Abstract
Since the first successful implementation of n-type doping, low-cost Mg3Sb2-xBix alloys have been rapidly developed as excellent thermoelectric materials in recent years. An average figure of merit zT above unity over the temperature range 300–700 K makes this new system become a promising alternative to the commercially used n-type Bi2Te3-xSex alloys for either refrigeration or low-grade heat power generation near room temperature. In this review, with the structure-property-application relationship as the mainline, we first discuss how the crystallographic, electronic, and phononic structures lay the foundation of the high thermoelectric performance. Then, optimization strategies, including the physical aspects of band engineering with Sb/Bi alloying and carrier scattering mechanism with grain boundary modification and the chemical aspects of Mg defects and aliovalent doping, are extensively reviewed. Mainstream directions targeting the improvement of zT near room temperature are outlined. Finally, device applications and related engineering issues are discussed. We hope this review could help to promote the understanding and future developments of low-cost Mg3Sb2-xBix alloys for practical thermoelectric applications.
1. Introduction
Thermoelectric (TE) materials, which can convert heat into electric energy or vice versa without any moving parts, provide a promising solution to the current energy crisis [1]. In order to enable more practical applications in power generation and solid-state cooling, the key challenge is to improve the performance of TE materials, which is usually gauged by the dimensionless figure of merit, zT, zT = S2σT/(κL + κe), where S, σ, T, κL, and κe are the Seebeck coefficient, electrical conductivity, absolute temperature, and lattice and electronic components of thermal conductivity κ, respectively [2]. Aiming at obtaining a high zT, there are two primary directions in the TE research: one is to optimize the TE properties of established good materials mainly through the band and phonon engineering strategies [3, 4]; the other is to discover novel promising candidates based on the instruction of theoretical predictions or trial and error [5, 6].
With a constant endeavor of thermoelectricians in the past decades, more than ten semiconductor systems have been exploited with high zT above unity, some even higher than 2, e.g., V2VI3 compounds [7–10], IV-VI compounds [11–18], transition metal chalcogenides [19, 20], half-Heusler compounds [21–26], skutterudites [27–29], Zintl compounds [30–33], clathrates [34, 35], metal silicides [36–38], and Si1-xGex alloys [39, 40]. Among these good TE systems, most of them exhibit peak zT in the moderate-to-high temperature range (≥600 K), of which the practical applications would be power generation. In contrast, TE materials with high zT near room temperature (RT) are superior candidates for both low-grade heat power generation and solid-state cooling. Regarding cooling applications, there is a big market demand in a variety of industries, such as healthcare, automotive, semiconductors, and electronics. The global solid-state cooling market is estimated to be valued at USD 395 million in 2019 and is predicted to reach USD 641 million by 2024 [41]. Hence, the development of low-cost TE materials with high zT near RT is highly desirable.
Since the discovery in the mid-twentieth century [42], V2VI3 compounds [9, 43], including Bi2Te3, Sb2Te3, and Bi2Se3, and their solid solutions, have long been the best TE materials near RT, being the primary choice for the commercial cooling application. However, the relatively scarce element tellurium (see Table 1) is a potential impediment for the large-scale TE applications of V2VI3 compounds. In recent years, Mg-based semiconductors, e.g., p-type α-MgAgSb [44–46] and n-type Mg3Sb2-xBix [33, 47, 48], have been discovered with good TE performance near RT, comparable to V2VI3 compounds. Magnesium is the 8th most abundant element in the earth's crust [49]. TE materials containing low-cost magnesium could thus have a better prospect for large-scale applications.
Table 1.
Basic physical and chemical information for the elements in the two n-type near-RT Mg3Sb2-xBix and Bi2Te3-xSex thermoelectrics. Oxygen is also included since it is often found in the synthesized samples. Most data are obtained from WebElements [101] while Shannon-Prewitt radii from KnowledgeDoor [102].
| Element | Mg | Sb | Bi | Te | Se | O |
|---|---|---|---|---|---|---|
| Atomic number | 12 | 51 | 83 | 52 | 34 | 8 |
| Density (g/cm3) | 1.738 | 6.697 | 9.78 | 6.24 | 4.819 | — |
| Abundances (ppm by weight) | 29000 | 0.2 | 0.025 | 0.001 | 0.05 | 460000 |
| Melting point (°C) | 650 | 630.63 | 271.3 | 449.51 | 221 | -218.3 |
| Boiling point (°C) | 1090 | 1387 | 1564 | 988 | 685 | -182.9 |
| Electronic configuration | 3s2 | 5s2 5p3 | 6s2 6p3 | 5s2 5p4 | 4s2 4p4 | 2s2 2p4 |
| Electronegativity (Pauling) | 1.31 | 2.05 | 2.02 | 2.1 | 2.55 | 3.44 |
| Atomic radius (Å) | 1.5 | 1.45 | 1.6 | 1.4 | 1.15 | 0.6 |
| Pauling ionic radius (Å) | 0.65 Mg2+ | — | — | 2.21 Te2- | 1.98 Se2- | 1.4 O2- |
| Shannon-Prewitt radius (Å) (coordination number) | 0.72 Mg2+(6) | 0.76 Sb3+(6) | 1.03 Bi3+(6) | 2.21 Te2-(6) | 1.98 Se2-(6) | 1.40 O2-(6) |
Historically, the exploration of Mg-based TE materials began with Mg2Si-based compounds in the 1960s [50]. By alloying with Sn or Ge, the resulted band convergence and point defect scattering contribute to a peak zT above unity in Mg2Si1-xSnx and Mg2Ge1-xSnx solid solutions [36, 37, 51, 52]. A series of work has also been conducted to optimize their TE performance, including introducing dislocations and point defects [53–55]. In 2012, the TE properties of another Mg-based compound α-MgAgSb, which belongs to the Nowotny-Juza family [56], were reported by Kirkham et al. [44]. The synthesized α-MgAgSb sample with unignorable impurity phases still exhibits a potential zT of 0.56 at around 400 K. Later, by improving phase purity, a peak zT above unity was obtained in p-type MgAgSb samples by several groups [45, 46, 57, 58]. The hierarchical chemical bonding in α-MgAgSb leads to strong anharmonicity and intrinsically low sound velocity [59], which are responsible for the low thermal conductivity κ and high zT near RT [45, 46, 57]. However, MgAgSb has a complex phase transition process when cooling from its liquid state with two phase transition points at around 360°C and 300°C, respectively [60]. Additionally, the binary impurity phase, for instance, Ag3Sb, is also easily formed during the synthesis. These make it difficult to synthesize the high-purity α-MgAgSb (room temperature phase); particularly, the single crystal is not yet reported. Moreover, the high TE performance in α-MgAgSb is only obtained when doping it as p-type, and there is still no experimental report on successful electron doping.
Intermetallic compound Mg3X2 (X = Sb, Bi) was first reported by Zintl and Husemann in the 1930s [61], later being classified as a member of Zintl phase compounds [62]. The electrical transport behavior of Mg3X2 was studied by Pincherle and Radcliffe in the 1950s [63]. Mg3Sb2 exhibited a p-type semiconducting behavior with an energy gap (Eg) of 0.8 eV while Mg3Bi2 showed metallic behavior. The study on the TE properties of Mg3X2 began in the 2000s [64]. Despite the low κ and inexpensive raw elements, its p-type semiconducting behavior and inferior TE performance do not make it a good TE material [65–69]. In 2016, Mg3Sb2-xBix compounds were discovered to be a new promising n-type TE system by exhibiting a peak zT of 1.5 at around 700 K [70–73], more than twice higher than that of the p-type counterparts (Figure 1(a)). This exciting result also makes n-type Mg3Sb2-xBix standing out of the Zintl phase TE family, of which the family members are generally exhibiting good p-type TE properties, even rarely been electron-doped [74]. The successful realization of n-type Mg3Sb2-xBix results from the understanding of the Mg defect chemistry, and the Bi alloying promoted electron doping by reducing the bandgap [70, 71]. That is, only in the Mg-rich environment, for instance, adding slightly excess Mg in the raw experimental design, can n-type Mg3Sb2-xBix be obtained. Actually, this excess Mg supportive electron doping has been recognized in synthesizing n-type Mg2Si1-xSnx TE materials previously, where excess Mg was thought to compensate the loss of Mg during the synthesis, and additionally, some excess Mg atoms could enter into the interstitial sites facilitating electron doping [54, 75, 76]. The role of excess Mg in Mg3Sb2-xBix is mainly suppressing the Mg vacancies while Mg interstitial is difficult to form, which will be discussed in detail in the later section.
Figure 1.
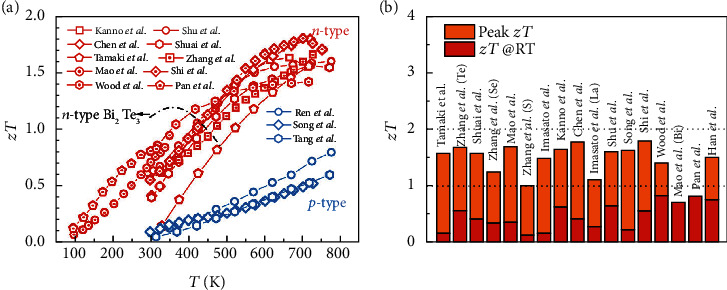
(a) Temperature-dependent zT for both n-type and p-type Mg3Sb2-xBix-based TE materials. The data are taken from Refs. [33, 48, 70–73, 77–83]. As a comparison, the zT of n-type Bi2Te3-xSex is also shown [8]. (b) The peak and room temperature zT for n-type Mg3Sb2-xBix. The data are taken from Refs. [33, 48, 70–73, 80, 82, 84–89].
Despite exhibiting a high zT of 1.5 at around 700 K, the power generators made from n-type Mg3Sb2-xBix might be challenging owing to the decomposition of the compounds and possible deterioration of electrical properties at elevated temperatures [90, 91]. Fortunately, even near RT, n-type Mg3Sb2-xBix also shows good zT of around 0.8 (Figure 1(b)), making it a promising alternative to the state-of-the-art n-type Bi2Te3-xSex for solid-state cooling application. Although only 4 years have passed since the discovery of n-type Mg3Sb2-xBix, there are already many important advances achieved, including the improvement of TE performance [33, 48, 70–73, 78–80, 82, 92, 93], the understanding of the origin for good power factor [70, 71] and intrinsically low κ [94], the revelation of the carrier scattering mechanism near RT [82, 95, 96], the increasing awareness of Mg defect chemistry [70, 97, 98], and even the successful attempt of TE module [33].
The advances in n-type Mg3Sb2-xBix demonstrate a good paradigm of how a new TE material can be rapidly developed and even transferred into the lab-scale module verification by the current TE community. In the past two years, there have been two timely review articles discussing the recent progress of Mg3Sb2 and its derivatives: one is focusing on the design principle with a combination of theory and experiment [99] and the other on the manipulation of defects and electronic transport properties [100]. Readers who are interested in more details may refer to these two reviews. In this review, we focus on recent progress and perspective of the TE performance enhancement near RT. Started with the understanding of basic information of Mg3Sb2-xBix, the bonding and crystal structure, electronic structure, and phonon dispersion are discussed to reveal the intrinsic foundation of the transport properties. Then, several important aspects, including band engineering with Sb/Bi alloying, carrier scattering mechanism near room temperature, Mg defect chemistry, and extrinsic doping which affect the TE performance near RT, are summarized. Finally, we discuss the thermal stability of this system, which is a key step towards practical applications. We hope this review could help to promote the development of Mg3Sb2-xBix for future TE applications near RT.
2. Chemical Bonding and Structure
2.1. Basic Information about the Constituent Elements
The structure-property relationship is essential to understand and design advanced materials for practical applications. Here, before the comprehensive discussions of the structure-property relationship in the Mg3Sb2-xBix system, we first make a list of the basic physical and chemical properties of the elements Mg, Sb, Bi, Te, and Se (Table 1), making up the two near-RT TE systems, Mg3Sb2-xBix and Bi2Te3-xSex. Several important points, which will be frequently referred to in the following sections, are first highlighted here. (i) The density of Mg is much smaller than that of Sb, Bi, Te, and Se. This could be an advantage of lightweight for n-type Mg3Sb2-xBix used for TE modules, compared to commercial Bi2Te3-xSex. (ii) The melting point of Mg is the highest one among the listed elements. However, its lower boiling point, compared to Sb and Bi, suggests a higher vapor pressure and a potentially larger loss of Mg for Mg3Sb2-xBix at elevated temperatures [103]. (iii) The Pauling electronegativity (EN) of Mg is only 1.31, much smaller compared to O (3.44), suggesting the high reactivity of Mg metal. In the atmosphere, the surface of Mg metal is soon coated with a thin layer of oxide that partly inhibits reactivity, the so-called passivation. The passivation and high vapor pressure of Mg could be reasons why the actual compositions of the synthesized Mg3Sb2-xBix compounds are generally Mg-deficient if being nominally designed. (iv) The electronic configuration of Mg is 3s2, and hence, Mg usually has a +2 oxidation state in the ambient environment. The Pauling ionic radius of Mg2+ is 0.65 Å, which is much smaller than that of the Sb and Bi anions (their Pauling ionic radii are not available but should be larger than their atomic radii, by referring to the difference in the atomic and ionic radii of Te, Se, and O). This diminutive radius of Mg2+, even comparable to that of Li+ (0.6 Å), was thought to be one of the structural reasons leading to weak interlayer bonding and thus intrinsically low κ in Mg3Sb2 and Mg3Bi2 [94]. Additionally, this is perhaps also why Mg3Bi2 has also attracted attention as candidates for superionic conductor [104, 105] and Mg-ion battery [106, 107]. Furthermore, although having not been thoroughly studied, this diminutive radius of Mg2+ might also relate to the thermal instability of this system when working at elevated temperatures. (v) The difference in Pauling EN for the constituent elements of Mg3Sb2 is 0.74 (0.71 for Mg3Bi2), which is much larger that of Bi2Te3 (0.08) and the other good thermoelectrics, such as Sb2Te3 (0.05), PbTe (0.23), PbSe (0.22), GeTe (0.09), SnTe (0.14), SnSe (0.59), SiGe (0.11), Mg2Si (0.59), and ZnSb (0.4). This is an indication that the chemical bond of Mg3Sb2 and Mg3Bi2 has a larger component of ionic character, compared to above mentioned good thermoelectrics.
2.2. Crystal Structure
Mg3Sb2 and Mg3Bi2 crystallize in a trigonal anti-α-La2O3 type structure (space group Pm1) below 1203 K and 976 K, respectively [108]. A cubic Mn2O3-type structure (space group Ia) was reported in some literature for Mg3X2 above the transition temperatures [109]. For Mg3Bi2, a neutron powder diffraction was carried out to determine the high-temperature phase by Barnes et al. [104]. A phase transition from the α phase (low temperature) to the β phase was observed at T = 730 ± 10°C. The β-Mg3Bi2 was determined to be a body-centered cubic structure. However, to our best survey of the literature, there is still no convincing structural information of β-Mg3Sb2. We tried to quench Mg3Sb2 and Mg3Bi2 samples into liquid nitrogen from temperature above 1203 K and 976 K, respectively. However, with the XRD measurement, the quenched crystals still show a trigonal structure without a trace of cubic structure, suggesting the phase transition might be fairly quick.
The crystal structure of trigonal Mg3X2 and the corresponding lattice parameters and bond lengths are shown in Figure 2(a) and Table 2, respectively. Under the Zintl concept, Mg3X2 can also be classified into the CaAl2Si2-type structure by rewriting it as MgMg2X2 [94]. Mg atoms take two different sites in the lattice: one is at the octahedral site surrounded by six X atoms, named as the Mg1 atom, and the other is at the tetrahedral site surrounded by four X atoms, named as the Mg2 atom. The Mg1 atom shows the most electropositive character and provides 2 electrons to the covalent bounded Mg2X22- network. This configuration not only provides a rough diagram to understand the structure-property relationship of Mg3X2 but also gives instruction about the site preference when alloying with other elements [113]. The Mg1 sites are favorably occupied when alloying with more electropositive alkaline earth metals and lanthanides, like Ca, Sr, Ba, La, and Yb [114–118], whereas Mg2 sites with more electronegative Zn, Mn, and Cd metals [119, 120].
Figure 2.
