Abstract
Introduction: Corynebacteria represent often-neglected etiological agents of post-traumatic and/or post-operative bone and joint infection (BJI). We describe here clinical characteristics and bacteriological determinants of this condition.
Methods: A retrospective cohort study described characteristics, outcome and determinants of treatment failure of all patients with proven Corynebacterium spp. BJI (i.e., ≥2 culture-positive gold-standard samples). Available strains were further characterized regarding their antibiotic susceptibilies, abilities to form early (BioFilm Ring Test®) and mature (crystal violet staining method) biofilms and to invade osteoblasts (gentamicin protection assay).
Results: The 51 included BJI were mostly chronic (88.2%), orthopedic device-related (74.5%) and polymicrobial (78.4%). After a follow-up of 60.7 weeks (IQR, 30.1–115.1), 20 (39.2%) treatment failures were observed, including 4 Corynebacterium-documented relapses, mostly associated with non-optimal surgical management (OR 7.291; p = 0.039). Internalization rate within MG63 human osteoblasts was higher for strains isolated from delayed (>3 months) BJI (p < 0.001). Infection of murine osteoblasts deleted for the β1-integrin resulted in a drastic reduction in the internalization rate. No difference was observed regarding biofilm formation.
Conclusions: Surgical management plays a crucial role in outcome of BJI involving corynebacteria, as often chronic and device-associated infections. Sanctuarisation within osteoblasts, implicating the β1 cellular integrin, may represent a pivotal virulence factor associated with BJI chronicity.
Keywords: Corynebacterium, osteoblasts, biofilm, bone and joint infection, intracellular
Introduction
Bone joint infection (BJI), and especially prosthetic joint infection (PJI), represents a major public health concern (1), due to: (i) their prevalence, complicating 1 to 2% of arthroplasty procedures, with an important upcoming increase due to the projected rise in prosthetic joint replacement indications in the coming years (2, 3); (ii) their severity, associated with a 5% mortality rate and responsible for permanent disabilities in up to 40% of patients; and iii) their substantial economic burden estimated to be as high as 75,000 to 100,000 USD per episode attributed to protracted hospital course, re-operations, lengthened rehabilitation time and extended use of antimicrobials (4–7). Consequently, BJI has been pointed out as a priority axis of clinical and scientific research in many countries. The optima management requires a multidisciplinary approach combining both surgical procedure and extended antimicrobial therapy (8). Despite this complex management, they are associated with a high failure rate, exceeding 20% in some series, with frequent relapses and transition to a chronic state (9–14). This propensity to chronicity and relapse has been related to specific bacterial phenotypes responsible for subsequent emergence of bacterial reservoirs, protecting the pathogen from the extracellular host defenses and most antimicrobials (15, 16). These mechanisms have been well-characterized among Staphylococcus aureus, the main etiological agent of BJI (17–19), and consist in: (i) biofilm formation, an surface-adherant bacterial community living in a matrix of self-generated polymeric substances (20, 21); (ii) internalization and persistence within non-phagocytic bone cells, triggered by the interaction of staphylococcal fibronectin binding proteins (FnBP) with host Fibronectin that acts as a bridge with cellular α5β1 integrin to prompted bacterial endocytosis by an active cellular process (22–24); and (iii) phenotype switching to small colony variants (SCVs), a slow-growing bacterial phenotype which can emerge during intracellular or biofilm-associated lifestyles, and conferring enhanced resistance to antimicrobials (25, 26).
Corynebacteria are a highly heterogeneous group of Gram positive rods containing more than 110 species. Their pathogenic potential is species-dependent: some of them, as Corynebacterium glutamicum or Cladosporium halotolerans, have never been described in human pathology, when others have been implicated in various infectious disease, from urinary tract infection to infective endocarditis (27). Two type of virulence factor have been well-characterized in this genus. First, exotoxin production has been described in Corynebacterium diphteriae, Corynebacterium ulcerans, and Corynebacterium pseudotuberculosis. These three pathogenic strains can product diphteria toxin and/or phospholipase B, and therefore cause diphtheria, which is the best known corynebacteria-associated disease (27). Interestingly, even non-toxinogenic strains of C. diphteriae can cause invasive infections such as endocarditis, brain abscess or BJI (28). Secondly, some species have been shown to produce various adhesion molecules allowing interaction with eukaryote cells. A fibrinogen and fibronectin binding-like activity has been demonstrated from invasive strains of Corynebacterium pseudodiphtericum (29), interaction with fibronectin determines corynebacteria adhesion to vaginal epithelial cells (30), and C. diphteriae can invade epithelial cells, with an important role of a transmembrane protein called DIP0733, which possesses a fibrinogen and collagen binding activity (31–33).
As part of normal human skin microbiota, corynebacteria can be implicated in inoculation disease. They are especially involved in up to 3% of BJI (34–36). However, little is known about the specific aspects of Corynebacterium spp. BJI: epidemiologic data are lacking, their specific management is not addressed in current guidelines, and the pathophysiology of Corynebacterium spp. BJI has not been investigated so far (18, 37, 38). We report here the experience of our regional reference center with the management of Corynebacterium spp. BJI, aiming to describe patients' characteristics and treatment failure's determinants. Clinical isolates were further characterized for species distribution, antimicrobial susceptibility profile, ability to form biofilm and to invade bone cells.
Patients and Methods
Ethical Statements
This study (ClinicalTrials.gov registration number NCT03081273) received the approval of the French South-East Ethics Committee (reference number QH20/2014). All patients received written information about the study. The requirement for written informed consent was waived by the Committee for the protection of persons (CPP) according to French legislation at time of the study.
Inclusion Criteria and Data Collection
This retrospective cohort study (2007–2016) included all patients followed-up in the infectious disease department of our tertiary care center for a proven Corynebacterium spp. BJI, i.e., with clinical, biological and/or radiological symptoms consistent with the diagnosis of BJI, with at least two per operative culture-positive samples yielding the same isolate (same species and same antibiotic susceptibility profile), and treated as such (1, 37, 38). Patients with diabetic foot- or pressure ulcer-related osteomyelitis were excluded because of their specific pathophysiology and management. For each patient, data were extracted from medical records by two of the study authors (infectious diseases specialists).
Microbiological diagnosis was performed according to international standards. For each patient, three to five intraoperative bone and/or periprosthetic tissue samples were collected under sterile conditions. They were then inoculated onto a Columbia sheep's blood agar plate (with reading at days 1, 2, and 3 before being thrown away), two PolyVitex chocolate agar plates (with reading at days 1, 2, and 3 before being thrown away for the first one and with reading at days 7 and 10 for the second one), two blood agar plates for anaerobic incubation (with reading at days 3 and 5 before being thrown away for the first one and with reading at days 7 and 10 for the second one) and into a Schaedler anaerobic liquid broth for which a daily reading was performed. If not cloudy, the broth was systematically subcultured on day 10 onto chocolate and blood agar plates for anaerobic incubation, incubated for 5 days in 5% CO2 and anaerobic atmosphere, respectively. Isolated bacteria were identified according to standard laboratory procedures (VITEK 2 system or VITEK matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; bioMerieux, Marcy l'Etoile, France). When several specimens were positive, the identification of each type of colony was performed for all specimens. Antimicrobial susceptibility profiles were determined at least twice for each type of bacteria after a random selection among the positive specimens. Results of superficial and/or soft tissue samples were excluded.
Definitions
BJIs were classified according to: (i) the potential presence of an orthopedic implant (i.e., joint prosthesis or osteosynthesis device); and (ii) the duration of progression from the presumed date of inoculation (i.e., date of device implantation for post-operative ODI, or date of symptom onset for native BJI) up to diagnosis, differentiating acute ( ≤ 4 weeks) vs. chronic (>4 weeks), and early (≤ 3months) vs. delayed (>3 months) infections (1, 19).
The surgical strategies considered as optimal were: (i) surgical debridement for chronic osteomyelitis; (ii) debridement with implant retention for acute ODI; and (iii) implant removal for chronic ODI. One-time exchange for chronic ODI was accepted if bacterial identification was previously known, without compromised local conditions (sinus tract, abscess and/or flap coverage requirement) (3, 11).
Treatment failure consisted in: (i) clinically persisting infection under appropriate antibiotherapy; or (ii) clinical relapse after the end of antibiotherapy; or (iii) septic indication for unplanned surgical revision more than 5 days after primary procedure; or (iv) superinfection; or (v) death related to the BJI or to a complication of its management.
Biological inflammatory syndrome referred to a plasmatic CRP level > 10 mg/L.
