ABSTRACT
Background: Pharmacological-assisted psychotherapies, using conventional and novel drug agents, are increasingly being used both in clinical and experimental research settings, respectively.
Objective: To determine the efficacy of conventional and novel pharmacological-assisted psychotherapies in reducing PTSD symptom severity.
Method: A systematic review and meta-analysis of randomised-controlled trials were undertaken; 21 studies were included.
Results: MDMA-assisted therapy was found to statistically superior to active and inactive placebo-assisted therapy in reduction of PTSD symptoms (standardised mean difference −1.09, 95% CI −1.60 to −0.58). There was no evidence of superiority over placebo for any other intervention.
Conclusions: MDMA-assisted therapy demonstrated an impressive effect size; however, it is difficult to have confidence at this stage in this intervention due to the small numbers of participants included, and more research in this area is needed. There was no evidence to support the efficacy of any other drug-assisted interventions.
KEYWORDS: PTSD, pharmacological therapy, drug-assisted therapy, MDMA, D-cycloserine, SSRI, SNRI
HIGHLIGHTS
We found that MDMA-assisted therapy is a novel and promising new treatment for severe PTSD.
Despite demonstrating an impressive effect size, more research is needed due to the small numbers of participants included.
We did not find evidence to recommend any other drug-assisted psychological intervention.
Short abstract
Antecedentes: Las psicoterapias asistidas farmacológicamente, que utilizan fármacos convencionales y nuevos, están siendo cada vez más utilizadas tanto en contextos clínicos como de investigación experimental, respectivamente.
Objetivos: Determinar la eficacia de las psicoterapias asistidas con fármacos convencionales y nuevos para reducir la severidad de los síntomas TEPT.
Método: Se llevó a cabo una revisión sistemática y un metanálisis de estudios controlados aleatorizados; se incluyeron 21 estudios.
Resultados: Se encontró que la terapia asistida por MDMA era estadísticamente superior a terapia asistida por placebo activo e inactivo en la reducción de los síntomas de TEPT (diferencia de medias estandarizada −1.09, IC del 95%: −1.60 a −0.58). No hubo evidencia de superioridad sobre placebo para ninguna otra intervención.
Conclusiones: La terapia asistida por MDMA demostró un tamaño de efecto impresionante; sin embargo es difícil tener confianza en esta etapa en esta intervención debido al pequeño número de participantes incluidos, y se necesita más investigación en esta área. No hubo evidencia para apoyar la eficacia de ninguna otra intervención asistida por fármacos.
PALABRAS CLAVE: Terapia farmacológica, terapia asistida por fármacos, MDMA, D-cicloserina, ISRS, ISRN
背景: 使用传统药物和新型药物的药物辅助心理治疗分别越来越多地用于临床和实验研究环境中。
目的: 确定传统和新型药物辅助心理治疗在降低PTSD症状严重程度方面的效能。
方法: 对随机对照试验进行了系统综述和元分析。纳入了21项研究。
结果: 发现在降低PTSD症状方面, MDMA辅助治疗在统计学上优于阳性对照和安慰剂辅助治疗 (标准平均差-1.09, 95%CI −1.60至-0.58) 。没有证据表明任何其他干预措施优于安慰剂的证据。
结论:MDMA辅助治疗展现出了令人印象深刻的效应量。但是, 由于参与人数少, 目前对这种干预措施很难有信心, 因此需要这一领域的更多研究。没有证据支持任何其他药物辅助干预措施的效能。
关键词: PTSD, 药物治疗, 药物辅助治疗, MDMA, D-环丝氨酸, SSRI, SNRI
1. Background
Post-traumatic stress disorder is a common, serious and debilitating illness, that often runs a chronic course (Santiago et al., 2013). Trauma-focused psychological therapies (TFPT) are well established as the treatments of choice for PTSD (National Collaborating Centre for Mental Health, 2005) and often utilise narrative imagery to reprocess traumatic memories (Bisson et al., 2007). Manualized, evidence-based, trauma-focused psychotherapies include prolonged exposure (PE), cognitive processing therapy (CPT), cognitive therapy for PTSD (CT-PTSD), and eye movement desensitization and reprocessing (EMDR) (Cusack et al., 2016).
For exposure-based TFPTs to be effective, people with PTSD need to remain emotionally engaged with the traumatic memory during exposure (Foa, Hembree, & Rothbaum, 2007) to facilitate fear extinction (McNally, 2007; Myers & Davis, 2007). For cognitive-based TFPTs to be effective, people with PTSD need to re-appraise the distorted beliefs that are often attached to emotionally distressing memories. Successful TFPT can lead to improved inhibition of fear responses and improved emotional regulation (Myers & Davis, 2007). However, people with PTSD are prone to extremes of overwhelming distress and emotional numbing (Foa et al., 2007), making it difficult to both tolerate and engage with TFPT, within what is called the ‘optimal arousal zone’ (Myers & Davis, 2007; Ogden, Minton, & Pain, 2006).
Moreover, the quality of the relationship between therapist and patient (the therapeutic alliance) is crucial to a positive outcome in TFPT (Charuvastra & Cloitre, 2008); mitigating against this are characteristic feelings of social detachment (Charuvastra & Cloitre, 2008; Johansen & Krebs, 2009) and hypervigilance in PTSD (Santiago et al., 2013). In clinical trials, 67% of patients who complete a course of TFPT no longer meet the criteria for PTSD (Brady, Green, Russ, Dutra, & Westen, 2005). Unfortunately, clinical trials of TFPT are associated with high dropout rates (up to 54% has been reported) (Schottenbauer, Glass, Arnkoff, Tendick, & Gray, 2008), and are ineffective in nearly half of patients who are able to tolerate it (Brady et al., 2005); with higher PTSD severity predicting a poor response (Blanchard et al., 2003).
Pharmacological treatments have also been shown to reduce PTSD symptom severity, albeit with a relatively small effect size (Hoskins et al., in press). We have found evidence to support the monotherapy use of the selective serotonin reuptake inhibitors (SSRIs) paroxetine, fluoxetine, sertraline, the serotonin noradrenaline reuptake inhibitor (SNRI) venlafaxine and the atypical antipsychotic quetiapine (Hoskins et al., in press). We also found evidence for the augmentation of mono pharmacotherapy with the alpha-1 adrenoceptor antagonist prazosin and the atypical antipsychotic risperidone (Hoskins et al., in press).
The overall disappointing results of either psychological or pharmacological treatment approaches for PTSD has led to a small but growing body of literature assessing the efficacy of combining pharmacological agents with psychotherapies. This can be divided into two categories; conventional pharmacological agents, such as SSRIs and SNRIs, which are taken daily, alongside a course of therapy; and unconventional agents, such as d-cycloserine (DCS), a partial NMDA agonist, and the empathogenic psychedelic 3,4-methylenedioxymethamphetamine (MDMA), which are taken immediately prior to a limited number of psychotherapy sessions in order to enhance a therapeutic process. We will explore the details of these interventions in our discussion.
Recent and previous reviews have investigated MDMA (Bahji, Forsyth, Groll, & Hawken, 2019) and DCS-assisted therapy (Mataix-Cols et al., 2017) in PTSD; this review seeks to investigate and compare all drug-assisted interventions with a unified methodology, as part of a series of two reviews by the authors to investigate the evidence base for all pharmacological approaches when treating PTSD. Our other review focused on pharmacological monotherapy, augmentation, pharmacotherapy versus pharmacotherapy, and pharmacotherapy versus psychotherapy (EJPT ref to be inserted).
2. Method
This was a systematic review and meta-analysis adhering to the Cochrane Collaboration’s standard methodology (Higgins & Green, 2011).
2.1. Participants
All studies where at least 70% of participants diagnosed with PTSD according to ICD or DSM criteria by means of a structured interview or diagnosis by a clinician were eligible. The lower age limit was 18 years with no restriction on the upper age limit. There was no restriction on the basis of gender or of comorbidity but PTSD was required to be the primary diagnosis. The duration of PTSD symptoms was required to be at least 3 months. There was no restriction on the basis of severity of PTSD symptoms or the type of traumatic event. There was no minimum sample size and unpublished studies were eligible. Only studies published in English were eligible.
2.2. Interventions
Any randomised-controlled trial evaluating the efficacy of pharmacological-assisted psychotherapy aimed at reducing the symptoms of PTSD in adults, in which the comparator of at least one arm was a psychotherapy, medication, or psychotherapy plus placebo.
