Abstract
Vascular ATP-sensitive K+ (KATP) channels support skeletal muscle blood flow and microvascular oxygen delivery-to-utilization matching during exercise. However, oral sulphonylurea treatment for diabetes inhibits pancreatic KATP channels to enhance insulin release. Herein we tested the hypotheses that: i) systemic KATP channel inhibition via glibenclamide (GLI; 10 mg kg−1 i.p.) would decrease cardiac output at rest (echocardiography), maximal aerobic capacity and the speed–duration relationship (i.e. lower critical speed (CS)) during treadmill running; and ii) local KATP channel inhibition (5 mg kg−1 GLI superfusion) would decrease blood flow (15 μm microspheres), interstitial space oxygen pressures (PO2is; phosphorescence quenching) and convective and diffusive O2 transport ( and DO2, respectively; Fick Principle and Law of Diffusion) in contracting fast-twitch oxidative mixed gastrocnemius muscle (MG: 9% type I+IIa fibres). At rest, GLI slowed left ventricular relaxation (2.11 ± 0.59 vs. 1.70 ± 0.23 cm s−1) and decreased heart rate (321 ± 23 vs. 304 ± 22 bpm, both P < 0.05) while cardiac output remained unaltered (219 ± 64 vs. 197 ± 39 ml min−1, P > 0.05). During exercise, GLI reduced (71.5 ± 3.1 vs. 67.9 ± 4.8 ml kg−1 min−1) and CS (35.9 ± 2.4 vs. 31.9 ± 3.1 m min−1, both P < 0.05). Local KATP channel inhibition decreased MG blood flow (52 ± 25 vs. 34 ± 13 ml min−1 100 g tissue−1) and PO2isnadir (5.9 ± 0.9 vs. 4.7 ± 1.1 mmHg) during twitch contractions. Furthermore, MG was reduced via impaired and DO2 (P < 0.05 for each). Collectively, these data support that vascular KATP channels help sustain submaximal exercise tolerance in healthy rats. For patients taking sulfonylureas, KATP channel inhibition may exacerbate exercise intolerance.
Keywords: exercise tolerance, gastrocnemius, glibenclamide, interstitial space oxygen pressure
Introduction
Sulphonylureas are the most popular second-line anti-diabetic drug prescribed to patients with Type 2 diabetes mellitus (T2DM, Montvida et al. 2018), enhancing insulin release from pancreatic beta cells by inhibition of ATP-sensitive potassium (KATP) channels. This is true irrespective of the increased risk of adverse cardiovascular events (Simpson et al. 2006, 2015; Abdelmoneim et al. 2016), developing heart failure (HF; McAlister et al. 2008; Kristiansen et al. 2011) and all-cause mortality (Simpson et al. 2015). KATP channels are metabolic sensors that are also present in neural, vascular (endothelial) and muscle (smooth, cardiac and skeletal) tissue, contributing significantly to the hyperpolarization of membrane potentials via K+ efflux and subsequent reductions in calcium ion influx. Attention to this category of K+ channels, and their physiological significance in metabolic control during exercise, has increased with the use of genetic knockout/down models (Flagg et al. 2010). However, the use of genetically altered animal models may result in unknown/unrelated systemic modifications and confound the translatability of KATP channel function to humans (Kane et al. 2004).
Current data from animal and human studies modulating KATP channel function with inhibitors (i.e. glibenclamide (GLI), tolbutamide) and activators (pinacidil) suggest that normal KATP channel function plays a significant role in limiting myocardial damage following ischaemic events in sedentary and exercise-trained animals (cardiac; Brown et al. 2005a,b), enhance reactive and functional hyperaemia to skeletal muscle (vascular; Banitt et al. 1996; Bijlstra et al. 1996; Saito et al. 1996; Hammer et al. 2001; Keller et al. 2004; Lu et al. 2013; Holdsworth et al. 2015; but not all, Farouque & Meredith, 2003), and reduce skeletal muscle tension between contractions (myocyte; Gong et al. 2000; Matar et al. 2000). Whereas systemic administration of GLI has been shown to decrease exercising limb blood flow (Keller et al. 2004; Holdsworth et al. 2015) and maximal aerobic capacity (; Lu et al. 2013), it remains unknown whether these cardiovascular impairments are mediated through reductions in cardiac function, vascular function within skeletal muscle, or both. As adequate energy production via oxidative metabolism dictates contractile function during fatiguing activity and depends on heterogeneous oxygen transport within muscle (Wilson et al. 1977; Hogan et al. 1992; Richardson et al. 1998), maximal oxygen uptake ( difference) relies on a prodigious increase in cardiac output combined with a highly effective red blood cell distribution and O2 extraction within active skeletal muscle (arterial–venous O2 content) (reviewed by Laughlin et al. 2012; Poole & Jones, 2012). Notwithstanding the importance of , the ability to sustain high-intensity exercise and daily physical tasks are more appropriately determined via a submaximal threshold (i.e. critical speed (CS) or critical power) where oxidative metabolism meets metabolic demand below this threshold but, above this threshold, increases infast-twitch fibre recruitment, fatigue-related metabolite production, and O2 consumption leading to and task failure (Monod & Scherrer, 1965; Poole et al. 1988, 2016; Jones et al. 2008; Copp et al. 2010). Importantly, it remains unknown how vascular KATP channels contribute to O2 transport within highly oxidative fast-twitch muscles and their role in supporting fatiguing exercise, especially as the proportional contribution of these channels to the overall vascular response may increase in disease (Holdsworth et al. 2017).
Therefore the current investigation was designed to assess the effect of systemic KATP channel inhibition via GLI on: i) resting cardiac function; ii) maximal aerobic capacity ; and iii) submaximal exercise tolerance (CS). Local KATP channel inhibition via GLI superfusion was used to assess: iv) skeletal muscle blood flow ; and v) interstitial space O2 pressures (PO2is; established by O2 delivery-to-utilization matching immediately proximal to myocytes) within contracting fast-twitch muscle of high oxidative capacity. Incorporating the Fick principle and law of diffusion direct measurements were used to estimate convective and diffusive (DO2) O2 conductances within microvascular and interstitial compartments where the convergence of and DO2 establish (Wagner, 1992, 1996). Considering that vascular function and exercise assessments of KATP channels have been performed in male rats (Lu et al. 2013; Holdsworth et al. 2015, 2016, 2017) when females may be more adversely affected by sulphonylurea treatment (Brown et al. 2005b; Johnson et al. 2006), the current investigation sought to bridge the translatability of KATP channel function to females. It was hypothesized that KATP channel inhibition would impair resting cardiac output and decrease and CS. It was further hypothesized that local KATP channel inhibition would reduce skeletal muscle blood flow and PO2is during twitch contractions, and slow the recovery of PO2is following contractions, effectively decreasing by impairing O2 conductance ( and DO2). Data in support of these hypotheses would reveal a heretofore under-appreciated peripheral vascular role for KATP channels in the maintenance of O2 delivery and contractile function.
Methods
Ethical approval
All protocols and procedures were approved by the Institutional Animal Care and Use Committee of Kansas State University and conducted according to the guidelines and ethical standards put forth by the National Institutes of Health and Journal of Physiology (Grundy, 2015). Ten female Sprague–Dawley rats (~8 months old during terminal experiments) were maintained in animal facilities accredited by the Association for the Assessment and Accreditation of Laboratory and Animal Care on a 12:12 h light:dark cycle with food and water provided ad libitum. Vaginal lavages were conducted for a minimum of 10 days to monitor menstrual cycles (Marcondes et al. 2002; Smith et al. 2017) with all testing performed during the pro-oestrus phase. In the initial 14–21 days, while menstrual cycles were monitored, acclimation to running was conducted on a custom-built treadmill for ~5 min day−1 at ~25 m min−1 up a 5% incline. During the final acclimation days the treadmill speed was increased progressively in the last 2–3 min up to 50–60 m min−1 to familiarize the rats with high-speed running (Copp et al. 2010; Craig et al. 2019a, Poole et al. 2020). Importantly, these brief duration acclimation runs do not elicit training adaptations (Dudley et al. 1982; Armstrong & Laughlin, 1984; Musch et al. 1992).
Drug dosing
KATP channel inhibition was administered via the pharmacological sulphonylurea derivative glibenclamide (GLI: 494 g mol−1, 5-chloro-N-{4-[N-(cyclohexylcarbamoyl)sulfamoyl]phenthyl}−2-methoxybenzamide, Sigma-Aldrich, St. Louis, MO). For acute systemic inhibition via an intraperitoneal injection on experimental days, a 10 ml stock solution was made by GLI dissolved in 9 ml saline (0.9% NaCl), 900 μl NaOH (0.1 m), and 100 μl DMSO and briefly sonicated. The amount of GLI dissolved in solution was determined on experimental days to obtain a final 1 ml dose of 10 mg kg−1 (Lu et al. 2013). For local inhibition via superfusion, the stock solution utilized distilled water in place of saline and GLI was dissolved to obtain a final 5 mg kg−1 dose, with 0.5 ml GLI stock solution diluted in 2.5 ml of warmed Krebs–Hensleit bicarbonate-buffered solution equilibrated with 5% CO2–95% N2 (pH 7.4; in mm, 4.7 KCl, 2.0 CaCl2, 2.4 MgSO4, 131 NaCl and 22 NaHCO3).
GLI injections (10 mg kg−1, i.p.) occurred ~30–60 min prior to echocardiographic assessment and treadmill exercise testing ( and CS; Lu et al. 2013) to align with peak plasma concentration (i.e. ~60–85 min after oral administration of 10 mg kg−1 GLI, Li et al. 2012). Thus, each rat underwent at least six GLI injections over ~7–8 weeks. During interstitial PO2 measurements, inhibition was administered locally via GLI superfusion (5 mg kg−1 in Krebs–Hensleit solution, Holdsworth et al. 2017).
