Abstract
Fibroblast growth factor receptor (FGFR) tyrosine kinases, which are expressed on the cell membrane, are involved in a wide range of biological functions such as cell proliferation, survival, migration, and differentiation. The identification of FGFR fusions and other alterations in a wide range of solid tumors, including cholangiocarcinoma and bladder cancer, has resulted in the development of several selective FGFR inhibitors for use in these indications, for example, infigratinib, erdafitinib, derazantinib, pemigatinib, and futibatinib. In addition to the typical adverse events associated with tyrosine kinases, the FGFR inhibitors appear to give rise to a number of adverse events affecting the skin. Here we describe these skin events, which include the more common nail adverse events (e.g., onycholysis), palmar–plantar erythrodysesthesia syndrome, and stomatitis, as well as less common reactions such as calciphylaxis. This review aims to provide oncologists with an understanding of these dermatologic events and proposes guidelines for the management of treatment‐emergent dermatologic adverse events. Awareness of possible adverse events associated with specific drugs should allow physicians to educate patients as to what to expect and implement effective management plans at the earliest possible opportunity, thereby preventing premature discontinuation while maintaining patient quality of life.
Implications for Practice
Identification of fibroblast growth factor receptor (FGFR) aberrations in cholangiocarcinoma and bladder cancer led to development of selective FGFR inhibitors for these indications, based on clinical benefit and safety profiles. The most frequent adverse events (AEs) include those affecting skin, hair, and nails, a unique class effect of these agents. These are usually mild to moderate in severity. This work reviewed skin AEs reported with FGFR inhibitors and provides management guidelines for physicians, aiming to increase awareness of skin events and provide effective treatment strategies. Early intervention and effective management may improve treatment adherence, optimize outcomes, and improve quality of life.
Keywords: Dermatologic, Fibroblast growth factor receptor, Drug‐related side effects and adverse events, Guidelines
Short abstract
This review provides oncologists with an understanding of dermatologic adverse events related to the use of FGFR inhibitors and proposes guidelines for treatment.
Introduction
Fibroblast growth factors (FGFs) and their receptors control a wide range of biological functions, regulating cellular proliferation, survival, migration, and differentiation [1]. Twenty‐two mammalian FGFs have been identified to date, many of which depend on interaction with FGF receptors (FGFRs) for their biological effects [2]. The human FGFR family comprises five members: FGFR1, FGFR2, FGFR3, FGFR4, and FGFR5. FGFRs 1–4 are receptor tyrosine kinases consisting of an extracellular ligand binding domain and a tyrosine kinase domain, which are expressed on the cell membrane [3]. FGFR fusions and other alterations have been reported in a wide range of solid tumors, including cholangiocarcinoma [4], bladder cancer [5], lung cancer [6], and glioblastoma [7]. Identification of targetable genomic alterations has resulted in the development of several FGF/FGFR‐directed therapies, primarily small‐molecule tyrosine kinase inhibitors (TKIs) or multikinase inhibitors, with differing profiles (Table 1). A number of selective FGFR TKIs—infigratinib, erdafitinib, derazantinib, pemigatinib, and futibatinib (TAS‐120)—are in advanced stages of development in patients with cholangiocarcinoma and urothelial cancer. Ongoing phase II and III trials of these agents are summarized in Table 2.
Table 1.
Selective FGFR‐directed tyrosine kinase inhibitors
| Agent | Infigratinib (BGJ398) | Pemigatinib (INCB054828) | Derazantinib (ARQ 087) | Futibatinib (TAS‐120) | Erdafitinib (JNJ‐42756493) | Rogaratinib (BAY 1163877) | Debio 1347 |
|---|---|---|---|---|---|---|---|
| Chemical structure |
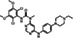
|
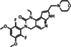
|
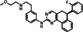
|
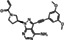
|
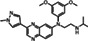
|
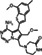
|
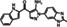
|
| Company | QED | Incyte | ArQule | Taiho | Janssen | Bayer | Debiopharm |
| Source | [65] | [66] | [67] | [68] | [69] | [70] | [71] |
| IC50, nM | |||||||
| FGFR1 | 0.9 | 0.4 | 4.5 | 1.8 | 1.2 | 11.2 | 9.3 |
| FGFR2 | 1.4 | 0.5 | 1.8 | 1.4 | 2.5 | <1 | 7.6 |
| FGFR3 | 0.9 | 1.2 | 4.5 | 1.6 | 3 | 19 | 22 |
| FGFR4 | 60 | 30 | 34 | 3.7 | 5.7 | 201 | — |
Abbreviations: FGFR, fibroblast growth factor receptor; IC50, median inhibitory concentration.
Table 2.
Current ongoing phase II and III trials with key selective FGFR tyrosine kinase inhibitors
| Agent and study ID | Phase | Indication | Regimen | No. of patients |
|---|---|---|---|---|
| Infigratinib (BGJ398) | ||||
| NCT03773302 | III | Cholangiocarcinoma | Infigratinib vs. gemcitabine/cisplatin | 384 |
| NCT04197986 | III | Urothelial cancer | Infigratinib vs. placebo | 218 |
| NCT02150967 | II | Cholangiocarcinoma | Infigratinib | 160 |
| NCT04233567 | II | Solid tumors | Infigratinib | 50 |
| Pemigatinib (INCB054828) | ||||
| NCT02872714 (FIGHT‐201) | II | Urothelial cancer | Pemigatinib | 240 |
| NCT04003610 (FIGHT‐205) | II | Urothelial cancer | Pemigatinib + pembrolizumab vs. pemigatinib vs. standard of care | 378 |
| NCT03914794 | II | Urothelial cancer | Pemigatinib | 43 |
| NCT03822117 (FIGHT‐207) | II | Solid tumors | Pemigatinib | 170 |
| NCT03011372 (FIGHT‐203) | II | Myeloproliferative neoplasms | Pemigatinib | 46 |
| NCT02924376 (FIGHT‐202) | II | Cholangiocarcinoma | Pemigatinib | 140 |
| NCT03656536 (FIGHT‐302) | III | Cholangiocarcinoma | Pemigatinib vs. gemcitabine/cisplatin | 432 |
| NCT04256980 | II | Cholangiocarcinoma | Pemigatinib | 54 |
| NCT04003623 | II | Solid tumors | Pemigatinib | 50 |
| NCT02393248 (FIGHT‐101) | I/II | Solid tumors | Pemigatinib; combination therapy | 325 |
| Derazantinib (ARQ 087) | ||||
| NCT03230318 | II | Cholangiocarcinoma | Derazantinib | 143 |
| NCT04045613 | Ib/II | Urothelial cancer | Derazantinib vs. derazantinib + atezolizumab | 303 |
| Futibatinib (TAS‐120) | ||||
| NCT04024436 | II | Breast cancer | Futibatinib or futibatinib + fulvestrant | 168 |
| NCT02052778 | I/II | Solid tumors | Futibatinib | 371 |
| Erdafitinib (JNJ‐42756493) | ||||
| NCT03390504 | III | Urothelial cancer | Erdafitinib vs. vinflunine or docetaxel or pembrolizumab | 631 |
| NCT03210714 | II | Solid tumors, non‐Hodgkin lymphoma, or histiocytic disorders | Erdafitinib | 49 (age >21 years) |
| NCT04083976 | II | Solid tumors | Erdafitinib | 280 |
| NCT02699606 | II | Urothelial cancer | Erdafitinib | 63 (Asian) |
| NCT03827850 (FIND) | II | NSCLC | Erdafitinib | 50 |
| NCT02365597 | II | Urothelial cancer | Erdafitinib | 217 |
| NCT02952573 | II | Multiple myeloma | Erdafitinib | 20 |
| NCT03999515 | II | Prostate cancer | Erdafitinib + abiraterone acetate or enzalutamide | 25 |
| NCT04172675 | II | Urothelial cancer | Erdafitinib vs. investigator choice intravesical chemotherapy | 280 |
| NCT03473743 | I/II | Urothelial cancer | Erdafitinib in combination with cetrelimab and/or platinum | 160 |
Abbreviations: FGFR, fibroblast growth factor receptor; NSCLC, non‐small cell lung cancer.
