Abstract
Functional neuroimaging studies in obesity have identified alterations in the connectivity within the reward network leading to decreased homeostatic control of ingestive behavior. However, the neural mechanisms underlying sex differences in the prevalence of food addiction in obesity is unknown. The aim of the study was to identify functional connectivity alterations associated with: (1) Food addiction, (2) Sex- differences in food addiction, (3) Ingestive behaviors. 150 participants (females: N = 103, males: N = 47; food addiction: N = 40, no food addiction: N = 110) with high BMI ≥ 25 kg/m2 underwent functional resting state MRIs. Participants were administered the Yale Food Addiction Scale (YFAS), to determine diagnostic criteria for food addiction (YFAS Symptom Count ≥ 3 with clinically significant impairment or distress), and completed ingestive behavior questionnaires. Connectivity differences were analyzed using a general linear model in the CONN Toolbox and images were segmented using the Schaefer 400, Harvard–Oxford Subcortical, and Ascending Arousal Network atlases. Significant connectivities and clinical variables were correlated. Statistical significance was corrected for multiple comparisons at q < .05. (1) Individuals with food addiction had greater connectivity between brainstem regions and the orbital frontal gyrus compared to individuals with no food addiction. (2) Females with food addiction had greater connectivity in the salience and emotional regulation networks and lowered connectivity between the default mode network and central executive network compared to males with food addiction. (3) Increased connectivity between regions of the reward network was positively associated with scores on the General Food Cravings Questionnaire-Trait, indicative of greater food cravings in individuals with food addiction. Individuals with food addiction showed greater connectivity between regions of the reward network suggesting dysregulation of the dopaminergic pathway. Additionally, greater connectivity in the locus coeruleus could indicate that the maladaptive food behaviors displayed by individuals with food addiction serve as a coping mechanism in response to pathological anxiety and stress. Sex differences in functional connectivity suggest that females with food addiction engage more in emotional overeating and less cognitive control and homeostatic processing compared to males. These mechanistic pathways may have clinical implications for understanding the sex-dependent variability in response to diet interventions.
Subject terms: Reward, Neuroscience, Insula, Limbic system, Prefrontal cortex, Striatum
Introduction
As the obesity epidemic progresses, with 42% of the adult U.S. population being obese, rising healthcare costs of over 700 billion dollars annually have been observed1–3. Studies have shown associations between obesity and abnormal ingestive behaviors, primarily “food addiction”, in 40% of individuals seeking bariatric surgery4,5. Food addiction describes an addictive response in some individuals, where unintended overeating, the increased intake of ultra-processed/hyperpalatable foods beyond homeostatic needs or eating primarily for pleasure occur despite negative consequences6–8. The Yale Food Addiction Scale (YFAS) is a validated and psychometrically sound measure that uses the DSM-IV diagnostic criteria for substance abuse to operationalize food addiction9,10. While it is believed that food addiction is distinct from other behavioral eating disorders, it does share the characteristics or withdrawal, tolerance, impulsivity, and emotional reactivity seen with substance-use disorders and other addictive behaviors6,7,11.
Research depicting sex differences in obesity and food addiction have gained momentum to increase treatment efficacy12. Although prevalence rates in obesity are similar between the sexes, females report nearly double the rates of food addiction compared to males (12.2–6.4% respectively), with obese females being more likely to encounter loss of control while eating12,13. This stems from the increased frequency and cravings towards food as well as the increased reactivity to food cues experienced by females with obesity compared to males12,14,15.
Past literature has used magnetic resonance imaging (MRI) as a tool to understand the underlying neurobiology of both obesity and food addiction16–18. Individuals with obesity show greater activation in the reward and salience networks, particularly in the basal ganglia, in response to visual stimuli compared to normal weight individuals19,20. This has been associated with an increased drive for cravings and greater food consumption, which could explain the prevalence of overeating in obese individuals21,22. Studies have also indicated brain connectivity differences related to food addiction between regions of the reward network due to its role in controlling voluntary behavior23–25. Altered connectivity in the basal ganglia occurs as a result of the decreased expression of dopamine (D2) receptors which mediate reward-seeking behavior4,26,27. The reduction in the availability of D2 receptors creates a perpetual hypodopaminergic state in affected individuals leading to decreasing reward sensitivity, as greater amounts of dopamine must be released to receive the same stimulatory effect25. Similar to other addictive behaviors, individuals with food addiction exhibit increased alterations in the regions of the reward network in response to food cues28–31. Decreased sensitivity of the reward system translates into decreased inhibitory control and cognitive modulation in food addiction4,26,27. Dysregulation of the reward network in conjunction with persistent activation of the dopaminergic pathway supports the compulsive, unregulated food consumption of palatable and high calorie foods seen in obese individuals with food addiction32,33.
Sex differences have also been observed in the brain signatures of individuals with obesity. Compared to males, females display an increased reactivity to visual food cues and higher incidences of food cravings, and greater activations in brain regions associated with visual stimuli identification, such as the fusiform gyrus34. Additionally, females with obesity showed positive associations between increased BMI and lower connectivity of core reward network regions with cortical and emotion regulation regions35,36. This has been linked to the decreased ability to control the physiological response to negative-emotion-inducing stimuli leading to increased emotional eating and food addictive behaviors in females with obesity compared to their male counterparts37,38. Sex differences in food addiction have implications for treatments outcomes, as sex differences to naltrexone responsivity have also been observed in other substance abuse disorders, with women generally reporting greater levels of nausea, and poorer treatment adherence and outcomes39,40.
Although past literature has addressed the association between food addiction and brain responses to stimuli, few, if any, studies have investigated sex differences in brain signatures, in individuals with and without food addiction. In this study, we aim to examine the neural substrates of food addiction using resting state fMRI and determine sex differences in network connectivity in order to test the following hypotheses: (1) Individuals with food addiction show greater connectivity with regions of the reward network, such as the basal ganglia, compared to individuals with no food addiction. (2) Females with food addiction show greater connectivity with regions of the brainstem, reward, and emotional regulation networks, but lowered connectivity with regions of the brainstem, sensorimotor, and central executive regions compared to males with food addiction. (3) Higher scores on questionnaires measuring altered ingestive eating behaviors are associated with greater connectivity of reward and emotional regulation regions, especially in females.
Materials and methods
Subjects
A total of 150 participants (male: N = 47, female: N = 103), ages 18–55 years old, were recruited with the use of flyers and community advertisements and enrolled in the study at the G. Oppenheimer Center for Stress and Resilience. All participants had a BMI greater than 25 kg/m2 (obese/overweight; referred to as high BMI henceforth). Participants were excluded for the following: major medical/neurological conditions, current or past psychiatric illness, comorbidities (vascular disease and diabetes), weight loss/abdominal surgeries, pregnancy or breastfeeding, substance use, extreme strenuous exercise (> 8 h of continuous exercise per week), substance use, tobacco dependence (half a pack or more daily), and metal implants. Participants taking medications that interfere with the central nervous system or regular use of analgesic drugs were also excluded. No participants exceeded 400lbs due to magnetic resonance imaging scanning weight limits. Since female sex hormones such as estrogen are known to effect brain structure and function, only premenopausal females were included. All procedures were in compliance with institutional guidelines and were approved by the Institutional Review Board at UCLA’s Office of Protection for Research Subjects. All participants provided written informed consent.
Questionnaires
Participants were asked to fill out the Yale Food Addiction (YFAS) questionnaire, a 25-item scale developed to measure “food addiction” by assessing signs of substance-dependence symptoms in eating behavior41. The Yale Food Addiction Scale (YFAS) has been the commonly utilized measure of food addiction to highly palatable (high fat and high sugar) foods, as these foods have been linked with excess consumption and lowered appetite modulation4,41. This scale is based upon the substance dependence criteria found in the DSM-IV42 (e.g., tolerance [marked increase in amount; marked decrease in effect], withdrawal [agitation, anxiety, physical symptoms], and loss of control [eating to the point of feeling physical ill])41. A symptom count of ≥ 3 together with endorsement of clinically significant impairment or distress on the YFAS denotes diagnostic criteria food addiction (FA). Clinically significant impairment or distress was defined as having a at least one positive response to the following two questions in the YFAS questionnaire: “My behavior with respect to food and eating causes significant distress” and “I experience significant problems in my ability to function effectively (daily routine, job/school, social activities, family activities, health difficulties) because of food and eating,” similar to previously published works10. Based on a 353-respondent exploratory survey, the YFAS has displayed a good internal reliability α = 0.8641. With these measures, participants were placed into one of the following groups based on diagnostic criteria of food addiction (FA): 1. High BMI and with FA (N = 40) 2. High BMI and without FA (N = 110). 3. Females with high BMI and with FA (N = 30), 4. Females with high BMI and without FA (N = 73), 5. Males with high BMI and with FA (N = 10), and 6. Males with high BMI and without FA (N = 37).
Another questionnaire that measures abnormal ingestive behavior was also administered to participants. The General Food Cravings Questionnaire—Traits (GFCQT-r), comprised of 15 items, measures the frequency and intensity of food cravings as a way to study eating patterns and behavior43. Higher scores on the GFCQT-r are positively associated with eating pathology, low dieting success, BMI, and increased food cravings43. The GFCQT-r has shown high internal consistency (α = 0.94) and reliability in its ability to assess food cravings as a trait44.
The Hospital Anxiety/Depression Scale (HADS) is a 14-item questionnaire that attempts to identify both possible and probable cases of both anxiety disorders and depression among patients in a non-psychiatric setting45. When applied to samples of primary care, psychiatric, and somatic patients, the Hospital Anxiety/Depression Scale has shown strong internal reliability and good concurrent validity45.
