Abstract
Background:
Risk factor-based equations are used to predict risk of kidney disease progression in patients with type 2 diabetes order to guide treatment decisions. It is, however, unknown whether these models can also be used to predict the effects of drugs on clinical outcomes.
Methods:
The previously developed Parameter Response Efficacy (PRE) score, which integrates multiple short-term drug effects, was first compared with the existing risk scores, Kidney Failure Risk Equation (KFRE) and The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) renal risk score, in its performance to predict end-stage renal disease (ESRD; KFRE) and doubling of serum creatinine or ESRD (ADVANCE). Second, changes in the risk scores were compared after 6 months’ treatment to predict the long-term effects of losartan on these renal outcomes in patients with type 2 diabetes and chronic kidney disease.
Results:
The KFRE, ADVANCE and PRE scores showed similarly good performance in predicting renal risk. However, for prediction of the effect of losartan, the KFRE risk score predicted a relative risk change in the occurrence of ESRD of 3.1% [95% confidence interval (CI) −5 to 12], whereas the observed risk change was −28.8% (95% CI −42.0 to −11.5). For the composite endpoint of doubling of serum creatinine or ESRD, the ADVANCE score predicted a risk change of −12.4% (95% CI −17 to −7), which underestimated the observed risk change −21.8% (95% CI −34 to −6). The PRE score predicted renal risk changes that were close to the observed risk changes with losartan treatment [−24.0% (95% CI −30 to −17) and −22.6% (95% CI −23 to −16) for ESRD and the composite renal outcome, respectively].
Conclusion:
A drug response score such as the PRE score may assist in improving clinical decision making and implement precision medicine strategies.
Keywords: diabetic kidney disease, drug response, prediction models, risk factors
Introduction
Diabetic kidney disease (DKD) is a heterogeneous disease involving various pathophysiological processes and exhibiting different rates of progression of the disease.1 Renal risk scores consisting of multiple risk markers have been developed to estimate the risk of kidney failure for patients with chronic kidney disease (CKD) or more specifically for patients with DKD.2,3 These individual predictions can be used to identify patients at high risk of disease in whom intensification of treatment is required.
Patients with DKD not only show a large variation in renal risk, but also show a large variation in drug response. Although the existing renal risk scores have been shown to predict the risk of kidney failure, it is unknown whether changes in these risk scores can predict the long term renal protection of an intervention. We previously developed and validated a drug response score [multiple Parameter Response Efficacy (PRE) score] that translates the short-term drug effects on multiple cardiorenal risk factors into a predicted drug effect on long-term renal outcomes.4–8 In this study we compared the PRE score with existing risk scores in their ability to predict renal risk and drug response on long-term renal outcomes.
Research design and methods
Included risk scores
We searched the literature for risk scores which were developed in patients with type 2 diabetes and/or chronic kidney disease to predict clinically relevant renal endpoints [end-stage renal disease (ESRD), doubling of serum creatinine] and had their risk equations and regression coefficients published. We found a total of 10 risk scores, of which two scores fulfilled our criteria and were included in the analysis.2,3 The risk scores identified by the literature search are shown in Supplemental Material Table 1 online. The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) risk engine was developed in 11,140 patients with type 2 diabetes to predict the 5-year probability of major kidney-related events (doubling of serum creatinine to ⩾200 μmol/L or ESRD).3 Predictors included in the ADVANCE risk engine are estimated glomerular filtration rate (eGFR), retinopathy, sex, urine albumin:urine creatinine ratio (UACR), systolic blood pressure (SBP), waist circumference, glycated hemoglobin and age at completion of formal education. The other risk score was the Kidney Failure Risk Equation (KFRE), which was developed and validated in patients with stage 3–5 CKD to predict the risk of ESRD.2,9–11 We used the eight-variable KFRE that included eGFR, age, sex, UACR, albumin, phosphate, bicarbonate and calcium as predictors.