2.3. Outcome measures
The primary outcomes of interest were clinician-administered continuous measures of PTSD symptom severity such as the Clinician Administered PTSD Scale (CAPS). Self-rated PTSD symptom scales were considered if clinician-administered scales were not reported.
2.4. Search strategy
This review used a common search strategy with the Cochrane review of early psychological interventions (Hoskins et al., in press). Following on from this previous search, we undertook a systematic computerized literature search of the Cochrane Common Mental Disorders Group clinical trials registers databases for studies published from January 2008 to May 2016 using the search terms PTSD or posttrauma* or post-trauma* or ‘post trauma*’ or ‘combat disorder*’ or ‘stress disorder*’. These databases are collated and updated on a weekly basis from MEDLINE, EMBASE and PsycINFO. A further search was undertaken in May 2018. Studies were additionally sought from the inclusion/exclusion list from a previous systematic review of pharmacotherapy (Bahji et al., 2019).
Searches were undertaken as part of a search process to support development of new PTSD treatment guidelines for the International Society for Traumatic Stress Studies (ISTSS). We checked the reference lists of studies identified in the search, related review articles and management guidelines. We contacted authors of unpublished studies that had completed recruitment where there was a registered protocol on a trial register, such as Clinical Trials. We posted a list of identified studies on the website of the International Society for Traumatic Stress website and asked the membership to identify studies that we might have missed.
2.5. Study selection
The lead author received the Cochrane database pharmacological search hits in an EndNoteX4 file. Studies identified from our previous review were added and duplicates were removed. A small team of secondary reviewers were allocated segments of the search hits and, alongside the lead author, independently screened the titles, and then abstracts. Studies that were clearly irrelevant were excluded and potentially relevant ones were assessed for inclusion as full texts. The full texts of included studies were read and then sorted into five categories; monotherapy; augmentation; pharmacological-assisted therapy; pharmacotherapy versus pharmacotherapy; pharmacotherapy versus psychotherapy. Any discrepancies between reviewers’ decisions were resolved by discussion with a third reviewer.
2.6. Data extraction and risk of bias assessment
All data from newly identified studies were double extracted by the lead author and a second independent reviewer (co-authors) into a standard table and any discrepancies were discussed with a third reviewer. Data for change from baseline to endpoint were extracted where possible, otherwise endpoint data were used. Continuous data were extracted for clinician-administered PTSD symptom severity using the Clinician Administered PTSD Scale as the gold standard; for self-rated PTSD, the Davidson Trauma Scale was used as the gold standard. If these scales were not used, data from alternative scales were extracted.
The lead author entered the outcome data in Review Manager 5 software, which was then checked by an independent second reviewer.
2.7. Risk of bias
The lead author and a small team of independent second reviewers assessed the risk of bias for each study, using the domain-based evaluation method recommended by the Cochrane Collaboration (Higgins & Green, 2011). This method considers the following domains: adequate random sequence generation; allocation concealment; blinding of participants and personnel; blinding of outcome assessors; incomplete outcome reporting; selective reporting; and any other sources of bias. Any discrepancies between the reviewers’ decisions were discussed with a third reviewer.
3. Statistical analysis
Review Manager 5 was used to synthesise data using meta-analysis and to provide forest plots for continuous data. Confidence intervals were set at 95% for all analyses and standard mean differences were used (SMD). The degree of heterogeneity was calculated using the I2 statistic, and where this was less than 30%, a fixed effects model was used; otherwise where I2 was over 30% a random effects model was used. Data were analysed from the intention to treat (ITT) sample, where possible, to avoid the effects of bias from completers-only analyses. A number of studies used a modified intention to treat (mITT) method, where participants were analysed, provided they had been randomised and received at least one post-baseline assessment (sometimes before or after the first dose of a study medication or placebo). Whilst this does not adhere to the ITT principle of ‘once randomised, always analysed’, because of the number of studies that employed this method it was necessary to allow it in order to conduct a meaningful review.
3.1. Results
The initial search yielded 10,317 records, with an additional 51 identified from our previous review. A total of 19 duplicates were removed, leaving 10,349 titles that were screened. 460 full abstracts were reviewed, with 306 excluded as irrelevant. This then left 154 full-text articles which were read and 39 were removed as not meeting the inclusion criteria. A total of 115 studies were included for our series of pharmacological reviews, with 21 studies (Buhmann, Nordentoft, Ekstroem, Carlsson, & Mortensen, 2016; De Kleine, Hendriks, Kusters, Broekman, & van Minnen, 2012; Difede et al., 2014; Flanagan, Sippel, Wahlquist, Moran-Santa Maria, & Back, 2018; Hien et al., 2015; Litz et al., 2012; Mithoefer et al., 2018; Mithoefer, Wagner, Mithoefer, Jerome, & Doblin, 2011; Mithoefer et al., 2013; Oehen, Traber, Widmer, & Schnyder, 2013; Ot’alora et al., 2018; Otto et al., 2003; Popiel, Zawadzki, Pragłowska, & Teichman, 2015; Rothbaum et al., 2006, 2014; Schneier et al., 2012; Simon et al., 2008; Sonne, Carlsson, Bech, Elklit, & Mortensen, 2016; Tuerk et al., 2018; Yehuda et al., 2015; Young et al., 2017; Zoellner, Telch, Foa, Farach, & McLean, 2017) included for this systematic review of pharmacotherapy-assisted psychotherapy approaches (Figure 1).
Figure 1.
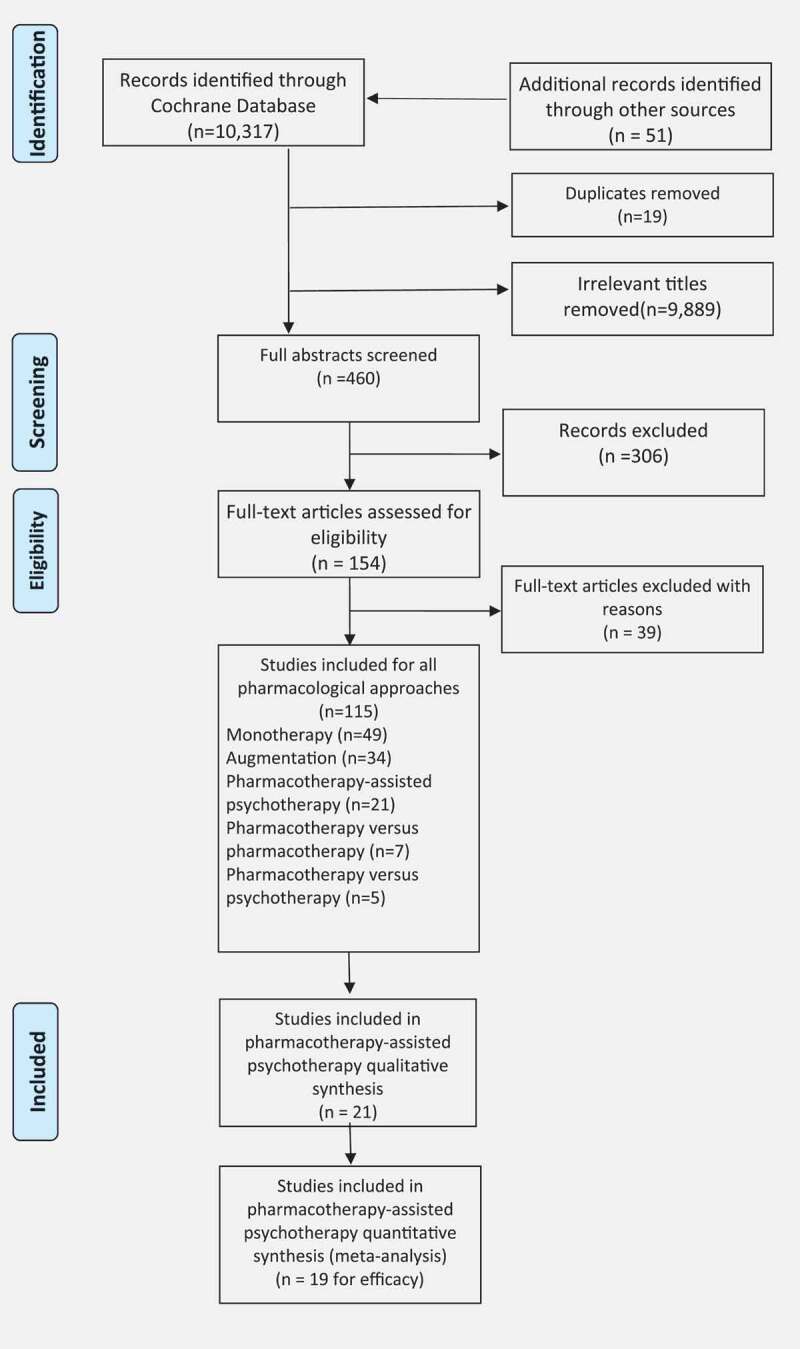
Flow diagram of included studies
3.2. Description of studies
The characteristics of the included 21 studies are detailed in Table 1. Eight studies (Buhmann et al., 2016; Hien et al., 2015; Otto et al., 2003; Popiel et al., 2015; Rothbaum et al., 2006; Simon et al., 2008; Sonne et al., 2016) assessed the use of an SSRI or SNRI in combination with a therapeutic approach. Of those eight studies, six (Buhmann et al., 2016; Hien et al., 2015; Popiel et al., 2015; Simon et al., 2008; Sonne et al., 2016) compared an SSRI/SNRI plus therapy approach to therapy plus placebo, with the remaining two employing an SSRI comparator (Otto et al., 2003; Rothbaum et al., 2006). Four studies assessed the use of DCS prior to exposure therapy (De Kleine et al., 2012; Difede et al., 2014; Litz et al., 2012; Rothbaum et al., 2014). Four studies assessed the use of MDMA-assisted psychotherapy (Mithoefer et al., 2018, 2011; Oehen et al., 2013; Ot’alora et al., 2018). Four single studies assessed the use of a therapeutic intervention assisted by propranolol (Brunet et al., 2014), yohimbine (Tuerk et al., 2018), cortisol (Yehuda et al., 2015) and methylene blue (Zoellner et al., 2017).