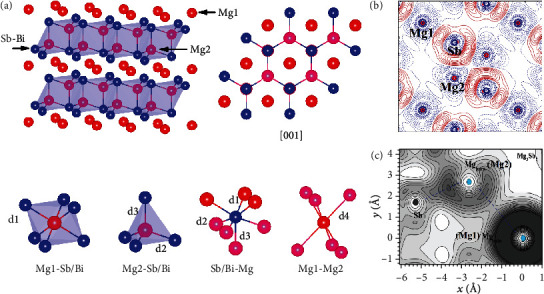
(a) Crystal structure of Mg3X2, the right figure is viewed from [001] direction. Drawn using VESTA [125]. The coordination of Mg1, Mg2, and X is presented below the crystal structure. (b) Static deformation electron density map on (110) plane of Mg3Sb2. (c) The partial charge densities for Mg3Sb2. Panel (b) is reproduced with permission from Ref. [121]. CC-BY-4.0. Panel (c) is reproduced with permission from Ref. [116]. Copyright 2019 Wiley Periodicals, Inc.
Table 2.
Lattice parameter and bond length between adjacent atoms for Mg3X2. The data are taken from Refs. [61, 108, 110–112].
| Mg3Sb2 | Mg3Bi2 | ||||
|---|---|---|---|---|---|
| Lattice parameter (Å) | Reference | Lattice parameter (Å) | Reference | ||
| a | c | a | c | ||
| 4.573 | 7.229 | [61] | 4.671 | 7.403 | [61] |
| 4.568 ± 0.003 | 7.229 ± 0.004 | [108] | 4.666 | 7.401 | [110] |
| 4.547(5) | 7.235(3) | [111] | 4.645(3) | 7.380(3) | [111] |
| Bond length (Å) | |||||
| Mg1-Sb(d1) | 3.111 | [108] | Mg1-Bi(d1) | 3.1403 | [112] |
| Mg2-Sb(d2) | 2.819 | Mg2-Bi(d2) | 2.9132 | ||
| Mg2-Sb(d3) | 2.933 | Mg2-Bi(d3) | 2.9900 | ||
| Mg1-Mg2(d4) | 3.736 | Mg1-Mg2(d4) | — | ||
Recently, Zhang et al. [99] performed a quantitative chemical bonding analysis of Mg3Sb2, from which they found that Mg1 and Mg2 atoms show close bonding character with the Sb atom with the atomic charge of +1.51 and +1.47, respectively (Figure 2(b)). The interlayer interaction in Mg3Sb2 is largely ionic with partial covalent nature, which is comparable to the intralayer interaction with the same type of bond [121]. These calculations give a rational explanation of the nearly isotropic thermal properties of Mg3X2 along the ab-plane and c-axis. These nearly isotropic thermal properties make Mg3X2 different from the other CaAl2Si2-type Zintl compounds, such as CaZn2Sb2 and SrZn2Sb2, showing anisotropic thermal properties [121]. With these findings, Zhang et al. argued the breakdown of the Zintl formalism in the AMg2X2 system (A is an alkaline earth or a divalent rare earth element) [99], arousing the debate as to whether Mg3Sb2 is a Zintl phase [121, 122]. To address the debate, it is necessary to recall the definition of a Zintl phase. AB2X2 compounds with CaAl2Si2-type structure were defined, by Hoffmann et al. [113, 123], as Zintl phases since the structure can be described as the covalently bonded [B2X2]δ− networks receiving electrons donated by the ionic Aδ+ cations. In these compounds, the clear coexistence of the ionic cation and covalent anionic network is therefore the key feature of the Zintl phase and widely applied Zintl concept. Moreover, Kauzlarich et al. have also given a comprehensive discussion and historical review on the definition of Zintl phases [124], which can be referred to as the intermetallic compounds with mixed chemical bonding character of ionic and covalent bonds, generally composed of electropositive metal (alkali metal, alkaline earth element, and lanthanide) and posttransition metal or metalloid (i.e., from groups 13, 14, 15, or 16). From this point of review, it is not appropriate to consider Mg3Sb2 as Zintl phases as both the cationic and anionic parts are nearly ionic [99] although they are charge-balanced and the electron-counting rule is still applicable. It should be noted that the definition of the Zintl phase and Zintl concept discussed here is, in the narrow sense, the definition made by Hoffmann et al. [113, 123] and Kauzlarich et al. [124].
In addition to the Mg-Sb interactions, the calculations by Sun et al. [116] show that there exists a bonding interaction between the Mg1 and Mg2 atoms, in the conduction band minimum (CBM) region (Figure 2(c)). Since the distance between Mg1 and Mg2 is fairly large (Table 2) and also their close atomic charge [121], this interaction between 3s levels of Mg1 and Mg2 would be much weaker than that of Mg-Sb bonds. In short, compared to the typical CaAl2Si2-type Zintl compounds, the chemical bonding of Mg3X2 shows a nearly isotropic character in the Mg-Sb bonds between the interlayer and intralayer and an additional Mg1-Mg2 interaction at the CBM. This unique bonding character in Mg3X2, partly originating from the similar chemical environment of Mg1 and Mg2 atoms, is crucial to understand their electronic and phononic structures and thus the electrical and thermal transport properties.
2.3. Electronic Structure
We now move to understand the electronic structure of Mg3X2. The calculated band structures of Mg3Sb2 and Mg3Bi2 are juxtaposed in Figure 3(a). Mg3Sb2 is an indirect semiconductor with the calculated bandgap Eg ranging from 0.4 to 0.7 eV based on different calculation methods [70, 71, 110, 116, 126, 127], while Mg3Bi2 is predicted to be a topological nodal line semimetal with the coexistence of electron and hole pockets near Fermi level EF [128–130]. Experimentally, an Eg of about 0.18 eV was estimated from the resistivity below 390 K for undoped Mg3Sb2 [131], while a much larger value of about 0.8 eV was reported by estimating the gap of the liquid phase of Mg3Sb2 [132]. A similar Eg of 0.8 eV was also reported in the 1950s [63]. The Fourier transform infrared spectroscopy measurement was performed to estimate the optical gap of Mg3Sb2-xBix solid solutions, yielding a similar value of about 0.28 eV for Mg3Sb1.5Bi0.5 [80] and Mg3SbBi [92]. Additionally, a direct revelation of the Eg for a semiconductor is possible by carrying out the angle-resolved photoemission spectroscopy (ARPES) on its n-type single crystals [26]. Pan et al. recently reported the ARPES study on n-type Mg3Sb2 single crystal, from which a forbidden gap is clearly observed (Figure 3(b)) and an Eg > 0.6eV is estimated for Mg3Sb2 [48]. But since the conduction band was not observed, further study is necessary to accurately determine the real Eg. The ARPES study was also performed for Mg3Bi2 single crystal, and its semimetal feature was confirmed [129].
Figure 3.
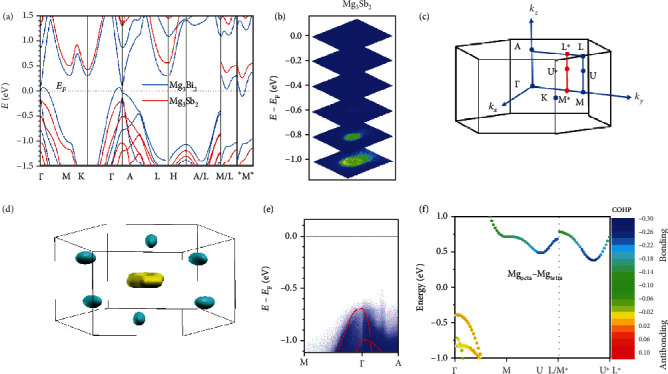
(a) Band structure of Mg3Sb2 and Mg3Bi2. (b) A series of constant energy maps of Mg3Sb2 by ARPES. (c) Brillouin zone (BZ) and high-symmetry points in BZ of the trigonal structure of Mg3X2. (d) The diagram of Fermi surfaces of Mg3Bi2. (e) ARPES spectra along the high-symmetry directions of Mg3Sb2, the red line is the corresponding calculated band structures. (f) The band-resolved projected Crystal Orbital Hamiltonian Population (COHP) for the interaction between Mg1 and Mg2 in Mg3Sb2. Panels (a, b, d, e) are reproduced with permission from Ref. [48]. Published by The Royal Society of Chemistry. Panel (f) is reproduced with permission from Ref. [116]. Copyright 2019 Wiley Periodicals, Inc.
From Figure 3(a), it is easily observed that the valence band maximum (VBM) of both Mg3Sb2 and Mg3Bi2 is near the Γ point, the center of the first Brillouin zone (Figure 3(c)). In contrast, the conduction band minimum (CBM) of Mg3X2 locates at the U∗ point in the M∗L∗ direction, away from the high-symmetry points. To simultaneously demonstrate the shape and valley number of VBM and CBM, the Fermi surfaces of Mg3Bi2 are provided (Figure 3(d)). There are two distinct features in the VBM and CBM of Mg3Bi2. First, there are six electron pockets but only one hole pocket, suggesting a high band degeneracy Nv of 6 in CBM and a low Nv of 1 in VBM. High Nv is usually thought to be beneficial for high TE performance since the zT is proportional to the expression μNvmb∗3/2/κL [11, 21], where μ is the carrier mobility and mb∗ is the single-band effective mass. The difference in band degeneracy of CBM and VBM might explain why Mg3(Sb,Bi)2 shows better n-type TE performance [70, 71], as shown in Figure 1(a).
Another distinct feature is that the six electron pockets are nearly spherical while the hole pocket shows an obvious anisotropy along the kx‐ky plane and kz direction. This anisotropy in VBM is well embodied in the calculated effective masses along the two directions [71, 133, 134]. For example, in the calculation by Zhang et al., mxx∗(myy∗) was estimated to be 1.15me and mzz∗ is 0.15me [71]. It is worth mentioning that the band anisotropy has recently been experimentally confirmed via the ARPES study on the Mg3Sb2 single crystal (Figure 3(e)), giving different effective masses of 0.9me and 0.16me along the two directions, respectively [48]. Moreover, a single crystal study on Mg3Bi2 had also confirmed this valence band anisotropy by measuring the electrical resistivity ρ along the ab-plane and c-axis, where the ab-plane was found to show a twice larger ρ [135]. The ρ along the ab-plane and c-axis was also reported for the Mg3Sb2 single crystals. However, since the studied single crystals were undoped ones and the very high ρ indicates that the Fermi level still lies in the forbidden gap, the band anisotropy on the VBM of Mg3Sb2 cannot be concluded [135]. The hole-doped Mg3Sb2 single crystals are required to observe the anisotropy in electrical transport along the ab-plane and c-axis.
Regarding the high Nv in the CBM of Mg3X2, another interesting question arises as to why the CBM locates off the high-symmetry points. This is an important question awaiting answers. If CBM locates at the U point, the electron pockets will be shared by the second Brillouin zone, and thus, the band degeneracy in the CBM will halve. One possible answer is traced to the electron structure calculation by Sun et al. [116], where they reported on a bonding interaction between the Mg1 and Mg2 atoms in the CBM region of Mg3Sb2, which is particularly stronger in the U∗ point than the other positions (Figure 3(f)). Even further, Han et al. recently found that this Mg1-Mg2 interaction in Mg3Sb2 can be even weakened by replacing Sb with Bi to increase the Mg1-Mg2 distance, leading to a more dispersive CBM although its position is still at the U∗ point [89]. Therefore, the unique Mg1-Mg2 interaction in Mg3X2 might be related to the origin of the CBM position, but further studies, both theoretically and experimentally, are still necessary before drawing a convincing conclusion. An experimental study of the CBM of Mg3X2 and the solid solution Mg3Sb0.75Bi1.25 by ARPES was attempted by Pan et al. [48]. Although their single crystals are all electron-doped with the carrier concentration n in the magnitude of 1019 cm−3, there is still no obvious electron pocket observed in the first Brillouin zone by ARPES. This indicates that higher electron-doped single crystals with n over 1020 cm−3 are necessary to experimentally reveal the CBM by ARPES.
2.4. Phononic Band Structure
Besides the high Nv, the other key reason leading to the high TE performance of n-type Mg3Sb2-xBix is their low κL. Figure 4(a) presents the experimental κL for both single-crystalline and polycrystalline Mg3X2 and their solid solutions. In most experimental reports, the κL of Mg3X2 at room temperature is smaller than 2 Wm−1 K−1, which is comparable to that of Bi2Te3. This “anomalously” low κL of Mg3X2 is to some extent unexpected, considering its simple crystal structure and the light element Mg. In general, compounds with a complex crystal structure, i.e., a large number of atoms in the unit cell, often have intrinsically low κL, because the considerable optical phonons in complex structures suppress the frequency of the acoustic phonons in the phase space and result in low group velocity of acoustic modes [136]. Compounds with heavy elements can also have low κL due to the low sound velocity of the acoustic phonons [137]. However, Mg3X2 has a simple crystal structure with only five atoms in the unit cell, and thus, the origin of its low κL becomes a very important question. The phononic band structure of pure Mg3Sb2 was reported by Peng et al. [94]. As shown in Figure 4(b), the thickness of the lines with colors represents the degree of anharmonicity. For the low-frequency acoustic branch, Mg3Sb2 shows a large degree of anharmonicity. If only taking the phonon-phonon Umklapp scattering into account, κL is proportional to the expression , where A is a collection of physical constant, the average mass of atoms of a crystal, θD the Debye temperature, δ3 the volume per atom, N the number of atoms in the primitive unit cell, and γ the Grüneisen parameter reflecting the anharmonicity of the lattice [138]. The calculated γ for Mg3Sb2 is 1.83, which is higher than that of CaMg2Bi2 (γ = 1.48) and CaMg2Sb2 (γ = 1.44) having the same crystal structure [94]. The strong anharmonicity had also been confirmed by studying the κL of Mg3X2 using the Debye-Callaway model [135]. Comparatively, the other good TE materials with intrinsically low κL also show a large γ, such as PbTe (1.45) [139], AgSbTe2 (2.05) [138], BiCuSeO (1.5) [140], MgAgSb (1.93) [59], and SnSe (3.13) [141].
Figure 4.
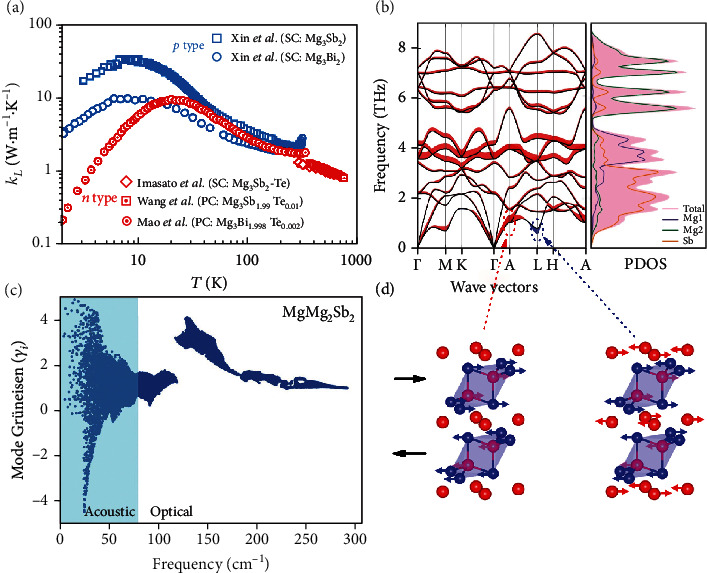
(a) The experimental κL of Mg3X2 and their alloys, where SC and PC denote single-crystalline and polycrystalline samples, respectively. The data are taken from Refs. [33, 96, 135, 142]. (b) Phonon dispersion and partial density of states (PDOS) of Mg3Sb2. (c) Mode Grüneisen parameter of Mg3Sb2. (d) The diagram of atomic displacement corresponding to the transverse at the A point (large positive γi) and at the L point (large negative γi). Panel (b) is reproduced with permission from Ref. [99]. CC-BY-4.0. Panels (c, d) are reproduced with permission from Ref. [94]. Copyright 2018 Elsevier Ltd.
The calculated mode Grüneisen parameter of Mg3Sb2 by Peng et al. [94] is presented in Figure 4(c). It is found that the phonons of Mg3Sb2 with frequency below 1 THz show both large positive and negative mode Grüneisen parameters, reflected by the red shadow at A point and blue shadow at L point, respectively. Both A and L points involve the shear movement of atoms in the ab-plane, as shown in Figure 4(d). More specifically, the same atoms in different layers move towards totally inverse direction at both A and L points, which could be one of the reasons for large shear stress between layers at these points [94]. Except for the large anharmonicity, Mg3Sb2 also shows a low sound velocity of 2587 m·s−1, while CaMg2Sb2 has an average sound velocity of 3317 m·s−1 [94], which also contributes to their low κL. Compared to Mg3Sb2, there is an obvious softening in the phonon density of states of Mg3Bi2 [143], owing to the heavier element Bi. This is why the κL of Mg3Bi2 is smaller than that of Mg3Sb2 [135].
