Abstract
-
•
In patients with diabetes hospitalized for COVID-19 in CORONADO study, 2.8% had a newly discovered.
-
•
2.8% had a newly discovered diabetes (NDD): mean age 60.2 ± 12.5 years and HbA1C 9.0 ± 2.5%. When compared with center, age and sex-matched patients with established type 2 diabetes, NDD was not significantly associated with a more severe COVID-19 prognosis.
Keywords: COVID-19, New-onset diabetes, Hospital, Death, Tracheal intubation
1. Introduction
It is now well-established that diabetes is a risk factor for increased coronavirus disease 2019 (COVID-19) severity, including higher mortality rate [1], [2], [3], [4]. Since the beginning of the pandemic some cases of newly-diagnosed diabetes (NDD) had been observed in patients with COVID-19 [5], [6], [7], highlighting a bidirectional relationship between COVID-19 and diabetes [8]. Moreover, it has been suggested that patients with NDD had a worsen prognosis than those with previously established diabetes [3], [9], [10].
The aim of the current post-hoc analysis is to describe the phenotypic characteristics of subjects with NDD in the nationwide CORONADO study [11].
2. Material and methods
2.1. Study design and participants
The aim of the CORONADO study (clinicaltrials.gov NCT04324736) was to describe the phenotypic characteristics and prognosis of patients with diabetes admitted with COVID-19 between March 10 and April 10, 2020 in 68 French centers. The protocol obtained all regulatory approvals and the design was previously reported [11]. Briefly, inclusion criteria were (i) hospitalization for biologically (positive SARS-CoV-2 PCR) and/or clinically/radiologically confirmed COVID-19; (ii) personal history of diabetes (based on medical record and/or routine use of antidiabetic drugs) or NDD. NDD definition was based on HbA1c ≥ 6.5% [48 mmol/mol] measured during the first week of hospitalization and the absence of personal history of diabetes or diabetic retinopathy (Flow Chart in Figure S1).
2.2. Data collection and outcomes
Data collection was retrospectively performed from medical files of all COVID-19 inpatients and by contacting the patient’s general and/or specialist practitioners, regular pharmacist and biomedical laboratory. The primary outcome combined tracheal intubation for mechanical ventilation and/or death within 7 days of admission. Secondary outcomes notably included death and tracheal intubation taken separately, and hospital discharge. A secondary time point was considered at day 28 for all patients alive and not discharged within 7 days.
2.3. Statistical analysis
NDD patients were matched with up to three patients with T2D on center, sex and age (±5 y). Characteristics are presented as mean ± SD, median [25th-75th percentile] or N (%). P-values were computed using Student-t tests, Mann-Whitney-Wilcoxon tests or Fisher tests. In the matched sample, we used logistic regression models to estimate ORs with 95%CI. Analyses were performed using statistical software R, version 4.0.2.
3. Results
3.1. Phenotypic characteristics of patients with NDD
Among 2,951 CORONADO participants, 2,820 could be analyzed regarding the primary outcome. After a careful review of the medical files, 80 patients (2.8%) were identified as having NDD (Figure S1).
The main clinical characteristics of all NDD patients are shown in supplemental Table 1. Median BMI (25th-75th percentile) was 27.7 (24.9–31.2) kg/m2 and mean HbA1C was 9.0 ± 2.5% [75.1 ± 27.2 mmol/mol]. Compared to patients with established T2D (n = 2237), those with NDD were younger (60.2 ± 12.5 vs 70.5 ± 12.3 yrs, p < 0.001) and more frequently from African or Caribbean origin (31.1 vs 16.7%, p = 0.0055).
In order to identify specific phenotypic characteristics associated to NDD, we have been able to match 67 patients with NDD to 176 patients with established T2D on age, sex and center. As shown in Table 1 , the median diabetes duration of patients with T2D was 9 years, with 35.8% under insulin therapy. Patients with NDD had less comorbidities than those with T2D with significantly less hypertension, dyslipidemia and CV diseases, as well as anti-hypertensive drugs and statins. Median BMI was numerically lower in NDD (27.9 vs 29.9 kg/m2 in T2D, p = 0.11), while HbA1C was numerically higher (8.9% vs 8.4% in T2D, p = 0.29). The time between the onset of COVID-19 symptoms and hospitalization was longer in NDD compared to T2D (6 vs 5 days, P = 0.019). The CRP level was significantly higher in patients with NDD, while plasma glucose levels on admission was not different (Table 1).
