Abstract
The exact molecular mechanisms associated with Alzheimer’s disease (AD) pathology continue to represent a mystery. In the past decades, comprehensive data were generated on the involvement of different signaling pathways in the AD pathogenesis. However, the utilization of signaling pathways as potential targets for the development of drugs against AD is rather limited due to the immense complexity of the brain and intricate molecular links between these pathways. Therefore, finding a correlation and cross-talk between these signaling pathways and establishing different therapeutic targets within and between those pathways are needed for better understanding of the biological events responsible for the AD-related neurodegeneration. For example, autophagy is a conservative cellular process that shows link with many other AD-related pathways and is crucial for maintenance of the correct cellular balance by degrading AD-associated pathogenic proteins. Considering the central role of autophagy in AD and its interplay with many other pathways, the finest therapeutic strategy to fight against AD is the use of autophagy as a target. As an essential step in this direction, this comprehensive review represents recent findings on the individual AD-related signaling pathways, describes key features of these pathways and their cross-talk with autophagy, represents current drug development, and introduces some of the multi-target beneficial approaches and strategies for the therapeutic intervention of AD.
Keywords: Alzheimer’s disease, Amyloid beta, Tau, Senile plaque, Neurofibrillary tangle, Autophagy, Signaling pathways
1. Introduction
Alzheimer’s disease (AD) is the most common form of age-related dementia with no curative therapy available as of yet due to the limited knowledge on the molecular basis of its pathobiology.1,2 AD was described for the first time by Dr. Alois Alzheimer in 1906, who reported that his patient Auguste D. started developing personality changes and cognitive deficits (such as aggression, confusion, paranoia, progressive memory decline, and sleep disturbance) in her late 40s, she succumbed to disease 5 years after admission to the clinic, and showed specific extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs) in the brain histology.3,4 Despite the painstaking attempts of multiple researchers working in this field for almost 115 years after the first report, and despite the existence of numerous hypothesis and theories, the exact pathological mechanisms of the AD remain poorly understood.5 This is because the molecular pathogenesis of AD is complex and involves multiple, often interconnected, factors.5
The numbers of older peoples are increasing rapidly in the developed and many developing countries, such as China, India, and Latin America. This dominating and ever-increasing aging of the world population enhances the chances of the global increase in the number of AD patients and dramatically increases the related burden. According to worlds Alzheimer’s report 2018, about 50 million peoples were living with dementia worldwide in 2018, and this number is expected to reach 152 million by 2050.6 Furthermore, the total worldwide cost of dealing with dementia in 2018 was US$1 trillion, which is estimated to rise to US$ 2 trillion by 2030.6 It has also been estimated that 40 million people, mostly older than 60 years, have dementia, and this number is projected to double every 20 years.
The AD is associated with the progressive neurodegeneration caused by the death of neurons in the hippocampus and surrounding parahippocampal region of brain and abnormal accumulation of specific amyloidogenic proteins or protein fragments in the affected brain areas, with these features being commonly considered as the hallmarks of AD. Early-onset AD (EOAD) and late-onset AD (LOAD) are etiologically and epidemiologically different forms of AD.3,7–9 The EOAD is less common than LOAD, which is the most frequent form of AD with complex etiology that constitute over 95% of all cases.3,10 EOAD, which is also known as familial AD (FAD), may be inherited in an autosomal dominant manner and associated with a non-dominant cause, such as mutations in APOE4 allele. LOAD, being sporadic AD (SAD) form, is the most common type of the age-related neurodegenerative diseases caused by various (often environmental factors) and affecting 5 million people in the USA alone.11,12 Compelling evidence generated by numerous experimental, biochemical, neuropathological, and genetic studies revealed that the pathobiology of AD is attributed to many factors of different nature, giving rise to multiple theories and hypotheses on the diverse mechanisms of the AD pathogenesis. However, aging is considered as the most crucial AD risk factor. Next, amyloid cascade hypothesis postulates that the deposition of the β-amyloid (Aβ, which is a 39–43 amino acid peptide derived from the amyloid precursor protein (APP) by β- and γ-secretase cleavage) or senile plaques in the brain plays a vital role in the AD pathology.13,14 In addition to secretase, several proteins have been identified recently that affect Aβ production and clearance. Furthermore, the FAD is associated with several mutations in genes, such as APP, PSEN1, PSEN2, and APOE4 that are directly involved in the Aβ generation and formation of toxic senile plaques.4,15 Studies have shown that mutation in presenilin gene promotes aberrant APP processing that leads to the formation of longer and more hydrophobic Aβ proteins.16 Aβ is produced by the processing of a parent protein APP that contributes to the proper neuronal and cerebral function development. However, aberrant APP processing and Aβ deposition are considered as the first events associated with the AD development.17,18 Among several other proteins involved in the AD pathogenesis, serious attention is given to the abnormally phosphorylated (hyperphosphorylated) Tau protein that aggregates to paired-helical filaments forming neurofibrillary tangles.19–25 Therefore, it is not surprising that Aβ42 and Tau proteins are considered as well-established cerebrospinal biomarkers for AD.1 In addition to several factors promoting AD, numerous mechanisms protect the brain from AD, such as clearing of abnormal/misfolded proteins, repairing of cells, and better energy metabolism. Typically, the failure of these protective mechanisms may lead to the emergence of AD phenotype. Furthermore, since Aβ, Tau, ApoE, PSEN1, and GSK3β are associated with diverse signaling cascades that regulate neuronal cell survival and activity, failure of these proteins and misbehavior of their associated signaling pathways are also related to the AD etiology.1 The autophagy is highly dynamic process, which is inducible by stress stimuli and environmental changes, such as appearance of protein aggregates or damaged organelles, nutrient starvation, and pathogen infection that can resolve cellular demands.26 Importantly, autophagy is linked to many other signaling pathways, have crucial role in AD pathogenesis, and can be considered as a prominent future drug target.
Therefore, it is evident from the past research that AD represents a multi-factorial disease that involves several cell-signaling pathways. Understandably, the interconnection between these signaling pathways is essential for finding suitable druggable targets. The goal of this review is to highlight different signaling pathways associated with AD pathology, discuss networks interconnecting these signaling pathways, describe interlink between autophagy and other pathways, and consider critical features responsible for the neurodegeneration and their implications for the AD management. Furthermore, we also discuss therapeutic opportunities to enhance the development of new drugs against AD pathogenesis. We hope that this review will help in better understanding of the molecular mechanisms of various pathways involved in AD pathogenesis and their cross-talk with autophagy along with the highlighting of several drug-designing strategies currently employed in this field. Since this review describes a whole cohort of the AD-related signaling pathways, by no means it aims at the exhaustive coverage of all the peculiarities of each pathway. Each of the pathways included in this compendium is itself a subject of numerous focused reviews, and the interested readers are encouraged to look for those specialized reviews.
2. Molecular signaling pathways in Alzheimer’s disease
AD is not only the illness of the brain itself, but is also closely associated with whole human body.1 The complexity of the human brain and unavailability of reasonable animal models are the roots for the unclear pathobiology of AD. Numerous signaling pathways explaining AD pathogenesis have been developed.27 Among those is the amyloid cascade hypothesis that describes the widely accepted pathway for AD pathology, suggesting that the Aβ deposition is the primary cause of AD.28 In addition to Aβ, abnormal Tau aggregation and formation of neurofibrillary tangles cause toxic effect and lead to the death of neurons.29 The genetic factors of AD are very complicated. Autosomal dominant familial EOAD is linked to the genetic mutations in at least three genes, such as APP, PSEN1, and PSEN2. On the other hand, mutations in APOEε4 allele represent a genetic risk factor of LOAD.30 The collapse of various neurotransmitter systems, such as those found in cholinergic, glutaminergic, and serotonergic neurons, might lead to the neurochemical abnormalities in the brain that end with the AD development.31–34 Although healthy mitochondria play various functions in the body, disturbing mitochondria have crucial connections with the AD pathogenesis.35 Endoplasmic reticulum stress and oxidative stress are strongly associated with neuronal injury in AD.36,37 Similarly, uncontrolled neuroinflammation generated by the release of inflammatory mediators can be associated with the dysfunction, necrosis, and apoptosis of neurons.38–40 Neuronal survival depends on the elimination of damaged, toxic, and aggregated proteins that are responsible for the neuronal cell death and cognitive decline.41 The ubiquitin-proteasomal system, together with the autophagy/endosomal-lysosomal system and management of protein misfolding by molecular chaperones are three central protein quality control systems of the cell that play a fundamental role in handling consequences of protein misfolding and aggregation.42,43 Autophagy is a primary intracellular means of removing aggregated proteins and damaged organelles by their degradation.44 In fact, autophagy is a main regulator of generation and clearance of Aβ and Tau proteins. Thus, perturbed autophagy is a well-established contributing mechanism in the pathogenesis of AD.45,46 Although in the norm, insulin regulates various central nervous system (CNS) functions, such as neuronal survival, learning, and memory, and also controls peripheral metabolism, the impaired neuronal insulin signaling triggers many events associated with the AD pathogenesis.47 Similarly, cellular Ca2+ regulates multiple processes crucial for the neuronal function in the norm. However, abnormal Ca2+signaling has been extensively observed in various neurodegenerative diseases, including AD.48,49 Neurotrophic factors (NTFs) are critical components for the maintenance of functions and survival of neurons, but, loss of activity of NTFs exaggerate the effects of Aβ toxicity.50,51 The brain contains 20% of the total body cholesterol that helps in the neuronal physiology and development; however, disturbance in the cholesterol metabolism in the midlife increases the risk of AD pathology.52,53 Regular activity of β-catenin/Wnt is associated with neuronal connectivity, survival, adhesion, proliferation, and differentiation. On the other hand, the perturbed β-catenin/Wnt signaling pathway is commonly reported in AD.54–56 The peptide hormone leptin is involved in the normal glucose homeostasis, control of obesity, food intake, and energy expenditure, and has diverse actions throughout the CNS, such as neuroprotection by various mechanisms, whereas aberrant leptin signaling is also related to AD pathogenesis.57,58 Excitotoxicity caused by excessive activation of N-methyl-D-aspartate (NMDA) receptors causes neuronal death, enhances the production of Aβ and Tau protein aggregation, and finally causes AD.59 Blood-Brain Barrier (BBB) leakage has also been repeatedly reported in AD.60 Finally, bidirectional communication between the gastrointestinal tract and brain is strongly correlated with AD pathogenesis. Alterations in gut microbiota composition influence not only various gut disorders but also central nervous system maladies, such as AD.61 The more in-depth understanding of the molecular mechanism in all the signaling pathways involved in AD has been reported here with their therapeutic targets (Table 2) and treatment strategies.
Table 2:
Drugs in clinical trials for AD based on molecular signaling pathways
| Sr no. | Agents in clinical trial | Mechanism of action | Company | Trial status | References |
|---|---|---|---|---|---|
| 1. Aβ signaling | |||||
| 1 | ACI-24 | Anti-Aβ | AC Immune’s Supramolecular Technology | Phase-I | 483 |
| 2 | ALZ-801 | Prevent Aβ aggregation | Alzheon | Phase-I | 484 |
| 3 | BI-1181181 | BACE-1 inhibitor | Boehringer Inhelneim | Phase-I | 485 |
| 4 | BMS-932481 | BACE-1 inhibitor | Bristol-myers Squibb | Phase-I | 486 |
| 5 | Exebryl-1 | Modulate α and β secretase activity | Proteo Tech | Phase-I | 487 |
| 6 | KHK-6640 | Anti-Aβ | Kyowa Hakko Kirin | Phase-I | 488 |
| 7 | MEDI-1814 | Anti-Aβ | Astrazeneca | Phase-I | 489 |
| 8 | Lu-AF-20513 | Anti-Aβ | Lundbeck | Phase-I | 490 |
| 9 | SAN-61 | Stimulate proliferation of neural stem cell acting simultaneously on Amyloid plaques and aggregates | Diamedica company | Phase-I | 491 |
| 10 | SAR-228810 | Anti-Aβ | Sanofi company | Phase-I | 492 |
| 11 | TTP-400 | Recombinant proteins affecting Aβ | Trans Tech Pharma | Phase-I | 493 |
| 12 | AD-02 | Anti-Aβ | Affiris | Phase-II | 494 |
| 13 | AD-04 | Anti-Aβ | Affiris | Phase-II | 103 |
| 14 | BAN-2401 | Anti-Aβ | Eisai | Phase-II | 495 |
| 15 | Bexorotene | Anti-Aβ | Eisai | Phase-II | 496 |
| 16 | BLU-8499 | Antiamyloidogenic agent | Alzheon | Phase-II | 497 |
| 17 | E-2609 | BACE1 inhibitor | Eisai Co | Phase-II | 498 |
| 18 | EVP-0962 | BACE1 inhibitor | FORUM Pharma | Phase-II | 499 |
| 19 | Octagam | Anti-Aβ | Octapharma AG | Phase-II | 500 |
| 20 | PN-1219 | Anti-Aβ | PFizer | Phase-II | 501 |
| 21 | Posiphen | Antiamyloidogenic agent | QR Pharma | Phase-II | 502 |
| 22 | Ro-63–8695 | Anti-human serum Amyloid P (Anti-SAP) | GalaxaSmithKline | Phase-II | 503 |
| 23 | UB-311 | Anti-Aβ | United Biomedical | Phase-II | 504 |
| 24 | Aducanumab | Anti-Aβ | Biogen Co. | Phase-III | 505 |
| 25 | ALZT-OP1 | Prevent Aβ aggregation | AZ Therapeutics | Phase-III | 506 |
| 26 | AZD-3293 | BACE1 inhibitor | AstraZeneca, EliLilly | Phase-III | 507 |
| 27 | Bapineuzumab | Acting on soluble form of Aβ | Oop & Johnson | Phase-III | 508 |
| 28 | Crenezumab | Anti-Aβ | AC Immune & Genetech | Phase-III | 509 |
| 29 | EGCG | Amyloid related | TaiyoInternational | Phase-III | 510 |
| 30 | Gammagard | Anti-Aβ | Baxalta | Phase-III | 511 |
| 31 | Gantenerumab | Anti-Aβ | Hoffman-La Roche | Phase-III | 512 |
| 32 | GV-971 | Inhibit Aβ aggregation | Shanghai Green Valley | Phase-III | 513 |
| 33 | JNJ-54861911 | BACE1 inhibitor | Janssen | Phase-III | 514 |
| 34 | MK8931 | BACE1 inhibitor | Merck & Co | Phase-III | 515 |
| 35 | ELND005 | Inhibit Aβ aggregation | Transition Therapeutics | Phase-III | 516 |
| 36 | Solanezumab | Reduce Aβ burden in brain by peripheral sink hypothesis | Ely Lilly | Phase-III | 517 |
| 37 | Tramiprosate | Anti-Aβ | Neurochem | Phase-III | 518 |
| 38 | TRx-00237 | Amyloid-related | Janssen, Pfizer, TauRx Therapeutics | Phase-III | 519 |
| 2. Tau Signaling | |||||
| 1 | ACI-35 | Tau-aggregation | ACImmune | Phase-I | 520 |
| 2 | BMS-986168 | Tau-aggregation | Bristol-myers squibb | Phase-I | 521 |
| 3 | RG-7345 | Tau-aggregation | Genetech, Hoffmann-La Roche | Phase-I | 522 |
| 4 | TPI-287 | Tau-aggregation | Cortice Biosciences | Phase-I | 523 |
| 5 | AADvac-1 | Tau-aggregation | Axon Neuroscience | Phase-II | 524 |
| 6 | Methylene Blue | Tau-aggregation | TauRx Therapeutics | Phase-II | 525 |
| 7 | TRx-0014 | Tau-aggregation | TauRx Therapeutics | Phase-II | 526 |
| 8 | TRx-00237 | Tau-aggregation | Janssen, Pfizer, TauRx Therapeutics | Phase-III | 519 |
| 3. Neurotransmitter Signaling | |||||
| 1 | AVN-322 | 5-HT6 receptor antagonist | Avineuro | Phase-I | 527 |
| 2 | Basmisanil (RG-1662) | GABA (A) receptor agonist | Roche | Phase-I | 528 |
| 3 | Bisnorcymserine | Selective inhibitor of butyryl-cholinesterase (BuChE) | QR Pharma | Phase-I | 529 |
| 4 | Huperzine A, Cerebra | NMDA R Antagonist, AChEI, Signal transduction modulator | NutriHerb | Phase-I | 530 |
| 5 | Memogain | Nonselective inhibitor of butyryl and acetylcholinesterases | Neurodyn Life Sciences | Phase-I | 531 |
| 6 | PQ-912 | Glutaminyl cyclise inhibitor | Probiodrug AG | Phase-I | 137 |
| 7 | SUVN-G3031 | H3R antagonist | Suven Life Sciences | Phase-I | 532 |
| 8 | AN2/AVex-73 | Block sodium channels and act as agonist of sigma-1 receptors and muscarinic (M1) receptors, and AChE inhibitor | Anavex Life Science | Phase-II | 533 |
| 9 | AZD-3480 | Agonist of alpha-4-beta-2-nAChR | Targacept | Phase-II | 534 |
| 10 | Ladostigil | Inhibitor of Acetylcholinesterase and monoamine oxidase A nad B | Avraham | Phase-II | 535 |
| 11 | Nelonicline (ABT-126) | Nicotinic alpha-7-nAChR agonist | AbbVie | Phase-II | 536 |
| 12 | ORM-12741 | Alpha 2C adrenoceptor antagonist | Orion | Phase-II | 537 |
| 13 | PXT-864 | Multitarget: NMDAR and GABA (B) antagonist, modulate metabotropic glutamate receptor | Pharnext | Phase-II | 538 |
| 14 | Rasagiline | Monoamine oxidase type B (MAO-B) inhibitor | Teva | Phase-II | 539 |
| 15 | Riluzole | Multitarget: group of targets in the glutamatergic system and different types of ion channels | Rockefeller University | Phase-II | 540 |
| 16 | S-38093 | Ionic channel modulator, antagonist of H3-histamine receptors (H3R) | Servier | Phase-II | 541 |
| 17 | S-47445 | Inotropic glutamate receptor | RespireRx Pharmaceuticals | Phase-II | 542,543 |
| 18 | SUVN-502 | 5-HT6 receptor antagonist | Suven Life Sciences | Phase-II | 544 |
| 19 | AVP-786 | NMDAR antagonist | Avanir Pharmaceuticals | Phase-III | 545 |
| 20 | Encenicline hydrochloride | Acetylcholine (nicotinic) receptors Ligand, nicotinic alpha-7-nAChR agonist | Bayer | Phase-III | 546 |
| 21 | Intepirdine | 5-HT6 receptor antagonist | GlaxoSmithKline | Phase-III | 544 |
| 22 | Lu-AE-58054 | Serotonin receptors | Lundbeck | Phase-III | 547 |
| 23 | Dexpramipexole | Dopamine receptor agonist | Biogen | Phase-II | 528 |
| 4. Neuroinflammation signaling | |||||
| 1 | AAD-2004 | Cytokines inhibitor | GNT Pharma | Phase-I | 548 |
| 2 | Entanercept | Inflammation | Amgen, Inc., Pfizer | Phase-II | 549 |
| 3 | Pioglitazone | Inflammation | Takeda | Phase-III | 550 |
| 5. Lipid/Cholesterol Signaling | |||||
| 1 | GSK-2647544 | Inhibitor of phospholipase A2 | GSK | Phase-I | 551 |
| 2 | Atorvastatin | Lowers cholesterol level | PFizer | Phase-II | 552,553 |
| 3 | Pitavastatin | Statin | Kowa | Phase-II | 553 |
| 4 | Rilapladib | Inhibitor of phospholipase A2 | Glaxo Smith Kline | Phase-II | 554 |
| 5 | Simvastatin | Statin | NTA | Phase-II | 555,556 |
| 6. Oxidative stress Signaling | |||||
| 1 | ARC-031 | Antioxidant | Archer Pharmaceuticals | Phase-I | 557 |
| 2 | Curcumin | Antioxidant | Now Foods | Phase-II | 558 |
| 3 | Lu-AF-20513 | Antioxidant | Lundbeck | Phase-II | 559 |
| 4 | Quercetin | Antioxidant | Twinlab | Phase-II | 558 |
| 5 | EGCG | Antioxidant | Taiyo International | Phase-III | 510 |
| 6 | HX-106 | Antioxidant | VitroMed | Phase-III | 558 |
| 7 | Nilvadipine | Antioxidant | Astellas Pharma | Phase-III | 560 |
| 8 | SK-PC-B70M | Antioxidant | SK Chemicals | Phase-III | 561 |
| 7. Ca 2+ Signaling | |||||
| 1 | ARC-031 | Calcium channel blocker | Archer pharmaceuticals | Phase-I | 557 |
| 2 | Levetiracetam | N-type calcium channel blocker | Agene-Bio | Phase-II | 562,563 |
| 3 | Nilvadipine | Calcium channel blocker | Astellas pharma | Phase-III | 560 |
| 8. Neurotrophic-factor Signaling | |||||
| 1 | FGL-2 | Stimulates the secretion of nerve growth factor (NGF) | Enkam | Phase-I | 564 |
| 2 | NSG-0202 | Stimulates the secretion NGF | Ns Gene | Phase-I | 565 |
| 3 | T-817MA | Neurotrophic agent | Toyama, FUJIFILM | Phase-II | 566 |
| 9. Endosomal-lysosomal System | |||||
| 1 | GC-021109 | Inducer of phagocytosis | Glia cure | Phase-I | 567 |
| 10. Ubiquitin Proteasomal System | |||||
| 1 | Resveratrol | Restore proteasomal activity | Solgar, Country life, MRM | Phase-III | 203,568 |
| 11. Insulin Signaling | |||||
| 1 | Exenatide | Agonist of human glucagon-like peptide-1 (Amino acids 7–37) | NIH | Phase-II | 569 |
| 2 | Liraglutide | Agonist of human glucagon-like peptide-1 (Amino acids 7–37) | Imperial college | Phase-II | 570 |
| 3 | MSDC-0160 | Antidiabetic | Metabolic solutions | Phase-II | 571 |
| 4 | Humulin | Insulin signaling | NIH | Phase-III | 524 |
| 12. Miscellaneous | |||||
| 1 | AUS-131 | Non-hormonal selective estrogen receptor beta (ERbeta) agonist | Ausio pharmaceuticals | Phase-I | 572 |
| 2 | BPN14770 | Phosphodiesterase inhibitor | Tetra discovery partners | Phase-I | 573 |
| 3 | Copaxone | Immunomodulator | Cedar-sinai medical center | Phase-I | 574 |
| 4 | RP-5063 | Multitarget | Reviva Pharma | Phase-I | 575 |
| 5 | Telmisartan | PPARalpha agonist, signal transduction modulator, PPARgamma modulator | Astellas Pharma | Phase-I | 576,577 |
| 6 | AVN-101 | Not-disclosed | Avineuro | Phase-II | 578 |
| 7 | AVN-397 | Not-disclosed | Avineuro | Phase-II | 579 |
| 8 | Benfotiamine | Neuroprotector | Burke Medical Research Institute | Phase-II | 580 |
| 9 | BI-409306 | Phosphodiesterase inhibitor | Boehringer Ingelheim | Phase-II | 581 |
| 10 | Bryostatin-1 | Activator of protein kinase C (PKC) isozymes | Blanchette Rockefeller Neuroscience institute | Phase-II | 582 |
| 11 | DAOI-B | Inhibits D-amino acid oxidases | Chang Gung Memorial Hospital | Phase-II | 583 |
| 12 | Davunetide | Glial cell mediator of vasoactive intestinal peptide (VIP) induced neuroprotection | Allon therapeutics Inc., paladin Labs INC. | Phase-II | 584,585 |
| 13 | Isotretinoin | Retinoid receptors | Hexal AG | Phase-II | 586 |
| 14 | LND-101001 | Not-disclosed | Lupin | Phase-II | 587 |
| 15 | MK-7622 | Not-disclosed | Merk & Co. | Phase-II | 588 |
| 16 | Rph-201 | Not-disclosed | Regenera pharma | Phase-II | 589 |
| 17 | Sargramostim | Granulocyte macrophage-stimulating agent | Perrigo | Phase-II | 590 |
| 18 | Tamibarotene | Retinoic acid receptor (RAR) alpha agonist | Osaca City University | Phase-II | 591 |
| 19 | UE-2343 | Inhibitor of 11-beta-hydroxysteroid dehydrogenase type-1 | Actinogen | Phase-II | 592 |
| 20 | VX-745 | MAPK P38 inhibitor | EIP pharma | Phase-II | 593 |
| 21 | Xanamen | Inhibitor of 11-beta-hydroxysteroid dehydrogenase type-1 | Actinogen | Phase-II | 592 |
| 22 | AC-1202 | Undisclosed | Accera | Phase-III | 594 |
| 23 | AC-1204 | Stimulation of metabolic process | Accera | Phase-III | 595 |
| 24 | Insulin detemir | Not-disclosed | Novo Nordisk | Phase-III | 596 |
| 25 | Masitinib mesylate | Inhibitor of c-KIT receptor, growth factor receptor (PDGFR), Fibroblast growth factor receptor-3 (FGFR-3) tyrosine kinases | AB Science | Phase-III | 597 |
| 26 | Memryte | Durin-leuprolide acetate and GnRH (LHRH) receptor agonist | Durect, Curaxis pharmaceutical | Phase-III | 598 |
2.1. Amyloid cascade signaling in AD
The Amyloid Cascade Hypothesis that was firstly proposed by Hardy and Higgins, in 1992,28 represents the best defined and widely accepted framework of AD pathogenesis. It postulates that the amyloid beta (Aβ) deposition represents the primary and sole event leading to AD by accelerating downstream deleterious events, such as formation of senile plaques and NFTs thereby leading to the neuronal death that results in the memory loss and finally clinical dementia.28,62 Although β- and γ-secretase-mediated cleavage of the APP generates several Aβ proteins ranging in length from 39 to 43 amino acids, Aβ40 and Aβ42 found in the AD brain seem to be the major constituents of the senile plaque.63,64 Substantial evidence suggested that cognitive decline in the AD might be due to the direct toxic effect of Aβ oligomers on synapses and neuronal network.65,66
Aβ is a 4-kDa protein originated from an APP,67 which is metabolized by competing pathways, such as α-secretase (non-amyloidogenic) pathway and β-secretase (amyloidogenic) pathway. In the α-secretase pathway, the initial cleavage of APP is made by the α-secretase followed by γ-secretase. By contrast, in the Aβ pathway, initial cleavage is made by the β-secretase followed by γ-secretase. In the β-secretase pathway, γ-cleavage mostly generates Aβ40 and sometimes Aβ42, which is characterized by high cytotoxicity.68 Cleavage by α-secretase generates large N-terminal peptide called sAPPα and smaller C-terminal fragment called C83. Cleavage with β-secretase forms a large N-terminal sAAPβ and a smaller C99 fragment, which is further cleaved by γ-secretase leading to the release of Aβ40/Aβ42 to the extracellular space and the APP intracellular C-terminal domain (AICD) into the intracellular space.69 After synthesis, Aβ is secreted outside the cell where it binds to various isoforms of ApoE, which allow them to undertake degradation and clearance by distinct pathways (proteolysis by the Aβ degrading enzyme (such as insulin-degrading enzyme (IDE) and a neutral endopeptidase neprilysin (NEO)), clearance through BBB, trafficking into the cell).68 Evidence have been reported that aberrant autophagy leads to disturbance in the processing of APP and aggravate AD pathology.70 The enhanced autophagy induction increases APP processing that creates conditions favorable for Aβ accumulation in AD brain.71,72 Therefore, targeting autophagy along with utilization of Aβ aggregation inhibitors may provide better therapy for AD.
Amyloidosis is caused by the gathering and aggregation of amyloidogenic proteins. It has been reported that the self-oligomerization of Aβ molecules might contribute to the neuronal injury and neurodegeneration.2,73,74 Accumulation and aggregation of Aβ proteins are due to the increase in the Aβ production in neurons and the decrease in the activity of the Aβ degrading enzymes.11 In normal physiological condition, production and clearance of Aβ are balanced. Conversely, under the pathological condition, increased Aβ production, or decreased Aβ degradation/clearance could result in the raised levels of Aβ1–42.73 It has been reported that soluble oligomers of Aβ are more toxic than fibrillar Aβ at a very early stage of AD.75,76 Interestingly, the soluble Aβ oligomers formation starts within cells and appearance of such oligomers is strongly associated with the development of dementia-like symptoms in AD brain. These oligomeric species of Aβ interact with the cell membrane and forms pore channel inside the membrane. Also, oligomers induces oxidative stress, mitochondrial alterations, and glial activation.66 In a recent finding, Aβ oligomers have been shown to disrupt the blood supply by constricting blood capillaries via interfering with pericytes. In normal physiological condition, pericytes control blood flow by controlling flexibility of capillary walls.77 This new effect of Aβ oligomers could be explored to develop new therapeutic strategies to cure early AD.
Aβ40 is the predominant Aβ species generated by the β-secretase pathway, whereas Aβ42 is a less abundant, but noticeably more amyloidogenic form of the Aβ protein. Indeed, Aβ42 is the primary Aβ species that contribute to the amyloid plaque formation in all forms of AD.2,74,78 Senile plaque consists of soluble and insoluble assemblies of Aβ, including soluble dimers, trimers, dodecamers, etc.2,74 β-Secretase (BACE1) is highly expressed in neurons and cleaves APP at the β-site, which is the first step of Aβ generation. Typically, a high level of BACE1 enhances the production of Aβ42 and initiates amyloidogenesis.79–81 β-cleavage of APP may be up-regulated in LOAD. Interestingly, β-cleavage mainly occurs in endosomes.3,82,83 PSEN1 and PSEN2 provide catalytic subunit to γ-secretase for APP cleavage. However, cleavage is mediated either by PSEN1 or PSEN2.4,15,16 Kimberly et al. reported that PSEN1 deficiency decreases the production of Aβ, suggesting that PSEN1 serves as the crucial mediator of APP cleavage by γ-secretase.84 Additionally, presenilin enhancer protein 2 (PEN-2, also known as γ-secretase subunit PEN-2), presenilin-stabilization factor APH-1 (also known as γ-secretase subunit APH-1A), and nicastrin (NCT) are also involved in interaction with presenilin leading to the formation of active γ-secretase.85–88 PSEN1 mutation generates more toxic Aβ42 that leads to the early-onset FAD.89 Neprilysin and IDE degrade Aβ, whereas their down-regulation raises the levels of Aβ production.90 Interestingly, both of these Aβ degrading enzymes are reported to be decreased in AD.68,91,92
Aberrant levels of Aβ may induce numerous pathological conditions, such as abnormalities in the synapse and neuronal network,68 distorted hippocampal synaptic plasticity,93 neuronal network dysfunction.94 They also can affect synaptic transmission95 and show inhibitory effects on the presynaptic P/Q Ca2+ current necessary for the synaptic plasticity,96 as well as can affect synaptic structure, composition, and density. Libro et al. reported that cannabidiol (CBD) potentiates Aβ and Tau degradation by inhibiting Tau phosphorylation and inhibiting enzymes involved in Aβ processing.97 Many β- and γ-secretase inhibitors are in phase-II and phase-III clinical trial (Table 2). Additionally, active immunization with the Aβ peptides and their derivatives is in the phase-II and phase-III clinical trial.11,98–104 This approach was used to develop serum antibody titers to the Aβ peptide that can help in the removal of Aβ from the brain and was shown to cause cognitive improvement in some patients.11,98–104 Furthermore, passive immunization strategies using antibodies targeting different regions of the Aβ1–42 peptide or Aβ oligomers are also currently being tested.99,105–116 Unfortunately, numerous attempts were made to develop Aβ-targeting drugs for AD have failed. Therefore, for the development of new medication against AD need to consider other targets along with Aβ.
