Abstract
Trace Amine-Associated Receptors (TAARs) are a family of G protein-coupled receptors that function as odorant receptors in the main olfactory system of vertebrates. TAARs are monoallelically expressed in primary sensory neurons where they couple to the same transduction cascade as canonical olfactory receptors and are mapped onto glomeruli within a specific region of the olfactory bulb. TAARs have a high affinity for volatile amines, a class of chemicals that are generated during the decomposition of proteins and are ubiquitous physiological metabolites that are found in body fluids. Thus, amines are proposed to play an important role in intra- and interspecific communication such as signaling the sex of the conspecific, the quality of the food source, or even the proximity of a predator. TAARs have a crucial role in the perception of these behaviorally relevant compounds as the genetic deletion of all or even individual olfactory TAARs can alter the behavioral response and reduce the sensitivity to amines. The small size of this receptor family combined with the ethological relevance of their ligands, makes the TAARs an attractive model system for probing olfactory perception. This review will summarize the current knowledge on the olfactory TAARs and discuss whether they represent a unique subsystem within the main olfactory system.
Keywords: Olfaction, G protein-coupled receptor (GCPR), olfactory receptor, perception, volatile amines
Introduction
Trace amine-associated receptors (TAARs) are a distinct subfamily of G protein-coupled receptors (GPCRs). Originally discovered in 2001 by two separate groups (Borowsky et al., 2001; Bunzow et al., 2001), these receptors were initially termed “trace amine receptors” as two TAAR members (TA1/TAAR1 and TA2/TAAR4) recognized several endogenous amine compounds termed trace amines (Borowsky et al., 2001; Bunzow et al., 2001; Lindemann and Hoener, 2005). Trace amines are structurally similar to biogenic amines (e.g. serotonin, noradrenaline, adrenaline, dopamine and histamine) but are maintained at lower endogenous tissue concentrations and thus were given the moniker “trace” (Lindemann and Hoener, 2005; Gainetdinov et al., 2018). Further work determined that mammalian Taar genes form a single cluster in the genome and that not all members of this family respond to trace amines, so a new nomenclature was proposed, adding “-associated” to their family name (TAARs) to account for this variation in ligand binding (Lindemann et al., 2005). The identification of this family of receptors generated significant interest as one member (TAAR1) exhibited a high affinity for several psychotropic agents and was expressed in the brain (Borowsky et al., 2001). The function of the remaining members of this family were a mystery.
In 2006, it was discovered that the majority of TAARs (other than TAAR1) have a chemosensory role in the main olfactory system, where they function as odorant receptors with a high affinity to volatile amines (Liberles and Buck, 2006). In terrestrial vertebrates, the main olfactory system is specialized for the detection of volatile chemicals. This diverse and complex set of stimuli is perceived in a combinatorial fashion by canonical odorant receptors (OR), which form the largest multigene family in vertebrates (Buck and Axel, 1991; Touhara and Vosshall, 2009; Niimura, 2012). The large size of the OR gene repertoire and its combinatorial code would seemingly allow animals to detect and discriminate much, if not all, of odor space. However, the persistence of these receptors across vertebrate evolution argues that TAARs occupy a unique niche in odor perception that is not filled by canonical ORs.
This review summarizes the current knowledge on the olfactory TAARs and discusses two central questions. First, what is the unique contribution that this enigmatic receptor family makes to odor perception? Second, whether the TAARs are expressed by neurons that are a separate and functionally distinct subsystem of the main olfactory pathway (e.g. GC-D / ‘necklace’ subsystem) or if they are expressed in a subset of neurons that function in a similar manner to canonical ORs. While this last question may seem semantic, the answer is actually very important, as it determines the applicability of TAAR-related research for addressing fundamental principles of the main olfactory system. In particular, there are several properties (including the small size of the family and the behavioral importance of their ligands) that could make TAARs an attractive model system for probing odor coding. This review will compare TAARs to the canonical OR system to provide a preliminary analysis of the applicability of TAAR-related research to the entire main olfactory system.
TAAR gene family
TAARs are thought to have arisen early in vertebrate evolution as ancestral Taar-like genes are present in lampreys (Libants et al., 2009; Eyun et al., 2016) and appear to have a chemosensory function (Scott et al., 2019). These Taar-like genes of the lamprey lack the typical TAAR motif in the transmembrane α-helix VII that is characteristic of Taar genes present in jawed vertebrates (Hussain et al., 2009; Liberles, 2015; Eyun et al., 2016; Scott et al., 2019; Xu and Li, 2020). Across the species examined, the number of functional Taar genes varies considerably (see Gainetdinov et al., 2018 for a thorough review). In mammals, the highest number of Taars identified thus far is in the flying fox (26), while the fewest is in the bottlenose dolphin (0), which has no functional Taars (Eyun et al., 2016). Mice are known to have 15 Taar (mTaars), while rats have 17 (rTaars), and humans have 6 (hTaars) functional TAAR genes (Liberles and Buck, 2006; Liberles, 2009; Hashiguchi and Nishida, 2007; Saraiva et al., 2015a; Eyun et al., 2016; Saraiva et al., 2019). These small Taar gene repertoires of tetrapods (0-26) are in stark contrast to teleost fishes, which have a greatly expanded the number of Taar genes (12-118) (Hashiguchi and Nishida, 2007; Hussain et al., 2009; Tessarolo et al., 2014; Azzouzi et al., 2015; Eyun et al., 2016; Eyun et al., 2018; Gao et al., 2017; Saraiva et al., 2015b). Another difference is that Taar genes are frequently clustered in a single genomic region in tetrapods, while the Taar genes of teleost fishes are usually located on multiple chromosomes (Hashiguchi and Nishida, 2007; Hussain et al., 2009; Eyun et al., 2016).
Phylogenetic analyses have classified Taar genes into three different clades (Hussain et al., 2009; Ferrero et al., 2012; Li et al., 2015). Mammalian Taars belong to Clade I and II while Clade III is specific to teleost fishes. Clade I (Taar 1-4) is thought to be evolutionarily older, more conserved, evolving under negative evolutionary pressure, and is generally represented by a single isoform in most species (Hussain et al., 2009; Ferrero et al., 2012; Eyun et al., 2016). In contrast Clade II (Taar5-9; E1, M1-3) appears to be evolving under the influence of positive selection pressures and has arisen more recently with multiple species-specific isoforms present (Ferrero et al., 2012; Eyun et al., 2016). Clade III Taars are teleost-specific and have undergone a rapid and more recent evolutionary expansion (Hussain et al., 2009) that may be associated with a change in the ligand binding site (Li et al., 2015).
The TAARs, as well as the canonical ORs and biogenic amine receptors, belong to the Class A (rhodopsin-like) class of GCPRs (Buck and Axel 1991; Borowsky et al., 2001; de March et al., 2015). Although members of the TAAR family have a chemosensory role, they are more structurally similar to biogenic amine receptors than they are to other canonical ORs.
TAAR gene expression
The majority of TAARs are thought to serve a chemosensory function (Liberles and Buck, 2006; Hussain et al., 2009; Horowitz et al., 2014). In their seminal paper, Liberles and Buck (2006) found that 14 of the 15 TAARs in the mouse were expressed in the main olfactory epithelium, where they function as chemosensory receptors. One member of this family (TAAR1) is entirely absent from the olfactory epithelium and is instead expressed in various brain regions as well as several peripheral tissues (see Gainetdinov et al., 2018 for a thorough review). Later work revealed chemosensory roles for TAARs in other species as well (e.g. Scott et al., 2019; Horowitz et al., 2014; Li et al., 2015; Hussain et al., 2013; Ferrero et al., 2012; Ferrero et al., 2011; Wallrabenstein et al., 2013; Staubert et al., 2010; Syed et al., 2015). Each Taar allele defines a unique sensory neuron population that does not express other Taars or canonical odorant receptor (Olfr) genes (Pacifico et al., 2012). Thus, the “one-receptor-one-neuron rule” of canonical ORs appears to be true for TAARs as well. However, TAARs may have a different gene choice mechanism than canonical ORs. The epigenetic heterochromatin signature that is characteristic of silenced Olfr genes is absent in Taar genes (Johnson et al., 2012). In addition, inactive Taar genes are sequestered in a different nuclear compartment than inactive Olfr genes. Olfr genes are usually found at or near large central aggregates of heterochromatin (i.e. chromocenters), while Taars are found with a thin rim of heterochromatin at the nuclear periphery (Yoon et al., 2015). The proposed mechanism for permissible Taar gene expression is a movement into a more interior location escaping the repressive heterochromatin environment in the nuclear periphery (Yoon et al., 2015).