Echocardiography determination of left ventricular function
Transthoracic echocardiography was performed with a commercially available system (Logiq S8; GE Health Care, Milwaukee, WI) using an 18 MHz linear transducer (L8–18i). Rats were anaesthetized initially on a 5% isoflurane–O2 mixture and then maintained on a 1.5–2% isoflurane–O2 mixture while positioned supine on a heating pad (42°C) to maintain core temperature. Standard two-dimensional and M-mode images were obtained from the midpapillary level with frame rates >50 frames s−1. Ventricular dimensions were obtained from M-mode measurements over four consecutive cardiac cycles. Left ventricular (LV) internal dimensions were measured at end diastole (LVIDd) and end systole (LVIDs). Fractional shortening (FS) was calculated from LV chamber diameters: FS = [(LVIDd – LVIDs)/LVIDd] × 100. Left end-systolic (LVESV) and end-diastolic (LVEDV) volumes were estimated using the Teichholz formula: LV volume = [7.0/(2.4 + LV dimension)] × LV dimension3. Stroke volume (SV) was calculated as: SV = LVEDV–LVESV. Ejection fraction (EF) was calculated using LV volume measurements: EF = [(LVEDV–LVESV)/LVEDV] × 100. Rates of contraction (+V) and relaxation (−V) of the posterior LV wall were also measured in M-mode by integrating the slope from end-diastolic and end-systolic internal diameter locations used for assessing LVIDd and LVIDs. Heart rate (HR) was estimated using the average contraction and relaxation times across the four cardiac cycles: HR = 60/(contraction time + relaxation time). Cardiac output (CO) was calculated using HR and SV values: CO = HR × SV.
Determination of maximal oxygen uptake and critical speed
Maximal oxygen uptake tests were performed in a plexiglass metabolic chamber placed on the treadmill (Musch et al. 1988) and connected to O2 (model S-3A/I) and CO2 (model CD-3A; AEI Technologies; Pittsburg, PA) analysers. Gas measurements were performed in real time and recorded in the final 5–10 s of each stage. Treadmill speed was initially set to 25 m min−1 for 2 min, increased to 40 m min−1 for an additional 2 min, and then increased progressively ~5 m min−1 each minute until the rat was unable to maintain pace with the treadmill or no further increases in were recorded despite increases in speed. High reproducibility of measurements has been established previously in our laboratory (Copp et al. 2009).
Following testing, the speed–duration relationship was determined via the multiple constant-speed method (Copp et al. 2010; Craig et al. 2019a). Critical speed tests consisted of five runs-to-exhaustion at predetermined speeds estimated to elicit exhaustion between 2 and 20 min. Each test began with a 2 min warm-up at 20 m min−1, followed by 1 min of quiet rest, and then rapid increase in treadmill speed (<10 s) toward the target speed to be maintained for the duration of the test. Timing began when the investigator adjusting treadmill speed verified the attainment of the target speed. When rats drifted toward the back of the running lane a separate investigator provided encouragement via manual bursts of air toward the hindlimbs. Tests were terminated immediately when rats were unable to keep up with the treadmill speed despite apparent exertion and encouragement. The termination of all tests was determined by the same investigators who were blinded to the overall exercise time. Successful runs-to-exhaustion were verified by the absence of a righting reflex (i.e. unwilling/unable to right themselves within 2 s of being placed on their backs). The initial run was set at 60 m min−1 and subsequent speeds were selected at ~5 m min−1 increments to obtain the appropriate range of run durations (i.e. 2–20 min). When successful constant-speed tests were completed, the speed–duration parameters were determined by: 1) the hyperbolic speed–time model (time = D′/(speed - CS), where the asymptote of this curve is CS and the curvature constant is D′; and 2) the linear 1/time model (speed = D′ × 1/time + CS), where speed is plotted as a function of the inverse of time (s) to exhaustion, D′ is the slope, and CS is the intercept of the regression line (Copp et al. 2010, 2013; Poole et al. 2016). To mitigate any potential influence of training (increased CS) or weight gain (decreased CS) on the speed–duration relationship, the slowest of the constant-speed runs were performed early (i.e. run 2–4) under control conditions and the final runs overall consisted of control and GLI runs at the slowest speeds. Preliminary data showed that times-to-exhaustion of the slowest speed under control conditions, and thus CS, were either maintained or decreased compared with the initial slowest run. Therefore the shorter of the two was used to model the speed–duration relationship.
Phosphorescence quenching determination of PO2is
On the final day of experimentation, rats were anaesthetized initially on a 5% isoflurane–O2 mixture and maintained on 2–2.5% isoflurane–O2 mixture for the duration of carotid and caudal (tail) artery catheterizations and surgical exposure of hindlimb muscles. Rats were placed on a heating pad to maintain core temperature at ~37–38°C, measured via rectal thermometer. Following a midline incision of the skin covering the neck, the right carotid artery was isolated and cannulated (PE-10 connected to PE-50; Intra-Medic polyethylene tubing; BD, Franklin Lakes, NJ, USA) for continuous measurements of mean arterial pressure (MAP) and HR, and infusion of fluorescent-labelled microspheres for blood flow measurements (see Fluorescent microsphere assessment of blood flow). The caudal artery was cannulated for infusion of pentobarbital sodium anaesthesia and blood sampling (i.e. blood gases and blood flow reference sample). Arterial blood samples were collected following the final contraction protocol for determination of O2 saturation, systemic haematocrit and plasma lactate (Nova Stat Profile M; Nova Biomedical, Waltham, MA, USA).
Following catheterization, an incision was made above the lateral malleolus of the left hindlimb and the overlaying skin and fascia reflected to expose the biceps femoris. Upon tying off the lateral great saphenous artery (6–0 silk suture) the distal portion of the biceps femoris was reflected to expose the mixed gastrocnemius (MG). The MG muscle was selected for its fast-twitch fibre composition (97% type IIA+IID/X+IIB), oxidative capacity (citrate synthase: ~25 μmol min−1 g−1; Armstrong & Phelps, 1984; Delp & Duan, 1996), and most importantly its recruitment at and above the fatigue threshold (i.e. CS; Copp et al. 2010). The MG was left attached to its anatomical origin and insertion while variations in muscle length were minimized throughout the experimental protocol with knee and ankle joints stabilized ~90° angles. Rats were then progressively transitioned off isoflurane and onto pentobarbital sodium anaesthesia (50 mg ml−1) with the depth of anaesthesia continuously monitored via toe pinch and corneal sensitivity reflexes, and additional anaesthesia provided as necessary (0.03–0.05 ml of 50 mg ml−1 diluted to 0.3 ml of heparinized saline). Platinum iridium electrodes were attached (6–0 silk suture) to the proximal (cathode) and distal (anode) regions of the muscles to produce electrically induced muscle contractions. Surrounding exposed tissue was covered with Saran Wrap (Dow Brands, Indianapolis, IN) to reduce tissue dehydration and exposure to superfused solutions. Exposed muscle was superfused regularly with warmed Krebs–Henseleit bicarbonate-buffered solution equilibrated with 5% CO2–95% N2.
Experimental protocol.
Two separate contraction bouts were performed on the MG under control (Krebs–Heneseleit) and KATP channel inhibition (5 mg kg−1 GLI in Krebs–Henseleit) superfused conditions. GLI superfusion was performed second, due to the long half-life of GLI, and with >20 min of recovery between contraction bouts to prevent any potential priming effect of repeated contraction bouts on PO2is profiles. Interstitial space PO2 (PO2is) was measured via phosphorescence quenching at rest and during 180 s twitch contractions (1 Hz, 7 V, 2 ms pulse duration; Grass stimulator model S88, Quincy, MA) and recorded at 2 s intervals (Craig et al. 2018, 2019a,b, Hirai et al. 2018a). Recovery PO2is was measured for an additional 240 s to ensure that PO2is returned and stabilized at baseline prior to subsequent GLI superfusion and contractions. With PO2is measured continuously, GLI was superfused (3 ml total volume) onto the MG for 180 s and allowed an additional 180 s before the same contraction protocol was repeated (i.e. total of >23 min elapsed between contraction bouts).
Measurement of interstitial PO2.
A frequency domain phosphorometer (PMOD 5000; Oxygen Enterprises, Philadelphia, PA) was used to measure PO2is as described previously (Craig et al. 2018, 2019a,b, Hirai et al. 2018a). The Oxyphor G4 (Pd-meso-tetra-(3,5-dicarboxyphenyl)-tetrabenzoporphyrin) was injected locally (2–4 10 μl injections at 10 μm concentration) with a 29 gauge needle, with care taken to avoid any visible vasculature. Following injection, the muscle was covered in Saran Wrap and allowed >20 min to allow the G4 to thoroughly diffuse throughout the interstitial space. This oxyphor is well suited for use in biological tissues because it does not cross membranes and is stable across the physiological pH range (Esipova et al. 2011). Muscle surface temperature was measured via non-contact infrared thermometer, since this oxyphor is temperature sensitive. The exposed MG temperatures were 31.6 ± 0.2°C. Previous studies have shown that the present twitch contraction protocol does not significantly change muscle temperature (Craig et al. 2018, 2019a).
Phosphorescence quenching applies the Stern–Volmer relationship (Rumsey et al. 1988; Esipova et al. 2011) describing the quantitative O2 dependence of the phosphorescent probe G4 via the equation PO2is=[(τ0/τ) – 1]/(kQ·τ0), where kQ is the quenching constant and τ and τ0 are the phosphorescence lifetimes at the ambient O2 concentration and in the absence of O2, respectively. For G4 in tissue at ~32°C, kQ is ~258 mmHg/s and τ0 is ~226 μs (Esipova et al. 2011). Because muscle temperature does not change appreciably throughout the contraction protocol used herein (Craig et al. 2018, 2019a), the phosphorescence lifetime is determined exclusively by the O2 partial pressure. Following G4 injection, the common end of the bifurcated light guide was positioned 3–4 mm above the exposed muscle surface. All PO2is measurements were performed in a dark room to minimize extraneous exposure to light.
Analysis of interstitial PO2 kinetics.
