Abstract
Background:
Chr17q12-21.2 region is the strongest and most consistently associated region with asthma susceptibility. The functional genes or single nucleotide polymorphisms (SNPs) are not obvious due to linkage disequilibrium.
Objectives:
Whole-genome sequence and RNAseq from human bronchial epithelial cells (BEC) were comprehensively investigated to dissect functional genes/SNPs for asthma severity in the Severe Asthma Research Program (SARP).
Methods:
eQTL analysis (n=114), correlation analysis (n=156) of gene expression and asthma phenotypes, and pathway analysis were performed in BEC and replicated. Genetic association for asthma severity (426 severe vs. 531 non-severe asthma) and longitudinal asthma exacerbations (n=273) was performed.
Results:
Multiple SNPs in GSDMB associated with asthma severity (odds ratio>1.25) and longitudinal asthma exacerbations (p<0.05). eQTL analyses identified multiple SNPs associated with expression levels of PGAP3, GSDMB, or GSDMA (3.1x10−9<p<1.8x10−4). Higher expression levels of GSDMB correlated with asthma and greater number of exacerbations (p<0.05). Expression levels of GSDMB correlated with genes involved in interferon signaling, MHC class I antigen presentation, and immune system pathways (FDR-p<0.05). rs1031458 and rs3902920 in GSDMB colocalized with interferon regulatory factor (IRF) binding sites and associated with GSDMB expression, asthma severity, and asthma exacerbations (p<0.05).
Conclusions:
By using a unique set of gene expression data from lung cells obtained using bronchoscopy from comprehensively characterized asthma subjects, we show that SNPs in GSDMB associated with asthma severity, exacerbations, and GSDMB expression levels. Furthermore, its expression levels correlated with asthma exacerbations and antiviral pathways. Thus, GSDMB is a functional gene for both asthma susceptibility and severity.
Keywords: Antiviral pathways, asthma exacerbations, asthma severity, eQTL, genetics, GSDMA, GSDMB, PGAP3, whole-genome sequence, RNAseq
Capsule summary
By using a unique dataset of gene expression from lung cells of asthmatics, we show strong evidence for GSDMB as a gene for asthma severity and asthma exacerbations probably through antiviral pathways.
INTRODUCTION
Asthma is a common inflammatory airway disease. ORMDL3 in chr17q12-21.2 region was the first gene identified through genome-wide association study (GWAS) of asthma.1 Since then, GWAS, candidate gene replication, and gene expression studies have consistently identified or confirmed SNPs in multiple genes in this region that are associated with asthma susceptibility, including PGAP32–4 ERBB2,5 IKZF3,6–9 ZPBP2,10–11 GSDMB,1,5,8,12–20 ORMDL3,11,21–25 and GSDMA.9,14–15,26 SNPs in IKZF327 ZPBP2,28 GSDMB,10,29–30 and PSMD329 have also been associated with allergic responses. However, partially due to linkage disequilibrium (LD), it has been difficult to determine the specific genes or SNPs responsible for those association. In addition, most published GWAS of asthma has tested the association of SNPs with asthma susceptibility (mild or severe asthma vs. healthy controls), not asthma severity. To analyze asthma severity, we performed a genetic association analysis for severe asthma compared to non-severe asthma and asthma exacerbations longitudinally over a 3 year period.
Autoimmune diseases (AD) arise from abnormal immune responses to self-antigens. SNPs in ERBB2,31 IKZF3,32–44 ZPBP2,45–46 GSDMB,47–58 and GSDMA59–60 have been associated with a variety of AD. In a previously published GWAS, we were the first to report that the opposite risk alleles in ILI3, TNIPI, HLA-DRA, and GSDMB associated with asthma and AD.61 In this study, we comprehensively compared all the GWAS-identified SNPs associated with asthma, allergy, and AD in chr17q12-21 region to reveal genetic effects on the immunopathogenesis of asthma, allergy, and AD.
In a recent review, genetic association, expression quantitative trait loci (eQTL), and epigenetics of 17 SNPs in chr17q12-21.2 region with asthma have been summarized.62 Proximal (PGAP3-ERBB2), core (IKZF3-ZPBP2-GSDMB-ORMDL3), and distal (GSDMA) regions have been suggested as independent regions associated with asthma.62
In order to delineate the functional genes/SNPs for asthma severity in this region, we utilized a unique dataset of lung gene expression data obtained from bronchial brushing during investigational bronchoscopy in extensively characterized patients with current asthma plus healthy controls. We hypothesize that combing SNP with RNA gene expression data from lung cells of asthmatics, we will be able to determine the functional asthma genes/SNPs in this complicated chromosomal region.
METHODS
Study subjects
SARP is a currently active multicenter program funded for the last 18 years by the NHLBI. Mild to severe subjects with asthma (enriched for severe) and a subset of controls have been extensively studied using standardized protocols. The earlier SARP cohort was cross-sectional (n=1,644). In a subset of subjects with mild to severe asthma, RNA was isolated from epithelial cells (BEC; n=155) that were obtained from brush biopsies (Table I and Table E1).63–65 The current SARP cohort is an ongoing longitudinal study (n=714).66–68 Bronchoscopy was performed on a subset of the longitudinal cohort to obtain epithelial cells from brush biopsies (n=156) for RNAseq (Table I and Table E1). All studies were approved by the appropriate institutional review board at the participating sites including informed consent.
TABLE I.
Demographics (mean±SDs) of subjects in SARP
| RNAseq (BEC) Longitudinal | Microarray (BEC) Cross-sectional | WGS† | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | WGS | All | GWAS | Severe Asthma | Non-severe Asthma | Longitudinal Exacerbations | Non-Hispanic White | African American | |
| n | 156 | 114 | 155 | 120 | 426 | 531 | 273 | 1,016 | 622 |
| Age | 41±13 | 41±13 | 37±13 | 36±13 | 46±15 | 37±15 | 47±16 | 39±17 | 29±17 |
| Female, n (%) | 99 (63) | 74 (65) | 101 (65) | 80 (67) | 269 (63) | 353 (66) | 176 (64) | 656 (65) | 369 (59) |
| BMI | 30±8.1 | 30±8.7 | 30±6.8 | 30±7.0 | 31±7.7 | 28±7.6 | 31±7.9 | 29±7.9 | 30±11 |
| Race (Non-Hispanic White/African American/Other*), % | 67/24/9 | 65/26/9 | 62/29/9 | 60/29/11 | 100/0/0 | 100/0/0 | 100/0/0 | 100/0/0 | 0/100/0 |
| Baseline % predicted FEV1 | 82±21 | 76±20 | 76±22 | 76±23 | 66±22 | 84±17 | 73±21 | 77±21 | 78±20 |
| Baseline FEV1/FVC | 0.73±0.10 | 0.70±0.10 | 0.72±0.12 | 0.71±0.13 | 0.66±0.13 | 0.75±0.09 | 0.69±0.11 | 0.72±0.12 | 0.72±0.11 |
| Asthma status (Control/Non-severe/Severe), n | 42/49/65 | 0/49/65 | 27/78/50 | 19/60/41 | 0/0/426 | 0/531/0 | 0/111/162 | 0/564/451 | 0/270/252 |
| Age Onset of Asthma<18 yrs, n (%) | 77 (68) | 77 (68) | 88 (71) | 69 (68) | 238 (56) | 348 (66) | 164 (60) | 645 (64) | 394 (75) |
Note: BEC: bronchial epithelial cell brushing; Microarray: Agilent Whole Human Genome Microarray 4x44K v2; WGS: whole-genome sequence; GWAS: genome-wide association study.
Other races include Hispanic, Asian, American Indian, and mixed.
1,016 non-Hispanic Whites and 622 African Americans in SARP longitudinal and cross-sectional cohorts with WGS were used for LD calculation; Among 1,016 non-Hispanic Whites, 957 adults (age ⩾ 12 yrs) (426 with severe asthma vs. 531 with non-severeasthma) were included in the genetic association analysis of asthma severity; 273 adults in the longitudinal cohort were included in the genetic association analysis of longitudinal asthma exacerbations.
Statistical analysis
Selection of SNPs and RNAseq Data.
Whole genome sequencing (WGS) in SARP (n=1,888; version Freeze 6; dbGaP accession: phs001446) was performed through NHLBI-sponsored TOPMed Program (www.nhlbiwgs.org). Standard quality control (QC) was performed. All SNPs in chr17q12-21.2 region were extracted (hg38: PPP1R1B to CSF3; chr17:39,626,924-40,017,813) in the longitudinal cohort with WGS using PLINK 1.9 software,69 and further QC were performed as described.61,70 Similarly, SNPs were extracted from the cross-sectional cohort with GWAS data and imputed based on TOPMed reference panel using the Michigan Imputation Server.71
RNAseq data from BEC in the longitudinal cohort were extracted for 14 candidate genes (except for ZPBP2 and LRRC3C which failed QC) in chr17q12-21.2 region. In brief, Illumina HiSeq RNAseq reads were quality filtered and mapped to human genome hg38 using STAR package.72 Read counts were regularized logarithm transformed using DESeq2 package.73 The RNAseq data will be deposited and accessible through GEO (www.ncbi.nlm.nih.gov/geo/). Agilent Whole Human Genome Microarray expression data of these 16 genes were extracted from BEC in the cross-sectional cohort as described.74–75 The microarray expression data have been deposited and can be accessed through GSE63142 and GSE43696.74,76–77
Genetic Association Analysis.
Logistic or linear regression, assuming a genetic additive model, was used for genetic association analysis of asthma severity (426 severe asthma vs. 531 non-severe asthma) and the number of exacerbations (n=273) due to asthma in three years in non-Hispanic White adults (age>12 years old) in the longitudinal cohort (Table I), adjusted for age, sex, and the first five components from the multidimensional scaling analysis of genome.
We first investigated a set of 48 candidate SNPs identified by previous GWAS of asthma, allergy, and AD (NHGRI-EBI GWAS catalog;78 www.ebi.ac.uk/gwas/) incorporated in UCSC genome browser (genome.ucsc.edu; accessed on August 12, 2019)79 for association with asthma severity and longitudinal exacerbations in SARP (Figure 1). To reduce multiple tests due to SNPs with strong LD, the numbers of independent tests were calculated using GEC.80 14.4 independent tests of 48 candidate SNPs were indicated by GEC, and thus SNPs with p-value<0.0035 (0.05/14.4 tests) were considered significant. SNPs with p-value<0.05 were considered as nominally significant. From all sequenced SNPs in the chr17q12-21.2 region, we extracted 1,266 common SNPs (MAF≥0.01) to test for association and p-value<0.05 was considered as nominally significant due to relatively small sample size. Note that all of the 48 candidate SNPs were included in the set of 1,266 common SNPs. LD was estimated with 95% confidence intervals of D’ to define LD blocks and LD plots of candidate SNPs in chr17q12-21.2 region were generated separately for 1,016 non-Hispanic Whites and 622 African Americans (Table I) using Haploview.81
FIG 1.