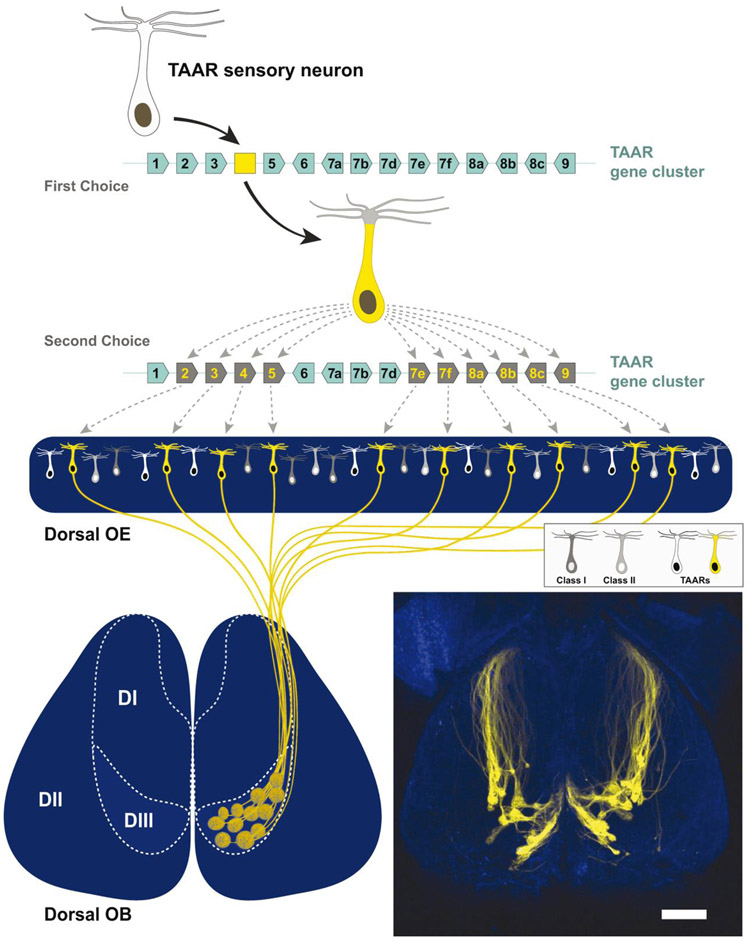
The expressed TAAR proteins are found in the olfactory cilia (the site of odor transduction) and within the axons of olfactory sensory neurons (OSN), where they contribute to axon guidance (Liberles and Buck, 2006; Pacifico et al., 2012; Johnson et al., 2012; Dieris et al., 2017). TAAR expression appears to be limited to a distinct OSN type that is biased to express Taar rather than Olfr genes. One key observation to support this hypothesis is that neurons that initially select a Taar pseudogene (e.g. fluorescent protein or beta-galactosidase) appear to be restricted in their second choice to another Taar allele (Figure 1; Pacifico et al., 2012; Yoon et al., 2015). This second choice preferentially occurs in trans as the removal of olfactory Taars on one chromosome results in a dramatic reduction in the total number of these pseudogene expressing OSNs (Pacifico et al., 2012). This provides evidence that the biased expression of TAARs is not due to a cluster effect. Another key observation to support this ‘TAAR cell type’ hypothesis is that biased expression will even occur in the absence of olfactory Taars on the other chromosome, resulting a doubling of expression of the remaining intact cis Taar genes (Pacifico et al., 2012). TAAR-expressing OSNs appear to be stochastically distributed within specific spatial zones. In the mouse, 10 of the 14 Taars (mTaar2, 3, 4, 5, 7e, 7f, 8a, 8b, 8c, and 9) are specifically expressed in OSNs within the dorsal epithelium, 2 Taars (mTaar7a and 7b) are expressed predominantly in the ventral epithelium, and the remaining Taars (mTaar6 and 7d) are broadly expressed in both zones (Pacifico et al., 2012). Interestingly, Taar4 in the macaque also appears to be broadly expressed in both zones (Horowitz et al., 2014), perhaps highlighting some species-specific expression patterns. The function of these spatial zones is still unknown but may relate to odor absorption (Mozell and Jagodowicz, 1973; Scott et al., 2014) or even innate odor processing (Kobayakawa et al., 2007). In addition, the relative expression of individual TAARs can also vary between species (Saraiva et al., 2019). For example, the most abundantly expressed TAAR in humans and dogs is TAAR5, in macaques and marmosets is TAAR2, in mice is TAAR6 and in rats is TAAR7 (Saraiva et al., 2019). It is tempting to conclude that these differences could result in enhanced sensitivity to specific ethologically relevant odorants. However, it is important to note that increasing the expression of a threshold-setting TAAR (beyond normal levels) does not enhance behavioral sensitivity (Dewan et al., 2018). Thus, while interesting, the perceptual impact of these species-level differences in receptor expression is unknown.
Figure 1.
The TAAR glomerular domain (DIII) is presumably formed by a sensory neuron population that is restricted to express TAARs. Top: Neurons that select a Taar pseudogene (e.g. a yellow fluorescent protein (YFP) that replaced the Taar4 coding sequence) will express YFP before choosing a second receptor. Middle: The expression of a second receptor is biased towards selection of a dorsally expressed Taar gene. Bottom: Both YFP-labeled and unlabeled TAAR-expressing sensory neurons converge to glomeruli in the olfactory bulb according to their expressed receptor. These TAAR glomeruli form a discrete cluster that is distinct from the glomeruli associated with class I (DI) or class II (DII) olfactory receptors. Bottom Right: Dorsal view of a heterozygous ΔT4-YFP mouse showing a cluster of glomeruli (yellow) located in the DIII domain. Scale bar is 500 μm. Figure is modified from Pacifico et al., 2012.
While this review is focused on the chemosensory function of TAARs, it is important to note that many of these so-called olfactory TAARs have also been found outside of the main olfactory epithelium. TAAR expression has been reported in the neonatal Grueneberg ganglion (Fleischer et al., 2007) as well several peripheral tissues and various brain regions at low levels (see Gainetdinov et al., 2018 for a thorough list). In fact, a recent study reported expression of TAAR5 in many brain regions of mice and proposed that this receptor may function to regulate emotional behaviors by modulating the serotonergic system (Espinoza et al., 2020). While intriguing, the gene targeted allele used to report TAAR5 expression retained a neomycin resistance gene selection cassette, which has been shown in previous studies to cause the abnormal gene expression in mTaar5 (Yoon et al., 2015; Dewan and Bozza, unpublished) and other genes (Kim et al., 1992; Fiering et al., 1993; Bouabe and Okkenhaug, 2013). Thus, further work is needed before a clear function can be ascribed to the atypical expression of olfactory TAARs.
Olfactory circuitry of TAARs
Olfactory sensory neurons that express the same TAAR converge to form a medial and lateral glomerulus within the adult mouse olfactory bulb (Pacifico et al., 2012). In the zebrafish, a putative glomerulus corresponding to a single TAAR was also observed in the adult fish (Dieris et al., 2017). However, TAAR-expressing OSNs in the larval zebrafish appear to innervate multiple protoglomeruli, while other OR-expressing OSNs innervate only a single protoglomerulus (Shao et al., 2017). This discrepancy has yet to be resolved but could be due to a developmental delay in the pruning of TAAR axonal projections in the zebrafish. The ontogeny of TAAR-expressing OSNs in the mouse has yet to be described.
The majority of TAAR-expressing OSNs project to a discrete group of glomeruli in the dorsal olfactory bulb of the mouse (Pacifico et al., 2012; Yoon et al., 2015) (Figure 1). This TAAR glomerular domain is distinct from the dorsal glomerular domains associated with either class I or class II canonical ORs (Pacifico et al., 2012). Each of these glomerular domains appears to be formed by a sensory neuron population that is restricted to express the genes of a particular OR class (class I ORs, class II ORs, or TAARs) (Bozza et al., 2009; Pacifico et al., 2012; Yoon et al., 2015). In the zebrafish, known TAAR ligands activate a cluster of neighboring glomeruli, providing some potential evidence for a TAAR domain in teleost fishes as well (Dieris et al., 2017). The function of these glomerular domain for olfactory perception and the innervation pattern of higher order projections from TAAR glomeruli are currently unknown.