As FGFs act with other signaling molecules to orchestrate processes such as tissue regeneration and healing, inhibition of FGFR signaling has the potential to lead to on‐target adverse events such as hyperphosphatemia, which is believed to result from inhibition of FGFR signaling in the proximal renal tubule, as well as others associated with off‐target effects, including alopecia, dry mouth/xerostomia, nail changes, and other dermatologic events [8, 9]. Depending on the breadth of their inhibitory targets, adverse events associated with anti‐FGFR TKIs can also include those related to vascular endothelial growth factor receptor (VEGFR) inhibition (e.g., hypertension, cardiovascular events, and proteinuria), as seen with earlier‐generation multikinase inhibitors, and others commonly reported with TKIs (e.g., gastrointestinal disorders, such as vomiting and diarrhea, skin reactions, and ocular effects, such as dry eye and retinal pigment epithelium detachment).
The aim of this review is to provide oncologists with an understanding of the dermatologic events associated with FGFR inhibitors currently in clinical development or approved by regulatory agencies for the treatment of cholangiocarcinoma and urothelial cancers.
Rationale for Use of FGFR Inhibitors in Cholangiocarcinoma and Other Malignancies
Cholangiocarcinoma
Cholangiocarcinoma is a heterogeneous grouping of malignancies arising from the biliary epithelium between the canals of Hering and the main bile duct. These are uncommon cancers, accounting for only 3% of gastrointestinal cancers [10]; however, the mortality rate is high and only 8%–10% of patients are alive at 5 years after diagnosis [11].
The incidence of cholangiocarcinoma varies greatly, with the highest rates seen in Asian countries and lower rates in Western countries [12], although rates of intrahepatic cholangiocarcinoma are increasing in Western countries [13]. In their analysis of SEER data, Saha and colleagues reported an increase in rates of intrahepatic cholangiocarcinoma, from 0.44/100,000 in 1973 to 1.18/100,000 in 2012 [14]. This corresponds to an estimated 8,000 new cases of cholangiocarcinoma per year in the U.S. [15].
Treatment options are limited for patients with metastatic cholangiocarcinoma and outcomes are poor. The gemcitabine + cisplatin doublet is the standard of care in the first‐line setting, resulting in median overall and progression‐free survivals of 11.7 and 8.0 months, respectively [16]. After first‐line therapy, there are no established systemic options [17, 18]. However, the practice‐changing ABC‐06 study demonstrated that treatment with a modified 5‐fluorouracil/folinic acid + oxaliplatin regimen and active symptom control was superior to active symptom control alone in patients with cholangiocarcinoma whose disease had progressed during or after treatment with gemcitabine + cisplatin [19]. Despite this, there remains a need for targeted agents with the potential to improve survival in selected patient populations.
Alterations in genes encoding FGFRs are common in patients with cholangiocarcinoma, the most common being FGFR2 fusions, FGFR19 amplifications, and FGFR2 mutations [20]. FGFR2 fusions are present in 13%–25% of patients with cholangiocarcinoma [20, 21] and therefore represent a promising target for therapy in enriched patient populations.
Key small‐molecule FGFR TKIs currently under clinical development for the treatment of cholangiocarcinoma include multikinase and tyrosine kinase inhibitors such as infigratinib, erdafitinib, derazantinib, futibatinib, pazopanib, and Debio 1347. Pemigatinib was approved for use in patients with FGFR2 fusion or rearrangement in April 2020, based on the results of the phase II FIGHT‐202 study [22]. Other agents with a broader spectrum of activity, for example, the multikinase inhibitors pazopanib and dovitinib, are also in development for this indication but are not included in this review.
Urothelial Cancer
An estimated 80,000 new cases of bladder cancer will be diagnosed in the U.S. in 2019, three quarters of which will be in men [23].
Approximately 12% of patients have regional or distant metastases at diagnosis [24]. Five‐year survival rates are 36% for regional and 5% for distant metastases [24]. Treatment options for locally advanced disease include surgery followed by cisplatin‐based chemotherapy if no neoadjuvant treatment has been given [25]. For those with metastatic disease, preferred options include gemcitabine + cisplatin for cisplatin‐eligible patients and gemcitabine + carboplatin for those who are not eligible [25]. Targeted therapies currently available for patients whose disease progressed on cisplatin‐based therapies include atezolizumab and pembrolizumab, which are approved by the U.S. Food and Drug Administration for use in patients whose tumors express programmed cell death ligand 1 (PD‐L1) [26], enfortumab vedotin for patients who have previously received a programmed cell death‐1 or PD‐L1 inhibitor [27], and erdafitinib for patients with FGFR2‐ and FGFR3‐altered disease, based on the results of the BLC2001 study [28].
Selective small‐molecule FGFR TKIs currently in development for use in patients with urothelial carcinoma include infigratinib, pemigatinib, erdafitinib, and rogaratinib.
Dermatologic Events in Patients Treated with FGFR TKIs: Skin, Hair, Nails, and Oral Mucosa
Dermatologic adverse events, including hair loss/alopecia, hand–foot skin reaction or palmar–plantar erythrodysesthesia syndrome (PPES), stomatitis (oral mucositis), and nail changes, have been reported in phase II studies in patients with cholangiocarcinoma and urothelial carcinoma treated with FGFR inhibitors (Fig. 1; Tables 3, 4). The pathophysiological mechanisms behind these adverse events are not yet fully elucidated. Several possible mechanisms have been proposed, including inhibition of FGFR in keratinocytes, inducing dysregulation of hair‐follicle homeostasis and epidermal proliferation and/or differentiation with downregulation of tight junction gene expression, as demonstrated in FGFR‐deficient mice [29] and by inhibiting hormonal (nonpathological) FGF signaling by FGF19, FGF21, and FGF23 [30]. FGF2 expression has been shown to be upregulated in the nail epithelium after digit amputation in the mouse, suggesting a role for FGF signaling in digit regeneration [31].
Figure 1.
