Key Points
Question
What is the prevalence of bicuspid aortic valve among newborns and what is the extent of associated disease of the aorta?
Findings
This cross-sectional study included 25 556 newborns in Denmark between 2016 and 2018 who underwent transthoracic echocardiography. The prevalence of bicuspid aortic valve was 0.77%; of these, aortopathy was present in 33%.
Meaning
Among newborns in Copenhagen, the prevalence of bicuspid aortic valve was 0.77% and associated aortic disease was common, suggesting that it was a fetal malformation.
Abstract
Importance
The prevalence and characteristics of bicuspid aortic valve (BAV) are mainly reported from selected cohorts. BAV is associated with aortopathy, but it is unclear if it represents a fetal developmental defect or is secondary to abnormal valve dynamics.
Objective
To determine the prevalence of BAV and BAV subtypes and to describe the associated aortopathy in a large, population-based cohort of newborns.
Design, Setting, and Participants
The Copenhagen Baby Heart Study was a cross-sectional, population-based study open to all newborns born in Copenhagen between April 1, 2016, and October 31, 2018. Newborns with BAV were matched 1:2 to newborns with a tricuspid aortic valve (non-BAV group) on sex, singleton/twin pregnancy, gestational age, weight, and age at time of examination.
Exposures
Transthoracic echocardiography within 60 days after birth.
Main Outcomes and Measures
Primary outcome was BAV prevalence and types, ie, number of raphes and spatial orientation of raphes or cusps (no raphes), according to the classification system of Sievers and Schmidtke (classified as type 0, 1, or 2, with numbers indicating the number of raphes). Secondary outcome was valve function and BAV-associated aortopathy, defined as aortic diameter z score of 3 or greater or coarctation.
Results
In total, 25 556 newborns (51.7% male; mean age, 12 [SD, 8] days) underwent echocardiography. BAV was diagnosed in 196 newborns (prevalence, 0.77% [95% CI, 0.67%-0.88%]), with male-female ratio 2.1:1. BAV was classified as type 0 in 17 newborns (8.7% [95% CI, 5.5%-13.5%]), type 1 in 178 (90.8% [95% CI, 86.0%-94.1%]) (147 [75.0% {95% CI, 68.5%-80.5%}] right-left coronary raphe, 27 [13.8% {95% CI, 9.6%-19.3%}] right coronary–noncoronary raphe, 4 [2.0% {95% CI, 0.8%-5.1%}] left coronary–noncoronary raphe), and type 2 in 1 (0.5% [95% CI, 0.1%-2.8%]). Aortic regurgitation was more prevalent in newborns with BAV (n = 29 [14.7%]) than in those without BAV (1.3%) (absolute % difference, 13.4% [95% CI, 7.8%-18.9%]; P < .001). Newborns with BAV had higher flow velocities across the valve (0.67 [95% CI, 0.65-0.69] m/s vs 0.61 [95% CI, 0.60-0.62] m/s; mean difference, 0.06 m/s [95% CI, 0-0.1]) and larger aortic root and tubular ascending aortic diameters than those without BAV (10.7 [95% CI, 10.7-10.9] mm vs 10.3 [95% CI, 10.2-10.4] mm; mean difference, 0.43 mm [95% CI, 0.2-0.6 mm] and 9.8 [95% CI, 9.6-10.0] mm vs 9.4 [95% CI, 9.3-9.5] mm; mean difference, 0.46 mm [95% CI, 0.30-0.70], respectively) (P < .001 for all). Aortopathy was seen in 65 newborns (33.2%) with BAV (62 with aortic z score ≥3; 3 with coarctation).
Conclusions and Relevance
Among newborns in Copenhagen, the prevalence of BAV was 0.77%. Aortopathy was common in newborns with BAV, suggesting that it also represents a fetal malformation.
This population epidemiology study describes the prevalence of bicuspid aortic valve (BAV) and BAV subtypes, and of accompanying enlarged aortic diameter or coarctation, in Danish newborns born in Copenhagen 2016-2018.
Introduction
Bicuspid aortic valve (BAV) is the most common congenital heart disease. BAV is generally considered to affect 0.5% to 1.4% of the population, based on autopsy studies and small echocardiographic studies.1,2 BAV is a complex and heterogeneous disease accounting for more premature deaths than all other congenital heart diseases combined.3,4
The pathology of BAV is mainly related to varying degrees of malformation of the valve, from complete absence of a commissure to underdevelopment of 1 or 2 commissures and adjacent cusps.5 The strong association with aortopathy, seen in 20% to 84% of patients with BAV, suggests that BAV is a disease involving the aorta.4,6,7 The etiology of BAV has not been fully elucidated, but there is familial clustering of BAV with linkage to several genes.8,9,10
In childhood, BAV is typically asymptomatic and diagnosed incidentally, whereas in adulthood, the diagnosis is often identified because of complications, eg, valvular dysfunction (aortic valve stenosis, aortic valve regurgitation, or both) and/or aortic aneurysm or aortic dissection.2,4,8,11 Interventions are eventually required in the majority of patients with BAV.11,12 Patients diagnosed early with BAV and followed up on a regular basis have the same life expectancy as the general population.13
Because of limited data on patients diagnosed with normally or near-normally functioning BAV, there is uncertainty about the true risk of complications related to BAV in the general population.13 The purpose of this study was to determine the prevalence of BAV and BAV subtypes among newborns in the general population and describe the associated aortopathy in newborns with BAV.
