Abstract
Upper respiratory tract infection (URTI)-associated acute cough is the most common symptom both in children and adults worldwide and causes economic and social problems with significant implications for the patient, the patient's family, and the health care system. New pathogenic mechanisms in acute cough, including the urge to cough (UTC) mechanisms, have been recently identified. The brainstem neural network, pharyngeal sensory innervation, airway mechanical stimulation, inflammatory mediators, and postnasal drip actively participate in the onset and maintenance of acute cough and the urge to cough phenomenon. However, there is still no effective pharmacological treatment capable of interfering with the pathophysiologic mechanisms involved in URTI-associated acute cough. Moreover, severe adverse events frequently occur in administering such cough medications, mainly in children. New evidence has been provided concerning polysaccharides, resins, and honey as potential cough relievers with high antitussive efficiency, effect on the UTC, and minimal side effects.
Keywords: acute cough, upper respiratory tract infection, urge-to-cough, children, pathophysiology, polysaccharides, honey, treatment
Introduction
Cough is scholastically defined as a forced expulsive maneuver, usually against a closed glottis, and is associated with a characteristic sound.1 Noteworthy, cough is a protective physiological reflex deputed to clear secretions from the airways or remove inhaled materials. As a physiological reflex, healthy children usually cough on average 11 times (range 1–34) daily.2 The cough may be pragmatically classified considering its duration and its characteristics.3 Conventionally, acute cough lasts <4 weeks, whereas chronic cough lasts more than 4 weeks.4 Cough may be wet, such as productive cough associated with secretion, or dry, such as irritative cough.5,6
Acute cough is usually caused by upper respiratory tract infections (URTIs), mainly sustained by a virus. Therefore, postviral acute cough is the most common respiratory symptom worldwide.7 It is important to remember that other triggers may cause acute cough, such as exposure to irritants (including tobacco smoke, pollutants, smells, aerosols, and dust), cold and/or dry air, and allergens, typically pollens in subjects with hay fever. Notably, it has to be kept in mind that if URTI symptoms, except cough, last more than 10 days, acute rhinosinusitis should be also suspected.8
Acute cough places a relevant burden on the socio-economy because children miss daycare or school and their parents miss workdays.9 In addition, acute cough is responsible for over 50% of new patient attendance in the primary care setting and is the major source of consultation in pharmacy practice.10 Consistently, URTI is very frequent as adult subjects experience 2–5 episodes yearly and school children suffer from 7 to 10 episodes per year and can cough for up to 140 days/year.11 Children are almost 4 times more likely to experience URTI-associated acute cough than adults; females are more frequently affected than males.12 URTI-associated acute cough is particularly intense and causes discomfort in children and elderly patients. Epidemiological surveys have shown that acute cough in otherwise healthy subjects has an average duration of 14 days.9 In children, however, acute cough can resolve in 50% of children by 10 days and in 90% by 25 days.13 On the other hand, prolonged cough substantially affects the quality of life and psychosocial well-being, particularly in children and in their families. Even though acute cough is usually a trivial and self-limiting problem, it is very troubling for children and the whole family. It often results in discomfort to the child and loss of sleep for both the child and all cohabitants, as recently reported by an Italian survey showing that cough disturbed sleep in 88% of children and 72% of parents.14 Moreover, cough entails social embarrassment as the coughing person is soon isolated and confined.
Cough treatment frequently requires self-medication or medical consultation.15 Costs of medical visits and over-the-counter products constitute a relevant burden. It has been estimated that $4 billion is spent worldwide on antitussive drugs per year. In the United Kingdom, the economic burden is expected to be at least £979 million per year, comprising £875 million in lost productivity and £104 million in costs to the health care system.7 Taking these issues into account, it is evident that acute cough management represents a relevant challenge in clinical practice.
Clinical Aspects of URTI-Associated Acute Cough
Acute cough consequent to URTI may persist in children for many days.16,17 Studies, using questionnaires for parents, demonstrated that long-standing cough, in the absence of asthma, is present in 5%–10% of children, independently of the moment at which the data were collected.18 Early entry into daycare makes very young children susceptible to acute respiratory infections; in these cases, parents' discomfort and anxiety are high when the children have a URTI episode as the practical approach is difficult. Indeed, when a child contracts the URTI and coughs, the parents encounter serious problems and awkwardness in taking care of him/her. In this regard, a review analyzed the natural history of cough, such as characteristics and duration, in children from birth to 4 years of age; the results showed that cough in infants and toddlers was almost always caused by a respiratory infection, two-thirds of children were visited by the pediatrician at least once per year for an acute respiratory infection, and three-fourths presented cough at the medical office.19 The peak incidence of acute cough is usually observed in January and February (8 times higher than in August).20 An Australian prospective study in a cohort of children under 10 years of age reported a yearly frequency of 2.2–5.3 respiratory infections and an average duration of the episodes of 5.5–6.8 days.21 A primary care study found that the yearly number of respiratory tract infections ranged between 5.0 and 7.95 in 0–3-year-old children and 2.4 and 5.02 in 10–14-year-old ones; it was therefore considered normal that a preschooler suffers as many as 6–8 episodes per year.22
From an etiological point of view, cough in children, differently from adults, is divisible into 3 main groups: expected cough, specific cough, and nonspecific cough; these groups may also overlap. Specific cough presents with signs that lead to the possibility of an underlying etiology, which must be identified through thorough investigations, while nonspecific cough is a dry, nonproductive chronic cough usually not accompanied by any other signs of respiratory ailments such as to allow a precise diagnosis.23 The most frequent type of cough experienced by children is expected cough, such as the cough associated with an acute respiratory infection. This cough is quite intense even at night and greatly disturbs sleep.24 The parents' worries about their children's cough can reach extreme levels, including the fear that the child may suffocate and suffer from pulmonary damage or infective complications affecting the lower airways.23 Besides anxiety and fear, parents do not sleep well when there is a child in the home who coughs during the night, and the sense of unease persists throughout the whole day.
About 66% of 0–4-year-old children with acute cough improve by 2 days, even if the cough itself and the nasal secretions persist for at least 1 week in 50% of children and for 3 weeks or more in 10%–20%.19 From the parental perspective, acute cough is therefore not a trivial illness, considering that some children remain unwell for 3–4 weeks.