Contracting PO2is responses were analysed using 30 s of resting data and the 180 s contraction bouts using a monoexponential plus time delay model (one component) or a monoexponential plus time delay with a secondary component (two component) model when necessary,
One component
Two component
where PO2is t represents the PO2is at any point in time, PO2is BL is the baseline before the onset of contractions, Δ1PO2is and Δ2PO2is are the primary and secondary amplitudes, TD and TD2 are the time delays before the fall and secondary rise in PO2is, and τ and τ2 are the time constants (i.e. the time required to reach 63% of the amplitude) for the primary and secondary amplitudes. The mean response time (MRT) was calculated as the sum of the model-derived TD and τ. When the secondary component model was necessary, the primary amplitude was constrained to the nadir value in order to maximize the accuracy of the primary response kinetics (Craig et al. 2018, 2019a,b). The goodness of model fit was determined using the following criteria: 1) coefficient of determination, 2) sum of the squared residuals, and 3) visual inspection and analysis of the model fits to the data and the residuals. Because Δ2PO2is (i.e. undershoot of PO2is; Δ2PO2is = PO2is end – PO2is nadir) was often non-exponential in nature, Δ2PO2is was determined manually by calculating the difference between the PO2is at the end of contractions (PO2is end, average of 172–180 s) minus the nadir value of PO2is during contractions (PO2is nadir = PO2is BL – Δ1PO2is). Rate of PO2is recovery was calculated in eight rats as the time taken to reach 63% of the overall response (i.e. T63) between PO2is end and recovery PO2is (average of 232–240 s).
Fluorescent microsphere determination of blood flow
The microsphere technique was used to determine MG blood flow as described previously (Musch et al. 1986; Van Oosterhout et al. 1998; Deveci & Egginton, 1999). Two fluorescent microspheres (blue-green (430/465 nm) and red (580/605 nm), Invitrogen FluoSpheres polystyrene microspheres, ThermoFisher Scientific) were injected in random order at the end of MG and MG GLI contractions. Following 180 s contractions, blood withdrawal from the tail catheter was initiated at 0.25 ml min−1 while 0.25–0.30 × 106 15.5 μm diameter fluorescent microspheres were injected into the aortic arch via the carotid artery catheter. Muscle contractions and blood withdrawal were terminated 30 s after the microsphere injection. Following the final contraction protocol, rats were killed via pentobarbital sodium overdose (>100 mg kg−1 i.a.), proper catheter placement in the aortic arch was confirmed, and tissues (left and right kidneys, left and right mixed gastrocnemei) dissected and stored (−80°C) for later analyses. For the final analyses, kidney and muscle tissues were weighed and placed directly in 15 ml screw cap polypropylene tubes with a conical base. Five ml of 2 M KOH in 99% ethanol with 0.5% Tween-80 were added to the tubes, vortexed, and placed in a dry heating block (60°C) with intermittent vortexing until tissue digestion was complete. Tubes were then centrifuged at 3000 rpm (1500 g) for 15 min. Supernatant was carefully aspirated until <500 μl remained to minimize the possibility of accidental microsphere loss. One ml of deionized H2O was added and tubes quickly vortexed to resuspend the remaining pellet, followed by the addition of 9 ml ethanoic Tween-80, vortexing and another 15 min of centrifuging. Tubes were aspirated as previously described, before 5 ml of 100 mm phosphate buffer (pH 7.0) was added to neutralize the pellet and solution, followed by 4 ml of absolute ethanol. The tubes were further vortexed, centrifuged, and aspirated to <300 μl. To ensure complete resuspension of microspheres, the tubes were vortexed again before being placed in an oven (60°C) to evaporate to 100–150 μl. To improve solvent extraction from the microspheres, which would be less efficient in a dry pellet, the tubes were periodically removed from the oven and vortexed. To dissolve the polystyrene microspheres and release the fluorescent dye, 2 ml of solvent (di(ethylene glycol) ethyl ether acetate, 98%; Sigma-Aldrich Corporation, St. Louis, MO, USA) was added and vortexed several times over 3–5 min and left for 30 min before being sonicated (5 min) in a water bath to ensure complete dye extraction. Once the solvent was added, all remaining steps were conducted in dim lighting to prevent signal decay prior to fluorescent intensity measurements. After sonication the tubes were centrifuged once more (10 min) before supernatant was pipetted (300 μl) into 96 well plates in quadruplicates for the measurement of fluorescent intensity (SpectraMax i3 Multi-Mode Platform, Molecular Devices, San Jose, CA). Total tissue blood flows were calculated according to the reference sample method (Ishise et al. 1980; Musch & Terrell, 1992) and expressed mass specifically in ml min−1 100g tissue−1. Adequate mixing of microspheres prior to infusion were determined by <20% difference in left and right kidney and/or muscle blood flows.
Muscle oxygen consumption
The Fick equation was used to calculate microvascular oxygen consumption with the assumptions that microvascular PO2 (PO2mv) can be calculated from interstitial measurements (i.e. PO2mv = PO2is + transcapillary PO2; Colburn et al. 2020a) and is an appropriate analogue for venous PO2 (McDonough et al. 2001) and, by extension from the O2 dissociation curve, venous blood O2 content (Roca et al. 1992). Therefore mixed venous O2 content (CvO2) was calculated from PO2mv using the rat O2 dissociation curve (constructed using the Hill coefficient (n) of 2.6, the measured [Hb], P50 (the PO2 at which Hb is 50% saturated with O2) of 38 mmHg, and an O2-carrying capacity of 1.34 ml O2 (gHb)−1). Arterial O2 content (CaO2) was measured directly via arterial blood sample and, when combined with blood flow and CvO2 values, was used to calculate via the principle of mass balance using the Fick Equation (i.e. ). Microvascular O2 diffusion conductance (DO2mv) was defined as which provides an index of diffusive O2 transport per unit of driving pressure. Interstitial O2 diffusion conductance was assessed utilizing the present PO2is and calculated (i.e. was presumed to be equal to considering the absence of storage for O2 in the interstitial fluid and, thus, O2 leaving the microvascular compartment must equal O2 leaving the interstitial compartment).
Statistical analyses
The effect of systemic GLI on resting LV function, exercise parameters (, CS and D′), and local GLI superfusion on contracting MG measurements (MAP, HR, PO2is kinetics parameters, , microvascular and and microvascular and interstitial DO2) were assessed using two-tail paired t tests. PO2is profiles were assessed via two-way repeated measure ANOVA (Time × Drug) with Tukey’s post hoc analyses. Data are presented as means ± SD. Significance was accepted at P < 0.05.
Results
Two rats were unwilling to complete all runs needed to assess the speed–duration relationship; therefore, comparisons between control and KATP channel inhibition were conducted on eight rats.
Resting echocardiography
Left ventricular echocardiographic measurements are presented in Table 1. Compared with control, GLI did not alter LVEDV (0.83 ± 0.24 vs. 0.83 ± 0.18, P = 0.976) nor LVESV (0.15 ± 0.07 vs. 0.19 ± 0.09 ml, P = 0.172) and thus stroke volume remained unchanged (0.68 ± 0.19 vs. 0.65 ± 0.11 ml, P = 0.354). LV fractional shortening (47 ± 6 vs. 42 ± 5, P = 0.084) and EF (83 ± 5 vs. 78 ± 6%, P = 0.088) were also not significantly altered. However, the rate of LV relaxation (2.11 ± 0.59 vs. 1.70 ± 0.23, P = 0.048), but not contraction (2.76 ± 0.49 vs. 2.44 ± 0.43 cm s−1, P = 0.079), was significantly slowed resulting in a decreased HR (321 ± 23 vs. 304 ± 22 bpm, P = 0.043) during maintained cardiac output (219 ± 64 vs. 197 ± 39 ml min−1, P = 0.105).
Table 1.
Doppler echocardiographic assessment of left ventricular function during control and systemic KATP channel inhibition
| Control | Glibenclamide | |
|---|---|---|
| LVIDd (cm) | 0.71 ± 0.08 | 0.72 ± 0.05 |
| LVIDs (cm) | 0.38 ± 0.08 | 0.41 ± 0.06 |
| FS (%) | 47 ± 6 | 42 ± 5 |
| LVEDV (ml) | 0.83 ± 0.24 | 0.83 ± 0.18 |
| LVESV (ml) | 0.15 ± 0.07 | 0.19 ± 0.09 |
| SV (ml) | 0.68 ± 0.19 | 0.65 ± 0.11 |
| EF (%) | 83 ± 5 | 78 ± 6 |
| +V (cm s−1) | 2.76 ± 0.49 | 2.44 ± 0.43 |
| −V (cm s−1) | 2.11 ± 0.59 | 1.70 ± 0.23 * |
| HR (bpm) | 321 ± 23 | 304 ± 22 * |
| CO (ml min−1) | 219 ± 64 | 197 ± 39 |
LVIDd, left ventricular end-diastole internal diameter; LVIDs, left ventricular end-systole internal diameter; FS, fractional shortening; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; SV, stroke volume; EF, ejection fraction; +V, rate of contraction; −V, rate of relaxation; HR, heart rate; CO, cardiac output. Data are means ± SD (n = 10) and compared via two-tail paired t test.
P < 0.05 vs. control.
