Abstract
Background
The Gerbode defect is a rare abnormal communication between the left ventricle (LV) and right atrium (RA). The lesion is either congenital or acquired. Acquired defects are largely iatrogenic or infective in origin. We present two cases of acquired Gerbode defects with similar clinical presentations but very different outcomes.
Case summaries
Patient 1 A 64-year-old male presented with features of decompensated cardiac failure and a low-grade temperature. Dehiscence of a recently implanted bioprosthetic aortic valve and high-velocity LV to RA jet (Gerbode defect) was found on echocardiography. Blood cultures grew Staphylococcus warneri and the diagnosis of infective endocarditis was established. The patient was treated with intravenous antibiotics and the aortic valve and Gerbode defect were successfully surgically repaired.
Patient 2 An 81-year-old male presented after being found on the floor at home. On admission, he was clinically septic with evidence of decompensated heart failure. No clear infective focus was initially found. Transthoracic echocardiography revealed severe left ventricular impairment, with a normal bioprosthetic aortic valve. He was treated with intravenous antibiotics, but later deteriorated with evidence of embolic phenomena. Repeat echocardiography revealed a complex infective aortic root lesion with bioprosthetic valve dehiscence and flow demonstrated from the LV to RA. Unfortunately, the patient succumbed to the infection and cardiac complications.
Discussion
The Gerbode defect is a rare but important complication of infective endocarditis and valve surgery. Care needs to be taken to assess for Gerbode defect shunts on echocardiogram, especially in the context of previous cardiac surgery.
Keywords: Case series, Gerbode defect, LV-RA fistula, Endocarditis
Learning points
An acquired Gerbode defect is a rare but important complication of aortic valve endocarditis and valve surgery associated with a high mortality.
Care should be taken to assess for Gerbode defect shunts on echocardiogram, as they can be misinterpreted as more common cardiac pathology.
Introduction
The Gerbode defect is a rare abnormal communication between the left ventricle (LV) and right atrium (RA). It was originally described in 1838 by Thurnam J, but went on to be named after Professor Frank Gerbode after he published the first case series of five successful surgical repairs.1
The lesion is either congenital or acquired. Isolated congenital Gerbode defects are rare, and whilst sequence variations in the NKX2-5, GATA4, and TBX5 genes have been implicated in the pathogenesis of some cases,2 the basis of the majority of cases remains poorly understood. Acquired Gerbode defects now outnumber congenital cases, largely due to an increase in predisposing risk factors such as previous cardiac surgery. One systematic review collated the case report experience from 1958 to 2015, finding a total of 234 patients with acquired defects.3 Of these, the majority (51%) were iatrogenic in aetiology, followed by infective (37%), traumatic (9%), and ischaemic (3%) aetiologies.
Left ventricle to RA shunts can be further categorized anatomically. Type 1 (indirect or infravalvular) defects are a combination of a membranous interventricular septal defect and tricuspid regurgitation, either due to poor leaflet apposition or septal leaflet perforation. Type 2 (direct or supravalvular) defects are a direct connection from the LV to RA through the superior part of the membranous interventricular septum. Direct defects are possible owing to the relative apical displacement of the tricuspid valve compared with the mitral valve. Type 3 (intermediate) defects comprise a combination of direct and indirect routes. The relative incidence of the different types is 12%, 81%, and 7%, respectively.3
The Gerbode defect results in a high-velocity jet, with shunting of blood from the LV to the RA due to large pressure gradients. Increased pressure and blood volume within the RA and right ventricle (RV) results in dilation of both chambers. Care needs to be taken during echocardiographic assessment to avoid misinterpreting these findings as suggestive of more common alternative pathology, such as tricuspid regurgitation.4,5
Herein, we present two cases of acquired Gerbode defects with similar clinical presentations but very different outcomes.