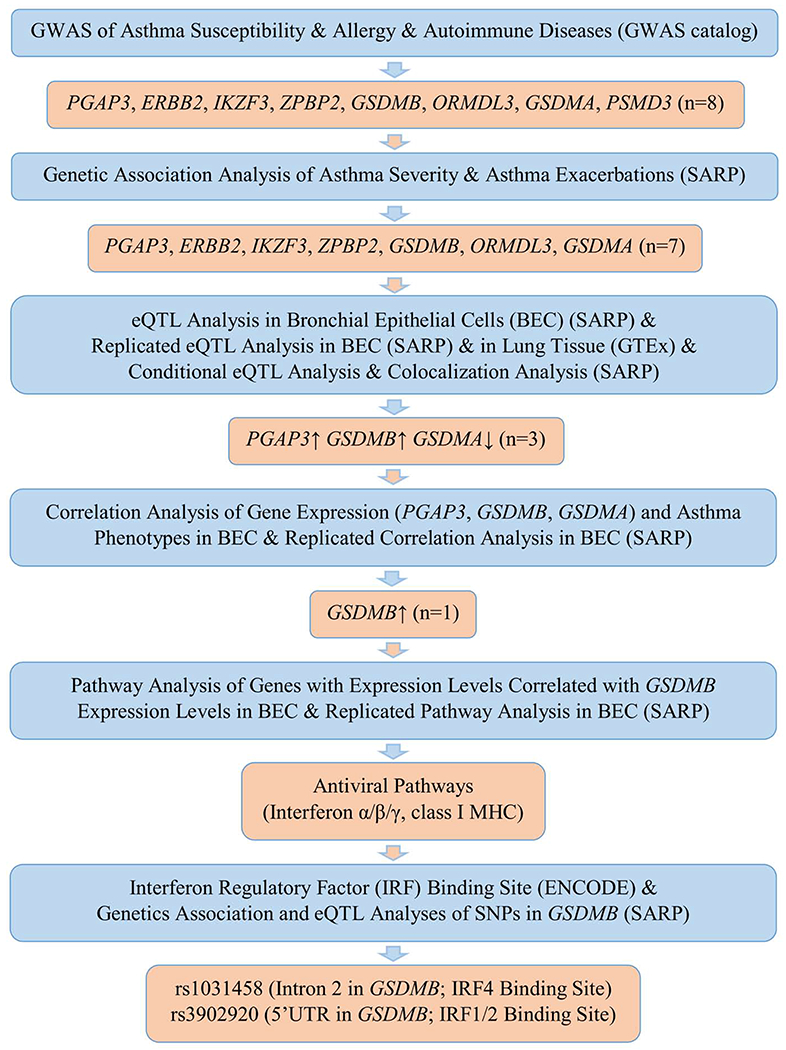
Flow chart of genetic analyses in chr17q12-21.2 region
eQTL Analysis.
A linear additive genetic model was used to test the association between SNPs and inverse normalized expression data as described before.74–75 The longitudinal and cross-sectional cohorts were used as discovery and replication datasets, respectively (Figure 1). Significant eQTL SNPs identified in the lung tissue (n=383) from Genotype-Tissue Expression (GTEx) database26 were also evidence for replication (Figure 1). In the longitudinal cohort with WGS and RNAseq in BEC (n=114), 252.6 independent tests of 862 common SNPs (MAF≥0.05) in chr17q12-21.2 region were indicated by GEC,80 and thus, SNPs with p-value<1.98x10−4 (0.05/252.6 tests) were considered as significant eQTL SNPs. SNPs with p-value<0.05 were considered as nominally significant. Conditional eQTL analysis of PGAP3, GSDMB, and GSDMA in the longitudinal cohort was performed to identify independent eQTL SNPs by stepwise adjusting the most significant eQTL SNP.
Colocalization Analysis.
To test whether the same SNP (n=862) is responsible for the genetic association of asthma severity and eQTL of PGAP3, GSDMB, or GSDMA in the longitudinal cohort (Figure 1), a Bayesian-based colocalization analysis was performed using coloc package.82 A posterior probability of 75% or greater was considered as strong evidence of colocalization. Colocalization analysis of SNPs associated with asthma severity or longitudinal asthma exacerbations and with gene expression of PGAP3, GSDMB, or GSDMA in the longitudinal cohort (Figure 1) was also performed through conditional eQTL analysis by adjusting the most significant SNP associated with asthma severity or longitudinal asthma exacerbations.
Correlation Analysis of Gene Expression and Asthma Phenotypes.
Correlation analysis of gene expression and asthma-related phenotypes was performed as described (Figure 1).74–75 In brief, a generalized linear model was used to test the correlation between expression levels of 16 candidate genes and asthma-related phenotypes with adjustment of age, sex, race (dummy variables for non-Hispanic Whites and African Americans), BMI, and batch effect. P-value<0.05 was considered as nominally significant.
Pathway Analysis.
Correlation analysis of gene expression levels of 16,068 genes in the longitudinal cohort or 19,567 genes in the cross-sectional cohort was performed using Spearman’s rank correlation. The genes with expression levels significantly correlated with PGAP3, GSDMB, or GSDMA (p<0.05/16,067=3.1x10−6 in the longitudinal cohort and p<0.05/19,566=2.5x10−6 in the cross-sectional cohort) were input into Reactome software for pathway analysis83 (Figure 1). Enriched biological pathways were identified using a hypergeometric distribution test with false discovery rate (FDR) adjusted p-value<0.05.
IRF Binding Site Analysis.
Interferon regulatory factor (IRF) binding sites were checked for GSDMB based on ENCODE database (Figure 1).84 Genetic association and eQTL analyses were performed for two common SNPs and four rare SNPs (MAF<0.01) in the identified IRF binding sites of GSDMB.
RESULTS
Genetic Association Analysis
16 candidate genes in chr17q12-21.2 region (Figure E1) were selected based on the published GWAS of asthma, allergy, or AD.78 To elucidate shared genetic variants for immune diseases, 48 SNPs in this region identified through GWAS of asthma, allergy, and AD78–79 or associated with asthma as reported by Stein et al.62 were investigated (Table II).
TABLE II.
Genetic association and eQTL results of 48 GWAS-identified SNPs associated with expression levels of PGAP3, GSDMB, or GSDMA in the longitudinal cohort
| SNP | Position (hg38) | SNP Type | Gene | Allelet‡ | Associated Trait** | LD†† (NHW) | LD†† (AA) | Asthma Severity*** | Number of Exacerbations in 3 years††† | eQTL of SARP3 in BEC (n=114) | eQTL of GTEx database Lung Tissue (n=383) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect Allele | OR | P | β | P | PGAP3 β (P) | GSDMB β (P) | GSDMA β (P) | |||||||||
| rs2941504* | 39674647 | synonymous | PGAP3 | A/G | Asthma2; eQTL for PGAP33 | 1 | 1 | A | 1.05 | 0.64 | 0.97 | 0.011 | 0.77 (5E-9) | 0.30 (0.03) | −0.32 (0.02) | A ~ PGAP3↑ (2E-10); ORMDL3↑ (1E-7); GSDMA↓ (4E-6) |
| rs2517955* | 39687428 | intronic | PGAP3 | C/T | eQTL for ORMDL34 | C | 1.10 | 0.35 | 1.05 | 0.0034 | 0.56 (5E-6) | 0.28 (0.03) | −0.11 (0.4) | C ~ PGAP3↑ (1E-12); ORMDL3↑ (2E-6) | ||
| rs2952156*† | 39720582 | intronic | ERBB2 | A/G | Asthma5 | 2 | A | 1.06 | 0.56 | 0.99 | 0.0081 | 0.76 (4E-8) | 0.30 (0.04) | −0.33 (0.02) | A ~ PGAP3↑ (4E-10); ORMDL3↑ (4E-9); GSDMA↓ (7E-6); GSDMB↑ (2E-5) | |
| rs4252665† | 39729130 | intronic | ERBB2 | T/C | SLE31 | T | 0.69 | 0.15 | −0.40 | 0.69 | −0.02 (1.0) | 0.04 (0.9) | −0.28 (0.5) | NS | ||
| rs2941522† | 39754115 | intergenic | GRB7-IKZF3 | T/C | Asthma6 | T | 1.24 | 0.028 | 0.84 | 0.019 | 0.45 (8E-4) | 0.33 (0.02) | −0.54 (6E-5) | T ~ GSDMB↑ (3E-15); ORMDL3↑ (4E-15); GSDMA↓ (1E-6); PGAP3↑ (1E-6) | ||
| rs12946510† | 39756124 | integenic | GRB7-IKZF3 | T/C | UC32–33; CD32–33; MS34; IBD32–33,35 | 2 | T | 0.86 | 0.12 | −0.72 | 0.046 | −0.43 (0.002) | −0.43 (0.002) | 0.44 (0.002) | T ~ GSDMB↓ (2E-9); ORMDL3↓ (9E-9); GSDMA↑ (1E-6) | |
| rs2941509† | 39764941 | 3′ UTR | IKZF3 | A/G | SLE36–37 | A | 0.81 | 0.44 | 0.46 | 0.65 | −0.08 (0.7) | 0.04 (0.9) | 0.02 (0.9) | A ~ GSDMB↓ (3E-5) | ||
| rs907092*† | 39766006 | synonymous | IKZF3 | G/A A/G | Asthma7–8; PBCh38 | G | 1.24 | 0.027 | 0.66 | 0.069 | 0.47 (8E-4) | 0.50 (4E-4) | −0.43 (0.003) | G ~ GSDMB↑ (2E-9); ORMDL3↑ (5E-9); GSDMA↓ (2E-7) | ||
| rs10445308† | 39781794 | intronic | IKZF3 | C/T | Atopy27 | C | 1.23 | 0.033 | 0.76 | 0.034 | 0.47 (7E-4) | 0.51 (2E-4) | −0.45 (1E-3) | C ~ GSDMB↑ (7E-10); ORMDL3↑ (3E-9); GSDMA↓ (3E-8) | ||
| rs12450323† | 39816455 | intronic | IKZF3 | T/G | Asthma9 | T | 1.15 | 0.24 | 0.96 | 0.023 | 0.63 (2E-4) | 0.43 (0.01) | 0.13 (0.4) | T ~ ORMDL3↑ (1E-5); GSDMB↑ (2E-5); PGAP3↑ (2E-5) | ||
| rs9303277*† | 39820216 | intronic | IKZF3 | T/C | PBCh39–42; SS43; SLE43 | T | 0.79 | 0.017 | −0.84 | 0.017 | −0.48 (4E-4) | −0.40 (0.004) | 0.49 (3E-4) | T ~ GSDMB↓ (7E-15); ORMDL3↓ (6E-14); GSDMA↑ (2E-7); PGAP3↓ (7E-6) | ||
| rs143123127† | 39850937 | intronic | IKZF3 | A/G | SLE37 | A | 0.80 | 0.43 | −0.38 | 0.73 | −0.02 (1.0) | 0.30 (0.5) | −0.77 (0.06) | NA | ||
| rs9635726† | 39863888 | intronic | IKZF3 | C/T | PBCh44 | C | 0.88 | 0.27 | −1.14 | 0.0078 | −0.63 (1E-4) | −0.47 (0.005) | −0.07 (0.7) | C ~ GSDMB↓ (1E-5); ORMDL3↓ (2E-5); PGAP3↓ (2E-5) | ||
| rs4795397† | 39867492 | intergenic | IKZF3-ZPBP2 | A/G (G/A) | Asthma8 IBD33 | A | 1.17 | 0.027 | 0.81 | 0.022 | 0.40 (0.003) | 0.52 (1E-4) | −0.42 (0.002) | A ~ ORMDL3↑ (4E-9); GSDMA↓ (2E-8); GSDMB↑ (7E-8) | ||
| rs11655198† | 39869916 | intronic | ZPBP2 | (C/T) | Asthma10 | 3 | C | 1.29 | 0.0097 | 0.82 | 0.019 | 0.40 (0.003) | 0.44 (0.001) | −0.30 (0.03) | C ~ GSDMB↑ (6E-15); ORMDL3↑ (8E-12); GSDMA↓ (8E-7); PGAP3↑ (3E-6) | |
| rs12936231* | 39872867 | intronic | ZPBP2 | (C/G) | ORMDL3 promoter11 | C | 1.29 | 0.0089 | 0.87 | 0.013 | 0.40 (0.004) | 0.42 (0.002) | −0.37 (0.008) | C ~ GSDMB↑ (5E-15); ORMDL3↑ (1E-14); GSDMA↓ (1E-7); PGAP3↑ (8E-6) | ||
