes, Research and Education (CORE), Evanston Northwestern Healthcare and Northwestern University; 1997. [Google Scholar]
- 19.Huang HQ, Brady MF, Cella D, Fleming G. Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer. 2007;17:387–393. [DOI] [PubMed] [Google Scholar]
- 20.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf. Accessed January 7, 2016.
- 21.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, 2018. 2018. https://www.R-project.org/.
- 22.Tremblay S, Shields A, Alloway R, et al. A prospective iterative trial of carfizomib-based desensitization trial: initial comparative observations. Am J Transplant. 2017;17(suppl 3):243–244. [Google Scholar]
- 23.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt N, Alloway RR, Walsh RC, et al. Prospective evaluation of the toxicity profile of proteasome inhibitor-based therapy in renal transplant candidates and recipients. Transplantation. 2012;94:352–361. [DOI] [PubMed] [Google Scholar]
- 25.Kozlowski T, Andreoni K. Limitations of rituximab/IVIg desensitization protocol in kidney transplantation; is this better than a tincture of time? Ann Transplant. 2011;16:19–25. [DOI] [PubMed] [Google Scholar]
- 26.Hakim RM, Milford E, Himmelfarb J, Wingard R, Lazarus JM, Watt RM. Extracorporeal removal of anti-HLA antibodies in transplant candidates. Am J Kidney Dis. 1990;16:423–431. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–242. [DOI] [PubMed] [Google Scholar]
- 28.Fairfax KA, Kallies A, Nutt SL, Tarlinton DM. Plasma cell development: from B-cell subsets to long-term survival niches. Semin Immunol. 2008;20:49–58. [DOI] [PubMed] [Google Scholar]
- 29.Kim J, Hayton WL, Robinson JM, Anderson CL. Kinetics of FcRnmediated recycling of IgG and albumin in human: pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin Immunol. 2007;122:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwun J, Burghuber C, Manook M, et al. Successful desensitization with proteasome inhibition and costimulation blockade in sensitized nonhuman primates. Blood Adv. 2017;1:2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwun J, Burghuber C, Manook M, et al. Humoral compensation after bortezomib treatment of allosensitized recipients. J Am Soc Nephrol. 2017;28:1991–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.