These existing risk scores were compared with the PRE score. The PRE score was developed in a pooled population of the DIAMETRIC database, which is a large individual patient database of clinical trials involving patients with type 2 diabetes and CKD. To create the PRE score we used from the DIAMETRIC database the individual patient data from the IDNT, SUN-Macro and ALTITUDE trials (N = 5454).12–14 These clinical trials included patients with type 2 diabetes at high renal and cardiovascular risk. Predictors used in the PRE score were UACR, SBP, hemoglobin, glycated hemoglobin, uric acid, total cholesterol, body mass index and potassium. Additional details about the studies that were used to develop the ADVANCE, KFRE and PRE scores are summarized in Supplemental Table 2. The details of the scores and data used to develop the scores have been published previously.2,3,8
The existing risk scores were first compared with the PRE score in their performance to predict the occurrence of renal events, and second, to predict the long-term effects of the angiotensin receptor blocker (ARB) losartan on renal outcomes in subjects with type 2 diabetes and CKD who participated in the RENAAL trial. The detailed study design and outcomes of this trial have been previously published.15
Outcomes
The composite endpoint of doubling of serum creatinine to ⩾200 μmol/L or ESRD, defined by the need for long-term dialysis or renal transplantation, was used to assess the predictive performance of the ADVANCE renal risk engine since the score was developed for this specific endpoint. Similarly, the outcome of ESRD was used for predictions by KFRE since the KFRE was developed and validated to predict this endpoint. Since the individual patient data were available to develop the PRE score, predictions by the PRE score were performed for both renal endpoints.
Statistical analysis
We first compared the PRE score with the ADVANCE and KFRE scores to assess their model performances in predicting renal risk. Model performance was examined by calibration and discrimination. To assess calibration, we compared the observed versus predicted risk of renal outcomes for each quintile of predicted risk and determined the magnitude of the deviation using the Greenwood–Nam–D’Agostino (GND) χ2 statistic.16 The survival function applied to the risk score coefficients was recalibrated on the baseline hazard observed in RENAAL. Receiver operating characteristic curves and C-statistics were computed to assess discrimination.
We subsequently compared the PRE score with the existing risk scores in their ability to predict the renoprotective effects of the ARB losartan. The regression coefficients that were calculated for the PRE score and those reported for the KFRE and ADVANCE risk engine were applied to the baseline and 6-month risk marker measurements of patients in RENAAL to estimate risk of renal outcomes at both time points, as previously described.5,7,8 The mean difference in the predicted risk in the ARB arm was adjusted for the mean difference in the predicted risk in the placebo arm to calculate the expected renal risk reduction conferred by ARB treatment.
The regression coefficients of each of the predictors included in the PRE score may vary depending on the characteristics of the background population the coefficients are derived from. We therefore performed an additional analysis to assess the consistency of the regression coefficients in various subgroups of the background population. If the regression coefficients are similar regardless of the characteristics of the population it would improve the generalizability and applicability of the score to various different populations. To this end, regression coefficients in subgroups varying by UACR, eGFR, SBP, age, gender, smoker and cardiovascular history were compared. In addition, the performance of the PRE score to predict the effect of losartan on renal outcome was compared according to the characteristics of the background population. The regression coefficients used to calculate the PRE score for the composite endpoint and the ESRD endpoint are described in Supplemental Appendix 1 and Supplemental Appendix 2, respectively.
The observed drug-induced reduction in risk of renal endpoints was calculated using a Cox proportional hazards model with ARB treatment as explanatory variable. Two-sided p-values <0.05 indicated statistical significance. All statistical analyses were conducted with R version 3.4.0 (R Project for Statistical Computing, http://www.r-project.org).
