Abstract
Background
Recent revision significantly changed the American Joint Committee on Cancer (AJCC) staging criteria for differentiated thyroid cancer (DTC). To quantitatively evaluate resulting changes in patient stage distribution and the associated disease‐specific survival (DSS) incorporating diverse populations, we performed a meta‐analysis of studies comparing the AJCC 7th edition (AJCC‐7) with 8th edition (AJCC‐8) staging for DTC.
Materials and Methods
After PROSPERO registration (#CRD42019123657), publications in English reporting DSS of DTC with AJCC‐7 and AJCC‐8 from inception to June 2019 were identified by search of MEDLINE and PubMed. Random‐effects meta‐analyses were conducted to compare differences in survival between AJCC‐7 and AJCC‐8. Pooled hazard ratios, 10‐year DSS, and corresponding interval estimates were calculated for AJCC subgroups. Differences in survival between editions were assessed using subgroup analysis with nonoverlapping confidence intervals indicating statistical significance.
Results
Final analysis included six studies with 10,850 subjects and median follow‐up from 55 to 148 months. Use of AJCC‐8 shifted classification to earlier stages: stage I, from 60% to 81%; stage II, from 5% to 13%; stage III, from 21% to 2%; stage IV, from 10% to 3%. Ten‐year DSS was significantly lower in AJCC‐8 versus AJCC‐7 in patients with stage II (88.6%, 95% confidence interval [CI] 82.7–94.6% vs. 98.1%, 95% CI 96.6–99.6%, respectively) and stage III disease (70.5%, 95% CI 59.1–83.9% vs. 96.8%, 95% CI 94.1–99.64%, respectively).
Conclusion
Meta‐analysis of revised AJCC staging for DTC, incorporating diverse populations, demonstrates redistribution of patients toward earlier clinical stages and better stratification of disease‐specific mortality risk, specifically among patients now classified with stage II and III disease.
Implications for Practice
This study provides updated estimates of disease‐specific survival for patients with differentiated thyroid cancer determined by the American Joint Committee on Cancer staging system that are generalizable to broader populations and support improved stratification using the recently revised criteria.
Keywords: Thyroid neoplasms, Mortality, Neoplasm staging, Meta‐analysis
Short abstract
This article evaluates recent revisions to the AJCC staging criteria for differentiated thyroid cancer.
Introduction
Thyroid cancer is the most common endocrine malignancy and is frequently encountered in clinical practice across medical and surgical specialties. Differentiated thyroid cancer (DTC), including papillary, follicular, and Hürthle cell subtypes, composes the majority of cases. The overall disease‐specific mortality for patients with DTC is low, although some patients are at risk for both locoregional recurrence and greater mortality when more aggressive disease is present [1]. To estimate mortality risk, a number of staging and prognosis systems are used in the care of patients with DTC, including the American Joint Committee on Cancer (AJCC) [2]; the metastasis, age, completeness of resection, invasion, and size (MACIS) model [3]; Age, Grade, Extent, Size (AGES) score; and the Age, Metastasis, Extent, Size (AMES) score [4]. Although the AJCC system is commonly used by oncologists and endocrinologists to estimate mortality in patients with thyroid cancer, many experts in the field felt that the clinical utility of prior AJCC staging iterations was limited because patients were not adequately stratified into high and low risk groups for mortality by the staging criteria [5, 6, 7].
In 2016, a revised 8th edition of the AJCC Tumor‐Node‐Metastasis (TNM) staging system for DTC was published and reflected updated data regarding the influence of age and extrathyroidal spread [2, 7]. Recent data clarified increased age as a continuous risk factor for disease‐specific mortality from DTC [8]. Furthermore, data from new studies emphasized adverse survival outcomes in patients with gross thyroid cancer invasion into surrounding structures, compared with patients with only central neck lymph node metastases or microscopic extrathyroidal extension who are at relatively low risk of dying from thyroid cancer [9, 10, 11, 12]. Therefore, major changes in AJCC 8th edition included the following: (a) the age‐at‐diagnosis cutoff used for staging increased from 45 to 55 years, (b) minor histological extrathyroidal extension was removed from the classification, (c) N1 disease was downstaged to stage I or II, (d) T3 status was divided into T3a (tumor size >4 cm) and T3b (gross extrathyroidal extension), and (e) upper mediastinal compartment (level VII) nodal involvement was reclassified to N1a from previous N1b.
The goal of these changes was to improve disease‐specific mortality prediction in patients with DTC, incorporating new data about risk factors for worse survival to better target increased surveillance and adjuvant therapy to patients with higher mortality risk and limit the burden of unnecessary care in lower risk patients [5, 7]. Since publication, several reports have compared the performance of the 7th edition of AJCC (AJCC‐7) and 8th edition of AJCC (AJCC‐8) [13, 14, 15, 16, 17, 18, 19]. Because thyroid cancer patient populations and practice patterns vary geographically and among institutions, we anticipated that this heterogeneity would be reflected in single‐center studies evaluating the new staging criteria. Thus, we performed a meta‐analysis of these data to provide a more generalizable summary of the stage migrations and differences in mortality prediction between AJCC‐7 and AJCC‐8.
Materials and Methods
Prior to study initiation, this protocol was registered and published online with PROSPERO, an international database of prospectively registered systematic reviews in health and social care maintained by the University of York (York, United Kingdom); the PROSPERO identifier is CRD42019123657. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines for meta‐analysis were applied [20].