Strain Characterization and Susceptibility Testing
Baseline strain characterization was routinely performed at time of diagnosis and retrieved from patients' medical records, including (i) species identification using VITEK®2 MS (bioMérieux, version 2.8.4.20081127, Shimadzu Biotech) (39); and (ii) antimicrobial susceptibility profile using the disk diffusion method on Mueller-Hinton agar supplemented with 5% sheep blood, as recommended by the European Committee on Antimicrobial Susceptibility Testing. Most clinical isolates responsible for a BJI diagnosed at our institution had been stored in cryotubes at −80°C since 2007. Available Corynebacterium spp. strains isolated from the included patients were subcultivated on Colombia agar supplemented with 5% sheep blood (COS, bioMérieux, Marcy l'Etoile, France) at 37°C for 48 h for further bacteriological assessments.
Biofilm Formation
Early-stage biofilm formation was assessed using a protocol based on the BioFilm Ring test®, relying on the immobilization of magnetic beads by the growing biofilm matrix (13). Briefly, 96-well microplates were inoculated with a set of 10-fold serial dilution of standardized bacterial suspension in BHI mixed with 1% (v/v) toner solution containing magnetic beads (Biofilm Control, Saint Beauzire, France). A well without bacteria was used as negative control. After 5 h of static incubation at 37°C, each well was covered with 100 μL of white opaque oil (contrast liquid) and plates were placed for 1 min on a dedicated block for magnetization before being scanned with a specific plate reader (Pack BIOFILM, Biofilm Control): free beads were attracted at the center of the well to form a spot, of which intensity dropped down as beads were immobilized during biofilm formation. The adhesion strength of each strain was expressed as BioFilm Index (BFI), as previously described (40). The biofilm-forming potential (BP) was calculated using the formula: BP = [1 – (BFI sample/average BFI of negative control)] for each well. The cut-off value corresponded to three standard deviations above the mean of the negative control wells (BFIc = 0.53). Isolates with values of BP above 0.53 were considered significant biofilm formers. The last dilution above 2BFIc identifies the ability of the microorganism to form biofilm: poor (BP < 2BFIc at 10−1 dilution), weak (BP > 2BFIc at 10−1 and/or 10−2 dilution), moderate (BP > 2BFIc at 10−3 and/or 10−4 dilution), and high (BP > 2BFIc at 10−5 and/or 10−6 dilution) biofilm producers (41).
Ability to form mature biofilm was evaluated using the crystal violet staining test, as previously described (42). Briefly, 96-well microplates were inoculated with standardized bacterial suspension in BHI supplemented with 1% glucose, and incubated for 24 h at 37°C. After being washed, biofilm was colored with 100 μL of 0.1% crystal violet (Merck, Fontenay-sous-Bois, France). After new wash, dye bound to the biofilm was resolubilized with 100 μL of 33% acetic acid (VWR International) per well. The optical density at 490 nm, measured with a micro ELISA Auto Reader, Model 680 (BioRad, Hercules, USA), allows a quantitative measurement of formed biofilm. S. aureus 6850 was used as positive control in each experiment.
Invasion of Human Osteoblasts
The ability of gentamicin-susceptible isolates to invade osteoblasts was evaluated in a gentamicin-protection assay. MG63 osteoblastic cells (CRL-1427; LGC standard, USA) were seeded at 40,000 cells per well into 48-well tissue culture plates and cultured for 24 h. Osteoblasts were infected with bacterial suspensions standardized in BHI at a multiplicity of infection of 1:100. After 2 h of co-culture, cells were treated for 1 h with gentamicin (200 mg/L) to kill the remaining extracellular bacteria and subsequently lysed by a 10-min incubation in sterile water. Dilutions of cell lysates were spiral-plated on COS using an easySpiral® automated plater (Interscience, Saint-Nom-la-Bretèche, France). Colonies were enumerated using a Scan®1200 automated plate reader (Interscience).
Given that the internalization of S. aureus within osteoblasts requires bacterial binding to the cellular α5β1 integrin via fibronectin (43, 44), Corynebacterium internalization was further investigated by infecting two murine osteoblastic cell lines with isolates able to invade MG63 cells in the above model: (i) OB-β1+/+ expressing a functional integrin β1 subunit, (ii) OB-β1−/− deficient in the expression of the β1 integrin subunit after the conditional deletion of the itgb1 gene by transfection (45, 46).
S. aureus laboratory strain 6850 was used as positive control in each experiment while S. aureus DU5883 strain, deleted for the fnbA/B genes (and so unable to invade osteoblasts), was used as negative control (47).
Statistical Analysis
Studied variables were described as percentages for dichotomous variables and as medians with interquartile range (IQR) for continuous variables. In percentage calculation, the number of missing values was excluded from the denominator. Non-parametric tests were used to compare groups (Fisher exact and Mann-Whitney U tests), as appropriate. Kaplan-Meier curves were compared between groups using the log-rank (Mantel-Cox) test. Determinants of treatment failure were assessed using stepwise binary logistic regression, and expressed as odd ratios (ORs) with their 95% confidence intervals (95%CI). Non-interacting variables with medical meaning and p-values obtained in univariate analysis < 0.15 were included in the final multivariate model. Bacteriological data provide from three independent experiments in triplicate, and results are expressed as mean of the nine measure points and its 95%CI. Results were expressed relatively to S. aureus 6850. A value of p < 0.05 was considered significant. All analyses were performed using SPSS v19.0 (SPSS, Chicago, IL, USA) and GraphPad-Prism v5.03 (GraphPad, San Diego, CA, USA) softwares.
Results
Characteristics of the Included Population
Fifty-one Corynebacterium spp. BJIs occurring in 49 patients were included, as two patients presented two consecutives independent BJI episodes (Table 1). All infections resulted from an inoculation mechanism, and were mostly chronic (n = 45, 88.2%) and ODI (n = 38, 74.5%). ODI included 23 (60.5%) osteosynthesis devices and 15 (39.5%) prosthetic joint infections (PJI) (Table 2).