2.2. Tau and neurofibrillary tangles in AD
Tau is the microtubule-binding, intrinsically disordered phosphoprotein abundantly found in central and peripheral nervous systems, where it concentrates predominantly in the axons of nerve cells and serves as the major constituent of neurofibrillary inclusions.117,118 Under physiological conditions, Tau binds and stabilizes microtubule in axons, whereas under pathological conditions, this protein gets hyperphosphorylated, and this leads to its detachment from the microtubules and initiates formation of insoluble Tau aggregates followed by the generation of specific neuronal inclusions, paired helical filaments (PHFs) assembled into neurofibrillary tangles (NFTs). The hyperphosphorylation of Tau occurs due to decreased activity of phosphatases and increased activity of kinases.119 Epigenetic modifications such as histone modification, non-coding RNA regulation, and DNA methylation can regulate tau phosphorylation and AD progression.120 There are six isoforms of Tau in the brain,121 containing 352, 381, 383, 410, 412, and 441 amino acids that are expressed in the adult human brain, whereas only one isoform composed of 352 amino acids is expressed in the human fetal brain.122,123 It has been reported that Tau oligomers with a prefilamentous structure represent the main culprit in the early AD stages.29,124 Tau pathology is the key driver of the disease progression not only in AD, but also in multiple other neurodegenerative diseases associated with the accumulation of Tau inclusions, which are collectively known as Tauopathies (e.g., primary age-related tauopathy (PART), chronic traumatic encephalopathy (CTE), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17), Lytico-bodig disease (Parkinson-dementia complex of Guam), ganglioglioma and gangliocytoma, meningioangiomatosis, postencephalitic parkinsonism, subacute sclerosing panencephalitis (SSPE), encephalopathy, tuberous sclerosis, pantothenate kinase-associated neurodegeneration, and lipofuscinosis).125 However, the molecular basis of the Tau-mediated neurotoxicity is not completely understood.126 In AD, neurofibrillary lesions are abundantly found that consist of paired helical and straight Tau filaments, whereas other Tauopathies might contain Tau filament with diverse morphologies.127
A variety of etiological factors might lead to the abnormal hyperphosphorylation of Tau. This includes diverse pathways, such as Aβ cascade, perturbed glucose metabolism, and impaired phosphorylation and dephosphorylation pathways. Hyperphosphorylated Tau loses its biological activity and disassociates from microtubules. As a result, Tau hyperphosphorylation promotes homooligomerization and intracellular accumulation of this protein. Abnormal Tau is then polymerizing into PHFs and NFTs that accumulate in the body of the neuronal cells, frustrate the affected neurons, and facilitate cell death.128,129 Prior to the NFTs formation, Tau self-assemble into various aggregation species, such as Tau oligomers, granules, and fibrils.130 Loss of function of the normal Tau protein and gain of toxic properties by generating multimeric species are strongly associated with the neuronal loss in CNS.131 Tau undergoes abnormal post-translational modifications during its fibrillation, resulting in altered microtubule-stabilizing properties and decreased solubility.123 NFTs and other Tau aggregates cause toxic effects to neurons by producing neurotoxic signaling defects and by impeding the normal cell function.29 PHFs consist of two fibrillar strands of which twist around one another in a helical manner.122 Goedert et al. indicated that multiple human Tauopathies are characterized by different Tau fibril morphologies, suggesting the existence of different molecular strains of aggregated Tau.132 Although, Tau pathology shows a better correlation with neurodegeneration and cognitive impairment in AD, still Tau-based approaches have not gained much more attention.133 Several possible Tau-based therapeutic strategies could be implied in AD, such as: i) Lowering the production of Tau aggregates or efficient removal of misfolded/oligomeric Tau aggregates; ii) Inhibition of toxic oligomers formation; iii) Reduction of the levels of Tau phosphorylation; iv) Increase of the PHFs/NFTs degradation; v) Microtubule stabilization; vi) Maintaining normal Tau conformations; and vii) Reducing levels of mutated Tau. Currently, few drugs have been reported to target Tauopathies (including AD) as shown in Table 2.
2.3. Genetic mutations in AD
In the brain, Aβ generation is influenced by the mutation in known genes associated with EOAD such as APP, PSEN1, and PSEN2,134 along with genes encoding β-secretase and proteins in the γ-secretase complex, such as NCSTN, APH1A, and PEN2.2 In addition to Aβ peptides, diffuse Aβ plaques abundantly contain various proteins, such as ApoE, clusterin, ACT, and complement proteins, that are playing a critical role in the dynamic balance between Aβ deposition and clearance.135–139 For example, the ApoE4 interference with the Aβ clearance from brain (which instead processed into neurotoxic fragments and contributed to SAD) was suggested as a major genetic risk factor for an AD.1,74,140,141 Typically, proteins, such as APP, Tau, and ApoE4 are involved in axonal transport.51 ApoE2 and ApoE3 have a higher affinity for Aβ, which helps the Aβ clearance by transportation and degradation. By contrast, ApoE4 possesses lower affinity to Aβ, and mainly the mutated forms of this protein induce Aβ accumulation in the brain.68,142 It has also been observed that genetic mutation in Tau protein generates frontotemporal dementia in the absence of senile plaques, suggesting that mutations in Tau can act as another factor causing AD.1
In the 1990s, the first pathogenic mutation was found in the amyloid precursor protein (APP). It is recognized now that in addition to APP, there are two more genes (presenilin 1 (PSEN1), and presenilin 2 (PSEN2)), mutations of which have been implicated in FAD. In fact, these three genes have been described to contain more than 160 highly penetrant but rare FAD-related mutations.143 The first of these genes is an APP, which is located on chromosome 21q21.3, and mutations in which are found in 25 extended families. Despite its overall importance for the pathogenesis of both familial and sporadic forms of AD, the functional repertoire of APP is far from being completely understood (although this protein has been shown to be related to neuronal development and formation and repair of synapses, e.g., being upregulated after neuronal injury). The second FAD-related gene, PSEN1, resides on chromosome 14q24.2 and its FAD-associated mutations are found in 315 families, whereas 18 families are known with FAD-related mutations in the PSEN2 gene, which is located on the chromosome 1q42.13 and has high sequence homology and very similar structural organization to PSEN1.4,144 Scheuner et al. reported that the FAD-linked mutations in all cases might increase the extracellular concentration of Aβ42, thereby leading to the enhanced aggregation of this protein and triggering the AD.15 The major biological function of presenilin is control γ-secretase activity which is responsible for proteolytic cleavage of the APP and NOTCH receptor proteins.145 It has been demonstrated that GSK-3β and β-catenin are the components of the PSEN1 protein complex, where PSEN1 regulates the pool of β-catenin, which is critical for neurogenesis as well as synaptogenesis.146–148 Intriguingly, the healthy subjects can get AD early, in case if they have inherited two ApoE4 genes or aggressive PSEN1 mutant genes. More importantly, since these genes are primary risk factors for AD, genetic testing (genotyping) is highly advisable to look for mutated ApoE4 and other susceptible genes.11 Recently, another pathological role for mutant PSEN1 was reported, which includes reduction in the autophagic cargo elimination that reduces the efficiency of autophagic process.149 Triggering receptor expressed on myeloid cells 2 (TREM2) specifically expressed in brain microglia have been identified as risk gene in AD.150 The partial functional loss in TREM2 protein and alteration in microglial cell behavior were reported in LOAD.151 Susceptibility to LOAD is associated to 20 disease-associated genomic loci, identified in the large-scale genome-wide association studies (GWAS) which was carried out in larger datasets.152,153 GWAS identifies novel disease-associated genetic variants by simultaneous testing of large number of genetic markers such as single nucleotide polymorphisms (SNPs) throughout the genome.154,155 Recent GWAS studies identified AD-associated genes such as PICALM, PTK2B, SORL1, BIN1, CLU, ABCA7, CD2AP, CR1, SLC24A4 RIN3 locus, EPHA1, MS4A4/MS4A6E, INPP5D, MEF2C, NME8, FERMT2, CD33, CASS4, ZCWPW1, CELF1, and HLA-DRB5 HLA-DRB1 locus.156 CD2AP, PICALM, BIN1 EPHA1, and CD33 are involved in synaptic functioning, ABCA7, MS4A, CLU, CR1, CD33, and EPHA1 are associated with immune system, CLU and ABCA7 are associated with lipid metabolism.157
2.4. Altered neurotransmitter signaling in AD
The AD is associated with the collapse of various neurotransmitter systems caused by the malfunction of cholinergic, glutaminergic, and serotonergic neurons. Therefore, decreased brain levels of neurotransmitters and neuromodulators are among the main neurochemical abnormalities of AD.31–34 Altered dopamine concentration in the brain is closely associated with the neurotoxicity.158,159 Nobili et al. recently reported the loss of neurons in ventral tegmental area (VTA) at pre-plaque stages in the mouse model of AD and observed memory impairment and disturbed synaptic plasticity.159 In vitro studies on norepinephrine reported that its low concentration can produce neuroprotective effect by acting as antioxidant and decreasing neuroinflammation.158
Synapses of the glutamatergic and GABAergic neurons provide excitatory and inhibitory outputs in CNS.160,161 Alterations in their function and distortions of the related signaling could promote the AD pathogenesis. Dysfunctional glutamatergic signaling increases cellular Ca2+ level followed by the promotion of the excitatory pathway that leads to the nerve cell death. The dysfunctional GABAergic transmission leads to an increase in the intracellular level of Cl− ions, which decreases the long-term potentiation (LTP), finally resulting in the impairment of cognition.162 The loss of presynaptic neuronal structure is responsible for the reduction in the brain level of neurotransmitters (mostly acetylcholine in cholinergic neurons) eventually makes cells to become deafferented due to synaptic and connectivity loss. Importantly, this loss of synapses and connectivity along with reduced levels of neurotransmitters and neuromodulators has been reported to become rather extensive in AD. Furthermore, in AD, failure in rapid ion channel signaling has been reported due to disconnection of the nerve cells, which reduces the release of secondary messengers by G protein-coupled receptors (GPCRs). These signaling events regulate numerous functions in the nerve cells.31 Lee et al. reported that APP cleavage might be regulated by various signals, such as acetylcholine, neuropeptides, glutamate, and serotonin.163,164 Therefore, the Aβ peptide generation in the brains of AD patients may cause impaired neurotransmission or reduced neuronal activity.31 It is extensively observed that GPCRs are involved in neurotransmitter system, which is damaged in the AD brain. Furthermore, the functions of GPCRs have been reported to be disturbed by Aβ peptide.165 The cholinergic neurons are present in high amount in basal forebrain region of brain that may be reduced or lost in AD. Cholinergic integrity and activity is neuroprotective and essential for cognitive processes.158 Furthermore, cholinergic transmission has a pivotal role in memory, cognition, and synaptic plasticity. Disruption of acetylcholine-containing neurons substantially contributes to the cognitive decline in AD.166 It has also been observed that Aβ is more toxic to cholinergic neurons than to other neuron types. Indeed, Aβ efficiently inhibits the cholinergic signaling.167 M1mAChRs (postsynaptic) and M2mAChRs (presynaptic) receptors are major mAChR subtypes involved in AD.168,169 Interestingly, neuronal autophagic process is directly regulated by neurotransmitter receptors. The coupling of neurotransmitter receptors with autophagy is crucial for regulation of neuronal function.170 Aβ-deposition in synapses results in the decreased release of the presynaptic acetylcholine leading to inhibition of the signal transduction and results in the impaired memory and cognition.165,171 Additionally, reduction in neurotransmitter synthesizing enzymes, such as glutamic acid decarboxylase (GAD) and choline acetyltransferase (CAT) has been found in dementia and AD.172 Acetylcholinesterase inhibition is the most extensively developed treatment strategy for an AD, which revamps the release of acetylcholine as well as modification of acetylcholine receptors.173 M1 agonists, such as xanomeline and AF102B, are in the clinical trial where they show a reduction in the Aβ levels in CSF, as well as the development of memory and cognition.174,175
Currently, there are two classes of FDA-approved drugs available for AD treatment, which are associated with the regulation of the neurotransmitter signaling.165 The first class of these drugs includes memantine that acts as a low-affinity voltage-dependent uncompetitive antagonist of the glutaminergic NMDA receptors that inhibit the prolonged influx of Ca2+ ions and gives rise to the symptomatic improvement in AD and restores functional glutaminergic transmission, which was disturbed in the AD.176 Furthermore, memantine can act as a non-competitive antagonist for serotonergic (5-HT3),177 cholinergic (nicotinic acetylcholine),178 dopaminergic (D2),179 and sigmaergic (σ1) receptors.180 The second class of drugs affecting neurotransmitter systems includes acetylcholinesterase inhibitors (such as rivastigmine, galantamine, and donepezil) that raises the levels of acetylcholine, which are reduced in AD,181 thereby stimulating presynaptic and postsynaptic muscarinic and nicotinic receptors and leading to an improvement of cognitive function in mild to moderate AD patients.182,183 However, still, new drugs are under clinical trials, which target neurotransmitter signaling (Table 2 and Figure 2).
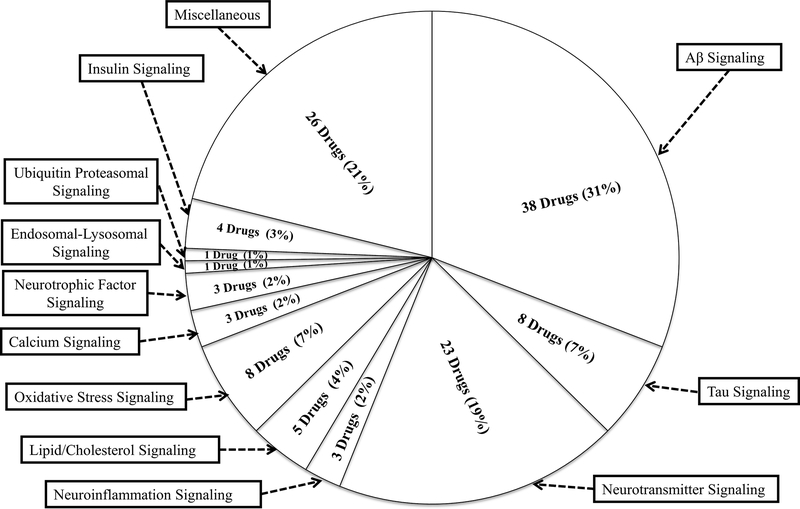
Figure 2: Distribution of drugs in clinical trials by signaling pathways in AD;
Numerous drugs are in different phases of clinical trials that targeting different signaling pathways. Few drugs have targets in more than one pathway. Most of the drugs (38 drugs) have been targeted against Aβ signaling. In addition, some drugs are in the miscellaneous category with an unknown mechanism of action/undisclosed information.
2.5. Mitochondrial dysfunction in AD
The mitochondrion is the critical organelle in neurons, which has numerous functions, such as ATP generation, control of energy efficiency, reactive oxygen species (ROS) generation, apoptotic signaling, and calcium homeostasis.35,184 It has been reported that mitochondrial transport decreased in AD brain.35,185 As a result, mitochondrial dysfunction is a primary and early event in pathological cascade of AD.35,185 The normal physiological function of mitochondria depends on their intact structure needed to maintain the electrochemical gradient. However, structurally damaged mitochondria with the lost internal structure and change in morphology have been observed in AD brain.186–188 Furthermore, mitochondria found in AD brain are characterized by the reduced efficiency of ATP production, increased levels of ROS and oxidative stress, lost Ca2+ buffering capacity, and released pro-apoptotic factors eventually leading to the induction of apoptosis. All these factors trigger apoptosis of neuronal cell by associating with various cytoplasmic factors.184 Studies in yeast have been reported that cellular proteostasis is maintained by mitophagy and mitochondrial proteases and their dysfunction leads to protein misfolding disease.187 Mitochondria may combine growth signaling and nutrient signaling pathways to regulate energy production and Ca2+ homeostasis and regulate apoptosis.189 Functional mitochondrial abnormalities, which include the aberrant respiratory chain, defective enzyme activity, impaired energy metabolism, generation and accumulation of ROS, increase in the BACE-mediated cleavage of Aβ, increased brain amyloidosis, increase in generation of NFTs, dendritic arborization, disturbance in oxidative phosphorylation (OXPHOS), impaired Ca2+-signaling, increased mtDNA mutations, defective mitochondrial bioenergetics, mitochondrial dynamics, and mitochondrial trafficking, are all known to play a key role in AD pathogenesis.49,184,187,190–200 Evidence suggested that progressive accumulation of mitochondrial Aβ can be associated with the mitochondria-mediated toxicity. It has also been suggested that mitochondria may play a central role in Aβ-induced oxidative damage and neuronal damage. Transfer of the intracellular Aβ into mitochondria occurs via a receptor-dependent pathway. Furthermore, intra-mitochondrial Aβ accumulation directly affects mitochondrial respiratory enzyme activity (Table 1) and leads to the mitochondrial dysfunction followed by the neuronal damage.192 It has also been reported that hyperphosphorylated Tau and NFTs in AD patient causes mitochondrial dysfunction and axonal transport inhibition.201
Table 1:
Therapeutic targets for AD based on molecular signaling pathways
| Sr. No. | Molecular Signaling Pathways | Therapeutic targets | References |
|---|---|---|---|
| 1 | Amyloid cascade signaling | Aβ, APP, BACE1, PSEN1, PSEN2, ApoE | 480 |
| 2 | Tau and Neurofibrillary tangles | Tau protein, kinases | 29 |
| 3 | Genetic mutation | APP, PSEN1, PSEN2, ApoE4 | 2 |
| 4 | Neurotransmitter signaling | NMDA receptor, acetyl-cholinesterase (AchE), Glutamatergic and GABAergic receptors | 162,181 |
| 5 | Mitochondrial dysfunction | Mitochondrial respiratory enzymes, mitochondrial ROS | 201,202 |
| 6 | Endoplasmic reticulum stress | Unfolded Protein Response (UPR), Stress sensors (IREI, PERK, and ATFE6) | 481 |
| 7 | Oxidative stress | Reactive oxygen species (ROS), Mitochondrial dysfunction, Advanced glycation end products (AGEs) | 224,226 |
| 8 | Neuroinflammatory signaling | Proinflammatory elements such as chemokines, IL-2β, IL-6, IL-12, INF-γ, TNF-α, NO, ROS, IL-1β | 39,40,251 |
| 9 | Ubiquitin-Proteasomal System | Ubiquitin activating enzyme (E1), Ubiquitin conjugating enzyme (E2), UCHL1, 26 S Proteasome | 42 |
| 10 | Autophagy/Endosomal-lysosomal system | Mammalian target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), PSEN1, SNCA, UBQLN1, UCHL1 ATG7, CDK5, CLU, CTSDITPR1, MAPT, BCL2, BECN1, FOXO1, and GFAP | 282,291,294,434 |
| 11 | Protein misfolding and molecular chaperones | Heat Shock Proteins (HSPs), HSP60 and HSP70 | 296 |
| 12 | Insulin Signaling | Insulin Receptors (IRs), Insulin degrading Enzymes (IDE), Akt, GSK-3β | 305 |
| 13 | Lipid/Cholesterol metabolism | ApoE, HMG-CoA reductase, Acyl CoA-Cholesterol O-acyl transferases (ACAT) | 52,74,482 |
| 14 | Calcium (Ca2+) signaling | Ca2+ homeostasis | 365 |
| 15 | Excitotoxicity | Glutamate, N-methyl-D-aspartate (NMDA) receptors, | 59 |
| 16 | Neurotrophic factors signaling | Brain-Derived Neurotrophic Factor (BDNF), Nerve Growth Factor (NGF), Neurotrophin-3 (NT-3), Neurotrophin-4/5, Trk receptors (TrkA, TrkB, TrkC) | 51 |
| 17 | Wnt/β-catenin signaling | β-catenin, GSK-3β | 391 |
| 18 | Leptin signaling | Leptin, Leptin receptor (ObR) | 58,399 |
| 19 | Blood-Brain Barrier (BBB) dysfunction | Receptor for advanced glycation end products (RAGE), Low-density lipoprotein receptor-related protein 1 (LRP-1) | 419 |
| 20 | Gut Microbiota and Nutrients | Diet and Nutritional interventions (prebiotics and probiotics), Gut microbes | 61 |
Mitochondrial ROS (Table 1) can increase the Aβ level, and Aβ can interact with mitochondria, which eventually cause mitochondrial dysfunction.202 In EOAD, mitochondria produce O2* and H2O2 free radicals that inhibit cellular ATP generation, whereas in LOAD, Aβ generation increased by the enhanced BACE activity followed by the Aβ entry to the mitochondria generates free radicals and disturbs electron transport chain (ETC), which decreases ATP, leading to the nerve cell damage and cognitive impairment.194 In addition to Aβ, overexpressed and aggregated Tau are also involved in disturbed mitochondrial transport.203 Mitochondria play a critical role in both necrotic and apoptotic cell death, and its dysfunction is an early feature of AD.192 Indeed, mitochondrial trafficking is crucial for the function and development of synapses and dendrites growth, which are observed to be impaired in the AD brain.204,205 Furthermore, AD brains have been observed with the increased mitochondrial autophagy due to the autophagosome accumulation.206,207 Mitochondrial fission and fusion are critical for the normal function of this organelle, which seems to be disturbed in AD.196,208 The components of γ-secretase complex have been found in mitochondria and further studies are required to confirm its association with AD pathogenesis.188 During mitochondrial dysfunction, autophagy plays important role for maintaining cellular homeostasis through the clearance of dysfunctional mitochondria through autophagic process (mitophagy).209 The agents that improve mitochondrial function and protect the neuronal cell from the death caused by the mitochondrial dysfunction could be a potential therapy against AD.
2.6. Endoplasmic reticulum stress in AD
The endoplasmic reticulum (ER) is involved in various cellular processes, such as maintenance of Ca2+ balance, protein folding, and quality control of nerve cells. ER stress can induce environmental and genetic insults. Interestingly, all the elements of ER stress can alleviate AD pathology.210 ER consists of stress sensors (such as serine/threonine-protein kinase/endoribonuclease IRE1, PRKR-like endoplasmic reticulum kinase (PERK), and cyclic AMP-dependent transcription factor ATF-6 alpha (ATF6)) that recognize protein misfolding in ER and produce unfolded protein response (UPR). UPR is a protective cellular mechanism of ER against stress response. Conversely, prolonged UPR activation leads to the apoptotic neuronal death. Extensive activation of UPR has been observed in the AD brain. Interestingly, UPR is activated in nerve cells, but not in the glial cells of the AD brain.210 It was observed that phosphorylated UPR proteins, such as pPERK, peIF2α (eukaryotic translation initiation factor 2 subunit 1), and pIRE1α were increased in the AD brain neurons.211 Under ER stress condition, the UPR-related proteins are hyperphosphorylated that leads to their dysfunction and death of nerve cell.212,213 Furthermore, ER stress may aggravate the effects of a mutation in PSEN1, APP processing, Aβ production, and accumulation, as well as tau phosphorylation followed by the neuronal death.214–216 Typically, PSEN1 mutation generates Ca2+ imbalance in the ER lumen and disturb ER homeostasis.212 The reduced Ca2+ level in ER results in the dysfunction of the protein folding process that elicits prolonged UPR activation.217 In agreement with these findings, Mota et al. observed the presence of ER stress markers and impaired ER Ca2+ homeostasis in the neurons of AD brain.216 Additionally, ER stress also induces inflammatory responses via the inflammatory caspase-induced signaling pathways and causes inflammation of nerve cell.210,212 A large body of evidence indicates that pathological condition of the AD, such as impaired Ca2+ homeostasis, oxidative stress, apoptotic cell death, intracellular Tau, and extracellular Aβ deposition may be caused by the ER stress and vice versa.210 Study by Abisambra et al. demonstrated that the accumulation of tau interferes with ER-associated degradation and triggers activation of the UPR that represents tau disrupts protein quality control in the ER.218 Furthermore, Murakami et al. reported that response to ER stress could be localized to the dendrites that may provide a link to axonal degeneration and synaptic loss.219
There is also a connection between ER stress/UPR activation and autophagic pathology in AD brain. A recent report shows that ER stress activates autophagy but not the UPS in the neuronal cells suggesting that autophagy is the key degradational pathway following the activation of UPR.220 ER stress and autophagy play an important role in regulating brain function. Recent studies have suggested that these UPR signals may be linked to autophagy. There is evidence that autophagy ameliorates ER stress by eliminating accumulated misfolded proteins. Both abnormal UPR and impaired autophagy have been implicated as a causative mechanism in the development of various neurodegenerative diseases.221 Nijholt et al. observed the connection between UPR activation and autophagy in AD brain. Furthermore, they also reported that ER stress activates autophagy but not the proteasome in neuronal cells suggest that autophagy is the key degradational pathway following UPR activation.220
2.7. Oxidative stress-related signaling in AD
Oxidative stress is the condition of imbalance in the production of antioxidants and free radicals, such as ROS and reactive nitrogen species (RNS), that plays a critical role in neurodegeneration and cognitive impairment.222–224 Importantly, brain is highly prone to oxidative stress since it is highly rich in lipid content and consumes a large amount of oxygen which leads to increased ROS production. Additionally, neuronal membranes are rich in polyunsaturated fatty acid that makes them more susceptible to ROS.225 At the cellular level, oxidative stress occurs from a variety of sources, such as mitochondrial dysfunction, and leads to oxidation of lipid, protein, and DNA damage.226 An indication of oxidative stress in the AD has been displayed through oxidation of nuclear and mitochondrial DNA, oxidation of lipids and proteins, advanced glycation end products and the formation of toxic species such as peroxides, alcohols, and aldehydes.227–231 However, it is still unclear what can serve as an exact source of the oxidative source in AD,232 although mitochondrial abnormalities in the AD are considered as the main source of oxidative stress.232 Memory impairment is associated with a decrease in the defense activity of the brain and plasma antioxidants.233 Evidence shows that there is a strong correlation between the levels of antioxidant enzymes, lipid peroxides, Aβ plaques, and NFTs in AD.234,235 Oxidative stress mediates abnormal aggregation of Aβ and Tau proteins, facilitates Tau hyperphosphorylation, which further produces neuronal damage by additional ROS generation. Furthermore, it was also reported that cells with the excess levels of Tau protein have a higher susceptibility to the oxidative stress.236 The effect of the presence of imbalanced oxygen radicals is massive oxidative damage of various biological molecules, which is found in AD, such as lipid peroxidation, the formation of adduction products, free carbonyls, advanced glycation end products (AGEs) and nitration.235,237,238 AGEs are commonly found in amyloid plaques and are responsible for the nerve damage and pathogenesis of AD. Furthermore, extracellular aggregation may be caused by the enhanced oxidation of the glycated proteins.226,239 AGEs are responsible for cross-linking of Aβ peptide that leads to the formation of Aβ fibril and eventually plaque formation, followed by the neuronal cell death by ROS and cytokines. Additionally, AGEs are also involved in nerve cell death by direct (chemical) and indirect (cellular) free radical production, which further elevates oxidative stress levels.226 DNA strand damage was also reported to increase the levels of free carbonyls in neurons and glia of AD brain.240 Fascinatingly, numerous antioxidants produce a significant improvement of memory and cognition in an animal model of AD.232 Neuronal stress produces ApoE4, which is cleaved into neurotoxic fragments that induce an effect on neuronal Aβ production and clearance, also produces mitochondrial dysfunction.11 Accumulation of misfolded amyloid protein activates UPR through ER stress kinase. Phosphorylation of signaling protein eIF2α affects synaptic function and cognitive process, whereas the IRE1α/JNK pathway may feed forward to the enhanced deposition of the amyloid protein.241 Oxidative stress is associated with mitophagy in AD and other neurodegenerative diseases. Reactive nitrogen species (RNS) and ROS act as inducer of autophagy or mitophagy which has protective roles in cell survival.242 Therefore, regulating the balance between oxidative stress and autophagy may provide better treatment for AD. The drugs have completed preclinical assessment for AD, such as Dantrolene, Edavorene, and GSK2606414, which act by inhibiting activation of PERK to ameliorate proteotoxic reactions.203 Some drugs are in different phases of clinical trials that targeting oxidative stress (Figure 2) such as ARC-031, Lu-AF-20513, curcumin, quercetin, nilvadipine, SK-PC-B70M, HX-106, EGCG (Table 2). These drugs are showing effective treatment in AD patients and more drugs must enter in clinical trial targeting oxidative stress.