TAAR signal transduction cascade
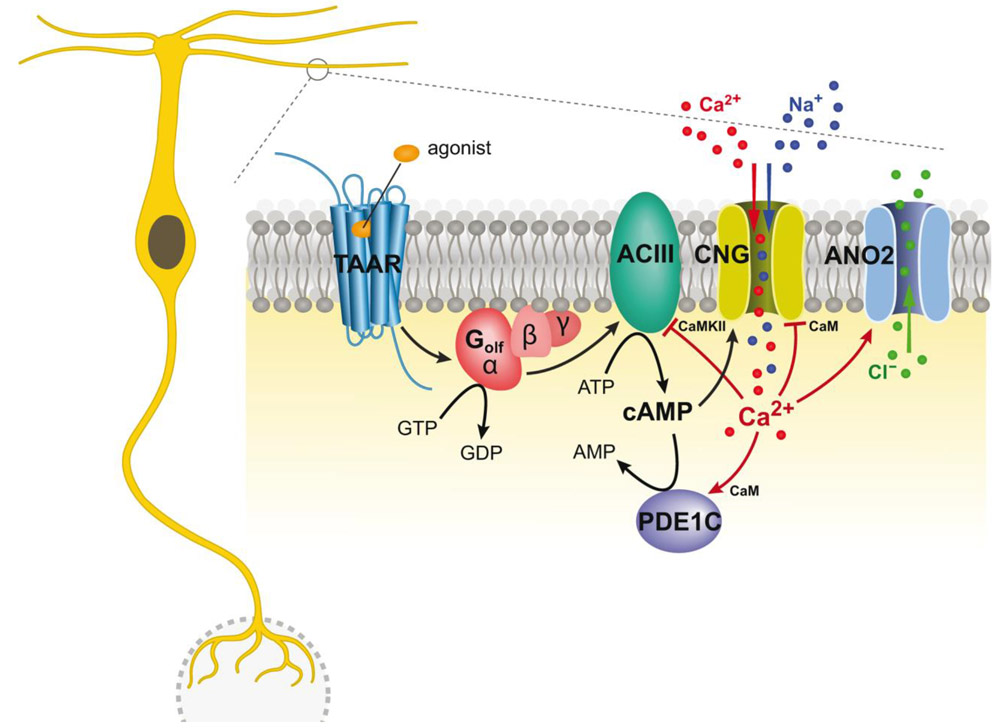
Despite being phylogenetically distinct from canonical ORs, TAARs transduce their respective ligands using the canonical olfactory signal transduction cascade (Figure 2). ORs couple to an olfactory-specific G-protein (Golf) that activates an adenylate cyclase (ACIII). The resulting increase in cAMP opens cyclic nucleotide-gated (CNG) channels (consisting of CNGA2, CNGB1b, and CNGA4 subunits) allowing Na+ and Ca2+ ions to depolarize the neuron and open a calcium-gated chloride channel (e.g. anoctamin 2 or Ano2). Phosphodiesterase 1C (PDE1C) hydrolyzes the cAMP, terminating its action (Bhandawat et al., 2005; Reisert et al., 2005; Kleene, 2008; Stephan et al., 2009; Reisert and Zhao, 2011). TAAR OSNs express the key components of the canonical odorant transduction cascade including Gαolf, ACIII, CNGA2, CNGB1b, PDE1c, and Ano2 (Liberles and Buck, 2006; Zhang et al., 2013). In addition, modulators of this signal transduction cascade including an adenylyl cyclase blocker and a phosphodiesterase inhibitor (that increases intracellular cAMP) modulate the transduction current of TAAR-expressing OSNs (Zhang et al., 2013), while a Gαolf knockout mouse strain eliminated the odor-evoked responses of TAAR-expressing OSNs (Zhang and Bozza, unpublished). It is important to note that not all main olfactory sensory neurons employ this signal transduction cascade (Munger et al., 2009). The guanylyl cyclase (GC-D) neurons of the ‘necklace’ subsystem do not express Golf, ACIII, PDE4a, or CNGA2 and instead employ a very different transduction cascade (Julifs et al., 1997; Meyer et al., 2000; Zufall and Munger, 2010). While the transient receptor potential (TRP) channel expressing OSNs have components of both the canonical signaling transduction cascade and the phospholipase C (PLC) signaling pathway (Lin et al., 2007; Elsaesser et al., 2005). In summary, TAARs are more closely aligned with canonical ORs than other main olfactory subsystems in terms of transducing odorants.
Figure 2.
The TAAR signal transduction cascade. Activation of a TAAR triggers an increase in cyclic adenosine monophosphate (cAMP) through the activation of the receptor coupled G-protein (Golf) and adenyl cyclase type III (ACIII). Activated by the cAMP, cyclic nucleotide-gated channels (CNG) cause a depolarizing influx of both calcium (Ca2+) and sodium (N+) ions. This transient increase in Ca2+ opens a calcium-activated chloride channel anoctamin 2 (ANO2) causing an efflux of chloride (Cl−) ions that further amplifies the response. Phosphodiesterase 1C (PDE1C) hydrolyzes the cAMP, terminating its action.
The recognition motifs of olfactory TAARs and their agonists
The shared homology between biogenic amine receptors and TAARs initially provided some clues to potential TAAR ligands. Biogenic amine receptors bind amines through a key salt bridge that contains an aspartic acid on the third transmembrane α-helix (Asp3.32) (Shi and Javitch, 2002). TAARs share this structural basis for amine recognition as the Asp3.32 residue is retained in clade I and II TAARs (Li et al., 2015; Ferrero et al., 2012). Using an in vitro heterologous system to perform a high-throughput screening of several mouse TAARs, Liberles and Buck (2006) determined that volatile amines are ligands for olfactory TAARs. Additional studies have confirmed this initial finding in many different species groups including rodents (mice, rats, and the northern treeshrew), canines (dog), primates (humans, macaque and ring-tailed lemur), amphibians (Xenopus), teleost fishes (zebrafish), and even in a TAAR-like receptor in the lamprey (Scott et al., 2019; Saraiva et al., 2016; Li et al., 2015; Horowitz et al., 2014; Li et al., 2013; Hussain et al., 2013; Ferrero et al., 2012; Ferrero et al., 2011; Wallrabenstein et al., 2013; Staubert et al., 2010; Zucchi et al., 2006; Zhang et al., 2013; Dewan et al., 2013; Syed et al., 2015). Based on their ligand specificity in vitro, clade I (Taars1-4) and II (Taars5-9) TAARs are referred to as primary or tertiary amine detectors, respectively (Ferrero et al., 2012). For a detailed comparison of the ligand specificity and sensitivity of individual TAARs using in vitro approaches, please see a recent review on TAAR agonists (Xu and Li, 2020).
Analyses of TAAR-expressing sensory neurons are thus far limited to mouse TAAR3-5. These studies have verified many of the agonists determined using heterologous assays, but also observed TAARs to be more broadly tuned and considerably more sensitive (Table 1). For example, cadaverine, N-methyl piperidine, and trimethylamine robustly activate genetically defined TAAR3 and TAAR4-expressing OSNs but fail to activate these receptors in heterologous assays (Zhang et al., 2013; Saraiva et al., 2016; Xu and Li, 2020). In addition, in vivo (and ex-vivo) analyses of TAAR-expressing sensory neurons also yield significantly higher amine sensitivities (i.e. lower thresholds) (Table 1). For example, TAAR4-expressing OSNs are 4-5 orders of magnitude more sensitive to β-phenylethylamine than heterologous assays using the same receptor (Table 1). It is important to note that the increased tuning breadth and sensitivity of TAAR-expressing sensory neurons do not appear to be the result of multiple expressed receptors (Zhang et al., 2013). However, the possibility that multiple TAARs are co-expressed at immature stages cannot be completely ruled out (Tan et al., 2015). Instead these discrepancies are more likely attributed to a variety of factors that differ between the expression of the receptor within their native cell type and heterologous expression in a non-native cell type. Analyses of TAAR-expressing sensory neurons highlight that the terms ‘primary or tertiary amine detectors’ should be used carefully as individual TAARs are relatively broadly tuned and can respond to a variety of different amines depending on the concentration.
Table 1.