Table 1.
Comparison of patients with favorable and unfavorable outcome and determinants of treatment failure in all patients with Corynebacterium spp. BJI (univariate analysis).
| Outcome | Univariate analysis | |||||
|---|---|---|---|---|---|---|
| Total population | Favorable | Failure | p-value | OR (95%CI) | p-value | |
| n | 51 | 31 | 20 | |||
| Demographics | ||||||
| Male gender | 36 (70.6%) | 19 (61.3%) | 17 (85.0%) | 0.115 | 3.579 (0.861–14.871) | 0.079 |
| Age (median, 95%CI), years | 54.2 (44.2–68.8) | 52.5 (46.3–67.6) | 56 (41.6–69.0) | 0.862 | 0.996 (0.963–1.030)* | 0.810 |
| Comorbidities | ||||||
| BMI (median, 95%CI), kg/m2 | 26.9 (23.5–28.6) | 25.3 (23.7–27.5) | 27.3 (23.3–28.8) | 0.378 | 1.112 (0.938–1.319) | 0.220 |
| ASA score (median, 95%CI) | 1 (1–2) | 2 (1–2) | 1 (1–2) | 0.697 | 0.908 (0.477–1.729) | 0.770 |
| CCI (median, 95%CI) | 0 (0–2) | 0 (0–2) | 0.5 (0–2) | 0.687 | 1.068 (0.737–1.547) | 0.728 |
| Corynebacterium species | ||||||
| C. striatum | 18 (37.5%) | 12 (38.7%) | 6 (36%) | 0.764 | 0.7731 (0.218–2.444) | 0.611 |
| C. tuberculostearicum | 6 (12.5%) | 3 (9.7%) | 3 (17.6%) | 0.661 | 1.750 (0.315–9.716) | 0.522 |
| C. simulans | 5 (10.4%) | 4 (12.9%) | 1 (5.9%) | 0.637 | 0.375 (0.039–3.633) | 0.397 |
| C. jekeium | 4 (8.3%) | 2 (6.5%) | 2 (11.8%) | 0.629 | 1.706 (0.220–13.243) | 0.610 |
| C. minutissimum | 4 (8.3%) | 2 (6.5%) | 2 (11.8%) | 0.629 | 1.706 (0.220–13.243) | 0.610 |
| C. amycolatum | 3 (6.3%) | 3 (9.7%) | 0 (0.0%) | 1.000 | 0.519 (0.050–5.379) | 0.582 |
| Corynebacterium urealyticum | 2 (4.2%) | 1 (3.2%) | 1 (5.9%) | 1.000 | 1.667 (0.098–28.320) | 0.724 |
| Others | 5 (10.4%) | 3 (9.7%) | 2 (11.8%) | 0.661 | 1.750 (0.315–9.716) | 0.522 |
| Type of BJI | ||||||
| Native chronic osteomyelitis | 13 (25.5%) | 9 (29.0%) | 4 (20.0%) | 0.529 | 0.611 (0.160–2.339) | 0.472 |
| ODI | ||||||
| PJI | 15 (39.5%) | 6 (27.3%) | 9 (56.3%) | 0.099 | 3.429 (0.078–13.390) | 0.076 |
| Osteosynthsesis device | 23 (60.5%) | 16 (72.2%) | 7 (48.3%) | 0.099 | 0.292 (0.075–1.139) | 0.076 |
| BJI mechanism | ||||||
| Superinfection | 24 (47.1%) | 13 (41.9%) | 11 (55.0%) | 0.402 | 1.692 (0.545–5.257) | 0.363 |
| Inoculation mechanism | 51 (100%) | 31 (100%) | 20 (100%) | 1.000 | NC | NC |
| Post-operative | 48 (94.1%) | 30 (96.8%) | 18 (90.0%) | 1.000 | 0.300 (0.025–3.549) | 0.340 |
| Post-traumatic | 23 (45.1%) | 14 (45.2%) | 9 (45.0%) | 0.553 | 0.994 (0.321–3.075) | 0.991 |
| BJI chronology | ||||||
| Early infection (< 3 months) | 34 (69.4%) | 21 (70.0%) | 13 (68.4%) | 1.000 | 0.929 (0.268–3.219) | 0.907 |
| Chronic infection (>4 weeks) | 45 (88.2%) | 26 (83.9%) | 19 (95.0%) | 0.384 | 3.654 (0.394–33.880) | 0.254 |
| Diagnostic features | ||||||
| Sinus tract | 29 (63.0%) | 18 (60.0%) | 11 (68.8%) | 0.750 | 1.467 (0.406–5.301) | 0.559 |
| Abscess | 9 (20.0%) | 5 (17.2%) | 4 (25.0%) | 0.700 | 1.600 (0.362–7.073) | 0.535 |
| Biological inflammatory syndrome | 30 (69.8%) | 17 (58.6%) | 13 (92.9%) | 0.033 | 9.176 (1.054–79.892) | 0.045 |
| Initial plasmatic CRP level (mg/L) | 37.3 (15.3–96.2) | 30.0 (14.5–91.5) | 45.0 (21.7–93.9) | 0.565 | 0.998 (0.990–1.007) | 0.724 |
| Polymicrobial infection | 40 (78.4%) | 26 (83.9%) | 14 (70.0%) | 0.304 | 0.449 (0.116–1.736) | 0.246 |
| Surgical management | 47 (92.2%) | 29 (93.5%) | 18 (90.0%) | 0.640 | 0.621 (0.080–4.804) | 0.648 |
| Inappropriate surgical management | 12 (23.5%) | 5 (16.1%) | 8 (35.0%) | 0.178 | 2.800 (0.743–10.553) | 0.128 |
| Flap coverage requirement | 8 (15.7%) | 3 (9.7%) | 5 (25.0%) | 0.237 | 3.111 (0.652–14.845) | 0.155 |
| Medical management | ||||||
| Antimicrobial therapy duration | ||||||
| Total treatment duration (weeks) | 24.7 (14.1–54.4) | 18.1 (13.1–33.9) | 37.1 (22.4–59.4) | 0.080 | 1.018 (0.997–1.039) | 0.088 |
| Corynebacterium-specific treatment duration | 16.3 (13.1–22.8) | 14.9 (12.9–18.9) | 20.0 (16.0–33.9) | 0.039 | 1.070 (1.004–1.141) | 0.038 |
| Corynebacterium-specific intravenous treatment | 48 (94.1%) | 29 (93.5%) | 19 (95.0%) | 1.000 | 0.310 (0.111–15.479) | 0.830 |
| Intravenous treatment duration | 14.1 (6.5–18.3) | 13.1 (5.9–15.0) | 18.1 (14.9–27.9) | 0.130 | 1.095 (1.007–1.190) | 0.034 |
| Oral switch | 26 (54.2%) | 20 (69.0%) | 6 (31.6%) | 0.018 | 0.208 (0.060–0.723) | 0.013 |
| Corynebacterium-specific combination therapy | 36 (75.0%) | 24 (82.8%) | 12 (63.2%) | 0.176 | 0.357 (0.093–1.365) | 0.132 |
| Combination therapy duration | 12.9 (6.8–16.6) | 12.1 (4.3–13.9) | 19.3 (10.6–22.5) | 0.491 | 1.107 (0.977–1.255) | 0.112 |
| First line antimicrobial regimen | ||||||
| Initial oral antimicrobial therapy | 18 (35.3%) | 14 (45.2%) | 4 (20.0%) | 0.080 | 0.304 (0.082–1.119) | 0.073 |
| Betalactam | 24 (50.0%) | 15 (50%) | 9 (50.0%) | 1.000 | 1.000 (0.311–3.218) | 1.000 |
| Glycopeptide | 35 (68.6%) | 23 (74.2%) | 12 (60.0%) | 0.360 | 0.522 (0.157–1.738) | 0.289 |
| Clindamycin | 5 (10.0%) | 4 (13.3%) | 1 (5.0%) | 0.636 | 0.342 (0.035–3.311) | 0.354 |
| Linezolid | 0 (0.0%) | 0 (0.0%) | 0 (0%) | NC | NC | NC |
| Daptomycin | 3 (5.9%) | 0 (0.0%) | 3 (15.0%) | 0.055 | NC | NC |
| Posterior antimicrobial regimen | ||||||
| Betalactam | 20 (40.8%) | 11 (36.7%) | 9 (47.4%) | 0.555 | 1.555 (0.484–4.995) | 0.459 |
| Glycopeptide | 19 (37.3%) | 10 (32.2%) | 9 (47.4%) | 0.358 | 1.718 (0.539–5.475) | 0.360 |
| Clindamycin | 8 (15.7%) | 5 (16.1%) | 3 (15.0%) | 0.496 | 0.555 (0.125–2.469) | 0.439 |
| Linezolid | 9 (17.6%) | 7 (22.6%) | 2 (10.0%) | 0.512 | 1.643 (0.442–6.102) | 0.458 |
| Daptomycin | 5 (9.8%) | 4 (12.9%) | 1 (5.0%) | 1.000 | 0.722 (0.119–4.372) | 0.723 |
| Daptomycin-containing regimen | 8 (15.7%) | 4 (12.9%) | 3 (15.0%) | 0.696 | 1.687 (0.370–7.697) | 0.499 |
95%CI, 95% confidence interval; ASA, American society of anesthesiologists; BMI, Body mass index; CCI, Charlson comorbidity index; CRP, C-reactive protein;NC, Not calculable; ODI, Orthopedic device-related infection; OR, Odd ratio; PJI, Prosthetic joint infection.
Calculated for 10 additional years.
Table 2.
Comparison of patients with favorable and unfavorable outcome and determinants of treatment failure in patients with Corynebacterium spp. orthopedic device-related infection (univariate analysis).