Literature Search
Electronic databases PubMed and MEDLINE were searched in June 2019 for relevant English language articles on the subject of revised AJCC staging in differentiated thyroid cancer and survival. Search terms were included for the AJCC staging system, thyroid cancer type, and primary outcome of disease‐specific survival (DSS). The following terms were used to search PubMed/MEDLINE: “American Joint Committee on Cancer”; AND “Thyroid cancer”; AND “survival”; NOT “medullary”; NOT “anaplastic”; AND [publication date] 01/01/2016 to present; AND [Language] English. One additional article was added manually for consideration by the review of references in articles and clinical expertise of the study authors.
Study Selection
Studies identified by the search strategy were evaluated for inclusion or exclusion by two independent reviewers (M.G.L. and A.L.). Initially, articles were screened using the title and abstract, and then articles were read in entirety to determine whether full inclusion and exclusion criteria were met. Included studies had to evaluate subjects with DTC, including papillary, follicular, or undefined subtype of DTC, and include staging data by both AJCC‐7 and AJCC‐8. Furthermore, studies had to report thyroid cancer–specific mortality. Language was restricted to English. AJCC staging for thyroid cancer is used for both adult and pediatric populations, and studies were not limited to adults to allow inclusion of greater geographic diversity. Additionally, although some studies were limited to individuals aged 18 years or older [14, 19], multiple published studies outside of the U.S. and Europe provided only interquartile range or standard deviation for age without specific age range, precluding determination of minimum included age [13, 16, 17], and one study reported an age range of 5.8 to 89.5 years [15]. Studies of medullary, poorly differentiated, or anaplastic thyroid cancer were excluded. After initial selection, full texts were reviewed and further excluded if DSS by stage for both AJCC‐7 and AJCC‐8 was not reported. Duplicates or studies on the same population were identified by review of published methods or known composition of national databases (e.g., the Surveillance, Epidemiology, and End Results database) and were excluded as well with only the largest cohort included in the analysis [21, 22]. Included studies from the same region, namely, reports by M. Kim et al. [17] and T.H. Kim et al. [16], were confirmed to comprise patients from unique populations. Any discrepancies were discussed by all authors and resolved by consensus. Table 1 provides an overview of studies included in the final analysis. Determination of AJCC‐7 and ‐8 staging was determined retrospectively in all included studies by review of final pathology reports, with supplementation from operating room reports [16] and clinical notation in some studies. Studies reported staging assignment by clinicians and study authors, including surgeons, endocrinologists, and pathologists, with expertise in thyroid cancer. Review of primary data [13, 14, 15] and/or direct communication with original study authors [13, 14, 15, 16, 17, 19] provided additional confirmation of staging completeness and process. All studies except for one included all information needed for assignment of both AJCC‐7 and ‐8 stage for all patients, including data on tumor size, extrathyroidal extension, and nodal and metastatic spread. From the study by Verburg et al. [15] 200 of 2,257 patients lacked complete staging information and were excluded from this meta‐analysis. Individual studies were assessed for risk of bias by two independent reviewers (S.S.P. and T.E.A.) using the National Heart, Lung, and Blood Institute (NHLB) Quality Assessment Tool for Observational Cohort and Cross‐Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools). We considered forms of bias including selection bias, performance bias, detection bias, attrition bias, and reporting bias in a systematic manner in order to estimate the likely magnitude of impact in relation to observed effect.
Table 1.
Study populations included in the meta‐analysis
| Study first author, year published | Location | Study design | n | Age, median, years | Sex | Follow‐up, median, months | DTC subtype, n (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| F | M | Papillary | Follicular | Other | ||||||
| M. Kim, 2017 | Korea | Retrospective | 1,613 | 44 | 1,414 (88) | 199 (12) | 134 | 1,526 (95) | 87 (5) | 0 (0) |
| T.H. Kim, 2017 | Korea | Retrospective | 3,176 | 46 | 2,747 (86) | 429 (14) | 148 | 3,091 (97) | 85 (3) | 0 (0) |
| Shteinshnaider, 2018 | Israel | Retrospective | 433 | 47 | 327 (76) | 106 (24) | 116 | 386 (89) | 29 (7) | 18 (4) |
| Tam, 2018 | U.S. | Retrospective | 2,579 | 48 | 1,819 (71) | 760 (29) | 55 | 2,422 (94) | 126 (5) | 31 (1) |
| van Velsen, 2018 | The Netherlands | Retrospective | 792 | 49 | 545 (69) | 247 (31) | 86 | 628 (79) | 164 (21) | 0 (0) |
| Verburg, 2018 | Germany | Retrospective | 2,257 | 48 | 1,571 (70) | 686 (30) | 86 | 593 (26) | 1,663 (74) | 0 (0) |
| Pooled data | 10,850 | 44–49 years | 8,423 (78) | 2,427 (22) | 55–148 months | 8,646 (80) | 2,154 (19) | 49 (0.5) | ||
| Study first author, year published | Primary tumor size, n (%) | Extrathyroidal extension, n (%) | Lymph node metastasis, n (%) | Distant metastasis, n (%) | Surgery, n (%) | RAI, n (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <2 cm | 2–4 cm | >4 cm | Present | None | Present | None | Present | None | Total | Partial | Yes | No | |
| M. Kim, 2017 | 1,020 (63) | 473 (29) | 1,]20 (7) | 816 (51) | 797 (49) | 901 (56) | 712 (44) | 39 (2) | 1,574 (98) | 1,312 (81) | 301 (19) | 1,264 (78) | 349 (22) |
| T.H. Kim, 2017 | 2,521 (79) | 536 (17) | 119 (4) | 1,814 (57) | 1,362 (43) | 1,116 (35) | 2,060 (65) | 43 (1) | 3,133 (99) | 2,922 (92) | 254 (8) | 2,724 (86) | 452 (14) |
| Shteinshnaider, 2018 | ND | ND | ND | 346 (80) | 87 (20) | 119 (27) | 314 (73) | 18 (4) | 415 (96) | 375 (87) | 58 (13) | 373 (86) | 60 (14) |
| Tam, 2018 | 1,720 (67) | 607 (24) | 228 (9) | 1,082 (42) | 1,497 (58) | 1,135 (44) | 1,444 (56) | 97 (4) | 2,482 (96) | 2,348 (91) | 231 (9) | 1,442 (58) | 1,054 (42) |
| van Velsen, 2018 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 748 (97) | 25 (3) | 736 (93) | 56 (7) |
| Verburg, 2018 | ND | ND | ND | ND | ND | 1,767 (78) | 490 (22) | 203 (9) | 2,054 (91) | ND | ND | ND | ND |
| Pooled data | 5,261 (72) | 1,616 (22) | 467 (6) | 4,058 (52) | 3,743 (48) | 5,038 (50) | 5,020 (50) | 400 (4) | 9,658 (96) | 7,705 (90) | 869 (10) | 6,539 (77) | 1,971 (23) |
Clinical data in some categories were not available for all subjects in published studies, and therefore the sum of all columns will be less than the total study population.
