Abstract
Background
Three vascular endothelial growth factor (VEGF) inhibitors, Bevacizumab (BEV), ramucirumab (RAM), and aflibercept (AFL), are widely used for metastatic colorectal cancer (mCRC) patients who are treated with second‐line chemotherapy. The difference in outcome between the three drugs has not been evaluated. In contrast to epidermal growth factor receptor inhibitors, VEGF inhibitors have few candidate predictors of efficacy.
Methods
Consecutive mCRC patients who were treated with second‐line chemotherapy were retrospectively enrolled. Overall response rate (ORR), progression‐free survival (PFS), overall survival (OS), and safety were assessed. Subgroup analyses of prognostic and predictive efficacy markers were performed.
Results
A total of 119 (41.2%), 107 (37.0%), and 63 patients (21.8%) were treated with FOLFIRI +BEV, RAM, or AFL, respectively. ORR, PFS, and OS showed no significant differences between three groups. However, the frequency of grade 3 or 4 adverse events (AEs) in the FOLFIRI +AFL group was significantly higher than that in the other groups (p < 0.001). Patients with grade 3 or 4 AEs, especially hypertension and neutropenia within the first four cycles of treatment had significantly longer PFS and OS than those without AEs, irrespective of treatment with VEGF inhibitors (p < 0.001). PFS in patients without prior BEV exposure was also significantly longer than that in patients with prior BEV exposure (p = 0.003).
Conclusions
Chemotherapeutic efficacy did not differ between the groups. Grade 3 or 4 AEs within the first four cycles of treatment and prior BEV exposure may be an effective predictor of treatment efficacy in mCRC patients administered VEGF inhibitors as second‐line chemotherapy.
Keywords: angiogenesis inhibitors, colorectal cancer, drug response biomarkers, vascular endothelial growth factors
Chemotherapeutic efficacy did not differ between the groups. Grade 3 or 4 AEs within the first four cycles of treatment may be an effective predictor of treatment efficacy in mCRC patients administered VEGF inhibitors as second‐line chemotherapy

1. INTRODUCTION
Angiogenesis is induced by the release of angiogenic factors in tissues with ischemia, such as cancer or wound healing, due to hypoxia and growth factors. 1 One of the glycoproteins involved in angiogenesis is vascular endothelial growth factor (VEGF) and excessive secretion of VEGF in cancer leads to abnormal angiogenesis. 2 , 3 Since tumors are accompanied by abnormal vascular structure, tumor fluid interstitial pressure increases due to vascular leakage and is accompanied by hypoxia. 4 , 5 These factors are related to tumor progression and treatment resistance. 6 , 7 Antiangiogenic drugs can inhibit the supply of oxygen and nutrients to the cancer, therefore suppressing the growth of the cancer. In addition, by reducing vascular permeability and normalizing interstitial pressure, concomitant cytotoxic chemotherapy can be delivered to cancer more easily. 8 Combination treatment of antiangiogenic drugs and cytotoxic chemotherapy are recommended for metastatic colorectal cancer (mCRC) patients as a second‐line chemotherapy. 9 , 10 because the addition of these drugs significantly increases the overall survival (OS) compared to cytotoxic chemotherapy alone. 11 , 12 , 13 Bevacizumab (BEV) is a monoclonal antibody against VEGF, which inhibits the action of VEGF, therefore suppressing angiogenesis and tumor growth and metastasis. 14 The phase III ML18147 trial reported BEV improves survival in patients who had already received BEV as first‐line therapy (HR 0.83, p = 0.021). 11 Ramucirumab (RAM), an anti‐VEGF receptor 2 (VEGFR‐2) fully human monoclonal IgG1 antibody, is a member of the antibody class of molecularly targeted therapies that works to inhibit tumor growth by preventing VEGF from binding to VEGFR‐2 and sending angiogenic signals downstream. 15 The phase III RAISE trial showed a significantly survival benefit for patients who were treated with RAM +FOLFIRI (HR 0.84, p = 0.0219). 16 Aflibercept (AFL) is a recombinant fusion protein consisting of the extracellular domain of the human VEGF receptor 1 and 2 proteins and the Fc portion of the human antibody IgG1. 17 , 18 The phase III VELOUR study revealed that compared to the placebo, AFL addition resulted in a significantly increased survival rate (HR 0.817, p = 0.0032). 13 According to these results, these antiangiogenic drugs were approved in Japan as combination therapy with FOLFIRI in a second‐line setting. 10 However, at present, there is no randomized trial directly comparing the three antiangiogenic drugs (BEV, RAM, and AFL) with FOLFIRI in second‐line mCRC treatment. Furthermore, there are only a few reports about predictive and/or surrogate biomarkers of treatment efficacy for second‐line VEGF inhibitor containing chemotherapy 12 , 19 although there is several reports that hypertension may be surrogate marker of clinical outcome of first‐line chemotherapy with BEV in mCRC. 20 , 21 The present study evaluated both the efficacy and safety among mCRC patients who were treated with FOLFIRI +BEV, RAM, or AFL as second‐line chemotherapy, and explored the predictive biomarkers for treatment efficacy to contribute information required for appropriate clinical decision‐making processes.