Functional magnetic resonance imaging acquisition
Whole brain resting-state functional data was acquired using a 3.0 T Siemens Prisma MRI scanner (Siemens, Erlangen, Germany). Detailed information on the standardized acquisition protocols, quality control measures, and image preprocessing are provided in previously published studies35,46–50. Resting-state scans were acquired with eyes closed and an echo planar sequence with the following parameters: TE/TR = 28 ms/2000 ms, flip angle = 77 degrees, scan duration = 8m6s–10m6s, FOV = 220 mm, slices = 40 and slice thickness = 4.0 mm, and slices were obtained with whole-brain coverage. Preprocessing and quality control was done using Statistical Parametric Mapping-12 (SPM12) software and involved bias field correction, co-registration, motion correction, spatial normalization, tissue segmentation, and Fourier transformation for frequency distribution. Data was then spatially normalized to the Montreal Neurological Institute (MNI) template using the structural scans, and then smoothed using a 4 mm isotropic Gaussian kernel.
Functional network construction
Functional brain networks were constructed as previously described in35. To summarize, measures of region-to-region functional connectivity (Fisher transformed Pearson’s correlations) were computed using the CONN toolbox and the aCompCor method in Matlab51. Confounding factors such as white matter, cerebrospinal fluid, the six motion realignment parameters, and the root mean squared (RMS) values of the detrended realignment estimates were regressed out for each voxel using ordinary least squares (OLS) regression on the normalized, smoothed resting-state images52. Subjects with RMS values over 0.25 were not included. Images were then filtered using a band-pass filter (0.008/s < f < 0.08/s) to reduce the low- and high-frequency noises. Although the influence of head motion cannot be completely removed, CompCor has been shown to be particularly effective for dealing with residual motion relative to other methods53. Regions of interest were segmented with the Harvard–Oxford Subcortical atlases, the Schaefer 400 cortical atlas, and the Ascending Arousal Network brainstem atlas54,55. These atlases parceled into a total of 430 brain regions. The ROI-ROI functional connectivity between the brain regions was indexed by a matrix of Fisher Z transformed correlation coefficients reflecting the association between average temporal BOLD time series signals across all voxels in each brain region. The magnitude of the Z value represents the weights in the functional network. Permuted statistical values from ROI-to-ROI analyses were further corrected using the false discovery rate (FDR) to measure significance with p(FDR) < 0.05.
Statistical analyses
Descriptive statistical analyses were performed on the 150 subject dataset using SPSS Statistics software on the four groupings listed earlier as well as a combined FA (N = 40) and no FA (N = 110) groups. The General Linear Model (GLM) procedure in SPSS was utilized to analyze the variance and significance between the four groups across all the clinical variables. Second level connectivity analyses were run on CONN to discern sex differences in functional connectivity using GLMs. For all analyses, the disease-dependent analyses (FA vs. no FA) were controlled for age and sex, and all sex difference analyses were controlled for age. The following 5 planned contrasts were used in the statistical analyses for both the clinical variables and connectivity analyses: FA > No FA, Females with FA > Males with FA, Females with FA > Females with no FA, Males with FA > Males with no FA, Females with no FA > Males with no FA.
Results
Subject characteristics
All participants had a BMI ≥ 25 kg/m2 and were divided into two groups based on diagnostic criteria of FA by their YFAS Symptom Count scores cut off ≥ 3 together with endorsing clinically significant impairment or distress. A complete summary of all the group differences in clinical variables are summarized in Tables 1 and 2.
Table 1.
Summary of Study Demographics and Clinical Behavioral Measures.
| Food addiction (YFAS Diagnostic Score: ≥ 3, and clinical impairment and distress) | No food addiction (YFAS Diagnostic Score: < 3, and low clinical impairment and distress) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement | Females (N = 30) | Males (N = 10) | Total (N = 40) | Females (N = 73) | Males (N = 37) | Total (N = 110) | ||||||||||||
| Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | Mean | SD | N | |
| Age | 31.10 | 10.73 | 30 | 28.20 | 6.29 | 10 | 30.35 | 9.82 | 40 | 33.25 | 9.81 | 72 | 33.51 | 12.38 | 37 | 33.34 | 10.69 | 110 |
| BMI | 31.40 | 5.13 | 30 | 30.11 | 2.35 | 10 | 31.08 | 4.60 | 40 | 31.78 | 4.46 | 73 | 31.36 | 4.95 | 37 | 31.64 | 4.61 | 110 |
| Yale Food Addiction Survey (YFAS) | ||||||||||||||||||
| YFAS withdrawal | 0.73 | 0.94 | 30 | 0.90 | 1.29 | 10 | 0.78 | 1.03 | 40 | 0.05 | 0.23 | 73 | 0.08 | 0.28 | 37 | 0.06 | 0.25 | 110 |
| YFAS tolerance | 0.72 | 0.80 | 29 | 0.14 | 0.92 | 10 | 0.74 | 0.82 | 39 | 0.03 | 0.17 | 71 | 0.06 | 0.33 | 36 | 0.04 | 0.24 | 107 |
| YFAS continued use | 0.73 | 0.45 | 30 | 0.60 | 0.52 | 10 | 0.70 | 0.46 | 40 | 0.08 | 0.28 | 73 | 0.03 | 0.16 | 37 | 0.06 | 0.25 | 110 |
| YFAS given up | 0.63 | 1.16 | 30 | 1.30 | 1.25 | 10 | 0.80 | 1.20 | 40 | 0.04 | 0.26 | 72 | 0.00 | 0.00 | 37 | 0.03 | 0.21 | 109 |
| YFAS time spent | 1.00 | 0.91 | 30 | 0.90 | 0.88 | 10 | 0.98 | 0.89 | 40 | 0.05 | 0.23 | 73 | 0.03 | 0.16 | 37 | 0.05 | 0.21 | 110 |
| YFAS loss of control | 0.40 | 0.77 | 30 | 0.80 | 0.92 | 10 | 0.50 | 0.82 | 40 | 0.00 | 0.00 | 72 | 0.00 | 0.00 | 37 | 0.00 | 0.00 | 109 |
| YFAS unsuccessful cut down | 2.30 | 0.79 | 30 | 1.90 | 0.99 | 10 | 2.20 | 0.85 | 40 | 1.43 | 0.74 | 68 | 1.5 | 0.98 | 32 | 1.45 | 0.82 | 100 |
| YFAS clinical significant impairment | 0.40 | 0.72 | 30 | 0.70 | 0.82 | 10 | 0.48 | 0.75 | 40 | 0.00 | 0.00 | 73 | 0.00 | 0.00 | 37 | 0.00 | 0.00 | 110 |
| YFAS symptom count | 4.03 | 1.40 | 30 | 4.30 | 1.42 | 10 | 4.10 | 1.39 | 40 | 1.11 | 0.59 | 73 | 0.97 | 0.55 | 37 | 1.06 | 0.58 | 110 |
| General Food Craving Questionnaire (GFCQT) | ||||||||||||||||||
| GFCQT trigger | 3.78 | 1.09 | 27 | 4.20 | 0.84 | 5 | 3.84 | 1.05 | 32 | 2.37 | 1.29 | 46 | 2.11 | 1.13 | 18 | 2.30 | 1.24 | 64 |
| GFCQT control | 16.60 | 5.32 | 27 | 17.6 | 1.52 | 5 | 16.75 | 4.91 | 32 | 9.89 | 4.64 | 45 | 8.72 | 3.97 | 18 | 9.56 | 4.45 | 63 |
| GFCQT intentions | 7.04 | 1.99 | 26 | 7.40 | 1.14 | 5 | 7.10 | 1.87 | 31 | 4.73 | 2.45 | 45 | 3.83 | 2.36 | 18 | 4.48 | 2.44 | 63 |
| GFCQT preoccupation | 15.20 | 5.42 | 27 | 16.40 | 3.72 | 5 | 15.41 | 5.16 | 32 | 8.74 | 4.09 | 46 | 7.00 | 3.11 | 18 | 8.25 | 3.89 | 64 |
| GFCQT emotions | 6.70 | 2.22 | 27 | 7.80 | 1.30 | 5 | 6.88 | 2.12 | 32 | 4.04 | 1.86 | 46 | 3.28 | 1.84 | 18 | 3.83 | 1.87 | 64 |
| GFCQT total | 49.50 | 15.36 | 26 | 53.40 | 5.94 | 5 | 50.10 | 14.27 | 31 | 29.93 | 12.92 | 45 | 24.94 | 11.34 | 18 | 28.51 | 12.61 | 63 |
| Hospital Anxiety/Depression Scale (HAD) | ||||||||||||||||||
| HAD anxiety | 6.26 | 4.37 | 23 | 5.40 | 3.03 | 10 | 6,00 | 3.98 | 33 | 4.39 | 3.55 | 72 | 3.76 | 3.08 | 37 | 4.17 | 3.39 | 109 |
| HAD depression | 3.17 | 3.27 | 23 | 3.70 | 3.30 | 10 | 3.33 | 3.24 | 33 | 1.97 | 2.34 | 72 | 1.62 | 1.74 | 37 | 1.85 | 2.15 | 109 |
Demonstrates study demographics and clinical behavioral measures for individuals with and without food addiction.
BMI, Body Mass Index; GFCQT, General Food Cravings Questionnaire – Trait; HAD, Hospital Anxiety and Depression Scale; YFAS, Yale Food Addiction Scale; sd, standard deviation; p-value significant < .05.
Table 2.