Maximal aerobic capacity and speed–duration relationship
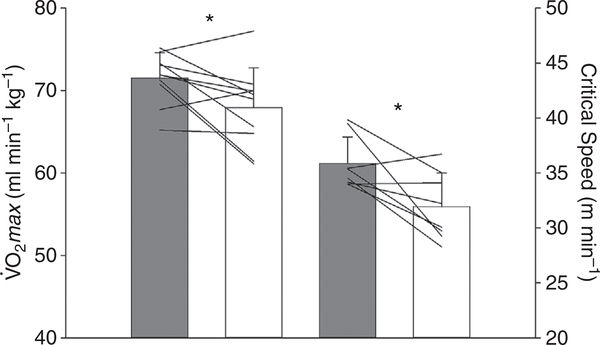
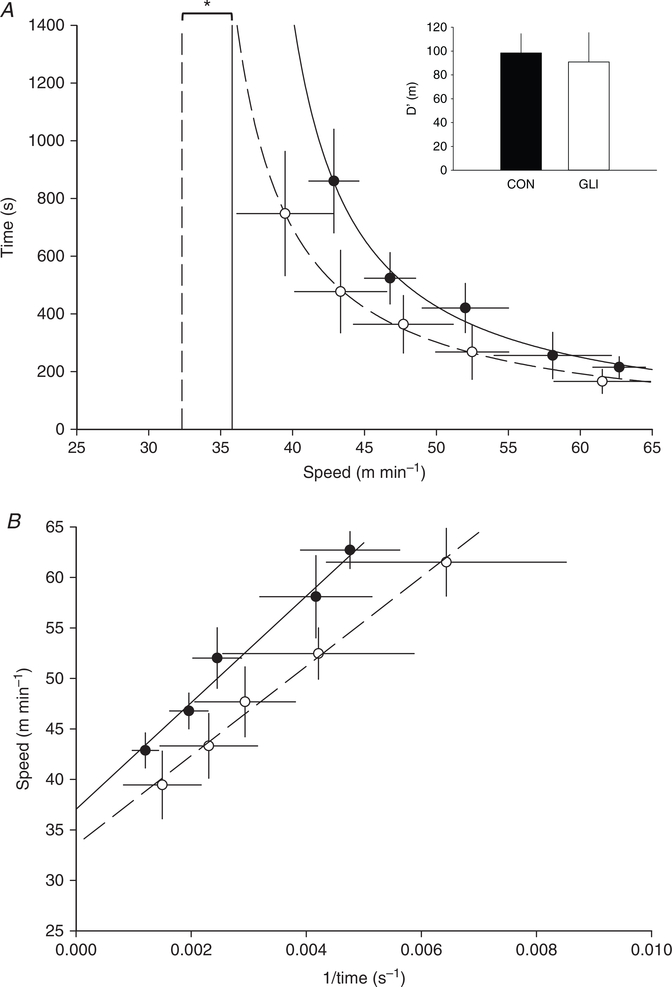
Table 2 and Figs 1 and 2 demonstrate that GLI reduced (71.5 ± 3.1 vs. 67.9 ± 4.8 ml min−1 kg−1, P = 0.034) and CS (35.9 ± 2.4 vs. 31.9 ± 3.1 m min−1, P = 0.020) whereas D′ remained unchanged (98 ± 16 vs. 91 ± 25 m, P = 0.532).
Table 2.
Individual maximal oxygen uptake and speed–duration relationship parameters during control and systemic KATP channel inhibition
| Control |
Glibenclamide |
|||||
|---|---|---|---|---|---|---|
| CS (m min−1) | D′ (m) | CS (m min−1) | D′ (m) | |||
| 1 | 67.7 | 39.5 | 84 | 70.0 | 29.3 | 124 |
| 2 | 71.3 | 34.2 | 127 | 61.4 | 32.2 | 79 |
| 3 | 75.2 | 34.5 | 93 | 69.5 | 28.3 | 95 |
| 4 | 65.2 | – | – | 64.8 | – | – |
| 5 | 70.8 | 34.0 | 92 | 61.1 | 34.1 | 83 |
| 6 | 73.3 | 39.8 | 75 | 65.5 | 35.0 | 79 |
| 7 | 74.7 | 35.4 | 101 | 77.2 | 29.7 | 127 |
| 8 | 73.1 | – | – | 70.8 | – | – |
| 9 | 71.9 | 34.0 | 111 | 69.9 | 30.1 | 89 |
| 10 | 71.9 | 35.4 | 105 | 69.0 | 36.7 | 52 |
| Mean ± SD | 71.5 ± 3.1 | 35.9 ± 2.4 | 98 ± 16 | 67.9 ± 4.8 * | 31.9 ± 3.1 * | 91 ± 25 |
, maximal oxygen uptake; CS, critical speed; D′, curvature constant. The speed–duration relationship parameters are presented from the hyperbolic model. Data were compared via two-tail paired t tests.
P < 0.05 vs. control.
Figure 1. Effect of systemic KATP channel inhibition on maximal and submaximal exercise.
Note the significant reduction in maximal oxygen uptake (; n = 10) and critical speed (n = 8) following glibenclamide (open bars) compared with control (grey bars). Data are means ± SD with individual data plotted and compared via two-tail paired t tests.
*P < 0.05 vs. control.
Figure 2. Speed–duration relationship following systemic KATP channel inhibition.
The hyperbolic (A) and 1/time linear (B) speed–duration relationships are modelled under control (closed circle, continuous line) and systemic KATP channel inhibition (GLI; open circle, dashed line) conditions to determine critical speed (vertical lines (A) and y-intercept (B)) and D′ (inset). These mean data fits are for illustrative purposes only, with individually determined critical speed and D′ and subsequent group means presented in Table 2. Data are means ± SD and compared via two-tail paired t tests.
*P < 0.05 vs. control.
Blood sample analysis and central haemodynamics during phosphorescence quenching
Arterial pH (7.39 ± 0.03), O2 saturation (90.7 ± 2.6%), haematocrit (34.9 ± 4.2%) and lactate concentration (1.5 ± 0.5 mm) were assessed following the GLI contraction. GLI superfusion did not alter MAP or HR (both P > 0.346); therefore, MAP (102 ± 10 and 99 ± 10 mmHg, P = 0.096) and HR (361 ± 23 and 364 ± 38 bpm, P = 0.831) were not different at the start of the control and GLI contractions, respectively.
MG blood flow and interstitial PO2
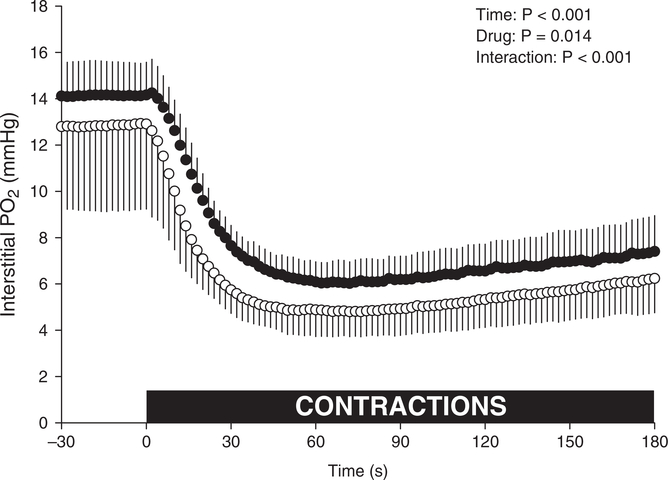
GLI superfusion impaired MG blood flow during contractions (52 ± 25 vs. 34 ± 13 ml min−1 100 g−1, P = 0.015). The effect of GLI superfusion on PO2is during the rest–contraction transient is presented in Table 3 and Fig. 3. GLI reduced MG PO2is BL (−1.1 ± 1.1 mmHg, P = 0.020). At the onset of contractions, there was a shortening in TD (P = 0.005) with a statistically non-significant change in τ (14.1 ± 2.7 vs. 12.2 ± 3.0, P = 0.053) yet a faster overall PO2is fall (MRT: 22.2 ± 4.7 vs. 17.3 ± 4.3 s, P = 0.002) to a lower PO2is nadir (5.9 ± 0.9 vs. 4.7 ± 1.1, P = 0.013) but not a different PO2is end (7.3 ± 1.5 vs. 6.1 ± 1.4 mmHg, P = 0.073) compared with control (see Fig. 3; all 2 s measurements, two-way repeated measures ANOVA with Tukey’s post hoc analyses, P < 0.062). Following contractions, GLI PO2is recovered more slowly (T63: 95 ± 19 vs. 118 ± 20 s, P = 0.047) but to a similar end recovery PO2is (14.6 ± 4.0 vs. 15.3 ± 6.5 mmHg, P = 0.556) during the observed window.
Table 3.
Interstitial PO2 kinetics parameters during 180 s twitch contractions and 240 s recovery during control and local KATP channel inhibition
| Mixed gastrocnemius |
||
|---|---|---|
| Control | Glibenclamide | |
| Pre-superfusion PO2is (mmHg) | – | 13.8 ± 3.4 |
| PO2isBL (mmHg) | 14.1 ± 1.4 | 12.8 ± 3.6 * |
| Δ1PO2is (mmHg) | 8.2 ± 1.5 | 8.1 ± 3.1 |
| TD (s) | 8.0 ± 4.3 | 5.2 ± 3.2 * |
| τ (s) | 14.1 ± 2.7 | 12.2 ± 3.0 |
| MRT (s) | 22.2 ± 4.7 | 17.3 ± 4.3 * |
| PO2isnadir (mmHg) | 5.9 ± 0.9 | 4.7 ± 1.1 * |
| Δ2PO2is (mmHg) | 1.4 ± 0.9 | 1.4 ± 0.8 |
| PO2isend (mmHg) | 7.3 ± 1.5 | 6.1 ± 1.4 |
| Δ1PO2is/τ (mmHg s−1) | 0.60 ± 0.16 | 0.72 ± 0.36 |
| Recovery T63 (s) | 95 ± 19 | 118 ± 20 * |
| Recovery PO2is (mmHg) | 14.6 ± 4.0 | 15.3 ± 6.5 |
| Δ3PO2is/T63 (mmHg s−1) | 0.07 ± 0.03 | 0.08 ± 0.04 |
PO2is BL, resting baseline; PO2is; Δ1PO2is and Δ2PO2is, amplitude of the first and second components, respectively; TD, time delay; τ, time constant; MRT, mean response time; PO2is nadir, lowest response prior to secondary rise in PO2is; PO2is end, PO2is at the end of contractions; Δ1PO2is/τ, rate of PO2is fall; T63, time to reach 63% of final response; Δ3PO2is/T63, rate of PO2is recovery. Data are means ± SD and compared via two-tail paired t tests.
P < 0.05 vs. control.
Figure 3. Interstitial PO2 of fast-twitch oxidative muscle following local KATP channel inhibition.
Note the difference in mixed gastrocnemius PO2is following glibenclamide (GLI, open symbols, n = 10) superfusion compared with control (closed symbols). Dashed line denotes the onset of twitch contractions at time zero. Data are means ± SD and compared via two-way (Time × Drug) repeated measure ANOVA with Tukey’s post hoc analyses.
