Abstract
Background
Purulent pericarditis, a rare disease with a high associated mortality rate in patients without adequate treatment, can cause serious complications, such as perforation of the surrounding tissue and organs. Parvimonas micra is a very rare cause of purulent pericarditis.
Case summary
A 70-year-old male patient presented to our emergency room with chest pain of 10 days’ duration. An electrocardiogram showed ST-segment elevation and PR-segment depression on multiple leads. A transthoracic echocardiogram showed normal left ventricular function and a large amount of pericardial effusion. Acute pericarditis was diagnosed, and anti-inflammatory drug therapy was initiated. Due to the lack of improvement in the symptoms, pericardiocentesis was performed on Day 8 and revealed about 800 cc of the bloody fluid. Parvimonas micra was detected in a culture of the pericardial effusion and blood. Although intravenous antibiotic therapy was initiated for purulent pericarditis, his fever persisted. Computed tomography of the chest performed on Day 14 showed an abscess cavity in the pericardial space around the right atrium (RA). Furthermore, transoesophageal echocardiography revealed vegetation in the RA. Emergency surgery confirmed the presence of vegetation and minor perforation of the RA with communication to the abscess cavity. After surgical therapy, the patient clinically improved and was discharged on Day 51.
Discussion
In cases of acute pericarditis, purulent pericarditis should be considered if clinical improvement is not observed after initial treatment with anti-inflammatory drugs. Once the diagnosis of purulent pericarditis is made, aggressive source control is necessary for improved clinical outcomes.
Keywords: Purulent pericarditis, Infective endocarditis, Intracardiac perforation, Parvimonas micra, Case report
Learning points
Purulent pericarditis is rare and difficult to diagnose.
Purulent pericarditis can cause serious complications, such as intracardiac perforation and infective endocarditis.
Aggressive source control, such as pericardiocentesis and surgical drainage, should be performed immediately to improve clinical outcomes.
Introduction
Purulent pericarditis reportedly accounts for only 0.7% of acute pericarditis cases.1 If appropriate treatment is not performed, the disease can progress very rapidly, and inflammation is likely to spread to surrounding tissues and organs. Staphylococcus aureus is the most commonly detected microorganism in purulent pericarditis while Parvimonas micra is very rarely implicated. The present report described a case of purulent pericarditis-induced intracardiac perforation and infective endocarditis due to P. micra.
Timeline
| Day 0 | The patient presented with chest pain, and acute pericarditis was diagnosed. |
| Day 7 | The patient had a fever, and a blood culture was performed. |
| Day 8 | Pericardiocentesis was performed and revealed bloody fluid. |
| Day 11 | Parvimonas micra was detected in the blood and pericardial effusion cultures. |
| Day 14 | Chest computed tomography (CT) revealed an abscess cavity in the pericardial space around the RA and a trabecular shadow suggesting vegetation in the right atrium (RA). |
| Day 15 | Transoesophageal echocardiography revealed vegetation in the RA, and emergency surgery was performed. |
| Day 51 | The patient was discharged after complete recovery. |
| 4 months post-operatively | Chest CT and transthoracic echocardiogram denied any recurrence of the abscess cavity and vegetation. |
| 6 months post-operatively | The patient had no recurrence of infection or signs of constrictive pericarditis. |
Case presentation