Group differences in demographics and clinical behavioral measures.
| Measurement | Food addiction versus no food addiction | Females with food addiction versus males with food addiction | Females with food addiction versus females with no food addiction | Males with food addiction versus males with no food addiction | ||||
|---|---|---|---|---|---|---|---|---|
| F-statistic | p-value | F-statistic | p-value | F-statistic | p-value | F-statistic | p-value | |
| Age | 2.92 | 0.09 | 0.56 | 4.00E−03 | 6.00E−03 | 6.00E−03 | 2.01 | 0.01 |
| BMI | 9.58 | 2.00E−03 | 0.70 | 0.41 | 9.62 | 2.00E−03 | 3.01 | 0.09 |
| Height | 0.60 | 0.44 | 16.33 | 8.59E−05 | 0.15 | 0.70 | 1.29 | 0.26 |
| Weight | 5.23 | 0.02 | 2.16 | 0.14 | 7.07 | 9.00E−03 | 1.12 | 0.29 |
| Hip | 2.44 | 0.12 | 1.75 | 0.19 | 7.45 | 8.00E−03 | 0.40 | 0.84 |
| Yale Food Addiction Survey (YFAS) | ||||||||
| YFAS withdrawal | 39.76 | 3.22E−09 | 0.64 | 0.42 | 30.21 | 1.68E−07 | 16.29 | 8.72E−05 |
| YFAS tolerance | 53.50 | 1.73E−11 | 0.20 | 0.66 | 45.53 | 3.78E−10 | 19.72 | 1.79E−05 |
| YFAS continued use | 85.23 | 2.84E−16 | 1.32 | 0.26 | 89.25 | 7.95E−17 | 25.59 | 1.25E−06 |
| YFAS given up | 51.39 | 3.56E−11 | 8.34 | 4.00E−03 | 18.55 | 3.03E−05 | 33.29 | 4.62E−08 |
| YFAS time spent | 77.77 | 3.20E−15 | 0.31 | 0.58 | 77.79 | 3.18E−15 | 24.57 | 1.96E−06 |
| YFAS loss of control | 48.32 | 1.14E−10 | 7.02 | 0.01 | 19.81 | 1.69E−05 | 29.46 | 2.34E−07 |
| YFAS unsuccessful cut down | 13.11 | 4.13E−04 | 1.74 | 0.19 | 23.02 | 4.16E−06 | 1.77 | 0.19 |
| YFAS clinical significant impairment | 47.65 | 1.45E−10 | 4.63 | 0.03 | 23.32 | 3.43E−06 | 26.44 | 8.60E−07 |
| YFAS symptom count | 294.83 | 7.44E−37 | 0.70 | 0.40 | 238.71 | 1.60E−32 | 114.45 | 4.41E−20 |
| General Food Craving Questionnaire (GFCQT) | ||||||||
| GFCQT trigger | 27.54 | 9.81E−07 | 0.53 | 0.47 | 23.88 | 4.31E−06 | 12.09 | 1.00E−03 |
| GFCQT control | 35.85 | 4.19E−08 | 0.20 | 0.66 | 35.25 | 5.22E−08 | 14.34 | 2.74E−04 |
| GFCQT intentions | 21.17 | 1.37E−05 | 0.11 | 0.75 | 17.00 | 8.29E−05 | 9.67 | 0.003 |
| GFCQT preoccupation | 42.61 | 3.59E−09 | 0.31 | 0.58 | 37.97 | 1.88E−08 | 18.36 | 4.50E−05 |
| GFCQT emotions | 43.39 | 2.74E−09 | 1.34 | 0.25 | 31.84 | 1.84E−07 | 21.16 | 1.35E−05 |
| GFCQT total | 42.07 | 4.65E−09 | 0.38 | 0.54 | 36.31 | 3.62E−08 | 18.31 | 4.68E−05 |
| Hospital Anxiety/Depression Scale (HAD) | ||||||||
| HAD anxiety | 5.33 | 0.02 | 0.41 | 0.52 | 4.85 | 0.03 | 1.69 | 0.12 |
| HAD depression | 9.70 | 2.00E−03 | 0.32 | 0.57 | 4.19 | 0.04 | 5.65 | 0.02 |
BMI, Body Mass Index; GFCQT, General Food Cravings Questionnaire—Trait; HAD, Hospital Anxiety and Depression Scale; YFAS, Yale Food Addiction Scale.
p-value significant < .05.
All significant group differences in demographics and clinical behavioral measures for individuals with food addiction (females and males) and individuals with no food addiction (females and males). All bolded values are significant p < 0.05.
Individuals with high BMI and food addiction had higher reported scores on the General Food Craving Questionnaire (GFCQT) (p < 0.05), and higher anxiety (p = 0.02) and depression (p = 2.00E−3) compared to individuals with no food addiction.
Compared to females with food addiction, males with food addiction had higher scores on the following YFAS subscales: Given Up (p = 4.00E−03), Loss of Control (p = 9.00E−03), and Clinical Significant Impairment components (p = 0.03).
Within females, females with food addiction had higher scores on all components of the GFCQT (p < 0.05) and higher anxiety (p = 0.03) and depression (p = 0.04) compared to females without food addiction. Compared to males without food addiction, males with food addiction had higher scores on all components of the GFCQT (p < 0.05), and depression (p = 0.02) scales.
Food addiction dependent effects on brain connectivity
Results are detailed in Table 3 and summarized in Table 5, and depicted in Fig. 1.
Table 3.
Resting state pairwise connections in individuals with food addiction compared to individuals with no food addiction.
| Food addiction versus no food addiction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Network | Analysis unit | Network | Analysis unit | df | t | p-value | q-value | Interpretation |
| Brainstem 113 connections | ||||||||
| Bst | L MRF | CEN | L_MFG (R_ContB_PFClv_3) | 146 | 4.05 | 8.28E−05 | 0.02 | Greater |
| Bst | L_MRF | CAN | R_OrG, (R_ContB_PFClv_2) | 146 | 3.98 | 1.08E−04 | 0.02 | Greater |
| Bst | L_MRF | CAN | L_OrG, (L_ContB_PFClv_1) | 146 | 3.89 | 1.52E−04 | 0.02 | Greater |
| Emotional regulation (ERN) network connections | ||||||||
| ERN | L_InfFS, (R_ContA_PFCl_2) | SMN | R_PosCG, (R_SomMotA_16) | 146 | − 4.00 | 1.00E−04 | 0.04 | Lower |
| Sensorimotor (SMN) network connections | ||||||||
| SMN | R_PosCG, (R_SomMotA_16) | DMN | R_MTG, (R_TempPar_6) | 146 | − 4.11 | 6.57E−05 | 0.03 | Lower |
Summarizes significant disease-related differences in functional connectivity (individuals with food addiction vs. individuals with no food addiction). All connections are significant q < 0.05.
Bst, Brainstem; CAN, Central Autonomic Network; CEN, Central Executive Network; DMN, Default Mode Network; ERN, Emotional Regulation Network; InfFS, Inferior frontal sulcus; MFG, Middle frontal gyrus; MRF, Mesencephalic reticular formation; MTG, Middle Temporal Gyrus; OrG, Orbital gyri; PosCG, Postcentral Gyrus; SMN, Sensorimotor Network.
df: degrees of freedom; p value significant < .05, q value (corrected for multiple comparisons) < .05.
Table 5.
Summary of all group comparisons in network connectivity (food addiction and sex).
| Network | Food addiction versus no food addiction | Females with food addiction versus males with food addiction | Females with food addiction versus females with no food addiction | Males with food addiction vs. males with no food addiction |
|---|---|---|---|---|
| Brainstem | Food addiction ↑ | Females with food addiction ↑ | Males with food addiction ↑↓ | |
| Emotional regulation | Food addiction ↓ | Females with food addiction ↑ | Females with food addiction ↓ | |
| Salience | Females with food addiction ↑ | Males with food addiction ↑ | ||
| Sensorimotor | Food addiction ↓ | Females with food addiction ↑ | Females with food addiction ↓ | Males with food addiction ↓ |
| Central autonomic | Females with food addiction ↑ | |||
| Central executive | Females with food addiction ↓ | Females with food addiction ↑ | ||
| Default mode | Females with food addiction ↓ | Males with food addiction ↑ |
Summarizes the group (disease effect, sex effect, and within-sex contrasts) network connectivity differences.
↑, Greater connectivity; ↓, Lower Connectivity.
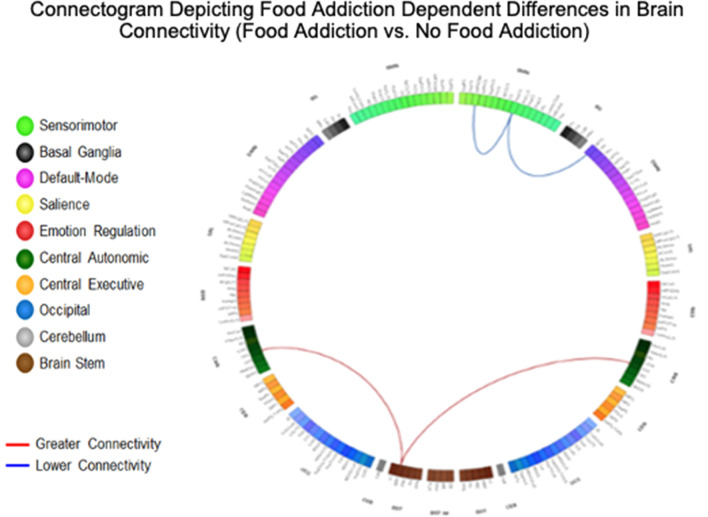
Figure 1.
Connectogram depicting food addiction dependent differences in brain connectivity (food addiction vs. no food addiction). Demonstrates significant differences in functional connectivity between individuals with food addiction and individuals with no food addiction. Analysis was performed Harvard–Oxford Subcortical atlases, the Schaefer 400 cortical atlas, and the Ascending Arousal Network brainstem atlas. Labels on the diagram are Destrieux, Harvard–Oxford Subcortical atlases, and the Ascending Arousal Network brainstem atlas equivalents. Red lines between two networks indicate greater functional connectivity, and blue lines indicate lowered functional connectivity. All connections are significant q < 0.05. Light Green: SMN (Sensorimotor Network); Black: BG (Basal Ganglia); Purple: DMN (Default Mode Network); Yellow: SAL (Salience); Red: ERN (Emotional Regulation Network); Dark Green: CAN (Central Autonomic Network); Orange: CEN (Central Executive Network); Blue: OCC (Occipital); Gray: CeB (Cerebellum); Brown: BST (Brain Stem). Bst, Brainstem; CAN, Central Autonomic Network; CEN, Central Executive Network; DMN, Default Mode Network; ERN, Emotional Regulation Network; InfFS, Inferior frontal sulcus; MFG, Middle frontal gyrus; MRF, Mesencephalic reticular formation; MTG, Middle Temporal Gyrus; OrG, Orbital gyri; PosCG, Postcentral Gyrus; SMN, Sensorimotor Network.