Methods
Written consent was obtained from the parents of all participants included in the study. The Copenhagen Baby Heart Study (CBHS [NCT02753348]) complies with the Declaration of Helsinki and was approved by the Regional Ethics Committee of the Capital City Region of Denmark (H-16001518) and by the Danish Data Protection Agency (I-Suite No. 04546, ID-No. HGH-2016-53).
This study was cross-sectional, with data collected prospectively from newborns included in the CBHS. The CBHS was a multicenter, population-based cohort study focusing on cardiac structure and function in newborns included between April 1, 2016, and October 31, 2018, in the Copenhagen Area of Denmark. The CBHS was open to all pregnant mothers in the catchment area of the 3 largest maternity wards in Copenhagen. Parents were encouraged to participate regardless of prenatal or neonatal conditions or findings and regardless of gestational age. The primary aim of the CBHS was to identify all newborns with BAV and establish the first large unselected BAV cohort for longitudinal follow-up.
A detailed description of the design and methodology of the CBHS has been published.14,15 In brief, participants were enrolled prenatally, and data regarding pregnancy, delivery, and parents’ health, predispositions, lifestyle, and socioeconomic status were collected. Examination of the newborn by sonographers systematically trained in neonatal echocardiography included systematic transthoracic echocardiography (TTE), electrocardiography, and pulse oximetry testing within 60 days after birth. Moreover, cord blood procedures (biochemical analyses, DNA purification, and biobank storage) were performed immediately after birth.
All newborns diagnosed with BAV were included in this study (BAV group) and matched 1:2 to newborns with a tricuspid aortic valve (non-BAV group) on sex; singleton/twin pregnancy; gestational age (≤5 days); weight (≤100 g) and age (≤1 day) at time of TTE.
Parents were approached with study information at the routine second-trimester ultrasound scan.
Echocardiography
The primary outcome was BAV prevalence and types, ie, number of raphes and spatial orientation of raphes or cusps (no raphes), according to the classification system of Sievers and Schmidtke.5 The secondary outcome was valve function and BAV-associated aortopathy, defined as aortic diameter z score of 3 or greater or coarctation.
Echocardiographic Examination
The TTE protocol (eTable 1 in the Supplement) in the CBHS included standard subxiphoid, apical, parasternal, and suprasternal views obtained in accordance with American Society of Echocardiography guidelines.16 The aortic valve and aorta were assessed from several views and with different Doppler modalities; parasternal long-axis (2D, color Doppler, M-Mode), short axis (2D, color Doppler), apical 5-chamber (2D, color Doppler, continuous- and pulsed-wave Doppler), and suprasternal (2D, color Doppler, continuous-wave Doppler). Echocardiographic images were acquired using Vivid E9 ultrasound machines (General Electric) equipped with cardiac sector probes 12S-D and 6S-D. The echocardiograms were stored digitally and analyzed offline using EchoPac software version 113 (General Electric).
Systematic Assessment of BAV
BAV was defined as an aortic valve with a partial or complete obliteration of the commissure between 2 adjacent cusps with or without a raphe, resulting in a “fish mouth”–like systolic opening of the valve. In addition, an aortic valve with commissural obliteration between adjacent cusps on both sides of 1 cusp was classified as bicuspid if 2 raphes could be detected; otherwise, it was classified as unicuspid (eFigure 1 in the Supplement). During all TTE examinations, the sonographer consistently evaluated whether BAV could be ruled out. If BAV could not be excluded, a specialist reviewed the potentially abnormal echocardiographic findings; if the aortic valve was not definite tricuspid nor bicuspid, the newborn underwent a TTE reexamination, including views and imaging modalities described in the previous section (flowchart illustrated in eFigure 2 in the Supplement). When BAV was detected, a second specialist reviewed and confirmed the diagnosis, and the newborn was included in the BAV group. Disagreement between the 2 specialists necessitated reexamination. All BAVs were classified according to the classification system of Sievers and Schmidtke,5 which reflects the number of raphes (type 0, 1, or 2, with numbers indicating the number of raphes) and the spatial orientation of raphe(s) or cusps (no raphe) (eFigure 1 in the Supplement).
Echocardiographic Analyses
Quantitative analyses included measurements of structural and functional parameters for the left ventricle, aortic valve, aorta, and main pulmonary artery, obtained in all newborns in the BAV and non-BAV groups according to published guidelines17 (eTable 2 in the Supplement). Measurements were obtained unblinded to valve morphology. All Doppler measurements were averaged over 3 cardiac cycles per view (if possible). All other measurements were obtained from 1 cardiac cycle. Aortic valve area (AVA) was calculated using the continuity equation, AVA = left ventricular outflow tractarea × left ventricular outflow tractvelocity time integral)/aortic valvevelocity time integral.