Table 1.
Characteristics of included studies.
| Methods |
Participants |
Outcomes |
Interventions |
Notes |
Risk of bias (low/unclear/high) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SSRI plus therapy | Sequence generation | Allocation concealment | Blinding participants/personnel | Blinding outcome assessors | Incomplete outcome data | Free of selective reporting | Any other bias | ||||
| Study ID: Buhman 2018 Denmark Study type: Multicentre, pragmatic, randomised controlled trial with 2 × 2 factorial design Duration: 6 months |
N = 280 Mean age: 49 years Sex: 41% female Diagnosis: DSM-IV PTSD Predominant trauma type: Asylum experience Mean duration of Sx: 14.7 years |
HTQ HSCL-25 HRSD HRSA SCL-90 VAS SDS WHO-5 |
Group 1: Sertraline (25–200 mg, mean dose 132.1 mg) ± minaserin (10–30 mg, mean dose 20 mg) n = 71 Group 2: Sertraline (25–200 mg, mean dose 132.1 mg) ± minaserin (10–30 mg, mean dose 20 mg) plus therapy n = 71 Group 3: Therapy (16 sessions CBT over 6 months) n = 70 Group 4: Waiting list n = 68 |
54% of sessions were translated. 25% in therapy actually received exposure treatment. Additionally, 27% of the therapy group received another antidepressant. |
Low | Low | High | Low | Unclear | Low | High |
| Study ID: Hien 2015 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm, fixed dose Duration: 12 weeks |
N = 69 Mean age:unknown Sex: 81% female Diagnosis: DSM-IV-TR PTSD and AUD Predominant trauma type: CSA (46%) Mean duration of Sx: 14 years |
CAPS SCID-I TLFB |
Group 1: Sertraline (50–200 mg, mean dose) plus 12 weekly sessions of Safety Seeking (SS) therapy n = 32 Group 2: Placebo plus SS n = 37 |
Medication titrated over 2 weeks prior to therapy. | Low | Low | Low | Low | Low | Low | High |
| Study ID: Otto 2003 Cambodia Study type: Single centre, randomised, open label, FLEXIBLE DOSE Duration: 10 weeks of therapy. Unclear total treatment duration. |
N = 10 Mean age: 47.2 years Sex: 100% female Diagnosis: DSM-IV Predominant trauma type: survivors of Pol Pot regime Mean duration of Sx: 24 years |
CAPS ASI SCL-90-R |
Group 1: open label sertraline 25–200 mg plus group CBT n = 5 Group 2: sertraline 25–200 mg n = 5 |
Open label. | Unclear | High | High | High | High | Unclear | High |
| Study ID: Popiel 2015 Poland Study type: Single centre, randomised, double blind, three parallel arms Duration: 12 weeks |
N = 228 Mean age: 36.9 years Sex: unclear Diagnosis: DSM-IV-TR PTSD Predominant trauma type: Motor Vehicle Accident (100%) Mean duration of Sx: 17.7 months |
SCID-I PDS BDI-II |
Group 1: Prolonged exposure (PE) x 12 weekly sessions n = 114 Group 2: Paroxetine 20 mg x 12 weeks n = 57 Group 3: PE plus paroxetine 20 mg x 12 weeks n = 57 |
Unclear | High | High | Low | High | Unclear | Low | |
| Study ID: Rothbaum 2006 USA Study type: Multicentre, randomised, double blind, parallel arm, flexible dose Duration: 16 weeks |
N = 65 (88 completed 10 week open label) Mean age: 39.3 Sex: 64.6% female Diagnosis: DSM-IV PTSD Predominant trauma type: Sexual assault Mean duration of Sx: 8.1 years |
SIP BDI STAI-S |
Group 1: Sertraline 50–200 mg x 10 weeks open label followed by 5 weeks double blind sertraline plus 10 sessions of twice-weekly Prolonged exposure (PE) n = 34 Group 2: Sertraline 50–200 mg x 10 weeks open label followed by 5 weeks sertraline alone n = 31 |
Unclear | Unclear | High | High | Unclear | Unclear | High | |
| Study ID: Schneier 2012 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm Duration: 10 weeks |
N = 37 Mean age: 50.3 years Sex: 54% female Diagnosis: DSM-IV PTSD Predominant trauma type: WTC attacks Mean duration of Sx: 6.5 years |
CAPS HAMD QLESQ |
Group 1: Paroxetine (12.5–50 mg, mean dose 32.2 mg) plus 10 weekly sessions of PE n = 19 Group 2: Placebo plus 10 weekly sessions of PE n = 18 |
Unclear | Unclear | Low | Low | High | Low | High | |
| Study ID: Simon 2008 USA Study type: Multicentre, randomised, double blind, placebo controlled, parallel arm, flexible dose Duration: 10 weeks |
N = 68 Mean age: 46 years Sex: 56% female Diagnosis: DSM-IV PTSD Predominant trauma type: physical and sexual abuse (78%) Mean duration of Sx: unclear |
SPRINT CGI-S |
Group 1: paroxetine (12.5–62.5 mg) + Prolonged Exposure (PE) n = 9 Group 2: placebo + PE n = 14 |
Therapy course: - 8 sessions of open label PE (90–120 minutes) - non-responders randomised to treatment arms - 5 sessions of double blind PE plus paroxetine/placebo (90–120 minutes, fortnightly) x 10 weeks |
Unclear | Unclear | Unclear | Low | High | Unclear | High |
| Study ID: Sonne 2016 Denmark Study type: Single centre, randomised, pragmatic, parallel arm Duration: 6–7 months |
N = 207 Mean age: 43.7 years Sex: 40% female Diagnosis: ICD-10 PTSD Predominant trauma type: Refugee experience Mean duration of Sx: 41.6 years |
HTQ HAMD HAMA HSCL-25 SAS-SR CSS SDS WHO-5 SCL-90 GAF |
Group 1: venlafaxine (37.5–375 mg, mean dose 125.41 mg) (± mianserin) plus therapy n = 98 Group 2: sertraline (25–200 mg, mean dose 96.21 mg (± mianserin) plus therapy n = 109 |
Therapy = 16 sessions manualised flexible CBT (TF-CBT, ACT, stress Mx and MM) and social counselling | Low | Low | High | Low | Low | Low | High |
| DCS-assisted therapy | |||||||||||
| Study ID: De Kleine 2012 Netherlands Study type: Single centre, randomised, double blind, placebo controlled, parallel arm Duration: 10 weeks |
N = 67 Mean age: 38.3 years Sex: 80.6% female Diagnosis: DSM-IV PTSD Predominant trauma type: sexual abuse (52%) Mean duration of Sx: unknown |
CAPS BDI STAI |
Group 1: 50 mg DCS administered orally 1 hour prior to each face-to-face session of manualized PE n = 33 Group 2: administered orally 1 hour prior to each face-to-face session of manualized PE N = 34 |
No take-home DCS for homework. Concomitant psychotropics were allowed. |
Low | Low | Low | Low | Low | Low | Unclear |
| Study ID: Difede 2014 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm Duration: 12 weeks |
N = 25 Mean age: 45.8 years Sex: 24% female Diagnosis: DSM-IV PTSD Predominant trauma type: WTC attacks Mean duration of Sx: unclear |
CAPS SCID-MDD BDI-II STAXI-2 PCL |
Group 1: 12 weekly sessions of CBT including prolonged exposure enhanced by virtual reality (VRE)with 100 mg DCS 90 mins before sessions 2–11 n = 13 Group 2: 12 weekly sessions of CBT including prolonged exposure enhanced by virtual reality with placebo 90 mins before sessions 2–11 n = 12 |
Pharmacotherapy (on a stable dose for 2 months) allowed during therapy. | Unclear | Unclear | Low | Low | Low | High | High |
| Study ID: Litz 2012 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm Duration: 6 weeks |
N = 26 Mean age: 32 years Sex: 100~% male Diagnosis: DSM-IV PTSD Predominant trauma type: combat Mean duration of Sx: unclear |
CAPS BDI |
Group 1: 6 weekly exposure sessions with 50 mg DCS given 30 mins prior to sessions 2–5 n = 13 Group 2: 6 weekly exposure sessions with placebo given 30 mins prior to sessions 2–5 n = 13 |
Trial stopped before planned recruitment of 68 was reached. | Low | Low | Unclear | Low | Low | Unclear | High |
