Abstract
Background:
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and dosage-limited oxaliplatin-related toxicity. To date, there are no successful interventions for CIPN prevention or treatment. A therapeutic role for cannabis in diabetic and HIV-related peripheral neuropathy and a protective role in CIPN have been suggested. We examined the effect of cannabis on oncologic patients with CIPN.
Methods:
Medical records of 768 consecutive patients treated with oxaliplatin and 5-fluorouracil-based combinations at a tertiary medical center from October 2015 to January 2018 were reviewed. Excluded patients were those with pre-existing neuropathy or patients who received fewer than two cycles of oxaliplatin treatment. CIPN grade, oxaliplatin cumulative dose, and neuropathy-free survival were evaluated. The patients were divided based upon the exposure to cannabis: prior to oxaliplatin (cannabis-first), cannabis following the initiation of oxaliplatin treatment (oxaliplatin-first), and no exposure (control).
Results:
In total, 513 patients met the inclusion criteria, of whom 248 were treated with cannabis and 265 served as controls. The cannabis-first group included 116 (46.7%) patients and the oxaliplatin-first group included 132 (53.3%) patients. Demographic parameters were comparable between groups. There was a significant difference in CIPN grade 2–3 between cannabis-exposed patients and controls (15.3% and 27.9%, respectively, p < 0.001). The protective effect of cannabis was more pronounced among cannabis-first patients compared to oxaliplatin-first patients (75% and 46.2%, respectively, p < 0.001). The median oxaliplatin cumulative doses were higher in the cannabis-first versus the oxaliplatin-first versus the control groups (545 mg/m2, 340 mg/m2, and 425 mg/m2 respectively, p < 0.001).
Conclusion:
The rate of neuropathy was reduced among patients treated with cannabis and oxaliplatin. This reduction was more significant in patients who received cannabis prior to treatment with oxaliplatin, suggesting a protective effect. A large prospective trial is planned.
Keywords: cannabis, neuropathy, oxaliplatin, palliation, side effects
Introduction
Oxaliplatin is a platinum-based chemotherapy widely used for the treatment of gastrointestinal (GI) malignancies and is part of the well-established regimen in the adjuvant1–3 and metastatic settings.4,5 Chemotherapy-induced peripheral neuropathy (CIPN), a well-known toxicity associated with the treatment of oxaliplatin, has a strong impact on the quality of life of cancer patients.6 Acute oxaliplatin-induced neuropathy is evident in up to 90% of oxaliplatin-treated patients, and continued exposure may lead to severe chronic neuropathy in approximately 31%.7
The mechanisms through which oxaliplatin causes neuropathy include damage to the cell body of the sensory nerves in the dorsal root ganglion, and at higher doses, damage to the anterior horn cells within the spinal cord.8 Underlying the neurotoxicity mechanism of platinum analogs involves the accumulation of the drug within the neuronal cell bodies and subsequent DNA damage, altering cellular activities, such as ion channel dysfunction, dysregulation of calcium homeostasis, and impaired function of transient receptor potential channels.9,10
To date, there are no conventional treatments aimed at neuroprotection or neuroregeneration in CIPN. Numerous trials have been conducted on a number of drugs, including vitamin E,11 glutathione,12 and anti-epileptic drugs,13 which are provided in attempts at neuropathy prevention, as well as tricyclic anti-depressants14 and gabapentin,15 which are administered for symptomatic analgesia related to neuropathy treatment. All of these investigations, however, have yielded inconclusive evidence. The use of intravenous calcium and magnesium has also been tested for the prevention of CIPN,16 but there are no large prospective trials that show a substantial decrease in the occurrence of CIPN and specifically oxaliplatin-induced neurotoxicity by any of these measures.17 The latest American Society of Clinical Oncology (ASCO) guidelines18,19 also support this paradigm highlighting the absence of agents for neurotoxicity prevention.