| Outcome | Univariate analysis | |||||
|---|---|---|---|---|---|---|
| ODI | Favorable | Failure | p-value | OR (IC95%) | p-value | |
| Demographics | ||||||
| Male gender | 25 (65.8%) | 12 (54.5%) | 13 (81.3%) | 0.165 | 3.611 (0.798; 16.347) | 0.096 |
| Age (median, 95%CI), years | 53.1 (44.1;69.0) | 52.2 (47.5;69.0) | 54.0 (41.6;69.0) | 0.679 | 0.991 (0.956; 1.027) | 0.615 |
| Comorbidities | ||||||
| BMI (median, 95%CI), kg/m2 | 25.4 (22.9;28.6) | 24.5 (22.5;26.0) | 28.0 (23.5;28.9) | 0.100 | 1.199 (0.969; 1.484) | 0.095 |
| ASA score (median, 95%CI) | 1 (1.0;2.0) | 1 (1.0;2.0) | 1.5 (1.0; 2.3) | 0.589 | 1.250 (0.605; 2.584) | 0.547 |
| CCI (median, 95%CI) | 0 (0.0;1.8) | 0 (0.0;1.0) | 0.5 (0.0;2.0) | 0.453 | 1.325 (0.809; 2.171) | 0.263 |
| BJI mechanism | ||||||
| Superinfection | 16 (42.1%) | 8 (36.4%) | 8 (50.0%) | 0.511 | 1.750 (0.472; 6.483) | 0.402 |
| Inoculation mechanism | 38 (100%) | 22 (100%) | 16 (100%) | |||
| Post-operative | 37 (97.4%) | 22 (100%) | 15 (93.8%) | 0.421 | NC | NC |
| Post-traumatic | 16 (42.1%) | 10 (45.5%) | 6 (37.5%) | 0.744 | 0.720 (0.193; 2.681) | 0.624 |
| BJI chronology | ||||||
| Early infection (< 3 months) | 25 (69.4%) | 13 (61.9%) | 12 (80.0%) | 0.295 | 2.462 (0.527; 11.500) | 0.252 |
| Chronic infection (>4 weeks) | 33 (86.8%) | 18 (81.8%) | 15 (93.8%) | 0.374 | 3.333 (0.336; 33.113) | 0.304 |
| Diagnostic features | ||||||
| Sinus tract | 21 (63.6%) | 13 (61.9%) | 8 (66.7%) | 1.000 | 1.231 (0.278; 5.454) | 0.785 |
| Abscess | 6 (18.8%) | 2 (10.0%) | 4 (33.3%) | 0.165 | 4.500 (0.679; 29.808) | 0.119 |
| Biological inflammatory syndrome | 26 (78.8%) | 14 (70.0%) | 12 (92.3%) | 0.202 | 5.143 (0.540; 48.943) | 0.154 |
| Initial plasmatic CRP level (mg/L) | 30.0 (15.0;93.9) | 20.0 (14.0;46.0) | 52.4 (20.1;96.2) | 0.414 | 0.999 (0.991; 1.007) | 0.824 |
| Surgical management | 36 (94.7%) | 22 (100%) | 14 (87.5%) | 0.171 | NC | NC |
| Inappropriate surgical strategy | 9 (25.0%) | 4 (18.2%) | 5 (35.7%) | 0.147 | 3.500 (0.808–15.163) | 0.094 |
| Surgical strategy | ||||||
| DAIR/debridement | 14 (38.9%) | 7 (31.8%) | 7 (50.0%) | 0.314 | 2.143 (0.539; 8.512) | 0.279 |
| One-stage exchange | 1 (2.8%) | 1 (4.5%) | 0 (0.0%) | 1.000 | NC | NC |
| Two-stage exchange | 11 (30.6%) | 9 (40.9%) | 2 (14.3%) | 0.142 | 0.241 (0.043; 1.346) | 0.105 |
| Definitive device ablation | 10 (27.8%) | 5 (22.7%) | 5 (37.5%) | 0.462 | 1.889 (0.430; 8.295) | 0.400 |
| Two-stage exchange OR definitive device ablation | 21 (58.3%) | 14 (63.6%) | 7 (50.0%) | 0.499 | 0.571 (0.147; 2.228) | 0.420 |
| Flap coverage requirement | 5 (13.2%) | 2 (9.1%) | 3 (18.8%) | 0.632 | 2.308 (0.338; 15.750) | 0.393 |
95%CI, 95% confidence interval; ASA, American society of anesthesiologists; BMI, Body mass index; CCI, Charlson comorbidity index; CRP, C-reactive protein; NC, Not calculable; ODI, Orthopedic device-related infection; OR, Odd ratio; PJI, Prosthetic joint infection.
Surgery was performed in 47 (92.2%) patients and considered as optimal in 39 (76.5%) cases. The total duration of antibiotherapy specifically directed against corynebacteria was 18.1 (IQR, 13.1–29.3) weeks, initially administrated intravenously for 14.1 weeks (IQR, 6.5–18.3) in 48 patients (94.1%).
Bacteriological Findings
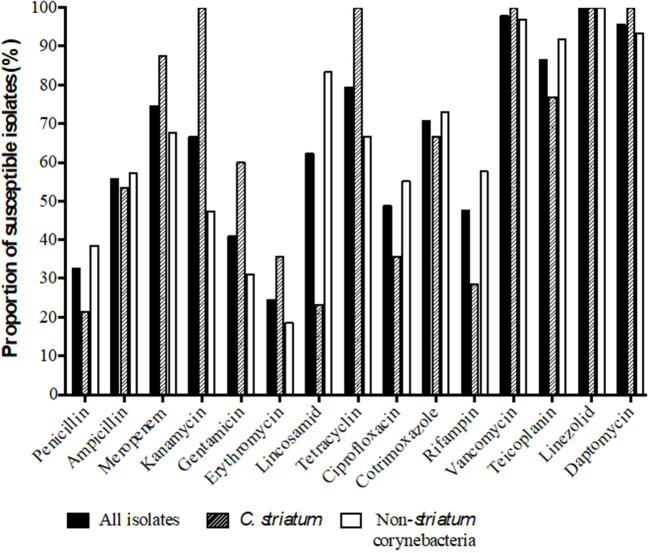
As one patient presented a co-infection with two different Corynebacteria, 52 strains were considered for inclusion. Species identification and antimicrobial susceptibility testing were available in patients' medical records for 45 of them. The most frequent species were Corynebacterium striatum (n = 18, 37.5%) and Corynebacterium tuberculostearicum (n = 6, 12.5%). Antimicrobial susceptibility profiles are presented in Figure 1. Most infections were polymicrobial (n = 40, 78.4%), including co-infections with coagulase-negative staphylococci (n = 20, 50.0%), Enterobacteriaceae (n = 14, 35.0%), S. aureus (n = 8, 20.0%), anaerobes (n = 7, 17.5%), enterococci (n = 5, 12.5%), P. aeruginosa (n = 4, 10.0%), streptococci (n = 4, 10.0%) and/or Candida (n = 1, 2.5%). A detailed description of the eleven patients with a monomicrobial Corynebacterium spp. BJI is provided in Supplementary Table 1.
Figure 1.
Susceptibility profile of the 51 BJI Corynebacterium spp. isolates.
Outcome and Determinants of Treatment Failure
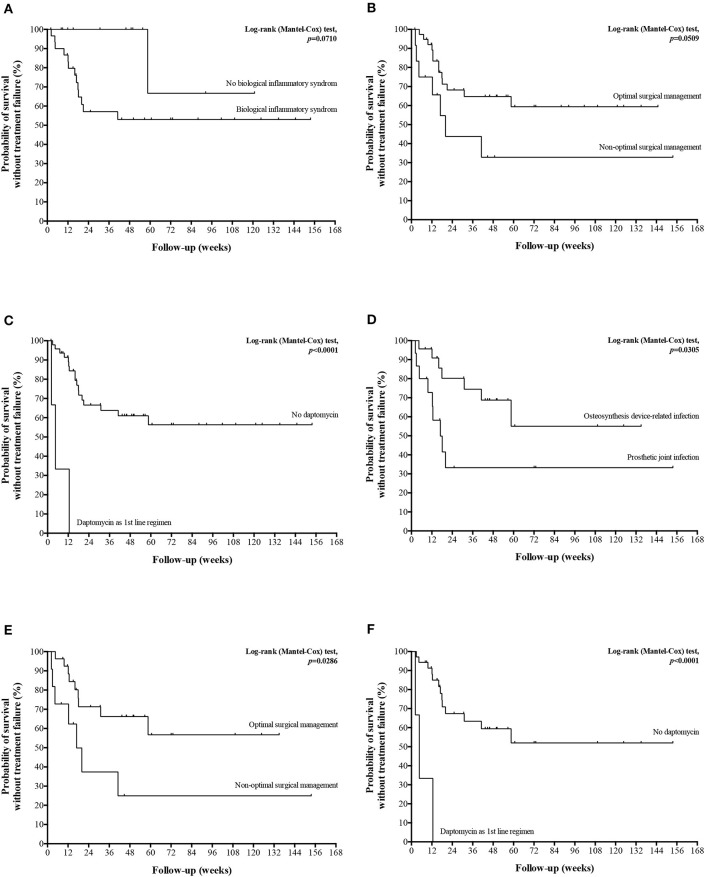
After a median follow-up of 60.7 weeks (IQR, 30.1–115.1) including 38.0 weeks (IQR, 10.1–85.7) after completion of the antibiotherapy, 20 (39.2%) treatment failures were observed in a median delay of 14.3 weeks (IQR, 9.1–18.6) after treatment initiation, including 13 (65.0%) persistent infections, 6 (30%) relapses, 10 (50%) superinfections and one infection-related death. Seventeen (85.0%) cases required an additional surgical procedure, including one limb amputation. Four (20.0%) treatment failures were documented with the same Corynebacterium spp. strain; no documentation was obtained in 8 (40.0%) patients. Comparison of patients with and without treatment failure is presented in Table 1, as well as univariate analysis for risk factor for treatment failure. In multivariate analysis, among male gender, initial biological inflammatory syndrome, non-optimal surgical management, and corynebacteria-directed combination therapy, independent determinants for treatment failure were an initial biological inflammatory syndrome (OR, 15.119; 95%CI, 1.189–192.205; p = 0.036) and non-optimal surgical management (OR, 7.291; 95%CI, 1.107–48.016; p = 0.039) (Figures 2A,B). Interestingly, the 3 (5.9%) patients who received daptomycin (6 to 8 mg/kg/day) as first-line regimen relapsed (Figure 2C), despite an optimal surgical management. Of note, two of these patients had a polymicrobial infection. The three Corynebacterium spp. Isolates were fully susceptible do daptomycin, with MICs of 0.5, 0.094, and 0.032 mg/L. The choice of daptomycin was based on the polymicrobial nature of the infection in one patient, and previous antimicrobial intolerances in the two others. Finally, daptomycin was used as part of a combination therapy in two of these three patients.