MG muscle oxygen delivery, consumption and diffusive conductance
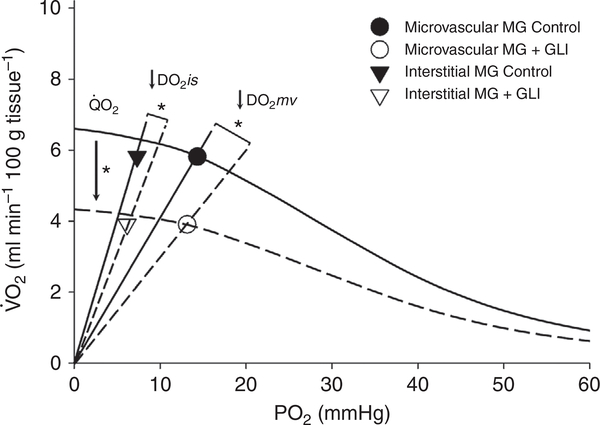
Compared with control, Fig. 4 illustrates that GLI decreased MG oxygen delivery (: 6.4 ± 3.2 vs. 4.2 ± 1.7 ml O2 min−1 100 g−1, P = 0.015) and oxygen consumption from the microvascular compartment (: 5.8 ± 2.9 vs. 3.9 ± 1.6 ml O2 min−1 100 g−1, P = 0.016). Consequently, microvascular (DO2mv: 0.40 ± 0.20 vs. 0.30 ± 0.11, P = 0.023) and interstitial diffusive conductances (DO2is: 0.80 ± 0.38 vs. 0.66 ± 0.25 ml O2 min−1 mmHg−1 100 g−1, P = 0.040) were significantly reduced. Furthermore, DO2is was significantly greater than DO2mv (P = 0.0002 and 0.0001, control and GLI, respectively), with the reduction in DO2mv following GLI (−22 ± 20%) trending towards being proportionally greater than the reduction in DO2is (−15 ± 17%; P = 0.070).
Figure 4. The effect of local KATP channel inhibition on the convective and diffusive determinants of oxygen transport.
Graphical representation of the relationship between convective ( difference; curved line) and diffusive (; slope from origin) determinants of oxygen transport in the microvascular (circles) and interstitial (down triangles) compartments of the fast-twitch oxidative mixed gastrocnemius (MG, n = 10) muscle following vascular KATP channel inhibition via glibenclamide (GLI, open symbols). Importantly, unlike haemoglobin–O2 transport in the microvasculature, the lack of haeme–O2 storage in interstitial fluid dictates that interstitial must equal microvascular allowing DO2is to be assessed with the present data . Note the reductions in both convective (QO2mv, curved lines y-intercept) and diffusive (DO2mv and DO2is) components of MG muscle compared with control (closed symbols). *P < 0.05 vs. control with two-tail paired t tests
Discussion
The main original finding of this investigation is that, in female rats, GLI-induced impairment of maximal (i.e. ) and submaximal (i.e. CS) exercise can be attributed, in part, to reductions in peripheral vascular KATP channel function. Systemic GLI administration did not change cardiac output at rest, assessed via Doppler echocardiography. However, the slowed LV relaxation and reduced HR at rest following GLI highlights a peripheral insult to KATP channels that is likely compensated, in part, by baroreflex-mediated changes in left ventricular function. Accordingly, local inhibition of vascular KATP channels during contractions resulted in decreased blood flow and interstitial space O2 delivery-to-utilization matching (i.e. PO2is) of fast-twitch oxidative skeletal muscle and slowed the recovery of PO2is following cessation of contractions. Using the Fick principle and law of diffusion, estimations of MG convective and diffusive conductances ( and DO2, respectively), and thus , were impaired following GLI. Therefore, the exercise intolerance that is symptomatic of patient populations (i.e. diabetes, HF), which have a greater proportion of, and blood flow to, fast-twitch fibres, may be exacerbated by oral sulphonylurea medications impairing vascular KATP channel-mediated vasodilation.
KATP channel function on maximal aerobic capacity and speed–duration relationship
Consistent with our hypothesis, systemic KATP channel inhibition impaired maximal aerobic capacity and submaximal exercise tolerance (CS). Lu and colleagues (2013) previously demonstrated impaired (~54 to 36 ml O2 min−1 kg−1, ~33%) in rats following GLI injections; however, utilizing a comparatively steeper ramp protocol, the current data exhibited higher baseline and a far smaller effect of GLI (~5%, Table 2). This discrepancy likely stems directly from the slower ramp protocol wherein longer durations at submaximal speeds would be expected to enhance glycogen depletion in skeletal muscle leading to exhaustion at lower levels. Supporting the need for steeper ramp protocols, Richardson et al. (1993) demonstrated greater and maximum work rate within 13–15 min of knee extension exercise compared with those previously measured using a slower, longer ramp protocol (~40–60 min; Andersen & Saltin, 1985). Accordingly, GLI-induced reductions in HR in the face of elevated MAP occurred at slower speeds, but not at 60 m min−1 (Holdsworth et al. 2015). Coupled with decreased hindlimb muscle blood flows throughout all speeds, this suggests that vascular control of O2 delivery is impaired especially at submaximal speeds while metabolite build-up at supra-CS speeds may activate group III/IV afferents to increase HR at near-maximal speed/intensity (Holdsworth et al. 2015). This notion is highlighted in the current investigation (Fig. 1) where GLI-induced reductions in CS were greater than those seen in (~12 vs. ~5%, respectively).
KATP channel function on cardiac function
Assessed via Doppler ultrasound under anaesthesia, GLI significantly reduced the rates of LV relaxation and HR (Table 1) but not cardiac output. Decreased HR has been demonstrated in GLI-treated rats during conscious rest which occurred simultaneously with increased MAP and decreased sympathetic activity to hindlimb muscles (Colburn et al. 2020b). Although resting cardiac function is not expected to relate directly to cardiac function during high-intensity exercise (Fig. 1), we believe that the present changes in LV relaxation and HR at rest help to emphasize a peripheral insult following systemic KATP channel inhibition (Holdsworth et al. 2015, 2016; Colburn et al. 2020b) that manifests changes in cardiac function likely to minimize MAP increases (Colburn et al. 2020b) and/or decrease the work of the heart during elevated MAP. Additionally, as direct KATP channel inhibition of cardiomyocytes would enhance contractility and hinder relaxation (Flagg et al. 2010; Kane et al. 2005; Zingman et al. 2007), it is most likely that the primary effect of systemic GLI administration herein results from a peripheral, and not cardiomyocyte-mediated, alteration in KATP channel function (i.e. vascular KATP channel inhibition leading to vasoconstriction and increased MAP with a baroreceptor-mediated secondary reduction in sympathetic activity to decrease cardiac output, Colburn et al. 2020b). These adjustments in cardiac function are likely removed at near-maximal exercise intensities when sympathetic activity is enhanced and HR is not different between control and GLI conditions (Holdsworth et al. 2015). However, future experimental designs where possible should measure SV and cardiac output during high-intensity exercise to assess the primary, or secondary, effect of KATP channel inhibition on cardiac function. Nevertheless, reductions in herein are considered to result, in part, from mismatch at the level of skeletal muscle and impaired systemic arterial–venous O2 difference (i.e. difference).
KATP channel function on skeletal muscle blood flow and PO2is
To assess the contribution of vascular KATP channels supporting skeletal muscle O2 transport, and thus oxidative phosphorylation, the current investigation measured the interstitial space driving pressure of O2 (PO2is) during the rest–contractions transient during submaximal contractions, muscle blood flow at the end of contractions, and PO2is during recovery. Following local GLI administration, PO2is in the MG fast-twitch oxidative muscle fell faster (i.e. MRT) and to a lower PO2is (PO2is nadir; Table 3, Fig. 3) when was reduced. Interestingly, because PO2is BL was also reduced following GLI, the magnitude of PO2is fall (i.e. Δ1PO2is) was unaltered, possibly due to a lowering of intracellular to preserve interstitial-myocyte PO2 and prevent muscle damage, as proposed by Richmond et al. (1999). Therein the ‘critical PO2’, the point at which PO2is ceased to continue falling and the NADH fluorescence signal increased, was ~2.4–2.9 mmHg for mixed-fibre spinotrapezius muscle and may be greater in the more oxidative MG (citrate synthase activity: ~26 vs. ~14 μmol min−1 g−1; Delp & Duan, 1996). While the exact contribution of lowered (Fig. 4) is unable to be separated completely between low - (via decreased which lowered PO2is and sped the fall in PO2is) and/or myocyte-mediated lowering, the end result is indeed lower which would increase the reliance on glycolytic energy sources for contractions and production of fatigue-related metabolites (Wilson et al. 1977; Hogan et al. 1992; Richardson et al. 1998).
In addition, despite recovering to a similar PO2is, GLI significantly slowed the recovery compared with control. All of these findings during local KATP channel inhibition provide evidence supporting the hypothesis that the reductions in exercise tolerance resulting from systemic GLI administration were due, in part, to impaired O2 transport at the microvascular level hindering aerobic metabolism within the contracting myocyte (see Convective and diffusive determinants of O2 transport below) and, especially for repeated bouts of physical activity, increasing the amount of time needed to re-establish muscle PO2 and replenish muscle energy stores (Haseler et al. 1999; Kindig et al. 2005).
Convective and diffusive determinants of O2 transport
Utilizing direct measurements of , arterial O2 content (CaO2), and the O2 pressures nearest the contracting myocytes (PO2is) to calculate PO2mv (PO2mv = PO2is + transcapillary PO2; Colburn et al. 2020a), the authors conflated the Fick principle ; i.e. convective O2 transport) and Fick’s law of diffusion ; i.e. diffusive O2 transport) to estimate muscle convective O2 delivery , diffusive conductance for O2 (DO2mv), and the resulting from the microvascular compartment. Importantly, the convergence of the convective and diffusive determinants to O2 transport describes the rate of O2 consumption by skeletal muscle (Wagner, 1992, 1996).