Comparison of EC50 values for odorants tested both in vitro and in vivo for mouse TAAR3 and TAAR4
| Odorant | Mouse TAAR3 EC50 (M) |
Mouse TAAR4 EC50 (M) |
|||
|---|---|---|---|---|---|
| Primay Amines | |||||
 |
in vitro | 1.0 x 10 −5 | c | n.r. | c |
| OSN | 1.5 x 10 −8 | a | 1.0 x 10−8 * | a | |
| Glomerulus | 4.1 x 10−10 | b | 9.9 x 10 −9 | b | |
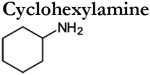 |
in vitro | 7.0 x 10 −6 | c | n.r. | c-d |
| OSN: | 2.7 x 10−7 | a | 7.7 x 10−10 | a | |
| Glomerulus | 5.0 x 10−7* | a | 5.0 x 10−7* | a | |
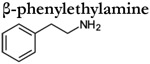 |
in vitro | 4.0 x 10 −5 | c | 7.0 x 10−7 | c |
| OSN: | 1.0 x 10−6* | a | 1.0 x 10−12 | a | |
| Glomerulus | 1.9 x 10−10 | b | 1.4 x 10−11 | b | |
| Tertiay Amines | |||||
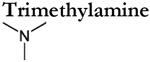 |
in vitro | n.r. | c-e | n.r. | c-e |
| OSN: | n.t | 1.0 x 10−8 * | a | ||
| Glomerulus | 7.9 x 10 −7 | b | 4.7 x 10 −7 | b | |
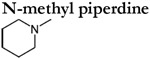 |
in vitro | n.r. | c-e | n.r. | c-e |
| OSN: | 1.0 x 10−6* | a | 5.1 x 10−10 | a | |
| Glomerulus | n.t. | n.t. | |||
| Diamine | |||||
| in vitro | n.r. | c | n.r. | c | |
| OSN: | 1.0 x 10−6* | a | 1.0 x 10−8 * | a | |
| Glomerulus | n.t. | n.t. | |||
Data is from the following studies:
responded at this concentration.
n.r. - no response. n.t. – not tested.
Although many of the teleost-specific clade III TAARs lack the Asp3.32 residue that is characteristic of clade I and II TAARs (and biogenic amine receptors), several still respond to amines (Hussain et al., 2009; Li et al., 2015). These clade III TAARs have retained amine selectivity through a different recognition motif involving an aspartic acid on the fifth transmembrane α-helix (Asp5.42) (Li et al., 2015). Interestingly, there are several clade III TAARs that have both the Asp3.32 and Asp5.42 residues, presumably promoting the detection of diamines (e.g. cadaverine) (Li et al., 2015). Mammalian TAAR6 and TAAR8 have similar residues (Asp3.32 and Asp5.43) and have also been proposed to have high affinities for diamines (Izquierdo et al., 2018; Li et al., 2015). However, mouse TAAR4 and TAAR9 retain the Asp3.32 but lack either Asp5.42 or Asp5.43 and respond to both monamines and diamines (Saraiva et al., 2016; Zhang et al., 2013). Similarly, the TAAR-like receptor in the sea lamprey only has the Asp3.32 residue but binds both polyamines and diamines (Scott et al., 2019). Thus, further work is needed to understand the structural basis of amine selectivity in TAARs.
Homology and mutagenesis experiments in mouse and zebrafish TAARs have identified several key residues that constitute the ligand binding pocket and help infer amine selectivity / sensitivity. Immediately adjacent to the third transmembrane α-helix and in close proximity to the conserved Asp3.32 residue are two key amino acids at position 1323.37 and 1333.38 (Ferrero et al., 2012). Swapping the amino acids at these two locations, altered the ligand responsiveness of TAAR7e to be similar to TAAR7f and vice versa (Ferrero et al., 2012). The sensitivity of zebrafish TAAR13c for cadaverine appears to be dependent upon two different binding sites. A typical binding /activation site that is located within the transmembrane domain cavity and a weaker binding site in the outer vestibule. Interestingly, mutant receptors that lack this outer binding site have enhanced sensitivity for cadaverine (Sharma et al., 2016). This has led to the hypothesis that this outer allosteric binding site limits access to the internal binding/activation site, decreasing cadaverine sensitivity (Sharma et al., 2016; Sharma et al., 2018). It is possible that this ligand gating site is present on other TAARs and could be an evolutionary mechanism to alter the physiological sensitivity towards specific amines.
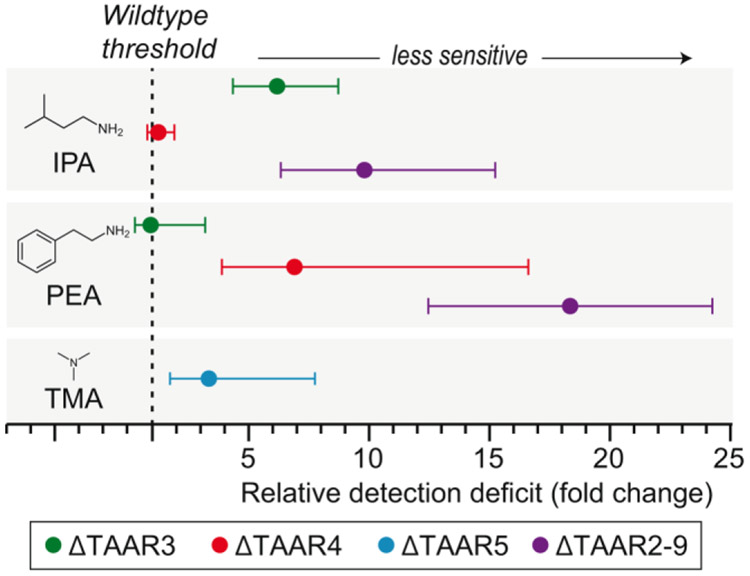
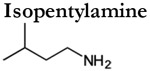
Interestingly, TAARs are not the only olfactory receptors that respond to amines. The genetic deletion of all olfactory TAARs resulted in only a moderate reduction in the behavioral detection of phenylethylamine and isopentylamine, rather than a complete specific anosmia (Figure 3; Dewan et al., 2018). Thus, canonical ORs alone (i.e. in the absence of TAARs) can still support the detect of relatively low concentrations of isopentylamine and phenylethylamine. Accordingly, a large-scale analysis of odor responses in dissociated OSNs identified broadly tuned neurons that respond to both amine and non-amine odorants (Nara et al., 2011). While many of these amine-responsive ORs have not been identified, one canonical OR (MOR256-17) responds to multiple different amines including isopentylamine and phenylethylamine (Tazir et al., 2016). Thus, care should be taken before automatically assigning amine responses or amine-dependent behaviors to the TAARs.
Figure 3.
Removing olfactory TAARs causes detection deficits to amines in a receptor/ligand specific manner. Amine behavioral thresholds were previously determined using a head-fixed Go/ No-Go detection task in several TAAR knockout mouse strains and their respective wildtype littermate controls. Differences in sensitivity attributed to individual or all olfactory TAARs are plotted. Please note that although mice lacking TAAR3 or TAAR4 did not differ in their sensitivity to phenylethylamine (PEA) or isopentylamine (IPA) respectively, these receptors still respond to low concentrations of these odorants. Plots show mean threshold +/− 95% confidence interval. Trimethylamine (TMA). Data from Dewan et al., 2018.
Amines are a behaviorally relevant class of odorants
Amines are produced by the decarboxylation of amino acids and are therefore enriched during the decomposition of proteins and concentrated in certain body fluids. Multiple amines including (but not limited to) isobutylamine, isopentylamine, trimethylamine, cadaverine, putrescine, tyramine, phenylethylamine, and tryptamine are key indicators of the spoilage or putrefaction of meats or fishes (Barger and Walpole 1909; Tarr, 1938; Bai et al., 2019; Veciana-Nogues et al., 1996). In fact, cadaverine and putrescine, two diamines associated with decaying tissue, promoted the burying of conspecifics in rats (Pinel et al., 1981). Thus, for many species several of these amines are highly aversive and may trigger innate avoidance (e.g. Ferrero et al., 2011; Dewan et al., 2013; Li et al., 2013; Hussain et al., 2013; Saraiva et al., 2016).