2. MATERIALS AND METHODS
2.1. Patients and treatment schedule
Two hundred and eighty‐nine mCRC patients who were treated with second‐line chemotherapies at our hospital, from January 2017 to December 2019 were retrospectively enrolled in the current study. BEV was administered at the recommended dose of 5 mg/kg. RAM was administered at the recommended dose of 8 mg/kg. AFL was administered at the recommended dose of 4 mg/kg. The concomitant chemotherapy was FOLFIRI (irinotecan 150–180 mg/m2, L‐leucovorin 200 mg/m2, bolus 5‐FU 400 mg/ m2, 46‐h infusion of 5‐FU 2,400 mg/ m2). Prophylactic treatments and dose reduction were performed based on recommendations of guidelines and physician's decisions.
2.2. Assessments
We collected the data identified by medical record and/or imaging. We confirmed age, sex, primary site, metastatic site, RAS status in tissue, prior BEV exposure in first‐line chemotherapy, first‐line progression‐free survival (patients treated with BEV only), patients who experienced relapse within 6 months of completing oxaliplatin‐based adjuvant therapy, and tumor markers (CEA and CA19‐9). Complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) were defined based on RECIST guidelines, v1.1. Objective response rate (ORR) denoted the proportion of patients who had a CR or PR to second‐line chemotherapy, and disease control rate (DCR) indicated the proportion of patients who had a CR, PR, or SD response to therapy. We defined progression‐free survival (PFS) as the time from the first day of second‐line treatment to either the first objective evidence of disease progression or death from any cause. We also defined OS as the time from the first day of second‐line treatment until the time of death. We assessed the grade of adverse events (AEs) using the Common Toxicity Criteria for Adverse Events (CTCAE) v4.0.
2.3. Statistical analyses
We estimated PFS and OS using the Kaplan–Meier method and also assessed the statistical significance of the correlation between the clinical outcome and clinical parameters using the log‐rank test. The t‐test, chi‐squared test, and Cox proportional hazard analysis were used for statistics tests. A value of p < 0.05 was considered statistically significant. In the Cox proportional hazard analysis, factors with p < 0.05 in the univariate analysis were included in the multivariate analysis (backward stepwise methods). Statistical analyses were performed using the EZR statistical software 1.41. 22
3. RESULTS
3.1. Patient characteristics
The characteristics of 289 mCRC patients who were treated with second‐line chemotherapy in our hospital are shown in Table 1. Median age was 63.0 years (range, 31.0–84.0 years). A total of 119 (41.2%), 107 (37.0%), and 63 patients (21.8%) were treated with FOLFIRI +BEV, RAM, and AFL, respectively. No significant differences were observed about RAS status, location of primary tumor, or the ratio of BEV exposure in pretreatment among the three groups.
TABLE 1.
Patient demographics and clinical characteristics.
| Characteristics |
Total (N = 289) No. of patients (%) |
FOLFIRI + Bevacizumab (N = 119) | FOLFIRI + Ramucirumab (N = 107) | FOLFIRI + Aflibercept (N = 63) | p value |
|---|---|---|---|---|---|
| Age at enrollment, years | |||||
| Median [range] | 63.0 [31.0–84.0] | 63.0 [32.0–82.0] | 64.0 [31–84.0] | 62.0 [43.0–80.0] | 0.87 |
| Sex | |||||
| Male | 137 (47.4) | 57 (47.9) | 46 (43.0) | 34 (54.0) | 0.39 |
| Female | 152 (52.6) | 62 (52.1) | 61 (57.0) | 29 (46.0) | |
| Primary site | |||||
| Right‐sided colon | 93 (32.2) | 39 (32.8) | 22 (20.6) | 17 (27.0) | 0.12 |
| Left‐sided colon | 196 (67.8) | 80 (67.2) | 85 (79.4) | 46 (73.0) | |
| Metastatic site | |||||
| Liver | 148 (51.2) | 58 (48.7) | 59 (55.1) | 31 (49.2) | 0.59 |
| Lung | 145 (50.2) | 66 (55.5) | 49 (45.8) | 36 (57.1) | 0.25 |