| rs59716545† | 39875604 | intronic | ZPBP2 | (G/T) | RA45 | G | 0.82 | 0.042 | −0.89 | 0.012 | −0.38 (0.005) | −0.52 (1E-4) | 0.31 (0.02) | NA | ||
| rs12939457† | 39875935 | intronic | ZPBP2 | T/C | Allergic rhinitis28 | T | 1.21 | 0.054 | 0.90 | 0.011 | 0.44 (0.002) | 0.50 (3E-4) | −0.40 (0.004) | NA | ||
| rs35736272† | 39876427 | intronic | ZPBP2 | (C/T) | AD46 | C | 0.81 | 0.033 | −0.89 | 0.012 | −0.40 (0.003) | −0.53 (6E-5) | 0.35 (0.01) | C ~ ORMDL3↓ (7E-10); GSDMB↓ (3E-9); GSDMA↑ (4E-9); | ||
| rs12232497† | 39883866 | intergenic | ZPBP2-GSDMB | C/T | AD47 | C | 0.81 | 0.033 | −0.89 | 0.012 | −0.40 (0.003) | −0.53 (6E-5) | 0.35 (0.01) | C ~ ORMDL3↓ (7E-10); GSDMA↑ (3E-9); GSDMB↓ (3E-9) | ||
| rs2872507† | 39884510 | intergenic | ZPBP2-GSDMB | A/G | RA48–49; T1D50; UC51; CD52–53 | A | 0.81 | 0.035 | −0.89 | 0.012 | −0.42 (0.002) | −0.51 (2E-4) | 0.35 (0.01) | A ~ ORMDL31 (3E-10); GSDMB 1 (5E-10); GSDMA1 (2E-9) | ||
| rs12936409† | 39887396 | intergenic | ZPBP2-GSDMB | T/C | RA48,54 | T | 0.81 | 0.030 | −0.88 | 0.014 | −0.43 (0.002) | −0.52 (1E-4) | 0.36 (0.009) | T ~ ORMDL3↓ (8E-10); GSDMA↑ (4E-9); GSDMB↓ (9E-9) | ||
| rs8067378† | 39895095 | intergenic | ZPBP2-GSDMB | G/A | PBCi55 | G | 0.76 | 0.0054 | −0.88 | 0.011 | −0.43 (0.002) | −0.42 (0.003) | 0.36 (0.01) | G ~ GSDMB↓ (5E-15); ORMDL3↓ (1E-14); GSDMA↑ (1E-7); PGAP3↓ (8E-6) | ||
| rs12453507† | 39896954 | intergenic | ZPBP2-GSDMB | (G/C) | T1D56 | G | 0.76 | 0.0050 | −0.78 | 0.024 | −0.36 (0.007) | −0.43 (1E-3) | 0.29 (0.03) | G ~ GSDMB↓ (2E-15); ORMDL3↓ (2E-12); GSDMA↑ (2E-8); PGAP3↓ (1E-6) | ||
| rs8069176*† | 39900944 | intergenic | ZPBP2-GSDMB | G/A | Asthma5 | G | 1.26 | 0.020 | 0.86 | 0.015 | 0.39 (0.003) | 0.57 (9E-6) | −0.33 (0.01) | G ~ ORMDL3↑ (6E-11); GSDMA↓ (1E-10); GSDMB↑ (2E-10) | ||
| rs4795399† | 39905186 | intronic | GSDMB | T/C | Asthma12†13 | T | 1.26 | 0.019 | 0.88 | 0.013 | 0.35 (0.007) | 0.55 (2E-5) | −0.31 (0.02) | T ~ ORMDL3↑ (1E-10); GSDMA↓ (2E-10); GSDMB↑ (7E-10) | ||
| rs2305480*† | 39905943 | missense | GSDMB | G/A A/G | Asthma14–15 RA48; UC57 | G | 1.27 | 0.015 | 0.93 | 0.0086 | 0.35 (0.007) | 0.55 (3E-5) | −0.31 (0.02) | G ~ ORMDL3↑ (1E-10); GSDMA↓ (2E-10); GSDMB↑ (2E-9) | ||
| rs2305479† | 39905964 | missense | GSDMB | (C/T) | Asthma5 | C | 1.34 | 0.0029 | 0.85 | 0.014 | 0.33 (0.01) | 0.46 (3E-4) | −0.26 (0.05) | C ~ GSDMB↑ (6E-15); ORMDL3↑ (4E-15); GSDMA↓ (1E-7); PGAP3↑ (5E-6) | ||
| rs62067034† | 39907485 | intronic | GSDMB | (C/T) | Asthma16 | C | 1.34 | 0.0029 | 0.85 | 0.014 | 0.33 (0.01) | 0.46 (3E-4) | −0.26 (0.05) | C ~ GSDMB↑ (2E-15); ORMDL3↑ (1E-12); GSDMA↓ (1E-7); PGAP3↑ (7E-6) | ||
| rs11078927*† | 39908152 | intronic | GSDMB | C/T | Asthma8,17 | C | 1.26 | 0.018 | 0.95 | 0.0073 | 0.37 (0.005) | 0.56 (2E-5) | −0.35 (0.009) | C ~ ORMDL3↑ (1E-10); GSDMA↓ (3E-10); GSDMB↑ (9E-10) | ||
| rs11078928* | 39908216 | receptor | GSDMB | T/C | eQTL for GSDMB18 | T | 1.26 | 0.018 | 0.95 | 0.0073 | 0.37 (0.005) | 0.56 (2E-5) | −0.35 (0.009) | T ~ ORMDL3↑ (8E-11); GSDMA↓ (4E-10); GSDMB↑ (8E-10) | ||
| rs117097909† | 39908718 | intronic | GSDMB | A/G | Asthma13 | A | 1.04 | 0.860 | −0.04 | 0.95 | 0.27 (0.37) | 0.52 (0.09) | 0.21 (0.49) | NA | ||
| rs2290400*† | 39909987 | intronic | GSDMB | T/C (C/T) | Asthma19 T1D58 | 3 | 4 | T | 1.32 | 0.0044 | 0.84 | 0.016 | 0.40 (0.003) | 0.41 (0.003) | −0.35 (0.01) | T ~ GSDMB↑ (8E-16); ORMDL3↑ (7E-15); GSDMA↓ (1E-8); PGAP3↑ (3E-6) |
| rs4795400† | 39910767 | intronic | GSDMB | (C/T) | Allergy10 | C | 1.27 | 0.015 | 0.91 | 0.0093 | 0.35 (0.007) | 0.55 (3E-5) | −0.31 (0.02) | C ~ ORMDL3↑ (2E-10); GSDMB↑ (3E-10); GSDMA↓ (5E-10) | ||
| rs869402† | 39911790 | intronic | GSDMB | (C/T) | Asthma20 | C | 1.33 | 0.0039 | 0.81 | 0.019 | 0.31 (0.01) | 0.41 (1E-3) | −0.24 (0.06) | C ~ GSDMB↑ (1E-15); ORMDL3↑ (2E-12); GSDMA↓ (2E-7); PGAP3↑ (3E-6) | ||
| rs921650† | 39912823 | intronic | GSDMB | A/G | Allergy29 | A | 1.32 | 0.0045 | 0.80 | 0.021 | 0.33 (0.009) | 0.47 (2E-4) | −0.27 (0.03) | A ~ GSDMB↑ (1E-15); ORMDL3↑ (8E-13); GSDMA↓ (2E-7); PGAP3↑ (3E-6) | ||
| rs7216389*† | 39913696 | intronic | GSDMB | T/C | Asthma1 | T | 1.32 | 0.0045 | 0.80 | 0.021 | 0.33 (0.009) | 0.47 (2E-4) | −0.27 (0.03) | T ~ GSDMB↑ (1E-15); ORMDL3↑ (8E-13); GSDMA↓ (2E-7); PGAP3↑ (3E-6) | ||
| rs9303280† | 39917778 | intronic | GSDMB | C/T | Allergy30 | C | 1.32 | 0.0049 | 0.77 | 0.028 | 0.37 (0.004) | 0.49 (1E-4) | −0.33 (0.01) | C ~ GSDMB↑ (3E-15); ORMDL3↑ (2E-12); GSDMA↓ (3E-9); PGAP3↑ (9E-6) | ||
| rs4065275* | 39924612 | intronic | ORMDL3 | G/A | ORMDL3 promoter11 | G | 1.31 | 0.0064 | 0.82 | 0.020 | 0.31 (0.02) | 0.36 (0.008) | −0.29 (0.04) | G ~ GSDMB↑ (1E-13); ORMDL3↑ (1E-13); GSDMA↓ (6E-11) | ||
| rs8076131* | 39924659 | intronic | ORMDL3 | A/G | Early wheeze21 | A | 1.25 | 0.022 | 0.85 | 0.015 | 0.36 (0.007) | 0.47 (3E-4) | −0.37 (0.006) | A ~ GSDMA↓ (1E-11); ORMDL3↑ (7E-11); GSDMB↑ (1E-9) | ||
| rs12603332* | 39926554 | 5′ UTR | ORMDL3 | C/T | eQTL and meQTL for O RMD L3/GSDMB22–23 | 4 | 5 | C | 1.31 | 0.0060 | 0.83 | 0.018 | 0.29 (0.03) | 0.40 (0.003) | −0.44 (9E-4) | C ~ ORMDL3↑ (2E-14); GSDMB↑ (2E-13); GSDMA↓ (6E-12); PGAP3↑ (2E-5) |
| rs4794820† | 39933091 | intronic | ORMDL3 | (G/A) | Asthma24 | G | 1.26 | 0.021 | 0.94 | 0.0077 | 0.28 (0.04) | 0.40 (0.002) | −0.38 (0.004) | G ~ GSDMA↓ (2E-17); ORMDL3↑ (2E-11); GSDMB↑ (2E-8) | ||
| rs6503525† | 39938921 | intergenic | ORMDL3-LRRC3C | (C/G) | Asthma25 | 5 | C | 1.23 | 0.038 | 0.57 | 0.12 | 0.25 (0.06) | 0.076 (0.6) | −0.37 (0.005) | C ~ GSDMA↓ (6E-21); GSDMB↑ (9E-8); ORMDL3↑ (2E-5) | |
| rs3902025† | 39963001 | 5′ UTR | GSDMA | C/A | SS59 | 6 | C | 0.81 | 0.033 | −0.65 | 0.072 | −0.30 (0.02) | −0.23 (0.09) | 0.48 (3E-4) | C ~ GSDMA↑ (2E-21); ORMDL3↓ (9E-7); GSDMB↓ (1E-5) | |
| rs3894194*† | 39965740 | missense | GSDMA | A/G G/A | Asthma14–15 SS60 | A | 1.14 | 0.20 | 0.33 | 0.35 | 0.36 (0.006) | 0.10 (0.4) | −0.46 (4E-4) | A ~ GSDMA↓ (1E-21); GSDMB↑ (4E-9); ORMDL3↑ (2E-7) | ||
| rs7212938† | 39966427 | missense | GSDMA | G/T | Asthma9 | G | 1.14 | 0.19 | 0.28 | 0.44 | 0.27 (0.04) | 0.14 (0.3) | −0.28 (0.03) | G ~ GSDMA↓ (3E-18); GSDMB↑ (1E-7); ORMDL3↑ (5E-5) | ||
| rs3859192* | 39972395 | intronic | GSDMA | T/C | eQTL for GSDMA26 | T | 0.96 | 0.68 | 0.44 | 0.23 | 0.41 (0.002) | 0.19 (0.2) | −0.42 (0.001) | T ~ GSDMA↓ (5E-52) | ||
| rs11652139† | 39992780 | intronic | PSMD3 | (A/G) | Allergy29 | A | 1.01 | 0.90 | 0.47 | 0.19 | 0.32 (0.02) | 0.29 (0.03) | −0.17 (0.2) | NA | ||
Note: entries with p-value<0.0035 for genetic association analysis of asthma severity or longitudinal asthma exacerbations were labeled in red color. NS: non-significant; NA: non-available.
17 SNPs associated with asthma and reported by Stein et al.62
SNPs associated with asthma, allergy, and autoimmune diseases from NHGRI-EBI GWAS catalog (www.ebi.ac.uk/gwas/),78 incorporated in UCSC genome browser (genome.ucsc.edu; accessed on March 1, 2019).79
Risk allele/Other allele: parenthesis indicates risk allele was not reported in the original study but predicted based on available data.
AD: autoimmune diseases; CD: Crohn’s disease; IBD: inflammatory bowel disease; MS: multiple sclerosis; PBCh: primary biliary cholangitis; PBCi: primary biliary cirrhosis; RA: rheumatoid arthritis; SLE: systemic lupus erythematosus; SS: systemic sclerosis; T1D: type I diabetes; UC: ulcerative colitis.