Table 1.
Clinical characteristics of CORONADO participants in the matched sample.
| Patient characteristics | NDD patients (n = 80) |
Matched Sample (n = 243) | OR or delta (95%CI) | p-value | SMD | |
|---|---|---|---|---|---|---|
| Exposed: NDD patients (n = 67) |
Controls: patients with T2D (n = 176) |
|||||
| Sex | 26/80 (32.5%) | 24/67 (35.8%) | 62/176 (35.2%) | 1.03 (0.57–1.85) | 0.9311 | 1.2 |
| Age | 60.2+/-12.5 | 61.9+/-11.3 | 64.1+/-10.7 | 0.98 (0.96–1.01) | 0.1711 | 19.2 |
| Ethnical origin | 0.1982 | 138.7 | ||||
| EU | 29/61 (47.5%) | 27/51 (52.9%) | 55/146 (37.7%) | 1.00 | ||
| MENA | 11/61 (18%) | 9/51 (17.6%) | 38/146 (26%) | 0.48 (0.20–1.14) | 0.0968 | |
| AC | 19/61 (31.1%) | 13/51 (25.5%) | 40/146 (27.4%) | 0.66 (0.30–1.44) | 0.2981 | |
| AS | 2/61 (3.3%) | 2/51 (3.9%) | 13/146 (8.9%) | 0.31 (0.07–1.49) | 0.1445 | |
| BMI (kg/m2) | 27.7 [24.9; 31.2] | 27.9 [25.2; 31.7] | 29.9 [26.2; 33.9] | 0.77 (0.56–1.06) | 0.1092 | 27.9 |
| HbA1c (mmol) | 75.1+/-27.2 | 73.8+/-27.4 | 68.8+/-26.2 | 1.01 (0.99–1.02) | 0.2912 | 18.6 |
| HbA1c (%) | 9.0+/-2.5 | 8.9+/-2.5 | 8.4+/-2.4 | 1.07 (0.94–1.23) | 0.2912 | 18.6 |
| Hypertension | 30/78 (38.5%) | 27/65 (41.5%) | 139/175 (79.4%) | 0.18 (0.10–0.34) | <10-4 | 84.1 |
| Dyslipidemia | 10/78 (12.8%) | 8/65 (12.3%) | 85/173 (49.1%) | 0.15 (0.07–0.32) | <10-4 | 87.1 |
| Current tobacco use | 3/66 (4.5%) | 2/55 (3.6%) | 12/148 (8.1%) | 0.43 (0.09–1.98) | 0.2766 | 19.1 |
| Cardiovascular diseasesa | 9/69 (13.0%) | 8/58 (13.8%) | 62/170 (36.5%) | 0.28 (0.12–0.63) | 0.002 | 54.2 |
| Active cancer | 3/76 (3.9%) | 3/64 (4.7%) | 10/172 (5.8%) | 0.80 (0.21–2.99) | 0.7364 | 5.1 |
| COPD | 3/77 (3.9%) | 3/64 (4.7%) | 21/173 (12.1%) | 0.36 (0.10–1.24) | 0.1041 | 27.1 |
| Treated OSA | 3/72 (4.2%) | 2/60 (3.3%) | 25/164 (15.2%) | 0.19 (0.04–0.84) | 0.0279 | 41.9 |
| Diabetes duration (years) | – | – | 9 [5; 15] | – | – | – |
| Routine medication | ||||||
| Metformin | – | – | 121/176 (68.8%) | – | – | – |
| Sulfonylureas | – | – | 47/176 (26.7%) | – | – | – |
| DPP4 inhibitors | – | – | 42/176 (23.9%) | – | – | – |
| GLP-1 RA | – | – | 26/176 (14.8%) | – | – | – |
| Insulin therapy | – | – | 63/176 (35.8%) | – | – | – |
| Thiazide diuretics | 7/80 (8.8%) | 5/67 (7.5%) | 41/176 (23.3%) | 0.27 (0.1–0.7) | 0.0077 | 45 |
| Loop diuretics | 3/80 (3.8%) | 2/67 (3.0%) | 24/176 (13.6%) | 0.19 (0.04–0.85) | 0.0294 | 39.3 |
| Potassium-sparing diuretics | 1/80 (1.2%) | 1/67 (1.5%) | 10/176 (5.7%) | 0.25 (0.03–2.00) | 0.1923 | 22.7 |