| Peritoneal | 97 (33.6) | 38 (31.9) | 31 (29.0) | 25 (39.7) | 0.34 |
| Lymph node | 99 (34.3) | 40 (33.6) | 40 (37.4) | 17 (27.0) | 0.38 |
| Other | 41 (14.2) | 14 (11.8) | 16 (15.0) | 11 (17.5) | 0.54 |
| RAS status in tissue | |||||
| Wild type | 134 (46.4) | 47 (39.5) | 57 (53.3) | 30 (47.6) | 0.11 |
| Mutant | 155 (53.6) | 72 (60.5) | 50 (46.7) | 33 (52.4) | |
| Prior bevacizumab exposure in first‐line chemotherapy | |||||
| Yes | 159 (55.0) | 68 (57.1) | 54 (50.5) | 37 (58.7) | 0.55 |
| No | 130 (45.0) | 51 (42.9) | 53 (49.5) | 26 (41.3) | |
| First‐line progression‐free survival (Patients treated with Bevacizumab only) | |||||
| ≤9 months | 71 (44.7) | 25 (36.8) | 29 (53.7) | 17 (45.9) | 0.16 |
| >9 months | 88 (55.3) | 43 (63.2) | 25 (46.3) | 20 (54.1) | |
| Patients who experienced relapse within 6 months of completing oxaliplatin‐based adjuvant therapy | |||||
| Yes | 40 (13.8) | 15 (12.6) | 15 (14.0) | 10 (15.9) | 0.84 |
| No | 249 (86.2) | 104 (87.4) | 92 (86.0) | 53 (84.1) | |
| Tumor markers (at initiation of second‐line chemotherapy) | |||||
| CEA median, [range] | 17.3 [0.5–17056.1] | 16.8 [1.0–1501.4] | 28.3 [0.5–17056.1] | 14.9 [1.0–7415] | 0.30 |
| CA19‐9 median, [range] | 32.7 [2.0–50000] | 27.6 [2.0–29210.2] | 33.0 [2.0–50000] | 33.6 [2.0–50000] | 0.71 |
| RAS:rat sarcoma viral oncogene homolog | |||||
| CEA: carcinoembryonic antigen | |||||
| CA19‐9: carbohydrate antigen 19–9 | |||||
3.2. Survival endpoints and factors associated with survival
To assess the clinical efficacy of FOLFIRI +each antiangiogenic drug in mCRC patients, we compared PFS, OS, and ORR among patients treated with FOLFIRI +BEV, FOLFIRI +RAM, and FOLFIRI +AFL. The median PFS values were 7.2 months (6.0–9.0), 5.8 months (4.6–6.8), and 8.2 months (5.2–12.8), respectively (p = 0.21; Figure S1A). The median OS was 18.6 months (17.4–21.3), 23.0 months (16.7–31.3), and NA, respectively (p = 0.47; Figure S1B). The ORR from each group was 15.1%, 11.2%, and 17.4%, respectively (p = 0.48, Table S1). No significant differences were observed between the groups based on RAS status and primary tumor location (RAS status: RAS wild type vs. RAS mutant; 6.5 months vs. 6.7 months, p = 0.93; primary tumor location: left side vs. right side; 7.1 months vs. 6.0 months, p = 0.09). Patients with grade 3 or 4 AEs within the first four cycles of treatment were related to significantly longer PFS (p = 0.0002; Figure 1A) and OS (p = 0.0001; Figure 1B); the ORR from each group was 16.9% and 12.2%, respectively (p = 0.32). In analysis of each AE, treatment‐induced neutropenia and hypertension within the first four cycles of treatment were related to significantly longer PFS (Neutropenia: p = 0.006, Hypertension: p = 0.001; Figure 2A,C) and OS (Neutropenia: p = 0.0005, Hypertension: p = 0.02; Figure 2B,D). Figure S2 shows that grade 3 or 4 AEs within the first four cycles of treatment were related to significantly longer PFS in patients both with and without prior BEV exposure (Non‐prior BEV: p = 0.009; prior BEV: p = 0.00001). Mean dose intensities of irinotecan within the first four cycles were 61.2 mg/m2/week, 60.5 mg/m2/week, and 59.0 mg/m2/week, respectively (p = 0.76). PFS in non‐prior BEV patients was also significantly longer than prior BEV patients (p = 0.003; Figure 3A); the ORR from each group was 21.5% and 7.5%, respectively (p = 0.0009).
FIGURE 1.
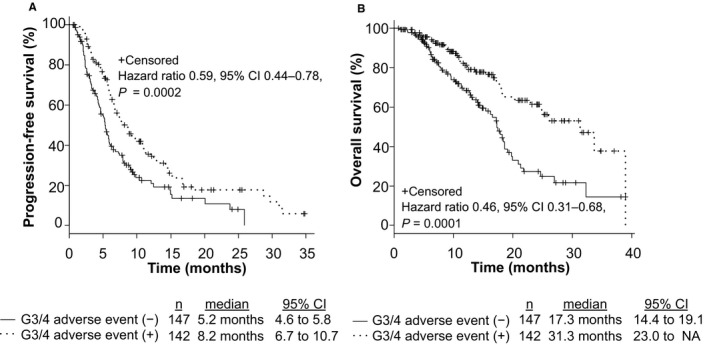
Progression‐free survival (PFS) and overall survival (OS) with respect to grade 3 or 4 adverse events (AEs) within the first four cycles of treatment in metastatic colorectal cancer (mCRC) patients who were treated with second‐line chemotherapy. This figure shows PFS (A) and OS (B) in patients with grade 3 or 4 AEs within the first four cycles of treatment compared to those without grade 3 or 4 AEs
FIGURE 2.