The cannabis plant and cannabinoid products contain hundreds of active compounds, among them Δ-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), which are considered the most clinically relevant. These compounds are known to reduce chemotherapy toxicities and to contain additive value in palliative care. Multiple trials and meta-analyses have evaluated the efficacy of cannabis in cancer patients for various indications. Prospective double-blind studies have examined the role of cannabis as an analgesic compound,20–23 and several meta-analyses have examined its role in chemotherapy-induced nausea and vomiting.24 In 2010, the Israeli Ministry of Health began to issue permits for cannabis use by cancer patients according to recommendations of the treating oncologists, and the cannabis is provided by an authorized physician at each cancer center.25
The therapeutic role of cannabis for peripheral neuropathy has been demonstrated for diabetes26,27 and for HIV-related neuropathy, with up to a 34% reduction of daily pain after cannabis smoking.28 The use of cannabis for CIPN has been examined in laboratory mice, and both CBD and THC alone attenuated mechanical allodynia in mice treated with paclitaxel.29 Other pre-clinical data showed efficacy in preventing the development of CIPN.30
To the best of our knowledge, the role of cannabis in the prevention or treatment of CIPN has not yet been explored. Given the lack of clinical evidence on the effect of cannabis use on oncologic patients with CIPN, we designed this study to explore such an effect on oxaliplatin-induced peripheral neuropathy in patients with malignancies of the GI tract.
Methods
Setting and patients
This is a retrospective study from one tertiary cancer center in Israel. The medical records of consecutive patients treated at the Tel Aviv Sourasky Medical Center (TASMC) with 5-fluorouracil (5FU) and oxaliplatin-based regimens for GI malignancies between October 2015 and January 2018 were reviewed. The regimens included FOLFOX (continuous 5FU 2400 mg/m2 for 44 h, oxaliplatin 85 mg/m2 for 90 min, push 5FU 400 mg/m2 for 10 min, and leucovorin 400 mg/m2 for 90 min D1Q14), FOLFIRINOX/FOLFOXIRI (continuous 5FU 2400 mg/m2 for 44 h, oxaliplatin 85 mg/m2 for 90 min, push 5FU 400 mg/m2 for 10 min, leucovorin 400 mg/m2 for 90 min, and irinotecan 185 mg/m2 for 90 min, D1Q14), and FLOX (push 5FU 500 mg/m2 for 5 min, leucovorin 500 mg/m2 for 120 min, and oxaliplatin 85 mg/m2 for 120 min D1,8,15,22,29,36 Q49 days). Exclusion criteria involved treatment of oral 5FU (capecitabine), patients who received only one cycle of oxaliplatin or had pre-existing neuropathy at baseline prior to the onset of oxaliplatin treatment.
Data review
Demographic and clinical data, including oncological diagnosis, intent of treatment, chemotherapy protocol, oxaliplatin treatment duration, and cumulative dose were collected from the patients’ medical records. We also collected data regarding the highest neuropathy grade as was evaluated by the treating oncologist, based on the Common Terminology Criteria for Adverse Event (CTCAE version 5).31 Medicinal cannabis use was evaluated by retrieving data from the institutional cannabis registration system. The study patient population was divided according to cannabis treatment and its use in relation to the initiation of oxaliplatin treatment as follows: the cannabis-first group included the patients who were treated with cannabis prior to oxaliplatin (the cannabis was prescribed to this subset of patients for various indications, such as nausea, anorexia, pain, mood disturbance) or within 1 month of the first administration of oxaliplatin. The oxaliplatin-first group included the patients who received oxaliplatin prior to the cannabis treatment. The control group consisted of patients who did not receive cannabis during oxaliplatin treatment.
The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation for Good Clinical Practice. The study was approved by the local ethics committee in Tel Aviv Sourasky Medical Center Institutional Review Board (Helsinki Committee). Given the retrospective nature of this study, a waiver had been given for patients’ informed consent for using the data from their medical records.
Objectives
The primary objective of this study was to assess the neuropathy rates in the three treatment groups. We also analyzed the functionally disturbing (defined as grade 2–3) neuropathy-free survival (FdNFS), defined as the time from the first cannabis treatment to the time of first appearance of Fd neuropathy.