The persistence of cough, its intensity, its impact on the quality of life, nasal obstruction, and rhinorrhea often lead parents to consult their pediatrician even when the child's general physical condition has improved. However, it is important to consider that cough and nasal secretions, even though they persist at length, usually tend to resolve spontaneously, thus second/third consultations and abuse of self-medication should be intensely discouraged by the pediatrician in clinical practice. The most important recommendation is to inform and reassure parents about the self-limiting characteristic of this issue.
What Is Cough?
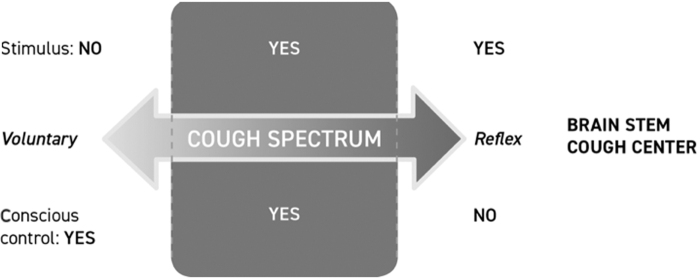
Cough is a physiological response to airway irritation, serving a fundamental role in airway protection and maintenance of airway patency. Cough has 2 main functions: (1) to prevent the entry of food and fluids into the lower airways and (2) to favor removal of material, which (due to its quantity, size, or rheological characteristics) exceeds the transport capacity of the mucociliary system. Cough can occur voluntarily or in response to a stimulus and can be both reflexive and nonreflexive, such as behavioral.25
Recently, increasing interest has been focused on the sensation of irritation that precedes the motor act of coughing, the so-called urge to cough (UTC) sensation, which in turn triggers the cough. It was initially thought that cough could be only generated by a brainstem neural network. Instead, cough can also be initiated by pharyngeal stimulation and this indicates that sensory nerves in the pharynx may be also involved in generation of the cough reflex associated with URTI.26 Chemical and mechanical stimulation of the airway can elicit the cough reflex and also a cognitive sensation, the UTC. Sensing the UTC is an important component for maintaining adequate airway protection. The UTC is the motivational component of voluntary cough and it is induced by stimuli that motivate subjects to protect their airway by coughing. In this regard, it has been hypothesized that there might be 2 separate pathways involved in the control of cough, a reflex pathway and a voluntary pathway (Fig. 1).27 It has been, therefore, supposed that URTI-associated cough is mainly elicited by the voluntary pathway, such as the cognitive recognition of the necessity to cough to protect airways (the UTC). Upper airway irritative stimuli, caused by mucosal inflammation, reach the cerebral cortex and elicit cough under voluntary control. The cortical response to afferent information from the pharynx is important because tussigenic stimuli in turn increase the UTC.28 This mechanism can explain why cough, associated with URTI or other types of oropharyngeal irritations, may be scarcely affected by codeine and common antitussive synthetic drugs, which usually interact with the reflex pathway of cough: in fact, codeine has been demonstrated to be no more effective than a syrup vehicle in controlling URTI-associated cough.29 Purely voluntary cough is consciously generated from the motor cortex in the brain independently of any peripheral sensory input from the airways.29 Moreover, purely reflex cough requires peripheral sensory input to the brainstem and occurs independently of any conscious control from the higher brain. This type of cough can be evoked under decerebrated or heavily anesthetized conditions and represents the basic defensive cough reflex. Between these 2 extremes on this spectrum (denoted by the shaded area), coughing is dependent not only on sensory input but also on various levels of behavioral control that can be enhanced or suppressed consciously, as depicted in Fig. 2.29
FIG. 1.
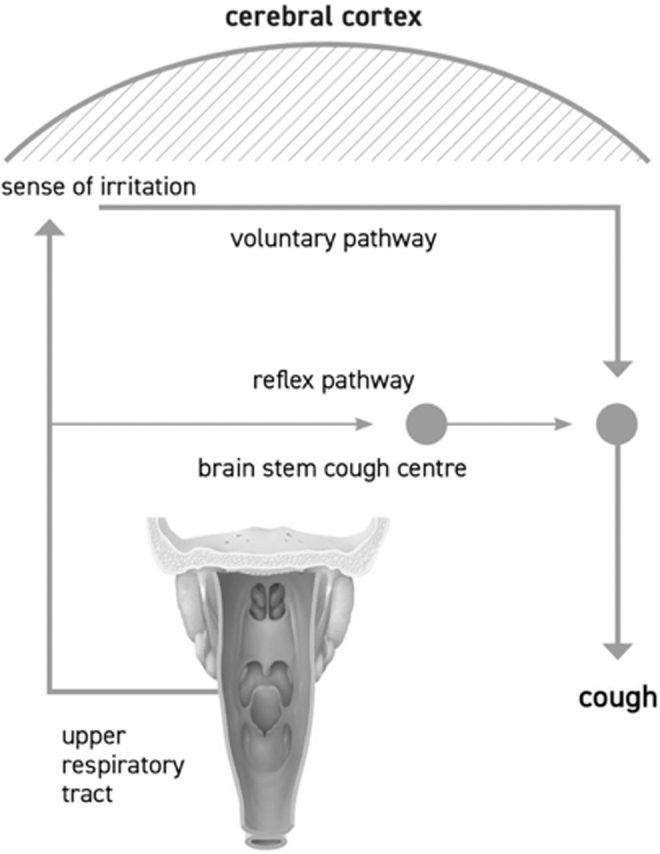
The reflex of the cough.
FIG. 2.
The spectrum of the cough.
The cough that accompanies URTIs may be probably situated in the intermediate area of the spectrum, mediated by the sense of irritation generated by inflammatory stimuli of pharyngeal origin, and partly of reflex origin. The ability of healthy subjects and individuals with URTI to voluntary suppress cough elicited by capsaicin has been demonstrated by a survey aiming at characterizing cough and UTC symptoms associated with the common cold: 98% of the respondents recognized the UTC phenomenon.30 Nearly two-thirds of the respondents with the UTC were unable to control cough. The ability to voluntarily control the UTC could be directly related to the intensity and duration of the stimuli and health conditions of the oropharyngeal mucosa. In healthy subjects, there may be better control of the UTC than in subjects with URTI. The normal protective cough reflex is exaggerated in some ways during URTI so that cough occurs spontaneously even without the risk of any material entering into the airways.