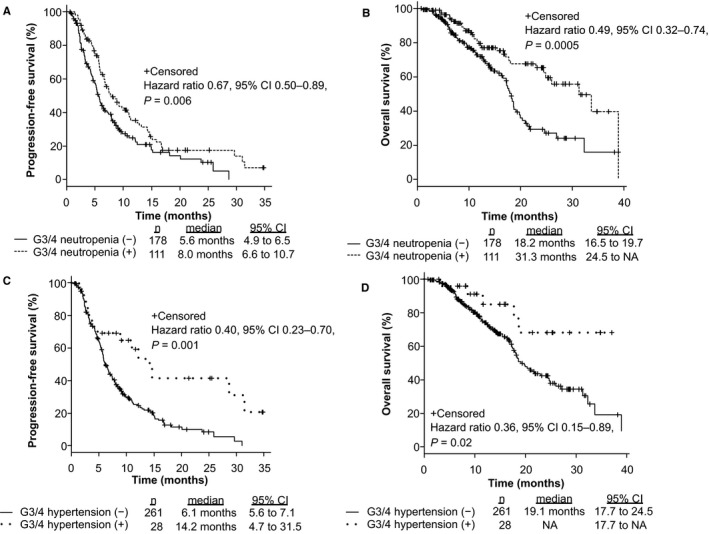
Progression‐free survival (PFS) and overall survival (OS) with respect to grade 3 or 4 neutropenia and hypertension within the first four cycles of treatment in mCRC patients who were treated with second‐line chemotherapy. This figure shows PFS (A, C) and OS (B, D) in patients with grade 3 or 4 neutropenia and hypertension within the first four cycles of treatment compared to those without grade 3 or 4 neutropenia and hypertension
FIGURE 3.
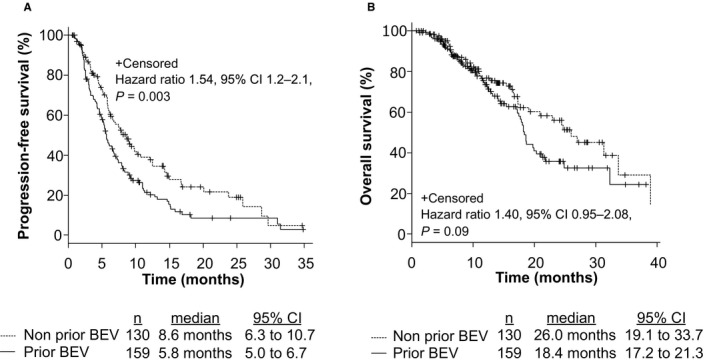
Progression‐free survival (PFS) and overall survival (OS) with respect to prior bevacizumab (BEV) exposure in mCRC patients who were treated with second‐line chemotherapy. This figure shows PFS (A) and OS (B) in patients with prior BEV exposure compared to those without prior BEV exposure
3.3. Toxicity
We next analyzed the AEs of mCRC patients who were treated with FOLFIRI +antiangiogenic drugs. Grade 3 or 4 AEs occurred in 168 patients (58.1%). AE occurrences in 168 patients with mCRC are summarized in Table 2. No treatment‐related death was observed. The most common grade 3 or 4 AEs were neutropenia (49.1%), hypertension (10.0%), and proteinuria (8.0%), respectively. Febrile neutropenia was observed in five patients (1.7%). The frequency of grade 3 or 4 AEs in the FOLFIRI +AFL group was significantly higher than those of the FOLFIRI +BEV group (any grade 3 or 4 AEs: 46.2%, 57.0% and 82.5%, p < 0.001; neutropenia: 38.7%, 56.1%, and 57.1%, p = 0.01; nausea: 0%, 0.9%, 4.8%, p = 0.02; hypertension: 1.7%, 11.2%, and 23.8%, p = 0.000007 in the FOLFIRI +BEV, RAM, and AFL groups, respectively).
TABLE 2.
Incidence of grade 3 or 4 of adverse events according to second‐line chemotherapy
| Adverse event | FOLFIRI + Bevacizumab (N = 119) | FOLFIRI + Ramucirumab (N = 107) | FOLFIRI + Aflibercept (N = 63) | p value |
|---|---|---|---|---|
| Neutropenia, (%) | 46 (38.7) | 60 (56.1) | 36 (57.1) | 0.01 |
| Febrile neutropenia, (%) | 1 (0.8) | 3 (2.8) | 1 (1.6) | 0.64 |
| Nausea, (%) | 0 (0) | 1 (0.9) | 3 (4.8) | 0.02 |
| Diarrhea, (%) | 5 (4.2) | 5 (4.7) | 5 (7.9) | 0.55 |
| Hypertension, (%) | 2 (1.7) | 12 (11.2) | 15 (23.8) | 0.000007 |
| Thromboembolic events, (%) | 4 (3.4) | 2 (1.9) | 1 (1.6) | 0.79 |
| Proteinuria, (%) | 6 (5.0) | 9 (8.4) | 8 (12.7) | 0.18 |
| Bleeding, (%) | 0 (0) | 0 (0) | 2 (3.2) | 0.047 |
| Mucotitis, (%) | 2 (1.7) | 2 (1.9) | 1 (1.6) | 1 |
| Infection, (%) | 1 (0.8) | 2 (1.9) | 1 (1.6) | 0.83 |
| Fatigue, (%) | 4 (3.4) | 2 (1.9) | 0 (0) | 0.38 |
| Edema, (%) | 1 (0.8) | 2 (1.9) | 0 (0) | 0.61 |
| Renal failure, (%) | 0 (0) | 1 (0.9) | 1 (1.6) | 0.35 |
The frequency of dose reduction or delayed treatment was 68.1% (81/119), 68.8% (75/109), and 68.3% (43/63) in the FOLFIRI +BEV, RAM, and AFL groups, respectively (p = 1). The relative dose intensity of each VEGF inhibitor was 72.7%, 72.1%, and 65.0%, respectively (p = 0.42), while the duration of administration of each VEGF inhibitor was 4.9 months, 4.4 months, and 5.2 months, respectively, in the same groups (p = 0.28). The frequency of grade 3 or 4 AEs within the first four cycles of treatment in mCRC patients treated with second‐line chemotherapy is summarized in Table 3. The most common grade 3 or 4 AEs within the first four cycles of treatment were neutropenia (38.4%), hypertension (9.7%), and proteinuria (6.9%) in the FOLFIRI +BEV, RAM, and AFL groups, respectively. The frequency of grade 3 or 4 AEs in the FOLFIRI +AFL group was significantly higher than that in the FOLFIRI +BEV group (any grade 3 or 4 AEs: 37.0%, 49.5%, and 71.4%, p = 0.00005; neutropenia: 26.9%, 43.9%, and 50.8%, p = 0.002; nausea: 0%, 0%, and 4.8%, p = 0.01; hypertension: 1.7%, 11.2%, and 22.2%, p = 0.00002 in the FOLFIRI +BEV, RAM, and AFL groups, respectively).