LD was estimated with 95% confidence intervals of D’ to define LD blocks of 48 SNPs for 1,016 non-Hispanic Whites (NHW) and 622 African Americans (AA) in SARP longitudinal cohort and cross-sectional cohort with WGS using Haploview.81
OR and P were odds ratio and p-value for genetic association analysis of asthma severity (426 severe vs. 531 non-severe asthma) in non-Hispanic White in SARP.
β and P were correlation coefficient and p-value for genetic association analysis of the number of longitudinal exacerbations due to asthma in 3 years in the longitudinal cohort in 273 asthmatics with longitudinal asthma exacerbations in non-Hispanic White in SARP.
Most of the SNPs previously associated with asthma susceptibility were associated with asthma severity at the nominal p-value of 0.05 (Table II). rs2305479 and rs62067034 in GSDMB were significantly associated with asthma severity after multiple-test adjustment (odds ratio=1.34; p=0.0029<0.0035). When testing 1,266 common SNPs, several independent signals were associated with asthma severity though no SNP reached a more stringent significance (p<0.05/1266) (Table E2), including five SNPs in GSDMB (odds ratio>1.3; p<0.0035) with the risk alleles associated with increased GSDMB expression.
Most of the SNPs previously associated with asthma susceptibility were also associated with longitudinal asthma exacerbations at the nominal p-value of 0.05 (Table II). rs2517955 in PGAP3 was significantly associated with longitudinal asthma exacerbations after multiple-test adjustment (p=0.0034). When testing 1,266 common SNPs, several independent signals were associated with longitudinal asthma exacerbations though no SNP reached stringent significance (p<0.05/1266) (Table E3), including four SNPs in PGAP3-ERBB2 region (p<0.0035) with the risk alleles associated with increased PGAP3 expression.
Multiple SNPs in this region were associated with asthma, allergy, and AD, however, the risk alleles were opposite between asthma and AD (Table II). For example, the G allele of rs907092 in IKZF3 was the risk allele for asthma (p<5x10−8)7–8 and asthma severity (p=0.027), and associated with higher expression levels of GSDMB (p=3.7x10−4) and PGAP3 (p=7.9x10−4), but was the protective allele for primary biliary cholangitis (PBCh) (p<5x10−8).38 The G allele of rs2305480 (a missense mutation in GSDMB) was the risk allele for asthma (p<5x10−8),14–15 asthma severity (p=0.015), longitudinal asthma exacerbations (p=0.0086) and associated with higher expression levels of GSDMB (p=2.5x10−5), but was the protective allele for rheumatoid arthritis (RA) and ulcerative colitis (UC) (p<5x10−8).48,57 The A allele of rs3894194 (a missense mutation in GSDMA) was the risk allele for asthma (p<5x10−8)14–15 and associated with lower expression levels of GSDMA (p=4.3x10−4), but was the protective allele for systemic sclerosis (SS) (p<5x10−8).59 All 48 candidate SNPs identified by previous GWAS (Table II) were common SNPs (MAF>0.01), and thus, belonged to 1,266 common SNPs analyzed in this study. When ranking genetic association of asthma severity p-values of 1,266 SNPs, 35 (73%), 6 (13%), 3 (6%), and 4 (8%) of 48 candidate SNPs were distributed in the 1st to 4th quartile, respectively.
eQTL Analysis and Colocalization Analysis
Expression of 14 genes (except ZPBP2 and LRRC3C) in the longitudinal cohort (n=114 BEC) and 16 gene in the cross-sectional cohort (n=120 BEC) passed QC (Table I and Table E1). LD pruning (r2≥0.8) of 862 common SNPs (MAF≥0.05) belonging to these 16 candidate genes generated 273 SNPs. The complete eQTL results of 862 SNPs were summarized in Table E4. 26 of 273 SNPs were significantly associated with the gene expression levels of PGAP3, GSDMB, or GSDMA, but not associated with the other genes in the longitudinal cohort (Table III and Table E4–E5). The eQTL findings of 26 SNPs in the longitudinal cohort were generally replicated in BEC in the cross-sectional cohort at nominal p-value of 0.05 (Table E6). Considering stringent replication (p<0.05/26=1.9x10−3), 16 of 26 SNPs in PGAP3 or GSDMB were replicated in BEC in the cross-sectional cohort; 21 of 26 SNPs in PGAP3, GSDMB, or GSDMA were replicated in GTEx lung tissue (Table III); all together, 22 of 26 SNPs were replicated. Three and six LD blocks were formed for these 26 SNPs in non-Hispanic Whites and African Americans, respectively (Table III, Figure E2–E3). SNPs in PPP1R1B, PGAP3, and ERBB2 were associated with PGAP3 expression. SNPs in IKZF3 region were associated with the expression levels of PGAP3, GSDMB, or GSDMA. SNPs in ZPBP2, GSDMB, and ORMDL3 were associated with GSDMB expression. SNPs in GSDMA were associated with GSDMA expression. Most of these 26 eQTL SNPs were associated with asthma severity or longitudinal asthma exacerbations at a nominal p-value of 0.05 (Table E7).
TABLE III.
eQTL results of 26 SNPs significantly associated with expression levels of PGAP3, GSDMB, or GSDMA in the longitudinal cohort
| SNP | Position (hg38) | Gene | LD* (NHW) | LD* (AA) | SARP3 BEC (n=114) | eQTL of GTEx database Lung Tissue (n=383)† | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | PGAP3 | GSDMB | GSDMA | |||||||||
| β | P | β | P | β | P | |||||||
| rs3751903 | 39627534 | PPP1R1B | 1 | 1 | C | 0.43 | 0.002 | 0.55 | 1.0E-4 | −0.10 | 0.5 | C ~ PGAP3↑ (1E-7) |
| rs3794712 | 39635234 | PPP1R1B | A | 0.48 | 0.007 | 0.78 | 6.5E-6 | 0.06 | 0.7 | NS | ||
| rs10558975 | 39675051 | PGAP3 | G | −0.54 | 1.1E-5 | −0.32 | 0.01 | 0.13 | 0.3 | G ~ PGAP3↓ (1E-13); ORMDL3↓ (2E-5); GSDMB↓ (6E-5) | ||
| rs907088 | 39677314 | PGAP3 | 2 | G | 0.69 | 3.3E-7 | 0.32 | 0.02 | −0.27 | 0.06 | G ~ PGAP3↑ (1E-12); ORMDL3↑ (8E-8) | |
| rs2517954 | 39687297 | PGAP3 | T | 0.77 | 3.1E-9 | 0.25 | 0.08 | −0.29 | 0.04 | T ~ PGAP3↑ (9E-10); ORMDL3↑ (3E-8) | ||
| rs2904765 | 39692422 | PGAP3-ERBB2 | T | 0.70 | 8.7E-6 | 0.17 | 0.3 | −0.34 | 0.04 | NS | ||
| rs56328874 | 39694273 | PGAP3-ERBB2 | A | −0.72 | 1.0E-4 | 0.16 | 0.4 | 0.26 | 0.2 | NS | ||
| rs2517951 | 39696844 | PGAP3-ERBB2 | 2 | 3 | T | −0.58 | 1.6E-6 | −0.29 | 0.02 | 0.13 | 0.3 | T ~ PGAP3↓ (1E-13); ORMDL3↓ (2E-6); GSDMB↓ (2E-5) |
| rs2952155 | 39705465 | ERBB2 | T | 0.72 | 4.6E-7 | 0.31 | 0.04 | −0.23 | 0.1 | T ~ PGAP3↑ (2E-8); ORMDL3↑ (1E-6) | ||
| rs2934967 | 39714125 | ERBB2 | G | 0.79 | 9.9E-9 | 0.25 | 0.08 | −0.29 | 0.05 | G ~ PGAP3↑ (5E-11); ORMDL3↑ (2E-8); GSDMB↑ (4E-5); GSDMA↓ (6E-6) | ||
| rs2941520 | 39747477 | GRB-IKZF3 | 4 | T | 0.70 | 1.7E-6 | 0.30 | 0.05 | 0.01 | 0.9 | T ~ PGAP3↑ (2E-10); ORMDL3↑ (3E-9); GSDMB↑ (1E-6) | |
| rs2941519 | 39747478 | GRB-IKZF3 | G | −0.36 | 0.01 | −0.15 | 0.3 | 0.52 | 1.6E-4 | G ~ ORMDL3↓ (5E-14); GSDMB↓ (6E-13); PGAP3↓ (5E-6); GSDMA↑ (3E-5) | ||
| rs9747973 | 39748854 | GRB-IKZF3 | C | −0.41 | 0.003 | −0.34 | 0.01 | 0.53 | 1.0E-4 | C ~ GSDMB↓ (2E-15); ORMDL3↓ (7E-15); GSDMA↑ (6E-7); PGAP3↓ (1E-6) | ||
| rs12450323 | 39816455 | IKZF3 | T | 0.63 | 1.5E-4 | 0.43 | 0.01 | 0.13 | 0.4 | T ~ ORMDL3↑ (1E-5); GSDMB↑ (2E-5); PGAP3↑ (2E-5) | ||
| rs114211283 | 39819840 | IKZF3 | A | 1.11 | 3.5E-5 | 0.13 | 0.6 | 0.12 | 0.7 | NS | ||
| rs62066988 | 39836028 | IKZF3 | T | −0.35 | 0.02 | −0.56 | 1.4E-4 | 0.31 | 0.04 | T ~ ORMDL3↓ (2E-6); GSDMB↓ (2E-5); GSDMA↑ (4E-5) | ||
| rs9635726 | 39863888 | IKZF3 | 3 | T | 0.63 | 1.3E-4 | 0.47 | 0.005 | 0.07 | 0.7 | T ~ GSDMB↑ (1E-5); ORMDL3↑ (2E-5); PGAP3↓ (2E-5) | |
| rs4795397 | 39867492 | IKZF3-ZPBP2 | 5 | G | −0.40 | 0.003 | −0.52 | 1.0E-4 | 0.42 | 0.002 | G ~ ORMDL3↓ (4E-9); GSDMA↑ (2E-8); GSDMB↓ (7E-8) | |
| rs12150079 | 39869164 | ZPBP2 | A | −0.38 | 0.01 | −0.59 | 5.7E-5 | 0.32 | 0.03 | A ~ ORMDL3↓ (3E-7); GSDMB↓ (6E-6); GSDMA↑ (7E-6) | ||
| rs11651596 | 39899863 | ZPBP2-GSDMB | 6 | C | −0.36 | 0.008 | −0.62 | 1.6E-6 | 0.32 | 0.02 | C ~ ORMDL3↓ (2E-10); GSDMA↑ (3E-10); GSDMB↓ (2E-9) | |
| rs11657449 | 39901588 | ZPBP2-GSDMB | C | −0.34 | 0.02 | −0.69 | 7.5E-7 | 0.32 | 0.03 | C ~ ORMDL3↓ (2E-7); GSDMA↑ (7E-7); GSDMB↓ (6E-6) | ||
| rs1011082 | 39912261 | GSDMB | T | −0.33 | 0.01 | −0.51 | 6.6E-5 | 0.31 | 0.02 | T ~ GSDMB↓ (8E-16); ORMDL3↓ (6E-13); GSDMA↑ (1E-7); PGAP3↓ (4E-7) | ||
| rs201413617 | 39917590 | GSDMB-ORMDL3 | G | −0.33 | 0.01 | −0.48 | 1.6E-4 | 0.27 | 0.04 | NS | ||
| rs4795405 | 39932164 | ORMDL3-LRRC3C | T | −0.35 | 0.01 | −0.51 | 1.6E-4 | 0.45 | 0.0009 | T ~ GSDMA↑ (3E-14); ORMDL3↓ (1E-10); GSDMB↓ (p=6E-10) | ||
| rs9914973 | 39966455 | GSDMA | C | −0.42 | 0.002 | −0.22 | 0.1 | 0.52 | 1.7E-4 | C ~ GSDMA↑ (9E-17) | ||
| rs3859193 | 39969603 | GSDMA | A | 0.31 | 0.02 | 0.13 | 0.3 | −0.50 | 7.2E-5 | A ~ GSDMA↓ (9E-30); GSDMB↑ (5E-7) | ||
Note: entries with p-value<1.98x10−4 were labeled in red color. β and P were correlation coefficient and p-value of eQTL analysis. BEC: bronchial epithelial cells brushing. NS: non-significant.