2.8. Neuroinflammatory signaling in AD
Although neuroinflammation is known to play a remarkable role in the AD, the debate is still going on whether it is harmful or protective. In the body, inflammation is generally intended to be protective. However, excessive inflammation and chronic response may lead to cell damage and various pathological developments.243,244 The ROS formation, enhanced activation of microglia and other immune cells, nuclear factor kappa B (NF-κB), and expression of cytokines are associated with neuroinflammation in AD.244,245 Misfolded/aggregated proteins trigger innate immune response characterized by the release of inflammatory mediators by binding to microglia/astroglia. Importantly, Aβ peptide promotes microglial and astrocytes activation through the action of scavenger receptors, chemokine receptors, and generates inflammatory mediators.246,247 Microglia and astrocytes play a pivotal role in neuroinflammation by releasing various pro-inflammatory elements, such as chemokines, IL-2β, IL-6, IL-12, INF-γ, TNF-α, NO, ROS, and O2− (Table 2) causing oxidative stress, nerve cell necrosis, apoptosis, and dysfunction of neurons. Hence, this pathway could represent a risk factor for SAD.38–40,248 Senile plaques and NFTs are physically associated with inflammatory markers, such as C-reactive protein (CRP) and transforming growth factor beta (TGFβ), that generate neuroinflammatory stress.249,250 The chronic activation of microglia is associated with the increased Aβ level followed by hyperphosphorylation of Tau and production of NFTs.247 Excitotoxicity or neuronal stress leads to the increased APP expression and increased release of sAPP. Eventually, enhanced production of Aβ and sAPP activates microglia to release IL-12 and IL-23, which bind to microglial receptors P19 and P40 that enhance the release of IL-1β. IL-1β again causes an increase in the APP level, which further enhances Aβ plaque production in the brain.251 Glial cells express a family of TLRs and CD14, which play a central role in the innate immunity that activates transcription factor NF-Kβ and leads to the release of the pro-inflammatory cytokines. Interestingly, both receptors are critical for the interaction of glial cells with Aβ and the release of pro-inflammatory cytokines.252–254 The pro-inflammatory cytokines, such as IL-1, enhance the APP synthesis and amyloid deposits and stimulate further cytokine production by activation of microglia. The specific cytokines IL-1, IL-3, IL-6, and TNF-α, have been reported to be elevated in brain tissue homogenates from the AD patients, and heightened IL-1 immunoreactivity has been detected in the CSF of the AD-afflicted individuals.255,256 Reports also suggest that autophagy/mitophagy reduces neuroinflammation in the brain and increases microglial phagocytosis.257 Thus, agents acting via modulation of autophagy may reduce autophagy and act as a promising therapy for AD. Several drugs investigated for AD, such as Ginkgo biloba, resveratrol, and cerebrolysin, have been examined for their anti-inflammatory activity in AD.258–260 Furthermore, the preclinical studies on the animal models of the AD and numerous clinical trials with the long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) show reduced Aβ accumulation and are associated with the decreased risk of AD.247,261 Human intravenous immunoglobulins (IVIgs) obtained from the pooled plasma of healthy blood donors, which are in phase-III clinical trial, were shown to reduce the harmful inflammatory processes as well as enhance Aβ clearance.11,262
2.9. Ubiquitin-dependent proteasomal system in AD
Abnormal/misfolded proteins are processed by cellular quality control systems such as molecular chaperones, ubiquitin-proteasome system (UPS), and Autophagy.42,263 UPS is the ubiquitin-dependent proteolytic system, which degrades 80–90% of normal as well as abnormal/misfolded intracellular proteins.264,265 The inhibition of proteasome function leads to the accumulation of cellular waste/misfolded proteins such as Aβ and Tau (Figure 1A right panel).266 Conversely, accumulated misfolded proteins also inhibit proteasome function, thereby leading to AD.42,267 Aβ self-aggregates into oligomers that inhibit the proteasomal pathway and stimulate the formation and accumulation of hyperphosphorylated Tau.42 Recently, different studies have reported that UPS affects the AD pathogenesis.268,269 UPS works with three families of the ubiquitination-related enzymes, such as E1 (ubiquitin activating), E2 (ubiquitin conjugating), and E3 (ubiquitin ligating) enzymes.42 In the neuronal cell, UPS is a large ATP-dependent proteolytic machine having an important role in the progression of the protein-misfolding-related diseases. The 26S proteasome consist of one 20S core particle and two 19S regulatory particles.264 Importantly, misfolded proteins are recognized and degraded by the 26S proteasome via the sequential process that includes misfolded protein ubiquitination, deubiquitination, and unfolding followed by cleavage in the 20S core with the proteolytic enzyme activity (Figure 1A left panel).263,270 Proteasome degrades Aβ and Tau. However, it has also been reported that Aβ40 binds inside the 20S proteasome and inhibit its chymotrypsin-like activity. Additionally, Tau accumulation also inhibits 26S proteasome and reduces abnormal protein degradation.42,271–273 In AD brain, the accumulation of ubiquitin-protein-conjugates (mutant UBB+1) is first observed in NFTs.274,275 Furthermore, PHFs of Tau proteins are also reported as being involved in the impairment of proteasomal activity.276 It has also been proposed that the accumulation of mutant ubiquitin, oxidation of specific deubiquitinating enzymes (DUBs), and down-regulation of E1 and E2 enzymes may lead to the neurodegeneration and AD.42,277 Recent study has been reported that the disturbance in polyubiquitination of the protein is associated with neurodegeneration in AD.278 Importantly, UPS plays a critical role in synaptic plasticity, and evidence also proved synaptic dysfunction in AD brain.42 Over-expression of APP mutant isoforms is associated with the decrease in UPS activity.279 UPS is also responsible for the degradation of mutated presenilin, which is the main culprit in the development of AD.280 Moreover, evidence suggest that altered proteasome activity activates autophagic process.257 The ubiquitin-specific hydrolase UCHL1 involved in the modulation of autophagosome-lysosome fusion281 indicates that these both pathways are depending on each other’s effect. Thus, targeting drugs for activation of UPS may provide better therapeutics against AD. Resveratrol is in Phase III clinical trial that restores proteasomal activity against Aβ accumulation (Table 2). Furthermore, Betulinic acid, IU1, and PAP1 stimulate proteasomal activity, which has completed preclinical assessment against AD.203
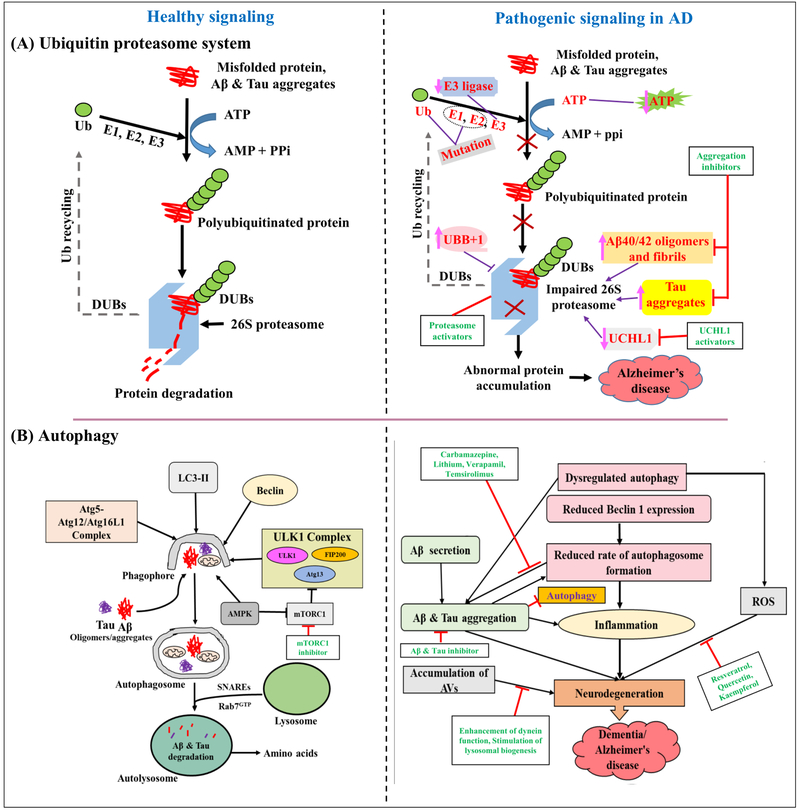
Figure 1: As an example, schematic representation of healthy and pathogenic signaling in AD;
(A) Ubiquitin proteasome system. Left panel shows healthy UPS. Misfolded proteins and protein (Aβ and Tau) aggregates are degraded by 26S proteasome with the help of ubiquitinating (E1, E2, and E3) and deubiquitinating enzymes (DUBs) in ATP-dependent manner. Right panel shows pathogenic UPS in AD. Impaired proteasomal function leads to inhibition of the misfolded proteins and aggregated Aβ and Tau degradation. This leads to accumulation of Aβ and Tau aggregates in neurons, nerve cell death, and finally AD pathogenesis. Enhanced level of mutant UBB+1, Aβ40/42 oligomers and/fibrils, Tau aggregates, mutation in E1 and E2 enzymes, reduced E3 ligase/CHIP and UCHL1 are responsible for aberrant UPS. Use of proteasome activators, aggregation inhibitors, and UCHL1 activators may provide management of AD. (B) Autophagy. Left panel shows healthy autophagy. Beclin 1, LC3-II, ULK1 complex, and Atg5-Atg12/Atg16L1 complex are involved in the formation of autophagosomes. Cytoplasmic contents such as protein aggregates (Aβ and Tau) and defective organelles are sequestered into a double-membrane-bound autophagosome. Further, these are transported and fuse with lysosomes in a Rab7- and SNARE-dependent manner. Aβ and Tau aggregates are then degraded by lysosomal hydrolases. Right panel shows defective autophagy in AD. Failure in autophagic process can be caused by protein aggregates, reduced expression of Beclin1, presenilin 1 dysfunction, disturbed lysosomal pH. Further, autophagic failure leads to accumulation of toxic proteins that subsequently affect cell health and survival. Finally, neurodegeneration affects cognitive function that leads to AD. Various drug targets and agents acting at different stages are shown in green color. The “→” refers to induction/activation and the “⊢” refers to inhibition. “↑” refers to increase and “↓” refers to decrease. Ub: Ubiquitin, DUBs: deubiquitinating enzymes, E1: Ub-activating enzyme, E2: Ub-conjugating enzyme, E3: Ub-ligase enzyme. mTOR: mammalian target of rapamycin; AMPK: AMP-dependent protein kinase; ROS: Reactive oxygen species; AVs: Autophagic vacuoles; ULK1: Unc-51 like autophagy activating kinase; Atg: Autophagy-related protein; SNAREs: Soluble NSF attachment protein receptor.
2.10. Autophagy and endosomal-lysosomal system in AD
The lysosome is a digestive organelle that facilitates the clearance of mutant and aggregated Aβ and reduces its aggregation.282 In this pathway, Aβ and Tau aggregates are entrapped or generated in endosomes, and then autophagic vacuole is delivered to the lysosome for its degradation by lysosomal enzymes (Figure 1B left panel).283 Interestingly, increase aggregation of Aβ and Tau inhibits autophagy and endosomal-lysosomal system and, subsequently, decreases the clearance of Aβ and Tau (Figure 1B right panel).284 Alteration in the endocytic pathway leads to increase in the cellular density of lysosomes and alters expression of genes, which enhance the level of lysosomal hydrolases.282 Endocytic vacuoles enriched with PSEN1 and APP can produce Aβ. Typically, the elevated APP levels can alter endosomal function.285 Lysosomal degradation has two different routes. One is the endocytic pathway, which degrades extracellular abnormal/aggregated proteins, and second is the autophagic pathway, which degrades intracellular aggregated proteins.41
It was also pointed out that the macroautophagy function is impaired during the AD. PSEN1 play a key role in autophagy, and some FAD-linked PSEN1 mutations have loss of function effect on macroautophagy leading to the accumulation of autophagy vacuoles (AVs) and to the impaired protein turnover. Typically, increased autophagy induction and defective clearance of Aβ-aggregates create conditions favorable for Aβ accumulation in AD.207,286 Abnormally accumulated Aβ peptide has been reported in the autophagy-endosomal-lysosomal vesicles. Importantly, the lysosomal activity modulators show a good effect in the treatment of neurodegeneration.42,287 Defective endosomal sorting and lysosomal function may work together with intracellular (endosomal) Aβ accumulation. Therefore, dysfunction in endocytosis seen in the AD brain represents a possible basis for the accelerated β-amyloidogenesis in the more than 90% of all AD cases.288,289 It has been observed that stimulation of lysosomal activity in TgCRND8 mice caused a noticeable reduction of the Aβ levels by enhancing Aβ clearance via the autophagic pathway.279 One of the key players in the regulation of autophagy is the serine/threonine kinase known as mammalian target of rapamycin (mTOR). Activation of mTOR is associated with inhibition of autophagy, decreased Aβ clearance, enhanced Aβ generation and deposition by modulating the APP metabolism, upregulating β- and γ-secretases, and thereby causing the AD pathogenesis.290,291 In fact, alteration of mTOR signaling and autophagy have been reported to occurs at the early stages of AD.292 mTOR is the downstream effector in Akt/PTEN signal transduction pathway and disturbance in the coordination of Akt and PTEN signal transduction is linked with AD.293 Rapamycin is a mTOR inhibitor that ameliorates the AD-like cognitive deficit by activating autophagy and inhibiting Aβ accumulation.282 AMP-activated protein kinase (AMPK) is the regulator of cellular energy homeostasis is also reported to be engaged in the AD pathogenesis. AMPK activation decreases mTOR activity leading to facilitation of autophagy and promoting lysosomal degradation of Aβ (Figure 1B left panel).294 Maintaining cellular homeostasis by boosting autophagy-lysosome pathway, enhancing lysosomal hydrolase activity, and improving retrograde axonal transport of endolysosomes may protect brain against neurodegeneration. Nilotinib is currently being tested in a phase II trial for AD that can augment the autophagic machinery by increasing levels of parkin, E3 ubiquitin ligase.295 Small molecules associated with regulation of autophagy and endosomal-lysosomal pathways, such as Rapamycin, Temsirolimus, Arctigenin, GTM‐1 (act by mTOR inhibition), and Trehalose (act by AMPK activation), have completed preclinical assessment for AD.203
2.11. Protein misfolding and molecular chaperones in AD
Molecular chaperones are the most decisive components of the cellular protein quality control system that acts as a first-line defense against protein misfolding and aggregation.42 Molecular chaperones assist in the folding and refolding of damaged proteins, prevent protein misfolding, and target severely damaged proteins to degradation.296 Perturbed proteostasis manifested in protein misfolding and formation of toxic aggregates within affected neurons is the important feature of AD pathology. In fact, the misfolding of proteins leads to the formation of toxic aggregates that produces neurotoxicity via cellular stress pathways.297 In response to that, the ER activates the UPR that leads to the up-regulation of ER molecular chaperones and folding enzymes that play a vital role in unfolding and refolding of the misfolded proteins to their natural states or directing them to degradation. In this way, molecular chaperones have a very crucial role in the diseases associated with neurodegeneration and protein misfolding.296,298 The heat shock proteins (HSPs), such as HSP60 and HSP70, are the main classes of chaperones that prevent the accumulation of misfolded conformers and inhibit intermolecular aggregation.296 The mammalian brain expresses over 200 different chaperones and co-chaperones that are found mainly in the nucleus, cytoplasm, and cellular organelles, which are then tailored due to cellular stress. In addition to that, each cell type expresses different sets of chaperones and co-chaperones.299,300 Chaperones are classified into six conserved classes according to their molecular size or function: HSP40, HSP60, HSP70, HSP90, HSP100, and the small HSPs (HSP10, HSP27, 15 to 30 kDa).299 HSP70 and HSP90 inhibit the formation of Aβ and its aggregation.296 HSP40 acts as co-chaperones of HSP70 and maintains ATPase activity important for HSP70. HSP90 is the most abundant molecular chaperone in the cell that plays a vital role in controlling protein misfolding and maintains cell cycle, as well as cell growth and development, and other essential activities of the cell. Furthermore, HSP90 activates more than 200 HSP90-client proteins required for cell signaling. HSP100 is also bound to the aggregated or misfolded protein to ensure correct folding. Aβ and Tau are the two misfolded proteins in AD, and HSP70 and HSP90 interact with both of these proteins and degrade them through UPS.299 Surprisingly, few protein aggregates are degraded by the lysosome based on the size of aggregates, the degree of misfolding, and peptide sequence and other aggregates are selectively tagged by ubiquitin for the proteasomal degradation. HSPs help to degrade the proteins by carrying them to the UPS.299 Dou et al. reported the reduced formation of NFTs and reduced accumulation of misfolded and aggregated proteins after up-regulation of molecular chaperones in the brain.301
Extracellular chaperones such as clusterin, haptoglobin, and alpha-2-macroglobulin (α2M) have been reported to inhibits misfolded protein aggregation.302 Clusterin interact with amyloidogenic proteins at their aggregation states such as prefibrillar states and mature fibrils. Further studies have shown that clusterin suppresses especially the elongation step of Aβ42 aggregation.303 Additionally, the molecular chaperone of Brichos family (proSP-C Brichos) shows Aβ42 aggregation inhibition by suppressing secondary nucleation. Combined used of Clusterin and Brichos shows additive effect on Aβ42 aggregation inhibition303 Anavex 2–73 is a small molecule that inhibits the intracellular sigma-1 chaperone protein and has completed phase II clinical trial for the treatment of AD [https://www.labome.com/method/Alzheimer-s-Disease-Clinical-Trials.html]. Curcumin was shown to promote the increase in the Hsp70 and Hsp90 activity that inhibited or delayed amyloid formation and reduced neurodegeneration42 PU‐DZ8 (HSP90 inhibitor), and YM‐08 (HSP70 activator), which acts by reducing accumulation of Tau protein in the brain, have completed preclinical assessment for an AD.203 Minimizing protein misfolding and aggregation by targeting chaperones and components of the UPR system is the promising therapeutic approach for the development of anti-AD drugs.
2.12. Insulin signaling in AD
Insulin resistance is associated with insulin receptor (IR) internalization, reduced IR activity, tumor necrosis factor receptor (TNFR) activation, glycogen synthase kinase three beta (GSK-3β) activation, reduced IDE activity, and increased levels of the inhibitory serine phosphorylation.304,305 Impaired insulin signaling and glucose distribution, as well as hyperinsulinemia, are among the critical risk factors for the AD. The decrease in glucose metabolism and increased radio-ligand uptake detect abnormal deposits of Aβ protein on Positron Emission Tomography (PET).11 However, debates are still going whether AD impairs insulin signaling or insulin resistance produces AD.306 Additionally, altered insulin signaling, inflammation, and distorted metabolism are key features of both AD and diabetes mellitus (DM).307 Many pieces of evidence have shown that decreased level of insulin in cerebrospinal fluid (CSF) and reduced expression of IRs are observed in AD.308,309 Insulin and insulin-like growth factor 1 (IGF-1) plays a pivotal role in CNS by regulating learning, memory, and neuronal survival. The condition of hyperglycemia increase peripheral insulin utilization and results in the reduction of the levels and transport of insulin in the brain. Defective insulin signaling also leads to the neuronal energy deficit and generates other metabolic insults. Insulin resistance stimulates GSK-3β activity that enhances the hyperphosphorylation of Tau and causes oxidative stress. In its turn, oxidative stress again generates mtDNA damage and mitochondrial dysfunction, and triggers the nerve cell apoptosis, and leads to dementia.47 Brain is an insulin-sensitive organ.310 Intranasal administration of insulin reported with improvement in learning and memory in animal AD models and humans.311,312 In a normal brain, insulin binds to its receptor, which causes phosphorylation of insulin receptor substrate 1 (IRS-1). This triggers activation of PI3-K and AKT that inhibits activation of GSK-3β and results in the synaptic plasticity, memory and cognition. Moreover, IDEs also degrades accumulated Aβ.304 In the AD, aggregation of Aβ leads to the TNF-α activation that activates TNFRs, followed by activation of stress kinases, such as c-Jun N-terminal kinases (JNKs), resulting in the inhibitory serine phosphorylation of IRS-1, which inhibit PI-3K and AKT activation leading to the GSK-3β activation followed by the Tau hyperphosphorylation and NFTs formation.304 Interestingly, Aβ oligomers also facilitate internalization of IRs through CK2/CaMKII pathway, resulting in reduced levels of IRs on the cell surface.304,313–316 Typically, insulin resistance decreases the level of IDE, which further reduces Aβ degradation. Collectively, all these events result in the impairment of nerve growth, learning, memory, and synaptic plasticity.304 It was reported that decrease PI3K/AKT-mediated activation of glucose transporters (GLUTs) and their decreased expression in the AD could produce glucose hypometabolism.47 In addition to the removal IRs from cell surfaces, Aβ oligomers increase TNF-α and activate various stress kinases, such as JNK, protein kinase R (PKR), and IκB kinase (IKK), cause inhibitory serine phosphorylation.317 Faulty activation of N-methyl-D-aspartate receptors (NMDARs) by Aβ-oligomers leads to the increase in the Ca2+influx that results in the augmented excitotoxicity, oxidative stress, disturb signaling, and impairs synaptic plasticity.307 Inhibition of IRS-1 has been observed in a transgenic mouse model of AD.318 On the other hand, glucagon-like peptide-1 receptor (GLP-1R) activation may provide neuroprotection against the AD.319 Hence, insulin and GLP-1R agonists are now in the clinical trial for the AD treatment. Exendin-4 is one of the GLP-1R agonists that has been reported to restore impaired brain insulin signaling and decrease Aβ accumulation, as well as improves cognition.318,320,321 The administration of intranasal insulin (INI) to reduce brain insulin resistance is under evaluation. Disturbance in biliverdin reductase-A (BVR-A) level leads to insulin resistance in AD.322 Recent study by Barone et al. reported that INI stimulates insulin signaling by activating BVR-A.323 Impaired glucose metabolism associated with the accumulation of advanced glycation end products (AGEs) stimulates Aβ accumulation and NFTs formation, which further causes mitochondrial dysfunction and oxidative stress.306
Normal insulin signaling is important for neuronal development, survival, and synaptogenesis.324 In AD, insulin signaling is defective and coincident with the abnormalities associated with reduced levels of IRS mRNA, Tau mRNA, IRS-associated PI3-K, phospho-Akt (activated), increased GSK-3β activity, and formation of NFTs and Aβ plaques.25,304,325–327 The decrease in the neuronal glucose metabolism particularly in temporoparietal and frontal association area, due to the blood-brain barrier and cerebral microvasculature alterations, reduces energy production in the brain, reduces levels of Tau O-Glc-N-acylation, affects the production and clearance of Aβ and Tau phosphorylation, and leads to neurodegeneration and AD.328–330 The insulin resistance is linked to autophagy, where autophagy protects pancreatic β-cells from apoptotic cell death and preserves their structure and function. Therefore, insulin resistance can be inhibited by activating autophagy.331
Furthermore, insulin deficiency may distort cholesterol metabolism in the brain.332 Cholesterol regulates both generation and deposition of Aβ, with elevated cholesterol increasing Aβ levels in the AD.333 The ε4 variant of the apolipoprotein E (APOE4) is the important component and regulator of the very-low-density lipoprotein (VLDL) homeostasis, serve as principal cholesterol carrier in the brain and are among the major genetic risk factors for the AD.334,335 Soluble Aβ interacts with APOE associated with VLDL particle, and this interaction promotes Aβ aggregation into the amyloid fibrils52 (see below for further discussion of a correlation between the distorted cholesterol metabolism and AD).
2.13. Altered lipid/cholesterol metabolism in AD
The brain contains highest (20% of the total body) cholesterol level, where it serves as the key component of the nerve cell membrane and is required for the receptor-ligand interactions, synaptic function, normal neuronal development, and physiology.52,53 The disturbance in lipid metabolism results into abnormal levels of certain lipids in the plasma, brain and cerebrospinal fluid.336 Importantly, the formation and clearance of Aβ are regulated by cholesterol.52 It has been reported that the high levels of cholesterol in the midlife may increase the risk of AD pathology.337 Studies of the cellular and animal AD models with high cholesterol levels revealed the increased production and aggregation of Aβ peptide.52 Various biochemical, molecular, AD model data suggested that alterations in ApoE, which is the key apolipoprotein in plasma and brain, may induce AD pathology.52 Kuo et al. reported that individuals with ApoE4 genotype demonstrated an increase in susceptibility to AD due to high cholesterol level.338 It is not surprising that hypercholesterolemia and atherosclerosis increase the risk of the AD due to the presence of high cholesterol levels in these conditions.52,339 ApoE and cholesterol metabolism are strongly involved in deregulation, biogenesis, and assembly of Aβ protein. Typically, ApoE interacting with the high-density lipoprotein (HDL)-like particles (which are a heterogeneous group of lipoproteins composed of various lipids and proteins and that act as an important player in the reverse cholesterol transport pathway) inhibits aggregation of Aβ. Conversely, free ApoE enhances Aβ aggregation.74 LRP (low-density lipoprotein (LDL) receptor-related protein) is a multi-ligand receptor that interacts with the Kunitz-type proteinase inhibitor (KPI) domain of APP751/770 and enhances the internalization of Aβ.340 ApoE binds to Aβ and stimulates internalization of Aβ by binding with the LDL receptor.341,342 Cholesterol is first released into a cell, then, is incorporated into intracellular membranes, where it stimulates machinery responsible for the APP processing leading to Aβ production and aggregation.52 Habchi et al. recently demonstrated that cholesterol associated with lipid membranes can efficiently accelerate Aβ42 aggregation by increasing its primary nucleation rate by up to 20-fold.343 In addition, recent study on FAD PS1 ΔE9 cells shown that the elevated cellular cholesterol level is associated with the disturbed APP processing.344 Jin et al. reported the presence of AD symptoms such as brain atrophy, brain metabolic and structural changes in high cholesterol fed rabbit.345
ApoE-linked Aβ moves into the endocytic lumen. Typically, Aβ entry inside the cell does not necessarily lead to its breakdown.52 Aβ gets released from ApoE and aggregates in the endocytic lumen and then is secreted outside the cell in the more toxic form.342 In comparison with other ApoE isoforms, lipid-free ApoE4 significantly enhances Aβ fibril formation.346 Furthermore, lipid rafts, which are membrane domains enriched in the cholesterol, promote interaction of APP with β-secretase, which is considered as the primary culprit for Aβ generation. Therefore, disturbance in the integrity of lipid rafts may affect numerous signaling pathways and generates neuronal death.53 For maintaining the cholesterol homeostasis in brain, it is converted into 24(S)-hydroxycholesterol (24OHC). However, several studies reported enhancement in level of 27(S)-hydroxycholesterol (27OHC) and reduction of 24OHC in the AD brain.347 Enhanced cholesterol levels reduce the efficiency of degradation processes, such as autophagy. Fusogenic ability of autophagosome and lysosome is disturbed due to the cholesterol-enrichment within the endosomes-lysosomes.348 Consequently, maintaining normal cholesterol level can enhance autophagy-mediated degradation of Aβ aggregates in AD brain.
Recently, a clinical trial has been started to test the effects of lowering of neuronal cholesterol levels as a means for the treatment of AD (Table 2).52 The corresponding agents reducing cholesterol levels are showing promising results against the AD. Therefore, cholesterol-lowering agents, such as statins and Acyl CoA: Cholesterol O-acyl transferase (ACAT) inhibitors could become valid agents for the prevention and treatment of AD.52 For example, statins were shown low cellular cholesterol levels that favored the α-secretase APP processing pathway leading to the decreased Aβ production and secretion.349–355 Thus, targeting statins to reduce cholesterol level could be a potential target for AD.
2.14. Calcium signaling in AD
Perturbed cellular Ca2+ regulation is the critical and early event associated with the AD pathogenesis.356 Ca2+ is an important regulator of the various neuronal processes associated with extracellular stimuli and intracellular responses.357 The Ca2+ regulates numerous cellular processes that are crucial for the normal neuronal function, such as neuronal physiology, growth, differentiation, neurotransmitter release, generation of the action potential, membrane excitability modulation, ATP production, cognition, as well as short-term and long-term synaptic plasticity.49 The Ca2+ signals are maintained by process such as store-operated Ca2+ entry (SOCE) and its dysregulation leads to perturbed intracellular Ca2+ signaling in neuronal and glial cells.358 Recent studies in AD mouse models reported that the downregulation of neuronal store-operated calcium entry (nSOCE) is associated with disrupted postsynaptic contacts and memory formation.359 Thus, activation of nSOCE could be a potential target for AD therapy. Additionally, deregulation of the Ca2+ homeostasis creates the Ca2+ overload in the nerve cell, which results in the mitochondrial dysfunction and neuronal cell death. Furthermore, abnormal Ca2+ efflux from ER and mitochondria in various neurodegenerative diseases has been reported.48,49 The abnormal accumulation of Ca2+ in the synaptic region was also linked to the impaired Ca2+ regulation.360 Increased Aβ42 level, disturbed Ca2+signaling, and aberrant expression of Ca2+signaling proteins have been reported in the animal models of a FAD as well as found in the samples of the post-mortem sporadic AD brain.49 Collectively, inhibitors and stabilizers of Ca2+ signaling are potential drug candidates in AD treatment.49
Presenilin interacts with the inositol trisphosphate receptor (InsP3R),361 which is a membrane glycoprotein complex acting as an inositol trisphosphate (InsP3)-activated Ca2+ channel. This presenilin-InsP3R interaction modulates the gating activity of this Ca2+ release channel.361 FAD-associated mutant forms of PSEN1 and PSEN2 exert stimulatory effects on InsP3R channel activity that result in perturbed cellular Ca2+signaling.361 Evidence also suggests that the Aβ oligomers initiate neuronal cell damage by channel-dependent disruption of the integrity of plasma and intracellular membranes leading to the Ca2+dyshomeostasis.362 Disturbed amyloid metabolism leads to disturbance in Ca2+ signaling followed by neuronal hyperactivity.363,364 Aβ fibrils generate glutamate-independent inward current, which further dysregulates Ca2+ homeostasis that induces neuronal cell death.365 Blocking Ca2+ channels to reduce neuronal hyperactivity showing effective treatment in AD patients and few Ca2+ channel blockers are in clinical trial for AD (Table 2).
2.15. Excitotoxicity in AD
Glutamate is a principle excitatory neurotransmitter in the brain that, being released from presynaptic terminals, mainly acts on the post-synaptic NMDA receptors. Excitotoxicity mainly occurs due to increased release and decreased uptake of glutamate neurotransmitter.366 All the neurons in CNS contain the NMDA subtype of ionotropic L-glutamate receptors that can affect post-synaptic influx of Ca2+.367 Neurochemical and neuropathological studies of the early-stage AD brain showed the distortion in the L-Glu-mediated pathway.367 Excessive activation of NMDA receptors by glutamate causes excitotoxicity followed by nerve cell death due to the excess release of glutamate in synapse and influx of Ca2+ inside the cell.368 This results in the activation of catabolic enzymes, free radical generation, and mitochondrial dysfunction. Furthermore, hyperactive NMDA receptors and excessive release of glutamate enhance the production of pathogenic Aβ and Tau protein.59 Extracellular Aβ and intracellular NFT deposition lead to the modification of the glutamate receptors and causes the excitotoxic neurodegeneration.369 Therefore, although NMDA receptors are generally neuroprotective, their over-activation causes cell death. NMDA receptors can be found in both presynaptic and postsynaptic locations on the neurons. These differently located NMDA receptors might have very different physiological roles. One illustrative example of this phenomenon is given by the cAMP response element binding (CREB) protein, which is one of the cellular targets affected by the dysregulation of NMDA receptors.370 CREB is the prototypical signal-regulated transcription factor that plays an essential role in the maintenance of LTP, learning, memory, and synaptic plasticity.371,372 The activity of CREB is regulated by phosphorylation, changes in which can be triggered by a variety of cellular signaling events, such as an increase in intracellular Ca2+ via activation of NMDA receptors, or activation of receptor tyrosine kinase by growth factors. Here, synaptic NMDA receptors promote calcium entry leading to the induction of the CREB phosphorylation, whereas extrasynaptic NMDA receptors show the opposite effects and initiate the CREB shut-off pathway.370 Transcriptional activity of CREB and associated LTP generation are decreased in AD mostly due to the Aβ-induced inactivation of PKA that leads to the decreased phosphorylation of CERB at residue Ser133.370 Madeira et al. recently reported the increased in level of glutamate and glutamine in the CSF of AD patients. Thus, CSF measurements of glutamate/glutamine level may act as a biomarker for the detection of AD.373
The hypothesis in which Glutamate-mediated neurotoxicity is involved in the pathogenesis of AD is widely accepted, and drugs, such as memantine, which reduce the neurotoxic effects of glutamate, have therapeutic potential for AD.374 Memantine is a non-competitive NMDA antagonist currently prescribed for the moderate-to-severe cases of AD.76 The combined use of memantine with acetylcholinesterase inhibitor drugs found to be beneficial, since cholinergic and glutamatergic dysfunction occurs at early stages of AD.375,376 Memantine is approved by USFDA and is in extensive clinical use in Europe since 1982 for the treatment of moderately severe to severe cases of AD.377 Excitotoxicity is also linked to autophagy, in which hyperstimulation of non-NMDA glutamate receptors activates lysosomal enzymes and induces autophagic process.378 The drugs which can modulate different aspects of excitotoxic mechanism along with autophagic activation can prevent neurodegeneration and may provide beneficial therapy against AD.