Figure 2.
Kaplan-Meier curves for the cumulative risk of treatment failure in all patients (A–C) and in patients with ODI panel (D–F) according to the major determinants of treatment failure.
Concerning specifically ODI, 16 (42.1%) treatment failures were observed. No significant risk factors was highlighted (Table 2), but treatment failure-free survival curve analysis suggested a significantly poorer outcome in patients with PJI compared to osteosynthesis device infection, in case of non-optimal surgical management, and if daptomycin was used as first-line regimen (Figures 2D–F).
Bone Cell Invasion
Among the 52 potential strains isolated from the patient study, 22 had not been conserved, five had been isolated in other institutions before patient referral to our reference center, seven were resistant to gentamicin preventing to perform gentamicin-protection assay, three could not be formally identified at the species level, and two C. tuberculostearicum strains had cultural aspect with tiny colonies preventing their enumeration on blood agar plates. Consequently, ability to invade human osteoblasts could be assessed for 13 corynebacteria strains (seven C. striatum, three Corynebacterium simulans, two Corynebacterium amycolatum/xerosis, and one Corynebacterium minutissimum) isolated from different patients (Table 3).
Table 3.
Description of the isolates evaluated in the osteoblastic cell infection model and biofilm formation assays.
| Strain identification | Corynebacterium species | Type of BJI | Chronology of infection | Internalization rate (95%CI)* | Biofilm-forming potential | Mature biofilm formation (95%CI)* |
|---|---|---|---|---|---|---|
| Cor 1b# | C. striatum | Osteosynthesis infection | Early | 0.34% (0.06–0.62) | POOR | 0.36% (−0.08–0.79) |
| Cor 4 | C. striatum | Native osteomyelitis | Early | 3.63% (1.47–5.80) | POOR | 35.71% (26.26–45.17) |
| Cor 5# | C. simulans | Prosthetic joint infection | Early | 0.60% (0.30–0.89) | WEAK | 1.64% (−0.84–4.13) |
| Cor 8b | C. striatum | Osteosynthesis infection | Delayed | 4.29% (0.71–7.88) | WEAK | 42.39% (30.65–54.13) |
| Cor 9b | C. striatum | Prosthetic joint infection | Early | 1.30% (0.36–2.25) | POOR | 5.66% (1.79–9.52) |
| Cor 10 | C. amycolatum/xerosis | Prosthetic joint infection | Delayed | 1.35% (0.37–2.32) | N/A | 8.58% (3.78–13.38) |
| Cor 11a | C. minutissimum | Native osteomyelitis | Delayed | 55.6% (28.99–82.24) | POOR | 1.04% (−0.27–2.34) |
| Cor 12# | C. simulans | Prosthetic joint infection | Delayed | 5.99% (3.11–8.87) | POOR | 4.61% (−1.70–10.92) |
| Cor 13 | C. striatum | Native osteomyelitis | Early | 15.3% (9.67–20.91) | N/A | 13.59% (6.11–21.06) |
| Cor 14 | C. simulans | Native osteomyelitis | Delayed | 2.84% (0.75–4.93) | POOR | 8.56% (−0.04–17.15) |
| Cor 15 | C. amycolatum/xerosis | Osteosynthesis infection | Delayed | 206% (131.08–281.86) | POOR | 18.06% (2.89–33.22) |
| Cor 16 | C. striatum | Osteosynthesis infection | Early | 35.2% (-3.23–73.647) | POOR | 2.38% (−0.17–4.93) |
| Cor 18 | C. striatum | Prosthetic joint infection | Delayed | 7.29% (3.03–11.54) | POOR | 0.01% (−0.01–0.04) |
95%CI, 95% confidence interval; BJI, Bone and joint infection; CO, Chronic osteomyelitis; ODI, Osteosynthesis device-associated infection; PJI, Prosthetic joint infection.
Results are given as mean and its 95% confidence interval (95%CI), compared to S. aureus 6850.
Designated monomicrobial infections.
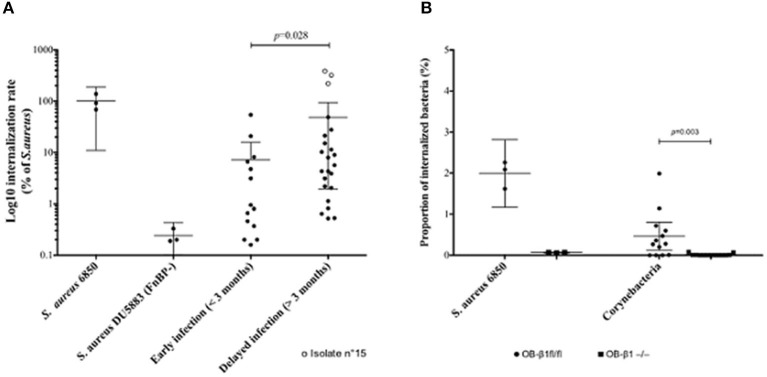
In comparison with S. aureus DU5883, all but one strain were significantly able to invade MG63 osteoblasts (Figure 3). The internalization rate was roughly comprised between 1 and 10% of positive control (S. aureus 6850). One C. amycolatum/xerosis strain (n°15) isolated from a delayed BJI even presented a very high internalization rate (200% of positive control).
Figure 3.
Ability of Corynebacterium spp. isolates to invade osteoblastic cells. (A) Internalization rates of Corynebacterium isolates in MG63 human osteoblasts according to bone and joint infection (BJI) evolution delay, in comparison with S. aureus 6850 (positive control) and S. aureus DU5883 strain, inactivated for the fnbA/B genes (FnBP, negative control). (B) Internalization rates of the Corynebacterium isolates in murine osteoblasts with functional (OB-β1fl/fl) or deficient (OB-β1−/−) expression of the integrin β1 subunit, in comparison with S. aureus 6850.
Strains isolated from delayed BJI had a significantly higher internalization rate compared to early ones (Figure 3A).
The internalization rate of each species are provided in Supplementary Figure 1A. The little number of isolates per species did not allow to provide pertinent statistical comparison.
Strains able to invade MG63 human osteoblasts, including isolate n°15, were challenged in OB-β1−/− murine osteoblasts, resulting in a drastic reduction of the internalization rate compared to OB-β1+/+ cells (Figure 3B).
Biofilm Formation
Early-stage biofilm formation was assessed for 11 of the 13 isolates used in the cellular infection model. The two other Cor 10 and 13° formed aggregates under the culture conditions specifically required for the BioFilm Ring test®. All but two corynebacteria had a poor BP. The two last strains Cor 5 and Cor 8b had a weak BP (Table 3).
All the 13 isolates were evaluable regarding their mature biofilm formation by the crystal violet staining method. Six of them formed mature biofilm, with a rate ranging from 8.6 to 42.4% compared to S. aureus 6850 (Table 3).
The little number of isolates per species prevented providing relevant interspecies comparison (Supplementary Figure 1B).
Early or mature biofilm formation abilities were not correlated with any relevant clinical feature.
Of note, neither internalization nor biofilm formation ability had a significant impact on patient outcome.
Discussion
Representing more than 3% of PJI etiologic agents (34–36), Corynebacterium spp. have been largely neglected in this field. Despite the limitations inherent to the retrospective and unicentric nature of our study, it provides major clinical and therapeutic insights regarding corynebacteria BJI. Our results are reinforced by the attempt to minimize the risk of considering commensal Corynebacterium spp. strains isolated as contaminants by including only BJI with at least two concordant positive per operative samples and excluding contiguous infections such as decubitus ulcer- and diabetic foot-related osteomyelitis that are associated with a high risk of sample contamination. Indeed, conclusions of some previously published series must be interpreted with caution as including more than 50% of patients with contiguous BJI and based on the culture results of superficial samples (48, 49).
Our results confirm that proven Corynebacterium BJI occur mainly by inoculation after trauma, mostly after road crash-related open fractures. Indeed, a predominance of young men was noted, with up to 70% of chronic osteomyelitis. PJI were less frequent than previously described (48), and mostly corresponded to superinfections during complex PJI managements. These differences can be explained by our stringent bacteriological definition of cases. Finally, the species distribution slightly differed from previous studies, with a predominance of C. striatum and C. tuberculostearicum, and less C. amycolatum and Corynebacterium jekeium than previously described (36, 48).