In the current investigation, impaired PO2is was due, in part, to reductions in . As a result, the rate of O2 able to be consumed from the microvascular compartment was significantly reduced . As demonstrated in Fig. 4, this change in was not only a consequence of impaired but also lowered microvascular-myocyte diffusing conductance (DO2mv ↓25%). Traditionally interpreted in the context of microvascular-myocyte O2 transport, DO2mv is altered via changes in capillary haematocrit, red blood cell (RBC) flux and RBC velocity (reviewed by Poole et al. 2013; Poole, 2019). More recently, with the advent of PO2is measurements during contractions (Hirai et al. 2018a; Colburn et al. 2020a), interstitial DO2 (DO2is, interstitial-myocyte) can be estimated presuming that O2 leaving the microvascular compartment equals O2 leaving the interstitial compartment (; i.e. negligible change in storage of O2 in interstitial fluid) during steady-state contractions and can be calculated from and the present PO2is (i.e. when ). Interestingly, since must be equivalent in both compartments, DO2 was greater in the interstitial compartment compared with microvascular compartment and both decreased with GLI. However, with GLI, DO2 was almost reduced to a significantly greater extent when O2 diffused out of the microvascular compartment compared with the subsequent O2 diffusion out of the interstitium (↓25% and 19%, DO2mv vs. DO2is, respectively). These disparate magnitudes of, and potential reductions in, DO2 between compartments are likely to result from: i) divergent surface areas for O2 flux (i.e. across capillary wall < into myocyte); and ii) fluid dynamics wherein impaired buffering of RBCs following GLI (i.e. ↓ percent capillaries flowing, RBC flux and RBC velocity, Hirai et al. 2018b) would yield greater decrements in DO2mv than DO2is considering interstitial fluid volume is expected to remain relatively constant during contractions and unaffected by GLI. Furthermore, since PO2mv for control and GLI conditions were calculated using the same transcapillary PO2 (PO2mv = PO2is + transcapillary PO2), the reduction in DO2mv following GLI is potentially underestimated as a result of increased transcapillary PO2. Crucially, increased and RBC dynamics increase DO2mv and reduce transcapillary PO2 (PO2mv-PO2is) in the contracting fast-twitch oxidative ; Colburn et al. 2020a) whereas the GLI-induced reduction in herein and impaired RBC dynamics (Hirai et al. 2018b) would serve to reduce DO2mv compared with control and therefore increasing the actual PO2mv following GLI (MG+GLI compared with MG Control: ).
The interplay between convective and diffusive O2 delivery on muscle has been assessed directly in healthy skeletal muscle across fibre types (Behnke et al. 2003; McDonough et al. 2005), during handgrip exercise (Rosenberry et al. 2019) and in disease populations during isolated knee extensor and cycling exercise (chronic obstructive pulmonary disease: Broxterman et al. 2020; HFrEF: Esposito et al. 2010, 2011 (exercise trained)). To our knowledge, the current investigation is the first to assess changes in convective and diffusive O2 conductance following specific channel/enzyme inhibition and provides evidence that this approach can be utilized in future studies examining O2 transport in health, dysfunction related to a range of cardiovascular diseases (i.e. diabetes, sickle cell anaemia, pulmonary hypertension, HF; Padilla et al. 2006, 2007; Hirai et al. 2015; Ferguson et al. 2018), and potential therapeutic interventions aimed at increasing O2 delivery (i.e. nitrate and nitrite supplementation; Ferguson et al. 2013a,b, 2015, 2016a,b; Glean et al. 2015; Colburn et al. 2017; Craig et al. 2019b).
Experimental considerations
Glycolytic muscle fibres experience the greatest metabolic perturbations during exercise and accordingly contain a greater content of pore-forming KATP channel subunit Kir6.2 (type IIB > IIX > IIA > I; Banas et al. 2011) which depresses force production and resting tension and limits intracellular calcium-mediated fibre damage (Gong et al. 2003; Thabet et al. 2005; Cifelli et al. 2008). When skeletal muscle KATP channels are inhibited via GLI, this could potentially lead to greater myocyte contraction and . Although augmenting KATP channels via pinacidil impairs force production and increases the rate of skeletal muscle fatigue ex vivo, KATP channel inhibition via GLI does not decrease skeletal muscle fatigue or alter force production during tetanic contractions yet appears to increase resting muscle tension between contractions and could, therefore, increase accordingly (Gong et al. 2000; Matar et al. 2000). Nonetheless, the topical application of GLI and related disturbance of matching (i.e. PO2is) of the fast-twitch oxidative glycolytic MG are, principally, consequent to impaired blood flow (decreased herein, and also in Holdsworth et al. 2015) rather than increased metabolic demand (; see Fig. 4).
Whereas the present experimental design precluded the assessment of blood flow at rest and during treadmill running (i.e. utilizing fluorescent microspheres to assess resting/running blood flow would prevent blood flow assessment during PO2is in the same rat), prior investigations from our laboratory have demonstrated reduced muscle blood flow following GLI at rest and a wide range of speeds (20, 40 and 60 m min−1, the latter of which yields on the inclined treadmill in rats, Colburn et al. 2020b and Holdsworth et al. 2015). Therefore, the authors are assured that skeletal muscle blood flow is reduced following systemic GLI administration herein and during the cardiac assessment via Doppler ultrasound.
Clinically, a primary concern of sulphonylurea use in patient populations is the potential for hypoglycaemia. Prior to exercise, hypoglycaemia following GLI-mediated insulin release and systemic glucose uptake would inherently limit exercise duration by restricting blood glucose stores available for energy production. To minimize this concern, the authors performed all exercise testing within 30–90 min of systemic GLI administration to target KATP channel inhibition and not incur the confounding effects on glucose availability that would result from a longer duration GLI-mediated insulin release (Li et al. 2012). Importantly, while assessing the speed–duration relationship (Table 2 and Fig. 2), the curvature constant (D′) remained unchanged following KATP channel inhibition. While CS is better understood and reflects the upper threshold of oxidative phosphorylation to support metabolic demand, D′ is considered to reflect principally the contributions of finite non-aerobic energy stores and fatigue-resistant muscular properties supporting exercise above CS (reviewed by Poole et al. 2016). Additionally, exercise measurements were performed every 4 days to target the pro-oestrus phase. Repeated acute doses of GLI, which has a half-life of up to 10 h, is not anticipated to have a cumulative effect across testing days. Nevertheless, if a chronic KATP channel inhibition effect was captured, it would mirror more directly the use of oral sulphonylureas in T2DM patients and the present investigation may actually underestimate the long-term effects of KATP channel inhibition that is associated with elevated risk for adverse cardiovascular events, developing HF, and all-cause mortality (Simpson et al. 2006, 2015; McAlister et al. 2008; Kristiansen et al. 2011; Abdelmoneim et al. 2016).
Conclusions
These data emphasize the important role that vascular ATP-sensitive K+ (KATP) channels have in supporting exercise tolerance. Crucially, systemic inhibition of KATP channels via GLI reduces and submaximal exercise tolerance (CS). These impairments during treadmill running are reflected in fast-twitch oxidative glycolytic MG muscle where local inhibition of vascular KATP channels reduces skeletal muscle blood flow and O2 delivery during twitch contractions (i.e. PO2is). As a result, by reducing convective O2 and diffusive O2 conductances , KATP channel inhibition lowered muscle . Therefore, the exercise (in)tolerance of disease patients taking oral sulphonylurea medication may be, in part, due to pharmacologically mediated impairments in vascular O2 transport and muscle O2 utilization.
Supplementary Material
Key points.
Oral sulphonylureas, widely prescribed for diabetes, inhibit pancreatic ATP-sensitive K+ (KATP) channels to increase insulin release. However, KATP channels are also located within vascular (endothelium and smooth muscle) and muscle (cardiac and skeletal) tissue.
We evaluated left ventricular function at rest, maximal aerobic capacity and submaximal exercise tolerance (i.e. speed–duration relationship) during treadmill running in rats, before and after systemic KATP channel inhibition via glibenclamide.
Glibenclamide impaired critical speed proportionally more than but did not alter resting cardiac output.
Vascular KATP channel function (topical glibenclamide superfused onto hindlimb skeletal muscle) resolved a decreased blood flow and interstitial PO2 during twitch contractions reflecting impaired O2 delivery-to-utilization matching.
Our findings demonstrate that systemic KATP channel inhibition reduces and critical speed during treadmill running in rats due, in part, to impaired convective and diffusive O2 delivery, and thus , especially within fast-twitch oxidative skeletal muscle.
Acknowledgements
We thank Dr Mark Weiss for technical instruction and assistance with vaginal lavage imaging.
Funding
This work was supported by National Institutes of Health (NIH) grants HL-137156-01 (BJB and DCP) and HL-2-108328 (DCP) and Ruth L. Kirschstein National Research Service Award F31HL145981 (TDC).
Biography
Trenton D. Colburn is a Predoctoral Fellow in the Cardiorespiratory Physiology Laboratory at Kansas State University, mentored by Drs Timothy I. Musch and David C. Poole. His research focuses on the regulation of oxygen delivery and utilization within the microvascular and interstitial compartments of skeletal muscle in health and disease, with particular interest in nitric oxide bioavailability and ATP-sensitive potassium channel function during exercise.