Brainstem connectivity
Individuals with food addiction had greater connectivity between the brainstem and middle frontal gyrus (q = 0.02), and with bilateral orbital gyri (Left and Right q = 0.02) in compared to those without food addiction.
Emotional regulation network connectivity
Individuals with food addiction had lowered connectivity between the inferior frontal sulcus and posterior central gyrus compared to those without food addiction (q = 0.04).
Sensorimotor network connectivity
Lowered connectivity was found between the postcentral gyrus and middle temporal gyrus in those with food addiction compared to those without food addiction (q = 0.03).
Sex differences in the food addiction dependent effects on brain connectivity
Results are detailed in Table 4 and summarized in Table 5, and depicted in Fig. 2.
Table 4.
Resting state pairwise connections in females with food addiction compared to males with food addiction.
| Females with food addiction versus males with food addiction | ||||||||
|---|---|---|---|---|---|---|---|---|
| Network | Analysis unit | Network | Analysis unit | df | t | p-value | q-value | Interpretation |
| Emotional regulation (ERN) network connections | ||||||||
| ERN | L_InfFGTrip (L_DefaultB_PFCv_4) | SMN | L_PaCL_S (L_SomMotA_6) | 147 | 3.99 | 1.04E-04 | 0.04 | Greater |
| Salience (SAL) network connections | ||||||||
| SAL | L_ShoInG (L_SalVentAttnA_Ins_1) | SMN | L_PaCL_S (L_SomMotA_13) | 147 | 4.31 | 2.97E-05 | 0.01 | Greater |
| SAL | R_MPosCgG_S, (R_SalVentAttnA_ParMed_1) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | 3.64 | 3.77E-04 | 0.03 | Greater |
| Sensorimotor (SMN) network connections | ||||||||
| SMN | R_SupFG, (R_DefaultA_PFCm_5) | SMN | L_MOcS_LuS, (L_VisCent_ExStr_8) | 147 | − 3.98 | 1.08E−04 | 0.04 | Lower |
| SMN | L_PaCL_S (L_SomMotA_19) | CEN | L_MFG (L_ContB_PFCd_1) | 147 | 4.54 | 1.16E−05 | 0.01 | Greater |
| SMN | L_PaCL_S (L_SomMotA_19) | CEN | L_MFG (L_DefaultB_PFCl_2) | 147 | 3.96 | 1.16E−04 | 0.03 | Greater |
| SMN | R_PosCG (R_DorsAttnB_PostC_1) | CEN | L_IntPS_TrPS (L_ContB_IPL_3) | 147 | − 3.66 | 3.51E−04 | 0.04 | Lower |
| SMN | L_PRCG, (L_SomMotA_13) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | 3.67 | 3.39E−04 | 0.03 | Greater |
| SMN | R_CS, (R_SomMotA_10) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | 3.23 | 1.53E−03 | 0.04 | Greater |
| SMN | L_PaCL_S, (L_SomMotA_19) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | 3.50 | 6.16E−04 | 0.03 | Greater |
| SMN | R_PRCG, (R_SomMotA_14) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | 3.44 | 7.57E−04 | 0.03 | Greater |
| SMN | R_PosCG, (R_SomMotA_6) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | 3.33 | 1.10E−03 | 0.04 | Greater |
| SMN | R_SupFG, (R_ContB_PFCmp_1) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | − 3.98 | 1.08E−04 | 0.04 | Lower |
| SMN | L_SupFG, (L_ContB_PFCmp_1) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | − 3.56 | 5.00E−04 | 0.03 | Lower |
| Central executive network (CEN) connections | ||||||||
| CEN | R_MFG, (R_ContB_PFCld_4) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | − 3.63 | 3.91E−04 | 0.03 | Lower |
| CEN | L_SbPS, (L_DefaultA_pCunPCC_7) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | − 3.38 | 9.28E−04 | 0.04 | Lower |
| CEN | L_IntPS_TrPS, (L_ContB_IPL_3) | DMN | R_SuMarG, (R_SalVentAttnA_ParOper_2) | 147 | − 3.96 | 1.16E−04 | 0.04 | Lower |
| CEN | L_IntPS_TrPS, (L_ContB_IPL_3) | DMN | R_SuMarG, (R_SalVentAttnA_ParOper_1) | 147 | − 3.68 | 3.27E−04 | 0.04 | Lower |
| CEN | L_IntPS_TrPS, (L_ContB_IPL_3) | DMN | R_SuMarG, (R_SalVentAttnA_ParOper_3) | 147 | − 3.60 | 4.34E−04 | 0.04 | Lower |
| CEN | L_IntPS_TrPS, (L_ContB_IPL_3) | DMN | L_SuMarG, (L_SalVentAttnA_ParOper_1) | 147 | − 3.63 | 3.91E−04 | 0.04 | Lower |
| Default mode network (DMN) connections | ||||||||
| DMN | R_SuMarG, (R_TempPar_10) | DMN | R_PrCun, (R_ContC_pCun_3) | 147 | − 4.37 | 2.34E−05 | 0.01 | Lower |
| DMN | R_PrCun, (R_ContC_pCun_3) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | − 3.70 | 3.04E−04 | 0.03 | Lower |
| DMN | L_PrCun, (L_ContC_pCun_3) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | − 3.62 | 4.05E−04 | 0.03 | Lower |
| DMN | R_AngG, (R_ContB_IPL_2) | DMN | L_SupTGLp, (L_TempPar_6) | 147 | − 3.59 | 4.50E−04 | 0.03 | Lower |
| DMN | R_Tpo, (R_LimbicA_TempPole_1) | DMN | R_SuMarG, (R_SalVentAttnA_ParOper_2) | 147 | 3.87 | 1.63E−04 | 0.04 | Greater |
| DMN | L_SupTGLp, (L_TempPar_6) | DMN | L_MPosCgG_S, (L_SomMotA_1) | 147 | 3.31 | 1.17E−03 | 0.04 | Greater |
Summarizes significant sex-related differences in functional connectivity (females with food addiction vs. males with food addiction). All connections are significant q < 0.05.
AngG, Angular gyrus; CEN, Central Executive Network; CS, Central sulcus (Rolando's fissure); DMN, Default Mode Network; ERN, Emotional Regulation Network; InfFGTrip, Triangular part of the inferior frontal gyrus; IntPS_TrPS, Intraparietal sulcus(interparietal sulcus) and transverse parietal sulci; MFG, Middle frontal gyrus; MOcS_LuS, Middle occipital sulcus and lunatus sulcus; MPosCgG_S, Middle-posterior part of the cingulate gyrus and sulcus; PaCL_S, Paracentral lobule and sulcus; PosCG, Postcentral Gyrus; PRCG, Precentral gyrus; PrCun, Precuneus; ShoInG, Short insular gyri; Tpo, temporal pole; SAL, Salience Network SbPS, Subparietal sulcus; SMN, Sensorimotor Network; SupFG, Superior frontal gyrus; SuMarG, Supramarginal gyrus; SupTGLp, Lateral aspect of the superior temporal gyrus.
df: degrees of freedom; p value significant < .05, q value (corrected for multiple comparisons) < .05.
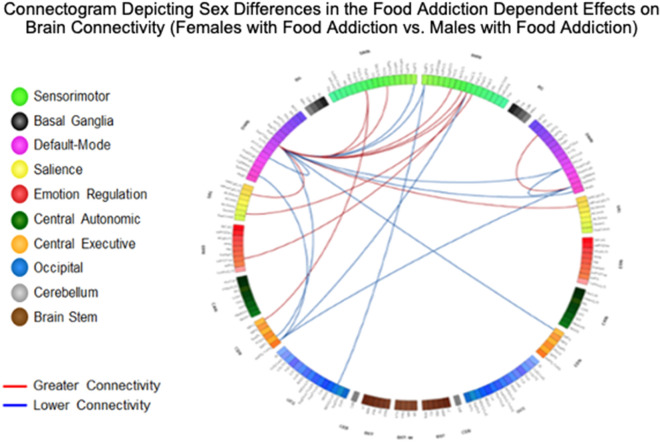
Figure 2.
Connectogram Depicting Sex Differences in the Food Addiction Dependent Effects on Brain Connectivity (Females with Food Addiction vs. Males with Food Addiction). Demonstrates significant differences in functional connectivity between females with food addiction and males with food addiction. Analysis was performed Harvard–Oxford Subcortical atlases, the Schaefer 400 cortical atlas, and the Ascending Arousal Network brainstem atlas. Labels on the diagram are Destrieux, Harvard–Oxford Subcortical atlases, and the Ascending Arousal Network brainstem atlas equivalents. Red lines between two networks indicate greater functional connectivity, and blue lines indicate lowered functional connectivity. All connections are significant q < 0.05. Light Green: SMN (Sensorimotor Network); Black: BG (Basal Ganglia); Purple: DMN (Default Mode Network); Yellow: SAL (Salience); Red: ERN (Emotional Regulation Network); Dark Green: CAN (Central Autonomic Network); Orange: CEN (Central Executive Network); Blue: OCC (Occipital); Gray: CeB (Cerebellum); Brown: BST (Brain Stem). AngG, Angular gyrus; CEN, Central Executive Network; CS, Central sulcus (Rolando's fissure); DMN, Default Mode Network; ERN, Emotional Regulation Network; InfFGTrip, Triangular part of the inferior frontal gyrus; IntPS_TrPS, Intraparietal sulcus(interparietal sulcus) and transverse parietal sulci; MFG, Middle frontal gyrus; MOcS_LuS, Middle occipital sulcus and lunatus sulcus; MPosCgG_S, Middle-posterior part of the cingulate gyrus and sulcus; PaCL_S, Paracentral lobule and sulcus; PosCG, Postcentral Gyrus; PRCG, Precentral gyrus; PrCun, Precuneus; ShoInG, Short insular gyri; Tpo, temporal pole; SAL, Salience Network SbPS, Subparietal sulcus; SMN, Sensorimotor Network; SupFG, Superior frontal gyrus; SuMarG, Supramarginal gyrus; SupTGLp, Lateral aspect of the superior temporal gyrus.