Qualitative analyses included classification of BAVs and evaluation of valvular function and associated lesions. Congenital aortic stenosis was defined as peak flow velocity greater than 2 m/s across the aortic valve. Aortic regurgitation was registered as trivial, mild, moderate, or severe (visual impression). Newborns diagnosed with BAV were referred to the pediatric cardiology outpatient clinic for follow-up with intervals depending on valvular function, aortic dimensions, and associated congenital heart diseases. Newborns with associated severe congenital heart diseases, such as severe coarctation, were immediately admitted to the hospital.
Quantitative and qualitative analyses of all newborns in the BAV and non-BAV groups were performed by a single physician (A.-S. S.), systematically trained in neonatal echocardiography, overseen by specialists in the field. The imaging protocol and the quality control protocol (systematic assessment of BAV) were determined before initiation of the CBHS.
Statistical Analyses
Categorical variables are presented as numbers and percentages. Continuous variables were tested for normality using the Shapiro-Wilk test and visually by the data distribution in histograms and are presented as mean (SD). Independent t test was used for comparison of normally distributed continuous variables between the BAV and non-BAV groups. Fisher exact test was used to compare dichotomous variables between the groups. Mean difference (continuous variables) and absolute % difference (dichotomous variables) are presented with 95% CIs. P < .05 (2-sided) was considered statistically significant. The Bonferroni correction was applied to correct for multiple testing for all echocardiographic parameters presented (n = 14), ie, P < .05/14 = .004.
The prevalence of BAV and BAV subtypes is presented with a 95% CI based on the Wilson score method without continuity correction.18 Body surface area was approximated with the Haycock formula using weight and length at time of TTE. z Scores according to body surface area were determined for each individual based on published z score models from the Pediatric Heart Network Normal Echocardiogram Database, which correlates well with the Boston Children’s Hospital system and other previous single-center models.19,20 Associated aortopathy was defined as significant dilatation (z score ≥3) of any segment of the ascending aorta or presence of coarctation.
Statistical analyses were performed using SPSS version 24 (SPSS Inc). Graphic illustrations were created using R version 3.4.4 (R Foundation for Statistical Computing).
Results
In total, 25 556 newborns (51.7% male; mean age, 12 [SD, 8] days) underwent TTE examination in the CBHS. BAV was identified in 196 newborns, corresponding to a prevalence of 0.77% (95% CI, 0.67%-0.88%) (eFigure 2 in the Supplement). Male-female ratio of BAV was 2.1:1. Demographic characteristics of the BAV and non-BAV groups show well-matched groups (Table). None of the newborns with BAV had undergone perinatal intervention prior to inclusion and TTE examination in the CBHS.
Table. Demographic Characteristics for the BAV Group and for the CBHS Cohort and the BAV and Non-BAV Groupsa.
| Characteristic | Mean (SD) | Difference (95% CI)c | |
|---|---|---|---|
| BAV group (n = 196) | CBHS cohort (n = 25 360)b | ||
| Sex, No. (%) | |||
| Male | 133 (67.9) | 13 068 (51.5) | 16.3 (9.5 to 23.2) |
| Female | 63 (32.1) | 12 292 (48.5) | −16.3 (−23.2 to 9.5) |
| Singleton/twin pregnancy, No. (%) | |||
| Singleton | 190 (96.9) | 24 458 (96.9) | 0.5 (−2.2 to 3.2) |
| Gestational age, mean (SD), d | 280.0 (10.9) | 278.9 (11.4) | 1.1 (−2.7 to 0.5) |
| Preterm (<37 wk), No. (%) | 6 (3.1) | 1236 (4.9) | −1.8 (−4.5 to 0.9) |
| Weight at birth, kg | 3.5 (0.5) | 3.5 (0.5) | 0 (−0.8 to 0.7) |
| Length at birth, cm | 51.7 (2.3) | 51.5 (2.5) | 0.2 (−0.5 to 0.2) |
| Apgar score <10, No. (%) | 17 (8.7) | 1511 (6.0) | 2.7 (−1.5 to 6.9) |
| Age at transthoracic echocardiography, d | 10.5 (6.8) | 11.9 (7.6) | −1.4 (0.3 to 2.4) |
| Weight at transthoracic echocardiography, kg | 3.6 (0.5) | 3.6 (0.6) | 0 (−0.1 to 0.1) |
| Length at transthoracic echocardiography, cm | 52.4 (2.5) | 52.3 (2.6) | 0.1 (−0.5 to 0.3) |
| Maternal age at delivery, y | 31.7 (4.8) | 31.7 (4.6) | 0 (−0.6 to 0.7) |
| Maternal prepregnancy BMId | 23.9 (4.2) | 23.6 (4.4) | 0.3 (−0.9 to 0.3) |
| Maternal smoking history, No. (%) | |||
| Yes | 15 (8.1) | 835 (3.4) | 4.7 (2.1 to 7.3) |
| Maternal race, No. (%) | |||
| White | 164 (91.1) | 21 399 (92.2) | −1.1 (−5.5 to 3.4) |
| Black | 2 (1.1) | 255 (1.1) | 0.0 (−1.5 to 1.6) |
| Asian | 11 (6.1) | 1190 (5.1) | 1.0 (−2.8 to 4.8) |
| Othere | 3 (1.7) | 406 (1.7) | −0.1 (−2.0 to 1.9) |
| Characteristic | BAV group (n = 191)f | Non-BAV group (n = 382) | Difference (95% CI)c |
| Sex, No. (%) | |||
| Male | 131 (68.6) | 262 (68.6) | 0 (−8.1 to 8.1) |
| Female | 60 (31.4) | 120 (31.4) | 0 (−8.1 to 8.1) |
| Singleton/twin pregnancy, No. (%) | |||
| Singleton | 188 (98.4) | 376 (98.4) | 0 (−2.2 to 2.2) |
| Gestational age, d | 280.5 (10.4) | 280.4 (10.1) | 0.03 (−1.7 to 1.8) |
| Age at transthoracic echocardiography, d | 10.3 (5.9) | 10.3 (5.9) | 0.02 (−1.1 to 1.0) |
| Weight at transthoracic echocardiography, kg | 3.6 (0.6) | 3.6 (0.5) | 0.004 (−0.1 to 0.1) |
Abbreviations: Apgar, appearance, pulse, grimace, activity, and respiration; BAV, bicuspid aortic valve; BMI, body mass index; CBHS, Copenhagen Baby Heart Study.