Comparisons among study populations demonstrated significant differences for the following clinical parameters (p < .001, by chi‐square with Bonferroni correction for multiple comparisons): sex, DTC subtype, primary tumor size, extrathyroidal extension, lymph node metastasis, distant metastasis, surgery, and RAI.
Abbreviations: DTC, differentiated thyroid cancer; F, female; M, male; ND, no data; RAI, radioactive iodine therapy.
Data Analysis and Extraction
For data collection, two study reviewers (M.G.L. and A.L.) independently abstracted data in duplicate for all included articles. The following elements were extracted from each included study: author and year of publication, PubMed identifier, study design, population of study (country and institution), type of DTC included, type of study, number of patients included in control (AJCC‐7) and intervention (AJCC‐8), and outcomes evaluated (e.g., DSS, overall survival, recurrence). Data were also collected on the number of patients categorized in each clinical stage under AJCC‐7 compared with AJCC‐8 and the specific groups transitioning stage with the revision. Clinical variables collected for each study included median age, sex distribution, follow‐up time, surgery (e.g., total vs. partial thyroidectomy), radioactive iodine treatment, primary tumor size (<2 cm, 2–4 cm, >4 cm), presence or absence of extrathyroidal extension, and lymph node or distant metastasis. For variables or outcomes with incomplete data, additional information was requested from the original study authors in writing to allow inclusion and optimize the completeness of data collection. Specifically, outcome data for hazard ratios and 10‐year DSS were requested from authors of studies for which only a Kaplan‐Meier curve for DSS was shown in the published literature [13, 14, 15, 16, 17, 18, 19]. Of such studies, one [18] was excluded because original study authors were unable to provide data for 10‐year DSS and hazard ratios (HRs), and therefore data necessary for this meta‐analysis could not be obtained.
Statistical Analysis
Descriptive statistics for clinical parameters for each study and for the pooled study population were determined using GraphPad Prism8 (GraphPad Software, San Diego, CA). Statistically significant differences among included studies for clinical parameters were evaluated by chi‐square test (α = .05), with Bonferroni correction for multiple comparisons. Random‐effects meta‐analyses were conducted to compare differences in survival between AJCC‐7 and AJCC‐8. Pooled HRs, 10‐year DSS, and corresponding interval estimates were calculated for AJCC subgroups. Differences in survival between editions were assessed using subgroup analysis with nonoverlapping confidence intervals indicating statistical significance. Data were harmonized for studies that did not provide estimates. Ten‐year DSSs and HRs were estimated for two studies (Shteinshnaider et al. [13] and Verburg et al. [15]; supplemental online Appendix 1). HR was calculated for one study (T.H. Kim et al. [16]) that only provided finer strata estimates (supplemental online Appendix 1). Some studies are missing estimates because no individuals fell into that stage category (Tam et al. [14] does not have AJCC‐7 stage I DSS and HR, and van Velsen et al. [19] does not have AJCC‐7 stage III DSS and HR).
Random‐effects meta‐analysis on proportions was conducted to assess stage migration between AJCC editions. Additional random‐effects models were conducted to compare differences in survival between AJCC‐7 and AJCC‐8. Pooled HRs and 10‐year DSS and corresponding interval estimates were calculated for AJCC subgroups. Differences in survival between editions were also assessed using meta‐regression and subgroup analysis, with nonoverlapping confidence intervals indicating statistical significance.