Results
Model performance at baseline
A total of 263 (34.5%) patients in the placebo arm experienced the composite renal outcome in the RENAAL trial during a median follow-up of 3.4 years. The observed versus predicted probability for the composite renal outcome of doubling of serum creatinine to ⩾200 μmol/L or ESRD and for the separate ESRD endpoint in RENAAL at median follow-up are shown in Supplemental Figure 1. Observed and predicted risks over quintiles of predicted risks of the doubling of serum creatinine or ESRD endpoint significantly differed for the ADVANCE score (χ2 statistic 124.5, p < 0.01), indicating inadequate calibration. The observed and predicted risks were fairly similar for the PRE score (χ2 statistic 8.8, p = 0.07). A total of 194 (25.5%) placebo-treated patients progressed to ESRD in the RENAAL trial. Observed and predicted risks over quintiles of predicted risk of the ESRD endpoint based on the KFRE and the PRE scores were similar and represented good calibration (GND χ2 statistic 4.7, p = 0.32 and χ2 statistic 8.9, P = 0.06, respectively).
The ADVANCE score showed good discrimination for the composite renal outcome (C-statistic 0.79) but was outperformed by the PRE score (C-statistic 0.82; p for difference 0.02). The KFRE and the PRE score showed similar discrimination for the ESRD endpoint (C-statistic 0.83 and 0.81, p for difference 0.24; Supplemental Figure 2).
Observed and predicted effects of ARB treatment on renal outcomes
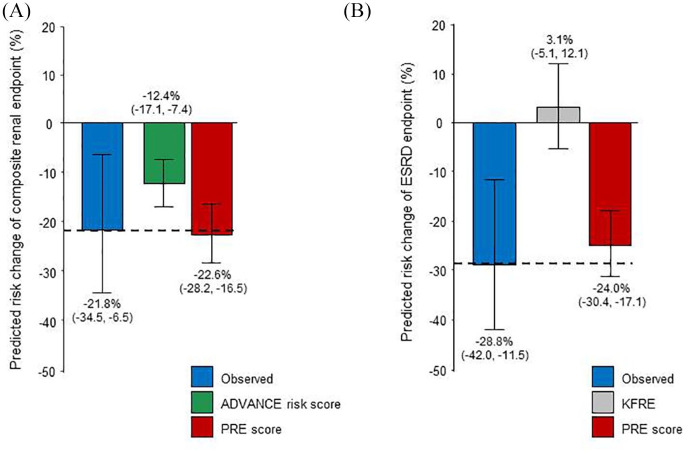
In the RENAAL trial, 226 (30.1%) patients in the losartan arm and 263 (34.5%) in the placebo arm reached the composite renal endpoint, resulting in a relative risk reduction of −21.8% (95% confidence interval (CI) −34% to −6%, p = 0.01). The predicted risk change for this endpoint based on the ADVANCE risk score was −12.4% (95% CI −17% to −7%), whereas the PRE score predicted a renal risk reduction of −22.6% (95% CI −23 to −16), close to the actual observed relative risk reduction [Figure 1(A)].
Figure 1.
Observed and predicted drug-induced changes in risk of the composite endpoint of doubling of serum creatinine to ⩾200 μmol/L or ESRD (A) and the ESRD endpoint (B) in the RENAAL dataset. Predictions based on The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) risk score are presented for only the composite renal endpoint since the ADVANCE risk score was developed using this specific endpoint. Predictions based on the KFRE are presented for only the ESRD endpoint since the KFRE was developed to predict ESRD events. PRE predictions are shown for both endpoints.
ESRD, end-stage renal disease; ADVANCE, The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation; KFRE, Kidney Failure Risk Equation; PRE, Parameter Response Efficacy.
A total of 147 (19.6%) in the losartan group and 194 (25.5%) patients in the placebo group in the RENAAL trial progressed to ESRD, relative risk reduction (−28.8%; 95% CI −42.0% to −11.5%; p = 0.002 compared with placebo). The predicted risk change for ESRD by the KFRE was 3.1% (95% CI −5% to 12%). The PRE score predicted a risk change of −24.0% [95% CI −30% to −17%, Figure 1(B)], again close to the actual observed relative risk reduction.