Results from a sensitivity analysis excluding all newborns with coexisting cardiac structural or functional abnormal findings (ventricular septal defect, coarctation, congenital aortic stenosis, reduced left ventricular systolic function requiring follow-up) showed no clinically important differences compared with results for the total cohort.
Excluding newborns with BAV (all included newborns had tricuspid aortic valves, except for 2 with quadricuspid aortic valves).
Mean difference for continuous variables; absolute percent difference for categorical variables.
Calculated as weight in kilograms divided by height in meters squared.
Other race is defined as mixed origin and races not included in the categories above.
Matching criteria for 5 newborns with BAV were not fulfilled and were excluded (3 newborns from twin pregnancies and 2 from single pregnancies; all deviated considerably in gestational age, weight [2.1-2.9 kg and 4.0 kg] and/or age [15-47 days] from average at time of examination).
BAV Subtypes
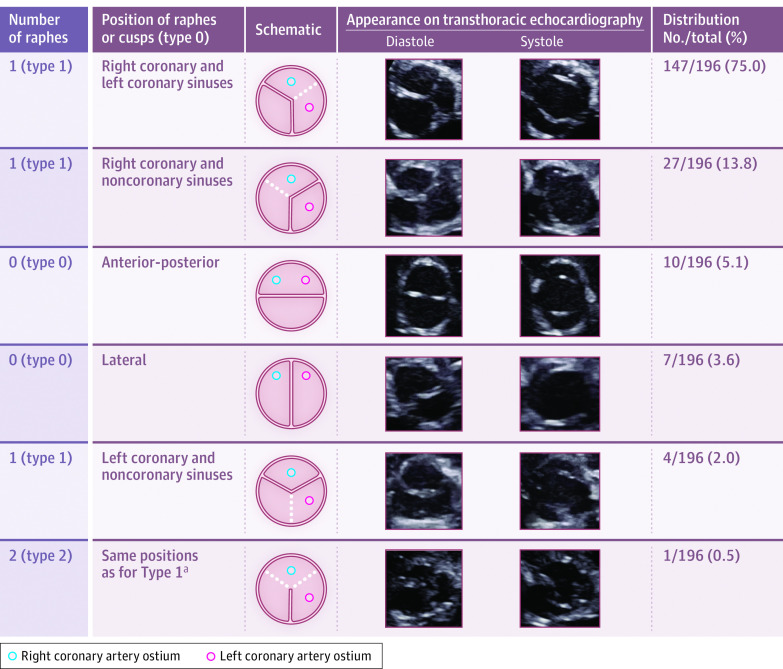
All subtypes of BAV described by Sievers and Schmidtke5 were represented. The most frequent subtype was type 1 (1 raphe), with the raphe positioned between the right and left coronary sinuses (75.0% [95% CI, 68.5%-80.5%] of all diagnosed BAVs), whereas type 2 (2 raphes) was the least frequent subtype (0.5% [95% CI, 0.1%-2.8%] of all diagnosed BAVs). The appearance of BAV from the parasternal short-axis view and the distribution of BAV subtypes are shown in the Figure.
Figure. Echocardiographic Appearance and Distribution of the Different Subtypes of BAV in the Group of Newborns With BAV, Classified According to Sievers and Schmidtke5.
Prevalence and 95% CIs for the bicuspid aortic valve (BAV) subtypes: type 0, anterior-posterior in 10 (5.1% [95% CI, 5.1%-27.9%]) and lateral in 7 (3.6% [95% CI, 3.6%-17.4%]); type 1 in 178 (90.8% [95% CI, 86.0%-94.1%]) (147 [75.0% {95% CI, 68.5%-80.5%}] right-left coronary raphe; 27 [13.8% {95% CI, 9.6%-19.3%}] right noncoronary raphe; 4 [2.0% {95% CI, 0.8%-5.1%}] nonleft coronary raphe); and type 2 in 1 (0.5% [95% CI, 0.1%-2.8%]).
aThe other types are not shown; see eFigure 1 in the Supplement for complete schematic.