Sensory Innervation of the Pharynx
The pharyngeal epithelium is richly innervated with sensory fibers, but deep receptors are less common than in the oral cavity.31 The sensory supply to the lining of the upper airways consists of bare nerve endings without any specialized form of the terminal receptor.32 The terminal nerve branches, derived from the vagal nerve (X) and the glossopharyngeal nerve (IX), form an extremely dense plexus in the mucosa at the junction of the rhinopharynx and oropharynx, where the greatest density of pharyngeal sensory receptors has been found. The branches of these nerves extend from the lateral wall to the posterior wall of the oropharynx and their terminals in the posterior pharyngeal wall connect, forming a neural network. These densely innervated areas correspond to a potent reflexogenic area triggering the pharyngeal swallowing and upper airway protective reflexes.33 The sensory mechanisms that regulate cough above the level of the glottis remain unknown. Because cough is an important mechanism for protection and clearance of the lower respiratory tract, it may be that sensory areas important for physiological initiation of swallowing are equally important and sensitive in generation of cough.34
Cough Can Be Triggered by Pharyngeal Sensory and Mechanical Stimulation
Many studies investigated the oropharyngeal sensory areas that can elicit or modify swallowing and cough using different sensory modalities, including mechanical, gustatory, and thermal stimuli. Oropharyngeal sensory inputs are specifically important for initiating and modifying the motor events comprising swallowing, and some evidence suggests that stimulation of this area can induce coughing.18 If stimulated, the pharyngeal plexus of nerve fibers can provoke swallowing or coughing, as demonstrated in an elegant study.28 Gentle air puffs directed onto the posterior wall of the oropharynx (of intensity such as to not cause a sensation of pressure, but only of a delicate stimulus) can trigger an intense need to cough or, in some cases, actual coughing.28
Mechanical stimulation, postnasal drip (PND), and water bolus placed into the pharynx evoke vigorous coughing in animals and humans, while capsaicin may be unable to initiate coughing when applied selectively to the pharynx. Infusion of fluid into the pharynx or pharyngeal stimulation with air puffs in awake or anesthetized subjects can initiate coughing or the UTC.35 Both swallowing and coughing are motor actions that depend upon sensory input.
Mechanisms that Trigger Acute Cough and Facilitate Its Persistence
URTI and other exogenous detriments can elicit acute cough by irritative stimuli acting on reflexogenic areas of the pharyngeal mucosa. URTI-associated cough may be determined by inflammatory mediators acting on the sensory terminations of upper airways and enhancing the UTC sensation.36 Information coming from the pharynx can be encoded into a conscious awareness of airway irritations leading to generation of the UTC, which in turn may facilitate behavioral or evoked coughing finalized to clear the airways.
It has been hypothesized that the possible mechanisms by which cough may be triggered and maintained during URTI could be as follows: (1) the inflammatory process caused by a respiratory infection that (through inflammatory mediators) would determine hyperactivity, both local and at a distance, and (2) irritation caused by PND, namely the presence of mucus drainage in the rhinopharynx.36 These elements could mutually contribute to elicit and maintain cough. In particular, 3 main pathogenic mechanisms may be involved: (1) local effects: in upper airway inflammation, the larynx and trachea are always involved in upper airway inflammation, as close to the mouth, pharynx, and nose; (2) humoral effects: the inflammatory mediators, produced at the site of infection, can be transported elsewhere through the bloodstream, triggering an inflammatory response; in fact, cytokines, produced during viral or bacterial infection, affect the central nervous system, determining the onset of fever, as well as mediators could determine airway hyperresponsiveness eliciting cough; and (3) PND: it is caused by the presence of mucus, often in the form of viscous filaments, difficult to expel from the nasopharynx, which when descending through the pharynx, causes a persistent mechanical stimulation of the upper portion of the larynx, provoking a continuous irritating cough. The application of mechanical stimuli, that is, air puffs, to the lateral posterior oropharyngeal walls elicits a cough response preceded by the UTC.34 Therefore, 2 mechanisms could elicit cough, including repetitive mechanical stimulation of the lateral posterior oropharynx wall and pharyngeal irritation secondary to drying and evaporative cooling of the mucosa. This cough may include a motivational component (sensing the UTC) important to maintain adequate airway protection.34
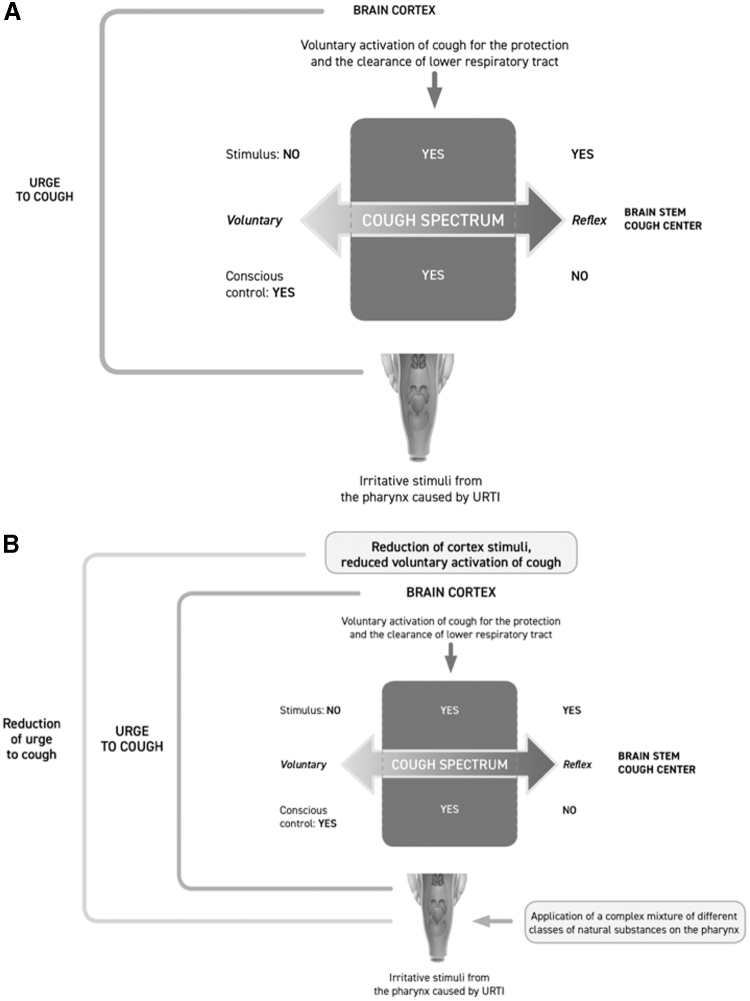
The persistence of cough in a portion of subjects with URTI could be due to lowering of the stimulus threshold dose necessary to cause the UTC and consequently actual cough. This condition can be induced by irritant agents, such as viruses, bacteria, or other inhaled substances, or by unfavorable environmental conditions, such as cold, dry, and/or polluted air. The UTC may therefore be activated and maintained even by stimuli that in normal conditions would be innocuous.37 Irritant stimuli in the pharynx are more involved than classical cough receptors, and the pharynx has a more important role in triggering cough, as patients with a long-lasting cough often complain of a persistent tickling or irritating sensation in the throat (similar to feeling an itch) or a choking sensation.38 The possible spectrum of cough associated with URTI is depicted in Fig. 3A.