Volatiles amines are also present in body fluids (e.g. urine and semen) of many animals, where they have been proposed to mediate intra-and interspecific communication. Isopentylamine and isobutylamine are found in male mouse urine and were initially reported to induce puberty in female mice; however, this effect was not replicated (Nishimura et al., 1989; Price and Vandenbergh, 1992). Isobutylamine is also found in female mouse urine, where urinary levels of this compound along with diaminopropane and dimethylamine do not differ according to sex (Harmeier et al., 2018). However, female mice were found to have higher concentrations of diethylamine and cinnamide in their urine than male mice (Harmeier et al., 2018). Trimethylamine also appears to be a sex-specific odor cue as male mice produce approximately 30-fold higher levels than female mice (Li et al., 2013). Humans who suffer from trimethylaminuria (a genetic disease also known as ‘fish malodor syndrome’) also produce abnormally high levels of trimethylamine (excreted in urine, sweat and their breath) and can have serious difficulties in social contexts (Fennema et al., 2016). Lastly, spermine and spermidine are found in the semen of many vertebrates and have been proposed to act as a pheromone for ovulatory lamprey females (Lefevre et al., 2011; Scott et al., 2019). In contrast to the previous examples, β-phenylethylamine is thought to function as a predator-derived odor or kairomone as the urine of carnivorous species is highly enriched with this compound (Ferrero et al., 2011). Consistent with this function, this odorant is highly aversive and alters the exploratory behavior of prey species such as mice and rats (Ferrero et al., 2011; Dewan et al., 2013; Saraiva et al., 2016; Francesconi et al., 2020). In addition, β-phenylethylamine exposure increased the activation of several higher brain regions that are associated with fear, anxiety, panic, and defensive behaviors in rodents and even differed according to sex (Perez-Gomez et al., 2015; Francesconi et al., 2020). It is possible that β-phenylethylamine can also function as an intraspecific cue, since increased urinary levels have been linked to stress in both mice, rats and humans (Grimsby et al., 1997; Paulos and Tessel, 1982; Snoddy et al., 1985). These examples highlight that amines likely play a critical role in various social behavior including sexual attraction, predator avoidance, social communication, the avoidance of rotting food, and generalized aversive responses. Thus, the evolutionary maintenance of receptors that are specialized to bind these compounds (ie TAARs) would seemingly be advantageous for many species.
The role of TAARs in amine perception.
TAARs play a crucial role in the perception of amines (Table 2). Using odor investigation assays, TAARs have been shown to be necessary for the appropriate behavioral response to specific amines. For example, mice lacking all olfactory TAARs (ΔT2-9) fail to avoid several different amines including the volatiles of a predator urine, which is enriched with β-phenylethylamine (Dewan et al., 2013). Likewise, olfactory TAARs are also crucial for male mice to maintain normal levels of investigation towards female urine and isobutylamine (Harmeier et al., 2018). While these two examples highlight the contribution of the entire repertoire of olfactory TAARs, these perceptual changes can even be linked to individual TAARs. The genetic deletion of TAAR5 abolished the attraction to trimethylamine, a putative murine sex specific cue (Li et al., 2013; Saraiva et al., 2016). While the genetic deletion of TAAR4 abolished the aversion to β-phenylethylamine and two different predator urines but not to isopentylamine or N-methyl piperidine, which are also TAAR4 ligands (Dewan et al., 2013; Zhang et al., 2013). Additional studies have provided correlative evidence for the role of individual TAARs in amine investigation. Saraiva et al., (2016) assayed the behavioral response of mice to a suite of TAAR ligands identified in vitro. They observed that individual TAAR ligands could be attractive, aversive, or even neutral and that both attractive and aversive ligands can activate the same TAAR. In the zebrafish, cadaverine activates both TAAR13c and TAAR13d and is known to elicit robust avoidance (Hussain et al., 2013; Li et al., 2015). While in the sea lamprey, the TAAR-like receptor TAAR348 is activated by spermine, a putative sex pheromone that attracts ovulatory females (Scott et al., 2019). The importance of individual TAARs for these species-specific behaviors might suggest that these instinctive odor-responses are part of a genetically programmed (ie ‘hard-wired’) neural circuit. However, the contribution of a single TAAR can be context dependent, as TAAR5 was necessary to neutralize the behavioral response towards a mixture of the highly aversive 2,5-dihydro-2,4,5-trimethylthiazoline (TMT) and the attractive TAAR5-ligand, trimethylamine (Saraiva et al., 2016). In addition, the observation that both attractive and aversive ligands can activate the same TAAR in vitro (Saraiva et al., 2016), provides some further evidence against a hard-wired model.
Table 2.
Summary of the odor-guided behaviors attributed to TAARs
| Receptor | Behavioral Function |
|---|---|
| Mammals | |
| Mouse TAAR3 | Isopentylamine sensitivity (not anosmia)1 |
| Bat TAAR3 | Association between TAAR3 genotype in females and MHC-dependent mate choice in males8++ |
| Mouse TAAR4 | Phenylethylamine sensitivity (not anosmia)1 Aversion to phenylethylamine as well as to puma and lynx urine volatiles2 |
| Rat TAAR4 | Aversion to phenylethylamine15-16++ |
| Mouse TAAR5 | Trimethylamine sensitivity (not anosmia)1 Attraction to trimethylamine3-4 Attraction to n,n,-dimethylethylamine4++ Reduction in anxiety- and depressive-like behaviors5** Decrease in serotonin levels in the brain5** |
| Rat TAAR5 | Trimethylamine aversion 13-14++ |
| Human TAAR5 | Trimethylamine sensitivity (not ansomia)7++ Assessment of spoiled food12++ |
| Mouse TAAR9 | Attraction to spermidine4++ |
| Mouse TAARs2-9 | Phenylethylamine, isopentylamine, and trimethylamine+ sensitivity (not anosmia)1 Aversion to phenylethylamine, isopentylamine, n-methyl piperidine, cadaverine, as well as puma and lynx+ urine volatiles2 Aversion to 2-methylbutylamine4++ Attraction to 3-amino-s-triazole, 1-(2-aminoethyl) piperidine, n,n-dimethylbutylamine, and octylamine4++ Investigation of isobutylamine and female urine by males6 |
| Raccoon TAARs2-9 | Association between the number of TAAR alleles in females and MHC-dependent mate choice9++ |
| Teleost Fishes | |
| Zebrafish TAAR13c | Aversion to cadaverine10++ |
| Jawless Fishes | |
| Lamprey TAAR348 (TAAR-like) | Attraction to spermine11++ |
Several associations are listed where receptor sensitivity and the behavioral effect were determined in different studies.
References:
Wallrabenstein et al., 2015;
Santos et al., 2016;
Santos et al., 2020;
Inferred due to effect in single TAAR deletion animal.
Function is based on correlative evidence
The mouse strain tested has an intact neomycin cassette, which could cause misexpression (e.g. Yoon et al., 2015).
To date, most of the evidence for TAAR-mediated behavioral effects, are species-specific — including predator avoidance, mate attraction, or social communication. The evolutionary maintenance of TAARs across vertebrate evolution would suggest a more universal function. Individual TAARs are highly sensitive to amines. In fact, low concentrations of amines selectively activate TAAR glomeruli in the mouse, without eliciting a response in other dorsal glomeruli (Pacifico et al., 2012; Dewan et al., 2013; Dewan et al., 2018). While the genetic deletion of all olfactory TAARs was able to abolish these high affinity amine responses in the dorsal olfactory bulb (Dewan et al., 2013) and cause amine detection deficits (Figure 3; Dewan et al., 2018). Most surprisingly, these amine thresholds were set by a single TAAR as the genetic deletion of TAAR3, TAAR4, or TAAR5 elicited significant odor detection deficits (but not anosmia) for isopentylamine, phenylethylamine, and trimethylamine, respectively (Dewan et al., 2018). TAAR5 may also be important for trimethylamine detection in humans. Timberol® inhibits trimethylamine activation of TAAR5 in vitro and was found to reduce trimethylamine sensitivity in humans by almost 10 fold (Wallrabenstein et al., 2015). However, the specific anosmia to trimethylamine in humans appears to be unrelated to polymorphisms in the coding sequence of the TAAR5 gene (Wallrabenstein et al., 2013). Interestingly, the genetic deletion of TAAR5 in the mouse also does not cause trimethylamine anosmia, resulting in only a ~4-fold decrease in sensitivity (Dewan et al., 2018). In summary, TAARs may be evolutionarily conserved because they are the most sensitive amine receptors and set the detection threshold of the animal to amines, a biologically relevant class of chemicals.