| Study ID: Rothbaum 2014 USA Study type: Randomised, double blind, placebo controlled, parallel arm Duration: 6 weeks |
N = 106 Mean age: 34.6 years Sex: 6% female Diagnosis: DSM-IV PTSD Predominant trauma type: combat (100%) Mean duration of Sx: unknown |
CAPS Startle response Salivary cortisol |
Group 1: 6 weekly 90 minute Virtual Reality Exposure (VRE) sessions with DCS 50 mg given 30 minutes prior to each session n = 53 Group 2: 6 weekly 90 minute VRE sessions with alprazolam given 30 minutes prior to each session n = 50 Group 3: 6 weekly 90 minute VRE sessions with placebo given 30 minutes prior to each session n = 53 |
56% were on a stable dose of psychotropic medications. | Unclear | Unclear | Unclear | Unclear | High | High | High |
| MDMA-assisted therapy | |||||||||||
| Study ID: Mithoefer 2010 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm, flexible dose Duration: 12 weeks |
N = 23 Mean age: 40.4 years Sex: 85% female Diagnosis: DSM-IV PTSD Predominant trauma type: crime related (95%) Mean duration of Sx: 19 years |
CAPS IES-R SCL-90-R RBANS PASAT RCFT |
Group 1: 12 therapy sessions with 125 mg MDMA given prior to sessions 3 and 7 n = 15 Group 2: 12 therapy sessions with placebo given prior to sessions 3 and 8 n = 8 |
MDMA-assisted therapy model includes male-female therapist dyad. Drug-assisted sessions last 6–8 hours, are non-directive and include music, eye mask and overnight stay. All participants were deemed to be treatment-resistant, although this was not clearly defined. Funded and managed by MAPS. |
Low | Low | High | Low | Low | Low | High |
| Study ID: Mithoefer 2018 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm, flexible dose Duration: 12 weeks |
N = 24 Mean age: 37.2 years Sex: 27% female Diagnosis: DSM-IV PTSD Predominant trauma type: service personnel Mean duration of Sx: 85.4 months |
CAPS-IV BDI-II PSQI PTGI NEO-PI-R DES-II GAF C-SSRS |
Group 1: 12 therapy sessions with 30 mg (active placebo) MDMA given prior to sessions 4 and 8 n = 7 Group 2: 12 therapy sessions with 75 mg MDMA given prior to sessions 4 and 8 n = 7 Group 3: 12 therapy sessions with 125 mg MDMA given prior to sessions 4 and 8 n = 12 |
MDMA-assisted therapy model includes male-female therapist dyad. Drug-assisted sessions last 6–8 hours, are non-directive and include music, eye mask and overnight stay. All participants were deemed to have chronic PTSD of at least 6 months duration or more. Modified ITT used; participants were included after one MDMA session. Funded and managed by MAPS. |
Low | Low | Low | Low | Low | Low | High |
| Study ID: Oehen 2013 Switzerland Study type: Single centre, randomised, double blind, placebo controlled, parallel arm, flexible dose Duration: 12 weeks |
N = 14 Mean age: 41.4 years Sex: 83% female Diagnosis: DSM-IV PTSD Predominant trauma type: CSA (50%) Mean duration of Sx: 18.3 years |
CAPS PDS |
Group 1: 12 therapy sessions with 25 mg (active placebo) MDMA given prior to sessions 3, 6 and 9 n = 5 Group 2: 12 therapy sessions with 125 mg (active placebo) MDMA given prior to sessions 3, 6 and 9 n = 9 |
MDMA-assisted therapy model includes male-female therapist dyad. Drug-assisted sessions last 6–8 hours, are non-directive and include music, eye mask and overnight stay. All participants were deemed to be treatment-resistant, defined as ‘having previously undergone at least 6 months of psychotherapy and 3 months of treatment with an SSRI’. Funded by MAPS. |
Unclear | Unclear | Low | Low | High | Low | High |
| Study ID: Ot’alora 2018 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm, flexible dose Duration: 12 weeks |
N = 28 Mean age: 42 years Sex: 67.9% female Diagnosis: DSM-IV PTSD Predominant trauma type: Mean duration of Sx: 29.4 years |
CAPS BDI-II PSQI DES-II |
Group 1: 12 therapy sessions with 100 mg MDMA given prior to sessions 4 and 8 n = 9 Group 2: 12 therapy sessions with 125 mg MDMA given prior to sessions 4 and 8 n = 13 Group3: 12 therapy sessions with 40 mg MDMA given prior to sessions 4 and 8 (active placebo) n = 6 |
MDMA-assisted therapy model includes male-female therapist dyad. Drug-assisted sessions last 6–8 hours, are non-directive and include music, eye mask and overnight stay. All participants were deemed to have chronic PTSD of at least 6 months duration or more. Modified ITT used; participants were included after one MDMA session plus one post-baseline assessment. Funded and managed by MAPS |
Low | Low | Low | Low | Low | Low | High |
| Other agents/approaches | |||||||||||
| Study ID: Brunet 2018 Canada Study type: Single centre, randomised, double blind, placebo controlled, parallel arm Duration: 6 weeks |
N = 61 Mean age:39.4 years Sex:58% female Diagnosis: DSM-IV-TR PTSD Predominant trauma type: physical and sexual (57%) Mean duration of Sx: unknown |
CAPS PCL-S |
Group 1: 6 weekly trauma memory reactivation sessions with propranolol (0.67 mg/kg short-acting plus 1 mg/kg long-acting preparations with food) n = 30 Group 2: 6 weekly trauma memory reactivation sessions with placebo n = 30 |
Brief intervention. First session 30 mins of written narrative. Subsequent sessions 10–20 minutes. | Low | Low | Low | Low | Low | Low | Low |
| Study ID: Tuerk 2018 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm, fixed dose Duration: 15 weeks |
N = 26 Mean age: 32.4 years Sex: 100% male Diagnosis: DSM-IV PTSD Predominant trauma type: combat Mean duration of Sx: unclear |
trauma-cued heart rate reactivity PCL-M BDI-II |
Group 1: Yohimbine 21.6 mg taken one hour prior to each Prolonged Exposure (PE) session n = 14 Group 2: Placebo taken one hour prior to each PE session n = 12 |
Low | Low | Low | Low | Low | Low | Unclear | |
| Study ID: Yehuda 2015 USA Study type: Single centre, randomised, double blind, placebo controlled, parallel arm, fixed dose Duration: 6 weeks |
N = 24 Mean age: 49.6 years Sex: unknown Diagnosis: DSM-IV PTSD Predominant trauma type: combat Mean duration of Sx: unknown |
CAPS PSS-SR BDI |
Group 1: 10 sessions of manualised prolonged exposure with 30 mg cortisol administered 20 minutes prior to sessions 3–10 n = 12 Group 2: 10 sessions of manualised prolonged exposure with placebo administered 20 minutes prior to sessions 3–10 n = 12 |
Insufficient data reported. | Low | Low | Low | Low | Unclear | Unclear | High |
| Study ID: Zoellner 2017 USA Study type: Duration: 6 weeks |
N = 42 Mean age: 37.5 years Sex: 100% female Diagnosis: DSM-IV-TR PTSD Predominant trauma type: physical assault (28.6%) Mean duration of Sx: |
PSS-I CGI SCID-IV PSS-SR QIDS-SR PTCI OSPAN SF-36 SUDS MEF |
Group 1: Six sessions of imaginal exposure (IE) plus 260 mg methylene blue(MB) administered post IE n = 15 Group 2: Six sessions of imaginal exposure (IE) plus placebo administered post IE n = 16 Group 3: Waiting list n = 11 |
Unclear | Unclear | Low | Unclear | Low | Low | Low | |
The average duration of the trials was 11.7 (±5.1) weeks, with an average age of 41.2 (±5.5) years and an average sample size of 72 (±74.8) participants. Thirteen of the studies took place in the USA, with two in Denmark, and single studies in Canada, the Netherlands, Poland and Switzerland.