Footnotes
Competing interests
The authors declare that there are no competing interests.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Statistical Summary Document
References
- Abdelmoneim AS, Eurich DT, Senthilselvan A, Qiu W & Simpson SH (2016). Dose-response relationship between sulphonylureas and major adverse cardiovascular events in elderly patients with type 2 diabetes. Pharmacoepidemiol Drug Saf 25, 1186–1195. [DOI] [PubMed] [Google Scholar]
- Andersen P & Saltin B (1985). Maximal perfusion of skeletal muscle in man. J Physiol 366, 233–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB & Laughlin MH (1984). Exercise blood flow patterns within and among rat muscles after training. Am J Physiol 246, H59–H68. [DOI] [PubMed] [Google Scholar]
- Banas K, Clow C, Jasmin BJ & Renaud JM (2011). The KATP channel Kir6.2 subunit content is higher in glycolytic than oxidative skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol 301, R916–R925. [DOI] [PubMed] [Google Scholar]
- Banitt PF, Smits P, Williams SB, Ganz P & Creager MA (1996). Activation of ATP-sensitive potassium channels contributes to reactive hyperemia in humans. Am J Physiol 271, H1594–1598. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, McDonough P, Padilla DJ, Musch TI & Poole DC (2003). Oxygen exchange profile in rat muscles of contrasting fibre types. J Physiol 549, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlstra PJ, den Arend JACJ, Lutterman JA, Russel FGM, Thien T & Smits P (1996). Blockade of vascular ATP-sensitive potassium channels reduces the vasodilator response to ischaemia in humans. Diabetologia 39, 1562–1568. [DOI] [PubMed] [Google Scholar]
- Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA & Moore RL (2005a). Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not mitochondrial, isoform of the KATP channel in the rat. J Physiol 569, 913–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Lynch JM, Armstrong CJ, Caruso NM, Ehlers LB, Johnson MS & Moore RL (2005b). Susceptibility of the heart to ischaemia-reperfusion injury and exercise-induced cardioprotection are sex-dependent in the rat. J Physiol 564, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman RM, Hoff J, Wagner PD & Richardson RS (2020). Determinants of the diminished exercise capacity in patients with chronic obstructive pulmonary disease: looking beyond the lungs. J Physiol 598, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifelli C, Boudreault L, Gong B, Bercier JP & Renaud JM (2008). Contractile dysfunctions in ATP-dependent K+ channel-deficient mouse muscle during fatigue involve excessive depolarization and Ca2+ influx through L-type Ca2+ channels. Exp Physiol 93, 1126–1138. [DOI] [PubMed] [Google Scholar]
- Colburn TD, Ferguson SK, Holdsworth CT, Craig JC, Musch TI & Poole DC (2017). Effect of sodium nitrite on local control of contracting skeletal muscle microvascular oxygen pressure in healthy rats. J Appl Physiol 122, 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn TD, Hirai DM, Craig JC, Ferguson SK, Weber RE, Schulze KM, Behnke BJ, Musch TI & Poole DC (2020a). Transcapillary PO2 gradients in contracting muscles across the fibre type and oxidative continuum. J Physiol 598, 3187–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colburn TD, Holdsworth CT, Craig JC, Hirai DM, Montgomery S, Poole DC, Musch TI & Kenney MJ (2020b). ATP-sensitive K+ channel inhibition in rats decreases kidney and skeletal muscle blood flow without increasing sympathetic nerve discharge. Respir Physiol Neurobiol 278, 103444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Davis RT, Poole DC & Musch TI (2009). Reproducibility of endurance capacity and peak in male Sprague-Dawley rats. J Appl Physiol 106, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Copp SW, Hirai DM, Musch TI & Poole DC (2010). Critical speed in the rat: implications for hindlimb muscle blood flow distribution and fibre recruitment. J Physiol 588, 5077–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp SW, Holdsworth CT, Ferguson SK, Hirai DM, Poole DC & Musch TI (2013). Muscle fibre-type dependence of neuronal nitric oxide synthase-mediated vascular control in the rat during high speed treadmill running. J Physiol 591, 2885–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JC, Colburn TD, Caldwell JT, Hirai DM, Tabuchi A, Baumfalk DR, Behnke BJ, Ade CJ, Musch TI & Poole DC (2019a). Central and peripheral factors mechanistically linked to exercise intolerance in heart failure with reduced ejection fraction. Am J Physiol Heart Circ Physiol 317, H434–H444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JC, Colburn TD, Hirai DM, Musch TI & Poole DC (2019b). Sexual dimorphism in the control of skeletal muscle interstitial PO2 of heart failure rats: effects of dietary nitrate supplementation. J Appl Physiol 126, 1184–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JC, Colburn TD, Hirai DM, Schettler MG, Musch TI & Poole DC (2018). Sex and nitric oxide bioavailability interact to modulate interstitial PO2 in healthy rat skeletal muscle. J Appl Physiol 124, 1558–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD & Duan C (1996). Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol, 80, 261–270. [DOI] [PubMed] [Google Scholar]
- Deveci D & Egginton S (1999). Development of the fluorescent microsphere technique for quantifying regional blood flow in small mammals. Exp Physiol 84, 615–630. [PubMed] [Google Scholar]
- Dudley GA, Abraham WM & Terjung RL (1982). Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol Respirat Environ Exercise Physiol 53, 844–850. [DOI] [PubMed] [Google Scholar]
- Esipova TV, Karagodov A, Miller J, Wilson DF, Busch TM & Vinogradov SA (2011). Two new “protected” oxyphors for biological oximetry: properties and application in tumor imaging. Anal Chem 83, 8756–8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD & Richardson RS (2010). Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol 55, 1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Reese V, Shabetai R, Wagner PD & Richardson RS (2011). Isolated quadriceps training increases maximal exercise capacity in chronic heart failure: the role of skeletal muscle convective and diffusive oxygen transport. J Am Coll Cardiol 58, 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farouque HMO & Meredith IT (2003). Effects of inhibition of ATP-sensitive potassium channels on metabolic vasodilation in the human forearm. Clin Sci 104, 39–46. [DOI] [PubMed] [Google Scholar]
- Ferguson SK, Glean AA, Holdsworth CT, Wright JL, Fees AJ, Colburn TD, Stabler T, Allen JD, Jones AM, Musch TI & Poole DC (2016a). Skeletal muscle vascular control during exercise: impact of nitrite infusion during nitric oxide synthase inhibition in healthy rats. J Cardiovasc Pharmacol Ther 21, 201–208. [DOI] [PubMed] [Google Scholar]
- Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI & Poole DC (2013a). Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Respir Physiol Neurobiol 187, 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SK, Hirai DM, Copp SW, Holdsworth CT, Allen JD, Jones AM, Musch TI & Poole DC (2013b). Impact of dietary nitrate supplemtation via beetroot juice on exercising muscle vascular control in rats. J Physiol 591, 547–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SK, Harral JW, Pak DI, Redinius KM, Stenmark KR, Schaer DJ, Buehler PW & Irwin DC (2018). Impact of cell-free hemoglobin on contracting skeletal muscle microvascular oxygen pressure dynamics. Nitric Oxide 76, 29–36. [DOI] [PubMed] [Google Scholar]
- Ferguson SK, Holdsworth CT, Colburn TD, Wright JL, Craig JC, Fees A, Jones AM, Allen JD, Musch TI & Poole DC (2016b). Dietary nitrate supplementation: impact on skeletal muscle vascular control in exercising rats with chronic heart failure. J Appl Physiol 121, 661–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SK, Holdsworth CT, Wright JL, Fees AJ, Allen JD, Jones AM, Musch TI & Poole DC (2015). Microvascular oxygen pressures in muscles comprised of different fiber types: impact of dietary nitrate supplementation. Nitric Oxide 48, 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagg TP, Enkvetchakul D, Koster JC & Nichols CG (2010). Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev, 90, 799–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glean AA, Ferguson SK, Holdsworth CT, Colburn TD, Wright JL, Fees AJ, Hageman KS, Poole DC & Musch TI (2015). Effects of nitrite infusion on skeletal muscle vascular control during exercise in rats with chronic heart failure. Am J Physiol-Heart C 309, H1354–H1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Miki T, Seino S & Renaud JM (2000). A KATP channel deficiency affects resting tension, not contractile force, during fatigue in skeletal muscle. Am J Physiol Cell Physiol 279, C1351–C1358. [DOI] [PubMed] [Google Scholar]
- Gong B, Legault D, Miki T, Seino S & Renaud JM (2003). KATP channels depress force production by reducing action potential amplitude in mouse EDL and soleus muscle. Am J Physiol Cell Physiol 285, C1464–C1474. [DOI] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology. J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer LW, Ligon AL & Hester RL (2001). Differential inhibition of functional dilation of small arterioles by indomethacin and glibenclamide. Hypertension 37, 599–603. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Hogan MC & Richardson RS (1999). Skeletal muscle phosphocreatine recovery in exercise-trained humans is dependent on O2 availability. J Appl Physiol 86, 2013–2018. [DOI] [PubMed] [Google Scholar]
- Hirai DM, Craig JC, Colburn TD, Eshima H, Kano Y, Sexton WL, Musch TI & Poole DC (2018a). Skeletal muscle microvascular and interstitial PO2 from rest to contractions. J Physiol 596, 869–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai DM, Craig JC, Colburn TD, Tabuchi A, Hageman KS, Musch TI & Poole DC (2018b). Regulation of capillary hemodynamics by KATP channels in resting skeletal muscle. FASEB J 32, 581.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai DM, Musch TI & Poole DC (2015). Exercise training in chronic heart failure: improving skeletal muscle O2 transport and utilization. Am J Physiol Heart Circ Physiol 309, H1419–H1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Arthur PG, Bebout DE, Hochachka PW & Wagner PD (1992). Role of O2 in regulating tissue respiration in dog muscle working in situ. J Appl Physiol 73, 728–736. [DOI] [PubMed] [Google Scholar]