LD was estimated with 95% confidence intervals of D’ to define LD blocks of 26 SNPs for 1,016 non-Hispanic Whites (NHW) and 622 African Americans (AA) in SARP longitudinal cohort and cross-sectional cohort with WGS using Haploview.81
eQTL of GTEx database: eQTL SNPs identified in the lung tissue (n=383) from Genotype-Tissue Expression (GTEx) database.26 ↑indicated up-regulation of gene expression and ↓indicated down-regulation of gene expression.
Five and six LD blocks were identified for 48 GWAS-identified SNPs in non-Hispanic Whites and African Americans, respectively (Table II, Figure E4–E5). Significant eQTL SNPs (p<0.0035) were associated with the expression levels of three genes (PGAP3, GSDMB, or GSDMA) in the longitudinal cohort and were generally replicated in the cross-sectional cohort at nominal p-value of 0.05 (Table II and Table E8). GTEx lung tissue eQTL in this region identified four genes (PGAP3, GSDMB, ORMDL3, and GSDMA) (Table II and III).
Conditional eQTL analysis was performed by stepwise adjusting the most significant eQTL SNP (Table E9), and indicated that two SNPs (rs2517954 in PGAP3 and rs114211283 in IKZF3), two SNPs (rs11657449 in ZPBP2-GSDMB and rs3794712 in PPP1R1B), and one SNP (rs3859193 in GSDMA) were independent eQTL SNPs for PGAP3, GSDMB, and GSDMA, respectively.
Colocalization analysis82 of the signals from genetic association of asthma severity and eQTL was performed, and indicated no significant colocalization SNP based on the criterion of posterior probability>75% (Table E10). rs2517954 in PGAP3, rs11657449 in ZPBP2-GSDMB, and rs2941522 in GRB7-IKZF3 were top colocalization SNPs for PGAP3, GSDMB, and GSDMA, respectively (Table E11). Colocalization analysis between SNPs associated with asthma severity or longitudinal asthma exacerbations and gene expression of PGAP3, GSDMB, and GSDMA was also performed through conditional eQTL analysis by adjusting the most significant SNP associated with asthma severity or longitudinal asthma exacerbations (Table E12, Table II). With adjustment of rs2952156 in ERBB2, rs2305479 in GSDMB, and rs3902025 in GSDMA, all eQTL SNPs for PGAP3 (except for rs114211283 in IKZF3), for GSDMB (except for two SNPs in PPP1R1B and ZPBP2-GSDMB), and for GSDMA became non-significant. For example, the association between GSDMB expression and rs11657449 in ZPBP2-GSDMB or rs3794712 in PPP1R1B was weakened when adjusting for rs2305479, indicating that rs2305479 partly accounted for the eQTL association but not completely. In summary, the colocalization analyses did not show strong evidence for colocalization.
Expression Analysis and Pathway Analysis
The risk alleles associated with asthma, asthma severity, and longitudinal asthma exacerbations were associated with higher expression levels of PGAP3 and GSDMB or the lower expression levels of GSDMA (Table II), which indicated that expression levels of PGAP3, GSDMB, and GSDMA may be correlated with asthma phenotypes.
Correlation analysis of gene expression (PGAP3, GSDMB, and GSDMA) and asthma phenotypes was performed in BEC in the longitudinal cohort (n=156) and replicated in BEC (n=155) in the cross-sectional cohort (Table IV). Higher expression levels of GSDMB were correlated with asthma (p=0.05), greater number of exacerbations in the last 12 months (p=0.02), and higher reduction of ACQ-6 after steroid treatment (p=0.0008) in the longitudinal cohort. Higher expression levels of GSDMB were correlated with emergency room (ER) visits or hospitalizations due to asthma in the last 12 months (p=0.03) in the cross-sectional cohort. Other asthma-related phenotypes were not correlated with expression levels of PGAP3, GSDMB, or GSDMA (Table E13), except that higher expression levels of GSDMB were correlated with higher FeNO (p=0.03) in the longitudinal cohort. Although correlation analysis was focused on PGAP3, GSDMB, and GSDMA, the other 13 genes were also analyzed (Table E14–E15). Higher expression of PNMT and lower expression of CSF3 were associated with asthma susceptibility in BEC in the longitudinal and cross-sectional cohorts.
TABLE IV.
Correlation of the expression levels of PGAP3, GSDMB, or GSDMA and asthma phenotypes in SARP
| RNAseq (156 BEC) in the longitudinal cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Asthma Susceptibility | Asthma Severity | Number of Exacerbations (last 12 months) | ΔACQ6 (After-Before steroid trt) | ||||||
| Healthy Controls (n=42) | Asthma (n=114) | P value | Non-severe Asthma (n=49) | Severe Asthma (n=65) | P value | Correlation Coefficient (β) | P value (n=114) | Correlation Coefficient (β) | P value (n=109) | |
| PGAP3 | 8.81±0.22 | 8.88±0.20 | 0.08 | 8.90±0.21 | 8.87±0.20 | 0.33 | −0.84 | 0.57 | −0.12 | 0.77 |
| GSDMB | 9.94±0.25 | 10.1±0.32 | 0.05 | 10.1±0.28 | 10.1±0.35 | 0.89 | 2.11 | 0.019 | −0.81 | 0.0008 |
| GSDMA | 0.50±0.15 | 0.55±0.22 | 0.26 | 0.52±0.19 | 0.57±0.24 | 0.29 | −1.40 | 0.27 | 0.14 | 0.69 |
| Microarray (155 BEC) in the cross-sectional cohort | ||||||||||
| Gene | Asthma Susceptibility | Asthma Severity | ER or Hospitalization (last 12 months) | |||||||
| Healthy Controls (n=27) | Asthma (n=128) | P value | Non-severe Asthma (n=78) | Severe Asthma (n=50) | P value | No (n=77) | Yes (n=47) | P value | ||
| PGAP3 | 10.8±0.22 | 10.8±0.33 | 0.43 | 10.8±0.34 | 10.8±0.31 | 0.63 | 10.8±0.35 | 10.8±0.29 | 0.57 | |
| GSDMB | 10.5±0.42 | 10.4±0.47 | 0.15 | 10.4±0.45 | 10.4±0.51 | 0.23 | 10.3±0.46 | 10.5±0.49 | 0.03 | |
| GSDMA | 6.26±0.15 | 6.21±0.13 | 0.02 | 6.21±0.14 | 6.21±0.12 | 0.56 | 6.21±0.13 | 6.20±0.13 | 0.41 | |
Note: a general linear model was used to test the correlation between gene expression levels (natural logarithm transformed in the longitudinal cohort or log2 transformed in the cross-sectional cohort) and asthma phenotypes with adjustment of age, sex, race, BMI, and batch effect.
Pathway analyses were performed on the genes with expression levels significantly correlated with PGAP3, GSDMB, or GSDMA. No biological pathways were identified for the genes correlated with PGAP3 or GSDMA after FDR adjustment (data not shown). 435 and 677 genes were positively and negatively correlated with GSDMB (p<3.1x10−6) in BEC in the longitudinal cohort, among which 636 genes were replicated in BEC in the cross-sectional cohort (p<0.05) (Table E16). Pathway analysis83 was performed on 1,112 and 462 genes with expression levels significantly correlated with GSDMB expression in BEC in the longitudinal cohort (p<3.1x10−6) and cross-sectional cohort (p<2.5x10−6), respectively. Expression levels of GSDMB were correlated with genes involved in interferon alpha/beta/gamma signaling, MHC class I antigen presentation, and immune system pathways (FDR-p<0.05) (Table V and Table E17).
Table V.
Biological pathways enriched for genes with expression levels correlated with GSDMB expression in BEC in the longitudinal cohort
| Pathway | Gene | P value (FDR) |
|---|---|---|
| Interferon gamma signaling | CIITA, HLA-A, HLA-B, HLA-C, HLA-F, IFNG, IFNGR2, IL20RA, IRF9, MID1, OAS2, OAS3, PML, SP110, SP140L, STAT 1, SUMO1, TRIM14, TRIM22, TRIM25, TRIM26, TRIM38 | 2.3E-14 |
| Interferon alpha/beta signaling | BST2, HLA-A, HLA-B, HLA-C, HLA-F, IFI27, IRF9, MX2, OAS2, OAS3, STAT1, STAT2, USP18, XAF1 | 2.3E-14 |
| Antigen Presentation (Folding, assembly and peptide loading of class I MHC) | HLA-A, HLA-B, HLA-C, HLA-F, SEC24B, TAP1, TAP2, TAPBP | 2.3E-14 |
| ER-Phagosome pathway | HLA-A, HLA-B, HLA-C, HLA-F, IKBKB, PSMB9, PSMD8, PSME2, RAPSN, TAP1, TAP2, TAPBP | 2.3E-14 |
| Interferon Signaling | BST2, CIITA, HLA-F, IFI27, IFNG, IFNGR2, IL20RA, IRF9, MAPK1, MID1, MX2, NUP210, OAS2, OAS3, PML, SP110, SP140L, STAT1, STAT2, SUMO1, TRIM14, TRIM22, TRIM25, TRIM26, TRIM38, UBA7, USP18, XAF1 | 2.3E-14 |
| Endosomal/Vacuolar pathway | HLA-A, HLA-B, HLA-C, HLA-F | 2.3E-14 |
| Antigen processing-Cross presentation | HLA-A, HLA-B, HLA-C, HLA-F, IKBKB, ITGB5, PSMB9, PSMD8, PSME2, RAPSN, TAP1, TAP2, TAPBP | 2.3E-14 |
| Class I MHC mediated antigen processing & presentation | ASB14, ASB4, ASB8, FBXL3, FBXO2, FBXO21, FBXO41, HLA-A, HLA-B, HLA-C, HLA-F, IKBKB, ITGB5, NARF, PJA2, POLL, PSMB9, PSMD8, PSME2, RAPSN, RBX1, RNF213, SEC24B, SIAH1, SKP1, TAP1, TAP2, TAPBP, TRIM36, TRIM39, UBA7, UBE2D2, UBE2D4, UBE2E3 | 3.5E-13 |
| Immunoregulatory interactions (between a Lymphoid and a non-Lymphoid cell) | CD226, CLEC2D, HLA-A, HLA-B, HLA-C, HLA-F, RAET1E | 6.3E-11 |
| Cytokine Signaling in Immune system | ATF1, ATF2, CALM2, CIITA, CRKL, DUSP16, FASLG, HLA-A, HLA-B, HLA-C, HLA-F, IFI27, IKBKB, IL18BP, IL20RA, IL37, IL6ST, IRF9, LAMTOR3, LGALS9, LIFR, MID1, MX2, NDN, PDGFA, PML, PSME2, PTPN14, PTPN4, RBX1, SKP1, SP110, STAT1, STAT2, STAT6, TRIM14, TRIM22, TRIM25, TRIM26, TRIM38 | 7.9E-08 |
| Adaptive Immune System | ACTR10, ASB4, ASB8, ASB14, BLNK, BTF3, BTN2A1, BTN2A2, BTN3A1, CALM2, CARD11, CD74, CLEC2D, DCTN3, DCTN6, FBXL3, FBXO41, GRAP2, HLA-A, HLA-B, HLA-C, IKBKB, MAP3K14, POLL, PSMD8, RAET1E, SEC24B, SIAH1, SKP1, TAP1, TAP2, TBCB, TEP1, TRIM36, TRIM39, UBA7, UBE2D2, UBE2D4, UBE2E3, ZAP70 | 9.7E-03 |
Note: pathways with false discovery rate (FDR)-adjusted p-value less than 0.05 were included.
IRF Binding Site Analysis
Interferon regulatory factor (IRF) binding sites were checked for GSDMB and two regions were identified based on ENCODE database (Figure E6).84 One IRF1/2 biding site was located at 5’UTR-exon 1-intron 1 region of GSDMB (Figure E7) and one IRF4 biding site was located at intron 2 of GSDMB (Figure E8). Two common SNPs and four rare SNPs were found in these two IRF biding sites based on SARP WGS (Table VI). Two common SNPs (rs1031458 and rs3902920) were associated with GSDMB expression, asthma severity, and longitudinal asthma exacerbations (p<0.05), making them potential functional SNPs.