Going further, there is still an interesting question concerning the thermal properties of Mg3X2. That is, why the large anharmonicity occurs in Mg3X2 but not in isoelectronic substituted CaMg2X2. Peng et al. [94] argued that the undersized Mg ions do not obey the octahedral bonding rules, leading to the instability of the octahedral site. Zhang et al. [99] thought that the higher formation enthalpy of Mg3X2 might lead to their relative instability and thus the soft mode and low κL. Because there are few experimental works focusing on the understanding of the phononic structure of Mg3X2 at present, future studies, for example, using inelastic phonon scattering [144, 145], might help to reveal the origin of large anharmonicity in Mg3X2.
The anisotropy of κL is also a worthy question requiring a detailed discussion. In the original work of Tamaki et al. [70], the sound velocities of longitudinal acoustic mode along the ab-plane and c-axis are calculated to be 4160 m·s−1 and 4730 m·s−1, respectively, from which they concluded a nearly isotropic character in thermal conduction of Mg3Sb2. This conclusion is further supported by the chemical bonding analysis by Zhang et al. [121] where they found that Mg3Sb2 exhibits a nearly isotropic three-dimensional bonding network with the interlayer and intralayer bonds being mostly ionic and similar. The calculated κL along the ab-plane and c-axis is very close. Experimentally, Song et al. [146] prepared the texture-enhanced Mg3Sb2-xBix samples, from which they found that the lattice thermal conductivities are almost the same along the pressing direction and in-plane one. In short, the advantage of the nearly isotropic thermal and electrical conductions in n-type Mg3Sb2-xBix is that one does not need to specially take care of the direction of polycrystalline samples when doing the high-temperature thermal and electrical properties. This is actually not the case for the other layered TE materials, for example, Bi2Te3-based compounds, for which the zT can be overestimated if not measuring all the transport properties in the same direction [10, 147, 148].
3. Optimization of TE Properties
The chemical bonding, crystal, electronic, and phononic structures, discussed in the above section, lay the foundation for Mg3X2 as good thermoelectric materials. In practical experiments, the optimization of carrier concentration, engineering of the band and phononic structures, and regulation of electron and phonon scattering mechanisms are effective strategies to achieve high TE performance. Forming Mg3Sb2-xBix solid solutions is the most prevailing means to achieve a high zT (Figure 1). This is because Bi alloying could generate multiple beneficial effects, synergistically regulating both the electrical and thermal transport properties. Mg vacancy defects, which can directly affect the type of carriers, together with extrinsic chemical doping, are crucial for tuning the electrical transport properties of Mg3X2. Moreover, the electron scattering mechanism is another knob for achieving high electrical conductivity and good TE performance near RT. In this section, we focus on the discussions of Sb/Bi alloying, Mg vacancy defects, chemical doping, and carrier scattering mechanism, from which we will try to point out the ways towards high TE performance near RT.
3.1. Sb/Bi Alloying
Isoelectronic alloying to form solid solutions is a widely used strategy in TE research, of which the main purpose is to suppress the κL by introducing strong point defect scattering of phonons [149–151]. One key issue underlying the usage of this strategy is whether the solid solution can be formed in a full composition range (or whether there is a miscibility gap). The miscibility gap occurs in several TE systems, such as Mg2Si1-xSnx [152–154], Ti1-xZrxNiSn [155, 156], and Ti1-xZrxCoSb [157, 158]. Luckily, there seems no miscibility gap in the Mg3Sb2-xBix system [33, 47, 73]. Intuitively, this can be understood partly from the mismatch in the lattice parameter of pristine compounds. For Mg3Sb2 and Mg3Bi2, the differences in the lattice parameter of both a and c directions are less than 2.5% (Table 1), while in the Mg2Si1-xSnx system, the difference is larger than 6% [159]. This relatively smaller mismatch in the lattice parameter could to some extent explain the almost unaffected electron mobility of n-type Mg3Sb2-xBix, which will be discussed in detail later together with the change of electronic structure.
Similar to the other TE solid solution systems, such as PbTe1-xSex [160], Zr1-xHfxNiSn [150], and Zr1-xHfxCoSb [159], the experimental κL for the Mg3Sb2-xBix system also presents a “U-shape” curve (Figure 5(a)), suggesting the efficacy in suppressing the lattice thermal transport with the enhanced point defect scattering of phonons. Near the pristine Mg3Sb2 and Mg3Bi2 sides, Bi/Sb alloying leads to a quick decay of κL, while in the region with 0.4 ≤ x ≤ 1.6, the κL does not change obviously. Since the radius difference between Sb and Bi is relatively small, we tried to calculate the κL of the solid solution by only considering the mass fluctuation-induced point defect scattering of phonons [149]. As shown in Figure 5(a), the calculated line matches relatively well with the experimental results near the Mg3Sb2 side, indicating that the phonon scattering mainly comes from the mass fluctuation. Besides the effect of alloying on lattice thermal conductivity κL, the change of sound velocity, probably originating from the alloying-induced alternation of phonon dispersion, could also influence κL [161]. When alloying with Mg3Bi2, the sound velocity will decrease gradually from 2587 m/s of Mg3Sb2 to 2055 m/s of Mg3Bi2 [94, 143], indicating lattice softening and probably lower κL. This may be one of the reasons why the experimental results deviate from the calculated line in Figure 5(a). Additionally, there is a problem that needs to be paid attention to when comparing the κL and zT from different groups. Since Mg3Sb2-xBix alloy has been widely studied, the choices of heat capacity, either from the measurement or using the Dulong-Petit value, could to some extent influence the estimated κL and zT. Regarding this point, an empirical heat capacity equation was summarized by Agne et al. by including the Dulong-Petit value and temperature-dependent effects based on theory and experiment, which can be used without running Cp experiments on each sample: Cp[J · g−1 · K−1] = 3NR/Mw(1 + 1.3 × 10−4T − 4 × 103T−2) (for temperatures 200K ≤ T ≤ 800K), where 3NR = 124.71J · mol−1 · K−1, Mw is the molecular weight of the formula unit being considered, and T is the absolute temperature [143]. To directly use this formula or take it as a standard reference for future studies is recommended so that a relatively fair comparison in the κL and zT can be reached.
Figure 5.
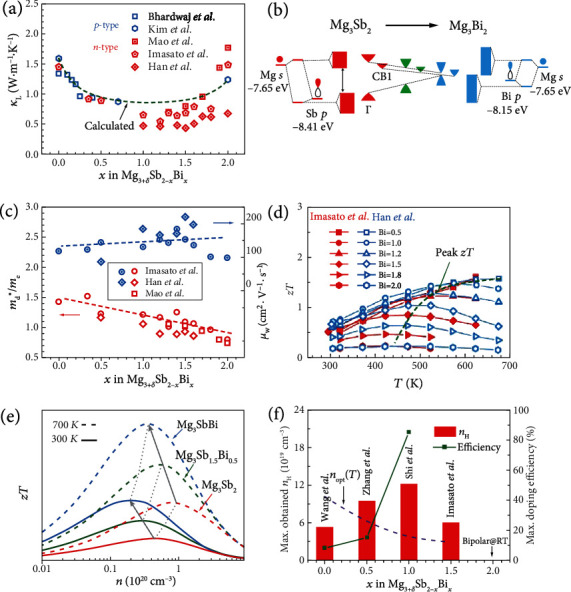
(a) κL at RT versus Bi alloying content for the Mg3+δSb2-xBix system. The data are taken from Refs. [33, 47, 67, 68, 89]; the dashed line is modeled only considering the mass fluctuation-induced point defect scattering of phonons. (b) Schematic illustration of the hybridization in Mg3X2 and the evolution of band structure, inspired by the work of Zhang et al. and Pan et al. [48, 99]. These energies of valence atomic orbitals are taken from the Periodic Table of Atomic Orbital Energies [172]. (c) The density of states (DOS) effective mass and weighted mobility versus Bi content. The data are taken from Refs. [33, 47, 89]. (d) Temperature dependence of zT for the Mg3+δSb2-xBix system with different Bi contents. The data are taken from Refs. [47, 89]. (e) Schematic carrier concentration dependence of zT for Mg3Sb2-xBix using the SPB model [164, 165]. (f) The experimentally obtained maximum Hall carrier concentration and the corresponding doping efficiency versus Bi content for the Mg3+δSb2-xBix system. The purple dashed line indicates the estimated nopt at 700 K obtained from Figure 5(e). The experimental data are taken from Refs. [47, 73, 118, 173].
In addition to the suppression of κL, there is also a tremendous change in the electronic structure of the Mg3Sb2-xBix system when changing x from 0 to 2 since Mg3Sb2 and Mg3Bi2 are semiconductor and semimetal, respectively (Figure 3(a)). This offers the opportunity to modulate the band structure of the Mg3Sb2-xBix solid solutions enabling desirable TE performance at different temperatures. Before going into the performance modulation, we first try to qualitatively understand the band structure evolution from Mg3Sb2 to Mg3Bi2 based on the molecular orbital analysis (Figure 5(b)). The valence electrons of Mg and Sb elements are 3s and 5s5p, respectively, mainly contributing to the formation of chemical bonding. The energy level of the Mg 3s orbital is higher than that of the Sb 5p orbital. When the Mg 3s and Sb 5p orbitals come together to form the two molecular orbitals, the upper antibonding state is mainly composed of the Mg 3s orbital while the lower bonding state mainly with Sb 5p. This real-space analysis of bonding formation can directly reflect the composition of the band structure in reciprocal space. As shown from the projected DOS analysis [127, 162, 163], the CBM is mainly contributed by the Mg s orbital while the Sb p orbital dominates the VBM. When the Sb atom is substituted by the Bi atom, it will lead to two main changes. On the one hand, the energy difference between the Mg 3s and Bi 6p orbitals is smaller than that between the Mg 3s and Sb 5p orbitals, leading to the reduced Eg in Mg3Bi2. On the other hand, the Bi 6p orbital is more dispersive than Sb 5p, which can further reduce the Eg and also result in lighter conduction and valence bands. These qualitative analyses suggest that both Eg and conduction band effective mass will become smaller in the band evolution from Mg3Sb2 to Mg3Bi2.
Experimentally, the change in the density of state effective mass md∗, md∗ = Nv3/2mb∗, which was derived from the measured S and nH using the single parabolic band (SPB) model [164, 165], is presented in Figure 5(c), which indeed decreases with increasing Bi content. Moreover, the mb∗ obtained from the calculated electronic structure of the Mg3Sb2-xBix system was also reported by Han et al. [89], exhibiting the same trend. Beneficial from the reduced mb∗, the weighted mobility μw = μ0(md∗/me)3/2 increases, where μ0 is the mobility of a carrier at kBT higher than the band edge [166, 167]. Weighted mobility is also influenced by the scattering process when alloying with Bi. However, the alloying scattering is not so strong to decrease the carrier mobility. The reasons for this could be twofold: (i) the conduction band is dominated by the 3s states of Mg, and thus, the anion substitution does not significantly affect the conduction band transport, (ii) similar to what happens in the half-Heusler TE solid solutions [24, 159], where the small radius difference in the alloying and host atoms owing to lanthanide contraction does not induce a strong alloy scattering potential responsible for the negligible change in the carrier mobility. Hence, besides the suppressed κL, the weak alloy scattering of carriers and the reduced mb∗ are additional advantages for Mg3Sb2-xBix solid solutions to achieve better TE performance.
Going further, we now discuss the effect of the reduced Eg on the TE properties of Mg3Sb2-xBix. In most of the previous TE studies, relatively large Eg, which can suppress the thermal excitation of minority carriers [3, 168], is generally desirable for obtaining a high peak zT at elevated temperatures. Since the possible TE applications of Mg3Sb2-xBix solid solutions are near RT, a too-large Eg is not really necessary as long as it can still effectively suppress the bipolar effect near RT. The optimum Eg of a TE material given by Sofo and Mahan is around 6–10 kBT [169], corresponding to 0.15–0.26 eV at 300 K. Figure 5(d) shows the temperature-dependent zT for n-type Mg3Sb2-xBix with different Bi contents [47, 89]. It is obvious to see that the temperature at which the peak zT occurs decreases with increasing Bi content. This gives an additional advantage for practical experiments. Namely, the optimal carrier concentration nopt will become smaller (Figure 5(e)) and thus easier to be achieved experimentally [168, 170].
In short, Bi alloying in Mg3Sb2 has exhibited multiple beneficial effects on the TE properties. First, it can significantly suppress the phonon transport but weakly affect the carrier transport. Second, the lightened mb∗ contributes to higher μw. Third, the reduced Eg makes the nopt easy to be achieved experimentally. Moreover, there is a large room to tune the peak zT of Mg3Sb2-xBix for applications at different temperatures. If aiming at power generation application, Sb-rich Mg3Sb2-xBix alloys have the advantage to achieve a high peak zT at elevated temperatures [70–73, 92]. However, owing to the solubility and efficiency of dopants, in the Sb-rich Mg3Sb2-xBix alloys, the experimentally maximum carrier concentration might still be lower than the nopt, particularly for Mg3Sb2 (Figure 5(f)). Therefore, more efficient dopants are waiting to be found [171]. In contrast, to get high TE performance near RT, Bi-rich Mg3Sb2-xBix alloys with smaller Eg are enough, in which the smaller nopt is easier to be reached, as recently demonstrated by Imasato et al. [47], Mao et al. [33], and Pan et al. [48].
3.2. Carrier Scattering Mechanism
The carrier scattering mechanism is a very important aspect to understand the electrical transport of TE materials, which helps to find suitable ways to improve TE performance by enhancing the carrier mobility and electrical conductivity. Since the discovery of n-type Mg3Sb2-xBix alloys [70, 71], an “anomalous” increase trend of μ(T) and σ(T) near RT is frequently observed in the doped samples with small grain sizes (Figure 6(a)), which was initially explained as ionized impurity scattering [70, 72, 79, 85]. Ionized impurity scattering-dominated carrier transport is commonly observed in the lightly doped semiconductors near and below RT [174], which is quickly weakened with increasing carrier concentration that induces a strong screen of the ionized impurity centers [175]. That is why in most good TE materials (typically heavily doped semiconductors), the μ(T) and σ(T) above RT generally decrease monotonously due to the dominated acoustic phonon scattering [176].
Figure 6.
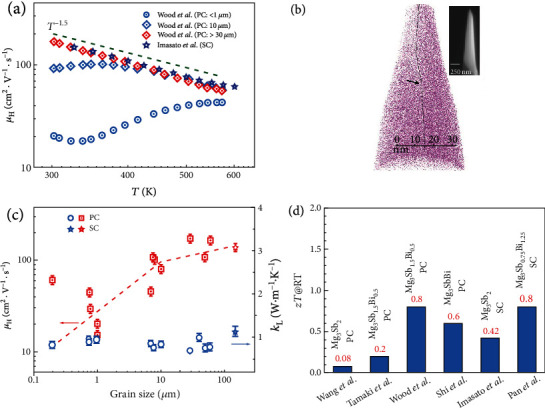
(a) Temperature-dependent μH for Mg3Sb2-xBix synthesized with different grain sizes. The data are taken from Refs. [78, 82]. (b) 3D reconstruction of the atom distribution of the microtip of n-type Mg3Sb2. The upper-right inset is a scanning electron microscope image of the APT specimen. (c) The μH and κL at RT versus grain size for Mg3(Sb,Bi)2 solid solutions, adapted from Ref. [48] with the data taken from Refs. [33, 47, 48, 78, 79, 166, 178]. (d) zT at RT for SC and PC samples with different compositions and preparing methods. The data are taken from Refs. [48, 70, 73, 82, 96, 136]. Panel (b) is reproduced with permission from Ref. [98]. Copyright 2019 Wiley-VCH.