Timeline
| Time | Patient 1 |
|---|---|
| Five months prior | Uncomplicated bioprosthetic aortic valve replacement and coronary artery bypass graft (CABG) |
| Three months prior | Routine echocardiogram showed an appropriately functioning aortic valve but new tricuspid regurgitation |
| Initial event | Patient presents with fatigue, shortness of breath, and bilateral leg swelling. |
| Day 1 | Echocardiogram reveals a left ventricle (LV) to right atrium (RA) shunt and partially dehisced aortic valve. Intravenous antibiotics commenced and surgery planned. |
| Day 9 | Redo aortic valve replacement and fistula closure |
| Day 28 | Discharged from hospital |
| Ten weeks after discharge | Feeling well at clinic review |
| Time | Patient 2 |
|---|---|
| Five years prior | Bioprosthetic aortic valve replacement and CABG |
| Initial event | Admitted to hospital following a collapse. Clinically septic. Bedside echocardiogram revealed severe left ventricular systolic dysfunction (LVSD) but no evidence of infective endocarditis. |
| Day 1 | Transferred to the intensive care unit (ICU) for inotropic support and haemofiltration. Klebsiella isolated from blood cultures. |
| Day 4 | Formal echocardiogram again showed severe LVSD but no evidence of endocarditis. |
| Day 10 | Weaned off inotropic support and stepped down to the general ward. |
| Day 18 | Increasing inflammatory markers and persistent pyrexia. New occlusion of superior mesenteric artery and right lower lobe pneumonia on computerized tomography scan. |
| Day 25 | New petechial rash. Repeat echocardiogram showed a partially dehisced aortic valve and LV to RA shunt. |
| Day 30 | Further clinical deterioration and decision to move to palliative care. Died in the evening. |
Case presentation
Patient 1
A 64-year-old male presented to the emergency department with a 10-day history of fatigue, shortness of breath, and peripheral oedema.
His medical history included: bioprosthetic aortic valve replacement (AVR) and coronary artery bypass graft (CABG) surgery, 5 months prior, for critical aortic stenosis and significant coronary artery disease, hypertension, type-2 diabetes mellitus, hypercholesterolaemia, nephrotic syndrome, and pulmonary embolism. He was an active gentleman, often completing several rounds of golf per week.
Medications included: warfarin, clopidogrel 75 mg OD, losartan 25 mg OD, atorvastatin 20 mg OD, prednisolone 60 mg OD, gliclazide 240 mg/40 mg BD, and linagliptin 5 mg OD.
At presentation, his blood pressure was 110/60 mmHg, heart rate 72 b.p.m., respiratory rate 25 b.p.m., oxygen saturations 100% on air, and he had a temperature of 37.7°C. On physical examination, he appeared breathless on minimal exertion, had fine right basal crepitations, a pan-systolic and early diastolic murmur, and bilateral mild pitting oedema. Initial laboratory investigations revealed a haemoglobin of 117 g/L, white cell count of 11.57 × 109/L (neutrophil count 9.26 × 109/L), C-reactive protein 11 mg/L, and creatinine 96 μmol/L [estimated glomerular filtration rate (eGFR) 72 mL/min].
The primary components of the admission differential diagnosis included: infective endocarditis, aortic valve dehiscence or thrombosis, congestive cardiac failure of alternative cause, and pneumonia.
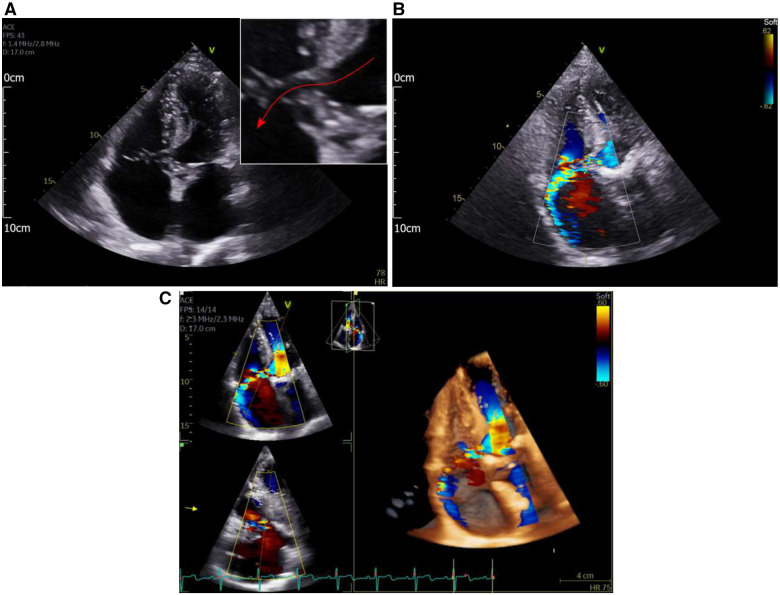
Urgent echocardiography was performed which demonstrated dehiscence of the aortic valve with mild aortic regurgitation. On 2D echo imaging, a hypoechoic tract could be visualized from the left ventricular outflow tract (LVOT) to the RA (Figure 1A). The suspicion of a Gerbode defect was confirmed on colour flow mapping, with a large jet demonstrated between the two chambers (Figure 1B and C).
Figure 1.