Comparison with canonical olfactory receptors
Despite their function as chemosensory receptors, members of the TAAR family are more closely related to biogenic amine receptors than any other chemosensory receptor. This has led to the question of whether TAARs feed into a distinct and separate main olfactory subsystem or represent functionally typical olfactory inputs that simply employ a phylogenetically divergent class of receptors? While this question may seem semantic, it is central to the applicability of TAAR-related research for addressing the function of the main olfactory system. Specific examples include the respective roles of the medial vs lateral glomeruli (Sato et al., 2020) or the impact of supernumerary glomeruli on behavioral sensitivity (Dewan et al., 2018). While the answer to this question is still up for debate, the following section will summarize the evidence on both sides of the argument by focusing on research in mice.
In several aspects, TAARs appear to function in a similar manner as canonical ORs. Each Taar allele defines a unique sensory neuron population that has several of the hallmarks of canonical OR expression. These include punctate expression in sparse sensory neurons, distribution within specific spatial zones of the epithelium, trafficking of the protein to the cilia where it binds volatile odorants and coupling to the same transduction cascade (Liberles and Buck, 2006; Pacifico et al., 2012; Johnson et al., 2012; Zhang et al., 2013; Liberles, 2015). OSNs that express an individual TAAR converge to form typical glomeruli within each olfactory bub with the receptor contributing to axon guidance (Pacifico et al., 2012). Lastly, TAARs have an associated dorsal glomerular domain that is presumably formed by an OR class-specific population of sensory neurons (Pacifico et al., 2012; Yoon et al., 2015). It is important to note that these similarities are not shared with all main olfactory sensory neurons. For example, GC-D neurons express a non-GCPR receptor, couple to a different signal transduction cascade, and project to a ring of 12-40 interconnected glomeruli that encircle the caudal olfactory bulb (Julifs et al., 1997; Shinoda et al., 1989; Meyer et al., 2000; Munger et al., 2009; Zufall and Munger, 2010; Greer et al., 2016). Thus, despite their phylogenetic divergence, TAARs appear to function in a manner that is more similar to canonical ORs than other subsystems within the main olfactory system.
Despite their similarities, TAARs also differ from canonical ORs. Firstly, TAARs appear to have at least a slightly different gene choice mechanism as Taar genes lack the epigenetic heterochromatin signature and are sequestered in a different nuclear compartment than Olfr genes (Johnson et al., 2012; Yoon et al., 2015). Secondly, TAAR projections to the bulb form medial and lateral glomeruli that are closer together than is typically observed for dorsal ORs, including a somewhat increased preponderance of fused or supernumerary glomeruli (Pacifico et al., 2012). Lastly, TAARs play a crucial role in various types of social behavior including social communication, mate choice, predator avoidance, sexual identity or receptive signals, and potentially even contribute to the evaluation of food sources (e.g. Ferrero et al., 2011; Dewan et al., 2013; Hussain et al., 2013; Li et al., 2013; Horowitz et al., 2014; Harmeier et al., 2018). However, one could also argue that the ethological importance of the odorant underlies these effects. Accordingly, the photoactivation of a single canonical OR (ofr1019), one of the receptors for the fox odor kairomone, TMT, induces immobility in mice (Saito et al., 2017). However, it should be noted that genetically abolishing this receptor only reduces the freezing behavior, but does not eliminate it (Saito et al., 2017). The innate attraction or aversion to several odorants (e.g. muscone and (Z)-5-tetradecen-1-ol) was attributed to single canonical ORs (Horio et al., 2019). Lastly, a canonical OR (MOR215-1) can set the behavioral sensitivity of the animal (Dewan et al., 2018). Thus, individual canonical ORs can also mediate innate behaviors and determine the behavioral sensitivity of an animal. These results bring into question the uniqueness of TAARs in this respect.
In summary, TAARs share many of the fundamental characteristics of canonical ORs. This preliminary analysis would seemingly lend support for the perspective that we should consider TAARs as functionally typical olfactory inputs that employ a phylogenetically divergent class of receptors. This view is further supported by the fact that both individual canonical ORs and TAARs can significantly impact odor-guided behavioral responses. On the other hand, we do not yet know the importance of the observed differences in the TAAR gene choice mechanism and how TAAR information is integrated and processed within higher cortical regions. Thus, further work is needed before the view that TAARs represent a unique subsystem within the main olfactory system can be dispelled.
Conclusions and Future Perspectives
TAARs occupy a unique niche in the main olfactory system of vertebrates. Their structural similarities to biogenic amine receptors, appears to confer high affinity to amines. As key indicators of spoilage or decay, amines are behaviorally relevant to most species and the ability to detect low levels of these compounds would seemingly be advantageous. In support of this view, TAARs appear to set the behavioral detection threshold of mice (Dewan et al., 2018), perhaps explaining their evolutionary maintenance. However, odor investigation assays in mice attribute larger perceptual consequences to the TAARs than could be explained by the modest detection deficits that have been observed. Thus, it seems possible that TAARs alter amine perception beyond enhanced sensitivity. One possibility is that the TAARs feed into a genetically programmed neural circuit that encodes innate or even species-specific amine responses. In fact, mice born without TAARs are repeatedly exposed to many of these amines after birth (as they are prominent urinary components) but fail to develop normal behavioral responses to these odorants as adults (Dewan et al., 2013; Li et al., 2013; Harmeier et al., 2018). These data provide some evidence against a stochastic neural circuit that is only influenced by odor experience. However, this TAAR neural circuit is also not ‘hard-wired’ as the behavioral response to amines can be context specific (Saraiva et al., 2016) and may modified by learning. Future work on how information from the TAARs is represented in more central projections and the coding logic of amines in olfactory cortical regions would be very informative. In addition, defining differences in cell type specific (i.e. TAAR, class I OR, or class II OR -expressing) OSNs would provide insight into the molecular and functional organization of olfactory inputs. Lastly, only a very few TAARs have been studied in-depth (mouse TAAR3-5 and zebrafish TAAR13c). Thus, additional studies examining TAARs in a wide range of species is needed before a clear function for the TAARs in olfactory signaling and perception will emerge.
Acknowledgements:
I would like to thank Annika Cichy and Thomas Bozza for their input on the manuscript and Charles Badlands for graphic design support.
Funding: This work was supported by National Institute of Health [grant number: DC014565]
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interests: The author has no conflict of interests.
Ethical Approval: This article does not contain any studies with human participants or animals performed by the author.