Within sex results detailed in Supplemental Tables S1, S2, and depicted in Supplemental Figures S1, S2.
Brainstem connectivity
Compared to females without food addiction, females with food addiction had greater connectivity between the brainstem and middle-anterior part of the cingulate gyrus/sulcus (q = 0.04).
Compared to males without food addiction, males with food addiction had greater connectivity between the brainstem and bilateral middle frontal gyrus, orbital gyrus, and middle occipital gyrus (q = 0.04), but lower connectivity between the brainstem and parahippocampal gyrus (q = 0.03), brainstem and precentral gyrus (q = 0.04), and the brainstem and anterior traverse collateral sulcus (q = 0.04).
Emotional regulation network connectivity
Compared to males with food addiction, females with food addiction had greater connectivity between the inferior frontal gyrus and paracentral lobule (q = 0.04).
Compared to females with no food addiction, females with food addiction had lower connectivity between the inferior frontal sulcus and subcentral gyrus (q = 0.01) and between the inferior frontal gyrus and superior parietal lobule (q = 0.03).
Salience network connectivity
Compared to males with food addiction, females with food addiction had greater connectivity between the short insular gyrus and paracentral lobule (q = 0.01) and between the middle/posterior part of the cingulate gyrus and superior temporal gyrus (q = 0.03).
Compared to males without food addiction, males with food addiction had greater connectivity between the opercular part of the inferior frontal gyrus and the inferior part of the precentral sulcus (q = 0.03).
Sensorimotor network connectivity
Compared to males with food addiction, females with food addiction had greater connectivity between the paracentral lobule/sulcus and the middle frontal gyrus (q = 0.01–0.03), and between the central sulcus, precentral gyrus and superior temporal gyrus (q = 0.03–0.04).
Compared to females without food addiction, females with food addiction had lowered connectivity between the inferior frontal gyrus and subcentral gyrus (q = 0.02) and between the precentral gyrus and lingual gyrus (q = 0.04).
Compared to males with no food addiction, males with food addiction had lowered connectivity between the paracentral lobule and middle frontal gyrus (q = 0.04).
Central autonomic network connectivity
Compared to females without food addiction, females with food addiction had greater connectivity between the anterior cingulate gyrus and sulcus and paracentral lobule (q = 3.00E-03).
Central executive network connectivity
Compared to males with food addiction, females with food addiction had lowered connectivity between the middle frontal gyrus and superior temporal gyrus (q = 0.03), intraparietal sulcus and supramarginal gyrus (q = 0.04), and superior frontal gyrus and superior temporal gyrus (q = 0.03–0.04).
Compared to females without food addiction, females with food addiction had greater connectivity between the intraparietal sulcus and orbital gyrus (q = 0.03).
Default mode network connectivity
Compared to males with food addiction, females with food addiction had lowered connectivity between the supramarginal gyrus and the precuneus (q = 0.01), precuneus and superior temporal gyrus (q = 0.03), and superior temporal gyrus and angular gyrus (q = 0.03).
Compared to males without food addiction, males with food addiction had greater connectivity between the supramarginal gyrus and the precuneus (q = 0.02) and between the temporal pole and orbital sulcus (q = 0.03).
Associations between brain connectivity and clinical variables
Food addiction associations
Results are summarized in Table 6.
Table 6.
Associations between significant functional connectivities and clinical variables.
| Food addiction versus no food addiction | |||||
|---|---|---|---|---|---|
| Food addiction | |||||
| No significant results | |||||
| No food addiction | |||||
| Functional Connectivity | Clinical variables | r | p | q | df |
| MRF to right OrG* | BMI | 0.35 | 2.10E−04 | 0.01 | 106 |
| MRF to left OrG | GFCQT intentions | − 0.38 | 2.00E−03 | 0.04 | 59 |
| Females with food addiction versus males with food addiction | |||||
| Females with food addiction | |||||
| No significant results | |||||
| Males with food addiction | |||||
| No significant results | |||||
| Females with food addiction vs females with no food addiction | |||||
| Females with food addiction | |||||
| Functional connectivity | Clinical variables | r | p | q | df |
| LC to left ACgG_S | BMI | 0.61 | 4.00E-04 | 0.04 | 27 |
| Females with no food addiction | |||||
| No significant results | |||||
| Males with food addiction versus males with no food addiction | |||||
| Males with food addiction | |||||
| No significant results | |||||
| Males with no food addiction | |||||
| No significant results | |||||
Summarizes correlations between functional connectivity and clinical variables. Comparisons include disease differences (individuals with food addiction vs. individuals with no food addiction), sex differences (females with food addiction vs. males with food addiction), disease effect within females (females with food addiction vs. females with no food addiction), and disease effect within males (males with food addiction vs. males with no food addiction). All connections are significant q < 0.05.
*Right ContB_PFClv_3.
ACgG_S, Middle-anterior part of thecingulate gyrus and sulcus; BMI, Body Mass Index; GFCQT, General Food Cravings Questionaire—Trait; LC, locus coeruleus; MRF, Mesencephalic reticular formation; OrG, Orbital gyri.
r: correlation, df: degrees of freedom; p value significant < .05, q value (corrected for multiple comparisons) < .05.
For individuals with no food addiction, connectivity between the brainstem (mesencephalic reticular formation) and central autonomic network (orbital gyrus) was positively associated with BMI (r = 0.35, q = 0.01) and negatively associated with the Intention component of the GFCQT (r = − 0.38, q = 0.04).
Sex difference associations
Results are summarized in Table 6.
In females with food addiction, connectivity between the brainstem (locus coeruleus) and emotional regulation network (middle-anterior part of the cingulate gyrus and sulcus) was positively associated with BMI (r = 0.61, q = 0.04).
Discussion
The goal of this study was to identify sex-related differences in the connectivity of brain networks in individuals meeting diagnostic criteria for food addiction. The main findings of the study were: 1) Food addiction was associated with greater connectivity among the reward regions and between the brainstem and central autonomic networks, and lower connectivity among the emotional regulation, sensorimotor, and default mode networks compared to those with no food addiction. 2. Sex differences were observed with females showing greater connectivity in the emotional regulation and salience networks and lower connectivity in the brainstem, central executive, and default mode networks. Our results support the hypothesis that altered connectivity in reward regions could increase the risk for addictive ingestive behaviors, resulting in the uncontrollable overeating patterns seen in individuals with clinically significant impairment or distress with food addiction. Additionally, our results indicate greater connectivity in particular resting-state networks, such as the emotional regulation and salience networks, which could explain the higher rates of emotional eating and food addiction seen in females. To our knowledge, this is the first study to investigate sex-related differences in resting-state connectivity in individuals with food addiction.
Food addiction dependent effects on brain connectivity
Uncontrollable eating seen in food addiction, can be explained by the reward deficiency hypothesis which states that a decreased availability of dopamine receptors, specifically D2 receptors, creates a less responsive reward system that is susceptible to addictive pathologies35,56–60. Drug-addiction studies have associated drug-dependence with changes in the dopamine receptor availability, with individuals with decreased receptors seeking greater and more frequent reward stimulation57. In individuals with food addiction, high sugar/high fat foods act as this source of stimulation, as such these ultra-processed foods serve as potent reward triggers that increase synaptic dopamine concentration overriding internal satiety cues57,61. Hence, the constant hypodopaminergic state of these individuals results in increased levels of food cravings and overindulgence of ultra-processed foods as a compensatory attempt to derive the euphoric effects of reward network stimulation62,63. Studies have also suggested that disruptions in the mesolimbic pathways associated with addiction behaviors effects both the DA reward circuits and DA pathways that lead to increased stress reactivity and disruption in interoceptive awareness29,57,64.
Consistent with the reward deficiency hypothesis, our results indicated greater connectivity between the brainstem and reward regions in individuals with food addiction. This could be associated with dopaminergic dysregulation, as the increased intake of ultra-processed foods, in those with food addiction leads to a more persistent stimulation of the reward pathway as a compensatory mechanism for the decreased receptor sensitivity and availability19,33,65. On the other hand, in those with no food addiction, a greater connectivity between the brainstem and the central autonomic network (orbital gyrus) was negatively associated with GFCQT scores, a measure of food cravings. Perhaps, in individuals with food addiction, increases in brainstem connectivity could be a result of an increased frequency of dopamine release in response to select salient inputs, such as ultra-processed foods, causing heightened reactions and cravings to consume larger quantities of such foods66,67. This increased brainstem connectivity, could be a counterbalancing mechanism for the reduction in dopamine receptors in the reward circuit per the reward deficiency hypothesis26,32,57,68.
In addition to decreased reward sensitivity, hyperactivation of the locus coeruleus could also contribute to the habitual overconsumption of ultra-processed foods seen in individuals with food addiction. The locus coeruleus plays a major role in an individual’s response to external stressors, with greater activation being linked to consistent higher levels of norepinephrine seen in individuals with anxiety69. Individuals in a sustained state of anxiety, consistently have elevated levels of norepinephrine which result in cortical atrophy leading to reduced cognitive and attentional control70. Thus, greater activation of the locus coeruleus could translate to a greater susceptibility to maladaptive, habitual behaviors such as food addiction due a combination of decreased internal regulation capabilities and perpetual anxiety in individuals with food addiction69,70.