A 70-year-old male patient with no remarkable medical history presented to our emergency department with chest pain of 10 days’ duration. His initial vital signs showed sinus tachycardia (100 to 110 b.p.m.), a blood pressure of 134/85 mmHg, and a body temperature of 36.7°C. No heart murmur, no crackle and no rub detected by physical examination during first day of admission. An electrocardiogram (ECG) showed a sinus rhythm with ST-segment elevation and PR-segment depression on multiple leads (Figure 1). A transthoracic echocardiogram (TTE) showed normal left ventricular function and pericardial effusion without vegetations. The amount of pericardial effusion was large (>500 mL). Nevertheless, no compression of the right side of the heart due to pericardial effusion was observed (Figure 2). Laboratory data showed elevated inflammation markers [white blood cell count 13 600/μL; neutrophils 87% (upper limit of normal ≤9000/μL); C-reactive protein, 16.1 mg/dL (normal range ≤0.3 mg/dL)], and normal cardiac enzyme levels [creatine kinase 40 U/L (upper limit of normal ≤150 U/L); high-sensitivity troponin T 0.007 ng/mL (normal range ≤0.014 ng/mL)]. Chest X-ray showed mild cardiomegaly. The patient’s symptoms and the TTE and ECG findings led to a diagnosis of viral acute pericarditis, and colchicine and loxoprofen were initiated. His fever worsened on Day 7, and a blood culture was performed. Pericardiocentesis was performed on Day 8, draining 800 cc of bloody fluid, which on analysis showed 15 000 white blood cells (62% polymorphonuclear cells, 38% mononuclear cells), total protein 6.1 g/dL, lactate dehydrogenase 998 U/L, and glucose 20 mg/dL. Cytology, histology (cell block), and a mycobacterial culture showed no evidence of malignant pericardial effusion and tuberculous pericarditis. On Day 11, the blood and pericardial fluid cultures were positive for P. micra, and purulent pericarditis was diagnosed. A dental examination revealed decayed teeth, and intravenous antibiotic therapy (ampicillin-sulbactam 3 g every 6 h) and dental treatment were initiated. Although pericardial drainage was performed, the spiking fever persisted. Chest computed tomography (CT) performed on Day 14 showed an abscess cavity in the pericardial space around the right atrium (RA) and a trabecular shadow suggesting vegetation in the RA (Figure 3). Transoesophageal echocardiography (TOE) on the following day revealed vegetation with severe mobility (19.2 mm × 9.2 mm) in the RA near the abscess cavity (Figure 4 and Videos 1 and 2). No vegetations were observed at the tricuspid valve or in the left side of the heart. Emergency surgery was performed via a median sternotomy. The surgical findings demonstrated frank pus in the abscess cavity and on the RA surface. After a pericardiotomy was performed and the abscess cavity communicating with the RA was removed, minor bleeding from the RA was observed. The surface of the RA was dented, very fragile, and easily penetrated. A cardiopulmonary bypass was therefore established, and large vegetation was observed in the RA (Figure 5). The vegetation, infected RA wall, and trabeculae carneae were removed, and the tricuspid valve and right ventricle were inspected carefully but showed no vegetations. The culture of the pus in the abscess cavity detected P. micra. Post-operatively, the patient improved clinically and was given intravenous ampicillin-sulbactam 3 g every 6 h for 4 weeks. Coronary CT angiography performed due to transient changes in ST-segment on the ECG monitor during the surgery showed no stenotic lesions. During follow-up, chest CT and TTE showed no recurrence of the abscess cavity or vegetations at 4 months post-operatively (Video 3), and the patient experienced no recurrence of infection or signs of constrictive pericarditis at 6 months post-operatively.
Figure 1.
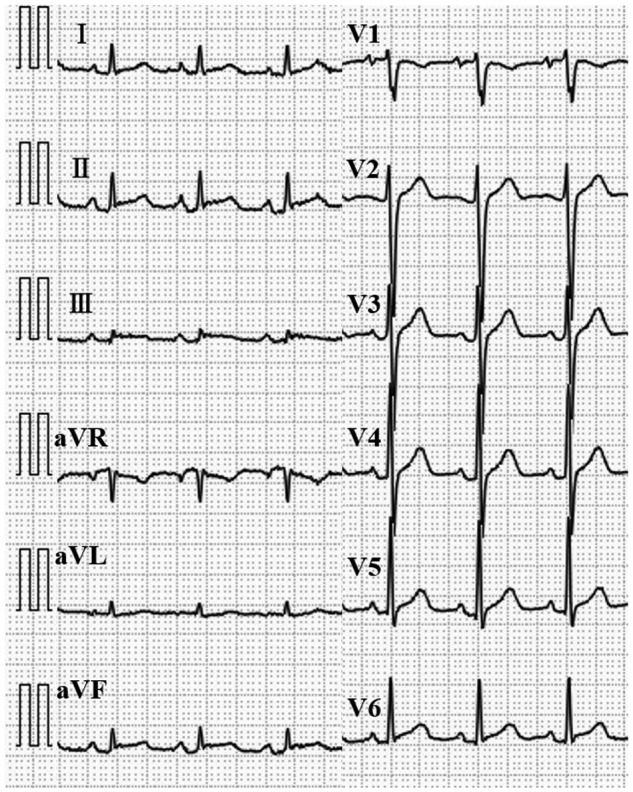
Electrocardiography at admission showing ST-segment elevation and PR- segment depression on the II, III, aVF, and V2 to V6 leads.