References
- Azzouzi N, Barloy-Hubler F, Galibert F (2015) Identification and characterization of cichlid TAAR genes and comparison with other teleost TAAR repertoires. BMC genomics 16:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat Y, Reisert J, Yau K (2005) Elementary response of olfactory receptor neurons to odorants. Science 308:1931–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouabe H, Okkenhaug K (2013) Gene targeting in mice: a review. Methods Mol Biol 1064:315–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Adham N, Jones K, Raddatz R, Artymyshyn R, Ogozalek K, Durkin M, Lakhlani P, Bonini J, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa F, Branchek T, Gerald C (2001) Trace amines: identification of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA 98:8966–8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Baker S, Goodrich-Schneider R, Montazeri N, Sarnoski P (2019) Aroma profile characterization of mahi-mahi and tuna for determining spoilage using purge and trap gas chromatography mass spectrometry. J Food Sci 84:481–489. [DOI] [PubMed] [Google Scholar]
- Barger G, Walpole G (1909) Isolation of the pressor principles of putrid meat. J Physiol 38:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, Vassalli A, Fuss S, Zhang J, Weiland B, Pacifico R, Feinstein P, Mombaerts P (2009) Mapping class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron 61:220–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck T, Axel R (1991) A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 65:175–187. [DOI] [PubMed] [Google Scholar]
- Bunzow J, Sonders M, Arttamangkul S, Harrison T, Zhang G, Quigley D, Darland T, Suchland K, Pasumamula S, Kennedy J, Olson S, Magenis R, Amara S, Grandy D (2001) Ampehtamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol pharmacol 60:1181–1188. [DOI] [PubMed] [Google Scholar]
- De March C, Kim S, Antoncazk S, Goddard W, Golebiowski J (2015) G protein-coupled receptors: From sequence to structure. Protein Sci 24:1543–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan A, Pacfico R, Zhan R, Rinberg D, Bozza T (2013) Non-redundant coding of aversive odours in the main olfactory pathway. Nature 497:486–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewan A, Cichy A, Zhang J, Miguel K, Feinstein P, Rinberg D, Bozza T (2018) Single olfactory receptors set odor detection thresholds. Nat Commun 9:2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieris M, Ahuja G, Krishna V, Korsching S (2017) A single identified glomerulus in the zebrafish olfactory bulb carries the high-affinity response to death-associated odor cadaverine. Sci Reports 7:40892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaesser R, Montani G, Tirindelli R, Paysan J (2005) Phosphatidyl-inositide signaling proteins in a novel class of sensory cells in the mammalian olfactory epithelium. Eur J Neurosci 21:2692–2700 [DOI] [PubMed] [Google Scholar]
- Espinoza S, Sukhanov I, Efimova E, Kozlova A, Antonova K, Illiano P, Teo D, Merkulyeva N, Kalinina D, Musienko P, Rocchi A, Mus T, Sotnikova T, Gainetdinov R (2020) Trace amine-associated receptor 5 provides olfactory input into limbic brain areas and modulates emotional behaviors and serotonin transmission. Front Mol Neurosci 13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyun S (2018) Accelerated pseudogenization of trace amine-associated receptors in primates. Genes Brain Behav 18:e12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyun S, Moriyama H, Hoffman F, Moriyama E (2016) Molecular evolution and functional divergence of trace amine-associated receptors. PLOS one 11:e0151023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema D, Phillips I, Shephard E (2016) Trimethylamine and trimethylamine N-Oxide, a flavin-containing monooxygenase 3 (FMOS)-mediated host microbiome metabolic axis implicated in health and disease. Drug Metab Dispos 44:1839–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero D, Lemon J, Fluegge D, Pashkovski S, Korzan W, Datta S, Spehr M, Fendt M, Liberles S (2011) Detection and avoidance of a carnivore odor by prey. PNAS 108:11235–11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero D, Wacker D, Roque M, Baldwin M, Stevens R, and Liberles S. (2012) Agonists for 13 trace amine-associated receptors provide insight into the molecular basis of odor selectivity. ACS Chem Biol. 7:1184–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiering S, Kim C, Epner E, Groudine M (1993) An “in-out” strategy using gene targeting and FLP recombinase for the functional dissection of complex DNA regulatory elements: Analysis of the beta-globin locus control region. Proc Natl Acad Sci USA 90:8469–8473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer J, Schwarzenbacher K, Breer H (2007) Expression of trace amine-associated receptors in the Grueneberg ganglion. Chem Senses 32:623–631. [DOI] [PubMed] [Google Scholar]
- Francesconi J, Macaroy C, Sawant S, Hamrick H, Wahab S, Klein I, McGann J (2018) Sexually dimorphic behavioral and neural responses to a predator scent Behav Brain Res 382:112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Liu S, Yao J, Li N, Yuan Z, Zhou T, Li Q, Liu Z (2017) Genomic organization and evolution of olfactory receptors and trace amine-associated receptors in channel catfish, Ictalurus puncatus. Biochim Biophys Acta 1861:644–651. [DOI] [PubMed] [Google Scholar]
- Gainetdinov R, Hoener M, Berry M (2018) Trace amines and their receptors. Pharmacol Rev 70:549–620. [DOI] [PubMed] [Google Scholar]
- Greer P, Bear D, Lassance J, Bloom M, Tsukhara T, Pashvoski S, Masuda F, Nowlan A, Kirchner R, Hoekstra H, Datta S (2016) A family of non-GPCR chemosensors defines an alternative logic for mammalian olfaction. Cell 165:1734–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman T, Adams J, Karoum F, Gal J, Shih J (1997) Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat Genet 17:206–210. [DOI] [PubMed] [Google Scholar]
- Harmeier A, Meyer C, Staempfli A, Casagrande F, Petrinovic M, Zhang Y, Kunnecke B, Iglesias A, Honer O, Hoener M (2018) How female mice attract males: A urinary volatile amine activates a trace amine-associated receptor that induces male sexual interest. Front Pharmacol 9:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiguchi Y, Nishida M (2007) Evolution of trace amine-associated receptor (TAAR) gene family in vertebrates: Lineage-specific expansions and degradations of a second class of vertebrate chemosensory receptors expressed in olfactory epithelium. Mol. Biol. Evol 24:2099–2107 [DOI] [PubMed] [Google Scholar]
- Horio N, Murata K, Yosikawa K, Yoshihara Y, Touhara K (2019) Contribution of individual olfactory receptors to odor-induced attractive or aversive behavior in mice. Nat Commun 10:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz L, Saraiva L, Kuang D, Yoon K, Buck L (2014) Olfactory receptor patterning in a higher primate. J Neurosci 34:12241–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Saraiva L, Ferrero D, Ahuja G, Krishna V, Liberles S, Korsching S (2013) High affinity olfactory receptor for the death-associated odor cadaverine. PNAS 110:19579–19584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain A, Saraiva L, Korsching S (2009) Positive Darwinian selection and the birth of an olfactory receptor clade in teleosts. PNAS 106:4313–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo C, Gomez-Tamayo J, Nebel J, Pardo L, Gonzalex A (2018) Identifying human diamine sensors for death related putrescine and cadaverine molecules. PLOS Comput Biol 14:e1005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Tsai L, Roy D, Valenzuela D, Mosley C, Magklara A, Lomvardas S, Liberles S, Barnea G (2012) Neurons expressing trace amine-associated receptors project to discrete glomeruli and constitute an olfactory subsystem. PNAS 109:13410–13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juilfs D, Fulle H, Zhao A, Houslay M, Garbers D, Beavo J (1997) A subset of olfactory neurons that selectively express cGMP-stimulated phosphodiesterase (PDE2) and guanylyl cyclase-D define a unique olfactory signal transduction pathway. Proc Natl Acad Sci 94:3388–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Epner E, Forester W, Groudine M (1992) Inactivation of the human beta-globin gene by targeted insertion into the beta-globin locus control region. Genes Dev 6:928–938. [DOI] [PubMed] [Google Scholar]
- Kleene S (2008) The electrochemical basis of odor transduction in vertebrate olfactory cilia. Chem Senses 33:839–859. [DOI] [PubMed] [Google Scholar]
- Kobayakawa K, Kobayakawa R, Matsumoto H, Oka Y, Imai T, Ikawa M, Okabe M, Ikeda T, Itohara S, Kikusui T, Mori K, Sakano S (2007) Innate versus learned odour processing in the mouse olfactory bulb. Nature 450:503–508. [DOI] [PubMed] [Google Scholar]
- Lefevre P, Palin M, Murphy B (2011) Polyamine on the reproductive landscape. Endocr Rev 32:694–712. [DOI] [PubMed] [Google Scholar]
- Li Q, Tachie-Baffour Y, Liu Z, Baldwin M, Kruse A, Liberles S (2015) Non-classical amine recognition evolved in a large clade of olfactory receptors. eLIFE 4:e10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Korzan W, Ferrero D, Chang R, Roy D, Buchi M, Lemon J, Kaur A, Stowers L, Fendt M, Liberles S (2013) Synchronous evolution of an odor biosynthesis pathway and behavioral response. Curr Biol 23:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libants S, Carr K, Wu H, Teeter J, Chung-Davidson Y, Zhang Z, Wilkerson C, Li W (2009) The sea lamprey Petromyzon marinus genome reveals the early origin of several chemosensory receptor families in the vertebrate lineage. BMC Evol. Biol 9:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles S (2015) Trace amine-associated receptors: ligands, neural circuits, and behaviors. Curr. Neurobiol 34:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles S (2009) Trace amine-associated receptors are olfactory receptors in vertebrates. Ann. N.Y. Acad. Sci 1170:168–172. [DOI] [PubMed] [Google Scholar]