The influence of individual studies was assessed by calculating the summary HR estimates in which estimates of each study were excluded one by one. The proportion of variation across studies due to heterogeneity was assessed using the I2 statistic (<30%, mild heterogeneity; 30%–50%, moderate heterogeneity; >50%, notable heterogeneity). Meta‐analyses were implemented using Stata 15 (StataCorp, College Station, TX), and survival analyses of individual studies were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Study Characteristics
Our search identified a total of 38 studies, of which 16 were excluded based upon title and abstract and 2 others excluded as reviews. Of the 20 full texts that were evaluated, 6 met all inclusion and exclusion criteria (Fig. 1). Therefore, the final analysis included six studies with 10,850 subjects and median follow‐up from 55 to 148 months. The pooled population consisted of 8,423 (78%) female and 2,427 (22%) male subjects and a median age range of 44 to 49 years (Table 1). The DTC cancer types were papillary (8,646/10,849, 80%), follicular (2,154/10,849, 19%), or unspecified (49/10,849, 0.5%). In all studies in which Hürthle cell histology was specified, these cases were grouped with follicular thyroid cancer [17, 19] or no Hürthle cell cases were reported [16]. As shown in Table 1, extrathyroidal extension was noted in 4,058 of 7,801 (52%) patients. Furthermore, 50% (5,038/10,058) of patients had lymph node metastasis, and 4% (400/10,058) had distant metastasis at diagnosis. For patients with treatment data available, most were treated with total (7,705/8,574, 90%) versus partial thyroidectomy (869/8,574, 10%), and 77% (6,539/8,510) received radioactive iodine therapy. Some clinical parameters were not available for all subjects, as reflected in group denominators. Notably, there was significant heterogeneity across study populations within these clinical parameters. The study by Verburg et al. [15] included significantly more patients with follicular thyroid cancer, compared with the predominantly papillary thyroid cancer DTC subtype in other studies (p < .001). Studies also varied significantly in the proportion of patients with cancer extrathyroidal extension (p < .001), ranging from 82% in the report by Shteinshnaider et al. [13] to 42% in the study by Tam et al. [14]; notably, these data were not available from two studies. The presence of lymph node and distant metastasis varied significantly (p < .001 for both), with the highest proportion of patients with metastatic disease in the population described by Verburg et al. [15]. Treatment approaches were available for five of six included studies and also demonstrated significant differences in the proportion of subjects treated with total versus partial thyroidectomy and the proportion receiving radioactive iodine ablation therapy (p < .001 for both). Risk of bias in individual studies assessed using the NHLB Quality Assessment Tool found a lack of impactful bias in the studies reviewed. All studies were retrospective, single‐center chart reviews. The exposure in all studies was AJCC staging, and the outcome was mortality. The most relevant areas to assess included clear definition of study population including inclusion and exclusion criteria, sample size, and time frame sufficient to assess mortality. Less important in these studies were questions that assessed exposure, blinding, and loss to follow‐up.
Figure 1.
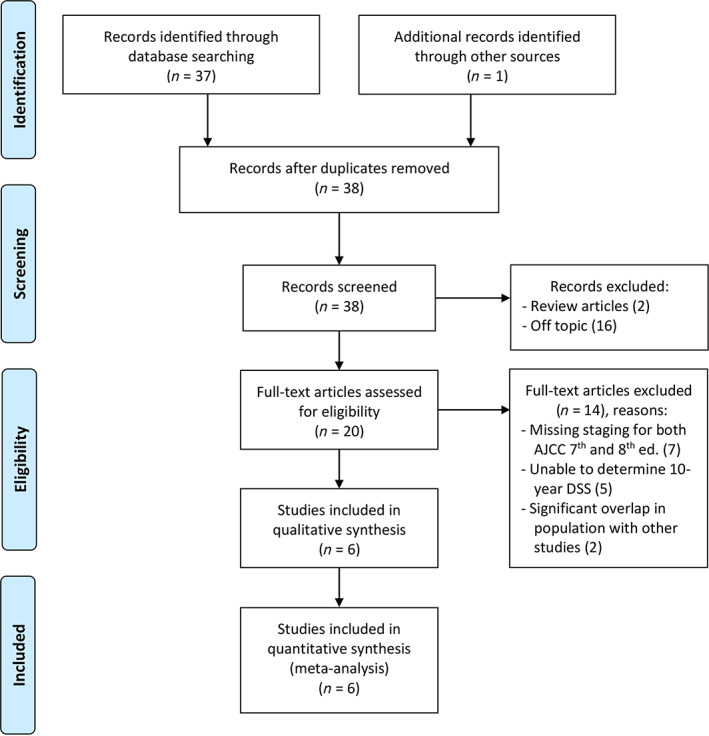
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow chart of study selection.Abbreviations: AJCC, American Joint Committee on Cancer; DSS, disease‐specific survival.
Stage Migration Patterns from AJCC Staging System 7th to 8th Edition
Revised staging criteria resulted in reclassification of many groups of patients based upon age, disease extension, and tumor size [7]. Figure 2 shows the pattern of stage migration for the pooled study population. To quantify these changes and test for differences in the patterns of stage migration among institutions, we performed a meta‐analysis for stage migration, as shown in Figure 3. Patients either remained in the original stage category or were staged to a lower category. No patients moved up to a higher stage category. Some subgroups do not have summary estimates because there was no variability: all patients in stage I remained in stage I, and all patients in stage III migrated out of stage III. For patients that were originally categorized in stage II (AJCC‐7), more than three‐fourths migrated to stage I (0.76, 95% confidence interval [CI]: 0.71–0.82), and about a quarter remained in stage II (0.24, 95% CI: 0.18–0.29). For patients originally in stage III, more than half moved to stage I (0.58, 95% CI: 0.51, 0.65), and the rest moved to stage II (0.41, 0.34–0.48). For patients originally in stage IV, a quarter moved to stage I (0.26, 95% CI: 0.18, 0.34), a third moved to stage II (0.32, 95% CI: 0.27–0.36), less than 20% moved to stage III (0.18, 95% CI: 0.11–0.24), and a quarter remained in stage IV (0.24, 95% CI: 0.10–0.37). Interestingly, notable heterogeneity (>50%) was found for migration proportion outcomes among studies (Fig. 3).
Figure 2.