TABLE VI.
Genetic association and eQTL results of 6 SNPs in IRF binding site of GSDMB in SARP.
| SNP | Position (hg38) | SNP Type | IRF Binding Sites | Allele* (MAF) | Asthma Severity† | Number of Exacerbations in 3 years‡ | eQTL in BEC (n=114) Longitudinal Cohort** | eQTL GTEx database Lung Tissue (n=383)†† | ||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (P) | β (P) | PGAP3 β (P) | GSDMB β (P) | GSDMA β (P) | ||||||
| rs1031458 | 39915920 | intronic | IRF4 | G/T (0.45) | 0.76 (0.0053) | −0.77 (0.028) | −0.36 (5.1E-3) | −0.47 (2.4E-4) | 0.31 (0.018) | GSDMB↓
(1E-20) ORMDL3↓ (3E-19) GSDMA↑ (1E-14) PGAP3↓ (1E-7) |
| rs3902920 | 39918763 | 5′UTR | IRF1/2 | T/C (0.46) | 0.75 (0.0027) | −0.88 (0.012) | −0.29 (0.036) | −0.45 (0.0011) | 0.27 (0.047) | NA |
| rs77929191 | 39915767 | intronic | IRF4 | A/G (0.0021) | 0.37 (0.40) | −2.9 (0.47) | −0.44 (0.54) | −1.26 (0.078) | −0.045 (0.95) | NS |
| rs536439445 | 39918670 | 5′UTR | IRF1/2 | A/G (0.0052) | 0.57 (0.41) | −1.8 (0.53) | 0.77 (0.45) | −1.40 (0.17) | 0.011 (0.99) | NS |
| rs549170154 | 39918764 | 5′UTR | IRF1/2 | C/T (0.0021) | 0.68 (0.74) | 5.1 (0.21) | 0.20 (0.78) | −0.18 (0.80) | −0.012 (0.99) | NA |
| rs540139228 | 39918797 | 5′UTR | IRF1/2 | G/A (0.0010) | 1.3 (0.86) | −2.6 (0.52) | NA | NA | NA | NA |
Minor allele (effect allele)/major allele (minor allele frequency).
OR and P were odds ratio and p-value for genetic association analysis of asthma severity (426 severe vs. 531 non-severe asthma) in non-Hispanic White in SARP.
β and P were correlation coefficient and p-value for genetic association analysis of the number of longitudinal exacerbations due to asthma in 3 years in the longitudinal cohort in 273 asthmatics with longitudinal asthma exacerbations in non-Hispanic White in SARP.
β and P were correlation coefficient and p-value for eQTL analysis in 114 subjects with RNAseq of bronchial epithelial cells in SARP longitudinal cohort.
eQTL of GTEx database: eQTL SNPs identified in the lung tissue (n=383) from Genotype-Tissue Expression (GTEx) database.26 ↑indicated up-regulation of gene expression and ↓indicated down-regulation of gene expression. NA: non-avaliable and NS: non-significant.
T allele of rs1031458 or C allele of rs3902920 were risk alleles for asthma severity and longitudinal asthma exacerbations (Table VI), and they were also associated with early onset of asthma (p<0.005) (Table E18) especially atopic early onset (age onset of asthma<6 yrs) asthma (p<0.00001) (Table E19 and Figure 2). Similarly, most of the top 10 SNPs associated with asthma severity (including rs3902920; Table E2) were also associated with asthma severity in the subjects with early onset asthma (onset<6 yrs) (Table E20). rs1031458 and rs3902920 were in strong LD (r2≥0.8) with multiple neighboring SNPs (Table II–III) in non-Hispanic Whites (Table E21). In African Americans, rs1031458 and rs3902920 were in strong LD with three (rs921650, rs7216389, and rs201413617) and zero neighboring SNPs, respectively.
FIG 2.
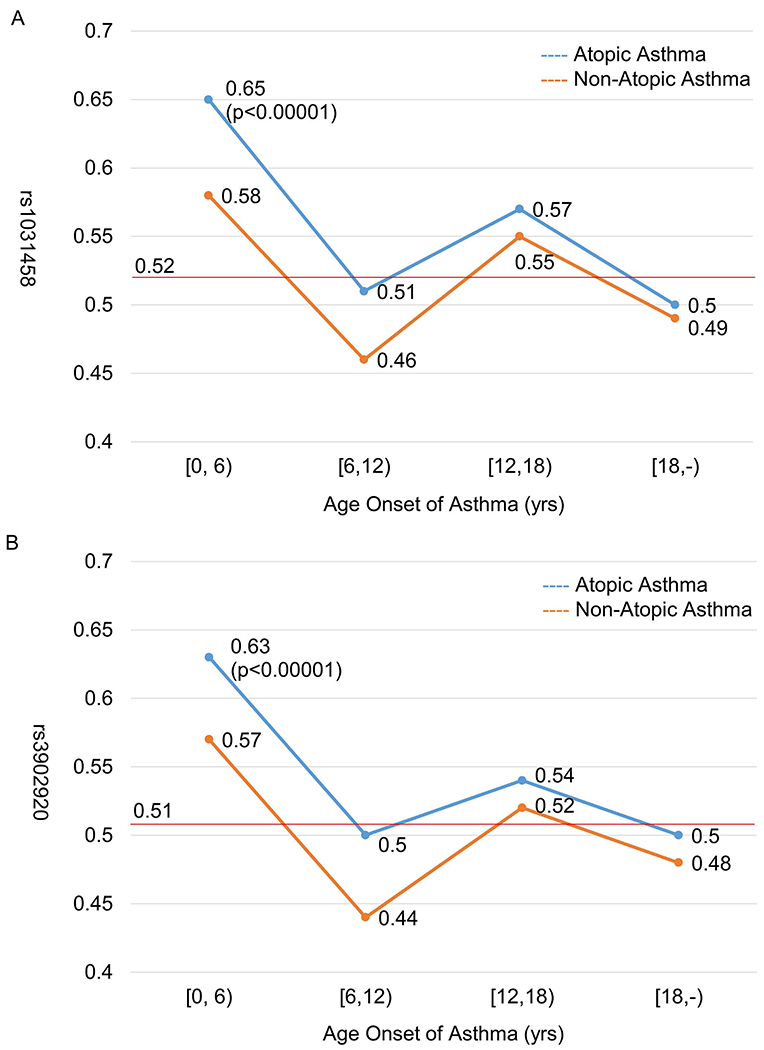
Risk allele frequency of rs1031458 (A) and rs3902920 (B) in GSDMB stratified by age onset of asthma and atopic status. Chi-square test was performed by comparing each asthma group with general North-Western European controls shown in red line (gnomAD V2.1.1; https://gnomad.broadinstitute.org/).
In summary, by using a unique set of gene expression data from lung cells of asthmatics obtained using investigative bronchoscopy and by performing comprehensive genetic association, expression correlation, eQTL, and pathway analyses, we have narrowed down chr17q12-21.2 region (16 candidate genes; 390 kbp) to two SNPs in GSDMB associated with asthma severity and asthma exacerbations potentially through antiviral pathways (Figure 1).
DISCUSSION
Almost all the SNPs identified by previous GWAS in GSDMB now show to be associated with asthma severity and longitudinal asthma exacerbations, indicating that SNPs in GSDMB are associated with asthma susceptibility, asthma severity, and asthma exacerbations. Asthma and AD share extensive immunological pathways, however, the risk alleles of the same associated SNPs in this region are consistently opposite for asthma and AD, which may indicate distinct immunopathogenesis processes. In addition to SNPs with MAF≥0.01, we also investigated rare SNPs (MAF<0.01; n=4,006) for association with asthma severity. 14 rare SNPs were associated with asthma severity at nominal p-value of 0.05 with large effect size (2.9<odds ratio<12) (Table E22). Replication of these rare SNPs is needed in larger cohorts with sequence data and asthma phenotypes. In conclusion, findings from genetic association of asthma susceptibility, asthma severity, and asthma exacerbations in this region are generally consistent, however, genetic association analysis can not narrow down the 16 candidate genes due to strong and complicated LD structure in this region.
Gene expression is dependent on cell type or tissue, time, and environmental factors such as disease status. It is critical that cells are obtained from the appropriate organ (lung for asthma) and from living subjects with the disease being investigated instead of from surgical specimens (usually from cancer patients) or autopsy specimens. Even findings of eQTL analyses in lung cells are not always consistent (Table E23). The most significant eQTL genes were GSDMA followed by GSDMB and ORMDL3 in two eQTL studies in lung tissue.26,85 Nicodemus-Johnson et al. identified ORMDL3 but not GSDMB in an eQTL analysis in BEC.4 Our eQTL analysis in BEC in both longitudinal and cross-sectional cohorts74 identified GSDMB but not ORMDL3.
Similarly, a recent genetic association and eQTL study has shown that eQTL SNPs for GSDMB (but not ORMDL3) in BEC play a major role in childhood asthma in African Americans.86 BEC obtained from brush biopsies are mainly composed of epithelial cells, although small proportion of basal cells and immune cells also exist. A flow cytometry study showed that 95% to 97% of the cells from bronchial brushings were epithelial cells.87 In this study, cell populations were not available for every subject, and thus, were not adjusted. Future eQTL and expression analyses by adjusting cell composition or single-cell RNAseq may reveal interesting results.
SNPs in PGAP3-ERBB2 region were associated with PGAP3 expression and longitudinal asthma exacerbations. In a previous GWAS, rs2941504 in PGAP3 has been associated with asthma.2 Another GWAS has identified rs2952156 in ERBB2 associated with asthma5 and PGAP3 expression in lung tissue.26 Thus, SNPs in PGAP3-ERBB2 are associated with asthma phenotypes by up-regulating PGAP3 gene expression. The first GWAS of asthma has identified rs7216389 in GSDMB associated with childhood asthma and the expression levels of ORMDL3 and GSDMB in lymphoblastoid cell lines.1 In this study, rs7216389 was significantly associated with GSDMB expression (p=1.7x10−4) but not ORMDL3 (p=0.22) in BEC. Thus, SNPs in ZPBP2-GSDMB-ORMDL3 are associated with asthma phenotypes by up-regulating GSDMB gene expression. rs3894194 in GSDMA has been associated with asthma14–15 and the expression levels of GSDMA in lung tissue.26 In this study, rs3894194 was significantly associated with GSDMA expression (p=4.3x10−4). Thus, SNPs in GSDMA are associated with asthma phenotypes by down-regulating GSDMA gene expression. Interestingly, SNPs in IKZF3 were not consistently associated with a specific gene expression, instead, associated with the expression levels of PGAP3, GSDMB, or GSDMA, which may indicate long-distance gene expression regulation. Interaction between gene regulatory elements and genes shown by GeneHancer88 also indicated IKZF3 was involved in complicated long-distance regulation of GSDMB, GSDMA, ORMDL3, and ERBB2 (Figure E9). In summary, our findings confirm the hypothesis that there are proximal, core, and distal regions independently associated with asthma.62 In addition, IKZF3 forms a long-distance regulation region. More importantly, we narrowed down 16 candidate genes to three genes (PGAP3, GSDMB, and GSDMA).
We attempted to identify functional SNPs using colocalization and conditional eQTL analyses. rs2517954 for PGAP3 and rs11657449 for GSDMB were identified by both colocalization analysis and conditional eQTL analysis, though the posterior probability of colocalization was not high. The probable reason is that the signals of genetic association are not strong due to sample size, and thus, eQTL signals drive the colocalization findings in SARP. Colocalization analysis through conditional eQTL analysis (Table E12) further indicates that the colocalization analysis based on the Bayesian approach does not show strong evidence for colocalization.