Figure 2.
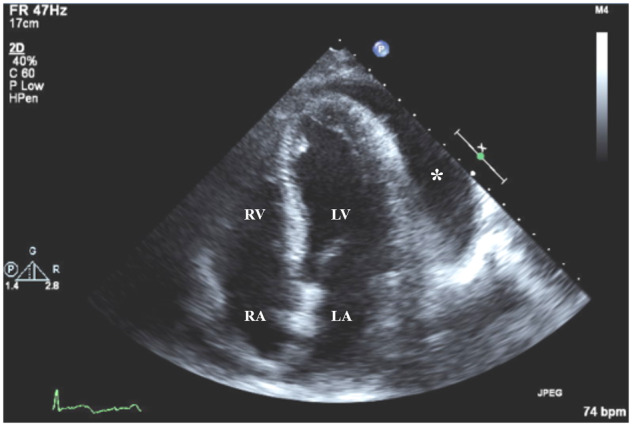
Transthoracic echocardiogram of apical four-chamber view at admission showing a large amount of pericardial effusion (asterisk) without compression of right side of the heart. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Figure 3.
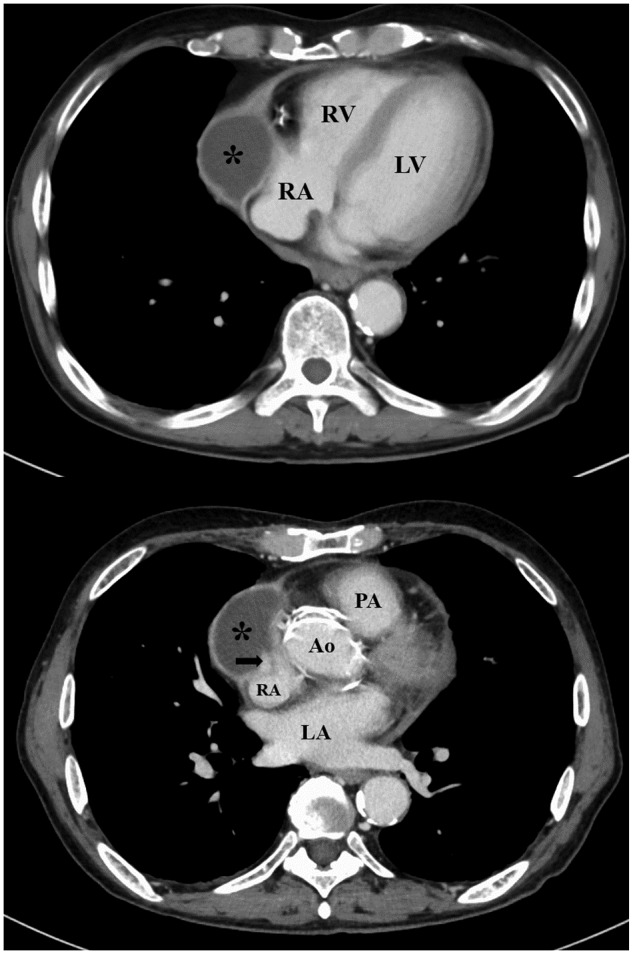
Computed tomography of chest showing abscess cavity in the pericardial space around the right atrium (asterisk) and trabecular shadow in the right atrium (arrow). Ao, ascending aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RA, right atrium; RV, right ventricle.
Figure 4.
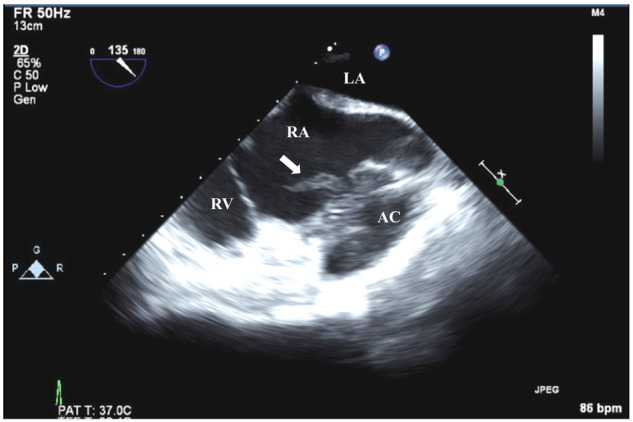
Transoesophageal echocardiographic mid-oesophageal 135˚ view of the right side of the heart. Vegetation with severe mobility (19.2 mm × 9.2 mm) was observed in the right atrium (arrow) near the abscess cavity in the pericardial space. AC, abscess cavity; LA, left atrium; RA, right atrium; RV, right ventricle.