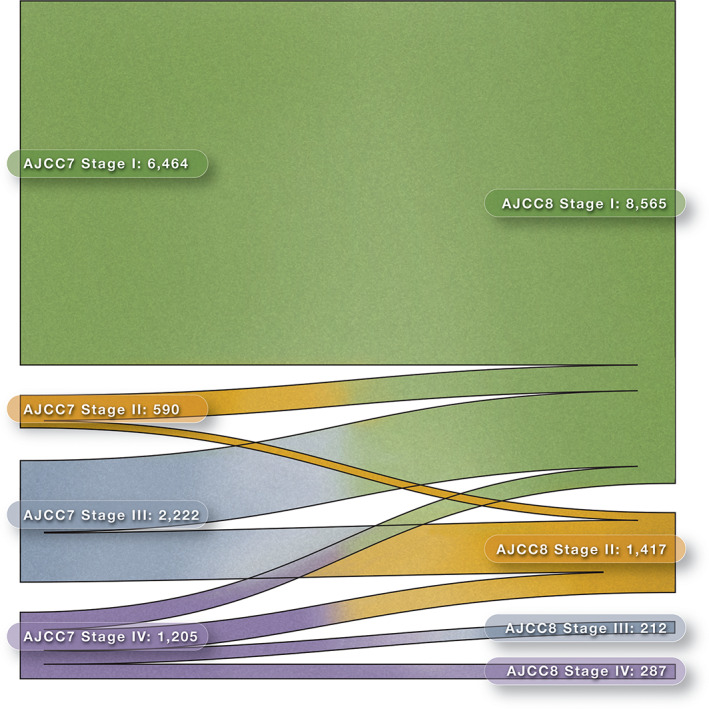
Alluvial diagram of stage migration with American Joint Committee on Cancer revision.Abbreviations: AJCC7, American Joint Committee on Cancer 7th edition; AJCC8, American Joint Committee on Cancer 8th edition.
Figure 3.
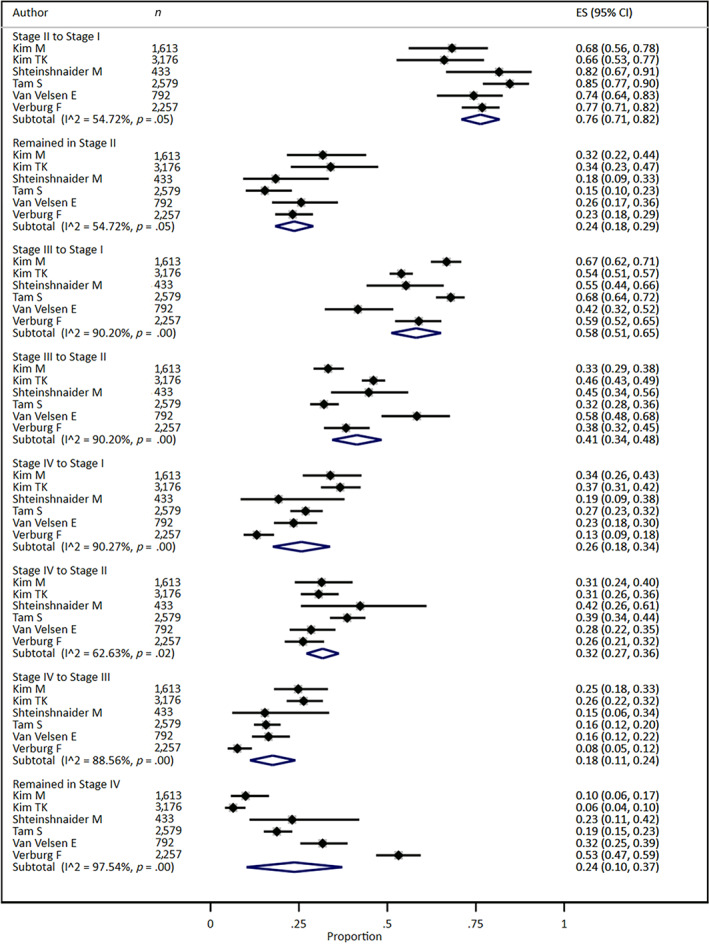
Stage migration from American Joint Committee on Cancer 7th to 8th edition staging for differentiated thyroid cancer.Abbreviations: CI, confidence interval; ES, effect size.
Disease‐Specific Survival from AJCC 7th to AJCC 8th Edition
A goal of the updated AJCC‐8 staging for DTC was to better distinguish patient disease groups with poor prognosis from the majority of patients with good long‐term outcomes. To test whether AJCC‐8 staging achieved this aim, we compared differences in disease‐specific survival across stages of disease when patients were classified according to AJCC‐7 versus AJCC‐8 (Fig. 4 and Table 2). As shown in Figure 4, AJCC‐8 better stratifies disease‐specific survival risk among stages. Specifically, the summary disease‐specific survival HRs in AJCC‐8 increase progressively with higher clinical stage. For AJCC‐8 classification, the summary HR for stage II was 10.6 (95% CI: 6.68–15.77), 43.81 (95% CI: 18.70–102.61) for stage III, and 85.39 (95% CI: 41.01–177.78) for stage IV relative to stage I. By comparison, the summary HRs in the prior AJCC‐7 system were approximately 4 for both stage II (4.36, 95% CI: 1.92–9.94) and stage III (4.27, 95% CI: 2.85–6.38) disease compared with stage I; the summary HR was higher for stage IV disease at 36.55 (95% CI: 18.30–73.01). Across all stages, the summary HRs were higher in AJCC‐8 compared with AJCC‐7, although these differences were not statistically significant (Table 2 and Fig. 4).
Figure 4.