Our results mirrored these predicted differences, with individuals with food addiction displaying significantly higher rates of anxiety, particularly in females. Additionally, greater connectivity between the locus coeruleus and the emotional regulation network was positively associated with BMI in females with food addiction. These results suggest the role of the locus coeruleus in weight management may contribute to the food addictive behaviors in an attempt to cope with anxiety.
Sex differences in the food addiction dependent effects on brain connectivity
Compared to males with food addiction, females with food addiction displayed significantly higher rates of emotional overeating37,38. Emotional overeating is related to cognitive alterations in two aspects: the inability to regulate emotional states and the inability to limit consumption of especially ultra-processed foods when in a compromised emotional state71. According to the self-medication hypothesis, individuals with difficulties in self-regulation turn to specific actions or external substances to help relieve the negative emotional impact of stimuli, resulting in substance abuse disorders72,73. In the case of food addiction, females with food addiction utilize high sugar/high fat foods as their sources of stimulation, in an attempt to lessen their emotional load57. The second aspect of emotional overeating, the reduced capability to limit intake of ultra-processed foods, is a result of lowered cognitive inhibitions created by heightened reactivity to food cues and attenuated satiety responses74–76 .
According to the incentive salience model, the motivational value of a “reward” is based on trigger cues, such as the sight, smell, and taste of ultra-processed foods77. In cases of addiction, other rewards are perceived to have a diminished incentive value relative to the drug causing compulsive behavior, reorientation of attentional resources, and downregulation of cognitive control regions57,78. This creates an increased reward being placed on ultra-processed foods, resulting in individuals seeking out these types of food beyond their basic homeostatic needs and even when their consumption leads to negative consequences57. In addition, due to the chronic consumption of high sugar/high fat foods, satiety signals in these individuals are compromised, as the palatability of ultra-processed food stimuli overrides an individual’s energy need79. These altered satiety cues in conjunction with increased response to food cues translate into more frequent and greater overeating behaviors.
Our results support this emotional overeating model, with females with food addiction exhibiting greater connectivity between the emotional regulation and salience networks compared to males with food addiction. Greater activation of the emotional regulation network aligns with the self-medication hypothesis, as females with food addiction engage in uncontrollable eating behaviors as an artificial coping mechanism to manage their emotional response to negative stimuli, similar to that of a drug46,80–84. The tendency of females with food addiction to actively seek out and consume ultra-processed foods in response to negative emotional stimuli, could refer to the relationship between greater emotional instability and increased food-seeking behavior as predicted in our model67,85.
The greater connectivity observed in the salience network, particularly between the salience and default mode network, is similarly consistent with our hypothesis and the incentive salience model77,86. The salience network adjusts attentional resources to salient sensory stimuli, with the observed greater activation suggesting an increased attentional value on food cues12,81,87–90. As females with food addiction place greater value and attention towards ultra-processed foods, the “incentive value” of this stimuli increases, resulting in a greater subconscious food focus and food cravings77,86,91. Additionally, this greater preoccupation towards ultra-processed foods, seen in females with food addiction, may also cause inhibition of cognitive regions in areas of the default-mode network leading to increased decisional impulsivity and lowered inhibitory responses in response to salient stimuli92,93.
Compared to males with food addiction, females with food addiction had greater connectivity among the sensorimotor network, particularly in the regions controlling evaluation of external stimuli94–97. These regions, namely the precentral gyrus and paracentral lobule, have been linked with altered explicit memory and inappropriate cognitive evaluations, potentially resulting in an increased motivational reward placed on food-related stimuli in females with food addiction98,99.
Additionally, when compared to females with no food addiction, those with food addiction exhibited lower connectivity in the sensorimotor network in regions involved in inhibition and attentional control, such as the inferior frontal gyrus100. Hypoactivation in the inferior frontal gyrus suggests increased impulsivity and the reinforcement of habit-forming systems, leading to weakened attempts to disengage with compulsive eating behaviors101.
Consistent with our model, females with food addiction had lowered connectivity within the default mode network compared to males with food addiction, potentially explaining the reduced cognitive control and greater preoccupation with food-related stimuli predicted in females with food addiction102. The default mode network plays a major role in self-generated and subconscious thought, displaying greater activation when resting103,104. Specifically, the dorsal medial prefrontal cortex subsystem has shown to preferentially activate during subconscious decision-making processes regarding one’s present mental state or situation105,106. Lower activation in this subsystem could translate to decreased subconscious inhibitions and altered attentional processing as a result of the top-down inhibition on cognitive control regions102,107,108. Weakened cognitive control circuits, upon interacting with the reward and emotional regulation system, reorient attentional resources in those with food addiction and create a vicious cycle of chronic overconsumption, impaired appetite regulation, and heightened food focus109. Additionally, consistent with the incentive salience model, our results showed lowered connectivity between the default mode network and the central executive network in females with food addiction, indicating that the lowered awareness of the present state results in less sensitive internal body cues and chronic food consumption behaviors as predicted in our hypothesis74–76.
Limitations and future research
Due to the cross-sectional design of the study, we were unable to address questions of causality between functional connectivity differences and food addiction. Future longitudinal studies are needed to determine if the observed connectivity differences between brain networks in individuals with food addiction are a premorbid state, or if they are a consequence of food addiction and associated metabolic changes. The integration of systemic inflammatory markers and metabolites derived from gut microbiota as mediators can help gain a more comprehensive understanding of food addiction in future mechanistic studies. While this study focused on resting-state connectivity differences, future research should consider investigating connectivity differences in response to ultra-processed food cues in individuals with food addiction. In order to be able to combine data to obtain a large sample size for subgroup analyses, all participants in this study completed the earlier version of the YFAS vs. the updated YFAS 2.0 questionnaire which is not only more psychometrically sound but has a stronger threshold of FA associated with obesity. This may have contributed to a reduced sensitivity in observing significant results, which will need to be validated in future studies using the YFAS 2.0.
Conclusions and clinical implications
When viewed together with previous findings, our results show greater connectivity in reward regions, indicative of the altered function of the reward circuit in individuals with food addiction. Sex differences in functional connectivity reveal that females with food addiction engage in more emotional eating behaviors while males with food addiction exhibit greater cognitive control and homeostatic processing. Since connectivity differences differ among males and females, this study contributes to the understanding of the nuances driving the sex-specific pathophysiology of food addiction. These mechanistic pathways may have clinical implications for understanding the variability in response to diet interventions and the need for more effective, targeted treatments for those with food addiction, especially females. Most clinical trials do not report sex differences related to treatment responses, but a few existing reports suggest that women are less likely to complete treatment, tend to lose less weight than men, have greater unsuccessful attempts to maintain weight loss resulting in the well-known “YoYo” diet phenomenon, and have limited responses to pharmacological treatments12,110,111. Therefore, treatments need to focus on the different sex-related eating patterns, such as women gaining weight via eating ultra-processed foods more frequently during emotional or stressful times, as compared to men, who gain weight via the consumption of larger meals. Therapeutic approaches would need to target different nodes of the brain related to food addiction, and individualization of treatments would need to be based on sex-related differences in order to improve greater clinical benefits13,112.
Supplementary Information
Acknowledgements
This research was supported by grants from the National Institutes of Health including K23 DK106528 (AG), R01 DK048351 (EAM), P50 DK064539 (EAM), and pilot funds were provided for brain scanning by the Ahmanson-Lovelace Brain Mapping Center.
Author contributions
S.R.: analysis, drafting of the manuscript, critical revision of the manuscript for important intellectual content. R.B., B.P.: analysis, drafting of the manuscript. V.O., A.A., B.N.: data interpretation, critical revision of the manuscript for important intellectual content. P.V.: statistical analysis. J.S.: data collection. E.A.M.: funding, study concept and design, critical revision of the manuscript for important intellectual content. A.G.: funding, study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, study supervision, statistical analysis, technical support.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83116-0.