(A) Apical four-chamber view demonstrating a hypoechoic tract between the left ventricular outflow tract and the RA (red arrow on inset magnified view). (B) Apical four-chamber view with colour flow mapping. A high-velocity jet can be seen from the left ventricular outflow tract into the right atrium. (C) Reconstructed 3D echo images demonstrating the high-velocity left ventricular to right atrial jet.
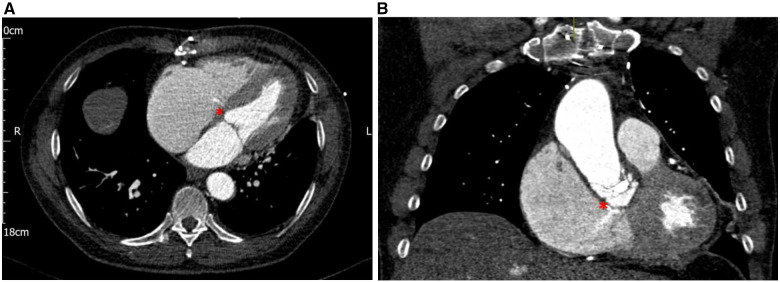
Computerized tomography (CT) revealed a complex aortic root pseudoaneurysm with LVOT to RA fistulation and a large left-to-right shunt (Figure 2A and B).
Figure 2.
(A) Transverse arterial phase-contrast computerized tomography image demonstrating a thin jet of contrast entering a dilated right atrium (red asterisk). (B) Coronal arterial phase-contrast computerized tomography image demonstrating a thin jet of contrast entering a dilated right atrium through a defect in the wall of the left ventricular outflow tract (red asterisk).
Three peripheral blood cultures taken on admission returned positive for Staphylococcus warneri, a coagulase-negative Staphylococcus. Treatment with intravenous gentamicin, vancomycin, and rifampicin was initiated and after 10 days of therapy, he underwent a surgical aortic valve replacement and LV–RA fistula repair. After 1 month in hospital, he was discharged home, and 2 months later was back on the golf-course, albeit with a very limited swing on account of his recent sternotomy.
Patient 2
An 81-year-old male presented to the emergency department after being found collapsed. He was delirious, pyrexial, and dyspnoeic. Five years prior to this, he had undergone bioprosthetic AVR and CABG, for critical aortic stenosis and significant coronary artery disease.
His medical history also included: moderate left ventricular systolic dysfunction (LVSD), psoriasis, and iron deficiency anaemia.
Medications included: furosemide 40 mg BD, lansoprazole 30 mg OD, atorvastatin 80 mg OD, saubitril/valsartan 49/51 mg BD, bisoprolol 1.25 mg OD, epleronone 25 mg OD, ferrous Sulphate 200 mg BD, and aspirin 75 mg OD.
At presentation, the blood pressure was 78/60 mmHg, heart rate 120 b.p.m., respiratory rate 38 b.p.m., oxygen saturations 77% on air, and he had a temperature of 37.8°C. On physical examination, he appeared breathless at rest, had bi-basal fine crepitations, a soft systolic murmur, and bilateral pitting oedema. Initial laboratory investigations revealed a haemoglobin of 113 g/L, white cell count of 11.14 × 109/L (neutrophil count 9.81 × 109/L), C-reactive protein 150 mg/L, creatinine 506 μmol/L (eGFR 9 mL/min; baseline creatinine 100–160 μmol/L), and creatinine kinase 74 U/L. Twelve-lead electrocardiogram (ECG) showed 1st degree heart block and old left bundle branch block.
The admission differential diagnosis was broad; however, the presence of sepsis in the context of cardiac valve surgery and new 1st degree heart block made infective endocarditis and possible aortic root abscess the primary concern.
Urgent echocardiography showed severe LVSD, although no clear evidence of endocarditis or valvular vegetations. The overall impression was that of decompensated heart failure with reduced ejection fraction, secondary to sepsis of unknown source. The patient was urgently transferred to the intensive care unit (ICU) for inotropic support and haemofiltration.
Three peripheral blood cultures returned positive for Klebsiella pneumoniae, a Gram-negative bacterium. Treatment with intravenous Piperacillin with Tazobactam was initiated. A CT abdomen and pelvis did not reveal a specific source for the Gram-negative bacteraemia.