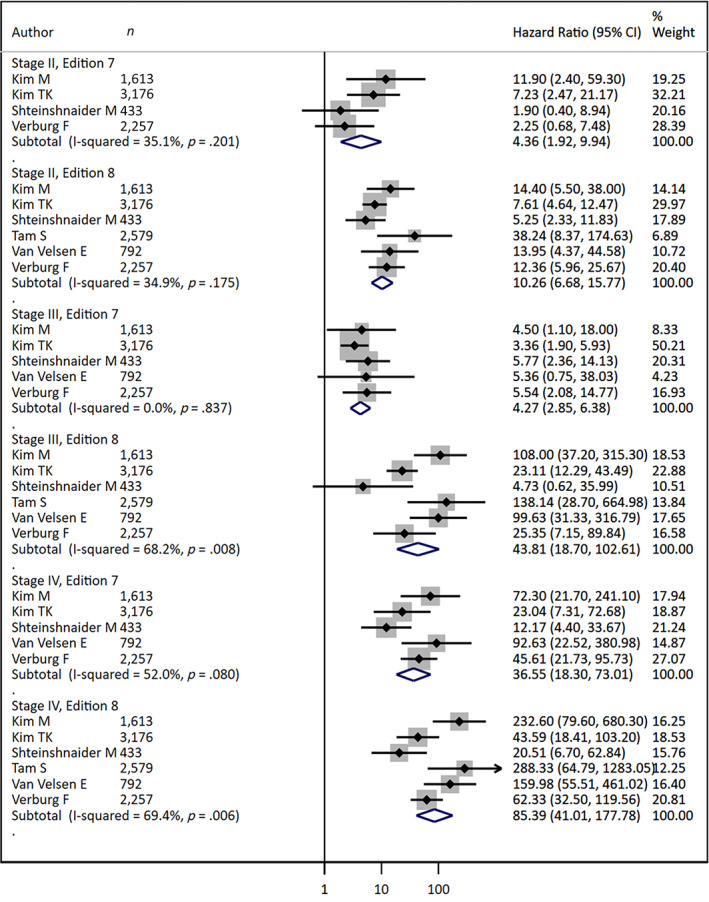
Stratified forest plot comparing 10‐year disease‐specific survival of American Joint Committee on Cancer 7th and 8th edition staging for differentiated thyroid cancer.Abbreviation: CI, confidence interval.
Table 2.
Meta‐analyses comparing summary HR of American Joint Committee on Cancer 7th and 8th edition staging for differentiated thyroid cancer
| Stage | 7th edition | 8th edition | ||
|---|---|---|---|---|
| Pooled HR (95% CI) | I2 (%) | Pooled HR (95% CI) | I2 (%) | |
| I | (ref) | |||
| II |
4.36 (1.92–9.94) |
35.1 |
10.26 (6.68–15.77) |
34.9 |
| III |
4.27 (2.85–6.38) |
0.0 |
43.81 (18.70–102.61) |
68.2 a |
| IV |
36.55 (18.30–73.01) |
52.0 |
85.39 (41.01–177.78) |
69.4 a |
p < .05.
Abbreviations: CI, confidence interval; HR, hazard ratio.
In addition, Figure 4 shows that the heterogeneity among studies is greater for stages III and IV with the new classification, whereas stage II heterogeneity remained similar between AJCC‐7 and AJCC‐8. Shteinshnaider et al. [13] and T.H. Kim et al. [16] tended to have lower HR across all stages with AJCC‐8, whereas M. Kim et al. [17], Tam et al. [14], and van Velsen et al. [19] had higher HRs compared with the summary HR across all stages with AJCC‐8. This variability across studies is reflected in the increased I2 measure for stage III and IV and emphasizes the importance of variations in local patient populations and practice patterns in the reported validation studies and the utility of meta‐analyses to provide more generalizable disease information. Ten‐year disease‐specific survival was significantly lower in AJCC‐8 compared with AJCC‐7 in stage II (88.58, 95% CI: 82.73–94.85 vs. 98.09, 95% CI: 96.59–99.60, respectively) and stage III (70.45, 95% CI: 59.13–83.93 vs. 96.84, 95% CI: 94.12, 99.64, respectively; Table 3). Meta‐regression analysis resulted in similar conclusions to the stratified meta‐analyses (not shown). There was mild to notable heterogeneity found among studies for DSS and HR outcomes (Tables 2 and 3).
Table 3.
Meta‐analyses comparing summary 10‐year DSS of American Joint Committee on Cancer 7th and 8th edition staging for differentiated thyroid cancer
| Stage | 7th edition | 8th edition | ||
|---|---|---|---|---|
| Pooled DSS (95% CI) | I2 (%) | Pooled DSS (95% CI) | I2 (%) | |
| I |
99.39 (98.85–99.93) |
57.1 |
99.28 (98.67–99.90) |
73.5 a |
| II |
98.09 (96.59–99.60) |
0.0 |
88.58 (82.73–94.85) |
70.1 |
| III |
96.84 (94.12–99.64) |
67.7 a |
70.45 (59.13–83.93) |
0.0 |
| IV |
72.59 (63.53–82.94) |
79.9 a | 65.49 (57.19–75.00) | 66.8 a |
p < .05.
Abbreviations: CI, confidence interval; DSS, disease‐specific survival.
Funnel plots for stage migration and disease‐specific survival analyses did not show evidence of publication bias (supplemental online Fig. 1). Sensitivity analysis indicates that the removal of studies one by one does not significantly change summary estimates, which makes us confident that the results are robust (supplemental online Fig. 2).