FIG. 3.
(A, B) The spectrum of the cough during URTI. (A) Urge to cough elicited by URTIs is probably situated in the intermediate area of the spectrum, mediated by the sense of irritation generated by mechanical and irritative stimuli caused by mucus, inflammatory mediators, and dryness. Oropharyngeal irritative sensory inputs travel to the brain cortex that elicits the urge to cough and actual cough. The urge to cough and cough may be initiated and inhibited by voluntary control from areas in the cerebral cortex. However, nearly two-thirds of the subjects with the urge to cough during common cold seem to be unable to control cough. That is what makes cough during URTIs so bothersome and long-lasting (image by Keller et al.,25 modified). (B) The spectrum of cough during URTIs modulated by application of complex mixtures of natural substances on the pharynx. Polysaccharides and flavonoids exert a mechanical barrier effect, in addition to a demulcent effect, and indirect inflammatory activity that decrease the urge to cough and support the patient's ability to inhibit cough by voluntary control (Image by Keller et al.,25 modified). URTI, upper respiratory tract infection.
Treatment of Cough: A Challenge in Clinical Practice
In children and adults, many of the most commonly used medications for treating acute cough (over-the-counter and prescription medications) failed to prove real efficacy for relieving cough, as reported by recent studies and meta-analyses.7,39–42 Much of the evidence supporting drug therapy in acute cough is old and of poor quality. There is, therefore, scarce randomized, controlled trial-based evidence on this topic.1 A recent review indicated that for subacute cough also there is no beneficial treatment,43 and even for chronic cough, there are no safe and effective therapies.44
The available treatments usually aim to suppress the symptom (cough suppressant therapy) with pharmacological agents that play an inhibitory effect on the cough reflex or act as mucolytic drugs, with the simple intent to reduce both intensity and frequency of cough in the short term. Moreover, some of these medications, including sedatives, mucolytic drugs, first-generation antihistamines, and vasoconstrictors, are burdened by significant side effects, sometimes even fatal in children, thus many health agencies proscribed their use in pediatrics worldwide.45–47 On the other hand, over-the-counter products, mostly cough and common cold formulations, have poor scientific evidence of real efficacy, but significantly account for cost exposures in children.48
A well-designed study investigating URTI-associated cough compared codeine, 30 mg (4 times a day for 4 days), with placebo; codeine had no greater effect than the placebo syrup either on an objective initial cough recording or on a subsequent self-reported cough.29 Another study compared oral codeine (50 mg) with placebo syrup in 82 participants in a parallel-group design; again, no effect greater than that of placebo was observed.27
A Paradigm Change: From Pharmacological Receptor Blockade to Protection of the Pharynx Mucosa
Despite pharmaceutical companies trying continuously to discover novel cough suppressant drugs, there are no new and effective antitussives on the market. There is shared awareness that an efficacious and safe cough drug is still an unmet medical need.49,50 Peripheral and central antitussive agents could be useful in patients with chronic bronchitis, but can have little efficacy in patients with URTI-associated acute cough.51 Indeed, it is exceedingly difficult to prevent cough by an antitussive drug; the lack of effectiveness likely stems from our incomplete understanding of the tussive reflex and involved mediators.52 However, interest has recently emerged in the perception of irritation that precedes the motor event of coughing, such as the UTC. Studies employing functional magnetic resonance imaging in healthy volunteers have demonstrated that the UTC sensation evoked by capsaicin is associated with activation in a variety of brain regions (eg, insular cortex, primary sensory cortex, and orbitofrontal cortex), thus suggesting a cortical neural network in sensing airway irritation and modulating cough.53 Components of this higher brain circuitry also comprise inhibitory mechanisms that can be consciously or subconsciously recruited to suppress cough neural processing in the brain.54 Therefore, the motor act of coughing should not be considered only a protective brainstem reflex, mainly elicited by receptors situated in an area extending from the larynx to the lower airway, which can be blocked or relieved by a pharmacological action. The UTC and the consequent motor act of coughing can be elicited also by irritative sensations acting on reflexogenic areas in the pharynx, and this type of cough does not seem to be modulated or blocked by pharmacological treatment, as evidenced by the fact that no drug available today can effectively block it.52 This concept is consistent with the placebo effect on cough. In most placebo-controlled trials investigating treatments of cough associated with the common cold, the placebo effect is almost as great as that caused by the tested active treatment. The real efficacy of drugs for cough associated with common cold is mainly related to the nonpharmacological properties of the medications, such as the sensory impact and the placebo effect.55 As coughing and UTC are markedly reduced by placebo, it suggests the presence of inhibitory neural pathways that can modify the processing of airway sensory inputs in a nonpharmacological manner. Reduced UTC is associated with decreased activation in some brain regions that respond to capsaicin inhalation. An interesting study demonstrated that the perceptual component of airway sensory irritation, involved in the UTC phenomenon, is significantly modifiable by a placebo.56 The magnitude of brain activation in the number of central loci involved in generating the UTC was significantly reduced by the placebo. Increased activity in the prefrontal cortex likely contributes to the placebo antitussive effects. There is also an emerging body of evidence confirming that perceptive mechanisms are an important component of the cough response and can be modified by sensory feedback.57 During URTI, the level of noxious sensations, traveling from the pharynx to the cortical areas involved in the UTC control, is probably very high as the need to cough is intense in many subjects and can last up to 10–25 days. Thus, reducing the level of irritative stimuli, elicited in the pharynx by the inflammatory process and directed to the cortical neural network, could be an effective intervention choice to limit generation of the UTC and consequently reduce the need for coughing. Therefore, a new, innovative therapeutic approach should be pursued; it could consist in protecting and hydrating the pharynx mucosa and modulating the sensory feedback from the upper airways to the cortical neural network. As a consequence, the goal will be to consciously or subconsciously control the UTC.