Aortic Valve Function
Aortic valve function is reported for both groups in eTable 3 in the Supplement. Aortic regurgitation was detected in 29 newborns (14.7%) with BAV and was found to have a higher prevalence than in the non-BAV group (1.3%) (absolute % difference, 13.4% [95% CI, 7.8%-18.9%; P < .001). For both groups, all cases of aortic regurgitation were trivial central regurgitation except for in 1 newborn with BAV with mild eccentric regurgitation. Mean and peak flow velocities across the aortic valve were higher in newborns with BAV than in those without BAV (mean flow velocity, 0.67 [95% CI, 0.65-0.69] m/s vs 0.61 [95% CI, 0.60-0.62] m/s; mean difference, 0.06 m/s [95% CI, 0-0.1]; peak flow velocity, 0.98 [95% CI, 0.95-1.01] m/s vs 0.89 [95% CI, 0.88-0.90] m/s; mean difference, 0.09 m/s [95% CI, 0-0.1]; both P < .001) (eFigure 3A in the Supplement). One newborn with BAV was diagnosed with congenital aortic stenosis (peak velocity across the aortic valve, 2.62 m/s).
Aortopathy
Aortic dimensions are presented in eTable 3 in the Supplement. Newborns with BAV had larger sinuses of Valsalva and tubular ascending aortic diameters than those without BAV (10.7 [95% CI, 10.5-10.9] mm vs 10.3 [95% CI, 10.2-10.4] mm; mean difference, 0.43 mm [95% CI, 0.2-0.6] and 9.8 [95% CI, 9.6-10.0] mm vs 9.4 [95% CI, 9.3-9.5] mm; mean difference, 0.46 mm [95% CI, 0.3-0.7], respectively; both P < .001) (eFigure 3C and 3D in the Supplement). z Scores for aortic dimensions in newborns with BAV (eFigure 4 in the Supplement) indicate that the enlargement was most pronounced for the tubular ascending aorta. Significant aortic dilatation (z score ≥3) was seen in 62 newborns (31.6%) with BAV. When excluding associated congenital heart diseases (including coarctation, aortic stenosis, and ventricular septal defect), significant aortic dilatation (z score ≥3) was seen in 58 newborns (29.6%) with BAV. The degree of ascending aortic dilatation in newborns with BAV did not seem to relate to the peak flow velocity across the aortic valve (eFigure 5 in the Supplement).
Coarctation was diagnosed in 3 newborns (1.5%) with BAV but not in any newborns without BAV (P = .01). In addition, descending aortic flow velocities were significantly higher in newborns with BAV compared with those without BAV (1.31 [SD, 0.22] m/s vs 1.24 [SD, 0.19] m/s; mean difference, 0.08 m/s [95% CI, 0-0.1]; P = .002) (eTable 3 and eFigure 3B in the Supplement).
Other Echocardiographic Findings
Left ventricular end-systolic and end-diastolic diameters and ejection fraction were not significantly different in the 2 groups. Ventricular septal defect was diagnosed in 8 newborns (4.2%) with BAV and in 16 (4.2%) without BAV [absolute % difference, 0% [95% CI, −3.5% to 3.5%]; P > .99 (eTable 3 in the Supplement). Newborns with BAV had larger main pulmonary artery diameters than those without BAV (9.2 [95% CI, 9.0-9.4] mm vs 8.6 [95% CI, 8.5-8.7] mm; mean difference, 0.59 mm [95% CI, 0.4-0.8]; P < .001). z Score for the main pulmonary artery diameter (eFigure 4 in the Supplement) was ≥3 in 2 newborns (1.0%) with BAV.
Comparing newborns with BAV (n = 196) with the rest of the CBHS cohort (n = 25 360) yielded similar results, ie, statistically significant wider sinuses of Valsalva and tubular ascending aorta and higher peak flow velocity through the aortic valve, whereas there was no significant difference in sinotubular junction or ejection fraction.
Discussion
This study provides an estimate of prevalence of BAV and BAV subtypes among newborns in Copenhagen. Aortopathy was common in newborns with BAV, suggesting that it also represents a fetal malformation.