Statistical analysis
Descriptive statistics was applied, reflecting the median and range for continuous variables and frequencies for categorical variables. Baseline characteristics were compared between the control and the two groups of cannabis-treated patients, as well as between the control, the cannabis-first, and the oxaliplatin-first groups. The Mann–Whitney test was used for comparing continuous variables in two groups. An omnibus Kruskal–Wallis non-parametric ANOVA was performed for continuous variables, followed, when relevant, by a pairwise comparison with the Mann–Whitney test, corrected for multiplicity by Tukey’s method for the comparison of three groups. The Chi-squared independence test was used for categorical variables for comparisons among both two and three groups. If relevant, pairwise comparisons were performed using Pearson’s residuals, and the results were compared to the relevant critical value. Survival analysis was performed with Kaplan–Meier estimators, and the Cox-proportional hazard model was used to detect the crude effect of cannabis use as well as its effect adjusted for other important covariates. A p-value < 0.05 was considered significant. All analyses were performed with RStudio Version 1.1.383.
Results
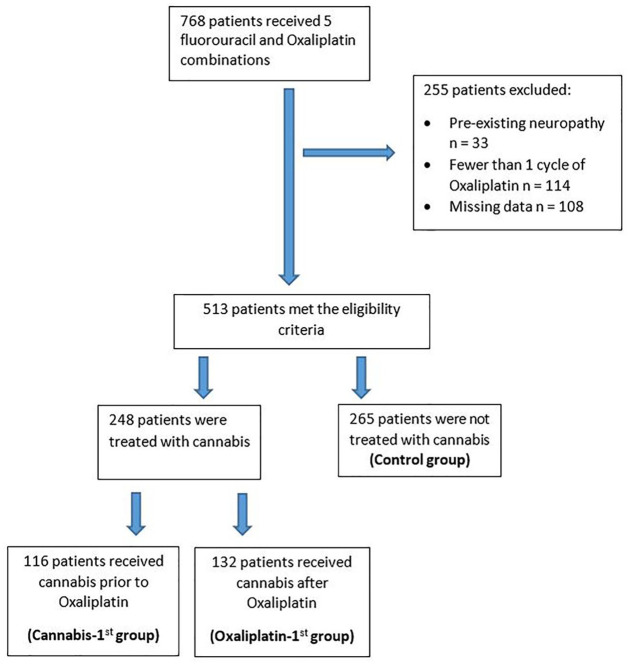
Consecutive patients treated in the TASMC GI oncology clinic between October 2015 and January 2018 were identified in the electronic database. In total, 513 patients met the inclusion criteria for the study, and were treated with combinations of oxaliplatin and 5FU regimens (Table 1, Figure 1).
Table 1.
Patient demographics, diagnoses, and treatment regimens.
| Control group | Cannabis-first group | Oxaliplatin-first group | p-value | |
|---|---|---|---|---|
| No. | 265 | 116 | 132 | |
| Age, years, median | 64 | 62.5 | 61 | 0.18 |
| IQR (58, 72) | IQR (54.7, 70) | IQR (57, 70) | ||
| (27–90) | (24–80) | (26–84) | ||
| Gender | 0.510 | |||
| Male (%) | 139 (52.5) | 54 (46.6) | 70 (53.0) | |
| Female (%) | 126 (47.5) | 62 (53.4) | 62 (46.7) | |
| Diagnosis (%) | 0.3 | |||
| Colon | 155 (58.5) | 47 (40.5) | 62 (47) | |
| Upper GI | 39 (14.7) | 18 (15.5) | 19 (14.4) | |
| Pancreatic | 70 (26.4) | 47 (40.5) | 49 (37.1) | |
| Other | 1 (0.4) | 4 (3.44) | 2 (1.5) | |
| Metastatic disease | 168 (63.4) | 99 (85.3) | 104 (78.8) | <0.001 |
| Protocol Tx (%) | 0.001 | |||
| FLOX | 19 (7.2) | 3 (2.6) | 4 (3.0) | |
| FOLFIRINOX | 51 (19.2) | 38 (32.8) | 37 (28.0) | |
| FOLFOX | 195 (73.6) | 72 (62.1) | 91 (68.9) | |
| FOLFOXIRI | 0 (0.0) | 3 (2.6) | 0 (0.0) | |
| Cumulative Oxaliplatin dose (mg/m2, median) | 425 | 595 | 340 | <0.001 |
| IQR (170, 680) | IQR (417, 765) | IQR (195, 591) |
IQR, interquartile range, GI, gastrointestinal; NaN non-available; Tx, treatment.
Figure 1.
Study flow chart.