Figure 5.
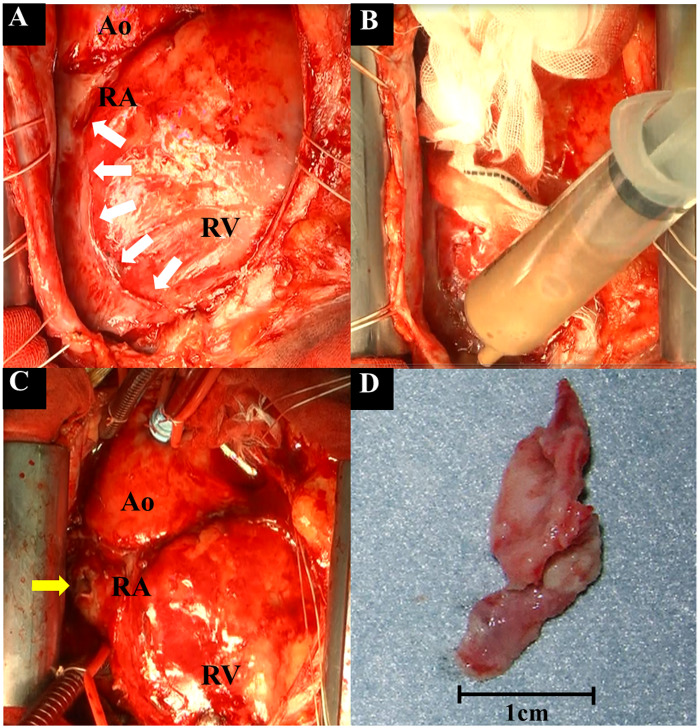
Intraoperative photos. (A) An abscess cavity was observed around the right side of the heart (white arrows). (B) Pus was removed from the abscess cavity. (C) After abscess cavity removal, subsidence in the right atrium and minor bleeding were observed (yellow arrow). (D) The vegetation (22 mm × 12 mm × 2 mm) removed from the right atrium. Ao, ascending aorta; RA, right atrium; RV, right ventricle.
Discussion
Purulent pericarditis is a rare, life-threatening infection of the pericardial space. Although fever is the most frequent presenting sign, our patient did not have fever at admission.2 As a result, no blood culture was performed on admission, and diagnosis and appropriate antimicrobial therapy were therefore delayed. Although TOE is strongly recommended in the guidelines for diagnosing infective endocarditis (IE), in the present case the procedure was delayed due to the absence of fever and murmur on admission.3 Because a pericardial fluid culture is important for early diagnosis and exclusion of purulent pericarditis, early pericardiocentesis should be performed to avoid delaying the diagnosis regardless of whether a fever is present.4
The patient’s tooth decay was found to be the cause of the bacteraemia and purulent pericarditis via haematogeneous dissemination as there was no other source of infection. Purulent pericarditis development is most frequently associated with an extension of an infection within the thorax and mediastinum or direct inoculation via trauma.5 A few cases of purulent pericarditis due to haematogeneous dissemination of pathogens without another primary source of infection have been reported.6
Purulent pericarditis is rarely caused by P. micra, an anaerobic Gram-positive coccus indigenous to the oral cavity and gastrointestinal tract and often associated with periodontal disease, sinusitis, pulmonary suppuration, and deep tissue infections, such as spinal inflammation.7 In general, an infection due to anaerobic Gram-positive cocci progresses slowly. Although standard antimicrobial therapy for P. micra infection has yet to be established, the organism is generally sensitive to beta-lactam antimicrobials.8Staphylococcus aureus is the most commonly detected microorganism in purulent pericarditis.9 Patients with a S. aureus infection can experience a rapid development of a broad array of complications. It is likely that the disease developed slowly in our patient because the pathogenic bacterium was P. micra. The slow development made diagnosis difficult until the disease worsened sufficiently to cause an intracardiac perforation.