Necropsy studies (n = 8800-21 417) have reported the prevalence of BAV to be 0.5% to 1.37%.21,22,23 However, necropsy studies are subject to selection bias, and, depending on the applied protocol, BAV can easily be overlooked.24 Additionally, the study population in several reports on the BAV prevalence was composed of more males than females,2,21,25 potentially leading to an overestimation of the prevalence as BAV is more frequent in males.2 With appropriate image quality, BAV can be detected using TTE with high sensitivity (92%) and specificity (96%).26 Previous TTE studies of BAV in schoolchildren and consecutive neonates report a prevalence of 0.46% to 0.49%, with a male-female distribution of 3 to 4:1; however, these studies were based on smaller cohorts (n = 817-1075).27,28
The observed male predominance of BAV is in accordance with the common assumption of male predominance, although less pronounced than previously reported.2 Potential sex-specific implications of BAV have not been fully clarified, but higher risks of complications in men with BAV have been reported.29
Previous larger studies reporting the distribution of BAV subtypes have been based on highly selected populations.3,5,30 Sievers et al reported that type 1 was present in 88% (right-left coronary raphe [71%], right coronary–noncoronary raphe [15%], left coronary–noncoronary raphe [3%]), type 0 in 7%, and type 2 in 5%, based on pathological findings in BAV surgical specimens (n = 304). Based on cardiac surgery intraoperative findings in patients with BAV (n = 828), the observed prevalences were type 1 in 78%, type 0 in 7%, and type 2/unicuspid in 14%.3,5 A clinical study from a tertiary referral center reported type 1/type 2 in 89% of patients with BAV and type 0 in 11% (n = 2118;mean age, 47 [SD, 18] years).30 The subtype distribution found in this study differs slightly from that found in tertiary clinical referral cohorts and surgical studies. These findings may indicate a referral bias in the symptomatic populations in previous studies, thus corroborating the assumption of an association between BAV subtype and outcome, as previously reported.3,30,31 Follow-up of this newborn cohort may help to further clarify this.13
Considering that congenital aortic stenosis is reported in 4 per 10 000 live births and assuming coexisting BAV in 77% of cases, 8 newborns with BAV and congenital aortic stenosis were expected to be diagnosed in total.32,33
Although the aortic valve in the vast majority of newborns with BAV was nonstenotic, the higher flow velocities across the valve indicate a smaller functional valve orifice size than in those without BAV, as seen in congenital aortic stenosis.11 Trivial or mild aortic valve regurgitation was observed in a considerable fraction of newborns with BAV, which indicates incomplete coaptation and possibly suggests discrete prolapse or redundancy in leaflets, as seen in children with BAV with significant aortic regurgitation.11 These observations could support increased hemodynamic stress on BAV valve leaflets.34
Several studies report a higher growth rate of the ascending aorta in children with BAV compared with controls and an increasing prevalence of tubular aortic dilatation through adulthood.4,35,36 This study demonstrates that BAV-associated aortopathy is prevalent neonatally, suggesting fetal commencement of dilatation, ie, earlier in life than previously described.
The development of BAV-associated aortopathy has been attributed to a hemodynamic theory and an intrinsic aortic wall abnormality theory.4,37 The neonatally present enlargement of the ascending aorta, despite normal or near-normal BAV function, supports the theory of an underlying disease of the aorta, whereas the observed higher flow velocities across the BAV without significantly smaller AVAs indicates abnormal valve dynamics.
Coarctation of the aorta is reported in 4 per 10 000 live births.32 Under the assumption of coexisting BAV in 50%,37 approximately 5 cases with BAV and coarctation were expected to be diagnosed in our cohort.
Limitations
This study has several limitations. First, included newborns had a mean age of 12 (SD, 8) days. With the comprehensive prenatal and postnatal strategies in Denmark, some newborns with severe congenital heart disease may already have come to medical attention prenatally or shortly after birth and entered routine clinical management, and, although encouraged to participate, might not have undergone TTE in the CBHS. This may have resulted in a slight underestimation of the prevalence of BAV. It is possible that other groups of patients (those with, eg, left and right ventricular size discrepancy, other congenital anomalies) may have been missed. Second, participants in the CBHS cohort were predominantly White. Findings of this study may vary for other races, as previously suggested.38
Third, despite optimal conditions in newborns and the comprehensive quality protocol setup, there is a possibility that some BAVs were overlooked. Fourth, all quantitative and qualitative analyses were performed by 1 physician. Although this increases the consistency of data, it adds a risk of systematic bias. Fifth, it was not possible to conduct the measurements of aortic dimensions blinded to the aortic valve morphology, as several features specific for BAV can be present (eg, doming, eccentric valve closure line).11
Conclusions
Among newborns in Copenhagen, the prevalence of BAV was 0.77%. Aortopathy was common in newborns with BAV, suggesting that it also represents a fetal malformation.