Discussion
The recently revised criteria in the 8th edition of AJCC staging for DTC introduced key modifications to improve stratification of mortality between stages. Expert consensus opinion was that previous versions of AJCC did not adequately distinguish those patients with a poor survival prognosis from the majority of patients diagnosed with DTC who have excellent long‐term survival, which limited its use for making treatment decisions. Although DTC has favorable prognosis, accurate prediction is important to clinical management of patients. Since the introduction of AJCC‐8, a number of groups have reported its performance compared with AJCC‐7, but because patient populations and practice patterns can vary significantly across institutions, the generalizability of these results remained unclear. Therefore, we performed a meta‐analysis of studies reporting the comparative performance of these AJCC editions, comprising data from multiple institutions in geographically diverse areas. We show that AJCC‐8 revision improved stratification of disease‐specific survival among clinical stages of disease compared with AJCC‐7. Specifically, summary HRs for disease‐specific survival with AJCC‐8 progressively increase with higher clinical stage, as shown in Figure 4 and Table 2. This is in contrast to the summary HR with AJCC‐7, in which stage II and III disease both had HR near 4 compared with stage I disease. We expect that patients who are moving from a higher stage using AJCC‐7 to a lower stage using AJCC‐8 had a lower risk of mortality. For example, using the pooled study population, all patients originally categorized as stage I remained in stage I, and approximately 20% of the total number of patients moved to stage I. We postulate that those patients who moved to stage I actually had a lower risk of mortality and expect the HRs to be greater in AJCC‐8 versus AJCC‐7. This is exactly what we observe for stages II, III, and IV with HRs of 4, 4, and 37 versus 10, 44, and 85, respectively, for AJCC‐7 versus AJCC‐8. The improved mortality risk stratification in AJCC‐8 may enable more appropriate patient counseling and optimal selection of patients for systematic therapies or more frequent tumor surveillance.
Although there was relative consistency between all included studies, the results of this analysis showed mild to notable heterogeneity in DSS estimates, which may have been affected by individual study differences in characteristics considered to influence thyroid cancer survival, including sex, DTC subtype (papillary vs. follicular), distant metastasis, surgery, and radioactive iodine therapy. These underlying differences in the individual study populations of published reports highlight the need for the present summative analysis providing broader validation of the revised AJCC criteria.
Meta‐analysis of stage migration showed that many patients were reclassified to lower stages when changing from AJCC‐7 to AJCC‐8. Importantly, significant heterogeneity was seen among studies for stage migration, particularly for stage III and IV disease. Significant differences in cancer characteristics between studies, which included primary tumor size, extrathyroidal extension, lymph node metastasis, and distant metastasis, likely influenced variation in stage migration. Patients aged ≥55 years with minimal extrathyroidal extension or central lymph node metastases would be classified as stage III in AJCC‐7, but in AJCC‐8 would be stage I or II, respectively. Although patients aged ≥55 years with distant metastases would be stage IV in AJCC‐7 and AJCC‐8, those with lateral lymph node metastases or gross extrathyroidal extension not involving the prevertebral fascia or encasing major vessels would be downstaged from stage IV to stage II or III when comparing AJCC‐7 with AJCC‐8. Given that the rate of distant metastasis varied from 1% to 9% between studies, this may have had significant influence over the variability in stage migration. Patients aged 45–55 years with stage III–IV disease in AJCC‐7 would be classified in AJCC‐8 as stage I (without distant metastases) or II (with distant metastases), and, therefore, differences in patient age between studies might affect differences in stage migration. Although the median patient age was similar between studies, making this less likely to account for the observed difference, it is possible that age differences in specific stage III–IV patients contributed to our findings.
Limitations of this study include the incomplete retrieval of identified research from one study (Ghaznavi et al. [18]), for which we were unable to obtain sufficient granularity of data to complete planned analyses. Our analysis was also limited largely by the available published data for clinical variables, such as age, which precluded patient level adjustment for these variables or subgroup analyses. Specifically, with the shift in age threshold from 45 to 55 years within the AJCC criteria, analysis within this age range would have been valuable but was not possible with the available data. Our analysis did not find evidence of publication bias, and sensitivity analysis suggested robust results with our conclusions not dependent upon data from any single study. Lastly, in addition to disease‐specific mortality, local disease recurrence remains a significant challenge for patients with thyroid cancer and their providers, which is not the goal of the AJCC system. Future research into risk factors for disease recurrence may further improve care by identifying patients most likely to benefit from aggressive adjuvant therapies and posttreatment surveillance.
Conclusion
The revised AJCC criteria more effectively identified patients with DTC with worse cancer‐specific survival. Notably, the clinical characteristics of each study population, including DTC subtype, extent of disease, and treatment, varied significantly among the included reports, and differences between studies were seen with respect to the proportion of patients migrating between stages and disease‐specific survival estimates. These data provide updated summary estimates of disease‐specific survival for the current AJCC stages that are generalizable to broader populations and provide further validation of the improved stratification of patients with increased mortality risk from DTC using AJCC 8th edition.
Author Contributions
Conception/design: Melissa G. Lechner, Alyssa Lampe, Stephanie Smooke Praw, Trevor E. Angell
Provision of study material or patients: Samantha H. Tam
Collection and/or assembly of data: Melissa G. Lechner, Alyssa Lampe
Data analysis and interpretation: Melissa G. Lechner, Angeli C. Bernardo, Alyssa Lampe, Stephanie Smooke Praw, Samantha H. Tam, Trevor E. Angell
Manuscript writing: Melissa G. Lechner, Angeli C. Bernardo, Alyssa Lampe, Stephanie Smooke Praw, Samantha H. Tam, Trevor E. Angell
Final approval of manuscript: Melissa G. Lechner, Angeli C. Bernardo, Alyssa Lampe, Stephanie Smooke Praw, Samantha H. Tam, Trevor E. Angell
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supplemental Methods and Data
Figure S1 Funnel plots did not show evidence of publication bias for mortality estimation (Hazard Ratio, HR) or stage migration.
Figure S2. Sensitivity analysis for disease specific mortality Hazard Ratio (HR) and stage migration indicates that the removal of studies one‐by‐one does not significantly change summary estimates.