In the present patient, the purulent pericarditis led to an intracardiac perforation and secondary infective endocarditis. At admission, he already had acute pericarditis with signs of intense inflammation, but TTE showed no vegetation. A TTE performed by a sonographer on Day 1 likewise revealed no vegetation. Isolated, right-sided IE accounts for approximately only 5–10% of all IE cases, and there are few case reports of IE with vegetation only at the RA.10 The major risk factors specific to right-sided IE are intravenous drug use, the presence of an implantable electronic cardiac device or other intravascular device, a prosthetic valve, and congenital heart disease in the right side of the heart, none of which were present in our patient.11 Furthermore, the vegetation was attached to the abscess cavity, and it was thought that the inflammation from the purulent pericarditis and the abscess cavity spread to the RA, causing perforation and IE. To the best of our knowledge, the present study is the first to report purulent pericarditis due to P. micra causing intracardiac perforation.
The standard treatment for purulent pericarditis consists of antimicrobial therapy and source control. The overall survival rate is about 30% with antimicrobial therapy alone and 50% when antimicrobial therapy is combined with early surgical drainage.12 In the present case, due to the presence of the abscess cavity and vegetation, source control only by pericardiocentensis was inadequate for complete improvement.
Conclusion
Because purulent pericarditis can cause serious complications, optimal antimicrobial therapy following detection of the pathogenic organism and immediate source control are indispensable for a favourable clinical outcome.
Lead author biography

Dr Hiroaki Morinaga was born in Tokyo, Japan in 1984. He received his MD from Juntendo University School of Medicine (Tokyo, Japan) in 2009 and finished his residency (2009–11) and fellowship in Internal medicine and Cardiology in 2016 in Japan. His research interests are mainly focused on echocardiography and heart failure.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for the submission and publication of this case report, including the images and associated text, was obtained from the patient in line with the COPE guidelines.
Conflict of interest: none declared.
Funding: none declared.
Supplementary Material
Contributor Information
Hiroaki Morinaga, Department of Cardiology, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan.
Ken Kato, Department of Cardiology, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan.
Motoyuki Hisagi, Department of Cardiovascular Surgery, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan.
Hiroyuki Tanaka, Department of Cardiology, Tokyo Metropolitan Tama Medical Center, Tokyo, Japan.
References
- 1. Imazio M, Cecchi E, Demichelis B, Ierna S, Demarie D, Ghisio A. et al. Indicators of poor prognosis of acute pericarditis. Circulation 2007;115:2739–2744. [DOI] [PubMed] [Google Scholar]
- 2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC.. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore) 2009;88:52–65. [DOI] [PubMed] [Google Scholar]
- 3. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del ZF. et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 4. Imazio M, Adler Y.. Management of pericardial effusion. Eur Heart J 2013;34:1186–1197. [DOI] [PubMed] [Google Scholar]
- 5. Brook L. Pericarditis caused by anaerobic bacteria. Int J Antimicrob Agents 2009;33:279–300. [DOI] [PubMed] [Google Scholar]
- 6. Farhat-Sabet A, Hull R, Thomas D.. Cardiac tamponade from purulent pericarditis due to cutibacterium acnes. Case Rep Cardiol 2018;2018:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cobo F, Rodriguez-Granger J, Sampedro A, Aliga-Martinez L, Navarro-Mari JM.. Pleural effusion due to Parvimonas micra. A case report and a literature review of 30 cases. Rev Esp Quimioter 2017;30:285–292. [PubMed] [Google Scholar]
- 8. Veloo AC, Welling GW, Degener JE.. Antimicrobial susceptibility of clinically relevant Gram-positive anaerobic cocci collected over a three-year period in the Netherlands. Antimicrob Agents Chemother 2011;55:1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Klacsmann PG, Bulkley BH, Hutchins GM.. The changed spectrum of purulent pericarditis: an 86 year autopsy experience in 200 patients. Am J Med 1977;63:666–673. [DOI] [PubMed] [Google Scholar]
- 10. Akinosoglou K, Apostolakis E, Marangos M, Pasvol G.. Native valve right sided infective endocarditis. Eur J Intern Med 2013;24:510–519. [DOI] [PubMed] [Google Scholar]
- 11. Chahoud J, Sharif Yakan A, Saad H, Kanj SS.. Right-sided infective endocarditis and pulmonary infiltrates: an update. Cardiol Rev 2016;24:230–237. [DOI] [PubMed] [Google Scholar]
- 12. Natrajsetty HS, Vijayalakshmi IB, Narasimhan C, Manjunath CN.. Purulent pericarditis with quadruple valve endocarditis. Am J Case Rep 2015;16:236–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.