eTable 1. Echocardiographic Protocol in the CBHS
eTable 2. Views and Modalities Employed for the Quantitative Echocardiographic Parameters
eTable 3. Echocardiographic Parameters for BAV and Non-BAV Groups
eFigure 1. Schematic Illustration of BAV Subtypes, Classified According to Sievers and Schmidtke
eFigure 2. Flowchart for the Identification of Newborns With BAV on Transthoracic Echocardiography
eFigure 3. Overlapping Kernel Density Plots for the BAV and Control Groups
eFigure 4. Z Scores for Ascending Aortic Dimensions and Main Pulmonary Artery Diameter in Newborns With BAV According to the Pediatric Heart Network Normal Echocardiogram Database
eFigure 5. Scatter Plots Displaying Ascending Aortic Diameter Expressed as Z score and the Peak Flow Velocity Across the Aortic Valve for Newborns in the BAV Group
eReferences
References
- 1.Masri A, Svensson LG, Griffin BP, Desai MY. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart. 2017;103(17):1323-1330. doi: 10.1136/heartjnl-2016-309916 [DOI] [PubMed] [Google Scholar]
- 2.Losenno KL, Goodman RL, Chu MWA. Bicuspid aortic valve disease and ascending aortic aneurysms: gaps in knowledge. Cardiol Res Pract. 2012;2012:145202. doi: 10.1155/2012/145202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sievers H-H, Stierle U, Hachmann RMS, Charitos EI. New insights in the association between bicuspid aortic valve phenotype, aortic configuration and valve haemodynamics. Eur J Cardiothorac Surg. 2016;49(2):439-446. doi: 10.1093/ejcts/ezv087 [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Siu SC. Aortic dilatation in patients with bicuspid aortic valve. N Engl J Med. 2014;370(20):1920-1929. doi: 10.1056/NEJMra1207059 [DOI] [PubMed] [Google Scholar]
- 5.Sievers H-H, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133(5):1226-1233. doi: 10.1016/j.jtcvs.2007.01.039 [DOI] [PubMed] [Google Scholar]
- 6.Morosin M, Leonelli V, Piazza R, et al. . Clinical and echocardiographic predictors of long-term outcome of a large cohort of patients with bicuspid aortic valve. J Cardiovasc Med (Hagerstown). 2017;18(2):74-82. doi: 10.2459/JCM.0000000000000430 [DOI] [PubMed] [Google Scholar]
- 7.Nishimura RA, Otto CM, Bonow RO, et al. ; American College of Cardiology; American College of Cardiology/American Heart Association; American Heart Association . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2014;148(1):e1-e132. doi: 10.1016/j.jtcvs.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 8.Andreassi MG, Della Corte A. Genetics of bicuspid aortic valve aortopathy. Curr Opin Cardiol. 2016;31(6):585-592. doi: 10.1097/HCO.0000000000000328 [DOI] [PubMed] [Google Scholar]
- 9.Prakash SK, Bossé Y, Muehlschlegel JD, et al. ; BAVCon Investigators . A roadmap to investigate the genetic basis of bicuspid aortic valve and its complications: insights from the International BAVCon (Bicuspid Aortic Valve Consortium). J Am Coll Cardiol. 2014;64(8):832-839. doi: 10.1016/j.jacc.2014.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martínez-Micaelo N, Beltrán-Debón R, Baiges I, Faiges M, Alegret JM. Specific circulating microRNA signature of bicuspid aortic valve disease. J Transl Med. 2017;15(1):76-85. doi: 10.1186/s12967-017-1176-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siu SC, Silversides CK. Bicuspid aortic valve disease. J Am Coll Cardiol. 2010;55(25):2789-2800. doi: 10.1016/j.jacc.2009.12.068 [DOI] [PubMed] [Google Scholar]
- 12.Tzemos N, Therrien J, Yip J, et al. . Outcomes in adults with bicuspid aortic valves. JAMA. 2008;300(11):1317-1325. doi: 10.1001/jama.300.11.1317 [DOI] [PubMed] [Google Scholar]
- 13.Michelena HI, Desjardins VA, Avierinos J-F, et al. . Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117(21):2776-2784. doi: 10.1161/CIRCULATIONAHA.107.740878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sillesen A-S, Raja AA, Pihl C, et al. . Copenhagen Baby Heart Study: a population study of newborns with prenatal inclusion. Eur J Epidemiol. 2019;34(1):79-90. doi: 10.1007/s10654-018-0448-y [DOI] [PubMed] [Google Scholar]
- 15.Sillesen A-S, Pihl C, Raja AA, et al. . Repeatability and reproducibility of neonatal echocardiography: the Copenhagen Baby Heart Study. J Am Soc Echocardiogr. 2019;32(7):895-905. doi: 10.1016/j.echo.2019.02.015 [DOI] [PubMed] [Google Scholar]
- 16.Lai WW, Geva T, Shirali GS, et al. ; Task Force of the Pediatric Council of the American Society of Echocardiography; Pediatric Council of the American Society of Echocardiography . Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2006;19(12):1413-1430. doi: 10.1016/j.echo.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 17.Lopez L, Colan SD, Frommelt PC, et al. . Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23(5):465-495. doi: 10.1016/j.echo.2010.03.019 [DOI] [PubMed] [Google Scholar]
- 18.Newcombe RG Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17(8):857-872. doi: [DOI] [PubMed] [Google Scholar]