| ARBs and/or ACE inhibitors | 18/80 (22.5%) | 15/67 (22.4%) | 100/176 (56.8%) | 0.22 (0.11–0.42) | <10-4 | 75.2 |
| Beta-blockers | 5/80 (6.2%) | 4/67 (6.0%) | 54/176 (30.7%) | 0.14 (0.05–0.41) | < 0.001 | 67.4 |
| Calcium-channel inhibitor | 8/80 (10.0%) | 6/67 (9.0%) | 57/176 (32.4%) | 0.21 (0.08–0.50) | < 0.001 | 60.4 |
| Statins | 8/80 (10.0%) | 7/67 (10.4%) | 83/176 (47.2%) | 0.13 (0.06–0.30) | < 0.001 | 88.7 |
| Anti-platelet agent | 8/80 (10.0%) | 7/67 (10.4%) | 70/176 (39.8%) | 0.18 (0.08–0.41) | < 0.001 | 71.9 |
| Anticoagulant | 1/80 (1.2%) | 1/67 (1.5%) | 23/176 (13.1%) | 0.10 (0.01–0.76) | 0.0262 | 45.7 |
| Oral corticosteroids | 3/80 (3.8%) | 3/67 (4.5%) | 11/176 (6.2%) | 0.70 (0.19–2.6) | 0.5979 | 7.9 |
| Symptoms on admission | ||||||
| Positive SARS-CoV-2 PCR | 71/78 (91.0%) | 59/65 (90.8%) | 166/175 (94.9%) | 0.53 (0.18–1.56) | 0.2514 | 15.9 |
| COVID-related symptoms | 77/80 (96.2%) | 64/67 (95.5%) | 174/176 (98.9%) | 0.25 (0.04–1.5) | 0.1284 | 20.3 |
| Duration of symptoms on admission | 7 [4; 9] | 6 [4; 8] | 5 [3; 7.2] | 1.08 (1.01–1.16) | 0.0185 | 34.1 |
| Fever | 67/78 (85.9%) | 56/66 (84.8%) | 133/173 (76.9%) | 1.68 (0.79–3.60) | 0.1789 | 20.4 |
| Asthenia | 51/77 (66.2%) | 43/65 (66.2%) | 112/165 (67.9%) | 0.92 (0.5–1.70) | 0.8016 | 3.7 |
| Cough | 64/78 (82.1%) | 53/65 (81.5%) | 117/172 (68.0%) | 2.08 (1.03–4.20) | 0.0419 | 31.5 |
| Cephalalgia | 14/76 (18.4%) | 11/64 (17.2%) | 34/162 (21.0%) | 0.78 (0.37–1.66) | 0.5199 | 9.7 |
| Dyspnoea | 58/79 (73.4%) | 50/66 (75.8%) | 126/173 (72.8%) | 1.17 (0.61–2.24) | 0.6465 | 6.7 |
| Rhinitis and/or pharyngeal symptoms | 8/71 (11.3%) | 7/62 (11.3%) | 19/161 (11.8%) | 0.95 (0.38–2.39) | 0.9152 | 1.6 |
| Ageusia and/or anosmia | 17/69 (24.6%) | 14/59 (23.7%) | 31/159 (19.5%) | 1.28 (0.63–2.63) | 0.4934 | 10.3 |
| Digestive disorder | 24/76 (31.6%) | 19/63 (30.2%) | 46/169 (27.2%) | 1.15 (0.61–2.18) | 0.6576 | 6.5 |
| Biological parameters | ||||||
| Plasma glucose on admission (mg/dL) | 173 [129; 28] | 170 [128; 256] | 173 [131; 231] | 1.25 (0.91–1.71) | 0.1623 | 28.8 |
| eGFR (CKD-EPI, mL/min) | 87.4 [68.9; 99.1] | 87.8 [68.3; 99.5] | 77.4 [51.8; 96.3] | 1.59 (1.1–2.29) | 0.0131 | 39 |
| ALT (% ULN) | 0.79 [0.50; 1.34] | 0.84 [0.52; 1.36] | 0.77 [0.48; 1.21] | 1.05 (0.79–1.39) | 0.7495 | 7.7 |
| AST (% ULN) | 1.27 [0.85; 2.3] | 1.26 [0.85; 2.3] | 1.2 [0.91; 1.75] | 1.06 (0.8–1.4) | 0.702 | 9.4 |
| Hemoglobin (g/dL) | 14.0 [13; 15.3] | 13.9 [13; 15.2] | 13.1 [11.7; 14.4] | 1.88 (1.31–2.69) | 0.0006 | 56 |