Combat trauma was the predominant trauma type in six of the studies, with sexual assault and childhood sexual abuse being the predominant trauma type in three studies each. Physical assault and refugee/asylum seeker-related trauma were the predominant trauma type in two studies each, leaving three single studies where participants had experienced motor vehicle accidents, terrorist attacks and war crimes.
3.3. Risk of bias assessments
Risk of bias assessments is included in Table 1. The overall quality of included studies was higher than those included in our monotherapy and augmentation reviews, and may be related to the recency of publication of those included here. Eleven studies adequately described their method of randomisation and allocation concealment. Blinding of participants and personnel was deemed to be of low risk of bias in 11 studies, and blinding of outcome assessors in 16 studies. Incomplete outcome data were addressed adequately in 11 studies. All pre-specified outcome variables were adequately reported in three studies, where protocols were available.
3.4. Efficacy of pharmacotherapy-assisted psychotherapy
Data from four studies (Hien et al., 2015, Popiel et al., 2015, Schneier et al., 2012, Simon et al., 2008) (n = 267) were available for inclusion in a meta-analysis of reduction in PTSD symptoms for an SSRI plus therapy versus placebo plus therapy (Figure 2). Three studies used prolonged exposure and one used Safety Seeking. The standard mean difference was −0.22 (95% CI −0.58 to 0.14) and I2 = 38%.
Figure 2.
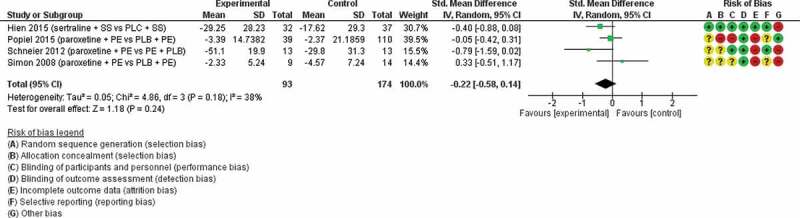
SSRI-assisted therapy versus placebo-assisted therapy
Ss = safety seeking therapy, PLC = placebo, PE = prolonged exposure.
Four studies (De Kleine et al., 2012, Difede et al., 2014, Litz et al., 2012, Rothbaum et al., 2014), (n = 224) were available for inclusion in a meta-analysis of reduction in PTSD symptoms for DCS-assisted exposure therapy versus placebo-assisted exposure therapy (Figure 3). The standard mean difference was 0.00 (95% CI −0.45 to 0.46) and I2 = 59%.
Figure 3.
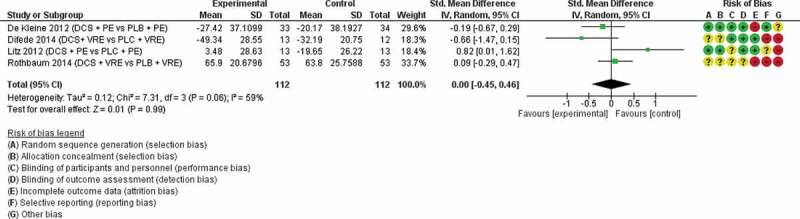
DCS-assisted therapy versus placebo-assisted therapy
DCS = d-cycloserine, PE = prolonged exposure, VRE = virtual reality exposure, PLB = placebo
Four studies (Mithoefer et al., 2010, Mithoefer et al., 2018, Oehen et al., 2013, Ot’alora et al., 2018) (n = 85) were available for inclusion in a meta-analysis of reduction in PTSD symptoms for MDMA-assisted therapy versus placebo/MDMA active placebo-assisted therapy (Figure 4). The standard mean difference was −1.09 (95% CI −1.60 to −0.58) and I2 = 0%.
Figure 4.
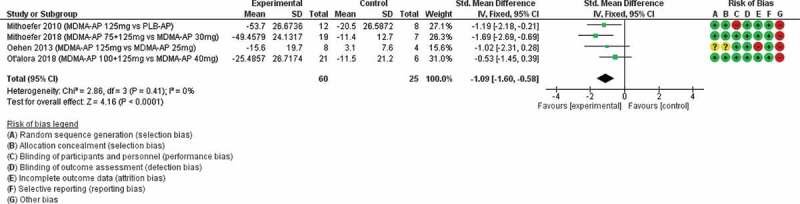
MDMA-assisted therapy versus placebo/active placebo-assisted therapy
MDMA-AP = MDMA-assisted therapy, PLB = placebo
Two studies (Otto et al., 2003, Rothbaum et al., 2006) (n = 75) were available for inclusion in a meta-analysis of reduction in PTSD symptoms for an SSRI plus therapy versus an SSRI alone (Figure 5). The standard mean difference was 0.2 (95% CI −1.01 to 1.04) and I2 = 58%.
Figure 5.
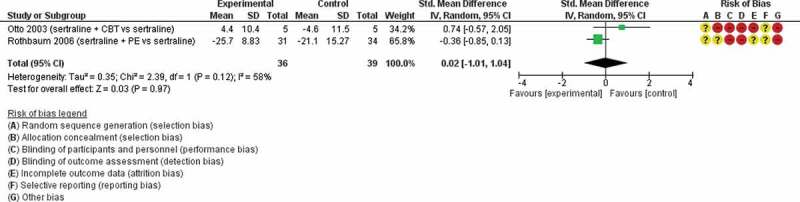
SSRI plus PE versus SSRI
CBT = cognitive-behavioural therapy, PE = prolonged exposure
4. Discussion
Only MDMA-assisted therapy was superior to placebo/active placebo-assisted therapy on reducing clinician-rated PTSD symptom severity. There was no evidence found to suggest that any of the other agents were superior to placebo/active placebo-assisted therapy on reducing clinician-rated PTSD symptom severity although a very small positive effect was found when all pharmacotherapy-assisted psychotherapies were considered together.
Several studies examined the efficacy of combining evidence based psychological therapies and medications. The absence of evidence to support the use of SSRI or SNRI medication in addition to psychotherapy was based upon a small number of RCTs with mixed methodologies and results. Evidenced based medications such as specific SSRIs sertraline, paroxetine, fluoxetine and the SNRI venlafaxine share in common a therapeutic lag in both clinical trials and clinical practice. Typically, patients are told to wait 6–8 weeks after initiation and then after each dose escalation before the full clinical effect might be felt.
Across the included several studies below, there was a difference in approach to combining medication and therapy; some started both simultaneously, some started therapy for several weeks first, then randomised non-responders to continue therapy or add in a medication or placebo, and some started medication in an open-label fashion before randomising and adding in therapy. There is an intuitive mechanism of action, whereby the small positive effect of medication when used as monotherapy, may allow participants to begin therapy with a smaller symptom load and perhaps tolerate and engage in therapy more effectively. In those studies that started medication and therapy simultaneously, or started medication after open-label therapy, this mechanism may not have been allowed time to work effectively.
Buhman et al. (2018) used a pragmatic study design in an asylum seeker population (n = 280) of mixed traumas to investigate four parallel arms; receiving sertraline with or without mianserin augmentation; receiving sertraline with or without mianserin augmentation plus therapy; therapy alone; and waitlist control. There was no significant difference found between each group, and of note, only 25% of participants who received a form of therapy actually received a trauma-focussed therapy. Additionally, 27% of participants in the therapy only group also received concomitant psychotropic medication.