TABLE 3.
Incidence of grade 3 or 4 adverse events within the first four cycles of treatment in patients with mCRC treated with second‐line chemotherapy
| Adverse event | FOLFIRI + Bevacizumab (N = 119) | FOLFIRI + Ramucirumab (N = 107) | FOLFIRI + Aflibercept (N = 63) | p value |
|---|---|---|---|---|
| Neutropenia, (%) | 32 (26.9) | 47 (43.9) | 32 (50.8) | 0.002 |
| Febrile neutropenia, (%) | 1 (0.8) | 2 (1.9) | 1 (1.6) | 0.83 |
| Nausea, (%) | 0 (0) | 0 (0) | 3 (4.8) | 0.01 |
| Diarrhea, (%) | 5 (4.2) | 2 (1.9) | 5 (7.9) | 0.17 |
| Hypertension, (%) | 2 (1.7) | 12 (11.2) | 14 (22.2) | 0.00002 |
| Thromboembolic events, (%) | 1 (0.8) | 1 (0.9) | 1 (1.6) | 1 |
| Proteinuria, (%) | 6 (5.0) | 8 (7.5) | 6 (9.5) | 0.50 |
| Bleeding, (%) | 0 (0) | 0 (0) | 2 (3.2) | 0.047 |
| Mucotitis, (%) | 1 (0.8) | 2 (1.9) | 1 (1.6) | 0.83 |
| Infection, (%) | 0 (0) | 2 (1.9) | 1 (1.6) | 0.33 |
| Fatigue, (%) | 4 (3.4) | 2 (1.9) | 0 (0) | 0.38 |
| Edema, (%) | 0 (0) | 1 (0.9) | 0 (0) | 0.59 |
| Renal failure, (%) | 0 (0) | 0 (0) | 1 (1.6) | 0.22 |
3.4. Univariate and multivariate analyses
In the univariate Cox proportional hazard analysis, liver metastasis, prior BEV exposure, and grade 3 or 4 AEs within the first four cycles were predictors for PFS (Table 4). Similarly, liver metastasis and grade 3 or 4 AEs within the first four cycles were predictors for OS (Table 4). Moreover, all of these were independent predictors for PFS (liver metastasis: HR 1.47, 95% CI 1.10–1.97, p = 0.01; prior BEV exposure: HR 1.52, 95% CI 1.13–2.05, p = 0.006; AEs: HR 0.57, 95% CI 0.43–0.77, p = 0.0002; Table 4) and OS (liver metastasis: HR 2.34, 95% CI 1.55–3.54, p = 0.00006; AEs: HR 0.44, 95% CI 0.29–0.66, p = 0.00009; Table 4) in the multivariate analysis.
TABLE 4.
Cox proportional hazard analysis for PFS and OS in mCRC patients treated with second‐line chemotherapy
| PFS | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | Lower 95% CI | Upper 95% CI | p value | HR | Lower 95% CI | Upper 95% CI | p value | ||
| Sex (Female* or Male) | 0.85 | 0.63 | 1.15 | 0.29 | |||||
| Age (<65* or ≥65) | 0.96 | 0.71 | 1.29 | 0.76 | |||||
| Primary tumor location (Left* or Right) | 1.35 | 0.99 | 1.86 | 0.06 | |||||
| Liver metastasis (Negative* or Positive) | 1.50 | 1.11 | 2.02 | 0.008 | 1.47 | 1.10 | 1.97 | 0.01 | |
| Lung metastasis (Negative* or Positive) | 0.92 | 0.68 | 1.24 | 0.58 | |||||
| Peritoneal metastasis (Negative* or Positive) | 1.21 | 0.89 | 1.65 | 0.22 | |||||
| Lymph node metastasis (Negative* or Positive) | 1.15 | 0.84 | 1.57 | 0.37 | |||||
| Tissue RAS mutation (Negative* or Positive) | 0.91 | 0.67 | 1.23 | 0.54 | |||||
| Prior bevacizumab exposure in first‐line chemotherapy (Negative* or Positive) | 1.51 | 1.11 | 2.04 | 0.008 | 1.52 | 1.13 | 2.05 | 0.006 | |
| Grade 3 or 4 adverse events within the first four cycles of treatment (Negative* or Positive) | 0.58 | 0.44 | 0.78 | 0.0003 | 0.57 | 0.43 | 0.77 | 0.0002 | |
| Treatment regimen (Bevacizumab or other*) | 1.10 | 0.83 | 1.46 | 0.51 | |||||
| OS | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | Lower 95% CI | Upper 95% CI | p value | HR | Lower 95% CI | Upper 95% CI | p value | |
| Sex (Female* or Male) | 1.04 | 0.70 | 1.55 | 0.85 | ||||
| Age (<65* or ≥65) | 0.93 | 0.62 | 1.39 | 0.73 | ||||
| Primary tumor location (Left* or Right) | 1.33 | 0.87 | 2.04 | 0.18 | ||||
| Liver metastasis (Negative* or Positive) | 2.26 | 1.45 | 3.44 | 0.0001 | 2.34 | 1.55 | 3.54 | 0.00006 |
| Lung metastasis (Negative* or Positive) | 0.87 | 0.58 | 0.51 | |||||
| Peritoneal metastasis (Negative* or Positive) | 0.92 | 0.60 | 1.42 | 0.72 | ||||
| Tissue RAS mutation (Negative* or Positive) | 1.19 | 0.78 | 1.82 | 0.41 | ||||
| Prior bevacizumab exposure in first‐line chemotherapy (Negative* or Positive) | 1.45 | 0.96 | 2.18 | 0.07 | ||||