| White cells count (G/L) | 6960 [5440; 8500] | 7040 [5810; 8890] | 6490 [5000; 8480] | 1.28 (0.96–1.70) | 0.0955 | 22.7 |
| Lymphocyte count (G/L) | 1020 [785; 1300] | 1000 [740; 1300] | 1150 [820; 1622] | 0.79 (0.58–1.07) | 0.1345 | 31.1 |
| Platelelet count (G/L) | 204 [159; 258] | 204 [161; 256] | 203 [166; 246] | 1.12 (0.84–1.5) | 0.4316 | 13.8 |
| CRP (mg/L) | 107 [2; 179] | 108 [6; 189] | 95 [4; 153] | 1.37 (1.00–1.88) | 0.0492 | 20 |
| LDH (UI/L) | 484 [363; 647] | 484 [359; 647] | 397 [300; 534] | 1.66 (0.93–2.94) | 0.0855 | 2.5 |
| CPK (UI/L) | 139 [87; 290] | 136 [82; 304] | 154 [87; 421] | 0.87 (0.6–1.26) | 0.4555 | 22.6 |
Characteristics are presented as N (%) for categorical variables and as mean ± SD. for continuous variables or median [IQR] if non-normally distributed. Univariable associations were estimated using Fisher exact tests, Student-t tests or Mann-Whitney-Wilcoxon tests, respectively.
Abbreviations: EU (Europid); MENA (Middle East North Africa); AC (African or Caribbean); AS (Asian); HbA1c corresponds to the glycated hemoglobin determined in the 6 months prior to or in the first 7 days following hospital admission; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnea; DPP4-inhibitors, Dipeptidyl peptidase 4-inhibitors; GLP-1RA, Glucagon-Like Peptide 1-Receptor Agonist; CCB, calcium channel blocker; ARB, angiotensin-2 receptor blocker; ACE-Inhibitors, angiotensin converting enzyme-inhibitors; eGFR, estimated glomerular filtration rate, according to the CKD-EPI formula; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; LDH, lactate dehydrogenase; CPK, creatine phosphokinase; ULN, Upper limit of normal; SMD, Standardized Mean Difference.
aCardiovascular diseases were defined as history of one or more of the following comorbidities: acute coronary syndrome, coronary artery disease revascularization, transient ischemic attack and/or lower limber artery revascularization
3.2. COVID-19-related outcomes in patients with NDD
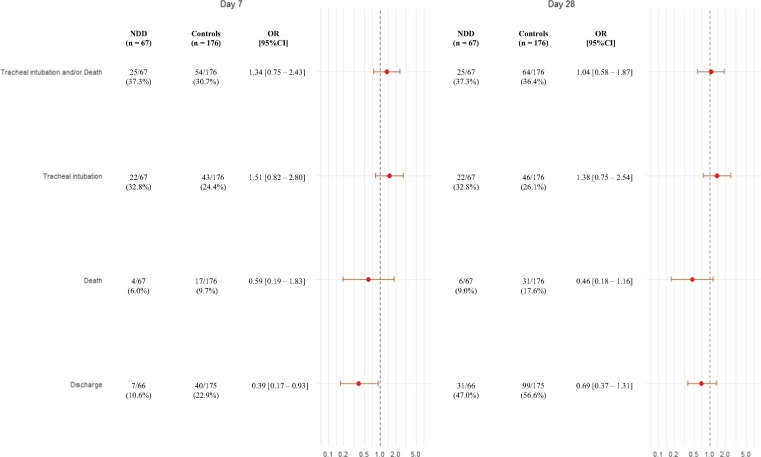
Finally, we compared the occurrence of major clinical outcomes within 7 and 28 days between patients with NDD and with established T2D. As shown in Figure 1 , there was no statistically significant difference regarding the composite primary outcome, as well as tracheal intubation or death, between the two groups. There was significantly less discharge in NDD group within 7 days (OR: 0.39 [0.17–0.96]), but the difference was not significant within 28 days (OR: 0.69 [0.37–1.31]).