- 19.Lopez L, Colan S, Stylianou M, et al. ; Pediatric Heart Network Investigators . Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging. 2017;10(11):e006979. doi: 10.1161/CIRCIMAGING.117.006979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez L, Frommelt PC, Colan SD, et al. . Pediatric Heart Network echocardiographic Z scores: comparison with other published models. J Am Soc Echocardiogr. Published online November 11, 2020. doi:10.1016/j.echo.2020.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wauchope GM The clinical importance of variations in the number of cusps forming the aortic and pulmonary valves. QJM Int J Med. 1928;21(83):383-399. [Google Scholar]
- 22.Larson EW, Edwards WD. Risk factors for aortic dissection: a necropsy study of 161 cases. Am J Cardiol. 1984;53(6):849-855. doi: 10.1016/0002-9149(84)90418-1 [DOI] [PubMed] [Google Scholar]
- 23.Datta BN, Bhusnurmath B, Khattri HN, Sapru RP, Bidwai PS, Wahi PL. Anatomically isolated aortic valve disease: morphologic study of 100 cases at autopsy. Jpn Heart J. 1988;29(5):661-670. doi: 10.1536/ihj.29.661 [DOI] [PubMed] [Google Scholar]
- 24.Roberts WC The congenitally bicuspid aortic valve: a study of 85 autopsy cases. Am J Cardiol. 1970;26(1):72-83. doi: 10.1016/0002-9149(70)90761-7 [DOI] [PubMed] [Google Scholar]
- 25.Pauperio HM, Azevedo AC, Ferreira CS. The aortic valve with two leaflets—a study in 2,000 autopsies. Cardiol Young. 1999;9(5):488-498. doi: 10.1017/S1047951100005400 [DOI] [PubMed] [Google Scholar]
- 26.Chan KL, Stinson WA, Veinot JP. Reliability of transthoracic echocardiography in the assessment of aortic valve morphology: pathological correlation in 178 patients. Can J Cardiol. 1999;15(1):48-52. [PubMed] [Google Scholar]
- 27.Basso C, Boschello M, Perrone C, et al. . An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol. 2004;93(5):661-663. doi: 10.1016/j.amjcard.2003.11.031 [DOI] [PubMed] [Google Scholar]
- 28.Tutar E, Ekici F, Atalay S, Nacar N. The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. Am Heart J. 2005;150(3):513-515. doi: 10.1016/j.ahj.2004.10.036 [DOI] [PubMed] [Google Scholar]
- 29.Michelena HI, Suri RM, Katan O, et al. . Sex differences and survival in adults with bicuspid aortic valves: verification in 3 contemporary echocardiographic cohorts. J Am Heart Assoc. 2016;5(10):e004211. doi: 10.1161/JAHA.116.004211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong WKF, Delgado V, Poh KK, et al. . Prognostic implications of raphe in bicuspid aortic valve anatomy. JAMA Cardiol. 2017;2(3):285-292. doi: 10.1001/jamacardio.2016.5228 [DOI] [PubMed] [Google Scholar]
- 31.Ruzmetov M, Shah JJ, Fortuna RS, Welke KF. The association between aortic valve leaflet morphology and patterns of aortic dilation in patients with bicuspid aortic valves. Ann Thorac Surg. 2015;99(6):2101-2107. doi: 10.1016/j.athoracsur.2015.02.036 [DOI] [PubMed] [Google Scholar]
- 32.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890-1900. doi: 10.1016/S0735-1097(02)01886-7 [DOI] [PubMed] [Google Scholar]
- 33.Jijeh AMZ, Ismail M, Al-Bahanta A, Alomrani A, Tamimi O. Percutaneous balloon dilatation for congenital aortic stenosis during infancy: a 15-year single-center experience. Ann Pediatr Cardiol. 2018;11(2):143-147. doi: 10.4103/apc.APC_171_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robicsek F, Thubrikar MJ, Cook JW, Fowler B. The congenitally bicuspid aortic valve: how does it function? why does it fail? Ann Thorac Surg. 2004;77(1):177-185. doi: 10.1016/S0003-4975(03)01249-9 [DOI] [PubMed] [Google Scholar]
- 35.Mahle WT, Sutherland JL, Frias PA. Outcome of isolated bicuspid aortic valve in childhood. J Pediatr. 2010;157(3):445-449. doi: 10.1016/j.jpeds.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 36.Merkx R, Duijnhouwer AL, Vink E, Roos-Hesselink JW, Schokking M. Aortic diameter growth in children with a bicuspid aortic valve. Am J Cardiol. 2017;120(1):131-136. doi: 10.1016/j.amjcard.2017.03.245 [DOI] [PubMed] [Google Scholar]
- 37.Gelpi G, Romagnoni C. Bicuspid aortic valve: a complex aortopathy rather than a simple valvulopathy. Int J Cardiol. 2018;250:120-121. doi: 10.1016/j.ijcard.2017.09.168 [DOI] [PubMed] [Google Scholar]
- 38.Chandra S, Lang RM, Nicolarsen J, et al. . Bicuspid aortic valve: inter-racial difference in frequency and aortic dimensions. JACC Cardiovasc Imaging. 2012;5(10):981-989. doi: 10.1016/j.jcmg.2012.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Echocardiographic Protocol in the CBHS
eTable 2. Views and Modalities Employed for the Quantitative Echocardiographic Parameters
eTable 3. Echocardiographic Parameters for BAV and Non-BAV Groups
eFigure 1. Schematic Illustration of BAV Subtypes, Classified According to Sievers and Schmidtke
eFigure 2. Flowchart for the Identification of Newborns With BAV on Transthoracic Echocardiography
eFigure 3. Overlapping Kernel Density Plots for the BAV and Control Groups
eFigure 4. Z Scores for Ascending Aortic Dimensions and Main Pulmonary Artery Diameter in Newborns With BAV According to the Pediatric Heart Network Normal Echocardiogram Database
eFigure 5. Scatter Plots Displaying Ascending Aortic Diameter Expressed as Z score and the Peak Flow Velocity Across the Aortic Valve for Newborns in the BAV Group
eReferences