2.16. Aberrant neurotrophic factor signaling in AD
The AD is also strongly associated with the deficits in the axonal transport and deregulation of several neurotrophic factors (NTFs). NTFs are small proteins that maintain nerve cell morphology, neurogenesis, synapse formation, neuronal survival, axonal guidance, as well as proper architecture and function of the brain during embryonic development.51,379 NTFs are produced in neocortex and hippocampus then are reversibly transported to the basal forebrain containing cholinergic neurons, where they play a number of roles in maintaining functionality and survival of neurons.51 Brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-3 (NT-3), and neurotrophin-4/5 are the most eminent members of the mammalian neurotrophin family. However, reduced in the level of BDNF, NGF, and NT4/5 were reported in the hippocampus of AD patients.379
Pro-neurotrophins are first synthesized and bind to their receptor P75NTR. Cleavage of pro-neurotrophins generates active neurotrophins, which bind and activate selectively one of three types of tyrosine kinase (Trk) receptors (TrkA, TrkB, or TrkC). NGF binds to TrkA receptor, BDNF and NT-4/5 bind to TrkB receptor, and NT-3 binds to TrkC receptors, which then lead to the accelerated downstream cell signaling.51 Numerous studies demonstrated the involvement of BDNF and NGF in AD pathology.51,380,381 It has also been reported that loss of the activity of NTFs may aggravate the effects of Aβ on the production of pathological conditions of dementia.50 BDNF is widely distributed throughout the CNS and is a critical component required for the function and survival of cortical, hippocampal, dopaminergic and cholinergic neurons.382,383 BDNF and TrkB stimulate LTP in the hippocampus, which is found to be reduced in the AD. LTP involves the formation of synapses at the dendrite spines of axonal collaterals.50,384,385 It was recently shown that up-regulation of BDNF might rescue learning deficits and cognitive impairment in Aβ42-induced AD.385 Xiang et al. recently reported that the reduction in BDNF level induces Tau pathology by activating asparagine endopeptidase (delta-secretase) in AD.386 Combined use of BDNF, NGF, and NT4/5 for the treatment of AD may be an attractive therapeutic perspective.
2.17. Alterations in Wnt/β-catenin signaling in AD
Wnt signaling is involved in various cellular key processes, such as survival, adhesion, proliferation, differentiation, and apoptosis taking place in neuronal as well as glial cells. Importantly, Wnt signaling regulates neuronal connectivity by controlling the formation of the synapses, axon remodeling, and dendrite morphogenesis.54–56 Recent studies revealed that the demolition of the Wnt signaling components is prominently occurring in AD.387–393 Granno et al. recently identified the downregulation of Wnt/β-catenin signaling in down syndrome hippocampus.386 Wnt binds to its Frizzled (Fz) transmembrane receptors associated with the LRP5/6 or interacts with the atypical receptor tyrosine kinases RYK or ROR2, and IGF1R (tyrosine kinase receptor type 1 or IGF (insulin-like growth factor) receptor) and activates three different pathways, which play various roles in different neuronal functions.55,189 The first of these pathways is the Wnt/β-catenin or canonical Wnt pathway, which regulates transcription through β-catenin. The second pathway is the Wnt/Ca2+ or non-canonical pathway, which is modulated by intracellular Ca2+ release that regulates gene transcription. Finally, the third Wnt-based pathway is the Wnt/PcP-JNK or Wnt cell polarity pathway, in which the JNK plays a crucial role in cytoskeleton remodelling, dendrites development, and complexity.394,395 It has been reported that Wnt modulates both pre- and postsynaptic regions of the mammalian nervous system and affects synaptic wiring and plasticity. Furthermore, Wnt signaling is responsible for the neuroprotection from the toxic effects of Aβ oligomers.395 The FAD patient has been reported with the inherited form of AD caused by the PSEN1 mutation leading to the reduced β-catenin level.148 The increased Wnt antagonist levels have been observed in the brain of AD mouse model.396 Wnt secretes glycoprotein and regulates diverse function in neuronal development by receptor-mediated signaling pathways.
Typically, Wnt protein is required for cellular proliferation, development, neurogenesis, axon guidance, neuronal migration, dendrite development, and synaptic formation.391,392,395 Interestingly, the neurotoxic effects of Aβ oligomers can be reversed by the Wnt ligands, such as Wnt-3a.395 Direct binding of Aβ to CRD-Fz5, a Wnt-binding receptor, inhibited canonical Wnt signaling pathways and thereby revealed that Aβ is directly associated with the Wnt impairment.393 Killick et al. reported that AD developed after the Aβ-driven up-regulation of various genes induced Clusterin/p53/Dkk1/Wnt-Pcp-JNK pathway. Evidence also suggests that Wnt inhibitor DKK-1 overexpression results in Tau hyperphosphorylation, β-amyloid toxicity, and cognitive deficits.397 ApoE4 was reported to be inhibited in the canonical Wnt signaling pathway in the PC12 cells.398 Wnt signaling activation inhibits GSK-3β that causes accumulation of β-catenin in the cytoplasm eventually leading to an increase in transcription of the Wnt target genes, which results in the neuroprotection.387,391 Interestingly, Wnt signaling involves GSK-3β activation/inhibition, and therefore it is linked to autophagy. GSK3β inhibition leads to AMPK activation that decreases mTOR signaling and further enhances the activation of autophagy.189 Several antioxidants, anti-inflammatory compounds, PPARα and γ agonists, and agents leading to the activation of the M1 muscarinic receptor and PKCare known to regulate Wnt/β-catenin signaling and promote neuroprotection against Aβ-driven pathology.392
2.18. Leptin signaling and AD
Recent studies revealed that the pleiotropic endocrine hormone leptin has diverse actions throughout the CNS and can be related to the link between obesity and AD.57 Leptin is a peptide hormone secreted from peripheral adipocytes and brain is involved in glucose homeostasis and regulation of obesity, energy expenditure, and food intake.58 Leptin produces neuroprotective effects by improving cognitive function, inhibiting Aβ production by inhibiting BACE1 and reducing GSK-3β-mediated Tau hyperphosphorylation. Additionally, leptin plays a role in controlling the neuronal morphology, hippocampal synaptic plasticity, synaptic transmission, and neurogenesis.399 Under the normal conditions, leptin receptors are highly expressed in the brain areas associated with learning and memory, such as the hippocampus. Leptin produces its function by binding to leptin receptor (LEPR or OBR) that facilitates numerous signaling pathways, such as ERK pathway, PI3K/Akt/mTOR pathway, and JAK/STAT pathway and regulates various cellular functions. In the hypothalamus, leptin regulates glucose homeostasis and food intake that reduces the risk of diabetes, obesity, and AD. Furthermore, in the hippocampus, leptin increases neurogenesis, synaptogenesis, and neuronal survival. Besides, leptin downregulates BACE1, reduces Tau phosphorylation, and increases Aβ clearance. All these leptin effects occur by activation of JAK/STAT, PI3K/Akt, and AMK/SIRT pathways.58
Also, leptin is involved in neuroprotection by shielding against oxidative stress and glutamatergic cytotoxicity, improving cell survival, inhibiting apoptotic cell death, and by enhancing the proliferation of hippocampal progenitor cells.400 Circulating leptin is critical for reducing the risk of dementia and AD.401 However, low circulating leptin levels are associated with energy impairment, as well as affect the AMPK activity, Aβ processing and Tau phosphorylation by the action of GSK-3β.402 A significant increase in the leptin levels in CSF and hippocampus has been observed in AD patients. Furthermore, leptin receptor proteins have also been observed in NFTs. Disruption of leptin-induced signal transduction occurs due to the presence of free leptin that is unable to bind OBRs since it gets reduced due to NFT-induced disturbances in intracellular trafficking network. This leads to cognitive decline.403 Maioli et al. reported that although the leptin signaling is disturbed in AD, the leptin levels in CSF remained intact.404
Leptin regulates body weight and systemic metabolism, and changes in the body weight and obesity increase the risk of the AD. Leptin helps degrading Aβ protein by increasing IDE involved in Aβ degradation. Additionally, leptin increases Aβ clearance by LRP-1 mediated uptake of ApoE-bound Aβ.405 Furthermore, leptin reduces the expression of BACE1, which is the main culprit in the synthesis of Aβ protein and senile plaque by activation of SIRT1 signaling pathway.58,406 It has been reported that leptin injection in a transgenic mouse model of AD reduced brain pathology and improved cognitive performance.402 Studies in the cell cultures as well as in animal models revealed that leptin reduces the amount of extracellular Aβ and intracellular Tau phosphorylation in neuronal cells.407 It was also shown that leptin supplementation reduced Aβ production and Tau phosphorylation.58 Corem et al. recently reported that deficiency of leptin receptors induces the disturbance in blood-brain barrier (BBB) function,408 that may further associate with AD pathogenesis. All these observations suggest that modulation of leptin signaling can be considered as a potential target for the development of the anti-AD drugs.
2.19. Blood-brain barrier (BBB) and cerebrovascular dysfunction in AD
The blood-brain barrier (BBB) regulates the homeostasis of the CNS by controlling the molecular exchange between the brain parenchyma and blood flow and keeps neurotoxic plasma-derived components, pathogens, and cells out of the brain.409 Leakage of the BBB leads to an influx of cells, microbial pathogens, and neurotoxic blood-derived debris that generate immune response and inflammation in CNS.60 Numerous studies on animal models suggest that Aβ and Tau lead to damage of BBB and abnormalities in blood vessels.410 Importantly, in the pathogenesis of AD, BBB dysfunction and cerebrovascular lesions coexist with senile plaques and NFTs. The disruption of BBB can be detected by measuring the appearance of the plasma- or serum-derived molecules in brain parenchyma. In fact, the presence of various plasma proteins (albumin, IgG), increased levels of the hemoglobin-derived peptides, and increased prothrombin quantities have been observed in the brain of AD patient, suggesting BBB dysfunction in AD. Furthermore, increased CSF/serum or CSF/plasma ratios of albumin serve as a critical hallmark of the BBB damage. Pericytes regulate BBB integrity and clearance of metabolite. However, coverage of microvessels by pericytes was shown to be significantly reduced in AD brain, again indicating the BBB disruption.60,411–413 BBB operates with the neurovascular unit (NVU) and its dysfunction, such as pericyte degeneration, astrocyte depolarization, loss of tight junction (TJ) integrity, diminished endothelial transport, thickening of basement membrane, are the hallmarks for BBB dysfunction. It has been reported that the BBB dysfunction reduces Aβ clearance from AD brain. BBB damage creates devastating consequences in the affected brain, such as neuroinflammation, plasma protein leakage, and reduced brain glucose uptake.414 BBB dysfunction in AD can be initiated by the presence and absence of Aβ pathology.415 BBB dysfunction occurs due to the presence of Aβ oligomers, truncated Tau, oxidative stress, inflammation, diabetes, vascular disease, and pathogenic ApoE4 allele. Furthermore, decreased GLUT-1 levels in the AD-affected BBB result in the decreased transport of glucose, essentially leading to the brain starvation.415 Finally, Aβ accumulation in CNS represents the most common consequence of BBB dysfunction.415 Here, altered Aβ transport is associated with the reduced LRP-1activity, dysfunction of P-glycoprotein (Pgp, which is an efflux transporter involved in transport of several compounds through BBB),415 and increased activity of the receptor for advanced glycation end products (RAGE) that serves as one of the mediators of Aβ transport across the BBB.416 Therefore, BBB impairment leads to the neuroinflammation and oxidative stress, enhances the activity of β-secretase and γ-secretase, and promotes Aβ generation.415 Additionally, it causes failure of Aβ transport from brain to periphery due to decreased levels of LRP-1 and increased levels of RAGE at the BBB, which consequently gives rise to cognitive impairment.417 Aβ putative receptor at BBB controls the level of soluble isoforms of Aβ in the brain by influx of circulating Aβ into brain via specific RAGE and gp330/megalin-mediated transcytosis and efflux of brain-derived Aβ into the circulation across the vascular system via BBB is occur by LRP-1.418
During aging and in AD, many other alterations, such as losing tight junction, increase barrier permeability, increase pinocytic vesicle, and ROS accumulation are observed in the endothelial cells of the BBB.419 Interestingly, Wnt/β-catenin signaling is associated with the formation, induction, and maturation of BBB function, whereas dysfunctional Wnt/β-catenin signaling leads to the BBB damages.420
2.20. Gut microbiota and nutrients in AD
The human microbiota is made up of trillions of complex communities of commensal, symbiotic, and pathogenic microorganisms that reside in the human body and include various bacteria, archaea, protists, fungi, and viruses. The microbiome is the collective genomes of all these microorganisms. Fascinatingly, the largest populations of microbes are located in the gut, where they play various important roles in nutrition and immunity, and also have numerous effects on the brain and behaviour in human.421–423 The alterations in gut microbiota are associated not only with several gut disorders but are also related to various neurological disorders.421 Zhuang et al. reported that gut microbiota composition is disturbed in AD patients and may be involved in the AD pathogenesis, suggesting that brain health is closely associated with the gut microbiota.424 The altered composition of the gut microbiota leads to the onset of aggregation and accumulation of Aβ in AD.61 Furthermore, aging and AD pathogenesis may occur due to the increased permeability of the gut and BBB, which is induced by microbiota dysbiosis.421 These observations reflect the existence of the microbiota-gut-brain axis, which includes multiple immune, endocrine, neural, and metabolic pathways.421 The disturbance in gut microbiota associated with inhibition of autophagy-mediated protein degradation, change in neurotransmitter levels of the brain, and bacterial amyloid mediated inflammation of CNS.425 Interestingly, gut microbiota can release proteins, peptides, and lipopolysaccharides that might play a role in the production of proinflammatory cytokines and modulation of signaling pathways associated with the AD pathogenesis.61 Indeed, alterations in the gut microbiome may activate pro-inflammatory cytokines, increase intestinal permeability, and affect the occurrence of insulin resistance, which is strongly associated with AD pathology.61 Additionally, bacteria from gut microbiota might excrete components, such as lipopolysaccharides (LPSs), immunogenic mixtures of proteins/peptides, bacterial amyloids, and other microbial exudates into their neighboring milieu. Excretion of these components induces signaling pathways and produces pro-inflammatory cytokines linked with the AD pathogenesis.421,426,427 Activation of inflammatory responses by gut microbiome might leads to cerebral accumulation of Aβ.428 Furthermore, bacterial amyloids might cross-seed formation of cerebral amyloids (such as Aβ, Tau protein, and α-synuclein) and also can activate signaling pathways that lead to the nerve cell death and progression of AD.428 Human gut microbiota is involved in the structural integrity of intestinal mucosa by producing short-chain fatty acids (SCFAs), such as acetate, butyrate, and propionate, which are the end products of fermentation of dietary fibers by the anaerobic intestinal microbiota.429 The disturbances in microbiota lead to the production of immunogenic endotoxins, increases intestinal permeability, and triggers an inflammatory response.430–432 The diet and some nutrients can disturb the composition of the gut microbiota that induces production and aggregation of cerebral amyloid proteins.61 In addition to that, the use of antibiotics, probiotics intervention, diet alterations, and fecal microbiota transplantation (FMT) were shown to bring modifications in the gut physiology and gut microbiota that increased the risks of AD.433 Therefore, modulation of the gut microbiome using specific nutritional interventions such as prebiotics and probiotics might be an effective strategy to reduce chronic inflammation and amyloid load in AD.61 Further studies of the interaction between the microbial exudates, such as lipopolysaccharides (LPSs), immunogenic mixtures of proteins/peptides, bacterial amyloids and their host environments are needed for the complete understanding of the complex relationships between the gut microbiota and AD. Personalized diet or utilization of beneficial microbiota may provide a great strategy for the management of AD.
2.21. Targeting autophagy: The finest therapeutic target for AD
As aforementioned, autophagy is the evolutionarily conserved major cellular pathway that degrades damaged organelles and unnecessary misfolded proteins and maintains correct cellular balance.331,434 Today, autophagy is one of the most rapidly growing fields in biomedical research. Belgian biochemist Christian de Duve has introduced term autophagy in 1963,435 and then Japanese cell biologist Yoshinori Ohsumi has worked on the discovery of the mechanisms of autophagy and identification of autophagy-related genes. For this revolutionary work, Yoshinori Ohsumi was awarded Nobel Prize in 2016 in Physiology and Medicine [https://www.nobelprize.org/nobel_prizes/medicine/laureates/2016/].
In contrast to the UPS, autophagy degrades a much more extensive range of substrates, which tend to be bulkier, including whole cellular organelles, protein oligomers, aggregates, and large protein complexes.26 Importantly, autophagic dysregulation has been strongly associated with many disease manifestations, including neurodegenerative disorders, cancer, inflammation, aging, and metabolic diseases.331 Based on the mechanisms, by which intracellular components are supplied into lysosome for degradation, autophagy has three types: microautophagy, macroautophagy, and chaperone-mediated autophagy (CMA).434 Macroautophagy is the primary autophagy type, in which the cytoplasmic material is absorbed by direct invagination of the lysosomal membrane into lysosome. In macroautophagy, cytoplasmic materials are engulfed in double-membrane vesicles called “autophagosomes”. These autophagosomes fuse with lysosomes and transports the cell “waste” inside lysosomes for degradation.436 In CMA, via coordinated action of chaperones of both sides of the membrane and a dedicated protein translocation complex, cytosolic substrate proteins are selectively targeted and translocated into the lysosomal lumen for their degradation.437 How autophagy is associated with Aβ-induced neurotoxicity was not completely known. However, recent studies reported that the defects in autophagic regulation may disturb the clearance of Aβ and increase death of nerve cells, suggesting that autophagy plays a neuroprotective role against Aβ-induced neurotoxicity.438
The protein homeostasis in autophagy is mediated by a complex molecular machinery that consists of more than 30 autophagy proteins (ATGs) and 50 lysosomal hydrolases.439 The genes which are linked with and common to autophagy and AD are PSEN1, SNCA, UBQLN1, UCHL1 ATG7, CDK5, CLU, CTSDITPR1, MAPT, BCL2, BECN1, FOXO1, and GFAP.434 The ATG7 gene is the key to regulate autophagic conjugation systems in mammalians. Interestingly, ATG7 protein is also associated with the memory functions.440 Furthermore, it regulates the level of Aβ and Tau proteins.441,442 Therefore, disturbed ATG7 expression (causing reduction in the cellular ATG7 protein levels) is associated with AD-like pathology.440 Ohsumi’s group found that the ATG1 protein (also known as ULK1) combines with the products of the genes ATG13 and ATG17 (RB1CC1/FIP200) to form autophagic complex, which is the first stage in autophagosome genesis, and the whole process is controlled by the target of rapamycin (TOR) kinase.434 The anti-apoptotic factor BCL2 interacts with BECN1 protein and regulate autophagic process, and its overexpression protects neuronal cells in vitro against Aβ-related death, reduces APP processing and Aβ deposition.443 The BECN1 protein facilitates the initiation of autophagy and autophagosomes, which are found to be reduced in AD patients. Indeed, reduced levels of BECN1 increase the Aβ levels in brain.444 CDK5 is an autophagy-regulating kinase, and studies in the AD mouse models reported that enhanced CDK5 activity protects against Aβ accumulation, Tau hyperphosphorylation, neuroinflammation, and memory loss.445 Clusterin (CLU) is a chaperone protein that contributes to the biogenesis of autophagosome, and mutations in CLU gene have been strongly associated with AD pathology.446 CLU protects brain against Aβ toxic effects by interacting with Aβ protein and reducing its aggregation.447 Lee et al. has reported that PSEN1 is essential for controlling acidification and proteolysis of lysosome throughout autophagy.448 α-Synuclein (SNCA) is abundantly found in brain, where it regulates the formation of autophagosome and is negatively regulated by autophagy.434 Mutation in SNCA gene are strongly associated with the risk of AD.449 SNCA regulates many events in AD pathology, such as induces expression of Aβ peptides and vice versa, stimulates Aβ peptide aggregation, and modulates the activity of BACE1.434 Interestingly, the interaction of SNCA with Tau and SNCA with Aβ peptides bring each other fibrillation.450 Ubiquilin 1 (UBQLN1) is strongly linked to the AD pathology, since APP processing, APP intracellular trafficking, and Aβ40/ Aβ42 secretion have been reported to be increased due to the reduced UBQLN1 expression in frontal and temporal cortices of AD patients.450 UCHL1 is an ubiquitin-specific hydrolase that modulates autophagosome-lysosome fusion by interaction with LAMP2.281 Studies in AD mouse model showed that UCHL1 plays crucial role in the memory and synaptic function.451 Decreased expression and activity of UCHL1 was found in cerebral cortex of AD patients. However, studies in the AD mouse models reported that overexpression of UCHL1 reduces Aβ production and protects AD brain against memory impairment.452,453
The CMA is involved in the removal of aberrant proteins. However, it also becomes victim of the toxic effect of these pathogenic proteins. Therefore, it is essential to analyze whether enhancing CMA activity could be of potential value or not.437 Transcriptional activity also plays crucial role in autophagic processes. For example, Transcription factor E3 (TFE3) and Transcription factor EB (TFEB) are the critical regulators of lysosomal biogenesis and autophagy.454 Enhanced activity of autophagy suppressers, such as mTORC, and reduced expression of autophagy-inducing molecules, such as beclin 1, Atg, AMPK, and ULK1 complex proteins leads to inhibition of autophagic induction.455 Inappropriate lysosomal fusion or pH due to increased autophagic vacuoles (AVs) containing inactive cathepsin and undigested content leads to neurodegeneration in AD neurons (Figure 1B right panel).455
Autophagy can be targeted for the degradation of pathogenic peptide aggregates such as Aβ and Tau protein. Autophagy-stimulating drugs may provide better treatment of AD, and many related drugs have been reported to show protective effects against AD in preclinical studies and clinical trials. These include rapamycin, nicotinamide, protein phosphatase 2A agonists, carbamazepine (CBZ), lithium, memantine, and latrepirdine.434 Studies performed by Li et al. and Zhang et al. in the AD mouse models showed that autophagy enhancer carbamazepine was able to protect brain against memory dysfunction and an increase in Aβ content.456,457 Mechanistic Target of Rapamycin (mTOR) pathway regulates autophagy and is proved to be strongly associated with the pathology of AD. Studies in the AD mouse models reported that mTOR signaling is inhibited in hippocampus and cortex.458 Spilman and colleagues has reported that blocking the mTOR signaling by rapamycin reduced cognitive deficits and amyloid pathology in mouse model of AD.459 It has also been reported that mTOR stimulates Tau pathology by facilitating intra- and extra-cellular distribution of Tau, its phosphorylation, and accumulation.460,461 Rapamycin is a selective inhibitor of target-of-rapamycin complex 1 (TORC1) that was reported to show reduced Aβ and Tau pathology and improved learning and memory in mouse model of AD.462 Temsirolimus is the prodrug of rapamycin that induces autophagy-dependent Aβ clearance, shows memory improvement in cellular and animal AD models,463 and reduces Tau accumulation in Tau mutant mice.464 The FDA approved drug memantine act by antagonizing NMDA receptors is also reported to stimulate autophagy in either mTOR-dependent or mTOR-independent way.465 Resveratrol and its derivatives are bioactive polyphenols that reduce Aβ aggregation via AMPK signaling pathway by activating autophagy.466 Porquet et al. has reported that long-term treatment of AD mouse model with resveratrol reduces memory loss and Aβ levels.467 The randomized, placebo-controlled trials reported that resveratrol penetrated the blood-brain barrier and was safe and well-tolerated.468 According to US FDA report, resveratrol is now in phase 3 clinical trial for AD treatment, and the outputs of study are not published as of yet [https://www.alzforum.org/therapeutics/resveratrol]. A study by Steele et al. in AD mouse model reported that latrepirdine reduces Aβ peptides level by stimulating autophagy in mTOR- and Atg5- dependent manner.469 Phase II trial in AD patients reported significant improvement over placebo. However, phase III clinical trial showed no effect, and therefore this clinical trial was discontinued by U.S. Food and Drug Administration (USFDA) [https://www.alzforum.org/therapeutics/dimebon]. Also, numerous autophagy-regulating compounds have shown protective effect against Aβ and Tau induced toxicity and improve memory in AD animal models. These studied compounds include Tetrahydrohyperforin, β-Asarone, Arctigenin, GTM-1, Trehalose, and Oleuropein Aglycone.434
2.22. Interplay between autophagy and other signaling pathways in AD
To maintain cellular homeostasis, protein quality control (PQC) system is controlled through multi-levels of interactions.470 Autophagy displays cross-talk with other signaling pathways, which are significantly involved in AD pathogenesis. The Aβ peptide is generated as a result of the APP processing during autophagic turnover of APP-rich organelles supplied by both endocytosis and autophagy. The enhanced autophagy induction is also reported to create conditions favorable for Aβ accumulation in AD brain.71 As discussed in genetic mutation part of this review, PSEN1 is a component of γ-secretase complex which is involved in APP cleavage471 and proteolysis via autophagy.472 Genetic mutations in PSEN1 lead to alterations in APP processing. However, recent studies suggested that PSEN1 mutation inhibits autophagy by reducing autophagic cargo elimination.149 Francois et al. has reported interplay between autophagy, inflammation, and AD, suggesting the presence of a link connecting these processes.473 It has also been reported that autophagy is directly regulated by neurotransmitter receptor.170 The link between autophagy and neurotransmitter receptors is essential for the regulation of neuronal function.170 Mitochondria and Mitochondrial autophagy play crucial role in cell health and its dysfunction leads to insufficient clearance of dysfunctional mitochondria by autophagy.209,474 Nijholt et al. reported that Endoplasmic reticulum stress activated autophagy in neuronal cells, however UPS was not activated, suggesting that autophagy is the foremost degradational pathway after UPR activation.220 Studies also reported that UPR signals in ER are linked to autophagy, since both abnormal UPR and impaired autophagy have been observed in AD.221 Oxidative stress/ROS (mainly mitochondrial H2O2 and O2−) modulate and induces autophagy via mTOR-dependent pathways. Also, autophagy can reduce oxidative stress/ROS-induced nerve cell damage by removing abnormal protein aggregates and improves their survival.475 Autophagy also plays an essential role in controlling the Aβ-induced neuroinflammation in brain.476 CDK5, an autophagy-regulating kinase, protects against neuroinflammation, Aβ accumulation, Tau hyperphosphorylation, and memory loss.445
Although the autophagy and UPS have independent degradation process mechanisms (Figure 1), wide range of studies have reported the presence of cooperation and cross-talk between these two pathways. These pathways share common substrates, such as CTFβ, Aβ, and α-synuclein, which are among the key harmful proteins for neuronal function. In addition, enzymes of the ubiquitylation machinery, such as E3 ligase Parkin, contribute to both degradation pathways.26 Thus, any disturbances in autophagy and UPS will affect their ability to effectively counteract proteotoxic stressors that provoke the accumulation of aberrant proteins.477
Furthermore, evidence also suggests that perturbations in proteasome activity activate autophagic process indicates that these both pathways are depending on each other’s effect.478 The insulin resistance (type 2 diabetes) that involves increase in blood sugar level shows strong correlation with autophagy. Autophagy protects pancreatic β-cells from apoptotic cell death, protects the insulin target organs from hyperglycemia-induced oxidative stress, and preserves their structure and function. Therefore, by activating autophagic process, insulin resistance can be avoided along with AD.331 The high intracellular cholesterol augments the Aβ-induced autophagosome formation. However, it affects lysosomal fusion capacity by changing the content and distribution of RAB7A and SNAP receptors (SNAREs). Reducing cellular cholesterol levels may enhance the delivery of Aβ to lysosomes and degradation.348 Autophagy is also linked to the neurotransmitter-induced excitotoxicity. Excessive stimulation of non-NMDA glutamate receptors activates lysosomal enzymes and induces autophagy. Autophagic activation by excitotoxic neuronal injury is crucial in order to prevent neurodegeneration.378
The drugs which targets more than one pathway must be tested for AD treatment such as resveratrol not only act via autophagy stimulation but also shows anti-inflammatory, anti-oxidant, and amyloid inhibition activity.479 Activation of autophagy is a crucial part of the cellular response to oxidative stress induced by ROS and RNS.475 Therefore, a large number of resveratrol derivatives can be prepared and studied for Aβ aggregation inhibition and synaptic and memory development. The transcription factor EB (TFEB) regulates lysosomal biogenesis and autophagy. The curcumin, a polyphenol antioxidant compound inhibits the mTOR signaling pathway and activates the TFEB, reduces cognitive impairments, and Aβ formation in AD models.26 mTOR inhibitor rapamycin exerts its neuroprotective actions and inhibition of Aβ and Tau via its autophagy inducing capability along with antioxidant and anti-inflammatory activity.476
3. Some Unanswered Questions
The decades of intensive research on various aspects of AD have generated massive knowledge on both the clinical and basic levels. As many with many other neurodegenerative diseases, AD is recognized now as a multifactorial malady in the broadest essence of this word. AD affects the body on multiple levels, hits different systems, produces various symptoms, and generates diverse biochemical outputs. AD is a disease with a multifactorial etiology, where any of a broad set of intrinsic and extrinsic factors (protein misfolding and aggregation, mutations, genetic predisposition, oxidative damage, impairment of the protein quality control system, environmental toxins, mitochondiapathy, microbiota dysbiosis, etc.), or their various combinations, can trigger the development of pathology. As a result, the AD field is intensively developing in different directions. New factors potentially involved in the AD pathogenesis are frequently found. To some extent, studies on AD resemble peeling of an onion, where removing one layer uncovers another layer, which in turn hides a new level of complexity. We are slowly going through this multilayer problem, trying to reach its core; trying to understand what the primary cause of AD is, if there is one; trying to get closer to the beginning of all the beginnings. Does “many reasons” necessarily mean “no reason at all”? Such a scenario is very unlikely in the case of AD, as this malady is known to be provoked by multiple individual factors, or by their various combinations. However, the current state of our understanding of AD clearly resembles The Never Ending Story, where a new study discovers a new player, the introduction of which initiates a new round of intensive studies, which produce a new “chronicle” culminating in suggesting of a new logical explanation of the “unique” and almost always “crucial” role of this new player in the development of general story. With all these newly discovered players, all these neatly described “chronicles”, and all these intriguing developments, the question is: are we any closer to understanding the AD aetiology today than we were 30, 20 or 10 years ago? The answer is a definite yes, as we now know more and understand some aspects of the disease better. So, just how far are we from the end of this journey? This question is harder to answer, as we are still searching for the beginning of this never-ending story. The hope is that one day all these individual chronicles will merge together and the puzzle will be solved.