- Liberles S Buck L (2006) A second class of chemosensory receptors in the olfactory epithelium. Nature 442:645–650. [DOI] [PubMed] [Google Scholar]
- Lin W, Margolskee R, Donnert G, Hell S, Restrepo D. (2007) Olfactory neurons expressing transient receptor potential channel M5 (TRPM5) are involved in sensing semiochemicals. Proc Natl Acad Sci USA 104:2471–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann L, Hoener M (2005) A renaissance in trace amines inspired by a novel GPCR family. Trends Pharm. Sci 26:274–281. [DOI] [PubMed] [Google Scholar]
- Meyer M, Angele A, Kremmer E, Kaupp U, Mueller F (2000) A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc Natl Acad Sci 97:10595–10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozell M, Jagodowicz M (1973) Chromatographic separation of odorants by the nose: retention times measured across in vivo olfactory mucosa. Science 181:1247–1249. [DOI] [PubMed] [Google Scholar]
- Munger S, Leinders-Zufall T, Zufall F (2009) Subsystem organization of the mammalian sense of smell. Annu Rev Physiol 71:115–140. [DOI] [PubMed] [Google Scholar]
- Nara K, Saraiva L, Ye X, Buck L (2011) A large-scale analysis of odor coding in the olfactory epithelium. J Neurosci 31:9179–9191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimura Y (2012) Olfactory receptor multigene family in vertebrates: From the viewpoint of evolutionary genetics. Curr Genomics 13:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Utsumi K, Yuhara M, Fujitani Y, Iritani A (1989) Identification of puberty-accelerating pheromones in male mouse urine. J Exp Zool 251:300–305. [DOI] [PubMed] [Google Scholar]
- Pacifico R, Dewan A, Cawley D, Guo C, Bozza T (2012) An olfactory subsystem that mediates high sensitivity detection of volatile amines. Cell Rep 2:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulos M, Tessel R (1982) Excretion of beta-phenylethylamine is elevated in humans after profound stress. Science 215:1127–1129. [DOI] [PubMed] [Google Scholar]
- Perez-Gomez A, Bleymehl K, Stein B, Pyrski M, Birnbaumer L, Munger S, Leiders-Zufall T, Zufall F, and Chamero P (2015) Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr Biol 25:1340–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinel E, Gorzalka B, Ladak F (1981) Cadaverine and putrescine initiate the burial od dead conspecifics by rats. Phys Behav 27:819–824. [DOI] [PubMed] [Google Scholar]
- Price M, Vandenbergh J (1992) Analysis of puberty-accelerating pheromones. J Exp Zool 264:42–45. [DOI] [PubMed] [Google Scholar]
- Reisert J, Lai J, Yau K, Bradley J (2005) Mechanism of the excitatory Cl-response in mouse olfactory receptor neurons. Neuron. 45:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Zhao H (2011) Response kinetics of olfactory receptor neurons and the implications in olfactory coding. J Gen Physiol 138:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Nishizumi H, Suzuki S, Matsumoto H, Ieki N, Abe T, Kiyonari H, Morita M, Yokota H, Hirayama N, Yamazaki T, Kikusui T, Mori K, Sakano H (2017) Immobility responses are induced by photoactivation of single glomerular species responsive to fox odour TMT. Nat Commun 8:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva L, Ibarra-Soria X, Klian M, Omura M, Scialdone A, Mombaerts P, Marioni J, Logan D (2015a) Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq. Sci Reports 5:18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva L, Ahuja G, Ivandic I, Syed A, Marioni J, Korsching S, Logan D (2015b) Molecular and neuronal homology between the olfactory systems of zebrafish and mouse. Sci Reports 5:11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva L, Kondoh K, Ye X, Yoon K, Hernandez M, Buck L (2016) Combinatorial effects of odorants on mouse behavior. PNAS 113: E3300–E3306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva L, Riveros-McKay F, Mezzavilla M, Abou-Moussa E, Arayata C, Makhlouf M, Trimmer C, Ibarra-Soria X, Khan M, Van Gervan L, Jorissen M, Gibbs M, O’Flynn C, McGrane S, Mombaerts P, Marioni J, Mainland J, Logan D (2019). A transcriptomic atlas of mammalian olfactory mucosae reveals an evolutionary influence on food odor detection in humans. Sci Adv 5:eaax0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Homma R, Nagayama S (2020) Direct comparison of odor responses of homologous glomeruli in the medial and lateral maps of the mouse olfactory bulb. eNeuro 7:eneuro.0449–19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J, Sherrill L, Jian J, Zhao K (2014) Tuning to odor solubility and sorption pattern in olfactory epithelial responses. J Neurosci 34:2025–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Zhang Z, Jia L, Li K, Zhang Q, Dexheimer T, Ellsworth E, Ren J, Chung-Davidson Y, Zu Y, Neubig R, Li W (2019) Spermine in semen of male sea lamprey acts as a sex pheromone. PLOS Biol 17:e3000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Lakhina V, Cheng R, Marcaccio C, Raper J (2017) Olfactory sensory axons target specific protoglomeruli in the olfactory bulb of zebrafish. Neural Dev 12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Ahuja G, Hussain A, Balfanz S, Baumann A, Korsching S (2016) Elimination of a ligand gating site generates a supersensitive olfactory receptor. Sci Reports 6:28359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Balfanz S, Baumann A, Korsching S (2018) Full rescue of an inactive olfactory receptor mutant by elimination of an allosteric ligand-gating site. Sci Reports 8:9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Javitch J (2002) The binding site of aminergic g protein-coupled receptors: The transmembrane segments and second extracellular loop. Annu Rev Pharmacol Toxicol. 42:437–467. [DOI] [PubMed] [Google Scholar]
- Shinoda K, Shiotani Y, Osawa Y (1989) Necklace olfactory glomeruli: form a unique components of the rat primary olfactory system. J Comp neurol 284:362–373. [DOI] [PubMed] [Google Scholar]
- Snoddy A, Heckathorn D, Tessel R (1985) Cod-restraint stress and urinary endogenous β-phenylethylamine in rats. Pharm Biochem Behav 22:497–500. [DOI] [PubMed] [Google Scholar]
- Staubert C, Boselt I, Bohnekamp J, Rompler H, Enard W, Schoneberg T (2010) Structural and functional evolution of trace amine-associated receptors TAAR3, TAAR4, and TAAR5 in primates. PLOS ONE 56:e11133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan A, Shum E, Hirsh S, Cyngar K, Reisert J, Zhao H (2009) ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci 106:11776–11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed A, Sansone A, Roner S, Nia S, Manzini I, Korsching S (2015) Different expression domains for two closely related amphibian TAARs generate a bimodal distribution similar to neuronal responses to amine odors. Sci Reports 5:13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L, Li Q, Xie X (2015) Olfactory sensory neurons transiently express multiple olfactory receptors during development. Mol Syst Biol 11:844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr H (1938) Trimethylamine formation in relation to the viable bacterial population of spoiling fish muscle. Nature 142:1078. [Google Scholar]
- Tazir B, Khan M, Mombaerts P, Grosmaitre X (2016) The extremely broad odorant response profile of mouse olfactory sensory neurons expressing the odorant receptor MOR256-17 includes trace amine-associated receptor ligands. Eur J Neurosci 43:608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarolo J, Tabesh M, Nesbit M, Davidson W (2014) Genomic organization and evolution of the trace amine-associated receptor (TAAR) repertoire in Atlantic salmon (Salmo salar). G3(Bethesda) 4:1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touhara K, Vosshall L (2009) Sensing odorants and pheromones with chemosensor receptors. Ann Rev Physiol 71:307–332. [DOI] [PubMed] [Google Scholar]
- Veciana-Nogues M, Albala-Hurtado S, Marine-Font A, Vidal-Carou M (1996) Changes in biogenic amines during the manufacture and storage of semipreserved anchovies. J Food Prot 59:1218–1222. [DOI] [PubMed] [Google Scholar]
- Wallrabenstein I, Kuklan J, Weber L, Zborala S, Werner M, Altmuller J, Becker C, Schmidt A, Hatt H, Hummel T (2013) Human trace amine-associated receptor TAAR5 can be activated by trimethylamine. PLoS ONE 8:e54950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Li Q (2020) TAAR agonists. Cell Mol. Neurobio 40:257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K, Ragoczy T, Lu Z, Kondoh K, Kuang D, Groudine M, Buck L (2015) Olfactory receptor genes expressed in distinct lineages are sequestered in different nuclear compartments. Proc Natl Acad Sci 112:E2403–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Pacifico R, Cawley D, Feinstein P, Bozza T (2013) Ultrasensitive detection of amines by a trace amine-associated receptor. J Neurosci 33:3228–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Chiellin G, Scalan T, Grandy D (2006) Trace amine-associated receptors and their ligands. Br J Pharmacol 149:967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall F, Munger S (2010) Receptor guanylyl cyclases in mammalian olfactory function. Mol Cell Biochem 334:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]