| Grade 3 or 4 adverse events within the first four cycles of treatment (Negative* or Positive) | 0.44 | 0.29 | 0.67 | 0.0001 | 0.44 | 0.29 | 0.66 | 0.00009 |
| Treatment regimen (Bevacizumab or other*) | 0.93 | 0.63 | 1.36 | 0.69 | ||||
4. DISCUSSION
To the best of our knowledge, this is the first report to evaluate safety and efficacy among FOLFIRI combined with BEV, RAM, or AFL as second‐line chemotherapy treatments in mCRC patients. No significant difference in chemotherapeutic efficacy was observed among the three groups. However, the AE rate was significantly higher, especially in the FOLFIRI +AFL group than in the FOLFIRI +BEV group. Furthermore, grade 3 or 4 AEs within the first four cycles were a surrogate marker for both PFS and OS, while prior BEV exposure was a predictor for PFS.
At present, there are no established efficacy biomarkers for any antiangiogenic drugs in mCRC. In this study a strong correlation was observed between the AEs, especially neutropenia and hypertension, and treatment efficacy. A previous report showed an association between the grade 3 or 4 diarrhea after the first cycle of irinotecan and the disease control. 23 This result suggests an association between antitumor effect and irinotecan exposure and/or its metabolites. 23 Indeed, neutropenia and delayed diarrhea have been shown to be related to both irinotecan and SN‐38 AUCs. 24 However, the correlation between those parameters and tumor response has not been clearly clarified 25 ; further study will be needed to clear this hypothesis. Furthermore, AEs related to antiangiogenic drugs as a surrogate marker of efficacy have shown in other cancers. Previous reports showed that VEGF binding to the VEGF receptor caused the induction of endothelial cells to increase nitric oxide production, which leads to vasodilation and reduced blood pressure. 19 , 26 Thus, hypertension is associated with impaired angiogenesis and represents bypass signaling pathway blockade of angiogenesis, especially in patients treated with BEV in first‐line chemotherapy. Furthermore, because angiogenesis inhibitors block the VEGF signaling, which is essential for the survival and maintenance of normal vascular endothelium, AEs of VEGF inhibitor may be derived from disorder to normal blood vessels. 27 Thus, several single nucleotide polymorphisms (SNPs) that relate to VEGF pathways or drug metabolism/transport may be related to the risk of VEGF inhibitor‐related hypertension. 28 , 29 Retrospective studies have reported an association between the development of grade 2 or 3 hypertension with BEV in first‐line mCRC treatment with regard to ORR and PFS. 21 In addition, the development of hypertension during RAM or AFL treatment has been related to improved efficacy in advanced cancers. 19 , 30 Although these results demonstrate that the emergence of grade 3 or 4 AEs after second‐line chemotherapy could be a surrogate marker of treatment efficacy in mCRC patients, further prospective study or subanalysis using a prospective study cohort is needed to verify our hypothesis. Although these factors may contribute to predicting the efficacy of second‐line chemotherapy, they did not serve as biomarkers contributing to the proper use of the three antiangiogenic drugs. In this study, as there was similar chemotherapeutic efficacy among the three groups and promising outcome was observed in patients with hypertension and neutropenia after second‐line chemotherapy, detrimental effects could be expected in patients without hypertension or neutropenia who were treated with FOLFIRI+RAM or AFL. Therefore, it is important to identify pretreatment biomarkers derived from the host rather than the tumor (e.g., SNPs) to identify these groups, especially in patients treated with FOLFIRI+RAM or AFL.