The cohort consisted of 250 (49%) females and 263 (51%) males. They were all diagnosed with GI malignancies; among them 217 patients (42%) had colon cancer. Nearly one-half of the patients (248, 48.3%) were treated with medical cannabis for palliative reasons. Of those cannabis-treated patients, the cannabis-first group included 116 (46.7%) patients, and the oxaliplatin-first group included 132 (53.2%) patients. The remaining 265 patients were included in the control group.
The demographic characteristics were comparable between the groups (including age and gender), with the exception of the stage of the disease: there were fewer patients with metastatic disease in the control group (n = 168, 63.4%) compared to those in the cannabis-first group (n = 99, 85.3%) and in the oxaliplatin-first group (n = 104, 78.8%) (p < 0.001). The most common treatment protocol was FOLFOX, which was administered to 73% of the patients in the control group, 62.1% in the cannabis-first group, and 68.9% in the oxaliplatin-first group. Significantly fewer patients in the control group were treated with FOLFIRINOX (19.2% versus 32.8% and 28%, respectively, p = 0.001).
Examining for the presence of CIPN in all cannabis-treated patients compared to the control group revealed CIPN of lower grade among the cannabis-treated patients. While 148 (59.7%) patients from all those treated with cannabis were free from neuropathy (grade 0), only 84 (31.7%) patients of the control group had grade 0 neuropathy (p < 0.001). This neuropathy-sparing effect was more pronounced among those treated with cannabis first compared to those treated with oxaliplatin first [87 patients (75%) and 61 patients (46.2%), respectively (p < 0.001)] (Table 2).
Table 2.
Neuropathy grade.
| Grade | Control group | Cannabis-first group | Oxaliplatin-first group |
|---|---|---|---|
| 265 (100%) | 116 (100%) | 132 (100%) | |
| 0 | 84 (31.7) | 87 (75.0) | 61 (46.2) |
| 1 | 107 (40.4) | 20 (17.2) | 42 (31.8) |
| 2 | 60 (22.6) | 8 (6.9) | 23 (17.4) |
| 3 | 14 (5.3) | 1 (0.9) | 6 (4.5) |
We also analyzed the cumulative oxaliplatin dose in each of the study groups. The cannabis-first group received a median cumulative oxaliplatin dose of 595 mg/m2, which represents a higher dose of oxaliplatin compared with both the control group (425 mg/m2, p < 0.001) and the oxaliplatin-first group (340 mg/m2, p < 0.001).
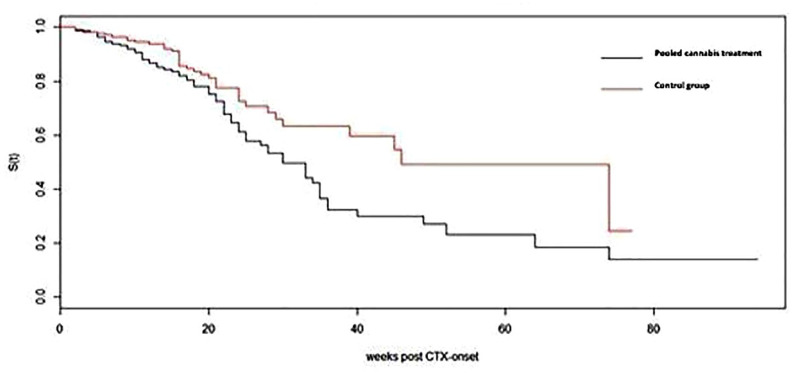
We next analyzed the parameter of FdNFS as the time from the start of the chemotherapy to the time that symptomatically compromised neuropathy occurred. The median FdNFS was significantly longer among the cannabis-treated patients compared to the control group (46 versus 30 weeks, unadjusted Cox hazard ratio 0.5871, 95% confidence interval (0.39–0.86) pooled cannabis group/control p = 0.007) (Figure 2).
Figure 2.
Functionally disturbing neuropathy-free survival in pooled cannabis treatment group versus control group. CTX onset is the date chemotherapy starts.