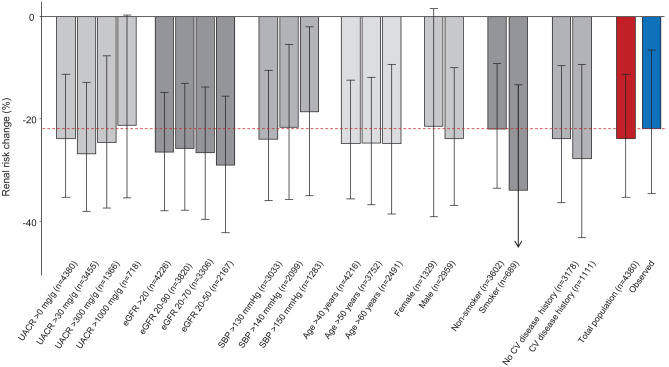
To assess the impact of the characteristics of the population on the regression coefficients included in the PRE score and predictive performance of the PRE score we performed various subgroup analyses. These analyses revealed that regression coefficients were remarkably stable regardless of the characteristics of the background population used to develop the PRE score (Supplemental Figure 3). As a result, the PRE score predicted that renal risk reduction in the RENAAL trial was consistent independent of the characteristics of the background population (Figure 2).
Figure 2.
Observed (blue bar) and PRE score predicted effect (red bar) of losartan on the composite endpoint of doubling of serum creatinine or end-stage renal disease. The gray bars show the PRE score predicted effect according to various subgroups of the background population to develop the PRE score. The consistency of the PRE score predicted effect across these subgroups indicates that the characteristics of the background population do not modify the PRE score predictions. The numbers in brackets indicate the number of participants in the background population used to develop the PRE score.
UACR, urinary albumin creatinine ratio; eGFR, estimated glomerular filtration rate; PRE, Parameter Response Efficacy; SBP, systolic blood pressure; CV, cardiovascular.
Conclusion
In this study we compared two existing risk prediction scores, the ADVANCE score and the KFRE, with the PRE score in predicting renal risk and in predicting the renoprotective effect of treatment with losartan in patients with type 2 diabetes and CKD. The three scores showed generally equal good performance to predict renal risk. However, therapy response predictions using the ADVANCE score and KFRE score markedly underestimated the actual observed renoprotective effect of losartan, whereas renoprotective drug efficacy estimates based on the PRE score were similar to those actually observed.
Why did the PRE score seem to perform better in predicting the effect of losartan than existing risk scores? While the ADVANCE risk score and the KFRE are based on baseline demographic and/or disease characteristics, of which some are not influenced by treatment (e.g. age or gender), the PRE score incorporates risk markers that change during drug intervention (e.g. blood pressure or hemoglobin). Interestingly, the different choice of parameters in the three scores had only modest influence on the performance to predict renal risk. It should also be noted that the PRE score was developed using clinical trial data which enhances internal validity given careful registration and adjudication of clinical endpoints.17 In contrast, most risk scores, like KFRE, are developed in observational studies with less stringent outcome recording and adjudication.