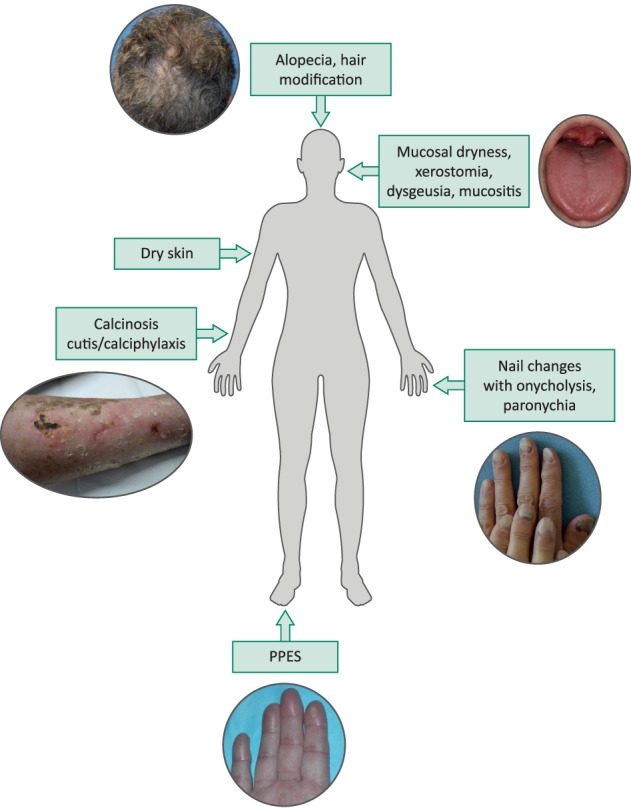
Schematic representation of dermatologic adverse events associated with fibroblast growth factor receptor inhibition. Suggested dose modifications for dermatologic adverse events: Grade 1/2: continue drug at standard dose. Grade 3, first occurrence: hold drug until resolved to grade ≤ 1 or baseline and reduce drug to the next dose level; second occurrence: interrupt drug until grade ≤ 1 or baseline. Once recovered, reduce drug to the next dose level; third occurrence: interrupt drug until grade ≤ 1 or baseline. Once recovered, reduce drug to the next dose level, if available as dose level –2. If already at dose level –2 at time of occurrence, permanently discontinue drug; fourth occurrence: permanently discontinue drug. Package insert to be consulted in the event of emergence of dermatologic adverse events and doses modified as recommended. Abbreviation: PPES, palmar–plantar erythrodysesthesia syndrome.
Table 3.
Dermatologic AEs associated with selective FGFR tyrosine kinase inhibitors in cholangiocarcinoma
| Agent | Infigratinib (BGJ398) | Pemigatinib (INCB054828) | Erdafitinib (JNJ‐42756493) | Derazantinib (ARQ 087) | Futibatinib (TAS‐120) | Debio 1347 |
|---|---|---|---|---|---|---|
| Reference | [35] | [22] | [42] | [39] | [43] | [72] |
| No. of patients | 61 | 146 | 17 | 29 | 67 | 8 |
| AE, all grade/grade ≥ 3, % | ||||||
| Stomatitis | 30/7 | 32/5 | 65/18 | 7/3 | 16/3 | 38 |
| Alopecia | 26/0 | 46/0 | — | 24/0 | 30/0 | — |
| Dry skin | 18/0 | 16/1 | 35/6 | 10/0 | 27/0 | — |
| PPES | 21/5 | 15/4 | 29/0 | — | 18/1 | — |
| Dry mouth | 23/0 | 29/0 | 59/6 | 45/0 | 33/0 | 50 |
| Nail discoloration | 8/0 | 8/1 | 18/6 | — | — | — |
| Nail‐bed disorder | 7/0 | — | — | — | — | — |
| Nail ridging | 8/0 | — | — | — | — | — |
| Paronychia | 7/0 | 6/1 | 24/6 | — | — | — |
| Onychomadesis | 18/0 | — | — | — | — | — |
| Nail disorder/changes | — | 3/1 | 29/6 | — | 16/0 | 63 |
| Mucosal dryness | 7/0 | — | — | — | — | — |
| Conjunctivitis | — | — | — | 14/0 | — | — |
| Pruritus | — | — | — | 10/0 | — | — |
| Rash | 7/0 | — | — | — | 10/0 | — |
| Rash maculopapular | 7/2 | — | — | — | — | — |
| Dermatitis | — | — | — | 7/0 | — | — |
| AEs leading to, % | ||||||
| Interruptions | 70 | 42 | 94 | — | 55 | — |
| Dose reductions | 38 | 14 | 47 | — | 51 | — |
| Discontinuations | 8 | 9 | — | 14 | 1 | — |
Abbreviations: —, not reported; AE, adverse event; FGFR, fibroblast growth factor receptor; PPES, palmar–plantar erythrodysesthesia syndrome.
Table 4.
Dermatologic AEs associated with selective FGFR tyrosine kinase inhibitors in urothelial carcinoma
| Agent | Infigratinib (BGJ398) | Pemigatinib (INCB054828) | Erdafitinib (JNJ‐42756493) | Rogaratinib (BAY 1163877) |
|---|---|---|---|---|
| Reference | [40] | [41] | [28] | [49] |
| No. of patients | 67 | 108 | 99 | 86 |
| AE, all grade/grade ≥ 3, % | ||||
| Stomatitis | 25/3 | 34/7 | 58/10 | 12/1 |
| Alopecia | 31/0 | 40/1 | 29/0 | 22/0 |
| Dry skin | 12/0 | — | 32/0 | — |
| PPES | 12/8 | — | 23/5 | — |
| Dry mouth | 31/2 | 32/1 | 46/0 | — |
| Nail disorder | 21/0 | — | 8/3 | — |
| Paronychia | — | — | 17/3 | — |
| Onycholysis | — | — | 18/2 | — |
| Nail dystrophy | — | — | 16/6 | — |
| Mucositis | — | — | — | — |
| AEs leading to, % | ||||
| Interruptions | — | 37 | — | — |
| Dose reductions | 46 | 14 | 56 | — |
| Discontinuations | 15 | 6 | 13 | 16 |
Abbreviations: —, not reported; AE, adverse event; FGFR, fibroblast growth factor receptor; PPES, palmar–plantar erythrodysesthesia syndrome.
Nail Changes
Nail changes are common in patients undergoing treatment with FGFR TKIs [32]. Patients can develop significant adverse events, the most important of which is onycholysis [33], and dose adjustment may be required as a result of this adverse event. Other less common nail events include paronychia, Beau's lines/onychomadesis, and brittle nails (onychoschizia). Paronychia was reported in 24% and 17% of patients with cholangiocarcinoma and urothelial carcinoma, respectively, treated with erdafitinib [28, 34]; furthermore, onycholysis and nail dystrophy were observed in 18% and 16%, respectively, of patients with urothelial carcinoma [28]. Paronychia and onychomadesis were reported in 7% and 18%, respectively, of patients with cholangiocarcinoma who received infigratinib [35].