Fig. 1.
Incidence of the different outcomes after 7 and 28 days, NDD vs. controls in the matched sample.
NDD patients were matched with up to three controls on center, sex and age (+/- 5 years). Odds ratios (NDD/controls) and corresponding 95% confidence intervals are calculated using logistic regression.
4. Discussion
In the present analysis of the CORONADO study, we found that NDD occurred in only 2.8% of patients with diabetes hospitalized for COVID-19. This frequency is much lower than those previously reported in Chinese studies, ranging from 16% [12] to 21% [9], but closer to that of 5% reported in a study from Italy [13]. Of note, ethnicity data showed that NDD was more prevalent than T2D in African-Caribbean people but this needs to be confirmed in larger cohorts.
Importantly, we found that the severity of COVID-19 was not different in patients with NDD and age-, center- and sex-matched subjects with established T2D. In contrast to previous reports [9], [13], our results did not confirm a worsen COVID-19 prognosis in NDD compared to established T2D. It should be underlined, however, that patients with NDD had significantly less comorbidities (i.e. CV diseases, hypertension or dyslipidemia) than those with established T2D. These potential confounding factors were not taken into account in our matching process. Another potential explanation for this discrepancy is the severity of the COVID-19 in T2D which appears more pronounced in CORONADO with a mortality rate of 17.6% within 28 days compared to 11.2% in the study of Li et al. [9] and 14.0% in those of Fadini et al. [13]. It should be underlined however that the mortality rate was also similar in NDD and established diabetes in the latter study in which the severity was mainly driven by admission in ICU [13]. We also found that tracheal intubation was numerically, but not statistically, higher and that early discharge was significantly lower in NDD compared to established T2D. In accordance with the prominent role of hyperglycemia per se as a prognosis factor for COVID-19 [14], the admission plasma glucose levels were not different between NDD and T2D groups. Some differences regarding routine treatments between patients with NDD or T2D might also have impacted the outcomes. For instance, metformin was associated with a reduced risk of death in patients with T2D in CORONADO [15]. Conversely, statin use was reported to be associated with a worse prognosis in CORONADO [16].
Our study displays some limitations, notably its observational and post-hoc design. Since we cannot guarantee that biological screening with HbA1C was systematically performed in all patients, an underestimation of the NDD frequency cannot be excluded. Finally, information on diabetes management and evolution following the admission are lacking, especially regarding the use and the dose of glucose lowering therapies as well as the quality of glycemic control.
As highlighted by some authors [8], [17], some dedicated prospective studies are warranted to assess more precisely the link between NDD and COVID-19 and to limit confounding biases due to observational design.
Acknowledgments
Acknowledgments
We thank the sponsor of the study (DRCI [délégation à la recherche clinique et à l'innovation] CHU Nantes), the Clinical Project Manager (M. Saignes) and assistant (J. Saunier), Clinical Research Associates (S. El Andaloussi, J. Martin-Gauthier, E. Rebouilleau) and data-managers (B. Guyomarc’h, T. Roman). We acknowledge all medical and clinical research staff involved in the diagnosis and treatment of patients with COVID-19 in participating centers. We thank all of the general practitioners, specialists, pharmacists and biological laboratories responsible for hospitalized patients for providing additional medical information to investigators. We thank the Société Francophone du Diabète (SFD) and Société Française d’Endocrinologie (SFE) for disseminating the study design and organization, and the Fédération Française des Diabétiques (FFD) for participating in the organization of the study.