Previous studies have shown inconsistent relationship between gene expression in this region and asthma susceptibility.62 The mRNA levels of ORMDL3 in lymphoblastoid cell lines have not been significantly different in children with or without asthma.1 An immunohistochemistry study has found that GSDMB protein levels are significantly higher in subjects with asthma than controls.89 In this study, higher mRNA levels of GSDMB were correlated with asthma and asthma exacerbations, though the correlation was not strong and not always consistently significant. Although our findings are based on relevant tissues (BEC) in relevant subjects (healthy controls, non-severe and severe asthma), subjects involved in this study are all adults (age≥12 years old). Typical of adult asthma cohorts, the SARP cohort consists of those with early onset of asthma and those with older age onset.63,67 Since asthma is often an early-onset disease, expression or eQTL analyses in children would be interesting but, of course, research bronchoscopies are not performed in children. In this study, gene expression correlation and eQTL analyses were performed in all SARP subjects with mixed races to increase sample size and power. Although gene expression is less influenced by population stratification than genetic association, the findings may still be biased due to different allele frequencies and LD structures in different ethnic groups. Correlation analysis of gene expression (PGAP3, GSDMB, and GSDMA) and asthma phenotypes (Table IV) and eQTL analysis of top five eQTL SNPs for these three genes (Table E4) were also performed in SARP non-Hispanic Whites (Table E24 and Table E25). The findings of gene expression correlation and eQTL analyses were similar between non-Hispanic Whites and all subjects with mixed races. In summary, the association of SNPs in GSDMB, the expression levels of GSDMB, and asthma phenotypes make GSDMB a strong candidate for severe asthma.
The function of PGAP3, GSDMB, or GSDMA is not totally understood. PGAP3 may have a role in controlling autoimmunity and Th1/Th2 balance.90 GSDMA may regulate or be regulated by TGF-β1 and mediate immune defense by inducing pyroptosis.91 GSDMB may regulate apoptosis of epithelial cells and upregulate expression of airway remodeling genes, chemokines, and heat-shock proteins.89,91 In this study, the expression levels of GSDMB are positively correlated with MHC class I molecules (HLA-A/-B/-C/-F), type I interferon (STAT1, STAT2, and IRF9) and type II interferon pathway genes (IFN-γ and STAT1), and Th1 pathway genes (IFN-γ, STAT1, IL18R1, and IL18BP). All these biological pathways are related to antiviral process, indicating that virus infection and expression of antiviral pathway genes may lead to severe asthma and asthma exacerbations. rs7216389 in GSDMB has been associated with human rhinovirus (HRV) induced wheezing illnesses in children and increased expression of GSDMB and ORMDL3 in HRV-stimulated peripheral-blood mononuclear cells, which further indicates the potential interaction of GSDMB and virus infection in asthma pathogenesis.92 In a previous gene expression analysis in human nasal epithelial cells, GSDMB expression can be induced by IFN-α stimulation.93 In this study, two SNPs (rs1031458 and rs3902920) in the promoter region of GSDMB are colocalized with IRF binding sites and associated with GSDMB expression, atopic early onset asthma, asthma severity, and longitudinal asthma exacerbations, making them potential functional SNPs.
One main disadvantage of this study is the relatively small sample size. In genetic association, eQTL, and gene expression correlation analyses, nominal p-values of 0.05 in addition to adjusted p-values have been used. Furthermore, the replication results in several datasets are not always consistently significant. Thus, it requires careful interpretation as for significance and replication. One main advantage of this study is that multi-level evidence point to the same gene (GSDMB).
In conclusion, we identified that three independent signals (PGAP3, GSDMB, and GSDMA) were associated with asthma susceptibility and GSDMB was also associated with asthma severity, asthma exacerbations, and antiviral pathways. Future candidate gene studies in large, multiethnic, or children with asthma and functional experiments may further reveal functional SNPs/genes for asthma including rare variants in this important region.
Supplementary Material
Key Messages.
SNPs in GSDMB were associated with asthma, asthma severity, asthma exacerbations, and GSDMB expression levels, and its expression levels were correlated with asthma, asthma exacerbations, and antiviral pathways.
SNPs in PGAP3-ERBB2, ZPBP2-GSDMB-ORMDL3, and GSDMA regions were associated with the expression levels of PGAP3, GSDMB, and GSDMA, respectively; SNPs in IKZF3 were associated with the expression levels of PGAP3, GSDMB, or GSDMA.
SNPs identified by GWAS of asthma or autoimmune diseases (AD) were also eQTL SNPs for PGAP3, GSDMB, or GSDMA, but showed opposite effect alleles between asthma and AD.
ACKNOWLEDGMENTS
We acknowledge all investigators, staff, and participants in the SARP studies. TOPMed Acknowledgements: Molecular data for the Trans-Omics in Precision Medicine (TOPMed) program was supported by the National Heart, Lung and Blood Institute (NHLBI). Genome sequencing for “NHLBI TOPMed: Severe Asthma Research Program” (phs001446) was performed at the New York Genome Center (HHSN268201500016C). Core support including centralized genomic read mapping and genotype calling, along with variant quality metrics and filtering were provided by the TOPMed Informatics Research Center (3R01HL-117626-02S1; contract HHSN268201800002I). Core support including phenotype harmonization, data management, sample-identity QC, and general program coordination were provided by the TOPMed Data Coordinating Center (R01HL-120393; U01HL-120393; contract HHSN268201800001I). We gratefully acknowledge the studies and participants who provided biological samples and data for TOPMed.
Declaration of all sources of funding: SARP cross-sectional cohort was supported by NIH grants HL69116, HL69130, HL69149, HL69155, HL69167, HL69170, HL69174, HL69349, UL1RR024992, M01RR018390, M01RR07122, M01RR03186, HL087665, and HL091762. SARP longitudinal cohort was funded by the NHLBI U10 HL109172, HL109168, HL109152, HL109257, HL109146, HL109250, HL109164, and HL109086. Genetic studies for SARP cross-sectional cohort were funded by NIH HL87665 and Go Grant RC2HL101487. SARP whole-genome sequencing was supported by NHLBI Trans-Omics for Precision Medicine (TOPMed) X01 grant.
Disclosure of potential conflict of interest: B. Modena reports grants from NHLBI, personal fees from Sanofi, Regeneron, GSK, Circassia, and AstraZeneca. W. W. Busse has received consulting fees from AstraZeneca, Genentech, Regeneron, Novartis, Sanofi, and GlaxoSmithKline. M. Castro receives University Grant Funding from NIH, American Lung Association, and PCORI, receives Pharmaceutical Grant Funding from AstraZeneca, Chiesi, Novartis, GSK, and Sanofi-Aventis, serves as a consultant for Genentech, Theravance, VIDA, Teva, Sanofi-Aventis and also a speaker for AstraZeneca, Genentech, GSK, Regeneron, Sanofi, Teva, and receives Royalties from Elsevier. L. C. Denlinger has active grant funding from NHLBI and has consulted in the last three years with AstraZeneca. A. T. Hastie reports grants from NIH and grant support from Genentech during the conduct of the study. B. D. Levy reports grants from NHLBI, personal fees from AstraZeneca, Bayer, Gossamer Bio, Merck, Nocion Therapeutics, Pieris Pharmaceuticals, Teva Pharmaceuticals, SRA, and Sanofi. P. G. Woodruff reports personal fees from Sanofi, Glenmark Pharmaceuticals, Theravance, GSK, NGM Pharma, Amgen, and Genentech. S. E. Wenzel has consulted for AstraZeneca, Genentech, GSK, Sanofi-Aventis in the last 3 yrs, in matters unrelated to the content of this manuscript, participated in multicenter clinical trial for AstraZeneca, GSK, Novartis and Sanofi Aventis in the last 3 yrs, unrelated to this manuscript, and received financial support for the last 2 yrs of SARP from an unrestricted grant from Boehringer-Ingelheim. E. R. Bleecker has performed clinical trials through his employer, Wake Forest School of Medicine and University of Arizona, for AstraZeneca, MedImmune, Boehringer Ingelheim, Genentech, Novartis, Regeneron, and Sanofi Genzyme, and has also served as a paid consultant for ALK-Abello, AstraZeneca, MedImmune, Glaxo Smith Kline, Novartis, Regeneron, Sanofi Genzyme, and TEVA, outside the submitted work. All other authors have nothing to disclose.
Abbreviations used
- ACQ-6
asthma control questionnaire-6
- AD
autoimmune diseases
- BEC
bronchial epithelial cells
- CD
Crohn’s disease
- eQTL
expression quantitative trait loci
- ER
emergency room
- FDR
false discovery rate
- GSDMA
gasdermin A
- GSDMB
gasdermin B
- GTEx
Genotype-Tissue Expression database
- GWAS
genome-wide association study
- HRV
human rhinovirus
- IBD
inflammatory bowel disease
- LD
linkage disequilibrium
- MAF
minor allele frequency
- MS
multiple sclerosis
- ORMDL3
ORMDL sphingolipid biosynthesis regulator 3
- PGAP3
post-GPI attachment to proteins 3
- PBCh
primary biliary cholangitis
- PBCi
primary biliary cirrhosis
- QC
quality control
- RA
rheumatoid arthritis
- RNAseq
RNA sequence
- SARP
Severe Asthma Research Program
- SLE
systemic lupus erythematosus
- SNP
Single nucleotide polymorphism
- SS
systemic sclerosis
- TOPMed
Trans-Omics for Precision Medicine
- T1D
type I diabetes
- UC
ulcerative colitis
- WGS
whole-genome sequence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–3. [DOI] [PubMed] [Google Scholar]
- 2.Anantharaman R, Andiappan AK, Nilkanth PP, Suri BK, Wang de Y, Chew FT. Genome-wide association study identifies PERLD1 as asthma candidate gene. BMC Med Genet. 2011;12:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halapi E, Gudbjartsson DF, Jonsdottir GM, Bjornsdottir US, Thorleifsson G, Helgadottir H, et al. A sequence variant on 17q21 is associated with age at onset and severity of asthma. Eur J Hum Genet. 2010;18(8):902–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicodemus-Johnson J, Myers RA, Sakabe NJ, Sobreira DR, Hogarth DK, Naureckas ET, et al. DNA methylation in lung cells is associated with asthma endotypes and genetic risk. JCI Insight. 2016;1(20):e90151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demenais F, Margaritte-Jeannin P, Barnes KC, Cookson WOC, Altmuller J, Ang W, et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet. 2018;50(1):42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrine N, Portelli MA, John C, Soler Artigas M, Bennett N, Hall R, et al. Moderate-to-severe asthma in individuals of European ancestry: a genome-wide association study. Lancet Respir Med. 2019;7(1):20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daya M, Rafaels N, Brunetti TM, Chavan S, Levin AM, Shetty A, et al. Association study in African-admixed populations across the Americas recapitulates asthma risk loci in non-African populations. Nat Commun. 2019;10(1):880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan Q, Brehm J, Pino-Yanes M, Forno E, Lin J, Oh SS, et al. A meta-analysis of genome-wide association studies of asthma in Puerto Ricans. Eur Respir J. 2017;49(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira MA, Matheson MC, Tang CS, Granell R, Ang W, Hui J, et al. Genome-wide association analysis identifies 11 risk variants associated with the asthma with hay fever phenotype. J Allergy Clin Immunol. 2014;133(6):1564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickrell JK, Berisa T, Liu JZ, Segurel L, Tung JY, Hinds DA. Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet. 2016;48(7):709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmiedel BJ, Seumois G, Samaniego-Castruita D, Cayford J, Schulten V, Chavez L, et al. 17q21 asthma-risk variants switch CTCF binding and regulate IL-2 production by T cells. Nat Commun. 2016;7:13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pividori M, Schoettler N, Nicolae DL, Ober C, Im HK. Shared and distinct genetic risk factors for childhood-onset and adult-onset asthma: genome-wide and transcriptome-wide studies. Lancet Respir Med. 2019;7(6):509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira MAR, Mathur R, Vonk JM, Szwajda A, Brumpton B, Granell R, et al. Genetic Architectures of Childhood- and Adult-Onset Asthma Are Partly Distinct. Am J Hum Genet. 2019;104(4):665–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnelykke K, Sleiman P, Nielsen K, Kreiner-Moller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46(1):51–5. [DOI] [PubMed] [Google Scholar]
- 15.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Almoguera B, Vazquez L, Mentch F, Connolly J, Pacheco JA, Sundaresan AS, et al. Identification of Four Novel Loci in Asthma in European American and African American Populations. Am J Respir Crit Care Med. 2017;195(4):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43(9):887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison FS, Locke JM, Wood AR, Tuke M, Pasko D, Murray A, et al. The splice site variant rs11078928 may be associated with a genotype-dependent alteration in expression of GSDMB transcripts. BMC Genomics. 2013;14:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieuwenhuis MA, Siedlinski M, van den Berge M, Granell R, Li X, Niens M, et al. Combining genomewide association study and lung eQTL analysis provides evidence for novel genes associated with asthma. Allergy. 2016;71(12):1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Z, Lee PH, Chaffin MD, Chung W, Loh PR, Lu Q, et al. A genome-wide cross-trait analysis from UK Biobank highlights the shared genetic architecture of asthma and allergic diseases. Nat Genet. 2018;50(6):857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loss GJ, Depner M, Hose AJ, Genuneit J, Karvonen AM, Hyvarinen A, et al. The Early Development of Wheeze. Environmental Determinants and Genetic Susceptibility at 17q21. Am J Respir Crit Care Med. 2016;193(8):889–97. [DOI] [PubMed] [Google Scholar]