Nail adverse events, which typically develop within 1–2 months of treatment initiation, can be prolonged and debilitating [32, 36], and in severe cases can cause pain and discomfort, which can lead to treatment discontinuation [37].
Alopecia
Alopecia is a psychosocially impactful consequence of cytotoxic chemotherapy and treatment with kinase inhibitors [38]. Alopecia, which includes the textural changes, thinning, or patchy hair categorized as grade 1 alopecia, and the complete hair loss categorized as grade 2 alopecia, has been reported in patients with cholangiocarcinoma treated with infigratinib. Specifically, 26% of patients treated with infigratinib [35], 46% of those treated with pemigatinib [22], and 24% of patients treated with derazantinib [39] experienced grade 1 or 2 alopecia. In patients with urothelial carcinoma, grade 1 or 2 alopecia occurred in 31%, 39%, and 29% of patients treated with infigratinib [40], pemigatinib [41], and erdafitinib [34].
Other body hair can also be adversely affected in patients undergoing treatment with FGFR inhibitors (e.g., eyelash trichomegaly has been reported with infigratinib) [33].
Palmar–Plantar Erythrodysesthesia Syndrome (Hand–Foot Skin Reaction; Hand–Foot Syndrome)
PPES has been reported with chemotherapy and TKI treatment. It is characterized by hyperkeratosis and focal calluses, which result in diffuse xerosis and erythema combined with fissures, mostly localized to digits. This skin reaction was reported in 21%, 29%, and 18% of patients with cholangiocarcinoma receiving infigratinib [35], erdafitinib [42], and futibatinib [43], respectively, and in 12% and 23% of patients with urothelial carcinoma receiving infigratinib [40] and erdafitinib [28], respectively. Among patients with cholangiocarcinoma, grade 3/4 PPES was reported in 5% of patients treated with infigratinib [35] and 4% of those treated with pemigatinib [22], whereas 8% of infigratinib‐treated [40] and 5% of erdafitinib‐treated patients with urothelial cancer [28] reported this event. Of note, this adverse event differs from that seen with traditional chemotherapeutic agents. PPES with cytotoxic agents such as capecitabine and doxorubicin is characterized by diffuse erythema, edema, and pain of the entire surface of the palms and soles [44]. With VEGFR/platelet‐derived growth factor receptor multikinase inhibitors, painful blisters located in areas of friction or pressure in the palms and soles are observed [44, 45]. Conversely, with FGFR inhibitors, the ventral aspect of the distal digits and lateral aspects of the palms and soles are affected by erythema and pain, accompanied by onycholysis and secondary paronychia, reminiscent of changes observed with microtubule inhibitors (i.e., taxanes). PPES often presents as a mild to moderate cutaneous edema, erythema, and hyperkeratosis with FGFR inhibitors; this evolves into painful digits that can impact patients' quality of life [46, 47] and can ultimately limit daily functioning and lead to a reduction of the duration and intensity of treatment or its discontinuation [48].
Stomatitis
Stomatitis is one of the most commonly observed adverse events in patients treated with FGFR inhibitors, with lesions appearing rapidly after treatment initiation. In contrast to radiation‐ or cytotoxic therapy‐induced oral mucositis, stomatitis is characterized by painful, well‐defined lesions. The incidence of stomatitis among patients with cholangiocarcinoma ranged from 7% with derazantinib [39] to 65% with erdafitinib [42]; furthermore, 18% of patients treated with erdafitinib experienced grade ≥ 3 stomatitis [42]. Among patients with urothelial carcinoma, the incidence of stomatitis ranged from 12% with rogaratinib [49] to 58% with erdafitinib [28]. Although usually self‐limiting, stomatitis can be very painful and can significantly impact patients' quality of life.
Dry Skin (Xerosis)
Xerosis is a common side effect of treatment with FGFR inhibitors, reported in 18% of patients in a systematic review of 58 targeted agents [50]. Xerosis may manifest as pruritus, fine scaling, and fissures. It may also progress to xerotic dermatitis and can lead to bacterial or viral superinfection with Staphylococcus aureus, herpes simplex, or other bacterial and viral agents. Although severe or life‐threatening complications are uncommon, low‐grade xerosis can result in dose delays or discontinuations, potentially impacting the overall efficacy of treatment.
The incidence of dry skin in patients with cholangiocarcinoma treated with the FGFR inhibitors ranged from 10% in derazantinib‐treated patients [39] to 35% in erdafitinib‐treated patients [42], whereas for those with urothelial cancer, dry skin was reported in 12% of infigratinib‐treated patients [40] and 32% of erdafitinib‐treated patients [34]. The dry skin associated with FGFR inhibition was generally mild to moderate (grade 1 or 2) in nature.
Dry Mouth/Xerostomia
Dry mouth, or xerostomia, is a subjective complaint that can be very severe and represents a significant burden for patients if speech, chewing, swallowing, and general wellbeing are affected [51]. Dry mouth can be associated with dysgeusia, which can occasionally be very severe [36]. FGFs and FGFRs play a central role in salivary gland branching morphogenesis and disruption of these factors or their receptors has been shown to have implications for salivary gland function [52]. Dry mouth, generally grade 1 or 2, was common in patients treated with FGFR inhibitors, occurring in 23%–59% of patients with cholangiocarcinoma and 31%–46% of patients with urothelial cancers (Tables 3, 4).
Calcinosis Cutis/Calciphylaxis
A rare skin/soft tissue reaction that has been observed in patients undergoing treatment with FGFR inhibitors is calcinosis cutis, a condition in which calcium salts are deposited in the skin and subcutaneous tissues. This has been reported in one patient treated with infigratinib [53] and another treated with pemigatinib [54]. Of further interest is the risk of nonuremic calciphylaxis, or intimal vascular calcifications, resulting in vascular thrombosis and extensive skin necrosis resulting in grade 3 and 4 cutaneous ulcerations. These conditions may be related to changes in underlying serum phosphatase known to be associated with these agents [55], or to the role of FGF/FGFR signaling in skeletal development [56]. Expression of FGF2 and its coreceptor syndecan‐4 is increased at sites of calcification in human atherosclerotic plaques, suggesting a role for FGFR inhibition in vascular calcification, a major cause of morbidity and mortality [57].
With the exception of calciphylaxis, the dermatologic adverse events described above are predominantly grade 1 and 2 in severity, but these adverse events have the potential to disrupt treatment, as reflected by the extent of dose modification shown in Tables 3 and 4. The time to onset of dermatologic adverse events associated with pan‐FGFR inhibition is summarized in Figure 2. Awareness and anticipation of these adverse events is critical in order to ensure patient adherence to FGFR‐targeted therapies.
Figure 2.
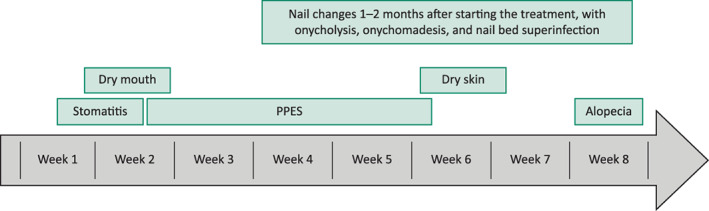
Onset over time of dermatologic adverse events associated with fibroblast growth factor receptor tyrosine kinase inhibitors. Abbreviation: PPES, palmar–plantar erythrodysesthesia syndrome.