Hien et al. (2015) compared two groups of participants with PTSD with alcohol use disorder, who received either sertraline plus 12 weeks of Safety Seeking (SS) therapy (n = 32), or placebo plus SS (n = 37). There were significant improvements in PTSD symptom severity in the sertraline plus therapy group compared to the placebo plus SS group. Interestingly, medication was titrated over two weeks prior to therapy commencing, a window that is well within the expected therapeutic lag of 6–8 weeks. Further research to explore this combination of sertraline plus SS is warranted, and researchers may wish to consider allowing for more time to establish participants on a therapeutic dose prior to commencing therapy, as their clinical gains might theoretically improve.
Popiel et al. (2015) compared three groups of PTSD participants with motor-vehicle accident-related trauma, receiving either 12 weeks of prolonged exposure (PE) (n = 114), paroxetine (n = 57), or PE plus paroxetine (n = 57). There were significant improvements in PTSD symptom severity across all three groups, with higher rates of remission in the PE alone group than in the paroxetine alone group. There was again a rapid dose titration in those participants receiving paroxetine, with maximum doses achieved after three to seven days; it could be reasoned that the therapeutic lag may not have clinically synchronised with the start of PE in the combined PE plus paroxetine group.
Otto et al. (2003) compared two groups of pharmacotherapy refractory female survivors of the Pol Pot regime Cambodia who received either sertraline plus five sessions of group cognitive-behavioural therapy (CBT) (n = 5), or CBT alone (n = 5). The authors reported that the combined group was superior to medication alone, but unfortunately, this small drug company-sponsored study was poorly designed with high rates of bias and incomplete outcome reporting.
Rothbaum et al. (2006) compared a population of PTSD participants with predominantly sexual assault traumas across two groups; sertraline treatment plus 10 sessions of twice weekly PE (n = 34) versus sertraline alone (n = 31). The study design allowed for 10 weeks of open-label sertraline prior to randomisation, which is commendable in that this will allow for any therapeutic lag effect to be negated prior to augmentation with therapy. Whilst there was no significant difference in PTSD symptom severity between these two groups, the combined sertraline plus PE group was superior to sertraline alone for weaker medication responders in a post-hoc analysis.
Schneier et al. (2012) investigated survivors of the World Trade Centre attack across two groups; paroxetine plus 10 weekly sessions of PE (n = 19) versus placebo plus 10 weekly sessions of PE (n = 18). There was greater reduction in PTSD symptom severity and higher rates of remission in the combined paroxetine and PE group at the immediate post-treatment point, but these differences were not maintained at follow up. The dose of paroxetine was started at 12.5 mg and increased in 12.5 mg increments per day for the first two weeks, then in 25 mg increments in week three and up to a maximum of 50 mg. Again, the simultaneous administration of paroxetine and PE without allowing for therapeutic lag may negatively affect the maximum theoretical benefit from combining approaches.
Simon et al. (2008) compared two groups of predominantly physical and sexual abuse survivors who received either paroxetine plus 10 sessions of PE (n = 9) or placebo plus PE (n = 14). All participants first received eight sessions of PE and non-responders were then randomised to either treatment arms. There was no significant difference between the groups, but there was a small trend towards greater reduction of PTSD symptom severity in the placebo group.
D-cycloserine (DCS) is a partial NMDA receptor agonist, and was investigated in four studies included for meta-analysis; the absence of evidence for the use of DCS to augment exposure-based therapies is, perhaps, more surprising as DCS, a partial NMDA receptor antagonist, has been found to facilitate fear extinction in animal models. There were mixed results across the four included studies and DCS was given at two different doses and at two different time points across the studies; either 50 mg or 100 mg, and given either 30 minutes or 90 minutes prior to therapy.
de Kleine et al. (2012) randomised 67 mostly female PTSD participants with sexual abuse as the predominant trauma type to two groups; 50 mg DCS given orally 1 hour prior to 10 weekly PE sessions (n = 33) versus placebo given in the same manner plus PE. Concomitant psychotropics were allowed, and DCS was only administered for face to face PE sessions rather than given as a take-home medication to bs used prior to any homework. There were no significant differences between the two groups, but in a post hoc analysis, there was greater PTSD symptom reduction in those participants with higher pre-treatment PTSD scores.
Difede et al. (2014) investigated 25 survivors of the World Trade Centre attacks across two groups; those receiving 100 mg DCS 90 minutes prior to sessions 2–11 of 12 weekly sessions of CBT, using virtual reality exposure (VRE) (n = 13), versus placebo given in place of DCS plus the same treatment modality (n = 12). Pharmacotherapy was allowed across both groups, if already on a stable dose of 2 months. There were significantly greater remission rates in the DCS plus VRE group.
Litz et al. (2012) investigated 26 male combat veterans across two groups; those receiving 50 mg of DCS given 30 minutes prior to sessions 2–5 of 6 weekly sessions of exposure, versus those receiving placebo given with an identical treatment modality. Interestingly, there was significantly less symptom reduction in the DCS plus exposure group compared to the placebo plus exposure group.
Finally, Rothbaum et al. (2014) randomised 106 mostly male combat veterans to three groups; group 1 received 6 weekly sessions of VRE with 50 mg DCS given 30 minutes prior to each session (n = 53); group 2 received alprazolam plus VRE (n = 50); and group 3 received placebo plus VRE (n = 53). Fifty-six percent of participants were on a stable dose of psychotropic medications. There were significant improvements in PTSD symptom severity across all VRE groups, but no significant difference between them.
Four single studies assessed the use of propranolol, yohimbine, cortisol and methylene blue and as such, it was not possible to combine the disparate agents into any meaningful meta-analysis. Brunet et al. (2018) compared brief, weekly trauma reactivation written narrative work using placebo or short and long-acting propranolol, which showed significant improvements in PTSD symptom severity in the propranolol group compared to placebo. This approach will require further research replication.
Tuerk et al. (2018) compared yohimbine and placebo when given 1 hour prior to prolonged exposure settings over 15 weeks, but found no significant difference between the comparison arms. Yehuda et al. (2015) compared 10 sessions of manualised prolonged exposure with either 30 mg cortisol or placebo administered 20 minutes prior to sessions 3–10, and found veterans administered cortisol were more likely to respond to treatment, but there were no significant differences between those who had placebo on PTSD symptom outcomes.
The positive finding for MDMA-assisted psychotherapy supports the FDA’s decision to grant it breakthrough therapy status in 2018 and can potentially be explained by its unique psychopharmacological profile. MDMA ingestion is typically characterized by 2–6 hours of subjective feelings of well-being, sociability, and positive mood. It exerts its effect mainly through both an increased release and reuptake inhibition of presynaptic serotonin, and to a lesser effect, dopamine. MDMA is also associated with a robust release of the neuropeptide oxytocin which precipitates pro-social subjective experiences in healthy controls. MDMA stimulates release of cortisol and noradrenaline, which may enhance both emotional engagement and extinction learning (Yehuda et al., 2015). Given its potential to reduce fear responding, enhance fear extinction, and increase prosocial emotional states, MDMA has been proposed as a candidate for assisting psychological therapies in traumatized people. MDMA is currently an illegal drug in all countries of the world and its research is tightly controlled.
A novel MDMA-Assisted Psychotherapy (MDMA-AP) approach was developed and manualized by the non-profit Multidisciplinary Association for Psychedelic Studies (MAPS) which includes a female-male therapist dyad, weekly 90-min preparation and integration sessions and 2–3 eight-hour-long drug-assisted sessions. During the drug-assisted sessions the participants are encouraged to focus their attention inwards in a comfortable setting, making use of eye shades and earphones with music.
The effects of MDMA-AP have been evaluated in four published RCTs of patients with chronic PTSD and our meta-analysis of MDMA-AP demonstrated an impressive effect size for this emerging intervention. Two of the included studies also included participants who were treatment-resistant, which was defined in one study as having previously undergone at least 6 months of therapy or 3 months of SSRI treatment. Notably, one long-term follow up study (Zoellner et al., 2017) found that clinical gains were largely sustained over time, with no evidence of addiction or abuse of MDMA over time and a positive adverse effect profile.
It is, however, difficult to have ultimate confidence in the effect size found because of the small numbers of participants in published studies (n = 85), as well as several methodological and other issues. A key difficulty in this research is the ability to maintain the blind, as MDMA demonstrates acute effects likely noticeable compared to placebo. However, all but one (Hien et al., 2015) of the four MDMA studies utilised a low-dose MDMA active placebo to assist in maintaining the blind.