- Holdsworth CT, Copp SW, Ferguson SK, Sims GE, Poole DC & Musch TI (2015). Acute inhibition of ATP-sensitive K+ channels impairs skeletal muscle vascular control in rats during treadmill exercise. Am J Physiol Heart Circ Physiol 308, H1434–H1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth CT, Ferguson SK, Colburn TD, Fees AJ, Craig JC, Hirai DM, Poole DC & Musch TI (2017). Vascular KATP channels mitigate severe muscle O2 delivery-utilization mismatch during contractions in chronic heart failure rats. Respir Physiol Neurobiol 238, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth CT, Ferguson SK, Poole DC & Musch TI (2016). Modulation of rat skeletal muscle microvascular O2 pressure via KATP channel inhibition following the onset of contractions. Respir Physiol Neurobiol 222, 48–54. [DOI] [PubMed] [Google Scholar]
- Ishise S, Pegram BL, Yamamoto J, Kitamura Y & Frohlich ED (1980). Reference sample microsphere method: cardiac output and blood flows in conscious rat. Am J Physiol Heart Circ Physiol 239, H443–H449. [DOI] [PubMed] [Google Scholar]
- Johnson MS, Moore RL & Brown DA (2006). Sex differences in myocardial infarct size are abolished by sarcolemmal KATP channel blockade in rat. Am J Physiol-Heart C 290, H2644–H2647. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, DiMenna F, Fulford J & Poole DC (2008). Muscle metabolic responses to exercise above and below the “critical power” assessed using 31P-MRS. Am J Physiol Regul Integr Comp Physiol 294, R585–R593. [DOI] [PubMed] [Google Scholar]
- Kane GC, Behfar A, Yamada S, Perez-Terzic C, O’Cochlain F, Reyes S, Dzeja PP, Miki T, Seino S & Terzic A (2004). ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes 53, S169–S175. [DOI] [PubMed] [Google Scholar]
- Kane GC, Liu XK, Yamada S, Olson TM & Terzic A (2005). Cardiac KATP channels in health and disease. J Mol Cell Cardiol 38, 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller DM, Ogoh S, Greene S & Raven PB (2004). Inhibition of KATP channel activity augments baroreflex-mediated vasoconstriction in exercising human skeletal muscle. J Physiol 561, 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig CA, Walsh B, Howlett RA, Stary CM & Hogan MC (2005). Relationship between intracellular PO2 recovery kinetics and fatigability in isolated single frog myocytes. J Appl Physiol 98, 2316–2319. [DOI] [PubMed] [Google Scholar]
- Kristiansen SB, Løfgren B, Nielsen JM, Støttrup NM, Buhl ES, Nielsen-Kudsk JE, Nielsen TT, Rungby J, Flyvbjerg A & Bøtker HE (2011). Comparison of two sulfonylureas with high and low myocardial KATP channel affinity on myocardial infarct size and metabolism in a rat model of type 2 diabetes. Diabetologia 54, 451–458. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Davis MJ, Secher NH, van Lieshout JJ, Arce-Esquivel AA, Simmons GH, Bender SB, Padilla J, Bache RJ, Merkus D & Duncker DJ (2012). Peripheral circulation. Compr Physiol 2, 321–447. [DOI] [PubMed] [Google Scholar]
- Li Y, Wei Y, Zhang F, Wang D & Wu X (2012). Changes in the pharmacokinetics of glibenclamide in rats with streptozotocin-induced diabetes mellitus. Acta Pharmaceutica Sinica B 2, 198–204. [Google Scholar]
- Lu S, Xiang L, Clemmer JS, Gowdey AR, Mittwede PN & Hester RL (2013). Impaired vascular KATP function attenuates exercise capacity in obese Zucker rats. Microcirculation 20, 662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcondes FJ, Bianchi FJ & Tanno AP (2002). Determination of the estrous cycle phases of rats: some helpful considerations. Brazilian J Biol 62, 609–614. [DOI] [PubMed] [Google Scholar]
- Matar W, Nosek TM, Wong D & Renaud JM (2000). Pinacidil suppresses contractility and preserves energy but glibenclamide has no effect during muscle fatigue. Am J Physiol Cell Physiol 278, C404–C416. [DOI] [PubMed] [Google Scholar]
- McAlister FA, Eurich DT, Majumdar SR & Johnson JA (2008). The risk of heart failure in patients with type 2 diabetes treated with oral agent monotherapy. Eur J Heart Fail 10, 703–708. [DOI] [PubMed] [Google Scholar]
- McDonough P, Behnke BJ, Kindig CA & Poole DC (2001). Rat muscle microvascular PO2 kinetics during the exercise off-transient. Exp Physiol 86, 349–356. [DOI] [PubMed] [Google Scholar]
- McDonough P, Behnke BJ, Padilla DJ, Musch TI & Poole DC (2005). Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J Physiol 563, 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod H & Scherrer J (1965). The work capacity of a synergic muscular group. Ergonomics 8, 329–338. [Google Scholar]
- Montvida O, Shaw J, Atherton JJ, Stringer F & Paul SK (2018). Long-term trends in antidiabetes drug usage in the U.S.: real-world evidence in patients newly diagnosed with type 2 diabetes. Diabetes Care 41, 69–78. [DOI] [PubMed] [Google Scholar]
- Musch TI (1988). Measurements of metabolic rate in rats: a comparison of techniques. J Appl Physiol 65, 964–970. [DOI] [PubMed] [Google Scholar]
- Musch TI (1992). Training effects on the regional blood flow response to exercise in myocardial infarcted rats. Am J Physiol-Heart C 262, H1846–H1852. [DOI] [PubMed] [Google Scholar]
- Musch TI & Terrell JA (1992). Skeletal muscle blood flow abnormalities in rats with a chronic myocardial infarction: rest and exercise. Am J Physiol Heart Circ Physiol 262, H411–H419. [DOI] [PubMed] [Google Scholar]
- Musch TI, Moore RL, Leathers DJ, Bruno A & Zelis R (1986). Endurance training in rats with chronic heart failure induced by myocardial infarction. Circulation 74, 431–441. [DOI] [PubMed] [Google Scholar]
- Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI & Poole DC (2006). Effects of type II diabetes on capillary hemodynamics in skeletal muscle. Am J Physiol Heart Circ Physiol 291, H2439–H2444. [DOI] [PubMed] [Google Scholar]
- Padilla DJ, McDonough P, Behnke BJ, Kano Y, Hageman KS, Musch TI & Poole DC (2007). Effects of type II diabetes on muscle microvascular oxygen pressures. Respir Physiol Neurobiol 156, 187–195. [DOI] [PubMed] [Google Scholar]
- Poole DC (2019). Edward F. Adolph distinguished lecture. Contemporary model of muscle microcirculation: gateway to function and dysfunction. J Appl Physiol 127, 1012–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Burnley M, Vanhatalo A, Rossiter HB & Jones AM (2016). Critical power: an important fatigue threshold in exercise physiology. Med Sci Sports Exerc 48, 2320–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Copp SW, Colburn TD, Craig JC, Allen DL, Sturek M, O’Leary DS, Zucker IH & Musch TI (2020). Guidelines for animal exercise and training protocols for cardiovascular studies. Am J Physiol Heart Circ Physiol 318, H1100–H1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC, Copp SW, Ferguson SK & Musch TI (2013). Skeletal muscle capillary function: contemporary observations and novel hypotheses. Exp Physiol 98, 1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole DC & Jones AM (2012). Oxygen uptake kinetics. Compr Physiol 2, 933–996. [DOI] [PubMed] [Google Scholar]
- Poole DC, Ward SA, Gardner GW & Whipp BJ (1988). Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics 31, 1265–1279. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Leigh JS & Wagner PD (1998). Lactate efflux from exercising human skeletal muscle: role of intracellular PO2. J Appl Physiol 85, 627–634. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Poole DC, Knight DR, Kurdak SS, Hogan MC, Grassi B, Johnson EC, Kendrick KF, Erickson BK & Wagner PD (1993). High muscle blood flow in man: is maximal O2 extraction compromised? J Appl Physiol 75, 1911–1916. [DOI] [PubMed] [Google Scholar]
- Richmond KN, Shonat RD, Lynch RM & Johnson PC (1999). Critical PO2 of skeletal muscle in vivo. Am J Physiol Heart Circ Physiol 277, H1831–H1840. [DOI] [PubMed] [Google Scholar]
- Roca J, Agusti AG, Alonsa A, Poole DC, Viegas C, Barbera JA, Rodriguez-Roisin R, Ferrer A & Wagner PD (1992). Effect of training on muscle O2 transport at VO2max. J Appl Physiol 73, 1067–1076. [DOI] [PubMed] [Google Scholar]
- Rosenberry R, Tucker WJ, Haykowsky MJ, Trojacek D, Chamseddine HH, Arena-Marshall CA, Zhu Y, Wang J, Kellawan JM, Tian F & Nelson MD (2019). Determinants of skeletal muscle oxygen consumption assessed by near-infrared diffuse correlation spectroscopy during incremental handgrip exercise. J Appl Physiol 127, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumsey WL, Vanderkooi JM & Wilson DF (1988). Imaging of phosphorescence: a novel method for measuring oxygen distribution in perfused tissue. Science 241, 1649–1651, 1988. [DOI] [PubMed] [Google Scholar]
- Saito Y, McKay M, Eraslan A & Hester RL (1996). Functional hyperemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. Am J Physiol Heart Circ Physiol 270, H1649–H1654. [DOI] [PubMed] [Google Scholar]
- Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS & Featherstone TR (2015). Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol 3, 43–51. [DOI] [PubMed] [Google Scholar]
- Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT & Johnson JA (2006). Dose-response relation between sulphonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ 174, 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Hageman KS, Harms CA, Poole DC & Musch TI (2017). Respiratory muscle blood flow during exercise: Effects of sex and ovarian cycle. J Appl Physiol 122, 918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thabet M, Miki T, Seino S & Renaud TM (2005). Treadmill running causes significant fiber damage in skeletal muscle of KATP channel-deficient mice. Physiol Genomics 22, 204–212. [DOI] [PubMed] [Google Scholar]
- Van Oosterhout MFM, Prinzen FW, Sakurada S, Glenny RW & Hales JRS (1998). Fluorescent microspheres are superior to radioactive microspheres in chronic blood flow measurements. Am J Physiol-Heart C 275, H110–H115. [DOI] [PubMed] [Google Scholar]
- Wagner PD (1992). Gas exchange and peripheral diffusion limitation. Med Sci Sports Exerc 24, 54–58. [PubMed] [Google Scholar]
- Wagner PD (1996). Determinants of maximal oxygen transport and utilization. Annu Rev Physiol 58, 21–50. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Erecinska M, Drown C & Silver IA (1977). Effect of oxygen tension on cellular energetics. Am J Physiol Cell Physiol 233, C135–C140. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Alekseev AE, Hodgson-Zingman DM & Terzic A (2007). ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol 103, 1888–1893. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.