Management and Supportive Care for Dermatologic Adverse Events: Proposed Guidelines
Prevention and early treatment of dermatologic adverse events are key to maximizing adherence to therapy and optimizing outcomes in patients undergoing treatment with FGFR inhibitors; however, data specific to preventive therapies for use with FGFR‐targeted therapy are scarce. When preventive measures are unsuccessful and adverse events emerge, effective management strategies can ensure continuation of treatment, particularly if used at the earliest appearance of grade 1 symptoms. Management approaches are shown in Figure 3 and summarized below. Notably, although treatment for skin toxicities will be initiated by the oncologic team, referral to a dermatologist for consultation is recommended for patients with grade 3/intolerable grade 2 events, or grade 2 events that have not responded to ≥ 4 weeks of therapy.
Figure 3.
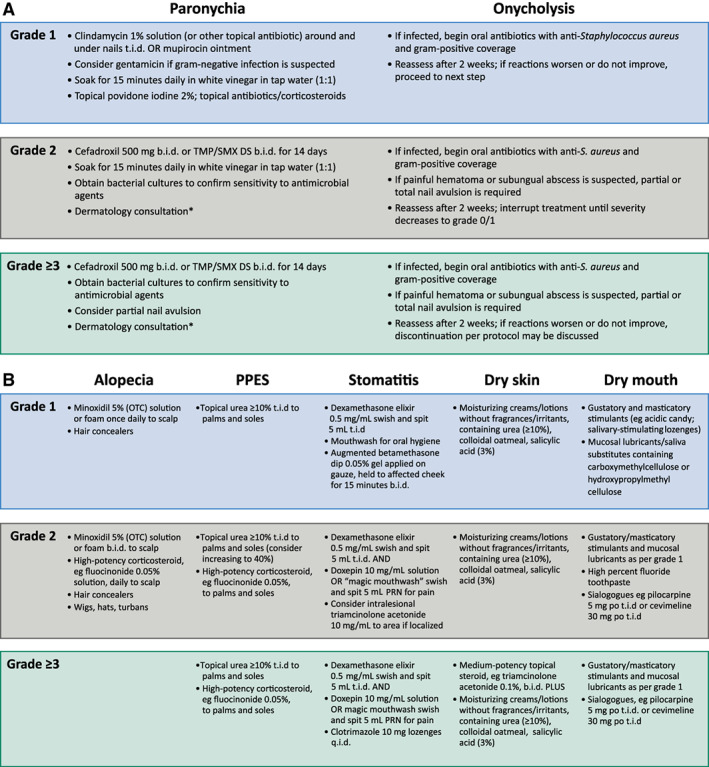
Management of fibroblast growth factor receptor‐related adverse events. (A): Nail changes. (B): Other dermatologic events. *Referral to a dermatologist for consultation is recommended for grade 3 and intolerable grade 2 events, or grade 2 events that have not responded to 4 weeks of therapy. Abbreviations: OTC, over the counter; PPES, palmar–plantar erythrodysesthesia syndrome; PRN, as needed; TMP/SMX DS, trimethoprim/sulfamethoxazole double strength.
Nail Changes
Counseling and education on the potential for nail changes are essential before initiation of treatment with FGFR inhibitors. Preventive strategies include avoidance of prolonged contact with water, repeated trauma, friction, and pressure on nails and nail beds. The use of protective gloves and limiting use of nail polish removers and nail hardeners is also helpful. Patients are also advised to avoid biting nails or cutting nails too short and to use topical emollients and loose‐fitting socks and footwear. Preventive correction of nail curvature may be considered.
Paronychia
Recommended treatments for grade 1 paronychia include topical povidone iodine 2%–10% applied twice daily [58] or daily nail soaking in 1:1 vinegar:water for 15 minutes a day. Patients with grade 2 or 3 paronychia should be treated with a 14‐day course of oral antibiotics in addition to daily nail soaking in 1:1 vinegar:water; bacterial cultures should be obtained to confirm sensitivity to antimicrobial agents. Dermatology consultation is recommended for grade ≥ 2 paronychia, given the potential chronicity of this event.
Onycholysis
Recommended management options for onycholysis consist of trimming the raised distal nails, clipping of the nails, and application of topical povidone iodine 2%–10% b.i.d. solution [58] around and under the nails. Oral antibiotics should be started if infection is suspected (bacterial cultures and sensitivities should be obtained prior to initiating antibiotics), and nail avulsion may be needed if the patient has painful hematoma or subungual abscess.
Alopecia
Preventive measures normally considered for patients undergoing traditional chemotherapy regimens, for example, scalp compression, scalp cooling, and medications, are not applicable to patients receiving FGFR inhibitors, and the health care provider's attention should be focused on early identification and management of symptoms.
Management of alopecia consists of prophylactic or reactive topical minoxidil 5% applied once daily to the scalp to encourage hair regrowth, and a high‐potency topical corticosteroid (e.g., fluocinonide 0.05% solution). In addition, hair camouflaging methods, which create the appearance of naturally thicker, fuller hair, may be considered. Alopecia typically reverses when treatment is discontinued.
PPES
Prevention strategies for PPES include prophylactic removal of hyperkeratotic areas, application of moisturizing cream containing urea ≥10%, pedicures, and cushioning of callused areas using soft or padded shoes [48]. Other preventive tactics include avoidance of activities that cause force or rubbing on the hands and feet during the first 6 weeks of treatment and limiting contact with harsh chemicals and sources of heat, such as sitting in saunas or the sun.
Management of PPES consists of keratolytic agents such as urea ≥ 10% for grade ≥ 1 PPES, with addition of high‐potency topical steroids such as fluocinonide 0.05% for grade ≥ 2 symptoms.
Stomatitis
Preventive strategies include undertaking dental work aimed at eliminating existing tooth and gum disease before the start of treatment and education regarding the importance of thorough and frequent cleaning of the oral cavity. Avoidance of salty, spicy, or citrus‐based foods, as well as hot beverages, may help prevent stomatitis.
Upon emergence of grade 1 or 2 stomatitis, dexamethasone 0.5 mg/5 mL elixir is recommended; an augmented betamethasone dipropionate 0.05% gel applied to gauze and held against the affected surface may also assist in alleviating symptoms.
Dry Skin
Patients should be advised to moisturize skin to minimize the risk of skin adverse events and to avoid excessive exposure to detergents and soaps containing fragrances. Urea preparations have been shown to prevent transepidermal water loss, and salicylic acid preparations are helpful for their keratolytic, bacteriostatic, and fungicidal effects [50]. Exfoliation of scaly areas of xerosis is recommended. For more severe grade 3 xerosis, which results in asteatotic dermatitis, treatment can be initiated with low‐potency topical steroids such as hydrocortisone 2.5% cream/ointment or triamcinolone 0.1% cream.