Our study also showed a strong correlation between prior Bev exposure and treatment efficacy. Considering prior BEV exposure, there were no phase III trials to validate the additional effect of BEV or RAM to FOLFIRI alone because all patients were treated with chemotherapy +BEV as first‐line treatment in the ML18147 11 and RAISE 16 trials. The E3200 trial reported the additional effect of BEV(10 mg/m2/2 weekly) compared to FOLFOX4 alone 31 ; the median PFS and OS for the group treated with FOLFOX4 + BEV were significantly longer than those of the group treated with FOLFOX4 alone (PFS: HR 0.61, p < 0.0001; OS: HR 0.75, p = 0.0011). 31 Furthermore, the ORR was 22.7% and 8.6%, respectively (p < 0.0001). 31 Suzuki et al. reported the clinical outcomes of second‐line FOLFIRI +RAM for mCRC patients by prior BEV exposure. 32 In that study, the median PFS in BEV‐naive patients was longer than those of prior BEV patients; the response rates were 23.0% and 3.0%, respectively (p = 0.0286). 32 Furthermore, subanalysis of the VELOUR trial showed response rate in non‐prior BEV patients better than prior BEV patients treated with FOLFIRI +AFL (HR = 0.79 vs. 0.86). 13 These results suggest that an additional effect of combination treatment with antiangiogenic drugs can be expected, especially in BEV‐naive patients, regardless of antiangiogenic drug type.
To date, although phase III trial data have been reported in antiangiogenic drugs combined with FOLFIRI, 11 , 13 , 16 the differences in the ratio of cytotoxic chemotherapy (oxaliplatin or irinotecan) and BEV exposure in prior chemotherapy among the three trials make interpretation and comparison of the results difficult. Furthermore, there are no randomized studies to select the best antiangiogenic drugs after the first‐line chemotherapy in mCRC patients. In the current study, no significant difference in chemotherapeutic efficacy was observed among the three groups, similar to the comparison of the phase III trial results. However, the frequency of AEs in the current study was higher, especially neutropenia in the FOLFIRI +RAM group and both hematological and nonhematological toxicity in the FOLFIRI +AFL group than in the FOLFIRI +BEV group. In particular, the frequency of severe neutropenia in the FOLFIRI +AFL group exceeded 50%. In a Japanese phase II study to evaluate the safety and efficacy of FOLFIRI +AFL (EFC11885), grade 3 or 4 neutropenia was occurred in 61.2% of patients (38/61). 33 These results suggest that the frequency of severe neutropenia in FOLFIRI +AFL treatment in Japan may be higher than those of western countries (i.e., VELOUR trial, 39%). 13 Thus, confirmation of the UGT1A1 polymorphism that is a determinant of neutropenia 34 , 35 is required before considering chemotherapy and supportive treatments, such as granulocyte colony‐stimulating factor, before or during second‐line treatment, especially in FOLFIRI +RAM and AFL. Based on the above results, treatment decisions regarding antiangiogenic drugs should be made according to patient characteristics and background factors.
The results of study were limited because the relatively small number of patients and retrospective study. Randomized clinical trials will be needed to determine the superiority or inferiority of these three drugs. However, it is difficult to perform a prospective comparison of the three drugs. Despite these limitations, the results of this study provided important and novel insights into the clinical use of these drugs and research prospects of second‐line chemotherapy with antiangiogenic agents.
In conclusion, no significant difference in chemotherapeutic efficacy was observed among the three antiangiogenic agents with FOLFIRI. In contrast, we confirmed significant differences in terms of both the frequency and presence of grade 3 or 4 AEs. Grade 3 or 4 AEs within the first four cycles of treatment and prior BEV exposure may be helpful predictor of efficacy in mCRC patients treated with second‐line chemotherapy with VEGF inhibitor.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Hiroki Osumi and Eiji Shinozaki conceptualized and designed the study. Hiroki Osumi, Eiji Shinozaki, and Akira Ooki analyzed and interpreted the data. Hiroki Osumi drafted the manuscript and performed statistical analysis. Eiji Shinozaki, Akira Ooki, Takeru Wakatsuki, Daisaku Kamiimabeppu, Taro Sato, Izuma Nakayama, Mariko Ogura, Daisuke Takahari, Keisho Chin, and Kensei Yamaguchi involved in critical revision of the manuscript for important intellectual content. Eiji Shinozaki and Kensei Yamaguchi involved in study supervision.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Institutional Review Board, approval number 2020–1017) and with the Helsinki Declaration.
CONSENT FOR PUBLICATION
The protocol was described on the hospital website and subjects were provided the opportunity to opt out; therefore, no new consent was required from patients.
Supporting information
Figure S1
Figure S2
Table S1
ACKNOWLEDGMENT
The authors thank Ms. Yuki Horiike for providing data management.