Developing drug response tools in patients with type 2 diabetes is an area of active ongoing research. A recent study proposed five clusters of individuals with different rates of diabetes progression and risk of complications. The authors suggested that this subclassification might help to tailor early treatment to patients who would benefit most.18 A subsequent analysis assessing the clinical utility of this cluster-based strategy indicated that for predicting response to glucose lowering drugs, models incorporating simple clinical features outperformed the cluster approach.19 However, the performance of all models, both clusters and clinical features, to predict response to glucose lowering drugs was low.19 Hence, these studies show that clinical features measured before drug exposure are insufficient to accurately and precisely predict drug response. Alternative strategies should thus be explored such as using the observed change in clinical features during the first weeks or months of therapy to predict a drug’s efficacy to reduce the risk of long-term clinical outcomes. We showed that the PRE score, by integrating changes in risk markers after a short period of treatment, can be utilized to predict long-term drug response, providing a promising alternative to predictions based on clinical features before drug exposure.4–8
This study should be interpreted with the following limitations in mind. We note that the ADVANCE score included the presence of retinopathy and the age at which formal education was completed as predictors for renal outcomes. These data were not available in the RENAAL study, which may have resulted in an underestimation of the performance to predict renal events, although it is unlikely that drug response estimations by the ADVANCE risk score were influenced since these features do not change over a short-term period. We compared the performance of the three risk scores to predict the effect in a single trial with a specific drug, losartan. Other studies demonstrated that the PRE score accurately predicts the effect of other drugs such as a glucagon like peptide receptor agonist, sodium glucose co-transporter 2 inhibitor and endothelin receptor antagonist.5,20–22 The risk scores compared in the present study were derived from different populations. The ADVANCE score was developed in a cohort of patients with type 2 diabetes at high cardiovascular risk, the KFRE was developed in a cohort of patients with severe kidney disease while the PRE score was developed in a broad population of patients with type 2 diabetes at early and advanced stage of disease. These differences should be taken into account when interpreting the findings and conclusions of our study. This study demonstrated that the PRE score adequately predicted response to ARB therapy. Further prospective studies are needed to assess whether a PRE score guided therapy approach compared with standard of care improves long-term clinical outcomes.
In conclusion, this study showed that the PRE-score, a composite score of multiple short-term drug effects, provided a more accurate prediction of long-term effects of ARBs on renal outcomes than existing risk scores, while it showed similar performance on estimating renal risk. This response score may aid in personalizing treatment in patients with type 2 diabetes to guide therapy towards more favorable outcomes.
Supplemental Material
Supplemental material, sj-pdf-1-tae-10.1177_2042018820974191 for A novel drug response score more accurately predicts renoprotective drug effects than existing renal risk scores by Nienke M. A. Idzerda, Sok Cin Tye, Dick de Zeeuw and Hiddo J. L. Heerspink in Therapeutic Advances in Endocrinology and Metabolism
Footnotes
Conflict of interest statement: NMAI and SCT report no conflicts of interest. HJLH serves as a consultant for AbbVie, Astellas, AstraZeneca, Boehringer Ingelheim, Fresenius, Gilead, Janssen, Merck, Mundi Pharma, and Mitsubishi Tanabe. DdZ is consultant for and received honoraria from AbbVie, Bayer, Boehringer Ingelheim, Fresenius, Janssen, Mundipharma, Mitsubishi-Tanabe.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NMAI is supported by a grant from the Innovative Medicines Initiative BEAt-DKD programme. The BEAt-DKD project has received funding from the IMI2 Joint Undertaking under grant agreement 115974. This joint undertaking receives support from the European Union’s Horizon 2020 research and innovation program and European Federation of Pharmaceutical Industries and Associations. SCT received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 754425. HJLH is supported by a VIDI grant from the Netherlands Organisation for Scientific Research (917.15.306).
ORCID iD: Nienke M. A. Idzerda  https://orcid.org/0000-0001-6912-3429
https://orcid.org/0000-0001-6912-3429
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Nienke M. A. Idzerda, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands
Sok Cin Tye, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Dick de Zeeuw, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Hiddo J. L. Heerspink, Department of Clinical Pharmacy and Pharmacology, University of Groningen, University Medical Center Groningen, Hanzeplein 1, PO Box 30001, Groningen, 9700 RB, The Netherlands.