Dry Mouth/Xerostomia
Patient education is an important component of dry mouth prevention. The importance of good oral hygiene, regular dentist visits, and other strategies for preventing oral disease should be stressed.
Treatment may include systemic and topical salivary stimulants, such as cevimeline and pilocarpine, and intraoral topical agents, such as chewing gums and saliva stimulants and substitutes [59]. High‐fluoride toothpaste is also recommended to prevent cavities.
Calcinosis Cutis/Calciphylaxis
Owing to the potential for ulcerations to develop and expand rapidly, as well as an extremely poor 1‐year mortality rate [60], drug discontinuation should be recommended for patients with calcinosis cutis. Treatment with oral or topical calcium channel blockers, intravenous immunoglobulin, and compounded or intralesional sodium thiosulfate may be initiated. For calciphylaxis, treatments generally include three‐times‐a‐week dosing at 3‐ to 4‐week intervals with intravenous sodium thiosulfate or intralesional sodium thiosulfate diluted 1:1 with 1% lidocaine to minimize the pain [60, 61, 62]. Patients should be screened for additional hypercoagulation disorders [63]. In addition, monitoring calcium and phosphate levels with phosphate binders, consideration for anticoagulation, and use of bisphosphonates may be considered. Dermatologic or endocrine consultations are warranted upon occurrence of grade 3 events and for patients who do not respond to therapy.
Dose Modifications
Dose modification in the event of dermatologic adverse events should be performed as recommended in the relevant package insert.
Unless otherwise recommended, treatment should be continued in cases of grade 1 and 2 adverse events and interrupted for grade 3 adverse events. When dermatologic events improve to grade ≤ 1, a rechallenge at a reduced dose is recommended.
Conclusion
The FGFR inhibitors have a distinctive adverse‐event profile that includes a range of dermatologic adverse events, the incidences of which vary between agents. The events are seldom severe or life threatening but can nonetheless limit the delivery of treatment through dose holds and may lead to premature drug discontinuation. In order to optimize patient outcomes, physicians should be mindful of possible untoward events associated with the drug being used, educate their patients, and be ready to implement effective management plans in a timely fashion. Prescribing information for erdafitinib should be consulted if appropriate [64]. Intervention and treatment at the earliest possible opportunity may prevent premature discontinuation while maintaining patients' quality of life.
Author Contributions
Conception/design: Mario E. Lacouture, Kathleen Guindon
Provision of study material or patients: Mario E. Lacouture, Vincent Sibaud, Milan J. Anadkat, Benjamin Kaffenberger, Jonathan Leventhal, Kathleen Guindon, Ghassan Abou‐Alfa
Collection and/or assembly of data: Mario E. Lacouture, Vincent Sibaud, Milan J. Anadkat, Benjamin Kaffenberger, Jonathan Leventhal, Kathleen Guindon, Ghassan Abou‐Alfa
Data analysis and interpretation: Mario E. Lacouture, Vincent Sibaud, Milan J. Anadkat, Benjamin Kaffenberger, Jonathan Leventhal, Kathleen Guindon, Ghassan Abou‐Alfa
Manuscript writing: Mario E. Lacouture, Vincent Sibaud, Milan J. Anadkat, Benjamin Kaffenberger, Jonathan Leventhal, Kathleen Guindon, Ghassan Abou‐Alfa
Final approval of manuscript: Mario E. Lacouture, Vincent Sibaud, Milan J. Anadkat, Benjamin Kaffenberger, Jonathan Leventhal, Kathleen Guindon, Ghassan Abou‐Alfa
Disclosures
Mario E. Lacouture: Johnson & Johnson, QED Therapeutics, Novartis, Deciphera, Loxo, Roche, AstraZeneca (C/A, H), Johnson & Johnson (RF); Vincent Sibaud: Novartis, Incyte, Pierre Fabre, Bristol‐Myers Squibb, Bayer, Bioderma (C/A, H, SAB); Benjamin Kaffenberger: Biogen, Celgene, Eli Lilly and Company, InflaRx, OnQuality, Veloce Biopharmaceuticals (RF); Jonathan Leventhal: Regeneron, Sanofi, Bristol‐Myers Squibb (SAB), Azitra, Inc. (RF); Kathleen Guindon: QED Therapeutics (E, OI); Ghassan Abou‐Alfa: Agios, AstraZeneca, Autem, Bayer, Beigene, Berry Genomics, Bioline, Bristol‐Myers Squibb, Celgene, CytomX, Debiopharm, Eisai, Eli Lilly and Company, Exelixis, Flatiron, Genoscience, Incyte, Ipsen, Janssen, Helio, Loxo, Merck, MINA, Pfizer, Polaris, QED Therapeutics, Redhill, Silenseed, Sillajen, Sobi, Targovax, Therabionics, Twoxar, Yiviva (C/A); ActaBiologica, Agios, Array, AstraZeneca, Bayer, Beigene, Bristol‐Myers Squibb, CASI, Celgene, Exelixis, Genentech, Halozyme, Incyte, Mabvax, Polaris, Puma Biotechnology, QED Therapeutics, Roche (RF); Agios, AstraZeneca, Autem, Bayer, Beigene, Berry Genomics, Bioline, Bristol‐Myers Squibb, Celgene, CytomX, Debiopharm, Eisai, Eli Lilly and Company, Exelixis, Flatiron, Genoscience, Incyte, Ipsen, Janssen, LAM, Loxo, Merck, MINA, Pfizer, Polaris, QED Therapeutics, Redhill, Silenseed, Sillajen, Sobi, Targovax, Therabionics, Twoxar, Yiviva (H); Articles and Methods for Preventing and Treating Dermatologic Adverse Events, identified by International Patent Application No. PCT/US2014/031545 filed on March 24, 2014, and priority application Serial No.: 61/804,907; Filed: March 25, 2013 (IP). Milan J. Anadkat indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
We thank Lee Miller and Deirdre Carman of Miller Medical Communications Ltd for copyediting and editorial/production assistance. This work was funded by QED Therapeutics, Inc. M.E.L. is funded by the NIH/National Cancer Institute Cancer Center Support Grant P30 CA008748.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Turner N, Grose R. Fibroblast growth factor signalling: From development to cancer. Nat Rev Cancer 2010;10:116–129. [DOI] [PubMed] [Google Scholar]
- 2. Hebert JM. FGFs: Neurodevelopment's Jack‐of‐all‐trades ‐ How do they do it? Front Neurosci 2011;5:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Regeenes R, Silva PN, Chang HH et al. Fibroblast growth factor receptor 5 (FGFR5) is a co‐receptor for FGFR1 that is up‐regulated in beta‐cells by cytokine‐induced inflammation. J Biol Chem 2018;293:17218–17228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borad MJ, Champion MD, Egan JB et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet 2014;10:e1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ross JS, Wang K, Khaira D et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer 2016;122:702–711. [DOI] [PubMed] [Google Scholar]
- 6. Wang R, Wang L, Li Y et al. FGFR1/3 tyrosine kinase fusions define a unique molecular subtype of non‐small cell lung cancer. Clin Cancer Res 2014;20:4107–4114. [DOI] [PubMed] [Google Scholar]
- 7. Singh D, Chan JM, Zoppoli P et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012;337:1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole‐body homeostasis (review). Int J Mol Med 2016;38:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Touat M, Ileana E, Postel‐Vinay S et al. Targeting FGFR signaling in cancer. Clin Cancer Res 2015;21:2684–2694. [DOI] [PubMed] [Google Scholar]
- 10. Kirstein MM, Vogel A. Epidemiology and risk factors of cholangiocarcinoma. Visc Med 2016;32:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noone AM, Howlader N, Krapcho M et al. SEER Cancer Statistics Review, 1975‐2015, National Cancer Institute. Based on November 2017 SEER data submission, posted to the SEER web site, April 2018. 2018. Available at https://seer.cancer.gov/csr/1975_2015/. Accessed March 9, 2020.