Back to n-type Mg3Sb2-xBix alloys, Kuo et al. [95] carefully analyzed the initially published experimental results and argued that the change from the ionized impurity scattering to acoustic phonon scattering near RT happens too fast to be satisfactorily explained by the conventional models using Matthiessen's rule. By considering the grain boundary region as an effectively separate phase rather than a scattering center, they put forward a two-phase model, which could successfully reproduce the experimentally observed σ(T). Moreover, they predicted a large improvement in the room temperature zT if the grain boundary resistance is eliminated. Later, using atom probe tomography, the grain boundary phase is identified to be Mg-deficient (approximately 5 at%) (Figure 6(b)), which is attributed to be responsible for the high grain boundary resistance [98]. Aiming at improving the μ and σ near RT by suppressing the grain boundary resistance, one direct way is to simply reduce the number of grain boundaries to get coarse-grained polycrystalline samples, which can be achieved by either increasing the sintering temperature [78, 82] or post-annealing under Mg atmosphere if initial grain sizes are small [82]. Furthermore, melting followed by a sintering process has recently been reported as an effective way to prepare samples with large grain sizes [73, 92, 177]. Some experimental results also indicate that Mg3Sb2-xBix samples with high Bi content can have large grain sizes under the same preparing condition [47], which is probably due to their low melting points.
Beyond the coarse-grained polycrystalline samples, the prediction of Kuo et al. [95] directly points out that the single-crystalline Mg3Sb2-xBix samples could have the ceiling μ and σ near RT due to the almost-completely eliminated grain boundary resistance. In experiments, Imasato et al. [96] reported on the TE properties of n-type Te-doped Mg3Sb2 single crystals, synthesized by the Sb-flux method [135] and Mg-vapor annealing [82], of which the σ indeed exhibits a metallic behavior with a typical T−1.5 dependence (Figure 6(a)), indicating the acoustic phonon scattering-dominated carrier transport and the absence of ionized impurity scattering [96]. Later, Pan et al. [48] reported on a direct growth of n-type Y-doped Mg3Sb0.75Bi1.25 single crystals by the Mg-flux method, which exhibits a high zT of ~0.82 at 315 K that is comparable to the state-of-the-art n-type room temperature Bi2Te3-xSex-based TE alloys [8, 10, 147]. This series of work demonstrates a good research relay towards high-performance near-RT Mg3Sb2-xBix thermoelectrics based on the accurate understanding of the carrier scattering mechanism.
As extended discussions, we think there are another two points relating to this grain boundary resistance that are worth noting. On the one hand, the μH of polycrystalline samples at RT tends to have a slower increase when the grain size reaches above ~10 μm, while the κL only changes slightly with grain size (Figure 6(c)). Kuo et al. recently pointed out that there will be an overestimation of κL for small grain polycrystalline samples because of high electrical resistance at the grain boundaries [179]. All of these indicate coarse-grained polycrystalline samples of tens of μm are preferred to have a similar TE performance to the single crystals with the same composition. As summarized in Figure 6(d), the coarse-grained samples and the single crystals indeed exhibit a close zT of approximately 0.8 at RT for Mg3Sb2-xBix solid solutions [48, 135]. Since the growth of centimeter-sized n-type single crystals is still challenging, coarse-grained polycrystalline samples could currently be preferable choice for practical applications.
On the other hand, it is the grain boundary phase which has a high resistance that leads to the “anomalous” μ(T) and σ(T) near RT. Ideally, the grain boundary is thought of as a kind of very thin interface between two grains in a polycrystalline material. However, in real materials, the grain boundary region could be more complicated than a thin interface due to the segregation of second phases or the change of composition [180, 181]. Good TE materials could particularly be the case since they always need to be alloyed or doped to tune the electrical and thermal properties for high performance, which unavoidably leads to complicated microstructures [8, 182, 183]. Hence, a deep microstructure analysis to know the grain boundary region better with advanced techniques is highly necessary [184, 185]. For n-type Mg3Sb2 polycrystalline samples, the grain boundary phase is found to be a 10 nm region with Mg deficiency [98], resulting in the high electrical resistance. One possible indication thereby is if there are ways to make the grain boundary phase have an equal or even lower resistance than the matrix, the “anomalous” μ(T) and σ(T) near RT might vanish. This might explain why in some experiments, even with the same preparation method, using highly metallic dopants or codoping, like Sc, Y, and some other transition metals, could to some extent tune the “anomalous” μ(T) and σ(T) near RT [72, 85, 93]. The underlying reason might be that these highly metallic cation dopants make the grain boundary region have lower resistance compared to the less metallic anion dopants Te, Se, and S.
3.3. The Excess Mg and Chemical Doping
Intrinsic point defects, including vacancy, interstitial atoms, and antisite defects, could have important effects on both the electrical and thermal transport properties of TE materials [9, 186] and hence were studied extensively, such as BiTe− antisite defect in Bi2Te3 [9, 187, 188], interstitial Ni in ZrNiSn [26, 189, 190], and Ag vacancy in MgAgSb [58, 191]. Mg vacancy and interstitial Mg are two commonly observed point defects in Mg2(Si,Ge,Sn)-based TE materials [54, 55, 76], which could serve as acceptor and donor, respectively. For Mg2Ge and Mg2Sn, the type of carrier depends on the chemical environment of synthesis. A Mg-excess environment will lead to n-type transport behavior, while Mg deficiency to p-type [76]. For Mg2Si, interstitial Mg is the dominant point defect in both Mg-excess and Mg-deficient environments, and hence, it always shows an n-type transport behavior [76].
Similarly, the Mg3Sb2-xBix system is also vitally affected by the chemical environment of synthesis [48, 70, 97]. To explore the dominant defect type, first-principles calculations are commonly used to give a possible prediction [70, 97]. As shown in Figure 7(a), in the Sb-excess environment, Mg vacancy is the most stable defect near CBM. The n-doping by replacing Sb with Te fails to work since its formation energy is higher than that of Mg vacancy. Therefore, Te doping cannot shift the EF into CBM if the chemical environment of synthesis is Sb-excess. In contrast, in the Mg-excess environment (Figure 7(b)), the formation energy of Mg vacancy is significantly increased, enabling the success of n-doping by substituting Te on the Sb site. Moreover, interstitial Mg has higher formation energy in both Sb-excess and Mg-excess conditions, indicating that only excess Mg in the synthesis process cannot help to shift theEFinto CBM.
Figure 7.
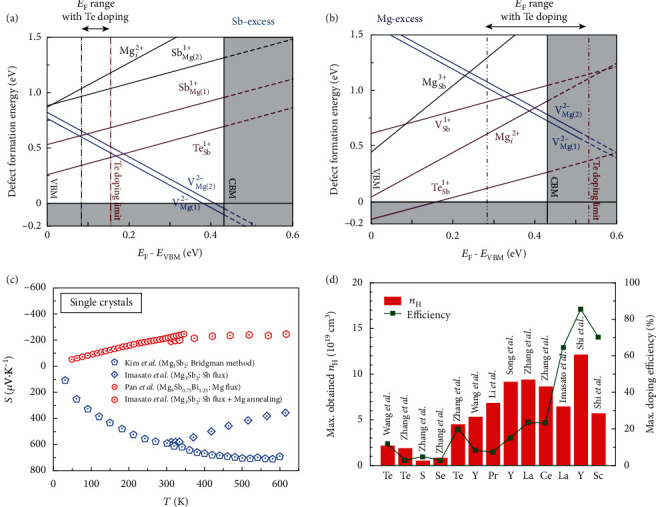
Defect formation energy in Mg3Sb2 in (a) Sb-excess and (b) Mg-excess conditions, respectively. (c) The Seebeck coefficient of Mg3Sb2 and Mg3(Sb,Bi)2 single crystals, grown under different chemical conditions. The data are taken from Refs. [48, 68, 96]. (d) The experimentally obtained maximum nH in Mg3(Sb,Bi)2 samples and the calculated doping efficiency for different dopants. The blue color columns represent dopants at anion sites and the red at cation sites. The doping efficiency is calculated using the measured nH divided by the theoretical one obtained assuming each doping atom offers one electron. The data are taken from Refs. [71, 73, 84, 86, 88, 92, 115, 118, 136, 173, 192, 193]. Panels (a, b) are reproduced with permission from Ref. [97]. Copyright 2018 Elsevier Ltd.
Experimentally, the transport properties of single crystals grown from the liquid flux methods (Sb-excess and Mg-excess) match well the prediction of the defect calculations, as summarized in Figure 7(c). Mg3Sb2 single crystals synthesized by using the vertical Bridgman method [68] and Sb-flux method [135] show the transport behavior of an intrinsic semiconductor with a large positive Seebeck coefficient. Te-doped Mg3Sb2 single crystals had also been successfully grown from the Sb-flux method, which however still shows the positive Seebeck coefficient, indicating Te doping itself cannot shift the EF into CBM if the crystals are Mg-deficient [96]. Imasato et al. did a post-annealing of those Te-doped Mg3Sb2 single crystals under Mg-vapor, which successfully changed the transport behavior of the single crystals from p-type to n-type [96]. Very recently, using Mg as the flux, Pan et al. first reported a direct growth of Y-doped n-type Mg3Sb2 and Mg3Sb0.75Bi1.25 single crystals [48]. Additionally, they found that the undoped Mg3Sb2 single crystal shows a weak p-type transport behavior despite grown out from the Mg-flux [48]. This suggests that interstitial Mg is difficult to enter into the lattice of Mg3Sb2, in agreement with the calculated phase diagram by Ohno et al. [97], where they found that the solubility of both excess Mg and Sb is less than 0.1%.
Excess Mg is then believed to be a prerequisite to obtain n-type Mg3Sb2-xBix. One interesting question arises as to how much excess Mg should be added when synthesizing the polycrystalline samples. Both the defect calculations and single-crystal studies give a simple answer [48, 97]. That is, only slightly excess Mg (less than 0.1%) is necessary to guarantee the system to be n-type if efficient dopants are chosen [171, 195, 196]. However, for the experimental preparation of polycrystalline samples, the real conditions are more complicated. As highlighted in the very beginning, Mg is a very reactive element whose surface in the air will soon be covered by MgO. Moreover, in the mixture of raw elements and the high-temperature sintering process, Mg loss can happen and it varies with different milling methods (hand milling or ball milling) and different sintering temperatures and atmospheres. These make it very difficult to accurately control the content of excess Mg in practical experiments. As summarized in Table 3, excess Mg with a range from 0 to even as high as 16% had been used in the initial synthesis by different groups to compensate for the potential loss of Mg. However, since excess Mg is too difficult to enter into the lattice structure to form interstitial Mg defect [48, 97], adding too much excess Mg could result in the Mg-rich phase, as found by Shuai et al. [178]. In another experiment by Imasato et al. [87], they found that too much excess Mg could lead to an increase in the thermal conductivity and thus is detrimental for the overall TE performance. Moreover, too much excess Mg might also affect the stability of the synthesized polycrystalline samples, which will be discussed in the next section. It is worth noting that alloying Bi can lower the temperatures in either the melting or sintering process, which might be helpful to control the loss of Mg.
Table 3.
The nominal element contents used by different groups to synthesize Mg3Sb2-xBix alloys. The preparation method, Hall carrier concentration, and peak zT are also shown. The data are taken from Refs. [33, 47, 48, 70–73, 80, 82, 84–88, 92, 93, 115, 192, 194].
| Nominal content | Method | n H (1019 cm−3) | zT | Reference | ||
|---|---|---|---|---|---|---|
| Mg | Bi | Dopant | ||||
| 3.2 | 0.49 | Te | BM+HP (Ar) | 2 | 1.5@700 K | [70] |
| 0.01 | ||||||
| 3 | 0.48 | Te | AM+BM+SPS (Vac.) | 2.2 | 1.65@725 K | [71] |
| 0.04 | ||||||
| 3.07 | 0.48 | Se | AM+BM+SPS (Vac.) | 0.91 | 1.23@725 K | [84] |
| 0.02 | ||||||
| 3.1 | 0.49 | TM&Te | BM+HP | 3-4.4 | 1.5-1.7@773 K | [72, 85] |
| 0.1&0.01 | ||||||
| 3.2 | 0.49 | Te | BM+HP | 2.0-3.5 | 1.4-1.5@773 K | [192] |
| 0.01 | ||||||
| 3 | 0.49 | S | AM+BM+SPS (Vac.) | 0.576 | 1.0@725 K | [86] |
| 0.01 | ||||||
| 3.02 | 0.49-0.89 | Mn&Te | BM+SPS | \ | 1.5-1.6@773 K | [80] |
| 0.01&0.01 | ||||||
| 3.01-3.2 | 0.49 | Te | BM+HP (Ar) | 2.8-3.5 | 1.2-1.4@600 K | [87] |
| 0.01 | ||||||
| 3.05 | 0.5 | La | BM+HP (Ar) | 5 | 1.0@600 K | [114] |
| 0.005 | ||||||
| 3.2 | 0.49 | Se | BM+HP | 1.9 | 1.4@723 K | [93] |
| 0.01 | ||||||
| 3.15 | 0.49 | Mn&Se | BM+HP | 2.1 | 1.7@623 K | [93] |
| 0.05&0.01 | ||||||
| 3.032 | 1 | Y | Melting+cut+HP | 7.1 | 1.8@700 K | [73] |
| 0.018 | ||||||
| 3.045 | 1 | Sc | Melting+cut+HP | 3.4 | 1.3@500 K | [92] |
| 0.005 | ||||||
| 3.05 | 1.39825-1.393 | Te | BM+HP (Ar) | 2.3-6 | 1.0-1.2@400-500 K | [47] |
| 0.01 | ||||||
| 3.02 | 0.5 | Y | BM+HP | 3.6 | 1.8@773 K | [88] |
| 0.02 | ||||||
| 3.2 | 1.298-1.498 | Te | BM+HP | 1.4 | 0.9@350 K | [33] |
| 0.002 | ||||||
| 3.5 | 0 | Sc&Te | Powder mix+SPS | 2 | 1.5@725 K | [190] |
| 0.03&0.04 | ||||||
| 3.01 | 0.49 | Te | BM+HP (Ar)+Mg-vapor annealing | 2.8 | 0.8@300 K, 1.4@700 K | [82] |
| 0.01 | ||||||
| >3 | 1.25 | Y | Mg-flux | 1.6 | 0.82@315 K | [48] |
| \ | ||||||
BM: ball milling; HP: hot pressing; AM: arc melting; SPS: spark plasma sintering; Vac.: vacuum; TM: transition metal.
Under the Mg-excess condition, it is possible to achieve n-type Mg3Sb2-xBix by extrinsic doping. The selection of dopants is another key point determining the electrical performance of this TE material. Generally, several aspects need to be considered to find a good dopant: (i) whether a dopant enables the realization of nopt since the doping efficiency varies for different elements; (ii) the dopant will induce defects or disorder in the host lattice, which may be detrimental to the carrier mobility. An empirical rule given by Ioffe that was summarized by Wang et al. [197] is as follows: the electron mobility is weakly affected if the dopant is introduced to the sub-lattice that has less contribution to the conduction band [197].
In the historical development of n-type Mg3Sb2-xBix, the selection of dopants also follows such empirical criteria. Initially, the anion dopant Te was first selected to realize n-type transport [70, 71]. Subsequently, its isoelectronic chalcogen dopants S [86] and Se [84, 93] were also studied, which however are found to be less efficient due to the decreased carrier concentration and mobility [86]. Despite being the most efficient anion dopant, Te doping itself is difficult to realize the nopt (up to 1 × 1020cm−3, Figure 5(e)) in Sb-rich Mg3Sb2-xBix, owing to the limited solubility and doping efficiency (Figure 7(d)). The experimentally obtained maximum Hall carrier concentration is just 2 × 1019cm−3 through Te doping at anion sites in Mg3Sb2 [136].
After the significant role of Mg-excess for realizing n-type doping was found [97], defect calculations predicted that Mg substitution with trivalent (or higher) cations can be even more effective than Se and Te doping to achieve high electron density above 1 × 1020cm−3 [171, 195, 196]. In experiments, group 3 elements (Sc and Y) were found to be the most effective cation dopants which not only enable the realization of high carrier concentration (Figure 7(d)) but also have a weak effect on carrier transport [48, 73, 92, 173, 192]. The latter might originate from the smaller differences in the electronegativity and ionic radius between Mg and the dopants [86, 89, 159, 197]. The lanthanide dopants with a larger ionic radius, such as La, Pr, and Ce [115, 118, 193], were also found to be effective in achieving higher electron density than Te doping (Figure 7(d)) buthad an adverse effect on the carrier mobility. Moreover, the cation dopants on the Mg sites seem to have one more advantage than the anion dopants. That is, it can improve the thermal stability as will be discussed in the following section [73, 115].