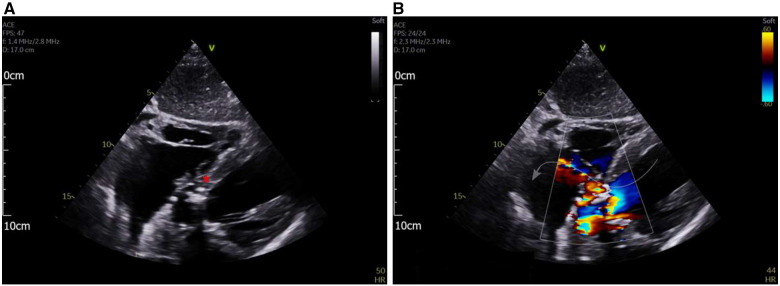
After initially stabilizing in ICU for several days, the patient deteriorated again with worsening dyspnoea and evidence of decompensated heart failure. Serial laboratory investigations revealed rising inflammatory markers. A repeat CT scan of the thorax, abdomen, and pelvis showed new occlusion of the superior mesenteric artery and right lower lobe pneumonia. Repeat transthoracic echocardiogram was organized due to the identification of a new petechial rash, a new harsh systolic murmur, and lengthening PR interval on ECG. This demonstrated a complex aortic root lesion, with aortic valve dehiscence and RA septal wall vegetations (Figure 3A). On colour flow mapping, there was communication between the LV and RA—the Gerbode defect (Figure 3B).
Figure 3.
(A) Subcostal four-chamber view showing a complex hypo and hyper-echoic area in the basal septum and aortic root (red asterisk). (B) Subcostal four-chamber view with colour flow mapping demonstrating flow from the left ventricle into the right atrium, through the aortic root lesion.
Transoesophageal echocardiogram and CT of the aortic root were arranged, however, the patient deteriorated rapidly before these were completed. Following discussion with the patient and his family, the decision was made for symptom control and palliative management. He died peacefully shortly after.
Discussion
The acquired Gerbode defect is a rare but increasingly reported complication associated with modern cardiac intervention. Both of our cases had a history of bioprosthetic aortic valve replacement and later developed infective endocarditis of the valve—the first case was identified shortly after surgery whereas the second case presented years later. These cases are in line with the limited aetiological data for the Gerbode defect. The majority of reported cases are related to either cardiac valve surgery or infective endocarditis.3 From the previously published case reports, 37% develop secondary to infective endocarditis, of which the majority (46%) are associated with the aortic valve (13% prosthetic vs. 87% native).3
Our first case had a routine post-operative echocardiogram on which new tricuspid regurgitation was reported, with an eccentric regurgitant jet noted within the RA. It is possible that the eccentric regurgitant component on this early scan represented the Gerbode shunt. Compounding matters in this case was concomitant true tricuspid regurgitation as well as high-velocity flow into the RA through a direct Gerbode defect.
The second case was extremely unwell at presentation. Two echocardiographic studies were undertaken within the first 9 days of hospital treatment, these showed severe LVSD but no clear evidence of valvular lesions. After a period of gradual improvement and clinical stability, the patient deteriorated again with classical signs of aortic valve infective endocarditis: embolic phenomena, clinical features of decompensated heart failure, and new 1st-degree heart block. A repeat echocardiogram revealed a dehisced bioprosthetic aortic valve implant and flow from the LV outflow tract into the RA. The aortic root appeared thickened and diastolic flow from the aortic root into the RA was demonstrated, suggesting a complex infective process with communicating channels from above and below the aortic valve. Transthoracic echocardiography has limited capacity to rule out infective endocarditis and subsequent transoesophageal echocardiographic assessment is usually recommended.6 Unfortunately, the patient deteriorated quickly once the diagnosis had become clear, and passed away before the aortic valve and root could be interrogated with a transoesophageal echocardiogram and CT aorta. In the setting of a prosthetic valve, it is important to have a low threshold for further cardiac imaging with transoesophageal echocardiography and CT, especially in the context of bacteraemia and a prosthetic valve implant.