References
- 1.Craig, M. D., Hales, M., Carroll, M. D., Cheryl, M.S.P.H., Fryar, D., & Ogden, C. L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018, NCHS Data Brief (2020). [PubMed]
- 2.Panuganti KK, Kshirsagar RK. Obesity. Treasure Island (FL): StatPearls; 2020. [Google Scholar]
- 3.Zhou L, Zeng Q, Jin S, Cheng G. The impact of changes in dietary knowledge on adult overweight and obesity in China. PLoS ONE. 2017;12:e0179551. doi: 10.1371/journal.pone.0179551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lerma-Cabrera JM, Carvajal F, Lopez-Legarrea P. Food addiction as a new piece of the obesity framework. Nutr. J. 2016;15:5. doi: 10.1186/s12937-016-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pedram P, Wadden D, Amini P, Gulliver W, Randell E, Cahill F, Vasdev S, Goodridge A, Carter JC, Zhai G, Ji Y, Sun G. Food addiction: its prevalence and significant association with obesity in the general population. PLoS ONE. 2013;8:e74832. doi: 10.1371/journal.pone.0074832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold MS, Graham NA, Cocores JA, Nixon SJ. Food addiction? J. Addict. Med. 2009;3:42–45. doi: 10.1097/ADM.0b013e318199cd20. [DOI] [PubMed] [Google Scholar]
- 7.Schulte EM, Avena NM, Gearhardt AN. Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS ONE. 2015;10:e0117959. doi: 10.1371/journal.pone.0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carter A, Hendrikse J, Lee N, Yucel M, Verdejo-Garcia A, Andrews ZB, Hall W. The neurobiology of "food addiction" and its implications for obesity treatment and policy. Annu. Rev. Nutr. 2016;36:105–128. doi: 10.1146/annurev-nutr-071715-050909. [DOI] [PubMed] [Google Scholar]
- 9.Gearhardt, A. N., Corbin, W. R., & Brownell, K. D. Development of the Yale Food Addiction Scale Version 2.0. Psychol. Addict. Behav.30, 113–121 (2016). [DOI] [PubMed]
- 10.Gordon, E. L., Ariel-Donges, A. H., Bauman, V., & Merlo, L. J. What is the evidence for "food addiction”? A systematic review. Nutrients10 (2018). [DOI] [PMC free article] [PubMed]
- 11.Gearhardt AN, Davis C, Kuschner R, Brownell KD. The addiction potential of hyperpalatable foods. Curr. Drug Abuse Rev. 2011;4:140–145. doi: 10.2174/1874473711104030140. [DOI] [PubMed] [Google Scholar]
- 12.Hallam J, Boswell RG, DeVito EE, Kober H. Gender-related differences in food craving and obesity. Yale J. Biol. Med. 2016;89:161–173. [PMC free article] [PubMed] [Google Scholar]
- 13.Striegel-Moore RH, Rosselli F, Perrin N, DeBar L, Wilson GT, May A, Kraemer HC. Gender difference in the prevalence of eating disorder symptoms. Int. J. Eat. Disord. 2009;42:471–474. doi: 10.1002/eat.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengor G, Gezer C. Food addiction and its relationship with disordered eating behaviours and obesity. Eat Weight Disord. 2019;24:1031–1039. doi: 10.1007/s40519-019-00662-3. [DOI] [PubMed] [Google Scholar]
- 15.Yu Z, Indelicato NA, Fuglestad P, Tan M, Bane L, Stice C. Sex differences in disordered eating and food addiction among college students. Appetite. 2018;129:12–18. doi: 10.1016/j.appet.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 16.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 18.van den Heuvel, M. P., & Hulshoff Pol, H. E. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur. Neuropsychopharmacol.20, 519–534 (2010). [DOI] [PubMed]
- 19.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogenkamp PS, Zhou W, Dahlberg LS, Stark J, Larsen AL, Olivo G, Wiemerslage L, Larsson EM, Sundbom M, Benedict C, Schioth HB. Higher resting-state activity in reward-related brain circuits in obese versus normal-weight females independent of food intake. Int. J. Obes. (Lond.) 2016;40:1687–1692. doi: 10.1038/ijo.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Borsook D, Upadhyay J, Chudler EH, Becerra L. A key role of the basal ganglia in pain and analgesia—insights gained through human functional imaging. Mol. Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Contreras-Rodriguez O, Martin-Perez C, Vilar-Lopez R, Verdejo-Garcia A. Ventral and dorsal striatum networks in obesity: link to food craving and weight gain. Biol. Psychiatry. 2017;81:789–796. doi: 10.1016/j.biopsych.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Tomasi D, Volkow ND. Resting functional connectivity of language networks: characterization and reproducibility. Mol. Psychiatry. 2012;17:841–854. doi: 10.1038/mp.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baik JH. Dopamine signaling in reward-related behaviors. Front. Neural Circuits. 2013;7:152. doi: 10.3389/fncir.2013.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelchat ML. Food addiction in humans. J. Nutr. 2009;139:620–622. doi: 10.3945/jn.108.097816. [DOI] [PubMed] [Google Scholar]
- 28.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch. Gen. Psychiatry. 2011;68:808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R. Food and drug reward: overlapping circuits in human obesity and addiction. Curr. Top. Behav. Neurosci. 2012;11:1–24. doi: 10.1007/7854_2011_169. [DOI] [PubMed] [Google Scholar]
- 30.Lindgren E, Gray K, Miller G, Tyler R, Wiers CE, Volkow ND, Wang GJ. Food addiction: a common neurobiological mechanism with drug abuse. Front. Biosci. (Landmark Ed) 2018;23:811–836. doi: 10.2741/4618. [DOI] [PubMed] [Google Scholar]
- 31.Volkow ND, Wise RA, Baler R. The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci. 2017;18:741–752. doi: 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- 32.Adinoff B. Neurobiologic processes in drug reward and addiction. Harv. Rev. Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Volkow ND, Wang GJ, Fowler JS, Tomasi D. Addiction circuitry in the human brain. Annu. Rev. Pharmacol. Toxicol. 2012;52:321–336. doi: 10.1146/annurev-pharmtox-010611-134625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao AM, Loughead J, Bakizada ZM, Hopkins CM, Geliebter A, Gur RC, Wadden TA. sex/gender differences in neural correlates of food stimuli: a systematic review of functional neuroimaging studies. Obes. Rev. 2017;18:687–699. doi: 10.1111/obr.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta A, Mayer EA, Acosta JR, Hamadani K, Torgerson C, van Horn JD, Chang L, Naliboff B, Tillisch K, Labus JS. Early adverse life events are associated with altered brain network architecture in a sex- dependent manner. Neurobiol. Stress. 2017;7:16–26. doi: 10.1016/j.ynstr.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haase L, Green E, Murphy C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite. 2011;57:421–434. doi: 10.1016/j.appet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steward T, Pico-Perez M, Mata F, Martinez-Zalacain I, Cano M, Contreras-Rodriguez O, Fernandez-Aranda F, Yucel M, Soriano-Mas C, Verdejo-Garcia A. Emotion regulation and excess weight: impaired affective processing characterized by dysfunctional insula activation and connectivity. PLoS ONE. 2016;11:e0152150. doi: 10.1371/journal.pone.0152150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Zhu W, Wong CT, Thanos PK, Geliebter A, Biegon A, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc. Natl. Acad. Sci. USA. 2009;106:1249–1254. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suh JJ, Pettinati HM, Kampman KM, O'Brien CP. Gender differences in predictors of treatment attrition with high dose naltrexone in cocaine and alcohol dependence. Am. J. Addict. 2008;17:463–468. doi: 10.1080/10550490802409074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pettinati HM, Kampman KM, Lynch KG, Suh JJ, Dackis CA, Oslin DW, O'Brien CP. Gender differences with high-dose naltrexone in patients with co-occurring cocaine and alcohol dependence. J. Substain. Abuse Treat. 2008;34:378–390. doi: 10.1016/j.jsat.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite. 2009;52:430–436. doi: 10.1016/j.appet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 42.A.P. Association, Diagnostic and Statistical Manual of Mental Disorders, 5th ed. edn. (Washington, DC, 2013).
- 43.Meule A, Teran CB, Berker J, Grundel T, Mayerhofer M, Platte P. On the differentiation between trait and state food craving: half-year retest-reliability of the Food Cravings Questionnaire-Trait-reduced (FCQ-T-r) and the Food Cravings Questionnaire-State (FCQ-S) J. Eat Disord. 2014;2:25. doi: 10.1186/s40337-014-0025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meule A, Hermann T, Kubler A. A short version of the Food Cravings Questionnaire-Trait: the FCQ-T-reduced. Front. Psychol. 2014;5:190. doi: 10.3389/fpsyg.2014.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale: an updated literature review. J. Psychosom. Res. 2002;52:69–77. doi: 10.1016/S0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 46.Gupta A, Mayer EA, Hamadani K, Bhatt R, Fling C, Alaverdyan M, Torgerson C, Ashe-McNalley C, Van Horn JD, Naliboff B, Tillisch K, Sanmiguel CP, Labus JS. Sex differences in the influence of body mass index on anatomical architecture of brain networks. Int. J. Obes. (Lond.) 2017;41:1185–1195. doi: 10.1038/ijo.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta A, Mayer EA, Labus JS, Bhatt RR, Ju T, Love A, Bal A, Tillisch K, Naliboff B, Sanmiguel CP, Kilpatrick LA. Sex commonalities and differences in obesity-related alterations in intrinsic brain activity and connectivity. Obesity (Silver Spring) 2018;26:340–350. doi: 10.1002/oby.22060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gupta A, Mayer EA, Sanmiguel CP, Van Horn JD, Woodworth D, Ellingson BM, Fling C, Love A, Tillisch K, Labus JS. Patterns of brain structural connectivity differentiate normal weight from overweight subjects. Neuroimage Clin. 2015;7:506–517. doi: 10.1016/j.nicl.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Labus JS, Naliboff B, Kilpatrick L, Liu C, Ashe-McNalley C, Dos Santos IR, Alaverdyan M, Woodworth D, Gupta A, Ellingson BM, Tillisch K, Mayer EA. Pain and Interoception Imaging Network (PAIN): a multimodal, multisite, brain-imaging repository for chronic somatic and visceral pain disorders. Neuroimage. 2016;124:1232–1237. doi: 10.1016/j.neuroimage.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Labus JS, Van Horn JD, Gupta A, Alaverdyan M, Torgerson C, Ashe-McNalley C, Irimia A, Hong JY, Naliboff B, Tillisch K, Mayer EA. Multivariate morphological brain signatures predict patients with chronic abdominal pain from healthy control subjects. Pain. 2015;156:1545–1554. doi: 10.1097/j.pain.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- 52.Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, Davatzikos C, Gur RC, Gur RE, Bassett DS, Satterthwaite TD. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage. 2017;154:174–187. doi: 10.1016/j.neuroimage.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irimia A, Chambers MC, Torgerson CM, Van Horn JD. Circular representation of human cortical networks for subject and population-level connectomic visualization. Neuroimage. 2012;60:1340–1351. doi: 10.1016/j.neuroimage.2012.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo XN, Holmes AJ, Eickhoff SB, Yeo BTT. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb. Cortex. 2018;28:3095–3114. doi: 10.1093/cercor/bhx179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dang LC, Samanez-Larkin GR, Castrellon JJ, Perkins SF, Cowan RL, Zald DH. Associations between dopamine D2 receptor availability and BMI depend on age. Neuroimage. 2016;138:176–183. doi: 10.1016/j.neuroimage.2016.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volkow ND, Wang GJ, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn. Sci. 2011;15:37–46. doi: 10.1016/j.tics.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang GJ, Tomasi D, Backus W, Wang R, Telang F, Geliebter A, Korner J, Bauman A, Fowler JS, Thanos PK, Volkow ND. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Gold MS, Badgaiyan RD, Blum K. A shared molecular and genetic basis for food and drug addiction: overcoming hypodopaminergic trait/state by incorporating dopamine agonistic therapy in psychiatry. Psychiatr. Clin. N. Am. 2015;38:419–462. doi: 10.1016/j.psc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 60.Blum K, Thanos PK, Wang GJ, Febo M, Demetrovics Z, Modestino EJ, Braverman ER, Baron D, Badgaiyan RD, Gold MS. The food and drug addiction epidemic: targeting dopamine homeostasis. Curr. Pharm. Des. 2018;23:6050–6061. doi: 10.2174/1381612823666170823101713. [DOI] [PubMed] [Google Scholar]