The management of BJI involving Corynebacterium is complex. First, optimal surgical management appeared as a crucial determinant for treatment outcome, as previously described for chronic and/or ODI (50, 51), including removal of orthopedic device and extensive bone curettage when necessary (37, 38). However, this theoretical optimal management can be impaired by fracture stabilization requirements, and sometimes leads to major tissue loss. Choice of antimicrobial therapy is also challenging. As shown by our results and previous series (48), Corynebacterium isolates can be resistant to most of the antibiotics commonly used in BJI, including amoxicillin which remains the drug of choice for susceptible isolates. Moreover, polymicrobial infection prevalence, vancomycin toxicity and/or patient's antibiotic intolerances can raise the need of off-label use of alternative drugs. In this setting, daptomycin has been increasingly used in Gram positive BJI (52). Interestingly, all patients treated by daptomycin experienced treatment failure, leading to highlight the use of this antimicrobial as a significant risk factor for poor outcome. Although based on a limited number of patients, this finding is coherent with treatment failures and daptomycin resistance selection observed during other chronic conditions such as infective endocarditis (53–55) and raises the question of reconsidering the use of daptomycin as a first-line agent. Finally, the higher rate of treatment failure of ODI compared to PJI was not explained by statistically significant differences between patients or their management. However, ODI mostly occurred following the management of severe limb trauma, requiring flap coverage in more than 20% of cases. Even if not highlighted by our results, the complexity of orthopedic situations observed in such kind of patients might have led to this poor outcome.
Overall, and despite a complex surgical and medical management, BJI with Corynebacterium spp. are difficult-to-treat infections, as evidenced by (i) the failure rate approaching 40%, (ii) the frequent need of iterative surgical procedures including surgical flap reconstruction in 15% of patients, and (iii) the prolonged courses of antimicrobial therapies. If polymicrobism has been highlighted as a risk factor for treatment failure in some studies (56), this point is still controversial (57), and polymicrobial infections were not associated with a poorer outcome in our series. Associated with a dramatically increase of morbidity and medical/societal cost (58), BJI chronicity and relapse have consequently to be investigated, including underlying mechanisms leading to bacterial escape from the action of the host immune system and/or the antibiotics. The extensive evaluation of Staphylococcus aureus BJI pathomechanisms highlighted three main phenotypic bacterial factors associated with BJI chronicity (59): (i)internalization and persistence in non-professional phagocytic bone cells (osteoblasts), which had been confirmed to be clinically associated with BJI chronicity (15), (ii) biofilm formation (60), and (iii) emergence of small colony variants (25). We provide here the first assessment of these mechanisms toward a collection of clinical Corynebacterium isolates responsible for BJI. We demonstrated that almost all Corynebacterium isolates were able to invade osteoblasts and that their internalization rate was correlated with BJI chronicity, even if in fine the cure rate was not impacted. This ability to sanctuarize in bone cells emphasized the importance of surgical debridement in chronic BJI with Corynebacterium spp. and pleads for including the ability of antibiotics to eradicate the intracellular reservoir of corynebacteria in the choice of antimicrobial therapy strategies, as suggested for S. aureus (15, 61, 62). Interestingly, the infection of murine osteoblasts deficient in the expression of β1 integrin abolished the cellular invasion ability of the evaluated strains. This strongly suggest that corynebacteria osteoblastic invasion relies on mechanisms similar to S. aureus, of which fibronectin binding proteins A and B link to fibronectin of the bone matrix that acts as bridges between S. aureus and osteoblasts through the cellular α5β1 integrin (43, 44). The ligand of the cellular β1 integrin remains to be described in corynebacteria, as representing a future potential therapeutic target. Regarding biofilm formation, all investigated strains of our study were poor biofilm formers as most Gram positive bacteria except S. aureus (40). A few studies have however suggested that biofilm formation could be a determinant of Corynebacterium spp. hospital acquired infections (63, 64). Unfortunately, we were not able to perform the biofilm and intracellular assays on the whole series, which might represent a bias. Indeed, the ability of corynebacteria to form biofilm seems strain-related, as shown by the differences observed toward a same species according to their sequence types (ST) (63, 65). However, no clinical differences were noted in our series between the patients for which the strain was available and the others (data not shown). Additionally, the comparison of isolates coming from mono and polymicrobial infection would have been interesting, but only three strains of our series were isolated from monomicrobial infection, making the comparison irrelevant.
The short time of follow-up (less than a year) of patients without treatment failure is not enough to affirm treatment success and represent another limitation to this study. However, even if relapses have been described several months/years after the end of therapy, this represents a rare event.
This series of proven Corynebacterium BJI allows to better understand this neglected disease. Most often presenting as a post-traumatic or post-surgical chronic infection, this difficult-to-treat condition requires a complex and collaborative medical-surgical management due to its poor prognosis which is mostly driven by the initial surgical debridement. Furthermore, if biofilm formation did not appear as a pivotal physiopathological mechanism of Corynebacterium in BJI, bone cells invasion via the cellular β1 integrin allows the formation of an intracellular reservoir that leads to chronic infection.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Ethics Statement
The requirement for written informed consent from participants for the usage of clinical isolates in the study was waived by the Committee for the protection of persons (CPP) according to French legislation at time of the study.
Author Contributions
PC collected the data, conducted most of the experiment, and wrote the manuscript under the supervision of FV. FV designed the study, analyzed the results and helped to perform the experiments. TF and FL helped to design the study. VT and AD reviewed the manuscript. JT provided the protocols for biofilm experiments. AC, CC, EB, and SL provided clinical data. All authors revised and edited the manuscript and read and approved the final manuscript.
Conflict of Interest
Biofilm Control provided support in the form of salaries for JT but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Lyon Bone and Joint Infection Study Group
Coordinator – TF; Infectious Diseases Specialists – TF, FV, Thomas Perpoint, Patrick Miailhes, Florence Ader, Agathe Becker, Sandrine Roux, Claire Triffault-Fillit, AC, Alexie Bosch, Marielle Perry, Fatiha Daoud, Johanna Lippman, EB, CC; Surgeons – SL, Elvire Servien, Romain Gaillard, Antoine Schneider, Stanislas Gunst, Cécile Batailler, Michel-Henry Fessy, Yannick Herry, Anthony Viste, Philippe Chaudier, Cyril Courtin, Lucie Louboutin, Sébastien Martres, Franck Trouillet, Cédric Barrey, Emmanuel Jouanneau, Timothée Jacquesson, Ali Mojallal, Fabienne Braye, Fabien Boucher, Hristo Shipkov, Joseph Chateau, Philippe Céruse, Carine Fuchsmann, Arnaud Gleizal; Anesthesiologists – Frédéric Aubrun, Mikhail Dziadzko, Caroline Macabéo; Microbiologists – FL, Jean-Philippe Rasigade, Laetitia Beraut, Céline Dupieux, Camille Kolenda, Jérôme Josse; Imaging – Fabien Craighero, Loic Boussel, Jean-Baptiste Pialat; Nuclear Medicine – Isabelle Morelec, Marc Janier, Francesco Giammarile; PK/PD specialists – Michel Tod, Marie-Claude Gagnieu, Sylvain Goutelle; Clinical research assistant and database manager – Eugénie Mabrut.
Footnotes
Funding. The clinical part of the study was carried out as part of our routine work. The bacteriological work was supported by an institutional grant to FV by the Hospices Civils de Lyon.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2020.539501/full#supplementary-material
Comparison of Corynebacterium species regarding their ability to invade MG63 human osteoblasts (A) and to form mature biofilm (B).
Clinical characteristics of the eleven patients with monomicrobial Corynebacterium spp. BJI.
Description of patients according to the BJI types, and comparison of osteosynthesis device-related and prosthetic joint infection.