Infective endocarditis is rare, with a incidence of around 1.7–6.2 per 100 000 patient-years in the general population.7 However, prosthetic valve endocarditis (PVE) is much more common, occurring in 1–6% of patients with valve prostheses (incidence 0.3–1.2% per patient-year).8 Our first patient had a prosthetic aortic valve and a complex medical background with several factors that increase the risk of infection, namely type 2 diabetes mellitus, nephrotic syndrome, and recent high-dose steroid use. Infective symptoms developed 5 months after aortic valve replacement, representing intermediate-onset (60 days—1 year) PVE. Coagulase-negative Staphylococci (CNS), as in this case, represent 17% of all PVE and are the most frequent causative organism in the intermediate period (40%).9,10
Although fortunately this patient ultimately had a successful outcome, there were multiple high-risk aspects to the case. In a large cohort of 2781 infective endocarditis cases, PVE, CNS infection, and paravalvular complications were associated with a significantly higher in-hospital mortality (odds ratio: 1.47, 1.50, and 2.25, respectively). Whereas surgical intervention was associated with a significantly lower in-hospital mortality (odds ratio 0.61), being performed in 49% of cases.9
This patient had the aortic valve replaced and the LV–RA defect closed which undoubtedly resulted in his successful recovery. Other reported strategies include interventional techniques such as the Amplatzer duct occluder or vascular plugs. Although very likely subject to patient selection and reporting bias, a surgical management strategy has been associated with a 90% chance of survival at a median follow-up of 13 months, compared with 57% with no intervention.3
The Gerbode defect presents several pitfalls in the diagnostic work up. Firstly, care must be taken to differentiate an RA regurgitant jet secondary to a Gerbode defect, from pure tricuspid regurgitation.4,5 Secondly, in order to detect the defect during echocardiographic assessment, one must ensure adequate coverage of the colour-flow mapping area to include the membranous interventricular septum, LVOT, and RA, in several views. Features that should prompt a specific assessment for a Gerbode defect include very high-velocity tricuspid regurgitation, an eccentric triscupid regurgitant jet, and clinical history of cardiac surgery and/or infective endocarditis. Finally, transoesophageal echocardiography and cardiac CT imaging are indicated if uncertainty remains after transthoracic echocardiography.
Conclusion
The Gerbode defect is a rare but important complication of aortic valve endocarditis and valve surgery associated with a high mortality. Compared with the more stable congenital Gerbode defects, acute fistulation between the LV and RA as a result of infective endocarditis or cardiac surgery is a life-threatening complication that often requires urgent definitive intervention. Extra care needs to be taken to assess for LV–RA shunts at echocardiography, especially when there is an eccentric RA jet on colour flow mapping, history of recent cardiac valve surgery, or symptoms suggestive of infective endocarditis.
Lead author biography

Dr Nick Sunderland is a specialist registrar in cardiology and NIHR academic clinical fellow at the Bristol Heart Institute. He graduated in medicine from the University of Oxford in 2013. His research interests are in cardiovascular autonomic control, cardiac arrhythmia, and electrophysiological mapping.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report, including images and associated text, has been obtained from both patients in line with COPE guidance.
Conflict of interest: none declared.
Funding: none declared.
Supplementary Material
References
- 1. Gerbode F, Hultgren H, Melrose D, Osborn J.. Syndrome of left ventricular-right atrial shunt; successful surgical repair of defect in five cases, with observation of bradycardia on closure. Ann Surg 1958;148:433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borkar Y, Nayak K, Shetty RK, Bhat G, Moka R.. Gerbode ventricular septal defect—a rare cardiac anomaly associated with genetic variants in Indian population—a case series. J Clin Diagn Res 2017;11:GR01–GR04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yuan SM. A systematic review of acquired left ventricle to right atrium shunts (Gerbode defects). Hellenic J Cardiol 2015;56:357–372. [PubMed] [Google Scholar]
- 4. Xhabija N, Prifti E, Allajbeu I, Sula F.. Gerbode defect following endocarditis and misinterpreted as severe pulmonary arterial hypertension. Cardiovasc Ultrasound 2010;8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tehrani F, Movahed MR.. How to prevent echocardiographic misinterpretation of Gerbode type defect as pulmonary arterial hypertension. Eur J Echocardiogr 2007;8:494–497. [DOI] [PubMed] [Google Scholar]
- 6. Bai AD, Steinberg M, Showler A, Burry L, Bhatia RS, Tomlinson A. et al. Diagnostic accuracy of transthoracic echocardiography for infective endocarditis findings using transesophageal echocardiography as the reference standard: a meta-analysis. J Am Soc Echocardiogr 2017;30:639–646.e8. [DOI] [PubMed] [Google Scholar]
- 7. Mylonakis E, Calderwood SB.. Infective endocarditis in adults. N Engl J Med 2001;345:1318–1330. [DOI] [PubMed] [Google Scholar]
- 8. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J, Del Zotti F. et al. 2015 ESC Guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 9. Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG, Bayer AS. et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 2009;169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JH, Burner KD, Fealey ME, Edwards WD, Tazelaar HD, Orszulak TA. et al. Prosthetic valve endocarditis: clinicopathological correlates in 122 surgical specimens from 116 patients (1985-2004). Cardiovasc Pathol 2011;20:26–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.