- 12. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int 2019;(39 suppl 1):19–31. [DOI] [PubMed] [Google Scholar]
- 13. Bridgewater JA, Goodman KA, Kalyan A et al. Biliary tract cancer: Epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book 2016;35:e194–e203. [DOI] [PubMed] [Google Scholar]
- 14. Saha SK, Zhu AX, Fuchs CS et al. Forty‐year trends in cholangiocarcinoma incidence in the U.S.: Intrahepatic disease on the rise. The Oncologist 2016;21:594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Cancer Society . Key statistics for bile duct cancer. 2018. Available at https://www.cancer.org/cancer/bile-duct-cancer/about/key-statistics.html. Accessed March 9, 2020.
- 16. Valle J, Wasan H, Palmer DH et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273–1281. [DOI] [PubMed] [Google Scholar]
- 17. Valle JW, Borbath I, Khan SA et al. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27:v28–v37. [DOI] [PubMed] [Google Scholar]
- 18. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology. Hepatobiliary Cancers. Version 4.2019. 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed March 9, 2020.
- 19. Lamarca A, Palmer DH, Wasan HS et al. ABC‐06 | A randomised phase III, multi‐centre, open‐label study of active symptom control (ASC) alone or ASC with oxaliplatin / 5‐FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously‐treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol 2019;37(suppl 15):4003a. [Google Scholar]
- 20. Jain A, Borad M, Kelley RK et al. Cholangiocarcinoma with FGFR genetic aberrations: A unique clinical phenotype. JCO Precis Oncol 2018:1–12. [DOI] [PubMed] [Google Scholar]
- 21. Churi CR, Shroff R, Wang Y et al. Mutation profiling in cholangiocarcinoma: Prognostic and therapeutic implications. PLoS One 2014;9:e115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abou‐Alfa GK, Sahai V, Hollebecque A et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open‐label, phase 2 study. Lancet Oncol 2020;21:671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Cancer Society . Cancer Facts & Figures 2019. 2019. Available at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2019/cancer-facts-and-figures-2019.pdf. Accessed March 9, 2020.
- 24. National Cancer Institute . Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Bladder Cancer. 2019. Available at https://seer.cancer.gov/statfacts/html/urinb.html. Accessed March 9, 2020.
- 25. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology Bladder Cancer v 3.2020. 2019. Available at https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed March 9, 2020.
- 26. Suzman DL, Agrawal S, Ning YM et al. FDA Approval Summary: Atezolizumab or pembrolizumab for the treatment of patients with advanced urothelial carcinoma ineligible for cisplatin‐containing chemotherapy. The Oncologist 2019;24:563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosenberg JE, O'Donnell PH, Balar AV et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti‐programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019;37:2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loriot Y, Necchi A, Park SH et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med 2019;381:338–348. [DOI] [PubMed] [Google Scholar]
- 29. Yang J, Meyer M, Muller AK et al. Fibroblast growth factor receptors 1 and 2 in keratinocytes control the epidermal barrier and cutaneous homeostasis. J Cell Biol 2010;188:935–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dieci MV, Arnedos M, Andre F et al. Fibroblast growth factor receptor inhibitors as a cancer treatment: From a biologic rationale to medical perspectives. Cancer Discov 2013;3:264–279. [DOI] [PubMed] [Google Scholar]
- 31. Takeo M, Chou WC, Sun Q et al. Wnt activation in nail epithelium couples nail growth to digit regeneration. Nature 2013;499:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robert C, Sibaud V, Mateus C et al. Nail toxicities induced by systemic anticancer treatments. Lancet Oncol 2015;16:e181–e189. [DOI] [PubMed] [Google Scholar]
- 33. Betrian S, Gomez‐Roca C, Vigarios E et al. Severe onycholysis and eyelash trichomegaly following use of new selective pan‐FGFR inhibitors. JAMA Dermatol 2017;153:723–725. [DOI] [PubMed] [Google Scholar]
- 34. Siefker‐Radtke AO, Necchi A, Park SH et al. First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ‐42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt). J Clin Oncol 2018;36(suppl 15):4503a. [Google Scholar]
- 35. Javle M, Lowery M, Shroff RT et al. Phase II study of BGJ398 in patients with FGFR‐altered advanced cholangiocarcinoma. J Clin Oncol 2018;36:276–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lacouture M, Sibaud V. Toxic side effects of targeted therapies and immunotherapies affecting the skin, oral mucosa, hair, and nails. Am J Clin Dermatol 2018;19:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosen AC, Case EC, Dusza SW et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: A questionnaire study in a dermatology referral clinic. Am J Clin Dermatol 2013;14:327–333. [DOI] [PubMed] [Google Scholar]
- 38. Freites‐Martinez A, Chan D, Sibaud V et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol 2019;155:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mazzaferro V, El‐Rayes BF, Droz Dit Busset M et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion‐positive intrahepatic cholangiocarcinoma. Br J Cancer 2019;120:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pal SK, Rosenberg JE, Hoffman‐Censits JH et al. Efficacy of BGJ398, a fibroblast growth factor receptor 1‐3 inhibitor, in patients with previously treated advanced urothelial carcinoma with FGFR3 alterations. Cancer Discov 2018;8:812–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Necchi A, Pouessel D, Leibowitz‐Amit R et al. Interim results of FIGHT‐201, a phase II, open‐label, multicenter study of INCB054828 in patients (pts) with metastatic or surgically unresectable urothelial carcinoma (UC) harboring fibroblast growth factor (FGF)/FGF receptor (FGFR) genetic alterations (GA). Ann Oncol 2018;29(suppl 8):viii319–viii320. [Google Scholar]
- 42. Park JO, Feng Y‐H, Chen Y‐Y et al. Updated results of a phase IIa study to evaluate the clinical efficacy and safety of erdafitinib in Asian advanced cholangiocarcinoma (CCA) patients with FGFR alterations. J Clin Oncol 2019;37(suppl 15):4117a. [Google Scholar]
- 43. Goyal L, Meric‐Bernstam F, Hollebecque A et al. FOENIX‐CCA2: A phase II, open‐label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J Clin Oncol 2020;38(suppl 15):108a. [Google Scholar]