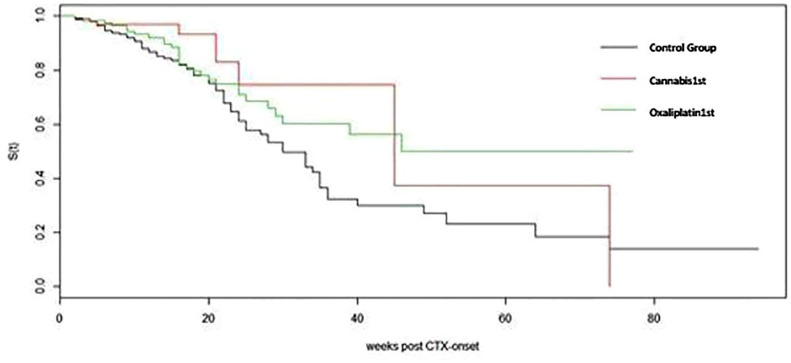
The FdNFS was similar for both the cannabis-first and oxaliplatin-first groups (median 45 versus 46 weeks, respectively, unadjusted hazard ratio = 0.753 95% confidence interval 0.09–2.62, p = 0.461) (Figure 3).
Figure 3.
Functionally disturbing neuropathy-free survival among cannabis-first, oxaliplatin-first, and control group. CTX onset is the date chemotherapy starts.
Multivariate Cox proportional hazard analysis for FdNFS was performed with cannabis treatment (Tx) and purpose of treatment (PoT) (adjuvant versus metastatic) as independent variables. We also included the interaction between the variables to evaluate different effects of cannabis between the PoT groups. The interaction of Tx * PoT was highly significant (hazard ratio 0.42, p = 0.0167), suggesting that patients with metastases benefit considerably more than patients in the adjuvant setting.
Discussion
In this retrospective study, we evaluated the influence of cannabis on platinum-induced neuropathy in GI cancer patients in a tertiary referral center in Israel.
Oxaliplatin-induced peripheral neuropathy is an adverse effect with substantial implications for patients’ quality of life.32,33 The accumulation of platinum in the neuronal nucleus leads to a progressive neuropathy which can, in turn, impair functional ability. Several trials have been conducted to study oxaliplatin-induced peripheral neuropathy. The IDEA trial34 was an attempt to reduce functional impairment due to neural toxicity by reducing the duration of oxaliplatin treatment in the adjuvant setting for colon cancer. It was a non-inferiority trial, in which colon cancer patients were randomly assigned to 3 and 6 months of FOLFOX versus capecitabine plus oxaliplatin (CAPOX) by physician choice. The trial did not meet its statistical boundaries for primary endpoint (disease-free survival) for the FOLFOX regimen, but it clearly demonstrated a decrease in the neuropathy grade in the shorter-duration (3 months) treatment group.
The mechanism through which cannabis and cannabinoid products affect neuropathy and neuropathic pain is considered to be through their effect on the peripheral nervous system. The influence may be related to CB2 receptors. CB2 receptors have been identified on peripheral nerve terminals,35,36 as well as throughout the immune system.37 In animal models of tissue and nerve injury-induced nociception, CB2-selective agonists suppressed hyperalgesia and allodynia, and normalized nociceptive thresholds without inducing analgesia. The effect of cannabis on peripheral neuron CB2 receptors is the presumed primary method of action of cannabis in the present study. The influence of cannabis on CB1 receptors is less well established in the literature in the context of CIPN, but it may have a role in the analgesic effect.38
Other attempts to address the relief of CIPN by means of various supplements or by the prevention of neurotoxicity have not proven any therapeutic benefit.11,12 Efforts to influence the pathological neuronal mechanism with anti-epileptic medications have resulted in only minimal palliation.13,14 Additional attempts shortening treatment time with reduced oxaliplatin cumulative dose minimizing the likelihood of neurotoxicity in the adjuvant setting,34 as well as “stop and go” approach in the metastatic setting.39
The main strength of this study is the reliability of the data on cannabis use, since all of the participating patients received cannabis by regulated licensure. It was possible to follow the dates, dosages, and indications of cannabis treatment by reviewing the patients’ cannabis approval documentation. Additionally, the data were retrieved from a large and high-quality tertiary care center database that includes medical records of patients with various GI malignancies and several treating physicians over a period of more than 2 years. The main limitation of this trial is that the comparison of cannabis use was not quantitative but qualitative: it was not possible to compare the amount of licensed cannabis or the types and indications for its use since these parameters were not specified. Also, neuropathy assessment was retrospective and relied on the doctors’ records of patients’ complaints and physical examinations.