DATA AVAILABILITY STATEMENT
This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
REFERENCES
- 1. Folkman J, Merler E, Abernathy C, Williams G. Isolation of a tumor factor responsible for angiogenesis. J Exp Med. 1971;133:275‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194‐204. [DOI] [PubMed] [Google Scholar]
- 3. Battaglin F, Puccini A, Intini R, et al. The role of tumor angiogenesis as a therapeutic target in colorectal cancer. Expert Rev Anticancer Ther. 2018;18:251‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 5. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873‐887. [DOI] [PubMed] [Google Scholar]
- 6. Jain RK. 1995 Whitaker Lecture: delivery of molecules, particles, and cells to solid tumors. Ann Biomed Eng. 1996;24:457‐473. [DOI] [PubMed] [Google Scholar]
- 7. Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high‐resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177‐182. [DOI] [PubMed] [Google Scholar]
- 8. Bottsford‐Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J Clin Oncol. 2012;30:4026‐4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshino T, Arnold D, Taniguchi H, et al. Pan‐Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO‐ESMO initiative endorsed by CSCO, KACO, MOS. SSO and TOS. Ann Oncol. 2018;29:44‐70. [DOI] [PubMed] [Google Scholar]
- 10. Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29‐37. [DOI] [PubMed] [Google Scholar]
- 12. Tabernero J, Hozak RR, Yoshino T, et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double‐blind, phase III study. Ann Oncol. 2018;29:602‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. J Clin Oncol. 2012;30:3499‐3506. [DOI] [PubMed] [Google Scholar]
- 14. Rosen LS. Clinical experience with angiogenesis signaling inhibitors: focus on vascular endothelial growth factor (VEGF) blockers. Cancer Control. 2002;9:36‐44. [DOI] [PubMed] [Google Scholar]
- 15. Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC‐1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor‐2. J Clin Oncol. 2010;28:780‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tabernero J, Yoshino T, Cohn AL, et al. Ramucirumab versus placebo in combination with second‐line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first‐line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double‐blind, multicentre, phase 3 study. Lancet Oncol. 2015;16:499‐508. [DOI] [PubMed] [Google Scholar]
- 17. Holash J, Davis S, Papadopoulos N, et al. VEGF‐Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393‐11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim ES, Serur A, Huang J, et al. Potent VEGF blockade causes regression of coopted vessels in a model of neuroblastoma. Proc Natl Acad Sci USA. 2002;99:11399‐11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Facemire CS, Nixon AB, Griffiths R, Hurwitz H, Coffman TM. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54:652‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Österlund P, Soveri LM, Isoniemi H, Poussa T, Alanko T, Bono P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab‐containing chemotherapy. Br J Cancer. 2011;104:599‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scartozzi M, Galizia E, Chiorrini S, et al. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first‐line bevacizumab. Ann Oncol. 2009;20:227‐230. [DOI] [PubMed] [Google Scholar]
- 22. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freyer G, Rougier P, Bugat R, et al. Prognostic factors for tumour response, progression‐free survival and toxicity in metastatic colorectal cancer patients given irinotecan (CPT‐11) as second‐line chemotherapy after 5FU failure. CPT‐11 F205, F220, F221 and V222 study groups. Br J Cancer. 2000;83:431‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chabot GG, Abigerges D, Catimel G, et al. Population pharmacokinetics and pharmacodynamics of irinotecan (CPT‐11) and active metabolite SN‐38 during phase I trials. Ann Oncol. 1995;6:141‐151. [DOI] [PubMed] [Google Scholar]
- 25. Canal P, Gay C, Dezeuze A, et al. Pharmacokinetics and pharmacodynamics of irinotecan during a phase II clinical trial in colorectal cancer. Pharmacology and Molecular Mechanisms Group of the European Organization for Research and Treatment of Cancer. J Clin Oncol. 1996;14:2688‐2695. [DOI] [PubMed] [Google Scholar]
- 26. Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11:1172‐1183. [DOI] [PubMed] [Google Scholar]
- 27. Steingart RM, Bakris GL, Chen HX, et al. Management of cardiac toxicity in patients receiving vascular endothelial growth factor signaling pathway inhibitors. Am Heart J. 2012;163:156‐163. [DOI] [PubMed] [Google Scholar]
- 28. Morita S, Uehara K, Nakayama G, et al. Association between bevacizumab‐related hypertension and vascular endothelial growth factor (VEGF) gene polymorphisms in Japanese patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2013;71:405‐411. [DOI] [PubMed] [Google Scholar]
- 29. Berger MD, Yamauchi S, Cao S, et al. Autophagy‐related polymorphisms predict hypertension in patients with metastatic colorectal cancer treated with FOLFIRI and bevacizumab: Results from TRIBE and FIRE‐3 trials. Eur J Cancer. 2017;77:13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fukuda N, Takahari D, Wakatsuki T, et al. Early hypertension is associated with better clinical outcomes in gastric cancer patients treated with ramucirumab plus paclitaxel. Oncotarget. 2018;9:15219‐15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539‐1544. [DOI] [PubMed] [Google Scholar]
- 32. Suzuki T, Shinozaki E, Osumi H, et al. Second‐line FOLFIRI plus ramucirumab with or without prior bevacizumab for patients with metastatic colorectal cancer. Cancer Chemother Pharmacol. 2019;84:307‐313. [DOI] [PubMed] [Google Scholar]
- 33. Denda T, Sakai D, Hamaguchi T, et al. Phase II trial of aflibercept with FOLFIRI as a second‐line treatment for Japanese patients with metastatic colorectal cancer. Cancer Sci. 2019;110:1032‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu ZY, Yu Q, Zhao YS. Dose‐dependent association between UGT1A1*28 polymorphism and irinotecan‐induced diarrhoea: a meta‐analysis. Eur J Cancer. 2010;46:1856‐1865. [DOI] [PubMed] [Google Scholar]
- 35. Ichikawa W, Uehara K, Minamimura K, et al. An internally and externally validated nomogram for predicting the risk of irinotecan‐induced severe neutropenia in advanced colorectal cancer patients. Br J Cancer. 2015;112:1709‐1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S2
Table S1
Data Availability Statement
This work is licensed under the Creative Commons Attribution 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.