4. TE Device and Thermal Stability
The rapid breakthrough in the TE performance of n-type Mg3Sb2-xBix from RT to 700 K ignited the research interest for practical device applications. Mao et al. [33] first reported on a unicouple consisting of n-type Mg3.2Bi1.498Sb0.5Te0.002 and p-type Bi0.5Sb1.5Te3, as shown in the inset of Figure 8(a). When an input electrical current of 9 A is applied and the hot side is maintained at 350 K, the Peltier effect generated by this unicouple gives a maximum temperature difference of 91 K, which is even larger than the commercial Bi2Te3-based device [33]. This encouraging result suggests the potential of low-cost Mg3Sb2-xBix as a substitute for the current state-of-the-art n-type Bi2Te3-xSex. However, for practical device applications, there are still important engineering challenges awaiting further studies, including the design principle of the module, electrode fabrication, interface optimization, and protective coating [198–200]. The solutions to these engineering problems rely on the basic chemical and physical properties of Mg3Sb2-xBix alloys. Above all, the stability issues of Mg3Sb2-xBix, including Mg loss and oxidization [65, 177], the decomposition and possible deterioration of TE performance [90, 91] at elevated temperatures, and possible deliquescence under humid environment, need to be well addressed before long-term usage.
Figure 8.
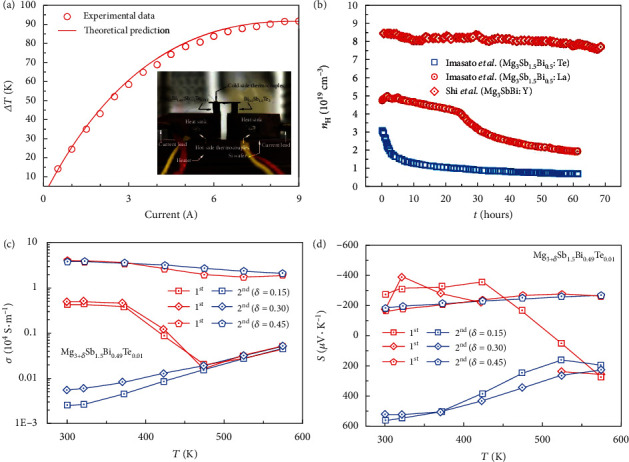
(a) Electrical current dependence of temperature difference (ΔT) between the hot and cold sides at the hot-side T of 350 K. The inset shows optical image of the experimental setup for the TE cooling measurement with a unicouple consisting of p-type Bi0.5Sb1.5Te3 and n-type Mg3.2Bi1.498Sb0.5Te0.002. (b) Hall carrier concentration versus measurement time for La-doped samples under dynamic vacuum and Y-doped samples under vacuum; the data are taken from Refs. [73, 115]. (c, d) Temperature-dependent electrical conductivity and Seebeck coefficient of Mg3+δSb1.5Bi0.49Te0.01 with different excess Mg in our experimental work. 1st and 2nd denote the first and second heating measurements in the helium atmosphere, respectively. Panel (a) is reproduced with permission from Ref. [33]. Copyright 2019 American Association for the Advancement of Science.
The stability problems for Mg3Sb2 came into attention even in the very early publication by Condron et al. in 2006, in which the studied samples exhibited a p-type semiconducting behavior [65]. They found oxygen present at the grain boundaries of the hot-pressed Mg3Sb2 samples. Mg loss and oxidization were also observed at temperatures above 900 K. In the case of n-type Mg3Sb2-xBix alloys, the oxidization of Mg was also observed. Shi et al. have tried to grind the melted Mg3Sb2-xBix ingots into powders under different atmospheres before hot pressing [177]. They found that, if the powders were ground in the glove box with the inert atmosphere, the obtained pellets will show a relatively low level of oxidization. Moreover, Mg deficiency was found at the grain boundary region in the n-type Mg3Sb2 samples by Kuo et al. [98]. In a temperature-dependent powder X-ray diffraction study, Song et al. [91] found that a secondary phase Sb appears even in the first heating process at temperatures above 500 K in both densified bulk samples and powdered samples of p-type Mg2.985Ag0.015Sb2. A similar phenomenon also happened to n-type Mg3Sb1.475Bi0.475Te0.05, in which Jørgensen et al. found an approximately 11 wt.% of elemental bismuth crystallizing as a secondary phase after the first thermal cycle from 300 K to 725 K [90]. In another study by Chen et al. [79], a Bi-rich secondary phase was directly observed using TEM. Furthermore, Mao et al. [33] found that n-type Mg3Sb2-xBix in the air will deteriorate if Bi content is increased. This can be understood from the view of chemical bonding. Since the bond length of Mg-Bi is larger than that of Mg-Sb, the diminutive Mg1 atom will more severely deviate the octahedral rule with increasing Bi content in Mg3Sb2-xBix [94], making the interlayer bonding weaker and thus the thermal stability worse.
Luckily, the thermal stability of Mg3Sb2-xBix could be improved if suitable doping elements were chosen. Compared to doping at anion sites, some studies found that both donors and acceptors replacing Mg sites are beneficial for improving the thermal stability, for example, doping Ag for p-type Mg3Sb2 [91] and Y [73], La [115], and Sc [92] for n-type Mg3Sb2-xBix (as shown in Figure 8(b)). This might be due to the larger sizes of these doping ions as the structural instability caused by the small size of Mg ions [97] can be improved after cationic doping. The thermal stability could also be improved by increasing nominal Mg content. Song et al. found that the Mg-excess p-type Mg3Sb2 show better stability than Mg-deficient ones [91]. We also checked the role of excess Mg on the electrical performance of Te-doped Mg3+δSb2-xBix during thermal cycling in the helium atmosphere. As shown in Figures 8(c) and 8(d), the less Mg-excess samples show less stability than the more Mg-excess ones, and the former even show a change of the sign of the Seebeck coefficient during thermal cycling. The excess Mg is demanded to suppress the formation of Mg vacancies enabling n-type conduction and also better thermal stability. However, too much excess Mg, as discussed in the above section, will also lead to the oxidization and the increase of thermal conductivity [87], which might be detrimental for the long-term use of Mg3+δSb2-xBix TE device.
All in all, it can be concluded that Mg plays a crucial role in the stability of this TE system. The small ionic radius of Mg makes it relatively unstable in the lattice of Mg3X2 [97], and Mg loss [70, 192] and the precipitation of the Sb/Bi phase [90, 91] can occur at elevated temperatures owing to the high vapor pressure of Mg. Luckily, doping at the Mg site could stabilize the lattice structure and balance the Mg loss [73, 115]. Considering that these problems are induced by the active Mg, the synthesis of Mg3+δSb2-xBix materials and the assembly of TE devices need to control strictly the temperature and atmosphere to guarantee stability and reproducibility, particularly for practical applications. These might make the process cost of Mg3+δSb2-xBix higher than that of Bi2Te3-based materials, although the cost of the raw elements in the former is lower. Moreover, for practical TE modules, there are additional demands for the exploration of matched p-type legs, suitable electrode materials, long-term interface stability, etc. All these aspects need to be comprehensively considered to finally answer the question of whether n-type Mg3+δSb2-xBix can substitute Bi2Te3-xSex for low-grade heat power generation and refrigeration.
5. Summary and Outlook
The past four years have witnessed the rapid development of n-type Mg3Sb2-xBix TE alloys with high zT values above 1.5 at 700 K and 0.8 at 300 K. These cheering results make low-cost Mg3Sb2-xBix a promising substitute for state-of-the-art n-type Bi2Te3-xSex for power generation and refrigeration near RT. The reasons underlying the high TE performance are high band degeneracy and weighted mobility enabling large power factor and strong anharmonicity guaranteeing low lattice thermal conductivity. These good electrical and thermal transport properties are fundamentally determined by the intrinsic crystallographic, electronic, and phononic structures of Mg3X2 strongly linked to the element Mg. The interaction between the Mg1 and Mg2 atoms is a possible origin leading to the high conduction band degeneracy. The diminutive size of Mg, which is too small for the octahedrally coordinated site, leads to weak interlayer bonding and low thermal conductivity but also thermal instability. The close interactions of Mg1-Sb and Mg2-Sb are attributed to the nearly isotropic thermal properties. Moreover, only under the Mg-excess condition can n-type doping be realized for the Mg3Sb2-xBix system. However, active Mg also brings problems for both current laboratory research and future applications. That is, Mg loss and oxidation at elevated temperatures could lead to the decomposition of the compounds and deterioration of electrical performance.
With the deep understanding of band structure, the carrier scattering mechanism, and roles of cation dopants, the TE performance of n-type Mg3Sb2-xBix is already comparable to that of the state-of-the-art Bi2Te3-xSex, laying a good foundation for practical TE applications near RT. Further studies on device design and fabrication are now calling on. In the meantime, the thermal and chemical stabilities of n-type Mg3Sb2-xBix alloys, which are strongly related to the active Mg element, will bring real challenges. All the processes, including material synthesis, transfer, joint connection, module assembly, and packaging, might need to be strictly controlled. Suitable interface materials, mechanical property tests, and large-scale production of TE modules are awaiting further studies. Moreover, despite the currently developed unicouple, Mg3Sb2-xBix and Bi2-xSbxTe3 as the n-type and p-type legs, respectively, give a very promising cooling performance, and whether Bi2-xSbxTe3 is the best p-type material for multipair TE module still needs further studies. The exploration of matched p-type material, based on Mg3Sb2-xBix or the other isoelectronic Zintl phase, should be put on the agenda.
Acknowledgments
A. Li acknowledges Prof. Claudia Felser for offering him a visiting opportunity at Max Planck Institute for Chemical Physics of Solids and is very grateful for Jiazhan Xin, Yu Pan, Kaiyang Xia, and Qi Zhang for suggestions on manuscript writing. C. Fu acknowledges the useful discussions with Jiawei Zhang. This work was supported by the National Natural Science Foundation of China (51761135127, 51861145305), the National Science Fund for Distinguished Young Scholars (51725102), and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (392228380).
Contributor Information
Chenguang Fu, Email: chenguang.fu@cpfs.mpg.de.
Tiejun Zhu, Email: zhutj@zju.edu.cn.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
A. Li collected and analyzed the data, did the investigation, and wrote the original draft; C. Fu, X. Zhao, and T. Zhu conceived the idea and reviewed and edited the manuscript.
References
- 1.He J., Tritt T. M. Advances in thermoelectric materials research: looking back and moving forward. Science. 2017;357(6358):p. eaak9997. doi: 10.1126/science.aak9997. [DOI] [PubMed] [Google Scholar]
- 2.Snyder G. J., Toberer E. S. Complex thermoelectric materials. Nature Materials. 2008;7(2):105–114. doi: 10.1038/nmat2090. [DOI] [PubMed] [Google Scholar]
- 3.Pei Y., Wang H., Snyder G. J. Band engineering of thermoelectric materials. Advanced Materials. 2012;24(46):6125–6135. doi: 10.1002/adma.201202919. [DOI] [PubMed] [Google Scholar]
- 4.Zhu T., Liu Y., Fu C., Heremans J. P., Snyder J. G., Zhao X. Compromise and synergy in high-efficiency thermoelectric materials. Advanced Materials. 2017;29(14) doi: 10.1002/adma.201605884.1605884 [DOI] [PubMed] [Google Scholar]
- 5.Yang J., Xi L., Qiu W., et al. On the tuning of electrical and thermal transport in thermoelectrics: an integrated theory–experiment perspective. NPJ Computational Materials. 2016;2(1) doi: 10.1038/npjcompumats.2015.15.15015 [DOI] [Google Scholar]
- 6.Gorai P., Stevanović V., Toberer E. S. Computationally guided discovery of thermoelectric materials. Nature Reviews Materials. 2017;2(9) doi: 10.1038/natrevmats.2017.53.17053 [DOI] [Google Scholar]
- 7.Poudel B., Hao Q., Ma Y., et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science. 2008;320(5876):634–638. doi: 10.1126/science.1156446. [DOI] [PubMed] [Google Scholar]
- 8.Hu L., Wu H., Zhu T., et al. Tuning multiscale microstructures to enhance thermoelectric performance of n-type bismuth-telluride-based solid solutions. Advanced Energy Materials. 2015;5(17) doi: 10.1002/aenm.201500411.1500411 [DOI] [Google Scholar]
- 9.Zhu T., Hu L., Zhao X., He J. New insights into intrinsic point defects in V2VI3Thermoelectric materials. Advanced Science. 2016;3(7) doi: 10.1002/advs.201600004.1600004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Y., Qiu Y., Witting I., et al. Synergistic modulation of mobility and thermal conductivity in (Bi,Sb)2Te3 towards high thermoelectric performance. Energy & Environmental Science. 2019;12(2):624–630. doi: 10.1039/C8EE03225D. [DOI] [Google Scholar]
- 11.Pei Y., Shi X., LaLonde A., Wang H., Chen L., Snyder G. J. Convergence of electronic bands for high performance bulk thermoelectrics. Nature. 2011;473(7345):66–69. doi: 10.1038/nature09996. [DOI] [PubMed] [Google Scholar]
- 12.Wang H., Pei Y., LaLonde A. D., Snyder G. J. Weak electron–phonon coupling contributing to high thermoelectric performance in n-type PbSe. Proceedings of the National Academy of Sciences. 2012;109(25):9705–9709. doi: 10.1073/pnas.1111419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas K., He J., Blum I. D., et al. High-performance bulk thermoelectrics with all-scale hierarchical architectures. Nature. 2012;489(7416):414–418. doi: 10.1038/nature11439. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Gibbs Z. M., Takagiwa Y., Snyder G. J. Tuning bands of PbSe for better thermoelectric efficiency. Energy & Environmental Science. 2014;7(2):804–811. doi: 10.1039/C3EE43438A. [DOI] [Google Scholar]
- 15.Zhao L.-D., Tan G., Hao S., et al. Ultrahigh power factor and thermoelectric performance in hole-doped single-crystal SnSe. Science. 2016;351(6269):141–144. doi: 10.1126/science.aad3749. [DOI] [PubMed] [Google Scholar]
- 16.Chang C., Wu M., He D., et al. 3D charge and 2D phonon transports leading to high out-of-planeZTin n-type SnSe crystals. Science. 2018;360(6390):778–783. doi: 10.1126/science.aaq1479. [DOI] [PubMed] [Google Scholar]
- 17.Hong M., Zou J., Chen Z.-G. Thermoelectric GeTe with diverse degrees of freedom having secured superhigh performance. Advanced Materials. 2019;31(14):p. 1807071. doi: 10.1002/adma.201807071. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Bu Z., Lin S., Chen Z., Li W., Pei Y. GeTe Thermoelectrics. Joule. 2020;4(5):986–1003. doi: 10.1016/j.joule.2020.03.004. [DOI] [Google Scholar]
- 19.Liu H., Shi X., Xu F., et al. Copper ion liquid-like thermoelectrics. Nature Materials. 2012;11(5):422–425. doi: 10.1038/nmat3273. [DOI] [PubMed] [Google Scholar]
- 20.He Y., Day T., Zhang T., et al. High thermoelectric performance in non-toxic earth-abundant copper sulfide. Advanced Materials. 2014;26(23):3974–3978. doi: 10.1002/adma.201400515. [DOI] [PubMed] [Google Scholar]
- 21.Fu C., Zhu T., Pei Y., et al. High band degeneracy contributes to high thermoelectric performance in p-type half-Heusler compounds. Advanced Energy Materials. 2014;4(18) doi: 10.1002/aenm.201400600.1400600 [DOI] [Google Scholar]
- 22.Fu C., Bai S., Liu Y., et al. Realizing high figure of merit in heavy-band p-type half-Heusler thermoelectric materials. Nature Communications. 2015;6(1) doi: 10.1038/ncomms9144.8144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu T., Fu C., Xie H., Liu Y., Zhao X. High efficiency half-Heusler thermoelectric materials for energy harvesting. Advanced Energy Materials. 2015;5(19) doi: 10.1002/aenm.201500588.1500588 [DOI] [Google Scholar]
- 24.Yu J., Fu C., Liu Y., et al. Unique role of refractory Ta alloying in enhancing the figure of merit of NbFeSb thermoelectric materials. Advanced Energy Materials. 2018;8(1) doi: 10.1002/aenm.201701313.1701313 [DOI] [Google Scholar]
- 25.Zhu H., Mao J., Li Y., et al. Discovery of TaFeSb-based half-Heuslers with high thermoelectric performance. Nature Communications. 2019;10(1):p. 270. doi: 10.1038/s41467-018-08223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu C., Yao M., Chen X., et al. Revealing the intrinsic electronic structure of 3D half-Heusler thermoelectric materials by angle-resolved photoemission spectroscopy. Advanced Science. 2020;7(1) doi: 10.1002/advs.201902409.1902409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi X., Yang J., Salvador J. R., et al. Multiple-filled skutterudites: high thermoelectric figure of merit through separately optimizing electrical and thermal transports. Journal of the American Chemical Society. 2011;133(20):7837–7846. doi: 10.1021/ja111199y. [DOI] [PubMed] [Google Scholar]
- 28.Tang Y., Gibbs Z. M., Agapito L. A., et al. Convergence of multi-valley bands as the electronic origin of high thermoelectric performance in CoSb3 skutterudites. Nature Materials. 2015;14(12):1223–1228. doi: 10.1038/nmat4430. [DOI] [PubMed] [Google Scholar]
- 29.Zhao W., Liu Z., Sun Z., et al. Superparamagnetic enhancement of thermoelectric performance. Nature. 2017;549(7671):247–251. doi: 10.1038/nature23667. [DOI] [PubMed] [Google Scholar]
- 30.Caillat T., Fleurial J. P., Borshchevsky A. Preparation and thermoelectric properties of semiconducting Zn4Sb3. Journal of Physics and Chemistry of Solids. 1997;58(7):1119–1125. doi: 10.1016/S0022-3697(96)00228-4. [DOI] [Google Scholar]
- 31.Snyder G. J., Christensen M., Nishibori E., Caillat T., Iversen B. B. Disordered zinc in Zn4Sb3 with phonon-glass and electron-crystal thermoelectric properties. Nature Materials. 2004;3(7):458–463. doi: 10.1038/nmat1154. [DOI] [PubMed] [Google Scholar]
- 32.Brown S. R., Kauzlarich S. M., Gascoin F., Snyder G. J. Yb14MnSb11: new high efficiency thermoelectric material for power generation. Chemistry of Materials. 2006;18(7):1873–1877. doi: 10.1021/cm060261t. [DOI] [Google Scholar]
- 33.Mao J., Zhu H., Ding Z., et al. High thermoelectric cooling performance of n-type Mg3Bi2-based materials. Science. 2019;365(6452):495–498. doi: 10.1126/science.aax7792. [DOI] [PubMed] [Google Scholar]
- 34.Shi X., Yang J., Bai S., et al. On the design of high-efficiency thermoelectric clathrates through a systematic cross-substitution of framework elements. Advanced Functional Materials. 2010;20(5):755–763. doi: 10.1002/adfm.200901817. [DOI] [Google Scholar]
- 35.Takabatake T., Suekuni K., Nakayama T., Kaneshita E. Phonon-glass electron-crystal thermoelectric clathrates: experiments and theory. Reviews of Modern Physics. 2014;86(2):669–716. doi: 10.1103/RevModPhys.86.669. [DOI] [Google Scholar]
- 36.Liu W., Tan X., Yin K., et al. Convergence of conduction bands as a means of enhancing thermoelectric performance ofn-TypeMg2Si1−xSnx Solid solutions. Physical Review Letters. 2012;108(16, article 166601) doi: 10.1103/PhysRevLett.108.166601. [DOI] [PubMed] [Google Scholar]
- 37.Liu W., Kim H. S., Chen S., et al. n-Type Thermoelectric Material Mg2Sn0.75Ge0.25 for High Power Generation. Proceedings of the National Academy of Sciences. 2015;112(11):3269–3274. doi: 10.1073/pnas.1424388112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen X., Weathers A., Carrete J., et al. Twisting phonons in complex crystals with quasi-one-dimensional substructures. Nature Communications. 2015;6(1) doi: 10.1038/ncomms7723.6723 [DOI] [PubMed] [Google Scholar]
- 39.Joshi G., Lee H., Lan Y., et al. Enhanced thermoelectric figure-of-merit in nanostructured p-type silicon germanium bulk alloys. Nano Letters. 2008;8(12):4670–4674. doi: 10.1021/nl8026795. [DOI] [PubMed] [Google Scholar]
- 40.Yu B., Zebarjadi M., Wang H., et al. Enhancement of thermoelectric properties by modulation-doping in silicon germanium alloy nanocomposites. Nano Letters. 2012;12(4):2077–2082. doi: 10.1021/nl3003045. [DOI] [PubMed] [Google Scholar]
- 41.Solid-state cooling market by product, type, end-user industry, region- Global Forecast to 2024. June 2020, https://www.reportlinker.com/p05804676/Solid-State-Cooling-Market-by-Product-Type-End-User-Industry-Region-Global-Forecast-to.html?utm_source=GNW.