This review summarized the current knowledge on the molecular mechanisms of various signaling pathways related to the AD pathogenesis. Although significant information is accumulated in this field, there is still a number of unanswered fundamental questions about the key mechanisms associated with the AD pathogenesis. Even if one focuses just on the major AD-related culprits, Aβ or Tau, many questions are still waiting for their answers. Among these important questions are: What are the exact molecular mechanisms of AD pathology and is there a single mechanism invariantly causing AD? Is amyloid cascade signaling central to all the AD-related pathways? Are misfolded, oligomerized, aggregated, and fibrillated Aβ and Tau always causing neurodegeneration and cognitive impairment? If so, are Aβ or Tau sufficient to be used as targets for AD therapy? Are there other factors in the brain that can enhance Aβ formation? How do we target signaling pathways to resolve problems leading to the neurodegeneration? Does Aβ and Tau aggregation impair other processes in body? Does Aβ aggregation affect the other signaling pathways in the body? Are the Aβ concentration increases or decreases in the organs of the body other than the brain? What are the mechanisms, by which senile plaques and NFT lead to the brain cell death? Whether these aggregates damage cells other than nerve cells? What could be the reason for autophagic failure with age? What are the mechanisms behind autophagic diminishing caused by Aβ aggregates? If the PQC system activated then how long this activation can be effectively maintained?
Although it is reported that metabolic syndrome, type 2 diabetes, and AD might be interconnected,61 it is also possible that other pathways are also linked with this. Autophagic pathway shows cross-link with many other pathways of AD. So, is targeting only autophagy represents an effective way to fight against AD? Do we know all signaling pathways that can lead to AD? Is linking of all the pathogenic signaling pathways to each is required for the pathogenesis of AD? If so, in which order and by what means? How do different pathogenic signaling pathways affect neuronal cell damage and memory loss? Are there new signaling pathways that can be associated with nerve cell death? Are all these pathological pathways always linked to the Aβ aggregation? How do aberrant signaling pathways increase the risk of dementia? What are the clinical links between AD and pathogenic signaling pathways? Answering these questions may provide the platform for the effective development of drugs against AD. This review represents the major signaling pathways associated with the AD pathobiology and emphasizes that consideration of these signaling pathways may help to solve several unanswered questions of AD pathology.
4. Current advances and therapeutic strategies against AD
Till now, there is no any mechanism-based therapy available for the AD. In this review, we have discussed key therapeutic strategies independently at each molecular signaling pathway of AD. USFDA approved five drugs that are able to maintain neurotransmitter levels in the brain but are not able to cure AD. Furthermore, to date, no treatment is available to reverse the symptoms of the AD. For significant breakthroughs in the treatment of any malady, precise knowledge of the underlying pathophysiology is important. For a better understanding of AD pathogenesis, all the related pathways must be checked for interlink between them.
There is still a question of whether targeting Aβ or Tau alone is sufficient for effective AD therapy. Currently, researchers are mainly focusing on developing strategies for the efficient elimination of Aβ. However, targeting only one component for AD therapy may not be sufficient, since numerous drugs targeting Aβ pathway failed in a clinical trial. Therefore, new insights into AD mechanisms and new therapeutic strategies can be helpful. It is very likely that targeting multiple pathways may be more effective than targeting a single pathway and that this complex approach might be beneficial for AD patients. Complete success in AD treatment requires an understanding of the exact molecular mechanisms of this disease. Multi-target therapy aiming at multiple common nodes from various signaling pathways, such as Aβ, Tau, GSK3β, acetylcholinesterase, as well as players related to inflammation, insulin signaling, controlling Ca2+ levels, cellular protein quality control systems, and oxidative stress could be more successful than any monotherapy. For example, promising strategies for the design of the effective therapeutics against AD might include various combinations of means for 1) regulating the APP processing by increasing α-secretase activity or blocking the β- or γ-secretase pathways; 2) utilizing various anti-aggregation molecules; 3) increasing Aβ and Tau clearance with antibodies or by activating ApoE, IDE, autophagy/ELS, and UPS; 4) improving Aβ and Tau proteostasis with chaperones, such as HSP70; 5) blocking signaling pathways, such as GSK3β and glutamate; 6) regulating APP processing by modulating cholesterol and lipid metabolism; 7) using anti-oxidant and anti-inflammatory agents; 8) maintaining neurotransmitter balance by reducing their degradation; 9) Enhance the activity/expression of autophagy-inducing molecules such as ATG proteins and beclin 1; 10) Inhibit the activity of autophagy suppressor such as mTOR; 11) Activating proteasomal degradation of Aβ and Tau; 12) improving neuronal growth by activating NGF and BDNF; and 13) modulating the gut microbiota with specific nutritional interventions. The agents acting through multiple pathways that may show potential in AD therapy. Thus, clinical trial must be undertaken on the drugs that are having multiple targets.
5. Concluding Remarks
Intensive research proposed the existence of numerous signaling pathways that can be related to the pathobiology of AD. Since currently, results of clinical trials for anti-AD drugs are mostly unsatisfactory; there is a strong demand for new drugs that can show some promise in the cure of this devastating disorder. In fact, a detailed mechanism of the pathogenesis needs to know key players associated with AD pathogenesis in order to tackle this pathological event. Therefore, the better understanding of the key cellular signaling pathways related to the propagation of neurodegeneration and various interconnections between them can provide new insights for the evaluation and development of new therapeutic strategies for cure of AD in near future. Indeed, looking at multiple signaling pathways provides valuable information and important insights into the novel pathological mechanisms. Systematic investigation, comparative analysis of individual pathway, finding correlations between them, and how all these pathways are causatively associated with AD could provide a novel perspective for understanding the pathogenesis, successful diagnosis, and management of AD. Identifying common nodes in multiple pathways might offer new opportunities for the designing of specific treatment strategies for AD. Considering cross talk of autophagy with many other pathways, it should be the focus of attention for AD research in the near future. Autophagic upregulation may be a promising therapeutic strategy for AD due to approachability of druggable targets described in this review. Finally, we expect that the next decade will generate additional information on the molecular mechanisms and common nodes of various signaling pathways associated with the AD pathophysiology, which will open new grounds for drug targeting.
Acknowledgments:
The authors are thankful to the Indian Institute of Technology (IIT) Mandi for providing facilities. RG and KG is grateful to the Department of Biotechnology (DBT- BT/PR16871/NER/95/329/2015), India for providing fellowship. DK is thankful to ICMR fellowship. Work of VNU was supported in part by the National Institute on Aging of the National Institutes of Health under Award Number RF1AG055088.
Abbreviations
- Aβ
amyloid beta
- ABACA1
ATP-binding cassette, sub-family A, member1
- ACAT
Acyl CoA: Cholesterol O-acyl transferase
- AD
Alzheimer’s disease
- AGE
advanced glycation end product
- AICD
APP intracellular C-terminal domain
- AMPK
AMP-activated protein kinase
- APOE4
ε4 variant of the apolipoprotein E
- APP
amyloid precursor protein
- ATF6
AMP-dependent transcription factor ATF-6 alpha
- ATG1
autophagy related 1
- AV
autophagy vacuole
- BACE1
β-secretase
- BBB
blood-brain barrier
- BDNF
brain-derived neurotrophic factor
- BIN1
bridging integrator 1
- BVR-A
biliverdin reductase-A
- CAT
choline acetyltransferase
- CBD
cannabidiol
- CD2AP
CD2-associated protein
- CLU
clusterin
- CMA
chaperone-mediated autophagy
- CNS
central nervous system
- CR1
complement receptor 1
- CREB
cAMP response element binding
- CRP
C-reactive protein
- CSF
cerebrospinal fluid
- DM
diabetes mellitus
- DUB
deubiquitinating enzyme
- eIF2α
eukaryotic translation initiation factor 2 subunit 1
- EOAD
early-onset AD
- ER
endoplasmic reticulum
- ETC
electron transport chain
- FAD
familial AD
- FMT
fecal microbiota transplantation
- Fz
frizzled
- GAD
glutamic acid decarboxylase
- GLP-1R
glucagon-like peptide-1 receptor
- GLUT
glucose transporter
- GPCR
G protein coupled receptor
- GSK-3β
glycogen synthase kinase three beta
- GWAS
genome wide association studies
- HDL
high-density lipoprotein
- HSP
heat shock protein
- IDE
insulin-degrading enzyme
- IGF
insulin-like growth factor
- IKK
IκB kinase
- IGF1R
tyrosine kinase receptor type 1 receptor (IGF receptor)
- InsP3
inositol trisphosphate
- InsP3R
InsP3 receptor
- INI
intranasal insulin
- IR
insulin receptor
- IRE1α
serine/threonine-protein kinase/endoribonuclease inositol-requiring enzyme 1 α
- IRS-1
insulin receptor substrate 1
- IVIg
intravenous immunoglobulins
- JNK
c-Jun N-terminal kinase
- KPI
Kunitz-type proteinase inhibitor
- LDL
low-density lipoprotein
- LOAD
late-onset AD
- LPS
lipopolysaccharide
- LRP
LDL receptor-related protein
- LTP
long-term potentiation
- mTOR
mammalian target of rapamycin
- NCT
nicastrin
- NEO
neutral endopeptidase neprilysin
- NFT
neurofibrillary tangle
- NF-κB
nuclear factor kappa B
- NGF
nerve growth factor
- NT-3
neurotrophin-3
- NTF
neurotrophic factor
- NMDA
N-methyl-D-aspartate
- NMDAR
NMDA receptor
- NSAID
non-steroidal anti-inflammatory drug
- NVU
neurovascular unit
- OBR
(or LEPR) leptin receptor
- OXPHOS
oxidative phosphorylation
- PEN-2
presenilin enhancer protein 2
- PERK
PRKR-like endoplasmic reticulum kinase
- PET
positron emission tomography
- Pgp
P-glycoprotein
- PHF
paired helical filament
- PICALM
phosphatidylinositol binding clathrin assembly protein
- PKR
protein kinase R
- PSEN
presenilin
- PTK2B
protein tyrosine kinase 2β
- RAGE
receptor for advanced glycation end products
- RIN3
Ras and Rab interactor 3
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- RYK
receptor tyrosine kinases
- SAD
sporadic AD
- SCFA
short-chain fatty acid
- SIRT
sirtuin
- SLC24A4
solute carrier family 24 (sodium/potassium/calcium exchanger), member 4
- SNAREs
soluble NSF attachment protein receptors
- SNPs
single nucleotide polymorphisms
- SORL1
sortilin-related receptor1
- TFEB
transcription factor EB
- TGFβ
transforming growth factor beta
- TJ
tight junction
- TNFR
tumor necrosis factor receptor
- TREM2
triggering receptor expressed on myeloid cells 2
- Trk
tyrosine kinase
- UCHL1
ubiquitin C-terminal hydrolase L1
- UPR
unfolded protein response
- UPS
ubiquitin-proteasome system
- VLDL
very-low-density lipoprotein
- VTA
ventral tegmental area
Author Biosketches
Kundlik Gadhave, Ph.D. is currently a senior project scientist at Indian Institute of Technology Mandi (IIT Mandi), Himachal Pradesh, India. He obtained his Ph.D. in biotechnology from the IIT Mandi (June 2020). He received his M.S. in pharmacology and toxicology from National Institute of Pharmaceutical Education and Research (NIPER) Guwahati, India in 2016. During M.S., he worked on drug development against mental disorder ‘Depression’ and Alzheimer’s disease-like complications of diabetes using animal models. After obtaining M.S., he joined Institute of Nanoscience and Technology, India where he worked on the formulation of nanoparticles for Parkinson’s disease. In February 2017, he joined IIT Mandi as a Ph.D. student, where he worked on understanding the unstructured biology of proteins from various signaling pathways associated with Alzheimer’s disease. His current research is focusing on amyloid formation of signal peptides and their roles in Alzheimer’s disease.
Deepak Kumar, Ph.D. is a senior research fellow at School of Basic Sciences, Indian Institute of Technology Mandi, India. His current research revolves around understanding the roles of intrinsically disordered proteins (IDPs) in a variety of biological processes. More specifically, he is focusing on neglected tropical diseases, such as Zika virus, and taking up the area of neurodegenerative diseases. He is using basic molecular biology, biophysics, and computational tools to characterize the functions of IDPs. In addition, he is focusing on the finding and analysis of druggable sites on flexible IDPs that could be inhibited by small molecules. He is using virtual screening approaches along with the enzymatic assays to find out active molecules.
Vladimir N. Uversky, Ph.D., DSc, Professor, obtained his Ph.D. in biophysics from the Moscow Institute of Physics and Technology (1991) and D.Sc. in biophysics from the Institute of Experimental and Theoretical Biophysics, Russian Academy of Sciences (1998). He spent his early career working on protein folding at the Institute of Protein Research and the Institute for Biological Instrumentation (Russian Academy of Sciences). Here, he has found that some mostly unstructured proteins can be biologically active. These findings forced him to reconsider the generality of the protein structure-function paradigm and to suggest that intrinsically disordered proteins represent a new important realm of the protein kingdom. In 1998, he moved to the University of California Santa Cruz to work on protein folding, misfolding, and protein intrinsic disorder. In 2004, he moved to the Center for Computational Biology and Bioinformatics at the Indiana University-Purdue University Indianapolis to work on the intrinsically disordered proteins. Since 2010, he has been with the Department of Molecular Biology at the University of South Florida, where he is now a Professor. He participated in the establishment of the Intrinsically Disordered Proteins Subgroup at the Biophysical Society and the Intrinsically Disordered Proteins Gordon Research Conference. Dr. Uversky has published over 850 peer-reviewed articles and book chapters on various aspects of protein intrinsic disorder phenomenon and on analysis of protein folding and misfolding. He was included by the Thomson Reuters to the list of the world Highly Cited Researchers in 2014, 2015, 2016, 2017, 2018, and 2019.
Rajanish Giri, Ph.D. is an Assistant Professor of Biotechnology at Indian Institute of Technology Mandi (IIT Mandi), Himachal Pradesh, India. He received his Master’s degree in Biotechnology from Banaras Hindu University, India in 2007, and Ph.D. from The Sapienza University of Rome, Italy in 2013. He was Post-doctoral researcher at the ‘Istituto Pasteur-Fondazioni Cenci Bolognetti’, Universita Degli Studi di Roma ‘La Sapienza’ (2012–2013) and McMaster University, Hamilton, Canada (2013–2015). After postdoctoral studies, he established his own research group at IIT Mandi, India in 2015, where he is working on the different aspects of intrinsically disordered proteins, including analysis of the disorder status of viral proteomes, as well as on the elucidation of the roles of protein misfolding and aggregation in neurodegenerative diseases, such as Alzheimer’s disease. He is the recipient of Har Govind Khorana-Innovative Young Biotechnologist Award (IYBA), Department of Biotechnology, Government of India in 2019.
Footnotes
Conflict of interest: All the authors declare that there is no potential conflict of interest.
References
- 1.Scheltens P, Blennow K, Breteler MMB, et al. Alzheimer’s disease. Lancet (London, England). 2016;388:505–517. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. [DOI] [PubMed] [Google Scholar]
- 3.Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clinical interventions in aging. 2007;2:347–359. [PMC free article] [PubMed] [Google Scholar]
- 5.Sanabria-Castro A, Alvarado-Echeverría I, Monge-Bonilla C. Molecular Pathogenesis of Alzheimer’s Disease: An Update. Annals of neurosciences. 2017;24:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer’s_Disease_International. World Alzheimer Report 2018 - The state of the art of dementia research: New frontiers. 2018; https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf.Accessed 04/01/2019, 2019.
- 7.Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. The Journal of clinical investigation. 2005;115:1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayeux R Epidemiology of neurodegeneration. Annual review of neuroscience. 2003;26:81–104. [DOI] [PubMed] [Google Scholar]
- 9.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60(4):534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayeux R Epidemiology of neurodegeneration. Annu Rev Neurosci. 2003;26:81–104. [DOI] [PubMed] [Google Scholar]
- 11.Mucke L Neuroscience: Alzheimer’s disease. Nature. 2009;461:895–897. [DOI] [PubMed] [Google Scholar]
- 12.Kauwe JSK, Wang J, Mayo K, et al. Alzheimer’s disease risk variants show association with cerebrospinal fluid amyloid beta. Neurogenetics. 2009;10:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nature reviews Drug discovery. 2011;10:698–712. [DOI] [PubMed] [Google Scholar]
- 14.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biological psychiatry. 2015;77:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chávez-Gutiérrez L, Bammens L, Benilova I, et al. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. The EMBO journal. 2012;31:2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends in pharmacological sciences. 1991;12:383–388. [DOI] [PubMed] [Google Scholar]
- 18.Zheng H, Jiang M, Trumbauer ME, et al. beta-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81:525–531. [DOI] [PubMed] [Google Scholar]
- 19.Bancher C, Brunner C, Lassmann H, et al. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain research. 1989;477:90–99. [DOI] [PubMed] [Google Scholar]
- 20.Braak E, Braak H, Mandelkow EM. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta neuropathologica. 1994;87:554–567. [DOI] [PubMed] [Google Scholar]
- 21.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. The Journal of biological chemistry. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 22.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandelkow EM, Mandelkow EM. Tau in Alzheimer’s disease. Trends in cell biology. 1998;8:425–427. [DOI] [PubMed] [Google Scholar]
- 24.Mandelkow E-ME, Mandelkow E-ME. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor perspectives in medicine. 2012;2:a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Mandelkow E. Tau in physiology and pathology. Nature reviews Neuroscience. 2016;17:5–21. [DOI] [PubMed] [Google Scholar]
- 26.Bustamante HA, Gonzalez AE, Cerda-Troncoso C, et al. Interplay Between the Autophagy-Lysosomal Pathway and the Ubiquitin-Proteasome System: A Target for Therapeutic Development in Alzheimer’s Disease. Front Cell Neurosci. 2018;12:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du X, Wang X, Geng M. Alzheimer’s disease hypothesis and related therapies. Translational neurodegeneration. 2018;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science (New York, NY). 1992;256:184–185. [DOI] [PubMed] [Google Scholar]
- 29.Navarrete LP, Pérez P, Morales I, Maccioni RB. Novel drugs affecting tau behavior in the treatment of Alzheimer’s disease and tauopathies. Current Alzheimer research. 2011;8:678–685. [DOI] [PubMed] [Google Scholar]
- 30.Bekris LM, Yu C-E, Bird TD, Tsuang DW. Genetics of Alzheimer disease. Journal of geriatric psychiatry and neurology. 2010;23:213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nitsch RM. From acetylcholine to amyloid: neurotransmitters and the pathology of Alzheimer’s disease. Neurodegeneration : a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration. 1996;5:477–482. [DOI] [PubMed] [Google Scholar]
- 32.Palmer AM, Francis PT, Benton JS, et al. Presynaptic serotonergic dysfunction in patients with Alzheimer’s disease. Journal of neurochemistry. 1987;48:8–15. [DOI] [PubMed] [Google Scholar]
- 33.Procter AW, Palmer AM, Francis PT, et al. Evidence of glutamatergic denervation and possible abnormal metabolism in Alzheimer’s disease. Journal of neurochemistry. 1988;50:790–802. [DOI] [PubMed] [Google Scholar]
- 34.Sims NR, Bowen DM, Allen SJ, et al. Presynaptic cholinergic dysfunction in patients with dementia. Journal of neurochemistry. 1983;40:503–509. [DOI] [PubMed] [Google Scholar]
- 35.Swerdlow RH, Burns JM, Khan SM. The Alzheimer’s disease mitochondrial cascade hypothesis. Journal of Alzheimer’s disease : JAD. 2010;20Suppl 2:S265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto S, Saido TC. Critical review: involvement of endoplasmic reticulum stress in the aetiology of Alzheimer’s disease. Open biology. 2018;8:180024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull. 2014;30(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gummalla M, Galetti S, Maeda RK, Karch F. Hox gene regulation in the central nervous system of Drosophila. Frontiers in cellular neuroscience. 2014;8:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. The Lancet Neurology. 2015;14:388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng C, Zhou X-W, Wang J-Z. The dual roles of cytokines in Alzheimer’s disease: update on interleukins, TNF-α, TGF-β and IFN-γ. Translational neurodegeneration. 2016;5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ihara Y, Morishima-Kawashima M, Nixon R. The ubiquitin-proteasome system and the autophagic-lysosomal system in Alzheimer disease. Cold Spring Harbor perspectives in medicine. 2012;2:a006361–a006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gadhave K, Bolshette N, Ahire A, et al. The ubiquitin proteasomal system: a potential target for the management of Alzheimer’s disease. Journal of cellular and molecular medicine. 2016;20:1392–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nedelsky NB, Todd PK, Taylor JP. Autophagy and the ubiquitin-proteasome system: collaborators in neuroprotection. Biochimica et biophysica acta. 2008;1782:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng Q, Siu W, Li L, et al. Autophagy in Alzheimer’s disease and promising modulatory effects of herbal medicine. Exp Gerontol. 2019;119:100–110. [DOI] [PubMed] [Google Scholar]
- 45.Nilsson P, Saido TC. Dual roles for autophagy: degradation and secretion of Alzheimer’s disease Abeta peptide. Bioessays. 2014;36(6):570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nilsson P, Loganathan K, Sekiguchi M, et al. Abeta secretion and plaque formation depend on autophagy. Cell Rep. 2013;5(1):61–69. [DOI] [PubMed] [Google Scholar]
- 47.Bosco D, Fava A, Plastino M, Montalcini T, Pujia A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. Journal of cellular and molecular medicine. 2011;15:1807–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marambaud P, Dreses-Werringloer U, Vingtdeux V. Calcium signaling in neurodegeneration. Molecular neurodegeneration. 2009;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Supnet C, Bezprozvanny I. Neuronal calcium signaling, mitochondrial dysfunction, and Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2010;20Suppl 2:S487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen SJ, Watson JJ, Dawbarn D. The neurotrophins and their role in Alzheimer’s disease. Current neuropharmacology. 2011;9:559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schindowski K, Belarbi K, Buée L. Neurotrophic factors in Alzheimer’s disease: role of axonal transport. Genes, brain, and behavior. 2008;7Suppl 1:43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nature neuroscience. 2003;6:345–351. [DOI] [PubMed] [Google Scholar]
- 53.Hicks DA, Nalivaeva NN, Turner AJ. Lipid rafts and Alzheimer’s disease: protein-lipid interactions and perturbation of signaling. Frontiers in physiology. 2012;3:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nature reviews Neuroscience. 2005;6:351–362. [DOI] [PubMed] [Google Scholar]
- 55.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. [DOI] [PubMed] [Google Scholar]
- 56.Nusse R, Varmus H. Three decades of Wnts: a personal perspective on how a scientific field developed. The EMBO journal. 2012;31:2670–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harvey J Leptin: the missing link in Alzheimer disease? Clinical chemistry. 2010;56:696–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marwarha G, Ghribi O. Leptin signaling and Alzheimer’s disease. American journal of neurodegenerative disease. 2012;1:245–265. [PMC free article] [PubMed] [Google Scholar]
- 59.Velliquette RA, O’Connor T, Vassar R. Energy inhibition elevates beta-secretase levels and activity and is potentially amyloidogenic in APP transgenic mice: possible early events in Alzheimer’s disease pathogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10874–10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nature reviews Neurology. 2018;14:133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutrition reviews. 2016;74:624–634. [DOI] [PubMed] [Google Scholar]
- 62.Selkoe DJ. Alzheimer’s disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. Journal of Alzheimer’s disease : JAD. 2001;3:75–80. [DOI] [PubMed] [Google Scholar]
- 63.Miller DL, Papayannopoulos IA, Styles J, et al. Peptide compositions of the cerebrovascular and senile plaque core amyloid deposits of Alzheimer’s disease. Archives of biochemistry and biophysics. 1993;301:41–52. [DOI] [PubMed] [Google Scholar]
- 64.Roher AE, Lowenson JD, Clarke S, et al. beta-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10836–10840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: from synapses toward neural networks. Nature neuroscience. 2010;13:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forloni G, Balducci C. Alzheimer’s Disease, Oligomers, and Inflammation. J Alzheimers Dis. 2018;62(3):1261–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mokhtar SH, Kim MJ, Magee KA, et al. Amyloid-beta-dependent phosphorylation of collapsin response mediator protein-2 dissociates kinesin in Alzheimer’s disease. Neural Regen Res. 2018;13(6):1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong S, Duan Y, Hu Y, Zhao Z, Zheng Z. Advances in the pathogenesis of Alzheimer’s disease: a re-evaluation of amyloid cascade hypothesis. Translational neurodegeneration. 2012;1:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salminen A, Kaarniranta K, Kauppinen A, et al. Impaired autophagy and APP processing in Alzheimer’s disease: The potential role of Beclin 1 interactome. Prog Neurobiol. 2013;106–107: 33–54. [DOI] [PubMed] [Google Scholar]
- 71.Nixon RA. Autophagy, amyloidogenesis and Alzheimer disease. J Cell Sci. 2007;120(Pt 23):4081–4091. [DOI] [PubMed] [Google Scholar]
- 72.Yu WH, Cuervo AM, Kumar A, et al. Macroautophagy--a novel Beta-amyloid peptide-generating pathway activated in Alzheimer’s disease. J Cell Biol. 2005;171(1):87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gupta A, Goyal R. Amyloid beta plaque: a culprit for neurodegeneration. Acta neurologica Belgica. 2016;116:445–450. [DOI] [PubMed] [Google Scholar]
- 74.Di Paolo G, Kim T-W. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nature reviews Neuroscience. 2011;12:284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mroczko B, Groblewska M, Litman-Zawadzka A, Kornhuber J, Lewczuk P. Amyloid beta oligomers (AbetaOs) in Alzheimer’s disease. J Neural Transm (Vienna). 2018;125(2):177–191. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Chang L, Song Y, Li H, Wu Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front Neurosci. 2019;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nortley R, Korte N, Izquierdo P, et al. Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes. Science. 2019;365(6450). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nature reviews Molecular cell biology. 2007;8:101–112. [DOI] [PubMed] [Google Scholar]
- 79.Faghihi MA, Modarresi F, Khalil AM, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nature medicine. 2008;14:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vassar R BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimer’s research & therapy. 2014;6:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou L, Barão S, Laga M, et al. The neural cell adhesion molecules L1 and CHL1 are cleaved by BACE1 protease in vivo. The Journal of biological chemistry. 2012;287:25927–25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grbovic OM, Mathews PM, Jiang Y, et al. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. The Journal of biological chemistry. 2003;278:31261–31268. [DOI] [PubMed] [Google Scholar]
- 83.Selkoe DJ, Yamazaki T, Citron M, et al. The role of APP processing and trafficking pathways in the formation of amyloid beta-protein. Annals of the New York Academy of Sciences. 1996;777:57–64. [DOI] [PubMed] [Google Scholar]
- 84.Kimberly WT, Xia W, Rahmati T, Wolfe MS, Selkoe DJ. The transmembrane aspartates in presenilin 1 and 2 are obligatory for gamma-secretase activity and amyloid beta-protein generation. The Journal of biological chemistry. 2000;275:3173–3178. [DOI] [PubMed] [Google Scholar]
- 85.Capell A, Beher D, Prokop S, et al. Gamma-secretase complex assembly within the early secretory pathway. The Journal of biological chemistry. 2005;280:6471–6478. [DOI] [PubMed] [Google Scholar]
- 86.Rajendran L, Honsho M, Zahn TR, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:11172–11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Runz H, Rietdorf J, Tomic I, et al. Inhibition of intracellular cholesterol transport alters presenilin localization and amyloid precursor protein processing in neuronal cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1679–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.De Strooper B Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38:9–12. [DOI] [PubMed] [Google Scholar]
- 89.Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin-1 gene. Journal of neurology. 2006;253:139–158. [DOI] [PubMed] [Google Scholar]
- 90.Maarouf CL, Walker JE, Sue LI, Dugger BN, Beach TG, Serrano GE. Impaired hepatic amyloid-beta degradation in Alzheimer’s disease. PLoS One. 2018;13(9):e0203659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cook DG, Leverenz JB, McMillan PJ, et al. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer’s disease is associated with the apolipoprotein E-epsilon4 allele. The American journal of pathology. 2003;162:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer’s disease. Brain pathology (Zurich, Switzerland). 2008;18:240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walsh DM, Klyubin I, Fadeeva JV, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. [DOI] [PubMed] [Google Scholar]
- 94.Palop JJ, Chin J, Roberson ED, et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tampellini D, Gouras GK. Analysis of vesicular trafficking in primary neurons by live imaging. Methods in molecular biology (Clifton, NJ). 2011;793:343–350. [DOI] [PubMed] [Google Scholar]
- 96.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:2866–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Libro R, Diomede F, Scionti D, et al. Cannabidiol Modulates the Expression of Alzheimer’s Disease-Related Genes in Mesenchymal Stem Cells. International journal of molecular sciences. 2016;18:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cribbs DH. Abeta DNA vaccination for Alzheimer’s disease: focus on disease prevention. CNS & neurological disorders drug targets. 2010;9:207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Delrieu J, Ousset PJ, Caillaud C, Vellas B. ‘Clinical trials in Alzheimer’s disease’: immunotherapy approaches. Journal of neurochemistry. 2012;120Suppl186–193. [DOI] [PubMed] [Google Scholar]
- 100.Ghochikyan A, Mkrtichyan M, Petrushina I, et al. Prototype Alzheimer’s disease epitope vaccine induced strong Th2-type anti-Abeta antibody response with Alum to Quil A adjuvant switch. Vaccine. 2006;24:2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lemere CA. Developing novel immunogens for a safe and effective Alzheimer’s disease vaccine. Progress in brain research. 2009;175:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nicoll JAR, Barton E, Boche D, et al. Abeta species removal after abeta42 immunization. Journal of neuropathology and experimental neurology. 2006;65:1040–1048. [DOI] [PubMed] [Google Scholar]
- 103.Schneeberger A, Mandler M, Otawa O, Zauner W, Mattner F, Schmidt W. Development of AFFITOPE vaccines for Alzheimer’s disease (AD)--from concept to clinical testing. The journal of nutrition, health & aging. 2009;13:264–267. [DOI] [PubMed] [Google Scholar]
- 104.Tabira T Immunization therapy for Alzheimer disease: a comprehensive review of active immunization strategies. The Tohoku journal of experimental medicine. 2010;220:95–106. [DOI] [PubMed] [Google Scholar]
- 105.Bard F, Barbour R, Cannon C, et al. Epitope and isotype specificities of antibodies to beta -amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Camboni M, Wang C-M, Miranda C, et al. Active and passive immunization strategies based on the SDPM1 peptide demonstrate pre-clinical efficacy in the APPswePSEN1dE9 mouse model for Alzheimer’s disease. Neurobiology of disease. 2014;62:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carty NC, Wilcock DM, Rosenthal A, et al. Intracranial administration of deglycosylated C-terminal-specific anti-Abeta antibody efficiently clears amyloid plaques without activating microglia in amyloid-depositing transgenic mice. Journal of neuroinflammation. 2006;3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.DeMattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A beta antibody alters CNS and plasma A beta clearance and decreases brain A beta burden in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8850–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Laskowitz DT, Kolls BJ, Salloway SP, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2010;74:2026; author reply2026–2027. [DOI] [PubMed] [Google Scholar]