MDMA-AP uses a non-evidence-based psychotherapy model, which, to our knowledge, has not been evaluated against other TFPTs in a medication-free setting. Lastly, all four published studies have been funded and managed by a single sponsor (MAPS), which potentially increases the risk of biased results. There are currently several ongoing Phase 2 studies in the USA, Israel and Canada, with more open-label feasibility studies planned across nearly a dozen EU sites. In the USA, the regulatory body (Food and Drug Administration) has granted Breakthrough Therapy Designation for MDMA-AP, with Phase 3 studies planned in the coming years. Future research will benefit from follow-up studies replicating and extending this work in additional research groups.
4.1. Study limitations
This study set out to examine the effects of drug-assisted therapies, and delineated approaches into three groups; conventional pharmacotherapy plus therapy, MDMA-AP and DCS-AP. A major strength of the study was adherence to the robust and state of the art systematic review and meta-analysis methodology advocated by The Cochrane Collaboration. There were, however, relatively few studies with small numbers per group with which to perform meta-analyses in each group, raising power and other issues with respect to the results. As the field progresses, future studies should broaden our knowledge and allow more confidence in the results of meta-analyses. Unfortunately, a significant proportion of studies conducted their analyses on a modified intention to treat (mITT) population, which does not adhere to the ‘once randomised, always analysed’ principle of an intention to treat analysis and therefore may bias the outcome. However, due to the number of studies which employed mITT analyses, we chose to include them in order to be able to conduct a meaningful review.
In the conventional SSRI/SNRI pharmacotherapy plus therapy group, in addition to the small number of studies, there was a wide variation in methodology and overall quality. Many of the included studies initiated an SSRI or SNRI followed by therapy after as little as two weeks and many did not report a measure of response to medication prior to therapy. This approach has several problems; it is unlikely to allow participants to feel the most benefit from the medication prior to therapy and it does not allow reviewers to understand differences between medication responders and non-responders who then go on to receive therapy. An alternative and preferable approach in future research would be to augment an established pharmacological treatment with psychotherapy. Further research in this area is, therefore, warranted, not least because combining conventional medications with TFPT is very common in clinical practice.
In the DCS-AP group, DCS was given at various doses and intervals prior to therapy, which was also given for a wide durational range (6–12 weeks). DCS was only given before formal therapy sessions; the therapists and authors did not adequately explore the possibility of giving ‘take home’ doses of DCS for use in completing homework between sessions. DCS-AP was given to participants who were not identified as treatment-resistant or severe, in comparison to MDMA-AP, begging the question who would DCS-AP be suitable for. Additionally, concomitant psychotropics were allowed in the majority of DCS studies, further confounding the true efficacy of this drug.
In the MDMA-AP studies, the very novel subjective experience of the drug is difficult to completely blind, but the use of active low-dose placebo seems to have been effective and should be employed in future research.
The benefit of all studies being funded and managed by a single sponsor (in terms of consistency, personnel training and high quality of reporting) is potentially offset by the risk of bias we associated with authors who are affiliated with one of the interventions. However, given the difficulties inherent in funding research into an experimental drug that is tightly regulated across the globe, very few groups are in a position to conduct research on MDMA.
4.2. Implications
Overall, there are no immediate clinical implications resulting from this study. MDMA-AP is the most promising novel treatment for chronic and often treatment-resistant PTSD, but cannot be recommended as a clinical treatment yet; larger scale Phase 3 studies are required to determine whether this is a safe and effective approach that can be recommended.
Combination approaches using SSRIs and SNRIs with exposure therapies are a common clinical practice, but are not currently supported by a sparse literature that addresses them; further research of conventional agents, which are initiated and titrated up to the highest tolerable dose prior to initiated therapy (pharmacological therapy augmented by psychotherapy), is clearly needed and of great clinical interest. Likewise, further research of DCS-assisted exposure therapy is required to assess whether higher doses given at a longer duration prior to therapy sessions would be superior to placebo.
Acknowledgments
We wish to thank the ISTSS and the Cochrane Collaboration for their support.
Funding Statement
The International Society for Traumatic Stress Studies supported this work to inform the development of their pharmacological treatment guidelines. The views expressed in this article are those of the authors solely and do not necessarily reflect the views, policies or decisions of their employers.
Disclosure statement
Dr Hoskins has received MDMA-assisted psychotherapy training and travel expenses from MAPS. Professor Bisson and Dr Hoskins are co-applicants on a MAPS-sponsored Phase 2 clinical trial of MDMA-assisted psychotherapy taking place with Cardiff University and Cardiff and Vale University Health Board (NHS).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- Bahji, A., Forsyth, A., Groll, D., & Hawken, E. R. (2019). Efficacy of 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for posttraumatic stress disorder: A systematic review and meta-analysis. Progress in Neuro-psychopharmacology & Biological Psychiatry, 10,96.109735. [DOI] [PubMed] [Google Scholar]
- Bisson, J. I., Ehlers, A., Matthews, R., Pilling, S., Richards, D., & Turner, S. (2007). Psychological treatments for chronic post-traumatic stress disorder: Systematic review and meta-analysis. BJPsychiatry, 190(97–104). doi: 10.1192/bjp.bp.106.021402 [DOI] [PubMed] [Google Scholar]
- Blanchard, E. B., Hickling, E. J., Malta, L. S., Jaccard, J., Devineni, T., Veazey, C. H., & Galovski, T. E. (2003). Prediction of response to psychological treatment among motor vehicle accident survivors with PTSD. Behavior Therapy, 34, 351–17. [Google Scholar]
- Brady, R., Green, J., Russ, E., Dutra, L., & Westen, D. (2005). A multidimensional meta-analysis of psychotherapy for PTSD. American Journal of Psychiatry, 162, 214–227. [DOI] [PubMed] [Google Scholar]
- Brunet, A., Thomas, É., Saumier, D., Ashbaugh, A. R., Azzoug, A., Pitman, R. K., … Tremblay, J. (2014). Trauma reactivation plus propranolol is associated with durably low physiological responding during subsequent script-driven traumatic imagery. Canadian Journal of Psychiatry, 59(4), 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhmann, C. B., Nordentoft, M., Ekstroem, M., Carlsson, J., & Mortensen, E. L. (2016). The effect of flexible cognitive-behavioural therapy and medical treatment, including antidepressants on post-traumatic stress disorder and depression in traumatised refugees: Pragmatic randomised controlled clinical trial. British Journal of Psychiatry, 208(3), 252–259. [DOI] [PubMed] [Google Scholar]
- Charuvastra, A., & CLoitre, M. (2008). Social bonds and posttraumatic stress disorder. Annual Review of Psychology, 59(1), 301–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack, K., Jonas, D. E., Forneris, C. A., Wines, C., Sonis, J., Middleton, J. C., … Gaynes, B. N. (2016). Psychological treatments for adults with posttraumatic stress disorder: A systematic review and meta-analysis. Clinical Psychology Review, 43, 128–141. [DOI] [PubMed] [Google Scholar]
- De Kleine, R. A., Hendriks, G. J., Kusters, W. J., Broekman, T. G., & van Minnen, A. (2012). A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biological Psychiatry, 1;71(11), 962–968. [DOI] [PubMed] [Google Scholar]
- Difede, J., Cukor, J., Wyka, K., Olden, M., Hoffman, H., Lee, F. S., & Altemus, M. (2014). D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: A pilot randomized clinical trial. Neuropsychopharmacology, 39, 1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan, J. C., Sippel, L. M., Wahlquist, A., Moran-Santa Maria, M. M., & Back, S. E. (2018). Augmenting prolonged exposure therapy for PTSD with intranasal oxytocin: A randomized, placebo-controlled pilot trial. Journal of Psychiatric Research, 98, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa, E. B., Hembree, E., & Rothbaum, B. (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences, therapist guide. UK: Oxford University Press. doi: 10.1093/med:psych/9780195308501.001.0001 [DOI] [Google Scholar]
- Hien, D. A., Levin, F. R., Ruglass, L. M., López-Castro, T., Papini, S., Hu, M. C., … Herron, A. (2015). Combining seeking safety with sertraline for PTSD and alcohol use disorders: A randomized controlled trial. Journal of Consulting and Clinical Psychology Journal of Consulting Psychology, 83, 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T., & Green, S. (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0. Cochrane Collaboration.