- 42.Ioffe A. F., Stil'bans L. S., Iordanishvili E. K., Stavitskaya T. S., Gelbtuch A., Vineyard G. Semiconductor thermoelements and thermoelectric cooling. Physics Today. 1959;12(5):p. 42. doi: 10.1063/1.3060810. [DOI] [Google Scholar]
- 43.Heremans J. P., Cava R. J., Samarth N. Tetradymites as thermoelectrics and topological insulators. Nature Reviews Materials. 2017;2(10) doi: 10.1038/natrevmats.2017.49.17049 [DOI] [Google Scholar]
- 44.Kirkham M. J., dos Santos A. M., Rawn C. J., Lara-Curzio E., Sharp J. W., Thompson A. J. Abinitiodetermination of crystal structures of the thermoelectric material MgAgSb. Physical Review B. 2012;85(14) doi: 10.1103/PhysRevB.85.144120.144120 [DOI] [Google Scholar]
- 45.Zhao H., Sui J., Tang Z., et al. High thermoelectric performance of MgAgSb-based materials. Nano Energy. 2014;7:97–103. doi: 10.1016/j.nanoen.2014.04.012. [DOI] [Google Scholar]
- 46.Ying P., Liu X., Fu C., et al. High performance α-MgAgSb thermoelectric materials for low temperature power generation. Chemistry of Materials. 2015;27(3):909–913. doi: 10.1021/cm5041826. [DOI] [Google Scholar]
- 47.Imasato K., Kang S. D., Snyder G. J. Exceptional thermoelectric performance in Mg3Sb0.6Bi1.4 for low-grade waste heat recovery. Energy & Environmental Science. 2019;12(3):965–971. doi: 10.1039/C8EE03374A. [DOI] [Google Scholar]
- 48.Pan Y., Yao M., Hong X., et al. Mg3(Bi,Sb)2 single crystals towards high thermoelectric performance. Energy & Environmental Science. 2020;13(6):1717–1724. doi: 10.1039/D0EE00838A. [DOI] [Google Scholar]
- 49.Lidde D. R. CRC Handbook of Chemistry and Physics. 89th. Taylor & Francis Group; 2008. [Google Scholar]
- 50.Nikitin E. N. Thermoelectric properties of solid solutions Mg2Si-Mg2Sn. Soviet Physics-Solid State. 1962;3:p. 2648. [Google Scholar]
- 51.Zaitsev V. K., Fedorov M. I., Gurieva E. A., et al. Highly effective Mg2Si1−xSnx thermoelectrics. Physical Review B. 2006;74(4) doi: 10.1103/PhysRevB.74.045207.045207 [DOI] [Google Scholar]
- 52.Liu W., Zhou J., Jie Q., et al. New insight into the material parameter B to understand the enhanced thermoelectric performance of Mg2Sn1−x−yGexSby. Energy & Environmental Science. 2016;9(2):530–539. doi: 10.1039/C5EE02600H. [DOI] [Google Scholar]
- 53.Liu W., Yin K., Zhang Q., Uher C., Tang X. Eco-friendly high-performance silicide thermoelectric materials. National Science Review. 2017;4(4):611–626. doi: 10.1093/nsr/nwx011. [DOI] [Google Scholar]
- 54.Jiang G., He J., Zhu T., et al. High performance Mg2(Si,Sn) solid solutions: a point defect chemistry approach to enhancing thermoelectric properties. Advanced Functional Materials. 2014;24(24):3776–3781. doi: 10.1002/adfm.201400123. [DOI] [Google Scholar]
- 55.Xin J., Wu H., Liu X., Zhu T., Yu G., Zhao X. Mg vacancy and dislocation strains as strong phonon scatterers in Mg2Si1−xSbx thermoelectric materials. Nano Energy. 2017;34:428–436. doi: 10.1016/j.nanoen.2017.03.012. [DOI] [Google Scholar]
- 56.Nowotny W. S. H. Ternäre Valenzverbindungen in den Systemen Kupfer (Silber)-Arsen (Antimon, Wismut)-Magnesium. Zeitschrift für Metallkunde. 1941;33:391–394. [Google Scholar]
- 57.Liu Z., Gao W., Meng X., et al. Mechanical properties of nanostructured thermoelectric materials α-MgAgSb. Scripta Materialia. 2017;127:72–75. doi: 10.1016/j.scriptamat.2016.08.037. [DOI] [Google Scholar]
- 58.Liu Z., Mao J., Sui J., Ren Z. High thermoelectric performance of α-MgAgSb for power generation. Energy & Environmental Science. 2018;11(1):23–44. doi: 10.1039/c7ee02504a. [DOI] [Google Scholar]
- 59.Ying P., Li X., Wang Y., et al. Hierarchical chemical bonds contributing to the intrinsically low thermal conductivity in α-MgAgSb thermoelectric materials. Advanced Functional Materials. 2017;27(1) doi: 10.1002/adfm.201604145.1604145 [DOI] [Google Scholar]
- 60.Mi J.-L., Ying P.-J., Sist M., et al. Elaborating the crystal structures of MgAgSb thermoelectric compound: polymorphs and atomic disorders. Chemistry of Materials. 2017;29(15):6378–6388. doi: 10.1021/acs.chemmater.7b01768. [DOI] [Google Scholar]
- 61.Zintl E., Husemann E. Bindungsart und Gitterbau binärer Magnesiumverbindungen. Zeitschrift für physikalische Chemie. 1933;21B(1):138–155. doi: 10.1515/zpch-1933-2112. [DOI] [Google Scholar]
- 62.Schäfer H., Eisenmann B., Müller W. Zintl phases: transitions between metallic and ionic bonding. Angewandte Chemie International Edition in English. 1973;12(9):694–712. doi: 10.1002/anie.197306941. [DOI] [Google Scholar]
- 63.Pincherle L., Radcliffe J. M. Semiconducting intermetallic compounds. Advances in Physics. 1956;5(19):271–322. doi: 10.1080/00018735600101195. [DOI] [Google Scholar]
- 64.Kajikawa T., Kimura N., Yokoyama T. Thermoelectric properties of intermetallic compounds: Mg3Bi2 and Mg3Sb2 for medium temperature range thermoelectric elements. 22nd International Conference on Thermoelectrics (ICT); August 2003; La Grande Motte, France. pp. 305–308. [DOI] [Google Scholar]
- 65.Condron C. L., Kauzlarich S. M., Gascoin F., Snyder G. J. Thermoelectric properties and microstructure of Mg3Sb2. Journal of Solid State Chemistry. 2006;179(8):2252–2257. doi: 10.1016/j.jssc.2006.01.034. [DOI] [Google Scholar]
- 66.Xin H. X., Qin X. Y. Electrical and thermoelectric properties of nanocrystal substitutional semiconductor alloys Mg3(BixSb1−x)2 prepared by mechanical alloying. Journal of Physics D: Applied Physics. 2006;39(24):5331–5337. doi: 10.1088/0022-3727/39/24/035. [DOI] [Google Scholar]
- 67.Bhardwaj A., Rajput A., Shukla A. K., et al. Mg3Sb2-based Zintl compound: a non-toxic, inexpensive and abundant thermoelectric material for power generation. RSC Advances. 2013;3(22):8504–8516. doi: 10.1039/C3RA40457A. [DOI] [Google Scholar]
- 68.Kim S., Kim C., Hong Y.-K., et al. Thermoelectric properties of Mn-doped Mg–Sb single crystals. Journal of Materials Chemistry A. 2014;2(31):12311–12316. doi: 10.1039/C4TA02386B. [DOI] [Google Scholar]
- 69.Shuai J., Wang Y., Kim H. S., et al. Thermoelectric properties of Na-doped Zintl compound: Mg3−xNaxSb2. Acta Materialia. 2015;93:187–193. doi: 10.1016/j.actamat.2015.04.023. [DOI] [Google Scholar]
- 70.Tamaki H., Sato H. K., Kanno T. Isotropic conduction network and defect chemistry in Mg3+δSb2-based layered Zintl compounds with high thermoelectric performance. Advanced Materials. 2016;28(46):10182–10187. doi: 10.1002/adma.201603955. [DOI] [PubMed] [Google Scholar]
- 71.Zhang J., Song L., Pedersen S. H., Yin H., Hung L. T., Iversen B. B. Discovery of high-performance low-cost n-type Mg3Sb2-based thermoelectric materials with multi-valley conduction bands. Nature Communications. 2017;8(1) doi: 10.1038/ncomms13901.13901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shuai J., Mao J., Song S., et al. Tuning the carrier scattering mechanism to effectively improve the thermoelectric properties. Energy & Environmental Science. 2017;10(3):799–807. doi: 10.1039/C7EE00098G. [DOI] [Google Scholar]
- 73.Shi X., Zhao T., Zhang X., et al. Extraordinary n-type Mg3SbBi thermoelectrics enabled by yttrium doping. Advanced Materials. 2019;31(36) doi: 10.1002/adma.201903387.1903387 [DOI] [PubMed] [Google Scholar]
- 74.Gorai P., Ganose A., Faghaninia A., Jain A., Stevanović V. Computational discovery of promising new n-type dopable ABX Zintl thermoelectric materials. Materials Horizons. 2020;7(7):1809–1818. doi: 10.1039/D0MH00197J. [DOI] [Google Scholar]
- 75.Liu W., Tang X., Li H., Sharp J., Zhou X., Uher C. Optimized thermoelectric properties of Sb-doped Mg2(1+z)Si0.5–ySn0.5Sby through adjustment of the Mg content. Chemistry of Materials. 2011;23(23):5256–5263. doi: 10.1021/cm202445d. [DOI] [Google Scholar]
- 76.Liu X., Xi L., Qiu W., et al. Significant roles of intrinsic point defects in Mg2X (X = Si, Ge, Sn) thermoelectric materials. Advanced Electronic Materials. 2016;2(2) doi: 10.1002/aelm.201500284.1500284 [DOI] [Google Scholar]
- 77.Song L., Zhang J., Iversen B. B. Simultaneous improvement of power factor and thermal conductivity via Ag doping in p-type Mg3Sb2 thermoelectric materials. Journal of Materials Chemistry A. 2017;5(10):4932–4939. doi: 10.1039/c6ta08316a. [DOI] [Google Scholar]
- 78.Kanno T., Tamaki H., Sato H. K., et al. Enhancement of average thermoelectric figure of merit by increasing the grain-size of Mg3.2Sb1.5Bi0.49Te0.01. Applied Physics Letters. 2018;112(3) doi: 10.1063/1.5016488.033903 [DOI] [Google Scholar]
- 79.Chen X., Wu H., Cui J., et al. Extraordinary thermoelectric performance in n-type manganese doped Mg3Sb2 Zintl: high band degeneracy, tuned carrier scattering mechanism and hierarchical microstructure. Nano Energy. 2018;52:246–255. doi: 10.1016/j.nanoen.2018.07.059. [DOI] [Google Scholar]
- 80.Shu R., Zhou Y., Wang Q., et al. Mg3+δSbxBi2−x Family: a promising substitute for the state-of-the-art n-type thermoelectric materials near room temperature. Advanced Functional Materials. 2019;29(4) doi: 10.1002/adfm.201807235.1807235 [DOI] [Google Scholar]
- 81.Ren Z., Shuai J., Mao J., et al. Significantly enhanced thermoelectric properties of p-type Mg3Sb2 via co-doping of Na and Zn. Acta Materialia. 2018;143:265–271. doi: 10.1016/j.actamat.2017.10.015. [DOI] [Google Scholar]
- 82.Wood M., Kuo J. J., Imasato K., Snyder G. J. Improvement of Low‐TemperaturezTin a Mg3Sb2–Mg3Bi2 Solid solution via Mg-vapor annealing. Advanced Materials. 2019;31(35) doi: 10.1002/adma.201902337.1902337 [DOI] [PubMed] [Google Scholar]
- 83.Tang X., Zhang B., Zhang X., et al. Enhancing the thermoelectric performance of p-type Mg3Sb2 via codoping of Li and Cd. ACS Applied Materials & Interfaces. 2020;12(7):8359–8365. doi: 10.1021/acsami.9b23059. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J., Song L., Mamakhel A., Jørgensen M. R. V., Iversen B. B. High-performance low-cost n-type se-doped Mg3Sb2-based Zintl compounds for thermoelectric application. Chemistry of Materials. 2017;29(12):5371–5383. doi: 10.1021/acs.chemmater.7b01746. [DOI] [Google Scholar]
- 85.Mao J., Shuai J., Song S., et al. Manipulation of ionized impurity scattering for achieving high thermoelectric performance in n-type Mg3Sb2-based materials. Proceedings of the National Academy of Sciences. 2017;114(40):10548–10553. doi: 10.1073/pnas.1711725114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J., Song L., Borup K. A., Jørgensen M. R. V., Iversen B. B. New insight on tuning electrical transport properties via chalcogen doping in n-type Mg3Sb2-based thermoelectric materials. Advanced Energy Materials. 2018;8(16) doi: 10.1002/aenm.201702776.1702776 [DOI] [Google Scholar]
- 87.Imasato K., Ohno S., Kang S. D., Snyder G. J. Improving the thermoelectric performance in Mg3+xSb1.5Bi0.49Te0.01 by reducing excess Mg. APL Materials. 2018;6(1) doi: 10.1063/1.5011379.016106 [DOI] [Google Scholar]
- 88.Song S. W., Mao J., Bordelon M., et al. Joint effect of magnesium and yttrium on enhancing thermoelectric properties of n-type Zintl Mg3+δY0.02Sb1.5Bi0.5. Materials Today Physics. 2019;8:25–33. doi: 10.1016/j.mtphys.2018.12.004. [DOI] [Google Scholar]
- 89.Han Z., Gui Z., Zhu Y. B., et al. The electronic transport channel protection and tuning in real space to boost the thermoelectric performance of Mg3+δSb2-yBiy near room temperature. Research. 2020;2020:12. doi: 10.34133/2020/1672051.1672051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jørgensen L. R., Zhang J., Zeuthen C. B., Iversen B. B. Thermal stability of Mg3Sb1.475Bi0.475Te0.05 high performance n-type thermoelectric investigated through powder X-ray diffraction and pair distribution function analysis. Journal of Materials Chemistry A. 2018;6(35):17171–17176. doi: 10.1039/c8ta06544f. [DOI] [Google Scholar]
- 91.Song L., Zhang J., Iversen B. B. Thermal stability of p-type Ag-doped Mg3Sb2thermoelectric materials investigated by powder X-ray diffraction. Physical Chemistry Chemical Physics. 2019;21(8):4295–4305. doi: 10.1039/c8cp07648k. [DOI] [PubMed] [Google Scholar]
- 92.Shi X., Sun C., Zhang X., et al. Efficient Sc-doped Mg3.05–xScxSbBi thermoelectrics near room temperature. Chemistry of Materials. 2019;31(21):8987–8994. doi: 10.1021/acs.chemmater.9b03156. [DOI] [Google Scholar]
- 93.Zhang F., Chen C., Yao H., et al. High-performance N-type Mg3Sb2 towards thermoelectric application near room temperature. Advanced Functional Materials. 2019;30(5) doi: 10.1002/adfm.201906143.1906143 [DOI] [Google Scholar]