Nonpharmacological Modulation of Unpleasant Sensations and Irritative Stimulus Transmission from the Pharynx to the Cortical Neural Network
Mucosal dehydration and mechanical stimuli, caused by pharyngeal dripping of viscous nasal mucus, viruses, bacteria, inflammatory mediators, and irritant substances, play a decisive role in triggering the UTC and sustaining URTI-associated acute cough. In this regard, up to 85% of the benefit of cough syrups depends on the physical and chemical effects of the syrup itself that exert a demulcent action, namely smoothing.26 A demulcent substance can form a soothing film over a mucous membrane, indirectly reducing pain and inflammation.
From a practical point of view, the ideal physical and chemical characteristics of cough syrup can be obtained by a complex mixture of natural substances exerting lubricant, demulcent, and protective barrier effects. This ideal compound can act as a humectant and can, thanks to the natural sweetness of a component such as honey, stimulate salivation and send sweet gustatory stimuli to the brain. The sweet taste and the viscous nature of the syrup are fundamental properties of ideal cough medication. In fact, sweet substances stimulate C fiber sensory neurons through transient receptor vanilloid-1 channels that are involved in cough reflex.58 These features can be better obtained with complex natural substances such as honey and polysaccharides with bioadhesive and demulcent activity. The physical and chemical properties of a specific formulation containing these natural substances can reduce the damage caused by the mucosal contact of microorganisms and other irritant agents. This inhibitory activity can limit production of inflammatory mediators and, consequently, generation of the UTC. These natural substances adhere to the pharynx mucosa and exert a mechanical barrier effect in addition to the demulcent activity, ultimately protecting the mucosa from irritative stimuli (Fig. 3B).
Bioadhesive and Demulcent Properties of Polysaccharides (Mucilage)
The class of compounds, named mucilage, is constituted by heterogeneous acidic polysaccharides. They are large and highly branched polymeric structures built from many different sugars and glucuronic acid units. They are very hydrophilic structures, capable of trapping water (and other molecules) in their cage-like structures to form a gel. These natural hydrocolloids, comprising complex carbohydrate molecules, have a wide scale of physical–chemical properties. The adhesive and protective properties of polysaccharides on mucosae are well known.59,60 Mucilage does adhere to both oral61–63 and gastric mucosae.64 Mucilage acts by accelerating healing of mucosa ulcers through 2 mechanisms: reinforcement of the resistance barrier and the oxygen radical scavenging activity.65
Bioadhesion is a well-known physical phenomenon whereby 2 materials are held together for an extended time by interfacial forces; if it concerns a mucosal tissue, it is named mucoadhesion.66 Histological studies of membranes, incubated with a fluorescence-labeled rhamnogalacturonan, indicated the presence of distinct polysaccharide layers on the apical membrane surface.62 Aqueous extracts of polysaccharide-rich plants are used for treating cough due to their mucoadhesive properties.67 Such properties allow the formation of a polysaccharide layer on the upper airway mucous membrane. In this way, polysaccharides can indirectly modulate the sensitivity of cough receptors and suppress cough. Polysaccharides are too large to be absorbed in the pharynx or to be transported to the bronchial mucosa.60 As a result, their effect, when applied to the pharynx, can be explained simply by a local protective and soothing action. The European Scientific Cooperative on Phytotherapy stated that the mucilage from marshmallow root and mallow flower can cover the mucosa, especially of the mouth and pharynx, protecting them from local irritation.63 Such protective effect is confirmed by the reported efficacy of polysaccharides, including Plantago lanceolata, in cough management.68
The Demulcent Activity of Honey
Honey is a natural substance with a complex composition: it is a supersaturated sugar solution, where carbohydrates are the main constituents accounting for about 95% dry matter.69–71 Other components are proteins, amino acids, enzymes, vitamins, minerals, and phenolic compounds. Potential therapeutic properties of honey have been attributed to these latter substances, which provide this food with antioxidant, radical scavenging activities.72 Honey features vary depending on the botanical source and geographical origin, as well as climatic, processing, and storage conditions.68 Honey exerts multiple activities, including anti-inflammatory, antibacterial, metabolic, and antioxidant properties, and has therefore been positively used in athletes,73 metabolic syndrome,74 and atherosclerosis.75
Current accumulating evidence also suggests that honey may have a role in treating cough and common cold in children.76–78 Notably, the World Health Organization regarded honey as a potentially valuable demulcent for treatment of URTI-associated cough in children.79 Demulcent syrups have an important quantity of sugars. Honey exerts its actions by increasing saliva production and swallowing and (thanks to its viscosity) by coating the peripheral sensory receptors that send irritative stimuli to the cortical neural network and thus interfering with the UTC.