- Hoskins, M., Pearce, J., Bethell, A., Dankova, L., Barbui, C., Tol, W., & Bisson, J. (2015). Pharmacotherapy for post-traumatic stress disorder: Systematic review and meta-analysis. British Journal of Psychiatry, 206(2), 93–100. [DOI] [PubMed] [Google Scholar]
- Hoskins, M. D., Bridges, J., Sinnerton, R., Nakamura, A., Underwood, J. F. G., Slater, A., … Bisson, J. I. (in press). Pharmacological therapy for post-traumatic stress disorder: A systematic review and meta-analysis of monotherapy, augmentation and head-to-head approaches. Journal of Traumatic Stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen, P. Ø., & Krebs, T. S. (2009). How could MDMA (ecstasy) help anxiety disorders? A neurobiological rationale. Journal of Psychopharmacology, 23(4), 389–391. [DOI] [PubMed] [Google Scholar]
- Litz, B. T., Salters-Pedneault, K., Steenkamp, M. M., Hermos, J. A., Bryant, R. A., Otto, M. W., & Hofmann, S. G. (2012). Randomized placebo-controlled trial of d-cycloserine and exposure therapy for posttraumatic stress disorder. Journal of Psychiatric Research, 46(9), 184–1190. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols, D., De La Cruz, L. F., Monzani, B., Rosenfield, D., Andersson, E., Pérez-Vigil, A., … Farrell, L. J. (2017). D-cycloserine augmentation of exposure-based cognitive behavior therapy for anxiety, obsessive-compulsive, and posttraumatic stress disorders: A systematic review and meta-analysis of individual participant data. JAMA Psychiatry, 74(5), 501–510. [DOI] [PubMed] [Google Scholar]
- McNally, R. J. (2007). Mechanisms of exposure therapy: How neuroscience can improve psychological treatments for anxiety disorders. Clinical Psychology Review, 27(6), 750–759. [DOI] [PubMed] [Google Scholar]
- Mithoefer, M. C., Mithoefer, A. T., Feduccia, A. A., Jerome, L., Wagner, M., Wymer, J., … Doblin, R. (2018). 3,4-methylendioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers; a randomised, double-blind, phase 2 clinical trial. Lancet Psychiatry, 5, 486–497. [DOI] [PubMed] [Google Scholar]
- Mithoefer, M. C., Wagner, M. T., Mithoefer, A. T., Jerome, L., & Doblin, R. (2011). The safety and efficacy of {±}3,4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: The first randomized controlled pilot study. Journal of Psychopharmacology, 25(4), 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithoefer, M. C., Wagner, M. T., Mithoefer, A. T., Jerome, L., Martin, S. F., Yazar-Klosinski, B., … Doblin, R. (2013). Durability of improvement in post-traumatic stress disorder symptoms and absence of harmful effects or drug dependency after 3,4-methylenedioxymethamphetamine-assisted psychotherapy: A prospective long-term follow-up study. Journal of Psychopharmacology, 27(1), 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, K. M., & Davis, M. (2007). Mechanisms of fear extinction. Molecular Psychiatry, 12, 120–150. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Mental Health . (2005). Post-traumatic stress disorder: The management of PTSD in adults and children in primary and secondary care. Clinical Guideline, 26. ISBN 1-904671-25-X. [PubMed] [Google Scholar]
- Oehen, P., Traber, R., Widmer, V., & Schnyder, U. (2013). A randomized, controlled pilot study of MDMA (± 3,4-Methylenedioxymethamphetamine)-assisted psychotherapy for treatment of resistant, chronic Post-Traumatic Stress Disorder (PTSD). Journal of Psychopharmacology, 27(1), 40–52. [DOI] [PubMed] [Google Scholar]
- Ogden, P. K. M., Minton, K., & Pain, C. (2006). Trauma and the body. Ney York: Norton press. ISBN 978-0-393-70457–0. [Google Scholar]
- Ot’alora, M. G., Grigsby, J., Poulter, B., Van Derveer, J. W., III, Giron, S. G., Jerome, L., … Doblin, R. (2018). 3,4-methylenedioxymethamphetamineassisted psychotherapy for treatment of chronic posttraumatic stress disorder: A randomized phase 2 controlled trial. Journal of Psychopharmacology, 32(12), 1295–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto, M. W., Hinton, D., Korbly, N. B., Chea, A., Ba, P., Gershuny, B. S., & Pollack, M. H. (2003). Treatment of pharmacotherapy-refractory post traumatic stress disorder among Cambodian refugees: A pilot study of combination treatment with cognitive behavior therapy vs sertraline alone. Behaviour Research and Therapy, 41, 1271–1276. [DOI] [PubMed] [Google Scholar]
- Popiel, A., Zawadzki, B., Pragłowska, E., & Teichman, Y. (2015). Prolonged exposure, paroxetine and the combination in the treatment of PTSD following a motor vehicle accident. A randomized clinical trial - The tRAKT study. Journal of Behavior Therapy and Experimental Psychiatry, 48, 17–26. [DOI] [PubMed] [Google Scholar]
- Review Manager (RevMan) . (2014). [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. [Google Scholar]
- Roberts, N. P., Kitchiner, N. J., Kenardy, J., & Bisson, J. I. (2010). Early psychological interventions to treat acute traumatic stress symptoms. Cochrane Database of Systematic Reviews, 17(3), CD007944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum, B. O., Cahil, S. P., Foa, E. B., Davidson, J. R. T., Compton, J., Connor, K. M., … Hahn, C. G. (2006). Augmentation of sertraline with prolonged exposure in the treatment of Posttraumatic Stress Disorder. Journal of Traumatic Stress, 19(5), 625–638. [DOI] [PubMed] [Google Scholar]
- Rothbaum, B. O., Price, M., Jovanovic, T., Norrholm, S. D., Gerardi, M., Dunlop, B., … Ressler, K. J. (2014). A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. American Journal of Psychiatry, 171(6), 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago, P. N., Ursano, R. J., Gray, C. L., Pynoos, R. S., Spiegel, D., Lewis-Fernandez, R., … Fullerton, C. S. (2013). A systematic review of PTSD prevalence and trajectories in DSM-5 defined trauma exposed populations: Intentional and non-intentional traumatic events. PLoS One, 8(4), e59236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier, F. R., Neria, Y., Pavlicova, M., Hembree, E., Suh, E. J., Amsel, L., & Marshall, R. D. (2012). Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: A randomized controlled trial. American Journal of Psychiatry, 169, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenbauer, M. A., Glass, C. R., Arnkoff, D. B., Tendick, V., & Gray, S. H. (2008). Nonresponse and dropout rates in outcome studies on PTSD: Review and methodological considerations. Psychiatry, 71(2), 134–168. [DOI] [PubMed] [Google Scholar]
- Simon, N. M., Connor, K. M., Lang, A. J., Rauch, S., Krulewicz, S., LeBeau, R. T., … Pollack, M. H. (2008). Paroxetine CR augmentation for posttraumatic stress disorder refractory to prolonged exposure therapy. The Journal of Clinical Psychiatry, 69(3), 400–405. [DOI] [PubMed] [Google Scholar]
- Sonne, C., Carlsson, J., Bech, P., Elklit, A., & Mortensen, E. L. (2016). Treatment of trauma-affected refugees with venlafaxine versus sertraline combined with psychotherapy - a randomised study. BMC Psychiatry, 16, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuerk, P. W., Wangelin, B. C., Powers, M. B., Smits, J. A. J., Acierno, R., Myers, U. S., … Hamner, M. B. (2018). Augmenting treatment efficiency in exposure therapy for PTSD: A randomized double-blind placebo-controlled trial of yohimbine HCl. Cognitive Behaviour Therapy, 2018, 1–2. [DOI] [PubMed] [Google Scholar]
- Yehuda, R., Bierer, L. M., Pratchett, L. C., Lehrner, A., Koch, E. C., Van Manen, J. A., … Hildebrandt, T. (2015). Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology, 51, 589–597. [DOI] [PubMed] [Google Scholar]
- Young, M. B., Norrholm, S. D., Khoury, L. M., Jovanovic, T., Rauch, S., Reiff, C. M., … Howell, L. L. (2017). Inhibition of serotonin transporters disrupts the enhancement of fear memory extinction by 3,4-methylenedioxymethamphetamine (MDMA). Psychopharmacology, 234(19), 2883–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoellner, L. A., Telch, M., Foa, E. B., Farach, F. J., & McLean, C. P. (2017). Enhancing extinction learning in PTSD with brief daily imaginal exposure and methylene blue: A randomized control trial. Journal of Clinical Psychiatry, 78(7), 782–789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