- 110.Levites Y, Das P, Price RW, et al. Anti-Abeta42- and anti-Abeta40-specific mAbs attenuate amyloid deposition in an Alzheimer disease mouse model. The Journal of clinical investigation. 2006;116:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levites Y, Jansen K, Smithson LA, et al. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:11923–11928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ostrowitzki S, Deptula D, Thurfjell L, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Archives of neurology. 2012;69:198–207. [DOI] [PubMed] [Google Scholar]
- 113.Panza F, Frisardi V, Solfrizzi V, et al. Immunotherapy for Alzheimer’s disease: from anti-β-amyloid to tau-based immunization strategies. Immunotherapy. 2012;4:213–238. [DOI] [PubMed] [Google Scholar]
- 114.Pride M, Seubert P, Grundman M, Hagen M, Eldridge J, Black RS. Progress in the active immunotherapeutic approach to Alzheimer’s disease: clinical investigations into AN1792-associated meningoencephalitis. Neuro-degenerative diseases. 2008;5:194–196. [DOI] [PubMed] [Google Scholar]
- 115.Wilcock DM, DiCarlo G, Henderson D, et al. Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3745–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamada K, Yabuki C, Seubert P, et al. Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11393–11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu S, Shala A, Bezginov A, Sljoka A, Audette G, Wilson DJ. Hyperphosphorylation of intrinsically disordered tau protein induces an amyloidogenic shift in its conformational ensemble. PLoS One. 2015;10(3):e0120416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimers Dis. 2012;2012:731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li C, Gotz J. Tau-based therapies in neurodegeneration: opportunities and challenges. Nat Rev Drug Discov. 2017;16(12):863–883. [DOI] [PubMed] [Google Scholar]
- 120.Yu CC, Jiang T, Yang AF, Du YJ, Wu M, Kong LH. Epigenetic Modulation on Tau Phosphorylation in Alzheimer’s Disease. Neural Plast. 2019;2019:6856327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kametani F, Hasegawa M. Reconsideration of Amyloid Hypothesis and Tau Hypothesis in Alzheimer’s Disease. Front Neurosci. 2018;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goedert M Tau protein and the neurofibrillary pathology of Alzheimer’s disease. Annals of the New York Academy of Sciences. 1996;777:121–131. [DOI] [PubMed] [Google Scholar]
- 123.Irwin DJ, Cohen TJ, Grossman M, et al. Acetylated tau, a novel pathological signature in Alzheimer’s disease and other tauopathies. Brain : a journal of neurology. 2012;135:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shafiei SS, Guerrero-Munoz MJ, Castillo-Carranza DL. Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage. Front Aging Neurosci. 2017;9:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao YL, Wang N, Sun FR, Cao XP, Zhang W, Yu JT. Tau in neurodegenerative disease. Ann Transl Med. 2018;6(10):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Holtzman DM, Carrillo MC, Hendrix JA, et al. Tau: From research to clinical development. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2016;12:1033–1039. [DOI] [PubMed] [Google Scholar]
- 127.Fitzpatrick AWP, Falcon B, He S, et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature. 2017;547:185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bancher C, Grundke-Iqbal I, Iqbal K, Fried VA, Smith HT, Wisniewski HM. Abnormal phosphorylation of tau precedes ubiquitination in neurofibrillary pathology of Alzheimer disease. Brain research. 1991;539:11–18. [DOI] [PubMed] [Google Scholar]
- 129.Gong C-X, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Current medicinal chemistry. 2008;15:2321–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takashima A Tau aggregation is a therapeutic target for Alzheimer’s disease. Current Alzheimer research. 2010;7:665–669. [DOI] [PubMed] [Google Scholar]
- 131.Badiola N, Suárez-Calvet M, Lleó A. Tau phosphorylation and aggregation as a therapeutic target in tauopathies. CNS & neurological disorders drug targets. 2010;9:727–740. [DOI] [PubMed] [Google Scholar]
- 132.Goedert M, Eisenberg DS, Crowther RA. Propagation of Tau Aggregates and Neurodegeneration. Annual review of neuroscience. 2017;40:189–210. [DOI] [PubMed] [Google Scholar]
- 133.Medina M An Overview on the Clinical Development of Tau-Based Therapeutics. Int J Mol Sci. 2018;19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dong Y, Li X, Cheng J, Hou L. Drug Development for Alzheimer’s Disease: Microglia Induced Neuroinflammation as a Target? Int J Mol Sci. 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bales KR, Verina T, Dodel RC, et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nature genetics. 1997;17:263–264. [DOI] [PubMed] [Google Scholar]
- 136.DeMattos RB, O’dell MA, Parsadanian M, et al. Clusterin promotes amyloid plaque formation and is critical for neuritic toxicity in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10843–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lues I, Weber F, Meyer A, et al. A phase 1 study to evaluate the safety and pharmacokinetics of PQ912, a glutaminyl cyclase inhibitor, in healthy subjects. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2015;1:182–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nilsson LN, Bales KR, DiCarlo G, et al. Alpha-1-antichymotrypsin promotes beta-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhan SS, Veerhuis R, Kamphorst W, Eikelenboom P. Distribution of beta amyloid associated proteins in plaques in Alzheimer’s disease and in the non-demented elderly. Neurodegeneration : a journal for neurodegenerative disorders, neuroprotection, and neuroregeneration. 1995;4:291–297. [DOI] [PubMed] [Google Scholar]
- 140.Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-β peptide clearance. Science translational medicine. 2011;3:89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mahley RW, Huang Y. Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76:871–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kanekiyo T, Xu H, Bu G. ApoE and Abeta in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81(4):740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Barber RC. The genetics of Alzheimer’s disease. Scientifica. 2012;2012:246210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kosik KS. Alzheimer’s disease: a cell biological perspective. Science (New York, NY). 1992;256:780–783. [DOI] [PubMed] [Google Scholar]
- 145.Lee S-F, Shah S, Li H, Yu C, Han W, Yu G. Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-beta precursor protein and Notch. The Journal of biological chemistry. 2002;277:45013–45019. [DOI] [PubMed] [Google Scholar]
- 146.Stone RD, Irvine AR, O’Connor GR. Candida endophthalmitis: report of an unusual case with isolation of the etiologic agent by vitreous biopsy. Annals of ophthalmology. 1975;7:757–762. [PubMed] [Google Scholar]
- 147.Takashima A, Murayama M, Murayama O, et al. Presenilin 1 associates with glycogen synthase kinase-3beta and its substrate tau. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9637–9641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang Z, Hartmann H, Do VM, et al. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. [DOI] [PubMed] [Google Scholar]
- 149.Fujikake N, Shin M, Shimizu S. Association Between Autophagy and Neurodegenerative Diseases. Front Neurosci. 2018;12:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zheng H, Cheng B, Li Y, Li X, Chen X, Zhang YW. TREM2 in Alzheimer’s Disease: Microglial Survival and Energy Metabolism. Front Aging Neurosci. 2018;10:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Carmona S, Zahs K, Wu E, Dakin K, Bras J, Guerreiro R. The role of TREM2 in Alzheimer’s disease and other neurodegenerative disorders. Lancet Neurol. 2018;17(8):721–730. [DOI] [PubMed] [Google Scholar]
- 152.Herold C, Hooli BV, Mullin K, et al. Family-based association analyses of imputed genotypes reveal genome-wide significant association of Alzheimer’s disease with OSBPL6, PTPRG, and PDCL3. Mol Psychiatry. 2016;21(11):1608–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jansen IE, Savage JE, Watanabe K, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet. 2019;51(3):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shen L, Jia J. An Overview of Genome-Wide Association Studies in Alzheimer’s Disease. Neurosci Bull 2016;32(2):183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bertram L, Tanzi RE. Genome-wide association studies in Alzheimer’s disease. Hum Mol Genet. 2009;18(R2):R137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kamboh MI, Demirci FY, Wang X, et al. Genome-wide association study of Alzheimer’s disease. Transl Psychiatry. 2012;2:e117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cuyvers E, Sleegers K. Genetic variations underlying Alzheimer’s disease: evidence from genome-wide association studies and beyond. Lancet Neurol. 2016;15(8):857–868. [DOI] [PubMed] [Google Scholar]
- 158.Kumar A, Yegla B, Foster TC. Redox Signaling in Neurotransmission and Cognition During Aging. Antioxid Redox Signal. 2018;28(18):1724–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nobili A, Latagliata EC, Viscomi MT, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun. 2017;8:14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li Y, Sun H, Chen Z, Xu H, Bu G, Zheng H. Implications of GABAergic Neurotransmission in Alzheimer’s Disease. Front Aging Neurosci. 2016;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron. 2019;102(1):75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li Y, Sun H, Chen Z, Xu H, Bu G, Zheng H. Implications of GABAergic Neurotransmission in Alzheimer’s Disease. Frontiers in aging neuroscience. 2016;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee RK, Wurtman RJ, Cox AJ, Nitsch RM. Amyloid precursor protein processing is stimulated by metabotropic glutamate receptors. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8083–8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science (New York, NY). 1992;258:304–307. [DOI] [PubMed] [Google Scholar]
- 165.Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nature reviews Neuroscience. 2011;12:73–87. [DOI] [PubMed] [Google Scholar]
- 166.Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science (New York, NY). 1982;217:408–414. [DOI] [PubMed] [Google Scholar]
- 167.Kelly JF, Furukawa K, Barger SW, et al. Amyloid beta-peptide disrupts carbachol-induced muscarinic cholinergic signal transduction in cortical neurons. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:6753–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tzavara ET, Bymaster FP, Felder CC, et al. Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Molecular psychiatry. 2003;8:673–679. [DOI] [PubMed] [Google Scholar]
- 169.Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rubinsztein DC, DiFiglia M, Heintz N, et al. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1(1):11–22. [DOI] [PubMed] [Google Scholar]
- 171.Thathiah A, De Strooper B. G protein-coupled receptors, cholinergic dysfunction, and Abeta toxicity in Alzheimer’s disease. Science signaling. 2009;2:re8. [DOI] [PubMed] [Google Scholar]
- 172.Perry EK, Gibson PH, Blessed G, Perry RH, Tomlinson BE. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. Journal of the neurological sciences. 1977;34:247–265. [DOI] [PubMed] [Google Scholar]
- 173.Benzi G, Moretti A. Is there a rationale for the use of acetylcholinesterase inhibitors in the therapy of Alzheimer’s disease? European journal of pharmacology. 1998;346:1–13. [DOI] [PubMed] [Google Scholar]
- 174.Bodick NC, Offen WW, Levey AI, et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Archives of neurology. 1997;54:465–473. [DOI] [PubMed] [Google Scholar]
- 175.Nitsch RM, Deng M, Tennis M, Schoenfeld D, Growdon JH. The selective muscarinic M1 agonist AF102B decreases levels of total Abeta in cerebrospinal fluid of patients with Alzheimer’s disease. Annals of neurology. 2000;48:913–918. [PubMed] [Google Scholar]
- 176.Parsons CG, Stöffler A, Danysz W. Memantine: a NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropharmacology. 2007;53:699–723. [DOI] [PubMed] [Google Scholar]
- 177.Rammes G, Rupprecht R, Ferrari U, Zieglgänsberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neuroscience letters. 2001;306:81–84. [DOI] [PubMed] [Google Scholar]
- 178.Aracava Y, Pereira EFR, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. The Journal of pharmacology and experimental therapeutics. 2005;312:1195–1205. [DOI] [PubMed] [Google Scholar]
- 179.Seeman P, Caruso C, Lasaga M. Memantine agonist action at dopamine D2High receptors. Synapse (New York, NY). 2008;62:149–153. [DOI] [PubMed] [Google Scholar]
- 180.Peeters M, Romieu P, Maurice T, Su T-P, Maloteaux J-M, Hermans E. Involvement of the sigma 1 receptor in the modulation of dopaminergic transmission by amantadine. The European journal of neuroscience. 2004;19:2212–2220. [DOI] [PubMed] [Google Scholar]
- 181.Scarpini E, Scheltens P, Feldman H. Treatment of Alzheimer’s disease: current status and new perspectives. The Lancet Neurology. 2003;2:539–547. [DOI] [PubMed] [Google Scholar]
- 182.Giacobini E From molecular structure to Alzheimer therapy. Japanese journal of pharmacology. 1997;74:225–241. [DOI] [PubMed] [Google Scholar]
- 183.Jann MW. Preclinical pharmacology of metrifonate. Pharmacotherapy. 1998;18(2 (Pt 2)):55–67; discussion 79–82. [PubMed] [Google Scholar]
- 184.Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochimica et biophysica acta. 2010;1802:2–10. [DOI] [PubMed] [Google Scholar]
- 185.Dautremepuits C, Betoulle S, Vernet G. Antioxidant response modulated by copper in healthy or parasitized carp (Cyprinus carpio L.) by Ptychobothrium sp. (Cestoda). Biochimica et biophysica acta. 2002;1573:4–8. [DOI] [PubMed] [Google Scholar]
- 186.Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Weidling I, Swerdlow RH. Mitochondrial Dysfunction and Stress Responses in Alzheimer’s Disease. Biology (Basel). 2019;8(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Area-Gomez E, de Groof A, Bonilla E, et al. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018;9(3):335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Godoy JA, Rios JA, Zolezzi JM, Braidy N, Inestrosa NC. Signaling pathway cross talk in Alzheimer’s disease. Cell communication and signaling : CCS. 2014;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Caspersen C, Wang N, Yao J, et al. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:2040–2041. [DOI] [PubMed] [Google Scholar]
- 191.Castellani R, Hirai K, Aliev G, et al. Role of mitochondrial dysfunction in Alzheimer’s disease. Journal of neuroscience research. 2002;70:357–360. [DOI] [PubMed] [Google Scholar]
- 192.Chen JX, Yan SS. Role of mitochondrial amyloid-beta in Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2010;20Suppl 2:S569–578. [DOI] [PubMed] [Google Scholar]
- 193.Hutchin TP, Heath PR, Pearson RC, Sinclair AJ. Mitochondrial DNA mutations in Alzheimer’s disease. Biochemical and biophysical research communications. 1997;241:221–225. [DOI] [PubMed] [Google Scholar]
- 194.Kumar A, Singh A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Frontiers in pharmacology. 2015;6:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Manczak M, Anekonda TS, Henson E, Park BS, Quinn J, Reddy PH. Mitochondria are a direct site of A beta accumulation in Alzheimer’s disease neurons: implications for free radical generation and oxidative damage in disease progression. Human molecular genetics. 2006;15:1437–1449. [DOI] [PubMed] [Google Scholar]
- 196.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Human molecular genetics. 2011;20:2495–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Parker WD, Parks J, Filley CM, Kleinschmidt-DeMasters BK. Electron transport chain defects in Alzheimer’s disease brain. Neurology. 1994;44:1090–1096. [DOI] [PubMed] [Google Scholar]
- 198.Salminen A, Haapasalo A, Kauppinen A, Kaarniranta K, Soininen H, Hiltunen M. Impaired mitochondrial energy metabolism in Alzheimer’s disease: Impact on pathogenesis via disturbed epigenetic regulation of chromatin landscape. Progress in neurobiology. 2015;131:1–20. [DOI] [PubMed] [Google Scholar]
- 199.Selfridge JE, E L, Lu J, Swerdlow RH. Role of mitochondrial homeostasis and dynamics in Alzheimer’s disease. Neurobiology of disease. 2013;51:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yao J, Irwin RW, Zhao L, Nilsen J, Hamilton RT, Brinton RD. Mitochondrial bioenergetic deficit precedes Alzheimer’s pathology in female mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Smith MA, Drew KL, Nunomura A, et al. Amyloid-beta, tau alterations and mitochondrial dysfunction in Alzheimer disease: the chickens or the eggs? Neurochemistry international. 2002;40:527–531. [DOI] [PubMed] [Google Scholar]
- 202.Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural regeneration research. 2013;8:2003–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cheng J, North BJ, Zhang T, et al. The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging cell. 2018;17:e12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang X, Su B, Fujioka H, Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. The American journal of pathology. 2008;173:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nixon RA, Wegiel J, Kumar A, et al. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. Journal of neuropathology and experimental neurology. 2005;64:113–122. [DOI] [PubMed] [Google Scholar]
- 207.Nixon RA, Yang D-S. Autophagy failure in Alzheimer’s disease--locating the primary defect. Neurobiology of disease. 2011;43:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang X, Su B, Siedlak SL, et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19318–19323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Garcia-Escudero V, Martin-Maestro P, Perry G, Avila J. Deconstructing mitochondrial dysfunction in Alzheimer disease. Oxid Med Cell Longev. 2013;2013:162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: a novel neuronal trigger for inflammation and Alzheimer’s pathology. Journal of neuroinflammation. 2009;6:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hoozemans JJM, van Haastert ES, Nijholt DAT, Rozemuller AJM, Eikelenboom P, Scheper W. The unfolded protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. The American journal of pathology. 2009;174:1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Plácido AI, Pereira CMF, Duarte AI, et al. The role of endoplasmic reticulum in amyloid precursor protein processing and trafficking: implications for Alzheimer’s disease. Biochimica et biophysica acta. 2014;1842:1444–1453. [DOI] [PubMed] [Google Scholar]
- 213.Zheng Z, Shang Y, Tao J, Zhang J, Sha B. Endoplasmic Reticulum Stress Signaling Pathways: Activation and Diseases. Curr Protein Pept Sci. 2019. [DOI] [PubMed] [Google Scholar]
- 214.Endres K, Reinhardt S. ER-stress in Alzheimer’s disease: turning the scale? American journal of neurodegenerative disease. 2013;2:247–265. [PMC free article] [PubMed] [Google Scholar]
- 215.Ho Y-S, Yang X, Lau JC-F, et al. Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: implication in Alzheimer’s disease pathogenesis. Journal of Alzheimer’s disease : JAD. 2012;28:839–854. [DOI] [PubMed] [Google Scholar]
- 216.Li J-Q, Yu J-T, Jiang T, Tan L. Endoplasmic reticulum dysfunction in Alzheimer’s disease. Molecular neurobiology. 2015;51:383–395. [DOI] [PubMed] [Google Scholar]
- 217.Volgyi K, Juhász G, Kovacs Z, Penke B. Dysfunction of Endoplasmic Reticulum (ER) and Mitochondria (MT) in Alzheimer’s Disease: The Role of the ER-MT Cross-Talk. Current Alzheimer research. 2015;12:655–672. [DOI] [PubMed] [Google Scholar]
- 218.Abisambra JF, Jinwal UK, Blair LJ, et al. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci. 2013;33(22):9498–9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Murakami T, Hino S-I, Saito A, Imaizumi K. Endoplasmic reticulum stress response in dendrites of cultured primary neurons. Neuroscience. 2007;146:1–8. [DOI] [PubMed] [Google Scholar]
- 220.Nijholt DA, de Graaf TR, van Haastert ES, et al. Endoplasmic reticulum stress activates autophagy but not the proteasome in neuronal cells: implications for Alzheimer’s disease. Cell Death Differ. 2011;18(6):1071–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12(2):225–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nature reviews Drug discovery. 2004;3:205–214. [DOI] [PubMed] [Google Scholar]
- 223.Sayre LM, Moreira PI, Smith MA, Perry G. Metal ions and oxidative protein modification in neurological disease. Annali dell’Istituto superiore di sanita. 2005;41:143–164. [PubMed] [Google Scholar]
- 224.Sayre LM, Perry G, Smith MA. Oxidative stress and neurotoxicity. Chemical research in toxicology. 2008;21:172–188. [DOI] [PubMed] [Google Scholar]
- 225.Singh A, Kukreti R, Saso L, Kukreti S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules. 2019;24(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gella A, Durany N. Oxidative stress in Alzheimer disease. Cell adhesion & migration. 2009;3:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Abd El Mohsen MM, Iravani MM, Spencer JPE, et al. Age-associated changes in protein oxidation and proteasome activities in rat brain: modulation by antioxidants. Biochemical and biophysical research communications. 2005;336:386–391. [DOI] [PubMed] [Google Scholar]
- 228.Cini M, Moretti A. Studies on lipid peroxidation and protein oxidation in the aging brain. Neurobiology of aging. 1995;16(1):53–57. [DOI] [PubMed] [Google Scholar]
- 229.Gupta A, Hasan M, Chander R, Kapoor NK. Age-related elevation of lipid peroxidation products: diminution of superoxide dismutase activity in the central nervous system of rats. Gerontology. 1991;37:305–309. [DOI] [PubMed] [Google Scholar]
- 230.Hamilton ML, Van Remmen H, Drake JA, et al. Does oxidative damage to DNA increase with age? Proceedings of the National Academy of Sciences of the United States of America. 2001;98:10469–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Mecocci P, Beal MF, Cecchetti R, et al. Mitochondrial membrane fluidity and oxidative damage to mitochondrial DNA in aged and AD human brain. Molecular and chemical neuropathology. 1997;31:53–64. [DOI] [PubMed] [Google Scholar]
- 232.Perry G, Cash AD, Smith MA. Alzheimer Disease and Oxidative Stress. Journal of biomedicine & biotechnology. 2002;2:120–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Perrig WJ, Perrig P, Stähelin HB. The relation between antioxidants and memory performance in the old and very old. Journal of the American Geriatrics Society. 1997;45:718–724. [DOI] [PubMed] [Google Scholar]
- 234.Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology. 1995;45:1594–1601. [DOI] [PubMed] [Google Scholar]
- 235.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. Journal of neurochemistry. 1997;68:2092–2097. [DOI] [PubMed] [Google Scholar]
- 236.Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow E-M. Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. The Journal of cell biology. 2002;156:1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Smith CD, Carney JM, Starke-Reed PE, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:10540–10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Smith MA, Taneda S, Richey PL, et al. Advanced Maillard reaction end products are associated with Alzheimer disease pathology. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:5710–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Thome J, Kornhuber J, Münch G, et al. [New hypothesis on etiopathogenesis of Alzheimer syndrome. Advanced glycation end products (AGEs)]. Der Nervenarzt. 1996;67:924–929. [DOI] [PubMed] [Google Scholar]
- 240.Lovell MA, Markesbery WR. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic acids research. 2007;35:7497–7504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Duran-Aniotz C, Martínez G, Hetz C. Memory loss in Alzheimer’s disease: are the alterations in the UPR network involved in the cognitive impairment? Frontiers in aging neuroscience. 2014;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shefa U, Jeong NY, Song IO, et al. Mitophagy links oxidative stress conditions and neurodegenerative diseases. Neural Regen Res. 2019;14(5):749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Calsolaro V, Edison P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2016;12:719–732. [DOI] [PubMed] [Google Scholar]
- 244.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement (N Y). 2018;4:575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Shal B, Ding W, Ali H, Kim YS, Khan S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front Pharmacol. 2018;9:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lorton D, Schaller J, Lala A, De Nardin E. Chemotactic-like receptors and Abeta peptide induced responses in Alzheimer’s disease. Neurobiology of aging. 2000;21(3):463–473. [DOI] [PubMed] [Google Scholar]
- 247.Meraz-Ríos MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernández J, Campos-Peña V. Inflammatory process in Alzheimer’s Disease. Frontiers in integrative neuroscience. 2013;7:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Subramaniyan S, Terrando N. Neuroinflammation and Perioperative Neurocognitive Disorders. Anesth Analg. 2019;128(4):781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Duong T, Nikolaeva M, Acton PJ. C-reactive protein-like immunoreactivity in the neurofibrillary tangles of Alzheimer’s disease. Brain research. 1997;749:152–156. [DOI] [PubMed] [Google Scholar]
- 250.van der Wal EA, Gómez-Pinilla F, Cotman CW. Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport. 1993;4:69–72. [DOI] [PubMed] [Google Scholar]
- 251.Griffin WST. Neuroinflammatory cytokine signaling and Alzheimer’s disease. The New England journal of medicine. 2013;368:770–771. [DOI] [PubMed] [Google Scholar]
- 252.Fassbender K, Walter S, Kühl S, et al. The LPS receptor (CD14) links innate immunity with Alzheimer’s disease. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:203–205. [DOI] [PubMed] [Google Scholar]
- 253.Tahara K, Kim H-D, Jin J-J, Maxwell JA, Li L, Fukuchi K-i. Role of toll-like receptor signalling in Abeta uptake and clearance. Brain : a journal of neurology. 2006;129:3006–3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. [DOI] [PubMed] [Google Scholar]
- 255.Eikelenboom P, Bate C, Van Gool WA, et al. Neuroinflammation in Alzheimer’s disease and prion disease. Glia. 2002;40:232–239. [DOI] [PubMed] [Google Scholar]
- 256.Pachter JS. Inflammatory mechanisms in Alzheimer disease: the role of beta-amyloid/glial interactions. Molecular psychiatry. 1997;2:91–95. [DOI] [PubMed] [Google Scholar]
- 257.Lautrup S, Lou G, Aman Y, Nilsen H, Tao J, Fang EF. Microglial mitophagy mitigates neuroinflammation in Alzheimer’s disease. Neurochem Int. 2019;129:104469. [DOI] [PubMed] [Google Scholar]
- 258.Antón Álvarez X, Fuentes P. Cerebrolysin in Alzheimer’s disease. Drugs of today (Barcelona, Spain : 1998). 2011;47:487–513. [DOI] [PubMed] [Google Scholar]
- 259.Canevelli M, Adali N, Kelaiditi E, et al. Effects of Gingko biloba supplementation in Alzheimer’s disease patients receiving cholinesterase inhibitors: data from the ICTUS study. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2014;21:888–892. [DOI] [PubMed] [Google Scholar]
- 260.Lu C, Guo Y, Li J, et al. Design, synthesis, and evaluation of resveratrol derivatives as Aß1–42 aggregation inhibitors, antioxidants, and neuroprotective agents. Bioorganic & Medicinal Chemistry Letters. 2012;22:7683–7687. [DOI] [PubMed] [Google Scholar]
- 261.Vlad SC, Miller DR, Kowall NW, Felson DT. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yiannopoulou KG, Papageorgiou SG. Current and future treatments for Alzheimer’s disease. Therapeutic advances in neurological disorders. 2013;6:19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ciechanover A, Kwon YT. Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Experimental & molecular medicine. 2015;47:e147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jang HH. Regulation of Protein Degradation by Proteasomes in Cancer. J Cancer Prev. 2018;23(4):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wang J, Maldonado MA. The ubiquitin-proteasome system and its role in inflammatory and autoimmune diseases. Cellular & molecular immunology. 2006;3:255–261. [PubMed] [Google Scholar]
- 266.Cao J, Zhong MB, Toro CA, Zhang L, Cai D. Endo-lysosomal pathway and ubiquitin-proteasome system dysfunction in Alzheimer’s disease pathogenesis. Neurosci Lett. 2019;703:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Wang J, Maldonado MA. The ubiquitin-proteasome system and its role in inflammatory and autoimmune diseases. Cellular & molecular immunology. 2006;3:255–261. [PubMed] [Google Scholar]
- 268.Koulich E, Li X, DeMartino GN. Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Molecular biology of the cell. 2008;19:1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Upadhya SC, Hegde AN. Role of the ubiquitin proteasome system in Alzheimer’s disease. BMC biochemistry. 2007;8Suppl 1:S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. [DOI] [PubMed] [Google Scholar]
- 271.Dal Vechio FH, Cerqueira F, Augusto O, Lopes R, Demasi M. Peptides that activate the 20S proteasome by gate opening increased oxidized protein removal and reduced protein aggregation. Free radical biology & medicine. 2014;67:304–313. [DOI] [PubMed] [Google Scholar]
- 272.Gregori L, Hainfeld JF, Simon MN, Goldgaber D. Binding of amyloid beta protein to the 20 S proteasome. The Journal of biological chemistry. 1997;272:58–62. [DOI] [PubMed] [Google Scholar]
- 273.Tseng BP, Green KN, Chan JL, Blurton-Jones M, LaFerla FM. Abeta inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiology of aging. 2008;29:1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Fergusson J, Landon M, Lowe J, Ward L, van Leeuwen FW, Mayer RJ. Neurofibrillary tangles in progressive supranuclear palsy brains exhibit immunoreactivity to frameshift mutant ubiquitin-B protein. Neuroscience letters. 2000;279:69–72. [DOI] [PubMed] [Google Scholar]
- 275.Mori H, Kondo J, Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer’s disease. Science (New York, NY). 1987;235:1641–1644. [DOI] [PubMed] [Google Scholar]
- 276.Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. The Journal of cell biology. 1998;143:1883–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hol EM, van Leeuwen FW, Fischer DF. The proteasome in Alzheimer’s disease and Parkinson’s disease: lessons from ubiquitin B+1. Trends in molecular medicine. 2005;11:488–495. [DOI] [PubMed] [Google Scholar]
- 278.Tramutola A, Triani F, Di Domenico F, et al. Poly-ubiquitin profile in Alzheimer disease brain. Neurobiol Dis. 2018;118:129–141. [DOI] [PubMed] [Google Scholar]
- 279.Cecarini V, Bonfili L, Cuccioloni M, et al. Crosstalk between the ubiquitin-proteasome system and autophagy in a human cellular model of Alzheimer’s disease. Biochimica et biophysica acta. 2012;1822:1741–1751. [DOI] [PubMed] [Google Scholar]
- 280.Keller JN, Hanni KB, Markesbery WR. Impaired proteasome function in Alzheimer’s disease. Journal of neurochemistry. 2000;75:436–439. [DOI] [PubMed] [Google Scholar]
- 281.Costes S, Gurlo T, Rivera JF, Butler PC. UCHL1 deficiency exacerbates human islet amyloid polypeptide toxicity in beta-cells: evidence of interplay between the ubiquitin/proteasome system and autophagy. Autophagy. 2014;10(6):1004–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Cai Z, Yan L-J. Rapamycin, Autophagy, and Alzheimer’s Disease. Journal of biochemical and pharmacological research. 2013;1:84–90. [PMC free article] [PubMed] [Google Scholar]
- 283.Yang D-S, Stavrides P, Mohan PS, et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain : a journal of neurology. 2011;134:258–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Eskelinen E-L, Saftig P. Autophagy: a lysosomal degradation pathway with a central role in health and disease. Biochimica et biophysica acta. 2009;1793:664–673. [DOI] [PubMed] [Google Scholar]
- 285.Orr ME, Oddo S. Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimer’s research & therapy. 2013;5:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Rubinsztein DC, DiFiglia M, Heintz N, et al. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. [DOI] [PubMed] [Google Scholar]
- 287.Ling D, Magallanes M, Salvaterra PM. Accumulation of amyloid-like Aβ1–42 in AEL (autophagy-endosomal-lysosomal) vesicles: potential implications for plaque biogenesis. ASN neuro. 2014;6:AN20130044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nixon RA, Cataldo AM, Mathews PM. The endosomal-lysosomal system of neurons in Alzheimer’s disease pathogenesis: a review. Neurochemical research. 2000;25:1161–1172. [DOI] [PubMed] [Google Scholar]
- 289.Peric A, Annaert W. Early etiology of Alzheimer’s disease: tipping the balance toward autophagy or endosomal dysfunction? Acta neuropathologica. 2015;129:363–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Cai Z, Chen G, He W, Xiao M, Yan L-J. Activation of mTOR: a culprit of Alzheimer’s disease? Neuropsychiatric disease and treatment. 2015;11:1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Camboni M, Wang C-M, Miranda C, et al. Active and passive immunization strategies based on the SDPM1 peptide demonstrate pre-clinical efficacy in the APPswePSEN1dE9 mouse model for Alzheimer’s disease. Neurobiology of disease. 2014;62:31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tramutola A, Triplett JC, Di Domenico F, et al. Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem. 2015;133(5):739–749. [DOI] [PubMed] [Google Scholar]
- 293.Griffin RJ, Moloney A, Kelliher M, et al. Activation of Akt/PKB, increased phosphorylation of Akt substrates and loss and altered distribution of Akt and PTEN are features of Alzheimer’s disease pathology. J Neurochem. 2005;93(1):105–117. [DOI] [PubMed] [Google Scholar]
- 294.Cai Z, Yan L-J, Li K, Quazi SH, Zhao B. Roles of AMP-activated protein kinase in Alzheimer’s disease. Neuromolecular medicine. 2012;14:1–14. [DOI] [PubMed] [Google Scholar]