- 94.Peng W., Petretto G., Rignanese G.-M., Hautier G., Zevalkink A. An unlikely route to low lattice thermal conductivity: small atoms in a simple layered structure. Joule. 2018;2(9):1879–1893. doi: 10.1016/j.joule.2018.06.014. [DOI] [Google Scholar]
- 95.Kuo J. J., Kang S. D., Imasato K., et al. Grain boundary dominated charge transport in Mg3Sb2-based compounds. Energy & Environmental Science. 2018;11(2):429–434. doi: 10.1039/C7EE03326E. [DOI] [Google Scholar]
- 96.Imasato K., Fu C., Pan Y., et al. Metallic n-type Mg3Sb2 Single crystals demonstrate the absence of ionized impurity scattering and enhanced thermoelectric performance. Advanced Materials. 2020;32(16) doi: 10.1002/adma.201908218.e1908218 [DOI] [PubMed] [Google Scholar]
- 97.Ohno S., Imasato K., Anand S., et al. Phase boundary mapping to obtain n-type Mg3Sb2-based thermoelectrics. Joule. 2018;2(1):141–154. doi: 10.1016/j.joule.2017.11.005. [DOI] [Google Scholar]
- 98.Kuo J. J., Yu Y., Kang S. D., Cojocaru-Mirédin O., Wuttig M., Snyder G. J. Mg deficiency in grain boundaries of n-type Mg3Sb2 Identified by atom probe tomography. Advanced Materials Interfaces. 2019;6(13) doi: 10.1002/admi.201900429.1900429 [DOI] [Google Scholar]
- 99.Zhang J., Song L., Iversen B. B. Insights into the design of thermoelectric Mg3Sb2 and its analogs by combining theory and experiment. Npj Computational Materials. 2019;5(1) doi: 10.1038/s41524-019-0215-y. [DOI] [Google Scholar]
- 100.Shi X., Wang X., Li W., Pei Y. Advances in thermoelectric Mg3Sb2and its derivatives. Small Methods. 2018;2(10) doi: 10.1002/smtd.201800022.1800022 [DOI] [Google Scholar]
- 101.The periodic table of the elements. June 2020, https://www.webelements.com/
- 102.Shannon-Prewitt effective ionic radius navigation. September 2020, http://www.knowledgedoor.com/2/elements_handbook/shannon-prewitt_effective_ionic_radius.html.
- 103.Perry R. H. In: Perry's Chemical Engineers' Handbook. 7th. Green D. W., editor. McGraw-Hill; 1997. [Google Scholar]
- 104.Barnes A. C., Guo C., Howells W. S. Fast-ion conduction and the structure of beta -Mg3Bi2. Journal of Physics: Condensed Matter. 1994;6(32):L467–L471. doi: 10.1088/0953-8984/6/32/002. [DOI] [Google Scholar]
- 105.Howells W. S., Barnes A. C., Hamilton M. Quasielastic neutron scattering and the dynamics of Mg2+ in the fast ion and liquid phases of Mg3Bi2. Physica B: Condensed Matter. 1999;266(1-2):97–99. doi: 10.1016/S0921-4526(98)01499-9. [DOI] [Google Scholar]
- 106.Lee J., Monserrat B., Seymour I. D., Liu Z., Dutton S. E., Grey C. P. Anab initioinvestigation on the electronic structure, defect energetics, and magnesium kinetics in Mg3Bi2. Journal of Materials Chemistry A. 2018;6(35):16983–16991. doi: 10.1039/c7ta11181a. [DOI] [Google Scholar]
- 107.Meng Z., Foix D., Brun N., et al. Alloys to replace Mg anodes in efficient and practical Mg-ion/sulfur batteries. ACS Energy Letters. 2019;4(9):2040–2044. doi: 10.1021/acsenergylett.9b01389. [DOI] [Google Scholar]
- 108.Martinez-Ripoll M., Haase A., Brauer G. The crystal structure of α-Mg3Sb2. Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 1974;30(8):2006–2009. doi: 10.1107/S0567740874006285. [DOI] [Google Scholar]
- 109.Predel F. Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys. Berlin Heidelberg: Springer-Verlag; 2016. [Google Scholar]
- 110.Imai Y., Watanabe A. Electronic structures of Mg3Pn2 (Pn = N, P, As, Sb and Bi) and Ca3N2 calculated by a first-principle pseudopotential method. Journal of Materials Science. 2006;41(8):2435–2441. doi: 10.1007/s10853-006-5181-3. [DOI] [Google Scholar]
- 111.Sevast’yanova L. G., Kravchenko O. V., Gulish O. K., Stupnikov V. A., Leonova M. E., Zhizhin M. G. Binary and ternary compounds in the Mg-Sb-B and Mg-Bi-B systems as catalysts for the synthesis of cubic BN. Inorganic Materials. 2006;42(8):863–866. doi: 10.1134/s0020168506080115. [DOI] [Google Scholar]
- 112.Sedighi M., Arghavani Nia B., Zarringhalam H., Moradian R. Density functional theory study of the structural and electronic properties of Mg3Bi2 in hexagonal and cubic phases. The European Physical Journal Applied Physics. 2013;61(1):p. 10103. doi: 10.1051/epjap/2012120207.10103 [DOI] [Google Scholar]
- 113.Zheng C., Hoffmann R., Nesper R., von Schnering H. G. Site preferences and bond length differences in CaAl2Si2-type Zintl compounds. Journal of the American Chemical Society. 1986;108(8):1876–1884. doi: 10.1021/ja00268a027. [DOI] [Google Scholar]
- 114.Sun J., Singh D. J. Thermoelectric properties of AMg2X2, AZn2Sb2 (A = Ca, Sr, Ba; X = Sb, Bi), and Ba2ZnX2 (X = Sb, Bi) Zintl compounds. Journal of Materials Chemistry A. 2017;5(18):8499–8509. doi: 10.1039/C6TA11234J. [DOI] [Google Scholar]
- 115.Imasato K., Wood M., Kuo J. J., Snyder G. J. Improved stability and high thermoelectric performance through cation site doping in n-type La-doped Mg3Sb1.5Bi0.5. Journal of Materials Chemistry A. 2018;6(41):19941–19946. doi: 10.1039/C8TA08975B. [DOI] [Google Scholar]
- 116.Sun X., Li X., Yang J., et al. Achieving band convergence by tuning the bonding ionicity in n-type Mg3Sb2. Journal of Computational Chemistry. 2019;40(18):1693–1700. doi: 10.1002/jcc.25822. [DOI] [PubMed] [Google Scholar]
- 117.Wood M., Imasato K., Anand S., Yang J., Snyder G. J. The importance of the Mg–Mg interaction in Mg3Sb2–Mg3Bi2 shown through cation site alloying. Journal of Materials Chemistry A. 2020;8(4):2033–2038. doi: 10.1039/C9TA11328B. [DOI] [Google Scholar]
- 118.Zhang F., Chen C., Li S., et al. Enhanced thermoelectric performance in N-type Mg3.2Sb1.5Bi0.5 by La or Ce doping into Mg. Advanced Electronic Materials. 2020;6(3) doi: 10.1002/aelm.201901391.1901391 [DOI] [Google Scholar]
- 119.Xia S.-Q., King P., Bobev S. Mg and Zn disorder in Mg1.59(1)Zn1.41(1)Sb2. Acta Crystallographica Section E: Structure Reports Online. 2006;62(9):i184–i186. doi: 10.1107/S1600536806029953. [DOI] [Google Scholar]
- 120.Xia S.-Q., Myers C., Bobev S. Combined experimental and density functional theory studies on the crystal structures and magnetic properties of Mg(Mg1–xMnx)2Sb2 (x ≈0.25) and BaMn2Sb2. European Journal of Inorganic Chemistry. 2008;2008(27):4262–4269. doi: 10.1002/ejic.200800572. [DOI] [Google Scholar]
- 121.Zhang J., Song L., Sist M., Tolborg K., Iversen B. B. Chemical bonding origin of the unexpected isotropic physical properties in thermoelectric Mg3Sb2 and related materials. Nature Communications. 2018;9(1) doi: 10.1038/s41467-018-06980-x.4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ortiz B. R., Gorai P., Braden T., et al. Discovery of n-type Zintl phases RbAlSb4, RbGaSb4, CsAlSb4, and CsGaSb4. ACS Applied Energy Materials. 2020;3(3):2182–2191. doi: 10.1021/acsaem.0c00048. [DOI] [Google Scholar]
- 123.Zheng C., Hoffmann R. Complementary local and extended views of bonding in the ThCr2Si2 and CaAl2Si2 structures. Journal of Solid State Chemistry. 1988;72(1):58–71. doi: 10.1016/0022-4596(88)90009-6. [DOI] [Google Scholar]
- 124.Kauzlarich S. M., Zevalkink A., Toberer E., Snyder G. J. Zintl phases: recent developments in thermoelectrics and future outlook. In: Nandhakumar I., White N. M., Beeby S., editors. Thermoelectric Materials and Devices. The Royal Society of Chemistry; 2017. pp. 1–26. [Google Scholar]
- 125.Momma K., Izumi F. VESTA3for three-dimensional visualization of crystal, volumetric and morphology data. Journal of Applied Crystallography. 2011;44(6):1272–1276. doi: 10.1107/S0021889811038970. [DOI] [Google Scholar]
- 126.Li J., Zhang S., Wang B., et al. Designing high-performance n-type Mg3Sb2-based thermoelectric materials through forming solid solutions and biaxial strain. Journal of Materials Chemistry A. 2018;6(41):20454–20462. doi: 10.1039/C8TA07285J. [DOI] [Google Scholar]
- 127.Tan X., Liu G.-Q., Hu H., Shao H., Xu J., Jiang J. Band engineering and crystal field screening in thermoelectric Mg3Sb2. Journal of Materials Chemistry A. 2019;7(15):8922–8928. doi: 10.1039/c9ta00288j. [DOI] [Google Scholar]
- 128.Zhang X., Jin L., Dai X., Liu G. Topological type-II nodal line semimetal and Dirac semimetal state in stable kagome compound Mg3Bi2. The Journal of Physical Chemistry Letters. 2017;8(19):4814–4819. doi: 10.1021/acs.jpclett.7b02129. [DOI] [PubMed] [Google Scholar]
- 129.Chang T.-R., Pletikosic I., Kong T., et al. Realization of a type-II nodal-line semimetal in Mg3Bi2. Advanced Science. 2019;6(4) doi: 10.1002/advs.201800897.1800897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhou T., Zhu X.-G., Tong M., et al. Experimental evidence of topological surface states in Mg3Bi2 Films grown by molecular beam epitaxy. Chinese Physics Letters. 2019;36(11) doi: 10.1088/0256-307x/36/11/117303.117303 [DOI] [Google Scholar]
- 131.Ahmadpour F., Kolodiazhnyi T., Mozharivskyj Y. Structural and physical properties of Mg3−xZnxSb2 (x =0–1.34) Journal of Solid State Chemistry. 2007;180(9):2420–2428. doi: 10.1016/j.jssc.2007.06.011. [DOI] [Google Scholar]
- 132.Verbrugge D. M., Van Zytveld J. B. Electronic properties of liquid MgSb. Journal of Non-Crystalline Solids. 1993;156-158:736–739. doi: 10.1016/0022-3093(93)90058-6. [DOI] [Google Scholar]
- 133.Huang S., Wang Z., Xiong R., Yu H., Shi J. Significant enhancement in thermoelectric performance of Mg3Sb2 from bulk to two-dimensional mono layer. Nano Energy. 2019;62:212–219. doi: 10.1016/j.nanoen.2019.05.028. [DOI] [Google Scholar]
- 134.Meng F., Sun S., Ma J., Chronister C., He J., Li W. Anisotropic thermoelectric figure-of-merit in Mg3Sb2. Materials Today Physics. 2020;13 doi: 10.1016/j.mtphys.2020.100217.100217 [DOI] [Google Scholar]
- 135.Xin J., Li G., Auffermann G., et al. Growth and transport properties of Mg3X2 (X = Sb, Bi) single crystals. Materials Today Physics. 2018;7:61–68. doi: 10.1016/j.mtphys.2018.11.004. [DOI] [Google Scholar]
- 136.Toberer E. S., Zevalkink A., Snyder G. J. Phonon engineering through crystal chemistry. Journal of Materials Chemistry. 2011;21(40):15843–15852. doi: 10.1039/C1JM11754H. [DOI] [Google Scholar]
- 137.Tritt T. M. Thermal Conductivity: Theory, Properties, and Applications. Springer; 2004. [Google Scholar]
- 138.Morelli D. T., Jovovic V., Heremans J. P. Intrinsically minimal thermal conductivity in CubicI−V−VI2Semiconductors. Physical Review Letters. 2008;101(3) doi: 10.1103/PhysRevLett.101.035901.035901 [DOI] [PubMed] [Google Scholar]
- 139.Roufosse M., Klemens P. G. Thermal conductivity of complex dielectric crystals. Physical Review B. 1973;7(12):5379–5386. doi: 10.1103/PhysRevB.7.5379. [DOI] [Google Scholar]
- 140.Zhao L.-D., He J., Berardan D., et al. BiCuSeO oxyselenides: new promising thermoelectric materials. Energy & Environmental Science. 2014;7(9):2900–2924. doi: 10.1039/C4EE00997E. [DOI] [Google Scholar]
- 141.Xiao Y., Chang C., Pei Y., et al. Origin of low thermal conductivity in SnSe. Physical Review B. 2016;94(12) doi: 10.1103/PhysRevB.94.125203.125203 [DOI] [Google Scholar]
- 142.Wang Y., Zhang X., Wang Y., Liu H., Zhang J. Enhanced thermoelectric properties of n-type Mg3Sb2 by excess magnesium and tellurium doping. Physica Status Solidi (A) 2019;216(6) doi: 10.1002/pssa.201800811.1800811 [DOI] [Google Scholar]
- 143.Agne M. T., Imasato K., Anand S., et al. Heat capacity of Mg3Sb2, Mg3Bi2, and their alloys at high temperature. Materials Today Physics. 2018;6:83–88. doi: 10.1016/j.mtphys.2018.10.001. [DOI] [Google Scholar]
- 144.Delaire O., Ma J., Marty K., et al. Giant anharmonic phonon scattering in PbTe. Nature Materials. 2011;10(8):614–619. doi: 10.1038/nmat3035. [DOI] [PubMed] [Google Scholar]
- 145.Ma J., Delaire O., May A. F., et al. Glass-like phonon scattering from a spontaneous nanostructure in AgSbTe2. Nature Nanotechnology. 2013;8(6):445–451. doi: 10.1038/nnano.2013.95. [DOI] [PubMed] [Google Scholar]
- 146.Song S., Mao J., Shuai J., et al. Study on anisotropy of n-type Mg3Sb2-based thermoelectric materials. Applied Physics Letters. 2018;112(9) doi: 10.1063/1.5000053.092103 [DOI] [Google Scholar]
- 147.Hu L., Zhu T., Liu X., Zhao X. Point defect engineering of high-performance bismuth-telluride-based thermoelectric materials. Advanced Functional Materials. 2014;24(33):5211–5218. doi: 10.1002/adfm.201400474. [DOI] [Google Scholar]