The results of several clinical trials have demonstrated that honey-based syrups may have a beneficial effect for symptomatic relief of nocturnal cough associated with URTIs.9,80–82 The available evidence shows that honey is better than placebo in reducing cough duration for children with acute cough. Honey also reduces cough severity, resolves bothersome cough, and improves sleep quality for both children and their parents to a greater extent than placebo, as recently evidenced by a meta-analysis.83 The use of honey has been demonstrated to be beneficial and safe, with no or minimal risk of side effects such as abdominal pain, nausea, and vomiting, which are common unspecific side effects observable even when using a placebo.84 However, the use of honey is not advisable in children under 12 months of age for the potential risk of infantile botulism.85
A specific, high-quality, polysaccharide–resin–honey-based cough syrup has been investigated in 2 randomized, controlled pediatric trials: this compound was superior to placebo in nocturnal and more intense cough86 and carbocysteine in the diurnal and nocturnal cough score.87
Conclusions
Acute cough caused by URTI is a bothersome, sometimes long-lasting, symptom in children and adults and it is the most common reason why patients seek medical attention. Acute cough, caused by URTI or other exogenous noxious agents, is sustained by irritative stimuli acting on reflexogenic areas of the pharyngeal mucosa that in turn elicit the UTC, a sensation of irritation that precedes the motor act of coughing. This type of cough falls under various levels of behavioral control and can be enhanced, limited, or suppressed consciously, but it is difficult to efficaciously suppress it during URTI only by sheer force of will. Despite huge research efforts, cough is yet an unmet clinical need. However, the efficacy of cough medications for URTI-associated cough is mainly related to nonpharmacological properties, such as the sensory impact and placebo effect. However, the main characteristics of an ideal cough syrup for acute cough, elicited by irritative stimuli in the pharynx, include (1) the ability to form a soothing film over the mucous membrane (a demulcent action), which indirectly reduces pain and inflammation thanks to a protective barrier effect; (2) the lubricant effect reducing repetitive mechanical stimulation of cough bouts and PND; (3) the humectant effect to reduce mucosal dryness; (4) sweetness and viscosity to stimulate salivation and swallowing and send sweet gustatory stimuli to the brain, involved in cough reflex; and (5) the radical scavenger activity for reducing the oxidative status of the inflamed mucosa. Interestingly, these effects can be nonpharmacologically exerted by complex natural substances such as mucilage (acidic polysaccharides), gums, resins, polyphenols, and honey. From a clinical point of view, this therapeutic option, represented by natural substances, could be the first choice, particularly in children, in the light of well-documented efficacy and the elevated profile of safety.
Directions for Future Research
Future research must include the following:
More detailed studies to investigate the pathophysiology of URTI-associated cough and the UTC.
Definition of characteristics of natural substances able to modulate cough mechanisms.
Further clinical studies to explore the preventive and curative activities of natural products.
Disclaimer
The authors declare that the article was written in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Author Contributions
All authors made a substantial contribution to the conception of the work. V.M., M.D.F., and S.M. reviewed the literature on the subject. V.M. and A.L. drafted the final version of the manuscript. G.C. edited the final version. A.L., G.C., and G.L.M. discussed and revised it critically for important intellectual content. All authors finally approved the version to be published and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Shields MD, Bush A, Everard ML, et al. Recommendations for the assessment and management of cough in children. Thorax 2008; 63(Suppl III):1–15 [DOI] [PubMed] [Google Scholar]
- 2. Gibson PG, Simpson JL, Ryan NM, et al. Mechanisms of cough. Curr Opin Allergy Clin Immunol 2014; 14:55–61 [DOI] [PubMed] [Google Scholar]
- 3. Munyard P, Bush A. How much coughing is normal? Arch Dis Child 1996; 74:531–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang AB, Glomb WB. Guidelines for evaluating chronic cough in pediatrics: ACCP evidence-based clinical practice guidelines. Chest 2006; 129:260S–283S [DOI] [PubMed] [Google Scholar]
- 5. Galway NC, Shields MD. The child with an incessantr dry cough. Paediatr Respir Rev 2019; 30:58–64 [DOI] [PubMed] [Google Scholar]
- 6. Gilchrist FJ. An approach to the child with a wet cough. Paediatr Respir Rev 2019; 31:75–81 [DOI] [PubMed] [Google Scholar]
- 7. Morice A, Kardos P. Comprehensive evidence-based review on European antitussives. BMJ Open Resp Res 2016; 3:e000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marseglia GL, Pagella F, Klersy C, et al. The 10-day mark is a good way to diagnose not only acute rhinosinusitis but also adenoiditis, as confirmed by endoscopy. Int J Pediatr Otorhinolaryngol 2007; 71:581–583 [DOI] [PubMed] [Google Scholar]
- 9. Cohen HA, Rozen J, Kristal H, et al. Effect of honey on nocturnal cough and sleep quality: a double-blind, randomized, placebo-controlled study. Pediatrics 2012; 130:465–471 [DOI] [PubMed] [Google Scholar]
- 10. Morice AH, McGarvey L, Pavord I, et al. Recommendations for the management of cough in adults. Thorax 2006; 61(Suppl):1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang AB, Harrhy VA, Simpson J, et al. Cough, airway inflammation and mild asthma exacerbation. Arch Dis Child 2002; 86:270–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hay AD, Wilson A, Fahey T, et al. The duration of acute cough in pre-school children presenting to primary care: a prospective cohort study. Fam Pract 2003; 20:6. [DOI] [PubMed] [Google Scholar]
- 13. Thompson M, Vodicka TA, Blair PS, et al. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ 2013; 347:f7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Blasio F, Dicpinigaitis PV, Rubin BK, et al. An onservational study on cough in children: epidemiology, impact on quality of sleep and treatment outcome. Cough 2012; 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence. Cough (acute): antimicrobial prescribing. NICE guideline. London: NICE National Institute for Health and Care Excellence, 2019. [Google Scholar]