- 44. Nagore E, Insa A, Sanmartín O. Antineoplastic therapy‐induced palmar plantar erythrodysesthesia ('hand‐foot') syndrome. Incidence, recognition and management. Am J Clin Dermatol 2000;1:225–234. [DOI] [PubMed] [Google Scholar]
- 45. Gomez P, Lacouture ME. Clinical presentation and management of hand‐foot skin reaction associated with sorafenib in combination with cytotoxic chemotherapy: Experience in breast cancer. The Oncologist 2011;16:1508–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nardone B, Hensley JR, Kulik L et al. The effect of hand‐foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health‐related quality of life. J Drugs Dermatol 2012;11:e61–e65. [PubMed] [Google Scholar]
- 47. Huggins RH, Kuzel TM, Anderson RT et al. Hand foot skin reaction (HFSR) by the multikinase inhibitors (MKIs) sorafenib and sunitinib: Impact on quality of life (QoL). J Clin Oncol 2008;26(suppl 15):16122a. [Google Scholar]
- 48. Anderson R, Jatoi A, Robert C et al. Search for evidence‐based approaches for the prevention and palliation of hand‐foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs). The Oncologist 2009;14:291–302. [DOI] [PubMed] [Google Scholar]
- 49. Quinn DI, Petrylak DP, Bellmunt J et al. FORT‐1: Phase II/III study of rogaratinib versus chemotherapy (CT) in patients (pts) with locally advanced or metastatic urothelial carcinoma (UC) selected based on FGFR1/3 mRNA expression. J Clin Oncol 2020;38(suppl 6):489a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Valentine J, Belum VR, Duran J et al. Incidence and risk of xerosis with targeted anticancer therapies. J Am Acad Dermatol 2015;72:656–667. [DOI] [PubMed] [Google Scholar]
- 51. Cassolato SF, Turnbull RS. Xerostomia: Clinical aspects and treatment. Gerodontology 2003;20:64–77. [DOI] [PubMed] [Google Scholar]
- 52. Prochazkova M, Prochazka J, Marangoni P et al. Bones, glands, ears and more: The multiple roles of FGF10 in craniofacial development. Front Genet 2018;9:542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Carr DR, Pootrakul L, Chen HZ et al. Metastatic calcinosis cutis associated with a selective FGFR inhibitor. JAMA Dermatol 2019;155:122–123. [DOI] [PubMed] [Google Scholar]
- 54. Arudra K, Patel R, Tetzlaff MT et al. Calcinosis cutis dermatologic toxicity associated with fibroblast growth factor receptor inhibitor for the treatment of Wilms tumor. J Cutan Pathol 2018;45:786–790. [DOI] [PubMed] [Google Scholar]
- 55. Chae YK, Ranganath K, Hammerman PS et al. Inhibition of the fibroblast growth factor receptor (FGFR) pathway: The current landscape and barriers to clinical application. Oncotarget 2017;8:16052–16074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Su N, Jin M, Chen L. Role of FGF/FGFR signaling in skeletal development and homeostasis: Learning from mouse models. Bone Res 2014;2:14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Borland SJ, Morris TG, Borland SC et al. Regulation of vascular smooth muscle cell calcification by syndecan‐4/FGF‐2/PKCalpha signalling and cross‐talk with TGFbeta. Cardiovasc Res 2017;113:1639–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Capriotti KD, Anadkat M, Choi J et al. A randomized phase 2 trial of the efficacy and safety of a novel topical povidone‐iodine formulation for cancer therapy‐associated paronychia. Invest New Drugs 2019;37:1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jensen SB, Pedersen AM, Vissink A et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Management strategies and economic impact. Support Care Cancer 2010;18:1061–1079. [DOI] [PubMed] [Google Scholar]
- 60. Weenig RH, Sewell LD, Davis MD et al. Calciphylaxis: Natural history, risk factor analysis, and outcome. J Am Acad Dermatol 2007;56:569–579. [DOI] [PubMed] [Google Scholar]
- 61. Vedvyas C, Winterfield LS, Vleugels RA. Calciphylaxis: A systematic review of existing and emerging therapies. J Am Acad Dermatol 2012;67:e253–e260. [DOI] [PubMed] [Google Scholar]
- 62. Strazzula L, Nigwekar SU, Steele D et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol 2013;149:946–949. [DOI] [PubMed] [Google Scholar]
- 63. Dobry AS, Ko LN, St John J et al. Association between hypercoagulable conditions and calciphylaxis in patients with renal disease: A case‐control study. JAMA Dermatol 2018;154:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janssen Pharmaceutical Companies. BALVERSA Highlights of Prescribing Information. 2019. Available at http://www.janssenlabels.com/package‐insert/product‐monograph/prescribing‐information/BALVERSA‐pi.pdf. Accessed March 9, 2020.
- 65. Guagnano V, Furet P, Spanka C et al. Discovery of 3‐(2,6‐dichloro‐3,5‐dimethoxy‐phenyl)‐1‐{6‐[4‐(4‐ethyl‐piperazin‐1‐yl)‐phenylamin o]‐pyrimidin‐4‐yl}‐1‐methyl‐urea (NVP‐BGJ398), a potent and selective inhibitor of the fibroblast growth factor receptor family of receptor tyrosine kinase. J Med Chem 2011;54:7066–7083. [DOI] [PubMed] [Google Scholar]
- 66. Roskoski R Jr . The role of fibroblast growth factor receptor (FGFR) protein‐tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder. Pharmacol Res 2020. Jan;151:104567. [DOI] [PubMed] [Google Scholar]
- 67. Hall TG, Yu Y, Eathiraj S et al. Preclinical activity of ARQ 087, a novel inhibitor targeting FGFR dysregulation. PLoS One 2016;11:e0162594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sootome H, Fujita H, Ito K et al. Futibatinib is a novel irreversible FGFR 1‐4 inhibitor that shows selective antitumor activity against FGFR‐deregulated tumors. Cancer Res 2020 Sep 24:canres.2568.2019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 69. Perera TPS, Jovcheva E, Mevellec L et al. Discovery and pharmacological characterization of JNJ‐42756493 (erdafitinib), a functionally selective small‐molecule FGFR family inhibitor. Mol Cancer Ther 2017;16:1010–1020. [DOI] [PubMed] [Google Scholar]
- 70. Heroult M, Ellinghaus P, Sieg C et al. Preclinical profile of BAY 1163877 ‐ A selective pan‐FGFR inhibitor in phase 1 clinical trial. Cancer Res 2014;74(suppl 19):1739a. [Google Scholar]
- 71. Nakanishi Y, Akiyama N, Tsukaguchi T et al. The fibroblast growth factor receptor genetic status as a potential predictor of the sensitivity to CH5183284/Debio 1347, a novel selective FGFR inhibitor. Mol Cancer Ther 2014;13:2547–2558. [DOI] [PubMed] [Google Scholar]
- 72. Cleary JM, Voss MH, Meric‐Bernstam F et al. Safety and efficacy of the selective FGFR inhibitor Debio 1347 in phase I study patients with FGFR genomically activated advanced biliary tract cancer (BTC). J Clin Oncol 2018;36(suppl 4):447a. [Google Scholar]