References
- 1.Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. (2016) 387:386–94. 10.1016/S0140-6736(14)61798-0 [DOI] [PubMed] [Google Scholar]
- 2.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. (2007) 89:780–5. 10.2106/JBJS.F.00222 [DOI] [PubMed] [Google Scholar]
- 3.Grammatico-Guillon L, Baron S, Gettner S, Lecuyer A-I, Gaborit C, Rosset P, et al. Bone and joint infections in hospitalized patients in France, 2008: clinical and economic outcomes. J Hosp Infect. (2012) 82:40–8. 10.1016/j.jhin.2012.04.025 [DOI] [PubMed] [Google Scholar]
- 4.Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN, Bozic KJ. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplasty. (2010) 25(Suppl. 6):103–7. 10.1016/j.arth.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. (2005) 87:1746–51. 10.2106/00004623-200508000-00012 [DOI] [PubMed] [Google Scholar]
- 6.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. (2012) 27(Suppl. 8):61–5.e1. 10.1016/j.arth.2012.02.022 [DOI] [PubMed] [Google Scholar]
- 7.Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. (2014) 29:929–32. 10.1016/j.arth.2013.09.017 [DOI] [PubMed] [Google Scholar]
- 8.Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sánchez-Somolinos M, Baraia-Etxaburu JM, et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis. (2013) 56:182–94. 10.1093/cid/cis746 [DOI] [PubMed] [Google Scholar]
- 9.Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJG, Armstrong DG, et al. 2012 infectious diseases society of america clinical practice guideline for the diagnosis and treatment of diabetic foot infections. J Am Podiatr Med Assoc. (2013) 103:2–7. 10.1093/cid/cis346 [DOI] [PubMed] [Google Scholar]
- 10.Zeller V, Kerroumi Y, Meyssonnier V, Heym B, Metten M-A, Desplaces N, et al. Analysis of postoperative and hematogenous prosthetic joint-infection microbiological patterns in a large cohort. J Infect. (2018) 76:328–34. 10.1016/j.jinf.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 11.Mylona E, Samarkos M, Kakalou E, Fanourgiakis P, Skoutelis A. Pyogenic vertebral osteomyelitis: a systematic review of clinical characteristics. Semin Arthritis Rheum. (2009) 39:10–7. 10.1016/j.semarthrit.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 12.Berbari EF, Kanj SS, Kowalski TJ, Darouiche RO, Widmer AF, Schmitt SK, et al. 2015 infectious diseases society of America (IDSA) clinical practice guidelines for the diagnosis and treatment of native vertebral osteomyelitis in adults. Clin Infect Dis. (2015) 61:e26-46. 10.1093/cid/civ482 [DOI] [PubMed] [Google Scholar]
- 13.Bosse MJ, Gruber HE, Ramp WK. Internalization of bacteria by osteoblasts in a patient with recurrent, long-term osteomyelitis. A case report. J Bone Joint Surg Am. (2005) 87:1343–7. 10.2106/JBJS.D.02649 [DOI] [PubMed] [Google Scholar]
- 14.Stevens QEJ, Seibly JM, Chen YH, Dickerman RD, Noel J, Kattner KA. Reactivation of dormant lumbar methicillin-resistant Staphylococcus aureus osteomyelitis after 12 years. J Clin Neurosci. (2007) 14:585–9. 10.1016/j.jocn.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 15.Valour F, Rasigade J-P, Trouillet-Assant S, Gagnaire J, Bouaziz A, Karsenty J, et al. Delta-toxin production deficiency in Staphylococcus aureus: a diagnostic marker of bone and joint infection chronicity linked with osteoblast invasion and biofilm formation. Clin Microbiol Infect. (2015) 21:568.e1–11. 10.1016/j.cmi.2015.01.026 [DOI] [PubMed] [Google Scholar]
- 16.Trouillet-Assant S, Lelièvre L, Martins-Simões P, Gonzaga L, Tasse J, Valour F, et al. Adaptive processes of Staphylococcus aureus isolates during the progression from acute to chronic bone and joint infections in patients. Cell Microbiol. (2016) 18:1405–14. 10.1111/cmi.12582 [DOI] [PubMed] [Google Scholar]
- 17.Ross JJ. Septic arthritis of native joints. Infect Dis Clin North Am. (2017) 31:203–18. 10.1016/j.idc.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Spilf [Primary infectious spondylitis, and following intradiscal procedure, without prothesis. Recommendations]. Med Mal Infect. (2007) 37:573–83. 10.1016/j.medmal.2007.03.007 [DOI] [PubMed] [Google Scholar]
- 19.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. (2014) 27:302–45. 10.1128/CMR.00111-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurlow J, Couch K, Laforet K, Bolton L, Metcalf D, Bowler P. Clinical biofilms: a challenging frontier in wound care. Adv Wound Care. (2015) 4:295–301. 10.1089/wound.2014.0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. (2001) 358:135–8. 10.1016/S0140-6736(01)05321-1 [DOI] [PubMed] [Google Scholar]
- 22.Fraunholz M, Sinha B. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol. (2012) 2:43. 10.3389/fcimb.2012.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson MC, Ramp WK, Nicholson NC, Williams AS, Nousiainen MT. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. (1995) 19:409–19. 10.1006/mpat.1995.0075 [DOI] [PubMed] [Google Scholar]
- 24.Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K, et al. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J Bone Joint Surg Br. (2003) 85:918–21. 10.1302/0301-620X.85B6.13509 [DOI] [PubMed] [Google Scholar]
- 25.Tuchscherr L, Heitmann V, Hussain M, Viemann D, Roth J, von Eiff C, et al. Staphylococcus aureus small-colony variants are adapted phenotypes for intracellular persistence. J Infect Dis. (2010) 202:1031–40. 10.1086/656047 [DOI] [PubMed] [Google Scholar]
- 26.Proctor RA, von Eiff C, Kahl BC, Becker K, McNamara P, Herrmann M, et al. Small colony variants: a pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat Rev Microbiol. (2006) 4:295–305. 10.1038/nrmicro1384 [DOI] [PubMed] [Google Scholar]
- 27.Oliveira A, Oliveira LC, Aburjaile F, Benevides L, Tiwari S, Jamal SB, et al. Insight of genus corynebacterium: ascertaining the role of pathogenic and non-pathogenic species. Front Microbiol. (2017) 8:1937. 10.3389/fmicb.2017.01937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funke G, von Graevenitz A, Clarridge JE, Bernard KA. Clinical microbiology of coryneform bacteria. Clin Microbiol Rev. (1997) 10:125–59. 10.1128/CMR.10.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souza MC, dos Santos LS, Sousa LP, Faria YV, Ramos JN, Sabbadini PS, et al. Biofilm formation and fibrinogen and fibronectin binding activities by Corynebacterium pseudodiphtheriticum invasive strains. Antonie Van Leeuwenhoek. (2015) 107:1387–99. 10.1007/s10482-015-0433-3 [DOI] [PubMed] [Google Scholar]
- 30.Gladysheva IV, Cherkasov SV. [The role of fibronectin in adhesion of corynebacteria to vaginal epitheliocytes]. Zh Mikrobiol Epidemiol Immunobiol. (2014) 6:67–73. [PubMed] [Google Scholar]
- 31.Antunes CA, Sanches dos Santos L, Hacker E, Köhler S, Bösl K, Ott L, et al. Characterization of DIP0733, a multi-functional virulence factor of Corynebacterium diphtheriae. Microbiology. (2015) 161:639–47. 10.1099/mic.0.000020 [DOI] [PubMed] [Google Scholar]
- 32.Sabbadini PS, Assis MC, Trost E, Gomes DLR, Moreira LO, Dos Santos CS, et al. Corynebacterium diphtheriae 67-72p hemagglutinin, characterized as the protein DIP0733, contributes to invasion and induction of apoptosis in HEp-2 cells. Microb Pathog. (2012) 52:165–76. 10.1016/j.micpath.2011.12.003 [DOI] [PubMed] [Google Scholar]
- 33.Weerasekera D, Stengel F, Sticht H, de Mattos Guaraldi AL, Burkovski A, Azevedo Antunes C. The C-terminal coiled-coil domain of Corynebacterium diphtheriae DIP0733 is crucial for interaction with epithelial cells and pathogenicity in invertebrate animal model systems. BMC Microbiol. (2018) 18:106. 10.1186/s12866-018-1247-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Triffault-Fillit C, Ferry T, Laurent F, Pradat P, Dupieux C, Conrad A, et al. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: a prospective cohort study. Clin Microbiol Infect. (2018) 25:353–58. 10.1016/j.cmi.2018.04.035 [DOI] [PubMed] [Google Scholar]
- 35.Benito N, Franco M, Ribera A, Soriano A, Rodriguez-Pardo D, Sorlí L, et al. Time trends in the aetiology of prosthetic joint infections: a multicentre cohort study. Clin Microbiol Infect. (2016) 22:732.e1–8. 10.1016/j.cmi.2016.05.004 [DOI] [PubMed] [Google Scholar]