- 148.Deng R., Su X., Zheng Z., et al. Thermal conductivity in Bi0.5Sb1.5Te3+x and the role of dense dislocation arrays at grain boundaries. Science Advances. 2018;4(6) doi: 10.1126/sciadv.aar5606.eaar5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang J., Meisner G. P., Chen L. Strain field fluctuation effects on lattice thermal conductivity of ZrNiSn-based thermoelectric compounds. Applied Physics Letters. 2004;85(7):1140–1142. doi: 10.1063/1.1783022. [DOI] [Google Scholar]
- 150.Liu Y., Xie H., Fu C., Snyder G. J., Zhao X., Zhu T. Demonstration of a phonon-glass electron-crystal strategy in (Hf,Zr)NiSn half-Heusler thermoelectric materials by alloying. Journal of Materials Chemistry A. 2015;3(45):22716–22722. doi: 10.1039/C5TA04418A. [DOI] [Google Scholar]
- 151.Gurunathan R., Hanus R., Dylla M., Katre A., Snyder G. J. Analytical models of phonon-point-defect scattering. Physical Review Applied. 2020;13(3) doi: 10.1103/PhysRevApplied.13.034011.034011 [DOI] [Google Scholar]
- 152.Zhang Q., Zhu T. J., Zhou A. J., Yin H., Zhao X. B. Preparation and thermoelectric properties of Mg2Si1−xSnx. Physica Scripta. 2007;T129(T129):123–126. doi: 10.1088/0031-8949/2007/T129/028. [DOI] [Google Scholar]
- 153.Yi S.-I., Attari V., Jeong M., et al. Strain-induced suppression of the miscibility gap in nanostructured Mg2Si–Mg2Sn solid solutions. Journal of Materials Chemistry A. 2018;6(36):17559–17570. doi: 10.1039/C8TA05798B. [DOI] [Google Scholar]
- 154.Yasseri M., Sankhla A., Kamila H., et al. Solid solution formation in Mg2(Si,Sn) and shape of the miscibility gap. Acta Materialia. 2020;185:80–88. doi: 10.1016/j.actamat.2019.11.054. [DOI] [Google Scholar]
- 155.Schwall M., Balke B. Phase separation as a key to a thermoelectric high efficiency. Physical Chemistry Chemical Physics. 2013;15(6):1868–1872. doi: 10.1039/C2CP43946H. [DOI] [PubMed] [Google Scholar]
- 156.Page A., Van der Ven A., Poudeu P. F. P., Uher C. Origins of phase separation in thermoelectric (Ti, Zr, Hf)NiSn half-Heusler alloys from first principles. Journal of Materials Chemistry A. 2016;4(36):13949–13956. doi: 10.1039/C6TA04957E. [DOI] [Google Scholar]
- 157.Rausch E., Balke B., Stahlhofen J. M., Ouardi S., Burkhardt U., Felser C. Fine tuning of thermoelectric performance in phase-separated half-Heusler compounds. Journal of Materials Chemistry C. 2015;3(40):10409–10414. doi: 10.1039/C5TC01196E. [DOI] [Google Scholar]
- 158.Mena J. M., Rausch E., Ouardi S., et al. Miscibility gap in the phase diagrams of thermoelectric half-Heusler materials CoTi1-xYxSb (Y = Sc, V, Mn, Fe) Journal of Electronic Materials. 2016;45(3):1382–1388. doi: 10.1007/s11664-015-4041-9. [DOI] [Google Scholar]
- 159.Liu Y., Fu C., Xia K., et al. Lanthanide contraction as a design factor for high-performance half-Heusler thermoelectric materials. Advanced Materials. 2018;30(32) doi: 10.1002/adma.201800881.1800881 [DOI] [PubMed] [Google Scholar]
- 160.Wang H., LaLonde A. D., Pei Y., Snyder G. J. The criteria for beneficial disorder in thermoelectric solid solutions. Advanced Functional Materials. 2013;23(12):1586–1596. doi: 10.1002/adfm.201201576. [DOI] [Google Scholar]
- 161.Hanus R., Agne M. T., Rettie A. J. E., et al. Lattice softening Significantly reduces thermal conductivity and leads to high thermoelectric Efficiency. Advanced Materials. 2019;31(21) doi: 10.1002/adma.201900108.1900108 [DOI] [PubMed] [Google Scholar]
- 162.Zhang J., Song L., Madsen G. K. H., et al. Designing high-performance layered thermoelectric materials through orbital engineering. Nature Communications. 2016;7(1) doi: 10.1038/ncomms10892.10892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li J., Zheng S., Fang T., Yue L., Zhang S., Lu G. Computational prediction of a highZTof n-type Mg3Sb2-based compounds with isotropic thermoelectric conduction performance. Physical Chemistry Chemical Physics. 2018;20(11):7686–7693. doi: 10.1039/C7CP08680F. [DOI] [PubMed] [Google Scholar]
- 164.May A. F., Snyder G. J. Introduction to modeling thermoelectric transport at high temperatures. In: Rowe D. M., editor. Materials, Preparation, and Characterization in Thermoelectrics. Taylor & Francis Group; 2017. pp. 1–18. [Google Scholar]
- 165.May A. F., Toberer E. S., Saramat A., Snyder G. J. Characterization and analysis of thermoelectric transport inn-typeBa8Ga16−xGe30+x. Physical Review B. 2009;80(12) doi: 10.1103/PhysRevB.80.125205.125205 [DOI] [Google Scholar]
- 166.Imasato K., Kang S. D., Ohno S., Snyder G. J. Band engineering in Mg3Sb2 by alloying with Mg3Bi2for enhanced thermoelectric performance. Materials Horizons. 2018;5(1):59–64. doi: 10.1039/C7MH00865A. [DOI] [Google Scholar]
- 167.Snyder G. J., Snyder A. H., Wood M., Gurunathan R., Snyder B. H., Niu C. Weighted mobility. Advanced Materials. 2020;32(25) doi: 10.1002/adma.202001537.2001537 [DOI] [PubMed] [Google Scholar]
- 168.Fu C., Zhu T., Liu Y., Xie H., Zhao X. Band engineering of high performance p-type FeNbSb based half-Heusler thermoelectric materials for figure of merit zT > 1. Energy & Environmental Science. 2015;8(1):216–220. doi: 10.1039/C4EE03042G. [DOI] [Google Scholar]
- 169.Sofo J. O., Mahan G. D. Optimum band gap of a thermoelectric material. Physical Review B. 1994;49(7):4565–4570. doi: 10.1103/PhysRevB.49.4565. [DOI] [PubMed] [Google Scholar]
- 170.Pei Y., Gibbs Z. M., Gloskovskii A., Balke B., Zeier W. G., Snyder G. J. Optimum carrier concentration in n-type PbTe thermoelectrics. Advanced Energy Materials. 2014;4(13) doi: 10.1002/aenm.201400486.1400486 [DOI] [Google Scholar]
- 171.Gorai P., Toberer E. S., Stevanović V. Effective n-type doping of Mg3Sb2 with group-3 elements. Journal of Applied Physics. 2019;125(2) doi: 10.1063/1.5081833.025105 [DOI] [Google Scholar]
- 172.Periodic table of atomic orbital energies. June 2020, http://www.graylark.com/eve/orbital-energies-table.html.
- 173.Wang Y., Zhang X., Liu Y., Wang Y., Liu H., Zhang J. Enhanced electrical transport performance through cation site doping in Y-doped Mg3.2Sb2. Journal of Materiomics. 2020;6(1):216–223. doi: 10.1016/j.jmat.2019.12.007. [DOI] [Google Scholar]
- 174.Böer K. W., Pohl U. W. Semiconductor Physics. Springer; 2018. [Google Scholar]
- 175.Ren Q., Fu C., Qiu Q., et al. Establishing the carrier scattering phase diagram for ZrNiSn-based half-Heusler thermoelectric materials. Nature Communication. 2020;11(1) doi: 10.1038/s41467-020-16913-2.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang H., Pei Y., LaLonde A. D., Snyder G. J. Material design considerations based on thermoelectric quality factor. In: TMs K. K., editor. Thermoelectric Nanomaterials: Materials Design and Applications. Springer; 2013. pp. 3–32. [DOI] [Google Scholar]
- 177.Shi X., Sun C., Bu Z., et al. Revelation of inherently high mobility enables Mg3Sb2 as a sustainable alternative to n‐Bi2Te3Thermoelectrics. Advanced Science. 2019;6(16, article 1802286) doi: 10.1002/advs.201802286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shuai J., Ge B., Mao J., Song S., Wang Y., Ren Z. Significant role of Mg stoichiometry in designing high thermoelectric performance for Mg3(Sb,Bi)2-based n-type Zintls. Journal of American Chemical Society. 2018;140(5):1910–1915. doi: 10.1021/jacs.7b12767. [DOI] [PubMed] [Google Scholar]
- 179.Kuo J. J., Wood M., Slade T. J., Kanatzidis M. G., Snyder G. J. Systematic over-estimation of lattice thermal conductivity in materials with electrically-resistive grain boundaries. Energy & Environmental Science. 2020;13(4):1250–1258. doi: 10.1039/C9EE03921J. [DOI] [Google Scholar]
- 180.Watanabe T. Grain boundary engineering: historical perspective and future prospects. Journal of Materials Science. 2011;46(12):4095–4115. doi: 10.1007/s10853-011-5393-z. [DOI] [Google Scholar]
- 181.Cantwell P. R., Tang M., Dillon S. J., Luo J., Rohrer G. S., Harmer M. P. Grain boundary complexions. Acta Materialia. 2014;62:1–48. doi: 10.1016/j.actamat.2013.07.037. [DOI] [Google Scholar]
- 182.Su X., Wei P., Li H., et al. Multi-scale microstructural thermoelectric materials: transport behavior, non-equilibrium preparation, and applications. Advanced Materials. 2017;29(20) doi: 10.1002/adma.201602013.1602013 [DOI] [PubMed] [Google Scholar]
- 183.Chen Z., Jian Z., Li W., et al. Lattice dislocations enhancing thermoelectric PbTe in addition to band convergence. Advanced Materials. 2017;29(23) doi: 10.1002/adma.201606768.1606768 [DOI] [PubMed] [Google Scholar]
- 184.Yu Y., Zhou C., Zhang S., et al. Revealing nano-chemistry at lattice defects in thermoelectric materials using atom probe tomography. Materials Today. 2020;32:260–274. doi: 10.1016/j.mattod.2019.11.010. [DOI] [Google Scholar]
- 185.Wu H., Zhang Y., Ning S., Zhao L. D., Pennycook S. J. Seeing atomic-scale structural origins and foreseeing new pathways to improved thermoelectric materials. Materials Horizons. 2019;6(8):1548–1570. doi: 10.1039/C9MH00543A. [DOI] [Google Scholar]
- 186.Zhao C., Li Z., Fan T., Xiao C., Xie Y. Defects engineering with multiple dimensions in thermoelectric materials. Research. 2020;2020, article 9652749:23. doi: 10.34133/2020/9652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Horák J., Čermák K., Koudelka L. Energy formation of antisite defects in doped Sb2Te3 and Bi2Te3 crystals. Journal of Physics and Chemistry of Solids. 1986;47(8):805–809. doi: 10.1016/0022-3697(86)90010-7. [DOI] [Google Scholar]
- 188.Cho S., Kim Y., DiVenere A., Wong G. K., Ketterson J. B., Meyer J. R. Antisite defects of Bi2Te3 thin films. Applied Physics Letters. 1999;75(10):1401–1403. doi: 10.1063/1.124707. [DOI] [Google Scholar]
- 189.Xie H., Wang H., Fu C., et al. The intrinsic disorder related alloy scattering in ZrNiSn half-Heusler thermoelectric materials. Scientific Reports. 2015;4(1) doi: 10.1038/srep06888.6888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zeier W. G., Schmitt J., Hautier G., et al. Engineering half-Heusler thermoelectric materials using Zintl chemistry. Nature Reviews Materials. 2016;1(6) doi: 10.1038/natrevmats.2016.32.16032 [DOI] [Google Scholar]
- 191.Liu Z., Geng H., Mao J., et al. Understanding and manipulating the intrinsic point defect in α-MgAgSb for higher thermoelectric performance. Journal of Materials Chemistry A. 2016;4(43):16834–16840. doi: 10.1039/C6TA06832D. [DOI] [Google Scholar]
- 192.Zhang J., Song L., Iversen B. B. Rapid one-step synthesis and compaction of high-performance n-type Mg3Sb2 Thermoelectrics. Angewandte Chemie International Edition in English. 2020;59(11):4278–4282. doi: 10.1002/anie.201912909. [DOI] [PubMed] [Google Scholar]
- 193.Li J., Jia F., Zhang S., et al. The manipulation of substitutional defects for realizing high thermoelectric performance in Mg3Sb2-based Zintl compounds. Journal of Materials Chemistry A. 2019;7(33):19316–19323. doi: 10.1039/c9ta06889a. [DOI] [Google Scholar]
- 194.Mao J., Wu Y., Song S., et al. Defect engineering for realizing high thermoelectric performance in n-type Mg3Sb2-based materials. ACS Energy Letters. 2017;2(10):2245–2250. doi: 10.1021/acsenergylett.7b00742. [DOI] [Google Scholar]
- 195.Gorai P., Ortiz B. R., Toberer E. S., Stevanović V. Investigation of n-type doping strategies for Mg3Sb2. Journal of Materials Chemistry A. 2018;6(28):13806–13815. doi: 10.1039/C8TA03344G. [DOI] [Google Scholar]
- 196.Li J., Zhang S., Zheng S., et al. Defect chemistry for N-type doping of Mg3Sb2-based thermoelectric materials. The Journal of Physical Chemistry C. 2019;123(34):20781–20788. doi: 10.1021/acs.jpcc.9b05859. [DOI] [Google Scholar]
- 197.Wang H., Cao X., Takagiwa Y., Snyder G. J. Higher mobility in bulk semiconductors by separating the dopants from the charge-conducting band – a case study of thermoelectric PbSe. Materials Horizons. 2015;2(3):323–329. doi: 10.1039/C5MH00021A. [DOI] [Google Scholar]
- 198.Zhang Q. H., Huang X. Y., Bai S. Q., Shi X., Uher C., Chen L. D. Thermoelectric devices for power generation: recent progress and future challenges. Advanced Engineering Materials. 2016;18(2):194–213. doi: 10.1002/adem.201500333. [DOI] [Google Scholar]
- 199.Liu W., Jie Q., Kim H. S., Ren Z. Current progress and future challenges in thermoelectric power generation: from materials to devices. Acta Materialia. 2015;87:357–376. doi: 10.1016/j.actamat.2014.12.042. [DOI] [Google Scholar]
- 200.He R., Schierning G., Nielsch K. Thermoelectric devices: a review of devices, architectures, and contact optimization. Advanced Materials Technologies. 2018;3(4) doi: 10.1002/admt.201700256.1700256 [DOI] [Google Scholar]