- 61.Davis C. From passive overeating to "food addiction": a spectrum of compulsion and severity. ISRN Obes. 2013;2013:435027. doi: 10.1155/2013/435027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo L, Wu K, Lu Y, Gao S, Kong X, Lu F, Wu F, Wu H, Wang J. Increased functional connectivity between medulla and inferior parietal cortex in medication-free major depressive disorder. Front. Neurosci. 2018;12:926. doi: 10.3389/fnins.2018.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meule A, Kubler A. Food cravings in food addiction: the distinct role of positive reinforcement. Eat Behav. 2012;13:252–255. doi: 10.1016/j.eatbeh.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. USA. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leyton M. What's deficient in reward deficiency? J. Psychiatry Neurosci. 2014;39:291–293. doi: 10.1503/jpn.140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis M. Addiction and the brain: development, not disease. Neuroethics. 2017;10:7–18. doi: 10.1007/s12152-016-9293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Siciliano, C. A., Noamany, H., Chang, C. J., Brown, A. R., Chen, X., Leible, D., Lee, J. J., Wang, J., Vernon, A. N., Vander Weele, C. M., Kimchi, E. Y., Heiman, M., & Tye, K. M. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science366, 1008–1012 (2019). [DOI] [PMC free article] [PubMed]
- 68.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morris LS, McCall JG, Charney DS, Murrough JW. The role of the locus coeruleus in the generation of pathological anxiety. Brain Neurosci. Adv. 2020;4:2398212820930321. doi: 10.1177/2398212820930321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Valentino RJ, Volkow ND. Drugs, sleep, and the addicted brain. Neuropsychopharmacology. 2020;45:3–5. doi: 10.1038/s41386-019-0465-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiedemann AA, Ivezaj V, Barnes RD. Characterizing emotional overeating among patients with and without binge-eating disorder in primary care. Gen. Hosp. Psychiatry. 2018;55:38–43. doi: 10.1016/j.genhosppsych.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Azizi A, Borjali A, Golzari M. The effectiveness of emotion regulation training and cognitive therapy on the emotional and addictional problems of substance abusers. Iran. J. Psychiatry. 2010;5:60–65. [PMC free article] [PubMed] [Google Scholar]
- 73.Khantzian EJ. The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv. Rev. Psychiatry. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- 74.James GA, Gold MS, Liu Y. Interaction of satiety and reward response to food stimulation. J. Addict. Dis. 2004;23:23–37. doi: 10.1300/J069v23n03_03. [DOI] [PubMed] [Google Scholar]
- 75.Nijs IM, Muris P, Euser AS, Franken IH. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite. 2010;54:243–254. doi: 10.1016/j.appet.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, von Deneen KM, Tian J, Gold MS, Liu Y. Food addiction and neuroimaging. Curr. Pharm. Des. 2011;17:1149–1157. doi: 10.2174/138161211795656855. [DOI] [PubMed] [Google Scholar]
- 77.Berridge KC. 'Liking' and 'wanting' food rewards: brain substrates and roles in eating disorders. Physiol. Behav. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith DG, Robbins TW. The neurobiological underpinnings of obesity and binge eating: a rationale for adopting the food addiction model. Biol. Psychiatry. 2013;73:804–810. doi: 10.1016/j.biopsych.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 80.Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front. Neuroendocrinol. 2014;35:303–319. doi: 10.1016/j.yfrne.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Geliebter, A., Pantazatos, S. P., McOuatt, H., Puma, L., Gibson, C. D., & Atalayer, D. Sex-based fMRI differences in obese humans in response to high vs. low energy food cues. Behav. Brain Res.243, 91–96 (2013). [DOI] [PMC free article] [PubMed]
- 82.Gianini LM, White MA, Masheb RM. Eating pathology, emotion regulation, and emotional overeating in obese adults with Binge Eating Disorder. Eat Behav. 2013;14:309–313. doi: 10.1016/j.eatbeh.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horstmann A, Busse FP, Mathar D, Muller K, Lepsien J, Schlogl H, Kabisch S, Kratzsch J, Neumann J, Stumvoll M, Villringer A, Pleger B. Obesity-related differences between women and men in brain structure and goal-directed behavior. Front. Hum. Neurosci. 2011;5:58. doi: 10.3389/fnhum.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mariani JJ, Khantzian EJ, Levin FR. The self-medication hypothesis and psychostimulant treatment of cocaine dependence: an update. Am. J. Addict. 2014;23:189–193. doi: 10.1111/j.1521-0391.2013.12086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, Qian RB, Xu HS, Hu X, Zhang DR. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS ONE. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016;71:670–679. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Connolly L, Coveleskie K, Kilpatrick LA, Labus JS, Ebrat B, Stains J, Jiang Z, Tillisch K, Raybould HE, Mayer EA. Differences in brain responses between lean and obese women to a sweetened drink. Neurogastroenterol. Motil. 2013;25:579–e460. doi: 10.1111/nmo.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park S, Shin WS. Differences in eating behaviors and masticatory performances by gender and obesity status. Physiol. Behav. 2015;138:69–74. doi: 10.1016/j.physbeh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 89.Peters SK, Dunlop K, Downar J. Cortico-striatal-thalamic loop circuits of the salience network: a central pathway in psychiatric disease and treatment. Front. Syst. Neurosci. 2016;10:104. doi: 10.3389/fnsys.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stice E, Burger KS, Yokum S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am. J. Clin. Nutr. 2013;98:1377–1384. doi: 10.3945/ajcn.113.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang J, Berridge KC, Tindell AJ, Smith KS, Aldridge JW. A neural computational model of incentive salience. PLoS Comput. Biol. 2009;5:e1000437. doi: 10.1371/journal.pcbi.1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hong JS, Kim SM, Bae S, Han DH. Impulsive internet game play is associated with increased functional connectivity between the default mode and salience networks in depressed patients with short allele of serotonin transporter gene. Front. Psychiatry. 2018;9:125. doi: 10.3389/fpsyt.2018.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McFadden KL, Tregellas JR, Shott ME, Frank GK. Reduced salience and default mode network activity in women with anorexia nervosa. J. Psychiatry Neurosci. 2014;39:178–188. doi: 10.1503/jpn.130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Furlong PL, Hobson AR, Aziz Q, Barnes GR, Singh KD, Hillebrand A, Thompson DG, Hamdy S. Dissociating the spatio-temporal characteristics of cortical neuronal activity associated with human volitional swallowing in the healthy adult brain. Neuroimage. 2004;22:1447–1455. doi: 10.1016/j.neuroimage.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 95.Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs—a quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- 96.McCaffery JM, Haley AP, Sweet LH, Phelan S, Raynor HA, Del Parigi A, Cohen R, Wing RR. Differential functional magnetic resonance imaging response to food pictures in successful weight-loss maintainers relative to normal-weight and obese controls. Am. J. Clin. Nutr. 2009;90:928–934. doi: 10.3945/ajcn.2009.27924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/S0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 98.Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS ONE. 2013;8:e60393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang L, Wang K, Liu JH, Wang YP. Altered default mode and sensorimotor network connectivity with striatal subregions in primary insomnia: a resting-state multi-band fMRI study. Front. Neurosci. 2018;12:917. doi: 10.3389/fnins.2018.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3:760–773. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Syan SK, Owens MM, Goodman B, Epstein LH, Meyre D, Sweet LH, MacKillop J. Deficits in executive function and suppression of default mode network in obesity. NeuroImage Clin. 2019;24:102015. doi: 10.1016/j.nicl.2019.102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss AL, Menon V. Persistent default-mode network connectivity during light sedation. Hum. Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qi H, Liu H, Hu H, He H, Zhao X. Primary disruption of the memory-related subsystems of the default mode network in Alzheimer's disease: resting-state functional connectivity MRI study. Front. Aging Neurosci. 2018;10:344. doi: 10.3389/fnagi.2018.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann. N. Y. Acad. Sci. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalon E, Hong JY, Tobin C, Schulte T. Psychological and neurobiological correlates of food addiction. Int. Rev. Neurobiol. 2016;129:85–110. doi: 10.1016/bs.irn.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Robertson C, Avenell A, Boachie C, Stewart F, Archibald D, Douglas F, Hoddinott P, van Teijlingen E, Boyers D. Should weight loss and maintenance programmes be designed differently for men? A systematic review of long-term randomised controlled trials presenting data for men and women: the ROMEO project. Obes. Res. Clin. Pract. 2016;10:70–84. doi: 10.1016/j.orcp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 111.Schulte EM, Gearhardt AN. Associations of food addiction in a sample recruited to be nationally representative of the United States. Eur. Eat Disord. Rev. 2018;26:112–119. doi: 10.1002/erv.2575. [DOI] [PubMed] [Google Scholar]
- 112.Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL. The prevalence of food addiction as assessed by the Yale Food Addiction Scale: a systematic review. Nutrients. 2014;6:4552–4590. doi: 10.3390/nu6104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hollemann, C. Haase, F., Rienacker, M., Barnscheidt, V., Krugener, J., Folchert, N., Brendel, R., Richter, S., Grosser, S., Sauter, E., Hubner, J., Oestreich, M., & Peibst, R. Separating the two polarities of the POLO contacts of an 26.1%-efficient IBC solar cell. Sci. Rep.10, 658 (2020). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.