- 22.Acevedo N, Reinius LE, Greco D, Gref A, Orsmark-Pietras C, Persson H, et al. Risk of childhood asthma is associated with CpG-site polymorphisms, regional DNA methylation and mRNA levels at the GSDMB/ORMDL3 locus. Hum Mol Genet. 2015;24(3):875–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galanter J, Choudhry S, Eng C, Nazario S, Rodriguez-Santana JR, Casal J, et al. ORMDL3 gene is associated with asthma in three ethnically diverse populations. Am J Respir Crit Care Med. 2008;177(11):1194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan YI, Shrine NR, Soler Artigas M, Wain LV, Blakey JD, Moffatt MF, et al. Genome-wide association study to identify genetic determinants of severe asthma. Thorax. 2012;67(9):762–8. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira MA, McRae AF, Medland SE, Nyholt DR, Gordon SD, Wright MJ, et al. Association between ORMDL3, IL1RL1 and a deletion on chromosome 17q21 with asthma risk in Australia. Eur J Hum Genet. 2011;19(4):458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consortium GTEx. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348(6235):648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marenholz I, Esparza-Gordillo J, Ruschendorf F, Bauerfeind A, Strachan DP, Spycher BD, et al. Meta-analysis identifies seven susceptibility loci involved in the atopic march. Nat Commun. 2015;6:8804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waage J, Standl M, Curtin JA, Jessen LE, Thorsen J, Tian C, et al. Genome-wide association and HLA fine-mapping studies identify risk loci and genetic pathways underlying allergic rhinitis. Nat Genet. 2018;50(8):1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira MA, Vonk JM, Baurecht H, Marenholz I, Tian C, Hoffman JD, et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet. 2017;49(12):1752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hinds DA, McMahon G, Kiefer AK, Do CB, Eriksson N, Evans DM, et al. A genome-wide association meta-analysis of self-reported allergy identifies shared and allergy-specific susceptibility loci. Nat Genet. 2013;45(8):907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langefeld CD, Ainsworth HC, Cunninghame Graham DS, Kelly JA, Comeau ME, Marion MC, et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat Commun. 2017;8:16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Lange KM, Moutsianas L, Lee JC, Lamb CA, Luo Y, Kennedy NA, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet. 2017;49(2):256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Multiple Sclerosis Genetics Consortium (IMSGC), Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45(11):1353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris DL, Sheng Y, Zhang Y, Wang YF, Zhu Z, Tombleson P, et al. Genome-wide association meta-analysis in Chinese and European individuals identifies ten new loci associated with systemic lupus erythematosus. Nat Genet. 2016;48(8):940–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bentham J, Morris DL, Graham DSC, Pinder CL, Tombleson P, Behrens TW, et al. Genetic association analyses implicate aberrant regulation of innate and adaptive immunity genes in the pathogenesis of systemic lupus erythematosus. Nat Genet. 2015;47(12):1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Lu Y, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360(24):2544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawashima M, Hitomi Y, Aiba Y, Nishida N, Kojima K, Kawai Y, et al. Genome-wide association studies identify PRKCB as a novel genetic susceptibility locus for primary biliary cholangitis in the Japanese population. Hum Mol Genet. 2017;26(3):650–9. [DOI] [PubMed] [Google Scholar]
- 40.Cordell HJ, Han Y, Mells GF, Li Y, Hirschfield GM, Greene CS, et al. International genome-wide meta-analysis identifies new primary biliary cirrhosis risk loci and targetable pathogenic pathways. Nat Commun. 2015;6:8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura M, Nishida N, Kawashima M, Aiba Y, Tanaka A, Yasunami M, et al. Genome-wide association study identifies TNFSF15 and POU2AF1 as susceptibility loci for primary biliary cirrhosis in the Japanese population. Am J Hum Genet. 2012;91(4):721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Invernizzi P, Lu Y, Kosoy R, Lu Y, Bianchi I, et al. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42(8):658–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin JE, Assassi S, Diaz-Gallo LM, Broen JC, Simeon CP, Castellvi I, et al. A systemic sclerosis and systemic lupus erythematosus pan-meta-GWAS reveals new shared susceptibility loci. Hum Mol Genet. 2013;22(19):4021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiu F, Tang R, Zuo X, Shi X, Wei Y, Zheng X, et al. A genome-wide association study identifies six novel risk loci for primary biliary cholangitis. Nat Commun. 2017;8:14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waage J, Standl M, Curtin JA, Jessen LE, Thorsen J, Tian C, et al. Genome-wide association and HLA fine-mapping studies identify risk loci and genetic pathways underlying allergic rhinitis. Nat Genet. 2018;50(8):1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ellinghaus D, Jostins L, Spain SL, Cortes A, Bethune J, Han B, et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet. 2016;48(5): 510–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li YR, Li J, Zhao SD, Bradfield JP, Mentch FD, Maggadottir SM, et al. Meta-analysis of shared genetic architecture across ten pediatric autoimmune diseases. Nat Med. 2015;21(9):1018–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44(12):1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, et al. Genome wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42(6):508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plagnol V, Howson JM, Smyth DJ, Walker N, Hafler JP, Wallace C, et al. Genome-wide association analysis of autoantibody positivity in type 1 diabetes cases. PLoS Genet. 2011;7(8):e1002216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson CA, Boucher G, Lees CW, Franke A, D’Amato M, Taylor KD, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43(3):246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42(12): 1118–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet. 2008;40(8):955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim K, Bang SY, Lee HS, Cho SK, Choi CB, Sung YK, et al. High-density genotyping of immune loci in Koreans and Europeans identifies eight new rheumatoid arthritis risk loci. Ann Rheum Dis. 2015;74(3):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu JZ, Almarri MA, Gaffney DJ, Mells GF, Jostins L, Cordell HJ, et al. Dense fine-mapping study identifies new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2012;44(10):1137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47(4):381–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42(4):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41(6):703–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayes MD, Bossini-Castillo L, Gorlova O, Martin JE, Zhou X, Chen WV, et al. Immunochip analysis identifies multiple susceptibility loci for systemic sclerosis. Am J Hum Genet. 2014;94(1):47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terao C, Kawaguchi T, Dieude P, Varga J, Kuwana M, Hudson M, et al. Transethnic meta-analysis identifies GSDMA and PRDM1 as susceptibility genes to systemic sclerosis. Ann Rheum Dis. 2017;76(6):1150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li X, Ampleford EJ, Howard TD, Moore WC, Torgerson DG, Li H, et al. Genome-wide association studies of asthma indicate opposite immunopathogenesis direction from autoimmune diseases. J Allergy Clin Immunol. 2012;130(4):861–8 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stein MM, Thompson EE, Schoettler N, Helling BA, Magnaye KM, Stanhope C, et al. A decade of research on the 17q12-21 asthma locus: Piecing together the puzzle. J Allergy Clin Immunol. 2018;142(3):749–64 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2):405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185(4):356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denlinger LC, Phillips BR, Ramratnam S, Ross K, Bhakta NR, Cardet JC, et al. Inflammatory and Comorbid Features of Patients with Severe Asthma and Frequent Exacerbations. Am J Respir Crit Care Med. 2017;195(3):302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline Features of the Severe Asthma Research Program (SARP III) Cohort: Differences with Age. J Allergy Clin Immunol Pract. 2018;6(2):545–54 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu W, Bang S, Bleecker ER, Castro M, Denlinger L, Erzurum SC, et al. Multiview Cluster Analysis Identifies Variable Corticosteroid Response Phenotypes in Severe Asthma. Am J Respir Crit Care Med. 2019;199(11):1358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Ortega VE, Ampleford EJ, Graham Barr R, Christenson SA, Cooper CB, et al. Genome-wide association study of lung function and clinical implication in heavy smokers. BMC Med Genet. 2018;19(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Das S, Forer L, Schonherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48(10):1284–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X, Hastie AT, Hawkins GA, Moore WC, Ampleford EJ, Milosevic J, et al. eQTL of bronchial epithelial cells and bronchial alveolar lavage deciphers GWAS-identified asthma genes. Allergy. 2015;70(10):1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Hawkins GA, Moore WC, Hastie AT, Ampleford EJ, Milosevic J, et al. Expression of asthma susceptibility genes in bronchial epithelial cells and bronchial alveolar lavage in the Severe Asthma Research Program (SARP) cohort. J Asthma. 2016;53(8):775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Modena BD, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Wu W, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med. 2014;190(12):1363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Modena BD, Bleecker ER, Busse WW, Erzurum SC, Gaston BM, Jarjour NN, et al. Gene Expression Correlated with Severe Asthma Characteristics Reveals Heterogeneous Mechanisms of Severe Disease. Am J Respir Crit Care Med. 2017;195(11):1449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buniello A, MacArthur JAL, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019;47(D1):D1005–D12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li MX, Yeung JM, Cherny SS, Sham PC. Evaluating the effective numbers of independent tests and significant p-value thresholds in commercial genotyping arrays and public imputation reference datasets. Hum Genet. 2012;131(5):747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–5. [DOI] [PubMed] [Google Scholar]
- 82.Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10(5):e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fabregat A, Jupe S, Matthews L, Sidiropoulos K, Gillespie M, Garapati P, et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018;46(D1):D649–D55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489: 57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hao K, Bossé Y, Nickle DC, Paré PD, Postma DS, Laviolette M, et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet. 2012;8(11):e1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ober C, McKennan CG, Magnaye KM, Altman MC, Washington C 3rd, Stanhope C, et al. Expression quantitative trait locus fine mapping of the 17q12-21 asthma locus in African American children: a genetic association and gene expression study. Lancet Respir Med. 2020;8(5):482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sandra JH, Greg LH, Mark H, Paul NR. Flow Cytometric Characterization of Cell Populations in Bronchoalveolar Lavage and Bronchial Brushings From Patients With Chronic Obstructive Pulmonary Disease. Cytometry B Clin Cytom. 2004;61(1):27–34. [DOI] [PubMed] [Google Scholar]
- 88.Fishilevich S, Nudel R, Rappaport N, Hadar R, Plaschkes I, Iny Stein T, et al. GeneHancer: genome-wide integration of enhancers and target genes in GeneCards. Database (Oxford). 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Das S, Miller M, Beppu AK, Mueller J, McGeough MD, Vuong C, et al. GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc Natl Acad Sci U S A. 2016;113(46):13132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Murakami Y, Yasui T, Wakana S, Kikutani H, Kinoshita T, et al. Significance of glycosylphosphatidylinositol-anchored protein enrichment in lipid rafts for the control of autoimmunity. J Biol Chem. 2013;288(35):25490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Das S, Miller M, Broide DH. Chromosome 17q21 Genes ORMDL3 and GSDMB in Asthma and Immune Diseases. Adv Immunol. 2017;135:1–52. [DOI] [PubMed] [Google Scholar]
- 92.Caliskan M, Bochkov YA, Kreiner-Moller E, Bonnelykke K, Stein MM, Du G, et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med. 2013;368(15):1398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giovannini-Chami L, Marcet B, Moreilhon C, et al. Distinct epithelial gene expression phenotypes in childhood respiratory allergy. Eur Respir J 2012; 39: 1197–1205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