- 295.Hara Y, McKeehan N, Fillit HM. Translating the biology of aging into novel therapeutics for Alzheimer disease. Neurology. 2019;92(2):84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bobori C, Theocharopoulou G, Vlamos P. Molecular Chaperones in Neurodegenerative Diseases: A Short Review. Advances in experimental medicine and biology. 2017;987:219–231. [DOI] [PubMed] [Google Scholar]
- 297.Gandhi J, Antonelli AC, Afridi A, et al. Protein misfolding and aggregation in neurodegenerative diseases: a review of pathogeneses, novel detection strategies, and potential therapeutics. Rev Neurosci. 2019;30(4):339–358. [DOI] [PubMed] [Google Scholar]
- 298.Garcia-Huerta P, Bargsted L, Rivas A, Matus S, Vidal RL. ER chaperones in neurodegenerative disease: Folding and beyond. Brain research. 2016;1648:580–587. [DOI] [PubMed] [Google Scholar]
- 299.Maiti P, Manna J, Veleri S, Frautschy S. Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. BioMed research international. 2014;2014:495091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Sweeney P, Park H, Baumann M, et al. Protein misfolding in neurodegenerative diseases: implications and strategies. Translational neurodegeneration. 2017;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Dou F, Netzer WJ, Tanemura K, et al. Chaperones increase association of tau protein with microtubules. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cater JH, Kumita JR, Zeineddine Abdallah R, et al. Human pregnancy zone protein stabilizes misfolded proteins including preeclampsia- and Alzheimer’s-associated amyloid beta peptide. Proc Natl Acad Sci U S A. 2019;116(13):6101–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Scheidt T, Lapinska U, Kumita JR, et al. Secondary nucleation and elongation occur at different sites on Alzheimer’s amyloid-beta aggregates. Sci Adv. 2019;5(4):eaau3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Bedse G, Di Domenico F, Serviddio G, Cassano T. Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Frontiers in neuroscience. 2015;9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Pardeshi R, Bolshette N, Gadhave K, et al. Insulin signaling: An opportunistic target to minify the risk of Alzheimer’s disease. Psychoneuroendocrinology. 2017;83:159–171. [DOI] [PubMed] [Google Scholar]
- 306.Candeias E, Duarte AI, Carvalho C, et al. The impairment of insulin signaling in Alzheimer’s disease. IUBMB life. 2012;64:951–957. [DOI] [PubMed] [Google Scholar]
- 307.De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer’s disease? Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014;10:S26–32. [DOI] [PubMed] [Google Scholar]
- 308.Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. Journal of aging research. 2012;2012:384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiology of aging. 2010;31:224–243. [DOI] [PubMed] [Google Scholar]
- 310.Ferreira LSS, Fernandes CS, Vieira MNN, De Felice FG. Insulin Resistance in Alzheimer’s Disease. Front Neurosci. 2018;12:830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Frazier HN, Ghoweri AO, Anderson KL, Lin RL, Porter NM, Thibault O. Broadening the definition of brain insulin resistance in aging and Alzheimer’s disease. Exp Neurol. 2019;313:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Caberlotto L, Nguyen TP, Lauria M, et al. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci Rep. 2019;9(1):3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Folch J, Ettcheto M, Busquets O, et al. The Implication of the Brain Insulin Receptor in Late Onset Alzheimer’s Disease Dementia. Pharmaceuticals (Basel, Switzerland). 2018;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Di Guglielmo GM, Drake PG, Baass PC, Authier F, Posner BI, Bergeron JJ. Insulin receptor internalization and signalling. Molecular and cellular biochemistry. 1998;182:59–63. [PubMed] [Google Scholar]
- 315.Liao F-F, Xu H. Insulin signaling in sporadic Alzheimer’s disease. Science signaling. 2009;2:p e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zhao W-Q, Lacor PN, Chen H, et al. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric a{beta}. The Journal of biological chemistry. 2009;284:18742–18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. [DOI] [PubMed] [Google Scholar]
- 318.Bomfim TR, Forny-Germano L, Sathler LB, et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer’s disease- associated Aβ oligomers. The Journal of clinical investigation. 2012;122:1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yildirim Simsir I, Soyaltin UE, Cetinkalp S. Glucagon like peptide-1 (GLP-1) likes Alzheimer’s disease. Diabetes & metabolic syndrome. 2018;12:469–475. [DOI] [PubMed] [Google Scholar]
- 320.Corbett A, Pickett J, Burns A, et al. Drug repositioning for Alzheimer’s disease. Nature reviews Drug discovery. 2012;11:833–846. [DOI] [PubMed] [Google Scholar]
- 321.Wadman M US government sets out Alzheimer’s plan. Nature. 2012;485:426–427. [DOI] [PubMed] [Google Scholar]
- 322.Barone E, Di Domenico F, Cassano T, et al. Impairment of biliverdin reductase-A promotes brain insulin resistance in Alzheimer disease: A new paradigm. Free Radic Biol Med. 2016;91:127–142. [DOI] [PubMed] [Google Scholar]
- 323.Barone E, Tramutola A, Triani F, et al. Biliverdin Reductase-A Mediates the Beneficial Effects of Intranasal Insulin in Alzheimer Disease. Mol Neurobiol. 2019;56(4):2922–2943. [DOI] [PubMed] [Google Scholar]
- 324.Pardeshi R, Bolshette N, Gadhave K, et al. Docosahexaenoic Acid Increases the Potency of Soluble Epoxide Hydrolase Inhibitor in Alleviating Streptozotocin-Induced Alzheimer’s Disease-Like Complications of Diabetes. Front Pharmacol. 2019;10:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer’s Disease. Experimental gerontology. 2007;42:10–21. [DOI] [PubMed] [Google Scholar]
- 326.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2005;7:45–61. [DOI] [PubMed] [Google Scholar]
- 327.Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong C-X. Deficient brain insulin signalling pathway in Alzheimer’s disease and diabetes. The Journal of pathology. 2011;225:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Liu F, Shi J, Tanimukai H, et al. Reduced O-GlcNAcylation links lower brain glucose metabolism and tau pathology in Alzheimer’s disease. Brain : a journal of neurology. 2009;132:1820–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Messier C, Gagnon M. Glucose regulation and cognitive functions: relation to Alzheimer’s disease and diabetes. Behavioural brain research. 1996;75:1–11. [DOI] [PubMed] [Google Scholar]
- 330.Sato N, Morishita R. The roles of lipid and glucose metabolism in modulation of β-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Frontiers in aging neuroscience. 2015;7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Condello M, Pellegrini E, Caraglia M, Meschini S. Targeting Autophagy to Overcome Human Diseases. Int J Mol Sci. 2019;20(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Suzuki R, Lee K, Jing E, et al. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell metabolism. 2010;12:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ghribi O Potential mechanisms linking cholesterol to Alzheimer’s disease-like pathology in rabbit brain, hippocampal organotypic slices, and skeletal muscle. Journal of Alzheimer’s disease : JAD. 2008;15:673–684. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 334.Evans RM, Emsley CL, Gao S, et al. Serum cholesterol, APOE genotype, and the risk of Alzheimer’s disease: a population-based study of African Americans. Neurology. 2000;54:240–242. [DOI] [PubMed] [Google Scholar]
- 335.Hall K, Murrell J, Ogunniyi A, et al. Cholesterol, APOE genotype, and Alzheimer disease: an epidemiologic study of Nigerian Yoruba. Neurology. 2006;66:223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Zarrouk A, Debbabi M, Bezine M, et al. Lipid Biomarkers in Alzheimer’s Disease. Curr Alzheimer Res. 2018;15(4):303–312. [DOI] [PubMed] [Google Scholar]
- 337.Kivipelto M, Helkala EL, Laakso MP, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ (Clinical research ed). 2001;322:1447–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Kuo YM, Emmerling MR, Bisgaier CL, et al. Elevated low-density lipoprotein in Alzheimer’s disease correlates with brain abeta 1–42 levels. Biochemical and biophysical research communications. 1998;252:711–715. [DOI] [PubMed] [Google Scholar]
- 339.Xue-Shan Z, Juan P, Qi W, et al. Imbalanced cholesterol metabolism in Alzheimer’s disease. Clinica chimica acta; international journal of clinical chemistry. 2016;456:107–114. [DOI] [PubMed] [Google Scholar]
- 340.Ulery PG, Beers J, Mikhailenko I, et al. Modulation of beta-amyloid precursor protein processing by the low density lipoprotein receptor-related protein (LRP). Evidence that LRP contributes to the pathogenesis of Alzheimer’s disease. The Journal of biological chemistry. 2000;275:7410–7415. [DOI] [PubMed] [Google Scholar]
- 341.LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. The Journal of biological chemistry. 1994;269:23403–23406. [PubMed] [Google Scholar]
- 342.Van Uden E, Sagara Y, Van Uden J, et al. A protective role of the low density lipoprotein receptor-related protein against amyloid beta-protein toxicity. The Journal of biological chemistry. 2000;275:30525–30530. [DOI] [PubMed] [Google Scholar]
- 343.Habchi J, Chia S, Galvagnion C, et al. Cholesterol catalyses Aβ42 aggregation through a heterogeneous nucleation pathway in the presence of lipid membranes. Nature chemistry. 2018;10:673–683. [DOI] [PubMed] [Google Scholar]
- 344.Cho YY, Kwon OH, Park MK, Kim TW, Chung S. Elevated cellular cholesterol in Familial Alzheimer’s presenilin 1 mutation is associated with lipid raft localization of beta-amyloid precursor protein. PLoS One. 2019;14(1):e0210535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Jin P, Pan Y, Pan Z, et al. Alzheimer-like brain metabolic and structural features in cholesterol-fed rabbit detected by magnetic resonance imaging. Lipids Health Dis. 2018;17(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Sanan DA, Weisgraber KH, Russell SJ, et al. Apolipoprotein E associates with beta amyloid peptide of Alzheimer’s disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. The Journal of clinical investigation. 1994;94:860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Costa AC, Joaquim HPG, Nunes VS, et al. Donepezil effects on cholesterol and oxysterol plasma levels of Alzheimer’s disease patients. Eur Arch Psychiatry Clin Neurosci. 2018;268(5):501–507. [DOI] [PubMed] [Google Scholar]
- 348.Barbero-Camps E, Roca-Agujetas V, Bartolessis I, et al. Cholesterol impairs autophagy-mediated clearance of amyloid beta while promoting its secretion. Autophagy. 2018;14(7):1129–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Buxbaum JD, Geoghagen NSM, Friedhoff LT. Cholesterol depletion with physiological concentrations of a statin decreases the formation of the Alzheimer amyloid Abeta peptide. Journal of Alzheimer’s disease : JAD. 2001;3:221–229. [DOI] [PubMed] [Google Scholar]
- 350.Cole SL, Grudzien A, Manhart IO, Kelly BL, Oakley H, Vassar R. Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. The Journal of biological chemistry. 2005;280:18755–18770. [DOI] [PubMed] [Google Scholar]
- 351.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. The Journal of cell biology. 2003;160:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Fassbender K, Simons M, Bergmann C, et al. Simvastatin strongly reduces levels of Alzheimer’s disease beta -amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM. The role of cholesterol in the biosynthesis of beta-amyloid. Neuroreport. 1999;10:1699–1705. [DOI] [PubMed] [Google Scholar]
- 354.Kojro E, Gimpl G, Lammich S, Marz W, Fahrenholz F. Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the alpha -secretase ADAM 10. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Camandola S, Mattson MP. Aberrant subcellular neuronal calcium regulation in aging and Alzheimer’s disease. Biochimica et biophysica acta. 2011;1813:965–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. [DOI] [PubMed] [Google Scholar]
- 358.Secondo A, Bagetta G, Amantea D. On the Role of Store-Operated Calcium Entry in Acute and Chronic Neurodegenerative Diseases. Front Mol Neurosci. 2018;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Popugaeva E, Pchitskaya E, Bezprozvanny I. Dysregulation of Intracellular Calcium Signaling in Alzheimer’s Disease. Antioxid Redox Signal. 2018;29(12):1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB reports. 2009;42:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Cheung K-H, Shineman D, Müller M, et al. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Arbel-Ornath M, Hudry E, Boivin JR, et al. Soluble oligomeric amyloid-β induces calcium dyshomeostasis that precedes synapse loss in the living mouse brain. Molecular neurodegeneration. 2017;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Ranjan B, Chong KH, Zheng J. Composite mathematical modeling of calcium signaling behind neuronal cell death in Alzheimer’s disease. BMC Syst Biol. 2018;12(Suppl 1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Lerdkrai C, Asavapanumas N, Brawek B, et al. Intracellular Ca(2+) stores control in vivo neuronal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2018;115(6):E1279–E1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Lin H, Bhatia R, Lal R. Amyloid beta protein forms ion channels: implications for Alzheimer’s disease pathophysiology. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:2433–2444. [DOI] [PubMed] [Google Scholar]
- 366.Zadori D, Veres G, Szalardy L, Klivenyi P, Vecsei L. Alzheimer’s Disease: Recent Concepts on the Relation of Mitochondrial Disturbances, Excitotoxicity, Neuroinflammation, and Kynurenines. J Alzheimers Dis. 2018;62(2):523–547. [DOI] [PubMed] [Google Scholar]
- 367.Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochemistry international. 2004;45:583–595. [DOI] [PubMed] [Google Scholar]
- 368.Olloquequi J, Cornejo-Cordova E, Verdaguer E, et al. Excitotoxicity in the pathogenesis of neurological and psychiatric disorders: Therapeutic implications. J Psychopharmacol. 2018;32(3):265–275. [DOI] [PubMed] [Google Scholar]
- 369.Parameshwaran K, Dhanasekaran M, Suppiramaniam V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Experimental neurology. 2008;210:7–13. [DOI] [PubMed] [Google Scholar]
- 370.Zhang Y, Li P, Feng J, Wu M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2016;37:1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Kamat PK, Kalani A, Rai S, et al. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Molecular neurobiology. 2016;53:648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Saura CA, Valero J. The role of CREB signaling in Alzheimer’s disease and other cognitive disorders. Reviews in the Neurosciences. 2011;22. [DOI] [PubMed] [Google Scholar]
- 373.Madeira C, Vargas-Lopes C, Brandao CO, et al. Elevated Glutamate and Glutamine Levels in the Cerebrospinal Fluid of Patients With Probable Alzheimer’s Disease and Depression. Front Psychiatry. 2018;9:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Molinuevo JL, Lladó A, Rami L. Memantine: targeting glutamate excitotoxicity in Alzheimer’s disease and other dementias. American journal of Alzheimer’s disease and other dementias. 2005;20:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Esposito Z, Belli L, Toniolo S, Sancesario G, Bianconi C, Martorana A. Amyloid β, glutamate, excitotoxicity in Alzheimer’s disease: are we on the right track? CNS neuroscience & therapeutics. 2013;19:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Matsunaga S, Kishi T, Nomura I, et al. The efficacy and safety of memantine for the treatment of Alzheimer’s disease. Expert Opin Drug Saf. 2018;17(10):1053–1061. [DOI] [PubMed] [Google Scholar]
- 377.Doraiswamy PM. Alzheimer’s disease and the glutamate NMDA receptor. Psychopharmacology bulletin. 2003;37:41–49. [PubMed] [Google Scholar]
- 378.Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30(4):379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Choi J, Kwon HJ, Lee JE, Lee Y, Seoh JY, Han PL. Hyperoxygenation revitalizes Alzheimer’s disease pathology through the upregulation of neurotrophic factors. Aging Cell. 2019;18(2):e12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Budni J, Bellettini-Santos T, Mina F, Garcez ML, Zugno AI. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging and disease. 2015;6:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Sampaio TB, Savall AS, Gutierrez MEZ, Pinton S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: implications for pathogenesis and therapy. Neural regeneration research. 2017;12:549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Baydyuk M, Xu B. BDNF signaling and survival of striatal neurons. Front Cell Neurosci. 2014;8:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Mitra S, Behbahani H, Eriksdotter M. Innovative Therapy for Alzheimer’s Disease-With Focus on Biodelivery of NGF. Front Neurosci. 2019;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain research Molecular brain research. 1997;49:71–81. [DOI] [PubMed] [Google Scholar]
- 385.Zhang L, Fang Y, Lian Y, et al. Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer’s disease induced by aβ1–42. PloS one. 2015;10:e0122415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Xiang J, Wang ZH, Ahn EH, et al. Delta-secretase-cleaved Tau antagonizes TrkB neurotrophic signalings, mediating Alzheimer’s disease pathologies. Proc Natl Acad Sci U S A. 2019;116(18):9094–9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Alvarez AR, Godoy JA, Mullendorff K, Olivares GH, Bronfman M, Inestrosa NC. Wnt-3a overcomes beta-amyloid toxicity in rat hippocampal neurons. Experimental cell research. 2004;297:186–196. [DOI] [PubMed] [Google Scholar]
- 388.Caricasole A, Copani A, Caraci F, et al. Induction of Dickkopf-1, a negative modulator of the Wnt pathway, is associated with neuronal degeneration in Alzheimer’s brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6021–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.De Ferrari GV, Papassotiropoulos A, Biechele T, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9434–9439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Ghanevati M, Miller CA. Phospho-beta-catenin accumulation in Alzheimer’s disease and in aggresomes attributable to proteasome dysfunction. Journal of molecular neuroscience : MN. 2005;25:79–94. [DOI] [PubMed] [Google Scholar]
- 391.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nature reviews Neuroscience. 2010;11:77–86. [DOI] [PubMed] [Google Scholar]
- 392.Inestrosa NC, Toledo EM. The role of Wnt signaling in neuronal dysfunction in Alzheimer’s Disease. Molecular neurodegeneration. 2008;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Magdesian MH, Carvalho MMVF, Mendes FA, et al. Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. The Journal of biological chemistry. 2008;283:9359–9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nature reviews Molecular cell biology. 2009;10:468–477. [DOI] [PubMed] [Google Scholar]
- 395.Rosso SB, Inestrosa NC. WNT signaling in neuronal maturation and synaptogenesis. Frontiers in cellular neuroscience. 2013;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Rosi MC, Luccarini I, Grossi C, et al. Increased Dickkopf-1 expression in transgenic mouse models of neurodegenerative disease. Journal of neurochemistry. 2010;112:1539–1551. [DOI] [PubMed] [Google Scholar]
- 397.Killick R, Ribe EM, Al-Shawi R, et al. Clusterin regulates β-amyloid toxicity via Dickkopf-1-driven induction of the wnt-PCP-JNK pathway. Molecular psychiatry. 2014;19:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Motti C, Funke H, Rust S, Dergunov A, Assmann G. Using mutagenic polymerase chain reaction primers to detect carriers of familial defective apolipoprotein B-100. Clinical chemistry. 1991;37:1762–1766. [PubMed] [Google Scholar]
- 399.Magalhães CA, Carvalho MG, Sousa LP, Caramelli P, Gomes KB. Leptin in Alzheimer’s disease. Clinica chimica acta; international journal of clinical chemistry. 2015;450:162–168. [DOI] [PubMed] [Google Scholar]
- 400.Paz-Filho G, Wong M-L, Licinio J. The procognitive effects of leptin in the brain and their clinical implications. International journal of clinical practice. 2010;64:1808–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Johnston JM, Greco SJ, Hamzelou A, Ashford JW, Tezapsidis N. Repositioning leptin as a therapy for Alzheimer’s disease. Therapy (London, England : 2004). 2011;8:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Bonda DJ, Stone JG, Torres SL, et al. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. Journal of neurochemistry. 2014;128:162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Maioli S, Lodeiro M, Merino-Serrais P, et al. Alterations in brain leptin signalling in spite of unchanged CSF leptin levels in Alzheimer’s disease. Aging cell. 2015;14:122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.McGuire MJ, Ishii M. Leptin Dysfunction and Alzheimer’s Disease: Evidence from Cellular, Animal, and Human Studies. Cellular and molecular neurobiology. 2016;36:203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Marwarha G, Raza S, Meiers C, Ghribi O. Leptin attenuates BACE1 expression and amyloid-β genesis via the activation of SIRT1 signaling pathway. Biochimica et biophysica acta. 2014;1842:1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 407.Tezapsidis N, Johnston JM, Smith MA, et al. Leptin: a novel therapeutic strategy for Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2009;16:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Corem N, Anzi S, Gelb S, Ben-Zvi A . Leptin receptor deficiency induces early, transient and hyperglycaemia-independent blood-brain barrier dysfunction. Sci Rep. 2019;9(1):2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Montagne A, Zhao Z, Zlokovic BV. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? The Journal of experimental medicine. 2017;214:3151–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Nation DA, Sweeney MD, Montagne A, et al. Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25(2):270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nature neuroscience. 2016;19:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain pathology (Zurich, Switzerland). 2014;24:371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Yamazaki Y, Kanekiyo T. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. International journal of molecular sciences. 2017;18:1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Erickson MA, Banks WA. Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2013;33:1500–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Deane R, Du Yan S, Submamaryan RK, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nature medicine. 2003;9:907–913. [DOI] [PubMed] [Google Scholar]
- 417.Cai Z, Qiao P-F, Wan C-Q, Cai M, Zhou N-K, Li Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. 2018;63:1223–1234. [DOI] [PubMed] [Google Scholar]
- 418.Sharma HS, Castellani RJ, Smith MA, Sharma A. The blood-brain barrier in Alzheimer’s disease: novel therapeutic targets and nanodrug delivery. International review of neurobiology. 2012;102:47–90. [DOI] [PubMed] [Google Scholar]
- 419.Marques F, Sousa JC, Sousa N, Palha JA. Blood-brain-barriers in aging and in Alzheimer’s disease. Molecular neurodegeneration. 2013;8:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Liu L, Wan W, Xia S, Kalionis B, Li Y. Dysfunctional Wnt/β-catenin signaling contributes to blood-brain barrier breakdown in Alzheimer’s disease. Neurochemistry international. 2014;75:19–25. [DOI] [PubMed] [Google Scholar]
- 421.Jiang C, Li G, Huang P, Liu Z, Zhao B. The Gut Microbiota and Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. 2017;58:1–15. [DOI] [PubMed] [Google Scholar]
- 422.NIH HMP Working Group J, Peterson J, Garges S, et al. The NIH Human Microbiome Project. Genome research. 2009;19:2317–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Zhuang Z-Q, Shen L-L, Li W-W, et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. 2018;63:1337–1346. [DOI] [PubMed] [Google Scholar]
- 425.Bostanciklioglu M The role of gut microbiota in pathogenesis of Alzheimer’s disease. J Appl Microbiol. 2019. [DOI] [PubMed] [Google Scholar]
- 426.Bhattacharjee S, Lukiw WJ. Alzheimer’s disease and the microbiome. Frontiers in cellular neuroscience. 2013;7:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Schwartz K, Boles BR. Microbial amyloids--functions and interactions within the host. Current opinion in microbiology. 2013;16:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Friedland RP. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. Journal of Alzheimer’s disease : JAD. 2015;45:349–362. [DOI] [PubMed] [Google Scholar]
- 429.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. Journal of lipid research. 2013;54:2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.La Rosa F, Clerici M, Ratto D, et al. The Gut-Brain Axis in Alzheimer’s Disease and Omega-3. A Critical Overview of Clinical Trials. Nutrients. 2018;10:1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Sochocka M, Donskow-Lysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol Neurobiol. 2019;56(3):1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Kowalski K, Mulak A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J Neurogastroenterol Motil. 2019;25(1):48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Hu X, Wang T, Jin F. Alzheimer’s disease and gut microbiota. Science China Life sciences. 2016;59:1006–1023. [DOI] [PubMed] [Google Scholar]
- 434.Uddin MS, Stachowiak A, Mamun AA, et al. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front Aging Neurosci. 2018;10:04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. [DOI] [PubMed] [Google Scholar]
- 436.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14(5):283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24(1):92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Hung SY, Huang WP, Liou HC, Fu WM. Autophagy protects neuron from Abeta-induced cytotoxicity. Autophagy. 2009;5(4):502–510. [DOI] [PubMed] [Google Scholar]
- 439.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441(2):523–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Carvalho C, Santos MS, Oliveira CR, Moreira PI. Alzheimer’s disease and type 2 diabetes-related alterations in brain mitochondria, autophagy and synaptic markers. Biochim Biophys Acta. 2015;1852(8):1665–1675. [DOI] [PubMed] [Google Scholar]
- 441.Cho SJ, Yun SM, Jo C, et al. SUMO1 promotes Abeta production via the modulation of autophagy. Autophagy. 2015;11(1):100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Inoue K, Rispoli J, Kaphzan H, et al. Macroautophagy deficiency mediates age-dependent neurodegeneration through a phospho-tau pathway. Mol Neurodegener. 2012;7:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Decuypere JP, Parys JB, Bultynck G. Regulation of the autophagic bcl-2/beclin 1 interaction. Cells. 2012;1(3):284–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Pickford F, Masliah E, Britschgi M, et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J Clin Invest. 2008;118(6):2190–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Shukla V, Zheng YL, Mishra SK, et al. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. FASEB J. 2013;27(1):174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Liu G, Wang H, Liu J, et al. The CLU gene rs11136000 variant is significantly associated with Alzheimer’s disease in Caucasian and Asian populations. Neuromolecular Med. 2014;16(1):52–60. [DOI] [PubMed] [Google Scholar]
- 447.Beeg M, Stravalaci M, Romeo M, et al. Clusterin Binds to Abeta1–42 Oligomers with High Affinity and Interferes with Peptide Aggregation by Inhibiting Primary and Secondary Nucleation. J Biol Chem. 2016;291(13):6958–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Lee JH, Yu WH, Kumar A, et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141(7):1146–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Wang Q, Tian Q, Song X, Liu Y, Li W. SNCA Gene Polymorphism may Contribute to an Increased Risk of Alzheimer’s Disease. J Clin Lab Anal. 2016;30(6):1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Giasson BI, Forman MS, Higuchi M, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300(5619):636–640. [DOI] [PubMed] [Google Scholar]
- 451.Gong B, Cao Z, Zheng P, et al. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126(4):775–788. [DOI] [PubMed] [Google Scholar]
- 452.Zhang M, Cai F, Zhang S, Zhang S, Song W. Overexpression of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1) delays Alzheimer’s progression in vivo. Sci Rep. 2014;4:7298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Guglielmotto M, Monteleone D, Boido M, et al. Abeta1–42-mediated down-regulation of Uch-L1 is dependent on NF-kappaB activation and impaired BACE1 lysosomal degradation. Aging Cell. 2012;11(5):834–844. [DOI] [PubMed] [Google Scholar]
- 454.Raben N, Puertollano R. TFEB and TFE3: Linking Lysosomes to Cellular Adaptation to Stress. Annu Rev Cell Dev Biol. 2016;32:255–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Orr ME, Oddo S. Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res Ther. 2013;5(5):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Zhang L, Wang L, Wang R, et al. Evaluating the Effectiveness of GTM-1, Rapamycin, and Carbamazepine on Autophagy and Alzheimer Disease. Med Sci Monit. 2017;23:801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Li L, Zhang S, Zhang X, et al. Autophagy enhancer carbamazepine alleviates memory deficits and cerebral amyloid-beta pathology in a mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2013;10(4):433–441. [DOI] [PubMed] [Google Scholar]
- 458.Sun YX, Ji X, Mao X, et al. Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J Alzheimers Dis. 2014;38(2):437–444. [DOI] [PubMed] [Google Scholar]
- 459.Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5(4):e9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Tang Z, Ioja E, Bereczki E, et al. mTor mediates tau localization and secretion: Implication for Alzheimer’s disease. Biochim Biophys Acta. 2015;1853(7):1646–1657. [DOI] [PubMed] [Google Scholar]
- 461.Caccamo A, Magri A, Medina DX, et al. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell. 2013;12(3):370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Jiang T, Yu JT, Zhu XC, et al. Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer’s disease. Pharmacol Res. 2014;81:54–63. [DOI] [PubMed] [Google Scholar]
- 464.Frederick C, Ando K, Leroy K, et al. Rapamycin ester analog CCI-779/Temsirolimus alleviates tau pathology and improves motor deficit in mutant tau transgenic mice. J Alzheimers Dis. 2015;44(4):1145–1156. [DOI] [PubMed] [Google Scholar]
- 465.Song G, Li Y, Lin L, Cao Y. Anti-autophagic and anti-apoptotic effects of memantine in a SH-SY5Y cell model of Alzheimer’s disease via mammalian target of rapamycin-dependent and -independent pathways. Mol Med Rep. 2015;12(5):7615–7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Vingtdeux V, Giliberto L, Zhao H, et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J Biol Chem. 2010;285(12):9100–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Porquet D, Grinan-Ferre C, Ferrer I, et al. Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J Alzheimers Dis. 2014;42(4):1209–1220. [DOI] [PubMed] [Google Scholar]
- 468.Turner RS, Thomas RG, Craft S, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Steele JW, Gandy S. Latrepirdine (Dimebon(R)), a potential Alzheimer therapeutic, regulates autophagy and neuropathology in an Alzheimer mouse model. Autophagy. 2013;9(4):617–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Koga H, Martinez-Vicente M, Arias E, Kaushik S, Sulzer D, Cuervo AM. Constitutive upregulation of chaperone-mediated autophagy in Huntington’s disease. J Neurosci. 2011;31(50):18492–18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.De Strooper B, Saftig P, Craessaerts K, et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391(6665):387–390. [DOI] [PubMed] [Google Scholar]
- 472.Neely KM, Green KN. Presenilins mediate efficient proteolysis via the autophagosome-lysosome system. Autophagy. 2011;7(6):664–665. [DOI] [PubMed] [Google Scholar]
- 473.Francois A, Terro F, Janet T, Rioux Bilan A, Paccalin M, Page G. Involvement of interleukin-1beta in the autophagic process of microglia: relevance to Alzheimer’s disease. J Neuroinflammation. 2013;10:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Kumar A, Dhawan A, Kadam A, Shinde A. Autophagy and Mitochondria: Targets in Neurodegenerative Disorders. CNS Neurol Disord Drug Targets. 2018;17(9):696–705. [DOI] [PubMed] [Google Scholar]
- 475.Fang C, Gu L, Smerin D, Mao S, Xiong X. The Interrelation between Reactive Oxygen Species and Autophagy in Neurological Disorders. Oxid Med Cell Longev. 2017;2017:8495160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476.Ghavami S, Shojaei S, Yeganeh B, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol. 2014;112:24–49. [DOI] [PubMed] [Google Scholar]
- 477.Vernace VA, Schmidt-Glenewinkel T, Figueiredo-Pereira ME. Aging and regulated protein degradation: who has the UPPer hand? Aging Cell. 2007;6(5):599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Korolchuk VI, Menzies FM, Rubinsztein DC. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584(7):1393–1398. [DOI] [PubMed] [Google Scholar]
- 479.Gomes BAQ, Silva JPB, Romeiro CFR, et al. Neuroprotective Mechanisms of Resveratrol in Alzheimer’s Disease: Role of SIRT1. Oxid Med Cell Longev. 2018;2018:8152373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annual review of neuroscience. 2011;34:185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Crisp AH. Clinical and therapeutic aspects of anorexia nervosa—a study of 30 cases. Journal of Psychosomatic Research. 1965;9:67–78. [DOI] [PubMed] [Google Scholar]
- 482.Vega GL, Weiner MF, Lipton AM, et al. Reduction in levels of 24S-hydroxycholesterol by statin treatment in patients with Alzheimer disease. Archives of neurology. 2003;60:510–515. [DOI] [PubMed] [Google Scholar]
- 483.Hickman DT, López-Deber MP, Ndao DM, et al. Sequence-independent control of peptide conformation in liposomal vaccines for targeting protein misfolding diseases. The Journal of biological chemistry. 2011;286:13966–13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Alzheon. 2019; https://alzheon.com/alzheon-pipeline/.Accessed 04/02/2019, 2019.