- 16. Dicpinigaitis PV. Effect of viral upper respiratory tract infection on cough reflex sensitivity. J Thorac Dis 2014; 6(Suppl 7):S708–S711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pappas DE, Hendley JO, Hayden FG, et al. Symptom profile of common colds in school-aged children. Pediatr Infect Dis J 2008; 271:8–11 [DOI] [PubMed] [Google Scholar]
- 18. Brodlie M, Graham C, McKean MC. Childhood cough. BMJ 2012; 344:e1177. [DOI] [PubMed] [Google Scholar]
- 19. Hay AD, Wilson AD. The natural history of acute cough in children aged 0 to 4 years in primary care: a systematic review. Br J Gen Pract 2002; 52:401–409 [PMC free article] [PubMed] [Google Scholar]
- 20. Hay AD, Schroeder K, Fahey T. Acute cough in children. 10-minute consultation. BMJ 2004; 328:1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chang AB, Landau LI, Van Asperen PP, et al. Cough in children: definitions and clinical evaluation. Med J Aust 2006; 184:398–403 [DOI] [PubMed] [Google Scholar]
- 22. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev 1994; 16:351–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang AB. Cough: are children really different to adults? Cough 2005; 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Urkin J, Ishay Y, Bilenko N, et al. Night-time cough in children with acute wheezing and with upper respiratory tract infection. Prim Care Respir J 2008; 17:217–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keller JA, McGovern AE, Mazzone SB. Translating cough mechanisms into better cough suppressants. Chest 2017; 152:833–841 [DOI] [PubMed] [Google Scholar]
- 26. Eccles R, Mallefet P. Soothing properties of glycerol in cough syrups for acute cough due to common cold. Pharmacy (Basel) 2017; 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Freestone C, Eccles R. Assessment of the antitussive efficacy of codeine in cough associated with common cold. J Pharm Pharmacol 1997; 49:1045–1049 [DOI] [PubMed] [Google Scholar]
- 28. Wheeler-Hegland K, Pitts T, Davenport PW. Cortical gating of oropharyngeal sensory stimuli. Front Physiol 2010; 1:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eccles R, Morris S, Jawad M. Lack of effect of codeine in the treatment of cough associated with acute upper respiratory tract infection. J Clin Pharm Ther 1992; 17:175–180 [DOI] [PubMed] [Google Scholar]
- 30. Eccles R, Dicpinigaitis P, Turner RB, et al. Characterization of urge to cough and cough symptoms associated with the common cold: results of a US internet survey. Postgrad Med 2016; 128:485–491 [DOI] [PubMed] [Google Scholar]
- 31. Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 2010,25:323–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eccles in Redington AE, Morice H. Acute and chronic cough. London: Taylor and Francis, 2005, pp. 215–236. [Google Scholar]
- 33. Mu L, Sanders I. Sensory nerve supply of the human oro- and laryngopharynx: a preliminary study. Anat Rec 2000; 258:406–420 [DOI] [PubMed] [Google Scholar]
- 34. Hegland KW, Pitts T, Bolser DC, et al. Urge to cough with voluntary suppression following mechanical pharyngeal stimulation. Bratisl Lek Listy 2011; 112:109–114 [PMC free article] [PubMed] [Google Scholar]
- 35. Canning BJ, Chang AB, Bolser DC, et al. Anatomy and neurophysiology of cough. Chest 2014; 146:1633–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dicpinigaitis PV, Bhat R, Rhoton WA, et al. Effect of viral upper respiratory tract infection on the urge-to-cough sensation. Respir Med 2011; 105:615–618 [DOI] [PubMed] [Google Scholar]
- 37. Mazzone SB. An overview of the sensory receptors regulating cough. Cough 2005; 1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther 2011; 24:267–271 [DOI] [PubMed] [Google Scholar]
- 39. Yoder KE, Shaffer ML, La Tournous SJ, et al. Child assessment of dextromethorphan, diphenhydramine, and placebo for nocturnal cough due to upper respiratory infection. Clin Pediatr (Phila) 2006; 45:633–640 [DOI] [PubMed] [Google Scholar]
- 40. Anderson-James S, Marchant JM, Acworth JP, et al. Inhaled corticosteroids for subacute cough in children. Cochrane Database Syst Rev 2013; CT008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith SM, Schroeder K, Fahley T.. Over-the-counter (OTC) medications for acute cough in children and adults in community setting (Review) Cochrane Database Syst Rev 2014; CD001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldman RD. Codeine for acute cough in children. Can Fam Phys 2010; 56:1293–1294 [PMC free article] [PubMed] [Google Scholar]
- 43. Speich B, Thomer A, Aghlmandi S, et al. Treatments for subacute cough in primary care: systematic review and meta-analyses of randomised clinical trials. Br J Gen Pract 2018; 68:e694–e702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonvini SJ, Belvisi MG. Cough and airway disease: the role of ion channels. Pulm Pharmacol Ther 2017; 47:21–28 [DOI] [PubMed] [Google Scholar]
- 45. Rimsza ME, Newberry S. Unexpected infant deaths associated with use of cough and cold medications. Pediatrics 2008; 122:e318–e322 [DOI] [PubMed] [Google Scholar]
- 46. Agence française de sécurité sanitaire des produits de santé (Afssaps, 2010). Retrait des spécialités mucolytiques chez le nourrisson: dernière étape de l'opération de rappel. http://ansm.sante.fr/S-informer/Informations-de-securite-Retraits-de-lots-et-de produits (accessed January22, 2020)
- 47. AIFA. Nota Informativa Dell'agenzia Italiana Del Farmaco. Ufficio di Farmacovigilanza. Rome: Agenzia Italiana del Farmaco, 2010. [Google Scholar]
- 48. Vernacchio L, Kelly JP, Kaufman DW, et al. Medication use among children <12 years of age in the United States: results from the Slone Survey. Pediatrics 2009; 124:446–454 [DOI] [PubMed] [Google Scholar]
- 49. Dicpinigaitis PV. Cough: an unmet clinical need. Br J Pharmacol 2011; 163:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dicpinigaitis PV, Morice AH, Birring SS, et al. Antitussive drugs-past, present, and future. Pharmacol Rev 2014; 66:468–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bolser DC. Cough suppressant and pharmacologic protussive therapy: ACCP evidence-based clinical practice guidelines. Chest 2006; 129(Suppl 1):238S–249S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bolser DC. Pharmacologic management of cough. Otolaryngol Clin North Am 2010; 43:147–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Grace MS, Dubuis E, Birrell MA, et al. Pre-clinical studies in cough research: role of Transient Receptor Potential (TRP) channels. Pulm Pharmacol Ther 2013; 26:498–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mazzone SB, McLennan L, McGovern AE, et al. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med 2007; 176:327–332 [DOI] [PubMed] [Google Scholar]