Acknowledgments
The authors would like to acknowledge the generous sharing of data and analyses requested to allow inclusion of studies in this meta‐analysis—Dr. Frederick Verburg (University Hospital Marburg, Marburg, Germany), Dr. Robin Peeters and Dr. Evert van Velsen (Erasmus Medical Center, Rotterdam, The Netherlands), Dr. Carlos Benbassat (Tel‐Aviv University, Tel‐Aviv, Israel), Dr. Tae Hyuk Kim (Sungkyunkwan University School of Medicine, Seoul, Korea), and Dr. Won Gu Kim (University of Ulsan College of Medicine, Seoul, Korea)—as well as Dr. Jien Shim (University of California Los Angeles, Los Angeles, CA) for Korean translation of correspondence. This work was supported in part by grants UL1TR001855 and UL1TR000130 through the University of Southern California (USC) Clinical and Translational Science Institute (CTSI) from the National Center for Advancing Translational Science (NCATS) of the U.S. National Institutes of Health (A.C.B. and T.E.A.).
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com
References
- 1. Haugen BR, Alexander EK, Bible KC et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016;26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brierley JD, Gospodarowicz MK, Wittekind C, eds. TNM Classification of Malignant Tumours. 8th ed Hoboken, NJ: John Wiley & Sons, 2017. [Google Scholar]
- 3. Hay ID, Bergstralh EJ, Goellner JR et al. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery 1993;114:1050–1057. [PubMed] [Google Scholar]
- 4. Davis NL, Bugis SP, McGregor GI et al. An evaluation of prognostic scoring systems in patients with follicular thyroid cancer. Am J Surg 1995;170:476–480. [DOI] [PubMed] [Google Scholar]
- 5. Omry‐Orbach G. Risk stratification in differentiated thyroid cancer: An ongoing process. Rambam Maimonides Med J 2016;7:e0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papaleontiou M, Haymart MR. New insights in risk stratification of differentiated thyroid cancer. Curr Opin Oncol 2014;26:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor‐Node‐Metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): What changed and why? Thyroid 2017;27:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bischoff LA, Curry J, Ahmed I et al. Is above age 45 appropriate for upstaging well‐differentiated papillary thyroid cancer? Endocr Pract 2013;19:995–997. [DOI] [PubMed] [Google Scholar]
- 9. Ito Y, Tomoda C, Uruno T et al. Prognostic significance of extrathyroid extension of papillary thyroid carcinoma: Massive but not minimal extension affects the relapse‐free survival. World J Surg 2006;30:780–786. [DOI] [PubMed] [Google Scholar]
- 10. Radowsky JS, Howard RS, Burch HB et al. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 2014;24:241–244.23713855 [Google Scholar]
- 11. Fukushima M, Ito Y, Hirokawa M et al. Prognostic impact of extrathyroid extension and clinical lymph node metastasis in papillary thyroid carcinoma depend on carcinoma size. World J Surg 2010;34:3007–3014. [DOI] [PubMed] [Google Scholar]
- 12. Diker‐Cohen T, Hirsch D, Shimon I et al. Impact of minimal extrathyroid extension in differentiated thyroid cancer: Systematic Review and meta‐analysis. J Clin Endocrinol Metab 2018;103:2100–2106. [DOI] [PubMed] [Google Scholar]
- 13. Shteinshnaider M, Kalmovich LM, Koren S et al. Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: A comparative study. Thyroid 2018;28:201–209. [DOI] [PubMed] [Google Scholar]
- 14. Tam S, Boonsripitayanon M, Amit M et al. Survival in differentiated thyroid cancer: Comparing the AJCC cancer staging seventh and eighth editions. Thyroid 2018;28:1301–1310. [DOI] [PubMed] [Google Scholar]
- 15. Verburg FA, Mäder U, Luster M et al. The effects of the Union for International Cancer Control/American Joint Committee on Cancer Tumour, Node, Metastasis system version 8 on staging of differentiated thyroid cancer: A comparison to version 7. Clin Endocrinol (Oxf) 2018;88:950–956. [DOI] [PubMed] [Google Scholar]
- 16. Kim TH, Kim YN, Kim HI et al. Prognostic value of the eighth edition AJCC TNM classification for differentiated thyroid carcinoma. Oral Oncol 2017;71:81–86. [DOI] [PubMed] [Google Scholar]
- 17. Kim M, Kim WG, Oh HS et al. Comparison of the seventh and eighth editions of the American Joint Committee on Cancer/Union for International Cancer Control Tumor‐Node‐Metastasis staging system for differentiated thyroid cancer. Thyroid 2017;27:1149–1155. [DOI] [PubMed] [Google Scholar]
- 18. Ghaznavi SA, Ganly I, Shaha AR et al. Using the American Thyroid Association risk‐stratification system to refine and individualize the American Joint Committee on Cancer eighth edition disease‐specific survival estimates in differentiated thyroid cancer. Thyroid 2018;28:1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Velsen EFS, Stegenga MT, van Kemenade FJ et al. Comparing the prognostic value of the eighth edition of the American Joint Committee on Cancer/Tumor Node Metastasis staging system between papillary and follicular thyroid cancer. Thyroid 2018;28:976–981. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pontius LN, Oyekunle TO, Thomas SM et al. Projecting survival in papillary thyroid cancer: A comparison of the seventh and eighth editions of the American Joint Commission on Cancer/Union for International Cancer Control staging systems in two contemporary national patient cohorts. Thyroid 2017;27:1408–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shaha AR, Migliacci JC, Nixon IJ et al. Stage migration with the new American Joint Committee on Cancer (AJCC) staging system (8th edition) for differentiated thyroid cancer. Surgery 2019;165:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Appendix S1. Supplemental Methods and Data
Figure S1 Funnel plots did not show evidence of publication bias for mortality estimation (Hazard Ratio, HR) or stage migration.
Figure S2. Sensitivity analysis for disease specific mortality Hazard Ratio (HR) and stage migration indicates that the removal of studies one‐by‐one does not significantly change summary estimates.