- 485.Nicolas LKK-P, Schaible J, Link J, Kleiner O, Borta A, Podhorna J, Scholpp J. Pharmacokinetics, pharmacodynamics, and safety of the novel bace inhibitor bi1181181 after oral administration of single ascending doses in healthy subjects. Alzheimers Dement. 2015;11:740–741.26194310 [Google Scholar]
- 486.Navarro D, Castaner R, Fernandez-Forner D, Cole P. NME digest. Drugs of the Future. 2015;40:667. [Google Scholar]
- 487.Snow AD, Cummings J, Thomas Lake T, et al. Exebryl-1: A novel small molecule currently in human clinical trials as a disease-modifying drug for the treatment of Alzheimer’s disease. Alzheimer’s & Dimentia. 2019;5(4):p418. [Google Scholar]
- 488.Pharma KHK. A Study of Single and Multiple Doses of KHK6640 in Subjects With Prodromal or Mild to Moderate Alzheimer’s Disease - Full Text View - ClinicalTrials.gov. 2014; https://clinicaltrials.gov/ct2/show/NCT02127476.Accessed 04/02/2019, 2019.
- 489.Tolerability and preliminary pharmacodynamics after single doses of MEDI1814, a Beta-Amyloid 42 (Aß42)-specific antibody, in mild-moderate Alzheimer’s disease. 2015.
- 490.Davtyan H, Ghochikyan A, Petrushina I, et al. Immunogenicity, efficacy, safety, and mechanism of action of epitope vaccine (Lu AF20513) for Alzheimer’s disease: prelude to a clinical trial. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4923–4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Vartiainen NE WM, Charles ML, Stenius T, Nurmi A, Yrjanheikki J. SAN-61 protects against Ab1–42, OGD and H2O2 in rat mixed cortical cultures and inhibits GSK- 3b (Abst. 336.16/L15). Society for Neuroscience Meeting. 2009. [Google Scholar]
- 492.Pradier L, Cohen C, Blanchard V, et al. SAR228810: An antiprotofibrillar beta-amyloid antibody designed to reduce risk of amyloid-related imaging abnormalities (ARIA). Alzheimer’s & Dementia. 2013;9:P808–P809. [Google Scholar]
- 493.Zhou B, Rothlein R, Shen J, et al. TTP4000, a soluble fusion protein inhibitor of Receptor for Advanced Glycation End Products (RAGE) is an effective therapy in animal models of Alzheimer’s disease. Faseb Journal. 2013;27. [Google Scholar]
- 494.Schneeberger A, Hendrix S, Ellison N, Bürger V, Dubois B. Additional results from a phase ii study to assess the clinical and immunological activity, safety, and tolerability of affitope® ad02 in patients with early Alzheimer’s disease (AD). Alzheimer’s & Dementia. 2015;11:P276. [DOI] [PubMed] [Google Scholar]
- 495.Tucker S, Möller C, Tegerstedt K, et al. The murine version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe mice. Journal of Alzheimer’s disease : JAD. 2015;43:575–588. [DOI] [PubMed] [Google Scholar]
- 496.Cramer PE, Cirrito JR, Wesson DW, et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science (New York, NY). 2012;335:1503–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Bellus. BELLUS Health Announces Partnership for the Development of BLU8499. 2018; https://www.newswire.ca/news-releases/bellus-health-announces-partnership-for-the-development-of-blu8499-510667701.html.Accessed 12.10.2018, 2018.
- 498.Matijevic M, Watanabe H, Sato Y, et al. A single dose of the beta-secretase inhibitor, e2609, decreases CSF bace1 enzymatic activity in cynomolgus monkeys. Alzheimer’s & Dementia. 2015;11:P841. [Google Scholar]
- 499.Forum_Pharmaceuticals_Inc. Safety, Tolerability, Pharmacokinetics of EVP-0962 and Effects of EVP-0962 on Cerebral Spinal Fluid Amyloid Concentrations in Healthy Subjects and in Subjects With Mild Cognitive Impairment or Early Alzheimer’s Disease. 2014; https://clinicaltrials.gov/ct2/show/NCT01661673.Accessed 04/02/2019, 2019.
- 500.Dodel R, Rominger A, Blennow K, et al. A randomized, double-blind, placebo-controlled dose-finding trial of intravenous immunoglobulin (IVIG; Octagam® 10%, Octapharma AG) in patients with mild to moderate Alzheimer’s disease (GAM10–04). Alzheimer’s & Dementia. 2011;7:e55–e56. [Google Scholar]
- 501.Landen JW, Zhao Q, Cohen S, et al. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clinical neuropharmacology. 2013;36:14–23. [DOI] [PubMed] [Google Scholar]
- 502.Maccecchini ML, Chang MY, Pan C, John V, Zetterberg H, Greig NH. Posiphen as a candidate drug to lower CSF amyloid precursor protein, amyloid-β peptide and τ levels: target engagement, tolerability and pharmacokinetics in humans. Journal of neurology, neurosurgery, and psychiatry. 2012;83:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503.Pepys MB, Herbert J, Hutchinson WL, et al. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417:254–259. [DOI] [PubMed] [Google Scholar]
- 504.Wang CY, Finstad CL, Walfield AM, et al. Site-specific UBITh amyloid-beta vaccine for immunotherapy of Alzheimer’s disease. Vaccine. 2007;25:3041–3052. [DOI] [PubMed] [Google Scholar]
- 505.Sevigny J, Chiao P, Williams L, et al. Aducanumab (BIIB037), an anti-amyloid beta monoclonal antibody, in patients with prodromal or mild Alzheimer’s disease: Interim results of a randomized, double-blind, placebo-controlled, phase 1b study. Alzheimer’s & Dementia. 2015;11:P277. [Google Scholar]
- 506.Aztherapies. Safety and Efficacy Study of ALZT-OP1 in Subjects With Evidence of Early Alzheimer’s Disease - Full Text View - ClinicalTrials.gov. 2015; https://clinicaltrials.gov/ct2/show/NCT02547818.Accessed 04/02.2019, 2019.
- 507.Alexander R, Budd S, Russell M, et al. AZD3293 A novel BACE1 inhibitor: safety, tolerability, and effects on plasma and CSF Aβ peptides following single- and multiple-dose administration. Neurobiology of Aging. 2014;35:S2. [Google Scholar]
- 508.Hu C, Adedokun O, Ito K, Raje S, Lu M. Confirmatory population pharmacokinetic analysis for bapineuzumab phase 3 studies in patients with mild to moderate Alzheimer’s disease. Journal of clinical pharmacology. 2015;55:221–229. [DOI] [PubMed] [Google Scholar]
- 509.Adolfsson O, Pihlgren M, Toni N, et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique aβ binding properties promotes neuroprotection and glial engulfment of Aβ. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:9677–9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Shen C, Chen L, Jiang L, Lai TYY. Neuroprotective effect of epigallocatechin-3-gallate in a mouse model of chronic glaucoma. Neuroscience letters. 2015;600:132–136. [DOI] [PubMed] [Google Scholar]
- 511.Baxalta. Phase III Efficacy, Safety, and Tolerability Study of HYQVIA/HyQvia and GAMMAGARD LIQUID/KIOVIG in CIDP. 2015; https://clinicaltrials.gov/ct2/show/NCT02549170.Accessed 04/02/2019, 2019.
- 512.Jacobsen H, Ozmen L, Caruso A, et al. Combined treatment with a BACE inhibitor and anti-Aβ antibody gantenerumab enhances amyloid reduction in APPLondon mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:11621–11630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Shanghai Greenvalley Pharmaceutical Co. L. Safety, Efficacy and Dose Titration of Sodium Oligo-mannurarate Capsule on Mild to Moderate Alzheimer’s Disease - Full Text View - ClinicalTrials.gov. 2014; https://clinicaltrials.gov/ct2/show/NCT01453569.Accessed 04/02/2019, 2019.
- 514.Neumann U, Rueeger H, Machauer R, et al. A novel BACE inhibitor NB-360 shows a superior pharmacological profile and robust reduction of amyloid-β and neuroinflammation in APP transgenic mice. Molecular neurodegeneration. 2015;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 515.Yan R, Vassar R. Targeting the β secretase BACE1 for Alzheimer’s disease therapy. The Lancet Neurology. 2014;13:319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Sinha S, Du Z, Maiti P, et al. Comparison of three amyloid assembly inhibitors: the sugar scyllo-inositol, the polyphenol epigallocatechin gallate, and the molecular tweezer CLR01. ACS chemical neuroscience. 2012;3:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517.Salloway S, Sperling R, Brashear HR. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer’s disease. The New England journal of medicine. 2014;370:1460. [DOI] [PubMed] [Google Scholar]
- 518.Bellus. Open-Label Extension of the Phase III Study With Tramiprosate (3APS) in Patients With Mild to Moderate Alzheimer’s Disease. 2007; https://clinicaltrials.gov/ct2/show/NCT00314912.Accessed 04/02/2019, 2019.
- 519.Baddeley TC, McCaffrey J, Storey JMD, et al. Complex disposition of methylthioninium redox forms determines efficacy in tau aggregation inhibitor therapy for Alzheimer’s disease. The Journal of pharmacology and experimental therapeutics. 2015;352:110–118. [DOI] [PubMed] [Google Scholar]
- 520.Sheridan C Pivotal trials for β-secretase inhibitors in Alzheimer’s. Nature biotechnology. 2015;33:115–116. [DOI] [PubMed] [Google Scholar]
- 521.Biogen. A Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose Study of Intravenously Administered BMS-986168 in Healthy Subjects. 2014; https://clinicaltrials.gov/ct2/show/NCT02294851.Accessed 04/02/2019, 2019.
- 522.Valera E, Spencer B, Masliah E. Immunotherapeutic Approaches Targeting Amyloid-β, α-Synuclein, and Tau for the Treatment of Neurodegenerative Disorders. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2016;13:179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Muggia F, Kudlowitz D. Novel taxanes. Anti-cancer drugs. 2014;25:593–598. [DOI] [PubMed] [Google Scholar]
- 524.Novak M Tau vaccine: Active immunization with misfolded tau protein attenuates tau pathology in the transgenic rat model of tauopathy. Alzheimers Dement. 2009;5(4):P93. [Google Scholar]
- 525.Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11213–11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Wischik CM, Staff RT, Wischik DJ, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2015;44:705–720. [DOI] [PubMed] [Google Scholar]
- 527.Tkachenko SIA, Khvat A, Okun I, Lavrovsky Y, Salimov R. Discovery and preclinical studies of AVN-322 highly selective and potent 5HT6 antagonist for cognition enhancement in treating neurodegerative diseases. 2009. [Google Scholar]
- 528.Cudkowicz M, Bozik ME, Ingersoll EW, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nature medicine. 2011;17:1652–1656. [DOI] [PubMed] [Google Scholar]
- 529.Macdonald IR, Rockwood K, Martin E, Darvesh S. Cholinesterase inhibition in Alzheimer’s disease: is specificity the answer? Journal of Alzheimer’s disease : JAD. 2014;42:379–384. [DOI] [PubMed] [Google Scholar]
- 530.Qian ZM, Ke Y. Huperzine A: Is it an Effective Disease-Modifying Drug for Alzheimer’s Disease? Frontiers in aging neuroscience. 2014;6:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 531.Bhattacharya S, Maelicke A, Montag D. Nasal Application of the Galantamine Pro-drug Memogain Slows Down Plaque Deposition and Ameliorates Behavior in 5X Familial Alzheimer’s Disease Mice. Journal of Alzheimer’s disease : JAD. 2015;46:123–136. [DOI] [PubMed] [Google Scholar]
- 532.Benade VS, Daripelli S, Thentu JB, et al. Suvn-g3031, an h3 receptor inverse agonist, produces procognitive effects without affecting sleep in preclinical models. Alzheimer’s & Dementia. 2015;11:P475. [Google Scholar]
- 533.Lahmy V, Meunier J, Malmström S, et al. Blockade of Tau hyperphosphorylation and Aβ₁₋₄₂ generation by the aminotetrahydrofuran derivative ANAVEX2–73, a mixed muscarinic and σ₁ receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1706–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Frölich L, Ashwood T, Nilsson J, Eckerwall G, Investigators S. Effects of AZD3480 on cognition in patients with mild-to-moderate Alzheimer’s disease: a phase IIb dose-finding study. Journal of Alzheimer’s disease : JAD. 2011;24:363–374. [DOI] [PubMed] [Google Scholar]
- 535.Weinreb O, Amit T, Bar-Am O, Youdim MBH. Ladostigil: a novel multimodal neuroprotective drug with cholinesterase and brain-selective monoamine oxidase inhibitory activities for Alzheimer’s disease treatment. Current drug targets. 2012;13:483–494. [DOI] [PubMed] [Google Scholar]
- 536.Othman A, Meier A, Ritchie CW, Florian H, Gault LM, Tang Q. EFFICACY AND SAFETY OF THE ALPHA7 AGONIST ABT-126 AS A MONOTHERAPY TREATMENT IN MILD-TO-MODERATE ALZHEIMER’S DEMENTIA: RESULTS OF A PHASE 2B TRIAL. Alzheimer’s & Dementia. 2014;10:P137. [Google Scholar]
- 537.Rinne JO, Wesnes K, Hänninen J, et al. Safety and efficacy of ORM-12741 on cognitive and behavioral symptoms in patients with Alzheimer’s disease: A randomized, double-blind, proof-of-concept study. Journal of the Neurological Sciences. 2013;333:e322. [Google Scholar]
- 538.Chumakov I, Nabirotchkin S, Cholet N, et al. Combining two repurposed drugs as a promising approach for Alzheimer’s disease therapy. Scientific reports. 2015;5:7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 539.Weinreb O, Badinter F, Amit T, Bar-Am O, Youdim MBH. Effect of long-term treatment with rasagiline on cognitive deficits and related molecular cascades in aged mice. Neurobiology of aging. 2015;36:2628–2636. [DOI] [PubMed] [Google Scholar]
- 540.Hunsberger HC, Weitzner DS, Rudy CC, et al. Riluzole rescues glutamate alterations, cognitive deficits, and tau pathology associated with P301L tau expression. Journal of neurochemistry. 2015;135:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Sors A, Panayi F, Bert L, et al. Mechanistic characterization of S 38093, a novel inverse agonist at histamine H3 receptors. European journal of pharmacology. 2017;803:11–23. [DOI] [PubMed] [Google Scholar]
- 542.Grigoriev VV, Proshin AN, Kinzirsky AS, Bachurin SO. Modern approaches to the design of memory and cognitive function stimulants based on AMPA receptor ligands. Russian Chemical Reviews. 2009;78:485–494. [Google Scholar]
- 543.Bretin S, Louis C, Seguin L, et al. Pharmacological characterisation of S 47445, a novel positive allosteric modulator of AMPA receptors. PLOS ONE. 2017;12:e0184429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Maher-Edwards G, Dixon R, Hunter JJ, et al. SB-742457 and donepezil in Alzheimer disease: a randomized, placebo-controlled study. International journal of geriatric psychiatry. 2011;26:536–544. [DOI] [PubMed] [Google Scholar]
- 545.Avanir. Avanir Pharmaceuticals Announces Initiation of Phase II Study of AVP-786 for the Adjunctive Treatment of Major Depressive Disorder | Avanir Pharmaceuticals Inc. 2014; https://www.avanir.com/press/avanir-pharmaceuticals-announces-initiation-phase-ii-study-avp-786-adjunctive-treatment-major. Accessed 04/02/2019, 2019. [Google Scholar]
- 546.Lombardo S, Maskos U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology. 2015;96:255–262. [DOI] [PubMed] [Google Scholar]
- 547.Wilkinson D, Windfeld K, Colding-Jørgensen E. Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer’s disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. The Lancet Neurology. 2014;13:1092–1099. [DOI] [PubMed] [Google Scholar]
- 548.Shin JH, Lee YA, Lee JK, et al. Concurrent blockade of free radical and microsomal prostaglandin E synthase-1-mediated PGE2 production improves safety and efficacy in a mouse model of amyotrophic lateral sclerosis. Journal of neurochemistry. 2012;122:952–961. [DOI] [PubMed] [Google Scholar]
- 549.Butchart JBL, Hopkins V, Teeling J, Puntener U, Culliford D, Sharples R, Sharif S, McFarlane BRR, Thomas R, Passmore P, Perry VH, Holmes C. Etanercept in Alzheimer disease: A randomized, placebo-controlled, double-blind, phase 2 trial. Neurology. 2015;85:2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiology of aging. 2011;32:1626–1633. [DOI] [PubMed] [Google Scholar]
- 551.GlaxoSmithKline. GSK2647544 RD, DDI in Healthy Young and Elderly Volunteers. 2014; https://clinicaltrials.gov/ct2/show/NCT01978327.Accessed 04/02/2019, 2019.
- 552.Barone E, Mancuso C, Di Domenico F, et al. Biliverdin reductase-A: a novel drug target for atorvastatin in a dog pre-clinical model of Alzheimer disease. Journal of neurochemistry. 2012;120:135–146. [DOI] [PubMed] [Google Scholar]
- 553.Zhang Y-Y, Fan Y-C, Wang M, Wang D, Li X-H. Atorvastatin attenuates the production of IL-1β, IL-6, and TNF-α in the hippocampus of an amyloid β1–42-induced rat model of Alzheimer’s disease. Clinical interventions in aging. 2013;8:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Shaddinger BC, Xu Y, Roger JH, et al. Platelet aggregation unchanged by lipoprotein-associated phospholipase A₂ inhibition: results from an in vitro study and two randomized phase I trials. PloS one. 2014;9:e83094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Sano M, Bell KL, Galasko D, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology. 2011;77:556–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Tong X-K, Lecrux C, Rosa-Neto P, Hamel E. Age-dependent rescue by simvastatin of Alzheimer’s disease cerebrovascular and memory deficits . The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:4705–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Ferguson SBA, Mouzon B, Phillips J, Crynen G, Reed J, Chaytow H, Mullan M, Mathura VMM, Crawford F. Time course of inflammatory marker response after TBI (Abst #. 254.07/Z8). Society for Neuroscience Meeting. 2011. [Google Scholar]
- 558.Lee DS, Choi J, Kim SS-H, Kim SS-H. Ameliorating effects of HX106N, a water-soluble botanical formulation, on Aβ25–35-induced memory impairment and oxidative stress in mice. Biological & pharmaceutical bulletin. 2014;37:954–960. [DOI] [PubMed] [Google Scholar]
- 559.Huang W-J, Zhang X, Chen W-W. Role of oxidative stress in Alzheimer’s disease. Biomedical reports. 2016;4:519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Meulenbroek O, O’Dwyer S, de Jong D, et al. European multicentre double-blind placebo-controlled trial of Nilvadipine in mild-to-moderate Alzheimer’s disease-the substudy protocols: NILVAD frailty; NILVAD blood and genetic biomarkers; NILVAD cerebrospinal fluid biomarkers; NILVAD cerebral blood flo. BMJ open. 2016;6:e011584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 561.SK Chemicals Co. L. A Confirmatory Trial of SK-PC-B70M in Mild to Moderate Alzheimer’s Disease. 2010; https://clinicaltrials.gov/ct2/show/NCT01249196.Accessed 04/02/2019, 2019.
- 562.Sanchez PE, Zhu L, Verret L, et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2895–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.Xiao R Levetiracetam might act as an efficacious drug to attenuate cognitive deficits of Alzheimer’s disease. Current topics in medicinal chemistry. 2016;16:565–573. [DOI] [PubMed] [Google Scholar]
- 564.Anand R, Seiberling M, Kamtchoua T, Pokorny R. Tolerability, safety and pharmacokinetics of the FGLL peptide, a novel mimetic of neural cell adhesion molecule, following intranasal administration in healthy volunteers. Clinical pharmacokinetics. 2007;46:351–358. [DOI] [PubMed] [Google Scholar]
- 565.Eriksdotter-Jönhagen M, Linderoth B, Lind G, et al. Encapsulated cell biodelivery of nerve growth factor to the Basal forebrain in patients with Alzheimer’s disease. Dementia and geriatric cognitive disorders. 2012;33:18–28. [DOI] [PubMed] [Google Scholar]
- 566.Takamura Y, Ono K, Matsumoto J, Yamada M, Nishijo H. Effects of the neurotrophic agent T-817MA on oligomeric amyloid-β-induced deficits in long-term potentiation in the hippocampal CA1 subfield. Neurobiology of aging. 2014;35:532–536. [DOI] [PubMed] [Google Scholar]
- 567.GliaCure I Safety, Tolerability, and Pharmacokinetic Study of Single Ascending Doses of GC021109 in Healthy Subjects 2014; https://clinicaltrials.gov/ct2/show/NCT02254369.Accessed 04/02/2019, 2019.
- 568.Turner RS, Thomas RG, Craft S, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Bomba M, Ciavardelli D, Silvestri E, et al. Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1-KI mice but has no effects in 3xTg-AD animals. Cell death & disease. 2013;4:e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Egefjord L, Gejl M, Møller A, et al. Effects of liraglutide on neurodegeneration, blood flow and cognition in Alzheimeŕs disease - protocol for a controlled, randomized double-blinded trial. Danish medical journal. 2012;59:A4519. [PubMed] [Google Scholar]
- 571.Shah RC, Matthews DC, Andrews RD, et al. An evaluation of MSDC-0160, a prototype mTOT modulating insulin sensitizer, in patients with mild Alzheimer’s disease. Current Alzheimer research. 2014;11:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572.Yao J, Zhao L, Mao Z, et al. Potentiation of brain mitochondrial function by S-equol and R/S-equol estrogen receptor β-selective phytoSERM treatments. Brain research. 2013;1514:128–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 573.Nih. New NIH-funded memory drug moves into Phase 1 clinical study. 2015; https://www.nih.gov/news-events/news-releases/new-nih-funded-memory-drug-moves-into-phase-1-clinical-study.Accessed 04/02/2019, 2019.
- 574.Jelinek GA, Weiland TJ, Hadgkiss EJ, Marck CH, Pereira N, van der Meer DM. Medication use in a large international sample of people with multiple sclerosis: associations with quality of life, relapse rate and disability. Neurological research. 2015;37:662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575.Kwon SRL, Huang M, Michael E, Meltzer H. RP5063 reverses and prevents sub-chronic phencyclidine-induced declarative memory deficits and increased dopamine efflux in the prefrontal cortex region in C57BL/6J mice (Abst/823.04). Society for Neuroscience Meeting. 2015. [Google Scholar]
- 576.Kume K, Hanyu H, Sakurai H, Takada Y, Onuma T, Iwamoto T. Effects of telmisartan on cognition and regional cerebral blood flow in hypertensive patients with Alzheimer’s disease. Geriatrics & gerontology international. 2012;12:207–214. [DOI] [PubMed] [Google Scholar]
- 577.Shindo T, Takasaki K, Uchida K, et al. Ameliorative effects of telmisartan on the inflammatory response and impaired spatial memory in a rat model of Alzheimer’s disease incorporating additional cerebrovascular disease factors. Biological & pharmaceutical bulletin. 2012;35:2141–2147. [DOI] [PubMed] [Google Scholar]
- 578.Ivachtchenko AV, Lavrovsky Y, Okun I. AVN-101: A Multi-Target Drug Candidate for the Treatment of CNS Disorders. Journal of Alzheimer’s disease : JAD. 2016;53:583–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 579.Garakani A, Murrough JW, Iosifescu DV. Advances in Psychopharmacology for Anxiety Disorders. FOCUS. 2014;12:152–162. [Google Scholar]
- 580.Sun X-J, Zhao L, Zhao N, et al. Benfotiamine prevents increased β-amyloid production in HEK cells induced by high glucose. Neuroscience bulletin. 2012;28:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581.Rosenbrock H, Marti A, Koros E, et al. Improving synaptic plasticity and cognitive function in rodents by the novel phosphodiesterase 9A inhibitor bi 409306. Alzheimer’s & Dementia. 2015;11:P612. [Google Scholar]
- 582.Sun M-K, Hongpaisan J, Lim CS, Alkon DL. Bryostatin-1 restores hippocampal synapses and spatial learning and memory in adult fragile x mice. The Journal of pharmacology and experimental therapeutics. 2014;349:393–401. [DOI] [PubMed] [Google Scholar]
- 583.Lin C-H, Chen P-K, Chang Y-C, et al. Benzoate, a D-amino acid oxidase inhibitor, for the treatment of early-phase Alzheimer disease: a randomized, double-blind, placebo-controlled trial. Biological psychiatry. 2014;75:678–685. [DOI] [PubMed] [Google Scholar]
- 584.Boxer AL, Lang AE, Grossman M, et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. The Lancet Neurology. 2014;13:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585.Jouroukhin Y, Ostritsky R, Assaf Y, Pelled G, Giladi E, Gozes I. NAP (davunetide) modifies disease progression in a mouse model of severe neurodegeneration: protection against impairments in axonal transport. Neurobiology of disease. 2013;56:79–94. [DOI] [PubMed] [Google Scholar]
- 586.Ormerod AD, Thind CK, Rice SA, Reid IC, Williams JHG, McCaffery PJA. Influence of isotretinoin on hippocampal-based learning in human subjects. Psychopharmacology. 2012;221:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Lupin. A Randomized, Double-blind, Placebo-controlled, Parallel Group, Comparative, Multicenter, Phase 2 Clinical Study to Evaluate Efficacy and Safety of Two Doses of LND101001 Monotherapy in Patients with Mild to Moderate Alzheimer’s Disease. 2014; https://adisinsight.springer.com/trials/700238658.Accessed 04/02/2019, 2019.
- 588.Merk_&_Co. Efficacy and safety of MK-7622 as adjunct therapy in participants with Alzheimer’s disease. 2015; https://clinicaltrials.gov/ct2/show/NCT01852110.Accessed 04/02/2019, 2019.
- 589.Regenera_Pharma_Ltd. A Randomized SAD and MAD Study Evaluating the Safety and Tolerability of RPh201 in Healthy Subjects and in Adults With Alzheimer’s Disease. 2012; https://clinicaltrials.gov/ct2/show/NCT01513967.Accessed 04/02/2019, 2019.
- 590.Lawson DH, Lee S, Zhao F, et al. Randomized, Placebo-Controlled, Phase III Trial of Yeast-Derived Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Versus Peptide Vaccination Versus GM-CSF Plus Peptide Vaccination Versus Placebo in Patients With No Evidence of Disease After Complete Surgical Resection of Locally Advanced and/or Stage IV Melanoma: A Trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697). Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:4066–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 591.Fukasawa H, Nakagomi M, Yamagata N, et al. Tamibarotene: a candidate retinoid drug for Alzheimer’s disease. Biological & pharmaceutical bulletin. 2012;35:1206–1212. [DOI] [PubMed] [Google Scholar]
- 592.Webster SPD. Discovery of UE2343, a potent inhibitor of 11beta-HSD1 for the treatment of Alzheimer’s disease. 2015. [Google Scholar]
- 593.Alam JJ. Selective Brain-Targeted Antagonism of p38 MAPKα Reduces Hippocampal IL-1β Levels and Improves Morris Water Maze Performance in Aged Rats. Journal of Alzheimer’s disease : JAD. 2015;48:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Farías GA, Guzmán-Martínez L, Delgado C, Maccioni RB. Nutraceuticals: a novel concept in prevention and treatment of Alzheimer’s disease and related disorders. Journal of Alzheimer’s disease : JAD. 2014;42:357–367. [DOI] [PubMed] [Google Scholar]
- 595.Accera I. AC-1204 26-Week Long Term Efficacy Response Trial With Optional Open-label Ext. 2012; https://clinicaltrials.gov/ct2/show/NCT01741194.Accessed 04/02/2019, 2019.
- 596.Claxton A, Baker LD, Hanson A, et al. Long Acting Intranasal Insulin Detemir Improves Cognition for Adults with Mild Cognitive Impairment or Early-Stage Alzheimer’s Disease Dementia. Journal of Alzheimer’s disease : JAD. 2015;45:1269–1270. [DOI] [PubMed] [Google Scholar]
- 597.Piette F, Belmin J, Vincent H, et al. Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimer’s research & therapy. 2011;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Bachurin SO, Bovina EV, Ustyugov AA. Drugs in Clinical Trials for Alzheimer’s Disease: The Major Trends. Med Res Rev. 2017;37(5):1186–1225. [DOI] [PubMed] [Google Scholar]