Oxaliplatin-induced neurotoxicity is a profound adverse effect which, according to the results of our investigation, may be mitigated and prevented by cannabis treatment. A randomized placebo-controlled trial of cannabis use in the setting of oxaliplatin chemotherapy is being planned to further investigate its potentially important neuroprotective effect.
Footnotes
Conflict of interest statement: Barliz Waissengrin: no conflict of interest. Dan Mirelman: no conflict of interest. Sharon Pelles: no conflict of interest. Felix Bukstein: no conflict of interest. Deborah T. Blumenthal: medical advisor: VBL, ViruCure; honoraria: Takeda, AstraZeneca. Ido Wolf: honoraria: BMS; lectures/research grant: Novartis, BMS, Roche; consulting and advisory: Roche. Ravit Geva: options: BOL Pharma; honoraria: MSD, Novartis, BMS, Roche, Janssen, Medison, Lilly, Bayer, Pfizer; consulting and advisory: BOL Pharma, MSD, Bayer, Novartis, Boehringer Ingelheim; travel, accommodations, expenses: Bayer, Merck, Medison, BMS.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Ravit Geva  https://orcid.org/0000-0002-5380-4948
https://orcid.org/0000-0002-5380-4948
Contributor Information
Barliz Waissengrin, Division of Oncology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Dan Mirelman, Division of Oncology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel.
Sharon Pelles, Division of Oncology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Felix Bukstein, Division of Oncology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Deborah T. Blumenthal, Division of Oncology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Ido Wolf, Division of Oncology, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel.
Ravit Geva, Oncology Division, Tel-Aviv Sourasky Medical Center, 6 Weizmann Street, Tel Aviv, 6423906, Israel.
References
- 1. André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 23: 2343–2351. [DOI] [PubMed] [Google Scholar]
- 2. André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 19: 3109–3116. [DOI] [PubMed] [Google Scholar]
- 3. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011; 28: 3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Gramont AD, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 16: 2938–2947. [DOI] [PubMed] [Google Scholar]
- 5. Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol 2003; 11: 2059–2069. [DOI] [PubMed] [Google Scholar]
- 6. Farquhar-Smith P, Brown MRD. Persistent pain in cancer survivors: pathogenesis and treatment options. Pain 2016; 24: 1–8. [Google Scholar]
- 7. Selvy M, Pereira B, Kerckhove N, et al. Long-term prevalence of sensory chemotherapy-induced peripheral neuropathy for 5 years after adjuvant FOLFOX chemotherapy to treat colorectal cancer: a multicenter cross-sectional study. J Clin Med 2020; 9: 2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci 2017; 31: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett 2015; 596: 90–107. [DOI] [PubMed] [Google Scholar]
- 10. Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol 2002; 249: 9–17. [DOI] [PubMed] [Google Scholar]
- 11. Pace A, Carpano S, Galie E, et al. Vitamin E in the neuroprotection of cisplatin induced peripheral neurotoxicity and ototoxicity. J Clin Oncol 2007; 25(Suppl. 18): 9114–9115. [Google Scholar]
- 12. Cascinu S, Catalano V, Cordella L, et al. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2002; 20: 3478–3483. [DOI] [PubMed] [Google Scholar]
- 13. Argyriou AA, Chroni E, Polychronopoulos P, et al. Efficacy of oxcarbazepine for prophylaxis against cumulative oxaliplatin-induced neuropathy. Neurology 2006; 67: 2253–2255. [DOI] [PubMed] [Google Scholar]
- 14. Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain 2002; 98: 195–203. [DOI] [PubMed] [Google Scholar]
- 15. Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3). Cancer 2007; 110: 2110–2118. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong CM, Cota G. Calcium block of Na+ channels and its effect on closing rate. Proc Natl Acad Sci U S A 1999; 96: 4154–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiladze IZ, Sharikadze N, Esakia T. Calcium gluconate and magnesium sulfate in preventing neurotoxicity in patients receiving oxaliplatin-based combination chemotherapy-capeOX. Ann Oncol 2016; 27: vi166. [Google Scholar]