- 55. Eccles R. Mechanisms of the placebo effect of sweet cough syrups. Respir Physiol Neurobiol 2006; 152:340–348 [DOI] [PubMed] [Google Scholar]
- 56. Leech J, Mazzone SB, Farrell M. Brain activity associated with placebo suppression of the urge-to-cough in humans. Am J Respir Crit Care Med 2013; 188:1069–1075 [DOI] [PubMed] [Google Scholar]
- 57. Bolser DC. A streetcar named urge-to-cough. J Appl Physiol 2010; 108:1030–1031 [DOI] [PubMed] [Google Scholar]
- 58. Smith JA, Badri H. Cough: new pharmacology. J Allergy Clin Immunol Pract 2019; 7:1731–1738 [DOI] [PubMed] [Google Scholar]
- 59. Thirawong N, Nunthanid J, Puttipipatkhachorn S, et al. Mucoadhesive properties of various pectins on gastrointestinal mucosa: an in vitro evaluation using texture analyzer. Eur J Pharm Biopharm 2007; 67:132–140 [DOI] [PubMed] [Google Scholar]
- 60. Bone K, Mills S. Principles and practices of phytotherapy modern herbal medicine, second ed. London: Churchill Livingstone-Elsevier, 2013, pp. 25–248. [Google Scholar]
- 61. Avachat AM, Dash RR, Shrotriya SN. Recent investigations of plant based natural gums, mucilages and resins in novel drug delivery systems. Indian J Pharm Educ Res 2011; 45:1 [Google Scholar]
- 62. Schmidgall J, Schnetz E, Hensel A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med 2000; 66:48–53 [DOI] [PubMed] [Google Scholar]
- 63. ESCOP Monographs. Altheae radix. Second edition. New York: Thieme, 2003, pp. 32–35. [Google Scholar]
- 64. Galati EM, Monforte MT, Miceli N, et al. Opuntia ficus indica (L.) Mill. mucilages show cytoprotective effect on gastric mucosa in rat. Phytother Res 2007; 21:344–346 [DOI] [PubMed] [Google Scholar]
- 65. Yang Q, Huang B, Li H, et al. Gastroprotective activities of a polysaccharide from the fruiting bodies of Pleurotus ostreatus in rats. Int J Biol Macromol 2012; 50:1224–1228 [DOI] [PubMed] [Google Scholar]
- 66. Smart JD. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev 2005; 57:1556–1568 [DOI] [PubMed] [Google Scholar]
- 67. Nosalova G, Fleskova D, Jurecek L, et al. Herbal polysaccharides and cough reflex. Respir Physiol Neurobiol 2013; 187:47–51 [DOI] [PubMed] [Google Scholar]
- 68. Najafian Y, Hamedi SS, Farshchi MK, et al. Plantago major in traditional persian medicine and modern phytotherapy: a narrative review. Electron Physician 2018; 10:6390–6399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Meo SA, Al-Asiri SA, Mahesa AL, et al. Role of honey in modern medicine. Saudi J Biol Sci 2017; 24:975–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pasupuleti VR, Sammugam L, Ramesh N, et al. Honey, propolis, and royal gelly: a comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev 2017; 2017:1259510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ahmed S, Sulaiman SA, Baig AA, et al. Honey as a potential natural antioxidant medicine: an insight into its molecular mechanism of action. Oxid Med Cell Longev 2018; 2018:ID8367846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cianciosi D, Forbes-Hermandez TY, Afrin S, et al. Phenolic compounds in honey and their associated health benefits: a review. Molecules 2018; 23:2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hills SP, Mitchell P, Wells C, et al. Honey supplementation and exercise: a systematic review. Nutrients 2019; 11:1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ramli NZ, Chin KY, Zarkasi KA, et al. A review of the protective effects of honey against metabolic síndrome. Nutrients 2018; 10:1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lan Nguyen HT, Panyoyai N, Kasapis S, et al. Honey and its role in relieving multiple facets of aterosclerosis. Nutrients 2019; 11:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Does a spoonful of honey make the medicine go down? Editorial. Lancet 2018; 392:712. [DOI] [PubMed] [Google Scholar]
- 77. Barker SJ. Honey for acute cough in children. Pediatr Child Health 2016; 21:199–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Goldman RD. Honey for treatment of cough in children. Can Fam Physician 2014; 60:1107–1110 [PMC free article] [PubMed] [Google Scholar]
- 79. WHO. Cough and cold remedies for the treatment of acute respiratory infections in young children 2001. Geneva, Switzerland: World Health Organization, 2001 [Google Scholar]
- 80. Ayazi P, Mahyar A, Yousef-Zanjani M, et al. Comparison of the effect of two kinds of iranian honey and diphenhydramine on nocturnal cough and the sleep quality in coughing children and their parents. PLoS One 2017; 12:e0170277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Paul IM, Beiler J, McMonagle A, et al. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med 2007; 161:1140–1146 [DOI] [PubMed] [Google Scholar]
- 82. Shadkam MN, Mozaffari-Khosravi H, Mozayan MR. A comparison of the effect of honey, dextromethorphan, and diphenhydramine on nightly cough and sleep quality in children and their parents. J Altern Complement Med 2010; 16:787–793 [DOI] [PubMed] [Google Scholar]
- 83. Oduwole O, Udoh EE, Oyo-Ita A, et al. Honey for acute cough in children. Cochrane Database Syst Rev 2018; CD007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nitsche MP, Carreño M. Is honey an effective treatment for acute cough in children? Medwave 2016; 16(Suppl 2):e6454. [DOI] [PubMed] [Google Scholar]
- 85. Cox N, Hinkle R. Infant botulism. Am Fam Physician 2002; 65:1388–1392 [PubMed] [Google Scholar]
- 86. Canciani M, Murgia V, Caimmi D, et al. Efficacy of Grintuss® pediatric syrup in treating cough in children: a randomized, multicenter, double blind, placebo-controlled clinical trial. Ital J Pediatr 2014; 40:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cohen HA, Hoshen M, Gur S, et al. Efficacy and tolerability of a polysaccharide-resin-honey based cough syrup as compared to carbocysteine syrup for children with colds: a randomized, single-blinded, multicenter study. World J Pediatr 2017; 13:27–33 [DOI] [PubMed] [Google Scholar]