5. Funding Sources
This study received the following funding: the Fondation Francophone de Recherche sur le Diabète (FFRD), supported by Novo Nordisk, MSD, Abbott, AstraZeneca, Lilly and FFD (Fédération Française des Diabétiques) – CORONADO initiative emergency grant; Société Francophone du Diabète (SFD) – CORONADO initiative emergency grant; Air Liquide Health Care international. CORONADO initiative emergency grant; Allergan. CORONADO initiative emergency grant; AstraZeneca. CORONADO initiative emergency grant; ELIVIE. CORONADO initiative emergency grant; FORTIL. CORONADO initiative emergency grant; Lifescan. CORONADO initiative emergency grant; Nantes Metropoôle. CORONADO initiative emergency grant; NHC. CORONADO initiative emergency grant; Novo Nordisk. CORONADO initiative emergency grant; Sanofi. CORONADO emergency grant; PHRC National COVID-19 Hospitalization and Care Organization Division (DHOS) as part of the Hospital Clinical Research Program (PHRC COVID-19-20-0138). All research facilities are acknowledged for providing research associates and research technicians for clinical investigations pro bono. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the study.
Conflict Of Interest
BC reports grants, non-financial support or personal fees from Abbott, Air Liquid, Allergan, Amgen, Akcea AstraZeneca, Elivie, Fortil, Pierre Fabre, Genfit, Gilead, Lifescan, Eli Lilly, Merck Sharpe Dome, NHC, Novo Nordisk, Regeneron and Sanofi. MP reports grants, non-financial support or personal fees from Air Liquid, Allergan, Amgen, Elivie, Fortil, Lifescan, NHC, Novo Nordisk, and Sanofi. MW reports grants, personal fees from Air Liquid, Allergan, Elivie, Fortis, Lifescan, NHC, Novo Nordisk, and Sanofi. SH reports grants, non-financial support or personal fees from Air Liquid, Allergan, Astra Zeneca, Bayer, Boehringer Ingelheim, Dinno Santé, Elivie, Fortil, Eli Lilly, Lifescan, LVL, Merck Sharpe Dome, NHC, Novartis, Pierre Fabre Santé, Sanofi, Servier, and Valbiotis. PG reports grants or personal fees from Abbott, Air Liquid, Allergan, Amgen, Astra-Zeneca, Boehringer Ingelheim, Elivie, Fortil, Eli Lilly, Lifescan, Merck Sharp and Dohme, Mundipharma, NHC, Novo Nordisk, Sanofi, and Servier. The other authors had nothing to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2021.108695.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of blood glucose and outcomes in patients with COVID-19 an pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubjane M., McCreedy N., Cariou B., Rubio M.A., Panton U.H., Hvid C., et al. Association of diabetes and severe COVID-19 outcomes: a rapid review and meta-analysis. J Endocrinol Metab. 2020;10:118–130. [Google Scholar]
- 5.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164 doi: 10.1016/j.diabres.2020.108166. 108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armeni E., Aziz U., Qamar S., Nasir S., Nethaji C., Negus R., et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet Diabetes Endocrinol. 2020;8:660–663. doi: 10.1016/S2213-8587(20)30221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubino F., Amiel S.A., Zimmet P., Alberti G., Bornstein S., Eckel R.H., et al. New-onset diabetes in Covid-19. N Engl J Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H., Tian S., Chen T., Cui Z., Shi N., Zhong X., et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020 doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh A.K., Singh R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract. 2020;167 doi: 10.1016/j.diabres.2020.108382. 108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y., Li H., Zhang J., Cao Y., Zhao X., Yu N., et al. A single-centre, retrospective, observational study in Wuhan. Diabetes Obes Metab. 2020;22:1443–1454. doi: 10.1111/dom.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fadini G.P., Morieri M.L., Boscari F., Fioretto P., Maran A., Busetto L., et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168 doi: 10.1016/j.diabres.2020.108374. 108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceriello A., Nigris V.D., Prattichizzo F. Why is hyperglycemia worsening COVID-19 and its prognosis? Diabetes Obes Metab. 2020 doi: 10.1111/dom.14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lalau J.D., Al-Salameh A., Hadjadj S., et al. Metformin use is associated with a reduced risk of mortality in patients with diabetes hospitalised for COVID-19. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cariou B., Goronflot T., Rimbert A., et al. Routine use of statins and increased mortality related to COVID-19 in inpatients with type 2 diabetes: results from the CORONADO study. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentile S., Strollo F., Mambro A., Ceriello A. COVID-19, ketoacidosis and new-onset diabetes: are there possible cause and effect relationship among them? Diabetes Obes Metab. 2020 doi: 10.1111/dom.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.