References
- 1. Tuomi T, Santoro N, Caprio S, et al. The many faces of diabetes: a disease with increasing heterogeneity. Lancet 2014; 383: 1084–1094. [DOI] [PubMed] [Google Scholar]
- 2. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011; 305: 1553–1559. [DOI] [PubMed] [Google Scholar]
- 3. Jardine MJ, Hata J, Woodward M, et al. Prediction of kidney-related outcomes in patients with type 2 diabetes. Am J Kidney Dis 2012; 60: 770–778. [DOI] [PubMed] [Google Scholar]
- 4. Schievink B, Grobbee D, Michael Lincoff A. Heart failure induced by aleglitazar treatment can be predicted based on short-term response in multiple risk markers. Submitted for publication. [Google Scholar]
- 5. Schievink B, de Zeeuw D, Smink PA, et al. Prediction of the effect of atrasentan on renal and heart failure outcomes based on short-term changes in multiple risk markers. Eur J Prev Cardiol 2016; 23: 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schievink B, de Zeeuw D, Parving HH, et al. The renal protective effect of angiotensin receptor blockers depends on intra-individual response variation in multiple risk markers. Br J Clin Pharmacol 2015; 80: 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smink PA, Hoekman J, Grobbee DE, et al. A prediction of the renal and cardiovascular efficacy of aliskiren in ALTITUDE using short-term changes in multiple risk markers. Eur J Prev Cardiol 2014; 21: 434–441. [DOI] [PubMed] [Google Scholar]
- 8. Smink PA, Miao Y, Eijkemans MJ, et al. The importance of short-term off-target effects in estimating the long-term renal and cardiovascular protection of angiotensin receptor blockers. Clin Pharmacol Ther 2014; 95: 208–215. [DOI] [PubMed] [Google Scholar]
- 9. Peeters MJ, van Zuilen AD, van den Brand JA, et al. Validation of the kidney failure risk equation in European CKD patients. Nephrol Dial Transplant 2013; 28: 1773–1779. [DOI] [PubMed] [Google Scholar]
- 10. Whitlock RH, Chartier M, Komenda P, et al. Validation of the kidney failure risk equation in Manitoba. Can J Kidney Health Dis 2017; 4: 2054358117705372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Winnicki E, McCulloch CE, Mitsnefes MM, et al. Use of the kidney failure risk equation to determine the risk of progression to end-stage renal disease in children with chronic kidney disease. JAMA Pediatr 2018; 172: 174–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367: 2204–2213. [DOI] [PubMed] [Google Scholar]
- 13. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860. [DOI] [PubMed] [Google Scholar]
- 14. Packham DK, Wolfe R, Reutens AT, et al. Sulodexide fails to demonstrate renoprotection in overt type 2 diabetic nephropathy. J Am Soc Nephrol 2012; 23: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 16. Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med 2015; 34: 1659–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basu S, Sussman JB, Berkowitz SA, et al. Development and validation of Risk Equations for Complications Of type 2 Diabetes (RECODe) using individual participant data from randomised trials. Lancet Diabetes Endocrinol 2017; 5: 788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahlqvist E, Storm P, Karajamaki A, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol 2018; 6: 361–369. [DOI] [PubMed] [Google Scholar]
- 19. Dennis JM, Shields BM, Henley WE, et al. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol 2019; 7: 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Idzerda NMA, Stefansson BV, Pena MJ, et al. Prediction of the effect of dapagliflozin on kidney and heart failure outcomes based on short-term changes in multiple risk markers. Nephrol Dial Transplant 2020; 35: 1570–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Idzerda NMA, Clegg LE, Hernandez AF, et al. Prediction and validation of exenatide risk marker effects on progression of renal disease: insights from EXSCEL. Diabetes Obes Metab 2020; 22: 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heerspink HJL, Parving HH, Andress DL, et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 2019; 393: 1937–1947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tae-10.1177_2042018820974191 for A novel drug response score more accurately predicts renoprotective drug effects than existing renal risk scores by Nienke M. A. Idzerda, Sok Cin Tye, Dick de Zeeuw and Hiddo J. L. Heerspink in Therapeutic Advances in Endocrinology and Metabolism