- 18. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014; 32: 1941–1967. [DOI] [PubMed] [Google Scholar]
- 19. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol 2020; 38: 3325–3348. [DOI] [PubMed] [Google Scholar]
- 20. Johnson JR, Burnell-Nugent M, Lossignol D, et al. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 2010; 39: 167–179. [DOI] [PubMed] [Google Scholar]
- 21. Portenoy RK, Ganae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain 2012; 13: 438–449. [DOI] [PubMed] [Google Scholar]
- 22. Johnson JR, Lossignol D, Burnell-Nugent M, et al. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage 2013; 46: 207–218. [DOI] [PubMed] [Google Scholar]
- 23. Hall W, Christie M, Currow D. Cannabinoids and cancer: causation, remediation, and palliation. Lancet Oncol 2005; 6: 35–42. [DOI] [PubMed] [Google Scholar]
- 24. Machado Rocha FC, Stéfano SC, De Cássia Haiek R, et al. Therapeutic use of cannabis sativa on chemotherapy induced nausea and vomiting among cancer patients: systematic review and meta-analysis. Eur J Cancer Care (Engl) 2008; 17: 431–443. [DOI] [PubMed] [Google Scholar]
- 25. Waissengrin B, Urban D, Leshem Y, et al. Patterns of use of medical cannabis among Israeli cancer patients: a single institution experience. J Pain Symptom Manage 2015; 49: 223–230. [DOI] [PubMed] [Google Scholar]
- 26. Comelli F, Bettoni I, Colleoni M, et al. Beneficial effects of a cannabis sativa extract treatment on diabetes-induced neuropathy and oxidative stress. Phytother Res 2009; 23: 1678–1684. [DOI] [PubMed] [Google Scholar]
- 27. Wallace MS, Marcotte TD, Umlauf A, et al. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16: 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007; 68: 515–521. [DOI] [PubMed] [Google Scholar]
- 29. King KM, Myers AM, Soroka-Monzo AJ, et al. Single and combined effects of Δ9 tetrahydrocannabinol and cannabidiol in a mouse model of chemotherapy induced neuropathic pain. Br J Pharmacol 2017; 174: 2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ward SJ, McAllister SD, Kawamura R, et al. Cannabidiol inhibits paclitaxel induced neuropathic pain through 5 HT1A receptors without diminishing nervous system function or chemotherapy efficacy. Br J Pharmacol 2014; 171: 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0, 2018, https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 32. Mols F, Beijers T, Vreugdenhil G, et al. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer 2014; 22: 2261–2269. [DOI] [PubMed] [Google Scholar]
- 33. Stefansson M, Nygren P. Oxaliplatin added to fluoropyrimidine for adjuvant treatment of colorectal cancer is associated with long-term impairment of peripheral nerve sensory function and quality of life. Acta Oncol 2016; 55: 1227–1235. [DOI] [PubMed] [Google Scholar]
- 34. Shi Q, Sobrero AF, Shields AF, et al. Prospective pooled analysis of six phase III trials investigating duration of adjuvant (adjuv) oxaliplatin-based therapy (3 vs 6 months) for patients (pts) with stage III colon cancer (CC): the IDEA (International Duration Evaluation of Adjuvant chemotherapy) collaboration. J Clin Oncol 2017; 28(Suppl. 5): v605–v649. [Google Scholar]
- 35. Pertwee R, Griffin G, Fernando S, et al. AM630, a competitive cannabinoid receptor antagonist. Life Sci 1995; 56: 1949–1955. [DOI] [PubMed] [Google Scholar]
- 36. Griffin G, Fernando SR, Ross RA, et al. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol 1997; 339: 53–61. [DOI] [PubMed] [Google Scholar]
- 37. Berry A, Cirillo PF, Riether D, et al. Compounds which modulate the CB2 receptor. Patent US7928123, USA, 2011. [Google Scholar]
- 38. Blanton HL, Brelsfoard J, DeTurk N, et al. Cannabinoids: current and future options to treat chronic and chemotherapy-induced neuropathic pain. Drugs 2019; 79: 969–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Adams RA, Meade AM, Seymour MT, et al. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 2019; 12: 642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]