- 36.Rizvi M, Khan F, Raza A, Shukla I, Sabir AB. Emergence of coryneforms in osteomyelitis and orthopaedic surgical site infections. Australas Med J. (2011) 4:412–7. 10.4066/AMJ.2011.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious diseases society of America. Clin Infect Dis. (2013) 56:e1–25. 10.1093/cid/cis803 [DOI] [PubMed] [Google Scholar]
- 38.la Société de Pathologie Infectieuse de Langue Française (SPILF) Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT) Groupe de Pathologie Infectieuse Pédiatrique (GPIP) Société Française d'Anesthésie et de Réanimation (SFAR) SociétéFrançaise de Chirurgie Orthopédique et Traumatologique (SOFCOT) Société Française d'Hygiène Hospitalière (SFHH) et al. [Clinical practice recommendations. Osteoarticular infections on materials (prosthesis, implant, osteosynthesis]. Med Mal Infect. (2009) 39:815–63. 10.1016/j.medmal.2009.05.004 [DOI] [PubMed] [Google Scholar]
- 39.Alatoom AA, Cazanave CJ, Cunningham SA, Ihde SM, Patel R. Identification of non-diphtheriae corynebacterium by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. (2012) 50:160–3. 10.1128/JCM.05889-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavant P, Gaillard-Martinie B, Talon R, Hébraud M, Bernardi T. A new device for rapid evaluation of biofilm formation potential by bacteria. J Microbiol Methods. (2007) 68:605–12. 10.1016/j.mimet.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 41.Di Domenico EG, Farulla I, Prignano G, Gallo MT, Vespaziani M, Cavallo I, et al. Biofilm is a major virulence determinant in bacterial colonization of chronic skin ulcers independently from the multidrug resistant phenotype. Int J Mol Sci. (2017) 18:1077. 10.3390/ijms18051077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. (2000) 40:175–9. 10.1016/S0167-7012(00)00122-6 [DOI] [PubMed] [Google Scholar]
- 43.Ellington JK, Reilly SS, Ramp WK, Smeltzer MS, Kellam JF, Hudson MC. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog. (1999) 26:317–23. 10.1006/mpat.1999.0272 [DOI] [PubMed] [Google Scholar]
- 44.Sinha B, François PP, Nüsse O, Foti M, Hartford OM, Vaudaux P, et al. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin alpha5beta1. Cell Microbiol. (1999) 1:101–17. 10.1046/j.1462-5822.1999.00011.x [DOI] [PubMed] [Google Scholar]
- 45.Brunner M, Millon-Frémillon A, Chevalier G, Nakchbandi IA, Mosher D, Block MR, et al. Osteoblast mineralization requires beta1 integrin/ICAP-1-dependent fibronectin deposition. J Cell Biol. (2011) 194:307–22. 10.1083/jcb.201007108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maali Y, Martins-Simões P, Valour F, Bouvard D, Rasigade J-P, Bes M, et al. Pathophysiological mechanisms of Staphylococcus Non-aureus bone and joint infection: interspecies homogeneity and specific behavior of S. pseudintermedius. Front Microbiol. (2016) 7:1063. 10.3389/fmicb.2016.01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed S, Meghji S, Williams RJ, Henderson B, Brock JH, Nair SP. Staphylococcus aureus fibronectin binding proteins are essential for internalization by osteoblasts but do not account for differences in intracellular levels of bacteria. Infect Immun. (2001) 69:2872–7. 10.1128/IAI.69.5.2872-2877.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Graevenitz A, Frommelt L, Pünter-Streit V, Funke G. Diversity of coryneforms found in infections following prosthetic joint insertion and open fractures. Infection. (1998) 26:36–8. 10.1007/BF02768750 [DOI] [PubMed] [Google Scholar]
- 49.Roux V, Drancourt M, Stein A, Riegel P, Raoult D, La Scola B. Corynebacterium species isolated from bone and joint infections identified by 16S rRNA gene sequence analysis. J Clin Microbiol. (2004) 42:2231–3. 10.1128/JCM.42.5.2231-2233.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Betsch BY, Eggli S, Siebenrock KA, Täuber MG, Mühlemann K. Treatment of joint prosthesis infection in accordance with current recommendations improves outcome. Clin Infect Dis. (2008) 46:1221–6. 10.1086/529436 [DOI] [PubMed] [Google Scholar]
- 51.Bouaziz A, Uçkay I, Lustig S, Boibieux A, Lew D, Hoffmeyer P, et al. Non-compliance with IDSA guidelines for patients presenting with methicillin-susceptible Staphylococcus aureus prosthetic joint infection is a risk factor for treatment failure. Med Mal Infect. (2018) 48:207–11. 10.1016/j.medmal.2017.09.016 [DOI] [PubMed] [Google Scholar]
- 52.Roux S, Valour F, Karsenty J, Gagnieu M-C, Perpoint T, Lustig S, et al. Daptomycin > 6 mg/kg/day as salvage therapy in patients with complex bone and joint infection: cohort study in a regional reference center. BMC Infect Dis. (2016) 16:83. 10.1186/s12879-016-1420-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skiest DJ. Treatment failure resulting from resistance of Staphylococcus aureus to daptomycin. J Clin Microbiol. (2006) 44:655–6. 10.1128/JCM.44.2.655-656.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hinic V, Lang C, Weisser M, Straub C, Frei R, Goldenberger D. Corynebacterium tuberculostearicum: a potentially misidentified and multiresistant Corynebacterium species isolated from clinical specimens. J Clin Microbiol. (2012) 50:2561–7. 10.1128/JCM.00386-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schoen C, Unzicker C, Stuhler G, Elias J, Einsele H, Grigoleit GU, et al. Life-threatening infection caused by daptomycin-resistant Corynebacterium jeikeium in a neutropenic patient. J Clin Microbiol. (2009) 47:2328–31. 10.1128/JCM.00457-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wimmer MD, Friedrich MJ, Randau TM, Ploeger MM, Schmolders J, Strauss AA, et al. Polymicrobial infections reduce the cure rate in prosthetic joint infections: outcome analysis with two-stage exchange and follow-up ≥two years. Int Orthop. (2016) 40:1367–73. 10.1007/s00264-015-2871-y [DOI] [PubMed] [Google Scholar]
- 57.Marculescu CE, Cantey JR. Polymicrobial prosthetic joint infections: risk factors and outcome. Clin Orthop Relat Res. (2008) 466:1397–404. 10.1007/s11999-008-0230-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peel TN, Cheng AC, Lorenzo YP, Kong DCM, Buising KL, Choong PFM. Factors influencing the cost of prosthetic joint infection treatment. J Hosp Infect. (2013) 85:213–9. 10.1016/j.jhin.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 59.Wright JA, Nair SP. Interaction of staphylococci with bone. Int J Med Microbiol. (2010) 300:193–204. 10.1016/j.ijmm.2009.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacqueline C, Caillon J. Impact of bacterial biofilm on the treatment of prosthetic joint infections. J Antimicrob Chemother. (2014) 69(Suppl. 1):i37–40. 10.1093/jac/dku254 [DOI] [PubMed] [Google Scholar]
- 61.Dupieux C, Trouillet-Assant S, Camus C, Abad L, Bes M, Benito Y, et al. Intraosteoblastic activity of daptomycin in combination with oxacillin and ceftaroline against MSSA and MRSA. J Antimicrob Chemother. (2017) 72:3353–6. 10.1093/jac/dkx314 [DOI] [PubMed] [Google Scholar]
- 62.Becker SC, Roach DR, Chauhan VS, Shen Y, Foster-Frey J, Powell AM, et al. Triple-acting lytic enzyme treatment of drug-resistant and intracellular Staphylococcus aureus. Sci Rep. (2016) 6:25063. 10.1038/srep25063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin L, Sakai Y, Bao R, Xie H, Masunaga K, Miura M, et al. Characteristics of multidrug-resistant Corynebacterium spp. Isolated from blood cultures of hospitalized patients in Japan. Jpn J Infect Dis. (2017) 70:152–7. 10.7883/yoken.JJID.2015.530 [DOI] [PubMed] [Google Scholar]
- 64.Souza C de, Faria YV, Sant'Anna L de O, Viana VG, Seabra SH, Souza MC de, et al. Biofilm production by multiresistant Corynebacterium striatum associated with nosocomial outbreak. Mem Inst Oswaldo Cruz. (2015) 110:242–8. 10.1590/0074-02760140373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang SJ, Choi S-M, Choi J-A, Choi JU, Oh T-H, Kim SE, et al. Factors affecting the clinical relevance of Corynebacterium striatum isolated from blood cultures. PLoS ONE. (2018) 13:e0199454. 10.1371/journal.pone.0199454 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of Corynebacterium species regarding their ability to invade MG63 human osteoblasts (A) and to form mature biofilm (B).
Clinical characteristics of the eleven patients with monomicrobial Corynebacterium spp. BJI.
Description of patients according to the BJI types, and comparison of osteosynthesis device-related and prosthetic joint infection.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.